User login
Hysteroscopic electromechanical power morcellation
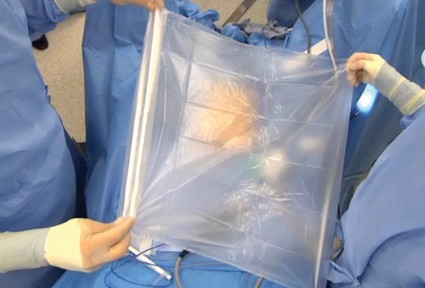
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
One of the hottest and most controversial topics in gynecologic surgery, at present, is laparoscopic electromechanical power morcellation.
In April of this year, the Food and Drug Administration sent out a news release regarding the potential risk of spread of sarcomatous tissue at the time of this procedure. In that release, the agency "discouraged" use of laparoscopic electromechanical power morcellation. Responses came from many societies, including the American College of Obstetricians and Gynecologists and the AAGL, which indicated that laparoscopic electromechanical power morcellation could be used if proper care was taken.
I am personally proud that the Master Class in Gynecologic Surgery has been very proactive and diligent in its discussion of laparoscopic electromechanical power morcellation. This is the third in our series regarding this topic.
In our first segment, I discussed the issue of electromechanical power morcellation relative to the inadvertent spread of sarcomatous tissue. In our second in the series, Dr. Ceana Nezhat, Dr. Bernard Taylor, and Dr. Tony Shibley discussed ways to minimize this risk – including morcellation in a bag. Videos of their individual techniques of electromechanical power morcellation, as well as that of Dr. Douglas Brown, can be viewed on SurgeryU. In addition, my partner, Dr. Aarathi Cholkeri-Singh, and I have a video on SurgeryU illustrating our technique of morcellation in a bag.
This current Master Class in Gynecologic Surgery is now devoted to hysteroscopic electromechanical power morcellation. In my discussions with physicians throughout the country relative to this technique, it has become evident that some institutions have not only banned the use of electromechanical power morcellation at time of laparoscopy, but have also stopped usage of hysteroscopic electromechanical power morcellation. While neither the FDA nor the lay press has ever questioned the use of hysteroscopic morcellators, I believe it is imperative that this topic be reviewed. I am sure that it will be obvious that hysteroscopic electromechanical power morcellation has thus far proved to be a safe and effective treatment option for various pathologic entities, including submucosal uterine fibroids.
To review hysteroscopic electromechanical power morcellation, I have invited Dr. Joseph S. Sanfilippo, professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh.
Dr. Sanfilippo is a lecturer and educator. He has written an extensive number of peer-reviewed articles, and has been a contributor to several textbooks. In addition, Dr. Sanfilippo has been and remains a very active member of the AAGL.
It is a pleasure and honor to welcome Dr. Sanfilippo to this edition of the Master Class in Gynecologic Surgery, the third installment on morcellation.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant to Hologic and is on the speakers bureau for Smith & Nephew. Videos for this and past Master Class in Gynecology Surgery articles can be viewed on SurgeryU.
Hysteroscopic morcellation – a very different entity
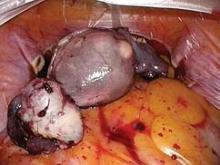
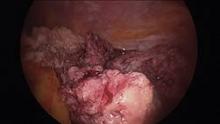
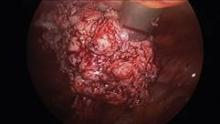
Submucous leiomyomas are the most problematic type of fibroid and have been associated with abnormal uterine bleeding, infertility, and other clinical issues. Treatment has been shown to be effective in improving fertility and success rates with assisted reproduction.
Newer hysteroscopic surgical techniques and morcellation technology allow us to remove not only polyps, but selected submucous myomas, in a fashion that is not only minimally invasive but that also raises few if any concerns about spreading or upstaging an unsuspected leiomyosarcoma. In this respect, the controversy over laparoscopic power morcellation does not extend to hysteroscopic morcellation.
Such a distinction was made during opening remarks at a meeting in June 2014 of the Obstetrics & Gynecology Devices Panel of the Food and Drug Administration’s Medical Devices Advisory Committee, which was charged with addressing such concerns.
Dr. Aron Yustein, deputy director of clinical affairs and chief medical officer of the FDA’s Office of Surveillance and Biometrics, explained that the panel would not address hysteroscopic morcellators "as we do not believe that when used [as intended], they pose the same risk" as that of laparoscopic morcellation in terms of potentially disseminating and upstaging an undetected uterine malignancy.
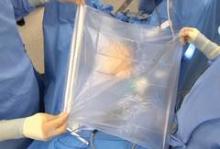
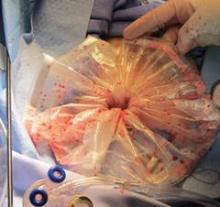
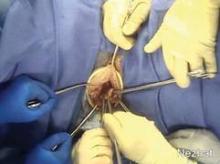
In hysteroscopic morcellation, tissue is contained and delivered through the morcellation system into a trap, or collecting pouch. This allows for complete capture and histopathologic assessment of all fragments extracted from the uterine cavity.
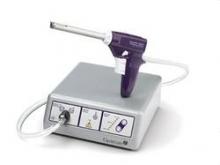
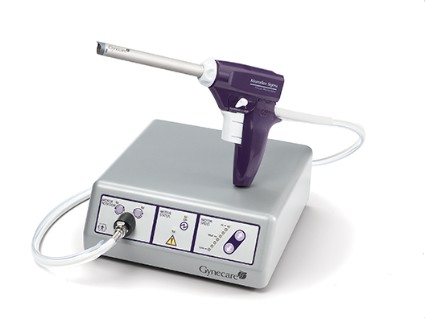
Numerous equipment options are currently available to gynecologic surgeons for hysteroscopically-guided myomectomy: Newer systems such as the Gynecare VersaPoint (Ethicon Endo-Surgery), and the Symphion system (Boston Scientific) facilitate bipolar electrosurgical resection. MyoSure (Hologic) and TRUCLEAR (Smith & Nephew), on the other hand, are hysteroscopic morcellators; they both use mechanical energy rather than high-frequency electrical energy to simultaneously cut and aspirate tissue.
Common to each of these options are advanced, automated fluid management systems that continuously measure distending media input and output, intrauterine distension pressure, and fluid deficit volume throughout the procedure. Such monitoring is critical to preventing excess fluid absorption and its associated complications. The new fluid management systems allow excellent visualization of the intrauterine cavity.
Benefit of Treatment
Leiomyomas, synonymously known as myomas, are among the uterine bleeding abnormalities included in a new classification system introduced in 2011 by the International Federation of Gynecology and Obstetrics. The system classifies the causes of abnormal uterine bleeding in reproductive-aged women; it is known by the acronym PALM-COEIN, for polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified.
In a practice bulletin published in 2012, the American College of Obstetricians and Gynecologists endorsed the nomenclature system and provided guidelines for evaluating reproductive-aged patients with abnormal uterine bleeding (Obstet. Gynecol. 2012;120:197-206).
The diagnosis and management of submucous leiomyomas is particularly important in cases of infertility, as these types of myomas (compared with intramural or subserosal) appear to have the greatest impact on pregnancy and implantation rates.
In general, uterine myomas are found in 5%-10% of women with infertility. In 1%-3% of infertility patients, myomas are the only abnormal findings. As described in a literature review, it is believed that myomas may interfere with sperm transport or access, and with implantation. Endometrial cavity deformity, cornual ostia obstruction, altered uterine contractility, and altered endometrial development may each play a role (Obstet. Gynecol. Clin. North Am. 2006;33:145-52).
Studies evaluating the impact of myomectomy on fertility outcomes provide evidence that submucous myomas should be removed before assisted reproductive technology/in vitro fertilization. According to the AAGL’s practice guidelines on the diagnosis and management of submucous leiomyomas, it "seems clear from high-quality studies that pregnancy rates are higher after myomectomy than no or ‘placebo’ procedures" (J. Minim. Invasive Gynecol. 2012;19:152-71).
The most widely used system for categorizing submucous myomas, developed by the European Society of Gynecological Endoscopy (ESGE), breaks them into three subtypes according to how much of the lesion’s diameter is contained within the myometrium: Type 0 myomas are entirely within the endometrial cavity, while type I have less than 50% myometrial extension, and type II are 50% or more within the myometrium.
It is the ESGE type 0 submucous myomas that are appropriate for resectoscopic surgery.
(Another system known as the STEPW classification system adds other categories, taking into account factors such as topography, extension of the base, and penetration. This system is becoming more recognized and may be useful in the future for evaluating patients for resectoscopic surgery and predicting outcomes, but it is not being used as often as the ESGE classification system.)
As the AAGL guidelines state, diagnosis is generally achieved with one or a combination of hysteroscopic and radiological techniques that may include transvaginal ultrasonography, saline infusion sonohysterography, and magnetic resonance imaging.
Research on safety
Hysteroscopic morcellation is the most recent operative hysteroscopic technique to be employed for the removal of submucous leiomyomas. In lieu of concerns about laparoscopic power morcellation, the question arises: Should we be concerned about cancer and hysteroscopy?
Numerous studies have looked at the question of whether hysteroscopic procedures produce intraperitoneal spread of endometrial cancer cells and, if so, whether this results in the "upstaging" of unsuspected cancer. Much of the research has involved diagnostic hysteroscopy, which includes the use of intrauterine cavity distension with fluid media, similar to that of operative hysteroscopy.
Investigators at Memorial Sloan-Kettering Cancer Center in New York, for instance, looked retrospectively at whether initial diagnostic procedures were associated with abnormal peritoneal washings (PW) in almost 300 women who were treated for endometrial carcinoma with hysterectomy and intraoperative PW. They found no association between the initial diagnostic procedures, including hysteroscopy, and the results of peritoneal cytology (Cancer 2000;90:143-7).
Similarly, physicians in the Czech Republic compared PW done at the start of surgery in 134 patients whose endometrial carcinoma had been diagnosed by hysteroscopy with 61 patients whose cancer had been diagnosed by dilation and curettage. The results, they said, suggest that hysteroscopy does not increase the risk of penetration of tumor cells into the peritoneal cavity more than does D&C (Eur. J. Gynaecol. Oncol. 2001; 22:342-4).
Another retrospective study of 146 patients with endometrial cancer who underwent either D&C or office hysteroscopy showed that diagnostic hysteroscopy did not increase the risk of adnexal, abdominal, or retroperitoneal lymph node metastases, compared with D&C, although there was an increase in positive peritoneal cytology (Gynecol. Oncol. 2007;107:94-8).
At least two broader reviews/meta-analyses also show no evidence for an upstaging of cancer from hysteroscopic procedures performed in the presence of cancer.
A meta-analysis of 19 studies suggests that preoperative hysteroscopy resulted in a statistically significant higher risk of positive peritoneal cytology compared with no hysteroscopy, but there was no evidence to support avoiding diagnostic hysteroscopy prior to surgical intervention for endometrial cancer (Fertil. Steril. 2011;96:957-61).
A literature review covering studies published between 1980 and 2001 showed that while there might be an increased risk of peritoneal contamination by cancer cells after hysteroscopy, there is no evidence that these patients fare worse compared with patients who have undergone other diagnostic procedures (Obstet. Gynecol. Surv. 2004;59:280-4).
Surgical rather than diagnostic hysterectomy was the focus of one recent case report from Italy. The patient was a 52-year-old nulliparous woman with a leiomyosarcoma detected 2 months after a hysteroscopic resection of a presumed myoma. After resection, the myoma was determined to be an atypical "mitotically active" leiomyoma (Eur. J. Gynaecol. Oncol. 2012;33:656-7).
The authors emphasize the "rarity" of this particular finding, and the available data overall offer no evidence for an upstaging of unsuspected endometrial cancer with hysteroscopic procedures. While hysteroscopy should not be used in cases of known cancer, as it does not facilitate treatment, there are no data that should lead us to be concerned about adverse effects in the presence of cancer.
Current systems
Traditionally, resectoscopy has posed numerous challenges for the removal of intracavitary lesions: Tissue removal has been difficult and time consuming. Visibility has been disrupted by gas bubbles, tissue fragments, blood clots, and cervical mucus. Multiple insertions have been required, raising the risk of embolism (a "piston effect"). There also have been concerns about the risk of perforation and about the learning curve.
Older resectoscopes – loop-electrode resectoscopes – were designed for monopolar electrosurgery, which requires the use of nonconductive, electrolyte-free solutions for uterine distension. This limited the amount of fluid absorption that could occur before procedures needed to be stopped.
The incorporation of bipolar instrumentation – and more recently, the development of hysteroscopic morcellation systems that use reciprocating blades driven by mechanical energy rather radiofrequency electrical energy – have enabled the use of electrolyte-containing distending media (saline or Ringer’s Lactate) and, consequently, a higher allowable amount of fluid absorption.
Saline is an ideal medium: It is isotonic, nonhemolytic, nonconductive, nontoxic, and rapidly cleared. The AAGL’s Practice Guidelines for the Management of Hysteroscopic Distending Media lists an intravasation safety limit of 2,500 cc for isotonic solution, compared with a maximum limit of 1,000 cc when using hypotonic solutions (J. Minim. Invasive Gynecol. 2013;20:137-48). This higher cut-off means we can achieve the vast majority of myoma resections in one sitting.
Hysteroscopic morcellators have additional advantages, in my experience. They allow for the use of smaller-diameter hysteroscopes, which in turn requires less cervical dilation. They also have improved reciprocating blades that enable the resection of myomas in addition to endometrial polyps. Previously, the focus was primarily on hysteroscopic polypectomy.
As technology has advanced with tissue removal being instantaneous, there is simultaneous cutting and extraction, and resections are therefore quicker. Overall, there is better visualization and a lower risk of perforation. The learning curve is quicker.
In a randomized trial focused on polypectomy, hysteroscopic mechanical morcellation was superior to electrosurgical resection. The multicenter trial from the United Kingdom compared the two modalities for removal of endometrial polyps in 121 women, and found that hysteroscopic morcellation with a mechanical-based morcellator was significantly quicker for polyp removal (a median time of 5½ minutes, versus 10 minutes, approximately), less painful and more acceptable to women, and more likely to completely remove the polyps (98% compared with 83%), the investigators reported (Obstet. Gynecol. 2014;123:745-51).
The only surgical complications in either group were vasovagal reactions, which occurred in 2% (1 out of 62) and 10% (6 out of 59) of the hysteroscopic morcellation and electrosurgical resection procedures, respectively. There was one serious adverse event, with a woman treated 2 weeks after morcellation for endomyometritis.
Indeed, infection, perforation and cervical trauma, mechanical complications, and media-related complications (intravasation and gas embolism) are risks with all modalities of operative hysteroscopy and all indications. Bleeding appears rarely to be a problem with mechanical morcellation, however, as does perforation. Certainly, perforation that occurs with a nonelectrical morcellator will be significantly less complicating than when energy is engaged.
Our experience overall with resections of intracavitary polyps and small myomas via hysteroscopic morcellation in 50 cases indicates a mean operating time of 9.4 min, a mean fluid deficit of 329 milliliters, and a mean surgeon rating of 9, with 10 representing an excellent rating. We have had no intra-or postoperative hemorrhage, no obvious electrolyte changes, and uneventful recoveries.
The majority of our hysteroscopic morcellations are done under conscious sedation with the addition of a local anesthetic in the form of a paracervical block. A 200-mcg vaginal tablet of misoprostol (Cytotec) off label the night before surgery is the pretreatment strategy I most often employ for cervical preparation. To prevent infection, I prescribe one dose of a broad-spectrum antibiotic, such as a cephalosporin, to my patients receiving myomectomies.
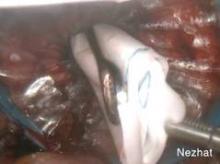
To learn hysteroscopic morcellation, one should begin with polypectomy and move to myomectomy once comfortable. With the TRUCLEAR system, the system I use most frequently, the hysteroscopic sheath should be inserted with the obturator in place to lessen cervical trauma.
The early flow of saline will not only aid the insertion process, it will assist in achieving good visualization quickly, as will increasing the uterine pressure setting at the start of the process. After the beginning of the procedure, however, pressure is maintained at the lowest setting capable of achieving adequate distension and providing good visualization.
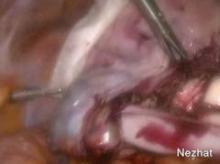
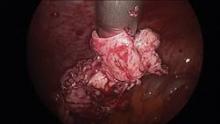
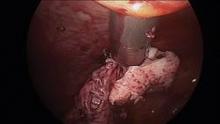
When morcellating pathology, one should work from the periphery to the base. The pathology is kept between the morcellator blade opening and the optics of the camera. Large myomas can be split in half, with each half approached from distal to proximal.
Running the morcellator in the open cavity for a short time will aid in clearing the visual field of debris. Overall, however, visualization with today’s hysteroscopic morcellators and advancements in fluid management is excellent. In our experience, hysteroscopic morcellation is proving to be a safe and effective tool for performing myomectomy and addressing problems of infertility and abnormal uterine bleeding.
Dr. Sanfilippo is professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh. He is on the advisory board for Bayer Healthcare and Smith &Nephew. A lecturer and educator, Dr. Sanfilippo has written peer-reviewed articles and has been a contributor to several textbooks. He is a member of the AAGL.
Submucous leiomyomas are the most problematic type of fibroid and have been associated with abnormal uterine bleeding, infertility, and other clinical issues. Treatment has been shown to be effective in improving fertility and success rates with assisted reproduction.
Newer hysteroscopic surgical techniques and morcellation technology allow us to remove not only polyps, but selected submucous myomas, in a fashion that is not only minimally invasive but that also raises few if any concerns about spreading or upstaging an unsuspected leiomyosarcoma. In this respect, the controversy over laparoscopic power morcellation does not extend to hysteroscopic morcellation.
Such a distinction was made during opening remarks at a meeting in June 2014 of the Obstetrics & Gynecology Devices Panel of the Food and Drug Administration’s Medical Devices Advisory Committee, which was charged with addressing such concerns.
Dr. Aron Yustein, deputy director of clinical affairs and chief medical officer of the FDA’s Office of Surveillance and Biometrics, explained that the panel would not address hysteroscopic morcellators "as we do not believe that when used [as intended], they pose the same risk" as that of laparoscopic morcellation in terms of potentially disseminating and upstaging an undetected uterine malignancy.
In hysteroscopic morcellation, tissue is contained and delivered through the morcellation system into a trap, or collecting pouch. This allows for complete capture and histopathologic assessment of all fragments extracted from the uterine cavity.
Numerous equipment options are currently available to gynecologic surgeons for hysteroscopically-guided myomectomy: Newer systems such as the Gynecare VersaPoint (Ethicon Endo-Surgery), and the Symphion system (Boston Scientific) facilitate bipolar electrosurgical resection. MyoSure (Hologic) and TRUCLEAR (Smith & Nephew), on the other hand, are hysteroscopic morcellators; they both use mechanical energy rather than high-frequency electrical energy to simultaneously cut and aspirate tissue.
Common to each of these options are advanced, automated fluid management systems that continuously measure distending media input and output, intrauterine distension pressure, and fluid deficit volume throughout the procedure. Such monitoring is critical to preventing excess fluid absorption and its associated complications. The new fluid management systems allow excellent visualization of the intrauterine cavity.
Benefit of Treatment
Leiomyomas, synonymously known as myomas, are among the uterine bleeding abnormalities included in a new classification system introduced in 2011 by the International Federation of Gynecology and Obstetrics. The system classifies the causes of abnormal uterine bleeding in reproductive-aged women; it is known by the acronym PALM-COEIN, for polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified.
In a practice bulletin published in 2012, the American College of Obstetricians and Gynecologists endorsed the nomenclature system and provided guidelines for evaluating reproductive-aged patients with abnormal uterine bleeding (Obstet. Gynecol. 2012;120:197-206).
The diagnosis and management of submucous leiomyomas is particularly important in cases of infertility, as these types of myomas (compared with intramural or subserosal) appear to have the greatest impact on pregnancy and implantation rates.
In general, uterine myomas are found in 5%-10% of women with infertility. In 1%-3% of infertility patients, myomas are the only abnormal findings. As described in a literature review, it is believed that myomas may interfere with sperm transport or access, and with implantation. Endometrial cavity deformity, cornual ostia obstruction, altered uterine contractility, and altered endometrial development may each play a role (Obstet. Gynecol. Clin. North Am. 2006;33:145-52).
Studies evaluating the impact of myomectomy on fertility outcomes provide evidence that submucous myomas should be removed before assisted reproductive technology/in vitro fertilization. According to the AAGL’s practice guidelines on the diagnosis and management of submucous leiomyomas, it "seems clear from high-quality studies that pregnancy rates are higher after myomectomy than no or ‘placebo’ procedures" (J. Minim. Invasive Gynecol. 2012;19:152-71).
The most widely used system for categorizing submucous myomas, developed by the European Society of Gynecological Endoscopy (ESGE), breaks them into three subtypes according to how much of the lesion’s diameter is contained within the myometrium: Type 0 myomas are entirely within the endometrial cavity, while type I have less than 50% myometrial extension, and type II are 50% or more within the myometrium.
It is the ESGE type 0 submucous myomas that are appropriate for resectoscopic surgery.
(Another system known as the STEPW classification system adds other categories, taking into account factors such as topography, extension of the base, and penetration. This system is becoming more recognized and may be useful in the future for evaluating patients for resectoscopic surgery and predicting outcomes, but it is not being used as often as the ESGE classification system.)
As the AAGL guidelines state, diagnosis is generally achieved with one or a combination of hysteroscopic and radiological techniques that may include transvaginal ultrasonography, saline infusion sonohysterography, and magnetic resonance imaging.
Research on safety
Hysteroscopic morcellation is the most recent operative hysteroscopic technique to be employed for the removal of submucous leiomyomas. In lieu of concerns about laparoscopic power morcellation, the question arises: Should we be concerned about cancer and hysteroscopy?
Numerous studies have looked at the question of whether hysteroscopic procedures produce intraperitoneal spread of endometrial cancer cells and, if so, whether this results in the "upstaging" of unsuspected cancer. Much of the research has involved diagnostic hysteroscopy, which includes the use of intrauterine cavity distension with fluid media, similar to that of operative hysteroscopy.
Investigators at Memorial Sloan-Kettering Cancer Center in New York, for instance, looked retrospectively at whether initial diagnostic procedures were associated with abnormal peritoneal washings (PW) in almost 300 women who were treated for endometrial carcinoma with hysterectomy and intraoperative PW. They found no association between the initial diagnostic procedures, including hysteroscopy, and the results of peritoneal cytology (Cancer 2000;90:143-7).
Similarly, physicians in the Czech Republic compared PW done at the start of surgery in 134 patients whose endometrial carcinoma had been diagnosed by hysteroscopy with 61 patients whose cancer had been diagnosed by dilation and curettage. The results, they said, suggest that hysteroscopy does not increase the risk of penetration of tumor cells into the peritoneal cavity more than does D&C (Eur. J. Gynaecol. Oncol. 2001; 22:342-4).
Another retrospective study of 146 patients with endometrial cancer who underwent either D&C or office hysteroscopy showed that diagnostic hysteroscopy did not increase the risk of adnexal, abdominal, or retroperitoneal lymph node metastases, compared with D&C, although there was an increase in positive peritoneal cytology (Gynecol. Oncol. 2007;107:94-8).
At least two broader reviews/meta-analyses also show no evidence for an upstaging of cancer from hysteroscopic procedures performed in the presence of cancer.
A meta-analysis of 19 studies suggests that preoperative hysteroscopy resulted in a statistically significant higher risk of positive peritoneal cytology compared with no hysteroscopy, but there was no evidence to support avoiding diagnostic hysteroscopy prior to surgical intervention for endometrial cancer (Fertil. Steril. 2011;96:957-61).
A literature review covering studies published between 1980 and 2001 showed that while there might be an increased risk of peritoneal contamination by cancer cells after hysteroscopy, there is no evidence that these patients fare worse compared with patients who have undergone other diagnostic procedures (Obstet. Gynecol. Surv. 2004;59:280-4).
Surgical rather than diagnostic hysterectomy was the focus of one recent case report from Italy. The patient was a 52-year-old nulliparous woman with a leiomyosarcoma detected 2 months after a hysteroscopic resection of a presumed myoma. After resection, the myoma was determined to be an atypical "mitotically active" leiomyoma (Eur. J. Gynaecol. Oncol. 2012;33:656-7).
The authors emphasize the "rarity" of this particular finding, and the available data overall offer no evidence for an upstaging of unsuspected endometrial cancer with hysteroscopic procedures. While hysteroscopy should not be used in cases of known cancer, as it does not facilitate treatment, there are no data that should lead us to be concerned about adverse effects in the presence of cancer.
Current systems
Traditionally, resectoscopy has posed numerous challenges for the removal of intracavitary lesions: Tissue removal has been difficult and time consuming. Visibility has been disrupted by gas bubbles, tissue fragments, blood clots, and cervical mucus. Multiple insertions have been required, raising the risk of embolism (a "piston effect"). There also have been concerns about the risk of perforation and about the learning curve.
Older resectoscopes – loop-electrode resectoscopes – were designed for monopolar electrosurgery, which requires the use of nonconductive, electrolyte-free solutions for uterine distension. This limited the amount of fluid absorption that could occur before procedures needed to be stopped.
The incorporation of bipolar instrumentation – and more recently, the development of hysteroscopic morcellation systems that use reciprocating blades driven by mechanical energy rather radiofrequency electrical energy – have enabled the use of electrolyte-containing distending media (saline or Ringer’s Lactate) and, consequently, a higher allowable amount of fluid absorption.
Saline is an ideal medium: It is isotonic, nonhemolytic, nonconductive, nontoxic, and rapidly cleared. The AAGL’s Practice Guidelines for the Management of Hysteroscopic Distending Media lists an intravasation safety limit of 2,500 cc for isotonic solution, compared with a maximum limit of 1,000 cc when using hypotonic solutions (J. Minim. Invasive Gynecol. 2013;20:137-48). This higher cut-off means we can achieve the vast majority of myoma resections in one sitting.
