User login
Towards Scalable Hospital-Based Palliative Care: Challenges and Opportunities for Hospitalists
There is growing evidence that supports the ability of specialty palliative care to achieve the Triple Aim in healthcare: (1) improve patient and family experience of care, (2) improve health outcomes, and (3) reduce healthcare costs.1,2 However, the full realization of this value remains elusive due, in large part, to the increasing demand for specialty palliative care services outpacing the supply of specialists.3 Because expansion of the specialty palliative care workforce will never be sufficient to meet the needs of seriously ill patients, and nonspecialist physicians often fail to recognize palliative care needs in a timely manner,4 innovative and systematic solutions are needed to provide high-quality palliative care in a manner that is sustainable.5
To close the gap between workforce and patient needs, experts have largely advocated for two care delivery models that aim to improve the organization and allocation of limited palliative care resources: (1) a tier-based approach in which primary palliative care (basic skills for all clinicians) and specialty palliative care (advanced skills requiring additional training) have distinct but supportive roles, and (2) a need-based approach where different types of palliative care clinicians are deployed based on specific needs.5,6 In this issue, Abedini and Chopra propose a “Palliative Care Redistribution Integrated System Model” (PRISM) that combines these two approaches, with need-based care delivery that escalates through skill tiers to improve hospital-based palliative care.7
PRISM is attractive because it leverages the skill sets of clinicians across disciplines and is designed for the hospital, where the vast majority of specialty palliative care is provided in the United States. Moreover, it employs hospitalists who routinely care for a high volume of seriously ill patients, and are therefore well positioned to expand the palliative care workforce. The authors suggest several approaches to implement PRISM, such as designating certain hospitalist teams for palliative care, more interdisciplinary support, automated patient risk stratification or mandatory screening checklists, and strategic use of bedside nurses and social workers to facilitate early basic needs assessments. Although sound in principle, there are several foreseeable barriers to each of these approaches and potential unintended consequences of PRISM in the fields of hospital and palliative medicine.
Applying insights from behavioral economics will be essential for the successful implementation and dissemination of PRISM. Changing clinician behavior is not a challenge unique to palliative care interventions, but it may be particularly difficult due to misperceptions that palliative care is synonymous with end-of-life care and that such conversations are always time-intensive. Indeed, Abedini and Chopra acknowledge that all clinicians need to be well versed in basic palliative care skills for PRISM to succeed, yet most educational initiatives have shown modest results at best. The most promising clinician education programs, such as the Serious Illness Care Program and VitalTalk require intensive training simulations and are most effective when implemented on a system level to promote cultural change.8.9 Thus, training hospitalists in preparation for PRISM will require considerable upfront investment by hospitals. While policy efforts to improve palliative care training in medical education are progressing (Palliative Care and Hospice Education and Training Act, H.R.1676), any evidence of impact is nearly a generation away.
The authors also advocate for a technology-driven solution for systematic and early identification of palliative care needs. However, ideal clinical decision support would not rely on checklists to be completed by bedside clinicians or “hard stop” alerts in the electronic health record, as both of these approaches rely heavily upon consistent and accurate data entry by busy clinicians. Rather, innovative predictive analytics with machine learning and natural language processing methods hold great promise to support an electronic precision medicine approach for palliative care delivery. Even after such prediction models are developed, rigorous studies are needed to understand how they can change clinician behavior and impact the quality and cost of care.
Shifting palliative care tasks to nonspecialists has implications beyond quality and access. First, there are likely to be reimbursement implications as nonbillable clinicians such as social workers provide palliative care services that were previously provided by physicians and advance practice providers. As value-based payment models grow, healthcare systems may be wise to invest in innovative palliative care delivery models such as PRISM, but obtaining financial support will require rigorous evidence of value. Second, it will be important to monitor the already high rates of burnout and emotional exhaustion among palliative care clinicians10 when implementing care delivery models that select only the most complex patients for referral to specialty palliative care. Finally, new palliative care delivery models must fit within a larger national strategy to grow palliative care across the care continuum.11 This is of particular importance with hospital-focused solutions such as PRISM due to concerns about the growing split in care coordination between inpatient and outpatient care. Since seriously ill patients spend the majority of time outside the hospital and evidence for the value of palliative care is most robust in home and ambulatory settings,1 an important role for hospitalists could be to systematically identify and refer high-risk patients to community-based palliative care services after discharge from a sentinel hospitalization.
In conclusion, innovative palliative care delivery models such as PRISM are critical to ensuring that seriously ill patients have access to high-quality palliative care; however, more work is still needed to create the training programs, patient identification tools, scalable implementation, and evaluation processes necessary for success.
Disclosures
Dr. Courtright and Dr. O’Connor have nothing to disclose.
Funding
This work was funded in part by a career development award from the National Palliative Care Research Center (KRC). The views expressed herein solely represent those of the authors.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes. Jama. 2016;316(20):2104. doi: 10.1001/jama.2016.16840. PubMed
2. May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820. doi: 10.1001/jamainternmed.2018.0750. PubMed
3. Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med. 2016;19(1):8-15. doi: 10.1089/jpm.2015.0351. PubMed
4. Heyland DK. Failure to Engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778. doi: 10.1001/jamainternmed.2013.180. PubMed
5. Courtright KR, Cassel JB, Halpern SD. A research agenda for high-value palliative care. Ann Intern Med. 2017;168(1):71. doi: 10.7326/m17-2164. PubMed
6. Billings JA, Bernacki R. Strategic targeting of advance care planning interventions. JAMA Intern Med. 2014;174(4):620. doi: 10.1001/jamainternmed.2013.14384. PubMed
7. Abedini NC, Chopra V. A Model to Improve Hospital-Based Palliative Care: The Palliative Care Redistribution Integrated System Model (PRISM). J Hosp Med. 2018;13(12):868-871. doi: 10.12788/jhm.3065 PubMed
8. Bernacki R, Hutchings M, Vick J, et al. Development of the Serious Illness Care Program: a randomized controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032. doi: 10.1136/bmjopen-2015-009032. PubMed
9. Clayton JM, Butow PN, Waters A, et al. Evaluation of a novel individualized communication-skills training intervention to improve doctors’ confidence and skills in end-of-life communication. Palliat Med. 2012;27(3):236-243. doi: 10.1177/0269216312449683. PubMed
10. Kamal AH, Bull JH, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manag. 2016;51(4):690-696. doi: 10.1016/j.jpainsymman.2015.10.020. PubMed
11. Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood). 2017;36(7):1265-1273. doi: 10.1377/hlthaff.2017.0164. PubMed
There is growing evidence that supports the ability of specialty palliative care to achieve the Triple Aim in healthcare: (1) improve patient and family experience of care, (2) improve health outcomes, and (3) reduce healthcare costs.1,2 However, the full realization of this value remains elusive due, in large part, to the increasing demand for specialty palliative care services outpacing the supply of specialists.3 Because expansion of the specialty palliative care workforce will never be sufficient to meet the needs of seriously ill patients, and nonspecialist physicians often fail to recognize palliative care needs in a timely manner,4 innovative and systematic solutions are needed to provide high-quality palliative care in a manner that is sustainable.5
To close the gap between workforce and patient needs, experts have largely advocated for two care delivery models that aim to improve the organization and allocation of limited palliative care resources: (1) a tier-based approach in which primary palliative care (basic skills for all clinicians) and specialty palliative care (advanced skills requiring additional training) have distinct but supportive roles, and (2) a need-based approach where different types of palliative care clinicians are deployed based on specific needs.5,6 In this issue, Abedini and Chopra propose a “Palliative Care Redistribution Integrated System Model” (PRISM) that combines these two approaches, with need-based care delivery that escalates through skill tiers to improve hospital-based palliative care.7
PRISM is attractive because it leverages the skill sets of clinicians across disciplines and is designed for the hospital, where the vast majority of specialty palliative care is provided in the United States. Moreover, it employs hospitalists who routinely care for a high volume of seriously ill patients, and are therefore well positioned to expand the palliative care workforce. The authors suggest several approaches to implement PRISM, such as designating certain hospitalist teams for palliative care, more interdisciplinary support, automated patient risk stratification or mandatory screening checklists, and strategic use of bedside nurses and social workers to facilitate early basic needs assessments. Although sound in principle, there are several foreseeable barriers to each of these approaches and potential unintended consequences of PRISM in the fields of hospital and palliative medicine.
Applying insights from behavioral economics will be essential for the successful implementation and dissemination of PRISM. Changing clinician behavior is not a challenge unique to palliative care interventions, but it may be particularly difficult due to misperceptions that palliative care is synonymous with end-of-life care and that such conversations are always time-intensive. Indeed, Abedini and Chopra acknowledge that all clinicians need to be well versed in basic palliative care skills for PRISM to succeed, yet most educational initiatives have shown modest results at best. The most promising clinician education programs, such as the Serious Illness Care Program and VitalTalk require intensive training simulations and are most effective when implemented on a system level to promote cultural change.8.9 Thus, training hospitalists in preparation for PRISM will require considerable upfront investment by hospitals. While policy efforts to improve palliative care training in medical education are progressing (Palliative Care and Hospice Education and Training Act, H.R.1676), any evidence of impact is nearly a generation away.
The authors also advocate for a technology-driven solution for systematic and early identification of palliative care needs. However, ideal clinical decision support would not rely on checklists to be completed by bedside clinicians or “hard stop” alerts in the electronic health record, as both of these approaches rely heavily upon consistent and accurate data entry by busy clinicians. Rather, innovative predictive analytics with machine learning and natural language processing methods hold great promise to support an electronic precision medicine approach for palliative care delivery. Even after such prediction models are developed, rigorous studies are needed to understand how they can change clinician behavior and impact the quality and cost of care.
Shifting palliative care tasks to nonspecialists has implications beyond quality and access. First, there are likely to be reimbursement implications as nonbillable clinicians such as social workers provide palliative care services that were previously provided by physicians and advance practice providers. As value-based payment models grow, healthcare systems may be wise to invest in innovative palliative care delivery models such as PRISM, but obtaining financial support will require rigorous evidence of value. Second, it will be important to monitor the already high rates of burnout and emotional exhaustion among palliative care clinicians10 when implementing care delivery models that select only the most complex patients for referral to specialty palliative care. Finally, new palliative care delivery models must fit within a larger national strategy to grow palliative care across the care continuum.11 This is of particular importance with hospital-focused solutions such as PRISM due to concerns about the growing split in care coordination between inpatient and outpatient care. Since seriously ill patients spend the majority of time outside the hospital and evidence for the value of palliative care is most robust in home and ambulatory settings,1 an important role for hospitalists could be to systematically identify and refer high-risk patients to community-based palliative care services after discharge from a sentinel hospitalization.
In conclusion, innovative palliative care delivery models such as PRISM are critical to ensuring that seriously ill patients have access to high-quality palliative care; however, more work is still needed to create the training programs, patient identification tools, scalable implementation, and evaluation processes necessary for success.
Disclosures
Dr. Courtright and Dr. O’Connor have nothing to disclose.
Funding
This work was funded in part by a career development award from the National Palliative Care Research Center (KRC). The views expressed herein solely represent those of the authors.
There is growing evidence that supports the ability of specialty palliative care to achieve the Triple Aim in healthcare: (1) improve patient and family experience of care, (2) improve health outcomes, and (3) reduce healthcare costs.1,2 However, the full realization of this value remains elusive due, in large part, to the increasing demand for specialty palliative care services outpacing the supply of specialists.3 Because expansion of the specialty palliative care workforce will never be sufficient to meet the needs of seriously ill patients, and nonspecialist physicians often fail to recognize palliative care needs in a timely manner,4 innovative and systematic solutions are needed to provide high-quality palliative care in a manner that is sustainable.5
To close the gap between workforce and patient needs, experts have largely advocated for two care delivery models that aim to improve the organization and allocation of limited palliative care resources: (1) a tier-based approach in which primary palliative care (basic skills for all clinicians) and specialty palliative care (advanced skills requiring additional training) have distinct but supportive roles, and (2) a need-based approach where different types of palliative care clinicians are deployed based on specific needs.5,6 In this issue, Abedini and Chopra propose a “Palliative Care Redistribution Integrated System Model” (PRISM) that combines these two approaches, with need-based care delivery that escalates through skill tiers to improve hospital-based palliative care.7
PRISM is attractive because it leverages the skill sets of clinicians across disciplines and is designed for the hospital, where the vast majority of specialty palliative care is provided in the United States. Moreover, it employs hospitalists who routinely care for a high volume of seriously ill patients, and are therefore well positioned to expand the palliative care workforce. The authors suggest several approaches to implement PRISM, such as designating certain hospitalist teams for palliative care, more interdisciplinary support, automated patient risk stratification or mandatory screening checklists, and strategic use of bedside nurses and social workers to facilitate early basic needs assessments. Although sound in principle, there are several foreseeable barriers to each of these approaches and potential unintended consequences of PRISM in the fields of hospital and palliative medicine.
