User login
Study: CMV doesn’t lower risk of relapse, death
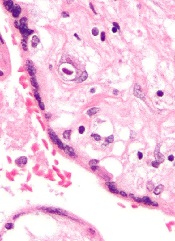
Small studies have suggested that early cytomegalovirus (CMV) reactivation may protect against leukemia relapse and even death after hematopoietic stem cell transplant.
However, a new study, based on data from about 9500 patients, suggests otherwise.
Results showed no association between CMV reactivation and relapse but suggested CMV reactivation increases the risk of non-relapse mortality.
Researchers reported these findings in Blood.
“The original purpose of the study was to confirm that CMV infection may prevent leukemia relapse, prevent death, and become a major therapeutic tool for improving patient survival rates,” said study author Pierre Teira, MD, of the University of Montreal in Quebec, Canada.
“However, we found the exact opposite. Our results clearly show that . . . the virus not only does not prevent leukemia relapse [it] also remains a major factor associated with the risk of death. Monitoring of CMV after transplantation remains a priority for patients.”
For this study, Dr Teira and his colleagues analyzed data from 9469 patients who received a transplant between 2003 and 2010.
The patients had acute myeloid leukemia (AML, n=5310), acute lymphoblastic leukemia (ALL, n=1883), chronic myeloid leukemia (CML, n=1079), or myelodysplastic syndromes (MDS, n=1197).
The median time to initial CMV reactivation was 41 days (range, 1-362 days).
The researchers found no significant association between CMV reactivation and disease relapse for AML (P=0.60), ALL (P=0.08), CML (P=0.94), or MDS (P=0.58).
However, CMV reactivation was associated with a significantly higher risk of nonrelapse mortality for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0004), and MDS (P=0.0002).
Therefore, CMV reactivation was associated with significantly lower overall survival for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0005), and MDS (P=0.003).
“Deaths due to uncontrolled CMV reactivation are virtually zero in this study, so uncontrolled CMV reactivation is not what reduces survival rates after transplantation,” Dr Teira noted. “The link between this common virus and increased risk of death remains a biological mystery.”
One possible explanation is that CMV decreases the ability of the patient’s immune system to fight against other types of infection. This is supported by the fact that death rates from infections other than CMV are higher in patients infected with CMV or patients whose donors were.
For researchers, the next step is therefore to verify whether the latest generation of anti-CMV treatments can prevent both reactivation of the virus and weakening of the patient’s immune system against other types of infection in the presence of CMV infection.
“CMV has a complex impact on the outcomes for transplant patients, and, each year, more than 30,000 patients around the world receive bone marrow transplants from donors,” Dr Teira said.
“It is therefore essential for future research to better understand the role played by CMV after bone marrow transplantation and improve the chances of success of the transplant. This will help to better choose the right donor for the right patient.” ![]()

Small studies have suggested that early cytomegalovirus (CMV) reactivation may protect against leukemia relapse and even death after hematopoietic stem cell transplant.
However, a new study, based on data from about 9500 patients, suggests otherwise.
Results showed no association between CMV reactivation and relapse but suggested CMV reactivation increases the risk of non-relapse mortality.
Researchers reported these findings in Blood.
“The original purpose of the study was to confirm that CMV infection may prevent leukemia relapse, prevent death, and become a major therapeutic tool for improving patient survival rates,” said study author Pierre Teira, MD, of the University of Montreal in Quebec, Canada.
“However, we found the exact opposite. Our results clearly show that . . . the virus not only does not prevent leukemia relapse [it] also remains a major factor associated with the risk of death. Monitoring of CMV after transplantation remains a priority for patients.”
For this study, Dr Teira and his colleagues analyzed data from 9469 patients who received a transplant between 2003 and 2010.
The patients had acute myeloid leukemia (AML, n=5310), acute lymphoblastic leukemia (ALL, n=1883), chronic myeloid leukemia (CML, n=1079), or myelodysplastic syndromes (MDS, n=1197).
The median time to initial CMV reactivation was 41 days (range, 1-362 days).
The researchers found no significant association between CMV reactivation and disease relapse for AML (P=0.60), ALL (P=0.08), CML (P=0.94), or MDS (P=0.58).
However, CMV reactivation was associated with a significantly higher risk of nonrelapse mortality for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0004), and MDS (P=0.0002).
Therefore, CMV reactivation was associated with significantly lower overall survival for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0005), and MDS (P=0.003).
“Deaths due to uncontrolled CMV reactivation are virtually zero in this study, so uncontrolled CMV reactivation is not what reduces survival rates after transplantation,” Dr Teira noted. “The link between this common virus and increased risk of death remains a biological mystery.”
One possible explanation is that CMV decreases the ability of the patient’s immune system to fight against other types of infection. This is supported by the fact that death rates from infections other than CMV are higher in patients infected with CMV or patients whose donors were.
For researchers, the next step is therefore to verify whether the latest generation of anti-CMV treatments can prevent both reactivation of the virus and weakening of the patient’s immune system against other types of infection in the presence of CMV infection.
“CMV has a complex impact on the outcomes for transplant patients, and, each year, more than 30,000 patients around the world receive bone marrow transplants from donors,” Dr Teira said.
“It is therefore essential for future research to better understand the role played by CMV after bone marrow transplantation and improve the chances of success of the transplant. This will help to better choose the right donor for the right patient.” ![]()

Small studies have suggested that early cytomegalovirus (CMV) reactivation may protect against leukemia relapse and even death after hematopoietic stem cell transplant.
However, a new study, based on data from about 9500 patients, suggests otherwise.
Results showed no association between CMV reactivation and relapse but suggested CMV reactivation increases the risk of non-relapse mortality.
Researchers reported these findings in Blood.
“The original purpose of the study was to confirm that CMV infection may prevent leukemia relapse, prevent death, and become a major therapeutic tool for improving patient survival rates,” said study author Pierre Teira, MD, of the University of Montreal in Quebec, Canada.
“However, we found the exact opposite. Our results clearly show that . . . the virus not only does not prevent leukemia relapse [it] also remains a major factor associated with the risk of death. Monitoring of CMV after transplantation remains a priority for patients.”
For this study, Dr Teira and his colleagues analyzed data from 9469 patients who received a transplant between 2003 and 2010.
The patients had acute myeloid leukemia (AML, n=5310), acute lymphoblastic leukemia (ALL, n=1883), chronic myeloid leukemia (CML, n=1079), or myelodysplastic syndromes (MDS, n=1197).
The median time to initial CMV reactivation was 41 days (range, 1-362 days).
The researchers found no significant association between CMV reactivation and disease relapse for AML (P=0.60), ALL (P=0.08), CML (P=0.94), or MDS (P=0.58).
However, CMV reactivation was associated with a significantly higher risk of nonrelapse mortality for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0004), and MDS (P=0.0002).
Therefore, CMV reactivation was associated with significantly lower overall survival for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0005), and MDS (P=0.003).
“Deaths due to uncontrolled CMV reactivation are virtually zero in this study, so uncontrolled CMV reactivation is not what reduces survival rates after transplantation,” Dr Teira noted. “The link between this common virus and increased risk of death remains a biological mystery.”
One possible explanation is that CMV decreases the ability of the patient’s immune system to fight against other types of infection. This is supported by the fact that death rates from infections other than CMV are higher in patients infected with CMV or patients whose donors were.
For researchers, the next step is therefore to verify whether the latest generation of anti-CMV treatments can prevent both reactivation of the virus and weakening of the patient’s immune system against other types of infection in the presence of CMV infection.
“CMV has a complex impact on the outcomes for transplant patients, and, each year, more than 30,000 patients around the world receive bone marrow transplants from donors,” Dr Teira said.
“It is therefore essential for future research to better understand the role played by CMV after bone marrow transplantation and improve the chances of success of the transplant. This will help to better choose the right donor for the right patient.” ![]()
FDA reports shortage of doxorubicin for injection, initiates importation
A critical shortage of doxorubicin hydrochloride 50 mg powder for injection has been reported to the Food and Drug Administration.
Doxorubicin is approved to treat acute lymphoblastic leukemia, acute myeloid leukemia, breast cancer, gastric cancer, ovarian cancer, neuroblastoma, and other cancer types.
To increase availability, the pharmaceutical company Hospira (a Pfizer company) is coordinating with the FDA to import the drug from Ahmedabad, India, where it is manufactured by Zydus Hospira Oncology Private Ltd. at an FDA-inspected facility that is in compliance with current good manufacturing practice requirements.
“It is important to note that there are substantive differences in the format and content of the labeling between the U.S.-approved doxorubicin hydrochloride for injection, USP and the Hospira Limited’s doxorubicin hydrochloride 50 mg powder for injection,” Hospira reported in a letter to health care providers.
To place an order or to get questions answered, contact Hospira directly by calling customer care at 1-877-946-7747 (Mondays-Fridays, 7 a.m.-6 p.m. Central time).
For clinical inquiries, contact Hospira Medical Communications at 1-800-615-0187 or email medcom@hospira.com.
According to the letter, adverse events or quality problems associated with use of this product should be reported by calling Hospira Global Complaint Management by phone, 1-800-441-4100; by sending an email to ProductComplaintsPP@hospira.com; or by submitting a report online to Medwatch.
On Twitter @jessnicolecraig
A critical shortage of doxorubicin hydrochloride 50 mg powder for injection has been reported to the Food and Drug Administration.
Doxorubicin is approved to treat acute lymphoblastic leukemia, acute myeloid leukemia, breast cancer, gastric cancer, ovarian cancer, neuroblastoma, and other cancer types.
To increase availability, the pharmaceutical company Hospira (a Pfizer company) is coordinating with the FDA to import the drug from Ahmedabad, India, where it is manufactured by Zydus Hospira Oncology Private Ltd. at an FDA-inspected facility that is in compliance with current good manufacturing practice requirements.
“It is important to note that there are substantive differences in the format and content of the labeling between the U.S.-approved doxorubicin hydrochloride for injection, USP and the Hospira Limited’s doxorubicin hydrochloride 50 mg powder for injection,” Hospira reported in a letter to health care providers.
To place an order or to get questions answered, contact Hospira directly by calling customer care at 1-877-946-7747 (Mondays-Fridays, 7 a.m.-6 p.m. Central time).
For clinical inquiries, contact Hospira Medical Communications at 1-800-615-0187 or email medcom@hospira.com.
According to the letter, adverse events or quality problems associated with use of this product should be reported by calling Hospira Global Complaint Management by phone, 1-800-441-4100; by sending an email to ProductComplaintsPP@hospira.com; or by submitting a report online to Medwatch.
On Twitter @jessnicolecraig
A critical shortage of doxorubicin hydrochloride 50 mg powder for injection has been reported to the Food and Drug Administration.
Doxorubicin is approved to treat acute lymphoblastic leukemia, acute myeloid leukemia, breast cancer, gastric cancer, ovarian cancer, neuroblastoma, and other cancer types.
To increase availability, the pharmaceutical company Hospira (a Pfizer company) is coordinating with the FDA to import the drug from Ahmedabad, India, where it is manufactured by Zydus Hospira Oncology Private Ltd. at an FDA-inspected facility that is in compliance with current good manufacturing practice requirements.
“It is important to note that there are substantive differences in the format and content of the labeling between the U.S.-approved doxorubicin hydrochloride for injection, USP and the Hospira Limited’s doxorubicin hydrochloride 50 mg powder for injection,” Hospira reported in a letter to health care providers.
To place an order or to get questions answered, contact Hospira directly by calling customer care at 1-877-946-7747 (Mondays-Fridays, 7 a.m.-6 p.m. Central time).
For clinical inquiries, contact Hospira Medical Communications at 1-800-615-0187 or email medcom@hospira.com.
According to the letter, adverse events or quality problems associated with use of this product should be reported by calling Hospira Global Complaint Management by phone, 1-800-441-4100; by sending an email to ProductComplaintsPP@hospira.com; or by submitting a report online to Medwatch.
On Twitter @jessnicolecraig
Better ways to drive CAR T-cell therapy

© ASCO/Brian Powers
CHICAGO—Treatment dose and schedule, as well as a patient’s tumor burden, influence the outcome of therapy with chimeric antigen receptor (CAR) T cells, according to research presented at the 2016 ASCO Annual Meeting.
One presentation suggested the dose and schedule of CTL019 can impact both complete response (CR) rates and the incidence of cytokine release syndrome (CRS).
Another presentation indicated that disease burden may determine the risk of toxicity and correlate with the efficacy of JCAR015.
CTL019
Noelle Frey, MD, of the University of Pennsylvania in Philadelphia, presented results observed with CTL019 in 30 adults with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) treated on 2 trials (NCT02030847 and NCT01029366) as abstract 7002.*
Dr Frey noted that CAR T-cell therapies, including CTL019, have led to “unprecedented remission rates of between 70% and 90%.” However, immune activation that leads to high response rates also confers significant treatment-related toxicity—namely, CRS.
