User login
VIDEO: Age, race influence understanding of the term ‘Pap smear’
SAN FRANCISCO – Deaths from cervical cancer have declined, but disparities – particularly among the uninsured – remain a concern, as nearly 29% of uninsured white women and more than 17% of uninsured Hispanic women have not been screened in the past 5 years, according to Dr. David Leighton Howard.
To assess understanding of the term “Pap smear” as a possible contributor to low screening rates, Dr. Howard and his colleagues surveyed both English-speaking and Spanish-speaking women and found that about two-thirds of the 160 English-speaking and 123 Spanish-speaking respondents were unable to distinguish between a pelvic exam and a Pap smear, but that a comparable percentage in both groups (74% and 70%, respectively), were able to identify at least one correct descriptor of the term. Those able to identify correct descriptors were more likely to be older (37 years vs. 29 years for English-speaking women and 36 years vs. 30 years for Spanish-speaking women), Dr. Howard of Joint Base Langley-Eustis, U.S. Air Force, Hampton, Va., reported at the annual meeting of the American College of Obstetricians and Gynecologists.
Paradoxically, Dr. Howard said, Spanish-speaking women were significantly less likely than were English-speaking women to use incorrect descriptors for the term Pap smear. Fewer said a Pap smear is the same as a pelvic examination (43% vs. 74%), is a test for a sexually transmitted disease (24% vs. 56%), is a pregnancy test (17% vs. 36%), or is a checkup (20% vs. 53%).
In a video interview, Dr. Howard discussed his findings, as well as the need for more research into the factors behind the age and ethnicity associations in his study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Deaths from cervical cancer have declined, but disparities – particularly among the uninsured – remain a concern, as nearly 29% of uninsured white women and more than 17% of uninsured Hispanic women have not been screened in the past 5 years, according to Dr. David Leighton Howard.
To assess understanding of the term “Pap smear” as a possible contributor to low screening rates, Dr. Howard and his colleagues surveyed both English-speaking and Spanish-speaking women and found that about two-thirds of the 160 English-speaking and 123 Spanish-speaking respondents were unable to distinguish between a pelvic exam and a Pap smear, but that a comparable percentage in both groups (74% and 70%, respectively), were able to identify at least one correct descriptor of the term. Those able to identify correct descriptors were more likely to be older (37 years vs. 29 years for English-speaking women and 36 years vs. 30 years for Spanish-speaking women), Dr. Howard of Joint Base Langley-Eustis, U.S. Air Force, Hampton, Va., reported at the annual meeting of the American College of Obstetricians and Gynecologists.
Paradoxically, Dr. Howard said, Spanish-speaking women were significantly less likely than were English-speaking women to use incorrect descriptors for the term Pap smear. Fewer said a Pap smear is the same as a pelvic examination (43% vs. 74%), is a test for a sexually transmitted disease (24% vs. 56%), is a pregnancy test (17% vs. 36%), or is a checkup (20% vs. 53%).
In a video interview, Dr. Howard discussed his findings, as well as the need for more research into the factors behind the age and ethnicity associations in his study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Deaths from cervical cancer have declined, but disparities – particularly among the uninsured – remain a concern, as nearly 29% of uninsured white women and more than 17% of uninsured Hispanic women have not been screened in the past 5 years, according to Dr. David Leighton Howard.
To assess understanding of the term “Pap smear” as a possible contributor to low screening rates, Dr. Howard and his colleagues surveyed both English-speaking and Spanish-speaking women and found that about two-thirds of the 160 English-speaking and 123 Spanish-speaking respondents were unable to distinguish between a pelvic exam and a Pap smear, but that a comparable percentage in both groups (74% and 70%, respectively), were able to identify at least one correct descriptor of the term. Those able to identify correct descriptors were more likely to be older (37 years vs. 29 years for English-speaking women and 36 years vs. 30 years for Spanish-speaking women), Dr. Howard of Joint Base Langley-Eustis, U.S. Air Force, Hampton, Va., reported at the annual meeting of the American College of Obstetricians and Gynecologists.
Paradoxically, Dr. Howard said, Spanish-speaking women were significantly less likely than were English-speaking women to use incorrect descriptors for the term Pap smear. Fewer said a Pap smear is the same as a pelvic examination (43% vs. 74%), is a test for a sexually transmitted disease (24% vs. 56%), is a pregnancy test (17% vs. 36%), or is a checkup (20% vs. 53%).
In a video interview, Dr. Howard discussed his findings, as well as the need for more research into the factors behind the age and ethnicity associations in his study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACOG ANNUAL CLINICAL MEETING
NASPAG: Teens largely support OTC oral contraceptive access
ORLANDO – Most adolescents expressed interest in and support for access to over-the-counter oral contraceptives, a survey found.
Data from a separate cross-sectional study showed that adolescents are skilled at self-screening for contraindications to combined OCs, demonstrating preliminary support for the safety of over-the-counter access.
Of 348 girls aged 14-17 years who completed the survey in the first study, 73% reported supporting over-the-counter (OTC) OC access for teens, and 61% reported being likely to use OCs if they were available OTC. Significant associations were found between support for OTC access and having used birth control and having had sex. An association was also found between the likelihood of OTC use and having used birth control, having had sex, and being white, Ruth Manski of the Jane Fonda Center for Adolescent Reproductive Health, Atlanta, reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
Those who had never been tested for sexually transmitted infections were more likely to support OTC access (91% vs. 76%).
Support for, and the likelihood of, using OTC OCs were not influenced by age, geographic region, rural/urban location, health insurance status, or pregnancy history. Race played a factor in that white participants were more likely than nonwhite participants to be interested in OTC access. The study also showed that participants understood an average of 7.1 of 8 key product-labeling concepts, and that most would be willing to pay up to $20 per month for OTC OCs, with the largest percentage (42%) willing to pay between $11 and $20. About a third said they would pay $21 or more.
Survey respondents were girls recruited via Facebook advertisements in 2014. Nearly a third (32%) were aged 17 years, 31% were aged 16 years, 24% were aged 15, and 13% were aged 14. Most (79%) were white, Ms. Manski said, adding that the respondents represented 44 states, 53% lived in a suburban area, 41% had private health insurance, and 33% had public health insurance.
About 90% reported having used contraception, and 44% reported having had sex. Of those who had sex, 60% reported having had unprotected sex, 58% used OCs, and 12% reported having been pregnant.
“So results from this study show that participants are supportive of over-the-counter access and that they’re interested in obtaining oral contraceptives over the counter. The findings also suggest that teenagers understand how to use oral contraceptives based on independent label review, which offers some evidence in response to concerns raised in other studies about teenagers’ ability to understand how to use over-the-counter products,” Ms. Manski said.
The findings suggest that while cost is a concern for teens and could impact their contraceptive choices, making OCs available OTC without age restrictions may help increase adolescents’ contraceptive access and use, she said, noting that this is important given the unique barriers that adolescents face with respect to accessing contraception.
The high levels of interest, and the perception of benefit, highlight the potential of this strategy to increase contraceptive access and reduce unintended pregnancy, she added.
Although some respondents expressed concerns about safety and the potential impact on sexual behavior, the evidence with respect to currently available OTC emergency contraception demonstrates that easier access does not increase sexual risk taking, and that teens can safely use OTC emergency contraception, she said.
However, the behavioral effects of OTC OC availability are not known and should be evaluated in actual use studies, Ms. Manski added.
