User login
Spare the nerves in deep infiltrative endometriosis surgery
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
Indications and techniques for multifetal pregnancy reduction
Multifetal pregnancy reduction (MPR) was developed in the 1980s in the wake of significant increases in the incidence of triplets and other higher-order multiples emanating from assisted reproductive technologies (ART). It was offered to reduce fetal number and improve outcomes for remaining fetuses by reducing rates of preterm delivery, fetal growth restriction, and other adverse perinatal outcomes, as well as maternal complications such as preeclampsia and postpartum hemorrhage.
In recent years, improvements in ART – mainly changes in ovulation induction practices and limitations in the number of embryos implanted to two at most – have reversed the increase in higher-order multiples. However, with intrauterine insemination, higher-order multiples still occur, and even without any reproductive assistance, the reality is that multiple pregnancies – particularly twins – continue to exist. In 2018, twins comprised about 3% of births in the United States.1
Twin pregnancies have a significantly higher risk than singleton gestations of preterm birth, maternal complications, and neonatal morbidity and mortality. The pregnancies are complicated more often by preterm premature rupture of membranes, fetal growth restriction, and hypertensive disorders of pregnancy.
Monochorionic diamniotic twin pregnancies face additional, unique risks of twin-to-twin transfusion syndrome, twin reversed arterial perfusion sequence, and twin-anemia polycythemia sequence. These pregnancies account for about 20% of all twin gestations, and decades of experience with ART have shown us that monochorionic diamniotic gestations occur at a higher rate after in-vitro fertilization.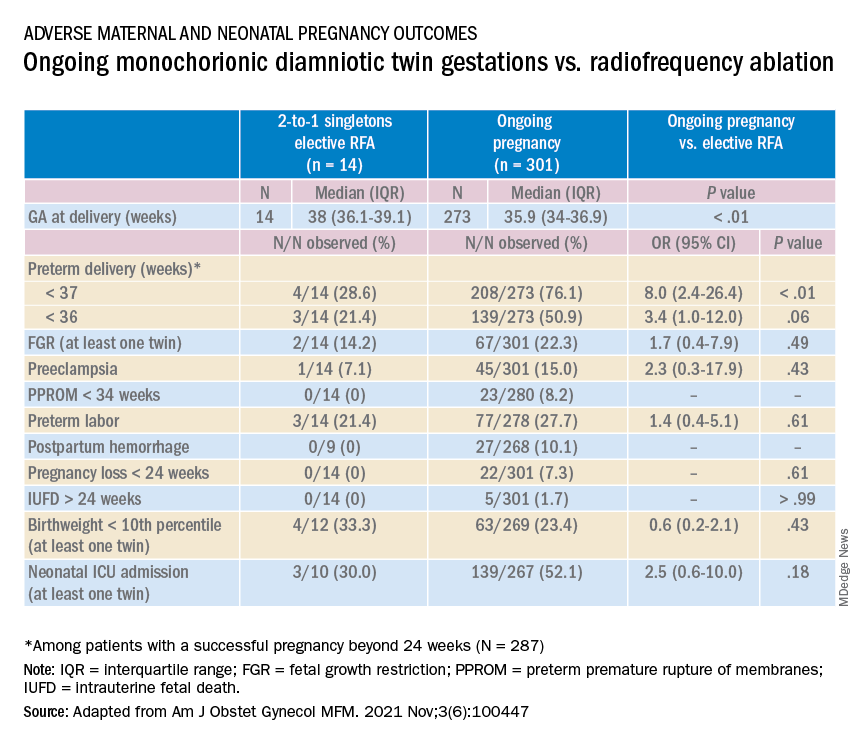
Although advances have improved the outcomes of multiple births, risks remain and elective MPR is still very relevant for twin gestations. Patients routinely receive counseling about the risks of twin gestations, but they often are not made aware of the option of elective fetal reduction.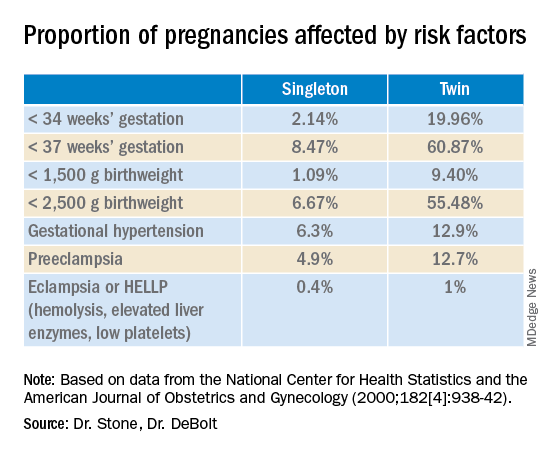
We have offered elective reduction (of nonanomalous fetuses) to a singleton for almost 30 years and have published several reports documenting that MPR in dichorionic diamniotic pregnancies reduces the risk of preterm delivery and other complications without increasing the risk of pregnancy loss.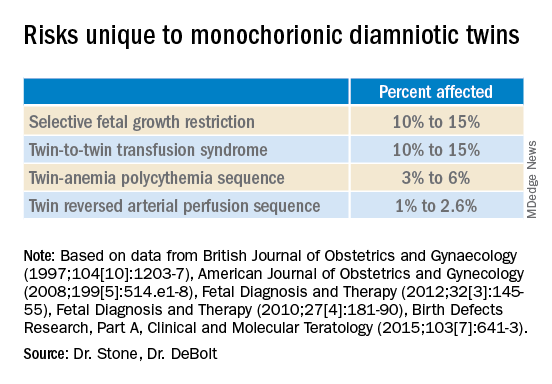
Most recently, we also published data comparing the outcomes of patients with monochorionic diamniotic gestations who underwent elective MPR by radiofrequency ablation (RFA) vs. those with ongoing monochorionic diamniotic gestations.2 While the numbers were small, the data show significantly lower rates of preterm birth without an increased risk of pregnancy loss.
Experience with dichorionic diamniotic twins, genetic testing
Our most recent review3 of outcomes in dichorionic diamniotic gestations covered 855 patients, 29% of whom underwent planned elective MPR at less than 15 weeks, and 71% of whom had ongoing twin gestations. Those with ongoing twin gestations had adjusted odds ratios of preterm delivery at less than 37 weeks and less than 34 weeks of 5.62 and 2.22, respectively (adjustments controlled for maternal characteristics such as maternal age, BMI, use of chorionic villus sampling [CVS], and history of preterm birth).
Ongoing twin pregnancies were also more likely to have preeclampsia (AOR, 3.33), preterm premature rupture of membranes (3.86), and low birthweight (under the 5th and 10th percentiles). There were no significant differences in the rate of unintended pregnancy loss (2.4% vs. 2.3%), and rates for total pregnancy loss at less than 24 weeks and less than 20 weeks were similar.
An important issue in the consideration of MPR is that prenatal diagnosis of chromosomal abnormalities is very safe in twins. Multiple gestations are at greater risk of chromosomal abnormalities, so performing MPR selectively – if a chromosomally abnormal fetus is present – is desirable for many parents.
A recent meta-analysis and systematic review of studies reporting fetal loss following amniocentesis or CVS in twin pregnancies found an exceedingly low risk of loss. Procedure-related fetal loss (the primary outcome) was lower than previously reported, and the rate of fetal loss before 24 weeks gestation or within 4 weeks after the procedure (secondary outcomes), did not differ from the background risk in twin pregnancies not undergoing invasive prenatal testing.4
Our data have shown no significant differences in pregnancy loss between patients who underwent CVS prior to MPR and those who did not. Looking specifically at reduction to a singleton gestation, patients who underwent CVS prior to MPR had a fourfold reduction in loss.5 Therefore, we counsel patients that CVS provides useful information – especially now with the common use of chromosomal microarray – at almost negligible risk.
MPR for monochorionic diamniotic twins
Most of the literature on MPR from twin to singleton gestations reports on intrathoracic potassium chloride injection used in dichorionic diamniotic twins.
MPR in monochorionic diamniotic twins is reserved in the United States for monochorionic pregnancies in which there are severe fetal anomalies, severe growth restriction, or other significant complications. It is performed in such cases around 20 weeks gestation. However, given the significant risks of monochorionic twin pregnancies, we also have been offering MPR electively and earlier in pregnancy. While many modalities of intrafetal cord occlusion exist, RFA at the cord insertion site into the fetal abdomen is our preferred technique.
In our retrospective review of 315 monochorionic diamniotic twin gestations, the 14 patients who had RFA electively had no pregnancy losses and a significantly lower rate of preterm birth at less than 37-weeks gestation, compared with 301 ongoing monochorionic diamniotic twin pregnancies (29% vs. 76%).5 Reduction with RFA, performed at a mean gestational age of 15 weeks, also eliminated the risks unique to monochorionic twins, such as twin-to-twin transfusion syndrome and twin-anemia polycythemia sequence. (Of the ongoing twin gestations, 12% required medically indicated RFA, fetoscopic laser ablation, and/or amnioreduction; 4% had unintended loss of one fetus; and 4% had unintended loss of both fetuses before 24 weeks’ gestation. Fewer than 70% of the ongoing twin gestations had none of the significant adverse outcomes unique to monochorionic twins.)
