User login
Pembrolizumab enhances CAR T-cell persistence in relapsed ALL
CHICAGO—Three of 6 pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL) whose CD19 chimeric antigen receptor (CAR) T cells did not persist even after reinfusion demonstrated persistence when the PD-1 checkpoint inhibitor pembrolizumab was added to the regimen.
CAR T cells can persist for months or even years and have the potential to mediate long-term disease control.
But some patients recover their normal B cells, which is a marker of loss of functional CAR T cells. These patients are at a higher risk of disease relapse.
Investigators, therefore, undertook a pilot study to determine whether PD-1 checkpoint pathway inhibition can improve CAR T persistence in these patients.
Shannon L. Maude, MD, of the Children’s Hospital of Philadelphia and the University of Pennsylvania Perelman School of Medicine, shared some of the patient cases in this pilot study at the ASCO 2017 Annual Meeting (Abstract 103*).
The investigators hypothesized that possible anti-murine immunogenicity could be causing poor CAR T-cell persistence, since the first CAR T developed used scFv domains of murine origin.
If T-cell exhaustion caused poor CAR T-cell persistence, immune checkpoints might play a role. In this case, combination with PD-1 checkpoint blockade could improve persistence, they hypothesized.
The investigators proposed to administer a repeat CAR T-cell infusion for relapsed or refractory ALL patients with poor persistence and add pembrolizumab after retreatment if the patients still had poor persistence. Patients were offered the option of another reinfusion prior to treatment with pembrolizumab if their CAR T-cell persistence continued to be poor.
Investigators added pembrolizumab no earlier than 14 days after infusion and only after patients recovered from cytokine release syndrome (CRS).
The infusions in the pilot study were humanized CART19 (huCART19, CTL119), unless otherwise specified.
Patient 1 – Pembrolizumab for partial response
This patient had no response to the prior murine CD19 CAR infusion, but responded well to infusion with huCART19 with good CAR T-cell proliferation.
By day 28, the patient had achieved a complete response (CR) in bone marrow but had a minimal residual disease (MRD) level of 1.2%.
At 7 weeks, the patient had a CD19+ relapse with low levels of huCART19.
Investigators added pembrolizumb on day 52 after infusion, and the patient had a modest increase in huCART19.
The patient had a temporary clearance of peripheral blasts followed by disease progression.
Patient 2 – Pembrolizumab for no response
This patient had a CD19+ relapse at 12 months after prior murine CD19 CAR infusion.
The patient was treated with huCART19, had good proliferation, but a rapid drop of CART19, and no response of the disease with a CD19+ relapse.
The patient had a reinfusion of huCART19 at 6 weeks and investigators added pembrolizumab on day 14 after reinfusion.
The patient experienced good huCART19 proliferation and prolonged persistence, but by day 28 had persistent disease, this time with decreased expression of CD19+ cells.
Patient 3 – Pembrolizumab for poor persistence
This patient had a CR with a prior murine CD19 CAR, but had poor persistence and early B cell recovery at 2 months.
The patient had a CD19+ relapse, was treated with huCART19, and had good proliferation again.
The patient achieved an MRD-negative CR, but because of short persistence and early B-cell recovery at 2 months, relapsed at 15 months.
The patient was reinfused at 17 months and pembrolizumab was added on day 14. The patient entered CR with prolonged persistence, but ultimately B-cell recovery occurred.
The patient was reinfused a third time, and pembrolizumab was added to the regimen every 3 weeks. The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 4 – Pembrolizumab for poor persistence
This patient had achieved a CR with murine CAR19, but had a CD19+ relapse at 9 months. The patient then received huCART19, had good proliferation and achieved a CR, but had short persistence and B-cell recovery at 2 months.
The patient relapsed at 12 months and was reinfused with huCART19 at 14 months.
Pembrolizumab was added on day 14 after reinfusion, but the patient had no huCART19 proliferation, no response, and CD19+ MRD.
Patient 5 – Pembrolizumab for poor persistence
The patient responded to a prior murine CART19, but had a CD19+ relapse at 12 months.
The patient was infused with huCART19 and had good proliferation but short persistence. The patient was reinfused at 6 months, but again had short persistence.
The patient was reinfused again at 8 months because of B-cell recovery, and pembrolizumab was added on day 14 and administered every 3 weeks thereafter.
The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 6 – Pembrolizumab for lymphomatous disease
This patient, who had widespread lymphadenopathy and M3 bone marrow, had not received prior CAR T cells and was treated for the first time with a murine CART19 for r/r ALL.
The patient had good expansion of CART19, but by day 28, PET scan showed widespread lymph node disease despite CR in the bone marrow.
The patient was given pembrolizumab on day 32 after infusion and every 2-3 weeks thereafter. After the addition of pembrolizumab, the patient had increased CART19 cells in blood and a significant decrease in PET-avid disease.
Summary
Three of six patients achieved objective clinical responses with pembrolizumab: 2 had prolonged B-cell aplasia, and another had a decrease in PET-avid lymphomatous disease.
The addition of pembrolizumab was also well tolerated by the patients, with minimal side effects of fever in 2 patients, cytopenias in 2 patients, and no instances of severe CRS.
The investigators believe the addition of checkpoint pathway inhibitors has the potential to prolong CAR T-cell persistence and warrants further investigation. ![]()
CHICAGO—Three of 6 pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL) whose CD19 chimeric antigen receptor (CAR) T cells did not persist even after reinfusion demonstrated persistence when the PD-1 checkpoint inhibitor pembrolizumab was added to the regimen.
CAR T cells can persist for months or even years and have the potential to mediate long-term disease control.
But some patients recover their normal B cells, which is a marker of loss of functional CAR T cells. These patients are at a higher risk of disease relapse.
Investigators, therefore, undertook a pilot study to determine whether PD-1 checkpoint pathway inhibition can improve CAR T persistence in these patients.
Shannon L. Maude, MD, of the Children’s Hospital of Philadelphia and the University of Pennsylvania Perelman School of Medicine, shared some of the patient cases in this pilot study at the ASCO 2017 Annual Meeting (Abstract 103*).
The investigators hypothesized that possible anti-murine immunogenicity could be causing poor CAR T-cell persistence, since the first CAR T developed used scFv domains of murine origin.
If T-cell exhaustion caused poor CAR T-cell persistence, immune checkpoints might play a role. In this case, combination with PD-1 checkpoint blockade could improve persistence, they hypothesized.
The investigators proposed to administer a repeat CAR T-cell infusion for relapsed or refractory ALL patients with poor persistence and add pembrolizumab after retreatment if the patients still had poor persistence. Patients were offered the option of another reinfusion prior to treatment with pembrolizumab if their CAR T-cell persistence continued to be poor.
Investigators added pembrolizumab no earlier than 14 days after infusion and only after patients recovered from cytokine release syndrome (CRS).
The infusions in the pilot study were humanized CART19 (huCART19, CTL119), unless otherwise specified.
Patient 1 – Pembrolizumab for partial response
This patient had no response to the prior murine CD19 CAR infusion, but responded well to infusion with huCART19 with good CAR T-cell proliferation.
By day 28, the patient had achieved a complete response (CR) in bone marrow but had a minimal residual disease (MRD) level of 1.2%.
At 7 weeks, the patient had a CD19+ relapse with low levels of huCART19.
Investigators added pembrolizumb on day 52 after infusion, and the patient had a modest increase in huCART19.
The patient had a temporary clearance of peripheral blasts followed by disease progression.