Concerns about the safety of OTC OC access were specifically addressed in the second study, which sought to determine whether adolescents are capable of self-screening for contraindications to OC use.
Prior studies have shown that adults are able to self-screen adequately, but little is known about adolescents’ ability to do so, Dr. Rebekah L. Williams of Riley Hospital for Children, Indianapolis, said at the meeting.
Findings in the first 61 teens aged 14-21 years enrolled in the study showed that the teens were actually more likely than their providers to report potential contraindications.
The teens completed screening questionnaires prior to their office visit, and the provider completed the medical history questionnaire after the visit.
Perfectly concordant responses between providers and patients were seen for six potential contraindications: diabetes (which was present in two cases) and heavy smoking, breastfeeding, wheelchair use, surgery within 4 weeks, and current HIV medication use (which were not present in any cases). Discordant responses were seen for the remaining potential contraindications, including breast cancer, liver disease, medication interactions, smoking, migraine, hypertension, gallbladder disease, thromboembolism/heart disease, having a first-degree relative with thromboembolism, weight over 200 pounds, and having been told/having the perception that OCs should not be used.
Potential contraindications reported only by the participant were breast cancer, liver disease, medication interaction, any smoking, hypertension, personal history of thromboembolism or heart disease, and having a first-degree relative with thromboembolism.
Participants were adolescents with a mean age of 17 years from a primary care medicine clinic in a Midwest urban setting. Of the 61 subjects, 62% were black, 62% reported having ever used contraceptives, 44% were currently using contraceptives, and 62% said they would be interested in OTC OC access. Among the 38 who reported having penile/vaginal sex, 87% had ever used OCs, and 60% were currently using them.
“In summary, adolescent women are interested in over-the-counter access to combined oral contraceptive pills, they’re skilled at self-screening, and they’re mostly more likely than providers to report potential contraindications. So these data really provide some preliminary support for the over-the-counter provision of oral contraceptive pills to adolescents,” Dr. Williams said, noting that additional research is needed to assess adolescents’ ability to use combined oral contraceptive pills correctly.
In addition, larger studies in groups with a higher prevalence of contraindications are needed, as are studies with recruitment from nonclinical settings and more diverse populations, she said.
Ms. Manski and Dr. Williams both reported having no relevant financial disclosures.
ORLANDO – Most adolescents expressed interest in and support for access to over-the-counter oral contraceptives, a survey found.
Data from a separate cross-sectional study showed that adolescents are skilled at self-screening for contraindications to combined OCs, demonstrating preliminary support for the safety of over-the-counter access.
Of 348 girls aged 14-17 years who completed the survey in the first study, 73% reported supporting over-the-counter (OTC) OC access for teens, and 61% reported being likely to use OCs if they were available OTC. Significant associations were found between support for OTC access and having used birth control and having had sex. An association was also found between the likelihood of OTC use and having used birth control, having had sex, and being white, Ruth Manski of the Jane Fonda Center for Adolescent Reproductive Health, Atlanta, reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
Those who had never been tested for sexually transmitted infections were more likely to support OTC access (91% vs. 76%).
Support for, and the likelihood of, using OTC OCs were not influenced by age, geographic region, rural/urban location, health insurance status, or pregnancy history. Race played a factor in that white participants were more likely than nonwhite participants to be interested in OTC access. The study also showed that participants understood an average of 7.1 of 8 key product-labeling concepts, and that most would be willing to pay up to $20 per month for OTC OCs, with the largest percentage (42%) willing to pay between $11 and $20. About a third said they would pay $21 or more.
Survey respondents were girls recruited via Facebook advertisements in 2014. Nearly a third (32%) were aged 17 years, 31% were aged 16 years, 24% were aged 15, and 13% were aged 14. Most (79%) were white, Ms. Manski said, adding that the respondents represented 44 states, 53% lived in a suburban area, 41% had private health insurance, and 33% had public health insurance.
About 90% reported having used contraception, and 44% reported having had sex. Of those who had sex, 60% reported having had unprotected sex, 58% used OCs, and 12% reported having been pregnant.
“So results from this study show that participants are supportive of over-the-counter access and that they’re interested in obtaining oral contraceptives over the counter. The findings also suggest that teenagers understand how to use oral contraceptives based on independent label review, which offers some evidence in response to concerns raised in other studies about teenagers’ ability to understand how to use over-the-counter products,” Ms. Manski said.
The findings suggest that while cost is a concern for teens and could impact their contraceptive choices, making OCs available OTC without age restrictions may help increase adolescents’ contraceptive access and use, she said, noting that this is important given the unique barriers that adolescents face with respect to accessing contraception.
The high levels of interest, and the perception of benefit, highlight the potential of this strategy to increase contraceptive access and reduce unintended pregnancy, she added.
Although some respondents expressed concerns about safety and the potential impact on sexual behavior, the evidence with respect to currently available OTC emergency contraception demonstrates that easier access does not increase sexual risk taking, and that teens can safely use OTC emergency contraception, she said.
However, the behavioral effects of OTC OC availability are not known and should be evaluated in actual use studies, Ms. Manski added.
Concerns about the safety of OTC OC access were specifically addressed in the second study, which sought to determine whether adolescents are capable of self-screening for contraindications to OC use.
Prior studies have shown that adults are able to self-screen adequately, but little is known about adolescents’ ability to do so, Dr. Rebekah L. Williams of Riley Hospital for Children, Indianapolis, said at the meeting.
Findings in the first 61 teens aged 14-21 years enrolled in the study showed that the teens were actually more likely than their providers to report potential contraindications.
The teens completed screening questionnaires prior to their office visit, and the provider completed the medical history questionnaire after the visit.
Perfectly concordant responses between providers and patients were seen for six potential contraindications: diabetes (which was present in two cases) and heavy smoking, breastfeeding, wheelchair use, surgery within 4 weeks, and current HIV medication use (which were not present in any cases). Discordant responses were seen for the remaining potential contraindications, including breast cancer, liver disease, medication interactions, smoking, migraine, hypertension, gallbladder disease, thromboembolism/heart disease, having a first-degree relative with thromboembolism, weight over 200 pounds, and having been told/having the perception that OCs should not be used.
Potential contraindications reported only by the participant were breast cancer, liver disease, medication interaction, any smoking, hypertension, personal history of thromboembolism or heart disease, and having a first-degree relative with thromboembolism.
Participants were adolescents with a mean age of 17 years from a primary care medicine clinic in a Midwest urban setting. Of the 61 subjects, 62% were black, 62% reported having ever used contraceptives, 44% were currently using contraceptives, and 62% said they would be interested in OTC OC access. Among the 38 who reported having penile/vaginal sex, 87% had ever used OCs, and 60% were currently using them.
“In summary, adolescent women are interested in over-the-counter access to combined oral contraceptive pills, they’re skilled at self-screening, and they’re mostly more likely than providers to report potential contraindications. So these data really provide some preliminary support for the over-the-counter provision of oral contraceptive pills to adolescents,” Dr. Williams said, noting that additional research is needed to assess adolescents’ ability to use combined oral contraceptive pills correctly.
In addition, larger studies in groups with a higher prevalence of contraindications are needed, as are studies with recruitment from nonclinical settings and more diverse populations, she said.
Ms. Manski and Dr. Williams both reported having no relevant financial disclosures.
ORLANDO – Most adolescents expressed interest in and support for access to over-the-counter oral contraceptives, a survey found.