Applying insights from behavioral economics will be essential for the successful implementation and dissemination of PRISM. Changing clinician behavior is not a challenge unique to palliative care interventions, but it may be particularly difficult due to misperceptions that palliative care is synonymous with end-of-life care and that such conversations are always time-intensive. Indeed, Abedini and Chopra acknowledge that all clinicians need to be well versed in basic palliative care skills for PRISM to succeed, yet most educational initiatives have shown modest results at best. The most promising clinician education programs, such as the Serious Illness Care Program and VitalTalk require intensive training simulations and are most effective when implemented on a system level to promote cultural change.8.9 Thus, training hospitalists in preparation for PRISM will require considerable upfront investment by hospitals. While policy efforts to improve palliative care training in medical education are progressing (Palliative Care and Hospice Education and Training Act, H.R.1676), any evidence of impact is nearly a generation away.
The authors also advocate for a technology-driven solution for systematic and early identification of palliative care needs. However, ideal clinical decision support would not rely on checklists to be completed by bedside clinicians or “hard stop” alerts in the electronic health record, as both of these approaches rely heavily upon consistent and accurate data entry by busy clinicians. Rather, innovative predictive analytics with machine learning and natural language processing methods hold great promise to support an electronic precision medicine approach for palliative care delivery. Even after such prediction models are developed, rigorous studies are needed to understand how they can change clinician behavior and impact the quality and cost of care.
Shifting palliative care tasks to nonspecialists has implications beyond quality and access. First, there are likely to be reimbursement implications as nonbillable clinicians such as social workers provide palliative care services that were previously provided by physicians and advance practice providers. As value-based payment models grow, healthcare systems may be wise to invest in innovative palliative care delivery models such as PRISM, but obtaining financial support will require rigorous evidence of value. Second, it will be important to monitor the already high rates of burnout and emotional exhaustion among palliative care clinicians10 when implementing care delivery models that select only the most complex patients for referral to specialty palliative care. Finally, new palliative care delivery models must fit within a larger national strategy to grow palliative care across the care continuum.11 This is of particular importance with hospital-focused solutions such as PRISM due to concerns about the growing split in care coordination between inpatient and outpatient care. Since seriously ill patients spend the majority of time outside the hospital and evidence for the value of palliative care is most robust in home and ambulatory settings,1 an important role for hospitalists could be to systematically identify and refer high-risk patients to community-based palliative care services after discharge from a sentinel hospitalization.
In conclusion, innovative palliative care delivery models such as PRISM are critical to ensuring that seriously ill patients have access to high-quality palliative care; however, more work is still needed to create the training programs, patient identification tools, scalable implementation, and evaluation processes necessary for success.
Disclosures
Dr. Courtright and Dr. O’Connor have nothing to disclose.
Funding
This work was funded in part by a career development award from the National Palliative Care Research Center (KRC). The views expressed herein solely represent those of the authors.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes. Jama. 2016;316(20):2104. doi: 10.1001/jama.2016.16840. PubMed
2. May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820. doi: 10.1001/jamainternmed.2018.0750. PubMed
3. Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med. 2016;19(1):8-15. doi: 10.1089/jpm.2015.0351. PubMed
4. Heyland DK. Failure to Engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778. doi: 10.1001/jamainternmed.2013.180. PubMed
5. Courtright KR, Cassel JB, Halpern SD. A research agenda for high-value palliative care. Ann Intern Med. 2017;168(1):71. doi: 10.7326/m17-2164. PubMed
6. Billings JA, Bernacki R. Strategic targeting of advance care planning interventions. JAMA Intern Med. 2014;174(4):620. doi: 10.1001/jamainternmed.2013.14384. PubMed
7. Abedini NC, Chopra V. A Model to Improve Hospital-Based Palliative Care: The Palliative Care Redistribution Integrated System Model (PRISM). J Hosp Med. 2018;13(12):868-871. doi: 10.12788/jhm.3065 PubMed
8. Bernacki R, Hutchings M, Vick J, et al. Development of the Serious Illness Care Program: a randomized controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032. doi: 10.1136/bmjopen-2015-009032. PubMed
9. Clayton JM, Butow PN, Waters A, et al. Evaluation of a novel individualized communication-skills training intervention to improve doctors’ confidence and skills in end-of-life communication. Palliat Med. 2012;27(3):236-243. doi: 10.1177/0269216312449683. PubMed
10. Kamal AH, Bull JH, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manag. 2016;51(4):690-696. doi: 10.1016/j.jpainsymman.2015.10.020. PubMed
11. Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood). 2017;36(7):1265-1273. doi: 10.1377/hlthaff.2017.0164. PubMed
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes. Jama. 2016;316(20):2104. doi: 10.1001/jama.2016.16840. PubMed
2. May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820. doi: 10.1001/jamainternmed.2018.0750. PubMed
3. Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med. 2016;19(1):8-15. doi: 10.1089/jpm.2015.0351. PubMed
4. Heyland DK. Failure to Engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778. doi: 10.1001/jamainternmed.2013.180. PubMed
5. Courtright KR, Cassel JB, Halpern SD. A research agenda for high-value palliative care. Ann Intern Med. 2017;168(1):71. doi: 10.7326/m17-2164. PubMed
6. Billings JA, Bernacki R. Strategic targeting of advance care planning interventions. JAMA Intern Med. 2014;174(4):620. doi: 10.1001/jamainternmed.2013.14384. PubMed
7. Abedini NC, Chopra V. A Model to Improve Hospital-Based Palliative Care: The Palliative Care Redistribution Integrated System Model (PRISM). J Hosp Med. 2018;13(12):868-871. doi: 10.12788/jhm.3065 PubMed
8. Bernacki R, Hutchings M, Vick J, et al. Development of the Serious Illness Care Program: a randomized controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032. doi: 10.1136/bmjopen-2015-009032. PubMed
9. Clayton JM, Butow PN, Waters A, et al. Evaluation of a novel individualized communication-skills training intervention to improve doctors’ confidence and skills in end-of-life communication. Palliat Med. 2012;27(3):236-243. doi: 10.1177/0269216312449683. PubMed
10. Kamal AH, Bull JH, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manag. 2016;51(4):690-696. doi: 10.1016/j.jpainsymman.2015.10.020. PubMed
11. Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood). 2017;36(7):1265-1273. doi: 10.1377/hlthaff.2017.0164. PubMed
© 2018 Society of Hospital Medicine
Healthcare Quality for Children and Adolescents with Suicidality Admitted to Acute Care Hospitals in the United States
Suicide is the second most common cause of death among children, adolescents, and young adults in the United States. In 2016, over 6,000 children and youth 5 to 24 years of age succumbed to suicide, thus reflecting a mortality rate nearly three times higher than deaths from malignancies and 28 times higher than deaths from sepsis in this age group.1 Suicidal ideation and suicide attempts are even more common, with 17% of high school students reporting seriously considering suicide and 8% reporting suicide attempts in the previous 12 months.2 These tragic statistics are reflected in our health system use, emergency department (ED) utilization for suicide attempts and suicidal ideation is growing at a tremendous rate, and over 50% of the children seen in EDs are subsequently admitted to the hospital for ongoing care.3,4
In this issue of Journal of Hospital Medicine, Doupnik and colleagues present an analysis of pediatric hospitalizations for suicide attempts and suicidal ideation at acute care hospitals contained within the 2013 and 2014 National Readmissions Dataset.5 This dataset reflects a nationally representative sample of pediatric hospitalizations, weighted to allow for national estimates. Although their focus was on hospital readmission, their analysis yielded additional valuable data about suicide attempts and suicidal ideation in American youth. The investigators identified 181,575 pediatric acute care hospitalizations for suicide attempts and suicidal ideation over the two-year study period, accounting for 9.5% of all acute care hospitalizations among children and adolescents 6 to 17 years of age nationally. This number exceeds the biennial number of pediatric hospitalizations for cellulitis, dehydration, and urinary tract infections, all of which are generally considered the “bread and butter” of pediatric hospital medicine.6
Doupnik and colleagues rightly pointed out that hospital readmission is not a nationally endorsed measure to evaluate the quality of pediatric mental health hospitalizations. At the same time, their work highlights that acute care hospitals need strategies to measure the quality of pediatric hospitalizations for suicide attempts and suicidal ideation. Beyond readmissions, how should the quality of these hospital stays be evaluated? A recent review of 15 national quality measure sets identified 257 unique measures to evaluate pediatric quality of care.7 Of these, only one focused on mental health hospitalization. This measure, which was endorsed by the National Quality Forum, determines the percentage of discharges for patients six years of age and older who were hospitalized for mental health diagnoses and who had a follow-up visit with a mental health practitioner within 7 and 30 days of hospital discharge.8 Given Doupnik et al.’s finding that one-third of all 30-day hospital readmissions occurred within seven days of hospital discharge, early follow-up visits with mental health practitioners is arguably essential.
Although evidence-based quality measures to evaluate hospital-based mental healthcare are limited, quality measure development is ongoing, facilitated by recent federal health policy and associated research efforts. Four newly developed measures focus on the quality of inpatient care for suicidality, including two evaluated using data from health records and two derived from caregiver surveys. The first medical records-based measure identifies whether caregivers of patients admitted to hospital for dangerous self-harm or suicidality have documentation that they were counseled on how to restrict their child’s or adolescent’s access to potentially lethal means of suicide before discharge. The second record-based measure evaluates documentation in the medical record of discussion between the hospital provider and the patient’s outpatient provider regarding the plan for follow-up.9 The two survey-based measures ask caregivers whether they were counseled on how to restrict access to potentially lethal means of suicide, and, for children and adolescents started on a new antidepressant medication or dose, whether they were counseled regarding the potential benefits and risks of the medication.10 All measures were field-tested at children’s hospitals to ensure feasibility in data collection. However, as shown by Doupnik et al., only 7.4% of acute care hospitalizations for suicide attempts and suicidal ideation occurred at freestanding children’s hospitals; most occurred at urban nonteaching centers. Evaluation of these new quality measures across structurally diverse hospitals is an important next step.
Beyond the healthcare constructs evaluated by these quality measures, many foundational questions about what constitutes high quality inpatient healthcare for suicide attempts and suicidal ideation remain. An American Academy of Child and Adolescent Psychiatry (AACAP) practice parameter, which was published in 2001, established minimal standards for the assessment and treatment of children and adolescents with suicidal behavior.11 This guideline recommends inpatient treatment until the mental state or level of suicidality has stabilized, with discharge considered only when the clinician is satisfied that adequate supervision and support will be available and when a responsible adult has agreed to secure or dispose of potentially lethal medications and firearms. It further recommends that the clinician treating the child or adolescent during the days following a suicide attempt be available to the patient and family – for example, to receive and make telephone calls outside of regular clinic hours. Recognizing the growing prevalence of suicidality in American children and youth, coupled with critical shortages in pediatric psychiatrists and fragmentation of inpatient and outpatient care, these minimal standards may be difficult to implement across the many settings where children receive their mental healthcare.4,12,13
The large number of children and adolescents being hospitalized for suicide attempts and suicidal ideation at acute care hospitals demands that we take stock of how we manage this vulnerable population. Although Doupnik and colleagues suggest that exclusion of specialty psychiatric hospitals from their dataset is a limitation, their presentation of suicide attempts and suicidal ideation epidemiology at acute care hospitals provides valuable data for pediatric hospitalists. Given the presence of pediatric hospitalists at many acute care hospitals, comanagement by hospital medicine and psychiatry services may prove both efficient and effective while breaking down the silos that traditionally separate these specialties. Alternatively, extending the role of collaborative care teams, which are increasingly embedded in pediatric primary care, into inpatient settings may enable continuity of care and improve healthcare quality.14 Finally, nearly 20 years have passed since the AACAP published its practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. An update to reflect contemporary suicide attempts and suicidal ideation statistics and evidence-based practices is needed, and collaboration between professional pediatric and psychiatric organizations in the creation of this update would recognize the growing role of pediatricians, including hospitalists, in the provision of mental healthcare for children.
Updated guidelines must take into account the transitions of care experienced by children and adolescents throughout their hospital stay: at admission, at discharge, and during their hospitalization if they move from medical to psychiatric care. Research is needed to determine what proportion of children and adolescents receive evidence-based mental health therapies while in hospital and how many are connected with wraparound mental health services before hospital discharge.15 Doupnik et al. excluded children and adolescents who were transferred to other hospitals, which included over 18,000 youth. How long did these patients spend “boarding,” and did they receive any mental health assessment or treatment during this period? Although the Joint Commission recommends that holding times for patients awaiting bed placement should not exceed four4 hours, hospitals have described average pediatric inpatient boarding times of 2-3 days while awaiting inpatient psychiatric care.16,17 In one study of children and adolescents awaiting transfer for inpatient psychiatric care, mental health counseling was received by only 6%, which reflects lost time that could have been spent treating this highly vulnerable population.16 Multidisciplinary collaboration is needed to address these issues and inform best practices.
Although mortality is a rare outcome for most conditions we treat in pediatric hospital medicine, mortality following suicide attempts is all too common. The data presented by Doupnik and colleagues provide a powerful call to improve healthcare quality across the diverse settings where children with suicidality receive their care.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
Funding
Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999-2016 on CDC WONDER Online Database, released December, 2017.
