User login
Generalized anxiety disorder: 8 studies of biological interventions
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
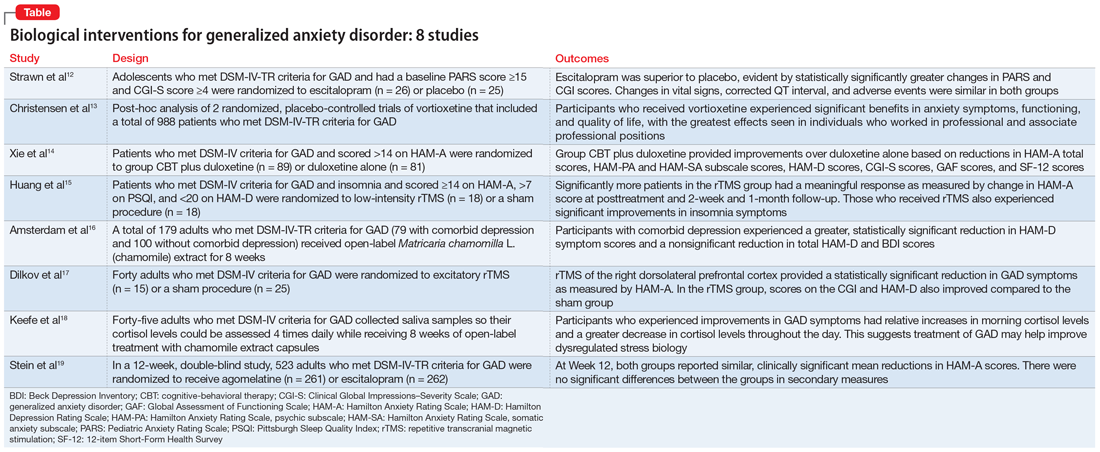
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
Paraphilic disorders and sexual criminality
Mr. J, age 23, presents to an outpatient mental health clinic for treatment of anxiety. He has no psychiatric history, is dressed neatly, and recently finished graduate school with a degree in accounting. Mr. J is reserved during the initial psychiatric evaluation and provides only basic facts about his developmental history.
Mr. J comes from a middle-class household with no history of trauma or substance use. He does not report any symptoms consistent with anxiety, but discloses a history of sexual preoccupations. Mr. J says that during adolescence he developed a predilection for observing others engage in sexual activity. In his late teens, he began following couples to their homes in the hope of witnessing sexual intimacy. In the rare instance that his voyeuristic fantasy comes to fruition, he masturbates and achieves sexual gratification he is incapable of experiencing otherwise. Mr. J notes that he has not yet been caught, but he expresses concern and embarrassment related to his actions. He concludes by noting that he seeks help because the frequency of this behavior has steadily increased.
How would you treat Mr. J? Where does the line exist between a normophilic sexual interest, fantasy or urge, and a paraphilia? Does Mr. J qualify as a sexually violent predator?
From The Rocky Horror Picture Show to Fifty Shades of Grey, sensationalized portrayals of sexual deviancy have long been present in popular culture. The continued popularity of serial killers years after their crimes seems in part related to the extreme sexual torture their victims often endure. However, a sexual offense does not always qualify as a paraphilic disorder.1 In fact, many individuals with paraphilic disorders never engage in illegal activity. Additionally, experiencing sexually deviant thoughts alone does not qualify as a paraphilic disorder.1
A thorough psychiatric evaluation should include a discussion of the patient’s sexual history, including the potential of sexual dysfunction and abnormal desires or behaviors. Most individuals with sexual dysfunction do not have a paraphilic disorder.2 DSM-5 and ICD-11 classify sexual dysfunction and paraphilic disorders in different categories. However, previous editions grouped them together under sexual and gender identity disorders. Individuals with paraphilic disorders may not originally present to the outpatient setting for a paraphilic disorder, but instead may first seek treatment for a more common comorbid disorder, such as a mood disorder, personality disorder, or substance use disorder.3
Diagnostically speaking, if individuals do not experience distress or issues with functionality and lack legal charges (suggesting that they have not violated the rights of others), they are categorized as having an atypical sexual interest but do not necessarily meet the criteria for a disorder.4 This article provides an overview of paraphilic disorders as well as forensic considerations when examining individuals with sexually deviant behaviors.
Overview of paraphilic disorders
DSM-5 characterizes a paraphilic disorder as “recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving nonhuman objects or nonconsenting partners for at least 6 months. The individual must have acted on the thought and/or it caused clinically significant distress or impairment in social, occupational, or other important areas of functioning.” DSM-5 outlines 9 categories of paraphilic disorders, which are described in Table 1.4,5
Continue to: Paraphilic disorders are more common...
Paraphilic disorders are more common in men than in women; the 2 most prevalent are voyeuristic disorder and frotteuristic disorder.6 The incidence of paraphilias in the general outpatient setting varies by disorder. Approximately 45% of individuals with pedophilic disorder seek treatment, whereas only 1% of individuals with zoophilia seek treatment.6 The incidence of paraphilic acts also varies drastically; individuals with exhibitionistic disorder engaged in an average of 50 acts vs only 3 for individuals with sexual sadism.6 Not all individuals with paraphilic disorders commit crimes. Approximately 58% of sexual offenders meet the criteria for a paraphilic disorder, but antisocial personality disorder is a far more common diagnosis.7
Sexual psychopath statutes: Phase 1
In 1937, Michigan became the first state to enact sexual psychopath statutes, allowing for indeterminate sentencing and the civil commitment/treatment of sex offenders with repeated convictions. By the 1970s, more than 30 states had enacted similar statutes. It was not until 1967, in Specht v Patterson,8 that the United States Supreme Court unanimously ruled that the Fourteenth Amendment Due Process Clause was violated when Francis Eddie Specht faced life in prison following his conviction for indecent liberties under the Colorado Sex Offenders Act.
Specht was convicted in 1959 for indecent liberties after pleading guilty to enticing a child younger than age 16 into an office and engaging in sexual activities with them. At the time of Specht’s conviction, the crime of indecent liberties carried a punishment of 10 years. However, Specht was sentenced under the Sexual Offenders Act, which allowed for an indeterminate sentence of 1 day to life in prison. The Supreme Court noted that Specht was denied the right to be present with counsel, to confront the evidence against him, to cross-examine witnesses, and to offer his own evidence, which was a violation of his constitutionally guaranteed Fourteenth Amendment right to Procedural Due Process. The decision led most states to repeal early sexual psychopath statutes.8
Sexually violent predator laws: Phase 2
After early sexual psychopath statutes were repealed, many states pushed to update sex offender laws in response to the Earl Shriner case.9 In 1989, Shriner was released from prison after serving a 10-year sentence for sexually assaulting 2 teenage girls. At the time, he did not meet the criteria for civil commitment in the state of Washington. On the day he was released, Shriner cut off a young boy’s penis and left him to die. Washington subsequently became the first of many states to enact sexually violent predator (SVP) laws. Table 210 shows states and districts that have SVP civil commitment laws.
A series of United States Supreme Court cases solidified current sexual offender civil commitment laws (Table 38,11-15).
Continue to: Allen v Illinois
Allen v Illinois (1986).11 The Court ruled that forcing an individual to participate in a psychiatric evaluation prior to a sexually dangerous person’s commitment hearing did not violate the individual’s Fifth Amendment right against self-incrimination because the purpose of the evaluation was to provide treatment, not punishment.
Kansas v Hendricks (1997).12 The Court upheld that the Kansas Sexually Violent Predator Act was constitutional and noted that the use of the broad term “mental abnormality” (in lieu of the more specific term “mental illness”) does not violate an individual’s Fourteenth Amendment right to substantive due process. Additionally, the Court opined that the constitutional ban on double jeopardy and ex post facto lawmaking does not apply because the procedures are civil, not criminal.
Kansas v Crane (2002).13 The Court upheld the Kansas Sexually Violent Predator Act, stating that mental illness and dangerousness are essential elements to meet the criteria for civil commitment. The Court added that proof of partial (not total) “volitional impairment” is all that is required to meet the threshold of sexual dangerousness.
McKune v Lile (2002).14 The Court ruled that a policy requiring participation in polygraph testing, which would lead to the disclosure of sexual crimes (even those that have not been prosecuted), does not violate an individual’s Fifth Amendment rights because it serves a vital penological purpose.
Adam Walsh Child Protection and Safety Act of 200616; United States v Comstock (2010).15 This act and subsequent case reinforced the federal government’s right to civilly commit sexually dangerous persons approaching the end of their prison sentences.
Continue to: What is requiried for civil commitment?
What is required for civil commitment?
SVP laws require 4 conditions to be met for the civil commitment of sexual offenders (Table 417). In criteria 1, “charges” is a key word, because this allows individuals found Not Guilty by Reason of Insanity or Incompetent to Stand Trial to be civilly committed. Criteria 2 defines “mental abnormality” as a “congenital or acquired condition affecting the emotional or volitional capacity which predisposes the person to commit criminal sexual acts in a degree constituting such person a menace to the health and safety of others.”18 This is a broad definition, and allows individuals with personality disorders to be civilly committed (although most sexual offenders are committed for having a paraphilic disorder). To determine risk, various actuarial instruments are used to assess for sexually violent recidivism, including (but not limited to) the Static-99R, Sexual Violence Risk-20, and the Sex Offender Risk Appraisal Guide.19
Although the percentages vary, sex offenders rarely are civilly committed following their criminal sentence. In California, approximately 1.5% of sex offenders are civilly committed.17 The standard of proof for civil commitment varies by state between “clear and convincing evidence” and “beyond a reasonable doubt.” As sex offenders approach the end of their sentence, sexually violent offenders are identified to the general population and referred for a psychiatric evaluation. If the individual meets the 4 criteria for commitment (Table 417), their case is sent to the prosecuting attorney’s office. If accepted, the court holds a probable cause hearing, followed by a full trial.
Pornography and sex offenders
Pornography has long been considered a risk factor for sexual offending, and the role of pornography in influencing sexual behavior has drawn recent interest in research towards predicting future offenses. However, a 2019 systematic review by Mellor et al20 on the relationship between pornography and sexual offending suggested that early exposure to pornography is not a risk factor for sexual offending, nor is the risk of offending increased shortly after pornography exposure. Additionally, pornography use did not predict recidivism in low-risk sexual offenders, but did in high-risk offenders.
The use of child pornography presents a set of new risk factors. Prohibited by federal and state law, child pornography is defined under Section 2256 of Title 18, United States Code, as any visual depiction of sexually explicit conduct involving a minor (someone <age 18). Visual depictions include photographs, videos, digital or computer-generated images indistinguishable from an actual minor, and images created to depict a minor. The law does not require an image of a child engaging in sexual activity for the image to be characterized as child pornography. Offenders are also commonly charged with the distribution of child pornography. A conviction of child pornography possession carries a 15- to 30-year sentence, and distribution carries a 5- to 20-year sentence.21 The individual must also file for the sex offender registry, which may restrict their employment and place of residency.
It is unclear what percentage of individuals charged with child pornography have a history of prior sexual offenses. Numerous studies suggest there is a low risk of online offenders without prior offenses becoming contact offenders. Characteristics of online-only offenders include being White, a single male, age 20 to 30, well-educated, and employed, and having antisocial traits and a history of sexual deviancy.22 Contact offenders tend to be married with easy access to children, unemployed, uneducated, and to have a history of mental illness or criminal offenses.22
Continue to: Recidivism and treatment
Recidivism and treatment
The recidivism rate among sexual offenders averages 13.7% at 3- to 6-year follow-up,although rates vary by type of sexual offense.23 Individuals who committed rape have the highest rate of recidivism, while those who engaged in incest have the lowest. Three key points about sexual offender recidivism are:
- it declines over time and with increased age.
- sexual offenders are more like to commit a nonsexual offense than a sexual offense.
- sexual offenders who have undergone treatment are 26.3% less likely to reoffend.23
Although there is no standard of treatment, current interventions include external control, reduction of sexual drive, treatment of comorbid conditions, cognitive-behavioral therapy (CBT), and dynamic psychotherapy. External control relies on an outside entity that affects the individual’s behavior. For sexually deviant behaviors, simply making the act illegal or involving the law may inhibit many individuals from acting on a thought. Additional external control may include pharmacotherapy, which ranges from nonhormonal options such as selective serotonin reuptake inhibitors (SSRIs) to hormonal options. Therapy tends to focus on social skills training, sex education, cognitive restructuring, and identifying triggers, as well as victim empathy. The best indicators for successful treatment include an absence of comorbidities, increased age, and adult interpersonal relationships.24
Treatment choice may be predicated on the severity of the paraphilia. Psychotherapy alone is recommended for individuals able to maintain functioning if it does not affect their conventional sexual activity. Common treatment for low-risk individuals is psychotherapy and an SSRI. As risk increases, so does treatment with pharmacologic agents. Beyond SSRIs, moderate offenders may be treated with an SSRI and a low-dose antiandrogen. This is escalated in high-risk violent offenders to long-acting gonadotropin-releasing hormone analogs and synthetic steroidal analogs.25
An evolving class of disorders
With the evolution and accessibility of pornography, uncommon sexual practices have become more common, gaining notoriety and increased social acceptance. As a result, mental health professionals may be tasked with evaluating patients for possible paraphilic disorders. A common misconception is that individuals with sexually deviant thoughts, sexual offenders, and patients with paraphilic disorders are all the same. However, more commonly, sexual offenders do not have a paraphilic disorder. In the case of SVPs, outside of imprisonment, civil commitment remains a consideration for possible treatment. To meet the threshold of civil commitment, a sexual offender must have a “mental abnormality,” which is most commonly a paraphilic disorder. The treatment of paraphilic disorders remains a difficult task and includes a mixture of psychotherapy and medication options.
CASE CONTINUED
Mr. J begins weekly CBT to gain control of his voyeuristic fantasies without impacting his conventional sexual activity and desire. He responds well to treatment, and after 18 months, begins a typical sexual relationship with a woman. Although his voyeuristic thoughts remain, the urge to act on the thoughts decreases as Mr. J develops coping mechanisms. He does not require pharmacologic treatment.
Bottom Line
Individuals with paraphilic disorders are too often portrayed as sexual deviants or criminals. Psychiatrists must review each case with careful consideration of individual risk factors, such as the patient’s sexual history, to evaluate potential treatment options while determining if they pose a threat to the public.
Related Resources
- Sorrentino R, Abramowitz J. Minor-attracted persons: a neglected population. Current Psychiatry. 2021;20(7):21-27. doi:10.12788/cp.0149
- Berlin FS. Paraphilic disorders: a better understanding. Current Psychiatry. 2019;18(4):22-26,28.
1. Federoff JP. The paraphilias. In: Gelder MG, Andreasen NC, López-Ibor JJ Jr, Geddes JR, eds. New Oxford Textbook of Psychiatry. 2nd ed. Oxford University Press; 2012:832-842.
2. Grubin D. Medical models and interventions in sexual deviance. In: Laws R, O’Donohue WT, eds. Sexual Deviance: Theory, Assessment and Treatment. 2nd ed. Guilford Press; 2008:594-610.
3. Guidry LL, Saleh FM. Clinical considerations of paraphilic sex offenders with comorbid psychiatric conditions. Sex Addict Compulsivity. 2004;11(1-2):21-34.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Balon R. Paraphilic disorders. In: Roberts LW, Hales RE, Yudofsky SC, eds. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019:749-770.
6. Sadock BJ, Sadock VA, Ruiz P. Paraphilic disorders. Kaplan and Sadock’s Synopsis of Psychiatry. 11th ed. Wolters Kluwer; 2015:593-599.
7. First MB, Halon RL. Use of DSM paraphilia diagnosis in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
8. Specht v Patterson, 386 US 605 (1967).
9. Ra EP. The civil confinement of sexual predators: a delicate balance. J Civ Rts Econ Dev. 2007;22(1):335-372.
10. Felthous AR, Ko J. Sexually violent predator law in the United States. East Asian Arch Psychiatry. 2018;28(4):159-173.
11. Allen v Illinois, 478 US 364 (1986).
12. Kansas v Hendricks, 521 US 346 (1997).
13. Kansas v Crane, 534 US 407 (2002).
14. McKune v Lile, 536 US 24 (2002).
15. United States v Comstock, 560 US 126 (2010).
16. Adam Walsh Child Protection and Safety Act of 2006, HR 4472, 109th Cong (2006). Accessed April 25, 2022. https://www.congress.gov/bill/109th-congress/house-bill/4472
17. Tucker DE, Brakel SJ. Sexually violent predator laws. In: Rosner R, Scott C, eds. Principles and Practice of Forensic Psychiatry. 3rd ed. CRC Press; 2017:823-831.
18. Wash. Rev. Code. Ann. §71.09.020(8)
19. Bradford J, de Amorim Levin GV, Booth BD, et al. Forensic assessment of sex offenders. In: Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2017:382-397.
20. Mellor E, Duff S. The use of pornography and the relationship between pornography exposure and sexual offending in males: a systematic review. Aggress Violent Beh. 2019;46:116-126.
21. Failure To Register, 18 USC § 2250 (2012). Accessed April 25, 2022. https://www.govinfo.gov/app/details/USCODE-2011-title18/USCODE-2011-title18-partI-chap109B-sec2250
22. Hirschtritt ME, Tucker D, Binder RL. Risk assessment of online child sexual exploitation offenders. J Am Acad Psychiatry Law. 2019;47(2):155-164.
23. Blasko BL. Overview of sexual offender typologies, recidivism, and treatment. In: Jeglic EL, Calkins C, eds. Sexual Violence: Evidence Based Policy and Prevention. Springer; 2016:11-29.
24. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490.
25. Holoyda B. Paraphilias: from diagnosis to treatment. Psychiatric Times. 2019;36(12).
Mr. J, age 23, presents to an outpatient mental health clinic for treatment of anxiety. He has no psychiatric history, is dressed neatly, and recently finished graduate school with a degree in accounting. Mr. J is reserved during the initial psychiatric evaluation and provides only basic facts about his developmental history.
Mr. J comes from a middle-class household with no history of trauma or substance use. He does not report any symptoms consistent with anxiety, but discloses a history of sexual preoccupations. Mr. J says that during adolescence he developed a predilection for observing others engage in sexual activity. In his late teens, he began following couples to their homes in the hope of witnessing sexual intimacy. In the rare instance that his voyeuristic fantasy comes to fruition, he masturbates and achieves sexual gratification he is incapable of experiencing otherwise. Mr. J notes that he has not yet been caught, but he expresses concern and embarrassment related to his actions. He concludes by noting that he seeks help because the frequency of this behavior has steadily increased.
How would you treat Mr. J? Where does the line exist between a normophilic sexual interest, fantasy or urge, and a paraphilia? Does Mr. J qualify as a sexually violent predator?
From The Rocky Horror Picture Show to Fifty Shades of Grey, sensationalized portrayals of sexual deviancy have long been present in popular culture. The continued popularity of serial killers years after their crimes seems in part related to the extreme sexual torture their victims often endure. However, a sexual offense does not always qualify as a paraphilic disorder.1 In fact, many individuals with paraphilic disorders never engage in illegal activity. Additionally, experiencing sexually deviant thoughts alone does not qualify as a paraphilic disorder.1
A thorough psychiatric evaluation should include a discussion of the patient’s sexual history, including the potential of sexual dysfunction and abnormal desires or behaviors. Most individuals with sexual dysfunction do not have a paraphilic disorder.2 DSM-5 and ICD-11 classify sexual dysfunction and paraphilic disorders in different categories. However, previous editions grouped them together under sexual and gender identity disorders. Individuals with paraphilic disorders may not originally present to the outpatient setting for a paraphilic disorder, but instead may first seek treatment for a more common comorbid disorder, such as a mood disorder, personality disorder, or substance use disorder.3
Diagnostically speaking, if individuals do not experience distress or issues with functionality and lack legal charges (suggesting that they have not violated the rights of others), they are categorized as having an atypical sexual interest but do not necessarily meet the criteria for a disorder.4 This article provides an overview of paraphilic disorders as well as forensic considerations when examining individuals with sexually deviant behaviors.
Overview of paraphilic disorders
DSM-5 characterizes a paraphilic disorder as “recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving nonhuman objects or nonconsenting partners for at least 6 months. The individual must have acted on the thought and/or it caused clinically significant distress or impairment in social, occupational, or other important areas of functioning.” DSM-5 outlines 9 categories of paraphilic disorders, which are described in Table 1.4,5
Continue to: Paraphilic disorders are more common...
Paraphilic disorders are more common in men than in women; the 2 most prevalent are voyeuristic disorder and frotteuristic disorder.6 The incidence of paraphilias in the general outpatient setting varies by disorder. Approximately 45% of individuals with pedophilic disorder seek treatment, whereas only 1% of individuals with zoophilia seek treatment.6 The incidence of paraphilic acts also varies drastically; individuals with exhibitionistic disorder engaged in an average of 50 acts vs only 3 for individuals with sexual sadism.6 Not all individuals with paraphilic disorders commit crimes. Approximately 58% of sexual offenders meet the criteria for a paraphilic disorder, but antisocial personality disorder is a far more common diagnosis.7
Sexual psychopath statutes: Phase 1
In 1937, Michigan became the first state to enact sexual psychopath statutes, allowing for indeterminate sentencing and the civil commitment/treatment of sex offenders with repeated convictions. By the 1970s, more than 30 states had enacted similar statutes. It was not until 1967, in Specht v Patterson,8 that the United States Supreme Court unanimously ruled that the Fourteenth Amendment Due Process Clause was violated when Francis Eddie Specht faced life in prison following his conviction for indecent liberties under the Colorado Sex Offenders Act.
Specht was convicted in 1959 for indecent liberties after pleading guilty to enticing a child younger than age 16 into an office and engaging in sexual activities with them. At the time of Specht’s conviction, the crime of indecent liberties carried a punishment of 10 years. However, Specht was sentenced under the Sexual Offenders Act, which allowed for an indeterminate sentence of 1 day to life in prison. The Supreme Court noted that Specht was denied the right to be present with counsel, to confront the evidence against him, to cross-examine witnesses, and to offer his own evidence, which was a violation of his constitutionally guaranteed Fourteenth Amendment right to Procedural Due Process. The decision led most states to repeal early sexual psychopath statutes.8
Sexually violent predator laws: Phase 2
After early sexual psychopath statutes were repealed, many states pushed to update sex offender laws in response to the Earl Shriner case.9 In 1989, Shriner was released from prison after serving a 10-year sentence for sexually assaulting 2 teenage girls. At the time, he did not meet the criteria for civil commitment in the state of Washington. On the day he was released, Shriner cut off a young boy’s penis and left him to die. Washington subsequently became the first of many states to enact sexually violent predator (SVP) laws. Table 210 shows states and districts that have SVP civil commitment laws.
A series of United States Supreme Court cases solidified current sexual offender civil commitment laws (Table 38,11-15).
Continue to: Allen v Illinois
Allen v Illinois (1986).11 The Court ruled that forcing an individual to participate in a psychiatric evaluation prior to a sexually dangerous person’s commitment hearing did not violate the individual’s Fifth Amendment right against self-incrimination because the purpose of the evaluation was to provide treatment, not punishment.
Kansas v Hendricks (1997).12 The Court upheld that the Kansas Sexually Violent Predator Act was constitutional and noted that the use of the broad term “mental abnormality” (in lieu of the more specific term “mental illness”) does not violate an individual’s Fourteenth Amendment right to substantive due process. Additionally, the Court opined that the constitutional ban on double jeopardy and ex post facto lawmaking does not apply because the procedures are civil, not criminal.
Kansas v Crane (2002).13 The Court upheld the Kansas Sexually Violent Predator Act, stating that mental illness and dangerousness are essential elements to meet the criteria for civil commitment. The Court added that proof of partial (not total) “volitional impairment” is all that is required to meet the threshold of sexual dangerousness.
McKune v Lile (2002).14 The Court ruled that a policy requiring participation in polygraph testing, which would lead to the disclosure of sexual crimes (even those that have not been prosecuted), does not violate an individual’s Fifth Amendment rights because it serves a vital penological purpose.
Adam Walsh Child Protection and Safety Act of 200616; United States v Comstock (2010).15 This act and subsequent case reinforced the federal government’s right to civilly commit sexually dangerous persons approaching the end of their prison sentences.
Continue to: What is requiried for civil commitment?
What is required for civil commitment?
SVP laws require 4 conditions to be met for the civil commitment of sexual offenders (Table 417). In criteria 1, “charges” is a key word, because this allows individuals found Not Guilty by Reason of Insanity or Incompetent to Stand Trial to be civilly committed. Criteria 2 defines “mental abnormality” as a “congenital or acquired condition affecting the emotional or volitional capacity which predisposes the person to commit criminal sexual acts in a degree constituting such person a menace to the health and safety of others.”18 This is a broad definition, and allows individuals with personality disorders to be civilly committed (although most sexual offenders are committed for having a paraphilic disorder). To determine risk, various actuarial instruments are used to assess for sexually violent recidivism, including (but not limited to) the Static-99R, Sexual Violence Risk-20, and the Sex Offender Risk Appraisal Guide.19
Although the percentages vary, sex offenders rarely are civilly committed following their criminal sentence. In California, approximately 1.5% of sex offenders are civilly committed.17 The standard of proof for civil commitment varies by state between “clear and convincing evidence” and “beyond a reasonable doubt.” As sex offenders approach the end of their sentence, sexually violent offenders are identified to the general population and referred for a psychiatric evaluation. If the individual meets the 4 criteria for commitment (Table 417), their case is sent to the prosecuting attorney’s office. If accepted, the court holds a probable cause hearing, followed by a full trial.
Pornography and sex offenders
Pornography has long been considered a risk factor for sexual offending, and the role of pornography in influencing sexual behavior has drawn recent interest in research towards predicting future offenses. However, a 2019 systematic review by Mellor et al20 on the relationship between pornography and sexual offending suggested that early exposure to pornography is not a risk factor for sexual offending, nor is the risk of offending increased shortly after pornography exposure. Additionally, pornography use did not predict recidivism in low-risk sexual offenders, but did in high-risk offenders.
The use of child pornography presents a set of new risk factors. Prohibited by federal and state law, child pornography is defined under Section 2256 of Title 18, United States Code, as any visual depiction of sexually explicit conduct involving a minor (someone <age 18). Visual depictions include photographs, videos, digital or computer-generated images indistinguishable from an actual minor, and images created to depict a minor. The law does not require an image of a child engaging in sexual activity for the image to be characterized as child pornography. Offenders are also commonly charged with the distribution of child pornography. A conviction of child pornography possession carries a 15- to 30-year sentence, and distribution carries a 5- to 20-year sentence.21 The individual must also file for the sex offender registry, which may restrict their employment and place of residency.
It is unclear what percentage of individuals charged with child pornography have a history of prior sexual offenses. Numerous studies suggest there is a low risk of online offenders without prior offenses becoming contact offenders. Characteristics of online-only offenders include being White, a single male, age 20 to 30, well-educated, and employed, and having antisocial traits and a history of sexual deviancy.22 Contact offenders tend to be married with easy access to children, unemployed, uneducated, and to have a history of mental illness or criminal offenses.22
Continue to: Recidivism and treatment
Recidivism and treatment
The recidivism rate among sexual offenders averages 13.7% at 3- to 6-year follow-up,although rates vary by type of sexual offense.23 Individuals who committed rape have the highest rate of recidivism, while those who engaged in incest have the lowest. Three key points about sexual offender recidivism are:
- it declines over time and with increased age.
- sexual offenders are more like to commit a nonsexual offense than a sexual offense.
