User login
Derm Research Unsettles Ped's Treatment of Acne
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
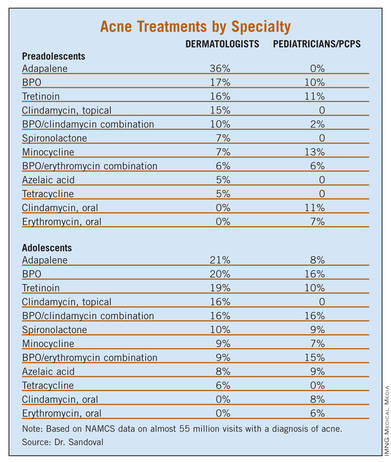
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.
This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.

This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.

This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
AT THE AMERICAN ACADEMY OF DERMATOLOGY'S SUMMER ACADEMY MEETING
Major Finding: Most (75.6%) neonatal and infantile acne was managed by a pediatrician, while most (67.1%) adolescent acne was managed by a dermatologist.
Data Source: NAMCS data was collected for outpatient visits by children receiving a diagnosis of acne vulgaris from 1993 to 2009.
Disclosures: The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
Is it Acne or Is it Rosacea? An Important Distinction
Randomized, Observer-Blind, Split-Face Study to Compare the Irritation Potential of 2 Topical Acne Formulations Over a 14-Day Treatment Period
Combined Oral Contraceptives for the Treatment of Acne: A Practical Guide
Hormonal therapy in the form of combined oral contraceptives (COCs) can be a useful tool in the treatment of patients with mild, moderate, or severe acne vulgaris.1,2 Eligible patients include postmenarcheal to premenopausal women who desire contraception or simply do not intend to become pregnant. Combined oral contraceptives may be used in combination with other acne treatments, and they can be considered as first-line agents in patients with a strong association between acne severity and their menstrual cycle or in the presence of menstrual cycle irregularities. Although there has been concern that the contraceptive ability of COCs is decreased by concomitant use of antibiotics, failure rates were no higher than the typical COC failure rates of 1% to 3%.3 There is no pharmacokinetic evidence to indicate that any antibiotics other than rifampin lower levels of COCs.4
Adrenal or ovarian neoplasms should be excluded by history and physical examination prior to initiating treatment with COCs. Hirsutism, alopecia, acanthosis nigricans, cushingoid symptoms, irregular menses, clitoromegaly, or severe acne of sudden onset warrant further endocrinologic testing.5-7 Menstrual irregularity in the first 2 to 3 years of postmenarcheal females is common and usually does not require further workup.8
Drug Selection
Combined oral contraceptives are effective in the treatment of acne but vary in their formulation.9-12 All COCs combine an estrogen, typically ethinyl estradiol (EE), and a progestin. First-generation COCs consisted of 50 to 150 µg of estrogens as well as progestins called estranes (eg, norethindrone, ethynodiol diacetate). Second-generation COCs dosed estrogen at less than 50 µg combined with gonane progestins (eg, levonorgestrel, norgestimate). Third-generation COCs also used less androgenic gonane progestins (eg, desogestrel, gestodene). A fourth generation has been proposed that consists of COCs using progestins, such as drospirenone (DRSP) and chlormadinone acetate, which are not testosterone derived.13 Theoretically, less androgenic COCs should be better acne treatments.5,14-16 However, all COCs effectively treat acne, and there is little evidence to suggest one is substantially more effective than the next.17-19 Therefore, it is believed that the estrogen component may be more responsible for the therapeutic effects of COCs via their ability to decrease androgen secretion from the ovaries and adrenal glands, reduce 5 α-reductase activity, and increase sex hormone–binding globulin.1,5,7,17,20
With more than 40 COCs now on the market, choosing the right one for a patient can be daunting.
There are 3 COCs that have been approved by the US Food and Drug Administration (FDA) to treat acne vulgaris: (1) Ortho Tri-Cyclen®: norgestimate (white tablets, 0.180 mg; light blue tablets, 0.215 mg; blue tablets, 0.250 mg); EE (0.035 mg); (2) Yaz®: DRSP (3 mg) combined with EE (0.02 mg); and (3) Estrostep® Fe: norethindrone acetate (1 mg) combined with EE (white triangular tablets, 20 µg; white square tablets, 30 µg; white round tablets, 35 µg) and ferrous fumarate (75 mg).21 Because most COCs are effective for most women, drug selection may be more appropriately determined by cost, side-effect profile, lifestyle, and patient preference (Tables 1 and 2, see PDF). Tolerability of the traditional 7-day placebo hormone-free interval (HFI) plays a critical role. Women who do not have difficulty with this HFI and who are affected by dysmenorrhea and other menstrual irregularities may benefit from traditional, less expensive COCs that simply combine 20 to 50 µg EE and a progestin in a 21 active-tablet and 7 placebo-tablet combination.21
Women who experience exaggerated premenstrual symptoms or menstrual headaches during the HFI may be better served by the newest generation of COCs, which have a shorter HFI (Tables 1and 2, see PDF). This group of COCs tends to be more expensive than traditional COCs but provides less frequent withdrawal bleeds and similar protection. Specifically, Lybrel® (levonorgestrel [90 µg] combined with EE [20 µg]) entirely eliminates monthly menses and is the first FDA-approved yearlong COC. Yasmin® (DRSP [3 mg] and EE [0.03 mg]) and Yaz are particularly unique because they use antiandrogenic DRSP, a spironolactone analogue, and specifically are marketed to have antiacne effects.22 Drospirenone is the only FDA-approved progestin with direct androgen receptor–blocking properties.21 Although the majority of women do well with their first COC, switching drugs occasionally is helpful.16
Associated Risks
All COCs have associated risks, but the risks can be minimized with careful patient screening (Tables 3 and 4, see PDF).19,23 Although serious adverse events are possible, overall risks for adverse events from COCs are low.9,19,24-26 Given the low age and generally good health of the patient population for which COCs typically are prescribed, even an elevated relative risk translates into a low absolute risk for notable side effects.19
The most substantial risks are cardiovascular.25 The risk for venous thromboembolism (VTE), deep vein thrombosis, and pulmonary embolism increases with age and is dose related.9,24 There is a 10-fold increased risk for VTE in the first year and a 2-fold risk in the second year of use and beyond.26,27 Additionally, there have been concerns that DRSP-containing COCs place patients at a higher risk for VTE compared to traditional COCs, but these concerns have not been supported by more recent studies.28 Discontinuation of the COC returns a patient to baseline risk within 3 months.29 Although the risk for VTE while using COCs is widely noted in the literature, the overall risk is relatively low.9,19
Myocardial infarction (MI) and ischemic stroke also are rare, even more so than venous events. There is an approximate 2-fold increase in risk for MI and ischemic stroke in women using COCs,24 but these adverse events are rare in reproductive-aged women, making the incidence low.9,24 The newer third-generation COCs have no increased risk for MI; some studies even showed a slight reduction in risk.13,30 However, the risk for MI increases 10-fold in women who smoke and also is higher for women older than 35 years.24 Guidelines caution physicians against prescribing COCs to women who smoke and are older than 35 years.13
The risk for localized breast cancer in patients taking COCs has been debated.9,19,20,24 One study found a relative risk for breast cancer of 1.24 while taking the drug and an increased risk for up to 4 years following cessation of treatment.31 However, the risk completely disappeared within 10 years, there was no increased lifetime risk, and the type of cancer was less advanced.24 None of the data show an increased risk for breast cancer in women aged 35 to 64 years who previously used or currently use COCs.32 Mortality rates from breast cancer do not increase with the use of COCs.33
Combined oral contraceptives only increase the risk for cervical cancer after 5 years of use.19,24 The risk for cervical cancer immediately begins to decline following cessation of use and is returned to baseline by 10 years.34 One study found no association between the use of COCs and cervical intraepithelial neoplasia grades 1 or higher among women with human papillomavirus, even beyond 5 years of COC use.35
It is important to stress to patients that the risks for COCs have been fairly well elucidated and are substantially less concerning than previously believed if the patient is otherwise healthy.4 Both patients and physicians may believe that these medications are inherently unsafe,16,36,37 but these fears are unfounded and contribute to the underutilization of COCs.
