User login
Pain in the United States: Time for a Culture Shift in Expectations, Messaging, and Management
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
© 2019 Society of Hospital Medicine
High-Goal ‘Lytes: Repletion Gone Awry?
Electrolyte imbalances, per se, predispose to ventricular ectopy and, in extreme cases, sudden cardiac death.1 As these outcomes are more common in the presence of intrinsic heart disease, serum electrolytes—particularly potassium and magnesium—are routinely monitored and made replete in patients with myocardial infarction (MI) or acute decompensated heart failure (ADHF).
Patients hospitalized with ADHF often present with metabolic derangements and varying degrees of chronic adaptations in their renin–angiotensin–aldosterone system.1,2 In addition, during an ADHF hospitalization, they are subjected to guideline-directed medical therapy (GDMT), commonly in escalating doses, that exhibit well-established effects on serum potassium levels, including diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, beta blockers, and mineralocorticoid receptor antagonists. Thus, there are myriad ways patients hospitalized for ADHF might experience electrolyte abnormalities.
In this issue of the Journal of Hospital Medicine, O’Sullivan et al. explore the associations between mean 72-hour serum potassium and important clinical outcomes—in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS)—among patients with normal admission serum potassium hospitalized for ADHF.3 Through a retrospective review of electronic records from 116 hospitals, the authors identified 4,995 initially normokalemic heart failure (HF; identified by ICD-9 codes) patients and grouped them into low-normal (3.5-4.0 mEq/L), normal (4.0-4.5 mEq/L), and high-normal (4.5-5.0 mEq/L) potassium groups.3 Adjustments were made for composite scores encapsulating other lab abnormalities and comorbidities.
Over the 72-hour exposure window, the authors observed no statistically significant difference in mortality, ICU transfer, or LOS between the low-normal and normal potassium groups.3 Moreover, in a sensitivity analysis of patients who did not receive potassium supplementation, there remained statistically similar rates of mortality, ICU transfer, and LOS.3 Together, these findings suggest that maintenance of potassium >4 mEq/L may not be efficacious for preventing in-hospital complications of ADHF.3 In fact, they observed more frequent mortality and ICU transfer in patients who had high-normal potassium. This group, however, had a higher burden of chronic kidney disease and illness severity on presentation and was less likely to receive supplemental potassium.3
ADHF accounts for more than one million hospital admissions annually with one in four patients readmitted within 30 days; estimated costs surpass $30 billion.2 Reducing unnecessary expenditures in the management of HF through evidence-based guidelines is paramount. Electrolyte repletion in the setting of ADHF may represent one such opportunity by reducing excess phlebotomy, laboratory services, and potassium supplementation. Patient experience may also improve from curbing these cumbersome practices. While society guidelines endorse potassium repletion in MI to reduce the risk of ventricular arrhythmia,4 there is no uniform consensus in ADHF. As the authors cite, existing data regarding ideal potassium levels in patients with ADHF is lacking, with current evidence drawn from small observational studies. The present study, being much larger in size and being linked with observed rates of active potassium supplementation, provides some of the strongest evidence to date that a potassium goal of >4 mEq/L may not be efficacious at reducing ADHF-related complications in the generalized HF population.
While it remains uncertain if avoiding low-normal potassium levels in ADHF is beneficial, over the long term, intermediate-range potassium levels are clearly associated with the lowest HF-related mortality. In a study of over 2,000 HF patients who underwent longitudinal potassium monitoring, mortality was distributed along a U-shaped curve with highest mortality at the extremes of kalemia and a nadir at a level of 4.3 mEq/L.5
A major limitation of the present study is that it does not account for variability within the ADHF population. Firstly, knowledge regarding the use of GDMT, which not only affects serum potassium (all GDMTs) but also reduces the likelihood of arrhythmias (beta blockers), would have been informative. Moreover, the authors do not have access to data regarding incident arrhythmia and instead use ICU admission as a surrogate. In addition, ADHF patients in this study varied greatly in illness severity, ranging from those receiving initial therapy with loop diuretics alone to those requiring augmentation with thiazides and even the use of temporary mechanical circulatory support.3 Escalating loop diuretic or metolazone use not only is associated with increased mortality6 but often results in impressive natriuresis and, potentially dangerous, kaliuresis secondary to the sequential nephron blockade.7 Those who underwent extensive potassium swings in the study may not be appropriately captured using 72-hour serum potassium averages. Additionally, this study did not assess for quantity of diuresis, which is known to affect serum potassium values. It is possible that those with low-normal potassium represent patients who underwent more effective diuresis and therefore were discharged sooner. Adding to the variability, ADHF in this study encompassed both systolic (HF with a reduced ejection fraction) and diastolic (HF with a preserved ejection fraction) HF although, perhaps not surprisingly, there were marked differences in the HF subtype by potassium group—the proportions with only diastolic dysfunction were 37.1%, 39.0%, and 45.8% in the low-normal, normal, and high-normal groups, respectively (P = .0174).3 Given the known heterogeneity between these two HF subtypes,8 particularly with respect to their response to mortality-reducing GDMT,2,8 the results may be significantly confounded.
Relatedly, by excluding initially hypokalemic patients, the authors have lost considerable power and broad generalizability as these patients likely represent those at greatest risk of recurrent hypokalemia and its attendant complications during admission.
This study should be lauded for critically appraising the ubiquitous practice of electrolyte repletion. The authors present compelling preliminary data suggesting that maintenance of potassium >4 mEq/L in the general ADHF population is not efficacious at preventing ADHF complications and, as a corollary, is likely not cost-effective. However, we agree with the authors that a randomized controlled trial will be needed to change clinical practice. Ideally, such a study would account for HF subtype and GDMT use and could compare rates of arrhythmia, AHDF-related death, and all-cause mortality in patients maintained to goal normokalemia (>3.5 mEq/L) versus “high
Disclosures
Dr. Blaha reports grants from NIH, grants from FDA, grants from AHA, grants and personal fees from Amgen Foundation, grants from Aetna Foundation, personal fees from Sanofi, personal fees from Regeneron, and personal fees from Novartis, from Novo Nordisk, and from Bayer, outside the submitted work. Dr. Dudum and Dr. Lahti have nothing to disclose.
1. Packer M, Gottlieb SS, Blum MA. Immediate and long-term pathophysiologic mechanisms underlying the genesis of sudden cardiac death in patients with congestive heart failure. Am J Med. 1987;82(3):4-10. https://doi.org/10.1016/0002-9343(87)90126-4.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019.
3. O’Sullivan KF, Kashef MA, Knee AB, et al. Examining the “Repletion Reflex”: the association between serum potassium and outcomes in hospitalized patients with HF. J Hosp Med. 14(12);729-736. https://doi.org/10.12788/jhm.3270.
4. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110(5):588-636. https://doi.org/10.1161/01.CIR.0000134791.68010.FA
5. Nunez J, Bayes-Genis A, Zannad F, et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
6. Neuberg GW, Miller AB, O’Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144(1):31-38. https://doi.org/10.1067/mhj.2002.123144
7. Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527-1534. https://doi.org/10.1016/j.jacc.2010.06.034.
8. Triposkiadis F, Butler J, Abboud FM, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 40(26):2155-2163. https://doi.org/10.1093/eurheartj/ehz158.
Electrolyte imbalances, per se, predispose to ventricular ectopy and, in extreme cases, sudden cardiac death.1 As these outcomes are more common in the presence of intrinsic heart disease, serum electrolytes—particularly potassium and magnesium—are routinely monitored and made replete in patients with myocardial infarction (MI) or acute decompensated heart failure (ADHF).
Patients hospitalized with ADHF often present with metabolic derangements and varying degrees of chronic adaptations in their renin–angiotensin–aldosterone system.1,2 In addition, during an ADHF hospitalization, they are subjected to guideline-directed medical therapy (GDMT), commonly in escalating doses, that exhibit well-established effects on serum potassium levels, including diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, beta blockers, and mineralocorticoid receptor antagonists. Thus, there are myriad ways patients hospitalized for ADHF might experience electrolyte abnormalities.
In this issue of the Journal of Hospital Medicine, O’Sullivan et al. explore the associations between mean 72-hour serum potassium and important clinical outcomes—in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS)—among patients with normal admission serum potassium hospitalized for ADHF.3 Through a retrospective review of electronic records from 116 hospitals, the authors identified 4,995 initially normokalemic heart failure (HF; identified by ICD-9 codes) patients and grouped them into low-normal (3.5-4.0 mEq/L), normal (4.0-4.5 mEq/L), and high-normal (4.5-5.0 mEq/L) potassium groups.3 Adjustments were made for composite scores encapsulating other lab abnormalities and comorbidities.
Over the 72-hour exposure window, the authors observed no statistically significant difference in mortality, ICU transfer, or LOS between the low-normal and normal potassium groups.3 Moreover, in a sensitivity analysis of patients who did not receive potassium supplementation, there remained statistically similar rates of mortality, ICU transfer, and LOS.3 Together, these findings suggest that maintenance of potassium >4 mEq/L may not be efficacious for preventing in-hospital complications of ADHF.3 In fact, they observed more frequent mortality and ICU transfer in patients who had high-normal potassium. This group, however, had a higher burden of chronic kidney disease and illness severity on presentation and was less likely to receive supplemental potassium.3
ADHF accounts for more than one million hospital admissions annually with one in four patients readmitted within 30 days; estimated costs surpass $30 billion.2 Reducing unnecessary expenditures in the management of HF through evidence-based guidelines is paramount. Electrolyte repletion in the setting of ADHF may represent one such opportunity by reducing excess phlebotomy, laboratory services, and potassium supplementation. Patient experience may also improve from curbing these cumbersome practices. While society guidelines endorse potassium repletion in MI to reduce the risk of ventricular arrhythmia,4 there is no uniform consensus in ADHF. As the authors cite, existing data regarding ideal potassium levels in patients with ADHF is lacking, with current evidence drawn from small observational studies. The present study, being much larger in size and being linked with observed rates of active potassium supplementation, provides some of the strongest evidence to date that a potassium goal of >4 mEq/L may not be efficacious at reducing ADHF-related complications in the generalized HF population.
While it remains uncertain if avoiding low-normal potassium levels in ADHF is beneficial, over the long term, intermediate-range potassium levels are clearly associated with the lowest HF-related mortality. In a study of over 2,000 HF patients who underwent longitudinal potassium monitoring, mortality was distributed along a U-shaped curve with highest mortality at the extremes of kalemia and a nadir at a level of 4.3 mEq/L.5
A major limitation of the present study is that it does not account for variability within the ADHF population. Firstly, knowledge regarding the use of GDMT, which not only affects serum potassium (all GDMTs) but also reduces the likelihood of arrhythmias (beta blockers), would have been informative. Moreover, the authors do not have access to data regarding incident arrhythmia and instead use ICU admission as a surrogate. In addition, ADHF patients in this study varied greatly in illness severity, ranging from those receiving initial therapy with loop diuretics alone to those requiring augmentation with thiazides and even the use of temporary mechanical circulatory support.3 Escalating loop diuretic or metolazone use not only is associated with increased mortality6 but often results in impressive natriuresis and, potentially dangerous, kaliuresis secondary to the sequential nephron blockade.7 Those who underwent extensive potassium swings in the study may not be appropriately captured using 72-hour serum potassium averages. Additionally, this study did not assess for quantity of diuresis, which is known to affect serum potassium values. It is possible that those with low-normal potassium represent patients who underwent more effective diuresis and therefore were discharged sooner. Adding to the variability, ADHF in this study encompassed both systolic (HF with a reduced ejection fraction) and diastolic (HF with a preserved ejection fraction) HF although, perhaps not surprisingly, there were marked differences in the HF subtype by potassium group—the proportions with only diastolic dysfunction were 37.1%, 39.0%, and 45.8% in the low-normal, normal, and high-normal groups, respectively (P = .0174).3 Given the known heterogeneity between these two HF subtypes,8 particularly with respect to their response to mortality-reducing GDMT,2,8 the results may be significantly confounded.
