User login
Diagnosis and treatment of uterine isthmocele
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
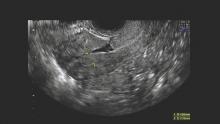
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
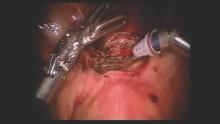
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
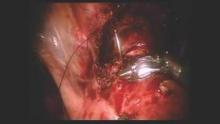
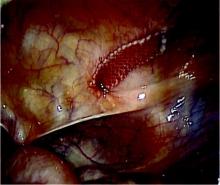
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
Using cervical length screening to predict preterm birth
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
The benefits, costs of universal cervical length screening
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.
Dos and don’ts for handling common sling complications
Large-scale randomized trials have not only documented the efficacy of minimally invasive midurethral slings for stress urinary continence, they have also provided more adequate data on the incidence of complications. In practice, meanwhile, we are seeing more complications as the number of midurethral sling placements increases.
Often times, complications can be significantly more impactful than the original urinary incontinence. It is important to take the complications of sling placement seriously. Let patients know that their symptoms matter, and that there are ways to manage complications.
With more long-term data and experience, we have learned more about what to do, and what not to do, to prevent, diagnose, and manage the complications associated with midurethral slings. Here is my approach to the complications most commonly encountered, including bladder perforation, voiding dysfunction, erosion, pain, and recurrent stress urinary incontinence.
I will not address vascular injury in this article, but certainly, this is a surgical emergency that needs to be handled as such. As described in the February 2015 edition of Master Class on midurethral sling technique, accurate visualization toward the ipsilateral shoulder during needle passage is an essential part of preventing vascular injuries during retropubic sling placement.
Bladder perforation
Bladder perforation has consistently been shown to be significantly more common with retropubic slings than with transobturator slings. Reported incidence has ranged from 0.8% to 34% for tension-free vaginal tape (TVT) procedures, with the higher rates seen mainly in teaching institutions. Most commonly, the reported incidence is less than 10%.
Bladder perforation has no effect on the efficacy of the treatment, and no apparent long-term consequences, as long as the injury is identified. Especially with a retropubic sling, cystoscopy should be performed after both needles are placed but prior to advancing the needles all the way through the retropubic space. Simply withdrawing a needle will cause little bladder injury while retracting deployed mesh is significantly more consequential.
I recommend filling the bladder to approximately 300 cc, or to the point where you can see evidence of full distension such as flattened urethral orifices. This confirms that the bladder is under enough distension to preclude any mucosal wrinkles or folds that can hide a trocar injury.
The first step upon recognition of a perforation is to stay calm. In the vast majority of cases, simply withdrawing the needle, replacing it, and verifying correct replacement will prevent any long-term consequences. On the other hand, you must be fully alert to the possibility that the needle wandered away from the pubic bone, and consequently may have entered a space such as the peritoneum. Suspicion for visceral injury should be increased.
Resist the temptation to replace the needle more laterally. This course correction is often an unhelpful instinct, because a more lateral replacement will not move the needle farther from the bladder; it will instead bring it closer to the iliac vessels. Vascular injuries resulting from the surgeon’s attempts at needle replacement are unfortunate, as a minor complication becomes a major one. The key is to be as distal as possible – as close to the pubic bone as possible – and not to replace the needles more laterally.
Postoperative drainage for 1-2 days may be considered, but there is nothing in the literature to require this, and many surgeons do not employ any sort of extra catheterization after surgery where perforation has been observed.
Voiding dysfunction
Some degree of voiding dysfunction is not uncommon in the short term, but when a patient is still unable to void normally or completely after several days, an evaluation is warranted. As with bladder perforation, reported incidence of voiding dysfunction has varied widely, from 2% to 45% with the newer midurethral slings. Generally, the need for surgical revision is about 2%.
There are two reasons for urinary retention: Insufficient contraction force in the bladder or too much resistance. If retention persists beyond a week – in the 7-10 day postop time period – I assess whether the problem is resulting from too much obstruction from the sling, some form of hypotonic bladder, other surgery performed in conjunction with sling placement, medications, or something else.
Difficulty in passing a small urethra catheter in the office may indicate excessive obstruction, for instance, and there may be indications on vaginal examination or through cystoscopy that the sling is too tight. A midurethral “speed bump,” or elevation at the midpoint, with either catheterization or the scope is consistent with over-correction.
Do not dilate or pull down on the sling with any kind of urethra dilator. The sling is more robust than the urethral mucosa, and we now appreciate that this practice is associated with urethral erosion.
If the problem is deemed to be excessive obstruction or over-resistance, and it is fewer than 10 days postop, the patient may be offered a minor revision; the original incision is reopened, the sling material is identified, and the sling arms (lateral to the urethra) are grasped with clamps. Gentle downward traction can loosen the sling.
The sling should be grasped laterally and not at the midpoint; some sling materials will stretch and fracture where the force is applied. A little bit of gentle downward traction (3-5 mm) will often give you the needed amount of space for relieving some of the obstruction.
Beyond 10 days postop, tissue in-growth makes such a sling adjustment difficult, if not impossible. At this point, I recommend transecting the entire sling in the midline.There is differing opinion about whether a portion of the mesh should be resected; I believe that such a resection is usually unnecessary, and that a simple midline release procedure is the best approach.
A study we performed more than a decade ago on surgical release of TVT showed that persistent post-TVT voiding dysfunction can be successfully managed with a simple midline release. Of 1,175 women who underwent TVT placement for stress urinary incontinence and/or intrinsic sphincter deficiency, 23 (1.9%) had persistent voiding dysfunction. All cases of impaired emptying were completely resolved with a release of the tape, and the majority remained cured in terms of their continence or went from “cured” to “improved” over baseline. Three patients (13%) had recurrence of stress incontinence (Obstet. Gynecol. 2002;100:898-902).
