User login
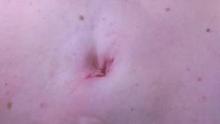
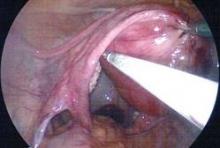
da Vinci Single-Site, the virtually scarless intra-abdominal hysterectomy
When the Food and Drug Administration cleared the da Vinci Single-Site Technology for use in benign hysterectomy and salpingo-oophorectomy in February 2013, I wondered how these procedures could possibly be performed with instruments that lack articulating wrists. I came to realize that with a stepwise approach to initiating this virtually scarless surgery, an appreciation of its nuances, and repetition of best practices found in my learning curve, Single-Site hysterectomy is indeed achievable and reproducible.
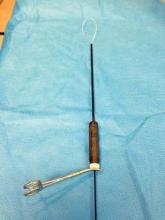
Single-Site tools are flexible and semirigid, allowing them to fit through the port’s curved cannulas. The port accommodates insufflation tubing, an 8.5-mm endoscope, two 5-mm operating instruments, and an assistant instrument. A 30-degree endoscope, facing downward to view the operating instruments underneath, is essential for achieving necessary triangulation.
I perform dissection with the fenestrated bipolar grasper in arm 1 and the monopolar cautery hook in arm 2. For vaginal cuff closure, I keep the fenestrated bipolar in arm 1 and place a needle holder in arm 2.
The fenestrated bipolar grasper for Single-Site became available only recently. Until then I utilized the Maryland bipolar forceps for dissection and tried various instruments, including the crocodile grasper, for vaginal cuff closure. Use of the fenestrated bipolar has been a game-changer for vessel sealing and has worked beautifully for cuff closure as well.
Single-port robotic hysterectomy requires deliberate transition. One must be comfortable first with minimal or no bedside assistance during multiport robotic surgery. The surgeon should take a stepwise approach to minimizing assistance – moving, for instance, from the use of five ports, to four ports, to four ports with no bedside assistance, to three ports, and lastly a "single-plus one" approach in which an extra port is placed as a precaution. I used a "single-plus one" approach for my first 10 cases before moving to a pure Single-Site approach.
I also began with two cases of bilateral salpingo-oophorectomy before performing Single-Site hysterectomy, and advise others to start with such easier cases as well. Patients selected for initial Single-Site surgery also should be younger than 70, with few comorbidities and a good performance status (American Society of Anesthesiologists classification I-II). They should have BMI less than 30, a history of little or no previous intra-abdominal or pelvic surgery, and a reasonably sized uterus (less than 12 weeks), and be candidates for vaginal or laparoscopic hysterectomy.
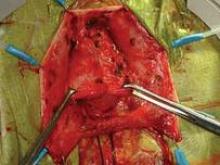
The Single-Site approach requires meticulous dissection, one side at a time. The surgeon must skeletonize vessels before coagulation, and must coagulate each vessel individually. Selective coagulation/sealing of vessels is key, as the surgeon does not have the luxury of using a harmonic scalpel, vessel sealer, or other such tools. Too much bleeding can make a case difficult, if not impossible, to complete without conversion. Similarly, because assistance is limited, coagulation must be done in a cephalad direction in order to minimize back-bleeding.
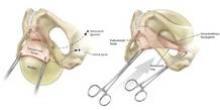
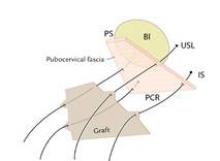
Uterine manipulation is always an important task, but it is especially important for the Single-Site hysterectomy. It is the assistant’s main role. He or she must know not only how to turn the uterus for vessel coagulation and visualization of anatomy, but also the nuances of traction and countertraction for dissection and the colpotomy. Various uterine manipulators will work; I prefer the VCare uterine manipulator.
Setup and entry
While steep Trendelenburg positioning is used for multiport hysterectomy, it must be lessened for the single-port approach. Trendelenburg changes the distance of the cannulae to the uterus, so individual adjustments are necessary. I start the patient in steep Trendelenburg, then lessen it just enough to displace the small bowel while maintaining an appropriate distance for the instruments to reach the uterus.
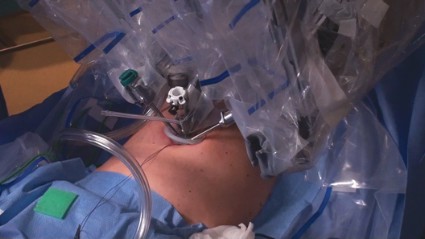
Center docking provides greater range of motion and is therefore often preferred, especially during early cases. However, side docking on the dominant-hand side, with the camera arm toward the uterus, is my preferred method. It provides the assistant maximal access to the vaginal area and best affords the surgeon the ability to add another port if it is necessary later.
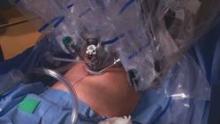
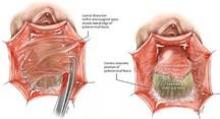
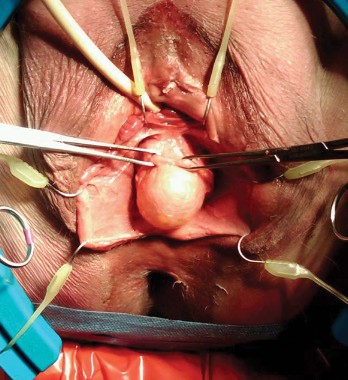
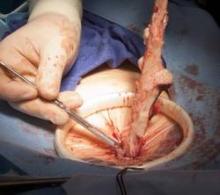
Entry and port placement are critical aspects of the Single-Site approach. The Single-Site Port requires a skin incision of 2.5 cm. I make this incision transversely, following the natural creases of the umbilicus. To get to the fascia, the umbilical stalk is transected, and a small hole – a hernia defect, in essence – is identified and lifted. A 3-cm vertical fascial incision is made to accommodate the Single-Site Port. It is important that this incision size is exact: If it is too short, the port will not expand to its natural state and there will be crowding of instruments, and if it is too large, leaking air will be a problem. I have found that placing stay sutures on the fascia at this point helps facilitate later closure.
Cannulas for the Single-Site Port come in two lengths: There is a set of short, 250-mm cannulas and a set of long, 300-mm cannulas. When to use these cannulas depends on the working area and the patient’s size. Generally, the shorter cannulas are used for everything but the cuff closure. Then arm 2 is replaced with the longer cannula for the extra rigidity needed to suture. My use of the different cannula lengths has varied depending on the patient’s body size, and with growing experience I have sometimes gone without exchanging them.
Dissection through cuff closure
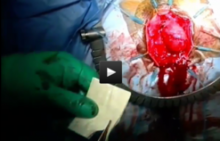
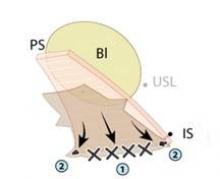
I start all my cases on the right side, holding the infundibulopelvic (IP) or utero-ovarian ligament on tension and skeletonizing it. I then coagulate the IP ligament with the fenestrated bipolar grasper (setting: 35 watts).
At this point and throughout the surgery, consistent use of the "cut" mode on the da Vinci console makes both dissection and sealing cleaner and more precise, with significantly less charring.
I use AirSeal for insufflation and automatic smoke evacuation.
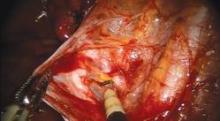
As I do with all minimally invasive hysterectomies, regardless of modality, I work to find and develop the pararectal space. This space is bordered by the ureter medially, the hypogastric or internal iliac artery laterally, and the base of the cardinal ligament anteriorly, with the levator ani muscle as the floor. Defining and developing the space enable me to visualize the ureter so that I can dissect it off the peritoneum and drop it out of harm’s way. It puts me in a much better position, moreover, to handle dissection in more complex cases involving large fibroids or endometriosis.
Once the pararectal space is developed and the ureter dissected, I proceed with transection of the round ligament. Dissecting the round ligament any earlier would be particularly troublesome in a Single-Site robotic hysterectomy because it would be challenging to achieve the necessary degree of uterine manipulation.
In developing the bladder flap, one must be conscious of the highly magnified view, as the bladder appears closer with the 30-degree 8.5-mm endoscope than it would with a 0-degree scope. A combination of traction and countertraction, small bites, and selective coagulation – one vessel at a time – is critical as dissection proceeds and the bladder flap is created.
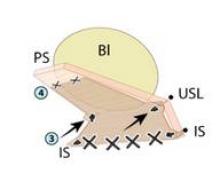
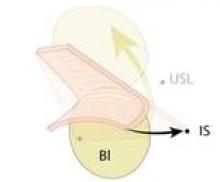
Because the pararectal space has been developed early on, the ureter can be seen throughout the dissection and there is no need to coagulate the vessels from the level of the internal os and downward, as many surgeons are traditionally taught. Vessels are coagulated right near the location of the VCare cup. It is important that all bleeding vessels are coagulated prior to colpotomy. Again, using the "cut" mode on the console during the colpotomy minimizes charring.
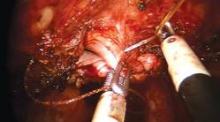
Vaginal cuff closure is the most technically challenging aspect of Single-Site hysterectomy. I tried numerous instruments and approaches and found a technique that works for me. The fenestrated bipolar grasper has been just as effective as the crocodile grasper, which is cost-effective because I use this instrument for dissection as well. Use of the fenestrated bipolar at this point also allows me to cauterize any small vessels that were not coagulated earlier.
I advise use of 2-0, barbed, 9-12\" absorbable suture on a 24- to 35-mm reverse-cutting needle. I recommend the 2-0 V-LOC reverse-cutting P-14 needle; this is the one that has worked for me with minimal difficulty. Because the suture is barbed, there is no need to tie.
I closed the cuff vertically in my initial cases – as I do in multiport cases – but at my 13th case I began horizontal cuff closures. Effectively grasping the needle, finding the correct combination of traction and countertraction, and achieving the correct angles take time and practice. I move in a transverse direction, right to left, in every case. The anterior cuff requires angled insertion of the needle, whereas the posterior cuff requires vertical insertion of the needle. The assistant may hold the bladder peritoneum as necessary throughout the closure to expose the cuff, but should not grasp and suspend the cuff itself.
I deliver the needle transvaginally, but alternatively it may be delivered through the camera port or the 10-mm assistant port. The tip of the needle should not be grasped in any circumstance, or it will be dulled.
Learning curve, outcomes
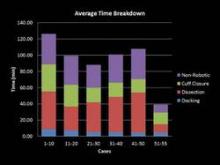
My learning curve for Single-Site hysterectomy was marked by two turning points: At case 20, I felt noticeably more proficient, with improved operative times and significantly less difficulty. At the 50th case, my comfort and skill levels jumped again, and I began to feel at times like I was performing multiport surgery.
From the first 20 cases to cases 51-55, my average total operating room time fell from approximately 113 minutes to 60 minutes, my average console time dropped from 71 minutes to 36 minutes, and my average cuff closure time fell from 29 minutes to 14 minutes. The most challenging part of the journey involved vaginal cuff closure; my initial closures took 60-70 minutes as I tried different needles and methods to close. By incorporating what I learned early on, cuff closure times dropped to 20-30 minutes by case 10 and continued to decrease further as I performed more cases.
There were no complications in my first 20 Single-Site surgeries. After that, I had one case of incisional hernia, two cases of pelvic hematoma, and one case of ureterovaginal fistula. I have yet to see cuff dehiscence. My average estimated blood loss has been 60 mL. The ureterovaginal fistula occurred in a patient with a 430-gram uterus and stage IV endometriosis, in whom I encountered a lot of bleeding and struggled with coagulation. My lesson from this case: If extensive coagulation is needed, consider being proactive and place a ureteral stent.
Single-Site hysterectomy makes minimally invasive surgery even more minimally invasive – without sacrificing the quality of care. My patients uniformly have been extremely happy with the cosmesis, and anecdotally they have reported less pain than those undergoing multiport robotic hysterectomy. I have no doubt this is just the beginning of what robotic surgery will enable in the coming years. The journey has been worth it.
Dr. Im is director of the Gynecologic Oncology Center at Mercy Medical Center, and clinical assistant professor of obstetrics, gynecology, and reproductive services at the University of Maryland, both in Baltimore. Dr. Im reports that he is an educational consultant for Intuitive Surgical, manufacturer of the da Vinci Single-Site Technology, and SurgiQuest, manufacturer of AirSeal.
When the Food and Drug Administration cleared the da Vinci Single-Site Technology for use in benign hysterectomy and salpingo-oophorectomy in February 2013, I wondered how these procedures could possibly be performed with instruments that lack articulating wrists. I came to realize that with a stepwise approach to initiating this virtually scarless surgery, an appreciation of its nuances, and repetition of best practices found in my learning curve, Single-Site hysterectomy is indeed achievable and reproducible.
Single-Site tools are flexible and semirigid, allowing them to fit through the port’s curved cannulas. The port accommodates insufflation tubing, an 8.5-mm endoscope, two 5-mm operating instruments, and an assistant instrument. A 30-degree endoscope, facing downward to view the operating instruments underneath, is essential for achieving necessary triangulation.
I perform dissection with the fenestrated bipolar grasper in arm 1 and the monopolar cautery hook in arm 2. For vaginal cuff closure, I keep the fenestrated bipolar in arm 1 and place a needle holder in arm 2.
The fenestrated bipolar grasper for Single-Site became available only recently. Until then I utilized the Maryland bipolar forceps for dissection and tried various instruments, including the crocodile grasper, for vaginal cuff closure. Use of the fenestrated bipolar has been a game-changer for vessel sealing and has worked beautifully for cuff closure as well.
Single-port robotic hysterectomy requires deliberate transition. One must be comfortable first with minimal or no bedside assistance during multiport robotic surgery. The surgeon should take a stepwise approach to minimizing assistance – moving, for instance, from the use of five ports, to four ports, to four ports with no bedside assistance, to three ports, and lastly a "single-plus one" approach in which an extra port is placed as a precaution. I used a "single-plus one" approach for my first 10 cases before moving to a pure Single-Site approach.
I also began with two cases of bilateral salpingo-oophorectomy before performing Single-Site hysterectomy, and advise others to start with such easier cases as well. Patients selected for initial Single-Site surgery also should be younger than 70, with few comorbidities and a good performance status (American Society of Anesthesiologists classification I-II). They should have BMI less than 30, a history of little or no previous intra-abdominal or pelvic surgery, and a reasonably sized uterus (less than 12 weeks), and be candidates for vaginal or laparoscopic hysterectomy.
The Single-Site approach requires meticulous dissection, one side at a time. The surgeon must skeletonize vessels before coagulation, and must coagulate each vessel individually. Selective coagulation/sealing of vessels is key, as the surgeon does not have the luxury of using a harmonic scalpel, vessel sealer, or other such tools. Too much bleeding can make a case difficult, if not impossible, to complete without conversion. Similarly, because assistance is limited, coagulation must be done in a cephalad direction in order to minimize back-bleeding.
Uterine manipulation is always an important task, but it is especially important for the Single-Site hysterectomy. It is the assistant’s main role. He or she must know not only how to turn the uterus for vessel coagulation and visualization of anatomy, but also the nuances of traction and countertraction for dissection and the colpotomy. Various uterine manipulators will work; I prefer the VCare uterine manipulator.
Setup and entry
While steep Trendelenburg positioning is used for multiport hysterectomy, it must be lessened for the single-port approach. Trendelenburg changes the distance of the cannulae to the uterus, so individual adjustments are necessary. I start the patient in steep Trendelenburg, then lessen it just enough to displace the small bowel while maintaining an appropriate distance for the instruments to reach the uterus.
Center docking provides greater range of motion and is therefore often preferred, especially during early cases. However, side docking on the dominant-hand side, with the camera arm toward the uterus, is my preferred method. It provides the assistant maximal access to the vaginal area and best affords the surgeon the ability to add another port if it is necessary later.
Entry and port placement are critical aspects of the Single-Site approach. The Single-Site Port requires a skin incision of 2.5 cm. I make this incision transversely, following the natural creases of the umbilicus. To get to the fascia, the umbilical stalk is transected, and a small hole – a hernia defect, in essence – is identified and lifted. A 3-cm vertical fascial incision is made to accommodate the Single-Site Port. It is important that this incision size is exact: If it is too short, the port will not expand to its natural state and there will be crowding of instruments, and if it is too large, leaking air will be a problem. I have found that placing stay sutures on the fascia at this point helps facilitate later closure.
Cannulas for the Single-Site Port come in two lengths: There is a set of short, 250-mm cannulas and a set of long, 300-mm cannulas. When to use these cannulas depends on the working area and the patient’s size. Generally, the shorter cannulas are used for everything but the cuff closure. Then arm 2 is replaced with the longer cannula for the extra rigidity needed to suture. My use of the different cannula lengths has varied depending on the patient’s body size, and with growing experience I have sometimes gone without exchanging them.
Dissection through cuff closure
I start all my cases on the right side, holding the infundibulopelvic (IP) or utero-ovarian ligament on tension and skeletonizing it. I then coagulate the IP ligament with the fenestrated bipolar grasper (setting: 35 watts).
At this point and throughout the surgery, consistent use of the "cut" mode on the da Vinci console makes both dissection and sealing cleaner and more precise, with significantly less charring.
I use AirSeal for insufflation and automatic smoke evacuation.
As I do with all minimally invasive hysterectomies, regardless of modality, I work to find and develop the pararectal space. This space is bordered by the ureter medially, the hypogastric or internal iliac artery laterally, and the base of the cardinal ligament anteriorly, with the levator ani muscle as the floor. Defining and developing the space enable me to visualize the ureter so that I can dissect it off the peritoneum and drop it out of harm’s way. It puts me in a much better position, moreover, to handle dissection in more complex cases involving large fibroids or endometriosis.
Once the pararectal space is developed and the ureter dissected, I proceed with transection of the round ligament. Dissecting the round ligament any earlier would be particularly troublesome in a Single-Site robotic hysterectomy because it would be challenging to achieve the necessary degree of uterine manipulation.
In developing the bladder flap, one must be conscious of the highly magnified view, as the bladder appears closer with the 30-degree 8.5-mm endoscope than it would with a 0-degree scope. A combination of traction and countertraction, small bites, and selective coagulation – one vessel at a time – is critical as dissection proceeds and the bladder flap is created.
Because the pararectal space has been developed early on, the ureter can be seen throughout the dissection and there is no need to coagulate the vessels from the level of the internal os and downward, as many surgeons are traditionally taught. Vessels are coagulated right near the location of the VCare cup. It is important that all bleeding vessels are coagulated prior to colpotomy. Again, using the "cut" mode on the console during the colpotomy minimizes charring.
Vaginal cuff closure is the most technically challenging aspect of Single-Site hysterectomy. I tried numerous instruments and approaches and found a technique that works for me. The fenestrated bipolar grasper has been just as effective as the crocodile grasper, which is cost-effective because I use this instrument for dissection as well. Use of the fenestrated bipolar at this point also allows me to cauterize any small vessels that were not coagulated earlier.
I advise use of 2-0, barbed, 9-12\" absorbable suture on a 24- to 35-mm reverse-cutting needle. I recommend the 2-0 V-LOC reverse-cutting P-14 needle; this is the one that has worked for me with minimal difficulty. Because the suture is barbed, there is no need to tie.
I closed the cuff vertically in my initial cases – as I do in multiport cases – but at my 13th case I began horizontal cuff closures. Effectively grasping the needle, finding the correct combination of traction and countertraction, and achieving the correct angles take time and practice. I move in a transverse direction, right to left, in every case. The anterior cuff requires angled insertion of the needle, whereas the posterior cuff requires vertical insertion of the needle. The assistant may hold the bladder peritoneum as necessary throughout the closure to expose the cuff, but should not grasp and suspend the cuff itself.
I deliver the needle transvaginally, but alternatively it may be delivered through the camera port or the 10-mm assistant port. The tip of the needle should not be grasped in any circumstance, or it will be dulled.
Learning curve, outcomes
My learning curve for Single-Site hysterectomy was marked by two turning points: At case 20, I felt noticeably more proficient, with improved operative times and significantly less difficulty. At the 50th case, my comfort and skill levels jumped again, and I began to feel at times like I was performing multiport surgery.
From the first 20 cases to cases 51-55, my average total operating room time fell from approximately 113 minutes to 60 minutes, my average console time dropped from 71 minutes to 36 minutes, and my average cuff closure time fell from 29 minutes to 14 minutes. The most challenging part of the journey involved vaginal cuff closure; my initial closures took 60-70 minutes as I tried different needles and methods to close. By incorporating what I learned early on, cuff closure times dropped to 20-30 minutes by case 10 and continued to decrease further as I performed more cases.
There were no complications in my first 20 Single-Site surgeries. After that, I had one case of incisional hernia, two cases of pelvic hematoma, and one case of ureterovaginal fistula. I have yet to see cuff dehiscence. My average estimated blood loss has been 60 mL. The ureterovaginal fistula occurred in a patient with a 430-gram uterus and stage IV endometriosis, in whom I encountered a lot of bleeding and struggled with coagulation. My lesson from this case: If extensive coagulation is needed, consider being proactive and place a ureteral stent.
Single-Site hysterectomy makes minimally invasive surgery even more minimally invasive – without sacrificing the quality of care. My patients uniformly have been extremely happy with the cosmesis, and anecdotally they have reported less pain than those undergoing multiport robotic hysterectomy. I have no doubt this is just the beginning of what robotic surgery will enable in the coming years. The journey has been worth it.
Dr. Im is director of the Gynecologic Oncology Center at Mercy Medical Center, and clinical assistant professor of obstetrics, gynecology, and reproductive services at the University of Maryland, both in Baltimore. Dr. Im reports that he is an educational consultant for Intuitive Surgical, manufacturer of the da Vinci Single-Site Technology, and SurgiQuest, manufacturer of AirSeal.
When the Food and Drug Administration cleared the da Vinci Single-Site Technology for use in benign hysterectomy and salpingo-oophorectomy in February 2013, I wondered how these procedures could possibly be performed with instruments that lack articulating wrists. I came to realize that with a stepwise approach to initiating this virtually scarless surgery, an appreciation of its nuances, and repetition of best practices found in my learning curve, Single-Site hysterectomy is indeed achievable and reproducible.
Single-Site tools are flexible and semirigid, allowing them to fit through the port’s curved cannulas. The port accommodates insufflation tubing, an 8.5-mm endoscope, two 5-mm operating instruments, and an assistant instrument. A 30-degree endoscope, facing downward to view the operating instruments underneath, is essential for achieving necessary triangulation.
I perform dissection with the fenestrated bipolar grasper in arm 1 and the monopolar cautery hook in arm 2. For vaginal cuff closure, I keep the fenestrated bipolar in arm 1 and place a needle holder in arm 2.
The fenestrated bipolar grasper for Single-Site became available only recently. Until then I utilized the Maryland bipolar forceps for dissection and tried various instruments, including the crocodile grasper, for vaginal cuff closure. Use of the fenestrated bipolar has been a game-changer for vessel sealing and has worked beautifully for cuff closure as well.
Single-port robotic hysterectomy requires deliberate transition. One must be comfortable first with minimal or no bedside assistance during multiport robotic surgery. The surgeon should take a stepwise approach to minimizing assistance – moving, for instance, from the use of five ports, to four ports, to four ports with no bedside assistance, to three ports, and lastly a "single-plus one" approach in which an extra port is placed as a precaution. I used a "single-plus one" approach for my first 10 cases before moving to a pure Single-Site approach.
I also began with two cases of bilateral salpingo-oophorectomy before performing Single-Site hysterectomy, and advise others to start with such easier cases as well. Patients selected for initial Single-Site surgery also should be younger than 70, with few comorbidities and a good performance status (American Society of Anesthesiologists classification I-II). They should have BMI less than 30, a history of little or no previous intra-abdominal or pelvic surgery, and a reasonably sized uterus (less than 12 weeks), and be candidates for vaginal or laparoscopic hysterectomy.
The Single-Site approach requires meticulous dissection, one side at a time. The surgeon must skeletonize vessels before coagulation, and must coagulate each vessel individually. Selective coagulation/sealing of vessels is key, as the surgeon does not have the luxury of using a harmonic scalpel, vessel sealer, or other such tools. Too much bleeding can make a case difficult, if not impossible, to complete without conversion. Similarly, because assistance is limited, coagulation must be done in a cephalad direction in order to minimize back-bleeding.
Uterine manipulation is always an important task, but it is especially important for the Single-Site hysterectomy. It is the assistant’s main role. He or she must know not only how to turn the uterus for vessel coagulation and visualization of anatomy, but also the nuances of traction and countertraction for dissection and the colpotomy. Various uterine manipulators will work; I prefer the VCare uterine manipulator.
Setup and entry
While steep Trendelenburg positioning is used for multiport hysterectomy, it must be lessened for the single-port approach. Trendelenburg changes the distance of the cannulae to the uterus, so individual adjustments are necessary. I start the patient in steep Trendelenburg, then lessen it just enough to displace the small bowel while maintaining an appropriate distance for the instruments to reach the uterus.
Center docking provides greater range of motion and is therefore often preferred, especially during early cases. However, side docking on the dominant-hand side, with the camera arm toward the uterus, is my preferred method. It provides the assistant maximal access to the vaginal area and best affords the surgeon the ability to add another port if it is necessary later.
Entry and port placement are critical aspects of the Single-Site approach. The Single-Site Port requires a skin incision of 2.5 cm. I make this incision transversely, following the natural creases of the umbilicus. To get to the fascia, the umbilical stalk is transected, and a small hole – a hernia defect, in essence – is identified and lifted. A 3-cm vertical fascial incision is made to accommodate the Single-Site Port. It is important that this incision size is exact: If it is too short, the port will not expand to its natural state and there will be crowding of instruments, and if it is too large, leaking air will be a problem. I have found that placing stay sutures on the fascia at this point helps facilitate later closure.
Cannulas for the Single-Site Port come in two lengths: There is a set of short, 250-mm cannulas and a set of long, 300-mm cannulas. When to use these cannulas depends on the working area and the patient’s size. Generally, the shorter cannulas are used for everything but the cuff closure. Then arm 2 is replaced with the longer cannula for the extra rigidity needed to suture. My use of the different cannula lengths has varied depending on the patient’s body size, and with growing experience I have sometimes gone without exchanging them.
Dissection through cuff closure
I start all my cases on the right side, holding the infundibulopelvic (IP) or utero-ovarian ligament on tension and skeletonizing it. I then coagulate the IP ligament with the fenestrated bipolar grasper (setting: 35 watts).
At this point and throughout the surgery, consistent use of the "cut" mode on the da Vinci console makes both dissection and sealing cleaner and more precise, with significantly less charring.
I use AirSeal for insufflation and automatic smoke evacuation.
As I do with all minimally invasive hysterectomies, regardless of modality, I work to find and develop the pararectal space. This space is bordered by the ureter medially, the hypogastric or internal iliac artery laterally, and the base of the cardinal ligament anteriorly, with the levator ani muscle as the floor. Defining and developing the space enable me to visualize the ureter so that I can dissect it off the peritoneum and drop it out of harm’s way. It puts me in a much better position, moreover, to handle dissection in more complex cases involving large fibroids or endometriosis.
Once the pararectal space is developed and the ureter dissected, I proceed with transection of the round ligament. Dissecting the round ligament any earlier would be particularly troublesome in a Single-Site robotic hysterectomy because it would be challenging to achieve the necessary degree of uterine manipulation.
In developing the bladder flap, one must be conscious of the highly magnified view, as the bladder appears closer with the 30-degree 8.5-mm endoscope than it would with a 0-degree scope. A combination of traction and countertraction, small bites, and selective coagulation – one vessel at a time – is critical as dissection proceeds and the bladder flap is created.
Because the pararectal space has been developed early on, the ureter can be seen throughout the dissection and there is no need to coagulate the vessels from the level of the internal os and downward, as many surgeons are traditionally taught. Vessels are coagulated right near the location of the VCare cup. It is important that all bleeding vessels are coagulated prior to colpotomy. Again, using the "cut" mode on the console during the colpotomy minimizes charring.
Vaginal cuff closure is the most technically challenging aspect of Single-Site hysterectomy. I tried numerous instruments and approaches and found a technique that works for me. The fenestrated bipolar grasper has been just as effective as the crocodile grasper, which is cost-effective because I use this instrument for dissection as well. Use of the fenestrated bipolar at this point also allows me to cauterize any small vessels that were not coagulated earlier.
I advise use of 2-0, barbed, 9-12\" absorbable suture on a 24- to 35-mm reverse-cutting needle. I recommend the 2-0 V-LOC reverse-cutting P-14 needle; this is the one that has worked for me with minimal difficulty. Because the suture is barbed, there is no need to tie.
I closed the cuff vertically in my initial cases – as I do in multiport cases – but at my 13th case I began horizontal cuff closures. Effectively grasping the needle, finding the correct combination of traction and countertraction, and achieving the correct angles take time and practice. I move in a transverse direction, right to left, in every case. The anterior cuff requires angled insertion of the needle, whereas the posterior cuff requires vertical insertion of the needle. The assistant may hold the bladder peritoneum as necessary throughout the closure to expose the cuff, but should not grasp and suspend the cuff itself.
I deliver the needle transvaginally, but alternatively it may be delivered through the camera port or the 10-mm assistant port. The tip of the needle should not be grasped in any circumstance, or it will be dulled.
Learning curve, outcomes
My learning curve for Single-Site hysterectomy was marked by two turning points: At case 20, I felt noticeably more proficient, with improved operative times and significantly less difficulty. At the 50th case, my comfort and skill levels jumped again, and I began to feel at times like I was performing multiport surgery.
From the first 20 cases to cases 51-55, my average total operating room time fell from approximately 113 minutes to 60 minutes, my average console time dropped from 71 minutes to 36 minutes, and my average cuff closure time fell from 29 minutes to 14 minutes. The most challenging part of the journey involved vaginal cuff closure; my initial closures took 60-70 minutes as I tried different needles and methods to close. By incorporating what I learned early on, cuff closure times dropped to 20-30 minutes by case 10 and continued to decrease further as I performed more cases.
There were no complications in my first 20 Single-Site surgeries. After that, I had one case of incisional hernia, two cases of pelvic hematoma, and one case of ureterovaginal fistula. I have yet to see cuff dehiscence. My average estimated blood loss has been 60 mL. The ureterovaginal fistula occurred in a patient with a 430-gram uterus and stage IV endometriosis, in whom I encountered a lot of bleeding and struggled with coagulation. My lesson from this case: If extensive coagulation is needed, consider being proactive and place a ureteral stent.
Single-Site hysterectomy makes minimally invasive surgery even more minimally invasive – without sacrificing the quality of care. My patients uniformly have been extremely happy with the cosmesis, and anecdotally they have reported less pain than those undergoing multiport robotic hysterectomy. I have no doubt this is just the beginning of what robotic surgery will enable in the coming years. The journey has been worth it.
Dr. Im is director of the Gynecologic Oncology Center at Mercy Medical Center, and clinical assistant professor of obstetrics, gynecology, and reproductive services at the University of Maryland, both in Baltimore. Dr. Im reports that he is an educational consultant for Intuitive Surgical, manufacturer of the da Vinci Single-Site Technology, and SurgiQuest, manufacturer of AirSeal.
Developments and issues in prenatal screening and diagnosis
Prenatal assessments for major chromosomal abnormalities have, like a pendulum, swung over the last 50 years between advancements in screening tests and diagnostic procedures.
In the 1960s, screening for advanced maternal age gave way to diagnostic amniocentesis. Maternal serum alpha-fetoprotein screening for neural tube defects came on the scene in the 1970s, and Down syndrome screening and chorionic villus sampling (CVS) followed in the 1980s. Better ultrasound screening markers were used in combination with biochemistry to advance first-trimester screening in the 1990s and 2000s, leading to a significant decline in diagnostic procedures. Now, free fetal DNA measurement, known as noninvasive prenatal screening, or NIPS, has entered the scene. This development, along with advances in the accuracy of diagnostic lab testing through microarray analysis, will soon lead the pendulum to swing back toward more definitive diagnostic procedures that require either CVS or amniocentesis.
Screening tests provide us with odds adjustments, not definitive answers, and are meant for everyone. Diagnostic tests are meant to give us definitive answers, may have risks, and therefore have traditionally been done on "at-risk" patients. Fundamentally, a screening test gives us an impression, while a diagnostic test gives us harsh reality. As always, there will be trade-offs. No approach is perfect, and no one size fits everyone.
Risks beyond Down syndrome
During the prenatal period, patients will often say, "I’m concerned about having a baby with Down syndrome." What they really mean is that they’re concerned about having a baby with a serious problem, and Down syndrome is the name they know.
Serious problems – a Mendelian disorder, a multifactorial disorder, or a major chromosomal abnormality – affect 2%-3% of all births. Less serious chromosomal abnormalities affect 5%-6% of births. Although advanced maternal age is no longer the sole criterion for deciding who should be offered diagnostic testing, age still is a principal factor for risk determination.
At age 35, the chance of having a baby with Down syndrome is 1 in 380, but the chance of having any chromosomal abnormality detectable by karyotype is 1 in 190. For a 30-year-old, the chance of having a baby with any chromosomal abnormality is 1 in 380, and for a 40-year-old, the risk is 1 in 65.
With the first-trimester screening approach that combines maternal serum free beta-human chorionic gonadotropin (free beta-HCG) and pregnancy-associated plasma protein A (PAPP-A) with fetal nuchal translucency measurement, we are able to detect upward of 85% of fetuses with Down syndrome, or trisomy 21. Yet the disorder is only one of a large number of chromosomal abnormalities observed.
In a recent single-center study of more than 20,000 first-trimester screenings, 5.6% were positive for Down syndrome risk. Of those who subsequently had an amniocentesis or CVS, we found 4% had an abnormal karyotype. Interestingly, 40% of the time the abnormality was not Down syndrome, but another chromosomal abnormality. Similar analyses for trisomies 13 and 18 – the other major abnormalities targeted in first-trimester combined screening – yielded similar statistics (Prenat. Diagn. 2013;33:251-6).
All told, of the screen-positive pregnancies found to have an abnormal karyotype, at least 30% had chromosomal abnormalities outside of those for which they were screen positive. Such findings speak to the limitations of screening as opposed to diagnostic testing, and have implications for patient counseling. Patients should be counseled about the possibility of all chromosome abnormalities – not just Down syndrome.
