User login
Medication Reconciliation Perspectives
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P<0.001), or educate patients on the postdischarge medication regimen (P<0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P<0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P<0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P<0.001), or educate patients on the postdischarge medication regimen (P<0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P<0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P<0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P<0.001), or educate patients on the postdischarge medication regimen (P<0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P<0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P<0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
Electronic Cigarettes
Electronic cigarettes are increasingly prevalent battery‐operated devices that heat a solution to generate an inhalable nicotine‐containing aerosol.[1, 2] Despite a diverse array of devices on the market, the US Food and Drug Administration (FDA) has only recently proposed expanding its regulatory ability to include electronic cigarettes.[3] States, municipalities, and institutions have enacted variable regulations on electronic nicotine delivery systems.[4, 5] Advocates of electronic cigarettes propose that they are a less‐toxic alternative to tobacco cigarettes, with potential for use as a nicotine replacement therapy (NRT).[6, 7, 8] Opponents argue that electronic cigarettes may undermine tobacco cessation goals and potentially expose nonusers to secondhand nicotine vapor.[9, 10]
Hospital providers frequently care for nicotine‐dependent patients.[11] We investigated inpatient healthcare providers' knowledge, perceptions, and experience with electronic cigarettes, with the goals of informing educational efforts and guiding policy decisions around hospital‐based use of electronic nicotine delivery systems.
METHODS
The study was conducted at a 183‐bed urban safety‐net medical center affiliated with a residency training program using a cross‐sectional survey to query a diverse array of inpatient providers (Table 1). Respondents who had not cared for an inpatient in the past 5 years were excluded. Surveys were designed based on prior literature, personal experience, and expert suggestions.[12] Surveys were disseminated in March 2014 via e‐mail, with embedded informed consent and a link that connected anonymously to the online survey (Qualtrics, Provo, UT). We did not collect unique identifiers and offered no incentive for participation. Data were downloaded to a secure database and analyzed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) and GraphPad Prism version 6.04 (GraphPad Software, Inc., La Jolla, CA). The study was approved by the institutional review board.
| Group (No.) | Do you know what an electronic cigarette is?* | Has a hospitalized patient ever asked you if he or she could use an electronic cigarette on hospital grounds?* | Do you see electronic cigarettes as a nicotine replacement option for hospitalized patients? | If you were caring for a patient, would you be okay with the patient using an electronic cigarette while hospitalized? | If you were hospitalized in a shared hospital room, would you be okay with your roommate using an electronic cigarette? | Should electronic cigarettes be banned from healthcare settings? | Should electronic cigarettes be banned in the same locations as traditional cigarettes? | Should electronic cigarettes be regulated by the US Food and Drug Administration? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Faculty MD (32) | 96.9% | 12.5% | 28.1% | 34.4% | 12.5% | 37.5% | 53.1% | 100% |
| Resident MD (33) | 97.0% | 9.1% | 27.3% | 45.5% | 24.2% | 45.5% | 36.4% | 93.9% |
| Registered nurse (35) | 94.3% | 42.9% | 25.7% | 28.6% | 25.7% | 40.0% | 54.3% | 68.6% |
| Rehabilitation staff (18) | 88.9% | 11.1% | 11.1% | 5.6% | 5.6% | 66.7% | 55.6% | 88.9% |
| Social worker (6) | 100% | 33.3% | 16.7% | 16.7% | 0.0% | 50.0% | 50.0% | 83.3% |
| Pharmacist (18) | 100% | 5.6% | 11.1% | 27.8% | 22.2% | 61.1% | 50.0% | 83.3% |
| All respondents (142) | 95.8% | 19.0% | 22.5% | 30.3% | 18.3% | 47.2% | 49.3% | 86.6% |
RESULTS
Study Participants
There were 242 survey respondents (response rate of 41%), of whom 100 were excluded based on study criteria. The median age of the 142 included participants was 34.0 years. There were significantly more female respondents (69%, P=0.001, 2 test), equally over‐represented across all inpatient provider groups. Only 1.4% of all respondents reported personal active tobacco use, whereas 24.6% of study participants reported prior tobacco use. Tobacco use history was similar across inpatient provider groups and gender. Respondents over 50 years of age demonstrated a higher rate of current or prior tobacco use compared with participants from other age groups combined (53% vs 23%, P=0.01, 2 test).
Electronic Cigarette Familiarity and Patient Requests
Of the participants, 95.8% reported familiarity with electronic cigarettes, without differences across age or gender. Of all of the providers, 19.0% reported being asked by a hospitalized patient for permission to use an electronic cigarette in the hospital. Registered nurses were significantly more likely to have been asked by patients compared to all other study participants (43% vs 11%, P<0.001, 2 test).
Electronic Cigarettes as NRT
Whereas 22.5% of study participants felt that electronic cigarettes could serve as a viable in‐hospital NRT, 48.6% felt that electronic cigarettes should not be used, and 28.9% were unsure (Table 1), irrespective of demographics or personal tobacco use history. One‐third of respondents would allow an inpatient under their care to use an electronic cigarette. Groups most likely to permit use were faculty (34.4%) and resident physicians (45.5%), though this difference was not statistically significant.
Perspectives on Exposure
Only 18.3% of study participants would agree to share a hospital room with a patient using an electronic cigarette. Of all participants, 47.2% and 49.3% felt that electronic cigarettes should be banned from healthcare settings and from the same locations as traditional cigarettes, respectively. There were no significant differences in perspectives when stratified by age or gender. Current or prior tobacco users were more likely to be accepting of the use of electronic cigarettes in healthcare settings compared to nonusers (50% vs 29%, P=0.02, Fisher exact test).
FDA Regulation
Of all study participants, 86.6% responded that electronic cigarettes should be regulated by the FDA. Physicians most strongly agreed with this statement compared with all other provider groups (97% vs 78%, P=0.004, 2 test). Conversely, registered nurses were least likely to feel that electronic cigarettes should be FDA‐regulated compared to all other provider groups (69% vs 93%, P<0.005, 2 test).
DISCUSSION
Our study is the first to provide hospital‐based providers' experience and perspectives surrounding electronic cigarette use. The vast majority of participants reported familiarity with electronic cigarettes, consistent with prior findings.[13] Though electronic cigarettes have yet to achieve a use in the hospital setting, 19% of our respondents reported receiving requests from hospitalized patients to use these devices. With increasing patient demand for electronic cigarettes, hospitals will need to update their tobacco policies to include these novel devices as well as target educational efforts toward front‐line providers, such as nurses, who receive the majority of requests.
Participants perceived traditional cigarettes to be significantly more harmful than electronic cigarettes, while established forms of NRT were felt to be less harmful than electronic cigarettes (data not shown). Concern about the health effects of electronic cigarettes is further reflected in providers' hesitancy to view these devices as an NRT option in the hospital, reluctance to consider sharing a room with an electronic cigarette user, and near majority opinion that electronic cigarettes should be banned from healthcare settings altogether. Current regulation by the US Department of Transportation bans electronic cigarette use on airplanes, whereas a host of states currently ban electronic cigarette use in similarly enclosed spaces such as correctional facilities and commuter trains.[14] More knowledge is needed on the health effects of electronic cigarettes on the primary user, secondhand exposure range, and their potential to aid in short‐ and long‐term nicotine cessation before providers and hospitals can make an informed risk‐benefit analysis for appropriate inpatient use. As current or past tobacco users were more accepting of the use of electronic cigarettes in hospital settings, these providers' opinions should be sought for a unique understanding of the interplay between electronic cigarettes and the healthcare environment.
Concern over the unknown safety effects can also be seen in the overwhelming provider support for FDA regulation. Healthcare advocacy groups, such as the American Heart Association, the American Lung Association, and the Legacy Foundation already support federal regulation.[15, 16, 17] FDA regulation may lead to the ability to standardize device content, regulate purchasing and marketing requirements, and ensure that claims to health effects are supported by scientific evidence, though agency involvement may also slow the process of integration into hospital use. Perhaps reflective of the immediacy of the problem, nurses who receive the majority of requests for electronic cigarettes from patients are least likely to want FDA regulation. Until more is known, patients and staff may benefit from pairing vaporizing patients in shared rooms or providing users with designated inhaling spaces.
Nicotine addiction is a strong driving force and, due to a strict no‐smoking policy at our institution, we have witnessed patients making unsafe decisions to leave the hospital (in some cases against medical advice) in an effort to continue smoking. Patients may be starting to look toward electronic cigarettes as an NRT option that more closely satisfies nicotine cravings as well as the ritualistic and tactile components of cigarette use. Electronic cigarettes could have the potential to act as a harm reduction method for nicotine‐dependent inpatients by decreasing the nicotine‐withdrawal related impetus for unsafe hospital discharges. Institutions should take this into account when formulating new policy.
Our study has several limitations. First, it was a single‐center study that may not be representative of provider perspectives at other institutions. Second, the survey was a cross‐sectional sample, missing providers who did not receive the e‐mail during the enrollment period. Third, responses may not accurately reflect perspectives of smaller responding groups such as social workers. Fourth, the survey did not include all types of physicians who deal with smoking cessation, though internal and family medicine physicians provide the majority of care for hospitalized patients at our institution. Fifth, we recorded self‐reported familiarity with electronic cigarettes and did not formally test providers' knowledge of the subject.
Our study provides new perspectives and data on electronic cigarettes to inform future research as well as hospital and healthcare policy. Hospitals should educate patients and front‐line providers around the paucity of health information on these novel devices, while formulating policy that acknowledges patient demand for electronic cigarettes and their potential for cessation therapy and harm reduction. Further research should focus on the effects of nicotine vapor inhalation on patients, the consequences of secondhand nicotine vapor, and the potential for electronic nicotine delivery systems to act as a novel NRT for hospital use.
Disclosure
Nothing to report.
- , , . E‐cigarettes: a scientific review. Circulation. 2014;129:1972–1986.
- , , , , . E‐Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766.
- U.S. Food and Drug Administration. FDA proposes to extend its tobacco authority to additional tobacco products, including e‐cigarettes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394667.htm. Accessed May 5, 2014.
- . Electronic cigarettes: smoke‐free laws, sale restrictions, and the public health. Am J Public Health. 2014;104(6):e17–e18.
- American Nonsmokers' Rights Foundation. US state and local laws regulating use of electronic cigarettes. Available at: http://www.no‐smoke.org/pdf/ecigslaws.pdf. Accessed September 2, 2014.
- , . Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028.
- , , , , , . Effect of an electronic nicotine delivery device (e‐cigarette) on smoking reduction and cessation: a prospective 6‐month pilot study. BMC Public Health. 2011;11:786.
- , , , , . Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction. 2014;109(9):1531–1540.
- , . The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–686.
- . Promise and peril of e‐Cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–136.
- , , , et al. Electronic cigarette awareness, use history, and expected future use among hospitalized cigarette smokers. Nicotine Tob Res. 2014;16(11):1512–1517.
- Global Adult Tobacco Survey Collaborative Group. Tobacco Questions for Surveys: A Subset of Key Questions From the Global Adult Tobacco Survey (GATS). 2nd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available at: http://www.who.int/tobacco/surveillance/en_tfi_tqs.pdf. Accessed April 23, 2014.
- , , . Healthcare providers' beliefs and attitudes about electronic cigarettes and preventive counseling for adolescent patients. J Adolesc Health. 2014;54(6):678–683.
- U.S. Department of Transportation. DOT policy on e‐cigarettes. Available at: http://www.dot.gov/sites/dot.gov/files/docs/PolicyOnECigarettes.pdf. Accessed September 2, 2014.
- American Heart Association. AHA: E‐cigarettes threaten to addict next generation of smokers; regulation, further study needed. Available at: http://blog.heart.org/aha‐e‐cigarettes‐threaten‐to‐addict‐next‐generation‐of‐smokers‐regulation‐further‐study‐needed/. Accessed August 25, 2014.
- American Lung Association. American Lung Association statement on e‐cigarettes. Available at: http://www.lung.org/stop‐smoking/tobacco‐control‐advocacy/federal/e‐cigarettes.html. Accesses August 25, 2014.
- Legacy for Health. E‐cigarette policy: the FDA should promptly exercise regulatory authority and over e‐cigarettes. Available at: http://www.legacyforhealth.org/content/download/3962/56088/version/1/file/LEG‐Policy_Statement‐ECigarette‐JAN2014.pdf. Accessed August 25, 2014.
Electronic cigarettes are increasingly prevalent battery‐operated devices that heat a solution to generate an inhalable nicotine‐containing aerosol.[1, 2] Despite a diverse array of devices on the market, the US Food and Drug Administration (FDA) has only recently proposed expanding its regulatory ability to include electronic cigarettes.[3] States, municipalities, and institutions have enacted variable regulations on electronic nicotine delivery systems.[4, 5] Advocates of electronic cigarettes propose that they are a less‐toxic alternative to tobacco cigarettes, with potential for use as a nicotine replacement therapy (NRT).[6, 7, 8] Opponents argue that electronic cigarettes may undermine tobacco cessation goals and potentially expose nonusers to secondhand nicotine vapor.[9, 10]
Hospital providers frequently care for nicotine‐dependent patients.[11] We investigated inpatient healthcare providers' knowledge, perceptions, and experience with electronic cigarettes, with the goals of informing educational efforts and guiding policy decisions around hospital‐based use of electronic nicotine delivery systems.
METHODS
The study was conducted at a 183‐bed urban safety‐net medical center affiliated with a residency training program using a cross‐sectional survey to query a diverse array of inpatient providers (Table 1). Respondents who had not cared for an inpatient in the past 5 years were excluded. Surveys were designed based on prior literature, personal experience, and expert suggestions.[12] Surveys were disseminated in March 2014 via e‐mail, with embedded informed consent and a link that connected anonymously to the online survey (Qualtrics, Provo, UT). We did not collect unique identifiers and offered no incentive for participation. Data were downloaded to a secure database and analyzed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) and GraphPad Prism version 6.04 (GraphPad Software, Inc., La Jolla, CA). The study was approved by the institutional review board.
| Group (No.) | Do you know what an electronic cigarette is?* | Has a hospitalized patient ever asked you if he or she could use an electronic cigarette on hospital grounds?* | Do you see electronic cigarettes as a nicotine replacement option for hospitalized patients? | If you were caring for a patient, would you be okay with the patient using an electronic cigarette while hospitalized? | If you were hospitalized in a shared hospital room, would you be okay with your roommate using an electronic cigarette? | Should electronic cigarettes be banned from healthcare settings? | Should electronic cigarettes be banned in the same locations as traditional cigarettes? | Should electronic cigarettes be regulated by the US Food and Drug Administration? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Faculty MD (32) | 96.9% | 12.5% | 28.1% | 34.4% | 12.5% | 37.5% | 53.1% | 100% |
| Resident MD (33) | 97.0% | 9.1% | 27.3% | 45.5% | 24.2% | 45.5% | 36.4% | 93.9% |
| Registered nurse (35) | 94.3% | 42.9% | 25.7% | 28.6% | 25.7% | 40.0% | 54.3% | 68.6% |
| Rehabilitation staff (18) | 88.9% | 11.1% | 11.1% | 5.6% | 5.6% | 66.7% | 55.6% | 88.9% |
| Social worker (6) | 100% | 33.3% | 16.7% | 16.7% | 0.0% | 50.0% | 50.0% | 83.3% |
| Pharmacist (18) | 100% | 5.6% | 11.1% | 27.8% | 22.2% | 61.1% | 50.0% | 83.3% |
| All respondents (142) | 95.8% | 19.0% | 22.5% | 30.3% | 18.3% | 47.2% | 49.3% | 86.6% |
RESULTS
Study Participants
There were 242 survey respondents (response rate of 41%), of whom 100 were excluded based on study criteria. The median age of the 142 included participants was 34.0 years. There were significantly more female respondents (69%, P=0.001, 2 test), equally over‐represented across all inpatient provider groups. Only 1.4% of all respondents reported personal active tobacco use, whereas 24.6% of study participants reported prior tobacco use. Tobacco use history was similar across inpatient provider groups and gender. Respondents over 50 years of age demonstrated a higher rate of current or prior tobacco use compared with participants from other age groups combined (53% vs 23%, P=0.01, 2 test).
Electronic Cigarette Familiarity and Patient Requests
Of the participants, 95.8% reported familiarity with electronic cigarettes, without differences across age or gender. Of all of the providers, 19.0% reported being asked by a hospitalized patient for permission to use an electronic cigarette in the hospital. Registered nurses were significantly more likely to have been asked by patients compared to all other study participants (43% vs 11%, P<0.001, 2 test).
Electronic Cigarettes as NRT
Whereas 22.5% of study participants felt that electronic cigarettes could serve as a viable in‐hospital NRT, 48.6% felt that electronic cigarettes should not be used, and 28.9% were unsure (Table 1), irrespective of demographics or personal tobacco use history. One‐third of respondents would allow an inpatient under their care to use an electronic cigarette. Groups most likely to permit use were faculty (34.4%) and resident physicians (45.5%), though this difference was not statistically significant.
Perspectives on Exposure
Only 18.3% of study participants would agree to share a hospital room with a patient using an electronic cigarette. Of all participants, 47.2% and 49.3% felt that electronic cigarettes should be banned from healthcare settings and from the same locations as traditional cigarettes, respectively. There were no significant differences in perspectives when stratified by age or gender. Current or prior tobacco users were more likely to be accepting of the use of electronic cigarettes in healthcare settings compared to nonusers (50% vs 29%, P=0.02, Fisher exact test).
FDA Regulation
Of all study participants, 86.6% responded that electronic cigarettes should be regulated by the FDA. Physicians most strongly agreed with this statement compared with all other provider groups (97% vs 78%, P=0.004, 2 test). Conversely, registered nurses were least likely to feel that electronic cigarettes should be FDA‐regulated compared to all other provider groups (69% vs 93%, P<0.005, 2 test).
DISCUSSION
Our study is the first to provide hospital‐based providers' experience and perspectives surrounding electronic cigarette use. The vast majority of participants reported familiarity with electronic cigarettes, consistent with prior findings.[13] Though electronic cigarettes have yet to achieve a use in the hospital setting, 19% of our respondents reported receiving requests from hospitalized patients to use these devices. With increasing patient demand for electronic cigarettes, hospitals will need to update their tobacco policies to include these novel devices as well as target educational efforts toward front‐line providers, such as nurses, who receive the majority of requests.
Participants perceived traditional cigarettes to be significantly more harmful than electronic cigarettes, while established forms of NRT were felt to be less harmful than electronic cigarettes (data not shown). Concern about the health effects of electronic cigarettes is further reflected in providers' hesitancy to view these devices as an NRT option in the hospital, reluctance to consider sharing a room with an electronic cigarette user, and near majority opinion that electronic cigarettes should be banned from healthcare settings altogether. Current regulation by the US Department of Transportation bans electronic cigarette use on airplanes, whereas a host of states currently ban electronic cigarette use in similarly enclosed spaces such as correctional facilities and commuter trains.[14] More knowledge is needed on the health effects of electronic cigarettes on the primary user, secondhand exposure range, and their potential to aid in short‐ and long‐term nicotine cessation before providers and hospitals can make an informed risk‐benefit analysis for appropriate inpatient use. As current or past tobacco users were more accepting of the use of electronic cigarettes in hospital settings, these providers' opinions should be sought for a unique understanding of the interplay between electronic cigarettes and the healthcare environment.
Concern over the unknown safety effects can also be seen in the overwhelming provider support for FDA regulation. Healthcare advocacy groups, such as the American Heart Association, the American Lung Association, and the Legacy Foundation already support federal regulation.[15, 16, 17] FDA regulation may lead to the ability to standardize device content, regulate purchasing and marketing requirements, and ensure that claims to health effects are supported by scientific evidence, though agency involvement may also slow the process of integration into hospital use. Perhaps reflective of the immediacy of the problem, nurses who receive the majority of requests for electronic cigarettes from patients are least likely to want FDA regulation. Until more is known, patients and staff may benefit from pairing vaporizing patients in shared rooms or providing users with designated inhaling spaces.
Nicotine addiction is a strong driving force and, due to a strict no‐smoking policy at our institution, we have witnessed patients making unsafe decisions to leave the hospital (in some cases against medical advice) in an effort to continue smoking. Patients may be starting to look toward electronic cigarettes as an NRT option that more closely satisfies nicotine cravings as well as the ritualistic and tactile components of cigarette use. Electronic cigarettes could have the potential to act as a harm reduction method for nicotine‐dependent inpatients by decreasing the nicotine‐withdrawal related impetus for unsafe hospital discharges. Institutions should take this into account when formulating new policy.
Our study has several limitations. First, it was a single‐center study that may not be representative of provider perspectives at other institutions. Second, the survey was a cross‐sectional sample, missing providers who did not receive the e‐mail during the enrollment period. Third, responses may not accurately reflect perspectives of smaller responding groups such as social workers. Fourth, the survey did not include all types of physicians who deal with smoking cessation, though internal and family medicine physicians provide the majority of care for hospitalized patients at our institution. Fifth, we recorded self‐reported familiarity with electronic cigarettes and did not formally test providers' knowledge of the subject.
Our study provides new perspectives and data on electronic cigarettes to inform future research as well as hospital and healthcare policy. Hospitals should educate patients and front‐line providers around the paucity of health information on these novel devices, while formulating policy that acknowledges patient demand for electronic cigarettes and their potential for cessation therapy and harm reduction. Further research should focus on the effects of nicotine vapor inhalation on patients, the consequences of secondhand nicotine vapor, and the potential for electronic nicotine delivery systems to act as a novel NRT for hospital use.
Disclosure
Nothing to report.
Electronic cigarettes are increasingly prevalent battery‐operated devices that heat a solution to generate an inhalable nicotine‐containing aerosol.[1, 2] Despite a diverse array of devices on the market, the US Food and Drug Administration (FDA) has only recently proposed expanding its regulatory ability to include electronic cigarettes.[3] States, municipalities, and institutions have enacted variable regulations on electronic nicotine delivery systems.[4, 5] Advocates of electronic cigarettes propose that they are a less‐toxic alternative to tobacco cigarettes, with potential for use as a nicotine replacement therapy (NRT).[6, 7, 8] Opponents argue that electronic cigarettes may undermine tobacco cessation goals and potentially expose nonusers to secondhand nicotine vapor.[9, 10]
Hospital providers frequently care for nicotine‐dependent patients.[11] We investigated inpatient healthcare providers' knowledge, perceptions, and experience with electronic cigarettes, with the goals of informing educational efforts and guiding policy decisions around hospital‐based use of electronic nicotine delivery systems.
METHODS
The study was conducted at a 183‐bed urban safety‐net medical center affiliated with a residency training program using a cross‐sectional survey to query a diverse array of inpatient providers (Table 1). Respondents who had not cared for an inpatient in the past 5 years were excluded. Surveys were designed based on prior literature, personal experience, and expert suggestions.[12] Surveys were disseminated in March 2014 via e‐mail, with embedded informed consent and a link that connected anonymously to the online survey (Qualtrics, Provo, UT). We did not collect unique identifiers and offered no incentive for participation. Data were downloaded to a secure database and analyzed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) and GraphPad Prism version 6.04 (GraphPad Software, Inc., La Jolla, CA). The study was approved by the institutional review board.
| Group (No.) | Do you know what an electronic cigarette is?* | Has a hospitalized patient ever asked you if he or she could use an electronic cigarette on hospital grounds?* | Do you see electronic cigarettes as a nicotine replacement option for hospitalized patients? | If you were caring for a patient, would you be okay with the patient using an electronic cigarette while hospitalized? | If you were hospitalized in a shared hospital room, would you be okay with your roommate using an electronic cigarette? | Should electronic cigarettes be banned from healthcare settings? | Should electronic cigarettes be banned in the same locations as traditional cigarettes? | Should electronic cigarettes be regulated by the US Food and Drug Administration? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Faculty MD (32) | 96.9% | 12.5% | 28.1% | 34.4% | 12.5% | 37.5% | 53.1% | 100% |
| Resident MD (33) | 97.0% | 9.1% | 27.3% | 45.5% | 24.2% | 45.5% | 36.4% | 93.9% |
| Registered nurse (35) | 94.3% | 42.9% | 25.7% | 28.6% | 25.7% | 40.0% | 54.3% | 68.6% |
| Rehabilitation staff (18) | 88.9% | 11.1% | 11.1% | 5.6% | 5.6% | 66.7% | 55.6% | 88.9% |
| Social worker (6) | 100% | 33.3% | 16.7% | 16.7% | 0.0% | 50.0% | 50.0% | 83.3% |
| Pharmacist (18) | 100% | 5.6% | 11.1% | 27.8% | 22.2% | 61.1% | 50.0% | 83.3% |
| All respondents (142) | 95.8% | 19.0% | 22.5% | 30.3% | 18.3% | 47.2% | 49.3% | 86.6% |
RESULTS
Study Participants
There were 242 survey respondents (response rate of 41%), of whom 100 were excluded based on study criteria. The median age of the 142 included participants was 34.0 years. There were significantly more female respondents (69%, P=0.001, 2 test), equally over‐represented across all inpatient provider groups. Only 1.4% of all respondents reported personal active tobacco use, whereas 24.6% of study participants reported prior tobacco use. Tobacco use history was similar across inpatient provider groups and gender. Respondents over 50 years of age demonstrated a higher rate of current or prior tobacco use compared with participants from other age groups combined (53% vs 23%, P=0.01, 2 test).
Electronic Cigarette Familiarity and Patient Requests
Of the participants, 95.8% reported familiarity with electronic cigarettes, without differences across age or gender. Of all of the providers, 19.0% reported being asked by a hospitalized patient for permission to use an electronic cigarette in the hospital. Registered nurses were significantly more likely to have been asked by patients compared to all other study participants (43% vs 11%, P<0.001, 2 test).
Electronic Cigarettes as NRT
Whereas 22.5% of study participants felt that electronic cigarettes could serve as a viable in‐hospital NRT, 48.6% felt that electronic cigarettes should not be used, and 28.9% were unsure (Table 1), irrespective of demographics or personal tobacco use history. One‐third of respondents would allow an inpatient under their care to use an electronic cigarette. Groups most likely to permit use were faculty (34.4%) and resident physicians (45.5%), though this difference was not statistically significant.
