User login
Team identifies potential biomarkers for AML therapy

Photo by Kristin Gladney
New research published in Cell Reports has revealed biomarkers that may help predict which acute myeloid leukemia (AML) patients will respond to treatment with PU-H71.
This drug targets a tumor-enriched form of the protein Hsp90 called teHSP90, which is critical to the growth of cancer cells.
However, previous preclinical experiments showed that PU-H71 only kills some AML cells.
“We observed that only a subset of leukemia patient samples were sensitive to the drug,” said study author Monica Guzman, PhD, of Weill Cornell Medical College in New York, New York.
“We wanted to be able to identify which patients with leukemia would respond to this drug.”
So Dr Guzman and her colleagues focused on groups of proteins that function within signaling networks in leukemia cells.
The researchers found that 2 of these networks, JAK-STAT and PI3K-AKT, were important for leukemia cells to function. These signaling pathways are critical for the survival of AML cells, and they, in turn, are dependent on teHsp90.
The team treated AML cells with PU-H71 and found that cells with greater JAK-STAT and PI3K-AKT activity were killed by the drug. Cells with less active signaling networks did not respond to PU-H71.
“Higher activation of these networks makes the leukemia cells more dependent on Hsp90,” Dr Guzman said. “Since PU-H71 targets teHsp90, leukemia samples with these features are good targets for treatment with the drug.”
The next step for Dr Guzman’s team is to test their findings in patients. Ideally, the JAK-STAT and PI3K-AKT signaling pathways will serve as biomarkers for identifying patients whose leukemias will be sensitive to PU-H71.
“We are working on a tool that will quickly and easily identify patients whose cancers will respond to PU-H71,” Dr Guzman said. “We are really looking forward to seeing this in leukemic patients and being able to offer them a new treatment.” ![]()

Photo by Kristin Gladney
New research published in Cell Reports has revealed biomarkers that may help predict which acute myeloid leukemia (AML) patients will respond to treatment with PU-H71.
This drug targets a tumor-enriched form of the protein Hsp90 called teHSP90, which is critical to the growth of cancer cells.
However, previous preclinical experiments showed that PU-H71 only kills some AML cells.
“We observed that only a subset of leukemia patient samples were sensitive to the drug,” said study author Monica Guzman, PhD, of Weill Cornell Medical College in New York, New York.
“We wanted to be able to identify which patients with leukemia would respond to this drug.”
So Dr Guzman and her colleagues focused on groups of proteins that function within signaling networks in leukemia cells.
The researchers found that 2 of these networks, JAK-STAT and PI3K-AKT, were important for leukemia cells to function. These signaling pathways are critical for the survival of AML cells, and they, in turn, are dependent on teHsp90.
The team treated AML cells with PU-H71 and found that cells with greater JAK-STAT and PI3K-AKT activity were killed by the drug. Cells with less active signaling networks did not respond to PU-H71.
“Higher activation of these networks makes the leukemia cells more dependent on Hsp90,” Dr Guzman said. “Since PU-H71 targets teHsp90, leukemia samples with these features are good targets for treatment with the drug.”
The next step for Dr Guzman’s team is to test their findings in patients. Ideally, the JAK-STAT and PI3K-AKT signaling pathways will serve as biomarkers for identifying patients whose leukemias will be sensitive to PU-H71.
“We are working on a tool that will quickly and easily identify patients whose cancers will respond to PU-H71,” Dr Guzman said. “We are really looking forward to seeing this in leukemic patients and being able to offer them a new treatment.” ![]()