Interestingly, there were still a couple of cases of fetal growth restriction in patients who underwent elective MPR – a rate higher than that seen in singleton gestations – most likely because of the early timing of the procedure.
Our numbers of MPRs in this review were small, but the data offer at least preliminary evidence that planned elective RFA before 17 weeks gestation may be offered to patients who do not want to assume the risks of monochorionic diamniotic twin pregnancies.
Counseling in twin pregnancies
We perform thorough, early assessments of fetal anatomy in our twin pregnancies, and we undertake thorough medical and obstetrical histories to uncover birth complications or medical conditions that would increase risks of preeclampsia, preterm birth, fetal growth restriction, and other complications.
Because monochorionic gestations are at particularly high risk for heart defects, we also routinely perform fetal echocardiography in these pregnancies.
Genetic testing is offered to all twin pregnancies, and as mentioned above, we especially counsel those considering MPR that such testing provides useful information.
Patients are made aware of the option of MPR and receive nondirective counseling. It is the patient’s choice. We recognize that elective termination is a controversial procedure, but we believe that the option of MPR should be available to patients who want to improve outcomes for their pregnancy.
When anomalies are discovered and selective termination is chosen, we usually try to perform MPR as early as possible. After 16 weeks, we’ve found, the rate of pregnancy loss increases slightly.
Dr. Stone is the Ellen and Howard C. Katz Chairman’s Chair, and Dr. DeBolt is a clinical fellow in maternal-fetal medicine, in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Martin JA and Osterman MJK. National Center of Health Statistics. NCHS Data Brief, 2019;no 351.
2. Manasa GR et al. Am J Obstet Gynecol MFM 2021;3:100447.
3. Vieira LA et al. Am J. Obstet Gynecol. 2019;221:253.e1-8.
4. Di Mascio et al. Ultrasound Obstet Gynecol 2020 Nov;56(5):647-55.
5. Ferrara L et al. Am J Obstet Gynecol. 2008 Oct;199(4):408.e1-4.
Multifetal pregnancy reduction (MPR) was developed in the 1980s in the wake of significant increases in the incidence of triplets and other higher-order multiples emanating from assisted reproductive technologies (ART). It was offered to reduce fetal number and improve outcomes for remaining fetuses by reducing rates of preterm delivery, fetal growth restriction, and other adverse perinatal outcomes, as well as maternal complications such as preeclampsia and postpartum hemorrhage.
In recent years, improvements in ART – mainly changes in ovulation induction practices and limitations in the number of embryos implanted to two at most – have reversed the increase in higher-order multiples. However, with intrauterine insemination, higher-order multiples still occur, and even without any reproductive assistance, the reality is that multiple pregnancies – particularly twins – continue to exist. In 2018, twins comprised about 3% of births in the United States.1
Twin pregnancies have a significantly higher risk than singleton gestations of preterm birth, maternal complications, and neonatal morbidity and mortality. The pregnancies are complicated more often by preterm premature rupture of membranes, fetal growth restriction, and hypertensive disorders of pregnancy.
Monochorionic diamniotic twin pregnancies face additional, unique risks of twin-to-twin transfusion syndrome, twin reversed arterial perfusion sequence, and twin-anemia polycythemia sequence. These pregnancies account for about 20% of all twin gestations, and decades of experience with ART have shown us that monochorionic diamniotic gestations occur at a higher rate after in-vitro fertilization.
Although advances have improved the outcomes of multiple births, risks remain and elective MPR is still very relevant for twin gestations. Patients routinely receive counseling about the risks of twin gestations, but they often are not made aware of the option of elective fetal reduction.
We have offered elective reduction (of nonanomalous fetuses) to a singleton for almost 30 years and have published several reports documenting that MPR in dichorionic diamniotic pregnancies reduces the risk of preterm delivery and other complications without increasing the risk of pregnancy loss.
Most recently, we also published data comparing the outcomes of patients with monochorionic diamniotic gestations who underwent elective MPR by radiofrequency ablation (RFA) vs. those with ongoing monochorionic diamniotic gestations.2 While the numbers were small, the data show significantly lower rates of preterm birth without an increased risk of pregnancy loss.
Experience with dichorionic diamniotic twins, genetic testing
Our most recent review3 of outcomes in dichorionic diamniotic gestations covered 855 patients, 29% of whom underwent planned elective MPR at less than 15 weeks, and 71% of whom had ongoing twin gestations. Those with ongoing twin gestations had adjusted odds ratios of preterm delivery at less than 37 weeks and less than 34 weeks of 5.62 and 2.22, respectively (adjustments controlled for maternal characteristics such as maternal age, BMI, use of chorionic villus sampling [CVS], and history of preterm birth).
Ongoing twin pregnancies were also more likely to have preeclampsia (AOR, 3.33), preterm premature rupture of membranes (3.86), and low birthweight (under the 5th and 10th percentiles). There were no significant differences in the rate of unintended pregnancy loss (2.4% vs. 2.3%), and rates for total pregnancy loss at less than 24 weeks and less than 20 weeks were similar.
An important issue in the consideration of MPR is that prenatal diagnosis of chromosomal abnormalities is very safe in twins. Multiple gestations are at greater risk of chromosomal abnormalities, so performing MPR selectively – if a chromosomally abnormal fetus is present – is desirable for many parents.
A recent meta-analysis and systematic review of studies reporting fetal loss following amniocentesis or CVS in twin pregnancies found an exceedingly low risk of loss. Procedure-related fetal loss (the primary outcome) was lower than previously reported, and the rate of fetal loss before 24 weeks gestation or within 4 weeks after the procedure (secondary outcomes), did not differ from the background risk in twin pregnancies not undergoing invasive prenatal testing.4
