User login
New anticancer drugs linked to increased costs, life expectancy
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
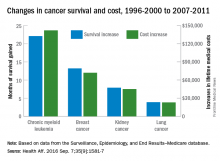
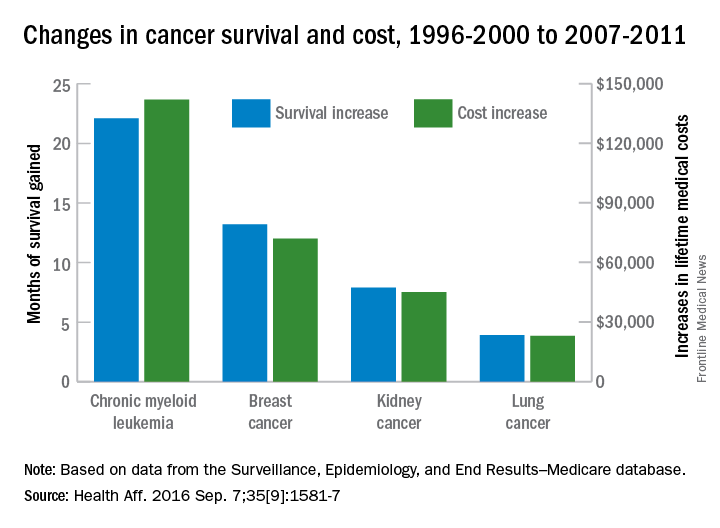
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
FROM HEALTH AFFAIRS
Drug combo shows promise for non–clear cell renal cell carcinoma
The combination of an mTOR complex 1 inhibitor (everolimus) plus a VEGF inhibitor (bevacizumab) showed promise against advanced non–clear cell renal cell carcinoma characterized by papillary features in a small manufacturer-sponsored phase II trial, according to a report published online Sept. 6 in the Journal of Clinical Oncology.
Non–clear cell renal cell carcinomas (ncRCCs) are a diverse mixture of heterogeneous malignancies and include papillary, chromophobe, medullary, collecting duct, and a variety of unclassified tumor types. Researchers performed a single-center trial to assess the effectiveness of combined everolimus plus bevacizumab in 35 treatment-naive patients who presented with advanced disease representing all of these histologic types. The unclassified subgroup (23 patients) included several tumors with prominent papillary architectural features that did not fulfill other criteria for papillary RCC, said Martin H. Voss, MD, of Memorial Sloan Kettering Cancer Center, New York, and his associates.
A total of 18 patients (53%) were alive and free of disease progression at 6 months, and 10 (29%) were alive and progression free at 12 months. Two patients still were receiving study treatment at the time of publication, after 20.2 and 30.4 months of therapy, respectively.
“Objective responses were observed in a sizable proportion of subjects with significant papillary (7 of 18) or chromophobe (2 of 5) tumor components but rarely in patients with unclassified RCC without papillary features (1 of 9) or those with medullary RCC (0 of 2),” Dr. Voss and his associates reported. Among patients with unclassified RCC, the 14 whose cancer had a major papillary component showed an objective response rate of 43%, a median progression-free survival of 12.9 months, and a median overall survival of 28.2 months. In contrast, the nine patients whose cancer did not have a major papillary component showed an objective response rate of 11%, a median progression-free survival of 1.9 months, and a median overall survival of 9.3 months, the investigators said (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.67.9084).
Treatment was generally well tolerated, even though there were frequent low-grade toxicities. High-grade toxicities known to be associated with mTOR complex 1 inhibitors or VEGF inhibitors included hyperglycemia (11%), hypertriglyceridemia (14%), lymphopenia (20%), hypertension (29%), and proteinuria (18%). There were two patient deaths from gastrointestinal hemorrhage, one of which was considered possibly related to bevacizumab.
Archived tissue samples were available for genetic analysis for some patients. Acquired mutations in the ARID1A gene were noted in 5 of 14 tumors with major papillary components, and all 5 of those patients achieved more than 6 months of progression-free survival with the combination therapy. In contrast, no ARID1A mutations were detected in any of the patients who had shorter progression-free survival, and none were detected in any of the tumors that did not have papillary components. This suggests that ARID1A “merits further study for its functional role in papillary RCC variants and as a candidate biomarker for future study of everolimus plus bevacizumab,” Dr. Voss and his associates said.
Novartis supported the study. Dr. Voss reported ties to Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Bristol-Myers Squibb, Genentech, and Takeda; his associates reported ties to numerous industry sources.
The signal of activity Voss et al. found in the subgroup of non–clear cell RCCs with papillary features is compelling, but studies with larger sample sizes are necessary. We need multicenter randomized trials that specifically focus on particular histologic subtypes and provide detailed molecular characterization.
Fortunately, another recent phase II study also found that pairing an mTOR inhibitor with a VEGF inhibitor (everolimus plus lenvatinib) improved the response rate, progression-free survival, and overall survival in clear cell RCC. The ensuing regulatory approval of this combination paves the way for further studies of similar regimens for tumors with papillary features.
Sumanta K. Pal, MD, is in the department of medical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. Financial disclosures for Dr. Pal and his associates are available at www.jco.org. Dr. Pal and his associates made these remarks in an editorial accompanying Dr. Voss’s report (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.69.3572).
The signal of activity Voss et al. found in the subgroup of non–clear cell RCCs with papillary features is compelling, but studies with larger sample sizes are necessary. We need multicenter randomized trials that specifically focus on particular histologic subtypes and provide detailed molecular characterization.
Fortunately, another recent phase II study also found that pairing an mTOR inhibitor with a VEGF inhibitor (everolimus plus lenvatinib) improved the response rate, progression-free survival, and overall survival in clear cell RCC. The ensuing regulatory approval of this combination paves the way for further studies of similar regimens for tumors with papillary features.
Sumanta K. Pal, MD, is in the department of medical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. Financial disclosures for Dr. Pal and his associates are available at www.jco.org. Dr. Pal and his associates made these remarks in an editorial accompanying Dr. Voss’s report (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.69.3572).
The signal of activity Voss et al. found in the subgroup of non–clear cell RCCs with papillary features is compelling, but studies with larger sample sizes are necessary. We need multicenter randomized trials that specifically focus on particular histologic subtypes and provide detailed molecular characterization.
Fortunately, another recent phase II study also found that pairing an mTOR inhibitor with a VEGF inhibitor (everolimus plus lenvatinib) improved the response rate, progression-free survival, and overall survival in clear cell RCC. The ensuing regulatory approval of this combination paves the way for further studies of similar regimens for tumors with papillary features.
Sumanta K. Pal, MD, is in the department of medical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. Financial disclosures for Dr. Pal and his associates are available at www.jco.org. Dr. Pal and his associates made these remarks in an editorial accompanying Dr. Voss’s report (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.69.3572).
The combination of an mTOR complex 1 inhibitor (everolimus) plus a VEGF inhibitor (bevacizumab) showed promise against advanced non–clear cell renal cell carcinoma characterized by papillary features in a small manufacturer-sponsored phase II trial, according to a report published online Sept. 6 in the Journal of Clinical Oncology.
Non–clear cell renal cell carcinomas (ncRCCs) are a diverse mixture of heterogeneous malignancies and include papillary, chromophobe, medullary, collecting duct, and a variety of unclassified tumor types. Researchers performed a single-center trial to assess the effectiveness of combined everolimus plus bevacizumab in 35 treatment-naive patients who presented with advanced disease representing all of these histologic types. The unclassified subgroup (23 patients) included several tumors with prominent papillary architectural features that did not fulfill other criteria for papillary RCC, said Martin H. Voss, MD, of Memorial Sloan Kettering Cancer Center, New York, and his associates.
A total of 18 patients (53%) were alive and free of disease progression at 6 months, and 10 (29%) were alive and progression free at 12 months. Two patients still were receiving study treatment at the time of publication, after 20.2 and 30.4 months of therapy, respectively.
“Objective responses were observed in a sizable proportion of subjects with significant papillary (7 of 18) or chromophobe (2 of 5) tumor components but rarely in patients with unclassified RCC without papillary features (1 of 9) or those with medullary RCC (0 of 2),” Dr. Voss and his associates reported. Among patients with unclassified RCC, the 14 whose cancer had a major papillary component showed an objective response rate of 43%, a median progression-free survival of 12.9 months, and a median overall survival of 28.2 months. In contrast, the nine patients whose cancer did not have a major papillary component showed an objective response rate of 11%, a median progression-free survival of 1.9 months, and a median overall survival of 9.3 months, the investigators said (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.67.9084).