Patient 2 – Pembrolizumab for no response
This patient had a CD19+ relapse at 12 months after prior murine CD19 CAR infusion.
The patient was treated with huCART19, had good proliferation, but a rapid drop of CART19, and no response of the disease with a CD19+ relapse.
The patient had a reinfusion of huCART19 at 6 weeks and investigators added pembrolizumab on day 14 after reinfusion.
The patient experienced good huCART19 proliferation and prolonged persistence, but by day 28 had persistent disease, this time with decreased expression of CD19+ cells.
Patient 3 – Pembrolizumab for poor persistence
This patient had a CR with a prior murine CD19 CAR, but had poor persistence and early B cell recovery at 2 months.
The patient had a CD19+ relapse, was treated with huCART19, and had good proliferation again.
The patient achieved an MRD-negative CR, but because of short persistence and early B-cell recovery at 2 months, relapsed at 15 months.
The patient was reinfused at 17 months and pembrolizumab was added on day 14. The patient entered CR with prolonged persistence, but ultimately B-cell recovery occurred.
The patient was reinfused a third time, and pembrolizumab was added to the regimen every 3 weeks. The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 4 – Pembrolizumab for poor persistence
This patient had achieved a CR with murine CAR19, but had a CD19+ relapse at 9 months. The patient then received huCART19, had good proliferation and achieved a CR, but had short persistence and B-cell recovery at 2 months.
The patient relapsed at 12 months and was reinfused with huCART19 at 14 months.
Pembrolizumab was added on day 14 after reinfusion, but the patient had no huCART19 proliferation, no response, and CD19+ MRD.
Patient 5 – Pembrolizumab for poor persistence
The patient responded to a prior murine CART19, but had a CD19+ relapse at 12 months.
The patient was infused with huCART19 and had good proliferation but short persistence. The patient was reinfused at 6 months, but again had short persistence.
The patient was reinfused again at 8 months because of B-cell recovery, and pembrolizumab was added on day 14 and administered every 3 weeks thereafter.
The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 6 – Pembrolizumab for lymphomatous disease
This patient, who had widespread lymphadenopathy and M3 bone marrow, had not received prior CAR T cells and was treated for the first time with a murine CART19 for r/r ALL.
The patient had good expansion of CART19, but by day 28, PET scan showed widespread lymph node disease despite CR in the bone marrow.
The patient was given pembrolizumab on day 32 after infusion and every 2-3 weeks thereafter. After the addition of pembrolizumab, the patient had increased CART19 cells in blood and a significant decrease in PET-avid disease.
Summary
Three of six patients achieved objective clinical responses with pembrolizumab: 2 had prolonged B-cell aplasia, and another had a decrease in PET-avid lymphomatous disease.
The addition of pembrolizumab was also well tolerated by the patients, with minimal side effects of fever in 2 patients, cytopenias in 2 patients, and no instances of severe CRS.
The investigators believe the addition of checkpoint pathway inhibitors has the potential to prolong CAR T-cell persistence and warrants further investigation. ![]()
CHICAGO—Three of 6 pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL) whose CD19 chimeric antigen receptor (CAR) T cells did not persist even after reinfusion demonstrated persistence when the PD-1 checkpoint inhibitor pembrolizumab was added to the regimen.
CAR T cells can persist for months or even years and have the potential to mediate long-term disease control.
But some patients recover their normal B cells, which is a marker of loss of functional CAR T cells. These patients are at a higher risk of disease relapse.
Investigators, therefore, undertook a pilot study to determine whether PD-1 checkpoint pathway inhibition can improve CAR T persistence in these patients.
Shannon L. Maude, MD, of the Children’s Hospital of Philadelphia and the University of Pennsylvania Perelman School of Medicine, shared some of the patient cases in this pilot study at the ASCO 2017 Annual Meeting (Abstract 103*).
The investigators hypothesized that possible anti-murine immunogenicity could be causing poor CAR T-cell persistence, since the first CAR T developed used scFv domains of murine origin.
If T-cell exhaustion caused poor CAR T-cell persistence, immune checkpoints might play a role. In this case, combination with PD-1 checkpoint blockade could improve persistence, they hypothesized.
The investigators proposed to administer a repeat CAR T-cell infusion for relapsed or refractory ALL patients with poor persistence and add pembrolizumab after retreatment if the patients still had poor persistence. Patients were offered the option of another reinfusion prior to treatment with pembrolizumab if their CAR T-cell persistence continued to be poor.
Investigators added pembrolizumab no earlier than 14 days after infusion and only after patients recovered from cytokine release syndrome (CRS).
The infusions in the pilot study were humanized CART19 (huCART19, CTL119), unless otherwise specified.
Patient 1 – Pembrolizumab for partial response
This patient had no response to the prior murine CD19 CAR infusion, but responded well to infusion with huCART19 with good CAR T-cell proliferation.
By day 28, the patient had achieved a complete response (CR) in bone marrow but had a minimal residual disease (MRD) level of 1.2%.
At 7 weeks, the patient had a CD19+ relapse with low levels of huCART19.
Investigators added pembrolizumb on day 52 after infusion, and the patient had a modest increase in huCART19.
The patient had a temporary clearance of peripheral blasts followed by disease progression.
Patient 2 – Pembrolizumab for no response
This patient had a CD19+ relapse at 12 months after prior murine CD19 CAR infusion.
The patient was treated with huCART19, had good proliferation, but a rapid drop of CART19, and no response of the disease with a CD19+ relapse.
The patient had a reinfusion of huCART19 at 6 weeks and investigators added pembrolizumab on day 14 after reinfusion.
The patient experienced good huCART19 proliferation and prolonged persistence, but by day 28 had persistent disease, this time with decreased expression of CD19+ cells.
Patient 3 – Pembrolizumab for poor persistence
This patient had a CR with a prior murine CD19 CAR, but had poor persistence and early B cell recovery at 2 months.
The patient had a CD19+ relapse, was treated with huCART19, and had good proliferation again.
The patient achieved an MRD-negative CR, but because of short persistence and early B-cell recovery at 2 months, relapsed at 15 months.
The patient was reinfused at 17 months and pembrolizumab was added on day 14. The patient entered CR with prolonged persistence, but ultimately B-cell recovery occurred.
The patient was reinfused a third time, and pembrolizumab was added to the regimen every 3 weeks. The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 4 – Pembrolizumab for poor persistence
This patient had achieved a CR with murine CAR19, but had a CD19+ relapse at 9 months. The patient then received huCART19, had good proliferation and achieved a CR, but had short persistence and B-cell recovery at 2 months.
The patient relapsed at 12 months and was reinfused with huCART19 at 14 months.
Pembrolizumab was added on day 14 after reinfusion, but the patient had no huCART19 proliferation, no response, and CD19+ MRD.
Patient 5 – Pembrolizumab for poor persistence
The patient responded to a prior murine CART19, but had a CD19+ relapse at 12 months.
The patient was infused with huCART19 and had good proliferation but short persistence. The patient was reinfused at 6 months, but again had short persistence.
The patient was reinfused again at 8 months because of B-cell recovery, and pembrolizumab was added on day 14 and administered every 3 weeks thereafter.
The patient is experiencing prolonged persistence and continued B-cell aplasia.
Patient 6 – Pembrolizumab for lymphomatous disease
This patient, who had widespread lymphadenopathy and M3 bone marrow, had not received prior CAR T cells and was treated for the first time with a murine CART19 for r/r ALL.
The patient had good expansion of CART19, but by day 28, PET scan showed widespread lymph node disease despite CR in the bone marrow.
