User login
Positive psychiatry: An introduction
Historically, psychology and psychiatry have mostly focused on negative emotions and pathological states. However, during the last few decades, new developments in both disciplines have created novel vistas for a more comprehensive understanding of human behavior.1,2 These developments have taken on the names of positive psychology and positive psychiatry, respectively. Positive psychiatry is the science and practice of psychiatry that focuses on psycho-bio-social study and promotion of well-being and health through enhancement of positive psychosocial factors (eg, resilience, optimism, wisdom, social support) in people with illnesses or disabilities as well as in the community at large.3 This new perspective is aimed at enhancing and enriching psychiatric practice and research rather than replacing our stated aim of providing reliable and valid diagnostic categories along with effective therapeutic interventions.
In this issue of
In Part 1, Boardman et al describe positive psychiatry tools to enhance clinical practice through positive interventions in several categories: adopting a positive orientation, harnessing strengths, mobilizing values, cultivating social connections, and optimizing health habits. The authors show how positive psychiatry aims to create a balance between pathogenesis (the study and understanding of diseases) and salutogenesis (the study and creation of health).4
In Part 2, Rettew discusses applying positive psychiatry principles and practices when working with children, adolescents, and their families. The author demonstrates how the principles and practices associated with positive psychiatry represent a natural and highly needed extension of the traditional work within child and adolescent psychiatry, and not a radical transformation of thought or effort. Rettew provides a case example in which he compares traditional and positive psychiatry approaches.
In Part 3, Oughli et al describe resilience in older adults with late-life depression, its clinical and neurocognitive correlates, and associated neurobiological and immunological biomarkers. The authors also narrate resilience-building interventions such as mind-body therapies, which have been reported to enhance resilience through promoting positive perceptions of various experiences and challenges. Evidence suggests that stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, resulting in greater resilience.
Finally, in Part 4, Hamid Peseschkian summarizes the ideas and practices of positive psychotherapy (PPT) as practiced in Germany since its introduction by Nossrat Peseschkian in 1977. Based on a resource-oriented conception of human beings, PPT combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. This short-term method can be readily understood by patients from diverse cultures and social backgrounds.
Taken together, these articles present recent advances in positive psychiatry, especially from an intervention perspective. This is a timely development in view of the evidence of rising global rates of suicide, substance use, anxiety, depression, and perceived stress. By uniting a positive perspective, along with studying its neurobiological underpinnings, and taking a life-long approach, we can now apply these innovations to children, young adults, and older adults, thus providing clinicians with tools to enhance well-being and promote mental health in people with and without mental or physical illnesses.
1. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
2. Jeste DV. A fulfilling year of APA presidency: from DSM-5 to positive psychiatry. Am J Psychiatry. 2013;170(10):1102-1105.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
Historically, psychology and psychiatry have mostly focused on negative emotions and pathological states. However, during the last few decades, new developments in both disciplines have created novel vistas for a more comprehensive understanding of human behavior.1,2 These developments have taken on the names of positive psychology and positive psychiatry, respectively. Positive psychiatry is the science and practice of psychiatry that focuses on psycho-bio-social study and promotion of well-being and health through enhancement of positive psychosocial factors (eg, resilience, optimism, wisdom, social support) in people with illnesses or disabilities as well as in the community at large.3 This new perspective is aimed at enhancing and enriching psychiatric practice and research rather than replacing our stated aim of providing reliable and valid diagnostic categories along with effective therapeutic interventions.
In this issue of
In Part 1, Boardman et al describe positive psychiatry tools to enhance clinical practice through positive interventions in several categories: adopting a positive orientation, harnessing strengths, mobilizing values, cultivating social connections, and optimizing health habits. The authors show how positive psychiatry aims to create a balance between pathogenesis (the study and understanding of diseases) and salutogenesis (the study and creation of health).4
In Part 2, Rettew discusses applying positive psychiatry principles and practices when working with children, adolescents, and their families. The author demonstrates how the principles and practices associated with positive psychiatry represent a natural and highly needed extension of the traditional work within child and adolescent psychiatry, and not a radical transformation of thought or effort. Rettew provides a case example in which he compares traditional and positive psychiatry approaches.
In Part 3, Oughli et al describe resilience in older adults with late-life depression, its clinical and neurocognitive correlates, and associated neurobiological and immunological biomarkers. The authors also narrate resilience-building interventions such as mind-body therapies, which have been reported to enhance resilience through promoting positive perceptions of various experiences and challenges. Evidence suggests that stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, resulting in greater resilience.
Finally, in Part 4, Hamid Peseschkian summarizes the ideas and practices of positive psychotherapy (PPT) as practiced in Germany since its introduction by Nossrat Peseschkian in 1977. Based on a resource-oriented conception of human beings, PPT combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. This short-term method can be readily understood by patients from diverse cultures and social backgrounds.
Taken together, these articles present recent advances in positive psychiatry, especially from an intervention perspective. This is a timely development in view of the evidence of rising global rates of suicide, substance use, anxiety, depression, and perceived stress. By uniting a positive perspective, along with studying its neurobiological underpinnings, and taking a life-long approach, we can now apply these innovations to children, young adults, and older adults, thus providing clinicians with tools to enhance well-being and promote mental health in people with and without mental or physical illnesses.
Historically, psychology and psychiatry have mostly focused on negative emotions and pathological states. However, during the last few decades, new developments in both disciplines have created novel vistas for a more comprehensive understanding of human behavior.1,2 These developments have taken on the names of positive psychology and positive psychiatry, respectively. Positive psychiatry is the science and practice of psychiatry that focuses on psycho-bio-social study and promotion of well-being and health through enhancement of positive psychosocial factors (eg, resilience, optimism, wisdom, social support) in people with illnesses or disabilities as well as in the community at large.3 This new perspective is aimed at enhancing and enriching psychiatric practice and research rather than replacing our stated aim of providing reliable and valid diagnostic categories along with effective therapeutic interventions.
In this issue of
In Part 1, Boardman et al describe positive psychiatry tools to enhance clinical practice through positive interventions in several categories: adopting a positive orientation, harnessing strengths, mobilizing values, cultivating social connections, and optimizing health habits. The authors show how positive psychiatry aims to create a balance between pathogenesis (the study and understanding of diseases) and salutogenesis (the study and creation of health).4
In Part 2, Rettew discusses applying positive psychiatry principles and practices when working with children, adolescents, and their families. The author demonstrates how the principles and practices associated with positive psychiatry represent a natural and highly needed extension of the traditional work within child and adolescent psychiatry, and not a radical transformation of thought or effort. Rettew provides a case example in which he compares traditional and positive psychiatry approaches.
In Part 3, Oughli et al describe resilience in older adults with late-life depression, its clinical and neurocognitive correlates, and associated neurobiological and immunological biomarkers. The authors also narrate resilience-building interventions such as mind-body therapies, which have been reported to enhance resilience through promoting positive perceptions of various experiences and challenges. Evidence suggests that stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, resulting in greater resilience.
Finally, in Part 4, Hamid Peseschkian summarizes the ideas and practices of positive psychotherapy (PPT) as practiced in Germany since its introduction by Nossrat Peseschkian in 1977. Based on a resource-oriented conception of human beings, PPT combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. This short-term method can be readily understood by patients from diverse cultures and social backgrounds.
Taken together, these articles present recent advances in positive psychiatry, especially from an intervention perspective. This is a timely development in view of the evidence of rising global rates of suicide, substance use, anxiety, depression, and perceived stress. By uniting a positive perspective, along with studying its neurobiological underpinnings, and taking a life-long approach, we can now apply these innovations to children, young adults, and older adults, thus providing clinicians with tools to enhance well-being and promote mental health in people with and without mental or physical illnesses.
1. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
2. Jeste DV. A fulfilling year of APA presidency: from DSM-5 to positive psychiatry. Am J Psychiatry. 2013;170(10):1102-1105.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
1. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
2. Jeste DV. A fulfilling year of APA presidency: from DSM-5 to positive psychiatry. Am J Psychiatry. 2013;170(10):1102-1105.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
Using the tools of positive psychiatry to improve clinical practice
FIRST OF 4 PARTS
What does wellness mean to you? A 2018 survey posed this question to more than 6,000 people living with depression and bipolar disorder. In addition to better treatment and greater understanding of their illnesses, other priorities emerged: a longing for better days, a sense of purpose, and a longing to function well and be happy.1 As one respondent explained, “Wellness means stability; well enough to hold a job, well enough to enjoy activities, well enough to feel joy and hope.” Traditional treatment that focuses on alleviating symptoms may not sufficiently address outcomes patients value. When the focus is primarily deficit-based, clinicians and patients may miss opportunities for optimization and transformation.
Positive psychiatry is the science and practice of psychiatry that seeks to enhance and promote well-being and health through the enhancement of positive psychosocial factors such as resilience, optimism, wisdom, and social support in people with illnesses or disabilities as well as those in the community at large.2 It is based on the principles that there is no health without mental health, and that mental health can improve through preventive, therapeutic, and rehabilitative interventions.3
Positive interventions are defined as “treatment methods or intentional activities that aim to cultivate positive feelings, behaviors, or cognitions.”4 They are evidence-based intentional exercises designed to increase well-being and enhance flourishing. Although positive interventions were originally studied as activities for nonclinical populations and for helping healthy people thrive, they are increasingly being valued for their therapeutic role in treating psychopathology.5 By adding positive interventions to their toolbox, psychiatrists can expand the range of treatment options, better engage patients during the treatment process, and bolster positive mental health.
In this article, we provide practical ways to integrate the tools and principles of positive psychiatry into everyday clinical practice. The goal is to broaden how clinicians think about mental health and therapeutic options and, above all, enhance our patients’ everyday well-being. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits are strategies clinicians can apply not only to provide a counterweight to the traditional emphasis on illness, but also to enhance the range and richness of their patients’ everyday experience.
Adopt a positive orientation
When a clinician first meets a patient, “What’s wrong?” is a typical conversation starter, and conversations tend to revolve around problems, failures, and negative experiences. Positive psychiatry posits that there is therapeutic benefit to emphasizing and exploring a patient’s positive emotions, experiences, and aspirations. Questions such as “What was your sense of well-being this week? What is your goal for today’s session? What is your goal for the coming week?” can reorient a session towards an individual’s potential and promote exploration of what’s possible.
To promote a positive orientation, clinicians may consider integrating the Savoring and Three Good Things exercises—2 well-studied interventions—into their repertoire to activate and enhance positive emotional states such as gratitude and joy.6 An example of a Savoring activity is taking a 20-minute daily walk while trying to notice as many positive elements as possible. Similarly, the Three Good Things exercise, in which patients are asked to notice and write down 3 positive events and reflect on why they happened, promotes positive reflection and gratitude. A 14-day daily diary study conducted during the COVID-19 pandemic found that higher levels of gratitude were associated with higher levels of positive affect, lower levels of perceived stress related to COVID-19, and better subjective health.7 In addition to coping with life’s negative events, deliberately enhancing the impact of good things is a positive emotion amplifier. As French writer François de La Rochefoucauld argued, “Happiness does not consist in things themselves but in the relish we have of them.”8
Continue to: Harness strengths
Harness strengths
A growing body of evidence suggests that in addition to focusing on a patient’s chief concern, identifying and cultivating an individual’s signature strengths can mitigate stress and enhance well-being. Signature strengths are positive personality qualities that reflect our core identity and are morally valued. The VIA Character Strengths Survey is the most used and validated psychometric instrument to measure and identify signature strengths such as curiosity, self-regulation, honesty, and teamwork.9
To incorporate this tool into clinical practice, ask patients to complete a strengths survey using a validated assessment tool such as the VIA survey (www.viacharacter.org). After a patient identifies their signature strengths, encourage them to explore and apply these strengths in everyday life and in new ways. In addition to becoming aware of and using their signature strengths, encourage patients to “strengths spot” in others. “What strengths did you notice your coworker, family, or friend using today?” is a potential question to explore with patients. A strengths-based approach may be particularly helpful in uncovering motivation and fully engaging patients in treatment. Moreover, integrating strengths into the typically negatively skewed narrative underscores to patients that therapy isn’t only about untwisting distorted thinking, but also about harnessing one’s strengths, talents, and abilities. Strengths expressed through pragmatic actions can boost coping skills as well as enhance well-being.