The NIPS rollout
We have known for well over 100 years that fetal cells cross the placental barrier in small numbers, driving the development of what’s currently known as NIPS. The future of NIPS actually lies in an ever-expanding number of disorders, and will eventually end with sequencing the entire genome.
There are two main methods by which NIPS is done. The original and predominant method uses massive parallel shotgun sequencing, known as next-generation sequencing. This method involves whole-genome amplification and collects enormous amounts of information. Investigators are now attempting to direct amplification at the subchromosome level, mimicking some of what microarray analysis can do.
The second approach uses selected probes, or targeted sequencing, to focus on those sections of DNA that are of interest. Although this method may be cheaper in the short run, one drawback is that new probes will need to be created for each new disorder.
Initially, investigators attempted to isolate nucleated fetal cells from the maternal blood and use them for aneuploidy detection. However, a National Institutes of Health–funded fetal-cell isolation study that ended in 2002 reported disappointing results: Fetal-cell isolation methods had low sensitivity and other technological shortcomings. Subsequently, a number of companies attempted to replicate and improve the work, also without much success.
Concurrent with efforts to use fetal-cell isolation to perform NIPS was the discovery, in the late 1990s by Dr. Dennis Lo, upon whose work next-generation sequencing for NIPS is based, of the presence of circulating cell-free fetal DNA in maternal plasma. The concentration of cell-free fetal DNA may be as much as 5%-8% of the total circulating cell-free DNA in maternal plasma, making free fetal DNA a promising source of fetal genetic material for noninvasive prenatal investigation.
The first high-quality trials on the use of free fetal DNA measurement for detection of trisomy 21 were published in the fall of 2011, and demonstrated up to a 99% detection rate for Down syndrome (less for trisomies 18 and 13). Companies subsequently began to manufacture free fetal DNA tests as off-label products, The tests were initially designated as "noninvasive prenatal diagnosis" tools, but experts around the world objected to the "diagnosis" label, and the terminology shifted to "noninvasive prenatal testing" and finally to "noninvasive prenatal screening"– a designation that I believe accurately reflects its current role.
The uptake in utilization of NIPS has been faster than anyone could have predicted: In just over 2 years, several hundred thousand screening tests have been performed. With 98% to 99% sensitivity, NIPS is an excellent screening test for Down syndrome. However, 99% sensitivity does not equate to 99% positive predictive value, that is, the risk that a patient with a positive screen actually has Down syndrome is much lower.
The published studies of NIPS cite false positive rates of 0.2%-1.0%, but this rate will increase as more disorders are screened. Positive predictive value is directly proportional to the underlying risk. For example, a test with 99% sensitivity and 99% specificity (1% false positive rate) means that a 26-year-old woman who has a positive free fetal DNA test actually has as low as an 11% chance of having a baby with Down syndrome. In older women, who have a higher incidence of having a baby with Down syndrome, a positive NIPS result will have a higher positive predictive value of giving birth to a baby with Down syndrome. However, at the current time, NIPS is an excellent screening test for Down syndrome, but it is not ready for universal primary screening in younger women.
Falsely reassuring are the hyped marketing claims in the United States that NIPS "replaces amnio" and eliminates the need for a nuchal translucency (NT) test. When clinicians abandon performing or referring for a high-quality NT measurement, they can miss or significantly delay the diagnosis of twins/zygosity, growth abnormalities and placentation, cardiac defects, and numerous other anomalies. Similarly, when patients who otherwise would have opted to have CVS or amniocentesis forego having the procedure and have NIPS performed instead, they may regret this decision.
The danger that overreliance on screening tests may paradoxically increase the number of births of babies with otherwise detectable problems was raised in a study led by the late Dr. George Henry, an obstetrician-geneticist in Denver. Curious about declining rates of diagnostic testing in his own practice, Dr. Henry examined trends in his state and found that while the utilization of diagnostic tests had plummeted by 70% over about 15 years, the birth rate of babies born with Down syndrome during this time had doubled among mothers over age 35, and stayed the same among mothers under age 35.
A sociologist-researcher examined the trend that Dr. Henry identified and found that the rise in Down syndrome births was not due to an increase in women electing to keep these pregnancies, but to the fact that the abnormality was not detected in the first place (Fetal Diagn. Ther. 2008;23:308-15). The same risk of screening tests replacing definitive diagnosis exists today with the uptake of NIPS.
The impact of microarray
Microarray technologies have been developed over the past decade. The National Institutes of Health study on chromosomal microarray versus karyotyping, published a year ago, is a game changer. The microarray is analogous to a 15-fold magnifying glass on the karyotype. While the smallest piece of a chromosome that can be evaluated by karyotype analysis is about 5 million base pairs, microarray analysis zooms in on about 200,000 base pairs, allowing us to see small genomic deletions and duplications (copy number variants) that we’ve never seen before.
The trial looked at upward of 4,000 women undergoing CVS or amniocentesis at one of 29 centers. Each diagnostic sample was split in two, with standard karyotyping performed on one portion and chromosomal microarray on the other (N. Engl. J. Med. 2012;367:2175-84).
There were several significant findings: One is that almost one-third of patients have a copy variation now known to be benign. Another is that 2.5% of women who had ultrasound-identified anomalies and normal karyotypes had microdeletion/duplications on the microarray that were clearly associated with a known clinical problem. Moreover, another 3.2% had gains and losses of potential clinical significance. As such, close to 6% of women with ultrasound-identified anomalies and a normal karyotype had clinically relevant copy number variants that only the microarray could find.
When microarray analysis was performed in women whose only indication for prenatal diagnosis was advanced age or an abnormal result on Down syndrome screening, as opposed to an ultrasound-detected anomaly, 0.5%, or 1 in 200, were found to have a pathogenic abnormality that otherwise would have been missed. This is significant: Previously, with karyotyping, we quoted patients a minimum of 1/500 risk of finding a clinically relevant chromosomal anomaly even if the combined report suggested much lower risks of Down syndrome. Now, with microarray, that risk is 1/200. Other studies already published or about to be published show this same level of risk determined by microarray.
With any new technological advance, we get our numerators of the problem before our denominators. The first cases published are those in which clinical or laboratory findings are associated with an abnormality. Only then do researchers go back and look at cases with those findings to test these associations – and only then are the markers sometimes found not to be associated. It will take a number of years to acquire a sizable database on microarray-detected copy number variants. In the end, microarray may help us to explain many of the approximately 1% of serious problems that we have been unable to diagnose until now.
Current decision-making
Ultimately, the future lies with routine, complete genomic sequencing that provides a detailed view of the fetal genome. This is likely to be about 7-10 years away, and the main question on the table is whether it will be performed invasively or with a maternal blood sample.
Today, when a 35-year-old woman comes into my office early in her pregnancy, I will tell her that the risk of having a baby with a chromosomal abnormality is 1 in 190. If she wants to know more, I will explain that the second-trimester quad screen will detect 60% of Down syndrome cases, that the first-trimester combined screen can identify 85% or more, and that the free fetal DNA test will get closer to 99%.
Despite the significant advances in screening, all of these options are still the fundamental equivalent of a Gallup poll. If the patient wants a definitive answer, she will need either an amniocentesis or a CVS, which, in experienced hands, have been shown through an increasing number of studies to be of equal risk. Because there is no such thing as "no" risk when it comes to prenatal diagnostic testing, the question that each patient must answer is, "Where do you want to put that risk – in the test or gambling on the outcome?"
We also have a great cultural divide in the United States. Some people want to know everything, others want to know nothing. Our affluent patients are getting older. Our poorer patients are getting younger. Some people will pay whatever it takes to get the answers they want, while others can or will not pay a dime beyond what their insurance will cover.
There is no one algorithm that can handle these two extremes of patients. Right now, many programs around the country have seen a diminishing number of patients having diagnostic testing – a phenomenon I believe is the result of a false sense of confidence, of people being lulled by the faulty argument that screening protocols can find Down syndrome, so what else is there to know?
Ultimately, a model for younger women would start with "contingent screening," in which patients could start with first-trimester combined screening and move straight to CVS if found to be at "high risk," and end testing if found to be at "low risk." Women who fall in the middle could undergo either free fetal DNA analysis or CVS, and we are developing methods to improve the mathematical processing of data to improve the sensitivity and specificity of all screening programs.
In my program, CVS and microarray are now offered to all patients regardless of age, as the risk that a microarray will find a significant abnormality is at least 1/200, which is the risk we have been quoting to 35-year-old patients for nearly 50 years.
Dr. Evans is president of the Fetal Medicine Foundation and International Fetal Medicine and Surgery Society Foundation, and professor of obstetrics and gynecology at the Icahn School of Medicine at Mount Sinai, New York. Dr. Evans disclosed that he is a consultant for PerkinElmer, a genetics company based in Waltham, Mass.
Prenatal assessments for major chromosomal abnormalities have, like a pendulum, swung over the last 50 years between advancements in screening tests and diagnostic procedures.
In the 1960s, screening for advanced maternal age gave way to diagnostic amniocentesis. Maternal serum alpha-fetoprotein screening for neural tube defects came on the scene in the 1970s, and Down syndrome screening and chorionic villus sampling (CVS) followed in the 1980s. Better ultrasound screening markers were used in combination with biochemistry to advance first-trimester screening in the 1990s and 2000s, leading to a significant decline in diagnostic procedures. Now, free fetal DNA measurement, known as noninvasive prenatal screening, or NIPS, has entered the scene. This development, along with advances in the accuracy of diagnostic lab testing through microarray analysis, will soon lead the pendulum to swing back toward more definitive diagnostic procedures that require either CVS or amniocentesis.
Screening tests provide us with odds adjustments, not definitive answers, and are meant for everyone. Diagnostic tests are meant to give us definitive answers, may have risks, and therefore have traditionally been done on "at-risk" patients. Fundamentally, a screening test gives us an impression, while a diagnostic test gives us harsh reality. As always, there will be trade-offs. No approach is perfect, and no one size fits everyone.
Risks beyond Down syndrome
During the prenatal period, patients will often say, "I’m concerned about having a baby with Down syndrome." What they really mean is that they’re concerned about having a baby with a serious problem, and Down syndrome is the name they know.
Serious problems – a Mendelian disorder, a multifactorial disorder, or a major chromosomal abnormality – affect 2%-3% of all births. Less serious chromosomal abnormalities affect 5%-6% of births. Although advanced maternal age is no longer the sole criterion for deciding who should be offered diagnostic testing, age still is a principal factor for risk determination.
At age 35, the chance of having a baby with Down syndrome is 1 in 380, but the chance of having any chromosomal abnormality detectable by karyotype is 1 in 190. For a 30-year-old, the chance of having a baby with any chromosomal abnormality is 1 in 380, and for a 40-year-old, the risk is 1 in 65.
With the first-trimester screening approach that combines maternal serum free beta-human chorionic gonadotropin (free beta-HCG) and pregnancy-associated plasma protein A (PAPP-A) with fetal nuchal translucency measurement, we are able to detect upward of 85% of fetuses with Down syndrome, or trisomy 21. Yet the disorder is only one of a large number of chromosomal abnormalities observed.
In a recent single-center study of more than 20,000 first-trimester screenings, 5.6% were positive for Down syndrome risk. Of those who subsequently had an amniocentesis or CVS, we found 4% had an abnormal karyotype. Interestingly, 40% of the time the abnormality was not Down syndrome, but another chromosomal abnormality. Similar analyses for trisomies 13 and 18 – the other major abnormalities targeted in first-trimester combined screening – yielded similar statistics (Prenat. Diagn. 2013;33:251-6).
All told, of the screen-positive pregnancies found to have an abnormal karyotype, at least 30% had chromosomal abnormalities outside of those for which they were screen positive. Such findings speak to the limitations of screening as opposed to diagnostic testing, and have implications for patient counseling. Patients should be counseled about the possibility of all chromosome abnormalities – not just Down syndrome.
The NIPS rollout
We have known for well over 100 years that fetal cells cross the placental barrier in small numbers, driving the development of what’s currently known as NIPS. The future of NIPS actually lies in an ever-expanding number of disorders, and will eventually end with sequencing the entire genome.
There are two main methods by which NIPS is done. The original and predominant method uses massive parallel shotgun sequencing, known as next-generation sequencing. This method involves whole-genome amplification and collects enormous amounts of information. Investigators are now attempting to direct amplification at the subchromosome level, mimicking some of what microarray analysis can do.
The second approach uses selected probes, or targeted sequencing, to focus on those sections of DNA that are of interest. Although this method may be cheaper in the short run, one drawback is that new probes will need to be created for each new disorder.
Initially, investigators attempted to isolate nucleated fetal cells from the maternal blood and use them for aneuploidy detection. However, a National Institutes of Health–funded fetal-cell isolation study that ended in 2002 reported disappointing results: Fetal-cell isolation methods had low sensitivity and other technological shortcomings. Subsequently, a number of companies attempted to replicate and improve the work, also without much success.
Concurrent with efforts to use fetal-cell isolation to perform NIPS was the discovery, in the late 1990s by Dr. Dennis Lo, upon whose work next-generation sequencing for NIPS is based, of the presence of circulating cell-free fetal DNA in maternal plasma. The concentration of cell-free fetal DNA may be as much as 5%-8% of the total circulating cell-free DNA in maternal plasma, making free fetal DNA a promising source of fetal genetic material for noninvasive prenatal investigation.
The first high-quality trials on the use of free fetal DNA measurement for detection of trisomy 21 were published in the fall of 2011, and demonstrated up to a 99% detection rate for Down syndrome (less for trisomies 18 and 13). Companies subsequently began to manufacture free fetal DNA tests as off-label products, The tests were initially designated as "noninvasive prenatal diagnosis" tools, but experts around the world objected to the "diagnosis" label, and the terminology shifted to "noninvasive prenatal testing" and finally to "noninvasive prenatal screening"– a designation that I believe accurately reflects its current role.
The uptake in utilization of NIPS has been faster than anyone could have predicted: In just over 2 years, several hundred thousand screening tests have been performed. With 98% to 99% sensitivity, NIPS is an excellent screening test for Down syndrome. However, 99% sensitivity does not equate to 99% positive predictive value, that is, the risk that a patient with a positive screen actually has Down syndrome is much lower.
The published studies of NIPS cite false positive rates of 0.2%-1.0%, but this rate will increase as more disorders are screened. Positive predictive value is directly proportional to the underlying risk. For example, a test with 99% sensitivity and 99% specificity (1% false positive rate) means that a 26-year-old woman who has a positive free fetal DNA test actually has as low as an 11% chance of having a baby with Down syndrome. In older women, who have a higher incidence of having a baby with Down syndrome, a positive NIPS result will have a higher positive predictive value of giving birth to a baby with Down syndrome. However, at the current time, NIPS is an excellent screening test for Down syndrome, but it is not ready for universal primary screening in younger women.
Falsely reassuring are the hyped marketing claims in the United States that NIPS "replaces amnio" and eliminates the need for a nuchal translucency (NT) test. When clinicians abandon performing or referring for a high-quality NT measurement, they can miss or significantly delay the diagnosis of twins/zygosity, growth abnormalities and placentation, cardiac defects, and numerous other anomalies. Similarly, when patients who otherwise would have opted to have CVS or amniocentesis forego having the procedure and have NIPS performed instead, they may regret this decision.
The danger that overreliance on screening tests may paradoxically increase the number of births of babies with otherwise detectable problems was raised in a study led by the late Dr. George Henry, an obstetrician-geneticist in Denver. Curious about declining rates of diagnostic testing in his own practice, Dr. Henry examined trends in his state and found that while the utilization of diagnostic tests had plummeted by 70% over about 15 years, the birth rate of babies born with Down syndrome during this time had doubled among mothers over age 35, and stayed the same among mothers under age 35.
A sociologist-researcher examined the trend that Dr. Henry identified and found that the rise in Down syndrome births was not due to an increase in women electing to keep these pregnancies, but to the fact that the abnormality was not detected in the first place (Fetal Diagn. Ther. 2008;23:308-15). The same risk of screening tests replacing definitive diagnosis exists today with the uptake of NIPS.
The impact of microarray
Microarray technologies have been developed over the past decade. The National Institutes of Health study on chromosomal microarray versus karyotyping, published a year ago, is a game changer. The microarray is analogous to a 15-fold magnifying glass on the karyotype. While the smallest piece of a chromosome that can be evaluated by karyotype analysis is about 5 million base pairs, microarray analysis zooms in on about 200,000 base pairs, allowing us to see small genomic deletions and duplications (copy number variants) that we’ve never seen before.
The trial looked at upward of 4,000 women undergoing CVS or amniocentesis at one of 29 centers. Each diagnostic sample was split in two, with standard karyotyping performed on one portion and chromosomal microarray on the other (N. Engl. J. Med. 2012;367:2175-84).
There were several significant findings: One is that almost one-third of patients have a copy variation now known to be benign. Another is that 2.5% of women who had ultrasound-identified anomalies and normal karyotypes had microdeletion/duplications on the microarray that were clearly associated with a known clinical problem. Moreover, another 3.2% had gains and losses of potential clinical significance. As such, close to 6% of women with ultrasound-identified anomalies and a normal karyotype had clinically relevant copy number variants that only the microarray could find.
When microarray analysis was performed in women whose only indication for prenatal diagnosis was advanced age or an abnormal result on Down syndrome screening, as opposed to an ultrasound-detected anomaly, 0.5%, or 1 in 200, were found to have a pathogenic abnormality that otherwise would have been missed. This is significant: Previously, with karyotyping, we quoted patients a minimum of 1/500 risk of finding a clinically relevant chromosomal anomaly even if the combined report suggested much lower risks of Down syndrome. Now, with microarray, that risk is 1/200. Other studies already published or about to be published show this same level of risk determined by microarray.
With any new technological advance, we get our numerators of the problem before our denominators. The first cases published are those in which clinical or laboratory findings are associated with an abnormality. Only then do researchers go back and look at cases with those findings to test these associations – and only then are the markers sometimes found not to be associated. It will take a number of years to acquire a sizable database on microarray-detected copy number variants. In the end, microarray may help us to explain many of the approximately 1% of serious problems that we have been unable to diagnose until now.
Current decision-making
Ultimately, the future lies with routine, complete genomic sequencing that provides a detailed view of the fetal genome. This is likely to be about 7-10 years away, and the main question on the table is whether it will be performed invasively or with a maternal blood sample.
Today, when a 35-year-old woman comes into my office early in her pregnancy, I will tell her that the risk of having a baby with a chromosomal abnormality is 1 in 190. If she wants to know more, I will explain that the second-trimester quad screen will detect 60% of Down syndrome cases, that the first-trimester combined screen can identify 85% or more, and that the free fetal DNA test will get closer to 99%.
Despite the significant advances in screening, all of these options are still the fundamental equivalent of a Gallup poll. If the patient wants a definitive answer, she will need either an amniocentesis or a CVS, which, in experienced hands, have been shown through an increasing number of studies to be of equal risk. Because there is no such thing as "no" risk when it comes to prenatal diagnostic testing, the question that each patient must answer is, "Where do you want to put that risk – in the test or gambling on the outcome?"
We also have a great cultural divide in the United States. Some people want to know everything, others want to know nothing. Our affluent patients are getting older. Our poorer patients are getting younger. Some people will pay whatever it takes to get the answers they want, while others can or will not pay a dime beyond what their insurance will cover.
There is no one algorithm that can handle these two extremes of patients. Right now, many programs around the country have seen a diminishing number of patients having diagnostic testing – a phenomenon I believe is the result of a false sense of confidence, of people being lulled by the faulty argument that screening protocols can find Down syndrome, so what else is there to know?
Ultimately, a model for younger women would start with "contingent screening," in which patients could start with first-trimester combined screening and move straight to CVS if found to be at "high risk," and end testing if found to be at "low risk." Women who fall in the middle could undergo either free fetal DNA analysis or CVS, and we are developing methods to improve the mathematical processing of data to improve the sensitivity and specificity of all screening programs.
In my program, CVS and microarray are now offered to all patients regardless of age, as the risk that a microarray will find a significant abnormality is at least 1/200, which is the risk we have been quoting to 35-year-old patients for nearly 50 years.
Dr. Evans is president of the Fetal Medicine Foundation and International Fetal Medicine and Surgery Society Foundation, and professor of obstetrics and gynecology at the Icahn School of Medicine at Mount Sinai, New York. Dr. Evans disclosed that he is a consultant for PerkinElmer, a genetics company based in Waltham, Mass.
Prenatal assessments for major chromosomal abnormalities have, like a pendulum, swung over the last 50 years between advancements in screening tests and diagnostic procedures.
In the 1960s, screening for advanced maternal age gave way to diagnostic amniocentesis. Maternal serum alpha-fetoprotein screening for neural tube defects came on the scene in the 1970s, and Down syndrome screening and chorionic villus sampling (CVS) followed in the 1980s. Better ultrasound screening markers were used in combination with biochemistry to advance first-trimester screening in the 1990s and 2000s, leading to a significant decline in diagnostic procedures. Now, free fetal DNA measurement, known as noninvasive prenatal screening, or NIPS, has entered the scene. This development, along with advances in the accuracy of diagnostic lab testing through microarray analysis, will soon lead the pendulum to swing back toward more definitive diagnostic procedures that require either CVS or amniocentesis.
Screening tests provide us with odds adjustments, not definitive answers, and are meant for everyone. Diagnostic tests are meant to give us definitive answers, may have risks, and therefore have traditionally been done on "at-risk" patients. Fundamentally, a screening test gives us an impression, while a diagnostic test gives us harsh reality. As always, there will be trade-offs. No approach is perfect, and no one size fits everyone.
Risks beyond Down syndrome
During the prenatal period, patients will often say, "I’m concerned about having a baby with Down syndrome." What they really mean is that they’re concerned about having a baby with a serious problem, and Down syndrome is the name they know.
Serious problems – a Mendelian disorder, a multifactorial disorder, or a major chromosomal abnormality – affect 2%-3% of all births. Less serious chromosomal abnormalities affect 5%-6% of births. Although advanced maternal age is no longer the sole criterion for deciding who should be offered diagnostic testing, age still is a principal factor for risk determination.
At age 35, the chance of having a baby with Down syndrome is 1 in 380, but the chance of having any chromosomal abnormality detectable by karyotype is 1 in 190. For a 30-year-old, the chance of having a baby with any chromosomal abnormality is 1 in 380, and for a 40-year-old, the risk is 1 in 65.
With the first-trimester screening approach that combines maternal serum free beta-human chorionic gonadotropin (free beta-HCG) and pregnancy-associated plasma protein A (PAPP-A) with fetal nuchal translucency measurement, we are able to detect upward of 85% of fetuses with Down syndrome, or trisomy 21. Yet the disorder is only one of a large number of chromosomal abnormalities observed.
In a recent single-center study of more than 20,000 first-trimester screenings, 5.6% were positive for Down syndrome risk. Of those who subsequently had an amniocentesis or CVS, we found 4% had an abnormal karyotype. Interestingly, 40% of the time the abnormality was not Down syndrome, but another chromosomal abnormality. Similar analyses for trisomies 13 and 18 – the other major abnormalities targeted in first-trimester combined screening – yielded similar statistics (Prenat. Diagn. 2013;33:251-6).
All told, of the screen-positive pregnancies found to have an abnormal karyotype, at least 30% had chromosomal abnormalities outside of those for which they were screen positive. Such findings speak to the limitations of screening as opposed to diagnostic testing, and have implications for patient counseling. Patients should be counseled about the possibility of all chromosome abnormalities – not just Down syndrome.
The NIPS rollout
We have known for well over 100 years that fetal cells cross the placental barrier in small numbers, driving the development of what’s currently known as NIPS. The future of NIPS actually lies in an ever-expanding number of disorders, and will eventually end with sequencing the entire genome.
There are two main methods by which NIPS is done. The original and predominant method uses massive parallel shotgun sequencing, known as next-generation sequencing. This method involves whole-genome amplification and collects enormous amounts of information. Investigators are now attempting to direct amplification at the subchromosome level, mimicking some of what microarray analysis can do.
The second approach uses selected probes, or targeted sequencing, to focus on those sections of DNA that are of interest. Although this method may be cheaper in the short run, one drawback is that new probes will need to be created for each new disorder.
Initially, investigators attempted to isolate nucleated fetal cells from the maternal blood and use them for aneuploidy detection. However, a National Institutes of Health–funded fetal-cell isolation study that ended in 2002 reported disappointing results: Fetal-cell isolation methods had low sensitivity and other technological shortcomings. Subsequently, a number of companies attempted to replicate and improve the work, also without much success.
Concurrent with efforts to use fetal-cell isolation to perform NIPS was the discovery, in the late 1990s by Dr. Dennis Lo, upon whose work next-generation sequencing for NIPS is based, of the presence of circulating cell-free fetal DNA in maternal plasma. The concentration of cell-free fetal DNA may be as much as 5%-8% of the total circulating cell-free DNA in maternal plasma, making free fetal DNA a promising source of fetal genetic material for noninvasive prenatal investigation.
The first high-quality trials on the use of free fetal DNA measurement for detection of trisomy 21 were published in the fall of 2011, and demonstrated up to a 99% detection rate for Down syndrome (less for trisomies 18 and 13). Companies subsequently began to manufacture free fetal DNA tests as off-label products, The tests were initially designated as "noninvasive prenatal diagnosis" tools, but experts around the world objected to the "diagnosis" label, and the terminology shifted to "noninvasive prenatal testing" and finally to "noninvasive prenatal screening"– a designation that I believe accurately reflects its current role.
The uptake in utilization of NIPS has been faster than anyone could have predicted: In just over 2 years, several hundred thousand screening tests have been performed. With 98% to 99% sensitivity, NIPS is an excellent screening test for Down syndrome. However, 99% sensitivity does not equate to 99% positive predictive value, that is, the risk that a patient with a positive screen actually has Down syndrome is much lower.
The published studies of NIPS cite false positive rates of 0.2%-1.0%, but this rate will increase as more disorders are screened. Positive predictive value is directly proportional to the underlying risk. For example, a test with 99% sensitivity and 99% specificity (1% false positive rate) means that a 26-year-old woman who has a positive free fetal DNA test actually has as low as an 11% chance of having a baby with Down syndrome. In older women, who have a higher incidence of having a baby with Down syndrome, a positive NIPS result will have a higher positive predictive value of giving birth to a baby with Down syndrome. However, at the current time, NIPS is an excellent screening test for Down syndrome, but it is not ready for universal primary screening in younger women.
Falsely reassuring are the hyped marketing claims in the United States that NIPS "replaces amnio" and eliminates the need for a nuchal translucency (NT) test. When clinicians abandon performing or referring for a high-quality NT measurement, they can miss or significantly delay the diagnosis of twins/zygosity, growth abnormalities and placentation, cardiac defects, and numerous other anomalies. Similarly, when patients who otherwise would have opted to have CVS or amniocentesis forego having the procedure and have NIPS performed instead, they may regret this decision.
The danger that overreliance on screening tests may paradoxically increase the number of births of babies with otherwise detectable problems was raised in a study led by the late Dr. George Henry, an obstetrician-geneticist in Denver. Curious about declining rates of diagnostic testing in his own practice, Dr. Henry examined trends in his state and found that while the utilization of diagnostic tests had plummeted by 70% over about 15 years, the birth rate of babies born with Down syndrome during this time had doubled among mothers over age 35, and stayed the same among mothers under age 35.
A sociologist-researcher examined the trend that Dr. Henry identified and found that the rise in Down syndrome births was not due to an increase in women electing to keep these pregnancies, but to the fact that the abnormality was not detected in the first place (Fetal Diagn. Ther. 2008;23:308-15). The same risk of screening tests replacing definitive diagnosis exists today with the uptake of NIPS.
The impact of microarray
Microarray technologies have been developed over the past decade. The National Institutes of Health study on chromosomal microarray versus karyotyping, published a year ago, is a game changer. The microarray is analogous to a 15-fold magnifying glass on the karyotype. While the smallest piece of a chromosome that can be evaluated by karyotype analysis is about 5 million base pairs, microarray analysis zooms in on about 200,000 base pairs, allowing us to see small genomic deletions and duplications (copy number variants) that we’ve never seen before.
The trial looked at upward of 4,000 women undergoing CVS or amniocentesis at one of 29 centers. Each diagnostic sample was split in two, with standard karyotyping performed on one portion and chromosomal microarray on the other (N. Engl. J. Med. 2012;367:2175-84).
There were several significant findings: One is that almost one-third of patients have a copy variation now known to be benign. Another is that 2.5% of women who had ultrasound-identified anomalies and normal karyotypes had microdeletion/duplications on the microarray that were clearly associated with a known clinical problem. Moreover, another 3.2% had gains and losses of potential clinical significance. As such, close to 6% of women with ultrasound-identified anomalies and a normal karyotype had clinically relevant copy number variants that only the microarray could find.
When microarray analysis was performed in women whose only indication for prenatal diagnosis was advanced age or an abnormal result on Down syndrome screening, as opposed to an ultrasound-detected anomaly, 0.5%, or 1 in 200, were found to have a pathogenic abnormality that otherwise would have been missed. This is significant: Previously, with karyotyping, we quoted patients a minimum of 1/500 risk of finding a clinically relevant chromosomal anomaly even if the combined report suggested much lower risks of Down syndrome. Now, with microarray, that risk is 1/200. Other studies already published or about to be published show this same level of risk determined by microarray.
With any new technological advance, we get our numerators of the problem before our denominators. The first cases published are those in which clinical or laboratory findings are associated with an abnormality. Only then do researchers go back and look at cases with those findings to test these associations – and only then are the markers sometimes found not to be associated. It will take a number of years to acquire a sizable database on microarray-detected copy number variants. In the end, microarray may help us to explain many of the approximately 1% of serious problems that we have been unable to diagnose until now.
Current decision-making
Ultimately, the future lies with routine, complete genomic sequencing that provides a detailed view of the fetal genome. This is likely to be about 7-10 years away, and the main question on the table is whether it will be performed invasively or with a maternal blood sample.
Today, when a 35-year-old woman comes into my office early in her pregnancy, I will tell her that the risk of having a baby with a chromosomal abnormality is 1 in 190. If she wants to know more, I will explain that the second-trimester quad screen will detect 60% of Down syndrome cases, that the first-trimester combined screen can identify 85% or more, and that the free fetal DNA test will get closer to 99%.
Despite the significant advances in screening, all of these options are still the fundamental equivalent of a Gallup poll. If the patient wants a definitive answer, she will need either an amniocentesis or a CVS, which, in experienced hands, have been shown through an increasing number of studies to be of equal risk. Because there is no such thing as "no" risk when it comes to prenatal diagnostic testing, the question that each patient must answer is, "Where do you want to put that risk – in the test or gambling on the outcome?"
We also have a great cultural divide in the United States. Some people want to know everything, others want to know nothing. Our affluent patients are getting older. Our poorer patients are getting younger. Some people will pay whatever it takes to get the answers they want, while others can or will not pay a dime beyond what their insurance will cover.
There is no one algorithm that can handle these two extremes of patients. Right now, many programs around the country have seen a diminishing number of patients having diagnostic testing – a phenomenon I believe is the result of a false sense of confidence, of people being lulled by the faulty argument that screening protocols can find Down syndrome, so what else is there to know?
Ultimately, a model for younger women would start with "contingent screening," in which patients could start with first-trimester combined screening and move straight to CVS if found to be at "high risk," and end testing if found to be at "low risk." Women who fall in the middle could undergo either free fetal DNA analysis or CVS, and we are developing methods to improve the mathematical processing of data to improve the sensitivity and specificity of all screening programs.
In my program, CVS and microarray are now offered to all patients regardless of age, as the risk that a microarray will find a significant abnormality is at least 1/200, which is the risk we have been quoting to 35-year-old patients for nearly 50 years.
Dr. Evans is president of the Fetal Medicine Foundation and International Fetal Medicine and Surgery Society Foundation, and professor of obstetrics and gynecology at the Icahn School of Medicine at Mount Sinai, New York. Dr. Evans disclosed that he is a consultant for PerkinElmer, a genetics company based in Waltham, Mass.
Biologic mesh in pelvic organ prolapse
Adverse events associated with the use of synthetic mesh in pelvic organ prolapse (POP) reparative surgery – and the Food and Drug Administration’s safety communication in 2011 warning physicians and patients about transvaginal placement of mesh for the repair of POP and stress urinary incontinence – have had a significant impact on gynecologic surgery.
Many physicians have become reluctant to use synthetic mesh products because of the risks of mesh erosion, exposure, pain and dyspareunia, and litigation. Patients also are concerned or even alarmed by reported risks. Moreover, some manufacturers have become increasingly concerned with the production of mesh products for POP repair, lessening the availability of synthetic mesh, and possibly some biologic mesh as well, for POP reparative surgery. Overall, the future of mesh augmentation in POP repair is uncertain.
Vaginal mesh procedures had surged in the decade prior to 2011 despite the lack of good randomized studies to determine whether mesh augmentation was truly efficacious. And unfortunately, the FDA has grouped synthetic and biologic material together in its reviews and notices of mesh products for POP repair. Not classifying and investigating them separately can mislead patients and hinder the development of randomized controlled trials needed to determine if augmentation with biologic material is truly superior to traditional POP repair using native tissue.
Comparing biologic mesh products to synthetic materials for pelvic organ prolapse repairs is like comparing apples to oranges: Synthetic mesh is permanent, while most biologics break down and remain in the body no longer than 6 months. From the standpoint of complications, this gives biologic materials an advantage. In my practice, biologic grafts have not eroded or caused pain, dyspareunia, or postoperative infections in any of my patients who have had surgical repair for POP.
There remains a real need for augmentation of weakened collagen tissue in the repair of POP. The native tissue in these patients is faulty tissue. Without reinforcement of defective tissue, we cannot expect excellent repairs. Data from nonrandomized studies have borne this out. When I talk with my patients about the options for surgical correction of POP, I tell them that success rates without the use of any augmentation are low, and that only about 60% of patients achieve a satisfactory result with traditional repairs.
Increasingly, I and other gynecologic surgeons are having success with biologic materials in our POP repairs. Efficacy needs to be measured in well-controlled randomized clinical trials, but at this point it appears anecdotally and from nonrandomized case reports that we can achieve good anatomic outcomes – upwards of 80% success rates – with biologic grafts, without the complications of synthetic mesh.