Photo by Kristin Gladney
New research published in Cell Reports has revealed biomarkers that may help predict which acute myeloid leukemia (AML) patients will respond to treatment with PU-H71.
This drug targets a tumor-enriched form of the protein Hsp90 called teHSP90, which is critical to the growth of cancer cells.
However, previous preclinical experiments showed that PU-H71 only kills some AML cells.
“We observed that only a subset of leukemia patient samples were sensitive to the drug,” said study author Monica Guzman, PhD, of Weill Cornell Medical College in New York, New York.
“We wanted to be able to identify which patients with leukemia would respond to this drug.”
So Dr Guzman and her colleagues focused on groups of proteins that function within signaling networks in leukemia cells.
The researchers found that 2 of these networks, JAK-STAT and PI3K-AKT, were important for leukemia cells to function. These signaling pathways are critical for the survival of AML cells, and they, in turn, are dependent on teHsp90.
The team treated AML cells with PU-H71 and found that cells with greater JAK-STAT and PI3K-AKT activity were killed by the drug. Cells with less active signaling networks did not respond to PU-H71.
“Higher activation of these networks makes the leukemia cells more dependent on Hsp90,” Dr Guzman said. “Since PU-H71 targets teHsp90, leukemia samples with these features are good targets for treatment with the drug.”
The next step for Dr Guzman’s team is to test their findings in patients. Ideally, the JAK-STAT and PI3K-AKT signaling pathways will serve as biomarkers for identifying patients whose leukemias will be sensitive to PU-H71.
“We are working on a tool that will quickly and easily identify patients whose cancers will respond to PU-H71,” Dr Guzman said. “We are really looking forward to seeing this in leukemic patients and being able to offer them a new treatment.” ![]()
Expert panel issues guidelines for treatment of hematologic cancers in pregnancy
Consensus guidelines for the perinatal management of hematologic malignancies detected during pregnancy have been issued by a panel of international experts.
The guidelines, published online in the Journal of Clinical Oncology, aim to ensure that timely treatment of the cancers is not delayed in pregnant women (doi: 10.1200/JCO.2015.62.4445).
While rare, hematologic malignancies in pregnancy introduce clinical, social, ethical, and moral dilemmas. Evidence-based data are scarce, according to the researchers, who note the International Network on Cancer, Infertility and Pregnancy registers all cancers occurring during gestation.
“Patient accrual is ongoing and essential, because registration of new cases and long-term follow-up will improve clinical knowledge and increase the level of evidence,” Dr. Michael Lishner of Tel Aviv University and Meir Medical Center, Kfar Saba, Israel, and his fellow panelists wrote.
Hodgkin lymphoma
The researchers note that Hodgkin lymphoma is the most common hematologic cancer in pregnancy, and the prognosis for these patients is excellent. When diagnosed during the first trimester, a regimen based on vinblastine monotherapy has been used. ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy can be used postpartum and has been used in cases of progression during pregnancy, the panelists wrote.
“The limited data available suggest that ABVD may be administered safely and effectively during the latter phases of pregnancy,” the panel wrote. “Although it may be associated with prematurity and lower birth weights, studies have not reported significant disadvantages.”
Non-Hodgkin lymphoma
The second most common cancer in pregnancy is non-Hodgkin lymphoma. In the case of indolent disease, watchful waiting is possible, with the intent to treat with monoclonal antibodies – with or without chemotherapy – if symptoms or evidence of disease progression are noted. Steroids can be administered during the first trimester as a bridge to the second trimester, when chemotherapy can be used with relatively greater safety, the panelists noted.
Aggressive lymphomas diagnosed before 20 weeks’ gestation warrant pregnancy termination and treatment, they recommend. When diagnosed after 20 weeks, therapy should be comparable to that given a nonpregnant woman, including monoclonal antibodies (R-CHOP).
Chronic myeloid leukemia
Chronic myeloid leukemia occurs in approximately 1 in 100,000 pregnancies and is typically diagnosed during routine blood testing in an asymptomatic patient. As a result, treatment may not be needed until the patient’s white count or platelet count have risen to levels associated with the onset of symptoms. An approximate guideline is a white cell count greater than 100 X 109/L and a platelet count greater than 500 X 109/L.
Therapeutic approaches in pregnancy include interferon-a (INF-a), which does not inhibit DNA synthesis or readily cross the placenta, and leukapheresis, which is frequently required two to three times per week during the first and second trimesters. Counts tend to drop during the third trimester, allowing less frequent intervention.
Consideration should be given to adding aspirin or low-molecular-weight heparin (LMWH) when the platelet count exceeds 1,000 X 109/L.
Myeloproliferative neoplasms
The most common myeloproliferative neoplasm seen in women of childbearing age is essential thrombocytosis.
“A large meta-analysis of pregnant women with essential thrombocytosis reported a live birth rate of 50%-70%, first trimester loss in 25%-40%, late pregnancy loss in 10%, placental abruption in 3.6%, and intrauterine growth restriction in 4.5%. Maternal morbidity is rare, but stroke has been reported,” according to the panelists.
Limited literature suggests similar outcomes for pregnant women with polycythemia vera and primary myelofibrosis.
In low-risk pregnancies, aspirin (75 mg/day) should be offered unless clearly contraindicated. For women with polycythemia vera, venesection may be continued when tolerated to maintain the hematocrit within the gestation-appropriate range.
Fetal ultrasound scans should be performed at 20, 26, and 34 weeks of gestation and uterine artery Doppler should be performed at 20 weeks’ gestation. If the mean pulsatility index exceeds 1.4, the pregnancy may be considered high risk, and treatment and monitoring should be increased.
In high-risk pregnancies, additional treatment includes cytoreductive therapy with or without LMWH. If cytoreductive therapy is required, INF-a should be titrated to maintain a platelet count of less than 400 X 109/L and hematocrit within appropriate range.
Local protocols regarding interruption of LMWH should be adhered to during labor, and dehydration should be avoided. Platelet count and hematocrit may increase postpartum, requiring cytoreductive therapy. Thromboprophylaxis should be considered at 6 weeks’ postpartum because of the increased risk of thrombosis, the guidelines note.
Acute leukemia
“The remarkable anemia, thrombocytopenia, and neutropenia that characterize acute myeloid and lymphoblastic leukemia” require prompt treatment. Leukapheresis in the presence of clinically significant evidence of leukostasis can be considered, regardless of gestational stage. When patients are diagnosed with acute myeloid leukemia during the first trimester, pregnancy termination followed by conventional induction therapy (cytarabine/anthracycline) is recommended.
Those diagnosed later in pregnancy can receive conventional induction therapy, although this seems to be associated with increased risk of fetal growth restriction and even fetal loss. “Notably, neonates rarely experience neutropenia and cardiac impairment unless exposed to lipophilic idarubicin, which should not be used,” the panelists wrote.
When acute promyelocytic leukemia is diagnosed in the first trimester, pregnancy termination is recommended before initiating conventional ATRA-anthracycline therapy. Later in pregnancy, the regimen demonstrates low teratogenicity and can be used in women diagnosed after that stage. Arsenic treatment is highly teratogenic and is prohibited throughout gestation.
Acute lymphocytic leukemia (ALL) requires prophylactic CNS therapy, including methotrexate and L-asparaginase, which are fetotoxic. Methotrexate interferes with organogenesis and is prohibited before week 20 of gestation. L-asparaginase may increase the high risk for thromboembolic events attributed to the combination of pregnancy and malignancy.
Notably, tyrosine kinase inhibitors, essential for patients with Philadelphia chromosome–positive ALL, are teratogenic. Given these limitations, women diagnosed with ALL before 20 weeks’ gestation should undergo termination of the pregnancy and start conventional treatment. After week 20, conventional chemotherapy can be administered during pregnancy. Tyrosine kinase inhibitors can be initiated postpartum.
The guidelines also contain recommendations on diagnostic testing and radiotherapy, maternal supportive care, and perinatal and pediatric aspects of hematologic malignancies in pregnancy. An online appendix offers recommendations on the treatment of rare hematologic malignancies, including hairy cell leukemia, multiple myeloma, and myelodysplastic syndromes.
Dr. Lishner and nine of his coauthors had no financial relationships to disclose. Three coauthors received honoraria and research funding or are consultants to a wide variety of drug makers.
On Twitter @maryjodales
Consensus guidelines for the perinatal management of hematologic malignancies detected during pregnancy have been issued by a panel of international experts.
The guidelines, published online in the Journal of Clinical Oncology, aim to ensure that timely treatment of the cancers is not delayed in pregnant women (doi: 10.1200/JCO.2015.62.4445).
While rare, hematologic malignancies in pregnancy introduce clinical, social, ethical, and moral dilemmas. Evidence-based data are scarce, according to the researchers, who note the International Network on Cancer, Infertility and Pregnancy registers all cancers occurring during gestation.
“Patient accrual is ongoing and essential, because registration of new cases and long-term follow-up will improve clinical knowledge and increase the level of evidence,” Dr. Michael Lishner of Tel Aviv University and Meir Medical Center, Kfar Saba, Israel, and his fellow panelists wrote.
Hodgkin lymphoma
The researchers note that Hodgkin lymphoma is the most common hematologic cancer in pregnancy, and the prognosis for these patients is excellent. When diagnosed during the first trimester, a regimen based on vinblastine monotherapy has been used. ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy can be used postpartum and has been used in cases of progression during pregnancy, the panelists wrote.
“The limited data available suggest that ABVD may be administered safely and effectively during the latter phases of pregnancy,” the panel wrote. “Although it may be associated with prematurity and lower birth weights, studies have not reported significant disadvantages.”
Non-Hodgkin lymphoma
The second most common cancer in pregnancy is non-Hodgkin lymphoma. In the case of indolent disease, watchful waiting is possible, with the intent to treat with monoclonal antibodies – with or without chemotherapy – if symptoms or evidence of disease progression are noted. Steroids can be administered during the first trimester as a bridge to the second trimester, when chemotherapy can be used with relatively greater safety, the panelists noted.
Aggressive lymphomas diagnosed before 20 weeks’ gestation warrant pregnancy termination and treatment, they recommend. When diagnosed after 20 weeks, therapy should be comparable to that given a nonpregnant woman, including monoclonal antibodies (R-CHOP).
Chronic myeloid leukemia
Chronic myeloid leukemia occurs in approximately 1 in 100,000 pregnancies and is typically diagnosed during routine blood testing in an asymptomatic patient. As a result, treatment may not be needed until the patient’s white count or platelet count have risen to levels associated with the onset of symptoms. An approximate guideline is a white cell count greater than 100 X 109/L and a platelet count greater than 500 X 109/L.
Therapeutic approaches in pregnancy include interferon-a (INF-a), which does not inhibit DNA synthesis or readily cross the placenta, and leukapheresis, which is frequently required two to three times per week during the first and second trimesters. Counts tend to drop during the third trimester, allowing less frequent intervention.
Consideration should be given to adding aspirin or low-molecular-weight heparin (LMWH) when the platelet count exceeds 1,000 X 109/L.
Myeloproliferative neoplasms
The most common myeloproliferative neoplasm seen in women of childbearing age is essential thrombocytosis.
“A large meta-analysis of pregnant women with essential thrombocytosis reported a live birth rate of 50%-70%, first trimester loss in 25%-40%, late pregnancy loss in 10%, placental abruption in 3.6%, and intrauterine growth restriction in 4.5%. Maternal morbidity is rare, but stroke has been reported,” according to the panelists.
Limited literature suggests similar outcomes for pregnant women with polycythemia vera and primary myelofibrosis.
In low-risk pregnancies, aspirin (75 mg/day) should be offered unless clearly contraindicated. For women with polycythemia vera, venesection may be continued when tolerated to maintain the hematocrit within the gestation-appropriate range.
Fetal ultrasound scans should be performed at 20, 26, and 34 weeks of gestation and uterine artery Doppler should be performed at 20 weeks’ gestation. If the mean pulsatility index exceeds 1.4, the pregnancy may be considered high risk, and treatment and monitoring should be increased.
In high-risk pregnancies, additional treatment includes cytoreductive therapy with or without LMWH. If cytoreductive therapy is required, INF-a should be titrated to maintain a platelet count of less than 400 X 109/L and hematocrit within appropriate range.
Local protocols regarding interruption of LMWH should be adhered to during labor, and dehydration should be avoided. Platelet count and hematocrit may increase postpartum, requiring cytoreductive therapy. Thromboprophylaxis should be considered at 6 weeks’ postpartum because of the increased risk of thrombosis, the guidelines note.
Acute leukemia
“The remarkable anemia, thrombocytopenia, and neutropenia that characterize acute myeloid and lymphoblastic leukemia” require prompt treatment. Leukapheresis in the presence of clinically significant evidence of leukostasis can be considered, regardless of gestational stage. When patients are diagnosed with acute myeloid leukemia during the first trimester, pregnancy termination followed by conventional induction therapy (cytarabine/anthracycline) is recommended.
Those diagnosed later in pregnancy can receive conventional induction therapy, although this seems to be associated with increased risk of fetal growth restriction and even fetal loss. “Notably, neonates rarely experience neutropenia and cardiac impairment unless exposed to lipophilic idarubicin, which should not be used,” the panelists wrote.
When acute promyelocytic leukemia is diagnosed in the first trimester, pregnancy termination is recommended before initiating conventional ATRA-anthracycline therapy. Later in pregnancy, the regimen demonstrates low teratogenicity and can be used in women diagnosed after that stage. Arsenic treatment is highly teratogenic and is prohibited throughout gestation.
Acute lymphocytic leukemia (ALL) requires prophylactic CNS therapy, including methotrexate and L-asparaginase, which are fetotoxic. Methotrexate interferes with organogenesis and is prohibited before week 20 of gestation. L-asparaginase may increase the high risk for thromboembolic events attributed to the combination of pregnancy and malignancy.
Notably, tyrosine kinase inhibitors, essential for patients with Philadelphia chromosome–positive ALL, are teratogenic. Given these limitations, women diagnosed with ALL before 20 weeks’ gestation should undergo termination of the pregnancy and start conventional treatment. After week 20, conventional chemotherapy can be administered during pregnancy. Tyrosine kinase inhibitors can be initiated postpartum.
The guidelines also contain recommendations on diagnostic testing and radiotherapy, maternal supportive care, and perinatal and pediatric aspects of hematologic malignancies in pregnancy. An online appendix offers recommendations on the treatment of rare hematologic malignancies, including hairy cell leukemia, multiple myeloma, and myelodysplastic syndromes.
Dr. Lishner and nine of his coauthors had no financial relationships to disclose. Three coauthors received honoraria and research funding or are consultants to a wide variety of drug makers.
On Twitter @maryjodales
Consensus guidelines for the perinatal management of hematologic malignancies detected during pregnancy have been issued by a panel of international experts.
The guidelines, published online in the Journal of Clinical Oncology, aim to ensure that timely treatment of the cancers is not delayed in pregnant women (doi: 10.1200/JCO.2015.62.4445).
While rare, hematologic malignancies in pregnancy introduce clinical, social, ethical, and moral dilemmas. Evidence-based data are scarce, according to the researchers, who note the International Network on Cancer, Infertility and Pregnancy registers all cancers occurring during gestation.
“Patient accrual is ongoing and essential, because registration of new cases and long-term follow-up will improve clinical knowledge and increase the level of evidence,” Dr. Michael Lishner of Tel Aviv University and Meir Medical Center, Kfar Saba, Israel, and his fellow panelists wrote.
Hodgkin lymphoma
The researchers note that Hodgkin lymphoma is the most common hematologic cancer in pregnancy, and the prognosis for these patients is excellent. When diagnosed during the first trimester, a regimen based on vinblastine monotherapy has been used. ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy can be used postpartum and has been used in cases of progression during pregnancy, the panelists wrote.
“The limited data available suggest that ABVD may be administered safely and effectively during the latter phases of pregnancy,” the panel wrote. “Although it may be associated with prematurity and lower birth weights, studies have not reported significant disadvantages.”
Non-Hodgkin lymphoma
The second most common cancer in pregnancy is non-Hodgkin lymphoma. In the case of indolent disease, watchful waiting is possible, with the intent to treat with monoclonal antibodies – with or without chemotherapy – if symptoms or evidence of disease progression are noted. Steroids can be administered during the first trimester as a bridge to the second trimester, when chemotherapy can be used with relatively greater safety, the panelists noted.
Aggressive lymphomas diagnosed before 20 weeks’ gestation warrant pregnancy termination and treatment, they recommend. When diagnosed after 20 weeks, therapy should be comparable to that given a nonpregnant woman, including monoclonal antibodies (R-CHOP).
Chronic myeloid leukemia
Chronic myeloid leukemia occurs in approximately 1 in 100,000 pregnancies and is typically diagnosed during routine blood testing in an asymptomatic patient. As a result, treatment may not be needed until the patient’s white count or platelet count have risen to levels associated with the onset of symptoms. An approximate guideline is a white cell count greater than 100 X 109/L and a platelet count greater than 500 X 109/L.
Therapeutic approaches in pregnancy include interferon-a (INF-a), which does not inhibit DNA synthesis or readily cross the placenta, and leukapheresis, which is frequently required two to three times per week during the first and second trimesters. Counts tend to drop during the third trimester, allowing less frequent intervention.
Consideration should be given to adding aspirin or low-molecular-weight heparin (LMWH) when the platelet count exceeds 1,000 X 109/L.
Myeloproliferative neoplasms
The most common myeloproliferative neoplasm seen in women of childbearing age is essential thrombocytosis.
“A large meta-analysis of pregnant women with essential thrombocytosis reported a live birth rate of 50%-70%, first trimester loss in 25%-40%, late pregnancy loss in 10%, placental abruption in 3.6%, and intrauterine growth restriction in 4.5%. Maternal morbidity is rare, but stroke has been reported,” according to the panelists.
Limited literature suggests similar outcomes for pregnant women with polycythemia vera and primary myelofibrosis.
In low-risk pregnancies, aspirin (75 mg/day) should be offered unless clearly contraindicated. For women with polycythemia vera, venesection may be continued when tolerated to maintain the hematocrit within the gestation-appropriate range.
Fetal ultrasound scans should be performed at 20, 26, and 34 weeks of gestation and uterine artery Doppler should be performed at 20 weeks’ gestation. If the mean pulsatility index exceeds 1.4, the pregnancy may be considered high risk, and treatment and monitoring should be increased.
In high-risk pregnancies, additional treatment includes cytoreductive therapy with or without LMWH. If cytoreductive therapy is required, INF-a should be titrated to maintain a platelet count of less than 400 X 109/L and hematocrit within appropriate range.
Local protocols regarding interruption of LMWH should be adhered to during labor, and dehydration should be avoided. Platelet count and hematocrit may increase postpartum, requiring cytoreductive therapy. Thromboprophylaxis should be considered at 6 weeks’ postpartum because of the increased risk of thrombosis, the guidelines note.
Acute leukemia
“The remarkable anemia, thrombocytopenia, and neutropenia that characterize acute myeloid and lymphoblastic leukemia” require prompt treatment. Leukapheresis in the presence of clinically significant evidence of leukostasis can be considered, regardless of gestational stage. When patients are diagnosed with acute myeloid leukemia during the first trimester, pregnancy termination followed by conventional induction therapy (cytarabine/anthracycline) is recommended.
Those diagnosed later in pregnancy can receive conventional induction therapy, although this seems to be associated with increased risk of fetal growth restriction and even fetal loss. “Notably, neonates rarely experience neutropenia and cardiac impairment unless exposed to lipophilic idarubicin, which should not be used,” the panelists wrote.
When acute promyelocytic leukemia is diagnosed in the first trimester, pregnancy termination is recommended before initiating conventional ATRA-anthracycline therapy. Later in pregnancy, the regimen demonstrates low teratogenicity and can be used in women diagnosed after that stage. Arsenic treatment is highly teratogenic and is prohibited throughout gestation.
Acute lymphocytic leukemia (ALL) requires prophylactic CNS therapy, including methotrexate and L-asparaginase, which are fetotoxic. Methotrexate interferes with organogenesis and is prohibited before week 20 of gestation. L-asparaginase may increase the high risk for thromboembolic events attributed to the combination of pregnancy and malignancy.
Notably, tyrosine kinase inhibitors, essential for patients with Philadelphia chromosome–positive ALL, are teratogenic. Given these limitations, women diagnosed with ALL before 20 weeks’ gestation should undergo termination of the pregnancy and start conventional treatment. After week 20, conventional chemotherapy can be administered during pregnancy. Tyrosine kinase inhibitors can be initiated postpartum.
The guidelines also contain recommendations on diagnostic testing and radiotherapy, maternal supportive care, and perinatal and pediatric aspects of hematologic malignancies in pregnancy. An online appendix offers recommendations on the treatment of rare hematologic malignancies, including hairy cell leukemia, multiple myeloma, and myelodysplastic syndromes.
Dr. Lishner and nine of his coauthors had no financial relationships to disclose. Three coauthors received honoraria and research funding or are consultants to a wide variety of drug makers.
On Twitter @maryjodales
FROM JOURNAL OF CLINICAL ONCOLOGY
A different approach to AML treatment