Our data have shown no significant differences in pregnancy loss between patients who underwent CVS prior to MPR and those who did not. Looking specifically at reduction to a singleton gestation, patients who underwent CVS prior to MPR had a fourfold reduction in loss.5 Therefore, we counsel patients that CVS provides useful information – especially now with the common use of chromosomal microarray – at almost negligible risk.
MPR for monochorionic diamniotic twins
Most of the literature on MPR from twin to singleton gestations reports on intrathoracic potassium chloride injection used in dichorionic diamniotic twins.
MPR in monochorionic diamniotic twins is reserved in the United States for monochorionic pregnancies in which there are severe fetal anomalies, severe growth restriction, or other significant complications. It is performed in such cases around 20 weeks gestation. However, given the significant risks of monochorionic twin pregnancies, we also have been offering MPR electively and earlier in pregnancy. While many modalities of intrafetal cord occlusion exist, RFA at the cord insertion site into the fetal abdomen is our preferred technique.
In our retrospective review of 315 monochorionic diamniotic twin gestations, the 14 patients who had RFA electively had no pregnancy losses and a significantly lower rate of preterm birth at less than 37-weeks gestation, compared with 301 ongoing monochorionic diamniotic twin pregnancies (29% vs. 76%).5 Reduction with RFA, performed at a mean gestational age of 15 weeks, also eliminated the risks unique to monochorionic twins, such as twin-to-twin transfusion syndrome and twin-anemia polycythemia sequence. (Of the ongoing twin gestations, 12% required medically indicated RFA, fetoscopic laser ablation, and/or amnioreduction; 4% had unintended loss of one fetus; and 4% had unintended loss of both fetuses before 24 weeks’ gestation. Fewer than 70% of the ongoing twin gestations had none of the significant adverse outcomes unique to monochorionic twins.)
Interestingly, there were still a couple of cases of fetal growth restriction in patients who underwent elective MPR – a rate higher than that seen in singleton gestations – most likely because of the early timing of the procedure.
Our numbers of MPRs in this review were small, but the data offer at least preliminary evidence that planned elective RFA before 17 weeks gestation may be offered to patients who do not want to assume the risks of monochorionic diamniotic twin pregnancies.
Counseling in twin pregnancies
We perform thorough, early assessments of fetal anatomy in our twin pregnancies, and we undertake thorough medical and obstetrical histories to uncover birth complications or medical conditions that would increase risks of preeclampsia, preterm birth, fetal growth restriction, and other complications.
Because monochorionic gestations are at particularly high risk for heart defects, we also routinely perform fetal echocardiography in these pregnancies.
Genetic testing is offered to all twin pregnancies, and as mentioned above, we especially counsel those considering MPR that such testing provides useful information.
Patients are made aware of the option of MPR and receive nondirective counseling. It is the patient’s choice. We recognize that elective termination is a controversial procedure, but we believe that the option of MPR should be available to patients who want to improve outcomes for their pregnancy.
When anomalies are discovered and selective termination is chosen, we usually try to perform MPR as early as possible. After 16 weeks, we’ve found, the rate of pregnancy loss increases slightly.
Dr. Stone is the Ellen and Howard C. Katz Chairman’s Chair, and Dr. DeBolt is a clinical fellow in maternal-fetal medicine, in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Martin JA and Osterman MJK. National Center of Health Statistics. NCHS Data Brief, 2019;no 351.
2. Manasa GR et al. Am J Obstet Gynecol MFM 2021;3:100447.
3. Vieira LA et al. Am J. Obstet Gynecol. 2019;221:253.e1-8.
4. Di Mascio et al. Ultrasound Obstet Gynecol 2020 Nov;56(5):647-55.
5. Ferrara L et al. Am J Obstet Gynecol. 2008 Oct;199(4):408.e1-4.
Multifetal pregnancy reduction (MPR) was developed in the 1980s in the wake of significant increases in the incidence of triplets and other higher-order multiples emanating from assisted reproductive technologies (ART). It was offered to reduce fetal number and improve outcomes for remaining fetuses by reducing rates of preterm delivery, fetal growth restriction, and other adverse perinatal outcomes, as well as maternal complications such as preeclampsia and postpartum hemorrhage.
In recent years, improvements in ART – mainly changes in ovulation induction practices and limitations in the number of embryos implanted to two at most – have reversed the increase in higher-order multiples. However, with intrauterine insemination, higher-order multiples still occur, and even without any reproductive assistance, the reality is that multiple pregnancies – particularly twins – continue to exist. In 2018, twins comprised about 3% of births in the United States.1
Twin pregnancies have a significantly higher risk than singleton gestations of preterm birth, maternal complications, and neonatal morbidity and mortality. The pregnancies are complicated more often by preterm premature rupture of membranes, fetal growth restriction, and hypertensive disorders of pregnancy.
Monochorionic diamniotic twin pregnancies face additional, unique risks of twin-to-twin transfusion syndrome, twin reversed arterial perfusion sequence, and twin-anemia polycythemia sequence. These pregnancies account for about 20% of all twin gestations, and decades of experience with ART have shown us that monochorionic diamniotic gestations occur at a higher rate after in-vitro fertilization.
Although advances have improved the outcomes of multiple births, risks remain and elective MPR is still very relevant for twin gestations. Patients routinely receive counseling about the risks of twin gestations, but they often are not made aware of the option of elective fetal reduction.
We have offered elective reduction (of nonanomalous fetuses) to a singleton for almost 30 years and have published several reports documenting that MPR in dichorionic diamniotic pregnancies reduces the risk of preterm delivery and other complications without increasing the risk of pregnancy loss.