Perspectives on Exposure
Only 18.3% of study participants would agree to share a hospital room with a patient using an electronic cigarette. Of all participants, 47.2% and 49.3% felt that electronic cigarettes should be banned from healthcare settings and from the same locations as traditional cigarettes, respectively. There were no significant differences in perspectives when stratified by age or gender. Current or prior tobacco users were more likely to be accepting of the use of electronic cigarettes in healthcare settings compared to nonusers (50% vs 29%, P=0.02, Fisher exact test).
FDA Regulation
Of all study participants, 86.6% responded that electronic cigarettes should be regulated by the FDA. Physicians most strongly agreed with this statement compared with all other provider groups (97% vs 78%, P=0.004, 2 test). Conversely, registered nurses were least likely to feel that electronic cigarettes should be FDA‐regulated compared to all other provider groups (69% vs 93%, P<0.005, 2 test).
DISCUSSION
Our study is the first to provide hospital‐based providers' experience and perspectives surrounding electronic cigarette use. The vast majority of participants reported familiarity with electronic cigarettes, consistent with prior findings.[13] Though electronic cigarettes have yet to achieve a use in the hospital setting, 19% of our respondents reported receiving requests from hospitalized patients to use these devices. With increasing patient demand for electronic cigarettes, hospitals will need to update their tobacco policies to include these novel devices as well as target educational efforts toward front‐line providers, such as nurses, who receive the majority of requests.
Participants perceived traditional cigarettes to be significantly more harmful than electronic cigarettes, while established forms of NRT were felt to be less harmful than electronic cigarettes (data not shown). Concern about the health effects of electronic cigarettes is further reflected in providers' hesitancy to view these devices as an NRT option in the hospital, reluctance to consider sharing a room with an electronic cigarette user, and near majority opinion that electronic cigarettes should be banned from healthcare settings altogether. Current regulation by the US Department of Transportation bans electronic cigarette use on airplanes, whereas a host of states currently ban electronic cigarette use in similarly enclosed spaces such as correctional facilities and commuter trains.[14] More knowledge is needed on the health effects of electronic cigarettes on the primary user, secondhand exposure range, and their potential to aid in short‐ and long‐term nicotine cessation before providers and hospitals can make an informed risk‐benefit analysis for appropriate inpatient use. As current or past tobacco users were more accepting of the use of electronic cigarettes in hospital settings, these providers' opinions should be sought for a unique understanding of the interplay between electronic cigarettes and the healthcare environment.
Concern over the unknown safety effects can also be seen in the overwhelming provider support for FDA regulation. Healthcare advocacy groups, such as the American Heart Association, the American Lung Association, and the Legacy Foundation already support federal regulation.[15, 16, 17] FDA regulation may lead to the ability to standardize device content, regulate purchasing and marketing requirements, and ensure that claims to health effects are supported by scientific evidence, though agency involvement may also slow the process of integration into hospital use. Perhaps reflective of the immediacy of the problem, nurses who receive the majority of requests for electronic cigarettes from patients are least likely to want FDA regulation. Until more is known, patients and staff may benefit from pairing vaporizing patients in shared rooms or providing users with designated inhaling spaces.
Nicotine addiction is a strong driving force and, due to a strict no‐smoking policy at our institution, we have witnessed patients making unsafe decisions to leave the hospital (in some cases against medical advice) in an effort to continue smoking. Patients may be starting to look toward electronic cigarettes as an NRT option that more closely satisfies nicotine cravings as well as the ritualistic and tactile components of cigarette use. Electronic cigarettes could have the potential to act as a harm reduction method for nicotine‐dependent inpatients by decreasing the nicotine‐withdrawal related impetus for unsafe hospital discharges. Institutions should take this into account when formulating new policy.
Our study has several limitations. First, it was a single‐center study that may not be representative of provider perspectives at other institutions. Second, the survey was a cross‐sectional sample, missing providers who did not receive the e‐mail during the enrollment period. Third, responses may not accurately reflect perspectives of smaller responding groups such as social workers. Fourth, the survey did not include all types of physicians who deal with smoking cessation, though internal and family medicine physicians provide the majority of care for hospitalized patients at our institution. Fifth, we recorded self‐reported familiarity with electronic cigarettes and did not formally test providers' knowledge of the subject.
Our study provides new perspectives and data on electronic cigarettes to inform future research as well as hospital and healthcare policy. Hospitals should educate patients and front‐line providers around the paucity of health information on these novel devices, while formulating policy that acknowledges patient demand for electronic cigarettes and their potential for cessation therapy and harm reduction. Further research should focus on the effects of nicotine vapor inhalation on patients, the consequences of secondhand nicotine vapor, and the potential for electronic nicotine delivery systems to act as a novel NRT for hospital use.
Disclosure
Nothing to report.
- , , . E‐cigarettes: a scientific review. Circulation. 2014;129:1972–1986.
- , , , , . E‐Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766.
- U.S. Food and Drug Administration. FDA proposes to extend its tobacco authority to additional tobacco products, including e‐cigarettes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394667.htm. Accessed May 5, 2014.
- . Electronic cigarettes: smoke‐free laws, sale restrictions, and the public health. Am J Public Health. 2014;104(6):e17–e18.
- American Nonsmokers' Rights Foundation. US state and local laws regulating use of electronic cigarettes. Available at: http://www.no‐smoke.org/pdf/ecigslaws.pdf. Accessed September 2, 2014.
- , . Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028.
- , , , , , . Effect of an electronic nicotine delivery device (e‐cigarette) on smoking reduction and cessation: a prospective 6‐month pilot study. BMC Public Health. 2011;11:786.
- , , , , . Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction. 2014;109(9):1531–1540.
- , . The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–686.
- . Promise and peril of e‐Cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–136.
- , , , et al. Electronic cigarette awareness, use history, and expected future use among hospitalized cigarette smokers. Nicotine Tob Res. 2014;16(11):1512–1517.
- Global Adult Tobacco Survey Collaborative Group. Tobacco Questions for Surveys: A Subset of Key Questions From the Global Adult Tobacco Survey (GATS). 2nd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available at: http://www.who.int/tobacco/surveillance/en_tfi_tqs.pdf. Accessed April 23, 2014.
- , , . Healthcare providers' beliefs and attitudes about electronic cigarettes and preventive counseling for adolescent patients. J Adolesc Health. 2014;54(6):678–683.
- U.S. Department of Transportation. DOT policy on e‐cigarettes. Available at: http://www.dot.gov/sites/dot.gov/files/docs/PolicyOnECigarettes.pdf. Accessed September 2, 2014.
- American Heart Association. AHA: E‐cigarettes threaten to addict next generation of smokers; regulation, further study needed. Available at: http://blog.heart.org/aha‐e‐cigarettes‐threaten‐to‐addict‐next‐generation‐of‐smokers‐regulation‐further‐study‐needed/. Accessed August 25, 2014.
- American Lung Association. American Lung Association statement on e‐cigarettes. Available at: http://www.lung.org/stop‐smoking/tobacco‐control‐advocacy/federal/e‐cigarettes.html. Accesses August 25, 2014.
- Legacy for Health. E‐cigarette policy: the FDA should promptly exercise regulatory authority and over e‐cigarettes. Available at: http://www.legacyforhealth.org/content/download/3962/56088/version/1/file/LEG‐Policy_Statement‐ECigarette‐JAN2014.pdf. Accessed August 25, 2014.
- , , . E‐cigarettes: a scientific review. Circulation. 2014;129:1972–1986.
- , , , , . E‐Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766.
- U.S. Food and Drug Administration. FDA proposes to extend its tobacco authority to additional tobacco products, including e‐cigarettes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394667.htm. Accessed May 5, 2014.
- . Electronic cigarettes: smoke‐free laws, sale restrictions, and the public health. Am J Public Health. 2014;104(6):e17–e18.
- American Nonsmokers' Rights Foundation. US state and local laws regulating use of electronic cigarettes. Available at: http://www.no‐smoke.org/pdf/ecigslaws.pdf. Accessed September 2, 2014.
- , . Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028.
- , , , , , . Effect of an electronic nicotine delivery device (e‐cigarette) on smoking reduction and cessation: a prospective 6‐month pilot study. BMC Public Health. 2011;11:786.
- , , , , . Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction. 2014;109(9):1531–1540.
- , . The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–686.
- . Promise and peril of e‐Cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–136.
- , , , et al. Electronic cigarette awareness, use history, and expected future use among hospitalized cigarette smokers. Nicotine Tob Res. 2014;16(11):1512–1517.
- Global Adult Tobacco Survey Collaborative Group. Tobacco Questions for Surveys: A Subset of Key Questions From the Global Adult Tobacco Survey (GATS). 2nd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available at: http://www.who.int/tobacco/surveillance/en_tfi_tqs.pdf. Accessed April 23, 2014.
- , , . Healthcare providers' beliefs and attitudes about electronic cigarettes and preventive counseling for adolescent patients. J Adolesc Health. 2014;54(6):678–683.
- U.S. Department of Transportation. DOT policy on e‐cigarettes. Available at: http://www.dot.gov/sites/dot.gov/files/docs/PolicyOnECigarettes.pdf. Accessed September 2, 2014.
- American Heart Association. AHA: E‐cigarettes threaten to addict next generation of smokers; regulation, further study needed. Available at: http://blog.heart.org/aha‐e‐cigarettes‐threaten‐to‐addict‐next‐generation‐of‐smokers‐regulation‐further‐study‐needed/. Accessed August 25, 2014.
- American Lung Association. American Lung Association statement on e‐cigarettes. Available at: http://www.lung.org/stop‐smoking/tobacco‐control‐advocacy/federal/e‐cigarettes.html. Accesses August 25, 2014.
- Legacy for Health. E‐cigarette policy: the FDA should promptly exercise regulatory authority and over e‐cigarettes. Available at: http://www.legacyforhealth.org/content/download/3962/56088/version/1/file/LEG‐Policy_Statement‐ECigarette‐JAN2014.pdf. Accessed August 25, 2014.
Paracentesis in Cirrhosis Patients/
Ascites is the most common complication of cirrhosis leading to hospital admission.[1] Approximately 12% of hospitalized patients who present with decompensated cirrhosis and ascites have spontaneous bacterial peritonitis (SBP); half of these patients do not present with abdominal pain, fever, nausea, or vomiting.[2] Guidelines published by the American Association for the Study of Liver Diseases (AASLD) recommend paracentesis for all hospitalized patients with cirrhosis and ascites and also recommend long‐term antibiotic prophylaxis for survivors of an SBP episode.[3] Despite evidence that in‐hospital mortality is reduced in those patients who receive paracentesis in a timely manner,[4, 5] only 40% to 60% of eligible patients receive paracentesis.[4, 6, 7] We aimed to describe clinical predictors of paracentesis and use of antibiotics following an episode of SBP in patients with decompensated cirrhosis and ascites.
METHODS
We conducted a retrospective cohort study of adults admitted to a single tertiary care center between January 1, 2009 and December 31, 2009.7 We included patients with an International Classification of Diseases, Ninth Revision discharge code consistent with decompensated cirrhosis who met clinical criteria for decompensated cirrhosis (see
RESULTS
We identified 193 admissions for 103 patients with decompensated cirrhosis and ascites (Table 1). Of these, 41% (80/193) received diagnostic paracentesis. Mean/standard deviation for age was 53.6/12.4 years; 71% of patients were male and 63% were English speaking. Common comorbidities included diabetes mellitus (33%), psychiatric diagnosis (29%), substance abuse (18%), and renal failure (17%). Excluding SBP, 31% of patients had another documented infection. Gastroenterology was consulted in 50% of the admissions. Fever was present in 27% of patients, elevated white blood cell (WBC) count (ie, WBC >11 k/mm3) was present in 27% of patients, International Normalized Ratio (INR) was elevated (>1.1) in 92% of patients, and 16% of patients had a platelet count of <50,000/mm3. Patients who received paracentesis were less likely to have a fever on presentation (19% vs 32%, P=0.06), low (ie, <50,000/mm3) platelet count (11% vs 19%, P=0.14), or concurrent gastrointestinal (GI) bleed (6% vs 16%, P=0.05). In a multiple logistic regression model including characteristics associated at P0.2 with paracentesis, fever, low platelet count, and concurrent GI bleeding were associated with decreased odds of receiving paracentesis (Appendix 1).
| Overall, N=193, Mean/SD or N (%)* | Paracentesis (), n=113, Mean/SD or N (%) | Paracentesis (+), n=80, Mean/SD or N (%) | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| ||||
| Age, y | 53.6/12.4 | 54.1/13.4 | 53.2/11.7 | 1.00 (0.981.03) |
| Sex (male) | 137 (71.0%) | 78 (69.0%) | 59 (73.8%) | 1.26 (0.672.39) |
| English speaking | 122 (63.2%) | 69 (61.1%) | 53 (66.3%) | 1.25 (0.692.28) |
| Etiology | ||||
| Alcohol | 120 (62.2%) | 74 (65.5%) | 46 (57.5%) | 0.71 (0.401.29) |
| Hepatitis C | 94 (48.7%) | 57 (50.4%) | 37 (46.3%) | 0.85 (0.481.50) |
| Hepatitis B | 16 (8.3%) | 7 (6.2%) | 9 (11.3%) | 1.92 (0.685.39) |
| NASH | 8 (4.2%) | 4 (3.5%) | 4 (5.0%) | 1.43 (0.355.91) |
| Cryptogenic | 11 (5.7%) | 6 (5.3%) | 5 (6.3%) | 1.19 (0.354.04) |
| Comorbidities | ||||
| Substance abuse | 34 (17.6%) | 22 (19.5%) | 12 (15.0%) | 0.73 (0.341.58) |
| Psychiatric diagnosis | 55 (28.5%) | 38 (33.6%) | 17 (21.3%) | 0.53 (0.271.03) |
| Diabetes mellitus | 63 (32.6%) | 37 (32.7%) | 26 (32.5%) | 0.99 (0.541.82) |
| Renal failure | 33 (17.1%) | 20 (17.7%) | 13 (16.3%) | 0.90 (0.421.94) |
| GI bleed | 23 (11.9%) | 18 (15.9%) | 5 (6.3%) | 0.35 (0.120.99) |
| Admission MELD | 17.3/7.3 | 17.5/7.3 | 17.0/7.3 | 0.99 (0.951.03) |
| Creatinine, median/IQR | 0.9/0.7 | 0.9/0.7 | 0.9/0.8 | 1.02 (0.821.27) |
| Gastroenterology consult | 97 (50.3%) | 46 (40.7%) | 51 (63.8%) | 2.56 (1.424.63) |
| Infection, UTI, pneumonia, other | 60 (31.1%) | 38 (33.6%) | 22 (27.5%) | 0.75 (0.401.40) |
| Temperature 100.4F | 49 (26.8%) | 34 (32.4%) | 15 (19.2%) | 0.50 (0.251.00) |
| WBC >11 k/mm3 | 50 (27.3%) | 28 (26.7%) | 22 (28.2%) | 1.08 (0.562.08) |
| WBC <4 k/mm3 | 43 (23.5%) | 23 (21.9%) | 20 (25.6%) | 1.23 (0.622.44) |
| INR >1.1 | 149 (92.0%) | 83 (93.3%) | 66 (90.4%) | 0.68 (0.222.13) |
| Highest temperature, F | 98.9/1.1 | 99.1/1.3 | 98.8/0.8 | 0.82 (0.621.09) |
| Highest HR | 98.2/20.4 | 97.4/22.4 | 99.2/17.4 | 1.00 (0.991.02) |
| Highest RR | 24.5/13.7 | 25.2/16.8 | 23.5/7.8 | 0.99 (0.961.02) |
| Lowest SBP | 101.0/20.0 | 99.4/20.3 | 102.2/19.7 | 0.99 (0.981.01) |
| Lowest MAP | 73.0/12.2 | 73.2/13.3 | 72.7/10.6 | 1.00 (0.971.02) |
| Lowest O2Sat | 92.6/13.6 | 91.0/17.7 | 94.9/2.8 | 1.04 (0.991.10) |
| Highest PT | 15.8/3.8 | 15.9/3.7 | 15.7/3.9 | 0.98 (0.901.08) |
| Platelets 50 k/mm3 | 30 (15.9%) | 21 (19.3%) | 9 (11.3%) | 0.53 (0.231.23) |
Of the patients who received paracentesis (n=80), 14% were diagnosed with SBP. Of these, 55% received prophylaxis on discharge. Among the patients who did not receive paracentesis (n=113), 38 (34%) received antibiotics for another documented infection (eg, pneumonia), and 25 patients (22%) received antibiotics with no other documented infection or evidence of variceal bleeding. Of these 25 patients who were presumed to be empirically treated for SBP (Figure 1), only 20% were prescribed prophylactic antibiotics on discharge.
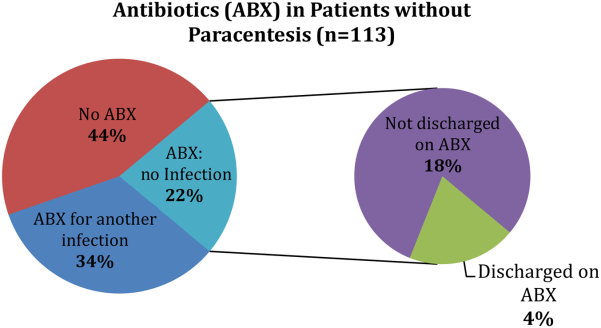
CONCLUSION
We found that many patients with decompensated cirrhosis and ascites did not receive paracentesis when hospitalized, which is similar to previously published data.[4, 6, 7] Clinical evidence of infection, such as fever or elevated WBC count, did not increase the odds of receiving paracentesis. Many patients treated for SBP were not discharged on prophylaxis.
This study is limited by its small single‐center design. We could only use data from 1 year (2009), because study data collection was part of a quality‐improvement project that took place for that year only. We did not adjust for the number of red blood cells in the ascitic fluid samples. We were also unable to determine the timing of gastroenterology consultation (whether it was done prior to paracentesis), admission venue (floor vs intensive care), or patient history of SBP.
Despite these limitations, there are important implications. First, the decision to perform paracentesis was not associated with symptoms of infection, although some clinical factors (eg, low platelets or GI bleeding) were associated with reduced odds of receiving paracentesis. Second, a majority of patients treated for SBP did not receive prophylactic antibiotics at discharge. These findings suggest a clear opportunity to increase awareness and acceptance of AASLD guidelines among hospital medicine practitioners. Quality‐improvement efforts should focus on the education of providers, and future research should identify barriers to paracentesis at both the practitioner and system levels (eg, availability of interventional radiology). Checklists or decision support within electronic order entry systems may also help reduce the low rates of paracentesis seen in our and prior studies.[4, 6, 7]
Disclosures: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu, Ghaoui, and Brooling had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Ghaoui, and Brooling conceived of the study. Dr. Ghaoui acquired the data. Ms. Friderici carried out the statistical analyses. Drs. Lagu, Ghaoui, Brooling, Lindenauer, and Ms. Friderici analyzed and interpreted the data, drafted the manuscript, and critically reviewed the manuscript for important intellectual content. The authors report no conflicts of interest.
- , , , , ; Spanish Collaborative Study Group On Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58(6):435–440.
- , , , et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33(1):41–48.
- , AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–1653.
- , , , . Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496–503.e1.
- , , , et al. Delayed paracentesis is associated with increased in‐hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436–1442.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143(1):70–77.
- , , , , , . Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014;34(2):204–210.
Ascites is the most common complication of cirrhosis leading to hospital admission.[1] Approximately 12% of hospitalized patients who present with decompensated cirrhosis and ascites have spontaneous bacterial peritonitis (SBP); half of these patients do not present with abdominal pain, fever, nausea, or vomiting.[2] Guidelines published by the American Association for the Study of Liver Diseases (AASLD) recommend paracentesis for all hospitalized patients with cirrhosis and ascites and also recommend long‐term antibiotic prophylaxis for survivors of an SBP episode.[3] Despite evidence that in‐hospital mortality is reduced in those patients who receive paracentesis in a timely manner,[4, 5] only 40% to 60% of eligible patients receive paracentesis.[4, 6, 7] We aimed to describe clinical predictors of paracentesis and use of antibiotics following an episode of SBP in patients with decompensated cirrhosis and ascites.
METHODS
We conducted a retrospective cohort study of adults admitted to a single tertiary care center between January 1, 2009 and December 31, 2009.7 We included patients with an International Classification of Diseases, Ninth Revision discharge code consistent with decompensated cirrhosis who met clinical criteria for decompensated cirrhosis (see
RESULTS
We identified 193 admissions for 103 patients with decompensated cirrhosis and ascites (Table 1). Of these, 41% (80/193) received diagnostic paracentesis. Mean/standard deviation for age was 53.6/12.4 years; 71% of patients were male and 63% were English speaking. Common comorbidities included diabetes mellitus (33%), psychiatric diagnosis (29%), substance abuse (18%), and renal failure (17%). Excluding SBP, 31% of patients had another documented infection. Gastroenterology was consulted in 50% of the admissions. Fever was present in 27% of patients, elevated white blood cell (WBC) count (ie, WBC >11 k/mm3) was present in 27% of patients, International Normalized Ratio (INR) was elevated (>1.1) in 92% of patients, and 16% of patients had a platelet count of <50,000/mm3. Patients who received paracentesis were less likely to have a fever on presentation (19% vs 32%, P=0.06), low (ie, <50,000/mm3) platelet count (11% vs 19%, P=0.14), or concurrent gastrointestinal (GI) bleed (6% vs 16%, P=0.05). In a multiple logistic regression model including characteristics associated at P0.2 with paracentesis, fever, low platelet count, and concurrent GI bleeding were associated with decreased odds of receiving paracentesis (Appendix 1).
| Overall, N=193, Mean/SD or N (%)* | Paracentesis (), n=113, Mean/SD or N (%) | Paracentesis (+), n=80, Mean/SD or N (%) | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| ||||
| Age, y | 53.6/12.4 | 54.1/13.4 | 53.2/11.7 | 1.00 (0.981.03) |
| Sex (male) | 137 (71.0%) | 78 (69.0%) | 59 (73.8%) | 1.26 (0.672.39) |
| English speaking | 122 (63.2%) | 69 (61.1%) | 53 (66.3%) | 1.25 (0.692.28) |
| Etiology | ||||
| Alcohol | 120 (62.2%) | 74 (65.5%) | 46 (57.5%) | 0.71 (0.401.29) |
| Hepatitis C | 94 (48.7%) | 57 (50.4%) | 37 (46.3%) | 0.85 (0.481.50) |
| Hepatitis B | 16 (8.3%) | 7 (6.2%) | 9 (11.3%) | 1.92 (0.685.39) |
| NASH | 8 (4.2%) | 4 (3.5%) | 4 (5.0%) | 1.43 (0.355.91) |
| Cryptogenic | 11 (5.7%) | 6 (5.3%) | 5 (6.3%) | 1.19 (0.354.04) |
| Comorbidities | ||||
| Substance abuse | 34 (17.6%) | 22 (19.5%) | 12 (15.0%) | 0.73 (0.341.58) |
| Psychiatric diagnosis | 55 (28.5%) | 38 (33.6%) | 17 (21.3%) | 0.53 (0.271.03) |
| Diabetes mellitus | 63 (32.6%) | 37 (32.7%) | 26 (32.5%) | 0.99 (0.541.82) |
| Renal failure | 33 (17.1%) | 20 (17.7%) | 13 (16.3%) | 0.90 (0.421.94) |
| GI bleed | 23 (11.9%) | 18 (15.9%) | 5 (6.3%) | 0.35 (0.120.99) |
| Admission MELD | 17.3/7.3 | 17.5/7.3 | 17.0/7.3 | 0.99 (0.951.03) |
| Creatinine, median/IQR | 0.9/0.7 | 0.9/0.7 | 0.9/0.8 | 1.02 (0.821.27) |
| Gastroenterology consult | 97 (50.3%) | 46 (40.7%) | 51 (63.8%) | 2.56 (1.424.63) |
| Infection, UTI, pneumonia, other | 60 (31.1%) | 38 (33.6%) | 22 (27.5%) | 0.75 (0.401.40) |
| Temperature 100.4F | 49 (26.8%) | 34 (32.4%) | 15 (19.2%) | 0.50 (0.251.00) |
| WBC >11 k/mm3 | 50 (27.3%) | 28 (26.7%) | 22 (28.2%) | 1.08 (0.562.08) |
| WBC <4 k/mm3 | 43 (23.5%) | 23 (21.9%) | 20 (25.6%) | 1.23 (0.622.44) |
| INR >1.1 | 149 (92.0%) | 83 (93.3%) | 66 (90.4%) | 0.68 (0.222.13) |
| Highest temperature, F | 98.9/1.1 | 99.1/1.3 | 98.8/0.8 | 0.82 (0.621.09) |
| Highest HR | 98.2/20.4 | 97.4/22.4 | 99.2/17.4 | 1.00 (0.991.02) |
| Highest RR | 24.5/13.7 | 25.2/16.8 | 23.5/7.8 | 0.99 (0.961.02) |
| Lowest SBP | 101.0/20.0 | 99.4/20.3 | 102.2/19.7 | 0.99 (0.981.01) |
| Lowest MAP | 73.0/12.2 | 73.2/13.3 | 72.7/10.6 | 1.00 (0.971.02) |
| Lowest O2Sat | 92.6/13.6 | 91.0/17.7 | 94.9/2.8 | 1.04 (0.991.10) |
| Highest PT | 15.8/3.8 | 15.9/3.7 | 15.7/3.9 | 0.98 (0.901.08) |
| Platelets 50 k/mm3 | 30 (15.9%) | 21 (19.3%) | 9 (11.3%) | 0.53 (0.231.23) |
Of the patients who received paracentesis (n=80), 14% were diagnosed with SBP. Of these, 55% received prophylaxis on discharge. Among the patients who did not receive paracentesis (n=113), 38 (34%) received antibiotics for another documented infection (eg, pneumonia), and 25 patients (22%) received antibiotics with no other documented infection or evidence of variceal bleeding. Of these 25 patients who were presumed to be empirically treated for SBP (Figure 1), only 20% were prescribed prophylactic antibiotics on discharge.

CONCLUSION
We found that many patients with decompensated cirrhosis and ascites did not receive paracentesis when hospitalized, which is similar to previously published data.[4, 6, 7] Clinical evidence of infection, such as fever or elevated WBC count, did not increase the odds of receiving paracentesis. Many patients treated for SBP were not discharged on prophylaxis.
This study is limited by its small single‐center design. We could only use data from 1 year (2009), because study data collection was part of a quality‐improvement project that took place for that year only. We did not adjust for the number of red blood cells in the ascitic fluid samples. We were also unable to determine the timing of gastroenterology consultation (whether it was done prior to paracentesis), admission venue (floor vs intensive care), or patient history of SBP.