We used to wait longer before revising the sling out of fear of losing the entire benefit of the sling. As it turns out, a simple midline release (leaving most, if not all, of the mesh in place) is usually just enough to treat the new complaint while still providing enough lateral support so that the patient retains most or all of the continence achieved with the sling.
Complaints of de novo urge incontinence, or overactive bladder, should be taken seriously. Urge incontinence has even more significant associations with depression and poor quality of life than stress incontinence. In the absence of retention, usual first-line therapies for overactive bladder can be employed, including anticholinergic medications, behavioral therapies, and physical therapy. Failing these interventions, my assessment for this complaint will be similar to that for retention; I’ll look for evidence of too much resistance, such as difficulty in passing a catheter, a “speed bump” cystoscopically, or an elevated pDet on pressure-flow studies, for instance.
If any of these are present, I usually offer sling release first. If, on the other hand, there is no evidence of over resistance in a patient who has de novo urge incontinence or overactive bladder and is refractory to conservative measures, a trial of sacral neuromodulation or botox injections is considered the next step.
Erosion
Erosion remains a difficult complication to understand. Long-term follow-up data show that it occurs after 3%-4% of sling placements, rather than 1% as originally believed. Data are inconsistent, but there probably is a slightly higher incidence of vaginal erosion with a transobturator sling, given more contact between the sling and the anterior vaginal wall.
There are hints in the literature that erosion may be related to technique – perhaps to the depth of dissection during surgery – but this is difficult to quantify. Moreover, many of the reported cases of erosion occur several years, or longer, after surgery. It is hard to blame surgical technique for such delayed erosion.
As we’ve seen with previous generations of mesh, there does not appear to be any window of time after which erosion is no longer a risk. We need to recognize that there is a medium- and long-term risk of erosion and appreciate its presenting symptoms: Recurrent urinary tract infection, pain with voiding, urgency, urinary incontinence, and microscopic hematuria of new onset.
Prevention may well entail preoperative estrogenization. The science looking at the effect of estrogen on sling placement is becoming more robust. While there are uncertainties, I believe that studies likely will show that topical estrogen in the preoperative and perioperative phases plays an important role in preventing erosion from occurring. Personally, I am using it much more than I was 10 years ago.
I like the convenience of the Vagifem tablet (Novo Nordisk Inc., Plainsboro, N.J.), and am reassured by data on systemic absorption with the 10-mcg dose, but any vaginal cream or compounded suppository can be used. I usually advise 4-6 weeks of preoperative preparation, with nightly use for 2 weeks followed by 2-3 nights per week thereafter. Smoking is also a likely risk factor. Data are not entirely consistent, but I believe we should provide counseling and encourage smoking cessation before the implant of mesh.
Management is dependent on when the erosion occurs or is recognized. When erosion occurs within 6 weeks post operatively, primary repair is an option. When erosion is detected after the 6-week window and is causing symptoms, a conservative trim of bristles poking through the vaginal mucosa is worth a try. I do not advise more than one such conservative trim, however, as repeated attempts and series of small resections can make the sling exceedingly difficult to remove if more complete resection is ultimately needed. After one unsuccessful trim, I usually remove the whole sling belly, or most of the vaginal part of the sling.
For slings made of type 1 macroporous mesh, resection of the retropubic or transobturator portions of the mesh usually is not required. In the more rare situation where those pelvic areas of the mesh are associated with pain, I favor a laparoscopic approach to the retropubic space to facilitate minimally invasive removal.
Postop pain, sling failure
Groin pain, or thigh pain, sometimes occurs after placement of a transobturator sling. As I discussed in the previous Master Class on midurethral sling technique, I have seen a significant decrease in groin pain in my patients – without any reduction in benefit – with the use of a shorter transobturator sling that does not leave mesh in the adductor compartment of the thigh and groin.
For persistent groin pain, I favor the use of trigger point injection. Sometimes one injection will impact the inflammatory cycle such that the patient derives long-term benefit. At other times, the trigger point injection will serve as a diagnostic; if pain returns after a period of benefit, I am inclined to resect that part of the mesh.
Pain inside the pelvis, especially on the pelvic sidewall (obturator or puborectalis complex) usually is related to mechanical tension. In my experience, this type of discomfort is slightly more likely to occur with the transobturator slings, which penetrate through the muscular pelvic sidewall and lead to more fibrosis and scar tissue formation.
In most cases of pain and discomfort, attempting to reproduce the patient’s symptoms by putting tension on particular parts of the sling during the office exam helps guide management. If I find that palpating or putting the sling on tension recreates her complaints, and conservative injections have provided temporary or inadequate relief, I usually advocate resecting the vaginal portion of the mesh to relieve that tension.
In cases of recurrent stress urinary incontinence (when the sling has failed), a TVT or repeat TVT is often warranted. The TVT sling has been demonstrated to work after nearly every other previous kind of anti-incontinence procedure, even after a previous retropubic sling. There is little data on mesh removal in such cases. I believe that unless a previously placed but failed sling is causing symptoms, there is no need to resect it. Mesh removal is significantly more traumatic than mesh placement, and in most cases it is not necessary.
Dr. Rardin reported that he has no relevant financial disclosures.
Large-scale randomized trials have not only documented the efficacy of minimally invasive midurethral slings for stress urinary continence, they have also provided more adequate data on the incidence of complications. In practice, meanwhile, we are seeing more complications as the number of midurethral sling placements increases.
