User login
Drug shows promise for treating resistant AML, MCL

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()
Nanoparticles deliver Aurora kinase inhibitor with increased safety and efficacy
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
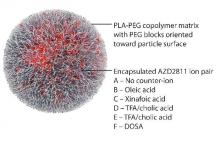
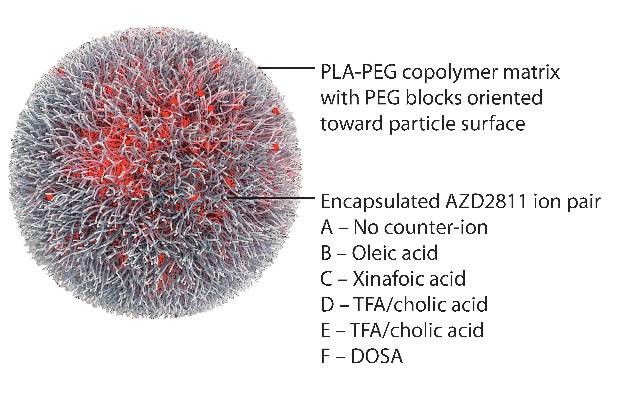
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Key clinical point: Aurora B kinase inhibitor nanoparticles displayed accumulation and retention in tumors with improved efficacy and minimal bone marrow pathology in animal models.
Major finding: Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity; the free drug was undetected in tumors 24 hours after administration, and nanoparticle-delivered drug was detectable up to 6 days.
Data sources: Nude rats and nude mice bearing human colorectal adenocarcinoma SW620 xenografts.
Disclosures: AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Olfactory receptor could be target for AML therapy

Investigators have discovered that an olfactory receptor in white blood cells responds to Sandalore, a synthetic odorant with a sandalwood note.
The team identified 7 olfactory receptors in a chronic myeloid leukemia (CML) cell line that were also present in white blood cells from patients with acute myeloid leukemia (AML).
One of the highest expressed receptors, OR2AT4, responded to Sandalore by fighting off the leukemia.
The investigators believe this finding could aid the development of new treatment for AML.
Hanns Hatt, PhD, DrMed, of the Ruhr-Universität Bochum in Germany, and his colleagues described this work in Cell Death Discovery.
The team found 7 olfactory receptors in the CML cell line K562—OR51B4, OR51B5, OR52D1, OR2W3, OR2B6, OR2AT4, and OR51I2. These receptors were also expressed in samples from AML patients.
The investigators then found that OR2AT4, one of the highest expressed olfactory receptors, is activated by Sandalore.
If Sandalore was used to activate the receptor, it inhibited leukemia cell proliferation and induced apoptosis in the leukemia cells. It also induced erythroid differentiation.
In 2014, Dr Hatt and his colleagues discovered that OR2AT4 is present in skin cells and that, by activating it with sandalwood aroma, wound healing is promoted. Through a series of tests, the team identified the signaling pathways underlying the observed effects.
With the current study, the investigators found that if Sandalore activates OR2AT4 in the context of CML or AML, processes similar to those in the olfactory cells in the nose start in blood cells.
The concentration of calcium ions in the cells increases. This, in turn, activates signaling pathways in which phosphate groups are transmitted to MAP kinases.
“This could be a new starting point for the development of leukemia treatment,” Dr Hatt said. “Acute myeloid leukemia, in particular, is a disease for which specific medication is not, as yet, available.” ![]()

Investigators have discovered that an olfactory receptor in white blood cells responds to Sandalore, a synthetic odorant with a sandalwood note.
The team identified 7 olfactory receptors in a chronic myeloid leukemia (CML) cell line that were also present in white blood cells from patients with acute myeloid leukemia (AML).
One of the highest expressed receptors, OR2AT4, responded to Sandalore by fighting off the leukemia.
The investigators believe this finding could aid the development of new treatment for AML.
Hanns Hatt, PhD, DrMed, of the Ruhr-Universität Bochum in Germany, and his colleagues described this work in Cell Death Discovery.
The team found 7 olfactory receptors in the CML cell line K562—OR51B4, OR51B5, OR52D1, OR2W3, OR2B6, OR2AT4, and OR51I2. These receptors were also expressed in samples from AML patients.
The investigators then found that OR2AT4, one of the highest expressed olfactory receptors, is activated by Sandalore.
If Sandalore was used to activate the receptor, it inhibited leukemia cell proliferation and induced apoptosis in the leukemia cells. It also induced erythroid differentiation.
In 2014, Dr Hatt and his colleagues discovered that OR2AT4 is present in skin cells and that, by activating it with sandalwood aroma, wound healing is promoted. Through a series of tests, the team identified the signaling pathways underlying the observed effects.
With the current study, the investigators found that if Sandalore activates OR2AT4 in the context of CML or AML, processes similar to those in the olfactory cells in the nose start in blood cells.
The concentration of calcium ions in the cells increases. This, in turn, activates signaling pathways in which phosphate groups are transmitted to MAP kinases.
“This could be a new starting point for the development of leukemia treatment,” Dr Hatt said. “Acute myeloid leukemia, in particular, is a disease for which specific medication is not, as yet, available.” ![]()

Investigators have discovered that an olfactory receptor in white blood cells responds to Sandalore, a synthetic odorant with a sandalwood note.
The team identified 7 olfactory receptors in a chronic myeloid leukemia (CML) cell line that were also present in white blood cells from patients with acute myeloid leukemia (AML).
One of the highest expressed receptors, OR2AT4, responded to Sandalore by fighting off the leukemia.
The investigators believe this finding could aid the development of new treatment for AML.
Hanns Hatt, PhD, DrMed, of the Ruhr-Universität Bochum in Germany, and his colleagues described this work in Cell Death Discovery.
The team found 7 olfactory receptors in the CML cell line K562—OR51B4, OR51B5, OR52D1, OR2W3, OR2B6, OR2AT4, and OR51I2. These receptors were also expressed in samples from AML patients.
The investigators then found that OR2AT4, one of the highest expressed olfactory receptors, is activated by Sandalore.
If Sandalore was used to activate the receptor, it inhibited leukemia cell proliferation and induced apoptosis in the leukemia cells. It also induced erythroid differentiation.
In 2014, Dr Hatt and his colleagues discovered that OR2AT4 is present in skin cells and that, by activating it with sandalwood aroma, wound healing is promoted. Through a series of tests, the team identified the signaling pathways underlying the observed effects.
With the current study, the investigators found that if Sandalore activates OR2AT4 in the context of CML or AML, processes similar to those in the olfactory cells in the nose start in blood cells.
The concentration of calcium ions in the cells increases. This, in turn, activates signaling pathways in which phosphate groups are transmitted to MAP kinases.
“This could be a new starting point for the development of leukemia treatment,” Dr Hatt said. “Acute myeloid leukemia, in particular, is a disease for which specific medication is not, as yet, available.” ![]()
Drug nets 3rd breakthrough designation from FDA

