User login
RFVTA system offers alternative to myomectomy
Uterine myomas cause heavy menstrual bleeding and other clinically significant symptoms in 35%-50% of affected women and have been shown to be the leading indication for hysterectomy in the United States among women aged 35-54 years.
Research has shown that a significant number of women who undergo hysterectomy for treatment of fibroids later regret the loss of their uterus and have other concerns and complications. Other options for therapy include various pharmacologic treatments, a progestin-releasing intrauterine device, uterine artery embolization, endometrial ablation, MRI-guided focused ultrasound surgery, and myomectomy performed laparoscopically, robotically, or hysteroscopically.
Myomectomy seems largely to preserve fertility, but rates of recurrence and additional procedures for bleeding and myoma symptoms are still high – upward of 30% in some studies. Overall, we need other more efficacious and minimally invasive options.
Radiofrequency volumetric thermal ablation (RFVTA) achieved through the Acessa System (Halt Medical) has been the newest addition to our armamentarium for treatment of symptomatic fibroids. It is suitable for every type of fibroid except for type 0 pedunculated intracavitary fibroids and type 7 pedunculated subserosal fibroids, which is significant because deep intramural fibroids have been difficult to target and treat by other methods.
Three-year outcome data show sustained improvements in fibroid symptoms and quality of life, with an incidence of recurrences and additional procedures – approximately 11% – that appears to be substantially lower than for other uterine-sparing fibroid treatments. In addition, while the technology is not indicated for women seeking future childbearing, successful pregnancies are being reported, suggesting that full-term pregnancies – and vaginal delivery in some cases – may be possible after RFVTA.
The principles
Radiofrequency ablation has been used for years in the treatment of liver and kidney tumors. The basic concept is that volumetric thermal ablation results in coagulative necrosis.
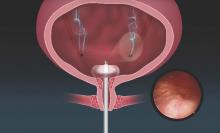
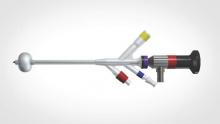
The Acessa System, approved by the Food and Drug Administration in late 2012, was designed to treat fibroids, which have much firmer tissue than the tissues being targeted in other radiofrequency ablation procedures. It uses a specially designed intrauterine ultrasound probe and radiofrequency probe, and it combines three fundamental gynecologic skills: Laparoscopy using two trocars and requiring no special suturing skills; ultrasound using a laparoscopic ultrasound probe to scan and manipulate; and probe placement under laparoscopic ultrasound guidance.
Specifically, the system allows for percutaneous, laparoscopic ultrasound–guided radiofrequency ablation of fibroids with a disposable 3.4-mm handpiece coupled to a dual-function radiofrequency generator. The handpiece contains a retractable array of electrodes, so that the fibroid may be ablated with one electrode or with the deployed electrode array.
The generator controls and monitors the ablation with real-time feedback from thermocouples. It monitors and displays the temperature at each needle tip, the average temperature of the array, and the return temperatures on two dispersive electrode pads that are placed on the anterior thighs. The electrode pads are designed to reduce the incidence of pad burns, which are a complication with other radiofrequency ablation devices. The system will automatically stop treatment if either of the pad thermocouples registers a skin temperature greater than 40° C (JSLS. 2014 Apr-Jun;18[2]:182-90).
The outcomes
Laparoscopic ultrasound–guided RFVTA has been studied in five prospective trials, including one multicenter international trial of 135 premenopausal women – the pivotal trial for FDA clearance – in which 104 women were followed for 3 years and found to have prolonged symptom relief and improved quality of life.
At baseline, the women had symptomatic uterine myomas and moderate to severe heavy menstrual bleeding measured by alkaline hematin analysis of returned sanitary products. Their mean symptom severity scores on the Uterine Fibroid Symptom and Quality-of-Life Questionnaire (UFS-QOL) decreased significantly from baseline to 3 months and changed little after that, for a total change of –32.6 over the study period.
The cumulative repeat intervention rate at 3 years was 11%, with 14 of the 135 participants having repeat interventions to treat bleeding and myoma symptoms. Seven of these women were found to have adenomyosis (J Minim Invasive Gynecol. 2014 Sep-Oct;21[5]:767-74).
The surprisingly low reintervention rates may stem from the benefits of direct contact imaging of the uterus. A comparison of images from the pivotal trial has shown that intraoperative ultrasound detected more than twice as many fibroids as did preoperative transvaginal ultrasound, and about one-third more than preoperative MRIs (J Minim Invasive Gynecol. 2013 Nov-Dec;20[6]:770-4).
Interestingly, four women became pregnant over the study’s 3-year follow-up, despite the inclusion requirement that women desire uterine conservation but not future childbearing.
We have followed reproductive outcomes in women after RFVTA of symptomatic fibroids in other studies as well. In our most recent analysis, presented in November at the 2015 American Association of Gynecologic Laparoscopists Global Congress, we identified 10 pregnancies among participants of the five prospective trials.
Of 232 women enrolled in premarket RFVTA studies – trials in which completing childbearing and continuing contraception were requirements – six conceived at 3.5-15 months post ablation. The number of myomas treated ranged from one to seven and included multiple types and dimensions. Five of these six women delivered full-term healthy babies – one by vaginal delivery and four by cesarean section. The sixth patient had a spontaneous abortion in the first trimester.
Of 43 women who participated in two randomized clinical trials undertaken after FDA clearance, four conceived at 4-23.5 months post ablation. Three of these women had uneventful, full-term pregnancies with vaginal births. The fourth had a cesarean section at 38 weeks.
Considering the theoretical advantages of the Acessa procedure – that it is less damaging to healthy myometrium – and the outcomes reported thus far, it appears likely that Acessa will be preferable to myomectomy. Early results from an ongoing 5-year German study that randomized 50 women to RFVTA or laparoscopic myomectomy show that RFVTA resulted in the treatment of more fibroids and involved a significantly shorter hospital stay and post-operative recovery (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
The technique
The patient is pretreated with a nonsteroidal anti-inflammatory agent and prophylactic antibiotic. She is placed in a supine position with arms tucked, and a single-toothed tenaculum is placed on the cervix from 12 to 6 o’clock, without any instrument for manipulation of the uterus. The system’s dispersive electrode pads are placed symmetrically just above the patella on the anterior thighs; asymmetrical placement could potentially increase the risk of a pad burn.
Two standard laparoscopic ports are placed. A 5-mm trocar for the camera and video laparoscope is placed through the umbilicus or at a supraumbilical or left upper–quadrant level, depending on the patient’s anatomy, her surgical history, and the size of the uterus. A thorough visual inspection of the abdomen should be performed to look for unsuspected findings.
A 10-mm trocar is then placed at the level of the top of the fundus for the intra-abdominal ultrasound probe. Laparoscopic ultrasound is used to survey the entire uterus, map the fibroids, and plan an approach. Once the fibroid to be treated first is identified, the ability to stabilize the uterus accordingly is assessed, and the dimensions of the fibroid are taken. The dimensions will be used by the surgeon with a volume algorithm to calculate the length of ablation time based on the size of the fibroid and electrode deployment.
Under ultrasound guidance, the Acessa radiofrequency ablation handpiece is inserted percutaneously at 12, 3, 6, or 9 o’clock relative to the ultrasound trocar, based on the location of the target fibroid. The uterus must be stabilized, with the handpiece and ultrasound probe parallel and in plane. The handpiece is then inserted 1 cm into the target fibroid through the uterine serosal surface, utilizing a combination of laparoscopic and ultrasound views. Care must be taken to use gentle rotation and minimal downward pressure as the tip of the handpiece is quite sharp.
The location of the tip is confirmed by laparoscopic ultrasound, and the 7-needle electrode array can then be deployed to the ablation site. All three dimensions of the fibroid should be viewed for placement and deployment of the electrodes. Care is taken to avoid large blood vessels and ensure that the electrodes are confined within the fibroid and within the uterus.
Radiofrequency ablation is carried out with a low-voltage, high-frequency alternating current. The radiofrequency waves heat the tissue to an average temperature of 95° C for a length of time determined by a treatment algorithm. The wattage automatically adjusts to maintain the treatment temperature for the calculated duration of ablation.
Small fibroids can be treated in a manual mode without deployment of the electrode array at a current output of 15 W.
At the conclusion of the ablation, the electrodes are withdrawn into the handpiece, the generator is changed to coagulation mode, and the handpiece is slowly withdrawn under ultrasound visualization. The tract is simultaneously coagulated. A bit of additional coagulation is facilitated by pausing at the serosal surface.
Additional fibroids can be ablated through another insertion of the handpiece, either through the same tract or through a new tract.
Larger fibroids may require multiple ablations. The maximum size of ablation is about 5 cm, so it is important to plan the treatment of larger fibroids. This can be accomplished by carefully scanning large fibroids and visualizing the number of overlapping ablations needed to treat the entire volume. I ask my assistant to record the size and location of each ablation; I find this helpful both for organizing the treatment of large fibroids and for dictating the operative report.
It is important to appreciate that treatment of one area can make it difficult to visualize nearby fibroids with ultrasound. The effect dissipates in about 30-45 minutes. It is one reason why having a fibroid map prior to treatment is so important.
Once all fibroids are treated, a final inspection is performed. We usually use a suction irrigator to clean out whatever small amounts of blood are present, and the laparoscopic and port sites are closed in standard fashion.
Patients are seen 1 week postoperatively and are instructed to call in cases of pain, fever, bleeding, or chills. Most patients require only NSAIDs for pain relief and return to work in 2-7 days.
Many patients experience a slightly heavier than normal first menses after treatment. Pelvic rest is recommended for 3 weeks as a precaution, and avoidance of intrauterine procedures is advised because the uterus will be soft and thus may be easily perforated. Patients who have had type 1, type 2, or type 2-5 fibroids ablated may experience drainage for several weeks as the fibroid tissue is reabsorbed.
Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist-in-chief for obstetrics and gynecology at the Detroit Medical Center. He was a principal investigator of the 3-year outcome study of Acessa sponsored by Halt Medical. He is a consultant for Halt Medical and directs physician training in the use of Acessa.
Uterine myomas cause heavy menstrual bleeding and other clinically significant symptoms in 35%-50% of affected women and have been shown to be the leading indication for hysterectomy in the United States among women aged 35-54 years.
Research has shown that a significant number of women who undergo hysterectomy for treatment of fibroids later regret the loss of their uterus and have other concerns and complications. Other options for therapy include various pharmacologic treatments, a progestin-releasing intrauterine device, uterine artery embolization, endometrial ablation, MRI-guided focused ultrasound surgery, and myomectomy performed laparoscopically, robotically, or hysteroscopically.
Myomectomy seems largely to preserve fertility, but rates of recurrence and additional procedures for bleeding and myoma symptoms are still high – upward of 30% in some studies. Overall, we need other more efficacious and minimally invasive options.
Radiofrequency volumetric thermal ablation (RFVTA) achieved through the Acessa System (Halt Medical) has been the newest addition to our armamentarium for treatment of symptomatic fibroids. It is suitable for every type of fibroid except for type 0 pedunculated intracavitary fibroids and type 7 pedunculated subserosal fibroids, which is significant because deep intramural fibroids have been difficult to target and treat by other methods.
Three-year outcome data show sustained improvements in fibroid symptoms and quality of life, with an incidence of recurrences and additional procedures – approximately 11% – that appears to be substantially lower than for other uterine-sparing fibroid treatments. In addition, while the technology is not indicated for women seeking future childbearing, successful pregnancies are being reported, suggesting that full-term pregnancies – and vaginal delivery in some cases – may be possible after RFVTA.
The principles
Radiofrequency ablation has been used for years in the treatment of liver and kidney tumors. The basic concept is that volumetric thermal ablation results in coagulative necrosis.
The Acessa System, approved by the Food and Drug Administration in late 2012, was designed to treat fibroids, which have much firmer tissue than the tissues being targeted in other radiofrequency ablation procedures. It uses a specially designed intrauterine ultrasound probe and radiofrequency probe, and it combines three fundamental gynecologic skills: Laparoscopy using two trocars and requiring no special suturing skills; ultrasound using a laparoscopic ultrasound probe to scan and manipulate; and probe placement under laparoscopic ultrasound guidance.
Specifically, the system allows for percutaneous, laparoscopic ultrasound–guided radiofrequency ablation of fibroids with a disposable 3.4-mm handpiece coupled to a dual-function radiofrequency generator. The handpiece contains a retractable array of electrodes, so that the fibroid may be ablated with one electrode or with the deployed electrode array.
The generator controls and monitors the ablation with real-time feedback from thermocouples. It monitors and displays the temperature at each needle tip, the average temperature of the array, and the return temperatures on two dispersive electrode pads that are placed on the anterior thighs. The electrode pads are designed to reduce the incidence of pad burns, which are a complication with other radiofrequency ablation devices. The system will automatically stop treatment if either of the pad thermocouples registers a skin temperature greater than 40° C (JSLS. 2014 Apr-Jun;18[2]:182-90).
The outcomes
Laparoscopic ultrasound–guided RFVTA has been studied in five prospective trials, including one multicenter international trial of 135 premenopausal women – the pivotal trial for FDA clearance – in which 104 women were followed for 3 years and found to have prolonged symptom relief and improved quality of life.
At baseline, the women had symptomatic uterine myomas and moderate to severe heavy menstrual bleeding measured by alkaline hematin analysis of returned sanitary products. Their mean symptom severity scores on the Uterine Fibroid Symptom and Quality-of-Life Questionnaire (UFS-QOL) decreased significantly from baseline to 3 months and changed little after that, for a total change of –32.6 over the study period.
The cumulative repeat intervention rate at 3 years was 11%, with 14 of the 135 participants having repeat interventions to treat bleeding and myoma symptoms. Seven of these women were found to have adenomyosis (J Minim Invasive Gynecol. 2014 Sep-Oct;21[5]:767-74).
The surprisingly low reintervention rates may stem from the benefits of direct contact imaging of the uterus. A comparison of images from the pivotal trial has shown that intraoperative ultrasound detected more than twice as many fibroids as did preoperative transvaginal ultrasound, and about one-third more than preoperative MRIs (J Minim Invasive Gynecol. 2013 Nov-Dec;20[6]:770-4).
Interestingly, four women became pregnant over the study’s 3-year follow-up, despite the inclusion requirement that women desire uterine conservation but not future childbearing.
We have followed reproductive outcomes in women after RFVTA of symptomatic fibroids in other studies as well. In our most recent analysis, presented in November at the 2015 American Association of Gynecologic Laparoscopists Global Congress, we identified 10 pregnancies among participants of the five prospective trials.