Mobilize values
Value affirmation exercises have been shown to generate lasting benefits in creating positive feelings and behaviors.10 Encouraging patients to think about what they genuinely value redirects their gaze towards possibility and diverts self-focus. For instance, ask a patient to identify 2 or 3 values and write about why they are important. By reflecting on their values in writing, they affirm their identity and self-worth, thus creating a virtuous cycle of confidence, effort, and achievement. People who put their values front and center are more attuned to the needs of others as well as their own needs, and they make better connections.11 Including a patient’s values in the treatment plan may increase problem-solving skills, boost motivation, and build better stress management skills.
The “life review” is another intervention that facilitates exploration of a patient’s values. This exercise involves asking patients to recount the story of their life and the experiences that were most meaningful to them. This process allows clinicians to gain a deeper understanding of the patient’s values, which can help guide treatment. Meta-analytic evidence has demonstrated these reminiscence-based interventions have significant effects on well-being.6 As Mahatma Gandhi famously said, “Happiness is when what you think, what you say, and what you do are in harmony.” Creating more overlap between a patient’s values and their everyday actions and behaviors bolsters resilience, buffers against stress, and can restore a healthier self-concept.
Cultivate social connections
Social connection is recognized as a core psychological need and essential for well-being. The opposite of connection—social isolation—has negative effects on overall health, including increases in inflammatory markers, depression rates, and even all-cause mortality.12 A 2015 meta-analytic review demonstrated that loneliness increased the likelihood of mortality by 26%—a similar increase as seen with smoking 15 cigarettes a day.13
Continue to: As with any vital sign...
As with any vital sign, exploring a patient’s number of social contacts, quantity of social visits per week, and quality of relationships is an important indicator of health. Giving patients tools to cultivate social connection and deepen their relationships can enhance therapeutic outcomes. Asking patients to perform acts of kindness is one example of a “social prescription.” Feeding a stranger’s parking meter, picking up litter, helping a friend with a chore, providing a meal to a person in need, and volunteering are potential ways for patients to engage in kind deeds. After each act, encourage the patient to write down what they did and how it made them feel.
“Prescribing” positive communication is another way to enhance a patient’s social connections. For instance, teaching them about active constructive responding (ACR)—responding with enthusiasm when another person shares information or good news—has been shown to strengthen bonds with friends and family.14 Making eye contact, giving the other person one’s full attention, inquiring about details, and responding with enthusiasm and interest are simple ways patients can apply ACR in their daily lives. Counseling a patient on increasing social connections, prescribing connections, and inquiring about quantity and quality of social interactions can help them not only add years to their life but also add health and well-being to those years.
Optimize healthy habits
Mounting research demonstrates that exercise, sleep, and nutrition are important for well-being. Evidence shows that therapeutic lifestyle changes can reduce depressive symptoms and boost positive feelings. Numerous meta-analyses have demonstrated the benefits of sleep and exercise interventions for reducing depressive symptoms in psychiatric patients.15,16 Longitudinal studies have provided evidence that healthy diets increase happiness, even after controlling for potential confounders such as socioeconomic factors.17 Other lifestyle factors—including financial stability, pet ownership, decreased social media use, and spending time in nature—have been shown to contribute to well-being.18
Despite the substantial evidence that lifestyle factors can improve health outcomes, few clinicians ask about, focus on, or promote positive habits.19 Positive psychiatry seeks to reorient clinicians towards lifestyle factors that enhance well-being. Clinicians can deploy a variety of strategies to support patients in making healthy and sustainable changes. Assessing readiness for change, motivational interviewing, setting SMART (specific, measurable, assignable, realistic, and time-related) goals, and referring patients to relevant community resources are ways to encourage and promote therapeutic lifestyle changes. Inquiring about a patient’s typical day—such as how they spend their free time, what they eat, when they go to bed, and how much time they spend outdoors—opens conversations about general well-being and shows the patient that therapy is about the whole person, and not only symptom management. Helping patients have better days can empower them to lead more satisfied lives.20
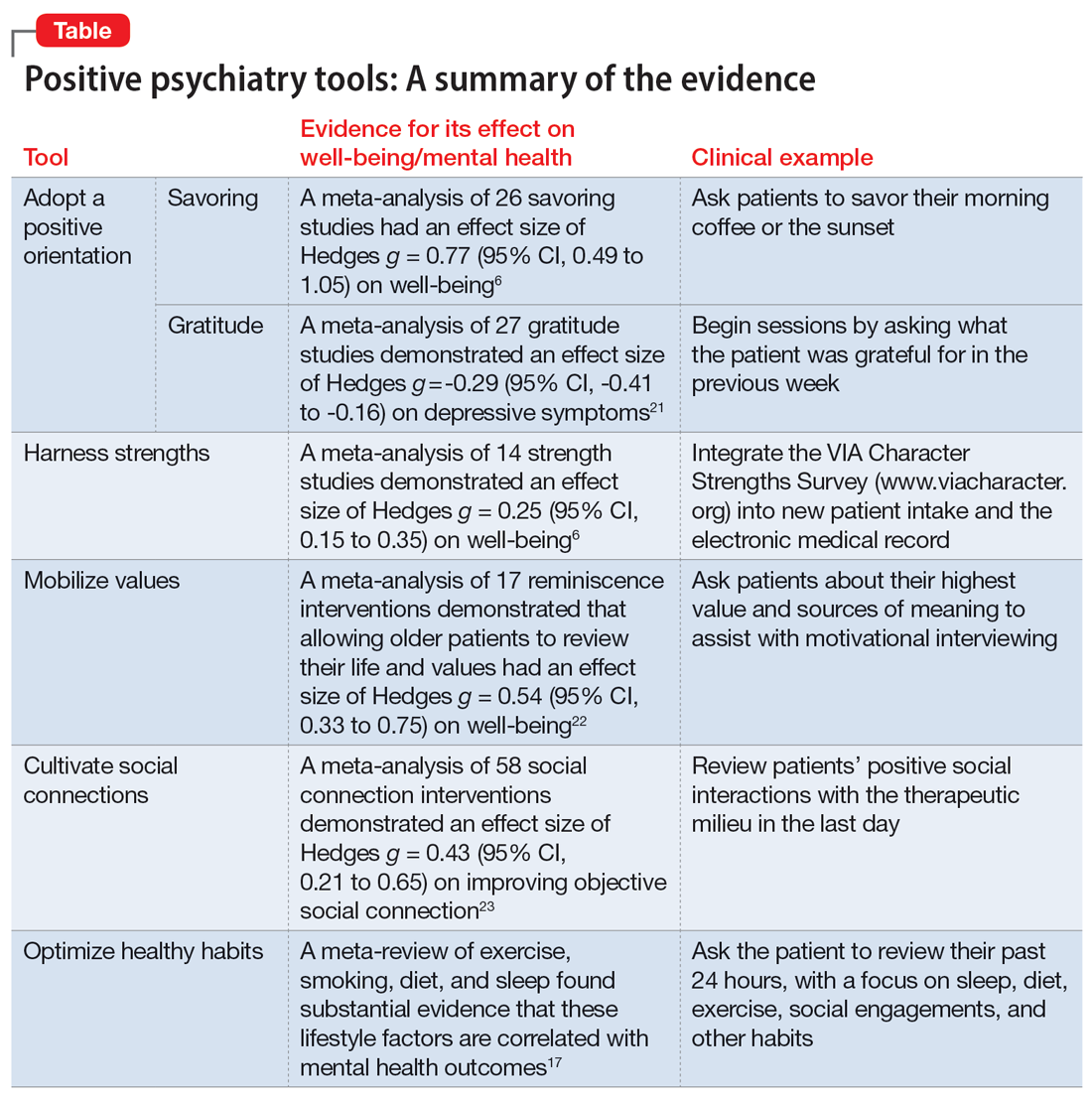
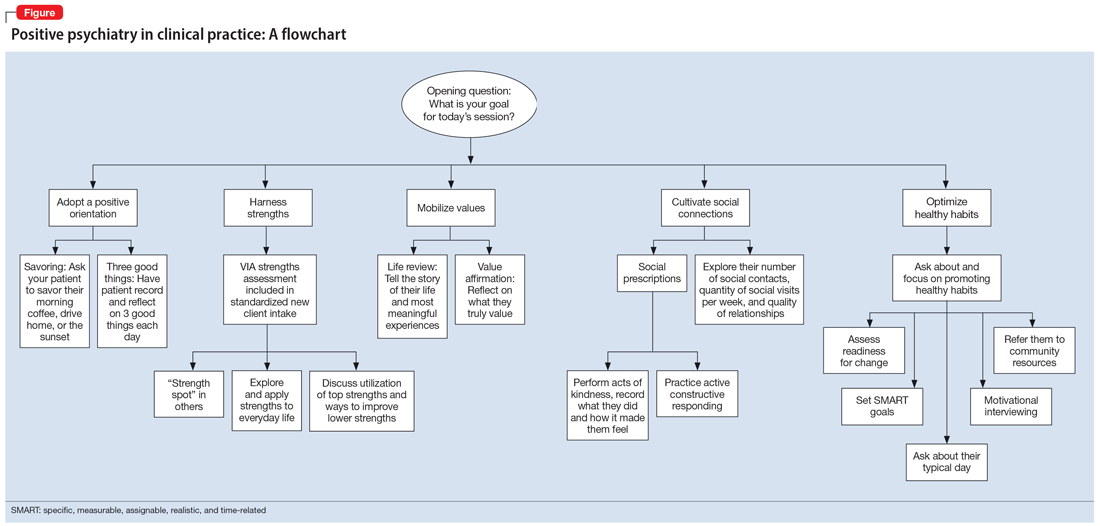
The Table6,17,21-23 summarizes the scientific evidence for the strategies described in this article. The Figure provides a flowchart for using these strategies in clinical practice.
Continue to: Balancing pathogenesis with salutogenesis
Balancing pathogenesis with salutogenesis
By exploring and emphasizing potential and possibility, positive psychiatry aims to create a balance between pathogenesis (the study and understanding of disease) with salutogenesis (the study and creation of health24). Clinicians are well positioned to manage symptoms and bolster positive states. Rather than an either/or approach to well-being, positive psychiatry strives for a both/and approach to well-being. By adding positive interventions to their toolbox, clinicians can expand the range of treatment options, better engage patients in the treatment process, and bolster mental health.
Bottom Line
Clinicians can integrate the tools and principles of positive psychiatry into clinical practice. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits can not only provide a counterweight to the traditional emphasis on illness, but also can enhance the range and richness of patients’ everyday experience.
Related Resources
- University of Pennsylvania. Authentic happiness. https://www.authentichappiness.sas.upenn.edu
- Jeste DV, Palmer BW (eds). Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
1. Morton E, Foxworth P, Dardess P, et al. “Supporting Wellness”: a depression and bipolar support alliance mixed-methods investigation of lived experience perspectives and priorities for mood disorder treatment. J Affect Disord. 2022;299:575-584.
2. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65(5):467-487.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Posit Psychol. 2021;16(6):749-769.
7. Jiang D. Feeling gratitude is associated with better well-being across the life span: a daily diary study during the COVID-19 outbreak. J Gerontol B Psychol Sci Soc Sci. 2022;77(4):e36-e45.
8. de La Rochefoucauld F. Maxims and moral reflections (1796). Gale ECCO: 2010.
9. Niemiec RM. VIA character strengths: Research and practice (The first 10 years). In: Knoop HH, Fave AD (eds). Well-being and Cultures. Springer;2013:11-29.
10. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Annu Rev Psychol. 2014;65:333-371.
