User login
Low Vitamin D Associated With Poor Prognostic Features in Breast Cancer
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
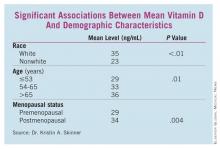
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Finding: Vitamin D deficiency was more than twice as common as normal levels in women undergoing surgery for breast cancer (OR, 2.4).
Data Source: A case-control study of vitamin D levels in 194 women with breast cancer matched 1:1 to a control population.
Disclosures: Dr. Skinner said she had no relevant disclosures.
CDER: Avastin Breast Cancer Claim Undermines Approval Process
The Food and Drug Administration’s accelerated approval process would operate as a lower approval standard if Genentech were allowed to maintain the metastatic breast cancer claim for Avastin, the Center for Drug Evaluation and Research states in a written summary of arguments to be presented at a June 28-29 hearing on the indication.
Current evidence does not justify allowing Avastin (bevacizumab) to retain an indication for combination use with paclitaxel in first-line metastatic breast cancer (MBC); doing so while Genentech designs and conducts additional confirmatory studies would jeopardize the integrity of the accelerated approval process, CDER says.
CDER also dispatches with Genentech’s argument that it should be allowed to demonstrate that the choice of chemotherapy partner is integral to Avastin’s benefit in MBC. Rather, the regulator asserts there is no proven scientific basis for Genentech’s view that Avastin’s effect is predicated upon substantive differences between paclitaxel and other chemotherapy agents.
The drug center stands by its view that as part of Avastin’s accelerated approval Genentech was required to confirm the magnitude of benefit seen in the E2100 pivotal trial, which the company has failed to do.
Making the Case
The document, released on May 16, summarizes the arguments CDER will make to presiding officer Dr. Karen Midthun and the Oncologic Drugs Advisory Committee at the June hearing.
In its written summary of arguments, Genentech said it does not plan to focus on the AVADO and RIBBON1 studies – which were intended to serve as confirmatory studies for Avastin’s accelerated approval in MBC. Rather, it will argue that accelerated approval should be maintained pending a confirmatory study of Avastin in combination with paclitaxel that also uses a biomarker to identify those patients most likely to benefit.
CDER’s filing makes clear the regulator’s focus will be both on the specific issues relating to the existing Avastin data as well as the bigger policy issue of accelerated approval standards.
CDER observes it took the "unprecedented step" of granting accelerated approval based solely on an interim analysis of progression-free survival in the E2100 trial, which studied Avastin in combination with paclitaxel. "This was the first approval of a nonhormonal agent in which evidence of a treatment effect on PFS [progression free survival] alone was viewed not as a surrogate end point, but rather as a clinical benefit because of the magnitude of improvement in PFS," CDER says.
Data from the E2100 trial showed a 5.5-month improvement in median PFS, and CDER granted accelerated approval because "in its best scientific judgment at that time, the magnitude of PFS in the single trial suggested that Avastin held promise for patients with MBC."
Two confirmatory trials studied Avastin in combination with chemotherapeutics other than paclitaxel. Avastin’s chemotherapy partner in AVADO was docetaxel, while RIBBON1 looked at Avastin in combination with taxanes, anthracyclines, or capecitabine. These studies showed statistically significant improvements in PFS ranging from less than one month to less than three months, well shy of the 5.5-month benefit seen with paclitaxel in E2100.
The "small effect" seen in the confirmatory trials was not enough to verify Avastin’s clinical benefit, CDER asserts.
"In order for the accelerated approval system to serve its purpose and not operate as a lower approval standard, CDER must be able to withdraw approvals when it determines, based upon careful consideration of the data, that the confirmatory trials have failed to verify clinical benefit," CDER says.
Accelerated Approval Encompasses "Accelerated Withdrawal"
"Accelerated withdrawal" is an integral part of the accelerated approval process, CDER maintains.
"Once postapproval trials fail to demonstrate the expected clinical benefit, the accelerated approval rubric does not contemplate – as Genentech argues – that the agency should ‘maintain’ approval while an applicant designs and conducts more trials with the hope of eventually verifying clinical benefit," CDER says. "If CDER were forced to allow products to stay on the market when the risk-benefit analysis shows that the product is not safe and effective for its intended use, the accelerated approval program would be significantly undermined."
The agency takes issue with Genentech’s assertion that the indication should be maintained as long as the data for Avastin in combination with paclitaxel continue to be reasonably likely to predict clinical benefit.
The "reasonably likely" language in the accelerated approval regulations refers to the relationship between a surrogate end point and clinical benefit, CDER explains. "In the case of Avastin’s MBC approval, there was no surrogate end point – CDER believed that 5.5 months of PFS could be deemed a clinical benefit, subject to confirmatory trials."
Genentech does not explain how it would enroll patients in a new, blinded, controlled trial of Avastin in combination with paclitaxel if this remains a labeled use, CDER says. Furthermore, such a study would likely take many years to complete.
"Rather than serving as a basis to ‘maintain’ approval, which is not part of the accelerated approval program, the new research proposed by Genentech, if completed favorably, could be used to support a new sBLA [Supplemental Biologics License Application] requesting an MBC indication."
Chemotherapy Partner Hypothesis Does Not Hold Water
Genentech has hypothesized that chemotherapy partners that provide for prolonged combined exposure with Avastin may result in the strongest treatment effect. Yet this hypothesis has not been substantiated by either clinical or nonclinical evidence, CDER says.
"To support this argument, CDER expects that there should be some proven scientific basis for substantial differences, such as evidence of drug interactions or synergistic/overlapping toxicity between Avastin and other chemotherapy drugs. There is none," CDER says. Evidence submitted to date, such as population pharmacokinetic analyses, suggest no unique interactions between Avastin and any of the chemotherapy agents administered in the trials.
"In the absence of a scientifically supported basis for chemotherapy-specific interactions the more likely explanation for the failure of the clinical trials to verify the results of the E2100 trial is that the magnitude of the PFS treatment effect observed in E2100 is an outlier."
Genentech’s argument on the importance of chemotherapy partner used also runs contrary to its prior position, CDER says, noting that the company initially sought broad approval of Avastin in combination with all taxane-based chemotherapies based solely on the E2100 data.
No One Is Moving Goal Posts at CDER
CDER further disputes Genentech’s argument that it has changed its approval standards for MBC treatments. The regulator consistently maintained in conversations with Genentech that the adequacy of PFS as an approval end point would depend upon the overall dataset and the magnitude of benefit, it says.
"CDER has not in any way sought to ‘move the goal posts,’ " the filing states. "CDER has not determined that a set magnitude of PFS improvement is needed to support an MBC indication; however, CDER has determined that the magnitude of PFS improvement shown in Avastin’s MBC post-approval studies, and shown in the totality of the relevant data, is so small that the risk-benefit balance is unfavorable."
This coverage is provided courtesy of "The Pink Sheet." "The Pink Sheet" and Internal Medicine News Digital Network are both owned by Elsevier.
The Food and Drug Administration’s accelerated approval process would operate as a lower approval standard if Genentech were allowed to maintain the metastatic breast cancer claim for Avastin, the Center for Drug Evaluation and Research states in a written summary of arguments to be presented at a June 28-29 hearing on the indication.
Current evidence does not justify allowing Avastin (bevacizumab) to retain an indication for combination use with paclitaxel in first-line metastatic breast cancer (MBC); doing so while Genentech designs and conducts additional confirmatory studies would jeopardize the integrity of the accelerated approval process, CDER says.
CDER also dispatches with Genentech’s argument that it should be allowed to demonstrate that the choice of chemotherapy partner is integral to Avastin’s benefit in MBC. Rather, the regulator asserts there is no proven scientific basis for Genentech’s view that Avastin’s effect is predicated upon substantive differences between paclitaxel and other chemotherapy agents.
The drug center stands by its view that as part of Avastin’s accelerated approval Genentech was required to confirm the magnitude of benefit seen in the E2100 pivotal trial, which the company has failed to do.
Making the Case
The document, released on May 16, summarizes the arguments CDER will make to presiding officer Dr. Karen Midthun and the Oncologic Drugs Advisory Committee at the June hearing.
In its written summary of arguments, Genentech said it does not plan to focus on the AVADO and RIBBON1 studies – which were intended to serve as confirmatory studies for Avastin’s accelerated approval in MBC. Rather, it will argue that accelerated approval should be maintained pending a confirmatory study of Avastin in combination with paclitaxel that also uses a biomarker to identify those patients most likely to benefit.
CDER’s filing makes clear the regulator’s focus will be both on the specific issues relating to the existing Avastin data as well as the bigger policy issue of accelerated approval standards.
CDER observes it took the "unprecedented step" of granting accelerated approval based solely on an interim analysis of progression-free survival in the E2100 trial, which studied Avastin in combination with paclitaxel. "This was the first approval of a nonhormonal agent in which evidence of a treatment effect on PFS [progression free survival] alone was viewed not as a surrogate end point, but rather as a clinical benefit because of the magnitude of improvement in PFS," CDER says.
Data from the E2100 trial showed a 5.5-month improvement in median PFS, and CDER granted accelerated approval because "in its best scientific judgment at that time, the magnitude of PFS in the single trial suggested that Avastin held promise for patients with MBC."
Two confirmatory trials studied Avastin in combination with chemotherapeutics other than paclitaxel. Avastin’s chemotherapy partner in AVADO was docetaxel, while RIBBON1 looked at Avastin in combination with taxanes, anthracyclines, or capecitabine. These studies showed statistically significant improvements in PFS ranging from less than one month to less than three months, well shy of the 5.5-month benefit seen with paclitaxel in E2100.
The "small effect" seen in the confirmatory trials was not enough to verify Avastin’s clinical benefit, CDER asserts.
"In order for the accelerated approval system to serve its purpose and not operate as a lower approval standard, CDER must be able to withdraw approvals when it determines, based upon careful consideration of the data, that the confirmatory trials have failed to verify clinical benefit," CDER says.
Accelerated Approval Encompasses "Accelerated Withdrawal"
"Accelerated withdrawal" is an integral part of the accelerated approval process, CDER maintains.
"Once postapproval trials fail to demonstrate the expected clinical benefit, the accelerated approval rubric does not contemplate – as Genentech argues – that the agency should ‘maintain’ approval while an applicant designs and conducts more trials with the hope of eventually verifying clinical benefit," CDER says. "If CDER were forced to allow products to stay on the market when the risk-benefit analysis shows that the product is not safe and effective for its intended use, the accelerated approval program would be significantly undermined."
The agency takes issue with Genentech’s assertion that the indication should be maintained as long as the data for Avastin in combination with paclitaxel continue to be reasonably likely to predict clinical benefit.
The "reasonably likely" language in the accelerated approval regulations refers to the relationship between a surrogate end point and clinical benefit, CDER explains. "In the case of Avastin’s MBC approval, there was no surrogate end point – CDER believed that 5.5 months of PFS could be deemed a clinical benefit, subject to confirmatory trials."
Genentech does not explain how it would enroll patients in a new, blinded, controlled trial of Avastin in combination with paclitaxel if this remains a labeled use, CDER says. Furthermore, such a study would likely take many years to complete.
"Rather than serving as a basis to ‘maintain’ approval, which is not part of the accelerated approval program, the new research proposed by Genentech, if completed favorably, could be used to support a new sBLA [Supplemental Biologics License Application] requesting an MBC indication."
Chemotherapy Partner Hypothesis Does Not Hold Water
Genentech has hypothesized that chemotherapy partners that provide for prolonged combined exposure with Avastin may result in the strongest treatment effect. Yet this hypothesis has not been substantiated by either clinical or nonclinical evidence, CDER says.
"To support this argument, CDER expects that there should be some proven scientific basis for substantial differences, such as evidence of drug interactions or synergistic/overlapping toxicity between Avastin and other chemotherapy drugs. There is none," CDER says. Evidence submitted to date, such as population pharmacokinetic analyses, suggest no unique interactions between Avastin and any of the chemotherapy agents administered in the trials.
"In the absence of a scientifically supported basis for chemotherapy-specific interactions the more likely explanation for the failure of the clinical trials to verify the results of the E2100 trial is that the magnitude of the PFS treatment effect observed in E2100 is an outlier."
Genentech’s argument on the importance of chemotherapy partner used also runs contrary to its prior position, CDER says, noting that the company initially sought broad approval of Avastin in combination with all taxane-based chemotherapies based solely on the E2100 data.
No One Is Moving Goal Posts at CDER
CDER further disputes Genentech’s argument that it has changed its approval standards for MBC treatments. The regulator consistently maintained in conversations with Genentech that the adequacy of PFS as an approval end point would depend upon the overall dataset and the magnitude of benefit, it says.
"CDER has not in any way sought to ‘move the goal posts,’ " the filing states. "CDER has not determined that a set magnitude of PFS improvement is needed to support an MBC indication; however, CDER has determined that the magnitude of PFS improvement shown in Avastin’s MBC post-approval studies, and shown in the totality of the relevant data, is so small that the risk-benefit balance is unfavorable."
This coverage is provided courtesy of "The Pink Sheet." "The Pink Sheet" and Internal Medicine News Digital Network are both owned by Elsevier.
The Food and Drug Administration’s accelerated approval process would operate as a lower approval standard if Genentech were allowed to maintain the metastatic breast cancer claim for Avastin, the Center for Drug Evaluation and Research states in a written summary of arguments to be presented at a June 28-29 hearing on the indication.
Current evidence does not justify allowing Avastin (bevacizumab) to retain an indication for combination use with paclitaxel in first-line metastatic breast cancer (MBC); doing so while Genentech designs and conducts additional confirmatory studies would jeopardize the integrity of the accelerated approval process, CDER says.
CDER also dispatches with Genentech’s argument that it should be allowed to demonstrate that the choice of chemotherapy partner is integral to Avastin’s benefit in MBC. Rather, the regulator asserts there is no proven scientific basis for Genentech’s view that Avastin’s effect is predicated upon substantive differences between paclitaxel and other chemotherapy agents.
The drug center stands by its view that as part of Avastin’s accelerated approval Genentech was required to confirm the magnitude of benefit seen in the E2100 pivotal trial, which the company has failed to do.
Making the Case
The document, released on May 16, summarizes the arguments CDER will make to presiding officer Dr. Karen Midthun and the Oncologic Drugs Advisory Committee at the June hearing.
In its written summary of arguments, Genentech said it does not plan to focus on the AVADO and RIBBON1 studies – which were intended to serve as confirmatory studies for Avastin’s accelerated approval in MBC. Rather, it will argue that accelerated approval should be maintained pending a confirmatory study of Avastin in combination with paclitaxel that also uses a biomarker to identify those patients most likely to benefit.
CDER’s filing makes clear the regulator’s focus will be both on the specific issues relating to the existing Avastin data as well as the bigger policy issue of accelerated approval standards.
CDER observes it took the "unprecedented step" of granting accelerated approval based solely on an interim analysis of progression-free survival in the E2100 trial, which studied Avastin in combination with paclitaxel. "This was the first approval of a nonhormonal agent in which evidence of a treatment effect on PFS [progression free survival] alone was viewed not as a surrogate end point, but rather as a clinical benefit because of the magnitude of improvement in PFS," CDER says.
Data from the E2100 trial showed a 5.5-month improvement in median PFS, and CDER granted accelerated approval because "in its best scientific judgment at that time, the magnitude of PFS in the single trial suggested that Avastin held promise for patients with MBC."
Two confirmatory trials studied Avastin in combination with chemotherapeutics other than paclitaxel. Avastin’s chemotherapy partner in AVADO was docetaxel, while RIBBON1 looked at Avastin in combination with taxanes, anthracyclines, or capecitabine. These studies showed statistically significant improvements in PFS ranging from less than one month to less than three months, well shy of the 5.5-month benefit seen with paclitaxel in E2100.
The "small effect" seen in the confirmatory trials was not enough to verify Avastin’s clinical benefit, CDER asserts.