2. Kann L, Kinchen S, Shanklin S, et al. Youth risk behavior surveillance-United States, 2013. MMWR. 2014;63(4):1-168. PubMed
3. Olfson M, Gameroff MJ, Marcus SC, Greenberg T, Shaffer D. Emergency treatment of young people following deliberate self-harm. Arch Gen Psychiatry. 2005;62(10):1122-1128. doi: 10.1001/archpsyc.62.10.1122 PubMed
4. Mercado MC, Holland K, Leemis RW, Stone DM, Wang J. Trends in emergency department visits for nonfatal self-inflicted injuries among youth aged 10 to 24 years in the United States, 2001-2015. JAMA. 2017;318(19):1931-1932. doi: 10.1001/jama.2017.13317 PubMed
5. Doupnik S, Rodean J, Zima B, et al. Readmissions after pediatric hospitalization for suicide ideation and suicide attempt [published online ahead of print October 31, 2018]. J Hosp Med. doi: 10.12788/jhm.3070
6. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. doi: 10.1002/jhm.2624 PubMed
7. House SA, Coon ER, Schroeder AR, Ralston SL. Categorization of national pediatric quality measures. Pediatrics. 2017;139(4):e20163269. PubMed
8. National Quality Forum. Follow-up after hospitalization for mental illness. Available at www.qualityforum.org. Accessed July 21, 2018.
9. Bardach N, Burkhart Q, Richardson L, et al. Hospital-based quality measures for pediatric mental health care. Pediatrics. 2018;141(6):e20173554. PubMed
10. Parast L, Bardach N, Burkhart Q, et al. Development of new quality measures for hospital-based care of suicidal youth. Acad Pediatr. 2018;18(3):248-255. doi: 10.1016/j.acap.2017.09.017 PubMed
11. Shaffer D, Pfeffer C. Practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7 Suppl):24-51. doi: 10.1097/00004583-200107001-00003
12. Thomas C, Holtzer C. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1023-1031. doi: 10.1097/01.chi.0000225353.16831.5d PubMed
13. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. 2018;141(6):e20172426. PubMed
14. Beach SR, Walker J, Celano CM, Mastromauro CA, Sharpe M, Huffman JC. Implementing collaborative care programs for psychiatric disorders in medical settings: a practical guide. Gen Hosp Psychiatry. 2015;37(6):522-527. doi: 10.1016/j.genhosppsych.2015.06.015 PubMed
15. Winters N, Pumariega A. Practice parameter on child and adolescent mental health care in community systems of care. J Am Acad Child Adolsc Psychiatry. 2007;46(2):284-299. DOI: 10.1097/01.chi.0000246061.70330.b8 PubMed
16. Claudius I, Donofrio J, Lam CN, Santillanes G. Impact of boarding pediatric psychiatric patients on a medical ward. Hosp Pediatr. 2014;4(3):125-131. doi: 10.1542/hpeds.2013-0079 PubMed
17. Gallagher KAS, Bujoreanu IS, Cheung P, Choi C, Golden S, Brodziak K. Psychiatric boarding in the pediatric inpatient medical setting: a retrospective analysis. Hosp Pediatr. 2013;7(8):444-450. doi: 10.1542/hpeds.2017-0005 PubMed
Suicide is the second most common cause of death among children, adolescents, and young adults in the United States. In 2016, over 6,000 children and youth 5 to 24 years of age succumbed to suicide, thus reflecting a mortality rate nearly three times higher than deaths from malignancies and 28 times higher than deaths from sepsis in this age group.1 Suicidal ideation and suicide attempts are even more common, with 17% of high school students reporting seriously considering suicide and 8% reporting suicide attempts in the previous 12 months.2 These tragic statistics are reflected in our health system use, emergency department (ED) utilization for suicide attempts and suicidal ideation is growing at a tremendous rate, and over 50% of the children seen in EDs are subsequently admitted to the hospital for ongoing care.3,4
In this issue of Journal of Hospital Medicine, Doupnik and colleagues present an analysis of pediatric hospitalizations for suicide attempts and suicidal ideation at acute care hospitals contained within the 2013 and 2014 National Readmissions Dataset.5 This dataset reflects a nationally representative sample of pediatric hospitalizations, weighted to allow for national estimates. Although their focus was on hospital readmission, their analysis yielded additional valuable data about suicide attempts and suicidal ideation in American youth. The investigators identified 181,575 pediatric acute care hospitalizations for suicide attempts and suicidal ideation over the two-year study period, accounting for 9.5% of all acute care hospitalizations among children and adolescents 6 to 17 years of age nationally. This number exceeds the biennial number of pediatric hospitalizations for cellulitis, dehydration, and urinary tract infections, all of which are generally considered the “bread and butter” of pediatric hospital medicine.6
Doupnik and colleagues rightly pointed out that hospital readmission is not a nationally endorsed measure to evaluate the quality of pediatric mental health hospitalizations. At the same time, their work highlights that acute care hospitals need strategies to measure the quality of pediatric hospitalizations for suicide attempts and suicidal ideation. Beyond readmissions, how should the quality of these hospital stays be evaluated? A recent review of 15 national quality measure sets identified 257 unique measures to evaluate pediatric quality of care.7 Of these, only one focused on mental health hospitalization. This measure, which was endorsed by the National Quality Forum, determines the percentage of discharges for patients six years of age and older who were hospitalized for mental health diagnoses and who had a follow-up visit with a mental health practitioner within 7 and 30 days of hospital discharge.8 Given Doupnik et al.’s finding that one-third of all 30-day hospital readmissions occurred within seven days of hospital discharge, early follow-up visits with mental health practitioners is arguably essential.
Although evidence-based quality measures to evaluate hospital-based mental healthcare are limited, quality measure development is ongoing, facilitated by recent federal health policy and associated research efforts. Four newly developed measures focus on the quality of inpatient care for suicidality, including two evaluated using data from health records and two derived from caregiver surveys. The first medical records-based measure identifies whether caregivers of patients admitted to hospital for dangerous self-harm or suicidality have documentation that they were counseled on how to restrict their child’s or adolescent’s access to potentially lethal means of suicide before discharge. The second record-based measure evaluates documentation in the medical record of discussion between the hospital provider and the patient’s outpatient provider regarding the plan for follow-up.9 The two survey-based measures ask caregivers whether they were counseled on how to restrict access to potentially lethal means of suicide, and, for children and adolescents started on a new antidepressant medication or dose, whether they were counseled regarding the potential benefits and risks of the medication.10 All measures were field-tested at children’s hospitals to ensure feasibility in data collection. However, as shown by Doupnik et al., only 7.4% of acute care hospitalizations for suicide attempts and suicidal ideation occurred at freestanding children’s hospitals; most occurred at urban nonteaching centers. Evaluation of these new quality measures across structurally diverse hospitals is an important next step.
Beyond the healthcare constructs evaluated by these quality measures, many foundational questions about what constitutes high quality inpatient healthcare for suicide attempts and suicidal ideation remain. An American Academy of Child and Adolescent Psychiatry (AACAP) practice parameter, which was published in 2001, established minimal standards for the assessment and treatment of children and adolescents with suicidal behavior.11 This guideline recommends inpatient treatment until the mental state or level of suicidality has stabilized, with discharge considered only when the clinician is satisfied that adequate supervision and support will be available and when a responsible adult has agreed to secure or dispose of potentially lethal medications and firearms. It further recommends that the clinician treating the child or adolescent during the days following a suicide attempt be available to the patient and family – for example, to receive and make telephone calls outside of regular clinic hours. Recognizing the growing prevalence of suicidality in American children and youth, coupled with critical shortages in pediatric psychiatrists and fragmentation of inpatient and outpatient care, these minimal standards may be difficult to implement across the many settings where children receive their mental healthcare.4,12,13
The large number of children and adolescents being hospitalized for suicide attempts and suicidal ideation at acute care hospitals demands that we take stock of how we manage this vulnerable population. Although Doupnik and colleagues suggest that exclusion of specialty psychiatric hospitals from their dataset is a limitation, their presentation of suicide attempts and suicidal ideation epidemiology at acute care hospitals provides valuable data for pediatric hospitalists. Given the presence of pediatric hospitalists at many acute care hospitals, comanagement by hospital medicine and psychiatry services may prove both efficient and effective while breaking down the silos that traditionally separate these specialties. Alternatively, extending the role of collaborative care teams, which are increasingly embedded in pediatric primary care, into inpatient settings may enable continuity of care and improve healthcare quality.14 Finally, nearly 20 years have passed since the AACAP published its practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. An update to reflect contemporary suicide attempts and suicidal ideation statistics and evidence-based practices is needed, and collaboration between professional pediatric and psychiatric organizations in the creation of this update would recognize the growing role of pediatricians, including hospitalists, in the provision of mental healthcare for children.
Updated guidelines must take into account the transitions of care experienced by children and adolescents throughout their hospital stay: at admission, at discharge, and during their hospitalization if they move from medical to psychiatric care. Research is needed to determine what proportion of children and adolescents receive evidence-based mental health therapies while in hospital and how many are connected with wraparound mental health services before hospital discharge.15 Doupnik et al. excluded children and adolescents who were transferred to other hospitals, which included over 18,000 youth. How long did these patients spend “boarding,” and did they receive any mental health assessment or treatment during this period? Although the Joint Commission recommends that holding times for patients awaiting bed placement should not exceed four4 hours, hospitals have described average pediatric inpatient boarding times of 2-3 days while awaiting inpatient psychiatric care.16,17 In one study of children and adolescents awaiting transfer for inpatient psychiatric care, mental health counseling was received by only 6%, which reflects lost time that could have been spent treating this highly vulnerable population.16 Multidisciplinary collaboration is needed to address these issues and inform best practices.
Although mortality is a rare outcome for most conditions we treat in pediatric hospital medicine, mortality following suicide attempts is all too common. The data presented by Doupnik and colleagues provide a powerful call to improve healthcare quality across the diverse settings where children with suicidality receive their care.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
Funding
Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
Suicide is the second most common cause of death among children, adolescents, and young adults in the United States. In 2016, over 6,000 children and youth 5 to 24 years of age succumbed to suicide, thus reflecting a mortality rate nearly three times higher than deaths from malignancies and 28 times higher than deaths from sepsis in this age group.1 Suicidal ideation and suicide attempts are even more common, with 17% of high school students reporting seriously considering suicide and 8% reporting suicide attempts in the previous 12 months.2 These tragic statistics are reflected in our health system use, emergency department (ED) utilization for suicide attempts and suicidal ideation is growing at a tremendous rate, and over 50% of the children seen in EDs are subsequently admitted to the hospital for ongoing care.3,4
In this issue of Journal of Hospital Medicine, Doupnik and colleagues present an analysis of pediatric hospitalizations for suicide attempts and suicidal ideation at acute care hospitals contained within the 2013 and 2014 National Readmissions Dataset.5 This dataset reflects a nationally representative sample of pediatric hospitalizations, weighted to allow for national estimates. Although their focus was on hospital readmission, their analysis yielded additional valuable data about suicide attempts and suicidal ideation in American youth. The investigators identified 181,575 pediatric acute care hospitalizations for suicide attempts and suicidal ideation over the two-year study period, accounting for 9.5% of all acute care hospitalizations among children and adolescents 6 to 17 years of age nationally. This number exceeds the biennial number of pediatric hospitalizations for cellulitis, dehydration, and urinary tract infections, all of which are generally considered the “bread and butter” of pediatric hospital medicine.6
Doupnik and colleagues rightly pointed out that hospital readmission is not a nationally endorsed measure to evaluate the quality of pediatric mental health hospitalizations. At the same time, their work highlights that acute care hospitals need strategies to measure the quality of pediatric hospitalizations for suicide attempts and suicidal ideation. Beyond readmissions, how should the quality of these hospital stays be evaluated? A recent review of 15 national quality measure sets identified 257 unique measures to evaluate pediatric quality of care.7 Of these, only one focused on mental health hospitalization. This measure, which was endorsed by the National Quality Forum, determines the percentage of discharges for patients six years of age and older who were hospitalized for mental health diagnoses and who had a follow-up visit with a mental health practitioner within 7 and 30 days of hospital discharge.8 Given Doupnik et al.’s finding that one-third of all 30-day hospital readmissions occurred within seven days of hospital discharge, early follow-up visits with mental health practitioners is arguably essential.
Although evidence-based quality measures to evaluate hospital-based mental healthcare are limited, quality measure development is ongoing, facilitated by recent federal health policy and associated research efforts. Four newly developed measures focus on the quality of inpatient care for suicidality, including two evaluated using data from health records and two derived from caregiver surveys. The first medical records-based measure identifies whether caregivers of patients admitted to hospital for dangerous self-harm or suicidality have documentation that they were counseled on how to restrict their child’s or adolescent’s access to potentially lethal means of suicide before discharge. The second record-based measure evaluates documentation in the medical record of discussion between the hospital provider and the patient’s outpatient provider regarding the plan for follow-up.9 The two survey-based measures ask caregivers whether they were counseled on how to restrict access to potentially lethal means of suicide, and, for children and adolescents started on a new antidepressant medication or dose, whether they were counseled regarding the potential benefits and risks of the medication.10 All measures were field-tested at children’s hospitals to ensure feasibility in data collection. However, as shown by Doupnik et al., only 7.4% of acute care hospitalizations for suicide attempts and suicidal ideation occurred at freestanding children’s hospitals; most occurred at urban nonteaching centers. Evaluation of these new quality measures across structurally diverse hospitals is an important next step.