Image by Tom Ellenberger
PARP inhibitors could prove useful for treating aggressive acute myeloid leukemia (AML), according to preclinical research published in Nature Medicine.
Experiments showed that AML driven by leukemia-associated transcription factors, including AML1-ETO and PML-RARα, was “extremely sensitive” to PARP inhibition.
Mixed-lineage leukemia (MLL) was initially resistant to treatment with PARP inhibitors, but inhibiting Hoxa9 changed that.
“PARP inhibitors could potentially offer a completely different approach to specifically target certain subtypes of acute myeloid leukemia,” said study author Chi Wai Eric So, PhD, of King’s College London in the UK.
He and his colleagues first tested the PARP inhibitor olaparib on primary mouse hematopoietic cells transformed by the leukemia-associated transcription factors AML1-ETO, PML-RARα, MLL-AF9, and E2A-PBX.
The drug suppressed the colony-forming ability of cells transformed by AML1-ETO or PML-RARα by about 90% but had little impact on MLL-AF9- or E2A-PBX-transformed cells. Olaparib also had minimal effects on normal bone marrow.
The investigators observed similar results in experiments with the PARP inhibitor veliparib.
They then tested PARP inhibitors in patient-derived leukemic cell lines carrying AML1-ETO (Kasumi), mutated PML-RARα that is resistant to standard all-trans retinoic acid treatment (NB4-LR2), or MLL-AF9 (THP1).
Similar to the previous experiments, PARP inhibition reduced the colony-forming ability of Kasumi and NB4-LR2 cells but not THP1 cells.
Next, the investigators transplanted the 3 cell lines into immunocompromised mice and treated the mice with olaparib. The drug delayed disease onset and prolonged survival in both the Kasumi and NB4-LR2 models but had no effect on disease onset or survival in the THP1 model.
Dr So and his colleagues said PARP inhibition was likely effective in AML driven by AML1-ETO and PML-RARα because of the suppressed expression of key homologous recombination-associated genes and the compromised DNA-damage response observed in these malignancies.
The team noted that PARP has proven critical in a variety of DNA repair processes, including as a sensor of single-strand breaks in base-excision repair, as a mediator for restarting stalled replication forks of homologous recombination-mediated double-strand break repair, and as a means of preventing the binding of Ku proteins to DNA ends in non-homologous end-joining pathways.
It therefore follows that MLL-driven AML was resistant to treatment with PARP inhibitors because DNA repair is not suppressed in this malignancy. The Hoxa9 protein activates other DNA damage repair pathways in these leukemia cells, keeping them alive.
So the investigators tested drugs that could (indirectly) inhibit Hoxa9—the GSK3 inhibitors LiCl and Li2CO3—in combination with olaparib. Both in vitro and in vivo, treatment with a GSK3 inhibitor and olaparib proved effective against MLL-driven AML.
“While this approach seems promising from these laboratory studies, it is still very early days,” said Matt Kaiser, PhD, head of research at Bloodwise, a charity that helped fund this study.
“We need to see how real patients respond to these drugs and how effective they are. PARP inhibitors such as olaparib are already being used to treat patients with other cancers and have been shown to be safe. This should speed up the time it takes for this potentially effective new treatment to enter clinical trials.” ![]()

Image by Tom Ellenberger
PARP inhibitors could prove useful for treating aggressive acute myeloid leukemia (AML), according to preclinical research published in Nature Medicine.
Experiments showed that AML driven by leukemia-associated transcription factors, including AML1-ETO and PML-RARα, was “extremely sensitive” to PARP inhibition.
Mixed-lineage leukemia (MLL) was initially resistant to treatment with PARP inhibitors, but inhibiting Hoxa9 changed that.
“PARP inhibitors could potentially offer a completely different approach to specifically target certain subtypes of acute myeloid leukemia,” said study author Chi Wai Eric So, PhD, of King’s College London in the UK.
He and his colleagues first tested the PARP inhibitor olaparib on primary mouse hematopoietic cells transformed by the leukemia-associated transcription factors AML1-ETO, PML-RARα, MLL-AF9, and E2A-PBX.
The drug suppressed the colony-forming ability of cells transformed by AML1-ETO or PML-RARα by about 90% but had little impact on MLL-AF9- or E2A-PBX-transformed cells. Olaparib also had minimal effects on normal bone marrow.
The investigators observed similar results in experiments with the PARP inhibitor veliparib.
They then tested PARP inhibitors in patient-derived leukemic cell lines carrying AML1-ETO (Kasumi), mutated PML-RARα that is resistant to standard all-trans retinoic acid treatment (NB4-LR2), or MLL-AF9 (THP1).
Similar to the previous experiments, PARP inhibition reduced the colony-forming ability of Kasumi and NB4-LR2 cells but not THP1 cells.
Next, the investigators transplanted the 3 cell lines into immunocompromised mice and treated the mice with olaparib. The drug delayed disease onset and prolonged survival in both the Kasumi and NB4-LR2 models but had no effect on disease onset or survival in the THP1 model.
Dr So and his colleagues said PARP inhibition was likely effective in AML driven by AML1-ETO and PML-RARα because of the suppressed expression of key homologous recombination-associated genes and the compromised DNA-damage response observed in these malignancies.
The team noted that PARP has proven critical in a variety of DNA repair processes, including as a sensor of single-strand breaks in base-excision repair, as a mediator for restarting stalled replication forks of homologous recombination-mediated double-strand break repair, and as a means of preventing the binding of Ku proteins to DNA ends in non-homologous end-joining pathways.
It therefore follows that MLL-driven AML was resistant to treatment with PARP inhibitors because DNA repair is not suppressed in this malignancy. The Hoxa9 protein activates other DNA damage repair pathways in these leukemia cells, keeping them alive.
So the investigators tested drugs that could (indirectly) inhibit Hoxa9—the GSK3 inhibitors LiCl and Li2CO3—in combination with olaparib. Both in vitro and in vivo, treatment with a GSK3 inhibitor and olaparib proved effective against MLL-driven AML.
“While this approach seems promising from these laboratory studies, it is still very early days,” said Matt Kaiser, PhD, head of research at Bloodwise, a charity that helped fund this study.
“We need to see how real patients respond to these drugs and how effective they are. PARP inhibitors such as olaparib are already being used to treat patients with other cancers and have been shown to be safe. This should speed up the time it takes for this potentially effective new treatment to enter clinical trials.” ![]()