"In order for the accelerated approval system to serve its purpose and not operate as a lower approval standard, CDER must be able to withdraw approvals when it determines, based upon careful consideration of the data, that the confirmatory trials have failed to verify clinical benefit," CDER says.
Accelerated Approval Encompasses "Accelerated Withdrawal"
"Accelerated withdrawal" is an integral part of the accelerated approval process, CDER maintains.
"Once postapproval trials fail to demonstrate the expected clinical benefit, the accelerated approval rubric does not contemplate – as Genentech argues – that the agency should ‘maintain’ approval while an applicant designs and conducts more trials with the hope of eventually verifying clinical benefit," CDER says. "If CDER were forced to allow products to stay on the market when the risk-benefit analysis shows that the product is not safe and effective for its intended use, the accelerated approval program would be significantly undermined."
The agency takes issue with Genentech’s assertion that the indication should be maintained as long as the data for Avastin in combination with paclitaxel continue to be reasonably likely to predict clinical benefit.
The "reasonably likely" language in the accelerated approval regulations refers to the relationship between a surrogate end point and clinical benefit, CDER explains. "In the case of Avastin’s MBC approval, there was no surrogate end point – CDER believed that 5.5 months of PFS could be deemed a clinical benefit, subject to confirmatory trials."
Genentech does not explain how it would enroll patients in a new, blinded, controlled trial of Avastin in combination with paclitaxel if this remains a labeled use, CDER says. Furthermore, such a study would likely take many years to complete.
"Rather than serving as a basis to ‘maintain’ approval, which is not part of the accelerated approval program, the new research proposed by Genentech, if completed favorably, could be used to support a new sBLA [Supplemental Biologics License Application] requesting an MBC indication."
Chemotherapy Partner Hypothesis Does Not Hold Water
Genentech has hypothesized that chemotherapy partners that provide for prolonged combined exposure with Avastin may result in the strongest treatment effect. Yet this hypothesis has not been substantiated by either clinical or nonclinical evidence, CDER says.
"To support this argument, CDER expects that there should be some proven scientific basis for substantial differences, such as evidence of drug interactions or synergistic/overlapping toxicity between Avastin and other chemotherapy drugs. There is none," CDER says. Evidence submitted to date, such as population pharmacokinetic analyses, suggest no unique interactions between Avastin and any of the chemotherapy agents administered in the trials.
"In the absence of a scientifically supported basis for chemotherapy-specific interactions the more likely explanation for the failure of the clinical trials to verify the results of the E2100 trial is that the magnitude of the PFS treatment effect observed in E2100 is an outlier."
Genentech’s argument on the importance of chemotherapy partner used also runs contrary to its prior position, CDER says, noting that the company initially sought broad approval of Avastin in combination with all taxane-based chemotherapies based solely on the E2100 data.
No One Is Moving Goal Posts at CDER
CDER further disputes Genentech’s argument that it has changed its approval standards for MBC treatments. The regulator consistently maintained in conversations with Genentech that the adequacy of PFS as an approval end point would depend upon the overall dataset and the magnitude of benefit, it says.
"CDER has not in any way sought to ‘move the goal posts,’ " the filing states. "CDER has not determined that a set magnitude of PFS improvement is needed to support an MBC indication; however, CDER has determined that the magnitude of PFS improvement shown in Avastin’s MBC post-approval studies, and shown in the totality of the relevant data, is so small that the risk-benefit balance is unfavorable."
This coverage is provided courtesy of "The Pink Sheet." "The Pink Sheet" and Internal Medicine News Digital Network are both owned by Elsevier.
FROM THE CENTER FOR DRUG EVALUATION AND RESEARCH
Genentech Appeals to FDAs Regulatory Flexibility on Avastin
Genentech’s advance summary of evidence and arguments for the Food and Drug Administration’s upcoming hearing on the agency’s proposed withdrawal of the metastatic breast cancer approval for Avastin shows that the company is trying to keep the focus on the additional confirmatory trial it proposes to conduct.
The document, released May 13, provides an overview of the case Genentech will make to support maintaining the accelerated approval of bevacizumab, in combination with paclitaxel, for first-line metastatic breast cancer (MBC) at the June 28-29 hearing, as requested by the presiding officer, Center for Biologics Evaluation and Research Director Karen Midthun.
Dr. Midthun recently released the agenda for the hearing and the issues that are to be discussed. These include whether the AVADO and RIBBON1 trials failed to verify Avastin’s clinical benefit in MBC as seen in the pivotal E2100 study, whether the available evidence shows it is safe and effective, and whether the FDA should withdraw the approval.
In its prehearing summary of evidence, Genentech notes it has recognized the view of the Center for Drug Evaluation and Research (CDER) and the Oncologic Drugs Advisory Committee that the AVADO and RIBBON1 trials failed to confirm the benefit and has responded by tailoring its proposal to maintaining accelerated approval for use with paclitaxel "subject to" an additional confirmatory trial.
Thus, the company does not plan to focus on AVADO and RIBBON1, the summary explains. "Instead, Genentech intends to focus on the question of whether accelerated approval should be maintained for Avastin and paclitaxel subject to a further confirmatory study of this combination, even in light of the views of CDER and the ODAC on AVADO and RIBBON1."
"The only open question is whether the magnitude of benefit observed in the E2100 study is reasonably likely to be confirmed in a second study of Avastin with paclitaxel," the company argues.
"Given the meaningful probability that the chemotherapy partner has an impact on the magnitude of benefit, and given that an additional study can resolve this question, accelerated approval should be maintained pending completion of Genentech’s further study."
The company also says that continuing the approval while gaining further evidence fits with the overall goals of the accelerated approval program and that the accelerated approval regime allows the FDA that regulatory flexibility.
The New Confirmatory Trial
Genentech first proposed conducting a new confirmatory trial in August 2010 after the FDA’s Oncologic Drugs Advisory Committee voted against continued approval in MBC, and that proposal was a pivotal point in its request for a hearing on FDA’s proposed withdrawal.
The company intends to conduct a new trial – a double-blind, randomized, multicenter phase III trial of Avastin in combination with weekly paclitaxel – using a biomarker to select for patients more likely to derive a greater benefit from treatment, which was an option alluded to in CDER’s memo on the proposed withdrawal.
"Based on the company’s extensive research, including a new assay sensitive to specific isoforms, plasma concentration of VEGF-A (Avastin’s target) has been identified as a potential predictive biomarker in breast, gastric and pancreatic cancers," the summary of evidence states.
An analysis of the AVADO trial, presented as an abstract at the San Antonio Breast Cancer Symposium in December, showed that patients with high levels of VEGF-A had a hazard ratio for progression-free survival analysis of 0.49, compared with 0.87 for patients with low levels of VEGF-A. "The data suggest that patients with high VEGF-A levels may be more likely to derive a substantial benefit from Avastin, and it is important and appropriate to validate this predictive biomarker in a prospective phase III trial," Genentech argued.
The company met with the agency to discuss the trial on Feb. 22, the preparatory documents for the hearing reveal, the day before the hearing was granted. At that meeting, Genentech reports, CDER stated that "PFS [progression free survival] results confirming the magnitude of treatment effect observed in E2100 without a detriment to OS [overall survival], coupled with the E2100 data, would support Avastin’s full approval with paclitaxel."
The company has proceeded with study planning and intends to submit the protocol under a Special Protocol Assessment, the summary states.
Nothing Wrong With E2100
Genentech presented an overview of the E2100 findings that emphasize their statistical rigor and clinical significance, an essential point of agreement to support continuing the accelerated approval based upon those findings while another confirmatory trial is conducted.
In the context of Dr. Midthun’s posed question on whether Avastin’s benefit-risk ratio remains favorable in MBC, the company notes that withdrawal would "necessarily rest on a determination that the findings from the E2100 study are no longer reliable and that Avastin presents unique toxicity concerns in the MBC setting."
"Respectfully, that determination would be contrary to the most reasonable interpretation of the scientific data."
In addition to defending E2100, the summary shows that Genentech will focus on FDA’s application of the accelerated approval standard, the external support for Avastin’s use in MBC (including that of other regulatory bodies), and the agency’s potential regulatory flexibility.
In the end, it’s that flexibility that Genentech is really appealing to. "Regulatory flexibility is intended for cases like this," the summary concludes, "where a formulaic application of the withdrawal standard would deprive patients and physicians of the choice intended by the accelerated approval program – and do so in the face of continued findings of benefit, a well-understood safety profile, and a viable option for providing a clearer answer to the remaining scientific questions."
Internal Medicine News Digital Network and "The Pink Sheet" are published by Elsevier.
Genentech’s advance summary of evidence and arguments for the Food and Drug Administration’s upcoming hearing on the agency’s proposed withdrawal of the metastatic breast cancer approval for Avastin shows that the company is trying to keep the focus on the additional confirmatory trial it proposes to conduct.
The document, released May 13, provides an overview of the case Genentech will make to support maintaining the accelerated approval of bevacizumab, in combination with paclitaxel, for first-line metastatic breast cancer (MBC) at the June 28-29 hearing, as requested by the presiding officer, Center for Biologics Evaluation and Research Director Karen Midthun.
Dr. Midthun recently released the agenda for the hearing and the issues that are to be discussed. These include whether the AVADO and RIBBON1 trials failed to verify Avastin’s clinical benefit in MBC as seen in the pivotal E2100 study, whether the available evidence shows it is safe and effective, and whether the FDA should withdraw the approval.
In its prehearing summary of evidence, Genentech notes it has recognized the view of the Center for Drug Evaluation and Research (CDER) and the Oncologic Drugs Advisory Committee that the AVADO and RIBBON1 trials failed to confirm the benefit and has responded by tailoring its proposal to maintaining accelerated approval for use with paclitaxel "subject to" an additional confirmatory trial.
Thus, the company does not plan to focus on AVADO and RIBBON1, the summary explains. "Instead, Genentech intends to focus on the question of whether accelerated approval should be maintained for Avastin and paclitaxel subject to a further confirmatory study of this combination, even in light of the views of CDER and the ODAC on AVADO and RIBBON1."
"The only open question is whether the magnitude of benefit observed in the E2100 study is reasonably likely to be confirmed in a second study of Avastin with paclitaxel," the company argues.
"Given the meaningful probability that the chemotherapy partner has an impact on the magnitude of benefit, and given that an additional study can resolve this question, accelerated approval should be maintained pending completion of Genentech’s further study."
The company also says that continuing the approval while gaining further evidence fits with the overall goals of the accelerated approval program and that the accelerated approval regime allows the FDA that regulatory flexibility.
The New Confirmatory Trial
Genentech first proposed conducting a new confirmatory trial in August 2010 after the FDA’s Oncologic Drugs Advisory Committee voted against continued approval in MBC, and that proposal was a pivotal point in its request for a hearing on FDA’s proposed withdrawal.
The company intends to conduct a new trial – a double-blind, randomized, multicenter phase III trial of Avastin in combination with weekly paclitaxel – using a biomarker to select for patients more likely to derive a greater benefit from treatment, which was an option alluded to in CDER’s memo on the proposed withdrawal.
"Based on the company’s extensive research, including a new assay sensitive to specific isoforms, plasma concentration of VEGF-A (Avastin’s target) has been identified as a potential predictive biomarker in breast, gastric and pancreatic cancers," the summary of evidence states.
An analysis of the AVADO trial, presented as an abstract at the San Antonio Breast Cancer Symposium in December, showed that patients with high levels of VEGF-A had a hazard ratio for progression-free survival analysis of 0.49, compared with 0.87 for patients with low levels of VEGF-A. "The data suggest that patients with high VEGF-A levels may be more likely to derive a substantial benefit from Avastin, and it is important and appropriate to validate this predictive biomarker in a prospective phase III trial," Genentech argued.
The company met with the agency to discuss the trial on Feb. 22, the preparatory documents for the hearing reveal, the day before the hearing was granted. At that meeting, Genentech reports, CDER stated that "PFS [progression free survival] results confirming the magnitude of treatment effect observed in E2100 without a detriment to OS [overall survival], coupled with the E2100 data, would support Avastin’s full approval with paclitaxel."
The company has proceeded with study planning and intends to submit the protocol under a Special Protocol Assessment, the summary states.
Nothing Wrong With E2100
Genentech presented an overview of the E2100 findings that emphasize their statistical rigor and clinical significance, an essential point of agreement to support continuing the accelerated approval based upon those findings while another confirmatory trial is conducted.
In the context of Dr. Midthun’s posed question on whether Avastin’s benefit-risk ratio remains favorable in MBC, the company notes that withdrawal would "necessarily rest on a determination that the findings from the E2100 study are no longer reliable and that Avastin presents unique toxicity concerns in the MBC setting."
"Respectfully, that determination would be contrary to the most reasonable interpretation of the scientific data."
In addition to defending E2100, the summary shows that Genentech will focus on FDA’s application of the accelerated approval standard, the external support for Avastin’s use in MBC (including that of other regulatory bodies), and the agency’s potential regulatory flexibility.
In the end, it’s that flexibility that Genentech is really appealing to. "Regulatory flexibility is intended for cases like this," the summary concludes, "where a formulaic application of the withdrawal standard would deprive patients and physicians of the choice intended by the accelerated approval program – and do so in the face of continued findings of benefit, a well-understood safety profile, and a viable option for providing a clearer answer to the remaining scientific questions."
Internal Medicine News Digital Network and "The Pink Sheet" are published by Elsevier.
Genentech’s advance summary of evidence and arguments for the Food and Drug Administration’s upcoming hearing on the agency’s proposed withdrawal of the metastatic breast cancer approval for Avastin shows that the company is trying to keep the focus on the additional confirmatory trial it proposes to conduct.
The document, released May 13, provides an overview of the case Genentech will make to support maintaining the accelerated approval of bevacizumab, in combination with paclitaxel, for first-line metastatic breast cancer (MBC) at the June 28-29 hearing, as requested by the presiding officer, Center for Biologics Evaluation and Research Director Karen Midthun.
Dr. Midthun recently released the agenda for the hearing and the issues that are to be discussed. These include whether the AVADO and RIBBON1 trials failed to verify Avastin’s clinical benefit in MBC as seen in the pivotal E2100 study, whether the available evidence shows it is safe and effective, and whether the FDA should withdraw the approval.
In its prehearing summary of evidence, Genentech notes it has recognized the view of the Center for Drug Evaluation and Research (CDER) and the Oncologic Drugs Advisory Committee that the AVADO and RIBBON1 trials failed to confirm the benefit and has responded by tailoring its proposal to maintaining accelerated approval for use with paclitaxel "subject to" an additional confirmatory trial.
Thus, the company does not plan to focus on AVADO and RIBBON1, the summary explains. "Instead, Genentech intends to focus on the question of whether accelerated approval should be maintained for Avastin and paclitaxel subject to a further confirmatory study of this combination, even in light of the views of CDER and the ODAC on AVADO and RIBBON1."
"The only open question is whether the magnitude of benefit observed in the E2100 study is reasonably likely to be confirmed in a second study of Avastin with paclitaxel," the company argues.
"Given the meaningful probability that the chemotherapy partner has an impact on the magnitude of benefit, and given that an additional study can resolve this question, accelerated approval should be maintained pending completion of Genentech’s further study."
The company also says that continuing the approval while gaining further evidence fits with the overall goals of the accelerated approval program and that the accelerated approval regime allows the FDA that regulatory flexibility.
The New Confirmatory Trial
Genentech first proposed conducting a new confirmatory trial in August 2010 after the FDA’s Oncologic Drugs Advisory Committee voted against continued approval in MBC, and that proposal was a pivotal point in its request for a hearing on FDA’s proposed withdrawal.
The company intends to conduct a new trial – a double-blind, randomized, multicenter phase III trial of Avastin in combination with weekly paclitaxel – using a biomarker to select for patients more likely to derive a greater benefit from treatment, which was an option alluded to in CDER’s memo on the proposed withdrawal.
