User login
Use of Intravenous Tranexamic Acid Improves Early Ambulation After Total Knee Arthroplasty and Anterior and Posterior Total Hip Arthroplasty
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
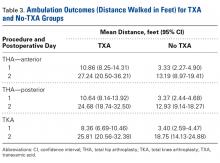
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
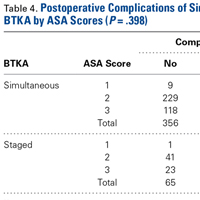
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
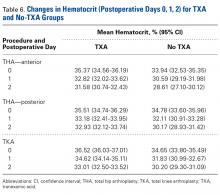
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
The Role of Synovial Cytokines in the Diagnosis of Periprosthetic Joint Infections: Current Concepts
Take-Home Points
- In cases of failed TJA, it is important to differentiate between septic and aseptic etiologies.
- Chronic and low-grade infections are challenging for orthopedic surgeons, as the symptoms often overlap with aseptic etiologies.
- Verification of infection eradication before beginning the second-stage reimplantation surgery is extremely important, but pre- and intraoperative findings can be unreliable.
- Synovial fluid cytokines have been shown to accurately diagnose PJIs.
- Synovial fluid cytokines may help surgeons differentiate between septic and aseptic cases of failed TJA.
Total joint arthroplasty (TJA) is an effective procedure that has been extensively used to relieve pain and improve quality of life in patients with various forms of joint disease. Although advances in technology and surgical technique have improved the success of TJA, periprosthetic joint infection (PJI) remains a serious complication. In the United States, it is estimated that PJI is the most common reason for total knee arthroplasty failure and the third most common reason for total hip arthroplasty revision.1 Although the incidence of PJI is 1% to 2%, the dramatic increase in TJA volume is expected to be accompanied by a similar rise in the number of infected TJAs; that number is expected to exceed 60,000 in the United States by 2020.2 Moreover, management of PJI is expensive and imposes a heavy burden on the healthcare system, with costs expected to hit $20 billion by 2020 in the US.2 Therefore, treating asepsis cases as infections imposes a heavy burden on the healthcare system and may result in excessive morbidity.3 At the same time, inadequate management of a PJI may result in recurrences that require infection treatment with morbid procedures, such as arthrodesis or amputation. Accurate diagnosis of PJI is of paramount importance in preventing potential implications of a misdiagnosed case. Unfortunately, the PJI diagnosis is extremely challenging, and the available diagnostic tests are often unreliable.4 Thus, research has recently focused on use of several synovial fluid cytokines in the detection of PJI.5-7 In this article, we provide an overview of the synovial biomarkers being used to diagnose PJI.
Diagnosis of Periprosthetic Joint Infection
Differentiating between septic and aseptic failed TJA is important, as the treatment options differ considerably. PJI can be broadly classified as acute or early postoperative (<6 weeks), late chronic (indolent onset), and acute-on-chronic (acute onset in well-functioning prosthesis, secondary to hematogenous spread).8 The acute and acute-on-chronic presentations are often associated with obvious signs of infection.9 However, chronic and low-grade infections pose a challenge to modern orthopedic practice, as the symptoms often overlap with that of aseptic causes of TJA failure.10 As a result, the International Consensus Group on Periprosthetic Joint Infection developed complex criteria using the Musculoskeletal Infection Society definition of PJI and involving a battery of tests for PJI diagnosis.11 According to these criteria, PJI is diagnosed when 1 of the 2 major criteria or 3 of the 5 minor criteria are met (Table 1).
Although these criteria constitute the most agreed on and widely used standard for PJI diagnosis, the definition is complex and often incomplete until surgical intervention. An ideal diagnostic test would aid in managing a PJI and provide results before a treatment decision is made. Many revision surgeries are being performed with insufficient information about the true diagnosis, and the diagnosis might change during or after surgery. About 10% of the revisions presumed to be aseptic may unexpectedly grow cultures during surgery and thereby satisfy the criteria for PJI after surgery.12 Moreover, with the use of novel methods such as polymerase chain reaction, microorganisms were identified in more than three-fourths of the presumed aseptic revisions.13 The optimal management of such cases is controversial, and it is unclear whether positive cultures should be treated as possible contaminants or true infection.12,14
Verification of Infection Eradication
A 2-stage revision procedure, widely accepted as the standard treatment for PJI, has success rates approaching 94%.15 In this procedure, it is important to verify infection eradication before beginning the second-stage reimplantation. Verification is crucial in avoiding reimplantation of an infected joint.16 After the first stage, patients are usually administered intravenous antibiotics for at least 6 weeks; these antibiotics are then withheld, and systemic inflammatory markers are evaluated for infection eradication. Although reliable criteria have been established for PJI diagnosis, guidelines for detecting eradication of infection are rudimentary. Most surgeons monitor the decrease in serologic markers, such as erythrocyte sedimentation rate and C-reactive protein (CRP) level, to assess the response to treatment. However, noninfectious etiologies may result in continued elevation of these markers.17 Even though aspirations are often performed to diagnose persistent infection before the second-stage procedure, their diagnostic utility may be limited.18 Use of cultures is also limited, as presence of antibiotic-loaded spacers can decrease the sensitivity of culture.19 Inadequate diagnosis often leads to unnecessary continuation of antimicrobial therapy or additional surgical débridement. Nuclear scans often remain positive because of aseptic inflammation related to surgery and are not useful in documenting sepsis arrest.20 Given the limitations of available tests, novel strategies for identifying the presence of infection at the second stage are being tested.
Synovial Fluid Cytokines
PJI pathogenesis begins with colonization of the implant surfaces with microorganisms and subsequent formation of biofilms.21 The human immune system is activated by the microbial products, cell wall components, and various biofilm proteins. Immune cells are recruited to the site, where they secrete a myriad of inflammatory biomarkers, such as cytokines, which promote further recruitment of inflammatory cells and aid in the eradication of pathogens.9 These inflammatory cytokines and cells are involved in aseptic inflammatory joint conditions, such as rheumatoid arthritis22,23; however, some are specifically involved in immune pathways combating pathogens.24 This action is the basis for increasing interest in using various synovial fluid cytokines and other biomarkers in the diagnosis of PJI. Here we describe some of the commonly studied cytokines.