Treatment was generally well tolerated, even though there were frequent low-grade toxicities. High-grade toxicities known to be associated with mTOR complex 1 inhibitors or VEGF inhibitors included hyperglycemia (11%), hypertriglyceridemia (14%), lymphopenia (20%), hypertension (29%), and proteinuria (18%). There were two patient deaths from gastrointestinal hemorrhage, one of which was considered possibly related to bevacizumab.
Archived tissue samples were available for genetic analysis for some patients. Acquired mutations in the ARID1A gene were noted in 5 of 14 tumors with major papillary components, and all 5 of those patients achieved more than 6 months of progression-free survival with the combination therapy. In contrast, no ARID1A mutations were detected in any of the patients who had shorter progression-free survival, and none were detected in any of the tumors that did not have papillary components. This suggests that ARID1A “merits further study for its functional role in papillary RCC variants and as a candidate biomarker for future study of everolimus plus bevacizumab,” Dr. Voss and his associates said.
Novartis supported the study. Dr. Voss reported ties to Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Bristol-Myers Squibb, Genentech, and Takeda; his associates reported ties to numerous industry sources.
The combination of an mTOR complex 1 inhibitor (everolimus) plus a VEGF inhibitor (bevacizumab) showed promise against advanced non–clear cell renal cell carcinoma characterized by papillary features in a small manufacturer-sponsored phase II trial, according to a report published online Sept. 6 in the Journal of Clinical Oncology.
Non–clear cell renal cell carcinomas (ncRCCs) are a diverse mixture of heterogeneous malignancies and include papillary, chromophobe, medullary, collecting duct, and a variety of unclassified tumor types. Researchers performed a single-center trial to assess the effectiveness of combined everolimus plus bevacizumab in 35 treatment-naive patients who presented with advanced disease representing all of these histologic types. The unclassified subgroup (23 patients) included several tumors with prominent papillary architectural features that did not fulfill other criteria for papillary RCC, said Martin H. Voss, MD, of Memorial Sloan Kettering Cancer Center, New York, and his associates.
A total of 18 patients (53%) were alive and free of disease progression at 6 months, and 10 (29%) were alive and progression free at 12 months. Two patients still were receiving study treatment at the time of publication, after 20.2 and 30.4 months of therapy, respectively.
“Objective responses were observed in a sizable proportion of subjects with significant papillary (7 of 18) or chromophobe (2 of 5) tumor components but rarely in patients with unclassified RCC without papillary features (1 of 9) or those with medullary RCC (0 of 2),” Dr. Voss and his associates reported. Among patients with unclassified RCC, the 14 whose cancer had a major papillary component showed an objective response rate of 43%, a median progression-free survival of 12.9 months, and a median overall survival of 28.2 months. In contrast, the nine patients whose cancer did not have a major papillary component showed an objective response rate of 11%, a median progression-free survival of 1.9 months, and a median overall survival of 9.3 months, the investigators said (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.67.9084).
Treatment was generally well tolerated, even though there were frequent low-grade toxicities. High-grade toxicities known to be associated with mTOR complex 1 inhibitors or VEGF inhibitors included hyperglycemia (11%), hypertriglyceridemia (14%), lymphopenia (20%), hypertension (29%), and proteinuria (18%). There were two patient deaths from gastrointestinal hemorrhage, one of which was considered possibly related to bevacizumab.
Archived tissue samples were available for genetic analysis for some patients. Acquired mutations in the ARID1A gene were noted in 5 of 14 tumors with major papillary components, and all 5 of those patients achieved more than 6 months of progression-free survival with the combination therapy. In contrast, no ARID1A mutations were detected in any of the patients who had shorter progression-free survival, and none were detected in any of the tumors that did not have papillary components. This suggests that ARID1A “merits further study for its functional role in papillary RCC variants and as a candidate biomarker for future study of everolimus plus bevacizumab,” Dr. Voss and his associates said.
Novartis supported the study. Dr. Voss reported ties to Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Bristol-Myers Squibb, Genentech, and Takeda; his associates reported ties to numerous industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The combination of everolimus plus bevacizumab showed promise against advanced non–clear cell renal cell carcinoma characterized by papillary features.
Major finding: Among patients with unclassified RCC, the 14 whose cancer had a major papillary component showed an objective response rate of 43%, a median progression-free survival of 12.9 months, and a median overall survival of 28.2 months.
Data source: A small prospective single-center phase II trial involving 35 adults with treatment-naive advanced non–clear cell RCC.
Disclosures: Novartis supported the study. Dr. Voss reported ties to Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Bristol-Myers Squibb, Genentech, and Takeda; his associates reported ties to numerous industry sources.
Study supports extending docetaxel therapy in metastatic castration-resistant prostate cancer
Extending docetaxel chemotherapy significantly improved overall survival in patients with metastatic castration-resistant prostate cancer (mCRPC), regardless of whether they received lenalidomide, according to a retrospective analysis of 1,059 patients from a randomized, phase III trial.
“We found a robust and independent effect on overall survival by the number of docetaxel cycles administered in the setting of mCRPC,” wrote Ellen de Morree of Erasmus MC Cancer Institute (Rotterdam, the Netherlands) and her associates (JAMA Oncol. 2016 Aug 25. doi: 10.1001/jamaoncol.2016.3000).
The association was independent of performance status (Eastern Cooperative Oncology Group score) or baseline levels of lactate dehydrogenase level, hemoglobin, and albumin, they noted. “These data indicate that patients who appear to have clinical, radiological, or biochemical benefit by docetaxel should continue beyond 6 cycles as long as they tolerate their treatment well,” they concluded.
This study, the first to investigate the optimal number of docetaxel cycles in mCRPC, analyzed data from the multicenter Mainsail trial, in which patients received docetaxel, prednisone, and lenalidomide (DPL) or docetaxel, prednisone, and a placebo (DP) until they developed progressive disease or unacceptable adverse effects. Although dose intensity was similar between the two trial arms, DPL patients developed myelotoxicity with lenalidomide and therefore received a median of only six treatment cycles, while DP patients received a median of eight cycles. That difference enabled this analysis, the investigators noted.
Cumulative dose of docetaxel, duration of lenalidomide treatment, and allocated treatment regimen were significant predictors of overall survival in the univariate analysis. Overall survival was associated with treatment arm in a multivariable analysis that did not account for number of docetaxel cycles (hazard ratio, 1.6; 95% confidence interval, 1.2 to 2.1; P less than .001). But that changed after the addition of a number of docetaxel cycles to the model, the researchers said. In this final model, treatment with eight or more cycles of docetaxel led to substantially improved overall survival (hazard ratio, 1.9; P less than .001), regardless of lenalidomide treatment (HR, 1.1; 95% CI, 0.9 to 1.2; P = .4). Sensitivity analyses confirmed the association – patients who received more than 10 cycles of docetaxel had a median overall survival of 33 months (30-37 months), versus 27 months (24-30 months) with 8-10 cycles and about 23 months (18-27 months) with 5-7 cycles (P less than .001).
Extending docetaxel chemotherapy significantly improved overall survival in patients with metastatic castration-resistant prostate cancer (mCRPC), regardless of whether they received lenalidomide, according to a retrospective analysis of 1,059 patients from a randomized, phase III trial.
“We found a robust and independent effect on overall survival by the number of docetaxel cycles administered in the setting of mCRPC,” wrote Ellen de Morree of Erasmus MC Cancer Institute (Rotterdam, the Netherlands) and her associates (JAMA Oncol. 2016 Aug 25. doi: 10.1001/jamaoncol.2016.3000).
The association was independent of performance status (Eastern Cooperative Oncology Group score) or baseline levels of lactate dehydrogenase level, hemoglobin, and albumin, they noted. “These data indicate that patients who appear to have clinical, radiological, or biochemical benefit by docetaxel should continue beyond 6 cycles as long as they tolerate their treatment well,” they concluded.
This study, the first to investigate the optimal number of docetaxel cycles in mCRPC, analyzed data from the multicenter Mainsail trial, in which patients received docetaxel, prednisone, and lenalidomide (DPL) or docetaxel, prednisone, and a placebo (DP) until they developed progressive disease or unacceptable adverse effects. Although dose intensity was similar between the two trial arms, DPL patients developed myelotoxicity with lenalidomide and therefore received a median of only six treatment cycles, while DP patients received a median of eight cycles. That difference enabled this analysis, the investigators noted.