Of 232 women enrolled in premarket RFVTA studies – trials in which completing childbearing and continuing contraception were requirements – six conceived at 3.5-15 months post ablation. The number of myomas treated ranged from one to seven and included multiple types and dimensions. Five of these six women delivered full-term healthy babies – one by vaginal delivery and four by cesarean section. The sixth patient had a spontaneous abortion in the first trimester.
Of 43 women who participated in two randomized clinical trials undertaken after FDA clearance, four conceived at 4-23.5 months post ablation. Three of these women had uneventful, full-term pregnancies with vaginal births. The fourth had a cesarean section at 38 weeks.
Considering the theoretical advantages of the Acessa procedure – that it is less damaging to healthy myometrium – and the outcomes reported thus far, it appears likely that Acessa will be preferable to myomectomy. Early results from an ongoing 5-year German study that randomized 50 women to RFVTA or laparoscopic myomectomy show that RFVTA resulted in the treatment of more fibroids and involved a significantly shorter hospital stay and post-operative recovery (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
The technique
The patient is pretreated with a nonsteroidal anti-inflammatory agent and prophylactic antibiotic. She is placed in a supine position with arms tucked, and a single-toothed tenaculum is placed on the cervix from 12 to 6 o’clock, without any instrument for manipulation of the uterus. The system’s dispersive electrode pads are placed symmetrically just above the patella on the anterior thighs; asymmetrical placement could potentially increase the risk of a pad burn.
Two standard laparoscopic ports are placed. A 5-mm trocar for the camera and video laparoscope is placed through the umbilicus or at a supraumbilical or left upper–quadrant level, depending on the patient’s anatomy, her surgical history, and the size of the uterus. A thorough visual inspection of the abdomen should be performed to look for unsuspected findings.
A 10-mm trocar is then placed at the level of the top of the fundus for the intra-abdominal ultrasound probe. Laparoscopic ultrasound is used to survey the entire uterus, map the fibroids, and plan an approach. Once the fibroid to be treated first is identified, the ability to stabilize the uterus accordingly is assessed, and the dimensions of the fibroid are taken. The dimensions will be used by the surgeon with a volume algorithm to calculate the length of ablation time based on the size of the fibroid and electrode deployment.
Under ultrasound guidance, the Acessa radiofrequency ablation handpiece is inserted percutaneously at 12, 3, 6, or 9 o’clock relative to the ultrasound trocar, based on the location of the target fibroid. The uterus must be stabilized, with the handpiece and ultrasound probe parallel and in plane. The handpiece is then inserted 1 cm into the target fibroid through the uterine serosal surface, utilizing a combination of laparoscopic and ultrasound views. Care must be taken to use gentle rotation and minimal downward pressure as the tip of the handpiece is quite sharp.
The location of the tip is confirmed by laparoscopic ultrasound, and the 7-needle electrode array can then be deployed to the ablation site. All three dimensions of the fibroid should be viewed for placement and deployment of the electrodes. Care is taken to avoid large blood vessels and ensure that the electrodes are confined within the fibroid and within the uterus.
Radiofrequency ablation is carried out with a low-voltage, high-frequency alternating current. The radiofrequency waves heat the tissue to an average temperature of 95° C for a length of time determined by a treatment algorithm. The wattage automatically adjusts to maintain the treatment temperature for the calculated duration of ablation.
Small fibroids can be treated in a manual mode without deployment of the electrode array at a current output of 15 W.
At the conclusion of the ablation, the electrodes are withdrawn into the handpiece, the generator is changed to coagulation mode, and the handpiece is slowly withdrawn under ultrasound visualization. The tract is simultaneously coagulated. A bit of additional coagulation is facilitated by pausing at the serosal surface.
Additional fibroids can be ablated through another insertion of the handpiece, either through the same tract or through a new tract.
Larger fibroids may require multiple ablations. The maximum size of ablation is about 5 cm, so it is important to plan the treatment of larger fibroids. This can be accomplished by carefully scanning large fibroids and visualizing the number of overlapping ablations needed to treat the entire volume. I ask my assistant to record the size and location of each ablation; I find this helpful both for organizing the treatment of large fibroids and for dictating the operative report.
It is important to appreciate that treatment of one area can make it difficult to visualize nearby fibroids with ultrasound. The effect dissipates in about 30-45 minutes. It is one reason why having a fibroid map prior to treatment is so important.
Once all fibroids are treated, a final inspection is performed. We usually use a suction irrigator to clean out whatever small amounts of blood are present, and the laparoscopic and port sites are closed in standard fashion.
Patients are seen 1 week postoperatively and are instructed to call in cases of pain, fever, bleeding, or chills. Most patients require only NSAIDs for pain relief and return to work in 2-7 days.
Many patients experience a slightly heavier than normal first menses after treatment. Pelvic rest is recommended for 3 weeks as a precaution, and avoidance of intrauterine procedures is advised because the uterus will be soft and thus may be easily perforated. Patients who have had type 1, type 2, or type 2-5 fibroids ablated may experience drainage for several weeks as the fibroid tissue is reabsorbed.
Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist-in-chief for obstetrics and gynecology at the Detroit Medical Center. He was a principal investigator of the 3-year outcome study of Acessa sponsored by Halt Medical. He is a consultant for Halt Medical and directs physician training in the use of Acessa.
Uterine myomas cause heavy menstrual bleeding and other clinically significant symptoms in 35%-50% of affected women and have been shown to be the leading indication for hysterectomy in the United States among women aged 35-54 years.
Research has shown that a significant number of women who undergo hysterectomy for treatment of fibroids later regret the loss of their uterus and have other concerns and complications. Other options for therapy include various pharmacologic treatments, a progestin-releasing intrauterine device, uterine artery embolization, endometrial ablation, MRI-guided focused ultrasound surgery, and myomectomy performed laparoscopically, robotically, or hysteroscopically.
Myomectomy seems largely to preserve fertility, but rates of recurrence and additional procedures for bleeding and myoma symptoms are still high – upward of 30% in some studies. Overall, we need other more efficacious and minimally invasive options.
Radiofrequency volumetric thermal ablation (RFVTA) achieved through the Acessa System (Halt Medical) has been the newest addition to our armamentarium for treatment of symptomatic fibroids. It is suitable for every type of fibroid except for type 0 pedunculated intracavitary fibroids and type 7 pedunculated subserosal fibroids, which is significant because deep intramural fibroids have been difficult to target and treat by other methods.
Three-year outcome data show sustained improvements in fibroid symptoms and quality of life, with an incidence of recurrences and additional procedures – approximately 11% – that appears to be substantially lower than for other uterine-sparing fibroid treatments. In addition, while the technology is not indicated for women seeking future childbearing, successful pregnancies are being reported, suggesting that full-term pregnancies – and vaginal delivery in some cases – may be possible after RFVTA.
The principles
Radiofrequency ablation has been used for years in the treatment of liver and kidney tumors. The basic concept is that volumetric thermal ablation results in coagulative necrosis.
The Acessa System, approved by the Food and Drug Administration in late 2012, was designed to treat fibroids, which have much firmer tissue than the tissues being targeted in other radiofrequency ablation procedures. It uses a specially designed intrauterine ultrasound probe and radiofrequency probe, and it combines three fundamental gynecologic skills: Laparoscopy using two trocars and requiring no special suturing skills; ultrasound using a laparoscopic ultrasound probe to scan and manipulate; and probe placement under laparoscopic ultrasound guidance.
Specifically, the system allows for percutaneous, laparoscopic ultrasound–guided radiofrequency ablation of fibroids with a disposable 3.4-mm handpiece coupled to a dual-function radiofrequency generator. The handpiece contains a retractable array of electrodes, so that the fibroid may be ablated with one electrode or with the deployed electrode array.
The generator controls and monitors the ablation with real-time feedback from thermocouples. It monitors and displays the temperature at each needle tip, the average temperature of the array, and the return temperatures on two dispersive electrode pads that are placed on the anterior thighs. The electrode pads are designed to reduce the incidence of pad burns, which are a complication with other radiofrequency ablation devices. The system will automatically stop treatment if either of the pad thermocouples registers a skin temperature greater than 40° C (JSLS. 2014 Apr-Jun;18[2]:182-90).
The outcomes
Laparoscopic ultrasound–guided RFVTA has been studied in five prospective trials, including one multicenter international trial of 135 premenopausal women – the pivotal trial for FDA clearance – in which 104 women were followed for 3 years and found to have prolonged symptom relief and improved quality of life.
At baseline, the women had symptomatic uterine myomas and moderate to severe heavy menstrual bleeding measured by alkaline hematin analysis of returned sanitary products. Their mean symptom severity scores on the Uterine Fibroid Symptom and Quality-of-Life Questionnaire (UFS-QOL) decreased significantly from baseline to 3 months and changed little after that, for a total change of –32.6 over the study period.
The cumulative repeat intervention rate at 3 years was 11%, with 14 of the 135 participants having repeat interventions to treat bleeding and myoma symptoms. Seven of these women were found to have adenomyosis (J Minim Invasive Gynecol. 2014 Sep-Oct;21[5]:767-74).
The surprisingly low reintervention rates may stem from the benefits of direct contact imaging of the uterus. A comparison of images from the pivotal trial has shown that intraoperative ultrasound detected more than twice as many fibroids as did preoperative transvaginal ultrasound, and about one-third more than preoperative MRIs (J Minim Invasive Gynecol. 2013 Nov-Dec;20[6]:770-4).
Interestingly, four women became pregnant over the study’s 3-year follow-up, despite the inclusion requirement that women desire uterine conservation but not future childbearing.
We have followed reproductive outcomes in women after RFVTA of symptomatic fibroids in other studies as well. In our most recent analysis, presented in November at the 2015 American Association of Gynecologic Laparoscopists Global Congress, we identified 10 pregnancies among participants of the five prospective trials.
Of 232 women enrolled in premarket RFVTA studies – trials in which completing childbearing and continuing contraception were requirements – six conceived at 3.5-15 months post ablation. The number of myomas treated ranged from one to seven and included multiple types and dimensions. Five of these six women delivered full-term healthy babies – one by vaginal delivery and four by cesarean section. The sixth patient had a spontaneous abortion in the first trimester.
Of 43 women who participated in two randomized clinical trials undertaken after FDA clearance, four conceived at 4-23.5 months post ablation. Three of these women had uneventful, full-term pregnancies with vaginal births. The fourth had a cesarean section at 38 weeks.
Considering the theoretical advantages of the Acessa procedure – that it is less damaging to healthy myometrium – and the outcomes reported thus far, it appears likely that Acessa will be preferable to myomectomy. Early results from an ongoing 5-year German study that randomized 50 women to RFVTA or laparoscopic myomectomy show that RFVTA resulted in the treatment of more fibroids and involved a significantly shorter hospital stay and post-operative recovery (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
The technique
The patient is pretreated with a nonsteroidal anti-inflammatory agent and prophylactic antibiotic. She is placed in a supine position with arms tucked, and a single-toothed tenaculum is placed on the cervix from 12 to 6 o’clock, without any instrument for manipulation of the uterus. The system’s dispersive electrode pads are placed symmetrically just above the patella on the anterior thighs; asymmetrical placement could potentially increase the risk of a pad burn.
Two standard laparoscopic ports are placed. A 5-mm trocar for the camera and video laparoscope is placed through the umbilicus or at a supraumbilical or left upper–quadrant level, depending on the patient’s anatomy, her surgical history, and the size of the uterus. A thorough visual inspection of the abdomen should be performed to look for unsuspected findings.
A 10-mm trocar is then placed at the level of the top of the fundus for the intra-abdominal ultrasound probe. Laparoscopic ultrasound is used to survey the entire uterus, map the fibroids, and plan an approach. Once the fibroid to be treated first is identified, the ability to stabilize the uterus accordingly is assessed, and the dimensions of the fibroid are taken. The dimensions will be used by the surgeon with a volume algorithm to calculate the length of ablation time based on the size of the fibroid and electrode deployment.
Under ultrasound guidance, the Acessa radiofrequency ablation handpiece is inserted percutaneously at 12, 3, 6, or 9 o’clock relative to the ultrasound trocar, based on the location of the target fibroid. The uterus must be stabilized, with the handpiece and ultrasound probe parallel and in plane. The handpiece is then inserted 1 cm into the target fibroid through the uterine serosal surface, utilizing a combination of laparoscopic and ultrasound views. Care must be taken to use gentle rotation and minimal downward pressure as the tip of the handpiece is quite sharp.
The location of the tip is confirmed by laparoscopic ultrasound, and the 7-needle electrode array can then be deployed to the ablation site. All three dimensions of the fibroid should be viewed for placement and deployment of the electrodes. Care is taken to avoid large blood vessels and ensure that the electrodes are confined within the fibroid and within the uterus.
Radiofrequency ablation is carried out with a low-voltage, high-frequency alternating current. The radiofrequency waves heat the tissue to an average temperature of 95° C for a length of time determined by a treatment algorithm. The wattage automatically adjusts to maintain the treatment temperature for the calculated duration of ablation.
Small fibroids can be treated in a manual mode without deployment of the electrode array at a current output of 15 W.
At the conclusion of the ablation, the electrodes are withdrawn into the handpiece, the generator is changed to coagulation mode, and the handpiece is slowly withdrawn under ultrasound visualization. The tract is simultaneously coagulated. A bit of additional coagulation is facilitated by pausing at the serosal surface.
Additional fibroids can be ablated through another insertion of the handpiece, either through the same tract or through a new tract.
Larger fibroids may require multiple ablations. The maximum size of ablation is about 5 cm, so it is important to plan the treatment of larger fibroids. This can be accomplished by carefully scanning large fibroids and visualizing the number of overlapping ablations needed to treat the entire volume. I ask my assistant to record the size and location of each ablation; I find this helpful both for organizing the treatment of large fibroids and for dictating the operative report.
It is important to appreciate that treatment of one area can make it difficult to visualize nearby fibroids with ultrasound. The effect dissipates in about 30-45 minutes. It is one reason why having a fibroid map prior to treatment is so important.
Once all fibroids are treated, a final inspection is performed. We usually use a suction irrigator to clean out whatever small amounts of blood are present, and the laparoscopic and port sites are closed in standard fashion.
Patients are seen 1 week postoperatively and are instructed to call in cases of pain, fever, bleeding, or chills. Most patients require only NSAIDs for pain relief and return to work in 2-7 days.
Many patients experience a slightly heavier than normal first menses after treatment. Pelvic rest is recommended for 3 weeks as a precaution, and avoidance of intrauterine procedures is advised because the uterus will be soft and thus may be easily perforated. Patients who have had type 1, type 2, or type 2-5 fibroids ablated may experience drainage for several weeks as the fibroid tissue is reabsorbed.
Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist-in-chief for obstetrics and gynecology at the Detroit Medical Center. He was a principal investigator of the 3-year outcome study of Acessa sponsored by Halt Medical. He is a consultant for Halt Medical and directs physician training in the use of Acessa.