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted a third breakthrough therapy designation for the BCL-2 inhibitor venetoclax (ABT-199).
This time, the designation is for venetoclax in combination with hypomethylating agents to treat patients with treatment-naïve acute myeloid leukemia (AML) who are ineligible for standard induction therapy.
Venetoclax previously received breakthrough designation as a single agent for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and in combination with rituximab to treat patients with relapsed or refractory CLL and 17p deletion.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
Venetoclax is currently under investigation in a phase 1/2 trial in combination with low-dose cytarabine for treatment-naïve patients with AML and in a phase 1b study in combination with decitabine or azacitidine for treatment-naïve AML patients.
A phase 2 study of single-agent venetoclax in AML has been completed. The results were presented at ASH 2014.
At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 patients achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3 and 4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche. ![]()

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted a third breakthrough therapy designation for the BCL-2 inhibitor venetoclax (ABT-199).
This time, the designation is for venetoclax in combination with hypomethylating agents to treat patients with treatment-naïve acute myeloid leukemia (AML) who are ineligible for standard induction therapy.
Venetoclax previously received breakthrough designation as a single agent for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and in combination with rituximab to treat patients with relapsed or refractory CLL and 17p deletion.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
Venetoclax is currently under investigation in a phase 1/2 trial in combination with low-dose cytarabine for treatment-naïve patients with AML and in a phase 1b study in combination with decitabine or azacitidine for treatment-naïve AML patients.
A phase 2 study of single-agent venetoclax in AML has been completed. The results were presented at ASH 2014.
At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 patients achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3 and 4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche. ![]()