A recent survey of members of the American Urogynecologic Society shows that the use of synthetic mesh in transvaginal POP surgery decreased significantly after the 2011 FDA safety update, while there was no significant change in the use of biologic graft for POP (Female Pelvic Med. Reconstr. Surg 2013;19:191-8). It may be that the tide will shift toward greater use of biologic grafts. At the least, gynecologic surgeons should appreciate the differences between the two types of materials.
In the meantime, transvaginal placement of synthetic mesh should be used carefully and sparingly, with proper attention paid to patient selection and technique to reduce as much as possible the risk of erosion and pain.
In either case, more attention should be paid to the prevention of postoperative infections. Postoperative infection is an underappreciated risk with pelvic reconstructive surgery overall, and the use of synthetic mesh significantly increases this risk. Biologics are safer from an infection standpoint, but proper prevention – including evaluating each patient’s vaginal microflora immediately preoperatively and treating patients accordingly – is important for any surgery.
My advice on synthetics
Selecting patients for POP repair with no mesh, biologic mesh, or synthetic mesh requires thorough patient counseling. This is best done over multiple visits, with the patient reviewing information and coming back for a subsequent visit 1-2 weeks later with questions. She must be prepared psychologically and have realistic expectations.
In its 2011 safety communication, the FDA stated that the main role for mesh with POP repair is in the anterior compartment, and that traditional apical or posterior repair with mesh does not appear to provide any added benefit, compared with traditional surgery without mesh. Rectocele repair should not preclude the use of mesh, however, especially when a prior nonmesh repair has failed. For a patient with a small rectocele, I would advise repair with native tissue, and a second surgery with augmentation if the initial repair fails.
Synthetic mesh should be reserved for patients who have had multiple failures with traditional repairs and who are not sexually active. Sexual behavior is key to the decision-making process I undertake with my patients because synthetic mesh can cause a loss of elasticity in the vagina and consequent dyspareunia. When synthetic meshes are selected, the surgeon should use a minimal amount of material to cover as small an area as possible. It should not be used concomitantly in both the anterior and posterior compartments, as the risk of mesh contraction, rigidity, and vaginal shrinkage is too great.
Incisions in a mesh-augmented anterior or posterior repair should be 3-4 mm thick, passing through the full thickness of the vagina. Posterior compartment incisions should be kept as small in length as possible to reduce the risk of erosion/exposure and hematoma. In the anterior compartment, for similar reasons, surgeons are increasingly moving toward using small semilunar incisions.
In addition to the well-reported risks of erosion, exposure, and extrusion, synthetic meshes pose a problem from an infection point of view. Not uncommonly, synthetic grafts are found upon removal to be covered with biofilms – matrices produced by bacteria or fungi that colonize the material and house the organisms. Biofilm formation can lead to both acute, significant infection and long-term chronic infection; it also can result in metastatic infection if the biofilm breaks off, fragments, and is transported to other areas of the body.
Minimizing infection risk
Biofilms have been known and studied for some time, but there is growing appreciation for the role they play in infections that are chronic, recurrent, or hard to detect and treat. It has been shown, for instance, that patients with recurrent bacterial vaginosis have Gardnerella vaginalis–generated biofilms that house the bacteria and keep it from being adequately penetrated by white blood cells or antibiotics.
Ongoing research is looking for agents to break down biofilms so that antibiotics can reach the infectious organisms embedded within them. At the current time, we do not have any tools available, other than the benefit of understanding how biofilms form, work, and can be prevented. Biofilms can form on a variety of surfaces, synthetic or natural, but clearly, permanent synthetic meshes are more likely to house biofilms than are biologic meshes.
In any case, every patient undergoing POP repair – any surgery, for that matter – should be evaluated prior to the procedure to determine if she is at higher risk of infection. The patient’s vaginal microflora should be evaluated, and conditions such as bacterial vaginosis or aerobic vaginitis should be treated presurgically to reduce her risk of postoperative infection.
Infections rates for POP surgery are not published, to my knowledge, but there is reason to believe the rate is substantive (the infection rate associated with hysterectomy, depending on the population, is 5%-9%, and we do know that most postsurgical pelvic infections are derived from the vaginal microflora.
I also advocate checking the vaginal pH in the operating room before the vagina is prepped. In my surgeries, if the pH is 4.5 or lower, a standard regimen for antibiotic prophylaxis (1-2 g cefazolin) is administered. If the pH is greater than 4.5, then 500 mg metronidazole is added to this standard regimen. This covers pathogenic obligate anaerobes, whose growth is favored in an environment with a higher pH. Antibiotic prophylaxis should be administered one time only.
It is important to recognize as early as possible the patient who is developing an infection or has an infection. There are no definitive signs of developing or early infection. Therefore, any patient who develops postoperative fever (101°F or higher) and has a pulse rate of 100 or higher and an elevated WBC count should be evaluated (physical examination including a pelvic exam). It is important to rule out infection involving the respiratory system, urinary tract, and pelvis.
If there is no evidence of infection, further observation is acceptable. If there is strong suspicion of infection, further evaluation is warranted (ultrasound or CT scan) and broad-spectrum antibiotics should be administered. The patient should be evaluated daily to determine the response to treatment.
Contrary to popular belief, some infections (such as group A streptococcus, group B streptococcus, Escherichia coli) can set in early, within 24-48 hours after surgery.
My experience with biologics
Pelvic reconstructive surgeons became interested in biologic material because both animal studies and clinical experience in other surgical areas, such as hernia repair, have demonstrated a high degree of neovascularization and reepithelialization at the implantation area. When non–cross-linked biologic material is implanted onto or near fascia, new fascia is generated. When it is placed at the site of skin dissection, skin is regenerated. Over 3-6 months, the graft materials break down and are excreted from the body. The risks of complications and infection with non–cross-linked biologic meshes are low in comparison with the synthetic nonabsorbable meshes.
Biologic materials that are cross linked, or treated in an effort to improve strength and durability, tend to have inadequate elasticity and are not porous enough for adequate transmission of white blood cells and macrophages. Cross-linked biologics also may become encapsulated, which makes them more permanent and prone to erosion and other problems seen with the synthetic graft materials.
The non–cross-linked biologics are porous and do not present a barrier to white blood cells and macrophages. At least one of the available biologics in this category has an active antimicrobial component.
Most importantly, the non–cross-linked biologic materials give us much of what we are trying to achieve with augmentation, which is not to have a permanent prosthetic device but rather an extracellular matrix that acts like a cluster of stem cells, stimulating the body to regenerate tissue at the site of implantation.
I have used biologic mesh in approximately 200 surgeries over the past 5 years; many of these surgeries have been POP repairs. My success rate in terms of anatomic outcome and symptom resolution (anecdotally, per nonrandomized evaluation) has been about 85%. I have not hesitated to place biologic mesh concomitantly in both the anterior and posterior compartments, and I have used it to lengthen the vagina. I have not seen any infection or any rejection or allergic issues, and there have been no cases of erosion/exposure.
On occasion, a suture migrates through the vaginal epithelium and creates chronic discharge and/or pain. When the granulation tissue and the suture are both removed, the patient’s symptoms resolve. I have had two patients in whom the suture line has spontaneously opened along the anterior-posterior wall. In both patients, the vaginal discharge resolved once the tissue reepithelialized in 6-8 weeks.
Many patients today tell me immediately in their initial visit that they do not want mesh. It takes some time and thorough explanation to help each patient understand that adverse outcomes are associated mainly with the synthetic meshes, and that biologic materials are worth considering.
Dr. Faro is professor and vice chairman of ob.gyn. at Lyndon Baines Johnson Hospital and professor of obstetrics, gynecology, and reproductive sciences at the University of Texas, both in Houston. He has led infectious disease sections at Baylor College of Medicine and Louisiana State University, is a past president of the Infectious Diseases Society for Obstetrics and Gynecology, has published on postoperative and other infections, and has otherwise been an expert and leader in this realm of gynecologic care. *Dr. Faro is a scientific advisor for the research arm of Medical Diagnostic Laboratories in Hamilton, N.J.
*CORRECTION (11/25/13): A previous version of this story reported an incorrect financial disclosure of Dr. Sebastian Faro. This article has been updated.
Adverse events associated with the use of synthetic mesh in pelvic organ prolapse (POP) reparative surgery – and the Food and Drug Administration’s safety communication in 2011 warning physicians and patients about transvaginal placement of mesh for the repair of POP and stress urinary incontinence – have had a significant impact on gynecologic surgery.
Many physicians have become reluctant to use synthetic mesh products because of the risks of mesh erosion, exposure, pain and dyspareunia, and litigation. Patients also are concerned or even alarmed by reported risks. Moreover, some manufacturers have become increasingly concerned with the production of mesh products for POP repair, lessening the availability of synthetic mesh, and possibly some biologic mesh as well, for POP reparative surgery. Overall, the future of mesh augmentation in POP repair is uncertain.
Vaginal mesh procedures had surged in the decade prior to 2011 despite the lack of good randomized studies to determine whether mesh augmentation was truly efficacious. And unfortunately, the FDA has grouped synthetic and biologic material together in its reviews and notices of mesh products for POP repair. Not classifying and investigating them separately can mislead patients and hinder the development of randomized controlled trials needed to determine if augmentation with biologic material is truly superior to traditional POP repair using native tissue.
Comparing biologic mesh products to synthetic materials for pelvic organ prolapse repairs is like comparing apples to oranges: Synthetic mesh is permanent, while most biologics break down and remain in the body no longer than 6 months. From the standpoint of complications, this gives biologic materials an advantage. In my practice, biologic grafts have not eroded or caused pain, dyspareunia, or postoperative infections in any of my patients who have had surgical repair for POP.
There remains a real need for augmentation of weakened collagen tissue in the repair of POP. The native tissue in these patients is faulty tissue. Without reinforcement of defective tissue, we cannot expect excellent repairs. Data from nonrandomized studies have borne this out. When I talk with my patients about the options for surgical correction of POP, I tell them that success rates without the use of any augmentation are low, and that only about 60% of patients achieve a satisfactory result with traditional repairs.
Increasingly, I and other gynecologic surgeons are having success with biologic materials in our POP repairs. Efficacy needs to be measured in well-controlled randomized clinical trials, but at this point it appears anecdotally and from nonrandomized case reports that we can achieve good anatomic outcomes – upwards of 80% success rates – with biologic grafts, without the complications of synthetic mesh.
A recent survey of members of the American Urogynecologic Society shows that the use of synthetic mesh in transvaginal POP surgery decreased significantly after the 2011 FDA safety update, while there was no significant change in the use of biologic graft for POP (Female Pelvic Med. Reconstr. Surg 2013;19:191-8). It may be that the tide will shift toward greater use of biologic grafts. At the least, gynecologic surgeons should appreciate the differences between the two types of materials.
In the meantime, transvaginal placement of synthetic mesh should be used carefully and sparingly, with proper attention paid to patient selection and technique to reduce as much as possible the risk of erosion and pain.
In either case, more attention should be paid to the prevention of postoperative infections. Postoperative infection is an underappreciated risk with pelvic reconstructive surgery overall, and the use of synthetic mesh significantly increases this risk. Biologics are safer from an infection standpoint, but proper prevention – including evaluating each patient’s vaginal microflora immediately preoperatively and treating patients accordingly – is important for any surgery.
My advice on synthetics
Selecting patients for POP repair with no mesh, biologic mesh, or synthetic mesh requires thorough patient counseling. This is best done over multiple visits, with the patient reviewing information and coming back for a subsequent visit 1-2 weeks later with questions. She must be prepared psychologically and have realistic expectations.
In its 2011 safety communication, the FDA stated that the main role for mesh with POP repair is in the anterior compartment, and that traditional apical or posterior repair with mesh does not appear to provide any added benefit, compared with traditional surgery without mesh. Rectocele repair should not preclude the use of mesh, however, especially when a prior nonmesh repair has failed. For a patient with a small rectocele, I would advise repair with native tissue, and a second surgery with augmentation if the initial repair fails.
Synthetic mesh should be reserved for patients who have had multiple failures with traditional repairs and who are not sexually active. Sexual behavior is key to the decision-making process I undertake with my patients because synthetic mesh can cause a loss of elasticity in the vagina and consequent dyspareunia. When synthetic meshes are selected, the surgeon should use a minimal amount of material to cover as small an area as possible. It should not be used concomitantly in both the anterior and posterior compartments, as the risk of mesh contraction, rigidity, and vaginal shrinkage is too great.
Incisions in a mesh-augmented anterior or posterior repair should be 3-4 mm thick, passing through the full thickness of the vagina. Posterior compartment incisions should be kept as small in length as possible to reduce the risk of erosion/exposure and hematoma. In the anterior compartment, for similar reasons, surgeons are increasingly moving toward using small semilunar incisions.
In addition to the well-reported risks of erosion, exposure, and extrusion, synthetic meshes pose a problem from an infection point of view. Not uncommonly, synthetic grafts are found upon removal to be covered with biofilms – matrices produced by bacteria or fungi that colonize the material and house the organisms. Biofilm formation can lead to both acute, significant infection and long-term chronic infection; it also can result in metastatic infection if the biofilm breaks off, fragments, and is transported to other areas of the body.
Minimizing infection risk
Biofilms have been known and studied for some time, but there is growing appreciation for the role they play in infections that are chronic, recurrent, or hard to detect and treat. It has been shown, for instance, that patients with recurrent bacterial vaginosis have Gardnerella vaginalis–generated biofilms that house the bacteria and keep it from being adequately penetrated by white blood cells or antibiotics.
Ongoing research is looking for agents to break down biofilms so that antibiotics can reach the infectious organisms embedded within them. At the current time, we do not have any tools available, other than the benefit of understanding how biofilms form, work, and can be prevented. Biofilms can form on a variety of surfaces, synthetic or natural, but clearly, permanent synthetic meshes are more likely to house biofilms than are biologic meshes.
In any case, every patient undergoing POP repair – any surgery, for that matter – should be evaluated prior to the procedure to determine if she is at higher risk of infection. The patient’s vaginal microflora should be evaluated, and conditions such as bacterial vaginosis or aerobic vaginitis should be treated presurgically to reduce her risk of postoperative infection.
Infections rates for POP surgery are not published, to my knowledge, but there is reason to believe the rate is substantive (the infection rate associated with hysterectomy, depending on the population, is 5%-9%, and we do know that most postsurgical pelvic infections are derived from the vaginal microflora.
I also advocate checking the vaginal pH in the operating room before the vagina is prepped. In my surgeries, if the pH is 4.5 or lower, a standard regimen for antibiotic prophylaxis (1-2 g cefazolin) is administered. If the pH is greater than 4.5, then 500 mg metronidazole is added to this standard regimen. This covers pathogenic obligate anaerobes, whose growth is favored in an environment with a higher pH. Antibiotic prophylaxis should be administered one time only.
It is important to recognize as early as possible the patient who is developing an infection or has an infection. There are no definitive signs of developing or early infection. Therefore, any patient who develops postoperative fever (101°F or higher) and has a pulse rate of 100 or higher and an elevated WBC count should be evaluated (physical examination including a pelvic exam). It is important to rule out infection involving the respiratory system, urinary tract, and pelvis.
If there is no evidence of infection, further observation is acceptable. If there is strong suspicion of infection, further evaluation is warranted (ultrasound or CT scan) and broad-spectrum antibiotics should be administered. The patient should be evaluated daily to determine the response to treatment.
Contrary to popular belief, some infections (such as group A streptococcus, group B streptococcus, Escherichia coli) can set in early, within 24-48 hours after surgery.
My experience with biologics
Pelvic reconstructive surgeons became interested in biologic material because both animal studies and clinical experience in other surgical areas, such as hernia repair, have demonstrated a high degree of neovascularization and reepithelialization at the implantation area. When non–cross-linked biologic material is implanted onto or near fascia, new fascia is generated. When it is placed at the site of skin dissection, skin is regenerated. Over 3-6 months, the graft materials break down and are excreted from the body. The risks of complications and infection with non–cross-linked biologic meshes are low in comparison with the synthetic nonabsorbable meshes.
Biologic materials that are cross linked, or treated in an effort to improve strength and durability, tend to have inadequate elasticity and are not porous enough for adequate transmission of white blood cells and macrophages. Cross-linked biologics also may become encapsulated, which makes them more permanent and prone to erosion and other problems seen with the synthetic graft materials.
The non–cross-linked biologics are porous and do not present a barrier to white blood cells and macrophages. At least one of the available biologics in this category has an active antimicrobial component.
Most importantly, the non–cross-linked biologic materials give us much of what we are trying to achieve with augmentation, which is not to have a permanent prosthetic device but rather an extracellular matrix that acts like a cluster of stem cells, stimulating the body to regenerate tissue at the site of implantation.
I have used biologic mesh in approximately 200 surgeries over the past 5 years; many of these surgeries have been POP repairs. My success rate in terms of anatomic outcome and symptom resolution (anecdotally, per nonrandomized evaluation) has been about 85%. I have not hesitated to place biologic mesh concomitantly in both the anterior and posterior compartments, and I have used it to lengthen the vagina. I have not seen any infection or any rejection or allergic issues, and there have been no cases of erosion/exposure.
On occasion, a suture migrates through the vaginal epithelium and creates chronic discharge and/or pain. When the granulation tissue and the suture are both removed, the patient’s symptoms resolve. I have had two patients in whom the suture line has spontaneously opened along the anterior-posterior wall. In both patients, the vaginal discharge resolved once the tissue reepithelialized in 6-8 weeks.
Many patients today tell me immediately in their initial visit that they do not want mesh. It takes some time and thorough explanation to help each patient understand that adverse outcomes are associated mainly with the synthetic meshes, and that biologic materials are worth considering.
Dr. Faro is professor and vice chairman of ob.gyn. at Lyndon Baines Johnson Hospital and professor of obstetrics, gynecology, and reproductive sciences at the University of Texas, both in Houston. He has led infectious disease sections at Baylor College of Medicine and Louisiana State University, is a past president of the Infectious Diseases Society for Obstetrics and Gynecology, has published on postoperative and other infections, and has otherwise been an expert and leader in this realm of gynecologic care. *Dr. Faro is a scientific advisor for the research arm of Medical Diagnostic Laboratories in Hamilton, N.J.
*CORRECTION (11/25/13): A previous version of this story reported an incorrect financial disclosure of Dr. Sebastian Faro. This article has been updated.
Adverse events associated with the use of synthetic mesh in pelvic organ prolapse (POP) reparative surgery – and the Food and Drug Administration’s safety communication in 2011 warning physicians and patients about transvaginal placement of mesh for the repair of POP and stress urinary incontinence – have had a significant impact on gynecologic surgery.
Many physicians have become reluctant to use synthetic mesh products because of the risks of mesh erosion, exposure, pain and dyspareunia, and litigation. Patients also are concerned or even alarmed by reported risks. Moreover, some manufacturers have become increasingly concerned with the production of mesh products for POP repair, lessening the availability of synthetic mesh, and possibly some biologic mesh as well, for POP reparative surgery. Overall, the future of mesh augmentation in POP repair is uncertain.
Vaginal mesh procedures had surged in the decade prior to 2011 despite the lack of good randomized studies to determine whether mesh augmentation was truly efficacious. And unfortunately, the FDA has grouped synthetic and biologic material together in its reviews and notices of mesh products for POP repair. Not classifying and investigating them separately can mislead patients and hinder the development of randomized controlled trials needed to determine if augmentation with biologic material is truly superior to traditional POP repair using native tissue.
Comparing biologic mesh products to synthetic materials for pelvic organ prolapse repairs is like comparing apples to oranges: Synthetic mesh is permanent, while most biologics break down and remain in the body no longer than 6 months. From the standpoint of complications, this gives biologic materials an advantage. In my practice, biologic grafts have not eroded or caused pain, dyspareunia, or postoperative infections in any of my patients who have had surgical repair for POP.
There remains a real need for augmentation of weakened collagen tissue in the repair of POP. The native tissue in these patients is faulty tissue. Without reinforcement of defective tissue, we cannot expect excellent repairs. Data from nonrandomized studies have borne this out. When I talk with my patients about the options for surgical correction of POP, I tell them that success rates without the use of any augmentation are low, and that only about 60% of patients achieve a satisfactory result with traditional repairs.
Increasingly, I and other gynecologic surgeons are having success with biologic materials in our POP repairs. Efficacy needs to be measured in well-controlled randomized clinical trials, but at this point it appears anecdotally and from nonrandomized case reports that we can achieve good anatomic outcomes – upwards of 80% success rates – with biologic grafts, without the complications of synthetic mesh.
A recent survey of members of the American Urogynecologic Society shows that the use of synthetic mesh in transvaginal POP surgery decreased significantly after the 2011 FDA safety update, while there was no significant change in the use of biologic graft for POP (Female Pelvic Med. Reconstr. Surg 2013;19:191-8). It may be that the tide will shift toward greater use of biologic grafts. At the least, gynecologic surgeons should appreciate the differences between the two types of materials.
In the meantime, transvaginal placement of synthetic mesh should be used carefully and sparingly, with proper attention paid to patient selection and technique to reduce as much as possible the risk of erosion and pain.
In either case, more attention should be paid to the prevention of postoperative infections. Postoperative infection is an underappreciated risk with pelvic reconstructive surgery overall, and the use of synthetic mesh significantly increases this risk. Biologics are safer from an infection standpoint, but proper prevention – including evaluating each patient’s vaginal microflora immediately preoperatively and treating patients accordingly – is important for any surgery.
My advice on synthetics
Selecting patients for POP repair with no mesh, biologic mesh, or synthetic mesh requires thorough patient counseling. This is best done over multiple visits, with the patient reviewing information and coming back for a subsequent visit 1-2 weeks later with questions. She must be prepared psychologically and have realistic expectations.
In its 2011 safety communication, the FDA stated that the main role for mesh with POP repair is in the anterior compartment, and that traditional apical or posterior repair with mesh does not appear to provide any added benefit, compared with traditional surgery without mesh. Rectocele repair should not preclude the use of mesh, however, especially when a prior nonmesh repair has failed. For a patient with a small rectocele, I would advise repair with native tissue, and a second surgery with augmentation if the initial repair fails.
Synthetic mesh should be reserved for patients who have had multiple failures with traditional repairs and who are not sexually active. Sexual behavior is key to the decision-making process I undertake with my patients because synthetic mesh can cause a loss of elasticity in the vagina and consequent dyspareunia. When synthetic meshes are selected, the surgeon should use a minimal amount of material to cover as small an area as possible. It should not be used concomitantly in both the anterior and posterior compartments, as the risk of mesh contraction, rigidity, and vaginal shrinkage is too great.
Incisions in a mesh-augmented anterior or posterior repair should be 3-4 mm thick, passing through the full thickness of the vagina. Posterior compartment incisions should be kept as small in length as possible to reduce the risk of erosion/exposure and hematoma. In the anterior compartment, for similar reasons, surgeons are increasingly moving toward using small semilunar incisions.
In addition to the well-reported risks of erosion, exposure, and extrusion, synthetic meshes pose a problem from an infection point of view. Not uncommonly, synthetic grafts are found upon removal to be covered with biofilms – matrices produced by bacteria or fungi that colonize the material and house the organisms. Biofilm formation can lead to both acute, significant infection and long-term chronic infection; it also can result in metastatic infection if the biofilm breaks off, fragments, and is transported to other areas of the body.
Minimizing infection risk
Biofilms have been known and studied for some time, but there is growing appreciation for the role they play in infections that are chronic, recurrent, or hard to detect and treat. It has been shown, for instance, that patients with recurrent bacterial vaginosis have Gardnerella vaginalis–generated biofilms that house the bacteria and keep it from being adequately penetrated by white blood cells or antibiotics.
Ongoing research is looking for agents to break down biofilms so that antibiotics can reach the infectious organisms embedded within them. At the current time, we do not have any tools available, other than the benefit of understanding how biofilms form, work, and can be prevented. Biofilms can form on a variety of surfaces, synthetic or natural, but clearly, permanent synthetic meshes are more likely to house biofilms than are biologic meshes.
In any case, every patient undergoing POP repair – any surgery, for that matter – should be evaluated prior to the procedure to determine if she is at higher risk of infection. The patient’s vaginal microflora should be evaluated, and conditions such as bacterial vaginosis or aerobic vaginitis should be treated presurgically to reduce her risk of postoperative infection.
Infections rates for POP surgery are not published, to my knowledge, but there is reason to believe the rate is substantive (the infection rate associated with hysterectomy, depending on the population, is 5%-9%, and we do know that most postsurgical pelvic infections are derived from the vaginal microflora.
I also advocate checking the vaginal pH in the operating room before the vagina is prepped. In my surgeries, if the pH is 4.5 or lower, a standard regimen for antibiotic prophylaxis (1-2 g cefazolin) is administered. If the pH is greater than 4.5, then 500 mg metronidazole is added to this standard regimen. This covers pathogenic obligate anaerobes, whose growth is favored in an environment with a higher pH. Antibiotic prophylaxis should be administered one time only.
It is important to recognize as early as possible the patient who is developing an infection or has an infection. There are no definitive signs of developing or early infection. Therefore, any patient who develops postoperative fever (101°F or higher) and has a pulse rate of 100 or higher and an elevated WBC count should be evaluated (physical examination including a pelvic exam). It is important to rule out infection involving the respiratory system, urinary tract, and pelvis.
If there is no evidence of infection, further observation is acceptable. If there is strong suspicion of infection, further evaluation is warranted (ultrasound or CT scan) and broad-spectrum antibiotics should be administered. The patient should be evaluated daily to determine the response to treatment.
Contrary to popular belief, some infections (such as group A streptococcus, group B streptococcus, Escherichia coli) can set in early, within 24-48 hours after surgery.
My experience with biologics
Pelvic reconstructive surgeons became interested in biologic material because both animal studies and clinical experience in other surgical areas, such as hernia repair, have demonstrated a high degree of neovascularization and reepithelialization at the implantation area. When non–cross-linked biologic material is implanted onto or near fascia, new fascia is generated. When it is placed at the site of skin dissection, skin is regenerated. Over 3-6 months, the graft materials break down and are excreted from the body. The risks of complications and infection with non–cross-linked biologic meshes are low in comparison with the synthetic nonabsorbable meshes.
Biologic materials that are cross linked, or treated in an effort to improve strength and durability, tend to have inadequate elasticity and are not porous enough for adequate transmission of white blood cells and macrophages. Cross-linked biologics also may become encapsulated, which makes them more permanent and prone to erosion and other problems seen with the synthetic graft materials.
The non–cross-linked biologics are porous and do not present a barrier to white blood cells and macrophages. At least one of the available biologics in this category has an active antimicrobial component.
Most importantly, the non–cross-linked biologic materials give us much of what we are trying to achieve with augmentation, which is not to have a permanent prosthetic device but rather an extracellular matrix that acts like a cluster of stem cells, stimulating the body to regenerate tissue at the site of implantation.
I have used biologic mesh in approximately 200 surgeries over the past 5 years; many of these surgeries have been POP repairs. My success rate in terms of anatomic outcome and symptom resolution (anecdotally, per nonrandomized evaluation) has been about 85%. I have not hesitated to place biologic mesh concomitantly in both the anterior and posterior compartments, and I have used it to lengthen the vagina. I have not seen any infection or any rejection or allergic issues, and there have been no cases of erosion/exposure.
On occasion, a suture migrates through the vaginal epithelium and creates chronic discharge and/or pain. When the granulation tissue and the suture are both removed, the patient’s symptoms resolve. I have had two patients in whom the suture line has spontaneously opened along the anterior-posterior wall. In both patients, the vaginal discharge resolved once the tissue reepithelialized in 6-8 weeks.
Many patients today tell me immediately in their initial visit that they do not want mesh. It takes some time and thorough explanation to help each patient understand that adverse outcomes are associated mainly with the synthetic meshes, and that biologic materials are worth considering.
Dr. Faro is professor and vice chairman of ob.gyn. at Lyndon Baines Johnson Hospital and professor of obstetrics, gynecology, and reproductive sciences at the University of Texas, both in Houston. He has led infectious disease sections at Baylor College of Medicine and Louisiana State University, is a past president of the Infectious Diseases Society for Obstetrics and Gynecology, has published on postoperative and other infections, and has otherwise been an expert and leader in this realm of gynecologic care. *Dr. Faro is a scientific advisor for the research arm of Medical Diagnostic Laboratories in Hamilton, N.J.
*CORRECTION (11/25/13): A previous version of this story reported an incorrect financial disclosure of Dr. Sebastian Faro. This article has been updated.
Universal carrier screening
Screening for fetal chromosomal anomalies such as Down syndrome has become a routine part of prenatal care, but it is only possible during pregnancy. On the other hand, identifying pregnancies at risk for single-gene autosomal recessive disorders, or "Mendelian" disorders, is possible as part of preconception care.
Although Mendelian disorders are individually rare, some are more prevalent than we have thought. When considered collectively, Mendelian disorders are among the most common causes of admissions to pediatric hospitals, a significant cause of infant mortality, and a considerable public health concern.
Screening for carriers of Mendelian disorders has traditionally focused on a limited number of diseases, as determined by the parents’ ethnicity and race. For example, Tay Sachs disease has been seen primarily in the Ashkenazi Jewish community, and sickle cell disease occurs most frequently among African Americans. Until very recently, carrier screening was offered only to members of these groups.
The problem with this approach is that we have become a significantly intermixed population. There has been so much intermarriage among different racial and ethnic groups, and assimilation of different populations, that it has become increasingly difficult to discern a single ethnicity for individuals to determine who is "high risk." An increasing number of people report mixed ancestry, and many patients often prefer not to categorize themselves by race or ethnicity.
For example, an increasing number of carriers of a mutation for Tay Sachs disease do not report Ashkenazi Jewish ancestry. Similarly, the ethnic lines that have traditionally defined risk for cystic fibrosis have changed, and more widespread carrier screening has been increasingly encouraged over the past decade.
In 2011, the American College of Obstetricians and Gynecologists’ (ACOG) Committee on Genetics issued an update on carrier screening for cystic fibrosis, in which it stressed that, although cystic fibrosis is still more common among non-Hispanic white individuals and those of Ashkenazi Jewish ancestry, it "is reasonable to offer CF carrier screening to all patients" (Committee Opinion 486, Obstet. Gynecol. 2011;117:1028-31). Pan-ethnic screening for spinal muscular atrophy also has increasingly been incorporated into routine practice.
With newly available high-throughput genomic methods, we can now screen all patients, regardless of ethnicity, using a universal, pan-ethnic approach, which can identify 100-plus disease-causing mutations at a fraction of the cost of ethnic targeted screening.
Currently, the majority of carrier screening is performed as part of prenatal care; however, preconception carrier screening would allow patients and couples to consider all reproductive options. Knowledge that they carry the gene for a recessive disease, and thus have a 1-in-4 chance of having an affected child, gives parents the opportunity to consider their options before conception. These choices can include early prenatal diagnostic testing with or without pregnancy termination, use of donor gametes or election not to conceive their own child, or preimplantation genetic diagnosis (PGD), wherein a monogenetic disorder is diagnosed using only a few cells from the developing zygote.
In our practice at Columbia, we have been performing pan-ethnic screening for less than a year, and have already identified four couples at risk for having a child with severe genetic diseases. Two of these couples were both carriers for spinal muscular atrophy, and two were found to be carriers for rare genetic diseases that would not have been screened for were it not for our new pan-ethnic screening approach.
Carrier frequency
There are new options available for pan-ethnic carrier screening provided by a number of commercial companies. One of the more frequently used panels covers 417 disease-causing mutations associated with 108 recessive diseases, nearly all of which are considered to be severe, associated with progressive disease and reduced life span, or requiring significant intervention or treatment. This panel includes all of the currently recommended disorders recommended by ACOG for routine obstetrical care of individuals of Eastern European Jewish descent (i.e., Tay-Sachs disease, Canavan disease, familial dysautonomia, and cystic fibrosis) as well as the 10 disorders recommended for routine screening by the American College of Medical Genetics (ACMG).
A challenge of the expanded panels is that as many as 1 in 4 individuals who are screened will be identified as a carrier of at least one autosomal recessive disease. However, when both partners are screened, relatively few couples are found to be at risk of having an affected child. Overall, fewer than 1% of couples who take the test will turn out to be carriers of the same disease.
In a recent study of carrier frequencies, 24% of more than 23,400 individuals were found to be heterozygous for at least one non-mild condition. Approximately 5% were carriers for multiple disorders. Not surprisingly, carrier rates varied by ethnicity, ranging from 43.6% of Ashkenazi Jewish individuals to 8.5% of East Asians.
Across all ethnic groups, the most common carrier frequencies for clinically significant disorders were for cystic fibrosis, DFNB1 nonsyndromic hearing loss and deafness, spinal muscular atrophy, familial Mediterranean fever, Smith-Lemli-Opitz syndrome, sickle cell disease/beta-thalassemia, and Gaucher disease. Among the carrier states detected in the study, almost 77% and 70% were for diseases not included in ACOG carrier screening guidelines or in ACMG guidelines, respectively.
Investigators calculated that 433 of the individuals found to be carriers would not have learned their carrier status under conventional ethnicity-based screening paradigms. For example, approximately 26% of familial dysautonomia carriers in the study did not report Jewish ancestry. There also were multiple instances in the study of carrier frequencies being higher than expected for particular populations. For instance, the carrier frequency for cystic fibrosis was 1 in 40 among South Asians – a rate that is significantly higher than other reported rates (Genet. Med. 2013;15:178-186).
The study confirms a number of findings, the two most important being that there are numerous severe Mendelian conditions that are more prevalent than commonly understood, and that a significant proportion of Mendelian diseases are present in individuals outside the populations that have traditionally been characterized as high risk.
Clinical considerations
Usage of pan-ethnic expanded carrier screening is rapidly increasing, with many reproductive endocrinologists now recommending it prior to in vitro fertilization, and a growing number of obstetricians, other physicians, and patients using the tests both prenatally and in the preconception stage.
For ob.gyns., pan-ethnic screening is a significant paradigm shift which will impact our interactions with patients. For example, we will need to develop new ways of providing genetic counseling to our patients. Traditionally, we have spent significant time, prior to screening, describing each major genetic disease for which screening is performed. With the new screening paradigm, a broader, more generic consent process will be more practical – one in which we talk with patients and provide written or Web-based information about the benefits and limitations of the multidisease carrier screening panel, and then reserve in-depth discussions of specific risks and disorders for a later time, as needed.