Despite these limitations, there are important implications. First, the decision to perform paracentesis was not associated with symptoms of infection, although some clinical factors (eg, low platelets or GI bleeding) were associated with reduced odds of receiving paracentesis. Second, a majority of patients treated for SBP did not receive prophylactic antibiotics at discharge. These findings suggest a clear opportunity to increase awareness and acceptance of AASLD guidelines among hospital medicine practitioners. Quality‐improvement efforts should focus on the education of providers, and future research should identify barriers to paracentesis at both the practitioner and system levels (eg, availability of interventional radiology). Checklists or decision support within electronic order entry systems may also help reduce the low rates of paracentesis seen in our and prior studies.[4, 6, 7]
Disclosures: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu, Ghaoui, and Brooling had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Ghaoui, and Brooling conceived of the study. Dr. Ghaoui acquired the data. Ms. Friderici carried out the statistical analyses. Drs. Lagu, Ghaoui, Brooling, Lindenauer, and Ms. Friderici analyzed and interpreted the data, drafted the manuscript, and critically reviewed the manuscript for important intellectual content. The authors report no conflicts of interest.
Ascites is the most common complication of cirrhosis leading to hospital admission.[1] Approximately 12% of hospitalized patients who present with decompensated cirrhosis and ascites have spontaneous bacterial peritonitis (SBP); half of these patients do not present with abdominal pain, fever, nausea, or vomiting.[2] Guidelines published by the American Association for the Study of Liver Diseases (AASLD) recommend paracentesis for all hospitalized patients with cirrhosis and ascites and also recommend long‐term antibiotic prophylaxis for survivors of an SBP episode.[3] Despite evidence that in‐hospital mortality is reduced in those patients who receive paracentesis in a timely manner,[4, 5] only 40% to 60% of eligible patients receive paracentesis.[4, 6, 7] We aimed to describe clinical predictors of paracentesis and use of antibiotics following an episode of SBP in patients with decompensated cirrhosis and ascites.
METHODS
We conducted a retrospective cohort study of adults admitted to a single tertiary care center between January 1, 2009 and December 31, 2009.7 We included patients with an International Classification of Diseases, Ninth Revision discharge code consistent with decompensated cirrhosis who met clinical criteria for decompensated cirrhosis (see
RESULTS
We identified 193 admissions for 103 patients with decompensated cirrhosis and ascites (Table 1). Of these, 41% (80/193) received diagnostic paracentesis. Mean/standard deviation for age was 53.6/12.4 years; 71% of patients were male and 63% were English speaking. Common comorbidities included diabetes mellitus (33%), psychiatric diagnosis (29%), substance abuse (18%), and renal failure (17%). Excluding SBP, 31% of patients had another documented infection. Gastroenterology was consulted in 50% of the admissions. Fever was present in 27% of patients, elevated white blood cell (WBC) count (ie, WBC >11 k/mm3) was present in 27% of patients, International Normalized Ratio (INR) was elevated (>1.1) in 92% of patients, and 16% of patients had a platelet count of <50,000/mm3. Patients who received paracentesis were less likely to have a fever on presentation (19% vs 32%, P=0.06), low (ie, <50,000/mm3) platelet count (11% vs 19%, P=0.14), or concurrent gastrointestinal (GI) bleed (6% vs 16%, P=0.05). In a multiple logistic regression model including characteristics associated at P0.2 with paracentesis, fever, low platelet count, and concurrent GI bleeding were associated with decreased odds of receiving paracentesis (Appendix 1).
| Overall, N=193, Mean/SD or N (%)* | Paracentesis (), n=113, Mean/SD or N (%) | Paracentesis (+), n=80, Mean/SD or N (%) | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| ||||
| Age, y | 53.6/12.4 | 54.1/13.4 | 53.2/11.7 | 1.00 (0.981.03) |
| Sex (male) | 137 (71.0%) | 78 (69.0%) | 59 (73.8%) | 1.26 (0.672.39) |
| English speaking | 122 (63.2%) | 69 (61.1%) | 53 (66.3%) | 1.25 (0.692.28) |
| Etiology | ||||
| Alcohol | 120 (62.2%) | 74 (65.5%) | 46 (57.5%) | 0.71 (0.401.29) |
| Hepatitis C | 94 (48.7%) | 57 (50.4%) | 37 (46.3%) | 0.85 (0.481.50) |
| Hepatitis B | 16 (8.3%) | 7 (6.2%) | 9 (11.3%) | 1.92 (0.685.39) |
| NASH | 8 (4.2%) | 4 (3.5%) | 4 (5.0%) | 1.43 (0.355.91) |
| Cryptogenic | 11 (5.7%) | 6 (5.3%) | 5 (6.3%) | 1.19 (0.354.04) |
| Comorbidities | ||||
| Substance abuse | 34 (17.6%) | 22 (19.5%) | 12 (15.0%) | 0.73 (0.341.58) |
| Psychiatric diagnosis | 55 (28.5%) | 38 (33.6%) | 17 (21.3%) | 0.53 (0.271.03) |
| Diabetes mellitus | 63 (32.6%) | 37 (32.7%) | 26 (32.5%) | 0.99 (0.541.82) |
| Renal failure | 33 (17.1%) | 20 (17.7%) | 13 (16.3%) | 0.90 (0.421.94) |
| GI bleed | 23 (11.9%) | 18 (15.9%) | 5 (6.3%) | 0.35 (0.120.99) |
| Admission MELD | 17.3/7.3 | 17.5/7.3 | 17.0/7.3 | 0.99 (0.951.03) |
| Creatinine, median/IQR | 0.9/0.7 | 0.9/0.7 | 0.9/0.8 | 1.02 (0.821.27) |
| Gastroenterology consult | 97 (50.3%) | 46 (40.7%) | 51 (63.8%) | 2.56 (1.424.63) |
| Infection, UTI, pneumonia, other | 60 (31.1%) | 38 (33.6%) | 22 (27.5%) | 0.75 (0.401.40) |
| Temperature 100.4F | 49 (26.8%) | 34 (32.4%) | 15 (19.2%) | 0.50 (0.251.00) |
| WBC >11 k/mm3 | 50 (27.3%) | 28 (26.7%) | 22 (28.2%) | 1.08 (0.562.08) |
| WBC <4 k/mm3 | 43 (23.5%) | 23 (21.9%) | 20 (25.6%) | 1.23 (0.622.44) |
| INR >1.1 | 149 (92.0%) | 83 (93.3%) | 66 (90.4%) | 0.68 (0.222.13) |
| Highest temperature, F | 98.9/1.1 | 99.1/1.3 | 98.8/0.8 | 0.82 (0.621.09) |
| Highest HR | 98.2/20.4 | 97.4/22.4 | 99.2/17.4 | 1.00 (0.991.02) |
| Highest RR | 24.5/13.7 | 25.2/16.8 | 23.5/7.8 | 0.99 (0.961.02) |
| Lowest SBP | 101.0/20.0 | 99.4/20.3 | 102.2/19.7 | 0.99 (0.981.01) |
| Lowest MAP | 73.0/12.2 | 73.2/13.3 | 72.7/10.6 | 1.00 (0.971.02) |
| Lowest O2Sat | 92.6/13.6 | 91.0/17.7 | 94.9/2.8 | 1.04 (0.991.10) |
| Highest PT | 15.8/3.8 | 15.9/3.7 | 15.7/3.9 | 0.98 (0.901.08) |
| Platelets 50 k/mm3 | 30 (15.9%) | 21 (19.3%) | 9 (11.3%) | 0.53 (0.231.23) |
Of the patients who received paracentesis (n=80), 14% were diagnosed with SBP. Of these, 55% received prophylaxis on discharge. Among the patients who did not receive paracentesis (n=113), 38 (34%) received antibiotics for another documented infection (eg, pneumonia), and 25 patients (22%) received antibiotics with no other documented infection or evidence of variceal bleeding. Of these 25 patients who were presumed to be empirically treated for SBP (Figure 1), only 20% were prescribed prophylactic antibiotics on discharge.

CONCLUSION
We found that many patients with decompensated cirrhosis and ascites did not receive paracentesis when hospitalized, which is similar to previously published data.[4, 6, 7] Clinical evidence of infection, such as fever or elevated WBC count, did not increase the odds of receiving paracentesis. Many patients treated for SBP were not discharged on prophylaxis.
This study is limited by its small single‐center design. We could only use data from 1 year (2009), because study data collection was part of a quality‐improvement project that took place for that year only. We did not adjust for the number of red blood cells in the ascitic fluid samples. We were also unable to determine the timing of gastroenterology consultation (whether it was done prior to paracentesis), admission venue (floor vs intensive care), or patient history of SBP.
Despite these limitations, there are important implications. First, the decision to perform paracentesis was not associated with symptoms of infection, although some clinical factors (eg, low platelets or GI bleeding) were associated with reduced odds of receiving paracentesis. Second, a majority of patients treated for SBP did not receive prophylactic antibiotics at discharge. These findings suggest a clear opportunity to increase awareness and acceptance of AASLD guidelines among hospital medicine practitioners. Quality‐improvement efforts should focus on the education of providers, and future research should identify barriers to paracentesis at both the practitioner and system levels (eg, availability of interventional radiology). Checklists or decision support within electronic order entry systems may also help reduce the low rates of paracentesis seen in our and prior studies.[4, 6, 7]
Disclosures: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu, Ghaoui, and Brooling had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Ghaoui, and Brooling conceived of the study. Dr. Ghaoui acquired the data. Ms. Friderici carried out the statistical analyses. Drs. Lagu, Ghaoui, Brooling, Lindenauer, and Ms. Friderici analyzed and interpreted the data, drafted the manuscript, and critically reviewed the manuscript for important intellectual content. The authors report no conflicts of interest.
- , , , , ; Spanish Collaborative Study Group On Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58(6):435–440.
- , , , et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33(1):41–48.
- , AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–1653.
- , , , . Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496–503.e1.
- , , , et al. Delayed paracentesis is associated with increased in‐hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436–1442.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143(1):70–77.
- , , , , , . Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014;34(2):204–210.
- , , , , ; Spanish Collaborative Study Group On Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58(6):435–440.
- , , , et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33(1):41–48.
- , AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–1653.
- , , , . Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496–503.e1.
- , , , et al. Delayed paracentesis is associated with increased in‐hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436–1442.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143(1):70–77.
- , , , , , . Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014;34(2):204–210.
Antipsychotics in Hospitalized Elders
Antipsychotic (AP) medications are often used in the hospitalized geriatric population for the treatment of delirium.[1] Because of adverse events associated with APs, efforts have been made to reduce their use in hospitalized elders,[2] but it is not clear if these recommendations have been widely adopted. We studied the use of APs in a cohort of hospitalized elders to better understand why APs are started and how often they are continued on discharge.
METHODS
We conducted a retrospective cohort study of patients aged 65 years or older admitted to a tertiary care hospital between October 1, 2012 and September 31, 2013. Using Stata's (StataCorp., College Station, TX) sample command,[3] we included a subset of randomly selected inpatients who received more than 1 dose of oral APs (determined using the electronic medication administration summary). We excluded patients admitted under observation status or to the psychiatric service, those who were on APs prior to admission, and those who only received prochloperazine for nausea. Using prior literature to identify terms frequently used to describe delirium (Figure 1), we created an algorithm and a chart abstraction form (see Supporting Information, Appendix 1, in the online version of this article).[4] We tested these instruments in a preliminary chart review involving 30 patients. Disagreements were discussed with coauthors and resolved through consensus, resulting in some algorithm changes (eg, excluding a large number of patients who received only 1 dose of haloperidol postoperatively, because we hypothesized that this use could be a prophylactic measure).[5] Two investigators extracted the remaining charts independently. We used descriptive statistics and performed cross‐tabulations on the selected variables.
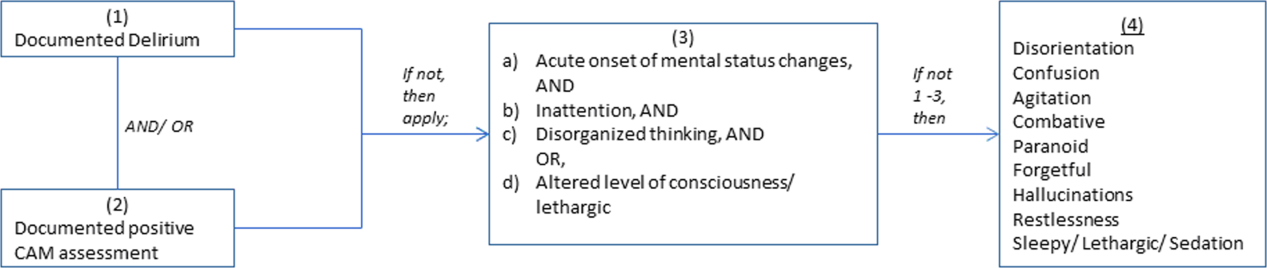
RESULTS
Of 12,817 geriatric hospitalizations during the study period, 1120 (9%) were treated with antipsychotics. We randomly selected 300 of these for extraction: 54% were male, and 67% were admitted to the medical service (Table 1). The inpatient mortality rate was 10% (30/300). The most frequent indication for AP use was delirium (83%, 249/300). Only 35% of delirious patients received a formal assessment with the Confusion Assessment Method (CAM). The most commonly used atypical antipsychotic was quetiapine (86%); 55% received more than 1 antipsychotic medication during hospitalization, and 48% (143/297) of patients were continued on APs at discharge (excluding 3 patients transferred to other acute care hospitals).
| Variable | N (%), Total=300 |
|---|---|
| |
| Gender | |
| Male | 161 (54) |
| Female | 139 (46) |
| Inpatient mortality rate | 30 (10) |
| Services | |
| Medicine | 202 (67) |
| Surgery | 98 (33) |
| Indication for APs use | |
| Delirium | 249 (83) |
| Hallucinations | 19 (6) |
| Anxiety | 20 (7) |
| Other | 38 (13) |
| Atypical APs | |
| Quetiapine | 257 (86) |
| Olanzapine | 29 (10) |
| Risperidone | 26 (9) |
| Typical APs | |
| Haloperidol | 166 (55) |
| Thorazine | 4 (1) |
| Use of CAM | 79 (32)a |
| Physical restraints | 89 (30) |
| Documented or suspected dementia | 134 (45) |
| Geriatrics consults | 120 (40) |
| Psychiatric consults | 29 (10) |
| ECG | |
| Prior to APs administration | 265 (88) |
| After APs administration | 157 (52) |
| QTc prolongation >500 ms | |
| Prior to APs administration | 41 (15)b |
| After APs administration | 39 (25)c |
| Admitted from SNF | 36 (12) |
| Discharge destination | |
| Home | 68 (23) |
| SNFs, short and long‐term rehabilitations | 199 (66) |
| Transfer to other acute care hospitals | 3 (1) |
| Continuation of APs at discharge | 143 (48)d |
Approximately 45% (134/300) had documented or suspected dementia, and 30% (89/300) were physically restrained during the hospital stay. Consultations with geriatrics were obtained in 40% (120/300) of the cases and with psychiatry in 10% (29/300) of the cases. Neurology is rarely consulted for delirium in our institution; thus, we did not collect data on those referrals. Electrocardiography (ECG) (recommended for patients at high cardiac risk[6]) was performed in 88% (265/300) of patients prior to AP administration and 52% (157/300) after. The corrected QT interval exceeded 500 ms in 15% (41/265) of patients prior to AP administration and 25% (39/157) after. Although few patients (12%) were admitted from nursing facilities, 66% (199/300) were eventually discharged to skilled nursing facilities (SNFs) or rehabilitation facilities; most of these patients (117/199, 59%) received AP treatment, compared to 38% of patients discharged to home (26/68).
DISCUSSION
In a cohort of hospitalized elders, we found that 9% were treated with APs. Most received APs for perceived delirium; in‐hospital ECG monitoring was suboptimal. Half of the patients started on APs remained on them at discharge; those discharged to SNFs were more likely to receive ongoing AP treatment.
Our study is limited by its retrospective, single‐center design, a lack of inter‐rater reliability measurement (although our training process was designed to standardize extraction methods), and the infrequent use of formal CAM assessment. Additionally, we were unable to determine how frequently APs were initiated in the intensive care unit. Any retrospective study is limited by the difficulty of distinguishing delirium from the behavioral and psychiatric symptoms of dementia, but we identified delirium using standard terms described in previous literature.
Our study also has a number of important implications. Because of a reported association between the use of APs and risk of death in the postacute setting,[7] national provider organizations have called for a reduction in AP initiation in hospitalized elders.[2] However, this study indicates that APs continue to be prescribed for delirium, which may be attributed to the lack of behavioral modification options in most hospitals, such as acute care for elders (ACE) units and hospital elder life programs (HELP).[8, 9] Our findings suggest that this problem would be further amplified in hospitals that lack access to geriatrics expertise.
Without alternative behavioral options, patients are at risk for prolonged delirium, which is associated with significant suffering and subsequent risk of further cognitive impairment and death.[10] Although evidence for the efficacy of APs in the treatment of delirium is limited and inconclusive, no better pharmacologic options exist. Hospitals that wish to reduce use of APs should therefore consider investing in environmental interventions (eg, ACE units, HELP), which lower the incidence of delirium and could, in turn, decrease the prescription and continuation of antipsychotics.[8, 9]
Acknowledgements
The authors acknowledge Mihaela Stefan, MD, FACP, for her comments on an earlier draft of this manuscript.
Disclosures: Drs. Lagu and Loh had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the analysis. Drs. Loh, Brennan, Lindenauer, and Lagu conceived of the study. Drs. Loh and Ramdass acquired the data. Ms. Garb analyzed and interpreted the data. Dr. Loh drafted the manuscript. Drs Brennan, Lindenauer, and Lagu, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. The authors report no conflicts of interest.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- . Off‐label use of antipsychotics for dementia patients discouraged. The Hospitalist. November 2012\http://www.the‐hospitalist.org/details/article/2785121/Off‐Label_Use_of_Antipsychotics_for_Dementia_Patients_Discouraged.html. Accessed June 29, 2014.
- STATA/MP [computer program]. Version 13.1 for Windows. College Station, TX: StataCorp; 2013.
- , , , , , . Association between sedating medications and delirium in older inpatients. J Am Geriatr Soc. 2013;61(6):923–930.
- , , , et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Crit Care Med. 2012;40(3):731–739.
- , , . QTc prolongation with antipsychotics: is routine ECG monitoring recommended? J Psychiatr Pract. 2014;20(3):196–206.
- , , , , . Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627–632.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60(12):2237–2245.
- , , , , . The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48(12):1697–1706.
- , , , . Persistent delirium in older hospital patients: a systematic review of frequency and prognosis. Age Ageing. 2009;38(1):19–26.
Antipsychotic (AP) medications are often used in the hospitalized geriatric population for the treatment of delirium.[1] Because of adverse events associated with APs, efforts have been made to reduce their use in hospitalized elders,[2] but it is not clear if these recommendations have been widely adopted. We studied the use of APs in a cohort of hospitalized elders to better understand why APs are started and how often they are continued on discharge.
METHODS
We conducted a retrospective cohort study of patients aged 65 years or older admitted to a tertiary care hospital between October 1, 2012 and September 31, 2013. Using Stata's (StataCorp., College Station, TX) sample command,[3] we included a subset of randomly selected inpatients who received more than 1 dose of oral APs (determined using the electronic medication administration summary). We excluded patients admitted under observation status or to the psychiatric service, those who were on APs prior to admission, and those who only received prochloperazine for nausea. Using prior literature to identify terms frequently used to describe delirium (Figure 1), we created an algorithm and a chart abstraction form (see Supporting Information, Appendix 1, in the online version of this article).[4] We tested these instruments in a preliminary chart review involving 30 patients. Disagreements were discussed with coauthors and resolved through consensus, resulting in some algorithm changes (eg, excluding a large number of patients who received only 1 dose of haloperidol postoperatively, because we hypothesized that this use could be a prophylactic measure).[5] Two investigators extracted the remaining charts independently. We used descriptive statistics and performed cross‐tabulations on the selected variables.

RESULTS
Of 12,817 geriatric hospitalizations during the study period, 1120 (9%) were treated with antipsychotics. We randomly selected 300 of these for extraction: 54% were male, and 67% were admitted to the medical service (Table 1). The inpatient mortality rate was 10% (30/300). The most frequent indication for AP use was delirium (83%, 249/300). Only 35% of delirious patients received a formal assessment with the Confusion Assessment Method (CAM). The most commonly used atypical antipsychotic was quetiapine (86%); 55% received more than 1 antipsychotic medication during hospitalization, and 48% (143/297) of patients were continued on APs at discharge (excluding 3 patients transferred to other acute care hospitals).
| Variable | N (%), Total=300 |
|---|---|
| |
| Gender | |
| Male | 161 (54) |
| Female | 139 (46) |
| Inpatient mortality rate | 30 (10) |
| Services | |
| Medicine | 202 (67) |
| Surgery | 98 (33) |
| Indication for APs use | |
| Delirium | 249 (83) |
| Hallucinations | 19 (6) |
| Anxiety | 20 (7) |
| Other | 38 (13) |
| Atypical APs | |
| Quetiapine | 257 (86) |
| Olanzapine | 29 (10) |
| Risperidone | 26 (9) |
| Typical APs | |
| Haloperidol | 166 (55) |
| Thorazine | 4 (1) |
| Use of CAM | 79 (32)a |
| Physical restraints | 89 (30) |
| Documented or suspected dementia | 134 (45) |
| Geriatrics consults | 120 (40) |
| Psychiatric consults | 29 (10) |
| ECG | |
| Prior to APs administration | 265 (88) |
| After APs administration | 157 (52) |
| QTc prolongation >500 ms | |
| Prior to APs administration | 41 (15)b |
| After APs administration | 39 (25)c |
| Admitted from SNF | 36 (12) |
| Discharge destination | |
| Home | 68 (23) |
| SNFs, short and long‐term rehabilitations | 199 (66) |
| Transfer to other acute care hospitals | 3 (1) |
| Continuation of APs at discharge | 143 (48)d |
Approximately 45% (134/300) had documented or suspected dementia, and 30% (89/300) were physically restrained during the hospital stay. Consultations with geriatrics were obtained in 40% (120/300) of the cases and with psychiatry in 10% (29/300) of the cases. Neurology is rarely consulted for delirium in our institution; thus, we did not collect data on those referrals. Electrocardiography (ECG) (recommended for patients at high cardiac risk[6]) was performed in 88% (265/300) of patients prior to AP administration and 52% (157/300) after. The corrected QT interval exceeded 500 ms in 15% (41/265) of patients prior to AP administration and 25% (39/157) after. Although few patients (12%) were admitted from nursing facilities, 66% (199/300) were eventually discharged to skilled nursing facilities (SNFs) or rehabilitation facilities; most of these patients (117/199, 59%) received AP treatment, compared to 38% of patients discharged to home (26/68).
DISCUSSION
In a cohort of hospitalized elders, we found that 9% were treated with APs. Most received APs for perceived delirium; in‐hospital ECG monitoring was suboptimal. Half of the patients started on APs remained on them at discharge; those discharged to SNFs were more likely to receive ongoing AP treatment.
Our study is limited by its retrospective, single‐center design, a lack of inter‐rater reliability measurement (although our training process was designed to standardize extraction methods), and the infrequent use of formal CAM assessment. Additionally, we were unable to determine how frequently APs were initiated in the intensive care unit. Any retrospective study is limited by the difficulty of distinguishing delirium from the behavioral and psychiatric symptoms of dementia, but we identified delirium using standard terms described in previous literature.
Our study also has a number of important implications. Because of a reported association between the use of APs and risk of death in the postacute setting,[7] national provider organizations have called for a reduction in AP initiation in hospitalized elders.[2] However, this study indicates that APs continue to be prescribed for delirium, which may be attributed to the lack of behavioral modification options in most hospitals, such as acute care for elders (ACE) units and hospital elder life programs (HELP).[8, 9] Our findings suggest that this problem would be further amplified in hospitals that lack access to geriatrics expertise.
Without alternative behavioral options, patients are at risk for prolonged delirium, which is associated with significant suffering and subsequent risk of further cognitive impairment and death.[10] Although evidence for the efficacy of APs in the treatment of delirium is limited and inconclusive, no better pharmacologic options exist. Hospitals that wish to reduce use of APs should therefore consider investing in environmental interventions (eg, ACE units, HELP), which lower the incidence of delirium and could, in turn, decrease the prescription and continuation of antipsychotics.[8, 9]
Acknowledgements
The authors acknowledge Mihaela Stefan, MD, FACP, for her comments on an earlier draft of this manuscript.
Disclosures: Drs. Lagu and Loh had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the analysis. Drs. Loh, Brennan, Lindenauer, and Lagu conceived of the study. Drs. Loh and Ramdass acquired the data. Ms. Garb analyzed and interpreted the data. Dr. Loh drafted the manuscript. Drs Brennan, Lindenauer, and Lagu, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. The authors report no conflicts of interest.
Antipsychotic (AP) medications are often used in the hospitalized geriatric population for the treatment of delirium.[1] Because of adverse events associated with APs, efforts have been made to reduce their use in hospitalized elders,[2] but it is not clear if these recommendations have been widely adopted. We studied the use of APs in a cohort of hospitalized elders to better understand why APs are started and how often they are continued on discharge.
METHODS
We conducted a retrospective cohort study of patients aged 65 years or older admitted to a tertiary care hospital between October 1, 2012 and September 31, 2013. Using Stata's (StataCorp., College Station, TX) sample command,[3] we included a subset of randomly selected inpatients who received more than 1 dose of oral APs (determined using the electronic medication administration summary). We excluded patients admitted under observation status or to the psychiatric service, those who were on APs prior to admission, and those who only received prochloperazine for nausea. Using prior literature to identify terms frequently used to describe delirium (Figure 1), we created an algorithm and a chart abstraction form (see Supporting Information, Appendix 1, in the online version of this article).[4] We tested these instruments in a preliminary chart review involving 30 patients. Disagreements were discussed with coauthors and resolved through consensus, resulting in some algorithm changes (eg, excluding a large number of patients who received only 1 dose of haloperidol postoperatively, because we hypothesized that this use could be a prophylactic measure).[5] Two investigators extracted the remaining charts independently. We used descriptive statistics and performed cross‐tabulations on the selected variables.

