User login
Does education to enhance maternal awareness of fetal movements help reduce stillbirth?
WHAT DOES THIS MEAN FOR PRACTICE?
- Data indicate that fetal movement counting does not help to reduce stillbirth incidence
- Continue to use fetal movement counting to maintain patient engagement in managing her own pregnancy
- Encourage fetal movement awareness but also counsel patients that awareness does not reduce stillbirth
- This may decrease a patient’s feelings of guilt (because she did not maintain fetal kick counting) should a stillbirth occur
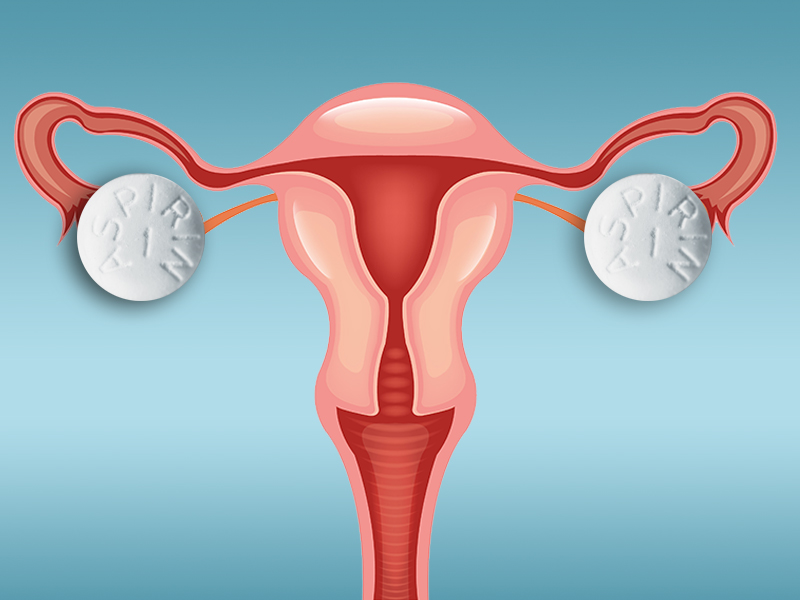
Does low-dose aspirin decrease a woman’s risk of ovarian cancer?
EXPERT COMMENTARY
Epidemiologic studies conducted in ovarian cancer suggest an association between chronic inflammation and incidence of disease.1 Nonsteroidal anti-inflammatory drugs (NSAIDs) work to decrease inflammation through the inhibition of cyclo-oxygenase (COX). Therefore, anti-inflammatory agents such as NSAIDs have been proposed to play a role in the pathophysiology of ovarian cancer.
Previous studies of this association show conflicting data. The majority of these studies are retrospective, and those that are prospective do not include detailed data regarding dosing and frequency of ASA use.2-6
_
Details of the study
This study by Barnard and colleagues is a prospective cohort study evaluating a total of 205,498 women from 1980–2015 from 2 separate cohorts (the Nurses’ Health Study and the Nurses’ Health Study II). The primary outcome was “to evaluate whether regular aspirin or nonaspirin NSAID use and patterns of use are associated with lower ovarian cancer risk.” Analgesic use and data regarding covariates were obtained via self-reported questionnaires. Ovarian cancer diagnosis was confirmed via medical records.
Results demonstrated that current low-dose aspirin use was associated with a decreased risk of ovarian cancer (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.61–0.96). This significance was not maintained upon further controlling for inflammatory factors (hypertension, autoimmune disease, inflammatory diet scores, smoking, etc) (HR, 0.94; 95% CI, 0.69–1.26). Other significant findings included an increased risk of developing ovarian cancer with standard-dose ASA use of ≥5 years or standard-dose use at 6 to 9 tablets per week (HR, 1.77; 95% CI, 1.13–2.77 and HR, 2.00; 95% CI, 1.27–3.15, respectively). An increased risk of developing ovarian cancer also was found for >10-year use or use of >10 tablets per week of nonaspirin NSAIDs (HR, 2.00; 95% CI, 1.27–3.15 and HR, 1.35; 95% CI, 1.02–1.79, respectively).
The authors concluded that there was a slight inverse association for low-dose aspirin and ovarian cancer risk and that standard aspirin or NSAID use actually may be associated with an increased risk of ovarian cancer.