Data from a separate cross-sectional study showed that adolescents are skilled at self-screening for contraindications to combined OCs, demonstrating preliminary support for the safety of over-the-counter access.
Of 348 girls aged 14-17 years who completed the survey in the first study, 73% reported supporting over-the-counter (OTC) OC access for teens, and 61% reported being likely to use OCs if they were available OTC. Significant associations were found between support for OTC access and having used birth control and having had sex. An association was also found between the likelihood of OTC use and having used birth control, having had sex, and being white, Ruth Manski of the Jane Fonda Center for Adolescent Reproductive Health, Atlanta, reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
Those who had never been tested for sexually transmitted infections were more likely to support OTC access (91% vs. 76%).
Support for, and the likelihood of, using OTC OCs were not influenced by age, geographic region, rural/urban location, health insurance status, or pregnancy history. Race played a factor in that white participants were more likely than nonwhite participants to be interested in OTC access. The study also showed that participants understood an average of 7.1 of 8 key product-labeling concepts, and that most would be willing to pay up to $20 per month for OTC OCs, with the largest percentage (42%) willing to pay between $11 and $20. About a third said they would pay $21 or more.
Survey respondents were girls recruited via Facebook advertisements in 2014. Nearly a third (32%) were aged 17 years, 31% were aged 16 years, 24% were aged 15, and 13% were aged 14. Most (79%) were white, Ms. Manski said, adding that the respondents represented 44 states, 53% lived in a suburban area, 41% had private health insurance, and 33% had public health insurance.
About 90% reported having used contraception, and 44% reported having had sex. Of those who had sex, 60% reported having had unprotected sex, 58% used OCs, and 12% reported having been pregnant.
“So results from this study show that participants are supportive of over-the-counter access and that they’re interested in obtaining oral contraceptives over the counter. The findings also suggest that teenagers understand how to use oral contraceptives based on independent label review, which offers some evidence in response to concerns raised in other studies about teenagers’ ability to understand how to use over-the-counter products,” Ms. Manski said.
The findings suggest that while cost is a concern for teens and could impact their contraceptive choices, making OCs available OTC without age restrictions may help increase adolescents’ contraceptive access and use, she said, noting that this is important given the unique barriers that adolescents face with respect to accessing contraception.
The high levels of interest, and the perception of benefit, highlight the potential of this strategy to increase contraceptive access and reduce unintended pregnancy, she added.
Although some respondents expressed concerns about safety and the potential impact on sexual behavior, the evidence with respect to currently available OTC emergency contraception demonstrates that easier access does not increase sexual risk taking, and that teens can safely use OTC emergency contraception, she said.
However, the behavioral effects of OTC OC availability are not known and should be evaluated in actual use studies, Ms. Manski added.
Concerns about the safety of OTC OC access were specifically addressed in the second study, which sought to determine whether adolescents are capable of self-screening for contraindications to OC use.
Prior studies have shown that adults are able to self-screen adequately, but little is known about adolescents’ ability to do so, Dr. Rebekah L. Williams of Riley Hospital for Children, Indianapolis, said at the meeting.
Findings in the first 61 teens aged 14-21 years enrolled in the study showed that the teens were actually more likely than their providers to report potential contraindications.
The teens completed screening questionnaires prior to their office visit, and the provider completed the medical history questionnaire after the visit.
Perfectly concordant responses between providers and patients were seen for six potential contraindications: diabetes (which was present in two cases) and heavy smoking, breastfeeding, wheelchair use, surgery within 4 weeks, and current HIV medication use (which were not present in any cases). Discordant responses were seen for the remaining potential contraindications, including breast cancer, liver disease, medication interactions, smoking, migraine, hypertension, gallbladder disease, thromboembolism/heart disease, having a first-degree relative with thromboembolism, weight over 200 pounds, and having been told/having the perception that OCs should not be used.
Potential contraindications reported only by the participant were breast cancer, liver disease, medication interaction, any smoking, hypertension, personal history of thromboembolism or heart disease, and having a first-degree relative with thromboembolism.
Participants were adolescents with a mean age of 17 years from a primary care medicine clinic in a Midwest urban setting. Of the 61 subjects, 62% were black, 62% reported having ever used contraceptives, 44% were currently using contraceptives, and 62% said they would be interested in OTC OC access. Among the 38 who reported having penile/vaginal sex, 87% had ever used OCs, and 60% were currently using them.
“In summary, adolescent women are interested in over-the-counter access to combined oral contraceptive pills, they’re skilled at self-screening, and they’re mostly more likely than providers to report potential contraindications. So these data really provide some preliminary support for the over-the-counter provision of oral contraceptive pills to adolescents,” Dr. Williams said, noting that additional research is needed to assess adolescents’ ability to use combined oral contraceptive pills correctly.
In addition, larger studies in groups with a higher prevalence of contraindications are needed, as are studies with recruitment from nonclinical settings and more diverse populations, she said.
Ms. Manski and Dr. Williams both reported having no relevant financial disclosures.
AT THE NASPAG ANNUAL MEETING
Key clinical point: Adolescents have an interest in access to over-the-counter OCs and appear capable of using them safely.
Major finding: 73% reported supporting OTC access for teens.
Data source: A survey of 348 subjects and a cross-sectional study involving 61 subjects.
Disclosures: Ms. Manski and Dr. Williams both reported having no relevant financial disclosures.
AACR: Targeted combo active in triple-negative breast cancer, ovarian cancer
Combining the poly(ADP-ribose) polymerase inhibitor olaparib and the investigational P13K inhibitor BKM 120 was safe and active in triple-negative breast cancer and ovarian cancer in a phase I trial.
Patients with both BRCA-mutant and BRCA-wildtype breast cancer responded to the combination. One patient with germline BRCA-wildtype triple-negative breast cancer (TNBC) and lung metastases had a partial response and remained on treatment for 20 cycles, or nearly 2 years, study author Dr. Ursula Matulonis reported at the annual meeting of the American Association for Cancer Research.
Rationale for the study lay in data from mouse models showing that combination olaparib and BKM120 was more effective than either drug alone in BRCA-mutant breast cancer and BRCA-wildtype TNBC. Similarities also exist between high-grade serous ovarian cancer and TNBC, including an association with germline BRCA mutations, sensitivity to platinum agents, and high copy number alteration rates, she said in a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly [ADP-ribose] polymerase) inhibitor, was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
The phase I, dose-escalation study enrolled 70 patients with a diagnosis of recurrent high-grade serous ovarian cancer or TNBC but also allowed documented germline BRCA mutation carriers regardless of histology.
The histology was high-grade serous in 90% of the 46 ovarian cancer patients, while 2% had high-grade endometrioid disease, 4% carcinosarcoma, and 4% poorly differentiated carcinoma. Most of the 24 breast cancer patients (63%) had TNBC, while 29% had estrogen receptor–positive/progesterone receptor–positive disease and 8% had ER+/PR– breast cancer.
Germline BRCA mutations were present in 77% of ovarian and 58% of breast cancer patients. The median age in the two groups was 60 years and 47.5 years, respectively. Prior PARP or P13kinase pathway inhibitors were allowed during dose escalation.
Among ovarian cancer patients, 12 (26%) had a partial response and 22 (48%) had stable disease. Responses were similar in the breast cancer group, with 5 (21%) partial responses and 12 (50%) patients with stable disease, said Dr. Matulonis of the Dana-Farber Cancer Center and Harvard Medical School, both in Boston.