Image by Tom Ellenberger
PARP inhibitors could prove useful for treating aggressive acute myeloid leukemia (AML), according to preclinical research published in Nature Medicine.
Experiments showed that AML driven by leukemia-associated transcription factors, including AML1-ETO and PML-RARα, was “extremely sensitive” to PARP inhibition.
Mixed-lineage leukemia (MLL) was initially resistant to treatment with PARP inhibitors, but inhibiting Hoxa9 changed that.
“PARP inhibitors could potentially offer a completely different approach to specifically target certain subtypes of acute myeloid leukemia,” said study author Chi Wai Eric So, PhD, of King’s College London in the UK.
He and his colleagues first tested the PARP inhibitor olaparib on primary mouse hematopoietic cells transformed by the leukemia-associated transcription factors AML1-ETO, PML-RARα, MLL-AF9, and E2A-PBX.
The drug suppressed the colony-forming ability of cells transformed by AML1-ETO or PML-RARα by about 90% but had little impact on MLL-AF9- or E2A-PBX-transformed cells. Olaparib also had minimal effects on normal bone marrow.
The investigators observed similar results in experiments with the PARP inhibitor veliparib.
They then tested PARP inhibitors in patient-derived leukemic cell lines carrying AML1-ETO (Kasumi), mutated PML-RARα that is resistant to standard all-trans retinoic acid treatment (NB4-LR2), or MLL-AF9 (THP1).
Similar to the previous experiments, PARP inhibition reduced the colony-forming ability of Kasumi and NB4-LR2 cells but not THP1 cells.
Next, the investigators transplanted the 3 cell lines into immunocompromised mice and treated the mice with olaparib. The drug delayed disease onset and prolonged survival in both the Kasumi and NB4-LR2 models but had no effect on disease onset or survival in the THP1 model.
Dr So and his colleagues said PARP inhibition was likely effective in AML driven by AML1-ETO and PML-RARα because of the suppressed expression of key homologous recombination-associated genes and the compromised DNA-damage response observed in these malignancies.
The team noted that PARP has proven critical in a variety of DNA repair processes, including as a sensor of single-strand breaks in base-excision repair, as a mediator for restarting stalled replication forks of homologous recombination-mediated double-strand break repair, and as a means of preventing the binding of Ku proteins to DNA ends in non-homologous end-joining pathways.
It therefore follows that MLL-driven AML was resistant to treatment with PARP inhibitors because DNA repair is not suppressed in this malignancy. The Hoxa9 protein activates other DNA damage repair pathways in these leukemia cells, keeping them alive.
So the investigators tested drugs that could (indirectly) inhibit Hoxa9—the GSK3 inhibitors LiCl and Li2CO3—in combination with olaparib. Both in vitro and in vivo, treatment with a GSK3 inhibitor and olaparib proved effective against MLL-driven AML.
“While this approach seems promising from these laboratory studies, it is still very early days,” said Matt Kaiser, PhD, head of research at Bloodwise, a charity that helped fund this study.
“We need to see how real patients respond to these drugs and how effective they are. PARP inhibitors such as olaparib are already being used to treat patients with other cancers and have been shown to be safe. This should speed up the time it takes for this potentially effective new treatment to enter clinical trials.” ![]()
Berry-derived compound can fight AML

A compound derived from the berries of the Bloodhorn tree has demonstrated activity against acute myeloid leukemia (AML), according to preclinical research published in Investigational New Drugs.
The compound, 7-formyl-10-methylisoellipticine, induced apoptosis in AML cells in a dose- and time-dependent manner.
It also significantly slowed tumor growth and reduced tumor mass in a mouse model of AML.
7-formyl-10-methylisoellipticine is derived from an ellipticine, which has been isolated from the berries of the Ochrosia Elliptica tree. The tree, also known as the Bloodhorn tree due to the shape and color of the berries, grows on the northeast coast of Australia and in the rainforests of Brazil.
“[We have] taken the natural product and restyled it with unique features to improve the potency and solubility,” explained Florence McCarthy, PhD, of University College Cork in Ireland.
“What is truly exceptional is that these features are not common in drugs, and so we aim to exploit this fully. There is also significant potential to apply this approach to other drugs in a similar fashion.”
For this study, Dr McCarthy and his colleagues first tested 7-formyl-10-methylisoellipticine in the AML cell line MV4-11. They tested a range of concentrations in an attempt to identify the minimum concentration that would cause significant cytotoxicity. It turned out to be 5 μM.
Over a period of 24 hours, 5 μM of 7-formyl-10-methylisoellipticine killed up to 40% of MV4-11 cells. And over 96 hours, 5 μM of 7-formyl-10-methylisoellipticine killed more than 90% of cells.
Further investigation revealed that 5 μM of 7-formyl-10-methylisoellipticine increases the sub-G1 phase of the MV4-11 cell cycle. And the compound functions, at least in part, by generating mitochondrial-derived reactive oxygen species.
The researchers then found that 7-formyl-10-methylisoellipticine is not toxic to BALB/c mice. The team injected the mice with 7-formyl-10-methylisoellipticine at a range of doses—5 mg/kg, 10 mg/kg, 25 mg/kg, and 50 mg/kg.
Regardless of the dose, the compound did not cause a change in body weight, significantly increase levels of alanine aminotransferase or aspartate aminotransferase relative to negative control, or significantly change cell morphology or tissue structure in specified major organs.
Finally, the researchers found that 7-formyl-10-methylisoellipticine has antitumor activity in an AML xenograft mouse model. Based on the toxicity experiments, the team used a dose of 25 mg/kg in these mice.
At this dose, 7-formyl-10-methylisoellipticine significantly slowed tumor growth and reduced tumor mass. Tumor growth was 4 times slower in mice treated with 7-formyl-10-methylisoellipticine than in control mice. And tumor mass was up to 7 times greater in controls than it was in treated mice.
Based on these results, the researchers said they plan to continue investigating the mechanism of action of ellipticines, which “have a clear potential clinical application.” ![]()

A compound derived from the berries of the Bloodhorn tree has demonstrated activity against acute myeloid leukemia (AML), according to preclinical research published in Investigational New Drugs.
The compound, 7-formyl-10-methylisoellipticine, induced apoptosis in AML cells in a dose- and time-dependent manner.
It also significantly slowed tumor growth and reduced tumor mass in a mouse model of AML.
7-formyl-10-methylisoellipticine is derived from an ellipticine, which has been isolated from the berries of the Ochrosia Elliptica tree. The tree, also known as the Bloodhorn tree due to the shape and color of the berries, grows on the northeast coast of Australia and in the rainforests of Brazil.
“[We have] taken the natural product and restyled it with unique features to improve the potency and solubility,” explained Florence McCarthy, PhD, of University College Cork in Ireland.
“What is truly exceptional is that these features are not common in drugs, and so we aim to exploit this fully. There is also significant potential to apply this approach to other drugs in a similar fashion.”
For this study, Dr McCarthy and his colleagues first tested 7-formyl-10-methylisoellipticine in the AML cell line MV4-11. They tested a range of concentrations in an attempt to identify the minimum concentration that would cause significant cytotoxicity. It turned out to be 5 μM.
Over a period of 24 hours, 5 μM of 7-formyl-10-methylisoellipticine killed up to 40% of MV4-11 cells. And over 96 hours, 5 μM of 7-formyl-10-methylisoellipticine killed more than 90% of cells.
Further investigation revealed that 5 μM of 7-formyl-10-methylisoellipticine increases the sub-G1 phase of the MV4-11 cell cycle. And the compound functions, at least in part, by generating mitochondrial-derived reactive oxygen species.
The researchers then found that 7-formyl-10-methylisoellipticine is not toxic to BALB/c mice. The team injected the mice with 7-formyl-10-methylisoellipticine at a range of doses—5 mg/kg, 10 mg/kg, 25 mg/kg, and 50 mg/kg.
Regardless of the dose, the compound did not cause a change in body weight, significantly increase levels of alanine aminotransferase or aspartate aminotransferase relative to negative control, or significantly change cell morphology or tissue structure in specified major organs.
Finally, the researchers found that 7-formyl-10-methylisoellipticine has antitumor activity in an AML xenograft mouse model. Based on the toxicity experiments, the team used a dose of 25 mg/kg in these mice.
At this dose, 7-formyl-10-methylisoellipticine significantly slowed tumor growth and reduced tumor mass. Tumor growth was 4 times slower in mice treated with 7-formyl-10-methylisoellipticine than in control mice. And tumor mass was up to 7 times greater in controls than it was in treated mice.
Based on these results, the researchers said they plan to continue investigating the mechanism of action of ellipticines, which “have a clear potential clinical application.” ![]()