The patient was given pembrolizumab on day 32 after infusion and every 2-3 weeks thereafter. After the addition of pembrolizumab, the patient had increased CART19 cells in blood and a significant decrease in PET-avid disease.
Summary
Three of six patients achieved objective clinical responses with pembrolizumab: 2 had prolonged B-cell aplasia, and another had a decrease in PET-avid lymphomatous disease.
The addition of pembrolizumab was also well tolerated by the patients, with minimal side effects of fever in 2 patients, cytopenias in 2 patients, and no instances of severe CRS.
The investigators believe the addition of checkpoint pathway inhibitors has the potential to prolong CAR T-cell persistence and warrants further investigation. ![]()
CAR T cells elicit durable, potent responses in kids with EM relapse of ALL
CHICAGO—Outcomes for pediatric patients with relapsed acute lymphoblastic leukemia (ALL) are dismal, with the probability of event-free survival ranging from 15% to 70% after a first relapse to 15% to 20% after a second relapse.
“So novel therapies are obviously urgently needed,” Mala Kiran Talekar, MD, of the Children's Hospital of Philadelphia in Pennsylvania, affirmed. “And herein comes the role of CAR T cells as a breakthrough therapy for relapsed/refractory pediatric ALL.”
She presented the outcome of chimeric antigen receptor (CAR) T-cell therapy in pediatric patients with non-CNS extramedullary (EM) relapse at the ASCO 2017 Annual meeting as abstract 10507.
The investigators had drawn the patient population for this analysis from 2 CAR studies, CTL019 and CTL119.
CTL019, which had already been completed, employed a murine CAR, and CTL119 is ongoing and uses a humanized CAR.
Of the 60 patients enrolled in CTL019, 56 (93%) achieved a complete response (CR) at day 28, and 100% had a CNS remission. Their 12-month overall survival (OS) was 79%.
“[K]eep in mind, when the study first started,” Dr Talekar said, “the patient population that had been referred to us was patients who had suffered a second or greater relapse or had been refractory to forms of treatment available to them, and the majority had been refractory to multiple therapies.”
The humanized CAR study, CTL119, is divided into 2 cohorts—one with CAR-naïve patients (n=22) and the other a CAR-retreatment arm (n=15) with patients who had received previous CAR therapy and relapsed.
Dr Talekar explained that the humanized CAR was made with the intention of decreasing rejection or loss of persistence of the T cells related to murine antigenicity.
Nine patients (60%) in the CAR-retreatment arm achieved a CR at day 28, and at 6 months, 78% experienced relapse-free survival (RFS) with a median follow-up of 12 months.
All of the CAR-naïve patients achieved CR at day 28, with 86% achieving RFS at 6 months, with a median follow-up of 10 months.
ALL with EM involvement
The investigators identified 10 pediatric patients treated in the murine (n=6) or humanized (n=4) trials who had received CAR therapy for isolated extramedullary disease or for combined bone marrow extramedullary (BM/EM) relapse of ALL.
They defined EM relapse as involvement of a non-CNS site confirmed by imaging with or without pathology within 12 months of CAR T-cell infusion. After infusion, patients had diagnostic imaging performed at 1, 3, 6, 9, and 12 months.
Of the 10 patients, 5 had active EM involvement at the time of infusion, 2 had isolated EM relapse—1 with parotid and multifocal bony lesions and 1 with testis and sinus lesions—and 5 had multiple sites of EM relapse.
The patients had 2 to 4 prior ALL relapses, 2 had prior local radiation to the EM site, and all 10 had received prior bone marrow transplants.
Three patients had an MLL rearrangement, 1 had hypodiploid ALL, and 1 had trisomy 21.
Nine of the 10 patients achieved MRD-negative CR at day 28.
One patient was not evaluable because his disease progressed within 2 weeks of CAR therapy in both the bone marrow and EM site. He died 6 weeks after the infusion.
Five patients evaluated by serial imaging had objective responses. Two had no evidence of EM disease by day 28, 2 had resolution by 3 months, and 1 had continued decrease in the size of her uterine mass at 3 and 6 months. She underwent hysterectomy at 8 months with no evidence of disease on pathology.
Four patients with a prior history of skin or testicular involvement had no evidence of disease by exam at day 28.
Three of the 9 patients relapsed with CD19+ disease. One had skin/medullary involvement and died at 38 months after CAR T-cell infusion. And 2 had medullary disease: 1 died at 17 months and 1 is alive at 28 months.
The remaining 6 patients are alive and well at a median follow-up of 10 months (range, 3 – 16 months) without recurrence of disease.
The investigators therefore concluded that single agent CAR T-cell immunotherapy can induce potent and durable response in patients with EM relapse of their ALL. ![]()
CHICAGO—Outcomes for pediatric patients with relapsed acute lymphoblastic leukemia (ALL) are dismal, with the probability of event-free survival ranging from 15% to 70% after a first relapse to 15% to 20% after a second relapse.
“So novel therapies are obviously urgently needed,” Mala Kiran Talekar, MD, of the Children's Hospital of Philadelphia in Pennsylvania, affirmed. “And herein comes the role of CAR T cells as a breakthrough therapy for relapsed/refractory pediatric ALL.”
She presented the outcome of chimeric antigen receptor (CAR) T-cell therapy in pediatric patients with non-CNS extramedullary (EM) relapse at the ASCO 2017 Annual meeting as abstract 10507.
The investigators had drawn the patient population for this analysis from 2 CAR studies, CTL019 and CTL119.
CTL019, which had already been completed, employed a murine CAR, and CTL119 is ongoing and uses a humanized CAR.
Of the 60 patients enrolled in CTL019, 56 (93%) achieved a complete response (CR) at day 28, and 100% had a CNS remission. Their 12-month overall survival (OS) was 79%.
“[K]eep in mind, when the study first started,” Dr Talekar said, “the patient population that had been referred to us was patients who had suffered a second or greater relapse or had been refractory to forms of treatment available to them, and the majority had been refractory to multiple therapies.”
The humanized CAR study, CTL119, is divided into 2 cohorts—one with CAR-naïve patients (n=22) and the other a CAR-retreatment arm (n=15) with patients who had received previous CAR therapy and relapsed.
Dr Talekar explained that the humanized CAR was made with the intention of decreasing rejection or loss of persistence of the T cells related to murine antigenicity.
Nine patients (60%) in the CAR-retreatment arm achieved a CR at day 28, and at 6 months, 78% experienced relapse-free survival (RFS) with a median follow-up of 12 months.
All of the CAR-naïve patients achieved CR at day 28, with 86% achieving RFS at 6 months, with a median follow-up of 10 months.
ALL with EM involvement
The investigators identified 10 pediatric patients treated in the murine (n=6) or humanized (n=4) trials who had received CAR therapy for isolated extramedullary disease or for combined bone marrow extramedullary (BM/EM) relapse of ALL.
They defined EM relapse as involvement of a non-CNS site confirmed by imaging with or without pathology within 12 months of CAR T-cell infusion. After infusion, patients had diagnostic imaging performed at 1, 3, 6, 9, and 12 months.
Of the 10 patients, 5 had active EM involvement at the time of infusion, 2 had isolated EM relapse—1 with parotid and multifocal bony lesions and 1 with testis and sinus lesions—and 5 had multiple sites of EM relapse.
The patients had 2 to 4 prior ALL relapses, 2 had prior local radiation to the EM site, and all 10 had received prior bone marrow transplants.
Three patients had an MLL rearrangement, 1 had hypodiploid ALL, and 1 had trisomy 21.