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted a third breakthrough therapy designation for the BCL-2 inhibitor venetoclax (ABT-199).
This time, the designation is for venetoclax in combination with hypomethylating agents to treat patients with treatment-naïve acute myeloid leukemia (AML) who are ineligible for standard induction therapy.
Venetoclax previously received breakthrough designation as a single agent for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and in combination with rituximab to treat patients with relapsed or refractory CLL and 17p deletion.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
Venetoclax is currently under investigation in a phase 1/2 trial in combination with low-dose cytarabine for treatment-naïve patients with AML and in a phase 1b study in combination with decitabine or azacitidine for treatment-naïve AML patients.
A phase 2 study of single-agent venetoclax in AML has been completed. The results were presented at ASH 2014.
At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 patients achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3 and 4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche. ![]()
Gemtuzumab ozogamicin boosts overall survival in older AML patients
Older patients with newly diagnosed acute myeloid leukemia who were unsuitable for intensive chemotherapy had significantly longer overall survival with gemtuzumab ozogamicin, compared with best supportive care, according to phase III trial results published Jan. 25.
The phase III EORTC-GIMEMA AML-19 trial randomly assigned 118 patients to receive gemtuzumab ozogamicin and 119 to receive best supportive care, including hydroxyurea. The median age was 77 years. In total, 104 of 118 patients in the gemtuzumab ozogamicin arm received the full induction course, and the median number of gemtuzumab ozogamicin infusions was three (range: 1 to 10).
Median overall survival (OS) for patients who received gemtuzumab ozogamicin was 4.9 months, compared with 3.6 months for those who received best supportive care, including hydroxyurea (hazard ratio, 0.69; 95% confidence interval, 0.53-0.90; P = .005).
One-year survival rates were 24.3% (95% CI, 16.9 to 32.4) for gemtuzumab ozogamicin and 9.7% (5.1 to 15.9) for best supportive care (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.0060).
The low intensity gemtuzumab ozogamicin regimen was generally well tolerated, with comparable toxicity between arms. Pancytopenia was observed in nearly all patients during gemtuzumab ozogamicin induction, however.
“Of importance, liver toxicity, a hallmark of [gemtuzumab ozogamicin] safety profile, was not increased in [gemtuzumab ozogamicin] recipients. Furthermore, it appeared to be less frequent and severe than previously reported by our group in a first-line trial, in which a more intensive [gemtuzumab ozogamicin] regimen was used in elderly patients with AML unfit for intensive chemotherapy,” wrote Dr. Sergio Amadori of the Tor Vergata University, Rome, and his colleagues.
Gemtuzumab ozogamicin therapy resulted in an overall complete response (CR) rate of 27% (15.3% CR and 11.7% CRi [incomplete recovery of peripheral blood counts]). The overall clinical benefit rate (CR + CRi+ partial response + stable disease for 30 days) was 56.7%.
Gemtuzumab ozogamicin combines a human monoclonal antibody specific for CD33 on myeloid cells with the DNA intercalator calicheamicin.
Patient characteristics that influenced gemtuzumab ozogamicin treatment effect were CD33 expression status, sex, and cytogenic profile. In patients with more than 80% CD33-positive blasts, gemtuzumab ozogamicin resulted in greater improvements over best supportive care (HR, 0.49; 95% CI, 0.32 to 0.76).
In women, OS was significantly improved (HR, 0.53; 95% CI, 0.35 to 0.79), whereas in men the hazard ratio was near 1. Patients with favorable/intermediate cytogenetic risk profiles had significant gemtuzumab ozogamicin benefit (HR, 0.52; 95% CI, 0.34 to 0.77), and those with adverse risk profiles had no treatment difference between arms.
The research was supported by Wyeth (Pfizer) and by the European Organisation for Research and Treatment of Cancer . Dr. Amadori reported having no disclosures. Several of his coauthors reported ties to industry.
Older patients with newly diagnosed acute myeloid leukemia who were unsuitable for intensive chemotherapy had significantly longer overall survival with gemtuzumab ozogamicin, compared with best supportive care, according to phase III trial results published Jan. 25.
The phase III EORTC-GIMEMA AML-19 trial randomly assigned 118 patients to receive gemtuzumab ozogamicin and 119 to receive best supportive care, including hydroxyurea. The median age was 77 years. In total, 104 of 118 patients in the gemtuzumab ozogamicin arm received the full induction course, and the median number of gemtuzumab ozogamicin infusions was three (range: 1 to 10).
Median overall survival (OS) for patients who received gemtuzumab ozogamicin was 4.9 months, compared with 3.6 months for those who received best supportive care, including hydroxyurea (hazard ratio, 0.69; 95% confidence interval, 0.53-0.90; P = .005).
One-year survival rates were 24.3% (95% CI, 16.9 to 32.4) for gemtuzumab ozogamicin and 9.7% (5.1 to 15.9) for best supportive care (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.0060).
The low intensity gemtuzumab ozogamicin regimen was generally well tolerated, with comparable toxicity between arms. Pancytopenia was observed in nearly all patients during gemtuzumab ozogamicin induction, however.
“Of importance, liver toxicity, a hallmark of [gemtuzumab ozogamicin] safety profile, was not increased in [gemtuzumab ozogamicin] recipients. Furthermore, it appeared to be less frequent and severe than previously reported by our group in a first-line trial, in which a more intensive [gemtuzumab ozogamicin] regimen was used in elderly patients with AML unfit for intensive chemotherapy,” wrote Dr. Sergio Amadori of the Tor Vergata University, Rome, and his colleagues.
Gemtuzumab ozogamicin therapy resulted in an overall complete response (CR) rate of 27% (15.3% CR and 11.7% CRi [incomplete recovery of peripheral blood counts]). The overall clinical benefit rate (CR + CRi+ partial response + stable disease for 30 days) was 56.7%.
Gemtuzumab ozogamicin combines a human monoclonal antibody specific for CD33 on myeloid cells with the DNA intercalator calicheamicin.
Patient characteristics that influenced gemtuzumab ozogamicin treatment effect were CD33 expression status, sex, and cytogenic profile. In patients with more than 80% CD33-positive blasts, gemtuzumab ozogamicin resulted in greater improvements over best supportive care (HR, 0.49; 95% CI, 0.32 to 0.76).
In women, OS was significantly improved (HR, 0.53; 95% CI, 0.35 to 0.79), whereas in men the hazard ratio was near 1. Patients with favorable/intermediate cytogenetic risk profiles had significant gemtuzumab ozogamicin benefit (HR, 0.52; 95% CI, 0.34 to 0.77), and those with adverse risk profiles had no treatment difference between arms.
The research was supported by Wyeth (Pfizer) and by the European Organisation for Research and Treatment of Cancer . Dr. Amadori reported having no disclosures. Several of his coauthors reported ties to industry.
Older patients with newly diagnosed acute myeloid leukemia who were unsuitable for intensive chemotherapy had significantly longer overall survival with gemtuzumab ozogamicin, compared with best supportive care, according to phase III trial results published Jan. 25.
The phase III EORTC-GIMEMA AML-19 trial randomly assigned 118 patients to receive gemtuzumab ozogamicin and 119 to receive best supportive care, including hydroxyurea. The median age was 77 years. In total, 104 of 118 patients in the gemtuzumab ozogamicin arm received the full induction course, and the median number of gemtuzumab ozogamicin infusions was three (range: 1 to 10).
Median overall survival (OS) for patients who received gemtuzumab ozogamicin was 4.9 months, compared with 3.6 months for those who received best supportive care, including hydroxyurea (hazard ratio, 0.69; 95% confidence interval, 0.53-0.90; P = .005).
One-year survival rates were 24.3% (95% CI, 16.9 to 32.4) for gemtuzumab ozogamicin and 9.7% (5.1 to 15.9) for best supportive care (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.0060).
The low intensity gemtuzumab ozogamicin regimen was generally well tolerated, with comparable toxicity between arms. Pancytopenia was observed in nearly all patients during gemtuzumab ozogamicin induction, however.
“Of importance, liver toxicity, a hallmark of [gemtuzumab ozogamicin] safety profile, was not increased in [gemtuzumab ozogamicin] recipients. Furthermore, it appeared to be less frequent and severe than previously reported by our group in a first-line trial, in which a more intensive [gemtuzumab ozogamicin] regimen was used in elderly patients with AML unfit for intensive chemotherapy,” wrote Dr. Sergio Amadori of the Tor Vergata University, Rome, and his colleagues.
Gemtuzumab ozogamicin therapy resulted in an overall complete response (CR) rate of 27% (15.3% CR and 11.7% CRi [incomplete recovery of peripheral blood counts]). The overall clinical benefit rate (CR + CRi+ partial response + stable disease for 30 days) was 56.7%.
Gemtuzumab ozogamicin combines a human monoclonal antibody specific for CD33 on myeloid cells with the DNA intercalator calicheamicin.
Patient characteristics that influenced gemtuzumab ozogamicin treatment effect were CD33 expression status, sex, and cytogenic profile. In patients with more than 80% CD33-positive blasts, gemtuzumab ozogamicin resulted in greater improvements over best supportive care (HR, 0.49; 95% CI, 0.32 to 0.76).
In women, OS was significantly improved (HR, 0.53; 95% CI, 0.35 to 0.79), whereas in men the hazard ratio was near 1. Patients with favorable/intermediate cytogenetic risk profiles had significant gemtuzumab ozogamicin benefit (HR, 0.52; 95% CI, 0.34 to 0.77), and those with adverse risk profiles had no treatment difference between arms.
The research was supported by Wyeth (Pfizer) and by the European Organisation for Research and Treatment of Cancer . Dr. Amadori reported having no disclosures. Several of his coauthors reported ties to industry.
Key clinical point: First-line, low-dose gemtuzumab ozogamicin significantly improved overall survival, compared with best supportive care in patients aged 61 years or older with AML.
Major finding: Median overall survival for patients who received gemtuzumab ozogamicin was 4.9 months, compared with 3.6 months for best supportive care, including hydroxyurea (hazard ratio, 0.69; 95% confidence interval, 0.53-0.90; P = .005).
Data source: The phase III EORTC-GIMEMA AML-19 trial randomly assigned 118 patients to receive gemtuzumab ozogamicin and 119 to receive best supportive care.
Disclosures: Research was supported by Wyeth (Pfizer) and by the European Organisation for Research and Treatment of Cancer. Dr. Amadori reported having no disclosures. Several of his coauthors reported ties to industry.
Minimal residual disease a powerful prognostic factor in AML
The presence of minimal residual disease predicts relapse in patients with NMP1-mutated acute myeloid leukemia and is superior to currently used molecular genetic markers in determining whether these patients should be considered for stem cell transplantation, a new study has found.
At 3 years, patients with minimal residual disease (MRD) had a significantly greater risk of relapse than those with no MRD (82% vs. 30%; univariate hazard ratio, 4.80; P less than .001) and a lower rate of survival (24% vs. 75%; univariate HR, 4.38; P less than .001), Adam Ivey of King’s College London reported (N Engl J Med. 2016; doi:10.1056/NEJMoa1507471).
In an editorial that accompanied the study Dr. Michael J. Burke from the Children’s Hospital of Wisconsin in Milwaukee wrote, “Time will tell, but this moment may prove to be a pivotal one in the assessment of minimal residual disease to assign treatment in patients with AML” (N Engl J Med. 2016; doi:10.1056/NEJMe1515525).
In adult AML, assessment of MRD has taken a back seat to analyses of cytogenetic and molecular lesions in determining a patient’s risk and treatment strategy. Typically, allogeneic stem cell transplantation is used for patients with high-risk features such as chromosome 3, 5, or 7 abnormalities or the FLT3-internal tandem duplication (ITD) mutation, while chemotherapy alone is used for low-risk disease.
The role of transplantation is unclear, however, for cytogenetically standard-risk patients, which includes those with a mutation in the gene encoding nucleophosmin (NPM1).
To address this issue, the investigators used a reverse-transcriptase quantitative polymerase chain reaction assay to evaluate 2,569 bone marrow and peripheral-blood samples from 346 patients with NPM1 mutations who had completed two cycles of induction chemotherapy in the U.K. National Cancer Research Institute AML17 trial.
MRD, defined as persistence of NPM1-mutated transcripts in peripheral blood, was present in 15% of patients after the second chemotherapy cycle.
Patients with MRD were significantly more likely than those without MRD to have a high U.K. Medical Research Council clinical risk score and to carry the FLT3-ITD mutation.
On univariate analysis, the risk of relapse was significantly higher with the presence of MRD in peripheral blood, an increased white cell count, and with the DNMT3A and FLT3-ITD mutations.
Only the presence of MRD and an elevated white cell count significantly predicted survival, Mr. Ivey reported.
“We could find no specific molecular subgroup consisting of 10 patients or more that had a rate of survival less than 52%; in contrast, the rate in the group with the presence of minimal residual disease was 24%,” he observed.
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse (HR, 5.09; P less than .001) or death (HR, 4.84; P less than .001).
The results were validated in an independent cohort of 91 AML17 study patients. It confirmed that MRD in peripheral blood predicts worse outcome at 2 years than the absence of MRD, with a cumulative incidence of relapse of 70% vs. 31% (P = .001) and overall survival rates of 40% vs. 87% (P = .001), reported the investigators, including senior author Professor David Grimwade, also from King’s College London.
The clinical implications of these results “are substantive” because NPM-1 mutated AML is the most common subtype of AML and because of the uncertainty over the best treatment strategy for patients typically classified as standard risk, editorialist Dr. Burke observed.
“Now with the ability to reclassify standard-risk or low-risk patients as high-risk on the basis of the persistent expression of mutant NPM1 transcripts, it may be possible that stem-cell transplantation is a better approach in patients who otherwise would be treated with chemotherapy alone and that transplantation may be avoidable in high-risk patients who have no evidence of minimal residual disease,” he wrote. “Such predictions will need to be tested prospectively.”
The presence of MRD is also known to be an important independent prognostic factor in acute lymphoblastic leukemia, but since AML has a greater molecular heterogeneity, routine MRD assessment has not been as quickly adopted in AML, Dr. Burke noted.
The Children’s Oncology Group, however, recently adopted MRD assessment by flow cytometry to further stratify children with newly diagnosed AML after first induction therapy into low-risk or high-risk groups.
The study was supported by grants from Bloodwise and the National Institute for Health Research. Mr. Ivey and Dr. Burke reported having no disclosures.
The presence of minimal residual disease predicts relapse in patients with NMP1-mutated acute myeloid leukemia and is superior to currently used molecular genetic markers in determining whether these patients should be considered for stem cell transplantation, a new study has found.
At 3 years, patients with minimal residual disease (MRD) had a significantly greater risk of relapse than those with no MRD (82% vs. 30%; univariate hazard ratio, 4.80; P less than .001) and a lower rate of survival (24% vs. 75%; univariate HR, 4.38; P less than .001), Adam Ivey of King’s College London reported (N Engl J Med. 2016; doi:10.1056/NEJMoa1507471).
In an editorial that accompanied the study Dr. Michael J. Burke from the Children’s Hospital of Wisconsin in Milwaukee wrote, “Time will tell, but this moment may prove to be a pivotal one in the assessment of minimal residual disease to assign treatment in patients with AML” (N Engl J Med. 2016; doi:10.1056/NEJMe1515525).
In adult AML, assessment of MRD has taken a back seat to analyses of cytogenetic and molecular lesions in determining a patient’s risk and treatment strategy. Typically, allogeneic stem cell transplantation is used for patients with high-risk features such as chromosome 3, 5, or 7 abnormalities or the FLT3-internal tandem duplication (ITD) mutation, while chemotherapy alone is used for low-risk disease.
The role of transplantation is unclear, however, for cytogenetically standard-risk patients, which includes those with a mutation in the gene encoding nucleophosmin (NPM1).
To address this issue, the investigators used a reverse-transcriptase quantitative polymerase chain reaction assay to evaluate 2,569 bone marrow and peripheral-blood samples from 346 patients with NPM1 mutations who had completed two cycles of induction chemotherapy in the U.K. National Cancer Research Institute AML17 trial.
MRD, defined as persistence of NPM1-mutated transcripts in peripheral blood, was present in 15% of patients after the second chemotherapy cycle.
Patients with MRD were significantly more likely than those without MRD to have a high U.K. Medical Research Council clinical risk score and to carry the FLT3-ITD mutation.
On univariate analysis, the risk of relapse was significantly higher with the presence of MRD in peripheral blood, an increased white cell count, and with the DNMT3A and FLT3-ITD mutations.
Only the presence of MRD and an elevated white cell count significantly predicted survival, Mr. Ivey reported.
“We could find no specific molecular subgroup consisting of 10 patients or more that had a rate of survival less than 52%; in contrast, the rate in the group with the presence of minimal residual disease was 24%,” he observed.
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse (HR, 5.09; P less than .001) or death (HR, 4.84; P less than .001).
The results were validated in an independent cohort of 91 AML17 study patients. It confirmed that MRD in peripheral blood predicts worse outcome at 2 years than the absence of MRD, with a cumulative incidence of relapse of 70% vs. 31% (P = .001) and overall survival rates of 40% vs. 87% (P = .001), reported the investigators, including senior author Professor David Grimwade, also from King’s College London.
The clinical implications of these results “are substantive” because NPM-1 mutated AML is the most common subtype of AML and because of the uncertainty over the best treatment strategy for patients typically classified as standard risk, editorialist Dr. Burke observed.
“Now with the ability to reclassify standard-risk or low-risk patients as high-risk on the basis of the persistent expression of mutant NPM1 transcripts, it may be possible that stem-cell transplantation is a better approach in patients who otherwise would be treated with chemotherapy alone and that transplantation may be avoidable in high-risk patients who have no evidence of minimal residual disease,” he wrote. “Such predictions will need to be tested prospectively.”
The presence of MRD is also known to be an important independent prognostic factor in acute lymphoblastic leukemia, but since AML has a greater molecular heterogeneity, routine MRD assessment has not been as quickly adopted in AML, Dr. Burke noted.
The Children’s Oncology Group, however, recently adopted MRD assessment by flow cytometry to further stratify children with newly diagnosed AML after first induction therapy into low-risk or high-risk groups.
The study was supported by grants from Bloodwise and the National Institute for Health Research. Mr. Ivey and Dr. Burke reported having no disclosures.
The presence of minimal residual disease predicts relapse in patients with NMP1-mutated acute myeloid leukemia and is superior to currently used molecular genetic markers in determining whether these patients should be considered for stem cell transplantation, a new study has found.
At 3 years, patients with minimal residual disease (MRD) had a significantly greater risk of relapse than those with no MRD (82% vs. 30%; univariate hazard ratio, 4.80; P less than .001) and a lower rate of survival (24% vs. 75%; univariate HR, 4.38; P less than .001), Adam Ivey of King’s College London reported (N Engl J Med. 2016; doi:10.1056/NEJMoa1507471).
In an editorial that accompanied the study Dr. Michael J. Burke from the Children’s Hospital of Wisconsin in Milwaukee wrote, “Time will tell, but this moment may prove to be a pivotal one in the assessment of minimal residual disease to assign treatment in patients with AML” (N Engl J Med. 2016; doi:10.1056/NEJMe1515525).
In adult AML, assessment of MRD has taken a back seat to analyses of cytogenetic and molecular lesions in determining a patient’s risk and treatment strategy. Typically, allogeneic stem cell transplantation is used for patients with high-risk features such as chromosome 3, 5, or 7 abnormalities or the FLT3-internal tandem duplication (ITD) mutation, while chemotherapy alone is used for low-risk disease.
The role of transplantation is unclear, however, for cytogenetically standard-risk patients, which includes those with a mutation in the gene encoding nucleophosmin (NPM1).
To address this issue, the investigators used a reverse-transcriptase quantitative polymerase chain reaction assay to evaluate 2,569 bone marrow and peripheral-blood samples from 346 patients with NPM1 mutations who had completed two cycles of induction chemotherapy in the U.K. National Cancer Research Institute AML17 trial.
MRD, defined as persistence of NPM1-mutated transcripts in peripheral blood, was present in 15% of patients after the second chemotherapy cycle.
Patients with MRD were significantly more likely than those without MRD to have a high U.K. Medical Research Council clinical risk score and to carry the FLT3-ITD mutation.
On univariate analysis, the risk of relapse was significantly higher with the presence of MRD in peripheral blood, an increased white cell count, and with the DNMT3A and FLT3-ITD mutations.
Only the presence of MRD and an elevated white cell count significantly predicted survival, Mr. Ivey reported.
“We could find no specific molecular subgroup consisting of 10 patients or more that had a rate of survival less than 52%; in contrast, the rate in the group with the presence of minimal residual disease was 24%,” he observed.
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse (HR, 5.09; P less than .001) or death (HR, 4.84; P less than .001).
The results were validated in an independent cohort of 91 AML17 study patients. It confirmed that MRD in peripheral blood predicts worse outcome at 2 years than the absence of MRD, with a cumulative incidence of relapse of 70% vs. 31% (P = .001) and overall survival rates of 40% vs. 87% (P = .001), reported the investigators, including senior author Professor David Grimwade, also from King’s College London.
The clinical implications of these results “are substantive” because NPM-1 mutated AML is the most common subtype of AML and because of the uncertainty over the best treatment strategy for patients typically classified as standard risk, editorialist Dr. Burke observed.
“Now with the ability to reclassify standard-risk or low-risk patients as high-risk on the basis of the persistent expression of mutant NPM1 transcripts, it may be possible that stem-cell transplantation is a better approach in patients who otherwise would be treated with chemotherapy alone and that transplantation may be avoidable in high-risk patients who have no evidence of minimal residual disease,” he wrote. “Such predictions will need to be tested prospectively.”
The presence of MRD is also known to be an important independent prognostic factor in acute lymphoblastic leukemia, but since AML has a greater molecular heterogeneity, routine MRD assessment has not been as quickly adopted in AML, Dr. Burke noted.
The Children’s Oncology Group, however, recently adopted MRD assessment by flow cytometry to further stratify children with newly diagnosed AML after first induction therapy into low-risk or high-risk groups.
The study was supported by grants from Bloodwise and the National Institute for Health Research. Mr. Ivey and Dr. Burke reported having no disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The presence of minimal residual disease provides powerful prognostic information independent of other risk factors in patients with NPM1-mutated AML.
Major finding: Minimal residual disease was associated with a significantly greater risk of relapse than absence of MRD (82% vs. 30%; hazard ratio, 4.80; P less than .001) and a lower rate of survival (24% vs. 75%; HR, 4.38, P less than .001).
Data source: Analysis of 346 patients with NPM1-mutated AML.
Disclosures: The study was supported by grants from Bloodwise and the National Institute for Health Research. Mr. Ivey and Dr. Burke reported having no disclosures.
MRD test can predict AML relapse