Lessons learned from the history of VBAC
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Barriers to VBAC remain in spite of evidence
The relative safety of vaginal birth after cesarean (VBAC) has been documented in several large-scale studies in the past 15 years, and was affirmed in 2010 through a National Institutes of Health consensus development conference and a practice bulletin from the American College of Obstetricians and Gynecologists. Yet, despite all this research and review, rates of a trial of labor after cesarean (TOLAC) have increased only modestly in the last several years.
Approximately 20% of all births in 2013 in women with a history of one cesarean section involved a trial of labor, according to a recent report from the Centers for Disease Control and Prevention. This represents only a small increase from 2006, when the TOLAC rate had plummeted to approximately 15%.
The limited change is concerning because up to two-thirds of women with a prior cesarean delivery are candidates for a trial of labor, and many of them are excellent candidates. In total, 70% of the women who attempted labor in 2013 after a previous cesarean had successful VBACs, the CDC data shows.
Several European countries have TOLAC rates between 50% and 70%, but in the United States, as evidenced by the recent CDC data, there continues to be an underutilization of attempted VBAC. We must ask ourselves, are women truly able to choose TOLAC, or are they being dissuaded by the health care system?
I believe that the barriers are still pervasive. Too often, women who are TOLAC candidates are not receiving appropriate counseling – and too often, women are not even being presented the option of a trial of labor, even when staff are immediately available to provide emergency care if needed.
Rupture concerns in perspective
When the NIH consensus development panel reviewed VBAC in 2010, it concluded that TOLAC is a reasonable option for many women with a prior cesarean. The panel found that restricted access to VBAC/TOLAC stemmed from existing practice guidelines and the medical liability climate, and it called upon providers and others to “mitigate or even eliminate” the barriers that women face in finding clinicians and facilities able and willing to offer TOLAC.
ACOG’s 2010 practice bulletin also acknowledged the problem of limited access. ACOG recommended, as it had in an earlier bulletin, that TOLAC-VBAC be undertaken in facilities where staff are immediately available for emergency care. It added, however, that when such resources are not available, the best alternative may be to refer patients to a facility with available resources. Health care providers and insurance carriers “should do all they can to facilitate transfer of care or comanagement in support of a desired TOLAC,” ACOG’s document states.
Why, given such recommendations, are we still falling so short of where we should be?
A number of nonclinical factors are involved, but clearly, as both the NIH and ACOG have stated, the fear of litigation in cases of uterine rupture is a contributing factor. A ruptured uterus is indeed the principal risk associated with TOLAC, and it can have serious sequelae including perinatal death, hypoxic ischemic encephalopathy (HIE), and hysterectomy.
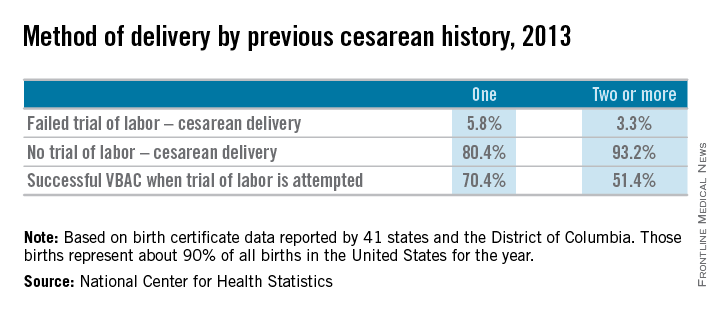
We must appreciate, however, that the absolute rates of uterine rupture and of serious adverse outcomes are quite low. The rupture rate in 2013 among women who underwent TOLAC but ultimately had a repeat cesarean section – the highest-risk group – was 495 per 100,000 live births, according to the CDC. This rate of approximately 0.5% is consistent with the level of risk reported in the literature for several decades.
In one of the two large observational studies done in the United States that have shed light on TOLAC outcomes, the rate of uterine rupture among women who underwent TOLAC was 0.7% for women with a prior low transverse incision, 2.0% for those with a prior low vertical incision, and 0.5% for those with an unknown type of prior incision. Overall, the rate of uterine rupture in this study’s cohort of 17,898 women who underwent TOLAC was 0.7% (N Engl J Med. 2004 Dec 16;351[25]:2581-9). The study was conducted at 19 medical centers belonging to the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medical Units (MFMU) Network.
The second large study conducted in the United States – a multicenter observational study in which records of approximately 25,000 women with a prior low-transverse cesarean section were reviewed – also showed rates of uterine rupture less than 1% (Am J Obstet Gynecol. 2005 Nov;193[5]:1656-62).
The attributable risk for perinatal death or HIE at term appears to be 1 per 2,000 TOLAC, according to the MFMU Network study.
Failed trials of labor resulting in repeat cesarean deliveries have consistently been associated with higher morbidity than scheduled repeat cesarean deliveries, with the greatest difference in rates for ruptured uterus. In the first MFMU Network study, there were no cases of uterine rupture among a cohort of 15,801 women who underwent elective repeat cesarean delivery, and in the second multicenter study of 25,000 women, this patient group had a rupture rate of 0.004%.
Yet, as ACOG points out, neither elective repeat cesarean deliveries nor TOLAC are without maternal or neonatal risk. Women who have successful VBAC delivery, on the other hand, have significantly lower morbidity and better outcomes than women who do not attempt labor. Women who undergo VBAC also avoid exposure to the significant risks of repeat cesarean deliveries in the long term.
Research unequivocally shows that the risk of placenta accreta, hysterectomy, hemorrhage, and other serious maternal morbidity increases progressively with each repeat cesarean delivery. Rates of placenta accreta have, in fact, been rising in the United States – a trend that should prompt us to think more about TOLAC.
Moreover, TOLAC is being shown to be a cost-effective strategy. In one analysis, TOLAC in a second pregnancy was cost-effective as long as the chance of VBAC exceeded approximately 74% (Obstet Gynecol. 2001 Jun;97[6]:932-41). More recently, TOLAC was found to be cost-effective across a wide variety of circumstances, including when a woman had a probability of VBAC as low as 43%. The model in this analysis, which used probability estimates from the MFMU Cesarean Registry, took a longer-term view by including probabilities of outcomes throughout a woman’s reproductive life that were contingent upon her initial choice regarding TOLAC (Am J Perinatol. 2013 Jan;30[1]:11-20).
Likelihood of success
Evaluating and discussing the likelihood of success with TOLAC is therefore key to the counseling process. The higher the likelihood of achieving VBAC, the more favorable the risk-benefit ratio will be and the more appealing it will be to consider.
According to one analysis, if a woman undergoing a TOLAC has at least a 60%-70% chance of VBAC, her chance of having major or minor morbidity is no greater than a woman undergoing a planned repeat cesarean delivery (Am J Obstet Gynecol 2009;200:56.e1-e6).
There are several prediction tools available that can be used at the first prenatal visit and in early labor to give a reasonably good estimate of success. One of these tools is available at the MFMU Network website (http://mfmu.bsc.gwu.edu). The tools take into account factors such as prior indication for cesarean delivery; history of vaginal delivery; demographic characteristics such as maternal age and body mass index; the occurrence of spontaneous labor; and cervical status at admission.
Prior vaginal delivery is one of the strongest predictors of a successful TOLAC. Research has consistently shown that women with a prior vaginal delivery – including a vaginal delivery predating an unsuccessful TOLAC – have significantly higher TOLAC success rates than women who did not have any prior vaginal delivery.
The indication for a prior cesarean delivery also clearly affects the likelihood of a successful TOLAC. Women whose first cesarean delivery was performed for a nonrecurring indication, such as breech presentation or low intolerance of labor, have TOLAC success rates that are similar to vaginal delivery rates for nulliparous women. Success rates for these women may exceed 85%. On the other hand, women who had a prior cesarean delivery for cephalopelvic disproportion or failure to progress have been shown to have lower TOLAC success rates ranging from 50%-67%.
Labor induction should be approached cautiously, as women who undergo induction of labor in TOLAC have an increased risk of repeat cesarean delivery. Still, success rates with induction are high. Data from the MFMU Cesarean Registry showed that about 66% of women undergoing induction after one prior cesarean delivery achieved VBAC versus 76% of women entering TOLAC spontaneously (Obstet Gynecol. 2007 Feb;109[2 Pt 1]:262-9). Another study of women undergoing induction after one prior cesarean reported an overall success rate of 78% (Obstet Gynecol. 2004 Mar;103[3]:534-8).
Whether induction specifically increases the risk for uterine rupture in TOLAC, compared with expectant management, is unclear. There also are conflicting data as to whether particular induction methods increase this risk.
Based on available data, ACOG considers induction of labor for either maternal or fetal indications to be an option for women undergoing TOLAC. Oxytocin may be used for induction as well as augmentation, but caution should be exercised at higher doses. While there is no clear dosing threshold for increased risk of rupture, research has suggested that higher doses of oxytocin are best avoided.
The use of prostaglandins is more controversial: Based on evidence from several small studies, ACOG concluded in its 2010 bulletin that misoprostol (prostaglandin E1) for cervical ripening is contraindicated in women undergoing TOLAC. It appears likely that rupture risk increases in patients who received both prostaglandins and oxytocin, so ACOG has advised avoiding their sequential use when prostaglandin E2 is used. This of course limits the options for the practitioner. Therefore, utilizing a Foley catheter followed by pitocin has been an approach advocated in some cases.
Uterine rupture is not predictable, and it is far more difficult to assess an individual’s risk of this complication than it is to assess the likelihood of VBAC. Still, there is value to discussing with the patient whether there are any other modifiers that could potentially influence the risk of rupture.
Since rates of uterine rupture are highest in women with previous classical or T-shaped incision, for example, it is important to try to ascertain what type of incision was previously used. It is widely appreciated that low-transverse uterine incisions are most favorable, but findings are mixed in regard to low-vertical incisions. Some research shows that women with a previous low-vertical incision do not have significantly lower VBAC success rates or higher risks of uterine rupture. TOLAC should therefore not be ruled out in these cases.
Additionally, TOLAC should not be ruled out for women who have had more than one cesarean delivery. Several studies have shown an increased risk of uterine rupture after two prior cesarean deliveries, compared with one, and one meta-analysis suggested a more than twofold increased risk (BJOG. 2010 Jan;117(1):5-19.).
In contrast, an analysis of the MFMU Cesarean Registry found no significant difference in rupture rates in women with one prior cesarean versus multiple prior cesareans (Obstet Gynecol. 2006 Jul;108[1]:12-20.).
It appears, therefore, that even if having more than one prior cesarean section is associated with an increased risk of rupture, the magnitude of this increase is small.
Just as women with a prior vaginal delivery have the highest chance of VBAC success, they also have the lowest rates of rupture among all women undergoing TOLAC.
Patient counseling
We must inform our patients who have had a cesarean section in the past of their options for childbirth in an unbiased manner.
The complications of both TOLAC and elective repeat cesarean section should be discussed, and every attempt should be made to individually assess both the likelihood of a successful VBAC and the comparative risk of maternal and perinatal morbidity. A shared decision-making process should be adopted, and whenever possible, the patient’s preference should be respected. In the end, a woman undergoing TOLAC should be truly motivated to pursue a trial of labor, because there are inherent risks.
One thing I’ve learned from my clinical practice and research on this issue is that the desire to undergo a vaginal delivery is powerful for some women. Many of my patients have self-referred for consultation about TOLAC after their ob.gyn. informed them that their hospital is not equipped, and they should therefore have a scheduled repeat operation. In many cases they discover that TOLAC is an option if they are willing to travel a half-hour or so.
We need to honor this desire and inform our patients of the option, and help facilitate delivery at another nearby hospital when our own facility is not equipped for TOLAC.
Dr. Landon is the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, Columbus. He served for more than 25 years as Ohio State’s coinvestigator for the National Institutes of Child Health and Human Development Maternal Fetal Medicine Units Network. He reported having no relevant financial disclosures.
The relative safety of vaginal birth after cesarean (VBAC) has been documented in several large-scale studies in the past 15 years, and was affirmed in 2010 through a National Institutes of Health consensus development conference and a practice bulletin from the American College of Obstetricians and Gynecologists. Yet, despite all this research and review, rates of a trial of labor after cesarean (TOLAC) have increased only modestly in the last several years.
Approximately 20% of all births in 2013 in women with a history of one cesarean section involved a trial of labor, according to a recent report from the Centers for Disease Control and Prevention. This represents only a small increase from 2006, when the TOLAC rate had plummeted to approximately 15%.
The limited change is concerning because up to two-thirds of women with a prior cesarean delivery are candidates for a trial of labor, and many of them are excellent candidates. In total, 70% of the women who attempted labor in 2013 after a previous cesarean had successful VBACs, the CDC data shows.
Several European countries have TOLAC rates between 50% and 70%, but in the United States, as evidenced by the recent CDC data, there continues to be an underutilization of attempted VBAC. We must ask ourselves, are women truly able to choose TOLAC, or are they being dissuaded by the health care system?
I believe that the barriers are still pervasive. Too often, women who are TOLAC candidates are not receiving appropriate counseling – and too often, women are not even being presented the option of a trial of labor, even when staff are immediately available to provide emergency care if needed.
Rupture concerns in perspective
When the NIH consensus development panel reviewed VBAC in 2010, it concluded that TOLAC is a reasonable option for many women with a prior cesarean. The panel found that restricted access to VBAC/TOLAC stemmed from existing practice guidelines and the medical liability climate, and it called upon providers and others to “mitigate or even eliminate” the barriers that women face in finding clinicians and facilities able and willing to offer TOLAC.
ACOG’s 2010 practice bulletin also acknowledged the problem of limited access. ACOG recommended, as it had in an earlier bulletin, that TOLAC-VBAC be undertaken in facilities where staff are immediately available for emergency care. It added, however, that when such resources are not available, the best alternative may be to refer patients to a facility with available resources. Health care providers and insurance carriers “should do all they can to facilitate transfer of care or comanagement in support of a desired TOLAC,” ACOG’s document states.
Why, given such recommendations, are we still falling so short of where we should be?
A number of nonclinical factors are involved, but clearly, as both the NIH and ACOG have stated, the fear of litigation in cases of uterine rupture is a contributing factor. A ruptured uterus is indeed the principal risk associated with TOLAC, and it can have serious sequelae including perinatal death, hypoxic ischemic encephalopathy (HIE), and hysterectomy.

We must appreciate, however, that the absolute rates of uterine rupture and of serious adverse outcomes are quite low. The rupture rate in 2013 among women who underwent TOLAC but ultimately had a repeat cesarean section – the highest-risk group – was 495 per 100,000 live births, according to the CDC. This rate of approximately 0.5% is consistent with the level of risk reported in the literature for several decades.