Benefits
There also are benefits of COCs that extend beyond their positive effect on acne.19 Combined oral contraceptives decrease the risk for endometrial and ovarian cancer.24 This protection continues for at least 20 years following discontinuation of the medication.
Combined oral contraceptives also may protect women from colorectal cancer.9,37 Additionally, there is less risk for symptomatic pelvic inflammatory disease, ectopic pregnancy, and benign breast disease, along with improved cycle control, less frequent instances of dysmenorrhea, and increased bone density. Combined oral contraceptives prevent unintended pregnancy, which can pose a substantial health risk, as well as psychologic, social, and financial distress.9,19,24,25,38
Another benefit of COCs is for women with severe acne who may be future candidates for isotretinoin therapy. In this situation, COCs arguably are the appropriate preceding step, considering the requirement of 2 forms of contraception while taking isotretinoin. By exclusively trying a patient on COCs prior to initiation of isotretinoin therapy, the physician may be better equipped to differentiate the causal medication of mood symptoms, as both medications are cited to produce mood-related side effects.39,40 In addition, a sufficiently satisfactory response to COCs could occur, which would render treatment with isotretinoin unnecessary.
Initial Evaluation and Monitoring
Initial evaluation should include a measurement of blood pressure and a detailed patient history.19,23 Physical examination that extends beyond the skin is not necessary or indicated.16,25,41 Pelvic examination, screening for cervical intraepithelial neoplasia, and breast examination are not required. Although these examinations are valuable as screening tools, they are not helpful in determining which patients should or should not undergo treatment with COCs.41-44 The American Congress of Obstetricians and Gynecologists guidelines recommend that patients initiate pelvic examination screening at 21 years of age and biennially thereafter, irrespective of the onset of sexual activity or contraception use.45 Therefore, there is no need for follow-up with a gynecologist after initiation of COCs for acne vulgaris. However, with any medication, return visits to the dermatologist should be scheduled to monitor clinical progress and side effects. Periodic blood pressure measurements also are recommended.19
The dermatologist may elect to perform a pregnancy test to enable the patient to start the medication that same day.16,38 Described as the quick-start approach, beginning to use COCs in the health care provider’s office under direct observation has been found to improve short-term compliance compared to initiation after next menses. The rate of pregnancy within the first 6 months of initiation of COCs was lower for those who used the quick-start approach.46 However, patients must be advised to use a separate method of contraception for the first week to avoid an unintended pregnancy.16 Alternatively, if the clinician and patient decide to initiate treatment at or immediately after the onset of the next menstrual cycle, a pregnancy test is not needed.38 The longer the patient delays the initiation of the COC, the less chance she will be adherent.38,41 No other routine laboratory testing is indicated in an otherwise healthy patient with an unremarkable personal and family history.16
Counseling
When prescribing COCs, appropriate counseling regarding when to start is critical. The patient must take the pill each day and be aware of what to do if she skips 1 or more pills (Figure, see PDF).47 The chance of pregnancy (1%–3%), the lack of protection against sexually transmitted diseases, and the dangers of concomitant smoking should be discussed.16,19 Patients should establish a daily routine for taking the COC to maximize compliance.48 Misconceptions surrounding COCs and pelvic examinations, future fertility or teratogenicity, and risk for weight gain should be dispelled.16,36,38,49 Finally, the patient should know that it might take several months to see antiacne results,5,7 and she should not discontinue the medication if it does not work right away. The patient should be given ample time to ask questions and the issues discussed should be documented.
Conclusion
Although some dermatologists may be hesitant to prescribe hormonal therapies, COCs can be safely and appropriately used in eligible female patients with acne. We provided guidelines for the selection of COCs, reviewed the risks and benefits, and described ways to monitor and counsel patients.
- Layton AM. A review on the treatment of acne vulgaris. Int J Clin Pract. 2006;60:64-72.
- Tan J. Hormonal treatment of acne: review of current best evidence. J Cutan Med Surg. 2004;(8, suppl 4):S11-S15.
- Helms SE, Bredle DL, Zajic J, et al. Oral contraceptive failure rates and oral antibiotics. J Am Acad Dermatol. 1997;36(5, pt 1):705-710.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin. no. 73: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
- Katsambas A, Papakonstantinou A. Acne: systemic treatment. Clin Dermatol. 2004;22:412-418.
- Essah PA, Wickham EP 3rd, Nunley JR, et al. Dermatology of androgen-related disorders. Clin Dermatol. 2006;24: 289-298.
- Johnson BA, Nunley JR. Use of systemic agents in the treatment of acne vulgaris [published correction in Am Fam Physician. 2001;63:following 1295]. Am Fam Physician. 2000;62:1823-1830.
- Golden NH, Carlson JL. The pathophysiology of amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:163-178.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Lucky AW, Henderson TA, Olson WH, et al. Effectiveness of norgestimate and ethinyl estradiol in treating moderate acne vulgaris. J Am Acad Dermatol. 1997;37 (5, pt 1):746-754.
- Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89:615-622.
- Estrostep Fe [package insert]. Rockaway, NJ: Warner Chilcott US; 2009.
- Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease [published correction in J Am Coll Cardiol. 2009;53:904]. J Am Coll Cardiol. 2009; 53:221-231.
- Goodman G. Managing acne vulgaris effectively. Aust Fam Physician. 2006;35:705-709.
- Haroun M. Hormonal therapy of acne. J Cutan Med Surg. 2004;(8, suppl 4):S6-S10.
- Rimsza ME. Counseling the adolescent about contraception. Pediatr Rev. 2003;24:162-170.
- Strauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management [published online ahead of print February 5, 2007]. J Am Acad Dermatol. 2007;56:651-663.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2007;1:CD004425.
- Petitti DB. Clinical practice. combination estrogen-progestin oral contraceptives [published correction in N Engl J Med. 2004;350:92]. N Engl J Med. 2003;349:1443-1450.
- Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22:419-428.
- Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
- Spencer AL, Bonnema R, McNamara MC. Helping women choose appropriate hormonal contraception: update on risks, benefits, and indications. Am J Med. 2009;122: 497-506.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva, Switzerland: World Health Organization; 2004. http://whqlibdoc.who.int/publications/2004/9241562668.pdf. Accessed July 25, 2012.
- Practice Committee of the American Society for Reproductive Medicine. Hormonal contraception: recent advances and controversies. Fertil Steril. 2006;86(5, suppl 1):S229-S235.
- Deligeoroglou E, Christopoulos P, Creatsas G. Contraception in adolescence. Ann N Y Acad Sci. 2006;1092:78-90.
- Lawrenson R, Farmer R. Venous thromboembolism and combined oral contraceptives: does the type of progestogen make a difference? Contraception. 2000;62 (suppl 2):21S-28S.
- Suissa S, Blais L, Spitzer WO, et al. First-time use of newer oral contraceptives and the risk of venous thromboembolism. Contraception. 1997;56:141-146.
- Tan JK, Ediriweera C. Efficacy and safety of combined ethinyl estradiol/drospirenone oral contraceptives in the treatment of acne. Int J Womens Health. 2010;1:213-221.
- Martinez F, Avecilla A. Combined hormonal contraception and venous thromboembolism. Eur J Contracept Reprod Health Care. 2007;12:97-106.