Often times, complications can be significantly more impactful than the original urinary incontinence. It is important to take the complications of sling placement seriously. Let patients know that their symptoms matter, and that there are ways to manage complications.
With more long-term data and experience, we have learned more about what to do, and what not to do, to prevent, diagnose, and manage the complications associated with midurethral slings. Here is my approach to the complications most commonly encountered, including bladder perforation, voiding dysfunction, erosion, pain, and recurrent stress urinary incontinence.
I will not address vascular injury in this article, but certainly, this is a surgical emergency that needs to be handled as such. As described in the February 2015 edition of Master Class on midurethral sling technique, accurate visualization toward the ipsilateral shoulder during needle passage is an essential part of preventing vascular injuries during retropubic sling placement.
Bladder perforation
Bladder perforation has consistently been shown to be significantly more common with retropubic slings than with transobturator slings. Reported incidence has ranged from 0.8% to 34% for tension-free vaginal tape (TVT) procedures, with the higher rates seen mainly in teaching institutions. Most commonly, the reported incidence is less than 10%.
Bladder perforation has no effect on the efficacy of the treatment, and no apparent long-term consequences, as long as the injury is identified. Especially with a retropubic sling, cystoscopy should be performed after both needles are placed but prior to advancing the needles all the way through the retropubic space. Simply withdrawing a needle will cause little bladder injury while retracting deployed mesh is significantly more consequential.
I recommend filling the bladder to approximately 300 cc, or to the point where you can see evidence of full distension such as flattened urethral orifices. This confirms that the bladder is under enough distension to preclude any mucosal wrinkles or folds that can hide a trocar injury.
The first step upon recognition of a perforation is to stay calm. In the vast majority of cases, simply withdrawing the needle, replacing it, and verifying correct replacement will prevent any long-term consequences. On the other hand, you must be fully alert to the possibility that the needle wandered away from the pubic bone, and consequently may have entered a space such as the peritoneum. Suspicion for visceral injury should be increased.
Resist the temptation to replace the needle more laterally. This course correction is often an unhelpful instinct, because a more lateral replacement will not move the needle farther from the bladder; it will instead bring it closer to the iliac vessels. Vascular injuries resulting from the surgeon’s attempts at needle replacement are unfortunate, as a minor complication becomes a major one. The key is to be as distal as possible – as close to the pubic bone as possible – and not to replace the needles more laterally.
Postoperative drainage for 1-2 days may be considered, but there is nothing in the literature to require this, and many surgeons do not employ any sort of extra catheterization after surgery where perforation has been observed.
Voiding dysfunction
Some degree of voiding dysfunction is not uncommon in the short term, but when a patient is still unable to void normally or completely after several days, an evaluation is warranted. As with bladder perforation, reported incidence of voiding dysfunction has varied widely, from 2% to 45% with the newer midurethral slings. Generally, the need for surgical revision is about 2%.
There are two reasons for urinary retention: Insufficient contraction force in the bladder or too much resistance. If retention persists beyond a week – in the 7-10 day postop time period – I assess whether the problem is resulting from too much obstruction from the sling, some form of hypotonic bladder, other surgery performed in conjunction with sling placement, medications, or something else.
Difficulty in passing a small urethra catheter in the office may indicate excessive obstruction, for instance, and there may be indications on vaginal examination or through cystoscopy that the sling is too tight. A midurethral “speed bump,” or elevation at the midpoint, with either catheterization or the scope is consistent with over-correction.
Do not dilate or pull down on the sling with any kind of urethra dilator. The sling is more robust than the urethral mucosa, and we now appreciate that this practice is associated with urethral erosion.
If the problem is deemed to be excessive obstruction or over-resistance, and it is fewer than 10 days postop, the patient may be offered a minor revision; the original incision is reopened, the sling material is identified, and the sling arms (lateral to the urethra) are grasped with clamps. Gentle downward traction can loosen the sling.
The sling should be grasped laterally and not at the midpoint; some sling materials will stretch and fracture where the force is applied. A little bit of gentle downward traction (3-5 mm) will often give you the needed amount of space for relieving some of the obstruction.
Beyond 10 days postop, tissue in-growth makes such a sling adjustment difficult, if not impossible. At this point, I recommend transecting the entire sling in the midline.There is differing opinion about whether a portion of the mesh should be resected; I believe that such a resection is usually unnecessary, and that a simple midline release procedure is the best approach.
A study we performed more than a decade ago on surgical release of TVT showed that persistent post-TVT voiding dysfunction can be successfully managed with a simple midline release. Of 1,175 women who underwent TVT placement for stress urinary incontinence and/or intrinsic sphincter deficiency, 23 (1.9%) had persistent voiding dysfunction. All cases of impaired emptying were completely resolved with a release of the tape, and the majority remained cured in terms of their continence or went from “cured” to “improved” over baseline. Three patients (13%) had recurrence of stress incontinence (Obstet. Gynecol. 2002;100:898-902).
We used to wait longer before revising the sling out of fear of losing the entire benefit of the sling. As it turns out, a simple midline release (leaving most, if not all, of the mesh in place) is usually just enough to treat the new complaint while still providing enough lateral support so that the patient retains most or all of the continence achieved with the sling.
Complaints of de novo urge incontinence, or overactive bladder, should be taken seriously. Urge incontinence has even more significant associations with depression and poor quality of life than stress incontinence. In the absence of retention, usual first-line therapies for overactive bladder can be employed, including anticholinergic medications, behavioral therapies, and physical therapy. Failing these interventions, my assessment for this complaint will be similar to that for retention; I’ll look for evidence of too much resistance, such as difficulty in passing a catheter, a “speed bump” cystoscopically, or an elevated pDet on pressure-flow studies, for instance.
