User login
FDA approves first ready-to-use oral solution of methotrexate
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
Treatment-related hypertension, kidney injury are undertreated in kids with leukemia
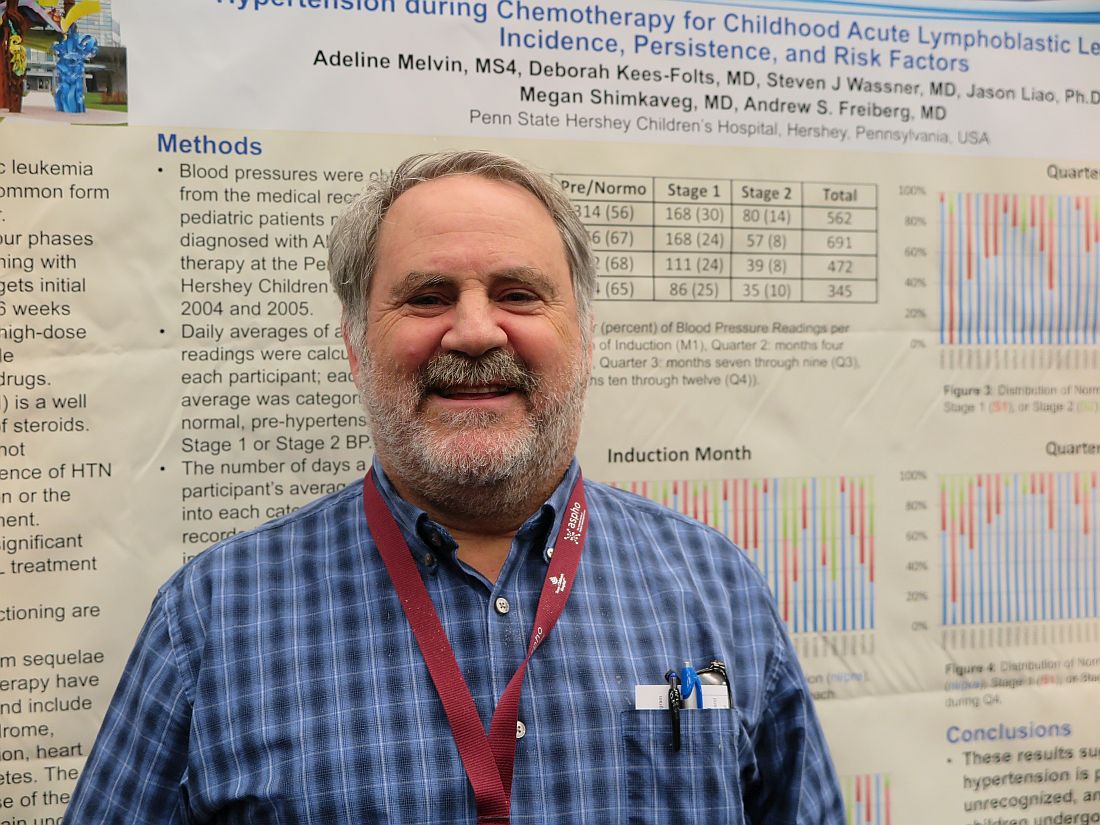
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
At ASPHO 2017
Key clinical point:
Major finding: The combined incidence of stage 1 or 2 hypertension in children during induction therapy for acute lymphoblastic leukemia was 44%.
Data source: Two retrospective institutional studies.
Disclosures: The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
CHMP recommends inotuzumab ozogamicin for adult ALL
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of inotuzumab ozogamicin (Besponsa®).
The CHMP is recommending approval of inotuzumab ozogamicin for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL), including patients with Philadelphia chromosome-positive ALL who have failed treatment with at least one tyrosine kinase inhibitor.
The CHMP’s opinion will be reviewed by the European Commission, which is expected to issue a decision on approval within 67 days from adoption of the opinion.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of inotuzumab ozogamicin (Besponsa®).
The CHMP is recommending approval of inotuzumab ozogamicin for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL), including patients with Philadelphia chromosome-positive ALL who have failed treatment with at least one tyrosine kinase inhibitor.
The CHMP’s opinion will be reviewed by the European Commission, which is expected to issue a decision on approval within 67 days from adoption of the opinion.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of inotuzumab ozogamicin (Besponsa®).
The CHMP is recommending approval of inotuzumab ozogamicin for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL), including patients with Philadelphia chromosome-positive ALL who have failed treatment with at least one tyrosine kinase inhibitor.
The CHMP’s opinion will be reviewed by the European Commission, which is expected to issue a decision on approval within 67 days from adoption of the opinion.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
ALL, HL guidelines added to radiation therapy resource
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
Second cancers take greater toll on younger patients
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Nanoparticles allow for creation of CAR T cells in vivo
Researchers say they have developed biodegradable nanoparticles that can be used to genetically reprogram T cells while they are still in the body.
The nanoparticles were able to program T cells with genes encoding leukemia-specific chimeric antigen receptors (CARs).
The resulting CAR T cells were able to eliminate leukemia or slow the progression of disease in a mouse model of B-cell acute lymphoblastic leukemia (B-ALL).
Researchers reported these results in Nature Nanotechnology.
“Our technology is the first that we know of to quickly program tumor-recognizing capabilities into T cells without extracting them for laboratory manipulation,” said Matthias Stephan, MD, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“The reprogrammed cells begin to work within 24 to 48 hours and continue to produce these receptors for weeks. This suggests that our technology has the potential to allow the immune system to quickly mount a strong enough response to destroy cancerous cells before the disease becomes fatal.”