"Based on the company’s extensive research, including a new assay sensitive to specific isoforms, plasma concentration of VEGF-A (Avastin’s target) has been identified as a potential predictive biomarker in breast, gastric and pancreatic cancers," the summary of evidence states.
An analysis of the AVADO trial, presented as an abstract at the San Antonio Breast Cancer Symposium in December, showed that patients with high levels of VEGF-A had a hazard ratio for progression-free survival analysis of 0.49, compared with 0.87 for patients with low levels of VEGF-A. "The data suggest that patients with high VEGF-A levels may be more likely to derive a substantial benefit from Avastin, and it is important and appropriate to validate this predictive biomarker in a prospective phase III trial," Genentech argued.
The company met with the agency to discuss the trial on Feb. 22, the preparatory documents for the hearing reveal, the day before the hearing was granted. At that meeting, Genentech reports, CDER stated that "PFS [progression free survival] results confirming the magnitude of treatment effect observed in E2100 without a detriment to OS [overall survival], coupled with the E2100 data, would support Avastin’s full approval with paclitaxel."
The company has proceeded with study planning and intends to submit the protocol under a Special Protocol Assessment, the summary states.
Nothing Wrong With E2100
Genentech presented an overview of the E2100 findings that emphasize their statistical rigor and clinical significance, an essential point of agreement to support continuing the accelerated approval based upon those findings while another confirmatory trial is conducted.
In the context of Dr. Midthun’s posed question on whether Avastin’s benefit-risk ratio remains favorable in MBC, the company notes that withdrawal would "necessarily rest on a determination that the findings from the E2100 study are no longer reliable and that Avastin presents unique toxicity concerns in the MBC setting."
"Respectfully, that determination would be contrary to the most reasonable interpretation of the scientific data."
In addition to defending E2100, the summary shows that Genentech will focus on FDA’s application of the accelerated approval standard, the external support for Avastin’s use in MBC (including that of other regulatory bodies), and the agency’s potential regulatory flexibility.
In the end, it’s that flexibility that Genentech is really appealing to. "Regulatory flexibility is intended for cases like this," the summary concludes, "where a formulaic application of the withdrawal standard would deprive patients and physicians of the choice intended by the accelerated approval program – and do so in the face of continued findings of benefit, a well-understood safety profile, and a viable option for providing a clearer answer to the remaining scientific questions."
Internal Medicine News Digital Network and "The Pink Sheet" are published by Elsevier.
FROM THE FDA
Radioactive Seeds Guide Surgeons to Nonpalpable Breast Lesions
WASHINGTON – Radioactive seeds were a safe and effective method of pinpointing nonpalpable breast lesions for surgery, with an 85% rate of negative margins on the first excision and an ipsilateral recurrence rate of less than 2% over 33 months, according to a large retrospective study.
Close margins occurred in 12% of the 767 patients with nonpalpable breast lesions, and positive margins occurred in 3%. The overall re-excision rate was 15%, Dr. Lee McGhan of the Mayo Clinic, Scottsdale, Ariz., said at the annual meeting of the American Society of Breast Surgeons.
Performing the procedure is "almost intuitive," with a very low learning curve, he said. "It is now our method of choice when dealing with preoperative localization of nonpalpable breast lesions."
His retrospective review of 978 prospectively collected patient records comprised 1,000 radioactive in 2003-2010. Almost 1,150 seeds were deployed.
The patients’ mean age was 65 years; their mean lesion size was 1.2 cm. Most (550) had an invasive carcinoma; 217 had DCIS (ductal carcinoma in situ); 115 had atypical hyperplasia, and the remainder, uncertain or suspicious percutaneous biopsy results.
Dr. McGhan reported 33-month follow-up results on the 767 women with either invasive carcinoma or DCIS. Most patients (910) received just one seed; 84 received two seeds; 5 received three seeds; and 1 patient received four of the devices.
Most (76%) underwent the procedure at least 1 day before surgery. Typically, Dr. McGhan said, patients came to the clinic a few days before surgery for an evaluation. Many chose to have the localization on a Friday, stayed over the weekend, and had the seeds removed early on Monday morning.
The 4- to 5-mm seeds containing radioactive iodine-125 can be placed up to 5 days before surgery. They were deployed through an 18-gauge spinal needle under image guidance; post deployment, a mammogram or ultrasound confirmed their position near the lesion. "We used a handheld gamma probe to identify the area of greatest radioactivity at the skin surface, marking the optimal site of skin incision," Dr. McGhan said.
Intraoperative complications included 30 displaced seeds – including 3 suctioned up by operative tubing and 3 that were improperly deployed during radiology – as well as one instance of an incorrect incision site resulting from a miscommunication between the radiologist and the surgeon, Dr. McGhan said. All of these seeds were retrieved with no patient harm.
All of the localized lesions were successfully removed, along with their associated seeds; the specimens were sent to pathology. Among the 550 invasive cancers, margins were negative in 87%, close in 9%, and positive in 3%. Re-excision was required in 13% (69).
Among the 217 DCIS lesions, margins were negative in 77%, close in 19%, and positive in 3%. Re-excision was necessary in 23% (49).
Sentinel node biopsies occurred in 544 cases, and were successful in all but one, Dr. McGhan said. "There was no blue dye detected, which was determined to be due to tumor invasion of the lymphatics rather than a direct complication of the procedure."
The mean follow-up period was 33 months. Over this time, the overall ipsilateral recurrence rate was 1.6% (12 patients). The rate was slightly higher among patients with DCIS (3%; seven patients). The local recurrence rate was 1% (five patients) among those with invasive cancer. By the end of the follow-up period, there were six mastectomies secondary to recurrence: three (0.5%) in the invasive cancer group and three (1%) in the DCIS group.
Dr. McGhan had no financial declarations.
WASHINGTON – Radioactive seeds were a safe and effective method of pinpointing nonpalpable breast lesions for surgery, with an 85% rate of negative margins on the first excision and an ipsilateral recurrence rate of less than 2% over 33 months, according to a large retrospective study.
Close margins occurred in 12% of the 767 patients with nonpalpable breast lesions, and positive margins occurred in 3%. The overall re-excision rate was 15%, Dr. Lee McGhan of the Mayo Clinic, Scottsdale, Ariz., said at the annual meeting of the American Society of Breast Surgeons.
Performing the procedure is "almost intuitive," with a very low learning curve, he said. "It is now our method of choice when dealing with preoperative localization of nonpalpable breast lesions."
His retrospective review of 978 prospectively collected patient records comprised 1,000 radioactive in 2003-2010. Almost 1,150 seeds were deployed.
The patients’ mean age was 65 years; their mean lesion size was 1.2 cm. Most (550) had an invasive carcinoma; 217 had DCIS (ductal carcinoma in situ); 115 had atypical hyperplasia, and the remainder, uncertain or suspicious percutaneous biopsy results.
Dr. McGhan reported 33-month follow-up results on the 767 women with either invasive carcinoma or DCIS. Most patients (910) received just one seed; 84 received two seeds; 5 received three seeds; and 1 patient received four of the devices.
Most (76%) underwent the procedure at least 1 day before surgery. Typically, Dr. McGhan said, patients came to the clinic a few days before surgery for an evaluation. Many chose to have the localization on a Friday, stayed over the weekend, and had the seeds removed early on Monday morning.
The 4- to 5-mm seeds containing radioactive iodine-125 can be placed up to 5 days before surgery. They were deployed through an 18-gauge spinal needle under image guidance; post deployment, a mammogram or ultrasound confirmed their position near the lesion. "We used a handheld gamma probe to identify the area of greatest radioactivity at the skin surface, marking the optimal site of skin incision," Dr. McGhan said.
Intraoperative complications included 30 displaced seeds – including 3 suctioned up by operative tubing and 3 that were improperly deployed during radiology – as well as one instance of an incorrect incision site resulting from a miscommunication between the radiologist and the surgeon, Dr. McGhan said. All of these seeds were retrieved with no patient harm.
All of the localized lesions were successfully removed, along with their associated seeds; the specimens were sent to pathology. Among the 550 invasive cancers, margins were negative in 87%, close in 9%, and positive in 3%. Re-excision was required in 13% (69).
Among the 217 DCIS lesions, margins were negative in 77%, close in 19%, and positive in 3%. Re-excision was necessary in 23% (49).
Sentinel node biopsies occurred in 544 cases, and were successful in all but one, Dr. McGhan said. "There was no blue dye detected, which was determined to be due to tumor invasion of the lymphatics rather than a direct complication of the procedure."
The mean follow-up period was 33 months. Over this time, the overall ipsilateral recurrence rate was 1.6% (12 patients). The rate was slightly higher among patients with DCIS (3%; seven patients). The local recurrence rate was 1% (five patients) among those with invasive cancer. By the end of the follow-up period, there were six mastectomies secondary to recurrence: three (0.5%) in the invasive cancer group and three (1%) in the DCIS group.
Dr. McGhan had no financial declarations.
WASHINGTON – Radioactive seeds were a safe and effective method of pinpointing nonpalpable breast lesions for surgery, with an 85% rate of negative margins on the first excision and an ipsilateral recurrence rate of less than 2% over 33 months, according to a large retrospective study.
Close margins occurred in 12% of the 767 patients with nonpalpable breast lesions, and positive margins occurred in 3%. The overall re-excision rate was 15%, Dr. Lee McGhan of the Mayo Clinic, Scottsdale, Ariz., said at the annual meeting of the American Society of Breast Surgeons.
Performing the procedure is "almost intuitive," with a very low learning curve, he said. "It is now our method of choice when dealing with preoperative localization of nonpalpable breast lesions."
His retrospective review of 978 prospectively collected patient records comprised 1,000 radioactive in 2003-2010. Almost 1,150 seeds were deployed.
The patients’ mean age was 65 years; their mean lesion size was 1.2 cm. Most (550) had an invasive carcinoma; 217 had DCIS (ductal carcinoma in situ); 115 had atypical hyperplasia, and the remainder, uncertain or suspicious percutaneous biopsy results.
Dr. McGhan reported 33-month follow-up results on the 767 women with either invasive carcinoma or DCIS. Most patients (910) received just one seed; 84 received two seeds; 5 received three seeds; and 1 patient received four of the devices.
Most (76%) underwent the procedure at least 1 day before surgery. Typically, Dr. McGhan said, patients came to the clinic a few days before surgery for an evaluation. Many chose to have the localization on a Friday, stayed over the weekend, and had the seeds removed early on Monday morning.
The 4- to 5-mm seeds containing radioactive iodine-125 can be placed up to 5 days before surgery. They were deployed through an 18-gauge spinal needle under image guidance; post deployment, a mammogram or ultrasound confirmed their position near the lesion. "We used a handheld gamma probe to identify the area of greatest radioactivity at the skin surface, marking the optimal site of skin incision," Dr. McGhan said.
Intraoperative complications included 30 displaced seeds – including 3 suctioned up by operative tubing and 3 that were improperly deployed during radiology – as well as one instance of an incorrect incision site resulting from a miscommunication between the radiologist and the surgeon, Dr. McGhan said. All of these seeds were retrieved with no patient harm.
All of the localized lesions were successfully removed, along with their associated seeds; the specimens were sent to pathology. Among the 550 invasive cancers, margins were negative in 87%, close in 9%, and positive in 3%. Re-excision was required in 13% (69).
Among the 217 DCIS lesions, margins were negative in 77%, close in 19%, and positive in 3%. Re-excision was necessary in 23% (49).
Sentinel node biopsies occurred in 544 cases, and were successful in all but one, Dr. McGhan said. "There was no blue dye detected, which was determined to be due to tumor invasion of the lymphatics rather than a direct complication of the procedure."
The mean follow-up period was 33 months. Over this time, the overall ipsilateral recurrence rate was 1.6% (12 patients). The rate was slightly higher among patients with DCIS (3%; seven patients). The local recurrence rate was 1% (five patients) among those with invasive cancer. By the end of the follow-up period, there were six mastectomies secondary to recurrence: three (0.5%) in the invasive cancer group and three (1%) in the DCIS group.
Dr. McGhan had no financial declarations.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Major Finding: Radioactive seed localization resulted in initial negative margins in 85% of cases and an overall re-excision rate of 2%.
Data Source: A retrospective study of 767 women with invasive cancer or DCIS who were followed for a mean of 33 months.
Disclosures: Dr. McGhan had no financial declarations.
Sequencing Reveals MAP3K1 Mutation in Luminal-Type Breast Cancer
ORLANDO – Massively parallel sequencing of DNA from tumor samples in 50 patients with luminal-type breast cancer revealed a novel mutation in the breast cancer tumor suppressor gene MAP3K1, which normally controls programmed cell death.
Presumably, the knockout mutation – which affected about 10% of estrogen receptor–positive breast cancers in the study and which "unequivocally destroys the function of the gene" – allows cells to survive when they would normally die, Dr. Matthew J. Ellis said at the annual meeting of the American Association for Cancer Research.
This finding, along with others from the sequencing of more than 10 trillion chemical bases of DNA in this extensive genomics investigation (one of the largest to date), marks an important early step toward personalized therapy for breast cancer patients who fail to respond to estrogen-lowering therapy prior to surgery, said Dr. Ellis, professor of medicine and chief of breast oncology at the Washington University in St. Louis.
Luminal-type breast cancer is the most common form of the disease, accounting for 70%-80% of hormone receptor–positive breast cancers. Many patients have a good prognosis, but a subset has this very aggressive type of disease. Indeed, more patients die of aggressive luminal-type breast cancer than do all other breast cancer subtypes combined, he said.
"So we set out to find a molecular basis for poor outcome in receptor-positive disease," he said.
DNA from tumor samples of patients who were enrolled in ongoing neoadjuvant endocrine clinical trials – 24 of whom were resistant to estrogen receptor–targeted therapy – was used for the supercomputer-conducted analysis. The whole genomes of the tumors were compared with the matched DNA of the same patients’ healthy cells, allowing identification of mutations occurring only in the cancer cells.
In all, 1,700 mutations were identified, and most of these were unique to individuals. In addition to two previously identified, relatively common mutations (PIK3CA and TP53), Dr. Ellis and his colleagues found only three others – including MAP3K1 – that recurred at a frequency of at least 10%; the other two were ATR and MYST3.
PIK3CA and TP53 were the most frequently mutated genes in estrogen receptor–positive breast cancer in this study, occurring in about 50% and 20% of tumors, respectively. MAP3K1 was the third most commonly mutated gene.
Considering the large number of mutations found, it was "a rather shocking result" to find only three new gene mutations at the 10% recurrence level, Dr. Ellis said. "What it says is that breast cancer is highly complex, that the genetic make-up involves a large number of mutations that averages about 20 tier-1 [or coding region] mutations in each tumor, and there’s a wide range," he added.
But the findings do offer a glimpse into how therapy can be personalized.
Using a "very, very simple model" produced by this analysis, Dr. Ellis illustrated how a constellation of mutations could be used to predict response or resistance patterns: The three-gene cluster of mutated MAP3K1, mutated PIK3CA, and wild type TP53, which occurred in a small subset of patients, was shown to be significantly associated with "luminal A status [indicative of good prognosis], suppressed proliferation, and favorable small tumors at the end of neoadjuvant treatment," he said.
Although there remains "a great sea of unknown," the findings – when considered in the context of the growing list of "druggable mutations" and treatments approved for other diseases – allow for a vision of therapy that involves obtaining the genetic information in advance of treatment to allow for the design of individually appropriate therapy to address the problem of resistance.
"Very clearly, this is a big problem clinically ... and only a tailored approach will lead to a solution to the problem," he said.
Dr. Ellis disclosed that he has received grant or research support from and/or served on the speakers bureau for Novartis, AstraZeneca, and Bioclassifier LLC.
ORLANDO – Massively parallel sequencing of DNA from tumor samples in 50 patients with luminal-type breast cancer revealed a novel mutation in the breast cancer tumor suppressor gene MAP3K1, which normally controls programmed cell death.
