User login
Does a Prior Hip Arthroscopy Affect Clinical Outcomes in Metal-on-Metal Hip Resurfacing Arthroplasty?
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
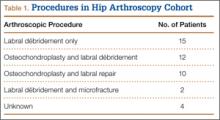
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
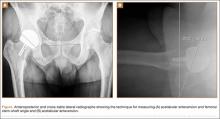
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
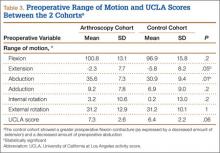
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
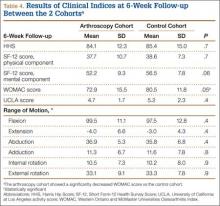
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
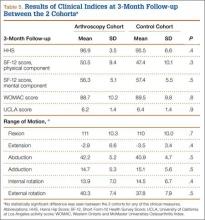
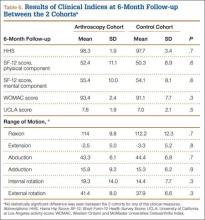
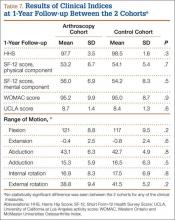
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.
Visualization and Reduction of a Meniscal Capsular Junction Tear in the Knee: An Arthroscopic Surgical Technique
The annual incidence of anterior cruciate ligament (ACL) injury in the general US population is estimated at 1 in 3000, or approximately 100,000 ACL injuries per year.1 The incidence of meniscal injuries after ACL tears ranges from 34% to 92%,2 with peripheral posterior horn tears of the medial meniscus accounting for 40% of the meniscal pathology.3
Although several meniscal tear patterns and their treatments have been described in the literature, posterior medial meniscal capsular junction (MCJ) tears have not been adequately addressed. Thijn4 found the accuracy of routine anterior portal arthroscopy in identifying medial meniscus tears was only 81%. Gillies and Seligson5 found a 25% arthroscopic false-negative rate caused by failure to detect peripheral tears in the posterior horn of the medial meniscus.
We reviewed 781 (517 male, 264 female) patients who underwent ACL reconstruction at our clinic and found a 12.3% incidence of MCJ tear with primary ACL injury and a 23.6% incidence of MCJ tear with revision ACL reconstruction. We believe this is a specific injury pattern. If not looked for during arthroscopy, it can be missed. Whether this tear pattern behaves differently from a posterior medial meniscus tear is yet to be determined.
To address such tear patterns, with or without ACL reconstruction, we use an arthroscopic repair technique that shows direct visualization of the tear and its reduction.
Materials and Methods
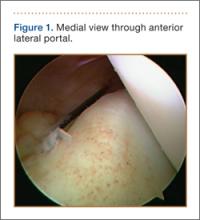
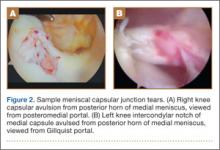
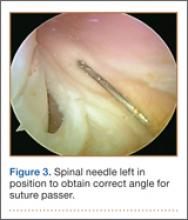
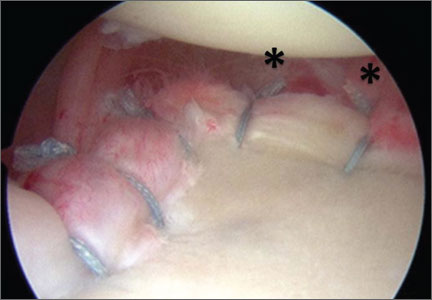
The standard anterior medial and lateral arthroscopic portals are established. A 30° scope is placed in the anterior lateral portal, and an arthroscopic shaver is used to débride the ACL remnants, including the footprint and the femoral insertion site. The camera is then adjusted to look straight down. Next, it is placed between the posterior cruciate ligament (PCL) and the medial femoral condyle and advanced toward the posterior capsule. It is then adjusted to view medially (Figure 1). If there is a tear (Figures 2A, 2B), a posterior medial portal (described by Gillquist and colleagues6) is established using an 18-gauge spinal needle for localization followed by a small stab incision through the skin. The spinal needle is left in position to obtain the correct angle for the suture passer (Figure 3). A 70° Hewson suture passer (Smith & Nephew, Memphis, Tennessee) is passed through the posterior medial portal.