- Carr BR, Ory H. Estrogen and progestin components of oral contraceptives: relationship to vascular disease. Contraception. 1997;55:267-272.
- Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1996;347: 1713-1727.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
- Faculty of Family Planning and Reproductive Health Care, Clinical Effectiveness Unit. FFPRHC Guidance (October 2003): first prescription of combined oral contraception. J Fam Plann Reprod Health Care. 2003;29:209-222.
- Appleby P, Beral V, Berrington de González A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370: 1609-1621.
- Harris TG, Miller L, Kulasingam SL, et al. Depotmedroxyprogesterone acetate and combined oral contraceptive use and cervical neoplasia among women with oncogenic human papillomavirus infection. Am J Obstet Gynecol. 2009;200:489, e1-e8.
- Lee J, Jezewski MA. Attitudes toward oral contraceptive use among women of reproductive age: a systematic review. ANS Adv Nurs Sci. 2007;30:e85-e103.
- Fernandez E, La Vecchia C, Balducci A, et al. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
- Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
- Segebladh B, Borgström A, Odlind V, et al. Prevalence of psychiatric disorders and premenstrual dysphoric symptoms in patients with experience of adverse mood during treatment with combined oral contraceptives [published online ahead of print September 11, 2008]. Contraception. 2009;79:50-55.
- Brelsford M, Beute TC. Preventing and managing the side effects of isotretinoin. Semin Cutan Med Surg. 2008;27: 197-206.
- Gardner J, Miller L. Promoting the safety and use of hormonal contraceptives. J Womens Health (Larchmt). 2005;14:53-60.
- Meckstroth KR. Physical examination before initiating hormonal contraception: what is necessary? Am Fam Physician. 2006;74:32, 34.
- Scott A, Glasier AF. Are routine breast and pelvic examinations necessary for women starting combined oral contraception [published online ahead of print June 10, 2004]? Hum Reprod Update. 2004;10:449-452.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 109: cervical cytology screening. Obstet Gynecol. 2009;114:1409-1420.
- Westhoff C, Heartwell S, Edwards S, et al. Initiation of oral contraceptives using a quick start compared with a conventional start: a randomized controlled trial. Obstet Gynecol. 2007;109:1270-1276.
- World Health Organization. Selected Practice Recommendations for Contraceptive Use. 2nd ed. Geneva, Switzerland: World Health Organization; 2005.
- Rosenberg MJ, Burnhill MS, Waugh MS, et al. Compliance and oral contraceptives: a review. Contraception. 1995;52:137-141.
- Cheung E, Free C. Factors influencing young women’s decision making regarding hormonal contraceptives: a qualitative study. Contraception. 2005;71:426-431.
Hormonal therapy in the form of combined oral contraceptives (COCs) can be a useful tool in the treatment of patients with mild, moderate, or severe acne vulgaris.1,2 Eligible patients include postmenarcheal to premenopausal women who desire contraception or simply do not intend to become pregnant. Combined oral contraceptives may be used in combination with other acne treatments, and they can be considered as first-line agents in patients with a strong association between acne severity and their menstrual cycle or in the presence of menstrual cycle irregularities. Although there has been concern that the contraceptive ability of COCs is decreased by concomitant use of antibiotics, failure rates were no higher than the typical COC failure rates of 1% to 3%.3 There is no pharmacokinetic evidence to indicate that any antibiotics other than rifampin lower levels of COCs.4
Adrenal or ovarian neoplasms should be excluded by history and physical examination prior to initiating treatment with COCs. Hirsutism, alopecia, acanthosis nigricans, cushingoid symptoms, irregular menses, clitoromegaly, or severe acne of sudden onset warrant further endocrinologic testing.5-7 Menstrual irregularity in the first 2 to 3 years of postmenarcheal females is common and usually does not require further workup.8
Drug Selection
Combined oral contraceptives are effective in the treatment of acne but vary in their formulation.9-12 All COCs combine an estrogen, typically ethinyl estradiol (EE), and a progestin. First-generation COCs consisted of 50 to 150 µg of estrogens as well as progestins called estranes (eg, norethindrone, ethynodiol diacetate). Second-generation COCs dosed estrogen at less than 50 µg combined with gonane progestins (eg, levonorgestrel, norgestimate). Third-generation COCs also used less androgenic gonane progestins (eg, desogestrel, gestodene). A fourth generation has been proposed that consists of COCs using progestins, such as drospirenone (DRSP) and chlormadinone acetate, which are not testosterone derived.13 Theoretically, less androgenic COCs should be better acne treatments.5,14-16 However, all COCs effectively treat acne, and there is little evidence to suggest one is substantially more effective than the next.17-19 Therefore, it is believed that the estrogen component may be more responsible for the therapeutic effects of COCs via their ability to decrease androgen secretion from the ovaries and adrenal glands, reduce 5 α-reductase activity, and increase sex hormone–binding globulin.1,5,7,17,20
With more than 40 COCs now on the market, choosing the right one for a patient can be daunting.
There are 3 COCs that have been approved by the US Food and Drug Administration (FDA) to treat acne vulgaris: (1) Ortho Tri-Cyclen®: norgestimate (white tablets, 0.180 mg; light blue tablets, 0.215 mg; blue tablets, 0.250 mg); EE (0.035 mg); (2) Yaz®: DRSP (3 mg) combined with EE (0.02 mg); and (3) Estrostep® Fe: norethindrone acetate (1 mg) combined with EE (white triangular tablets, 20 µg; white square tablets, 30 µg; white round tablets, 35 µg) and ferrous fumarate (75 mg).21 Because most COCs are effective for most women, drug selection may be more appropriately determined by cost, side-effect profile, lifestyle, and patient preference (Tables 1 and 2, see PDF). Tolerability of the traditional 7-day placebo hormone-free interval (HFI) plays a critical role. Women who do not have difficulty with this HFI and who are affected by dysmenorrhea and other menstrual irregularities may benefit from traditional, less expensive COCs that simply combine 20 to 50 µg EE and a progestin in a 21 active-tablet and 7 placebo-tablet combination.21
Women who experience exaggerated premenstrual symptoms or menstrual headaches during the HFI may be better served by the newest generation of COCs, which have a shorter HFI (Tables 1and 2, see PDF). This group of COCs tends to be more expensive than traditional COCs but provides less frequent withdrawal bleeds and similar protection. Specifically, Lybrel® (levonorgestrel [90 µg] combined with EE [20 µg]) entirely eliminates monthly menses and is the first FDA-approved yearlong COC. Yasmin® (DRSP [3 mg] and EE [0.03 mg]) and Yaz are particularly unique because they use antiandrogenic DRSP, a spironolactone analogue, and specifically are marketed to have antiacne effects.22 Drospirenone is the only FDA-approved progestin with direct androgen receptor–blocking properties.21 Although the majority of women do well with their first COC, switching drugs occasionally is helpful.16
Associated Risks
All COCs have associated risks, but the risks can be minimized with careful patient screening (Tables 3 and 4, see PDF).19,23 Although serious adverse events are possible, overall risks for adverse events from COCs are low.9,19,24-26 Given the low age and generally good health of the patient population for which COCs typically are prescribed, even an elevated relative risk translates into a low absolute risk for notable side effects.19
The most substantial risks are cardiovascular.25 The risk for venous thromboembolism (VTE), deep vein thrombosis, and pulmonary embolism increases with age and is dose related.9,24 There is a 10-fold increased risk for VTE in the first year and a 2-fold risk in the second year of use and beyond.26,27 Additionally, there have been concerns that DRSP-containing COCs place patients at a higher risk for VTE compared to traditional COCs, but these concerns have not been supported by more recent studies.28 Discontinuation of the COC returns a patient to baseline risk within 3 months.29 Although the risk for VTE while using COCs is widely noted in the literature, the overall risk is relatively low.9,19
Myocardial infarction (MI) and ischemic stroke also are rare, even more so than venous events. There is an approximate 2-fold increase in risk for MI and ischemic stroke in women using COCs,24 but these adverse events are rare in reproductive-aged women, making the incidence low.9,24 The newer third-generation COCs have no increased risk for MI; some studies even showed a slight reduction in risk.13,30 However, the risk for MI increases 10-fold in women who smoke and also is higher for women older than 35 years.24 Guidelines caution physicians against prescribing COCs to women who smoke and are older than 35 years.13
The risk for localized breast cancer in patients taking COCs has been debated.9,19,20,24 One study found a relative risk for breast cancer of 1.24 while taking the drug and an increased risk for up to 4 years following cessation of treatment.31 However, the risk completely disappeared within 10 years, there was no increased lifetime risk, and the type of cancer was less advanced.24 None of the data show an increased risk for breast cancer in women aged 35 to 64 years who previously used or currently use COCs.32 Mortality rates from breast cancer do not increase with the use of COCs.33
Combined oral contraceptives only increase the risk for cervical cancer after 5 years of use.19,24 The risk for cervical cancer immediately begins to decline following cessation of use and is returned to baseline by 10 years.34 One study found no association between the use of COCs and cervical intraepithelial neoplasia grades 1 or higher among women with human papillomavirus, even beyond 5 years of COC use.35
It is important to stress to patients that the risks for COCs have been fairly well elucidated and are substantially less concerning than previously believed if the patient is otherwise healthy.4 Both patients and physicians may believe that these medications are inherently unsafe,16,36,37 but these fears are unfounded and contribute to the underutilization of COCs.