Hysteroscopic morcellators have additional advantages, in my experience. They allow for the use of smaller-diameter hysteroscopes, which in turn requires less cervical dilation. They also have improved reciprocating blades that enable the resection of myomas in addition to endometrial polyps. Previously, the focus was primarily on hysteroscopic polypectomy.
As technology has advanced with tissue removal being instantaneous, there is simultaneous cutting and extraction, and resections are therefore quicker. Overall, there is better visualization and a lower risk of perforation. The learning curve is quicker.
In a randomized trial focused on polypectomy, hysteroscopic mechanical morcellation was superior to electrosurgical resection. The multicenter trial from the United Kingdom compared the two modalities for removal of endometrial polyps in 121 women, and found that hysteroscopic morcellation with a mechanical-based morcellator was significantly quicker for polyp removal (a median time of 5½ minutes, versus 10 minutes, approximately), less painful and more acceptable to women, and more likely to completely remove the polyps (98% compared with 83%), the investigators reported (Obstet. Gynecol. 2014;123:745-51).
The only surgical complications in either group were vasovagal reactions, which occurred in 2% (1 out of 62) and 10% (6 out of 59) of the hysteroscopic morcellation and electrosurgical resection procedures, respectively. There was one serious adverse event, with a woman treated 2 weeks after morcellation for endomyometritis.
Indeed, infection, perforation and cervical trauma, mechanical complications, and media-related complications (intravasation and gas embolism) are risks with all modalities of operative hysteroscopy and all indications. Bleeding appears rarely to be a problem with mechanical morcellation, however, as does perforation. Certainly, perforation that occurs with a nonelectrical morcellator will be significantly less complicating than when energy is engaged.
Our experience overall with resections of intracavitary polyps and small myomas via hysteroscopic morcellation in 50 cases indicates a mean operating time of 9.4 min, a mean fluid deficit of 329 milliliters, and a mean surgeon rating of 9, with 10 representing an excellent rating. We have had no intra-or postoperative hemorrhage, no obvious electrolyte changes, and uneventful recoveries.
The majority of our hysteroscopic morcellations are done under conscious sedation with the addition of a local anesthetic in the form of a paracervical block. A 200-mcg vaginal tablet of misoprostol (Cytotec) off label the night before surgery is the pretreatment strategy I most often employ for cervical preparation. To prevent infection, I prescribe one dose of a broad-spectrum antibiotic, such as a cephalosporin, to my patients receiving myomectomies.
To learn hysteroscopic morcellation, one should begin with polypectomy and move to myomectomy once comfortable. With the TRUCLEAR system, the system I use most frequently, the hysteroscopic sheath should be inserted with the obturator in place to lessen cervical trauma.
The early flow of saline will not only aid the insertion process, it will assist in achieving good visualization quickly, as will increasing the uterine pressure setting at the start of the process. After the beginning of the procedure, however, pressure is maintained at the lowest setting capable of achieving adequate distension and providing good visualization.
When morcellating pathology, one should work from the periphery to the base. The pathology is kept between the morcellator blade opening and the optics of the camera. Large myomas can be split in half, with each half approached from distal to proximal.
Running the morcellator in the open cavity for a short time will aid in clearing the visual field of debris. Overall, however, visualization with today’s hysteroscopic morcellators and advancements in fluid management is excellent. In our experience, hysteroscopic morcellation is proving to be a safe and effective tool for performing myomectomy and addressing problems of infertility and abnormal uterine bleeding.
Dr. Sanfilippo is professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh. He is on the advisory board for Bayer Healthcare and Smith &Nephew. A lecturer and educator, Dr. Sanfilippo has written peer-reviewed articles and has been a contributor to several textbooks. He is a member of the AAGL.
Submucous leiomyomas are the most problematic type of fibroid and have been associated with abnormal uterine bleeding, infertility, and other clinical issues. Treatment has been shown to be effective in improving fertility and success rates with assisted reproduction.
Newer hysteroscopic surgical techniques and morcellation technology allow us to remove not only polyps, but selected submucous myomas, in a fashion that is not only minimally invasive but that also raises few if any concerns about spreading or upstaging an unsuspected leiomyosarcoma. In this respect, the controversy over laparoscopic power morcellation does not extend to hysteroscopic morcellation.
Such a distinction was made during opening remarks at a meeting in June 2014 of the Obstetrics & Gynecology Devices Panel of the Food and Drug Administration’s Medical Devices Advisory Committee, which was charged with addressing such concerns.
Dr. Aron Yustein, deputy director of clinical affairs and chief medical officer of the FDA’s Office of Surveillance and Biometrics, explained that the panel would not address hysteroscopic morcellators "as we do not believe that when used [as intended], they pose the same risk" as that of laparoscopic morcellation in terms of potentially disseminating and upstaging an undetected uterine malignancy.
In hysteroscopic morcellation, tissue is contained and delivered through the morcellation system into a trap, or collecting pouch. This allows for complete capture and histopathologic assessment of all fragments extracted from the uterine cavity.
Numerous equipment options are currently available to gynecologic surgeons for hysteroscopically-guided myomectomy: Newer systems such as the Gynecare VersaPoint (Ethicon Endo-Surgery), and the Symphion system (Boston Scientific) facilitate bipolar electrosurgical resection. MyoSure (Hologic) and TRUCLEAR (Smith & Nephew), on the other hand, are hysteroscopic morcellators; they both use mechanical energy rather than high-frequency electrical energy to simultaneously cut and aspirate tissue.
Common to each of these options are advanced, automated fluid management systems that continuously measure distending media input and output, intrauterine distension pressure, and fluid deficit volume throughout the procedure. Such monitoring is critical to preventing excess fluid absorption and its associated complications. The new fluid management systems allow excellent visualization of the intrauterine cavity.
Benefit of Treatment
Leiomyomas, synonymously known as myomas, are among the uterine bleeding abnormalities included in a new classification system introduced in 2011 by the International Federation of Gynecology and Obstetrics. The system classifies the causes of abnormal uterine bleeding in reproductive-aged women; it is known by the acronym PALM-COEIN, for polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified.
In a practice bulletin published in 2012, the American College of Obstetricians and Gynecologists endorsed the nomenclature system and provided guidelines for evaluating reproductive-aged patients with abnormal uterine bleeding (Obstet. Gynecol. 2012;120:197-206).
The diagnosis and management of submucous leiomyomas is particularly important in cases of infertility, as these types of myomas (compared with intramural or subserosal) appear to have the greatest impact on pregnancy and implantation rates.
In general, uterine myomas are found in 5%-10% of women with infertility. In 1%-3% of infertility patients, myomas are the only abnormal findings. As described in a literature review, it is believed that myomas may interfere with sperm transport or access, and with implantation. Endometrial cavity deformity, cornual ostia obstruction, altered uterine contractility, and altered endometrial development may each play a role (Obstet. Gynecol. Clin. North Am. 2006;33:145-52).
Studies evaluating the impact of myomectomy on fertility outcomes provide evidence that submucous myomas should be removed before assisted reproductive technology/in vitro fertilization. According to the AAGL’s practice guidelines on the diagnosis and management of submucous leiomyomas, it "seems clear from high-quality studies that pregnancy rates are higher after myomectomy than no or ‘placebo’ procedures" (J. Minim. Invasive Gynecol. 2012;19:152-71).
The most widely used system for categorizing submucous myomas, developed by the European Society of Gynecological Endoscopy (ESGE), breaks them into three subtypes according to how much of the lesion’s diameter is contained within the myometrium: Type 0 myomas are entirely within the endometrial cavity, while type I have less than 50% myometrial extension, and type II are 50% or more within the myometrium.
It is the ESGE type 0 submucous myomas that are appropriate for resectoscopic surgery.
(Another system known as the STEPW classification system adds other categories, taking into account factors such as topography, extension of the base, and penetration. This system is becoming more recognized and may be useful in the future for evaluating patients for resectoscopic surgery and predicting outcomes, but it is not being used as often as the ESGE classification system.)
As the AAGL guidelines state, diagnosis is generally achieved with one or a combination of hysteroscopic and radiological techniques that may include transvaginal ultrasonography, saline infusion sonohysterography, and magnetic resonance imaging.
Research on safety
Hysteroscopic morcellation is the most recent operative hysteroscopic technique to be employed for the removal of submucous leiomyomas. In lieu of concerns about laparoscopic power morcellation, the question arises: Should we be concerned about cancer and hysteroscopy?
Numerous studies have looked at the question of whether hysteroscopic procedures produce intraperitoneal spread of endometrial cancer cells and, if so, whether this results in the "upstaging" of unsuspected cancer. Much of the research has involved diagnostic hysteroscopy, which includes the use of intrauterine cavity distension with fluid media, similar to that of operative hysteroscopy.
Investigators at Memorial Sloan-Kettering Cancer Center in New York, for instance, looked retrospectively at whether initial diagnostic procedures were associated with abnormal peritoneal washings (PW) in almost 300 women who were treated for endometrial carcinoma with hysterectomy and intraoperative PW. They found no association between the initial diagnostic procedures, including hysteroscopy, and the results of peritoneal cytology (Cancer 2000;90:143-7).
Similarly, physicians in the Czech Republic compared PW done at the start of surgery in 134 patients whose endometrial carcinoma had been diagnosed by hysteroscopy with 61 patients whose cancer had been diagnosed by dilation and curettage. The results, they said, suggest that hysteroscopy does not increase the risk of penetration of tumor cells into the peritoneal cavity more than does D&C (Eur. J. Gynaecol. Oncol. 2001; 22:342-4).
Another retrospective study of 146 patients with endometrial cancer who underwent either D&C or office hysteroscopy showed that diagnostic hysteroscopy did not increase the risk of adnexal, abdominal, or retroperitoneal lymph node metastases, compared with D&C, although there was an increase in positive peritoneal cytology (Gynecol. Oncol. 2007;107:94-8).
At least two broader reviews/meta-analyses also show no evidence for an upstaging of cancer from hysteroscopic procedures performed in the presence of cancer.
A meta-analysis of 19 studies suggests that preoperative hysteroscopy resulted in a statistically significant higher risk of positive peritoneal cytology compared with no hysteroscopy, but there was no evidence to support avoiding diagnostic hysteroscopy prior to surgical intervention for endometrial cancer (Fertil. Steril. 2011;96:957-61).
A literature review covering studies published between 1980 and 2001 showed that while there might be an increased risk of peritoneal contamination by cancer cells after hysteroscopy, there is no evidence that these patients fare worse compared with patients who have undergone other diagnostic procedures (Obstet. Gynecol. Surv. 2004;59:280-4).
Surgical rather than diagnostic hysterectomy was the focus of one recent case report from Italy. The patient was a 52-year-old nulliparous woman with a leiomyosarcoma detected 2 months after a hysteroscopic resection of a presumed myoma. After resection, the myoma was determined to be an atypical "mitotically active" leiomyoma (Eur. J. Gynaecol. Oncol. 2012;33:656-7).
The authors emphasize the "rarity" of this particular finding, and the available data overall offer no evidence for an upstaging of unsuspected endometrial cancer with hysteroscopic procedures. While hysteroscopy should not be used in cases of known cancer, as it does not facilitate treatment, there are no data that should lead us to be concerned about adverse effects in the presence of cancer.
Current systems
Traditionally, resectoscopy has posed numerous challenges for the removal of intracavitary lesions: Tissue removal has been difficult and time consuming. Visibility has been disrupted by gas bubbles, tissue fragments, blood clots, and cervical mucus. Multiple insertions have been required, raising the risk of embolism (a "piston effect"). There also have been concerns about the risk of perforation and about the learning curve.
Older resectoscopes – loop-electrode resectoscopes – were designed for monopolar electrosurgery, which requires the use of nonconductive, electrolyte-free solutions for uterine distension. This limited the amount of fluid absorption that could occur before procedures needed to be stopped.
The incorporation of bipolar instrumentation – and more recently, the development of hysteroscopic morcellation systems that use reciprocating blades driven by mechanical energy rather radiofrequency electrical energy – have enabled the use of electrolyte-containing distending media (saline or Ringer’s Lactate) and, consequently, a higher allowable amount of fluid absorption.
Saline is an ideal medium: It is isotonic, nonhemolytic, nonconductive, nontoxic, and rapidly cleared. The AAGL’s Practice Guidelines for the Management of Hysteroscopic Distending Media lists an intravasation safety limit of 2,500 cc for isotonic solution, compared with a maximum limit of 1,000 cc when using hypotonic solutions (J. Minim. Invasive Gynecol. 2013;20:137-48). This higher cut-off means we can achieve the vast majority of myoma resections in one sitting.
Hysteroscopic morcellators have additional advantages, in my experience. They allow for the use of smaller-diameter hysteroscopes, which in turn requires less cervical dilation. They also have improved reciprocating blades that enable the resection of myomas in addition to endometrial polyps. Previously, the focus was primarily on hysteroscopic polypectomy.
As technology has advanced with tissue removal being instantaneous, there is simultaneous cutting and extraction, and resections are therefore quicker. Overall, there is better visualization and a lower risk of perforation. The learning curve is quicker.
In a randomized trial focused on polypectomy, hysteroscopic mechanical morcellation was superior to electrosurgical resection. The multicenter trial from the United Kingdom compared the two modalities for removal of endometrial polyps in 121 women, and found that hysteroscopic morcellation with a mechanical-based morcellator was significantly quicker for polyp removal (a median time of 5½ minutes, versus 10 minutes, approximately), less painful and more acceptable to women, and more likely to completely remove the polyps (98% compared with 83%), the investigators reported (Obstet. Gynecol. 2014;123:745-51).
The only surgical complications in either group were vasovagal reactions, which occurred in 2% (1 out of 62) and 10% (6 out of 59) of the hysteroscopic morcellation and electrosurgical resection procedures, respectively. There was one serious adverse event, with a woman treated 2 weeks after morcellation for endomyometritis.
Indeed, infection, perforation and cervical trauma, mechanical complications, and media-related complications (intravasation and gas embolism) are risks with all modalities of operative hysteroscopy and all indications. Bleeding appears rarely to be a problem with mechanical morcellation, however, as does perforation. Certainly, perforation that occurs with a nonelectrical morcellator will be significantly less complicating than when energy is engaged.
Our experience overall with resections of intracavitary polyps and small myomas via hysteroscopic morcellation in 50 cases indicates a mean operating time of 9.4 min, a mean fluid deficit of 329 milliliters, and a mean surgeon rating of 9, with 10 representing an excellent rating. We have had no intra-or postoperative hemorrhage, no obvious electrolyte changes, and uneventful recoveries.
The majority of our hysteroscopic morcellations are done under conscious sedation with the addition of a local anesthetic in the form of a paracervical block. A 200-mcg vaginal tablet of misoprostol (Cytotec) off label the night before surgery is the pretreatment strategy I most often employ for cervical preparation. To prevent infection, I prescribe one dose of a broad-spectrum antibiotic, such as a cephalosporin, to my patients receiving myomectomies.
To learn hysteroscopic morcellation, one should begin with polypectomy and move to myomectomy once comfortable. With the TRUCLEAR system, the system I use most frequently, the hysteroscopic sheath should be inserted with the obturator in place to lessen cervical trauma.
The early flow of saline will not only aid the insertion process, it will assist in achieving good visualization quickly, as will increasing the uterine pressure setting at the start of the process. After the beginning of the procedure, however, pressure is maintained at the lowest setting capable of achieving adequate distension and providing good visualization.
When morcellating pathology, one should work from the periphery to the base. The pathology is kept between the morcellator blade opening and the optics of the camera. Large myomas can be split in half, with each half approached from distal to proximal.
Running the morcellator in the open cavity for a short time will aid in clearing the visual field of debris. Overall, however, visualization with today’s hysteroscopic morcellators and advancements in fluid management is excellent. In our experience, hysteroscopic morcellation is proving to be a safe and effective tool for performing myomectomy and addressing problems of infertility and abnormal uterine bleeding.
Dr. Sanfilippo is professor of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh and director of the division of reproductive endocrinology and infertility at Magee-Womens Hospital in Pittsburgh. He is on the advisory board for Bayer Healthcare and Smith &Nephew. A lecturer and educator, Dr. Sanfilippo has written peer-reviewed articles and has been a contributor to several textbooks. He is a member of the AAGL.
Diabesity – Fattening the U.S. health care budget
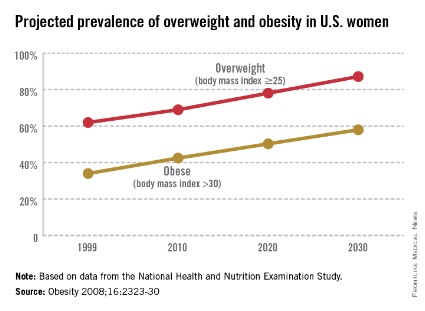
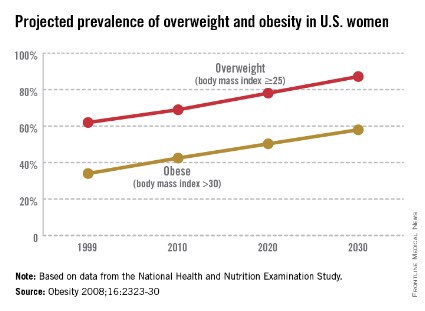
The world is not only getting older and warmer, but it’s getting heavier and more unfit each year. According to the World Health Organization’s most recent data, more than 1.4 billion adults were overweight in 2008, and 500 million of these adults were obese. In 2012, more than 40 million young children were overweight or obese. Diabetes, a disorder closely linked with obesity, affects about 347 million people worldwide – approximately ten times more people than those with HIV/AIDS. Although the number of AIDS-related deaths has steadily decreased over the last decade, even in developing countries, the number of diabetes-related deaths has steadily increased. The WHO projects that diabetes-related deaths will double by 2030, making it the seventh leading cause of death worldwide. In the United States, diabetes already is the seventh leading cause of death.
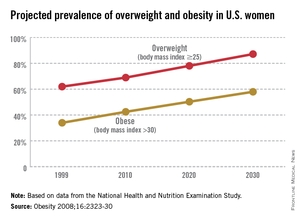
According to the Centers for Disease Control and Prevention, obesity-related diseases cost $147 billion annually, a number which dwarfs the health care costs associated with smoking ($96 billion). In addition to the link with type 2 diabetes, there are strong links between obesity and heart disease, kidney disease, depression, and hypertension. From 1987 to 2007, obesity was estimated to have caused more than a 20% increase in total health care spending.
The American Diabetes Association estimates that people diagnosed with diabetes have average yearly medical expenditures of over $13,000, which is over two times higher than the expenditures of a person without diagnosed diabetes. The 2012 estimated annual cost of care for diagnosed diabetes was $245 billion, which includes $176 billion in direct medical costs and $69 billion in reduced productivity (Diabetes Care 2013 [doi:10.2337/dc12-2625]). These figures, while staggering, do not include projected expenditures for people who have yet to receive a diabetes diagnosis.

The federal government has chosen to take dramatic steps to help Americans lose weight. Since 2011, the Centers for Medicare & Medicaid Services has covered screening and intensive behavioral therapy for obesity by primary care physicians during office visits or outpatient hospital care. Additionally, the Affordable Care Act (ACA) now requires insurance companies to help overweight and obese patients try to lose weight and be healthier. The 2012 Institute of Medicine (IOM) report, Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation also has made major recommendations for health care practitioners, schools, and the food and beverage industry to take a more active part in improving our overall health.
Simple steps make a big impact on health
Despite the daunting – and perhaps somewhat disheartening – statistics on obesity and diabetes in the United States and around the world, research has shown that small steps to achieving a healthy weight and maintaining an active lifestyle can make a dramatic difference on the course of a person’s life. According to the Department of Agriculture, healthier diets could prevent about $71 billion in yearly health care costs, lost productivity, and premature deaths. This number is staggering, when you consider that the change could be as small as choosing a salad instead of fries for a side dish.
Medical research backs up the well-worn adage that "an apple a day keeps the doctor away." The Diabetes Prevention Program study, conducted in the early 2000s, found that lifestyle changes, such as getting more exercise and eating a balanced diet, had a major impact on whether a patient who is overweight with prediabetes developed type 2 diabetes (N. Engl. J. Med. 2002;346:393-403).
Lifestyle changes can also reduce the onset of diabetes in high-risk groups such as Asian Indians, where the incidence of diabetes is the highest in the world. The Indian Diabetes Prevention Programme showed that weight loss and healthy eating reduced the incidence of type 2 diabetes in this population at a rate similar to the use of metformin, one of the most common oral antidiabetic drugs (Diabetologia 2006;49:289-97).
The 16th U.S. Surgeon General, David Satcher, M.D., Ph.D., is famous for giving his "Prescription for Great Health" when he addresses colleges and universities, which includes not smoking, staying away from illicit drug use, and abstaining from unsafe sex. Importantly, the first two key points of his "prescription" are exercising at least five times a week for 30 minutes and eating at least five servings of fruits and vegetables daily. Again, very simple recommendations, but his advice has lasting and profound ramifications.
Do obstetrician/gynecologists have a role?
As ob.gyns., we always have played an incredibly critical role in maintaining the health and well-being of our patients. Now, more than ever, we have a significant opportunity to set our patients on a path to better eating, incorporating exercise into their daily routines and passing down these good habits to their children.
In the "old days," the ob.gyn. focused on a limited period in a patient’s life. Perhaps we only saw a patient for annual exams and then for a more intense time prior to and during pregnancy, and then for a checkup post partum where we may have examined our patients only for complications of the pregnancy and delivery and not much more. Although we may have included some counseling on maintaining a healthy pregnancy, many of us relied on a patient’s primary care physician to provide ongoing support.
Today, however, we must take a more active role in helping our patients establish and maintain a healthy lifestyle. Despite the increased insurance coverage under the ACA and the expansion of Medicaid, a woman’s ob.gyn. may be the only health care practitioner she will see on a routine basis. Many women do not visit a general practitioner for routine physical examinations, but women will see their ob.gyn. for regular exams. We can use these annual or biannual office visits to help women set goals to live a healthy life, approaching each patient as a whole person who needs comprehensive care throughout her reproductive life and beyond.
For patients who are overweight or obese, we may focus on helping them reduce their body mass index and blood pressure and encourage them to stay fit. We also should do everything we can to ensure that if a woman has had gestational diabetes, she’s doing what she can to reduce her risk of developing type 2 diabetes after pregnancy. For these patients, we should consider testing their blood glucose every 1-2 years during the annual checkup.
Healthy weight in pregnancy: to gain or to lose?
Whether or not an ob.gyn. practice implements a screening program and more intensive obesity and diabetes counseling, we all will face the same question: How much weight should my patient gain to have a healthy baby? Interestingly, in the first half of the 20th century, ob.gyns. were discouraged from recommending that their pregnant patients gain very much weight. Indeed, the 13th edition of "Williams Obstetrics" (New York: Appleton-Century-Crofts, 1966, p. 326) stated that obstetricians should limit their patients from gaining more than 25 pounds during gestation, and that the ideal weight gain was 15 pounds.
This guidance was called into question by a 1970 National Academy of Sciences report, "Maternal Nutrition and the Course of Pregnancy," which indicated a strong link between infant mortality and low maternal pregnancy weight. Further evidence suggested a need for new standards and, in 1990, the IOM issued recommendations on women’s nutrition during pregnancy (Nutrition During Pregnancy, Weight Gain and Nutrient Supplements. Washington, D.C.: National Academy Press, 1990). (See table.)
Americans consume 31% more calories today than they did 40 years ago. Because of this, a woman’s need to gain weight to improve the outcome of her pregnancy is significantly reduced. The calories that many people include in their diets often come from high-fat, sodium-loaded, processed foods. We also have become a more sedentary society, spending our days at a computer, browsing the internet, watching TV, and opting to drive rather than to walk. Taking these factors into account, revising the recommendations for weight gain seemed crucial. In 2009, the IOM revised its guidance on healthy weight gain in pregnancy, and these ranges are currently widely accepted by obstetricians today (iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx). (See table.)
With the obesity and diabetes epidemics on the rise, we may need to update the 2009 IOM guidelines again – and very soon. Isolated studies have indicated that, for women who are severely obese, moderate weight loss during pregnancy may improve pregnancy outcomes. These findings remain controversial, but the "heavy" burden of diabetes and obesity on the U.S. health care system in general, and the need to reduce obstetrical complications that accompany deliveries in patients who are overweight or obese and diabetic, means that we as a community may need to reexamine our practices and approaches much more closely.
"Food" for thought
We all know of patients who, once they become pregnant, begin justifying a greater intake of food as "eating for two." Many women may use their pregnancy as an excuse to overindulge in unhealthy foods or to forgo the gym and other regular exercise regimens. Recommending basic steps to change a patient’s lifestyle can make an incredible difference in improving maternal and fetal health outcomes.
Summary recommendations for healthy pregnancy
• A low-glycemic diet, combined with moderate exercise, can reduce or eliminate many of the negative consequences of obesity on pregnant women and their babies.
• Proper weight management during pregnancy can improve birth outcomes.
• Weight loss during pregnancy is not recommended, except, potentially, for morbidly obese women (BMI greater than 40).
• For women who are normal weight, overweight or obese, leading healthy lifestyles can greatly improve maternal and fetal health outcomes. These include physical exercise, balanced diet, and weight loss, in combination with medication in some cases.
• It is never too late to begin healthy habits!
If we microfocus only on a woman’s predelivery and postdelivery health, then we’re losing a big opportunity to improve her whole self and prevent future health complications during and outside of pregnancy. The good news for ob.gyns. is that this complex problem has a simple, well-documented, and proven solution.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
The world is not only getting older and warmer, but it’s getting heavier and more unfit each year. According to the World Health Organization’s most recent data, more than 1.4 billion adults were overweight in 2008, and 500 million of these adults were obese. In 2012, more than 40 million young children were overweight or obese. Diabetes, a disorder closely linked with obesity, affects about 347 million people worldwide – approximately ten times more people than those with HIV/AIDS. Although the number of AIDS-related deaths has steadily decreased over the last decade, even in developing countries, the number of diabetes-related deaths has steadily increased. The WHO projects that diabetes-related deaths will double by 2030, making it the seventh leading cause of death worldwide. In the United States, diabetes already is the seventh leading cause of death.