Genetic counseling should be provided mainly on a post-test basis, and probably most often after both parents have been tested and found to be carriers. Ideally, both spouses/partners would be screened at the same time, but because most women visit their physicians by themselves, it is more practical overall to perform initial carrier screening for the woman first and then test her spouse/partner if she is found to be a carrier. Separate spouse/partner screening would require providers to contact carrier women quickly and reliably, and to obtain samples from their partners as soon as possible. The lab also should be made aware of the pre-identified carrier status of the patients before testing the spouses/partners.
A crucial message we should share with our patients during any counseling is that broader screening does not eliminate the risk of having an affected child, but reduces the risk. At present, many of the panels test only for established disease-causing mutations. There will always be patients who have a rare mutation that has not previously been described. This is particularly true as we begin screening populations not previously evaluated for Mendelian disorders. It is likely that the assortment of mutations causing specific diseases will be different in these groups, and that the residual risk of being a carrier will be higher than in well-studied populations.
With the cost for screening becoming more affordable, sequencing will become a major tool for determining carrier status of our patients. However, ob.gyns. must keep in mind that expanded screening should not substitute for obtaining a family history and referring a patient for genetic counseling when inheritable risk seems possible. Pan-ethnic screening may be able to identify patients’ risks for genetic disorders, minimizing the risk of having a child with a serious birth defect, but it has limitations. For example, not all pan-ethnic panels include analysis for premutation carriers, making it important to inquire about a history of unexplained mental retardation and autism in males, since this may stem from Fragile X syndrome.
Additionally, screening opens up the possibility of finding mutations with unknown or uncertain clinical significance. At present, when one parent is identified as a carrier of a disease-causing gene, the other can choose to have the disease-causing gene sequenced. This option should be offered to all carriers, but for many genes the additional information may be minimal and not worth the extra cost.
As we increase the frequency of carrier screening, we will identify individuals with mild forms of some diseases. For example, some mutations causing Gaucher disease are associated with a mild phenotype, and adults with these mutations may go undiagnosed for a lifetime. Patients should be made aware of this possibility and informed about the fact that some Mendelian disorders may only become evident in adulthood.
All of these issues must be carefully considered as expanded carrier screening moves into routine clinical care. The ACMG recently published a position statement on preconception and prenatal expanded carrier screening in which it listed criteria that must be met by a disorder to be included in a panel (Genet. Med. 2013;15:482-83). Presently, a work group composed of members of the obstetrical, maternal-fetal medicine, pediatric, genetics, and counseling communities is developing practice guidelines to advise the medical community on the use of expanded carrier screening panels.
Dr. Wapner, a professor in the department of obstetrics and gynecology at Columbia University Medical Center, New York, is recognized as an expert in reproductive genetics. He reported that he has no relevant disclosures.
Screening for fetal chromosomal anomalies such as Down syndrome has become a routine part of prenatal care, but it is only possible during pregnancy. On the other hand, identifying pregnancies at risk for single-gene autosomal recessive disorders, or "Mendelian" disorders, is possible as part of preconception care.
Although Mendelian disorders are individually rare, some are more prevalent than we have thought. When considered collectively, Mendelian disorders are among the most common causes of admissions to pediatric hospitals, a significant cause of infant mortality, and a considerable public health concern.
Screening for carriers of Mendelian disorders has traditionally focused on a limited number of diseases, as determined by the parents’ ethnicity and race. For example, Tay Sachs disease has been seen primarily in the Ashkenazi Jewish community, and sickle cell disease occurs most frequently among African Americans. Until very recently, carrier screening was offered only to members of these groups.
The problem with this approach is that we have become a significantly intermixed population. There has been so much intermarriage among different racial and ethnic groups, and assimilation of different populations, that it has become increasingly difficult to discern a single ethnicity for individuals to determine who is "high risk." An increasing number of people report mixed ancestry, and many patients often prefer not to categorize themselves by race or ethnicity.
For example, an increasing number of carriers of a mutation for Tay Sachs disease do not report Ashkenazi Jewish ancestry. Similarly, the ethnic lines that have traditionally defined risk for cystic fibrosis have changed, and more widespread carrier screening has been increasingly encouraged over the past decade.
In 2011, the American College of Obstetricians and Gynecologists’ (ACOG) Committee on Genetics issued an update on carrier screening for cystic fibrosis, in which it stressed that, although cystic fibrosis is still more common among non-Hispanic white individuals and those of Ashkenazi Jewish ancestry, it "is reasonable to offer CF carrier screening to all patients" (Committee Opinion 486, Obstet. Gynecol. 2011;117:1028-31). Pan-ethnic screening for spinal muscular atrophy also has increasingly been incorporated into routine practice.
With newly available high-throughput genomic methods, we can now screen all patients, regardless of ethnicity, using a universal, pan-ethnic approach, which can identify 100-plus disease-causing mutations at a fraction of the cost of ethnic targeted screening.
Currently, the majority of carrier screening is performed as part of prenatal care; however, preconception carrier screening would allow patients and couples to consider all reproductive options. Knowledge that they carry the gene for a recessive disease, and thus have a 1-in-4 chance of having an affected child, gives parents the opportunity to consider their options before conception. These choices can include early prenatal diagnostic testing with or without pregnancy termination, use of donor gametes or election not to conceive their own child, or preimplantation genetic diagnosis (PGD), wherein a monogenetic disorder is diagnosed using only a few cells from the developing zygote.
In our practice at Columbia, we have been performing pan-ethnic screening for less than a year, and have already identified four couples at risk for having a child with severe genetic diseases. Two of these couples were both carriers for spinal muscular atrophy, and two were found to be carriers for rare genetic diseases that would not have been screened for were it not for our new pan-ethnic screening approach.
Carrier frequency
There are new options available for pan-ethnic carrier screening provided by a number of commercial companies. One of the more frequently used panels covers 417 disease-causing mutations associated with 108 recessive diseases, nearly all of which are considered to be severe, associated with progressive disease and reduced life span, or requiring significant intervention or treatment. This panel includes all of the currently recommended disorders recommended by ACOG for routine obstetrical care of individuals of Eastern European Jewish descent (i.e., Tay-Sachs disease, Canavan disease, familial dysautonomia, and cystic fibrosis) as well as the 10 disorders recommended for routine screening by the American College of Medical Genetics (ACMG).
A challenge of the expanded panels is that as many as 1 in 4 individuals who are screened will be identified as a carrier of at least one autosomal recessive disease. However, when both partners are screened, relatively few couples are found to be at risk of having an affected child. Overall, fewer than 1% of couples who take the test will turn out to be carriers of the same disease.
In a recent study of carrier frequencies, 24% of more than 23,400 individuals were found to be heterozygous for at least one non-mild condition. Approximately 5% were carriers for multiple disorders. Not surprisingly, carrier rates varied by ethnicity, ranging from 43.6% of Ashkenazi Jewish individuals to 8.5% of East Asians.
Across all ethnic groups, the most common carrier frequencies for clinically significant disorders were for cystic fibrosis, DFNB1 nonsyndromic hearing loss and deafness, spinal muscular atrophy, familial Mediterranean fever, Smith-Lemli-Opitz syndrome, sickle cell disease/beta-thalassemia, and Gaucher disease. Among the carrier states detected in the study, almost 77% and 70% were for diseases not included in ACOG carrier screening guidelines or in ACMG guidelines, respectively.
Investigators calculated that 433 of the individuals found to be carriers would not have learned their carrier status under conventional ethnicity-based screening paradigms. For example, approximately 26% of familial dysautonomia carriers in the study did not report Jewish ancestry. There also were multiple instances in the study of carrier frequencies being higher than expected for particular populations. For instance, the carrier frequency for cystic fibrosis was 1 in 40 among South Asians – a rate that is significantly higher than other reported rates (Genet. Med. 2013;15:178-186).
The study confirms a number of findings, the two most important being that there are numerous severe Mendelian conditions that are more prevalent than commonly understood, and that a significant proportion of Mendelian diseases are present in individuals outside the populations that have traditionally been characterized as high risk.
Clinical considerations
Usage of pan-ethnic expanded carrier screening is rapidly increasing, with many reproductive endocrinologists now recommending it prior to in vitro fertilization, and a growing number of obstetricians, other physicians, and patients using the tests both prenatally and in the preconception stage.
For ob.gyns., pan-ethnic screening is a significant paradigm shift which will impact our interactions with patients. For example, we will need to develop new ways of providing genetic counseling to our patients. Traditionally, we have spent significant time, prior to screening, describing each major genetic disease for which screening is performed. With the new screening paradigm, a broader, more generic consent process will be more practical – one in which we talk with patients and provide written or Web-based information about the benefits and limitations of the multidisease carrier screening panel, and then reserve in-depth discussions of specific risks and disorders for a later time, as needed.
Genetic counseling should be provided mainly on a post-test basis, and probably most often after both parents have been tested and found to be carriers. Ideally, both spouses/partners would be screened at the same time, but because most women visit their physicians by themselves, it is more practical overall to perform initial carrier screening for the woman first and then test her spouse/partner if she is found to be a carrier. Separate spouse/partner screening would require providers to contact carrier women quickly and reliably, and to obtain samples from their partners as soon as possible. The lab also should be made aware of the pre-identified carrier status of the patients before testing the spouses/partners.
A crucial message we should share with our patients during any counseling is that broader screening does not eliminate the risk of having an affected child, but reduces the risk. At present, many of the panels test only for established disease-causing mutations. There will always be patients who have a rare mutation that has not previously been described. This is particularly true as we begin screening populations not previously evaluated for Mendelian disorders. It is likely that the assortment of mutations causing specific diseases will be different in these groups, and that the residual risk of being a carrier will be higher than in well-studied populations.
With the cost for screening becoming more affordable, sequencing will become a major tool for determining carrier status of our patients. However, ob.gyns. must keep in mind that expanded screening should not substitute for obtaining a family history and referring a patient for genetic counseling when inheritable risk seems possible. Pan-ethnic screening may be able to identify patients’ risks for genetic disorders, minimizing the risk of having a child with a serious birth defect, but it has limitations. For example, not all pan-ethnic panels include analysis for premutation carriers, making it important to inquire about a history of unexplained mental retardation and autism in males, since this may stem from Fragile X syndrome.
Additionally, screening opens up the possibility of finding mutations with unknown or uncertain clinical significance. At present, when one parent is identified as a carrier of a disease-causing gene, the other can choose to have the disease-causing gene sequenced. This option should be offered to all carriers, but for many genes the additional information may be minimal and not worth the extra cost.
As we increase the frequency of carrier screening, we will identify individuals with mild forms of some diseases. For example, some mutations causing Gaucher disease are associated with a mild phenotype, and adults with these mutations may go undiagnosed for a lifetime. Patients should be made aware of this possibility and informed about the fact that some Mendelian disorders may only become evident in adulthood.
All of these issues must be carefully considered as expanded carrier screening moves into routine clinical care. The ACMG recently published a position statement on preconception and prenatal expanded carrier screening in which it listed criteria that must be met by a disorder to be included in a panel (Genet. Med. 2013;15:482-83). Presently, a work group composed of members of the obstetrical, maternal-fetal medicine, pediatric, genetics, and counseling communities is developing practice guidelines to advise the medical community on the use of expanded carrier screening panels.
Dr. Wapner, a professor in the department of obstetrics and gynecology at Columbia University Medical Center, New York, is recognized as an expert in reproductive genetics. He reported that he has no relevant disclosures.
Screening for fetal chromosomal anomalies such as Down syndrome has become a routine part of prenatal care, but it is only possible during pregnancy. On the other hand, identifying pregnancies at risk for single-gene autosomal recessive disorders, or "Mendelian" disorders, is possible as part of preconception care.
Although Mendelian disorders are individually rare, some are more prevalent than we have thought. When considered collectively, Mendelian disorders are among the most common causes of admissions to pediatric hospitals, a significant cause of infant mortality, and a considerable public health concern.
Screening for carriers of Mendelian disorders has traditionally focused on a limited number of diseases, as determined by the parents’ ethnicity and race. For example, Tay Sachs disease has been seen primarily in the Ashkenazi Jewish community, and sickle cell disease occurs most frequently among African Americans. Until very recently, carrier screening was offered only to members of these groups.
The problem with this approach is that we have become a significantly intermixed population. There has been so much intermarriage among different racial and ethnic groups, and assimilation of different populations, that it has become increasingly difficult to discern a single ethnicity for individuals to determine who is "high risk." An increasing number of people report mixed ancestry, and many patients often prefer not to categorize themselves by race or ethnicity.
For example, an increasing number of carriers of a mutation for Tay Sachs disease do not report Ashkenazi Jewish ancestry. Similarly, the ethnic lines that have traditionally defined risk for cystic fibrosis have changed, and more widespread carrier screening has been increasingly encouraged over the past decade.
In 2011, the American College of Obstetricians and Gynecologists’ (ACOG) Committee on Genetics issued an update on carrier screening for cystic fibrosis, in which it stressed that, although cystic fibrosis is still more common among non-Hispanic white individuals and those of Ashkenazi Jewish ancestry, it "is reasonable to offer CF carrier screening to all patients" (Committee Opinion 486, Obstet. Gynecol. 2011;117:1028-31). Pan-ethnic screening for spinal muscular atrophy also has increasingly been incorporated into routine practice.
With newly available high-throughput genomic methods, we can now screen all patients, regardless of ethnicity, using a universal, pan-ethnic approach, which can identify 100-plus disease-causing mutations at a fraction of the cost of ethnic targeted screening.
Currently, the majority of carrier screening is performed as part of prenatal care; however, preconception carrier screening would allow patients and couples to consider all reproductive options. Knowledge that they carry the gene for a recessive disease, and thus have a 1-in-4 chance of having an affected child, gives parents the opportunity to consider their options before conception. These choices can include early prenatal diagnostic testing with or without pregnancy termination, use of donor gametes or election not to conceive their own child, or preimplantation genetic diagnosis (PGD), wherein a monogenetic disorder is diagnosed using only a few cells from the developing zygote.
In our practice at Columbia, we have been performing pan-ethnic screening for less than a year, and have already identified four couples at risk for having a child with severe genetic diseases. Two of these couples were both carriers for spinal muscular atrophy, and two were found to be carriers for rare genetic diseases that would not have been screened for were it not for our new pan-ethnic screening approach.
Carrier frequency
There are new options available for pan-ethnic carrier screening provided by a number of commercial companies. One of the more frequently used panels covers 417 disease-causing mutations associated with 108 recessive diseases, nearly all of which are considered to be severe, associated with progressive disease and reduced life span, or requiring significant intervention or treatment. This panel includes all of the currently recommended disorders recommended by ACOG for routine obstetrical care of individuals of Eastern European Jewish descent (i.e., Tay-Sachs disease, Canavan disease, familial dysautonomia, and cystic fibrosis) as well as the 10 disorders recommended for routine screening by the American College of Medical Genetics (ACMG).
A challenge of the expanded panels is that as many as 1 in 4 individuals who are screened will be identified as a carrier of at least one autosomal recessive disease. However, when both partners are screened, relatively few couples are found to be at risk of having an affected child. Overall, fewer than 1% of couples who take the test will turn out to be carriers of the same disease.
In a recent study of carrier frequencies, 24% of more than 23,400 individuals were found to be heterozygous for at least one non-mild condition. Approximately 5% were carriers for multiple disorders. Not surprisingly, carrier rates varied by ethnicity, ranging from 43.6% of Ashkenazi Jewish individuals to 8.5% of East Asians.
Across all ethnic groups, the most common carrier frequencies for clinically significant disorders were for cystic fibrosis, DFNB1 nonsyndromic hearing loss and deafness, spinal muscular atrophy, familial Mediterranean fever, Smith-Lemli-Opitz syndrome, sickle cell disease/beta-thalassemia, and Gaucher disease. Among the carrier states detected in the study, almost 77% and 70% were for diseases not included in ACOG carrier screening guidelines or in ACMG guidelines, respectively.
Investigators calculated that 433 of the individuals found to be carriers would not have learned their carrier status under conventional ethnicity-based screening paradigms. For example, approximately 26% of familial dysautonomia carriers in the study did not report Jewish ancestry. There also were multiple instances in the study of carrier frequencies being higher than expected for particular populations. For instance, the carrier frequency for cystic fibrosis was 1 in 40 among South Asians – a rate that is significantly higher than other reported rates (Genet. Med. 2013;15:178-186).
The study confirms a number of findings, the two most important being that there are numerous severe Mendelian conditions that are more prevalent than commonly understood, and that a significant proportion of Mendelian diseases are present in individuals outside the populations that have traditionally been characterized as high risk.
Clinical considerations
Usage of pan-ethnic expanded carrier screening is rapidly increasing, with many reproductive endocrinologists now recommending it prior to in vitro fertilization, and a growing number of obstetricians, other physicians, and patients using the tests both prenatally and in the preconception stage.
For ob.gyns., pan-ethnic screening is a significant paradigm shift which will impact our interactions with patients. For example, we will need to develop new ways of providing genetic counseling to our patients. Traditionally, we have spent significant time, prior to screening, describing each major genetic disease for which screening is performed. With the new screening paradigm, a broader, more generic consent process will be more practical – one in which we talk with patients and provide written or Web-based information about the benefits and limitations of the multidisease carrier screening panel, and then reserve in-depth discussions of specific risks and disorders for a later time, as needed.
Genetic counseling should be provided mainly on a post-test basis, and probably most often after both parents have been tested and found to be carriers. Ideally, both spouses/partners would be screened at the same time, but because most women visit their physicians by themselves, it is more practical overall to perform initial carrier screening for the woman first and then test her spouse/partner if she is found to be a carrier. Separate spouse/partner screening would require providers to contact carrier women quickly and reliably, and to obtain samples from their partners as soon as possible. The lab also should be made aware of the pre-identified carrier status of the patients before testing the spouses/partners.
A crucial message we should share with our patients during any counseling is that broader screening does not eliminate the risk of having an affected child, but reduces the risk. At present, many of the panels test only for established disease-causing mutations. There will always be patients who have a rare mutation that has not previously been described. This is particularly true as we begin screening populations not previously evaluated for Mendelian disorders. It is likely that the assortment of mutations causing specific diseases will be different in these groups, and that the residual risk of being a carrier will be higher than in well-studied populations.
With the cost for screening becoming more affordable, sequencing will become a major tool for determining carrier status of our patients. However, ob.gyns. must keep in mind that expanded screening should not substitute for obtaining a family history and referring a patient for genetic counseling when inheritable risk seems possible. Pan-ethnic screening may be able to identify patients’ risks for genetic disorders, minimizing the risk of having a child with a serious birth defect, but it has limitations. For example, not all pan-ethnic panels include analysis for premutation carriers, making it important to inquire about a history of unexplained mental retardation and autism in males, since this may stem from Fragile X syndrome.
Additionally, screening opens up the possibility of finding mutations with unknown or uncertain clinical significance. At present, when one parent is identified as a carrier of a disease-causing gene, the other can choose to have the disease-causing gene sequenced. This option should be offered to all carriers, but for many genes the additional information may be minimal and not worth the extra cost.
As we increase the frequency of carrier screening, we will identify individuals with mild forms of some diseases. For example, some mutations causing Gaucher disease are associated with a mild phenotype, and adults with these mutations may go undiagnosed for a lifetime. Patients should be made aware of this possibility and informed about the fact that some Mendelian disorders may only become evident in adulthood.
All of these issues must be carefully considered as expanded carrier screening moves into routine clinical care. The ACMG recently published a position statement on preconception and prenatal expanded carrier screening in which it listed criteria that must be met by a disorder to be included in a panel (Genet. Med. 2013;15:482-83). Presently, a work group composed of members of the obstetrical, maternal-fetal medicine, pediatric, genetics, and counseling communities is developing practice guidelines to advise the medical community on the use of expanded carrier screening panels.
Dr. Wapner, a professor in the department of obstetrics and gynecology at Columbia University Medical Center, New York, is recognized as an expert in reproductive genetics. He reported that he has no relevant disclosures.
The new anterior repair
For more than 100 years, gynecologic surgeons have been taught that the vaginal defects causing anterior and posterior vaginal prolapse result either from generalized midline stretching or thinning of the pubocervical fascia, or from lateral or paravaginal injuries.
The pubocervical fascia is a common surgical term for the fibromuscular coat of the vaginal epithelium. Histologically, it is indistinguishable from the deep vaginal wall and does not look like a distinct fascial layer. Clinically, however, it can be identified both abdominally and vaginally, and surgically, it can be dissected from the underlying fibromuscular tissue of the vagina in the vesicovaginal space via the vaginal approach.
A trapezoid-shaped structure of pubocervical fascia serves as a kind of hammock on which the bladder is believed to passively rest. Some believe that visceral bladder connective tissue is the supportive tissue for the bladder, but most refer to this connective tissue as pubocervical fascia – even though usage of the term is not quite anatomically correct and is not used consistently in the literature.
The true pubocervical fascia extends from the pubic bone anteriorly and laterally to the arcus tendineus fascia pelvis. Its proximal posterior edge is attached to the pericervical ring. This supportive fascia is where the defects responsible for anterior-wall prolapse have traditionally been believed to occur.
Midline plication, as well as paravaginal repair involving lateral attachment of the vagina to the arcus, have thus been the surgical approaches of choice for anterior-wall prolapse and cystocele. We have relied on these approaches despite recurrence rates of 40%-60% with traditional colporrhaphy and up to 50% with paravaginal repairs.
For many years, these two theories about the etiology of anterior vaginal-wall prolapse and cystoceles seemed to me to be in disagreement with each other when it comes to inherent mechanisms of bladder prolapse and anterior vaginal-wall prolapse. I have wondered, what really causes bladder prolapse and why has recurrence of cystocele been the Achilles’ heel of pelvic reconstructive surgery?
In the past several decades, Dr. A.C. Richardson described several defects in the pubocervical fascia that he believed were associated with cystoceles. Later on in his career and life, he taught that bladder prolapse was the result of distinct detachments or site-specific defects in the support structures from the pericervical ring.
Reconstructing the pericervical ring and its attachments, he reasoned, would restore normal anatomy and repair bladder prolapse. Dr. Richardson’s later conclusions were never published, however, and midline plication and paravaginal repairs have remained the mainstays of bladder prolapse and anterior vaginal wall prolapse.
In the meantime, a firm understanding of exactly how vaginal birth affects the supportive structure of the bladder eluded us. It has long been believed that vaginal birth trauma contributes to the likelihood of symptomatic prolapse occurring, yet there was never any proof as to how and when the stress of vaginal birth causes pubocervical fascial injury. Nor was there any proof as to the location and direction of tears. Without accurately identifying specific damage patterns, one cannot recognize true defects that need to be repaired.
With the help of biomechanical engineers and biomechanical modeling, my colleagues at Atlanta’s Emory University and I found that the superior-to-inferior direction of the sheer stress and strain caused by both fetal descent and internal rotation of the fetal head causes tears in the pubocervical fascia that run in the transverse direction at the level of and from the pericervical ring – not vertically in the midline or laterally (paravaginally) as many had theorized.
Then, during cadaver dissections and surgical repairs of bladder prolapse, we identified the bladder protruding/herniating through the separation of the pubocervical fascia from the pericervical ring.
A true cystocele, we now know, is the result of a transverse defect that separates the pubocervical fascia from the pericervical ring. A paravaginal defect, on the other hand, can cause the anterior vaginal wall to prolapse, but it is not the cause of bladder prolapse. There is an important distinction to be made between these two entities.
Our approach to bladder prolapse, therefore, requires a transverse defect repair. We have developed a surgical technique that appears to successfully correct the defect by reattaching the pubocervical fascia to its original supportive structures, the pericervical ring, and the retroperitoneal uterosacral ligaments as they insert into the sacral peritoneum.
Surgical Technique
Our vaginal surgical procedure was developed to 1) correctly identify the defect causing a cystocele and to identify the bladder protruding through the defect, and 2) repair the defect by reattaching the pubocervical fascia to its original supportive structures.
A full-thickness midline incision of the anterior vaginal epithelium is performed to enter the vesicovaginal space. The incision is carried up to the posterior urethral fold, and the vaginal epithelium is retracted laterally using a self-retaining ring retractor with hooks; this fully exposes the vesicovaginal space and the pubocervical fascia. The hooks attached to the retractor provide a level of traction and counter retraction for dissection and continual exposure of the surgical field that is hard to achieve with Allis clamps held by assistants. (We use a Lone Star ring retractor made by CooperSurgical, Trumbull, Conn.)
The pubocervical fascia is dissected and separated from the underside of the vaginal epithelium without splitting the vagina, as is done using traditional methods for midline plication. (See figure 8A.) The scissors are spread apart in the avascular lateral retroperitoneal space beneath the pubocervical fascia, and used to separate the fascia from the undersurface of the vagina to the pubic ramus. This is repeated on the other side of the dissection.
The surgeon can recognize the pubocervical fascia by its trapezoidal shape. Once the entire trapezoidal pubocervical fascia is identified, the presence of midline, paravaginal, or transverse defects can be identified and confirmed. (See figure 8B.)
In the absence of any midline or paravaginal defects with bladder protrusion or herniation, the possibility of a transverse defect can be evaluated by identifying the base of the pubocervical fascia. At this point, connective tissue adhesions are often seen between the pubocervical fascia and the prolapsed bladder and vagina. (See figure 9A.) We believe this is adhesive scar tissue that forms naturally after the trauma and mechanical damage of vaginal birth; its existence has not been truly recognized and/or well appreciated in the past.
When the adhesive scar is excised, the pubocervical fascia is freed up and can be easily separated from the bladder and vagina. (See figure 9B.) Allis clamps are placed on the lateral aspects of the pubocervical fascia and are lifted superiorly to expose the prolapsed bladder. (See figure 9C.) It is apparent at this point where the pubocervical fascia was originally attached to the pericervical ring. Correction of the bladder herniation can be demonstrated if the pubocervical fascia is elevated apically toward the pericervical ring (its previous attachment) and the retroperitoneal uterosacral ligaments. (See figure 9D.)
Permanent sutures of #0 Ethibond (Ethicon, Johnson & Johnson, Somerville, N.J.) are placed into each iliococcygeus fascia, or prespinous fascia, inferior to the ischial spine.
A suture of # 2-0 Ethibond is placed into each retroperitoneal uterosacral ligament as it attaches to the sacral periosteum at the level of S-2 or S-3 – a retroperitoneal uterosacral colpopexy. To do this, we use a pelvic reconstructive rectal pouch (American Medical Systems, Minnetonka, Minn.) that enables the index finger to be placed into the rectum for examination and palpation without contamination. The retroperitoneal uterosacral ligament is palpated at the level of the sacrum, where it attaches to the sacral periosteum, and a long Allis clamp is used to grasp the ligament. Each suture is placed from lateral to medial either through or around the ligament to avoid any entrapment of the ureter.
These sutures are held for placement into a piece of Surgisis Biodesign graft for anterior repair (Cook Medical, Bloomington, Ind.) that has been precut into a trapezoidal shape to fit the trapezoidal dissection of the pubocervical fascia.
The uterosacral sutures are placed into the superior nubbins on the lateral side of the graft and are held, and the prespinous (iliococcygeus) sutures of #0 Ethibond are placed through the graft’s lower nubbins and held. (See figure 11A.). We then place several sutures of #2-0 Ethibond into the base of the graft, then through the pubocervical fascia, and then the pericervical ring; tying these sutures repairs the transverse defect. (See figure 11B.) The lateral sutures are then tied, beginning with the prespinous sutures.
The graft is attached distally to the periurethral connective tissue with #2-0 Vicryl so that the graft lays flat against the pubocervical fascia. The bladder is now back in its normal anatomic position. (See figures 11C and 11D.)
Excess vaginal mucosa is then excised, and the vagina is closed in a running fashion with #2-0 Vicryl (Ethicon, Johnson & Johnson, Somerville, N.J.). An intraoperative cystoscopy is performed to document ureteral patency.
The Surgisis graft material is a biologic, recently Food and Drug Administration–approved graft that promotes tissue remodeling. I like using it because it safely provides more strength to the pubocervical fascia before it dissolves in 3-6 months, but the repair can be done alternatively without the graft – with sutures alone.
In our patient population of more than 500 patients followed for 24 months, we have had excellent results with 92% cure rate with sutures and 95% cure rates with Surgisis Biodesign. Some of these patients had previously failed midline plication and paravaginal repairs; the others had stage III or stage IV prolapse and had not undergone prior surgical repairs.
Surgeons who have been taught this new anterior repair have been excited and have found the technique easy to learn. However, only multicenter studies, currently in progress, and time will tell if this new technique will replace traditional anterior colporrhaphy.
Dr. Kovac is emeritus distinguished professor of gynecologic surgery at Emory University, Atlanta. He said he has no disclosures. To view a video of the surgery, visit SurgeryU at www.aagl.org/obgyn-news.
For more than 100 years, gynecologic surgeons have been taught that the vaginal defects causing anterior and posterior vaginal prolapse result either from generalized midline stretching or thinning of the pubocervical fascia, or from lateral or paravaginal injuries.
The pubocervical fascia is a common surgical term for the fibromuscular coat of the vaginal epithelium. Histologically, it is indistinguishable from the deep vaginal wall and does not look like a distinct fascial layer. Clinically, however, it can be identified both abdominally and vaginally, and surgically, it can be dissected from the underlying fibromuscular tissue of the vagina in the vesicovaginal space via the vaginal approach.
A trapezoid-shaped structure of pubocervical fascia serves as a kind of hammock on which the bladder is believed to passively rest. Some believe that visceral bladder connective tissue is the supportive tissue for the bladder, but most refer to this connective tissue as pubocervical fascia – even though usage of the term is not quite anatomically correct and is not used consistently in the literature.
The true pubocervical fascia extends from the pubic bone anteriorly and laterally to the arcus tendineus fascia pelvis. Its proximal posterior edge is attached to the pericervical ring. This supportive fascia is where the defects responsible for anterior-wall prolapse have traditionally been believed to occur.
Midline plication, as well as paravaginal repair involving lateral attachment of the vagina to the arcus, have thus been the surgical approaches of choice for anterior-wall prolapse and cystocele. We have relied on these approaches despite recurrence rates of 40%-60% with traditional colporrhaphy and up to 50% with paravaginal repairs.
For many years, these two theories about the etiology of anterior vaginal-wall prolapse and cystoceles seemed to me to be in disagreement with each other when it comes to inherent mechanisms of bladder prolapse and anterior vaginal-wall prolapse. I have wondered, what really causes bladder prolapse and why has recurrence of cystocele been the Achilles’ heel of pelvic reconstructive surgery?
In the past several decades, Dr. A.C. Richardson described several defects in the pubocervical fascia that he believed were associated with cystoceles. Later on in his career and life, he taught that bladder prolapse was the result of distinct detachments or site-specific defects in the support structures from the pericervical ring.
Reconstructing the pericervical ring and its attachments, he reasoned, would restore normal anatomy and repair bladder prolapse. Dr. Richardson’s later conclusions were never published, however, and midline plication and paravaginal repairs have remained the mainstays of bladder prolapse and anterior vaginal wall prolapse.
In the meantime, a firm understanding of exactly how vaginal birth affects the supportive structure of the bladder eluded us. It has long been believed that vaginal birth trauma contributes to the likelihood of symptomatic prolapse occurring, yet there was never any proof as to how and when the stress of vaginal birth causes pubocervical fascial injury. Nor was there any proof as to the location and direction of tears. Without accurately identifying specific damage patterns, one cannot recognize true defects that need to be repaired.
With the help of biomechanical engineers and biomechanical modeling, my colleagues at Atlanta’s Emory University and I found that the superior-to-inferior direction of the sheer stress and strain caused by both fetal descent and internal rotation of the fetal head causes tears in the pubocervical fascia that run in the transverse direction at the level of and from the pericervical ring – not vertically in the midline or laterally (paravaginally) as many had theorized.
Then, during cadaver dissections and surgical repairs of bladder prolapse, we identified the bladder protruding/herniating through the separation of the pubocervical fascia from the pericervical ring.
A true cystocele, we now know, is the result of a transverse defect that separates the pubocervical fascia from the pericervical ring. A paravaginal defect, on the other hand, can cause the anterior vaginal wall to prolapse, but it is not the cause of bladder prolapse. There is an important distinction to be made between these two entities.
Our approach to bladder prolapse, therefore, requires a transverse defect repair. We have developed a surgical technique that appears to successfully correct the defect by reattaching the pubocervical fascia to its original supportive structures, the pericervical ring, and the retroperitoneal uterosacral ligaments as they insert into the sacral peritoneum.
Surgical Technique
Our vaginal surgical procedure was developed to 1) correctly identify the defect causing a cystocele and to identify the bladder protruding through the defect, and 2) repair the defect by reattaching the pubocervical fascia to its original supportive structures.
A full-thickness midline incision of the anterior vaginal epithelium is performed to enter the vesicovaginal space. The incision is carried up to the posterior urethral fold, and the vaginal epithelium is retracted laterally using a self-retaining ring retractor with hooks; this fully exposes the vesicovaginal space and the pubocervical fascia. The hooks attached to the retractor provide a level of traction and counter retraction for dissection and continual exposure of the surgical field that is hard to achieve with Allis clamps held by assistants. (We use a Lone Star ring retractor made by CooperSurgical, Trumbull, Conn.)
The pubocervical fascia is dissected and separated from the underside of the vaginal epithelium without splitting the vagina, as is done using traditional methods for midline plication. (See figure 8A.) The scissors are spread apart in the avascular lateral retroperitoneal space beneath the pubocervical fascia, and used to separate the fascia from the undersurface of the vagina to the pubic ramus. This is repeated on the other side of the dissection.
The surgeon can recognize the pubocervical fascia by its trapezoidal shape. Once the entire trapezoidal pubocervical fascia is identified, the presence of midline, paravaginal, or transverse defects can be identified and confirmed. (See figure 8B.)
In the absence of any midline or paravaginal defects with bladder protrusion or herniation, the possibility of a transverse defect can be evaluated by identifying the base of the pubocervical fascia. At this point, connective tissue adhesions are often seen between the pubocervical fascia and the prolapsed bladder and vagina. (See figure 9A.) We believe this is adhesive scar tissue that forms naturally after the trauma and mechanical damage of vaginal birth; its existence has not been truly recognized and/or well appreciated in the past.