Interleukin 1β
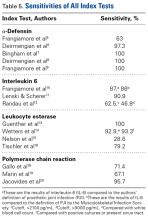
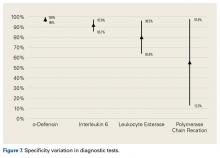
Interleukin 1β (IL-1β) is a major proinflammatory cytokine that is synthesized by multiple cells, including macrophages and monocytes.25 IL-1β is produced in response to microorganisms, other cytokines, antigen-presenting cells, and immune complexes; stimulates production of acute-phase proteins by the liver; and is an important pyrogen.25 Deirmengian and colleagues5 found that synovial IL-1β increased 258-fold in patients with a PJI. Studies have found that synovial IL-1β has sensitivity ranging from 66.7% to 100% and specificity ranging from 87% to 100%, with 1 study reporting an accuracy of 100%.5,6,26,27
Interleukin 6
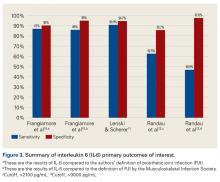
Also produced by macrophages and monocytes, interleukin 6 (IL-6) is a potent stimulator of acute-phase proteins.28,29 IL-6 has a role as a chemoattractant and helps with cell differentiation when changing from innate to acquired immunity.30 It is also used as an aid in diagnosing PJI; it has sensitivity ranging from 62% to 100% and specificity ranging from 85% to 100%.5,6,26,31,32 Synovial IL-6 measurements were more accurate than serum IL-6 measurements.26 Furthermore, synovial IL-6 can be increased up to 27-fold in PJI cases.5 In one study, synovial IL-6 levels >2100 pg/mL had sensitivity of 62.5% and specificity of 85.7% in PJI diagnosis26; in another study, an IL-6 threshold of 4270 pg/mL had sensitivity of 87.1%, specificity of 100%, and accuracy of 94.6%.31
C-Reactive Protein
CRP is an acute-phase reactant. Blood levels increase in response to aseptic inflammatory processes and systemic infection.33 CRP plays an important role in host defense by activating complement and helping mediate phagocytosis.33,34 Although serum CRP levels have been used in diagnosing PJIs,6 they can yield false-negative results.35,36 Therefore, attention turned to synovial CRP levels, which were found to be increased 13-fold in PJI cases.5 It has been shown that synovial CRP levels are significantly higher in infected vs noninfected prosthetic joints34 and had diagnostic accuracy better than that of serum CRP levels in diagnosing PJI.37 One study found that CRP at a threshold of 3.7 mg/L had sensitivity of 84%, specificity of 97.1%, and accuracy of 91.5%,37 whereas another study found that CRP at a threshold of 3.61 mg/L had sensitivity of 87.1%, specificity of 97.7%, and accuracy of 93.3%.31
α-Defensin
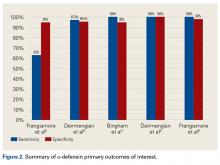
α-Defensin, a natural peptide produced and secreted by neutrophils in response to pathogens, has antimicrobial and cytotoxic properties,38-40 signals for the secretion of various cytokines, and acts as a chemoattractant for various immune cells.41 Deirmengian and colleagues6 found that α-defensin was consistently elevated in patients with PJI. α-Defensin is extremely accurate in diagnosing PJI; it has sensitivity ranging from 97% to 100% and specificity ranging from 96% to 100%.6,27,42 Moreover, α-defensin was effective in diagnosing PJI caused by a wide spectrum of organisms, including various low-virulence bacteria and fungi.43
Leukocyte Esterase
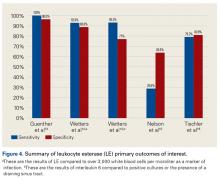
Leukocyte esterase is an enzyme produced and secreted by neutrophils at sites of active infection.7,44 Testing for this enzyme is performed with a colorimetric strip and was originally performed for the diagnosis of urinary tract infections.44,45 In a study conducted by Parvizi and colleagues,7 this strip was used to test for leukocyte esterase in synovial fluid samples; a ++ reading was found to have sensitivity of 80.6% and specificity of 100% in diagnosing knee PJI. Similarly, De Vecchi and colleagues45 found sensitivity of 92.6% and specificity of 97%.
Other Synovial Markers
Research has identified numerous molecular biomarkers that may be associated with the pathogenesis of PJI. Although several (eg, cytokines) have demonstrated higher levels in synovial fluid in patients with PJI than in normal controls, only a few have had clinically relevant diagnostic utility.6 Deirmengian and colleagues6 screened 43 synovial fluid biomarkers that potentially could be used in the diagnosis of PJI. Besides the cytokine α-defensin, 4 other biomarkers—lactoferrin, neutrophil gelatinase-associated lipocalcin, neutrophil elastase 2, and bactericidal/permeability-increasing protein—had accuracy of 100%. In addition, 8 cytokines and biomarkers (IL-8, CRP, resistin, thrombospondin, IL-1β, IL-6, IL-10, IL-1α) had area under the curve values higher than 0.9. Studies have also evaluated the diagnostic utility of metabolic products such as lactate, lactate dehydrogenase, and glucose; their accuracy was comparable to that of serum CRP.32
Serum Markers
In addition to the synovial fluid cytokines, several serum inflammatory cytokines have been studied as potential targets in diagnosing infection. Serum IL-6 has had excellent diagnostic accuracy46 and, when combined with CRP, could increase sensitivity in diagnosing PJI; such a combination (vs either test alone) could be useful in screening patients.47,48 Biomarkers such as tumor necrosis factor α and procalcitonin are considered very specific for PJI and may be useful in confirmatory testing.48 Evidence also suggests that toll-like receptor 2 proteins are elevated in the serum of patients with PJI and therefore are a potential diagnostic tool.49
Limitations of Synovial Cytokines
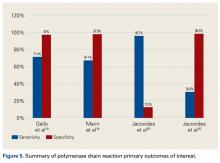
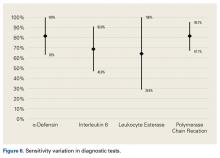
The literature suggests that some synovial fluid cytokines have promise.6 However, the best biomarker or combination of biomarkers is yet to be determined. Results have been consistent with α-defensin and other cytokines but mixed with IL-6 and still others32,42,50 (Table 2).