A compound derived from the berries of the Bloodhorn tree has demonstrated activity against acute myeloid leukemia (AML), according to preclinical research published in Investigational New Drugs.
The compound, 7-formyl-10-methylisoellipticine, induced apoptosis in AML cells in a dose- and time-dependent manner.
It also significantly slowed tumor growth and reduced tumor mass in a mouse model of AML.
7-formyl-10-methylisoellipticine is derived from an ellipticine, which has been isolated from the berries of the Ochrosia Elliptica tree. The tree, also known as the Bloodhorn tree due to the shape and color of the berries, grows on the northeast coast of Australia and in the rainforests of Brazil.
“[We have] taken the natural product and restyled it with unique features to improve the potency and solubility,” explained Florence McCarthy, PhD, of University College Cork in Ireland.
“What is truly exceptional is that these features are not common in drugs, and so we aim to exploit this fully. There is also significant potential to apply this approach to other drugs in a similar fashion.”
For this study, Dr McCarthy and his colleagues first tested 7-formyl-10-methylisoellipticine in the AML cell line MV4-11. They tested a range of concentrations in an attempt to identify the minimum concentration that would cause significant cytotoxicity. It turned out to be 5 μM.
Over a period of 24 hours, 5 μM of 7-formyl-10-methylisoellipticine killed up to 40% of MV4-11 cells. And over 96 hours, 5 μM of 7-formyl-10-methylisoellipticine killed more than 90% of cells.
Further investigation revealed that 5 μM of 7-formyl-10-methylisoellipticine increases the sub-G1 phase of the MV4-11 cell cycle. And the compound functions, at least in part, by generating mitochondrial-derived reactive oxygen species.
The researchers then found that 7-formyl-10-methylisoellipticine is not toxic to BALB/c mice. The team injected the mice with 7-formyl-10-methylisoellipticine at a range of doses—5 mg/kg, 10 mg/kg, 25 mg/kg, and 50 mg/kg.
Regardless of the dose, the compound did not cause a change in body weight, significantly increase levels of alanine aminotransferase or aspartate aminotransferase relative to negative control, or significantly change cell morphology or tissue structure in specified major organs.
Finally, the researchers found that 7-formyl-10-methylisoellipticine has antitumor activity in an AML xenograft mouse model. Based on the toxicity experiments, the team used a dose of 25 mg/kg in these mice.
At this dose, 7-formyl-10-methylisoellipticine significantly slowed tumor growth and reduced tumor mass. Tumor growth was 4 times slower in mice treated with 7-formyl-10-methylisoellipticine than in control mice. And tumor mass was up to 7 times greater in controls than it was in treated mice.
Based on these results, the researchers said they plan to continue investigating the mechanism of action of ellipticines, which “have a clear potential clinical application.” ![]()
RIC can improve HSCT outcomes in older AML patients

Photo by Chad McNeeley
Results of a phase 2 study suggest that reduced-intensity conditioning (RIC) may allow older patients with acute myeloid leukemia (AML) to remain in long-term remission after allogeneic hematopoietic stem cell transplant (HSCT).
The study included patients age 60 and older who were in their first complete remission (CR1) at transplant.
Two years later, the probability of overall survival was 48%, and the probability of disease-free survival was 42%.
“This new data offers strong support against using biological age as a limiting factor for stem cell transplantation in AML patients who are otherwise well positioned to tolerate and achieve long-term remission with this approach,” said Steven Devine, MD, of The Ohio State University in Columbus.
He and his colleagues shared the data in the Journal of Clinical Oncology.
The researchers wanted to determine whether RIC could improve long-term remission rates after HSCT for older patients with AML.
So the team studied 114 patients with a median age of 65 (range, 60-74) who were treated at 21 US hospitals between November 2004 and November 2011. Most patients were male (62%), and most had intermediate cytogenetics (70%).
All of the patients were in CR1 according to International Working Group criteria. The CR had to be achieved after no more than 2 cycles of induction chemotherapy, and patients were required to undergo HSCT within 6 months of the initial documentation of morphologic CR.
The median time from CR1 documentation to HSCT was 85 days (range, 9-184), and the median time from AML diagnosis to HSCT was 138 days (range, 61-265).
All patients received RIC (fludarabine followed by busulfan) prior to HSCT, which essentially cut treatment strength by half compared to traditional high-dose conditioning. The patients received tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis.
Forty-eight percent of patients underwent HSCT from a matched, related donor, and 52% had a matched, unrelated donor.
At 2 years, disease-free survival was 42%, and overall survival was 48%. Among patients with unrelated donors, disease-free survival was 40%, and overall survival was 50%.
The cumulative incidence of relapse was 44%, and non-relapse mortality was 15%. Twenty-eight percent of patients had chronic GVHD, and 9.6% had grade 2-4 acute GVHD.
“Close to half of the patients treated in this study achieved long-term, cancer-free survival after 2 years,” Dr Devine noted. “These outcomes are similar to what we would expect to see in younger patients and appear to be better results than those that can be achieved with conventional chemotherapy-based approaches typically used in AML patients over 60.” ![]()

Photo by Chad McNeeley
Results of a phase 2 study suggest that reduced-intensity conditioning (RIC) may allow older patients with acute myeloid leukemia (AML) to remain in long-term remission after allogeneic hematopoietic stem cell transplant (HSCT).
The study included patients age 60 and older who were in their first complete remission (CR1) at transplant.
Two years later, the probability of overall survival was 48%, and the probability of disease-free survival was 42%.
“This new data offers strong support against using biological age as a limiting factor for stem cell transplantation in AML patients who are otherwise well positioned to tolerate and achieve long-term remission with this approach,” said Steven Devine, MD, of The Ohio State University in Columbus.
He and his colleagues shared the data in the Journal of Clinical Oncology.
The researchers wanted to determine whether RIC could improve long-term remission rates after HSCT for older patients with AML.
So the team studied 114 patients with a median age of 65 (range, 60-74) who were treated at 21 US hospitals between November 2004 and November 2011. Most patients were male (62%), and most had intermediate cytogenetics (70%).
All of the patients were in CR1 according to International Working Group criteria. The CR had to be achieved after no more than 2 cycles of induction chemotherapy, and patients were required to undergo HSCT within 6 months of the initial documentation of morphologic CR.
The median time from CR1 documentation to HSCT was 85 days (range, 9-184), and the median time from AML diagnosis to HSCT was 138 days (range, 61-265).
All patients received RIC (fludarabine followed by busulfan) prior to HSCT, which essentially cut treatment strength by half compared to traditional high-dose conditioning. The patients received tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis.
Forty-eight percent of patients underwent HSCT from a matched, related donor, and 52% had a matched, unrelated donor.
At 2 years, disease-free survival was 42%, and overall survival was 48%. Among patients with unrelated donors, disease-free survival was 40%, and overall survival was 50%.
The cumulative incidence of relapse was 44%, and non-relapse mortality was 15%. Twenty-eight percent of patients had chronic GVHD, and 9.6% had grade 2-4 acute GVHD.
“Close to half of the patients treated in this study achieved long-term, cancer-free survival after 2 years,” Dr Devine noted. “These outcomes are similar to what we would expect to see in younger patients and appear to be better results than those that can be achieved with conventional chemotherapy-based approaches typically used in AML patients over 60.” ![]()

Photo by Chad McNeeley
Results of a phase 2 study suggest that reduced-intensity conditioning (RIC) may allow older patients with acute myeloid leukemia (AML) to remain in long-term remission after allogeneic hematopoietic stem cell transplant (HSCT).
The study included patients age 60 and older who were in their first complete remission (CR1) at transplant.
Two years later, the probability of overall survival was 48%, and the probability of disease-free survival was 42%.
“This new data offers strong support against using biological age as a limiting factor for stem cell transplantation in AML patients who are otherwise well positioned to tolerate and achieve long-term remission with this approach,” said Steven Devine, MD, of The Ohio State University in Columbus.
He and his colleagues shared the data in the Journal of Clinical Oncology.
The researchers wanted to determine whether RIC could improve long-term remission rates after HSCT for older patients with AML.
So the team studied 114 patients with a median age of 65 (range, 60-74) who were treated at 21 US hospitals between November 2004 and November 2011. Most patients were male (62%), and most had intermediate cytogenetics (70%).
All of the patients were in CR1 according to International Working Group criteria. The CR had to be achieved after no more than 2 cycles of induction chemotherapy, and patients were required to undergo HSCT within 6 months of the initial documentation of morphologic CR.
The median time from CR1 documentation to HSCT was 85 days (range, 9-184), and the median time from AML diagnosis to HSCT was 138 days (range, 61-265).
All patients received RIC (fludarabine followed by busulfan) prior to HSCT, which essentially cut treatment strength by half compared to traditional high-dose conditioning. The patients received tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis.
Forty-eight percent of patients underwent HSCT from a matched, related donor, and 52% had a matched, unrelated donor.
At 2 years, disease-free survival was 42%, and overall survival was 48%. Among patients with unrelated donors, disease-free survival was 40%, and overall survival was 50%.
The cumulative incidence of relapse was 44%, and non-relapse mortality was 15%. Twenty-eight percent of patients had chronic GVHD, and 9.6% had grade 2-4 acute GVHD.
“Close to half of the patients treated in this study achieved long-term, cancer-free survival after 2 years,” Dr Devine noted. “These outcomes are similar to what we would expect to see in younger patients and appear to be better results than those that can be achieved with conventional chemotherapy-based approaches typically used in AML patients over 60.” ![]()
EC expands indication for azacitidine in AML

The European Commission (EC) has expanded the approved indication for azacitidine for injection (Vidaza) in acute myeloid leukemia (AML).
Now, the drug is approved to treat AML patients age 65 and older who are ineligible for hematopoietic stem cell transplant (HSCT) and have more than 30% myeloblasts according to the WHO classification.
Previously, HSCT-ineligible elderly AML patients could only receive azacitidine if they had less than 30% blasts.
Because this new indication for azacitidine is thought to bring significant clinical benefit in comparison with existing therapies, the drug will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
In addition to the aforementioned AML indications, azacitidine is approved in the European Economic Area to treat HSCT-ineligible adults with intermediate-2- and high-risk myelodysplastic syndromes and HSCT-ineligible adults who have chronic myelomonocytic leukemia and 10%-29% marrow blasts without myeloproliferative disorder.
Azacitidine is marketed as Vidaza by Celgene.
AML-001 trial
The EC’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle), or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33% of patients, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()