Photo by William Weinert
A test measuring minimal residual disease (MRD) can help predict relapse in patients with acute myeloid leukemia (AML), according to a study published in NEJM.
Investigators used the test to detect MRD in samples from patients with NPM1-mutated AML who were deemed standard-risk by conventional methods.
The team said the presence of MRD after treatment provided powerful prognostic information independent of other risk factors.
“What we have been able to identify is a group of patients who otherwise would be thought to do quite well, who, in fact, have a very poor prognosis, and who are not well served currently,” said study author Robert Hills, DPhil, of Cardiff University School of Medicine in the UK.
“This opens up the exciting prospect that we can do the same for other groups of patients as well.”
For this study, Dr Hills and his colleagues used a reverse-transcriptase quantitative polymerase-chain-reaction assay to detect MRD in 2569 samples (902 bone marrow samples and 1667 peripheral blood samples) from 346 patients with NPM1-mutated AML.
The patients had received 2 cycles of chemotherapy as part of the National Cancer Research Institute AML17 trial. They were treated at centers in the UK, Denmark, and New Zealand. All patients were shown to be at standard risk of relapse using conventional tests.
The investigators found that NPM1-mutated transcripts persisted in the blood of 15% of patients after the second cycle of chemotherapy.
This finding was associated with a greater risk of relapse after 3 years of follow-up.
Eighty-two percent of patients with NPM1-mutated transcripts had relapsed within 3 years, compared to 30% of patients who had no detectable NPM1. The hazard ratio was 4.80 (P<0.001).
Patients with traces of NPM1 also had a lower rate of survival—24%, compared to 75% for patients without detectable NPM1. The hazard ratio was 4.38 (P<0.001).
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse or death. The hazard ratios were 5.09 and 4.84, respectively (P<0.001 for both).
The investigators validated these findings in a cohort of 91 patients with NPM1-mutated AML.
The presence of MRD in the blood of these patients was associated with a higher cumulative incidence of relapse—70% vs 31% (P=0.001)—and a lower rate of survival—40% vs 87% (P=0.001)—at 2 years.
The investigators also found that, with sequential monitoring of MRD, a rising level of NPM1-mutated transcripts could predict relapse.
“Conventional methods for guiding treatment for this aggressive type of leukemia are inadequate,” said study author David Grimwade, PhD, of King’s College London in the UK.
“The MRD test is an invaluable tool to assess treatment response and identify those patients for whom chemotherapy is not sufficient and require stem cell transplantation or new treatments.” ![]()