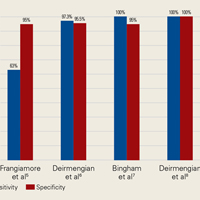
Information on the utility of synovial biomarkers in detecting persistent infection is limited. Frangiamore and colleagues50 found that IL-1 and IL-6 levels decreased between the stages of 2-stage revision. Unfortunately, none of the synovial fluid cytokines investigated (IL-1, IL-2, IL-6, IL-8, Il-10, interferon γ, granulocyte macrophage-colony stimulating factor, tumor necrosis factor α, IL-12p70) satisfactorily detected resolution of infection in the setting of prior treatment for PJI. Although cytokines are expected to be elevated in the presence of infection, the internal milieu at the time of stage 2 of the revision makes diagnosis of infection difficult. In addition, presence of spacer particles and recent surgery may activate immune pathways and yield false-positive results. Furthermore, antibiotic cement spacers may suppress the microorganisms to very low levels and yield false-negative results even if these organisms remain virulent.19
Even though the synovial molecular markers can detect the presence of infection, they are unable to identify pathogens. As identifying the pathogen is important in the treatment of PJI, there has been interest in using polymerase chain reaction (PCR) techniques.51 These tests may also provide specific information about the pathogen, such as its antibiotic sensitivity. A recently developed technology, the Ibis T5000 Universal Biosensor (Ibis Biosciences), uses novel pan-domain primers in a series of PCRs. This biosensor is useful in diagnosing infections when cultures are negative and appears to be more accurate than conventional PCR.13 As reported by Jacovides and colleagues,13 this novel PCR technique identified an organism in about 88% of presumed cases of aseptic revision.
Conclusion
PJI poses an extreme challenge to the healthcare system. Given the morbidity associated with improper management of PJI, accurate diagnosis is of paramount importance. Given the limitations of current tests, synovial fluid cytokines hold promise in the diagnosis of PJIs. However, these cytokines are expensive, and their clinical utility in PJI management is not well established. More research is needed before guidelines for synovial fluid cytokines and biomarkers can replace or be incorporated into guidelines for the treatment of PJIs.
1 Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
3. Sierra RJ, Trousdale RT, Pagnano MW. Above-the-knee amputation after a total knee replacement: prevalence, etiology, and functional outcome. J Bone Joint Surg Am. 2003;85(6):1000-1004.
4. Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88(4):869-882.
5. Deirmengian C, Hallab N, Tarabishy A, et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468(8):2017-2023.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
7. Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93(24):2242-2248.
8. Kuiper JW, Willink RT, Moojen DJF, van den Bekerom MP, Colen S. Treatment of acute periprosthetic infections with prosthesis retention: review of current concepts. World J Orthop. 2014;5(5):667-676.
9. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-1654.
10. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25.
11. Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331.
12. Saleh A, Guirguis A, Klika AK, Johnson L, Higuera CA, Barsoum WK. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty. 2014;29(11):2181-2186.
13. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)–based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
14. Barrack RL, Aggarwal A, Burnett RS, et al. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):94-99.
15. Macheras GA, Koutsostathis SD, Kateros K, Papadakis S, Anastasopoulos P. A two stage re-implantation protocol for the treatment of deep periprosthetic hip infection. Mid to long-term results. Hip Int. 2012;22(suppl 8):S54-S61.
16. George J, Kwiecien G, Klika AK, et al. Are frozen sections and MSIS criteria reliable at the time of reimplantation of two-stage revision arthroplasty? Clin Orthop Relat Res. 2016;474(7):1619-1626.
17. Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469(4):1002-1008.
18. Lonner JH, Siliski JM, Della Valle C, DiCesare P, Lotke PA. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop. 2001;30(4):305-309.
19. Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82(11):1552-1557.
20. Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39(1):66-78.
21. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158-168.
22. Fontana A, Hengartner H, Weber E, Fehr K, Grob PJ, Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49-53.
23. Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83(2):585-592.
24. Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel). 2014;7(5):545-594.
25. Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075-1079.
26. Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95(7):644-651.
27. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
28. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
29. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621-636.
30. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878-888.
31. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
32. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
33. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24(2):163-176.
34. Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplasty. 2012;27(8 suppl):12-16.
35. Ghanem E, Antoci V, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13(6):e444-e449.
36. Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35(11):1621-1626.
37. Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470(1):54-60.
38. Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105-128.
39. Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76(4):1427-1435.
40. Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104(6):1778-1783.
41. Ulm H, Wilmes M, Shai Y, Sahl HG. Antimicrobial host defensins—specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249.
42. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
43. Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473(7):2229-2235.
44. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
45. De Vecchi E, Villa F, Bortolin M, et al. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: a prospective study. Clin Microbiol Infect. 2016;22(6):555-560.
46. Di Cesare PE, Chang E, Preston CF, Liu C. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87(9):1921-1927.
47. Ettinger M, Calliess T, Kielstein JT, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis. 2015;61(3):332-341.
48. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89(1):94-99.
49. Galliera E, Drago L, Vassena C, et al. Toll-like receptor 2 in serum: a potential diagnostic marker of prosthetic joint infection? J Clin Microbiol. 2014;52(2):620-623.
50. Frangiamore SJ, Siqueira MB, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res. 2016;474(7):1630-1639.
51. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
52. Omar M, Ettinger M, Reichling M, et al. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97(2):173-176.
53. Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472(12):3997-4003.
54. Omar M, Ettinger M, Reichling M, et al. Preliminary results of a new test for rapid diagnosis of septic arthritis with use of leukocyte esterase and glucose reagent strips. J Bone Joint Surg Am. 2014;96(24):2032-2037.
Take-Home Points
- In cases of failed TJA, it is important to differentiate between septic and aseptic etiologies.
- Chronic and low-grade infections are challenging for orthopedic surgeons, as the symptoms often overlap with aseptic etiologies.
- Verification of infection eradication before beginning the second-stage reimplantation surgery is extremely important, but pre- and intraoperative findings can be unreliable.
- Synovial fluid cytokines have been shown to accurately diagnose PJIs.
- Synovial fluid cytokines may help surgeons differentiate between septic and aseptic cases of failed TJA.
Total joint arthroplasty (TJA) is an effective procedure that has been extensively used to relieve pain and improve quality of life in patients with various forms of joint disease. Although advances in technology and surgical technique have improved the success of TJA, periprosthetic joint infection (PJI) remains a serious complication. In the United States, it is estimated that PJI is the most common reason for total knee arthroplasty failure and the third most common reason for total hip arthroplasty revision.1 Although the incidence of PJI is 1% to 2%, the dramatic increase in TJA volume is expected to be accompanied by a similar rise in the number of infected TJAs; that number is expected to exceed 60,000 in the United States by 2020.2 Moreover, management of PJI is expensive and imposes a heavy burden on the healthcare system, with costs expected to hit $20 billion by 2020 in the US.2 Therefore, treating asepsis cases as infections imposes a heavy burden on the healthcare system and may result in excessive morbidity.3 At the same time, inadequate management of a PJI may result in recurrences that require infection treatment with morbid procedures, such as arthrodesis or amputation. Accurate diagnosis of PJI is of paramount importance in preventing potential implications of a misdiagnosed case. Unfortunately, the PJI diagnosis is extremely challenging, and the available diagnostic tests are often unreliable.4 Thus, research has recently focused on use of several synovial fluid cytokines in the detection of PJI.5-7 In this article, we provide an overview of the synovial biomarkers being used to diagnose PJI.