She reported that, in the 2 trials of adult ALL patients, a high dose of CTL019 led to a 100% response rate and a 100% CRS rate. Splitting the dose over 3 days led to an 86% response rate and a 66% CRS rate. A single low dose reduced efficacy to 33% and CRS to 66%.
Three of 6 patients who received a single high dose developed CRS and died within weeks. All of these patients had infections or sepsis that likely led to their deaths, Dr Frey said. She suggested clinicians aggressively monitor infections and treat with antimicrobials prior to CAR T-cell therapy.
“The infusion dose and schedule of CTL019 correlate with toxicity and response,” she observed. “A fractionated dosing scheme allows for real-time intra-patient dose modification in response to toxicity and the maintenance of high response rates. Concurrent sepsis and CRS confers a poor outcome.”
Dr Frey said future studies will determine the optimal approach to minimize toxicity while maintaining high efficacy. A fractionated dosing scheme is only one way to mitigate CRS. Other attractive approaches include inverse dosing based on disease burden and varying the timing of anti-cytokine-directed therapy.
JCAR015
Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, reported data from a phase 1 clinical trial (NCT01044069) of JCAR015 in 51 adults with relapsed/refractory ALL as abstract 7003.*
Treatment with JCAR015 led to high CR rates and minimal residual disease (MRD)-negativity, regardless of the amount of disease at baseline. However, outcomes were superior among patients with minimal disease at baseline.
For patients with morphologic disease, 77% achieved a CR by 20 days after treatment, and 90% were MRD-negative.
For those with minimal disease, 90% achieved a CR by 25 days after treatment, and 78% were MRD-negative. These patients experienced significantly less CRS and neurotoxicity than patients with morphologic disease.
“High CR and MRD-negativity rates were observed regardless of pre-T-cell burden,” Dr Park noted. “We observed durable responses and survivals in a subset of patients with no subsequent allotransplant in both morphological and minimal disease cohorts.” ![]()
*Data in the abstracts differ from the presentations.

© ASCO/Brian Powers
CHICAGO—Treatment dose and schedule, as well as a patient’s tumor burden, influence the outcome of therapy with chimeric antigen receptor (CAR) T cells, according to research presented at the 2016 ASCO Annual Meeting.
One presentation suggested the dose and schedule of CTL019 can impact both complete response (CR) rates and the incidence of cytokine release syndrome (CRS).
Another presentation indicated that disease burden may determine the risk of toxicity and correlate with the efficacy of JCAR015.
CTL019
Noelle Frey, MD, of the University of Pennsylvania in Philadelphia, presented results observed with CTL019 in 30 adults with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) treated on 2 trials (NCT02030847 and NCT01029366) as abstract 7002.*
Dr Frey noted that CAR T-cell therapies, including CTL019, have led to “unprecedented remission rates of between 70% and 90%.” However, immune activation that leads to high response rates also confers significant treatment-related toxicity—namely, CRS.
She reported that, in the 2 trials of adult ALL patients, a high dose of CTL019 led to a 100% response rate and a 100% CRS rate. Splitting the dose over 3 days led to an 86% response rate and a 66% CRS rate. A single low dose reduced efficacy to 33% and CRS to 66%.
Three of 6 patients who received a single high dose developed CRS and died within weeks. All of these patients had infections or sepsis that likely led to their deaths, Dr Frey said. She suggested clinicians aggressively monitor infections and treat with antimicrobials prior to CAR T-cell therapy.
“The infusion dose and schedule of CTL019 correlate with toxicity and response,” she observed. “A fractionated dosing scheme allows for real-time intra-patient dose modification in response to toxicity and the maintenance of high response rates. Concurrent sepsis and CRS confers a poor outcome.”
Dr Frey said future studies will determine the optimal approach to minimize toxicity while maintaining high efficacy. A fractionated dosing scheme is only one way to mitigate CRS. Other attractive approaches include inverse dosing based on disease burden and varying the timing of anti-cytokine-directed therapy.
JCAR015
Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, reported data from a phase 1 clinical trial (NCT01044069) of JCAR015 in 51 adults with relapsed/refractory ALL as abstract 7003.*
Treatment with JCAR015 led to high CR rates and minimal residual disease (MRD)-negativity, regardless of the amount of disease at baseline. However, outcomes were superior among patients with minimal disease at baseline.
For patients with morphologic disease, 77% achieved a CR by 20 days after treatment, and 90% were MRD-negative.
For those with minimal disease, 90% achieved a CR by 25 days after treatment, and 78% were MRD-negative. These patients experienced significantly less CRS and neurotoxicity than patients with morphologic disease.
“High CR and MRD-negativity rates were observed regardless of pre-T-cell burden,” Dr Park noted. “We observed durable responses and survivals in a subset of patients with no subsequent allotransplant in both morphological and minimal disease cohorts.” ![]()
*Data in the abstracts differ from the presentations.

© ASCO/Brian Powers
CHICAGO—Treatment dose and schedule, as well as a patient’s tumor burden, influence the outcome of therapy with chimeric antigen receptor (CAR) T cells, according to research presented at the 2016 ASCO Annual Meeting.
One presentation suggested the dose and schedule of CTL019 can impact both complete response (CR) rates and the incidence of cytokine release syndrome (CRS).
Another presentation indicated that disease burden may determine the risk of toxicity and correlate with the efficacy of JCAR015.
CTL019
Noelle Frey, MD, of the University of Pennsylvania in Philadelphia, presented results observed with CTL019 in 30 adults with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) treated on 2 trials (NCT02030847 and NCT01029366) as abstract 7002.*
Dr Frey noted that CAR T-cell therapies, including CTL019, have led to “unprecedented remission rates of between 70% and 90%.” However, immune activation that leads to high response rates also confers significant treatment-related toxicity—namely, CRS.
She reported that, in the 2 trials of adult ALL patients, a high dose of CTL019 led to a 100% response rate and a 100% CRS rate. Splitting the dose over 3 days led to an 86% response rate and a 66% CRS rate. A single low dose reduced efficacy to 33% and CRS to 66%.
Three of 6 patients who received a single high dose developed CRS and died within weeks. All of these patients had infections or sepsis that likely led to their deaths, Dr Frey said. She suggested clinicians aggressively monitor infections and treat with antimicrobials prior to CAR T-cell therapy.
“The infusion dose and schedule of CTL019 correlate with toxicity and response,” she observed. “A fractionated dosing scheme allows for real-time intra-patient dose modification in response to toxicity and the maintenance of high response rates. Concurrent sepsis and CRS confers a poor outcome.”
Dr Frey said future studies will determine the optimal approach to minimize toxicity while maintaining high efficacy. A fractionated dosing scheme is only one way to mitigate CRS. Other attractive approaches include inverse dosing based on disease burden and varying the timing of anti-cytokine-directed therapy.
JCAR015
Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York, reported data from a phase 1 clinical trial (NCT01044069) of JCAR015 in 51 adults with relapsed/refractory ALL as abstract 7003.*
Treatment with JCAR015 led to high CR rates and minimal residual disease (MRD)-negativity, regardless of the amount of disease at baseline. However, outcomes were superior among patients with minimal disease at baseline.
For patients with morphologic disease, 77% achieved a CR by 20 days after treatment, and 90% were MRD-negative.
For those with minimal disease, 90% achieved a CR by 25 days after treatment, and 78% were MRD-negative. These patients experienced significantly less CRS and neurotoxicity than patients with morphologic disease.
“High CR and MRD-negativity rates were observed regardless of pre-T-cell burden,” Dr Park noted. “We observed durable responses and survivals in a subset of patients with no subsequent allotransplant in both morphological and minimal disease cohorts.” ![]()
*Data in the abstracts differ from the presentations.
No kidding: Adults fare better with pediatric ALL regimen
COPENHAGEN – A study from seven Nordic and Baltic countries adds to the growing body of evidence that young adults with acute lymphoblastic leukemia do better when they are treated like children – that is, with a standard pediatric chemotherapy regimen.
In a study of 221 patients from the age of 18 to 45 with acute lymphoblastic leukemia (ALL) treated with a pediatric regimen and followed for a median of 4 years, the event-free survival rate was 73%, compared with 42% for historical controls, reported Dr. Nina Toft from Herlev (Denmark) University Hospital.
“We have improved the survival for ALL patients 18 to 45 years, and we show that the cure rates are close to those of children. If you compare an adult in the standard-risk group with a child in the standard-risk group, there’s no difference,” she said at a briefing at the annual congress of the European Hematology Association (EHA).
The findings support those from an earlier study reported at the 2014 annual meeting of the American Society of Hematology. That study, conducted by Dr. Wendy Stock of the University of Chicago Medical Center and her colleagues showed that among 296 adolescents and young adults with ALL treated with an intensive pediatric chemotherapy combination regimen rather than a less-intensive adult regimen, the rate of overall survival (OS) at 2 years was 78%, and the event-free survival (EFS) rate was 66%.
In the current study, Dr. Toft and her colleagues looked at event-free survival among 1,509 children and adults (up to age 45) diagnosed with Philadelphia chromosome-negative ALL from July 2008 through December 2014.
The patients were treated in Sweden, Norway, Iceland, Finland Denmark, Lithuania, and Estonia, and all received the Nordic Society for Pediatric Hematology and Oncology (NOPHO)–ALL 2008 protocol, consisting of induction with prednisolone, vincristine, doxorubicin, and pegylated (PEG) asparaginase; consolidation for patients with standard- and intermediate-risk disease, with mercaptopurine, vincristine, PEG asparaginase, and methotrexate; and additional intensive combination chemotherapy for patients with high-risk disease, followed by maintenance with mercaptopurine and/or methotrexate, PEG asparaginase, vincristine, and dexamethasone.
Of the 1,509 patients, 1,022 were children from 1 to 9 years, 266 were preteens/adolescents (10-17 years), and 221 were adults up to age 45.
All patients were stratified by clinical, cytogenetic, and other factors into one of four risk groups: standard, intermediate, high risk, or high risk in first remission after a hematopoietic stem cell transplant, and all were treated with the NOPHO-ALL 2008 protocol.
The investigators found that in general older patients had higher-risk disease. Nonetheless, treatment intervals and severe toxicities, with the exception of osteonecrosis and thrombosis, were “almost identical” between adults and children, Dr. Toft said.
After a median of 4 years of follow-up, 16 patients, 3 of whom were adults, had died during the induction phase, and 50 patients (12 adults) had died in first remission.
There were a total of 123 relapses (36 among adults), and 1 adult and 12 children were diagnosed with a second malignancy.
Overall 5-year EFS rates were 88% for patients aged 1-9 years, 79% for those 10-17 years, and 73% for those 18-45, and these differences were significant (P less than .001). However, when EFS was stratified by risk group, EFS rates were lower only for adults with intermediate-risk disease (78% vs. 90% for 1- to 9-year-olds and 82% for 10- to 17-year-olds, P = .002).
Although the investigators did not formally report overall survival data, it was approximately 95% among all children in the cohort, and approximately 76% among the adults, Dr. Toft said in response to a reporter.
“What we need now is further cooperation, because we need to unite and get more patients so that we can show new developments faster. We also need to find new risk criteria, because of the intermediate-risk group; something is missing for the adults. We need more cytogenetics, we need something to define the patients as high risk or not,” Dr. Toft said.
“We also need new drugs, because with this method we’ve reached the limit of toxicity,” she added.
The study was sponsored by health authorities in the participating countries. Dr. Toft reported no relevant financial disclosures.
COPENHAGEN – A study from seven Nordic and Baltic countries adds to the growing body of evidence that young adults with acute lymphoblastic leukemia do better when they are treated like children – that is, with a standard pediatric chemotherapy regimen.
In a study of 221 patients from the age of 18 to 45 with acute lymphoblastic leukemia (ALL) treated with a pediatric regimen and followed for a median of 4 years, the event-free survival rate was 73%, compared with 42% for historical controls, reported Dr. Nina Toft from Herlev (Denmark) University Hospital.
“We have improved the survival for ALL patients 18 to 45 years, and we show that the cure rates are close to those of children. If you compare an adult in the standard-risk group with a child in the standard-risk group, there’s no difference,” she said at a briefing at the annual congress of the European Hematology Association (EHA).
The findings support those from an earlier study reported at the 2014 annual meeting of the American Society of Hematology. That study, conducted by Dr. Wendy Stock of the University of Chicago Medical Center and her colleagues showed that among 296 adolescents and young adults with ALL treated with an intensive pediatric chemotherapy combination regimen rather than a less-intensive adult regimen, the rate of overall survival (OS) at 2 years was 78%, and the event-free survival (EFS) rate was 66%.
In the current study, Dr. Toft and her colleagues looked at event-free survival among 1,509 children and adults (up to age 45) diagnosed with Philadelphia chromosome-negative ALL from July 2008 through December 2014.
The patients were treated in Sweden, Norway, Iceland, Finland Denmark, Lithuania, and Estonia, and all received the Nordic Society for Pediatric Hematology and Oncology (NOPHO)–ALL 2008 protocol, consisting of induction with prednisolone, vincristine, doxorubicin, and pegylated (PEG) asparaginase; consolidation for patients with standard- and intermediate-risk disease, with mercaptopurine, vincristine, PEG asparaginase, and methotrexate; and additional intensive combination chemotherapy for patients with high-risk disease, followed by maintenance with mercaptopurine and/or methotrexate, PEG asparaginase, vincristine, and dexamethasone.