- sexual offenders who have undergone treatment are 26.3% less likely to reoffend.23
Although there is no standard of treatment, current interventions include external control, reduction of sexual drive, treatment of comorbid conditions, cognitive-behavioral therapy (CBT), and dynamic psychotherapy. External control relies on an outside entity that affects the individual’s behavior. For sexually deviant behaviors, simply making the act illegal or involving the law may inhibit many individuals from acting on a thought. Additional external control may include pharmacotherapy, which ranges from nonhormonal options such as selective serotonin reuptake inhibitors (SSRIs) to hormonal options. Therapy tends to focus on social skills training, sex education, cognitive restructuring, and identifying triggers, as well as victim empathy. The best indicators for successful treatment include an absence of comorbidities, increased age, and adult interpersonal relationships.24
Treatment choice may be predicated on the severity of the paraphilia. Psychotherapy alone is recommended for individuals able to maintain functioning if it does not affect their conventional sexual activity. Common treatment for low-risk individuals is psychotherapy and an SSRI. As risk increases, so does treatment with pharmacologic agents. Beyond SSRIs, moderate offenders may be treated with an SSRI and a low-dose antiandrogen. This is escalated in high-risk violent offenders to long-acting gonadotropin-releasing hormone analogs and synthetic steroidal analogs.25
An evolving class of disorders
With the evolution and accessibility of pornography, uncommon sexual practices have become more common, gaining notoriety and increased social acceptance. As a result, mental health professionals may be tasked with evaluating patients for possible paraphilic disorders. A common misconception is that individuals with sexually deviant thoughts, sexual offenders, and patients with paraphilic disorders are all the same. However, more commonly, sexual offenders do not have a paraphilic disorder. In the case of SVPs, outside of imprisonment, civil commitment remains a consideration for possible treatment. To meet the threshold of civil commitment, a sexual offender must have a “mental abnormality,” which is most commonly a paraphilic disorder. The treatment of paraphilic disorders remains a difficult task and includes a mixture of psychotherapy and medication options.
CASE CONTINUED
Mr. J begins weekly CBT to gain control of his voyeuristic fantasies without impacting his conventional sexual activity and desire. He responds well to treatment, and after 18 months, begins a typical sexual relationship with a woman. Although his voyeuristic thoughts remain, the urge to act on the thoughts decreases as Mr. J develops coping mechanisms. He does not require pharmacologic treatment.
Bottom Line
Individuals with paraphilic disorders are too often portrayed as sexual deviants or criminals. Psychiatrists must review each case with careful consideration of individual risk factors, such as the patient’s sexual history, to evaluate potential treatment options while determining if they pose a threat to the public.
Related Resources
- Sorrentino R, Abramowitz J. Minor-attracted persons: a neglected population. Current Psychiatry. 2021;20(7):21-27. doi:10.12788/cp.0149
- Berlin FS. Paraphilic disorders: a better understanding. Current Psychiatry. 2019;18(4):22-26,28.
Mr. J, age 23, presents to an outpatient mental health clinic for treatment of anxiety. He has no psychiatric history, is dressed neatly, and recently finished graduate school with a degree in accounting. Mr. J is reserved during the initial psychiatric evaluation and provides only basic facts about his developmental history.
Mr. J comes from a middle-class household with no history of trauma or substance use. He does not report any symptoms consistent with anxiety, but discloses a history of sexual preoccupations. Mr. J says that during adolescence he developed a predilection for observing others engage in sexual activity. In his late teens, he began following couples to their homes in the hope of witnessing sexual intimacy. In the rare instance that his voyeuristic fantasy comes to fruition, he masturbates and achieves sexual gratification he is incapable of experiencing otherwise. Mr. J notes that he has not yet been caught, but he expresses concern and embarrassment related to his actions. He concludes by noting that he seeks help because the frequency of this behavior has steadily increased.
How would you treat Mr. J? Where does the line exist between a normophilic sexual interest, fantasy or urge, and a paraphilia? Does Mr. J qualify as a sexually violent predator?
From The Rocky Horror Picture Show to Fifty Shades of Grey, sensationalized portrayals of sexual deviancy have long been present in popular culture. The continued popularity of serial killers years after their crimes seems in part related to the extreme sexual torture their victims often endure. However, a sexual offense does not always qualify as a paraphilic disorder.1 In fact, many individuals with paraphilic disorders never engage in illegal activity. Additionally, experiencing sexually deviant thoughts alone does not qualify as a paraphilic disorder.1
A thorough psychiatric evaluation should include a discussion of the patient’s sexual history, including the potential of sexual dysfunction and abnormal desires or behaviors. Most individuals with sexual dysfunction do not have a paraphilic disorder.2 DSM-5 and ICD-11 classify sexual dysfunction and paraphilic disorders in different categories. However, previous editions grouped them together under sexual and gender identity disorders. Individuals with paraphilic disorders may not originally present to the outpatient setting for a paraphilic disorder, but instead may first seek treatment for a more common comorbid disorder, such as a mood disorder, personality disorder, or substance use disorder.3
Diagnostically speaking, if individuals do not experience distress or issues with functionality and lack legal charges (suggesting that they have not violated the rights of others), they are categorized as having an atypical sexual interest but do not necessarily meet the criteria for a disorder.4 This article provides an overview of paraphilic disorders as well as forensic considerations when examining individuals with sexually deviant behaviors.
Overview of paraphilic disorders
DSM-5 characterizes a paraphilic disorder as “recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving nonhuman objects or nonconsenting partners for at least 6 months. The individual must have acted on the thought and/or it caused clinically significant distress or impairment in social, occupational, or other important areas of functioning.” DSM-5 outlines 9 categories of paraphilic disorders, which are described in Table 1.4,5
Continue to: Paraphilic disorders are more common...
Paraphilic disorders are more common in men than in women; the 2 most prevalent are voyeuristic disorder and frotteuristic disorder.6 The incidence of paraphilias in the general outpatient setting varies by disorder. Approximately 45% of individuals with pedophilic disorder seek treatment, whereas only 1% of individuals with zoophilia seek treatment.6 The incidence of paraphilic acts also varies drastically; individuals with exhibitionistic disorder engaged in an average of 50 acts vs only 3 for individuals with sexual sadism.6 Not all individuals with paraphilic disorders commit crimes. Approximately 58% of sexual offenders meet the criteria for a paraphilic disorder, but antisocial personality disorder is a far more common diagnosis.7
Sexual psychopath statutes: Phase 1
In 1937, Michigan became the first state to enact sexual psychopath statutes, allowing for indeterminate sentencing and the civil commitment/treatment of sex offenders with repeated convictions. By the 1970s, more than 30 states had enacted similar statutes. It was not until 1967, in Specht v Patterson,8 that the United States Supreme Court unanimously ruled that the Fourteenth Amendment Due Process Clause was violated when Francis Eddie Specht faced life in prison following his conviction for indecent liberties under the Colorado Sex Offenders Act.
Specht was convicted in 1959 for indecent liberties after pleading guilty to enticing a child younger than age 16 into an office and engaging in sexual activities with them. At the time of Specht’s conviction, the crime of indecent liberties carried a punishment of 10 years. However, Specht was sentenced under the Sexual Offenders Act, which allowed for an indeterminate sentence of 1 day to life in prison. The Supreme Court noted that Specht was denied the right to be present with counsel, to confront the evidence against him, to cross-examine witnesses, and to offer his own evidence, which was a violation of his constitutionally guaranteed Fourteenth Amendment right to Procedural Due Process. The decision led most states to repeal early sexual psychopath statutes.8
Sexually violent predator laws: Phase 2
After early sexual psychopath statutes were repealed, many states pushed to update sex offender laws in response to the Earl Shriner case.9 In 1989, Shriner was released from prison after serving a 10-year sentence for sexually assaulting 2 teenage girls. At the time, he did not meet the criteria for civil commitment in the state of Washington. On the day he was released, Shriner cut off a young boy’s penis and left him to die. Washington subsequently became the first of many states to enact sexually violent predator (SVP) laws. Table 210 shows states and districts that have SVP civil commitment laws.
A series of United States Supreme Court cases solidified current sexual offender civil commitment laws (Table 38,11-15).
Continue to: Allen v Illinois
Allen v Illinois (1986).11 The Court ruled that forcing an individual to participate in a psychiatric evaluation prior to a sexually dangerous person’s commitment hearing did not violate the individual’s Fifth Amendment right against self-incrimination because the purpose of the evaluation was to provide treatment, not punishment.
Kansas v Hendricks (1997).12 The Court upheld that the Kansas Sexually Violent Predator Act was constitutional and noted that the use of the broad term “mental abnormality” (in lieu of the more specific term “mental illness”) does not violate an individual’s Fourteenth Amendment right to substantive due process. Additionally, the Court opined that the constitutional ban on double jeopardy and ex post facto lawmaking does not apply because the procedures are civil, not criminal.
Kansas v Crane (2002).13 The Court upheld the Kansas Sexually Violent Predator Act, stating that mental illness and dangerousness are essential elements to meet the criteria for civil commitment. The Court added that proof of partial (not total) “volitional impairment” is all that is required to meet the threshold of sexual dangerousness.
McKune v Lile (2002).14 The Court ruled that a policy requiring participation in polygraph testing, which would lead to the disclosure of sexual crimes (even those that have not been prosecuted), does not violate an individual’s Fifth Amendment rights because it serves a vital penological purpose.
Adam Walsh Child Protection and Safety Act of 200616; United States v Comstock (2010).15 This act and subsequent case reinforced the federal government’s right to civilly commit sexually dangerous persons approaching the end of their prison sentences.
Continue to: What is requiried for civil commitment?
What is required for civil commitment?
SVP laws require 4 conditions to be met for the civil commitment of sexual offenders (Table 417). In criteria 1, “charges” is a key word, because this allows individuals found Not Guilty by Reason of Insanity or Incompetent to Stand Trial to be civilly committed. Criteria 2 defines “mental abnormality” as a “congenital or acquired condition affecting the emotional or volitional capacity which predisposes the person to commit criminal sexual acts in a degree constituting such person a menace to the health and safety of others.”18 This is a broad definition, and allows individuals with personality disorders to be civilly committed (although most sexual offenders are committed for having a paraphilic disorder). To determine risk, various actuarial instruments are used to assess for sexually violent recidivism, including (but not limited to) the Static-99R, Sexual Violence Risk-20, and the Sex Offender Risk Appraisal Guide.19
Although the percentages vary, sex offenders rarely are civilly committed following their criminal sentence. In California, approximately 1.5% of sex offenders are civilly committed.17 The standard of proof for civil commitment varies by state between “clear and convincing evidence” and “beyond a reasonable doubt.” As sex offenders approach the end of their sentence, sexually violent offenders are identified to the general population and referred for a psychiatric evaluation. If the individual meets the 4 criteria for commitment (Table 417), their case is sent to the prosecuting attorney’s office. If accepted, the court holds a probable cause hearing, followed by a full trial.
Pornography and sex offenders
Pornography has long been considered a risk factor for sexual offending, and the role of pornography in influencing sexual behavior has drawn recent interest in research towards predicting future offenses. However, a 2019 systematic review by Mellor et al20 on the relationship between pornography and sexual offending suggested that early exposure to pornography is not a risk factor for sexual offending, nor is the risk of offending increased shortly after pornography exposure. Additionally, pornography use did not predict recidivism in low-risk sexual offenders, but did in high-risk offenders.
The use of child pornography presents a set of new risk factors. Prohibited by federal and state law, child pornography is defined under Section 2256 of Title 18, United States Code, as any visual depiction of sexually explicit conduct involving a minor (someone <age 18). Visual depictions include photographs, videos, digital or computer-generated images indistinguishable from an actual minor, and images created to depict a minor. The law does not require an image of a child engaging in sexual activity for the image to be characterized as child pornography. Offenders are also commonly charged with the distribution of child pornography. A conviction of child pornography possession carries a 15- to 30-year sentence, and distribution carries a 5- to 20-year sentence.21 The individual must also file for the sex offender registry, which may restrict their employment and place of residency.
It is unclear what percentage of individuals charged with child pornography have a history of prior sexual offenses. Numerous studies suggest there is a low risk of online offenders without prior offenses becoming contact offenders. Characteristics of online-only offenders include being White, a single male, age 20 to 30, well-educated, and employed, and having antisocial traits and a history of sexual deviancy.22 Contact offenders tend to be married with easy access to children, unemployed, uneducated, and to have a history of mental illness or criminal offenses.22
Continue to: Recidivism and treatment
Recidivism and treatment
The recidivism rate among sexual offenders averages 13.7% at 3- to 6-year follow-up,although rates vary by type of sexual offense.23 Individuals who committed rape have the highest rate of recidivism, while those who engaged in incest have the lowest. Three key points about sexual offender recidivism are:
- it declines over time and with increased age.
- sexual offenders are more like to commit a nonsexual offense than a sexual offense.
- sexual offenders who have undergone treatment are 26.3% less likely to reoffend.23
Although there is no standard of treatment, current interventions include external control, reduction of sexual drive, treatment of comorbid conditions, cognitive-behavioral therapy (CBT), and dynamic psychotherapy. External control relies on an outside entity that affects the individual’s behavior. For sexually deviant behaviors, simply making the act illegal or involving the law may inhibit many individuals from acting on a thought. Additional external control may include pharmacotherapy, which ranges from nonhormonal options such as selective serotonin reuptake inhibitors (SSRIs) to hormonal options. Therapy tends to focus on social skills training, sex education, cognitive restructuring, and identifying triggers, as well as victim empathy. The best indicators for successful treatment include an absence of comorbidities, increased age, and adult interpersonal relationships.24
Treatment choice may be predicated on the severity of the paraphilia. Psychotherapy alone is recommended for individuals able to maintain functioning if it does not affect their conventional sexual activity. Common treatment for low-risk individuals is psychotherapy and an SSRI. As risk increases, so does treatment with pharmacologic agents. Beyond SSRIs, moderate offenders may be treated with an SSRI and a low-dose antiandrogen. This is escalated in high-risk violent offenders to long-acting gonadotropin-releasing hormone analogs and synthetic steroidal analogs.25
An evolving class of disorders
With the evolution and accessibility of pornography, uncommon sexual practices have become more common, gaining notoriety and increased social acceptance. As a result, mental health professionals may be tasked with evaluating patients for possible paraphilic disorders. A common misconception is that individuals with sexually deviant thoughts, sexual offenders, and patients with paraphilic disorders are all the same. However, more commonly, sexual offenders do not have a paraphilic disorder. In the case of SVPs, outside of imprisonment, civil commitment remains a consideration for possible treatment. To meet the threshold of civil commitment, a sexual offender must have a “mental abnormality,” which is most commonly a paraphilic disorder. The treatment of paraphilic disorders remains a difficult task and includes a mixture of psychotherapy and medication options.
CASE CONTINUED
Mr. J begins weekly CBT to gain control of his voyeuristic fantasies without impacting his conventional sexual activity and desire. He responds well to treatment, and after 18 months, begins a typical sexual relationship with a woman. Although his voyeuristic thoughts remain, the urge to act on the thoughts decreases as Mr. J develops coping mechanisms. He does not require pharmacologic treatment.
Bottom Line
Individuals with paraphilic disorders are too often portrayed as sexual deviants or criminals. Psychiatrists must review each case with careful consideration of individual risk factors, such as the patient’s sexual history, to evaluate potential treatment options while determining if they pose a threat to the public.
Related Resources
- Sorrentino R, Abramowitz J. Minor-attracted persons: a neglected population. Current Psychiatry. 2021;20(7):21-27. doi:10.12788/cp.0149
- Berlin FS. Paraphilic disorders: a better understanding. Current Psychiatry. 2019;18(4):22-26,28.
1. Federoff JP. The paraphilias. In: Gelder MG, Andreasen NC, López-Ibor JJ Jr, Geddes JR, eds. New Oxford Textbook of Psychiatry. 2nd ed. Oxford University Press; 2012:832-842.
2. Grubin D. Medical models and interventions in sexual deviance. In: Laws R, O’Donohue WT, eds. Sexual Deviance: Theory, Assessment and Treatment. 2nd ed. Guilford Press; 2008:594-610.
3. Guidry LL, Saleh FM. Clinical considerations of paraphilic sex offenders with comorbid psychiatric conditions. Sex Addict Compulsivity. 2004;11(1-2):21-34.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Balon R. Paraphilic disorders. In: Roberts LW, Hales RE, Yudofsky SC, eds. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019:749-770.
6. Sadock BJ, Sadock VA, Ruiz P. Paraphilic disorders. Kaplan and Sadock’s Synopsis of Psychiatry. 11th ed. Wolters Kluwer; 2015:593-599.
7. First MB, Halon RL. Use of DSM paraphilia diagnosis in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
8. Specht v Patterson, 386 US 605 (1967).
9. Ra EP. The civil confinement of sexual predators: a delicate balance. J Civ Rts Econ Dev. 2007;22(1):335-372.
10. Felthous AR, Ko J. Sexually violent predator law in the United States. East Asian Arch Psychiatry. 2018;28(4):159-173.
11. Allen v Illinois, 478 US 364 (1986).
12. Kansas v Hendricks, 521 US 346 (1997).
13. Kansas v Crane, 534 US 407 (2002).
14. McKune v Lile, 536 US 24 (2002).
15. United States v Comstock, 560 US 126 (2010).
16. Adam Walsh Child Protection and Safety Act of 2006, HR 4472, 109th Cong (2006). Accessed April 25, 2022. https://www.congress.gov/bill/109th-congress/house-bill/4472
17. Tucker DE, Brakel SJ. Sexually violent predator laws. In: Rosner R, Scott C, eds. Principles and Practice of Forensic Psychiatry. 3rd ed. CRC Press; 2017:823-831.
18. Wash. Rev. Code. Ann. §71.09.020(8)
19. Bradford J, de Amorim Levin GV, Booth BD, et al. Forensic assessment of sex offenders. In: Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2017:382-397.
20. Mellor E, Duff S. The use of pornography and the relationship between pornography exposure and sexual offending in males: a systematic review. Aggress Violent Beh. 2019;46:116-126.
21. Failure To Register, 18 USC § 2250 (2012). Accessed April 25, 2022. https://www.govinfo.gov/app/details/USCODE-2011-title18/USCODE-2011-title18-partI-chap109B-sec2250
22. Hirschtritt ME, Tucker D, Binder RL. Risk assessment of online child sexual exploitation offenders. J Am Acad Psychiatry Law. 2019;47(2):155-164.
23. Blasko BL. Overview of sexual offender typologies, recidivism, and treatment. In: Jeglic EL, Calkins C, eds. Sexual Violence: Evidence Based Policy and Prevention. Springer; 2016:11-29.
24. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490.
25. Holoyda B. Paraphilias: from diagnosis to treatment. Psychiatric Times. 2019;36(12).
1. Federoff JP. The paraphilias. In: Gelder MG, Andreasen NC, López-Ibor JJ Jr, Geddes JR, eds. New Oxford Textbook of Psychiatry. 2nd ed. Oxford University Press; 2012:832-842.
2. Grubin D. Medical models and interventions in sexual deviance. In: Laws R, O’Donohue WT, eds. Sexual Deviance: Theory, Assessment and Treatment. 2nd ed. Guilford Press; 2008:594-610.
3. Guidry LL, Saleh FM. Clinical considerations of paraphilic sex offenders with comorbid psychiatric conditions. Sex Addict Compulsivity. 2004;11(1-2):21-34.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Balon R. Paraphilic disorders. In: Roberts LW, Hales RE, Yudofsky SC, eds. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019:749-770.
6. Sadock BJ, Sadock VA, Ruiz P. Paraphilic disorders. Kaplan and Sadock’s Synopsis of Psychiatry. 11th ed. Wolters Kluwer; 2015:593-599.
7. First MB, Halon RL. Use of DSM paraphilia diagnosis in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
8. Specht v Patterson, 386 US 605 (1967).
9. Ra EP. The civil confinement of sexual predators: a delicate balance. J Civ Rts Econ Dev. 2007;22(1):335-372.
10. Felthous AR, Ko J. Sexually violent predator law in the United States. East Asian Arch Psychiatry. 2018;28(4):159-173.
11. Allen v Illinois, 478 US 364 (1986).
12. Kansas v Hendricks, 521 US 346 (1997).
13. Kansas v Crane, 534 US 407 (2002).
14. McKune v Lile, 536 US 24 (2002).
15. United States v Comstock, 560 US 126 (2010).
16. Adam Walsh Child Protection and Safety Act of 2006, HR 4472, 109th Cong (2006). Accessed April 25, 2022. https://www.congress.gov/bill/109th-congress/house-bill/4472
17. Tucker DE, Brakel SJ. Sexually violent predator laws. In: Rosner R, Scott C, eds. Principles and Practice of Forensic Psychiatry. 3rd ed. CRC Press; 2017:823-831.
18. Wash. Rev. Code. Ann. §71.09.020(8)
19. Bradford J, de Amorim Levin GV, Booth BD, et al. Forensic assessment of sex offenders. In: Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2017:382-397.
20. Mellor E, Duff S. The use of pornography and the relationship between pornography exposure and sexual offending in males: a systematic review. Aggress Violent Beh. 2019;46:116-126.
21. Failure To Register, 18 USC § 2250 (2012). Accessed April 25, 2022. https://www.govinfo.gov/app/details/USCODE-2011-title18/USCODE-2011-title18-partI-chap109B-sec2250
22. Hirschtritt ME, Tucker D, Binder RL. Risk assessment of online child sexual exploitation offenders. J Am Acad Psychiatry Law. 2019;47(2):155-164.
23. Blasko BL. Overview of sexual offender typologies, recidivism, and treatment. In: Jeglic EL, Calkins C, eds. Sexual Violence: Evidence Based Policy and Prevention. Springer; 2016:11-29.
24. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490.
25. Holoyda B. Paraphilias: from diagnosis to treatment. Psychiatric Times. 2019;36(12).
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 2)
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (
Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16
Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (
Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16
Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (
Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16
Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
Cats, toxoplasmosis, and psychosis: Understanding the risks
It has been clearly established that most human infectious diseases are caused by infectious agents that have been transmitted from animals to humans.1 Based on published estimates from the 2000s, 60% to 76% of emerging infectious disease events are transmitted from animals to humans.2
When we consider animals that cause human diseases, we usually think of rats and bats. We rarely think of the 90 million cats owned as pets in the United States, or the approximately 30 to 80 million feral cats. Many consider cats as family members, and three-fourths of cats owned in the United States are allowed to sleep on the beds of their owners.1 These cats may be a substantial source of human disease. Researchers at the University of Liverpool have identified 273 infectious agents carried by cats, of which 151 are known to be shared with humans.1 The most widely known of these agents are Lyssavirus, the virus that causes rabies; Bartonella henselae, the bacteria that causes cat scratch disease; and Toxoplasma gondii (T. gondii), the parasite that causes toxoplasmosis.
In my new open-access book Parasites, Pussycats and Psychosis (available at https://link.springer.com/book/10.1007/978-3-030-86811-6), I describe the relationship between cats, T. gondii, and toxoplasmosis, and detail the evidence linking T. gondii to some cases of schizophrenia, bipolar disorder, and other diseases.1 Though human T. gondii infection is typically asymptomatic or produces minor, flu-like symptoms, there are a few important exceptions. This article outlines those exceptions, and investigates evidence that implicates a link between T. gondii and psychosis.
How T. gondii can be transmitted
T. gondii has been called “one of the most successful parasites on earth.”3 Globally, approximately one-third of the human population is infected with T. gondii, though this varies widely by country and is dependent on dietary habits and exposure to cats. A 2014 survey reported that 11% of Americans—approximately 40 million people—have been infected, as evidenced by the presence of antibodies in their blood.1
T. gondii begins its life cycle when a cat becomes infected, usually as a kitten. Most infected cats are asymptomatic, but for approximately 8 days they excrete up to 50 million infectious oocysts in their feces daily. Depending on the temperature, these oocysts can live for 2 years or longer.It is thought that a single oocyst can cause human infection.1 Since cats like loose soil for defecation, the infective oocysts commonly end up in gardens, uncovered sandboxes, or animal feed piles in barns. After 24 hours, the oocysts dry out and may become aerosolized. For this reason, cat owners are advised to change their cat’s litter daily.
The number of ways T. gondii can be transmitted to humans is extensive. Farm animals can become infected from contaminated feed; this causes T. gondii oocysts in animals’ muscles, which later may cause human infection if eaten as undercooked meat. Many such family outbreaks of toxoplasmosis have been described.1
If infective oocysts get into the water supply, they may also cause outbreaks of disease. More than 200 such outbreaks have been described, including an instance in Victoria, British Columbia, in which 100 people became clinically infected.4
Continue to: Family outbreaks...
Family outbreaks have also been described that involve multiple children who played in an infected sandbox or dirt pile.5 Similarly, an outbreak has been reported in a riding stable that was home to infected cats. Infective oocysts were thought to have become aerosolized and breathed in by the patrons.6 Multiple other possible modes of transmission are being investigated, including sexual transmission among humans.7
Human infections are not always benign
In most human T. gondii cases, the infected individual experiences mild, flu-like symptoms, often with enlarged lymph nodes, or has no symptoms.1 Thus, most people who have been infected with
There are 3 exceptions to this otherwise benign clinical picture. The first is cerebral toxoplasmosis, which occurs in individuals who are immunosuppressed because they have AIDS or are receiving treatment for cancer or organ transplantation. Cerebral toxoplasmosis can be severe and was a common cause of death in patients with AIDS before the development of effective AIDS treatments.
The second exception is congenital toxoplasmosis, when an infection occurs in a pregnant woman. Such infections can cause severe damage to the developing fetus, including abortion, stillbirth, and brain damage. Congenital toxoplasmosis infections occur in approximately 1 of every 10,000 births in the United States, or approximately 3,800 each year.8 As a result, pregnant women are advised not to change their cat’s litter and to be tested for evidence of T. gondii infection.
The third exception is eye disease. Toxoplasmosis is one of the most common causes of eye disease, especially of the retina. Each year in the United States, approximately 4,800 individuals develop systematic ocular toxoplasmosis.9
Continue to: Toxoplasmosis and psychosis
Toxoplasmosis and psychosis: What evidence supports a link?
Until recently, cerebral infections, congenital infections, and eye disease were thought to be the main clinical problems associated with toxoplasmosis. However, accumulating evidence suggests that psychosis should be added to this list. Five lines of evidence support this.
1. T. gondii can cause psychotic symptoms. It has been known for decades that T. gondii can cause delusions, auditory hallucinations, and other psychotic symptoms.1 In one of the earliest publications (1966), Ladee10 concluded “The literature not infrequently focuses attention on psychosis with schizophrenia or schizophreniform features that accompany chronic toxoplasmosis.” Among the cases Ladee10 described was a laboratory worker who became infected with T. gondii and developed delusions and hallucinations.10
2. Patients with schizophrenia who are infected with T. gondii have more severe psychotic symptoms. This finding has been reported in at least 7 studies.1 Holub et al11 evaluated 251 patients with schizophrenia who were treated in Prague Psychiatric Centre between 2000 and 2010. Overall, 57 participants were infected with T. gondii and 194 were not infected. Compared to those who were not infected, the infected group:
- had significantly more severe symptoms (P = .032) as measured on the Positive and Negative Symptom Scale
- were prescribed higher doses of antipsychotic medications
- had been hospitalized longer.11
3. Compared with controls, patients with psychosis are significantly more likely to have antibodies against T. gondii, indicating previous infection. To date there have been approximately 100 such studies, of which at least three-fourths reported a positive association. In a 2012 meta-analysis of 38 such studies, Torrey et al12 reported an odds ratio (OR) of 2.7—compared to persons who have not been infected, those who have been infected with T. gondii were 2.7 times more likely to have schizophrenia.12 This study replicated the findings of a previous meta-analysis of 23 antibody studies, which also found an OR of 2.7.13
4. Compared with controls, individuals with schizophrenia or bipolar disorder are significantly more likely as a child to have lived in a home with a cat. Since 1995, 10 such studies have been published; 7 were positive, 2 were negative, and 1 was inconclusive.1 Torrey et al14 reviewed 2,025 individuals with schizophrenia or bipolar disorder and 4,847 controls and found that 51% of the cases and 43% of the controls had owned a cat before age 13; this difference was highly significant (P < .001). In fact, it is surprising that any study can find a statistically significant association between cat ownership and childhood psychosis. This is because a child who did not own a cat could become infected in many locations where cats have been present, including sandboxes at school, a babysitter’s or friend’s house, or a public park. And even if a child became infected at home, they would not necessarily have owned a cat, since the neighbor’s cat could have been responsible for the oocyst contamination.