Study strengths and weaknesses
This study has many strengths. It was a large prospective cohort investigation with adequate power to detect clinically significant differences. The authors collected detailed exposure data, which was novel. They also considered a latency period prior to the diagnosis of ovarian cancer during which a patient may increase their analgesic use in order to treat pain caused by the impending cancer.
However, the conclusions of the authors seem to be overstated in the setting of the data. Specifically, the deduction regarding a decreased risk of ovarian cancer with low-dose aspirin use given the loss of the statistical significance when controlling for pertinent cofounders. Further, the study authors did not evaluate adverse effects associated with low-dose aspirin use, which would be clinically applicable when determining whether the results from this study should become formal recommendations. Lastly, other important clinical factors, such as the presence of genetic mutations or endometriosis, were not considered, and these considerations would greatly affect results.
In the setting of previous large prospective studies that suggest no association between ASA use and ovarian cancer risk,4-6 data from this study are not compelling enough to recommend regular low-dose aspirin use to all women.
Based on these current data, there is insufficient evidence to suggest the use of low-dose aspirin for chemoprophylaxis of ovarian cancer. In order to suggest the use of a drug for prophylaxis the benefits must outweigh the risks, and in the case of NSAIDs, this has yet to be confirmed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Poole EM, Lee IM, Ridker PM, et al. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256-1264.
- Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
- Peres LC, Camacho F, Abbott SE, et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Br J Cancer. 2016;114(7):819-825.
- Murphy MA, Trabert B, Yang HP, et al. Non-steroidal antiinflammatory drug use and ovarian cancer risk: findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control. 2012;23:1839-1852.
- Brasky TM, Liu J, White E, et al. Non-steroidal antiinflammatory drugs and cancer risk in women: results from the Women’s Health Initiative. Int J Cancer. 2014;135:1869-1883.
- Lacey JV Jr, Sherman ME, Hartge P, et al. Medication use and risk of ovarian carcinoma: a prospective study. Int J Cancer. 2004;108:281-286.
EXPERT COMMENTARY
Epidemiologic studies conducted in ovarian cancer suggest an association between chronic inflammation and incidence of disease.1 Nonsteroidal anti-inflammatory drugs (NSAIDs) work to decrease inflammation through the inhibition of cyclo-oxygenase (COX). Therefore, anti-inflammatory agents such as NSAIDs have been proposed to play a role in the pathophysiology of ovarian cancer.
Previous studies of this association show conflicting data. The majority of these studies are retrospective, and those that are prospective do not include detailed data regarding dosing and frequency of ASA use.2-6
_
Details of the study
This study by Barnard and colleagues is a prospective cohort study evaluating a total of 205,498 women from 1980–2015 from 2 separate cohorts (the Nurses’ Health Study and the Nurses’ Health Study II). The primary outcome was “to evaluate whether regular aspirin or nonaspirin NSAID use and patterns of use are associated with lower ovarian cancer risk.” Analgesic use and data regarding covariates were obtained via self-reported questionnaires. Ovarian cancer diagnosis was confirmed via medical records.
Results demonstrated that current low-dose aspirin use was associated with a decreased risk of ovarian cancer (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.61–0.96). This significance was not maintained upon further controlling for inflammatory factors (hypertension, autoimmune disease, inflammatory diet scores, smoking, etc) (HR, 0.94; 95% CI, 0.69–1.26). Other significant findings included an increased risk of developing ovarian cancer with standard-dose ASA use of ≥5 years or standard-dose use at 6 to 9 tablets per week (HR, 1.77; 95% CI, 1.13–2.77 and HR, 2.00; 95% CI, 1.27–3.15, respectively). An increased risk of developing ovarian cancer also was found for >10-year use or use of >10 tablets per week of nonaspirin NSAIDs (HR, 2.00; 95% CI, 1.27–3.15 and HR, 1.35; 95% CI, 1.02–1.79, respectively).
The authors concluded that there was a slight inverse association for low-dose aspirin and ovarian cancer risk and that standard aspirin or NSAID use actually may be associated with an increased risk of ovarian cancer.