Relatedly, by excluding initially hypokalemic patients, the authors have lost considerable power and broad generalizability as these patients likely represent those at greatest risk of recurrent hypokalemia and its attendant complications during admission.
This study should be lauded for critically appraising the ubiquitous practice of electrolyte repletion. The authors present compelling preliminary data suggesting that maintenance of potassium >4 mEq/L in the general ADHF population is not efficacious at preventing ADHF complications and, as a corollary, is likely not cost-effective. However, we agree with the authors that a randomized controlled trial will be needed to change clinical practice. Ideally, such a study would account for HF subtype and GDMT use and could compare rates of arrhythmia, AHDF-related death, and all-cause mortality in patients maintained to goal normokalemia (>3.5 mEq/L) versus “high
Disclosures
Dr. Blaha reports grants from NIH, grants from FDA, grants from AHA, grants and personal fees from Amgen Foundation, grants from Aetna Foundation, personal fees from Sanofi, personal fees from Regeneron, and personal fees from Novartis, from Novo Nordisk, and from Bayer, outside the submitted work. Dr. Dudum and Dr. Lahti have nothing to disclose.
Electrolyte imbalances, per se, predispose to ventricular ectopy and, in extreme cases, sudden cardiac death.1 As these outcomes are more common in the presence of intrinsic heart disease, serum electrolytes—particularly potassium and magnesium—are routinely monitored and made replete in patients with myocardial infarction (MI) or acute decompensated heart failure (ADHF).
Patients hospitalized with ADHF often present with metabolic derangements and varying degrees of chronic adaptations in their renin–angiotensin–aldosterone system.1,2 In addition, during an ADHF hospitalization, they are subjected to guideline-directed medical therapy (GDMT), commonly in escalating doses, that exhibit well-established effects on serum potassium levels, including diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, beta blockers, and mineralocorticoid receptor antagonists. Thus, there are myriad ways patients hospitalized for ADHF might experience electrolyte abnormalities.
In this issue of the Journal of Hospital Medicine, O’Sullivan et al. explore the associations between mean 72-hour serum potassium and important clinical outcomes—in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS)—among patients with normal admission serum potassium hospitalized for ADHF.3 Through a retrospective review of electronic records from 116 hospitals, the authors identified 4,995 initially normokalemic heart failure (HF; identified by ICD-9 codes) patients and grouped them into low-normal (3.5-4.0 mEq/L), normal (4.0-4.5 mEq/L), and high-normal (4.5-5.0 mEq/L) potassium groups.3 Adjustments were made for composite scores encapsulating other lab abnormalities and comorbidities.
Over the 72-hour exposure window, the authors observed no statistically significant difference in mortality, ICU transfer, or LOS between the low-normal and normal potassium groups.3 Moreover, in a sensitivity analysis of patients who did not receive potassium supplementation, there remained statistically similar rates of mortality, ICU transfer, and LOS.3 Together, these findings suggest that maintenance of potassium >4 mEq/L may not be efficacious for preventing in-hospital complications of ADHF.3 In fact, they observed more frequent mortality and ICU transfer in patients who had high-normal potassium. This group, however, had a higher burden of chronic kidney disease and illness severity on presentation and was less likely to receive supplemental potassium.3
ADHF accounts for more than one million hospital admissions annually with one in four patients readmitted within 30 days; estimated costs surpass $30 billion.2 Reducing unnecessary expenditures in the management of HF through evidence-based guidelines is paramount. Electrolyte repletion in the setting of ADHF may represent one such opportunity by reducing excess phlebotomy, laboratory services, and potassium supplementation. Patient experience may also improve from curbing these cumbersome practices. While society guidelines endorse potassium repletion in MI to reduce the risk of ventricular arrhythmia,4 there is no uniform consensus in ADHF. As the authors cite, existing data regarding ideal potassium levels in patients with ADHF is lacking, with current evidence drawn from small observational studies. The present study, being much larger in size and being linked with observed rates of active potassium supplementation, provides some of the strongest evidence to date that a potassium goal of >4 mEq/L may not be efficacious at reducing ADHF-related complications in the generalized HF population.
While it remains uncertain if avoiding low-normal potassium levels in ADHF is beneficial, over the long term, intermediate-range potassium levels are clearly associated with the lowest HF-related mortality. In a study of over 2,000 HF patients who underwent longitudinal potassium monitoring, mortality was distributed along a U-shaped curve with highest mortality at the extremes of kalemia and a nadir at a level of 4.3 mEq/L.5
A major limitation of the present study is that it does not account for variability within the ADHF population. Firstly, knowledge regarding the use of GDMT, which not only affects serum potassium (all GDMTs) but also reduces the likelihood of arrhythmias (beta blockers), would have been informative. Moreover, the authors do not have access to data regarding incident arrhythmia and instead use ICU admission as a surrogate. In addition, ADHF patients in this study varied greatly in illness severity, ranging from those receiving initial therapy with loop diuretics alone to those requiring augmentation with thiazides and even the use of temporary mechanical circulatory support.3 Escalating loop diuretic or metolazone use not only is associated with increased mortality6 but often results in impressive natriuresis and, potentially dangerous, kaliuresis secondary to the sequential nephron blockade.7 Those who underwent extensive potassium swings in the study may not be appropriately captured using 72-hour serum potassium averages. Additionally, this study did not assess for quantity of diuresis, which is known to affect serum potassium values. It is possible that those with low-normal potassium represent patients who underwent more effective diuresis and therefore were discharged sooner. Adding to the variability, ADHF in this study encompassed both systolic (HF with a reduced ejection fraction) and diastolic (HF with a preserved ejection fraction) HF although, perhaps not surprisingly, there were marked differences in the HF subtype by potassium group—the proportions with only diastolic dysfunction were 37.1%, 39.0%, and 45.8% in the low-normal, normal, and high-normal groups, respectively (P = .0174).3 Given the known heterogeneity between these two HF subtypes,8 particularly with respect to their response to mortality-reducing GDMT,2,8 the results may be significantly confounded.
Relatedly, by excluding initially hypokalemic patients, the authors have lost considerable power and broad generalizability as these patients likely represent those at greatest risk of recurrent hypokalemia and its attendant complications during admission.
This study should be lauded for critically appraising the ubiquitous practice of electrolyte repletion. The authors present compelling preliminary data suggesting that maintenance of potassium >4 mEq/L in the general ADHF population is not efficacious at preventing ADHF complications and, as a corollary, is likely not cost-effective. However, we agree with the authors that a randomized controlled trial will be needed to change clinical practice. Ideally, such a study would account for HF subtype and GDMT use and could compare rates of arrhythmia, AHDF-related death, and all-cause mortality in patients maintained to goal normokalemia (>3.5 mEq/L) versus “high
Disclosures
Dr. Blaha reports grants from NIH, grants from FDA, grants from AHA, grants and personal fees from Amgen Foundation, grants from Aetna Foundation, personal fees from Sanofi, personal fees from Regeneron, and personal fees from Novartis, from Novo Nordisk, and from Bayer, outside the submitted work. Dr. Dudum and Dr. Lahti have nothing to disclose.
1. Packer M, Gottlieb SS, Blum MA. Immediate and long-term pathophysiologic mechanisms underlying the genesis of sudden cardiac death in patients with congestive heart failure. Am J Med. 1987;82(3):4-10. https://doi.org/10.1016/0002-9343(87)90126-4.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019.
3. O’Sullivan KF, Kashef MA, Knee AB, et al. Examining the “Repletion Reflex”: the association between serum potassium and outcomes in hospitalized patients with HF. J Hosp Med. 14(12);729-736. https://doi.org/10.12788/jhm.3270.
4. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110(5):588-636. https://doi.org/10.1161/01.CIR.0000134791.68010.FA
5. Nunez J, Bayes-Genis A, Zannad F, et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
6. Neuberg GW, Miller AB, O’Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144(1):31-38. https://doi.org/10.1067/mhj.2002.123144
7. Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527-1534. https://doi.org/10.1016/j.jacc.2010.06.034.
8. Triposkiadis F, Butler J, Abboud FM, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 40(26):2155-2163. https://doi.org/10.1093/eurheartj/ehz158.
1. Packer M, Gottlieb SS, Blum MA. Immediate and long-term pathophysiologic mechanisms underlying the genesis of sudden cardiac death in patients with congestive heart failure. Am J Med. 1987;82(3):4-10. https://doi.org/10.1016/0002-9343(87)90126-4.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019.
3. O’Sullivan KF, Kashef MA, Knee AB, et al. Examining the “Repletion Reflex”: the association between serum potassium and outcomes in hospitalized patients with HF. J Hosp Med. 14(12);729-736. https://doi.org/10.12788/jhm.3270.
4. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110(5):588-636. https://doi.org/10.1161/01.CIR.0000134791.68010.FA
5. Nunez J, Bayes-Genis A, Zannad F, et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
6. Neuberg GW, Miller AB, O’Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144(1):31-38. https://doi.org/10.1067/mhj.2002.123144
7. Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527-1534. https://doi.org/10.1016/j.jacc.2010.06.034.
8. Triposkiadis F, Butler J, Abboud FM, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 40(26):2155-2163. https://doi.org/10.1093/eurheartj/ehz158.
© 2019 Society of Hospital Medicine
You can observe a lot by watching
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
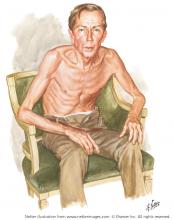
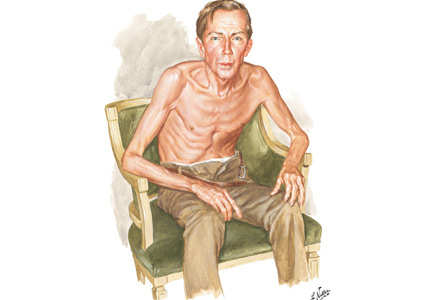
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
If a picture is worth a thousand words, a patient is worth ten thousand
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
Frailty Tools are Not Yet Ready for Prime Time in High-Risk Identification
In this issue of the Journal of Hospital Medicine, McAlister et al.1 compared the ability of the Clinical Frailty Scale (CFS) and the Hospital Frailty Risk Score (HFRS) to predict 30-day readmission or death. The authors prospectively assessed adult patients aged ≥18 years without cognitive impairment being discharged back to the community after medical admissions. They demonstrated only modest overlap in frailty designation between HFRS and CFS and concluded that CFS is better than HFRS for predicting the outcomes of interest.
Before a prediction rule is widely adopted for use in routine practice, robust external validation is needed.2 Factors such as the prevalence of disease in a population, the clinical competencies of a health system, the socioeconomic status, and the ethnicity of the population can all affect how well a clinical rule performs, but may not become apparent until a prospective validation in a different population is attempted.
In developing the HFRS, Gilbert et al. aimed to create a low-cost, highly generalizable method of identifying frailty using International Classification of Diseases (ICD) 10 billing codes.3 The derivation and validation cohorts for HFRS included older adults aged >75 years in the United Kingdom, many of whom had cognitive impairment. Therefore, it is not surprising that the tool behaved very differently in the younger Canadian cohort described by McAlister et al. where persons with cognitive impairment were excluded. That the HFRS had less predictability in the Canadian cohort may simply indicate that it performs better in an older population with cognitive vulnerabilities; given the frailty constructs of the CFS, it may provide less insights in older populations.
