User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Cell-Free DNA Blood Test Shows Strong Performance in Detecting Early-Stage CRC
Cell-Free DNA Blood Test Shows Strong Performance in Detecting Early-Stage CRC
TOPLINE:
A novel, blood-based test developed using fragmentomic features of cell-free DNA (cfDNA) detects colorectal cancer (CRC) with a 90.4% sensitivity and shows consistent performance across stages and tumor locations.
METHODOLOGY:
- Researchers conducted a prospective case-control study to develop and validate a noninvasive cfDNA-based screening test for CRC.
- Adults aged 40-89 years with CRC or advanced adenomas were enrolled at a tertiary center in South Korea between 2021 and 2024.
- Blood samples were drawn after colonoscopy, but prior to treatment, in patients with CRC, advanced adenomas, and asymptomatic controls with normal colonoscopy results.
- A model was trained on fragmentonic features derived from whole genome sequencing of cfDNA from 1250 participants and validated for its diagnostic performance in the remaining 427 participants, including all with advanced adenomas.
- The primary endpoint was the sensitivity of the cfDNA test for detecting CRC. The area under the receiver operating characteristic curve (AUROC) was also calculated.
TAKEAWAY:
- The cfDNA test detected CRC with 90.4% sensitivity and an AUROC of 0.978.
- Sensitivity by CRC stage was 84.2% for stage I, 85.0% for stage II, 94.4% for stage III, 100% for stage IV.
- Advanced adenomas were detected with 58.3% sensitivity and an AUROC of 0.862.
- Among individuals with normal colonoscopy findings, the test was correctly negative 94.7% of the time.
- Diagnostic sensitivities were consistent between left- and right-sided CRC tumors, among participants aged < 60 years and ≥ 60 years, and across left- and right-sided advanced adenomas.
IN PRACTICE:
"This highlights the potential clinical utility of the test in identifying candidates for minimally invasive therapeutic approaches tool for CRC," the authors wrote. "Notably, the high sensitivity observed for early-stage CRC and the favorable sensitivity for [advanced adenoma] suggest that this cfDNA test may offer benefits not only in diagnosis but also in prognosis and ultimately in CRC prevention."
SOURCE:
This study was led by Seung Wook Hong, MD, Asan Medical Center in Seoul, South Korea. It was published online on November 19, 2025, in the American Journal of Gastroenterology.
LIMITATIONS:
The case-control design introduced spectrum bias by comparing clearly defined CRC and advanced adenomas cases with individuals who had normal colonoscopy results. The CRC prevalence of 17% to 18% was higher than that observed in true screening populations, limiting generalizability. The exclusively Korean cohort limited extrapolation to non-Asian populations.
DISCLOSURES:
The study received support from GC Genome, Yongin, South Korea. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A novel, blood-based test developed using fragmentomic features of cell-free DNA (cfDNA) detects colorectal cancer (CRC) with a 90.4% sensitivity and shows consistent performance across stages and tumor locations.
METHODOLOGY:
- Researchers conducted a prospective case-control study to develop and validate a noninvasive cfDNA-based screening test for CRC.
- Adults aged 40-89 years with CRC or advanced adenomas were enrolled at a tertiary center in South Korea between 2021 and 2024.
- Blood samples were drawn after colonoscopy, but prior to treatment, in patients with CRC, advanced adenomas, and asymptomatic controls with normal colonoscopy results.
- A model was trained on fragmentonic features derived from whole genome sequencing of cfDNA from 1250 participants and validated for its diagnostic performance in the remaining 427 participants, including all with advanced adenomas.
- The primary endpoint was the sensitivity of the cfDNA test for detecting CRC. The area under the receiver operating characteristic curve (AUROC) was also calculated.
TAKEAWAY:
- The cfDNA test detected CRC with 90.4% sensitivity and an AUROC of 0.978.
- Sensitivity by CRC stage was 84.2% for stage I, 85.0% for stage II, 94.4% for stage III, 100% for stage IV.
- Advanced adenomas were detected with 58.3% sensitivity and an AUROC of 0.862.
- Among individuals with normal colonoscopy findings, the test was correctly negative 94.7% of the time.
- Diagnostic sensitivities were consistent between left- and right-sided CRC tumors, among participants aged < 60 years and ≥ 60 years, and across left- and right-sided advanced adenomas.
IN PRACTICE:
"This highlights the potential clinical utility of the test in identifying candidates for minimally invasive therapeutic approaches tool for CRC," the authors wrote. "Notably, the high sensitivity observed for early-stage CRC and the favorable sensitivity for [advanced adenoma] suggest that this cfDNA test may offer benefits not only in diagnosis but also in prognosis and ultimately in CRC prevention."
SOURCE:
This study was led by Seung Wook Hong, MD, Asan Medical Center in Seoul, South Korea. It was published online on November 19, 2025, in the American Journal of Gastroenterology.
LIMITATIONS:
The case-control design introduced spectrum bias by comparing clearly defined CRC and advanced adenomas cases with individuals who had normal colonoscopy results. The CRC prevalence of 17% to 18% was higher than that observed in true screening populations, limiting generalizability. The exclusively Korean cohort limited extrapolation to non-Asian populations.
DISCLOSURES:
The study received support from GC Genome, Yongin, South Korea. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A novel, blood-based test developed using fragmentomic features of cell-free DNA (cfDNA) detects colorectal cancer (CRC) with a 90.4% sensitivity and shows consistent performance across stages and tumor locations.
METHODOLOGY:
- Researchers conducted a prospective case-control study to develop and validate a noninvasive cfDNA-based screening test for CRC.
- Adults aged 40-89 years with CRC or advanced adenomas were enrolled at a tertiary center in South Korea between 2021 and 2024.
- Blood samples were drawn after colonoscopy, but prior to treatment, in patients with CRC, advanced adenomas, and asymptomatic controls with normal colonoscopy results.
- A model was trained on fragmentonic features derived from whole genome sequencing of cfDNA from 1250 participants and validated for its diagnostic performance in the remaining 427 participants, including all with advanced adenomas.