If any of these are present, I usually offer sling release first. If, on the other hand, there is no evidence of over resistance in a patient who has de novo urge incontinence or overactive bladder and is refractory to conservative measures, a trial of sacral neuromodulation or botox injections is considered the next step.
Erosion
Erosion remains a difficult complication to understand. Long-term follow-up data show that it occurs after 3%-4% of sling placements, rather than 1% as originally believed. Data are inconsistent, but there probably is a slightly higher incidence of vaginal erosion with a transobturator sling, given more contact between the sling and the anterior vaginal wall.
There are hints in the literature that erosion may be related to technique – perhaps to the depth of dissection during surgery – but this is difficult to quantify. Moreover, many of the reported cases of erosion occur several years, or longer, after surgery. It is hard to blame surgical technique for such delayed erosion.
As we’ve seen with previous generations of mesh, there does not appear to be any window of time after which erosion is no longer a risk. We need to recognize that there is a medium- and long-term risk of erosion and appreciate its presenting symptoms: Recurrent urinary tract infection, pain with voiding, urgency, urinary incontinence, and microscopic hematuria of new onset.
Prevention may well entail preoperative estrogenization. The science looking at the effect of estrogen on sling placement is becoming more robust. While there are uncertainties, I believe that studies likely will show that topical estrogen in the preoperative and perioperative phases plays an important role in preventing erosion from occurring. Personally, I am using it much more than I was 10 years ago.
I like the convenience of the Vagifem tablet (Novo Nordisk Inc., Plainsboro, N.J.), and am reassured by data on systemic absorption with the 10-mcg dose, but any vaginal cream or compounded suppository can be used. I usually advise 4-6 weeks of preoperative preparation, with nightly use for 2 weeks followed by 2-3 nights per week thereafter. Smoking is also a likely risk factor. Data are not entirely consistent, but I believe we should provide counseling and encourage smoking cessation before the implant of mesh.
Management is dependent on when the erosion occurs or is recognized. When erosion occurs within 6 weeks post operatively, primary repair is an option. When erosion is detected after the 6-week window and is causing symptoms, a conservative trim of bristles poking through the vaginal mucosa is worth a try. I do not advise more than one such conservative trim, however, as repeated attempts and series of small resections can make the sling exceedingly difficult to remove if more complete resection is ultimately needed. After one unsuccessful trim, I usually remove the whole sling belly, or most of the vaginal part of the sling.
For slings made of type 1 macroporous mesh, resection of the retropubic or transobturator portions of the mesh usually is not required. In the more rare situation where those pelvic areas of the mesh are associated with pain, I favor a laparoscopic approach to the retropubic space to facilitate minimally invasive removal.
Postop pain, sling failure
Groin pain, or thigh pain, sometimes occurs after placement of a transobturator sling. As I discussed in the previous Master Class on midurethral sling technique, I have seen a significant decrease in groin pain in my patients – without any reduction in benefit – with the use of a shorter transobturator sling that does not leave mesh in the adductor compartment of the thigh and groin.
For persistent groin pain, I favor the use of trigger point injection. Sometimes one injection will impact the inflammatory cycle such that the patient derives long-term benefit. At other times, the trigger point injection will serve as a diagnostic; if pain returns after a period of benefit, I am inclined to resect that part of the mesh.
Pain inside the pelvis, especially on the pelvic sidewall (obturator or puborectalis complex) usually is related to mechanical tension. In my experience, this type of discomfort is slightly more likely to occur with the transobturator slings, which penetrate through the muscular pelvic sidewall and lead to more fibrosis and scar tissue formation.
In most cases of pain and discomfort, attempting to reproduce the patient’s symptoms by putting tension on particular parts of the sling during the office exam helps guide management. If I find that palpating or putting the sling on tension recreates her complaints, and conservative injections have provided temporary or inadequate relief, I usually advocate resecting the vaginal portion of the mesh to relieve that tension.
In cases of recurrent stress urinary incontinence (when the sling has failed), a TVT or repeat TVT is often warranted. The TVT sling has been demonstrated to work after nearly every other previous kind of anti-incontinence procedure, even after a previous retropubic sling. There is little data on mesh removal in such cases. I believe that unless a previously placed but failed sling is causing symptoms, there is no need to resect it. Mesh removal is significantly more traumatic than mesh placement, and in most cases it is not necessary.
Dr. Rardin reported that he has no relevant financial disclosures.
Large-scale randomized trials have not only documented the efficacy of minimally invasive midurethral slings for stress urinary continence, they have also provided more adequate data on the incidence of complications. In practice, meanwhile, we are seeing more complications as the number of midurethral sling placements increases.
Often times, complications can be significantly more impactful than the original urinary incontinence. It is important to take the complications of sling placement seriously. Let patients know that their symptoms matter, and that there are ways to manage complications.
With more long-term data and experience, we have learned more about what to do, and what not to do, to prevent, diagnose, and manage the complications associated with midurethral slings. Here is my approach to the complications most commonly encountered, including bladder perforation, voiding dysfunction, erosion, pain, and recurrent stress urinary incontinence.
I will not address vascular injury in this article, but certainly, this is a surgical emergency that needs to be handled as such. As described in the February 2015 edition of Master Class on midurethral sling technique, accurate visualization toward the ipsilateral shoulder during needle passage is an essential part of preventing vascular injuries during retropubic sling placement.