Continue to: Epidemiologically...
5. Epidemiologically, there is a close temporal correlation between the rise of cats as pets and the rise of psychosis. This can be illustrated most clearly in England, where the rise of cat ownership has been documented by writers and where there is data on the rise of psychosis, especially in the 18th and 19th centuries.1
How many cases of psychosis might be caused by T. gondii?
In 2014, using data from the antibody studies discussed above,12,13 Smith15 sought to discover how many cases of psychosis might be caused by T. gondii. He concluded that 21% of cases of schizophrenia might have been caused by T. gondii. Based on the annual incidence of schizophrenia in the United States, this would mean an estimated >10,000 new cases of schizophrenia each year are attributable to this parasite.
Some researchers have found links between T. gondii and several nonpsychiatric diseases and conditions, including epilepsy and brain cancer (Box1,16-19).
Box
As interest in Toxoplasma gondii (T. gondii) has increased, researchers have looked for associations between this parasite with other diseases and conditions. Based on the literature, the following are of most interest:
Epilepsy. Since 1995, 16 studies1 have explored the relationship between T. gondii and epilepsy. A recent meta-analysis reported a statistically significant association between T. gondii and epilepsy.16
Brain cancer. Authors in 2 of 3 studies of meningiomas and 4 of 5 studies of gliomas reported statistically significant associations between these brain tumors and infection with T. gondii.1,17
Rheumatoid arthritis. Eight studies reported an increased prevalence of T. gondii antibodies in individuals with rheumatoid arthritis.1,18
Motor vehicle accidents. Infection with T. gondii is known to decrease motor reaction times in humans. At least 11 studies1 have examined whether infected individuals are more likely to have been involved in motor vehicle accidents. The results are mixed; the largest study reported a weak but statistically significant association.19
Clinical implications: What to tell patients about cats
What do these studies of toxoplasmosis imply for psychiatric care? As mental health professionals, part of our job is to educate our patients. Anything that appears to be a risk factor for the development of psychosis is thus of interest. Consider discussing the following with your patients.
Are cats safe? Cats that are kept exclusively indoors are safe pets because they are unlikely to become infected with T. gondii. However, cats that are allowed to go outdoors may not be safe, especially for children and young adults. What is needed is an effective vaccine that could be given to newborn kittens to prevent infection, but development of this type of vaccine has never been prioritized. At the community level, programs to decrease the number of stray and feral cats would also decrease the risk of infection.
Continue to: How to decrease risk
How to decrease risk. On a personal level, we can decrease T. gondii infections by not eating undercooked meat. Pregnant women and individuals who are immunocompromised should not change cat litter. When gardening, we should wear gloves because cats favor loose soil for depositing their feces. We should also protect children by covering sandboxes when not in use and by not allowing children to play in uncovered public sandboxes.
Treatment. Toxoplasmosis typically is treated with pyrimethamine, usually in combination with a sulfa drug. However, pyrimethamine does not cross the blood brain barrier and thus is ineffective when T. gondii infects the brain. The development of a drug that will effectively treat T. gondii in the brain should be a high priority.
For additional details on the studies discussed in this article as well as more resources on the impact T. gondii can have if proper precautions are not taken, see my open-access book at https://link.springer.com/book/10.1007/978-3-030-86811-6.
Bottom Line
Some evidence suggests that infection with Toxoplasma gondii (T. gondii) may cause psychotic symptoms, may increase an individual’s risk of developing psychosis, and may result in more severe psychotic symptoms. Cats can transmit T. gondii to humans. Educate patients that they can reduce their risk by keeping their cats inside, avoiding exposure to cat feces, particularly while pregnant or if immunocompromised, and not eating undercooked meat.
Related Resources
- Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
Drug Brand Names
Pyrimethamine • Daraprim
1. Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
2. Rohr JR, Barrett CB, Civitello DJ, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2(6):445-456.
3. Joynson DHM. Preface. In: Joynson DHM, Wreghitt TG, eds. Toxoplasmosis: A Comprehensive Clinical Guide. Cambridge University Press; 2001:xi.
4. Bowie WR, King AS, Werker DH, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350(9072):173-177.
5. Stagno S, Dykes AC, Amos CS, et al. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980;65(4):706-712.
6. Teutsch SM, Juranek DD, Sulzer A, et al. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300(13):695-699.
7. Kaňková Š, Hlaváčová J, Flegr J. Oral sex: a new, and possibly the most dangerous, route of toxoplasmosis transmission. Med Hypotheses. 2020;141:109725.
8. Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital T. gondii infection. N Engl J Med. 1994;330(26):1858-1863.
9. Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82(3):464-465.
10. Ladee GA. Diagnostic problems in psychiatry with regard to acquired toxoplasmosis. Psychiatr Neurol Neurochir. 1966;69(1):65-82.
11. Holub D, Flegr J, Dragomirecká E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
12. Torrey EF, Bartko JJ, Yolken RH. T. gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38(3):642-647.
13. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729-736.
14. Torrey EF, Simmons W, Yolken RH. Is childhood cat ownership a risk factor for schizophrenia later in life? Schizophr Res. 2015;165(1):1-2.
15. Smith G. Estimating the population attributable fraction for schizophrenia when T. gondii is assumed absent in human populations. Prev Vet Med. 2014;117(3-4):425-435.
16. Sadeghi M, Riahi SM, Mohammadi M, et al. An updated meta-analysis of the association between T. gondii infection and risk of epilepsy. Trans R Soc Trop Med Hyg. 2019;113(8):453-462.
17. Hodge JM, Coghill AE, Kim Y, et al. T. gondii infection and the risk of adult glioma in two prospective studies. Int J Cancer. 2021;148(10):2449-2456.
18. Hosseininejad Z, Sharif M, Sarvi S, et al. Toxoplasmosis seroprevalence in rheumatoid arthritis patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(6):e0006545.
19. Burgdorf KS, Trabjerg BB, Pedersen MG, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019; 79:152-158.
It has been clearly established that most human infectious diseases are caused by infectious agents that have been transmitted from animals to humans.1 Based on published estimates from the 2000s, 60% to 76% of emerging infectious disease events are transmitted from animals to humans.2
When we consider animals that cause human diseases, we usually think of rats and bats. We rarely think of the 90 million cats owned as pets in the United States, or the approximately 30 to 80 million feral cats. Many consider cats as family members, and three-fourths of cats owned in the United States are allowed to sleep on the beds of their owners.1 These cats may be a substantial source of human disease. Researchers at the University of Liverpool have identified 273 infectious agents carried by cats, of which 151 are known to be shared with humans.1 The most widely known of these agents are Lyssavirus, the virus that causes rabies; Bartonella henselae, the bacteria that causes cat scratch disease; and Toxoplasma gondii (T. gondii), the parasite that causes toxoplasmosis.
In my new open-access book Parasites, Pussycats and Psychosis (available at https://link.springer.com/book/10.1007/978-3-030-86811-6), I describe the relationship between cats, T. gondii, and toxoplasmosis, and detail the evidence linking T. gondii to some cases of schizophrenia, bipolar disorder, and other diseases.1 Though human T. gondii infection is typically asymptomatic or produces minor, flu-like symptoms, there are a few important exceptions. This article outlines those exceptions, and investigates evidence that implicates a link between T. gondii and psychosis.
How T. gondii can be transmitted
T. gondii has been called “one of the most successful parasites on earth.”3 Globally, approximately one-third of the human population is infected with T. gondii, though this varies widely by country and is dependent on dietary habits and exposure to cats. A 2014 survey reported that 11% of Americans—approximately 40 million people—have been infected, as evidenced by the presence of antibodies in their blood.1
T. gondii begins its life cycle when a cat becomes infected, usually as a kitten. Most infected cats are asymptomatic, but for approximately 8 days they excrete up to 50 million infectious oocysts in their feces daily. Depending on the temperature, these oocysts can live for 2 years or longer.It is thought that a single oocyst can cause human infection.1 Since cats like loose soil for defecation, the infective oocysts commonly end up in gardens, uncovered sandboxes, or animal feed piles in barns. After 24 hours, the oocysts dry out and may become aerosolized. For this reason, cat owners are advised to change their cat’s litter daily.
The number of ways T. gondii can be transmitted to humans is extensive. Farm animals can become infected from contaminated feed; this causes T. gondii oocysts in animals’ muscles, which later may cause human infection if eaten as undercooked meat. Many such family outbreaks of toxoplasmosis have been described.1
If infective oocysts get into the water supply, they may also cause outbreaks of disease. More than 200 such outbreaks have been described, including an instance in Victoria, British Columbia, in which 100 people became clinically infected.4
Continue to: Family outbreaks...
Family outbreaks have also been described that involve multiple children who played in an infected sandbox or dirt pile.5 Similarly, an outbreak has been reported in a riding stable that was home to infected cats. Infective oocysts were thought to have become aerosolized and breathed in by the patrons.6 Multiple other possible modes of transmission are being investigated, including sexual transmission among humans.7
Human infections are not always benign
In most human T. gondii cases, the infected individual experiences mild, flu-like symptoms, often with enlarged lymph nodes, or has no symptoms.1 Thus, most people who have been infected with
There are 3 exceptions to this otherwise benign clinical picture. The first is cerebral toxoplasmosis, which occurs in individuals who are immunosuppressed because they have AIDS or are receiving treatment for cancer or organ transplantation. Cerebral toxoplasmosis can be severe and was a common cause of death in patients with AIDS before the development of effective AIDS treatments.
The second exception is congenital toxoplasmosis, when an infection occurs in a pregnant woman. Such infections can cause severe damage to the developing fetus, including abortion, stillbirth, and brain damage. Congenital toxoplasmosis infections occur in approximately 1 of every 10,000 births in the United States, or approximately 3,800 each year.8 As a result, pregnant women are advised not to change their cat’s litter and to be tested for evidence of T. gondii infection.
The third exception is eye disease. Toxoplasmosis is one of the most common causes of eye disease, especially of the retina. Each year in the United States, approximately 4,800 individuals develop systematic ocular toxoplasmosis.9
Continue to: Toxoplasmosis and psychosis
Toxoplasmosis and psychosis: What evidence supports a link?
Until recently, cerebral infections, congenital infections, and eye disease were thought to be the main clinical problems associated with toxoplasmosis. However, accumulating evidence suggests that psychosis should be added to this list. Five lines of evidence support this.
1. T. gondii can cause psychotic symptoms. It has been known for decades that T. gondii can cause delusions, auditory hallucinations, and other psychotic symptoms.1 In one of the earliest publications (1966), Ladee10 concluded “The literature not infrequently focuses attention on psychosis with schizophrenia or schizophreniform features that accompany chronic toxoplasmosis.” Among the cases Ladee10 described was a laboratory worker who became infected with T. gondii and developed delusions and hallucinations.10
2. Patients with schizophrenia who are infected with T. gondii have more severe psychotic symptoms. This finding has been reported in at least 7 studies.1 Holub et al11 evaluated 251 patients with schizophrenia who were treated in Prague Psychiatric Centre between 2000 and 2010. Overall, 57 participants were infected with T. gondii and 194 were not infected. Compared to those who were not infected, the infected group:
- had significantly more severe symptoms (P = .032) as measured on the Positive and Negative Symptom Scale
- were prescribed higher doses of antipsychotic medications
- had been hospitalized longer.11
3. Compared with controls, patients with psychosis are significantly more likely to have antibodies against T. gondii, indicating previous infection. To date there have been approximately 100 such studies, of which at least three-fourths reported a positive association. In a 2012 meta-analysis of 38 such studies, Torrey et al12 reported an odds ratio (OR) of 2.7—compared to persons who have not been infected, those who have been infected with T. gondii were 2.7 times more likely to have schizophrenia.12 This study replicated the findings of a previous meta-analysis of 23 antibody studies, which also found an OR of 2.7.13
4. Compared with controls, individuals with schizophrenia or bipolar disorder are significantly more likely as a child to have lived in a home with a cat. Since 1995, 10 such studies have been published; 7 were positive, 2 were negative, and 1 was inconclusive.1 Torrey et al14 reviewed 2,025 individuals with schizophrenia or bipolar disorder and 4,847 controls and found that 51% of the cases and 43% of the controls had owned a cat before age 13; this difference was highly significant (P < .001). In fact, it is surprising that any study can find a statistically significant association between cat ownership and childhood psychosis. This is because a child who did not own a cat could become infected in many locations where cats have been present, including sandboxes at school, a babysitter’s or friend’s house, or a public park. And even if a child became infected at home, they would not necessarily have owned a cat, since the neighbor’s cat could have been responsible for the oocyst contamination.
Continue to: Epidemiologically...
5. Epidemiologically, there is a close temporal correlation between the rise of cats as pets and the rise of psychosis. This can be illustrated most clearly in England, where the rise of cat ownership has been documented by writers and where there is data on the rise of psychosis, especially in the 18th and 19th centuries.1
How many cases of psychosis might be caused by T. gondii?
In 2014, using data from the antibody studies discussed above,12,13 Smith15 sought to discover how many cases of psychosis might be caused by T. gondii. He concluded that 21% of cases of schizophrenia might have been caused by T. gondii. Based on the annual incidence of schizophrenia in the United States, this would mean an estimated >10,000 new cases of schizophrenia each year are attributable to this parasite.
Some researchers have found links between T. gondii and several nonpsychiatric diseases and conditions, including epilepsy and brain cancer (Box1,16-19).
Box
As interest in Toxoplasma gondii (T. gondii) has increased, researchers have looked for associations between this parasite with other diseases and conditions. Based on the literature, the following are of most interest:
Epilepsy. Since 1995, 16 studies1 have explored the relationship between T. gondii and epilepsy. A recent meta-analysis reported a statistically significant association between T. gondii and epilepsy.16
Brain cancer. Authors in 2 of 3 studies of meningiomas and 4 of 5 studies of gliomas reported statistically significant associations between these brain tumors and infection with T. gondii.1,17
Rheumatoid arthritis. Eight studies reported an increased prevalence of T. gondii antibodies in individuals with rheumatoid arthritis.1,18
Motor vehicle accidents. Infection with T. gondii is known to decrease motor reaction times in humans. At least 11 studies1 have examined whether infected individuals are more likely to have been involved in motor vehicle accidents. The results are mixed; the largest study reported a weak but statistically significant association.19
Clinical implications: What to tell patients about cats
What do these studies of toxoplasmosis imply for psychiatric care? As mental health professionals, part of our job is to educate our patients. Anything that appears to be a risk factor for the development of psychosis is thus of interest. Consider discussing the following with your patients.
Are cats safe? Cats that are kept exclusively indoors are safe pets because they are unlikely to become infected with T. gondii. However, cats that are allowed to go outdoors may not be safe, especially for children and young adults. What is needed is an effective vaccine that could be given to newborn kittens to prevent infection, but development of this type of vaccine has never been prioritized. At the community level, programs to decrease the number of stray and feral cats would also decrease the risk of infection.
Continue to: How to decrease risk
How to decrease risk. On a personal level, we can decrease T. gondii infections by not eating undercooked meat. Pregnant women and individuals who are immunocompromised should not change cat litter. When gardening, we should wear gloves because cats favor loose soil for depositing their feces. We should also protect children by covering sandboxes when not in use and by not allowing children to play in uncovered public sandboxes.
Treatment. Toxoplasmosis typically is treated with pyrimethamine, usually in combination with a sulfa drug. However, pyrimethamine does not cross the blood brain barrier and thus is ineffective when T. gondii infects the brain. The development of a drug that will effectively treat T. gondii in the brain should be a high priority.
For additional details on the studies discussed in this article as well as more resources on the impact T. gondii can have if proper precautions are not taken, see my open-access book at https://link.springer.com/book/10.1007/978-3-030-86811-6.
Bottom Line
Some evidence suggests that infection with Toxoplasma gondii (T. gondii) may cause psychotic symptoms, may increase an individual’s risk of developing psychosis, and may result in more severe psychotic symptoms. Cats can transmit T. gondii to humans. Educate patients that they can reduce their risk by keeping their cats inside, avoiding exposure to cat feces, particularly while pregnant or if immunocompromised, and not eating undercooked meat.
Related Resources
- Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
Drug Brand Names
Pyrimethamine • Daraprim
It has been clearly established that most human infectious diseases are caused by infectious agents that have been transmitted from animals to humans.1 Based on published estimates from the 2000s, 60% to 76% of emerging infectious disease events are transmitted from animals to humans.2
When we consider animals that cause human diseases, we usually think of rats and bats. We rarely think of the 90 million cats owned as pets in the United States, or the approximately 30 to 80 million feral cats. Many consider cats as family members, and three-fourths of cats owned in the United States are allowed to sleep on the beds of their owners.1 These cats may be a substantial source of human disease. Researchers at the University of Liverpool have identified 273 infectious agents carried by cats, of which 151 are known to be shared with humans.1 The most widely known of these agents are Lyssavirus, the virus that causes rabies; Bartonella henselae, the bacteria that causes cat scratch disease; and Toxoplasma gondii (T. gondii), the parasite that causes toxoplasmosis.
In my new open-access book Parasites, Pussycats and Psychosis (available at https://link.springer.com/book/10.1007/978-3-030-86811-6), I describe the relationship between cats, T. gondii, and toxoplasmosis, and detail the evidence linking T. gondii to some cases of schizophrenia, bipolar disorder, and other diseases.1 Though human T. gondii infection is typically asymptomatic or produces minor, flu-like symptoms, there are a few important exceptions. This article outlines those exceptions, and investigates evidence that implicates a link between T. gondii and psychosis.
How T. gondii can be transmitted
T. gondii has been called “one of the most successful parasites on earth.”3 Globally, approximately one-third of the human population is infected with T. gondii, though this varies widely by country and is dependent on dietary habits and exposure to cats. A 2014 survey reported that 11% of Americans—approximately 40 million people—have been infected, as evidenced by the presence of antibodies in their blood.1
T. gondii begins its life cycle when a cat becomes infected, usually as a kitten. Most infected cats are asymptomatic, but for approximately 8 days they excrete up to 50 million infectious oocysts in their feces daily. Depending on the temperature, these oocysts can live for 2 years or longer.It is thought that a single oocyst can cause human infection.1 Since cats like loose soil for defecation, the infective oocysts commonly end up in gardens, uncovered sandboxes, or animal feed piles in barns. After 24 hours, the oocysts dry out and may become aerosolized. For this reason, cat owners are advised to change their cat’s litter daily.
The number of ways T. gondii can be transmitted to humans is extensive. Farm animals can become infected from contaminated feed; this causes T. gondii oocysts in animals’ muscles, which later may cause human infection if eaten as undercooked meat. Many such family outbreaks of toxoplasmosis have been described.1
If infective oocysts get into the water supply, they may also cause outbreaks of disease. More than 200 such outbreaks have been described, including an instance in Victoria, British Columbia, in which 100 people became clinically infected.4
Continue to: Family outbreaks...
Family outbreaks have also been described that involve multiple children who played in an infected sandbox or dirt pile.5 Similarly, an outbreak has been reported in a riding stable that was home to infected cats. Infective oocysts were thought to have become aerosolized and breathed in by the patrons.6 Multiple other possible modes of transmission are being investigated, including sexual transmission among humans.7
Human infections are not always benign
In most human T. gondii cases, the infected individual experiences mild, flu-like symptoms, often with enlarged lymph nodes, or has no symptoms.1 Thus, most people who have been infected with
There are 3 exceptions to this otherwise benign clinical picture. The first is cerebral toxoplasmosis, which occurs in individuals who are immunosuppressed because they have AIDS or are receiving treatment for cancer or organ transplantation. Cerebral toxoplasmosis can be severe and was a common cause of death in patients with AIDS before the development of effective AIDS treatments.
The second exception is congenital toxoplasmosis, when an infection occurs in a pregnant woman. Such infections can cause severe damage to the developing fetus, including abortion, stillbirth, and brain damage. Congenital toxoplasmosis infections occur in approximately 1 of every 10,000 births in the United States, or approximately 3,800 each year.8 As a result, pregnant women are advised not to change their cat’s litter and to be tested for evidence of T. gondii infection.
The third exception is eye disease. Toxoplasmosis is one of the most common causes of eye disease, especially of the retina. Each year in the United States, approximately 4,800 individuals develop systematic ocular toxoplasmosis.9
Continue to: Toxoplasmosis and psychosis
Toxoplasmosis and psychosis: What evidence supports a link?
Until recently, cerebral infections, congenital infections, and eye disease were thought to be the main clinical problems associated with toxoplasmosis. However, accumulating evidence suggests that psychosis should be added to this list. Five lines of evidence support this.
1. T. gondii can cause psychotic symptoms. It has been known for decades that T. gondii can cause delusions, auditory hallucinations, and other psychotic symptoms.1 In one of the earliest publications (1966), Ladee10 concluded “The literature not infrequently focuses attention on psychosis with schizophrenia or schizophreniform features that accompany chronic toxoplasmosis.” Among the cases Ladee10 described was a laboratory worker who became infected with T. gondii and developed delusions and hallucinations.10
2. Patients with schizophrenia who are infected with T. gondii have more severe psychotic symptoms. This finding has been reported in at least 7 studies.1 Holub et al11 evaluated 251 patients with schizophrenia who were treated in Prague Psychiatric Centre between 2000 and 2010. Overall, 57 participants were infected with T. gondii and 194 were not infected. Compared to those who were not infected, the infected group:
- had significantly more severe symptoms (P = .032) as measured on the Positive and Negative Symptom Scale
- were prescribed higher doses of antipsychotic medications
- had been hospitalized longer.11
3. Compared with controls, patients with psychosis are significantly more likely to have antibodies against T. gondii, indicating previous infection. To date there have been approximately 100 such studies, of which at least three-fourths reported a positive association. In a 2012 meta-analysis of 38 such studies, Torrey et al12 reported an odds ratio (OR) of 2.7—compared to persons who have not been infected, those who have been infected with T. gondii were 2.7 times more likely to have schizophrenia.12 This study replicated the findings of a previous meta-analysis of 23 antibody studies, which also found an OR of 2.7.13
4. Compared with controls, individuals with schizophrenia or bipolar disorder are significantly more likely as a child to have lived in a home with a cat. Since 1995, 10 such studies have been published; 7 were positive, 2 were negative, and 1 was inconclusive.1 Torrey et al14 reviewed 2,025 individuals with schizophrenia or bipolar disorder and 4,847 controls and found that 51% of the cases and 43% of the controls had owned a cat before age 13; this difference was highly significant (P < .001). In fact, it is surprising that any study can find a statistically significant association between cat ownership and childhood psychosis. This is because a child who did not own a cat could become infected in many locations where cats have been present, including sandboxes at school, a babysitter’s or friend’s house, or a public park. And even if a child became infected at home, they would not necessarily have owned a cat, since the neighbor’s cat could have been responsible for the oocyst contamination.
Continue to: Epidemiologically...
5. Epidemiologically, there is a close temporal correlation between the rise of cats as pets and the rise of psychosis. This can be illustrated most clearly in England, where the rise of cat ownership has been documented by writers and where there is data on the rise of psychosis, especially in the 18th and 19th centuries.1
How many cases of psychosis might be caused by T. gondii?
In 2014, using data from the antibody studies discussed above,12,13 Smith15 sought to discover how many cases of psychosis might be caused by T. gondii. He concluded that 21% of cases of schizophrenia might have been caused by T. gondii. Based on the annual incidence of schizophrenia in the United States, this would mean an estimated >10,000 new cases of schizophrenia each year are attributable to this parasite.
Some researchers have found links between T. gondii and several nonpsychiatric diseases and conditions, including epilepsy and brain cancer (Box1,16-19).
Box
As interest in Toxoplasma gondii (T. gondii) has increased, researchers have looked for associations between this parasite with other diseases and conditions. Based on the literature, the following are of most interest:
Epilepsy. Since 1995, 16 studies1 have explored the relationship between T. gondii and epilepsy. A recent meta-analysis reported a statistically significant association between T. gondii and epilepsy.16
Brain cancer. Authors in 2 of 3 studies of meningiomas and 4 of 5 studies of gliomas reported statistically significant associations between these brain tumors and infection with T. gondii.1,17
Rheumatoid arthritis. Eight studies reported an increased prevalence of T. gondii antibodies in individuals with rheumatoid arthritis.1,18
Motor vehicle accidents. Infection with T. gondii is known to decrease motor reaction times in humans. At least 11 studies1 have examined whether infected individuals are more likely to have been involved in motor vehicle accidents. The results are mixed; the largest study reported a weak but statistically significant association.19
Clinical implications: What to tell patients about cats
What do these studies of toxoplasmosis imply for psychiatric care? As mental health professionals, part of our job is to educate our patients. Anything that appears to be a risk factor for the development of psychosis is thus of interest. Consider discussing the following with your patients.
Are cats safe? Cats that are kept exclusively indoors are safe pets because they are unlikely to become infected with T. gondii. However, cats that are allowed to go outdoors may not be safe, especially for children and young adults. What is needed is an effective vaccine that could be given to newborn kittens to prevent infection, but development of this type of vaccine has never been prioritized. At the community level, programs to decrease the number of stray and feral cats would also decrease the risk of infection.
Continue to: How to decrease risk
How to decrease risk. On a personal level, we can decrease T. gondii infections by not eating undercooked meat. Pregnant women and individuals who are immunocompromised should not change cat litter. When gardening, we should wear gloves because cats favor loose soil for depositing their feces. We should also protect children by covering sandboxes when not in use and by not allowing children to play in uncovered public sandboxes.
Treatment. Toxoplasmosis typically is treated with pyrimethamine, usually in combination with a sulfa drug. However, pyrimethamine does not cross the blood brain barrier and thus is ineffective when T. gondii infects the brain. The development of a drug that will effectively treat T. gondii in the brain should be a high priority.
For additional details on the studies discussed in this article as well as more resources on the impact T. gondii can have if proper precautions are not taken, see my open-access book at https://link.springer.com/book/10.1007/978-3-030-86811-6.
Bottom Line
Some evidence suggests that infection with Toxoplasma gondii (T. gondii) may cause psychotic symptoms, may increase an individual’s risk of developing psychosis, and may result in more severe psychotic symptoms. Cats can transmit T. gondii to humans. Educate patients that they can reduce their risk by keeping their cats inside, avoiding exposure to cat feces, particularly while pregnant or if immunocompromised, and not eating undercooked meat.
Related Resources
- Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
Drug Brand Names
Pyrimethamine • Daraprim
1. Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
2. Rohr JR, Barrett CB, Civitello DJ, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2(6):445-456.
3. Joynson DHM. Preface. In: Joynson DHM, Wreghitt TG, eds. Toxoplasmosis: A Comprehensive Clinical Guide. Cambridge University Press; 2001:xi.
4. Bowie WR, King AS, Werker DH, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350(9072):173-177.
5. Stagno S, Dykes AC, Amos CS, et al. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980;65(4):706-712.
6. Teutsch SM, Juranek DD, Sulzer A, et al. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300(13):695-699.
7. Kaňková Š, Hlaváčová J, Flegr J. Oral sex: a new, and possibly the most dangerous, route of toxoplasmosis transmission. Med Hypotheses. 2020;141:109725.
8. Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital T. gondii infection. N Engl J Med. 1994;330(26):1858-1863.
9. Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82(3):464-465.
10. Ladee GA. Diagnostic problems in psychiatry with regard to acquired toxoplasmosis. Psychiatr Neurol Neurochir. 1966;69(1):65-82.
11. Holub D, Flegr J, Dragomirecká E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
12. Torrey EF, Bartko JJ, Yolken RH. T. gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38(3):642-647.
13. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729-736.
14. Torrey EF, Simmons W, Yolken RH. Is childhood cat ownership a risk factor for schizophrenia later in life? Schizophr Res. 2015;165(1):1-2.
15. Smith G. Estimating the population attributable fraction for schizophrenia when T. gondii is assumed absent in human populations. Prev Vet Med. 2014;117(3-4):425-435.
16. Sadeghi M, Riahi SM, Mohammadi M, et al. An updated meta-analysis of the association between T. gondii infection and risk of epilepsy. Trans R Soc Trop Med Hyg. 2019;113(8):453-462.
17. Hodge JM, Coghill AE, Kim Y, et al. T. gondii infection and the risk of adult glioma in two prospective studies. Int J Cancer. 2021;148(10):2449-2456.
18. Hosseininejad Z, Sharif M, Sarvi S, et al. Toxoplasmosis seroprevalence in rheumatoid arthritis patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(6):e0006545.
19. Burgdorf KS, Trabjerg BB, Pedersen MG, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019; 79:152-158.
1. Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
2. Rohr JR, Barrett CB, Civitello DJ, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2(6):445-456.
3. Joynson DHM. Preface. In: Joynson DHM, Wreghitt TG, eds. Toxoplasmosis: A Comprehensive Clinical Guide. Cambridge University Press; 2001:xi.
4. Bowie WR, King AS, Werker DH, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350(9072):173-177.
5. Stagno S, Dykes AC, Amos CS, et al. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980;65(4):706-712.
6. Teutsch SM, Juranek DD, Sulzer A, et al. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300(13):695-699.
7. Kaňková Š, Hlaváčová J, Flegr J. Oral sex: a new, and possibly the most dangerous, route of toxoplasmosis transmission. Med Hypotheses. 2020;141:109725.
8. Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital T. gondii infection. N Engl J Med. 1994;330(26):1858-1863.
9. Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82(3):464-465.
10. Ladee GA. Diagnostic problems in psychiatry with regard to acquired toxoplasmosis. Psychiatr Neurol Neurochir. 1966;69(1):65-82.
11. Holub D, Flegr J, Dragomirecká E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
12. Torrey EF, Bartko JJ, Yolken RH. T. gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38(3):642-647.
13. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729-736.
14. Torrey EF, Simmons W, Yolken RH. Is childhood cat ownership a risk factor for schizophrenia later in life? Schizophr Res. 2015;165(1):1-2.
15. Smith G. Estimating the population attributable fraction for schizophrenia when T. gondii is assumed absent in human populations. Prev Vet Med. 2014;117(3-4):425-435.
16. Sadeghi M, Riahi SM, Mohammadi M, et al. An updated meta-analysis of the association between T. gondii infection and risk of epilepsy. Trans R Soc Trop Med Hyg. 2019;113(8):453-462.
17. Hodge JM, Coghill AE, Kim Y, et al. T. gondii infection and the risk of adult glioma in two prospective studies. Int J Cancer. 2021;148(10):2449-2456.
18. Hosseininejad Z, Sharif M, Sarvi S, et al. Toxoplasmosis seroprevalence in rheumatoid arthritis patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(6):e0006545.
19. Burgdorf KS, Trabjerg BB, Pedersen MG, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019; 79:152-158.
Psychodynamic factors in psychotropic prescribing
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.
Do no harm: Benztropine revisited
Ms. P, a 63-year-old woman with a history of schizophrenia whose symptoms have been stable on haloperidol 10 mg/d and ziprasidone 40 mg twice daily, presents to the outpatient clinic for a medication review. She mentions that she has noticed problems with her “memory.” She says she has had difficulty remembering names of people and places as well as difficulty concentrating while reading and writing, which she did months ago with ease. A Montreal Cognitive Assessment (MoCA) is conducted, and Ms. P scores 13/30, indicating moderate cognitive impairment. Visuospatial tasks and clock drawing are intact, but she exhibits impairments in working memory, attention, and concentration. One year ago, Ms. P’s MoCA score was 27/30. She agrees to a neurologic assessment and is referred to neurology for work-up.
Ms. P’s physical examination and routine laboratory tests are all within normal limits. The neurologic exam reveals deficits in working memory, concentration, and attention, but is otherwise unremarkable. MRI reveals mild chronic microvascular changes. The neurology service does not rule out cognitive impairment but recommends adjusting the dosage of Ms. P’s psychiatric medications to elucidate if her impairment of memory and attention is due to medications. However, Ms. P had been managed on her current regimen for several years and had not been hospitalized in many years. Previous attempts to taper her antipsychotics had resulted in worsening symptoms. Ms. P is reluctant to attempt a taper of her antipsychotics because she fears decompensation of her chronic illness. The treating team reviews Ms. P’s medication regimen, and notes that she is receiving benztropine 1 mg twice daily for prophylaxis of extrapyramidal symptoms (EPS). Ms. P denies past or present symptoms of drug-induced parkinsonism, dystonia, or akathisia as well as constipation, sialorrhea, blurry vision, palpitations, or urinary retention.
Benztropine is a tropane alkaloid that was synthetized by combining the tropine portion of atropine with the benzhydryl portion of diphenhydramine hydrochloride. It has anticholinergic and antihistaminic properties1 and seems to inhibit the dopamine transporter. Benztropine is indicated for all forms of parkinsonism, including antipsychotic-induced parkinsonism, but is also prescribed for many off-label uses, including sialorrhea and akathisia (although many authors do not recommend anticholinergics for this purpose2,3), and for prophylaxis of EPS. Benztropine can be administered intravenously, intramuscularly, or orally. Given orally, the typical dosing is twice daily with a maximum dose of 6 mg/d. Benztropine is preferred over diphenhydramine and trihexyphenidyl due to adverse effects of sedation or potential for misuse of the medication.1
Second-generation antipsychotics (SGAs) have been associated with lower rates of neurologic adverse effects compared with first-generation antipsychotics (FGAs). Because SGAs are increasingly prescribed, the use of benztropine (along with other agents such as trihexyphenidyl) for EPS prophylaxis is not an evidence-based practice. However, despite a movement away from prophylactic management of movement disorders, benztropine continues to be prescribed for EPS and/or cholinergic symptoms, despite the peripheral and cognitive adverse effects of this agent and, in many instances, the lack of clear indication for its use.
According to the most recent edition of the American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia,4 anticholinergics should only be used for preventing acute dystonia in conjunction with a long-acting injectable antipsychotic. Furthermore, the APA Guideline states anticholinergics may be used for drug-induced parkinsonism when the dose of an antipsychotic cannot be reduced and an alternative agent is required. However, the first-line agent for drug-induced parkinsonism is amantadine, and benztropine should only be considered if amantadine is contraindicated.4 The rationale for this guideline and for judicious use of anticholinergics is that like any pharmacologic treatment, anticholinergics (including benztropine) carry the potential for adverse effects. For benztropine, these range from mild effects such as tachycardia and constipation to paralytic ileus, increased falls, worsening of tardive dyskinesia (TD), and potential cognitive impairment. Literature suggests that the first step in managing cognitive concerns in a patient with schizophrenia should be a close review of medications, and avoidance of agents with anticholinergic properties.5
Prescribing benztropine for EPS
EPS, which include dystonia, akathisia, drug-induced parkinsonism, and TD, are very frequent adverse effects noted with antipsychotics. Benztropine has demonstrated benefit in managing acute dystonia and the APA Guideline recommends IM administration of either benztropine 1 mg or diphenhydramine 25 mg for this purpose.4 However, in our experience, the most frequent indication for long-term prescribing of benztropine is prophylaxis of antipsychoticinduced dystonia. This use was suggested by some older studies. In a 1987 study by Boyer et al,6 patients who were administered benztropine with haloperidol did not develop acute dystonia, while patients who received haloperidol alone developed dystonia. However, this was a small retrospective study with methodological issues. Boyer et al6 suggested discontinuing prophylaxis with benztropine within 1 week, as acute dystonia occurred within 2.5 days. Other researchers7,8 have argued that short-term prophylaxis with benztropine for 1 week may work, especially during treatment with high-potency antipsychotics. However, in a review of the use of anticholinergics in conjunction with antipsychotics, Desmarais et al5 concluded that there is no need for prophylaxis and recommended alternative treatments. As we have noticed in Ms. P and other patients treated in our facilities, benztropine is frequently continued indefinitely without a clinical indication for its continuous use. Assessment and indication for continued use of benztropine should be considered regularly, and it should be discontinued when there is no clear indication for its use or when adverse effects emerge.
Prescribing benztropine for TD
TD is a subtype of tardive syndromes associated with the use of antipsychotics. It is characterized by repetitive involuntary movements such as lip smacking, puckering, chewing, or tongue protrusion. Proposed pathophysiological mechanisms include dopamine receptor hypersensitivity, N-methyl-D-aspartate (NMDA) receptor excitotoxicity, and gamma-aminobutyric acid (GABA)-containing neuron activity.
According to the APA Guideline, evidence of benztropine’s efficacy for the prevention of TD is lacking.4 A 2018 Cochrane systematic review9 was unable to provide a definitive conclusion regarding the effectiveness of benztropine and other anticholinergics for the treatment of antipsychotic-induced TD. While many clinicians believe that benztropine can be used to treat all types of EPS, there are no clear instances in reviewed literature where the efficacy of benztropine for treating TD could be reliably demonstrated. Furthermore, some literature suggests that anticholinergics such as benztropine increase the risk of developing TD.5,10 The mechanism underlying benztropine’s ability to precipitate or exacerbate abnormal movements is unclear, though it is theorized that anticholinergic medications may inhibit dopamine reuptake into neurons, thus leading to an excess of dopamine in the synaptic cleft that manifests as dyskinesias.10 Some authors also recommend that the first step in the management of TD should be to gradually discontinue anticholinergics, as this has been associated with improvement in TD.11
Continue to: Prescribing anticholinergics in specific patient populations...
Prescribing anticholinergics in specific patient populations
In addition to the adverse effects described above, benztropine can affect cognition, as we observed in Ms. P. The cholinergic system plays a role in human cognition, and blockade of muscarinic receptors has been associated with impairments in working memory and prefrontal tasks.12 These adverse cognitive effects are more pronounced in certain populations, including patients with schizophrenia and older adults.
Schizophrenia is associated with declining cognitive function, and the cognitive faculties of patients with schizophrenia may be worsened by anticholinergics. In patients with schizophrenia, social interactions and social integration are often impacted by profound negative symptoms such as social withdrawal and poverty of thought and speech.13 In a double-blind study by Baker et al,14 benztropine was found to have an impact on attention and concentration in patients with chronic schizophrenia. Baker et al14 found that patients with schizophrenia who were switched from benztropine to placebo increased their overall Wechsler Memory Scale scores compared to those maintained on benztropine. One crosssectional analysis found that a higher anticholinergic burden was associated with impairments across all cognitive domains, including memory, attention/control, executive and visuospatial functioning, and motor speed domains.15 Importantly, a higher anticholinergic medication burden was associated with worse cognitive performance.15 In addition to impairments in cognitive processing, anticholinergics have been associated with a decreased ability to benefit from psychosocial programs and impaired abilities to manage activities of daily living.4 In another study exploring the effects of discontinuing anticholinergics and the impact on movement disorders, Desmarais et al16 found patients experienced a significant improvement in scores on the Brief Assessment of Cognition in Schizophrenia after discontinuing anticholinergics. Vinogradov et al17 noted that “serum anticholinergic activity in schizophrenia patients shows a significant association with impaired performance in measures of verbal working memory and verbal learning memory and was significantly associated with a lowered response to an intensive course of computerized cognitive training.” They felt their findings underscored the cognitive cost of medications with high anticholinergic burden.
Geriatric patients. Careful consideration should be given before starting benztropine in patients age ≥65. The 2019 American Geriatric Society’s Beers Criteria18 recommend avoiding benztropine in geriatric patients; the level of recommendation is strong. Furthermore, the American Geriatric Society designates benztropine as a medication that should be avoided, and a nondrug approach or alternative medication be prescribed independent of the patient’s condition or diagnosis. In a recently published case report, Esang et al19 highlighted several salient findings from previous studies on the risks associated with anticholinergic use:
- any medications a patient takes with anticholinergic properties contribute to the overall anticholinergic load of a patient’s medication regimen
- the higher the anticholinergic burden, the greater the cognitive deficits
- switching from an FGA to an SGA may decrease the risk of EPS and may limit the need for anticholinergic medications such as benztropine for a particular patient.
One must also consider that the effects of multiple medications with anticholinergic properties is probably cumulative.
Alternatives for treating drug-induced parkinsonism
Antipsychotics exert their effects through antagonism of the D2 receptor, and this is the same mechanism that leads to parkinsonism. Specifically, the mechanism is believed to be D2 receptor antagonism in the striatum leading to disinhibition of striatal neurons containing GABA.11 This disinhibition of medium spiny neurons is propagated when acetylcholine is released from cholinergic interneurons. Anticholinergics such as benztropine can remedy symptoms by blocking the signal of acetylcholine on the M1 receptors on medium spiny neurons. However, benztropine also has the propensity to decrease cholinergic transmission, thereby impairing storage of new information into long-term memory as well as impair perception of time—similar to effects seen with (for instance) diphenhydramine.20
The first step in managing drug-induced parkinsonism is to monitor symptoms. The APA Guideline recommends monitoring for acute-onset EPS at weekly intervals when beginning treatment and until stable for 2 weeks, and then monitoring at every follow-up visit thereafter.4 The next recommendation for long-term management of drug-induced parkinsonism is reducing the antipsychotic dose, or replacing the patient’s antipsychotic with an antipsychotic that is less likely to precipitate parkinsonism,4 such as quetiapine, iloperidone, or clozapine.11 If dose reduction is not possible, and the patient’s symptoms are severe, pharmacologic management is indicated. The APA Guideline recommends amantadine as a first-line agent because it is associated with fewer peripheral adverse effects and less impairment in cognition compared with benztropine.4 In a small (N = 60) doubleblind crossover trial, Gelenberg et al20 found benztropine 4 mg/d—but not amantadine 200 mg/d—impaired free recall and perception of time, and participants’ perception of their own memory impairment was significantly greater with benztropine. Amantadine has also been compared to biperiden, a relatively selective M1 muscarinic receptor muscarinic agent. In a separate double-blind crossover study of 26 patients with chronic schizophrenia, Silver and Geraisy21 found that compared to amantadine, biperiden was associated with worse memory performance. The recommended starting dose of amantadine for parkinsonism is 100 mg in the morning, increased to 100 mg twice a day and titrated to a maximum daily dose of 300 mg/d in divided doses.4
Continue to: Alternatives for treating drug-induced akathisia...
Alternatives for treating drug-induced akathisia
Akathisia remains a relatively common adverse effect of SGAs, and the profound physical distress and impaired functioning caused by akathisia necessitates pharmacologic treatment. Despite frequent use in practice for presumed benefit in akathisia, benztropine is not effective for the treatment of akathisia and the APA Guideline recommends that long-term management should begin with an antipsychotic dose reduction, followed by a switch to an agent with less propensity to incite akathisia.4 Acute manifestations of akathisia must be treated, and mirtazapine, propranolol, or clonazepam may be considered as alternatives.4 Mirtazapine is dosed 7.5 mg to 10 mg nightly for akathisia, though it should be used in caution in patients at risk for mania.4 Mirtazapine’s potent 5-HT2A blockade at low doses may contribute to its utility in treating akathisia.2 Propranolol, a nonselective lipophilic beta-adrenergic antagonist, also has demonstrated efficacy in managing akathisia, with recommended dosing of 40 mg to 80 mg twice daily.2 Benzodiazepines such as clonazepam require judicious use for akathisia because they may also precipitate or exacerbate cognitive impairment.4
Alternatives for treating TD
As mentioned above, benztropine is not recommended for the treatment of TD.1 The Box4,22,23 outlines potential treatment options for TD.
Box
Monitoring is the first step in the prevention of tardive dyskinesia (TD). The American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia recommends patients receiving first-generation antipsychotics (FGAs) be monitored every 6 months, those prescribed second-generation antipsychotics (SGAs) be monitored every 12 months, and twice as frequent monitoring for geriatric patients and those who developed involuntary movements rapidly after starting an antipsychotic.4
The APA Guideline recommends decreasing or gradually tapering antipsychotics as another strategy for preventing TD.4 However, these recommendations should be weighed against the risk of short-term antipsychotic withdrawal. Withdrawal of D2 antagonists is associated with worsening of dyskinesias or withdrawal dyskinesia and psychotic decompensation.22
Current treatment recommendations give preference to the importance of preventing development of TD by tapering to the lowest dose of antipsychotic needed to control symptoms for the shortest duration possible.22 Thereafter, if treatment intervention is needed, consideration should be given to the following pharmacological interventions in order from highest level of recommendation (Grade A) to lowest (Grade C):
A: vesicular monoamine transporter-2 inhibitors deutetrabenazine and valbenazine
B: clonazepam, ginkgo biloba
C: amantadine, tetrabenazine, and globus pallidus interna deep brain stimulation.22
There is insufficient evidence to support or refute withdrawing causative agents or switching from FGAs to SGAs to treat TD.22 Furthermore, for many patients with schizophrenia, a gradual discontinuation of their antipsychotic must be weighed against the risk of relapse.23
Valbenazine and deutetrabenazine have been demonstrated to be efficacious and are FDA-approved for managing TD. The initial dose of valbenazine is 40 mg/d. Common adverse effects include somnolence and fatigue/ sedation. Valbenazine should be avoided in patients with QT prolongation or arrhythmias. Deutetrabenazine has less impact on the cytochrome P450 2D6 enzyme and therefore does not require genotyping as would be the case for patients who are receiving >50 mg/d of tetrabenazine. The starting dose of deutetrabenazine is 6 mg/d. Adverse effects include depression, suicidality, neuroleptic malignant syndrome, parkinsonism, and QT prolongation. Deutetrabenazine is contraindicated in patients who are suicidal or have untreated depression, hepatic impairment, or concomitant use of monoamine oxidase inhibitors.22 Deutetrabenazine is an isomer of tetrabenazine; however, evidence supporting the parent compound suggests limited use due to increased risk of adverse effects compared with valbenazine and deutetrabenazine.23 Tetrabenazine may be considered as an adjunctive treatment or used as a single agent if valbenazine or deutetrabenazine are not accessible.22
Discontinuing benztropine
Benztropine is recommended as a firstline agent for the management of acute dystonia, and it may be used temporarily for drug-induced parkinsonism, but it is not recommended to prevent EPS or TD. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic agent such as benztropine. Based on their review of earlier studies, Desmarais et al5 suggest a gradual 3-month discontinuation of benztropine. Multiple studies have demonstrated an ability to taper anticholinergics in days to months.4 However, gradual discontinuation is advisable to avoid cholinergic rebound and the reemergence of EPS, and to decrease the risk of neuroleptic malignant syndrome associated with sudden discontinuation.5 One suggested taper regimen is a decrease of 0.5 mg benztropine every week. Amantadine may be considered if parkinsonism is noted during the taper. Patients on benztropine may develop rebound symptoms, such as vivid dreams/nightmares; if this occurs, the taper rate can be slowed to a decrease of 0.5 mg every 2 weeks.4
Continue to: First do no harm...
First do no harm
Psychiatrists commonly prescribe benztropine to prevent EPS and TD, but available literature does not support the efficacy of benztropine for mitigating drug-induced parkinsonism, and studies report benztropine may significantly worsen cognitive processes and exacerbate TD.16 In addition, benztropine misuse has been correlated with euphoria and psychosis.16 More than 3 decades ago, the World Health Organization Heads of Centres Collaborating in WHO-Coordinated Studies on Biological Aspects of Mental Illness issued a consensus statement24 discouraging the prophylactic use of anticholinergics for patients receiving antipsychotics, yet we still see patients on an indefinite regimen of benztropine.
As clinicians, our goals should be to optimize a patient’s functioning and quality of life, and to use the lowest dose of medication along with the fewest medications necessary to avoid adverse effects such as EPS. Benztropine is recommended as a first-line agent for the management of acute dystonia, but its continued or indefinite use to prevent antipsychotic-induced adverse effects is not recommended. While all pharmacologic interventions carry a risk of adverse effects, weighing the risk of those effects against the clinical benefits is the prerogative of a skilled clinician. Benztropine and other anticholinergics prescribed for prophylactic purposes have numerous adverse effects, limited clinical utility, and a deleterious effect on quality of life. Furthermore, benztropine prophylaxis of drug-induced parkinsonism does not seem to be warranted, and the risks do not seem to outweigh the harm benztropine may cause, with the possible exception of “prophylactic” treatment of dystonia that is discontinued in a few days, as some researchers have suggested.6-8 The preventive value of benztropine has not been demonstrated. It is time we took inventory of medications that might cause more harm than good, rely on current treatment guidelines instead of habit, and use these agents judiciously while considering replacement with novel, safer medications whenever possible.
CASE CONTINUED
The clinical team considers benztropine’s ability to cause cognitive effects, and decides to taper and discontinue it over 1 month. Ms. P is seen in an outpatient clinic within 1 month of discontinuing benztropine. She reports that her difficulty remembering words and details has improved. She also says that she is now able to concentrate on writing and reading. The consulting neurologist also notes improvement. Ms. P continues to report improvement in symptoms over the next 2 months of follow-up, and says that her mood improved and she has less apathy.
Bottom Line
Benztropine is a first-line medication for acute dystonia, but its continued or indefinite use for preventing antipsychotic-induced adverse effects is not recommended. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic medication such as benztropine.
1. Cogentin [package insert]. McPherson, KS: Lundbeck Inc; 2013.
2. Poyurovsky M, Weizman A. Treatment of antipsychoticrelated akathisia revisited. J Clin Psychopharmacol. 2015; 35(6):711-714.
3. Salem H, Nagpal C, Pigott T, et al. Revisiting antipsychoticinduced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
4. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd ed. American Psychiatric Association; 2021.
5. Desmarais JE, Beauclair L, Margolese HC. Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol. 2012;26(9):1167-1174.
6. Boyer WF, Bakalar NH, Lake CR. Anticholinergic prophylaxis of acute haloperidol-induced acute dystonic reactions. J Clin Psychopharmacol. 1987;7(3):164-166.
7. Winslow RS, Stillner V, Coons DJ, et al. Prevention of acute dystonic reactions in patients beginning high-potency neuroleptics. Am J Psychiatry. 1986;143(6):706-710.
8. Stern TA, Anderson WH. Benztropine prophylaxis of dystonic reactions. Psychopharmacology (Berl). 1979; 61(3):261-262.
9. Bergman H, Soares‐Weiser K. Anticholinergic medication for antipsychotic‐induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1(1):CD000204. doi:10.1002/ 14651858.CD000204.pub2
10. Howrie DL, Rowley AH, Krenzelok EP. Benztropineinduced acute dystonic reaction. Ann Emerg Med. 1986;15(5):594-596.
11. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs. tardive dyskinesia--key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2): 233-248.
12. Wijegunaratne H, Qazi H, Koola M. Chronic and bedtime use of benztropine with antipsychotics: is it necessary? Schizophr Res. 2014;153(1-3):248-249.
13. Möller HJ. The relevance of negative symptoms in schizophrenia and how to treat them with psychopharmaceuticals? Psychiatr Danub. 2016;28(4):435-440.
14. Baker LA, Cheng LY, Amara IB. The withdrawal of benztropine mesylate in chronic schizophrenic patients. Br J Psychiatry. 1983;143:584-590.
15. Joshi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry. 2021;178(9):838-847.
16. Desmarais JE, Beauclair E, Annable L, et al. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Ther Adv Psychopharmacol. 2014;4(6): 257-267.
17. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9): 1055-1062.
18. American Geriatrics Society 2019 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
19. Esang M, Person US, Izekor OO, et al. An unlikely case of benztropine misuse in an elderly schizophrenic. Cureus. 2021;13(2):e13434. doi:10.7759/cureus.13434
20. Gelenberg AJ, Van Putten T, Lavori PW, et al. Anticholinergic effects on memory: benztropine versus amantadine. J Clin Psychopharmacol. 1989;9(3):180-185.
21. Silver H, Geraisy N. Effects of biperiden and amantadine on memory in medicated chronic schizophrenic patients. A double-blind cross-over study. Br J Psychiatry. 1995; 166(2):241-243.
22. Bhidayasiri R, Jitkritsadakul O, Friedman J, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67-75.
23. Ricciardi L, Pringsheim T, Barnes TRE, et al. Treatment recommendations for tardive dyskinesia. Canadian J Psychiatry. 2019;64(6):388-399.
24. Prophylactic use of anticholinergics in patients on long-term neuroleptic treatment. A consensus statement. World Health Organization heads of centres collaborating in WHO coordinated studies on biological aspects of mental illness. Br J Psychiatry. 1990;156:412.
Ms. P, a 63-year-old woman with a history of schizophrenia whose symptoms have been stable on haloperidol 10 mg/d and ziprasidone 40 mg twice daily, presents to the outpatient clinic for a medication review. She mentions that she has noticed problems with her “memory.” She says she has had difficulty remembering names of people and places as well as difficulty concentrating while reading and writing, which she did months ago with ease. A Montreal Cognitive Assessment (MoCA) is conducted, and Ms. P scores 13/30, indicating moderate cognitive impairment. Visuospatial tasks and clock drawing are intact, but she exhibits impairments in working memory, attention, and concentration. One year ago, Ms. P’s MoCA score was 27/30. She agrees to a neurologic assessment and is referred to neurology for work-up.
Ms. P’s physical examination and routine laboratory tests are all within normal limits. The neurologic exam reveals deficits in working memory, concentration, and attention, but is otherwise unremarkable. MRI reveals mild chronic microvascular changes. The neurology service does not rule out cognitive impairment but recommends adjusting the dosage of Ms. P’s psychiatric medications to elucidate if her impairment of memory and attention is due to medications. However, Ms. P had been managed on her current regimen for several years and had not been hospitalized in many years. Previous attempts to taper her antipsychotics had resulted in worsening symptoms. Ms. P is reluctant to attempt a taper of her antipsychotics because she fears decompensation of her chronic illness. The treating team reviews Ms. P’s medication regimen, and notes that she is receiving benztropine 1 mg twice daily for prophylaxis of extrapyramidal symptoms (EPS). Ms. P denies past or present symptoms of drug-induced parkinsonism, dystonia, or akathisia as well as constipation, sialorrhea, blurry vision, palpitations, or urinary retention.
Benztropine is a tropane alkaloid that was synthetized by combining the tropine portion of atropine with the benzhydryl portion of diphenhydramine hydrochloride. It has anticholinergic and antihistaminic properties1 and seems to inhibit the dopamine transporter. Benztropine is indicated for all forms of parkinsonism, including antipsychotic-induced parkinsonism, but is also prescribed for many off-label uses, including sialorrhea and akathisia (although many authors do not recommend anticholinergics for this purpose2,3), and for prophylaxis of EPS. Benztropine can be administered intravenously, intramuscularly, or orally. Given orally, the typical dosing is twice daily with a maximum dose of 6 mg/d. Benztropine is preferred over diphenhydramine and trihexyphenidyl due to adverse effects of sedation or potential for misuse of the medication.1
Second-generation antipsychotics (SGAs) have been associated with lower rates of neurologic adverse effects compared with first-generation antipsychotics (FGAs). Because SGAs are increasingly prescribed, the use of benztropine (along with other agents such as trihexyphenidyl) for EPS prophylaxis is not an evidence-based practice. However, despite a movement away from prophylactic management of movement disorders, benztropine continues to be prescribed for EPS and/or cholinergic symptoms, despite the peripheral and cognitive adverse effects of this agent and, in many instances, the lack of clear indication for its use.