The European Commission (EC) has expanded the approved indication for azacitidine for injection (Vidaza) in acute myeloid leukemia (AML).
Now, the drug is approved to treat AML patients age 65 and older who are ineligible for hematopoietic stem cell transplant (HSCT) and have more than 30% myeloblasts according to the WHO classification.
Previously, HSCT-ineligible elderly AML patients could only receive azacitidine if they had less than 30% blasts.
Because this new indication for azacitidine is thought to bring significant clinical benefit in comparison with existing therapies, the drug will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
In addition to the aforementioned AML indications, azacitidine is approved in the European Economic Area to treat HSCT-ineligible adults with intermediate-2- and high-risk myelodysplastic syndromes and HSCT-ineligible adults who have chronic myelomonocytic leukemia and 10%-29% marrow blasts without myeloproliferative disorder.
Azacitidine is marketed as Vidaza by Celgene.
AML-001 trial
The EC’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle), or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33% of patients, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()

The European Commission (EC) has expanded the approved indication for azacitidine for injection (Vidaza) in acute myeloid leukemia (AML).
Now, the drug is approved to treat AML patients age 65 and older who are ineligible for hematopoietic stem cell transplant (HSCT) and have more than 30% myeloblasts according to the WHO classification.
Previously, HSCT-ineligible elderly AML patients could only receive azacitidine if they had less than 30% blasts.
Because this new indication for azacitidine is thought to bring significant clinical benefit in comparison with existing therapies, the drug will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
In addition to the aforementioned AML indications, azacitidine is approved in the European Economic Area to treat HSCT-ineligible adults with intermediate-2- and high-risk myelodysplastic syndromes and HSCT-ineligible adults who have chronic myelomonocytic leukemia and 10%-29% marrow blasts without myeloproliferative disorder.
Azacitidine is marketed as Vidaza by Celgene.
AML-001 trial
The EC’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle), or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33% of patients, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()
New test could help fight leukemia

Image courtesy of NIAID
Researchers say they have developed a test that can reveal how the immune system would respond to vaccines for leukemia.
To conduct this test, cancer-specific-proteins are spotted onto a microscope slide.
They are then incubated with a patient blood sample to show whether the immune system can recognize the proteins.
The researchers believe this test could inform immunotherapy trial development and eventually direct the treatment of leukemia.
They described the test in PLOS ONE.
The team explained that cellular arrays using peptide-MHC (pMHC) tetramers allow the simultaneous detection of different antigen-specific T-cell populations that are naturally circulating in leukemia patients and healthy individuals.
The researchers developed a pMHC array to detect CD8+ T-cell populations in leukemia patients that recognize epitopes within viral antigens and leukemia antigens.
Experiments showed this test was at least as sensitive as flow cytometry.
The pMHC array successfully identified more than 40 T-cell populations. It identified T cells that recognized various tumor antigen epitopes in patients with acute myeloid leukemia and acute lymphoblastic leukemia.
“This [test] would allow us to know how good a patients’ immune system is and potentially which proteins their immune system will react to, allowing us to prioritize which proteins we use to develop anticancer vaccines,” said study author Barbara Guinn, PhD, of the University of Southampton in the UK.
“In the future, we may be able to monitor patient immune responses as they are treated in clinical trials, helping us to direct the immune system more efficiently against cancer cells.”
Dr Guinn has spent a large part of her career investigating the differences between cancer cells and normal cells in terms of the proteins they make. She has been able to identify a number of proteins that are overexpressed in tumor cells but not healthy cells.
“Some of these proteins act as biomarkers for patient survival,” she said, “and some of them have helped us understand more about how cancer develops in subgroups of patients with leukemia.” ![]()

Image courtesy of NIAID
Researchers say they have developed a test that can reveal how the immune system would respond to vaccines for leukemia.
To conduct this test, cancer-specific-proteins are spotted onto a microscope slide.
They are then incubated with a patient blood sample to show whether the immune system can recognize the proteins.
The researchers believe this test could inform immunotherapy trial development and eventually direct the treatment of leukemia.
They described the test in PLOS ONE.
The team explained that cellular arrays using peptide-MHC (pMHC) tetramers allow the simultaneous detection of different antigen-specific T-cell populations that are naturally circulating in leukemia patients and healthy individuals.
The researchers developed a pMHC array to detect CD8+ T-cell populations in leukemia patients that recognize epitopes within viral antigens and leukemia antigens.
Experiments showed this test was at least as sensitive as flow cytometry.
The pMHC array successfully identified more than 40 T-cell populations. It identified T cells that recognized various tumor antigen epitopes in patients with acute myeloid leukemia and acute lymphoblastic leukemia.
“This [test] would allow us to know how good a patients’ immune system is and potentially which proteins their immune system will react to, allowing us to prioritize which proteins we use to develop anticancer vaccines,” said study author Barbara Guinn, PhD, of the University of Southampton in the UK.
“In the future, we may be able to monitor patient immune responses as they are treated in clinical trials, helping us to direct the immune system more efficiently against cancer cells.”
Dr Guinn has spent a large part of her career investigating the differences between cancer cells and normal cells in terms of the proteins they make. She has been able to identify a number of proteins that are overexpressed in tumor cells but not healthy cells.
“Some of these proteins act as biomarkers for patient survival,” she said, “and some of them have helped us understand more about how cancer develops in subgroups of patients with leukemia.” ![]()

Image courtesy of NIAID
Researchers say they have developed a test that can reveal how the immune system would respond to vaccines for leukemia.
To conduct this test, cancer-specific-proteins are spotted onto a microscope slide.
They are then incubated with a patient blood sample to show whether the immune system can recognize the proteins.
The researchers believe this test could inform immunotherapy trial development and eventually direct the treatment of leukemia.
They described the test in PLOS ONE.
The team explained that cellular arrays using peptide-MHC (pMHC) tetramers allow the simultaneous detection of different antigen-specific T-cell populations that are naturally circulating in leukemia patients and healthy individuals.
The researchers developed a pMHC array to detect CD8+ T-cell populations in leukemia patients that recognize epitopes within viral antigens and leukemia antigens.
Experiments showed this test was at least as sensitive as flow cytometry.
The pMHC array successfully identified more than 40 T-cell populations. It identified T cells that recognized various tumor antigen epitopes in patients with acute myeloid leukemia and acute lymphoblastic leukemia.
“This [test] would allow us to know how good a patients’ immune system is and potentially which proteins their immune system will react to, allowing us to prioritize which proteins we use to develop anticancer vaccines,” said study author Barbara Guinn, PhD, of the University of Southampton in the UK.
“In the future, we may be able to monitor patient immune responses as they are treated in clinical trials, helping us to direct the immune system more efficiently against cancer cells.”
Dr Guinn has spent a large part of her career investigating the differences between cancer cells and normal cells in terms of the proteins they make. She has been able to identify a number of proteins that are overexpressed in tumor cells but not healthy cells.
“Some of these proteins act as biomarkers for patient survival,” she said, “and some of them have helped us understand more about how cancer develops in subgroups of patients with leukemia.” ![]()
Group identifies potential target for AML
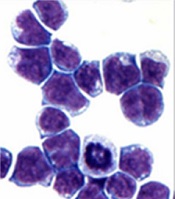
marrow cells generated by AE
Image courtesy of
The Rockefeller University
Preclinical research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the histone demethylase JMJD1C.
Investigators found that JMJD1C interacts with RUNX1–RUNX1T1 (formerly AML1-ETO and abbreviated here as AE), a transcription factor generated by the t(8;21) translocation in AML.
However, the team also found that JMJD1C is required for proliferation in many AML cell lines, not just those with AE.
Robert G. Roeder, PhD, of The Rockefeller University in New York, New York, and his colleagues detailed these findings in Genes and Development.
The investigators began this study by searching for proteins that interact with AE, and they identified JMJD1C. To investigate the relationship between JMJD1C and AE, the team explored the broader effects of removing JMJD1C.
“We found that numerous genes were downregulated upon loss of JMJD1C, and the set overlaps significantly with the genes that are normally activated by AE,” said study author Mo Chen, PhD, a researcher in Dr Roeder’s lab.
The investigators also found that AML cells are addicted to the presence of JMJD1C, and, without it, they cannot survive. The team observed an increase in apoptosis when JMJD1C was depleted from Kasumi-1 and SKNO-1 cell lines.
With subsequent experiments, the investigators confirmed that JMJD1C interacts with AE and demonstrated that JMJD1C is required for AE to exert its cancer-promoting effects. But they also found that JMJD1C plays an even broader role in AML.
“We were very surprised to find that JMJD1C is required for the proliferation of other acute myeloid leukemia cell lines, which do not have AE,” Dr Chen said.
The team found that depleting JMJD1C compromised the growth of the following cell lines: Kasumi-1 (AML1-ETO; AML M2), MOLM-13 (MLL-AF9, FLT3ITD; AML M5a), THP-1 (MLL-AF9, NRASmut; AML M5), HNT-34 (BCR-ABL1; AML M4), MV4-11 (MLL-AF4; AML M5), CMK (JAK3A527V; AML M7+ Down’s Syndrome), HEL (AML M6), NOMO-1 (MLL-AF9; AML M5a), NB4 (PML-RARα; AML M3), and HL-60 (MYC amplification; AML M2).
The only cell line that was not affected by depletion of JMJD1C was KG-1 (NRASmut; AML).
To build upon this discovery, the investigators looked for transcription factors aside from AE that might be responsible for JMJD1C addiction. The team found at least 2—LYL1 and HEB—that can recruit JMJD1C to target genes in diseased cells that lack AE, fueling leukemia growth.
The investigators said these results suggest JMJD1C may play a general role in promoting the growth of myeloid leukemias.
“We are excited because this type of general phenomena is an ideal target for drug development,” Dr Roeder said. “Our work will facilitate the development of selective inhibitors against JMJD1C, which is a highly promising therapeutic target for multiple types of leukemia.” ![]()