We applaud the efforts to find a way to better identify high-risk groups of adults. We also appreciate the increasing attention to function and other frailty-related domains in risk prediction models. Nevertheless, we recommend caution in using any of the many existing frailty indices4 in risk prediction tools unless it is clear what domains of frailty are most relevant for the predicted outcome and what population is the subject of interest.
One of the challenges of choosing an appropriate frailty tool is that different tools are measuring different domains or constructs of frailty. Most consider frailty either as a physical phenotype5 or as a more multifaceted construct with impairments in physical and mental health, function, and social interaction.6 There is often poor overlap between those individuals identified as frail by different measures, highlighting that they are in fact identifying different people within the population studied and have different predictive abilities.
An ideal frailty tool for clinical use would allow clinicians to identify high-risk patients relative to specific outcome(s) in real time prior to discharge from hospital or prior to a sentinel event in the community. CFS can be calculated at the bedside, but HFRS calculation can only be done retrospectively when medical records are coded for claims after discharge. This makes HFRS more suited to research or post hoc quality measure work and CFS more suited to clinical use as the authors describe.
Although using a frailty indicator to help determine those at high risk of early readmission is an important objective, the presence of frailty accounts for only part of a person’s risk for readmission or other untoward events. Reasons for readmissions are complex and often heavily weighted on a lack of social and community supports. A deeper understanding of the reasons for readmission is needed to establish whether readmission of these complex patients has more to do with frailty or other drivers such as poor transitions of care.
The prevalence of frailty will continue to increase as our population ages. Definitions of frailty vary, but there is a broad agreement that frailty, regardless of how it is constructed, increases with age, results in multisystem changes, and leads to increased healthcare utilization and costs. Preventing the development of frailty, identifying frailty, and developing interventions to address frailty in and out of the hospital setting are all vital. We welcome further research regarding the biopsychosocial constructs of frailty, how they overlap with the frailty phenotype, and how these constructs inform both our understanding of frailty and the use of frailty tools.
Disclosures
The authors have no conflicts of interest to report.
1. McAlister FA, Lin M, Bakal JA. Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions. J Hosp Med. 2019;14(7):407-410. doi: 10.12788/jhm.3174 PubMed
2. Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793-799. doi: 10.1056/NEJM198509263131306. PubMed
3. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8. PubMed
4. de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 0.1016/j.arr.2010.09.001. PubMed
5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3);M146-M156. PubMed
6. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10-12. doi: 10.1093/ageing/aft160. PubMed
In this issue of the Journal of Hospital Medicine, McAlister et al.1 compared the ability of the Clinical Frailty Scale (CFS) and the Hospital Frailty Risk Score (HFRS) to predict 30-day readmission or death. The authors prospectively assessed adult patients aged ≥18 years without cognitive impairment being discharged back to the community after medical admissions. They demonstrated only modest overlap in frailty designation between HFRS and CFS and concluded that CFS is better than HFRS for predicting the outcomes of interest.
Before a prediction rule is widely adopted for use in routine practice, robust external validation is needed.2 Factors such as the prevalence of disease in a population, the clinical competencies of a health system, the socioeconomic status, and the ethnicity of the population can all affect how well a clinical rule performs, but may not become apparent until a prospective validation in a different population is attempted.
In developing the HFRS, Gilbert et al. aimed to create a low-cost, highly generalizable method of identifying frailty using International Classification of Diseases (ICD) 10 billing codes.3 The derivation and validation cohorts for HFRS included older adults aged >75 years in the United Kingdom, many of whom had cognitive impairment. Therefore, it is not surprising that the tool behaved very differently in the younger Canadian cohort described by McAlister et al. where persons with cognitive impairment were excluded. That the HFRS had less predictability in the Canadian cohort may simply indicate that it performs better in an older population with cognitive vulnerabilities; given the frailty constructs of the CFS, it may provide less insights in older populations.
We applaud the efforts to find a way to better identify high-risk groups of adults. We also appreciate the increasing attention to function and other frailty-related domains in risk prediction models. Nevertheless, we recommend caution in using any of the many existing frailty indices4 in risk prediction tools unless it is clear what domains of frailty are most relevant for the predicted outcome and what population is the subject of interest.
One of the challenges of choosing an appropriate frailty tool is that different tools are measuring different domains or constructs of frailty. Most consider frailty either as a physical phenotype5 or as a more multifaceted construct with impairments in physical and mental health, function, and social interaction.6 There is often poor overlap between those individuals identified as frail by different measures, highlighting that they are in fact identifying different people within the population studied and have different predictive abilities.
An ideal frailty tool for clinical use would allow clinicians to identify high-risk patients relative to specific outcome(s) in real time prior to discharge from hospital or prior to a sentinel event in the community. CFS can be calculated at the bedside, but HFRS calculation can only be done retrospectively when medical records are coded for claims after discharge. This makes HFRS more suited to research or post hoc quality measure work and CFS more suited to clinical use as the authors describe.
Although using a frailty indicator to help determine those at high risk of early readmission is an important objective, the presence of frailty accounts for only part of a person’s risk for readmission or other untoward events. Reasons for readmissions are complex and often heavily weighted on a lack of social and community supports. A deeper understanding of the reasons for readmission is needed to establish whether readmission of these complex patients has more to do with frailty or other drivers such as poor transitions of care.
The prevalence of frailty will continue to increase as our population ages. Definitions of frailty vary, but there is a broad agreement that frailty, regardless of how it is constructed, increases with age, results in multisystem changes, and leads to increased healthcare utilization and costs. Preventing the development of frailty, identifying frailty, and developing interventions to address frailty in and out of the hospital setting are all vital. We welcome further research regarding the biopsychosocial constructs of frailty, how they overlap with the frailty phenotype, and how these constructs inform both our understanding of frailty and the use of frailty tools.
Disclosures
The authors have no conflicts of interest to report.
In this issue of the Journal of Hospital Medicine, McAlister et al.1 compared the ability of the Clinical Frailty Scale (CFS) and the Hospital Frailty Risk Score (HFRS) to predict 30-day readmission or death. The authors prospectively assessed adult patients aged ≥18 years without cognitive impairment being discharged back to the community after medical admissions. They demonstrated only modest overlap in frailty designation between HFRS and CFS and concluded that CFS is better than HFRS for predicting the outcomes of interest.
Before a prediction rule is widely adopted for use in routine practice, robust external validation is needed.2 Factors such as the prevalence of disease in a population, the clinical competencies of a health system, the socioeconomic status, and the ethnicity of the population can all affect how well a clinical rule performs, but may not become apparent until a prospective validation in a different population is attempted.
In developing the HFRS, Gilbert et al. aimed to create a low-cost, highly generalizable method of identifying frailty using International Classification of Diseases (ICD) 10 billing codes.3 The derivation and validation cohorts for HFRS included older adults aged >75 years in the United Kingdom, many of whom had cognitive impairment. Therefore, it is not surprising that the tool behaved very differently in the younger Canadian cohort described by McAlister et al. where persons with cognitive impairment were excluded. That the HFRS had less predictability in the Canadian cohort may simply indicate that it performs better in an older population with cognitive vulnerabilities; given the frailty constructs of the CFS, it may provide less insights in older populations.
We applaud the efforts to find a way to better identify high-risk groups of adults. We also appreciate the increasing attention to function and other frailty-related domains in risk prediction models. Nevertheless, we recommend caution in using any of the many existing frailty indices4 in risk prediction tools unless it is clear what domains of frailty are most relevant for the predicted outcome and what population is the subject of interest.
One of the challenges of choosing an appropriate frailty tool is that different tools are measuring different domains or constructs of frailty. Most consider frailty either as a physical phenotype5 or as a more multifaceted construct with impairments in physical and mental health, function, and social interaction.6 There is often poor overlap between those individuals identified as frail by different measures, highlighting that they are in fact identifying different people within the population studied and have different predictive abilities.
An ideal frailty tool for clinical use would allow clinicians to identify high-risk patients relative to specific outcome(s) in real time prior to discharge from hospital or prior to a sentinel event in the community. CFS can be calculated at the bedside, but HFRS calculation can only be done retrospectively when medical records are coded for claims after discharge. This makes HFRS more suited to research or post hoc quality measure work and CFS more suited to clinical use as the authors describe.
Although using a frailty indicator to help determine those at high risk of early readmission is an important objective, the presence of frailty accounts for only part of a person’s risk for readmission or other untoward events. Reasons for readmissions are complex and often heavily weighted on a lack of social and community supports. A deeper understanding of the reasons for readmission is needed to establish whether readmission of these complex patients has more to do with frailty or other drivers such as poor transitions of care.
The prevalence of frailty will continue to increase as our population ages. Definitions of frailty vary, but there is a broad agreement that frailty, regardless of how it is constructed, increases with age, results in multisystem changes, and leads to increased healthcare utilization and costs. Preventing the development of frailty, identifying frailty, and developing interventions to address frailty in and out of the hospital setting are all vital. We welcome further research regarding the biopsychosocial constructs of frailty, how they overlap with the frailty phenotype, and how these constructs inform both our understanding of frailty and the use of frailty tools.
Disclosures
The authors have no conflicts of interest to report.
1. McAlister FA, Lin M, Bakal JA. Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions. J Hosp Med. 2019;14(7):407-410. doi: 10.12788/jhm.3174 PubMed
2. Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793-799. doi: 10.1056/NEJM198509263131306. PubMed
3. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8. PubMed
4. de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 0.1016/j.arr.2010.09.001. PubMed
5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3);M146-M156. PubMed
6. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10-12. doi: 10.1093/ageing/aft160. PubMed
1. McAlister FA, Lin M, Bakal JA. Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions. J Hosp Med. 2019;14(7):407-410. doi: 10.12788/jhm.3174 PubMed
2. Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793-799. doi: 10.1056/NEJM198509263131306. PubMed
3. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8. PubMed
4. de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 0.1016/j.arr.2010.09.001. PubMed
5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3);M146-M156. PubMed
6. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10-12. doi: 10.1093/ageing/aft160. PubMed
© 2019 Society of Hospital Medicine
Restarting Anticoagulants after a Gastrointestinal Hemorrhage—Between Rockall and a Hard Place
Anticoagulant use to prevent ischemic strokes in patients with atrial fibrillation (AF) continues to be one of the most challenging decisions facing patients and their physicians, in large part due to significant patient-to-patient variation in both AF-related stroke risk and anticoagulant-associated hemorrhage risk. Now, add a layer of complexity—.how should one approach anticoagulant use following an adverse event such as an acute upper gastrointestinal (GI) hemorrhage? On the one side, the risk of ischemic stroke, and on the other, the risk of recurrent bleeding, either of which can lead to death or disability. Making this decision requires humility, clinical acumen, shared decision-making, and data.
Data on this subject are sparse.1,2 Observational studies show that patients who restart anticoagulants after GI hemorrhage experience fewer ischemic strokes. These studies also show that patients who restart anticoagulant therapy are healthier than those who do not—in measurable ways and, importantly, in unmeasurable ways. Thus far, observational studies have not sufficiently dealt with confounding by indication; that is, patients who restart anticoagulants are fundamentally different than patients who do not.