Most recently, we also published data comparing the outcomes of patients with monochorionic diamniotic gestations who underwent elective MPR by radiofrequency ablation (RFA) vs. those with ongoing monochorionic diamniotic gestations.2 While the numbers were small, the data show significantly lower rates of preterm birth without an increased risk of pregnancy loss.
Experience with dichorionic diamniotic twins, genetic testing
Our most recent review3 of outcomes in dichorionic diamniotic gestations covered 855 patients, 29% of whom underwent planned elective MPR at less than 15 weeks, and 71% of whom had ongoing twin gestations. Those with ongoing twin gestations had adjusted odds ratios of preterm delivery at less than 37 weeks and less than 34 weeks of 5.62 and 2.22, respectively (adjustments controlled for maternal characteristics such as maternal age, BMI, use of chorionic villus sampling [CVS], and history of preterm birth).
Ongoing twin pregnancies were also more likely to have preeclampsia (AOR, 3.33), preterm premature rupture of membranes (3.86), and low birthweight (under the 5th and 10th percentiles). There were no significant differences in the rate of unintended pregnancy loss (2.4% vs. 2.3%), and rates for total pregnancy loss at less than 24 weeks and less than 20 weeks were similar.
An important issue in the consideration of MPR is that prenatal diagnosis of chromosomal abnormalities is very safe in twins. Multiple gestations are at greater risk of chromosomal abnormalities, so performing MPR selectively – if a chromosomally abnormal fetus is present – is desirable for many parents.
A recent meta-analysis and systematic review of studies reporting fetal loss following amniocentesis or CVS in twin pregnancies found an exceedingly low risk of loss. Procedure-related fetal loss (the primary outcome) was lower than previously reported, and the rate of fetal loss before 24 weeks gestation or within 4 weeks after the procedure (secondary outcomes), did not differ from the background risk in twin pregnancies not undergoing invasive prenatal testing.4
Our data have shown no significant differences in pregnancy loss between patients who underwent CVS prior to MPR and those who did not. Looking specifically at reduction to a singleton gestation, patients who underwent CVS prior to MPR had a fourfold reduction in loss.5 Therefore, we counsel patients that CVS provides useful information – especially now with the common use of chromosomal microarray – at almost negligible risk.
MPR for monochorionic diamniotic twins
Most of the literature on MPR from twin to singleton gestations reports on intrathoracic potassium chloride injection used in dichorionic diamniotic twins.
MPR in monochorionic diamniotic twins is reserved in the United States for monochorionic pregnancies in which there are severe fetal anomalies, severe growth restriction, or other significant complications. It is performed in such cases around 20 weeks gestation. However, given the significant risks of monochorionic twin pregnancies, we also have been offering MPR electively and earlier in pregnancy. While many modalities of intrafetal cord occlusion exist, RFA at the cord insertion site into the fetal abdomen is our preferred technique.
In our retrospective review of 315 monochorionic diamniotic twin gestations, the 14 patients who had RFA electively had no pregnancy losses and a significantly lower rate of preterm birth at less than 37-weeks gestation, compared with 301 ongoing monochorionic diamniotic twin pregnancies (29% vs. 76%).5 Reduction with RFA, performed at a mean gestational age of 15 weeks, also eliminated the risks unique to monochorionic twins, such as twin-to-twin transfusion syndrome and twin-anemia polycythemia sequence. (Of the ongoing twin gestations, 12% required medically indicated RFA, fetoscopic laser ablation, and/or amnioreduction; 4% had unintended loss of one fetus; and 4% had unintended loss of both fetuses before 24 weeks’ gestation. Fewer than 70% of the ongoing twin gestations had none of the significant adverse outcomes unique to monochorionic twins.)
Interestingly, there were still a couple of cases of fetal growth restriction in patients who underwent elective MPR – a rate higher than that seen in singleton gestations – most likely because of the early timing of the procedure.
Our numbers of MPRs in this review were small, but the data offer at least preliminary evidence that planned elective RFA before 17 weeks gestation may be offered to patients who do not want to assume the risks of monochorionic diamniotic twin pregnancies.
Counseling in twin pregnancies
We perform thorough, early assessments of fetal anatomy in our twin pregnancies, and we undertake thorough medical and obstetrical histories to uncover birth complications or medical conditions that would increase risks of preeclampsia, preterm birth, fetal growth restriction, and other complications.
Because monochorionic gestations are at particularly high risk for heart defects, we also routinely perform fetal echocardiography in these pregnancies.
Genetic testing is offered to all twin pregnancies, and as mentioned above, we especially counsel those considering MPR that such testing provides useful information.
Patients are made aware of the option of MPR and receive nondirective counseling. It is the patient’s choice. We recognize that elective termination is a controversial procedure, but we believe that the option of MPR should be available to patients who want to improve outcomes for their pregnancy.
When anomalies are discovered and selective termination is chosen, we usually try to perform MPR as early as possible. After 16 weeks, we’ve found, the rate of pregnancy loss increases slightly.
Dr. Stone is the Ellen and Howard C. Katz Chairman’s Chair, and Dr. DeBolt is a clinical fellow in maternal-fetal medicine, in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Martin JA and Osterman MJK. National Center of Health Statistics. NCHS Data Brief, 2019;no 351.
2. Manasa GR et al. Am J Obstet Gynecol MFM 2021;3:100447.
3. Vieira LA et al. Am J. Obstet Gynecol. 2019;221:253.e1-8.
4. Di Mascio et al. Ultrasound Obstet Gynecol 2020 Nov;56(5):647-55.
5. Ferrara L et al. Am J Obstet Gynecol. 2008 Oct;199(4):408.e1-4.
Managing maternal mortality with multifetal pregnancy reduction
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
Left upper quadrant entry is often a reliable alternative to umbilicus
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.
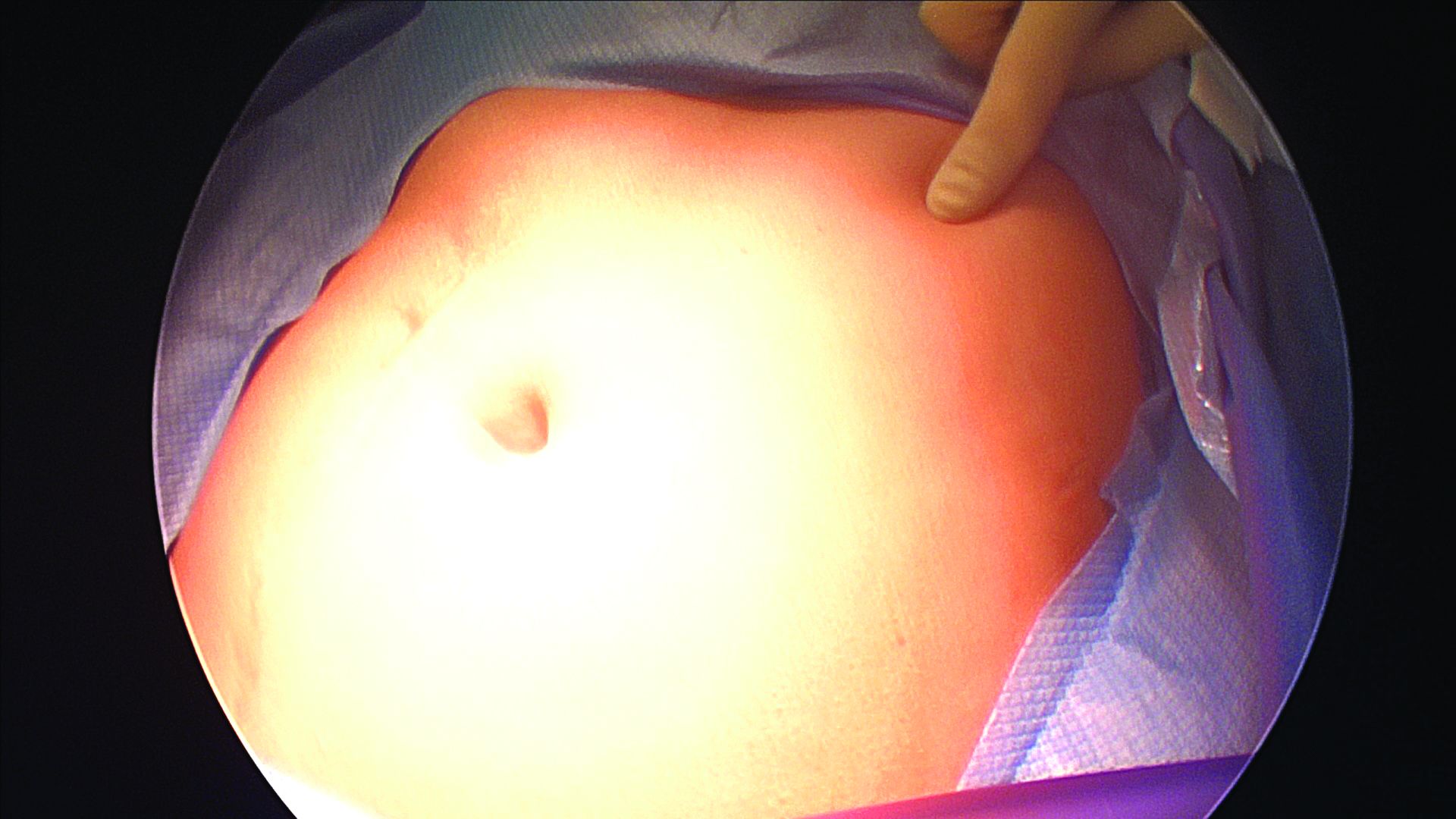
The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.
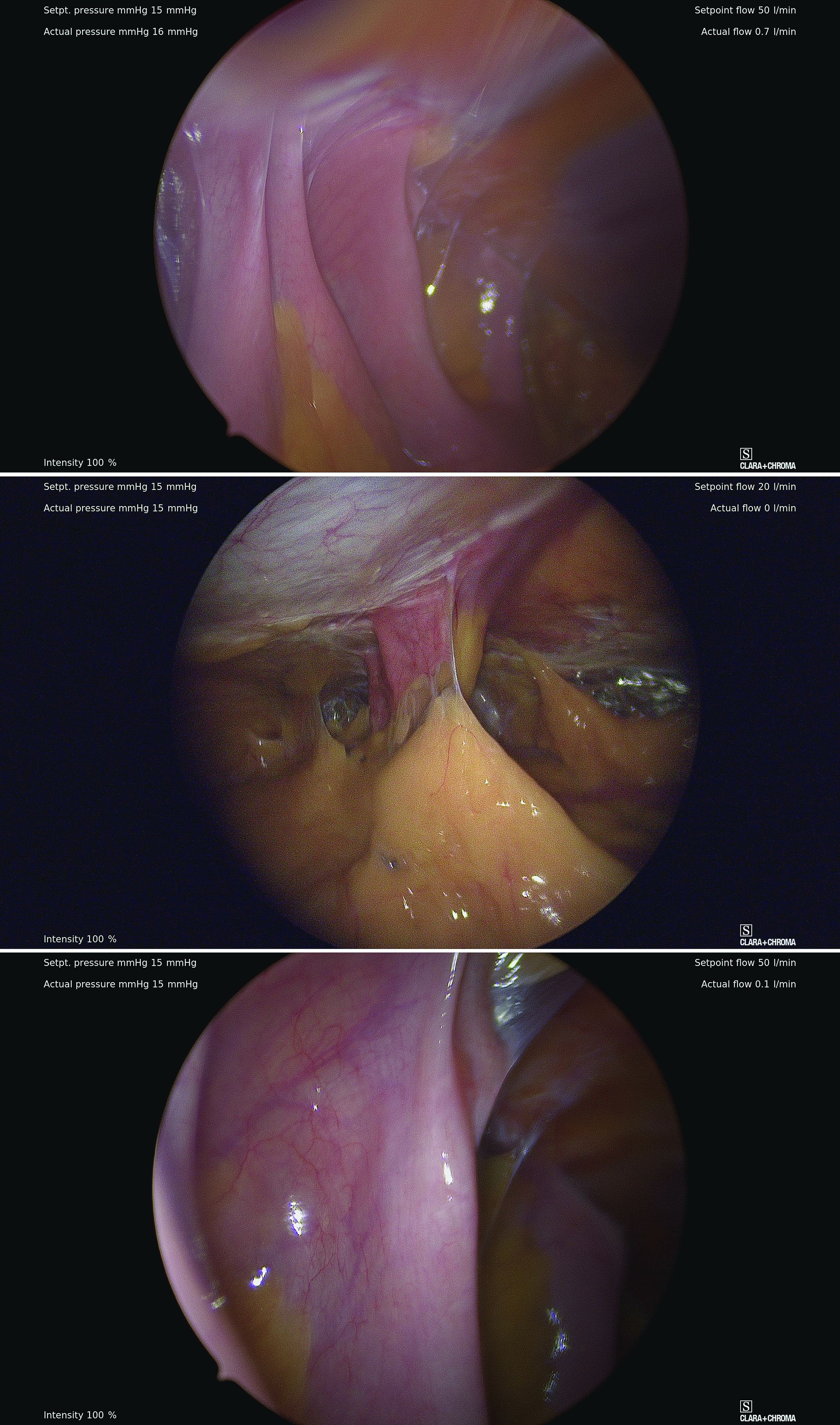
However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.