Cumulative dose of docetaxel, duration of lenalidomide treatment, and allocated treatment regimen were significant predictors of overall survival in the univariate analysis. Overall survival was associated with treatment arm in a multivariable analysis that did not account for number of docetaxel cycles (hazard ratio, 1.6; 95% confidence interval, 1.2 to 2.1; P less than .001). But that changed after the addition of a number of docetaxel cycles to the model, the researchers said. In this final model, treatment with eight or more cycles of docetaxel led to substantially improved overall survival (hazard ratio, 1.9; P less than .001), regardless of lenalidomide treatment (HR, 1.1; 95% CI, 0.9 to 1.2; P = .4). Sensitivity analyses confirmed the association – patients who received more than 10 cycles of docetaxel had a median overall survival of 33 months (30-37 months), versus 27 months (24-30 months) with 8-10 cycles and about 23 months (18-27 months) with 5-7 cycles (P less than .001).
Extending docetaxel chemotherapy significantly improved overall survival in patients with metastatic castration-resistant prostate cancer (mCRPC), regardless of whether they received lenalidomide, according to a retrospective analysis of 1,059 patients from a randomized, phase III trial.
“We found a robust and independent effect on overall survival by the number of docetaxel cycles administered in the setting of mCRPC,” wrote Ellen de Morree of Erasmus MC Cancer Institute (Rotterdam, the Netherlands) and her associates (JAMA Oncol. 2016 Aug 25. doi: 10.1001/jamaoncol.2016.3000).
The association was independent of performance status (Eastern Cooperative Oncology Group score) or baseline levels of lactate dehydrogenase level, hemoglobin, and albumin, they noted. “These data indicate that patients who appear to have clinical, radiological, or biochemical benefit by docetaxel should continue beyond 6 cycles as long as they tolerate their treatment well,” they concluded.
This study, the first to investigate the optimal number of docetaxel cycles in mCRPC, analyzed data from the multicenter Mainsail trial, in which patients received docetaxel, prednisone, and lenalidomide (DPL) or docetaxel, prednisone, and a placebo (DP) until they developed progressive disease or unacceptable adverse effects. Although dose intensity was similar between the two trial arms, DPL patients developed myelotoxicity with lenalidomide and therefore received a median of only six treatment cycles, while DP patients received a median of eight cycles. That difference enabled this analysis, the investigators noted.
Cumulative dose of docetaxel, duration of lenalidomide treatment, and allocated treatment regimen were significant predictors of overall survival in the univariate analysis. Overall survival was associated with treatment arm in a multivariable analysis that did not account for number of docetaxel cycles (hazard ratio, 1.6; 95% confidence interval, 1.2 to 2.1; P less than .001). But that changed after the addition of a number of docetaxel cycles to the model, the researchers said. In this final model, treatment with eight or more cycles of docetaxel led to substantially improved overall survival (hazard ratio, 1.9; P less than .001), regardless of lenalidomide treatment (HR, 1.1; 95% CI, 0.9 to 1.2; P = .4). Sensitivity analyses confirmed the association – patients who received more than 10 cycles of docetaxel had a median overall survival of 33 months (30-37 months), versus 27 months (24-30 months) with 8-10 cycles and about 23 months (18-27 months) with 5-7 cycles (P less than .001).
FROM JAMA ONCOLOGY
Key clinical point: The number of docetaxel cycles independently predicted overall survival in metastatic castration-resistant prostate cancer (mCRPC).
Major finding: Treatment with eight or more cycles of docetaxel led to improved overall survival (hazard ratio, 1.9; P less than .001) regardless of lenalidomide treatment.
Data source: A retrospective study of 1,059 patients from the Mainsail trial, a randomized phase III study that compared docetaxel, prednisone, and lenalidomide with docetaxel, prednisone, and placebo.
Disclosures: Celgene funded the study but had no role in its design, conduct, interpretation, or in manuscript preparation or review. Dr. Morree had no disclosures.
Prostate cancer incidence continues to decrease after recommendation against screening
Incidence rates for localized- and regional-stage prostate cancer continued to decline 2 years following the recommendation by the U.S. Preventive Services Task Force against prostate-specific antigen (PSA) testing in all men.
“Convincing evidence demonstrates that the PSA test often produces false-positive results [and] false-positive PSA test results are associated with negative psychological effects, including persistent worry about prostate cancer,” the task force stated in a recommendation published in October 2011 and finalized in May 2012.
From 2011 to 2012, immediately following the recommendation, there was a significant decline in early-stage cancer incidence rates among men 50 years or older, according to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) program.
For the current study, Ahmedin Jemal, DVM, PhD, and his associates at the American Cancer Society analyzed incidence data for invasive prostate cancer from 18 SEER registries, which, combined, represented about 28% of the U.S. population.
Investigators reported a continued decline in localized- and regional-stage prostate cancer incidence from 2012 to 2013. Specifically, the incidence rates per 100,000 men decreased from 356.5 to 335.4 in men aged 50-74 years and from 379.2 to 353.6 in men 75 years and older (JAMA Oncol. 2016 Aug 16. doi: 10.1001/jamaoncol.2016.2667).
Incidence rates for distant-stage disease were unchanged during the same time period for men of all ages.
Similar results were reported for non-Hispanic whites and non-Hispanic blacks.
“Whether this pattern will lead to a future increase in the diagnosis of distant-stage disease and prostate cancer mortality requires long-term monitoring because of the slow growing nature of this malignant neoplasm,” the investigators noted.
The American Cancer Society funded the study. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
Incidence rates for localized- and regional-stage prostate cancer continued to decline 2 years following the recommendation by the U.S. Preventive Services Task Force against prostate-specific antigen (PSA) testing in all men.
“Convincing evidence demonstrates that the PSA test often produces false-positive results [and] false-positive PSA test results are associated with negative psychological effects, including persistent worry about prostate cancer,” the task force stated in a recommendation published in October 2011 and finalized in May 2012.
From 2011 to 2012, immediately following the recommendation, there was a significant decline in early-stage cancer incidence rates among men 50 years or older, according to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) program.
For the current study, Ahmedin Jemal, DVM, PhD, and his associates at the American Cancer Society analyzed incidence data for invasive prostate cancer from 18 SEER registries, which, combined, represented about 28% of the U.S. population.
Investigators reported a continued decline in localized- and regional-stage prostate cancer incidence from 2012 to 2013. Specifically, the incidence rates per 100,000 men decreased from 356.5 to 335.4 in men aged 50-74 years and from 379.2 to 353.6 in men 75 years and older (JAMA Oncol. 2016 Aug 16. doi: 10.1001/jamaoncol.2016.2667).
Incidence rates for distant-stage disease were unchanged during the same time period for men of all ages.
Similar results were reported for non-Hispanic whites and non-Hispanic blacks.
“Whether this pattern will lead to a future increase in the diagnosis of distant-stage disease and prostate cancer mortality requires long-term monitoring because of the slow growing nature of this malignant neoplasm,” the investigators noted.
The American Cancer Society funded the study. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
Incidence rates for localized- and regional-stage prostate cancer continued to decline 2 years following the recommendation by the U.S. Preventive Services Task Force against prostate-specific antigen (PSA) testing in all men.
“Convincing evidence demonstrates that the PSA test often produces false-positive results [and] false-positive PSA test results are associated with negative psychological effects, including persistent worry about prostate cancer,” the task force stated in a recommendation published in October 2011 and finalized in May 2012.
From 2011 to 2012, immediately following the recommendation, there was a significant decline in early-stage cancer incidence rates among men 50 years or older, according to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) program.
For the current study, Ahmedin Jemal, DVM, PhD, and his associates at the American Cancer Society analyzed incidence data for invasive prostate cancer from 18 SEER registries, which, combined, represented about 28% of the U.S. population.
Investigators reported a continued decline in localized- and regional-stage prostate cancer incidence from 2012 to 2013. Specifically, the incidence rates per 100,000 men decreased from 356.5 to 335.4 in men aged 50-74 years and from 379.2 to 353.6 in men 75 years and older (JAMA Oncol. 2016 Aug 16. doi: 10.1001/jamaoncol.2016.2667).
Incidence rates for distant-stage disease were unchanged during the same time period for men of all ages.
Similar results were reported for non-Hispanic whites and non-Hispanic blacks.
“Whether this pattern will lead to a future increase in the diagnosis of distant-stage disease and prostate cancer mortality requires long-term monitoring because of the slow growing nature of this malignant neoplasm,” the investigators noted.
The American Cancer Society funded the study. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
FROM JAMA ONCOLOGY
Key clinical point: Incidence rates for localized- and regional-stage prostate cancer continue to decline.
Major finding: The incidence rates for localized- and regional-stage prostate cancer per 100,000 men decreased from 356.5 to 335.4 in men aged 50-74 years and from 379.2 to 353.6 in men 75 years and older.
Data source: Meta-analysis from 18 SEER registries.
Disclosures: The American Cancer Society funded the study. The authors had no relevant disclosures to report.