Ten dosing regimens were evaluated in the study, beginning with an initial dose of BKM120 60 mg once daily and olaparib 100 mg twice daily, both given orally on a continuous basis. This elicited two dose-limiting toxicities (DLTs) – grade 3 hyperglycemia and grade 3 transaminitis – and prompted the investigators to back down to dose levels of 40 mg and 50 mg, respectively.
No DLTs occurred until dosing reached BKM120 60 mg and olaparib 300 mg, at which point one grade-4 transaminitis and one grade-3 depression were reported in cycle 2, she said. Dose levels of 50 mg and 300 mg, respectively, were selected for the expansion cohort.
As for why the two DLTs occurred at the initial dose but the same doses were later used without incident, Dr. Matulonis said that one of the patients with a DLT fell out of well-controlled diabetes and the other had liver metastases that accelerated during treatment.
Overall, the most common nonhematologic toxicities of any grade were nausea (79.4%), fatigue (66%), and hyperglycemia (40%). Related hematologic toxicities of any grade were anemia in 23.5%, neutropenia in 12%, and thrombocytopenia and leukopenia, both in 10% of patients.
“Combinations of biologic agents will require establishment of target patient populations using biomarkers in order to predict sensitivity as well as determine mechanisms of resistance,” Dr. Matulonis concluded.
Next-generation sequencing is ongoing for BKM120/olaparib patients and mechanisms of response and resistance are being studied in human ovarian mouse models, she added.
The study was funded by Stand Up to Cancer, the Kathryn Fox Samway Foundation, and participating centers. Olaparib was provided by AstraZeneca and BKM120 by Novartis. Dr. Matulonis reported research funding from AstraZeneca, as well as renumeration for attending a speaker’s bureau.
On Twitter @pwendl
Combining the poly(ADP-ribose) polymerase inhibitor olaparib and the investigational P13K inhibitor BKM 120 was safe and active in triple-negative breast cancer and ovarian cancer in a phase I trial.
Patients with both BRCA-mutant and BRCA-wildtype breast cancer responded to the combination. One patient with germline BRCA-wildtype triple-negative breast cancer (TNBC) and lung metastases had a partial response and remained on treatment for 20 cycles, or nearly 2 years, study author Dr. Ursula Matulonis reported at the annual meeting of the American Association for Cancer Research.
Rationale for the study lay in data from mouse models showing that combination olaparib and BKM120 was more effective than either drug alone in BRCA-mutant breast cancer and BRCA-wildtype TNBC. Similarities also exist between high-grade serous ovarian cancer and TNBC, including an association with germline BRCA mutations, sensitivity to platinum agents, and high copy number alteration rates, she said in a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly [ADP-ribose] polymerase) inhibitor, was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
The phase I, dose-escalation study enrolled 70 patients with a diagnosis of recurrent high-grade serous ovarian cancer or TNBC but also allowed documented germline BRCA mutation carriers regardless of histology.
The histology was high-grade serous in 90% of the 46 ovarian cancer patients, while 2% had high-grade endometrioid disease, 4% carcinosarcoma, and 4% poorly differentiated carcinoma. Most of the 24 breast cancer patients (63%) had TNBC, while 29% had estrogen receptor–positive/progesterone receptor–positive disease and 8% had ER+/PR– breast cancer.
Germline BRCA mutations were present in 77% of ovarian and 58% of breast cancer patients. The median age in the two groups was 60 years and 47.5 years, respectively. Prior PARP or P13kinase pathway inhibitors were allowed during dose escalation.
Among ovarian cancer patients, 12 (26%) had a partial response and 22 (48%) had stable disease. Responses were similar in the breast cancer group, with 5 (21%) partial responses and 12 (50%) patients with stable disease, said Dr. Matulonis of the Dana-Farber Cancer Center and Harvard Medical School, both in Boston.
Ten dosing regimens were evaluated in the study, beginning with an initial dose of BKM120 60 mg once daily and olaparib 100 mg twice daily, both given orally on a continuous basis. This elicited two dose-limiting toxicities (DLTs) – grade 3 hyperglycemia and grade 3 transaminitis – and prompted the investigators to back down to dose levels of 40 mg and 50 mg, respectively.
No DLTs occurred until dosing reached BKM120 60 mg and olaparib 300 mg, at which point one grade-4 transaminitis and one grade-3 depression were reported in cycle 2, she said. Dose levels of 50 mg and 300 mg, respectively, were selected for the expansion cohort.
As for why the two DLTs occurred at the initial dose but the same doses were later used without incident, Dr. Matulonis said that one of the patients with a DLT fell out of well-controlled diabetes and the other had liver metastases that accelerated during treatment.
Overall, the most common nonhematologic toxicities of any grade were nausea (79.4%), fatigue (66%), and hyperglycemia (40%). Related hematologic toxicities of any grade were anemia in 23.5%, neutropenia in 12%, and thrombocytopenia and leukopenia, both in 10% of patients.
“Combinations of biologic agents will require establishment of target patient populations using biomarkers in order to predict sensitivity as well as determine mechanisms of resistance,” Dr. Matulonis concluded.
Next-generation sequencing is ongoing for BKM120/olaparib patients and mechanisms of response and resistance are being studied in human ovarian mouse models, she added.
The study was funded by Stand Up to Cancer, the Kathryn Fox Samway Foundation, and participating centers. Olaparib was provided by AstraZeneca and BKM120 by Novartis. Dr. Matulonis reported research funding from AstraZeneca, as well as renumeration for attending a speaker’s bureau.
On Twitter @pwendl
Combining the poly(ADP-ribose) polymerase inhibitor olaparib and the investigational P13K inhibitor BKM 120 was safe and active in triple-negative breast cancer and ovarian cancer in a phase I trial.
Patients with both BRCA-mutant and BRCA-wildtype breast cancer responded to the combination. One patient with germline BRCA-wildtype triple-negative breast cancer (TNBC) and lung metastases had a partial response and remained on treatment for 20 cycles, or nearly 2 years, study author Dr. Ursula Matulonis reported at the annual meeting of the American Association for Cancer Research.
Rationale for the study lay in data from mouse models showing that combination olaparib and BKM120 was more effective than either drug alone in BRCA-mutant breast cancer and BRCA-wildtype TNBC. Similarities also exist between high-grade serous ovarian cancer and TNBC, including an association with germline BRCA mutations, sensitivity to platinum agents, and high copy number alteration rates, she said in a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly [ADP-ribose] polymerase) inhibitor, was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
The phase I, dose-escalation study enrolled 70 patients with a diagnosis of recurrent high-grade serous ovarian cancer or TNBC but also allowed documented germline BRCA mutation carriers regardless of histology.
The histology was high-grade serous in 90% of the 46 ovarian cancer patients, while 2% had high-grade endometrioid disease, 4% carcinosarcoma, and 4% poorly differentiated carcinoma. Most of the 24 breast cancer patients (63%) had TNBC, while 29% had estrogen receptor–positive/progesterone receptor–positive disease and 8% had ER+/PR– breast cancer.
Germline BRCA mutations were present in 77% of ovarian and 58% of breast cancer patients. The median age in the two groups was 60 years and 47.5 years, respectively. Prior PARP or P13kinase pathway inhibitors were allowed during dose escalation.
Among ovarian cancer patients, 12 (26%) had a partial response and 22 (48%) had stable disease. Responses were similar in the breast cancer group, with 5 (21%) partial responses and 12 (50%) patients with stable disease, said Dr. Matulonis of the Dana-Farber Cancer Center and Harvard Medical School, both in Boston.