Nine of the 10 patients achieved MRD-negative CR at day 28.
One patient was not evaluable because his disease progressed within 2 weeks of CAR therapy in both the bone marrow and EM site. He died 6 weeks after the infusion.
Five patients evaluated by serial imaging had objective responses. Two had no evidence of EM disease by day 28, 2 had resolution by 3 months, and 1 had continued decrease in the size of her uterine mass at 3 and 6 months. She underwent hysterectomy at 8 months with no evidence of disease on pathology.
Four patients with a prior history of skin or testicular involvement had no evidence of disease by exam at day 28.
Three of the 9 patients relapsed with CD19+ disease. One had skin/medullary involvement and died at 38 months after CAR T-cell infusion. And 2 had medullary disease: 1 died at 17 months and 1 is alive at 28 months.
The remaining 6 patients are alive and well at a median follow-up of 10 months (range, 3 – 16 months) without recurrence of disease.
The investigators therefore concluded that single agent CAR T-cell immunotherapy can induce potent and durable response in patients with EM relapse of their ALL. ![]()
CHICAGO—Outcomes for pediatric patients with relapsed acute lymphoblastic leukemia (ALL) are dismal, with the probability of event-free survival ranging from 15% to 70% after a first relapse to 15% to 20% after a second relapse.
“So novel therapies are obviously urgently needed,” Mala Kiran Talekar, MD, of the Children's Hospital of Philadelphia in Pennsylvania, affirmed. “And herein comes the role of CAR T cells as a breakthrough therapy for relapsed/refractory pediatric ALL.”
She presented the outcome of chimeric antigen receptor (CAR) T-cell therapy in pediatric patients with non-CNS extramedullary (EM) relapse at the ASCO 2017 Annual meeting as abstract 10507.
The investigators had drawn the patient population for this analysis from 2 CAR studies, CTL019 and CTL119.
CTL019, which had already been completed, employed a murine CAR, and CTL119 is ongoing and uses a humanized CAR.
Of the 60 patients enrolled in CTL019, 56 (93%) achieved a complete response (CR) at day 28, and 100% had a CNS remission. Their 12-month overall survival (OS) was 79%.
“[K]eep in mind, when the study first started,” Dr Talekar said, “the patient population that had been referred to us was patients who had suffered a second or greater relapse or had been refractory to forms of treatment available to them, and the majority had been refractory to multiple therapies.”
The humanized CAR study, CTL119, is divided into 2 cohorts—one with CAR-naïve patients (n=22) and the other a CAR-retreatment arm (n=15) with patients who had received previous CAR therapy and relapsed.
Dr Talekar explained that the humanized CAR was made with the intention of decreasing rejection or loss of persistence of the T cells related to murine antigenicity.
Nine patients (60%) in the CAR-retreatment arm achieved a CR at day 28, and at 6 months, 78% experienced relapse-free survival (RFS) with a median follow-up of 12 months.
All of the CAR-naïve patients achieved CR at day 28, with 86% achieving RFS at 6 months, with a median follow-up of 10 months.
ALL with EM involvement
The investigators identified 10 pediatric patients treated in the murine (n=6) or humanized (n=4) trials who had received CAR therapy for isolated extramedullary disease or for combined bone marrow extramedullary (BM/EM) relapse of ALL.
They defined EM relapse as involvement of a non-CNS site confirmed by imaging with or without pathology within 12 months of CAR T-cell infusion. After infusion, patients had diagnostic imaging performed at 1, 3, 6, 9, and 12 months.
Of the 10 patients, 5 had active EM involvement at the time of infusion, 2 had isolated EM relapse—1 with parotid and multifocal bony lesions and 1 with testis and sinus lesions—and 5 had multiple sites of EM relapse.
The patients had 2 to 4 prior ALL relapses, 2 had prior local radiation to the EM site, and all 10 had received prior bone marrow transplants.
Three patients had an MLL rearrangement, 1 had hypodiploid ALL, and 1 had trisomy 21.
Nine of the 10 patients achieved MRD-negative CR at day 28.
One patient was not evaluable because his disease progressed within 2 weeks of CAR therapy in both the bone marrow and EM site. He died 6 weeks after the infusion.
Five patients evaluated by serial imaging had objective responses. Two had no evidence of EM disease by day 28, 2 had resolution by 3 months, and 1 had continued decrease in the size of her uterine mass at 3 and 6 months. She underwent hysterectomy at 8 months with no evidence of disease on pathology.
Four patients with a prior history of skin or testicular involvement had no evidence of disease by exam at day 28.
Three of the 9 patients relapsed with CD19+ disease. One had skin/medullary involvement and died at 38 months after CAR T-cell infusion. And 2 had medullary disease: 1 died at 17 months and 1 is alive at 28 months.
The remaining 6 patients are alive and well at a median follow-up of 10 months (range, 3 – 16 months) without recurrence of disease.
The investigators therefore concluded that single agent CAR T-cell immunotherapy can induce potent and durable response in patients with EM relapse of their ALL. ![]()
Severe health conditions decrease among childhood cancer survivors
CHICAGO—The 15-year cumulative incidence of severe health conditions for survivors of childhood cancer has decreased over the past 30 years, from 12.7% for those diagnosed in the 1970s to 10.1% and 8.9% for those diagnosed in the 1980s and 1990s, respectively. And the decreases were greatest for patients with Wilms’ tumor and Hodgkin lymphoma (HL), followed by patients with astrocytoma, non-Hodgkin lymphoma (NHL), and acute lymphoblastic leukemia (ALL).
Investigators of the Childhood Cancer Survivor Study (CCSS) undertook a retrospective cohort analysis of children aged 0 – 14 years diagnosed with cancer between 1970 and 1999. Their goal was to determine whether cancer therapy modifications have maintained cure rates while decreasing the risk of late effects of therapy.
Todd M. Gibson, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, presented the findings at the 2017 annual meeting of the American Society for Clinical Oncology (ASCO) as abstract LBA10500.
Researchers analyzed data from 23,600 childhood cancer survivors in the CCSS who were alive 5 years after diagnosis. The patients had leukemia, lymphoma, CNS malignancies, Wilms tumor, neuroblastoma, or soft-tissue/bone sarcoma.
Dr Gibson noted that while 83% of children with a malignancy achieve a 5-year survival, more than half develop at least one severe, disabling, life-threatening health condition by age 50.
The survivors were a median age at last follow-up of 28 years (range, 5-63) and the median time since diagnosis was 21 years (range, 5-43).
The investigators found significant decreases in severe health conditions in 6 diagnostic groups:
- Wilms tumor, decreased from 13% to 5% (P<0.0001)
- HL, decreased from 18% to 11% (P<0.0001)
- Astrocytoma, decreased from 15% to 9% (P=0.004)
- NHL, decreased from 10% to 6% (P=0.04)
- ALL, decreased from 9% to 7% (P=0.002)
- Ewings sarcoma, decreased from 19% to 10% (P=0.01)
They found no reductions in subsequent severe health conditions among survivors of neuroblastoma, acute myeloid leukemia (AML), soft tissue sarcoma, or osteosarcoma.
The investigators believe the decreases were driven mainly by a reduced incidence of endocrine conditions, subsequent malignant neoplasms, gastrointestinal and neurological conditions, but not cardiac or pulmonary conditions.
They also analyzed the reduction in treatment intensities by decade for different diseases and found they correlated with the reduced incidence of serious chronic health conditions by 15 years after diagnosis.