Presumably, the knockout mutation – which affected about 10% of estrogen receptor–positive breast cancers in the study and which "unequivocally destroys the function of the gene" – allows cells to survive when they would normally die, Dr. Matthew J. Ellis said at the annual meeting of the American Association for Cancer Research.
This finding, along with others from the sequencing of more than 10 trillion chemical bases of DNA in this extensive genomics investigation (one of the largest to date), marks an important early step toward personalized therapy for breast cancer patients who fail to respond to estrogen-lowering therapy prior to surgery, said Dr. Ellis, professor of medicine and chief of breast oncology at the Washington University in St. Louis.
Luminal-type breast cancer is the most common form of the disease, accounting for 70%-80% of hormone receptor–positive breast cancers. Many patients have a good prognosis, but a subset has this very aggressive type of disease. Indeed, more patients die of aggressive luminal-type breast cancer than do all other breast cancer subtypes combined, he said.
"So we set out to find a molecular basis for poor outcome in receptor-positive disease," he said.
DNA from tumor samples of patients who were enrolled in ongoing neoadjuvant endocrine clinical trials – 24 of whom were resistant to estrogen receptor–targeted therapy – was used for the supercomputer-conducted analysis. The whole genomes of the tumors were compared with the matched DNA of the same patients’ healthy cells, allowing identification of mutations occurring only in the cancer cells.
In all, 1,700 mutations were identified, and most of these were unique to individuals. In addition to two previously identified, relatively common mutations (PIK3CA and TP53), Dr. Ellis and his colleagues found only three others – including MAP3K1 – that recurred at a frequency of at least 10%; the other two were ATR and MYST3.
PIK3CA and TP53 were the most frequently mutated genes in estrogen receptor–positive breast cancer in this study, occurring in about 50% and 20% of tumors, respectively. MAP3K1 was the third most commonly mutated gene.
Considering the large number of mutations found, it was "a rather shocking result" to find only three new gene mutations at the 10% recurrence level, Dr. Ellis said. "What it says is that breast cancer is highly complex, that the genetic make-up involves a large number of mutations that averages about 20 tier-1 [or coding region] mutations in each tumor, and there’s a wide range," he added.
But the findings do offer a glimpse into how therapy can be personalized.
Using a "very, very simple model" produced by this analysis, Dr. Ellis illustrated how a constellation of mutations could be used to predict response or resistance patterns: The three-gene cluster of mutated MAP3K1, mutated PIK3CA, and wild type TP53, which occurred in a small subset of patients, was shown to be significantly associated with "luminal A status [indicative of good prognosis], suppressed proliferation, and favorable small tumors at the end of neoadjuvant treatment," he said.
Although there remains "a great sea of unknown," the findings – when considered in the context of the growing list of "druggable mutations" and treatments approved for other diseases – allow for a vision of therapy that involves obtaining the genetic information in advance of treatment to allow for the design of individually appropriate therapy to address the problem of resistance.
"Very clearly, this is a big problem clinically ... and only a tailored approach will lead to a solution to the problem," he said.
Dr. Ellis disclosed that he has received grant or research support from and/or served on the speakers bureau for Novartis, AstraZeneca, and Bioclassifier LLC.
ORLANDO – Massively parallel sequencing of DNA from tumor samples in 50 patients with luminal-type breast cancer revealed a novel mutation in the breast cancer tumor suppressor gene MAP3K1, which normally controls programmed cell death.
Presumably, the knockout mutation – which affected about 10% of estrogen receptor–positive breast cancers in the study and which "unequivocally destroys the function of the gene" – allows cells to survive when they would normally die, Dr. Matthew J. Ellis said at the annual meeting of the American Association for Cancer Research.
This finding, along with others from the sequencing of more than 10 trillion chemical bases of DNA in this extensive genomics investigation (one of the largest to date), marks an important early step toward personalized therapy for breast cancer patients who fail to respond to estrogen-lowering therapy prior to surgery, said Dr. Ellis, professor of medicine and chief of breast oncology at the Washington University in St. Louis.
Luminal-type breast cancer is the most common form of the disease, accounting for 70%-80% of hormone receptor–positive breast cancers. Many patients have a good prognosis, but a subset has this very aggressive type of disease. Indeed, more patients die of aggressive luminal-type breast cancer than do all other breast cancer subtypes combined, he said.
"So we set out to find a molecular basis for poor outcome in receptor-positive disease," he said.
DNA from tumor samples of patients who were enrolled in ongoing neoadjuvant endocrine clinical trials – 24 of whom were resistant to estrogen receptor–targeted therapy – was used for the supercomputer-conducted analysis. The whole genomes of the tumors were compared with the matched DNA of the same patients’ healthy cells, allowing identification of mutations occurring only in the cancer cells.
In all, 1,700 mutations were identified, and most of these were unique to individuals. In addition to two previously identified, relatively common mutations (PIK3CA and TP53), Dr. Ellis and his colleagues found only three others – including MAP3K1 – that recurred at a frequency of at least 10%; the other two were ATR and MYST3.
PIK3CA and TP53 were the most frequently mutated genes in estrogen receptor–positive breast cancer in this study, occurring in about 50% and 20% of tumors, respectively. MAP3K1 was the third most commonly mutated gene.
Considering the large number of mutations found, it was "a rather shocking result" to find only three new gene mutations at the 10% recurrence level, Dr. Ellis said. "What it says is that breast cancer is highly complex, that the genetic make-up involves a large number of mutations that averages about 20 tier-1 [or coding region] mutations in each tumor, and there’s a wide range," he added.
But the findings do offer a glimpse into how therapy can be personalized.
Using a "very, very simple model" produced by this analysis, Dr. Ellis illustrated how a constellation of mutations could be used to predict response or resistance patterns: The three-gene cluster of mutated MAP3K1, mutated PIK3CA, and wild type TP53, which occurred in a small subset of patients, was shown to be significantly associated with "luminal A status [indicative of good prognosis], suppressed proliferation, and favorable small tumors at the end of neoadjuvant treatment," he said.
Although there remains "a great sea of unknown," the findings – when considered in the context of the growing list of "druggable mutations" and treatments approved for other diseases – allow for a vision of therapy that involves obtaining the genetic information in advance of treatment to allow for the design of individually appropriate therapy to address the problem of resistance.
"Very clearly, this is a big problem clinically ... and only a tailored approach will lead to a solution to the problem," he said.
Dr. Ellis disclosed that he has received grant or research support from and/or served on the speakers bureau for Novartis, AstraZeneca, and Bioclassifier LLC.
Major Finding: A three-gene cluster of mutated MAP3K1, mutated PIK3CA, and wild-type TP53 was shown to be significantly associated with "luminal A status (indicative of good prognosis), suppressed proliferation, and favorable small tumors at the end of neoadjuvant treatment."
Data Source: Parallel sequencing of DNA from tumor samples of 50 patients with luminal-type breast cancer.
Disclosures: Dr. Ellis disclosed that he has received grant or research support from and/or served on the speakers bureau for Novartis, AstraZeneca, and Bioclassifier LLC.
NCCN Breast Guidelines Stand by Bevacizumab
HOLLYWOOD, FLA. – The National Comprehensive Cancer Network is sticking by its endorsement of bevacizumab in combination with paclitaxel for the treatment of metastatic breast cancer.
Dr. Robert W. Carlson announced that the network’s breast cancer panel voted to retain the guideline recommendation after holding multiple "marathon" meetings to review data behind the Food and Drug Administration’s controversial decision to withdraw marketing approval for bevacizumab (Avastin) in metastatic breast cancer.
"The data observed in the [E2100 trial] really had not changed from its approval previously, and we thought that if the data were compelling 2 years ago, why isn’t it compelling enough today?" explained Dr. Carlson of Stanford (Calif.) University, chair of the 27-member panel, at the annual conference of the National Comprehensive Cancer Network
Bevacizumab received accelerated approval in 2008 based on results of the E2100 trial, which showed a statistically significant increase in progression-free survival but not overall survival. When subsequent trials did not match these results or show an improvement in overall survival, the FDA’s Oncologic Drugs Advisory Committee voted unanimously that the indication ought to be withdrawn.
The Food and Drug Administration announced in December that it would do so. A more than 20% increase in grade 3-5 toxicity relative to paclitaxel alone suggests the benefits of the drug do not outweigh the risks, according to the agency, which has scheduled a hearing June 28-29, 2011, to consider an appeal by drugmaker Genentech (owned by Roche).
In a footnote to a listing of preferred chemotherapy regimens for metastatic breast cancer in the updated NCCN guidelines, the breast cancer panel states: "Randomized clinical trials in metastatic breast cancer document that the addition of bevacizumab to some first- or second-line chemotherapy agents modestly improves time to progression and response rates but does not improve overall survival. The time to progression impact may vary among cytotoxic agents and appears greatest with bevacizumab in combination with weekly paclitaxel."
Other important updates to the breast cancer guidelines follow.
Axillary Lymph Node Dissection
The panel stopped short of endorsing omission of complete axillary lymph node dissection (ALND) in patients with early breast cancer who are found to be node positive or have unidentified nodal involvement after sentinel lymph node dissection (SLND).
After reviewing the American College of Surgeons Oncology Group (ACOSOG) Z011 study, which found no improvement in outcomes with the additional procedure (J. Clin. Oncol. 28:18s, 2010 [suppl; abstr CRA506]), the NCCN panel issued a "guidance" via the following footnote in the new guidelines: "The data from a single randomized trial suggests complete axillary lymph node dissection in women with clinically node negative T/1-2 tumors, fewer than 3 involved sentinel lymph nodes, and undergoing breast conserving surgery and whole breast radiation results in more morbidity, no improvement in local regional recurrence rates, and no difference in overall survival compared with sentinel lymph node procedure alone."
While the statistical and absolute numerical advantage in the observed survival curves clearly favored skipping the additional procedure, the panel declined to recommend against ALND because the study accrued only 450 of the planned 1,900 patients, according to Dr. Carlson. Nonetheless, he acknowledged that results are already changing clinical practice.
"There’s no reason to suspect that the trend wouldn’t have continued with sufficient accrual," he said. "In fact, as a result of the study, there is at least one NCCN institution that has abandoned axillary node dissection in this highly selective group of patients."
Eribulin as Third-Line Monotherapy
Based on the findings of the phase III EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice vs. E7389) trial, the guidelines panel included eribulin (Halaven), a synthetic microtubule blocker, on the list of preferred single-agent treatments for metastatic or recurrent breast cancer. It is FDA approved in patients previously treated with both a taxane-based therapy and an anthracycline-based treatment.
The addition of this agent to the treatment algorithm is especially noteworthy, Dr. Carlson said, "considering the limited options for women with metastatic breast cancer who have already received other therapies."
The median overall survival of women randomized to eribulin in the EMBRACE trial was 13.12 months, compared with 10.65 in the comparator group of women treated with their doctors’ choice of chemotherapy (Lancet 2011 March 3 [doi:10.1016/S0140-6736(11)60070-6]). Despite the statistically significant improvement in overall survival, there was no progression-free survival advantage with eribulin treatment – a result that Dr. Carlson deemed "confusing."
The apparent contradiction "gives me a lot of concern that we may not know what we are measuring when we measure progression-free survival," he said.
Denosumab for Skeletal Protection
Recently approved by the FDA for the prevention of skeletal events in advanced breast cancer patients with bone metastases, the monoclonal antibody denosumab (Xgeva) joins zoledronic acid (Zometa) and pamidronate (Aredia) as supportive care options in the updated guidelines. The recommendation is based on the findings of a phase III trial in which denosumab reduced the risks of experiencing a first skeletal event by 18% and multiple skeletal-related events by 23% relative to zoledronic acid (J. Clin. Oncol. 2010;10:5132-9)
Although treatment with denosumab did not improve overall survival or progression-free survival, the impact of the drug in reducing such events as fractures and bone pain could potentially minimize the need for surgery or radiation for skeletal complications and as such substantially improves patients’ quality of life, Dr. Carlson stressed.
Role of Biomarkers
While affirming the importance of determining the status of the estrogen receptor (ER), progesterone receptor (PR), and HER2 in the initial or recurrent work-up for stage IV disease, the revised guidelines do not address testing for CYP2D6 biomarkers prior to prescribing tamoxifen.
Although it has been suggested that testing for variations in the CYP2D6 gene can identify poor metabolizers of tamoxifen who will not benefit from the drug’s protective effects, "the biologic evidence is inconsistent and confusing," Dr. Carlson stated. As such, he noted, "the guidelines are silent on the issue of [CYP2D6] testing, which clinicians should interpret as a recommendation not to do it."
The updated NCCN Guidelines for Breast Cancer are available free of charge at the NCCN website.
Dr. Carlson disclosed receiving grant and research support from AstraZeneca Pharmaceuticals, Genentech, Pfizer, and Sanofi-Aventis US.
HOLLYWOOD, FLA. – The National Comprehensive Cancer Network is sticking by its endorsement of bevacizumab in combination with paclitaxel for the treatment of metastatic breast cancer.
Dr. Robert W. Carlson announced that the network’s breast cancer panel voted to retain the guideline recommendation after holding multiple "marathon" meetings to review data behind the Food and Drug Administration’s controversial decision to withdraw marketing approval for bevacizumab (Avastin) in metastatic breast cancer.
"The data observed in the [E2100 trial] really had not changed from its approval previously, and we thought that if the data were compelling 2 years ago, why isn’t it compelling enough today?" explained Dr. Carlson of Stanford (Calif.) University, chair of the 27-member panel, at the annual conference of the National Comprehensive Cancer Network
Bevacizumab received accelerated approval in 2008 based on results of the E2100 trial, which showed a statistically significant increase in progression-free survival but not overall survival. When subsequent trials did not match these results or show an improvement in overall survival, the FDA’s Oncologic Drugs Advisory Committee voted unanimously that the indication ought to be withdrawn.
The Food and Drug Administration announced in December that it would do so. A more than 20% increase in grade 3-5 toxicity relative to paclitaxel alone suggests the benefits of the drug do not outweigh the risks, according to the agency, which has scheduled a hearing June 28-29, 2011, to consider an appeal by drugmaker Genentech (owned by Roche).
In a footnote to a listing of preferred chemotherapy regimens for metastatic breast cancer in the updated NCCN guidelines, the breast cancer panel states: "Randomized clinical trials in metastatic breast cancer document that the addition of bevacizumab to some first- or second-line chemotherapy agents modestly improves time to progression and response rates but does not improve overall survival. The time to progression impact may vary among cytotoxic agents and appears greatest with bevacizumab in combination with weekly paclitaxel."
Other important updates to the breast cancer guidelines follow.
Axillary Lymph Node Dissection
The panel stopped short of endorsing omission of complete axillary lymph node dissection (ALND) in patients with early breast cancer who are found to be node positive or have unidentified nodal involvement after sentinel lymph node dissection (SLND).
After reviewing the American College of Surgeons Oncology Group (ACOSOG) Z011 study, which found no improvement in outcomes with the additional procedure (J. Clin. Oncol. 28:18s, 2010 [suppl; abstr CRA506]), the NCCN panel issued a "guidance" via the following footnote in the new guidelines: "The data from a single randomized trial suggests complete axillary lymph node dissection in women with clinically node negative T/1-2 tumors, fewer than 3 involved sentinel lymph nodes, and undergoing breast conserving surgery and whole breast radiation results in more morbidity, no improvement in local regional recurrence rates, and no difference in overall survival compared with sentinel lymph node procedure alone."
While the statistical and absolute numerical advantage in the observed survival curves clearly favored skipping the additional procedure, the panel declined to recommend against ALND because the study accrued only 450 of the planned 1,900 patients, according to Dr. Carlson. Nonetheless, he acknowledged that results are already changing clinical practice.