marrow cells generated by AE
Image courtesy of
The Rockefeller University
Preclinical research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the histone demethylase JMJD1C.
Investigators found that JMJD1C interacts with RUNX1–RUNX1T1 (formerly AML1-ETO and abbreviated here as AE), a transcription factor generated by the t(8;21) translocation in AML.
However, the team also found that JMJD1C is required for proliferation in many AML cell lines, not just those with AE.
Robert G. Roeder, PhD, of The Rockefeller University in New York, New York, and his colleagues detailed these findings in Genes and Development.
The investigators began this study by searching for proteins that interact with AE, and they identified JMJD1C. To investigate the relationship between JMJD1C and AE, the team explored the broader effects of removing JMJD1C.
“We found that numerous genes were downregulated upon loss of JMJD1C, and the set overlaps significantly with the genes that are normally activated by AE,” said study author Mo Chen, PhD, a researcher in Dr Roeder’s lab.
The investigators also found that AML cells are addicted to the presence of JMJD1C, and, without it, they cannot survive. The team observed an increase in apoptosis when JMJD1C was depleted from Kasumi-1 and SKNO-1 cell lines.
With subsequent experiments, the investigators confirmed that JMJD1C interacts with AE and demonstrated that JMJD1C is required for AE to exert its cancer-promoting effects. But they also found that JMJD1C plays an even broader role in AML.
“We were very surprised to find that JMJD1C is required for the proliferation of other acute myeloid leukemia cell lines, which do not have AE,” Dr Chen said.
The team found that depleting JMJD1C compromised the growth of the following cell lines: Kasumi-1 (AML1-ETO; AML M2), MOLM-13 (MLL-AF9, FLT3ITD; AML M5a), THP-1 (MLL-AF9, NRASmut; AML M5), HNT-34 (BCR-ABL1; AML M4), MV4-11 (MLL-AF4; AML M5), CMK (JAK3A527V; AML M7+ Down’s Syndrome), HEL (AML M6), NOMO-1 (MLL-AF9; AML M5a), NB4 (PML-RARα; AML M3), and HL-60 (MYC amplification; AML M2).
The only cell line that was not affected by depletion of JMJD1C was KG-1 (NRASmut; AML).
To build upon this discovery, the investigators looked for transcription factors aside from AE that might be responsible for JMJD1C addiction. The team found at least 2—LYL1 and HEB—that can recruit JMJD1C to target genes in diseased cells that lack AE, fueling leukemia growth.
The investigators said these results suggest JMJD1C may play a general role in promoting the growth of myeloid leukemias.
“We are excited because this type of general phenomena is an ideal target for drug development,” Dr Roeder said. “Our work will facilitate the development of selective inhibitors against JMJD1C, which is a highly promising therapeutic target for multiple types of leukemia.” ![]()

marrow cells generated by AE
Image courtesy of
The Rockefeller University
Preclinical research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the histone demethylase JMJD1C.
Investigators found that JMJD1C interacts with RUNX1–RUNX1T1 (formerly AML1-ETO and abbreviated here as AE), a transcription factor generated by the t(8;21) translocation in AML.
However, the team also found that JMJD1C is required for proliferation in many AML cell lines, not just those with AE.
Robert G. Roeder, PhD, of The Rockefeller University in New York, New York, and his colleagues detailed these findings in Genes and Development.
The investigators began this study by searching for proteins that interact with AE, and they identified JMJD1C. To investigate the relationship between JMJD1C and AE, the team explored the broader effects of removing JMJD1C.
“We found that numerous genes were downregulated upon loss of JMJD1C, and the set overlaps significantly with the genes that are normally activated by AE,” said study author Mo Chen, PhD, a researcher in Dr Roeder’s lab.
The investigators also found that AML cells are addicted to the presence of JMJD1C, and, without it, they cannot survive. The team observed an increase in apoptosis when JMJD1C was depleted from Kasumi-1 and SKNO-1 cell lines.
With subsequent experiments, the investigators confirmed that JMJD1C interacts with AE and demonstrated that JMJD1C is required for AE to exert its cancer-promoting effects. But they also found that JMJD1C plays an even broader role in AML.
“We were very surprised to find that JMJD1C is required for the proliferation of other acute myeloid leukemia cell lines, which do not have AE,” Dr Chen said.
The team found that depleting JMJD1C compromised the growth of the following cell lines: Kasumi-1 (AML1-ETO; AML M2), MOLM-13 (MLL-AF9, FLT3ITD; AML M5a), THP-1 (MLL-AF9, NRASmut; AML M5), HNT-34 (BCR-ABL1; AML M4), MV4-11 (MLL-AF4; AML M5), CMK (JAK3A527V; AML M7+ Down’s Syndrome), HEL (AML M6), NOMO-1 (MLL-AF9; AML M5a), NB4 (PML-RARα; AML M3), and HL-60 (MYC amplification; AML M2).
The only cell line that was not affected by depletion of JMJD1C was KG-1 (NRASmut; AML).
To build upon this discovery, the investigators looked for transcription factors aside from AE that might be responsible for JMJD1C addiction. The team found at least 2—LYL1 and HEB—that can recruit JMJD1C to target genes in diseased cells that lack AE, fueling leukemia growth.
The investigators said these results suggest JMJD1C may play a general role in promoting the growth of myeloid leukemias.
“We are excited because this type of general phenomena is an ideal target for drug development,” Dr Roeder said. “Our work will facilitate the development of selective inhibitors against JMJD1C, which is a highly promising therapeutic target for multiple types of leukemia.”
Novel compound could treat leukemia
A small-molecule compound that has previously shown activity against Ewing sarcoma and prostate cancer may fight leukemia as well, according to preclinical research published in Oncotarget.
The compound, YK-4-279, inhibits the oncogenic activity of the fusion protein EWS-FLI1.
“EWS-FLI1 is already known to drive a rare but deadly bone cancer called Ewing sarcoma,” said study author Aykut Üren, MD, of Georgetown University Medical Center in Washington, DC.
“It also appears to drive cancer cell growth in some prostate cancers.”
ETS family fusion proteins are found in patients with acute myeloid leukemia and acute lymphoblastic leukemia as well.
So Dr Üren and his colleagues decided to create a mouse model of EWS-FLI1-induced leukemia and assess the activity of YK-4-279 in this model.
Mice with EWS-FLI1-induced leukemia presented with severe hepatomegaly, splenomegaly, and anemia, followed by rapid death.
The investigators treated these mice with injections of YK-4-279 five days a week for 2 weeks or vehicle intraperitoneal injections on the same schedule.
The team said treatment with YK-4-279 significantly reduced white blood cell counts, nucleated erythroblasts in the peripheral blood, splenomegaly, and hepatomegaly.
They noted that mice experienced reductions in the weight of their spleens and livers without experiencing reductions in total body weight.
In addition, mice that received YK-4-279 had significantly better overall survival than control mice. The median survival times were 60.5 days and 21 days, respectively.
The investigators also noted that treated mice did not exhibit overt toxicity in the liver, spleen, or bone marrow.
“The fact that treated mice did not get sick from the YK-4-279 gives us an early indication that it might be safe to use in humans, but that is a question that can’t be answered until we conduct clinical trials,” Dr Üren said.
Nevertheless, he and his colleagues believe these results support the continued preclinical development of YK-4-279 for Ewing sarcoma, prostate cancers, and leukemias with highly homologous translocation products or with a clear ETS-driven gene signature.

A small-molecule compound that has previously shown activity against Ewing sarcoma and prostate cancer may fight leukemia as well, according to preclinical research published in Oncotarget.
The compound, YK-4-279, inhibits the oncogenic activity of the fusion protein EWS-FLI1.
“EWS-FLI1 is already known to drive a rare but deadly bone cancer called Ewing sarcoma,” said study author Aykut Üren, MD, of Georgetown University Medical Center in Washington, DC.
“It also appears to drive cancer cell growth in some prostate cancers.”
ETS family fusion proteins are found in patients with acute myeloid leukemia and acute lymphoblastic leukemia as well.
So Dr Üren and his colleagues decided to create a mouse model of EWS-FLI1-induced leukemia and assess the activity of YK-4-279 in this model.
Mice with EWS-FLI1-induced leukemia presented with severe hepatomegaly, splenomegaly, and anemia, followed by rapid death.
The investigators treated these mice with injections of YK-4-279 five days a week for 2 weeks or vehicle intraperitoneal injections on the same schedule.
The team said treatment with YK-4-279 significantly reduced white blood cell counts, nucleated erythroblasts in the peripheral blood, splenomegaly, and hepatomegaly.
They noted that mice experienced reductions in the weight of their spleens and livers without experiencing reductions in total body weight.
In addition, mice that received YK-4-279 had significantly better overall survival than control mice. The median survival times were 60.5 days and 21 days, respectively.
The investigators also noted that treated mice did not exhibit overt toxicity in the liver, spleen, or bone marrow.
“The fact that treated mice did not get sick from the YK-4-279 gives us an early indication that it might be safe to use in humans, but that is a question that can’t be answered until we conduct clinical trials,” Dr Üren said.
Nevertheless, he and his colleagues believe these results support the continued preclinical development of YK-4-279 for Ewing sarcoma, prostate cancers, and leukemias with highly homologous translocation products or with a clear ETS-driven gene signature.