The National Institutes of Health funded the study.
CHICAGO—The 15-year cumulative incidence of severe health conditions for survivors of childhood cancer has decreased over the past 30 years, from 12.7% for those diagnosed in the 1970s to 10.1% and 8.9% for those diagnosed in the 1980s and 1990s, respectively. And the decreases were greatest for patients with Wilms’ tumor and Hodgkin lymphoma (HL), followed by patients with astrocytoma, non-Hodgkin lymphoma (NHL), and acute lymphoblastic leukemia (ALL).
Investigators of the Childhood Cancer Survivor Study (CCSS) undertook a retrospective cohort analysis of children aged 0 – 14 years diagnosed with cancer between 1970 and 1999. Their goal was to determine whether cancer therapy modifications have maintained cure rates while decreasing the risk of late effects of therapy.
Todd M. Gibson, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, presented the findings at the 2017 annual meeting of the American Society for Clinical Oncology (ASCO) as abstract LBA10500.
Researchers analyzed data from 23,600 childhood cancer survivors in the CCSS who were alive 5 years after diagnosis. The patients had leukemia, lymphoma, CNS malignancies, Wilms tumor, neuroblastoma, or soft-tissue/bone sarcoma.
Dr Gibson noted that while 83% of children with a malignancy achieve a 5-year survival, more than half develop at least one severe, disabling, life-threatening health condition by age 50.
The survivors were a median age at last follow-up of 28 years (range, 5-63) and the median time since diagnosis was 21 years (range, 5-43).
The investigators found significant decreases in severe health conditions in 6 diagnostic groups:
- Wilms tumor, decreased from 13% to 5% (P<0.0001)
- HL, decreased from 18% to 11% (P<0.0001)
- Astrocytoma, decreased from 15% to 9% (P=0.004)
- NHL, decreased from 10% to 6% (P=0.04)
- ALL, decreased from 9% to 7% (P=0.002)
- Ewings sarcoma, decreased from 19% to 10% (P=0.01)
They found no reductions in subsequent severe health conditions among survivors of neuroblastoma, acute myeloid leukemia (AML), soft tissue sarcoma, or osteosarcoma.
The investigators believe the decreases were driven mainly by a reduced incidence of endocrine conditions, subsequent malignant neoplasms, gastrointestinal and neurological conditions, but not cardiac or pulmonary conditions.
They also analyzed the reduction in treatment intensities by decade for different diseases and found they correlated with the reduced incidence of serious chronic health conditions by 15 years after diagnosis.
The National Institutes of Health funded the study.
CHICAGO—The 15-year cumulative incidence of severe health conditions for survivors of childhood cancer has decreased over the past 30 years, from 12.7% for those diagnosed in the 1970s to 10.1% and 8.9% for those diagnosed in the 1980s and 1990s, respectively. And the decreases were greatest for patients with Wilms’ tumor and Hodgkin lymphoma (HL), followed by patients with astrocytoma, non-Hodgkin lymphoma (NHL), and acute lymphoblastic leukemia (ALL).
Investigators of the Childhood Cancer Survivor Study (CCSS) undertook a retrospective cohort analysis of children aged 0 – 14 years diagnosed with cancer between 1970 and 1999. Their goal was to determine whether cancer therapy modifications have maintained cure rates while decreasing the risk of late effects of therapy.
Todd M. Gibson, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, presented the findings at the 2017 annual meeting of the American Society for Clinical Oncology (ASCO) as abstract LBA10500.
Researchers analyzed data from 23,600 childhood cancer survivors in the CCSS who were alive 5 years after diagnosis. The patients had leukemia, lymphoma, CNS malignancies, Wilms tumor, neuroblastoma, or soft-tissue/bone sarcoma.
Dr Gibson noted that while 83% of children with a malignancy achieve a 5-year survival, more than half develop at least one severe, disabling, life-threatening health condition by age 50.
The survivors were a median age at last follow-up of 28 years (range, 5-63) and the median time since diagnosis was 21 years (range, 5-43).
The investigators found significant decreases in severe health conditions in 6 diagnostic groups:
- Wilms tumor, decreased from 13% to 5% (P<0.0001)
- HL, decreased from 18% to 11% (P<0.0001)
- Astrocytoma, decreased from 15% to 9% (P=0.004)
- NHL, decreased from 10% to 6% (P=0.04)
- ALL, decreased from 9% to 7% (P=0.002)
- Ewings sarcoma, decreased from 19% to 10% (P=0.01)
They found no reductions in subsequent severe health conditions among survivors of neuroblastoma, acute myeloid leukemia (AML), soft tissue sarcoma, or osteosarcoma.
The investigators believe the decreases were driven mainly by a reduced incidence of endocrine conditions, subsequent malignant neoplasms, gastrointestinal and neurological conditions, but not cardiac or pulmonary conditions.
They also analyzed the reduction in treatment intensities by decade for different diseases and found they correlated with the reduced incidence of serious chronic health conditions by 15 years after diagnosis.
The National Institutes of Health funded the study.
Differences emerge in new guidelines for managing FN in kids
A multidisciplinary, international panel of experts has updated earlier clinical practice guidelines on managing fever and neutropenia (FN) in children with cancer and in those undergoing hematopoietic stem cell transplantation (HSCT). And while most of the recommendations remained unchanged from the 2012 guidelines, a few key differences emerged. The changes included addition of a 4th generation cephalosporin for empirical antifungal therapy and refinements in risk stratification for invasive fungal disease (IFD), among others.
The new guidelines were published by The International Pediatric Fever and Neutropenia Guideline Panel in the Journal of Clinical Oncology.
The recommendations were organized into 3 major sections: initial presentation, ongoing management, and empirical antifungal therapy. The guidelines panel followed procedures previously validated for creating evidence-based guidelines and used the Appraisal of Guidelines for Research & Evaluation II instrument as a framework.
For the initial presentation of FN, the panel increased the quality of evidence from low to moderate in the recommendation to obtain peripheral blood cultures concurrent with central venous catheter cultures.
In the treatment of FN, the panel added a 4th-generation cephalosporin as empirical therapy in high-risk FN.
The panel refined the IFD risk factors and decreased the quality of evidence from moderate to low. Children with acute myeloid leukemia (AML), high-risk acute lymphoblastic leukemia (ALL), relapsed acute leukemia, those undergoing allogeneic HSCT, those with prolonged neutropenia, and those receiving high-dose corticosteroids are at high risk of IFD. All others should be categorized as IFD low risk.
The panel suggested serum galactomannan not be used to guide empirical antifungal management for prolonged FN lasting 96 hours or more in high-risk IFD patients. GM does not rule out non-Aspergillus molds, and therefore high negative values provide less useful predictions. Previously, the use of galactomannan was a weak recommendation.
The panel added a new recommendation against using fungal polymerase chain reaction (PCR) testing in blood. They explained PCR testing provides poor positive predictive values and negative predictive values are not sufficiently high to be clinically useful. Also, PCR testing is not yet standardized.
Another new recommendation is the addition of imaging of the abdomen in patients without localizing signs or symptoms. Even though the ideal imaging modality is not known, ultrasound is readily available, not associated with radiation exposure, and usually does not require sedation. For these reasons, the panel said it is preferable to computed tomography or magnetic resonance imaging.
The panel also changed a previously weak recommendation to administer empirical therapy for IFD low-risk patients with prolonged FN to a weak recommendation against administering therapy for these patients.
The panel's recommendations and their rationale can be found in the JCO article.