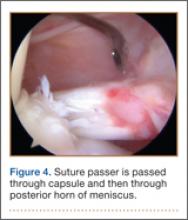
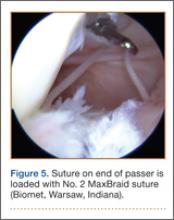
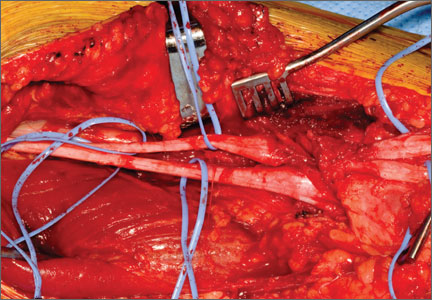
Once inside the joint, the suture passer is passed through the capsule and then through the posterior horn of the meniscus (Figure 4). A loop grasper is used to grab the suture on the end of the passer and then is brought out the posterior medial portal and loaded with a No. 2 MaxBraid suture (Biomet, Warsaw, Indiana) (Figure 5). In some cases, the suture passer’s wire goes out the notch toward the anterior aspect of the knee. If this occurs, the loop grasper can be used to grab this wire from the anterior medial portal and load with the MaxBraid suture.
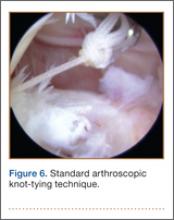
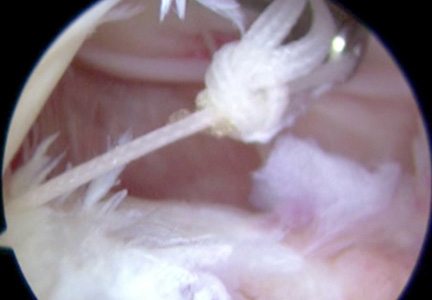
Standard arthroscopic knot-tying techniques are used under direct visualization showing the reduction of the capsule to the meniscus (Figure 6). This is done from the posterior medial portal. The excess suture is cut with an arthroscopic suture cutter in the standard fashion. In the rare case of an intact ACL with this same tear pattern, the same technique can be used. If there is difficulty moving past the intact ACL and PCL, a posterior lateral portal can be used as another accessory portal. The arthroscope can then be placed in the posterior lateral portal, while the posterior medial portal can be used as the working portal. Care must be taken in either technique to avoid soft-tissue bridges.
Discussion
Previous biomechanical studies have shown the meniscus to be important to knee stability. In an ACL-deficient knee, the posterior medial meniscus is important as a secondary stabilizer, and for that reason it is crucial to identify and repair tears there to avoid risking extra force on the ACL graft.7,8 We think an MCJ tear can potentially compromise knee stability as well, so there is a need to examine the posterior aspect of the knee during every knee arthroscopy. However, biomechanical studies must be performed to validate this theory.
To assess whether orthopedists in general are aware of and concerned about MCJ tears, a survey was e-mailed to members of the Arthroscopy Association of North America (AANA) and the American Sports Medicine Fellowship Society (ASMFS). Sixty-seven orthopedic surgeons who perform ACL reconstruction surgeries responded to some or all of the following questions. Nearly half (48%) of the surgeons said they always assess the posteromedial MCJ by placing the camera between the PCL and the medial femoral condyle. Only 25% said MCJ tears should be repaired always, but another 64% said these tears should be repaired sometimes. Thus, 89% responded that at least some MCJ tears should be repaired. Most (88%) said these tears could sometimes or always be a source of chronic pain. Also, 92% said these tears could sometimes or always change the contact pressures in the knee, and 66% said these tears could sometimes or always change the rotational stability of the knee. Finally, 60% said MCJ tears could sometimes or always affect ACL graft failure. These data show a need to determine an appropriate surgical technique that will help treat MCJ tears.
There is a vast amount of literature about the meniscus, but there are few current studies on the specific entity of MCJ tears. We think these tears act similarly to posterior meniscus tears and should be addressed similarly. MCJ tears are easily missed on anterior arthroscopy. In every knee arthroscopy, the posterior aspect of the knee should be checked for these injuries, particularly in ACL-deficient knees. A lesion found within the capsule can be repaired with the technique we have described.
1. Fu FH, Cohen SB. Current Concepts in ACL Reconstruction. Thorofare, NJ: Slack; 2008.
2. Simonian PT, Cole BJ, Bach BR. Sports Injuries of the Knee: Surgical Approaches. New York, NY: Thieme; 2006.
3. Smith JP 3rd, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415-419.
4. Thijn CJ. Accuracy of double-contrast arthrography and arthroscopy of the knee joint. Skeletal Radiol. 1982;8(3):187-192.