According to the Centers for Disease Control and Prevention, obesity-related diseases cost $147 billion annually, a number which dwarfs the health care costs associated with smoking ($96 billion). In addition to the link with type 2 diabetes, there are strong links between obesity and heart disease, kidney disease, depression, and hypertension. From 1987 to 2007, obesity was estimated to have caused more than a 20% increase in total health care spending.
The American Diabetes Association estimates that people diagnosed with diabetes have average yearly medical expenditures of over $13,000, which is over two times higher than the expenditures of a person without diagnosed diabetes. The 2012 estimated annual cost of care for diagnosed diabetes was $245 billion, which includes $176 billion in direct medical costs and $69 billion in reduced productivity (Diabetes Care 2013 [doi:10.2337/dc12-2625]). These figures, while staggering, do not include projected expenditures for people who have yet to receive a diabetes diagnosis.

The federal government has chosen to take dramatic steps to help Americans lose weight. Since 2011, the Centers for Medicare & Medicaid Services has covered screening and intensive behavioral therapy for obesity by primary care physicians during office visits or outpatient hospital care. Additionally, the Affordable Care Act (ACA) now requires insurance companies to help overweight and obese patients try to lose weight and be healthier. The 2012 Institute of Medicine (IOM) report, Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation also has made major recommendations for health care practitioners, schools, and the food and beverage industry to take a more active part in improving our overall health.
Simple steps make a big impact on health
Despite the daunting – and perhaps somewhat disheartening – statistics on obesity and diabetes in the United States and around the world, research has shown that small steps to achieving a healthy weight and maintaining an active lifestyle can make a dramatic difference on the course of a person’s life. According to the Department of Agriculture, healthier diets could prevent about $71 billion in yearly health care costs, lost productivity, and premature deaths. This number is staggering, when you consider that the change could be as small as choosing a salad instead of fries for a side dish.
Medical research backs up the well-worn adage that "an apple a day keeps the doctor away." The Diabetes Prevention Program study, conducted in the early 2000s, found that lifestyle changes, such as getting more exercise and eating a balanced diet, had a major impact on whether a patient who is overweight with prediabetes developed type 2 diabetes (N. Engl. J. Med. 2002;346:393-403).
Lifestyle changes can also reduce the onset of diabetes in high-risk groups such as Asian Indians, where the incidence of diabetes is the highest in the world. The Indian Diabetes Prevention Programme showed that weight loss and healthy eating reduced the incidence of type 2 diabetes in this population at a rate similar to the use of metformin, one of the most common oral antidiabetic drugs (Diabetologia 2006;49:289-97).
The 16th U.S. Surgeon General, David Satcher, M.D., Ph.D., is famous for giving his "Prescription for Great Health" when he addresses colleges and universities, which includes not smoking, staying away from illicit drug use, and abstaining from unsafe sex. Importantly, the first two key points of his "prescription" are exercising at least five times a week for 30 minutes and eating at least five servings of fruits and vegetables daily. Again, very simple recommendations, but his advice has lasting and profound ramifications.
Do obstetrician/gynecologists have a role?
As ob.gyns., we always have played an incredibly critical role in maintaining the health and well-being of our patients. Now, more than ever, we have a significant opportunity to set our patients on a path to better eating, incorporating exercise into their daily routines and passing down these good habits to their children.
In the "old days," the ob.gyn. focused on a limited period in a patient’s life. Perhaps we only saw a patient for annual exams and then for a more intense time prior to and during pregnancy, and then for a checkup post partum where we may have examined our patients only for complications of the pregnancy and delivery and not much more. Although we may have included some counseling on maintaining a healthy pregnancy, many of us relied on a patient’s primary care physician to provide ongoing support.
Today, however, we must take a more active role in helping our patients establish and maintain a healthy lifestyle. Despite the increased insurance coverage under the ACA and the expansion of Medicaid, a woman’s ob.gyn. may be the only health care practitioner she will see on a routine basis. Many women do not visit a general practitioner for routine physical examinations, but women will see their ob.gyn. for regular exams. We can use these annual or biannual office visits to help women set goals to live a healthy life, approaching each patient as a whole person who needs comprehensive care throughout her reproductive life and beyond.
For patients who are overweight or obese, we may focus on helping them reduce their body mass index and blood pressure and encourage them to stay fit. We also should do everything we can to ensure that if a woman has had gestational diabetes, she’s doing what she can to reduce her risk of developing type 2 diabetes after pregnancy. For these patients, we should consider testing their blood glucose every 1-2 years during the annual checkup.
Healthy weight in pregnancy: to gain or to lose?
Whether or not an ob.gyn. practice implements a screening program and more intensive obesity and diabetes counseling, we all will face the same question: How much weight should my patient gain to have a healthy baby? Interestingly, in the first half of the 20th century, ob.gyns. were discouraged from recommending that their pregnant patients gain very much weight. Indeed, the 13th edition of "Williams Obstetrics" (New York: Appleton-Century-Crofts, 1966, p. 326) stated that obstetricians should limit their patients from gaining more than 25 pounds during gestation, and that the ideal weight gain was 15 pounds.
This guidance was called into question by a 1970 National Academy of Sciences report, "Maternal Nutrition and the Course of Pregnancy," which indicated a strong link between infant mortality and low maternal pregnancy weight. Further evidence suggested a need for new standards and, in 1990, the IOM issued recommendations on women’s nutrition during pregnancy (Nutrition During Pregnancy, Weight Gain and Nutrient Supplements. Washington, D.C.: National Academy Press, 1990). (See table.)
Americans consume 31% more calories today than they did 40 years ago. Because of this, a woman’s need to gain weight to improve the outcome of her pregnancy is significantly reduced. The calories that many people include in their diets often come from high-fat, sodium-loaded, processed foods. We also have become a more sedentary society, spending our days at a computer, browsing the internet, watching TV, and opting to drive rather than to walk. Taking these factors into account, revising the recommendations for weight gain seemed crucial. In 2009, the IOM revised its guidance on healthy weight gain in pregnancy, and these ranges are currently widely accepted by obstetricians today (iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx). (See table.)
With the obesity and diabetes epidemics on the rise, we may need to update the 2009 IOM guidelines again – and very soon. Isolated studies have indicated that, for women who are severely obese, moderate weight loss during pregnancy may improve pregnancy outcomes. These findings remain controversial, but the "heavy" burden of diabetes and obesity on the U.S. health care system in general, and the need to reduce obstetrical complications that accompany deliveries in patients who are overweight or obese and diabetic, means that we as a community may need to reexamine our practices and approaches much more closely.
"Food" for thought
We all know of patients who, once they become pregnant, begin justifying a greater intake of food as "eating for two." Many women may use their pregnancy as an excuse to overindulge in unhealthy foods or to forgo the gym and other regular exercise regimens. Recommending basic steps to change a patient’s lifestyle can make an incredible difference in improving maternal and fetal health outcomes.
Summary recommendations for healthy pregnancy
• A low-glycemic diet, combined with moderate exercise, can reduce or eliminate many of the negative consequences of obesity on pregnant women and their babies.
• Proper weight management during pregnancy can improve birth outcomes.
• Weight loss during pregnancy is not recommended, except, potentially, for morbidly obese women (BMI greater than 40).
• For women who are normal weight, overweight or obese, leading healthy lifestyles can greatly improve maternal and fetal health outcomes. These include physical exercise, balanced diet, and weight loss, in combination with medication in some cases.
• It is never too late to begin healthy habits!
If we microfocus only on a woman’s predelivery and postdelivery health, then we’re losing a big opportunity to improve her whole self and prevent future health complications during and outside of pregnancy. The good news for ob.gyns. is that this complex problem has a simple, well-documented, and proven solution.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
The world is not only getting older and warmer, but it’s getting heavier and more unfit each year. According to the World Health Organization’s most recent data, more than 1.4 billion adults were overweight in 2008, and 500 million of these adults were obese. In 2012, more than 40 million young children were overweight or obese. Diabetes, a disorder closely linked with obesity, affects about 347 million people worldwide – approximately ten times more people than those with HIV/AIDS. Although the number of AIDS-related deaths has steadily decreased over the last decade, even in developing countries, the number of diabetes-related deaths has steadily increased. The WHO projects that diabetes-related deaths will double by 2030, making it the seventh leading cause of death worldwide. In the United States, diabetes already is the seventh leading cause of death.

According to the Centers for Disease Control and Prevention, obesity-related diseases cost $147 billion annually, a number which dwarfs the health care costs associated with smoking ($96 billion). In addition to the link with type 2 diabetes, there are strong links between obesity and heart disease, kidney disease, depression, and hypertension. From 1987 to 2007, obesity was estimated to have caused more than a 20% increase in total health care spending.
The American Diabetes Association estimates that people diagnosed with diabetes have average yearly medical expenditures of over $13,000, which is over two times higher than the expenditures of a person without diagnosed diabetes. The 2012 estimated annual cost of care for diagnosed diabetes was $245 billion, which includes $176 billion in direct medical costs and $69 billion in reduced productivity (Diabetes Care 2013 [doi:10.2337/dc12-2625]). These figures, while staggering, do not include projected expenditures for people who have yet to receive a diabetes diagnosis.

The federal government has chosen to take dramatic steps to help Americans lose weight. Since 2011, the Centers for Medicare & Medicaid Services has covered screening and intensive behavioral therapy for obesity by primary care physicians during office visits or outpatient hospital care. Additionally, the Affordable Care Act (ACA) now requires insurance companies to help overweight and obese patients try to lose weight and be healthier. The 2012 Institute of Medicine (IOM) report, Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation also has made major recommendations for health care practitioners, schools, and the food and beverage industry to take a more active part in improving our overall health.
Simple steps make a big impact on health
Despite the daunting – and perhaps somewhat disheartening – statistics on obesity and diabetes in the United States and around the world, research has shown that small steps to achieving a healthy weight and maintaining an active lifestyle can make a dramatic difference on the course of a person’s life. According to the Department of Agriculture, healthier diets could prevent about $71 billion in yearly health care costs, lost productivity, and premature deaths. This number is staggering, when you consider that the change could be as small as choosing a salad instead of fries for a side dish.
Medical research backs up the well-worn adage that "an apple a day keeps the doctor away." The Diabetes Prevention Program study, conducted in the early 2000s, found that lifestyle changes, such as getting more exercise and eating a balanced diet, had a major impact on whether a patient who is overweight with prediabetes developed type 2 diabetes (N. Engl. J. Med. 2002;346:393-403).
Lifestyle changes can also reduce the onset of diabetes in high-risk groups such as Asian Indians, where the incidence of diabetes is the highest in the world. The Indian Diabetes Prevention Programme showed that weight loss and healthy eating reduced the incidence of type 2 diabetes in this population at a rate similar to the use of metformin, one of the most common oral antidiabetic drugs (Diabetologia 2006;49:289-97).
The 16th U.S. Surgeon General, David Satcher, M.D., Ph.D., is famous for giving his "Prescription for Great Health" when he addresses colleges and universities, which includes not smoking, staying away from illicit drug use, and abstaining from unsafe sex. Importantly, the first two key points of his "prescription" are exercising at least five times a week for 30 minutes and eating at least five servings of fruits and vegetables daily. Again, very simple recommendations, but his advice has lasting and profound ramifications.
Do obstetrician/gynecologists have a role?
As ob.gyns., we always have played an incredibly critical role in maintaining the health and well-being of our patients. Now, more than ever, we have a significant opportunity to set our patients on a path to better eating, incorporating exercise into their daily routines and passing down these good habits to their children.
In the "old days," the ob.gyn. focused on a limited period in a patient’s life. Perhaps we only saw a patient for annual exams and then for a more intense time prior to and during pregnancy, and then for a checkup post partum where we may have examined our patients only for complications of the pregnancy and delivery and not much more. Although we may have included some counseling on maintaining a healthy pregnancy, many of us relied on a patient’s primary care physician to provide ongoing support.
Today, however, we must take a more active role in helping our patients establish and maintain a healthy lifestyle. Despite the increased insurance coverage under the ACA and the expansion of Medicaid, a woman’s ob.gyn. may be the only health care practitioner she will see on a routine basis. Many women do not visit a general practitioner for routine physical examinations, but women will see their ob.gyn. for regular exams. We can use these annual or biannual office visits to help women set goals to live a healthy life, approaching each patient as a whole person who needs comprehensive care throughout her reproductive life and beyond.
For patients who are overweight or obese, we may focus on helping them reduce their body mass index and blood pressure and encourage them to stay fit. We also should do everything we can to ensure that if a woman has had gestational diabetes, she’s doing what she can to reduce her risk of developing type 2 diabetes after pregnancy. For these patients, we should consider testing their blood glucose every 1-2 years during the annual checkup.
Healthy weight in pregnancy: to gain or to lose?
Whether or not an ob.gyn. practice implements a screening program and more intensive obesity and diabetes counseling, we all will face the same question: How much weight should my patient gain to have a healthy baby? Interestingly, in the first half of the 20th century, ob.gyns. were discouraged from recommending that their pregnant patients gain very much weight. Indeed, the 13th edition of "Williams Obstetrics" (New York: Appleton-Century-Crofts, 1966, p. 326) stated that obstetricians should limit their patients from gaining more than 25 pounds during gestation, and that the ideal weight gain was 15 pounds.
This guidance was called into question by a 1970 National Academy of Sciences report, "Maternal Nutrition and the Course of Pregnancy," which indicated a strong link between infant mortality and low maternal pregnancy weight. Further evidence suggested a need for new standards and, in 1990, the IOM issued recommendations on women’s nutrition during pregnancy (Nutrition During Pregnancy, Weight Gain and Nutrient Supplements. Washington, D.C.: National Academy Press, 1990). (See table.)
Americans consume 31% more calories today than they did 40 years ago. Because of this, a woman’s need to gain weight to improve the outcome of her pregnancy is significantly reduced. The calories that many people include in their diets often come from high-fat, sodium-loaded, processed foods. We also have become a more sedentary society, spending our days at a computer, browsing the internet, watching TV, and opting to drive rather than to walk. Taking these factors into account, revising the recommendations for weight gain seemed crucial. In 2009, the IOM revised its guidance on healthy weight gain in pregnancy, and these ranges are currently widely accepted by obstetricians today (iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx). (See table.)
With the obesity and diabetes epidemics on the rise, we may need to update the 2009 IOM guidelines again – and very soon. Isolated studies have indicated that, for women who are severely obese, moderate weight loss during pregnancy may improve pregnancy outcomes. These findings remain controversial, but the "heavy" burden of diabetes and obesity on the U.S. health care system in general, and the need to reduce obstetrical complications that accompany deliveries in patients who are overweight or obese and diabetic, means that we as a community may need to reexamine our practices and approaches much more closely.
"Food" for thought
We all know of patients who, once they become pregnant, begin justifying a greater intake of food as "eating for two." Many women may use their pregnancy as an excuse to overindulge in unhealthy foods or to forgo the gym and other regular exercise regimens. Recommending basic steps to change a patient’s lifestyle can make an incredible difference in improving maternal and fetal health outcomes.
Summary recommendations for healthy pregnancy
• A low-glycemic diet, combined with moderate exercise, can reduce or eliminate many of the negative consequences of obesity on pregnant women and their babies.
• Proper weight management during pregnancy can improve birth outcomes.
• Weight loss during pregnancy is not recommended, except, potentially, for morbidly obese women (BMI greater than 40).
• For women who are normal weight, overweight or obese, leading healthy lifestyles can greatly improve maternal and fetal health outcomes. These include physical exercise, balanced diet, and weight loss, in combination with medication in some cases.
• It is never too late to begin healthy habits!
If we microfocus only on a woman’s predelivery and postdelivery health, then we’re losing a big opportunity to improve her whole self and prevent future health complications during and outside of pregnancy. The good news for ob.gyns. is that this complex problem has a simple, well-documented, and proven solution.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Diabesity – Fattening the U.S. health care budget
The world is not only getting older and warmer, but it’s getting heavier and more unfit each year. According to the World Health Organization’s most recent data, more than 1.4 billion adults were overweight in 2008, and 500 million of these adults were obese. In 2012, more than 40 million young children were overweight or obese. Diabetes, a disorder closely linked with obesity, affects about 347 million people worldwide – approximately ten times more people than those with HIV/AIDS. Although the number of AIDS-related deaths has steadily decreased over the last decade, even in developing countries, the number of diabetes-related deaths has steadily increased. The WHO projects that diabetes-related deaths will double by 2030, making it the seventh leading cause of death worldwide. In the United States, diabetes already is the seventh leading cause of death.

According to the Centers for Disease Control and Prevention, obesity-related diseases cost $147 billion annually, a number which dwarfs the health care costs associated with smoking ($96 billion). In addition to the link with type 2 diabetes, there are strong links between obesity and heart disease, kidney disease, depression, and hypertension. From 1987 to 2007, obesity was estimated to have caused more than a 20% increase in total health care spending.
The American Diabetes Association estimates that people diagnosed with diabetes have average yearly medical expenditures of over $13,000, which is over two times higher than the expenditures of a person without diagnosed diabetes. The 2012 estimated annual cost of care for diagnosed diabetes was $245 billion, which includes $176 billion in direct medical costs and $69 billion in reduced productivity (Diabetes Care 2013 [doi:10.2337/dc12-2625]). These figures, while staggering, do not include projected expenditures for people who have yet to receive a diabetes diagnosis.

The federal government has chosen to take dramatic steps to help Americans lose weight. Since 2011, the Centers for Medicare & Medicaid Services has covered screening and intensive behavioral therapy for obesity by primary care physicians during office visits or outpatient hospital care. Additionally, the Affordable Care Act (ACA) now requires insurance companies to help overweight and obese patients try to lose weight and be healthier. The 2012 Institute of Medicine (IOM) report, Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation also has made major recommendations for health care practitioners, schools, and the food and beverage industry to take a more active part in improving our overall health.
Simple steps make a big impact on health
Despite the daunting – and perhaps somewhat disheartening – statistics on obesity and diabetes in the United States and around the world, research has shown that small steps to achieving a healthy weight and maintaining an active lifestyle can make a dramatic difference on the course of a person’s life. According to the Department of Agriculture, healthier diets could prevent about $71 billion in yearly health care costs, lost productivity, and premature deaths. This number is staggering, when you consider that the change could be as small as choosing a salad instead of fries for a side dish.
Medical research backs up the well-worn adage that "an apple a day keeps the doctor away." The Diabetes Prevention Program study, conducted in the early 2000s, found that lifestyle changes, such as getting more exercise and eating a balanced diet, had a major impact on whether a patient who is overweight with prediabetes developed type 2 diabetes (N. Engl. J. Med. 2002;346:393-403).
Lifestyle changes can also reduce the onset of diabetes in high-risk groups such as Asian Indians, where the incidence of diabetes is the highest in the world. The Indian Diabetes Prevention Programme showed that weight loss and healthy eating reduced the incidence of type 2 diabetes in this population at a rate similar to the use of metformin, one of the most common oral antidiabetic drugs (Diabetologia 2006;49:289-97).
The 16th U.S. Surgeon General, David Satcher, M.D., Ph.D., is famous for giving his "Prescription for Great Health" when he addresses colleges and universities, which includes not smoking, staying away from illicit drug use, and abstaining from unsafe sex. Importantly, the first two key points of his "prescription" are exercising at least five times a week for 30 minutes and eating at least five servings of fruits and vegetables daily. Again, very simple recommendations, but his advice has lasting and profound ramifications.
Do obstetrician/gynecologists have a role?
As ob.gyns., we always have played an incredibly critical role in maintaining the health and well-being of our patients. Now, more than ever, we have a significant opportunity to set our patients on a path to better eating, incorporating exercise into their daily routines and passing down these good habits to their children.
In the "old days," the ob.gyn. focused on a limited period in a patient’s life. Perhaps we only saw a patient for annual exams and then for a more intense time prior to and during pregnancy, and then for a checkup post partum where we may have examined our patients only for complications of the pregnancy and delivery and not much more. Although we may have included some counseling on maintaining a healthy pregnancy, many of us relied on a patient’s primary care physician to provide ongoing support.
Today, however, we must take a more active role in helping our patients establish and maintain a healthy lifestyle. Despite the increased insurance coverage under the ACA and the expansion of Medicaid, a woman’s ob.gyn. may be the only health care practitioner she will see on a routine basis. Many women do not visit a general practitioner for routine physical examinations, but women will see their ob.gyn. for regular exams. We can use these annual or biannual office visits to help women set goals to live a healthy life, approaching each patient as a whole person who needs comprehensive care throughout her reproductive life and beyond.
For patients who are overweight or obese, we may focus on helping them reduce their body mass index and blood pressure and encourage them to stay fit. We also should do everything we can to ensure that if a woman has had gestational diabetes, she’s doing what she can to reduce her risk of developing type 2 diabetes after pregnancy. For these patients, we should consider testing their blood glucose every 1-2 years during the annual checkup.
Healthy weight in pregnancy: to gain or to lose?
Whether or not an ob.gyn. practice implements a screening program and more intensive obesity and diabetes counseling, we all will face the same question: How much weight should my patient gain to have a healthy baby? Interestingly, in the first half of the 20th century, ob.gyns. were discouraged from recommending that their pregnant patients gain very much weight. Indeed, the 13th edition of "Williams Obstetrics" (New York: Appleton-Century-Crofts, 1966, p. 326) stated that obstetricians should limit their patients from gaining more than 25 pounds during gestation, and that the ideal weight gain was 15 pounds.
This guidance was called into question by a 1970 National Academy of Sciences report, "Maternal Nutrition and the Course of Pregnancy," which indicated a strong link between infant mortality and low maternal pregnancy weight. Further evidence suggested a need for new standards and, in 1990, the IOM issued recommendations on women’s nutrition during pregnancy (Nutrition During Pregnancy, Weight Gain and Nutrient Supplements. Washington, D.C.: National Academy Press, 1990). (See table.)
Americans consume 31% more calories today than they did 40 years ago. Because of this, a woman’s need to gain weight to improve the outcome of her pregnancy is significantly reduced. The calories that many people include in their diets often come from high-fat, sodium-loaded, processed foods. We also have become a more sedentary society, spending our days at a computer, browsing the internet, watching TV, and opting to drive rather than to walk. Taking these factors into account, revising the recommendations for weight gain seemed crucial. In 2009, the IOM revised its guidance on healthy weight gain in pregnancy, and these ranges are currently widely accepted by obstetricians today (iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx). (See table.)
With the obesity and diabetes epidemics on the rise, we may need to update the 2009 IOM guidelines again – and very soon. Isolated studies have indicated that, for women who are severely obese, moderate weight loss during pregnancy may improve pregnancy outcomes. These findings remain controversial, but the "heavy" burden of diabetes and obesity on the U.S. health care system in general, and the need to reduce obstetrical complications that accompany deliveries in patients who are overweight or obese and diabetic, means that we as a community may need to reexamine our practices and approaches much more closely.
"Food" for thought
We all know of patients who, once they become pregnant, begin justifying a greater intake of food as "eating for two." Many women may use their pregnancy as an excuse to overindulge in unhealthy foods or to forgo the gym and other regular exercise regimens. Recommending basic steps to change a patient’s lifestyle can make an incredible difference in improving maternal and fetal health outcomes.
Summary recommendations for healthy pregnancy
• A low-glycemic diet, combined with moderate exercise, can reduce or eliminate many of the negative consequences of obesity on pregnant women and their babies.
• Proper weight management during pregnancy can improve birth outcomes.
• Weight loss during pregnancy is not recommended, except, potentially, for morbidly obese women (BMI greater than 40).
• For women who are normal weight, overweight or obese, leading healthy lifestyles can greatly improve maternal and fetal health outcomes. These include physical exercise, balanced diet, and weight loss, in combination with medication in some cases.
• It is never too late to begin healthy habits!
If we microfocus only on a woman’s predelivery and postdelivery health, then we’re losing a big opportunity to improve her whole self and prevent future health complications during and outside of pregnancy. The good news for ob.gyns. is that this complex problem has a simple, well-documented, and proven solution.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
The world is not only getting older and warmer, but it’s getting heavier and more unfit each year. According to the World Health Organization’s most recent data, more than 1.4 billion adults were overweight in 2008, and 500 million of these adults were obese. In 2012, more than 40 million young children were overweight or obese. Diabetes, a disorder closely linked with obesity, affects about 347 million people worldwide – approximately ten times more people than those with HIV/AIDS. Although the number of AIDS-related deaths has steadily decreased over the last decade, even in developing countries, the number of diabetes-related deaths has steadily increased. The WHO projects that diabetes-related deaths will double by 2030, making it the seventh leading cause of death worldwide. In the United States, diabetes already is the seventh leading cause of death.

According to the Centers for Disease Control and Prevention, obesity-related diseases cost $147 billion annually, a number which dwarfs the health care costs associated with smoking ($96 billion). In addition to the link with type 2 diabetes, there are strong links between obesity and heart disease, kidney disease, depression, and hypertension. From 1987 to 2007, obesity was estimated to have caused more than a 20% increase in total health care spending.
The American Diabetes Association estimates that people diagnosed with diabetes have average yearly medical expenditures of over $13,000, which is over two times higher than the expenditures of a person without diagnosed diabetes. The 2012 estimated annual cost of care for diagnosed diabetes was $245 billion, which includes $176 billion in direct medical costs and $69 billion in reduced productivity (Diabetes Care 2013 [doi:10.2337/dc12-2625]). These figures, while staggering, do not include projected expenditures for people who have yet to receive a diabetes diagnosis.

The federal government has chosen to take dramatic steps to help Americans lose weight. Since 2011, the Centers for Medicare & Medicaid Services has covered screening and intensive behavioral therapy for obesity by primary care physicians during office visits or outpatient hospital care. Additionally, the Affordable Care Act (ACA) now requires insurance companies to help overweight and obese patients try to lose weight and be healthier. The 2012 Institute of Medicine (IOM) report, Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation also has made major recommendations for health care practitioners, schools, and the food and beverage industry to take a more active part in improving our overall health.