In this issue of the Journal of Hospital Medicine®, Pappas et al. focus on the optimal timing of resuming oral anticoagulation in patients who have sustained acute upper GI bleeds while receiving oral anticoagulation for AF.3 They use a microsimulation modeling approach to address this question, by creating a synthetic population of patients reflective of age, gender, and comorbidities in a United States population of patients with AF. Using data from epidemiologic studies that describe the risk of rebleeding, hemorrhagic complications, and ischemic stroke as well as the quality of life associated with each of these events, the authors have constructed a decision analytic model to determine the optimal day to restart anticoagulation. This modeling approach mitigates confounding by indication, a limitation of observational studies. They report that the optimal day to restart anticoagulant therapy is in the range of 32-51 days. As one would predict, when using direct-acting anticoagulants and for patients with high stroke risk, the investigators find that restarting therapy earlier is associated with greater benefit. These findings help to untangle a knot of risk and benefits facing patients with AF following an acute GI hemorrhage.
Interpreting the results relies on an understanding of the strengths and weaknesses of simulation modeling and the data used in the analysis. Like any research method, the devil is in the details. Stitching together event rates and outcomes from multiple studies, the results of a simulation model are only as good as the studies the model draws from. In particular, assumptions regarding the time-dependent decline in rebleeding risk are a critical component of determining the optimal time to resume anticoagulation. The authors had to make multiple assumptions to project the 24-hour risk of rebleeding determined from the Rockall score to estimate the risk of rebleeding over the next days to months.4 Consequently, the results are likely overly precise. Practically, 30-50 days or four to eight weeks may better reflect the precision of the study findings.
Results on optimal timing of resuming anticoagulation therapy are most applicable for patients when the decision to restart anticoagulants has already been made. We part ways with the authors in their conclusion that these results confirm that anticoagulants should be restarted. There are multiple appropriate reasons why anticoagulant therapy should not be restarted following an acute upper GI hemorrhage. For example, in observational studies, patients not restarted on anticoagulant therapy were more likely to have a history of falls and to have had severe bleeds.1 Furthermore, patients who do not restart therapy are more likely to die in follow-up. It is tempting to use this fact to support restarting anticoagulants. However, when the causes of death are examined, the vast majority of deaths were unrelated to thrombosis or hemorrhage.2 Patients with AF are older and have multiple comorbidities and life-limiting conditions. Accordingly, the results of this study are better used to engage patients in shared decision-making and contextualized in the broader picture of patients’ health and goals.5
Restarting anticoagulants after a GI hemorrhage is a difficult and high-stakes clinical decision. The study by Pappas et al. uses a simulation model to advance our understanding about the optimal timing to restart anticoagulants. By integrating the dynamic risk of ischemic stroke and recurrent hemorrhage following GI hemorrhage, they estimate the maximal benefit when anticoagulants are restarted between 30 days and 50 days after hemorrhage. The results of their analysis are best used to inform timing among patients where the decision to restart anticoagulants has already been made. The analysis also provides a useful starting point for shared decision-making by highlighting that the optimal net benefit is influenced by patient-to-patient variation in the underlying AF-related stroke risk and anticoagulant-associated rebleeding risk.
Disclosures: Dr. Shah has nothing to disclose. Dr. Eckman reports grants from Heart Rhythm Society/Boehringer-Ingelheim and grants from Bristol-Myers Squibb/Pfizer Education Consortium, outside the submitted work.
1. Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113(4):662-668. doi: 10.1016/j.amjcard.2013.10.044. PubMed
2. Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484-1491. doi: 10.1001/archinternmed.2012.4261. PubMed
3. Pappas MA, Evans N, Rizk MK, Rothberg MB. Resuming anticoagulation following upper gastrointestinal bleeding among patients with nonvalvular atrial fibrillation—a microsimulation analysis. J Hosp Med. 2019;14(7):394-400. doi: 10.12788/jhm.3189. PubMed
4. Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316-321. doi: 10.1136/gut.38.3.316. PubMed
5. Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals–directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1(1):9-10. doi: 10.1001/jamacardio.2015.0248. PubMed
Anticoagulant use to prevent ischemic strokes in patients with atrial fibrillation (AF) continues to be one of the most challenging decisions facing patients and their physicians, in large part due to significant patient-to-patient variation in both AF-related stroke risk and anticoagulant-associated hemorrhage risk. Now, add a layer of complexity—.how should one approach anticoagulant use following an adverse event such as an acute upper gastrointestinal (GI) hemorrhage? On the one side, the risk of ischemic stroke, and on the other, the risk of recurrent bleeding, either of which can lead to death or disability. Making this decision requires humility, clinical acumen, shared decision-making, and data.
Data on this subject are sparse.1,2 Observational studies show that patients who restart anticoagulants after GI hemorrhage experience fewer ischemic strokes. These studies also show that patients who restart anticoagulant therapy are healthier than those who do not—in measurable ways and, importantly, in unmeasurable ways. Thus far, observational studies have not sufficiently dealt with confounding by indication; that is, patients who restart anticoagulants are fundamentally different than patients who do not.
In this issue of the Journal of Hospital Medicine®, Pappas et al. focus on the optimal timing of resuming oral anticoagulation in patients who have sustained acute upper GI bleeds while receiving oral anticoagulation for AF.3 They use a microsimulation modeling approach to address this question, by creating a synthetic population of patients reflective of age, gender, and comorbidities in a United States population of patients with AF. Using data from epidemiologic studies that describe the risk of rebleeding, hemorrhagic complications, and ischemic stroke as well as the quality of life associated with each of these events, the authors have constructed a decision analytic model to determine the optimal day to restart anticoagulation. This modeling approach mitigates confounding by indication, a limitation of observational studies. They report that the optimal day to restart anticoagulant therapy is in the range of 32-51 days. As one would predict, when using direct-acting anticoagulants and for patients with high stroke risk, the investigators find that restarting therapy earlier is associated with greater benefit. These findings help to untangle a knot of risk and benefits facing patients with AF following an acute GI hemorrhage.
Interpreting the results relies on an understanding of the strengths and weaknesses of simulation modeling and the data used in the analysis. Like any research method, the devil is in the details. Stitching together event rates and outcomes from multiple studies, the results of a simulation model are only as good as the studies the model draws from. In particular, assumptions regarding the time-dependent decline in rebleeding risk are a critical component of determining the optimal time to resume anticoagulation. The authors had to make multiple assumptions to project the 24-hour risk of rebleeding determined from the Rockall score to estimate the risk of rebleeding over the next days to months.4 Consequently, the results are likely overly precise. Practically, 30-50 days or four to eight weeks may better reflect the precision of the study findings.
Results on optimal timing of resuming anticoagulation therapy are most applicable for patients when the decision to restart anticoagulants has already been made. We part ways with the authors in their conclusion that these results confirm that anticoagulants should be restarted. There are multiple appropriate reasons why anticoagulant therapy should not be restarted following an acute upper GI hemorrhage. For example, in observational studies, patients not restarted on anticoagulant therapy were more likely to have a history of falls and to have had severe bleeds.1 Furthermore, patients who do not restart therapy are more likely to die in follow-up. It is tempting to use this fact to support restarting anticoagulants. However, when the causes of death are examined, the vast majority of deaths were unrelated to thrombosis or hemorrhage.2 Patients with AF are older and have multiple comorbidities and life-limiting conditions. Accordingly, the results of this study are better used to engage patients in shared decision-making and contextualized in the broader picture of patients’ health and goals.5
Restarting anticoagulants after a GI hemorrhage is a difficult and high-stakes clinical decision. The study by Pappas et al. uses a simulation model to advance our understanding about the optimal timing to restart anticoagulants. By integrating the dynamic risk of ischemic stroke and recurrent hemorrhage following GI hemorrhage, they estimate the maximal benefit when anticoagulants are restarted between 30 days and 50 days after hemorrhage. The results of their analysis are best used to inform timing among patients where the decision to restart anticoagulants has already been made. The analysis also provides a useful starting point for shared decision-making by highlighting that the optimal net benefit is influenced by patient-to-patient variation in the underlying AF-related stroke risk and anticoagulant-associated rebleeding risk.
Disclosures: Dr. Shah has nothing to disclose. Dr. Eckman reports grants from Heart Rhythm Society/Boehringer-Ingelheim and grants from Bristol-Myers Squibb/Pfizer Education Consortium, outside the submitted work.
Anticoagulant use to prevent ischemic strokes in patients with atrial fibrillation (AF) continues to be one of the most challenging decisions facing patients and their physicians, in large part due to significant patient-to-patient variation in both AF-related stroke risk and anticoagulant-associated hemorrhage risk. Now, add a layer of complexity—.how should one approach anticoagulant use following an adverse event such as an acute upper gastrointestinal (GI) hemorrhage? On the one side, the risk of ischemic stroke, and on the other, the risk of recurrent bleeding, either of which can lead to death or disability. Making this decision requires humility, clinical acumen, shared decision-making, and data.
Data on this subject are sparse.1,2 Observational studies show that patients who restart anticoagulants after GI hemorrhage experience fewer ischemic strokes. These studies also show that patients who restart anticoagulant therapy are healthier than those who do not—in measurable ways and, importantly, in unmeasurable ways. Thus far, observational studies have not sufficiently dealt with confounding by indication; that is, patients who restart anticoagulants are fundamentally different than patients who do not.
In this issue of the Journal of Hospital Medicine®, Pappas et al. focus on the optimal timing of resuming oral anticoagulation in patients who have sustained acute upper GI bleeds while receiving oral anticoagulation for AF.3 They use a microsimulation modeling approach to address this question, by creating a synthetic population of patients reflective of age, gender, and comorbidities in a United States population of patients with AF. Using data from epidemiologic studies that describe the risk of rebleeding, hemorrhagic complications, and ischemic stroke as well as the quality of life associated with each of these events, the authors have constructed a decision analytic model to determine the optimal day to restart anticoagulation. This modeling approach mitigates confounding by indication, a limitation of observational studies. They report that the optimal day to restart anticoagulant therapy is in the range of 32-51 days. As one would predict, when using direct-acting anticoagulants and for patients with high stroke risk, the investigators find that restarting therapy earlier is associated with greater benefit. These findings help to untangle a knot of risk and benefits facing patients with AF following an acute GI hemorrhage.
Interpreting the results relies on an understanding of the strengths and weaknesses of simulation modeling and the data used in the analysis. Like any research method, the devil is in the details. Stitching together event rates and outcomes from multiple studies, the results of a simulation model are only as good as the studies the model draws from. In particular, assumptions regarding the time-dependent decline in rebleeding risk are a critical component of determining the optimal time to resume anticoagulation. The authors had to make multiple assumptions to project the 24-hour risk of rebleeding determined from the Rockall score to estimate the risk of rebleeding over the next days to months.4 Consequently, the results are likely overly precise. Practically, 30-50 days or four to eight weeks may better reflect the precision of the study findings.
Results on optimal timing of resuming anticoagulation therapy are most applicable for patients when the decision to restart anticoagulants has already been made. We part ways with the authors in their conclusion that these results confirm that anticoagulants should be restarted. There are multiple appropriate reasons why anticoagulant therapy should not be restarted following an acute upper GI hemorrhage. For example, in observational studies, patients not restarted on anticoagulant therapy were more likely to have a history of falls and to have had severe bleeds.1 Furthermore, patients who do not restart therapy are more likely to die in follow-up. It is tempting to use this fact to support restarting anticoagulants. However, when the causes of death are examined, the vast majority of deaths were unrelated to thrombosis or hemorrhage.2 Patients with AF are older and have multiple comorbidities and life-limiting conditions. Accordingly, the results of this study are better used to engage patients in shared decision-making and contextualized in the broader picture of patients’ health and goals.5
Restarting anticoagulants after a GI hemorrhage is a difficult and high-stakes clinical decision. The study by Pappas et al. uses a simulation model to advance our understanding about the optimal timing to restart anticoagulants. By integrating the dynamic risk of ischemic stroke and recurrent hemorrhage following GI hemorrhage, they estimate the maximal benefit when anticoagulants are restarted between 30 days and 50 days after hemorrhage. The results of their analysis are best used to inform timing among patients where the decision to restart anticoagulants has already been made. The analysis also provides a useful starting point for shared decision-making by highlighting that the optimal net benefit is influenced by patient-to-patient variation in the underlying AF-related stroke risk and anticoagulant-associated rebleeding risk.