11. Thomaes S, Bushman BJ, de Castro BO, et al. Arousing “gentle passions” in young adolescents: sustained experimental effects of value affirmations on prosocial feelings and behaviors. Dev Psychol. 2012;48(1):103-110.
12. Cacioppo JT, Cacioppo S, Capitanio JP, et al. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733-767.
13. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
14. Gable SL, Reis HT, Impett EA, et al. What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. J Pers Soc Psychol. 2004;87(2):228-245.
15. Gee B, Orchard F, Clarke E, et al. The effect of non-pharmacological sleep interventions on depression symptoms: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2019;43:118-128.
16. Krogh J, Hjorthøj C, Speyer H, et al. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2017;7(9):e014820. doi:10.1136/bmjopen-2016-014820
17. Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360-380.
18. Piotrowski MC, Lunsford J, Gaynes BN. Lifestyle psychiatry for depression and anxiety: beyond diet and exercise. Lifestyle Med. 2021;2(1):e21. doi:10.1002/lim2.21
19. Janney CA, Brzoznowski KF, Richardson Cret al. Moving towards wellness: physical activity practices, perspectives, and preferences of users of outpatient mental health service. Gen Hosp Psychiatry. 2017;49:63-66.
20. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
21. Cregg DR, Cheavens JS. Gratitude interventions: effective self-help? A meta-analysis of the impact on symptoms of depression and anxiety. J Happiness Stud. 2021;22(1):413-445.
22. Bohlmeijer E, Roemer M, Cuijpers P, et al. The effects of reminiscence on psychological well-being in older adults: a meta-analysis. Aging Ment Health. 2007;11(3):291-300.
23. Zagic D, Wuthrich VM, Rapee RM, et al. Interventions to improve social connections: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2022;57(5):885-906.
24. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
FIRST OF 4 PARTS
What does wellness mean to you? A 2018 survey posed this question to more than 6,000 people living with depression and bipolar disorder. In addition to better treatment and greater understanding of their illnesses, other priorities emerged: a longing for better days, a sense of purpose, and a longing to function well and be happy.1 As one respondent explained, “Wellness means stability; well enough to hold a job, well enough to enjoy activities, well enough to feel joy and hope.” Traditional treatment that focuses on alleviating symptoms may not sufficiently address outcomes patients value. When the focus is primarily deficit-based, clinicians and patients may miss opportunities for optimization and transformation.
Positive psychiatry is the science and practice of psychiatry that seeks to enhance and promote well-being and health through the enhancement of positive psychosocial factors such as resilience, optimism, wisdom, and social support in people with illnesses or disabilities as well as those in the community at large.2 It is based on the principles that there is no health without mental health, and that mental health can improve through preventive, therapeutic, and rehabilitative interventions.3
Positive interventions are defined as “treatment methods or intentional activities that aim to cultivate positive feelings, behaviors, or cognitions.”4 They are evidence-based intentional exercises designed to increase well-being and enhance flourishing. Although positive interventions were originally studied as activities for nonclinical populations and for helping healthy people thrive, they are increasingly being valued for their therapeutic role in treating psychopathology.5 By adding positive interventions to their toolbox, psychiatrists can expand the range of treatment options, better engage patients during the treatment process, and bolster positive mental health.
In this article, we provide practical ways to integrate the tools and principles of positive psychiatry into everyday clinical practice. The goal is to broaden how clinicians think about mental health and therapeutic options and, above all, enhance our patients’ everyday well-being. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits are strategies clinicians can apply not only to provide a counterweight to the traditional emphasis on illness, but also to enhance the range and richness of their patients’ everyday experience.
Adopt a positive orientation
When a clinician first meets a patient, “What’s wrong?” is a typical conversation starter, and conversations tend to revolve around problems, failures, and negative experiences. Positive psychiatry posits that there is therapeutic benefit to emphasizing and exploring a patient’s positive emotions, experiences, and aspirations. Questions such as “What was your sense of well-being this week? What is your goal for today’s session? What is your goal for the coming week?” can reorient a session towards an individual’s potential and promote exploration of what’s possible.
To promote a positive orientation, clinicians may consider integrating the Savoring and Three Good Things exercises—2 well-studied interventions—into their repertoire to activate and enhance positive emotional states such as gratitude and joy.6 An example of a Savoring activity is taking a 20-minute daily walk while trying to notice as many positive elements as possible. Similarly, the Three Good Things exercise, in which patients are asked to notice and write down 3 positive events and reflect on why they happened, promotes positive reflection and gratitude. A 14-day daily diary study conducted during the COVID-19 pandemic found that higher levels of gratitude were associated with higher levels of positive affect, lower levels of perceived stress related to COVID-19, and better subjective health.7 In addition to coping with life’s negative events, deliberately enhancing the impact of good things is a positive emotion amplifier. As French writer François de La Rochefoucauld argued, “Happiness does not consist in things themselves but in the relish we have of them.”8
Continue to: Harness strengths
Harness strengths
A growing body of evidence suggests that in addition to focusing on a patient’s chief concern, identifying and cultivating an individual’s signature strengths can mitigate stress and enhance well-being. Signature strengths are positive personality qualities that reflect our core identity and are morally valued. The VIA Character Strengths Survey is the most used and validated psychometric instrument to measure and identify signature strengths such as curiosity, self-regulation, honesty, and teamwork.9
To incorporate this tool into clinical practice, ask patients to complete a strengths survey using a validated assessment tool such as the VIA survey (www.viacharacter.org). After a patient identifies their signature strengths, encourage them to explore and apply these strengths in everyday life and in new ways. In addition to becoming aware of and using their signature strengths, encourage patients to “strengths spot” in others. “What strengths did you notice your coworker, family, or friend using today?” is a potential question to explore with patients. A strengths-based approach may be particularly helpful in uncovering motivation and fully engaging patients in treatment. Moreover, integrating strengths into the typically negatively skewed narrative underscores to patients that therapy isn’t only about untwisting distorted thinking, but also about harnessing one’s strengths, talents, and abilities. Strengths expressed through pragmatic actions can boost coping skills as well as enhance well-being.
Mobilize values
Value affirmation exercises have been shown to generate lasting benefits in creating positive feelings and behaviors.10 Encouraging patients to think about what they genuinely value redirects their gaze towards possibility and diverts self-focus. For instance, ask a patient to identify 2 or 3 values and write about why they are important. By reflecting on their values in writing, they affirm their identity and self-worth, thus creating a virtuous cycle of confidence, effort, and achievement. People who put their values front and center are more attuned to the needs of others as well as their own needs, and they make better connections.11 Including a patient’s values in the treatment plan may increase problem-solving skills, boost motivation, and build better stress management skills.
The “life review” is another intervention that facilitates exploration of a patient’s values. This exercise involves asking patients to recount the story of their life and the experiences that were most meaningful to them. This process allows clinicians to gain a deeper understanding of the patient’s values, which can help guide treatment. Meta-analytic evidence has demonstrated these reminiscence-based interventions have significant effects on well-being.6 As Mahatma Gandhi famously said, “Happiness is when what you think, what you say, and what you do are in harmony.” Creating more overlap between a patient’s values and their everyday actions and behaviors bolsters resilience, buffers against stress, and can restore a healthier self-concept.
Cultivate social connections
Social connection is recognized as a core psychological need and essential for well-being. The opposite of connection—social isolation—has negative effects on overall health, including increases in inflammatory markers, depression rates, and even all-cause mortality.12 A 2015 meta-analytic review demonstrated that loneliness increased the likelihood of mortality by 26%—a similar increase as seen with smoking 15 cigarettes a day.13
Continue to: As with any vital sign...
As with any vital sign, exploring a patient’s number of social contacts, quantity of social visits per week, and quality of relationships is an important indicator of health. Giving patients tools to cultivate social connection and deepen their relationships can enhance therapeutic outcomes. Asking patients to perform acts of kindness is one example of a “social prescription.” Feeding a stranger’s parking meter, picking up litter, helping a friend with a chore, providing a meal to a person in need, and volunteering are potential ways for patients to engage in kind deeds. After each act, encourage the patient to write down what they did and how it made them feel.
“Prescribing” positive communication is another way to enhance a patient’s social connections. For instance, teaching them about active constructive responding (ACR)—responding with enthusiasm when another person shares information or good news—has been shown to strengthen bonds with friends and family.14 Making eye contact, giving the other person one’s full attention, inquiring about details, and responding with enthusiasm and interest are simple ways patients can apply ACR in their daily lives. Counseling a patient on increasing social connections, prescribing connections, and inquiring about quantity and quality of social interactions can help them not only add years to their life but also add health and well-being to those years.
Optimize healthy habits
Mounting research demonstrates that exercise, sleep, and nutrition are important for well-being. Evidence shows that therapeutic lifestyle changes can reduce depressive symptoms and boost positive feelings. Numerous meta-analyses have demonstrated the benefits of sleep and exercise interventions for reducing depressive symptoms in psychiatric patients.15,16 Longitudinal studies have provided evidence that healthy diets increase happiness, even after controlling for potential confounders such as socioeconomic factors.17 Other lifestyle factors—including financial stability, pet ownership, decreased social media use, and spending time in nature—have been shown to contribute to well-being.18
Despite the substantial evidence that lifestyle factors can improve health outcomes, few clinicians ask about, focus on, or promote positive habits.19 Positive psychiatry seeks to reorient clinicians towards lifestyle factors that enhance well-being. Clinicians can deploy a variety of strategies to support patients in making healthy and sustainable changes. Assessing readiness for change, motivational interviewing, setting SMART (specific, measurable, assignable, realistic, and time-related) goals, and referring patients to relevant community resources are ways to encourage and promote therapeutic lifestyle changes. Inquiring about a patient’s typical day—such as how they spend their free time, what they eat, when they go to bed, and how much time they spend outdoors—opens conversations about general well-being and shows the patient that therapy is about the whole person, and not only symptom management. Helping patients have better days can empower them to lead more satisfied lives.20

The Table6,17,21-23 summarizes the scientific evidence for the strategies described in this article. The Figure provides a flowchart for using these strategies in clinical practice.

Continue to: Balancing pathogenesis with salutogenesis
Balancing pathogenesis with salutogenesis
By exploring and emphasizing potential and possibility, positive psychiatry aims to create a balance between pathogenesis (the study and understanding of disease) with salutogenesis (the study and creation of health24). Clinicians are well positioned to manage symptoms and bolster positive states. Rather than an either/or approach to well-being, positive psychiatry strives for a both/and approach to well-being. By adding positive interventions to their toolbox, clinicians can expand the range of treatment options, better engage patients in the treatment process, and bolster mental health.
Bottom Line
Clinicians can integrate the tools and principles of positive psychiatry into clinical practice. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits can not only provide a counterweight to the traditional emphasis on illness, but also can enhance the range and richness of patients’ everyday experience.
Related Resources
- University of Pennsylvania. Authentic happiness. https://www.authentichappiness.sas.upenn.edu
- Jeste DV, Palmer BW (eds). Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
FIRST OF 4 PARTS
What does wellness mean to you? A 2018 survey posed this question to more than 6,000 people living with depression and bipolar disorder. In addition to better treatment and greater understanding of their illnesses, other priorities emerged: a longing for better days, a sense of purpose, and a longing to function well and be happy.1 As one respondent explained, “Wellness means stability; well enough to hold a job, well enough to enjoy activities, well enough to feel joy and hope.” Traditional treatment that focuses on alleviating symptoms may not sufficiently address outcomes patients value. When the focus is primarily deficit-based, clinicians and patients may miss opportunities for optimization and transformation.
Positive psychiatry is the science and practice of psychiatry that seeks to enhance and promote well-being and health through the enhancement of positive psychosocial factors such as resilience, optimism, wisdom, and social support in people with illnesses or disabilities as well as those in the community at large.2 It is based on the principles that there is no health without mental health, and that mental health can improve through preventive, therapeutic, and rehabilitative interventions.3
Positive interventions are defined as “treatment methods or intentional activities that aim to cultivate positive feelings, behaviors, or cognitions.”4 They are evidence-based intentional exercises designed to increase well-being and enhance flourishing. Although positive interventions were originally studied as activities for nonclinical populations and for helping healthy people thrive, they are increasingly being valued for their therapeutic role in treating psychopathology.5 By adding positive interventions to their toolbox, psychiatrists can expand the range of treatment options, better engage patients during the treatment process, and bolster positive mental health.