Simple steps make a big impact on health
Despite the daunting – and perhaps somewhat disheartening – statistics on obesity and diabetes in the United States and around the world, research has shown that small steps to achieving a healthy weight and maintaining an active lifestyle can make a dramatic difference on the course of a person’s life. According to the Department of Agriculture, healthier diets could prevent about $71 billion in yearly health care costs, lost productivity, and premature deaths. This number is staggering, when you consider that the change could be as small as choosing a salad instead of fries for a side dish.
Medical research backs up the well-worn adage that "an apple a day keeps the doctor away." The Diabetes Prevention Program study, conducted in the early 2000s, found that lifestyle changes, such as getting more exercise and eating a balanced diet, had a major impact on whether a patient who is overweight with prediabetes developed type 2 diabetes (N. Engl. J. Med. 2002;346:393-403).
Lifestyle changes can also reduce the onset of diabetes in high-risk groups such as Asian Indians, where the incidence of diabetes is the highest in the world. The Indian Diabetes Prevention Programme showed that weight loss and healthy eating reduced the incidence of type 2 diabetes in this population at a rate similar to the use of metformin, one of the most common oral antidiabetic drugs (Diabetologia 2006;49:289-97).
The 16th U.S. Surgeon General, David Satcher, M.D., Ph.D., is famous for giving his "Prescription for Great Health" when he addresses colleges and universities, which includes not smoking, staying away from illicit drug use, and abstaining from unsafe sex. Importantly, the first two key points of his "prescription" are exercising at least five times a week for 30 minutes and eating at least five servings of fruits and vegetables daily. Again, very simple recommendations, but his advice has lasting and profound ramifications.
Do obstetrician/gynecologists have a role?
As ob.gyns., we always have played an incredibly critical role in maintaining the health and well-being of our patients. Now, more than ever, we have a significant opportunity to set our patients on a path to better eating, incorporating exercise into their daily routines and passing down these good habits to their children.
In the "old days," the ob.gyn. focused on a limited period in a patient’s life. Perhaps we only saw a patient for annual exams and then for a more intense time prior to and during pregnancy, and then for a checkup post partum where we may have examined our patients only for complications of the pregnancy and delivery and not much more. Although we may have included some counseling on maintaining a healthy pregnancy, many of us relied on a patient’s primary care physician to provide ongoing support.
Today, however, we must take a more active role in helping our patients establish and maintain a healthy lifestyle. Despite the increased insurance coverage under the ACA and the expansion of Medicaid, a woman’s ob.gyn. may be the only health care practitioner she will see on a routine basis. Many women do not visit a general practitioner for routine physical examinations, but women will see their ob.gyn. for regular exams. We can use these annual or biannual office visits to help women set goals to live a healthy life, approaching each patient as a whole person who needs comprehensive care throughout her reproductive life and beyond.
For patients who are overweight or obese, we may focus on helping them reduce their body mass index and blood pressure and encourage them to stay fit. We also should do everything we can to ensure that if a woman has had gestational diabetes, she’s doing what she can to reduce her risk of developing type 2 diabetes after pregnancy. For these patients, we should consider testing their blood glucose every 1-2 years during the annual checkup.
Healthy weight in pregnancy: to gain or to lose?
Whether or not an ob.gyn. practice implements a screening program and more intensive obesity and diabetes counseling, we all will face the same question: How much weight should my patient gain to have a healthy baby? Interestingly, in the first half of the 20th century, ob.gyns. were discouraged from recommending that their pregnant patients gain very much weight. Indeed, the 13th edition of "Williams Obstetrics" (New York: Appleton-Century-Crofts, 1966, p. 326) stated that obstetricians should limit their patients from gaining more than 25 pounds during gestation, and that the ideal weight gain was 15 pounds.
This guidance was called into question by a 1970 National Academy of Sciences report, "Maternal Nutrition and the Course of Pregnancy," which indicated a strong link between infant mortality and low maternal pregnancy weight. Further evidence suggested a need for new standards and, in 1990, the IOM issued recommendations on women’s nutrition during pregnancy (Nutrition During Pregnancy, Weight Gain and Nutrient Supplements. Washington, D.C.: National Academy Press, 1990). (See table.)
Americans consume 31% more calories today than they did 40 years ago. Because of this, a woman’s need to gain weight to improve the outcome of her pregnancy is significantly reduced. The calories that many people include in their diets often come from high-fat, sodium-loaded, processed foods. We also have become a more sedentary society, spending our days at a computer, browsing the internet, watching TV, and opting to drive rather than to walk. Taking these factors into account, revising the recommendations for weight gain seemed crucial. In 2009, the IOM revised its guidance on healthy weight gain in pregnancy, and these ranges are currently widely accepted by obstetricians today (iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx). (See table.)
With the obesity and diabetes epidemics on the rise, we may need to update the 2009 IOM guidelines again – and very soon. Isolated studies have indicated that, for women who are severely obese, moderate weight loss during pregnancy may improve pregnancy outcomes. These findings remain controversial, but the "heavy" burden of diabetes and obesity on the U.S. health care system in general, and the need to reduce obstetrical complications that accompany deliveries in patients who are overweight or obese and diabetic, means that we as a community may need to reexamine our practices and approaches much more closely.
"Food" for thought
We all know of patients who, once they become pregnant, begin justifying a greater intake of food as "eating for two." Many women may use their pregnancy as an excuse to overindulge in unhealthy foods or to forgo the gym and other regular exercise regimens. Recommending basic steps to change a patient’s lifestyle can make an incredible difference in improving maternal and fetal health outcomes.
Summary recommendations for healthy pregnancy
• A low-glycemic diet, combined with moderate exercise, can reduce or eliminate many of the negative consequences of obesity on pregnant women and their babies.
• Proper weight management during pregnancy can improve birth outcomes.
• Weight loss during pregnancy is not recommended, except, potentially, for morbidly obese women (BMI greater than 40).
• For women who are normal weight, overweight or obese, leading healthy lifestyles can greatly improve maternal and fetal health outcomes. These include physical exercise, balanced diet, and weight loss, in combination with medication in some cases.
• It is never too late to begin healthy habits!
If we microfocus only on a woman’s predelivery and postdelivery health, then we’re losing a big opportunity to improve her whole self and prevent future health complications during and outside of pregnancy. The good news for ob.gyns. is that this complex problem has a simple, well-documented, and proven solution.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
The world is not only getting older and warmer, but it’s getting heavier and more unfit each year. According to the World Health Organization’s most recent data, more than 1.4 billion adults were overweight in 2008, and 500 million of these adults were obese. In 2012, more than 40 million young children were overweight or obese. Diabetes, a disorder closely linked with obesity, affects about 347 million people worldwide – approximately ten times more people than those with HIV/AIDS. Although the number of AIDS-related deaths has steadily decreased over the last decade, even in developing countries, the number of diabetes-related deaths has steadily increased. The WHO projects that diabetes-related deaths will double by 2030, making it the seventh leading cause of death worldwide. In the United States, diabetes already is the seventh leading cause of death.

According to the Centers for Disease Control and Prevention, obesity-related diseases cost $147 billion annually, a number which dwarfs the health care costs associated with smoking ($96 billion). In addition to the link with type 2 diabetes, there are strong links between obesity and heart disease, kidney disease, depression, and hypertension. From 1987 to 2007, obesity was estimated to have caused more than a 20% increase in total health care spending.
The American Diabetes Association estimates that people diagnosed with diabetes have average yearly medical expenditures of over $13,000, which is over two times higher than the expenditures of a person without diagnosed diabetes. The 2012 estimated annual cost of care for diagnosed diabetes was $245 billion, which includes $176 billion in direct medical costs and $69 billion in reduced productivity (Diabetes Care 2013 [doi:10.2337/dc12-2625]). These figures, while staggering, do not include projected expenditures for people who have yet to receive a diabetes diagnosis.

The federal government has chosen to take dramatic steps to help Americans lose weight. Since 2011, the Centers for Medicare & Medicaid Services has covered screening and intensive behavioral therapy for obesity by primary care physicians during office visits or outpatient hospital care. Additionally, the Affordable Care Act (ACA) now requires insurance companies to help overweight and obese patients try to lose weight and be healthier. The 2012 Institute of Medicine (IOM) report, Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation also has made major recommendations for health care practitioners, schools, and the food and beverage industry to take a more active part in improving our overall health.
Simple steps make a big impact on health
Despite the daunting – and perhaps somewhat disheartening – statistics on obesity and diabetes in the United States and around the world, research has shown that small steps to achieving a healthy weight and maintaining an active lifestyle can make a dramatic difference on the course of a person’s life. According to the Department of Agriculture, healthier diets could prevent about $71 billion in yearly health care costs, lost productivity, and premature deaths. This number is staggering, when you consider that the change could be as small as choosing a salad instead of fries for a side dish.
Medical research backs up the well-worn adage that "an apple a day keeps the doctor away." The Diabetes Prevention Program study, conducted in the early 2000s, found that lifestyle changes, such as getting more exercise and eating a balanced diet, had a major impact on whether a patient who is overweight with prediabetes developed type 2 diabetes (N. Engl. J. Med. 2002;346:393-403).
Lifestyle changes can also reduce the onset of diabetes in high-risk groups such as Asian Indians, where the incidence of diabetes is the highest in the world. The Indian Diabetes Prevention Programme showed that weight loss and healthy eating reduced the incidence of type 2 diabetes in this population at a rate similar to the use of metformin, one of the most common oral antidiabetic drugs (Diabetologia 2006;49:289-97).
The 16th U.S. Surgeon General, David Satcher, M.D., Ph.D., is famous for giving his "Prescription for Great Health" when he addresses colleges and universities, which includes not smoking, staying away from illicit drug use, and abstaining from unsafe sex. Importantly, the first two key points of his "prescription" are exercising at least five times a week for 30 minutes and eating at least five servings of fruits and vegetables daily. Again, very simple recommendations, but his advice has lasting and profound ramifications.
Do obstetrician/gynecologists have a role?
As ob.gyns., we always have played an incredibly critical role in maintaining the health and well-being of our patients. Now, more than ever, we have a significant opportunity to set our patients on a path to better eating, incorporating exercise into their daily routines and passing down these good habits to their children.
In the "old days," the ob.gyn. focused on a limited period in a patient’s life. Perhaps we only saw a patient for annual exams and then for a more intense time prior to and during pregnancy, and then for a checkup post partum where we may have examined our patients only for complications of the pregnancy and delivery and not much more. Although we may have included some counseling on maintaining a healthy pregnancy, many of us relied on a patient’s primary care physician to provide ongoing support.
Today, however, we must take a more active role in helping our patients establish and maintain a healthy lifestyle. Despite the increased insurance coverage under the ACA and the expansion of Medicaid, a woman’s ob.gyn. may be the only health care practitioner she will see on a routine basis. Many women do not visit a general practitioner for routine physical examinations, but women will see their ob.gyn. for regular exams. We can use these annual or biannual office visits to help women set goals to live a healthy life, approaching each patient as a whole person who needs comprehensive care throughout her reproductive life and beyond.
For patients who are overweight or obese, we may focus on helping them reduce their body mass index and blood pressure and encourage them to stay fit. We also should do everything we can to ensure that if a woman has had gestational diabetes, she’s doing what she can to reduce her risk of developing type 2 diabetes after pregnancy. For these patients, we should consider testing their blood glucose every 1-2 years during the annual checkup.
Healthy weight in pregnancy: to gain or to lose?
Whether or not an ob.gyn. practice implements a screening program and more intensive obesity and diabetes counseling, we all will face the same question: How much weight should my patient gain to have a healthy baby? Interestingly, in the first half of the 20th century, ob.gyns. were discouraged from recommending that their pregnant patients gain very much weight. Indeed, the 13th edition of "Williams Obstetrics" (New York: Appleton-Century-Crofts, 1966, p. 326) stated that obstetricians should limit their patients from gaining more than 25 pounds during gestation, and that the ideal weight gain was 15 pounds.
This guidance was called into question by a 1970 National Academy of Sciences report, "Maternal Nutrition and the Course of Pregnancy," which indicated a strong link between infant mortality and low maternal pregnancy weight. Further evidence suggested a need for new standards and, in 1990, the IOM issued recommendations on women’s nutrition during pregnancy (Nutrition During Pregnancy, Weight Gain and Nutrient Supplements. Washington, D.C.: National Academy Press, 1990). (See table.)
Americans consume 31% more calories today than they did 40 years ago. Because of this, a woman’s need to gain weight to improve the outcome of her pregnancy is significantly reduced. The calories that many people include in their diets often come from high-fat, sodium-loaded, processed foods. We also have become a more sedentary society, spending our days at a computer, browsing the internet, watching TV, and opting to drive rather than to walk. Taking these factors into account, revising the recommendations for weight gain seemed crucial. In 2009, the IOM revised its guidance on healthy weight gain in pregnancy, and these ranges are currently widely accepted by obstetricians today (iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx). (See table.)
With the obesity and diabetes epidemics on the rise, we may need to update the 2009 IOM guidelines again – and very soon. Isolated studies have indicated that, for women who are severely obese, moderate weight loss during pregnancy may improve pregnancy outcomes. These findings remain controversial, but the "heavy" burden of diabetes and obesity on the U.S. health care system in general, and the need to reduce obstetrical complications that accompany deliveries in patients who are overweight or obese and diabetic, means that we as a community may need to reexamine our practices and approaches much more closely.
"Food" for thought
We all know of patients who, once they become pregnant, begin justifying a greater intake of food as "eating for two." Many women may use their pregnancy as an excuse to overindulge in unhealthy foods or to forgo the gym and other regular exercise regimens. Recommending basic steps to change a patient’s lifestyle can make an incredible difference in improving maternal and fetal health outcomes.
Summary recommendations for healthy pregnancy
• A low-glycemic diet, combined with moderate exercise, can reduce or eliminate many of the negative consequences of obesity on pregnant women and their babies.
• Proper weight management during pregnancy can improve birth outcomes.
• Weight loss during pregnancy is not recommended, except, potentially, for morbidly obese women (BMI greater than 40).
• For women who are normal weight, overweight or obese, leading healthy lifestyles can greatly improve maternal and fetal health outcomes. These include physical exercise, balanced diet, and weight loss, in combination with medication in some cases.
• It is never too late to begin healthy habits!
If we microfocus only on a woman’s predelivery and postdelivery health, then we’re losing a big opportunity to improve her whole self and prevent future health complications during and outside of pregnancy. The good news for ob.gyns. is that this complex problem has a simple, well-documented, and proven solution.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Electromechanical power morcellation confined to a bag
In the previous edition of Master Class in Gynecologic Surgery, I described the controversy concerning electromechanical power morcellation freely in the abdominopelvic cavity. I also discussed the risks, as noted in the literature, of spreading an unsuspected leiomyosarcoma, and thus, up-staging the disease and lowering both the length of the disease-free state and the overall survival. I also talked about the lack of an adequate diagnostic study to definitively separate a leiomyoma from a leiomyosarcoma, discussed the at-risk population group, and also noted the benefits of minimally invasive gynecologic surgery. At that time, I stated I was against a ban on the morcellator, and that I recommended that physicians provide proper informed consent and, when possible, consider other treatment options, especially in the at-risk population. These included continued use of minimally invasive gynecologic surgery utilizing a specimen bag for electromechanical power morcellation.
Following that publication, the Food and Drug Administration released a statement regarding electromechanical power morcellation on April 17, 2014. While "discouraging" the use of electromechanical power morcellation, they wisely did not call for a moratorium. Again, the FDA recommended that patients be properly informed as to risk and that alternative therapies be discussed, which included the use of power morcellation in a bag.
While the FDA did not ban electromechanical power morcellation, the phrase "discourages the use" sent widespread ripples throughout our specialty. Within a week, Ethicon Endo-Surgery pulled its morcellator off the market worldwide. My hospital system – Advocate Health Care – as well as virtually every hospital in Boston placed a moratorium on electromechanical power morcellation. Dr. Jim Tsaltas, president of the Australian Gynecological Endoscopy & Surgery Society (AGES), was contacted by his country’s FDA equivalent to discuss the use of power morcellation freely in the abdominopelvic cavity.
Since the letter from the FDA, position papers have come from both the world’s largest society focused on minimally invasive surgery, the AAGL, and the American College of Obstetricians and Gynecologists (ACOG). Both of the society’s statements agree that proper informed consent is imperative and that alternate treatment be considered, especially in the at-risk population. Although both papers discuss the use of electromechanical power morcellation in the confines of a bag, the ACOG statement accurately notes that there is very little data regarding morcellation in a bag placed in the abdominopelvic cavity.
Experience with electromechanical power morcellation in a bag placed in the abdominopelvic cavity is now quickly developing. To quote my coauthor, Dr. Ceana Nezhat, "My goal with this Master Class is to provide a forum for discussion and encourage gynecologic surgeons to continue to practice minimally invasive surgery for the benefit of their patients. Despite the current limitation on unprotected, intraperitoneal electromechanical morcellation, the hope is that surgeons will not revert back to laparotomy, but will continue to learn and find innovative ways to provide the least invasive surgical techniques or refer to centers that can provide these services to women."
In this edition of Master Class in Gynecologic Surgery, I am featuring techniques of electromechanical power morcellation in a specimen bag by early adapters and innovators, and other techniques by Dr. Tony Shibley, Dr. Bernard Taylor, and Dr. Ceana H. Nezhat.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for the past 20 years. He has a focus on single-site surgery and has been involved in minimally invasive surgical education both nationally and internationally, as well as in medical device development.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He lectures nationally and internationally on minimally invasive gynecologic surgery.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery.
Videos of our experts’ individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website. Also at SurgeryU is a video of the electromechanical power morcellation technique in a bag that my partner, Dr. Aarathi Cholkeri-Singh, and I utilize. We use a 3,100-cc ripstop nylon specimen bag from Espiner Medical and the 5 x 150 mm, extra-long, shielded-bladed balloon-tipped trocar from Applied Medical. Go to SurgeryU to view videos of the procedures.
In the previous edition of Master Class in Gynecologic Surgery, I described the controversy concerning electromechanical power morcellation freely in the abdominopelvic cavity. I also discussed the risks, as noted in the literature, of spreading an unsuspected leiomyosarcoma, and thus, up-staging the disease and lowering both the length of the disease-free state and the overall survival. I also talked about the lack of an adequate diagnostic study to definitively separate a leiomyoma from a leiomyosarcoma, discussed the at-risk population group, and also noted the benefits of minimally invasive gynecologic surgery. At that time, I stated I was against a ban on the morcellator, and that I recommended that physicians provide proper informed consent and, when possible, consider other treatment options, especially in the at-risk population. These included continued use of minimally invasive gynecologic surgery utilizing a specimen bag for electromechanical power morcellation.
Following that publication, the Food and Drug Administration released a statement regarding electromechanical power morcellation on April 17, 2014. While "discouraging" the use of electromechanical power morcellation, they wisely did not call for a moratorium. Again, the FDA recommended that patients be properly informed as to risk and that alternative therapies be discussed, which included the use of power morcellation in a bag.
While the FDA did not ban electromechanical power morcellation, the phrase "discourages the use" sent widespread ripples throughout our specialty. Within a week, Ethicon Endo-Surgery pulled its morcellator off the market worldwide. My hospital system – Advocate Health Care – as well as virtually every hospital in Boston placed a moratorium on electromechanical power morcellation. Dr. Jim Tsaltas, president of the Australian Gynecological Endoscopy & Surgery Society (AGES), was contacted by his country’s FDA equivalent to discuss the use of power morcellation freely in the abdominopelvic cavity.
Since the letter from the FDA, position papers have come from both the world’s largest society focused on minimally invasive surgery, the AAGL, and the American College of Obstetricians and Gynecologists (ACOG). Both of the society’s statements agree that proper informed consent is imperative and that alternate treatment be considered, especially in the at-risk population. Although both papers discuss the use of electromechanical power morcellation in the confines of a bag, the ACOG statement accurately notes that there is very little data regarding morcellation in a bag placed in the abdominopelvic cavity.
Experience with electromechanical power morcellation in a bag placed in the abdominopelvic cavity is now quickly developing. To quote my coauthor, Dr. Ceana Nezhat, "My goal with this Master Class is to provide a forum for discussion and encourage gynecologic surgeons to continue to practice minimally invasive surgery for the benefit of their patients. Despite the current limitation on unprotected, intraperitoneal electromechanical morcellation, the hope is that surgeons will not revert back to laparotomy, but will continue to learn and find innovative ways to provide the least invasive surgical techniques or refer to centers that can provide these services to women."
In this edition of Master Class in Gynecologic Surgery, I am featuring techniques of electromechanical power morcellation in a specimen bag by early adapters and innovators, and other techniques by Dr. Tony Shibley, Dr. Bernard Taylor, and Dr. Ceana H. Nezhat.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for the past 20 years. He has a focus on single-site surgery and has been involved in minimally invasive surgical education both nationally and internationally, as well as in medical device development.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He lectures nationally and internationally on minimally invasive gynecologic surgery.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery.
Videos of our experts’ individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website. Also at SurgeryU is a video of the electromechanical power morcellation technique in a bag that my partner, Dr. Aarathi Cholkeri-Singh, and I utilize. We use a 3,100-cc ripstop nylon specimen bag from Espiner Medical and the 5 x 150 mm, extra-long, shielded-bladed balloon-tipped trocar from Applied Medical. Go to SurgeryU to view videos of the procedures.
In the previous edition of Master Class in Gynecologic Surgery, I described the controversy concerning electromechanical power morcellation freely in the abdominopelvic cavity. I also discussed the risks, as noted in the literature, of spreading an unsuspected leiomyosarcoma, and thus, up-staging the disease and lowering both the length of the disease-free state and the overall survival. I also talked about the lack of an adequate diagnostic study to definitively separate a leiomyoma from a leiomyosarcoma, discussed the at-risk population group, and also noted the benefits of minimally invasive gynecologic surgery. At that time, I stated I was against a ban on the morcellator, and that I recommended that physicians provide proper informed consent and, when possible, consider other treatment options, especially in the at-risk population. These included continued use of minimally invasive gynecologic surgery utilizing a specimen bag for electromechanical power morcellation.
Following that publication, the Food and Drug Administration released a statement regarding electromechanical power morcellation on April 17, 2014. While "discouraging" the use of electromechanical power morcellation, they wisely did not call for a moratorium. Again, the FDA recommended that patients be properly informed as to risk and that alternative therapies be discussed, which included the use of power morcellation in a bag.
While the FDA did not ban electromechanical power morcellation, the phrase "discourages the use" sent widespread ripples throughout our specialty. Within a week, Ethicon Endo-Surgery pulled its morcellator off the market worldwide. My hospital system – Advocate Health Care – as well as virtually every hospital in Boston placed a moratorium on electromechanical power morcellation. Dr. Jim Tsaltas, president of the Australian Gynecological Endoscopy & Surgery Society (AGES), was contacted by his country’s FDA equivalent to discuss the use of power morcellation freely in the abdominopelvic cavity.
Since the letter from the FDA, position papers have come from both the world’s largest society focused on minimally invasive surgery, the AAGL, and the American College of Obstetricians and Gynecologists (ACOG). Both of the society’s statements agree that proper informed consent is imperative and that alternate treatment be considered, especially in the at-risk population. Although both papers discuss the use of electromechanical power morcellation in the confines of a bag, the ACOG statement accurately notes that there is very little data regarding morcellation in a bag placed in the abdominopelvic cavity.
Experience with electromechanical power morcellation in a bag placed in the abdominopelvic cavity is now quickly developing. To quote my coauthor, Dr. Ceana Nezhat, "My goal with this Master Class is to provide a forum for discussion and encourage gynecologic surgeons to continue to practice minimally invasive surgery for the benefit of their patients. Despite the current limitation on unprotected, intraperitoneal electromechanical morcellation, the hope is that surgeons will not revert back to laparotomy, but will continue to learn and find innovative ways to provide the least invasive surgical techniques or refer to centers that can provide these services to women."
In this edition of Master Class in Gynecologic Surgery, I am featuring techniques of electromechanical power morcellation in a specimen bag by early adapters and innovators, and other techniques by Dr. Tony Shibley, Dr. Bernard Taylor, and Dr. Ceana H. Nezhat.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for the past 20 years. He has a focus on single-site surgery and has been involved in minimally invasive surgical education both nationally and internationally, as well as in medical device development.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He lectures nationally and internationally on minimally invasive gynecologic surgery.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery.
Videos of our experts’ individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website. Also at SurgeryU is a video of the electromechanical power morcellation technique in a bag that my partner, Dr. Aarathi Cholkeri-Singh, and I utilize. We use a 3,100-cc ripstop nylon specimen bag from Espiner Medical and the 5 x 150 mm, extra-long, shielded-bladed balloon-tipped trocar from Applied Medical. Go to SurgeryU to view videos of the procedures.
Morcellation may be impractical
Over one-half million hysterectomies are performed annually in the United States. Approximately one-third of them are done using minimally invasive techniques. Electromechanical morcellation was introduced to gynecologic surgery in the 1990s. This allowed for women with larger uteri to be able to undergo minimally invasive surgery (MIS). Over time, there was a shift from laparoscopic-assisted vaginal hysterectomy to laparoscopic-assisted supracervical hysterectomy using the morcellator. This is done even in cases when the uterus is small enough to be easily delivered transvaginally.
Uncontained intraperitoneal morcellation, especially with the rotating blade morcellator, carries the risk of peritoneal spread of an unsuspected malignancy. This not only upstages the cancer and impacts the need for or type of adjuvant therapy, but also worsens the prognosis.
No one argues that intraperitoneal morcellation of a known malignancy should be avoided. At present, there is no accurate way of diagnosing a uterine leiomyosarcoma in a woman thought to have leiomyoma before the specimen is evaluated in the pathology laboratory. Endometrial sampling and imaging studies have poor sensitivity for detecting this pathology prior to surgery. The symptoms associated with uterine leiomyosarcoma can be similar to those experienced by women with leiomyomas. Several studies have shown that the median age of women who undergo surgery for removal of leiomyomas but are found to have a leiomyosarcoma is 50 years. The incidence of unsuspected leiomyosarcoma in women having uterine leiomyomas surgically removed increases with age. However, the age of these women with unsuspected uterine leiomyosarcoma ranges from 18 to 95 years. Therefore, it should be noted that uterine leiomyosarcoma can occur at almost any age in a woman’s lifetime.