Active surveillance may be feasible for some advanced RCC patients
For metastatic renal cell carcinoma patients with fewer adverse risk factors and fewer metastatic disease sites, initial active surveillance may be a safe and feasible approach to delay the toxicities of systemic therapy, investigators report.
Fifty-two patients with treatment-naive, asymptomatic, metastatic renal-cell carcinoma were enrolled in a prospective phase II trial and radiographically assessed at baseline, every 3 months for year 1, every 4 months for year 2, then every 6 months thereafter. Patients continued on observation until the treating physician and patient made the decision to initiate systemic therapy.
Median follow-up time was 38.1 months and median time on surveillance before treatment initiation – the primary endpoint of the study – was 14.9 months (95% confidence interval, 10.6-25.0), reported Brian Rini, MD, of the Cleveland Clinic Taussig Cancer Institute and his associates (Lancet Oncol. 2016. doi: 10.1016/S1470-2045(16)30196-6).
Forty-three (90%) of the 48 evaluable patients experienced disease progression during the study, median time to progression was 9.4 months, and 22 patients died from renal cell carcinoma. One patient developed brain metastases and died without receiving systemic therapy. In multivariable analysis, only the number of involved organs (P = .0414) and number of International Metastatic Database Consortium risk factors (P = .0403) were independently prognostic.
Using this analysis, Dr. Rini and associates identified two prognostic groups – a favorable group consisting of patients with no or one International Metastatic Database Consortium (IMDC) risk factors and two or fewer organs with metastatic disease, and an unfavorable group consisting of all other patients. The favorable group (n = 22) patients had an estimated median surveillance time of 22.2 months (95% CI, 13.8-33.3), whereas the unfavorable group (n = 19) had an estimated median surveillance time of 8.4 months (3.2-14.1; P = .0056).
Anxiety, depression, and quality of life did not change significantly over the period of surveillance, suggesting that living with untreated cancer did not cause psychological harm to patients in this study.
“Findings from our prospective trial show active surveillance to be a viable initial strategy in some patients with metastatic renal-cell carcinoma. The median surveillance period before start of systematic therapy was greater than 1 year, with no observed adverse effects on quality of life, anxiety and depression,” Dr. Rini and his associates said.
“Appropriate selection of patients and adequate monitoring, which should include CNS surveillance, is crucial in application of this approach,” they added.
This study was unfunded. One investigator reported receiving financial compensation from Pfizer and GlaxoSmithKline. All other investigators reported having no relevant disclosures.
On Twitter @jessnicolecraig
For metastatic renal cell carcinoma patients with fewer adverse risk factors and fewer metastatic disease sites, initial active surveillance may be a safe and feasible approach to delay the toxicities of systemic therapy, investigators report.
Fifty-two patients with treatment-naive, asymptomatic, metastatic renal-cell carcinoma were enrolled in a prospective phase II trial and radiographically assessed at baseline, every 3 months for year 1, every 4 months for year 2, then every 6 months thereafter. Patients continued on observation until the treating physician and patient made the decision to initiate systemic therapy.
Median follow-up time was 38.1 months and median time on surveillance before treatment initiation – the primary endpoint of the study – was 14.9 months (95% confidence interval, 10.6-25.0), reported Brian Rini, MD, of the Cleveland Clinic Taussig Cancer Institute and his associates (Lancet Oncol. 2016. doi: 10.1016/S1470-2045(16)30196-6).
Forty-three (90%) of the 48 evaluable patients experienced disease progression during the study, median time to progression was 9.4 months, and 22 patients died from renal cell carcinoma. One patient developed brain metastases and died without receiving systemic therapy. In multivariable analysis, only the number of involved organs (P = .0414) and number of International Metastatic Database Consortium risk factors (P = .0403) were independently prognostic.
Using this analysis, Dr. Rini and associates identified two prognostic groups – a favorable group consisting of patients with no or one International Metastatic Database Consortium (IMDC) risk factors and two or fewer organs with metastatic disease, and an unfavorable group consisting of all other patients. The favorable group (n = 22) patients had an estimated median surveillance time of 22.2 months (95% CI, 13.8-33.3), whereas the unfavorable group (n = 19) had an estimated median surveillance time of 8.4 months (3.2-14.1; P = .0056).
Anxiety, depression, and quality of life did not change significantly over the period of surveillance, suggesting that living with untreated cancer did not cause psychological harm to patients in this study.
“Findings from our prospective trial show active surveillance to be a viable initial strategy in some patients with metastatic renal-cell carcinoma. The median surveillance period before start of systematic therapy was greater than 1 year, with no observed adverse effects on quality of life, anxiety and depression,” Dr. Rini and his associates said.
“Appropriate selection of patients and adequate monitoring, which should include CNS surveillance, is crucial in application of this approach,” they added.
This study was unfunded. One investigator reported receiving financial compensation from Pfizer and GlaxoSmithKline. All other investigators reported having no relevant disclosures.
On Twitter @jessnicolecraig
For metastatic renal cell carcinoma patients with fewer adverse risk factors and fewer metastatic disease sites, initial active surveillance may be a safe and feasible approach to delay the toxicities of systemic therapy, investigators report.
Fifty-two patients with treatment-naive, asymptomatic, metastatic renal-cell carcinoma were enrolled in a prospective phase II trial and radiographically assessed at baseline, every 3 months for year 1, every 4 months for year 2, then every 6 months thereafter. Patients continued on observation until the treating physician and patient made the decision to initiate systemic therapy.
Median follow-up time was 38.1 months and median time on surveillance before treatment initiation – the primary endpoint of the study – was 14.9 months (95% confidence interval, 10.6-25.0), reported Brian Rini, MD, of the Cleveland Clinic Taussig Cancer Institute and his associates (Lancet Oncol. 2016. doi: 10.1016/S1470-2045(16)30196-6).
Forty-three (90%) of the 48 evaluable patients experienced disease progression during the study, median time to progression was 9.4 months, and 22 patients died from renal cell carcinoma. One patient developed brain metastases and died without receiving systemic therapy. In multivariable analysis, only the number of involved organs (P = .0414) and number of International Metastatic Database Consortium risk factors (P = .0403) were independently prognostic.
Using this analysis, Dr. Rini and associates identified two prognostic groups – a favorable group consisting of patients with no or one International Metastatic Database Consortium (IMDC) risk factors and two or fewer organs with metastatic disease, and an unfavorable group consisting of all other patients. The favorable group (n = 22) patients had an estimated median surveillance time of 22.2 months (95% CI, 13.8-33.3), whereas the unfavorable group (n = 19) had an estimated median surveillance time of 8.4 months (3.2-14.1; P = .0056).
Anxiety, depression, and quality of life did not change significantly over the period of surveillance, suggesting that living with untreated cancer did not cause psychological harm to patients in this study.
“Findings from our prospective trial show active surveillance to be a viable initial strategy in some patients with metastatic renal-cell carcinoma. The median surveillance period before start of systematic therapy was greater than 1 year, with no observed adverse effects on quality of life, anxiety and depression,” Dr. Rini and his associates said.
“Appropriate selection of patients and adequate monitoring, which should include CNS surveillance, is crucial in application of this approach,” they added.
This study was unfunded. One investigator reported receiving financial compensation from Pfizer and GlaxoSmithKline. All other investigators reported having no relevant disclosures.
On Twitter @jessnicolecraig
FROM LANCET ONCOLOGY
Key clinical point: For a subset of patients with metastatic renal cell carcinoma, initial active surveillance of metastasis may be a safe and feasible option.
Major finding: The favorable group (n = 22) patients and had an estimated median surveillance time of 22.2 months (95% CI, 13.8-33.3), whereas the unfavorable group (n = 19) had an estimated median surveillance time of 8.4 months (3.2-14.1; P = .0056)
Data source: A prospective phase II trial involving 52 patients with treatment-naive, metastatic renal cell carcinoma.
Disclosures: This study was unfunded. One investigator reported receiving financial compensation from Pfizer and GlaxoSmithKline. All other investigators reported having no relevant disclosures.
Salvage RT may reduce risk of prostate cancer metastasis even at low PSA levels
Increasing prostate-specific antigen (PSA) levels prior to salvage radiotherapy were significantly associated with an increased risk of distant metastasis and cause-specific mortality but not overall survival among men with detectable PSA following radical prostatectomy, according to a report published in the Journal of Clinical Oncology.
Investigators retrospectively studied 1,106 consecutive patients with surgically staged prostate cancer who received salvage radiotherapy (SRT) and who had a documented PSA of 0.1 ng/mL or greater. A total of 208 patients developed distant metastasis during median follow-up of 8.9 years.