Beyond the healthcare constructs evaluated by these quality measures, many foundational questions about what constitutes high quality inpatient healthcare for suicide attempts and suicidal ideation remain. An American Academy of Child and Adolescent Psychiatry (AACAP) practice parameter, which was published in 2001, established minimal standards for the assessment and treatment of children and adolescents with suicidal behavior.11 This guideline recommends inpatient treatment until the mental state or level of suicidality has stabilized, with discharge considered only when the clinician is satisfied that adequate supervision and support will be available and when a responsible adult has agreed to secure or dispose of potentially lethal medications and firearms. It further recommends that the clinician treating the child or adolescent during the days following a suicide attempt be available to the patient and family – for example, to receive and make telephone calls outside of regular clinic hours. Recognizing the growing prevalence of suicidality in American children and youth, coupled with critical shortages in pediatric psychiatrists and fragmentation of inpatient and outpatient care, these minimal standards may be difficult to implement across the many settings where children receive their mental healthcare.4,12,13
The large number of children and adolescents being hospitalized for suicide attempts and suicidal ideation at acute care hospitals demands that we take stock of how we manage this vulnerable population. Although Doupnik and colleagues suggest that exclusion of specialty psychiatric hospitals from their dataset is a limitation, their presentation of suicide attempts and suicidal ideation epidemiology at acute care hospitals provides valuable data for pediatric hospitalists. Given the presence of pediatric hospitalists at many acute care hospitals, comanagement by hospital medicine and psychiatry services may prove both efficient and effective while breaking down the silos that traditionally separate these specialties. Alternatively, extending the role of collaborative care teams, which are increasingly embedded in pediatric primary care, into inpatient settings may enable continuity of care and improve healthcare quality.14 Finally, nearly 20 years have passed since the AACAP published its practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. An update to reflect contemporary suicide attempts and suicidal ideation statistics and evidence-based practices is needed, and collaboration between professional pediatric and psychiatric organizations in the creation of this update would recognize the growing role of pediatricians, including hospitalists, in the provision of mental healthcare for children.
Updated guidelines must take into account the transitions of care experienced by children and adolescents throughout their hospital stay: at admission, at discharge, and during their hospitalization if they move from medical to psychiatric care. Research is needed to determine what proportion of children and adolescents receive evidence-based mental health therapies while in hospital and how many are connected with wraparound mental health services before hospital discharge.15 Doupnik et al. excluded children and adolescents who were transferred to other hospitals, which included over 18,000 youth. How long did these patients spend “boarding,” and did they receive any mental health assessment or treatment during this period? Although the Joint Commission recommends that holding times for patients awaiting bed placement should not exceed four4 hours, hospitals have described average pediatric inpatient boarding times of 2-3 days while awaiting inpatient psychiatric care.16,17 In one study of children and adolescents awaiting transfer for inpatient psychiatric care, mental health counseling was received by only 6%, which reflects lost time that could have been spent treating this highly vulnerable population.16 Multidisciplinary collaboration is needed to address these issues and inform best practices.
Although mortality is a rare outcome for most conditions we treat in pediatric hospital medicine, mortality following suicide attempts is all too common. The data presented by Doupnik and colleagues provide a powerful call to improve healthcare quality across the diverse settings where children with suicidality receive their care.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
Funding
Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999-2016 on CDC WONDER Online Database, released December, 2017.
2. Kann L, Kinchen S, Shanklin S, et al. Youth risk behavior surveillance-United States, 2013. MMWR. 2014;63(4):1-168. PubMed
3. Olfson M, Gameroff MJ, Marcus SC, Greenberg T, Shaffer D. Emergency treatment of young people following deliberate self-harm. Arch Gen Psychiatry. 2005;62(10):1122-1128. doi: 10.1001/archpsyc.62.10.1122 PubMed
4. Mercado MC, Holland K, Leemis RW, Stone DM, Wang J. Trends in emergency department visits for nonfatal self-inflicted injuries among youth aged 10 to 24 years in the United States, 2001-2015. JAMA. 2017;318(19):1931-1932. doi: 10.1001/jama.2017.13317 PubMed
5. Doupnik S, Rodean J, Zima B, et al. Readmissions after pediatric hospitalization for suicide ideation and suicide attempt [published online ahead of print October 31, 2018]. J Hosp Med. doi: 10.12788/jhm.3070
6. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. doi: 10.1002/jhm.2624 PubMed
7. House SA, Coon ER, Schroeder AR, Ralston SL. Categorization of national pediatric quality measures. Pediatrics. 2017;139(4):e20163269. PubMed
8. National Quality Forum. Follow-up after hospitalization for mental illness. Available at www.qualityforum.org. Accessed July 21, 2018.
9. Bardach N, Burkhart Q, Richardson L, et al. Hospital-based quality measures for pediatric mental health care. Pediatrics. 2018;141(6):e20173554. PubMed
10. Parast L, Bardach N, Burkhart Q, et al. Development of new quality measures for hospital-based care of suicidal youth. Acad Pediatr. 2018;18(3):248-255. doi: 10.1016/j.acap.2017.09.017 PubMed
11. Shaffer D, Pfeffer C. Practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7 Suppl):24-51. doi: 10.1097/00004583-200107001-00003
12. Thomas C, Holtzer C. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1023-1031. doi: 10.1097/01.chi.0000225353.16831.5d PubMed
13. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. 2018;141(6):e20172426. PubMed
14. Beach SR, Walker J, Celano CM, Mastromauro CA, Sharpe M, Huffman JC. Implementing collaborative care programs for psychiatric disorders in medical settings: a practical guide. Gen Hosp Psychiatry. 2015;37(6):522-527. doi: 10.1016/j.genhosppsych.2015.06.015 PubMed
15. Winters N, Pumariega A. Practice parameter on child and adolescent mental health care in community systems of care. J Am Acad Child Adolsc Psychiatry. 2007;46(2):284-299. DOI: 10.1097/01.chi.0000246061.70330.b8 PubMed
16. Claudius I, Donofrio J, Lam CN, Santillanes G. Impact of boarding pediatric psychiatric patients on a medical ward. Hosp Pediatr. 2014;4(3):125-131. doi: 10.1542/hpeds.2013-0079 PubMed
17. Gallagher KAS, Bujoreanu IS, Cheung P, Choi C, Golden S, Brodziak K. Psychiatric boarding in the pediatric inpatient medical setting: a retrospective analysis. Hosp Pediatr. 2013;7(8):444-450. doi: 10.1542/hpeds.2017-0005 PubMed
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999-2016 on CDC WONDER Online Database, released December, 2017.
2. Kann L, Kinchen S, Shanklin S, et al. Youth risk behavior surveillance-United States, 2013. MMWR. 2014;63(4):1-168. PubMed
3. Olfson M, Gameroff MJ, Marcus SC, Greenberg T, Shaffer D. Emergency treatment of young people following deliberate self-harm. Arch Gen Psychiatry. 2005;62(10):1122-1128. doi: 10.1001/archpsyc.62.10.1122 PubMed
4. Mercado MC, Holland K, Leemis RW, Stone DM, Wang J. Trends in emergency department visits for nonfatal self-inflicted injuries among youth aged 10 to 24 years in the United States, 2001-2015. JAMA. 2017;318(19):1931-1932. doi: 10.1001/jama.2017.13317 PubMed
5. Doupnik S, Rodean J, Zima B, et al. Readmissions after pediatric hospitalization for suicide ideation and suicide attempt [published online ahead of print October 31, 2018]. J Hosp Med. doi: 10.12788/jhm.3070
6. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. doi: 10.1002/jhm.2624 PubMed
7. House SA, Coon ER, Schroeder AR, Ralston SL. Categorization of national pediatric quality measures. Pediatrics. 2017;139(4):e20163269. PubMed
8. National Quality Forum. Follow-up after hospitalization for mental illness. Available at www.qualityforum.org. Accessed July 21, 2018.
9. Bardach N, Burkhart Q, Richardson L, et al. Hospital-based quality measures for pediatric mental health care. Pediatrics. 2018;141(6):e20173554. PubMed
10. Parast L, Bardach N, Burkhart Q, et al. Development of new quality measures for hospital-based care of suicidal youth. Acad Pediatr. 2018;18(3):248-255. doi: 10.1016/j.acap.2017.09.017 PubMed
11. Shaffer D, Pfeffer C. Practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7 Suppl):24-51. doi: 10.1097/00004583-200107001-00003
12. Thomas C, Holtzer C. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1023-1031. doi: 10.1097/01.chi.0000225353.16831.5d PubMed
13. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. 2018;141(6):e20172426. PubMed
14. Beach SR, Walker J, Celano CM, Mastromauro CA, Sharpe M, Huffman JC. Implementing collaborative care programs for psychiatric disorders in medical settings: a practical guide. Gen Hosp Psychiatry. 2015;37(6):522-527. doi: 10.1016/j.genhosppsych.2015.06.015 PubMed
15. Winters N, Pumariega A. Practice parameter on child and adolescent mental health care in community systems of care. J Am Acad Child Adolsc Psychiatry. 2007;46(2):284-299. DOI: 10.1097/01.chi.0000246061.70330.b8 PubMed
16. Claudius I, Donofrio J, Lam CN, Santillanes G. Impact of boarding pediatric psychiatric patients on a medical ward. Hosp Pediatr. 2014;4(3):125-131. doi: 10.1542/hpeds.2013-0079 PubMed
17. Gallagher KAS, Bujoreanu IS, Cheung P, Choi C, Golden S, Brodziak K. Psychiatric boarding in the pediatric inpatient medical setting: a retrospective analysis. Hosp Pediatr. 2013;7(8):444-450. doi: 10.1542/hpeds.2017-0005 PubMed
© 2018 Society of Hospital Medicine
The Virtual Hospitalist: The Future is Now
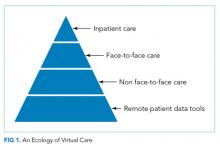
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
© 2018 Society of Hospital Medicine
The Continued Quest for Pediatric Readmission Risk Prediction
While the use of pediatric readmission rates as a quality metric remains controversial, pediatric hospital-to-home transitions need improvement.1 As many as a third of pediatric readmissions are preventable,2 but the multifactorial and complex nature of factors that contribute to pediatric readmissions presents a challenge in tackling readmission. Several factors are associated with increased risk of readmission; these factors include both clinical and sociodemographic characteristics;3 however, we still have much to learn. Further, the only large trial of an intervention to prevent pediatric readmissions across all comers (nontargeted) was unsuccessful in decreasing reutilization.4 By contrast, various studies have succeeded in reducing readmission and/or emergency department revisit rates associated with inpatient interventions in select populations.5 Currently, however, no standardized or validated pediatric risk prediction tool can reliably identify the high-risk patients who may benefit from interventions. In the Journal of Hospital Medicine, Brittan and colleagues add to the literature base exploring the factors associated with an increased 30-day readmission risk by trialing an electronic health record (EHR)-based tool composed of three components: presence of home health, polypharmacy in the form of ≥6 medications, and presence of a caregiver who prefers a language other than English.6
This brief report contributes significantly to the literature. First, the presence of a tool embedded within the pediatric EHR and readily accessible at the point of clinical care is novel. Study authors purposefully chose components easily extractable from the EHR which update automatically. This infrastructure generates an automated score that is easily accessible to clinicians in real-time. Second, the transparency of the tool is notable given its display via the EHR’s “Discharge Readiness Report,” where a clinician can view not only the total composite score (1 point for each component) but also the specific components for which a point was allocated. Although a composite score in and of itself is potentially helpful, understanding specific factors that contribute to a patient’s increased risk of readmission allows for better targeting of interventions. For example, in Brittan’s simple, three-component model, the presence of polypharmacy might trigger a pharmacist to meet with the family prior to discharge to discuss indications for and how to properly administer medications. Finally, a multidisciplinary team composed of clinicians, nurse-family educators, case managers, social workers, and informatics experts developed and implemented this tool. Although the roll-out and longitudinal use of this tool is not described, the engagement of these multiple provider-types is likely to increase successful roll-out and utilization of the tool.
Unfortunately, the utility of this tool in predicting readmission is limited as evidenced by its low c-statistic. This limitation may be due to several reasons. The tool was not originally built as a tool to predict readmissions but rather an instrument to identify complex discharge care as part of a quality improvement initiative to improve discharge processes. Given the questions about readmission risk prediction, the authors explored the potential for the tool to predict readmission risk. The authors acknowledge that the tool excluded many known readmission risk factors based upon inconsistent documentation within the EHR and the desire to emphasize only modifiable factors. Thus, variables, including prior hospitalization which is a well-documented risk factor for readmissions (but not modifiable) and social determinants of health (which are not consistently documented), were excluded. Additionally, the included variable of “language preference” may have been a considerably broad characteristic. Limited English proficiency has been increasingly recognized as a construct placing patients at higher risk for adverse outcomes. However, caregivers with high English proficiency also exhibit varying degrees of health literacy. The inclusion of health literacy may be additive to a readmission risk prediction tool. Finally, the outcome is not well-described with regard to identification of “unplanned” events. Thus, their outcome measure may have included planned admissions for which the readmission risk prediction tool would be irrelevant.