In one of the two large observational studies done in the United States that have shed light on TOLAC outcomes, the rate of uterine rupture among women who underwent TOLAC was 0.7% for women with a prior low transverse incision, 2.0% for those with a prior low vertical incision, and 0.5% for those with an unknown type of prior incision. Overall, the rate of uterine rupture in this study’s cohort of 17,898 women who underwent TOLAC was 0.7% (N Engl J Med. 2004 Dec 16;351[25]:2581-9). The study was conducted at 19 medical centers belonging to the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medical Units (MFMU) Network.
The second large study conducted in the United States – a multicenter observational study in which records of approximately 25,000 women with a prior low-transverse cesarean section were reviewed – also showed rates of uterine rupture less than 1% (Am J Obstet Gynecol. 2005 Nov;193[5]:1656-62).
The attributable risk for perinatal death or HIE at term appears to be 1 per 2,000 TOLAC, according to the MFMU Network study.
Failed trials of labor resulting in repeat cesarean deliveries have consistently been associated with higher morbidity than scheduled repeat cesarean deliveries, with the greatest difference in rates for ruptured uterus. In the first MFMU Network study, there were no cases of uterine rupture among a cohort of 15,801 women who underwent elective repeat cesarean delivery, and in the second multicenter study of 25,000 women, this patient group had a rupture rate of 0.004%.
Yet, as ACOG points out, neither elective repeat cesarean deliveries nor TOLAC are without maternal or neonatal risk. Women who have successful VBAC delivery, on the other hand, have significantly lower morbidity and better outcomes than women who do not attempt labor. Women who undergo VBAC also avoid exposure to the significant risks of repeat cesarean deliveries in the long term.
Research unequivocally shows that the risk of placenta accreta, hysterectomy, hemorrhage, and other serious maternal morbidity increases progressively with each repeat cesarean delivery. Rates of placenta accreta have, in fact, been rising in the United States – a trend that should prompt us to think more about TOLAC.
Moreover, TOLAC is being shown to be a cost-effective strategy. In one analysis, TOLAC in a second pregnancy was cost-effective as long as the chance of VBAC exceeded approximately 74% (Obstet Gynecol. 2001 Jun;97[6]:932-41). More recently, TOLAC was found to be cost-effective across a wide variety of circumstances, including when a woman had a probability of VBAC as low as 43%. The model in this analysis, which used probability estimates from the MFMU Cesarean Registry, took a longer-term view by including probabilities of outcomes throughout a woman’s reproductive life that were contingent upon her initial choice regarding TOLAC (Am J Perinatol. 2013 Jan;30[1]:11-20).
Likelihood of success
Evaluating and discussing the likelihood of success with TOLAC is therefore key to the counseling process. The higher the likelihood of achieving VBAC, the more favorable the risk-benefit ratio will be and the more appealing it will be to consider.
According to one analysis, if a woman undergoing a TOLAC has at least a 60%-70% chance of VBAC, her chance of having major or minor morbidity is no greater than a woman undergoing a planned repeat cesarean delivery (Am J Obstet Gynecol 2009;200:56.e1-e6).
There are several prediction tools available that can be used at the first prenatal visit and in early labor to give a reasonably good estimate of success. One of these tools is available at the MFMU Network website (http://mfmu.bsc.gwu.edu). The tools take into account factors such as prior indication for cesarean delivery; history of vaginal delivery; demographic characteristics such as maternal age and body mass index; the occurrence of spontaneous labor; and cervical status at admission.
Prior vaginal delivery is one of the strongest predictors of a successful TOLAC. Research has consistently shown that women with a prior vaginal delivery – including a vaginal delivery predating an unsuccessful TOLAC – have significantly higher TOLAC success rates than women who did not have any prior vaginal delivery.
The indication for a prior cesarean delivery also clearly affects the likelihood of a successful TOLAC. Women whose first cesarean delivery was performed for a nonrecurring indication, such as breech presentation or low intolerance of labor, have TOLAC success rates that are similar to vaginal delivery rates for nulliparous women. Success rates for these women may exceed 85%. On the other hand, women who had a prior cesarean delivery for cephalopelvic disproportion or failure to progress have been shown to have lower TOLAC success rates ranging from 50%-67%.
Labor induction should be approached cautiously, as women who undergo induction of labor in TOLAC have an increased risk of repeat cesarean delivery. Still, success rates with induction are high. Data from the MFMU Cesarean Registry showed that about 66% of women undergoing induction after one prior cesarean delivery achieved VBAC versus 76% of women entering TOLAC spontaneously (Obstet Gynecol. 2007 Feb;109[2 Pt 1]:262-9). Another study of women undergoing induction after one prior cesarean reported an overall success rate of 78% (Obstet Gynecol. 2004 Mar;103[3]:534-8).
Whether induction specifically increases the risk for uterine rupture in TOLAC, compared with expectant management, is unclear. There also are conflicting data as to whether particular induction methods increase this risk.
Based on available data, ACOG considers induction of labor for either maternal or fetal indications to be an option for women undergoing TOLAC. Oxytocin may be used for induction as well as augmentation, but caution should be exercised at higher doses. While there is no clear dosing threshold for increased risk of rupture, research has suggested that higher doses of oxytocin are best avoided.
The use of prostaglandins is more controversial: Based on evidence from several small studies, ACOG concluded in its 2010 bulletin that misoprostol (prostaglandin E1) for cervical ripening is contraindicated in women undergoing TOLAC. It appears likely that rupture risk increases in patients who received both prostaglandins and oxytocin, so ACOG has advised avoiding their sequential use when prostaglandin E2 is used. This of course limits the options for the practitioner. Therefore, utilizing a Foley catheter followed by pitocin has been an approach advocated in some cases.
Uterine rupture is not predictable, and it is far more difficult to assess an individual’s risk of this complication than it is to assess the likelihood of VBAC. Still, there is value to discussing with the patient whether there are any other modifiers that could potentially influence the risk of rupture.
Since rates of uterine rupture are highest in women with previous classical or T-shaped incision, for example, it is important to try to ascertain what type of incision was previously used. It is widely appreciated that low-transverse uterine incisions are most favorable, but findings are mixed in regard to low-vertical incisions. Some research shows that women with a previous low-vertical incision do not have significantly lower VBAC success rates or higher risks of uterine rupture. TOLAC should therefore not be ruled out in these cases.
Additionally, TOLAC should not be ruled out for women who have had more than one cesarean delivery. Several studies have shown an increased risk of uterine rupture after two prior cesarean deliveries, compared with one, and one meta-analysis suggested a more than twofold increased risk (BJOG. 2010 Jan;117(1):5-19.).
In contrast, an analysis of the MFMU Cesarean Registry found no significant difference in rupture rates in women with one prior cesarean versus multiple prior cesareans (Obstet Gynecol. 2006 Jul;108[1]:12-20.).
It appears, therefore, that even if having more than one prior cesarean section is associated with an increased risk of rupture, the magnitude of this increase is small.
Just as women with a prior vaginal delivery have the highest chance of VBAC success, they also have the lowest rates of rupture among all women undergoing TOLAC.
Patient counseling
We must inform our patients who have had a cesarean section in the past of their options for childbirth in an unbiased manner.
The complications of both TOLAC and elective repeat cesarean section should be discussed, and every attempt should be made to individually assess both the likelihood of a successful VBAC and the comparative risk of maternal and perinatal morbidity. A shared decision-making process should be adopted, and whenever possible, the patient’s preference should be respected. In the end, a woman undergoing TOLAC should be truly motivated to pursue a trial of labor, because there are inherent risks.
One thing I’ve learned from my clinical practice and research on this issue is that the desire to undergo a vaginal delivery is powerful for some women. Many of my patients have self-referred for consultation about TOLAC after their ob.gyn. informed them that their hospital is not equipped, and they should therefore have a scheduled repeat operation. In many cases they discover that TOLAC is an option if they are willing to travel a half-hour or so.
We need to honor this desire and inform our patients of the option, and help facilitate delivery at another nearby hospital when our own facility is not equipped for TOLAC.
Dr. Landon is the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, Columbus. He served for more than 25 years as Ohio State’s coinvestigator for the National Institutes of Child Health and Human Development Maternal Fetal Medicine Units Network. He reported having no relevant financial disclosures.
The relative safety of vaginal birth after cesarean (VBAC) has been documented in several large-scale studies in the past 15 years, and was affirmed in 2010 through a National Institutes of Health consensus development conference and a practice bulletin from the American College of Obstetricians and Gynecologists. Yet, despite all this research and review, rates of a trial of labor after cesarean (TOLAC) have increased only modestly in the last several years.
Approximately 20% of all births in 2013 in women with a history of one cesarean section involved a trial of labor, according to a recent report from the Centers for Disease Control and Prevention. This represents only a small increase from 2006, when the TOLAC rate had plummeted to approximately 15%.
The limited change is concerning because up to two-thirds of women with a prior cesarean delivery are candidates for a trial of labor, and many of them are excellent candidates. In total, 70% of the women who attempted labor in 2013 after a previous cesarean had successful VBACs, the CDC data shows.
Several European countries have TOLAC rates between 50% and 70%, but in the United States, as evidenced by the recent CDC data, there continues to be an underutilization of attempted VBAC. We must ask ourselves, are women truly able to choose TOLAC, or are they being dissuaded by the health care system?
I believe that the barriers are still pervasive. Too often, women who are TOLAC candidates are not receiving appropriate counseling – and too often, women are not even being presented the option of a trial of labor, even when staff are immediately available to provide emergency care if needed.
Rupture concerns in perspective
When the NIH consensus development panel reviewed VBAC in 2010, it concluded that TOLAC is a reasonable option for many women with a prior cesarean. The panel found that restricted access to VBAC/TOLAC stemmed from existing practice guidelines and the medical liability climate, and it called upon providers and others to “mitigate or even eliminate” the barriers that women face in finding clinicians and facilities able and willing to offer TOLAC.
ACOG’s 2010 practice bulletin also acknowledged the problem of limited access. ACOG recommended, as it had in an earlier bulletin, that TOLAC-VBAC be undertaken in facilities where staff are immediately available for emergency care. It added, however, that when such resources are not available, the best alternative may be to refer patients to a facility with available resources. Health care providers and insurance carriers “should do all they can to facilitate transfer of care or comanagement in support of a desired TOLAC,” ACOG’s document states.
Why, given such recommendations, are we still falling so short of where we should be?
A number of nonclinical factors are involved, but clearly, as both the NIH and ACOG have stated, the fear of litigation in cases of uterine rupture is a contributing factor. A ruptured uterus is indeed the principal risk associated with TOLAC, and it can have serious sequelae including perinatal death, hypoxic ischemic encephalopathy (HIE), and hysterectomy.

We must appreciate, however, that the absolute rates of uterine rupture and of serious adverse outcomes are quite low. The rupture rate in 2013 among women who underwent TOLAC but ultimately had a repeat cesarean section – the highest-risk group – was 495 per 100,000 live births, according to the CDC. This rate of approximately 0.5% is consistent with the level of risk reported in the literature for several decades.
In one of the two large observational studies done in the United States that have shed light on TOLAC outcomes, the rate of uterine rupture among women who underwent TOLAC was 0.7% for women with a prior low transverse incision, 2.0% for those with a prior low vertical incision, and 0.5% for those with an unknown type of prior incision. Overall, the rate of uterine rupture in this study’s cohort of 17,898 women who underwent TOLAC was 0.7% (N Engl J Med. 2004 Dec 16;351[25]:2581-9). The study was conducted at 19 medical centers belonging to the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medical Units (MFMU) Network.
The second large study conducted in the United States – a multicenter observational study in which records of approximately 25,000 women with a prior low-transverse cesarean section were reviewed – also showed rates of uterine rupture less than 1% (Am J Obstet Gynecol. 2005 Nov;193[5]:1656-62).
The attributable risk for perinatal death or HIE at term appears to be 1 per 2,000 TOLAC, according to the MFMU Network study.
Failed trials of labor resulting in repeat cesarean deliveries have consistently been associated with higher morbidity than scheduled repeat cesarean deliveries, with the greatest difference in rates for ruptured uterus. In the first MFMU Network study, there were no cases of uterine rupture among a cohort of 15,801 women who underwent elective repeat cesarean delivery, and in the second multicenter study of 25,000 women, this patient group had a rupture rate of 0.004%.
Yet, as ACOG points out, neither elective repeat cesarean deliveries nor TOLAC are without maternal or neonatal risk. Women who have successful VBAC delivery, on the other hand, have significantly lower morbidity and better outcomes than women who do not attempt labor. Women who undergo VBAC also avoid exposure to the significant risks of repeat cesarean deliveries in the long term.
Research unequivocally shows that the risk of placenta accreta, hysterectomy, hemorrhage, and other serious maternal morbidity increases progressively with each repeat cesarean delivery. Rates of placenta accreta have, in fact, been rising in the United States – a trend that should prompt us to think more about TOLAC.
Moreover, TOLAC is being shown to be a cost-effective strategy. In one analysis, TOLAC in a second pregnancy was cost-effective as long as the chance of VBAC exceeded approximately 74% (Obstet Gynecol. 2001 Jun;97[6]:932-41). More recently, TOLAC was found to be cost-effective across a wide variety of circumstances, including when a woman had a probability of VBAC as low as 43%. The model in this analysis, which used probability estimates from the MFMU Cesarean Registry, took a longer-term view by including probabilities of outcomes throughout a woman’s reproductive life that were contingent upon her initial choice regarding TOLAC (Am J Perinatol. 2013 Jan;30[1]:11-20).
Likelihood of success
Evaluating and discussing the likelihood of success with TOLAC is therefore key to the counseling process. The higher the likelihood of achieving VBAC, the more favorable the risk-benefit ratio will be and the more appealing it will be to consider.
According to one analysis, if a woman undergoing a TOLAC has at least a 60%-70% chance of VBAC, her chance of having major or minor morbidity is no greater than a woman undergoing a planned repeat cesarean delivery (Am J Obstet Gynecol 2009;200:56.e1-e6).
There are several prediction tools available that can be used at the first prenatal visit and in early labor to give a reasonably good estimate of success. One of these tools is available at the MFMU Network website (http://mfmu.bsc.gwu.edu). The tools take into account factors such as prior indication for cesarean delivery; history of vaginal delivery; demographic characteristics such as maternal age and body mass index; the occurrence of spontaneous labor; and cervical status at admission.
Prior vaginal delivery is one of the strongest predictors of a successful TOLAC. Research has consistently shown that women with a prior vaginal delivery – including a vaginal delivery predating an unsuccessful TOLAC – have significantly higher TOLAC success rates than women who did not have any prior vaginal delivery.