According to the most recent edition of the American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia,4 anticholinergics should only be used for preventing acute dystonia in conjunction with a long-acting injectable antipsychotic. Furthermore, the APA Guideline states anticholinergics may be used for drug-induced parkinsonism when the dose of an antipsychotic cannot be reduced and an alternative agent is required. However, the first-line agent for drug-induced parkinsonism is amantadine, and benztropine should only be considered if amantadine is contraindicated.4 The rationale for this guideline and for judicious use of anticholinergics is that like any pharmacologic treatment, anticholinergics (including benztropine) carry the potential for adverse effects. For benztropine, these range from mild effects such as tachycardia and constipation to paralytic ileus, increased falls, worsening of tardive dyskinesia (TD), and potential cognitive impairment. Literature suggests that the first step in managing cognitive concerns in a patient with schizophrenia should be a close review of medications, and avoidance of agents with anticholinergic properties.5
Prescribing benztropine for EPS
EPS, which include dystonia, akathisia, drug-induced parkinsonism, and TD, are very frequent adverse effects noted with antipsychotics. Benztropine has demonstrated benefit in managing acute dystonia and the APA Guideline recommends IM administration of either benztropine 1 mg or diphenhydramine 25 mg for this purpose.4 However, in our experience, the most frequent indication for long-term prescribing of benztropine is prophylaxis of antipsychoticinduced dystonia. This use was suggested by some older studies. In a 1987 study by Boyer et al,6 patients who were administered benztropine with haloperidol did not develop acute dystonia, while patients who received haloperidol alone developed dystonia. However, this was a small retrospective study with methodological issues. Boyer et al6 suggested discontinuing prophylaxis with benztropine within 1 week, as acute dystonia occurred within 2.5 days. Other researchers7,8 have argued that short-term prophylaxis with benztropine for 1 week may work, especially during treatment with high-potency antipsychotics. However, in a review of the use of anticholinergics in conjunction with antipsychotics, Desmarais et al5 concluded that there is no need for prophylaxis and recommended alternative treatments. As we have noticed in Ms. P and other patients treated in our facilities, benztropine is frequently continued indefinitely without a clinical indication for its continuous use. Assessment and indication for continued use of benztropine should be considered regularly, and it should be discontinued when there is no clear indication for its use or when adverse effects emerge.
Prescribing benztropine for TD
TD is a subtype of tardive syndromes associated with the use of antipsychotics. It is characterized by repetitive involuntary movements such as lip smacking, puckering, chewing, or tongue protrusion. Proposed pathophysiological mechanisms include dopamine receptor hypersensitivity, N-methyl-D-aspartate (NMDA) receptor excitotoxicity, and gamma-aminobutyric acid (GABA)-containing neuron activity.
According to the APA Guideline, evidence of benztropine’s efficacy for the prevention of TD is lacking.4 A 2018 Cochrane systematic review9 was unable to provide a definitive conclusion regarding the effectiveness of benztropine and other anticholinergics for the treatment of antipsychotic-induced TD. While many clinicians believe that benztropine can be used to treat all types of EPS, there are no clear instances in reviewed literature where the efficacy of benztropine for treating TD could be reliably demonstrated. Furthermore, some literature suggests that anticholinergics such as benztropine increase the risk of developing TD.5,10 The mechanism underlying benztropine’s ability to precipitate or exacerbate abnormal movements is unclear, though it is theorized that anticholinergic medications may inhibit dopamine reuptake into neurons, thus leading to an excess of dopamine in the synaptic cleft that manifests as dyskinesias.10 Some authors also recommend that the first step in the management of TD should be to gradually discontinue anticholinergics, as this has been associated with improvement in TD.11
Continue to: Prescribing anticholinergics in specific patient populations...
Prescribing anticholinergics in specific patient populations
In addition to the adverse effects described above, benztropine can affect cognition, as we observed in Ms. P. The cholinergic system plays a role in human cognition, and blockade of muscarinic receptors has been associated with impairments in working memory and prefrontal tasks.12 These adverse cognitive effects are more pronounced in certain populations, including patients with schizophrenia and older adults.
Schizophrenia is associated with declining cognitive function, and the cognitive faculties of patients with schizophrenia may be worsened by anticholinergics. In patients with schizophrenia, social interactions and social integration are often impacted by profound negative symptoms such as social withdrawal and poverty of thought and speech.13 In a double-blind study by Baker et al,14 benztropine was found to have an impact on attention and concentration in patients with chronic schizophrenia. Baker et al14 found that patients with schizophrenia who were switched from benztropine to placebo increased their overall Wechsler Memory Scale scores compared to those maintained on benztropine. One crosssectional analysis found that a higher anticholinergic burden was associated with impairments across all cognitive domains, including memory, attention/control, executive and visuospatial functioning, and motor speed domains.15 Importantly, a higher anticholinergic medication burden was associated with worse cognitive performance.15 In addition to impairments in cognitive processing, anticholinergics have been associated with a decreased ability to benefit from psychosocial programs and impaired abilities to manage activities of daily living.4 In another study exploring the effects of discontinuing anticholinergics and the impact on movement disorders, Desmarais et al16 found patients experienced a significant improvement in scores on the Brief Assessment of Cognition in Schizophrenia after discontinuing anticholinergics. Vinogradov et al17 noted that “serum anticholinergic activity in schizophrenia patients shows a significant association with impaired performance in measures of verbal working memory and verbal learning memory and was significantly associated with a lowered response to an intensive course of computerized cognitive training.” They felt their findings underscored the cognitive cost of medications with high anticholinergic burden.
Geriatric patients. Careful consideration should be given before starting benztropine in patients age ≥65. The 2019 American Geriatric Society’s Beers Criteria18 recommend avoiding benztropine in geriatric patients; the level of recommendation is strong. Furthermore, the American Geriatric Society designates benztropine as a medication that should be avoided, and a nondrug approach or alternative medication be prescribed independent of the patient’s condition or diagnosis. In a recently published case report, Esang et al19 highlighted several salient findings from previous studies on the risks associated with anticholinergic use:
- any medications a patient takes with anticholinergic properties contribute to the overall anticholinergic load of a patient’s medication regimen
- the higher the anticholinergic burden, the greater the cognitive deficits
- switching from an FGA to an SGA may decrease the risk of EPS and may limit the need for anticholinergic medications such as benztropine for a particular patient.
One must also consider that the effects of multiple medications with anticholinergic properties is probably cumulative.
Alternatives for treating drug-induced parkinsonism
Antipsychotics exert their effects through antagonism of the D2 receptor, and this is the same mechanism that leads to parkinsonism. Specifically, the mechanism is believed to be D2 receptor antagonism in the striatum leading to disinhibition of striatal neurons containing GABA.11 This disinhibition of medium spiny neurons is propagated when acetylcholine is released from cholinergic interneurons. Anticholinergics such as benztropine can remedy symptoms by blocking the signal of acetylcholine on the M1 receptors on medium spiny neurons. However, benztropine also has the propensity to decrease cholinergic transmission, thereby impairing storage of new information into long-term memory as well as impair perception of time—similar to effects seen with (for instance) diphenhydramine.20
The first step in managing drug-induced parkinsonism is to monitor symptoms. The APA Guideline recommends monitoring for acute-onset EPS at weekly intervals when beginning treatment and until stable for 2 weeks, and then monitoring at every follow-up visit thereafter.4 The next recommendation for long-term management of drug-induced parkinsonism is reducing the antipsychotic dose, or replacing the patient’s antipsychotic with an antipsychotic that is less likely to precipitate parkinsonism,4 such as quetiapine, iloperidone, or clozapine.11 If dose reduction is not possible, and the patient’s symptoms are severe, pharmacologic management is indicated. The APA Guideline recommends amantadine as a first-line agent because it is associated with fewer peripheral adverse effects and less impairment in cognition compared with benztropine.4 In a small (N = 60) doubleblind crossover trial, Gelenberg et al20 found benztropine 4 mg/d—but not amantadine 200 mg/d—impaired free recall and perception of time, and participants’ perception of their own memory impairment was significantly greater with benztropine. Amantadine has also been compared to biperiden, a relatively selective M1 muscarinic receptor muscarinic agent. In a separate double-blind crossover study of 26 patients with chronic schizophrenia, Silver and Geraisy21 found that compared to amantadine, biperiden was associated with worse memory performance. The recommended starting dose of amantadine for parkinsonism is 100 mg in the morning, increased to 100 mg twice a day and titrated to a maximum daily dose of 300 mg/d in divided doses.4
Continue to: Alternatives for treating drug-induced akathisia...
Alternatives for treating drug-induced akathisia
Akathisia remains a relatively common adverse effect of SGAs, and the profound physical distress and impaired functioning caused by akathisia necessitates pharmacologic treatment. Despite frequent use in practice for presumed benefit in akathisia, benztropine is not effective for the treatment of akathisia and the APA Guideline recommends that long-term management should begin with an antipsychotic dose reduction, followed by a switch to an agent with less propensity to incite akathisia.4 Acute manifestations of akathisia must be treated, and mirtazapine, propranolol, or clonazepam may be considered as alternatives.4 Mirtazapine is dosed 7.5 mg to 10 mg nightly for akathisia, though it should be used in caution in patients at risk for mania.4 Mirtazapine’s potent 5-HT2A blockade at low doses may contribute to its utility in treating akathisia.2 Propranolol, a nonselective lipophilic beta-adrenergic antagonist, also has demonstrated efficacy in managing akathisia, with recommended dosing of 40 mg to 80 mg twice daily.2 Benzodiazepines such as clonazepam require judicious use for akathisia because they may also precipitate or exacerbate cognitive impairment.4
Alternatives for treating TD
As mentioned above, benztropine is not recommended for the treatment of TD.1 The Box4,22,23 outlines potential treatment options for TD.
Box
Monitoring is the first step in the prevention of tardive dyskinesia (TD). The American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia recommends patients receiving first-generation antipsychotics (FGAs) be monitored every 6 months, those prescribed second-generation antipsychotics (SGAs) be monitored every 12 months, and twice as frequent monitoring for geriatric patients and those who developed involuntary movements rapidly after starting an antipsychotic.4
The APA Guideline recommends decreasing or gradually tapering antipsychotics as another strategy for preventing TD.4 However, these recommendations should be weighed against the risk of short-term antipsychotic withdrawal. Withdrawal of D2 antagonists is associated with worsening of dyskinesias or withdrawal dyskinesia and psychotic decompensation.22
Current treatment recommendations give preference to the importance of preventing development of TD by tapering to the lowest dose of antipsychotic needed to control symptoms for the shortest duration possible.22 Thereafter, if treatment intervention is needed, consideration should be given to the following pharmacological interventions in order from highest level of recommendation (Grade A) to lowest (Grade C):
A: vesicular monoamine transporter-2 inhibitors deutetrabenazine and valbenazine
B: clonazepam, ginkgo biloba
C: amantadine, tetrabenazine, and globus pallidus interna deep brain stimulation.22
There is insufficient evidence to support or refute withdrawing causative agents or switching from FGAs to SGAs to treat TD.22 Furthermore, for many patients with schizophrenia, a gradual discontinuation of their antipsychotic must be weighed against the risk of relapse.23
Valbenazine and deutetrabenazine have been demonstrated to be efficacious and are FDA-approved for managing TD. The initial dose of valbenazine is 40 mg/d. Common adverse effects include somnolence and fatigue/ sedation. Valbenazine should be avoided in patients with QT prolongation or arrhythmias. Deutetrabenazine has less impact on the cytochrome P450 2D6 enzyme and therefore does not require genotyping as would be the case for patients who are receiving >50 mg/d of tetrabenazine. The starting dose of deutetrabenazine is 6 mg/d. Adverse effects include depression, suicidality, neuroleptic malignant syndrome, parkinsonism, and QT prolongation. Deutetrabenazine is contraindicated in patients who are suicidal or have untreated depression, hepatic impairment, or concomitant use of monoamine oxidase inhibitors.22 Deutetrabenazine is an isomer of tetrabenazine; however, evidence supporting the parent compound suggests limited use due to increased risk of adverse effects compared with valbenazine and deutetrabenazine.23 Tetrabenazine may be considered as an adjunctive treatment or used as a single agent if valbenazine or deutetrabenazine are not accessible.22
Discontinuing benztropine
Benztropine is recommended as a firstline agent for the management of acute dystonia, and it may be used temporarily for drug-induced parkinsonism, but it is not recommended to prevent EPS or TD. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic agent such as benztropine. Based on their review of earlier studies, Desmarais et al5 suggest a gradual 3-month discontinuation of benztropine. Multiple studies have demonstrated an ability to taper anticholinergics in days to months.4 However, gradual discontinuation is advisable to avoid cholinergic rebound and the reemergence of EPS, and to decrease the risk of neuroleptic malignant syndrome associated with sudden discontinuation.5 One suggested taper regimen is a decrease of 0.5 mg benztropine every week. Amantadine may be considered if parkinsonism is noted during the taper. Patients on benztropine may develop rebound symptoms, such as vivid dreams/nightmares; if this occurs, the taper rate can be slowed to a decrease of 0.5 mg every 2 weeks.4
Continue to: First do no harm...
First do no harm
Psychiatrists commonly prescribe benztropine to prevent EPS and TD, but available literature does not support the efficacy of benztropine for mitigating drug-induced parkinsonism, and studies report benztropine may significantly worsen cognitive processes and exacerbate TD.16 In addition, benztropine misuse has been correlated with euphoria and psychosis.16 More than 3 decades ago, the World Health Organization Heads of Centres Collaborating in WHO-Coordinated Studies on Biological Aspects of Mental Illness issued a consensus statement24 discouraging the prophylactic use of anticholinergics for patients receiving antipsychotics, yet we still see patients on an indefinite regimen of benztropine.
As clinicians, our goals should be to optimize a patient’s functioning and quality of life, and to use the lowest dose of medication along with the fewest medications necessary to avoid adverse effects such as EPS. Benztropine is recommended as a first-line agent for the management of acute dystonia, but its continued or indefinite use to prevent antipsychotic-induced adverse effects is not recommended. While all pharmacologic interventions carry a risk of adverse effects, weighing the risk of those effects against the clinical benefits is the prerogative of a skilled clinician. Benztropine and other anticholinergics prescribed for prophylactic purposes have numerous adverse effects, limited clinical utility, and a deleterious effect on quality of life. Furthermore, benztropine prophylaxis of drug-induced parkinsonism does not seem to be warranted, and the risks do not seem to outweigh the harm benztropine may cause, with the possible exception of “prophylactic” treatment of dystonia that is discontinued in a few days, as some researchers have suggested.6-8 The preventive value of benztropine has not been demonstrated. It is time we took inventory of medications that might cause more harm than good, rely on current treatment guidelines instead of habit, and use these agents judiciously while considering replacement with novel, safer medications whenever possible.
CASE CONTINUED
The clinical team considers benztropine’s ability to cause cognitive effects, and decides to taper and discontinue it over 1 month. Ms. P is seen in an outpatient clinic within 1 month of discontinuing benztropine. She reports that her difficulty remembering words and details has improved. She also says that she is now able to concentrate on writing and reading. The consulting neurologist also notes improvement. Ms. P continues to report improvement in symptoms over the next 2 months of follow-up, and says that her mood improved and she has less apathy.
Bottom Line
Benztropine is a first-line medication for acute dystonia, but its continued or indefinite use for preventing antipsychotic-induced adverse effects is not recommended. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic medication such as benztropine.
Ms. P, a 63-year-old woman with a history of schizophrenia whose symptoms have been stable on haloperidol 10 mg/d and ziprasidone 40 mg twice daily, presents to the outpatient clinic for a medication review. She mentions that she has noticed problems with her “memory.” She says she has had difficulty remembering names of people and places as well as difficulty concentrating while reading and writing, which she did months ago with ease. A Montreal Cognitive Assessment (MoCA) is conducted, and Ms. P scores 13/30, indicating moderate cognitive impairment. Visuospatial tasks and clock drawing are intact, but she exhibits impairments in working memory, attention, and concentration. One year ago, Ms. P’s MoCA score was 27/30. She agrees to a neurologic assessment and is referred to neurology for work-up.
Ms. P’s physical examination and routine laboratory tests are all within normal limits. The neurologic exam reveals deficits in working memory, concentration, and attention, but is otherwise unremarkable. MRI reveals mild chronic microvascular changes. The neurology service does not rule out cognitive impairment but recommends adjusting the dosage of Ms. P’s psychiatric medications to elucidate if her impairment of memory and attention is due to medications. However, Ms. P had been managed on her current regimen for several years and had not been hospitalized in many years. Previous attempts to taper her antipsychotics had resulted in worsening symptoms. Ms. P is reluctant to attempt a taper of her antipsychotics because she fears decompensation of her chronic illness. The treating team reviews Ms. P’s medication regimen, and notes that she is receiving benztropine 1 mg twice daily for prophylaxis of extrapyramidal symptoms (EPS). Ms. P denies past or present symptoms of drug-induced parkinsonism, dystonia, or akathisia as well as constipation, sialorrhea, blurry vision, palpitations, or urinary retention.
Benztropine is a tropane alkaloid that was synthetized by combining the tropine portion of atropine with the benzhydryl portion of diphenhydramine hydrochloride. It has anticholinergic and antihistaminic properties1 and seems to inhibit the dopamine transporter. Benztropine is indicated for all forms of parkinsonism, including antipsychotic-induced parkinsonism, but is also prescribed for many off-label uses, including sialorrhea and akathisia (although many authors do not recommend anticholinergics for this purpose2,3), and for prophylaxis of EPS. Benztropine can be administered intravenously, intramuscularly, or orally. Given orally, the typical dosing is twice daily with a maximum dose of 6 mg/d. Benztropine is preferred over diphenhydramine and trihexyphenidyl due to adverse effects of sedation or potential for misuse of the medication.1
Second-generation antipsychotics (SGAs) have been associated with lower rates of neurologic adverse effects compared with first-generation antipsychotics (FGAs). Because SGAs are increasingly prescribed, the use of benztropine (along with other agents such as trihexyphenidyl) for EPS prophylaxis is not an evidence-based practice. However, despite a movement away from prophylactic management of movement disorders, benztropine continues to be prescribed for EPS and/or cholinergic symptoms, despite the peripheral and cognitive adverse effects of this agent and, in many instances, the lack of clear indication for its use.
According to the most recent edition of the American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia,4 anticholinergics should only be used for preventing acute dystonia in conjunction with a long-acting injectable antipsychotic. Furthermore, the APA Guideline states anticholinergics may be used for drug-induced parkinsonism when the dose of an antipsychotic cannot be reduced and an alternative agent is required. However, the first-line agent for drug-induced parkinsonism is amantadine, and benztropine should only be considered if amantadine is contraindicated.4 The rationale for this guideline and for judicious use of anticholinergics is that like any pharmacologic treatment, anticholinergics (including benztropine) carry the potential for adverse effects. For benztropine, these range from mild effects such as tachycardia and constipation to paralytic ileus, increased falls, worsening of tardive dyskinesia (TD), and potential cognitive impairment. Literature suggests that the first step in managing cognitive concerns in a patient with schizophrenia should be a close review of medications, and avoidance of agents with anticholinergic properties.5
Prescribing benztropine for EPS
EPS, which include dystonia, akathisia, drug-induced parkinsonism, and TD, are very frequent adverse effects noted with antipsychotics. Benztropine has demonstrated benefit in managing acute dystonia and the APA Guideline recommends IM administration of either benztropine 1 mg or diphenhydramine 25 mg for this purpose.4 However, in our experience, the most frequent indication for long-term prescribing of benztropine is prophylaxis of antipsychoticinduced dystonia. This use was suggested by some older studies. In a 1987 study by Boyer et al,6 patients who were administered benztropine with haloperidol did not develop acute dystonia, while patients who received haloperidol alone developed dystonia. However, this was a small retrospective study with methodological issues. Boyer et al6 suggested discontinuing prophylaxis with benztropine within 1 week, as acute dystonia occurred within 2.5 days. Other researchers7,8 have argued that short-term prophylaxis with benztropine for 1 week may work, especially during treatment with high-potency antipsychotics. However, in a review of the use of anticholinergics in conjunction with antipsychotics, Desmarais et al5 concluded that there is no need for prophylaxis and recommended alternative treatments. As we have noticed in Ms. P and other patients treated in our facilities, benztropine is frequently continued indefinitely without a clinical indication for its continuous use. Assessment and indication for continued use of benztropine should be considered regularly, and it should be discontinued when there is no clear indication for its use or when adverse effects emerge.
Prescribing benztropine for TD
TD is a subtype of tardive syndromes associated with the use of antipsychotics. It is characterized by repetitive involuntary movements such as lip smacking, puckering, chewing, or tongue protrusion. Proposed pathophysiological mechanisms include dopamine receptor hypersensitivity, N-methyl-D-aspartate (NMDA) receptor excitotoxicity, and gamma-aminobutyric acid (GABA)-containing neuron activity.
According to the APA Guideline, evidence of benztropine’s efficacy for the prevention of TD is lacking.4 A 2018 Cochrane systematic review9 was unable to provide a definitive conclusion regarding the effectiveness of benztropine and other anticholinergics for the treatment of antipsychotic-induced TD. While many clinicians believe that benztropine can be used to treat all types of EPS, there are no clear instances in reviewed literature where the efficacy of benztropine for treating TD could be reliably demonstrated. Furthermore, some literature suggests that anticholinergics such as benztropine increase the risk of developing TD.5,10 The mechanism underlying benztropine’s ability to precipitate or exacerbate abnormal movements is unclear, though it is theorized that anticholinergic medications may inhibit dopamine reuptake into neurons, thus leading to an excess of dopamine in the synaptic cleft that manifests as dyskinesias.10 Some authors also recommend that the first step in the management of TD should be to gradually discontinue anticholinergics, as this has been associated with improvement in TD.11
Continue to: Prescribing anticholinergics in specific patient populations...
Prescribing anticholinergics in specific patient populations
In addition to the adverse effects described above, benztropine can affect cognition, as we observed in Ms. P. The cholinergic system plays a role in human cognition, and blockade of muscarinic receptors has been associated with impairments in working memory and prefrontal tasks.12 These adverse cognitive effects are more pronounced in certain populations, including patients with schizophrenia and older adults.
Schizophrenia is associated with declining cognitive function, and the cognitive faculties of patients with schizophrenia may be worsened by anticholinergics. In patients with schizophrenia, social interactions and social integration are often impacted by profound negative symptoms such as social withdrawal and poverty of thought and speech.13 In a double-blind study by Baker et al,14 benztropine was found to have an impact on attention and concentration in patients with chronic schizophrenia. Baker et al14 found that patients with schizophrenia who were switched from benztropine to placebo increased their overall Wechsler Memory Scale scores compared to those maintained on benztropine. One crosssectional analysis found that a higher anticholinergic burden was associated with impairments across all cognitive domains, including memory, attention/control, executive and visuospatial functioning, and motor speed domains.15 Importantly, a higher anticholinergic medication burden was associated with worse cognitive performance.15 In addition to impairments in cognitive processing, anticholinergics have been associated with a decreased ability to benefit from psychosocial programs and impaired abilities to manage activities of daily living.4 In another study exploring the effects of discontinuing anticholinergics and the impact on movement disorders, Desmarais et al16 found patients experienced a significant improvement in scores on the Brief Assessment of Cognition in Schizophrenia after discontinuing anticholinergics. Vinogradov et al17 noted that “serum anticholinergic activity in schizophrenia patients shows a significant association with impaired performance in measures of verbal working memory and verbal learning memory and was significantly associated with a lowered response to an intensive course of computerized cognitive training.” They felt their findings underscored the cognitive cost of medications with high anticholinergic burden.
Geriatric patients. Careful consideration should be given before starting benztropine in patients age ≥65. The 2019 American Geriatric Society’s Beers Criteria18 recommend avoiding benztropine in geriatric patients; the level of recommendation is strong. Furthermore, the American Geriatric Society designates benztropine as a medication that should be avoided, and a nondrug approach or alternative medication be prescribed independent of the patient’s condition or diagnosis. In a recently published case report, Esang et al19 highlighted several salient findings from previous studies on the risks associated with anticholinergic use:
- any medications a patient takes with anticholinergic properties contribute to the overall anticholinergic load of a patient’s medication regimen
- the higher the anticholinergic burden, the greater the cognitive deficits
- switching from an FGA to an SGA may decrease the risk of EPS and may limit the need for anticholinergic medications such as benztropine for a particular patient.
One must also consider that the effects of multiple medications with anticholinergic properties is probably cumulative.
Alternatives for treating drug-induced parkinsonism
Antipsychotics exert their effects through antagonism of the D2 receptor, and this is the same mechanism that leads to parkinsonism. Specifically, the mechanism is believed to be D2 receptor antagonism in the striatum leading to disinhibition of striatal neurons containing GABA.11 This disinhibition of medium spiny neurons is propagated when acetylcholine is released from cholinergic interneurons. Anticholinergics such as benztropine can remedy symptoms by blocking the signal of acetylcholine on the M1 receptors on medium spiny neurons. However, benztropine also has the propensity to decrease cholinergic transmission, thereby impairing storage of new information into long-term memory as well as impair perception of time—similar to effects seen with (for instance) diphenhydramine.20
The first step in managing drug-induced parkinsonism is to monitor symptoms. The APA Guideline recommends monitoring for acute-onset EPS at weekly intervals when beginning treatment and until stable for 2 weeks, and then monitoring at every follow-up visit thereafter.4 The next recommendation for long-term management of drug-induced parkinsonism is reducing the antipsychotic dose, or replacing the patient’s antipsychotic with an antipsychotic that is less likely to precipitate parkinsonism,4 such as quetiapine, iloperidone, or clozapine.11 If dose reduction is not possible, and the patient’s symptoms are severe, pharmacologic management is indicated. The APA Guideline recommends amantadine as a first-line agent because it is associated with fewer peripheral adverse effects and less impairment in cognition compared with benztropine.4 In a small (N = 60) doubleblind crossover trial, Gelenberg et al20 found benztropine 4 mg/d—but not amantadine 200 mg/d—impaired free recall and perception of time, and participants’ perception of their own memory impairment was significantly greater with benztropine. Amantadine has also been compared to biperiden, a relatively selective M1 muscarinic receptor muscarinic agent. In a separate double-blind crossover study of 26 patients with chronic schizophrenia, Silver and Geraisy21 found that compared to amantadine, biperiden was associated with worse memory performance. The recommended starting dose of amantadine for parkinsonism is 100 mg in the morning, increased to 100 mg twice a day and titrated to a maximum daily dose of 300 mg/d in divided doses.4
Continue to: Alternatives for treating drug-induced akathisia...