RESULTS
Of 12,817 geriatric hospitalizations during the study period, 1120 (9%) were treated with antipsychotics. We randomly selected 300 of these for extraction: 54% were male, and 67% were admitted to the medical service (Table 1). The inpatient mortality rate was 10% (30/300). The most frequent indication for AP use was delirium (83%, 249/300). Only 35% of delirious patients received a formal assessment with the Confusion Assessment Method (CAM). The most commonly used atypical antipsychotic was quetiapine (86%); 55% received more than 1 antipsychotic medication during hospitalization, and 48% (143/297) of patients were continued on APs at discharge (excluding 3 patients transferred to other acute care hospitals).
| Variable | N (%), Total=300 |
|---|---|
| |
| Gender | |
| Male | 161 (54) |
| Female | 139 (46) |
| Inpatient mortality rate | 30 (10) |
| Services | |
| Medicine | 202 (67) |
| Surgery | 98 (33) |
| Indication for APs use | |
| Delirium | 249 (83) |
| Hallucinations | 19 (6) |
| Anxiety | 20 (7) |
| Other | 38 (13) |
| Atypical APs | |
| Quetiapine | 257 (86) |
| Olanzapine | 29 (10) |
| Risperidone | 26 (9) |
| Typical APs | |
| Haloperidol | 166 (55) |
| Thorazine | 4 (1) |
| Use of CAM | 79 (32)a |
| Physical restraints | 89 (30) |
| Documented or suspected dementia | 134 (45) |
| Geriatrics consults | 120 (40) |
| Psychiatric consults | 29 (10) |
| ECG | |
| Prior to APs administration | 265 (88) |
| After APs administration | 157 (52) |
| QTc prolongation >500 ms | |
| Prior to APs administration | 41 (15)b |
| After APs administration | 39 (25)c |
| Admitted from SNF | 36 (12) |
| Discharge destination | |
| Home | 68 (23) |
| SNFs, short and long‐term rehabilitations | 199 (66) |
| Transfer to other acute care hospitals | 3 (1) |
| Continuation of APs at discharge | 143 (48)d |
Approximately 45% (134/300) had documented or suspected dementia, and 30% (89/300) were physically restrained during the hospital stay. Consultations with geriatrics were obtained in 40% (120/300) of the cases and with psychiatry in 10% (29/300) of the cases. Neurology is rarely consulted for delirium in our institution; thus, we did not collect data on those referrals. Electrocardiography (ECG) (recommended for patients at high cardiac risk[6]) was performed in 88% (265/300) of patients prior to AP administration and 52% (157/300) after. The corrected QT interval exceeded 500 ms in 15% (41/265) of patients prior to AP administration and 25% (39/157) after. Although few patients (12%) were admitted from nursing facilities, 66% (199/300) were eventually discharged to skilled nursing facilities (SNFs) or rehabilitation facilities; most of these patients (117/199, 59%) received AP treatment, compared to 38% of patients discharged to home (26/68).
DISCUSSION
In a cohort of hospitalized elders, we found that 9% were treated with APs. Most received APs for perceived delirium; in‐hospital ECG monitoring was suboptimal. Half of the patients started on APs remained on them at discharge; those discharged to SNFs were more likely to receive ongoing AP treatment.
Our study is limited by its retrospective, single‐center design, a lack of inter‐rater reliability measurement (although our training process was designed to standardize extraction methods), and the infrequent use of formal CAM assessment. Additionally, we were unable to determine how frequently APs were initiated in the intensive care unit. Any retrospective study is limited by the difficulty of distinguishing delirium from the behavioral and psychiatric symptoms of dementia, but we identified delirium using standard terms described in previous literature.
Our study also has a number of important implications. Because of a reported association between the use of APs and risk of death in the postacute setting,[7] national provider organizations have called for a reduction in AP initiation in hospitalized elders.[2] However, this study indicates that APs continue to be prescribed for delirium, which may be attributed to the lack of behavioral modification options in most hospitals, such as acute care for elders (ACE) units and hospital elder life programs (HELP).[8, 9] Our findings suggest that this problem would be further amplified in hospitals that lack access to geriatrics expertise.
Without alternative behavioral options, patients are at risk for prolonged delirium, which is associated with significant suffering and subsequent risk of further cognitive impairment and death.[10] Although evidence for the efficacy of APs in the treatment of delirium is limited and inconclusive, no better pharmacologic options exist. Hospitals that wish to reduce use of APs should therefore consider investing in environmental interventions (eg, ACE units, HELP), which lower the incidence of delirium and could, in turn, decrease the prescription and continuation of antipsychotics.[8, 9]
Acknowledgements
The authors acknowledge Mihaela Stefan, MD, FACP, for her comments on an earlier draft of this manuscript.
Disclosures: Drs. Lagu and Loh had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the analysis. Drs. Loh, Brennan, Lindenauer, and Lagu conceived of the study. Drs. Loh and Ramdass acquired the data. Ms. Garb analyzed and interpreted the data. Dr. Loh drafted the manuscript. Drs Brennan, Lindenauer, and Lagu, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. The authors report no conflicts of interest.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- . Off‐label use of antipsychotics for dementia patients discouraged. The Hospitalist. November 2012\http://www.the‐hospitalist.org/details/article/2785121/Off‐Label_Use_of_Antipsychotics_for_Dementia_Patients_Discouraged.html. Accessed June 29, 2014.
- STATA/MP [computer program]. Version 13.1 for Windows. College Station, TX: StataCorp; 2013.
- , , , , , . Association between sedating medications and delirium in older inpatients. J Am Geriatr Soc. 2013;61(6):923–930.
- , , , et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Crit Care Med. 2012;40(3):731–739.
- , , . QTc prolongation with antipsychotics: is routine ECG monitoring recommended? J Psychiatr Pract. 2014;20(3):196–206.
- , , , , . Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627–632.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60(12):2237–2245.
- , , , , . The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48(12):1697–1706.
- , , , . Persistent delirium in older hospital patients: a systematic review of frequency and prognosis. Age Ageing. 2009;38(1):19–26.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- . Off‐label use of antipsychotics for dementia patients discouraged. The Hospitalist. November 2012\http://www.the‐hospitalist.org/details/article/2785121/Off‐Label_Use_of_Antipsychotics_for_Dementia_Patients_Discouraged.html. Accessed June 29, 2014.
- STATA/MP [computer program]. Version 13.1 for Windows. College Station, TX: StataCorp; 2013.
- , , , , , . Association between sedating medications and delirium in older inpatients. J Am Geriatr Soc. 2013;61(6):923–930.
- , , , et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Crit Care Med. 2012;40(3):731–739.
- , , . QTc prolongation with antipsychotics: is routine ECG monitoring recommended? J Psychiatr Pract. 2014;20(3):196–206.
- , , , , . Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627–632.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60(12):2237–2245.
- , , , , . The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48(12):1697–1706.
- , , , . Persistent delirium in older hospital patients: a systematic review of frequency and prognosis. Age Ageing. 2009;38(1):19–26.
Telemetry Order Duration Reductions
The Society of Hospital Medicine's Adult Choosing Wisely measures include not ordering continuous telemetry monitoring outside of the ICU [intensive care unit] without using a protocol that governs continuation.[1] Current guidelines for cardiac monitoring use recommend minimum durations for all adult class I and most class II indications.[2] However, telemetry ordering often fails to include timing or criteria for discontinuation. We determined the impact of a reduction in telemetry order duration within our hospital, hypothesizing this reduction would lead to earlier reassessment of telemetry need and therefore decrease overall utilization.
METHODS
Setting
Durham Veterans Affairs Medical Center (DVAMC) is a 151‐bed tertiary care hospital within Veterans Affairs (VA) Integrated Services Network Region 6 (VISN 6) serving as the primary VA hospital for >54,000 patients and a referral hospital for VISN 6. Twenty‐five telemetry units are available for use on 2 wards with 48 potential telemetry beds. All nonintensive care wards contain general medical and surgical patients, without a primary inpatient cardiology service. Most orders are written by housestaff supervised by attending physicians.
Intervention
Prior to our intervention, the maximum allowable duration of telemetry orders was 72 hours. The duration was enforced by nursing staff automatically discontinuing telemetry not renewed within 72 hours. For our intervention, we reduced the duration of telemetry within our electronic ordering system in November 2013 so that orders had to be renewed within 48 hours or they were discontinued. No education regarding appropriate telemetry use was provided. This intervention was created as a quality‐improvement (QI) project affecting all telemetry use within DVAMC and was exempt from institutional review board review.
Outcomes
Outcomes included the mean number of telemetry orders per week, mean duration of telemetry orders, mean duration of telemetry per episode, and the ratio of time on telemetry relative to the total length of stay. As a balancing measure, we examined rates of rapid response and code blue events. All measures were compared for 12 weeks before and 16 weeks after the intervention. Telemetry orders and durations were obtained using the Corporate Data Warehouse.
Analysis
All outcome measurements were continuous variables and compared using the Student t test in Stata version 9.2 (StataCorp, College Station, TX).
RESULTS
Following the intervention, overall order duration decreased by 33% from 66.68.3 hours to 44.52.3 hours per order (P<0.01), mirroring the reduction in the maximum telemetry order duration from 72 to 48 hours (Table 1). However, an increase in telemetry order frequency after the intervention resulted in no significant change in telemetry duration per episode or the proportion of the hospitalization on telemetry (59.3 vs 56.3 hours per patient, P=0.43; and 66.4% vs 66.2% of hospitalization, P=0.58). Rapid response and code blue events did not differ significantly relative to the intervention (2.8 events per week before and 3.1 events per week after, P=0.63).
| Before Intervention | After Intervention | P Value | |
|---|---|---|---|
| |||
| No. of hospitalizations with telemetry ordered | 557 | 684 | NA |
| No. of telemetry orders | 952 | 1515 | NA |
| Average no. of orders per week (SD) | 79.3 (9.2) | 94.7 (25.9) | 0.06 |
| Hours of telemetry per order (SD) | 66.6 (8.3) | 44.5 (2.3) | <0.01 |
| Duration of telemetry per patient, h | 59.3 | 56.3 | 0.43 |
| % of hospitalizations receiving telemetry per patient | 66.4% | 66.2% | 0.90 |
| RRT/code blue events per week | 2.8 | 3.1 | 0.63 |
DISCUSSION
Overall, telemetry utilization was unchanged in spite of an intervention successfully reducing telemetry order duration. Providers responded to this decreased order duration by increasing renewal orders, leaving the amount of time patients spent on telemetry unchanged.
Little primary evidence underlies the American Heart Association recommendations for duration of telemetry in general ward patients.[2] The existing literature documents the timing in which arrhythmias occur after cardiac surgery or myocardial infarction, and therefore is limited in guiding patient care outside intensive care unit settings.[3, 4] As such, hospitalists and inpatient providers have little data directing additional telemetry decisions for these patients, and none for patients requiring telemetry for other indications.
As interventions focusing solely on telemetry duration may not lead to changes in usage patterns, reducing telemetry utilization may require active stewardship. For example, explicit justification may be needed for renewal of telemetry orders. Similarly, education on appropriate telemetry indications in tandem with electronic ordering changes may be more likely to change behavior. Alternatively, incorporating data identifying chest pain patients at very low risk of developing arrhythmias or cardiac complications, based on published risk scores at the time of ordering, may lead to better decision making in initiating telemetry.[5, 6]
This QI project had several limitations. First, the intervention occurred in a facility with a previous telemetry order duration limit. In hospitals without a current duration limitation, some reduction in overall telemetry utilization may be possible. Second, this project was a nonrandom before/after study and potentially subject to bias due to confounding. However, our limited number of telemetry resources, the relatively low number of inpatient teams at our facility, and the inability to target geographic locations for team admissions would have made a cluster‐randomized trial impractical. Third, rationales for telemetry ordering were unknown, as well as drivers for increased orders after the intervention. Better understanding these factors could lead to targeted interventions in some settings.
CONCLUSION
In conclusion, a QI initiative reducing telemetry order duration did not reduce overall telemetry utilization but increased the number of telemetry orders written. Interventions incorporating appropriate telemetry indications or event risks may be required to change ordering behaviors.
Disclosure: Nothing to report.
- Society of Hospital Medicine. Society of Hospital Medicine–adult hospital medicine: five things physicians and patients should question. Available at: http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed June 4, 2014.
- , , , et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical‐Care Nurses. Circulation. 2004;110(17):2721–2746.
- , , , . Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56(3):539–549.
- , , , et al. Time‐based risk assessment after myocardial infarction. Implications for timing of discharge and applications to medical decision‐making. Eur Heart J. 2003;24(2):182–189.
- , , , et al. Emergency department admissions to inpatient cardiac telemetry beds: a prospective cohort study of risk stratification and outcomes. Am J Med. 2001;110(1):7–11.
- , , , . Lack of utility of telemetry monitoring for identification of cardiac death and life‐threatening ventricular dysrhythmias in low‐risk patients with chest pain. Ann Emerg Med. 2004;43(1):71–76.
The Society of Hospital Medicine's Adult Choosing Wisely measures include not ordering continuous telemetry monitoring outside of the ICU [intensive care unit] without using a protocol that governs continuation.[1] Current guidelines for cardiac monitoring use recommend minimum durations for all adult class I and most class II indications.[2] However, telemetry ordering often fails to include timing or criteria for discontinuation. We determined the impact of a reduction in telemetry order duration within our hospital, hypothesizing this reduction would lead to earlier reassessment of telemetry need and therefore decrease overall utilization.
METHODS
Setting
Durham Veterans Affairs Medical Center (DVAMC) is a 151‐bed tertiary care hospital within Veterans Affairs (VA) Integrated Services Network Region 6 (VISN 6) serving as the primary VA hospital for >54,000 patients and a referral hospital for VISN 6. Twenty‐five telemetry units are available for use on 2 wards with 48 potential telemetry beds. All nonintensive care wards contain general medical and surgical patients, without a primary inpatient cardiology service. Most orders are written by housestaff supervised by attending physicians.
Intervention
Prior to our intervention, the maximum allowable duration of telemetry orders was 72 hours. The duration was enforced by nursing staff automatically discontinuing telemetry not renewed within 72 hours. For our intervention, we reduced the duration of telemetry within our electronic ordering system in November 2013 so that orders had to be renewed within 48 hours or they were discontinued. No education regarding appropriate telemetry use was provided. This intervention was created as a quality‐improvement (QI) project affecting all telemetry use within DVAMC and was exempt from institutional review board review.
Outcomes
Outcomes included the mean number of telemetry orders per week, mean duration of telemetry orders, mean duration of telemetry per episode, and the ratio of time on telemetry relative to the total length of stay. As a balancing measure, we examined rates of rapid response and code blue events. All measures were compared for 12 weeks before and 16 weeks after the intervention. Telemetry orders and durations were obtained using the Corporate Data Warehouse.
Analysis
All outcome measurements were continuous variables and compared using the Student t test in Stata version 9.2 (StataCorp, College Station, TX).
RESULTS
Following the intervention, overall order duration decreased by 33% from 66.68.3 hours to 44.52.3 hours per order (P<0.01), mirroring the reduction in the maximum telemetry order duration from 72 to 48 hours (Table 1). However, an increase in telemetry order frequency after the intervention resulted in no significant change in telemetry duration per episode or the proportion of the hospitalization on telemetry (59.3 vs 56.3 hours per patient, P=0.43; and 66.4% vs 66.2% of hospitalization, P=0.58). Rapid response and code blue events did not differ significantly relative to the intervention (2.8 events per week before and 3.1 events per week after, P=0.63).
| Before Intervention | After Intervention | P Value | |
|---|---|---|---|
| |||
| No. of hospitalizations with telemetry ordered | 557 | 684 | NA |
| No. of telemetry orders | 952 | 1515 | NA |
| Average no. of orders per week (SD) | 79.3 (9.2) | 94.7 (25.9) | 0.06 |
| Hours of telemetry per order (SD) | 66.6 (8.3) | 44.5 (2.3) | <0.01 |
| Duration of telemetry per patient, h | 59.3 | 56.3 | 0.43 |
| % of hospitalizations receiving telemetry per patient | 66.4% | 66.2% | 0.90 |
| RRT/code blue events per week | 2.8 | 3.1 | 0.63 |
DISCUSSION
Overall, telemetry utilization was unchanged in spite of an intervention successfully reducing telemetry order duration. Providers responded to this decreased order duration by increasing renewal orders, leaving the amount of time patients spent on telemetry unchanged.
Little primary evidence underlies the American Heart Association recommendations for duration of telemetry in general ward patients.[2] The existing literature documents the timing in which arrhythmias occur after cardiac surgery or myocardial infarction, and therefore is limited in guiding patient care outside intensive care unit settings.[3, 4] As such, hospitalists and inpatient providers have little data directing additional telemetry decisions for these patients, and none for patients requiring telemetry for other indications.
As interventions focusing solely on telemetry duration may not lead to changes in usage patterns, reducing telemetry utilization may require active stewardship. For example, explicit justification may be needed for renewal of telemetry orders. Similarly, education on appropriate telemetry indications in tandem with electronic ordering changes may be more likely to change behavior. Alternatively, incorporating data identifying chest pain patients at very low risk of developing arrhythmias or cardiac complications, based on published risk scores at the time of ordering, may lead to better decision making in initiating telemetry.[5, 6]
This QI project had several limitations. First, the intervention occurred in a facility with a previous telemetry order duration limit. In hospitals without a current duration limitation, some reduction in overall telemetry utilization may be possible. Second, this project was a nonrandom before/after study and potentially subject to bias due to confounding. However, our limited number of telemetry resources, the relatively low number of inpatient teams at our facility, and the inability to target geographic locations for team admissions would have made a cluster‐randomized trial impractical. Third, rationales for telemetry ordering were unknown, as well as drivers for increased orders after the intervention. Better understanding these factors could lead to targeted interventions in some settings.
CONCLUSION
In conclusion, a QI initiative reducing telemetry order duration did not reduce overall telemetry utilization but increased the number of telemetry orders written. Interventions incorporating appropriate telemetry indications or event risks may be required to change ordering behaviors.
Disclosure: Nothing to report.
The Society of Hospital Medicine's Adult Choosing Wisely measures include not ordering continuous telemetry monitoring outside of the ICU [intensive care unit] without using a protocol that governs continuation.[1] Current guidelines for cardiac monitoring use recommend minimum durations for all adult class I and most class II indications.[2] However, telemetry ordering often fails to include timing or criteria for discontinuation. We determined the impact of a reduction in telemetry order duration within our hospital, hypothesizing this reduction would lead to earlier reassessment of telemetry need and therefore decrease overall utilization.
METHODS
Setting
Durham Veterans Affairs Medical Center (DVAMC) is a 151‐bed tertiary care hospital within Veterans Affairs (VA) Integrated Services Network Region 6 (VISN 6) serving as the primary VA hospital for >54,000 patients and a referral hospital for VISN 6. Twenty‐five telemetry units are available for use on 2 wards with 48 potential telemetry beds. All nonintensive care wards contain general medical and surgical patients, without a primary inpatient cardiology service. Most orders are written by housestaff supervised by attending physicians.
Intervention
Prior to our intervention, the maximum allowable duration of telemetry orders was 72 hours. The duration was enforced by nursing staff automatically discontinuing telemetry not renewed within 72 hours. For our intervention, we reduced the duration of telemetry within our electronic ordering system in November 2013 so that orders had to be renewed within 48 hours or they were discontinued. No education regarding appropriate telemetry use was provided. This intervention was created as a quality‐improvement (QI) project affecting all telemetry use within DVAMC and was exempt from institutional review board review.
Outcomes
Outcomes included the mean number of telemetry orders per week, mean duration of telemetry orders, mean duration of telemetry per episode, and the ratio of time on telemetry relative to the total length of stay. As a balancing measure, we examined rates of rapid response and code blue events. All measures were compared for 12 weeks before and 16 weeks after the intervention. Telemetry orders and durations were obtained using the Corporate Data Warehouse.
Analysis
All outcome measurements were continuous variables and compared using the Student t test in Stata version 9.2 (StataCorp, College Station, TX).
RESULTS
Following the intervention, overall order duration decreased by 33% from 66.68.3 hours to 44.52.3 hours per order (P<0.01), mirroring the reduction in the maximum telemetry order duration from 72 to 48 hours (Table 1). However, an increase in telemetry order frequency after the intervention resulted in no significant change in telemetry duration per episode or the proportion of the hospitalization on telemetry (59.3 vs 56.3 hours per patient, P=0.43; and 66.4% vs 66.2% of hospitalization, P=0.58). Rapid response and code blue events did not differ significantly relative to the intervention (2.8 events per week before and 3.1 events per week after, P=0.63).
| Before Intervention | After Intervention | P Value | |
|---|---|---|---|
| |||
| No. of hospitalizations with telemetry ordered | 557 | 684 | NA |
| No. of telemetry orders | 952 | 1515 | NA |
| Average no. of orders per week (SD) | 79.3 (9.2) | 94.7 (25.9) | 0.06 |
| Hours of telemetry per order (SD) | 66.6 (8.3) | 44.5 (2.3) | <0.01 |
| Duration of telemetry per patient, h | 59.3 | 56.3 | 0.43 |
| % of hospitalizations receiving telemetry per patient | 66.4% | 66.2% | 0.90 |
| RRT/code blue events per week | 2.8 | 3.1 | 0.63 |
DISCUSSION
Overall, telemetry utilization was unchanged in spite of an intervention successfully reducing telemetry order duration. Providers responded to this decreased order duration by increasing renewal orders, leaving the amount of time patients spent on telemetry unchanged.
Little primary evidence underlies the American Heart Association recommendations for duration of telemetry in general ward patients.[2] The existing literature documents the timing in which arrhythmias occur after cardiac surgery or myocardial infarction, and therefore is limited in guiding patient care outside intensive care unit settings.[3, 4] As such, hospitalists and inpatient providers have little data directing additional telemetry decisions for these patients, and none for patients requiring telemetry for other indications.
As interventions focusing solely on telemetry duration may not lead to changes in usage patterns, reducing telemetry utilization may require active stewardship. For example, explicit justification may be needed for renewal of telemetry orders. Similarly, education on appropriate telemetry indications in tandem with electronic ordering changes may be more likely to change behavior. Alternatively, incorporating data identifying chest pain patients at very low risk of developing arrhythmias or cardiac complications, based on published risk scores at the time of ordering, may lead to better decision making in initiating telemetry.[5, 6]
This QI project had several limitations. First, the intervention occurred in a facility with a previous telemetry order duration limit. In hospitals without a current duration limitation, some reduction in overall telemetry utilization may be possible. Second, this project was a nonrandom before/after study and potentially subject to bias due to confounding. However, our limited number of telemetry resources, the relatively low number of inpatient teams at our facility, and the inability to target geographic locations for team admissions would have made a cluster‐randomized trial impractical. Third, rationales for telemetry ordering were unknown, as well as drivers for increased orders after the intervention. Better understanding these factors could lead to targeted interventions in some settings.
CONCLUSION
In conclusion, a QI initiative reducing telemetry order duration did not reduce overall telemetry utilization but increased the number of telemetry orders written. Interventions incorporating appropriate telemetry indications or event risks may be required to change ordering behaviors.
Disclosure: Nothing to report.
- Society of Hospital Medicine. Society of Hospital Medicine–adult hospital medicine: five things physicians and patients should question. Available at: http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed June 4, 2014.
- , , , et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical‐Care Nurses. Circulation. 2004;110(17):2721–2746.
- , , , . Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56(3):539–549.
- , , , et al. Time‐based risk assessment after myocardial infarction. Implications for timing of discharge and applications to medical decision‐making. Eur Heart J. 2003;24(2):182–189.
- , , , et al. Emergency department admissions to inpatient cardiac telemetry beds: a prospective cohort study of risk stratification and outcomes. Am J Med. 2001;110(1):7–11.
- , , , . Lack of utility of telemetry monitoring for identification of cardiac death and life‐threatening ventricular dysrhythmias in low‐risk patients with chest pain. Ann Emerg Med. 2004;43(1):71–76.
- Society of Hospital Medicine. Society of Hospital Medicine–adult hospital medicine: five things physicians and patients should question. Available at: http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed June 4, 2014.
- , , , et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical‐Care Nurses. Circulation. 2004;110(17):2721–2746.
- , , , . Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56(3):539–549.
- , , , et al. Time‐based risk assessment after myocardial infarction. Implications for timing of discharge and applications to medical decision‐making. Eur Heart J. 2003;24(2):182–189.
- , , , et al. Emergency department admissions to inpatient cardiac telemetry beds: a prospective cohort study of risk stratification and outcomes. Am J Med. 2001;110(1):7–11.
- , , , . Lack of utility of telemetry monitoring for identification of cardiac death and life‐threatening ventricular dysrhythmias in low‐risk patients with chest pain. Ann Emerg Med. 2004;43(1):71–76.
Intrateam Coverage and Handoffs
We have traditionally viewed continuity of care with a particular intern as important for high‐quality inpatient care, but this continuity is difficult to achieve. As we move to a model of team rather than individual continuity, information transfers between team members become critical.
When discontinuity between the primary team and a cross‐covering team occurs, this informational continuity is managed through formal handoffs.[1] Accordingly, there has been ample research on handoffs between different teams,[2, 3, 4, 5] but there has been little published literature to date to describe handoffs between members of the same team. Therefore, we set out (1) to learn how interns view intrateam handoffs and (2) to identify intern‐perceived problems with intrateam handoffs.
MATERIALS AND METHODS
This was a cross‐sectional survey study done at a 500‐bed academic medical center affiliated with a large internal medicine residency program. The survey was developed by the study team and reviewed for content and clarity by our chief residents and by 2 nationally known medical educators outside our institution. Study participants were internal medicine interns. Interns in this program rotate through 3 hospitals and do 7 to 8 ward months. The call schedules are different at each site (see Supporting Information, Appendix A, in the online version of this article). Opportunities for intrateam coverage of 1 intern by another include clinics (1/week), days off (1/week), some overnight periods, and occasional educational conferences. When possible, daily attending rounds include the entire team, but due to clinics, conferences, and days off, it is rare that the entire team is present. Bedside rounds are done at the discretion of the attending. The survey (see Supporting Information, Appendix B, in the online version of this article) included questions regarding situations when the respondent was covering his or her cointern's patients (cointern was defined as another intern on the respondent's same inpatient ward team). We also asked about situations when a cointern was covering the respondent's patients. For those questions, we considered answers of >60% to be a majority. We distributed this anonymous survey on 2 dates (January 2012 and March 2012) during regularly scheduled conferences. We mainly report descriptive findings. We also compared the percentage of study participants reporting problems when covering cointerns' patients to the percentage of study participants reporting problems when cointerns covered their (study participants') patients using 2, with significance set at P<0.05. This study was designated as exempt by the institutional review board.