Benefits
There also are benefits of COCs that extend beyond their positive effect on acne.19 Combined oral contraceptives decrease the risk for endometrial and ovarian cancer.24 This protection continues for at least 20 years following discontinuation of the medication.
Combined oral contraceptives also may protect women from colorectal cancer.9,37 Additionally, there is less risk for symptomatic pelvic inflammatory disease, ectopic pregnancy, and benign breast disease, along with improved cycle control, less frequent instances of dysmenorrhea, and increased bone density. Combined oral contraceptives prevent unintended pregnancy, which can pose a substantial health risk, as well as psychologic, social, and financial distress.9,19,24,25,38
Another benefit of COCs is for women with severe acne who may be future candidates for isotretinoin therapy. In this situation, COCs arguably are the appropriate preceding step, considering the requirement of 2 forms of contraception while taking isotretinoin. By exclusively trying a patient on COCs prior to initiation of isotretinoin therapy, the physician may be better equipped to differentiate the causal medication of mood symptoms, as both medications are cited to produce mood-related side effects.39,40 In addition, a sufficiently satisfactory response to COCs could occur, which would render treatment with isotretinoin unnecessary.
Initial Evaluation and Monitoring
Initial evaluation should include a measurement of blood pressure and a detailed patient history.19,23 Physical examination that extends beyond the skin is not necessary or indicated.16,25,41 Pelvic examination, screening for cervical intraepithelial neoplasia, and breast examination are not required. Although these examinations are valuable as screening tools, they are not helpful in determining which patients should or should not undergo treatment with COCs.41-44 The American Congress of Obstetricians and Gynecologists guidelines recommend that patients initiate pelvic examination screening at 21 years of age and biennially thereafter, irrespective of the onset of sexual activity or contraception use.45 Therefore, there is no need for follow-up with a gynecologist after initiation of COCs for acne vulgaris. However, with any medication, return visits to the dermatologist should be scheduled to monitor clinical progress and side effects. Periodic blood pressure measurements also are recommended.19
The dermatologist may elect to perform a pregnancy test to enable the patient to start the medication that same day.16,38 Described as the quick-start approach, beginning to use COCs in the health care provider’s office under direct observation has been found to improve short-term compliance compared to initiation after next menses. The rate of pregnancy within the first 6 months of initiation of COCs was lower for those who used the quick-start approach.46 However, patients must be advised to use a separate method of contraception for the first week to avoid an unintended pregnancy.16 Alternatively, if the clinician and patient decide to initiate treatment at or immediately after the onset of the next menstrual cycle, a pregnancy test is not needed.38 The longer the patient delays the initiation of the COC, the less chance she will be adherent.38,41 No other routine laboratory testing is indicated in an otherwise healthy patient with an unremarkable personal and family history.16
Counseling
When prescribing COCs, appropriate counseling regarding when to start is critical. The patient must take the pill each day and be aware of what to do if she skips 1 or more pills (Figure, see PDF).47 The chance of pregnancy (1%–3%), the lack of protection against sexually transmitted diseases, and the dangers of concomitant smoking should be discussed.16,19 Patients should establish a daily routine for taking the COC to maximize compliance.48 Misconceptions surrounding COCs and pelvic examinations, future fertility or teratogenicity, and risk for weight gain should be dispelled.16,36,38,49 Finally, the patient should know that it might take several months to see antiacne results,5,7 and she should not discontinue the medication if it does not work right away. The patient should be given ample time to ask questions and the issues discussed should be documented.
Conclusion
Although some dermatologists may be hesitant to prescribe hormonal therapies, COCs can be safely and appropriately used in eligible female patients with acne. We provided guidelines for the selection of COCs, reviewed the risks and benefits, and described ways to monitor and counsel patients.
Hormonal therapy in the form of combined oral contraceptives (COCs) can be a useful tool in the treatment of patients with mild, moderate, or severe acne vulgaris.1,2 Eligible patients include postmenarcheal to premenopausal women who desire contraception or simply do not intend to become pregnant. Combined oral contraceptives may be used in combination with other acne treatments, and they can be considered as first-line agents in patients with a strong association between acne severity and their menstrual cycle or in the presence of menstrual cycle irregularities. Although there has been concern that the contraceptive ability of COCs is decreased by concomitant use of antibiotics, failure rates were no higher than the typical COC failure rates of 1% to 3%.3 There is no pharmacokinetic evidence to indicate that any antibiotics other than rifampin lower levels of COCs.4
Adrenal or ovarian neoplasms should be excluded by history and physical examination prior to initiating treatment with COCs. Hirsutism, alopecia, acanthosis nigricans, cushingoid symptoms, irregular menses, clitoromegaly, or severe acne of sudden onset warrant further endocrinologic testing.5-7 Menstrual irregularity in the first 2 to 3 years of postmenarcheal females is common and usually does not require further workup.8
Drug Selection
Combined oral contraceptives are effective in the treatment of acne but vary in their formulation.9-12 All COCs combine an estrogen, typically ethinyl estradiol (EE), and a progestin. First-generation COCs consisted of 50 to 150 µg of estrogens as well as progestins called estranes (eg, norethindrone, ethynodiol diacetate). Second-generation COCs dosed estrogen at less than 50 µg combined with gonane progestins (eg, levonorgestrel, norgestimate). Third-generation COCs also used less androgenic gonane progestins (eg, desogestrel, gestodene). A fourth generation has been proposed that consists of COCs using progestins, such as drospirenone (DRSP) and chlormadinone acetate, which are not testosterone derived.13 Theoretically, less androgenic COCs should be better acne treatments.5,14-16 However, all COCs effectively treat acne, and there is little evidence to suggest one is substantially more effective than the next.17-19 Therefore, it is believed that the estrogen component may be more responsible for the therapeutic effects of COCs via their ability to decrease androgen secretion from the ovaries and adrenal glands, reduce 5 α-reductase activity, and increase sex hormone–binding globulin.1,5,7,17,20
With more than 40 COCs now on the market, choosing the right one for a patient can be daunting.