A Food and Drug Administration analysis released in April 2014 suggests that a uterine leiomyosarcoma can be found in 1 out of 350 women undergoing surgery for presumed leiomyomas. Leibsohn et al. (Am. J. Obstet. Gynecol. 1990;162:968-76) reviewed 1,432 cases of hysterectomy performed in women with symptoms thought to be due to uterine leiomyomas. A leiomyosarcoma was found in seven cases (0.49%). One of 511 (0.2%) women between the ages of 31 and 40 years had an unsuspected leiomyosarcoma.
We could ask, at what level of risk is peritoneal dissemination by uncontained morcellation of an unsuspected malignancy justified? Is the harm to one patient justified by the possible benefit to thousands of other patients? I will argue that any level of risk is too high if there is a way to achieve the same results without worsening the prognosis of women who harbor unsuspected leiomyosarcoma. The safe use of morcellation will not impede MIS. Women with smaller uteri can undergo transvaginal hysterectomy or laparoscopic-assisted vaginal hysterectomy. Leiomyomas could be removed intact through a colpotomy or through a small abdominal incision. Larger specimens could be morcellated within an isolation bag. In some situations the specimen may be too large or the surgeon lacks the necessary skills for MIS to be safely performed. In those cases a laparotomy is the safest approach. It is ingenuous to suggest that these cases should be referred to a gynecologist with the skill to perform these challenging procedures minimally invasively. This may be possible in some geographic locations and acceptable to some patients, but frequently it is impractical.
Dr. Enrique Hernandez is the Abraham Roth Professor and Chair of Obstetrics, Gynecology and Reproductive Sciences at Temple University, Philadelphia. Dr. Hernandez said he had no relevant financial disclosures.
Over one-half million hysterectomies are performed annually in the United States. Approximately one-third of them are done using minimally invasive techniques. Electromechanical morcellation was introduced to gynecologic surgery in the 1990s. This allowed for women with larger uteri to be able to undergo minimally invasive surgery (MIS). Over time, there was a shift from laparoscopic-assisted vaginal hysterectomy to laparoscopic-assisted supracervical hysterectomy using the morcellator. This is done even in cases when the uterus is small enough to be easily delivered transvaginally.
Uncontained intraperitoneal morcellation, especially with the rotating blade morcellator, carries the risk of peritoneal spread of an unsuspected malignancy. This not only upstages the cancer and impacts the need for or type of adjuvant therapy, but also worsens the prognosis.
No one argues that intraperitoneal morcellation of a known malignancy should be avoided. At present, there is no accurate way of diagnosing a uterine leiomyosarcoma in a woman thought to have leiomyoma before the specimen is evaluated in the pathology laboratory. Endometrial sampling and imaging studies have poor sensitivity for detecting this pathology prior to surgery. The symptoms associated with uterine leiomyosarcoma can be similar to those experienced by women with leiomyomas. Several studies have shown that the median age of women who undergo surgery for removal of leiomyomas but are found to have a leiomyosarcoma is 50 years. The incidence of unsuspected leiomyosarcoma in women having uterine leiomyomas surgically removed increases with age. However, the age of these women with unsuspected uterine leiomyosarcoma ranges from 18 to 95 years. Therefore, it should be noted that uterine leiomyosarcoma can occur at almost any age in a woman’s lifetime.
A Food and Drug Administration analysis released in April 2014 suggests that a uterine leiomyosarcoma can be found in 1 out of 350 women undergoing surgery for presumed leiomyomas. Leibsohn et al. (Am. J. Obstet. Gynecol. 1990;162:968-76) reviewed 1,432 cases of hysterectomy performed in women with symptoms thought to be due to uterine leiomyomas. A leiomyosarcoma was found in seven cases (0.49%). One of 511 (0.2%) women between the ages of 31 and 40 years had an unsuspected leiomyosarcoma.
We could ask, at what level of risk is peritoneal dissemination by uncontained morcellation of an unsuspected malignancy justified? Is the harm to one patient justified by the possible benefit to thousands of other patients? I will argue that any level of risk is too high if there is a way to achieve the same results without worsening the prognosis of women who harbor unsuspected leiomyosarcoma. The safe use of morcellation will not impede MIS. Women with smaller uteri can undergo transvaginal hysterectomy or laparoscopic-assisted vaginal hysterectomy. Leiomyomas could be removed intact through a colpotomy or through a small abdominal incision. Larger specimens could be morcellated within an isolation bag. In some situations the specimen may be too large or the surgeon lacks the necessary skills for MIS to be safely performed. In those cases a laparotomy is the safest approach. It is ingenuous to suggest that these cases should be referred to a gynecologist with the skill to perform these challenging procedures minimally invasively. This may be possible in some geographic locations and acceptable to some patients, but frequently it is impractical.
Dr. Enrique Hernandez is the Abraham Roth Professor and Chair of Obstetrics, Gynecology and Reproductive Sciences at Temple University, Philadelphia. Dr. Hernandez said he had no relevant financial disclosures.
Over one-half million hysterectomies are performed annually in the United States. Approximately one-third of them are done using minimally invasive techniques. Electromechanical morcellation was introduced to gynecologic surgery in the 1990s. This allowed for women with larger uteri to be able to undergo minimally invasive surgery (MIS). Over time, there was a shift from laparoscopic-assisted vaginal hysterectomy to laparoscopic-assisted supracervical hysterectomy using the morcellator. This is done even in cases when the uterus is small enough to be easily delivered transvaginally.
Uncontained intraperitoneal morcellation, especially with the rotating blade morcellator, carries the risk of peritoneal spread of an unsuspected malignancy. This not only upstages the cancer and impacts the need for or type of adjuvant therapy, but also worsens the prognosis.
No one argues that intraperitoneal morcellation of a known malignancy should be avoided. At present, there is no accurate way of diagnosing a uterine leiomyosarcoma in a woman thought to have leiomyoma before the specimen is evaluated in the pathology laboratory. Endometrial sampling and imaging studies have poor sensitivity for detecting this pathology prior to surgery. The symptoms associated with uterine leiomyosarcoma can be similar to those experienced by women with leiomyomas. Several studies have shown that the median age of women who undergo surgery for removal of leiomyomas but are found to have a leiomyosarcoma is 50 years. The incidence of unsuspected leiomyosarcoma in women having uterine leiomyomas surgically removed increases with age. However, the age of these women with unsuspected uterine leiomyosarcoma ranges from 18 to 95 years. Therefore, it should be noted that uterine leiomyosarcoma can occur at almost any age in a woman’s lifetime.
A Food and Drug Administration analysis released in April 2014 suggests that a uterine leiomyosarcoma can be found in 1 out of 350 women undergoing surgery for presumed leiomyomas. Leibsohn et al. (Am. J. Obstet. Gynecol. 1990;162:968-76) reviewed 1,432 cases of hysterectomy performed in women with symptoms thought to be due to uterine leiomyomas. A leiomyosarcoma was found in seven cases (0.49%). One of 511 (0.2%) women between the ages of 31 and 40 years had an unsuspected leiomyosarcoma.
We could ask, at what level of risk is peritoneal dissemination by uncontained morcellation of an unsuspected malignancy justified? Is the harm to one patient justified by the possible benefit to thousands of other patients? I will argue that any level of risk is too high if there is a way to achieve the same results without worsening the prognosis of women who harbor unsuspected leiomyosarcoma. The safe use of morcellation will not impede MIS. Women with smaller uteri can undergo transvaginal hysterectomy or laparoscopic-assisted vaginal hysterectomy. Leiomyomas could be removed intact through a colpotomy or through a small abdominal incision. Larger specimens could be morcellated within an isolation bag. In some situations the specimen may be too large or the surgeon lacks the necessary skills for MIS to be safely performed. In those cases a laparotomy is the safest approach. It is ingenuous to suggest that these cases should be referred to a gynecologist with the skill to perform these challenging procedures minimally invasively. This may be possible in some geographic locations and acceptable to some patients, but frequently it is impractical.
Dr. Enrique Hernandez is the Abraham Roth Professor and Chair of Obstetrics, Gynecology and Reproductive Sciences at Temple University, Philadelphia. Dr. Hernandez said he had no relevant financial disclosures.
Safety techniques regarding morcellation
Power morcellation within an insufflated bag
By Tony Shibley, M.D.
Morcellating safely in laparoscopic hysterectomy or myomectomy involves not only the use of a bag, but also the creation of an artificial pneumoperitoneum inside the bag. Inflation of the bag allows us to create a safe and completely contained working environment in the abdomen, with good visualization and adequate working space.
In the past, specimen containment bags were used for morcellation, but the bags would adhere to tissue and would be easily cut or would tear or rupture. Without proper space and visualization, surgeons could not break tissue into smaller fragments without endangering the bag or the structures behind the bag. We were safely and precisely disconnecting tissue in our surgeries, but falling short with tissue removal. There were calls for more durable bags, but the problems of visualization and safe distance were not addressed. Eventually, the goal of morcellating within a bag was largely abandoned in favor of open power morcellation.
I stopped performing open power morcellation about 2 years ago and developed a new approach to enclosed morcellation. By creating an artificial pneumoperitoneum in a bag, a good working space is developed within it. This meets the needs of containment while significantly lowering the risk of tissue dissemination and also reducing the risk of bowel injury, vascular injury, and other types of morcellator-related mechanical injuries. This approach to morcellation is safer on all fronts.
My method of enclosed morcellation has been utilized in both single-port and multiport hysterectomy (total and supracervical) and myomectomy performed with either traditional laparoscopy or the robotic platform.
The morcellation process is begun after surgery is complete except for tissue removal, and the patient is hemostatic. The specimen has been placed in a location where it can be easily retrieved; I prefer the right upper quadrant.
In a single-port approach, the bag is inserted into the abdomen through an open single-port cannula at the umbilicus. I use a Steri-Drape Isolation Bag (3M), 50 cm by 50 cm, with drawstrings. (See Figure 1.) Standing across from my assistant, I tightly fan-fold the bag and wring it to purge it of air. Using an atraumatic grasper, I position the bag linearly with the opening up; it is inserted into the pelvis first, with the superior end guided upward into the mid- and then upper-abdomen.
After the bag is placed, the cannula is recapped with the multiport cap. I use the Olympus TriPort 15 (Advanced Surgical Concepts), and the abdomen is reinsufflated. A camera is inserted into one of the 5-mm ports. I use the Olympus 5-mm articulating laparoscope.
Through the other 5-mm port, I maneuver the bag to form a pocket opening by pushing the upper corners of the bag against the respective abdominal sidewalls, and the body of the bag into the pelvis. I then elevate the specimen and, with a rotational movement, I guide the specimen along the right abdominal sidewall and into the pocket opening. I then elevate the sides of the bag and ensure that the specimen is well contained. (See Figure 2.)
The drawstrings are then brought up into the port hub. The cap is removed, and the opening of the bag is pulled up through the port and out approximately 15-20 cm, as symmetrically as possible.
The port cap is then replaced within the exteriorized neck of the bag, and the bag is insufflated with traditional laparoscopic pressure of 15-18 mm, creating an artificial pneumoperitoneum. (See Figure 3.) The camera is reinserted, followed by the morcellator, into the 15-mm port of the single-site tri-port. The morcellator must be lubricated so it does not catch the bag on the way in. Morcellation is carried out under direct vision.
After the morcellation process is complete, the morcellator is removed, followed by the camera and the tri-port cap. The bag is desufflated and removed through the open cannula, and the abdomen is closed as usual.
In a multiport approach, the umbilical incision is extended to 15 mm after completion of the hysterectomy or myomectomy. The bag is inserted and placed in a manner similar to the single-port approach, except that the lateral ports may be utilized to further position the bag once it is placed about halfway in. The bag is similarly opened, the specimen contained, and the neck of the bag exteriorized. A 15-mm port is similarly lubricated and placed inside the neck of the bag and through the umbilical incision.
As the bag is insufflated, the lateral ports are backed slightly out so that they're flush with the abdominal wall, and the trocars are opened to allow the release of any residual gas in the abdomen. The laparoscope is then placed into the 15-mm umbilical trocar.
Visualization will ultimately occur from another site, however. One of the lateral 5-mm trocars is advanced at a right angle to the insufflated bag, and an introducer is placed to puncture the bag. I recommend using a blunt plastic trocar; a balloon-tip trocar also can be used. Contrary to what one might think, the bag will not leak any gas. The laparoscope is now transferred to this 5-mm lateral port, and the umbilical port is removed. A lubricated morcellator is inserted directly through the umbilical incision, and morcellation is performed under direct visualization from the side port.
These techniques for single-port or multiport enclosed power morcellation have been shown to be reproducible and successful – with all bags intact and specimens contained – in a multicenter analysis that is being prepared for publication. The largest uterus in the study was 1,481 g.
To make the approach less cumbersome, I have designed a morcellation device consisting of a specialized bag that will open automatically and assist in capturing the specimen. The device, which has closure tabs and a retrieval lanyard, has been developed with the input of other minimally invasive gynecologic surgeons and the design and engineering expertise of Advanced Surgical Concepts. It will be manufactured by the company pending Food and Drug Administration approval.
Concealed power morcellation: Our take
By Bernard Taylor, M.D.
The approach to the use of power morcellation within a bag in my practice was not specifically driven by the desire to prevent the dispersion of malignant leiomyosarcoma – an issue that is now front and center and indeed, is important – but by a broader desire to perform as complete an extraction as possible.
For years we have used isolation bags to conceal and contain small uteri, ovaries, and specimens that we debulk using a scalpel and remove through a small umbilical incision. However, this approach is cumbersome for removing larger uteri. Consequently, over the past year we refined our methods for performing power morcellation within a bag.
This procedure can be performed after traditional laparoscopy or robotic-assisted hysterectomy or myomectomy to extract the specimen from the abdomen. A surgical tissue bag is placed into the abdomen and is insufflated within the abdomen. Morcellation is carried out within the concealed "pseudopneumoperitoneum," and the bag is exteriorized from the abdomen, typically through the umbilical incision.
The majority of my procedures utilize a multiport approach due to the nature of my practice as a urogynecologist. After hysterectomy or myomectomy, the specimen is placed in the upper abdomen. The cuff is closed, other procedures are completed in the usual fashion, and hemostasis is reassured. In robotic procedures, we undock the robot prior to morcellation. The umbilical port is removed, and the bag is placed into the abdomen with a ring forceps or atraumatic forceps without teeth. The umbilical port is then replaced to reestablish the pneumoperitoneum for visualization.
For larger uterine specimens, I use an Isodrape isolation bag (Microtek Medical). This 18 x 18 inch bag with a drawstring opening easily accommodates uteri larger than 1,000 g. The bag is placed into the pelvis with the open end at the pelvic brim and is opened wide. I use the term "open from iliac to iliac and sacrum to bladder." Once the bag is positioned, the specimen is grasped; the patient is slowly taken out of the Trendelenburg position to allow for gravity to assist in the placement of the specimen into the bag.
The drawstrings are then grasped and taken out of the umbilical trocar with simultaneous removal of the umbilical port. At this time, the open end of the bag is exteriorized, and the specimen technically is extraperitoneal and "concealed."
To insufflate the bag at this point and create the "pseudopneumoperitoneum," a 15-mm port is lubricated and placed through the opening in the bag. The bag is then insufflated to the usual pressure (15 mm Hg). The accessory ports are opened at this time to allow for the abdominal pressure to fall and for the bag to expand and fill the abdominal cavity.
To enable visualization throughout the morcellation procedure, I use one of the previously placed lower abdomen lateral ports to place a 5-mm camera. The camera is placed after a 5-mm balloon tip trocar is introduced and advanced to perforate the bag. The balloon tip is insufflated in the usual fashion, securing the bag against the abdominal wall and preventing a gas leak.
Alternatively, I have also used a SILSport (Covidien) at the umbilicus in cases when there is a leak at the umbilical incision and the 15-mm port does not seal properly. While use of a single-port technique has been shown to be feasible for both visualization and morcellation, it can present challenges in complex cases with a larger uterus or multiple fibroids.
Either way, morcellation can take place within the concealed "pseudo-peritoneal" cavity, with the morcellator lubricated and placed either through the umbilical incision or through the Tri-Port. After morcellation is complete, the bag containing the smaller tissue fragments and blood is simply removed through the umbilicus, and closure of the abdomen is completed in the usual fashion.
I also have adopted a simplified approach to remove the smaller uteri with supracervical hysterectomy and sacral colpopexy performed laparoscopically. Despite preoperative screening with endometrial biopsy and pelvic ultrasound, several of my patients in the past have been diagnosed with either early ovarian or endometrial neoplasias on final pathology. After completing the procedure, I place the small menopausal senile uterus and adnexa into a 15-mm endoscopic bag. The bag is brought through the umbilical port, and the specimen is removed via morcellation with a scalpel or scissors.
Surgeons have asked about additional time needed to place the bag and position the specimen. Technically, specimen placement is a learned skill; once one is proficient, the case times are not any different. In fact, cases may be shorter because of the time saved by not having to retrieve uterine fragments from the abdomen and pelvis.
Theoretically, the procedure addresses concerns associated with tissue fragmentation and dissemination within the abdominal cavity. To date, there are no trials showing prevention of cancer upstaging or benign conditions such as leiomyomatosis peritonealis disseminata or endometriosis.
Enclosed vaginal morcellation for enlarged uteri
By Ceana H. Nezhat, M.D.
Removal of large uteri via minimally invasive surgery poses challenges to gynecologic surgeons. Limitations on the use of intraperitoneal electromechanical morcellation are a step forward in terms of protecting patients from undue harm, but we also must acknowledge that minimally invasive surgery has been shown to be superior to laparotomy in the majority of cases.
While safer ways to extract large specimens without risk of spreading both benign and malignant tissue are being studied and developed, gynecologic surgeons need to find alternative minimally invasive approaches for removing large specimens.
Minimally invasive approaches to extirpate uteri and myomas have been described prior to the advent of electromechanical morcellation. A natural orifice such as the vagina for gynecologic procedures is a clear choice for removing specimens. Laparoscopic-assisted myomectomy is a combination of laparoscopy and minilaparotomy for tissue extraction (JSLS 2001;5:299-303). Another alternative is extracting myoma and other tissues through a posterior colpotomy. Rates of dyspareunia and adhesions in the cul-de-sac after tissue extraction through a colpotomy are low (J. Reprod. Med. 1993;38:534-6).
Vaginal hysterectomy was chronicled and performed by the Greek physician Soranus of Ephesus in 120 A.D. (Acta Chir. Iugosl. 2011:58:9-14), and it is still the preferred route of hysterectomy when it can be performed. Large uteri can be transvaginally morcellated and vaginally extracted; however, this technique still poses the risk, although low, of spilling fragments of uterine tissue in the abdomen.
By combining total laparoscopic hysterectomy and vaginal morcellation of enlarged uteri within an enclosed specimen bag, the risk of spilling uterine fragments is reduced, in addition to avoiding potential visceral and vascular complications associated with intraperitoneal electromechanical morcellation.
Enclosed vaginal morcellation is the technique of placing the enlarged hysterectomy specimen in an endoscopic specimen retrieval bag and then transvaginally morcellating the tissue.
In the development of this technique, I have experimented with different endoscopic sacs and retractors. In my experience, the optimal laparoscopic bag is the LapSac Surgical Tissue Pouch (Cook Medical). This endoscopic, one-time use, specimen retrieval bag is flexible, durable nylon with an integral polyurethane inner coating and polypropylene drawstring (Figure 4). The bag comes in four sizes, the largest (size 8 x 10 inch, volume 1,500 mL) of which I used to assist in removing the 18-week size uterus in the accompanying video.
The other key piece of equipment for vaginal extraction of large uteri is a transvaginally-placed wound retractor. As I have previously described, the self-retaining retractor provides optimal exposure in a narrow field and minimizes trauma to surrounding tissue (J. Minim. Inv. Gynecol. 2009:16:616-7). Two wound retractors are suitable for this surgical technique: the Alexis Wound Protector/Retractor (Applied Medical) (Figure 5) and the Mobius Elastic Abdominal Retractor (Cooper Surgical) (Figure 6).
After complete laparoscopic detachment of the uterus and cervix (and adnexa), the wound retractor is placed transvaginally. One semirigid ring is pushed superiorly through the vagina and into the peritoneal cavity, where is it placed in the cul-de-sac. In this manner, a uniform, circumferential orifice is created. In narrower vaginal vaults, the ring can be compressed between the thumb and index finger to reduce its diameter and thus be introduced into the peritoneal cavity atraumatically. With the small-size retraction systems, an orifice of 2.5 to 6 cm diameter can be created.
The LapSac is folded and then inserted with ring forceps transvaginally through the wound retractor. (See Figure 7.) The bag is unfolded intraperitoneally using the integral tabs to demarcate the edges of the bag. The bag should be unfolded in the pelvis with the opening of the bag cephalad. The fundus of the hysterectomy specimen is grasped with a tenaculum and placed inside the bag. (See Figure 8.)
It is an important technical point to grab the fundus or the heaviest point of the specimen to allow weight and gravity to facilitate placing the specimen securely in the bag. If the cervix or a lighter part of the specimen is grasped, placement in the bag is more difficult and the specimen will likely slip out of the bag.
Once the entire hysterectomy specimen is in the LapSac, the bag is cinched by grasping the blue drawstrings. The opening of the bag is pulled through the vagina. The opening of the bag is externalized and the edges are attached with Allis clamps to the external edge of the wound retractor. The specimen (typically the cervix first) is grasped with a tenaculum and vaginally morcellated and cored with a scalpel (Figure 9).
Metal vaginal retractors are placed within the bag to protect against sharp injury during morcellation. I prefer to amputate the cervix and adnexa separately and en bloc to preserve as much original architecture as possible so the pathologists can properly orient and examine the tissue. After completion of the morcellation, the wound retractor is removed, the cuff closed, and the procedure completed per surgeon preference.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for 20 years. He reported that he has a financial interest in the artificial pneumoperitoneum device under development, and that he is a consultant for Olympus.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He reported that he has no disclosures relevant to this Master Class.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University. He reported that he is a consultant for Karl Storz Endoscopy.
Videos of these experts' individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website.
Power morcellation within an insufflated bag
By Tony Shibley, M.D.
Morcellating safely in laparoscopic hysterectomy or myomectomy involves not only the use of a bag, but also the creation of an artificial pneumoperitoneum inside the bag. Inflation of the bag allows us to create a safe and completely contained working environment in the abdomen, with good visualization and adequate working space.
In the past, specimen containment bags were used for morcellation, but the bags would adhere to tissue and would be easily cut or would tear or rupture. Without proper space and visualization, surgeons could not break tissue into smaller fragments without endangering the bag or the structures behind the bag. We were safely and precisely disconnecting tissue in our surgeries, but falling short with tissue removal. There were calls for more durable bags, but the problems of visualization and safe distance were not addressed. Eventually, the goal of morcellating within a bag was largely abandoned in favor of open power morcellation.
I stopped performing open power morcellation about 2 years ago and developed a new approach to enclosed morcellation. By creating an artificial pneumoperitoneum in a bag, a good working space is developed within it. This meets the needs of containment while significantly lowering the risk of tissue dissemination and also reducing the risk of bowel injury, vascular injury, and other types of morcellator-related mechanical injuries. This approach to morcellation is safer on all fronts.
My method of enclosed morcellation has been utilized in both single-port and multiport hysterectomy (total and supracervical) and myomectomy performed with either traditional laparoscopy or the robotic platform.
The morcellation process is begun after surgery is complete except for tissue removal, and the patient is hemostatic. The specimen has been placed in a location where it can be easily retrieved; I prefer the right upper quadrant.
In a single-port approach, the bag is inserted into the abdomen through an open single-port cannula at the umbilicus. I use a Steri-Drape Isolation Bag (3M), 50 cm by 50 cm, with drawstrings. (See Figure 1.) Standing across from my assistant, I tightly fan-fold the bag and wring it to purge it of air. Using an atraumatic grasper, I position the bag linearly with the opening up; it is inserted into the pelvis first, with the superior end guided upward into the mid- and then upper-abdomen.
After the bag is placed, the cannula is recapped with the multiport cap. I use the Olympus TriPort 15 (Advanced Surgical Concepts), and the abdomen is reinsufflated. A camera is inserted into one of the 5-mm ports. I use the Olympus 5-mm articulating laparoscope.
Through the other 5-mm port, I maneuver the bag to form a pocket opening by pushing the upper corners of the bag against the respective abdominal sidewalls, and the body of the bag into the pelvis. I then elevate the specimen and, with a rotational movement, I guide the specimen along the right abdominal sidewall and into the pocket opening. I then elevate the sides of the bag and ensure that the specimen is well contained. (See Figure 2.)
The drawstrings are then brought up into the port hub. The cap is removed, and the opening of the bag is pulled up through the port and out approximately 15-20 cm, as symmetrically as possible.
The port cap is then replaced within the exteriorized neck of the bag, and the bag is insufflated with traditional laparoscopic pressure of 15-18 mm, creating an artificial pneumoperitoneum. (See Figure 3.) The camera is reinserted, followed by the morcellator, into the 15-mm port of the single-site tri-port. The morcellator must be lubricated so it does not catch the bag on the way in. Morcellation is carried out under direct vision.
After the morcellation process is complete, the morcellator is removed, followed by the camera and the tri-port cap. The bag is desufflated and removed through the open cannula, and the abdomen is closed as usual.
In a multiport approach, the umbilical incision is extended to 15 mm after completion of the hysterectomy or myomectomy. The bag is inserted and placed in a manner similar to the single-port approach, except that the lateral ports may be utilized to further position the bag once it is placed about halfway in. The bag is similarly opened, the specimen contained, and the neck of the bag exteriorized. A 15-mm port is similarly lubricated and placed inside the neck of the bag and through the umbilical incision.
As the bag is insufflated, the lateral ports are backed slightly out so that they're flush with the abdominal wall, and the trocars are opened to allow the release of any residual gas in the abdomen. The laparoscope is then placed into the 15-mm umbilical trocar.
Visualization will ultimately occur from another site, however. One of the lateral 5-mm trocars is advanced at a right angle to the insufflated bag, and an introducer is placed to puncture the bag. I recommend using a blunt plastic trocar; a balloon-tip trocar also can be used. Contrary to what one might think, the bag will not leak any gas. The laparoscope is now transferred to this 5-mm lateral port, and the umbilical port is removed. A lubricated morcellator is inserted directly through the umbilical incision, and morcellation is performed under direct visualization from the side port.