In this article, we provide practical ways to integrate the tools and principles of positive psychiatry into everyday clinical practice. The goal is to broaden how clinicians think about mental health and therapeutic options and, above all, enhance our patients’ everyday well-being. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits are strategies clinicians can apply not only to provide a counterweight to the traditional emphasis on illness, but also to enhance the range and richness of their patients’ everyday experience.
Adopt a positive orientation
When a clinician first meets a patient, “What’s wrong?” is a typical conversation starter, and conversations tend to revolve around problems, failures, and negative experiences. Positive psychiatry posits that there is therapeutic benefit to emphasizing and exploring a patient’s positive emotions, experiences, and aspirations. Questions such as “What was your sense of well-being this week? What is your goal for today’s session? What is your goal for the coming week?” can reorient a session towards an individual’s potential and promote exploration of what’s possible.
To promote a positive orientation, clinicians may consider integrating the Savoring and Three Good Things exercises—2 well-studied interventions—into their repertoire to activate and enhance positive emotional states such as gratitude and joy.6 An example of a Savoring activity is taking a 20-minute daily walk while trying to notice as many positive elements as possible. Similarly, the Three Good Things exercise, in which patients are asked to notice and write down 3 positive events and reflect on why they happened, promotes positive reflection and gratitude. A 14-day daily diary study conducted during the COVID-19 pandemic found that higher levels of gratitude were associated with higher levels of positive affect, lower levels of perceived stress related to COVID-19, and better subjective health.7 In addition to coping with life’s negative events, deliberately enhancing the impact of good things is a positive emotion amplifier. As French writer François de La Rochefoucauld argued, “Happiness does not consist in things themselves but in the relish we have of them.”8
Continue to: Harness strengths
Harness strengths
A growing body of evidence suggests that in addition to focusing on a patient’s chief concern, identifying and cultivating an individual’s signature strengths can mitigate stress and enhance well-being. Signature strengths are positive personality qualities that reflect our core identity and are morally valued. The VIA Character Strengths Survey is the most used and validated psychometric instrument to measure and identify signature strengths such as curiosity, self-regulation, honesty, and teamwork.9
To incorporate this tool into clinical practice, ask patients to complete a strengths survey using a validated assessment tool such as the VIA survey (www.viacharacter.org). After a patient identifies their signature strengths, encourage them to explore and apply these strengths in everyday life and in new ways. In addition to becoming aware of and using their signature strengths, encourage patients to “strengths spot” in others. “What strengths did you notice your coworker, family, or friend using today?” is a potential question to explore with patients. A strengths-based approach may be particularly helpful in uncovering motivation and fully engaging patients in treatment. Moreover, integrating strengths into the typically negatively skewed narrative underscores to patients that therapy isn’t only about untwisting distorted thinking, but also about harnessing one’s strengths, talents, and abilities. Strengths expressed through pragmatic actions can boost coping skills as well as enhance well-being.
Mobilize values
Value affirmation exercises have been shown to generate lasting benefits in creating positive feelings and behaviors.10 Encouraging patients to think about what they genuinely value redirects their gaze towards possibility and diverts self-focus. For instance, ask a patient to identify 2 or 3 values and write about why they are important. By reflecting on their values in writing, they affirm their identity and self-worth, thus creating a virtuous cycle of confidence, effort, and achievement. People who put their values front and center are more attuned to the needs of others as well as their own needs, and they make better connections.11 Including a patient’s values in the treatment plan may increase problem-solving skills, boost motivation, and build better stress management skills.
The “life review” is another intervention that facilitates exploration of a patient’s values. This exercise involves asking patients to recount the story of their life and the experiences that were most meaningful to them. This process allows clinicians to gain a deeper understanding of the patient’s values, which can help guide treatment. Meta-analytic evidence has demonstrated these reminiscence-based interventions have significant effects on well-being.6 As Mahatma Gandhi famously said, “Happiness is when what you think, what you say, and what you do are in harmony.” Creating more overlap between a patient’s values and their everyday actions and behaviors bolsters resilience, buffers against stress, and can restore a healthier self-concept.
Cultivate social connections
Social connection is recognized as a core psychological need and essential for well-being. The opposite of connection—social isolation—has negative effects on overall health, including increases in inflammatory markers, depression rates, and even all-cause mortality.12 A 2015 meta-analytic review demonstrated that loneliness increased the likelihood of mortality by 26%—a similar increase as seen with smoking 15 cigarettes a day.13
Continue to: As with any vital sign...
As with any vital sign, exploring a patient’s number of social contacts, quantity of social visits per week, and quality of relationships is an important indicator of health. Giving patients tools to cultivate social connection and deepen their relationships can enhance therapeutic outcomes. Asking patients to perform acts of kindness is one example of a “social prescription.” Feeding a stranger’s parking meter, picking up litter, helping a friend with a chore, providing a meal to a person in need, and volunteering are potential ways for patients to engage in kind deeds. After each act, encourage the patient to write down what they did and how it made them feel.
“Prescribing” positive communication is another way to enhance a patient’s social connections. For instance, teaching them about active constructive responding (ACR)—responding with enthusiasm when another person shares information or good news—has been shown to strengthen bonds with friends and family.14 Making eye contact, giving the other person one’s full attention, inquiring about details, and responding with enthusiasm and interest are simple ways patients can apply ACR in their daily lives. Counseling a patient on increasing social connections, prescribing connections, and inquiring about quantity and quality of social interactions can help them not only add years to their life but also add health and well-being to those years.
Optimize healthy habits
Mounting research demonstrates that exercise, sleep, and nutrition are important for well-being. Evidence shows that therapeutic lifestyle changes can reduce depressive symptoms and boost positive feelings. Numerous meta-analyses have demonstrated the benefits of sleep and exercise interventions for reducing depressive symptoms in psychiatric patients.15,16 Longitudinal studies have provided evidence that healthy diets increase happiness, even after controlling for potential confounders such as socioeconomic factors.17 Other lifestyle factors—including financial stability, pet ownership, decreased social media use, and spending time in nature—have been shown to contribute to well-being.18
Despite the substantial evidence that lifestyle factors can improve health outcomes, few clinicians ask about, focus on, or promote positive habits.19 Positive psychiatry seeks to reorient clinicians towards lifestyle factors that enhance well-being. Clinicians can deploy a variety of strategies to support patients in making healthy and sustainable changes. Assessing readiness for change, motivational interviewing, setting SMART (specific, measurable, assignable, realistic, and time-related) goals, and referring patients to relevant community resources are ways to encourage and promote therapeutic lifestyle changes. Inquiring about a patient’s typical day—such as how they spend their free time, what they eat, when they go to bed, and how much time they spend outdoors—opens conversations about general well-being and shows the patient that therapy is about the whole person, and not only symptom management. Helping patients have better days can empower them to lead more satisfied lives.20

The Table6,17,21-23 summarizes the scientific evidence for the strategies described in this article. The Figure provides a flowchart for using these strategies in clinical practice.

Continue to: Balancing pathogenesis with salutogenesis
Balancing pathogenesis with salutogenesis
By exploring and emphasizing potential and possibility, positive psychiatry aims to create a balance between pathogenesis (the study and understanding of disease) with salutogenesis (the study and creation of health24). Clinicians are well positioned to manage symptoms and bolster positive states. Rather than an either/or approach to well-being, positive psychiatry strives for a both/and approach to well-being. By adding positive interventions to their toolbox, clinicians can expand the range of treatment options, better engage patients in the treatment process, and bolster mental health.
Bottom Line
Clinicians can integrate the tools and principles of positive psychiatry into clinical practice. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits can not only provide a counterweight to the traditional emphasis on illness, but also can enhance the range and richness of patients’ everyday experience.
Related Resources
- University of Pennsylvania. Authentic happiness. https://www.authentichappiness.sas.upenn.edu
- Jeste DV, Palmer BW (eds). Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
1. Morton E, Foxworth P, Dardess P, et al. “Supporting Wellness”: a depression and bipolar support alliance mixed-methods investigation of lived experience perspectives and priorities for mood disorder treatment. J Affect Disord. 2022;299:575-584.
2. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65(5):467-487.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Posit Psychol. 2021;16(6):749-769.
7. Jiang D. Feeling gratitude is associated with better well-being across the life span: a daily diary study during the COVID-19 outbreak. J Gerontol B Psychol Sci Soc Sci. 2022;77(4):e36-e45.
8. de La Rochefoucauld F. Maxims and moral reflections (1796). Gale ECCO: 2010.
9. Niemiec RM. VIA character strengths: Research and practice (The first 10 years). In: Knoop HH, Fave AD (eds). Well-being and Cultures. Springer;2013:11-29.
10. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Annu Rev Psychol. 2014;65:333-371.
11. Thomaes S, Bushman BJ, de Castro BO, et al. Arousing “gentle passions” in young adolescents: sustained experimental effects of value affirmations on prosocial feelings and behaviors. Dev Psychol. 2012;48(1):103-110.
12. Cacioppo JT, Cacioppo S, Capitanio JP, et al. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733-767.
13. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
14. Gable SL, Reis HT, Impett EA, et al. What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. J Pers Soc Psychol. 2004;87(2):228-245.
15. Gee B, Orchard F, Clarke E, et al. The effect of non-pharmacological sleep interventions on depression symptoms: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2019;43:118-128.
16. Krogh J, Hjorthøj C, Speyer H, et al. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2017;7(9):e014820. doi:10.1136/bmjopen-2016-014820
17. Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360-380.
18. Piotrowski MC, Lunsford J, Gaynes BN. Lifestyle psychiatry for depression and anxiety: beyond diet and exercise. Lifestyle Med. 2021;2(1):e21. doi:10.1002/lim2.21
19. Janney CA, Brzoznowski KF, Richardson Cret al. Moving towards wellness: physical activity practices, perspectives, and preferences of users of outpatient mental health service. Gen Hosp Psychiatry. 2017;49:63-66.
20. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
21. Cregg DR, Cheavens JS. Gratitude interventions: effective self-help? A meta-analysis of the impact on symptoms of depression and anxiety. J Happiness Stud. 2021;22(1):413-445.
22. Bohlmeijer E, Roemer M, Cuijpers P, et al. The effects of reminiscence on psychological well-being in older adults: a meta-analysis. Aging Ment Health. 2007;11(3):291-300.
23. Zagic D, Wuthrich VM, Rapee RM, et al. Interventions to improve social connections: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2022;57(5):885-906.
24. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
1. Morton E, Foxworth P, Dardess P, et al. “Supporting Wellness”: a depression and bipolar support alliance mixed-methods investigation of lived experience perspectives and priorities for mood disorder treatment. J Affect Disord. 2022;299:575-584.
2. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65(5):467-487.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Posit Psychol. 2021;16(6):749-769.
7. Jiang D. Feeling gratitude is associated with better well-being across the life span: a daily diary study during the COVID-19 outbreak. J Gerontol B Psychol Sci Soc Sci. 2022;77(4):e36-e45.
8. de La Rochefoucauld F. Maxims and moral reflections (1796). Gale ECCO: 2010.
9. Niemiec RM. VIA character strengths: Research and practice (The first 10 years). In: Knoop HH, Fave AD (eds). Well-being and Cultures. Springer;2013:11-29.
10. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Annu Rev Psychol. 2014;65:333-371.
11. Thomaes S, Bushman BJ, de Castro BO, et al. Arousing “gentle passions” in young adolescents: sustained experimental effects of value affirmations on prosocial feelings and behaviors. Dev Psychol. 2012;48(1):103-110.
12. Cacioppo JT, Cacioppo S, Capitanio JP, et al. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733-767.
13. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
14. Gable SL, Reis HT, Impett EA, et al. What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. J Pers Soc Psychol. 2004;87(2):228-245.