Photo by William Weinert
A test measuring minimal residual disease (MRD) can help predict relapse in patients with acute myeloid leukemia (AML), according to a study published in NEJM.
Investigators used the test to detect MRD in samples from patients with NPM1-mutated AML who were deemed standard-risk by conventional methods.
The team said the presence of MRD after treatment provided powerful prognostic information independent of other risk factors.
“What we have been able to identify is a group of patients who otherwise would be thought to do quite well, who, in fact, have a very poor prognosis, and who are not well served currently,” said study author Robert Hills, DPhil, of Cardiff University School of Medicine in the UK.
“This opens up the exciting prospect that we can do the same for other groups of patients as well.”
For this study, Dr Hills and his colleagues used a reverse-transcriptase quantitative polymerase-chain-reaction assay to detect MRD in 2569 samples (902 bone marrow samples and 1667 peripheral blood samples) from 346 patients with NPM1-mutated AML.
The patients had received 2 cycles of chemotherapy as part of the National Cancer Research Institute AML17 trial. They were treated at centers in the UK, Denmark, and New Zealand. All patients were shown to be at standard risk of relapse using conventional tests.
The investigators found that NPM1-mutated transcripts persisted in the blood of 15% of patients after the second cycle of chemotherapy.
This finding was associated with a greater risk of relapse after 3 years of follow-up.
Eighty-two percent of patients with NPM1-mutated transcripts had relapsed within 3 years, compared to 30% of patients who had no detectable NPM1. The hazard ratio was 4.80 (P<0.001).
Patients with traces of NPM1 also had a lower rate of survival—24%, compared to 75% for patients without detectable NPM1. The hazard ratio was 4.38 (P<0.001).
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse or death. The hazard ratios were 5.09 and 4.84, respectively (P<0.001 for both).
The investigators validated these findings in a cohort of 91 patients with NPM1-mutated AML.
The presence of MRD in the blood of these patients was associated with a higher cumulative incidence of relapse—70% vs 31% (P=0.001)—and a lower rate of survival—40% vs 87% (P=0.001)—at 2 years.
The investigators also found that, with sequential monitoring of MRD, a rising level of NPM1-mutated transcripts could predict relapse.
“Conventional methods for guiding treatment for this aggressive type of leukemia are inadequate,” said study author David Grimwade, PhD, of King’s College London in the UK.
“The MRD test is an invaluable tool to assess treatment response and identify those patients for whom chemotherapy is not sufficient and require stem cell transplantation or new treatments.” ![]()