Disclosures: Dr. Shah has nothing to disclose. Dr. Eckman reports grants from Heart Rhythm Society/Boehringer-Ingelheim and grants from Bristol-Myers Squibb/Pfizer Education Consortium, outside the submitted work.
1. Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113(4):662-668. doi: 10.1016/j.amjcard.2013.10.044. PubMed
2. Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484-1491. doi: 10.1001/archinternmed.2012.4261. PubMed
3. Pappas MA, Evans N, Rizk MK, Rothberg MB. Resuming anticoagulation following upper gastrointestinal bleeding among patients with nonvalvular atrial fibrillation—a microsimulation analysis. J Hosp Med. 2019;14(7):394-400. doi: 10.12788/jhm.3189. PubMed
4. Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316-321. doi: 10.1136/gut.38.3.316. PubMed
5. Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals–directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1(1):9-10. doi: 10.1001/jamacardio.2015.0248. PubMed
1. Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113(4):662-668. doi: 10.1016/j.amjcard.2013.10.044. PubMed
2. Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484-1491. doi: 10.1001/archinternmed.2012.4261. PubMed
3. Pappas MA, Evans N, Rizk MK, Rothberg MB. Resuming anticoagulation following upper gastrointestinal bleeding among patients with nonvalvular atrial fibrillation—a microsimulation analysis. J Hosp Med. 2019;14(7):394-400. doi: 10.12788/jhm.3189. PubMed
4. Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316-321. doi: 10.1136/gut.38.3.316. PubMed
5. Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals–directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1(1):9-10. doi: 10.1001/jamacardio.2015.0248. PubMed
© 2019 Society of Hospital Medicine
Who Will Guard the Guardians? Preventing Drug Diversion in Hospitals
The patient safety field rightly focuses on identifying and addressing problems with systems of care. From the patient’s perspective, however, underlying systems issues might be less critical than another unspoken question: can I trust the people who are taking care of me? Last year, a popular podcast1 detailed the shocking story of Dallas neurosurgeon Christopher Duntsch, who was responsible for the death of two patients and severe injuries in dozens of other patients over two years. Although fellow surgeons had raised concerns about his surgical skill and professionalism almost immediately after he entered practice, multiple hospitals allowed him to continue operating until the Texas Medical Board revoked his license. Duntsch was ultimately prosecuted, convicted, and sentenced to life imprisonment, in what is believed to be the first case of a physician receiving criminal punishment for malpractice.
Only a small proportion of clinicians repeatedly harm patients as Duntsch did, and the harm they cause accounts for only a small share of the preventable adverse events that patients experience. Understandably, cases of individual clinicians who directly harm patients tend to capture the public’s attention, as they vividly illustrate how vulnerable patients are when they entrust their health to a clinician. As a result, these cases have a significant effect on the patient’s trust in healthcare institutions.
In this issue of the Journal of Hospital Medicine, Fan and colleagues2 describe the problem of controlled-substance diversion in hospitals and review the contributors and potential solutions to this issue. Their thorough and insightful review highlights a growing problem that is probably invisible to most hospitalists. Diversion of controlled substances can happen at any stage of the medication use process, from procurement to disposal and drugs can be diverted by healthcare workers, nonclinical staff, patients, and caregivers. Perhaps most concerning to hospitalists, diversion at the prescribing and administration stages can directly affect patient care. Strategies used to individualize pain control, such as using flexible dose ranges for opioids, can be manipulated to facilitate diversion at the expense of the patient’s suffering.
The review presents a comprehensive summary of safeguards against diversion at each stage of the medication use process and appropriately emphasizes system-level solutions. These include analyzing electronic health record data to identify unusual patterns of controlled substance use and developing dedicated diversion investigation teams. These measures, if implemented, are likely to be effective at reducing the risk of diversion. However, given the complexity of medication use, eliminating this risk is unrealistic. Opioids are used in more than half of all nonsurgical hospital admissions;3 although this proportion may be decreasing due to efforts to curb opioid overprescribing, many hospitalized patients still require opioids or other controlled substances for symptom control. The opportunity to divert controlled substances will always be present.
Eliminating the problem of drug diversion in hospitals will require addressing the individuals who divert controlled substances and strengthening the medication safety system. The term “impaired clinician” is used to describe clinicians who cannot provide competent care due to illness, mental health, or a substance-use disorder. In an influential 2006 commentary,Leape and Fromson made the case that physician performance impairment is often a symptom of underlying disorders, ranging from short-term, reversible issues (eg, an episode of burnout or depression) to long-term problems that can lead to permanent consequences (ie, physical illness or substance-use disorders).4 In this framework, a clinician who diverts controlled substances represents a particularly extreme example of the broader problem of physicians who are unable to perform their professional responsibilities.
Leape and Fromson called for proactively identifying clinicians at risk of performance failure and intervening to remediate or discipline them before patients are harmed. To accomplish this, they envisioned a system with three key characteristics:
- Fairness: All physicians should be subject to regular assessment, and the same standards should be applied to all physicians in the same discipline.
- Objectivity: Performance assessment should be based on objective data.
- Responsiveness: Physicians with performance issues should be identified and given feedback promptly, and provided with opportunities for remediation and assistance when underlying conditions are affecting their performance.
Some progress has been made toward this goal, especially in identifying underlying factors that predispose to performance problems.5 There is also greater awareness of underlying factors that may predispose to more subtle performance deterioration. The recent focus on burnout and well-being among physicians is long overdue, and the recent Charter on Physician Well-Being6 articulates important principles for healthcare organizations to address this epidemic. Substance-use disorder is a recognized risk factor for performance impairment. Physicians have a higher rate of prescription drug abuse and a similar overall rate of substance-use disorders compared to the general population. While there is limited research around the risk factors for drug diversion by physicians, qualitative studies7 of physicians undergoing treatment for substance-use disorders found that most began diverting drugs to manage physical pain, emotional or psychiatric distress, or acutely stressful situations. It is plausible that many burned out or depressed clinicians are turning to illicit substances to self-medicate increasing the risk of diversion.
However, 13 years after Leape and Fromson’s commentary was published, it is difficult to conclude that their vision has been achieved. Objectivity in physician performance assessment is still lacking, and most practicing physicians do not receive any form of regular assessment. This places the onus on members of the healthcare team to identify poorly performing colleagues before patients are harmed. Although nearly all states mandate that physicians report impaired colleagues to either the state medical board or a physician rehabilitation program, healthcare professionals are often reluctant8 to report colleagues with performance issues, and clinicians are also unlikely9 to self-report mental health or substance-use issues due to stigma and fear that their ability to practice may be at risk.
Even when colleagues do raise alarms—as was the case with Dr. Duntsch, who required treatment for a substance-use disorder during residency—existing regulatory mechanisms either lack evidence of effectiveness or are not applied consistently. State licensing boards play a crucial role in identifying problems with clinicians and have the power to authorize remediation or disciplinary measures. However, individual states vary widely10 in their likelihood of disciplining physicians for similar offenses. The board certification process is intended to ensure that only fully competent physicians can practice medicine independently. However, there is little evidence that the certification process ensures that clinicians maintain their skills, and significant controversy has accompanied efforts to revise the maintenance of certification process. The medical malpractice system aims to improve patient safety by ensuring compensation when patients are injured and by deterring substandard clinicians from practicing. Unfortunately, the system often fails to meet this goal, as malpractice claims are rarely filed even when patients are harmed due to negligent care.11
Given the widespread availability of controlled substances in hospitals, comprehensive solutions must incorporate the systems-based solutions proffered by Fan and colleagues and address individual clinicians (and staff) who divert drugs. These clinicians are likely to share some of the same risk factors as clinicians who cannot perform their professional responsibilities for other reasons. Major system changes are necessary to minimize the risk of short-term conditions that could affect physician performance (such as burnout) and develop robust methods to identify clinicians with longer-term issues affecting their performance (such as substance-use disorders).
Although individual clinician performance problems likely account for a small proportion of adverse events, these issues strike at the heart of the physician-patient relationship and have a profound impact on patients’ trust in the healthcare system. Healthcare organizations must maintain transparent and effective processes for addressing performance failures such as drug diversion by clinicians, even if these processes are rarely deployed.
Disclosures
The author does not have any conflict of interest to report.
1. “Dr. Death” (podcast). https://wondery.com/shows/dr-death/. Accessed May 16, 2019.
2. Fan M, Tscheng D, Hyland B, et al. Diversion of controlled drugs in hospitals: a scoping review of contributors and safeguards [published online ahead of printe June 12, 2019]. J Hosp Med. doi: 10.12788/jhm.3228. PubMed
3. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. doi: 10.1002/jhm.2102. PubMed
4. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Intern Med. 2006;144(2):107-115. doi: 10.7326/0003-4819-144-2-200601170-00008. PubMed
5. Studdert DM, Bismark MM, Mello MM, et al. Prevalence and characteristics of physicians prone to malpractice claims. N Engl J Med. 2016;374(4):354-362. doi: 10.1056/nejmsa1506137. PubMed
6. Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319(15):1541-1542. doi: 10.1001/jama.2018.1331. PubMed
7. Merlo LJ, Singhakant S, Cummings SM, Cottler LB. Reasons for misuse of prescription medication among physicians undergoing monitoring by a physician health program. J Addict Med. 2013;7(5):349-353. doi: 10.1097/adm.0b013e31829da074. PubMed
8. DesRoches CM, Fromson JA, Rao SR, et al. Physicians’ perceptions, preparedness for reporting, and experiences related to impaired and incompetent colleagues. JAMA. 2010;304(2):187-193. doi: 10.1001/jama.2010.921. PubMed
9. Samuel L. Doctors fear mental health disclosure could jeopardize their licenses. STAT. October 16, 2017. https://www.statnews.com/2017/10/16/doctors-mental-health-licenses/. Accessed May 16, 2019.
10. Harris JA, Byhoff E. Variations by the state in physician disciplinary actions by US medical licensure boards. BMJ Qual Saf. 2017;26(3):200-208. doi:10.1136/bmjqs-2015-004974. PubMed
11. Studdert DM, Thomas EJ, Burstin HR, et al. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. doi:10.1097/00005650-200003000-00002. PubMed
The patient safety field rightly focuses on identifying and addressing problems with systems of care. From the patient’s perspective, however, underlying systems issues might be less critical than another unspoken question: can I trust the people who are taking care of me? Last year, a popular podcast1 detailed the shocking story of Dallas neurosurgeon Christopher Duntsch, who was responsible for the death of two patients and severe injuries in dozens of other patients over two years. Although fellow surgeons had raised concerns about his surgical skill and professionalism almost immediately after he entered practice, multiple hospitals allowed him to continue operating until the Texas Medical Board revoked his license. Duntsch was ultimately prosecuted, convicted, and sentenced to life imprisonment, in what is believed to be the first case of a physician receiving criminal punishment for malpractice.
Only a small proportion of clinicians repeatedly harm patients as Duntsch did, and the harm they cause accounts for only a small share of the preventable adverse events that patients experience. Understandably, cases of individual clinicians who directly harm patients tend to capture the public’s attention, as they vividly illustrate how vulnerable patients are when they entrust their health to a clinician. As a result, these cases have a significant effect on the patient’s trust in healthcare institutions.