The indication for a prior cesarean delivery also clearly affects the likelihood of a successful TOLAC. Women whose first cesarean delivery was performed for a nonrecurring indication, such as breech presentation or low intolerance of labor, have TOLAC success rates that are similar to vaginal delivery rates for nulliparous women. Success rates for these women may exceed 85%. On the other hand, women who had a prior cesarean delivery for cephalopelvic disproportion or failure to progress have been shown to have lower TOLAC success rates ranging from 50%-67%.
Labor induction should be approached cautiously, as women who undergo induction of labor in TOLAC have an increased risk of repeat cesarean delivery. Still, success rates with induction are high. Data from the MFMU Cesarean Registry showed that about 66% of women undergoing induction after one prior cesarean delivery achieved VBAC versus 76% of women entering TOLAC spontaneously (Obstet Gynecol. 2007 Feb;109[2 Pt 1]:262-9). Another study of women undergoing induction after one prior cesarean reported an overall success rate of 78% (Obstet Gynecol. 2004 Mar;103[3]:534-8).
Whether induction specifically increases the risk for uterine rupture in TOLAC, compared with expectant management, is unclear. There also are conflicting data as to whether particular induction methods increase this risk.
Based on available data, ACOG considers induction of labor for either maternal or fetal indications to be an option for women undergoing TOLAC. Oxytocin may be used for induction as well as augmentation, but caution should be exercised at higher doses. While there is no clear dosing threshold for increased risk of rupture, research has suggested that higher doses of oxytocin are best avoided.
The use of prostaglandins is more controversial: Based on evidence from several small studies, ACOG concluded in its 2010 bulletin that misoprostol (prostaglandin E1) for cervical ripening is contraindicated in women undergoing TOLAC. It appears likely that rupture risk increases in patients who received both prostaglandins and oxytocin, so ACOG has advised avoiding their sequential use when prostaglandin E2 is used. This of course limits the options for the practitioner. Therefore, utilizing a Foley catheter followed by pitocin has been an approach advocated in some cases.
Uterine rupture is not predictable, and it is far more difficult to assess an individual’s risk of this complication than it is to assess the likelihood of VBAC. Still, there is value to discussing with the patient whether there are any other modifiers that could potentially influence the risk of rupture.
Since rates of uterine rupture are highest in women with previous classical or T-shaped incision, for example, it is important to try to ascertain what type of incision was previously used. It is widely appreciated that low-transverse uterine incisions are most favorable, but findings are mixed in regard to low-vertical incisions. Some research shows that women with a previous low-vertical incision do not have significantly lower VBAC success rates or higher risks of uterine rupture. TOLAC should therefore not be ruled out in these cases.
Additionally, TOLAC should not be ruled out for women who have had more than one cesarean delivery. Several studies have shown an increased risk of uterine rupture after two prior cesarean deliveries, compared with one, and one meta-analysis suggested a more than twofold increased risk (BJOG. 2010 Jan;117(1):5-19.).
In contrast, an analysis of the MFMU Cesarean Registry found no significant difference in rupture rates in women with one prior cesarean versus multiple prior cesareans (Obstet Gynecol. 2006 Jul;108[1]:12-20.).
It appears, therefore, that even if having more than one prior cesarean section is associated with an increased risk of rupture, the magnitude of this increase is small.
Just as women with a prior vaginal delivery have the highest chance of VBAC success, they also have the lowest rates of rupture among all women undergoing TOLAC.
Patient counseling
We must inform our patients who have had a cesarean section in the past of their options for childbirth in an unbiased manner.
The complications of both TOLAC and elective repeat cesarean section should be discussed, and every attempt should be made to individually assess both the likelihood of a successful VBAC and the comparative risk of maternal and perinatal morbidity. A shared decision-making process should be adopted, and whenever possible, the patient’s preference should be respected. In the end, a woman undergoing TOLAC should be truly motivated to pursue a trial of labor, because there are inherent risks.
One thing I’ve learned from my clinical practice and research on this issue is that the desire to undergo a vaginal delivery is powerful for some women. Many of my patients have self-referred for consultation about TOLAC after their ob.gyn. informed them that their hospital is not equipped, and they should therefore have a scheduled repeat operation. In many cases they discover that TOLAC is an option if they are willing to travel a half-hour or so.
We need to honor this desire and inform our patients of the option, and help facilitate delivery at another nearby hospital when our own facility is not equipped for TOLAC.
Dr. Landon is the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, Columbus. He served for more than 25 years as Ohio State’s coinvestigator for the National Institutes of Child Health and Human Development Maternal Fetal Medicine Units Network. He reported having no relevant financial disclosures.
Making cystoscopy accessible in gynecology
Gynecologists have used the cystoscope for decades to examine the urethra and bladder, despite urology’s traditional claim that the procedure falls under its purview.
The lines between urology and gynecology have blurred, and cystoscopy has become an even more important and natural part of gynecology’s realm.
During the past 2 decades, gynecologists have become even more involved both in evaluating problems such as overactive bladder symptoms, recurrent urinary tract infection, and bladder/pelvic pain, and in performing pelvic reconstruction procedures.
The American College of Obstetricians and Gynecologists has recommended adoption of cystoscopy by ob.gyns. for diagnostic purposes and some operative indications – most importantly for ruling out cystotomy and intravesical or intraurethral suture or mesh placement, and for verifying ureteral patency. ACOG’s 2007 committee opinion on the role of cystourethroscopy in the generalist obstetrican-gyncecologist practice was reaffirmed in 2015 (Obstet Gynecol. 2007 Jul;110[1]:221-24.).
Yet, to a large extent, cystoscopy has been a good fit in principle, rather than in practice. Training in residency programs has been limited, and traditional cystoscopy can be cumbersome and time consuming. It also is costly, requiring equipment – including a light source and camera – and service contracts that may make it too expensive for many gynecologists to set up and maintain in their offices.
Cystoscopy has therefore often required referral to urologists, resulting in additional appointments, patient inconvenience, and increased costs to the health care system. The learning curve for traditional cystoscopy has been relatively steep, and delays in diagnosis and management as a result of referrals are not uncommon.
Moreover, cystoscopes were never designed to be safe and comfortable for women. Men and women have different anatomy, yet there always has been a one-size-fits-all device. The flexible cystoscope commonly used by urologists was designed for the unique length and anatomy of the male urethra.
A new catheter-based system specifically for female cystoscopy and simple diagnostic visualization of the female bladder and ureters is now available. The system – called CystoSure (Emmy Medical) – comprises a single-use silicone access catheter (18 French today, 16 French in development) and a reusable 2.7 mm, 70-degree rigid-rod lens optic.
The CystoSure catheter is of shorter length than the traditional catheter is, and it adds a fourth self-sealing port; this fourth port allows it to function both as a three-way urinary catheter and as an access sheath for female cystoscopy. When the scope is not inserted, the port remains sealed. The catheter design allows for multiple passes of the Cystosure scope without additional trauma, infection risk, or discomfort.
Additionally, the distal tip of the catheter is open with a flat pancake-shaped balloon that ensures that the scope is consistently placed and fixed at the trigonal ridge. Since the scope tip cannot advance beyond the lower bladder segment, bladder perforation and trauma risk are negligible.
Comprehensive evaluation of the entire bladder lumen including the trigone and ureters is performed with a simple 360-degree rotation of the scope, with minimal manipulation, compared with the traditional in-and-out technique used to circumferentially view sections of the bladder surface.
Full evaluation of the bladder and ureters takes less than 1 minute, and the urethra can be visualized, if desired, by decompressing the distal balloon and removing the entire unit.
The new cystoscopy procedure involves no assembly and is safer, simpler and more consistent than traditional cystoscopy – factors that we hope will make it easier to perform more often in the office for evaluation of bladder conditions (with or without simple cystometrogram testing), as well as during laparoscopic surgery, hysterectomy, incontinence/prolapse surgery, and other urologic procedures to ensure that the bladder and ureters are uninjured and to verify bilateral ureteral flow.
From May 2015 through the mid-summer, we completed and reviewed 55 cases of cystoscopy with Cystosure at several Harvard hospitals, including Brigham and Women’s Faulkner Hospital, Boston, the majority of them in the operating room during sling procedures and other laparoscopic surgeries. We achieved complete bladder and ureter visualization in all cases – including a small number of procedures done in the office setting – with no complications and an extremely short learning curve. For most physicians, it was possible to learn how to perform comprehensive cystoscopy with Cystosure in just one case.
Intraoperative cystoscopy
Reported rates of ureteral and bladder injury during gynecologic procedures have varied by study, type of injury, and complexity of surgery.
In an early report on the usefulness of intra-operative cystoscopy, Dr. Sergio Ribeirio and his colleagues reported that the procedure enabled early recognition and treatment of ureteral injuries in four of a series of 118 patients (3.4%) undergoing total laparoscopic hysterectomy with vault suspension (Hum Reprod. 1999 Jul;14[7]:1727-9.)
A review of 236,392 patients who underwent various laparoscopic gynecologic operations during 1994-2000 showed a urinary tract injury rate ranging from 0.02% to 1.7% (Clin Obstet Gynecol. 2002 Jun;45[2]:469- 80.). And, in another review specifically of ureteral injury in laparoscopic pelvic surgery, incidences of injury ranged from less than 1% to 2% (Obstet Gynecol Surv. 2003 Dec;58[12]:794-9.).
Other studies on the use of cystoscopy have reported injury rates up to and above 3%. In most cases, such reports include the incidence of bladder injury, which is less uncommon. Intraoperative bladder perforation occurs in 3%-9% of cases of midurethral retropubic sling procedures, for instance, according to ACOG’s opinion paper.
In a recent chart review of almost 1,000 women who underwent uterosacral colpopexy for pelvic organ prolapse, on the other hand, the intraoperative bladder injury rate was only 1%, and the rate of ureteral kinking/obstruction requiring stitch removal was significantly higher at 4.5% (Am J Obstet Gynecol. 2015;212:603.e1-7.).
Urinary tract injuries can have serious implications in terms of morbidity and litigation. When an injury is detected intraoperatively, the surgeon can repair it immediately and reduce the risk of complications and readmissions. The ureteral kinking detected in the previously mentioned study would not have been diagnosed without routine cystoscopy; nor would most cases of inadvertent suture or mesh placement in the bladder or urethral lumen.
The advisability of performing cystoscopy routinely in all gynecologic surgical procedures has been debated and should be studied further. However, given the advantages of early detection and the new availability of relatively simple and inexpensive cystoscopy, it is now possible – and will likely be beneficial – to move toward more routine use.
Currently, cystoscopy is performed in only a minority of indicated cases. In the 2003 review cited above from Obstetrical & Gynecological Survey, the ureteral injuries that occurred were identified intraoperatively in only 8.6% of the cases. And in an additional systematic literature review of urinary tract injury from gynecologic surgery, only 17 of the 47 studies included in the review employed routine intraoperative cystoscopy (Obstet Gynecol. 2006 Jun;107[6]:1366-72.).
A survey of ob.gyn residents presented at the ACOG meeting in May 2015 similarly showed that for hysterectomy, universal cystoscopy (defined as being performed in more than 90% of cases) was performed in the residents’ training settings for only a fraction of various types of hysterectomies, from vaginal hysterectomy to total laparoscopic hysterectomy.
Yet, in looking toward their future practice, the residents indicated in the survey that they plan to perform universal cystoscopy more frequently. The majority of them – almost 80% – had been involved with a hysterectomy having a bladder or ureter injury, according to the survey.
The Cystosure system facilitates a complete check of ureteral patency and bladder integrity. The system’s three-way catheter can be placed once and used for multiple passes of the cystoscope as well as for intraoperative retrograde fill of the bladder, postoperative drainage, and IV-based hands-free backfill voiding trials prior to discharge. The catheter’s red balloon port accepts the standard 5 cc syringe, and the blue inflow port provides a universal IV/cysto tubing fitting. The yellow drainage port may be attached to a standardized urinary drainage bag.
With Cystosure, a postoperative voiding trial thus becomes simpler and more efficient than it has in the past. Our nurses can clamp the outflow port, attach the IV bag to the inflow port, and briefly turn their attention elsewhere while the bladder fills hands free. The catheter is then removed, and the patient is allowed to void.
In the office
In the office, Cystosure can similarly make the evaluation of conditions like overactive bladder, urinary incontinence, incomplete bladder emptying, and recurrent urinary tract infections much easier and less expensive, enabling more gynecologists to take the lead in diagnosis.
Currently, there are various methods for performing cystometric testing. One technique, sometimes called “poor man’s cystometry,” involves placing a Foley red rubber catheter in the bladder, attaching a large syringe with the plunger removed, filling the bladder by pour technique, and monitoring the patient’s described sensations of bladder fullness and urge to urinate. This basic test can provide useful information about bladder functioning; patients with overactive bladder feel sensation at much smaller volumes than do patients with neurogenic bladder, for instance.
Yet, while the technique is simple and cheap, it is far from precise and may be misleading. It provides for a fast fill of the bladder in that water enters the bladder as fast as gravity allows. The rapid infusion can sometimes cause an artifact in the patient’s sensation – a significant feeling of pressure or fullness that is premature.
The more-sophisticated technique, multichannel urodynamics, pumps fluid at a slower, controlled rate and provides more accurate information. Yet, it requires expensive equipment, more time, and special expertise. It has not been universally accessible and relevant to the ob.gyn.’s office.
Cystosure bridges the gap between the accurate but costly multichannel urodynamics and the simple but less accurate fast-fill testing method. The nurse can place the Cystosure catheter, attach IV tubing to the inflow port, and then control the drip rate, emulating the pump of the complex urodynamics equipment. When the patient indicates fullness and the overactive bladder/incontinence evaluation is completed, the physician may immediately proceed with simple diagnostic cystoscopy without any further urethral manipulation.
The system can also be coupled to an LED-based battery light source and/or attached to a smartphone/iPad, so that cystoscopy can be performed in any room or at bedside without large bulky equipment and cords. Images and video can be saved and shared from remote locations or used for documentation or teaching.
Dr. Kohli is medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham and Women's Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston. He serves as chief medical officer at Emmy Medical, Holliston, Mass., which manufactures Cystosure.
Gynecologists have used the cystoscope for decades to examine the urethra and bladder, despite urology’s traditional claim that the procedure falls under its purview.
The lines between urology and gynecology have blurred, and cystoscopy has become an even more important and natural part of gynecology’s realm.
During the past 2 decades, gynecologists have become even more involved both in evaluating problems such as overactive bladder symptoms, recurrent urinary tract infection, and bladder/pelvic pain, and in performing pelvic reconstruction procedures.