15. Gee B, Orchard F, Clarke E, et al. The effect of non-pharmacological sleep interventions on depression symptoms: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2019;43:118-128.
16. Krogh J, Hjorthøj C, Speyer H, et al. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2017;7(9):e014820. doi:10.1136/bmjopen-2016-014820
17. Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360-380.
18. Piotrowski MC, Lunsford J, Gaynes BN. Lifestyle psychiatry for depression and anxiety: beyond diet and exercise. Lifestyle Med. 2021;2(1):e21. doi:10.1002/lim2.21
19. Janney CA, Brzoznowski KF, Richardson Cret al. Moving towards wellness: physical activity practices, perspectives, and preferences of users of outpatient mental health service. Gen Hosp Psychiatry. 2017;49:63-66.
20. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
21. Cregg DR, Cheavens JS. Gratitude interventions: effective self-help? A meta-analysis of the impact on symptoms of depression and anxiety. J Happiness Stud. 2021;22(1):413-445.
22. Bohlmeijer E, Roemer M, Cuijpers P, et al. The effects of reminiscence on psychological well-being in older adults: a meta-analysis. Aging Ment Health. 2007;11(3):291-300.
23. Zagic D, Wuthrich VM, Rapee RM, et al. Interventions to improve social connections: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2022;57(5):885-906.
24. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
Generalized anxiety disorder: 8 studies of psychosocial interventions
SECOND OF 2 PARTS
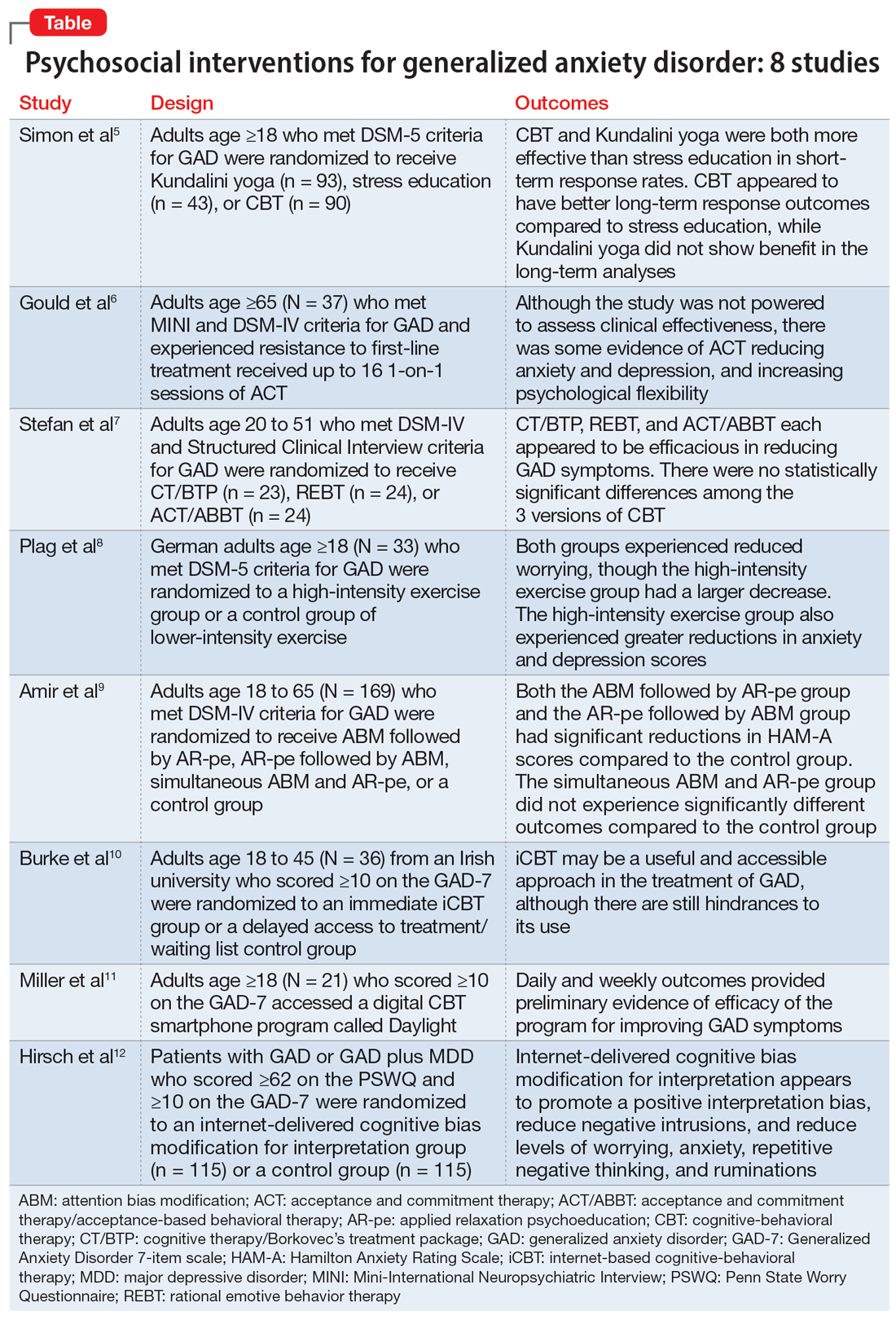
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (
1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
SECOND OF 2 PARTS
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (

1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
SECOND OF 2 PARTS
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (

1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
Neuropsychiatric symptoms after stroke
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.
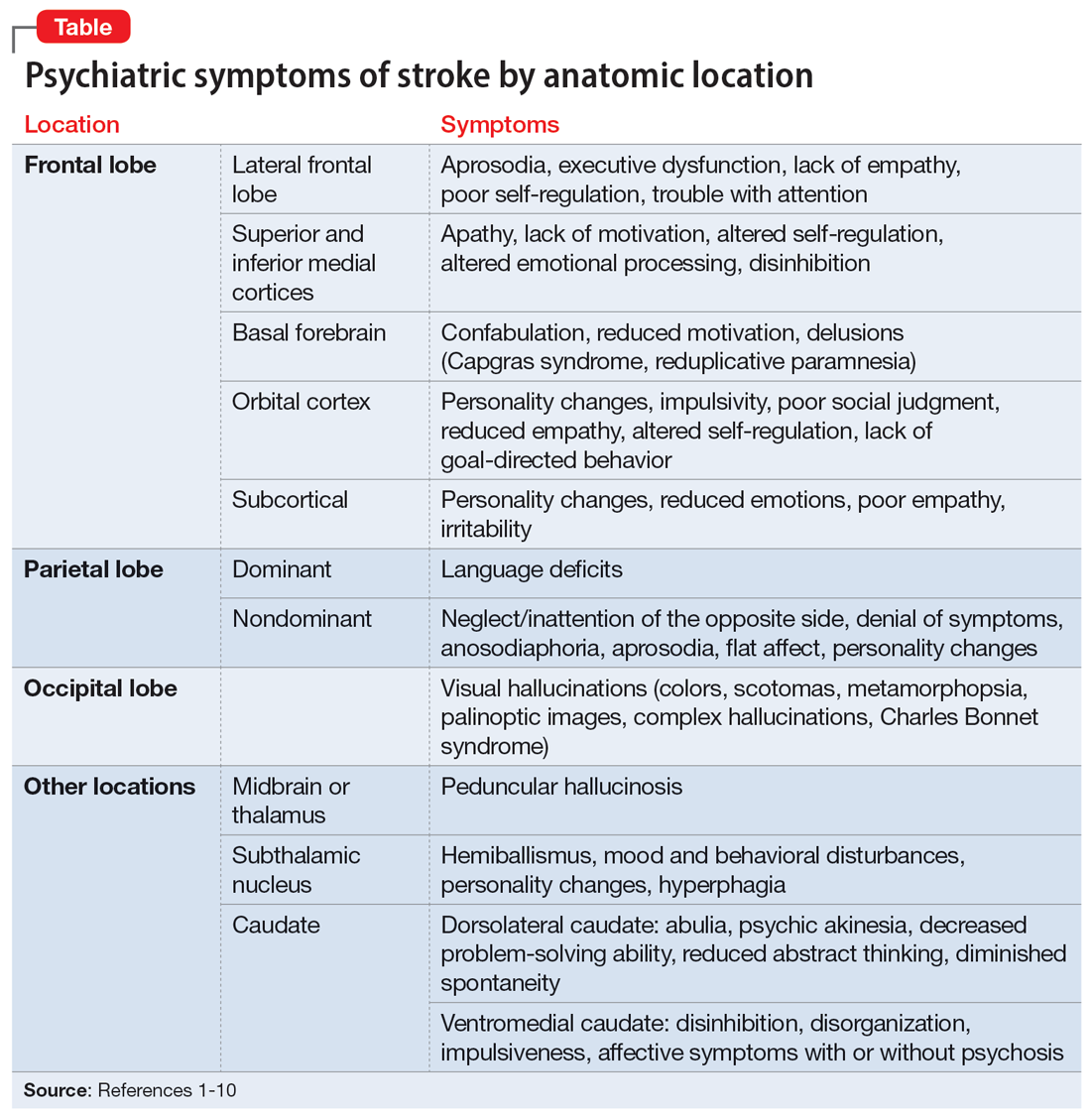
Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Laboratory monitoring for patients on buprenorphine: 10 questions
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
‘Med check’ appointments: How to minimize your malpractice risk
Medical malpractice claims can arise in any type of health care setting. The purpose of this article is to discuss the risk of medical malpractice suits in the context of brief “med checks,” which are 15- to 20-minute follow-up appointments for psychiatric outpatient medication management. Similar issues arise in brief new patient and transfer visits.
Malpractice hinges on ‘reasonableness’
Malpractice is an allegation of professional negligence.1 More specifically, it is an allegation that a clinician violated an existing duty by deviating from the standard of care, and that deviation caused damages.2 Medical malpractice claims involve questions about whether there was a deviation from the standard of care (whether the clinician failed to exercise a reasonable degree of skill and care given the context of the situation) and whether there was causation (whether a deviation caused a patient’s damages).3 These are fact-based determinations. Thus, the legal resolution of a malpractice claim is based on the facts of each specific case.
The advisability of 15-minute med checks and the associated limitation on a clinician’s ability to provide talk therapy are beyond the scope of this article. What is clear, however, is that not all brief med check appointments are created equal. Their safety and efficacy are dictated by the milieu in which they exist.
Practically speaking, although many factors need to be considered, the standard of care in a medical malpractice lawsuit is based on reasonableness.4-6 One strategy to proactively manage your malpractice risk is to consider—either for your existing job or before accepting a new position—whether your agency’s setup for brief med checks will allow you to practice reasonably. This article provides information to help you answer this question and describes a hypothetical case vignette to illustrate how certain factors might help lower the chances of facing a malpractice suit.
Established patients
In med check appointments for established patients, consider the patient population, the availability of pre- and postvisit support services, and contingency plans (Table).

Different patient populations require different levels of treatment. Consider, for example, a patient with anxiety and trauma who is actively engaged with a therapist who works at the same agency as their psychiatrist, where the medication management appointments are solely for selective serotonin reuptake inhibitor refills. Compare that to a dual-diagnosis patient—with a psychotic and substance use disorder—who has had poor medication compliance and frequent rehospitalizations. The first patient is more likely to be reasonably managed in a 15-minute med check. The second patient would need significantly more pre- and postvisit support services. This consideration is relevant from a clinical perspective, and if a bad outcome occurs, from a malpractice perspective. Patient populations are not homogeneous; the reasonableness of a clinician’s actions during a brief med check visit depends on the specific patient.
Pre- and postvisit support services vary greatly from clinic to clinic. They range from clerical support (eg, calling a pharmacy to ensure that a patient’s medication is available for same-day pickup) to nursing support (eg, an injection nurse who is on site and can immediately provide a patient with a missed injection) to case manager support (eg, a case manager to facilitate coordination of care, such as by having a patient fill out record releases and then working to ensure that relevant hospital records are received prior to the next visit). The real-world availability of these services can determine the feasibility of safely conducting a 15-minute med check visit.