Study strengths and weaknesses
This study has many strengths. It was a large prospective cohort investigation with adequate power to detect clinically significant differences. The authors collected detailed exposure data, which was novel. They also considered a latency period prior to the diagnosis of ovarian cancer during which a patient may increase their analgesic use in order to treat pain caused by the impending cancer.
However, the conclusions of the authors seem to be overstated in the setting of the data. Specifically, the deduction regarding a decreased risk of ovarian cancer with low-dose aspirin use given the loss of the statistical significance when controlling for pertinent cofounders. Further, the study authors did not evaluate adverse effects associated with low-dose aspirin use, which would be clinically applicable when determining whether the results from this study should become formal recommendations. Lastly, other important clinical factors, such as the presence of genetic mutations or endometriosis, were not considered, and these considerations would greatly affect results.
In the setting of previous large prospective studies that suggest no association between ASA use and ovarian cancer risk,4-6 data from this study are not compelling enough to recommend regular low-dose aspirin use to all women.
Based on these current data, there is insufficient evidence to suggest the use of low-dose aspirin for chemoprophylaxis of ovarian cancer. In order to suggest the use of a drug for prophylaxis the benefits must outweigh the risks, and in the case of NSAIDs, this has yet to be confirmed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Epidemiologic studies conducted in ovarian cancer suggest an association between chronic inflammation and incidence of disease.1 Nonsteroidal anti-inflammatory drugs (NSAIDs) work to decrease inflammation through the inhibition of cyclo-oxygenase (COX). Therefore, anti-inflammatory agents such as NSAIDs have been proposed to play a role in the pathophysiology of ovarian cancer.
Previous studies of this association show conflicting data. The majority of these studies are retrospective, and those that are prospective do not include detailed data regarding dosing and frequency of ASA use.2-6
_
Details of the study
This study by Barnard and colleagues is a prospective cohort study evaluating a total of 205,498 women from 1980–2015 from 2 separate cohorts (the Nurses’ Health Study and the Nurses’ Health Study II). The primary outcome was “to evaluate whether regular aspirin or nonaspirin NSAID use and patterns of use are associated with lower ovarian cancer risk.” Analgesic use and data regarding covariates were obtained via self-reported questionnaires. Ovarian cancer diagnosis was confirmed via medical records.
Results demonstrated that current low-dose aspirin use was associated with a decreased risk of ovarian cancer (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.61–0.96). This significance was not maintained upon further controlling for inflammatory factors (hypertension, autoimmune disease, inflammatory diet scores, smoking, etc) (HR, 0.94; 95% CI, 0.69–1.26). Other significant findings included an increased risk of developing ovarian cancer with standard-dose ASA use of ≥5 years or standard-dose use at 6 to 9 tablets per week (HR, 1.77; 95% CI, 1.13–2.77 and HR, 2.00; 95% CI, 1.27–3.15, respectively). An increased risk of developing ovarian cancer also was found for >10-year use or use of >10 tablets per week of nonaspirin NSAIDs (HR, 2.00; 95% CI, 1.27–3.15 and HR, 1.35; 95% CI, 1.02–1.79, respectively).
The authors concluded that there was a slight inverse association for low-dose aspirin and ovarian cancer risk and that standard aspirin or NSAID use actually may be associated with an increased risk of ovarian cancer.
Study strengths and weaknesses
This study has many strengths. It was a large prospective cohort investigation with adequate power to detect clinically significant differences. The authors collected detailed exposure data, which was novel. They also considered a latency period prior to the diagnosis of ovarian cancer during which a patient may increase their analgesic use in order to treat pain caused by the impending cancer.
However, the conclusions of the authors seem to be overstated in the setting of the data. Specifically, the deduction regarding a decreased risk of ovarian cancer with low-dose aspirin use given the loss of the statistical significance when controlling for pertinent cofounders. Further, the study authors did not evaluate adverse effects associated with low-dose aspirin use, which would be clinically applicable when determining whether the results from this study should become formal recommendations. Lastly, other important clinical factors, such as the presence of genetic mutations or endometriosis, were not considered, and these considerations would greatly affect results.
In the setting of previous large prospective studies that suggest no association between ASA use and ovarian cancer risk,4-6 data from this study are not compelling enough to recommend regular low-dose aspirin use to all women.