5. Gillies H, Seligson D. Precision in the diagnosis of meniscal lesions: a comparison of clinical evaluation, arthrography, and arthroscopy. J Bone Joint Surg Am. 1979;61(3):343-346.
6. Gillquist J, Hagberg G, Oretorp N. Arthroscopic examination of the posteromedial compartment of the knee joint. Int Orthop. 1979;3(1):13-18.
7. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883-888.
8. Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament–deficient knee. J Orthop Res. 2000;18(1):109-115.
The annual incidence of anterior cruciate ligament (ACL) injury in the general US population is estimated at 1 in 3000, or approximately 100,000 ACL injuries per year.1 The incidence of meniscal injuries after ACL tears ranges from 34% to 92%,2 with peripheral posterior horn tears of the medial meniscus accounting for 40% of the meniscal pathology.3
Although several meniscal tear patterns and their treatments have been described in the literature, posterior medial meniscal capsular junction (MCJ) tears have not been adequately addressed. Thijn4 found the accuracy of routine anterior portal arthroscopy in identifying medial meniscus tears was only 81%. Gillies and Seligson5 found a 25% arthroscopic false-negative rate caused by failure to detect peripheral tears in the posterior horn of the medial meniscus.
We reviewed 781 (517 male, 264 female) patients who underwent ACL reconstruction at our clinic and found a 12.3% incidence of MCJ tear with primary ACL injury and a 23.6% incidence of MCJ tear with revision ACL reconstruction. We believe this is a specific injury pattern. If not looked for during arthroscopy, it can be missed. Whether this tear pattern behaves differently from a posterior medial meniscus tear is yet to be determined.
To address such tear patterns, with or without ACL reconstruction, we use an arthroscopic repair technique that shows direct visualization of the tear and its reduction.
Materials and Methods
The standard anterior medial and lateral arthroscopic portals are established. A 30° scope is placed in the anterior lateral portal, and an arthroscopic shaver is used to débride the ACL remnants, including the footprint and the femoral insertion site. The camera is then adjusted to look straight down. Next, it is placed between the posterior cruciate ligament (PCL) and the medial femoral condyle and advanced toward the posterior capsule. It is then adjusted to view medially (Figure 1). If there is a tear (Figures 2A, 2B), a posterior medial portal (described by Gillquist and colleagues6) is established using an 18-gauge spinal needle for localization followed by a small stab incision through the skin. The spinal needle is left in position to obtain the correct angle for the suture passer (Figure 3). A 70° Hewson suture passer (Smith & Nephew, Memphis, Tennessee) is passed through the posterior medial portal.
Once inside the joint, the suture passer is passed through the capsule and then through the posterior horn of the meniscus (Figure 4). A loop grasper is used to grab the suture on the end of the passer and then is brought out the posterior medial portal and loaded with a No. 2 MaxBraid suture (Biomet, Warsaw, Indiana) (Figure 5). In some cases, the suture passer’s wire goes out the notch toward the anterior aspect of the knee. If this occurs, the loop grasper can be used to grab this wire from the anterior medial portal and load with the MaxBraid suture.
Standard arthroscopic knot-tying techniques are used under direct visualization showing the reduction of the capsule to the meniscus (Figure 6). This is done from the posterior medial portal. The excess suture is cut with an arthroscopic suture cutter in the standard fashion. In the rare case of an intact ACL with this same tear pattern, the same technique can be used. If there is difficulty moving past the intact ACL and PCL, a posterior lateral portal can be used as another accessory portal. The arthroscope can then be placed in the posterior lateral portal, while the posterior medial portal can be used as the working portal. Care must be taken in either technique to avoid soft-tissue bridges.
Discussion
Previous biomechanical studies have shown the meniscus to be important to knee stability. In an ACL-deficient knee, the posterior medial meniscus is important as a secondary stabilizer, and for that reason it is crucial to identify and repair tears there to avoid risking extra force on the ACL graft.7,8 We think an MCJ tear can potentially compromise knee stability as well, so there is a need to examine the posterior aspect of the knee during every knee arthroscopy. However, biomechanical studies must be performed to validate this theory.