Dr Stephan and his colleagues designed their nanoparticles to carry genes that encode for CARs intended to target and eliminate B-ALL. The nanoparticles are coated with ligands that make them seek out and bind to T cells.
When a nanoparticle binds to a T cell, the cell engulfs the particle. The nanoparticle then travels to the cell’s nucleus and dissolves.
The CAR genes integrate into chromosomes housed in the nucleus, making it possible for the T cells to begin decoding the new genes and producing CARs within 1 or 2 days.
Once they determined their CAR-carrying nanoparticles reprogrammed a noticeable percentage of T cells, the researchers tested the T cells’ efficacy in a mouse model of B-ALL.
The team infused the nanoparticles into 10 mice and found the treatment eradicated tumors in 7 of the animals. The other 3 mice “showed substantial regression” of leukemia, the researchers said.
On average, mice that received CAR-carrying nanoparticles had a 58-day improvement in survival compared to control mice.
Mice that received the nanoparticles also had “dramatically reduced” B-cell numbers in their spleens. The researchers noted that this is consistent with the reversible B-cell aplasia observed in patients who receive conventional CD19 CAR T-cell therapy.
Dr Stephan and his colleagues also tested conventional CAR T-cell therapy in the B-ALL mouse model. The mice received cyclophosphamide followed by CAR T cells created ex vivo.
These mice had significantly better survival than controls, but their survival was comparable to that of the mice that received the CAR-carrying nanoparticles.
Although these nanoparticles are several steps away from the clinic, Dr Stephan said he imagines a future in which nanoparticles transform cell-based immunotherapies into easily administered, off-the-shelf treatments that are available anywhere.
“I’ve never had cancer, but if I did get a cancer diagnosis, I would want to start treatment right away,” Dr Stephan said. “I want to make cellular immunotherapy a treatment option the day of diagnosis and have it able to be done in an outpatient setting near where people live.” ![]()
Researchers say they have developed biodegradable nanoparticles that can be used to genetically reprogram T cells while they are still in the body.
The nanoparticles were able to program T cells with genes encoding leukemia-specific chimeric antigen receptors (CARs).
The resulting CAR T cells were able to eliminate leukemia or slow the progression of disease in a mouse model of B-cell acute lymphoblastic leukemia (B-ALL).
Researchers reported these results in Nature Nanotechnology.
“Our technology is the first that we know of to quickly program tumor-recognizing capabilities into T cells without extracting them for laboratory manipulation,” said Matthias Stephan, MD, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“The reprogrammed cells begin to work within 24 to 48 hours and continue to produce these receptors for weeks. This suggests that our technology has the potential to allow the immune system to quickly mount a strong enough response to destroy cancerous cells before the disease becomes fatal.”
Dr Stephan and his colleagues designed their nanoparticles to carry genes that encode for CARs intended to target and eliminate B-ALL. The nanoparticles are coated with ligands that make them seek out and bind to T cells.
When a nanoparticle binds to a T cell, the cell engulfs the particle. The nanoparticle then travels to the cell’s nucleus and dissolves.
The CAR genes integrate into chromosomes housed in the nucleus, making it possible for the T cells to begin decoding the new genes and producing CARs within 1 or 2 days.
Once they determined their CAR-carrying nanoparticles reprogrammed a noticeable percentage of T cells, the researchers tested the T cells’ efficacy in a mouse model of B-ALL.
The team infused the nanoparticles into 10 mice and found the treatment eradicated tumors in 7 of the animals. The other 3 mice “showed substantial regression” of leukemia, the researchers said.
On average, mice that received CAR-carrying nanoparticles had a 58-day improvement in survival compared to control mice.
Mice that received the nanoparticles also had “dramatically reduced” B-cell numbers in their spleens. The researchers noted that this is consistent with the reversible B-cell aplasia observed in patients who receive conventional CD19 CAR T-cell therapy.
Dr Stephan and his colleagues also tested conventional CAR T-cell therapy in the B-ALL mouse model. The mice received cyclophosphamide followed by CAR T cells created ex vivo.
These mice had significantly better survival than controls, but their survival was comparable to that of the mice that received the CAR-carrying nanoparticles.
Although these nanoparticles are several steps away from the clinic, Dr Stephan said he imagines a future in which nanoparticles transform cell-based immunotherapies into easily administered, off-the-shelf treatments that are available anywhere.
“I’ve never had cancer, but if I did get a cancer diagnosis, I would want to start treatment right away,” Dr Stephan said. “I want to make cellular immunotherapy a treatment option the day of diagnosis and have it able to be done in an outpatient setting near where people live.” ![]()
Researchers say they have developed biodegradable nanoparticles that can be used to genetically reprogram T cells while they are still in the body.
The nanoparticles were able to program T cells with genes encoding leukemia-specific chimeric antigen receptors (CARs).
The resulting CAR T cells were able to eliminate leukemia or slow the progression of disease in a mouse model of B-cell acute lymphoblastic leukemia (B-ALL).
Researchers reported these results in Nature Nanotechnology.