Alternatives for treating drug-induced akathisia
Akathisia remains a relatively common adverse effect of SGAs, and the profound physical distress and impaired functioning caused by akathisia necessitates pharmacologic treatment. Despite frequent use in practice for presumed benefit in akathisia, benztropine is not effective for the treatment of akathisia and the APA Guideline recommends that long-term management should begin with an antipsychotic dose reduction, followed by a switch to an agent with less propensity to incite akathisia.4 Acute manifestations of akathisia must be treated, and mirtazapine, propranolol, or clonazepam may be considered as alternatives.4 Mirtazapine is dosed 7.5 mg to 10 mg nightly for akathisia, though it should be used in caution in patients at risk for mania.4 Mirtazapine’s potent 5-HT2A blockade at low doses may contribute to its utility in treating akathisia.2 Propranolol, a nonselective lipophilic beta-adrenergic antagonist, also has demonstrated efficacy in managing akathisia, with recommended dosing of 40 mg to 80 mg twice daily.2 Benzodiazepines such as clonazepam require judicious use for akathisia because they may also precipitate or exacerbate cognitive impairment.4
Alternatives for treating TD
As mentioned above, benztropine is not recommended for the treatment of TD.1 The Box4,22,23 outlines potential treatment options for TD.
Box
Monitoring is the first step in the prevention of tardive dyskinesia (TD). The American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia recommends patients receiving first-generation antipsychotics (FGAs) be monitored every 6 months, those prescribed second-generation antipsychotics (SGAs) be monitored every 12 months, and twice as frequent monitoring for geriatric patients and those who developed involuntary movements rapidly after starting an antipsychotic.4
The APA Guideline recommends decreasing or gradually tapering antipsychotics as another strategy for preventing TD.4 However, these recommendations should be weighed against the risk of short-term antipsychotic withdrawal. Withdrawal of D2 antagonists is associated with worsening of dyskinesias or withdrawal dyskinesia and psychotic decompensation.22
Current treatment recommendations give preference to the importance of preventing development of TD by tapering to the lowest dose of antipsychotic needed to control symptoms for the shortest duration possible.22 Thereafter, if treatment intervention is needed, consideration should be given to the following pharmacological interventions in order from highest level of recommendation (Grade A) to lowest (Grade C):
A: vesicular monoamine transporter-2 inhibitors deutetrabenazine and valbenazine
B: clonazepam, ginkgo biloba
C: amantadine, tetrabenazine, and globus pallidus interna deep brain stimulation.22
There is insufficient evidence to support or refute withdrawing causative agents or switching from FGAs to SGAs to treat TD.22 Furthermore, for many patients with schizophrenia, a gradual discontinuation of their antipsychotic must be weighed against the risk of relapse.23
Valbenazine and deutetrabenazine have been demonstrated to be efficacious and are FDA-approved for managing TD. The initial dose of valbenazine is 40 mg/d. Common adverse effects include somnolence and fatigue/ sedation. Valbenazine should be avoided in patients with QT prolongation or arrhythmias. Deutetrabenazine has less impact on the cytochrome P450 2D6 enzyme and therefore does not require genotyping as would be the case for patients who are receiving >50 mg/d of tetrabenazine. The starting dose of deutetrabenazine is 6 mg/d. Adverse effects include depression, suicidality, neuroleptic malignant syndrome, parkinsonism, and QT prolongation. Deutetrabenazine is contraindicated in patients who are suicidal or have untreated depression, hepatic impairment, or concomitant use of monoamine oxidase inhibitors.22 Deutetrabenazine is an isomer of tetrabenazine; however, evidence supporting the parent compound suggests limited use due to increased risk of adverse effects compared with valbenazine and deutetrabenazine.23 Tetrabenazine may be considered as an adjunctive treatment or used as a single agent if valbenazine or deutetrabenazine are not accessible.22
Discontinuing benztropine
Benztropine is recommended as a firstline agent for the management of acute dystonia, and it may be used temporarily for drug-induced parkinsonism, but it is not recommended to prevent EPS or TD. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic agent such as benztropine. Based on their review of earlier studies, Desmarais et al5 suggest a gradual 3-month discontinuation of benztropine. Multiple studies have demonstrated an ability to taper anticholinergics in days to months.4 However, gradual discontinuation is advisable to avoid cholinergic rebound and the reemergence of EPS, and to decrease the risk of neuroleptic malignant syndrome associated with sudden discontinuation.5 One suggested taper regimen is a decrease of 0.5 mg benztropine every week. Amantadine may be considered if parkinsonism is noted during the taper. Patients on benztropine may develop rebound symptoms, such as vivid dreams/nightmares; if this occurs, the taper rate can be slowed to a decrease of 0.5 mg every 2 weeks.4
Continue to: First do no harm...
First do no harm
Psychiatrists commonly prescribe benztropine to prevent EPS and TD, but available literature does not support the efficacy of benztropine for mitigating drug-induced parkinsonism, and studies report benztropine may significantly worsen cognitive processes and exacerbate TD.16 In addition, benztropine misuse has been correlated with euphoria and psychosis.16 More than 3 decades ago, the World Health Organization Heads of Centres Collaborating in WHO-Coordinated Studies on Biological Aspects of Mental Illness issued a consensus statement24 discouraging the prophylactic use of anticholinergics for patients receiving antipsychotics, yet we still see patients on an indefinite regimen of benztropine.
As clinicians, our goals should be to optimize a patient’s functioning and quality of life, and to use the lowest dose of medication along with the fewest medications necessary to avoid adverse effects such as EPS. Benztropine is recommended as a first-line agent for the management of acute dystonia, but its continued or indefinite use to prevent antipsychotic-induced adverse effects is not recommended. While all pharmacologic interventions carry a risk of adverse effects, weighing the risk of those effects against the clinical benefits is the prerogative of a skilled clinician. Benztropine and other anticholinergics prescribed for prophylactic purposes have numerous adverse effects, limited clinical utility, and a deleterious effect on quality of life. Furthermore, benztropine prophylaxis of drug-induced parkinsonism does not seem to be warranted, and the risks do not seem to outweigh the harm benztropine may cause, with the possible exception of “prophylactic” treatment of dystonia that is discontinued in a few days, as some researchers have suggested.6-8 The preventive value of benztropine has not been demonstrated. It is time we took inventory of medications that might cause more harm than good, rely on current treatment guidelines instead of habit, and use these agents judiciously while considering replacement with novel, safer medications whenever possible.
CASE CONTINUED
The clinical team considers benztropine’s ability to cause cognitive effects, and decides to taper and discontinue it over 1 month. Ms. P is seen in an outpatient clinic within 1 month of discontinuing benztropine. She reports that her difficulty remembering words and details has improved. She also says that she is now able to concentrate on writing and reading. The consulting neurologist also notes improvement. Ms. P continues to report improvement in symptoms over the next 2 months of follow-up, and says that her mood improved and she has less apathy.
Bottom Line
Benztropine is a first-line medication for acute dystonia, but its continued or indefinite use for preventing antipsychotic-induced adverse effects is not recommended. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic medication such as benztropine.
1. Cogentin [package insert]. McPherson, KS: Lundbeck Inc; 2013.
2. Poyurovsky M, Weizman A. Treatment of antipsychoticrelated akathisia revisited. J Clin Psychopharmacol. 2015; 35(6):711-714.
3. Salem H, Nagpal C, Pigott T, et al. Revisiting antipsychoticinduced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
4. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd ed. American Psychiatric Association; 2021.
5. Desmarais JE, Beauclair L, Margolese HC. Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol. 2012;26(9):1167-1174.
6. Boyer WF, Bakalar NH, Lake CR. Anticholinergic prophylaxis of acute haloperidol-induced acute dystonic reactions. J Clin Psychopharmacol. 1987;7(3):164-166.
7. Winslow RS, Stillner V, Coons DJ, et al. Prevention of acute dystonic reactions in patients beginning high-potency neuroleptics. Am J Psychiatry. 1986;143(6):706-710.
8. Stern TA, Anderson WH. Benztropine prophylaxis of dystonic reactions. Psychopharmacology (Berl). 1979; 61(3):261-262.
9. Bergman H, Soares‐Weiser K. Anticholinergic medication for antipsychotic‐induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1(1):CD000204. doi:10.1002/ 14651858.CD000204.pub2
10. Howrie DL, Rowley AH, Krenzelok EP. Benztropineinduced acute dystonic reaction. Ann Emerg Med. 1986;15(5):594-596.
11. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs. tardive dyskinesia--key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2): 233-248.
12. Wijegunaratne H, Qazi H, Koola M. Chronic and bedtime use of benztropine with antipsychotics: is it necessary? Schizophr Res. 2014;153(1-3):248-249.
13. Möller HJ. The relevance of negative symptoms in schizophrenia and how to treat them with psychopharmaceuticals? Psychiatr Danub. 2016;28(4):435-440.
14. Baker LA, Cheng LY, Amara IB. The withdrawal of benztropine mesylate in chronic schizophrenic patients. Br J Psychiatry. 1983;143:584-590.
15. Joshi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry. 2021;178(9):838-847.
16. Desmarais JE, Beauclair E, Annable L, et al. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Ther Adv Psychopharmacol. 2014;4(6): 257-267.
17. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9): 1055-1062.
18. American Geriatrics Society 2019 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
19. Esang M, Person US, Izekor OO, et al. An unlikely case of benztropine misuse in an elderly schizophrenic. Cureus. 2021;13(2):e13434. doi:10.7759/cureus.13434
20. Gelenberg AJ, Van Putten T, Lavori PW, et al. Anticholinergic effects on memory: benztropine versus amantadine. J Clin Psychopharmacol. 1989;9(3):180-185.
21. Silver H, Geraisy N. Effects of biperiden and amantadine on memory in medicated chronic schizophrenic patients. A double-blind cross-over study. Br J Psychiatry. 1995; 166(2):241-243.
22. Bhidayasiri R, Jitkritsadakul O, Friedman J, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67-75.
23. Ricciardi L, Pringsheim T, Barnes TRE, et al. Treatment recommendations for tardive dyskinesia. Canadian J Psychiatry. 2019;64(6):388-399.
24. Prophylactic use of anticholinergics in patients on long-term neuroleptic treatment. A consensus statement. World Health Organization heads of centres collaborating in WHO coordinated studies on biological aspects of mental illness. Br J Psychiatry. 1990;156:412.
1. Cogentin [package insert]. McPherson, KS: Lundbeck Inc; 2013.
2. Poyurovsky M, Weizman A. Treatment of antipsychoticrelated akathisia revisited. J Clin Psychopharmacol. 2015; 35(6):711-714.
3. Salem H, Nagpal C, Pigott T, et al. Revisiting antipsychoticinduced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
4. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd ed. American Psychiatric Association; 2021.
5. Desmarais JE, Beauclair L, Margolese HC. Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol. 2012;26(9):1167-1174.
6. Boyer WF, Bakalar NH, Lake CR. Anticholinergic prophylaxis of acute haloperidol-induced acute dystonic reactions. J Clin Psychopharmacol. 1987;7(3):164-166.
7. Winslow RS, Stillner V, Coons DJ, et al. Prevention of acute dystonic reactions in patients beginning high-potency neuroleptics. Am J Psychiatry. 1986;143(6):706-710.
8. Stern TA, Anderson WH. Benztropine prophylaxis of dystonic reactions. Psychopharmacology (Berl). 1979; 61(3):261-262.
9. Bergman H, Soares‐Weiser K. Anticholinergic medication for antipsychotic‐induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1(1):CD000204. doi:10.1002/ 14651858.CD000204.pub2
10. Howrie DL, Rowley AH, Krenzelok EP. Benztropineinduced acute dystonic reaction. Ann Emerg Med. 1986;15(5):594-596.
11. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs. tardive dyskinesia--key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2): 233-248.
12. Wijegunaratne H, Qazi H, Koola M. Chronic and bedtime use of benztropine with antipsychotics: is it necessary? Schizophr Res. 2014;153(1-3):248-249.
13. Möller HJ. The relevance of negative symptoms in schizophrenia and how to treat them with psychopharmaceuticals? Psychiatr Danub. 2016;28(4):435-440.
14. Baker LA, Cheng LY, Amara IB. The withdrawal of benztropine mesylate in chronic schizophrenic patients. Br J Psychiatry. 1983;143:584-590.
15. Joshi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry. 2021;178(9):838-847.
16. Desmarais JE, Beauclair E, Annable L, et al. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Ther Adv Psychopharmacol. 2014;4(6): 257-267.
17. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9): 1055-1062.
18. American Geriatrics Society 2019 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
19. Esang M, Person US, Izekor OO, et al. An unlikely case of benztropine misuse in an elderly schizophrenic. Cureus. 2021;13(2):e13434. doi:10.7759/cureus.13434
20. Gelenberg AJ, Van Putten T, Lavori PW, et al. Anticholinergic effects on memory: benztropine versus amantadine. J Clin Psychopharmacol. 1989;9(3):180-185.
21. Silver H, Geraisy N. Effects of biperiden and amantadine on memory in medicated chronic schizophrenic patients. A double-blind cross-over study. Br J Psychiatry. 1995; 166(2):241-243.
22. Bhidayasiri R, Jitkritsadakul O, Friedman J, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67-75.
23. Ricciardi L, Pringsheim T, Barnes TRE, et al. Treatment recommendations for tardive dyskinesia. Canadian J Psychiatry. 2019;64(6):388-399.
24. Prophylactic use of anticholinergics in patients on long-term neuroleptic treatment. A consensus statement. World Health Organization heads of centres collaborating in WHO coordinated studies on biological aspects of mental illness. Br J Psychiatry. 1990;156:412.
Autism spectrum disorder in children and adolescents: Treatment options
SECOND OF 2 PARTS
Evidence supports the crucial role of early intervention and nonpharmacologic approaches
A large percentage of individuals with autism spectrum disorder (ASD) experience persisting significant social deficits in adulthood,1 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.2,3 Childhood is a vital time for making the most significant and lasting changes that can improve functioning of individuals with ASD. Psychiatrists and other physicians who treat children are in a key role to influence outcomes of children at risk for or diagnosed with ASD.
This article provides updates on various aspects of ASD diagnosis and treatment (based on available evidence up to March 2020). Part 1 (
A comprehensive approach is essential
Multiple treatment modalities have been recommended for ASD.5 It is essential to address all aspects of ASD through cognitive, developmental, social-communication, sensory-motor, and behavioral interventions. Nonpharmacologic interventions are crucial in improving long-term outcomes of children with ASD.6
Nonpharmacologic treatments
Nonpharmacologic interventions commonly utilized for children with ASD include behavioral therapies, other psychological therapies, speech-language therapy, occupational therapy, educational interventions, parent coaching/training, developmental social interventions, and other modalities of therapy that are delivered in school, home, and clinic settings.5,7
A recent study examining ASD treatment trends via caregivers’ reports (N = 5,122) from the SPARK (Simons Foundation Powering Autism Research for Knowledge) cohort in the United States reported that 80% of children received speech-language therapy or occupational therapy; 52% got both.5 The study revealed that approximately one-quarter utilized 3 therapies simultaneously; two-thirds had utilized 3 or more therapies in the previous year.5
Interventions for children with ASD need to be individualized.1,8 Evidence-based behavioral interventions for ASD fall into 2 broad categories: Applied Behavior Analysis (ABA), and Naturalistic Developmental Behavioral Interventions (NDBI). Traditionally, ABA has been a key model, guiding treatment for enhancing social-communicating skills and lowering maladaptive behaviors in ASD.9 ABA follows a structured and prescribed format,10,11 and has been shown to be efficacious.1,7 More recently, NDBI, in which interventions are “embedded” in the natural environment of the young child and more actively incorporate a developmental perspective, has been shown to be beneficial in improving and generalizing social-communication skills in young children with ASD.7,11
Early Start Denver Model (ESDM) is an intensive, naturalistic behavioral intervention4 that has been shown to be efficacious for enhancing communication and adaptive behavior in children with ASD.7,8,12 A multisite randomized controlled trial (RCT) by Rogers et al12 that examined the efficacy of ESDM in 118 children (age 14 to 24 months) with ASD found the treatment was beneficial and superior compared with a “community intervention” group, in regards to language ability measured in time by group analyses.The ESDM intervention in this study involved weekly parent coaching for 3 months, along with 24 months of 15 hours/week of one-on-one treatment provided by therapy professionals.12
Reciprocal imitation training (RIT) is another naturalistic intervention that has shown benefit in training children with ASD in imitation skills during play.13 Studies have found that both RIT and ESDM can be parent-implemented, after parents receive training.13,14
Parent-mediated, parent-implemented interventions may have a role in improving outcomes in childhood ASD,7,15 particularly “better generalization and maintenance of skills than therapist-implemented intervention” for lowering challenging behaviors and enhancing verbal and nonverbal communication.16
Various social skills interventions have also been found effective for children with ASD.1 Such interventions are often provided in the school setting.7 Coordination with the child’s school to discuss and advocating for adequate and suitable interventions, educational services, and placement is an essential aspect of ASD treatment.7
Two other school-based, comprehensive treatment model interventions—Learning Experiences and Alternative Programs for Preschoolers and their Parents (LEAP), and TEACCH—have some evidence of leading to improvement in children with ASD.7,17
Some studies have found that music therapy may have high efficacy for children with ASD, even with smaller length and intensity of treatment, particularly in improving social interaction, engagement with parents, joint attention, and communication.3,18 Further research is needed to conclusively establish the efficacy of music therapy for ASD in children and adolescents.
A few studies have assessed the long-term outcomes of interventions for ASD; however, more research is needed.19 Pickles et al19 conducted a follow-up to determine the long-term effects of the Preschool Autism Communication Trial (PACT), an RCT of parent-mediated social communication therapy for children age 2 to 4 with ASD. The children’s average age at follow-up was 10 years. The authors found a significant long-term decrease in ASD symptoms and enhancement of social communication with parents (N = 152).
Technology-based interventions, including games and robotics, have been investigated in recent years, for treatment of children with ASD (eg, for improving social skills).20
Research suggests that the intensity (number of hours) and duration of nonpharmacologic treatments for ASD is critical to improving outcomes (Box1,3,5,7,10,16).
Box
A higher intensity of nonpharmacologic intervention (greater number of hours) has been associated with greater benefit for children with autism spectrum disorder (ASD), in the form of enhancements in IQ and adaptive behavior.1,10,16 In the United States, the intensity of interventions commonly ranges from 30 to 200 or more minutes per week.3 This may mean that a child with ASD who is receiving 30 minutes of speech therapy at school and continues to exhibit significant deficits in speech-language or social-communication may likely benefit from additional hours of speech therapy and/or social-communication skill training, and should be referred accordingly, even for private therapy services if needed and feasible.7 Guidelines created through a systematic review of evidence recommend at least 25 hours per week of comprehensive treatment interventions for children with ASD to address language, social deficits, and behavioral difficulties.1 The duration of intervention has also been shown to play a role in outcomes.1,3,10 Given the complexity and extent of impairment often associated with ASD, it is not surprising that in recent research examining trends in ASD treatment in the United States, most caregivers reported therapy as ongoing.5 The exact intensity and duration of nonpharmacologic interventions may depend on several factors, such as severity of ASD and of the specific deficit being targeted, type of intervention, and therapist skill. The quality of skills of the care provider has also been shown to affect the benefits gained from the intervention.3
Continue to: Pharmacotherapy...
Pharmacotherapy
Medications cannot resolve core features of ASD.21 However, certain medications may help address associated comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), depression, or others, when these conditions have not responded to nonpharmacologic interventions.7,22 Common symptoms that are often treated with pharmacotherapy include aggression, irritability, hyperactivity, attentional difficulties, tics, self-injurious behavior, obsessive-compulsive symptoms, and mood dysregulation/lability.23 Generally speaking, medications might be considered if symptoms are severe and markedly impair functioning. For mild to moderate conditions, psychotherapy and other nonpharmacologic interventions are generally considered first-line. Since none of the medications described below are specific to ASD and psychiatrists generally receive training in prescribing them for other indications, a comprehensive review of their risks and benefits is beyond the scope of this article. No psychotropic medications are known to have robust evidence for safety in preschool children with ASD, and thus are best avoided.
Antipsychotics. Risperidone (for age 5 and older) and aripiprazole (age 6 to 17) are the only medications FDA-approved for use in children and adolescents with ASD, specifically for irritability associated with ASD.21,24 These 2 second-generation antipsychotics may also assist in lowering aggression in patients with ASD.24 First-generation antipsychotics such as haloperidol have been shown to be effective for irritability and aggression in ASD, but the risk of significant adverse effects such as dyskinesias and extrapyramidal symptoms limit their use.24 Two studies (a double-blind study and an open-label extension of that study) in children and adolescents with ASD found that risperidone was more effective and better tolerated than haloperidol in behavioral measures, impulsivity, and even in the social domain.25,26 In addition to other adverse effects and risks, increased prolactin secondary to risperidone use requires close monitoring and caution.24-26 As is the case with the use of other psychotropic medications in children and adolescents, those with ASD who receive antipsychotics should also be periodically reassessed to determine the need for continued use of these medications.27 A multicenter relapse prevention RCT found no statistically significant difference in the time to relapse between aripiprazole and placebo.27 Metabolic syndrome, cardiac risks, and other risks need to be considered before prescribing an antipsychotic.28 Given their serious adverse effects profile, use should be considered only when there is severe impairment or risk of injury, after carefully weighing risks/benefits.
Medications for attentional difficulties. A multisite, randomized, placebo-controlled trial evaluating the use of extended-release guanfacine in children with ASD (N = 62) found the rate of positive response on the Clinical Global Impressions–Improvement scale was 50% for guanfacine vs 9.4% for placebo.29 Clinicians need to monitor for adverse effects of guanfacine, such as fatigue, drowsiness, lightheadedness, lowering of blood pressure and heart rate, and other effects.29 A randomized, double-blind trial of 97 children and adolescents with ASD and ADHD found that atomoxetine had moderate benefit for ADHD symptoms.30 The study reported no serious adverse effects.30 However, it is especially important to monitor for hepatic and cardiac adverse effects (in addition to monitoring for risk of increase in suicidal thoughts/behavior, as in the case of antidepressants) when using atomoxetine, in addition to other side effects and risks. Some evidence suggests that methylphenidate may be effective for attentional difficulties in children and adolescents with ASD21 but may pose a higher risk of adverse effects in this population compared with neurotypical patients.31
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are sometimes used to reduce obsessive-compulsive symptoms, repetitive behavior, or depressive symptoms in children with ASD, but are not FDA-approved for children or adolescents with ASD. In general, there is inadequate evidence to support the use of SSRIs for ASD in children.31-34 In addition, children with ASD may be at a greater risk of adverse effects from SSRIs.32,34 Despite this, SSRIs are the most commonly prescribed psychotropic medications in children with ASD.32
An RCT examining the efficacy of fluoxetine in 158 children and adolescents with ASD found no significant difference in Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) score after 14 weeks of treatment; activation was a common adverse effect.35 A 2005 randomized, double-blind, placebo-controlled trial of 45 children/adolescents with ASD found that low-dose liquid fluoxetine was more effective than placebo for reducing repetitive behaviors in this population.36 Larger studies are warranted to further evaluate the efficacy and safety of fluoxetine (and of SSRIs in general, particularly in the long term) for children and adolescents with ASD.36 A 2009 randomized, placebo-controlled trial of 149 children with ASD revealed no significant difference between citalopram and placebo as measured by Clinical Global Impressions scale or CY-BOCS scores, and noted a significantly elevated likelihood of adverse effects.37
Other antidepressants. There is insufficient evidence to support the use of any other antidepressants in children and adolescents with ASD. A few studies38,39 have examined the use of venlafaxine in children with ASD; however, further research and controlled studies with large sample sizes are required to conclusively establish its benefits. There is a dearth of evidence examining the use of the tetracyclic antidepressant mirtazapine, or other classes of medications such as tricyclic antidepressants or mood stabilizers, in children with ASD; only a few small studies have assessed the efficacy and adverse effects of these medications for such patients.31
Polypharmacy. Although there is no evidence to support polypharmacy in children and adolescents with ASD, the practice appears to be rampant in these patients.28,40 A 2013 retrospective, observational study of psychotropic medication use in children with ASD (N = 33,565) found that 64% were prescribed psychotropic medications, and 35% exhibited evidence of polypharmacy.40 In this study, the total duration of polypharmacy averaged 525 days.40 When addressing polypharmacy, systematic deprescribing or simplification of the psychotropic medication regimen may be needed,28 while taking into account the patient’s complete clinical situation, including (but not limited to) tolerability of the medication regimen, presence or absence of current stressors, presence or absence of adequate supports, use of nonpharmacologic treatments where appropriate, and other factors.
More studies assessing the efficacy and safety of psychotropic medications for children and adolescents with ASD are needed,32 especially studies that evaluate the effects of long-term use, because evidence for pharmacologic treatments for children with ASD is mixed and insufficient.33 There is also a need for evidence-based standards for prescribing psychotropic medications in children and adolescents with ASD.
Psychotropic medications, if used in ASD, should be used only in conjunction with other evidence-based treatment modalities, and not as monotherapy.21 Children and adolescents with ASD may be particularly susceptible to side effects or adverse effects of certain psychotropic medications.31 When considering medications, carefully weigh the risks and benefits.7,21,24,28 Starting low and going slow is generally the preferred strategy.31,32 As always, when recommending medications, discuss in detail with parents the potential side effects, benefits, risks, interactions, and alternatives.
Other agents. Several double-blind, placebo-controlled trials have evaluated using melatonin for sleep difficulties in children and adolescents with ASD.41 A randomized, placebo-controlled, 12-week trial that assessed 160 children with ASD and insomnia found that melatonin plus cognitive-behavioral therapy (CBT) was superior in efficacy to melatonin alone, CBT alone, or placebo.41
The evidence regarding oxytocin use for children with ASD is mixed.31 Some small studies have associated improvement in the social domain with its use. Guastella et al42 conducted a randomized, double-blind, placebo-controlled trial of oxytocin nasal spray for 16 participants (age 12 to 19) with ASD, and found oxytocin enhanced emotional identification. Gordon et al43 conducted a functional MRI study of brain activity with oxytocin use in children with high-functioning ASD (N = 17). They found that oxytocin may augment “salience and hedonic evaluations of socially meaningful stimuli in children with ASD” and thus help social attunement. Further research is needed to evaluate the impact of oxytocin on social behavior.
Complementary and alternative medicine. Although there is limited and inconclusive evidence about the use of complementary and alternative medicine in children and adolescents with ASD, these therapies continue to be commonly used.44-46 A recent survey of parents (N = 211) of children with ASD from academic ASD outpatient clinics in Germany found that 46% reported their child was using or had used some type of complementary and alternative medicine.44 There is inadequate evidence to support the use of a gluten-free, casein-free diet for children/adolescents with ASD.46 A recent cross-sectional study assessing supplement use in 210 children with ASD in Canada found that 75% used supplements, such as multivitamins (77.8%), vitamin D (44.9%), omega 3 (42.5%), probiotics (36.5%), and magnesium (28.1%), despite insufficient evidence to support their safety or efficacy for children with ASD.47 Importantly, 33.5% of parents in this study reported that they did not inform the physician about all their child’s supplements.47 Some of the reasons the parents in this study provided for not disclosing information about supplements to their physicians were “physician lack of knowledge,” “no benefit,” “too time-consuming,” and “scared of judgment.”47 Semi-structured interviews of parents of 21 children with ASD in Australia revealed that parents found information on complementary and alternative medicine and therapies complex and often conflicting.45 In addition to recommendations from health care professionals, evidence suggests that parents often consider the opinions of media, friends, and family when making a decision on using complementary and alternative medicine modalities for children/adolescents with ASD.46 Such findings can inform physician practices regarding supplement use, and highlight the need to educate parents about the evidence regarding these therapies and potential adverse effects and interactions of such therapies,46 along with the need to develop a centralized, evidence-based resource for parents regarding their use.45
Omega 3 supplementation has in general shown few adverse effects47; still, risks/benefits need to be weighed before use. Some evidence suggests that it may decrease hyperactivity in children with ASD.31,48 However, further research, particularly controlled trials with large sample sizes, are needed for a definitive determination of efficacy.31,48 A meta-analysis that included 27 RCTs assessing the efficacy of dietary interventions for various ASD symptoms found that omega 3 supplementation was more effective than placebo, but compared with placebo, the effect size was small.49 A RCT of 73 children with ASD in New Zealand found that omega 3 long chain polyunsaturated fatty acids may benefit some core symptoms of ASD; the authors suggested that further research is needed to conclusively establish efficacy.50
Continue to: A need for advocacy and research..
