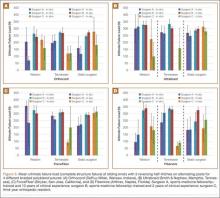
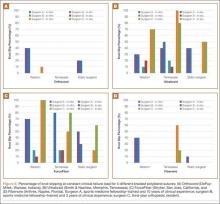
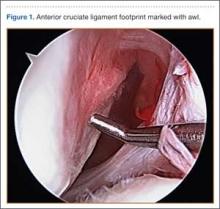
User login
Arthroscopic Posterior-Inferior Capsular Release in the Treatment of Overhead Athletes
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
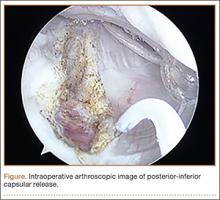
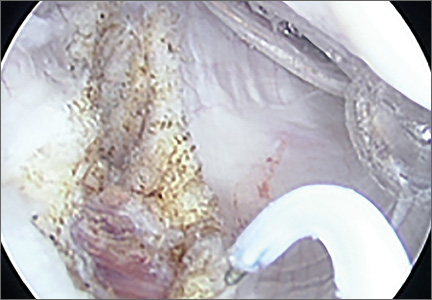
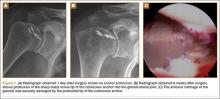
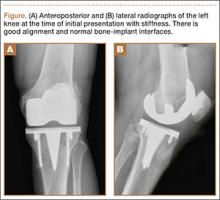
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
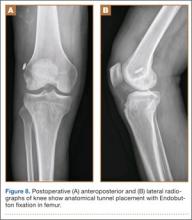
Revision Anterior Cruciate Ligament Reconstruction With Bone–Patellar Tendon–Bone Allograft and Extra-Articular Iliotibial Band Tenodesis
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
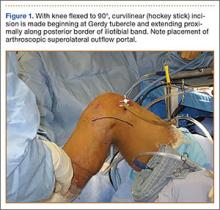
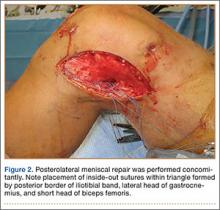
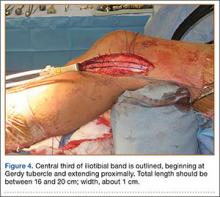
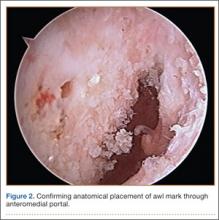
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
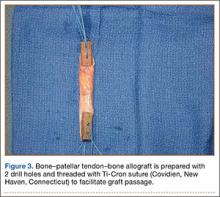
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
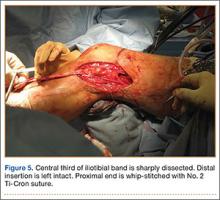
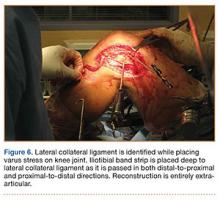
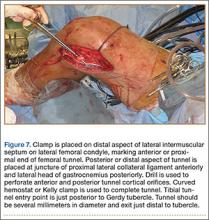
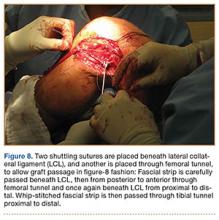
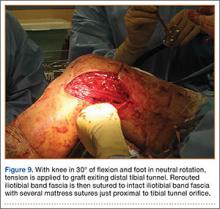
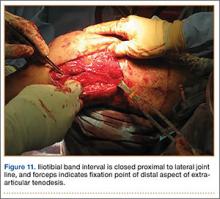
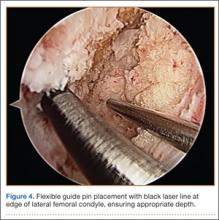
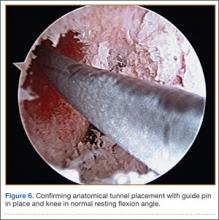
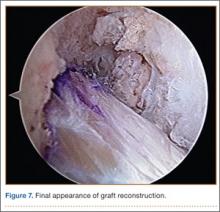
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
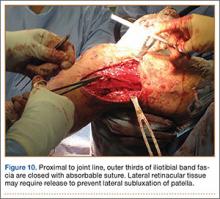
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
Greater Auricular Nerve Palsy After Arthroscopic Anterior-Inferior and Posterior-Inferior Labral Tear Repair Using Beach-Chair Positioning and a Standard Universal Headrest
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
In Vitro and In Situ Characterization of Arthroscopic Loop Security and Knot Security of Braided Polyblend Sutures: A Biomechanical Study
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
Arthroscopic Anterior Cruciate Ligament Reconstruction Using a Flexible Guide Pin With a Rigid Reamer
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
Glenoid Damage From Articular Protrusion of Metal Suture Anchor After Arthroscopic Rotator Cuff Repair
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Cutaneous Burn Caused by Radiofrequency Ablation Probe During Shoulder Arthroscopy
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Mycobacterium bovis Infection of Total Knee Arthroplasty After Bacillus Calmette-Guérin Therapy for Bladder Cancer
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Synovial Fistula After Tension Band Plating for Genu Valgum Correction
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
Anterior Hip Capsuloligamentous Reconstruction for Recurrent Instability After Hip Arthroscopy
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.