The guidelines update was supported by meeting grants from the Canadian Institutes of Health Research and the Garron Comprehensive Cancer Centre. ![]()
A multidisciplinary, international panel of experts has updated earlier clinical practice guidelines on managing fever and neutropenia (FN) in children with cancer and in those undergoing hematopoietic stem cell transplantation (HSCT). And while most of the recommendations remained unchanged from the 2012 guidelines, a few key differences emerged. The changes included addition of a 4th generation cephalosporin for empirical antifungal therapy and refinements in risk stratification for invasive fungal disease (IFD), among others.
The new guidelines were published by The International Pediatric Fever and Neutropenia Guideline Panel in the Journal of Clinical Oncology.
The recommendations were organized into 3 major sections: initial presentation, ongoing management, and empirical antifungal therapy. The guidelines panel followed procedures previously validated for creating evidence-based guidelines and used the Appraisal of Guidelines for Research & Evaluation II instrument as a framework.
For the initial presentation of FN, the panel increased the quality of evidence from low to moderate in the recommendation to obtain peripheral blood cultures concurrent with central venous catheter cultures.
In the treatment of FN, the panel added a 4th-generation cephalosporin as empirical therapy in high-risk FN.
The panel refined the IFD risk factors and decreased the quality of evidence from moderate to low. Children with acute myeloid leukemia (AML), high-risk acute lymphoblastic leukemia (ALL), relapsed acute leukemia, those undergoing allogeneic HSCT, those with prolonged neutropenia, and those receiving high-dose corticosteroids are at high risk of IFD. All others should be categorized as IFD low risk.
The panel suggested serum galactomannan not be used to guide empirical antifungal management for prolonged FN lasting 96 hours or more in high-risk IFD patients. GM does not rule out non-Aspergillus molds, and therefore high negative values provide less useful predictions. Previously, the use of galactomannan was a weak recommendation.
The panel added a new recommendation against using fungal polymerase chain reaction (PCR) testing in blood. They explained PCR testing provides poor positive predictive values and negative predictive values are not sufficiently high to be clinically useful. Also, PCR testing is not yet standardized.
Another new recommendation is the addition of imaging of the abdomen in patients without localizing signs or symptoms. Even though the ideal imaging modality is not known, ultrasound is readily available, not associated with radiation exposure, and usually does not require sedation. For these reasons, the panel said it is preferable to computed tomography or magnetic resonance imaging.
The panel also changed a previously weak recommendation to administer empirical therapy for IFD low-risk patients with prolonged FN to a weak recommendation against administering therapy for these patients.
The panel's recommendations and their rationale can be found in the JCO article.
The guidelines update was supported by meeting grants from the Canadian Institutes of Health Research and the Garron Comprehensive Cancer Centre. ![]()
A multidisciplinary, international panel of experts has updated earlier clinical practice guidelines on managing fever and neutropenia (FN) in children with cancer and in those undergoing hematopoietic stem cell transplantation (HSCT). And while most of the recommendations remained unchanged from the 2012 guidelines, a few key differences emerged. The changes included addition of a 4th generation cephalosporin for empirical antifungal therapy and refinements in risk stratification for invasive fungal disease (IFD), among others.
The new guidelines were published by The International Pediatric Fever and Neutropenia Guideline Panel in the Journal of Clinical Oncology.
The recommendations were organized into 3 major sections: initial presentation, ongoing management, and empirical antifungal therapy. The guidelines panel followed procedures previously validated for creating evidence-based guidelines and used the Appraisal of Guidelines for Research & Evaluation II instrument as a framework.
For the initial presentation of FN, the panel increased the quality of evidence from low to moderate in the recommendation to obtain peripheral blood cultures concurrent with central venous catheter cultures.
In the treatment of FN, the panel added a 4th-generation cephalosporin as empirical therapy in high-risk FN.
The panel refined the IFD risk factors and decreased the quality of evidence from moderate to low. Children with acute myeloid leukemia (AML), high-risk acute lymphoblastic leukemia (ALL), relapsed acute leukemia, those undergoing allogeneic HSCT, those with prolonged neutropenia, and those receiving high-dose corticosteroids are at high risk of IFD. All others should be categorized as IFD low risk.
The panel suggested serum galactomannan not be used to guide empirical antifungal management for prolonged FN lasting 96 hours or more in high-risk IFD patients. GM does not rule out non-Aspergillus molds, and therefore high negative values provide less useful predictions. Previously, the use of galactomannan was a weak recommendation.
The panel added a new recommendation against using fungal polymerase chain reaction (PCR) testing in blood. They explained PCR testing provides poor positive predictive values and negative predictive values are not sufficiently high to be clinically useful. Also, PCR testing is not yet standardized.
Another new recommendation is the addition of imaging of the abdomen in patients without localizing signs or symptoms. Even though the ideal imaging modality is not known, ultrasound is readily available, not associated with radiation exposure, and usually does not require sedation. For these reasons, the panel said it is preferable to computed tomography or magnetic resonance imaging.
The panel also changed a previously weak recommendation to administer empirical therapy for IFD low-risk patients with prolonged FN to a weak recommendation against administering therapy for these patients.
The panel's recommendations and their rationale can be found in the JCO article.
The guidelines update was supported by meeting grants from the Canadian Institutes of Health Research and the Garron Comprehensive Cancer Centre. ![]()
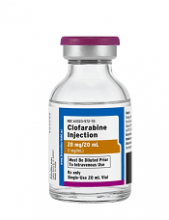
First generic version of clofarabine available in US
Clofarabine Injection, the first-to-market generic version of Sanofi Genzyme’s Clolar, is now available in the US.
The generic, a product of Fresenius Kabi, is available as a single dose vial containing 20 mg per 20 mL clofarabine.
Clofarabine is a purine nucleoside metabolic inhibitor indicated for the treatment of patients ages 1 to 21 with relapsed or refractory acute lymphoblastic leukemia (ALL) who received at least 2 prior treatment regimens.
Clolar was granted accelerated approval for this indication in the US in 2004.
The approval was based on response rates observed in ALL patients. There are no trials verifying that clofarabine confers improvement in survival or disease-related symptoms in ALL patients.
Clofarabine was assessed in a single-arm, phase 2 trial of 61 pediatric patients with relapsed/refractory ALL.
The patients’ median age was 12 (range, 1 to 20 years), and their median number of prior treatment regimens was 3 (range, 2 to 6).
The patients received clofarabine at 52 mg/m2 intravenously over 2 hours daily for 5 days, every 2 to 6 weeks.
The overall response rate was 30%. Seven patient achieved a complete response (CR), 5 had a CR without platelet recovery, and 6 patients had a partial response.
The median duration of CR in patients who did not go on to hematopoietic stem cell transplant was 6 weeks.
The most common grade 3 or higher adverse events were febrile neutropenia, anorexia, hypotension, and nausea.
These results were published in the Journal of Clinical Oncology in 2006. ![]()
Clofarabine Injection, the first-to-market generic version of Sanofi Genzyme’s Clolar, is now available in the US.
The generic, a product of Fresenius Kabi, is available as a single dose vial containing 20 mg per 20 mL clofarabine.
Clofarabine is a purine nucleoside metabolic inhibitor indicated for the treatment of patients ages 1 to 21 with relapsed or refractory acute lymphoblastic leukemia (ALL) who received at least 2 prior treatment regimens.
Clolar was granted accelerated approval for this indication in the US in 2004.
The approval was based on response rates observed in ALL patients. There are no trials verifying that clofarabine confers improvement in survival or disease-related symptoms in ALL patients.