Photo by William Weinert
A test measuring minimal residual disease (MRD) can help predict relapse in patients with acute myeloid leukemia (AML), according to a study published in NEJM.
Investigators used the test to detect MRD in samples from patients with NPM1-mutated AML who were deemed standard-risk by conventional methods.
The team said the presence of MRD after treatment provided powerful prognostic information independent of other risk factors.
“What we have been able to identify is a group of patients who otherwise would be thought to do quite well, who, in fact, have a very poor prognosis, and who are not well served currently,” said study author Robert Hills, DPhil, of Cardiff University School of Medicine in the UK.
“This opens up the exciting prospect that we can do the same for other groups of patients as well.”
For this study, Dr Hills and his colleagues used a reverse-transcriptase quantitative polymerase-chain-reaction assay to detect MRD in 2569 samples (902 bone marrow samples and 1667 peripheral blood samples) from 346 patients with NPM1-mutated AML.
The patients had received 2 cycles of chemotherapy as part of the National Cancer Research Institute AML17 trial. They were treated at centers in the UK, Denmark, and New Zealand. All patients were shown to be at standard risk of relapse using conventional tests.
The investigators found that NPM1-mutated transcripts persisted in the blood of 15% of patients after the second cycle of chemotherapy.
This finding was associated with a greater risk of relapse after 3 years of follow-up.
Eighty-two percent of patients with NPM1-mutated transcripts had relapsed within 3 years, compared to 30% of patients who had no detectable NPM1. The hazard ratio was 4.80 (P<0.001).
Patients with traces of NPM1 also had a lower rate of survival—24%, compared to 75% for patients without detectable NPM1. The hazard ratio was 4.38 (P<0.001).
In multivariate analysis, the presence of MRD was the only significant prognostic factor for relapse or death. The hazard ratios were 5.09 and 4.84, respectively (P<0.001 for both).
The investigators validated these findings in a cohort of 91 patients with NPM1-mutated AML.
The presence of MRD in the blood of these patients was associated with a higher cumulative incidence of relapse—70% vs 31% (P=0.001)—and a lower rate of survival—40% vs 87% (P=0.001)—at 2 years.
The investigators also found that, with sequential monitoring of MRD, a rising level of NPM1-mutated transcripts could predict relapse.
“Conventional methods for guiding treatment for this aggressive type of leukemia are inadequate,” said study author David Grimwade, PhD, of King’s College London in the UK.
“The MRD test is an invaluable tool to assess treatment response and identify those patients for whom chemotherapy is not sufficient and require stem cell transplantation or new treatments.” ![]()
Protein may be therapeutic target for AML

and Matt McCormack, PhD
Photo courtesy of the
Walter and Eliza Hall
Institute of Medical Research
Preclinical research suggests the Hhex protein could be a cancer-specific therapeutic target for acute myeloid leukemia (AML).
Investigators discovered that loss of the Hhex protein halted leukemia cell growth and division in vitro and in vivo, but normal cells were unaffected by the loss of Hhex.
Matt McCormack, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues relayed these findings in Genes and Development.
“There is an urgent need for new therapies to treat AML,” Dr McCormack said. “We showed blocking the Hhex protein could put the brakes on leukemia growth and completely eliminate AML in preclinical models. This could be targeted by new drugs to treat AML in humans.”
Specifically, the investigators found that Hhex was overexpressed in human AML, and the protein was essential for the maintenance of AML driven by the oncogenic fusion protein MLL-ENL and its downstream effectors, HoxA9 and Meis1.
However, Hhex was not required for normal myelopoiesis.
“Hhex is only essential for the leukemic cells, meaning we could target and treat leukemia without toxic effects on normal cells, avoiding many of the serious side effects that come with standard cancer treatments,” Dr McCormack said.
“We also know that most people with AML have increased levels of Hhex, often associated with adverse outcomes, further indicating it is an important target for new AML drugs.”
Dr McCormack and his colleagues also attempted to determine the mechanism by which Hhex promotes AML.
They found the protein represses the tumor suppressors p16INK4a and p19ARF in leukemic stem cells by regulating the Polycomb-repressive complex 2 (PRC2). They said that Hhex binds to the Cdkn2a locus and directly interacts with PRC2 to enable H3K27me3-mediated epigenetic repression.
“Hhex works by recruiting epigenetic factors to growth-control genes, effectively silencing them,” said author Ben Shields, PhD, also of the Walter and Eliza Hall Institute.
“This allows the leukemia cells to reproduce and accumulate more damage, contributing to the speed of AML progression.”
Dr McCormack said that although drugs inhibiting epigenetic modification have been tested against AML in the past, they have caused significant toxicity because their targets are also required for normal blood cell function.
“Unlike the epigenetic factors targeted previously, Hhex only regulates a small number of genes and is dispensable for normal blood cells,” Dr McCormack reiterated.
“This gives us a rare opportunity to kill AML cells without causing many side effects. We now hope to identify the critical regions of the Hhex protein that enable it to function, which will allow us to design much-needed new drugs to treat AML.” ![]()