In summary, Brittan and colleagues engaged a multidisciplinary group of providers to address discharge planning processes and leveraged the EHR to support their efforts in the form of a brief screening tool. Although this tool was not predictive of hospital readmissions, it highlights the opportunity to better utilize the EHR to gather meaningful, real-time data and subsequently use this information to positively impact our clinical care and allocation of resources. The tool should serve as a stepping stone to building a more extensive tool with inclusion of other known and potential readmission risk factors, thus resulting in a clinically relevant readmission risk prediction tool.
The authors have nothing to disclose.
1. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics 2015;136(6):e1539-1549. doi: 10.1542/peds.2015-2098. PubMed
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-Day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2). doi: 10.1542/peds.2015-4182. PubMed
3. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
4. Auger KA, Simmons JM, Tubbs-Cooley H, et al. Hospital to home outcomes (H2O) randomized trial of a post-discharge nurse home visit. Pediatrics. In press. PubMed
5. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. doi: 10.1002/jhm.2134. PubMed
6. Brittan MS, Martin SL, Anderson, Moss A,Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions [published online ahead of print August 29, 2018]. J Hosp Med. doi: 10.12788/jhm.3043. PubMed
While the use of pediatric readmission rates as a quality metric remains controversial, pediatric hospital-to-home transitions need improvement.1 As many as a third of pediatric readmissions are preventable,2 but the multifactorial and complex nature of factors that contribute to pediatric readmissions presents a challenge in tackling readmission. Several factors are associated with increased risk of readmission; these factors include both clinical and sociodemographic characteristics;3 however, we still have much to learn. Further, the only large trial of an intervention to prevent pediatric readmissions across all comers (nontargeted) was unsuccessful in decreasing reutilization.4 By contrast, various studies have succeeded in reducing readmission and/or emergency department revisit rates associated with inpatient interventions in select populations.5 Currently, however, no standardized or validated pediatric risk prediction tool can reliably identify the high-risk patients who may benefit from interventions. In the Journal of Hospital Medicine, Brittan and colleagues add to the literature base exploring the factors associated with an increased 30-day readmission risk by trialing an electronic health record (EHR)-based tool composed of three components: presence of home health, polypharmacy in the form of ≥6 medications, and presence of a caregiver who prefers a language other than English.6
This brief report contributes significantly to the literature. First, the presence of a tool embedded within the pediatric EHR and readily accessible at the point of clinical care is novel. Study authors purposefully chose components easily extractable from the EHR which update automatically. This infrastructure generates an automated score that is easily accessible to clinicians in real-time. Second, the transparency of the tool is notable given its display via the EHR’s “Discharge Readiness Report,” where a clinician can view not only the total composite score (1 point for each component) but also the specific components for which a point was allocated. Although a composite score in and of itself is potentially helpful, understanding specific factors that contribute to a patient’s increased risk of readmission allows for better targeting of interventions. For example, in Brittan’s simple, three-component model, the presence of polypharmacy might trigger a pharmacist to meet with the family prior to discharge to discuss indications for and how to properly administer medications. Finally, a multidisciplinary team composed of clinicians, nurse-family educators, case managers, social workers, and informatics experts developed and implemented this tool. Although the roll-out and longitudinal use of this tool is not described, the engagement of these multiple provider-types is likely to increase successful roll-out and utilization of the tool.
Unfortunately, the utility of this tool in predicting readmission is limited as evidenced by its low c-statistic. This limitation may be due to several reasons. The tool was not originally built as a tool to predict readmissions but rather an instrument to identify complex discharge care as part of a quality improvement initiative to improve discharge processes. Given the questions about readmission risk prediction, the authors explored the potential for the tool to predict readmission risk. The authors acknowledge that the tool excluded many known readmission risk factors based upon inconsistent documentation within the EHR and the desire to emphasize only modifiable factors. Thus, variables, including prior hospitalization which is a well-documented risk factor for readmissions (but not modifiable) and social determinants of health (which are not consistently documented), were excluded. Additionally, the included variable of “language preference” may have been a considerably broad characteristic. Limited English proficiency has been increasingly recognized as a construct placing patients at higher risk for adverse outcomes. However, caregivers with high English proficiency also exhibit varying degrees of health literacy. The inclusion of health literacy may be additive to a readmission risk prediction tool. Finally, the outcome is not well-described with regard to identification of “unplanned” events. Thus, their outcome measure may have included planned admissions for which the readmission risk prediction tool would be irrelevant.
In summary, Brittan and colleagues engaged a multidisciplinary group of providers to address discharge planning processes and leveraged the EHR to support their efforts in the form of a brief screening tool. Although this tool was not predictive of hospital readmissions, it highlights the opportunity to better utilize the EHR to gather meaningful, real-time data and subsequently use this information to positively impact our clinical care and allocation of resources. The tool should serve as a stepping stone to building a more extensive tool with inclusion of other known and potential readmission risk factors, thus resulting in a clinically relevant readmission risk prediction tool.
The authors have nothing to disclose.
While the use of pediatric readmission rates as a quality metric remains controversial, pediatric hospital-to-home transitions need improvement.1 As many as a third of pediatric readmissions are preventable,2 but the multifactorial and complex nature of factors that contribute to pediatric readmissions presents a challenge in tackling readmission. Several factors are associated with increased risk of readmission; these factors include both clinical and sociodemographic characteristics;3 however, we still have much to learn. Further, the only large trial of an intervention to prevent pediatric readmissions across all comers (nontargeted) was unsuccessful in decreasing reutilization.4 By contrast, various studies have succeeded in reducing readmission and/or emergency department revisit rates associated with inpatient interventions in select populations.5 Currently, however, no standardized or validated pediatric risk prediction tool can reliably identify the high-risk patients who may benefit from interventions. In the Journal of Hospital Medicine, Brittan and colleagues add to the literature base exploring the factors associated with an increased 30-day readmission risk by trialing an electronic health record (EHR)-based tool composed of three components: presence of home health, polypharmacy in the form of ≥6 medications, and presence of a caregiver who prefers a language other than English.6
This brief report contributes significantly to the literature. First, the presence of a tool embedded within the pediatric EHR and readily accessible at the point of clinical care is novel. Study authors purposefully chose components easily extractable from the EHR which update automatically. This infrastructure generates an automated score that is easily accessible to clinicians in real-time. Second, the transparency of the tool is notable given its display via the EHR’s “Discharge Readiness Report,” where a clinician can view not only the total composite score (1 point for each component) but also the specific components for which a point was allocated. Although a composite score in and of itself is potentially helpful, understanding specific factors that contribute to a patient’s increased risk of readmission allows for better targeting of interventions. For example, in Brittan’s simple, three-component model, the presence of polypharmacy might trigger a pharmacist to meet with the family prior to discharge to discuss indications for and how to properly administer medications. Finally, a multidisciplinary team composed of clinicians, nurse-family educators, case managers, social workers, and informatics experts developed and implemented this tool. Although the roll-out and longitudinal use of this tool is not described, the engagement of these multiple provider-types is likely to increase successful roll-out and utilization of the tool.
Unfortunately, the utility of this tool in predicting readmission is limited as evidenced by its low c-statistic. This limitation may be due to several reasons. The tool was not originally built as a tool to predict readmissions but rather an instrument to identify complex discharge care as part of a quality improvement initiative to improve discharge processes. Given the questions about readmission risk prediction, the authors explored the potential for the tool to predict readmission risk. The authors acknowledge that the tool excluded many known readmission risk factors based upon inconsistent documentation within the EHR and the desire to emphasize only modifiable factors. Thus, variables, including prior hospitalization which is a well-documented risk factor for readmissions (but not modifiable) and social determinants of health (which are not consistently documented), were excluded. Additionally, the included variable of “language preference” may have been a considerably broad characteristic. Limited English proficiency has been increasingly recognized as a construct placing patients at higher risk for adverse outcomes. However, caregivers with high English proficiency also exhibit varying degrees of health literacy. The inclusion of health literacy may be additive to a readmission risk prediction tool. Finally, the outcome is not well-described with regard to identification of “unplanned” events. Thus, their outcome measure may have included planned admissions for which the readmission risk prediction tool would be irrelevant.
In summary, Brittan and colleagues engaged a multidisciplinary group of providers to address discharge planning processes and leveraged the EHR to support their efforts in the form of a brief screening tool. Although this tool was not predictive of hospital readmissions, it highlights the opportunity to better utilize the EHR to gather meaningful, real-time data and subsequently use this information to positively impact our clinical care and allocation of resources. The tool should serve as a stepping stone to building a more extensive tool with inclusion of other known and potential readmission risk factors, thus resulting in a clinically relevant readmission risk prediction tool.
The authors have nothing to disclose.
1. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics 2015;136(6):e1539-1549. doi: 10.1542/peds.2015-2098. PubMed
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-Day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2). doi: 10.1542/peds.2015-4182. PubMed
3. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
4. Auger KA, Simmons JM, Tubbs-Cooley H, et al. Hospital to home outcomes (H2O) randomized trial of a post-discharge nurse home visit. Pediatrics. In press. PubMed
5. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. doi: 10.1002/jhm.2134. PubMed
6. Brittan MS, Martin SL, Anderson, Moss A,Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions [published online ahead of print August 29, 2018]. J Hosp Med. doi: 10.12788/jhm.3043. PubMed
1. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics 2015;136(6):e1539-1549. doi: 10.1542/peds.2015-2098. PubMed
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-Day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2). doi: 10.1542/peds.2015-4182. PubMed
3. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
4. Auger KA, Simmons JM, Tubbs-Cooley H, et al. Hospital to home outcomes (H2O) randomized trial of a post-discharge nurse home visit. Pediatrics. In press. PubMed
5. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. doi: 10.1002/jhm.2134. PubMed
6. Brittan MS, Martin SL, Anderson, Moss A,Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions [published online ahead of print August 29, 2018]. J Hosp Med. doi: 10.12788/jhm.3043. PubMed
© 2018 Society of Hospital Medicine
Transcatheter aortic valve replacement for bicuspid aortic valve stenosis
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
Perioperative Management of ACE Inhibitor Therapy: Challenges of Clinical Decision-Making Based on Surrogate Endpoints
Renin-angiotensin inhibitors, which include angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), have demonstrated benefits in the treatment of several common cardiovascular and renal conditions. For example, they are prescribed to individuals with hypertension, heart failure with reduced ejection fraction (HFrEF), prior myocardial infarction, and chronic kidney disease with proteinuria. Perhaps unsurprisingly, many individuals presenting for surgery are already on long-term ACE inhibitor or ARB therapy. For example, such individuals comprised approximately one-third of the sample in the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) multicenter prospective cohort study of major inpatient noncardiac surgery.1
There is considerable controversy regarding how best to manage these cardiovascular medications during the perioperative period. The critical question pertains to whether renin-angiotensin inhibitors should be temporarily withdrawn 24 hours before surgery or continued uninterrupted up to the day of surgery. The main argument for withdrawing these medications is concern that they cause perioperative hypotension. For example, a recent systematic review of randomized controlled trials (RCTs) and cohort studies found that preoperative continuation of renin-angiotensin inhibitor therapy led to a significantly increased risk of intraoperative hypotension, albeit without associated effects on rates of death, major adverse cardiac events, or postoperative hypotension.2 Notably, randomized trial evidence in this meta-analysis was limited to only five trials with a total of 774 participants. Conversely, preoperative interruption of renin-angiotensin inhibitor therapy also has risks. For example, there is a potential for unintended permanent discontinuation of medications with long-term benefits.3 Furthermore, some prior cohort studies have demonstrated that the failure to resume renin-angiotensin inhibitor therapy promptly after surgery is associated with an elevated risk of postoperative mortality.4,5 While these studies have methodological limitations related to survivorship bias and unmeasured confounders, they still raise concerns that the abrupt withdrawal of long-term cardiovascular therapy before major surgery can have adverse effects. While ACE inhibitor withdrawal has not shown adverse physiological effects in the perioperative setting, it has led to rebound myocardial ischemia in patients with prior myocardial infarction.6
Given this controversy, there is variation across hospitals1 and practice guidelines with respect to perioperative management of renin-angiotensin inhibitors. For example, the 2017 Canadian Cardiovascular Society guidelines recommend that renin-angiotensin inhibitors be stopped temporarily 24 hours before major inpatient surgery,7 and the 2014 European guidelines recommend continuing therapy in patients with HFrEF but temporarily interrupting therapy in patients with hypertension.8 The 2014 American Heart Association and American College of Cardiology guidelines suggest that either continuation or interruption are reasonable options, but any interrupted therapy should be restarted postoperatively as soon as clinically feasible.9
In this issue of the Journal of Hospital Medicine, Shiffermiller and colleagues present a single-center RCT that provides additional high-quality data to improve our understanding of this important clinical issue.10 In a sample of 275 patients undergoing nonvascular inpatient noncardiac surgery, omission of the final dose of preoperative ACE inhibitor therapy reduced the risk of intraoperative hypotension across multiple definitions, including any episode of systolic blood pressure less than 80 mmHg (number needed to treat: 8), any episode of a systolic blood pressure less than 80 mmHg necessitating vasopressor therapy (number needed to treat: 6), and total cumulative duration of intraoperative systolic blood pressure less than 80 mmHg. In addition, the investigators found that preoperative interruption of ACE inhibitor therapy reduced the risk of postoperative hypotension (number needed to treat: 9), increased the risk of severe postoperative hypertension (number needed to harm: 9), and had no effect on clinical outcomes (eg, acute kidney injury, major adverse cardiac events). In conjunction with a recent systematic review,2 these new data demonstrate that temporary preoperative discontinuation of renin-angiotensin inhibitors leads to reduced risks of intraoperative and postoperative hypotension, with the only major identified risk being episodes of postoperative hypertension.