Clofarabine was assessed in a single-arm, phase 2 trial of 61 pediatric patients with relapsed/refractory ALL.
The patients’ median age was 12 (range, 1 to 20 years), and their median number of prior treatment regimens was 3 (range, 2 to 6).
The patients received clofarabine at 52 mg/m2 intravenously over 2 hours daily for 5 days, every 2 to 6 weeks.
The overall response rate was 30%. Seven patient achieved a complete response (CR), 5 had a CR without platelet recovery, and 6 patients had a partial response.
The median duration of CR in patients who did not go on to hematopoietic stem cell transplant was 6 weeks.
The most common grade 3 or higher adverse events were febrile neutropenia, anorexia, hypotension, and nausea.
These results were published in the Journal of Clinical Oncology in 2006. ![]()
Clofarabine Injection, the first-to-market generic version of Sanofi Genzyme’s Clolar, is now available in the US.
The generic, a product of Fresenius Kabi, is available as a single dose vial containing 20 mg per 20 mL clofarabine.
Clofarabine is a purine nucleoside metabolic inhibitor indicated for the treatment of patients ages 1 to 21 with relapsed or refractory acute lymphoblastic leukemia (ALL) who received at least 2 prior treatment regimens.
Clolar was granted accelerated approval for this indication in the US in 2004.
The approval was based on response rates observed in ALL patients. There are no trials verifying that clofarabine confers improvement in survival or disease-related symptoms in ALL patients.
Clofarabine was assessed in a single-arm, phase 2 trial of 61 pediatric patients with relapsed/refractory ALL.
The patients’ median age was 12 (range, 1 to 20 years), and their median number of prior treatment regimens was 3 (range, 2 to 6).
The patients received clofarabine at 52 mg/m2 intravenously over 2 hours daily for 5 days, every 2 to 6 weeks.
The overall response rate was 30%. Seven patient achieved a complete response (CR), 5 had a CR without platelet recovery, and 6 patients had a partial response.
The median duration of CR in patients who did not go on to hematopoietic stem cell transplant was 6 weeks.
The most common grade 3 or higher adverse events were febrile neutropenia, anorexia, hypotension, and nausea.
These results were published in the Journal of Clinical Oncology in 2006. ![]()
Antibody shows potential for treating AML, B-ALL
Endoglin may be a promising therapeutic target in acute myeloid leukemia (AML) and B-cell acute lymphoblastic leukemia (B-ALL), according to researchers.
The group identified endoglin expression on the majority of blasts from patients with AML and B-ALL.
The team also found that an endoglin antibody, TRC105 (carotuximab), exhibited activity against AML and B-ALL in vivo, and combining the drug with chemotherapeutic agents enhanced this activity.
Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis, and her colleagues reported these findings in Blood.
The researchers first discovered that endoglin, which is also known as CD105, was “highly expressed” in leukemic blasts.
In samples from AML patients, 47.6% to 98.5% of blasts were CD105+. In samples from B-ALL patients, 92.6% to 99% of blasts were CD105+.
“We have been studying the function of endoglin in hematopoiesis for more than a decade, and the consistent expression of this receptor in the majority of acute leukemias was intriguing,” Dr Perlingeiro said.
She and her colleagues also found that CD105+ blasts had superior leukemogenic activity and reduced survival in mice when compared to CD105- blasts.
Mice injected with AML CD105+ blasts had all died at day 110 after injection, but mice injected with CD105- AML blasts survived until day 140.
Mice injected with CD105+ ALL blasts died 3 months after injection, but mice injected with CD105- ALL blasts were still alive and showed no signs of disease at the time of sacrifice, which was 5 months after injection.
TRC105 monotherapy
Several experiments showed that TRC105 could reduce leukemic activity in vivo.
TRC105 reduced blast counts in the peripheral blood of mice that had been injected with AML blasts. The drug also reduced blasts in the bone marrow initially, though blast counts were comparable in treated mice and controls by week 12.
On the other hand, mice treated with TRC105 did not experience the weight loss and splenomegaly observed in control mice. And TRC105 suppressed the ability of AML blasts to give rise to leukemia in secondary recipient mice.
In mice injected with ALL blasts, TRC105 initially decreased blast counts. However, by week 8, blast counts in the peripheral blood, bone marrow, and spleen of treated mice were similar to those observed in controls. The researchers said this suggests that TRC105 only slows the development of ALL.
The team then evaluated the effects of TRC105 after disease had been established. Mice with established AML received TRC105 for 8 weeks, and mice with established ALL received TRC105 for 4 weeks.
In mice with AML, TRC105 reduced blasts in the peripheral blood and spleen but not the bone marrow. The treatment also prevented splenomegaly and weight loss and prolonged survival.
“Our hypothesis that endoglin expression was linked to leukemia-forming activity was proven to be true, and it was even more rewarding to witness the robust anti-leukemogenic effect of blocking endoglin signaling with TRC105, even when leukemia had already been established in the mouse,” Dr Perlingeiro said.
However, in mice with established ALL, TRC105 had no effect on leukemia progression.
The researchers said this could be due to expression of soluble endoglin (sENG), which would titrate the TRC105 antibody, limiting its ability to bind to membrane-bound endoglin on leukemic cells. Results of additional experiments supported this idea.
TRC105 in combination
The researchers also tested TRC105 in combination with chemotherapy in the mouse models. The team combined the antibody with cytarabine to treat AML and cyclophosphamide to treat ALL.
In mice with AML, cytarabine and TRC105 significantly reduced levels of leukemic cells in the peripheral blood.
In mice with ALL, cyclophosphamide and TRC105 suppressed leukemia development more effectively and more quickly than cyclophosphamide alone.
The researchers detected high levels of sENG in untreated mice with ALL, but levels were lower in the TRC105-treated mice. And there was “no significant detection” of sENG in mice that received cyclophosphamide and TRC105 or cyclophosphamide alone.
Dr Perlingeiro and her colleagues said this suggests the inhibitory effects of sENG can be circumvented by suppressing tumor burden, which results in the combination therapy demonstrating potent antileukemic activity. ![]()
Endoglin may be a promising therapeutic target in acute myeloid leukemia (AML) and B-cell acute lymphoblastic leukemia (B-ALL), according to researchers.
The group identified endoglin expression on the majority of blasts from patients with AML and B-ALL.
The team also found that an endoglin antibody, TRC105 (carotuximab), exhibited activity against AML and B-ALL in vivo, and combining the drug with chemotherapeutic agents enhanced this activity.
Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis, and her colleagues reported these findings in Blood.
The researchers first discovered that endoglin, which is also known as CD105, was “highly expressed” in leukemic blasts.
In samples from AML patients, 47.6% to 98.5% of blasts were CD105+. In samples from B-ALL patients, 92.6% to 99% of blasts were CD105+.
“We have been studying the function of endoglin in hematopoiesis for more than a decade, and the consistent expression of this receptor in the majority of acute leukemias was intriguing,” Dr Perlingeiro said.
She and her colleagues also found that CD105+ blasts had superior leukemogenic activity and reduced survival in mice when compared to CD105- blasts.
Mice injected with AML CD105+ blasts had all died at day 110 after injection, but mice injected with CD105- AML blasts survived until day 140.
Mice injected with CD105+ ALL blasts died 3 months after injection, but mice injected with CD105- ALL blasts were still alive and showed no signs of disease at the time of sacrifice, which was 5 months after injection.
TRC105 monotherapy
Several experiments showed that TRC105 could reduce leukemic activity in vivo.