“Our technology is the first that we know of to quickly program tumor-recognizing capabilities into T cells without extracting them for laboratory manipulation,” said Matthias Stephan, MD, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“The reprogrammed cells begin to work within 24 to 48 hours and continue to produce these receptors for weeks. This suggests that our technology has the potential to allow the immune system to quickly mount a strong enough response to destroy cancerous cells before the disease becomes fatal.”
Dr Stephan and his colleagues designed their nanoparticles to carry genes that encode for CARs intended to target and eliminate B-ALL. The nanoparticles are coated with ligands that make them seek out and bind to T cells.
When a nanoparticle binds to a T cell, the cell engulfs the particle. The nanoparticle then travels to the cell’s nucleus and dissolves.
The CAR genes integrate into chromosomes housed in the nucleus, making it possible for the T cells to begin decoding the new genes and producing CARs within 1 or 2 days.
Once they determined their CAR-carrying nanoparticles reprogrammed a noticeable percentage of T cells, the researchers tested the T cells’ efficacy in a mouse model of B-ALL.
The team infused the nanoparticles into 10 mice and found the treatment eradicated tumors in 7 of the animals. The other 3 mice “showed substantial regression” of leukemia, the researchers said.
On average, mice that received CAR-carrying nanoparticles had a 58-day improvement in survival compared to control mice.
Mice that received the nanoparticles also had “dramatically reduced” B-cell numbers in their spleens. The researchers noted that this is consistent with the reversible B-cell aplasia observed in patients who receive conventional CD19 CAR T-cell therapy.
Dr Stephan and his colleagues also tested conventional CAR T-cell therapy in the B-ALL mouse model. The mice received cyclophosphamide followed by CAR T cells created ex vivo.
These mice had significantly better survival than controls, but their survival was comparable to that of the mice that received the CAR-carrying nanoparticles.
Although these nanoparticles are several steps away from the clinic, Dr Stephan said he imagines a future in which nanoparticles transform cell-based immunotherapies into easily administered, off-the-shelf treatments that are available anywhere.
“I’ve never had cancer, but if I did get a cancer diagnosis, I would want to start treatment right away,” Dr Stephan said. “I want to make cellular immunotherapy a treatment option the day of diagnosis and have it able to be done in an outpatient setting near where people live.” ![]()
Imbalance drives development of B-ALL, team says
Researchers say they have discovered an imbalance that drives the development of B-cell acute lymphoblastic leukemia (B-ALL).
The group’s study suggests that activation of STAT5 causes competition among other transcription factors that leads to B-ALL.
Therefore, the researchers believe that inhibiting the activation of STAT5 and restoring the natural balance of proteins could mean more effective treatment for B-ALL.
Seth Frietze, PhD, of the University of Vermont in Burlington, Vermont, and his colleagues conducted this research and reported the results in Nature Immunology.
The researchers first studied the role of STAT5 in B-ALL using mouse models.
The experiments revealed that STAT5 activation and defects in a signaling pathway worked together to promote B-ALL. The defects were in signaling components of the B-cell antigen receptor precursor—IKAROS, NF-κB, BLNK, BTK, and PKCβ.
With further investigation, the researchers found that STAT5 “antagonized NF-κB and IKAROS by opposing the regulation of shared target genes.”
The team also studied samples from patients with B-ALL and found that patients with a high ratio of active STAT5 to NF-κB or IKAROS had more aggressive disease.
Specifically, the ratio of active STAT5 to IKAROS was negatively correlated with patient survival and the duration of remission. The ratio of active STAT5 to the NF-κB subunit RELA correlated with remission duration but not survival.
“The major outcome of this story is that a signature emerged from looking at the level of activated proteins compared to other proteins that’s very predictive of how a patient will respond to therapy,” Dr Frietze said.
“That’s a novel finding. If we could find drugs to target that activation, that could be an incredibly effective way to treat leukemia.” ![]()
Researchers say they have discovered an imbalance that drives the development of B-cell acute lymphoblastic leukemia (B-ALL).
The group’s study suggests that activation of STAT5 causes competition among other transcription factors that leads to B-ALL.
Therefore, the researchers believe that inhibiting the activation of STAT5 and restoring the natural balance of proteins could mean more effective treatment for B-ALL.
Seth Frietze, PhD, of the University of Vermont in Burlington, Vermont, and his colleagues conducted this research and reported the results in Nature Immunology.
The researchers first studied the role of STAT5 in B-ALL using mouse models.
The experiments revealed that STAT5 activation and defects in a signaling pathway worked together to promote B-ALL. The defects were in signaling components of the B-cell antigen receptor precursor—IKAROS, NF-κB, BLNK, BTK, and PKCβ.
With further investigation, the researchers found that STAT5 “antagonized NF-κB and IKAROS by opposing the regulation of shared target genes.”
The team also studied samples from patients with B-ALL and found that patients with a high ratio of active STAT5 to NF-κB or IKAROS had more aggressive disease.
Specifically, the ratio of active STAT5 to IKAROS was negatively correlated with patient survival and the duration of remission. The ratio of active STAT5 to the NF-κB subunit RELA correlated with remission duration but not survival.
“The major outcome of this story is that a signature emerged from looking at the level of activated proteins compared to other proteins that’s very predictive of how a patient will respond to therapy,” Dr Frietze said.
“That’s a novel finding. If we could find drugs to target that activation, that could be an incredibly effective way to treat leukemia.” ![]()
Researchers say they have discovered an imbalance that drives the development of B-cell acute lymphoblastic leukemia (B-ALL).