These techniques for single-port or multiport enclosed power morcellation have been shown to be reproducible and successful – with all bags intact and specimens contained – in a multicenter analysis that is being prepared for publication. The largest uterus in the study was 1,481 g.
To make the approach less cumbersome, I have designed a morcellation device consisting of a specialized bag that will open automatically and assist in capturing the specimen. The device, which has closure tabs and a retrieval lanyard, has been developed with the input of other minimally invasive gynecologic surgeons and the design and engineering expertise of Advanced Surgical Concepts. It will be manufactured by the company pending Food and Drug Administration approval.
Concealed power morcellation: Our take
By Bernard Taylor, M.D.
The approach to the use of power morcellation within a bag in my practice was not specifically driven by the desire to prevent the dispersion of malignant leiomyosarcoma – an issue that is now front and center and indeed, is important – but by a broader desire to perform as complete an extraction as possible.
For years we have used isolation bags to conceal and contain small uteri, ovaries, and specimens that we debulk using a scalpel and remove through a small umbilical incision. However, this approach is cumbersome for removing larger uteri. Consequently, over the past year we refined our methods for performing power morcellation within a bag.
This procedure can be performed after traditional laparoscopy or robotic-assisted hysterectomy or myomectomy to extract the specimen from the abdomen. A surgical tissue bag is placed into the abdomen and is insufflated within the abdomen. Morcellation is carried out within the concealed "pseudopneumoperitoneum," and the bag is exteriorized from the abdomen, typically through the umbilical incision.
The majority of my procedures utilize a multiport approach due to the nature of my practice as a urogynecologist. After hysterectomy or myomectomy, the specimen is placed in the upper abdomen. The cuff is closed, other procedures are completed in the usual fashion, and hemostasis is reassured. In robotic procedures, we undock the robot prior to morcellation. The umbilical port is removed, and the bag is placed into the abdomen with a ring forceps or atraumatic forceps without teeth. The umbilical port is then replaced to reestablish the pneumoperitoneum for visualization.
For larger uterine specimens, I use an Isodrape isolation bag (Microtek Medical). This 18 x 18 inch bag with a drawstring opening easily accommodates uteri larger than 1,000 g. The bag is placed into the pelvis with the open end at the pelvic brim and is opened wide. I use the term "open from iliac to iliac and sacrum to bladder." Once the bag is positioned, the specimen is grasped; the patient is slowly taken out of the Trendelenburg position to allow for gravity to assist in the placement of the specimen into the bag.
The drawstrings are then grasped and taken out of the umbilical trocar with simultaneous removal of the umbilical port. At this time, the open end of the bag is exteriorized, and the specimen technically is extraperitoneal and "concealed."
To insufflate the bag at this point and create the "pseudopneumoperitoneum," a 15-mm port is lubricated and placed through the opening in the bag. The bag is then insufflated to the usual pressure (15 mm Hg). The accessory ports are opened at this time to allow for the abdominal pressure to fall and for the bag to expand and fill the abdominal cavity.
To enable visualization throughout the morcellation procedure, I use one of the previously placed lower abdomen lateral ports to place a 5-mm camera. The camera is placed after a 5-mm balloon tip trocar is introduced and advanced to perforate the bag. The balloon tip is insufflated in the usual fashion, securing the bag against the abdominal wall and preventing a gas leak.
Alternatively, I have also used a SILSport (Covidien) at the umbilicus in cases when there is a leak at the umbilical incision and the 15-mm port does not seal properly. While use of a single-port technique has been shown to be feasible for both visualization and morcellation, it can present challenges in complex cases with a larger uterus or multiple fibroids.
Either way, morcellation can take place within the concealed "pseudo-peritoneal" cavity, with the morcellator lubricated and placed either through the umbilical incision or through the Tri-Port. After morcellation is complete, the bag containing the smaller tissue fragments and blood is simply removed through the umbilicus, and closure of the abdomen is completed in the usual fashion.
I also have adopted a simplified approach to remove the smaller uteri with supracervical hysterectomy and sacral colpopexy performed laparoscopically. Despite preoperative screening with endometrial biopsy and pelvic ultrasound, several of my patients in the past have been diagnosed with either early ovarian or endometrial neoplasias on final pathology. After completing the procedure, I place the small menopausal senile uterus and adnexa into a 15-mm endoscopic bag. The bag is brought through the umbilical port, and the specimen is removed via morcellation with a scalpel or scissors.
Surgeons have asked about additional time needed to place the bag and position the specimen. Technically, specimen placement is a learned skill; once one is proficient, the case times are not any different. In fact, cases may be shorter because of the time saved by not having to retrieve uterine fragments from the abdomen and pelvis.
Theoretically, the procedure addresses concerns associated with tissue fragmentation and dissemination within the abdominal cavity. To date, there are no trials showing prevention of cancer upstaging or benign conditions such as leiomyomatosis peritonealis disseminata or endometriosis.
Enclosed vaginal morcellation for enlarged uteri
By Ceana H. Nezhat, M.D.
Removal of large uteri via minimally invasive surgery poses challenges to gynecologic surgeons. Limitations on the use of intraperitoneal electromechanical morcellation are a step forward in terms of protecting patients from undue harm, but we also must acknowledge that minimally invasive surgery has been shown to be superior to laparotomy in the majority of cases.
While safer ways to extract large specimens without risk of spreading both benign and malignant tissue are being studied and developed, gynecologic surgeons need to find alternative minimally invasive approaches for removing large specimens.
Minimally invasive approaches to extirpate uteri and myomas have been described prior to the advent of electromechanical morcellation. A natural orifice such as the vagina for gynecologic procedures is a clear choice for removing specimens. Laparoscopic-assisted myomectomy is a combination of laparoscopy and minilaparotomy for tissue extraction (JSLS 2001;5:299-303). Another alternative is extracting myoma and other tissues through a posterior colpotomy. Rates of dyspareunia and adhesions in the cul-de-sac after tissue extraction through a colpotomy are low (J. Reprod. Med. 1993;38:534-6).
Vaginal hysterectomy was chronicled and performed by the Greek physician Soranus of Ephesus in 120 A.D. (Acta Chir. Iugosl. 2011:58:9-14), and it is still the preferred route of hysterectomy when it can be performed. Large uteri can be transvaginally morcellated and vaginally extracted; however, this technique still poses the risk, although low, of spilling fragments of uterine tissue in the abdomen.
By combining total laparoscopic hysterectomy and vaginal morcellation of enlarged uteri within an enclosed specimen bag, the risk of spilling uterine fragments is reduced, in addition to avoiding potential visceral and vascular complications associated with intraperitoneal electromechanical morcellation.
Enclosed vaginal morcellation is the technique of placing the enlarged hysterectomy specimen in an endoscopic specimen retrieval bag and then transvaginally morcellating the tissue.
In the development of this technique, I have experimented with different endoscopic sacs and retractors. In my experience, the optimal laparoscopic bag is the LapSac Surgical Tissue Pouch (Cook Medical). This endoscopic, one-time use, specimen retrieval bag is flexible, durable nylon with an integral polyurethane inner coating and polypropylene drawstring (Figure 4). The bag comes in four sizes, the largest (size 8 x 10 inch, volume 1,500 mL) of which I used to assist in removing the 18-week size uterus in the accompanying video.
The other key piece of equipment for vaginal extraction of large uteri is a transvaginally-placed wound retractor. As I have previously described, the self-retaining retractor provides optimal exposure in a narrow field and minimizes trauma to surrounding tissue (J. Minim. Inv. Gynecol. 2009:16:616-7). Two wound retractors are suitable for this surgical technique: the Alexis Wound Protector/Retractor (Applied Medical) (Figure 5) and the Mobius Elastic Abdominal Retractor (Cooper Surgical) (Figure 6).
After complete laparoscopic detachment of the uterus and cervix (and adnexa), the wound retractor is placed transvaginally. One semirigid ring is pushed superiorly through the vagina and into the peritoneal cavity, where is it placed in the cul-de-sac. In this manner, a uniform, circumferential orifice is created. In narrower vaginal vaults, the ring can be compressed between the thumb and index finger to reduce its diameter and thus be introduced into the peritoneal cavity atraumatically. With the small-size retraction systems, an orifice of 2.5 to 6 cm diameter can be created.
The LapSac is folded and then inserted with ring forceps transvaginally through the wound retractor. (See Figure 7.) The bag is unfolded intraperitoneally using the integral tabs to demarcate the edges of the bag. The bag should be unfolded in the pelvis with the opening of the bag cephalad. The fundus of the hysterectomy specimen is grasped with a tenaculum and placed inside the bag. (See Figure 8.)
It is an important technical point to grab the fundus or the heaviest point of the specimen to allow weight and gravity to facilitate placing the specimen securely in the bag. If the cervix or a lighter part of the specimen is grasped, placement in the bag is more difficult and the specimen will likely slip out of the bag.
Once the entire hysterectomy specimen is in the LapSac, the bag is cinched by grasping the blue drawstrings. The opening of the bag is pulled through the vagina. The opening of the bag is externalized and the edges are attached with Allis clamps to the external edge of the wound retractor. The specimen (typically the cervix first) is grasped with a tenaculum and vaginally morcellated and cored with a scalpel (Figure 9).
Metal vaginal retractors are placed within the bag to protect against sharp injury during morcellation. I prefer to amputate the cervix and adnexa separately and en bloc to preserve as much original architecture as possible so the pathologists can properly orient and examine the tissue. After completion of the morcellation, the wound retractor is removed, the cuff closed, and the procedure completed per surgeon preference.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for 20 years. He reported that he has a financial interest in the artificial pneumoperitoneum device under development, and that he is a consultant for Olympus.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He reported that he has no disclosures relevant to this Master Class.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University. He reported that he is a consultant for Karl Storz Endoscopy.
Videos of these experts' individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website.
Power morcellation within an insufflated bag
By Tony Shibley, M.D.
Morcellating safely in laparoscopic hysterectomy or myomectomy involves not only the use of a bag, but also the creation of an artificial pneumoperitoneum inside the bag. Inflation of the bag allows us to create a safe and completely contained working environment in the abdomen, with good visualization and adequate working space.
In the past, specimen containment bags were used for morcellation, but the bags would adhere to tissue and would be easily cut or would tear or rupture. Without proper space and visualization, surgeons could not break tissue into smaller fragments without endangering the bag or the structures behind the bag. We were safely and precisely disconnecting tissue in our surgeries, but falling short with tissue removal. There were calls for more durable bags, but the problems of visualization and safe distance were not addressed. Eventually, the goal of morcellating within a bag was largely abandoned in favor of open power morcellation.
I stopped performing open power morcellation about 2 years ago and developed a new approach to enclosed morcellation. By creating an artificial pneumoperitoneum in a bag, a good working space is developed within it. This meets the needs of containment while significantly lowering the risk of tissue dissemination and also reducing the risk of bowel injury, vascular injury, and other types of morcellator-related mechanical injuries. This approach to morcellation is safer on all fronts.
My method of enclosed morcellation has been utilized in both single-port and multiport hysterectomy (total and supracervical) and myomectomy performed with either traditional laparoscopy or the robotic platform.
The morcellation process is begun after surgery is complete except for tissue removal, and the patient is hemostatic. The specimen has been placed in a location where it can be easily retrieved; I prefer the right upper quadrant.
In a single-port approach, the bag is inserted into the abdomen through an open single-port cannula at the umbilicus. I use a Steri-Drape Isolation Bag (3M), 50 cm by 50 cm, with drawstrings. (See Figure 1.) Standing across from my assistant, I tightly fan-fold the bag and wring it to purge it of air. Using an atraumatic grasper, I position the bag linearly with the opening up; it is inserted into the pelvis first, with the superior end guided upward into the mid- and then upper-abdomen.
After the bag is placed, the cannula is recapped with the multiport cap. I use the Olympus TriPort 15 (Advanced Surgical Concepts), and the abdomen is reinsufflated. A camera is inserted into one of the 5-mm ports. I use the Olympus 5-mm articulating laparoscope.
Through the other 5-mm port, I maneuver the bag to form a pocket opening by pushing the upper corners of the bag against the respective abdominal sidewalls, and the body of the bag into the pelvis. I then elevate the specimen and, with a rotational movement, I guide the specimen along the right abdominal sidewall and into the pocket opening. I then elevate the sides of the bag and ensure that the specimen is well contained. (See Figure 2.)
The drawstrings are then brought up into the port hub. The cap is removed, and the opening of the bag is pulled up through the port and out approximately 15-20 cm, as symmetrically as possible.
The port cap is then replaced within the exteriorized neck of the bag, and the bag is insufflated with traditional laparoscopic pressure of 15-18 mm, creating an artificial pneumoperitoneum. (See Figure 3.) The camera is reinserted, followed by the morcellator, into the 15-mm port of the single-site tri-port. The morcellator must be lubricated so it does not catch the bag on the way in. Morcellation is carried out under direct vision.
After the morcellation process is complete, the morcellator is removed, followed by the camera and the tri-port cap. The bag is desufflated and removed through the open cannula, and the abdomen is closed as usual.
In a multiport approach, the umbilical incision is extended to 15 mm after completion of the hysterectomy or myomectomy. The bag is inserted and placed in a manner similar to the single-port approach, except that the lateral ports may be utilized to further position the bag once it is placed about halfway in. The bag is similarly opened, the specimen contained, and the neck of the bag exteriorized. A 15-mm port is similarly lubricated and placed inside the neck of the bag and through the umbilical incision.
As the bag is insufflated, the lateral ports are backed slightly out so that they're flush with the abdominal wall, and the trocars are opened to allow the release of any residual gas in the abdomen. The laparoscope is then placed into the 15-mm umbilical trocar.
Visualization will ultimately occur from another site, however. One of the lateral 5-mm trocars is advanced at a right angle to the insufflated bag, and an introducer is placed to puncture the bag. I recommend using a blunt plastic trocar; a balloon-tip trocar also can be used. Contrary to what one might think, the bag will not leak any gas. The laparoscope is now transferred to this 5-mm lateral port, and the umbilical port is removed. A lubricated morcellator is inserted directly through the umbilical incision, and morcellation is performed under direct visualization from the side port.
These techniques for single-port or multiport enclosed power morcellation have been shown to be reproducible and successful – with all bags intact and specimens contained – in a multicenter analysis that is being prepared for publication. The largest uterus in the study was 1,481 g.
To make the approach less cumbersome, I have designed a morcellation device consisting of a specialized bag that will open automatically and assist in capturing the specimen. The device, which has closure tabs and a retrieval lanyard, has been developed with the input of other minimally invasive gynecologic surgeons and the design and engineering expertise of Advanced Surgical Concepts. It will be manufactured by the company pending Food and Drug Administration approval.
Concealed power morcellation: Our take
By Bernard Taylor, M.D.
The approach to the use of power morcellation within a bag in my practice was not specifically driven by the desire to prevent the dispersion of malignant leiomyosarcoma – an issue that is now front and center and indeed, is important – but by a broader desire to perform as complete an extraction as possible.
For years we have used isolation bags to conceal and contain small uteri, ovaries, and specimens that we debulk using a scalpel and remove through a small umbilical incision. However, this approach is cumbersome for removing larger uteri. Consequently, over the past year we refined our methods for performing power morcellation within a bag.
This procedure can be performed after traditional laparoscopy or robotic-assisted hysterectomy or myomectomy to extract the specimen from the abdomen. A surgical tissue bag is placed into the abdomen and is insufflated within the abdomen. Morcellation is carried out within the concealed "pseudopneumoperitoneum," and the bag is exteriorized from the abdomen, typically through the umbilical incision.
The majority of my procedures utilize a multiport approach due to the nature of my practice as a urogynecologist. After hysterectomy or myomectomy, the specimen is placed in the upper abdomen. The cuff is closed, other procedures are completed in the usual fashion, and hemostasis is reassured. In robotic procedures, we undock the robot prior to morcellation. The umbilical port is removed, and the bag is placed into the abdomen with a ring forceps or atraumatic forceps without teeth. The umbilical port is then replaced to reestablish the pneumoperitoneum for visualization.
For larger uterine specimens, I use an Isodrape isolation bag (Microtek Medical). This 18 x 18 inch bag with a drawstring opening easily accommodates uteri larger than 1,000 g. The bag is placed into the pelvis with the open end at the pelvic brim and is opened wide. I use the term "open from iliac to iliac and sacrum to bladder." Once the bag is positioned, the specimen is grasped; the patient is slowly taken out of the Trendelenburg position to allow for gravity to assist in the placement of the specimen into the bag.
The drawstrings are then grasped and taken out of the umbilical trocar with simultaneous removal of the umbilical port. At this time, the open end of the bag is exteriorized, and the specimen technically is extraperitoneal and "concealed."
To insufflate the bag at this point and create the "pseudopneumoperitoneum," a 15-mm port is lubricated and placed through the opening in the bag. The bag is then insufflated to the usual pressure (15 mm Hg). The accessory ports are opened at this time to allow for the abdominal pressure to fall and for the bag to expand and fill the abdominal cavity.
To enable visualization throughout the morcellation procedure, I use one of the previously placed lower abdomen lateral ports to place a 5-mm camera. The camera is placed after a 5-mm balloon tip trocar is introduced and advanced to perforate the bag. The balloon tip is insufflated in the usual fashion, securing the bag against the abdominal wall and preventing a gas leak.
Alternatively, I have also used a SILSport (Covidien) at the umbilicus in cases when there is a leak at the umbilical incision and the 15-mm port does not seal properly. While use of a single-port technique has been shown to be feasible for both visualization and morcellation, it can present challenges in complex cases with a larger uterus or multiple fibroids.
Either way, morcellation can take place within the concealed "pseudo-peritoneal" cavity, with the morcellator lubricated and placed either through the umbilical incision or through the Tri-Port. After morcellation is complete, the bag containing the smaller tissue fragments and blood is simply removed through the umbilicus, and closure of the abdomen is completed in the usual fashion.
I also have adopted a simplified approach to remove the smaller uteri with supracervical hysterectomy and sacral colpopexy performed laparoscopically. Despite preoperative screening with endometrial biopsy and pelvic ultrasound, several of my patients in the past have been diagnosed with either early ovarian or endometrial neoplasias on final pathology. After completing the procedure, I place the small menopausal senile uterus and adnexa into a 15-mm endoscopic bag. The bag is brought through the umbilical port, and the specimen is removed via morcellation with a scalpel or scissors.
Surgeons have asked about additional time needed to place the bag and position the specimen. Technically, specimen placement is a learned skill; once one is proficient, the case times are not any different. In fact, cases may be shorter because of the time saved by not having to retrieve uterine fragments from the abdomen and pelvis.
Theoretically, the procedure addresses concerns associated with tissue fragmentation and dissemination within the abdominal cavity. To date, there are no trials showing prevention of cancer upstaging or benign conditions such as leiomyomatosis peritonealis disseminata or endometriosis.
Enclosed vaginal morcellation for enlarged uteri
By Ceana H. Nezhat, M.D.
Removal of large uteri via minimally invasive surgery poses challenges to gynecologic surgeons. Limitations on the use of intraperitoneal electromechanical morcellation are a step forward in terms of protecting patients from undue harm, but we also must acknowledge that minimally invasive surgery has been shown to be superior to laparotomy in the majority of cases.
While safer ways to extract large specimens without risk of spreading both benign and malignant tissue are being studied and developed, gynecologic surgeons need to find alternative minimally invasive approaches for removing large specimens.
Minimally invasive approaches to extirpate uteri and myomas have been described prior to the advent of electromechanical morcellation. A natural orifice such as the vagina for gynecologic procedures is a clear choice for removing specimens. Laparoscopic-assisted myomectomy is a combination of laparoscopy and minilaparotomy for tissue extraction (JSLS 2001;5:299-303). Another alternative is extracting myoma and other tissues through a posterior colpotomy. Rates of dyspareunia and adhesions in the cul-de-sac after tissue extraction through a colpotomy are low (J. Reprod. Med. 1993;38:534-6).
Vaginal hysterectomy was chronicled and performed by the Greek physician Soranus of Ephesus in 120 A.D. (Acta Chir. Iugosl. 2011:58:9-14), and it is still the preferred route of hysterectomy when it can be performed. Large uteri can be transvaginally morcellated and vaginally extracted; however, this technique still poses the risk, although low, of spilling fragments of uterine tissue in the abdomen.
By combining total laparoscopic hysterectomy and vaginal morcellation of enlarged uteri within an enclosed specimen bag, the risk of spilling uterine fragments is reduced, in addition to avoiding potential visceral and vascular complications associated with intraperitoneal electromechanical morcellation.
Enclosed vaginal morcellation is the technique of placing the enlarged hysterectomy specimen in an endoscopic specimen retrieval bag and then transvaginally morcellating the tissue.
In the development of this technique, I have experimented with different endoscopic sacs and retractors. In my experience, the optimal laparoscopic bag is the LapSac Surgical Tissue Pouch (Cook Medical). This endoscopic, one-time use, specimen retrieval bag is flexible, durable nylon with an integral polyurethane inner coating and polypropylene drawstring (Figure 4). The bag comes in four sizes, the largest (size 8 x 10 inch, volume 1,500 mL) of which I used to assist in removing the 18-week size uterus in the accompanying video.
The other key piece of equipment for vaginal extraction of large uteri is a transvaginally-placed wound retractor. As I have previously described, the self-retaining retractor provides optimal exposure in a narrow field and minimizes trauma to surrounding tissue (J. Minim. Inv. Gynecol. 2009:16:616-7). Two wound retractors are suitable for this surgical technique: the Alexis Wound Protector/Retractor (Applied Medical) (Figure 5) and the Mobius Elastic Abdominal Retractor (Cooper Surgical) (Figure 6).
After complete laparoscopic detachment of the uterus and cervix (and adnexa), the wound retractor is placed transvaginally. One semirigid ring is pushed superiorly through the vagina and into the peritoneal cavity, where is it placed in the cul-de-sac. In this manner, a uniform, circumferential orifice is created. In narrower vaginal vaults, the ring can be compressed between the thumb and index finger to reduce its diameter and thus be introduced into the peritoneal cavity atraumatically. With the small-size retraction systems, an orifice of 2.5 to 6 cm diameter can be created.
The LapSac is folded and then inserted with ring forceps transvaginally through the wound retractor. (See Figure 7.) The bag is unfolded intraperitoneally using the integral tabs to demarcate the edges of the bag. The bag should be unfolded in the pelvis with the opening of the bag cephalad. The fundus of the hysterectomy specimen is grasped with a tenaculum and placed inside the bag. (See Figure 8.)
It is an important technical point to grab the fundus or the heaviest point of the specimen to allow weight and gravity to facilitate placing the specimen securely in the bag. If the cervix or a lighter part of the specimen is grasped, placement in the bag is more difficult and the specimen will likely slip out of the bag.
Once the entire hysterectomy specimen is in the LapSac, the bag is cinched by grasping the blue drawstrings. The opening of the bag is pulled through the vagina. The opening of the bag is externalized and the edges are attached with Allis clamps to the external edge of the wound retractor. The specimen (typically the cervix first) is grasped with a tenaculum and vaginally morcellated and cored with a scalpel (Figure 9).
Metal vaginal retractors are placed within the bag to protect against sharp injury during morcellation. I prefer to amputate the cervix and adnexa separately and en bloc to preserve as much original architecture as possible so the pathologists can properly orient and examine the tissue. After completion of the morcellation, the wound retractor is removed, the cuff closed, and the procedure completed per surgeon preference.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for 20 years. He reported that he has a financial interest in the artificial pneumoperitoneum device under development, and that he is a consultant for Olympus.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He reported that he has no disclosures relevant to this Master Class.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University. He reported that he is a consultant for Karl Storz Endoscopy.
Videos of these experts' individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website.
A new paradigm for preeclampsia
That preeclampsia is a growing problem, and one of the most significant causes of maternal-fetal morbidity and mortality today, is what drove the American College of Obstetricians and Gynecologists (ACOG) to convene a task force on hypertension in pregnancy in 2011. Indeed, the incidence of preeclampsia has increased approximately 10% over the last 3 decades in the United States, such that approximately 5%-7% of all pregnant women will develop the disorder.
The specific etiology of preeclampsia remains unclear, but the reasons for the increased incidence likely include the rise in delayed childbearing, the increased use of assisted reproductive technology, a rise in the number of twins, and the obesity pandemic.
Epidemiologically, older women with their first pregnancy are at higher risk of developing preeclampsia, whether or not they use assisted reproductive technology (ART). The not-infrequent use of ART among older women, namely IVF, has a compounding effect. So too, does the incidence of twinning. While we fortunately are seeing a lower rate of higher-order multiple gestations associated with IVF than we did in the 1990s, the incidence of twinning has increased dramatically. Women with multiple gestations of any order are at higher risk of developing preeclampsia.
The obesity pandemic is widely believed to be the most modifiable risk factor for preeclampsia. If we can help women to achieve a body mass index (BMI) that is as close to optimal as possible prior to conception, we will likely see significant reductions in the incidence of hypertensive disorders.
Prompt diagnosis of preeclampsia is critical, and on this front, ACOG\'s Task Force on Hypertension in Pregnancy report of 2013 sets forth an important new paradigm for thinking about the disorder and establishing its presence.
Managing preeclampsia remains challenging, however, as there are many areas in which evidence for guiding therapy and management is still insufficient. ACOG’s task force set out to review available data and to attempt to provide clarity on the management of preeclampsia as well as its diagnosis. This was no easy task, and in their culminating report, which lists 60 distinct recommendations, the task force clearly acknowledges the weak evidence base, giving relatively few of their recommendations top marks for both the quality of evidence and their strength of recommendation.
The report appropriately reminds us that there are few if any prescriptions or protocols when it comes to managing preeclampsia. My main concern with the task force’s coverage of management involves their recommendations that magnesium sulfate be used to treat patients with eclampsia and those with preeclampsia with severe features, but not necessarily those without severe features. Patients can progress so rapidly that unless every woman with preeclampsia is vigilantly scrutinized during labor and post delivery – a difficult, if not impossible, task – the window of opportunity to prevent convulsions through the use of magnesium sulfate may well be missed.