Of the 1,509 patients, 1,022 were children from 1 to 9 years, 266 were preteens/adolescents (10-17 years), and 221 were adults up to age 45.
All patients were stratified by clinical, cytogenetic, and other factors into one of four risk groups: standard, intermediate, high risk, or high risk in first remission after a hematopoietic stem cell transplant, and all were treated with the NOPHO-ALL 2008 protocol.
The investigators found that in general older patients had higher-risk disease. Nonetheless, treatment intervals and severe toxicities, with the exception of osteonecrosis and thrombosis, were “almost identical” between adults and children, Dr. Toft said.
After a median of 4 years of follow-up, 16 patients, 3 of whom were adults, had died during the induction phase, and 50 patients (12 adults) had died in first remission.
There were a total of 123 relapses (36 among adults), and 1 adult and 12 children were diagnosed with a second malignancy.
Overall 5-year EFS rates were 88% for patients aged 1-9 years, 79% for those 10-17 years, and 73% for those 18-45, and these differences were significant (P less than .001). However, when EFS was stratified by risk group, EFS rates were lower only for adults with intermediate-risk disease (78% vs. 90% for 1- to 9-year-olds and 82% for 10- to 17-year-olds, P = .002).
Although the investigators did not formally report overall survival data, it was approximately 95% among all children in the cohort, and approximately 76% among the adults, Dr. Toft said in response to a reporter.
“What we need now is further cooperation, because we need to unite and get more patients so that we can show new developments faster. We also need to find new risk criteria, because of the intermediate-risk group; something is missing for the adults. We need more cytogenetics, we need something to define the patients as high risk or not,” Dr. Toft said.
“We also need new drugs, because with this method we’ve reached the limit of toxicity,” she added.
The study was sponsored by health authorities in the participating countries. Dr. Toft reported no relevant financial disclosures.
COPENHAGEN – A study from seven Nordic and Baltic countries adds to the growing body of evidence that young adults with acute lymphoblastic leukemia do better when they are treated like children – that is, with a standard pediatric chemotherapy regimen.
In a study of 221 patients from the age of 18 to 45 with acute lymphoblastic leukemia (ALL) treated with a pediatric regimen and followed for a median of 4 years, the event-free survival rate was 73%, compared with 42% for historical controls, reported Dr. Nina Toft from Herlev (Denmark) University Hospital.
“We have improved the survival for ALL patients 18 to 45 years, and we show that the cure rates are close to those of children. If you compare an adult in the standard-risk group with a child in the standard-risk group, there’s no difference,” she said at a briefing at the annual congress of the European Hematology Association (EHA).
The findings support those from an earlier study reported at the 2014 annual meeting of the American Society of Hematology. That study, conducted by Dr. Wendy Stock of the University of Chicago Medical Center and her colleagues showed that among 296 adolescents and young adults with ALL treated with an intensive pediatric chemotherapy combination regimen rather than a less-intensive adult regimen, the rate of overall survival (OS) at 2 years was 78%, and the event-free survival (EFS) rate was 66%.
In the current study, Dr. Toft and her colleagues looked at event-free survival among 1,509 children and adults (up to age 45) diagnosed with Philadelphia chromosome-negative ALL from July 2008 through December 2014.
The patients were treated in Sweden, Norway, Iceland, Finland Denmark, Lithuania, and Estonia, and all received the Nordic Society for Pediatric Hematology and Oncology (NOPHO)–ALL 2008 protocol, consisting of induction with prednisolone, vincristine, doxorubicin, and pegylated (PEG) asparaginase; consolidation for patients with standard- and intermediate-risk disease, with mercaptopurine, vincristine, PEG asparaginase, and methotrexate; and additional intensive combination chemotherapy for patients with high-risk disease, followed by maintenance with mercaptopurine and/or methotrexate, PEG asparaginase, vincristine, and dexamethasone.
Of the 1,509 patients, 1,022 were children from 1 to 9 years, 266 were preteens/adolescents (10-17 years), and 221 were adults up to age 45.
All patients were stratified by clinical, cytogenetic, and other factors into one of four risk groups: standard, intermediate, high risk, or high risk in first remission after a hematopoietic stem cell transplant, and all were treated with the NOPHO-ALL 2008 protocol.
The investigators found that in general older patients had higher-risk disease. Nonetheless, treatment intervals and severe toxicities, with the exception of osteonecrosis and thrombosis, were “almost identical” between adults and children, Dr. Toft said.
After a median of 4 years of follow-up, 16 patients, 3 of whom were adults, had died during the induction phase, and 50 patients (12 adults) had died in first remission.
There were a total of 123 relapses (36 among adults), and 1 adult and 12 children were diagnosed with a second malignancy.
Overall 5-year EFS rates were 88% for patients aged 1-9 years, 79% for those 10-17 years, and 73% for those 18-45, and these differences were significant (P less than .001). However, when EFS was stratified by risk group, EFS rates were lower only for adults with intermediate-risk disease (78% vs. 90% for 1- to 9-year-olds and 82% for 10- to 17-year-olds, P = .002).
Although the investigators did not formally report overall survival data, it was approximately 95% among all children in the cohort, and approximately 76% among the adults, Dr. Toft said in response to a reporter.
“What we need now is further cooperation, because we need to unite and get more patients so that we can show new developments faster. We also need to find new risk criteria, because of the intermediate-risk group; something is missing for the adults. We need more cytogenetics, we need something to define the patients as high risk or not,” Dr. Toft said.
“We also need new drugs, because with this method we’ve reached the limit of toxicity,” she added.
The study was sponsored by health authorities in the participating countries. Dr. Toft reported no relevant financial disclosures.
AT THE EHA CONGRESS
Key clinical point: Younger adults with acute lymphoblastic leukemia have better outcomes when treated with more intensive chemotherapy regimens designed for children with ALL.
Major finding: No significant differences were found in event-free survival between children and adults, except for a slightly lower EFS among adults with intermediate risk.
Data source: A prospective study of 1,509 children and adults with ALL in seven Nordic and Baltic countries.
Disclosures: The study was sponsored by health authorities in the participating countries. Dr. Toft reported no relevant financial disclosures.
Immunotherapy ‘outcompetes’ chemo in rel/ref B-ALL

COPENHAGEN—Interim results from the phase 3 TOWER trial suggest blinatumomab can prolong overall survival (OS) when compared to standard care in adults with Ph-negative, relapsed/refractory B-cell precursor acute lymphoblastic leukemia (B-ALL).
The median OS for patients treated with blinatumomab was nearly double the median OS of patients who received standard chemotherapy (investigator’s choice of 4 different regimens).
Based on these results, Amgen, the company sponsoring the trial, decided to stop it early.
“This was the first study to show that immunotherapy can outcompete chemotherapy,” said Max S. Topp, MD, of University Hospital of Wuerzburg in Germany.
Dr Topp presented results from this study at the 21st Congress of the European Hematology Association (abstract S149).
Patients and treatment
The TOWER trial enrolled and randomized 405 patients with Ph-negative, relapsed/refractory B-ALL, and 376 of them ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of 1 of 4 protocol-defined standard chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Patients who received blinatumomab received it as a continuous infusion, 4 weeks on and 2 weeks off, at 9 µg/day for 7 days, then 28 µg/day on weeks 2-4. They received 2 cycles of induction, which was followed by 3 cycles of consolidation if they had ≤5% blasts.
If patients still had ≤5% blasts after consolidation, they received up to 12 months of blinatumomab maintenance. Maintenance was a continuous infusion, 4 weeks on and 8 weeks off, at 28 µg/day.
Patient characteristics were similar between the treatment arms. The median age was 37 in both arms (overall range, 18-80). About 40% of patients in the blinatumomab arm and 50% in the chemotherapy arm had not received any prior salvage regimens.
More than 30% of patients in both arms had undergone an allogeneic hematopoietic stem cell transplant (allo-HSCT), and about 20% were primary refractory. Roughly 30% of blinatumomab-treated patients were refractory to salvage therapy, as were more than 20% of chemotherapy-treated patients.
Response and survival
During induction, in the intent-to-treat population (n=271 in the blinatumomab arm and 134 in the chemotherapy arm), the overall response rate was 44% in the blinatumomab arm and 25% in the chemotherapy arm (P<0.001). The complete response rates were 34% and 16%, respectively.
Among patients who received their assigned treatment (n=267 in the blinatumomab arm and 109 in the chemotherapy arm), the overall response rates were 45% and 30%, respectively (P=0.007).
In the intent-to-treat population, the median OS was 7.7 months (95% CI, 5.6-9.6) in the blinatumomab arm and 4 months (95% CI, 2.9-5.3) in the chemotherapy arm (hazard ratio=0.71, P=0.012). The survival curves were similar in the as-treated population.
Dr Topp noted that the improvement in OS with blinatumomab was consistent across subgroups, regardless of age, prior salvage therapy, or prior allo-HSCT.
Dr Topp and his colleagues also considered the effect post-treatment allo-HSCT might have on OS. Sixty-five patients in the blinatumomab arm and 32 in the chemotherapy arm went on to receive an allo-HSCT (24% of patients in both arms).
When the researchers censored for post-treatment allo-HSCT, the median OS was 6.9 months in the blinatumomab arm and 3.9 months in the chemotherapy arm (hazard ratio=0.66, P=0.004).
Safety
In the as-treated population, 99% of patients in both arms experienced adverse events (AEs).
The incidence of grade 3 AEs was 37% in the blinatumomab arm and 30% in the chemotherapy arm. The incidence of grade 4 AEs was 31% and 44%, respectively. The incidence of grade 5 AEs was 19% and 17%, respectively.
Grade 3 or higher AEs of interest, according to Dr Topp, were infection (34% with blinatumomab and 52% with chemotherapy), neutropenia (38% and 58%, respectively), neurologic events (9% and 8%, respectively), and cytokine release syndrome (5% and 0%, respectively).
Seven patients—5 in the blinatumomab arm and 2 in the chemotherapy arm—who did not undergo allo-HSCT died during the study without documented relapse. ![]()

COPENHAGEN—Interim results from the phase 3 TOWER trial suggest blinatumomab can prolong overall survival (OS) when compared to standard care in adults with Ph-negative, relapsed/refractory B-cell precursor acute lymphoblastic leukemia (B-ALL).
The median OS for patients treated with blinatumomab was nearly double the median OS of patients who received standard chemotherapy (investigator’s choice of 4 different regimens).
Based on these results, Amgen, the company sponsoring the trial, decided to stop it early.
“This was the first study to show that immunotherapy can outcompete chemotherapy,” said Max S. Topp, MD, of University Hospital of Wuerzburg in Germany.
Dr Topp presented results from this study at the 21st Congress of the European Hematology Association (abstract S149).
Patients and treatment
The TOWER trial enrolled and randomized 405 patients with Ph-negative, relapsed/refractory B-ALL, and 376 of them ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of 1 of 4 protocol-defined standard chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Patients who received blinatumomab received it as a continuous infusion, 4 weeks on and 2 weeks off, at 9 µg/day for 7 days, then 28 µg/day on weeks 2-4. They received 2 cycles of induction, which was followed by 3 cycles of consolidation if they had ≤5% blasts.
If patients still had ≤5% blasts after consolidation, they received up to 12 months of blinatumomab maintenance. Maintenance was a continuous infusion, 4 weeks on and 8 weeks off, at 28 µg/day.
Patient characteristics were similar between the treatment arms. The median age was 37 in both arms (overall range, 18-80). About 40% of patients in the blinatumomab arm and 50% in the chemotherapy arm had not received any prior salvage regimens.
More than 30% of patients in both arms had undergone an allogeneic hematopoietic stem cell transplant (allo-HSCT), and about 20% were primary refractory. Roughly 30% of blinatumomab-treated patients were refractory to salvage therapy, as were more than 20% of chemotherapy-treated patients.
Response and survival
During induction, in the intent-to-treat population (n=271 in the blinatumomab arm and 134 in the chemotherapy arm), the overall response rate was 44% in the blinatumomab arm and 25% in the chemotherapy arm (P<0.001). The complete response rates were 34% and 16%, respectively.
Among patients who received their assigned treatment (n=267 in the blinatumomab arm and 109 in the chemotherapy arm), the overall response rates were 45% and 30%, respectively (P=0.007).
In the intent-to-treat population, the median OS was 7.7 months (95% CI, 5.6-9.6) in the blinatumomab arm and 4 months (95% CI, 2.9-5.3) in the chemotherapy arm (hazard ratio=0.71, P=0.012). The survival curves were similar in the as-treated population.
Dr Topp noted that the improvement in OS with blinatumomab was consistent across subgroups, regardless of age, prior salvage therapy, or prior allo-HSCT.