To assess whether orthopedists in general are aware of and concerned about MCJ tears, a survey was e-mailed to members of the Arthroscopy Association of North America (AANA) and the American Sports Medicine Fellowship Society (ASMFS). Sixty-seven orthopedic surgeons who perform ACL reconstruction surgeries responded to some or all of the following questions. Nearly half (48%) of the surgeons said they always assess the posteromedial MCJ by placing the camera between the PCL and the medial femoral condyle. Only 25% said MCJ tears should be repaired always, but another 64% said these tears should be repaired sometimes. Thus, 89% responded that at least some MCJ tears should be repaired. Most (88%) said these tears could sometimes or always be a source of chronic pain. Also, 92% said these tears could sometimes or always change the contact pressures in the knee, and 66% said these tears could sometimes or always change the rotational stability of the knee. Finally, 60% said MCJ tears could sometimes or always affect ACL graft failure. These data show a need to determine an appropriate surgical technique that will help treat MCJ tears.
There is a vast amount of literature about the meniscus, but there are few current studies on the specific entity of MCJ tears. We think these tears act similarly to posterior meniscus tears and should be addressed similarly. MCJ tears are easily missed on anterior arthroscopy. In every knee arthroscopy, the posterior aspect of the knee should be checked for these injuries, particularly in ACL-deficient knees. A lesion found within the capsule can be repaired with the technique we have described.
The annual incidence of anterior cruciate ligament (ACL) injury in the general US population is estimated at 1 in 3000, or approximately 100,000 ACL injuries per year.1 The incidence of meniscal injuries after ACL tears ranges from 34% to 92%,2 with peripheral posterior horn tears of the medial meniscus accounting for 40% of the meniscal pathology.3
Although several meniscal tear patterns and their treatments have been described in the literature, posterior medial meniscal capsular junction (MCJ) tears have not been adequately addressed. Thijn4 found the accuracy of routine anterior portal arthroscopy in identifying medial meniscus tears was only 81%. Gillies and Seligson5 found a 25% arthroscopic false-negative rate caused by failure to detect peripheral tears in the posterior horn of the medial meniscus.
We reviewed 781 (517 male, 264 female) patients who underwent ACL reconstruction at our clinic and found a 12.3% incidence of MCJ tear with primary ACL injury and a 23.6% incidence of MCJ tear with revision ACL reconstruction. We believe this is a specific injury pattern. If not looked for during arthroscopy, it can be missed. Whether this tear pattern behaves differently from a posterior medial meniscus tear is yet to be determined.
To address such tear patterns, with or without ACL reconstruction, we use an arthroscopic repair technique that shows direct visualization of the tear and its reduction.
Materials and Methods
The standard anterior medial and lateral arthroscopic portals are established. A 30° scope is placed in the anterior lateral portal, and an arthroscopic shaver is used to débride the ACL remnants, including the footprint and the femoral insertion site. The camera is then adjusted to look straight down. Next, it is placed between the posterior cruciate ligament (PCL) and the medial femoral condyle and advanced toward the posterior capsule. It is then adjusted to view medially (Figure 1). If there is a tear (Figures 2A, 2B), a posterior medial portal (described by Gillquist and colleagues6) is established using an 18-gauge spinal needle for localization followed by a small stab incision through the skin. The spinal needle is left in position to obtain the correct angle for the suture passer (Figure 3). A 70° Hewson suture passer (Smith & Nephew, Memphis, Tennessee) is passed through the posterior medial portal.
Once inside the joint, the suture passer is passed through the capsule and then through the posterior horn of the meniscus (Figure 4). A loop grasper is used to grab the suture on the end of the passer and then is brought out the posterior medial portal and loaded with a No. 2 MaxBraid suture (Biomet, Warsaw, Indiana) (Figure 5). In some cases, the suture passer’s wire goes out the notch toward the anterior aspect of the knee. If this occurs, the loop grasper can be used to grab this wire from the anterior medial portal and load with the MaxBraid suture.
Standard arthroscopic knot-tying techniques are used under direct visualization showing the reduction of the capsule to the meniscus (Figure 6). This is done from the posterior medial portal. The excess suture is cut with an arthroscopic suture cutter in the standard fashion. In the rare case of an intact ACL with this same tear pattern, the same technique can be used. If there is difficulty moving past the intact ACL and PCL, a posterior lateral portal can be used as another accessory portal. The arthroscope can then be placed in the posterior lateral portal, while the posterior medial portal can be used as the working portal. Care must be taken in either technique to avoid soft-tissue bridges.