The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.

However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.

The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.

However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
Safe abdominal laparoscopic entry
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
How 100 years of insulin have changed pregnancy for women with type 1 diabetes
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%
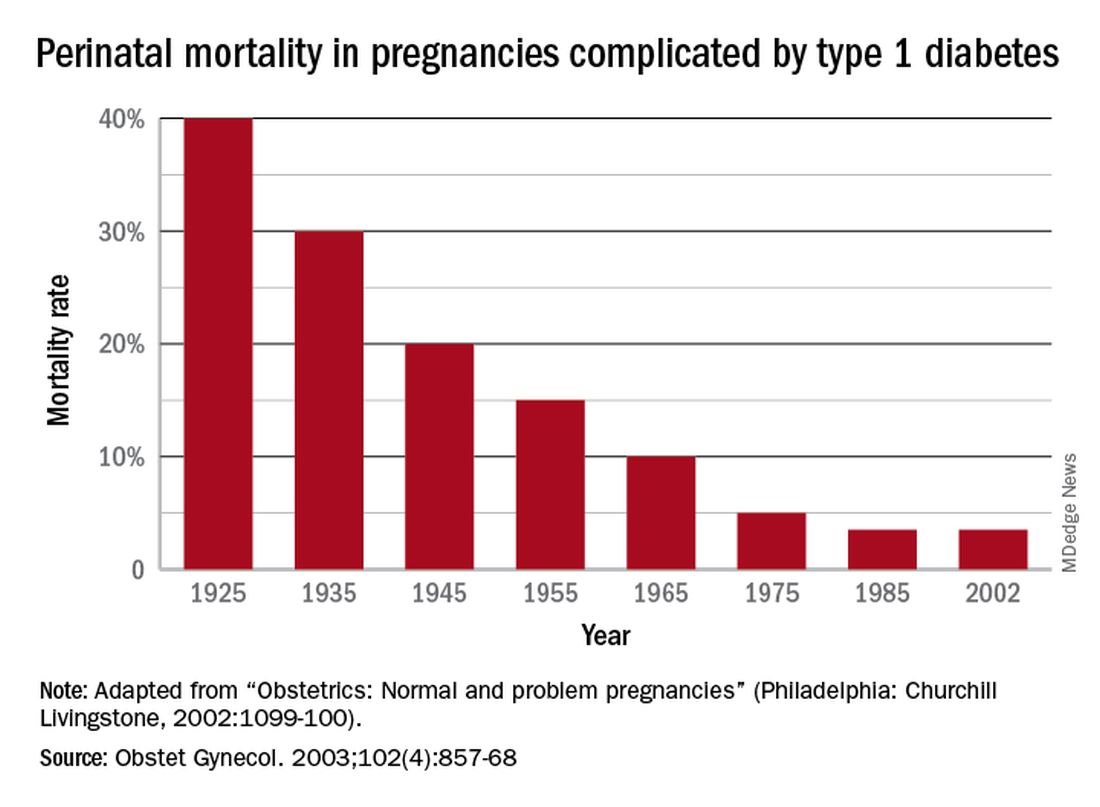
In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.
Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%

In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.

Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%

In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.

Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Insulin in pregnancy: A look back at history for Diabetes Awareness Month
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Laparoscopic abdominal cerclage: An effective, patient-sought approach for cervical insufficiency
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.
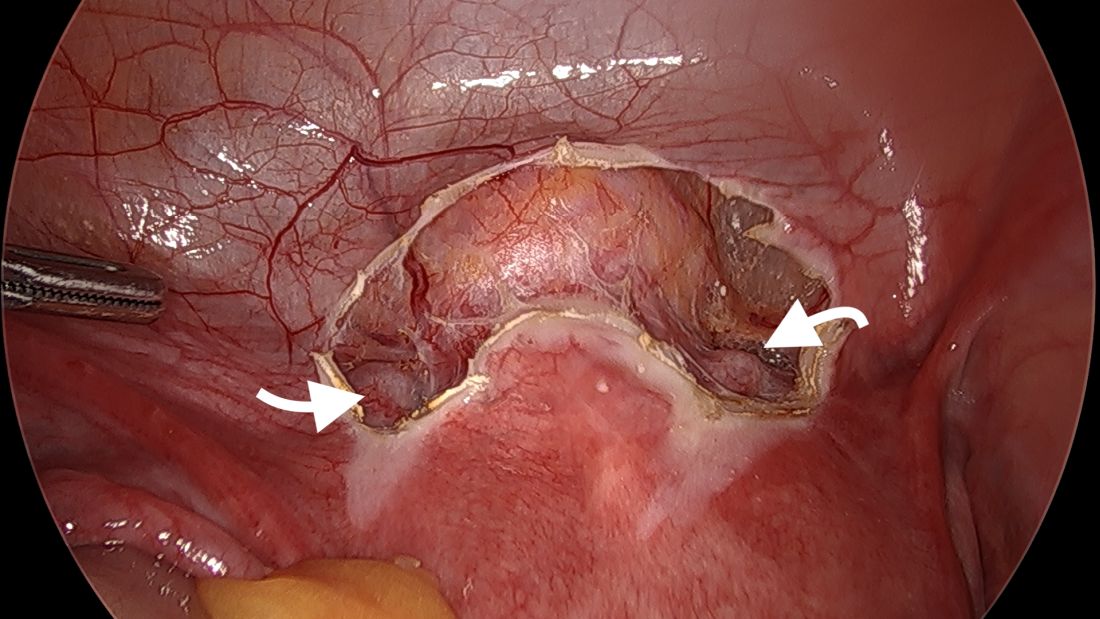
We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.
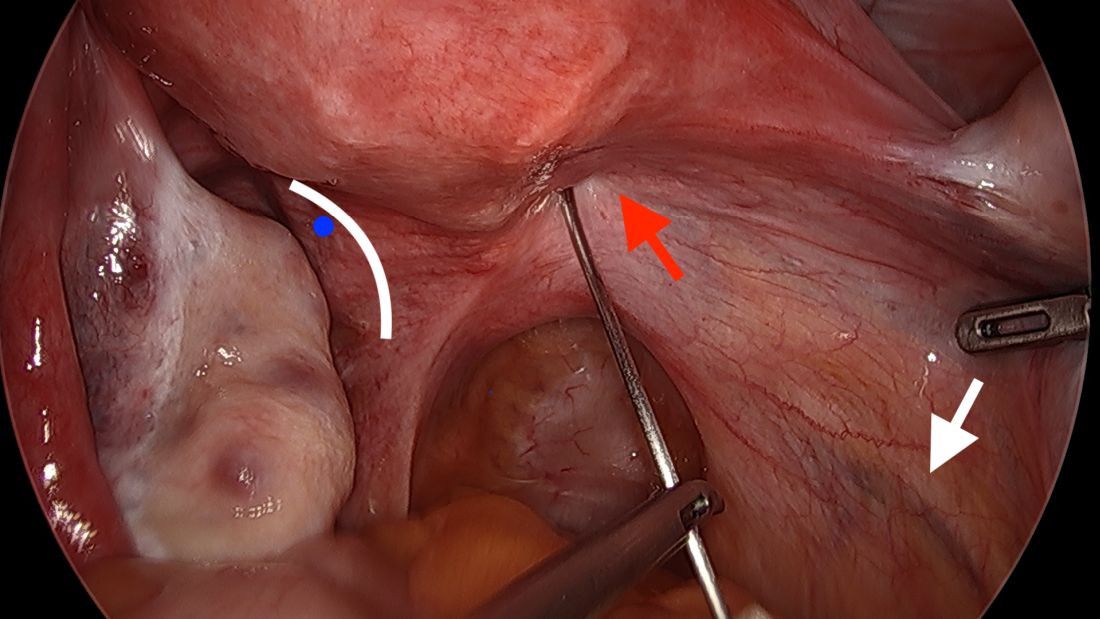
The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.