Dr Topp and his colleagues also considered the effect post-treatment allo-HSCT might have on OS. Sixty-five patients in the blinatumomab arm and 32 in the chemotherapy arm went on to receive an allo-HSCT (24% of patients in both arms).
When the researchers censored for post-treatment allo-HSCT, the median OS was 6.9 months in the blinatumomab arm and 3.9 months in the chemotherapy arm (hazard ratio=0.66, P=0.004).
Safety
In the as-treated population, 99% of patients in both arms experienced adverse events (AEs).
The incidence of grade 3 AEs was 37% in the blinatumomab arm and 30% in the chemotherapy arm. The incidence of grade 4 AEs was 31% and 44%, respectively. The incidence of grade 5 AEs was 19% and 17%, respectively.
Grade 3 or higher AEs of interest, according to Dr Topp, were infection (34% with blinatumomab and 52% with chemotherapy), neutropenia (38% and 58%, respectively), neurologic events (9% and 8%, respectively), and cytokine release syndrome (5% and 0%, respectively).
Seven patients—5 in the blinatumomab arm and 2 in the chemotherapy arm—who did not undergo allo-HSCT died during the study without documented relapse. ![]()

COPENHAGEN—Interim results from the phase 3 TOWER trial suggest blinatumomab can prolong overall survival (OS) when compared to standard care in adults with Ph-negative, relapsed/refractory B-cell precursor acute lymphoblastic leukemia (B-ALL).
The median OS for patients treated with blinatumomab was nearly double the median OS of patients who received standard chemotherapy (investigator’s choice of 4 different regimens).
Based on these results, Amgen, the company sponsoring the trial, decided to stop it early.
“This was the first study to show that immunotherapy can outcompete chemotherapy,” said Max S. Topp, MD, of University Hospital of Wuerzburg in Germany.
Dr Topp presented results from this study at the 21st Congress of the European Hematology Association (abstract S149).
Patients and treatment
The TOWER trial enrolled and randomized 405 patients with Ph-negative, relapsed/refractory B-ALL, and 376 of them ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of 1 of 4 protocol-defined standard chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Patients who received blinatumomab received it as a continuous infusion, 4 weeks on and 2 weeks off, at 9 µg/day for 7 days, then 28 µg/day on weeks 2-4. They received 2 cycles of induction, which was followed by 3 cycles of consolidation if they had ≤5% blasts.
If patients still had ≤5% blasts after consolidation, they received up to 12 months of blinatumomab maintenance. Maintenance was a continuous infusion, 4 weeks on and 8 weeks off, at 28 µg/day.
Patient characteristics were similar between the treatment arms. The median age was 37 in both arms (overall range, 18-80). About 40% of patients in the blinatumomab arm and 50% in the chemotherapy arm had not received any prior salvage regimens.
More than 30% of patients in both arms had undergone an allogeneic hematopoietic stem cell transplant (allo-HSCT), and about 20% were primary refractory. Roughly 30% of blinatumomab-treated patients were refractory to salvage therapy, as were more than 20% of chemotherapy-treated patients.
Response and survival
During induction, in the intent-to-treat population (n=271 in the blinatumomab arm and 134 in the chemotherapy arm), the overall response rate was 44% in the blinatumomab arm and 25% in the chemotherapy arm (P<0.001). The complete response rates were 34% and 16%, respectively.
Among patients who received their assigned treatment (n=267 in the blinatumomab arm and 109 in the chemotherapy arm), the overall response rates were 45% and 30%, respectively (P=0.007).
In the intent-to-treat population, the median OS was 7.7 months (95% CI, 5.6-9.6) in the blinatumomab arm and 4 months (95% CI, 2.9-5.3) in the chemotherapy arm (hazard ratio=0.71, P=0.012). The survival curves were similar in the as-treated population.
Dr Topp noted that the improvement in OS with blinatumomab was consistent across subgroups, regardless of age, prior salvage therapy, or prior allo-HSCT.
Dr Topp and his colleagues also considered the effect post-treatment allo-HSCT might have on OS. Sixty-five patients in the blinatumomab arm and 32 in the chemotherapy arm went on to receive an allo-HSCT (24% of patients in both arms).
When the researchers censored for post-treatment allo-HSCT, the median OS was 6.9 months in the blinatumomab arm and 3.9 months in the chemotherapy arm (hazard ratio=0.66, P=0.004).
Safety
In the as-treated population, 99% of patients in both arms experienced adverse events (AEs).
The incidence of grade 3 AEs was 37% in the blinatumomab arm and 30% in the chemotherapy arm. The incidence of grade 4 AEs was 31% and 44%, respectively. The incidence of grade 5 AEs was 19% and 17%, respectively.
Grade 3 or higher AEs of interest, according to Dr Topp, were infection (34% with blinatumomab and 52% with chemotherapy), neutropenia (38% and 58%, respectively), neurologic events (9% and 8%, respectively), and cytokine release syndrome (5% and 0%, respectively).
Seven patients—5 in the blinatumomab arm and 2 in the chemotherapy arm—who did not undergo allo-HSCT died during the study without documented relapse. ![]()
Blinatumomab doubles survival in relapsed Ph-negative ALL
Copenhagen – The monoclonal antibody blinatumomab nearly doubled overall survival compared with standard chemotherapy among patients with relapsed or refractory B-cell precursor leukemia negative for the Philadelphia chromosome, investigators reported.
Among 405 patients enrolled in the TOWER study, a multicenter, open-label phase III trial, median overall survival for patients treated with blinatumomab (Blincyto) was 7.7 months, compared with 4.0 months for patients treated with one of four standard chemotherapy regimens (P = .012) reported Dr. Max S. Topp of Universitätsklinikum in Würzburg, Germany.
“Blinatumomab is the first immunotherapy agent to demonstrate an overall survival benefit when compared to chemotherapy in patients relapsing with adult acute lymphoblastic leukemia. It increases almost twofold the overall survival when compared to standard care. This was consistent in all subgroups that we were looking at, regardless of age, prior salvage therapy, or patients relapsing after an allo-transplantation,” he said at a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
The trial was halted early, after a preplanned interim analysis showed a clear survival benefit with blinatumomab.
Blinatumomab is a bispecific T-cell engager (BiTE) antibody designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. As previously reported, it has induced high complete remission rates in patients with relapsed or refractory acute lymphoblastic leukemia (ALL).
In May 2015, the Food and Drug Administration granted blinatumomab accelerated approval for the treatment of adult patients with relapsed/refractory Philadelphia chromosome–negative B-cell precursor acute lymphoblastic leukemia (BCP-ALL).
In the TOWER trial, patients with relapsed/refractory BCP-ALL were randomly assigned on a 2:1 basis to receive either blinatumomab (271 patients) or standard chemotherapy (134), consisting of the investigator’s choice of one of four defined regimens (based on either anthracyclines, histone deacetylase inhibitors, high-dose methotrexate, or clofarabine).
The patients were further stratified by age, prior salvage therapy, and prior allogeneic stem cell transplant (alloSCT).
Patients assigned to receive blinatumomab received it in 6-week cycles consisting of continuous infusions of 9 mcg/day in week 1 of cycle 1, then 28 mcg/day for weeks 3-4, followed by 2 weeks off. Patients were pretreated with dexamethasone for prophylaxis against the cytokine release syndrome.
Patients whose disease was in remission following two induction cycles could be continued on therapy until relapse.
As noted, the trial was halted early, after 248 patients had died; the primary analysis had been planned to occur after 330 patients had died.
In addition to the superior survival rates, blinatumomab was associated with a higher rate of complete responses (39% vs. 19%, P less than .001) and combined complete responses, complete hematologic responses, and complete responses with incomplete recovery of counts (46% vs. 28%, P = .001).
In all, 19% of patients assigned to blinatumomab and 17% assigned to chemotherapy died on study. Grade 3 or greater adverse events included neutropenia (38% of patients and 58%, respectively), infections (34% and 52%), neurologic events (9% and 8%), and the cytokine release syndrome (5% vs. 0%).
Dr. Anton Hagenbeek, professor of hematology at the University of Amsterdam, who moderated the briefing, asked Dr. Topp whether there were plans to use blinatumomab earlier in the course of disease.
Dr. Topp agreed that it might be a valuable addition to upfront therapy, noting that blinatumomab has been shown in a small percentage of patients who are positive for minimal residual disease to convert to being negative for minimal residual disease, and that this conversion was associated with improved overall survival.
Copenhagen – The monoclonal antibody blinatumomab nearly doubled overall survival compared with standard chemotherapy among patients with relapsed or refractory B-cell precursor leukemia negative for the Philadelphia chromosome, investigators reported.
Among 405 patients enrolled in the TOWER study, a multicenter, open-label phase III trial, median overall survival for patients treated with blinatumomab (Blincyto) was 7.7 months, compared with 4.0 months for patients treated with one of four standard chemotherapy regimens (P = .012) reported Dr. Max S. Topp of Universitätsklinikum in Würzburg, Germany.
“Blinatumomab is the first immunotherapy agent to demonstrate an overall survival benefit when compared to chemotherapy in patients relapsing with adult acute lymphoblastic leukemia. It increases almost twofold the overall survival when compared to standard care. This was consistent in all subgroups that we were looking at, regardless of age, prior salvage therapy, or patients relapsing after an allo-transplantation,” he said at a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
The trial was halted early, after a preplanned interim analysis showed a clear survival benefit with blinatumomab.
Blinatumomab is a bispecific T-cell engager (BiTE) antibody designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. As previously reported, it has induced high complete remission rates in patients with relapsed or refractory acute lymphoblastic leukemia (ALL).
In May 2015, the Food and Drug Administration granted blinatumomab accelerated approval for the treatment of adult patients with relapsed/refractory Philadelphia chromosome–negative B-cell precursor acute lymphoblastic leukemia (BCP-ALL).
In the TOWER trial, patients with relapsed/refractory BCP-ALL were randomly assigned on a 2:1 basis to receive either blinatumomab (271 patients) or standard chemotherapy (134), consisting of the investigator’s choice of one of four defined regimens (based on either anthracyclines, histone deacetylase inhibitors, high-dose methotrexate, or clofarabine).
The patients were further stratified by age, prior salvage therapy, and prior allogeneic stem cell transplant (alloSCT).
Patients assigned to receive blinatumomab received it in 6-week cycles consisting of continuous infusions of 9 mcg/day in week 1 of cycle 1, then 28 mcg/day for weeks 3-4, followed by 2 weeks off. Patients were pretreated with dexamethasone for prophylaxis against the cytokine release syndrome.
Patients whose disease was in remission following two induction cycles could be continued on therapy until relapse.
As noted, the trial was halted early, after 248 patients had died; the primary analysis had been planned to occur after 330 patients had died.
In addition to the superior survival rates, blinatumomab was associated with a higher rate of complete responses (39% vs. 19%, P less than .001) and combined complete responses, complete hematologic responses, and complete responses with incomplete recovery of counts (46% vs. 28%, P = .001).
In all, 19% of patients assigned to blinatumomab and 17% assigned to chemotherapy died on study. Grade 3 or greater adverse events included neutropenia (38% of patients and 58%, respectively), infections (34% and 52%), neurologic events (9% and 8%), and the cytokine release syndrome (5% vs. 0%).
Dr. Anton Hagenbeek, professor of hematology at the University of Amsterdam, who moderated the briefing, asked Dr. Topp whether there were plans to use blinatumomab earlier in the course of disease.
Dr. Topp agreed that it might be a valuable addition to upfront therapy, noting that blinatumomab has been shown in a small percentage of patients who are positive for minimal residual disease to convert to being negative for minimal residual disease, and that this conversion was associated with improved overall survival.
Copenhagen – The monoclonal antibody blinatumomab nearly doubled overall survival compared with standard chemotherapy among patients with relapsed or refractory B-cell precursor leukemia negative for the Philadelphia chromosome, investigators reported.
Among 405 patients enrolled in the TOWER study, a multicenter, open-label phase III trial, median overall survival for patients treated with blinatumomab (Blincyto) was 7.7 months, compared with 4.0 months for patients treated with one of four standard chemotherapy regimens (P = .012) reported Dr. Max S. Topp of Universitätsklinikum in Würzburg, Germany.
“Blinatumomab is the first immunotherapy agent to demonstrate an overall survival benefit when compared to chemotherapy in patients relapsing with adult acute lymphoblastic leukemia. It increases almost twofold the overall survival when compared to standard care. This was consistent in all subgroups that we were looking at, regardless of age, prior salvage therapy, or patients relapsing after an allo-transplantation,” he said at a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
The trial was halted early, after a preplanned interim analysis showed a clear survival benefit with blinatumomab.
Blinatumomab is a bispecific T-cell engager (BiTE) antibody designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. As previously reported, it has induced high complete remission rates in patients with relapsed or refractory acute lymphoblastic leukemia (ALL).
In May 2015, the Food and Drug Administration granted blinatumomab accelerated approval for the treatment of adult patients with relapsed/refractory Philadelphia chromosome–negative B-cell precursor acute lymphoblastic leukemia (BCP-ALL).