TRC105 reduced blast counts in the peripheral blood of mice that had been injected with AML blasts. The drug also reduced blasts in the bone marrow initially, though blast counts were comparable in treated mice and controls by week 12.
On the other hand, mice treated with TRC105 did not experience the weight loss and splenomegaly observed in control mice. And TRC105 suppressed the ability of AML blasts to give rise to leukemia in secondary recipient mice.
In mice injected with ALL blasts, TRC105 initially decreased blast counts. However, by week 8, blast counts in the peripheral blood, bone marrow, and spleen of treated mice were similar to those observed in controls. The researchers said this suggests that TRC105 only slows the development of ALL.
The team then evaluated the effects of TRC105 after disease had been established. Mice with established AML received TRC105 for 8 weeks, and mice with established ALL received TRC105 for 4 weeks.
In mice with AML, TRC105 reduced blasts in the peripheral blood and spleen but not the bone marrow. The treatment also prevented splenomegaly and weight loss and prolonged survival.
“Our hypothesis that endoglin expression was linked to leukemia-forming activity was proven to be true, and it was even more rewarding to witness the robust anti-leukemogenic effect of blocking endoglin signaling with TRC105, even when leukemia had already been established in the mouse,” Dr Perlingeiro said.
However, in mice with established ALL, TRC105 had no effect on leukemia progression.
The researchers said this could be due to expression of soluble endoglin (sENG), which would titrate the TRC105 antibody, limiting its ability to bind to membrane-bound endoglin on leukemic cells. Results of additional experiments supported this idea.
TRC105 in combination
The researchers also tested TRC105 in combination with chemotherapy in the mouse models. The team combined the antibody with cytarabine to treat AML and cyclophosphamide to treat ALL.
In mice with AML, cytarabine and TRC105 significantly reduced levels of leukemic cells in the peripheral blood.
In mice with ALL, cyclophosphamide and TRC105 suppressed leukemia development more effectively and more quickly than cyclophosphamide alone.
The researchers detected high levels of sENG in untreated mice with ALL, but levels were lower in the TRC105-treated mice. And there was “no significant detection” of sENG in mice that received cyclophosphamide and TRC105 or cyclophosphamide alone.
Dr Perlingeiro and her colleagues said this suggests the inhibitory effects of sENG can be circumvented by suppressing tumor burden, which results in the combination therapy demonstrating potent antileukemic activity. ![]()
Endoglin may be a promising therapeutic target in acute myeloid leukemia (AML) and B-cell acute lymphoblastic leukemia (B-ALL), according to researchers.
The group identified endoglin expression on the majority of blasts from patients with AML and B-ALL.
The team also found that an endoglin antibody, TRC105 (carotuximab), exhibited activity against AML and B-ALL in vivo, and combining the drug with chemotherapeutic agents enhanced this activity.
Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis, and her colleagues reported these findings in Blood.
The researchers first discovered that endoglin, which is also known as CD105, was “highly expressed” in leukemic blasts.
In samples from AML patients, 47.6% to 98.5% of blasts were CD105+. In samples from B-ALL patients, 92.6% to 99% of blasts were CD105+.
“We have been studying the function of endoglin in hematopoiesis for more than a decade, and the consistent expression of this receptor in the majority of acute leukemias was intriguing,” Dr Perlingeiro said.
She and her colleagues also found that CD105+ blasts had superior leukemogenic activity and reduced survival in mice when compared to CD105- blasts.
Mice injected with AML CD105+ blasts had all died at day 110 after injection, but mice injected with CD105- AML blasts survived until day 140.
Mice injected with CD105+ ALL blasts died 3 months after injection, but mice injected with CD105- ALL blasts were still alive and showed no signs of disease at the time of sacrifice, which was 5 months after injection.
TRC105 monotherapy
Several experiments showed that TRC105 could reduce leukemic activity in vivo.
TRC105 reduced blast counts in the peripheral blood of mice that had been injected with AML blasts. The drug also reduced blasts in the bone marrow initially, though blast counts were comparable in treated mice and controls by week 12.
On the other hand, mice treated with TRC105 did not experience the weight loss and splenomegaly observed in control mice. And TRC105 suppressed the ability of AML blasts to give rise to leukemia in secondary recipient mice.
In mice injected with ALL blasts, TRC105 initially decreased blast counts. However, by week 8, blast counts in the peripheral blood, bone marrow, and spleen of treated mice were similar to those observed in controls. The researchers said this suggests that TRC105 only slows the development of ALL.
The team then evaluated the effects of TRC105 after disease had been established. Mice with established AML received TRC105 for 8 weeks, and mice with established ALL received TRC105 for 4 weeks.
In mice with AML, TRC105 reduced blasts in the peripheral blood and spleen but not the bone marrow. The treatment also prevented splenomegaly and weight loss and prolonged survival.
“Our hypothesis that endoglin expression was linked to leukemia-forming activity was proven to be true, and it was even more rewarding to witness the robust anti-leukemogenic effect of blocking endoglin signaling with TRC105, even when leukemia had already been established in the mouse,” Dr Perlingeiro said.
However, in mice with established ALL, TRC105 had no effect on leukemia progression.
The researchers said this could be due to expression of soluble endoglin (sENG), which would titrate the TRC105 antibody, limiting its ability to bind to membrane-bound endoglin on leukemic cells. Results of additional experiments supported this idea.
TRC105 in combination
The researchers also tested TRC105 in combination with chemotherapy in the mouse models. The team combined the antibody with cytarabine to treat AML and cyclophosphamide to treat ALL.
In mice with AML, cytarabine and TRC105 significantly reduced levels of leukemic cells in the peripheral blood.
In mice with ALL, cyclophosphamide and TRC105 suppressed leukemia development more effectively and more quickly than cyclophosphamide alone.
The researchers detected high levels of sENG in untreated mice with ALL, but levels were lower in the TRC105-treated mice. And there was “no significant detection” of sENG in mice that received cyclophosphamide and TRC105 or cyclophosphamide alone.
Dr Perlingeiro and her colleagues said this suggests the inhibitory effects of sENG can be circumvented by suppressing tumor burden, which results in the combination therapy demonstrating potent antileukemic activity. ![]()
Novel inhibitor proves ‘potent’ in hematologic malignancies
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
MRD better measure of ALL remission than morphology
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
FROM ASPHO 2017
Key clinical point: Patients with ALL determined to be in remission by both morphology and minimal residual disease had better outcomes than did those with discordant results.
Major finding: Event-free survival of B-ALL was 87% for patients with concordant remission findings vs. 59% for patients with discordant findings and 39% for concordant not-in-remission findings.
Data source: Retrospective review of data on 9,350 children and young adults with ALL.
Disclosures: The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
Could refractory T-ALL be daratumumab’s next frontier?
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
Key clinical point: CD38 may be a valid target for therapy against relapsed/refractory T-cell acute lymphoblastic leukemia.
Major finding: Six of seven models of early T-precursor T-ALL responded to daratumumab injections.
Data source: In vitro and in vivo studies evaluating the potential of daratumumab for treatment of T-ALL.
Disclosures: The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported having no conflicts of interest.
Cord blood/placental cell combo induces rapid immune recovery
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
Key clinical point: A combination of donor cord blood and human placenta–derived stem cells induced more rapid engraftment than cord blood alone.
Major finding: The probability of 12-month overall survival was 81%.
Data source: Open-label single-arm study in 16 children with severe malignant and nonmalignant diseases requiring hematopoietic stem cell transplants.
Disclosures: The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.