Ten dosing regimens were evaluated in the study, beginning with an initial dose of BKM120 60 mg once daily and olaparib 100 mg twice daily, both given orally on a continuous basis. This elicited two dose-limiting toxicities (DLTs) – grade 3 hyperglycemia and grade 3 transaminitis – and prompted the investigators to back down to dose levels of 40 mg and 50 mg, respectively.
No DLTs occurred until dosing reached BKM120 60 mg and olaparib 300 mg, at which point one grade-4 transaminitis and one grade-3 depression were reported in cycle 2, she said. Dose levels of 50 mg and 300 mg, respectively, were selected for the expansion cohort.
As for why the two DLTs occurred at the initial dose but the same doses were later used without incident, Dr. Matulonis said that one of the patients with a DLT fell out of well-controlled diabetes and the other had liver metastases that accelerated during treatment.
Overall, the most common nonhematologic toxicities of any grade were nausea (79.4%), fatigue (66%), and hyperglycemia (40%). Related hematologic toxicities of any grade were anemia in 23.5%, neutropenia in 12%, and thrombocytopenia and leukopenia, both in 10% of patients.
“Combinations of biologic agents will require establishment of target patient populations using biomarkers in order to predict sensitivity as well as determine mechanisms of resistance,” Dr. Matulonis concluded.
Next-generation sequencing is ongoing for BKM120/olaparib patients and mechanisms of response and resistance are being studied in human ovarian mouse models, she added.
The study was funded by Stand Up to Cancer, the Kathryn Fox Samway Foundation, and participating centers. Olaparib was provided by AstraZeneca and BKM120 by Novartis. Dr. Matulonis reported research funding from AstraZeneca, as well as renumeration for attending a speaker’s bureau.
On Twitter @pwendl
FROM THE AACR ANNUAL MEETING
Key clinical point: Combining the PARP inhibitor olaparib and the investigational P13K inhibitor BKM 120 is safe and active in triple-negative breast cancer and ovarian cancer in early studies.
Major finding: Partial responses occurred in 26% of patients with ovarian cancer and 21% with breast cancer.
Data source: Phase I study in 70 women with ovarian cancer or breast cancer.
Disclosures: The study was funded by Stand Up to Cancer, the Kathryn Fox Samway Foundation, and participating centers. Olaparib was provided by AstraZeneca and BKM120 by Novartis. Dr. Matulonis reported research funding from AstraZeneca, as well as renumeration for attending a speaker’s bureau.
ACP recommends cervical screening no more than every 3 years
BOSTON – New guidelines for cervical cancer screening in average-risk women issued today by the American College of Physicians are intended to standardize care and balance the harms and benefits of testing.
“Historically, physicians have initiated cervical cancer screening too early, and it is performed too frequently,” Dr. Tanveer P. Mir, chair-elect of the ACP’s Board of Regents, said during a media briefing. She noted that physicians also continue to screen patients at low or average risk, such as those who are older or who have had a hysterectomy. “There is much room for improvement.”
The ACP defines “average risk” as asymptomatic women with no prior history of precancerous lesions or cervical cancer, women who do not have HIV, and women who were not exposed in utero to certain synthetic estrogens.The potential harms of over testing range from false positives that require painful biopsies to unnecessary hysterectomies.
The American Congress of Obstetricians and Gynecologists and the American Society for Clinical Pathology have both endorsed the ACP’s new guidelines, which adhere closely to those released by the U.S. Preventive Services Task Force in 2012.
The ACP’s new guidelines advise that women at average risk for cervical cancer who are age 21 or older should undergo cytology testing every 3 years. For women age 30 and older who prefer less frequent screening, physicians should combine HPV (human papillomavirus) testing with the cytology tests every 5 years (Ann. Intern. Med. 2015 April 30 [doi:10.7326/M14-2426]).
Physicians should not screen women aged 65 years and older who have had three consecutive negative cytology results or two consecutive, combined negative cytology and HPV test results within 10 years, with the most recent test having been within 5 years, according to the new guidelines.
The guidelines also recommend that physicians should not screen average-risk women under 21 years of age for cervical cancer. For average-risk women who are 21 or older, cytology testing should not be done more than once every 3 years.
The ACP does not recommend screening in women at average risk who have had a hysterectomy with the removal of their cervix at any age.
“These are guidelines. It really is a clinical decision by the individual physician as to whether they’re going to follow guidelines, and which particular guidelines they’re going to follow,” ACP President David A. Fleming said.
Nearly one-third of all women aged 21-24 years will test positive for HPV, while 12% of women aged 30-34 years and 5% of women aged 60-64 years will also test positive, according to the ACP.
About 13% of all women aged 21-24 years, 7% of those aged 30-34 years, and 3% of women aged 60-64 years will have abnormal cytology. While cytologic abnormalities are common in female patients under 21 years of age, clinically important cervical lesions are not.
The paper’s release is part of the ACP’s effort to prepare its members for the transition from fee-for-service payment models to value-based ones that the U.S. Department of Health & Human Services has said will be the basis for at least 80% of all care by 2018.
“This is high-value care,” Dr. Robert M. Centor, chair of the ACP’s Board of Regents, said of the new guidelines. “Value is not merely cost. Some expensive tests and treatments have high value because they provide high benefit and low harm. Conversely, some inexpensive tests and treatments have low value because they do not have enough benefits to justify even their remote costs, and might even be harmful.”
More guidelines will follow as part of the ACP’s campaign to change what Dr. Centor called the “paradigm” of over treatment.
In anticipation of pushback from patients who would prefer to have the screening even if they are at low to average risk, Dr. Fleming said in an interview that the ACP will make educational materials available online for physicians to distribute to patients.
“I try to put it in human terms and explain the unintended consequences of [too much testing], and sometimes I do have a strong recommendation that we either do or do not do whatever the guideline is,” he said. “That’s where the trust relationship comes in.”
On Twitter @whitneymcknight
BOSTON – New guidelines for cervical cancer screening in average-risk women issued today by the American College of Physicians are intended to standardize care and balance the harms and benefits of testing.
“Historically, physicians have initiated cervical cancer screening too early, and it is performed too frequently,” Dr. Tanveer P. Mir, chair-elect of the ACP’s Board of Regents, said during a media briefing. She noted that physicians also continue to screen patients at low or average risk, such as those who are older or who have had a hysterectomy. “There is much room for improvement.”
The ACP defines “average risk” as asymptomatic women with no prior history of precancerous lesions or cervical cancer, women who do not have HIV, and women who were not exposed in utero to certain synthetic estrogens.The potential harms of over testing range from false positives that require painful biopsies to unnecessary hysterectomies.
The American Congress of Obstetricians and Gynecologists and the American Society for Clinical Pathology have both endorsed the ACP’s new guidelines, which adhere closely to those released by the U.S. Preventive Services Task Force in 2012.
The ACP’s new guidelines advise that women at average risk for cervical cancer who are age 21 or older should undergo cytology testing every 3 years. For women age 30 and older who prefer less frequent screening, physicians should combine HPV (human papillomavirus) testing with the cytology tests every 5 years (Ann. Intern. Med. 2015 April 30 [doi:10.7326/M14-2426]).
Physicians should not screen women aged 65 years and older who have had three consecutive negative cytology results or two consecutive, combined negative cytology and HPV test results within 10 years, with the most recent test having been within 5 years, according to the new guidelines.