RESULTS
Thirty‐four interns completed the survey out of a total of 44 interns present at the conferences (response rate=77%). There were 46 interns in the program, including categorical, medicine‐pediatrics, and preliminary interns. The mean age was 28 (standard deviation 2.8). Two‐thirds of respondents were female, and 65% were categorical.
Difference Between Intra‐ and Interteam Handoffs
Eighty‐eight percent felt that a handoff to a cointern was different than a handoff to an overnight cross‐cover intern; many interns said they assumed their cointerns had at least some knowledge of their patients, and therefore put less time and detail into their handoffs. When covering for their cointern, 47% reported feeling the same amount of responsibility as for their own patients, whereas 38% of interns reported feeling much or somewhat less responsible for their cointerns' patients and the remainder (15%) felt somewhat or much more responsible.
Knowledge of Cointern's Patients
Most (65%) interns reported at least 3 days in their last inpatient ward month when they covered a cointern's patient that had not been formally handed off to them. Forty‐five percent of respondents reported seldom or never receiving a written sign‐out on their cointern's patients.
Respondents were asked to think about times before they had covered their cointern's patients. Sixty‐eight percent of respondents reported knowing the number 1 problem for the majority of their cointern's patients. Twenty‐four percent reported having ever actually seen the majority of their cointern's patients. Only 3% of respondents said they had ever examined the majority of their cointern's patients prior to providing coverage.
Perceived Problems With Intrateam Coverage
While covering a cointern's patients, nearly half reported missing changes in patients' exams and forgetting to order labs or imaging. More than half reported unexpected family meetings or phone calls. In contrast, respondents noted more problems when their cointern had covered for them (Table 1). Seventy‐nine percent felt that patient care was at least sometimes delayed because of incomplete knowledge due to intrateam coverage.
| What Problems Have You Noticed | ||
|---|---|---|
| While Respondent Covers a Cointern's Patient? | After Respondent's Patients Were Covered by Cointern? | |
| ||
| Missed labs | 18% | 33% |
| Missed consult recommendations | 21% | 30% |
| Missed exam changes | 42% | 27% |
| Forgot to follow‐up imaging | 27% | 30% |
| Forgot to order labs or imaging | 42%a | 70%a |
| Failure to adjust meds | 27% | 27% |
| Unexpected family meeting/phone calls | 61%a | 30%a |
| Did not understand the plan from cointern's notes | 45% | 27% |
DISCUSSION
In our program, interns commonly cover for each other. This intrateam coverage frequently occurs without a formal handoff, and interns do not always know key information about their cointern's patients. Interns reported frequent problems with intrateam coverage such as missed lab results, consult recommendations, and changes in the physical exam. These missed items could result in delayed diagnoses and delayed treatment. These problems have been identified in interteam handoffs as well.[6, 7] Even in optimized interteam handoffs, receivers fail to identify the most important piece of information about 60% of the patients,[8] and our results mirror this finding.
The finding that fewer than a quarter of the respondents have ever seen the majority of their cointerns' patients is certainly of concern. This likely arises from several inter‐related factors: reduced hours for housestaff, schedules built to accommodate the reduced hours (eg, overlapping rather than simultaneous shifts), and the choice of some attendings to not take the entire team around to see every patient. In institutions where bedside rounds as a team are the norm, this finding will be less applicable, but others across the country have noticed this trend[9, 10] and have tried to counteract it.[11] This situation has both patient care and educational implications. The main patient care implication is that the other team members may be less able to seamlessly assume care when the primary intern is away or busy. Therefore, intrateam coverage becomes much more like traditional cross‐coverage of another team's patients, during which there is no expectation that the covering person will have ever seen the patients for whom they are assuming care. The main educational implication of not seeing the cointerns' patients is that the interns are seeing only half the patients that they could otherwise see. Learning medicine is experiential, and limiting opportunities for seeing and examining patients is unwise in this era of reduced time spent in the hospital.
Limitations of this study include being conducted in a single program. It will be important for other sites to assess their own practices with respect to intrateam handoffs. Another limitation is that it was a cross‐sectional survey subject to recall bias. We may have obtained more detailed information if we had conducted interviews. We also did not quantify the frequency of missed labs, consult recommendations, and physical examination changes that occurred during intrateam coverage. Finally, we did not independently verify the problems identified by the interns.
Some possible strategies to address this issue include (1) treating intrateam handoffs like interteam handoffs by implementing a formal system, (2) better utilizing senior residents/faculty when interns are covering for each other, (3) using bedside attending rounds to increase the exposure of all team members to the team's patients, (4) block scheduling to avoid absences due to clinics,[12] and (5) better communication and teamwork training to increase team awareness of all patients.[13]
Disclosures
Disclosures: There was no external funding for this work. However, this material is the result of work supported with resources and the use of facilities at the Clement J. Zablocki VA Medical Center, Milwaukee, WI. This work was presented in poster format at the national Society of Hospital Medicine meeting in National Harbor, Maryland in May 2013. The authors have no conflicts of interest to report.
- , , , et al. Residents' and attending physicians' handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775–1787.
- , , . Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , . Faculty member review and feedback using a sign‐out checklist: improving intern written sign‐out. Acad Med. 2012;87(8):1125–1131.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , . Transfers of patient care between house staff on internal medicine wards. Arch Intern Med. 2006;166:1173–1177.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care 2005;14(6):401–407.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , . Interns overestimate the effectiveness of their hand‐off communication. Pediatrics. 2010;125(3):491–496.
- . Culture shock—patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748–2751.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- , , , et al. The ambulatory long‐block: an accreditation council for graduate medical education (ACGME) educational innovations project (EIP). J Gen Intern Med. 2008;23(7):921–926.
- AHRQ. TeamSTEPPS: National Implementation. Available at: http://teamstepps.ahrq.gov/. Accessed June 19, 2014.
We have traditionally viewed continuity of care with a particular intern as important for high‐quality inpatient care, but this continuity is difficult to achieve. As we move to a model of team rather than individual continuity, information transfers between team members become critical.
When discontinuity between the primary team and a cross‐covering team occurs, this informational continuity is managed through formal handoffs.[1] Accordingly, there has been ample research on handoffs between different teams,[2, 3, 4, 5] but there has been little published literature to date to describe handoffs between members of the same team. Therefore, we set out (1) to learn how interns view intrateam handoffs and (2) to identify intern‐perceived problems with intrateam handoffs.
MATERIALS AND METHODS
This was a cross‐sectional survey study done at a 500‐bed academic medical center affiliated with a large internal medicine residency program. The survey was developed by the study team and reviewed for content and clarity by our chief residents and by 2 nationally known medical educators outside our institution. Study participants were internal medicine interns. Interns in this program rotate through 3 hospitals and do 7 to 8 ward months. The call schedules are different at each site (see Supporting Information, Appendix A, in the online version of this article). Opportunities for intrateam coverage of 1 intern by another include clinics (1/week), days off (1/week), some overnight periods, and occasional educational conferences. When possible, daily attending rounds include the entire team, but due to clinics, conferences, and days off, it is rare that the entire team is present. Bedside rounds are done at the discretion of the attending. The survey (see Supporting Information, Appendix B, in the online version of this article) included questions regarding situations when the respondent was covering his or her cointern's patients (cointern was defined as another intern on the respondent's same inpatient ward team). We also asked about situations when a cointern was covering the respondent's patients. For those questions, we considered answers of >60% to be a majority. We distributed this anonymous survey on 2 dates (January 2012 and March 2012) during regularly scheduled conferences. We mainly report descriptive findings. We also compared the percentage of study participants reporting problems when covering cointerns' patients to the percentage of study participants reporting problems when cointerns covered their (study participants') patients using 2, with significance set at P<0.05. This study was designated as exempt by the institutional review board.
RESULTS
Thirty‐four interns completed the survey out of a total of 44 interns present at the conferences (response rate=77%). There were 46 interns in the program, including categorical, medicine‐pediatrics, and preliminary interns. The mean age was 28 (standard deviation 2.8). Two‐thirds of respondents were female, and 65% were categorical.
Difference Between Intra‐ and Interteam Handoffs
Eighty‐eight percent felt that a handoff to a cointern was different than a handoff to an overnight cross‐cover intern; many interns said they assumed their cointerns had at least some knowledge of their patients, and therefore put less time and detail into their handoffs. When covering for their cointern, 47% reported feeling the same amount of responsibility as for their own patients, whereas 38% of interns reported feeling much or somewhat less responsible for their cointerns' patients and the remainder (15%) felt somewhat or much more responsible.
Knowledge of Cointern's Patients
Most (65%) interns reported at least 3 days in their last inpatient ward month when they covered a cointern's patient that had not been formally handed off to them. Forty‐five percent of respondents reported seldom or never receiving a written sign‐out on their cointern's patients.
Respondents were asked to think about times before they had covered their cointern's patients. Sixty‐eight percent of respondents reported knowing the number 1 problem for the majority of their cointern's patients. Twenty‐four percent reported having ever actually seen the majority of their cointern's patients. Only 3% of respondents said they had ever examined the majority of their cointern's patients prior to providing coverage.
Perceived Problems With Intrateam Coverage
While covering a cointern's patients, nearly half reported missing changes in patients' exams and forgetting to order labs or imaging. More than half reported unexpected family meetings or phone calls. In contrast, respondents noted more problems when their cointern had covered for them (Table 1). Seventy‐nine percent felt that patient care was at least sometimes delayed because of incomplete knowledge due to intrateam coverage.
| What Problems Have You Noticed | ||
|---|---|---|
| While Respondent Covers a Cointern's Patient? | After Respondent's Patients Were Covered by Cointern? | |
| ||
| Missed labs | 18% | 33% |
| Missed consult recommendations | 21% | 30% |
| Missed exam changes | 42% | 27% |
| Forgot to follow‐up imaging | 27% | 30% |
| Forgot to order labs or imaging | 42%a | 70%a |
| Failure to adjust meds | 27% | 27% |
| Unexpected family meeting/phone calls | 61%a | 30%a |
| Did not understand the plan from cointern's notes | 45% | 27% |
DISCUSSION
In our program, interns commonly cover for each other. This intrateam coverage frequently occurs without a formal handoff, and interns do not always know key information about their cointern's patients. Interns reported frequent problems with intrateam coverage such as missed lab results, consult recommendations, and changes in the physical exam. These missed items could result in delayed diagnoses and delayed treatment. These problems have been identified in interteam handoffs as well.[6, 7] Even in optimized interteam handoffs, receivers fail to identify the most important piece of information about 60% of the patients,[8] and our results mirror this finding.
The finding that fewer than a quarter of the respondents have ever seen the majority of their cointerns' patients is certainly of concern. This likely arises from several inter‐related factors: reduced hours for housestaff, schedules built to accommodate the reduced hours (eg, overlapping rather than simultaneous shifts), and the choice of some attendings to not take the entire team around to see every patient. In institutions where bedside rounds as a team are the norm, this finding will be less applicable, but others across the country have noticed this trend[9, 10] and have tried to counteract it.[11] This situation has both patient care and educational implications. The main patient care implication is that the other team members may be less able to seamlessly assume care when the primary intern is away or busy. Therefore, intrateam coverage becomes much more like traditional cross‐coverage of another team's patients, during which there is no expectation that the covering person will have ever seen the patients for whom they are assuming care. The main educational implication of not seeing the cointerns' patients is that the interns are seeing only half the patients that they could otherwise see. Learning medicine is experiential, and limiting opportunities for seeing and examining patients is unwise in this era of reduced time spent in the hospital.
Limitations of this study include being conducted in a single program. It will be important for other sites to assess their own practices with respect to intrateam handoffs. Another limitation is that it was a cross‐sectional survey subject to recall bias. We may have obtained more detailed information if we had conducted interviews. We also did not quantify the frequency of missed labs, consult recommendations, and physical examination changes that occurred during intrateam coverage. Finally, we did not independently verify the problems identified by the interns.
Some possible strategies to address this issue include (1) treating intrateam handoffs like interteam handoffs by implementing a formal system, (2) better utilizing senior residents/faculty when interns are covering for each other, (3) using bedside attending rounds to increase the exposure of all team members to the team's patients, (4) block scheduling to avoid absences due to clinics,[12] and (5) better communication and teamwork training to increase team awareness of all patients.[13]
Disclosures
Disclosures: There was no external funding for this work. However, this material is the result of work supported with resources and the use of facilities at the Clement J. Zablocki VA Medical Center, Milwaukee, WI. This work was presented in poster format at the national Society of Hospital Medicine meeting in National Harbor, Maryland in May 2013. The authors have no conflicts of interest to report.
We have traditionally viewed continuity of care with a particular intern as important for high‐quality inpatient care, but this continuity is difficult to achieve. As we move to a model of team rather than individual continuity, information transfers between team members become critical.
When discontinuity between the primary team and a cross‐covering team occurs, this informational continuity is managed through formal handoffs.[1] Accordingly, there has been ample research on handoffs between different teams,[2, 3, 4, 5] but there has been little published literature to date to describe handoffs between members of the same team. Therefore, we set out (1) to learn how interns view intrateam handoffs and (2) to identify intern‐perceived problems with intrateam handoffs.
MATERIALS AND METHODS
This was a cross‐sectional survey study done at a 500‐bed academic medical center affiliated with a large internal medicine residency program. The survey was developed by the study team and reviewed for content and clarity by our chief residents and by 2 nationally known medical educators outside our institution. Study participants were internal medicine interns. Interns in this program rotate through 3 hospitals and do 7 to 8 ward months. The call schedules are different at each site (see Supporting Information, Appendix A, in the online version of this article). Opportunities for intrateam coverage of 1 intern by another include clinics (1/week), days off (1/week), some overnight periods, and occasional educational conferences. When possible, daily attending rounds include the entire team, but due to clinics, conferences, and days off, it is rare that the entire team is present. Bedside rounds are done at the discretion of the attending. The survey (see Supporting Information, Appendix B, in the online version of this article) included questions regarding situations when the respondent was covering his or her cointern's patients (cointern was defined as another intern on the respondent's same inpatient ward team). We also asked about situations when a cointern was covering the respondent's patients. For those questions, we considered answers of >60% to be a majority. We distributed this anonymous survey on 2 dates (January 2012 and March 2012) during regularly scheduled conferences. We mainly report descriptive findings. We also compared the percentage of study participants reporting problems when covering cointerns' patients to the percentage of study participants reporting problems when cointerns covered their (study participants') patients using 2, with significance set at P<0.05. This study was designated as exempt by the institutional review board.
RESULTS
Thirty‐four interns completed the survey out of a total of 44 interns present at the conferences (response rate=77%). There were 46 interns in the program, including categorical, medicine‐pediatrics, and preliminary interns. The mean age was 28 (standard deviation 2.8). Two‐thirds of respondents were female, and 65% were categorical.
Difference Between Intra‐ and Interteam Handoffs
Eighty‐eight percent felt that a handoff to a cointern was different than a handoff to an overnight cross‐cover intern; many interns said they assumed their cointerns had at least some knowledge of their patients, and therefore put less time and detail into their handoffs. When covering for their cointern, 47% reported feeling the same amount of responsibility as for their own patients, whereas 38% of interns reported feeling much or somewhat less responsible for their cointerns' patients and the remainder (15%) felt somewhat or much more responsible.
Knowledge of Cointern's Patients
Most (65%) interns reported at least 3 days in their last inpatient ward month when they covered a cointern's patient that had not been formally handed off to them. Forty‐five percent of respondents reported seldom or never receiving a written sign‐out on their cointern's patients.
Respondents were asked to think about times before they had covered their cointern's patients. Sixty‐eight percent of respondents reported knowing the number 1 problem for the majority of their cointern's patients. Twenty‐four percent reported having ever actually seen the majority of their cointern's patients. Only 3% of respondents said they had ever examined the majority of their cointern's patients prior to providing coverage.
Perceived Problems With Intrateam Coverage
While covering a cointern's patients, nearly half reported missing changes in patients' exams and forgetting to order labs or imaging. More than half reported unexpected family meetings or phone calls. In contrast, respondents noted more problems when their cointern had covered for them (Table 1). Seventy‐nine percent felt that patient care was at least sometimes delayed because of incomplete knowledge due to intrateam coverage.
| What Problems Have You Noticed | ||
|---|---|---|
| While Respondent Covers a Cointern's Patient? | After Respondent's Patients Were Covered by Cointern? | |
| ||
| Missed labs | 18% | 33% |
| Missed consult recommendations | 21% | 30% |
| Missed exam changes | 42% | 27% |
| Forgot to follow‐up imaging | 27% | 30% |
| Forgot to order labs or imaging | 42%a | 70%a |
| Failure to adjust meds | 27% | 27% |
| Unexpected family meeting/phone calls | 61%a | 30%a |
| Did not understand the plan from cointern's notes | 45% | 27% |
DISCUSSION
In our program, interns commonly cover for each other. This intrateam coverage frequently occurs without a formal handoff, and interns do not always know key information about their cointern's patients. Interns reported frequent problems with intrateam coverage such as missed lab results, consult recommendations, and changes in the physical exam. These missed items could result in delayed diagnoses and delayed treatment. These problems have been identified in interteam handoffs as well.[6, 7] Even in optimized interteam handoffs, receivers fail to identify the most important piece of information about 60% of the patients,[8] and our results mirror this finding.
The finding that fewer than a quarter of the respondents have ever seen the majority of their cointerns' patients is certainly of concern. This likely arises from several inter‐related factors: reduced hours for housestaff, schedules built to accommodate the reduced hours (eg, overlapping rather than simultaneous shifts), and the choice of some attendings to not take the entire team around to see every patient. In institutions where bedside rounds as a team are the norm, this finding will be less applicable, but others across the country have noticed this trend[9, 10] and have tried to counteract it.[11] This situation has both patient care and educational implications. The main patient care implication is that the other team members may be less able to seamlessly assume care when the primary intern is away or busy. Therefore, intrateam coverage becomes much more like traditional cross‐coverage of another team's patients, during which there is no expectation that the covering person will have ever seen the patients for whom they are assuming care. The main educational implication of not seeing the cointerns' patients is that the interns are seeing only half the patients that they could otherwise see. Learning medicine is experiential, and limiting opportunities for seeing and examining patients is unwise in this era of reduced time spent in the hospital.
Limitations of this study include being conducted in a single program. It will be important for other sites to assess their own practices with respect to intrateam handoffs. Another limitation is that it was a cross‐sectional survey subject to recall bias. We may have obtained more detailed information if we had conducted interviews. We also did not quantify the frequency of missed labs, consult recommendations, and physical examination changes that occurred during intrateam coverage. Finally, we did not independently verify the problems identified by the interns.
Some possible strategies to address this issue include (1) treating intrateam handoffs like interteam handoffs by implementing a formal system, (2) better utilizing senior residents/faculty when interns are covering for each other, (3) using bedside attending rounds to increase the exposure of all team members to the team's patients, (4) block scheduling to avoid absences due to clinics,[12] and (5) better communication and teamwork training to increase team awareness of all patients.[13]
Disclosures
Disclosures: There was no external funding for this work. However, this material is the result of work supported with resources and the use of facilities at the Clement J. Zablocki VA Medical Center, Milwaukee, WI. This work was presented in poster format at the national Society of Hospital Medicine meeting in National Harbor, Maryland in May 2013. The authors have no conflicts of interest to report.
- , , , et al. Residents' and attending physicians' handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775–1787.
- , , . Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , . Faculty member review and feedback using a sign‐out checklist: improving intern written sign‐out. Acad Med. 2012;87(8):1125–1131.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , . Transfers of patient care between house staff on internal medicine wards. Arch Intern Med. 2006;166:1173–1177.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care 2005;14(6):401–407.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , . Interns overestimate the effectiveness of their hand‐off communication. Pediatrics. 2010;125(3):491–496.
- . Culture shock—patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748–2751.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- , , , et al. The ambulatory long‐block: an accreditation council for graduate medical education (ACGME) educational innovations project (EIP). J Gen Intern Med. 2008;23(7):921–926.
- AHRQ. TeamSTEPPS: National Implementation. Available at: http://teamstepps.ahrq.gov/. Accessed June 19, 2014.
- , , , et al. Residents' and attending physicians' handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775–1787.
- , , . Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , . Faculty member review and feedback using a sign‐out checklist: improving intern written sign‐out. Acad Med. 2012;87(8):1125–1131.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , . Transfers of patient care between house staff on internal medicine wards. Arch Intern Med. 2006;166:1173–1177.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care 2005;14(6):401–407.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , . Interns overestimate the effectiveness of their hand‐off communication. Pediatrics. 2010;125(3):491–496.
- . Culture shock—patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748–2751.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- , , , et al. The ambulatory long‐block: an accreditation council for graduate medical education (ACGME) educational innovations project (EIP). J Gen Intern Med. 2008;23(7):921–926.
- AHRQ. TeamSTEPPS: National Implementation. Available at: http://teamstepps.ahrq.gov/. Accessed June 19, 2014.
Hospitalists' Use of PPIs
Proton pump inhibitors (PPIs) are commonly used to treat acid‐related disorders but are associated with an increased risk of pneumonia and Clostridium difficile‐associated diarrhea.[1, 2] Initiation of PPIs in hospitalized patients should therefore be limited to specific clinical situations, such as upper gastrointestinal bleeding or stress ulcer prophylaxis in the critically ill.[3] Prior studies suggest significant overuse of PPIs in hospitalized patients exists,[4, 5, 6, 7] but these were published before the widespread implementation of local and national quality improvement efforts targeted at reducing PPI use in medical inpatients (eg, Society of Hospital Medicine's Choosing Wisely list[8]). We aimed to determine the frequency of inappropriate use of PPIs in a contemporary cohort of hospitalized patients in a tertiary care academic medical center.
METHODS
We conducted a retrospective cohort study of 297 patients admitted to a tertiary care center hospitalist service comprised of teaching and nonteaching medical patients who were not critically ill, were admitted between January 1, 2012 and March 31, 2012, and received a PPI during their hospital stay. Three internists used American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy and prior studies to develop criteria to identify appropriate and inappropriate PPI use (Table 1).[4, 5, 6, 7] Appropriate indications included gastrointestinal (GI) bleeding, esophagitis, gastritis, gastroesophageal reflux (GERD), and continuation of home PPI (abrupt discontinuation can trigger reflux symptoms).[9] We extracted the medical records of included patients, applying our prespecified criteria to determine whether use was appropriate. In patients in whom PPI was a continued home medication, we also extracted 2 years of data prior to the index date to determine if the medication was started during a prior hospital admission and, if so, whether this initiation was appropriate. We used descriptive statistics and [2] tests to compare patient characteristics and indications for PPI use.
| Appropriate PPI use | Inappropriate PPI use |
|---|---|
| |
| History of upper GI bleeding | No reason given |
| Endoscopic evidence of peptic ulcer disease | Unspecified GI prophylaxis |
| Esophagitis | Nonspecific abdominal pain |
| Gastritis and duodenitis | Heartburn (nonchronic) |
| Eradication of H pylori | Acute pancreatitis |
| GERD | Anemia |
| Barrett's esophagus | Heparin use for DVT prophylaxis |
| Continued on home PPI | Use of aspirin, NSAID, steroids or Coumadin (as a single agent) |
| Acute esophageal variceal bleeding | |
| NSAID used in patient >65 years‐old | |
| High‐risk groups; combination of 2 or more of aspirin, NSAID, clopidogrel, or Coumadin | |
RESULTS
Of 297 patients, the mean age was 64.4 years (standard deviation 16.3 years), most were white (69%), and 56% were women (Table 2). PPI use was appropriate in 231 (78%, 95% confidence interval: 72.6%‐82.4%) patients. Of these, a majority (172, 75%) of patients received a PPI because it was a continued home medication. Only 40 of the 172 patients had the medication started during a recent hospitalization, and in half of those cases (20) the PPI use was appropriate.
| Demographics | PPI Not Indicated, N=66 | PPI Indicated, N=231 | Total=297 | |||
|---|---|---|---|---|---|---|
| ||||||
| Age, y, mean (SD) | 62.5 | (16.2) | 64.9 | (16.3) | 64.4 | (16.3) |
| Sex, % No. | ||||||
| Female | 51.5% | 34 | 56.7% | 131 | 55.6% | 165 |
| Male | 48.5% | 32 | 43.3% | 100 | 44.4% | 132 |
| Race, % No. | ||||||
| Asian | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Black | 10.6% | 7 | 9.1% | 21 | 9.4% | 28 |
| Hispanic | 18.2% | 12 | 19.5% | 45 | 19.2% | 57 |
| Unknown | 0.0% | 0 | 2.2% | 5 | 1.7% | 5 |
| White | 71.2% | 47 | 68.4% | 158 | 69.0% | 205 |
| Insurance, % No. | ||||||
| Insured | 95.5% | 63 | 87.4% | 202 | 89.2% | 265 |
| Uninsured | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Unknown | 4.5% | 3 | 11.7% | 27 | 10.1% | 30 |
| Service, % No. | ||||||
| Teaching | 25.8% | 17 | 32.9% | 76 | 31.3% | 93 |
| Nonteaching | 74.2% | 49 | 66.7% | 154 | 68.4% | 203 |
| Unknown | 0.0% | 0 | 0.4% | 1 | 0.3% | 1 |
| Chronic disease, % No. | ||||||
| Cardiac disease | 16.7% | 11 | 13.4% | 31 | 14.1% | 42 |
| Pulmonary disease | 16.7% | 11 | 14.7% | 34 | 15.2% | 45 |
| Gastrointestinal disease | 13.6% | 9 | 19.5% | 45 | 18.2% | 54 |
| Hepatic disease | 7.6% | 5 | 3.9% | 9 | 4.7% | 14 |
| Stroke | 1.5% | 1 | 5.2% | 12 | 4.4% | 13 |
| Sepsis | 12.1% | 8 | 13.0% | 30 | 12.8% | 38 |
| Other | 33.3% | 22 | 29.4% | 68 | 30.3% | 90 |
| PPI status, % No. | ||||||
| Continued home PPI | 0.0% | 0 | 74.5% | 172 | 58.1% | 172 |
| Started on PPI in hospital | 100% | 65 | 25.5% | 59 | 41.9% | 124 |
| Discharged on AST, % No. | ||||||
| Yes | 36.4% | 24 | 89.6% | 207 | 22.2% | 231 |
| PPI | 87.5% | 21 | 96.6% | 200 | 95.7% | 221 |
| Brand | 52.4% | 11 | 59.5% | 119 | 58.8% | 130 |
| Generic | 47.6% | 10 | 40.5% | 81 | 41.2% | 91 |
| H2 blocker | 12.5% | 3 | 3.4% | 7 | 4.3% | 10 |
| Brand | 0.0% | 0 | 71.4% | 5 | 50.0% | 5 |
| Generic | 100.0% | 3 | 28.6% | 2 | 50.0% | 5 |
| Medications, % No. | ||||||
| Aspirin | 36.4% | 24 | 43.7% | 101 | 42.1% | 125 |
| NSAID | 10.6% | 4 | 6.5% | 15 | 6.4% | 19 |
| Corticosteroids | 13.6% | 9 | 16.9% | 39 | 16.2% | 48 |
| Warfarin | 0.0% | 5 | 19.0% | 44 | 16.5% | 49 |
| Clopidogrel | 12.1% | 8 | 10.8% | 25 | 11.1% | 33 |
The second most common appropriate diagnosis was GERD (31%), followed by history of GI bleeding (19%) and treatment for esophagitis or gastritis (18%). Among the 66 patients receiving a PPI inappropriately, the majority of patients (56%) had no documented reason for PPI use, and only 11 patients (17%) were receiving PPI for stress ulcer prophylaxis (Figure 1). Five patients (8%) were treated prophylactically because of steroid or anticoagulant use. We observed no differences in age, gender, race, or reason for admission between the patients treated appropriately versus inappropriately.