The group’s study suggests that activation of STAT5 causes competition among other transcription factors that leads to B-ALL.
Therefore, the researchers believe that inhibiting the activation of STAT5 and restoring the natural balance of proteins could mean more effective treatment for B-ALL.
Seth Frietze, PhD, of the University of Vermont in Burlington, Vermont, and his colleagues conducted this research and reported the results in Nature Immunology.
The researchers first studied the role of STAT5 in B-ALL using mouse models.
The experiments revealed that STAT5 activation and defects in a signaling pathway worked together to promote B-ALL. The defects were in signaling components of the B-cell antigen receptor precursor—IKAROS, NF-κB, BLNK, BTK, and PKCβ.
With further investigation, the researchers found that STAT5 “antagonized NF-κB and IKAROS by opposing the regulation of shared target genes.”
The team also studied samples from patients with B-ALL and found that patients with a high ratio of active STAT5 to NF-κB or IKAROS had more aggressive disease.
Specifically, the ratio of active STAT5 to IKAROS was negatively correlated with patient survival and the duration of remission. The ratio of active STAT5 to the NF-κB subunit RELA correlated with remission duration but not survival.
“The major outcome of this story is that a signature emerged from looking at the level of activated proteins compared to other proteins that’s very predictive of how a patient will respond to therapy,” Dr Frietze said.
“That’s a novel finding. If we could find drugs to target that activation, that could be an incredibly effective way to treat leukemia.” ![]()
Study reveals global inequalities in childhood leukemia survival
New research has revealed global inequalities in survival rates for pediatric patients with leukemia.
Investigators analyzed data on nearly 90,000 pediatric leukemia patients treated in 53 countries.
In most countries, patients with lymphoid leukemias or acute myeloid leukemia (AML) saw an increase in 5-year survival between 1995 and 2009.
However, there were wide variations in survival between the countries.
The investigators reported these findings in The Lancet Haematology.
They evaluated data from 89,828 leukemia patients (ages 0 to 14) included in 198 cancer registries in 53 countries.
The team estimated 5-year net survival for patients with AML or lymphoid leukemias (controlling for non-leukemia-related deaths) by calendar period of diagnosis—1995–1999, 2000–2004, and 2005–2009—in each country.
For children diagnosed with lymphoid leukemias between 1995 and 1999, 5-year survival rates ranged from 10.6% (in China) to 86.8% (in Austria). For children diagnosed between 2005 and 2009, the rates ranged from 52.4% (Colombia) to 91.6% (Germany).
For AML, 5-year survival rates ranged from 4.2% (China) to 72.2% (Sweden) in patients diagnosed between 1995 and 1999. For children diagnosed between 2005 and 2009, 5-year survival rates ranged from 33.3% (Bulgaria) to 78.2% (Germany).
The investigators noted that, in some countries, survival for both groups of leukemia patients was consistently high.
In Austria, for example, 5-year survival rates for lymphoid leukemias were 86.8% in 1995-1999 and 91.1% in 2005-2009. For AML, rates were 60.1% and 72.6%, respectively.
Other countries saw substantial increases in survival over time.
In China, the 5-year survival rate for patients with lymphoid leukemias increased from 10.6% in 1995-1999 to 69.2% in 2005-2009. For patients with AML, the rate increased from 4.2% to 41.1%.
“These findings show the extent of worldwide inequalities in access to optimal healthcare for children with cancer,” said study author Audrey Bonaventure, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“Providing additional resources, alongside evidence-based initiatives such as international collaborations and treatment guidelines, could improve access to efficient treatment and care for all children with leukemia. This would contribute substantially to reducing worldwide inequalities in survival.” ![]()
New research has revealed global inequalities in survival rates for pediatric patients with leukemia.
Investigators analyzed data on nearly 90,000 pediatric leukemia patients treated in 53 countries.
In most countries, patients with lymphoid leukemias or acute myeloid leukemia (AML) saw an increase in 5-year survival between 1995 and 2009.
However, there were wide variations in survival between the countries.
The investigators reported these findings in The Lancet Haematology.
They evaluated data from 89,828 leukemia patients (ages 0 to 14) included in 198 cancer registries in 53 countries.
The team estimated 5-year net survival for patients with AML or lymphoid leukemias (controlling for non-leukemia-related deaths) by calendar period of diagnosis—1995–1999, 2000–2004, and 2005–2009—in each country.
For children diagnosed with lymphoid leukemias between 1995 and 1999, 5-year survival rates ranged from 10.6% (in China) to 86.8% (in Austria). For children diagnosed between 2005 and 2009, the rates ranged from 52.4% (Colombia) to 91.6% (Germany).
For AML, 5-year survival rates ranged from 4.2% (China) to 72.2% (Sweden) in patients diagnosed between 1995 and 1999. For children diagnosed between 2005 and 2009, 5-year survival rates ranged from 33.3% (Bulgaria) to 78.2% (Germany).
The investigators noted that, in some countries, survival for both groups of leukemia patients was consistently high.
In Austria, for example, 5-year survival rates for lymphoid leukemias were 86.8% in 1995-1999 and 91.1% in 2005-2009. For AML, rates were 60.1% and 72.6%, respectively.
Other countries saw substantial increases in survival over time.
In China, the 5-year survival rate for patients with lymphoid leukemias increased from 10.6% in 1995-1999 to 69.2% in 2005-2009. For patients with AML, the rate increased from 4.2% to 41.1%.