- The primary endpoint was the sensitivity of the cfDNA test for detecting CRC. The area under the receiver operating characteristic curve (AUROC) was also calculated.
TAKEAWAY:
- The cfDNA test detected CRC with 90.4% sensitivity and an AUROC of 0.978.
- Sensitivity by CRC stage was 84.2% for stage I, 85.0% for stage II, 94.4% for stage III, 100% for stage IV.
- Advanced adenomas were detected with 58.3% sensitivity and an AUROC of 0.862.
- Among individuals with normal colonoscopy findings, the test was correctly negative 94.7% of the time.
- Diagnostic sensitivities were consistent between left- and right-sided CRC tumors, among participants aged < 60 years and ≥ 60 years, and across left- and right-sided advanced adenomas.
IN PRACTICE:
"This highlights the potential clinical utility of the test in identifying candidates for minimally invasive therapeutic approaches tool for CRC," the authors wrote. "Notably, the high sensitivity observed for early-stage CRC and the favorable sensitivity for [advanced adenoma] suggest that this cfDNA test may offer benefits not only in diagnosis but also in prognosis and ultimately in CRC prevention."
SOURCE:
This study was led by Seung Wook Hong, MD, Asan Medical Center in Seoul, South Korea. It was published online on November 19, 2025, in the American Journal of Gastroenterology.
LIMITATIONS:
The case-control design introduced spectrum bias by comparing clearly defined CRC and advanced adenomas cases with individuals who had normal colonoscopy results. The CRC prevalence of 17% to 18% was higher than that observed in true screening populations, limiting generalizability. The exclusively Korean cohort limited extrapolation to non-Asian populations.
DISCLOSURES:
The study received support from GC Genome, Yongin, South Korea. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Cell-Free DNA Blood Test Shows Strong Performance in Detecting Early-Stage CRC
Cell-Free DNA Blood Test Shows Strong Performance in Detecting Early-Stage CRC
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).
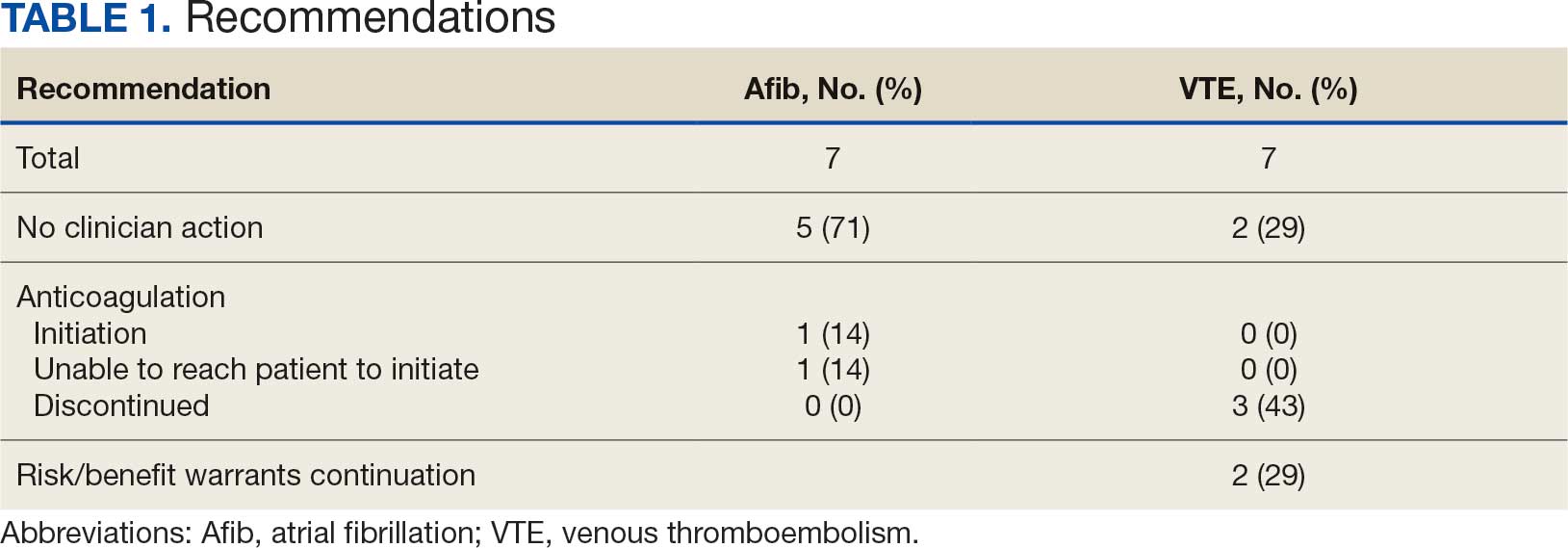
In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
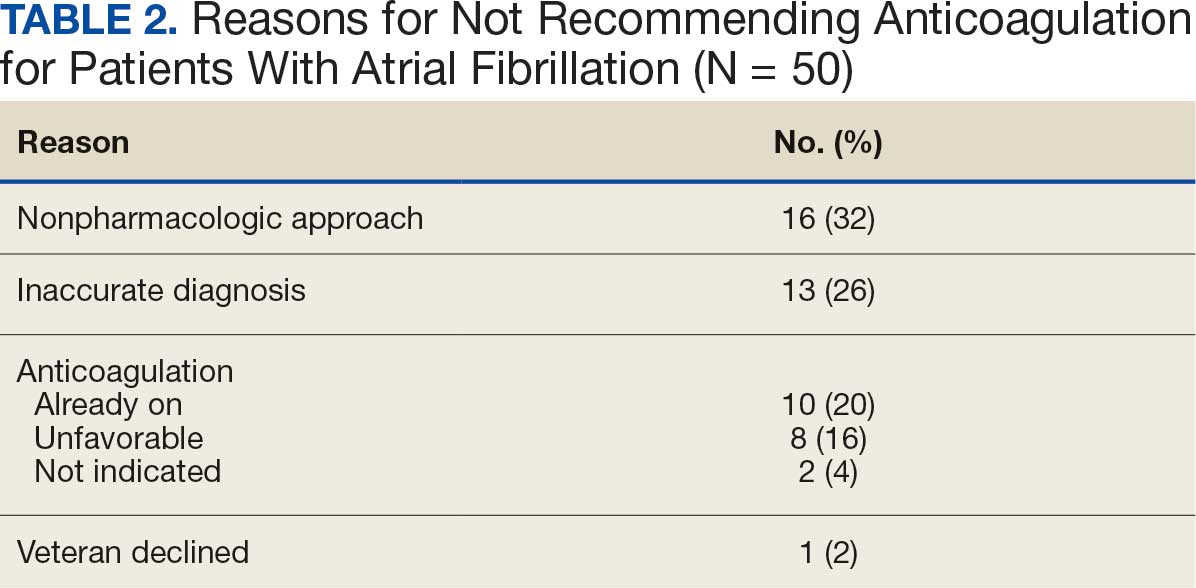
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).
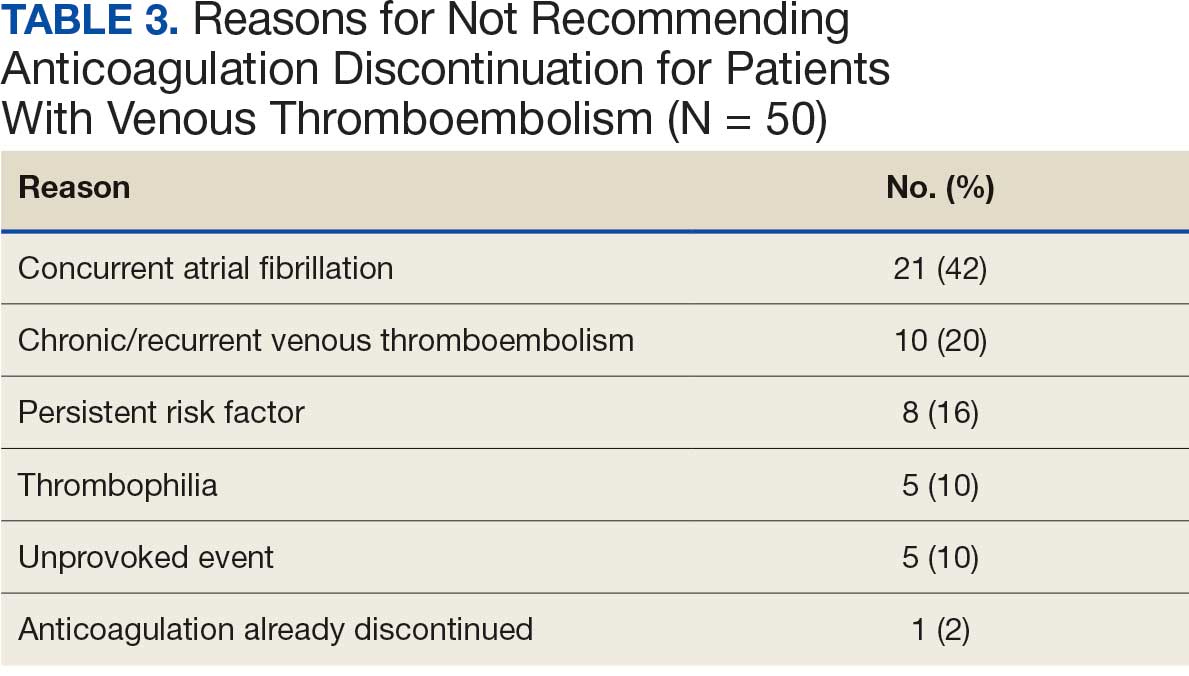
In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).
Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Geographic Clusters Show Uneven Cancer Screening in the US
Geographic Clusters Show Uneven Cancer Screening in the US
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Geographic Clusters Show Uneven Cancer Screening in the US
Geographic Clusters Show Uneven Cancer Screening in the US
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Artificial Intelligence Shows Promise in Detecting Missed Interval Breast Cancer on Screening Mammograms
TOPLINE:
An artificial intelligence (AI) system flagged high-risk areas on mammograms for potentially missed interval breast cancers (IBCs), which radiologists had also retrospectively identified as abnormal. Moreover, the AI detected a substantial number of IBCs that manual review had overlooked.
METHODOLOGY:
- Researchers conducted a retrospective analysis of 119 IBC screening mammograms of women (mean age, 57.3 years) with a high breast density (Breast Imaging Reporting and Data System [BI-RADS] c/d, 63.0%) using data retrieved from Cancer Registries of Eastern Switzerland and Grisons-Glarus databases.
- A recorded tumour was classified as IBC when an invasive or in situ BC was diagnosed within 24 months after a normal screening mammogram.
- Three radiologists retrospectively assessed the mammograms for visible signs of BC, which were then classified as either potentially missed IBCs or IBCs without retrospective abnormalities on the basis of consensus conference recommendations of radiologists.
- An AI system generated two scores (a scale of 0 to 100): a case score reflecting the likelihood that the mammogram currently harbours cancer and a risk score estimating the probability of a BC diagnosis within 2 years.
TAKEAWAY:
- Radiologists classified 68.9% of IBCs as those having no retrospective abnormalities and assigned significantly higher BI-RADS scores to the remaining 31.1% of potentially missed IBCs (P < .05).
- Potentially missed IBCs received significantly higher AI case scores (mean, 54.1 vs 23.1; P < .05) and were assigned to a higher risk category (48.7% vs 14.6%; P < .05) than IBCs without retrospective abnormalities.
- Of all IBC cases, 46.2% received an AI case score > 25, 25.2% scored > 50, and 13.4% scored > 75.
- Potentially missed IBCs scored widely between low and high risk and case scores, whereas IBCs without retrospective abnormalities scored low case and risk scores. Specifically, 73.0% of potentially missed IBCs vs 34.1% of IBCs without retrospective abnormalities had case scores > 25, 51.4% vs 13.4% had case scores > 50, and 29.7% vs 6.1% had case scores > 75.
IN PRACTICE:
“Our research highlights that an AI system can identify BC signs in relevant portions of IBC screening mammograms and thus potentially reduce the number of IBCs in an MSP [mammography screening program] that currently does not utilize an AI system,” the authors of the study concluded, adding that “it can identify some IBCs that are not visible to humans (IBCs without retrospective abnormalities).”
SOURCE:
This study was led by Jonas Subelack, Chair of Health Economics, Policy and Management, School of Medicine, University of St. Gallen, St. Gallen, Switzerland. It was published online in European Radiology.
LIMITATIONS:
The retrospective study design inherently limited causal conclusions. Without access to diagnostic mammograms or the detailed position of BC, researchers could not evaluate whether AI-marked lesions corresponded to later detected BCs.
DISCLOSURES:
This research was funded by the Cancer League of Eastern Switzerland. One author reported receiving consulting and speaker fees from iCAD.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI) system flagged high-risk areas on mammograms for potentially missed interval breast cancers (IBCs), which radiologists had also retrospectively identified as abnormal. Moreover, the AI detected a substantial number of IBCs that manual review had overlooked.
METHODOLOGY:
- Researchers conducted a retrospective analysis of 119 IBC screening mammograms of women (mean age, 57.3 years) with a high breast density (Breast Imaging Reporting and Data System [BI-RADS] c/d, 63.0%) using data retrieved from Cancer Registries of Eastern Switzerland and Grisons-Glarus databases.
- A recorded tumour was classified as IBC when an invasive or in situ BC was diagnosed within 24 months after a normal screening mammogram.
- Three radiologists retrospectively assessed the mammograms for visible signs of BC, which were then classified as either potentially missed IBCs or IBCs without retrospective abnormalities on the basis of consensus conference recommendations of radiologists.
- An AI system generated two scores (a scale of 0 to 100): a case score reflecting the likelihood that the mammogram currently harbours cancer and a risk score estimating the probability of a BC diagnosis within 2 years.
TAKEAWAY:
- Radiologists classified 68.9% of IBCs as those having no retrospective abnormalities and assigned significantly higher BI-RADS scores to the remaining 31.1% of potentially missed IBCs (P < .05).
- Potentially missed IBCs received significantly higher AI case scores (mean, 54.1 vs 23.1; P < .05) and were assigned to a higher risk category (48.7% vs 14.6%; P < .05) than IBCs without retrospective abnormalities.
- Of all IBC cases, 46.2% received an AI case score > 25, 25.2% scored > 50, and 13.4% scored > 75.
- Potentially missed IBCs scored widely between low and high risk and case scores, whereas IBCs without retrospective abnormalities scored low case and risk scores. Specifically, 73.0% of potentially missed IBCs vs 34.1% of IBCs without retrospective abnormalities had case scores > 25, 51.4% vs 13.4% had case scores > 50, and 29.7% vs 6.1% had case scores > 75.
IN PRACTICE:
“Our research highlights that an AI system can identify BC signs in relevant portions of IBC screening mammograms and thus potentially reduce the number of IBCs in an MSP [mammography screening program] that currently does not utilize an AI system,” the authors of the study concluded, adding that “it can identify some IBCs that are not visible to humans (IBCs without retrospective abnormalities).”
SOURCE:
This study was led by Jonas Subelack, Chair of Health Economics, Policy and Management, School of Medicine, University of St. Gallen, St. Gallen, Switzerland. It was published online in European Radiology.
LIMITATIONS:
The retrospective study design inherently limited causal conclusions. Without access to diagnostic mammograms or the detailed position of BC, researchers could not evaluate whether AI-marked lesions corresponded to later detected BCs.
DISCLOSURES:
This research was funded by the Cancer League of Eastern Switzerland. One author reported receiving consulting and speaker fees from iCAD.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI) system flagged high-risk areas on mammograms for potentially missed interval breast cancers (IBCs), which radiologists had also retrospectively identified as abnormal. Moreover, the AI detected a substantial number of IBCs that manual review had overlooked.
METHODOLOGY:
- Researchers conducted a retrospective analysis of 119 IBC screening mammograms of women (mean age, 57.3 years) with a high breast density (Breast Imaging Reporting and Data System [BI-RADS] c/d, 63.0%) using data retrieved from Cancer Registries of Eastern Switzerland and Grisons-Glarus databases.
- A recorded tumour was classified as IBC when an invasive or in situ BC was diagnosed within 24 months after a normal screening mammogram.
- Three radiologists retrospectively assessed the mammograms for visible signs of BC, which were then classified as either potentially missed IBCs or IBCs without retrospective abnormalities on the basis of consensus conference recommendations of radiologists.
- An AI system generated two scores (a scale of 0 to 100): a case score reflecting the likelihood that the mammogram currently harbours cancer and a risk score estimating the probability of a BC diagnosis within 2 years.
TAKEAWAY:
- Radiologists classified 68.9% of IBCs as those having no retrospective abnormalities and assigned significantly higher BI-RADS scores to the remaining 31.1% of potentially missed IBCs (P < .05).
- Potentially missed IBCs received significantly higher AI case scores (mean, 54.1 vs 23.1; P < .05) and were assigned to a higher risk category (48.7% vs 14.6%; P < .05) than IBCs without retrospective abnormalities.
- Of all IBC cases, 46.2% received an AI case score > 25, 25.2% scored > 50, and 13.4% scored > 75.
- Potentially missed IBCs scored widely between low and high risk and case scores, whereas IBCs without retrospective abnormalities scored low case and risk scores. Specifically, 73.0% of potentially missed IBCs vs 34.1% of IBCs without retrospective abnormalities had case scores > 25, 51.4% vs 13.4% had case scores > 50, and 29.7% vs 6.1% had case scores > 75.
IN PRACTICE:
“Our research highlights that an AI system can identify BC signs in relevant portions of IBC screening mammograms and thus potentially reduce the number of IBCs in an MSP [mammography screening program] that currently does not utilize an AI system,” the authors of the study concluded, adding that “it can identify some IBCs that are not visible to humans (IBCs without retrospective abnormalities).”
SOURCE:
This study was led by Jonas Subelack, Chair of Health Economics, Policy and Management, School of Medicine, University of St. Gallen, St. Gallen, Switzerland. It was published online in European Radiology.
LIMITATIONS:
The retrospective study design inherently limited causal conclusions. Without access to diagnostic mammograms or the detailed position of BC, researchers could not evaluate whether AI-marked lesions corresponded to later detected BCs.
DISCLOSURES:
This research was funded by the Cancer League of Eastern Switzerland. One author reported receiving consulting and speaker fees from iCAD.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.
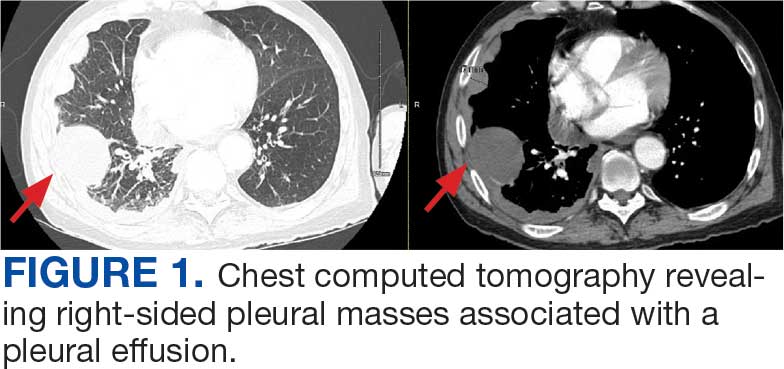
Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).
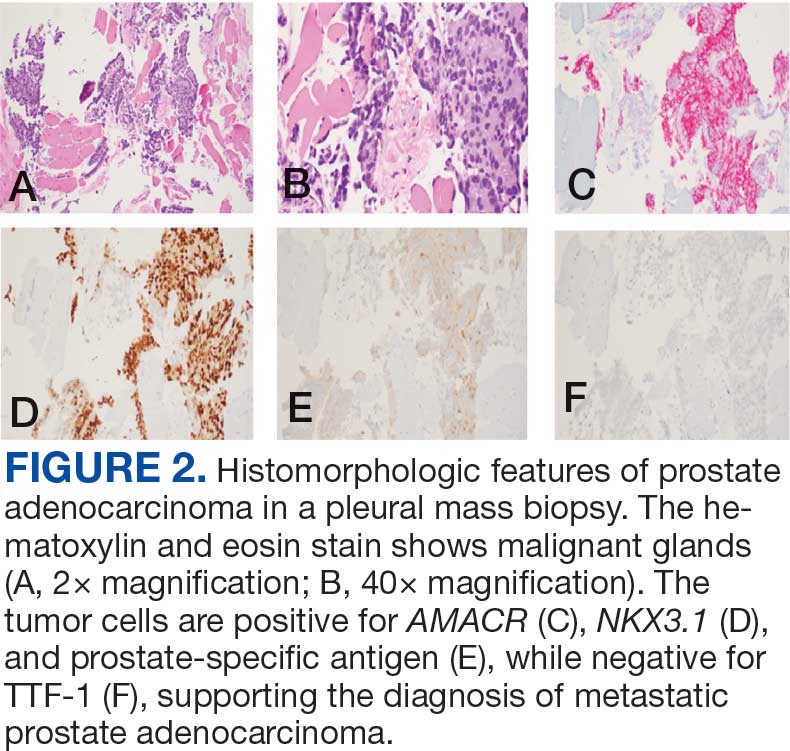
Case 2
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).
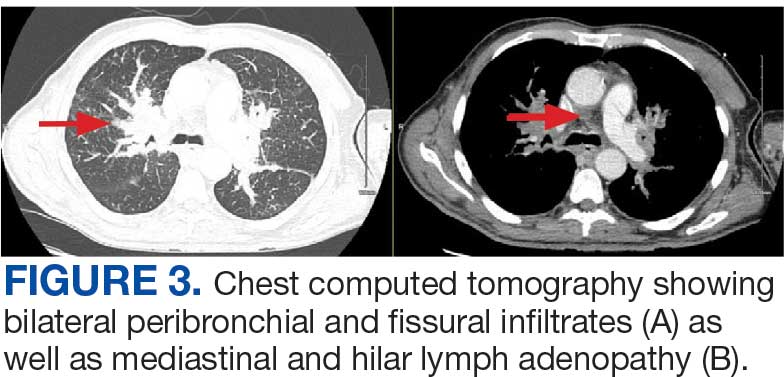
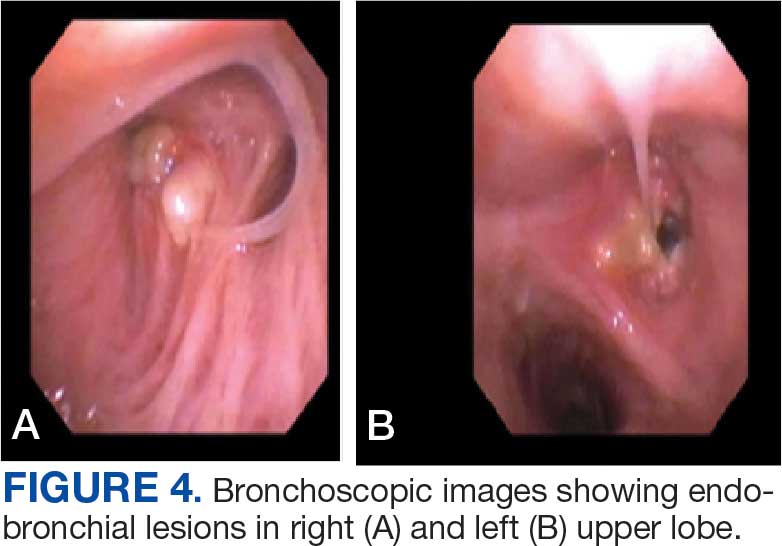
The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.
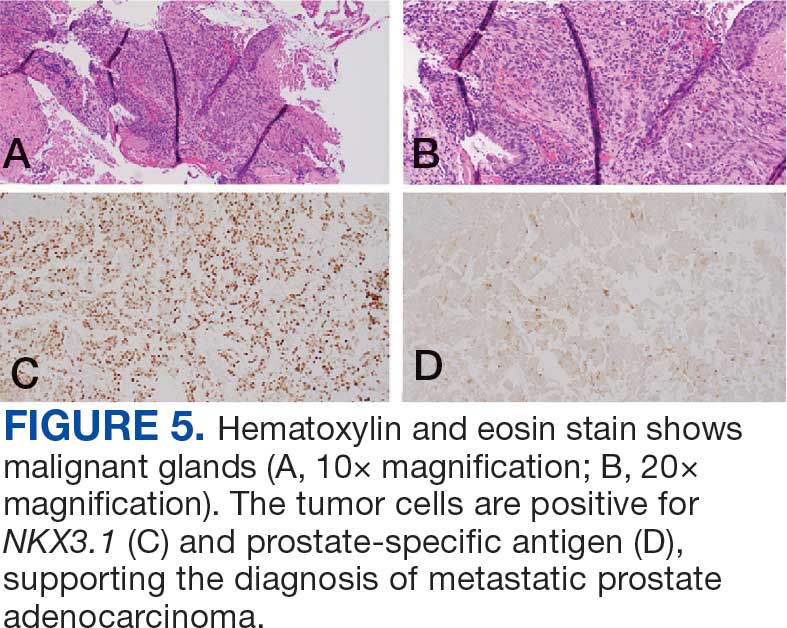
Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.
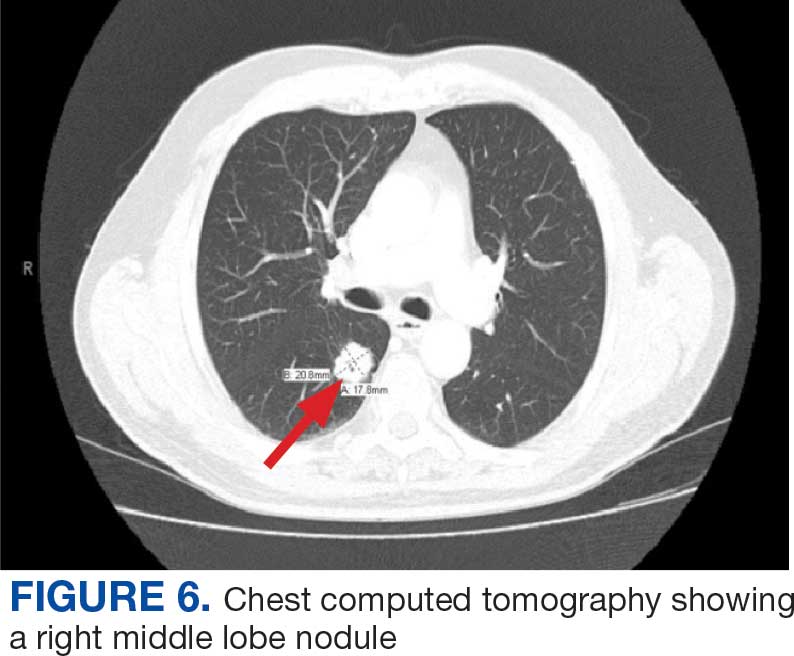
The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.

Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).

Case 2
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).


The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.

Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.

The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.

Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).

Case 2
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).


The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.

Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.

The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).
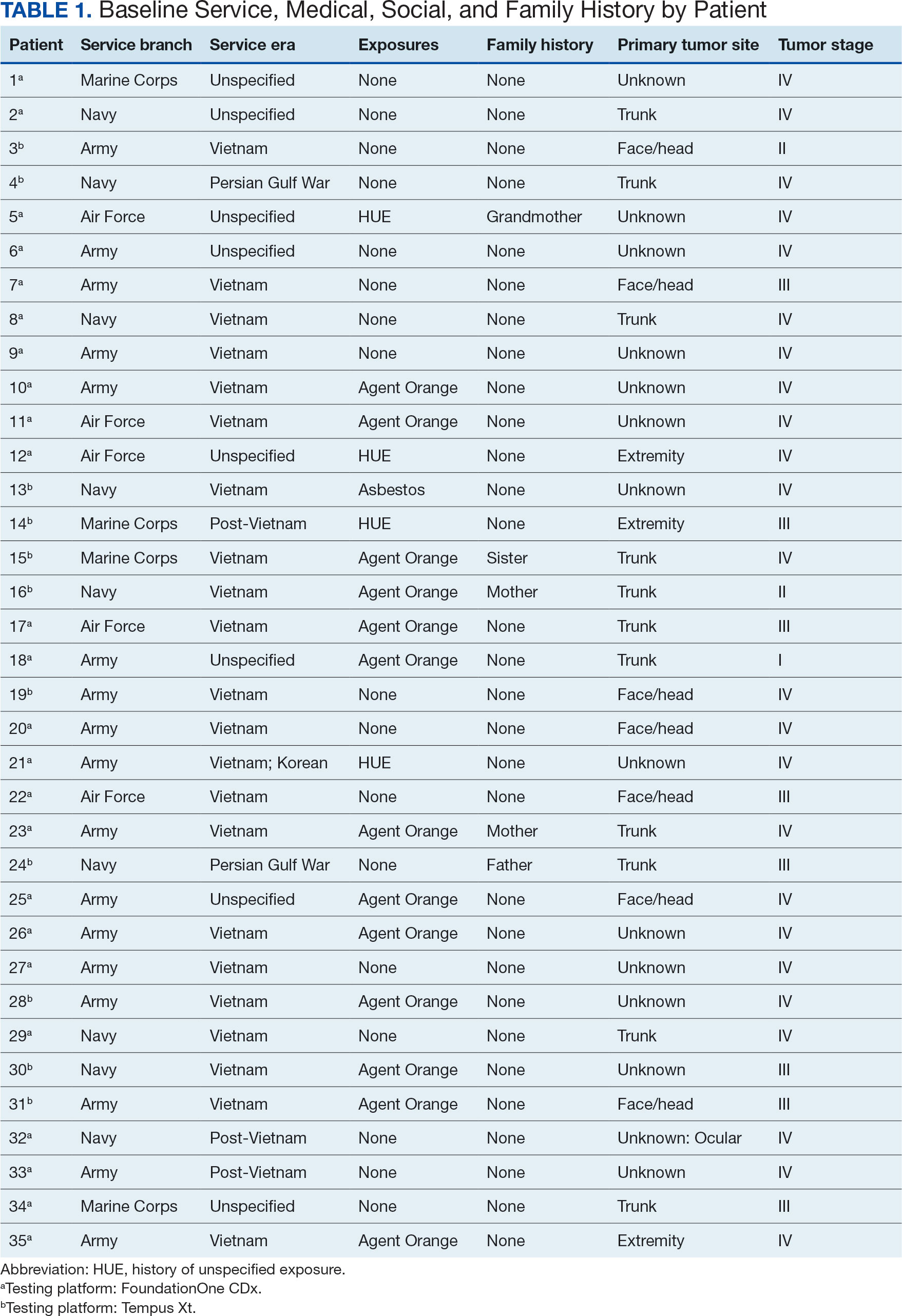
Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.
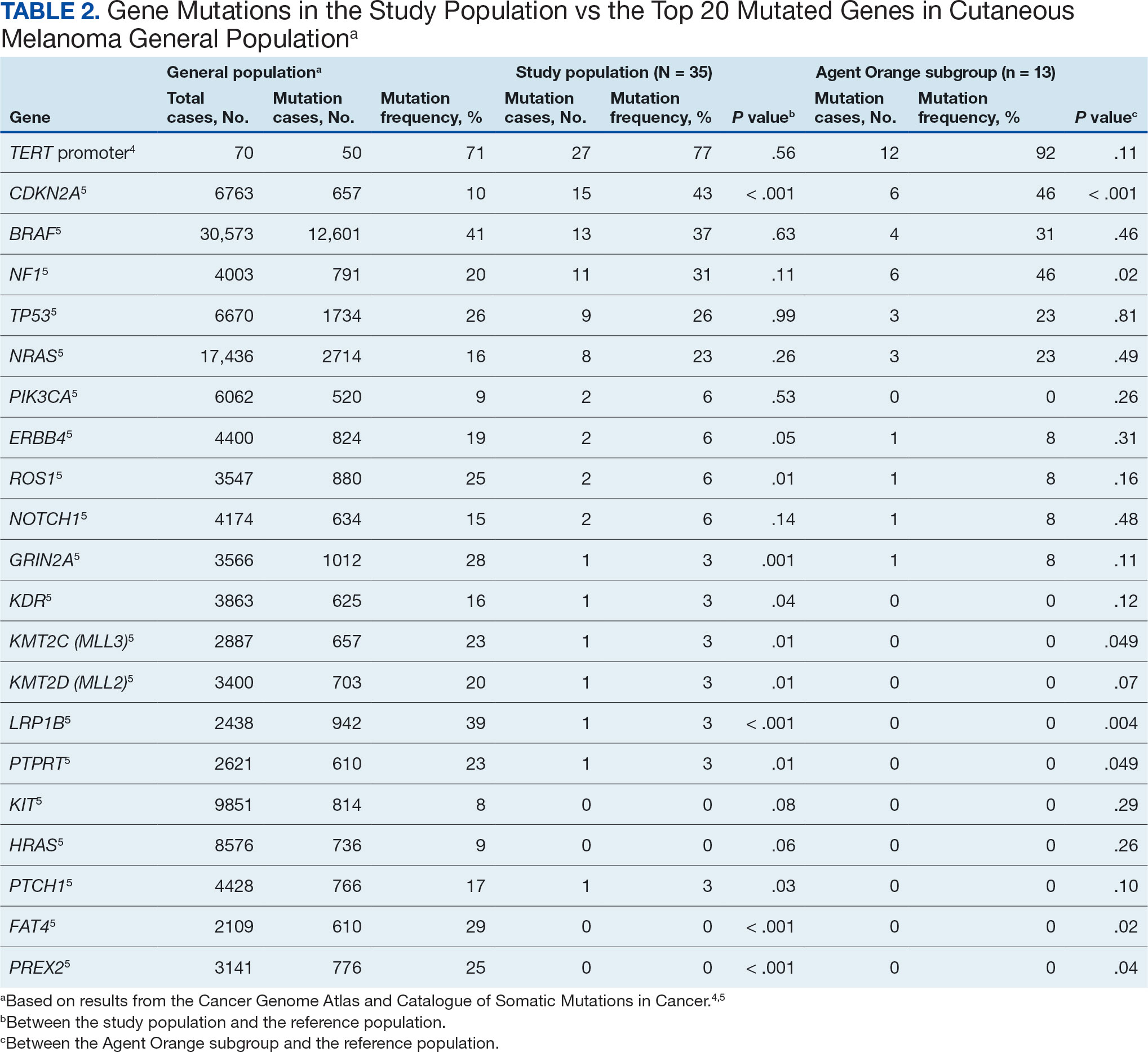
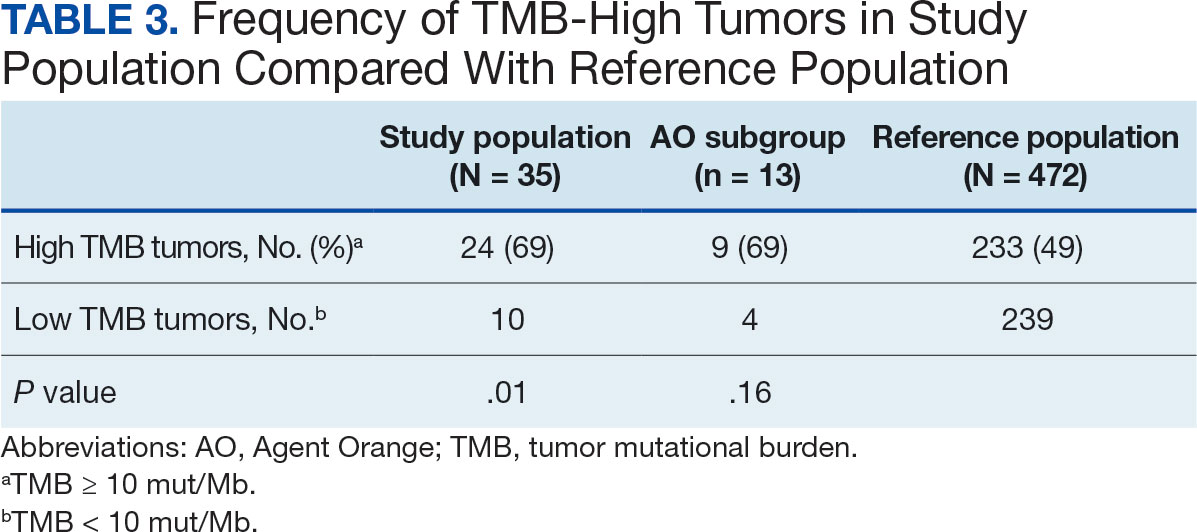
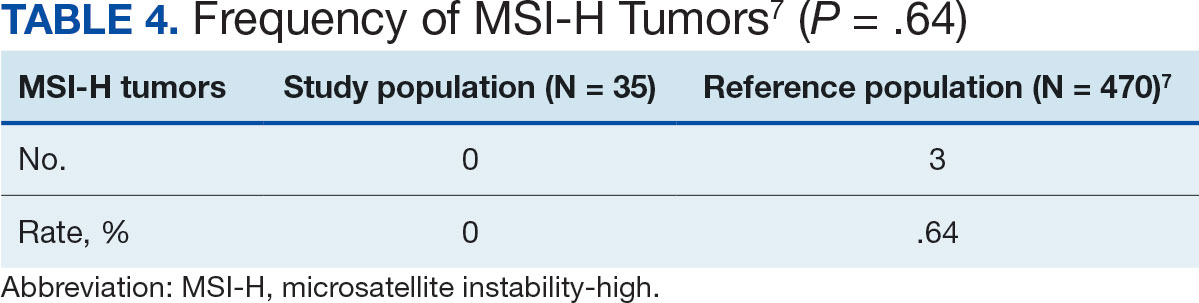
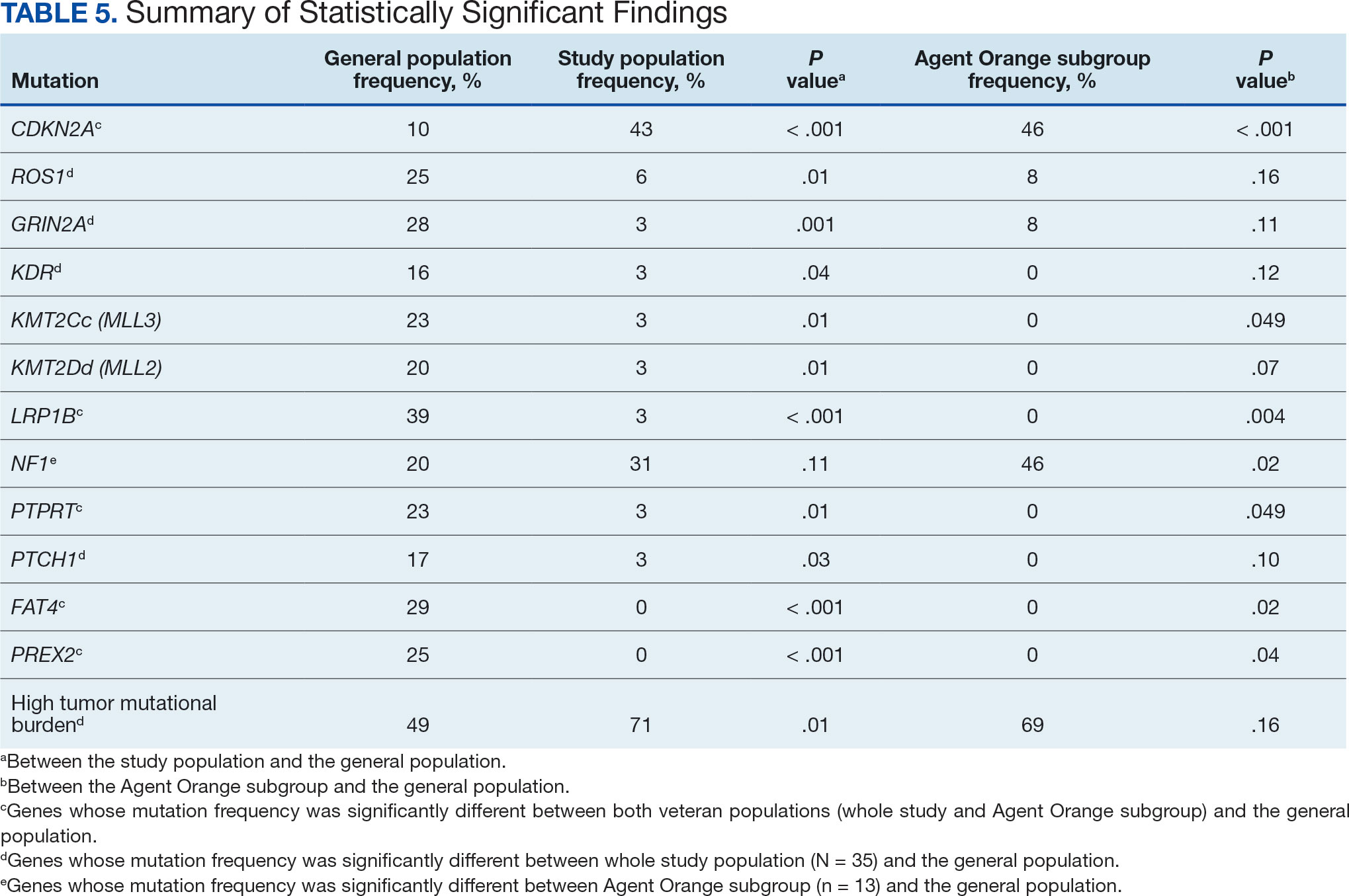
Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).

Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.




Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).

Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.




Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
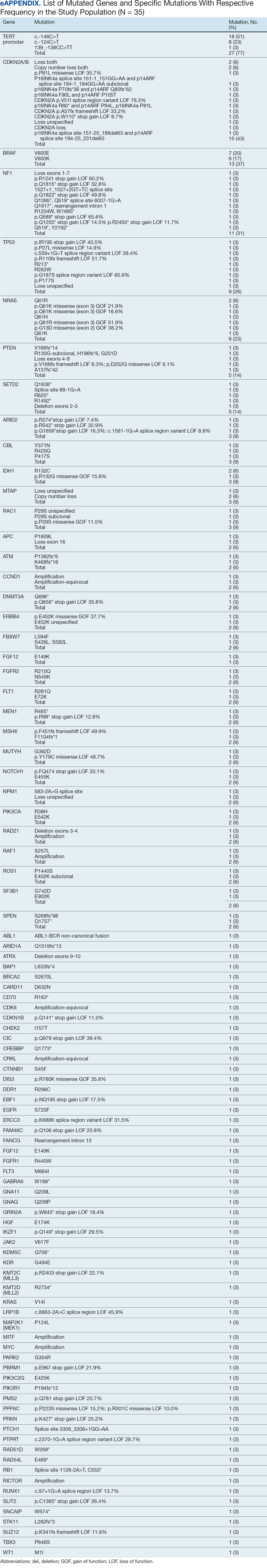
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
Rurality and Age May Shape Phone-Only Mental Health Care Access Among Veterans
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).
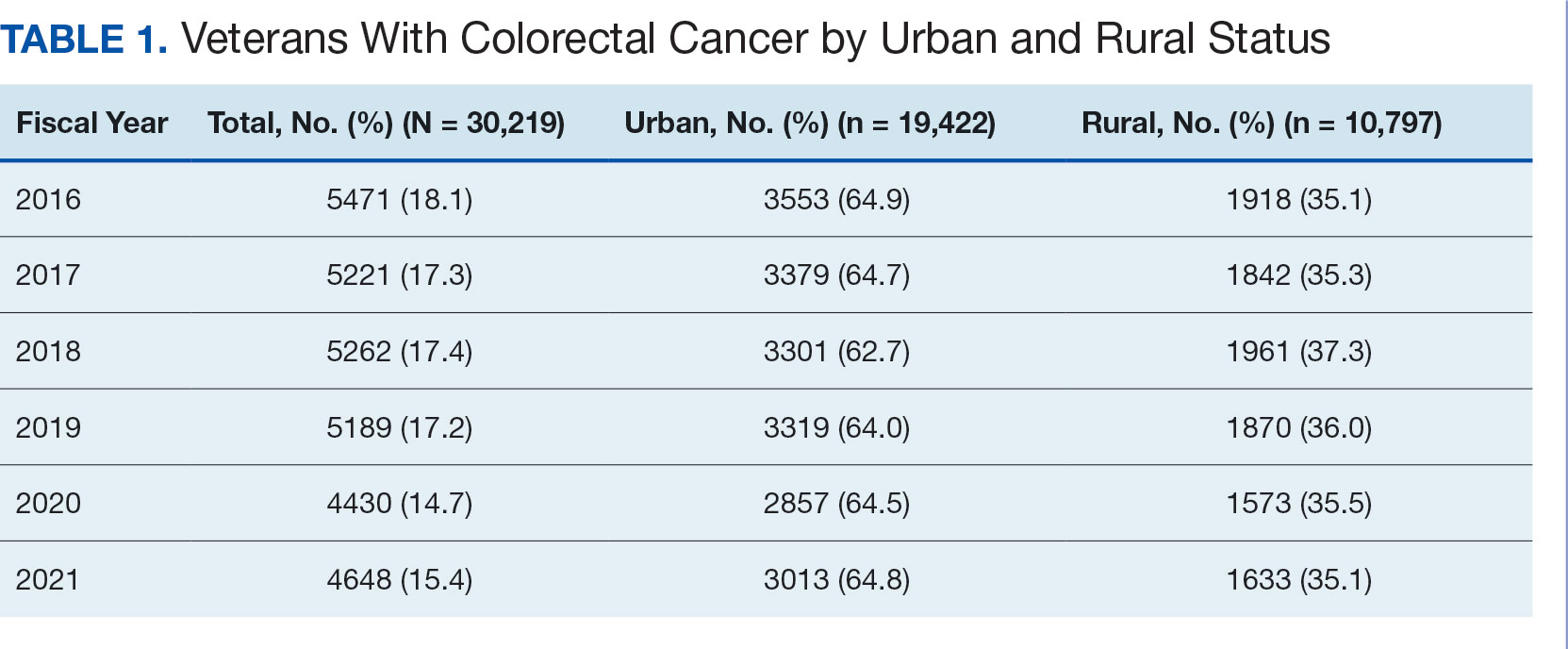
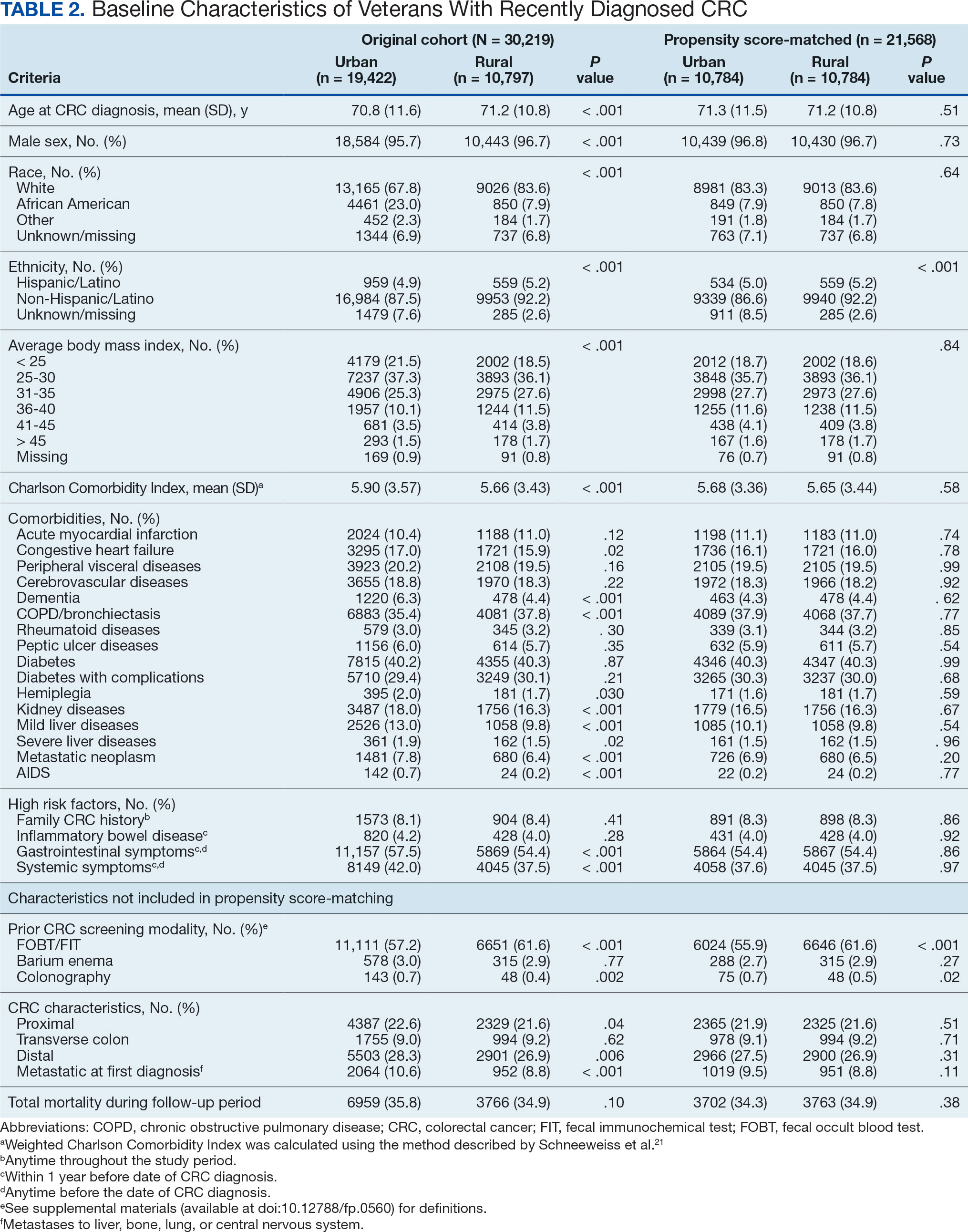
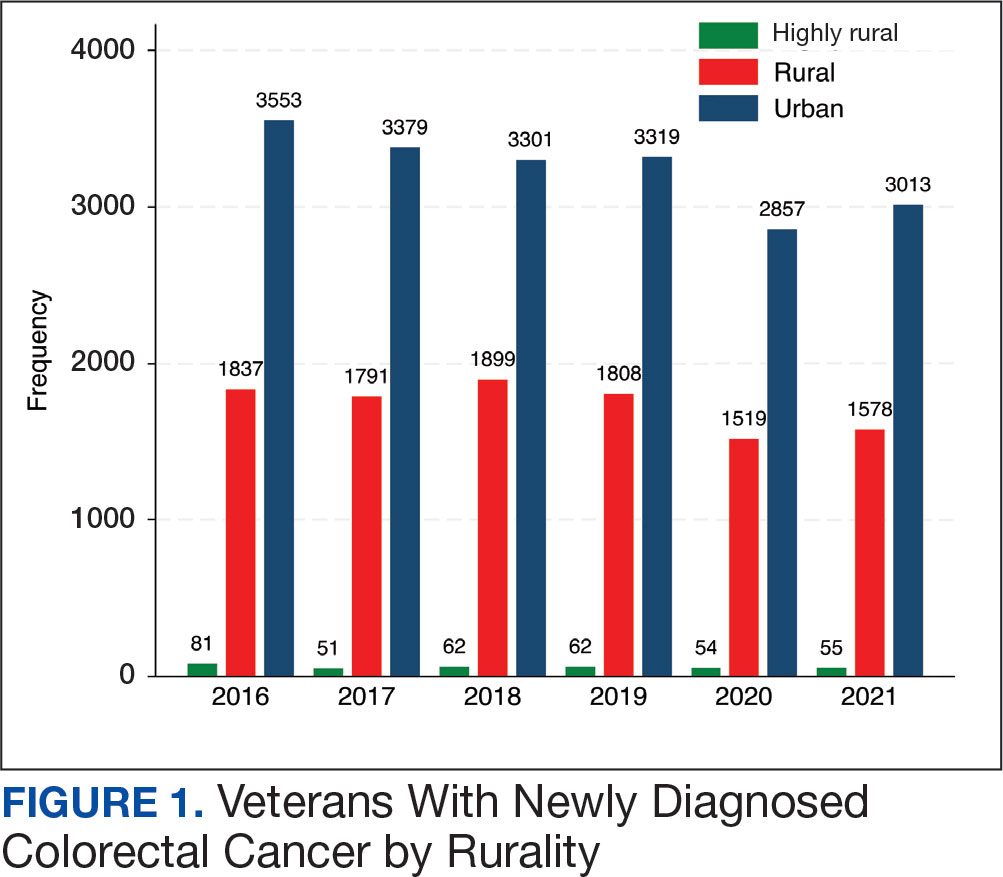