In this issue of the Journal of Hospital Medicine, Fan and colleagues2 describe the problem of controlled-substance diversion in hospitals and review the contributors and potential solutions to this issue. Their thorough and insightful review highlights a growing problem that is probably invisible to most hospitalists. Diversion of controlled substances can happen at any stage of the medication use process, from procurement to disposal and drugs can be diverted by healthcare workers, nonclinical staff, patients, and caregivers. Perhaps most concerning to hospitalists, diversion at the prescribing and administration stages can directly affect patient care. Strategies used to individualize pain control, such as using flexible dose ranges for opioids, can be manipulated to facilitate diversion at the expense of the patient’s suffering.
The review presents a comprehensive summary of safeguards against diversion at each stage of the medication use process and appropriately emphasizes system-level solutions. These include analyzing electronic health record data to identify unusual patterns of controlled substance use and developing dedicated diversion investigation teams. These measures, if implemented, are likely to be effective at reducing the risk of diversion. However, given the complexity of medication use, eliminating this risk is unrealistic. Opioids are used in more than half of all nonsurgical hospital admissions;3 although this proportion may be decreasing due to efforts to curb opioid overprescribing, many hospitalized patients still require opioids or other controlled substances for symptom control. The opportunity to divert controlled substances will always be present.
Eliminating the problem of drug diversion in hospitals will require addressing the individuals who divert controlled substances and strengthening the medication safety system. The term “impaired clinician” is used to describe clinicians who cannot provide competent care due to illness, mental health, or a substance-use disorder. In an influential 2006 commentary,Leape and Fromson made the case that physician performance impairment is often a symptom of underlying disorders, ranging from short-term, reversible issues (eg, an episode of burnout or depression) to long-term problems that can lead to permanent consequences (ie, physical illness or substance-use disorders).4 In this framework, a clinician who diverts controlled substances represents a particularly extreme example of the broader problem of physicians who are unable to perform their professional responsibilities.
Leape and Fromson called for proactively identifying clinicians at risk of performance failure and intervening to remediate or discipline them before patients are harmed. To accomplish this, they envisioned a system with three key characteristics:
- Fairness: All physicians should be subject to regular assessment, and the same standards should be applied to all physicians in the same discipline.
- Objectivity: Performance assessment should be based on objective data.
- Responsiveness: Physicians with performance issues should be identified and given feedback promptly, and provided with opportunities for remediation and assistance when underlying conditions are affecting their performance.
Some progress has been made toward this goal, especially in identifying underlying factors that predispose to performance problems.5 There is also greater awareness of underlying factors that may predispose to more subtle performance deterioration. The recent focus on burnout and well-being among physicians is long overdue, and the recent Charter on Physician Well-Being6 articulates important principles for healthcare organizations to address this epidemic. Substance-use disorder is a recognized risk factor for performance impairment. Physicians have a higher rate of prescription drug abuse and a similar overall rate of substance-use disorders compared to the general population. While there is limited research around the risk factors for drug diversion by physicians, qualitative studies7 of physicians undergoing treatment for substance-use disorders found that most began diverting drugs to manage physical pain, emotional or psychiatric distress, or acutely stressful situations. It is plausible that many burned out or depressed clinicians are turning to illicit substances to self-medicate increasing the risk of diversion.
However, 13 years after Leape and Fromson’s commentary was published, it is difficult to conclude that their vision has been achieved. Objectivity in physician performance assessment is still lacking, and most practicing physicians do not receive any form of regular assessment. This places the onus on members of the healthcare team to identify poorly performing colleagues before patients are harmed. Although nearly all states mandate that physicians report impaired colleagues to either the state medical board or a physician rehabilitation program, healthcare professionals are often reluctant8 to report colleagues with performance issues, and clinicians are also unlikely9 to self-report mental health or substance-use issues due to stigma and fear that their ability to practice may be at risk.
Even when colleagues do raise alarms—as was the case with Dr. Duntsch, who required treatment for a substance-use disorder during residency—existing regulatory mechanisms either lack evidence of effectiveness or are not applied consistently. State licensing boards play a crucial role in identifying problems with clinicians and have the power to authorize remediation or disciplinary measures. However, individual states vary widely10 in their likelihood of disciplining physicians for similar offenses. The board certification process is intended to ensure that only fully competent physicians can practice medicine independently. However, there is little evidence that the certification process ensures that clinicians maintain their skills, and significant controversy has accompanied efforts to revise the maintenance of certification process. The medical malpractice system aims to improve patient safety by ensuring compensation when patients are injured and by deterring substandard clinicians from practicing. Unfortunately, the system often fails to meet this goal, as malpractice claims are rarely filed even when patients are harmed due to negligent care.11
Given the widespread availability of controlled substances in hospitals, comprehensive solutions must incorporate the systems-based solutions proffered by Fan and colleagues and address individual clinicians (and staff) who divert drugs. These clinicians are likely to share some of the same risk factors as clinicians who cannot perform their professional responsibilities for other reasons. Major system changes are necessary to minimize the risk of short-term conditions that could affect physician performance (such as burnout) and develop robust methods to identify clinicians with longer-term issues affecting their performance (such as substance-use disorders).
Although individual clinician performance problems likely account for a small proportion of adverse events, these issues strike at the heart of the physician-patient relationship and have a profound impact on patients’ trust in the healthcare system. Healthcare organizations must maintain transparent and effective processes for addressing performance failures such as drug diversion by clinicians, even if these processes are rarely deployed.
Disclosures
The author does not have any conflict of interest to report.
The patient safety field rightly focuses on identifying and addressing problems with systems of care. From the patient’s perspective, however, underlying systems issues might be less critical than another unspoken question: can I trust the people who are taking care of me? Last year, a popular podcast1 detailed the shocking story of Dallas neurosurgeon Christopher Duntsch, who was responsible for the death of two patients and severe injuries in dozens of other patients over two years. Although fellow surgeons had raised concerns about his surgical skill and professionalism almost immediately after he entered practice, multiple hospitals allowed him to continue operating until the Texas Medical Board revoked his license. Duntsch was ultimately prosecuted, convicted, and sentenced to life imprisonment, in what is believed to be the first case of a physician receiving criminal punishment for malpractice.
Only a small proportion of clinicians repeatedly harm patients as Duntsch did, and the harm they cause accounts for only a small share of the preventable adverse events that patients experience. Understandably, cases of individual clinicians who directly harm patients tend to capture the public’s attention, as they vividly illustrate how vulnerable patients are when they entrust their health to a clinician. As a result, these cases have a significant effect on the patient’s trust in healthcare institutions.
In this issue of the Journal of Hospital Medicine, Fan and colleagues2 describe the problem of controlled-substance diversion in hospitals and review the contributors and potential solutions to this issue. Their thorough and insightful review highlights a growing problem that is probably invisible to most hospitalists. Diversion of controlled substances can happen at any stage of the medication use process, from procurement to disposal and drugs can be diverted by healthcare workers, nonclinical staff, patients, and caregivers. Perhaps most concerning to hospitalists, diversion at the prescribing and administration stages can directly affect patient care. Strategies used to individualize pain control, such as using flexible dose ranges for opioids, can be manipulated to facilitate diversion at the expense of the patient’s suffering.
The review presents a comprehensive summary of safeguards against diversion at each stage of the medication use process and appropriately emphasizes system-level solutions. These include analyzing electronic health record data to identify unusual patterns of controlled substance use and developing dedicated diversion investigation teams. These measures, if implemented, are likely to be effective at reducing the risk of diversion. However, given the complexity of medication use, eliminating this risk is unrealistic. Opioids are used in more than half of all nonsurgical hospital admissions;3 although this proportion may be decreasing due to efforts to curb opioid overprescribing, many hospitalized patients still require opioids or other controlled substances for symptom control. The opportunity to divert controlled substances will always be present.
Eliminating the problem of drug diversion in hospitals will require addressing the individuals who divert controlled substances and strengthening the medication safety system. The term “impaired clinician” is used to describe clinicians who cannot provide competent care due to illness, mental health, or a substance-use disorder. In an influential 2006 commentary,Leape and Fromson made the case that physician performance impairment is often a symptom of underlying disorders, ranging from short-term, reversible issues (eg, an episode of burnout or depression) to long-term problems that can lead to permanent consequences (ie, physical illness or substance-use disorders).4 In this framework, a clinician who diverts controlled substances represents a particularly extreme example of the broader problem of physicians who are unable to perform their professional responsibilities.
Leape and Fromson called for proactively identifying clinicians at risk of performance failure and intervening to remediate or discipline them before patients are harmed. To accomplish this, they envisioned a system with three key characteristics:
- Fairness: All physicians should be subject to regular assessment, and the same standards should be applied to all physicians in the same discipline.
- Objectivity: Performance assessment should be based on objective data.
- Responsiveness: Physicians with performance issues should be identified and given feedback promptly, and provided with opportunities for remediation and assistance when underlying conditions are affecting their performance.
Some progress has been made toward this goal, especially in identifying underlying factors that predispose to performance problems.5 There is also greater awareness of underlying factors that may predispose to more subtle performance deterioration. The recent focus on burnout and well-being among physicians is long overdue, and the recent Charter on Physician Well-Being6 articulates important principles for healthcare organizations to address this epidemic. Substance-use disorder is a recognized risk factor for performance impairment. Physicians have a higher rate of prescription drug abuse and a similar overall rate of substance-use disorders compared to the general population. While there is limited research around the risk factors for drug diversion by physicians, qualitative studies7 of physicians undergoing treatment for substance-use disorders found that most began diverting drugs to manage physical pain, emotional or psychiatric distress, or acutely stressful situations. It is plausible that many burned out or depressed clinicians are turning to illicit substances to self-medicate increasing the risk of diversion.
However, 13 years after Leape and Fromson’s commentary was published, it is difficult to conclude that their vision has been achieved. Objectivity in physician performance assessment is still lacking, and most practicing physicians do not receive any form of regular assessment. This places the onus on members of the healthcare team to identify poorly performing colleagues before patients are harmed. Although nearly all states mandate that physicians report impaired colleagues to either the state medical board or a physician rehabilitation program, healthcare professionals are often reluctant8 to report colleagues with performance issues, and clinicians are also unlikely9 to self-report mental health or substance-use issues due to stigma and fear that their ability to practice may be at risk.
Even when colleagues do raise alarms—as was the case with Dr. Duntsch, who required treatment for a substance-use disorder during residency—existing regulatory mechanisms either lack evidence of effectiveness or are not applied consistently. State licensing boards play a crucial role in identifying problems with clinicians and have the power to authorize remediation or disciplinary measures. However, individual states vary widely10 in their likelihood of disciplining physicians for similar offenses. The board certification process is intended to ensure that only fully competent physicians can practice medicine independently. However, there is little evidence that the certification process ensures that clinicians maintain their skills, and significant controversy has accompanied efforts to revise the maintenance of certification process. The medical malpractice system aims to improve patient safety by ensuring compensation when patients are injured and by deterring substandard clinicians from practicing. Unfortunately, the system often fails to meet this goal, as malpractice claims are rarely filed even when patients are harmed due to negligent care.11
Given the widespread availability of controlled substances in hospitals, comprehensive solutions must incorporate the systems-based solutions proffered by Fan and colleagues and address individual clinicians (and staff) who divert drugs. These clinicians are likely to share some of the same risk factors as clinicians who cannot perform their professional responsibilities for other reasons. Major system changes are necessary to minimize the risk of short-term conditions that could affect physician performance (such as burnout) and develop robust methods to identify clinicians with longer-term issues affecting their performance (such as substance-use disorders).
Although individual clinician performance problems likely account for a small proportion of adverse events, these issues strike at the heart of the physician-patient relationship and have a profound impact on patients’ trust in the healthcare system. Healthcare organizations must maintain transparent and effective processes for addressing performance failures such as drug diversion by clinicians, even if these processes are rarely deployed.