Based on these current data, there is insufficient evidence to suggest the use of low-dose aspirin for chemoprophylaxis of ovarian cancer. In order to suggest the use of a drug for prophylaxis the benefits must outweigh the risks, and in the case of NSAIDs, this has yet to be confirmed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Poole EM, Lee IM, Ridker PM, et al. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256-1264.
- Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
- Peres LC, Camacho F, Abbott SE, et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Br J Cancer. 2016;114(7):819-825.
- Murphy MA, Trabert B, Yang HP, et al. Non-steroidal antiinflammatory drug use and ovarian cancer risk: findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control. 2012;23:1839-1852.
- Brasky TM, Liu J, White E, et al. Non-steroidal antiinflammatory drugs and cancer risk in women: results from the Women’s Health Initiative. Int J Cancer. 2014;135:1869-1883.
- Lacey JV Jr, Sherman ME, Hartge P, et al. Medication use and risk of ovarian carcinoma: a prospective study. Int J Cancer. 2004;108:281-286.
- Poole EM, Lee IM, Ridker PM, et al. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256-1264.
- Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
- Peres LC, Camacho F, Abbott SE, et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Br J Cancer. 2016;114(7):819-825.
- Murphy MA, Trabert B, Yang HP, et al. Non-steroidal antiinflammatory drug use and ovarian cancer risk: findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control. 2012;23:1839-1852.
- Brasky TM, Liu J, White E, et al. Non-steroidal antiinflammatory drugs and cancer risk in women: results from the Women’s Health Initiative. Int J Cancer. 2014;135:1869-1883.
- Lacey JV Jr, Sherman ME, Hartge P, et al. Medication use and risk of ovarian carcinoma: a prospective study. Int J Cancer. 2004;108:281-286.
Is salpingectomy (vs standard tubal ligation) for sterilization a feasible option at cesarean delivery?
Are patients more satisfied with combination or monotherapy for hirsutism in PCOS?
EXPERT COMMENTARY
Ezeh and colleagues conducted a retrospective analysis to evaluate the effectiveness of long-term combination suppressive therapy on hirsutism, acne, and menstrual disturbances in patients with PCOS and to identify the elements that could predict therapeutic response.
Details of the study
This chart review examined data from 200 nondiabetic patients with PCOS who presented between October 1987 and June 2002. PCOS diagnosis was based on the National Institutes of Health (NIH) 1990 criteria. During the initial visit, patients underwent a detailed medical history and physical exam, including a modified Ferriman-Gallwey hirsutism score and hormonal evaluation.
Treatment regimens. Patients were treated with suppressive therapy that consisted of an oral contraceptive (OC) (35 µg ethinyl estradiol plus 1 mg ethynodiol diacetate), an antiandrogen (spironolactone 200 mg/day), or a combination of these drugs. They were followed every 4 to 12 months (mean follow-up time, 34.2 months; range, 6–155 months), and subjective therapy response was assessed from medical records and by improvements in hirsutism scores.
Study findings. The 138 patients treated with combination suppressive therapy reported higher rates of subjective improvement in hirsutism compared with patients treated with other regimens (89.9% vs 72.0%, P<.0001). They also had a significant objective reduction in their modified Ferriman-Gallwey hirsutism score (6.0 vs 3.2; P = .0001). The combination therapy was superior to either regimen alone; the response to therapy for symptom resolution took at least 6 months and continued for up to 60 months of combination suppressive therapy.
Adding electrolysis treatment to the combination regimen resulted in improved patient satisfaction, but the differences were not significant. Patients’ satisfaction with the therapeutic response could be predicted from their pretreatment hirsutism scores or circulating sex hormone–binding globulin levels.
Study strengths and weaknesses
The study’s major strengths are the large number of patients included, the uniformity of criteria for diagnosis, and the prolonged follow-up. This is one of the few studies to report the impact of therapy on health-related quality of life in patients with PCOS and to assess response to therapy with use of objective measures, such as changes in the modified Ferriman-Gallwey score.
However, the criteria used to diagnose PCOS—the NIH 1990 criteria—currently are used less commonly than the Rotterdam 2003 criteria, and they are less inclusive for the diagnosis of PCOS.