A small-molecule compound that has previously shown activity against Ewing sarcoma and prostate cancer may fight leukemia as well, according to preclinical research published in Oncotarget.
The compound, YK-4-279, inhibits the oncogenic activity of the fusion protein EWS-FLI1.
“EWS-FLI1 is already known to drive a rare but deadly bone cancer called Ewing sarcoma,” said study author Aykut Üren, MD, of Georgetown University Medical Center in Washington, DC.
“It also appears to drive cancer cell growth in some prostate cancers.”
ETS family fusion proteins are found in patients with acute myeloid leukemia and acute lymphoblastic leukemia as well.
So Dr Üren and his colleagues decided to create a mouse model of EWS-FLI1-induced leukemia and assess the activity of YK-4-279 in this model.
Mice with EWS-FLI1-induced leukemia presented with severe hepatomegaly, splenomegaly, and anemia, followed by rapid death.
The investigators treated these mice with injections of YK-4-279 five days a week for 2 weeks or vehicle intraperitoneal injections on the same schedule.
The team said treatment with YK-4-279 significantly reduced white blood cell counts, nucleated erythroblasts in the peripheral blood, splenomegaly, and hepatomegaly.
They noted that mice experienced reductions in the weight of their spleens and livers without experiencing reductions in total body weight.
In addition, mice that received YK-4-279 had significantly better overall survival than control mice. The median survival times were 60.5 days and 21 days, respectively.
The investigators also noted that treated mice did not exhibit overt toxicity in the liver, spleen, or bone marrow.
“The fact that treated mice did not get sick from the YK-4-279 gives us an early indication that it might be safe to use in humans, but that is a question that can’t be answered until we conduct clinical trials,” Dr Üren said.
Nevertheless, he and his colleagues believe these results support the continued preclinical development of YK-4-279 for Ewing sarcoma, prostate cancers, and leukemias with highly homologous translocation products or with a clear ETS-driven gene signature.
Antibody can turn AML cells against each other

Image by Joshua Stokes
Scientists say they have discovered an antibody that can transform leukemic cells into immune cells that target their former “siblings.”
This agonist antibody, which is highly specific for the thrombopoietin receptor, can turn acute myeloid leukemia (AML) cells into dendritic cells.
With extended exposure to the antibody, these dendritic cells can then transform into natural killer (NK)-like cells that target AML cells.
The scientists described these discoveries in PNAS.
“It’s a totally new approach to cancer, and we’re working to test it in human patients as soon as possible,” said study author Richard A. Lerner, MD, of The Scripps Research Institute in La Jolla, California.
Unexpected transformation
Dr Lerner’s lab has pioneered techniques to generate and screen large libraries of antibodies to find therapeutic antibodies that bind to a desired target or activate a desired receptor on cells.
During the course of this work, the team discovered a phenomenon they call “receptor pleiotropism,” in which agonist antibodies against known receptors cause cell fates that are extremely different from those induced by the natural agonist to the receptor.
Why this happens is unclear, but the discovery led the scientists to wonder if they could use the method to convert leukemia cells into non-cancerous cells.
To find out, they tested 20 of their recently discovered receptor-activating antibodies against AML cells from patients. This revealed the transformative properties of the agonist antibody that is highly specific for the thrombopoietin receptor.
When this antibody was applied to healthy, immature bone marrow cells, it caused them to mature into megakaryocytes. However, when the antibody was applied to AML cells, they were converted into dendritic cells.
With longer exposures to the antibody and certain other in vitro conditions, the induced dendritic cells morphed into cells that closely resemble NK cells.
“That antibody could have turned those acute myeloid leukemia cells into a lot of other cell types, but, somehow, we were lucky enough to get NK cells,” Dr Lerner said.
The team examined these induced NK cells with electron microscopy and observed that many of the cells had extended tendrils through the outer membranes of neighboring AML cells. In in vitro tests, a modest number of these NK cells wiped out about 15% of the surrounding AML cell population in 24 hours.
The scientists also noted that the induced NK cells’ cancer-killing effects appeared to be purely “fratricidal.” Unrelated breast cancer cells did not die off in large numbers in the presence of the NK cells.
Fratricidin therapy
Why the induced NK cells appear to target only closely related cells isn’t yet clear. However, the finding suggests there may be antibodies—and even small-molecule compounds—that would turn other cancerous cell types into fratricidal NK cells by activating other receptors expressed on those cells.
Such fratricidal therapies, which Dr Lerner calls “fratricidins,” would have several potential advantages. First, especially if they are antibodies, they could be clinically useful with little or no further modification.
Second, their high specificity for their target receptors, and the resulting NK cells’ specificity for related cancer cells, should reduce the likelihood of adverse effects.
Finally, the peculiar dynamics of fratricidin therapy, in which every cancerous cell is potentially convertible to a cancer-killing NK cell, suggests that—if the strategy works—it might not just reduce the targeted cancer-cell population in a patient but eliminate it altogether.
“We’re in discussions with pharmaceutical companies to take this straight into humans after the appropriate preclinical toxicity studies,” Dr Lerner said.
This research was supported by the JPB Foundation and Zebra Biologics.

Image by Joshua Stokes
Scientists say they have discovered an antibody that can transform leukemic cells into immune cells that target their former “siblings.”
This agonist antibody, which is highly specific for the thrombopoietin receptor, can turn acute myeloid leukemia (AML) cells into dendritic cells.
With extended exposure to the antibody, these dendritic cells can then transform into natural killer (NK)-like cells that target AML cells.
The scientists described these discoveries in PNAS.
“It’s a totally new approach to cancer, and we’re working to test it in human patients as soon as possible,” said study author Richard A. Lerner, MD, of The Scripps Research Institute in La Jolla, California.
Unexpected transformation
Dr Lerner’s lab has pioneered techniques to generate and screen large libraries of antibodies to find therapeutic antibodies that bind to a desired target or activate a desired receptor on cells.
During the course of this work, the team discovered a phenomenon they call “receptor pleiotropism,” in which agonist antibodies against known receptors cause cell fates that are extremely different from those induced by the natural agonist to the receptor.
Why this happens is unclear, but the discovery led the scientists to wonder if they could use the method to convert leukemia cells into non-cancerous cells.
To find out, they tested 20 of their recently discovered receptor-activating antibodies against AML cells from patients. This revealed the transformative properties of the agonist antibody that is highly specific for the thrombopoietin receptor.
When this antibody was applied to healthy, immature bone marrow cells, it caused them to mature into megakaryocytes. However, when the antibody was applied to AML cells, they were converted into dendritic cells.
With longer exposures to the antibody and certain other in vitro conditions, the induced dendritic cells morphed into cells that closely resemble NK cells.
“That antibody could have turned those acute myeloid leukemia cells into a lot of other cell types, but, somehow, we were lucky enough to get NK cells,” Dr Lerner said.
The team examined these induced NK cells with electron microscopy and observed that many of the cells had extended tendrils through the outer membranes of neighboring AML cells. In in vitro tests, a modest number of these NK cells wiped out about 15% of the surrounding AML cell population in 24 hours.
The scientists also noted that the induced NK cells’ cancer-killing effects appeared to be purely “fratricidal.” Unrelated breast cancer cells did not die off in large numbers in the presence of the NK cells.
Fratricidin therapy
Why the induced NK cells appear to target only closely related cells isn’t yet clear. However, the finding suggests there may be antibodies—and even small-molecule compounds—that would turn other cancerous cell types into fratricidal NK cells by activating other receptors expressed on those cells.
Such fratricidal therapies, which Dr Lerner calls “fratricidins,” would have several potential advantages. First, especially if they are antibodies, they could be clinically useful with little or no further modification.
Second, their high specificity for their target receptors, and the resulting NK cells’ specificity for related cancer cells, should reduce the likelihood of adverse effects.
Finally, the peculiar dynamics of fratricidin therapy, in which every cancerous cell is potentially convertible to a cancer-killing NK cell, suggests that—if the strategy works—it might not just reduce the targeted cancer-cell population in a patient but eliminate it altogether.
“We’re in discussions with pharmaceutical companies to take this straight into humans after the appropriate preclinical toxicity studies,” Dr Lerner said.
This research was supported by the JPB Foundation and Zebra Biologics.

Image by Joshua Stokes
Scientists say they have discovered an antibody that can transform leukemic cells into immune cells that target their former “siblings.”
This agonist antibody, which is highly specific for the thrombopoietin receptor, can turn acute myeloid leukemia (AML) cells into dendritic cells.
With extended exposure to the antibody, these dendritic cells can then transform into natural killer (NK)-like cells that target AML cells.
The scientists described these discoveries in PNAS.
“It’s a totally new approach to cancer, and we’re working to test it in human patients as soon as possible,” said study author Richard A. Lerner, MD, of The Scripps Research Institute in La Jolla, California.
Unexpected transformation
Dr Lerner’s lab has pioneered techniques to generate and screen large libraries of antibodies to find therapeutic antibodies that bind to a desired target or activate a desired receptor on cells.
During the course of this work, the team discovered a phenomenon they call “receptor pleiotropism,” in which agonist antibodies against known receptors cause cell fates that are extremely different from those induced by the natural agonist to the receptor.
Why this happens is unclear, but the discovery led the scientists to wonder if they could use the method to convert leukemia cells into non-cancerous cells.
To find out, they tested 20 of their recently discovered receptor-activating antibodies against AML cells from patients. This revealed the transformative properties of the agonist antibody that is highly specific for the thrombopoietin receptor.
When this antibody was applied to healthy, immature bone marrow cells, it caused them to mature into megakaryocytes. However, when the antibody was applied to AML cells, they were converted into dendritic cells.
With longer exposures to the antibody and certain other in vitro conditions, the induced dendritic cells morphed into cells that closely resemble NK cells.
“That antibody could have turned those acute myeloid leukemia cells into a lot of other cell types, but, somehow, we were lucky enough to get NK cells,” Dr Lerner said.
The team examined these induced NK cells with electron microscopy and observed that many of the cells had extended tendrils through the outer membranes of neighboring AML cells. In in vitro tests, a modest number of these NK cells wiped out about 15% of the surrounding AML cell population in 24 hours.
The scientists also noted that the induced NK cells’ cancer-killing effects appeared to be purely “fratricidal.” Unrelated breast cancer cells did not die off in large numbers in the presence of the NK cells.
Fratricidin therapy
Why the induced NK cells appear to target only closely related cells isn’t yet clear. However, the finding suggests there may be antibodies—and even small-molecule compounds—that would turn other cancerous cell types into fratricidal NK cells by activating other receptors expressed on those cells.
Such fratricidal therapies, which Dr Lerner calls “fratricidins,” would have several potential advantages. First, especially if they are antibodies, they could be clinically useful with little or no further modification.
Second, their high specificity for their target receptors, and the resulting NK cells’ specificity for related cancer cells, should reduce the likelihood of adverse effects.
Finally, the peculiar dynamics of fratricidin therapy, in which every cancerous cell is potentially convertible to a cancer-killing NK cell, suggests that—if the strategy works—it might not just reduce the targeted cancer-cell population in a patient but eliminate it altogether.
“We’re in discussions with pharmaceutical companies to take this straight into humans after the appropriate preclinical toxicity studies,” Dr Lerner said.
This research was supported by the JPB Foundation and Zebra Biologics.