In the TOWER trial, patients with relapsed/refractory BCP-ALL were randomly assigned on a 2:1 basis to receive either blinatumomab (271 patients) or standard chemotherapy (134), consisting of the investigator’s choice of one of four defined regimens (based on either anthracyclines, histone deacetylase inhibitors, high-dose methotrexate, or clofarabine).
The patients were further stratified by age, prior salvage therapy, and prior allogeneic stem cell transplant (alloSCT).
Patients assigned to receive blinatumomab received it in 6-week cycles consisting of continuous infusions of 9 mcg/day in week 1 of cycle 1, then 28 mcg/day for weeks 3-4, followed by 2 weeks off. Patients were pretreated with dexamethasone for prophylaxis against the cytokine release syndrome.
Patients whose disease was in remission following two induction cycles could be continued on therapy until relapse.
As noted, the trial was halted early, after 248 patients had died; the primary analysis had been planned to occur after 330 patients had died.
In addition to the superior survival rates, blinatumomab was associated with a higher rate of complete responses (39% vs. 19%, P less than .001) and combined complete responses, complete hematologic responses, and complete responses with incomplete recovery of counts (46% vs. 28%, P = .001).
In all, 19% of patients assigned to blinatumomab and 17% assigned to chemotherapy died on study. Grade 3 or greater adverse events included neutropenia (38% of patients and 58%, respectively), infections (34% and 52%), neurologic events (9% and 8%), and the cytokine release syndrome (5% vs. 0%).
Dr. Anton Hagenbeek, professor of hematology at the University of Amsterdam, who moderated the briefing, asked Dr. Topp whether there were plans to use blinatumomab earlier in the course of disease.
Dr. Topp agreed that it might be a valuable addition to upfront therapy, noting that blinatumomab has been shown in a small percentage of patients who are positive for minimal residual disease to convert to being negative for minimal residual disease, and that this conversion was associated with improved overall survival.
At THE EHA CONGRESS
Key clinical point: Single-agent blinatumomab nearly doubled overall survival compared to chemotherapy in relapsed/refractory ALL.
Major finding: Median overall survival was 7.7 months for patients on blinatumomab compared with 4.0 months for those on chemotherapy.
Data source: Randomized open-label phase III trial in 405 adults with relapsed/refractory Philadelphia chromosome–negative B-cell precursor ALL.
Disclosures: Amgen funded the study. Dr. Topp disclosed having a consultant or advisory role and receiving other remuneration from Micromet, which was acquired by Amgen. Dr. Hagenbeek reported no relevant disclosures.
Inotuzumab bests standard of care in adult ALL

Photo courtesy of MDACC
In multiple categories, the antibody-drug conjuagate inotuzumab ozogamicin achieved significantly better results than the standard of care in the treatment of adults with acute lymphoblastic leukemia (ALL).
Patients in the inotuzumab arm experienced a higher rate of complete remissions, a greater frequency of achieving minimal residual disease negativity, and longer progression-free survival and overall survival.
However, veno-occlusive liver disease occurred more frequently in the inotuzumab-treated patients.
Inotuzumab ozogamicin, an anti-CD22 antibody conjugated to calicheamicin, received breakthrough designation for ALL from the US Food and Drug Administration last October.
For this phase 3 trial, called INO-VATE, investigators randomized 326 patients to receive inotuzumab or the investigator’s choice of standard therapy. The first 218 patients, 109 in each arm, were included in the intent-to-treat analysis of complete remission.
Hagop M. Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, presented the findings at the European Hematology Association meeting as abstract LB2233*. The study was simultaneously published in NEJM. Data cited here are based on the published paper.
Patients had to be 18 years of age or older and had to have relapsed or refractory disease with 5% or more blasts in the bone marrow. They had to be CD22-positive and could be either Philadelphia chromosome positive or negative. Patients had to be scheduled for their first or second salvage therapy.
No cross-over between the groups was allowed.
The primary endpoints were complete remission including complete remission with incomplete hematologic recovery, and overall survival.
Treatments
Patients in the inotuzumab arm received the drug intravenously at a starting dose of 1.8 mg/m2 per cycle for up to 6 cycles. Once a patient achieved complete remission or remission with incomplete hematologic recovery, the dose per cycle was reduced to 1.5 mg/m2.
Patients in the standard therapy arm could receive one of three regimens: FLAG (fludarabine, cytarabine, and granulocyte colony-stimulating factor), cytarabine plus mitoxantrone, or high-dose cytarabine. These regimens were chosen because they are commonly used for the treatment of relapsed or refractory ALL.
Patient characteristics
Patients in both arms were a median age of 47, range 18 – 79. And a little more than a third in each arm were 55 or older. Most patients were white, and about half had an ECOG performance status of 1.
Almost three quarters of the patients in each arm had bone marrow blasts of 50% or more.
Results
Patients in the inotuzumab arm received a median of 3 cycles of therapy and those in the standard therapy arm received a median of 1 cycle.
More patients in the inotuzumab arm received treatment for 2 or more cycles (73%) compared to the standard therapy arm (22%), a finding the investigators said was expected.
Dose reductions were more common in the inotuzumab arm (12%) compared with the standard therapy arm (3%).
More inotuzumab-treated patients discontinued therapy due to achieving complete remission (35%) than in the standard arm (15%).
And fewer patients in the inotuzumab arm (10%) discontinued treatment because of resistant disease than in the standard arm (40%).
Efficacy
The rate of complete remission, including incomplete hematologic recovery, was significantly higher in the inotuzumab group (80.7%) than in the standard group (29.4%), P<0.001.
In both groups, patients who achieved complete remission, including those with incomplete hematologic recovery, did so at the end of cycle 1.
"Standard chemotherapy regimens result in complete remission in 31 to 41 percent of patients who relapse earlier,” Dr Kantarjian noted, “and just 18 to 25 percent in those who relapse later."
"Patients in the inotuzumab ozogamicin study,” he continued, “had remission rates of 58% higher than previously reported, possibly due to patients being treated later in the disease course."
Among the complete responders, significantly more patients achieved minimal residual disease (MRD) negativity in the inotuzumab arm (78.4%) than in the standard therapy group (28.1%), P<0.001.
The median duration of remission was 4.6 months in the inotuzumab arm and 3.1 months in the standard therapy group, P=0.03.
And more patients treated with inotuzumab (41%) proceeded to stem cell transplant directly after treatment than in the standard therapy group (11%), P<0.001.
"Given that stem cell transplant is considered the only curative treatment option,” Dr Kantarjian said, “the ability of inotuzumab ozogamicin to increase the number of patients able to bridge to transplant is encouraging."
Survival
The intention-to-treat survival analysis included 164 patients in the inotuzumab arm and 162 in the standard therapy arm.
Progression-free survival (PFS) was significantly longer in the inotuzumab arm than in the standard therapy arm, a median of 5.0 months compared to 1.8 months, respectively, P<0.001.
The second primary objective of longer overall survival at the prespecified boundary of P=0.0208 was not met. Median overall survival was 7.7 months in the inotuzumab arm and 6.7 months in the standard therapy group, P=0.04.
Safety
In both treatment groups, the most common hematologic adverse events of any cause occurring during treatment were cytopenias.
Thrombocytopenia of grade 3 or higher was lower in the inotuzumab arm (37%) than in the standard therapy arm (59%).
Febrile neutropenia of grade 3 or higher occurred in 24% of inotuzumab-treated patients compared with 49% of patients in the standard therapy group.
In the inotuzumab group, the most common non-hematologic adverse events of any grade included nausea (32%), headache (28%), and pyrexia (27%). Grade 3 or higher nausea, headache, and pyrexia occurred in 2%, 1%, and 4%, respectively.
In the standard therapy arm, the most common non-hematologic events of any grade included nausea (47%), pyrexia (43%), and diarrhea (40%). Grade 3 or higher nausea, pyrexia, and diarrhea occurred in 0%, 5%, and 1%, respectively.
Febrile neutropenia was the most frequently reported serious adverse event, occurring in 12% of the inotuzumab-treated patients and 18% in the standard therapy group.
And liver-related adverse events were more common in the inotuzumab arm.
The most frequent liver-related adverse event of any grade was increased aspartate aminotransferase level, 20% in the inotuzumab group and 10% in the standard therapy group, hyperbilirubinemia, 15% and 10%, respectively, and increased alanine aminotransferase level, 14% and 11%, respectively.
Veno-occlusive liver disease (VOD) occurred more frequently with inotuzumab (11%, 15 patients) compared with standard therapy (1%, 1 patient). And cases were reported up to 2 years after randomization.
Five of the 15 patients developed VOD during or shortly after inotuzumab treatment. No cases of VOD occurred during the administration of standard therapy.
Seventeen deaths occurred during treatment in the inotuzumab arm and 11 in the standard therapy arm. Four deaths in the inotuzumab group and 2 in the standard therapy group were believed to be treatment-related.
The study was funded by Pfizer. ![]()
*Data in the abstract differ from those published in NEJM.

Photo courtesy of MDACC
In multiple categories, the antibody-drug conjuagate inotuzumab ozogamicin achieved significantly better results than the standard of care in the treatment of adults with acute lymphoblastic leukemia (ALL).
Patients in the inotuzumab arm experienced a higher rate of complete remissions, a greater frequency of achieving minimal residual disease negativity, and longer progression-free survival and overall survival.
However, veno-occlusive liver disease occurred more frequently in the inotuzumab-treated patients.
Inotuzumab ozogamicin, an anti-CD22 antibody conjugated to calicheamicin, received breakthrough designation for ALL from the US Food and Drug Administration last October.
For this phase 3 trial, called INO-VATE, investigators randomized 326 patients to receive inotuzumab or the investigator’s choice of standard therapy. The first 218 patients, 109 in each arm, were included in the intent-to-treat analysis of complete remission.
Hagop M. Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, presented the findings at the European Hematology Association meeting as abstract LB2233*. The study was simultaneously published in NEJM. Data cited here are based on the published paper.
Patients had to be 18 years of age or older and had to have relapsed or refractory disease with 5% or more blasts in the bone marrow. They had to be CD22-positive and could be either Philadelphia chromosome positive or negative. Patients had to be scheduled for their first or second salvage therapy.
No cross-over between the groups was allowed.
The primary endpoints were complete remission including complete remission with incomplete hematologic recovery, and overall survival.
Treatments
Patients in the inotuzumab arm received the drug intravenously at a starting dose of 1.8 mg/m2 per cycle for up to 6 cycles. Once a patient achieved complete remission or remission with incomplete hematologic recovery, the dose per cycle was reduced to 1.5 mg/m2.
Patients in the standard therapy arm could receive one of three regimens: FLAG (fludarabine, cytarabine, and granulocyte colony-stimulating factor), cytarabine plus mitoxantrone, or high-dose cytarabine. These regimens were chosen because they are commonly used for the treatment of relapsed or refractory ALL.
Patient characteristics
Patients in both arms were a median age of 47, range 18 – 79. And a little more than a third in each arm were 55 or older. Most patients were white, and about half had an ECOG performance status of 1.
Almost three quarters of the patients in each arm had bone marrow blasts of 50% or more.
Results
Patients in the inotuzumab arm received a median of 3 cycles of therapy and those in the standard therapy arm received a median of 1 cycle.
More patients in the inotuzumab arm received treatment for 2 or more cycles (73%) compared to the standard therapy arm (22%), a finding the investigators said was expected.
Dose reductions were more common in the inotuzumab arm (12%) compared with the standard therapy arm (3%).
More inotuzumab-treated patients discontinued therapy due to achieving complete remission (35%) than in the standard arm (15%).
And fewer patients in the inotuzumab arm (10%) discontinued treatment because of resistant disease than in the standard arm (40%).
Efficacy
The rate of complete remission, including incomplete hematologic recovery, was significantly higher in the inotuzumab group (80.7%) than in the standard group (29.4%), P<0.001.
In both groups, patients who achieved complete remission, including those with incomplete hematologic recovery, did so at the end of cycle 1.
"Standard chemotherapy regimens result in complete remission in 31 to 41 percent of patients who relapse earlier,” Dr Kantarjian noted, “and just 18 to 25 percent in those who relapse later."
"Patients in the inotuzumab ozogamicin study,” he continued, “had remission rates of 58% higher than previously reported, possibly due to patients being treated later in the disease course."
Among the complete responders, significantly more patients achieved minimal residual disease (MRD) negativity in the inotuzumab arm (78.4%) than in the standard therapy group (28.1%), P<0.001.
The median duration of remission was 4.6 months in the inotuzumab arm and 3.1 months in the standard therapy group, P=0.03.
And more patients treated with inotuzumab (41%) proceeded to stem cell transplant directly after treatment than in the standard therapy group (11%), P<0.001.
"Given that stem cell transplant is considered the only curative treatment option,” Dr Kantarjian said, “the ability of inotuzumab ozogamicin to increase the number of patients able to bridge to transplant is encouraging."
Survival
The intention-to-treat survival analysis included 164 patients in the inotuzumab arm and 162 in the standard therapy arm.