Disclosures
The author does not have any conflict of interest to report.
1. “Dr. Death” (podcast). https://wondery.com/shows/dr-death/. Accessed May 16, 2019.
2. Fan M, Tscheng D, Hyland B, et al. Diversion of controlled drugs in hospitals: a scoping review of contributors and safeguards [published online ahead of printe June 12, 2019]. J Hosp Med. doi: 10.12788/jhm.3228. PubMed
3. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. doi: 10.1002/jhm.2102. PubMed
4. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Intern Med. 2006;144(2):107-115. doi: 10.7326/0003-4819-144-2-200601170-00008. PubMed
5. Studdert DM, Bismark MM, Mello MM, et al. Prevalence and characteristics of physicians prone to malpractice claims. N Engl J Med. 2016;374(4):354-362. doi: 10.1056/nejmsa1506137. PubMed
6. Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319(15):1541-1542. doi: 10.1001/jama.2018.1331. PubMed
7. Merlo LJ, Singhakant S, Cummings SM, Cottler LB. Reasons for misuse of prescription medication among physicians undergoing monitoring by a physician health program. J Addict Med. 2013;7(5):349-353. doi: 10.1097/adm.0b013e31829da074. PubMed
8. DesRoches CM, Fromson JA, Rao SR, et al. Physicians’ perceptions, preparedness for reporting, and experiences related to impaired and incompetent colleagues. JAMA. 2010;304(2):187-193. doi: 10.1001/jama.2010.921. PubMed
9. Samuel L. Doctors fear mental health disclosure could jeopardize their licenses. STAT. October 16, 2017. https://www.statnews.com/2017/10/16/doctors-mental-health-licenses/. Accessed May 16, 2019.
10. Harris JA, Byhoff E. Variations by the state in physician disciplinary actions by US medical licensure boards. BMJ Qual Saf. 2017;26(3):200-208. doi:10.1136/bmjqs-2015-004974. PubMed
11. Studdert DM, Thomas EJ, Burstin HR, et al. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. doi:10.1097/00005650-200003000-00002. PubMed
1. “Dr. Death” (podcast). https://wondery.com/shows/dr-death/. Accessed May 16, 2019.
2. Fan M, Tscheng D, Hyland B, et al. Diversion of controlled drugs in hospitals: a scoping review of contributors and safeguards [published online ahead of printe June 12, 2019]. J Hosp Med. doi: 10.12788/jhm.3228. PubMed
3. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. doi: 10.1002/jhm.2102. PubMed
4. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Intern Med. 2006;144(2):107-115. doi: 10.7326/0003-4819-144-2-200601170-00008. PubMed
5. Studdert DM, Bismark MM, Mello MM, et al. Prevalence and characteristics of physicians prone to malpractice claims. N Engl J Med. 2016;374(4):354-362. doi: 10.1056/nejmsa1506137. PubMed
6. Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319(15):1541-1542. doi: 10.1001/jama.2018.1331. PubMed
7. Merlo LJ, Singhakant S, Cummings SM, Cottler LB. Reasons for misuse of prescription medication among physicians undergoing monitoring by a physician health program. J Addict Med. 2013;7(5):349-353. doi: 10.1097/adm.0b013e31829da074. PubMed
8. DesRoches CM, Fromson JA, Rao SR, et al. Physicians’ perceptions, preparedness for reporting, and experiences related to impaired and incompetent colleagues. JAMA. 2010;304(2):187-193. doi: 10.1001/jama.2010.921. PubMed
9. Samuel L. Doctors fear mental health disclosure could jeopardize their licenses. STAT. October 16, 2017. https://www.statnews.com/2017/10/16/doctors-mental-health-licenses/. Accessed May 16, 2019.
10. Harris JA, Byhoff E. Variations by the state in physician disciplinary actions by US medical licensure boards. BMJ Qual Saf. 2017;26(3):200-208. doi:10.1136/bmjqs-2015-004974. PubMed
11. Studdert DM, Thomas EJ, Burstin HR, et al. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. doi:10.1097/00005650-200003000-00002. PubMed
© 2019 Society of Hospital Medicine
Ultrasound Guidance for Lumbar Puncture: A Consideration, Not an Obligation
Recognizing the increasingly important role of point-of-care ultrasound (POCUS) in advancing clinical care, the Society of Hospital Medicine (SHM) has published a valuable series of position statements to guide hospitalists and administrators on the safe and effective use of POCUS.1 In this issue of the Journal of Hospital Medicine, Soni et al. present a series of consensus-based recommendations on ultrasound guidance for lumbar puncture (LP).2 Among these are the recommendations that ultrasound “should be used” to map the lumbar spine and to select an appropriate puncture site to reduce insertion attempts, reduce needle redirections, and increase overall procedural success.
At first glance, the recommendations appear definitive. However, not immediately obvious is the authors’ clarification that “This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP.” Even with the authors’ caveat, this nuance may not be readily apparent to the readers who review only the executive summary of the guidelines or who omit the context provided in the background of the position statement.
The directive language of this position statement may be a result of an unmerited amplification. The SHM POCUS Task Force employed the Research and Development Appropriateness Method to quantify the degree of consensus and the strength of the recommendation assigned,3 reaching “very good” consensus for each of the recommendations espoused in its position statement. Procedurally, this implies that ≥80% of the 27 voting members rated each published recommendation statement as “appropriate”. Using wording assigned a priori by the committee to each level of consensus, appropriateness became magnified to the declaration “should be used”. In this manner, the strength of the recommendations in this position statement is not necessarily based on the experts’ convictions related to ultrasound-guided LP, nor the strength of the supporting evidence.
In the case of ultrasound-guided LP, we might choose different descriptors than “appropriate” or “should be used”. The evidence base for ultrasound guidance for LP, though growing, may be insufficient as a foundation to a position statement and is certainly insufficient to create a new standard of care for hospitalists. Although the SHM POCUS Task Force completed a thoughtful literature review, no systematic approach (eg, GRADE methodology4) was used to rate the quality of evidence. Furthermore, the literature reviewed was drawn predominantly from anesthesia and emergency medicine sources—not readily generalizable to the hospitalist. Notably, these studies examined all neuraxial procedures (most commonly epidural and spinal anesthesia), which employ different techniques and tools than LP and are performed by clinicians with vastly different procedural training backgrounds than most hospitalists. Altogether, this creates the potential for a gap between true evidence quality and the strength of recommendation.
At a high level, although the technique for ultrasound mapping of the lumbar spine may be similar, the use of ultrasound has been less well studied specifically for LP. When considering LP alone, the available literature is inadequate to recommend uniform ultrasound guidance. A 2018 meta-analysis by Gottlieb et al. included 12 studies focusing only on LP, totaling N = 957 patients.5 This showed some favorability of ultrasound guidance, with a success rate of 90% using ultrasound, 81.4% with a landmark-based approach, and an odds ratio of 2.22 favoring ultrasound guidance (95% CI: 1.03-4.77). Unfortunately, when focusing only on adult patients, the advantage of POCUS diminished, with 91.4% success in the ultrasound group, 87.7% success in the landmark group, and a nonsignificant odds ratio of 2.10 (95% CI: 0.66-7.44).
Unequivocally, POCUS has established itself as a transformative technology for the guidance of invasive bedside procedures, bringing increased procedural success, improved safety, and decreased complication rates.6 For some procedures, particularly central venous catheterization, ultrasound guidance is a clear standard of care.7,8 For LP, the greatest benefit has been observed in patients with anticipated procedural challenges, most commonly obese patients in whom landmarks are not easily palpable.9 Moreover, the harms ultrasound seeks to prevent are substantially different. The primary risk of deferring ultrasound guidance for LP is most often a failed procedure, whereas for other common ultrasound-guided procedures, the harms may include significant vascular injury, pneumothorax, or bowel perforation. Differences in the relative harms make risk-benefit assessments harder to quantify and studies harder to carry out.
Sonographic guidance for LP has a role in clinical practice and should always be considered. However, at present, there exist no guidelines in any other specialty regarding the routine use of ultrasound-guided LP, including anesthesia, emergency medicine, neurology, or interventional radiology.10-15 As a result, a conservative interpretation of the POCUS Task Force’s findings would be to consider the use of ultrasound guidance for LP in patients where landmark identification is particularly challenging, but not to consider it a standard requirement for accreditation, training, or practice as of yet. Saying “more studies are required” can be a cop-out in some cases, but in this situation, the old adage does seem to apply.
We have great respect for the work of the SHM POCUS Task Force in advancing the use of POCUS in hospital medicine. Though ultrasound is not currently mandated as a care standard for the performance of LP, we all can agree that POCUS does confer advantages for this procedure, particularly in a well-selected patient population. To continue to provide care of the highest quality, hospitalists must be encouraged to elevate their practice with POCUS and be supported with the equipment, training, credentialing, and quality assurance structures necessary to integrate bedside ultrasound safely and effectively into their diagnostic and procedural practice.
Disclosures
No conflicts of interest to disclose.
Funding
None.
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3079.
2. Soni NJ, Franco-Sadud R, Dobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
3. Fitch, K, Bernstein SJ, Aguilar MD et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation, 2001.
4. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;334(7650):924-926. PubMed
5. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
6. Moore CL, Copel JA. Point of care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
7. Shojania K, Duncan B, McDonald K, Wachter RM. Making health care safer: a critical analysis of patient safety practices. Rockville, MD: Agency for Healthcare Research and Quality, 2001. Evidence Report/Technology Assessment No. 43; AHRQ publication 01-E058. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catherization. Cochrane Database Syst Rev. 2015;Art. No.: 1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
9. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
11. Neal JM, Brull R, Horn JL, et al. The Second American Society of Regional Anesthesia and Pain Medicine Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia: executive summary. Reg Anesth Pain Med. 2016;41(2):181-194. doi: 10.1097/AAP.0000000000000331.
12. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270-300. doi: 10.1097/ALN.0000000000000935.
13. Engelborghs S, Sebastiaan E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111-126. doi: 10.1016/j.dadm.2017.04.007.
14. American College of Radiology. ACR-SPR-SRU Practice Parameter for the Performing and Interpreting Diagnostic Ultrasound Examinations. 2017; Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-perf-interpret.pdf. Accessed April 15, 2019.
15. American College of Radiology. ACR-AIUM-SPR-SRU Practice Parameter for the Performance of an Ultrasound Examination of the Neonatal and Infant Spine. 2016/ Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-NeonatalSpine.pdf. Accessed April 15, 2019.
Recognizing the increasingly important role of point-of-care ultrasound (POCUS) in advancing clinical care, the Society of Hospital Medicine (SHM) has published a valuable series of position statements to guide hospitalists and administrators on the safe and effective use of POCUS.1 In this issue of the Journal of Hospital Medicine, Soni et al. present a series of consensus-based recommendations on ultrasound guidance for lumbar puncture (LP).2 Among these are the recommendations that ultrasound “should be used” to map the lumbar spine and to select an appropriate puncture site to reduce insertion attempts, reduce needle redirections, and increase overall procedural success.
At first glance, the recommendations appear definitive. However, not immediately obvious is the authors’ clarification that “This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP.” Even with the authors’ caveat, this nuance may not be readily apparent to the readers who review only the executive summary of the guidelines or who omit the context provided in the background of the position statement.