In multivariate analysis, each doubling of pre-SRT PSA was associated with a 32% increased risk of distant metastasis (P less than .001), reported Bradley J. Stish, MD, and his associates at the Mayo Clinic (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3425).
Each pre-SRT PSA doubling also significantly increased the relative risk of cause-specific mortality (HR, 1.40; P less than .001) but not overall survival (HR, 1.12; P = .02).
More advanced tumor stages, higher Gleason scores, and higher PSA levels prior to salvage radiotherapy were significantly associated with an increased risk of biochemical recurrence. Salvage radiotherapy dose greater than 68 Gy and use of androgen suppression were associated with reduced risk of biochemical recurrence. Overall survival was 92.9% (95% confidence interval, 91.3%-94.5%) at 5 years and 77.3% (95% CI, 74.2%-80.5%) at 10 years. The cause-specific mortality rates were 3.0% (95% CI, 1.9%-4.0%) at 5 years and 10.4% (95% CI, 8.0%-12.6%) at 10 years.
“When taken together, these findings provide strong evidence supporting the clinical benefits attributable to early [salvage radiotherapy] in men with detectable PSA after [radical prostatectomy],” investigators wrote.
Beginning salvage radiotherapy treatment at the lowest PSA level is “most beneficial for long-term therapeutic efficacy,” they added.
On Twitter @jessnicolecraig
Increasing prostate-specific antigen (PSA) levels prior to salvage radiotherapy were significantly associated with an increased risk of distant metastasis and cause-specific mortality but not overall survival among men with detectable PSA following radical prostatectomy, according to a report published in the Journal of Clinical Oncology.
Investigators retrospectively studied 1,106 consecutive patients with surgically staged prostate cancer who received salvage radiotherapy (SRT) and who had a documented PSA of 0.1 ng/mL or greater. A total of 208 patients developed distant metastasis during median follow-up of 8.9 years.
In multivariate analysis, each doubling of pre-SRT PSA was associated with a 32% increased risk of distant metastasis (P less than .001), reported Bradley J. Stish, MD, and his associates at the Mayo Clinic (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3425).
Each pre-SRT PSA doubling also significantly increased the relative risk of cause-specific mortality (HR, 1.40; P less than .001) but not overall survival (HR, 1.12; P = .02).
More advanced tumor stages, higher Gleason scores, and higher PSA levels prior to salvage radiotherapy were significantly associated with an increased risk of biochemical recurrence. Salvage radiotherapy dose greater than 68 Gy and use of androgen suppression were associated with reduced risk of biochemical recurrence. Overall survival was 92.9% (95% confidence interval, 91.3%-94.5%) at 5 years and 77.3% (95% CI, 74.2%-80.5%) at 10 years. The cause-specific mortality rates were 3.0% (95% CI, 1.9%-4.0%) at 5 years and 10.4% (95% CI, 8.0%-12.6%) at 10 years.
“When taken together, these findings provide strong evidence supporting the clinical benefits attributable to early [salvage radiotherapy] in men with detectable PSA after [radical prostatectomy],” investigators wrote.
Beginning salvage radiotherapy treatment at the lowest PSA level is “most beneficial for long-term therapeutic efficacy,” they added.
On Twitter @jessnicolecraig
Increasing prostate-specific antigen (PSA) levels prior to salvage radiotherapy were significantly associated with an increased risk of distant metastasis and cause-specific mortality but not overall survival among men with detectable PSA following radical prostatectomy, according to a report published in the Journal of Clinical Oncology.
Investigators retrospectively studied 1,106 consecutive patients with surgically staged prostate cancer who received salvage radiotherapy (SRT) and who had a documented PSA of 0.1 ng/mL or greater. A total of 208 patients developed distant metastasis during median follow-up of 8.9 years.
In multivariate analysis, each doubling of pre-SRT PSA was associated with a 32% increased risk of distant metastasis (P less than .001), reported Bradley J. Stish, MD, and his associates at the Mayo Clinic (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3425).
Each pre-SRT PSA doubling also significantly increased the relative risk of cause-specific mortality (HR, 1.40; P less than .001) but not overall survival (HR, 1.12; P = .02).
More advanced tumor stages, higher Gleason scores, and higher PSA levels prior to salvage radiotherapy were significantly associated with an increased risk of biochemical recurrence. Salvage radiotherapy dose greater than 68 Gy and use of androgen suppression were associated with reduced risk of biochemical recurrence. Overall survival was 92.9% (95% confidence interval, 91.3%-94.5%) at 5 years and 77.3% (95% CI, 74.2%-80.5%) at 10 years. The cause-specific mortality rates were 3.0% (95% CI, 1.9%-4.0%) at 5 years and 10.4% (95% CI, 8.0%-12.6%) at 10 years.
“When taken together, these findings provide strong evidence supporting the clinical benefits attributable to early [salvage radiotherapy] in men with detectable PSA after [radical prostatectomy],” investigators wrote.
Beginning salvage radiotherapy treatment at the lowest PSA level is “most beneficial for long-term therapeutic efficacy,” they added.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Salvage radiotherapy should not be delayed by prolonged monitoring of detectable post radical prostatectomy PSA levels.
Major finding: A multivariate analysis revealed that prior to salvage radiotherapy, each doubling of prostate specific antigen was significantly associated with an increased risk of distant metastasis (hazard ratio, 1.32; 95% CI, 1.19-1.46; P less than .001) and cause-specific mortality (HR, 1.40; 95% CI, 1.21-1.63, P less than .001).
Data source: A retrospective study of 1,106 men with detectable prostate-specific antigen levels following radical prostatectomy for prostate cancer.
Disclosures: The Mayo Clinic funded this study. Six of the investigators had no disclosures to report; one investigator reported holding stock or ownership interest in Pfizer.
Gene profile predicts RCC response to nivolumab
Many patients with advanced renal cell carcinoma have tumors that do not respond to immune checkpoint inhibitors targeted against the programmed death-1 (PD-1) pathway, despite expression of the target PD ligand 1 (PD-L1) on their tumors. Now investigators think they know why, and hope to use the information to predict which patients are likely to benefit and identify potential new therapies or combinations.
A study of renal cell carcinoma (RCC) samples from tumors with both good and poor clinical responses to treatment with the anti–PD-1 agent nivolumab (Opdivo) showed that a tumor gene–expression profile tipped more toward genes for controlling metabolic functions rather than immune functions was associated with a lack of response to anti-PD-1 therapy, reported Suzanne L. Topalian, MD, and her colleagues from Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, both in Baltimore.
“These findings suggest that tumor cell–intrinsic metabolic factors may contribute to treatment resistance in RCC, thus serving as predictive markers for treatment outcomes and potential new targets for combination therapy regimens with anti–PD-1,” they wrote in a study published online in Cancer Immunology Research.
The investigators obtained tumor samples from 13 patients with unresectable metastatic RCC treated in one of four clinical trials. They used radiographic staging to classify each patient as either a responder or nonresponder to anti–PD-1 therapy according to RECIST (Response Evaluation Criteria in Solid Tumors). The samples were evaluated with whole genome microarray and multiplex quantitative reverse-transcription polymerase chain reaction (qRT-PCR) profiling and analysis, and the results were compared with those from eight renal cell carcinoma cell lines.
They looked for expression of nearly 30,000 gene targets in samples from responders and nonresponders and found a pattern of differential expression of genes encoding for metabolic pathways and immune functions.
Specifically, they found that the expression of genes involved in metabolic and solute transport functions (for example, UGT1A) were associated with poor response to nivolumab, whereas overexpression of genes for immune markers involved in T-cell differentiation (BACH2) and leukocyte migration (CCL3) were associated with a good response.
The investigators acknowledge that the study was retrospective and limited by the analysis of only a small number of tumor samples but suggest that their findings point the way to further investigations in larger groups of patients with RCC tumors, including those both positive and negative for PD-L1 expression.
“The general approach to identifying biomarkers of clinical response to PD-1–targeted therapies has so far focused on immunologic factors in the [tumor microenvironment]. However, a deeper level of investigation may be warranted for individual tumor types, and intersections of tumor cell–intrinsic factors with immunologic factors may be particularly revealing,” they wrote.
The study was supported by research grants from the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Bristol-Myers Squibb, the National Cancer Institute, and Stand Up To Cancer. Dr. Topalian has served as a consultant/advisory board member for Five Prime Therapeutics, MedImmune, Merck, and Pfizer, and has an ownership interest in Bristol-Myers Squibb, Five Prime Therapeutics,and Potenza Therapeutics. Other coauthors reported similar potential conflicts of interest.
Many patients with advanced renal cell carcinoma have tumors that do not respond to immune checkpoint inhibitors targeted against the programmed death-1 (PD-1) pathway, despite expression of the target PD ligand 1 (PD-L1) on their tumors. Now investigators think they know why, and hope to use the information to predict which patients are likely to benefit and identify potential new therapies or combinations.