ACOG’s new terminology, definitions
Importantly, ACOG’s Task Force on Hypertension in Pregnancy report emphasizes that preeclampsia is an evolving, dynamic, and multisystemic process. It recommends elimination of the terms "mild" and "severe" preeclampsia and encourages the use of new terminology, pushing us to think instead of preeclampsia as being a disorder with or without "severe features." According to the report, a diagnosis of "mild preeclampsia" applies only at the moment at which the diagnosis is established, making the phrase misleading.
Physicians and other providers who have long been in practice will have a hard time ridding their vocabulary of the terms mild and severe preeclampsia, but the intent of the recommendation – to foster appreciation of preeclampsia as an evolving disease – is important and should become entrenched in our approach to hypertension in pregnancy.
The report also downgrades the role of proteinuria in the diagnosis of preeclampsia. Proteinuria is defined as the excretion of 300 mg or more of protein in a 24-hour urine collection or a urine protein/creatinine ratio of at least 0.3 mg/dL. Although proteinuria may indeed be a primary diagnostic finding, it should not be required in order to make the diagnosis of preeclampsia if other severe features are present.
As described in the report, severe features of preeclampsia may include thrombocytopenia (platelet count less than 100,000/microliter), impaired liver function, a rise in serum creatinine indicating progressive renal insufficiency (a serum creatinine concentration greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease), central nervous system disturbances, pulmonary edema, and persistently high elevations in blood pressure (a systolic blood pressure of 160 mm Hg or higher or a diastolic reading of 110 mm Hg or higher on two occasions at least 4 hours apart).
The document states, in other words, that to establish preeclampsia, equal weight should be given to any of these so-called severe features – even in the absence of proteinuria – when they occur along with new-onset hypertension at 20 weeks’ gestation or beyond. (The blood pressure criteria stipulate hypertension as a systolic blood pressure of 140 mm Hg or higher and a diastolic of 90 mm Hg or higher, taken on at least two occasions 4 hours apart.)
This new approach to diagnosing preeclampsia is a major change. It reflects what the ACOG document rightly calls a "minimal relationship" between the quantity of urinary protein and maternal and fetal outcomes in preeclampsia, as well as the fact that preeclampsia may quickly evolve. We need these broader criteria and lower thresholds so that we will not miss patients who might present atypically, with proteinuria not yet a significant finding, but with disease quickly developing.
Another helpful change is the removal of significant fetal growth restriction (less than the fifth percentile) as a diagnostic feature for traditionally coined "severe preeclampsia." Fetal growth restriction is not included in ACOG’s recommended list of severe features of preeclampsia. This is a helpful clarification that should prevent potentially unnecessary deliveries. The problems of fetal growth restriction (FGR) and preeclampsia should be considered and managed separately, with the option of expectant management considered with respect to each entity alone, as opposed to the finding of FGR driving a diagnosis of severe preeclampsia and thus delivery within 48 hours.
Where evidence is strongest
ACOG’s report contains 60 distinct recommendations covering hypertensive disorders in pregnancy, but only 6 of these recommendations are graded as both "strong" and based on "high-quality" evidence. (A "strong" recommendation is one that the task force considered so well supported by the literature that it is applicable to "virtually all patients.")
The remaining recommendations are either graded as "qualified" or are based on evidence that is rated as "very low," "low," or "moderate," or some combination of the "qualified" grade and a quality-of-evidence ranking below "high."
The task force utilized a strategy developed by the Grading of Recommendations Assessment, Development and Evaluation Working Group that rates the quality of evidence based largely on what’s called "confidence in estimates of effect." Under this approach, randomized, controlled trials are important but may still be considered flawed and observational studies are usually but not necessarily classified as low quality.
The small number of strong, high-quality recommendations in the ACOG report reflects the fact that, despite advances in our understanding of preeclampsia, there are many areas in which we lack good evidence from randomized, controlled trials. Importantly, the task force emphasizes that it offers recommendations and not prescriptions, and that sound clinical judgment remains a key part of patient management.
The six strong recommendations in the report that are based on high-quality evidence are as follows:
• The administration of vitamin C or vitamin E to prevent preeclampsia is not recommended.
• Women with severe preeclampsia who are being expectantly managed at 34 weeks’ or less of gestation should receive corticosteroids for the benefit of accelerating fetal lung maturation.
• Women who have chronic hypertension with superimposed preeclampsia and are being expectantly managed at 34 weeks’ or less of gestation also should have corticosteroids administered for the purpose of accelerating fetal lung maturation.
• For women with eclampsia, the administration of parenteral magnesium sulfate is recommended.
• For women with severe preeclampsia, the administration of intrapartum-postpartum magnesium sulfate to prevent eclampsia is recommended.
• For women with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome who are before the gestational age of fetal viability, delivery should be undertaken shortly after initial maternal stabilization.
My take
Contrary to former recommendations, the task force’s new recommendations suggest that use of magnesium sulfate for preeclampsia without severe features (formerly called "mild preeclampsia") may not be needed. Although magnesium sulfate may not be warranted in every case, patients can progress so rapidly from having no severe symptoms to developing severe symptoms that it is difficult if not impossible to parse out who would or would not benefit from treatment. If we try to do so, we run the great risk of not providing the necessary medication to our patients, thereby increasing the chances of maternal morbidity and mortality.
In our institution, we continue to use magnesium sulfate intrapartum and post partum for every patient with a diagnosis of preeclampsia, whether or not she has severe features. Without high-quality evidence to the contrary, I do not believe that we should alter the former recommendation that magnesium sulfate be used in all cases of preeclampsia. We administer the agent for 24 hours post partum because studies looking at a 48-hour window have shown that most patients who have an eclamptic seizure within 48 hours after delivery will actually experience it within the first 24 hours.
There is a remaining conundrum. We know that approximately 50% of postpartum seizures occur more than 48 hours post delivery. It is vital, therefore, that patients who have hypertensive disorders during pregnancy be educated about the signs and symptoms of postpartum preeclampsia and be given clear directions about how to contact their provider if any signs or symptoms – such as headache, visual disturbances, and right-upper-quadrant pain – are noticed. Patients who are discharged with blood pressures that are still elevated (but not enough to require hospitalization) should be seen again within 72 hours or a week for an evaluation of their blood pressure. The threshold in our practices for arranging earlier postpartum visits, moreover, should be set very low for these women.
The need for patient education is mentioned in the ACOG report, which says, "It is suggested that health care providers convey information about preeclampsia in the context of prenatal care and postpartum care using proven health care communication practices."
The wording of the recommendation as well as its "qualified" strength and the designation of a "low" quality of evidence should not detract from the importance of the message that patient education is a key to successful recognition and management of preeclampsia. The awareness of and knowledge about postpartum preeclampsia have been shown in recent research to be disappointingly low. We need to do better.
With respect to prevention, there are two strategies that, despite not having strong recommendations and/or the backing of high-quality evidence, are still considered to be effective. One is the use of daily low-dose aspirin (60-80 mg) in women with a history of early-onset preeclampsia and preterm delivery prior to 34 weeks’ of gestation. The other is calcium supplementation for women who have an inadequate daily intake of calcium, although this practice has less relevance in the United States than in the developing world.
The goal of low-dose aspirin is really to reduce the likelihood of severe preeclampsia recurring. Almost half of patients with a history of preeclampsia will not develop the disorder in a subsequent pregnancy (without aspirin therapy), so the reduction in the incidence of preeclampsia with low-dose aspirin is unlikely to be significant. However, as aspirin therapy is a relatively benign and inexpensive intervention, it is worth considering. Indeed, meta-analyses of women in randomized trials of low-dose aspirin for preeclampsia prevention have shown small reductions in the incidence and morbidity of preeclampsia, without any evidence of adverse effects.
[Notably, the U.S. Preventive Services Task Force said in a draft recommendation statement issued in April that it recommends low-dose aspirin use after 12 weeks of pregnancy in women who are at high risk for preeclampsia. (See accompanying story.)]
Over the years a variety of predictive strategies – for predicting early-onset preeclampsia in particular – have been proposed and researched, including various biomarkers, blood tests, and imaging studies. These are all worthwhile endeavors at the research level, but for any screening strategy to be implemented there must be an effective intervention. At the current time, our only effective intervention is delivery based on clinical findings.
On the other end of the spectrum, it is clear today that preeclampsia is associated with later-life cardiovascular disease in women. It is only in the last 5-10 years that research findings have come to the fore and that we have thought about preeclampsia as a marker for increased disease risk, just as we did starting several decades ago with gestational diabetes and the risk of future type 2 diabetes.
ACOG’s task force suggests yearly assessment of blood pressure, lipids, fasting blood glucose, and BMI in women with a medical history of preeclampsia who delivered preterm or who have a history of recurrent preeclampsia. The recommendation is "qualified," with a low quality of evidence, and contains a cautionary footnote stating that "the value and appropriate timing of assessment is not yet established."
Indeed, the next step in research will be to follow patients with preeclampsia longitudinally and determine whether or not interventional strategies can be devised to reduce the cardiovascular risk to baseline or, ideally below baseline, in these patients. Until we have such information, we should still recognize that this group of patients may be at higher risk for cardiovascular disease later in life, and, at the very least, be even more vigilant about adhering to regular health maintenance examinations.
Dr. John T. Repke is professor and chair of obstetrics and gynecology at Pennsylvania State University, Hershey, and has published extensively on hypertension in pregnancy. Dr. Repke said he has no relevant financial disclosures.
That preeclampsia is a growing problem, and one of the most significant causes of maternal-fetal morbidity and mortality today, is what drove the American College of Obstetricians and Gynecologists (ACOG) to convene a task force on hypertension in pregnancy in 2011. Indeed, the incidence of preeclampsia has increased approximately 10% over the last 3 decades in the United States, such that approximately 5%-7% of all pregnant women will develop the disorder.
The specific etiology of preeclampsia remains unclear, but the reasons for the increased incidence likely include the rise in delayed childbearing, the increased use of assisted reproductive technology, a rise in the number of twins, and the obesity pandemic.
Epidemiologically, older women with their first pregnancy are at higher risk of developing preeclampsia, whether or not they use assisted reproductive technology (ART). The not-infrequent use of ART among older women, namely IVF, has a compounding effect. So too, does the incidence of twinning. While we fortunately are seeing a lower rate of higher-order multiple gestations associated with IVF than we did in the 1990s, the incidence of twinning has increased dramatically. Women with multiple gestations of any order are at higher risk of developing preeclampsia.
The obesity pandemic is widely believed to be the most modifiable risk factor for preeclampsia. If we can help women to achieve a body mass index (BMI) that is as close to optimal as possible prior to conception, we will likely see significant reductions in the incidence of hypertensive disorders.
Prompt diagnosis of preeclampsia is critical, and on this front, ACOG\'s Task Force on Hypertension in Pregnancy report of 2013 sets forth an important new paradigm for thinking about the disorder and establishing its presence.
Managing preeclampsia remains challenging, however, as there are many areas in which evidence for guiding therapy and management is still insufficient. ACOG’s task force set out to review available data and to attempt to provide clarity on the management of preeclampsia as well as its diagnosis. This was no easy task, and in their culminating report, which lists 60 distinct recommendations, the task force clearly acknowledges the weak evidence base, giving relatively few of their recommendations top marks for both the quality of evidence and their strength of recommendation.
The report appropriately reminds us that there are few if any prescriptions or protocols when it comes to managing preeclampsia. My main concern with the task force’s coverage of management involves their recommendations that magnesium sulfate be used to treat patients with eclampsia and those with preeclampsia with severe features, but not necessarily those without severe features. Patients can progress so rapidly that unless every woman with preeclampsia is vigilantly scrutinized during labor and post delivery – a difficult, if not impossible, task – the window of opportunity to prevent convulsions through the use of magnesium sulfate may well be missed.
ACOG’s new terminology, definitions
Importantly, ACOG’s Task Force on Hypertension in Pregnancy report emphasizes that preeclampsia is an evolving, dynamic, and multisystemic process. It recommends elimination of the terms "mild" and "severe" preeclampsia and encourages the use of new terminology, pushing us to think instead of preeclampsia as being a disorder with or without "severe features." According to the report, a diagnosis of "mild preeclampsia" applies only at the moment at which the diagnosis is established, making the phrase misleading.
Physicians and other providers who have long been in practice will have a hard time ridding their vocabulary of the terms mild and severe preeclampsia, but the intent of the recommendation – to foster appreciation of preeclampsia as an evolving disease – is important and should become entrenched in our approach to hypertension in pregnancy.
The report also downgrades the role of proteinuria in the diagnosis of preeclampsia. Proteinuria is defined as the excretion of 300 mg or more of protein in a 24-hour urine collection or a urine protein/creatinine ratio of at least 0.3 mg/dL. Although proteinuria may indeed be a primary diagnostic finding, it should not be required in order to make the diagnosis of preeclampsia if other severe features are present.
As described in the report, severe features of preeclampsia may include thrombocytopenia (platelet count less than 100,000/microliter), impaired liver function, a rise in serum creatinine indicating progressive renal insufficiency (a serum creatinine concentration greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease), central nervous system disturbances, pulmonary edema, and persistently high elevations in blood pressure (a systolic blood pressure of 160 mm Hg or higher or a diastolic reading of 110 mm Hg or higher on two occasions at least 4 hours apart).
The document states, in other words, that to establish preeclampsia, equal weight should be given to any of these so-called severe features – even in the absence of proteinuria – when they occur along with new-onset hypertension at 20 weeks’ gestation or beyond. (The blood pressure criteria stipulate hypertension as a systolic blood pressure of 140 mm Hg or higher and a diastolic of 90 mm Hg or higher, taken on at least two occasions 4 hours apart.)
This new approach to diagnosing preeclampsia is a major change. It reflects what the ACOG document rightly calls a "minimal relationship" between the quantity of urinary protein and maternal and fetal outcomes in preeclampsia, as well as the fact that preeclampsia may quickly evolve. We need these broader criteria and lower thresholds so that we will not miss patients who might present atypically, with proteinuria not yet a significant finding, but with disease quickly developing.
Another helpful change is the removal of significant fetal growth restriction (less than the fifth percentile) as a diagnostic feature for traditionally coined "severe preeclampsia." Fetal growth restriction is not included in ACOG’s recommended list of severe features of preeclampsia. This is a helpful clarification that should prevent potentially unnecessary deliveries. The problems of fetal growth restriction (FGR) and preeclampsia should be considered and managed separately, with the option of expectant management considered with respect to each entity alone, as opposed to the finding of FGR driving a diagnosis of severe preeclampsia and thus delivery within 48 hours.
Where evidence is strongest
ACOG’s report contains 60 distinct recommendations covering hypertensive disorders in pregnancy, but only 6 of these recommendations are graded as both "strong" and based on "high-quality" evidence. (A "strong" recommendation is one that the task force considered so well supported by the literature that it is applicable to "virtually all patients.")
The remaining recommendations are either graded as "qualified" or are based on evidence that is rated as "very low," "low," or "moderate," or some combination of the "qualified" grade and a quality-of-evidence ranking below "high."
The task force utilized a strategy developed by the Grading of Recommendations Assessment, Development and Evaluation Working Group that rates the quality of evidence based largely on what’s called "confidence in estimates of effect." Under this approach, randomized, controlled trials are important but may still be considered flawed and observational studies are usually but not necessarily classified as low quality.
The small number of strong, high-quality recommendations in the ACOG report reflects the fact that, despite advances in our understanding of preeclampsia, there are many areas in which we lack good evidence from randomized, controlled trials. Importantly, the task force emphasizes that it offers recommendations and not prescriptions, and that sound clinical judgment remains a key part of patient management.
The six strong recommendations in the report that are based on high-quality evidence are as follows:
• The administration of vitamin C or vitamin E to prevent preeclampsia is not recommended.
• Women with severe preeclampsia who are being expectantly managed at 34 weeks’ or less of gestation should receive corticosteroids for the benefit of accelerating fetal lung maturation.
• Women who have chronic hypertension with superimposed preeclampsia and are being expectantly managed at 34 weeks’ or less of gestation also should have corticosteroids administered for the purpose of accelerating fetal lung maturation.
• For women with eclampsia, the administration of parenteral magnesium sulfate is recommended.
• For women with severe preeclampsia, the administration of intrapartum-postpartum magnesium sulfate to prevent eclampsia is recommended.
• For women with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome who are before the gestational age of fetal viability, delivery should be undertaken shortly after initial maternal stabilization.
My take
Contrary to former recommendations, the task force’s new recommendations suggest that use of magnesium sulfate for preeclampsia without severe features (formerly called "mild preeclampsia") may not be needed. Although magnesium sulfate may not be warranted in every case, patients can progress so rapidly from having no severe symptoms to developing severe symptoms that it is difficult if not impossible to parse out who would or would not benefit from treatment. If we try to do so, we run the great risk of not providing the necessary medication to our patients, thereby increasing the chances of maternal morbidity and mortality.
In our institution, we continue to use magnesium sulfate intrapartum and post partum for every patient with a diagnosis of preeclampsia, whether or not she has severe features. Without high-quality evidence to the contrary, I do not believe that we should alter the former recommendation that magnesium sulfate be used in all cases of preeclampsia. We administer the agent for 24 hours post partum because studies looking at a 48-hour window have shown that most patients who have an eclamptic seizure within 48 hours after delivery will actually experience it within the first 24 hours.
There is a remaining conundrum. We know that approximately 50% of postpartum seizures occur more than 48 hours post delivery. It is vital, therefore, that patients who have hypertensive disorders during pregnancy be educated about the signs and symptoms of postpartum preeclampsia and be given clear directions about how to contact their provider if any signs or symptoms – such as headache, visual disturbances, and right-upper-quadrant pain – are noticed. Patients who are discharged with blood pressures that are still elevated (but not enough to require hospitalization) should be seen again within 72 hours or a week for an evaluation of their blood pressure. The threshold in our practices for arranging earlier postpartum visits, moreover, should be set very low for these women.
The need for patient education is mentioned in the ACOG report, which says, "It is suggested that health care providers convey information about preeclampsia in the context of prenatal care and postpartum care using proven health care communication practices."
The wording of the recommendation as well as its "qualified" strength and the designation of a "low" quality of evidence should not detract from the importance of the message that patient education is a key to successful recognition and management of preeclampsia. The awareness of and knowledge about postpartum preeclampsia have been shown in recent research to be disappointingly low. We need to do better.
With respect to prevention, there are two strategies that, despite not having strong recommendations and/or the backing of high-quality evidence, are still considered to be effective. One is the use of daily low-dose aspirin (60-80 mg) in women with a history of early-onset preeclampsia and preterm delivery prior to 34 weeks’ of gestation. The other is calcium supplementation for women who have an inadequate daily intake of calcium, although this practice has less relevance in the United States than in the developing world.
The goal of low-dose aspirin is really to reduce the likelihood of severe preeclampsia recurring. Almost half of patients with a history of preeclampsia will not develop the disorder in a subsequent pregnancy (without aspirin therapy), so the reduction in the incidence of preeclampsia with low-dose aspirin is unlikely to be significant. However, as aspirin therapy is a relatively benign and inexpensive intervention, it is worth considering. Indeed, meta-analyses of women in randomized trials of low-dose aspirin for preeclampsia prevention have shown small reductions in the incidence and morbidity of preeclampsia, without any evidence of adverse effects.
[Notably, the U.S. Preventive Services Task Force said in a draft recommendation statement issued in April that it recommends low-dose aspirin use after 12 weeks of pregnancy in women who are at high risk for preeclampsia. (See accompanying story.)]
Over the years a variety of predictive strategies – for predicting early-onset preeclampsia in particular – have been proposed and researched, including various biomarkers, blood tests, and imaging studies. These are all worthwhile endeavors at the research level, but for any screening strategy to be implemented there must be an effective intervention. At the current time, our only effective intervention is delivery based on clinical findings.
On the other end of the spectrum, it is clear today that preeclampsia is associated with later-life cardiovascular disease in women. It is only in the last 5-10 years that research findings have come to the fore and that we have thought about preeclampsia as a marker for increased disease risk, just as we did starting several decades ago with gestational diabetes and the risk of future type 2 diabetes.
ACOG’s task force suggests yearly assessment of blood pressure, lipids, fasting blood glucose, and BMI in women with a medical history of preeclampsia who delivered preterm or who have a history of recurrent preeclampsia. The recommendation is "qualified," with a low quality of evidence, and contains a cautionary footnote stating that "the value and appropriate timing of assessment is not yet established."
Indeed, the next step in research will be to follow patients with preeclampsia longitudinally and determine whether or not interventional strategies can be devised to reduce the cardiovascular risk to baseline or, ideally below baseline, in these patients. Until we have such information, we should still recognize that this group of patients may be at higher risk for cardiovascular disease later in life, and, at the very least, be even more vigilant about adhering to regular health maintenance examinations.
Dr. John T. Repke is professor and chair of obstetrics and gynecology at Pennsylvania State University, Hershey, and has published extensively on hypertension in pregnancy. Dr. Repke said he has no relevant financial disclosures.
That preeclampsia is a growing problem, and one of the most significant causes of maternal-fetal morbidity and mortality today, is what drove the American College of Obstetricians and Gynecologists (ACOG) to convene a task force on hypertension in pregnancy in 2011. Indeed, the incidence of preeclampsia has increased approximately 10% over the last 3 decades in the United States, such that approximately 5%-7% of all pregnant women will develop the disorder.
The specific etiology of preeclampsia remains unclear, but the reasons for the increased incidence likely include the rise in delayed childbearing, the increased use of assisted reproductive technology, a rise in the number of twins, and the obesity pandemic.
Epidemiologically, older women with their first pregnancy are at higher risk of developing preeclampsia, whether or not they use assisted reproductive technology (ART). The not-infrequent use of ART among older women, namely IVF, has a compounding effect. So too, does the incidence of twinning. While we fortunately are seeing a lower rate of higher-order multiple gestations associated with IVF than we did in the 1990s, the incidence of twinning has increased dramatically. Women with multiple gestations of any order are at higher risk of developing preeclampsia.
The obesity pandemic is widely believed to be the most modifiable risk factor for preeclampsia. If we can help women to achieve a body mass index (BMI) that is as close to optimal as possible prior to conception, we will likely see significant reductions in the incidence of hypertensive disorders.
Prompt diagnosis of preeclampsia is critical, and on this front, ACOG\'s Task Force on Hypertension in Pregnancy report of 2013 sets forth an important new paradigm for thinking about the disorder and establishing its presence.
Managing preeclampsia remains challenging, however, as there are many areas in which evidence for guiding therapy and management is still insufficient. ACOG’s task force set out to review available data and to attempt to provide clarity on the management of preeclampsia as well as its diagnosis. This was no easy task, and in their culminating report, which lists 60 distinct recommendations, the task force clearly acknowledges the weak evidence base, giving relatively few of their recommendations top marks for both the quality of evidence and their strength of recommendation.
The report appropriately reminds us that there are few if any prescriptions or protocols when it comes to managing preeclampsia. My main concern with the task force’s coverage of management involves their recommendations that magnesium sulfate be used to treat patients with eclampsia and those with preeclampsia with severe features, but not necessarily those without severe features. Patients can progress so rapidly that unless every woman with preeclampsia is vigilantly scrutinized during labor and post delivery – a difficult, if not impossible, task – the window of opportunity to prevent convulsions through the use of magnesium sulfate may well be missed.
ACOG’s new terminology, definitions
Importantly, ACOG’s Task Force on Hypertension in Pregnancy report emphasizes that preeclampsia is an evolving, dynamic, and multisystemic process. It recommends elimination of the terms "mild" and "severe" preeclampsia and encourages the use of new terminology, pushing us to think instead of preeclampsia as being a disorder with or without "severe features." According to the report, a diagnosis of "mild preeclampsia" applies only at the moment at which the diagnosis is established, making the phrase misleading.
Physicians and other providers who have long been in practice will have a hard time ridding their vocabulary of the terms mild and severe preeclampsia, but the intent of the recommendation – to foster appreciation of preeclampsia as an evolving disease – is important and should become entrenched in our approach to hypertension in pregnancy.
The report also downgrades the role of proteinuria in the diagnosis of preeclampsia. Proteinuria is defined as the excretion of 300 mg or more of protein in a 24-hour urine collection or a urine protein/creatinine ratio of at least 0.3 mg/dL. Although proteinuria may indeed be a primary diagnostic finding, it should not be required in order to make the diagnosis of preeclampsia if other severe features are present.
As described in the report, severe features of preeclampsia may include thrombocytopenia (platelet count less than 100,000/microliter), impaired liver function, a rise in serum creatinine indicating progressive renal insufficiency (a serum creatinine concentration greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease), central nervous system disturbances, pulmonary edema, and persistently high elevations in blood pressure (a systolic blood pressure of 160 mm Hg or higher or a diastolic reading of 110 mm Hg or higher on two occasions at least 4 hours apart).
The document states, in other words, that to establish preeclampsia, equal weight should be given to any of these so-called severe features – even in the absence of proteinuria – when they occur along with new-onset hypertension at 20 weeks’ gestation or beyond. (The blood pressure criteria stipulate hypertension as a systolic blood pressure of 140 mm Hg or higher and a diastolic of 90 mm Hg or higher, taken on at least two occasions 4 hours apart.)
This new approach to diagnosing preeclampsia is a major change. It reflects what the ACOG document rightly calls a "minimal relationship" between the quantity of urinary protein and maternal and fetal outcomes in preeclampsia, as well as the fact that preeclampsia may quickly evolve. We need these broader criteria and lower thresholds so that we will not miss patients who might present atypically, with proteinuria not yet a significant finding, but with disease quickly developing.
Another helpful change is the removal of significant fetal growth restriction (less than the fifth percentile) as a diagnostic feature for traditionally coined "severe preeclampsia." Fetal growth restriction is not included in ACOG’s recommended list of severe features of preeclampsia. This is a helpful clarification that should prevent potentially unnecessary deliveries. The problems of fetal growth restriction (FGR) and preeclampsia should be considered and managed separately, with the option of expectant management considered with respect to each entity alone, as opposed to the finding of FGR driving a diagnosis of severe preeclampsia and thus delivery within 48 hours.