The directive language of this position statement may be a result of an unmerited amplification. The SHM POCUS Task Force employed the Research and Development Appropriateness Method to quantify the degree of consensus and the strength of the recommendation assigned,3 reaching “very good” consensus for each of the recommendations espoused in its position statement. Procedurally, this implies that ≥80% of the 27 voting members rated each published recommendation statement as “appropriate”. Using wording assigned a priori by the committee to each level of consensus, appropriateness became magnified to the declaration “should be used”. In this manner, the strength of the recommendations in this position statement is not necessarily based on the experts’ convictions related to ultrasound-guided LP, nor the strength of the supporting evidence.
In the case of ultrasound-guided LP, we might choose different descriptors than “appropriate” or “should be used”. The evidence base for ultrasound guidance for LP, though growing, may be insufficient as a foundation to a position statement and is certainly insufficient to create a new standard of care for hospitalists. Although the SHM POCUS Task Force completed a thoughtful literature review, no systematic approach (eg, GRADE methodology4) was used to rate the quality of evidence. Furthermore, the literature reviewed was drawn predominantly from anesthesia and emergency medicine sources—not readily generalizable to the hospitalist. Notably, these studies examined all neuraxial procedures (most commonly epidural and spinal anesthesia), which employ different techniques and tools than LP and are performed by clinicians with vastly different procedural training backgrounds than most hospitalists. Altogether, this creates the potential for a gap between true evidence quality and the strength of recommendation.
At a high level, although the technique for ultrasound mapping of the lumbar spine may be similar, the use of ultrasound has been less well studied specifically for LP. When considering LP alone, the available literature is inadequate to recommend uniform ultrasound guidance. A 2018 meta-analysis by Gottlieb et al. included 12 studies focusing only on LP, totaling N = 957 patients.5 This showed some favorability of ultrasound guidance, with a success rate of 90% using ultrasound, 81.4% with a landmark-based approach, and an odds ratio of 2.22 favoring ultrasound guidance (95% CI: 1.03-4.77). Unfortunately, when focusing only on adult patients, the advantage of POCUS diminished, with 91.4% success in the ultrasound group, 87.7% success in the landmark group, and a nonsignificant odds ratio of 2.10 (95% CI: 0.66-7.44).
Unequivocally, POCUS has established itself as a transformative technology for the guidance of invasive bedside procedures, bringing increased procedural success, improved safety, and decreased complication rates.6 For some procedures, particularly central venous catheterization, ultrasound guidance is a clear standard of care.7,8 For LP, the greatest benefit has been observed in patients with anticipated procedural challenges, most commonly obese patients in whom landmarks are not easily palpable.9 Moreover, the harms ultrasound seeks to prevent are substantially different. The primary risk of deferring ultrasound guidance for LP is most often a failed procedure, whereas for other common ultrasound-guided procedures, the harms may include significant vascular injury, pneumothorax, or bowel perforation. Differences in the relative harms make risk-benefit assessments harder to quantify and studies harder to carry out.
Sonographic guidance for LP has a role in clinical practice and should always be considered. However, at present, there exist no guidelines in any other specialty regarding the routine use of ultrasound-guided LP, including anesthesia, emergency medicine, neurology, or interventional radiology.10-15 As a result, a conservative interpretation of the POCUS Task Force’s findings would be to consider the use of ultrasound guidance for LP in patients where landmark identification is particularly challenging, but not to consider it a standard requirement for accreditation, training, or practice as of yet. Saying “more studies are required” can be a cop-out in some cases, but in this situation, the old adage does seem to apply.
We have great respect for the work of the SHM POCUS Task Force in advancing the use of POCUS in hospital medicine. Though ultrasound is not currently mandated as a care standard for the performance of LP, we all can agree that POCUS does confer advantages for this procedure, particularly in a well-selected patient population. To continue to provide care of the highest quality, hospitalists must be encouraged to elevate their practice with POCUS and be supported with the equipment, training, credentialing, and quality assurance structures necessary to integrate bedside ultrasound safely and effectively into their diagnostic and procedural practice.
Disclosures
No conflicts of interest to disclose.
Funding
None.
Recognizing the increasingly important role of point-of-care ultrasound (POCUS) in advancing clinical care, the Society of Hospital Medicine (SHM) has published a valuable series of position statements to guide hospitalists and administrators on the safe and effective use of POCUS.1 In this issue of the Journal of Hospital Medicine, Soni et al. present a series of consensus-based recommendations on ultrasound guidance for lumbar puncture (LP).2 Among these are the recommendations that ultrasound “should be used” to map the lumbar spine and to select an appropriate puncture site to reduce insertion attempts, reduce needle redirections, and increase overall procedural success.
At first glance, the recommendations appear definitive. However, not immediately obvious is the authors’ clarification that “This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP.” Even with the authors’ caveat, this nuance may not be readily apparent to the readers who review only the executive summary of the guidelines or who omit the context provided in the background of the position statement.
The directive language of this position statement may be a result of an unmerited amplification. The SHM POCUS Task Force employed the Research and Development Appropriateness Method to quantify the degree of consensus and the strength of the recommendation assigned,3 reaching “very good” consensus for each of the recommendations espoused in its position statement. Procedurally, this implies that ≥80% of the 27 voting members rated each published recommendation statement as “appropriate”. Using wording assigned a priori by the committee to each level of consensus, appropriateness became magnified to the declaration “should be used”. In this manner, the strength of the recommendations in this position statement is not necessarily based on the experts’ convictions related to ultrasound-guided LP, nor the strength of the supporting evidence.
In the case of ultrasound-guided LP, we might choose different descriptors than “appropriate” or “should be used”. The evidence base for ultrasound guidance for LP, though growing, may be insufficient as a foundation to a position statement and is certainly insufficient to create a new standard of care for hospitalists. Although the SHM POCUS Task Force completed a thoughtful literature review, no systematic approach (eg, GRADE methodology4) was used to rate the quality of evidence. Furthermore, the literature reviewed was drawn predominantly from anesthesia and emergency medicine sources—not readily generalizable to the hospitalist. Notably, these studies examined all neuraxial procedures (most commonly epidural and spinal anesthesia), which employ different techniques and tools than LP and are performed by clinicians with vastly different procedural training backgrounds than most hospitalists. Altogether, this creates the potential for a gap between true evidence quality and the strength of recommendation.
At a high level, although the technique for ultrasound mapping of the lumbar spine may be similar, the use of ultrasound has been less well studied specifically for LP. When considering LP alone, the available literature is inadequate to recommend uniform ultrasound guidance. A 2018 meta-analysis by Gottlieb et al. included 12 studies focusing only on LP, totaling N = 957 patients.5 This showed some favorability of ultrasound guidance, with a success rate of 90% using ultrasound, 81.4% with a landmark-based approach, and an odds ratio of 2.22 favoring ultrasound guidance (95% CI: 1.03-4.77). Unfortunately, when focusing only on adult patients, the advantage of POCUS diminished, with 91.4% success in the ultrasound group, 87.7% success in the landmark group, and a nonsignificant odds ratio of 2.10 (95% CI: 0.66-7.44).
Unequivocally, POCUS has established itself as a transformative technology for the guidance of invasive bedside procedures, bringing increased procedural success, improved safety, and decreased complication rates.6 For some procedures, particularly central venous catheterization, ultrasound guidance is a clear standard of care.7,8 For LP, the greatest benefit has been observed in patients with anticipated procedural challenges, most commonly obese patients in whom landmarks are not easily palpable.9 Moreover, the harms ultrasound seeks to prevent are substantially different. The primary risk of deferring ultrasound guidance for LP is most often a failed procedure, whereas for other common ultrasound-guided procedures, the harms may include significant vascular injury, pneumothorax, or bowel perforation. Differences in the relative harms make risk-benefit assessments harder to quantify and studies harder to carry out.
Sonographic guidance for LP has a role in clinical practice and should always be considered. However, at present, there exist no guidelines in any other specialty regarding the routine use of ultrasound-guided LP, including anesthesia, emergency medicine, neurology, or interventional radiology.10-15 As a result, a conservative interpretation of the POCUS Task Force’s findings would be to consider the use of ultrasound guidance for LP in patients where landmark identification is particularly challenging, but not to consider it a standard requirement for accreditation, training, or practice as of yet. Saying “more studies are required” can be a cop-out in some cases, but in this situation, the old adage does seem to apply.
We have great respect for the work of the SHM POCUS Task Force in advancing the use of POCUS in hospital medicine. Though ultrasound is not currently mandated as a care standard for the performance of LP, we all can agree that POCUS does confer advantages for this procedure, particularly in a well-selected patient population. To continue to provide care of the highest quality, hospitalists must be encouraged to elevate their practice with POCUS and be supported with the equipment, training, credentialing, and quality assurance structures necessary to integrate bedside ultrasound safely and effectively into their diagnostic and procedural practice.
Disclosures
No conflicts of interest to disclose.
Funding
None.
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3079.
2. Soni NJ, Franco-Sadud R, Dobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
3. Fitch, K, Bernstein SJ, Aguilar MD et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation, 2001.
4. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;334(7650):924-926. PubMed
5. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
6. Moore CL, Copel JA. Point of care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
7. Shojania K, Duncan B, McDonald K, Wachter RM. Making health care safer: a critical analysis of patient safety practices. Rockville, MD: Agency for Healthcare Research and Quality, 2001. Evidence Report/Technology Assessment No. 43; AHRQ publication 01-E058. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catherization. Cochrane Database Syst Rev. 2015;Art. No.: 1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
9. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
11. Neal JM, Brull R, Horn JL, et al. The Second American Society of Regional Anesthesia and Pain Medicine Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia: executive summary. Reg Anesth Pain Med. 2016;41(2):181-194. doi: 10.1097/AAP.0000000000000331.
12. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270-300. doi: 10.1097/ALN.0000000000000935.
13. Engelborghs S, Sebastiaan E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111-126. doi: 10.1016/j.dadm.2017.04.007.
14. American College of Radiology. ACR-SPR-SRU Practice Parameter for the Performing and Interpreting Diagnostic Ultrasound Examinations. 2017; Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-perf-interpret.pdf. Accessed April 15, 2019.
15. American College of Radiology. ACR-AIUM-SPR-SRU Practice Parameter for the Performance of an Ultrasound Examination of the Neonatal and Infant Spine. 2016/ Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-NeonatalSpine.pdf. Accessed April 15, 2019.
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3079.
2. Soni NJ, Franco-Sadud R, Dobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
3. Fitch, K, Bernstein SJ, Aguilar MD et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation, 2001.
4. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;334(7650):924-926. PubMed
5. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
6. Moore CL, Copel JA. Point of care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
7. Shojania K, Duncan B, McDonald K, Wachter RM. Making health care safer: a critical analysis of patient safety practices. Rockville, MD: Agency for Healthcare Research and Quality, 2001. Evidence Report/Technology Assessment No. 43; AHRQ publication 01-E058. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catherization. Cochrane Database Syst Rev. 2015;Art. No.: 1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
9. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
11. Neal JM, Brull R, Horn JL, et al. The Second American Society of Regional Anesthesia and Pain Medicine Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia: executive summary. Reg Anesth Pain Med. 2016;41(2):181-194. doi: 10.1097/AAP.0000000000000331.
12. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270-300. doi: 10.1097/ALN.0000000000000935.
13. Engelborghs S, Sebastiaan E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111-126. doi: 10.1016/j.dadm.2017.04.007.
14. American College of Radiology. ACR-SPR-SRU Practice Parameter for the Performing and Interpreting Diagnostic Ultrasound Examinations. 2017; Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-perf-interpret.pdf. Accessed April 15, 2019.
15. American College of Radiology. ACR-AIUM-SPR-SRU Practice Parameter for the Performance of an Ultrasound Examination of the Neonatal and Infant Spine. 2016/ Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-NeonatalSpine.pdf. Accessed April 15, 2019.
© 2019 Society of Hospital Medicine