There are 3 COCs that have been approved by the US Food and Drug Administration (FDA) to treat acne vulgaris: (1) Ortho Tri-Cyclen®: norgestimate (white tablets, 0.180 mg; light blue tablets, 0.215 mg; blue tablets, 0.250 mg); EE (0.035 mg); (2) Yaz®: DRSP (3 mg) combined with EE (0.02 mg); and (3) Estrostep® Fe: norethindrone acetate (1 mg) combined with EE (white triangular tablets, 20 µg; white square tablets, 30 µg; white round tablets, 35 µg) and ferrous fumarate (75 mg).21 Because most COCs are effective for most women, drug selection may be more appropriately determined by cost, side-effect profile, lifestyle, and patient preference (Tables 1 and 2, see PDF). Tolerability of the traditional 7-day placebo hormone-free interval (HFI) plays a critical role. Women who do not have difficulty with this HFI and who are affected by dysmenorrhea and other menstrual irregularities may benefit from traditional, less expensive COCs that simply combine 20 to 50 µg EE and a progestin in a 21 active-tablet and 7 placebo-tablet combination.21
Women who experience exaggerated premenstrual symptoms or menstrual headaches during the HFI may be better served by the newest generation of COCs, which have a shorter HFI (Tables 1and 2, see PDF). This group of COCs tends to be more expensive than traditional COCs but provides less frequent withdrawal bleeds and similar protection. Specifically, Lybrel® (levonorgestrel [90 µg] combined with EE [20 µg]) entirely eliminates monthly menses and is the first FDA-approved yearlong COC. Yasmin® (DRSP [3 mg] and EE [0.03 mg]) and Yaz are particularly unique because they use antiandrogenic DRSP, a spironolactone analogue, and specifically are marketed to have antiacne effects.22 Drospirenone is the only FDA-approved progestin with direct androgen receptor–blocking properties.21 Although the majority of women do well with their first COC, switching drugs occasionally is helpful.16
Associated Risks
All COCs have associated risks, but the risks can be minimized with careful patient screening (Tables 3 and 4, see PDF).19,23 Although serious adverse events are possible, overall risks for adverse events from COCs are low.9,19,24-26 Given the low age and generally good health of the patient population for which COCs typically are prescribed, even an elevated relative risk translates into a low absolute risk for notable side effects.19
The most substantial risks are cardiovascular.25 The risk for venous thromboembolism (VTE), deep vein thrombosis, and pulmonary embolism increases with age and is dose related.9,24 There is a 10-fold increased risk for VTE in the first year and a 2-fold risk in the second year of use and beyond.26,27 Additionally, there have been concerns that DRSP-containing COCs place patients at a higher risk for VTE compared to traditional COCs, but these concerns have not been supported by more recent studies.28 Discontinuation of the COC returns a patient to baseline risk within 3 months.29 Although the risk for VTE while using COCs is widely noted in the literature, the overall risk is relatively low.9,19
Myocardial infarction (MI) and ischemic stroke also are rare, even more so than venous events. There is an approximate 2-fold increase in risk for MI and ischemic stroke in women using COCs,24 but these adverse events are rare in reproductive-aged women, making the incidence low.9,24 The newer third-generation COCs have no increased risk for MI; some studies even showed a slight reduction in risk.13,30 However, the risk for MI increases 10-fold in women who smoke and also is higher for women older than 35 years.24 Guidelines caution physicians against prescribing COCs to women who smoke and are older than 35 years.13
The risk for localized breast cancer in patients taking COCs has been debated.9,19,20,24 One study found a relative risk for breast cancer of 1.24 while taking the drug and an increased risk for up to 4 years following cessation of treatment.31 However, the risk completely disappeared within 10 years, there was no increased lifetime risk, and the type of cancer was less advanced.24 None of the data show an increased risk for breast cancer in women aged 35 to 64 years who previously used or currently use COCs.32 Mortality rates from breast cancer do not increase with the use of COCs.33
Combined oral contraceptives only increase the risk for cervical cancer after 5 years of use.19,24 The risk for cervical cancer immediately begins to decline following cessation of use and is returned to baseline by 10 years.34 One study found no association between the use of COCs and cervical intraepithelial neoplasia grades 1 or higher among women with human papillomavirus, even beyond 5 years of COC use.35
It is important to stress to patients that the risks for COCs have been fairly well elucidated and are substantially less concerning than previously believed if the patient is otherwise healthy.4 Both patients and physicians may believe that these medications are inherently unsafe,16,36,37 but these fears are unfounded and contribute to the underutilization of COCs.
Benefits
There also are benefits of COCs that extend beyond their positive effect on acne.19 Combined oral contraceptives decrease the risk for endometrial and ovarian cancer.24 This protection continues for at least 20 years following discontinuation of the medication.
Combined oral contraceptives also may protect women from colorectal cancer.9,37 Additionally, there is less risk for symptomatic pelvic inflammatory disease, ectopic pregnancy, and benign breast disease, along with improved cycle control, less frequent instances of dysmenorrhea, and increased bone density. Combined oral contraceptives prevent unintended pregnancy, which can pose a substantial health risk, as well as psychologic, social, and financial distress.9,19,24,25,38
Another benefit of COCs is for women with severe acne who may be future candidates for isotretinoin therapy. In this situation, COCs arguably are the appropriate preceding step, considering the requirement of 2 forms of contraception while taking isotretinoin. By exclusively trying a patient on COCs prior to initiation of isotretinoin therapy, the physician may be better equipped to differentiate the causal medication of mood symptoms, as both medications are cited to produce mood-related side effects.39,40 In addition, a sufficiently satisfactory response to COCs could occur, which would render treatment with isotretinoin unnecessary.
Initial Evaluation and Monitoring
Initial evaluation should include a measurement of blood pressure and a detailed patient history.19,23 Physical examination that extends beyond the skin is not necessary or indicated.16,25,41 Pelvic examination, screening for cervical intraepithelial neoplasia, and breast examination are not required. Although these examinations are valuable as screening tools, they are not helpful in determining which patients should or should not undergo treatment with COCs.41-44 The American Congress of Obstetricians and Gynecologists guidelines recommend that patients initiate pelvic examination screening at 21 years of age and biennially thereafter, irrespective of the onset of sexual activity or contraception use.45 Therefore, there is no need for follow-up with a gynecologist after initiation of COCs for acne vulgaris. However, with any medication, return visits to the dermatologist should be scheduled to monitor clinical progress and side effects. Periodic blood pressure measurements also are recommended.19
The dermatologist may elect to perform a pregnancy test to enable the patient to start the medication that same day.16,38 Described as the quick-start approach, beginning to use COCs in the health care provider’s office under direct observation has been found to improve short-term compliance compared to initiation after next menses. The rate of pregnancy within the first 6 months of initiation of COCs was lower for those who used the quick-start approach.46 However, patients must be advised to use a separate method of contraception for the first week to avoid an unintended pregnancy.16 Alternatively, if the clinician and patient decide to initiate treatment at or immediately after the onset of the next menstrual cycle, a pregnancy test is not needed.38 The longer the patient delays the initiation of the COC, the less chance she will be adherent.38,41 No other routine laboratory testing is indicated in an otherwise healthy patient with an unremarkable personal and family history.16
Counseling
When prescribing COCs, appropriate counseling regarding when to start is critical. The patient must take the pill each day and be aware of what to do if she skips 1 or more pills (Figure, see PDF).47 The chance of pregnancy (1%–3%), the lack of protection against sexually transmitted diseases, and the dangers of concomitant smoking should be discussed.16,19 Patients should establish a daily routine for taking the COC to maximize compliance.48 Misconceptions surrounding COCs and pelvic examinations, future fertility or teratogenicity, and risk for weight gain should be dispelled.16,36,38,49 Finally, the patient should know that it might take several months to see antiacne results,5,7 and she should not discontinue the medication if it does not work right away. The patient should be given ample time to ask questions and the issues discussed should be documented.