There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).
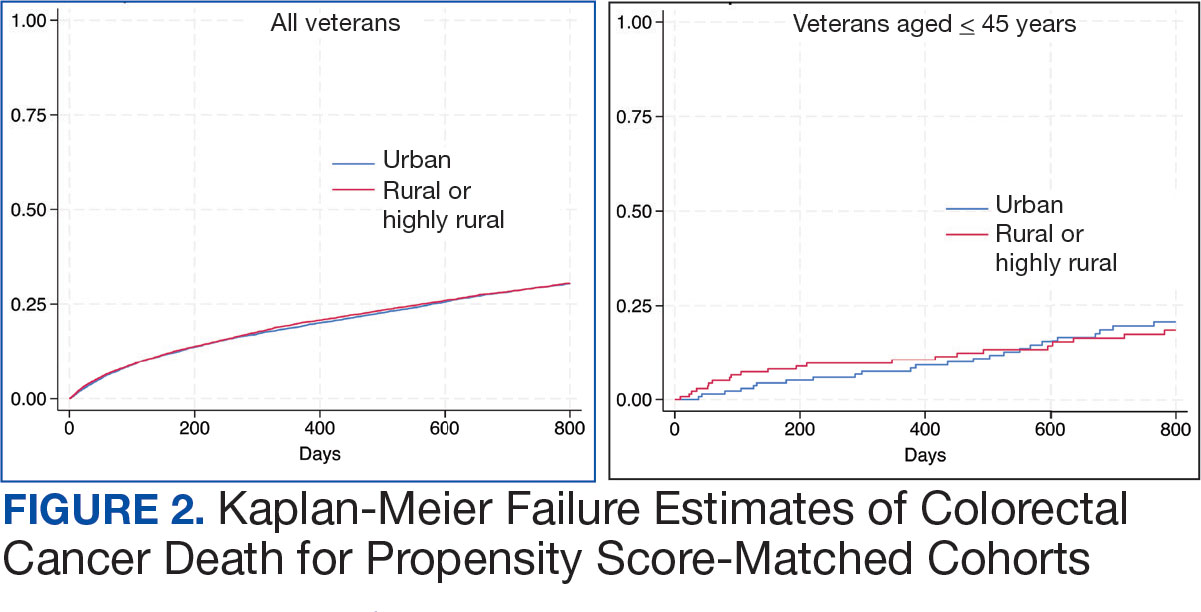
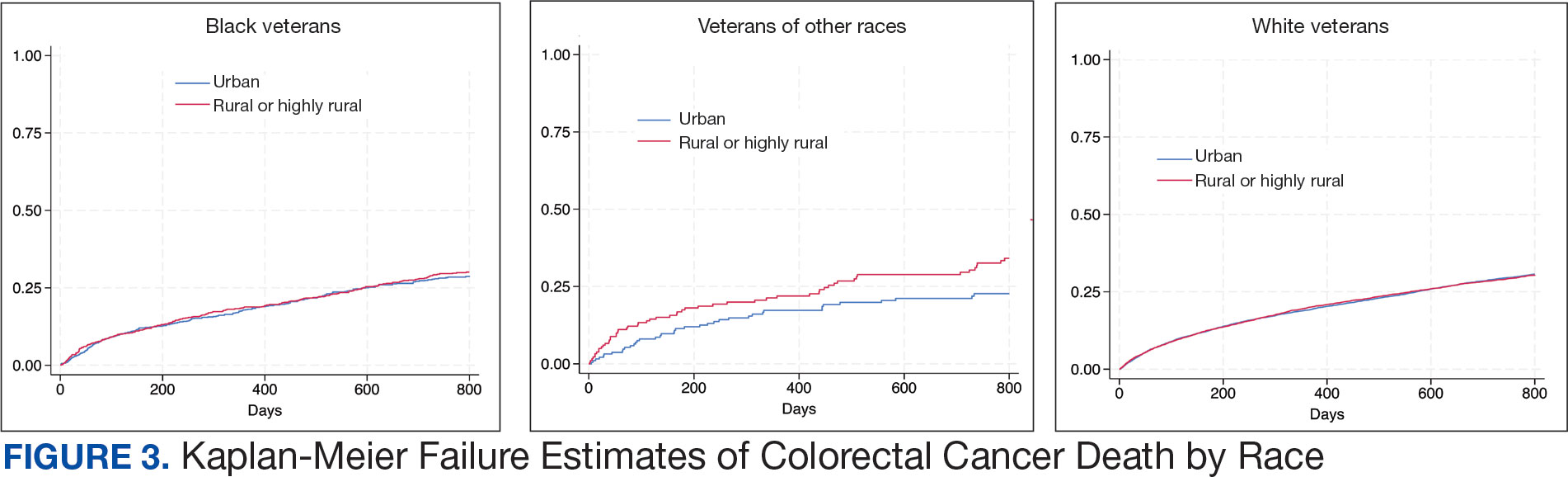
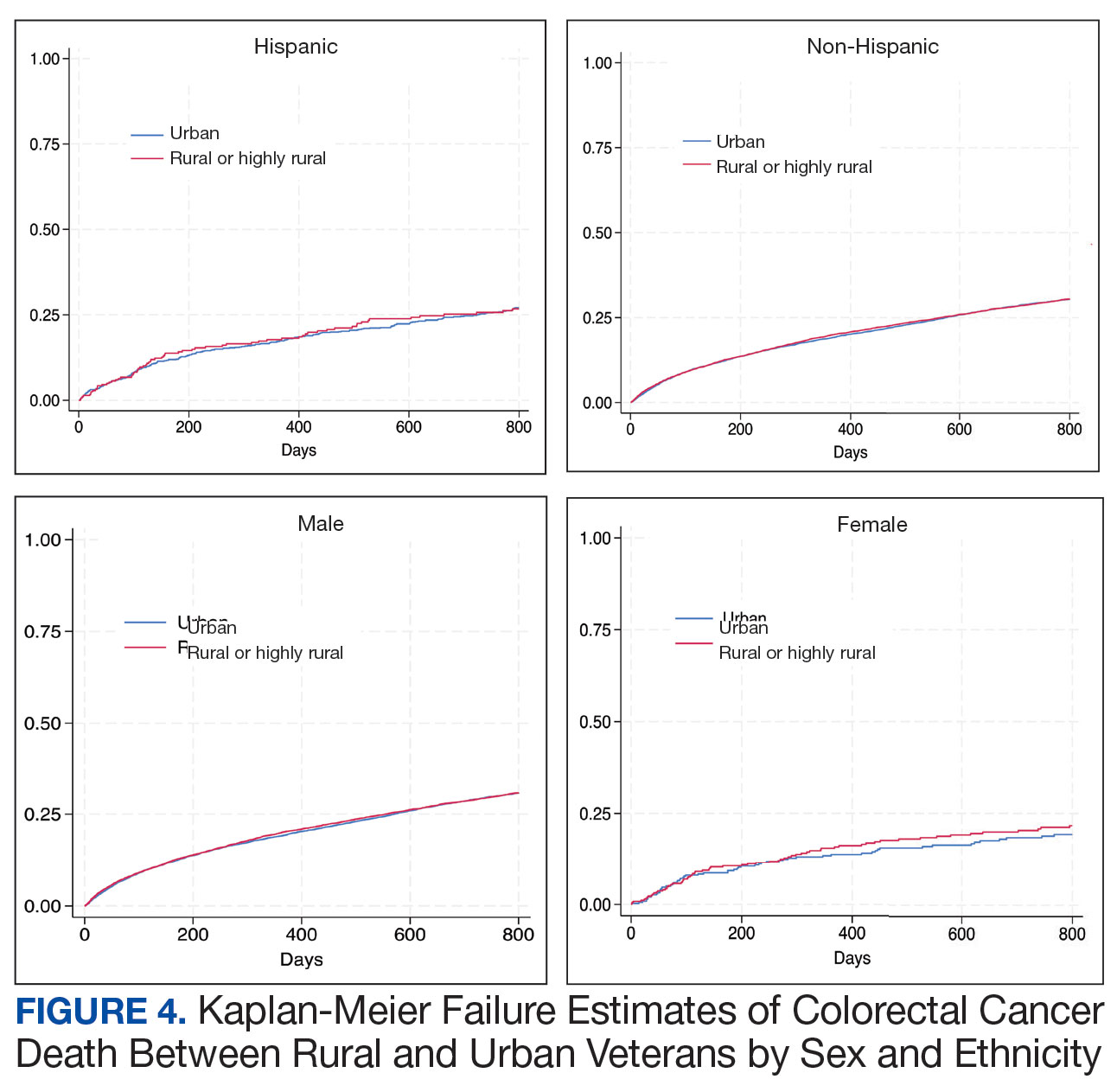
Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).
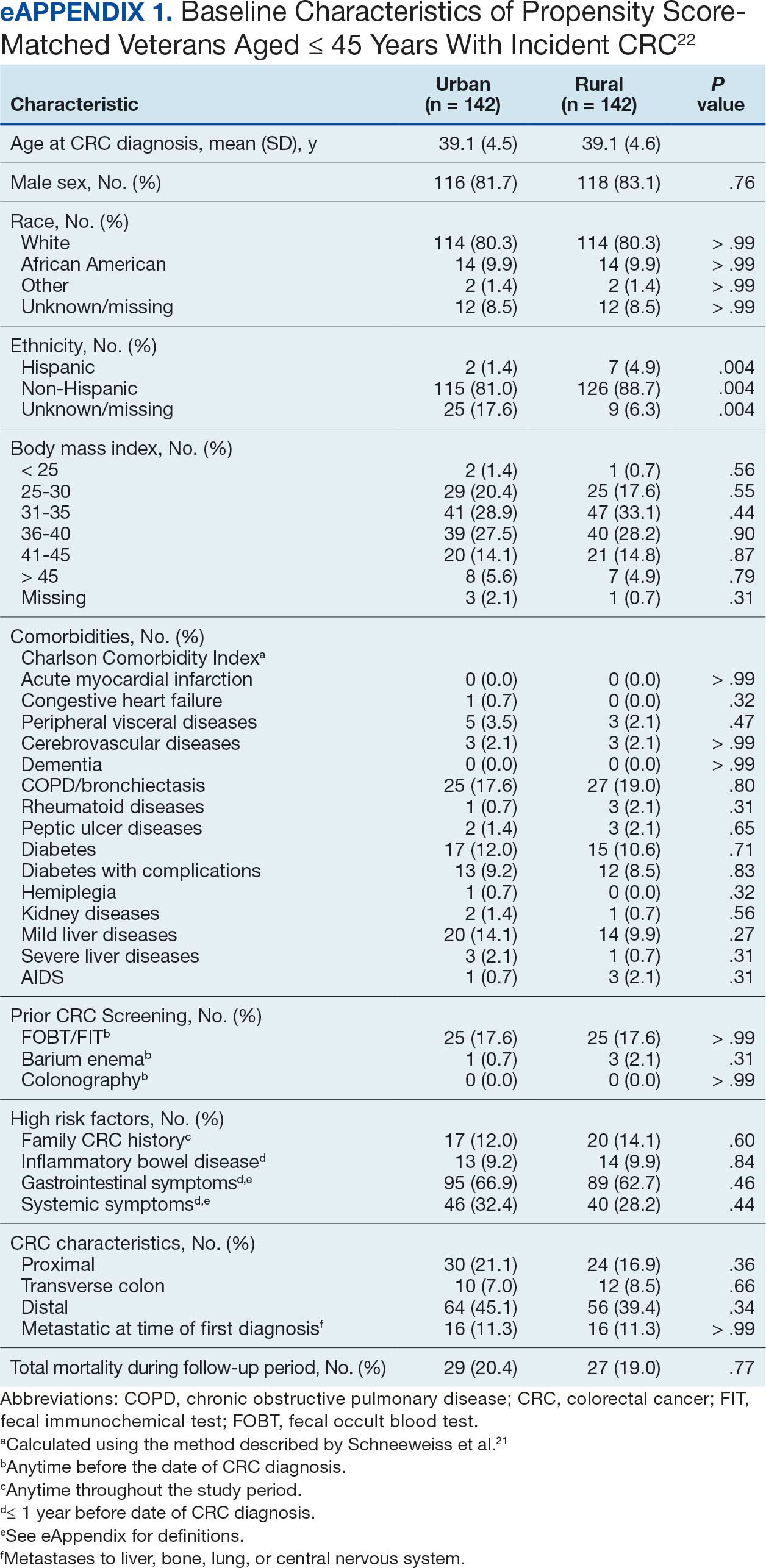
There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).
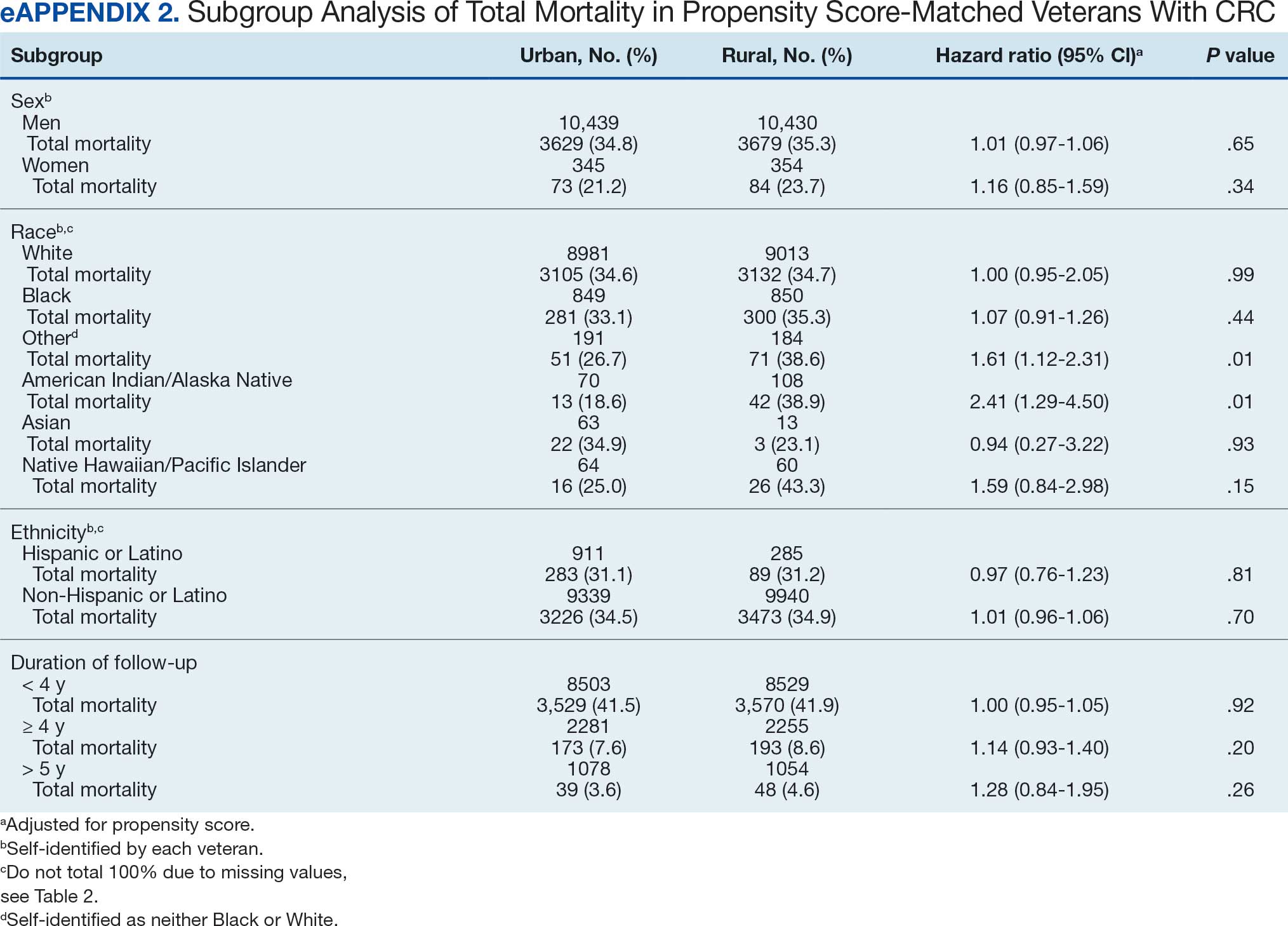
Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).