Continue to: Regardless of the patient population...
Regardless of the patient population, unexpected situations will arise. It could be a patient with posttraumatic stress disorder who was recently retraumatized and is in the midst of disclosing this new trauma at the end of a 15-minute visit. Or it could be a patient with dual diagnoses who comes to the agency intoxicated and manic, describing a plan to kill his neighbor with a shotgun. A clinician’s ability to meet the standard of care, and act reasonably within the confines of a brief med check structure, can thus depend on whether there are means of adequately managing such emergent situations.
Some clinics have fairly high no-show rates. Leaving no-show slots open for administrative time can provide a means of managing emergent situations. If, however, they are automatically rebooked with walk-ins, brief visits become more challenging. Thus, when assessing contingency plan logistics, consider the no-show rate, what happens when there are no-shows, how many other clinicians are available on a given day, and whether staff is available to provide support (eg, sitting with a patient while waiting for an ambulance).
New and transfer patients
Brief visits for new or transfer patients require the same assessment described above. However, there are additional considerations regarding previsit support services. Some clinics use clinical social workers to perform intake evaluations before a new patient sees the psychiatrist. A high-quality intake evaluation can allow a psychiatrist to focus, in a shorter amount of time, on a patient’s medication needs. An additional time saver is having support staff who will obtain relevant medical records before a patient’s first psychiatric visit. Such actions can greatly increase the efficacy of a new patient appointment for the prescribing clinician.
The reliability of and level of detail assessed in prior evaluations can be particularly relevant when considering a job providing coverage as locum tenens, when all patients will be new to you. Unfortunately, if you are not employed at a clinic, it can be hard to assess this ahead of time. If you know colleagues in the area where you are considering taking a locum position, ask for their opinions about the quality of work at the agency.
Case vignette
Mr. J is a 30-year-old man with schizoaffective disorder. For several years, he has been followed once every 4 weeks at the local clinic. During the first year of treatment, he had numerous hospitalizations due to medication noncompliance, psychotic episodes, and threats of violence against his mother. For the past year, he had been stable on the same dose of an oral antipsychotic medication (risperidone 2 mg twice a day). Then he stopped taking his medication, became increasingly psychotic, and, while holding a butcher knife, threatened to kill his mother. His mother called 911 and Mr. J was hospitalized.
Continue to: While in the hospital...
While in the hospital, Mr. J was restarted on risperidone 2 mg twice a day, and lithium 600 mg twice a day was added. As part of discharge planning, the hospital social worker set up an outpatient appointment with Dr. R, Mr. J’s treating psychiatrist at the clinic. That appointment was scheduled as a 15-minute med check. At the visit, Dr. R did not have or try to obtain a copy of the hospital discharge summary. Mr. J told Dr. R that he had been hospitalized because he had run out of his oral antipsychotic, and that it had been restarted during the hospitalization. Dr. R—who did not know about the recent incident involving a butcher knife or the subsequent medication changes—continued Mr. J’s risperidone, but did not continue his lithium because she did not know it had been added.
Dr. R scheduled a 4-week follow-up visit for Mr. J. Then she went on maternity leave. Because the agency was short-staffed, they hired Dr. C—a locum tenens—to see all of Dr. R’s established patients in 15-minute time slots.
At their first visit, Mr. J told Dr. C that he was gaining too much weight from his antipsychotic and wanted to know if it would be OK to decrease the dose. Dr. C reviewed Dr. R’s last office note but, due to limited time, did not review any other notes. Although Dr. C had 2 no-shows that day, the clinic had a policy that required Dr. C to see walk-ins whenever there was a no-show.
Dr. C did not know of Mr. J’s threats of violence or the medication changes associated with his recent hospitalization (they were not referenced in Dr. R’s last note). Dr. C decreased the dose of Mr. J’s risperidone from 2 mg twice a day to 0.5 mg twice a day. He did not do a violence risk assessment. Two weeks after the visit with Dr. C, Mr. J, who had become increasingly depressed and psychotic, killed his mother and died by suicide.
The estates of Mr. J and his mother filed a medical malpractice lawsuit against Dr. R and Dr. C. Both psychiatrists had a duty to Mr. J. Whether there was a duty to Mr. J’s mother would depend in part on the state’s duty to protect laws. Either way, the malpractice case would hinge on whether the psychiatrists’ conduct fell below the standard of care.
Continue to: In this case...
In this case, the critical issues were Dr. R’s failure to obtain and review the recent hospital records and Dr. C’s decision to decrease the antipsychotic dose. Of particular concern is Dr. C’s decision to decrease the antipsychotic dose without reviewing more information from past records, and the resultant failure to perform a violence risk assessment. These deviations cannot be blamed entirely on the brevity of the med check appointment. They could happen in a clinic that allotted longer time periods for follow-up visits, but they are, however, more likely to occur in a short med check appointment due to time constraints.
The likelihood of these errors could have been reduced by additional support services, as well as more time for Dr. C to see each patient who was new to him. For example, if there had been a support person available to obtain hospital records prior to the postdischarge appointment, Dr. R and Dr. C would have been more likely to be aware of the violent threat associated with Mr. J’s hospitalization. Additionally, if the busy clinicians had contingency plans to assess complicated patients, such as the ability to use no-show time to deal with difficult situations, Dr. C could have taken more time to review past records.
Bottom Line
When working in a setting that involves brief med check appointments, assess the agency structure, and whether it will allow you to practice reasonably. This will be relevant clinically and may reduce the risk of malpractice lawsuits. Reasonableness of a clinician’s actions is a fact-specific question and is influenced by multiple factors, including the patient population, the availability and quality of an agency’s support services, and contingency plans.
Related Resources
- Mossman D. Successfully navigating the 15-minute ‘med check.’ Current Psychiatry. 2010;9(6):40-43.
- Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
Drug Brand Names
Lithium • Eskalith, Lithobid
Risperidone • Risperdal
1. Malpractice. In: Garner BA, ed. Black’s Law Dictionary. 11th ed. Thomson West; 2019:1148.
2. Frierson RL, Joshi KG. Malpractice law and psychiatry: an overview. Focus. 2019;17:332-336. doi:10.1176/appi.focus.20190017
3. Negligence Based Claims. In: Boumil MM, Hattis PA, eds. Medical Liability in a Nutshell. 4th ed. West Academic Publishing; 2017:43-88
4. Peters PG. The quiet demise of deference to custom: malpractice law at the millennium. Washington and Lee Law Review. 2000;57(1):163-205. Accessed July 8, 2022. https://scholarlycommons.law.wlu.edu/wlulr/vol57/iss1/5
5. Simon RI. Standard-of-care testimony: best practices or reasonable care? J Am Acad Psychiatry Law. 2005;33(1):8-11. Accessed July 8, 2022. http://jaapl.org/content/33/1/8
6. Behrens SA. Call in Houdini: the time has come to be released from the geographic straightjacket known as the locality rule. Drake Law Review. 2008; 56(3):753-790. Accessed June 20, 2022. https://lawreviewdrake.files.wordpress.com/2015/06/lrvol56-3_behrens.pdf
Medical malpractice claims can arise in any type of health care setting. The purpose of this article is to discuss the risk of medical malpractice suits in the context of brief “med checks,” which are 15- to 20-minute follow-up appointments for psychiatric outpatient medication management. Similar issues arise in brief new patient and transfer visits.
Malpractice hinges on ‘reasonableness’
Malpractice is an allegation of professional negligence.1 More specifically, it is an allegation that a clinician violated an existing duty by deviating from the standard of care, and that deviation caused damages.2 Medical malpractice claims involve questions about whether there was a deviation from the standard of care (whether the clinician failed to exercise a reasonable degree of skill and care given the context of the situation) and whether there was causation (whether a deviation caused a patient’s damages).3 These are fact-based determinations. Thus, the legal resolution of a malpractice claim is based on the facts of each specific case.
The advisability of 15-minute med checks and the associated limitation on a clinician’s ability to provide talk therapy are beyond the scope of this article. What is clear, however, is that not all brief med check appointments are created equal. Their safety and efficacy are dictated by the milieu in which they exist.
Practically speaking, although many factors need to be considered, the standard of care in a medical malpractice lawsuit is based on reasonableness.4-6 One strategy to proactively manage your malpractice risk is to consider—either for your existing job or before accepting a new position—whether your agency’s setup for brief med checks will allow you to practice reasonably. This article provides information to help you answer this question and describes a hypothetical case vignette to illustrate how certain factors might help lower the chances of facing a malpractice suit.
Established patients
In med check appointments for established patients, consider the patient population, the availability of pre- and postvisit support services, and contingency plans (Table).

Different patient populations require different levels of treatment. Consider, for example, a patient with anxiety and trauma who is actively engaged with a therapist who works at the same agency as their psychiatrist, where the medication management appointments are solely for selective serotonin reuptake inhibitor refills. Compare that to a dual-diagnosis patient—with a psychotic and substance use disorder—who has had poor medication compliance and frequent rehospitalizations. The first patient is more likely to be reasonably managed in a 15-minute med check. The second patient would need significantly more pre- and postvisit support services. This consideration is relevant from a clinical perspective, and if a bad outcome occurs, from a malpractice perspective. Patient populations are not homogeneous; the reasonableness of a clinician’s actions during a brief med check visit depends on the specific patient.
Pre- and postvisit support services vary greatly from clinic to clinic. They range from clerical support (eg, calling a pharmacy to ensure that a patient’s medication is available for same-day pickup) to nursing support (eg, an injection nurse who is on site and can immediately provide a patient with a missed injection) to case manager support (eg, a case manager to facilitate coordination of care, such as by having a patient fill out record releases and then working to ensure that relevant hospital records are received prior to the next visit). The real-world availability of these services can determine the feasibility of safely conducting a 15-minute med check visit.
Continue to: Regardless of the patient population...
Regardless of the patient population, unexpected situations will arise. It could be a patient with posttraumatic stress disorder who was recently retraumatized and is in the midst of disclosing this new trauma at the end of a 15-minute visit. Or it could be a patient with dual diagnoses who comes to the agency intoxicated and manic, describing a plan to kill his neighbor with a shotgun. A clinician’s ability to meet the standard of care, and act reasonably within the confines of a brief med check structure, can thus depend on whether there are means of adequately managing such emergent situations.
Some clinics have fairly high no-show rates. Leaving no-show slots open for administrative time can provide a means of managing emergent situations. If, however, they are automatically rebooked with walk-ins, brief visits become more challenging. Thus, when assessing contingency plan logistics, consider the no-show rate, what happens when there are no-shows, how many other clinicians are available on a given day, and whether staff is available to provide support (eg, sitting with a patient while waiting for an ambulance).
New and transfer patients
Brief visits for new or transfer patients require the same assessment described above. However, there are additional considerations regarding previsit support services. Some clinics use clinical social workers to perform intake evaluations before a new patient sees the psychiatrist. A high-quality intake evaluation can allow a psychiatrist to focus, in a shorter amount of time, on a patient’s medication needs. An additional time saver is having support staff who will obtain relevant medical records before a patient’s first psychiatric visit. Such actions can greatly increase the efficacy of a new patient appointment for the prescribing clinician.
The reliability of and level of detail assessed in prior evaluations can be particularly relevant when considering a job providing coverage as locum tenens, when all patients will be new to you. Unfortunately, if you are not employed at a clinic, it can be hard to assess this ahead of time. If you know colleagues in the area where you are considering taking a locum position, ask for their opinions about the quality of work at the agency.
Case vignette
Mr. J is a 30-year-old man with schizoaffective disorder. For several years, he has been followed once every 4 weeks at the local clinic. During the first year of treatment, he had numerous hospitalizations due to medication noncompliance, psychotic episodes, and threats of violence against his mother. For the past year, he had been stable on the same dose of an oral antipsychotic medication (risperidone 2 mg twice a day). Then he stopped taking his medication, became increasingly psychotic, and, while holding a butcher knife, threatened to kill his mother. His mother called 911 and Mr. J was hospitalized.
