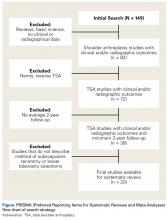
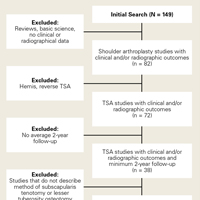
User login
Acute Intraprosthetic Dissociation of a Dual-Mobility Hip in the United States
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
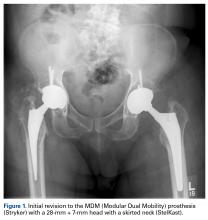
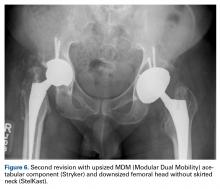
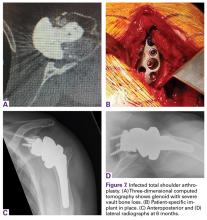
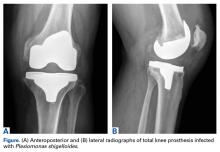
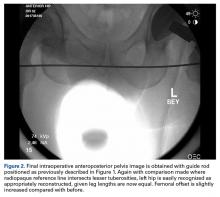
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
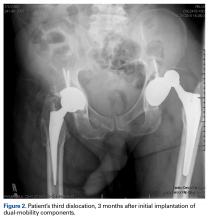
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
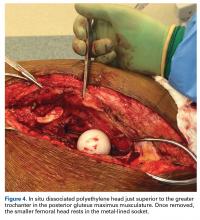
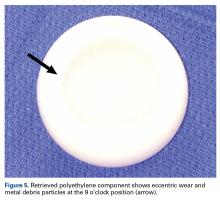
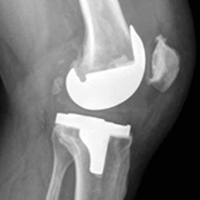
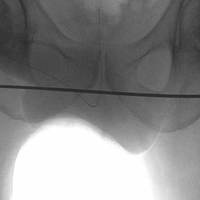
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
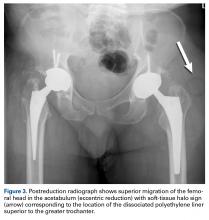
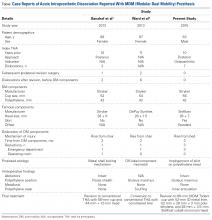
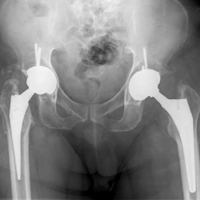
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
Joint-Preserving Osteotomies for Isolated Patellofemoral Osteoarthritis: Alternatives to Arthroplasty
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
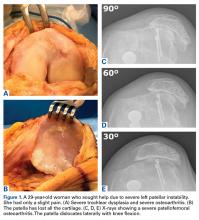
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
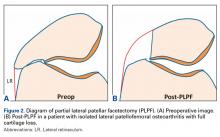
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
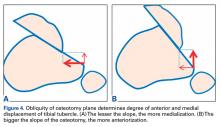
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
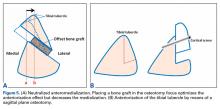
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
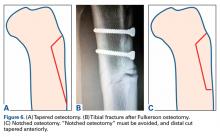
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
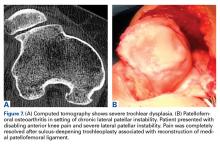
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
Subscapularis Tenotomy Versus Lesser Tuberosity Osteotomy for Total Shoulder Arthroplasty: A Systematic Review
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Novel Solution for Massive Glenoid Defects in Shoulder Arthroplasty: A Patient-Specific Glenoid Vault Reconstruction System
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
Robotic-Assisted Total Knee Arthroplasty
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Short-Term Projected Use of Reverse Total Shoulder Arthroplasty in Proximal Humerus Fracture Cases Recorded in Humana’s National Private-Payer Database
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.
Plesiomonas shigelloides Periprosthetic Knee Infection After Consumption of Raw Oysters
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
Using a Modified Ball-Tip Guide Rod to Equalize Leg Length and Restore Femoral Offset
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
Can a Total Knee Arthroplasty Perioperative Surgical Home Close the Gap Between Primary and Revision TKA Outcomes?
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
Comparing Cost, Efficacy, and Safety of Intravenous and Topical Tranexamic Acid in Total Hip and Knee Arthroplasty
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.