and Matt McCormack, PhD
Photo courtesy of the
Walter and Eliza Hall
Institute of Medical Research
Preclinical research suggests the Hhex protein could be a cancer-specific therapeutic target for acute myeloid leukemia (AML).
Investigators discovered that loss of the Hhex protein halted leukemia cell growth and division in vitro and in vivo, but normal cells were unaffected by the loss of Hhex.
Matt McCormack, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues relayed these findings in Genes and Development.
“There is an urgent need for new therapies to treat AML,” Dr McCormack said. “We showed blocking the Hhex protein could put the brakes on leukemia growth and completely eliminate AML in preclinical models. This could be targeted by new drugs to treat AML in humans.”
Specifically, the investigators found that Hhex was overexpressed in human AML, and the protein was essential for the maintenance of AML driven by the oncogenic fusion protein MLL-ENL and its downstream effectors, HoxA9 and Meis1.
However, Hhex was not required for normal myelopoiesis.
“Hhex is only essential for the leukemic cells, meaning we could target and treat leukemia without toxic effects on normal cells, avoiding many of the serious side effects that come with standard cancer treatments,” Dr McCormack said.
“We also know that most people with AML have increased levels of Hhex, often associated with adverse outcomes, further indicating it is an important target for new AML drugs.”
Dr McCormack and his colleagues also attempted to determine the mechanism by which Hhex promotes AML.
They found the protein represses the tumor suppressors p16INK4a and p19ARF in leukemic stem cells by regulating the Polycomb-repressive complex 2 (PRC2). They said that Hhex binds to the Cdkn2a locus and directly interacts with PRC2 to enable H3K27me3-mediated epigenetic repression.
“Hhex works by recruiting epigenetic factors to growth-control genes, effectively silencing them,” said author Ben Shields, PhD, also of the Walter and Eliza Hall Institute.
“This allows the leukemia cells to reproduce and accumulate more damage, contributing to the speed of AML progression.”
Dr McCormack said that although drugs inhibiting epigenetic modification have been tested against AML in the past, they have caused significant toxicity because their targets are also required for normal blood cell function.
“Unlike the epigenetic factors targeted previously, Hhex only regulates a small number of genes and is dispensable for normal blood cells,” Dr McCormack reiterated.
“This gives us a rare opportunity to kill AML cells without causing many side effects. We now hope to identify the critical regions of the Hhex protein that enable it to function, which will allow us to design much-needed new drugs to treat AML.” ![]()

and Matt McCormack, PhD
Photo courtesy of the
Walter and Eliza Hall
Institute of Medical Research
Preclinical research suggests the Hhex protein could be a cancer-specific therapeutic target for acute myeloid leukemia (AML).
Investigators discovered that loss of the Hhex protein halted leukemia cell growth and division in vitro and in vivo, but normal cells were unaffected by the loss of Hhex.
Matt McCormack, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues relayed these findings in Genes and Development.
“There is an urgent need for new therapies to treat AML,” Dr McCormack said. “We showed blocking the Hhex protein could put the brakes on leukemia growth and completely eliminate AML in preclinical models. This could be targeted by new drugs to treat AML in humans.”
Specifically, the investigators found that Hhex was overexpressed in human AML, and the protein was essential for the maintenance of AML driven by the oncogenic fusion protein MLL-ENL and its downstream effectors, HoxA9 and Meis1.
However, Hhex was not required for normal myelopoiesis.
“Hhex is only essential for the leukemic cells, meaning we could target and treat leukemia without toxic effects on normal cells, avoiding many of the serious side effects that come with standard cancer treatments,” Dr McCormack said.
“We also know that most people with AML have increased levels of Hhex, often associated with adverse outcomes, further indicating it is an important target for new AML drugs.”
Dr McCormack and his colleagues also attempted to determine the mechanism by which Hhex promotes AML.
They found the protein represses the tumor suppressors p16INK4a and p19ARF in leukemic stem cells by regulating the Polycomb-repressive complex 2 (PRC2). They said that Hhex binds to the Cdkn2a locus and directly interacts with PRC2 to enable H3K27me3-mediated epigenetic repression.
“Hhex works by recruiting epigenetic factors to growth-control genes, effectively silencing them,” said author Ben Shields, PhD, also of the Walter and Eliza Hall Institute.
“This allows the leukemia cells to reproduce and accumulate more damage, contributing to the speed of AML progression.”
Dr McCormack said that although drugs inhibiting epigenetic modification have been tested against AML in the past, they have caused significant toxicity because their targets are also required for normal blood cell function.
“Unlike the epigenetic factors targeted previously, Hhex only regulates a small number of genes and is dispensable for normal blood cells,” Dr McCormack reiterated.
“This gives us a rare opportunity to kill AML cells without causing many side effects. We now hope to identify the critical regions of the Hhex protein that enable it to function, which will allow us to design much-needed new drugs to treat AML.” ![]()
Predicting transformation from MDS to AML

Photo courtesy of
McMaster University
Research published in Cancer Cell suggests a molecular signature can be used to predict which patients with myelodysplastic syndromes (MDS) will develop acute myeloid leukemia (AML).
Investigators found that progressive removal of glycogen synthase kinase-3 (GSK-3) signaling via GSK-3β deletion in hematopoietic stem cells (HSCs) results in an MDS-like state.
And when both GSK-3β and GSK-3α are deleted, AML develops.
“We’ve found that the transition from healthy to cancerous blood stem cells happens in clear, compartmentalized steps,” said study author Mick Bhatia, PhD, of McMaster University in Hamilton, Ontario, Canada. “We’ve identified 2 steps in that staircase.”
Specifically, the investigators found that deleting GSK-3β in HSCs led to the generation of self-renewing cells dubbed MDS-initiating cells. These cells proved capable of sustaining MDS in vivo.
Next, the team found that GSK-3β deletion drives Wnt/Akt/mTOR signaling and can induce AML in the absence of GSK-3α.
They noted that GSK-3α has no biological impact on hematopoiesis, but GSK-3α deletion is necessary for the evolution of MDS to AML that occurs in the absence of GSK-3β.
The investigators then defined a molecular signature of GSK-3β-deficient HSCs that could predict transformation to AML in patients with MDS.
The team tested the utility of this 63-gene signature using blood samples that were previously collected from patients with MDS, some of whom ultimately developed AML. The results showed the signature could accurately predict which patients would develop AML and which would not.
“[O]ur next step is to go beyond better predictive measures for the development of a blood cancer and use this predictive gene expression as a target for drugs to prevent AML from developing altogether,” Dr Bhatia said. “This will be part of a new era of genetic-based drug discovery.” ![]()