This current evidence base suggests that, in most cases, perioperative physicians should temporarily interrupt renin-angiotensin inhibitor therapy before inpatient noncardiac surgery, provided that protocols are in place to resume treatment postoperatively as soon as clinically feasible. Nonetheless, clinicians must also be cognizant of the key limitations to current data, namely that hypotension, be it intraoperative or postoperative, remains essentially a surrogate endpoint.11,12 Stated otherwise, the clinical importance of perioperative hypotension is largely predicated on its close association with clinically important or patient-relevant outcomes such as cardiovascular complications, acute kidney injury, and death.13–16 There is an implicit assumption that a reduction in the risk of hypotension will necessarily lead to reduced rates of clinical adverse events. This assumption is unlikely to be true, especially since many different underlying mechanisms lead to hypotension in the dynamic perioperative environment, including decreased cardiac contractility, decreased heart rate, decreased intravascular volume status, and vasodilation. Consistent with this possibility, different perioperative interventions with similar effects on hypotension have shown quite different effects on clinical outcomes. For example, epidural analgesia invariably reduces perioperative blood pressure, yet it does not appear to increase the risk of postoperative complications.17 Similarly, both beta-blockers and clonidine increase the risk of significant perioperative hypotension and bradycardia, yet only beta-blockers appear to lead to increased rates of mortality after noncardiac surgery.18,19 Thus, the relationship between perioperative hypotension and outcomes is clearly complex. Unless a RCT demonstrates that a hypotension-reduction strategy leads to an improvement in clinical outcomes,20 perioperative physicians should not assume that prevention of hypotension will always lead to improvements in patient-relevant clinical outcomes. Similar assumptions about other surrogate endpoints in cardiovascular medicine have sometimes been spectacularly incorrect.12,21 To more definitively address this important clinical issue, RCTs must be specifically designed to compare the effects of renin-angiotensin inhibitor therapy withdrawal versus continuation on patient-relevant and clinically important outcomes, such as death, myocardial infarction, and stroke. Fortunately, some ongoing trials will address this question, either directly (ClinicalTrials.gov NCT03374449) or as a component of a hypotension-avoidance strategy (ClinicalTrials.gov NCT03505723).
Overall, perioperative physicians should now adopt the standard approach of temporarily withdrawing renin-angiotensin inhibitor therapy 24 hours before major inpatient noncardiac surgery. Nonetheless, they should do so cautiously, recognizing that the data underpinning this strategy remain weak. As with many aspects of perioperative medicine, more research remains needed.
Disclosures
The authors have nothing to report.Funding: DNW is supported in part by a New Investigator Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto.
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: An Analysis of the Vascular events In noncardiac Surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
2. Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery [published online ahead of print January 29, 2018]. Anesth Analg. doi: 10.1213/ANE.0000000000002837. PubMed
3. Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525-2531. doi: 10.1001/archinte.166.22.2525. PubMed
4. Mudumbai SC, Takemoto S, Cason BA, Au S, Upadhyay A, Wallace AW. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289-296. doi: 10.1002/jhm.2182. PubMed
5. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology. 2015;123(2):288-306. doi: 10.1097/ALN.0000000000000739. PubMed
6. van den Heuvel AF, van Gilst WH, van Veldhuisen DJ, de Vries RJ, Dunselman PH, Kingma JH. Long-term anti-ischemic effects of angiotensin-converting enzyme inhibition in patients after myocardial infarction. J Am Coll Cardiol. 1997;30(2):400-405. doi: 10.1016/S0735-1097(97)00183-6 PubMed
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. doi: 10.1016/j.cjca.2016.09.008. PubMed
8. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management./ The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282. PubMed
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. doi: 10.1161/CIR.0000000000000105. PubMed
10. Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin converting enzyme inhibition (PREOP-ACEI) [published online ahead of print July 25, 2018]. J Hosp Med. doi: 10.12788/jhm.3036. PubMed
11. Psaty BM, Weiss NS, Furberg CD, et al. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA. 1999;282(8):786-790. doi: 10.1001/jama.282.8.786. PubMed
12. Vanderweele TJ. Surrogate measures and consistent surrogates. Biometrics. 2013;69(3):561-569. doi: 10.1111/biom.12071. PubMed
13. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515-523. doi: 10.1097/ALN.0000000000000765. PubMed
14. van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35-44. doi: 10.1097/ALN.0000000000000922. PubMed
15. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319.doi: 10.1097/ALN.0000000000000756. PubMed
17. Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276-1282. doi: 10.1016/S0140-6736(02)08266-1. PubMed
18. POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. doi: 10.1016/S0140-6736(08)60601-7. PubMed
19. Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1504-1513. doi: 10.1056/NEJMoa1401106. PubMed
20. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346-1357. doi: 10.1001/jama.2017.14172. PubMed
21. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629 PubMed
Renin-angiotensin inhibitors, which include angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), have demonstrated benefits in the treatment of several common cardiovascular and renal conditions. For example, they are prescribed to individuals with hypertension, heart failure with reduced ejection fraction (HFrEF), prior myocardial infarction, and chronic kidney disease with proteinuria. Perhaps unsurprisingly, many individuals presenting for surgery are already on long-term ACE inhibitor or ARB therapy. For example, such individuals comprised approximately one-third of the sample in the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) multicenter prospective cohort study of major inpatient noncardiac surgery.1
There is considerable controversy regarding how best to manage these cardiovascular medications during the perioperative period. The critical question pertains to whether renin-angiotensin inhibitors should be temporarily withdrawn 24 hours before surgery or continued uninterrupted up to the day of surgery. The main argument for withdrawing these medications is concern that they cause perioperative hypotension. For example, a recent systematic review of randomized controlled trials (RCTs) and cohort studies found that preoperative continuation of renin-angiotensin inhibitor therapy led to a significantly increased risk of intraoperative hypotension, albeit without associated effects on rates of death, major adverse cardiac events, or postoperative hypotension.2 Notably, randomized trial evidence in this meta-analysis was limited to only five trials with a total of 774 participants. Conversely, preoperative interruption of renin-angiotensin inhibitor therapy also has risks. For example, there is a potential for unintended permanent discontinuation of medications with long-term benefits.3 Furthermore, some prior cohort studies have demonstrated that the failure to resume renin-angiotensin inhibitor therapy promptly after surgery is associated with an elevated risk of postoperative mortality.4,5 While these studies have methodological limitations related to survivorship bias and unmeasured confounders, they still raise concerns that the abrupt withdrawal of long-term cardiovascular therapy before major surgery can have adverse effects. While ACE inhibitor withdrawal has not shown adverse physiological effects in the perioperative setting, it has led to rebound myocardial ischemia in patients with prior myocardial infarction.6
Given this controversy, there is variation across hospitals1 and practice guidelines with respect to perioperative management of renin-angiotensin inhibitors. For example, the 2017 Canadian Cardiovascular Society guidelines recommend that renin-angiotensin inhibitors be stopped temporarily 24 hours before major inpatient surgery,7 and the 2014 European guidelines recommend continuing therapy in patients with HFrEF but temporarily interrupting therapy in patients with hypertension.8 The 2014 American Heart Association and American College of Cardiology guidelines suggest that either continuation or interruption are reasonable options, but any interrupted therapy should be restarted postoperatively as soon as clinically feasible.9
In this issue of the Journal of Hospital Medicine, Shiffermiller and colleagues present a single-center RCT that provides additional high-quality data to improve our understanding of this important clinical issue.10 In a sample of 275 patients undergoing nonvascular inpatient noncardiac surgery, omission of the final dose of preoperative ACE inhibitor therapy reduced the risk of intraoperative hypotension across multiple definitions, including any episode of systolic blood pressure less than 80 mmHg (number needed to treat: 8), any episode of a systolic blood pressure less than 80 mmHg necessitating vasopressor therapy (number needed to treat: 6), and total cumulative duration of intraoperative systolic blood pressure less than 80 mmHg. In addition, the investigators found that preoperative interruption of ACE inhibitor therapy reduced the risk of postoperative hypotension (number needed to treat: 9), increased the risk of severe postoperative hypertension (number needed to harm: 9), and had no effect on clinical outcomes (eg, acute kidney injury, major adverse cardiac events). In conjunction with a recent systematic review,2 these new data demonstrate that temporary preoperative discontinuation of renin-angiotensin inhibitors leads to reduced risks of intraoperative and postoperative hypotension, with the only major identified risk being episodes of postoperative hypertension.
This current evidence base suggests that, in most cases, perioperative physicians should temporarily interrupt renin-angiotensin inhibitor therapy before inpatient noncardiac surgery, provided that protocols are in place to resume treatment postoperatively as soon as clinically feasible. Nonetheless, clinicians must also be cognizant of the key limitations to current data, namely that hypotension, be it intraoperative or postoperative, remains essentially a surrogate endpoint.11,12 Stated otherwise, the clinical importance of perioperative hypotension is largely predicated on its close association with clinically important or patient-relevant outcomes such as cardiovascular complications, acute kidney injury, and death.13–16 There is an implicit assumption that a reduction in the risk of hypotension will necessarily lead to reduced rates of clinical adverse events. This assumption is unlikely to be true, especially since many different underlying mechanisms lead to hypotension in the dynamic perioperative environment, including decreased cardiac contractility, decreased heart rate, decreased intravascular volume status, and vasodilation. Consistent with this possibility, different perioperative interventions with similar effects on hypotension have shown quite different effects on clinical outcomes. For example, epidural analgesia invariably reduces perioperative blood pressure, yet it does not appear to increase the risk of postoperative complications.17 Similarly, both beta-blockers and clonidine increase the risk of significant perioperative hypotension and bradycardia, yet only beta-blockers appear to lead to increased rates of mortality after noncardiac surgery.18,19 Thus, the relationship between perioperative hypotension and outcomes is clearly complex. Unless a RCT demonstrates that a hypotension-reduction strategy leads to an improvement in clinical outcomes,20 perioperative physicians should not assume that prevention of hypotension will always lead to improvements in patient-relevant clinical outcomes. Similar assumptions about other surrogate endpoints in cardiovascular medicine have sometimes been spectacularly incorrect.12,21 To more definitively address this important clinical issue, RCTs must be specifically designed to compare the effects of renin-angiotensin inhibitor therapy withdrawal versus continuation on patient-relevant and clinically important outcomes, such as death, myocardial infarction, and stroke. Fortunately, some ongoing trials will address this question, either directly (ClinicalTrials.gov NCT03374449) or as a component of a hypotension-avoidance strategy (ClinicalTrials.gov NCT03505723).
Overall, perioperative physicians should now adopt the standard approach of temporarily withdrawing renin-angiotensin inhibitor therapy 24 hours before major inpatient noncardiac surgery. Nonetheless, they should do so cautiously, recognizing that the data underpinning this strategy remain weak. As with many aspects of perioperative medicine, more research remains needed.
Disclosures
The authors have nothing to report.Funding: DNW is supported in part by a New Investigator Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto.
Renin-angiotensin inhibitors, which include angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), have demonstrated benefits in the treatment of several common cardiovascular and renal conditions. For example, they are prescribed to individuals with hypertension, heart failure with reduced ejection fraction (HFrEF), prior myocardial infarction, and chronic kidney disease with proteinuria. Perhaps unsurprisingly, many individuals presenting for surgery are already on long-term ACE inhibitor or ARB therapy. For example, such individuals comprised approximately one-third of the sample in the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) multicenter prospective cohort study of major inpatient noncardiac surgery.1
There is considerable controversy regarding how best to manage these cardiovascular medications during the perioperative period. The critical question pertains to whether renin-angiotensin inhibitors should be temporarily withdrawn 24 hours before surgery or continued uninterrupted up to the day of surgery. The main argument for withdrawing these medications is concern that they cause perioperative hypotension. For example, a recent systematic review of randomized controlled trials (RCTs) and cohort studies found that preoperative continuation of renin-angiotensin inhibitor therapy led to a significantly increased risk of intraoperative hypotension, albeit without associated effects on rates of death, major adverse cardiac events, or postoperative hypotension.2 Notably, randomized trial evidence in this meta-analysis was limited to only five trials with a total of 774 participants. Conversely, preoperative interruption of renin-angiotensin inhibitor therapy also has risks. For example, there is a potential for unintended permanent discontinuation of medications with long-term benefits.3 Furthermore, some prior cohort studies have demonstrated that the failure to resume renin-angiotensin inhibitor therapy promptly after surgery is associated with an elevated risk of postoperative mortality.4,5 While these studies have methodological limitations related to survivorship bias and unmeasured confounders, they still raise concerns that the abrupt withdrawal of long-term cardiovascular therapy before major surgery can have adverse effects. While ACE inhibitor withdrawal has not shown adverse physiological effects in the perioperative setting, it has led to rebound myocardial ischemia in patients with prior myocardial infarction.6
Given this controversy, there is variation across hospitals1 and practice guidelines with respect to perioperative management of renin-angiotensin inhibitors. For example, the 2017 Canadian Cardiovascular Society guidelines recommend that renin-angiotensin inhibitors be stopped temporarily 24 hours before major inpatient surgery,7 and the 2014 European guidelines recommend continuing therapy in patients with HFrEF but temporarily interrupting therapy in patients with hypertension.8 The 2014 American Heart Association and American College of Cardiology guidelines suggest that either continuation or interruption are reasonable options, but any interrupted therapy should be restarted postoperatively as soon as clinically feasible.9
In this issue of the Journal of Hospital Medicine, Shiffermiller and colleagues present a single-center RCT that provides additional high-quality data to improve our understanding of this important clinical issue.10 In a sample of 275 patients undergoing nonvascular inpatient noncardiac surgery, omission of the final dose of preoperative ACE inhibitor therapy reduced the risk of intraoperative hypotension across multiple definitions, including any episode of systolic blood pressure less than 80 mmHg (number needed to treat: 8), any episode of a systolic blood pressure less than 80 mmHg necessitating vasopressor therapy (number needed to treat: 6), and total cumulative duration of intraoperative systolic blood pressure less than 80 mmHg. In addition, the investigators found that preoperative interruption of ACE inhibitor therapy reduced the risk of postoperative hypotension (number needed to treat: 9), increased the risk of severe postoperative hypertension (number needed to harm: 9), and had no effect on clinical outcomes (eg, acute kidney injury, major adverse cardiac events). In conjunction with a recent systematic review,2 these new data demonstrate that temporary preoperative discontinuation of renin-angiotensin inhibitors leads to reduced risks of intraoperative and postoperative hypotension, with the only major identified risk being episodes of postoperative hypertension.