When the adhesive scar is excised, the pubocervical fascia is freed up and can be easily separated from the bladder and vagina. (See figure 9B.) Allis clamps are placed on the lateral aspects of the pubocervical fascia and are lifted superiorly to expose the prolapsed bladder. (See figure 9C.) It is apparent at this point where the pubocervical fascia was originally attached to the pericervical ring. Correction of the bladder herniation can be demonstrated if the pubocervical fascia is elevated apically toward the pericervical ring (its previous attachment) and the retroperitoneal uterosacral ligaments. (See figure 9D.)
Permanent sutures of #0 Ethibond (Ethicon, Johnson & Johnson, Somerville, N.J.) are placed into each iliococcygeus fascia, or prespinous fascia, inferior to the ischial spine.
A suture of # 2-0 Ethibond is placed into each retroperitoneal uterosacral ligament as it attaches to the sacral periosteum at the level of S-2 or S-3 – a retroperitoneal uterosacral colpopexy. To do this, we use a pelvic reconstructive rectal pouch (American Medical Systems, Minnetonka, Minn.) that enables the index finger to be placed into the rectum for examination and palpation without contamination. The retroperitoneal uterosacral ligament is palpated at the level of the sacrum, where it attaches to the sacral periosteum, and a long Allis clamp is used to grasp the ligament. Each suture is placed from lateral to medial either through or around the ligament to avoid any entrapment of the ureter.
These sutures are held for placement into a piece of Surgisis Biodesign graft for anterior repair (Cook Medical, Bloomington, Ind.) that has been precut into a trapezoidal shape to fit the trapezoidal dissection of the pubocervical fascia.
The uterosacral sutures are placed into the superior nubbins on the lateral side of the graft and are held, and the prespinous (iliococcygeus) sutures of #0 Ethibond are placed through the graft’s lower nubbins and held. (See figure 11A.). We then place several sutures of #2-0 Ethibond into the base of the graft, then through the pubocervical fascia, and then the pericervical ring; tying these sutures repairs the transverse defect. (See figure 11B.) The lateral sutures are then tied, beginning with the prespinous sutures.
The graft is attached distally to the periurethral connective tissue with #2-0 Vicryl so that the graft lays flat against the pubocervical fascia. The bladder is now back in its normal anatomic position. (See figures 11C and 11D.)
Excess vaginal mucosa is then excised, and the vagina is closed in a running fashion with #2-0 Vicryl (Ethicon, Johnson & Johnson, Somerville, N.J.). An intraoperative cystoscopy is performed to document ureteral patency.
The Surgisis graft material is a biologic, recently Food and Drug Administration–approved graft that promotes tissue remodeling. I like using it because it safely provides more strength to the pubocervical fascia before it dissolves in 3-6 months, but the repair can be done alternatively without the graft – with sutures alone.
In our patient population of more than 500 patients followed for 24 months, we have had excellent results with 92% cure rate with sutures and 95% cure rates with Surgisis Biodesign. Some of these patients had previously failed midline plication and paravaginal repairs; the others had stage III or stage IV prolapse and had not undergone prior surgical repairs.
Surgeons who have been taught this new anterior repair have been excited and have found the technique easy to learn. However, only multicenter studies, currently in progress, and time will tell if this new technique will replace traditional anterior colporrhaphy.
Dr. Kovac is emeritus distinguished professor of gynecologic surgery at Emory University, Atlanta. He said he has no disclosures. To view a video of the surgery, visit SurgeryU at www.aagl.org/obgyn-news.
For more than 100 years, gynecologic surgeons have been taught that the vaginal defects causing anterior and posterior vaginal prolapse result either from generalized midline stretching or thinning of the pubocervical fascia, or from lateral or paravaginal injuries.
The pubocervical fascia is a common surgical term for the fibromuscular coat of the vaginal epithelium. Histologically, it is indistinguishable from the deep vaginal wall and does not look like a distinct fascial layer. Clinically, however, it can be identified both abdominally and vaginally, and surgically, it can be dissected from the underlying fibromuscular tissue of the vagina in the vesicovaginal space via the vaginal approach.
A trapezoid-shaped structure of pubocervical fascia serves as a kind of hammock on which the bladder is believed to passively rest. Some believe that visceral bladder connective tissue is the supportive tissue for the bladder, but most refer to this connective tissue as pubocervical fascia – even though usage of the term is not quite anatomically correct and is not used consistently in the literature.
The true pubocervical fascia extends from the pubic bone anteriorly and laterally to the arcus tendineus fascia pelvis. Its proximal posterior edge is attached to the pericervical ring. This supportive fascia is where the defects responsible for anterior-wall prolapse have traditionally been believed to occur.
Midline plication, as well as paravaginal repair involving lateral attachment of the vagina to the arcus, have thus been the surgical approaches of choice for anterior-wall prolapse and cystocele. We have relied on these approaches despite recurrence rates of 40%-60% with traditional colporrhaphy and up to 50% with paravaginal repairs.
For many years, these two theories about the etiology of anterior vaginal-wall prolapse and cystoceles seemed to me to be in disagreement with each other when it comes to inherent mechanisms of bladder prolapse and anterior vaginal-wall prolapse. I have wondered, what really causes bladder prolapse and why has recurrence of cystocele been the Achilles’ heel of pelvic reconstructive surgery?
In the past several decades, Dr. A.C. Richardson described several defects in the pubocervical fascia that he believed were associated with cystoceles. Later on in his career and life, he taught that bladder prolapse was the result of distinct detachments or site-specific defects in the support structures from the pericervical ring.
Reconstructing the pericervical ring and its attachments, he reasoned, would restore normal anatomy and repair bladder prolapse. Dr. Richardson’s later conclusions were never published, however, and midline plication and paravaginal repairs have remained the mainstays of bladder prolapse and anterior vaginal wall prolapse.
In the meantime, a firm understanding of exactly how vaginal birth affects the supportive structure of the bladder eluded us. It has long been believed that vaginal birth trauma contributes to the likelihood of symptomatic prolapse occurring, yet there was never any proof as to how and when the stress of vaginal birth causes pubocervical fascial injury. Nor was there any proof as to the location and direction of tears. Without accurately identifying specific damage patterns, one cannot recognize true defects that need to be repaired.
With the help of biomechanical engineers and biomechanical modeling, my colleagues at Atlanta’s Emory University and I found that the superior-to-inferior direction of the sheer stress and strain caused by both fetal descent and internal rotation of the fetal head causes tears in the pubocervical fascia that run in the transverse direction at the level of and from the pericervical ring – not vertically in the midline or laterally (paravaginally) as many had theorized.
Then, during cadaver dissections and surgical repairs of bladder prolapse, we identified the bladder protruding/herniating through the separation of the pubocervical fascia from the pericervical ring.
A true cystocele, we now know, is the result of a transverse defect that separates the pubocervical fascia from the pericervical ring. A paravaginal defect, on the other hand, can cause the anterior vaginal wall to prolapse, but it is not the cause of bladder prolapse. There is an important distinction to be made between these two entities.
Our approach to bladder prolapse, therefore, requires a transverse defect repair. We have developed a surgical technique that appears to successfully correct the defect by reattaching the pubocervical fascia to its original supportive structures, the pericervical ring, and the retroperitoneal uterosacral ligaments as they insert into the sacral peritoneum.
Surgical Technique
Our vaginal surgical procedure was developed to 1) correctly identify the defect causing a cystocele and to identify the bladder protruding through the defect, and 2) repair the defect by reattaching the pubocervical fascia to its original supportive structures.
A full-thickness midline incision of the anterior vaginal epithelium is performed to enter the vesicovaginal space. The incision is carried up to the posterior urethral fold, and the vaginal epithelium is retracted laterally using a self-retaining ring retractor with hooks; this fully exposes the vesicovaginal space and the pubocervical fascia. The hooks attached to the retractor provide a level of traction and counter retraction for dissection and continual exposure of the surgical field that is hard to achieve with Allis clamps held by assistants. (We use a Lone Star ring retractor made by CooperSurgical, Trumbull, Conn.)
The pubocervical fascia is dissected and separated from the underside of the vaginal epithelium without splitting the vagina, as is done using traditional methods for midline plication. (See figure 8A.) The scissors are spread apart in the avascular lateral retroperitoneal space beneath the pubocervical fascia, and used to separate the fascia from the undersurface of the vagina to the pubic ramus. This is repeated on the other side of the dissection.
The surgeon can recognize the pubocervical fascia by its trapezoidal shape. Once the entire trapezoidal pubocervical fascia is identified, the presence of midline, paravaginal, or transverse defects can be identified and confirmed. (See figure 8B.)
In the absence of any midline or paravaginal defects with bladder protrusion or herniation, the possibility of a transverse defect can be evaluated by identifying the base of the pubocervical fascia. At this point, connective tissue adhesions are often seen between the pubocervical fascia and the prolapsed bladder and vagina. (See figure 9A.) We believe this is adhesive scar tissue that forms naturally after the trauma and mechanical damage of vaginal birth; its existence has not been truly recognized and/or well appreciated in the past.
When the adhesive scar is excised, the pubocervical fascia is freed up and can be easily separated from the bladder and vagina. (See figure 9B.) Allis clamps are placed on the lateral aspects of the pubocervical fascia and are lifted superiorly to expose the prolapsed bladder. (See figure 9C.) It is apparent at this point where the pubocervical fascia was originally attached to the pericervical ring. Correction of the bladder herniation can be demonstrated if the pubocervical fascia is elevated apically toward the pericervical ring (its previous attachment) and the retroperitoneal uterosacral ligaments. (See figure 9D.)
Permanent sutures of #0 Ethibond (Ethicon, Johnson & Johnson, Somerville, N.J.) are placed into each iliococcygeus fascia, or prespinous fascia, inferior to the ischial spine.
A suture of # 2-0 Ethibond is placed into each retroperitoneal uterosacral ligament as it attaches to the sacral periosteum at the level of S-2 or S-3 – a retroperitoneal uterosacral colpopexy. To do this, we use a pelvic reconstructive rectal pouch (American Medical Systems, Minnetonka, Minn.) that enables the index finger to be placed into the rectum for examination and palpation without contamination. The retroperitoneal uterosacral ligament is palpated at the level of the sacrum, where it attaches to the sacral periosteum, and a long Allis clamp is used to grasp the ligament. Each suture is placed from lateral to medial either through or around the ligament to avoid any entrapment of the ureter.
These sutures are held for placement into a piece of Surgisis Biodesign graft for anterior repair (Cook Medical, Bloomington, Ind.) that has been precut into a trapezoidal shape to fit the trapezoidal dissection of the pubocervical fascia.
The uterosacral sutures are placed into the superior nubbins on the lateral side of the graft and are held, and the prespinous (iliococcygeus) sutures of #0 Ethibond are placed through the graft’s lower nubbins and held. (See figure 11A.). We then place several sutures of #2-0 Ethibond into the base of the graft, then through the pubocervical fascia, and then the pericervical ring; tying these sutures repairs the transverse defect. (See figure 11B.) The lateral sutures are then tied, beginning with the prespinous sutures.
The graft is attached distally to the periurethral connective tissue with #2-0 Vicryl so that the graft lays flat against the pubocervical fascia. The bladder is now back in its normal anatomic position. (See figures 11C and 11D.)
Excess vaginal mucosa is then excised, and the vagina is closed in a running fashion with #2-0 Vicryl (Ethicon, Johnson & Johnson, Somerville, N.J.). An intraoperative cystoscopy is performed to document ureteral patency.
The Surgisis graft material is a biologic, recently Food and Drug Administration–approved graft that promotes tissue remodeling. I like using it because it safely provides more strength to the pubocervical fascia before it dissolves in 3-6 months, but the repair can be done alternatively without the graft – with sutures alone.
In our patient population of more than 500 patients followed for 24 months, we have had excellent results with 92% cure rate with sutures and 95% cure rates with Surgisis Biodesign. Some of these patients had previously failed midline plication and paravaginal repairs; the others had stage III or stage IV prolapse and had not undergone prior surgical repairs.
Surgeons who have been taught this new anterior repair have been excited and have found the technique easy to learn. However, only multicenter studies, currently in progress, and time will tell if this new technique will replace traditional anterior colporrhaphy.
Dr. Kovac is emeritus distinguished professor of gynecologic surgery at Emory University, Atlanta. He said he has no disclosures. To view a video of the surgery, visit SurgeryU at www.aagl.org/obgyn-news.
An immunization update
As ob.gyns., we pride ourselves on being primary care providers as well as specialists. A central obligation of the primary care physician is the prevention of disease, and immunization of vaccine-preventable diseases is an essential component of prevention. In fact, nothing is more effective at preventing infectious diseases than immunization.
Immunization has not traditionally been as central to our role as it has been for pediatricians, who have long viewed vaccines as a core component of their care. However, although there are certain vaccines that pediatricians can give more easily than we can, such as the human papillomavirus vaccine, there are other vaccines that ob.gyns. can more easily provide. For example, we are better positioned than pediatricians to protect newborns from pertussis.
No other physician, moreover, is better situated to vaccinate vulnerable populations than is the ob.gyn. We are important sources of information and advice for adolescents, adults, and pregnant women. We therefore have a critically important opportunity to identify the diseases that put our patients and their progeny at greatest risk, and a responsibility to make immunization an integral part of our practices.
Numerous investigations and reports addressing vaccine implementation strategies have relevance for both obstetric and gynecologic patients, and studies addressing successful strategies for immunization of pregnant women in particular have increased since the 2009 H1N1 influenza pandemic.
Last spring, the Committee on Obstetric Practice and Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists published guidance on how to successfully incorporate immunizations into routine care and develop an immunization culture (Committee Opinion No. 558, Obstet. Gynecol. 2013;121:897-903).
Among the key points:
• Make direct recommendations. Talk to your patients directly. Recommend individual immunizations. A provider recommendation has been shown to be one of the strongest influences on patient acceptance. Tell patients, "You should have this vaccine," or "It is important for you," or "I’m telling you as your health care specialist that this vaccine is in your best interest."
Physician scripts for several immunizations are available on ACOG’s immunization website, immunizationforwomen.org, and numerous other sources.
• Designate a vaccine coordinator. As the "vaccine champion," this person orders and receives the vaccines, ensures that the vaccines are stored properly, and has knowledge of appropriate billing codes for vaccination services. He or she also maintains contact with the state health department’s immunization program manager, who can answer physicians’ questions and help practices.
• Institute standing orders. Such orders allow for an indicated vaccine to be administered to patients without an individual physician order. For example, every pregnant woman who shows up during flu season should have a standing order for the influenza vaccination.
Evaluate your prompts, paper or electronic, to remind providers and staff which patients need to be immunized. Hold everyone accountable.
• Get yourself and your staff immunized. Educate staff about the safety and efficacy of immunizations, and ensure that your office health care providers, your entire staff, and you are immunized as recommended. If your staff or you are not immunized, it can be hard to convince patients to receive a vaccine. As ACOG’s Committee Opinion on "Integrating Immunizations into Practice" highlights, moreover, office personnel who express their own uncertainty or lack of knowledge to patients can negatively affect patients’ willingness to receive a vaccine. Additionally, being a potential source of infection for your patients violates ethical obligations.
Research has shown that educational efforts for office staff can markedly increase office immunization rates. In one study of the H1N1 influenza pandemic of 2009, educational sessions for ob.gyns’ staff were part of a multifaceted approach that led to a high vaccine acceptance rate of 76% in an ethnically diverse population of 157 obstetrics patients (Infect. Dis. Obstet. Gynecol. 2011;2011:746214 [doi: 10.1155/2011/746214]). The educational sessions for staff were instituted proactively prior to availability of the vaccine.
Influenza vaccination
Influenza affects 10%-20% of the U.S. population annually, and pregnant women are more likely to have serious complications should they contract the virus. Pregnant women are at least 4-5 times more likely to be hospitalized and equally more likely to die from infection, and their infants are more likely to have influenza-related respiratory illnesses and die.
A 2010 study of the 2009 H1N1 pandemic showed that although pregnant women in the United States represent 1%-2% of the population, they accounted for up to 7%-10% of the hospitalized patients, 6%-9% of the ICU patients, and 6%-10% of the patients who died (N. Engl. J. Med. 2010:362:1708-19).
A study published in early 2013 showed that vaccination was 70% successful in preventing 2009 H1N1 influenza infection in pregnant women in Norway during the pandemic, and that the risk of fetal death nearly doubled among women who contracted influenza (N. Engl. J. Med. 2013;368:333-40). Of almost 120,000 pregnant women in the study, approximately half had received the flu vaccine.
Studies of earlier influenza pandemics and large epidemiologic studies of otherwise healthy pregnant women who contracted nonpandemic seasonal influenza have similarly demonstrated how pregnant women and their infants disproportionately experience severe sequelae.
We need to inform our pregnant patients that the influenza vaccine protects their newborns as well as themselves. We must also work harder to dispel misconceptions about the safety of the vaccine.
One barrier to pregnant women receiving the 2009 H1N1 influenza vaccine was perceived risks to the fetus (Am. J. Obstet. Gynecol. 2011;204:S124-7). The source of much of this concern stems from the fact that some influenza vaccines contain trace amounts of the preservative thimerosal.
Influenza vaccines that contain a mercury-free preservative are available, but pregnant women should be informed that the Centers for Disease Control and Prevention (CDC), the Institute of Medicine, and numerous other health organizations have concluded that the thimerosal used in the vaccine is safe. The only flu vaccine that pregnant women should not receive is the attenuated vaccine.
An additional concern for some is that the influenza vaccine contains chicken egg protein, an allergen for some individuals. However, the CDC’s Advisory Committee on Immunization Practices now recommends that individuals who have only had hives after exposure to egg should receive the influenza vaccine, though physicians should take extra precautions, such as observing these patients for at least 30 minutes after administering the vaccine (www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
With influenza immunization, we should celebrate our successes. As described in ACOG’s Committee Opinion on integrating vaccinations, vaccination of pregnant women increased nationwide to a level of approximately 50% in 2009, a significant increase over pre-pandemic rates of approximately 15%. Rates during the 2011-2012 influenza season remained approximately 47%.
Such improvement shows that immunization is achievable in our practices. However, rates hovering around half of all pregnant women are still just slightly north of mediocre. We should continue to make the benefits of vaccination clear to staff and patients, and the algorithm for implementation simple.
Given that the flu season begins in October and can run into spring – and that it takes about 2 weeks for production of protective antibody levels – it is rare that a pregnant woman will not need the vaccine.
Full recommendations for the prevention and control of influenza in 2013-2014 were expected at the time of this writing to be published in the Morbidity and Mortality Weekly Report.
Tdap
Pertussis is highly infectious, and infants who contract the bacterium have increased rates of whooping cough attacks and are at the greatest risk for severe disease and death. Pertussis outbreaks have become common in the United States, and can be difficult to identify and manage. Infants continue to have the highest reported rates.
When immunization is an integral part of one’s office (with standing orders, etc.), administering a dose of Tdap during each pregnancy to prevent pertussis in infants – as is recommended in the CDC immunization schedule released in January 2013 – should be relatively simple during prenatal office visits.
The postpartum "cocooning" approach recommended by the CDC in 2006 and supported by ACOG has been practically and logistically difficult to implement. While the concept is sound, it has proved too cumbersome overall to vaccinate every family member and caregiver who will have close contact with an infant. Merely having the parents vaccinated immediately postpartum – the other part of cocooning – has been difficult enough.
The new recommendations draw upon the proven paradigm of maternal vaccination for newborn benefit and the relative ease of immunization during prenatal care visits. Ob.gyns. should administer a dose of Tdap during each pregnancy – optimally between 27 and 36 weeks’ gestation – irrespective of the patient’s prior history of receiving Tdap.
Infants do not start their vaccination series against these pathogens until age 2 months; maternal immunization in late pregnancy leads to high transplacental antibody transfer, which will protect infants until they receive their own vaccines.
Although the optimal timing for maternal Tdap immunization is later in pregnancy, the vaccine may be given at any time if necessitated by clinical circumstance. For example, if a woman steps on a rusty nail during her first trimester and has not had a tetanus booster in the prior 5 years, or if a local school reports an epidemic, she should receive the Tdap vaccine immediately.
Cocooning is now the default; if Tdap is not administered during pregnancy for some reason, it should be administered immediately postpartum, with as much cocooning as possible.
The challenge with the Tdap vaccine is that few people who live outside areas where pertussis epidemics have occurred know someone who has had the bacterial disease. Education and a direct recommendation for the vaccine are therefore critical.
Human papillomavirus, hepatitis B
HPV vaccines are not recommended for use in pregnant women, and although ob.gyns. are not the central players with these vaccines, we still have an important role to play in HPV immunization. We can help backstop pediatricians and facilitate the recommended "catch-up" for females aged 13-26 years who were not immunized at the recommended starting age of 11 or 12 years.
Unfortunately, the three-dose HPV vaccine series was misframed in the United States as a vaccine to prevent a sexually transmitted infection, rather than being framed, as it was in other countries, as a vaccine to prevent cancer. The unintended consequence has been widespread unwillingness of many U.S. parents to vaccinate their young daughters – a phenomenon that has challenged pediatricians and limited uptake of the vaccine.
For ob.gyns., the catch-up role means that many of their patients who are potential candidates for the vaccines are already sexually active and carrying HPV. Still, ob.gyns. should review the vaccine history with their patients and administer remaining or all doses as needed.
Both of the available vaccines – the quadrivalent HPV vaccine and the bivalent HPV vaccine – protect against viral genotypes 16 and 18, which are associated with 70% of cervical cancers. The quadrivalent vaccine provides extra protection against genotypes 6 and 11, which are associated with 90% of genital warts cases. Both vaccines protect against vulvar, vaginal, anal, and penile dysplasias.
The HPV vaccines have been used broadly throughout the world. In Australia, where vaccine coverage has been high, there is now evidence of herd immunity, with the number of males presenting with new diagnoses of genital warts declining even though females are the ones being vaccinated.
With respect to hepatitis B infection, sexual transmission is the most common mode of transmission in the United States, and in this sense, ob.gyns. have an important opportunity to ensure that women at risk for hepatitis B infection are vaccinated. Ob.gyns. should take a history of a sexually transmitted infection, in particular, as a trigger for action. It should be second nature for us to tell a patient who had gonorrhea 2 years ago that we recommend the hepatitis B vaccine for her.
A history of a sexually transmitted infection is only one of the risk factors for hepatitis B – others include recurrent or current injection drug use, previous incarceration, and exposure to blood products – but it is the one that most clearly calls us into a public health role. A significant number of women who see us during any given year do not see any other physicians or health care providers, so we cannot depend on other providers to take the lead on immunization.
Remember, you cannot always learn of a history of a sexually transmitted infection by simply asking, have you ever had a sexually transmitted infection? Women should be given a list of specific sexually transmitted infections and asked whether they’re ever had any of them. Research has shown that women commonly do not equate pelvic inflammatory disease or Trichomonas vaginalis, for instance, with sexual transmission.
Dr. Minkoff serves as chairman of the department of obstetrics and gynecology at Maimonides Medical Center, and is a distinguished professor of obstetrics and gynecology at SUNY Downstate Medical Center, both in Brooklyn, N.Y. Dr. Minkoff reported that he has no disclosures relevant to this Master Class.
As ob.gyns., we pride ourselves on being primary care providers as well as specialists. A central obligation of the primary care physician is the prevention of disease, and immunization of vaccine-preventable diseases is an essential component of prevention. In fact, nothing is more effective at preventing infectious diseases than immunization.
Immunization has not traditionally been as central to our role as it has been for pediatricians, who have long viewed vaccines as a core component of their care. However, although there are certain vaccines that pediatricians can give more easily than we can, such as the human papillomavirus vaccine, there are other vaccines that ob.gyns. can more easily provide. For example, we are better positioned than pediatricians to protect newborns from pertussis.
No other physician, moreover, is better situated to vaccinate vulnerable populations than is the ob.gyn. We are important sources of information and advice for adolescents, adults, and pregnant women. We therefore have a critically important opportunity to identify the diseases that put our patients and their progeny at greatest risk, and a responsibility to make immunization an integral part of our practices.
Numerous investigations and reports addressing vaccine implementation strategies have relevance for both obstetric and gynecologic patients, and studies addressing successful strategies for immunization of pregnant women in particular have increased since the 2009 H1N1 influenza pandemic.
Last spring, the Committee on Obstetric Practice and Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists published guidance on how to successfully incorporate immunizations into routine care and develop an immunization culture (Committee Opinion No. 558, Obstet. Gynecol. 2013;121:897-903).
Among the key points:
• Make direct recommendations. Talk to your patients directly. Recommend individual immunizations. A provider recommendation has been shown to be one of the strongest influences on patient acceptance. Tell patients, "You should have this vaccine," or "It is important for you," or "I’m telling you as your health care specialist that this vaccine is in your best interest."
Physician scripts for several immunizations are available on ACOG’s immunization website, immunizationforwomen.org, and numerous other sources.
• Designate a vaccine coordinator. As the "vaccine champion," this person orders and receives the vaccines, ensures that the vaccines are stored properly, and has knowledge of appropriate billing codes for vaccination services. He or she also maintains contact with the state health department’s immunization program manager, who can answer physicians’ questions and help practices.
• Institute standing orders. Such orders allow for an indicated vaccine to be administered to patients without an individual physician order. For example, every pregnant woman who shows up during flu season should have a standing order for the influenza vaccination.
Evaluate your prompts, paper or electronic, to remind providers and staff which patients need to be immunized. Hold everyone accountable.
• Get yourself and your staff immunized. Educate staff about the safety and efficacy of immunizations, and ensure that your office health care providers, your entire staff, and you are immunized as recommended. If your staff or you are not immunized, it can be hard to convince patients to receive a vaccine. As ACOG’s Committee Opinion on "Integrating Immunizations into Practice" highlights, moreover, office personnel who express their own uncertainty or lack of knowledge to patients can negatively affect patients’ willingness to receive a vaccine. Additionally, being a potential source of infection for your patients violates ethical obligations.
Research has shown that educational efforts for office staff can markedly increase office immunization rates. In one study of the H1N1 influenza pandemic of 2009, educational sessions for ob.gyns’ staff were part of a multifaceted approach that led to a high vaccine acceptance rate of 76% in an ethnically diverse population of 157 obstetrics patients (Infect. Dis. Obstet. Gynecol. 2011;2011:746214 [doi: 10.1155/2011/746214]). The educational sessions for staff were instituted proactively prior to availability of the vaccine.
Influenza vaccination
Influenza affects 10%-20% of the U.S. population annually, and pregnant women are more likely to have serious complications should they contract the virus. Pregnant women are at least 4-5 times more likely to be hospitalized and equally more likely to die from infection, and their infants are more likely to have influenza-related respiratory illnesses and die.
A 2010 study of the 2009 H1N1 pandemic showed that although pregnant women in the United States represent 1%-2% of the population, they accounted for up to 7%-10% of the hospitalized patients, 6%-9% of the ICU patients, and 6%-10% of the patients who died (N. Engl. J. Med. 2010:362:1708-19).
A study published in early 2013 showed that vaccination was 70% successful in preventing 2009 H1N1 influenza infection in pregnant women in Norway during the pandemic, and that the risk of fetal death nearly doubled among women who contracted influenza (N. Engl. J. Med. 2013;368:333-40). Of almost 120,000 pregnant women in the study, approximately half had received the flu vaccine.
Studies of earlier influenza pandemics and large epidemiologic studies of otherwise healthy pregnant women who contracted nonpandemic seasonal influenza have similarly demonstrated how pregnant women and their infants disproportionately experience severe sequelae.
We need to inform our pregnant patients that the influenza vaccine protects their newborns as well as themselves. We must also work harder to dispel misconceptions about the safety of the vaccine.
One barrier to pregnant women receiving the 2009 H1N1 influenza vaccine was perceived risks to the fetus (Am. J. Obstet. Gynecol. 2011;204:S124-7). The source of much of this concern stems from the fact that some influenza vaccines contain trace amounts of the preservative thimerosal.
Influenza vaccines that contain a mercury-free preservative are available, but pregnant women should be informed that the Centers for Disease Control and Prevention (CDC), the Institute of Medicine, and numerous other health organizations have concluded that the thimerosal used in the vaccine is safe. The only flu vaccine that pregnant women should not receive is the attenuated vaccine.
An additional concern for some is that the influenza vaccine contains chicken egg protein, an allergen for some individuals. However, the CDC’s Advisory Committee on Immunization Practices now recommends that individuals who have only had hives after exposure to egg should receive the influenza vaccine, though physicians should take extra precautions, such as observing these patients for at least 30 minutes after administering the vaccine (www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
With influenza immunization, we should celebrate our successes. As described in ACOG’s Committee Opinion on integrating vaccinations, vaccination of pregnant women increased nationwide to a level of approximately 50% in 2009, a significant increase over pre-pandemic rates of approximately 15%. Rates during the 2011-2012 influenza season remained approximately 47%.
Such improvement shows that immunization is achievable in our practices. However, rates hovering around half of all pregnant women are still just slightly north of mediocre. We should continue to make the benefits of vaccination clear to staff and patients, and the algorithm for implementation simple.
Given that the flu season begins in October and can run into spring – and that it takes about 2 weeks for production of protective antibody levels – it is rare that a pregnant woman will not need the vaccine.
Full recommendations for the prevention and control of influenza in 2013-2014 were expected at the time of this writing to be published in the Morbidity and Mortality Weekly Report.
Tdap
Pertussis is highly infectious, and infants who contract the bacterium have increased rates of whooping cough attacks and are at the greatest risk for severe disease and death. Pertussis outbreaks have become common in the United States, and can be difficult to identify and manage. Infants continue to have the highest reported rates.
When immunization is an integral part of one’s office (with standing orders, etc.), administering a dose of Tdap during each pregnancy to prevent pertussis in infants – as is recommended in the CDC immunization schedule released in January 2013 – should be relatively simple during prenatal office visits.
The postpartum "cocooning" approach recommended by the CDC in 2006 and supported by ACOG has been practically and logistically difficult to implement. While the concept is sound, it has proved too cumbersome overall to vaccinate every family member and caregiver who will have close contact with an infant. Merely having the parents vaccinated immediately postpartum – the other part of cocooning – has been difficult enough.
The new recommendations draw upon the proven paradigm of maternal vaccination for newborn benefit and the relative ease of immunization during prenatal care visits. Ob.gyns. should administer a dose of Tdap during each pregnancy – optimally between 27 and 36 weeks’ gestation – irrespective of the patient’s prior history of receiving Tdap.
Infants do not start their vaccination series against these pathogens until age 2 months; maternal immunization in late pregnancy leads to high transplacental antibody transfer, which will protect infants until they receive their own vaccines.
Although the optimal timing for maternal Tdap immunization is later in pregnancy, the vaccine may be given at any time if necessitated by clinical circumstance. For example, if a woman steps on a rusty nail during her first trimester and has not had a tetanus booster in the prior 5 years, or if a local school reports an epidemic, she should receive the Tdap vaccine immediately.
Cocooning is now the default; if Tdap is not administered during pregnancy for some reason, it should be administered immediately postpartum, with as much cocooning as possible.
The challenge with the Tdap vaccine is that few people who live outside areas where pertussis epidemics have occurred know someone who has had the bacterial disease. Education and a direct recommendation for the vaccine are therefore critical.
Human papillomavirus, hepatitis B
HPV vaccines are not recommended for use in pregnant women, and although ob.gyns. are not the central players with these vaccines, we still have an important role to play in HPV immunization. We can help backstop pediatricians and facilitate the recommended "catch-up" for females aged 13-26 years who were not immunized at the recommended starting age of 11 or 12 years.
Unfortunately, the three-dose HPV vaccine series was misframed in the United States as a vaccine to prevent a sexually transmitted infection, rather than being framed, as it was in other countries, as a vaccine to prevent cancer. The unintended consequence has been widespread unwillingness of many U.S. parents to vaccinate their young daughters – a phenomenon that has challenged pediatricians and limited uptake of the vaccine.
For ob.gyns., the catch-up role means that many of their patients who are potential candidates for the vaccines are already sexually active and carrying HPV. Still, ob.gyns. should review the vaccine history with their patients and administer remaining or all doses as needed.
Both of the available vaccines – the quadrivalent HPV vaccine and the bivalent HPV vaccine – protect against viral genotypes 16 and 18, which are associated with 70% of cervical cancers. The quadrivalent vaccine provides extra protection against genotypes 6 and 11, which are associated with 90% of genital warts cases. Both vaccines protect against vulvar, vaginal, anal, and penile dysplasias.
The HPV vaccines have been used broadly throughout the world. In Australia, where vaccine coverage has been high, there is now evidence of herd immunity, with the number of males presenting with new diagnoses of genital warts declining even though females are the ones being vaccinated.
With respect to hepatitis B infection, sexual transmission is the most common mode of transmission in the United States, and in this sense, ob.gyns. have an important opportunity to ensure that women at risk for hepatitis B infection are vaccinated. Ob.gyns. should take a history of a sexually transmitted infection, in particular, as a trigger for action. It should be second nature for us to tell a patient who had gonorrhea 2 years ago that we recommend the hepatitis B vaccine for her.
A history of a sexually transmitted infection is only one of the risk factors for hepatitis B – others include recurrent or current injection drug use, previous incarceration, and exposure to blood products – but it is the one that most clearly calls us into a public health role. A significant number of women who see us during any given year do not see any other physicians or health care providers, so we cannot depend on other providers to take the lead on immunization.
Remember, you cannot always learn of a history of a sexually transmitted infection by simply asking, have you ever had a sexually transmitted infection? Women should be given a list of specific sexually transmitted infections and asked whether they’re ever had any of them. Research has shown that women commonly do not equate pelvic inflammatory disease or Trichomonas vaginalis, for instance, with sexual transmission.
Dr. Minkoff serves as chairman of the department of obstetrics and gynecology at Maimonides Medical Center, and is a distinguished professor of obstetrics and gynecology at SUNY Downstate Medical Center, both in Brooklyn, N.Y. Dr. Minkoff reported that he has no disclosures relevant to this Master Class.
As ob.gyns., we pride ourselves on being primary care providers as well as specialists. A central obligation of the primary care physician is the prevention of disease, and immunization of vaccine-preventable diseases is an essential component of prevention. In fact, nothing is more effective at preventing infectious diseases than immunization.