The OC pill formulation used in this study contained the progestogen ethynodiol diacetate, which is not used routinely in modern clinical practice. In addition, the majority of patients were non-Hispanic white, which limits extrapolating these findings to other races and ethnicities.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This retrospective study offers Level II evidence confirming the superiority of a combined OC plus spironolactone (compared with either agent alone) in the treatment of hirsutism in women with PCOS. In addition, this study emphasizes the importance of using combination suppressive therapy for at least 6 months to see a clinical response. Electrolysis may be helpful to patients especially during the initial 6 months of suppressive treatment. Finally, spironolactone alone could be reserved for cases in which OCs are contraindicated in women not interested in becoming pregnant.
In our practice, we treat patients with hirsutism using OC pills containing the progestogen levonorgestrel plus spironolactone at a lower dose of 100 mg/day, since patients treated with higher spironolactone doses report irregular bleeding and fatigue.
--ELIE HOBEIKA, MD, AND BERT SCOCCIA, MD
EXPERT COMMENTARY
Ezeh and colleagues conducted a retrospective analysis to evaluate the effectiveness of long-term combination suppressive therapy on hirsutism, acne, and menstrual disturbances in patients with PCOS and to identify the elements that could predict therapeutic response.
Details of the study
This chart review examined data from 200 nondiabetic patients with PCOS who presented between October 1987 and June 2002. PCOS diagnosis was based on the National Institutes of Health (NIH) 1990 criteria. During the initial visit, patients underwent a detailed medical history and physical exam, including a modified Ferriman-Gallwey hirsutism score and hormonal evaluation.
Treatment regimens. Patients were treated with suppressive therapy that consisted of an oral contraceptive (OC) (35 µg ethinyl estradiol plus 1 mg ethynodiol diacetate), an antiandrogen (spironolactone 200 mg/day), or a combination of these drugs. They were followed every 4 to 12 months (mean follow-up time, 34.2 months; range, 6–155 months), and subjective therapy response was assessed from medical records and by improvements in hirsutism scores.
Study findings. The 138 patients treated with combination suppressive therapy reported higher rates of subjective improvement in hirsutism compared with patients treated with other regimens (89.9% vs 72.0%, P<.0001). They also had a significant objective reduction in their modified Ferriman-Gallwey hirsutism score (6.0 vs 3.2; P = .0001). The combination therapy was superior to either regimen alone; the response to therapy for symptom resolution took at least 6 months and continued for up to 60 months of combination suppressive therapy.
Adding electrolysis treatment to the combination regimen resulted in improved patient satisfaction, but the differences were not significant. Patients’ satisfaction with the therapeutic response could be predicted from their pretreatment hirsutism scores or circulating sex hormone–binding globulin levels.
Study strengths and weaknesses
The study’s major strengths are the large number of patients included, the uniformity of criteria for diagnosis, and the prolonged follow-up. This is one of the few studies to report the impact of therapy on health-related quality of life in patients with PCOS and to assess response to therapy with use of objective measures, such as changes in the modified Ferriman-Gallwey score.
However, the criteria used to diagnose PCOS—the NIH 1990 criteria—currently are used less commonly than the Rotterdam 2003 criteria, and they are less inclusive for the diagnosis of PCOS.
The OC pill formulation used in this study contained the progestogen ethynodiol diacetate, which is not used routinely in modern clinical practice. In addition, the majority of patients were non-Hispanic white, which limits extrapolating these findings to other races and ethnicities.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This retrospective study offers Level II evidence confirming the superiority of a combined OC plus spironolactone (compared with either agent alone) in the treatment of hirsutism in women with PCOS. In addition, this study emphasizes the importance of using combination suppressive therapy for at least 6 months to see a clinical response. Electrolysis may be helpful to patients especially during the initial 6 months of suppressive treatment. Finally, spironolactone alone could be reserved for cases in which OCs are contraindicated in women not interested in becoming pregnant.
In our practice, we treat patients with hirsutism using OC pills containing the progestogen levonorgestrel plus spironolactone at a lower dose of 100 mg/day, since patients treated with higher spironolactone doses report irregular bleeding and fatigue.