Conclusion
Although some dermatologists may be hesitant to prescribe hormonal therapies, COCs can be safely and appropriately used in eligible female patients with acne. We provided guidelines for the selection of COCs, reviewed the risks and benefits, and described ways to monitor and counsel patients.
- Layton AM. A review on the treatment of acne vulgaris. Int J Clin Pract. 2006;60:64-72.
- Tan J. Hormonal treatment of acne: review of current best evidence. J Cutan Med Surg. 2004;(8, suppl 4):S11-S15.
- Helms SE, Bredle DL, Zajic J, et al. Oral contraceptive failure rates and oral antibiotics. J Am Acad Dermatol. 1997;36(5, pt 1):705-710.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin. no. 73: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
- Katsambas A, Papakonstantinou A. Acne: systemic treatment. Clin Dermatol. 2004;22:412-418.
- Essah PA, Wickham EP 3rd, Nunley JR, et al. Dermatology of androgen-related disorders. Clin Dermatol. 2006;24: 289-298.
- Johnson BA, Nunley JR. Use of systemic agents in the treatment of acne vulgaris [published correction in Am Fam Physician. 2001;63:following 1295]. Am Fam Physician. 2000;62:1823-1830.
- Golden NH, Carlson JL. The pathophysiology of amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:163-178.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Lucky AW, Henderson TA, Olson WH, et al. Effectiveness of norgestimate and ethinyl estradiol in treating moderate acne vulgaris. J Am Acad Dermatol. 1997;37 (5, pt 1):746-754.
- Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89:615-622.
- Estrostep Fe [package insert]. Rockaway, NJ: Warner Chilcott US; 2009.
- Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease [published correction in J Am Coll Cardiol. 2009;53:904]. J Am Coll Cardiol. 2009; 53:221-231.
- Goodman G. Managing acne vulgaris effectively. Aust Fam Physician. 2006;35:705-709.
- Haroun M. Hormonal therapy of acne. J Cutan Med Surg. 2004;(8, suppl 4):S6-S10.
- Rimsza ME. Counseling the adolescent about contraception. Pediatr Rev. 2003;24:162-170.
- Strauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management [published online ahead of print February 5, 2007]. J Am Acad Dermatol. 2007;56:651-663.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2007;1:CD004425.
- Petitti DB. Clinical practice. combination estrogen-progestin oral contraceptives [published correction in N Engl J Med. 2004;350:92]. N Engl J Med. 2003;349:1443-1450.
- Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22:419-428.
- Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
- Spencer AL, Bonnema R, McNamara MC. Helping women choose appropriate hormonal contraception: update on risks, benefits, and indications. Am J Med. 2009;122: 497-506.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva, Switzerland: World Health Organization; 2004. http://whqlibdoc.who.int/publications/2004/9241562668.pdf. Accessed July 25, 2012.
- Practice Committee of the American Society for Reproductive Medicine. Hormonal contraception: recent advances and controversies. Fertil Steril. 2006;86(5, suppl 1):S229-S235.
- Deligeoroglou E, Christopoulos P, Creatsas G. Contraception in adolescence. Ann N Y Acad Sci. 2006;1092:78-90.
- Lawrenson R, Farmer R. Venous thromboembolism and combined oral contraceptives: does the type of progestogen make a difference? Contraception. 2000;62 (suppl 2):21S-28S.
- Suissa S, Blais L, Spitzer WO, et al. First-time use of newer oral contraceptives and the risk of venous thromboembolism. Contraception. 1997;56:141-146.
- Tan JK, Ediriweera C. Efficacy and safety of combined ethinyl estradiol/drospirenone oral contraceptives in the treatment of acne. Int J Womens Health. 2010;1:213-221.
- Martinez F, Avecilla A. Combined hormonal contraception and venous thromboembolism. Eur J Contracept Reprod Health Care. 2007;12:97-106.
- Carr BR, Ory H. Estrogen and progestin components of oral contraceptives: relationship to vascular disease. Contraception. 1997;55:267-272.
- Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1996;347: 1713-1727.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
- Faculty of Family Planning and Reproductive Health Care, Clinical Effectiveness Unit. FFPRHC Guidance (October 2003): first prescription of combined oral contraception. J Fam Plann Reprod Health Care. 2003;29:209-222.
- Appleby P, Beral V, Berrington de González A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370: 1609-1621.
- Harris TG, Miller L, Kulasingam SL, et al. Depotmedroxyprogesterone acetate and combined oral contraceptive use and cervical neoplasia among women with oncogenic human papillomavirus infection. Am J Obstet Gynecol. 2009;200:489, e1-e8.
- Lee J, Jezewski MA. Attitudes toward oral contraceptive use among women of reproductive age: a systematic review. ANS Adv Nurs Sci. 2007;30:e85-e103.
- Fernandez E, La Vecchia C, Balducci A, et al. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
- Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
- Segebladh B, Borgström A, Odlind V, et al. Prevalence of psychiatric disorders and premenstrual dysphoric symptoms in patients with experience of adverse mood during treatment with combined oral contraceptives [published online ahead of print September 11, 2008]. Contraception. 2009;79:50-55.
- Brelsford M, Beute TC. Preventing and managing the side effects of isotretinoin. Semin Cutan Med Surg. 2008;27: 197-206.
- Gardner J, Miller L. Promoting the safety and use of hormonal contraceptives. J Womens Health (Larchmt). 2005;14:53-60.
- Meckstroth KR. Physical examination before initiating hormonal contraception: what is necessary? Am Fam Physician. 2006;74:32, 34.
- Scott A, Glasier AF. Are routine breast and pelvic examinations necessary for women starting combined oral contraception [published online ahead of print June 10, 2004]? Hum Reprod Update. 2004;10:449-452.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 109: cervical cytology screening. Obstet Gynecol. 2009;114:1409-1420.
- Westhoff C, Heartwell S, Edwards S, et al. Initiation of oral contraceptives using a quick start compared with a conventional start: a randomized controlled trial. Obstet Gynecol. 2007;109:1270-1276.
- World Health Organization. Selected Practice Recommendations for Contraceptive Use. 2nd ed. Geneva, Switzerland: World Health Organization; 2005.
- Rosenberg MJ, Burnhill MS, Waugh MS, et al. Compliance and oral contraceptives: a review. Contraception. 1995;52:137-141.
- Cheung E, Free C. Factors influencing young women’s decision making regarding hormonal contraceptives: a qualitative study. Contraception. 2005;71:426-431.
- Layton AM. A review on the treatment of acne vulgaris. Int J Clin Pract. 2006;60:64-72.
- Tan J. Hormonal treatment of acne: review of current best evidence. J Cutan Med Surg. 2004;(8, suppl 4):S11-S15.
- Helms SE, Bredle DL, Zajic J, et al. Oral contraceptive failure rates and oral antibiotics. J Am Acad Dermatol. 1997;36(5, pt 1):705-710.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin. no. 73: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
- Katsambas A, Papakonstantinou A. Acne: systemic treatment. Clin Dermatol. 2004;22:412-418.
- Essah PA, Wickham EP 3rd, Nunley JR, et al. Dermatology of androgen-related disorders. Clin Dermatol. 2006;24: 289-298.
- Johnson BA, Nunley JR. Use of systemic agents in the treatment of acne vulgaris [published correction in Am Fam Physician. 2001;63:following 1295]. Am Fam Physician. 2000;62:1823-1830.
- Golden NH, Carlson JL. The pathophysiology of amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:163-178.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Lucky AW, Henderson TA, Olson WH, et al. Effectiveness of norgestimate and ethinyl estradiol in treating moderate acne vulgaris. J Am Acad Dermatol. 1997;37 (5, pt 1):746-754.
- Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89:615-622.
- Estrostep Fe [package insert]. Rockaway, NJ: Warner Chilcott US; 2009.
- Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease [published correction in J Am Coll Cardiol. 2009;53:904]. J Am Coll Cardiol. 2009; 53:221-231.
- Goodman G. Managing acne vulgaris effectively. Aust Fam Physician. 2006;35:705-709.
- Haroun M. Hormonal therapy of acne. J Cutan Med Surg. 2004;(8, suppl 4):S6-S10.
- Rimsza ME. Counseling the adolescent about contraception. Pediatr Rev. 2003;24:162-170.
- Strauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management [published online ahead of print February 5, 2007]. J Am Acad Dermatol. 2007;56:651-663.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2007;1:CD004425.
- Petitti DB. Clinical practice. combination estrogen-progestin oral contraceptives [published correction in N Engl J Med. 2004;350:92]. N Engl J Med. 2003;349:1443-1450.
- Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22:419-428.
- Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
- Spencer AL, Bonnema R, McNamara MC. Helping women choose appropriate hormonal contraception: update on risks, benefits, and indications. Am J Med. 2009;122: 497-506.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva, Switzerland: World Health Organization; 2004. http://whqlibdoc.who.int/publications/2004/9241562668.pdf. Accessed July 25, 2012.
- Practice Committee of the American Society for Reproductive Medicine. Hormonal contraception: recent advances and controversies. Fertil Steril. 2006;86(5, suppl 1):S229-S235.
- Deligeoroglou E, Christopoulos P, Creatsas G. Contraception in adolescence. Ann N Y Acad Sci. 2006;1092:78-90.
- Lawrenson R, Farmer R. Venous thromboembolism and combined oral contraceptives: does the type of progestogen make a difference? Contraception. 2000;62 (suppl 2):21S-28S.
- Suissa S, Blais L, Spitzer WO, et al. First-time use of newer oral contraceptives and the risk of venous thromboembolism. Contraception. 1997;56:141-146.
- Tan JK, Ediriweera C. Efficacy and safety of combined ethinyl estradiol/drospirenone oral contraceptives in the treatment of acne. Int J Womens Health. 2010;1:213-221.
- Martinez F, Avecilla A. Combined hormonal contraception and venous thromboembolism. Eur J Contracept Reprod Health Care. 2007;12:97-106.
- Carr BR, Ory H. Estrogen and progestin components of oral contraceptives: relationship to vascular disease. Contraception. 1997;55:267-272.
- Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1996;347: 1713-1727.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
- Faculty of Family Planning and Reproductive Health Care, Clinical Effectiveness Unit. FFPRHC Guidance (October 2003): first prescription of combined oral contraception. J Fam Plann Reprod Health Care. 2003;29:209-222.
- Appleby P, Beral V, Berrington de González A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370: 1609-1621.
- Harris TG, Miller L, Kulasingam SL, et al. Depotmedroxyprogesterone acetate and combined oral contraceptive use and cervical neoplasia among women with oncogenic human papillomavirus infection. Am J Obstet Gynecol. 2009;200:489, e1-e8.
- Lee J, Jezewski MA. Attitudes toward oral contraceptive use among women of reproductive age: a systematic review. ANS Adv Nurs Sci. 2007;30:e85-e103.
- Fernandez E, La Vecchia C, Balducci A, et al. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
- Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
- Segebladh B, Borgström A, Odlind V, et al. Prevalence of psychiatric disorders and premenstrual dysphoric symptoms in patients with experience of adverse mood during treatment with combined oral contraceptives [published online ahead of print September 11, 2008]. Contraception. 2009;79:50-55.
- Brelsford M, Beute TC. Preventing and managing the side effects of isotretinoin. Semin Cutan Med Surg. 2008;27: 197-206.
- Gardner J, Miller L. Promoting the safety and use of hormonal contraceptives. J Womens Health (Larchmt). 2005;14:53-60.
- Meckstroth KR. Physical examination before initiating hormonal contraception: what is necessary? Am Fam Physician. 2006;74:32, 34.
- Scott A, Glasier AF. Are routine breast and pelvic examinations necessary for women starting combined oral contraception [published online ahead of print June 10, 2004]? Hum Reprod Update. 2004;10:449-452.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 109: cervical cytology screening. Obstet Gynecol. 2009;114:1409-1420.
- Westhoff C, Heartwell S, Edwards S, et al. Initiation of oral contraceptives using a quick start compared with a conventional start: a randomized controlled trial. Obstet Gynecol. 2007;109:1270-1276.
- World Health Organization. Selected Practice Recommendations for Contraceptive Use. 2nd ed. Geneva, Switzerland: World Health Organization; 2005.
- Rosenberg MJ, Burnhill MS, Waugh MS, et al. Compliance and oral contraceptives: a review. Contraception. 1995;52:137-141.
- Cheung E, Free C. Factors influencing young women’s decision making regarding hormonal contraceptives: a qualitative study. Contraception. 2005;71:426-431.
Acneiform Eruptions Induced by Epidermal Growth Factor Receptor Inhibitors: Treatment With Oral Isotretinoin
Whey Protein Precipitating Moderate to Severe Acne Flares in 5 Teenaged Athletes
Drug Samples Found to Sway Acne Prescribing
RALEIGH, N.C. – Offering free drug samples to newly diagnosed acne patients was found to increase the likelihood of prescribing more expensive medications, according to a new study.
"The benefits of free sample distribution in dermatology clinics must be weighed against the significant negative impact that free samples have on prescribing and prescription costs. Clinical trials comparing the efficacy of new branded generic drugs with existing alternatives should increasingly be used to justify their increased retail cost," said study investigator Michael Hurley, a Stanford (Calif.) University medical student.
The investigators analyzed all prescriptions written for newly diagnosed acne patients in 2010 at two dermatology clinics, one in an academic medical center that does not allow samples, and the second at an affiliated neighborhood clinic that does.
At the no-samples clinic, 17% of prescriptions written by office-based dermatologists for acne patients at their initial office visit were for branded or branded-generic drugs, compared with a 74% rate at the neighborhood clinic allowing free samples.
The average prescription costs were also higher at the clinic that uses free samples. The average retail cost of the top 20 prescribed acne medications at each site (which collectively accounted for roughly 70% of all acne prescriptions) was $204 at the neighborhood clinic, compared with $70.49 at the clinic with no free samples. After the average number of prescriptions written per visit was taken into account, this amounted to a cost difference of about $260 per office visit, reported Mr. Hurley.
In a multivariate regression analysis accounting for patient characteristics and other potential confounding factors, dermatologists who provided free samples of acne drugs were 3.4-fold more likely to prescribe a branded or branded generic drug than a less expensive generic.
After identifying the marked difference in dermatologists’ acne drug prescribing patterns at the two clinics in Northern California, Mr. Hurley and his coinvestigators analyzed national data to establish trends.
Data from the National Disease and Therapeutic Index – a national survey of physician self-reported office visits, diagnoses, and treatments conducted by IMS Health – were assessed. The data showed that in 2010, 79% of all prescriptions written by office-based dermatologists for acne patients at their initial visit were for branded or branded-generic drugs.
The data also found that the proportion of acne prescriptions written with a free sample increased from 38% to 51% in the last decade. Prescriptions for branded generic medications rose similarly, most likely because of the increased use of free samples of those drugs, said Mr. Hurley. Meanwhile the percentage of acne prescriptions for generic drugs has remained flat, and in absolute numbers has actually decreased, he said.
Upon close scrutiny of dermatologists’ acne prescribing nationally in 2010, 2005, and 2001, Mr. Hurley concluded that although dermatologists’ drug preferences do change over time as new medications enter the market, their prescribing consistently remains closely related to what’s available as a free sample.