Diagnosis of Periprosthetic Joint Infection
Differentiating between septic and aseptic failed TJA is important, as the treatment options differ considerably. PJI can be broadly classified as acute or early postoperative (<6 weeks), late chronic (indolent onset), and acute-on-chronic (acute onset in well-functioning prosthesis, secondary to hematogenous spread).8 The acute and acute-on-chronic presentations are often associated with obvious signs of infection.9 However, chronic and low-grade infections pose a challenge to modern orthopedic practice, as the symptoms often overlap with that of aseptic causes of TJA failure.10 As a result, the International Consensus Group on Periprosthetic Joint Infection developed complex criteria using the Musculoskeletal Infection Society definition of PJI and involving a battery of tests for PJI diagnosis.11 According to these criteria, PJI is diagnosed when 1 of the 2 major criteria or 3 of the 5 minor criteria are met (Table 1).
Although these criteria constitute the most agreed on and widely used standard for PJI diagnosis, the definition is complex and often incomplete until surgical intervention. An ideal diagnostic test would aid in managing a PJI and provide results before a treatment decision is made. Many revision surgeries are being performed with insufficient information about the true diagnosis, and the diagnosis might change during or after surgery. About 10% of the revisions presumed to be aseptic may unexpectedly grow cultures during surgery and thereby satisfy the criteria for PJI after surgery.12 Moreover, with the use of novel methods such as polymerase chain reaction, microorganisms were identified in more than three-fourths of the presumed aseptic revisions.13 The optimal management of such cases is controversial, and it is unclear whether positive cultures should be treated as possible contaminants or true infection.12,14
Verification of Infection Eradication
A 2-stage revision procedure, widely accepted as the standard treatment for PJI, has success rates approaching 94%.15 In this procedure, it is important to verify infection eradication before beginning the second-stage reimplantation. Verification is crucial in avoiding reimplantation of an infected joint.16 After the first stage, patients are usually administered intravenous antibiotics for at least 6 weeks; these antibiotics are then withheld, and systemic inflammatory markers are evaluated for infection eradication. Although reliable criteria have been established for PJI diagnosis, guidelines for detecting eradication of infection are rudimentary. Most surgeons monitor the decrease in serologic markers, such as erythrocyte sedimentation rate and C-reactive protein (CRP) level, to assess the response to treatment. However, noninfectious etiologies may result in continued elevation of these markers.17 Even though aspirations are often performed to diagnose persistent infection before the second-stage procedure, their diagnostic utility may be limited.18 Use of cultures is also limited, as presence of antibiotic-loaded spacers can decrease the sensitivity of culture.19 Inadequate diagnosis often leads to unnecessary continuation of antimicrobial therapy or additional surgical débridement. Nuclear scans often remain positive because of aseptic inflammation related to surgery and are not useful in documenting sepsis arrest.20 Given the limitations of available tests, novel strategies for identifying the presence of infection at the second stage are being tested.
Synovial Fluid Cytokines
PJI pathogenesis begins with colonization of the implant surfaces with microorganisms and subsequent formation of biofilms.21 The human immune system is activated by the microbial products, cell wall components, and various biofilm proteins. Immune cells are recruited to the site, where they secrete a myriad of inflammatory biomarkers, such as cytokines, which promote further recruitment of inflammatory cells and aid in the eradication of pathogens.9 These inflammatory cytokines and cells are involved in aseptic inflammatory joint conditions, such as rheumatoid arthritis22,23; however, some are specifically involved in immune pathways combating pathogens.24 This action is the basis for increasing interest in using various synovial fluid cytokines and other biomarkers in the diagnosis of PJI. Here we describe some of the commonly studied cytokines.
Interleukin 1β
Interleukin 1β (IL-1β) is a major proinflammatory cytokine that is synthesized by multiple cells, including macrophages and monocytes.25 IL-1β is produced in response to microorganisms, other cytokines, antigen-presenting cells, and immune complexes; stimulates production of acute-phase proteins by the liver; and is an important pyrogen.25 Deirmengian and colleagues5 found that synovial IL-1β increased 258-fold in patients with a PJI. Studies have found that synovial IL-1β has sensitivity ranging from 66.7% to 100% and specificity ranging from 87% to 100%, with 1 study reporting an accuracy of 100%.5,6,26,27
Interleukin 6
Also produced by macrophages and monocytes, interleukin 6 (IL-6) is a potent stimulator of acute-phase proteins.28,29 IL-6 has a role as a chemoattractant and helps with cell differentiation when changing from innate to acquired immunity.30 It is also used as an aid in diagnosing PJI; it has sensitivity ranging from 62% to 100% and specificity ranging from 85% to 100%.5,6,26,31,32 Synovial IL-6 measurements were more accurate than serum IL-6 measurements.26 Furthermore, synovial IL-6 can be increased up to 27-fold in PJI cases.5 In one study, synovial IL-6 levels >2100 pg/mL had sensitivity of 62.5% and specificity of 85.7% in PJI diagnosis26; in another study, an IL-6 threshold of 4270 pg/mL had sensitivity of 87.1%, specificity of 100%, and accuracy of 94.6%.31
C-Reactive Protein
CRP is an acute-phase reactant. Blood levels increase in response to aseptic inflammatory processes and systemic infection.33 CRP plays an important role in host defense by activating complement and helping mediate phagocytosis.33,34 Although serum CRP levels have been used in diagnosing PJIs,6 they can yield false-negative results.35,36 Therefore, attention turned to synovial CRP levels, which were found to be increased 13-fold in PJI cases.5 It has been shown that synovial CRP levels are significantly higher in infected vs noninfected prosthetic joints34 and had diagnostic accuracy better than that of serum CRP levels in diagnosing PJI.37 One study found that CRP at a threshold of 3.7 mg/L had sensitivity of 84%, specificity of 97.1%, and accuracy of 91.5%,37 whereas another study found that CRP at a threshold of 3.61 mg/L had sensitivity of 87.1%, specificity of 97.7%, and accuracy of 93.3%.31
α-Defensin
α-Defensin, a natural peptide produced and secreted by neutrophils in response to pathogens, has antimicrobial and cytotoxic properties,38-40 signals for the secretion of various cytokines, and acts as a chemoattractant for various immune cells.41 Deirmengian and colleagues6 found that α-defensin was consistently elevated in patients with PJI. α-Defensin is extremely accurate in diagnosing PJI; it has sensitivity ranging from 97% to 100% and specificity ranging from 96% to 100%.6,27,42 Moreover, α-defensin was effective in diagnosing PJI caused by a wide spectrum of organisms, including various low-virulence bacteria and fungi.43
Leukocyte Esterase
Leukocyte esterase is an enzyme produced and secreted by neutrophils at sites of active infection.7,44 Testing for this enzyme is performed with a colorimetric strip and was originally performed for the diagnosis of urinary tract infections.44,45 In a study conducted by Parvizi and colleagues,7 this strip was used to test for leukocyte esterase in synovial fluid samples; a ++ reading was found to have sensitivity of 80.6% and specificity of 100% in diagnosing knee PJI. Similarly, De Vecchi and colleagues45 found sensitivity of 92.6% and specificity of 97%.