“These findings show the extent of worldwide inequalities in access to optimal healthcare for children with cancer,” said study author Audrey Bonaventure, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“Providing additional resources, alongside evidence-based initiatives such as international collaborations and treatment guidelines, could improve access to efficient treatment and care for all children with leukemia. This would contribute substantially to reducing worldwide inequalities in survival.” ![]()
New research has revealed global inequalities in survival rates for pediatric patients with leukemia.
Investigators analyzed data on nearly 90,000 pediatric leukemia patients treated in 53 countries.
In most countries, patients with lymphoid leukemias or acute myeloid leukemia (AML) saw an increase in 5-year survival between 1995 and 2009.
However, there were wide variations in survival between the countries.
The investigators reported these findings in The Lancet Haematology.
They evaluated data from 89,828 leukemia patients (ages 0 to 14) included in 198 cancer registries in 53 countries.
The team estimated 5-year net survival for patients with AML or lymphoid leukemias (controlling for non-leukemia-related deaths) by calendar period of diagnosis—1995–1999, 2000–2004, and 2005–2009—in each country.
For children diagnosed with lymphoid leukemias between 1995 and 1999, 5-year survival rates ranged from 10.6% (in China) to 86.8% (in Austria). For children diagnosed between 2005 and 2009, the rates ranged from 52.4% (Colombia) to 91.6% (Germany).
For AML, 5-year survival rates ranged from 4.2% (China) to 72.2% (Sweden) in patients diagnosed between 1995 and 1999. For children diagnosed between 2005 and 2009, 5-year survival rates ranged from 33.3% (Bulgaria) to 78.2% (Germany).
The investigators noted that, in some countries, survival for both groups of leukemia patients was consistently high.
In Austria, for example, 5-year survival rates for lymphoid leukemias were 86.8% in 1995-1999 and 91.1% in 2005-2009. For AML, rates were 60.1% and 72.6%, respectively.
Other countries saw substantial increases in survival over time.
In China, the 5-year survival rate for patients with lymphoid leukemias increased from 10.6% in 1995-1999 to 69.2% in 2005-2009. For patients with AML, the rate increased from 4.2% to 41.1%.
“These findings show the extent of worldwide inequalities in access to optimal healthcare for children with cancer,” said study author Audrey Bonaventure, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“Providing additional resources, alongside evidence-based initiatives such as international collaborations and treatment guidelines, could improve access to efficient treatment and care for all children with leukemia. This would contribute substantially to reducing worldwide inequalities in survival.”
VIDEO: Blinatumomab, inotuzumab reshape relapsed ALL treatment
NEW YORK – A pair of new monoclonal antibodies have dramatically changed treatment for patients with acute lymphoblastic leukemia to prepare them for a stem cell transplant, Daniel J. DeAngelo, MD, said at a conference held by Imedex.
“We don’t use standard chemotherapy for reinduction anymore; we use blinatumomab or inotuzumab,” said Dr. DeAngelo, a hematologist oncologist at Dana-Farber Cancer Institute in Boston.
Blinatumomab (Blincyto), approved by the Food and Drug Administration in 2014, has produced “exceptional” response rates, becoming “standard of care” for patients with relapsed acute lymphoblastic leukemia (ALL) that does not have a Philadelphia chromosome, Dr. DeAngelo said in a video interview.
Approved based on results from a phase II study, blinatumomab’s efficacy and safety were recently further delineated in results from the first phase III trial (N Engl J Med. 2017 Mar 2;376[9]:836-74), with 376 treated patients. In that trial, blinatumomab more than doubled the complete remission rate, compared with control patients (34% vs. 16%), and nearly doubled median overall survival – 7.7 months with blinatumomab, compared with 4.0 months for control patients treated with standard chemotherapy.
These findings “further substantiated” blinatumomab’s role, he said.
Blinatumomab’s big limitations are certain adverse effects and the logistics of its dosing. The major adverse effect is “cytokine release syndrome,” which manifests as fever, low blood pressure, and neurologic toxicities that can range from tremors to encephalopathy and seizure. These are manageable by close observation of patients by experienced nurses, Dr. DeAngelo said.
Dosing involves 4 weeks of continuous infusion, starting with 10 days done entirely in the hospital, with the remaining 18 days with patients going home but needing to return every 48 hours to have their infusion bag changed. “Depending on how far the patient lives from the clinic, it can be a logistical challenge,” he said.
A second new antibody he has used on many patients is inotuzumab, which was accepted for review for approval by the FDA in February 2017, with action expected by August.
Dr. DeAngelo served as a coinvestigator in a phase III trial reported in 2016 with 218 evaluable patients. In that trial, investigators reported an 81% complete remission rate with inotuzumab treatment, compared with a 29% among control patients on chemotherapy (N Engl J Med. 2016 Aug 25;375[8]:740-53).
Inotuzumab was effective against patients with Philadelphia chromosome positive ALL, but it will not work for the roughly 5%-10% of ALL patients who lack CD-22 expression in their B-cell ALL.
Inotuzumab is easier to administer than blinatumomab, requiring a once a week infusion, and causes little immediate toxicity – although thrombocytopenia and liver-function abnormalities can occur with continued use, and the risk of veno-occlusive disease is increased when patients later receive a stem cell transplant, Dr. DeAngelo said.