"There’s no reason to suspect that the trend wouldn’t have continued with sufficient accrual," he said. "In fact, as a result of the study, there is at least one NCCN institution that has abandoned axillary node dissection in this highly selective group of patients."
Eribulin as Third-Line Monotherapy
Based on the findings of the phase III EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice vs. E7389) trial, the guidelines panel included eribulin (Halaven), a synthetic microtubule blocker, on the list of preferred single-agent treatments for metastatic or recurrent breast cancer. It is FDA approved in patients previously treated with both a taxane-based therapy and an anthracycline-based treatment.
The addition of this agent to the treatment algorithm is especially noteworthy, Dr. Carlson said, "considering the limited options for women with metastatic breast cancer who have already received other therapies."
The median overall survival of women randomized to eribulin in the EMBRACE trial was 13.12 months, compared with 10.65 in the comparator group of women treated with their doctors’ choice of chemotherapy (Lancet 2011 March 3 [doi:10.1016/S0140-6736(11)60070-6]). Despite the statistically significant improvement in overall survival, there was no progression-free survival advantage with eribulin treatment – a result that Dr. Carlson deemed "confusing."
The apparent contradiction "gives me a lot of concern that we may not know what we are measuring when we measure progression-free survival," he said.
Denosumab for Skeletal Protection
Recently approved by the FDA for the prevention of skeletal events in advanced breast cancer patients with bone metastases, the monoclonal antibody denosumab (Xgeva) joins zoledronic acid (Zometa) and pamidronate (Aredia) as supportive care options in the updated guidelines. The recommendation is based on the findings of a phase III trial in which denosumab reduced the risks of experiencing a first skeletal event by 18% and multiple skeletal-related events by 23% relative to zoledronic acid (J. Clin. Oncol. 2010;10:5132-9)
Although treatment with denosumab did not improve overall survival or progression-free survival, the impact of the drug in reducing such events as fractures and bone pain could potentially minimize the need for surgery or radiation for skeletal complications and as such substantially improves patients’ quality of life, Dr. Carlson stressed.
Role of Biomarkers
While affirming the importance of determining the status of the estrogen receptor (ER), progesterone receptor (PR), and HER2 in the initial or recurrent work-up for stage IV disease, the revised guidelines do not address testing for CYP2D6 biomarkers prior to prescribing tamoxifen.
Although it has been suggested that testing for variations in the CYP2D6 gene can identify poor metabolizers of tamoxifen who will not benefit from the drug’s protective effects, "the biologic evidence is inconsistent and confusing," Dr. Carlson stated. As such, he noted, "the guidelines are silent on the issue of [CYP2D6] testing, which clinicians should interpret as a recommendation not to do it."
The updated NCCN Guidelines for Breast Cancer are available free of charge at the NCCN website.
Dr. Carlson disclosed receiving grant and research support from AstraZeneca Pharmaceuticals, Genentech, Pfizer, and Sanofi-Aventis US.
HOLLYWOOD, FLA. – The National Comprehensive Cancer Network is sticking by its endorsement of bevacizumab in combination with paclitaxel for the treatment of metastatic breast cancer.
Dr. Robert W. Carlson announced that the network’s breast cancer panel voted to retain the guideline recommendation after holding multiple "marathon" meetings to review data behind the Food and Drug Administration’s controversial decision to withdraw marketing approval for bevacizumab (Avastin) in metastatic breast cancer.
"The data observed in the [E2100 trial] really had not changed from its approval previously, and we thought that if the data were compelling 2 years ago, why isn’t it compelling enough today?" explained Dr. Carlson of Stanford (Calif.) University, chair of the 27-member panel, at the annual conference of the National Comprehensive Cancer Network
Bevacizumab received accelerated approval in 2008 based on results of the E2100 trial, which showed a statistically significant increase in progression-free survival but not overall survival. When subsequent trials did not match these results or show an improvement in overall survival, the FDA’s Oncologic Drugs Advisory Committee voted unanimously that the indication ought to be withdrawn.
The Food and Drug Administration announced in December that it would do so. A more than 20% increase in grade 3-5 toxicity relative to paclitaxel alone suggests the benefits of the drug do not outweigh the risks, according to the agency, which has scheduled a hearing June 28-29, 2011, to consider an appeal by drugmaker Genentech (owned by Roche).
In a footnote to a listing of preferred chemotherapy regimens for metastatic breast cancer in the updated NCCN guidelines, the breast cancer panel states: "Randomized clinical trials in metastatic breast cancer document that the addition of bevacizumab to some first- or second-line chemotherapy agents modestly improves time to progression and response rates but does not improve overall survival. The time to progression impact may vary among cytotoxic agents and appears greatest with bevacizumab in combination with weekly paclitaxel."
Other important updates to the breast cancer guidelines follow.
Axillary Lymph Node Dissection
The panel stopped short of endorsing omission of complete axillary lymph node dissection (ALND) in patients with early breast cancer who are found to be node positive or have unidentified nodal involvement after sentinel lymph node dissection (SLND).
After reviewing the American College of Surgeons Oncology Group (ACOSOG) Z011 study, which found no improvement in outcomes with the additional procedure (J. Clin. Oncol. 28:18s, 2010 [suppl; abstr CRA506]), the NCCN panel issued a "guidance" via the following footnote in the new guidelines: "The data from a single randomized trial suggests complete axillary lymph node dissection in women with clinically node negative T/1-2 tumors, fewer than 3 involved sentinel lymph nodes, and undergoing breast conserving surgery and whole breast radiation results in more morbidity, no improvement in local regional recurrence rates, and no difference in overall survival compared with sentinel lymph node procedure alone."
While the statistical and absolute numerical advantage in the observed survival curves clearly favored skipping the additional procedure, the panel declined to recommend against ALND because the study accrued only 450 of the planned 1,900 patients, according to Dr. Carlson. Nonetheless, he acknowledged that results are already changing clinical practice.
"There’s no reason to suspect that the trend wouldn’t have continued with sufficient accrual," he said. "In fact, as a result of the study, there is at least one NCCN institution that has abandoned axillary node dissection in this highly selective group of patients."
Eribulin as Third-Line Monotherapy
Based on the findings of the phase III EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice vs. E7389) trial, the guidelines panel included eribulin (Halaven), a synthetic microtubule blocker, on the list of preferred single-agent treatments for metastatic or recurrent breast cancer. It is FDA approved in patients previously treated with both a taxane-based therapy and an anthracycline-based treatment.
The addition of this agent to the treatment algorithm is especially noteworthy, Dr. Carlson said, "considering the limited options for women with metastatic breast cancer who have already received other therapies."
The median overall survival of women randomized to eribulin in the EMBRACE trial was 13.12 months, compared with 10.65 in the comparator group of women treated with their doctors’ choice of chemotherapy (Lancet 2011 March 3 [doi:10.1016/S0140-6736(11)60070-6]). Despite the statistically significant improvement in overall survival, there was no progression-free survival advantage with eribulin treatment – a result that Dr. Carlson deemed "confusing."
The apparent contradiction "gives me a lot of concern that we may not know what we are measuring when we measure progression-free survival," he said.
Denosumab for Skeletal Protection
Recently approved by the FDA for the prevention of skeletal events in advanced breast cancer patients with bone metastases, the monoclonal antibody denosumab (Xgeva) joins zoledronic acid (Zometa) and pamidronate (Aredia) as supportive care options in the updated guidelines. The recommendation is based on the findings of a phase III trial in which denosumab reduced the risks of experiencing a first skeletal event by 18% and multiple skeletal-related events by 23% relative to zoledronic acid (J. Clin. Oncol. 2010;10:5132-9)
Although treatment with denosumab did not improve overall survival or progression-free survival, the impact of the drug in reducing such events as fractures and bone pain could potentially minimize the need for surgery or radiation for skeletal complications and as such substantially improves patients’ quality of life, Dr. Carlson stressed.
Role of Biomarkers
While affirming the importance of determining the status of the estrogen receptor (ER), progesterone receptor (PR), and HER2 in the initial or recurrent work-up for stage IV disease, the revised guidelines do not address testing for CYP2D6 biomarkers prior to prescribing tamoxifen.
Although it has been suggested that testing for variations in the CYP2D6 gene can identify poor metabolizers of tamoxifen who will not benefit from the drug’s protective effects, "the biologic evidence is inconsistent and confusing," Dr. Carlson stated. As such, he noted, "the guidelines are silent on the issue of [CYP2D6] testing, which clinicians should interpret as a recommendation not to do it."
The updated NCCN Guidelines for Breast Cancer are available free of charge at the NCCN website.
Dr. Carlson disclosed receiving grant and research support from AstraZeneca Pharmaceuticals, Genentech, Pfizer, and Sanofi-Aventis US.
FROM THE ANNUAL CONFERENCE OF THE NATIONAL COMPREHENSIVE CANCER NETWORK
Bone Micrometastases Show No Survival Impact in Early Breast Cancer
SAN ANTONIO – Survival is nearly identical in early-stage breast cancer regardless of whether or not bone marrow micrometastases are present, according to the prospective Swiss Multicenter Study Group trial.
Among 410 women with T1 and T2 disease and no palpable axillary lymph nodes, the 5-year overall survival was 92.5% with bone marrow micrometastases (BMM) and 92.7% without (P = .85).
The 5-year disease-free survival reached 92.2% in patients with BMM and 93.6% in those without (P = .50), Dr. Igor Langer said during a plenary session at a symposium sponsored by the Society of Surgical Oncology. Micrometastases were detected in 118 patients (29%).
Audience members asked Dr. Langer to explain his findings in light of those from the ACOSOG (American College of Surgeons Oncology Group) Z0010 trial, which also involved T1 and T2 disease. In that trial, BMMs were detected by immunohistochemistry in just 3% of women, and were associated with a significantly lower 5-year survival rate of 90% vs. 95% in patients without BMMs, according to data presented at the 2010 annual meeting of the American Society of Clinical Oncology.
Dr. Langer of the department of surgery at the Lindenhofspital in Bern, Switzerland, replied that most studies looking for BMM can identify them in 15%-40% of patients. "So I don’t know if it is a methodological difference, in terms of preparation of the bone marrow and identification of these cells," he said. "The difference in prognosis is multifactorial."
He pointed out that there is no standardized method to detect BMMs, that different methods were used to identify sentinel lymph nodes (SLNs), that patients received different adjuvant regimens, and that the number of cases who developed BMMs was so low, it could easily change outcomes.
Another attendee asked whether there is a particular cutoff for BMMs, noting that in many cancers the presence of micrometastases has no impact on survival, and that they are prognostic in melanoma only when they are at least 0.2 mm in the lymph nodes. Dr. Langer said that the median number of tumor cells in the analysis was two, but he balked at the possibility of using BMM testing in early breast cancer.
"You shouldn’t do the bone marrow aspiration," he said in an interview. "It might have prognostic impact, but if it doesn’t drive your therapy – if it doesn’t influence what you are doing – you have findings and you don’t know what to do with them. It’s too immature. We first have to standardize the testing."
Dr. Langer reported on 659 women from 13 Swiss centers who underwent intraoperative SLN examination by frozen section and paraffin serial sectioning at a cutting interval of 250 mcm with H&E (hematoxylin and eosin) staining and immunohistochemistry.
As previously reported, SLNs were identified in 98% of patients, including 71% with T1 disease and 29% with T2 disease (Breast Cancer Res. Treat. 2009;113:129-36). Their median age was 59 years, and 80% were postmenopausal. The median tumor size was 17 mm.
Of the 659 patients, 410 also underwent bone marrow aspiration from both iliac crests; these formed the basis of the current analysis. The mononuclear cells of the bone marrow aspirates were isolated by density gradient centrifugation through Ficoll. In all, 2 million bone marrow cells were evaluated, and the presence of one or more tumor cells was regarded as BMM positive, Dr. Langer said.
Cancer cells were stained with monoclonal antibodies A45-B/B3 against cytokeratin 8, 18, and 19 and were counted by an automated, computerized digital microscope. All results were reviewed by one pathologist.
Bone marrow micrometastases were detected in 118 (28.8%) of the 410 women. About 210 women were SLN negative and BMM negative, although considerable discordance was observed, Dr. Langer said. In all, 67 women (16.4%) were BMM positive and SLN negative, whereas 82 women (20%) were BMM negative and SLN positive.
In multivariate logistic regression analysis that included tumor size, tumor grade, tumor receptor status, and menopausal status, the presence of positive SLNs was the only significant independent predictor for the presence of BMM (P = .007; odds ratio, 1.860). T stage was not significant; only N stage was, he said.
In the earlier published analysis, the Swiss researchers identified SLN micrometastases or isolated tumor cells in 47 patients who underwent delayed ALND (axillary lymph node dissection). In 96% of these patients, the second operation was not beneficial because the ALND specimens were free of macrometastases. This finding – coupled with an overall accuracy of frozen section of 90% in the detection of SLN macrometastases – led the group to strongly recommend the routine use of SLN frozen section in early-stage breast cancer.
The recently published ACOSOG Z0011 trial concluded that ALND offers no survival advantage over SLN dissection alone in women with T1-T2 invasive breast cancer with no palpable adenopathy and one or two lymph nodes that contain metastases identified by frozen section, touch preparation, or H&E staining.
Sentinel node micrometastases (defined as H&E tumor deposits no greater than 2 mm in size) were identified in 37.5% of patients in the ALND group vs. 45% of those in the SLND group (JAMA 2011;305:569-75).
The Swiss Group for Clinical Cancer Research and the Cancer League of Basel-Stadt and Basel-Land funded the study. Dr. Langer and his coauthors disclosed no conflicts.
SAN ANTONIO – Survival is nearly identical in early-stage breast cancer regardless of whether or not bone marrow micrometastases are present, according to the prospective Swiss Multicenter Study Group trial.
Among 410 women with T1 and T2 disease and no palpable axillary lymph nodes, the 5-year overall survival was 92.5% with bone marrow micrometastases (BMM) and 92.7% without (P = .85).
The 5-year disease-free survival reached 92.2% in patients with BMM and 93.6% in those without (P = .50), Dr. Igor Langer said during a plenary session at a symposium sponsored by the Society of Surgical Oncology. Micrometastases were detected in 118 patients (29%).
Audience members asked Dr. Langer to explain his findings in light of those from the ACOSOG (American College of Surgeons Oncology Group) Z0010 trial, which also involved T1 and T2 disease. In that trial, BMMs were detected by immunohistochemistry in just 3% of women, and were associated with a significantly lower 5-year survival rate of 90% vs. 95% in patients without BMMs, according to data presented at the 2010 annual meeting of the American Society of Clinical Oncology.
Dr. Langer of the department of surgery at the Lindenhofspital in Bern, Switzerland, replied that most studies looking for BMM can identify them in 15%-40% of patients. "So I don’t know if it is a methodological difference, in terms of preparation of the bone marrow and identification of these cells," he said. "The difference in prognosis is multifactorial."
He pointed out that there is no standardized method to detect BMMs, that different methods were used to identify sentinel lymph nodes (SLNs), that patients received different adjuvant regimens, and that the number of cases who developed BMMs was so low, it could easily change outcomes.
Another attendee asked whether there is a particular cutoff for BMMs, noting that in many cancers the presence of micrometastases has no impact on survival, and that they are prognostic in melanoma only when they are at least 0.2 mm in the lymph nodes. Dr. Langer said that the median number of tumor cells in the analysis was two, but he balked at the possibility of using BMM testing in early breast cancer.
"You shouldn’t do the bone marrow aspiration," he said in an interview. "It might have prognostic impact, but if it doesn’t drive your therapy – if it doesn’t influence what you are doing – you have findings and you don’t know what to do with them. It’s too immature. We first have to standardize the testing."
Dr. Langer reported on 659 women from 13 Swiss centers who underwent intraoperative SLN examination by frozen section and paraffin serial sectioning at a cutting interval of 250 mcm with H&E (hematoxylin and eosin) staining and immunohistochemistry.