Other Synovial Markers
Research has identified numerous molecular biomarkers that may be associated with the pathogenesis of PJI. Although several (eg, cytokines) have demonstrated higher levels in synovial fluid in patients with PJI than in normal controls, only a few have had clinically relevant diagnostic utility.6 Deirmengian and colleagues6 screened 43 synovial fluid biomarkers that potentially could be used in the diagnosis of PJI. Besides the cytokine α-defensin, 4 other biomarkers—lactoferrin, neutrophil gelatinase-associated lipocalcin, neutrophil elastase 2, and bactericidal/permeability-increasing protein—had accuracy of 100%. In addition, 8 cytokines and biomarkers (IL-8, CRP, resistin, thrombospondin, IL-1β, IL-6, IL-10, IL-1α) had area under the curve values higher than 0.9. Studies have also evaluated the diagnostic utility of metabolic products such as lactate, lactate dehydrogenase, and glucose; their accuracy was comparable to that of serum CRP.32
Serum Markers
In addition to the synovial fluid cytokines, several serum inflammatory cytokines have been studied as potential targets in diagnosing infection. Serum IL-6 has had excellent diagnostic accuracy46 and, when combined with CRP, could increase sensitivity in diagnosing PJI; such a combination (vs either test alone) could be useful in screening patients.47,48 Biomarkers such as tumor necrosis factor α and procalcitonin are considered very specific for PJI and may be useful in confirmatory testing.48 Evidence also suggests that toll-like receptor 2 proteins are elevated in the serum of patients with PJI and therefore are a potential diagnostic tool.49
Limitations of Synovial Cytokines
The literature suggests that some synovial fluid cytokines have promise.6 However, the best biomarker or combination of biomarkers is yet to be determined. Results have been consistent with α-defensin and other cytokines but mixed with IL-6 and still others32,42,50 (Table 2).
Information on the utility of synovial biomarkers in detecting persistent infection is limited. Frangiamore and colleagues50 found that IL-1 and IL-6 levels decreased between the stages of 2-stage revision. Unfortunately, none of the synovial fluid cytokines investigated (IL-1, IL-2, IL-6, IL-8, Il-10, interferon γ, granulocyte macrophage-colony stimulating factor, tumor necrosis factor α, IL-12p70) satisfactorily detected resolution of infection in the setting of prior treatment for PJI. Although cytokines are expected to be elevated in the presence of infection, the internal milieu at the time of stage 2 of the revision makes diagnosis of infection difficult. In addition, presence of spacer particles and recent surgery may activate immune pathways and yield false-positive results. Furthermore, antibiotic cement spacers may suppress the microorganisms to very low levels and yield false-negative results even if these organisms remain virulent.19
Even though the synovial molecular markers can detect the presence of infection, they are unable to identify pathogens. As identifying the pathogen is important in the treatment of PJI, there has been interest in using polymerase chain reaction (PCR) techniques.51 These tests may also provide specific information about the pathogen, such as its antibiotic sensitivity. A recently developed technology, the Ibis T5000 Universal Biosensor (Ibis Biosciences), uses novel pan-domain primers in a series of PCRs. This biosensor is useful in diagnosing infections when cultures are negative and appears to be more accurate than conventional PCR.13 As reported by Jacovides and colleagues,13 this novel PCR technique identified an organism in about 88% of presumed cases of aseptic revision.
Conclusion
PJI poses an extreme challenge to the healthcare system. Given the morbidity associated with improper management of PJI, accurate diagnosis is of paramount importance. Given the limitations of current tests, synovial fluid cytokines hold promise in the diagnosis of PJIs. However, these cytokines are expensive, and their clinical utility in PJI management is not well established. More research is needed before guidelines for synovial fluid cytokines and biomarkers can replace or be incorporated into guidelines for the treatment of PJIs.
Take-Home Points
- In cases of failed TJA, it is important to differentiate between septic and aseptic etiologies.
- Chronic and low-grade infections are challenging for orthopedic surgeons, as the symptoms often overlap with aseptic etiologies.
- Verification of infection eradication before beginning the second-stage reimplantation surgery is extremely important, but pre- and intraoperative findings can be unreliable.
- Synovial fluid cytokines have been shown to accurately diagnose PJIs.
- Synovial fluid cytokines may help surgeons differentiate between septic and aseptic cases of failed TJA.
Total joint arthroplasty (TJA) is an effective procedure that has been extensively used to relieve pain and improve quality of life in patients with various forms of joint disease. Although advances in technology and surgical technique have improved the success of TJA, periprosthetic joint infection (PJI) remains a serious complication. In the United States, it is estimated that PJI is the most common reason for total knee arthroplasty failure and the third most common reason for total hip arthroplasty revision.1 Although the incidence of PJI is 1% to 2%, the dramatic increase in TJA volume is expected to be accompanied by a similar rise in the number of infected TJAs; that number is expected to exceed 60,000 in the United States by 2020.2 Moreover, management of PJI is expensive and imposes a heavy burden on the healthcare system, with costs expected to hit $20 billion by 2020 in the US.2 Therefore, treating asepsis cases as infections imposes a heavy burden on the healthcare system and may result in excessive morbidity.3 At the same time, inadequate management of a PJI may result in recurrences that require infection treatment with morbid procedures, such as arthrodesis or amputation. Accurate diagnosis of PJI is of paramount importance in preventing potential implications of a misdiagnosed case. Unfortunately, the PJI diagnosis is extremely challenging, and the available diagnostic tests are often unreliable.4 Thus, research has recently focused on use of several synovial fluid cytokines in the detection of PJI.5-7 In this article, we provide an overview of the synovial biomarkers being used to diagnose PJI.