DISCUSSION
In a contemporary cohort, chronic PPI use prior to admission was the most common reason PPIs were prescribed in the hospital. About 20% of hospitalized patients were started on a PPI for an inappropriate indication, the majority of whom lacked documentation concerning the reason for use. Among patients treated inappropriately, 36% were discharged on acid‐suppressive therapy.
The prior literature has reported a much higher percentages of unnecessary PPI use in hospitalized patients.[4, 5, 6, 7] Gupta et al. found that 70% of patients admitted to an internal medicine service received acid‐suppressive therapy, 73% of whom were treated unnecessarily.[5] Similarly, Nardino et al. found that 65% of acid‐suppressive therapy in hospitalized medical patients was not indicated.[4] If we had excluded patients on home PPIs from our study cohort, we would have found a higher rate of inappropriate use due to a smaller overall patient population. However, we chose to include these patients because they represented the vast majority of hospitalist‐prescribed PPIs. Notably, most of these prior prescriptions were not written during a recent hospital stay, indicating that the majority were initiated by outpatient physicians.
Our study is limited by its small sample size, single‐center design, and inability to determine the indications for outpatient PPI use. Still, it has important implications. Prior work has suggested that focusing efforts on PPI overuse may be premature in the absence of valid risk‐prediction models defining the patient populations that most benefit from PPI therapy.[10] Our work additionally suggests that hospital rates of inappropriate initiation may be relatively low, perhaps because hospitalist culture and practice have been affected by both local and national quality improvement efforts and by evidence dissemination.[8] Quality improvement efforts focused on reducing inpatient PPI use are likely to reveal diminishing returns, as admitting hospitalists are unlikely to abruptly discontinue PPIs prescribed in the outpatient setting.[9] Hospitalists should be encouraged to assess and document the need for PPIs during admission, hospitalization, and discharge processes. However, future efforts to reduce PPI overuse among hospitalized patients should predominately be focused on reducing inappropriate chronic PPI use in the outpatient setting.
Acknowledgements
The authors acknowledge Peter Lindenauer for his comments on an earlier draft of this manuscript.
Disclosures: The study was conducted with funding from the Department of Medicine at Baystate Medical Center. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Albugeaey had full access to all of the data in the study, and they take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Albugeaey, and Seiler conceived of the study. Drs. Albugeaey and Al Faraj acquired the data. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb analyzed and interpreted the data. Drs. Albugeaey and Lagu drafted the manuscript. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Albugeaey is a recipient of a scholarship from the Ministry of Higher Education, Kingdom of Saudi Arabia. The authors report no conflicts of interest.
- , , , , . Acid‐suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991–997.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , . Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–360; quiz 361.
- , , . Overuse of acid‐suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118–3122.
- , , , et al. Overuse of acid suppression therapy in hospitalized patients. South Med J. 2010;103(3):207–211.
- , , , , , . Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7(5):421–425.
- , , , . Inappropriate utilization of intravenous proton pump inhibitors in hospital practice—a prospective study of the extent of the problem and predictive factors. QJM. 2010;103(5):327–335.
- Choosing Wisely, Society of Hospital Medicine, Adult Hospital Medicine. Available at:http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed April 11, 2014.
- , , , . Safety of the long‐term use of proton pump inhibitors. World J Gastroenterol. 2010;16(19):2323–2330.
- , . Prophylaxis rates for venous thromboembolism and gastrointestinal bleeding in general medical patients: too low or too high? BMJ. 2012;344:e3248.
Proton pump inhibitors (PPIs) are commonly used to treat acid‐related disorders but are associated with an increased risk of pneumonia and Clostridium difficile‐associated diarrhea.[1, 2] Initiation of PPIs in hospitalized patients should therefore be limited to specific clinical situations, such as upper gastrointestinal bleeding or stress ulcer prophylaxis in the critically ill.[3] Prior studies suggest significant overuse of PPIs in hospitalized patients exists,[4, 5, 6, 7] but these were published before the widespread implementation of local and national quality improvement efforts targeted at reducing PPI use in medical inpatients (eg, Society of Hospital Medicine's Choosing Wisely list[8]). We aimed to determine the frequency of inappropriate use of PPIs in a contemporary cohort of hospitalized patients in a tertiary care academic medical center.
METHODS
We conducted a retrospective cohort study of 297 patients admitted to a tertiary care center hospitalist service comprised of teaching and nonteaching medical patients who were not critically ill, were admitted between January 1, 2012 and March 31, 2012, and received a PPI during their hospital stay. Three internists used American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy and prior studies to develop criteria to identify appropriate and inappropriate PPI use (Table 1).[4, 5, 6, 7] Appropriate indications included gastrointestinal (GI) bleeding, esophagitis, gastritis, gastroesophageal reflux (GERD), and continuation of home PPI (abrupt discontinuation can trigger reflux symptoms).[9] We extracted the medical records of included patients, applying our prespecified criteria to determine whether use was appropriate. In patients in whom PPI was a continued home medication, we also extracted 2 years of data prior to the index date to determine if the medication was started during a prior hospital admission and, if so, whether this initiation was appropriate. We used descriptive statistics and [2] tests to compare patient characteristics and indications for PPI use.
| Appropriate PPI use | Inappropriate PPI use |
|---|---|
| |
| History of upper GI bleeding | No reason given |
| Endoscopic evidence of peptic ulcer disease | Unspecified GI prophylaxis |
| Esophagitis | Nonspecific abdominal pain |
| Gastritis and duodenitis | Heartburn (nonchronic) |
| Eradication of H pylori | Acute pancreatitis |
| GERD | Anemia |
| Barrett's esophagus | Heparin use for DVT prophylaxis |
| Continued on home PPI | Use of aspirin, NSAID, steroids or Coumadin (as a single agent) |
| Acute esophageal variceal bleeding | |
| NSAID used in patient >65 years‐old | |
| High‐risk groups; combination of 2 or more of aspirin, NSAID, clopidogrel, or Coumadin | |
RESULTS
Of 297 patients, the mean age was 64.4 years (standard deviation 16.3 years), most were white (69%), and 56% were women (Table 2). PPI use was appropriate in 231 (78%, 95% confidence interval: 72.6%‐82.4%) patients. Of these, a majority (172, 75%) of patients received a PPI because it was a continued home medication. Only 40 of the 172 patients had the medication started during a recent hospitalization, and in half of those cases (20) the PPI use was appropriate.
| Demographics | PPI Not Indicated, N=66 | PPI Indicated, N=231 | Total=297 | |||
|---|---|---|---|---|---|---|
| ||||||
| Age, y, mean (SD) | 62.5 | (16.2) | 64.9 | (16.3) | 64.4 | (16.3) |
| Sex, % No. | ||||||
| Female | 51.5% | 34 | 56.7% | 131 | 55.6% | 165 |
| Male | 48.5% | 32 | 43.3% | 100 | 44.4% | 132 |
| Race, % No. | ||||||
| Asian | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Black | 10.6% | 7 | 9.1% | 21 | 9.4% | 28 |
| Hispanic | 18.2% | 12 | 19.5% | 45 | 19.2% | 57 |
| Unknown | 0.0% | 0 | 2.2% | 5 | 1.7% | 5 |
| White | 71.2% | 47 | 68.4% | 158 | 69.0% | 205 |
| Insurance, % No. | ||||||
| Insured | 95.5% | 63 | 87.4% | 202 | 89.2% | 265 |
| Uninsured | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Unknown | 4.5% | 3 | 11.7% | 27 | 10.1% | 30 |
| Service, % No. | ||||||
| Teaching | 25.8% | 17 | 32.9% | 76 | 31.3% | 93 |
| Nonteaching | 74.2% | 49 | 66.7% | 154 | 68.4% | 203 |
| Unknown | 0.0% | 0 | 0.4% | 1 | 0.3% | 1 |
| Chronic disease, % No. | ||||||
| Cardiac disease | 16.7% | 11 | 13.4% | 31 | 14.1% | 42 |
| Pulmonary disease | 16.7% | 11 | 14.7% | 34 | 15.2% | 45 |
| Gastrointestinal disease | 13.6% | 9 | 19.5% | 45 | 18.2% | 54 |
| Hepatic disease | 7.6% | 5 | 3.9% | 9 | 4.7% | 14 |
| Stroke | 1.5% | 1 | 5.2% | 12 | 4.4% | 13 |
| Sepsis | 12.1% | 8 | 13.0% | 30 | 12.8% | 38 |
| Other | 33.3% | 22 | 29.4% | 68 | 30.3% | 90 |
| PPI status, % No. | ||||||
| Continued home PPI | 0.0% | 0 | 74.5% | 172 | 58.1% | 172 |
| Started on PPI in hospital | 100% | 65 | 25.5% | 59 | 41.9% | 124 |
| Discharged on AST, % No. | ||||||
| Yes | 36.4% | 24 | 89.6% | 207 | 22.2% | 231 |
| PPI | 87.5% | 21 | 96.6% | 200 | 95.7% | 221 |
| Brand | 52.4% | 11 | 59.5% | 119 | 58.8% | 130 |
| Generic | 47.6% | 10 | 40.5% | 81 | 41.2% | 91 |
| H2 blocker | 12.5% | 3 | 3.4% | 7 | 4.3% | 10 |
| Brand | 0.0% | 0 | 71.4% | 5 | 50.0% | 5 |
| Generic | 100.0% | 3 | 28.6% | 2 | 50.0% | 5 |
| Medications, % No. | ||||||
| Aspirin | 36.4% | 24 | 43.7% | 101 | 42.1% | 125 |
| NSAID | 10.6% | 4 | 6.5% | 15 | 6.4% | 19 |
| Corticosteroids | 13.6% | 9 | 16.9% | 39 | 16.2% | 48 |
| Warfarin | 0.0% | 5 | 19.0% | 44 | 16.5% | 49 |
| Clopidogrel | 12.1% | 8 | 10.8% | 25 | 11.1% | 33 |
The second most common appropriate diagnosis was GERD (31%), followed by history of GI bleeding (19%) and treatment for esophagitis or gastritis (18%). Among the 66 patients receiving a PPI inappropriately, the majority of patients (56%) had no documented reason for PPI use, and only 11 patients (17%) were receiving PPI for stress ulcer prophylaxis (Figure 1). Five patients (8%) were treated prophylactically because of steroid or anticoagulant use. We observed no differences in age, gender, race, or reason for admission between the patients treated appropriately versus inappropriately.

DISCUSSION
In a contemporary cohort, chronic PPI use prior to admission was the most common reason PPIs were prescribed in the hospital. About 20% of hospitalized patients were started on a PPI for an inappropriate indication, the majority of whom lacked documentation concerning the reason for use. Among patients treated inappropriately, 36% were discharged on acid‐suppressive therapy.
The prior literature has reported a much higher percentages of unnecessary PPI use in hospitalized patients.[4, 5, 6, 7] Gupta et al. found that 70% of patients admitted to an internal medicine service received acid‐suppressive therapy, 73% of whom were treated unnecessarily.[5] Similarly, Nardino et al. found that 65% of acid‐suppressive therapy in hospitalized medical patients was not indicated.[4] If we had excluded patients on home PPIs from our study cohort, we would have found a higher rate of inappropriate use due to a smaller overall patient population. However, we chose to include these patients because they represented the vast majority of hospitalist‐prescribed PPIs. Notably, most of these prior prescriptions were not written during a recent hospital stay, indicating that the majority were initiated by outpatient physicians.
Our study is limited by its small sample size, single‐center design, and inability to determine the indications for outpatient PPI use. Still, it has important implications. Prior work has suggested that focusing efforts on PPI overuse may be premature in the absence of valid risk‐prediction models defining the patient populations that most benefit from PPI therapy.[10] Our work additionally suggests that hospital rates of inappropriate initiation may be relatively low, perhaps because hospitalist culture and practice have been affected by both local and national quality improvement efforts and by evidence dissemination.[8] Quality improvement efforts focused on reducing inpatient PPI use are likely to reveal diminishing returns, as admitting hospitalists are unlikely to abruptly discontinue PPIs prescribed in the outpatient setting.[9] Hospitalists should be encouraged to assess and document the need for PPIs during admission, hospitalization, and discharge processes. However, future efforts to reduce PPI overuse among hospitalized patients should predominately be focused on reducing inappropriate chronic PPI use in the outpatient setting.
Acknowledgements
The authors acknowledge Peter Lindenauer for his comments on an earlier draft of this manuscript.
Disclosures: The study was conducted with funding from the Department of Medicine at Baystate Medical Center. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Albugeaey had full access to all of the data in the study, and they take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Albugeaey, and Seiler conceived of the study. Drs. Albugeaey and Al Faraj acquired the data. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb analyzed and interpreted the data. Drs. Albugeaey and Lagu drafted the manuscript. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Albugeaey is a recipient of a scholarship from the Ministry of Higher Education, Kingdom of Saudi Arabia. The authors report no conflicts of interest.
Proton pump inhibitors (PPIs) are commonly used to treat acid‐related disorders but are associated with an increased risk of pneumonia and Clostridium difficile‐associated diarrhea.[1, 2] Initiation of PPIs in hospitalized patients should therefore be limited to specific clinical situations, such as upper gastrointestinal bleeding or stress ulcer prophylaxis in the critically ill.[3] Prior studies suggest significant overuse of PPIs in hospitalized patients exists,[4, 5, 6, 7] but these were published before the widespread implementation of local and national quality improvement efforts targeted at reducing PPI use in medical inpatients (eg, Society of Hospital Medicine's Choosing Wisely list[8]). We aimed to determine the frequency of inappropriate use of PPIs in a contemporary cohort of hospitalized patients in a tertiary care academic medical center.
METHODS
We conducted a retrospective cohort study of 297 patients admitted to a tertiary care center hospitalist service comprised of teaching and nonteaching medical patients who were not critically ill, were admitted between January 1, 2012 and March 31, 2012, and received a PPI during their hospital stay. Three internists used American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy and prior studies to develop criteria to identify appropriate and inappropriate PPI use (Table 1).[4, 5, 6, 7] Appropriate indications included gastrointestinal (GI) bleeding, esophagitis, gastritis, gastroesophageal reflux (GERD), and continuation of home PPI (abrupt discontinuation can trigger reflux symptoms).[9] We extracted the medical records of included patients, applying our prespecified criteria to determine whether use was appropriate. In patients in whom PPI was a continued home medication, we also extracted 2 years of data prior to the index date to determine if the medication was started during a prior hospital admission and, if so, whether this initiation was appropriate. We used descriptive statistics and [2] tests to compare patient characteristics and indications for PPI use.
| Appropriate PPI use | Inappropriate PPI use |
|---|---|
| |
| History of upper GI bleeding | No reason given |
| Endoscopic evidence of peptic ulcer disease | Unspecified GI prophylaxis |
| Esophagitis | Nonspecific abdominal pain |
| Gastritis and duodenitis | Heartburn (nonchronic) |
| Eradication of H pylori | Acute pancreatitis |
| GERD | Anemia |
| Barrett's esophagus | Heparin use for DVT prophylaxis |
| Continued on home PPI | Use of aspirin, NSAID, steroids or Coumadin (as a single agent) |
| Acute esophageal variceal bleeding | |
| NSAID used in patient >65 years‐old | |
| High‐risk groups; combination of 2 or more of aspirin, NSAID, clopidogrel, or Coumadin | |
RESULTS
Of 297 patients, the mean age was 64.4 years (standard deviation 16.3 years), most were white (69%), and 56% were women (Table 2). PPI use was appropriate in 231 (78%, 95% confidence interval: 72.6%‐82.4%) patients. Of these, a majority (172, 75%) of patients received a PPI because it was a continued home medication. Only 40 of the 172 patients had the medication started during a recent hospitalization, and in half of those cases (20) the PPI use was appropriate.
| Demographics | PPI Not Indicated, N=66 | PPI Indicated, N=231 | Total=297 | |||
|---|---|---|---|---|---|---|
| ||||||
| Age, y, mean (SD) | 62.5 | (16.2) | 64.9 | (16.3) | 64.4 | (16.3) |
| Sex, % No. | ||||||
| Female | 51.5% | 34 | 56.7% | 131 | 55.6% | 165 |
| Male | 48.5% | 32 | 43.3% | 100 | 44.4% | 132 |
| Race, % No. | ||||||
| Asian | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Black | 10.6% | 7 | 9.1% | 21 | 9.4% | 28 |
| Hispanic | 18.2% | 12 | 19.5% | 45 | 19.2% | 57 |
| Unknown | 0.0% | 0 | 2.2% | 5 | 1.7% | 5 |
| White | 71.2% | 47 | 68.4% | 158 | 69.0% | 205 |
| Insurance, % No. | ||||||
| Insured | 95.5% | 63 | 87.4% | 202 | 89.2% | 265 |
| Uninsured | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Unknown | 4.5% | 3 | 11.7% | 27 | 10.1% | 30 |
| Service, % No. | ||||||
| Teaching | 25.8% | 17 | 32.9% | 76 | 31.3% | 93 |
| Nonteaching | 74.2% | 49 | 66.7% | 154 | 68.4% | 203 |
| Unknown | 0.0% | 0 | 0.4% | 1 | 0.3% | 1 |
| Chronic disease, % No. | ||||||
| Cardiac disease | 16.7% | 11 | 13.4% | 31 | 14.1% | 42 |
| Pulmonary disease | 16.7% | 11 | 14.7% | 34 | 15.2% | 45 |
| Gastrointestinal disease | 13.6% | 9 | 19.5% | 45 | 18.2% | 54 |
| Hepatic disease | 7.6% | 5 | 3.9% | 9 | 4.7% | 14 |
| Stroke | 1.5% | 1 | 5.2% | 12 | 4.4% | 13 |
| Sepsis | 12.1% | 8 | 13.0% | 30 | 12.8% | 38 |
| Other | 33.3% | 22 | 29.4% | 68 | 30.3% | 90 |
| PPI status, % No. | ||||||
| Continued home PPI | 0.0% | 0 | 74.5% | 172 | 58.1% | 172 |
| Started on PPI in hospital | 100% | 65 | 25.5% | 59 | 41.9% | 124 |
| Discharged on AST, % No. | ||||||
| Yes | 36.4% | 24 | 89.6% | 207 | 22.2% | 231 |
| PPI | 87.5% | 21 | 96.6% | 200 | 95.7% | 221 |
| Brand | 52.4% | 11 | 59.5% | 119 | 58.8% | 130 |
| Generic | 47.6% | 10 | 40.5% | 81 | 41.2% | 91 |
| H2 blocker | 12.5% | 3 | 3.4% | 7 | 4.3% | 10 |
| Brand | 0.0% | 0 | 71.4% | 5 | 50.0% | 5 |
| Generic | 100.0% | 3 | 28.6% | 2 | 50.0% | 5 |
| Medications, % No. | ||||||
| Aspirin | 36.4% | 24 | 43.7% | 101 | 42.1% | 125 |
| NSAID | 10.6% | 4 | 6.5% | 15 | 6.4% | 19 |
| Corticosteroids | 13.6% | 9 | 16.9% | 39 | 16.2% | 48 |
| Warfarin | 0.0% | 5 | 19.0% | 44 | 16.5% | 49 |
| Clopidogrel | 12.1% | 8 | 10.8% | 25 | 11.1% | 33 |
The second most common appropriate diagnosis was GERD (31%), followed by history of GI bleeding (19%) and treatment for esophagitis or gastritis (18%). Among the 66 patients receiving a PPI inappropriately, the majority of patients (56%) had no documented reason for PPI use, and only 11 patients (17%) were receiving PPI for stress ulcer prophylaxis (Figure 1). Five patients (8%) were treated prophylactically because of steroid or anticoagulant use. We observed no differences in age, gender, race, or reason for admission between the patients treated appropriately versus inappropriately.

DISCUSSION
In a contemporary cohort, chronic PPI use prior to admission was the most common reason PPIs were prescribed in the hospital. About 20% of hospitalized patients were started on a PPI for an inappropriate indication, the majority of whom lacked documentation concerning the reason for use. Among patients treated inappropriately, 36% were discharged on acid‐suppressive therapy.
The prior literature has reported a much higher percentages of unnecessary PPI use in hospitalized patients.[4, 5, 6, 7] Gupta et al. found that 70% of patients admitted to an internal medicine service received acid‐suppressive therapy, 73% of whom were treated unnecessarily.[5] Similarly, Nardino et al. found that 65% of acid‐suppressive therapy in hospitalized medical patients was not indicated.[4] If we had excluded patients on home PPIs from our study cohort, we would have found a higher rate of inappropriate use due to a smaller overall patient population. However, we chose to include these patients because they represented the vast majority of hospitalist‐prescribed PPIs. Notably, most of these prior prescriptions were not written during a recent hospital stay, indicating that the majority were initiated by outpatient physicians.
Our study is limited by its small sample size, single‐center design, and inability to determine the indications for outpatient PPI use. Still, it has important implications. Prior work has suggested that focusing efforts on PPI overuse may be premature in the absence of valid risk‐prediction models defining the patient populations that most benefit from PPI therapy.[10] Our work additionally suggests that hospital rates of inappropriate initiation may be relatively low, perhaps because hospitalist culture and practice have been affected by both local and national quality improvement efforts and by evidence dissemination.[8] Quality improvement efforts focused on reducing inpatient PPI use are likely to reveal diminishing returns, as admitting hospitalists are unlikely to abruptly discontinue PPIs prescribed in the outpatient setting.[9] Hospitalists should be encouraged to assess and document the need for PPIs during admission, hospitalization, and discharge processes. However, future efforts to reduce PPI overuse among hospitalized patients should predominately be focused on reducing inappropriate chronic PPI use in the outpatient setting.
Acknowledgements
The authors acknowledge Peter Lindenauer for his comments on an earlier draft of this manuscript.
Disclosures: The study was conducted with funding from the Department of Medicine at Baystate Medical Center. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Albugeaey had full access to all of the data in the study, and they take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Albugeaey, and Seiler conceived of the study. Drs. Albugeaey and Al Faraj acquired the data. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb analyzed and interpreted the data. Drs. Albugeaey and Lagu drafted the manuscript. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Albugeaey is a recipient of a scholarship from the Ministry of Higher Education, Kingdom of Saudi Arabia. The authors report no conflicts of interest.
- , , , , . Acid‐suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991–997.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , . Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–360; quiz 361.
- , , . Overuse of acid‐suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118–3122.
- , , , et al. Overuse of acid suppression therapy in hospitalized patients. South Med J. 2010;103(3):207–211.
- , , , , , . Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7(5):421–425.
- , , , . Inappropriate utilization of intravenous proton pump inhibitors in hospital practice—a prospective study of the extent of the problem and predictive factors. QJM. 2010;103(5):327–335.
- Choosing Wisely, Society of Hospital Medicine, Adult Hospital Medicine. Available at:http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed April 11, 2014.
- , , , . Safety of the long‐term use of proton pump inhibitors. World J Gastroenterol. 2010;16(19):2323–2330.
- , . Prophylaxis rates for venous thromboembolism and gastrointestinal bleeding in general medical patients: too low or too high? BMJ. 2012;344:e3248.
- , , , , . Acid‐suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991–997.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , . Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–360; quiz 361.
- , , . Overuse of acid‐suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118–3122.
- , , , et al. Overuse of acid suppression therapy in hospitalized patients. South Med J. 2010;103(3):207–211.
- , , , , , . Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7(5):421–425.
- , , , . Inappropriate utilization of intravenous proton pump inhibitors in hospital practice—a prospective study of the extent of the problem and predictive factors. QJM. 2010;103(5):327–335.
- Choosing Wisely, Society of Hospital Medicine, Adult Hospital Medicine. Available at:http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed April 11, 2014.
- , , , . Safety of the long‐term use of proton pump inhibitors. World J Gastroenterol. 2010;16(19):2323–2330.
- , . Prophylaxis rates for venous thromboembolism and gastrointestinal bleeding in general medical patients: too low or too high? BMJ. 2012;344:e3248.