“It’s nice to have options” when choosing antibody-based treatment, he said. Blinatumomab is a good choice for patients with a lower tumor burden – either patients with early relapse or with minimal residual disease – while inotuzumab works better for patients with more bulky disease, as well as those who are not able to accommodate the logistic demands of blinatumomab infusions.
Dr. DeAngelo also highlighted several trials now underway that are testing the efficacy of both antibodies when used as part of first-line treatment.
Dr. DeAngelo has been a consultant to Amgen, the company that markets blinatumomab (Blincyto); to Pfizer, the company developing inotuzumab; and to Ariad, InCyte, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW YORK – A pair of new monoclonal antibodies have dramatically changed treatment for patients with acute lymphoblastic leukemia to prepare them for a stem cell transplant, Daniel J. DeAngelo, MD, said at a conference held by Imedex.
“We don’t use standard chemotherapy for reinduction anymore; we use blinatumomab or inotuzumab,” said Dr. DeAngelo, a hematologist oncologist at Dana-Farber Cancer Institute in Boston.
Blinatumomab (Blincyto), approved by the Food and Drug Administration in 2014, has produced “exceptional” response rates, becoming “standard of care” for patients with relapsed acute lymphoblastic leukemia (ALL) that does not have a Philadelphia chromosome, Dr. DeAngelo said in a video interview.
Approved based on results from a phase II study, blinatumomab’s efficacy and safety were recently further delineated in results from the first phase III trial (N Engl J Med. 2017 Mar 2;376[9]:836-74), with 376 treated patients. In that trial, blinatumomab more than doubled the complete remission rate, compared with control patients (34% vs. 16%), and nearly doubled median overall survival – 7.7 months with blinatumomab, compared with 4.0 months for control patients treated with standard chemotherapy.
These findings “further substantiated” blinatumomab’s role, he said.
Blinatumomab’s big limitations are certain adverse effects and the logistics of its dosing. The major adverse effect is “cytokine release syndrome,” which manifests as fever, low blood pressure, and neurologic toxicities that can range from tremors to encephalopathy and seizure. These are manageable by close observation of patients by experienced nurses, Dr. DeAngelo said.
Dosing involves 4 weeks of continuous infusion, starting with 10 days done entirely in the hospital, with the remaining 18 days with patients going home but needing to return every 48 hours to have their infusion bag changed. “Depending on how far the patient lives from the clinic, it can be a logistical challenge,” he said.
A second new antibody he has used on many patients is inotuzumab, which was accepted for review for approval by the FDA in February 2017, with action expected by August.
Dr. DeAngelo served as a coinvestigator in a phase III trial reported in 2016 with 218 evaluable patients. In that trial, investigators reported an 81% complete remission rate with inotuzumab treatment, compared with a 29% among control patients on chemotherapy (N Engl J Med. 2016 Aug 25;375[8]:740-53).
Inotuzumab was effective against patients with Philadelphia chromosome positive ALL, but it will not work for the roughly 5%-10% of ALL patients who lack CD-22 expression in their B-cell ALL.
Inotuzumab is easier to administer than blinatumomab, requiring a once a week infusion, and causes little immediate toxicity – although thrombocytopenia and liver-function abnormalities can occur with continued use, and the risk of veno-occlusive disease is increased when patients later receive a stem cell transplant, Dr. DeAngelo said.
“It’s nice to have options” when choosing antibody-based treatment, he said. Blinatumomab is a good choice for patients with a lower tumor burden – either patients with early relapse or with minimal residual disease – while inotuzumab works better for patients with more bulky disease, as well as those who are not able to accommodate the logistic demands of blinatumomab infusions.
Dr. DeAngelo also highlighted several trials now underway that are testing the efficacy of both antibodies when used as part of first-line treatment.
Dr. DeAngelo has been a consultant to Amgen, the company that markets blinatumomab (Blincyto); to Pfizer, the company developing inotuzumab; and to Ariad, InCyte, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW YORK – A pair of new monoclonal antibodies have dramatically changed treatment for patients with acute lymphoblastic leukemia to prepare them for a stem cell transplant, Daniel J. DeAngelo, MD, said at a conference held by Imedex.
“We don’t use standard chemotherapy for reinduction anymore; we use blinatumomab or inotuzumab,” said Dr. DeAngelo, a hematologist oncologist at Dana-Farber Cancer Institute in Boston.
Blinatumomab (Blincyto), approved by the Food and Drug Administration in 2014, has produced “exceptional” response rates, becoming “standard of care” for patients with relapsed acute lymphoblastic leukemia (ALL) that does not have a Philadelphia chromosome, Dr. DeAngelo said in a video interview.
Approved based on results from a phase II study, blinatumomab’s efficacy and safety were recently further delineated in results from the first phase III trial (N Engl J Med. 2017 Mar 2;376[9]:836-74), with 376 treated patients. In that trial, blinatumomab more than doubled the complete remission rate, compared with control patients (34% vs. 16%), and nearly doubled median overall survival – 7.7 months with blinatumomab, compared with 4.0 months for control patients treated with standard chemotherapy.
These findings “further substantiated” blinatumomab’s role, he said.
Blinatumomab’s big limitations are certain adverse effects and the logistics of its dosing. The major adverse effect is “cytokine release syndrome,” which manifests as fever, low blood pressure, and neurologic toxicities that can range from tremors to encephalopathy and seizure. These are manageable by close observation of patients by experienced nurses, Dr. DeAngelo said.