A need for advocacy and research
Physicians who treat children with ASD can not only make appropriate referrals and educate parents, but also educate their patients’ schools and advocate for their patients to get the level of services they need.23,28
A recent study in the United States found that behavior therapy and speech-language therapy were used less often in the treatment of children with ASD in rural areas compared with those in metro areas.5 This suggests that in addition to increasing parents’ awareness and use of ASD services and providing referrals where appropriate, physicians are in a unique position to advocate for public health policies to improve access, coverage, and training for the provision of such services in rural areas.
There is need for ongoing research to further examine the efficacy and nuances of effects of various treatment interventions for ASD, especially long-term studies with larger sample sizes.11,51 Additionally, research is warranted to better understand the underlying genetic and neurobiological mechanisms of ASD, which would help guide the development of biomarkers,52 innovative treatments, and disease-modifying agents for ASD.7,22 Exploring the effects of potential alliances or joint action between biological and psychosocial interventions for ASD is also an area that needs further research.51
Bottom Line
A combination of treatment modalities (such as speech-language therapy, social skills training, behavior therapy/other psychotherapy, and occupational therapy for sensory sensitivities) is generally needed to improve the long-term outcomes of children and adolescents with autism spectrum disorder (ASD). In addition to the importance of early intervention, the intensity and duration of nonpharmacologic treatments are vital to improving outcomes in ASD.
1. Maglione MA, Gans D, Das L, et al. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2):S169-S178.
2. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
3. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
4. Charman T. Editorial: trials and tribulations in early autism intervention research. J Am Acad Child Adolesc Psychiatry. 2019;58(9):846-848. doi:10.1016/j.jaac.2019.03.004
5. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):517-526. doi:10.1002/aur.2070
6. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi:10.3389/fnins.2016.00393
7. Hyman SL, Levy SE, Myers SM, et al. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
8. Contaldo A, Colombi C, Pierotti C, et al. Outcomes and moderators of Early Start Denver Model intervention in young children with autism spectrum disorder delivered in a mixed individual and group setting. Autism. 2020;24(3):718-729. doi:10.1177/1362361319888344
9. Lei J, Ventola P. Pivotal response treatment for autism spectrum disorder: current perspectives. Neuropsychiatr Dis Treat. 2017;13:1613-1626. doi:10.2147/NDT.S120710
10. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
11. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
12. Rogers SJ, Estes A, Lord C, et al. A multisite randomized controlled two-phase trial of the Early Start Denver Model compared to treatment as usual. J Am Acad Child Adolesc Psychiatry. 2019;58(9):853-865. doi:10.1016/j.jaac.2019.01.004
13. Ingersoll B, Gergans S. The effect of a parent-implemented imitation intervention on spontaneous imitation skills in young children with autism. Res Dev Disabil. 2007;28(2):163-175.
14. Waddington H, van der Meer L, Sigafoos J, et al. Examining parent use of specific intervention techniques during a 12-week training program based on the Early Start Denver Model. Autism. 2020;24(2):484-498. doi:10.1177/1362361319876495
15. Trembath D, Gurm M, Scheerer NE, et al. Systematic review of factors that may influence the outcomes and generalizability of parent‐mediated interventions for young children with autism spectrum disorder. Autism Res. 2019;12(9):1304-1321.
16. Rogers SJ, Estes A, Lord C, et al. Effects of a brief Early Start Denver Model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
17. Boyd BA, Hume K, McBee MT, et al. Comparative efficacy of LEAP, TEACCH and non-model-specific special education programs for preschoolers with autism spectrum disorders. J Autism Dev Disord. 2014;44(2):366-380. doi:10.1007/s10803-013-1877-9
18. Thompson GA, McFerran KS, Gold C. Family-centred music therapy to promote social engagement in young children with severe autism spectrum disorder: a randomized controlled study. Child Care Health Dev. 2014;40(6):840-852. doi:10.1111/cch.12121
19. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
20. Grossard C, Palestra G, Xavier J, et al. ICT and autism care: state of the art. Curr Opin Psychiatry. 2018;31(6):474-483. doi:10.1097/YCO.0000000000000455
21. Cukier S, Barrios N. Pharmacological interventions for intellectual disability and autism. Vertex. 2019;XXX(143)52-63.
22. Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91-104.
23. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(2):237-257.
24. LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. P T. 2015;40(6):389-397.
25. Gencer O, Emiroglu FN, Miral S, et al. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder. An open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217-225.
26. Miral S, Gencer O, Inal-Emiroglu FN, et al. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry. 2008;17(1):1-8.
27. Findling RL, Mankoski R, Timko K, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22-30. doi:10.4088/jcp.13m08500
28. McLennan JD. Deprescribing in a youth with an intellectual disability, autism, behavioural problems, and medication-related obesity: a case study. J Can Acad Child Adolesc Psychiatry. 2019;28(3):141-146.
29. Scahill L, McCracken JT, King B, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206. doi:10.1176/appi.ajp.2015.15010055
30. Harfterkamp M, van de Loo-Neus G, Minderaa RB, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733-741. doi:10.1016/j.jaac.2012.04.011
31. DeFilippis M, Wagner KD. Treatment of autism spectrum disorder in children and adolescents. Psychopharmacol Bull. 2016;46(2):18-41.
32. DeFilippis M. Depression in children and adolescents with autism spectrum disorder. Children (Basel). 2018;5(9):112. doi:10.3390/children5090112
33. Goel R, Hong JS, Findling RL, et al. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psychiatry. 2018;30(1):78-95. doi:10.1080/09540261.2018.1458706
34. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677. doi:10.1002/14651858.CD004677.pub3
35. Herscu P, Handen BL, Arnold LE, et al. The SOFIA study: negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord. 2020;50(9):3233-3244. doi:10.1007/s10803-019-04120-y
36. Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582-589.
37. King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583-590. doi:10.1001/archgenpsychiatry.2009.30
38. Hollander E, Kaplan A, Cartwright C, et al. Venlafaxine in children, adolescents, and young adults with autism spectrum disorders: an open retrospective clinical report. J Child Neurol. 2000;15(2):132-135.
39. Carminati GG, Deriaz N, Bertschy G. Low-dose venlafaxine in three adolescents and young adults with autistic disorder improves self-injurious behavior and attention deficit/hyperactivity disorders (ADHD)-like symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):312-315.
40. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840. doi:10.1542/peds.2012-3774
41. Cortesi F, Giannotti F, Sebastiani T, et al. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res. 2012;21(6):700-709. doi:10.1111/j.1365-2869.2012.01021.x
42. Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692-694. doi:10.1016/j.biopsych.2009.09.020
43. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
44. Höfer J, Bachmann C, Kamp-Becker I, et al. Willingness to try and lifetime use of complementary and alternative medicine in children and adolescents with autism spectrum disorder in Germany: a survey of parents. Autism. 2019;23(7):1865-1870. doi:10.1177/1362361318823545
45. Smith CA, Parton C, King M, et al. Parents’ experiences of information-seeking and decision-making regarding complementary medicine for children with autism spectrum disorder: a qualitative study. BMC Complement Med Ther. 2020;20(1):4. doi:10.1186/s12906-019-2805-0
46. Marsden REF, Francis J, Garner I. Use of GFCF diets in children with ASD. An investigation into parents’ beliefs using the theory of planned behaviour. J Autism Dev Disord. 2019;49(9):3716-3731. doi:10.1007/s10803-019-04035-8
47. Trudeau MS, Madden RF, Parnell JA, et al. Dietary and supplement-based complementary and alternative medicine use in pediatric autism spectrum disorder. Nutrients. 2019;11(8):1783. doi:10.3390/nu11081783
48. Bent S, Hendren RL, Zandi T, et al. Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J Am Acad Child Adolesc Psychiatry. 2014;53(6):658-666. doi:10.1016/j.jaac.2014.01.018
49. Fraguas D, Díaz-Caneja C, Pina-Camacho L, et al. Dietary interventions for autism spectrum disorder: a meta-analysis. Pediatrics. 144(5):e20183218.
50. Mazahery H, Conlon CA, Beck KL, et al. A randomised-controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children. J Autism Dev Disord. 2019;49(5):1778-1794. doi:10.1007/s10803-018-3860-y
51. Green J, Garg S. Annual research review: the state of autism intervention science: progress, target psychological and biological mechanisms and future prospects. J Child Psychol Psychiatry. 2018;59(4):424-443. doi:10.1111/jcpp.1289
52. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.53
SECOND OF 2 PARTS
Evidence supports the crucial role of early intervention and nonpharmacologic approaches
A large percentage of individuals with autism spectrum disorder (ASD) experience persisting significant social deficits in adulthood,1 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.2,3 Childhood is a vital time for making the most significant and lasting changes that can improve functioning of individuals with ASD. Psychiatrists and other physicians who treat children are in a key role to influence outcomes of children at risk for or diagnosed with ASD.
This article provides updates on various aspects of ASD diagnosis and treatment (based on available evidence up to March 2020). Part 1 (
A comprehensive approach is essential
Multiple treatment modalities have been recommended for ASD.5 It is essential to address all aspects of ASD through cognitive, developmental, social-communication, sensory-motor, and behavioral interventions. Nonpharmacologic interventions are crucial in improving long-term outcomes of children with ASD.6
Nonpharmacologic treatments
Nonpharmacologic interventions commonly utilized for children with ASD include behavioral therapies, other psychological therapies, speech-language therapy, occupational therapy, educational interventions, parent coaching/training, developmental social interventions, and other modalities of therapy that are delivered in school, home, and clinic settings.5,7
A recent study examining ASD treatment trends via caregivers’ reports (N = 5,122) from the SPARK (Simons Foundation Powering Autism Research for Knowledge) cohort in the United States reported that 80% of children received speech-language therapy or occupational therapy; 52% got both.5 The study revealed that approximately one-quarter utilized 3 therapies simultaneously; two-thirds had utilized 3 or more therapies in the previous year.5
Interventions for children with ASD need to be individualized.1,8 Evidence-based behavioral interventions for ASD fall into 2 broad categories: Applied Behavior Analysis (ABA), and Naturalistic Developmental Behavioral Interventions (NDBI). Traditionally, ABA has been a key model, guiding treatment for enhancing social-communicating skills and lowering maladaptive behaviors in ASD.9 ABA follows a structured and prescribed format,10,11 and has been shown to be efficacious.1,7 More recently, NDBI, in which interventions are “embedded” in the natural environment of the young child and more actively incorporate a developmental perspective, has been shown to be beneficial in improving and generalizing social-communication skills in young children with ASD.7,11
Early Start Denver Model (ESDM) is an intensive, naturalistic behavioral intervention4 that has been shown to be efficacious for enhancing communication and adaptive behavior in children with ASD.7,8,12 A multisite randomized controlled trial (RCT) by Rogers et al12 that examined the efficacy of ESDM in 118 children (age 14 to 24 months) with ASD found the treatment was beneficial and superior compared with a “community intervention” group, in regards to language ability measured in time by group analyses.The ESDM intervention in this study involved weekly parent coaching for 3 months, along with 24 months of 15 hours/week of one-on-one treatment provided by therapy professionals.12
Reciprocal imitation training (RIT) is another naturalistic intervention that has shown benefit in training children with ASD in imitation skills during play.13 Studies have found that both RIT and ESDM can be parent-implemented, after parents receive training.13,14
Parent-mediated, parent-implemented interventions may have a role in improving outcomes in childhood ASD,7,15 particularly “better generalization and maintenance of skills than therapist-implemented intervention” for lowering challenging behaviors and enhancing verbal and nonverbal communication.16
Various social skills interventions have also been found effective for children with ASD.1 Such interventions are often provided in the school setting.7 Coordination with the child’s school to discuss and advocating for adequate and suitable interventions, educational services, and placement is an essential aspect of ASD treatment.7
Two other school-based, comprehensive treatment model interventions—Learning Experiences and Alternative Programs for Preschoolers and their Parents (LEAP), and TEACCH—have some evidence of leading to improvement in children with ASD.7,17
Some studies have found that music therapy may have high efficacy for children with ASD, even with smaller length and intensity of treatment, particularly in improving social interaction, engagement with parents, joint attention, and communication.3,18 Further research is needed to conclusively establish the efficacy of music therapy for ASD in children and adolescents.
A few studies have assessed the long-term outcomes of interventions for ASD; however, more research is needed.19 Pickles et al19 conducted a follow-up to determine the long-term effects of the Preschool Autism Communication Trial (PACT), an RCT of parent-mediated social communication therapy for children age 2 to 4 with ASD. The children’s average age at follow-up was 10 years. The authors found a significant long-term decrease in ASD symptoms and enhancement of social communication with parents (N = 152).
Technology-based interventions, including games and robotics, have been investigated in recent years, for treatment of children with ASD (eg, for improving social skills).20
Research suggests that the intensity (number of hours) and duration of nonpharmacologic treatments for ASD is critical to improving outcomes (Box1,3,5,7,10,16).
Box
A higher intensity of nonpharmacologic intervention (greater number of hours) has been associated with greater benefit for children with autism spectrum disorder (ASD), in the form of enhancements in IQ and adaptive behavior.1,10,16 In the United States, the intensity of interventions commonly ranges from 30 to 200 or more minutes per week.3 This may mean that a child with ASD who is receiving 30 minutes of speech therapy at school and continues to exhibit significant deficits in speech-language or social-communication may likely benefit from additional hours of speech therapy and/or social-communication skill training, and should be referred accordingly, even for private therapy services if needed and feasible.7 Guidelines created through a systematic review of evidence recommend at least 25 hours per week of comprehensive treatment interventions for children with ASD to address language, social deficits, and behavioral difficulties.1 The duration of intervention has also been shown to play a role in outcomes.1,3,10 Given the complexity and extent of impairment often associated with ASD, it is not surprising that in recent research examining trends in ASD treatment in the United States, most caregivers reported therapy as ongoing.5 The exact intensity and duration of nonpharmacologic interventions may depend on several factors, such as severity of ASD and of the specific deficit being targeted, type of intervention, and therapist skill. The quality of skills of the care provider has also been shown to affect the benefits gained from the intervention.3
Continue to: Pharmacotherapy...
Pharmacotherapy
Medications cannot resolve core features of ASD.21 However, certain medications may help address associated comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), depression, or others, when these conditions have not responded to nonpharmacologic interventions.7,22 Common symptoms that are often treated with pharmacotherapy include aggression, irritability, hyperactivity, attentional difficulties, tics, self-injurious behavior, obsessive-compulsive symptoms, and mood dysregulation/lability.23 Generally speaking, medications might be considered if symptoms are severe and markedly impair functioning. For mild to moderate conditions, psychotherapy and other nonpharmacologic interventions are generally considered first-line. Since none of the medications described below are specific to ASD and psychiatrists generally receive training in prescribing them for other indications, a comprehensive review of their risks and benefits is beyond the scope of this article. No psychotropic medications are known to have robust evidence for safety in preschool children with ASD, and thus are best avoided.
Antipsychotics. Risperidone (for age 5 and older) and aripiprazole (age 6 to 17) are the only medications FDA-approved for use in children and adolescents with ASD, specifically for irritability associated with ASD.21,24 These 2 second-generation antipsychotics may also assist in lowering aggression in patients with ASD.24 First-generation antipsychotics such as haloperidol have been shown to be effective for irritability and aggression in ASD, but the risk of significant adverse effects such as dyskinesias and extrapyramidal symptoms limit their use.24 Two studies (a double-blind study and an open-label extension of that study) in children and adolescents with ASD found that risperidone was more effective and better tolerated than haloperidol in behavioral measures, impulsivity, and even in the social domain.25,26 In addition to other adverse effects and risks, increased prolactin secondary to risperidone use requires close monitoring and caution.24-26 As is the case with the use of other psychotropic medications in children and adolescents, those with ASD who receive antipsychotics should also be periodically reassessed to determine the need for continued use of these medications.27 A multicenter relapse prevention RCT found no statistically significant difference in the time to relapse between aripiprazole and placebo.27 Metabolic syndrome, cardiac risks, and other risks need to be considered before prescribing an antipsychotic.28 Given their serious adverse effects profile, use should be considered only when there is severe impairment or risk of injury, after carefully weighing risks/benefits.
Medications for attentional difficulties. A multisite, randomized, placebo-controlled trial evaluating the use of extended-release guanfacine in children with ASD (N = 62) found the rate of positive response on the Clinical Global Impressions–Improvement scale was 50% for guanfacine vs 9.4% for placebo.29 Clinicians need to monitor for adverse effects of guanfacine, such as fatigue, drowsiness, lightheadedness, lowering of blood pressure and heart rate, and other effects.29 A randomized, double-blind trial of 97 children and adolescents with ASD and ADHD found that atomoxetine had moderate benefit for ADHD symptoms.30 The study reported no serious adverse effects.30 However, it is especially important to monitor for hepatic and cardiac adverse effects (in addition to monitoring for risk of increase in suicidal thoughts/behavior, as in the case of antidepressants) when using atomoxetine, in addition to other side effects and risks. Some evidence suggests that methylphenidate may be effective for attentional difficulties in children and adolescents with ASD21 but may pose a higher risk of adverse effects in this population compared with neurotypical patients.31
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are sometimes used to reduce obsessive-compulsive symptoms, repetitive behavior, or depressive symptoms in children with ASD, but are not FDA-approved for children or adolescents with ASD. In general, there is inadequate evidence to support the use of SSRIs for ASD in children.31-34 In addition, children with ASD may be at a greater risk of adverse effects from SSRIs.32,34 Despite this, SSRIs are the most commonly prescribed psychotropic medications in children with ASD.32
An RCT examining the efficacy of fluoxetine in 158 children and adolescents with ASD found no significant difference in Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) score after 14 weeks of treatment; activation was a common adverse effect.35 A 2005 randomized, double-blind, placebo-controlled trial of 45 children/adolescents with ASD found that low-dose liquid fluoxetine was more effective than placebo for reducing repetitive behaviors in this population.36 Larger studies are warranted to further evaluate the efficacy and safety of fluoxetine (and of SSRIs in general, particularly in the long term) for children and adolescents with ASD.36 A 2009 randomized, placebo-controlled trial of 149 children with ASD revealed no significant difference between citalopram and placebo as measured by Clinical Global Impressions scale or CY-BOCS scores, and noted a significantly elevated likelihood of adverse effects.37
Other antidepressants. There is insufficient evidence to support the use of any other antidepressants in children and adolescents with ASD. A few studies38,39 have examined the use of venlafaxine in children with ASD; however, further research and controlled studies with large sample sizes are required to conclusively establish its benefits. There is a dearth of evidence examining the use of the tetracyclic antidepressant mirtazapine, or other classes of medications such as tricyclic antidepressants or mood stabilizers, in children with ASD; only a few small studies have assessed the efficacy and adverse effects of these medications for such patients.31
Polypharmacy. Although there is no evidence to support polypharmacy in children and adolescents with ASD, the practice appears to be rampant in these patients.28,40 A 2013 retrospective, observational study of psychotropic medication use in children with ASD (N = 33,565) found that 64% were prescribed psychotropic medications, and 35% exhibited evidence of polypharmacy.40 In this study, the total duration of polypharmacy averaged 525 days.40 When addressing polypharmacy, systematic deprescribing or simplification of the psychotropic medication regimen may be needed,28 while taking into account the patient’s complete clinical situation, including (but not limited to) tolerability of the medication regimen, presence or absence of current stressors, presence or absence of adequate supports, use of nonpharmacologic treatments where appropriate, and other factors.
More studies assessing the efficacy and safety of psychotropic medications for children and adolescents with ASD are needed,32 especially studies that evaluate the effects of long-term use, because evidence for pharmacologic treatments for children with ASD is mixed and insufficient.33 There is also a need for evidence-based standards for prescribing psychotropic medications in children and adolescents with ASD.
Psychotropic medications, if used in ASD, should be used only in conjunction with other evidence-based treatment modalities, and not as monotherapy.21 Children and adolescents with ASD may be particularly susceptible to side effects or adverse effects of certain psychotropic medications.31 When considering medications, carefully weigh the risks and benefits.7,21,24,28 Starting low and going slow is generally the preferred strategy.31,32 As always, when recommending medications, discuss in detail with parents the potential side effects, benefits, risks, interactions, and alternatives.
Other agents. Several double-blind, placebo-controlled trials have evaluated using melatonin for sleep difficulties in children and adolescents with ASD.41 A randomized, placebo-controlled, 12-week trial that assessed 160 children with ASD and insomnia found that melatonin plus cognitive-behavioral therapy (CBT) was superior in efficacy to melatonin alone, CBT alone, or placebo.41
The evidence regarding oxytocin use for children with ASD is mixed.31 Some small studies have associated improvement in the social domain with its use. Guastella et al42 conducted a randomized, double-blind, placebo-controlled trial of oxytocin nasal spray for 16 participants (age 12 to 19) with ASD, and found oxytocin enhanced emotional identification. Gordon et al43 conducted a functional MRI study of brain activity with oxytocin use in children with high-functioning ASD (N = 17). They found that oxytocin may augment “salience and hedonic evaluations of socially meaningful stimuli in children with ASD” and thus help social attunement. Further research is needed to evaluate the impact of oxytocin on social behavior.
Complementary and alternative medicine. Although there is limited and inconclusive evidence about the use of complementary and alternative medicine in children and adolescents with ASD, these therapies continue to be commonly used.44-46 A recent survey of parents (N = 211) of children with ASD from academic ASD outpatient clinics in Germany found that 46% reported their child was using or had used some type of complementary and alternative medicine.44 There is inadequate evidence to support the use of a gluten-free, casein-free diet for children/adolescents with ASD.46 A recent cross-sectional study assessing supplement use in 210 children with ASD in Canada found that 75% used supplements, such as multivitamins (77.8%), vitamin D (44.9%), omega 3 (42.5%), probiotics (36.5%), and magnesium (28.1%), despite insufficient evidence to support their safety or efficacy for children with ASD.47 Importantly, 33.5% of parents in this study reported that they did not inform the physician about all their child’s supplements.47 Some of the reasons the parents in this study provided for not disclosing information about supplements to their physicians were “physician lack of knowledge,” “no benefit,” “too time-consuming,” and “scared of judgment.”47 Semi-structured interviews of parents of 21 children with ASD in Australia revealed that parents found information on complementary and alternative medicine and therapies complex and often conflicting.45 In addition to recommendations from health care professionals, evidence suggests that parents often consider the opinions of media, friends, and family when making a decision on using complementary and alternative medicine modalities for children/adolescents with ASD.46 Such findings can inform physician practices regarding supplement use, and highlight the need to educate parents about the evidence regarding these therapies and potential adverse effects and interactions of such therapies,46 along with the need to develop a centralized, evidence-based resource for parents regarding their use.45
Omega 3 supplementation has in general shown few adverse effects47; still, risks/benefits need to be weighed before use. Some evidence suggests that it may decrease hyperactivity in children with ASD.31,48 However, further research, particularly controlled trials with large sample sizes, are needed for a definitive determination of efficacy.31,48 A meta-analysis that included 27 RCTs assessing the efficacy of dietary interventions for various ASD symptoms found that omega 3 supplementation was more effective than placebo, but compared with placebo, the effect size was small.49 A RCT of 73 children with ASD in New Zealand found that omega 3 long chain polyunsaturated fatty acids may benefit some core symptoms of ASD; the authors suggested that further research is needed to conclusively establish efficacy.50
Continue to: A need for advocacy and research..
A need for advocacy and research
Physicians who treat children with ASD can not only make appropriate referrals and educate parents, but also educate their patients’ schools and advocate for their patients to get the level of services they need.23,28
A recent study in the United States found that behavior therapy and speech-language therapy were used less often in the treatment of children with ASD in rural areas compared with those in metro areas.5 This suggests that in addition to increasing parents’ awareness and use of ASD services and providing referrals where appropriate, physicians are in a unique position to advocate for public health policies to improve access, coverage, and training for the provision of such services in rural areas.
There is need for ongoing research to further examine the efficacy and nuances of effects of various treatment interventions for ASD, especially long-term studies with larger sample sizes.11,51 Additionally, research is warranted to better understand the underlying genetic and neurobiological mechanisms of ASD, which would help guide the development of biomarkers,52 innovative treatments, and disease-modifying agents for ASD.7,22 Exploring the effects of potential alliances or joint action between biological and psychosocial interventions for ASD is also an area that needs further research.51
Bottom Line
A combination of treatment modalities (such as speech-language therapy, social skills training, behavior therapy/other psychotherapy, and occupational therapy for sensory sensitivities) is generally needed to improve the long-term outcomes of children and adolescents with autism spectrum disorder (ASD). In addition to the importance of early intervention, the intensity and duration of nonpharmacologic treatments are vital to improving outcomes in ASD.
SECOND OF 2 PARTS
Evidence supports the crucial role of early intervention and nonpharmacologic approaches
A large percentage of individuals with autism spectrum disorder (ASD) experience persisting significant social deficits in adulthood,1 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.2,3 Childhood is a vital time for making the most significant and lasting changes that can improve functioning of individuals with ASD. Psychiatrists and other physicians who treat children are in a key role to influence outcomes of children at risk for or diagnosed with ASD.
This article provides updates on various aspects of ASD diagnosis and treatment (based on available evidence up to March 2020). Part 1 (
A comprehensive approach is essential
Multiple treatment modalities have been recommended for ASD.5 It is essential to address all aspects of ASD through cognitive, developmental, social-communication, sensory-motor, and behavioral interventions. Nonpharmacologic interventions are crucial in improving long-term outcomes of children with ASD.6
Nonpharmacologic treatments
Nonpharmacologic interventions commonly utilized for children with ASD include behavioral therapies, other psychological therapies, speech-language therapy, occupational therapy, educational interventions, parent coaching/training, developmental social interventions, and other modalities of therapy that are delivered in school, home, and clinic settings.5,7
A recent study examining ASD treatment trends via caregivers’ reports (N = 5,122) from the SPARK (Simons Foundation Powering Autism Research for Knowledge) cohort in the United States reported that 80% of children received speech-language therapy or occupational therapy; 52% got both.5 The study revealed that approximately one-quarter utilized 3 therapies simultaneously; two-thirds had utilized 3 or more therapies in the previous year.5
Interventions for children with ASD need to be individualized.1,8 Evidence-based behavioral interventions for ASD fall into 2 broad categories: Applied Behavior Analysis (ABA), and Naturalistic Developmental Behavioral Interventions (NDBI). Traditionally, ABA has been a key model, guiding treatment for enhancing social-communicating skills and lowering maladaptive behaviors in ASD.9 ABA follows a structured and prescribed format,10,11 and has been shown to be efficacious.1,7 More recently, NDBI, in which interventions are “embedded” in the natural environment of the young child and more actively incorporate a developmental perspective, has been shown to be beneficial in improving and generalizing social-communication skills in young children with ASD.7,11
Early Start Denver Model (ESDM) is an intensive, naturalistic behavioral intervention4 that has been shown to be efficacious for enhancing communication and adaptive behavior in children with ASD.7,8,12 A multisite randomized controlled trial (RCT) by Rogers et al12 that examined the efficacy of ESDM in 118 children (age 14 to 24 months) with ASD found the treatment was beneficial and superior compared with a “community intervention” group, in regards to language ability measured in time by group analyses.The ESDM intervention in this study involved weekly parent coaching for 3 months, along with 24 months of 15 hours/week of one-on-one treatment provided by therapy professionals.12
Reciprocal imitation training (RIT) is another naturalistic intervention that has shown benefit in training children with ASD in imitation skills during play.13 Studies have found that both RIT and ESDM can be parent-implemented, after parents receive training.13,14
Parent-mediated, parent-implemented interventions may have a role in improving outcomes in childhood ASD,7,15 particularly “better generalization and maintenance of skills than therapist-implemented intervention” for lowering challenging behaviors and enhancing verbal and nonverbal communication.16
Various social skills interventions have also been found effective for children with ASD.1 Such interventions are often provided in the school setting.7 Coordination with the child’s school to discuss and advocating for adequate and suitable interventions, educational services, and placement is an essential aspect of ASD treatment.7
Two other school-based, comprehensive treatment model interventions—Learning Experiences and Alternative Programs for Preschoolers and their Parents (LEAP), and TEACCH—have some evidence of leading to improvement in children with ASD.7,17
Some studies have found that music therapy may have high efficacy for children with ASD, even with smaller length and intensity of treatment, particularly in improving social interaction, engagement with parents, joint attention, and communication.3,18 Further research is needed to conclusively establish the efficacy of music therapy for ASD in children and adolescents.