Trends in Blood‐Product Transfusion
Although potentially life saving, blood‐product transfusion is costly and associated with transfusion‐related adverse events, including death on rare occasions. Studies in varied patient populations have demonstrated that a restrictive red blood cell transfusion strategy reduces the number of transfusion‐related adverse effects and can result in improved short‐term survival.[1, 2, 3] In 2011, more than 20 million blood products were transfused in the United States, which resulted in more than 50,000 transfusion‐related adverse reactions (0.24%).[4] With a mean cost of greater than $50 per unit of plasma and $500 per unit of apheresis platelets,[4] the cost of blood transfusion is well in excess of $1 billion per year. Blood‐product transfusion is the most frequent inpatient procedure,[5] and inpatient blood‐product transfusion contributes to the bulk of transfusions nationwide. To study the utilization of blood‐product transfusion in the inpatient population, we studied the temporal trend of inpatient blood‐product transfusions in the United States from 2002 to 2011 using data from the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.[4] The NIS, the largest inpatient care database in the United States, includes approximately a 20% stratified sample of US community hospital admissions and is weighted at discharge level to permit population‐level estimates.[6] We utilized this database to identify the total number of blood‐product transfusions and discharges between 2002 and 2011. We calculated the rate of all blood‐product transfusions, which include packed red blood cell, platelets, and other blood components, using the International Classification of DiseasesNinth Revision, Clinical Modification Procedural Clinical Classification Software code 222.[7] Trend analysis and calculation of average annual percent change were done using the Joinpoint Regression Program version 4.0.4 (National Cancer Institute, Bethesda, MD).[8] This software uses trend data and calculates the best fit lines to create the simplest joinpoint model that the data allow. The model can be expressed as a figure where several different multisegmented trend lines are connected together at the joinpoints. Trend over a fixed prespecified interval was computed as average annual percent change, and the Monte Carlo permutation method was used to test for apparent change in the trends.[9, 10] The study was exempted by the institutional review board of the University of Nebraska Medical Center.
Between 2002 and 2011, there were a total of 24,641,581 blood‐product transfusions among 389,761,571 hospitalizations. The rate of transfusion per 100 hospitalizations increased by 2.9% from 2002 to 2011 (4.6% in 2002 [n=1,767,111] to 7.5% in 2011 [n=2,929,312]) (Figure 1). The average annual percent change from 2002 to 2011 was 5.6% (95% confidence interval [CI]: 3.7‐7.6), which was statistically significant at P<0.05. A statistically significant change in trend (joinpoint) was observed in 2004. The annual percent change was 11.2% (95% CI: 0.323.4) from 2002 to 2004 and 4.1% (95% CI: 3.05.1) from 2004 to 2011, both of which were statistically significant at P<0.05 (Figure 2).
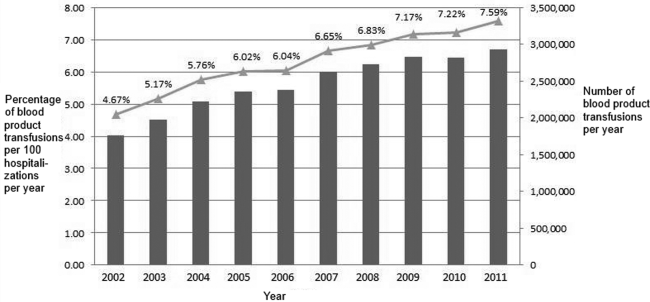
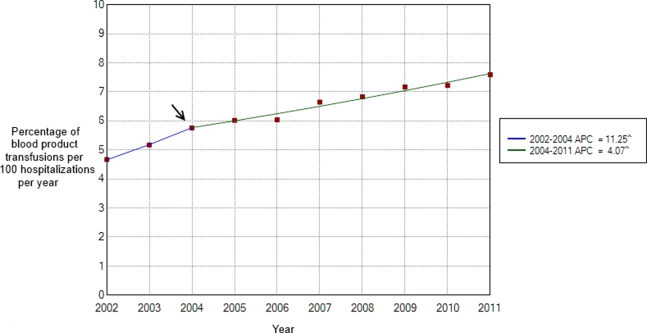
Our study demonstrates an overall increasing trend in the inpatient blood‐product transfusions over the past decade. However, the rate of increase seems to have slowed down since 2004. The National Blood Collection and Utilization Survey[4] demonstrated a decrease of 11.6% in the total number of all components transfused in the United States between 2008 and 2011. Our data are different from the survey, which also included blood transfusions in outpatient settings, emergency departments, and pediatric patients. The rising proportion of aging population with multiple comorbidities and cancers, increases in hematopoietic stem cell/solid organ transplants and chemotherapy, as well as widespread availability of blood products presumably contributed to the continued increase observed in our inpatient data after 2004. Nevertheless, the declining trend in the rate of the increased blood‐product transfusion usage seen after 2004 is encouraging. Increased awareness of restrictive transfusion strategy, coupled with efforts by professional bodies to improve the adoption of restrictive strategies, is most likely responsible for this.[3, 11, 12] As the clinical classification software procedure code 222 lumps together all the different types of blood products, we were unable to study the transfusion trend among each different type of blood products. In conclusion, further efforts need to be directed at increasing the awareness of clinicians, especially hospitalists, about the benefits of a restrictive transfusion policy and decreasing the rate of blood product use in the inpatient service. Furthermore, studies elaborating the patient population who are being transfused and the factors influencing the transfusion trends can provide useful insights to optimize blood‐product utilization and control resource consumption.
Disclosure
Nothing to report.
- , , . Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309(1):83–84.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
- , , , . Evidence review: periprocedural use of blood products. J Hosp Med. 2013;8(11):647–652.
- The 2011 National Blood Collection and Utilization Survey Report. Washington, DC: U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health; 2013.
- , , , et al. HCUP facts and figures: statistics on hospital‐based care in the United States. 2009. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed January 2, 2014.
- HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 15, 2013.
- HCUP Clinical Classifications Software (CCS) for ICD‐9‐CM. Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed December 15, 2013.
- Joinpoint Regression Program, Version 4.0.4, December, 2014. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Available at: https://surveillance.cancer.gov/joinpoint/download. Accessed December 25, 2013.
- , , , , . Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682.
- , , , . Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , . Patient‐centered blood management. J Hosp Med. 2014;9(1):60–65.
Although potentially life saving, blood‐product transfusion is costly and associated with transfusion‐related adverse events, including death on rare occasions. Studies in varied patient populations have demonstrated that a restrictive red blood cell transfusion strategy reduces the number of transfusion‐related adverse effects and can result in improved short‐term survival.[1, 2, 3] In 2011, more than 20 million blood products were transfused in the United States, which resulted in more than 50,000 transfusion‐related adverse reactions (0.24%).[4] With a mean cost of greater than $50 per unit of plasma and $500 per unit of apheresis platelets,[4] the cost of blood transfusion is well in excess of $1 billion per year. Blood‐product transfusion is the most frequent inpatient procedure,[5] and inpatient blood‐product transfusion contributes to the bulk of transfusions nationwide. To study the utilization of blood‐product transfusion in the inpatient population, we studied the temporal trend of inpatient blood‐product transfusions in the United States from 2002 to 2011 using data from the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.[4] The NIS, the largest inpatient care database in the United States, includes approximately a 20% stratified sample of US community hospital admissions and is weighted at discharge level to permit population‐level estimates.[6] We utilized this database to identify the total number of blood‐product transfusions and discharges between 2002 and 2011. We calculated the rate of all blood‐product transfusions, which include packed red blood cell, platelets, and other blood components, using the International Classification of DiseasesNinth Revision, Clinical Modification Procedural Clinical Classification Software code 222.[7] Trend analysis and calculation of average annual percent change were done using the Joinpoint Regression Program version 4.0.4 (National Cancer Institute, Bethesda, MD).[8] This software uses trend data and calculates the best fit lines to create the simplest joinpoint model that the data allow. The model can be expressed as a figure where several different multisegmented trend lines are connected together at the joinpoints. Trend over a fixed prespecified interval was computed as average annual percent change, and the Monte Carlo permutation method was used to test for apparent change in the trends.[9, 10] The study was exempted by the institutional review board of the University of Nebraska Medical Center.
Between 2002 and 2011, there were a total of 24,641,581 blood‐product transfusions among 389,761,571 hospitalizations. The rate of transfusion per 100 hospitalizations increased by 2.9% from 2002 to 2011 (4.6% in 2002 [n=1,767,111] to 7.5% in 2011 [n=2,929,312]) (Figure 1). The average annual percent change from 2002 to 2011 was 5.6% (95% confidence interval [CI]: 3.7‐7.6), which was statistically significant at P<0.05. A statistically significant change in trend (joinpoint) was observed in 2004. The annual percent change was 11.2% (95% CI: 0.323.4) from 2002 to 2004 and 4.1% (95% CI: 3.05.1) from 2004 to 2011, both of which were statistically significant at P<0.05 (Figure 2).


Our study demonstrates an overall increasing trend in the inpatient blood‐product transfusions over the past decade. However, the rate of increase seems to have slowed down since 2004. The National Blood Collection and Utilization Survey[4] demonstrated a decrease of 11.6% in the total number of all components transfused in the United States between 2008 and 2011. Our data are different from the survey, which also included blood transfusions in outpatient settings, emergency departments, and pediatric patients. The rising proportion of aging population with multiple comorbidities and cancers, increases in hematopoietic stem cell/solid organ transplants and chemotherapy, as well as widespread availability of blood products presumably contributed to the continued increase observed in our inpatient data after 2004. Nevertheless, the declining trend in the rate of the increased blood‐product transfusion usage seen after 2004 is encouraging. Increased awareness of restrictive transfusion strategy, coupled with efforts by professional bodies to improve the adoption of restrictive strategies, is most likely responsible for this.[3, 11, 12] As the clinical classification software procedure code 222 lumps together all the different types of blood products, we were unable to study the transfusion trend among each different type of blood products. In conclusion, further efforts need to be directed at increasing the awareness of clinicians, especially hospitalists, about the benefits of a restrictive transfusion policy and decreasing the rate of blood product use in the inpatient service. Furthermore, studies elaborating the patient population who are being transfused and the factors influencing the transfusion trends can provide useful insights to optimize blood‐product utilization and control resource consumption.
Disclosure
Nothing to report.
Although potentially life saving, blood‐product transfusion is costly and associated with transfusion‐related adverse events, including death on rare occasions. Studies in varied patient populations have demonstrated that a restrictive red blood cell transfusion strategy reduces the number of transfusion‐related adverse effects and can result in improved short‐term survival.[1, 2, 3] In 2011, more than 20 million blood products were transfused in the United States, which resulted in more than 50,000 transfusion‐related adverse reactions (0.24%).[4] With a mean cost of greater than $50 per unit of plasma and $500 per unit of apheresis platelets,[4] the cost of blood transfusion is well in excess of $1 billion per year. Blood‐product transfusion is the most frequent inpatient procedure,[5] and inpatient blood‐product transfusion contributes to the bulk of transfusions nationwide. To study the utilization of blood‐product transfusion in the inpatient population, we studied the temporal trend of inpatient blood‐product transfusions in the United States from 2002 to 2011 using data from the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.[4] The NIS, the largest inpatient care database in the United States, includes approximately a 20% stratified sample of US community hospital admissions and is weighted at discharge level to permit population‐level estimates.[6] We utilized this database to identify the total number of blood‐product transfusions and discharges between 2002 and 2011. We calculated the rate of all blood‐product transfusions, which include packed red blood cell, platelets, and other blood components, using the International Classification of DiseasesNinth Revision, Clinical Modification Procedural Clinical Classification Software code 222.[7] Trend analysis and calculation of average annual percent change were done using the Joinpoint Regression Program version 4.0.4 (National Cancer Institute, Bethesda, MD).[8] This software uses trend data and calculates the best fit lines to create the simplest joinpoint model that the data allow. The model can be expressed as a figure where several different multisegmented trend lines are connected together at the joinpoints. Trend over a fixed prespecified interval was computed as average annual percent change, and the Monte Carlo permutation method was used to test for apparent change in the trends.[9, 10] The study was exempted by the institutional review board of the University of Nebraska Medical Center.
Between 2002 and 2011, there were a total of 24,641,581 blood‐product transfusions among 389,761,571 hospitalizations. The rate of transfusion per 100 hospitalizations increased by 2.9% from 2002 to 2011 (4.6% in 2002 [n=1,767,111] to 7.5% in 2011 [n=2,929,312]) (Figure 1). The average annual percent change from 2002 to 2011 was 5.6% (95% confidence interval [CI]: 3.7‐7.6), which was statistically significant at P<0.05. A statistically significant change in trend (joinpoint) was observed in 2004. The annual percent change was 11.2% (95% CI: 0.323.4) from 2002 to 2004 and 4.1% (95% CI: 3.05.1) from 2004 to 2011, both of which were statistically significant at P<0.05 (Figure 2).


Our study demonstrates an overall increasing trend in the inpatient blood‐product transfusions over the past decade. However, the rate of increase seems to have slowed down since 2004. The National Blood Collection and Utilization Survey[4] demonstrated a decrease of 11.6% in the total number of all components transfused in the United States between 2008 and 2011. Our data are different from the survey, which also included blood transfusions in outpatient settings, emergency departments, and pediatric patients. The rising proportion of aging population with multiple comorbidities and cancers, increases in hematopoietic stem cell/solid organ transplants and chemotherapy, as well as widespread availability of blood products presumably contributed to the continued increase observed in our inpatient data after 2004. Nevertheless, the declining trend in the rate of the increased blood‐product transfusion usage seen after 2004 is encouraging. Increased awareness of restrictive transfusion strategy, coupled with efforts by professional bodies to improve the adoption of restrictive strategies, is most likely responsible for this.[3, 11, 12] As the clinical classification software procedure code 222 lumps together all the different types of blood products, we were unable to study the transfusion trend among each different type of blood products. In conclusion, further efforts need to be directed at increasing the awareness of clinicians, especially hospitalists, about the benefits of a restrictive transfusion policy and decreasing the rate of blood product use in the inpatient service. Furthermore, studies elaborating the patient population who are being transfused and the factors influencing the transfusion trends can provide useful insights to optimize blood‐product utilization and control resource consumption.
Disclosure
Nothing to report.
- , , . Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309(1):83–84.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
- , , , . Evidence review: periprocedural use of blood products. J Hosp Med. 2013;8(11):647–652.
- The 2011 National Blood Collection and Utilization Survey Report. Washington, DC: U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health; 2013.
- , , , et al. HCUP facts and figures: statistics on hospital‐based care in the United States. 2009. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed January 2, 2014.
- HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 15, 2013.
- HCUP Clinical Classifications Software (CCS) for ICD‐9‐CM. Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed December 15, 2013.
- Joinpoint Regression Program, Version 4.0.4, December, 2014. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Available at: https://surveillance.cancer.gov/joinpoint/download. Accessed December 25, 2013.
- , , , , . Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682.
- , , , . Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , . Patient‐centered blood management. J Hosp Med. 2014;9(1):60–65.
- , , . Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309(1):83–84.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
- , , , . Evidence review: periprocedural use of blood products. J Hosp Med. 2013;8(11):647–652.
- The 2011 National Blood Collection and Utilization Survey Report. Washington, DC: U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health; 2013.
- , , , et al. HCUP facts and figures: statistics on hospital‐based care in the United States. 2009. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed January 2, 2014.
- HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 15, 2013.
- HCUP Clinical Classifications Software (CCS) for ICD‐9‐CM. Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed December 15, 2013.
- Joinpoint Regression Program, Version 4.0.4, December, 2014. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Available at: https://surveillance.cancer.gov/joinpoint/download. Accessed December 25, 2013.
- , , , , . Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682.
- , , , . Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , . Patient‐centered blood management. J Hosp Med. 2014;9(1):60–65.
Accessing Online Medical Information
Online publication of medical research continues to grow at a rapid pace, with approximately 2,000 to 4,000 new citations indexed daily by the National Library of Medicine.[1] Prior studies suggest use of web‐based applications such as Google and electronic databases may improve accuracy and efficiency in clinical decision‐making compared to accessing primary sources of medical information.[2, 3, 4] To date, however, no analyses have examined longitudinal patterns of utilization associated with these online resources. Accordingly, we sought to describe temporal trends in the online use of select sources of primary medical literature and drug information compared to UpToDate (
METHODS
We obtained data from Google Trends (Google Inc., Menlo Park, CA;
Ordinary least‐squares linear regression was used to calculate coefficients of trend for each source of online medical information, and postestimation differences across all pair‐wise combinations of coefficients were assessed using the generalized Hausman specification test. We performed locally weighted least squares regression to produce smoothed curves of each search query for graphical visualization. All analyses were performed using Stata SE 13.1 (StataCorp, College Station, TX), and all statistical tests were 2‐tailed with equal to 0.05.
RESULTS
Since January 2004, relative search interest associated with UpToDate has increased steadily, whereas web‐based queries for other sources of online medical information have declined (Figure 1). Relative search interest in UpToDate has, on average, exceeded that of JAMA, NEJM, and PDR since approximately July 2011 (Figure 1), whereas PubMed has been associated with the greatest, albeit diminishing, relative search interest. Linear regression yielded the following significant (P<0.001) coefficients of trend for UpToDate (coefficient=0.010), JAMA (coefficient=0.012), NEJM (coefficient=0.030), PDR (coefficient=0.020), and PubMed (coefficient=0.011). Every coefficient differed significantly from each other (P<0.001).

DISCUSSION
Proliferation of medical researchin concert with expanding access to the Internethas dramatically magnified the amount and availability of medical information.[1] Our results support prior research indicating that medical information may be increasingly accessed by providers via interaction with online summary databases, rather than through electronic sources of primary medical literature or digital textbooks.[3, 6, 7]
Our study has implications for the practice of hospital‐based medicine. Our findings may reflect evolving provider preferences for synthesized medical information that can be translated efficiently to clinical practice.[8, 9] Use of summary databases may potentially lead to improved inpatient outcomes[10] by enhancing knowledge of current medical evidence, adherence to clinical guidelines, and subsequent consistency of care across providers. However, increased reliance on these resources necessitates that such databases are subject to ongoing evaluation and integration of novel research according to standardized criteria, such as those employed by the Cochrane Collaboration or the United States Preventive Services Task Force, to ensure the quality of the medical information they purport to deliver.
These results are also relevant to inpatient medical education. As summary databases are used more frequently, trainees may elect to memorize fewer medical facts and algorithms. Ideally, this transition would foster more opportunities to hone clinical reasoning skills and concentrate on delivering patient‐centered care. However, it may also create unwanted dependency on externalized expertise, which could impede the ability to critically evaluate primary medical literature, appropriately contextualize care options, and engage in real‐time problem solving.
Our study has several limitations. It is ecologic by design and cannot account for unknown secular trends. This analysis does not capture actual use or direct access of online medical resources, although we believe our observed results most likely mirror in‐person patterns of use. Additionally, because UpToDate is frequently incorporated into existing health information technology platforms (unlike journals), our results are biased conservatively. Finally, this study compares online medical information resources only, and we cannot account for concomitant use of printed/nondigital publications.
Our results signal an emergentand perhaps permanentshift in the utilization of online medical information in the United States. These trends may inform future efforts to optimize medical education and evidence‐based patient care as knowledge‐seeking behaviors continue to adapt to changes in technology and clinical demands.
- National Library of Medicine. MEDLINE factsheet. Available at: http://www.nlm.nih.gov/pubs/factsheets/medline.html. Accessed January 25, 2014.
- , , , . Speed, accuracy, and confidence in Google, Ovid, PubMed, and UpToDate: results of a randomised trial. Postgrad Med J. 2010;86:459–465.
- , , , . Should we Google it? Resource use by internal medicine residents for point‐of‐care clinical decision making. Acad Med. 2013;88:788–794.
- , , , , . A comparison of world wide web resources for identifying medical information. Acad Radiol. 2008;15:1165–1172.
- , . Predicting the present with Google Trends. Econ Rec. 2012;88:2–9.
- , , , , . Access of primary and secondary literature by health personnel in an academic health center: implications for open access. J Med Libr Assoc. 2013;101:205–212.
- , , . Public access and use health research: an exploratory study of the National Institutes of Health (NIH) public access policy using interviews and surveys of health personnel. J Med Internet Res. 2011;13:e97.
- , . Resource utilisation patterns of third‐year medical students. Clin Teach. 2011;8:43–47.
- , , , et al. A multi‐institutional survey of internal medicine residents' learning habits. Med Teach. 2010;32:773–775.
- , , . Use of UpToDate and outcomes in US hospitals. J Hosp Med. 2012;7:85–90.
Online publication of medical research continues to grow at a rapid pace, with approximately 2,000 to 4,000 new citations indexed daily by the National Library of Medicine.[1] Prior studies suggest use of web‐based applications such as Google and electronic databases may improve accuracy and efficiency in clinical decision‐making compared to accessing primary sources of medical information.[2, 3, 4] To date, however, no analyses have examined longitudinal patterns of utilization associated with these online resources. Accordingly, we sought to describe temporal trends in the online use of select sources of primary medical literature and drug information compared to UpToDate (
METHODS
We obtained data from Google Trends (Google Inc., Menlo Park, CA;
Ordinary least‐squares linear regression was used to calculate coefficients of trend for each source of online medical information, and postestimation differences across all pair‐wise combinations of coefficients were assessed using the generalized Hausman specification test. We performed locally weighted least squares regression to produce smoothed curves of each search query for graphical visualization. All analyses were performed using Stata SE 13.1 (StataCorp, College Station, TX), and all statistical tests were 2‐tailed with equal to 0.05.
RESULTS
Since January 2004, relative search interest associated with UpToDate has increased steadily, whereas web‐based queries for other sources of online medical information have declined (Figure 1). Relative search interest in UpToDate has, on average, exceeded that of JAMA, NEJM, and PDR since approximately July 2011 (Figure 1), whereas PubMed has been associated with the greatest, albeit diminishing, relative search interest. Linear regression yielded the following significant (P<0.001) coefficients of trend for UpToDate (coefficient=0.010), JAMA (coefficient=0.012), NEJM (coefficient=0.030), PDR (coefficient=0.020), and PubMed (coefficient=0.011). Every coefficient differed significantly from each other (P<0.001).

DISCUSSION
Proliferation of medical researchin concert with expanding access to the Internethas dramatically magnified the amount and availability of medical information.[1] Our results support prior research indicating that medical information may be increasingly accessed by providers via interaction with online summary databases, rather than through electronic sources of primary medical literature or digital textbooks.[3, 6, 7]
Our study has implications for the practice of hospital‐based medicine. Our findings may reflect evolving provider preferences for synthesized medical information that can be translated efficiently to clinical practice.[8, 9] Use of summary databases may potentially lead to improved inpatient outcomes[10] by enhancing knowledge of current medical evidence, adherence to clinical guidelines, and subsequent consistency of care across providers. However, increased reliance on these resources necessitates that such databases are subject to ongoing evaluation and integration of novel research according to standardized criteria, such as those employed by the Cochrane Collaboration or the United States Preventive Services Task Force, to ensure the quality of the medical information they purport to deliver.
These results are also relevant to inpatient medical education. As summary databases are used more frequently, trainees may elect to memorize fewer medical facts and algorithms. Ideally, this transition would foster more opportunities to hone clinical reasoning skills and concentrate on delivering patient‐centered care. However, it may also create unwanted dependency on externalized expertise, which could impede the ability to critically evaluate primary medical literature, appropriately contextualize care options, and engage in real‐time problem solving.
Our study has several limitations. It is ecologic by design and cannot account for unknown secular trends. This analysis does not capture actual use or direct access of online medical resources, although we believe our observed results most likely mirror in‐person patterns of use. Additionally, because UpToDate is frequently incorporated into existing health information technology platforms (unlike journals), our results are biased conservatively. Finally, this study compares online medical information resources only, and we cannot account for concomitant use of printed/nondigital publications.
Our results signal an emergentand perhaps permanentshift in the utilization of online medical information in the United States. These trends may inform future efforts to optimize medical education and evidence‐based patient care as knowledge‐seeking behaviors continue to adapt to changes in technology and clinical demands.
Online publication of medical research continues to grow at a rapid pace, with approximately 2,000 to 4,000 new citations indexed daily by the National Library of Medicine.[1] Prior studies suggest use of web‐based applications such as Google and electronic databases may improve accuracy and efficiency in clinical decision‐making compared to accessing primary sources of medical information.[2, 3, 4] To date, however, no analyses have examined longitudinal patterns of utilization associated with these online resources. Accordingly, we sought to describe temporal trends in the online use of select sources of primary medical literature and drug information compared to UpToDate (
METHODS
We obtained data from Google Trends (Google Inc., Menlo Park, CA;
Ordinary least‐squares linear regression was used to calculate coefficients of trend for each source of online medical information, and postestimation differences across all pair‐wise combinations of coefficients were assessed using the generalized Hausman specification test. We performed locally weighted least squares regression to produce smoothed curves of each search query for graphical visualization. All analyses were performed using Stata SE 13.1 (StataCorp, College Station, TX), and all statistical tests were 2‐tailed with equal to 0.05.
RESULTS
Since January 2004, relative search interest associated with UpToDate has increased steadily, whereas web‐based queries for other sources of online medical information have declined (Figure 1). Relative search interest in UpToDate has, on average, exceeded that of JAMA, NEJM, and PDR since approximately July 2011 (Figure 1), whereas PubMed has been associated with the greatest, albeit diminishing, relative search interest. Linear regression yielded the following significant (P<0.001) coefficients of trend for UpToDate (coefficient=0.010), JAMA (coefficient=0.012), NEJM (coefficient=0.030), PDR (coefficient=0.020), and PubMed (coefficient=0.011). Every coefficient differed significantly from each other (P<0.001).

DISCUSSION
Proliferation of medical researchin concert with expanding access to the Internethas dramatically magnified the amount and availability of medical information.[1] Our results support prior research indicating that medical information may be increasingly accessed by providers via interaction with online summary databases, rather than through electronic sources of primary medical literature or digital textbooks.[3, 6, 7]
Our study has implications for the practice of hospital‐based medicine. Our findings may reflect evolving provider preferences for synthesized medical information that can be translated efficiently to clinical practice.[8, 9] Use of summary databases may potentially lead to improved inpatient outcomes[10] by enhancing knowledge of current medical evidence, adherence to clinical guidelines, and subsequent consistency of care across providers. However, increased reliance on these resources necessitates that such databases are subject to ongoing evaluation and integration of novel research according to standardized criteria, such as those employed by the Cochrane Collaboration or the United States Preventive Services Task Force, to ensure the quality of the medical information they purport to deliver.
These results are also relevant to inpatient medical education. As summary databases are used more frequently, trainees may elect to memorize fewer medical facts and algorithms. Ideally, this transition would foster more opportunities to hone clinical reasoning skills and concentrate on delivering patient‐centered care. However, it may also create unwanted dependency on externalized expertise, which could impede the ability to critically evaluate primary medical literature, appropriately contextualize care options, and engage in real‐time problem solving.
Our study has several limitations. It is ecologic by design and cannot account for unknown secular trends. This analysis does not capture actual use or direct access of online medical resources, although we believe our observed results most likely mirror in‐person patterns of use. Additionally, because UpToDate is frequently incorporated into existing health information technology platforms (unlike journals), our results are biased conservatively. Finally, this study compares online medical information resources only, and we cannot account for concomitant use of printed/nondigital publications.