The guidelines also recommend that physicians should not screen average-risk women under 21 years of age for cervical cancer. For average-risk women who are 21 or older, cytology testing should not be done more than once every 3 years.
The ACP does not recommend screening in women at average risk who have had a hysterectomy with the removal of their cervix at any age.
“These are guidelines. It really is a clinical decision by the individual physician as to whether they’re going to follow guidelines, and which particular guidelines they’re going to follow,” ACP President David A. Fleming said.
Nearly one-third of all women aged 21-24 years will test positive for HPV, while 12% of women aged 30-34 years and 5% of women aged 60-64 years will also test positive, according to the ACP.
About 13% of all women aged 21-24 years, 7% of those aged 30-34 years, and 3% of women aged 60-64 years will have abnormal cytology. While cytologic abnormalities are common in female patients under 21 years of age, clinically important cervical lesions are not.
The paper’s release is part of the ACP’s effort to prepare its members for the transition from fee-for-service payment models to value-based ones that the U.S. Department of Health & Human Services has said will be the basis for at least 80% of all care by 2018.
“This is high-value care,” Dr. Robert M. Centor, chair of the ACP’s Board of Regents, said of the new guidelines. “Value is not merely cost. Some expensive tests and treatments have high value because they provide high benefit and low harm. Conversely, some inexpensive tests and treatments have low value because they do not have enough benefits to justify even their remote costs, and might even be harmful.”
More guidelines will follow as part of the ACP’s campaign to change what Dr. Centor called the “paradigm” of over treatment.
In anticipation of pushback from patients who would prefer to have the screening even if they are at low to average risk, Dr. Fleming said in an interview that the ACP will make educational materials available online for physicians to distribute to patients.
“I try to put it in human terms and explain the unintended consequences of [too much testing], and sometimes I do have a strong recommendation that we either do or do not do whatever the guideline is,” he said. “That’s where the trust relationship comes in.”
On Twitter @whitneymcknight
BOSTON – New guidelines for cervical cancer screening in average-risk women issued today by the American College of Physicians are intended to standardize care and balance the harms and benefits of testing.
“Historically, physicians have initiated cervical cancer screening too early, and it is performed too frequently,” Dr. Tanveer P. Mir, chair-elect of the ACP’s Board of Regents, said during a media briefing. She noted that physicians also continue to screen patients at low or average risk, such as those who are older or who have had a hysterectomy. “There is much room for improvement.”
The ACP defines “average risk” as asymptomatic women with no prior history of precancerous lesions or cervical cancer, women who do not have HIV, and women who were not exposed in utero to certain synthetic estrogens.The potential harms of over testing range from false positives that require painful biopsies to unnecessary hysterectomies.
The American Congress of Obstetricians and Gynecologists and the American Society for Clinical Pathology have both endorsed the ACP’s new guidelines, which adhere closely to those released by the U.S. Preventive Services Task Force in 2012.
The ACP’s new guidelines advise that women at average risk for cervical cancer who are age 21 or older should undergo cytology testing every 3 years. For women age 30 and older who prefer less frequent screening, physicians should combine HPV (human papillomavirus) testing with the cytology tests every 5 years (Ann. Intern. Med. 2015 April 30 [doi:10.7326/M14-2426]).
Physicians should not screen women aged 65 years and older who have had three consecutive negative cytology results or two consecutive, combined negative cytology and HPV test results within 10 years, with the most recent test having been within 5 years, according to the new guidelines.
The guidelines also recommend that physicians should not screen average-risk women under 21 years of age for cervical cancer. For average-risk women who are 21 or older, cytology testing should not be done more than once every 3 years.
The ACP does not recommend screening in women at average risk who have had a hysterectomy with the removal of their cervix at any age.
“These are guidelines. It really is a clinical decision by the individual physician as to whether they’re going to follow guidelines, and which particular guidelines they’re going to follow,” ACP President David A. Fleming said.
Nearly one-third of all women aged 21-24 years will test positive for HPV, while 12% of women aged 30-34 years and 5% of women aged 60-64 years will also test positive, according to the ACP.
About 13% of all women aged 21-24 years, 7% of those aged 30-34 years, and 3% of women aged 60-64 years will have abnormal cytology. While cytologic abnormalities are common in female patients under 21 years of age, clinically important cervical lesions are not.
The paper’s release is part of the ACP’s effort to prepare its members for the transition from fee-for-service payment models to value-based ones that the U.S. Department of Health & Human Services has said will be the basis for at least 80% of all care by 2018.
“This is high-value care,” Dr. Robert M. Centor, chair of the ACP’s Board of Regents, said of the new guidelines. “Value is not merely cost. Some expensive tests and treatments have high value because they provide high benefit and low harm. Conversely, some inexpensive tests and treatments have low value because they do not have enough benefits to justify even their remote costs, and might even be harmful.”
More guidelines will follow as part of the ACP’s campaign to change what Dr. Centor called the “paradigm” of over treatment.
In anticipation of pushback from patients who would prefer to have the screening even if they are at low to average risk, Dr. Fleming said in an interview that the ACP will make educational materials available online for physicians to distribute to patients.
“I try to put it in human terms and explain the unintended consequences of [too much testing], and sometimes I do have a strong recommendation that we either do or do not do whatever the guideline is,” he said. “That’s where the trust relationship comes in.”
On Twitter @whitneymcknight
AT ACP INTERNAL MEDICINE 2015
2015 Update on cervical disease: New ammo for HPV prevention and screening
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
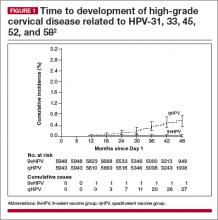
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
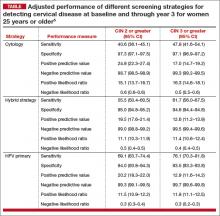
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
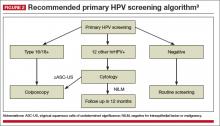
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
In this article
- Disease progression with 9-valent versus quadrivalent HPV vaccine
- How well do current screening strategies detect disease?
- Recommended primary HPV screening algorithm
New light shed on olaparib therapy in ovarian cancer
CHICAGO – Olaparib improves outcomes in women with ovarian cancer who have a germline BRCA mutation, but its use at least as maintenance therapy is not cost-effective, according to research reported at the annual meeting of the Society of Gynecologic Oncology.
The Food and Drug Administration approved olaparib, an oral inhibitor of poly-ADP-ribose polymerase (PARP), in December 2014 for the treatment of patients who have recurrent ovarian cancer with a germline BRCA mutation and have received at least three prior lines of therapy. It is currently not approved for maintenance therapy in the United States.
Subsequent therapy may mask a survival benefit
In the first of three studies, investigators led by Dr. Ursula A. Matulonis performed a post hoc, exploratory analysis of data from a pivotal randomized phase II trial in platinum-sensitive relapsed ovarian cancer (Study 19). The trial, sponsored by AstraZeneca, compared olaparib (Lynparza) with placebo as maintenance therapy.
A previous stratified analysis among 265 patients revealed that those with a BRCA mutation had a marked progression-free survival benefit with olaparib versus placebo (11.2 vs. 4.3 months; hazard ratio, 0.18) but no significant gain in overall survival (34.9 vs. 31.9 months; hazard ratio, 0.73) (Lancet Oncol. 2014;15:852-61), according to Dr. Matulonis, medical director and program leader of the medical gynecologic oncology program at the Dana-Farber Cancer Institute and an associate professor of medicine at Harvard Medical School, both in Boston.