Diagnosis of Periprosthetic Joint Infection
Differentiating between septic and aseptic failed TJA is important, as the treatment options differ considerably. PJI can be broadly classified as acute or early postoperative (<6 weeks), late chronic (indolent onset), and acute-on-chronic (acute onset in well-functioning prosthesis, secondary to hematogenous spread).8 The acute and acute-on-chronic presentations are often associated with obvious signs of infection.9 However, chronic and low-grade infections pose a challenge to modern orthopedic practice, as the symptoms often overlap with that of aseptic causes of TJA failure.10 As a result, the International Consensus Group on Periprosthetic Joint Infection developed complex criteria using the Musculoskeletal Infection Society definition of PJI and involving a battery of tests for PJI diagnosis.11 According to these criteria, PJI is diagnosed when 1 of the 2 major criteria or 3 of the 5 minor criteria are met (Table 1).
Although these criteria constitute the most agreed on and widely used standard for PJI diagnosis, the definition is complex and often incomplete until surgical intervention. An ideal diagnostic test would aid in managing a PJI and provide results before a treatment decision is made. Many revision surgeries are being performed with insufficient information about the true diagnosis, and the diagnosis might change during or after surgery. About 10% of the revisions presumed to be aseptic may unexpectedly grow cultures during surgery and thereby satisfy the criteria for PJI after surgery.12 Moreover, with the use of novel methods such as polymerase chain reaction, microorganisms were identified in more than three-fourths of the presumed aseptic revisions.13 The optimal management of such cases is controversial, and it is unclear whether positive cultures should be treated as possible contaminants or true infection.12,14
Verification of Infection Eradication
A 2-stage revision procedure, widely accepted as the standard treatment for PJI, has success rates approaching 94%.15 In this procedure, it is important to verify infection eradication before beginning the second-stage reimplantation. Verification is crucial in avoiding reimplantation of an infected joint.16 After the first stage, patients are usually administered intravenous antibiotics for at least 6 weeks; these antibiotics are then withheld, and systemic inflammatory markers are evaluated for infection eradication. Although reliable criteria have been established for PJI diagnosis, guidelines for detecting eradication of infection are rudimentary. Most surgeons monitor the decrease in serologic markers, such as erythrocyte sedimentation rate and C-reactive protein (CRP) level, to assess the response to treatment. However, noninfectious etiologies may result in continued elevation of these markers.17 Even though aspirations are often performed to diagnose persistent infection before the second-stage procedure, their diagnostic utility may be limited.18 Use of cultures is also limited, as presence of antibiotic-loaded spacers can decrease the sensitivity of culture.19 Inadequate diagnosis often leads to unnecessary continuation of antimicrobial therapy or additional surgical débridement. Nuclear scans often remain positive because of aseptic inflammation related to surgery and are not useful in documenting sepsis arrest.20 Given the limitations of available tests, novel strategies for identifying the presence of infection at the second stage are being tested.
Synovial Fluid Cytokines
PJI pathogenesis begins with colonization of the implant surfaces with microorganisms and subsequent formation of biofilms.21 The human immune system is activated by the microbial products, cell wall components, and various biofilm proteins. Immune cells are recruited to the site, where they secrete a myriad of inflammatory biomarkers, such as cytokines, which promote further recruitment of inflammatory cells and aid in the eradication of pathogens.9 These inflammatory cytokines and cells are involved in aseptic inflammatory joint conditions, such as rheumatoid arthritis22,23; however, some are specifically involved in immune pathways combating pathogens.24 This action is the basis for increasing interest in using various synovial fluid cytokines and other biomarkers in the diagnosis of PJI. Here we describe some of the commonly studied cytokines.
Interleukin 1β
Interleukin 1β (IL-1β) is a major proinflammatory cytokine that is synthesized by multiple cells, including macrophages and monocytes.25 IL-1β is produced in response to microorganisms, other cytokines, antigen-presenting cells, and immune complexes; stimulates production of acute-phase proteins by the liver; and is an important pyrogen.25 Deirmengian and colleagues5 found that synovial IL-1β increased 258-fold in patients with a PJI. Studies have found that synovial IL-1β has sensitivity ranging from 66.7% to 100% and specificity ranging from 87% to 100%, with 1 study reporting an accuracy of 100%.5,6,26,27
Interleukin 6
Also produced by macrophages and monocytes, interleukin 6 (IL-6) is a potent stimulator of acute-phase proteins.28,29 IL-6 has a role as a chemoattractant and helps with cell differentiation when changing from innate to acquired immunity.30 It is also used as an aid in diagnosing PJI; it has sensitivity ranging from 62% to 100% and specificity ranging from 85% to 100%.5,6,26,31,32 Synovial IL-6 measurements were more accurate than serum IL-6 measurements.26 Furthermore, synovial IL-6 can be increased up to 27-fold in PJI cases.5 In one study, synovial IL-6 levels >2100 pg/mL had sensitivity of 62.5% and specificity of 85.7% in PJI diagnosis26; in another study, an IL-6 threshold of 4270 pg/mL had sensitivity of 87.1%, specificity of 100%, and accuracy of 94.6%.31
C-Reactive Protein
CRP is an acute-phase reactant. Blood levels increase in response to aseptic inflammatory processes and systemic infection.33 CRP plays an important role in host defense by activating complement and helping mediate phagocytosis.33,34 Although serum CRP levels have been used in diagnosing PJIs,6 they can yield false-negative results.35,36 Therefore, attention turned to synovial CRP levels, which were found to be increased 13-fold in PJI cases.5 It has been shown that synovial CRP levels are significantly higher in infected vs noninfected prosthetic joints34 and had diagnostic accuracy better than that of serum CRP levels in diagnosing PJI.37 One study found that CRP at a threshold of 3.7 mg/L had sensitivity of 84%, specificity of 97.1%, and accuracy of 91.5%,37 whereas another study found that CRP at a threshold of 3.61 mg/L had sensitivity of 87.1%, specificity of 97.7%, and accuracy of 93.3%.31
α-Defensin
α-Defensin, a natural peptide produced and secreted by neutrophils in response to pathogens, has antimicrobial and cytotoxic properties,38-40 signals for the secretion of various cytokines, and acts as a chemoattractant for various immune cells.41 Deirmengian and colleagues6 found that α-defensin was consistently elevated in patients with PJI. α-Defensin is extremely accurate in diagnosing PJI; it has sensitivity ranging from 97% to 100% and specificity ranging from 96% to 100%.6,27,42 Moreover, α-defensin was effective in diagnosing PJI caused by a wide spectrum of organisms, including various low-virulence bacteria and fungi.43
Leukocyte Esterase
Leukocyte esterase is an enzyme produced and secreted by neutrophils at sites of active infection.7,44 Testing for this enzyme is performed with a colorimetric strip and was originally performed for the diagnosis of urinary tract infections.44,45 In a study conducted by Parvizi and colleagues,7 this strip was used to test for leukocyte esterase in synovial fluid samples; a ++ reading was found to have sensitivity of 80.6% and specificity of 100% in diagnosing knee PJI. Similarly, De Vecchi and colleagues45 found sensitivity of 92.6% and specificity of 97%.