For example, during 2010 the top five acne medications prescribed by dermatologists were (in descending order) Epiduo, doxycycline hyclate, Metrogel, Solodyn, and Differin. The top five prescribed with a free sample were Epiduo, Metrogel, Solodyn, Ziana, and Oracea.
The top five agents prescribed overall in 2005 were Differin, Benzaclin, Duac, Retin-A-Micro, and doxycycline hyclate, whereas the top five prescribed with a free sample during that year were Differin, Duac, Benzaclin, Retin-A-Micro, and Metrogel.
Mr. Hurley reported having no financial conflicts.
RALEIGH, N.C. – Offering free drug samples to newly diagnosed acne patients was found to increase the likelihood of prescribing more expensive medications, according to a new study.
"The benefits of free sample distribution in dermatology clinics must be weighed against the significant negative impact that free samples have on prescribing and prescription costs. Clinical trials comparing the efficacy of new branded generic drugs with existing alternatives should increasingly be used to justify their increased retail cost," said study investigator Michael Hurley, a Stanford (Calif.) University medical student.
The investigators analyzed all prescriptions written for newly diagnosed acne patients in 2010 at two dermatology clinics, one in an academic medical center that does not allow samples, and the second at an affiliated neighborhood clinic that does.
At the no-samples clinic, 17% of prescriptions written by office-based dermatologists for acne patients at their initial office visit were for branded or branded-generic drugs, compared with a 74% rate at the neighborhood clinic allowing free samples.
The average prescription costs were also higher at the clinic that uses free samples. The average retail cost of the top 20 prescribed acne medications at each site (which collectively accounted for roughly 70% of all acne prescriptions) was $204 at the neighborhood clinic, compared with $70.49 at the clinic with no free samples. After the average number of prescriptions written per visit was taken into account, this amounted to a cost difference of about $260 per office visit, reported Mr. Hurley.
In a multivariate regression analysis accounting for patient characteristics and other potential confounding factors, dermatologists who provided free samples of acne drugs were 3.4-fold more likely to prescribe a branded or branded generic drug than a less expensive generic.
After identifying the marked difference in dermatologists’ acne drug prescribing patterns at the two clinics in Northern California, Mr. Hurley and his coinvestigators analyzed national data to establish trends.
Data from the National Disease and Therapeutic Index – a national survey of physician self-reported office visits, diagnoses, and treatments conducted by IMS Health – were assessed. The data showed that in 2010, 79% of all prescriptions written by office-based dermatologists for acne patients at their initial visit were for branded or branded-generic drugs.
The data also found that the proportion of acne prescriptions written with a free sample increased from 38% to 51% in the last decade. Prescriptions for branded generic medications rose similarly, most likely because of the increased use of free samples of those drugs, said Mr. Hurley. Meanwhile the percentage of acne prescriptions for generic drugs has remained flat, and in absolute numbers has actually decreased, he said.
Upon close scrutiny of dermatologists’ acne prescribing nationally in 2010, 2005, and 2001, Mr. Hurley concluded that although dermatologists’ drug preferences do change over time as new medications enter the market, their prescribing consistently remains closely related to what’s available as a free sample.
For example, during 2010 the top five acne medications prescribed by dermatologists were (in descending order) Epiduo, doxycycline hyclate, Metrogel, Solodyn, and Differin. The top five prescribed with a free sample were Epiduo, Metrogel, Solodyn, Ziana, and Oracea.
The top five agents prescribed overall in 2005 were Differin, Benzaclin, Duac, Retin-A-Micro, and doxycycline hyclate, whereas the top five prescribed with a free sample during that year were Differin, Duac, Benzaclin, Retin-A-Micro, and Metrogel.
Mr. Hurley reported having no financial conflicts.
RALEIGH, N.C. – Offering free drug samples to newly diagnosed acne patients was found to increase the likelihood of prescribing more expensive medications, according to a new study.
"The benefits of free sample distribution in dermatology clinics must be weighed against the significant negative impact that free samples have on prescribing and prescription costs. Clinical trials comparing the efficacy of new branded generic drugs with existing alternatives should increasingly be used to justify their increased retail cost," said study investigator Michael Hurley, a Stanford (Calif.) University medical student.
The investigators analyzed all prescriptions written for newly diagnosed acne patients in 2010 at two dermatology clinics, one in an academic medical center that does not allow samples, and the second at an affiliated neighborhood clinic that does.
At the no-samples clinic, 17% of prescriptions written by office-based dermatologists for acne patients at their initial office visit were for branded or branded-generic drugs, compared with a 74% rate at the neighborhood clinic allowing free samples.
The average prescription costs were also higher at the clinic that uses free samples. The average retail cost of the top 20 prescribed acne medications at each site (which collectively accounted for roughly 70% of all acne prescriptions) was $204 at the neighborhood clinic, compared with $70.49 at the clinic with no free samples. After the average number of prescriptions written per visit was taken into account, this amounted to a cost difference of about $260 per office visit, reported Mr. Hurley.
In a multivariate regression analysis accounting for patient characteristics and other potential confounding factors, dermatologists who provided free samples of acne drugs were 3.4-fold more likely to prescribe a branded or branded generic drug than a less expensive generic.
After identifying the marked difference in dermatologists’ acne drug prescribing patterns at the two clinics in Northern California, Mr. Hurley and his coinvestigators analyzed national data to establish trends.
Data from the National Disease and Therapeutic Index – a national survey of physician self-reported office visits, diagnoses, and treatments conducted by IMS Health – were assessed. The data showed that in 2010, 79% of all prescriptions written by office-based dermatologists for acne patients at their initial visit were for branded or branded-generic drugs.
The data also found that the proportion of acne prescriptions written with a free sample increased from 38% to 51% in the last decade. Prescriptions for branded generic medications rose similarly, most likely because of the increased use of free samples of those drugs, said Mr. Hurley. Meanwhile the percentage of acne prescriptions for generic drugs has remained flat, and in absolute numbers has actually decreased, he said.
Upon close scrutiny of dermatologists’ acne prescribing nationally in 2010, 2005, and 2001, Mr. Hurley concluded that although dermatologists’ drug preferences do change over time as new medications enter the market, their prescribing consistently remains closely related to what’s available as a free sample.
For example, during 2010 the top five acne medications prescribed by dermatologists were (in descending order) Epiduo, doxycycline hyclate, Metrogel, Solodyn, and Differin. The top five prescribed with a free sample were Epiduo, Metrogel, Solodyn, Ziana, and Oracea.
The top five agents prescribed overall in 2005 were Differin, Benzaclin, Duac, Retin-A-Micro, and doxycycline hyclate, whereas the top five prescribed with a free sample during that year were Differin, Duac, Benzaclin, Retin-A-Micro, and Metrogel.
Mr. Hurley reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: At the no-samples clinic, 17% of prescriptions written by office-based dermatologists for acne patients at their initial office visit were for branded or branded-generic drugs, compared with a 74% rate at the neighborhood clinic allowing free samples.
Data Source: All prescriptions written for newly diagnosed acne patients in 2010 at two dermatology clinics were analyzed, one in an academic medical center that does not allow samples, and the second at an affiliated neighborhood clinic that does.
Disclosures: Mr. Hurley reported having no financial conflicts.
Acne and Rosacea: Epidemiology, Diagnosis, and Treatment [book review]
Effects of Topical Retinoid Therapy on Acne Lesions: A Psychometric Assessment
Ziana Proves Less Irritating Than Epiduo for Acne
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
Data Source: Results were taken from a double-blind, randomized, split-face comparative trial involving 24 patients with mild to moderate facial acne.
Disclosures: The study was sponsored by Medicis.