A study of renal cell carcinoma (RCC) samples from tumors with both good and poor clinical responses to treatment with the anti–PD-1 agent nivolumab (Opdivo) showed that a tumor gene–expression profile tipped more toward genes for controlling metabolic functions rather than immune functions was associated with a lack of response to anti-PD-1 therapy, reported Suzanne L. Topalian, MD, and her colleagues from Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, both in Baltimore.
“These findings suggest that tumor cell–intrinsic metabolic factors may contribute to treatment resistance in RCC, thus serving as predictive markers for treatment outcomes and potential new targets for combination therapy regimens with anti–PD-1,” they wrote in a study published online in Cancer Immunology Research.
The investigators obtained tumor samples from 13 patients with unresectable metastatic RCC treated in one of four clinical trials. They used radiographic staging to classify each patient as either a responder or nonresponder to anti–PD-1 therapy according to RECIST (Response Evaluation Criteria in Solid Tumors). The samples were evaluated with whole genome microarray and multiplex quantitative reverse-transcription polymerase chain reaction (qRT-PCR) profiling and analysis, and the results were compared with those from eight renal cell carcinoma cell lines.
They looked for expression of nearly 30,000 gene targets in samples from responders and nonresponders and found a pattern of differential expression of genes encoding for metabolic pathways and immune functions.
Specifically, they found that the expression of genes involved in metabolic and solute transport functions (for example, UGT1A) were associated with poor response to nivolumab, whereas overexpression of genes for immune markers involved in T-cell differentiation (BACH2) and leukocyte migration (CCL3) were associated with a good response.
The investigators acknowledge that the study was retrospective and limited by the analysis of only a small number of tumor samples but suggest that their findings point the way to further investigations in larger groups of patients with RCC tumors, including those both positive and negative for PD-L1 expression.
“The general approach to identifying biomarkers of clinical response to PD-1–targeted therapies has so far focused on immunologic factors in the [tumor microenvironment]. However, a deeper level of investigation may be warranted for individual tumor types, and intersections of tumor cell–intrinsic factors with immunologic factors may be particularly revealing,” they wrote.
The study was supported by research grants from the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Bristol-Myers Squibb, the National Cancer Institute, and Stand Up To Cancer. Dr. Topalian has served as a consultant/advisory board member for Five Prime Therapeutics, MedImmune, Merck, and Pfizer, and has an ownership interest in Bristol-Myers Squibb, Five Prime Therapeutics,and Potenza Therapeutics. Other coauthors reported similar potential conflicts of interest.
Many patients with advanced renal cell carcinoma have tumors that do not respond to immune checkpoint inhibitors targeted against the programmed death-1 (PD-1) pathway, despite expression of the target PD ligand 1 (PD-L1) on their tumors. Now investigators think they know why, and hope to use the information to predict which patients are likely to benefit and identify potential new therapies or combinations.
A study of renal cell carcinoma (RCC) samples from tumors with both good and poor clinical responses to treatment with the anti–PD-1 agent nivolumab (Opdivo) showed that a tumor gene–expression profile tipped more toward genes for controlling metabolic functions rather than immune functions was associated with a lack of response to anti-PD-1 therapy, reported Suzanne L. Topalian, MD, and her colleagues from Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, both in Baltimore.
“These findings suggest that tumor cell–intrinsic metabolic factors may contribute to treatment resistance in RCC, thus serving as predictive markers for treatment outcomes and potential new targets for combination therapy regimens with anti–PD-1,” they wrote in a study published online in Cancer Immunology Research.
The investigators obtained tumor samples from 13 patients with unresectable metastatic RCC treated in one of four clinical trials. They used radiographic staging to classify each patient as either a responder or nonresponder to anti–PD-1 therapy according to RECIST (Response Evaluation Criteria in Solid Tumors). The samples were evaluated with whole genome microarray and multiplex quantitative reverse-transcription polymerase chain reaction (qRT-PCR) profiling and analysis, and the results were compared with those from eight renal cell carcinoma cell lines.
They looked for expression of nearly 30,000 gene targets in samples from responders and nonresponders and found a pattern of differential expression of genes encoding for metabolic pathways and immune functions.
Specifically, they found that the expression of genes involved in metabolic and solute transport functions (for example, UGT1A) were associated with poor response to nivolumab, whereas overexpression of genes for immune markers involved in T-cell differentiation (BACH2) and leukocyte migration (CCL3) were associated with a good response.
The investigators acknowledge that the study was retrospective and limited by the analysis of only a small number of tumor samples but suggest that their findings point the way to further investigations in larger groups of patients with RCC tumors, including those both positive and negative for PD-L1 expression.
“The general approach to identifying biomarkers of clinical response to PD-1–targeted therapies has so far focused on immunologic factors in the [tumor microenvironment]. However, a deeper level of investigation may be warranted for individual tumor types, and intersections of tumor cell–intrinsic factors with immunologic factors may be particularly revealing,” they wrote.
The study was supported by research grants from the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Bristol-Myers Squibb, the National Cancer Institute, and Stand Up To Cancer. Dr. Topalian has served as a consultant/advisory board member for Five Prime Therapeutics, MedImmune, Merck, and Pfizer, and has an ownership interest in Bristol-Myers Squibb, Five Prime Therapeutics,and Potenza Therapeutics. Other coauthors reported similar potential conflicts of interest.
FROM CANCER IMMUNOLOGY RESEARCH
Key clinical point: Many renal cell carcinoma tumors do not respond to therapy with an anti-PD-1 agent, despite being positive for the PD-L1 target.
Major finding: A gene expression profile favoring genes associated with metabolic and solute transport functions was associated with poor response to nivolumab.
Data source: Retrospective study of tumor gene expression in 13 patients with advanced RCC and 8 RCC cell lines.
Disclosures: The study was supported by research grants from the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Baltimore; Bristol-Myers Squibb; the National Cancer Institute; and Stand Up To Cancer. Dr. Topalian has served as a consultant/advisory board member for Five Prime Therapeutics, MedImmune, Merck, and Pfizer, and has an ownership interest in Bristol-Myers Squibb, Five Prime Therapeutics,and Potenza Therapeutics. Other coauthors reported similar potential conflicts of interest.
First-in-class agent shows early promise in treating clear cell renal cell carcinoma
CHICAGO – Investigational agent PT2385, an inhibitor of hypoxia-inducible factor 2-alpha (HIF-2alpha), appears safe and tolerable and demonstrated early evidence of clinical activity in heavily pretreated patients with advanced clear cell renal cell carcinoma, investigators reported at the annual meeting of the American Society of Clinical Oncology.
In a small, phase I dose-escalation study, 39% of patients in the study achieved complete response, partial response, or stable disease lasting at least 16 weeks.
PT2385 is a first-in-class, orally administered selective small molecule inhibitor of HIF-2alpha, a transcription factor with a role in clear cell renal cell carcinoma.
“Hypoxia-inducible factor 2-alpha is a key oncogenic driver of clear cell renal cell carcinoma,” Kevin Courtney, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, said at the meeting.
A total of 51 patients (n = 26 for dose escalation cohort and n = 25 in dose expansion cohort) met the study’s eligibility requirements of having a diagnosis of clear cell renal cell carcinoma, an Eastern Cooperative Oncology Group score ranging from 0 to 1, and at least one prior anticancer therapy. The median age of the cohort was 65 years with the youngest participant being 29 years and the oldest being 80 years old. The majority of patients were male (71%), had received four or more prior systemic therapies (53%), and had undergone vascular endothelial growth factor therapy (98%). Patients were treated with continuous twice-daily oral dosing of PT2385 until progression or toxicity. A total of three patients were treated at the 100-mg dose, three at the 200-mg dose, four at the 400-mg dose, seven at the 800-mg dose, six at a dose of 1,200 mg, and three at a dose of 1,800 mg.
“No dose-limiting toxicity was observed at any dose level,” Dr. Courtney said.
Based on safety, pharmokinetic and pharmodynamic data, 800 mg twice daily was the selected dose for the expansion cohort, he reported.
Of 51 patients treated, 1 patient had a complete response, 3 achieved partial responses, and 16 patients achieved stable disease for at least 16 weeks. The most common grade one or two adverse events were anemia (n = 18), peripheral edema (n = 18), fatigue (n = 18), nausea (n = 15), and back pain (n = 12). Only two grade four adverse events (low lymphocyte count) were reported.
“Notably, hypertension was not seen,” Dr. Courtney said.
At the time the study concluded, the four patients who achieved complete or partial responses remained on the treatment, and five had received treatment for a year or longer.