Bladder perforation
Bladder perforation has consistently been shown to be significantly more common with retropubic slings than with transobturator slings. Reported incidence has ranged from 0.8% to 34% for tension-free vaginal tape (TVT) procedures, with the higher rates seen mainly in teaching institutions. Most commonly, the reported incidence is less than 10%.
Bladder perforation has no effect on the efficacy of the treatment, and no apparent long-term consequences, as long as the injury is identified. Especially with a retropubic sling, cystoscopy should be performed after both needles are placed but prior to advancing the needles all the way through the retropubic space. Simply withdrawing a needle will cause little bladder injury while retracting deployed mesh is significantly more consequential.
I recommend filling the bladder to approximately 300 cc, or to the point where you can see evidence of full distension such as flattened urethral orifices. This confirms that the bladder is under enough distension to preclude any mucosal wrinkles or folds that can hide a trocar injury.
The first step upon recognition of a perforation is to stay calm. In the vast majority of cases, simply withdrawing the needle, replacing it, and verifying correct replacement will prevent any long-term consequences. On the other hand, you must be fully alert to the possibility that the needle wandered away from the pubic bone, and consequently may have entered a space such as the peritoneum. Suspicion for visceral injury should be increased.
Resist the temptation to replace the needle more laterally. This course correction is often an unhelpful instinct, because a more lateral replacement will not move the needle farther from the bladder; it will instead bring it closer to the iliac vessels. Vascular injuries resulting from the surgeon’s attempts at needle replacement are unfortunate, as a minor complication becomes a major one. The key is to be as distal as possible – as close to the pubic bone as possible – and not to replace the needles more laterally.
Postoperative drainage for 1-2 days may be considered, but there is nothing in the literature to require this, and many surgeons do not employ any sort of extra catheterization after surgery where perforation has been observed.
Voiding dysfunction
Some degree of voiding dysfunction is not uncommon in the short term, but when a patient is still unable to void normally or completely after several days, an evaluation is warranted. As with bladder perforation, reported incidence of voiding dysfunction has varied widely, from 2% to 45% with the newer midurethral slings. Generally, the need for surgical revision is about 2%.
There are two reasons for urinary retention: Insufficient contraction force in the bladder or too much resistance. If retention persists beyond a week – in the 7-10 day postop time period – I assess whether the problem is resulting from too much obstruction from the sling, some form of hypotonic bladder, other surgery performed in conjunction with sling placement, medications, or something else.
Difficulty in passing a small urethra catheter in the office may indicate excessive obstruction, for instance, and there may be indications on vaginal examination or through cystoscopy that the sling is too tight. A midurethral “speed bump,” or elevation at the midpoint, with either catheterization or the scope is consistent with over-correction.
Do not dilate or pull down on the sling with any kind of urethra dilator. The sling is more robust than the urethral mucosa, and we now appreciate that this practice is associated with urethral erosion.
If the problem is deemed to be excessive obstruction or over-resistance, and it is fewer than 10 days postop, the patient may be offered a minor revision; the original incision is reopened, the sling material is identified, and the sling arms (lateral to the urethra) are grasped with clamps. Gentle downward traction can loosen the sling.
The sling should be grasped laterally and not at the midpoint; some sling materials will stretch and fracture where the force is applied. A little bit of gentle downward traction (3-5 mm) will often give you the needed amount of space for relieving some of the obstruction.
Beyond 10 days postop, tissue in-growth makes such a sling adjustment difficult, if not impossible. At this point, I recommend transecting the entire sling in the midline.There is differing opinion about whether a portion of the mesh should be resected; I believe that such a resection is usually unnecessary, and that a simple midline release procedure is the best approach.
A study we performed more than a decade ago on surgical release of TVT showed that persistent post-TVT voiding dysfunction can be successfully managed with a simple midline release. Of 1,175 women who underwent TVT placement for stress urinary incontinence and/or intrinsic sphincter deficiency, 23 (1.9%) had persistent voiding dysfunction. All cases of impaired emptying were completely resolved with a release of the tape, and the majority remained cured in terms of their continence or went from “cured” to “improved” over baseline. Three patients (13%) had recurrence of stress incontinence (Obstet. Gynecol. 2002;100:898-902).
We used to wait longer before revising the sling out of fear of losing the entire benefit of the sling. As it turns out, a simple midline release (leaving most, if not all, of the mesh in place) is usually just enough to treat the new complaint while still providing enough lateral support so that the patient retains most or all of the continence achieved with the sling.
Complaints of de novo urge incontinence, or overactive bladder, should be taken seriously. Urge incontinence has even more significant associations with depression and poor quality of life than stress incontinence. In the absence of retention, usual first-line therapies for overactive bladder can be employed, including anticholinergic medications, behavioral therapies, and physical therapy. Failing these interventions, my assessment for this complaint will be similar to that for retention; I’ll look for evidence of too much resistance, such as difficulty in passing a catheter, a “speed bump” cystoscopically, or an elevated pDet on pressure-flow studies, for instance.
If any of these are present, I usually offer sling release first. If, on the other hand, there is no evidence of over resistance in a patient who has de novo urge incontinence or overactive bladder and is refractory to conservative measures, a trial of sacral neuromodulation or botox injections is considered the next step.
Erosion
Erosion remains a difficult complication to understand. Long-term follow-up data show that it occurs after 3%-4% of sling placements, rather than 1% as originally believed. Data are inconsistent, but there probably is a slightly higher incidence of vaginal erosion with a transobturator sling, given more contact between the sling and the anterior vaginal wall.