Continue to: While in the hospital...
While in the hospital, Mr. J was restarted on risperidone 2 mg twice a day, and lithium 600 mg twice a day was added. As part of discharge planning, the hospital social worker set up an outpatient appointment with Dr. R, Mr. J’s treating psychiatrist at the clinic. That appointment was scheduled as a 15-minute med check. At the visit, Dr. R did not have or try to obtain a copy of the hospital discharge summary. Mr. J told Dr. R that he had been hospitalized because he had run out of his oral antipsychotic, and that it had been restarted during the hospitalization. Dr. R—who did not know about the recent incident involving a butcher knife or the subsequent medication changes—continued Mr. J’s risperidone, but did not continue his lithium because she did not know it had been added.
Dr. R scheduled a 4-week follow-up visit for Mr. J. Then she went on maternity leave. Because the agency was short-staffed, they hired Dr. C—a locum tenens—to see all of Dr. R’s established patients in 15-minute time slots.
At their first visit, Mr. J told Dr. C that he was gaining too much weight from his antipsychotic and wanted to know if it would be OK to decrease the dose. Dr. C reviewed Dr. R’s last office note but, due to limited time, did not review any other notes. Although Dr. C had 2 no-shows that day, the clinic had a policy that required Dr. C to see walk-ins whenever there was a no-show.
Dr. C did not know of Mr. J’s threats of violence or the medication changes associated with his recent hospitalization (they were not referenced in Dr. R’s last note). Dr. C decreased the dose of Mr. J’s risperidone from 2 mg twice a day to 0.5 mg twice a day. He did not do a violence risk assessment. Two weeks after the visit with Dr. C, Mr. J, who had become increasingly depressed and psychotic, killed his mother and died by suicide.
The estates of Mr. J and his mother filed a medical malpractice lawsuit against Dr. R and Dr. C. Both psychiatrists had a duty to Mr. J. Whether there was a duty to Mr. J’s mother would depend in part on the state’s duty to protect laws. Either way, the malpractice case would hinge on whether the psychiatrists’ conduct fell below the standard of care.
Continue to: In this case...
In this case, the critical issues were Dr. R’s failure to obtain and review the recent hospital records and Dr. C’s decision to decrease the antipsychotic dose. Of particular concern is Dr. C’s decision to decrease the antipsychotic dose without reviewing more information from past records, and the resultant failure to perform a violence risk assessment. These deviations cannot be blamed entirely on the brevity of the med check appointment. They could happen in a clinic that allotted longer time periods for follow-up visits, but they are, however, more likely to occur in a short med check appointment due to time constraints.
The likelihood of these errors could have been reduced by additional support services, as well as more time for Dr. C to see each patient who was new to him. For example, if there had been a support person available to obtain hospital records prior to the postdischarge appointment, Dr. R and Dr. C would have been more likely to be aware of the violent threat associated with Mr. J’s hospitalization. Additionally, if the busy clinicians had contingency plans to assess complicated patients, such as the ability to use no-show time to deal with difficult situations, Dr. C could have taken more time to review past records.
Bottom Line
When working in a setting that involves brief med check appointments, assess the agency structure, and whether it will allow you to practice reasonably. This will be relevant clinically and may reduce the risk of malpractice lawsuits. Reasonableness of a clinician’s actions is a fact-specific question and is influenced by multiple factors, including the patient population, the availability and quality of an agency’s support services, and contingency plans.
Related Resources
- Mossman D. Successfully navigating the 15-minute ‘med check.’ Current Psychiatry. 2010;9(6):40-43.
- Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
Drug Brand Names
Lithium • Eskalith, Lithobid
Risperidone • Risperdal
Medical malpractice claims can arise in any type of health care setting. The purpose of this article is to discuss the risk of medical malpractice suits in the context of brief “med checks,” which are 15- to 20-minute follow-up appointments for psychiatric outpatient medication management. Similar issues arise in brief new patient and transfer visits.
Malpractice hinges on ‘reasonableness’
Malpractice is an allegation of professional negligence.1 More specifically, it is an allegation that a clinician violated an existing duty by deviating from the standard of care, and that deviation caused damages.2 Medical malpractice claims involve questions about whether there was a deviation from the standard of care (whether the clinician failed to exercise a reasonable degree of skill and care given the context of the situation) and whether there was causation (whether a deviation caused a patient’s damages).3 These are fact-based determinations. Thus, the legal resolution of a malpractice claim is based on the facts of each specific case.
The advisability of 15-minute med checks and the associated limitation on a clinician’s ability to provide talk therapy are beyond the scope of this article. What is clear, however, is that not all brief med check appointments are created equal. Their safety and efficacy are dictated by the milieu in which they exist.
Practically speaking, although many factors need to be considered, the standard of care in a medical malpractice lawsuit is based on reasonableness.4-6 One strategy to proactively manage your malpractice risk is to consider—either for your existing job or before accepting a new position—whether your agency’s setup for brief med checks will allow you to practice reasonably. This article provides information to help you answer this question and describes a hypothetical case vignette to illustrate how certain factors might help lower the chances of facing a malpractice suit.
Established patients
In med check appointments for established patients, consider the patient population, the availability of pre- and postvisit support services, and contingency plans (Table).

Different patient populations require different levels of treatment. Consider, for example, a patient with anxiety and trauma who is actively engaged with a therapist who works at the same agency as their psychiatrist, where the medication management appointments are solely for selective serotonin reuptake inhibitor refills. Compare that to a dual-diagnosis patient—with a psychotic and substance use disorder—who has had poor medication compliance and frequent rehospitalizations. The first patient is more likely to be reasonably managed in a 15-minute med check. The second patient would need significantly more pre- and postvisit support services. This consideration is relevant from a clinical perspective, and if a bad outcome occurs, from a malpractice perspective. Patient populations are not homogeneous; the reasonableness of a clinician’s actions during a brief med check visit depends on the specific patient.
Pre- and postvisit support services vary greatly from clinic to clinic. They range from clerical support (eg, calling a pharmacy to ensure that a patient’s medication is available for same-day pickup) to nursing support (eg, an injection nurse who is on site and can immediately provide a patient with a missed injection) to case manager support (eg, a case manager to facilitate coordination of care, such as by having a patient fill out record releases and then working to ensure that relevant hospital records are received prior to the next visit). The real-world availability of these services can determine the feasibility of safely conducting a 15-minute med check visit.
Continue to: Regardless of the patient population...
Regardless of the patient population, unexpected situations will arise. It could be a patient with posttraumatic stress disorder who was recently retraumatized and is in the midst of disclosing this new trauma at the end of a 15-minute visit. Or it could be a patient with dual diagnoses who comes to the agency intoxicated and manic, describing a plan to kill his neighbor with a shotgun. A clinician’s ability to meet the standard of care, and act reasonably within the confines of a brief med check structure, can thus depend on whether there are means of adequately managing such emergent situations.
Some clinics have fairly high no-show rates. Leaving no-show slots open for administrative time can provide a means of managing emergent situations. If, however, they are automatically rebooked with walk-ins, brief visits become more challenging. Thus, when assessing contingency plan logistics, consider the no-show rate, what happens when there are no-shows, how many other clinicians are available on a given day, and whether staff is available to provide support (eg, sitting with a patient while waiting for an ambulance).
New and transfer patients
Brief visits for new or transfer patients require the same assessment described above. However, there are additional considerations regarding previsit support services. Some clinics use clinical social workers to perform intake evaluations before a new patient sees the psychiatrist. A high-quality intake evaluation can allow a psychiatrist to focus, in a shorter amount of time, on a patient’s medication needs. An additional time saver is having support staff who will obtain relevant medical records before a patient’s first psychiatric visit. Such actions can greatly increase the efficacy of a new patient appointment for the prescribing clinician.
The reliability of and level of detail assessed in prior evaluations can be particularly relevant when considering a job providing coverage as locum tenens, when all patients will be new to you. Unfortunately, if you are not employed at a clinic, it can be hard to assess this ahead of time. If you know colleagues in the area where you are considering taking a locum position, ask for their opinions about the quality of work at the agency.
Case vignette
Mr. J is a 30-year-old man with schizoaffective disorder. For several years, he has been followed once every 4 weeks at the local clinic. During the first year of treatment, he had numerous hospitalizations due to medication noncompliance, psychotic episodes, and threats of violence against his mother. For the past year, he had been stable on the same dose of an oral antipsychotic medication (risperidone 2 mg twice a day). Then he stopped taking his medication, became increasingly psychotic, and, while holding a butcher knife, threatened to kill his mother. His mother called 911 and Mr. J was hospitalized.
Continue to: While in the hospital...
While in the hospital, Mr. J was restarted on risperidone 2 mg twice a day, and lithium 600 mg twice a day was added. As part of discharge planning, the hospital social worker set up an outpatient appointment with Dr. R, Mr. J’s treating psychiatrist at the clinic. That appointment was scheduled as a 15-minute med check. At the visit, Dr. R did not have or try to obtain a copy of the hospital discharge summary. Mr. J told Dr. R that he had been hospitalized because he had run out of his oral antipsychotic, and that it had been restarted during the hospitalization. Dr. R—who did not know about the recent incident involving a butcher knife or the subsequent medication changes—continued Mr. J’s risperidone, but did not continue his lithium because she did not know it had been added.
Dr. R scheduled a 4-week follow-up visit for Mr. J. Then she went on maternity leave. Because the agency was short-staffed, they hired Dr. C—a locum tenens—to see all of Dr. R’s established patients in 15-minute time slots.
At their first visit, Mr. J told Dr. C that he was gaining too much weight from his antipsychotic and wanted to know if it would be OK to decrease the dose. Dr. C reviewed Dr. R’s last office note but, due to limited time, did not review any other notes. Although Dr. C had 2 no-shows that day, the clinic had a policy that required Dr. C to see walk-ins whenever there was a no-show.
Dr. C did not know of Mr. J’s threats of violence or the medication changes associated with his recent hospitalization (they were not referenced in Dr. R’s last note). Dr. C decreased the dose of Mr. J’s risperidone from 2 mg twice a day to 0.5 mg twice a day. He did not do a violence risk assessment. Two weeks after the visit with Dr. C, Mr. J, who had become increasingly depressed and psychotic, killed his mother and died by suicide.
The estates of Mr. J and his mother filed a medical malpractice lawsuit against Dr. R and Dr. C. Both psychiatrists had a duty to Mr. J. Whether there was a duty to Mr. J’s mother would depend in part on the state’s duty to protect laws. Either way, the malpractice case would hinge on whether the psychiatrists’ conduct fell below the standard of care.
Continue to: In this case...
In this case, the critical issues were Dr. R’s failure to obtain and review the recent hospital records and Dr. C’s decision to decrease the antipsychotic dose. Of particular concern is Dr. C’s decision to decrease the antipsychotic dose without reviewing more information from past records, and the resultant failure to perform a violence risk assessment. These deviations cannot be blamed entirely on the brevity of the med check appointment. They could happen in a clinic that allotted longer time periods for follow-up visits, but they are, however, more likely to occur in a short med check appointment due to time constraints.
The likelihood of these errors could have been reduced by additional support services, as well as more time for Dr. C to see each patient who was new to him. For example, if there had been a support person available to obtain hospital records prior to the postdischarge appointment, Dr. R and Dr. C would have been more likely to be aware of the violent threat associated with Mr. J’s hospitalization. Additionally, if the busy clinicians had contingency plans to assess complicated patients, such as the ability to use no-show time to deal with difficult situations, Dr. C could have taken more time to review past records.
Bottom Line
When working in a setting that involves brief med check appointments, assess the agency structure, and whether it will allow you to practice reasonably. This will be relevant clinically and may reduce the risk of malpractice lawsuits. Reasonableness of a clinician’s actions is a fact-specific question and is influenced by multiple factors, including the patient population, the availability and quality of an agency’s support services, and contingency plans.