As previously reported, SLNs were identified in 98% of patients, including 71% with T1 disease and 29% with T2 disease (Breast Cancer Res. Treat. 2009;113:129-36). Their median age was 59 years, and 80% were postmenopausal. The median tumor size was 17 mm.
Of the 659 patients, 410 also underwent bone marrow aspiration from both iliac crests; these formed the basis of the current analysis. The mononuclear cells of the bone marrow aspirates were isolated by density gradient centrifugation through Ficoll. In all, 2 million bone marrow cells were evaluated, and the presence of one or more tumor cells was regarded as BMM positive, Dr. Langer said.
Cancer cells were stained with monoclonal antibodies A45-B/B3 against cytokeratin 8, 18, and 19 and were counted by an automated, computerized digital microscope. All results were reviewed by one pathologist.
Bone marrow micrometastases were detected in 118 (28.8%) of the 410 women. About 210 women were SLN negative and BMM negative, although considerable discordance was observed, Dr. Langer said. In all, 67 women (16.4%) were BMM positive and SLN negative, whereas 82 women (20%) were BMM negative and SLN positive.
In multivariate logistic regression analysis that included tumor size, tumor grade, tumor receptor status, and menopausal status, the presence of positive SLNs was the only significant independent predictor for the presence of BMM (P = .007; odds ratio, 1.860). T stage was not significant; only N stage was, he said.
In the earlier published analysis, the Swiss researchers identified SLN micrometastases or isolated tumor cells in 47 patients who underwent delayed ALND (axillary lymph node dissection). In 96% of these patients, the second operation was not beneficial because the ALND specimens were free of macrometastases. This finding – coupled with an overall accuracy of frozen section of 90% in the detection of SLN macrometastases – led the group to strongly recommend the routine use of SLN frozen section in early-stage breast cancer.
The recently published ACOSOG Z0011 trial concluded that ALND offers no survival advantage over SLN dissection alone in women with T1-T2 invasive breast cancer with no palpable adenopathy and one or two lymph nodes that contain metastases identified by frozen section, touch preparation, or H&E staining.
Sentinel node micrometastases (defined as H&E tumor deposits no greater than 2 mm in size) were identified in 37.5% of patients in the ALND group vs. 45% of those in the SLND group (JAMA 2011;305:569-75).
The Swiss Group for Clinical Cancer Research and the Cancer League of Basel-Stadt and Basel-Land funded the study. Dr. Langer and his coauthors disclosed no conflicts.
SAN ANTONIO – Survival is nearly identical in early-stage breast cancer regardless of whether or not bone marrow micrometastases are present, according to the prospective Swiss Multicenter Study Group trial.
Among 410 women with T1 and T2 disease and no palpable axillary lymph nodes, the 5-year overall survival was 92.5% with bone marrow micrometastases (BMM) and 92.7% without (P = .85).
The 5-year disease-free survival reached 92.2% in patients with BMM and 93.6% in those without (P = .50), Dr. Igor Langer said during a plenary session at a symposium sponsored by the Society of Surgical Oncology. Micrometastases were detected in 118 patients (29%).
Audience members asked Dr. Langer to explain his findings in light of those from the ACOSOG (American College of Surgeons Oncology Group) Z0010 trial, which also involved T1 and T2 disease. In that trial, BMMs were detected by immunohistochemistry in just 3% of women, and were associated with a significantly lower 5-year survival rate of 90% vs. 95% in patients without BMMs, according to data presented at the 2010 annual meeting of the American Society of Clinical Oncology.
Dr. Langer of the department of surgery at the Lindenhofspital in Bern, Switzerland, replied that most studies looking for BMM can identify them in 15%-40% of patients. "So I don’t know if it is a methodological difference, in terms of preparation of the bone marrow and identification of these cells," he said. "The difference in prognosis is multifactorial."
He pointed out that there is no standardized method to detect BMMs, that different methods were used to identify sentinel lymph nodes (SLNs), that patients received different adjuvant regimens, and that the number of cases who developed BMMs was so low, it could easily change outcomes.
Another attendee asked whether there is a particular cutoff for BMMs, noting that in many cancers the presence of micrometastases has no impact on survival, and that they are prognostic in melanoma only when they are at least 0.2 mm in the lymph nodes. Dr. Langer said that the median number of tumor cells in the analysis was two, but he balked at the possibility of using BMM testing in early breast cancer.
"You shouldn’t do the bone marrow aspiration," he said in an interview. "It might have prognostic impact, but if it doesn’t drive your therapy – if it doesn’t influence what you are doing – you have findings and you don’t know what to do with them. It’s too immature. We first have to standardize the testing."
Dr. Langer reported on 659 women from 13 Swiss centers who underwent intraoperative SLN examination by frozen section and paraffin serial sectioning at a cutting interval of 250 mcm with H&E (hematoxylin and eosin) staining and immunohistochemistry.
As previously reported, SLNs were identified in 98% of patients, including 71% with T1 disease and 29% with T2 disease (Breast Cancer Res. Treat. 2009;113:129-36). Their median age was 59 years, and 80% were postmenopausal. The median tumor size was 17 mm.
Of the 659 patients, 410 also underwent bone marrow aspiration from both iliac crests; these formed the basis of the current analysis. The mononuclear cells of the bone marrow aspirates were isolated by density gradient centrifugation through Ficoll. In all, 2 million bone marrow cells were evaluated, and the presence of one or more tumor cells was regarded as BMM positive, Dr. Langer said.
Cancer cells were stained with monoclonal antibodies A45-B/B3 against cytokeratin 8, 18, and 19 and were counted by an automated, computerized digital microscope. All results were reviewed by one pathologist.
Bone marrow micrometastases were detected in 118 (28.8%) of the 410 women. About 210 women were SLN negative and BMM negative, although considerable discordance was observed, Dr. Langer said. In all, 67 women (16.4%) were BMM positive and SLN negative, whereas 82 women (20%) were BMM negative and SLN positive.
In multivariate logistic regression analysis that included tumor size, tumor grade, tumor receptor status, and menopausal status, the presence of positive SLNs was the only significant independent predictor for the presence of BMM (P = .007; odds ratio, 1.860). T stage was not significant; only N stage was, he said.
In the earlier published analysis, the Swiss researchers identified SLN micrometastases or isolated tumor cells in 47 patients who underwent delayed ALND (axillary lymph node dissection). In 96% of these patients, the second operation was not beneficial because the ALND specimens were free of macrometastases. This finding – coupled with an overall accuracy of frozen section of 90% in the detection of SLN macrometastases – led the group to strongly recommend the routine use of SLN frozen section in early-stage breast cancer.
The recently published ACOSOG Z0011 trial concluded that ALND offers no survival advantage over SLN dissection alone in women with T1-T2 invasive breast cancer with no palpable adenopathy and one or two lymph nodes that contain metastases identified by frozen section, touch preparation, or H&E staining.
Sentinel node micrometastases (defined as H&E tumor deposits no greater than 2 mm in size) were identified in 37.5% of patients in the ALND group vs. 45% of those in the SLND group (JAMA 2011;305:569-75).
The Swiss Group for Clinical Cancer Research and the Cancer League of Basel-Stadt and Basel-Land funded the study. Dr. Langer and his coauthors disclosed no conflicts.
FROM A SYMPOSIUM SPONSORED BY THE SOCIETY OF SURGICAL ONCOLOGY
Major Finding: Overall survival was 92.7% in patients without BMM and 92.5% in those with BMM.
Data Source: A prospective Swiss Multicenter Study Group trial of 410 women with early-stage breast cancer.
Disclosures: The Swiss Group for Clinical Cancer Research and Swiss Cancer League of Basel-Stadt and Basel-Land funded the study. Dr. Langer and his coauthors disclosed no conflicts.
Clinical Trial Participation Tied to Improved Breast Cancer Outcomes
SAN ANTONIO – The literature is somewhat checkered over who can claim bragging rights for improved breast cancer outcomes, but the overall trend has favored surgical oncologists over general surgeons.
What’s driving the improved outcomes isn’t entirely clear, but a new analysis suggests that a key mechanism is that patients treated by surgical oncologists participate in clinical trials at dramatically higher rates, lead author Dr. William Dooley said during a plenary session at a symposium sponsored by the Society of Surgical Oncology.
Among 1,220 women analyzed for the period of 2001-2008, 56% of 777 patients treated by a surgical oncologist participated in a clinical trial, compared with only 7% of 443 general surgery patients (P value = .000).
Patients in a clinical trial were more likely to stay connected with the system and followed by their treating physician, said Dr. Dooley, the G. Rainey Williams Professor and chair of surgical breast oncology at the University of Oklahoma Breast Institute, Oklahoma City. The average follow-up was 44.6 months for the 468 trial participants vs. 38.5 months for the 752 nonparticipants (P = .000).
Participation in a clinical trial was associated with a significant survival advantage, increasing overall survival from 26% to 31% at 5 years (P = .000).
The benefits of clinical trial involvement cut across the entire health care team, Dr. Dooley said. Radiologists and pathologists receive outside review of and feedback for their work, while outside monitoring tracks the actions of the treatment team, patient progress through the treatment plan, and adherence of all elements of care to the system.
"When we begin to think about clinical trials and groups like ACOSOG [American College of Surgeons Oncology Group], we need to bring forth the spectrum of clinical trials to cover the whole spectrum of breast disease and encourage all surgeons in the country to be involved," he said. "This may be a very dramatic way that we can improve the quality of breast cancer care nationally."
The entire analysis included 2,191 women who received primary breast cancer treatment from 1995 to 2008 at the Breast Institute, with 752 under the care of a surgical oncologist and 1,439 cared for by a general surgeon. The average age of the women was 54 years and 57 years, respectively.
When the researchers compared their outcomes using standardized measures, the case mix included 25% more late-stage disease than the national population. General surgeons performed right at the national average, while additional oncologic training for surgeons provided a 28% improvement in workup and treatment.
But most significantly, the additional training reduced deaths for stage I-III breast cancer from 39% for stage IIIb disease to 57% for stage I disease, Dr. Dooley said.
Another mechanism driving the improved outcomes is that patients treated by a surgical oncologist are far more likely to complete National Comprehensive Cancer Network guideline–compatible therapy in a timely fashion, he said. In all, 77% of stage I-III patients treated by a surgical oncologist completed chemotherapy or hormonal adjuvant therapy, compared with 68.5% of general surgery patients (P less than .02).
Breast conservation rates were significantly higher for surgical oncology patients than for general surgery patients overall (53% vs. 38%, P less than .001), and for stage 0-II disease (66% vs. 54%, P less than .01).
Radiation therapy for breast conservation was completed by 97% of patients treated by a surgical oncologist and 67% of general surgery patients, and by 99% vs. 74% for stage III disease (P less than .001 for each), Dr. Dooley said.
"Good intentions don’t get us very far," he said. "The Commission on Cancer is starting a program to help us monitor initiation of treatment. It’s not the initiation that counts. It’s whether the patients actually finish that treatment completely."
There were no survival differences for women with stage 0 or IV disease, and their data were excluded from the presentation.
During a discussion of the study, an audience member questioned the presence of comorbidities in each group, observing that patients enrolled in clinical trials tend to be healthier. Dr. Dooley responded that details on comorbidities were limited, but that Charlson Index scores were similar in both groups. In addition, he noted that the same beneficial effect of clinical trial participation has been observed even in trials with just tissue banking.
Rates of stage I, IIA, and IIB disease were similar at 20.5%, 30.5%, and 17% in the surgical oncology group and 19.6%, 32%, and 24% in the general surgery group. Chemotherapy, however, was significantly more common among patients treated by a surgical oncologist than among those in the general surgery group (69% vs. 56%).
Another attendee pointed out that the Rapid Reporting System sends out an alert if cancer patients are not completing therapy. Dr. Dooley said the system was not in place for the entire study period.
Dr. Dooley and his coauthors reported no relevant conflicts of interest.
SAN ANTONIO – The literature is somewhat checkered over who can claim bragging rights for improved breast cancer outcomes, but the overall trend has favored surgical oncologists over general surgeons.
What’s driving the improved outcomes isn’t entirely clear, but a new analysis suggests that a key mechanism is that patients treated by surgical oncologists participate in clinical trials at dramatically higher rates, lead author Dr. William Dooley said during a plenary session at a symposium sponsored by the Society of Surgical Oncology.
Among 1,220 women analyzed for the period of 2001-2008, 56% of 777 patients treated by a surgical oncologist participated in a clinical trial, compared with only 7% of 443 general surgery patients (P value = .000).
Patients in a clinical trial were more likely to stay connected with the system and followed by their treating physician, said Dr. Dooley, the G. Rainey Williams Professor and chair of surgical breast oncology at the University of Oklahoma Breast Institute, Oklahoma City. The average follow-up was 44.6 months for the 468 trial participants vs. 38.5 months for the 752 nonparticipants (P = .000).
Participation in a clinical trial was associated with a significant survival advantage, increasing overall survival from 26% to 31% at 5 years (P = .000).
The benefits of clinical trial involvement cut across the entire health care team, Dr. Dooley said. Radiologists and pathologists receive outside review of and feedback for their work, while outside monitoring tracks the actions of the treatment team, patient progress through the treatment plan, and adherence of all elements of care to the system.
"When we begin to think about clinical trials and groups like ACOSOG [American College of Surgeons Oncology Group], we need to bring forth the spectrum of clinical trials to cover the whole spectrum of breast disease and encourage all surgeons in the country to be involved," he said. "This may be a very dramatic way that we can improve the quality of breast cancer care nationally."
The entire analysis included 2,191 women who received primary breast cancer treatment from 1995 to 2008 at the Breast Institute, with 752 under the care of a surgical oncologist and 1,439 cared for by a general surgeon. The average age of the women was 54 years and 57 years, respectively.
When the researchers compared their outcomes using standardized measures, the case mix included 25% more late-stage disease than the national population. General surgeons performed right at the national average, while additional oncologic training for surgeons provided a 28% improvement in workup and treatment.
But most significantly, the additional training reduced deaths for stage I-III breast cancer from 39% for stage IIIb disease to 57% for stage I disease, Dr. Dooley said.
Another mechanism driving the improved outcomes is that patients treated by a surgical oncologist are far more likely to complete National Comprehensive Cancer Network guideline–compatible therapy in a timely fashion, he said. In all, 77% of stage I-III patients treated by a surgical oncologist completed chemotherapy or hormonal adjuvant therapy, compared with 68.5% of general surgery patients (P less than .02).
Breast conservation rates were significantly higher for surgical oncology patients than for general surgery patients overall (53% vs. 38%, P less than .001), and for stage 0-II disease (66% vs. 54%, P less than .01).
Radiation therapy for breast conservation was completed by 97% of patients treated by a surgical oncologist and 67% of general surgery patients, and by 99% vs. 74% for stage III disease (P less than .001 for each), Dr. Dooley said.
"Good intentions don’t get us very far," he said. "The Commission on Cancer is starting a program to help us monitor initiation of treatment. It’s not the initiation that counts. It’s whether the patients actually finish that treatment completely."
There were no survival differences for women with stage 0 or IV disease, and their data were excluded from the presentation.
During a discussion of the study, an audience member questioned the presence of comorbidities in each group, observing that patients enrolled in clinical trials tend to be healthier. Dr. Dooley responded that details on comorbidities were limited, but that Charlson Index scores were similar in both groups. In addition, he noted that the same beneficial effect of clinical trial participation has been observed even in trials with just tissue banking.
Rates of stage I, IIA, and IIB disease were similar at 20.5%, 30.5%, and 17% in the surgical oncology group and 19.6%, 32%, and 24% in the general surgery group. Chemotherapy, however, was significantly more common among patients treated by a surgical oncologist than among those in the general surgery group (69% vs. 56%).
Another attendee pointed out that the Rapid Reporting System sends out an alert if cancer patients are not completing therapy. Dr. Dooley said the system was not in place for the entire study period.