There are hints in the literature that erosion may be related to technique – perhaps to the depth of dissection during surgery – but this is difficult to quantify. Moreover, many of the reported cases of erosion occur several years, or longer, after surgery. It is hard to blame surgical technique for such delayed erosion.
As we’ve seen with previous generations of mesh, there does not appear to be any window of time after which erosion is no longer a risk. We need to recognize that there is a medium- and long-term risk of erosion and appreciate its presenting symptoms: Recurrent urinary tract infection, pain with voiding, urgency, urinary incontinence, and microscopic hematuria of new onset.
Prevention may well entail preoperative estrogenization. The science looking at the effect of estrogen on sling placement is becoming more robust. While there are uncertainties, I believe that studies likely will show that topical estrogen in the preoperative and perioperative phases plays an important role in preventing erosion from occurring. Personally, I am using it much more than I was 10 years ago.
I like the convenience of the Vagifem tablet (Novo Nordisk Inc., Plainsboro, N.J.), and am reassured by data on systemic absorption with the 10-mcg dose, but any vaginal cream or compounded suppository can be used. I usually advise 4-6 weeks of preoperative preparation, with nightly use for 2 weeks followed by 2-3 nights per week thereafter. Smoking is also a likely risk factor. Data are not entirely consistent, but I believe we should provide counseling and encourage smoking cessation before the implant of mesh.
Management is dependent on when the erosion occurs or is recognized. When erosion occurs within 6 weeks post operatively, primary repair is an option. When erosion is detected after the 6-week window and is causing symptoms, a conservative trim of bristles poking through the vaginal mucosa is worth a try. I do not advise more than one such conservative trim, however, as repeated attempts and series of small resections can make the sling exceedingly difficult to remove if more complete resection is ultimately needed. After one unsuccessful trim, I usually remove the whole sling belly, or most of the vaginal part of the sling.
For slings made of type 1 macroporous mesh, resection of the retropubic or transobturator portions of the mesh usually is not required. In the more rare situation where those pelvic areas of the mesh are associated with pain, I favor a laparoscopic approach to the retropubic space to facilitate minimally invasive removal.
Postop pain, sling failure
Groin pain, or thigh pain, sometimes occurs after placement of a transobturator sling. As I discussed in the previous Master Class on midurethral sling technique, I have seen a significant decrease in groin pain in my patients – without any reduction in benefit – with the use of a shorter transobturator sling that does not leave mesh in the adductor compartment of the thigh and groin.
For persistent groin pain, I favor the use of trigger point injection. Sometimes one injection will impact the inflammatory cycle such that the patient derives long-term benefit. At other times, the trigger point injection will serve as a diagnostic; if pain returns after a period of benefit, I am inclined to resect that part of the mesh.
Pain inside the pelvis, especially on the pelvic sidewall (obturator or puborectalis complex) usually is related to mechanical tension. In my experience, this type of discomfort is slightly more likely to occur with the transobturator slings, which penetrate through the muscular pelvic sidewall and lead to more fibrosis and scar tissue formation.
In most cases of pain and discomfort, attempting to reproduce the patient’s symptoms by putting tension on particular parts of the sling during the office exam helps guide management. If I find that palpating or putting the sling on tension recreates her complaints, and conservative injections have provided temporary or inadequate relief, I usually advocate resecting the vaginal portion of the mesh to relieve that tension.
In cases of recurrent stress urinary incontinence (when the sling has failed), a TVT or repeat TVT is often warranted. The TVT sling has been demonstrated to work after nearly every other previous kind of anti-incontinence procedure, even after a previous retropubic sling. There is little data on mesh removal in such cases. I believe that unless a previously placed but failed sling is causing symptoms, there is no need to resect it. Mesh removal is significantly more traumatic than mesh placement, and in most cases it is not necessary.
Dr. Rardin reported that he has no relevant financial disclosures.
Tackling midurethral sling complications
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Over the past 2 decades, midurethral slings, both via a retropubic and a transobturator approach have become the first-line therapy for the surgical correction of female stress urinary incontinence. Not only are cure rates excellent for both techniques, but the incidence of complications are low.
Intraoperatively, major concerns include vascular lesions, nerve injuries, and injuries to the bowel. More minor concerns are related to the bladder.
Perioperative complications include retropubic hematoma, blood loss, urinary tract infection, and spondylitis. Postoperative risks include transient versus permanent urinary retention, vaginal versus urethral erosion, de novo urgency, bladder erosion, and urethral obstruction.
In this edition of Master Class in gynecologic surgery, I am pleased to solicit the help of Dr. Charles Rardin, who will make recommendations regarding the management of some of the most common complications related to midurethral sling procedures.
Dr. Rardin is the director of the Robotic Surgery Program at Women & Infants Hospital of Rhode Island, in Providence; a surgeon in Women & Infants’ division of urogynecology and Reconstructive Pelvic Surgery; and is the director of the hospital’s fellowship urogynecology and reconstructive pelvic surgery.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Ob.gyns. can help end the HIV epidemic
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.
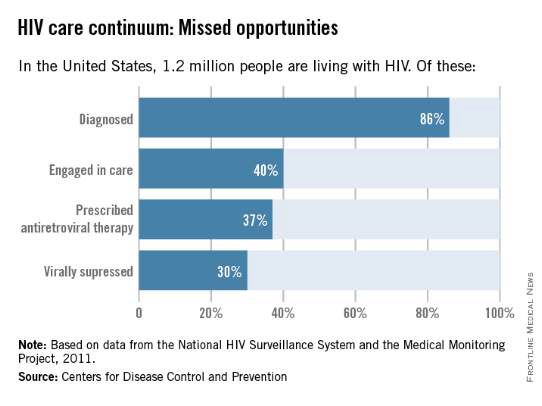
Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.

Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.

Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
HIV treatment adherence still a challenge
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Breaking down midurethral sling approaches
It has been nearly 20 years since the first minimally invasive midurethral sling was introduced. This development was followed 5 years later with the introduction of the transobturator midurethral sling. The advent of both ambulatory techniques has essentially changed the landscape in the surgical treatment of stress urinary incontinence; midurethral slings are certainly considered the procedure of choice for many women.
The midurethral sling has continued to evolve. Not only does the surgeon have the choice of placing a retropubic midurethral sling (bottom to top or top to bottom) and the transobturator midurethral sling (inside-out or outside-in), but, as of late, single incision midurethral slings (mini-slings or mini-tape) as well.
In the previous Master Class on urinary incontinence, Dr. Eric Sokol discussed issues of sling selection and the evidence in favor of various types of retropubic and transobturator slings. This month, we’ll discuss the technique behind these two approaches. I have elicited the assistance of Dr. Sokol, as well as Dr. Charles Rardin.
Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals.
Dr. Rardin is the director of the robotic surgery program at Women & Infants Hospital of Rhode Island, Providence, a surgeon in the division of urogynecology and reconstructive pelvic surgery, and is the director of the hospital’s fellowship in urogynecology and reconstructive pelvic surgery. He is also an assistant professor at Brown University, also in Providence. He has published numerous articles in peer-reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
It has been nearly 20 years since the first minimally invasive midurethral sling was introduced. This development was followed 5 years later with the introduction of the transobturator midurethral sling. The advent of both ambulatory techniques has essentially changed the landscape in the surgical treatment of stress urinary incontinence; midurethral slings are certainly considered the procedure of choice for many women.
The midurethral sling has continued to evolve. Not only does the surgeon have the choice of placing a retropubic midurethral sling (bottom to top or top to bottom) and the transobturator midurethral sling (inside-out or outside-in), but, as of late, single incision midurethral slings (mini-slings or mini-tape) as well.
In the previous Master Class on urinary incontinence, Dr. Eric Sokol discussed issues of sling selection and the evidence in favor of various types of retropubic and transobturator slings. This month, we’ll discuss the technique behind these two approaches. I have elicited the assistance of Dr. Sokol, as well as Dr. Charles Rardin.
Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals.
Dr. Rardin is the director of the robotic surgery program at Women & Infants Hospital of Rhode Island, Providence, a surgeon in the division of urogynecology and reconstructive pelvic surgery, and is the director of the hospital’s fellowship in urogynecology and reconstructive pelvic surgery. He is also an assistant professor at Brown University, also in Providence. He has published numerous articles in peer-reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
It has been nearly 20 years since the first minimally invasive midurethral sling was introduced. This development was followed 5 years later with the introduction of the transobturator midurethral sling. The advent of both ambulatory techniques has essentially changed the landscape in the surgical treatment of stress urinary incontinence; midurethral slings are certainly considered the procedure of choice for many women.
The midurethral sling has continued to evolve. Not only does the surgeon have the choice of placing a retropubic midurethral sling (bottom to top or top to bottom) and the transobturator midurethral sling (inside-out or outside-in), but, as of late, single incision midurethral slings (mini-slings or mini-tape) as well.
In the previous Master Class on urinary incontinence, Dr. Eric Sokol discussed issues of sling selection and the evidence in favor of various types of retropubic and transobturator slings. This month, we’ll discuss the technique behind these two approaches. I have elicited the assistance of Dr. Sokol, as well as Dr. Charles Rardin.
Dr. Sokol is an associate professor of obstetrics and gynecology, associate professor of urology (by courtesy), and cochief of the division of urogynecology and pelvic reconstructive surgery at Stanford (Calif.) University. He has published many articles regarding urogynecology and minimally invasive surgery. Dr. Sokol has been awarded numerous teaching awards, and he is a reviewer for multiple prestigious, peer-reviewed journals.
Dr. Rardin is the director of the robotic surgery program at Women & Infants Hospital of Rhode Island, Providence, a surgeon in the division of urogynecology and reconstructive pelvic surgery, and is the director of the hospital’s fellowship in urogynecology and reconstructive pelvic surgery. He is also an assistant professor at Brown University, also in Providence. He has published numerous articles in peer-reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speaker’s bureau for Ethicon.
Expert tips on retropubic vs. transobturator sling approaches
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Umbilical cord clamping
Embryogenesis is an incredible phenomenon. It begins with fertilization and implantation of the blastocyst into the uterine wall, followed by development of the extraembryonic membrane which gives rise to the placenta, which is, literally, the lifeblood of the fetus. Although physicians often focus on the health of the placenta during pregnancy, the umbilical cord is an equally important organ.
The umbilical cord acts as a conduit through which metabolic products and biproducts, such as nutrients, antibodies, iron, and blood, pass bidirectionally between a mother and her baby. Many problems can arise if the cord becomes altered. For example, true umbilical cord knot is associated with small-for-gestational-age fetuses, premature birth, neonatal intensive care unit admissions, and fetal death (Int. J. Gynaecol. Obstet. 2013;122:18-21). In addition, short or long cord length may lead to fetal heart rate anomalies and higher risk for birth asphyxia (J. Obstet. Gynaecol. India. 2012;62:520-5).
While the health of the umbilical cord during pregnancy closely correlates to successful outcomes, the cord’s functions conclude at birth. Physicians clamp the umbilical cord at parturition as a routine part of the delivery process. Many ob.gyns. may not even stop to think about cord clamping. To borrow the phrase from the old Nike slogan, they “just do it.”