--ELIE HOBEIKA, MD, AND BERT SCOCCIA, MD
EXPERT COMMENTARY
Ezeh and colleagues conducted a retrospective analysis to evaluate the effectiveness of long-term combination suppressive therapy on hirsutism, acne, and menstrual disturbances in patients with PCOS and to identify the elements that could predict therapeutic response.
Details of the study
This chart review examined data from 200 nondiabetic patients with PCOS who presented between October 1987 and June 2002. PCOS diagnosis was based on the National Institutes of Health (NIH) 1990 criteria. During the initial visit, patients underwent a detailed medical history and physical exam, including a modified Ferriman-Gallwey hirsutism score and hormonal evaluation.
Treatment regimens. Patients were treated with suppressive therapy that consisted of an oral contraceptive (OC) (35 µg ethinyl estradiol plus 1 mg ethynodiol diacetate), an antiandrogen (spironolactone 200 mg/day), or a combination of these drugs. They were followed every 4 to 12 months (mean follow-up time, 34.2 months; range, 6–155 months), and subjective therapy response was assessed from medical records and by improvements in hirsutism scores.
Study findings. The 138 patients treated with combination suppressive therapy reported higher rates of subjective improvement in hirsutism compared with patients treated with other regimens (89.9% vs 72.0%, P<.0001). They also had a significant objective reduction in their modified Ferriman-Gallwey hirsutism score (6.0 vs 3.2; P = .0001). The combination therapy was superior to either regimen alone; the response to therapy for symptom resolution took at least 6 months and continued for up to 60 months of combination suppressive therapy.
Adding electrolysis treatment to the combination regimen resulted in improved patient satisfaction, but the differences were not significant. Patients’ satisfaction with the therapeutic response could be predicted from their pretreatment hirsutism scores or circulating sex hormone–binding globulin levels.
Study strengths and weaknesses
The study’s major strengths are the large number of patients included, the uniformity of criteria for diagnosis, and the prolonged follow-up. This is one of the few studies to report the impact of therapy on health-related quality of life in patients with PCOS and to assess response to therapy with use of objective measures, such as changes in the modified Ferriman-Gallwey score.
However, the criteria used to diagnose PCOS—the NIH 1990 criteria—currently are used less commonly than the Rotterdam 2003 criteria, and they are less inclusive for the diagnosis of PCOS.
The OC pill formulation used in this study contained the progestogen ethynodiol diacetate, which is not used routinely in modern clinical practice. In addition, the majority of patients were non-Hispanic white, which limits extrapolating these findings to other races and ethnicities.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This retrospective study offers Level II evidence confirming the superiority of a combined OC plus spironolactone (compared with either agent alone) in the treatment of hirsutism in women with PCOS. In addition, this study emphasizes the importance of using combination suppressive therapy for at least 6 months to see a clinical response. Electrolysis may be helpful to patients especially during the initial 6 months of suppressive treatment. Finally, spironolactone alone could be reserved for cases in which OCs are contraindicated in women not interested in becoming pregnant.
In our practice, we treat patients with hirsutism using OC pills containing the progestogen levonorgestrel plus spironolactone at a lower dose of 100 mg/day, since patients treated with higher spironolactone doses report irregular bleeding and fatigue.
--ELIE HOBEIKA, MD, AND BERT SCOCCIA, MD
Are women seeking short-acting contraception satisfied with LARC after giving it a try?
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
New and promising GSM treatments, more clinical takeaways from NAMS 2018
Learn more about NAMS: http://www.menopause.org/home
Learn more about NAMS: http://www.menopause.org/home
Learn more about NAMS: http://www.menopause.org/home
How can we best use diagnostic brain imaging in pregnant women with severe headache?
WHAT DOES THIS MEAN FOR PRACTICE?
- Acute, severe headache in pregnancy needs immediate attention when it includes:
- seizures
- altered sensorium, or
- loss of consciousness
- An appropriate threshold utilizing history and clinical diagnosis must be set for obtaining neurologic consultation and for the consultant to obtain imaging
- Brain scans can identify symptomatic pathologic results (27.6% in this study)
- Theoretical concerns about imaging call for the OB to be very involved in evaluation and management
- OB and neurologist should discuss risks and benefits of imaging throughout care
Addressing your patient's sexual function after cancer
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.