Crossover to olaparib was not permitted in the trial, but patients may have accessed PARP inhibitors outside of the study, she noted. “It was hypothesized that patients switching treatments may have reduced the beneficial impact of olaparib on the overall survival results. We know that switching is common in randomized trials in oncology but difficult to prevent practically and ethically, and it certainly may make an impact on overall survival.”
In the new, exploratory analysis, she and her colleagues reassessed outcomes after excluding all trial sites at which at least one patient received a PARP inhibitor after progression, which left 198 patients.
Results still showed a dramatic progression-free survival benefit for olaparib over placebo for those with a BRCA mutation (12.4 vs. 4.4 months; hazard ratio, 0.14). But now, olaparib also conferred a significant overall survival benefit, halving the risk of death (34.9 vs. 26.6 months; hazard ratio, 0.52), reported Dr. Matulonis, who disclosed that she has received research funding and speakers bureau remuneration from AstraZeneca.
“This change in overall survival hazard ratio may suggest a confounding influence that reduced the beneficial impact of olaparib,” she said. “There remains a degree of uncertainty owing to small sample sizes and lack of data maturity, so further analysis on more mature data is required to confirm these findings.”
Analysis confirms efficacy, safety in heavily pretreated patients
In the second study, Dr. Matulonis and colleagues performed a pooled analysis to assess the impact of olaparib in patients with advanced relapsed ovarian cancer having a germline BRCA mutation. Results were based on 273 patients given olaparib monotherapy in six AstraZeneca-sponsored phase I and II trials.
In the entire cohort, the overall response rate was 36% and the median duration of response was 7.4 months. The corresponding values were 31% and 7.8 months in the three-fourths of patients who had received at least three prior lines of chemotherapy.
Benefit was seen whether patients’ disease was platinum sensitive, resistant, or unknown, Dr. Matulonis reported. However, the response rate fell as the number of previous lines of therapy increased.
The rate of grade 3 or worse adverse events was 50% in the total population and 54% in the subset who received three or more previous therapies, and the rate of serious adverse events was 30% and 34%, respectively. Eight patients (all in the heavily pretreated group) had a serious adverse event leading to death, but none were considered causally related to olaparib.
“Olaparib treatment benefits were observed in all the patient subgroups,” Dr. Matulonis said. “The safety profile of olaparib was acceptable in patients who had received three or more prior lines of therapy.”
She noted that an ongoing series of phase III trials of monotherapy in patients with germline BRCA mutations – the Studies of Olaparib in Ovarian Cancer (SOLO) 1, 2, and 3 trials – should provide more information on use of the drug in various settings.
Invited discussant Dr. Elizabeth Swisher, medical director of the breast and ovarian cancer prevention program at the Seattle Cancer Care Alliance and professor in the gynecologic oncology division at the University of Washington, noted the uncertainty surrounding the optimal use of PARP inhibitors in ovarian cancer and mentioned that olaparib is approved as maintenance therapy in Europe.
“Many of you have probably been in the same situation that I have, where I have a patient in front of me and have to say, ‘Yes, this would be a good drug for you, but you need to fail a couple more lines of chemotherapy first,’ ” she said. “And we all know that later in the disease course, GI symptoms become more prominent and an oral drug may not be tolerated, so we might lose the opportunity to treat these patients with these drugs.”
Therefore, the field should address some key unanswered questions about PARP inhibitor therapy, according to Dr. Swisher: “Really, what is the best time point for using it – is it at maintenance, or is it at the time of relapse? And if we use it as maintenance, is it in the primary setting or the recurrent setting?” she elaborated.
She noted that women with somatic BRCA mutations as opposed to germline ones seem to benefit similarly from olaparib, but as insurance companies in the United States often resist covering tumor testing, this subset of women is often missed. “Predictors of response and resistance other than BRCA mutations are needed,” she added.
Models suggest maintenance therapy is not cost-effective
In the third study, investigators led by Dr. Haller J. Smith, a resident in obstetrics and gynecology at the University of Alabama, Birmingham, constructed models to assess the cost-effectiveness of olaparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer.
They used as a target population 5,549 women who had a complete response to primary therapy, experienced a recurrence, and then had at least a partial response to second-line chemotherapy. Analyses assumed a germline BRCA mutation prevalence of 20%.
In the model, patients received six cycles of chemotherapy, followed by either observation or olaparib maintenance therapy. The cost of olaparib was set at $7,000 per month, while the cost of observation was based on national guidelines and Medicare reimbursement rates. “The cost of adverse events was not included in the model as the majority of these in the phase II trial were grade 1 or 2,” Dr. Smith noted.
Results of the base case analysis showed that among patients with a BRCA mutation, the incremental cost-effectiveness ratio (ICER) for olaparib relative to observation was $135,672 per progression-free life-year saved – more than double the $50,000 threshold the investigators considered to be cost-effective, Dr. Smith reported. Among patients with wild type for BRCA, the ICER was sharply higher, at $315,840.
Sensitivity analyses showed that olaparib therapy was still not cost-effective when the prevalence of BRCA mutations and progression-free survival were varied. But the ICER fell to $97,404 when the cost of the drug was reduced to $5,000 and fell to $49,584 when it was reduced to $2,500.
“In order to achieve an ICER of less than $50,000, the cost of olaparib would have to be $2,500 or less per month,” Dr. Smith said. However, “in the era of molecular targeted therapies, an ICER of less than $100,000 would be considered by many to be cost-effective,” she acknowledged.
“While PARP inhibitors and other molecular targeted therapies represent exciting new therapeutic options for our patients, the costs associated with these drugs remain a significant concern. As health care costs continue to increase, cost-effectiveness studies are likely to become a more important part of the drug development and approval process,” she concluded.
CHICAGO – Olaparib improves outcomes in women with ovarian cancer who have a germline BRCA mutation, but its use at least as maintenance therapy is not cost-effective, according to research reported at the annual meeting of the Society of Gynecologic Oncology.
The Food and Drug Administration approved olaparib, an oral inhibitor of poly-ADP-ribose polymerase (PARP), in December 2014 for the treatment of patients who have recurrent ovarian cancer with a germline BRCA mutation and have received at least three prior lines of therapy. It is currently not approved for maintenance therapy in the United States.
Subsequent therapy may mask a survival benefit
In the first of three studies, investigators led by Dr. Ursula A. Matulonis performed a post hoc, exploratory analysis of data from a pivotal randomized phase II trial in platinum-sensitive relapsed ovarian cancer (Study 19). The trial, sponsored by AstraZeneca, compared olaparib (Lynparza) with placebo as maintenance therapy.
A previous stratified analysis among 265 patients revealed that those with a BRCA mutation had a marked progression-free survival benefit with olaparib versus placebo (11.2 vs. 4.3 months; hazard ratio, 0.18) but no significant gain in overall survival (34.9 vs. 31.9 months; hazard ratio, 0.73) (Lancet Oncol. 2014;15:852-61), according to Dr. Matulonis, medical director and program leader of the medical gynecologic oncology program at the Dana-Farber Cancer Institute and an associate professor of medicine at Harvard Medical School, both in Boston.