This current evidence base suggests that, in most cases, perioperative physicians should temporarily interrupt renin-angiotensin inhibitor therapy before inpatient noncardiac surgery, provided that protocols are in place to resume treatment postoperatively as soon as clinically feasible. Nonetheless, clinicians must also be cognizant of the key limitations to current data, namely that hypotension, be it intraoperative or postoperative, remains essentially a surrogate endpoint.11,12 Stated otherwise, the clinical importance of perioperative hypotension is largely predicated on its close association with clinically important or patient-relevant outcomes such as cardiovascular complications, acute kidney injury, and death.13–16 There is an implicit assumption that a reduction in the risk of hypotension will necessarily lead to reduced rates of clinical adverse events. This assumption is unlikely to be true, especially since many different underlying mechanisms lead to hypotension in the dynamic perioperative environment, including decreased cardiac contractility, decreased heart rate, decreased intravascular volume status, and vasodilation. Consistent with this possibility, different perioperative interventions with similar effects on hypotension have shown quite different effects on clinical outcomes. For example, epidural analgesia invariably reduces perioperative blood pressure, yet it does not appear to increase the risk of postoperative complications.17 Similarly, both beta-blockers and clonidine increase the risk of significant perioperative hypotension and bradycardia, yet only beta-blockers appear to lead to increased rates of mortality after noncardiac surgery.18,19 Thus, the relationship between perioperative hypotension and outcomes is clearly complex. Unless a RCT demonstrates that a hypotension-reduction strategy leads to an improvement in clinical outcomes,20 perioperative physicians should not assume that prevention of hypotension will always lead to improvements in patient-relevant clinical outcomes. Similar assumptions about other surrogate endpoints in cardiovascular medicine have sometimes been spectacularly incorrect.12,21 To more definitively address this important clinical issue, RCTs must be specifically designed to compare the effects of renin-angiotensin inhibitor therapy withdrawal versus continuation on patient-relevant and clinically important outcomes, such as death, myocardial infarction, and stroke. Fortunately, some ongoing trials will address this question, either directly (ClinicalTrials.gov NCT03374449) or as a component of a hypotension-avoidance strategy (ClinicalTrials.gov NCT03505723).
Overall, perioperative physicians should now adopt the standard approach of temporarily withdrawing renin-angiotensin inhibitor therapy 24 hours before major inpatient noncardiac surgery. Nonetheless, they should do so cautiously, recognizing that the data underpinning this strategy remain weak. As with many aspects of perioperative medicine, more research remains needed.
Disclosures
The authors have nothing to report.Funding: DNW is supported in part by a New Investigator Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto.
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: An Analysis of the Vascular events In noncardiac Surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
2. Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery [published online ahead of print January 29, 2018]. Anesth Analg. doi: 10.1213/ANE.0000000000002837. PubMed
3. Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525-2531. doi: 10.1001/archinte.166.22.2525. PubMed
4. Mudumbai SC, Takemoto S, Cason BA, Au S, Upadhyay A, Wallace AW. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289-296. doi: 10.1002/jhm.2182. PubMed
5. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology. 2015;123(2):288-306. doi: 10.1097/ALN.0000000000000739. PubMed
6. van den Heuvel AF, van Gilst WH, van Veldhuisen DJ, de Vries RJ, Dunselman PH, Kingma JH. Long-term anti-ischemic effects of angiotensin-converting enzyme inhibition in patients after myocardial infarction. J Am Coll Cardiol. 1997;30(2):400-405. doi: 10.1016/S0735-1097(97)00183-6 PubMed
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. doi: 10.1016/j.cjca.2016.09.008. PubMed
8. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management./ The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282. PubMed
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. doi: 10.1161/CIR.0000000000000105. PubMed
10. Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin converting enzyme inhibition (PREOP-ACEI) [published online ahead of print July 25, 2018]. J Hosp Med. doi: 10.12788/jhm.3036. PubMed
11. Psaty BM, Weiss NS, Furberg CD, et al. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA. 1999;282(8):786-790. doi: 10.1001/jama.282.8.786. PubMed
12. Vanderweele TJ. Surrogate measures and consistent surrogates. Biometrics. 2013;69(3):561-569. doi: 10.1111/biom.12071. PubMed
13. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515-523. doi: 10.1097/ALN.0000000000000765. PubMed
14. van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35-44. doi: 10.1097/ALN.0000000000000922. PubMed
15. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319.doi: 10.1097/ALN.0000000000000756. PubMed
17. Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276-1282. doi: 10.1016/S0140-6736(02)08266-1. PubMed
18. POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. doi: 10.1016/S0140-6736(08)60601-7. PubMed
19. Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1504-1513. doi: 10.1056/NEJMoa1401106. PubMed
20. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346-1357. doi: 10.1001/jama.2017.14172. PubMed
21. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629 PubMed
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: An Analysis of the Vascular events In noncardiac Surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
2. Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery [published online ahead of print January 29, 2018]. Anesth Analg. doi: 10.1213/ANE.0000000000002837. PubMed
3. Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525-2531. doi: 10.1001/archinte.166.22.2525. PubMed
4. Mudumbai SC, Takemoto S, Cason BA, Au S, Upadhyay A, Wallace AW. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289-296. doi: 10.1002/jhm.2182. PubMed
5. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology. 2015;123(2):288-306. doi: 10.1097/ALN.0000000000000739. PubMed
6. van den Heuvel AF, van Gilst WH, van Veldhuisen DJ, de Vries RJ, Dunselman PH, Kingma JH. Long-term anti-ischemic effects of angiotensin-converting enzyme inhibition in patients after myocardial infarction. J Am Coll Cardiol. 1997;30(2):400-405. doi: 10.1016/S0735-1097(97)00183-6 PubMed
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. doi: 10.1016/j.cjca.2016.09.008. PubMed
8. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management./ The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282. PubMed
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. doi: 10.1161/CIR.0000000000000105. PubMed
10. Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin converting enzyme inhibition (PREOP-ACEI) [published online ahead of print July 25, 2018]. J Hosp Med. doi: 10.12788/jhm.3036. PubMed
11. Psaty BM, Weiss NS, Furberg CD, et al. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA. 1999;282(8):786-790. doi: 10.1001/jama.282.8.786. PubMed
12. Vanderweele TJ. Surrogate measures and consistent surrogates. Biometrics. 2013;69(3):561-569. doi: 10.1111/biom.12071. PubMed
13. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515-523. doi: 10.1097/ALN.0000000000000765. PubMed
14. van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35-44. doi: 10.1097/ALN.0000000000000922. PubMed
15. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319.doi: 10.1097/ALN.0000000000000756. PubMed
17. Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276-1282. doi: 10.1016/S0140-6736(02)08266-1. PubMed
18. POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. doi: 10.1016/S0140-6736(08)60601-7. PubMed
19. Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1504-1513. doi: 10.1056/NEJMoa1401106. PubMed
20. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346-1357. doi: 10.1001/jama.2017.14172. PubMed
21. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629 PubMed
© 2018 Society of Hospital Medicine
Coronary artery calcium scoring: A valuable tool in primary care
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
Postdischarge Emergency Department Visits: Good, Bad, or Ugly?
Once upon a time, discharges were easy to categorize: good, bad, or ugly. Good discharges allowed the patient to leave before noon, while bad discharges allowed the patient to leave without follow-up appointments. The worst discharges were defined by the two ugly cousins of acute care re-escalation: return emergency department (ED) visits and readmissions. Recently, however, much of this conventional wisdom has been turned on its head. For example, pre-noon discharges and provider-scheduled follow-up appointments may lead to unintended negative consequences and futility.1,2 In contrast, weekend discharges, which were often viewed to be unsafe, may reduce lengths of stay without compromising care even in high-risk patients.3
Having obfuscated the line between good and bad, we can now turn our attention to the ugly. Comparing return ED visits with readmissions, hospitalists may be forgiven for judging the latter cousin as uglier – and not just for reimbursement reasons. Readmitted patients are sicker, more vulnerable, and have poorer outcomes. In our healthcare system’s resultant quest to eliminate readmissions, return ED visits that do not end in readmission are generally either ignored or grouped with readmissions. Ignoring these treat-and-discharge ED visits is problematic because of their incidence, which rivals that of ED visits ending in readmission.4 On the other hand, grouping these visits with readmissions only makes sense if the two are considered to be equally ugly outcomes. Is this a valid assumption to make?
In this issue of the Journal of Hospital Medicine, Venkatesh et al5 tackle that question by studying Medicare beneficiaries hospitalized for acute myocardial infarction, heart failure, or pneumonia over a 1-year period. The authors differentiate 30-day treat-and-discharge ED visit rates from 30-day readmission rates before risk-standardizing these rates based on visit codes and hospital characteristics. Similar to the results of prior studies, the authors observe an 8%–9% overall incidence of treat-and-discharge ED visits within 30 days of hospital discharge.6 Mapping treat-and-discharge ED visit rates versus readmission rates for each hospital, the authors detect modest but noticeable inverse correlations between the two. Among hospitals discharging heart failure patients, for example, every 10% increase in postdischarge ED visit rates corresponds to a roughly 2% decrease in readmission rates.
The authors are correct to tread cautiously with their interpretation of this correlation. Dispositions for ED patients exist on a continuum, so hospitals with higher propensities to discharge patients from EDs (whether directly or from observation units) will inherently have lower admission rates. The authors hint at a causal relationship nonetheless, suggesting that ED providers may be able to intervene on high-risk patients earlier before Readmission Road becomes a one-way street. Proving this hypothesis will require careful research that controls for patient, disease, and ED factors as well as their complex interactions in the post-discharge timeline. That being said, most analyses of outpatient follow-up visits (except for heart failure patients) have failed to find any anti-readmission correlation analogous to that identified by Venkatesh et al. What powers do ED providers have that outpatient providers lack? Many, admittedly: stat phlebotomy services, on-demand consultations, and observation units. Additionally, while ED visits invariably require a patient’s presence in person, 25% of provider-scheduled posthospitalization outpatient visits end in no-shows.2 Whether patient-triggered follow-up through rapid access clinics or even urgent care centers can replicate ED functionality in recently discharged patients is unknown and warrants further study.
Venkatesh et al5 also find that reasons for postdischarge ED visits bear only a slight resemblance to reasons for index hospitalizations. For example, of all ED visits by patients recovering from hospitalizations for pneumonia, only 20% involve respiratory or pulmonary complaints. What explains the other 80%? Some variability may be attributable to the study’s use of visit codes instead of chart reviews or stakeholder interviews; in surveys of patients and ED physicians during these postdischarge visits, the two groups may have very different perceptions of why the encounter is occurring and whether it is preventable.7 Regardless of who is “right,” the heterogeneity of reasons that prompt care re-escalation lends further credence to the existence of a distinct posthospitalization syndrome:8 in the immediate postdischarge interval, patients experience many transient but real physiological risks for which they may identify the ED as their best recourse.
Whether the ED actually provides secondary prophylaxis against the posthospitalization syndrome is highly debatable, and Venkatesh et al wisely refrain from assigning a positive or negative valence to treat-and-discharge ED visits. Ultimately, postdischarge ED visits are neither inherently good nor bad (nor ugly, for that matter). Their unique nature is attracting newfound appreciation, and their potential ability to prevent readmission merits further research. If hospitals with high postdischarge ED visit rates can deliver high-quality care while truly arresting or reversing readmission-bound trajectories, then the strategies employed by these hospitals should inspire emulation, innovation, and dissemination.
Disclosures
The authors have nothing to disclose.
1. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. 10.1002/jhm.2529. PubMed
2. Banerjee R, Suarez A, Kier M, et al. If you book it, will they come? Attendance at postdischarge follow-up visits scheduled by inpatient providers. J Hosp Med. 2017;12(8):618-625. 10.12788/jhm.2777. PubMed
3. McAlister FA, Youngson E, Padwal RS, Majumdar SR. Similar outcomes among general medicine patients discharged on weekends. J Hosp Med. 2015;10(2):69-74. 10.1002/jhm.2310. PubMed
4. Rising KL, White LF, Fernandez WG, Boutwell AE. Emergency department visits after hospital discharge: a missing part of the equation. Ann Emerg Med. 2013;62(2):145-150. 10.1016/j.annemergmed.2013.01.024. PubMed
5. Venkatesh A, Wang C, Wang Y, Altaf F, Bernheim S, Horwitz L. Association between post-discharge emergency department visitation and readmission rates J Hosp Med. 2018;13(9):589-594. doi: 10.12788/jhm.2937.
6. Vashi AA, Fox JP, Carr BG, et al. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364-371. 10.1001/jama.2012.216219. PubMed
7. Suffoletto B, Hu J, Guyette M, Callaway C. Factors contributing to emergency department care within 30 days of hospital discharge and potential ways to prevent it: differences in perspectives of patients, caregivers, and emergency physicians. J Hosp Med. 2014;9(5):315-319. 10.1002/jhm.2167. PubMed
8. Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. 10.1056/NEJMp1212324. PubMed
Once upon a time, discharges were easy to categorize: good, bad, or ugly. Good discharges allowed the patient to leave before noon, while bad discharges allowed the patient to leave without follow-up appointments. The worst discharges were defined by the two ugly cousins of acute care re-escalation: return emergency department (ED) visits and readmissions. Recently, however, much of this conventional wisdom has been turned on its head. For example, pre-noon discharges and provider-scheduled follow-up appointments may lead to unintended negative consequences and futility.1,2 In contrast, weekend discharges, which were often viewed to be unsafe, may reduce lengths of stay without compromising care even in high-risk patients.3
Having obfuscated the line between good and bad, we can now turn our attention to the ugly. Comparing return ED visits with readmissions, hospitalists may be forgiven for judging the latter cousin as uglier – and not just for reimbursement reasons. Readmitted patients are sicker, more vulnerable, and have poorer outcomes. In our healthcare system’s resultant quest to eliminate readmissions, return ED visits that do not end in readmission are generally either ignored or grouped with readmissions. Ignoring these treat-and-discharge ED visits is problematic because of their incidence, which rivals that of ED visits ending in readmission.4 On the other hand, grouping these visits with readmissions only makes sense if the two are considered to be equally ugly outcomes. Is this a valid assumption to make?
In this issue of the Journal of Hospital Medicine, Venkatesh et al5 tackle that question by studying Medicare beneficiaries hospitalized for acute myocardial infarction, heart failure, or pneumonia over a 1-year period. The authors differentiate 30-day treat-and-discharge ED visit rates from 30-day readmission rates before risk-standardizing these rates based on visit codes and hospital characteristics. Similar to the results of prior studies, the authors observe an 8%–9% overall incidence of treat-and-discharge ED visits within 30 days of hospital discharge.6 Mapping treat-and-discharge ED visit rates versus readmission rates for each hospital, the authors detect modest but noticeable inverse correlations between the two. Among hospitals discharging heart failure patients, for example, every 10% increase in postdischarge ED visit rates corresponds to a roughly 2% decrease in readmission rates.
The authors are correct to tread cautiously with their interpretation of this correlation. Dispositions for ED patients exist on a continuum, so hospitals with higher propensities to discharge patients from EDs (whether directly or from observation units) will inherently have lower admission rates. The authors hint at a causal relationship nonetheless, suggesting that ED providers may be able to intervene on high-risk patients earlier before Readmission Road becomes a one-way street. Proving this hypothesis will require careful research that controls for patient, disease, and ED factors as well as their complex interactions in the post-discharge timeline. That being said, most analyses of outpatient follow-up visits (except for heart failure patients) have failed to find any anti-readmission correlation analogous to that identified by Venkatesh et al. What powers do ED providers have that outpatient providers lack? Many, admittedly: stat phlebotomy services, on-demand consultations, and observation units. Additionally, while ED visits invariably require a patient’s presence in person, 25% of provider-scheduled posthospitalization outpatient visits end in no-shows.2 Whether patient-triggered follow-up through rapid access clinics or even urgent care centers can replicate ED functionality in recently discharged patients is unknown and warrants further study.
Venkatesh et al5 also find that reasons for postdischarge ED visits bear only a slight resemblance to reasons for index hospitalizations. For example, of all ED visits by patients recovering from hospitalizations for pneumonia, only 20% involve respiratory or pulmonary complaints. What explains the other 80%? Some variability may be attributable to the study’s use of visit codes instead of chart reviews or stakeholder interviews; in surveys of patients and ED physicians during these postdischarge visits, the two groups may have very different perceptions of why the encounter is occurring and whether it is preventable.7 Regardless of who is “right,” the heterogeneity of reasons that prompt care re-escalation lends further credence to the existence of a distinct posthospitalization syndrome:8 in the immediate postdischarge interval, patients experience many transient but real physiological risks for which they may identify the ED as their best recourse.
Whether the ED actually provides secondary prophylaxis against the posthospitalization syndrome is highly debatable, and Venkatesh et al wisely refrain from assigning a positive or negative valence to treat-and-discharge ED visits. Ultimately, postdischarge ED visits are neither inherently good nor bad (nor ugly, for that matter). Their unique nature is attracting newfound appreciation, and their potential ability to prevent readmission merits further research. If hospitals with high postdischarge ED visit rates can deliver high-quality care while truly arresting or reversing readmission-bound trajectories, then the strategies employed by these hospitals should inspire emulation, innovation, and dissemination.
Disclosures
The authors have nothing to disclose.
Once upon a time, discharges were easy to categorize: good, bad, or ugly. Good discharges allowed the patient to leave before noon, while bad discharges allowed the patient to leave without follow-up appointments. The worst discharges were defined by the two ugly cousins of acute care re-escalation: return emergency department (ED) visits and readmissions. Recently, however, much of this conventional wisdom has been turned on its head. For example, pre-noon discharges and provider-scheduled follow-up appointments may lead to unintended negative consequences and futility.1,2 In contrast, weekend discharges, which were often viewed to be unsafe, may reduce lengths of stay without compromising care even in high-risk patients.3
Having obfuscated the line between good and bad, we can now turn our attention to the ugly. Comparing return ED visits with readmissions, hospitalists may be forgiven for judging the latter cousin as uglier – and not just for reimbursement reasons. Readmitted patients are sicker, more vulnerable, and have poorer outcomes. In our healthcare system’s resultant quest to eliminate readmissions, return ED visits that do not end in readmission are generally either ignored or grouped with readmissions. Ignoring these treat-and-discharge ED visits is problematic because of their incidence, which rivals that of ED visits ending in readmission.4 On the other hand, grouping these visits with readmissions only makes sense if the two are considered to be equally ugly outcomes. Is this a valid assumption to make?
In this issue of the Journal of Hospital Medicine, Venkatesh et al5 tackle that question by studying Medicare beneficiaries hospitalized for acute myocardial infarction, heart failure, or pneumonia over a 1-year period. The authors differentiate 30-day treat-and-discharge ED visit rates from 30-day readmission rates before risk-standardizing these rates based on visit codes and hospital characteristics. Similar to the results of prior studies, the authors observe an 8%–9% overall incidence of treat-and-discharge ED visits within 30 days of hospital discharge.6 Mapping treat-and-discharge ED visit rates versus readmission rates for each hospital, the authors detect modest but noticeable inverse correlations between the two. Among hospitals discharging heart failure patients, for example, every 10% increase in postdischarge ED visit rates corresponds to a roughly 2% decrease in readmission rates.
The authors are correct to tread cautiously with their interpretation of this correlation. Dispositions for ED patients exist on a continuum, so hospitals with higher propensities to discharge patients from EDs (whether directly or from observation units) will inherently have lower admission rates. The authors hint at a causal relationship nonetheless, suggesting that ED providers may be able to intervene on high-risk patients earlier before Readmission Road becomes a one-way street. Proving this hypothesis will require careful research that controls for patient, disease, and ED factors as well as their complex interactions in the post-discharge timeline. That being said, most analyses of outpatient follow-up visits (except for heart failure patients) have failed to find any anti-readmission correlation analogous to that identified by Venkatesh et al. What powers do ED providers have that outpatient providers lack? Many, admittedly: stat phlebotomy services, on-demand consultations, and observation units. Additionally, while ED visits invariably require a patient’s presence in person, 25% of provider-scheduled posthospitalization outpatient visits end in no-shows.2 Whether patient-triggered follow-up through rapid access clinics or even urgent care centers can replicate ED functionality in recently discharged patients is unknown and warrants further study.
Venkatesh et al5 also find that reasons for postdischarge ED visits bear only a slight resemblance to reasons for index hospitalizations. For example, of all ED visits by patients recovering from hospitalizations for pneumonia, only 20% involve respiratory or pulmonary complaints. What explains the other 80%? Some variability may be attributable to the study’s use of visit codes instead of chart reviews or stakeholder interviews; in surveys of patients and ED physicians during these postdischarge visits, the two groups may have very different perceptions of why the encounter is occurring and whether it is preventable.7 Regardless of who is “right,” the heterogeneity of reasons that prompt care re-escalation lends further credence to the existence of a distinct posthospitalization syndrome:8 in the immediate postdischarge interval, patients experience many transient but real physiological risks for which they may identify the ED as their best recourse.
Whether the ED actually provides secondary prophylaxis against the posthospitalization syndrome is highly debatable, and Venkatesh et al wisely refrain from assigning a positive or negative valence to treat-and-discharge ED visits. Ultimately, postdischarge ED visits are neither inherently good nor bad (nor ugly, for that matter). Their unique nature is attracting newfound appreciation, and their potential ability to prevent readmission merits further research. If hospitals with high postdischarge ED visit rates can deliver high-quality care while truly arresting or reversing readmission-bound trajectories, then the strategies employed by these hospitals should inspire emulation, innovation, and dissemination.
Disclosures
The authors have nothing to disclose.
1. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. 10.1002/jhm.2529. PubMed
2. Banerjee R, Suarez A, Kier M, et al. If you book it, will they come? Attendance at postdischarge follow-up visits scheduled by inpatient providers. J Hosp Med. 2017;12(8):618-625. 10.12788/jhm.2777. PubMed
3. McAlister FA, Youngson E, Padwal RS, Majumdar SR. Similar outcomes among general medicine patients discharged on weekends. J Hosp Med. 2015;10(2):69-74. 10.1002/jhm.2310. PubMed
4. Rising KL, White LF, Fernandez WG, Boutwell AE. Emergency department visits after hospital discharge: a missing part of the equation. Ann Emerg Med. 2013;62(2):145-150. 10.1016/j.annemergmed.2013.01.024. PubMed
5. Venkatesh A, Wang C, Wang Y, Altaf F, Bernheim S, Horwitz L. Association between post-discharge emergency department visitation and readmission rates J Hosp Med. 2018;13(9):589-594. doi: 10.12788/jhm.2937.
6. Vashi AA, Fox JP, Carr BG, et al. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364-371. 10.1001/jama.2012.216219. PubMed
7. Suffoletto B, Hu J, Guyette M, Callaway C. Factors contributing to emergency department care within 30 days of hospital discharge and potential ways to prevent it: differences in perspectives of patients, caregivers, and emergency physicians. J Hosp Med. 2014;9(5):315-319. 10.1002/jhm.2167. PubMed
8. Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. 10.1056/NEJMp1212324. PubMed
1. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. 10.1002/jhm.2529. PubMed
2. Banerjee R, Suarez A, Kier M, et al. If you book it, will they come? Attendance at postdischarge follow-up visits scheduled by inpatient providers. J Hosp Med. 2017;12(8):618-625. 10.12788/jhm.2777. PubMed
3. McAlister FA, Youngson E, Padwal RS, Majumdar SR. Similar outcomes among general medicine patients discharged on weekends. J Hosp Med. 2015;10(2):69-74. 10.1002/jhm.2310. PubMed
4. Rising KL, White LF, Fernandez WG, Boutwell AE. Emergency department visits after hospital discharge: a missing part of the equation. Ann Emerg Med. 2013;62(2):145-150. 10.1016/j.annemergmed.2013.01.024. PubMed
5. Venkatesh A, Wang C, Wang Y, Altaf F, Bernheim S, Horwitz L. Association between post-discharge emergency department visitation and readmission rates J Hosp Med. 2018;13(9):589-594. doi: 10.12788/jhm.2937.
6. Vashi AA, Fox JP, Carr BG, et al. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364-371. 10.1001/jama.2012.216219. PubMed
7. Suffoletto B, Hu J, Guyette M, Callaway C. Factors contributing to emergency department care within 30 days of hospital discharge and potential ways to prevent it: differences in perspectives of patients, caregivers, and emergency physicians. J Hosp Med. 2014;9(5):315-319. 10.1002/jhm.2167. PubMed
8. Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. 10.1056/NEJMp1212324. PubMed
© 2018 Society of Hospital Medicine