Dosing involves 4 weeks of continuous infusion, starting with 10 days done entirely in the hospital, with the remaining 18 days with patients going home but needing to return every 48 hours to have their infusion bag changed. “Depending on how far the patient lives from the clinic, it can be a logistical challenge,” he said.
A second new antibody he has used on many patients is inotuzumab, which was accepted for review for approval by the FDA in February 2017, with action expected by August.
Dr. DeAngelo served as a coinvestigator in a phase III trial reported in 2016 with 218 evaluable patients. In that trial, investigators reported an 81% complete remission rate with inotuzumab treatment, compared with a 29% among control patients on chemotherapy (N Engl J Med. 2016 Aug 25;375[8]:740-53).
Inotuzumab was effective against patients with Philadelphia chromosome positive ALL, but it will not work for the roughly 5%-10% of ALL patients who lack CD-22 expression in their B-cell ALL.
Inotuzumab is easier to administer than blinatumomab, requiring a once a week infusion, and causes little immediate toxicity – although thrombocytopenia and liver-function abnormalities can occur with continued use, and the risk of veno-occlusive disease is increased when patients later receive a stem cell transplant, Dr. DeAngelo said.
“It’s nice to have options” when choosing antibody-based treatment, he said. Blinatumomab is a good choice for patients with a lower tumor burden – either patients with early relapse or with minimal residual disease – while inotuzumab works better for patients with more bulky disease, as well as those who are not able to accommodate the logistic demands of blinatumomab infusions.
Dr. DeAngelo also highlighted several trials now underway that are testing the efficacy of both antibodies when used as part of first-line treatment.
Dr. DeAngelo has been a consultant to Amgen, the company that markets blinatumomab (Blincyto); to Pfizer, the company developing inotuzumab; and to Ariad, InCyte, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM A MEETING ON HEMATOLOGIC MALIGNANCIES
Parental smoking linked to genetic changes in kids with ALL
Smoking by either parent helps promote genetic deletions in children that are associated with the development and progression of acute lymphoblastic leukemia (ALL), according to a study published in Cancer Research.
The strongest associations in this study were found in children whose parents smoked while the children were in utero and during their infancy.
However, the genetic deletions were also noted in the offspring of parents who may have quit smoking even before conception.
The link between ALL and parental smoking has already been established, but this is the first study that points to specific genetic changes in children with ALL, according to study author Adam de Smith, PhD, of the University of California San Francisco.
“With more smoking among the parents, we saw more deletions within the child’s ALL cells at diagnosis,” Dr de smith said.
For this study, he and his colleagues looked at pre-treatment tumor samples from 559 ALL patients.
The team wanted to see if any of the 8 genes that are frequently deleted in ALL patients (CDKN2A, ETV6, IKZF1, PAX5, RB1, BTG1, PAR1 region, and EBF1) were missing in the samples.
Questionnaires were given to parents to find out if smoking habits impacted the number of genetic deletions. Data was corroborated by a biomarker in newborns’ blood samples that indicates exposure to maternal smoking during pregnancy.
The researchers found that approximately two-thirds of the tumor samples (n=353) contained at least 1 deletion.
Deletions were considerably more common in children whose mothers had smoked during pregnancy and after birth.
For each 5 cigarettes smoked daily during pregnancy, there was a 22% increase in the number of deletions. For each 5 cigarettes smoked daily during breastfeeding, there was a 74% increase in the number of deletions.
Smoking of 5 cigarettes daily by the mother or father before conception was associated with a 7% to 8% higher number of deletions.
Role of child age and sex
One discovery the researchers found intriguing was the link between the fathers’ pre-conception smoking and their child’s age at diagnosis.
“There was a significant effect on deletion numbers in cases where the patient was age 6 or younger,” Dr de Smith said. “Our results suggest that paternal pre-conception smoking, which is known to cause oxidative damage to sperm DNA, may lead to a higher propensity of deletions in children with earlier-onset ALL.”
“It is also true that some of those fathers who smoked before conception also continue to smoke in the presence of the mother and child, so more research is needed to explain the mechanism of smoking-related damage in all of the time periods of exposure to the child.”
In addition, male children were found to be more sensitive to the effects of maternal smoking, including smoking that occurred pre-conception.
The researchers said this could be explained by the fact that male fetuses grow more rapidly, which leads to increased vulnerability of developing lymphocytes to toxins that cause genetic damage.
“Our study indicates that the more tobacco exposure, the more cumulative DNA damage is evident in the ALL cell,” said study author Joseph Wiemels, PhD, of the University of California San Francisco.
“While causes of ALL are multifactorial—including the inborn genetic makeup of the child, patterns of infection, pesticides, and other environmental exposure—if there was no smoking in the environment, then there would likely be fewer children with the disease. We may add ALL to the long list of diseases impacted by smoking, and, in this case, affecting one of our most vulnerable populations—our children.”
Smoking by either parent helps promote genetic deletions in children that are associated with the development and progression of acute lymphoblastic leukemia (ALL), according to a study published in Cancer Research.
The strongest associations in this study were found in children whose parents smoked while the children were in utero and during their infancy.
However, the genetic deletions were also noted in the offspring of parents who may have quit smoking even before conception.
The link between ALL and parental smoking has already been established, but this is the first study that points to specific genetic changes in children with ALL, according to study author Adam de Smith, PhD, of the University of California San Francisco.