The American College of Obstetricians and Gynecologists has recommended adoption of cystoscopy by ob.gyns. for diagnostic purposes and some operative indications – most importantly for ruling out cystotomy and intravesical or intraurethral suture or mesh placement, and for verifying ureteral patency. ACOG’s 2007 committee opinion on the role of cystourethroscopy in the generalist obstetrican-gyncecologist practice was reaffirmed in 2015 (Obstet Gynecol. 2007 Jul;110[1]:221-24.).
Yet, to a large extent, cystoscopy has been a good fit in principle, rather than in practice. Training in residency programs has been limited, and traditional cystoscopy can be cumbersome and time consuming. It also is costly, requiring equipment – including a light source and camera – and service contracts that may make it too expensive for many gynecologists to set up and maintain in their offices.
Cystoscopy has therefore often required referral to urologists, resulting in additional appointments, patient inconvenience, and increased costs to the health care system. The learning curve for traditional cystoscopy has been relatively steep, and delays in diagnosis and management as a result of referrals are not uncommon.
Moreover, cystoscopes were never designed to be safe and comfortable for women. Men and women have different anatomy, yet there always has been a one-size-fits-all device. The flexible cystoscope commonly used by urologists was designed for the unique length and anatomy of the male urethra.
A new catheter-based system specifically for female cystoscopy and simple diagnostic visualization of the female bladder and ureters is now available. The system – called CystoSure (Emmy Medical) – comprises a single-use silicone access catheter (18 French today, 16 French in development) and a reusable 2.7 mm, 70-degree rigid-rod lens optic.
The CystoSure catheter is of shorter length than the traditional catheter is, and it adds a fourth self-sealing port; this fourth port allows it to function both as a three-way urinary catheter and as an access sheath for female cystoscopy. When the scope is not inserted, the port remains sealed. The catheter design allows for multiple passes of the Cystosure scope without additional trauma, infection risk, or discomfort.
Additionally, the distal tip of the catheter is open with a flat pancake-shaped balloon that ensures that the scope is consistently placed and fixed at the trigonal ridge. Since the scope tip cannot advance beyond the lower bladder segment, bladder perforation and trauma risk are negligible.
Comprehensive evaluation of the entire bladder lumen including the trigone and ureters is performed with a simple 360-degree rotation of the scope, with minimal manipulation, compared with the traditional in-and-out technique used to circumferentially view sections of the bladder surface.
Full evaluation of the bladder and ureters takes less than 1 minute, and the urethra can be visualized, if desired, by decompressing the distal balloon and removing the entire unit.
The new cystoscopy procedure involves no assembly and is safer, simpler and more consistent than traditional cystoscopy – factors that we hope will make it easier to perform more often in the office for evaluation of bladder conditions (with or without simple cystometrogram testing), as well as during laparoscopic surgery, hysterectomy, incontinence/prolapse surgery, and other urologic procedures to ensure that the bladder and ureters are uninjured and to verify bilateral ureteral flow.
From May 2015 through the mid-summer, we completed and reviewed 55 cases of cystoscopy with Cystosure at several Harvard hospitals, including Brigham and Women’s Faulkner Hospital, Boston, the majority of them in the operating room during sling procedures and other laparoscopic surgeries. We achieved complete bladder and ureter visualization in all cases – including a small number of procedures done in the office setting – with no complications and an extremely short learning curve. For most physicians, it was possible to learn how to perform comprehensive cystoscopy with Cystosure in just one case.
Intraoperative cystoscopy
Reported rates of ureteral and bladder injury during gynecologic procedures have varied by study, type of injury, and complexity of surgery.
In an early report on the usefulness of intra-operative cystoscopy, Dr. Sergio Ribeirio and his colleagues reported that the procedure enabled early recognition and treatment of ureteral injuries in four of a series of 118 patients (3.4%) undergoing total laparoscopic hysterectomy with vault suspension (Hum Reprod. 1999 Jul;14[7]:1727-9.)
A review of 236,392 patients who underwent various laparoscopic gynecologic operations during 1994-2000 showed a urinary tract injury rate ranging from 0.02% to 1.7% (Clin Obstet Gynecol. 2002 Jun;45[2]:469- 80.). And, in another review specifically of ureteral injury in laparoscopic pelvic surgery, incidences of injury ranged from less than 1% to 2% (Obstet Gynecol Surv. 2003 Dec;58[12]:794-9.).
Other studies on the use of cystoscopy have reported injury rates up to and above 3%. In most cases, such reports include the incidence of bladder injury, which is less uncommon. Intraoperative bladder perforation occurs in 3%-9% of cases of midurethral retropubic sling procedures, for instance, according to ACOG’s opinion paper.
In a recent chart review of almost 1,000 women who underwent uterosacral colpopexy for pelvic organ prolapse, on the other hand, the intraoperative bladder injury rate was only 1%, and the rate of ureteral kinking/obstruction requiring stitch removal was significantly higher at 4.5% (Am J Obstet Gynecol. 2015;212:603.e1-7.).
Urinary tract injuries can have serious implications in terms of morbidity and litigation. When an injury is detected intraoperatively, the surgeon can repair it immediately and reduce the risk of complications and readmissions. The ureteral kinking detected in the previously mentioned study would not have been diagnosed without routine cystoscopy; nor would most cases of inadvertent suture or mesh placement in the bladder or urethral lumen.
The advisability of performing cystoscopy routinely in all gynecologic surgical procedures has been debated and should be studied further. However, given the advantages of early detection and the new availability of relatively simple and inexpensive cystoscopy, it is now possible – and will likely be beneficial – to move toward more routine use.
Currently, cystoscopy is performed in only a minority of indicated cases. In the 2003 review cited above from Obstetrical & Gynecological Survey, the ureteral injuries that occurred were identified intraoperatively in only 8.6% of the cases. And in an additional systematic literature review of urinary tract injury from gynecologic surgery, only 17 of the 47 studies included in the review employed routine intraoperative cystoscopy (Obstet Gynecol. 2006 Jun;107[6]:1366-72.).
A survey of ob.gyn residents presented at the ACOG meeting in May 2015 similarly showed that for hysterectomy, universal cystoscopy (defined as being performed in more than 90% of cases) was performed in the residents’ training settings for only a fraction of various types of hysterectomies, from vaginal hysterectomy to total laparoscopic hysterectomy.
Yet, in looking toward their future practice, the residents indicated in the survey that they plan to perform universal cystoscopy more frequently. The majority of them – almost 80% – had been involved with a hysterectomy having a bladder or ureter injury, according to the survey.
The Cystosure system facilitates a complete check of ureteral patency and bladder integrity. The system’s three-way catheter can be placed once and used for multiple passes of the cystoscope as well as for intraoperative retrograde fill of the bladder, postoperative drainage, and IV-based hands-free backfill voiding trials prior to discharge. The catheter’s red balloon port accepts the standard 5 cc syringe, and the blue inflow port provides a universal IV/cysto tubing fitting. The yellow drainage port may be attached to a standardized urinary drainage bag.
With Cystosure, a postoperative voiding trial thus becomes simpler and more efficient than it has in the past. Our nurses can clamp the outflow port, attach the IV bag to the inflow port, and briefly turn their attention elsewhere while the bladder fills hands free. The catheter is then removed, and the patient is allowed to void.
In the office
In the office, Cystosure can similarly make the evaluation of conditions like overactive bladder, urinary incontinence, incomplete bladder emptying, and recurrent urinary tract infections much easier and less expensive, enabling more gynecologists to take the lead in diagnosis.
Currently, there are various methods for performing cystometric testing. One technique, sometimes called “poor man’s cystometry,” involves placing a Foley red rubber catheter in the bladder, attaching a large syringe with the plunger removed, filling the bladder by pour technique, and monitoring the patient’s described sensations of bladder fullness and urge to urinate. This basic test can provide useful information about bladder functioning; patients with overactive bladder feel sensation at much smaller volumes than do patients with neurogenic bladder, for instance.
Yet, while the technique is simple and cheap, it is far from precise and may be misleading. It provides for a fast fill of the bladder in that water enters the bladder as fast as gravity allows. The rapid infusion can sometimes cause an artifact in the patient’s sensation – a significant feeling of pressure or fullness that is premature.
The more-sophisticated technique, multichannel urodynamics, pumps fluid at a slower, controlled rate and provides more accurate information. Yet, it requires expensive equipment, more time, and special expertise. It has not been universally accessible and relevant to the ob.gyn.’s office.
Cystosure bridges the gap between the accurate but costly multichannel urodynamics and the simple but less accurate fast-fill testing method. The nurse can place the Cystosure catheter, attach IV tubing to the inflow port, and then control the drip rate, emulating the pump of the complex urodynamics equipment. When the patient indicates fullness and the overactive bladder/incontinence evaluation is completed, the physician may immediately proceed with simple diagnostic cystoscopy without any further urethral manipulation.
The system can also be coupled to an LED-based battery light source and/or attached to a smartphone/iPad, so that cystoscopy can be performed in any room or at bedside without large bulky equipment and cords. Images and video can be saved and shared from remote locations or used for documentation or teaching.
Dr. Kohli is medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham and Women's Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston. He serves as chief medical officer at Emmy Medical, Holliston, Mass., which manufactures Cystosure.
Gynecologists have used the cystoscope for decades to examine the urethra and bladder, despite urology’s traditional claim that the procedure falls under its purview.
The lines between urology and gynecology have blurred, and cystoscopy has become an even more important and natural part of gynecology’s realm.
During the past 2 decades, gynecologists have become even more involved both in evaluating problems such as overactive bladder symptoms, recurrent urinary tract infection, and bladder/pelvic pain, and in performing pelvic reconstruction procedures.
The American College of Obstetricians and Gynecologists has recommended adoption of cystoscopy by ob.gyns. for diagnostic purposes and some operative indications – most importantly for ruling out cystotomy and intravesical or intraurethral suture or mesh placement, and for verifying ureteral patency. ACOG’s 2007 committee opinion on the role of cystourethroscopy in the generalist obstetrican-gyncecologist practice was reaffirmed in 2015 (Obstet Gynecol. 2007 Jul;110[1]:221-24.).
Yet, to a large extent, cystoscopy has been a good fit in principle, rather than in practice. Training in residency programs has been limited, and traditional cystoscopy can be cumbersome and time consuming. It also is costly, requiring equipment – including a light source and camera – and service contracts that may make it too expensive for many gynecologists to set up and maintain in their offices.
Cystoscopy has therefore often required referral to urologists, resulting in additional appointments, patient inconvenience, and increased costs to the health care system. The learning curve for traditional cystoscopy has been relatively steep, and delays in diagnosis and management as a result of referrals are not uncommon.
Moreover, cystoscopes were never designed to be safe and comfortable for women. Men and women have different anatomy, yet there always has been a one-size-fits-all device. The flexible cystoscope commonly used by urologists was designed for the unique length and anatomy of the male urethra.
A new catheter-based system specifically for female cystoscopy and simple diagnostic visualization of the female bladder and ureters is now available. The system – called CystoSure (Emmy Medical) – comprises a single-use silicone access catheter (18 French today, 16 French in development) and a reusable 2.7 mm, 70-degree rigid-rod lens optic.
The CystoSure catheter is of shorter length than the traditional catheter is, and it adds a fourth self-sealing port; this fourth port allows it to function both as a three-way urinary catheter and as an access sheath for female cystoscopy. When the scope is not inserted, the port remains sealed. The catheter design allows for multiple passes of the Cystosure scope without additional trauma, infection risk, or discomfort.
Additionally, the distal tip of the catheter is open with a flat pancake-shaped balloon that ensures that the scope is consistently placed and fixed at the trigonal ridge. Since the scope tip cannot advance beyond the lower bladder segment, bladder perforation and trauma risk are negligible.
Comprehensive evaluation of the entire bladder lumen including the trigone and ureters is performed with a simple 360-degree rotation of the scope, with minimal manipulation, compared with the traditional in-and-out technique used to circumferentially view sections of the bladder surface.
Full evaluation of the bladder and ureters takes less than 1 minute, and the urethra can be visualized, if desired, by decompressing the distal balloon and removing the entire unit.
The new cystoscopy procedure involves no assembly and is safer, simpler and more consistent than traditional cystoscopy – factors that we hope will make it easier to perform more often in the office for evaluation of bladder conditions (with or without simple cystometrogram testing), as well as during laparoscopic surgery, hysterectomy, incontinence/prolapse surgery, and other urologic procedures to ensure that the bladder and ureters are uninjured and to verify bilateral ureteral flow.
From May 2015 through the mid-summer, we completed and reviewed 55 cases of cystoscopy with Cystosure at several Harvard hospitals, including Brigham and Women’s Faulkner Hospital, Boston, the majority of them in the operating room during sling procedures and other laparoscopic surgeries. We achieved complete bladder and ureter visualization in all cases – including a small number of procedures done in the office setting – with no complications and an extremely short learning curve. For most physicians, it was possible to learn how to perform comprehensive cystoscopy with Cystosure in just one case.
Intraoperative cystoscopy
Reported rates of ureteral and bladder injury during gynecologic procedures have varied by study, type of injury, and complexity of surgery.
In an early report on the usefulness of intra-operative cystoscopy, Dr. Sergio Ribeirio and his colleagues reported that the procedure enabled early recognition and treatment of ureteral injuries in four of a series of 118 patients (3.4%) undergoing total laparoscopic hysterectomy with vault suspension (Hum Reprod. 1999 Jul;14[7]:1727-9.)
A review of 236,392 patients who underwent various laparoscopic gynecologic operations during 1994-2000 showed a urinary tract injury rate ranging from 0.02% to 1.7% (Clin Obstet Gynecol. 2002 Jun;45[2]:469- 80.). And, in another review specifically of ureteral injury in laparoscopic pelvic surgery, incidences of injury ranged from less than 1% to 2% (Obstet Gynecol Surv. 2003 Dec;58[12]:794-9.).
Other studies on the use of cystoscopy have reported injury rates up to and above 3%. In most cases, such reports include the incidence of bladder injury, which is less uncommon. Intraoperative bladder perforation occurs in 3%-9% of cases of midurethral retropubic sling procedures, for instance, according to ACOG’s opinion paper.
In a recent chart review of almost 1,000 women who underwent uterosacral colpopexy for pelvic organ prolapse, on the other hand, the intraoperative bladder injury rate was only 1%, and the rate of ureteral kinking/obstruction requiring stitch removal was significantly higher at 4.5% (Am J Obstet Gynecol. 2015;212:603.e1-7.).
Urinary tract injuries can have serious implications in terms of morbidity and litigation. When an injury is detected intraoperatively, the surgeon can repair it immediately and reduce the risk of complications and readmissions. The ureteral kinking detected in the previously mentioned study would not have been diagnosed without routine cystoscopy; nor would most cases of inadvertent suture or mesh placement in the bladder or urethral lumen.