Other Synovial Markers
Research has identified numerous molecular biomarkers that may be associated with the pathogenesis of PJI. Although several (eg, cytokines) have demonstrated higher levels in synovial fluid in patients with PJI than in normal controls, only a few have had clinically relevant diagnostic utility.6 Deirmengian and colleagues6 screened 43 synovial fluid biomarkers that potentially could be used in the diagnosis of PJI. Besides the cytokine α-defensin, 4 other biomarkers—lactoferrin, neutrophil gelatinase-associated lipocalcin, neutrophil elastase 2, and bactericidal/permeability-increasing protein—had accuracy of 100%. In addition, 8 cytokines and biomarkers (IL-8, CRP, resistin, thrombospondin, IL-1β, IL-6, IL-10, IL-1α) had area under the curve values higher than 0.9. Studies have also evaluated the diagnostic utility of metabolic products such as lactate, lactate dehydrogenase, and glucose; their accuracy was comparable to that of serum CRP.32
Serum Markers
In addition to the synovial fluid cytokines, several serum inflammatory cytokines have been studied as potential targets in diagnosing infection. Serum IL-6 has had excellent diagnostic accuracy46 and, when combined with CRP, could increase sensitivity in diagnosing PJI; such a combination (vs either test alone) could be useful in screening patients.47,48 Biomarkers such as tumor necrosis factor α and procalcitonin are considered very specific for PJI and may be useful in confirmatory testing.48 Evidence also suggests that toll-like receptor 2 proteins are elevated in the serum of patients with PJI and therefore are a potential diagnostic tool.49
Limitations of Synovial Cytokines
The literature suggests that some synovial fluid cytokines have promise.6 However, the best biomarker or combination of biomarkers is yet to be determined. Results have been consistent with α-defensin and other cytokines but mixed with IL-6 and still others32,42,50 (Table 2).
Information on the utility of synovial biomarkers in detecting persistent infection is limited. Frangiamore and colleagues50 found that IL-1 and IL-6 levels decreased between the stages of 2-stage revision. Unfortunately, none of the synovial fluid cytokines investigated (IL-1, IL-2, IL-6, IL-8, Il-10, interferon γ, granulocyte macrophage-colony stimulating factor, tumor necrosis factor α, IL-12p70) satisfactorily detected resolution of infection in the setting of prior treatment for PJI. Although cytokines are expected to be elevated in the presence of infection, the internal milieu at the time of stage 2 of the revision makes diagnosis of infection difficult. In addition, presence of spacer particles and recent surgery may activate immune pathways and yield false-positive results. Furthermore, antibiotic cement spacers may suppress the microorganisms to very low levels and yield false-negative results even if these organisms remain virulent.19
Even though the synovial molecular markers can detect the presence of infection, they are unable to identify pathogens. As identifying the pathogen is important in the treatment of PJI, there has been interest in using polymerase chain reaction (PCR) techniques.51 These tests may also provide specific information about the pathogen, such as its antibiotic sensitivity. A recently developed technology, the Ibis T5000 Universal Biosensor (Ibis Biosciences), uses novel pan-domain primers in a series of PCRs. This biosensor is useful in diagnosing infections when cultures are negative and appears to be more accurate than conventional PCR.13 As reported by Jacovides and colleagues,13 this novel PCR technique identified an organism in about 88% of presumed cases of aseptic revision.
Conclusion
PJI poses an extreme challenge to the healthcare system. Given the morbidity associated with improper management of PJI, accurate diagnosis is of paramount importance. Given the limitations of current tests, synovial fluid cytokines hold promise in the diagnosis of PJIs. However, these cytokines are expensive, and their clinical utility in PJI management is not well established. More research is needed before guidelines for synovial fluid cytokines and biomarkers can replace or be incorporated into guidelines for the treatment of PJIs.
1 Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
3. Sierra RJ, Trousdale RT, Pagnano MW. Above-the-knee amputation after a total knee replacement: prevalence, etiology, and functional outcome. J Bone Joint Surg Am. 2003;85(6):1000-1004.
4. Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88(4):869-882.
5. Deirmengian C, Hallab N, Tarabishy A, et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468(8):2017-2023.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
7. Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93(24):2242-2248.
8. Kuiper JW, Willink RT, Moojen DJF, van den Bekerom MP, Colen S. Treatment of acute periprosthetic infections with prosthesis retention: review of current concepts. World J Orthop. 2014;5(5):667-676.
9. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-1654.
10. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25.
11. Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331.
12. Saleh A, Guirguis A, Klika AK, Johnson L, Higuera CA, Barsoum WK. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty. 2014;29(11):2181-2186.
13. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)–based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
14. Barrack RL, Aggarwal A, Burnett RS, et al. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):94-99.
15. Macheras GA, Koutsostathis SD, Kateros K, Papadakis S, Anastasopoulos P. A two stage re-implantation protocol for the treatment of deep periprosthetic hip infection. Mid to long-term results. Hip Int. 2012;22(suppl 8):S54-S61.
16. George J, Kwiecien G, Klika AK, et al. Are frozen sections and MSIS criteria reliable at the time of reimplantation of two-stage revision arthroplasty? Clin Orthop Relat Res. 2016;474(7):1619-1626.
17. Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469(4):1002-1008.