Photo courtesy of
McMaster University
Research published in Cancer Cell suggests a molecular signature can be used to predict which patients with myelodysplastic syndromes (MDS) will develop acute myeloid leukemia (AML).
Investigators found that progressive removal of glycogen synthase kinase-3 (GSK-3) signaling via GSK-3β deletion in hematopoietic stem cells (HSCs) results in an MDS-like state.
And when both GSK-3β and GSK-3α are deleted, AML develops.
“We’ve found that the transition from healthy to cancerous blood stem cells happens in clear, compartmentalized steps,” said study author Mick Bhatia, PhD, of McMaster University in Hamilton, Ontario, Canada. “We’ve identified 2 steps in that staircase.”
Specifically, the investigators found that deleting GSK-3β in HSCs led to the generation of self-renewing cells dubbed MDS-initiating cells. These cells proved capable of sustaining MDS in vivo.
Next, the team found that GSK-3β deletion drives Wnt/Akt/mTOR signaling and can induce AML in the absence of GSK-3α.
They noted that GSK-3α has no biological impact on hematopoiesis, but GSK-3α deletion is necessary for the evolution of MDS to AML that occurs in the absence of GSK-3β.
The investigators then defined a molecular signature of GSK-3β-deficient HSCs that could predict transformation to AML in patients with MDS.
The team tested the utility of this 63-gene signature using blood samples that were previously collected from patients with MDS, some of whom ultimately developed AML. The results showed the signature could accurately predict which patients would develop AML and which would not.
“[O]ur next step is to go beyond better predictive measures for the development of a blood cancer and use this predictive gene expression as a target for drugs to prevent AML from developing altogether,” Dr Bhatia said. “This will be part of a new era of genetic-based drug discovery.” ![]()

Photo courtesy of
McMaster University
Research published in Cancer Cell suggests a molecular signature can be used to predict which patients with myelodysplastic syndromes (MDS) will develop acute myeloid leukemia (AML).
Investigators found that progressive removal of glycogen synthase kinase-3 (GSK-3) signaling via GSK-3β deletion in hematopoietic stem cells (HSCs) results in an MDS-like state.
And when both GSK-3β and GSK-3α are deleted, AML develops.
“We’ve found that the transition from healthy to cancerous blood stem cells happens in clear, compartmentalized steps,” said study author Mick Bhatia, PhD, of McMaster University in Hamilton, Ontario, Canada. “We’ve identified 2 steps in that staircase.”
Specifically, the investigators found that deleting GSK-3β in HSCs led to the generation of self-renewing cells dubbed MDS-initiating cells. These cells proved capable of sustaining MDS in vivo.
Next, the team found that GSK-3β deletion drives Wnt/Akt/mTOR signaling and can induce AML in the absence of GSK-3α.
They noted that GSK-3α has no biological impact on hematopoiesis, but GSK-3α deletion is necessary for the evolution of MDS to AML that occurs in the absence of GSK-3β.
The investigators then defined a molecular signature of GSK-3β-deficient HSCs that could predict transformation to AML in patients with MDS.
The team tested the utility of this 63-gene signature using blood samples that were previously collected from patients with MDS, some of whom ultimately developed AML. The results showed the signature could accurately predict which patients would develop AML and which would not.
“[O]ur next step is to go beyond better predictive measures for the development of a blood cancer and use this predictive gene expression as a target for drugs to prevent AML from developing altogether,” Dr Bhatia said. “This will be part of a new era of genetic-based drug discovery.” ![]()
Genes may be targets for AML therapy

Two genes are critical to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Previous research suggested the genes, KDM4C and PRMT1, are key players in transcription regulation during both normal and disease development.
The new study showed that, during AML development, KDM4C and PRMT1 are recruited to enable the transformation of blood cells into cancer cells.
The genes work in tandem, and, if either is not fully active, AML does not develop.
The researchers made these discoveries by inhibiting KDM4C and PRMT1—either genetically or pharmacologically—in mice with AML.
When either gene was silenced via genetic means, the majority of the mice were still alive at the end of the researchers’ 60-day experiment. However, the majority of control mice died in less than 40 days.
The team observed similarly favorable results when they inhibited either gene with drugs—the PRMT1 inhibitor AMI-408 and the KDM4C inhibitor SD70.
The median disease latency was 48 days in mice that received AMI-408 and 36 days in control mice. The median disease latency was 62 days in mice that received SD70 and 55 days in control mice.
“The demonstration of how critical these genes are to cancer transformation and treatment could be highly significant for the design of new drugs,” said study author Eric So, PhD, of King’s College London in the UK.
“Further work is needed to develop and refine drugs to maximize their effects and so that they are suitable for patients. Clinical trials will then be needed to see how leukemia patients respond to these drugs and how use of them can be optimized.” ![]()

Two genes are critical to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Previous research suggested the genes, KDM4C and PRMT1, are key players in transcription regulation during both normal and disease development.
The new study showed that, during AML development, KDM4C and PRMT1 are recruited to enable the transformation of blood cells into cancer cells.
The genes work in tandem, and, if either is not fully active, AML does not develop.
The researchers made these discoveries by inhibiting KDM4C and PRMT1—either genetically or pharmacologically—in mice with AML.
When either gene was silenced via genetic means, the majority of the mice were still alive at the end of the researchers’ 60-day experiment. However, the majority of control mice died in less than 40 days.
The team observed similarly favorable results when they inhibited either gene with drugs—the PRMT1 inhibitor AMI-408 and the KDM4C inhibitor SD70.
The median disease latency was 48 days in mice that received AMI-408 and 36 days in control mice. The median disease latency was 62 days in mice that received SD70 and 55 days in control mice.
“The demonstration of how critical these genes are to cancer transformation and treatment could be highly significant for the design of new drugs,” said study author Eric So, PhD, of King’s College London in the UK.
“Further work is needed to develop and refine drugs to maximize their effects and so that they are suitable for patients. Clinical trials will then be needed to see how leukemia patients respond to these drugs and how use of them can be optimized.” ![]()

Two genes are critical to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Previous research suggested the genes, KDM4C and PRMT1, are key players in transcription regulation during both normal and disease development.
The new study showed that, during AML development, KDM4C and PRMT1 are recruited to enable the transformation of blood cells into cancer cells.
The genes work in tandem, and, if either is not fully active, AML does not develop.
The researchers made these discoveries by inhibiting KDM4C and PRMT1—either genetically or pharmacologically—in mice with AML.
When either gene was silenced via genetic means, the majority of the mice were still alive at the end of the researchers’ 60-day experiment. However, the majority of control mice died in less than 40 days.
The team observed similarly favorable results when they inhibited either gene with drugs—the PRMT1 inhibitor AMI-408 and the KDM4C inhibitor SD70.
The median disease latency was 48 days in mice that received AMI-408 and 36 days in control mice. The median disease latency was 62 days in mice that received SD70 and 55 days in control mice.
“The demonstration of how critical these genes are to cancer transformation and treatment could be highly significant for the design of new drugs,” said study author Eric So, PhD, of King’s College London in the UK.
“Further work is needed to develop and refine drugs to maximize their effects and so that they are suitable for patients. Clinical trials will then be needed to see how leukemia patients respond to these drugs and how use of them can be optimized.”