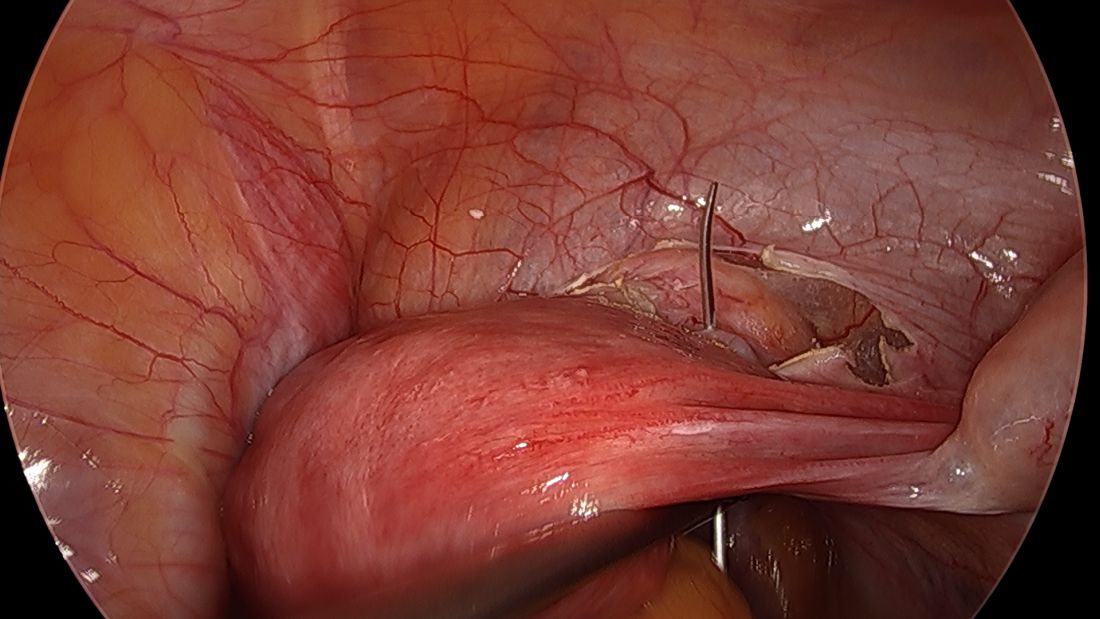
Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.
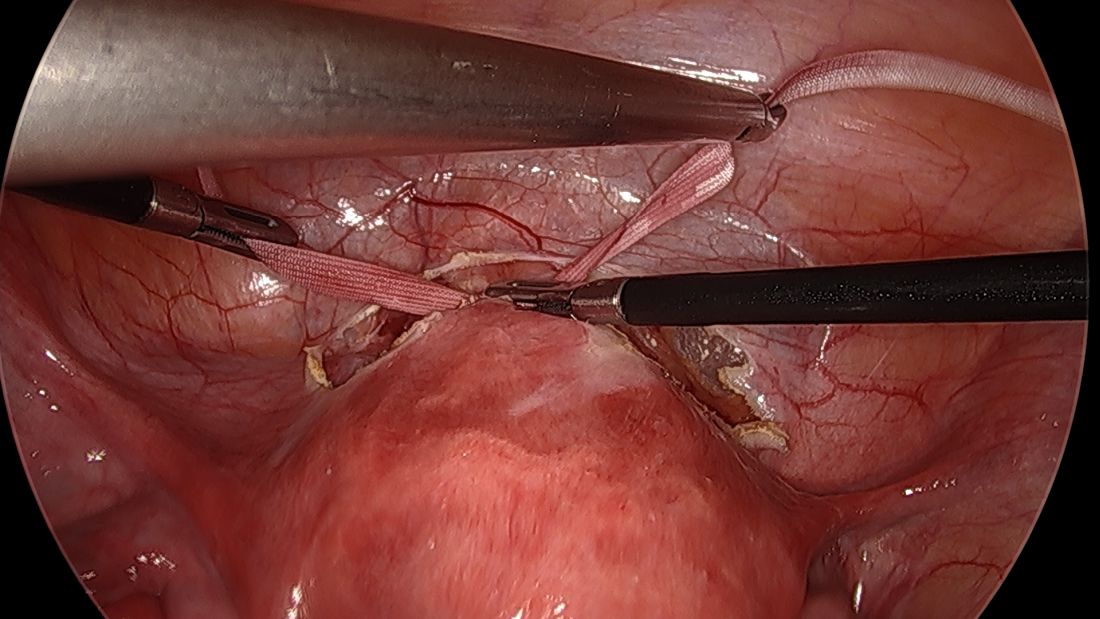
I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.
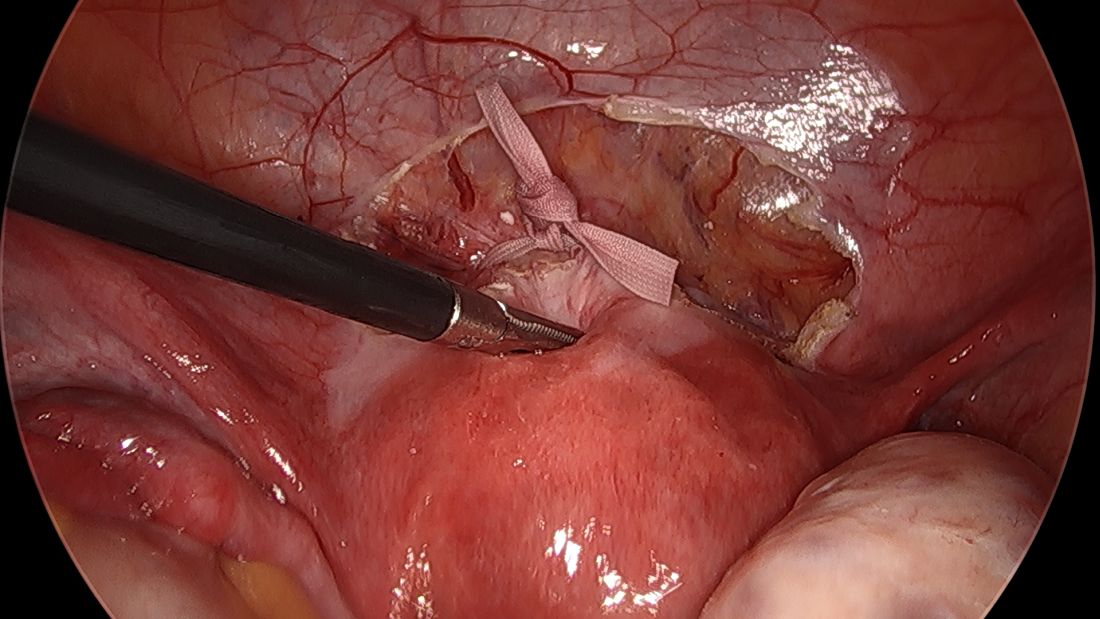
The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.
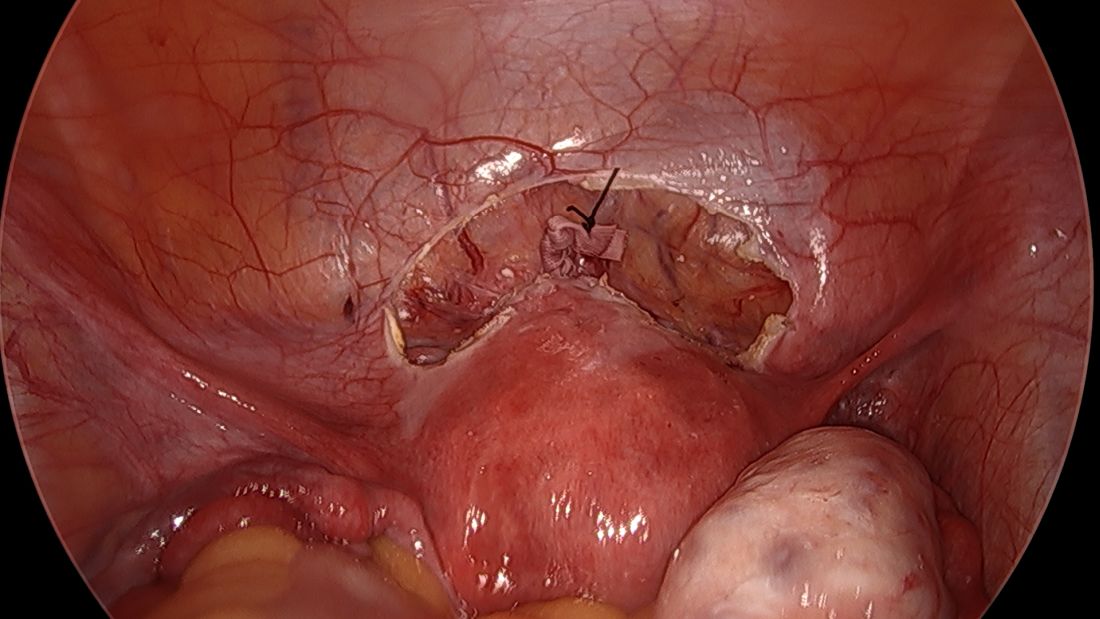
We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.
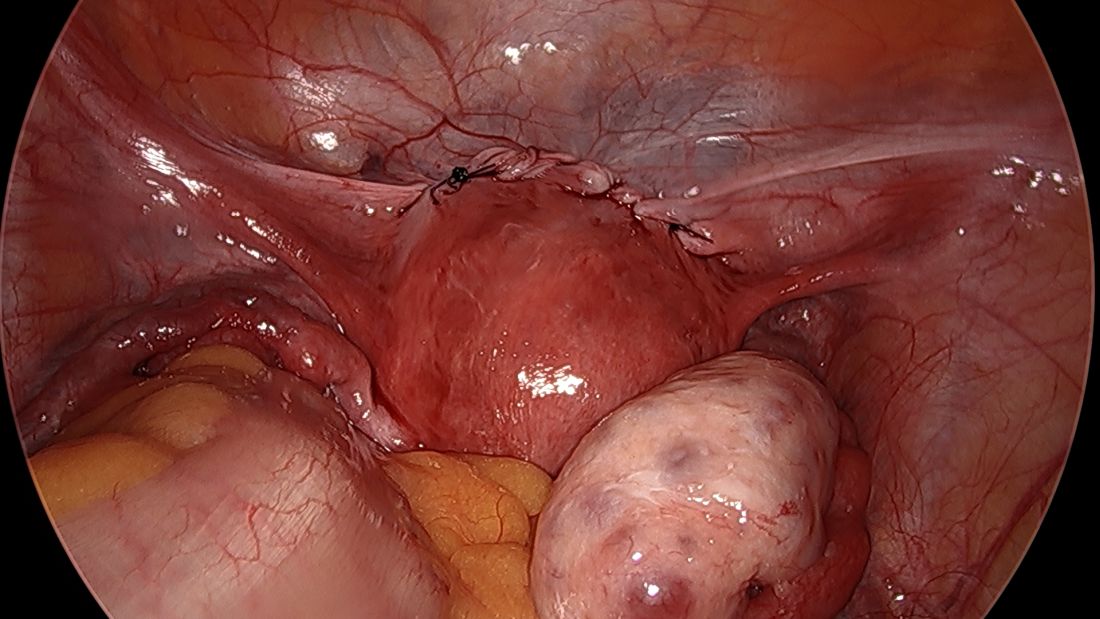
We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.