18. Lonner JH, Siliski JM, Della Valle C, DiCesare P, Lotke PA. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop. 2001;30(4):305-309.
19. Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82(11):1552-1557.
20. Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39(1):66-78.
21. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158-168.
22. Fontana A, Hengartner H, Weber E, Fehr K, Grob PJ, Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49-53.
23. Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83(2):585-592.
24. Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel). 2014;7(5):545-594.
25. Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075-1079.
26. Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95(7):644-651.
27. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
28. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
29. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621-636.
30. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878-888.
31. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
32. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
33. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24(2):163-176.
34. Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplasty. 2012;27(8 suppl):12-16.
35. Ghanem E, Antoci V, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13(6):e444-e449.
36. Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35(11):1621-1626.
37. Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470(1):54-60.
38. Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105-128.
39. Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76(4):1427-1435.
40. Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104(6):1778-1783.
41. Ulm H, Wilmes M, Shai Y, Sahl HG. Antimicrobial host defensins—specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249.
42. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
43. Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473(7):2229-2235.
44. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
45. De Vecchi E, Villa F, Bortolin M, et al. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: a prospective study. Clin Microbiol Infect. 2016;22(6):555-560.
46. Di Cesare PE, Chang E, Preston CF, Liu C. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87(9):1921-1927.
47. Ettinger M, Calliess T, Kielstein JT, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis. 2015;61(3):332-341.
48. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89(1):94-99.
49. Galliera E, Drago L, Vassena C, et al. Toll-like receptor 2 in serum: a potential diagnostic marker of prosthetic joint infection? J Clin Microbiol. 2014;52(2):620-623.
50. Frangiamore SJ, Siqueira MB, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res. 2016;474(7):1630-1639.
51. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
52. Omar M, Ettinger M, Reichling M, et al. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97(2):173-176.
53. Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472(12):3997-4003.
54. Omar M, Ettinger M, Reichling M, et al. Preliminary results of a new test for rapid diagnosis of septic arthritis with use of leukocyte esterase and glucose reagent strips. J Bone Joint Surg Am. 2014;96(24):2032-2037.
1 Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
3. Sierra RJ, Trousdale RT, Pagnano MW. Above-the-knee amputation after a total knee replacement: prevalence, etiology, and functional outcome. J Bone Joint Surg Am. 2003;85(6):1000-1004.
4. Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88(4):869-882.
5. Deirmengian C, Hallab N, Tarabishy A, et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468(8):2017-2023.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
7. Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93(24):2242-2248.
8. Kuiper JW, Willink RT, Moojen DJF, van den Bekerom MP, Colen S. Treatment of acute periprosthetic infections with prosthesis retention: review of current concepts. World J Orthop. 2014;5(5):667-676.
9. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-1654.
10. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25.
11. Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331.
12. Saleh A, Guirguis A, Klika AK, Johnson L, Higuera CA, Barsoum WK. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty. 2014;29(11):2181-2186.
13. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)–based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
14. Barrack RL, Aggarwal A, Burnett RS, et al. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):94-99.
15. Macheras GA, Koutsostathis SD, Kateros K, Papadakis S, Anastasopoulos P. A two stage re-implantation protocol for the treatment of deep periprosthetic hip infection. Mid to long-term results. Hip Int. 2012;22(suppl 8):S54-S61.
16. George J, Kwiecien G, Klika AK, et al. Are frozen sections and MSIS criteria reliable at the time of reimplantation of two-stage revision arthroplasty? Clin Orthop Relat Res. 2016;474(7):1619-1626.
17. Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469(4):1002-1008.
18. Lonner JH, Siliski JM, Della Valle C, DiCesare P, Lotke PA. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop. 2001;30(4):305-309.
19. Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82(11):1552-1557.
20. Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39(1):66-78.
21. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158-168.
22. Fontana A, Hengartner H, Weber E, Fehr K, Grob PJ, Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49-53.
23. Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83(2):585-592.
24. Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel). 2014;7(5):545-594.
25. Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075-1079.
26. Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95(7):644-651.
27. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
28. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
29. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621-636.
30. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878-888.
31. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
32. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
33. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24(2):163-176.
34. Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplasty. 2012;27(8 suppl):12-16.
35. Ghanem E, Antoci V, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13(6):e444-e449.
36. Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35(11):1621-1626.
37. Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470(1):54-60.
38. Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105-128.
39. Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76(4):1427-1435.
40. Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104(6):1778-1783.
41. Ulm H, Wilmes M, Shai Y, Sahl HG. Antimicrobial host defensins—specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249.
42. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
43. Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473(7):2229-2235.
44. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
45. De Vecchi E, Villa F, Bortolin M, et al. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: a prospective study. Clin Microbiol Infect. 2016;22(6):555-560.
46. Di Cesare PE, Chang E, Preston CF, Liu C. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87(9):1921-1927.
47. Ettinger M, Calliess T, Kielstein JT, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis. 2015;61(3):332-341.
48. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89(1):94-99.
49. Galliera E, Drago L, Vassena C, et al. Toll-like receptor 2 in serum: a potential diagnostic marker of prosthetic joint infection? J Clin Microbiol. 2014;52(2):620-623.
50. Frangiamore SJ, Siqueira MB, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res. 2016;474(7):1630-1639.
51. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
52. Omar M, Ettinger M, Reichling M, et al. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97(2):173-176.
53. Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472(12):3997-4003.
54. Omar M, Ettinger M, Reichling M, et al. Preliminary results of a new test for rapid diagnosis of septic arthritis with use of leukocyte esterase and glucose reagent strips. J Bone Joint Surg Am. 2014;96(24):2032-2037.
Reverse Shoulder Arthroplasty and Latissimus Dorsi Tendon Transfer
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
Reliability of 3-Dimensional Glenoid Component Templating and Correlation to Intraoperative Component Selection
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
Management and Prevention of Intraoperative Acetabular Fracture in Primary Total Hip Arthroplasty
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
Is Simultaneous Bilateral Total Knee Arthroplasty (BTKA) as Safe as Staged BTKA?
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
Systematic Review of Novel Synovial Fluid Markers and Polymerase Chain Reaction in the Diagnosis of Prosthetic Joint Infection
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
Difficult-to-Detect Low-Grade Infections Responsible for Poor Outcomes in Total Knee Arthroplasty
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Primary Total Knee Arthroplasty for Distal Femur Fractures: A Systematic Review of Indications, Implants, Techniques, and Results
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
Acute Intraprosthetic Dissociation of a Dual-Mobility Hip in the United States
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.