There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).



Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).

There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).

Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States, with an estimated 52,550 deaths in 2023.1 However, the disease burden varies among different segments of the population.2 While both CRC incidence and mortality have been decreasing due to screening and advances in treatment, there are disparities in incidence and mortality across the sociodemographic spectrum including race, ethnicity, education, and income.1-4 While CRC incidence is decreasing for older adults, it is increasing among those aged < 55 years.5 The incidence of CRC in adults aged 40 to 54 years has increased by 0.5% to 1.3% annually since the mid-1990s.6 The US Preventive Services Task Force now recommends starting CRC screening at age 45 years for asymptomatic adults with average risk.7
Disparities also exist across geographical boundaries and living environment. Rural Americans faces additional challenges in health and lifestyle that can affect CRC outcomes. Compared to their urban counterparts, rural residents are more likely to be older, have lower levels of education, higher levels of poverty, lack health insurance, and less access to health care practitioners (HCPs).8-10 Geographic proximity, defined as travel time or physical distance to a health facility, has been recognized as a predictor of inferior outcomes.11 These aspects of rural living may pose challenges for accessing care for CRC screening and treatment.11-13 National and local studies have shown disparities in CRC screening rates, incidence, and mortality between rural and urban populations.14-16
It is unclear whether rural/urban disparities persist under the Veterans Health Administration (VHA) health care delivery model. This study examined differences in baseline characteristics and mortality between rural and urban veterans newly diagnosed with CRC. We also focused on a subpopulation aged ≤ 45 years.
Methods
This study extracted national data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) hosted in the VA Informatics and Computing Infrastructure (VINCI) environment. VINCI is an initiative to improve access to VA data and facilitate the analysis of these data while ensuring veterans’ privacy and data security.17 CDW is the VHA business intelligence information repository, which extracts data from clinical and nonclinical sources following prescribed and validated protocols. Data extracted included demographics, diagnosis, and procedure codes for both inpatient and outpatient encounters, vital signs, and vital status. This study used data previously extracted from a national cohort of veterans that encompassed all patients who received a group of commonly prescribed medications, such as statins, proton pump inhibitors, histamine-2 blockers, acetaminophen-containing products, and hydrocortisone-containing skin applications. This cohort encompassed 8,648,754 veterans, from whom 2,460,727 had encounters during fiscal years (FY) 2016 to 2021 (study period). The cohort was used to ensure that subjects were VHA patients, allowing them to adequately capture their clinical profiles.
Patients were identified as rural or urban based on their residence address at the date of their first diagnosis of CRC. The Geospatial Service Support Center (GSSC) aggregates and updates veterans’ residence address records for all enrolled veterans from the National Change of Address database. The data contain 1 record per enrollee. GSSC Geocoded Enrollee File contains enrollee addresses and their rurality indicators, categorized as urban, rural, or highly rural.18 Rurality is defined by the Rural Urban Commuting Area (RUCA) categories developed by the Department of Agriculture and the Health Resources and Services Administration of the US Department of Health and Human Services.19 Urban areas had RUCA codes of 1.0 to 1.1, and highly rural areas had RUCA scores of 10.0. All other areas were classified as rural. Since the proportion of veterans from highly rural areas was small, we included residents from highly rural areas in the rural residents’ group.
Inclusion and Exclusion Criteria
All veterans newly diagnosed with CRC from FY 2016 to 2021 were included. We used the ninth and tenth clinical modification revisions of the International Classification of Diseases (ICD-9-CM and ICD-10-CM) to define CRC diagnosis (Supplemental materials).4,20 To ensure that patients were newly diagnosed with CRC, this study excluded patients with a previous ICD-9-CM code for CRC diagnosis since FY 2003.
Comorbidities were identified using diagnosis and procedure codes from inpatient and outpatient encounters, which were used to calculate the Charlson Comorbidity Index (CCI) at the time of CRC diagnosis using the weighted method described by Schneeweiss et al.21 We defined CRC high-risk conditions and CRC screening tests, including flexible sigmoidoscopy and stool tests, as described in previous studies (Supplemental materials).20
The main outcome was total mortality. The date of death was extracted from the VHA Death Ascertainment File, which contains mortality data from the Master Person Index file in CDW and the Social Security Administration Death Master File. We used the date of death from any cause, as cause of death was not available.
A propensity score (PS) was created to match rural (including highly rural) and urban residents at a ratio of 1:1. Using a standard procedure described in prior publications, multivariable logistic regression used all baseline characteristics to estimate the PS and perform nearest-number matching without replacement.22,23 A caliper of 0.01 maximized the matched cohort size and achieved balance (Supplemental materials). We then examined the balance of baseline characteristics between PS-matched groups.
Analyses
Cox proportional hazards regression analysis estimated the hazard ratio (HR) of death in rural residents compared to urban residents in the PS-matched cohort. The outcome event was the date of death during the study’s follow-up period (defined as period from first CRC diagnosis to death or study end), with censoring at the study’s end date (September 30, 2021). The proportional hazards assumption was assessed by inspecting the Kaplan-Meier curves. Multiple analyses examined the HR of total mortality in the PS-matched cohort, stratified by sex, race, and ethnicity. We also examined the HR of total mortality stratified by duration of follow-up.
Another PS-matching analysis among veterans aged ≤ 45 years was performed using the same techniques described earlier in this article. We performed a Cox proportional hazards regression analysis to compare mortality in PS-matched urban and rural veterans aged ≤ 45 years. The HR of death in all veterans aged ≤ 45 years (before PS-matching) was estimated using Cox proportional hazard regression analysis, adjusting for PS.
Dichotomous variables were compared using X2 tests and continuous variables were compared using t tests. Baseline characteristics with missing values were converted into categorical variables and the proportion of subjects with missing values was equalized between treatment groups after PS-matching. For subgroup analysis, we examined the HR of total mortality in each subgroup using separate Cox proportional hazards regression models similar to the primary analysis but adjusted for PS. Due to multiple comparisons in the subgroup analysis, the findings should be considered exploratory. Statistical tests were 2-tailed, and significance was defined as P < .05. Data management and statistical analyses were conducted from June 2022 to January 2023 using STATA, Version 17. The VA Orlando Healthcare System Institutional Review Board approved the study and waived requirements for informed consent because only deidentified data were used.
Results
After excluding 49 patients (Supplemental materials, available at doi:10.12788/fp.0560), we identified 30,219 veterans with newly diagnosed CRC between FY 2016 to 2021 (Table 1). Of these, 19,422 (64.3%) resided in urban areas and 10,797 (35.7%) resided in rural areas (Table 2). The mean (SD) duration from the first CRC diagnosis to death or study end was 832 (640) days, and the median (IQR) was 723 (246–1330) days. Overall, incident CRC diagnoses were numerically highest in FY 2016 and lowest in FY 2020 (Figure 1). Patients with CRC in rural areas vs urban areas were significantly older (mean, 71.2 years vs 70.8 years, respectively; P < .001), more likely to be male (96.7% vs 95.7%, respectively; P < .001), more likely to be White (83.6% vs 67.8%, respectively; P < .001) and more likely to be non-Hispanic (92.2% vs 87.5%, respectively; P < .001). In terms of general health, rural veterans with CRC were more likely to be overweight or obese (81.5% rural vs 78.5% urban; P < .001) but had fewer mean comorbidities as measured by CCI (5.66 rural vs 5.90 urban; P < .001). A higher proportion of rural veterans with CRC had received stool-based (fecal occult blood test or fecal immunochemical test) CRC screening tests (61.6% rural vs 57.2% urban; P < .001). Fewer rural patients presented with systemic symptoms or signs within 1 year of CRC diagnosis (54.4% rural vs 57.5% urban, P < .001). Among urban patients with CRC, 6959 (35.8%) deaths were observed, compared with 3766 (34.9%) among rural patients (P = .10).



There were 21,568 PS-matched veterans: 10,784 in each group. In the PS-matched cohort, baseline characteristics were similar between veterans in urban and rural communities, including age, sex, race/ethnicity, body mass index, and comorbidities. Among rural patients with CRC, 3763 deaths (34.9%) were observed compared with 3702 (34.3%) among urban veterans. There was no significant difference in the HR of mortality between rural and urban CRC residents (HR, 1.01; 95% CI, 0.97-1.06; P = .53) (Figure 2).