The advisability of performing cystoscopy routinely in all gynecologic surgical procedures has been debated and should be studied further. However, given the advantages of early detection and the new availability of relatively simple and inexpensive cystoscopy, it is now possible – and will likely be beneficial – to move toward more routine use.
Currently, cystoscopy is performed in only a minority of indicated cases. In the 2003 review cited above from Obstetrical & Gynecological Survey, the ureteral injuries that occurred were identified intraoperatively in only 8.6% of the cases. And in an additional systematic literature review of urinary tract injury from gynecologic surgery, only 17 of the 47 studies included in the review employed routine intraoperative cystoscopy (Obstet Gynecol. 2006 Jun;107[6]:1366-72.).
A survey of ob.gyn residents presented at the ACOG meeting in May 2015 similarly showed that for hysterectomy, universal cystoscopy (defined as being performed in more than 90% of cases) was performed in the residents’ training settings for only a fraction of various types of hysterectomies, from vaginal hysterectomy to total laparoscopic hysterectomy.
Yet, in looking toward their future practice, the residents indicated in the survey that they plan to perform universal cystoscopy more frequently. The majority of them – almost 80% – had been involved with a hysterectomy having a bladder or ureter injury, according to the survey.
The Cystosure system facilitates a complete check of ureteral patency and bladder integrity. The system’s three-way catheter can be placed once and used for multiple passes of the cystoscope as well as for intraoperative retrograde fill of the bladder, postoperative drainage, and IV-based hands-free backfill voiding trials prior to discharge. The catheter’s red balloon port accepts the standard 5 cc syringe, and the blue inflow port provides a universal IV/cysto tubing fitting. The yellow drainage port may be attached to a standardized urinary drainage bag.
With Cystosure, a postoperative voiding trial thus becomes simpler and more efficient than it has in the past. Our nurses can clamp the outflow port, attach the IV bag to the inflow port, and briefly turn their attention elsewhere while the bladder fills hands free. The catheter is then removed, and the patient is allowed to void.
In the office
In the office, Cystosure can similarly make the evaluation of conditions like overactive bladder, urinary incontinence, incomplete bladder emptying, and recurrent urinary tract infections much easier and less expensive, enabling more gynecologists to take the lead in diagnosis.
Currently, there are various methods for performing cystometric testing. One technique, sometimes called “poor man’s cystometry,” involves placing a Foley red rubber catheter in the bladder, attaching a large syringe with the plunger removed, filling the bladder by pour technique, and monitoring the patient’s described sensations of bladder fullness and urge to urinate. This basic test can provide useful information about bladder functioning; patients with overactive bladder feel sensation at much smaller volumes than do patients with neurogenic bladder, for instance.
Yet, while the technique is simple and cheap, it is far from precise and may be misleading. It provides for a fast fill of the bladder in that water enters the bladder as fast as gravity allows. The rapid infusion can sometimes cause an artifact in the patient’s sensation – a significant feeling of pressure or fullness that is premature.
The more-sophisticated technique, multichannel urodynamics, pumps fluid at a slower, controlled rate and provides more accurate information. Yet, it requires expensive equipment, more time, and special expertise. It has not been universally accessible and relevant to the ob.gyn.’s office.
Cystosure bridges the gap between the accurate but costly multichannel urodynamics and the simple but less accurate fast-fill testing method. The nurse can place the Cystosure catheter, attach IV tubing to the inflow port, and then control the drip rate, emulating the pump of the complex urodynamics equipment. When the patient indicates fullness and the overactive bladder/incontinence evaluation is completed, the physician may immediately proceed with simple diagnostic cystoscopy without any further urethral manipulation.
The system can also be coupled to an LED-based battery light source and/or attached to a smartphone/iPad, so that cystoscopy can be performed in any room or at bedside without large bulky equipment and cords. Images and video can be saved and shared from remote locations or used for documentation or teaching.
Dr. Kohli is medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham and Women's Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston. He serves as chief medical officer at Emmy Medical, Holliston, Mass., which manufactures Cystosure.
Cystoscopies are us
In 2012, the AAGL issued Guidelines for Intraoperative Cystoscopy in Laparoscopic Hysterectomy (J Minim Invasive Gynecol. 2012 Jul-Aug;19[4]:407-11.). In this AAGL report, a meta-analysis noted 27 published trials comprising 3,643 cases. Laparoscopic hysterectomy was associated with an increased risk of urinary tract injury when compared with abdominal hysterectomy (odds ratio, 2.61; 95% confidence interval, 1.22-5.60), according to the meta-analysis (BMJ. 2005 Jun 25;330[7506]:1478.).
As a result of this meta-analysis, as well as multiple other studies, the AAGL Guidelines Committee noted that “current evidence supports the conclusion that cystoscopic evaluation of the lower urinary tract should be readily available to gynecologic surgeons performing laparoscopic hysterectomy.” The resultant guidelines recommend that “a surgeon with appropriate education, training, and institutional privileges be available without delay to perform the task (cystoscopy).”
Besides the evaluation of the urinary tract for potential injury at hysterectomy, cystoscopy is useful in evaluation of various urogynecologic concerns, potential malignancy, and possible genitourinary fistula.
In this edition of the Master Class in Gynecologic Surgery, I have asked urogynecologist Dr. Neeraj Kohli to discuss the use of cystoscopy in gynecology, as well as to present new instrumentation to aide in the performance of the procedure.
Dr. Kohli is in private practice as medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham Women’s Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston.
Dr. Kohli is a nationally recognized leader in the field of urogynecology and reconstructive pelvic surgery, specializing in the treatment of pelvic prolapse, urinary incontinence, and advanced pelvic surgery. He has authored more than 100 scientific articles, book chapters, research abstracts, clinical presentations and multimedia educational tools.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this Master Class.
In 2012, the AAGL issued Guidelines for Intraoperative Cystoscopy in Laparoscopic Hysterectomy (J Minim Invasive Gynecol. 2012 Jul-Aug;19[4]:407-11.). In this AAGL report, a meta-analysis noted 27 published trials comprising 3,643 cases. Laparoscopic hysterectomy was associated with an increased risk of urinary tract injury when compared with abdominal hysterectomy (odds ratio, 2.61; 95% confidence interval, 1.22-5.60), according to the meta-analysis (BMJ. 2005 Jun 25;330[7506]:1478.).
As a result of this meta-analysis, as well as multiple other studies, the AAGL Guidelines Committee noted that “current evidence supports the conclusion that cystoscopic evaluation of the lower urinary tract should be readily available to gynecologic surgeons performing laparoscopic hysterectomy.” The resultant guidelines recommend that “a surgeon with appropriate education, training, and institutional privileges be available without delay to perform the task (cystoscopy).”
Besides the evaluation of the urinary tract for potential injury at hysterectomy, cystoscopy is useful in evaluation of various urogynecologic concerns, potential malignancy, and possible genitourinary fistula.
In this edition of the Master Class in Gynecologic Surgery, I have asked urogynecologist Dr. Neeraj Kohli to discuss the use of cystoscopy in gynecology, as well as to present new instrumentation to aide in the performance of the procedure.
Dr. Kohli is in private practice as medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham Women’s Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston.
Dr. Kohli is a nationally recognized leader in the field of urogynecology and reconstructive pelvic surgery, specializing in the treatment of pelvic prolapse, urinary incontinence, and advanced pelvic surgery. He has authored more than 100 scientific articles, book chapters, research abstracts, clinical presentations and multimedia educational tools.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this Master Class.
In 2012, the AAGL issued Guidelines for Intraoperative Cystoscopy in Laparoscopic Hysterectomy (J Minim Invasive Gynecol. 2012 Jul-Aug;19[4]:407-11.). In this AAGL report, a meta-analysis noted 27 published trials comprising 3,643 cases. Laparoscopic hysterectomy was associated with an increased risk of urinary tract injury when compared with abdominal hysterectomy (odds ratio, 2.61; 95% confidence interval, 1.22-5.60), according to the meta-analysis (BMJ. 2005 Jun 25;330[7506]:1478.).
As a result of this meta-analysis, as well as multiple other studies, the AAGL Guidelines Committee noted that “current evidence supports the conclusion that cystoscopic evaluation of the lower urinary tract should be readily available to gynecologic surgeons performing laparoscopic hysterectomy.” The resultant guidelines recommend that “a surgeon with appropriate education, training, and institutional privileges be available without delay to perform the task (cystoscopy).”
Besides the evaluation of the urinary tract for potential injury at hysterectomy, cystoscopy is useful in evaluation of various urogynecologic concerns, potential malignancy, and possible genitourinary fistula.
In this edition of the Master Class in Gynecologic Surgery, I have asked urogynecologist Dr. Neeraj Kohli to discuss the use of cystoscopy in gynecology, as well as to present new instrumentation to aide in the performance of the procedure.
Dr. Kohli is in private practice as medical director of Boston Urogyn in Wellesley, Mass., an ob.gyn. staff member at Brigham Women’s Hospital/Newton Wellesley Hospital, and assistant professor of ob.gyn. at Harvard Medical School in Boston.
Dr. Kohli is a nationally recognized leader in the field of urogynecology and reconstructive pelvic surgery, specializing in the treatment of pelvic prolapse, urinary incontinence, and advanced pelvic surgery. He has authored more than 100 scientific articles, book chapters, research abstracts, clinical presentations and multimedia educational tools.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this Master Class.
Keeping laparoscopy safe for the obese patient
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
Positioning obese patients for minimally invasive gynecologic surgery
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at obnews@frontlinemedcom.com.
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at obnews@frontlinemedcom.com.
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at obnews@frontlinemedcom.com.
Using maternal triglyceride levels as a marker for pregnancy risk
It is widely known and taught that maternal blood lipid levels increase slightly during pregnancy. Lipid values throughout pregnancy, however, have not been well described, making it difficult to ascertain which changes are normal and which changes may be potentially troubling for the mother and/or the baby.
Similarly, the association between pregnancy outcomes and lipid levels prior to conception and during pregnancy has been studied only minimally. In both areas, more research is needed.
Yet despite the need for more research, it now appears that the mother’s lipid profile – particularly her triglyceride levels before and during pregnancy – warrants our attention. Results from several clinical studies suggest that elevated maternal triglyceride (TG) levels may be associated with gestational diabetes mellitus (GDM) and preeclampsia. Since these conditions can contribute to the development of peri- and postpartum complications and increase the mother’s risk of developing subsequent type 2 diabetes and systemic hypertension, a mother’s lipid profile – just like her glucose levels – may help us define who is at high risk of pregnancy complications and later adverse effects.
Research findings
In addition to assessing fetal health during pregnancy, ob.gyns. routinely measure and monitor maternal blood pressure, weight gain, and blood sugar, which fluctuate during normal pregnancies.
We have found that lipid levels, notably maternal TG, total cholesterol, and the major particles of high-density lipoproteins (HDLs) and low-density lipoproteins (LDLs) also vary during pregnancy, with a nadir during the first trimester, followed by a gradual increase and a peaking before delivery.
It is well known that severely elevated blood pressure, gestational weight, or glucose can signify a pregnancy at risk for adverse outcomes. However, our research has shown that high levels of TGs, but not the levels of HDLs, LDLs, or total cholesterol, during pregnancy also are associated with an increased risk for preeclampsia and gestational diabetes mellitus (Am. J. Obstet. Gynecol. 2009;201:482.e1-8).
In our study, the rate of preeclampsia or GDM increased with maternal TG level, from 7.2% in women who had the lowest levels (<25th percentile) to 19.8% in women who had the highest levels (>75th percentile).
We found that TG levels in the upper quartile also were associated with a significantly higher risk of preeclampsia, compared with the lower quartile (relative risk, 1.87).
Similarly, among women without diagnosed GDM, those with TG levels in the upper quartile were more likely to have a fasting glucose level of 100 mg/dL or more, compared with the intermediate group (TG level between the 25th and 75th percentiles) and the lower quartile. Women with the highest TG levels also were more likely to have infants classified as large for gestational age.
Our findings are consistent with a review that showed a positive association between elevated maternal TG and the risk of preeclampsia (BJOG 2006;113:379-86), as well as a cohort study that found plasma TG levels in the first trimester were independently and linearly associated with pregnancy-induced hypertension, preeclampsia, and large-for-gestational age (J. Clin. Endocrinol. Metab. 2012;97:3917-25).
Interestingly, the cohort study did not show an association between elevated maternal TG levels, adverse pregnancy outcomes, and body mass index. This suggests that weight gain and TG may be independent risk factors.
In practice
At this point in time, without defined cut-off values and well-tested interventions, there is no recommendation regarding maternal lipid measurement during pregnancy. We have shown, however, that maternal TG levels above 140 mg/dL at 3 months’ gestation and TG levels of 200 mg/dL or more at 6 months’ gestation are very high and may indicate a high-risk pregnancy.
Like all ob.gyns., we advise women before pregnancy to lose weight and to normalize blood glucose levels before attempting to conceive to reduce pregnancy complications, but we also encourage our patients to lower their TG levels. Given the observed associations between higher TG levels and adverse pregnancy outcomes, we now routinely measure maternal lipids as well as blood glucose in our pregnant patients. We also test lipid levels in pregnant women who have other risk factors such as GDM in a prior pregnancy or chronic high blood pressure.
It is possible that lifestyle programs (such as those involving diet, weight reduction, and physical activity) prior to and during pregnancy, with a focus not only on maintaining a healthy weight but also on lowering TG levels, may help to further prevent complications during pregnancy and adverse birth outcomes. Although more research is needed, lowering TGs with cholesterol-reducing drugs also may help improve pregnancy outcomes. Indeed, there is currently a study investigating the pharmacologic treatment of high lipids during pregnancy.
For now, we should advise our patients who have higher TG levels in pregnancy to improve their diets and levels of physical activity. We also should monitor these patients for the increased likelihood of developing GDM and preeclampsia because higher lipid profiles appear to equate to a higher risk of adverse outcomes.
Finally, our attention to lipid profiles should extend beyond birth, since the long-term risk of cardiovascular disease may be influenced by preeclampsia and potentially by the lipid changes that escalate with the condition.
Dr. Wiznitzer reported having no financial disclosures related to this Master Class.
It is widely known and taught that maternal blood lipid levels increase slightly during pregnancy. Lipid values throughout pregnancy, however, have not been well described, making it difficult to ascertain which changes are normal and which changes may be potentially troubling for the mother and/or the baby.
Similarly, the association between pregnancy outcomes and lipid levels prior to conception and during pregnancy has been studied only minimally. In both areas, more research is needed.