Our results signal an emergentand perhaps permanentshift in the utilization of online medical information in the United States. These trends may inform future efforts to optimize medical education and evidence‐based patient care as knowledge‐seeking behaviors continue to adapt to changes in technology and clinical demands.
- National Library of Medicine. MEDLINE factsheet. Available at: http://www.nlm.nih.gov/pubs/factsheets/medline.html. Accessed January 25, 2014.
- , , , . Speed, accuracy, and confidence in Google, Ovid, PubMed, and UpToDate: results of a randomised trial. Postgrad Med J. 2010;86:459–465.
- , , , . Should we Google it? Resource use by internal medicine residents for point‐of‐care clinical decision making. Acad Med. 2013;88:788–794.
- , , , , . A comparison of world wide web resources for identifying medical information. Acad Radiol. 2008;15:1165–1172.
- , . Predicting the present with Google Trends. Econ Rec. 2012;88:2–9.
- , , , , . Access of primary and secondary literature by health personnel in an academic health center: implications for open access. J Med Libr Assoc. 2013;101:205–212.
- , , . Public access and use health research: an exploratory study of the National Institutes of Health (NIH) public access policy using interviews and surveys of health personnel. J Med Internet Res. 2011;13:e97.
- , . Resource utilisation patterns of third‐year medical students. Clin Teach. 2011;8:43–47.
- , , , et al. A multi‐institutional survey of internal medicine residents' learning habits. Med Teach. 2010;32:773–775.
- , , . Use of UpToDate and outcomes in US hospitals. J Hosp Med. 2012;7:85–90.
- National Library of Medicine. MEDLINE factsheet. Available at: http://www.nlm.nih.gov/pubs/factsheets/medline.html. Accessed January 25, 2014.
- , , , . Speed, accuracy, and confidence in Google, Ovid, PubMed, and UpToDate: results of a randomised trial. Postgrad Med J. 2010;86:459–465.
- , , , . Should we Google it? Resource use by internal medicine residents for point‐of‐care clinical decision making. Acad Med. 2013;88:788–794.
- , , , , . A comparison of world wide web resources for identifying medical information. Acad Radiol. 2008;15:1165–1172.
- , . Predicting the present with Google Trends. Econ Rec. 2012;88:2–9.
- , , , , . Access of primary and secondary literature by health personnel in an academic health center: implications for open access. J Med Libr Assoc. 2013;101:205–212.
- , , . Public access and use health research: an exploratory study of the National Institutes of Health (NIH) public access policy using interviews and surveys of health personnel. J Med Internet Res. 2011;13:e97.
- , . Resource utilisation patterns of third‐year medical students. Clin Teach. 2011;8:43–47.
- , , , et al. A multi‐institutional survey of internal medicine residents' learning habits. Med Teach. 2010;32:773–775.
- , , . Use of UpToDate and outcomes in US hospitals. J Hosp Med. 2012;7:85–90.
Actionability of TPAD Results
Effective communication between inpatient and primary care physicians (PCPs) is essential for safe, high‐quality transitions. Unfortunately, PCPs are often not meaningfully engaged in this process; communication is frequently challenging or nonexistent.[1, 2] Instead, information is suboptimally conveyed via lengthy, disorganized discharge summaries.[3] Consequently, timely knowledge is not transferred to PCPs, who instead must seek out and identify actionable information themselves. These deficiencies can lead to misinterpretation of information and patient harm.[4]
An important component of ideal transitions[5] is timely communication of results of tests pending at discharge (TPADs). TPADs are variably documented in discharge summaries, and physician awareness about them is strikingly poor.[3, 6, 7] Communication about TPADs should convey rationales for ordering tests and necessary actions to take in response to finalized results. Most often, this knowledge resides with the inpatient team.
Health information technology (HIT) is an effective strategy for improving test‐result management. We implemented an automated system that notifies inpatient attendings and PCPs of TPAD results via email and demonstrated increased awareness by these physicians at the time of required action.[8, 9] Nevertheless, without timely knowledge transfer, attendings and PCPs may have differing opinions regarding which TPAD results require action. We conducted a secondary analysis of survey respondents from our original clustered randomized controlled trial to measure the degree of agreement between inpatient and ambulatory physicians regarding actionability of TPAD results.
METHODS
The methods of our original study are described elsewhere.[9] In that study, the attending and PCP of each patient were independently surveyed (via email and then by fax if the electronic survey was not completed) to determine their awareness of finalized TPAD results, and to identify actionable results and the types of actions taken (or that would need to be taken). Discharge summaries were available in our electronic medical record (EMR) within 24 hours of discharge. Network physicians (affiliated with Partners HealthCare, Inc.) had access to all components of the EMR, including the discharge summary and test results. Non‐network PCPs were faxed discharge summaries within 48 hours of discharge per institutional policies. For this study, we identified all patients for whom the attending and PCP completed the survey and answered questions about TPAD actionability. We then compared the identified TPADs listed by the attending and PCP in that survey.
RESULTS
We enrolled 441 patients in our original study. We sent 441 surveys to 117 attendings and 353 surveys to 273 PCPs. Eighty‐eight patients did not have an identified PCP. We received 275 responses from 83 attendings (62% response rate), and 152 responses from 112 PCPs (43% response rate). Patient and physician characteristics are reported elsewhere.[9]
For this analysis, we identified the 98 patients (aged 6018 years, 44 male, 52 Caucasian, 46 non‐Caucasian, 85 network, 13 non‐network) cared for by 46 attendings (aged 4411 years, 33 male, 22 hospitalists, 24 nonhospitalists) and 79 PCPs (aged 4512.5, 33 male, 66 network, 13 non‐network) for whom we received completed surveys from both physicians. For 59 patients, both thought none of the TPAD results were actionable. For 12 patients, both thought at least 1 was actionable, and they identified the same actionable TPAD result for all 12. Overall, attendings and PCPs agreed on actionability in 72.5% (71/98) (Kappa 0.29, 95% confidence interval: 0.09‐0.50). Table 1 shows the type of action taken by responsible providers. There were 9 patients (9%) for whom the attending alone thought at least 1 TPAD result was actionable; of these, subsequent attending‐initiated communication occurred in 77.8% (7/9). There were 18 patients (18%) for whom the PCP alone thought at least 1 TPAD result was actionable; of these, subsequent PCP‐initiated communication occurred in 77.8% (14/18). Table 2 shows concordance of actionable TPAD by type. In instances of disagreement, the attending frequently reported microbiology TPADs (eg, culture data, viral serologies) as actionable, whereas the PCP reported all TPAD types (eg, culture data, colon biopsy, vitamin D, magnetic resonance imaging) as actionable.
| Inpatient Attending‐Initiated Action(s)a | PCP‐Initiated Action(s)a | |
|---|---|---|
| ||
| Patient was notifiedb | 11.1% (1/9) | 66.7% (12/18) |
| Subspecialist was contacted | 33.3% (3/9) | 16.7% (3/18) |
| PCP or inpatient team contacted | 33.3% (3/9) | 16.7% (3/18) |
| Further testing/modified treatment | 11.1% (1/9) | 33.3% (6/18) |
| Referred to ambulatory visit/emergency room | 0% (0/9) | 11.1% (2/18) |
| Documentation | 11.1% (1/9) | 16.7% (3/18) |
| Type of TPAD | Attending and PCP Agreed on Identity of Actionable TPADa | Attending and PCP Disagreed on Identity of Actionable TPADa | ||
|---|---|---|---|---|
| TPAD Identified | No TPAD Identified, n=59 | TPAD Identified by Attending Only | TPAD Identified by PCP Only | |
| ||||
| Microbiologyb | 25% (3/12) | N/A | 56% (5/9) | 17% (3/18) |
| Pathologyc | 17% (2/12) | N/A | 0% (0/9) | 17% (3/18) |
| Chemistry and hematologyd | 58% (7/12) | N/A | 11% (1/9) | 22% (4/18) |
| Radiologye | 0% (0/12) | N/A | 11% (1/9) | 39% (5/18) |
| Unclassified (left blank) | 0% (0/12) | N/A | 22% (1/9) | 17% (3/18) |
DISCUSSION
We found fair agreement between attendings and PCPs regarding actionability of TPAD results. In 27 patients (27.5%), either the attending or PCP considered TPAD results actionable when the other did not. Possible explanations for this include different thresholds for taking action (eg, inpatient physicians may view vitamin D levels as acceptable within broader ranges than PCPs, and PCPs may view negative results as actionable if they need to contact the patient whereas attendings may not), varying clinical context (eg, rationale for why microbiology culture data is actionable), and varying practices for escalating care (eg, referring patients back to the hospital).
Our study was limited by small sample size and low PCP response rate. Nonetheless, the findings suggest that poor concordance between inpatient and ambulatory physicians will persist without tools that promote more effective communication. Greater awareness alone may be insufficient to mitigate consequences of missed TPAD results if physicians are not on the same page regarding which results require action.
To better engage PCPs, healthcare systems require HIT infrastructure that facilitates seamless care team communication across care settings.[2] When optimally configured, HIT can facilitate greater PCP involvement in postdischarge communication. For example, our system promoted subsequent postdischarge communication in 78% of initial discordance in TPAD actionability; however, most of it was not between the attending and the PCP. Thus, improvements could be made to facilitate more effective communication among key inpatient and ambulatory providers. Furthermore, when configured to facilitate conversation among these providers regarding the discharge care plan throughout a patient's entire hospital course, HIT can promote effective knowledge transfer by virtue of adding clinical context to test ordering and follow‐up. Additional work is needed to understand whether such communication clarifies contingencies and facilitates appropriate postdischarge action. Nevertheless, current electronic solutions (eg, passive placement into results in‐baskets) will likely be ineffective because they do not reliably improve awareness and active communication about context, rationale, interpretation, suggested action, or transfer of responsibility.
In summary, discrepancies in TPAD actionability by inpatient and ambulatory providers still exist, even when awareness of TPAD results is improved by HIT. By fostering more effective communication among key care‐team members across care settings, HIT could mitigate the consequences of suboptimal care transitions. With regard to TPAD results, this may favorably impact unnecessary testing, diagnostic and therapeutic delays, and medical errors.
Disclosures: This article is based on research funded through AHRQ grant #R21HS018229; the authors have no other disclosures or conflicts or interest.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5:385–391.
- . A primary care physician's ideal transitions of care—where's the evidence? J Hosp Med. 2013;8(8):472–477.
- , , , et al. Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2012;8(2):102–109.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , , et al. Patient safety concerns rising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Design and implementation of an automated email notification system for results of tests pending at discharge. J Am Med Inform Assoc. 2012;19(4):523–528.
- , , , et al. Impact of an automated email notification system for results of rest pending at discharge: a cluster‐randomized controlled trial [published online ahead of print October 23, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2013‐002030.
Effective communication between inpatient and primary care physicians (PCPs) is essential for safe, high‐quality transitions. Unfortunately, PCPs are often not meaningfully engaged in this process; communication is frequently challenging or nonexistent.[1, 2] Instead, information is suboptimally conveyed via lengthy, disorganized discharge summaries.[3] Consequently, timely knowledge is not transferred to PCPs, who instead must seek out and identify actionable information themselves. These deficiencies can lead to misinterpretation of information and patient harm.[4]
An important component of ideal transitions[5] is timely communication of results of tests pending at discharge (TPADs). TPADs are variably documented in discharge summaries, and physician awareness about them is strikingly poor.[3, 6, 7] Communication about TPADs should convey rationales for ordering tests and necessary actions to take in response to finalized results. Most often, this knowledge resides with the inpatient team.
Health information technology (HIT) is an effective strategy for improving test‐result management. We implemented an automated system that notifies inpatient attendings and PCPs of TPAD results via email and demonstrated increased awareness by these physicians at the time of required action.[8, 9] Nevertheless, without timely knowledge transfer, attendings and PCPs may have differing opinions regarding which TPAD results require action. We conducted a secondary analysis of survey respondents from our original clustered randomized controlled trial to measure the degree of agreement between inpatient and ambulatory physicians regarding actionability of TPAD results.
METHODS
The methods of our original study are described elsewhere.[9] In that study, the attending and PCP of each patient were independently surveyed (via email and then by fax if the electronic survey was not completed) to determine their awareness of finalized TPAD results, and to identify actionable results and the types of actions taken (or that would need to be taken). Discharge summaries were available in our electronic medical record (EMR) within 24 hours of discharge. Network physicians (affiliated with Partners HealthCare, Inc.) had access to all components of the EMR, including the discharge summary and test results. Non‐network PCPs were faxed discharge summaries within 48 hours of discharge per institutional policies. For this study, we identified all patients for whom the attending and PCP completed the survey and answered questions about TPAD actionability. We then compared the identified TPADs listed by the attending and PCP in that survey.
RESULTS
We enrolled 441 patients in our original study. We sent 441 surveys to 117 attendings and 353 surveys to 273 PCPs. Eighty‐eight patients did not have an identified PCP. We received 275 responses from 83 attendings (62% response rate), and 152 responses from 112 PCPs (43% response rate). Patient and physician characteristics are reported elsewhere.[9]
For this analysis, we identified the 98 patients (aged 6018 years, 44 male, 52 Caucasian, 46 non‐Caucasian, 85 network, 13 non‐network) cared for by 46 attendings (aged 4411 years, 33 male, 22 hospitalists, 24 nonhospitalists) and 79 PCPs (aged 4512.5, 33 male, 66 network, 13 non‐network) for whom we received completed surveys from both physicians. For 59 patients, both thought none of the TPAD results were actionable. For 12 patients, both thought at least 1 was actionable, and they identified the same actionable TPAD result for all 12. Overall, attendings and PCPs agreed on actionability in 72.5% (71/98) (Kappa 0.29, 95% confidence interval: 0.09‐0.50). Table 1 shows the type of action taken by responsible providers. There were 9 patients (9%) for whom the attending alone thought at least 1 TPAD result was actionable; of these, subsequent attending‐initiated communication occurred in 77.8% (7/9). There were 18 patients (18%) for whom the PCP alone thought at least 1 TPAD result was actionable; of these, subsequent PCP‐initiated communication occurred in 77.8% (14/18). Table 2 shows concordance of actionable TPAD by type. In instances of disagreement, the attending frequently reported microbiology TPADs (eg, culture data, viral serologies) as actionable, whereas the PCP reported all TPAD types (eg, culture data, colon biopsy, vitamin D, magnetic resonance imaging) as actionable.
| Inpatient Attending‐Initiated Action(s)a | PCP‐Initiated Action(s)a | |
|---|---|---|
| ||
| Patient was notifiedb | 11.1% (1/9) | 66.7% (12/18) |
| Subspecialist was contacted | 33.3% (3/9) | 16.7% (3/18) |
| PCP or inpatient team contacted | 33.3% (3/9) | 16.7% (3/18) |
| Further testing/modified treatment | 11.1% (1/9) | 33.3% (6/18) |
| Referred to ambulatory visit/emergency room | 0% (0/9) | 11.1% (2/18) |
| Documentation | 11.1% (1/9) | 16.7% (3/18) |
| Type of TPAD | Attending and PCP Agreed on Identity of Actionable TPADa | Attending and PCP Disagreed on Identity of Actionable TPADa | ||
|---|---|---|---|---|
| TPAD Identified | No TPAD Identified, n=59 | TPAD Identified by Attending Only | TPAD Identified by PCP Only | |
| ||||
| Microbiologyb | 25% (3/12) | N/A | 56% (5/9) | 17% (3/18) |
| Pathologyc | 17% (2/12) | N/A | 0% (0/9) | 17% (3/18) |
| Chemistry and hematologyd | 58% (7/12) | N/A | 11% (1/9) | 22% (4/18) |
| Radiologye | 0% (0/12) | N/A | 11% (1/9) | 39% (5/18) |
| Unclassified (left blank) | 0% (0/12) | N/A | 22% (1/9) | 17% (3/18) |
DISCUSSION
We found fair agreement between attendings and PCPs regarding actionability of TPAD results. In 27 patients (27.5%), either the attending or PCP considered TPAD results actionable when the other did not. Possible explanations for this include different thresholds for taking action (eg, inpatient physicians may view vitamin D levels as acceptable within broader ranges than PCPs, and PCPs may view negative results as actionable if they need to contact the patient whereas attendings may not), varying clinical context (eg, rationale for why microbiology culture data is actionable), and varying practices for escalating care (eg, referring patients back to the hospital).
Our study was limited by small sample size and low PCP response rate. Nonetheless, the findings suggest that poor concordance between inpatient and ambulatory physicians will persist without tools that promote more effective communication. Greater awareness alone may be insufficient to mitigate consequences of missed TPAD results if physicians are not on the same page regarding which results require action.
To better engage PCPs, healthcare systems require HIT infrastructure that facilitates seamless care team communication across care settings.[2] When optimally configured, HIT can facilitate greater PCP involvement in postdischarge communication. For example, our system promoted subsequent postdischarge communication in 78% of initial discordance in TPAD actionability; however, most of it was not between the attending and the PCP. Thus, improvements could be made to facilitate more effective communication among key inpatient and ambulatory providers. Furthermore, when configured to facilitate conversation among these providers regarding the discharge care plan throughout a patient's entire hospital course, HIT can promote effective knowledge transfer by virtue of adding clinical context to test ordering and follow‐up. Additional work is needed to understand whether such communication clarifies contingencies and facilitates appropriate postdischarge action. Nevertheless, current electronic solutions (eg, passive placement into results in‐baskets) will likely be ineffective because they do not reliably improve awareness and active communication about context, rationale, interpretation, suggested action, or transfer of responsibility.
In summary, discrepancies in TPAD actionability by inpatient and ambulatory providers still exist, even when awareness of TPAD results is improved by HIT. By fostering more effective communication among key care‐team members across care settings, HIT could mitigate the consequences of suboptimal care transitions. With regard to TPAD results, this may favorably impact unnecessary testing, diagnostic and therapeutic delays, and medical errors.
Disclosures: This article is based on research funded through AHRQ grant #R21HS018229; the authors have no other disclosures or conflicts or interest.
Effective communication between inpatient and primary care physicians (PCPs) is essential for safe, high‐quality transitions. Unfortunately, PCPs are often not meaningfully engaged in this process; communication is frequently challenging or nonexistent.[1, 2] Instead, information is suboptimally conveyed via lengthy, disorganized discharge summaries.[3] Consequently, timely knowledge is not transferred to PCPs, who instead must seek out and identify actionable information themselves. These deficiencies can lead to misinterpretation of information and patient harm.[4]
An important component of ideal transitions[5] is timely communication of results of tests pending at discharge (TPADs). TPADs are variably documented in discharge summaries, and physician awareness about them is strikingly poor.[3, 6, 7] Communication about TPADs should convey rationales for ordering tests and necessary actions to take in response to finalized results. Most often, this knowledge resides with the inpatient team.
Health information technology (HIT) is an effective strategy for improving test‐result management. We implemented an automated system that notifies inpatient attendings and PCPs of TPAD results via email and demonstrated increased awareness by these physicians at the time of required action.[8, 9] Nevertheless, without timely knowledge transfer, attendings and PCPs may have differing opinions regarding which TPAD results require action. We conducted a secondary analysis of survey respondents from our original clustered randomized controlled trial to measure the degree of agreement between inpatient and ambulatory physicians regarding actionability of TPAD results.
METHODS
The methods of our original study are described elsewhere.[9] In that study, the attending and PCP of each patient were independently surveyed (via email and then by fax if the electronic survey was not completed) to determine their awareness of finalized TPAD results, and to identify actionable results and the types of actions taken (or that would need to be taken). Discharge summaries were available in our electronic medical record (EMR) within 24 hours of discharge. Network physicians (affiliated with Partners HealthCare, Inc.) had access to all components of the EMR, including the discharge summary and test results. Non‐network PCPs were faxed discharge summaries within 48 hours of discharge per institutional policies. For this study, we identified all patients for whom the attending and PCP completed the survey and answered questions about TPAD actionability. We then compared the identified TPADs listed by the attending and PCP in that survey.
RESULTS
We enrolled 441 patients in our original study. We sent 441 surveys to 117 attendings and 353 surveys to 273 PCPs. Eighty‐eight patients did not have an identified PCP. We received 275 responses from 83 attendings (62% response rate), and 152 responses from 112 PCPs (43% response rate). Patient and physician characteristics are reported elsewhere.[9]
For this analysis, we identified the 98 patients (aged 6018 years, 44 male, 52 Caucasian, 46 non‐Caucasian, 85 network, 13 non‐network) cared for by 46 attendings (aged 4411 years, 33 male, 22 hospitalists, 24 nonhospitalists) and 79 PCPs (aged 4512.5, 33 male, 66 network, 13 non‐network) for whom we received completed surveys from both physicians. For 59 patients, both thought none of the TPAD results were actionable. For 12 patients, both thought at least 1 was actionable, and they identified the same actionable TPAD result for all 12. Overall, attendings and PCPs agreed on actionability in 72.5% (71/98) (Kappa 0.29, 95% confidence interval: 0.09‐0.50). Table 1 shows the type of action taken by responsible providers. There were 9 patients (9%) for whom the attending alone thought at least 1 TPAD result was actionable; of these, subsequent attending‐initiated communication occurred in 77.8% (7/9). There were 18 patients (18%) for whom the PCP alone thought at least 1 TPAD result was actionable; of these, subsequent PCP‐initiated communication occurred in 77.8% (14/18). Table 2 shows concordance of actionable TPAD by type. In instances of disagreement, the attending frequently reported microbiology TPADs (eg, culture data, viral serologies) as actionable, whereas the PCP reported all TPAD types (eg, culture data, colon biopsy, vitamin D, magnetic resonance imaging) as actionable.
| Inpatient Attending‐Initiated Action(s)a | PCP‐Initiated Action(s)a | |
|---|---|---|
| ||
| Patient was notifiedb | 11.1% (1/9) | 66.7% (12/18) |
| Subspecialist was contacted | 33.3% (3/9) | 16.7% (3/18) |
| PCP or inpatient team contacted | 33.3% (3/9) | 16.7% (3/18) |
| Further testing/modified treatment | 11.1% (1/9) | 33.3% (6/18) |
| Referred to ambulatory visit/emergency room | 0% (0/9) | 11.1% (2/18) |
| Documentation | 11.1% (1/9) | 16.7% (3/18) |
| Type of TPAD | Attending and PCP Agreed on Identity of Actionable TPADa | Attending and PCP Disagreed on Identity of Actionable TPADa | ||
|---|---|---|---|---|
| TPAD Identified | No TPAD Identified, n=59 | TPAD Identified by Attending Only | TPAD Identified by PCP Only | |
| ||||
| Microbiologyb | 25% (3/12) | N/A | 56% (5/9) | 17% (3/18) |
| Pathologyc | 17% (2/12) | N/A | 0% (0/9) | 17% (3/18) |
| Chemistry and hematologyd | 58% (7/12) | N/A | 11% (1/9) | 22% (4/18) |
| Radiologye | 0% (0/12) | N/A | 11% (1/9) | 39% (5/18) |
| Unclassified (left blank) | 0% (0/12) | N/A | 22% (1/9) | 17% (3/18) |
DISCUSSION
We found fair agreement between attendings and PCPs regarding actionability of TPAD results. In 27 patients (27.5%), either the attending or PCP considered TPAD results actionable when the other did not. Possible explanations for this include different thresholds for taking action (eg, inpatient physicians may view vitamin D levels as acceptable within broader ranges than PCPs, and PCPs may view negative results as actionable if they need to contact the patient whereas attendings may not), varying clinical context (eg, rationale for why microbiology culture data is actionable), and varying practices for escalating care (eg, referring patients back to the hospital).
Our study was limited by small sample size and low PCP response rate. Nonetheless, the findings suggest that poor concordance between inpatient and ambulatory physicians will persist without tools that promote more effective communication. Greater awareness alone may be insufficient to mitigate consequences of missed TPAD results if physicians are not on the same page regarding which results require action.
To better engage PCPs, healthcare systems require HIT infrastructure that facilitates seamless care team communication across care settings.[2] When optimally configured, HIT can facilitate greater PCP involvement in postdischarge communication. For example, our system promoted subsequent postdischarge communication in 78% of initial discordance in TPAD actionability; however, most of it was not between the attending and the PCP. Thus, improvements could be made to facilitate more effective communication among key inpatient and ambulatory providers. Furthermore, when configured to facilitate conversation among these providers regarding the discharge care plan throughout a patient's entire hospital course, HIT can promote effective knowledge transfer by virtue of adding clinical context to test ordering and follow‐up. Additional work is needed to understand whether such communication clarifies contingencies and facilitates appropriate postdischarge action. Nevertheless, current electronic solutions (eg, passive placement into results in‐baskets) will likely be ineffective because they do not reliably improve awareness and active communication about context, rationale, interpretation, suggested action, or transfer of responsibility.
In summary, discrepancies in TPAD actionability by inpatient and ambulatory providers still exist, even when awareness of TPAD results is improved by HIT. By fostering more effective communication among key care‐team members across care settings, HIT could mitigate the consequences of suboptimal care transitions. With regard to TPAD results, this may favorably impact unnecessary testing, diagnostic and therapeutic delays, and medical errors.
Disclosures: This article is based on research funded through AHRQ grant #R21HS018229; the authors have no other disclosures or conflicts or interest.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5:385–391.
- . A primary care physician's ideal transitions of care—where's the evidence? J Hosp Med. 2013;8(8):472–477.
- , , , et al. Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2012;8(2):102–109.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , , et al. Patient safety concerns rising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Design and implementation of an automated email notification system for results of tests pending at discharge. J Am Med Inform Assoc. 2012;19(4):523–528.
- , , , et al. Impact of an automated email notification system for results of rest pending at discharge: a cluster‐randomized controlled trial [published online ahead of print October 23, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2013‐002030.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5:385–391.
- . A primary care physician's ideal transitions of care—where's the evidence? J Hosp Med. 2013;8(8):472–477.
- , , , et al. Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2012;8(2):102–109.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , , et al. Patient safety concerns rising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Design and implementation of an automated email notification system for results of tests pending at discharge. J Am Med Inform Assoc. 2012;19(4):523–528.
- , , , et al. Impact of an automated email notification system for results of rest pending at discharge: a cluster‐randomized controlled trial [published online ahead of print October 23, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2013‐002030.