Progression-free survival (PFS) was significantly longer in the inotuzumab arm than in the standard therapy arm, a median of 5.0 months compared to 1.8 months, respectively, P<0.001.
The second primary objective of longer overall survival at the prespecified boundary of P=0.0208 was not met. Median overall survival was 7.7 months in the inotuzumab arm and 6.7 months in the standard therapy group, P=0.04.
Safety
In both treatment groups, the most common hematologic adverse events of any cause occurring during treatment were cytopenias.
Thrombocytopenia of grade 3 or higher was lower in the inotuzumab arm (37%) than in the standard therapy arm (59%).
Febrile neutropenia of grade 3 or higher occurred in 24% of inotuzumab-treated patients compared with 49% of patients in the standard therapy group.
In the inotuzumab group, the most common non-hematologic adverse events of any grade included nausea (32%), headache (28%), and pyrexia (27%). Grade 3 or higher nausea, headache, and pyrexia occurred in 2%, 1%, and 4%, respectively.
In the standard therapy arm, the most common non-hematologic events of any grade included nausea (47%), pyrexia (43%), and diarrhea (40%). Grade 3 or higher nausea, pyrexia, and diarrhea occurred in 0%, 5%, and 1%, respectively.
Febrile neutropenia was the most frequently reported serious adverse event, occurring in 12% of the inotuzumab-treated patients and 18% in the standard therapy group.
And liver-related adverse events were more common in the inotuzumab arm.
The most frequent liver-related adverse event of any grade was increased aspartate aminotransferase level, 20% in the inotuzumab group and 10% in the standard therapy group, hyperbilirubinemia, 15% and 10%, respectively, and increased alanine aminotransferase level, 14% and 11%, respectively.
Veno-occlusive liver disease (VOD) occurred more frequently with inotuzumab (11%, 15 patients) compared with standard therapy (1%, 1 patient). And cases were reported up to 2 years after randomization.
Five of the 15 patients developed VOD during or shortly after inotuzumab treatment. No cases of VOD occurred during the administration of standard therapy.
Seventeen deaths occurred during treatment in the inotuzumab arm and 11 in the standard therapy arm. Four deaths in the inotuzumab group and 2 in the standard therapy group were believed to be treatment-related.
The study was funded by Pfizer. ![]()
*Data in the abstract differ from those published in NEJM.

Photo courtesy of MDACC
In multiple categories, the antibody-drug conjuagate inotuzumab ozogamicin achieved significantly better results than the standard of care in the treatment of adults with acute lymphoblastic leukemia (ALL).
Patients in the inotuzumab arm experienced a higher rate of complete remissions, a greater frequency of achieving minimal residual disease negativity, and longer progression-free survival and overall survival.
However, veno-occlusive liver disease occurred more frequently in the inotuzumab-treated patients.
Inotuzumab ozogamicin, an anti-CD22 antibody conjugated to calicheamicin, received breakthrough designation for ALL from the US Food and Drug Administration last October.
For this phase 3 trial, called INO-VATE, investigators randomized 326 patients to receive inotuzumab or the investigator’s choice of standard therapy. The first 218 patients, 109 in each arm, were included in the intent-to-treat analysis of complete remission.
Hagop M. Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, presented the findings at the European Hematology Association meeting as abstract LB2233*. The study was simultaneously published in NEJM. Data cited here are based on the published paper.
Patients had to be 18 years of age or older and had to have relapsed or refractory disease with 5% or more blasts in the bone marrow. They had to be CD22-positive and could be either Philadelphia chromosome positive or negative. Patients had to be scheduled for their first or second salvage therapy.
No cross-over between the groups was allowed.
The primary endpoints were complete remission including complete remission with incomplete hematologic recovery, and overall survival.
Treatments
Patients in the inotuzumab arm received the drug intravenously at a starting dose of 1.8 mg/m2 per cycle for up to 6 cycles. Once a patient achieved complete remission or remission with incomplete hematologic recovery, the dose per cycle was reduced to 1.5 mg/m2.
Patients in the standard therapy arm could receive one of three regimens: FLAG (fludarabine, cytarabine, and granulocyte colony-stimulating factor), cytarabine plus mitoxantrone, or high-dose cytarabine. These regimens were chosen because they are commonly used for the treatment of relapsed or refractory ALL.
Patient characteristics
Patients in both arms were a median age of 47, range 18 – 79. And a little more than a third in each arm were 55 or older. Most patients were white, and about half had an ECOG performance status of 1.
Almost three quarters of the patients in each arm had bone marrow blasts of 50% or more.
Results
Patients in the inotuzumab arm received a median of 3 cycles of therapy and those in the standard therapy arm received a median of 1 cycle.
More patients in the inotuzumab arm received treatment for 2 or more cycles (73%) compared to the standard therapy arm (22%), a finding the investigators said was expected.
Dose reductions were more common in the inotuzumab arm (12%) compared with the standard therapy arm (3%).
More inotuzumab-treated patients discontinued therapy due to achieving complete remission (35%) than in the standard arm (15%).
And fewer patients in the inotuzumab arm (10%) discontinued treatment because of resistant disease than in the standard arm (40%).
Efficacy
The rate of complete remission, including incomplete hematologic recovery, was significantly higher in the inotuzumab group (80.7%) than in the standard group (29.4%), P<0.001.
In both groups, patients who achieved complete remission, including those with incomplete hematologic recovery, did so at the end of cycle 1.
"Standard chemotherapy regimens result in complete remission in 31 to 41 percent of patients who relapse earlier,” Dr Kantarjian noted, “and just 18 to 25 percent in those who relapse later."
"Patients in the inotuzumab ozogamicin study,” he continued, “had remission rates of 58% higher than previously reported, possibly due to patients being treated later in the disease course."
Among the complete responders, significantly more patients achieved minimal residual disease (MRD) negativity in the inotuzumab arm (78.4%) than in the standard therapy group (28.1%), P<0.001.
The median duration of remission was 4.6 months in the inotuzumab arm and 3.1 months in the standard therapy group, P=0.03.
And more patients treated with inotuzumab (41%) proceeded to stem cell transplant directly after treatment than in the standard therapy group (11%), P<0.001.
"Given that stem cell transplant is considered the only curative treatment option,” Dr Kantarjian said, “the ability of inotuzumab ozogamicin to increase the number of patients able to bridge to transplant is encouraging."
Survival
The intention-to-treat survival analysis included 164 patients in the inotuzumab arm and 162 in the standard therapy arm.
Progression-free survival (PFS) was significantly longer in the inotuzumab arm than in the standard therapy arm, a median of 5.0 months compared to 1.8 months, respectively, P<0.001.
The second primary objective of longer overall survival at the prespecified boundary of P=0.0208 was not met. Median overall survival was 7.7 months in the inotuzumab arm and 6.7 months in the standard therapy group, P=0.04.
Safety
In both treatment groups, the most common hematologic adverse events of any cause occurring during treatment were cytopenias.
Thrombocytopenia of grade 3 or higher was lower in the inotuzumab arm (37%) than in the standard therapy arm (59%).
Febrile neutropenia of grade 3 or higher occurred in 24% of inotuzumab-treated patients compared with 49% of patients in the standard therapy group.
In the inotuzumab group, the most common non-hematologic adverse events of any grade included nausea (32%), headache (28%), and pyrexia (27%). Grade 3 or higher nausea, headache, and pyrexia occurred in 2%, 1%, and 4%, respectively.
In the standard therapy arm, the most common non-hematologic events of any grade included nausea (47%), pyrexia (43%), and diarrhea (40%). Grade 3 or higher nausea, pyrexia, and diarrhea occurred in 0%, 5%, and 1%, respectively.
Febrile neutropenia was the most frequently reported serious adverse event, occurring in 12% of the inotuzumab-treated patients and 18% in the standard therapy group.
And liver-related adverse events were more common in the inotuzumab arm.
The most frequent liver-related adverse event of any grade was increased aspartate aminotransferase level, 20% in the inotuzumab group and 10% in the standard therapy group, hyperbilirubinemia, 15% and 10%, respectively, and increased alanine aminotransferase level, 14% and 11%, respectively.
Veno-occlusive liver disease (VOD) occurred more frequently with inotuzumab (11%, 15 patients) compared with standard therapy (1%, 1 patient). And cases were reported up to 2 years after randomization.
Five of the 15 patients developed VOD during or shortly after inotuzumab treatment. No cases of VOD occurred during the administration of standard therapy.
Seventeen deaths occurred during treatment in the inotuzumab arm and 11 in the standard therapy arm. Four deaths in the inotuzumab group and 2 in the standard therapy group were believed to be treatment-related.
The study was funded by Pfizer. ![]()
*Data in the abstract differ from those published in NEJM.
Inotuzumab continues to wow against relapsed/refractory ALL
Copenhagen – The experimental antibody-drug conjugate inotuzumab ozogamicin was associated with a nearly threefold higher complete remission rate than standard intensive chemotherapy in patients with relapsed/refractory acute lymphoblastic leukemia, investigators reported.
Among 218 patients in a phase III clinical trial, rates of combined complete remissions (CR) and complete remission with incomplete recovery of counts (CRi) were 80.7% for patients randomly assigned to receive the anti-CD22 conjugated antibody inotuzumab ozogamicin, compared with 29.4% for patients assigned to receive a standard intensive chemotherapy regimen chosen by investigators (P less than .001).
In addition, among patients who had complete remissions in both groups, significantly more patients in the inotuzumab ozogamicin group had fewer than .01% marrow blasts, a level below the threshold for minimal residual disease (MRD) (78.4% vs. 28.1%, respectively, P less than .001), reported Dr. Hagop Kantarjian, professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
“In my opinion, inotuzumab is well tolerated with appropriate prevention measures, and my hope is that soon in the future, inotuzumab will be used not as a single agent, but in combination with chemotherapy or with other monoclonal antibodies targeting CD19,” he said at the annual congress of the European Hematology Association.
Inotuzumab ozogamicin is an anti-CD22 monoclonal antibody conjugated to calicheamicin, a cytotoxic antibiotic. CD22 is expressed on the surface of about 90% of B-cell ALL cells.
Dr. Daniel DeAngelo from the Dana-Farber Cancer Institute in Boston reported that in the same cohort of patients, the first 218 of 326 randomized in the INO-VATE trial, , the co-primary endpoint of CR/CRi by independent review was achieved by 80.7% of patients treated with inotuzumab and 33.3% treated with standard of care.The updated data presented at the meeting also are published online in the New England Journal of Medicine 2016 June doi:10.1056/NEJMoa1509277 .
The phase III trial randomized 326 patients with relapsed/refractory CD22-positive ALL due for salvage 1 or 2 therapy to inotuzumab or standard of care, consisting of either the FLAG regimen (fludarabine (Fludara)/cytarabine (Ara-C)/granulocyte colony-stimulating factor), Ara-C plus mitoxantrone (Novantrone), or high-dose Ara-C. The starting dose for inotuzumab was 1.8 mg/m2/cycle and was reduced to 1.5 mg/m2/cycle once a CR or CRi was achieved. Patients were stratified by duration of first remission, salvage 1 or 2, and age.
The first 218 randomized patients were included in the intention-to-treat CR/CRi analysis, which was modified after excluding 13 patients who refused to start treatment, all in the standard-of-care arm.
The patients’ median age was 47 years (ranging up to 79 years), two-thirds were in salvage 1, and more than half had a remission duration of less than 12 months, an adverse prognostic feature.
Dr. Kantarjian reported that in addition to the superior remission rates seen with inotuzumab ozogamicin, patients on the antibody-drug conjugate had significantly longer duration of remissions, at a median of 4.6 months vs. 3.1 months for patients on chemotherapy (P = .003).
An intention-to-treat survival analysis including all 326 patients randomized in the trial showed that progression-free survival was significantly longer in the inotuzumab ozogamicin group, at a median of 5.0 vs. 1.8 months (hazard ratio .45 P less than .001).
Overall survival was slightly better with inotuzumab, at a median of 7.7 months vs. 6.7 months, but this did not meet the study definition of significantly improved overall survival with a pre-specified boundary of P = .014 or lower.
In the safety population, which included 139 patients assigned to inotuzumab and 120 to chemotherapy who had received at least one dose of the assigned regimen before data cutoff, the most frequent grade 3 or higher non-hematologic adverse events with inotuzumab ozogamicin were liver-related. In all, 13% of patients assigned to inotuzumab in the safety population developed veno-occlusive liver disease of any grade, compared with 1% of those assigned to standard of care.
“This drug showed consistently high activity in several studies, so I think it’s very exciting, The real challenge will be how to proceed, how to incorporate it into clinical trials both in pediatrics and adults,” commented Dr. Shai Izraeli, head of the section of functional genomics and childhood leukemia and cancer research at Sheba Medical Center in Ramat Gan, Israel. Dr. Izraeli was co-moderator of the late-breaking abstract session where the data were presented, but was not involved in the study.
He said in an interview that the challenge for investigators will be to develop clinical trials that combine this agent with different immunotherapies from different companies, which would be a logistical nightmare, “but I think we need to do it.”