Where evidence is strongest
ACOG’s report contains 60 distinct recommendations covering hypertensive disorders in pregnancy, but only 6 of these recommendations are graded as both "strong" and based on "high-quality" evidence. (A "strong" recommendation is one that the task force considered so well supported by the literature that it is applicable to "virtually all patients.")
The remaining recommendations are either graded as "qualified" or are based on evidence that is rated as "very low," "low," or "moderate," or some combination of the "qualified" grade and a quality-of-evidence ranking below "high."
The task force utilized a strategy developed by the Grading of Recommendations Assessment, Development and Evaluation Working Group that rates the quality of evidence based largely on what’s called "confidence in estimates of effect." Under this approach, randomized, controlled trials are important but may still be considered flawed and observational studies are usually but not necessarily classified as low quality.
The small number of strong, high-quality recommendations in the ACOG report reflects the fact that, despite advances in our understanding of preeclampsia, there are many areas in which we lack good evidence from randomized, controlled trials. Importantly, the task force emphasizes that it offers recommendations and not prescriptions, and that sound clinical judgment remains a key part of patient management.
The six strong recommendations in the report that are based on high-quality evidence are as follows:
• The administration of vitamin C or vitamin E to prevent preeclampsia is not recommended.
• Women with severe preeclampsia who are being expectantly managed at 34 weeks’ or less of gestation should receive corticosteroids for the benefit of accelerating fetal lung maturation.
• Women who have chronic hypertension with superimposed preeclampsia and are being expectantly managed at 34 weeks’ or less of gestation also should have corticosteroids administered for the purpose of accelerating fetal lung maturation.
• For women with eclampsia, the administration of parenteral magnesium sulfate is recommended.
• For women with severe preeclampsia, the administration of intrapartum-postpartum magnesium sulfate to prevent eclampsia is recommended.
• For women with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome who are before the gestational age of fetal viability, delivery should be undertaken shortly after initial maternal stabilization.
My take
Contrary to former recommendations, the task force’s new recommendations suggest that use of magnesium sulfate for preeclampsia without severe features (formerly called "mild preeclampsia") may not be needed. Although magnesium sulfate may not be warranted in every case, patients can progress so rapidly from having no severe symptoms to developing severe symptoms that it is difficult if not impossible to parse out who would or would not benefit from treatment. If we try to do so, we run the great risk of not providing the necessary medication to our patients, thereby increasing the chances of maternal morbidity and mortality.
In our institution, we continue to use magnesium sulfate intrapartum and post partum for every patient with a diagnosis of preeclampsia, whether or not she has severe features. Without high-quality evidence to the contrary, I do not believe that we should alter the former recommendation that magnesium sulfate be used in all cases of preeclampsia. We administer the agent for 24 hours post partum because studies looking at a 48-hour window have shown that most patients who have an eclamptic seizure within 48 hours after delivery will actually experience it within the first 24 hours.
There is a remaining conundrum. We know that approximately 50% of postpartum seizures occur more than 48 hours post delivery. It is vital, therefore, that patients who have hypertensive disorders during pregnancy be educated about the signs and symptoms of postpartum preeclampsia and be given clear directions about how to contact their provider if any signs or symptoms – such as headache, visual disturbances, and right-upper-quadrant pain – are noticed. Patients who are discharged with blood pressures that are still elevated (but not enough to require hospitalization) should be seen again within 72 hours or a week for an evaluation of their blood pressure. The threshold in our practices for arranging earlier postpartum visits, moreover, should be set very low for these women.
The need for patient education is mentioned in the ACOG report, which says, "It is suggested that health care providers convey information about preeclampsia in the context of prenatal care and postpartum care using proven health care communication practices."
The wording of the recommendation as well as its "qualified" strength and the designation of a "low" quality of evidence should not detract from the importance of the message that patient education is a key to successful recognition and management of preeclampsia. The awareness of and knowledge about postpartum preeclampsia have been shown in recent research to be disappointingly low. We need to do better.
With respect to prevention, there are two strategies that, despite not having strong recommendations and/or the backing of high-quality evidence, are still considered to be effective. One is the use of daily low-dose aspirin (60-80 mg) in women with a history of early-onset preeclampsia and preterm delivery prior to 34 weeks’ of gestation. The other is calcium supplementation for women who have an inadequate daily intake of calcium, although this practice has less relevance in the United States than in the developing world.
The goal of low-dose aspirin is really to reduce the likelihood of severe preeclampsia recurring. Almost half of patients with a history of preeclampsia will not develop the disorder in a subsequent pregnancy (without aspirin therapy), so the reduction in the incidence of preeclampsia with low-dose aspirin is unlikely to be significant. However, as aspirin therapy is a relatively benign and inexpensive intervention, it is worth considering. Indeed, meta-analyses of women in randomized trials of low-dose aspirin for preeclampsia prevention have shown small reductions in the incidence and morbidity of preeclampsia, without any evidence of adverse effects.
[Notably, the U.S. Preventive Services Task Force said in a draft recommendation statement issued in April that it recommends low-dose aspirin use after 12 weeks of pregnancy in women who are at high risk for preeclampsia. (See accompanying story.)]
Over the years a variety of predictive strategies – for predicting early-onset preeclampsia in particular – have been proposed and researched, including various biomarkers, blood tests, and imaging studies. These are all worthwhile endeavors at the research level, but for any screening strategy to be implemented there must be an effective intervention. At the current time, our only effective intervention is delivery based on clinical findings.
On the other end of the spectrum, it is clear today that preeclampsia is associated with later-life cardiovascular disease in women. It is only in the last 5-10 years that research findings have come to the fore and that we have thought about preeclampsia as a marker for increased disease risk, just as we did starting several decades ago with gestational diabetes and the risk of future type 2 diabetes.
ACOG’s task force suggests yearly assessment of blood pressure, lipids, fasting blood glucose, and BMI in women with a medical history of preeclampsia who delivered preterm or who have a history of recurrent preeclampsia. The recommendation is "qualified," with a low quality of evidence, and contains a cautionary footnote stating that "the value and appropriate timing of assessment is not yet established."
Indeed, the next step in research will be to follow patients with preeclampsia longitudinally and determine whether or not interventional strategies can be devised to reduce the cardiovascular risk to baseline or, ideally below baseline, in these patients. Until we have such information, we should still recognize that this group of patients may be at higher risk for cardiovascular disease later in life, and, at the very least, be even more vigilant about adhering to regular health maintenance examinations.
Dr. John T. Repke is professor and chair of obstetrics and gynecology at Pennsylvania State University, Hershey, and has published extensively on hypertension in pregnancy. Dr. Repke said he has no relevant financial disclosures.
Managing acute pyelonephritis during pregnancy
Acute pyelonephritis is a serious and common medical complication of pregnancy. It is estimated to occur in up to 1%-2% of pregnancies and is a common nonobstetrical indication for antepartum hospital admissions. Its prevalence is probably even higher in obstetrical clinics serving underserved inner-city populations such as ours in Newark, N.J.
The diagnosis of acute pyelonephritis is based on clinical signs and symptoms. Patients usually feel ill and have fever, chills, flank pain (usually right-sided), dysuria, and urgency and frequency. Nausea and vomiting also may be present. Laboratory abnormalities may include pyuria and bacteriuria, with white blood cell counts often predictive of pyelonephritis. A urine culture and sensitivity will often reveal Escherichia coli, but other less commonly found causative organisms may be detected as well.
It is the prevailing view that most pregnant women with acute pyelonephritis should be hospitalized for careful monitoring, evaluated for possible sepsis, and treated with parenteral antibiotics. Recently published retrospective cohort studies, as well as our own experience, have emphasized that the risks of preterm labor and delivery in these patients can be significant, as can the risks of septic shock and other complications. Treatment, therefore, should be aggressive, with careful monitoring and charting of vital signs, including urinary output; and fetal monitoring and monitoring of uterine contractions. That way one can identify patients who are not responding to treatment or who may be developing septic shock or preterm labor.
Studies have shown that 10%-12% of all pregnant women have asymptomatic bacteriuria. Because physiologic changes associated with pregnancy encourage urinary stasis, there is an increased risk of progression to acute pyelonephritis with the potential for serious infectious complications, even in pregnant women who are otherwise healthy. By and large, however, pyelonephritis is usually a preventable problem given access to prenatal care. Screening for asymptomatic bacteriuria during the first prenatal visit is important, and repeat screening in each trimester in women who are at high risk for recurrent infection is critical for preventing symptomatic and possibly severe infection.
Our screening preference is to perform a urine culture and sensitivity test at the first prenatal visit. Other providers may utilize a urinalysis and leukocyte esterase test initially, but as this approach is not as sensitive or specific, it must be followed by a urine culture and sensitivity testing if the urinalysis results are positive. Obstetricians and others providing prenatal care should utilize whatever approach works best for their patients and environment. Most importantly, screening for asymptomatic bacteriuria must occur early in the pregnancy.
Additional urine culture and sensitivity testing are advisable for patients who are at high risk for urinary tract infections, such as those who have had frequent UTIs before pregnancy and those who have anemia, sickle cell trait, a history of renal stones, diabetes mellitus, obesity, or neurologic disorders (such as neurogenic bladder and multiple sclerosis). Considering the increase in prevalence of obesity and diabetes, these high-risk patients represent a growing proportion of the obstetric population and appear to be at increased risk of UTIs as well. Women of increasing age and increasing parity also may be at higher risk of developing UTIs during pregnancy.
Cranberry juice has been touted for years as an effective remedy for the prevention and treatment of UTIs in women, and I advise my patients who have a UTI during pregnancy, who have diabetes, or who have other risk factors, to drink a glass of unsweetened cranberry juice each day. No definitive mechanism of action has been established, but it appears that cranberry juice prevents or interferes with the adherence of bacteria (particularly E. coli) to uroepithelial cells. It is important to emphasize to patients to consume unsweetened cranberry juice and not cranberry juice cocktail because of the high sugar content in the latter.
Recent research has emphasized that pregnancies of women who develop pyelonephritis are more likely to be complicated by spontaneous preterm birth, septicemia, and other adverse outcomes. In a retrospective cohort study of more than 546,000 singleton pregnancies delivered in all Kaiser Permanente of Southern California hospitals from 1993 to 2010, women with pyelonephritis were almost 57 times more likely than those without pyelonephritis to develop septicemia and 1.3 times more likely to have spontaneous preterm birth.
In addition, pregnancies of women with pyelonephritis were 2.6 times more likely than those of the baseline obstetric population to be complicated by anemia and 16.5 times more likely to be complicated by acute renal failure (Am. J. Obstet. Gynecol. 2014;210:219.e1-6). The overall incidence of acute antepartum pyelonephritis in this cohort study was relatively low compared with the incidence in other populations – 0.5% – which is not surprising given that patients in Kaiser’s integrated health care system routinely receive prenatal screening for asymptomatic bacteriuria.
Another retrospective population-based study comparing almost 220,000 singleton pregnancies of patients with and without acute pyelonephritis concluded that the infection is an independent risk factor for preterm delivery (Eur. J. Obstet. Gynecol. Reprod. Biol 2012;162:24-7).
After admission to the hospital, patients must be carefully monitored for uterine contractions and changes in vital signs and fetal heart rate. Several years ago, in an effort to empirically and synergistically target E. coli, the most common cause of UTIs and pyelonephritis, we began administering both an extended-spectrum cephalosporin (intravenous ceftriaxone) and an antimicrobial that will target gram-negative organisms, such as an aminoglycoside (gentamicin) or aztreonam.
We established this protocol because reviews of the outcomes at our institution indicated that intravenous ceftriaxone alone had not prevented some of our patients from developing septic shock in the first 8-20 hours post admission, despite the fact that culture and sensitivity results later indicated that the organism was E. coli and sensitive to the antimicrobial.
While we have not yet done any formal data analysis since changing our protocol, the combination parenteral antimicrobial regimen prescribed on admission appears to be effective in preventing the development of septic shock. We prescribe ceftriaxone 2 g intravenously once a day and gentamicin 5 mg/kg per day. Both drugs are continued until the patient improves clinically and has been afebrile for 48 hours.
At discharge, patients are prescribed a 10- to 14-day oral antimicrobial regimen dependent upon the culture and sensitivity report. Because at least 50% of E. coli are resistant to penicillin-like antimicrobials, the initial treatment no longer involves the use of ampicillin or amoxicillin. A repeat urine culture test at the end of treatment to confirm clearance of the infection is essential.
The possibility of anatomical obstructions in the urinary system should be investigated in pregnant patients who have multiple UTIs or who are unresponsive to appropriate antibiotic therapy for pyelonephritis. In this group we have performed ultrasound of the urinary tract system and have diagnosed renal stones as the risk factor for recurrent UTI. These patients are prescribed antimicrobial prophylaxis for the duration of the pregnancy. After delivery, they are referred to a urologist for follow-up care and treatment.
Dr. Apuzzio reported that he has no disclosures relevant to this Master Class.
Dr. Apuzzio is a professor in the department of obstetrics, gynecology, and women’s health, director of prenatal diagnosis and infectious diseases, professor of radiology, and director of maternal-fetal medicine at Rutgers New Jersey Medical School, Newark.
Acute pyelonephritis is a serious and common medical complication of pregnancy. It is estimated to occur in up to 1%-2% of pregnancies and is a common nonobstetrical indication for antepartum hospital admissions. Its prevalence is probably even higher in obstetrical clinics serving underserved inner-city populations such as ours in Newark, N.J.
The diagnosis of acute pyelonephritis is based on clinical signs and symptoms. Patients usually feel ill and have fever, chills, flank pain (usually right-sided), dysuria, and urgency and frequency. Nausea and vomiting also may be present. Laboratory abnormalities may include pyuria and bacteriuria, with white blood cell counts often predictive of pyelonephritis. A urine culture and sensitivity will often reveal Escherichia coli, but other less commonly found causative organisms may be detected as well.
It is the prevailing view that most pregnant women with acute pyelonephritis should be hospitalized for careful monitoring, evaluated for possible sepsis, and treated with parenteral antibiotics. Recently published retrospective cohort studies, as well as our own experience, have emphasized that the risks of preterm labor and delivery in these patients can be significant, as can the risks of septic shock and other complications. Treatment, therefore, should be aggressive, with careful monitoring and charting of vital signs, including urinary output; and fetal monitoring and monitoring of uterine contractions. That way one can identify patients who are not responding to treatment or who may be developing septic shock or preterm labor.
Studies have shown that 10%-12% of all pregnant women have asymptomatic bacteriuria. Because physiologic changes associated with pregnancy encourage urinary stasis, there is an increased risk of progression to acute pyelonephritis with the potential for serious infectious complications, even in pregnant women who are otherwise healthy. By and large, however, pyelonephritis is usually a preventable problem given access to prenatal care. Screening for asymptomatic bacteriuria during the first prenatal visit is important, and repeat screening in each trimester in women who are at high risk for recurrent infection is critical for preventing symptomatic and possibly severe infection.
Our screening preference is to perform a urine culture and sensitivity test at the first prenatal visit. Other providers may utilize a urinalysis and leukocyte esterase test initially, but as this approach is not as sensitive or specific, it must be followed by a urine culture and sensitivity testing if the urinalysis results are positive. Obstetricians and others providing prenatal care should utilize whatever approach works best for their patients and environment. Most importantly, screening for asymptomatic bacteriuria must occur early in the pregnancy.
Additional urine culture and sensitivity testing are advisable for patients who are at high risk for urinary tract infections, such as those who have had frequent UTIs before pregnancy and those who have anemia, sickle cell trait, a history of renal stones, diabetes mellitus, obesity, or neurologic disorders (such as neurogenic bladder and multiple sclerosis). Considering the increase in prevalence of obesity and diabetes, these high-risk patients represent a growing proportion of the obstetric population and appear to be at increased risk of UTIs as well. Women of increasing age and increasing parity also may be at higher risk of developing UTIs during pregnancy.
Cranberry juice has been touted for years as an effective remedy for the prevention and treatment of UTIs in women, and I advise my patients who have a UTI during pregnancy, who have diabetes, or who have other risk factors, to drink a glass of unsweetened cranberry juice each day. No definitive mechanism of action has been established, but it appears that cranberry juice prevents or interferes with the adherence of bacteria (particularly E. coli) to uroepithelial cells. It is important to emphasize to patients to consume unsweetened cranberry juice and not cranberry juice cocktail because of the high sugar content in the latter.
Recent research has emphasized that pregnancies of women who develop pyelonephritis are more likely to be complicated by spontaneous preterm birth, septicemia, and other adverse outcomes. In a retrospective cohort study of more than 546,000 singleton pregnancies delivered in all Kaiser Permanente of Southern California hospitals from 1993 to 2010, women with pyelonephritis were almost 57 times more likely than those without pyelonephritis to develop septicemia and 1.3 times more likely to have spontaneous preterm birth.
In addition, pregnancies of women with pyelonephritis were 2.6 times more likely than those of the baseline obstetric population to be complicated by anemia and 16.5 times more likely to be complicated by acute renal failure (Am. J. Obstet. Gynecol. 2014;210:219.e1-6). The overall incidence of acute antepartum pyelonephritis in this cohort study was relatively low compared with the incidence in other populations – 0.5% – which is not surprising given that patients in Kaiser’s integrated health care system routinely receive prenatal screening for asymptomatic bacteriuria.
Another retrospective population-based study comparing almost 220,000 singleton pregnancies of patients with and without acute pyelonephritis concluded that the infection is an independent risk factor for preterm delivery (Eur. J. Obstet. Gynecol. Reprod. Biol 2012;162:24-7).
After admission to the hospital, patients must be carefully monitored for uterine contractions and changes in vital signs and fetal heart rate. Several years ago, in an effort to empirically and synergistically target E. coli, the most common cause of UTIs and pyelonephritis, we began administering both an extended-spectrum cephalosporin (intravenous ceftriaxone) and an antimicrobial that will target gram-negative organisms, such as an aminoglycoside (gentamicin) or aztreonam.
We established this protocol because reviews of the outcomes at our institution indicated that intravenous ceftriaxone alone had not prevented some of our patients from developing septic shock in the first 8-20 hours post admission, despite the fact that culture and sensitivity results later indicated that the organism was E. coli and sensitive to the antimicrobial.
While we have not yet done any formal data analysis since changing our protocol, the combination parenteral antimicrobial regimen prescribed on admission appears to be effective in preventing the development of septic shock. We prescribe ceftriaxone 2 g intravenously once a day and gentamicin 5 mg/kg per day. Both drugs are continued until the patient improves clinically and has been afebrile for 48 hours.
At discharge, patients are prescribed a 10- to 14-day oral antimicrobial regimen dependent upon the culture and sensitivity report. Because at least 50% of E. coli are resistant to penicillin-like antimicrobials, the initial treatment no longer involves the use of ampicillin or amoxicillin. A repeat urine culture test at the end of treatment to confirm clearance of the infection is essential.
The possibility of anatomical obstructions in the urinary system should be investigated in pregnant patients who have multiple UTIs or who are unresponsive to appropriate antibiotic therapy for pyelonephritis. In this group we have performed ultrasound of the urinary tract system and have diagnosed renal stones as the risk factor for recurrent UTI. These patients are prescribed antimicrobial prophylaxis for the duration of the pregnancy. After delivery, they are referred to a urologist for follow-up care and treatment.
Dr. Apuzzio reported that he has no disclosures relevant to this Master Class.
Dr. Apuzzio is a professor in the department of obstetrics, gynecology, and women’s health, director of prenatal diagnosis and infectious diseases, professor of radiology, and director of maternal-fetal medicine at Rutgers New Jersey Medical School, Newark.
Acute pyelonephritis is a serious and common medical complication of pregnancy. It is estimated to occur in up to 1%-2% of pregnancies and is a common nonobstetrical indication for antepartum hospital admissions. Its prevalence is probably even higher in obstetrical clinics serving underserved inner-city populations such as ours in Newark, N.J.
The diagnosis of acute pyelonephritis is based on clinical signs and symptoms. Patients usually feel ill and have fever, chills, flank pain (usually right-sided), dysuria, and urgency and frequency. Nausea and vomiting also may be present. Laboratory abnormalities may include pyuria and bacteriuria, with white blood cell counts often predictive of pyelonephritis. A urine culture and sensitivity will often reveal Escherichia coli, but other less commonly found causative organisms may be detected as well.
It is the prevailing view that most pregnant women with acute pyelonephritis should be hospitalized for careful monitoring, evaluated for possible sepsis, and treated with parenteral antibiotics. Recently published retrospective cohort studies, as well as our own experience, have emphasized that the risks of preterm labor and delivery in these patients can be significant, as can the risks of septic shock and other complications. Treatment, therefore, should be aggressive, with careful monitoring and charting of vital signs, including urinary output; and fetal monitoring and monitoring of uterine contractions. That way one can identify patients who are not responding to treatment or who may be developing septic shock or preterm labor.
Studies have shown that 10%-12% of all pregnant women have asymptomatic bacteriuria. Because physiologic changes associated with pregnancy encourage urinary stasis, there is an increased risk of progression to acute pyelonephritis with the potential for serious infectious complications, even in pregnant women who are otherwise healthy. By and large, however, pyelonephritis is usually a preventable problem given access to prenatal care. Screening for asymptomatic bacteriuria during the first prenatal visit is important, and repeat screening in each trimester in women who are at high risk for recurrent infection is critical for preventing symptomatic and possibly severe infection.
Our screening preference is to perform a urine culture and sensitivity test at the first prenatal visit. Other providers may utilize a urinalysis and leukocyte esterase test initially, but as this approach is not as sensitive or specific, it must be followed by a urine culture and sensitivity testing if the urinalysis results are positive. Obstetricians and others providing prenatal care should utilize whatever approach works best for their patients and environment. Most importantly, screening for asymptomatic bacteriuria must occur early in the pregnancy.
Additional urine culture and sensitivity testing are advisable for patients who are at high risk for urinary tract infections, such as those who have had frequent UTIs before pregnancy and those who have anemia, sickle cell trait, a history of renal stones, diabetes mellitus, obesity, or neurologic disorders (such as neurogenic bladder and multiple sclerosis). Considering the increase in prevalence of obesity and diabetes, these high-risk patients represent a growing proportion of the obstetric population and appear to be at increased risk of UTIs as well. Women of increasing age and increasing parity also may be at higher risk of developing UTIs during pregnancy.
Cranberry juice has been touted for years as an effective remedy for the prevention and treatment of UTIs in women, and I advise my patients who have a UTI during pregnancy, who have diabetes, or who have other risk factors, to drink a glass of unsweetened cranberry juice each day. No definitive mechanism of action has been established, but it appears that cranberry juice prevents or interferes with the adherence of bacteria (particularly E. coli) to uroepithelial cells. It is important to emphasize to patients to consume unsweetened cranberry juice and not cranberry juice cocktail because of the high sugar content in the latter.
Recent research has emphasized that pregnancies of women who develop pyelonephritis are more likely to be complicated by spontaneous preterm birth, septicemia, and other adverse outcomes. In a retrospective cohort study of more than 546,000 singleton pregnancies delivered in all Kaiser Permanente of Southern California hospitals from 1993 to 2010, women with pyelonephritis were almost 57 times more likely than those without pyelonephritis to develop septicemia and 1.3 times more likely to have spontaneous preterm birth.
In addition, pregnancies of women with pyelonephritis were 2.6 times more likely than those of the baseline obstetric population to be complicated by anemia and 16.5 times more likely to be complicated by acute renal failure (Am. J. Obstet. Gynecol. 2014;210:219.e1-6). The overall incidence of acute antepartum pyelonephritis in this cohort study was relatively low compared with the incidence in other populations – 0.5% – which is not surprising given that patients in Kaiser’s integrated health care system routinely receive prenatal screening for asymptomatic bacteriuria.
Another retrospective population-based study comparing almost 220,000 singleton pregnancies of patients with and without acute pyelonephritis concluded that the infection is an independent risk factor for preterm delivery (Eur. J. Obstet. Gynecol. Reprod. Biol 2012;162:24-7).
After admission to the hospital, patients must be carefully monitored for uterine contractions and changes in vital signs and fetal heart rate. Several years ago, in an effort to empirically and synergistically target E. coli, the most common cause of UTIs and pyelonephritis, we began administering both an extended-spectrum cephalosporin (intravenous ceftriaxone) and an antimicrobial that will target gram-negative organisms, such as an aminoglycoside (gentamicin) or aztreonam.
We established this protocol because reviews of the outcomes at our institution indicated that intravenous ceftriaxone alone had not prevented some of our patients from developing septic shock in the first 8-20 hours post admission, despite the fact that culture and sensitivity results later indicated that the organism was E. coli and sensitive to the antimicrobial.
While we have not yet done any formal data analysis since changing our protocol, the combination parenteral antimicrobial regimen prescribed on admission appears to be effective in preventing the development of septic shock. We prescribe ceftriaxone 2 g intravenously once a day and gentamicin 5 mg/kg per day. Both drugs are continued until the patient improves clinically and has been afebrile for 48 hours.
At discharge, patients are prescribed a 10- to 14-day oral antimicrobial regimen dependent upon the culture and sensitivity report. Because at least 50% of E. coli are resistant to penicillin-like antimicrobials, the initial treatment no longer involves the use of ampicillin or amoxicillin. A repeat urine culture test at the end of treatment to confirm clearance of the infection is essential.
The possibility of anatomical obstructions in the urinary system should be investigated in pregnant patients who have multiple UTIs or who are unresponsive to appropriate antibiotic therapy for pyelonephritis. In this group we have performed ultrasound of the urinary tract system and have diagnosed renal stones as the risk factor for recurrent UTI. These patients are prescribed antimicrobial prophylaxis for the duration of the pregnancy. After delivery, they are referred to a urologist for follow-up care and treatment.
Dr. Apuzzio reported that he has no disclosures relevant to this Master Class.
Dr. Apuzzio is a professor in the department of obstetrics, gynecology, and women’s health, director of prenatal diagnosis and infectious diseases, professor of radiology, and director of maternal-fetal medicine at Rutgers New Jersey Medical School, Newark.
Putting morcellation into perspective – ‘Just the facts, Ma’am, nothing but the facts’
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.