Discussion
Previous biomechanical studies have shown the meniscus to be important to knee stability. In an ACL-deficient knee, the posterior medial meniscus is important as a secondary stabilizer, and for that reason it is crucial to identify and repair tears there to avoid risking extra force on the ACL graft.7,8 We think an MCJ tear can potentially compromise knee stability as well, so there is a need to examine the posterior aspect of the knee during every knee arthroscopy. However, biomechanical studies must be performed to validate this theory.
To assess whether orthopedists in general are aware of and concerned about MCJ tears, a survey was e-mailed to members of the Arthroscopy Association of North America (AANA) and the American Sports Medicine Fellowship Society (ASMFS). Sixty-seven orthopedic surgeons who perform ACL reconstruction surgeries responded to some or all of the following questions. Nearly half (48%) of the surgeons said they always assess the posteromedial MCJ by placing the camera between the PCL and the medial femoral condyle. Only 25% said MCJ tears should be repaired always, but another 64% said these tears should be repaired sometimes. Thus, 89% responded that at least some MCJ tears should be repaired. Most (88%) said these tears could sometimes or always be a source of chronic pain. Also, 92% said these tears could sometimes or always change the contact pressures in the knee, and 66% said these tears could sometimes or always change the rotational stability of the knee. Finally, 60% said MCJ tears could sometimes or always affect ACL graft failure. These data show a need to determine an appropriate surgical technique that will help treat MCJ tears.
There is a vast amount of literature about the meniscus, but there are few current studies on the specific entity of MCJ tears. We think these tears act similarly to posterior meniscus tears and should be addressed similarly. MCJ tears are easily missed on anterior arthroscopy. In every knee arthroscopy, the posterior aspect of the knee should be checked for these injuries, particularly in ACL-deficient knees. A lesion found within the capsule can be repaired with the technique we have described.
1. Fu FH, Cohen SB. Current Concepts in ACL Reconstruction. Thorofare, NJ: Slack; 2008.
2. Simonian PT, Cole BJ, Bach BR. Sports Injuries of the Knee: Surgical Approaches. New York, NY: Thieme; 2006.
3. Smith JP 3rd, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415-419.
4. Thijn CJ. Accuracy of double-contrast arthrography and arthroscopy of the knee joint. Skeletal Radiol. 1982;8(3):187-192.
5. Gillies H, Seligson D. Precision in the diagnosis of meniscal lesions: a comparison of clinical evaluation, arthrography, and arthroscopy. J Bone Joint Surg Am. 1979;61(3):343-346.
6. Gillquist J, Hagberg G, Oretorp N. Arthroscopic examination of the posteromedial compartment of the knee joint. Int Orthop. 1979;3(1):13-18.
7. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883-888.
8. Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament–deficient knee. J Orthop Res. 2000;18(1):109-115.
1. Fu FH, Cohen SB. Current Concepts in ACL Reconstruction. Thorofare, NJ: Slack; 2008.
2. Simonian PT, Cole BJ, Bach BR. Sports Injuries of the Knee: Surgical Approaches. New York, NY: Thieme; 2006.
3. Smith JP 3rd, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415-419.
4. Thijn CJ. Accuracy of double-contrast arthrography and arthroscopy of the knee joint. Skeletal Radiol. 1982;8(3):187-192.
5. Gillies H, Seligson D. Precision in the diagnosis of meniscal lesions: a comparison of clinical evaluation, arthrography, and arthroscopy. J Bone Joint Surg Am. 1979;61(3):343-346.
6. Gillquist J, Hagberg G, Oretorp N. Arthroscopic examination of the posteromedial compartment of the knee joint. Int Orthop. 1979;3(1):13-18.
7. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883-888.
8. Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament–deficient knee. J Orthop Res. 2000;18(1):109-115.
Knee Surgery Not Necessary for Middle-Aged Patients with Mild Osteoarthritis
New meta-analysis evidence suggests that there is no benefit to having arthroscopic meniscal debridement for degenerative meniscal tears in comparison with nonoperative treatments or sham treatments in middle-aged patients with mild or no concomitant osteoarthritis. The findings were published August 25 online ahead of print in the Canadian Medical Association Journal.
“Doctors need to carefully weigh the costs and benefits when deciding who should undergo such surgery,” said Moin Khan, MD, lead author of the study and research fellow in orthopedic surgery in the Michael G. DeGroote School of Medicine at McMaster University in Hamilton, Ontario.