However, exactly when physicians should clamp the umbilical cord remains a topic for debate. Should ob.gyns. clamp the cord immediately or shortly after birth? Proponents of immediate clamping might argue that waiting too long could cause an influx of placental* blood in the neonate, leading to risk for jaundice. Those on the side of delayed cord clamping might say that waiting to terminate the cord prevents the baby’s oxygen supply from being prematurely cut off. Also, how long should the delay last: One minute? Two minutes? More? Less?
Clearly, the issue of cord clamping requires discussion. Therefore, we have devoted the first Master Class of 2015 to this important topic. We have invited Dr. George A. Macones, the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis, to explore the debate. As the recent vice chair for the Committee on Practice Bulletins – Obstetrics for the American College of Obstetricians and Gynecologists, Dr. Macones has a unique perspective on the arguments for and against immediate or delayed cord clamping, as well as significant experience as a practicing ob.gyn. and leader at a vibrant academic medical center.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Correction, 4/14/15: An earlier version of this article misstated the potential consequences of waiting too long to clamp the umbilical cord.
Embryogenesis is an incredible phenomenon. It begins with fertilization and implantation of the blastocyst into the uterine wall, followed by development of the extraembryonic membrane which gives rise to the placenta, which is, literally, the lifeblood of the fetus. Although physicians often focus on the health of the placenta during pregnancy, the umbilical cord is an equally important organ.
The umbilical cord acts as a conduit through which metabolic products and biproducts, such as nutrients, antibodies, iron, and blood, pass bidirectionally between a mother and her baby. Many problems can arise if the cord becomes altered. For example, true umbilical cord knot is associated with small-for-gestational-age fetuses, premature birth, neonatal intensive care unit admissions, and fetal death (Int. J. Gynaecol. Obstet. 2013;122:18-21). In addition, short or long cord length may lead to fetal heart rate anomalies and higher risk for birth asphyxia (J. Obstet. Gynaecol. India. 2012;62:520-5).
While the health of the umbilical cord during pregnancy closely correlates to successful outcomes, the cord’s functions conclude at birth. Physicians clamp the umbilical cord at parturition as a routine part of the delivery process. Many ob.gyns. may not even stop to think about cord clamping. To borrow the phrase from the old Nike slogan, they “just do it.”
However, exactly when physicians should clamp the umbilical cord remains a topic for debate. Should ob.gyns. clamp the cord immediately or shortly after birth? Proponents of immediate clamping might argue that waiting too long could cause an influx of placental* blood in the neonate, leading to risk for jaundice. Those on the side of delayed cord clamping might say that waiting to terminate the cord prevents the baby’s oxygen supply from being prematurely cut off. Also, how long should the delay last: One minute? Two minutes? More? Less?
Clearly, the issue of cord clamping requires discussion. Therefore, we have devoted the first Master Class of 2015 to this important topic. We have invited Dr. George A. Macones, the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis, to explore the debate. As the recent vice chair for the Committee on Practice Bulletins – Obstetrics for the American College of Obstetricians and Gynecologists, Dr. Macones has a unique perspective on the arguments for and against immediate or delayed cord clamping, as well as significant experience as a practicing ob.gyn. and leader at a vibrant academic medical center.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Correction, 4/14/15: An earlier version of this article misstated the potential consequences of waiting too long to clamp the umbilical cord.
Embryogenesis is an incredible phenomenon. It begins with fertilization and implantation of the blastocyst into the uterine wall, followed by development of the extraembryonic membrane which gives rise to the placenta, which is, literally, the lifeblood of the fetus. Although physicians often focus on the health of the placenta during pregnancy, the umbilical cord is an equally important organ.
The umbilical cord acts as a conduit through which metabolic products and biproducts, such as nutrients, antibodies, iron, and blood, pass bidirectionally between a mother and her baby. Many problems can arise if the cord becomes altered. For example, true umbilical cord knot is associated with small-for-gestational-age fetuses, premature birth, neonatal intensive care unit admissions, and fetal death (Int. J. Gynaecol. Obstet. 2013;122:18-21). In addition, short or long cord length may lead to fetal heart rate anomalies and higher risk for birth asphyxia (J. Obstet. Gynaecol. India. 2012;62:520-5).
While the health of the umbilical cord during pregnancy closely correlates to successful outcomes, the cord’s functions conclude at birth. Physicians clamp the umbilical cord at parturition as a routine part of the delivery process. Many ob.gyns. may not even stop to think about cord clamping. To borrow the phrase from the old Nike slogan, they “just do it.”
However, exactly when physicians should clamp the umbilical cord remains a topic for debate. Should ob.gyns. clamp the cord immediately or shortly after birth? Proponents of immediate clamping might argue that waiting too long could cause an influx of placental* blood in the neonate, leading to risk for jaundice. Those on the side of delayed cord clamping might say that waiting to terminate the cord prevents the baby’s oxygen supply from being prematurely cut off. Also, how long should the delay last: One minute? Two minutes? More? Less?
Clearly, the issue of cord clamping requires discussion. Therefore, we have devoted the first Master Class of 2015 to this important topic. We have invited Dr. George A. Macones, the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis, to explore the debate. As the recent vice chair for the Committee on Practice Bulletins – Obstetrics for the American College of Obstetricians and Gynecologists, Dr. Macones has a unique perspective on the arguments for and against immediate or delayed cord clamping, as well as significant experience as a practicing ob.gyn. and leader at a vibrant academic medical center.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Correction, 4/14/15: An earlier version of this article misstated the potential consequences of waiting too long to clamp the umbilical cord.