We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.

The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.

Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.

I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.

The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.

We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.

We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.

We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.

The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.

Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.

I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.

The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.

We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.

We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
Laparoscopic approach to abdominal cerclage
Preterm birth remains a significant cause of infant morbidity and mortality. A well-established cause of preterm birth is cervical insufficiency, which occurs in approximately 1% of pregnancies and up to 8% of recurrent miscarriages and midtrimester pregnancy loss. A cerclage, a purse-string suture around the cervix, is placed to treat cervical insufficiency and, thus, prevent second-trimester loss and preterm birth. While, traditionally, placement of the cerclage was performed via a vaginal route, over the past 50 years, abdominal cerclage has been utilized in cases in which a vaginal cerclage has failed or the cervix is extremely short. The advantage of the abdominal approach is the ability to place the suture at the level of the internal os. Moreover, there is no potential risk of ascending infection and resultant preterm labor or premature rupture of membranes secondary to a foreign body in the vagina, as in the case of vaginal cerclage. There has been a reluctance to perform abdominal cerclage as a first-time treatment secondary to the need for cesarean section, risk of hemorrhage at the uterine vessels, and in the past, the need for a laparotomy.
With the introduction of a laparoscopic or robot-assisted approach to abdominal cerclage in preterm birth prevention, there has been an upsurge in the popularity of abdominal cerclage as the first-line surgical procedure, especially after a failed vaginal cerclage. In 2018, Moawad et al., in a systematic review of laparoscopic abdominal cerclage, noted slight improvement in neonatal outcomes with laparoscopy vs. laparotomy.
For this edition of the Master Class in gynecologic surgery, I have enlisted the assistance of Jon I. Einarsson, MD, PhD, MPH, who is chief of the division of minimally invasive gynecology at Brigham and Women’s Hospital and professor of obstetrics/gynecology at Harvard Medical School, Boston. Dr. Einarsson is a past president of the American Association of Gynecologic Laparoscopists. He is a very well-known, published clinical researcher and surgical innovator. Dr. Einarsson is the founder of Freyja Healthcare, a privately held medical device company advancing women’s health through innovation.
It is a pleasure and honor to welcome my friend and colleague, Dr. Jon I. Einarsson, to this edition of the Master Class in gynecologic surgery.
Dr. Miller is professor of obstetrics and gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
Preterm birth remains a significant cause of infant morbidity and mortality. A well-established cause of preterm birth is cervical insufficiency, which occurs in approximately 1% of pregnancies and up to 8% of recurrent miscarriages and midtrimester pregnancy loss. A cerclage, a purse-string suture around the cervix, is placed to treat cervical insufficiency and, thus, prevent second-trimester loss and preterm birth. While, traditionally, placement of the cerclage was performed via a vaginal route, over the past 50 years, abdominal cerclage has been utilized in cases in which a vaginal cerclage has failed or the cervix is extremely short. The advantage of the abdominal approach is the ability to place the suture at the level of the internal os. Moreover, there is no potential risk of ascending infection and resultant preterm labor or premature rupture of membranes secondary to a foreign body in the vagina, as in the case of vaginal cerclage. There has been a reluctance to perform abdominal cerclage as a first-time treatment secondary to the need for cesarean section, risk of hemorrhage at the uterine vessels, and in the past, the need for a laparotomy.
With the introduction of a laparoscopic or robot-assisted approach to abdominal cerclage in preterm birth prevention, there has been an upsurge in the popularity of abdominal cerclage as the first-line surgical procedure, especially after a failed vaginal cerclage. In 2018, Moawad et al., in a systematic review of laparoscopic abdominal cerclage, noted slight improvement in neonatal outcomes with laparoscopy vs. laparotomy.
For this edition of the Master Class in gynecologic surgery, I have enlisted the assistance of Jon I. Einarsson, MD, PhD, MPH, who is chief of the division of minimally invasive gynecology at Brigham and Women’s Hospital and professor of obstetrics/gynecology at Harvard Medical School, Boston. Dr. Einarsson is a past president of the American Association of Gynecologic Laparoscopists. He is a very well-known, published clinical researcher and surgical innovator. Dr. Einarsson is the founder of Freyja Healthcare, a privately held medical device company advancing women’s health through innovation.
It is a pleasure and honor to welcome my friend and colleague, Dr. Jon I. Einarsson, to this edition of the Master Class in gynecologic surgery.
Dr. Miller is professor of obstetrics and gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
Preterm birth remains a significant cause of infant morbidity and mortality. A well-established cause of preterm birth is cervical insufficiency, which occurs in approximately 1% of pregnancies and up to 8% of recurrent miscarriages and midtrimester pregnancy loss. A cerclage, a purse-string suture around the cervix, is placed to treat cervical insufficiency and, thus, prevent second-trimester loss and preterm birth. While, traditionally, placement of the cerclage was performed via a vaginal route, over the past 50 years, abdominal cerclage has been utilized in cases in which a vaginal cerclage has failed or the cervix is extremely short. The advantage of the abdominal approach is the ability to place the suture at the level of the internal os. Moreover, there is no potential risk of ascending infection and resultant preterm labor or premature rupture of membranes secondary to a foreign body in the vagina, as in the case of vaginal cerclage. There has been a reluctance to perform abdominal cerclage as a first-time treatment secondary to the need for cesarean section, risk of hemorrhage at the uterine vessels, and in the past, the need for a laparotomy.
With the introduction of a laparoscopic or robot-assisted approach to abdominal cerclage in preterm birth prevention, there has been an upsurge in the popularity of abdominal cerclage as the first-line surgical procedure, especially after a failed vaginal cerclage. In 2018, Moawad et al., in a systematic review of laparoscopic abdominal cerclage, noted slight improvement in neonatal outcomes with laparoscopy vs. laparotomy.
For this edition of the Master Class in gynecologic surgery, I have enlisted the assistance of Jon I. Einarsson, MD, PhD, MPH, who is chief of the division of minimally invasive gynecology at Brigham and Women’s Hospital and professor of obstetrics/gynecology at Harvard Medical School, Boston. Dr. Einarsson is a past president of the American Association of Gynecologic Laparoscopists. He is a very well-known, published clinical researcher and surgical innovator. Dr. Einarsson is the founder of Freyja Healthcare, a privately held medical device company advancing women’s health through innovation.
It is a pleasure and honor to welcome my friend and colleague, Dr. Jon I. Einarsson, to this edition of the Master Class in gynecologic surgery.
Dr. Miller is professor of obstetrics and gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
How advances in genomics have informed obstetrics practice
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.