Dr. Dooley and his coauthors reported no relevant conflicts of interest.
SAN ANTONIO – The literature is somewhat checkered over who can claim bragging rights for improved breast cancer outcomes, but the overall trend has favored surgical oncologists over general surgeons.
What’s driving the improved outcomes isn’t entirely clear, but a new analysis suggests that a key mechanism is that patients treated by surgical oncologists participate in clinical trials at dramatically higher rates, lead author Dr. William Dooley said during a plenary session at a symposium sponsored by the Society of Surgical Oncology.
Among 1,220 women analyzed for the period of 2001-2008, 56% of 777 patients treated by a surgical oncologist participated in a clinical trial, compared with only 7% of 443 general surgery patients (P value = .000).
Patients in a clinical trial were more likely to stay connected with the system and followed by their treating physician, said Dr. Dooley, the G. Rainey Williams Professor and chair of surgical breast oncology at the University of Oklahoma Breast Institute, Oklahoma City. The average follow-up was 44.6 months for the 468 trial participants vs. 38.5 months for the 752 nonparticipants (P = .000).
Participation in a clinical trial was associated with a significant survival advantage, increasing overall survival from 26% to 31% at 5 years (P = .000).
The benefits of clinical trial involvement cut across the entire health care team, Dr. Dooley said. Radiologists and pathologists receive outside review of and feedback for their work, while outside monitoring tracks the actions of the treatment team, patient progress through the treatment plan, and adherence of all elements of care to the system.
"When we begin to think about clinical trials and groups like ACOSOG [American College of Surgeons Oncology Group], we need to bring forth the spectrum of clinical trials to cover the whole spectrum of breast disease and encourage all surgeons in the country to be involved," he said. "This may be a very dramatic way that we can improve the quality of breast cancer care nationally."
The entire analysis included 2,191 women who received primary breast cancer treatment from 1995 to 2008 at the Breast Institute, with 752 under the care of a surgical oncologist and 1,439 cared for by a general surgeon. The average age of the women was 54 years and 57 years, respectively.
When the researchers compared their outcomes using standardized measures, the case mix included 25% more late-stage disease than the national population. General surgeons performed right at the national average, while additional oncologic training for surgeons provided a 28% improvement in workup and treatment.
But most significantly, the additional training reduced deaths for stage I-III breast cancer from 39% for stage IIIb disease to 57% for stage I disease, Dr. Dooley said.
Another mechanism driving the improved outcomes is that patients treated by a surgical oncologist are far more likely to complete National Comprehensive Cancer Network guideline–compatible therapy in a timely fashion, he said. In all, 77% of stage I-III patients treated by a surgical oncologist completed chemotherapy or hormonal adjuvant therapy, compared with 68.5% of general surgery patients (P less than .02).
Breast conservation rates were significantly higher for surgical oncology patients than for general surgery patients overall (53% vs. 38%, P less than .001), and for stage 0-II disease (66% vs. 54%, P less than .01).
Radiation therapy for breast conservation was completed by 97% of patients treated by a surgical oncologist and 67% of general surgery patients, and by 99% vs. 74% for stage III disease (P less than .001 for each), Dr. Dooley said.
"Good intentions don’t get us very far," he said. "The Commission on Cancer is starting a program to help us monitor initiation of treatment. It’s not the initiation that counts. It’s whether the patients actually finish that treatment completely."
There were no survival differences for women with stage 0 or IV disease, and their data were excluded from the presentation.
During a discussion of the study, an audience member questioned the presence of comorbidities in each group, observing that patients enrolled in clinical trials tend to be healthier. Dr. Dooley responded that details on comorbidities were limited, but that Charlson Index scores were similar in both groups. In addition, he noted that the same beneficial effect of clinical trial participation has been observed even in trials with just tissue banking.
Rates of stage I, IIA, and IIB disease were similar at 20.5%, 30.5%, and 17% in the surgical oncology group and 19.6%, 32%, and 24% in the general surgery group. Chemotherapy, however, was significantly more common among patients treated by a surgical oncologist than among those in the general surgery group (69% vs. 56%).
Another attendee pointed out that the Rapid Reporting System sends out an alert if cancer patients are not completing therapy. Dr. Dooley said the system was not in place for the entire study period.
Dr. Dooley and his coauthors reported no relevant conflicts of interest.
FROM A SYMPOSIUM SPONSORED BY THE SOCIETY OF SURGICAL ONCOLOGY
Major Finding: Among 1,220 women, 56% of patients treated by a surgical oncologist participated in a clinical trial, compared with 7% of general surgery patients (P value = .000).
Data Source: Single-institution retrospective analysis of 2,191 women with breast cancer.
Disclosures: Dr. Dooley and his coauthors reported no relevant conflicts of interest.
Techniques Compared for Breast Tumor Detection
SAN ANTONIO – Positive margin and reoperation rates were similar for radio-guided seed localization and wire-guided localization of nonpalpable breast tumors in a prospective multicenter trial – in contrast to results from previous studies.
In an intent-to-treat analysis of 305 women with confirmed invasive or ductal carcinoma in situ who were undergoing localization and breast-conserving surgery, positive margin rates were 10.5% for radio-guided seed localization (RSL) and 11.9% for wire-guided localization (P = .990).
Rates of positive plus close margins (defined as disease within less than 1 mm) were 19% for the seed group and 22% for the wire group (P = .609), Dr. Peter Lovrics reported at a symposium sponsored by the Society of Surgical Oncology.
Rates of reoperation – either re-excision of margins or mastectomy – were also similar (19% and 15%, respectively) in the wire and seed groups, said Dr. Lovrics of the department of surgery at McMaster University in Hamilton, Ont.
The feasibility of RSL for identifying occult breast tumors was first reported a little more than a decade ago, and RSL has been rapidly adopted in many European countries. The procedure involves intratumoral injection of a radiotracer to identify the primary tumor and sentinel lymph nodes for intraoperative gamma probe–guided dissection.
In a recent literature review of 12 studies, Dr. Lovrics and his associates found that RSL yields lower positive margins (odds ratio, 0.367) and fewer reoperations (OR, 0.347) than does wire-guided localization, and three of those studies also suggested improved cosmesis (Eur. J. Surg. Oncol. 2011 Feb. 16 [E-pub ahead of print]).
When asked why RSL failed to produce significantly better outcomes, Dr. Lovrics said it may be that with a larger sample size, RSL would be potentially better, yielding results similar to those of other studies. Notably, overall positive margin rates were low for both groups and were much higher for wire-guided localization in most other studies.
"This may be partially attributed to the fact that all patients had a preoperative core biopsy and a confirmed diagnosis of cancer, so all of the procedures were definitive operations," he said in an interview. "In some other studies, not all patients had confirmed cancer and some operations were diagnostic in intent. Otherwise, the study population was typical – similar to other studies in tumor characteristics."
In the current study, there were 152 patients in the seed group and 153 in the wire group, with 6 patients crossing over to the wire group because the seed did not arrive at the hospital on the day of surgery. Mammogram findings included a mass in 47% of the wire group and in 50% of the seed group, and microcalcifications in 11% and 14%, respectively. Three-fourths of patients had a mass present on ultrasonography. Their average age was 60 years.
Secondary outcomes between groups were very similar, except for a significantly shorter operative time of 19 minutes with seed localization vs. 22 minutes in the wire group (P less than .001), Dr. Lovrics said. Excision difficulty was also ranked significantly easier for surgeons with seed localization (P = .008).
Factors such as skin removal and whether the dissection was taken to the chest wall were similar, as were the number of additional cavity margins excised and postoperative complications at 1 month, he said during a breast-focused plenary session.
Pain-reported anxiety during localization was similar in both groups, although patients in the seed group reported significantly less pain during the localization procedure than did those in the wire group (P = 0.38). An analysis of cosmetic results is ongoing.
Dr. Lovrics said that RSL allows exquisite and precise localization of the tumor, and that radiation exposure to patients and health care workers is negligible.
"For the surgeon, it provides very real-time, enhanced guidance for localization and is preferred by surgeons," he concluded. "Radio-seed localization is an acceptable alternative to wire-guided localization."
During a discussion of the study, audience members asked whether a cost analysis had been performed and whether the seeds were placed on the day of the surgery. Dr. Lovrics said the seeds and the wire both cost about $50, and that they currently place the seed on the day of surgery, but they hope to insert it earlier to further reduce OR time.
When asked whether a radiograph is still needed after seed localization, Dr. Lovrics said that a postoperative radiograph is still essential because of the risk of seed migration. Seeds were misplaced in four cases, one seed migrated, one wire migrated, and one wire fell out.
The Canadian Breast Cancer Foundation funded the study. Dr. Lovrics and his coauthors reported no conflicts of interest.
SAN ANTONIO – Positive margin and reoperation rates were similar for radio-guided seed localization and wire-guided localization of nonpalpable breast tumors in a prospective multicenter trial – in contrast to results from previous studies.
In an intent-to-treat analysis of 305 women with confirmed invasive or ductal carcinoma in situ who were undergoing localization and breast-conserving surgery, positive margin rates were 10.5% for radio-guided seed localization (RSL) and 11.9% for wire-guided localization (P = .990).
Rates of positive plus close margins (defined as disease within less than 1 mm) were 19% for the seed group and 22% for the wire group (P = .609), Dr. Peter Lovrics reported at a symposium sponsored by the Society of Surgical Oncology.
Rates of reoperation – either re-excision of margins or mastectomy – were also similar (19% and 15%, respectively) in the wire and seed groups, said Dr. Lovrics of the department of surgery at McMaster University in Hamilton, Ont.
The feasibility of RSL for identifying occult breast tumors was first reported a little more than a decade ago, and RSL has been rapidly adopted in many European countries. The procedure involves intratumoral injection of a radiotracer to identify the primary tumor and sentinel lymph nodes for intraoperative gamma probe–guided dissection.
In a recent literature review of 12 studies, Dr. Lovrics and his associates found that RSL yields lower positive margins (odds ratio, 0.367) and fewer reoperations (OR, 0.347) than does wire-guided localization, and three of those studies also suggested improved cosmesis (Eur. J. Surg. Oncol. 2011 Feb. 16 [E-pub ahead of print]).
When asked why RSL failed to produce significantly better outcomes, Dr. Lovrics said it may be that with a larger sample size, RSL would be potentially better, yielding results similar to those of other studies. Notably, overall positive margin rates were low for both groups and were much higher for wire-guided localization in most other studies.
"This may be partially attributed to the fact that all patients had a preoperative core biopsy and a confirmed diagnosis of cancer, so all of the procedures were definitive operations," he said in an interview. "In some other studies, not all patients had confirmed cancer and some operations were diagnostic in intent. Otherwise, the study population was typical – similar to other studies in tumor characteristics."
In the current study, there were 152 patients in the seed group and 153 in the wire group, with 6 patients crossing over to the wire group because the seed did not arrive at the hospital on the day of surgery. Mammogram findings included a mass in 47% of the wire group and in 50% of the seed group, and microcalcifications in 11% and 14%, respectively. Three-fourths of patients had a mass present on ultrasonography. Their average age was 60 years.
Secondary outcomes between groups were very similar, except for a significantly shorter operative time of 19 minutes with seed localization vs. 22 minutes in the wire group (P less than .001), Dr. Lovrics said. Excision difficulty was also ranked significantly easier for surgeons with seed localization (P = .008).
Factors such as skin removal and whether the dissection was taken to the chest wall were similar, as were the number of additional cavity margins excised and postoperative complications at 1 month, he said during a breast-focused plenary session.
Pain-reported anxiety during localization was similar in both groups, although patients in the seed group reported significantly less pain during the localization procedure than did those in the wire group (P = 0.38). An analysis of cosmetic results is ongoing.
Dr. Lovrics said that RSL allows exquisite and precise localization of the tumor, and that radiation exposure to patients and health care workers is negligible.
"For the surgeon, it provides very real-time, enhanced guidance for localization and is preferred by surgeons," he concluded. "Radio-seed localization is an acceptable alternative to wire-guided localization."
During a discussion of the study, audience members asked whether a cost analysis had been performed and whether the seeds were placed on the day of the surgery. Dr. Lovrics said the seeds and the wire both cost about $50, and that they currently place the seed on the day of surgery, but they hope to insert it earlier to further reduce OR time.
When asked whether a radiograph is still needed after seed localization, Dr. Lovrics said that a postoperative radiograph is still essential because of the risk of seed migration. Seeds were misplaced in four cases, one seed migrated, one wire migrated, and one wire fell out.
The Canadian Breast Cancer Foundation funded the study. Dr. Lovrics and his coauthors reported no conflicts of interest.
SAN ANTONIO – Positive margin and reoperation rates were similar for radio-guided seed localization and wire-guided localization of nonpalpable breast tumors in a prospective multicenter trial – in contrast to results from previous studies.
In an intent-to-treat analysis of 305 women with confirmed invasive or ductal carcinoma in situ who were undergoing localization and breast-conserving surgery, positive margin rates were 10.5% for radio-guided seed localization (RSL) and 11.9% for wire-guided localization (P = .990).
Rates of positive plus close margins (defined as disease within less than 1 mm) were 19% for the seed group and 22% for the wire group (P = .609), Dr. Peter Lovrics reported at a symposium sponsored by the Society of Surgical Oncology.
Rates of reoperation – either re-excision of margins or mastectomy – were also similar (19% and 15%, respectively) in the wire and seed groups, said Dr. Lovrics of the department of surgery at McMaster University in Hamilton, Ont.
The feasibility of RSL for identifying occult breast tumors was first reported a little more than a decade ago, and RSL has been rapidly adopted in many European countries. The procedure involves intratumoral injection of a radiotracer to identify the primary tumor and sentinel lymph nodes for intraoperative gamma probe–guided dissection.
In a recent literature review of 12 studies, Dr. Lovrics and his associates found that RSL yields lower positive margins (odds ratio, 0.367) and fewer reoperations (OR, 0.347) than does wire-guided localization, and three of those studies also suggested improved cosmesis (Eur. J. Surg. Oncol. 2011 Feb. 16 [E-pub ahead of print]).
When asked why RSL failed to produce significantly better outcomes, Dr. Lovrics said it may be that with a larger sample size, RSL would be potentially better, yielding results similar to those of other studies. Notably, overall positive margin rates were low for both groups and were much higher for wire-guided localization in most other studies.
"This may be partially attributed to the fact that all patients had a preoperative core biopsy and a confirmed diagnosis of cancer, so all of the procedures were definitive operations," he said in an interview. "In some other studies, not all patients had confirmed cancer and some operations were diagnostic in intent. Otherwise, the study population was typical – similar to other studies in tumor characteristics."
In the current study, there were 152 patients in the seed group and 153 in the wire group, with 6 patients crossing over to the wire group because the seed did not arrive at the hospital on the day of surgery. Mammogram findings included a mass in 47% of the wire group and in 50% of the seed group, and microcalcifications in 11% and 14%, respectively. Three-fourths of patients had a mass present on ultrasonography. Their average age was 60 years.
Secondary outcomes between groups were very similar, except for a significantly shorter operative time of 19 minutes with seed localization vs. 22 minutes in the wire group (P less than .001), Dr. Lovrics said. Excision difficulty was also ranked significantly easier for surgeons with seed localization (P = .008).
Factors such as skin removal and whether the dissection was taken to the chest wall were similar, as were the number of additional cavity margins excised and postoperative complications at 1 month, he said during a breast-focused plenary session.
Pain-reported anxiety during localization was similar in both groups, although patients in the seed group reported significantly less pain during the localization procedure than did those in the wire group (P = 0.38). An analysis of cosmetic results is ongoing.
Dr. Lovrics said that RSL allows exquisite and precise localization of the tumor, and that radiation exposure to patients and health care workers is negligible.
"For the surgeon, it provides very real-time, enhanced guidance for localization and is preferred by surgeons," he concluded. "Radio-seed localization is an acceptable alternative to wire-guided localization."