The study was funded by Pfizer. Dr. Kantarjian disclosed research support from the company. Dr. Izraeli reported having no relevant disclosures.
Copenhagen – The experimental antibody-drug conjugate inotuzumab ozogamicin was associated with a nearly threefold higher complete remission rate than standard intensive chemotherapy in patients with relapsed/refractory acute lymphoblastic leukemia, investigators reported.
Among 218 patients in a phase III clinical trial, rates of combined complete remissions (CR) and complete remission with incomplete recovery of counts (CRi) were 80.7% for patients randomly assigned to receive the anti-CD22 conjugated antibody inotuzumab ozogamicin, compared with 29.4% for patients assigned to receive a standard intensive chemotherapy regimen chosen by investigators (P less than .001).
In addition, among patients who had complete remissions in both groups, significantly more patients in the inotuzumab ozogamicin group had fewer than .01% marrow blasts, a level below the threshold for minimal residual disease (MRD) (78.4% vs. 28.1%, respectively, P less than .001), reported Dr. Hagop Kantarjian, professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
“In my opinion, inotuzumab is well tolerated with appropriate prevention measures, and my hope is that soon in the future, inotuzumab will be used not as a single agent, but in combination with chemotherapy or with other monoclonal antibodies targeting CD19,” he said at the annual congress of the European Hematology Association.
Inotuzumab ozogamicin is an anti-CD22 monoclonal antibody conjugated to calicheamicin, a cytotoxic antibiotic. CD22 is expressed on the surface of about 90% of B-cell ALL cells.
Dr. Daniel DeAngelo from the Dana-Farber Cancer Institute in Boston reported that in the same cohort of patients, the first 218 of 326 randomized in the INO-VATE trial, , the co-primary endpoint of CR/CRi by independent review was achieved by 80.7% of patients treated with inotuzumab and 33.3% treated with standard of care.The updated data presented at the meeting also are published online in the New England Journal of Medicine 2016 June doi:10.1056/NEJMoa1509277 .
The phase III trial randomized 326 patients with relapsed/refractory CD22-positive ALL due for salvage 1 or 2 therapy to inotuzumab or standard of care, consisting of either the FLAG regimen (fludarabine (Fludara)/cytarabine (Ara-C)/granulocyte colony-stimulating factor), Ara-C plus mitoxantrone (Novantrone), or high-dose Ara-C. The starting dose for inotuzumab was 1.8 mg/m2/cycle and was reduced to 1.5 mg/m2/cycle once a CR or CRi was achieved. Patients were stratified by duration of first remission, salvage 1 or 2, and age.
The first 218 randomized patients were included in the intention-to-treat CR/CRi analysis, which was modified after excluding 13 patients who refused to start treatment, all in the standard-of-care arm.
The patients’ median age was 47 years (ranging up to 79 years), two-thirds were in salvage 1, and more than half had a remission duration of less than 12 months, an adverse prognostic feature.
Dr. Kantarjian reported that in addition to the superior remission rates seen with inotuzumab ozogamicin, patients on the antibody-drug conjugate had significantly longer duration of remissions, at a median of 4.6 months vs. 3.1 months for patients on chemotherapy (P = .003).
An intention-to-treat survival analysis including all 326 patients randomized in the trial showed that progression-free survival was significantly longer in the inotuzumab ozogamicin group, at a median of 5.0 vs. 1.8 months (hazard ratio .45 P less than .001).
Overall survival was slightly better with inotuzumab, at a median of 7.7 months vs. 6.7 months, but this did not meet the study definition of significantly improved overall survival with a pre-specified boundary of P = .014 or lower.
In the safety population, which included 139 patients assigned to inotuzumab and 120 to chemotherapy who had received at least one dose of the assigned regimen before data cutoff, the most frequent grade 3 or higher non-hematologic adverse events with inotuzumab ozogamicin were liver-related. In all, 13% of patients assigned to inotuzumab in the safety population developed veno-occlusive liver disease of any grade, compared with 1% of those assigned to standard of care.
“This drug showed consistently high activity in several studies, so I think it’s very exciting, The real challenge will be how to proceed, how to incorporate it into clinical trials both in pediatrics and adults,” commented Dr. Shai Izraeli, head of the section of functional genomics and childhood leukemia and cancer research at Sheba Medical Center in Ramat Gan, Israel. Dr. Izraeli was co-moderator of the late-breaking abstract session where the data were presented, but was not involved in the study.
He said in an interview that the challenge for investigators will be to develop clinical trials that combine this agent with different immunotherapies from different companies, which would be a logistical nightmare, “but I think we need to do it.”
The study was funded by Pfizer. Dr. Kantarjian disclosed research support from the company. Dr. Izraeli reported having no relevant disclosures.
Copenhagen – The experimental antibody-drug conjugate inotuzumab ozogamicin was associated with a nearly threefold higher complete remission rate than standard intensive chemotherapy in patients with relapsed/refractory acute lymphoblastic leukemia, investigators reported.
Among 218 patients in a phase III clinical trial, rates of combined complete remissions (CR) and complete remission with incomplete recovery of counts (CRi) were 80.7% for patients randomly assigned to receive the anti-CD22 conjugated antibody inotuzumab ozogamicin, compared with 29.4% for patients assigned to receive a standard intensive chemotherapy regimen chosen by investigators (P less than .001).
In addition, among patients who had complete remissions in both groups, significantly more patients in the inotuzumab ozogamicin group had fewer than .01% marrow blasts, a level below the threshold for minimal residual disease (MRD) (78.4% vs. 28.1%, respectively, P less than .001), reported Dr. Hagop Kantarjian, professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
“In my opinion, inotuzumab is well tolerated with appropriate prevention measures, and my hope is that soon in the future, inotuzumab will be used not as a single agent, but in combination with chemotherapy or with other monoclonal antibodies targeting CD19,” he said at the annual congress of the European Hematology Association.
Inotuzumab ozogamicin is an anti-CD22 monoclonal antibody conjugated to calicheamicin, a cytotoxic antibiotic. CD22 is expressed on the surface of about 90% of B-cell ALL cells.
Dr. Daniel DeAngelo from the Dana-Farber Cancer Institute in Boston reported that in the same cohort of patients, the first 218 of 326 randomized in the INO-VATE trial, , the co-primary endpoint of CR/CRi by independent review was achieved by 80.7% of patients treated with inotuzumab and 33.3% treated with standard of care.The updated data presented at the meeting also are published online in the New England Journal of Medicine 2016 June doi:10.1056/NEJMoa1509277 .
The phase III trial randomized 326 patients with relapsed/refractory CD22-positive ALL due for salvage 1 or 2 therapy to inotuzumab or standard of care, consisting of either the FLAG regimen (fludarabine (Fludara)/cytarabine (Ara-C)/granulocyte colony-stimulating factor), Ara-C plus mitoxantrone (Novantrone), or high-dose Ara-C. The starting dose for inotuzumab was 1.8 mg/m2/cycle and was reduced to 1.5 mg/m2/cycle once a CR or CRi was achieved. Patients were stratified by duration of first remission, salvage 1 or 2, and age.
The first 218 randomized patients were included in the intention-to-treat CR/CRi analysis, which was modified after excluding 13 patients who refused to start treatment, all in the standard-of-care arm.
The patients’ median age was 47 years (ranging up to 79 years), two-thirds were in salvage 1, and more than half had a remission duration of less than 12 months, an adverse prognostic feature.
Dr. Kantarjian reported that in addition to the superior remission rates seen with inotuzumab ozogamicin, patients on the antibody-drug conjugate had significantly longer duration of remissions, at a median of 4.6 months vs. 3.1 months for patients on chemotherapy (P = .003).
An intention-to-treat survival analysis including all 326 patients randomized in the trial showed that progression-free survival was significantly longer in the inotuzumab ozogamicin group, at a median of 5.0 vs. 1.8 months (hazard ratio .45 P less than .001).
Overall survival was slightly better with inotuzumab, at a median of 7.7 months vs. 6.7 months, but this did not meet the study definition of significantly improved overall survival with a pre-specified boundary of P = .014 or lower.
In the safety population, which included 139 patients assigned to inotuzumab and 120 to chemotherapy who had received at least one dose of the assigned regimen before data cutoff, the most frequent grade 3 or higher non-hematologic adverse events with inotuzumab ozogamicin were liver-related. In all, 13% of patients assigned to inotuzumab in the safety population developed veno-occlusive liver disease of any grade, compared with 1% of those assigned to standard of care.
“This drug showed consistently high activity in several studies, so I think it’s very exciting, The real challenge will be how to proceed, how to incorporate it into clinical trials both in pediatrics and adults,” commented Dr. Shai Izraeli, head of the section of functional genomics and childhood leukemia and cancer research at Sheba Medical Center in Ramat Gan, Israel. Dr. Izraeli was co-moderator of the late-breaking abstract session where the data were presented, but was not involved in the study.
He said in an interview that the challenge for investigators will be to develop clinical trials that combine this agent with different immunotherapies from different companies, which would be a logistical nightmare, “but I think we need to do it.”
The study was funded by Pfizer. Dr. Kantarjian disclosed research support from the company. Dr. Izraeli reported having no relevant disclosures.
At THE EHA CONGRESS
Key clinical point:. The drug-antibody conjugate inotuzumab ozogamicin showed strong activity against advanced acute lymphoblastic leukemia.
Major finding: The complete remission rate with inotuzumab was 80.7% vs. 29.4% for standard intensive chemotherapy.
Data source: 216 of 326 patients in a randomized phase 3 clinical trial.
Disclosures: The study was funded by Pfizer. Dr. Kantarjian disclosed research support from the company. Dr. Izraeli reported having no relevant disclosures.
Inherited thrombocytopenia type is a risk factor for hematologic cancers
Copenhagen – Investigators in Italy have found that a recently identified form of inherited thrombocytopenia related to mutations in the gene ETV6 (ETV6-RT) appears to be largely asymptomatic, but puts patients at significantly increased risk for hematologic malignancies, particularly childhood B-cell acute lymphoblastic leukemia (ALL).
“When we are talking about ETV6-related thrombocytopenia patients, we must pay more attention to this hidden but very dangerous risk of developing blood cancer – so that we can define prognosis and plan a personalized follow-up – [rather] than to bleeding complications, which are rare and usually do not affect the quality of life,” said Dr. Federica Melazzini from the University of Pavia, Italy.
At a briefing at the annual congress of the European Hematology Association, Dr. Melazzini described a prospective study of the clinical and laboratory features of the newly identified disorder, with a focus on its association with hematologic cancers.
They enrolled 130 unrelated consecutive patients with inherited thrombocytopenias (IT) who did not have definitive diagnoses because they did exhibit criteria for any of the known forms of IT.
The investigators used whole exome sequencing or Sanger sequencing to look for mutations in ETV6, and when a mutation was identified, all available relatives of the affected participant (proband) also were evaluated. The authors also included data on five patients from two families known to have ETV6-RT from previous studies.
The participants were evaluated with complete blood counts, platelet sizing, flow cytometry studies of platelet membrane glycoproteins, as well as platelet aggregation, activation, adhesion and spreading. Other evaluations included differentiation of human megakaryocytes, morphological analysis of megakaryocytes, megakaryocytes flow cytometry, and evaluation of pro-platelet formation by megakaryocytes differentiated in vitro.
The investigators identified a total of 20 patients from 7 families bearing 5 different mutations in ETV6. Although these patients had only mild thrombocytopenias and a low bleeding tendency, 4 of the 20 had childhood ALL, supporting the association between ETV6 mutations and early leukemic transformation.
This translated into an incidence rate for hematologic malignancies of nearly 1 in 100 persons per year, compared with 1 in 10,000 per year in the general population, Dr. Melazzini said.
The investigators also noted that one patient developed Janus kinase 2 (JAK2)-positive polycythemia vera at age 37 years, “suggesting that this disease should be added to the list of malignancies to which the ETV6-RT predisposes.”
Dr. Melazzini said that clinicians should suspect ETV6-RT when a patient presents with an autosomal inherited thrombocytopenia without platelet macrocytosis, and should confirm the diagnosis with genetic analysis.
Patients also should be followed with whole blood cell counts and peripheral blood smear evaluations every 6-12 months, she recommended.
Dr Anton Hagenbeek, professor of hematology at the University of Amsterdam, who moderated the briefing but was not involved in the study, asked how clinicians should communicate the increased risk of hematologic malignancies to the patients.
Dr. Melazzini replied that patients need counseling about the risks of developing ALL or of passing on the trait if they plan to have children. Additionally, if patients develop ALL, the family should be cautioned against using a related donor for any allogeneic stem cell transplants, she said.
The study funding source was not disclosed. Dr. Melazzini and Dr. Hagenbeek reported no relevant disclosures.
Copenhagen – Investigators in Italy have found that a recently identified form of inherited thrombocytopenia related to mutations in the gene ETV6 (ETV6-RT) appears to be largely asymptomatic, but puts patients at significantly increased risk for hematologic malignancies, particularly childhood B-cell acute lymphoblastic leukemia (ALL).