A few studies have assessed the long-term outcomes of interventions for ASD; however, more research is needed.19 Pickles et al19 conducted a follow-up to determine the long-term effects of the Preschool Autism Communication Trial (PACT), an RCT of parent-mediated social communication therapy for children age 2 to 4 with ASD. The children’s average age at follow-up was 10 years. The authors found a significant long-term decrease in ASD symptoms and enhancement of social communication with parents (N = 152).
Technology-based interventions, including games and robotics, have been investigated in recent years, for treatment of children with ASD (eg, for improving social skills).20
Research suggests that the intensity (number of hours) and duration of nonpharmacologic treatments for ASD is critical to improving outcomes (Box1,3,5,7,10,16).
Box
A higher intensity of nonpharmacologic intervention (greater number of hours) has been associated with greater benefit for children with autism spectrum disorder (ASD), in the form of enhancements in IQ and adaptive behavior.1,10,16 In the United States, the intensity of interventions commonly ranges from 30 to 200 or more minutes per week.3 This may mean that a child with ASD who is receiving 30 minutes of speech therapy at school and continues to exhibit significant deficits in speech-language or social-communication may likely benefit from additional hours of speech therapy and/or social-communication skill training, and should be referred accordingly, even for private therapy services if needed and feasible.7 Guidelines created through a systematic review of evidence recommend at least 25 hours per week of comprehensive treatment interventions for children with ASD to address language, social deficits, and behavioral difficulties.1 The duration of intervention has also been shown to play a role in outcomes.1,3,10 Given the complexity and extent of impairment often associated with ASD, it is not surprising that in recent research examining trends in ASD treatment in the United States, most caregivers reported therapy as ongoing.5 The exact intensity and duration of nonpharmacologic interventions may depend on several factors, such as severity of ASD and of the specific deficit being targeted, type of intervention, and therapist skill. The quality of skills of the care provider has also been shown to affect the benefits gained from the intervention.3
Continue to: Pharmacotherapy...
Pharmacotherapy
Medications cannot resolve core features of ASD.21 However, certain medications may help address associated comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), depression, or others, when these conditions have not responded to nonpharmacologic interventions.7,22 Common symptoms that are often treated with pharmacotherapy include aggression, irritability, hyperactivity, attentional difficulties, tics, self-injurious behavior, obsessive-compulsive symptoms, and mood dysregulation/lability.23 Generally speaking, medications might be considered if symptoms are severe and markedly impair functioning. For mild to moderate conditions, psychotherapy and other nonpharmacologic interventions are generally considered first-line. Since none of the medications described below are specific to ASD and psychiatrists generally receive training in prescribing them for other indications, a comprehensive review of their risks and benefits is beyond the scope of this article. No psychotropic medications are known to have robust evidence for safety in preschool children with ASD, and thus are best avoided.
Antipsychotics. Risperidone (for age 5 and older) and aripiprazole (age 6 to 17) are the only medications FDA-approved for use in children and adolescents with ASD, specifically for irritability associated with ASD.21,24 These 2 second-generation antipsychotics may also assist in lowering aggression in patients with ASD.24 First-generation antipsychotics such as haloperidol have been shown to be effective for irritability and aggression in ASD, but the risk of significant adverse effects such as dyskinesias and extrapyramidal symptoms limit their use.24 Two studies (a double-blind study and an open-label extension of that study) in children and adolescents with ASD found that risperidone was more effective and better tolerated than haloperidol in behavioral measures, impulsivity, and even in the social domain.25,26 In addition to other adverse effects and risks, increased prolactin secondary to risperidone use requires close monitoring and caution.24-26 As is the case with the use of other psychotropic medications in children and adolescents, those with ASD who receive antipsychotics should also be periodically reassessed to determine the need for continued use of these medications.27 A multicenter relapse prevention RCT found no statistically significant difference in the time to relapse between aripiprazole and placebo.27 Metabolic syndrome, cardiac risks, and other risks need to be considered before prescribing an antipsychotic.28 Given their serious adverse effects profile, use should be considered only when there is severe impairment or risk of injury, after carefully weighing risks/benefits.
Medications for attentional difficulties. A multisite, randomized, placebo-controlled trial evaluating the use of extended-release guanfacine in children with ASD (N = 62) found the rate of positive response on the Clinical Global Impressions–Improvement scale was 50% for guanfacine vs 9.4% for placebo.29 Clinicians need to monitor for adverse effects of guanfacine, such as fatigue, drowsiness, lightheadedness, lowering of blood pressure and heart rate, and other effects.29 A randomized, double-blind trial of 97 children and adolescents with ASD and ADHD found that atomoxetine had moderate benefit for ADHD symptoms.30 The study reported no serious adverse effects.30 However, it is especially important to monitor for hepatic and cardiac adverse effects (in addition to monitoring for risk of increase in suicidal thoughts/behavior, as in the case of antidepressants) when using atomoxetine, in addition to other side effects and risks. Some evidence suggests that methylphenidate may be effective for attentional difficulties in children and adolescents with ASD21 but may pose a higher risk of adverse effects in this population compared with neurotypical patients.31
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are sometimes used to reduce obsessive-compulsive symptoms, repetitive behavior, or depressive symptoms in children with ASD, but are not FDA-approved for children or adolescents with ASD. In general, there is inadequate evidence to support the use of SSRIs for ASD in children.31-34 In addition, children with ASD may be at a greater risk of adverse effects from SSRIs.32,34 Despite this, SSRIs are the most commonly prescribed psychotropic medications in children with ASD.32
An RCT examining the efficacy of fluoxetine in 158 children and adolescents with ASD found no significant difference in Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) score after 14 weeks of treatment; activation was a common adverse effect.35 A 2005 randomized, double-blind, placebo-controlled trial of 45 children/adolescents with ASD found that low-dose liquid fluoxetine was more effective than placebo for reducing repetitive behaviors in this population.36 Larger studies are warranted to further evaluate the efficacy and safety of fluoxetine (and of SSRIs in general, particularly in the long term) for children and adolescents with ASD.36 A 2009 randomized, placebo-controlled trial of 149 children with ASD revealed no significant difference between citalopram and placebo as measured by Clinical Global Impressions scale or CY-BOCS scores, and noted a significantly elevated likelihood of adverse effects.37
Other antidepressants. There is insufficient evidence to support the use of any other antidepressants in children and adolescents with ASD. A few studies38,39 have examined the use of venlafaxine in children with ASD; however, further research and controlled studies with large sample sizes are required to conclusively establish its benefits. There is a dearth of evidence examining the use of the tetracyclic antidepressant mirtazapine, or other classes of medications such as tricyclic antidepressants or mood stabilizers, in children with ASD; only a few small studies have assessed the efficacy and adverse effects of these medications for such patients.31
Polypharmacy. Although there is no evidence to support polypharmacy in children and adolescents with ASD, the practice appears to be rampant in these patients.28,40 A 2013 retrospective, observational study of psychotropic medication use in children with ASD (N = 33,565) found that 64% were prescribed psychotropic medications, and 35% exhibited evidence of polypharmacy.40 In this study, the total duration of polypharmacy averaged 525 days.40 When addressing polypharmacy, systematic deprescribing or simplification of the psychotropic medication regimen may be needed,28 while taking into account the patient’s complete clinical situation, including (but not limited to) tolerability of the medication regimen, presence or absence of current stressors, presence or absence of adequate supports, use of nonpharmacologic treatments where appropriate, and other factors.
More studies assessing the efficacy and safety of psychotropic medications for children and adolescents with ASD are needed,32 especially studies that evaluate the effects of long-term use, because evidence for pharmacologic treatments for children with ASD is mixed and insufficient.33 There is also a need for evidence-based standards for prescribing psychotropic medications in children and adolescents with ASD.
Psychotropic medications, if used in ASD, should be used only in conjunction with other evidence-based treatment modalities, and not as monotherapy.21 Children and adolescents with ASD may be particularly susceptible to side effects or adverse effects of certain psychotropic medications.31 When considering medications, carefully weigh the risks and benefits.7,21,24,28 Starting low and going slow is generally the preferred strategy.31,32 As always, when recommending medications, discuss in detail with parents the potential side effects, benefits, risks, interactions, and alternatives.
Other agents. Several double-blind, placebo-controlled trials have evaluated using melatonin for sleep difficulties in children and adolescents with ASD.41 A randomized, placebo-controlled, 12-week trial that assessed 160 children with ASD and insomnia found that melatonin plus cognitive-behavioral therapy (CBT) was superior in efficacy to melatonin alone, CBT alone, or placebo.41
The evidence regarding oxytocin use for children with ASD is mixed.31 Some small studies have associated improvement in the social domain with its use. Guastella et al42 conducted a randomized, double-blind, placebo-controlled trial of oxytocin nasal spray for 16 participants (age 12 to 19) with ASD, and found oxytocin enhanced emotional identification. Gordon et al43 conducted a functional MRI study of brain activity with oxytocin use in children with high-functioning ASD (N = 17). They found that oxytocin may augment “salience and hedonic evaluations of socially meaningful stimuli in children with ASD” and thus help social attunement. Further research is needed to evaluate the impact of oxytocin on social behavior.
Complementary and alternative medicine. Although there is limited and inconclusive evidence about the use of complementary and alternative medicine in children and adolescents with ASD, these therapies continue to be commonly used.44-46 A recent survey of parents (N = 211) of children with ASD from academic ASD outpatient clinics in Germany found that 46% reported their child was using or had used some type of complementary and alternative medicine.44 There is inadequate evidence to support the use of a gluten-free, casein-free diet for children/adolescents with ASD.46 A recent cross-sectional study assessing supplement use in 210 children with ASD in Canada found that 75% used supplements, such as multivitamins (77.8%), vitamin D (44.9%), omega 3 (42.5%), probiotics (36.5%), and magnesium (28.1%), despite insufficient evidence to support their safety or efficacy for children with ASD.47 Importantly, 33.5% of parents in this study reported that they did not inform the physician about all their child’s supplements.47 Some of the reasons the parents in this study provided for not disclosing information about supplements to their physicians were “physician lack of knowledge,” “no benefit,” “too time-consuming,” and “scared of judgment.”47 Semi-structured interviews of parents of 21 children with ASD in Australia revealed that parents found information on complementary and alternative medicine and therapies complex and often conflicting.45 In addition to recommendations from health care professionals, evidence suggests that parents often consider the opinions of media, friends, and family when making a decision on using complementary and alternative medicine modalities for children/adolescents with ASD.46 Such findings can inform physician practices regarding supplement use, and highlight the need to educate parents about the evidence regarding these therapies and potential adverse effects and interactions of such therapies,46 along with the need to develop a centralized, evidence-based resource for parents regarding their use.45
Omega 3 supplementation has in general shown few adverse effects47; still, risks/benefits need to be weighed before use. Some evidence suggests that it may decrease hyperactivity in children with ASD.31,48 However, further research, particularly controlled trials with large sample sizes, are needed for a definitive determination of efficacy.31,48 A meta-analysis that included 27 RCTs assessing the efficacy of dietary interventions for various ASD symptoms found that omega 3 supplementation was more effective than placebo, but compared with placebo, the effect size was small.49 A RCT of 73 children with ASD in New Zealand found that omega 3 long chain polyunsaturated fatty acids may benefit some core symptoms of ASD; the authors suggested that further research is needed to conclusively establish efficacy.50
Continue to: A need for advocacy and research..
A need for advocacy and research
Physicians who treat children with ASD can not only make appropriate referrals and educate parents, but also educate their patients’ schools and advocate for their patients to get the level of services they need.23,28
A recent study in the United States found that behavior therapy and speech-language therapy were used less often in the treatment of children with ASD in rural areas compared with those in metro areas.5 This suggests that in addition to increasing parents’ awareness and use of ASD services and providing referrals where appropriate, physicians are in a unique position to advocate for public health policies to improve access, coverage, and training for the provision of such services in rural areas.
There is need for ongoing research to further examine the efficacy and nuances of effects of various treatment interventions for ASD, especially long-term studies with larger sample sizes.11,51 Additionally, research is warranted to better understand the underlying genetic and neurobiological mechanisms of ASD, which would help guide the development of biomarkers,52 innovative treatments, and disease-modifying agents for ASD.7,22 Exploring the effects of potential alliances or joint action between biological and psychosocial interventions for ASD is also an area that needs further research.51
Bottom Line
A combination of treatment modalities (such as speech-language therapy, social skills training, behavior therapy/other psychotherapy, and occupational therapy for sensory sensitivities) is generally needed to improve the long-term outcomes of children and adolescents with autism spectrum disorder (ASD). In addition to the importance of early intervention, the intensity and duration of nonpharmacologic treatments are vital to improving outcomes in ASD.
1. Maglione MA, Gans D, Das L, et al. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2):S169-S178.
2. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
3. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
4. Charman T. Editorial: trials and tribulations in early autism intervention research. J Am Acad Child Adolesc Psychiatry. 2019;58(9):846-848. doi:10.1016/j.jaac.2019.03.004
5. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):517-526. doi:10.1002/aur.2070
6. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi:10.3389/fnins.2016.00393
7. Hyman SL, Levy SE, Myers SM, et al. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
8. Contaldo A, Colombi C, Pierotti C, et al. Outcomes and moderators of Early Start Denver Model intervention in young children with autism spectrum disorder delivered in a mixed individual and group setting. Autism. 2020;24(3):718-729. doi:10.1177/1362361319888344
9. Lei J, Ventola P. Pivotal response treatment for autism spectrum disorder: current perspectives. Neuropsychiatr Dis Treat. 2017;13:1613-1626. doi:10.2147/NDT.S120710
10. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
11. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
12. Rogers SJ, Estes A, Lord C, et al. A multisite randomized controlled two-phase trial of the Early Start Denver Model compared to treatment as usual. J Am Acad Child Adolesc Psychiatry. 2019;58(9):853-865. doi:10.1016/j.jaac.2019.01.004
13. Ingersoll B, Gergans S. The effect of a parent-implemented imitation intervention on spontaneous imitation skills in young children with autism. Res Dev Disabil. 2007;28(2):163-175.
14. Waddington H, van der Meer L, Sigafoos J, et al. Examining parent use of specific intervention techniques during a 12-week training program based on the Early Start Denver Model. Autism. 2020;24(2):484-498. doi:10.1177/1362361319876495
15. Trembath D, Gurm M, Scheerer NE, et al. Systematic review of factors that may influence the outcomes and generalizability of parent‐mediated interventions for young children with autism spectrum disorder. Autism Res. 2019;12(9):1304-1321.
16. Rogers SJ, Estes A, Lord C, et al. Effects of a brief Early Start Denver Model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
17. Boyd BA, Hume K, McBee MT, et al. Comparative efficacy of LEAP, TEACCH and non-model-specific special education programs for preschoolers with autism spectrum disorders. J Autism Dev Disord. 2014;44(2):366-380. doi:10.1007/s10803-013-1877-9
18. Thompson GA, McFerran KS, Gold C. Family-centred music therapy to promote social engagement in young children with severe autism spectrum disorder: a randomized controlled study. Child Care Health Dev. 2014;40(6):840-852. doi:10.1111/cch.12121
19. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
20. Grossard C, Palestra G, Xavier J, et al. ICT and autism care: state of the art. Curr Opin Psychiatry. 2018;31(6):474-483. doi:10.1097/YCO.0000000000000455
21. Cukier S, Barrios N. Pharmacological interventions for intellectual disability and autism. Vertex. 2019;XXX(143)52-63.
22. Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91-104.
23. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(2):237-257.
24. LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. P T. 2015;40(6):389-397.
25. Gencer O, Emiroglu FN, Miral S, et al. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder. An open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217-225.
26. Miral S, Gencer O, Inal-Emiroglu FN, et al. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry. 2008;17(1):1-8.
27. Findling RL, Mankoski R, Timko K, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22-30. doi:10.4088/jcp.13m08500
28. McLennan JD. Deprescribing in a youth with an intellectual disability, autism, behavioural problems, and medication-related obesity: a case study. J Can Acad Child Adolesc Psychiatry. 2019;28(3):141-146.
29. Scahill L, McCracken JT, King B, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206. doi:10.1176/appi.ajp.2015.15010055
30. Harfterkamp M, van de Loo-Neus G, Minderaa RB, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733-741. doi:10.1016/j.jaac.2012.04.011
31. DeFilippis M, Wagner KD. Treatment of autism spectrum disorder in children and adolescents. Psychopharmacol Bull. 2016;46(2):18-41.
32. DeFilippis M. Depression in children and adolescents with autism spectrum disorder. Children (Basel). 2018;5(9):112. doi:10.3390/children5090112
33. Goel R, Hong JS, Findling RL, et al. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psychiatry. 2018;30(1):78-95. doi:10.1080/09540261.2018.1458706
34. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677. doi:10.1002/14651858.CD004677.pub3
35. Herscu P, Handen BL, Arnold LE, et al. The SOFIA study: negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord. 2020;50(9):3233-3244. doi:10.1007/s10803-019-04120-y
36. Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582-589.
37. King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583-590. doi:10.1001/archgenpsychiatry.2009.30
38. Hollander E, Kaplan A, Cartwright C, et al. Venlafaxine in children, adolescents, and young adults with autism spectrum disorders: an open retrospective clinical report. J Child Neurol. 2000;15(2):132-135.
39. Carminati GG, Deriaz N, Bertschy G. Low-dose venlafaxine in three adolescents and young adults with autistic disorder improves self-injurious behavior and attention deficit/hyperactivity disorders (ADHD)-like symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):312-315.
40. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840. doi:10.1542/peds.2012-3774
41. Cortesi F, Giannotti F, Sebastiani T, et al. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res. 2012;21(6):700-709. doi:10.1111/j.1365-2869.2012.01021.x
42. Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692-694. doi:10.1016/j.biopsych.2009.09.020
43. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
44. Höfer J, Bachmann C, Kamp-Becker I, et al. Willingness to try and lifetime use of complementary and alternative medicine in children and adolescents with autism spectrum disorder in Germany: a survey of parents. Autism. 2019;23(7):1865-1870. doi:10.1177/1362361318823545
45. Smith CA, Parton C, King M, et al. Parents’ experiences of information-seeking and decision-making regarding complementary medicine for children with autism spectrum disorder: a qualitative study. BMC Complement Med Ther. 2020;20(1):4. doi:10.1186/s12906-019-2805-0
46. Marsden REF, Francis J, Garner I. Use of GFCF diets in children with ASD. An investigation into parents’ beliefs using the theory of planned behaviour. J Autism Dev Disord. 2019;49(9):3716-3731. doi:10.1007/s10803-019-04035-8
47. Trudeau MS, Madden RF, Parnell JA, et al. Dietary and supplement-based complementary and alternative medicine use in pediatric autism spectrum disorder. Nutrients. 2019;11(8):1783. doi:10.3390/nu11081783
48. Bent S, Hendren RL, Zandi T, et al. Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J Am Acad Child Adolesc Psychiatry. 2014;53(6):658-666. doi:10.1016/j.jaac.2014.01.018
49. Fraguas D, Díaz-Caneja C, Pina-Camacho L, et al. Dietary interventions for autism spectrum disorder: a meta-analysis. Pediatrics. 144(5):e20183218.
50. Mazahery H, Conlon CA, Beck KL, et al. A randomised-controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children. J Autism Dev Disord. 2019;49(5):1778-1794. doi:10.1007/s10803-018-3860-y
51. Green J, Garg S. Annual research review: the state of autism intervention science: progress, target psychological and biological mechanisms and future prospects. J Child Psychol Psychiatry. 2018;59(4):424-443. doi:10.1111/jcpp.1289
52. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.53
1. Maglione MA, Gans D, Das L, et al. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2):S169-S178.
2. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
3. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
4. Charman T. Editorial: trials and tribulations in early autism intervention research. J Am Acad Child Adolesc Psychiatry. 2019;58(9):846-848. doi:10.1016/j.jaac.2019.03.004
5. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):517-526. doi:10.1002/aur.2070
6. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi:10.3389/fnins.2016.00393
7. Hyman SL, Levy SE, Myers SM, et al. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
8. Contaldo A, Colombi C, Pierotti C, et al. Outcomes and moderators of Early Start Denver Model intervention in young children with autism spectrum disorder delivered in a mixed individual and group setting. Autism. 2020;24(3):718-729. doi:10.1177/1362361319888344
9. Lei J, Ventola P. Pivotal response treatment for autism spectrum disorder: current perspectives. Neuropsychiatr Dis Treat. 2017;13:1613-1626. doi:10.2147/NDT.S120710
10. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
11. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
12. Rogers SJ, Estes A, Lord C, et al. A multisite randomized controlled two-phase trial of the Early Start Denver Model compared to treatment as usual. J Am Acad Child Adolesc Psychiatry. 2019;58(9):853-865. doi:10.1016/j.jaac.2019.01.004
13. Ingersoll B, Gergans S. The effect of a parent-implemented imitation intervention on spontaneous imitation skills in young children with autism. Res Dev Disabil. 2007;28(2):163-175.
14. Waddington H, van der Meer L, Sigafoos J, et al. Examining parent use of specific intervention techniques during a 12-week training program based on the Early Start Denver Model. Autism. 2020;24(2):484-498. doi:10.1177/1362361319876495
15. Trembath D, Gurm M, Scheerer NE, et al. Systematic review of factors that may influence the outcomes and generalizability of parent‐mediated interventions for young children with autism spectrum disorder. Autism Res. 2019;12(9):1304-1321.
16. Rogers SJ, Estes A, Lord C, et al. Effects of a brief Early Start Denver Model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
17. Boyd BA, Hume K, McBee MT, et al. Comparative efficacy of LEAP, TEACCH and non-model-specific special education programs for preschoolers with autism spectrum disorders. J Autism Dev Disord. 2014;44(2):366-380. doi:10.1007/s10803-013-1877-9
18. Thompson GA, McFerran KS, Gold C. Family-centred music therapy to promote social engagement in young children with severe autism spectrum disorder: a randomized controlled study. Child Care Health Dev. 2014;40(6):840-852. doi:10.1111/cch.12121
19. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
20. Grossard C, Palestra G, Xavier J, et al. ICT and autism care: state of the art. Curr Opin Psychiatry. 2018;31(6):474-483. doi:10.1097/YCO.0000000000000455
21. Cukier S, Barrios N. Pharmacological interventions for intellectual disability and autism. Vertex. 2019;XXX(143)52-63.
22. Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91-104.
23. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(2):237-257.
24. LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. P T. 2015;40(6):389-397.
25. Gencer O, Emiroglu FN, Miral S, et al. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder. An open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217-225.
26. Miral S, Gencer O, Inal-Emiroglu FN, et al. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry. 2008;17(1):1-8.
27. Findling RL, Mankoski R, Timko K, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22-30. doi:10.4088/jcp.13m08500
28. McLennan JD. Deprescribing in a youth with an intellectual disability, autism, behavioural problems, and medication-related obesity: a case study. J Can Acad Child Adolesc Psychiatry. 2019;28(3):141-146.
29. Scahill L, McCracken JT, King B, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206. doi:10.1176/appi.ajp.2015.15010055
30. Harfterkamp M, van de Loo-Neus G, Minderaa RB, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733-741. doi:10.1016/j.jaac.2012.04.011
31. DeFilippis M, Wagner KD. Treatment of autism spectrum disorder in children and adolescents. Psychopharmacol Bull. 2016;46(2):18-41.
32. DeFilippis M. Depression in children and adolescents with autism spectrum disorder. Children (Basel). 2018;5(9):112. doi:10.3390/children5090112
33. Goel R, Hong JS, Findling RL, et al. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psychiatry. 2018;30(1):78-95. doi:10.1080/09540261.2018.1458706
34. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677. doi:10.1002/14651858.CD004677.pub3
35. Herscu P, Handen BL, Arnold LE, et al. The SOFIA study: negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord. 2020;50(9):3233-3244. doi:10.1007/s10803-019-04120-y
36. Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582-589.
37. King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583-590. doi:10.1001/archgenpsychiatry.2009.30
38. Hollander E, Kaplan A, Cartwright C, et al. Venlafaxine in children, adolescents, and young adults with autism spectrum disorders: an open retrospective clinical report. J Child Neurol. 2000;15(2):132-135.
39. Carminati GG, Deriaz N, Bertschy G. Low-dose venlafaxine in three adolescents and young adults with autistic disorder improves self-injurious behavior and attention deficit/hyperactivity disorders (ADHD)-like symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):312-315.
40. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840. doi:10.1542/peds.2012-3774
41. Cortesi F, Giannotti F, Sebastiani T, et al. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res. 2012;21(6):700-709. doi:10.1111/j.1365-2869.2012.01021.x
42. Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692-694. doi:10.1016/j.biopsych.2009.09.020
43. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
44. Höfer J, Bachmann C, Kamp-Becker I, et al. Willingness to try and lifetime use of complementary and alternative medicine in children and adolescents with autism spectrum disorder in Germany: a survey of parents. Autism. 2019;23(7):1865-1870. doi:10.1177/1362361318823545
45. Smith CA, Parton C, King M, et al. Parents’ experiences of information-seeking and decision-making regarding complementary medicine for children with autism spectrum disorder: a qualitative study. BMC Complement Med Ther. 2020;20(1):4. doi:10.1186/s12906-019-2805-0
46. Marsden REF, Francis J, Garner I. Use of GFCF diets in children with ASD. An investigation into parents’ beliefs using the theory of planned behaviour. J Autism Dev Disord. 2019;49(9):3716-3731. doi:10.1007/s10803-019-04035-8
47. Trudeau MS, Madden RF, Parnell JA, et al. Dietary and supplement-based complementary and alternative medicine use in pediatric autism spectrum disorder. Nutrients. 2019;11(8):1783. doi:10.3390/nu11081783
48. Bent S, Hendren RL, Zandi T, et al. Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J Am Acad Child Adolesc Psychiatry. 2014;53(6):658-666. doi:10.1016/j.jaac.2014.01.018
49. Fraguas D, Díaz-Caneja C, Pina-Camacho L, et al. Dietary interventions for autism spectrum disorder: a meta-analysis. Pediatrics. 144(5):e20183218.
50. Mazahery H, Conlon CA, Beck KL, et al. A randomised-controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children. J Autism Dev Disord. 2019;49(5):1778-1794. doi:10.1007/s10803-018-3860-y
51. Green J, Garg S. Annual research review: the state of autism intervention science: progress, target psychological and biological mechanisms and future prospects. J Child Psychol Psychiatry. 2018;59(4):424-443. doi:10.1111/jcpp.1289
52. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.53