Immunization has not traditionally been as central to our role as it has been for pediatricians, who have long viewed vaccines as a core component of their care. However, although there are certain vaccines that pediatricians can give more easily than we can, such as the human papillomavirus vaccine, there are other vaccines that ob.gyns. can more easily provide. For example, we are better positioned than pediatricians to protect newborns from pertussis.
No other physician, moreover, is better situated to vaccinate vulnerable populations than is the ob.gyn. We are important sources of information and advice for adolescents, adults, and pregnant women. We therefore have a critically important opportunity to identify the diseases that put our patients and their progeny at greatest risk, and a responsibility to make immunization an integral part of our practices.
Numerous investigations and reports addressing vaccine implementation strategies have relevance for both obstetric and gynecologic patients, and studies addressing successful strategies for immunization of pregnant women in particular have increased since the 2009 H1N1 influenza pandemic.
Last spring, the Committee on Obstetric Practice and Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists published guidance on how to successfully incorporate immunizations into routine care and develop an immunization culture (Committee Opinion No. 558, Obstet. Gynecol. 2013;121:897-903).
Among the key points:
• Make direct recommendations. Talk to your patients directly. Recommend individual immunizations. A provider recommendation has been shown to be one of the strongest influences on patient acceptance. Tell patients, "You should have this vaccine," or "It is important for you," or "I’m telling you as your health care specialist that this vaccine is in your best interest."
Physician scripts for several immunizations are available on ACOG’s immunization website, immunizationforwomen.org, and numerous other sources.
• Designate a vaccine coordinator. As the "vaccine champion," this person orders and receives the vaccines, ensures that the vaccines are stored properly, and has knowledge of appropriate billing codes for vaccination services. He or she also maintains contact with the state health department’s immunization program manager, who can answer physicians’ questions and help practices.
• Institute standing orders. Such orders allow for an indicated vaccine to be administered to patients without an individual physician order. For example, every pregnant woman who shows up during flu season should have a standing order for the influenza vaccination.
Evaluate your prompts, paper or electronic, to remind providers and staff which patients need to be immunized. Hold everyone accountable.
• Get yourself and your staff immunized. Educate staff about the safety and efficacy of immunizations, and ensure that your office health care providers, your entire staff, and you are immunized as recommended. If your staff or you are not immunized, it can be hard to convince patients to receive a vaccine. As ACOG’s Committee Opinion on "Integrating Immunizations into Practice" highlights, moreover, office personnel who express their own uncertainty or lack of knowledge to patients can negatively affect patients’ willingness to receive a vaccine. Additionally, being a potential source of infection for your patients violates ethical obligations.
Research has shown that educational efforts for office staff can markedly increase office immunization rates. In one study of the H1N1 influenza pandemic of 2009, educational sessions for ob.gyns’ staff were part of a multifaceted approach that led to a high vaccine acceptance rate of 76% in an ethnically diverse population of 157 obstetrics patients (Infect. Dis. Obstet. Gynecol. 2011;2011:746214 [doi: 10.1155/2011/746214]). The educational sessions for staff were instituted proactively prior to availability of the vaccine.
Influenza vaccination
Influenza affects 10%-20% of the U.S. population annually, and pregnant women are more likely to have serious complications should they contract the virus. Pregnant women are at least 4-5 times more likely to be hospitalized and equally more likely to die from infection, and their infants are more likely to have influenza-related respiratory illnesses and die.
A 2010 study of the 2009 H1N1 pandemic showed that although pregnant women in the United States represent 1%-2% of the population, they accounted for up to 7%-10% of the hospitalized patients, 6%-9% of the ICU patients, and 6%-10% of the patients who died (N. Engl. J. Med. 2010:362:1708-19).
A study published in early 2013 showed that vaccination was 70% successful in preventing 2009 H1N1 influenza infection in pregnant women in Norway during the pandemic, and that the risk of fetal death nearly doubled among women who contracted influenza (N. Engl. J. Med. 2013;368:333-40). Of almost 120,000 pregnant women in the study, approximately half had received the flu vaccine.
Studies of earlier influenza pandemics and large epidemiologic studies of otherwise healthy pregnant women who contracted nonpandemic seasonal influenza have similarly demonstrated how pregnant women and their infants disproportionately experience severe sequelae.
We need to inform our pregnant patients that the influenza vaccine protects their newborns as well as themselves. We must also work harder to dispel misconceptions about the safety of the vaccine.
One barrier to pregnant women receiving the 2009 H1N1 influenza vaccine was perceived risks to the fetus (Am. J. Obstet. Gynecol. 2011;204:S124-7). The source of much of this concern stems from the fact that some influenza vaccines contain trace amounts of the preservative thimerosal.
Influenza vaccines that contain a mercury-free preservative are available, but pregnant women should be informed that the Centers for Disease Control and Prevention (CDC), the Institute of Medicine, and numerous other health organizations have concluded that the thimerosal used in the vaccine is safe. The only flu vaccine that pregnant women should not receive is the attenuated vaccine.
An additional concern for some is that the influenza vaccine contains chicken egg protein, an allergen for some individuals. However, the CDC’s Advisory Committee on Immunization Practices now recommends that individuals who have only had hives after exposure to egg should receive the influenza vaccine, though physicians should take extra precautions, such as observing these patients for at least 30 minutes after administering the vaccine (www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
With influenza immunization, we should celebrate our successes. As described in ACOG’s Committee Opinion on integrating vaccinations, vaccination of pregnant women increased nationwide to a level of approximately 50% in 2009, a significant increase over pre-pandemic rates of approximately 15%. Rates during the 2011-2012 influenza season remained approximately 47%.
Such improvement shows that immunization is achievable in our practices. However, rates hovering around half of all pregnant women are still just slightly north of mediocre. We should continue to make the benefits of vaccination clear to staff and patients, and the algorithm for implementation simple.
Given that the flu season begins in October and can run into spring – and that it takes about 2 weeks for production of protective antibody levels – it is rare that a pregnant woman will not need the vaccine.
Full recommendations for the prevention and control of influenza in 2013-2014 were expected at the time of this writing to be published in the Morbidity and Mortality Weekly Report.
Tdap
Pertussis is highly infectious, and infants who contract the bacterium have increased rates of whooping cough attacks and are at the greatest risk for severe disease and death. Pertussis outbreaks have become common in the United States, and can be difficult to identify and manage. Infants continue to have the highest reported rates.
When immunization is an integral part of one’s office (with standing orders, etc.), administering a dose of Tdap during each pregnancy to prevent pertussis in infants – as is recommended in the CDC immunization schedule released in January 2013 – should be relatively simple during prenatal office visits.
The postpartum "cocooning" approach recommended by the CDC in 2006 and supported by ACOG has been practically and logistically difficult to implement. While the concept is sound, it has proved too cumbersome overall to vaccinate every family member and caregiver who will have close contact with an infant. Merely having the parents vaccinated immediately postpartum – the other part of cocooning – has been difficult enough.
The new recommendations draw upon the proven paradigm of maternal vaccination for newborn benefit and the relative ease of immunization during prenatal care visits. Ob.gyns. should administer a dose of Tdap during each pregnancy – optimally between 27 and 36 weeks’ gestation – irrespective of the patient’s prior history of receiving Tdap.
Infants do not start their vaccination series against these pathogens until age 2 months; maternal immunization in late pregnancy leads to high transplacental antibody transfer, which will protect infants until they receive their own vaccines.
Although the optimal timing for maternal Tdap immunization is later in pregnancy, the vaccine may be given at any time if necessitated by clinical circumstance. For example, if a woman steps on a rusty nail during her first trimester and has not had a tetanus booster in the prior 5 years, or if a local school reports an epidemic, she should receive the Tdap vaccine immediately.
Cocooning is now the default; if Tdap is not administered during pregnancy for some reason, it should be administered immediately postpartum, with as much cocooning as possible.
The challenge with the Tdap vaccine is that few people who live outside areas where pertussis epidemics have occurred know someone who has had the bacterial disease. Education and a direct recommendation for the vaccine are therefore critical.
Human papillomavirus, hepatitis B
HPV vaccines are not recommended for use in pregnant women, and although ob.gyns. are not the central players with these vaccines, we still have an important role to play in HPV immunization. We can help backstop pediatricians and facilitate the recommended "catch-up" for females aged 13-26 years who were not immunized at the recommended starting age of 11 or 12 years.
Unfortunately, the three-dose HPV vaccine series was misframed in the United States as a vaccine to prevent a sexually transmitted infection, rather than being framed, as it was in other countries, as a vaccine to prevent cancer. The unintended consequence has been widespread unwillingness of many U.S. parents to vaccinate their young daughters – a phenomenon that has challenged pediatricians and limited uptake of the vaccine.
For ob.gyns., the catch-up role means that many of their patients who are potential candidates for the vaccines are already sexually active and carrying HPV. Still, ob.gyns. should review the vaccine history with their patients and administer remaining or all doses as needed.
Both of the available vaccines – the quadrivalent HPV vaccine and the bivalent HPV vaccine – protect against viral genotypes 16 and 18, which are associated with 70% of cervical cancers. The quadrivalent vaccine provides extra protection against genotypes 6 and 11, which are associated with 90% of genital warts cases. Both vaccines protect against vulvar, vaginal, anal, and penile dysplasias.
The HPV vaccines have been used broadly throughout the world. In Australia, where vaccine coverage has been high, there is now evidence of herd immunity, with the number of males presenting with new diagnoses of genital warts declining even though females are the ones being vaccinated.
With respect to hepatitis B infection, sexual transmission is the most common mode of transmission in the United States, and in this sense, ob.gyns. have an important opportunity to ensure that women at risk for hepatitis B infection are vaccinated. Ob.gyns. should take a history of a sexually transmitted infection, in particular, as a trigger for action. It should be second nature for us to tell a patient who had gonorrhea 2 years ago that we recommend the hepatitis B vaccine for her.
A history of a sexually transmitted infection is only one of the risk factors for hepatitis B – others include recurrent or current injection drug use, previous incarceration, and exposure to blood products – but it is the one that most clearly calls us into a public health role. A significant number of women who see us during any given year do not see any other physicians or health care providers, so we cannot depend on other providers to take the lead on immunization.
Remember, you cannot always learn of a history of a sexually transmitted infection by simply asking, have you ever had a sexually transmitted infection? Women should be given a list of specific sexually transmitted infections and asked whether they’re ever had any of them. Research has shown that women commonly do not equate pelvic inflammatory disease or Trichomonas vaginalis, for instance, with sexual transmission.
Dr. Minkoff serves as chairman of the department of obstetrics and gynecology at Maimonides Medical Center, and is a distinguished professor of obstetrics and gynecology at SUNY Downstate Medical Center, both in Brooklyn, N.Y. Dr. Minkoff reported that he has no disclosures relevant to this Master Class.
Single port laparoscopic hysterectomy
Several years ago, I adopted a single-incision approach to virtually all benign gynecologic laparoscopic procedures as the next logical step in the practice of minimally invasive surgery. In this time, my partner and I have performed more than 450 single-incision hysterectomies using a simple, reproducible technique that differs from other approaches to single-incision laparoscopy, or laparoendoscopic single-site surgery (LESS), as it is most commonly called.
Rather than using articulating instruments, we use a 30-degree 5-mm bariatric-length laparoscope and straight, extra-long instruments. With sequential placement of one instrument at a time and attention to the direction of extra-abdominal hand movements, we create a work space with unimpeded access to the uterus and prevent the clashing of instruments, or "sword fighting," that can come with inserting several instruments through a single port.
Morcellation of the uterine fundus is accomplished extraperitoneally with specimen removal through the umbilicus. Use of a wider port with the LESS technique allows for morcellation by hand under direct vision with a #15 scalpel. This makes morcellation easier, safer, and more efficient than using an automated morcellator placed through a smaller port as in traditional multiport laparoscopic hysterectomy. We have successfully removed multiple uteri in excess of 1,000 grams – including a 3,000-gram uterus – using this technique.
Overall surgical principles/technique
We routinely use either the SILS port (Covidien), which has a predetermined setup of channels as depicted in the illustrations, or the GelPoint (Applied Medical), which allows for various trocar arrangements. Other single-incision ports can be used, though; the key to the procedure lies not in the port used, but in the placement of the instruments. A right-angled light connector also is essential, as it deviates the light cord out of the surgical field.
Regardless of the single-incision port used, the camera and a laparoscopic single-tooth tenaculum are placed in the lateral trocars, in the same horizontal plane. The active, working instrument – either Kleppinger bipolar forceps or a 45-cm Harmonic ACE Shears (Ethicon Endo-Surgery) – is then placed in the middle trocar.
While the use of angled instruments may seem helpful for creating appropriate triangulation in LESS, we have found that such instrumentation is actually counterproductive. Angled instrumentation creates new angles, in essence, which complicates instrument placement and makes it more difficult to achieve enough intra-abdominal working space and avoid sword fighting.
One exception is the laparoscope. We have found that a 30-degree 5-mm bariatric-length laparoscope works best. When exposing the right side of the uterus, the laparoscope is first inserted into the far left trocar and directed toward the right lower quadrant of the abdomen. The camera is then angled to visualize the uterus from a slightly elevated position. In this position, the operator’s external hand – holding the camera – is now deviated inferiorly and to the left side of the patient.
A 5-mm single-tooth tenaculum is then inserted into the other lateral trocar and used to manipulate the uterus anteriorly and to the left. In a simple supracervical hysterectomy, once the uterus is properly positioned, the utero-ovarian ligament and fallopian tube are exposed. In surgeries involving oophorectomy, the ovary rather than the uterus is first grasped and deviated medially and elevated, exposing the infundibulopelvic ligament. In this position, the assistant’s external hand is now deviated slightly inferiorly and to the right side of the patient.
When properly placed, the camera and the tenaculum are crossed intra-abdominally and held in slightly elevated positions. This allows for insertion of the active instrument underneath both the camera and grasper and directly toward the tissue to be incised. The slight elevation and intra-abdominal crossing of the instruments, while at first confusing, serves to create the angles that give the surgeon good external work space as well as excellent internal range of motion. We apply a significant amount of tension on the uterus to create this working space. The greater the deviation of the uterus (and the longer the instruments), the more work space there is.
With correct instrument placement, the active trocar has direct access to the uterus, so insertion of an instrument is simple, ergonomic, and intuitive. With this positioning, the active instrument (bipolar forceps, harmonic scalpel, suction, or probe) also has complete freedom of movement internally and externally. The operator’s hand can move superiorly, inferiorly, or laterally as needed to access the uterus.
We utilize the Kleppinger forceps and the 45-cm Harmonic Ace Shears for all of our dissection. Because the uterus is held on some tension, we first coagulate all vascular pedicles with the Kleppinger forceps before cutting. For safety, the uterine arteries are transected fairly high, just cephalad to the internal os. This gives us plenty of room to safely grasp and recoagulate a bleeding pedicle without fear of injuring the ureter.
Sequential instrument placement is key to this technique. If sword fighting occurs, one must reexamine the sequence of instrument placement and pay greater attention to the direction of extra-abdominal hand movements and subsequent angles. Again, the laparoscope and grasper must be inserted, positioned, and frozen in place before the active instrument is introduced.
Once one side of the uterus has been dissected free and the uterine artery transected, then all instruments are removed and replaced in a mirror-image manner to access the opposite side. Essentially, the camera and grasper switch trocars, and the active instrument remains in the middle.
Abdominal entry is performed with the traditional open laparoscopy technique described by Dr. Harrith Hasson of Chicago (Am. J. Obstet. Gynecol. 1971;110:886-7). While single-channel trocars generally require a 5- to 10-mm incision, LESS multichannel ports require a slightly larger incision. We recommend a straight vertical incision of about 2 cm through the center of the umbilicus.
The position of the surgeon also differs from multipart laparoscopy. With LESS, the surgeon stands in line with the port and the pathology, usually between the patient’s head and shoulder, and holds either the grasper or camera while operating the active blade. To prevent sliding in a steep Trendelenburg position, the patient is placed on an egg-crate mattress, and both the patient and the mattress are taped to the table. The arms also are tucked to the sides in a manner more resembling robotic positioning than the positioning traditionally associated with laparoscopic surgery. For morcellation, the patient is brought to the standard supine position and then returned to steep Trendelenburg afterward.
Other surgical tips
• Uterine manipulation. In most of our cases of LESS hysterectomy, placement of a traditional uterine manipulator has been unnecessary and, in fact, detracts from the simplicity of our approach, which utilizes the single-tooth tenaculum to manipulate and expose the uterus. We quickly learned that even lightweight manipulators compromise uterine mobility and interfere with the creation of necessary angles. The manipulator becomes like a ball and chain on the cervix.
For total laparoscopic hysterectomy, however, a uterine manipulator can be helpful for the colpotomy. Late placement of the manipulator in these cases – after the lateral attachments are dissected and before colpotomy is performed – works perfectly.
• Control of the uterine vessels. Access to the uterine vessels can be difficult with LESS. It requires severely angulating the uterus to expose the uterine artery and vein, allowing a more direct approach from the umbilicus. With the uterus on significant tension, initial cauterization with the bipolar forceps is performed before ligating the vessels with the harmonic scalpel.
• Cervical amputation. We use a monopolar loop (LiNA Loop or Storz SupraLoop) for amputation of the uterine fundus in supracervical hysterectomies. We place the loop after we’ve carried our dissection down to the middle of the cardinal ligament. The loop is placed at the level of the internal os.
Placement of the LiNA Loop device was more challenging than we expected, as the camera and grasper must be removed and reinserted on opposite sides in order to visualize the entire loop, a process during which the loop can potentially loosen and trap small bowel or other tissue on the nonvisualized side.
Our solution has been to tighten the loop on the cervix and place a Kelly clamp on the shaft of the loop before switching our instruments from side to side. The clamp keeps the loop snug against the cervix. This technique allows us to visualize one side, remove the instruments, and then visualize the other side without worrying that the loop may loosen and cause damage.
• Vaginal cuff closure. When the vaginal apex is easily accessible, transvaginal closure is the simplest method. The vaginal cuff can be closed intracorporeally with a conventional straight needle driver and a curved grasper; however, intra-abdominal closure of the cuff can be technically challenging in LESS. Historically, I used the angulating 5-mm Endo Grasp and a 0-Vicryl suture on a CT-2 needle and secured the stitch on both ends with absorbable Lapra Ty anchors (Ethicon Endo-Surgery). Increasingly, however, I use the EndoStitch (Covidien, Mansfield, Mass.), a 10 mm suturing device, to place interrupted stitches, and I secure each one with the TK Ti-Knot Device (LSI Solutions), an automated knot-tying device.*
• Umbilical suturing. We close the umbilicus with one running 3-4-bite suture through the fascia and peritoneum of 0-Vicryl or 0-PDS (polydioxanone). The skin is closed with three to five sutures – one that reattaches the center of the umbilicus to the fascial plate and one or two inverted sutures on either side that reapproximate the lower and upper poles of the umbilicus.
• Postoperative care. Patients are discharged either the evening of the procedure or, if they choose, the following morning. We use 0.25% Marcaine with epinephrine in the umbilicus for preemptive analgesia. Xeroform gauze is placed in the umbilicus. The wound is then covered with an eye patch and Tegaderm for 3 days. The umbilicus has a poor nerve supply, which helps minimize pain, but it also has a poor blood supply, which slows the healing process. We have found that a 1-week course of broad-spectrum antibiotics promotes and speeds healing.
Outcomes/experience
In 2009, at a set point in time, I transitioned to LESS as the primary approach for all laparoscopic hysterectomies. As a result, I have been able to compare the LESS approach to traditional multiport laparoscopic hysterectomy using my own cases as retrospective controls, free of selection bias. Allowing for a short learning curve of about 20 cases, analysis of our data revealed no significant difference in operative times, blood loss, conversion to laparotomy, or complication rates.
Our patients have had significantly lower pain levels with LESS, which is consistent with the literature. The cosmetic advantages of LESS have also been significant. In addition, the lack of accessory ports in LESS eliminates any potential for port-site complications such as hematoma or hernia. Umbilical hernias have been rare in our patients, occurring in only one or two patients.
Interestingly, we found that as uterine weight increased, operating times with the LESS procedure increased at a lower rate than did operating times with traditional laparoscopy. Clearly, large uteri are more easily removed with a single-port technique using extraperitoneal morcellation than with a traditional four-port technique using an automated morcellator through an accessory port. Extracorporeal morcellation with LESS is also safer and less expensive.
Dr. Wagner has been in private practice in East Northport, N.Y., since 1991. He practices at Huntington (N.Y.) Hospital, where he is the codirector of minimally invasive surgery. He was instrumental in developing the gynecologic residency program at the institution. Dr. Wagner reported that he has no financial disclosures relevant to this Master Class.
*Correction 7/30/2013: This article was updated to reflect new information from the author.
Several years ago, I adopted a single-incision approach to virtually all benign gynecologic laparoscopic procedures as the next logical step in the practice of minimally invasive surgery. In this time, my partner and I have performed more than 450 single-incision hysterectomies using a simple, reproducible technique that differs from other approaches to single-incision laparoscopy, or laparoendoscopic single-site surgery (LESS), as it is most commonly called.
Rather than using articulating instruments, we use a 30-degree 5-mm bariatric-length laparoscope and straight, extra-long instruments. With sequential placement of one instrument at a time and attention to the direction of extra-abdominal hand movements, we create a work space with unimpeded access to the uterus and prevent the clashing of instruments, or "sword fighting," that can come with inserting several instruments through a single port.
Morcellation of the uterine fundus is accomplished extraperitoneally with specimen removal through the umbilicus. Use of a wider port with the LESS technique allows for morcellation by hand under direct vision with a #15 scalpel. This makes morcellation easier, safer, and more efficient than using an automated morcellator placed through a smaller port as in traditional multiport laparoscopic hysterectomy. We have successfully removed multiple uteri in excess of 1,000 grams – including a 3,000-gram uterus – using this technique.
Overall surgical principles/technique
We routinely use either the SILS port (Covidien), which has a predetermined setup of channels as depicted in the illustrations, or the GelPoint (Applied Medical), which allows for various trocar arrangements. Other single-incision ports can be used, though; the key to the procedure lies not in the port used, but in the placement of the instruments. A right-angled light connector also is essential, as it deviates the light cord out of the surgical field.
Regardless of the single-incision port used, the camera and a laparoscopic single-tooth tenaculum are placed in the lateral trocars, in the same horizontal plane. The active, working instrument – either Kleppinger bipolar forceps or a 45-cm Harmonic ACE Shears (Ethicon Endo-Surgery) – is then placed in the middle trocar.
While the use of angled instruments may seem helpful for creating appropriate triangulation in LESS, we have found that such instrumentation is actually counterproductive. Angled instrumentation creates new angles, in essence, which complicates instrument placement and makes it more difficult to achieve enough intra-abdominal working space and avoid sword fighting.
One exception is the laparoscope. We have found that a 30-degree 5-mm bariatric-length laparoscope works best. When exposing the right side of the uterus, the laparoscope is first inserted into the far left trocar and directed toward the right lower quadrant of the abdomen. The camera is then angled to visualize the uterus from a slightly elevated position. In this position, the operator’s external hand – holding the camera – is now deviated inferiorly and to the left side of the patient.
A 5-mm single-tooth tenaculum is then inserted into the other lateral trocar and used to manipulate the uterus anteriorly and to the left. In a simple supracervical hysterectomy, once the uterus is properly positioned, the utero-ovarian ligament and fallopian tube are exposed. In surgeries involving oophorectomy, the ovary rather than the uterus is first grasped and deviated medially and elevated, exposing the infundibulopelvic ligament. In this position, the assistant’s external hand is now deviated slightly inferiorly and to the right side of the patient.
When properly placed, the camera and the tenaculum are crossed intra-abdominally and held in slightly elevated positions. This allows for insertion of the active instrument underneath both the camera and grasper and directly toward the tissue to be incised. The slight elevation and intra-abdominal crossing of the instruments, while at first confusing, serves to create the angles that give the surgeon good external work space as well as excellent internal range of motion. We apply a significant amount of tension on the uterus to create this working space. The greater the deviation of the uterus (and the longer the instruments), the more work space there is.
With correct instrument placement, the active trocar has direct access to the uterus, so insertion of an instrument is simple, ergonomic, and intuitive. With this positioning, the active instrument (bipolar forceps, harmonic scalpel, suction, or probe) also has complete freedom of movement internally and externally. The operator’s hand can move superiorly, inferiorly, or laterally as needed to access the uterus.
We utilize the Kleppinger forceps and the 45-cm Harmonic Ace Shears for all of our dissection. Because the uterus is held on some tension, we first coagulate all vascular pedicles with the Kleppinger forceps before cutting. For safety, the uterine arteries are transected fairly high, just cephalad to the internal os. This gives us plenty of room to safely grasp and recoagulate a bleeding pedicle without fear of injuring the ureter.
Sequential instrument placement is key to this technique. If sword fighting occurs, one must reexamine the sequence of instrument placement and pay greater attention to the direction of extra-abdominal hand movements and subsequent angles. Again, the laparoscope and grasper must be inserted, positioned, and frozen in place before the active instrument is introduced.
Once one side of the uterus has been dissected free and the uterine artery transected, then all instruments are removed and replaced in a mirror-image manner to access the opposite side. Essentially, the camera and grasper switch trocars, and the active instrument remains in the middle.
Abdominal entry is performed with the traditional open laparoscopy technique described by Dr. Harrith Hasson of Chicago (Am. J. Obstet. Gynecol. 1971;110:886-7). While single-channel trocars generally require a 5- to 10-mm incision, LESS multichannel ports require a slightly larger incision. We recommend a straight vertical incision of about 2 cm through the center of the umbilicus.
The position of the surgeon also differs from multipart laparoscopy. With LESS, the surgeon stands in line with the port and the pathology, usually between the patient’s head and shoulder, and holds either the grasper or camera while operating the active blade. To prevent sliding in a steep Trendelenburg position, the patient is placed on an egg-crate mattress, and both the patient and the mattress are taped to the table. The arms also are tucked to the sides in a manner more resembling robotic positioning than the positioning traditionally associated with laparoscopic surgery. For morcellation, the patient is brought to the standard supine position and then returned to steep Trendelenburg afterward.
Other surgical tips
• Uterine manipulation. In most of our cases of LESS hysterectomy, placement of a traditional uterine manipulator has been unnecessary and, in fact, detracts from the simplicity of our approach, which utilizes the single-tooth tenaculum to manipulate and expose the uterus. We quickly learned that even lightweight manipulators compromise uterine mobility and interfere with the creation of necessary angles. The manipulator becomes like a ball and chain on the cervix.
For total laparoscopic hysterectomy, however, a uterine manipulator can be helpful for the colpotomy. Late placement of the manipulator in these cases – after the lateral attachments are dissected and before colpotomy is performed – works perfectly.
• Control of the uterine vessels. Access to the uterine vessels can be difficult with LESS. It requires severely angulating the uterus to expose the uterine artery and vein, allowing a more direct approach from the umbilicus. With the uterus on significant tension, initial cauterization with the bipolar forceps is performed before ligating the vessels with the harmonic scalpel.
• Cervical amputation. We use a monopolar loop (LiNA Loop or Storz SupraLoop) for amputation of the uterine fundus in supracervical hysterectomies. We place the loop after we’ve carried our dissection down to the middle of the cardinal ligament. The loop is placed at the level of the internal os.
Placement of the LiNA Loop device was more challenging than we expected, as the camera and grasper must be removed and reinserted on opposite sides in order to visualize the entire loop, a process during which the loop can potentially loosen and trap small bowel or other tissue on the nonvisualized side.
Our solution has been to tighten the loop on the cervix and place a Kelly clamp on the shaft of the loop before switching our instruments from side to side. The clamp keeps the loop snug against the cervix. This technique allows us to visualize one side, remove the instruments, and then visualize the other side without worrying that the loop may loosen and cause damage.
• Vaginal cuff closure. When the vaginal apex is easily accessible, transvaginal closure is the simplest method. The vaginal cuff can be closed intracorporeally with a conventional straight needle driver and a curved grasper; however, intra-abdominal closure of the cuff can be technically challenging in LESS. Historically, I used the angulating 5-mm Endo Grasp and a 0-Vicryl suture on a CT-2 needle and secured the stitch on both ends with absorbable Lapra Ty anchors (Ethicon Endo-Surgery). Increasingly, however, I use the EndoStitch (Covidien, Mansfield, Mass.), a 10 mm suturing device, to place interrupted stitches, and I secure each one with the TK Ti-Knot Device (LSI Solutions), an automated knot-tying device.*
• Umbilical suturing. We close the umbilicus with one running 3-4-bite suture through the fascia and peritoneum of 0-Vicryl or 0-PDS (polydioxanone). The skin is closed with three to five sutures – one that reattaches the center of the umbilicus to the fascial plate and one or two inverted sutures on either side that reapproximate the lower and upper poles of the umbilicus.
• Postoperative care. Patients are discharged either the evening of the procedure or, if they choose, the following morning. We use 0.25% Marcaine with epinephrine in the umbilicus for preemptive analgesia. Xeroform gauze is placed in the umbilicus. The wound is then covered with an eye patch and Tegaderm for 3 days. The umbilicus has a poor nerve supply, which helps minimize pain, but it also has a poor blood supply, which slows the healing process. We have found that a 1-week course of broad-spectrum antibiotics promotes and speeds healing.
Outcomes/experience
In 2009, at a set point in time, I transitioned to LESS as the primary approach for all laparoscopic hysterectomies. As a result, I have been able to compare the LESS approach to traditional multiport laparoscopic hysterectomy using my own cases as retrospective controls, free of selection bias. Allowing for a short learning curve of about 20 cases, analysis of our data revealed no significant difference in operative times, blood loss, conversion to laparotomy, or complication rates.
Our patients have had significantly lower pain levels with LESS, which is consistent with the literature. The cosmetic advantages of LESS have also been significant. In addition, the lack of accessory ports in LESS eliminates any potential for port-site complications such as hematoma or hernia. Umbilical hernias have been rare in our patients, occurring in only one or two patients.
Interestingly, we found that as uterine weight increased, operating times with the LESS procedure increased at a lower rate than did operating times with traditional laparoscopy. Clearly, large uteri are more easily removed with a single-port technique using extraperitoneal morcellation than with a traditional four-port technique using an automated morcellator through an accessory port. Extracorporeal morcellation with LESS is also safer and less expensive.
Dr. Wagner has been in private practice in East Northport, N.Y., since 1991. He practices at Huntington (N.Y.) Hospital, where he is the codirector of minimally invasive surgery. He was instrumental in developing the gynecologic residency program at the institution. Dr. Wagner reported that he has no financial disclosures relevant to this Master Class.
*Correction 7/30/2013: This article was updated to reflect new information from the author.
Several years ago, I adopted a single-incision approach to virtually all benign gynecologic laparoscopic procedures as the next logical step in the practice of minimally invasive surgery. In this time, my partner and I have performed more than 450 single-incision hysterectomies using a simple, reproducible technique that differs from other approaches to single-incision laparoscopy, or laparoendoscopic single-site surgery (LESS), as it is most commonly called.
Rather than using articulating instruments, we use a 30-degree 5-mm bariatric-length laparoscope and straight, extra-long instruments. With sequential placement of one instrument at a time and attention to the direction of extra-abdominal hand movements, we create a work space with unimpeded access to the uterus and prevent the clashing of instruments, or "sword fighting," that can come with inserting several instruments through a single port.
Morcellation of the uterine fundus is accomplished extraperitoneally with specimen removal through the umbilicus. Use of a wider port with the LESS technique allows for morcellation by hand under direct vision with a #15 scalpel. This makes morcellation easier, safer, and more efficient than using an automated morcellator placed through a smaller port as in traditional multiport laparoscopic hysterectomy. We have successfully removed multiple uteri in excess of 1,000 grams – including a 3,000-gram uterus – using this technique.
Overall surgical principles/technique
We routinely use either the SILS port (Covidien), which has a predetermined setup of channels as depicted in the illustrations, or the GelPoint (Applied Medical), which allows for various trocar arrangements. Other single-incision ports can be used, though; the key to the procedure lies not in the port used, but in the placement of the instruments. A right-angled light connector also is essential, as it deviates the light cord out of the surgical field.
Regardless of the single-incision port used, the camera and a laparoscopic single-tooth tenaculum are placed in the lateral trocars, in the same horizontal plane. The active, working instrument – either Kleppinger bipolar forceps or a 45-cm Harmonic ACE Shears (Ethicon Endo-Surgery) – is then placed in the middle trocar.
While the use of angled instruments may seem helpful for creating appropriate triangulation in LESS, we have found that such instrumentation is actually counterproductive. Angled instrumentation creates new angles, in essence, which complicates instrument placement and makes it more difficult to achieve enough intra-abdominal working space and avoid sword fighting.
One exception is the laparoscope. We have found that a 30-degree 5-mm bariatric-length laparoscope works best. When exposing the right side of the uterus, the laparoscope is first inserted into the far left trocar and directed toward the right lower quadrant of the abdomen. The camera is then angled to visualize the uterus from a slightly elevated position. In this position, the operator’s external hand – holding the camera – is now deviated inferiorly and to the left side of the patient.
A 5-mm single-tooth tenaculum is then inserted into the other lateral trocar and used to manipulate the uterus anteriorly and to the left. In a simple supracervical hysterectomy, once the uterus is properly positioned, the utero-ovarian ligament and fallopian tube are exposed. In surgeries involving oophorectomy, the ovary rather than the uterus is first grasped and deviated medially and elevated, exposing the infundibulopelvic ligament. In this position, the assistant’s external hand is now deviated slightly inferiorly and to the right side of the patient.
When properly placed, the camera and the tenaculum are crossed intra-abdominally and held in slightly elevated positions. This allows for insertion of the active instrument underneath both the camera and grasper and directly toward the tissue to be incised. The slight elevation and intra-abdominal crossing of the instruments, while at first confusing, serves to create the angles that give the surgeon good external work space as well as excellent internal range of motion. We apply a significant amount of tension on the uterus to create this working space. The greater the deviation of the uterus (and the longer the instruments), the more work space there is.
With correct instrument placement, the active trocar has direct access to the uterus, so insertion of an instrument is simple, ergonomic, and intuitive. With this positioning, the active instrument (bipolar forceps, harmonic scalpel, suction, or probe) also has complete freedom of movement internally and externally. The operator’s hand can move superiorly, inferiorly, or laterally as needed to access the uterus.