“When we are talking about ETV6-related thrombocytopenia patients, we must pay more attention to this hidden but very dangerous risk of developing blood cancer – so that we can define prognosis and plan a personalized follow-up – [rather] than to bleeding complications, which are rare and usually do not affect the quality of life,” said Dr. Federica Melazzini from the University of Pavia, Italy.
At a briefing at the annual congress of the European Hematology Association, Dr. Melazzini described a prospective study of the clinical and laboratory features of the newly identified disorder, with a focus on its association with hematologic cancers.
They enrolled 130 unrelated consecutive patients with inherited thrombocytopenias (IT) who did not have definitive diagnoses because they did exhibit criteria for any of the known forms of IT.
The investigators used whole exome sequencing or Sanger sequencing to look for mutations in ETV6, and when a mutation was identified, all available relatives of the affected participant (proband) also were evaluated. The authors also included data on five patients from two families known to have ETV6-RT from previous studies.
The participants were evaluated with complete blood counts, platelet sizing, flow cytometry studies of platelet membrane glycoproteins, as well as platelet aggregation, activation, adhesion and spreading. Other evaluations included differentiation of human megakaryocytes, morphological analysis of megakaryocytes, megakaryocytes flow cytometry, and evaluation of pro-platelet formation by megakaryocytes differentiated in vitro.
The investigators identified a total of 20 patients from 7 families bearing 5 different mutations in ETV6. Although these patients had only mild thrombocytopenias and a low bleeding tendency, 4 of the 20 had childhood ALL, supporting the association between ETV6 mutations and early leukemic transformation.
This translated into an incidence rate for hematologic malignancies of nearly 1 in 100 persons per year, compared with 1 in 10,000 per year in the general population, Dr. Melazzini said.
The investigators also noted that one patient developed Janus kinase 2 (JAK2)-positive polycythemia vera at age 37 years, “suggesting that this disease should be added to the list of malignancies to which the ETV6-RT predisposes.”
Dr. Melazzini said that clinicians should suspect ETV6-RT when a patient presents with an autosomal inherited thrombocytopenia without platelet macrocytosis, and should confirm the diagnosis with genetic analysis.
Patients also should be followed with whole blood cell counts and peripheral blood smear evaluations every 6-12 months, she recommended.
Dr Anton Hagenbeek, professor of hematology at the University of Amsterdam, who moderated the briefing but was not involved in the study, asked how clinicians should communicate the increased risk of hematologic malignancies to the patients.
Dr. Melazzini replied that patients need counseling about the risks of developing ALL or of passing on the trait if they plan to have children. Additionally, if patients develop ALL, the family should be cautioned against using a related donor for any allogeneic stem cell transplants, she said.
The study funding source was not disclosed. Dr. Melazzini and Dr. Hagenbeek reported no relevant disclosures.
Copenhagen – Investigators in Italy have found that a recently identified form of inherited thrombocytopenia related to mutations in the gene ETV6 (ETV6-RT) appears to be largely asymptomatic, but puts patients at significantly increased risk for hematologic malignancies, particularly childhood B-cell acute lymphoblastic leukemia (ALL).
“When we are talking about ETV6-related thrombocytopenia patients, we must pay more attention to this hidden but very dangerous risk of developing blood cancer – so that we can define prognosis and plan a personalized follow-up – [rather] than to bleeding complications, which are rare and usually do not affect the quality of life,” said Dr. Federica Melazzini from the University of Pavia, Italy.
At a briefing at the annual congress of the European Hematology Association, Dr. Melazzini described a prospective study of the clinical and laboratory features of the newly identified disorder, with a focus on its association with hematologic cancers.
They enrolled 130 unrelated consecutive patients with inherited thrombocytopenias (IT) who did not have definitive diagnoses because they did exhibit criteria for any of the known forms of IT.
The investigators used whole exome sequencing or Sanger sequencing to look for mutations in ETV6, and when a mutation was identified, all available relatives of the affected participant (proband) also were evaluated. The authors also included data on five patients from two families known to have ETV6-RT from previous studies.
The participants were evaluated with complete blood counts, platelet sizing, flow cytometry studies of platelet membrane glycoproteins, as well as platelet aggregation, activation, adhesion and spreading. Other evaluations included differentiation of human megakaryocytes, morphological analysis of megakaryocytes, megakaryocytes flow cytometry, and evaluation of pro-platelet formation by megakaryocytes differentiated in vitro.
The investigators identified a total of 20 patients from 7 families bearing 5 different mutations in ETV6. Although these patients had only mild thrombocytopenias and a low bleeding tendency, 4 of the 20 had childhood ALL, supporting the association between ETV6 mutations and early leukemic transformation.
This translated into an incidence rate for hematologic malignancies of nearly 1 in 100 persons per year, compared with 1 in 10,000 per year in the general population, Dr. Melazzini said.
The investigators also noted that one patient developed Janus kinase 2 (JAK2)-positive polycythemia vera at age 37 years, “suggesting that this disease should be added to the list of malignancies to which the ETV6-RT predisposes.”
Dr. Melazzini said that clinicians should suspect ETV6-RT when a patient presents with an autosomal inherited thrombocytopenia without platelet macrocytosis, and should confirm the diagnosis with genetic analysis.
Patients also should be followed with whole blood cell counts and peripheral blood smear evaluations every 6-12 months, she recommended.
Dr Anton Hagenbeek, professor of hematology at the University of Amsterdam, who moderated the briefing but was not involved in the study, asked how clinicians should communicate the increased risk of hematologic malignancies to the patients.
Dr. Melazzini replied that patients need counseling about the risks of developing ALL or of passing on the trait if they plan to have children. Additionally, if patients develop ALL, the family should be cautioned against using a related donor for any allogeneic stem cell transplants, she said.
The study funding source was not disclosed. Dr. Melazzini and Dr. Hagenbeek reported no relevant disclosures.
At THE EHA CONGRESS
Key clinical point: ETV6-related thrombocytopenia (EV6-RT) appears to predispose carriers to acute lymphoblastic leukemia and other hematologic cancers.
Major finding: Four of 20 patients with ETV6-RT had childhood ALL, and one developed JAK2-positive polycythemia vera at age 37.
Data source: Study of clinical and laboratory findings in 20 patients with EV6-RT.
Disclosures: The study funding source was not disclosed. Dr. Melazzini and Dr. Hagenbeek reported no relevant disclosures.
Team discovers 2 new subtypes of pediatric BCP ALL

Image courtesy Spencer Phillips
Using next-generation sequencing (NGS) technology, a team of researchers has discovered 2 new subtypes of pediatric B-cell precursor acute lymphoblastic leukemia (BCP ALL).
The 2 new subtypes, called DUX4-rearranged and ETV6/RUNX1-like, may improve risk stratification and targeted therapeutic options for treatment of the disease, the researchers say.
Previous studies have defined 6 major groups of ALL in children. The 2 new types can now be added to these groups.
The research team performed RNA sequencing in a population-based series of 195 pediatric BCP ALL cases, all under 18 years.
They found gene fusions present in 65% of BCP ALL cases. They also identified several new fusions along with the 2 novel subtypes.
The DUX4-rearranged subtype occurs “when a gene called DUX4, which is normally inactive in blood cells, becomes activated when the gene is relocated in the genome,” corresponding author Henrik Lilljebjörn, of Lund University in Sweden, said.
The DUX4-rearranged subtype was represented in 4% of the cases. It led to overexpression of DUX4 and frequently occurred together with intragenic ERG deletions.
The team observed a borderline significance (P=0.051) for age in cases with DUX4 rearrangements. Those with DUX4 rearrangements tended to be from older patients compared to cases lacking the fusion, with a median age of 6.5 year versus 4 years, respectively. The authors noted that this association needs to be confirmed in larger cohorts.
The ETV6/RUNX1-like subtype “resembles a previously known type of childhood leukemia,”Lilljebjörn said, “but is caused by other genetic mutations."
The ETV6/RUNX1-like subtype was represented in 3% of the BCP ALL cases.
According to the team, if all known subtypes are taken into consideration, “98% of the BCP ALL cases could be classified into distinct genetic subtypes with a known underlying driver mutation, or, less commonly, with a rare in-frame gene fusion,” they wrote.
“Finding the critical mutations in the diseased cells,” principal investigator Thoas Fioretos, MD, PhD, of Lund University, explained, “is an important condition for understanding the mechanisms of the disease and ultimately discovering new therapies.”
The investigators published their work in Nature Communications.
The research was funded mainly by The Swedish Childhood Cancer Foundation, The Swedish Cancer Society, The Swedish Research Council, the Faculty of Medicine at Lund University and Region Skåne. ![]()

Image courtesy Spencer Phillips
Using next-generation sequencing (NGS) technology, a team of researchers has discovered 2 new subtypes of pediatric B-cell precursor acute lymphoblastic leukemia (BCP ALL).
The 2 new subtypes, called DUX4-rearranged and ETV6/RUNX1-like, may improve risk stratification and targeted therapeutic options for treatment of the disease, the researchers say.
Previous studies have defined 6 major groups of ALL in children. The 2 new types can now be added to these groups.
The research team performed RNA sequencing in a population-based series of 195 pediatric BCP ALL cases, all under 18 years.
They found gene fusions present in 65% of BCP ALL cases. They also identified several new fusions along with the 2 novel subtypes.
The DUX4-rearranged subtype occurs “when a gene called DUX4, which is normally inactive in blood cells, becomes activated when the gene is relocated in the genome,” corresponding author Henrik Lilljebjörn, of Lund University in Sweden, said.
The DUX4-rearranged subtype was represented in 4% of the cases. It led to overexpression of DUX4 and frequently occurred together with intragenic ERG deletions.
The team observed a borderline significance (P=0.051) for age in cases with DUX4 rearrangements. Those with DUX4 rearrangements tended to be from older patients compared to cases lacking the fusion, with a median age of 6.5 year versus 4 years, respectively. The authors noted that this association needs to be confirmed in larger cohorts.
The ETV6/RUNX1-like subtype “resembles a previously known type of childhood leukemia,”Lilljebjörn said, “but is caused by other genetic mutations."
The ETV6/RUNX1-like subtype was represented in 3% of the BCP ALL cases.
According to the team, if all known subtypes are taken into consideration, “98% of the BCP ALL cases could be classified into distinct genetic subtypes with a known underlying driver mutation, or, less commonly, with a rare in-frame gene fusion,” they wrote.
“Finding the critical mutations in the diseased cells,” principal investigator Thoas Fioretos, MD, PhD, of Lund University, explained, “is an important condition for understanding the mechanisms of the disease and ultimately discovering new therapies.”
The investigators published their work in Nature Communications.
The research was funded mainly by The Swedish Childhood Cancer Foundation, The Swedish Cancer Society, The Swedish Research Council, the Faculty of Medicine at Lund University and Region Skåne. ![]()

Image courtesy Spencer Phillips
Using next-generation sequencing (NGS) technology, a team of researchers has discovered 2 new subtypes of pediatric B-cell precursor acute lymphoblastic leukemia (BCP ALL).
The 2 new subtypes, called DUX4-rearranged and ETV6/RUNX1-like, may improve risk stratification and targeted therapeutic options for treatment of the disease, the researchers say.
Previous studies have defined 6 major groups of ALL in children. The 2 new types can now be added to these groups.
The research team performed RNA sequencing in a population-based series of 195 pediatric BCP ALL cases, all under 18 years.
They found gene fusions present in 65% of BCP ALL cases. They also identified several new fusions along with the 2 novel subtypes.
The DUX4-rearranged subtype occurs “when a gene called DUX4, which is normally inactive in blood cells, becomes activated when the gene is relocated in the genome,” corresponding author Henrik Lilljebjörn, of Lund University in Sweden, said.
The DUX4-rearranged subtype was represented in 4% of the cases. It led to overexpression of DUX4 and frequently occurred together with intragenic ERG deletions.
The team observed a borderline significance (P=0.051) for age in cases with DUX4 rearrangements. Those with DUX4 rearrangements tended to be from older patients compared to cases lacking the fusion, with a median age of 6.5 year versus 4 years, respectively. The authors noted that this association needs to be confirmed in larger cohorts.
The ETV6/RUNX1-like subtype “resembles a previously known type of childhood leukemia,”Lilljebjörn said, “but is caused by other genetic mutations."
The ETV6/RUNX1-like subtype was represented in 3% of the BCP ALL cases.
According to the team, if all known subtypes are taken into consideration, “98% of the BCP ALL cases could be classified into distinct genetic subtypes with a known underlying driver mutation, or, less commonly, with a rare in-frame gene fusion,” they wrote.
“Finding the critical mutations in the diseased cells,” principal investigator Thoas Fioretos, MD, PhD, of Lund University, explained, “is an important condition for understanding the mechanisms of the disease and ultimately discovering new therapies.”
The investigators published their work in Nature Communications.
The research was funded mainly by The Swedish Childhood Cancer Foundation, The Swedish Cancer Society, The Swedish Research Council, the Faculty of Medicine at Lund University and Region Skåne. ![]()