Among veterans aged ≤ 45 years, 551 were diagnosed with CRC (391 urban and 160 rural). We PS-matched 142 pairs of urban and rural veterans without residual differences in baseline characteristics (eAppendix 1). There was no significant difference in the HR of mortality between rural and urban veterans aged ≤ 45 years (HR, 0.97; 95% CI, 0.57-1.63; P = .90) (Figure 2). Similarly, no difference in mortality was observed adjusting for PS between all rural and urban veterans aged ≤ 45 years (HR, 1.03; 95% CI, 0.67-1.59; P = .88).

There was no difference in total mortality between rural and urban veterans in any subgroup except for American Indian or Alaska Native veterans (HR, 2.41; 95% CI, 1.29-4.50; P = .006) (eAppendix 2).

Discussion
This study examined characteristics of patients with CRC between urban and rural areas among veterans who were VHA patients. Similar to other studies, rural veterans with CRC were older, more likely to be White, and were obese, but exhibited fewer comorbidities (lower CCI and lower incidence of congestive heart failure, dementia, hemiplegia, kidney diseases, liver diseases and AIDS, but higher incidence of chronic obstructive lung disease).8,16 The incidence of CRC in this study population was lowest in FY 2020, which was reported by the Centers for Disease Control and Prevention and is attributed to COVID-19 pandemic disruption of health services.24 The overall mortality in this study was similar to rates reported in other studies from the VA Central Cancer Registry.4 In the PS-matched cohort, where baseline characteristics were similar between urban and rural patients with CRC, we found no disparities in CRC-specific mortality between veterans in rural and urban areas. Additionally, when analysis was restricted to veterans aged ≤ 45 years, the results remained consistent.
Subgroup analyses showed no significant difference in mortality between rural and urban areas by sex, race or ethnicity, except rural American Indian or Alaska Native veterans who had double the mortality of their urban counterparts (HR, 2.41; 95% CI, 1.29-4.50; P = .006). This finding is difficult to interpret due to the small number of events and the wide CI. While with a Bonferroni correction the adjusted P value was .08, which is not statistically significant, a previous study found that although CRC incidence was lower overall in American Indian or Alaska Native populations compared to non-Hispanic White populations, CRC incidence was higher among American Indian or Alaska Native individuals in some areas such as Alaska and the Northern Plains.25,26 Studies have noted that rural American Indian/Alaska Native populations experience greater poverty, less access to broadband internet, and limited access to care, contributing to poorer cancer outcomes and lower survival.27 Thus, the finding of disparity in mortality between rural and urban American Indian or Alaska Native veterans warrants further study.
Other studies have raised concerns that CRC disproportionately affects adults in rural areas with higher mortality rates.14-16 These disparities arise from sociodemographic factors and modifiable risk factors, including physical activity, dietary patterns, access to cancer screening, and gaps in quality treatment resources.16,28 These factors operate at multiple levels: from individual, local health system, to community and policy.2,27 For example, a South Carolina study (1996–2016) found that residents in rural areas were more likely to be diagnosed with advanced CRC, possibly indicating lower rates of CRC screening in rural areas. They also had higher likelihood of death from CRC.15 However, the study did not include any clinical parameters, such as comorbidities or obesity. A statewide, population-based study in Utah showed that rural men experienced a lower CRC survival in their unadjusted analysis.16 However, the study was small, with only 3948 urban and 712 rural residents. Additionally, there was no difference in total mortality in the whole cohort (HR, 0.96; 95% CI, 0.86-1.07) or in CRC-specific death (HR, 0.93; 95% CI, 0.81-1.08). A nationwide study also showed that CRC mortality rates were 8% higher in nonmetropolitan or rural areas than in the most urbanized areas containing large metropolitan counties.29 However, this study did not include descriptions of clinical confounders, such as comorbidities, making it difficult to ascertain whether the difference in CRC mortality was due to rurality or differences in baseline risk characteristics.
In this study, the lack of CRC-specific mortality disparities may be attributed to the structures and practices of VHA health care. Recent studies have noted that mortality of several chronic medical conditions treated at the VHA was lower than at non-VHA hospitals.30,31 One study that measured the quality of nonmetastatic CRC care based on National Comprehensive Cancer Network guidelines showed that > 72% of VHA patients received guideline-concordant care for each diagnostic and therapeutic measure, except for follow-up colonoscopy timing, which appear to be similar or superior to that of the private sector.30,32,33 Some of the VA initiative for CRC screening may bypass the urban-rurality divide such as the mailed fecal immunochemical test program for CRC. This program was implemented at the onset of the COVID-19 pandemic to avoid disruptions of medical care.34 Rural patients are more likely to undergo fecal immunochemical testing when compared to urban patients in this data. Beyond clinical care, the VHA uses processes to tackle social determinants of health such as housing, food security, and transportation, promoting equal access to health care, and promoting cultural competency among HCPs.35-37
The results suggest that solutions to CRC disparities between rural and urban areas need to consider known barriers to rural health care, including transportation, diminished rural health care workforce, and other social determinants of health.9,10,27,38 VHA makes considerable efforts to provide equitable care to all enrolled veterans, including specific programs for rural veterans, including ongoing outreach.39 This study demonstrated lack of disparity in CRC-specific mortality in veterans receiving VHA care, highlighting the importance of these efforts.
Strengths and Limitations
This study used the VHA cohort to compare patient characteristics and mortality between patients with CRC residing in rural and urban areas. The study provides nationwide perspectives on CRC across the geographical spectrum and used a longitudinal cohort with prolonged follow-up to account for comorbidities.
However, the study compared a cohort of rural and urban veterans enrolled in the VHA; hence, the results may not reflect CRC outcomes in veterans without access to VHA care. Rurality has been independently associated with decreased likelihood of meeting CRC screening guidelines among veterans and military service members.38 This study lacked sufficient information to compare CRC staging or treatment modalities among veterans. Although the data cannot identify CRC stage, the proportions of patients with metastatic CRC at diagnosis and CRC location were similar between groups. The study did not have information on their care outside of VHA setting.
This study could not ascertain whether disparities existed in CRC treatment modality since rural residence may result in referral to community-based CRC care, which did not appear in the data. To address these limitations, we used death from any cause as the primary outcome, since death is a hard outcome and is not subject to ascertainment bias. The relatively short follow-up time is another limitation, though subgroup analysis by follow-up did not show significant differences. Despite PS matching, residual unmeasured confounding may exist between urban and rural groups. The predominantly White, male VHA population with high CCI may limit the generalizability of the results.
Conclusions
Rural VHA enrollees had similar survival rates after CRC diagnosis compared to their urban counterparts in a PS-matched analysis. The VHA models of care—including mailed CRC screening tools, several socioeconomic determinants of health (housing, food security, and transportation), and promoting equal access to health care, as well as cultural competency among HCPs—HCPs—may help alleviate disparities across the rural-urban spectrum. The VHA should continue efforts to enroll veterans and provide comprehensive coordinated care in community partnerships.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi:10.1053/j.gastro.2019.10.029
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668-75. doi:10.1002/ijc.25481
- Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs central cancer registry. Clin Colorectal Cancer. 2016;15(4):e199-e204. doi:10.1016/j.clcc.2016.04.005
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force. 2021. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews:Chapter 1. Agency for Healthcare Research and Quality (US); 2021. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK570917/
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw322
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
- Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63-e71. doi:10.2105/AJPH.2013.301572
- Cauwels J. The many barriers to high-quality rural health care. 2022;(9):1-32. NEJM Catal Innov Care Deliv. Accessed April 24, 2025. https://catalyst.nejm.org/doi/pdf/10.1056/CAT.22.0254
- Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Aff (Millwood);38(12):2003-2010. doi:10.1377/hlthaff.2019.00722
- Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg. 2014;149(6):537-543. doi:10.1001/jamasurg.2013.5062
- Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131-138. doi:10.1016/s1040-8428(01)00139-1
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341-378. doi:10.1146/annurev.publhealth.18.1.341
- Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
- Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996 - 2016. J Rural Health. 2022;38(1):34-39. doi:10.1111/jrh.12580
- Rogers CR, Blackburn BE, Huntington M, et al. Rural- urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes Control. 2020;31(3):241-253. doi:10.1007/s10552-020-01268-2
- US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. https://vincicentral.vinci.med.va.gov [Source not verified]
- US Department of Veterans Affairs Information Resource Center. VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition. US Department of Veterans Affairs, Health Services Research & Development Service, Information Resource Center; May. 2016. [source not verified]
- Goldsmith HF, Puskin DS, Stiles DJ. Improving the operational definition of “rural areas” for federal programs. US Department of Health and Human Services; 1993. Accessed February 27, 2025. https://www.ruralhealthinfo.org/pdf/improving-the-operational-definition-of-rural-areas.pdf
- Adams MA, Kerr EA, Dominitz JA, et al. Development and validation of a new ICD-10-based screening colonoscopy overuse measure in a large integrated healthcare system: a retrospective observational study. BMJ Qual Saf. 2023;32(7):414-424. doi:10.1136/bmjqs-2021-014236
- Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103-1120. doi:10.1111/1475-6773.00165
- Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2(4):358-377.
- Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical software components. Revised February 1, 2018. Accessed February 27, 2025. https://ideas.repec.org/c/boc/bocode/s432001.html.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool. Centers for Disease Control and Prevention. June 2024. Accessed February 27, 2025. https://www.cdc.gov/cancer/dataviz
- Cao J, Zhang S. Multiple Comparison Procedures. JAMA. 2014;312(5):543-544. doi:10.1001/jama.2014.9440
- Gopalani SV, Janitz AE, Martinez SA, et al. Trends in cancer incidence among American Indians and Alaska Natives and Non-Hispanic Whites in the United States, 1999-2015. Epidemiology. 2020;31(2):205-213. doi:10.1097/EDE.0000000000001140
- Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4). doi:10.3390/ijerph18041384
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi:10.1158/1055-9965.EPI-17-0092
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part iicolorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi:10.1155/2011/107497
- Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181. doi:10.1200/JCO.2009.26.7948
- Yoon J, Phibbs CS, Ong MK, et al. Outcomes of veterans treated in Veterans Affairs hospitals vs non-Veterans Affairs hospitals. JAMA Netw Open. 2023;6(12):e2345898. doi:10.1001/jamanetworkopen.2023.45898
- Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626-634. doi:10.1200/JCO.2005.03.3365
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595. doi:10.1053/j.gastro.2008.02.002
- Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4). doi:10.1136/bmjoq-2022-001927
- Yehia BR, Greenstone CL, Hosenfeld CB, Matthews KL, Zephyrin LC. The role of VA community care in addressing health and health care disparities. Med Care. 2017;55(Suppl 9 suppl 2):S4-S5. doi:10.1097/MLR.0000000000000768
- Wright BN, MacDermid Wadsworth S, Wellnitz A, Eicher- Miller HA. Reaching rural veterans: a new mechanism to connect rural, low-income US Veterans with resources and improve food security. J Public Health (Oxf). 2019;41(4):714-723. doi:10.1093/pubmed/fdy203
- Nelson RE, Byrne TH, Suo Y, et al. Association of temporary financial assistance with housing stability among US veterans in the supportive services for veteran families program. JAMA Netw Open. 2021;4(2):e2037047. doi:10.1001/jamanetworkopen.2020.37047
- McDaniel JT, Albright D, Lee HY, et al. Rural–urban disparities in colorectal cancer screening among military service members and Veterans. J Mil Veteran Fam Health. 2019;5(1):40-48. doi:10.3138/jmvfh.2018-0013
- US Department of Veterans Affairs, Office of Rural Health. The rural veteran outreach toolkit. Updated February 12, 2025. Accessed February 18, 2025. https://www.ruralhealth.va.gov/partners/toolkit.asp
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans
Colorectal Cancer Characteristics and Mortality From Propensity Score-Matched Cohorts of Urban and Rural Veterans