The study was funded by Peloton Therapeutics. Dr. Courtney reported having stock and ownership interest in, serving in advisory roles for, or receiving research funding from multiple companies. Several coinvestigators reported receiving research funding from or holding patents with multiple companies including Peloton Therapeutics.
On Twitter @jessnicolecraig
CHICAGO – Investigational agent PT2385, an inhibitor of hypoxia-inducible factor 2-alpha (HIF-2alpha), appears safe and tolerable and demonstrated early evidence of clinical activity in heavily pretreated patients with advanced clear cell renal cell carcinoma, investigators reported at the annual meeting of the American Society of Clinical Oncology.
In a small, phase I dose-escalation study, 39% of patients in the study achieved complete response, partial response, or stable disease lasting at least 16 weeks.
PT2385 is a first-in-class, orally administered selective small molecule inhibitor of HIF-2alpha, a transcription factor with a role in clear cell renal cell carcinoma.
“Hypoxia-inducible factor 2-alpha is a key oncogenic driver of clear cell renal cell carcinoma,” Kevin Courtney, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, said at the meeting.
A total of 51 patients (n = 26 for dose escalation cohort and n = 25 in dose expansion cohort) met the study’s eligibility requirements of having a diagnosis of clear cell renal cell carcinoma, an Eastern Cooperative Oncology Group score ranging from 0 to 1, and at least one prior anticancer therapy. The median age of the cohort was 65 years with the youngest participant being 29 years and the oldest being 80 years old. The majority of patients were male (71%), had received four or more prior systemic therapies (53%), and had undergone vascular endothelial growth factor therapy (98%). Patients were treated with continuous twice-daily oral dosing of PT2385 until progression or toxicity. A total of three patients were treated at the 100-mg dose, three at the 200-mg dose, four at the 400-mg dose, seven at the 800-mg dose, six at a dose of 1,200 mg, and three at a dose of 1,800 mg.
“No dose-limiting toxicity was observed at any dose level,” Dr. Courtney said.
Based on safety, pharmokinetic and pharmodynamic data, 800 mg twice daily was the selected dose for the expansion cohort, he reported.
Of 51 patients treated, 1 patient had a complete response, 3 achieved partial responses, and 16 patients achieved stable disease for at least 16 weeks. The most common grade one or two adverse events were anemia (n = 18), peripheral edema (n = 18), fatigue (n = 18), nausea (n = 15), and back pain (n = 12). Only two grade four adverse events (low lymphocyte count) were reported.
“Notably, hypertension was not seen,” Dr. Courtney said.
At the time the study concluded, the four patients who achieved complete or partial responses remained on the treatment, and five had received treatment for a year or longer.
The study was funded by Peloton Therapeutics. Dr. Courtney reported having stock and ownership interest in, serving in advisory roles for, or receiving research funding from multiple companies. Several coinvestigators reported receiving research funding from or holding patents with multiple companies including Peloton Therapeutics.
On Twitter @jessnicolecraig
CHICAGO – Investigational agent PT2385, an inhibitor of hypoxia-inducible factor 2-alpha (HIF-2alpha), appears safe and tolerable and demonstrated early evidence of clinical activity in heavily pretreated patients with advanced clear cell renal cell carcinoma, investigators reported at the annual meeting of the American Society of Clinical Oncology.
In a small, phase I dose-escalation study, 39% of patients in the study achieved complete response, partial response, or stable disease lasting at least 16 weeks.
PT2385 is a first-in-class, orally administered selective small molecule inhibitor of HIF-2alpha, a transcription factor with a role in clear cell renal cell carcinoma.
“Hypoxia-inducible factor 2-alpha is a key oncogenic driver of clear cell renal cell carcinoma,” Kevin Courtney, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, said at the meeting.
A total of 51 patients (n = 26 for dose escalation cohort and n = 25 in dose expansion cohort) met the study’s eligibility requirements of having a diagnosis of clear cell renal cell carcinoma, an Eastern Cooperative Oncology Group score ranging from 0 to 1, and at least one prior anticancer therapy. The median age of the cohort was 65 years with the youngest participant being 29 years and the oldest being 80 years old. The majority of patients were male (71%), had received four or more prior systemic therapies (53%), and had undergone vascular endothelial growth factor therapy (98%). Patients were treated with continuous twice-daily oral dosing of PT2385 until progression or toxicity. A total of three patients were treated at the 100-mg dose, three at the 200-mg dose, four at the 400-mg dose, seven at the 800-mg dose, six at a dose of 1,200 mg, and three at a dose of 1,800 mg.
“No dose-limiting toxicity was observed at any dose level,” Dr. Courtney said.
Based on safety, pharmokinetic and pharmodynamic data, 800 mg twice daily was the selected dose for the expansion cohort, he reported.
Of 51 patients treated, 1 patient had a complete response, 3 achieved partial responses, and 16 patients achieved stable disease for at least 16 weeks. The most common grade one or two adverse events were anemia (n = 18), peripheral edema (n = 18), fatigue (n = 18), nausea (n = 15), and back pain (n = 12). Only two grade four adverse events (low lymphocyte count) were reported.
“Notably, hypertension was not seen,” Dr. Courtney said.
At the time the study concluded, the four patients who achieved complete or partial responses remained on the treatment, and five had received treatment for a year or longer.
The study was funded by Peloton Therapeutics. Dr. Courtney reported having stock and ownership interest in, serving in advisory roles for, or receiving research funding from multiple companies. Several coinvestigators reported receiving research funding from or holding patents with multiple companies including Peloton Therapeutics.
On Twitter @jessnicolecraig
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: The first-in-class oral HIF-2alpha inhibitor PT2385 demonstrated early evidence of clinical activity in patients with advanced clear cell renal cell carcinoma. The drug also appears safe and tolerable.
Major finding: Overall, one patient had a complete response, three achieved partial responses, and 16 patients achieved stable disease for at least 16 weeks.
Data source: A phase I dose-escalation and expansion study of 51 heavily pretreated patients with advanced clear cell renal cell carcinoma.
Disclosures: The study was funded by Peloton Therapeutics. Dr. Courtney reported having stock and ownership interest in, serving in advisory roles for, or receiving research funding from multiple companies. Several coinvestigators reported receiving research funding from or holding patents with multiple companies including Peloton Therapeutics.
Disparities in prostate cancer treatment found at both academic and community centers
Academic and community cancer centers demonstrated similarly high rates of sociodemographic disparities in prostate cancer treatment patterns, a study showed.
Specifically, black, Hispanic, and uninsured patients were less likely to receive definitive therapy for high-risk prostate cancer at community and academic cancer centers than were white or privately insured patients. In addition, white patients began receiving therapy earlier than black and Hispanic patients at both community and academic medical centers.
“The finding that disparities exist across cancer care centers, regardless of academic versus community center structure, suggests that there may be something intrinsic about systemic, provider-level, or patient-level issues that may be interfering with treatment,” wrote Brandon Mahal, MD, of Brigham and Women’s Hospital in Boston and his associates (Cancer. July 2016. doi:10.1002/cncr.30205).
Dr. Mahal and associates identified 138,019 patients with high-risk prostate cancer who had a definitive treatment history available through the National Cancer Data Base. Definitive therapy was defined as either prostatectomy or radiation therapy plus androgen-deprivation therapy. High-risk disease was defined as having a prostate-specific antigen score above 20 ng/mL, a Gleason score of 8-10, or a clinical tumor classification of cT3a.
The investigators found black, Hispanic, and uninsured patients were less likely to receive definitive therapy at community cancer centers than were white or privately insured patients (adjusted odds ratios: 0.60, 0.69, and 0.25; 95% confidence intervals: 0.56-0.64, 0.61-0.78, and 0.22-0.30 for black, Hispanic, and uninsured patients; P less than .001). This finding was also true for patients who received treatment at academic cancer centers (AOR: 0.50, 0.56, and 0.31; 95% CI: 0.46-0.54, 0.50-0.64, and 0.28-0.36; P less than .001 for all).
At academic centers, the median time until receipt of definitive treatment was 83 days for white patients, 102 days for black patients, and 94 days for Hispanic patients. At community centers, the median time until receipt of treatment was 77 days for white patients, 92 days for black patients, and 87 days for Hispanic patients.
This study was supported by David and Cynthia Chapin, Hugh Simons, the Campbell Family, the Prostate Cancer Foundation, Fitz’s Cancer Warriors, the Scott Forbes and Gina Ventre Fund, and a grant from an anonymous foundation. Three investigators reported receiving financial compensation from multiple companies.
On Twitter @jessnicolecraig
Academic and community cancer centers demonstrated similarly high rates of sociodemographic disparities in prostate cancer treatment patterns, a study showed.