Dr. Khan and colleagues conducted a meta-analysis of 7 randomized controlled trials published between 1946 and January 20, 2014, on arthroscopic partial meniscectomy in patients with mild to no osteoarthritis compared with nonoperative treatments. Two reviewers independently screened all abstracts and titles for eligibility. In total, there were 811 knees in 805 patients with a mean age of 56 years. The pooled treatment effect of arthroscopic surgery did not show a significant or minimally important difference between treatment arms for long-term functional outcomes. Short-term functional outcomes between groups were significant but did not exceed the threshold for minimally important difference. Arthroscopic surgery did not result in a significant improvement in either short- or long-term pain scores.
“This study shows that surgery should not be the initial option for middle-age or older patients, as there is limited evidence supporting partial meniscectomy surgery for meniscus tears,” Dr. Khan said. “Other treatments should be used first.”
“Arthroscopic debridement or washout of knee osteoarthritis has come under lots of scrutiny based upon trials that suggest patients get no benefit from the procedure. We’re concerned that many surgeons worldwide may still be doing this procedure,” stated the researchers.
Suggested Reading
Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014 Aug 25. pii: cmaj.140433. [Epub ahead of print]
New meta-analysis evidence suggests that there is no benefit to having arthroscopic meniscal debridement for degenerative meniscal tears in comparison with nonoperative treatments or sham treatments in middle-aged patients with mild or no concomitant osteoarthritis. The findings were published August 25 online ahead of print in the Canadian Medical Association Journal.
“Doctors need to carefully weigh the costs and benefits when deciding who should undergo such surgery,” said Moin Khan, MD, lead author of the study and research fellow in orthopedic surgery in the Michael G. DeGroote School of Medicine at McMaster University in Hamilton, Ontario.
Dr. Khan and colleagues conducted a meta-analysis of 7 randomized controlled trials published between 1946 and January 20, 2014, on arthroscopic partial meniscectomy in patients with mild to no osteoarthritis compared with nonoperative treatments. Two reviewers independently screened all abstracts and titles for eligibility. In total, there were 811 knees in 805 patients with a mean age of 56 years. The pooled treatment effect of arthroscopic surgery did not show a significant or minimally important difference between treatment arms for long-term functional outcomes. Short-term functional outcomes between groups were significant but did not exceed the threshold for minimally important difference. Arthroscopic surgery did not result in a significant improvement in either short- or long-term pain scores.
“This study shows that surgery should not be the initial option for middle-age or older patients, as there is limited evidence supporting partial meniscectomy surgery for meniscus tears,” Dr. Khan said. “Other treatments should be used first.”
“Arthroscopic debridement or washout of knee osteoarthritis has come under lots of scrutiny based upon trials that suggest patients get no benefit from the procedure. We’re concerned that many surgeons worldwide may still be doing this procedure,” stated the researchers.
New meta-analysis evidence suggests that there is no benefit to having arthroscopic meniscal debridement for degenerative meniscal tears in comparison with nonoperative treatments or sham treatments in middle-aged patients with mild or no concomitant osteoarthritis. The findings were published August 25 online ahead of print in the Canadian Medical Association Journal.
“Doctors need to carefully weigh the costs and benefits when deciding who should undergo such surgery,” said Moin Khan, MD, lead author of the study and research fellow in orthopedic surgery in the Michael G. DeGroote School of Medicine at McMaster University in Hamilton, Ontario.
Dr. Khan and colleagues conducted a meta-analysis of 7 randomized controlled trials published between 1946 and January 20, 2014, on arthroscopic partial meniscectomy in patients with mild to no osteoarthritis compared with nonoperative treatments. Two reviewers independently screened all abstracts and titles for eligibility. In total, there were 811 knees in 805 patients with a mean age of 56 years. The pooled treatment effect of arthroscopic surgery did not show a significant or minimally important difference between treatment arms for long-term functional outcomes. Short-term functional outcomes between groups were significant but did not exceed the threshold for minimally important difference. Arthroscopic surgery did not result in a significant improvement in either short- or long-term pain scores.
“This study shows that surgery should not be the initial option for middle-age or older patients, as there is limited evidence supporting partial meniscectomy surgery for meniscus tears,” Dr. Khan said. “Other treatments should be used first.”
“Arthroscopic debridement or washout of knee osteoarthritis has come under lots of scrutiny based upon trials that suggest patients get no benefit from the procedure. We’re concerned that many surgeons worldwide may still be doing this procedure,” stated the researchers.
Suggested Reading
Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014 Aug 25. pii: cmaj.140433. [Epub ahead of print]
Suggested Reading
Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014 Aug 25. pii: cmaj.140433. [Epub ahead of print]