“With more smoking among the parents, we saw more deletions within the child’s ALL cells at diagnosis,” Dr de smith said.
For this study, he and his colleagues looked at pre-treatment tumor samples from 559 ALL patients.
The team wanted to see if any of the 8 genes that are frequently deleted in ALL patients (CDKN2A, ETV6, IKZF1, PAX5, RB1, BTG1, PAR1 region, and EBF1) were missing in the samples.
Questionnaires were given to parents to find out if smoking habits impacted the number of genetic deletions. Data was corroborated by a biomarker in newborns’ blood samples that indicates exposure to maternal smoking during pregnancy.
The researchers found that approximately two-thirds of the tumor samples (n=353) contained at least 1 deletion.
Deletions were considerably more common in children whose mothers had smoked during pregnancy and after birth.
For each 5 cigarettes smoked daily during pregnancy, there was a 22% increase in the number of deletions. For each 5 cigarettes smoked daily during breastfeeding, there was a 74% increase in the number of deletions.
Smoking of 5 cigarettes daily by the mother or father before conception was associated with a 7% to 8% higher number of deletions.
Role of child age and sex
One discovery the researchers found intriguing was the link between the fathers’ pre-conception smoking and their child’s age at diagnosis.
“There was a significant effect on deletion numbers in cases where the patient was age 6 or younger,” Dr de Smith said. “Our results suggest that paternal pre-conception smoking, which is known to cause oxidative damage to sperm DNA, may lead to a higher propensity of deletions in children with earlier-onset ALL.”
“It is also true that some of those fathers who smoked before conception also continue to smoke in the presence of the mother and child, so more research is needed to explain the mechanism of smoking-related damage in all of the time periods of exposure to the child.”
In addition, male children were found to be more sensitive to the effects of maternal smoking, including smoking that occurred pre-conception.
The researchers said this could be explained by the fact that male fetuses grow more rapidly, which leads to increased vulnerability of developing lymphocytes to toxins that cause genetic damage.
“Our study indicates that the more tobacco exposure, the more cumulative DNA damage is evident in the ALL cell,” said study author Joseph Wiemels, PhD, of the University of California San Francisco.
“While causes of ALL are multifactorial—including the inborn genetic makeup of the child, patterns of infection, pesticides, and other environmental exposure—if there was no smoking in the environment, then there would likely be fewer children with the disease. We may add ALL to the long list of diseases impacted by smoking, and, in this case, affecting one of our most vulnerable populations—our children.”
Smoking by either parent helps promote genetic deletions in children that are associated with the development and progression of acute lymphoblastic leukemia (ALL), according to a study published in Cancer Research.
The strongest associations in this study were found in children whose parents smoked while the children were in utero and during their infancy.
However, the genetic deletions were also noted in the offspring of parents who may have quit smoking even before conception.
The link between ALL and parental smoking has already been established, but this is the first study that points to specific genetic changes in children with ALL, according to study author Adam de Smith, PhD, of the University of California San Francisco.
“With more smoking among the parents, we saw more deletions within the child’s ALL cells at diagnosis,” Dr de smith said.
For this study, he and his colleagues looked at pre-treatment tumor samples from 559 ALL patients.
The team wanted to see if any of the 8 genes that are frequently deleted in ALL patients (CDKN2A, ETV6, IKZF1, PAX5, RB1, BTG1, PAR1 region, and EBF1) were missing in the samples.
Questionnaires were given to parents to find out if smoking habits impacted the number of genetic deletions. Data was corroborated by a biomarker in newborns’ blood samples that indicates exposure to maternal smoking during pregnancy.
The researchers found that approximately two-thirds of the tumor samples (n=353) contained at least 1 deletion.
Deletions were considerably more common in children whose mothers had smoked during pregnancy and after birth.
For each 5 cigarettes smoked daily during pregnancy, there was a 22% increase in the number of deletions. For each 5 cigarettes smoked daily during breastfeeding, there was a 74% increase in the number of deletions.
Smoking of 5 cigarettes daily by the mother or father before conception was associated with a 7% to 8% higher number of deletions.
Role of child age and sex
One discovery the researchers found intriguing was the link between the fathers’ pre-conception smoking and their child’s age at diagnosis.
“There was a significant effect on deletion numbers in cases where the patient was age 6 or younger,” Dr de Smith said. “Our results suggest that paternal pre-conception smoking, which is known to cause oxidative damage to sperm DNA, may lead to a higher propensity of deletions in children with earlier-onset ALL.”
“It is also true that some of those fathers who smoked before conception also continue to smoke in the presence of the mother and child, so more research is needed to explain the mechanism of smoking-related damage in all of the time periods of exposure to the child.”
In addition, male children were found to be more sensitive to the effects of maternal smoking, including smoking that occurred pre-conception.
The researchers said this could be explained by the fact that male fetuses grow more rapidly, which leads to increased vulnerability of developing lymphocytes to toxins that cause genetic damage.
“Our study indicates that the more tobacco exposure, the more cumulative DNA damage is evident in the ALL cell,” said study author Joseph Wiemels, PhD, of the University of California San Francisco.
“While causes of ALL are multifactorial—including the inborn genetic makeup of the child, patterns of infection, pesticides, and other environmental exposure—if there was no smoking in the environment, then there would likely be fewer children with the disease. We may add ALL to the long list of diseases impacted by smoking, and, in this case, affecting one of our most vulnerable populations—our children.”