Specifically, black, Hispanic, and uninsured patients were less likely to receive definitive therapy for high-risk prostate cancer at community and academic cancer centers than were white or privately insured patients. In addition, white patients began receiving therapy earlier than black and Hispanic patients at both community and academic medical centers.
“The finding that disparities exist across cancer care centers, regardless of academic versus community center structure, suggests that there may be something intrinsic about systemic, provider-level, or patient-level issues that may be interfering with treatment,” wrote Brandon Mahal, MD, of Brigham and Women’s Hospital in Boston and his associates (Cancer. July 2016. doi:10.1002/cncr.30205).
Dr. Mahal and associates identified 138,019 patients with high-risk prostate cancer who had a definitive treatment history available through the National Cancer Data Base. Definitive therapy was defined as either prostatectomy or radiation therapy plus androgen-deprivation therapy. High-risk disease was defined as having a prostate-specific antigen score above 20 ng/mL, a Gleason score of 8-10, or a clinical tumor classification of cT3a.
The investigators found black, Hispanic, and uninsured patients were less likely to receive definitive therapy at community cancer centers than were white or privately insured patients (adjusted odds ratios: 0.60, 0.69, and 0.25; 95% confidence intervals: 0.56-0.64, 0.61-0.78, and 0.22-0.30 for black, Hispanic, and uninsured patients; P less than .001). This finding was also true for patients who received treatment at academic cancer centers (AOR: 0.50, 0.56, and 0.31; 95% CI: 0.46-0.54, 0.50-0.64, and 0.28-0.36; P less than .001 for all).
At academic centers, the median time until receipt of definitive treatment was 83 days for white patients, 102 days for black patients, and 94 days for Hispanic patients. At community centers, the median time until receipt of treatment was 77 days for white patients, 92 days for black patients, and 87 days for Hispanic patients.
This study was supported by David and Cynthia Chapin, Hugh Simons, the Campbell Family, the Prostate Cancer Foundation, Fitz’s Cancer Warriors, the Scott Forbes and Gina Ventre Fund, and a grant from an anonymous foundation. Three investigators reported receiving financial compensation from multiple companies.
On Twitter @jessnicolecraig
Academic and community cancer centers demonstrated similarly high rates of sociodemographic disparities in prostate cancer treatment patterns, a study showed.
Specifically, black, Hispanic, and uninsured patients were less likely to receive definitive therapy for high-risk prostate cancer at community and academic cancer centers than were white or privately insured patients. In addition, white patients began receiving therapy earlier than black and Hispanic patients at both community and academic medical centers.
“The finding that disparities exist across cancer care centers, regardless of academic versus community center structure, suggests that there may be something intrinsic about systemic, provider-level, or patient-level issues that may be interfering with treatment,” wrote Brandon Mahal, MD, of Brigham and Women’s Hospital in Boston and his associates (Cancer. July 2016. doi:10.1002/cncr.30205).
Dr. Mahal and associates identified 138,019 patients with high-risk prostate cancer who had a definitive treatment history available through the National Cancer Data Base. Definitive therapy was defined as either prostatectomy or radiation therapy plus androgen-deprivation therapy. High-risk disease was defined as having a prostate-specific antigen score above 20 ng/mL, a Gleason score of 8-10, or a clinical tumor classification of cT3a.
The investigators found black, Hispanic, and uninsured patients were less likely to receive definitive therapy at community cancer centers than were white or privately insured patients (adjusted odds ratios: 0.60, 0.69, and 0.25; 95% confidence intervals: 0.56-0.64, 0.61-0.78, and 0.22-0.30 for black, Hispanic, and uninsured patients; P less than .001). This finding was also true for patients who received treatment at academic cancer centers (AOR: 0.50, 0.56, and 0.31; 95% CI: 0.46-0.54, 0.50-0.64, and 0.28-0.36; P less than .001 for all).
At academic centers, the median time until receipt of definitive treatment was 83 days for white patients, 102 days for black patients, and 94 days for Hispanic patients. At community centers, the median time until receipt of treatment was 77 days for white patients, 92 days for black patients, and 87 days for Hispanic patients.
This study was supported by David and Cynthia Chapin, Hugh Simons, the Campbell Family, the Prostate Cancer Foundation, Fitz’s Cancer Warriors, the Scott Forbes and Gina Ventre Fund, and a grant from an anonymous foundation. Three investigators reported receiving financial compensation from multiple companies.
On Twitter @jessnicolecraig
FROM CANCER
Key clinical point: Academic and community cancer centers demonstrated similarly high rates of sociodemographic disparities in prostate cancer treatment patterns.
Major finding: Black, Hispanic, and uninsured patients were less likely to receive definitive therapy at community cancer centers than were white or privately insured patients (adjusted odds ratios: 0.60, 0.69, and 0.25; 95% confidence intervals: 0.56-0.64, 0.61-0.78, and 0.22-0.30 for black, Hispanic, and uninsured patients; P less than .001), and were less likely to receive definitive therapy at academic cancer centers (AOR: 0.50, 0.56, and 0.31; 95% CI: 0.46-0.54, 0.50-0.64, 0.28-0.36; P less than .001 for all).
Data source: A retrospective analysis of 138,019 patients with high-risk prostate cancer who had a definitive treatment history available in the National Cancer Data Base.
Disclosures: This study was supported by David and Cynthia Chapin, Hugh Simons, the Campbell Family, the Prostate Cancer Foundation, Fitz’s Cancer Warriors, the Scott Forbes and Gina Ventre Fund, and a grant from an anonymous foundation. Three investigators reported receiving financial compensation from multiple companies.
FDA grants priority review to nivolumab for head and neck cancer
The Food and Drug Administration has granted a priority review for expanded use of nivolumab for patients with previously treated recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN), based on results of Checkmate-141.
CheckMate-141 was stopped early in January 2016 after the study met its primary endpoint of improved overall survival in SCCHN patients receiving nivolumab after platinum-based therapy, compared to investigator’s choice of therapy (methotrexate, docetaxel, or cetuximab).
The FDA is expected to act on the review by Nov. 11, 2016, according to a written statement from Bristol-Myers Squib, makers of nivolumab.
The PD-1 immune checkpoint inhibitor, marketed as Opdivo, was also granted breakthrough therapy designation by the FDA for the treatment of unresectable locally advanced or metastatic urothelial carcinoma that has progressed during or following a platinum-based regimen, according to another written statement from Bristol-Myers Squibb. This is the sixth breakthrough therapy designation for nivolumab.
The breakthrough therapy designation in bladder cancer is based on the results of the phase II CA209-275 trial and other supportive data.
On Twitter @jessnicolecraig
The Food and Drug Administration has granted a priority review for expanded use of nivolumab for patients with previously treated recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN), based on results of Checkmate-141.
CheckMate-141 was stopped early in January 2016 after the study met its primary endpoint of improved overall survival in SCCHN patients receiving nivolumab after platinum-based therapy, compared to investigator’s choice of therapy (methotrexate, docetaxel, or cetuximab).
The FDA is expected to act on the review by Nov. 11, 2016, according to a written statement from Bristol-Myers Squib, makers of nivolumab.
The PD-1 immune checkpoint inhibitor, marketed as Opdivo, was also granted breakthrough therapy designation by the FDA for the treatment of unresectable locally advanced or metastatic urothelial carcinoma that has progressed during or following a platinum-based regimen, according to another written statement from Bristol-Myers Squibb. This is the sixth breakthrough therapy designation for nivolumab.
The breakthrough therapy designation in bladder cancer is based on the results of the phase II CA209-275 trial and other supportive data.
On Twitter @jessnicolecraig
The Food and Drug Administration has granted a priority review for expanded use of nivolumab for patients with previously treated recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN), based on results of Checkmate-141.
CheckMate-141 was stopped early in January 2016 after the study met its primary endpoint of improved overall survival in SCCHN patients receiving nivolumab after platinum-based therapy, compared to investigator’s choice of therapy (methotrexate, docetaxel, or cetuximab).
The FDA is expected to act on the review by Nov. 11, 2016, according to a written statement from Bristol-Myers Squib, makers of nivolumab.
The PD-1 immune checkpoint inhibitor, marketed as Opdivo, was also granted breakthrough therapy designation by the FDA for the treatment of unresectable locally advanced or metastatic urothelial carcinoma that has progressed during or following a platinum-based regimen, according to another written statement from Bristol-Myers Squibb. This is the sixth breakthrough therapy designation for nivolumab.
The breakthrough therapy designation in bladder cancer is based on the results of the phase II CA209-275 trial and other supportive data.
On Twitter @jessnicolecraig