Yet despite the need for more research, it now appears that the mother’s lipid profile – particularly her triglyceride levels before and during pregnancy – warrants our attention. Results from several clinical studies suggest that elevated maternal triglyceride (TG) levels may be associated with gestational diabetes mellitus (GDM) and preeclampsia. Since these conditions can contribute to the development of peri- and postpartum complications and increase the mother’s risk of developing subsequent type 2 diabetes and systemic hypertension, a mother’s lipid profile – just like her glucose levels – may help us define who is at high risk of pregnancy complications and later adverse effects.
Research findings
In addition to assessing fetal health during pregnancy, ob.gyns. routinely measure and monitor maternal blood pressure, weight gain, and blood sugar, which fluctuate during normal pregnancies.
We have found that lipid levels, notably maternal TG, total cholesterol, and the major particles of high-density lipoproteins (HDLs) and low-density lipoproteins (LDLs) also vary during pregnancy, with a nadir during the first trimester, followed by a gradual increase and a peaking before delivery.
It is well known that severely elevated blood pressure, gestational weight, or glucose can signify a pregnancy at risk for adverse outcomes. However, our research has shown that high levels of TGs, but not the levels of HDLs, LDLs, or total cholesterol, during pregnancy also are associated with an increased risk for preeclampsia and gestational diabetes mellitus (Am. J. Obstet. Gynecol. 2009;201:482.e1-8).
In our study, the rate of preeclampsia or GDM increased with maternal TG level, from 7.2% in women who had the lowest levels (<25th percentile) to 19.8% in women who had the highest levels (>75th percentile).
We found that TG levels in the upper quartile also were associated with a significantly higher risk of preeclampsia, compared with the lower quartile (relative risk, 1.87).
Similarly, among women without diagnosed GDM, those with TG levels in the upper quartile were more likely to have a fasting glucose level of 100 mg/dL or more, compared with the intermediate group (TG level between the 25th and 75th percentiles) and the lower quartile. Women with the highest TG levels also were more likely to have infants classified as large for gestational age.
Our findings are consistent with a review that showed a positive association between elevated maternal TG and the risk of preeclampsia (BJOG 2006;113:379-86), as well as a cohort study that found plasma TG levels in the first trimester were independently and linearly associated with pregnancy-induced hypertension, preeclampsia, and large-for-gestational age (J. Clin. Endocrinol. Metab. 2012;97:3917-25).
Interestingly, the cohort study did not show an association between elevated maternal TG levels, adverse pregnancy outcomes, and body mass index. This suggests that weight gain and TG may be independent risk factors.
In practice
At this point in time, without defined cut-off values and well-tested interventions, there is no recommendation regarding maternal lipid measurement during pregnancy. We have shown, however, that maternal TG levels above 140 mg/dL at 3 months’ gestation and TG levels of 200 mg/dL or more at 6 months’ gestation are very high and may indicate a high-risk pregnancy.
Like all ob.gyns., we advise women before pregnancy to lose weight and to normalize blood glucose levels before attempting to conceive to reduce pregnancy complications, but we also encourage our patients to lower their TG levels. Given the observed associations between higher TG levels and adverse pregnancy outcomes, we now routinely measure maternal lipids as well as blood glucose in our pregnant patients. We also test lipid levels in pregnant women who have other risk factors such as GDM in a prior pregnancy or chronic high blood pressure.
It is possible that lifestyle programs (such as those involving diet, weight reduction, and physical activity) prior to and during pregnancy, with a focus not only on maintaining a healthy weight but also on lowering TG levels, may help to further prevent complications during pregnancy and adverse birth outcomes. Although more research is needed, lowering TGs with cholesterol-reducing drugs also may help improve pregnancy outcomes. Indeed, there is currently a study investigating the pharmacologic treatment of high lipids during pregnancy.
For now, we should advise our patients who have higher TG levels in pregnancy to improve their diets and levels of physical activity. We also should monitor these patients for the increased likelihood of developing GDM and preeclampsia because higher lipid profiles appear to equate to a higher risk of adverse outcomes.
Finally, our attention to lipid profiles should extend beyond birth, since the long-term risk of cardiovascular disease may be influenced by preeclampsia and potentially by the lipid changes that escalate with the condition.
Dr. Wiznitzer reported having no financial disclosures related to this Master Class.
It is widely known and taught that maternal blood lipid levels increase slightly during pregnancy. Lipid values throughout pregnancy, however, have not been well described, making it difficult to ascertain which changes are normal and which changes may be potentially troubling for the mother and/or the baby.
Similarly, the association between pregnancy outcomes and lipid levels prior to conception and during pregnancy has been studied only minimally. In both areas, more research is needed.
Yet despite the need for more research, it now appears that the mother’s lipid profile – particularly her triglyceride levels before and during pregnancy – warrants our attention. Results from several clinical studies suggest that elevated maternal triglyceride (TG) levels may be associated with gestational diabetes mellitus (GDM) and preeclampsia. Since these conditions can contribute to the development of peri- and postpartum complications and increase the mother’s risk of developing subsequent type 2 diabetes and systemic hypertension, a mother’s lipid profile – just like her glucose levels – may help us define who is at high risk of pregnancy complications and later adverse effects.
Research findings
In addition to assessing fetal health during pregnancy, ob.gyns. routinely measure and monitor maternal blood pressure, weight gain, and blood sugar, which fluctuate during normal pregnancies.
We have found that lipid levels, notably maternal TG, total cholesterol, and the major particles of high-density lipoproteins (HDLs) and low-density lipoproteins (LDLs) also vary during pregnancy, with a nadir during the first trimester, followed by a gradual increase and a peaking before delivery.
It is well known that severely elevated blood pressure, gestational weight, or glucose can signify a pregnancy at risk for adverse outcomes. However, our research has shown that high levels of TGs, but not the levels of HDLs, LDLs, or total cholesterol, during pregnancy also are associated with an increased risk for preeclampsia and gestational diabetes mellitus (Am. J. Obstet. Gynecol. 2009;201:482.e1-8).
In our study, the rate of preeclampsia or GDM increased with maternal TG level, from 7.2% in women who had the lowest levels (<25th percentile) to 19.8% in women who had the highest levels (>75th percentile).
We found that TG levels in the upper quartile also were associated with a significantly higher risk of preeclampsia, compared with the lower quartile (relative risk, 1.87).
Similarly, among women without diagnosed GDM, those with TG levels in the upper quartile were more likely to have a fasting glucose level of 100 mg/dL or more, compared with the intermediate group (TG level between the 25th and 75th percentiles) and the lower quartile. Women with the highest TG levels also were more likely to have infants classified as large for gestational age.
Our findings are consistent with a review that showed a positive association between elevated maternal TG and the risk of preeclampsia (BJOG 2006;113:379-86), as well as a cohort study that found plasma TG levels in the first trimester were independently and linearly associated with pregnancy-induced hypertension, preeclampsia, and large-for-gestational age (J. Clin. Endocrinol. Metab. 2012;97:3917-25).
Interestingly, the cohort study did not show an association between elevated maternal TG levels, adverse pregnancy outcomes, and body mass index. This suggests that weight gain and TG may be independent risk factors.
In practice
At this point in time, without defined cut-off values and well-tested interventions, there is no recommendation regarding maternal lipid measurement during pregnancy. We have shown, however, that maternal TG levels above 140 mg/dL at 3 months’ gestation and TG levels of 200 mg/dL or more at 6 months’ gestation are very high and may indicate a high-risk pregnancy.
Like all ob.gyns., we advise women before pregnancy to lose weight and to normalize blood glucose levels before attempting to conceive to reduce pregnancy complications, but we also encourage our patients to lower their TG levels. Given the observed associations between higher TG levels and adverse pregnancy outcomes, we now routinely measure maternal lipids as well as blood glucose in our pregnant patients. We also test lipid levels in pregnant women who have other risk factors such as GDM in a prior pregnancy or chronic high blood pressure.
It is possible that lifestyle programs (such as those involving diet, weight reduction, and physical activity) prior to and during pregnancy, with a focus not only on maintaining a healthy weight but also on lowering TG levels, may help to further prevent complications during pregnancy and adverse birth outcomes. Although more research is needed, lowering TGs with cholesterol-reducing drugs also may help improve pregnancy outcomes. Indeed, there is currently a study investigating the pharmacologic treatment of high lipids during pregnancy.
For now, we should advise our patients who have higher TG levels in pregnancy to improve their diets and levels of physical activity. We also should monitor these patients for the increased likelihood of developing GDM and preeclampsia because higher lipid profiles appear to equate to a higher risk of adverse outcomes.
Finally, our attention to lipid profiles should extend beyond birth, since the long-term risk of cardiovascular disease may be influenced by preeclampsia and potentially by the lipid changes that escalate with the condition.
Dr. Wiznitzer reported having no financial disclosures related to this Master Class.
Best lipid levels in pregnancy still unclear
As ob.gyns., we often focus on optimizing our patients’ reproductive health. Research has shown, however, that the condition of a woman’s health prior to conception can be just as – if not more – important to her pregnancy and her lifelong well-being. For example, we have established that women who take the daily recommended dose of folic acid (400 mcg), even outside of pregnancy, have a reduced risk for neural tube defects in their infants.
Last year, we devoted a series of Master Class columns to the crucial need to properly manage maternal weight gain and blood sugar levels before, during, and after gestation to improve pregnancy outcomes. We also have seen that intensive glycemic and weight control in women can reduce their risk of fetal and maternal complications.
However, the leading causes of morbidity and mortality remain cardiovascular diseases, both in the developing and developed world. One of the key contributors to poor heart and vascular health is high cholesterol. Although the body needs cholesterol, just as it needs sugar, excess lipids in the blood can lead to infarction and stroke.
According to the U.S. Centers for Disease Control and Prevention, the desirable total cholesterol levels, including low- and high-density lipids and triglycerides, for men and nonpregnant women fall below 200 mg/dL. What remain less clear are the desired lipid levels for pregnant women.
We have known for decades that cholesterol concentrations increase during pregnancy, possibly by as much as 50%. We do not, however, have a firm understanding of what may constitute normally higher lipid concentrations and what may signal risk to the health of the baby or mother. Additionally, while we may run a lipid panel when we order a blood test, ob.gyns. do not routinely monitor a women’s cholesterol.
Since excess lipids, obesity, and heart disease often occur in the same patient and have become increasingly prevalent in our society, it may be time to reexamine any correlations between maternal lipid levels and adverse pregnancy outcomes.
To comment on this reemerging area, we invited Dr. Arnon Wiznitzer, professor and chairman of the department of obstetrics and gynecology at Helen Schneider Hospital for Women and deputy director of the Rabin Medical Center, Sackler Faculty of Medicine, Tel Aviv University. Dr. Wiznitzer’s extensive experience working with women who have diabetes in pregnancy led him to examine other comorbidities, including lipids, which might confound good pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
As ob.gyns., we often focus on optimizing our patients’ reproductive health. Research has shown, however, that the condition of a woman’s health prior to conception can be just as – if not more – important to her pregnancy and her lifelong well-being. For example, we have established that women who take the daily recommended dose of folic acid (400 mcg), even outside of pregnancy, have a reduced risk for neural tube defects in their infants.
Last year, we devoted a series of Master Class columns to the crucial need to properly manage maternal weight gain and blood sugar levels before, during, and after gestation to improve pregnancy outcomes. We also have seen that intensive glycemic and weight control in women can reduce their risk of fetal and maternal complications.
However, the leading causes of morbidity and mortality remain cardiovascular diseases, both in the developing and developed world. One of the key contributors to poor heart and vascular health is high cholesterol. Although the body needs cholesterol, just as it needs sugar, excess lipids in the blood can lead to infarction and stroke.
According to the U.S. Centers for Disease Control and Prevention, the desirable total cholesterol levels, including low- and high-density lipids and triglycerides, for men and nonpregnant women fall below 200 mg/dL. What remain less clear are the desired lipid levels for pregnant women.
We have known for decades that cholesterol concentrations increase during pregnancy, possibly by as much as 50%. We do not, however, have a firm understanding of what may constitute normally higher lipid concentrations and what may signal risk to the health of the baby or mother. Additionally, while we may run a lipid panel when we order a blood test, ob.gyns. do not routinely monitor a women’s cholesterol.
Since excess lipids, obesity, and heart disease often occur in the same patient and have become increasingly prevalent in our society, it may be time to reexamine any correlations between maternal lipid levels and adverse pregnancy outcomes.
To comment on this reemerging area, we invited Dr. Arnon Wiznitzer, professor and chairman of the department of obstetrics and gynecology at Helen Schneider Hospital for Women and deputy director of the Rabin Medical Center, Sackler Faculty of Medicine, Tel Aviv University. Dr. Wiznitzer’s extensive experience working with women who have diabetes in pregnancy led him to examine other comorbidities, including lipids, which might confound good pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
As ob.gyns., we often focus on optimizing our patients’ reproductive health. Research has shown, however, that the condition of a woman’s health prior to conception can be just as – if not more – important to her pregnancy and her lifelong well-being. For example, we have established that women who take the daily recommended dose of folic acid (400 mcg), even outside of pregnancy, have a reduced risk for neural tube defects in their infants.
Last year, we devoted a series of Master Class columns to the crucial need to properly manage maternal weight gain and blood sugar levels before, during, and after gestation to improve pregnancy outcomes. We also have seen that intensive glycemic and weight control in women can reduce their risk of fetal and maternal complications.
However, the leading causes of morbidity and mortality remain cardiovascular diseases, both in the developing and developed world. One of the key contributors to poor heart and vascular health is high cholesterol. Although the body needs cholesterol, just as it needs sugar, excess lipids in the blood can lead to infarction and stroke.
According to the U.S. Centers for Disease Control and Prevention, the desirable total cholesterol levels, including low- and high-density lipids and triglycerides, for men and nonpregnant women fall below 200 mg/dL. What remain less clear are the desired lipid levels for pregnant women.
We have known for decades that cholesterol concentrations increase during pregnancy, possibly by as much as 50%. We do not, however, have a firm understanding of what may constitute normally higher lipid concentrations and what may signal risk to the health of the baby or mother. Additionally, while we may run a lipid panel when we order a blood test, ob.gyns. do not routinely monitor a women’s cholesterol.
Since excess lipids, obesity, and heart disease often occur in the same patient and have become increasingly prevalent in our society, it may be time to reexamine any correlations between maternal lipid levels and adverse pregnancy outcomes.
To comment on this reemerging area, we invited Dr. Arnon Wiznitzer, professor and chairman of the department of obstetrics and gynecology at Helen Schneider Hospital for Women and deputy director of the Rabin Medical Center, Sackler Faculty of Medicine, Tel Aviv University. Dr. Wiznitzer’s extensive experience working with women who have diabetes in pregnancy led him to examine other comorbidities, including lipids, which might confound good pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Putting isthmocele into perspective
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.