Related Resources
- Mossman D. Successfully navigating the 15-minute ‘med check.’ Current Psychiatry. 2010;9(6):40-43.
- Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
Drug Brand Names
Lithium • Eskalith, Lithobid
Risperidone • Risperdal
1. Malpractice. In: Garner BA, ed. Black’s Law Dictionary. 11th ed. Thomson West; 2019:1148.
2. Frierson RL, Joshi KG. Malpractice law and psychiatry: an overview. Focus. 2019;17:332-336. doi:10.1176/appi.focus.20190017
3. Negligence Based Claims. In: Boumil MM, Hattis PA, eds. Medical Liability in a Nutshell. 4th ed. West Academic Publishing; 2017:43-88
4. Peters PG. The quiet demise of deference to custom: malpractice law at the millennium. Washington and Lee Law Review. 2000;57(1):163-205. Accessed July 8, 2022. https://scholarlycommons.law.wlu.edu/wlulr/vol57/iss1/5
5. Simon RI. Standard-of-care testimony: best practices or reasonable care? J Am Acad Psychiatry Law. 2005;33(1):8-11. Accessed July 8, 2022. http://jaapl.org/content/33/1/8
6. Behrens SA. Call in Houdini: the time has come to be released from the geographic straightjacket known as the locality rule. Drake Law Review. 2008; 56(3):753-790. Accessed June 20, 2022. https://lawreviewdrake.files.wordpress.com/2015/06/lrvol56-3_behrens.pdf
1. Malpractice. In: Garner BA, ed. Black’s Law Dictionary. 11th ed. Thomson West; 2019:1148.
2. Frierson RL, Joshi KG. Malpractice law and psychiatry: an overview. Focus. 2019;17:332-336. doi:10.1176/appi.focus.20190017
3. Negligence Based Claims. In: Boumil MM, Hattis PA, eds. Medical Liability in a Nutshell. 4th ed. West Academic Publishing; 2017:43-88
4. Peters PG. The quiet demise of deference to custom: malpractice law at the millennium. Washington and Lee Law Review. 2000;57(1):163-205. Accessed July 8, 2022. https://scholarlycommons.law.wlu.edu/wlulr/vol57/iss1/5
5. Simon RI. Standard-of-care testimony: best practices or reasonable care? J Am Acad Psychiatry Law. 2005;33(1):8-11. Accessed July 8, 2022. http://jaapl.org/content/33/1/8
6. Behrens SA. Call in Houdini: the time has come to be released from the geographic straightjacket known as the locality rule. Drake Law Review. 2008; 56(3):753-790. Accessed June 20, 2022. https://lawreviewdrake.files.wordpress.com/2015/06/lrvol56-3_behrens.pdf
Risk factors for nonsuicidal self-injury: A review of the evidence
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.
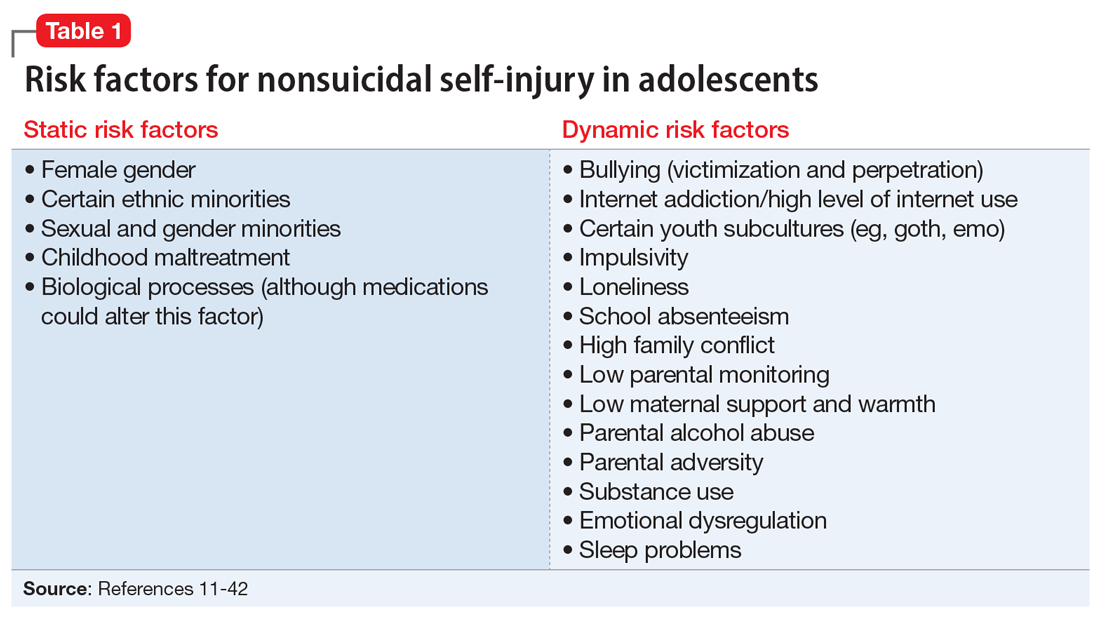
NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.
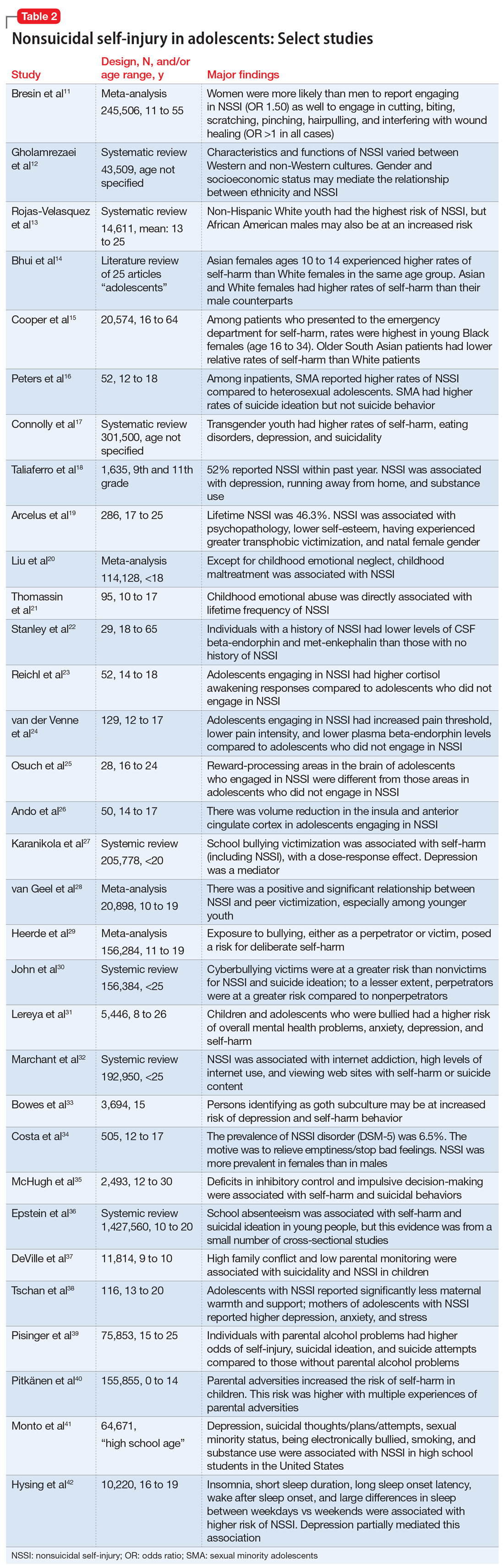
Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.
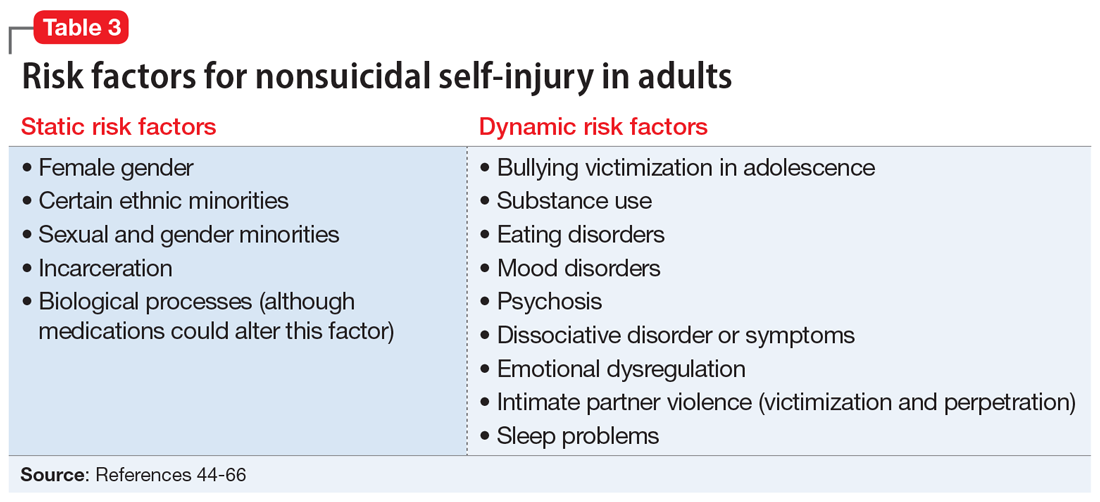
Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11
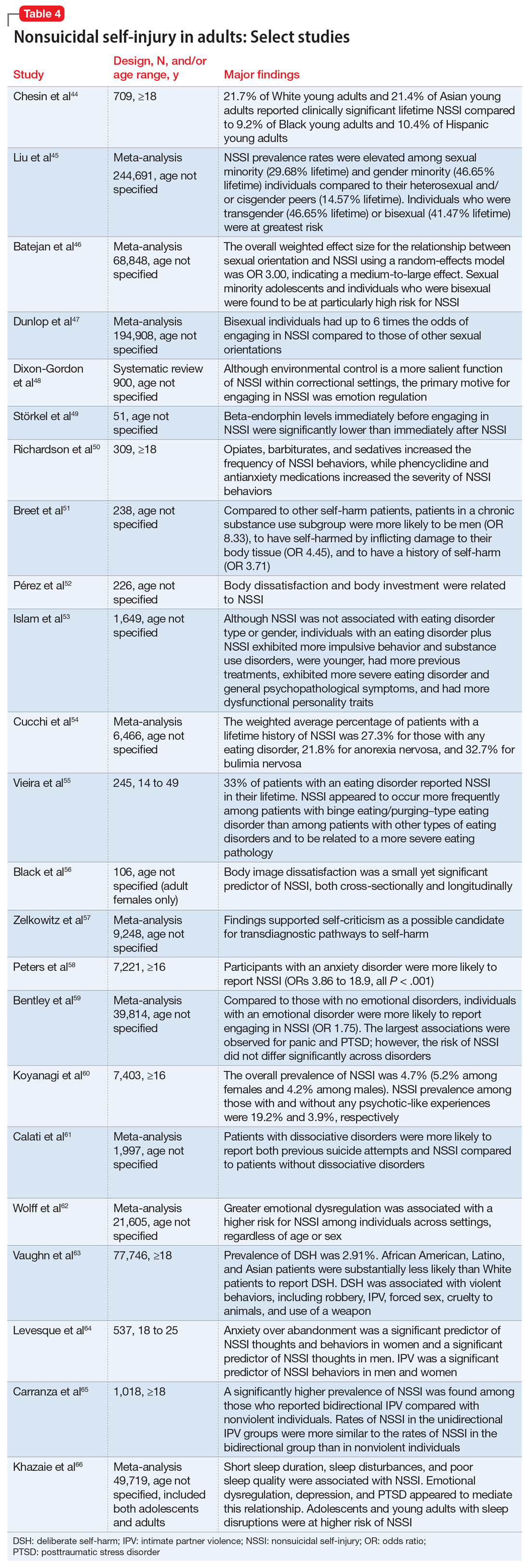
As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360