We utilize the Kleppinger forceps and the 45-cm Harmonic Ace Shears for all of our dissection. Because the uterus is held on some tension, we first coagulate all vascular pedicles with the Kleppinger forceps before cutting. For safety, the uterine arteries are transected fairly high, just cephalad to the internal os. This gives us plenty of room to safely grasp and recoagulate a bleeding pedicle without fear of injuring the ureter.
Sequential instrument placement is key to this technique. If sword fighting occurs, one must reexamine the sequence of instrument placement and pay greater attention to the direction of extra-abdominal hand movements and subsequent angles. Again, the laparoscope and grasper must be inserted, positioned, and frozen in place before the active instrument is introduced.
Once one side of the uterus has been dissected free and the uterine artery transected, then all instruments are removed and replaced in a mirror-image manner to access the opposite side. Essentially, the camera and grasper switch trocars, and the active instrument remains in the middle.
Abdominal entry is performed with the traditional open laparoscopy technique described by Dr. Harrith Hasson of Chicago (Am. J. Obstet. Gynecol. 1971;110:886-7). While single-channel trocars generally require a 5- to 10-mm incision, LESS multichannel ports require a slightly larger incision. We recommend a straight vertical incision of about 2 cm through the center of the umbilicus.
The position of the surgeon also differs from multipart laparoscopy. With LESS, the surgeon stands in line with the port and the pathology, usually between the patient’s head and shoulder, and holds either the grasper or camera while operating the active blade. To prevent sliding in a steep Trendelenburg position, the patient is placed on an egg-crate mattress, and both the patient and the mattress are taped to the table. The arms also are tucked to the sides in a manner more resembling robotic positioning than the positioning traditionally associated with laparoscopic surgery. For morcellation, the patient is brought to the standard supine position and then returned to steep Trendelenburg afterward.
Other surgical tips
• Uterine manipulation. In most of our cases of LESS hysterectomy, placement of a traditional uterine manipulator has been unnecessary and, in fact, detracts from the simplicity of our approach, which utilizes the single-tooth tenaculum to manipulate and expose the uterus. We quickly learned that even lightweight manipulators compromise uterine mobility and interfere with the creation of necessary angles. The manipulator becomes like a ball and chain on the cervix.
For total laparoscopic hysterectomy, however, a uterine manipulator can be helpful for the colpotomy. Late placement of the manipulator in these cases – after the lateral attachments are dissected and before colpotomy is performed – works perfectly.
• Control of the uterine vessels. Access to the uterine vessels can be difficult with LESS. It requires severely angulating the uterus to expose the uterine artery and vein, allowing a more direct approach from the umbilicus. With the uterus on significant tension, initial cauterization with the bipolar forceps is performed before ligating the vessels with the harmonic scalpel.
• Cervical amputation. We use a monopolar loop (LiNA Loop or Storz SupraLoop) for amputation of the uterine fundus in supracervical hysterectomies. We place the loop after we’ve carried our dissection down to the middle of the cardinal ligament. The loop is placed at the level of the internal os.
Placement of the LiNA Loop device was more challenging than we expected, as the camera and grasper must be removed and reinserted on opposite sides in order to visualize the entire loop, a process during which the loop can potentially loosen and trap small bowel or other tissue on the nonvisualized side.
Our solution has been to tighten the loop on the cervix and place a Kelly clamp on the shaft of the loop before switching our instruments from side to side. The clamp keeps the loop snug against the cervix. This technique allows us to visualize one side, remove the instruments, and then visualize the other side without worrying that the loop may loosen and cause damage.
• Vaginal cuff closure. When the vaginal apex is easily accessible, transvaginal closure is the simplest method. The vaginal cuff can be closed intracorporeally with a conventional straight needle driver and a curved grasper; however, intra-abdominal closure of the cuff can be technically challenging in LESS. Historically, I used the angulating 5-mm Endo Grasp and a 0-Vicryl suture on a CT-2 needle and secured the stitch on both ends with absorbable Lapra Ty anchors (Ethicon Endo-Surgery). Increasingly, however, I use the EndoStitch (Covidien, Mansfield, Mass.), a 10 mm suturing device, to place interrupted stitches, and I secure each one with the TK Ti-Knot Device (LSI Solutions), an automated knot-tying device.*
• Umbilical suturing. We close the umbilicus with one running 3-4-bite suture through the fascia and peritoneum of 0-Vicryl or 0-PDS (polydioxanone). The skin is closed with three to five sutures – one that reattaches the center of the umbilicus to the fascial plate and one or two inverted sutures on either side that reapproximate the lower and upper poles of the umbilicus.
• Postoperative care. Patients are discharged either the evening of the procedure or, if they choose, the following morning. We use 0.25% Marcaine with epinephrine in the umbilicus for preemptive analgesia. Xeroform gauze is placed in the umbilicus. The wound is then covered with an eye patch and Tegaderm for 3 days. The umbilicus has a poor nerve supply, which helps minimize pain, but it also has a poor blood supply, which slows the healing process. We have found that a 1-week course of broad-spectrum antibiotics promotes and speeds healing.
Outcomes/experience
In 2009, at a set point in time, I transitioned to LESS as the primary approach for all laparoscopic hysterectomies. As a result, I have been able to compare the LESS approach to traditional multiport laparoscopic hysterectomy using my own cases as retrospective controls, free of selection bias. Allowing for a short learning curve of about 20 cases, analysis of our data revealed no significant difference in operative times, blood loss, conversion to laparotomy, or complication rates.
Our patients have had significantly lower pain levels with LESS, which is consistent with the literature. The cosmetic advantages of LESS have also been significant. In addition, the lack of accessory ports in LESS eliminates any potential for port-site complications such as hematoma or hernia. Umbilical hernias have been rare in our patients, occurring in only one or two patients.
Interestingly, we found that as uterine weight increased, operating times with the LESS procedure increased at a lower rate than did operating times with traditional laparoscopy. Clearly, large uteri are more easily removed with a single-port technique using extraperitoneal morcellation than with a traditional four-port technique using an automated morcellator through an accessory port. Extracorporeal morcellation with LESS is also safer and less expensive.
Dr. Wagner has been in private practice in East Northport, N.Y., since 1991. He practices at Huntington (N.Y.) Hospital, where he is the codirector of minimally invasive surgery. He was instrumental in developing the gynecologic residency program at the institution. Dr. Wagner reported that he has no financial disclosures relevant to this Master Class.
*Correction 7/30/2013: This article was updated to reflect new information from the author.
The future of health care delivery
We have a real paradox in American health care.
We have superb medical schools and exceptionally well-trained physicians who are committed to our care. America is the envy of the world for its biomedical research prowess, funded largely by the National Institutes of Health and conducted in universities and medical schools across the country. The U.S. pharmaceutical industry continually brings forth lifesaving and disease-altering medications, and the medical device industry is incredibly innovative and entrepreneurial.
On the other hand, we have a very dysfunctional delivery system for this incredible care. We spend more per capita on health care than any other country, and yet, compared with the health of other countries, especially developed countries, our outcomes are not better. Our life spans are shorter than in Japan, for instance, and our infant mortality rates are higher than in England and France.
Our current delivery system concentrates on illness and on trauma, and focuses on treating acute medical problems, where it is reasonably effective, but works poorly to address most chronic medical illnesses. Chronic illnesses consume about 75%-85% of all dollars spent on medical care. The Milken Institute published a white paper a few years ago on chronic illnesses in which it noted that nearly one-half of Americans had one or more, mostly preventable, chronic illnesses. According to this report, these illnesses cost the economy over $1 trillion per year.
Chronic illnesses – from diabetes and coronary artery disease to cancer, and chronic lung and kidney disease – are increasing in frequency at a rapid rate. Moreover, they are largely preventable. One-third of Americans are overweight and more than one-third are obese. Chronic stress is prevalent, and 20% of Americans still smoke. Too many people are sedentary and either overeat or primarily eat a nonnutritious diet. The result is that high blood pressure, high cholesterol, and elevated blood glucose are extremely prevalent in the United States. These and other poor health factors lead to and exacerbate a host of chronic conditions that are difficult to manage, last a lifetime (some cancers excepted), and are expensive to treat.
What we need in America today is to focus on true health care on two fronts. First, not only do we need to diagnose and treat disease and injury when they occur, but we must promote wellness and disease prevention. Second, we need a health care delivery system that truly and effectively coordinates care for patients with chronic illnesses. Both of these shifts require primary care at the helm – with paid time for careful listening and attention to detail – and a multidisciplinary approach that appropriately integrates medical specialists along with nurse practitioners, physician assistants, and other nonphysician professionals.
Refocusing American health care involves and impacts obstetrician-gynecologists as much as any other physicians, because ob.gyns. are uniquely positioned to affect women’s lives and health behaviors from adolescence to childbearing years and early motherhood, and through aging.
Drivers of change
Adverse behaviors and lifestyles and the prevalence of chronic illness in our society are exerting a great force on the health care delivery system and will, therefore, drive substantial change in the system in coming years – more so than the current health care reform. Among the other drivers of change:
• Aging. American society is growing older, and just as in a car, "old parts wear out." Aging brings impaired vision, impaired hearing, impaired mobility, impaired dentition, impaired bone strength, and impaired cognition, all of which need to be managed.
• Consumerism. Patients no longer want to be patient. They are coming to want and expect to be treated as valued customers by primary care providers and specialists. They want good service and expect higher levels of respect. Patients expect their physicians to listen to them and treat their conditions with confidentiality. They also want short wait times in the office, short wait times when calling for an appointment, and short travel distances.
Patients increasingly understand that care is often not as high quality and safe as it should be, and they are expecting actions to make it better. If they perceive nothing is being done, they are increasingly likely to go elsewhere. They also want interaction by e-mail and other electronic methods, and they also are pressing for and expecting a more integrative approach from their providers – an approach that cares for the whole person and incorporates complementary medical modalities where appropriate.
• Professional shortages and expectations. Shortages of nurses and pharmacists have been noted for more than a decade. More and more, there are shortages of primary care physicians, general surgeons, and other physicians, including ob.gyns., especially in rural and urban poor areas. Newly graduated physicians increasingly want little or no administrative responsibility, less night and weekend call duties, a salaried position instead of a private practice, and family time assured.
• Technology. New technologies have made health care delivery much more nimble by embedding tools into smartphones, allowing providers to access information wirelessly, or miniaturizing equipment. Hand-held ultrasound is now available at a price that a single physician can afford. For hospitals, on the other hand, the cost of new technologies like MRIs, CT scanners, and equipment for radiation therapy is so high that in order to stay abreast of trends, hospitals need substantial hard-to-raise capital.
• Costs. The costs of care are rising with no end in sight, and none of the current reforms and other proposals offered thus far will effectively curb the increases. This is because most approaches offered by government and insurers do not address the real problems. Patients, in the meantime, are facing greater requirements from employers to share the cost of care. Among employer-sponsored plans, there is an increasing push toward high-deductible-plans, with deductibles in the $1,000-$2,000 range.
The most glaring problem in American health care, however – and the largest driver of change – is the limited time that primary care physicians actually spend with their patients. Most primary care physicians in the United State, including many ob.gyns., are trapped in a business model that forces them to see at least 24-25 patients per day (a total patient load of 2,000-plus). The model typically allows for about 12 minutes of face time with each patient and leaves no time for careful listening, for care coordination, for talking with specialists, or for thinking deeply about diagnostic dilemmas.
When patients have a slightly complex case that cannot be solved in 12 minutes or less, physicians are left no option but to refer these patients to a specialist, which dramatically increases the cost of care delivery.
The changes ahead
There are many pilot programs embedded in the Patient Protection and Affordable Health Care Act that attempt to address health care delivery cost and quality, and perhaps some will bear value in coming years. Overall, however, reform in Washington is largely about medical care financing and insurance coverage.
Accountable care organizations and medical homes are good alternatives to traditional care models and could provide outstanding care, but these options will not succeed unless productivity standards are lowered such that the generalist physician truly has the time to listen, think, prevent, and coordinate. Similarly, capitation (payment of a fixed sum for all care for one patient for 1 year) will succeed only if the rate per patient is sufficient enough so that the physician can sustain a practice while seeing fewer patients and hence spend enough time with each one. Thus far, this has rarely been the case.
In the absence of major changes on the horizon from the government or insurers that put incentives and funding in the right places, health care delivery can only transform in fits and starts in response to the major drivers of change. Although some changes to the current system will be truly transformational, many will only be incremental.
Among the transformative – and one could say disruptive – changes will be change in our hospitals. Certainly more and more can be accomplished in the outpatient setting, but as more people survive longer and have more chronic illnesses, there will be a need for more hospital beds, ICUs, operating rooms, and high technology – the reverse of the mantra of recent decades which proclaimed that we had "too many hospitals and too many beds." Hospitals also will need capital for renovations, new wings, and all the needed technology. With credit tight, smaller hospitals will merge into larger systems, and there will be few stand-alone community hospitals in the coming years.
To compensate for the shortage of nonspecialists and to allow generalists to do what is needed and what they are best at doing, there will be greater use of nurse practitioners, physician assistants, nutritionists, exercise physiologists, and other nonphysician professionals. Physicians will increasingly need to embrace, rather than marginalize, the work of adjunct providers in providing quality interaction with patients, augmenting preventive programs, and enhancing care coordination.
Ob.gyns. will likely find such team-building helpful as they strengthen their efforts to provide preventive care and promote healthy behaviors for women of all ages. The model of a health care team also can help ob.gyns. as they strive to deliver more preconception care and to work with women before and during pregnancy to create optimal intrauterine environments that will lead to healthier offspring. Like family physicians and internists, ob.gyns need time to spend with each patient to learn about her family and the environment in which she lives.
To provide comprehensive preventive care, and to adequately coordinate the care of patients with chronic illnesses, some physicians have decided to bill the patient directly for services and not accept commercial insurance or Medicare. Others have opted to convert their practices to "retainer-based" practices, or "concierge" practices, which are, in effect, a type of capitation without the intermediary of the insurance company. The patient pays a fixed annual fee (usually $1,500-$2,000) for all care provided by the physician. In turn, the physician drops the practice size to about 500 patients; guarantees appointments within 24 hours; provides 24/7 cell phone access; and offers appointments that last as long as necessary, the option of e-mail conversations, and visitations in the hospital and emergency room.
Physicians operating such retainer-based practices report working as many hours as they did before, but say that they are giving superior care and that their patients are reporting a much greater level of satisfaction. The total costs of care come way down because the physician now has the time needed to thoughtfully sort out issues, resulting in fewer referrals to specialists, more lifestyle modifications instead of prescriptions, and fewer tests and x-rays. Hospitalizations are reduced by one-half, and unplanned readmissions after hospital discharge also are significantly reduced.
These changes, while quite disruptive, are, in the long run, bright spots on the horizon. While the cost of joining a retainer-based practice is out of reach for some individuals, the retainer system gives proof-of-concept to insurers and the government, who thus far have been too short-sighted to pay more per patient for primary care, and consequently have paid more across the entire spectrum of care. If such models could be adapted for wider use through the reallocation of insurer dollars, it would improve value for everyone.
The extent to which the retainer model will prevail in terms of physicians’ desires and expectations – as opposed to the options of becoming employed or joining large group practices – is uncertain. It is likely that we will see multiple shifts. Certainly, however, the future for delivering care to complex, chronically ill patients lies largely in multidisciplinary team-based care, with primary care physicians – including ob.gyns – serving as the quarterbacks.
Ultimately, key players will need to make it all happen so that we can have a delivery system that serves us well and costs us less. In the meantime, just as is happening in many large clinics, in certain specialty care centers, and in some primary care practices today, there is much that individuals and a combination of the leaders in medicine can do to keep the transformation moving.
Dr. Schimpff is the former chief executive officer of the University of Maryland Medical Center, Baltimore, and is a voluntary professor of medicine at the University of Maryland School of Medicine. He consults for the U.S. Army, medical startups, and Fortune 500 companies. Dr. Schimpff said he has no financial disclosures.
We have a real paradox in American health care.
We have superb medical schools and exceptionally well-trained physicians who are committed to our care. America is the envy of the world for its biomedical research prowess, funded largely by the National Institutes of Health and conducted in universities and medical schools across the country. The U.S. pharmaceutical industry continually brings forth lifesaving and disease-altering medications, and the medical device industry is incredibly innovative and entrepreneurial.
On the other hand, we have a very dysfunctional delivery system for this incredible care. We spend more per capita on health care than any other country, and yet, compared with the health of other countries, especially developed countries, our outcomes are not better. Our life spans are shorter than in Japan, for instance, and our infant mortality rates are higher than in England and France.
Our current delivery system concentrates on illness and on trauma, and focuses on treating acute medical problems, where it is reasonably effective, but works poorly to address most chronic medical illnesses. Chronic illnesses consume about 75%-85% of all dollars spent on medical care. The Milken Institute published a white paper a few years ago on chronic illnesses in which it noted that nearly one-half of Americans had one or more, mostly preventable, chronic illnesses. According to this report, these illnesses cost the economy over $1 trillion per year.
Chronic illnesses – from diabetes and coronary artery disease to cancer, and chronic lung and kidney disease – are increasing in frequency at a rapid rate. Moreover, they are largely preventable. One-third of Americans are overweight and more than one-third are obese. Chronic stress is prevalent, and 20% of Americans still smoke. Too many people are sedentary and either overeat or primarily eat a nonnutritious diet. The result is that high blood pressure, high cholesterol, and elevated blood glucose are extremely prevalent in the United States. These and other poor health factors lead to and exacerbate a host of chronic conditions that are difficult to manage, last a lifetime (some cancers excepted), and are expensive to treat.
What we need in America today is to focus on true health care on two fronts. First, not only do we need to diagnose and treat disease and injury when they occur, but we must promote wellness and disease prevention. Second, we need a health care delivery system that truly and effectively coordinates care for patients with chronic illnesses. Both of these shifts require primary care at the helm – with paid time for careful listening and attention to detail – and a multidisciplinary approach that appropriately integrates medical specialists along with nurse practitioners, physician assistants, and other nonphysician professionals.
Refocusing American health care involves and impacts obstetrician-gynecologists as much as any other physicians, because ob.gyns. are uniquely positioned to affect women’s lives and health behaviors from adolescence to childbearing years and early motherhood, and through aging.
Drivers of change
Adverse behaviors and lifestyles and the prevalence of chronic illness in our society are exerting a great force on the health care delivery system and will, therefore, drive substantial change in the system in coming years – more so than the current health care reform. Among the other drivers of change:
• Aging. American society is growing older, and just as in a car, "old parts wear out." Aging brings impaired vision, impaired hearing, impaired mobility, impaired dentition, impaired bone strength, and impaired cognition, all of which need to be managed.
• Consumerism. Patients no longer want to be patient. They are coming to want and expect to be treated as valued customers by primary care providers and specialists. They want good service and expect higher levels of respect. Patients expect their physicians to listen to them and treat their conditions with confidentiality. They also want short wait times in the office, short wait times when calling for an appointment, and short travel distances.
Patients increasingly understand that care is often not as high quality and safe as it should be, and they are expecting actions to make it better. If they perceive nothing is being done, they are increasingly likely to go elsewhere. They also want interaction by e-mail and other electronic methods, and they also are pressing for and expecting a more integrative approach from their providers – an approach that cares for the whole person and incorporates complementary medical modalities where appropriate.
• Professional shortages and expectations. Shortages of nurses and pharmacists have been noted for more than a decade. More and more, there are shortages of primary care physicians, general surgeons, and other physicians, including ob.gyns., especially in rural and urban poor areas. Newly graduated physicians increasingly want little or no administrative responsibility, less night and weekend call duties, a salaried position instead of a private practice, and family time assured.
• Technology. New technologies have made health care delivery much more nimble by embedding tools into smartphones, allowing providers to access information wirelessly, or miniaturizing equipment. Hand-held ultrasound is now available at a price that a single physician can afford. For hospitals, on the other hand, the cost of new technologies like MRIs, CT scanners, and equipment for radiation therapy is so high that in order to stay abreast of trends, hospitals need substantial hard-to-raise capital.
• Costs. The costs of care are rising with no end in sight, and none of the current reforms and other proposals offered thus far will effectively curb the increases. This is because most approaches offered by government and insurers do not address the real problems. Patients, in the meantime, are facing greater requirements from employers to share the cost of care. Among employer-sponsored plans, there is an increasing push toward high-deductible-plans, with deductibles in the $1,000-$2,000 range.
The most glaring problem in American health care, however – and the largest driver of change – is the limited time that primary care physicians actually spend with their patients. Most primary care physicians in the United State, including many ob.gyns., are trapped in a business model that forces them to see at least 24-25 patients per day (a total patient load of 2,000-plus). The model typically allows for about 12 minutes of face time with each patient and leaves no time for careful listening, for care coordination, for talking with specialists, or for thinking deeply about diagnostic dilemmas.
When patients have a slightly complex case that cannot be solved in 12 minutes or less, physicians are left no option but to refer these patients to a specialist, which dramatically increases the cost of care delivery.
The changes ahead
There are many pilot programs embedded in the Patient Protection and Affordable Health Care Act that attempt to address health care delivery cost and quality, and perhaps some will bear value in coming years. Overall, however, reform in Washington is largely about medical care financing and insurance coverage.
Accountable care organizations and medical homes are good alternatives to traditional care models and could provide outstanding care, but these options will not succeed unless productivity standards are lowered such that the generalist physician truly has the time to listen, think, prevent, and coordinate. Similarly, capitation (payment of a fixed sum for all care for one patient for 1 year) will succeed only if the rate per patient is sufficient enough so that the physician can sustain a practice while seeing fewer patients and hence spend enough time with each one. Thus far, this has rarely been the case.
In the absence of major changes on the horizon from the government or insurers that put incentives and funding in the right places, health care delivery can only transform in fits and starts in response to the major drivers of change. Although some changes to the current system will be truly transformational, many will only be incremental.
Among the transformative – and one could say disruptive – changes will be change in our hospitals. Certainly more and more can be accomplished in the outpatient setting, but as more people survive longer and have more chronic illnesses, there will be a need for more hospital beds, ICUs, operating rooms, and high technology – the reverse of the mantra of recent decades which proclaimed that we had "too many hospitals and too many beds." Hospitals also will need capital for renovations, new wings, and all the needed technology. With credit tight, smaller hospitals will merge into larger systems, and there will be few stand-alone community hospitals in the coming years.
To compensate for the shortage of nonspecialists and to allow generalists to do what is needed and what they are best at doing, there will be greater use of nurse practitioners, physician assistants, nutritionists, exercise physiologists, and other nonphysician professionals. Physicians will increasingly need to embrace, rather than marginalize, the work of adjunct providers in providing quality interaction with patients, augmenting preventive programs, and enhancing care coordination.
Ob.gyns. will likely find such team-building helpful as they strengthen their efforts to provide preventive care and promote healthy behaviors for women of all ages. The model of a health care team also can help ob.gyns. as they strive to deliver more preconception care and to work with women before and during pregnancy to create optimal intrauterine environments that will lead to healthier offspring. Like family physicians and internists, ob.gyns need time to spend with each patient to learn about her family and the environment in which she lives.
To provide comprehensive preventive care, and to adequately coordinate the care of patients with chronic illnesses, some physicians have decided to bill the patient directly for services and not accept commercial insurance or Medicare. Others have opted to convert their practices to "retainer-based" practices, or "concierge" practices, which are, in effect, a type of capitation without the intermediary of the insurance company. The patient pays a fixed annual fee (usually $1,500-$2,000) for all care provided by the physician. In turn, the physician drops the practice size to about 500 patients; guarantees appointments within 24 hours; provides 24/7 cell phone access; and offers appointments that last as long as necessary, the option of e-mail conversations, and visitations in the hospital and emergency room.
Physicians operating such retainer-based practices report working as many hours as they did before, but say that they are giving superior care and that their patients are reporting a much greater level of satisfaction. The total costs of care come way down because the physician now has the time needed to thoughtfully sort out issues, resulting in fewer referrals to specialists, more lifestyle modifications instead of prescriptions, and fewer tests and x-rays. Hospitalizations are reduced by one-half, and unplanned readmissions after hospital discharge also are significantly reduced.
These changes, while quite disruptive, are, in the long run, bright spots on the horizon. While the cost of joining a retainer-based practice is out of reach for some individuals, the retainer system gives proof-of-concept to insurers and the government, who thus far have been too short-sighted to pay more per patient for primary care, and consequently have paid more across the entire spectrum of care. If such models could be adapted for wider use through the reallocation of insurer dollars, it would improve value for everyone.
The extent to which the retainer model will prevail in terms of physicians’ desires and expectations – as opposed to the options of becoming employed or joining large group practices – is uncertain. It is likely that we will see multiple shifts. Certainly, however, the future for delivering care to complex, chronically ill patients lies largely in multidisciplinary team-based care, with primary care physicians – including ob.gyns – serving as the quarterbacks.
Ultimately, key players will need to make it all happen so that we can have a delivery system that serves us well and costs us less. In the meantime, just as is happening in many large clinics, in certain specialty care centers, and in some primary care practices today, there is much that individuals and a combination of the leaders in medicine can do to keep the transformation moving.
Dr. Schimpff is the former chief executive officer of the University of Maryland Medical Center, Baltimore, and is a voluntary professor of medicine at the University of Maryland School of Medicine. He consults for the U.S. Army, medical startups, and Fortune 500 companies. Dr. Schimpff said he has no financial disclosures.
We have a real paradox in American health care.
We have superb medical schools and exceptionally well-trained physicians who are committed to our care. America is the envy of the world for its biomedical research prowess, funded largely by the National Institutes of Health and conducted in universities and medical schools across the country. The U.S. pharmaceutical industry continually brings forth lifesaving and disease-altering medications, and the medical device industry is incredibly innovative and entrepreneurial.
On the other hand, we have a very dysfunctional delivery system for this incredible care. We spend more per capita on health care than any other country, and yet, compared with the health of other countries, especially developed countries, our outcomes are not better. Our life spans are shorter than in Japan, for instance, and our infant mortality rates are higher than in England and France.
Our current delivery system concentrates on illness and on trauma, and focuses on treating acute medical problems, where it is reasonably effective, but works poorly to address most chronic medical illnesses. Chronic illnesses consume about 75%-85% of all dollars spent on medical care. The Milken Institute published a white paper a few years ago on chronic illnesses in which it noted that nearly one-half of Americans had one or more, mostly preventable, chronic illnesses. According to this report, these illnesses cost the economy over $1 trillion per year.
Chronic illnesses – from diabetes and coronary artery disease to cancer, and chronic lung and kidney disease – are increasing in frequency at a rapid rate. Moreover, they are largely preventable. One-third of Americans are overweight and more than one-third are obese. Chronic stress is prevalent, and 20% of Americans still smoke. Too many people are sedentary and either overeat or primarily eat a nonnutritious diet. The result is that high blood pressure, high cholesterol, and elevated blood glucose are extremely prevalent in the United States. These and other poor health factors lead to and exacerbate a host of chronic conditions that are difficult to manage, last a lifetime (some cancers excepted), and are expensive to treat.
What we need in America today is to focus on true health care on two fronts. First, not only do we need to diagnose and treat disease and injury when they occur, but we must promote wellness and disease prevention. Second, we need a health care delivery system that truly and effectively coordinates care for patients with chronic illnesses. Both of these shifts require primary care at the helm – with paid time for careful listening and attention to detail – and a multidisciplinary approach that appropriately integrates medical specialists along with nurse practitioners, physician assistants, and other nonphysician professionals.
Refocusing American health care involves and impacts obstetrician-gynecologists as much as any other physicians, because ob.gyns. are uniquely positioned to affect women’s lives and health behaviors from adolescence to childbearing years and early motherhood, and through aging.
Drivers of change
Adverse behaviors and lifestyles and the prevalence of chronic illness in our society are exerting a great force on the health care delivery system and will, therefore, drive substantial change in the system in coming years – more so than the current health care reform. Among the other drivers of change:
• Aging. American society is growing older, and just as in a car, "old parts wear out." Aging brings impaired vision, impaired hearing, impaired mobility, impaired dentition, impaired bone strength, and impaired cognition, all of which need to be managed.
• Consumerism. Patients no longer want to be patient. They are coming to want and expect to be treated as valued customers by primary care providers and specialists. They want good service and expect higher levels of respect. Patients expect their physicians to listen to them and treat their conditions with confidentiality. They also want short wait times in the office, short wait times when calling for an appointment, and short travel distances.
Patients increasingly understand that care is often not as high quality and safe as it should be, and they are expecting actions to make it better. If they perceive nothing is being done, they are increasingly likely to go elsewhere. They also want interaction by e-mail and other electronic methods, and they also are pressing for and expecting a more integrative approach from their providers – an approach that cares for the whole person and incorporates complementary medical modalities where appropriate.
• Professional shortages and expectations. Shortages of nurses and pharmacists have been noted for more than a decade. More and more, there are shortages of primary care physicians, general surgeons, and other physicians, including ob.gyns., especially in rural and urban poor areas. Newly graduated physicians increasingly want little or no administrative responsibility, less night and weekend call duties, a salaried position instead of a private practice, and family time assured.
• Technology. New technologies have made health care delivery much more nimble by embedding tools into smartphones, allowing providers to access information wirelessly, or miniaturizing equipment. Hand-held ultrasound is now available at a price that a single physician can afford. For hospitals, on the other hand, the cost of new technologies like MRIs, CT scanners, and equipment for radiation therapy is so high that in order to stay abreast of trends, hospitals need substantial hard-to-raise capital.
• Costs. The costs of care are rising with no end in sight, and none of the current reforms and other proposals offered thus far will effectively curb the increases. This is because most approaches offered by government and insurers do not address the real problems. Patients, in the meantime, are facing greater requirements from employers to share the cost of care. Among employer-sponsored plans, there is an increasing push toward high-deductible-plans, with deductibles in the $1,000-$2,000 range.
The most glaring problem in American health care, however – and the largest driver of change – is the limited time that primary care physicians actually spend with their patients. Most primary care physicians in the United State, including many ob.gyns., are trapped in a business model that forces them to see at least 24-25 patients per day (a total patient load of 2,000-plus). The model typically allows for about 12 minutes of face time with each patient and leaves no time for careful listening, for care coordination, for talking with specialists, or for thinking deeply about diagnostic dilemmas.
When patients have a slightly complex case that cannot be solved in 12 minutes or less, physicians are left no option but to refer these patients to a specialist, which dramatically increases the cost of care delivery.
The changes ahead
There are many pilot programs embedded in the Patient Protection and Affordable Health Care Act that attempt to address health care delivery cost and quality, and perhaps some will bear value in coming years. Overall, however, reform in Washington is largely about medical care financing and insurance coverage.
Accountable care organizations and medical homes are good alternatives to traditional care models and could provide outstanding care, but these options will not succeed unless productivity standards are lowered such that the generalist physician truly has the time to listen, think, prevent, and coordinate. Similarly, capitation (payment of a fixed sum for all care for one patient for 1 year) will succeed only if the rate per patient is sufficient enough so that the physician can sustain a practice while seeing fewer patients and hence spend enough time with each one. Thus far, this has rarely been the case.
In the absence of major changes on the horizon from the government or insurers that put incentives and funding in the right places, health care delivery can only transform in fits and starts in response to the major drivers of change. Although some changes to the current system will be truly transformational, many will only be incremental.
Among the transformative – and one could say disruptive – changes will be change in our hospitals. Certainly more and more can be accomplished in the outpatient setting, but as more people survive longer and have more chronic illnesses, there will be a need for more hospital beds, ICUs, operating rooms, and high technology – the reverse of the mantra of recent decades which proclaimed that we had "too many hospitals and too many beds." Hospitals also will need capital for renovations, new wings, and all the needed technology. With credit tight, smaller hospitals will merge into larger systems, and there will be few stand-alone community hospitals in the coming years.
To compensate for the shortage of nonspecialists and to allow generalists to do what is needed and what they are best at doing, there will be greater use of nurse practitioners, physician assistants, nutritionists, exercise physiologists, and other nonphysician professionals. Physicians will increasingly need to embrace, rather than marginalize, the work of adjunct providers in providing quality interaction with patients, augmenting preventive programs, and enhancing care coordination.
Ob.gyns. will likely find such team-building helpful as they strengthen their efforts to provide preventive care and promote healthy behaviors for women of all ages. The model of a health care team also can help ob.gyns. as they strive to deliver more preconception care and to work with women before and during pregnancy to create optimal intrauterine environments that will lead to healthier offspring. Like family physicians and internists, ob.gyns need time to spend with each patient to learn about her family and the environment in which she lives.
To provide comprehensive preventive care, and to adequately coordinate the care of patients with chronic illnesses, some physicians have decided to bill the patient directly for services and not accept commercial insurance or Medicare. Others have opted to convert their practices to "retainer-based" practices, or "concierge" practices, which are, in effect, a type of capitation without the intermediary of the insurance company. The patient pays a fixed annual fee (usually $1,500-$2,000) for all care provided by the physician. In turn, the physician drops the practice size to about 500 patients; guarantees appointments within 24 hours; provides 24/7 cell phone access; and offers appointments that last as long as necessary, the option of e-mail conversations, and visitations in the hospital and emergency room.
Physicians operating such retainer-based practices report working as many hours as they did before, but say that they are giving superior care and that their patients are reporting a much greater level of satisfaction. The total costs of care come way down because the physician now has the time needed to thoughtfully sort out issues, resulting in fewer referrals to specialists, more lifestyle modifications instead of prescriptions, and fewer tests and x-rays. Hospitalizations are reduced by one-half, and unplanned readmissions after hospital discharge also are significantly reduced.
These changes, while quite disruptive, are, in the long run, bright spots on the horizon. While the cost of joining a retainer-based practice is out of reach for some individuals, the retainer system gives proof-of-concept to insurers and the government, who thus far have been too short-sighted to pay more per patient for primary care, and consequently have paid more across the entire spectrum of care. If such models could be adapted for wider use through the reallocation of insurer dollars, it would improve value for everyone.
The extent to which the retainer model will prevail in terms of physicians’ desires and expectations – as opposed to the options of becoming employed or joining large group practices – is uncertain. It is likely that we will see multiple shifts. Certainly, however, the future for delivering care to complex, chronically ill patients lies largely in multidisciplinary team-based care, with primary care physicians – including ob.gyns – serving as the quarterbacks.