During a discussion of the study, audience members asked whether a cost analysis had been performed and whether the seeds were placed on the day of the surgery. Dr. Lovrics said the seeds and the wire both cost about $50, and that they currently place the seed on the day of surgery, but they hope to insert it earlier to further reduce OR time.
When asked whether a radiograph is still needed after seed localization, Dr. Lovrics said that a postoperative radiograph is still essential because of the risk of seed migration. Seeds were misplaced in four cases, one seed migrated, one wire migrated, and one wire fell out.
The Canadian Breast Cancer Foundation funded the study. Dr. Lovrics and his coauthors reported no conflicts of interest.
FROM SYMPOSIUM SPONSORED BY SOCIETY OF SURGICAL ONCOLOGY
Major Finding: Positive margin rates were 10.5% for radio-guided seed localization and 11.9% for wire-guided localization.
Data Source: Prospective randomized trial of 305 women with nonpalpable breast tumors.
Disclosures: The Canadian Breast Cancer Foundation funded the study. Dr. Lovrics and his coauthors reported no conflicts of interest.
Eribulin Extends Survival of Women With Heavily Pretreated Breast Cancer
Eribulin monotherapy achieved "a significant and clinically meaningful improvement" in overall survival, compared with other treatments of choice in women with heavily pretreated metastatic breast cancer, according to pivotal EMBRACE trial data published online by the Lancet on March 3.
Median overall survival rate reached 13.1 months in women treated with eribulin (Halaven), compared with 10.6 months in the control group (P = .041). Median progression-free survival was 3.7 months and 2.2 months, respectively (P = .137) based on an independent review of the intent-to-treat population. Fatal adverse events occurred in 4% of the eribulin patients vs. 7% of the control group.
"To our knowledge, EMBRACE is the first major single-agent study of a cytotoxic or biological agent to show significantly increased survival in patients with such heavily pretreated metastatic breast cancer," said Dr. Javier Cortes of Vall d’Hebron University Hospital and Institute of Oncology in Barcelona and colleagues (Lancet 2011 March 3 [doi: 10.1016/S0140-6736(11)60070-6]).
A non-taxane microtubule inhibitor, eribulin is a synthetic analogue of halichondrin B, a natural product isolated from a sea sponge. The U.S. Food & Drug Administration based its approval of eribulin for women who had received at least two prior treatments, including a taxane and an anthracycline, for metastatic breast cancer on results of the EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice vs. E7389) study. Findings were originally presented at the American Society of Clinical Oncology annual meeting in 2010.
The open-label study randomized 508 women to eribulin and 254 to a control group; 6 patients in each group discontinued before starting treatment, leaving 503 in the eribulin group and 247 in the control group. The women were aged 18 years and older, with confirmed breast cancer and a history of 2-5 previous chemotherapy regimens.
Patients in the eribulin group received 1.4 mg/m2 eribulin mesylate intravenously for 2-5 minutes on days 1 and 8 of a 21-day cycle. The control group received the physician’s treatment of choice, which could be any single-agent chemotherapy, hormonal therapy, or biologic therapy approved for cancer, and administered according to the local practice.
The researchers assessed tumor response every 8 weeks. Treatment continued until disease progression, serious noncompliance with protocol, unacceptable toxicity, or a doctor’s or patient’s request to stop treatment. The patients had a median of four previous chemotherapy regimens, of which capecitabine was the most common (73%). A total of 16% had HER2-positive disease, and 19% had triple-negative breast cancer.
The most common overall adverse event was asthenia or fatigue of all grades, which occurred in 54% in the eribulin group and 40% of the control group. The most common hematologic adverse event was neutropenia of all grades in 52% of the eribulin group and 30% of the control group.
About a fourth of both groups experienced serious adverse events. Peripheral neuropathy was the most common cause for discontinuing eribulin, occurring in 5% of 503 patients.
The findings were limited by the range of the therapies in the control group and by the use of overall survival as a primary end point, the researchers noted. But the diversity of the control group might make the findings more generalizable to clinical practice, they added.
"The benefit that eribulin had shown as a single agent in this setting suggests that this drug could become a new standard of care; further evaluation earlier in the natural history of breast cancer is warranted," the researchers wrote.
In an accompanying editorial, Dr. Nancy U. Lin and Dr. Harold J. Burstein wrote that he results of the EMBRACE study raise many questions, including whether some patients or tumor subtypes would benefit from ongoing therapy (Lancet 2011 March 3 [doi: 10.1016/S0140-6736(11)60280-8]).
Dr. Lin and Dr. Burstein, both of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, noted that the relatively modest clinical improvements suggest the need for more information on how the treatment affects symptom control and quality of life.
"In the meantime, EMBRACE provides much-needed, high-level evidence for chemotherapy use in patients with heavily pretreated breast cancer," they wrote. "And that evidence suggests that the methods to treat advanced breast cancer are growing," they wrote.
The study was funded by Eisai, maker of eribulin, and Eisai employees were involved in the study design, data collection, and data analysis. Dr. Cortes has received consultant fees from Eisai and Roche.
Dr. Lin and Dr. Burstein said they had no conflicts of interest.
The results of the EMBRACE study raise many questions, including whether some patients or tumor subtypes would benefit from ongoing therapy, Dr. Nancy U. Lin and Dr. Harold J. Burstein of Harvard Medical School, Boston, wrote in an accompanying editorial (Lancet 2011 March 3 [Epub doi: 10.1016/S0140-6736(11)60280-8]).
The editorialists noted that the relatively modest clinical improvements suggest the need for more information on how the treatment affects symptom control and quality of life.
"In the meantime, EMBRACE provides much-needed, high-level evidence for chemotherapy use in patients with heavily pretreated breast cancer," they wrote. "And that evidence suggests that the methods to treat advanced breast cancer are growing," they said.
Dr. Nancy U. Lin and Dr. Harold J. Burstein are oncologists at Dana-Farber Cancer Institute, and on the faculty of Harvard Medical School. They said they had no conflicts of interest.
The results of the EMBRACE study raise many questions, including whether some patients or tumor subtypes would benefit from ongoing therapy, Dr. Nancy U. Lin and Dr. Harold J. Burstein of Harvard Medical School, Boston, wrote in an accompanying editorial (Lancet 2011 March 3 [Epub doi: 10.1016/S0140-6736(11)60280-8]).
The editorialists noted that the relatively modest clinical improvements suggest the need for more information on how the treatment affects symptom control and quality of life.
"In the meantime, EMBRACE provides much-needed, high-level evidence for chemotherapy use in patients with heavily pretreated breast cancer," they wrote. "And that evidence suggests that the methods to treat advanced breast cancer are growing," they said.
Dr. Nancy U. Lin and Dr. Harold J. Burstein are oncologists at Dana-Farber Cancer Institute, and on the faculty of Harvard Medical School. They said they had no conflicts of interest.
The results of the EMBRACE study raise many questions, including whether some patients or tumor subtypes would benefit from ongoing therapy, Dr. Nancy U. Lin and Dr. Harold J. Burstein of Harvard Medical School, Boston, wrote in an accompanying editorial (Lancet 2011 March 3 [Epub doi: 10.1016/S0140-6736(11)60280-8]).
The editorialists noted that the relatively modest clinical improvements suggest the need for more information on how the treatment affects symptom control and quality of life.
"In the meantime, EMBRACE provides much-needed, high-level evidence for chemotherapy use in patients with heavily pretreated breast cancer," they wrote. "And that evidence suggests that the methods to treat advanced breast cancer are growing," they said.
Dr. Nancy U. Lin and Dr. Harold J. Burstein are oncologists at Dana-Farber Cancer Institute, and on the faculty of Harvard Medical School. They said they had no conflicts of interest.
Eribulin monotherapy achieved "a significant and clinically meaningful improvement" in overall survival, compared with other treatments of choice in women with heavily pretreated metastatic breast cancer, according to pivotal EMBRACE trial data published online by the Lancet on March 3.
Median overall survival rate reached 13.1 months in women treated with eribulin (Halaven), compared with 10.6 months in the control group (P = .041). Median progression-free survival was 3.7 months and 2.2 months, respectively (P = .137) based on an independent review of the intent-to-treat population. Fatal adverse events occurred in 4% of the eribulin patients vs. 7% of the control group.
"To our knowledge, EMBRACE is the first major single-agent study of a cytotoxic or biological agent to show significantly increased survival in patients with such heavily pretreated metastatic breast cancer," said Dr. Javier Cortes of Vall d’Hebron University Hospital and Institute of Oncology in Barcelona and colleagues (Lancet 2011 March 3 [doi: 10.1016/S0140-6736(11)60070-6]).
A non-taxane microtubule inhibitor, eribulin is a synthetic analogue of halichondrin B, a natural product isolated from a sea sponge. The U.S. Food & Drug Administration based its approval of eribulin for women who had received at least two prior treatments, including a taxane and an anthracycline, for metastatic breast cancer on results of the EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice vs. E7389) study. Findings were originally presented at the American Society of Clinical Oncology annual meeting in 2010.
The open-label study randomized 508 women to eribulin and 254 to a control group; 6 patients in each group discontinued before starting treatment, leaving 503 in the eribulin group and 247 in the control group. The women were aged 18 years and older, with confirmed breast cancer and a history of 2-5 previous chemotherapy regimens.
Patients in the eribulin group received 1.4 mg/m2 eribulin mesylate intravenously for 2-5 minutes on days 1 and 8 of a 21-day cycle. The control group received the physician’s treatment of choice, which could be any single-agent chemotherapy, hormonal therapy, or biologic therapy approved for cancer, and administered according to the local practice.
The researchers assessed tumor response every 8 weeks. Treatment continued until disease progression, serious noncompliance with protocol, unacceptable toxicity, or a doctor’s or patient’s request to stop treatment. The patients had a median of four previous chemotherapy regimens, of which capecitabine was the most common (73%). A total of 16% had HER2-positive disease, and 19% had triple-negative breast cancer.
The most common overall adverse event was asthenia or fatigue of all grades, which occurred in 54% in the eribulin group and 40% of the control group. The most common hematologic adverse event was neutropenia of all grades in 52% of the eribulin group and 30% of the control group.
About a fourth of both groups experienced serious adverse events. Peripheral neuropathy was the most common cause for discontinuing eribulin, occurring in 5% of 503 patients.
The findings were limited by the range of the therapies in the control group and by the use of overall survival as a primary end point, the researchers noted. But the diversity of the control group might make the findings more generalizable to clinical practice, they added.
"The benefit that eribulin had shown as a single agent in this setting suggests that this drug could become a new standard of care; further evaluation earlier in the natural history of breast cancer is warranted," the researchers wrote.
In an accompanying editorial, Dr. Nancy U. Lin and Dr. Harold J. Burstein wrote that he results of the EMBRACE study raise many questions, including whether some patients or tumor subtypes would benefit from ongoing therapy (Lancet 2011 March 3 [doi: 10.1016/S0140-6736(11)60280-8]).
Dr. Lin and Dr. Burstein, both of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, noted that the relatively modest clinical improvements suggest the need for more information on how the treatment affects symptom control and quality of life.
"In the meantime, EMBRACE provides much-needed, high-level evidence for chemotherapy use in patients with heavily pretreated breast cancer," they wrote. "And that evidence suggests that the methods to treat advanced breast cancer are growing," they wrote.
The study was funded by Eisai, maker of eribulin, and Eisai employees were involved in the study design, data collection, and data analysis. Dr. Cortes has received consultant fees from Eisai and Roche.
Dr. Lin and Dr. Burstein said they had no conflicts of interest.
Eribulin monotherapy achieved "a significant and clinically meaningful improvement" in overall survival, compared with other treatments of choice in women with heavily pretreated metastatic breast cancer, according to pivotal EMBRACE trial data published online by the Lancet on March 3.
Median overall survival rate reached 13.1 months in women treated with eribulin (Halaven), compared with 10.6 months in the control group (P = .041). Median progression-free survival was 3.7 months and 2.2 months, respectively (P = .137) based on an independent review of the intent-to-treat population. Fatal adverse events occurred in 4% of the eribulin patients vs. 7% of the control group.
"To our knowledge, EMBRACE is the first major single-agent study of a cytotoxic or biological agent to show significantly increased survival in patients with such heavily pretreated metastatic breast cancer," said Dr. Javier Cortes of Vall d’Hebron University Hospital and Institute of Oncology in Barcelona and colleagues (Lancet 2011 March 3 [doi: 10.1016/S0140-6736(11)60070-6]).
A non-taxane microtubule inhibitor, eribulin is a synthetic analogue of halichondrin B, a natural product isolated from a sea sponge. The U.S. Food & Drug Administration based its approval of eribulin for women who had received at least two prior treatments, including a taxane and an anthracycline, for metastatic breast cancer on results of the EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice vs. E7389) study. Findings were originally presented at the American Society of Clinical Oncology annual meeting in 2010.
The open-label study randomized 508 women to eribulin and 254 to a control group; 6 patients in each group discontinued before starting treatment, leaving 503 in the eribulin group and 247 in the control group. The women were aged 18 years and older, with confirmed breast cancer and a history of 2-5 previous chemotherapy regimens.
Patients in the eribulin group received 1.4 mg/m2 eribulin mesylate intravenously for 2-5 minutes on days 1 and 8 of a 21-day cycle. The control group received the physician’s treatment of choice, which could be any single-agent chemotherapy, hormonal therapy, or biologic therapy approved for cancer, and administered according to the local practice.
The researchers assessed tumor response every 8 weeks. Treatment continued until disease progression, serious noncompliance with protocol, unacceptable toxicity, or a doctor’s or patient’s request to stop treatment. The patients had a median of four previous chemotherapy regimens, of which capecitabine was the most common (73%). A total of 16% had HER2-positive disease, and 19% had triple-negative breast cancer.
The most common overall adverse event was asthenia or fatigue of all grades, which occurred in 54% in the eribulin group and 40% of the control group. The most common hematologic adverse event was neutropenia of all grades in 52% of the eribulin group and 30% of the control group.
About a fourth of both groups experienced serious adverse events. Peripheral neuropathy was the most common cause for discontinuing eribulin, occurring in 5% of 503 patients.
The findings were limited by the range of the therapies in the control group and by the use of overall survival as a primary end point, the researchers noted. But the diversity of the control group might make the findings more generalizable to clinical practice, they added.
"The benefit that eribulin had shown as a single agent in this setting suggests that this drug could become a new standard of care; further evaluation earlier in the natural history of breast cancer is warranted," the researchers wrote.
In an accompanying editorial, Dr. Nancy U. Lin and Dr. Harold J. Burstein wrote that he results of the EMBRACE study raise many questions, including whether some patients or tumor subtypes would benefit from ongoing therapy (Lancet 2011 March 3 [doi: 10.1016/S0140-6736(11)60280-8]).
Dr. Lin and Dr. Burstein, both of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, noted that the relatively modest clinical improvements suggest the need for more information on how the treatment affects symptom control and quality of life.
"In the meantime, EMBRACE provides much-needed, high-level evidence for chemotherapy use in patients with heavily pretreated breast cancer," they wrote. "And that evidence suggests that the methods to treat advanced breast cancer are growing," they wrote.
The study was funded by Eisai, maker of eribulin, and Eisai employees were involved in the study design, data collection, and data analysis. Dr. Cortes has received consultant fees from Eisai and Roche.
Dr. Lin and Dr. Burstein said they had no conflicts of interest.
FROM THE LANCET
Major Finding: Median overall survival rate reached 13.1 months in women treated with eribulin, compared with 10.6 months in the control group (P = .041).
Data Source: The EMBRACE trial in 764 women with heavily pretreated advanced and metastatic breast cancer.
Disclosures: The study was funded by Eisai, maker of eribulin, and Eisai employees were involved in the study design, data collection, and data analysis. Dr. Cortes has received consultant fees from Eisai and Roche.