Ultimately, key players will need to make it all happen so that we can have a delivery system that serves us well and costs us less. In the meantime, just as is happening in many large clinics, in certain specialty care centers, and in some primary care practices today, there is much that individuals and a combination of the leaders in medicine can do to keep the transformation moving.
Dr. Schimpff is the former chief executive officer of the University of Maryland Medical Center, Baltimore, and is a voluntary professor of medicine at the University of Maryland School of Medicine. He consults for the U.S. Army, medical startups, and Fortune 500 companies. Dr. Schimpff said he has no financial disclosures.
HIV in Pregnancy: An Update
Remarkable progress has been made over the past 30-plus years in the identification of the human immunodeficiency virus, in the development of drugs to treat HIV infection, and in providing access and assuring adherence to these increasingly effective therapies. As a result, we can now offer women with HIV infection a significantly improved prognosis as well as a very high likelihood of having children who will not be infected with HIV.
While HIV infection is still an incurable, lifelong disease, it has in many ways become a chronic disease just like many other chronic diseases – a status that just a generation ago would have been a pipe dream. Today when I see a patient who has hypertension, diabetes, and HIV infection, I am often more worried about managing the hypertension and diabetes than I am about managing the HIV.
In my state of New York, for example, the number of babies born with HIV infection 15 years ago was approximately 100; in 2010, this number was 3.
Whether we practice in an area of high or low prevalence, we each have a responsibility to identify HIV-infected women and, in the context of pregnancy, to ensure that each patient’s prognosis is optimized, and perinatal transmission is prevented by bringing her viral load to an undetectable level. In cases in which an uninfected woman is considering pregnancy and her partner is HIV infected, periconception administration of antiretroviral preexposure prophylaxis is a new and promising tool.
Identifying patients
None of these benefits can be provided to HIV-infected women if their status is unknown. Just as we have to measure blood pressure in order to be able to treat hypertension, we must ensure that women in our practices know their HIV status so that they can avail themselves of the remarkable advances that have been made in the care of HIV infection and the prevention of mother-to-child transmission. Testing appropriately is part of our role.
Certainly, HIV incidence and risk for infection vary across the United States; there are dramatic differences in risk, for instance, between Des Moines, where prevalence is low, and Washington, D.C., where almost 3% of the population is infected with HIV. Still, regardless of geography, we must appreciate that the impact of the epidemic on women has grown significantly over time, such that women now account for approximately one in four people living with HIV, according to the CDC.
What is especially important for us to appreciate is the fact that a sizable portion of all people with HIV still do not know their HIV status. The CDC estimates that approximately 18% of all HIV-infected individuals are unaware of their status.
A significant percentage of the children who are infected, moreover, were born to women whose status was unknown. According to the CDC, approximately 27% of the mothers of HIV-infected infants reported from 2003 to 2007 were diagnosed with HIV after delivery, and only 29% of the mothers of infected infants received antiretroviral therapy (ART) during pregnancy.
The CDC, the American College of Obstetricians and Gynecologists (ACOG), the American Academy of Pediatrics, and other national organizations have long called for universal prenatal HIV testing to improve the health outcomes of the mother and infant.
On a broader level, the approach to testing has evolved. In 2006, the CDC moved away from targeted risk-based HIV testing recommendations and advised routine opt-out testing for all patients aged 13-64 years. The agency concluded that universal testing is more effective largely because many patients found to be infected did not consider themselves to be at risk. ACOG weighed in the following year, also recommending universal testing with patient notification and an opt-out option, which removes the need for detailed, testing-related informed consent.
Some state laws do not allow opt-out testing and still require affirmative consent, however, so it is important to know your state’s laws. Regardless of your state’s situation, however, failing to test because you deem a patient unlikely to be infected is no longer acceptable.
Testing should be approached just as a blood pressure check is approached – as part of a routine battery of tests that is performed unless the patient declines.
Although the adverse consequences of being identified as HIV positive remain to some extent, it is by no means as severe as it was in the 1980s, when women with positive test results were stigmatized and discriminated against. As primary care providers, we should be reassuring in this regard and much more assertive in making sure patients understand the benefits of testing.
In a related move this year, the U.S. Preventive Services Task Force is recommending that all patients aged 15-65 years should be screened for HIV, regardless of their risk level.
Treating infection
Strategies for preventing perinatal transmission and managing HIV disease in pregnancy are evolving so rapidly that it is often best for obstetricians who see only a few HIV-infected patients a year to work in consultation with an obstetrician with expertise in HIV in pregnancy, an infectious disease specialist or HIV specialty care provider, or an internist with expert knowledge of the antiretroviral drugs. The National Institutes of Health’s guidelines for the use of antiretroviral (ARV) drugs in pregnant HIV-infected women, last updated in July 2012, can be a useful reference.
The latest generation of ARV drugs are not only more effective, but also many require only once-a-day dosing schedules, which can improve patient adherence. On the flip side, there are dozens of choices for individualizing treatment, making treatment decisions much more complex than a decade ago.
Among the important trends and changes for ob.gyns to be aware of are the following:
• A "test and treat" paradigm. We are entering a "test and treat" era in the treatment of HIV infection overall, with the paradigm shifting to a much more liberal and immediate recourse to therapy for HIV-infected adults.
Previously, regular monitoring of virologic status would help guide the timing of therapy initiation. For example, CD4 T-lymphocyte counts of less than 350 mm3 or plasma HIV RNA levels that exceeded certain thresholds were the recommended triggers for initiation of ARV therapy. Although there remains some disagreement among experts who support treating a patient when CD4 counts reach 550 mm3 and experts who support treating a patient regardless of CD4 or viral load, I believe the scale is tipping toward treating almost everyone upon diagnosis. Waiting until patients have become more immunocompromised and reached a chronic infection–induced inflammatory state can drive the development of cardiovascular disease and other long-term health care problems.
While some debate persists as to whether all HIV-infected individuals should begin treatment regardless of CD4 count, there is no question as to the appropriate approach for the pregnant woman.
For HIV-infected pregnant women, it is currently recommended that a combination ARV drug regimen be implemented as early as possible antepartum to prevent perinatal transmission, regardless of HIV RNA copy number or CD4 T-lymphocyte count. Evidence that transmission may be lowered with earlier initiation of ART, combined with growing evidence regarding the safety of ARV drugs, has shifted the mindset from a paradigm of avoiding therapy in the first trimester unless absolutely necessary, to a slightly more aggressive approach with earlier initiation of ARV drugs.
Women who enter pregnancy on an effective ARV regimen should continue this regimen without disruption, as drugs changes during pregnancy may be associated with loss of viral control and increased risk of perinatal transmission.
• Lessening concern about risks. Certainly, the known benefits of ARV drugs for a pregnant woman must be weighed against the potential risks of adverse events to the woman, fetus, and newborn. The NIH clinical guidelines categorize various antiretroviral agents for use in pregnancy as preferred, alternative, or for "use in special circumstances."
In general, however, we have acquired more information on the safety of many of these drugs in pregnancy. As data have accumulated, concerns about potential teratogenic effects and other adverse effects of ARV drugs on fetuses and newborns have lessened.
For example, long-standing concerns about tenofovir decreasing fetal bone porosity and potentially causing other anomalies have lessened with recent studies suggesting that adverse events from tenofovir occur infrequently. The use of other drugs, such as efavirenz, also has been liberalized with recent evidence showing that the risk of adverse events is much lower than once believed; recent guidelines now advise that efavirenz rarely needs to be discontinued among women who were receiving it prior to pregnancy.
Obstetricians’ chief role in working with consultants is to ensure that a less-effective drug is not prescribed merely because a patient is pregnant. An effective Class C drug should not be replaced by an ineffective Class B drug solely because an internist does not understand drug use in pregnancy. Obstetricians understand that risks of drugs must be balanced against the risks of untreated diseases.
We sometimes prescribe antiseizure drugs and other category D medications during pregnancy when the benefits outweigh the risks, and preventing HIV transmission from mother to fetus is no different. Suppressing the virus is the primary goal in HIV treatment today, and pregnancy should not preclude the use of therapeutics that can attain that goal.
• New intrapartum approach. Probably the most significant recent change to the NIH guidelines concerns the use of intravenous zidovudine (AZT) during labor in women infected with HIV.
Parenteral AZT had been the standard of care since 1994; however, the 2012 guidelines state that AZT is no longer required during labor for HIV-infected women who are receiving combination antiretroviral regimens and who have an undetectable viral load (HIV RNA less than 400 copies/mL near delivery). Studies have shown that with these two criteria met, the transmission rate is low enough that there is no reason to believe that AZT would provide any additional benefit.
Utilizing the Ob. skill set
The choice of drug regiments for HIV-infected pregnant women should be based on the same principles used to choose regimens for nonpregnant individuals, and it is the obstetrician’s job – not the job of the internist or other expert – to decide whether there are compelling pregnancy-specific reasons that should cause modifications.
When there is concern about an elevated risk of preterm birth, as there is with the use of protease inhibitors in the first trimester, obstetricians can utilize cervical length screening and/or appropriate techniques and approaches for monitoring patients, just as they would monitor other patients with elevated risk.
Obstetricians also can also bring their expertise and knowledge of current obstetrical technologies to the table when it comes to the use of amniocentesis in HIV-infected pregnant women. The risks of amniocentesis are minimal (no perinatal transmissions have been reported after amniocentesis in women without detectable virus and on effective ART), but any small potential or perceived risk can be further reduced by using new tests that allow a determination of the likelihood of chromosomal abnormalities through peripheral blood sampling. If a patient’s peripheral blood status is indicative of lower-than-expected risks of aneuploidy, for instance, the patient may decide she can forego amniocentesis.
Additionally, the field of safe reproduction for HIV-discordant couples is progressing. Data from the NIH-supported HPTN 052 randomized clinical trial show that early initiation of ART in the infected partner significantly reduces HIV transmission to the uninfected partner.
Although not as well studied, periconception administration of antiretroviral pre-exposure prophylaxis (PrEP) for HIV-uninfected partners may offer an additional tool for reducing the risk of sexual transmission. (The NIH guidelines for use of ARV drugs in pregnant HIV-infected women include a section on "Reproductive Options of HIV-Concordant and Serodiscordant Couples.")
Uninfected women should be regularly counseled about consistent condom use, but especially in cases in which couples are opposed to protected intercourse and the use of assisted reproductive techniques (sperm preparation techniques coupled with either intrauterine insemination or in vitro fertilization), the use of antiretroviral medications by uninfected women may provide for safer conception.
Dr. Minkoff serves as chairman of the department of obstetrics and gynecology at Maimonides Medical Center in New York and is a distinguished professor of obstetrics and gynecology at State University of New York–Health Science Center. Hef has published extensively on HIV detection and treatment and has been involved over the years on numerous panels, task forces, and guideline committees dealing with the care of HIV-infected women. He currently serves on the panel for the National Institutes of Health’s guidelines for the use of antiretroviral drugs in pregnant HIV-infected women.
Dr. Minkoff reported that he has no disclosures relevant to this Master Class.
Remarkable progress has been made over the past 30-plus years in the identification of the human immunodeficiency virus, in the development of drugs to treat HIV infection, and in providing access and assuring adherence to these increasingly effective therapies. As a result, we can now offer women with HIV infection a significantly improved prognosis as well as a very high likelihood of having children who will not be infected with HIV.
While HIV infection is still an incurable, lifelong disease, it has in many ways become a chronic disease just like many other chronic diseases – a status that just a generation ago would have been a pipe dream. Today when I see a patient who has hypertension, diabetes, and HIV infection, I am often more worried about managing the hypertension and diabetes than I am about managing the HIV.
In my state of New York, for example, the number of babies born with HIV infection 15 years ago was approximately 100; in 2010, this number was 3.
Whether we practice in an area of high or low prevalence, we each have a responsibility to identify HIV-infected women and, in the context of pregnancy, to ensure that each patient’s prognosis is optimized, and perinatal transmission is prevented by bringing her viral load to an undetectable level. In cases in which an uninfected woman is considering pregnancy and her partner is HIV infected, periconception administration of antiretroviral preexposure prophylaxis is a new and promising tool.
Identifying patients
None of these benefits can be provided to HIV-infected women if their status is unknown. Just as we have to measure blood pressure in order to be able to treat hypertension, we must ensure that women in our practices know their HIV status so that they can avail themselves of the remarkable advances that have been made in the care of HIV infection and the prevention of mother-to-child transmission. Testing appropriately is part of our role.
Certainly, HIV incidence and risk for infection vary across the United States; there are dramatic differences in risk, for instance, between Des Moines, where prevalence is low, and Washington, D.C., where almost 3% of the population is infected with HIV. Still, regardless of geography, we must appreciate that the impact of the epidemic on women has grown significantly over time, such that women now account for approximately one in four people living with HIV, according to the CDC.
What is especially important for us to appreciate is the fact that a sizable portion of all people with HIV still do not know their HIV status. The CDC estimates that approximately 18% of all HIV-infected individuals are unaware of their status.
A significant percentage of the children who are infected, moreover, were born to women whose status was unknown. According to the CDC, approximately 27% of the mothers of HIV-infected infants reported from 2003 to 2007 were diagnosed with HIV after delivery, and only 29% of the mothers of infected infants received antiretroviral therapy (ART) during pregnancy.
The CDC, the American College of Obstetricians and Gynecologists (ACOG), the American Academy of Pediatrics, and other national organizations have long called for universal prenatal HIV testing to improve the health outcomes of the mother and infant.
On a broader level, the approach to testing has evolved. In 2006, the CDC moved away from targeted risk-based HIV testing recommendations and advised routine opt-out testing for all patients aged 13-64 years. The agency concluded that universal testing is more effective largely because many patients found to be infected did not consider themselves to be at risk. ACOG weighed in the following year, also recommending universal testing with patient notification and an opt-out option, which removes the need for detailed, testing-related informed consent.
Some state laws do not allow opt-out testing and still require affirmative consent, however, so it is important to know your state’s laws. Regardless of your state’s situation, however, failing to test because you deem a patient unlikely to be infected is no longer acceptable.
Testing should be approached just as a blood pressure check is approached – as part of a routine battery of tests that is performed unless the patient declines.
Although the adverse consequences of being identified as HIV positive remain to some extent, it is by no means as severe as it was in the 1980s, when women with positive test results were stigmatized and discriminated against. As primary care providers, we should be reassuring in this regard and much more assertive in making sure patients understand the benefits of testing.
In a related move this year, the U.S. Preventive Services Task Force is recommending that all patients aged 15-65 years should be screened for HIV, regardless of their risk level.
Treating infection
Strategies for preventing perinatal transmission and managing HIV disease in pregnancy are evolving so rapidly that it is often best for obstetricians who see only a few HIV-infected patients a year to work in consultation with an obstetrician with expertise in HIV in pregnancy, an infectious disease specialist or HIV specialty care provider, or an internist with expert knowledge of the antiretroviral drugs. The National Institutes of Health’s guidelines for the use of antiretroviral (ARV) drugs in pregnant HIV-infected women, last updated in July 2012, can be a useful reference.
The latest generation of ARV drugs are not only more effective, but also many require only once-a-day dosing schedules, which can improve patient adherence. On the flip side, there are dozens of choices for individualizing treatment, making treatment decisions much more complex than a decade ago.
Among the important trends and changes for ob.gyns to be aware of are the following:
• A "test and treat" paradigm. We are entering a "test and treat" era in the treatment of HIV infection overall, with the paradigm shifting to a much more liberal and immediate recourse to therapy for HIV-infected adults.
Previously, regular monitoring of virologic status would help guide the timing of therapy initiation. For example, CD4 T-lymphocyte counts of less than 350 mm3 or plasma HIV RNA levels that exceeded certain thresholds were the recommended triggers for initiation of ARV therapy. Although there remains some disagreement among experts who support treating a patient when CD4 counts reach 550 mm3 and experts who support treating a patient regardless of CD4 or viral load, I believe the scale is tipping toward treating almost everyone upon diagnosis. Waiting until patients have become more immunocompromised and reached a chronic infection–induced inflammatory state can drive the development of cardiovascular disease and other long-term health care problems.
While some debate persists as to whether all HIV-infected individuals should begin treatment regardless of CD4 count, there is no question as to the appropriate approach for the pregnant woman.
For HIV-infected pregnant women, it is currently recommended that a combination ARV drug regimen be implemented as early as possible antepartum to prevent perinatal transmission, regardless of HIV RNA copy number or CD4 T-lymphocyte count. Evidence that transmission may be lowered with earlier initiation of ART, combined with growing evidence regarding the safety of ARV drugs, has shifted the mindset from a paradigm of avoiding therapy in the first trimester unless absolutely necessary, to a slightly more aggressive approach with earlier initiation of ARV drugs.
Women who enter pregnancy on an effective ARV regimen should continue this regimen without disruption, as drugs changes during pregnancy may be associated with loss of viral control and increased risk of perinatal transmission.
• Lessening concern about risks. Certainly, the known benefits of ARV drugs for a pregnant woman must be weighed against the potential risks of adverse events to the woman, fetus, and newborn. The NIH clinical guidelines categorize various antiretroviral agents for use in pregnancy as preferred, alternative, or for "use in special circumstances."
In general, however, we have acquired more information on the safety of many of these drugs in pregnancy. As data have accumulated, concerns about potential teratogenic effects and other adverse effects of ARV drugs on fetuses and newborns have lessened.
For example, long-standing concerns about tenofovir decreasing fetal bone porosity and potentially causing other anomalies have lessened with recent studies suggesting that adverse events from tenofovir occur infrequently. The use of other drugs, such as efavirenz, also has been liberalized with recent evidence showing that the risk of adverse events is much lower than once believed; recent guidelines now advise that efavirenz rarely needs to be discontinued among women who were receiving it prior to pregnancy.
Obstetricians’ chief role in working with consultants is to ensure that a less-effective drug is not prescribed merely because a patient is pregnant. An effective Class C drug should not be replaced by an ineffective Class B drug solely because an internist does not understand drug use in pregnancy. Obstetricians understand that risks of drugs must be balanced against the risks of untreated diseases.
We sometimes prescribe antiseizure drugs and other category D medications during pregnancy when the benefits outweigh the risks, and preventing HIV transmission from mother to fetus is no different. Suppressing the virus is the primary goal in HIV treatment today, and pregnancy should not preclude the use of therapeutics that can attain that goal.
• New intrapartum approach. Probably the most significant recent change to the NIH guidelines concerns the use of intravenous zidovudine (AZT) during labor in women infected with HIV.
Parenteral AZT had been the standard of care since 1994; however, the 2012 guidelines state that AZT is no longer required during labor for HIV-infected women who are receiving combination antiretroviral regimens and who have an undetectable viral load (HIV RNA less than 400 copies/mL near delivery). Studies have shown that with these two criteria met, the transmission rate is low enough that there is no reason to believe that AZT would provide any additional benefit.
Utilizing the Ob. skill set
The choice of drug regiments for HIV-infected pregnant women should be based on the same principles used to choose regimens for nonpregnant individuals, and it is the obstetrician’s job – not the job of the internist or other expert – to decide whether there are compelling pregnancy-specific reasons that should cause modifications.
When there is concern about an elevated risk of preterm birth, as there is with the use of protease inhibitors in the first trimester, obstetricians can utilize cervical length screening and/or appropriate techniques and approaches for monitoring patients, just as they would monitor other patients with elevated risk.
Obstetricians also can also bring their expertise and knowledge of current obstetrical technologies to the table when it comes to the use of amniocentesis in HIV-infected pregnant women. The risks of amniocentesis are minimal (no perinatal transmissions have been reported after amniocentesis in women without detectable virus and on effective ART), but any small potential or perceived risk can be further reduced by using new tests that allow a determination of the likelihood of chromosomal abnormalities through peripheral blood sampling. If a patient’s peripheral blood status is indicative of lower-than-expected risks of aneuploidy, for instance, the patient may decide she can forego amniocentesis.
Additionally, the field of safe reproduction for HIV-discordant couples is progressing. Data from the NIH-supported HPTN 052 randomized clinical trial show that early initiation of ART in the infected partner significantly reduces HIV transmission to the uninfected partner.
Although not as well studied, periconception administration of antiretroviral pre-exposure prophylaxis (PrEP) for HIV-uninfected partners may offer an additional tool for reducing the risk of sexual transmission. (The NIH guidelines for use of ARV drugs in pregnant HIV-infected women include a section on "Reproductive Options of HIV-Concordant and Serodiscordant Couples.")
Uninfected women should be regularly counseled about consistent condom use, but especially in cases in which couples are opposed to protected intercourse and the use of assisted reproductive techniques (sperm preparation techniques coupled with either intrauterine insemination or in vitro fertilization), the use of antiretroviral medications by uninfected women may provide for safer conception.
Dr. Minkoff serves as chairman of the department of obstetrics and gynecology at Maimonides Medical Center in New York and is a distinguished professor of obstetrics and gynecology at State University of New York–Health Science Center. Hef has published extensively on HIV detection and treatment and has been involved over the years on numerous panels, task forces, and guideline committees dealing with the care of HIV-infected women. He currently serves on the panel for the National Institutes of Health’s guidelines for the use of antiretroviral drugs in pregnant HIV-infected women.
Dr. Minkoff reported that he has no disclosures relevant to this Master Class.
Remarkable progress has been made over the past 30-plus years in the identification of the human immunodeficiency virus, in the development of drugs to treat HIV infection, and in providing access and assuring adherence to these increasingly effective therapies. As a result, we can now offer women with HIV infection a significantly improved prognosis as well as a very high likelihood of having children who will not be infected with HIV.
While HIV infection is still an incurable, lifelong disease, it has in many ways become a chronic disease just like many other chronic diseases – a status that just a generation ago would have been a pipe dream. Today when I see a patient who has hypertension, diabetes, and HIV infection, I am often more worried about managing the hypertension and diabetes than I am about managing the HIV.
In my state of New York, for example, the number of babies born with HIV infection 15 years ago was approximately 100; in 2010, this number was 3.
Whether we practice in an area of high or low prevalence, we each have a responsibility to identify HIV-infected women and, in the context of pregnancy, to ensure that each patient’s prognosis is optimized, and perinatal transmission is prevented by bringing her viral load to an undetectable level. In cases in which an uninfected woman is considering pregnancy and her partner is HIV infected, periconception administration of antiretroviral preexposure prophylaxis is a new and promising tool.
Identifying patients
None of these benefits can be provided to HIV-infected women if their status is unknown. Just as we have to measure blood pressure in order to be able to treat hypertension, we must ensure that women in our practices know their HIV status so that they can avail themselves of the remarkable advances that have been made in the care of HIV infection and the prevention of mother-to-child transmission. Testing appropriately is part of our role.
Certainly, HIV incidence and risk for infection vary across the United States; there are dramatic differences in risk, for instance, between Des Moines, where prevalence is low, and Washington, D.C., where almost 3% of the population is infected with HIV. Still, regardless of geography, we must appreciate that the impact of the epidemic on women has grown significantly over time, such that women now account for approximately one in four people living with HIV, according to the CDC.
What is especially important for us to appreciate is the fact that a sizable portion of all people with HIV still do not know their HIV status. The CDC estimates that approximately 18% of all HIV-infected individuals are unaware of their status.
A significant percentage of the children who are infected, moreover, were born to women whose status was unknown. According to the CDC, approximately 27% of the mothers of HIV-infected infants reported from 2003 to 2007 were diagnosed with HIV after delivery, and only 29% of the mothers of infected infants received antiretroviral therapy (ART) during pregnancy.
The CDC, the American College of Obstetricians and Gynecologists (ACOG), the American Academy of Pediatrics, and other national organizations have long called for universal prenatal HIV testing to improve the health outcomes of the mother and infant.
On a broader level, the approach to testing has evolved. In 2006, the CDC moved away from targeted risk-based HIV testing recommendations and advised routine opt-out testing for all patients aged 13-64 years. The agency concluded that universal testing is more effective largely because many patients found to be infected did not consider themselves to be at risk. ACOG weighed in the following year, also recommending universal testing with patient notification and an opt-out option, which removes the need for detailed, testing-related informed consent.
Some state laws do not allow opt-out testing and still require affirmative consent, however, so it is important to know your state’s laws. Regardless of your state’s situation, however, failing to test because you deem a patient unlikely to be infected is no longer acceptable.
Testing should be approached just as a blood pressure check is approached – as part of a routine battery of tests that is performed unless the patient declines.
Although the adverse consequences of being identified as HIV positive remain to some extent, it is by no means as severe as it was in the 1980s, when women with positive test results were stigmatized and discriminated against. As primary care providers, we should be reassuring in this regard and much more assertive in making sure patients understand the benefits of testing.
In a related move this year, the U.S. Preventive Services Task Force is recommending that all patients aged 15-65 years should be screened for HIV, regardless of their risk level.
Treating infection
Strategies for preventing perinatal transmission and managing HIV disease in pregnancy are evolving so rapidly that it is often best for obstetricians who see only a few HIV-infected patients a year to work in consultation with an obstetrician with expertise in HIV in pregnancy, an infectious disease specialist or HIV specialty care provider, or an internist with expert knowledge of the antiretroviral drugs. The National Institutes of Health’s guidelines for the use of antiretroviral (ARV) drugs in pregnant HIV-infected women, last updated in July 2012, can be a useful reference.
The latest generation of ARV drugs are not only more effective, but also many require only once-a-day dosing schedules, which can improve patient adherence. On the flip side, there are dozens of choices for individualizing treatment, making treatment decisions much more complex than a decade ago.
Among the important trends and changes for ob.gyns to be aware of are the following:
• A "test and treat" paradigm. We are entering a "test and treat" era in the treatment of HIV infection overall, with the paradigm shifting to a much more liberal and immediate recourse to therapy for HIV-infected adults.
Previously, regular monitoring of virologic status would help guide the timing of therapy initiation. For example, CD4 T-lymphocyte counts of less than 350 mm3 or plasma HIV RNA levels that exceeded certain thresholds were the recommended triggers for initiation of ARV therapy. Although there remains some disagreement among experts who support treating a patient when CD4 counts reach 550 mm3 and experts who support treating a patient regardless of CD4 or viral load, I believe the scale is tipping toward treating almost everyone upon diagnosis. Waiting until patients have become more immunocompromised and reached a chronic infection–induced inflammatory state can drive the development of cardiovascular disease and other long-term health care problems.
While some debate persists as to whether all HIV-infected individuals should begin treatment regardless of CD4 count, there is no question as to the appropriate approach for the pregnant woman.
For HIV-infected pregnant women, it is currently recommended that a combination ARV drug regimen be implemented as early as possible antepartum to prevent perinatal transmission, regardless of HIV RNA copy number or CD4 T-lymphocyte count. Evidence that transmission may be lowered with earlier initiation of ART, combined with growing evidence regarding the safety of ARV drugs, has shifted the mindset from a paradigm of avoiding therapy in the first trimester unless absolutely necessary, to a slightly more aggressive approach with earlier initiation of ARV drugs.
Women who enter pregnancy on an effective ARV regimen should continue this regimen without disruption, as drugs changes during pregnancy may be associated with loss of viral control and increased risk of perinatal transmission.
• Lessening concern about risks. Certainly, the known benefits of ARV drugs for a pregnant woman must be weighed against the potential risks of adverse events to the woman, fetus, and newborn. The NIH clinical guidelines categorize various antiretroviral agents for use in pregnancy as preferred, alternative, or for "use in special circumstances."
In general, however, we have acquired more information on the safety of many of these drugs in pregnancy. As data have accumulated, concerns about potential teratogenic effects and other adverse effects of ARV drugs on fetuses and newborns have lessened.
For example, long-standing concerns about tenofovir decreasing fetal bone porosity and potentially causing other anomalies have lessened with recent studies suggesting that adverse events from tenofovir occur infrequently. The use of other drugs, such as efavirenz, also has been liberalized with recent evidence showing that the risk of adverse events is much lower than once believed; recent guidelines now advise that efavirenz rarely needs to be discontinued among women who were receiving it prior to pregnancy.
Obstetricians’ chief role in working with consultants is to ensure that a less-effective drug is not prescribed merely because a patient is pregnant. An effective Class C drug should not be replaced by an ineffective Class B drug solely because an internist does not understand drug use in pregnancy. Obstetricians understand that risks of drugs must be balanced against the risks of untreated diseases.
We sometimes prescribe antiseizure drugs and other category D medications during pregnancy when the benefits outweigh the risks, and preventing HIV transmission from mother to fetus is no different. Suppressing the virus is the primary goal in HIV treatment today, and pregnancy should not preclude the use of therapeutics that can attain that goal.
• New intrapartum approach. Probably the most significant recent change to the NIH guidelines concerns the use of intravenous zidovudine (AZT) during labor in women infected with HIV.
Parenteral AZT had been the standard of care since 1994; however, the 2012 guidelines state that AZT is no longer required during labor for HIV-infected women who are receiving combination antiretroviral regimens and who have an undetectable viral load (HIV RNA less than 400 copies/mL near delivery). Studies have shown that with these two criteria met, the transmission rate is low enough that there is no reason to believe that AZT would provide any additional benefit.
Utilizing the Ob. skill set
The choice of drug regiments for HIV-infected pregnant women should be based on the same principles used to choose regimens for nonpregnant individuals, and it is the obstetrician’s job – not the job of the internist or other expert – to decide whether there are compelling pregnancy-specific reasons that should cause modifications.
When there is concern about an elevated risk of preterm birth, as there is with the use of protease inhibitors in the first trimester, obstetricians can utilize cervical length screening and/or appropriate techniques and approaches for monitoring patients, just as they would monitor other patients with elevated risk.
Obstetricians also can also bring their expertise and knowledge of current obstetrical technologies to the table when it comes to the use of amniocentesis in HIV-infected pregnant women. The risks of amniocentesis are minimal (no perinatal transmissions have been reported after amniocentesis in women without detectable virus and on effective ART), but any small potential or perceived risk can be further reduced by using new tests that allow a determination of the likelihood of chromosomal abnormalities through peripheral blood sampling. If a patient’s peripheral blood status is indicative of lower-than-expected risks of aneuploidy, for instance, the patient may decide she can forego amniocentesis.
Additionally, the field of safe reproduction for HIV-discordant couples is progressing. Data from the NIH-supported HPTN 052 randomized clinical trial show that early initiation of ART in the infected partner significantly reduces HIV transmission to the uninfected partner.
Although not as well studied, periconception administration of antiretroviral pre-exposure prophylaxis (PrEP) for HIV-uninfected partners may offer an additional tool for reducing the risk of sexual transmission. (The NIH guidelines for use of ARV drugs in pregnant HIV-infected women include a section on "Reproductive Options of HIV-Concordant and Serodiscordant Couples.")
Uninfected women should be regularly counseled about consistent condom use, but especially in cases in which couples are opposed to protected intercourse and the use of assisted reproductive techniques (sperm preparation techniques coupled with either intrauterine insemination or in vitro fertilization), the use of antiretroviral medications by uninfected women may provide for safer conception.
Dr. Minkoff serves as chairman of the department of obstetrics and gynecology at Maimonides Medical Center in New York and is a distinguished professor of obstetrics and gynecology at State University of New York–Health Science Center. Hef has published extensively on HIV detection and treatment and has been involved over the years on numerous panels, task forces, and guideline committees dealing with the care of HIV-infected women. He currently serves on the panel for the National Institutes of Health’s guidelines for the use of antiretroviral drugs in pregnant HIV-infected women.
Dr. Minkoff reported that he has no disclosures relevant to this Master Class.
Master Class named 'Best Regular Column'
Ob.Gyn. News has received the Gold Award from the American Society of Healthcare Publication Editors for having the "best regular column." Master Class, a column that has run regularly in the print publication since January 2004 and is featured on Ob.Gyn. News Digital Network, earned the prestigious award for demonstrating editorial excellence and achievement.
The column is written by Christine Kilgore. The medical editors are Dr. Charles E. Miller and Dr. E. Albert Reece. Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
Dr. Reece is vice president for medical affairs, the John Z. and Akiko K. Bowers Distinguished Professor, and dean of the University of Maryland School of Medicine in Baltimore. He is also professor in the departments of obstetrics and gynecology, medicine, and biochemistry and molecular biology.
ASHPE, "is dedicated to enhancing the knowledge and skills of healthcare publication editors, and thereby expanding their value to, and contribution of, the publications they serve," according to a statement on their website. The organization is "committed to fostering the highest ethical standards in management; rewarding excellence in publications development and editorial performance; and serving as an authority on evolving trends in the healthcare publishing sector."
Ob.Gyn. News has received the Gold Award from the American Society of Healthcare Publication Editors for having the "best regular column." Master Class, a column that has run regularly in the print publication since January 2004 and is featured on Ob.Gyn. News Digital Network, earned the prestigious award for demonstrating editorial excellence and achievement.
The column is written by Christine Kilgore. The medical editors are Dr. Charles E. Miller and Dr. E. Albert Reece. Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
Dr. Reece is vice president for medical affairs, the John Z. and Akiko K. Bowers Distinguished Professor, and dean of the University of Maryland School of Medicine in Baltimore. He is also professor in the departments of obstetrics and gynecology, medicine, and biochemistry and molecular biology.
ASHPE, "is dedicated to enhancing the knowledge and skills of healthcare publication editors, and thereby expanding their value to, and contribution of, the publications they serve," according to a statement on their website. The organization is "committed to fostering the highest ethical standards in management; rewarding excellence in publications development and editorial performance; and serving as an authority on evolving trends in the healthcare publishing sector."
Ob.Gyn. News has received the Gold Award from the American Society of Healthcare Publication Editors for having the "best regular column." Master Class, a column that has run regularly in the print publication since January 2004 and is featured on Ob.Gyn. News Digital Network, earned the prestigious award for demonstrating editorial excellence and achievement.
The column is written by Christine Kilgore. The medical editors are Dr. Charles E. Miller and Dr. E. Albert Reece. Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
Dr. Reece is vice president for medical affairs, the John Z. and Akiko K. Bowers Distinguished Professor, and dean of the University of Maryland School of Medicine in Baltimore. He is also professor in the departments of obstetrics and gynecology, medicine, and biochemistry and molecular biology.
ASHPE, "is dedicated to enhancing the knowledge and skills of healthcare publication editors, and thereby expanding their value to, and contribution of, the publications they serve," according to a statement on their website. The organization is "committed to fostering the highest ethical standards in management; rewarding excellence in publications development and editorial performance; and serving as an authority on evolving trends in the healthcare publishing sector."