Crossover to olaparib was not permitted in the trial, but patients may have accessed PARP inhibitors outside of the study, she noted. “It was hypothesized that patients switching treatments may have reduced the beneficial impact of olaparib on the overall survival results. We know that switching is common in randomized trials in oncology but difficult to prevent practically and ethically, and it certainly may make an impact on overall survival.”
In the new, exploratory analysis, she and her colleagues reassessed outcomes after excluding all trial sites at which at least one patient received a PARP inhibitor after progression, which left 198 patients.
Results still showed a dramatic progression-free survival benefit for olaparib over placebo for those with a BRCA mutation (12.4 vs. 4.4 months; hazard ratio, 0.14). But now, olaparib also conferred a significant overall survival benefit, halving the risk of death (34.9 vs. 26.6 months; hazard ratio, 0.52), reported Dr. Matulonis, who disclosed that she has received research funding and speakers bureau remuneration from AstraZeneca.
“This change in overall survival hazard ratio may suggest a confounding influence that reduced the beneficial impact of olaparib,” she said. “There remains a degree of uncertainty owing to small sample sizes and lack of data maturity, so further analysis on more mature data is required to confirm these findings.”
Analysis confirms efficacy, safety in heavily pretreated patients
In the second study, Dr. Matulonis and colleagues performed a pooled analysis to assess the impact of olaparib in patients with advanced relapsed ovarian cancer having a germline BRCA mutation. Results were based on 273 patients given olaparib monotherapy in six AstraZeneca-sponsored phase I and II trials.
In the entire cohort, the overall response rate was 36% and the median duration of response was 7.4 months. The corresponding values were 31% and 7.8 months in the three-fourths of patients who had received at least three prior lines of chemotherapy.
Benefit was seen whether patients’ disease was platinum sensitive, resistant, or unknown, Dr. Matulonis reported. However, the response rate fell as the number of previous lines of therapy increased.
The rate of grade 3 or worse adverse events was 50% in the total population and 54% in the subset who received three or more previous therapies, and the rate of serious adverse events was 30% and 34%, respectively. Eight patients (all in the heavily pretreated group) had a serious adverse event leading to death, but none were considered causally related to olaparib.
“Olaparib treatment benefits were observed in all the patient subgroups,” Dr. Matulonis said. “The safety profile of olaparib was acceptable in patients who had received three or more prior lines of therapy.”
She noted that an ongoing series of phase III trials of monotherapy in patients with germline BRCA mutations – the Studies of Olaparib in Ovarian Cancer (SOLO) 1, 2, and 3 trials – should provide more information on use of the drug in various settings.
Invited discussant Dr. Elizabeth Swisher, medical director of the breast and ovarian cancer prevention program at the Seattle Cancer Care Alliance and professor in the gynecologic oncology division at the University of Washington, noted the uncertainty surrounding the optimal use of PARP inhibitors in ovarian cancer and mentioned that olaparib is approved as maintenance therapy in Europe.
“Many of you have probably been in the same situation that I have, where I have a patient in front of me and have to say, ‘Yes, this would be a good drug for you, but you need to fail a couple more lines of chemotherapy first,’ ” she said. “And we all know that later in the disease course, GI symptoms become more prominent and an oral drug may not be tolerated, so we might lose the opportunity to treat these patients with these drugs.”
Therefore, the field should address some key unanswered questions about PARP inhibitor therapy, according to Dr. Swisher: “Really, what is the best time point for using it – is it at maintenance, or is it at the time of relapse? And if we use it as maintenance, is it in the primary setting or the recurrent setting?” she elaborated.
She noted that women with somatic BRCA mutations as opposed to germline ones seem to benefit similarly from olaparib, but as insurance companies in the United States often resist covering tumor testing, this subset of women is often missed. “Predictors of response and resistance other than BRCA mutations are needed,” she added.
Models suggest maintenance therapy is not cost-effective
In the third study, investigators led by Dr. Haller J. Smith, a resident in obstetrics and gynecology at the University of Alabama, Birmingham, constructed models to assess the cost-effectiveness of olaparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer.
They used as a target population 5,549 women who had a complete response to primary therapy, experienced a recurrence, and then had at least a partial response to second-line chemotherapy. Analyses assumed a germline BRCA mutation prevalence of 20%.
In the model, patients received six cycles of chemotherapy, followed by either observation or olaparib maintenance therapy. The cost of olaparib was set at $7,000 per month, while the cost of observation was based on national guidelines and Medicare reimbursement rates. “The cost of adverse events was not included in the model as the majority of these in the phase II trial were grade 1 or 2,” Dr. Smith noted.
Results of the base case analysis showed that among patients with a BRCA mutation, the incremental cost-effectiveness ratio (ICER) for olaparib relative to observation was $135,672 per progression-free life-year saved – more than double the $50,000 threshold the investigators considered to be cost-effective, Dr. Smith reported. Among patients with wild type for BRCA, the ICER was sharply higher, at $315,840.
Sensitivity analyses showed that olaparib therapy was still not cost-effective when the prevalence of BRCA mutations and progression-free survival were varied. But the ICER fell to $97,404 when the cost of the drug was reduced to $5,000 and fell to $49,584 when it was reduced to $2,500.
“In order to achieve an ICER of less than $50,000, the cost of olaparib would have to be $2,500 or less per month,” Dr. Smith said. However, “in the era of molecular targeted therapies, an ICER of less than $100,000 would be considered by many to be cost-effective,” she acknowledged.
“While PARP inhibitors and other molecular targeted therapies represent exciting new therapeutic options for our patients, the costs associated with these drugs remain a significant concern. As health care costs continue to increase, cost-effectiveness studies are likely to become a more important part of the drug development and approval process,” she concluded.
CHICAGO – Olaparib improves outcomes in women with ovarian cancer who have a germline BRCA mutation, but its use at least as maintenance therapy is not cost-effective, according to research reported at the annual meeting of the Society of Gynecologic Oncology.
The Food and Drug Administration approved olaparib, an oral inhibitor of poly-ADP-ribose polymerase (PARP), in December 2014 for the treatment of patients who have recurrent ovarian cancer with a germline BRCA mutation and have received at least three prior lines of therapy. It is currently not approved for maintenance therapy in the United States.
Subsequent therapy may mask a survival benefit
In the first of three studies, investigators led by Dr. Ursula A. Matulonis performed a post hoc, exploratory analysis of data from a pivotal randomized phase II trial in platinum-sensitive relapsed ovarian cancer (Study 19). The trial, sponsored by AstraZeneca, compared olaparib (Lynparza) with placebo as maintenance therapy.
A previous stratified analysis among 265 patients revealed that those with a BRCA mutation had a marked progression-free survival benefit with olaparib versus placebo (11.2 vs. 4.3 months; hazard ratio, 0.18) but no significant gain in overall survival (34.9 vs. 31.9 months; hazard ratio, 0.73) (Lancet Oncol. 2014;15:852-61), according to Dr. Matulonis, medical director and program leader of the medical gynecologic oncology program at the Dana-Farber Cancer Institute and an associate professor of medicine at Harvard Medical School, both in Boston.
Crossover to olaparib was not permitted in the trial, but patients may have accessed PARP inhibitors outside of the study, she noted. “It was hypothesized that patients switching treatments may have reduced the beneficial impact of olaparib on the overall survival results. We know that switching is common in randomized trials in oncology but difficult to prevent practically and ethically, and it certainly may make an impact on overall survival.”
In the new, exploratory analysis, she and her colleagues reassessed outcomes after excluding all trial sites at which at least one patient received a PARP inhibitor after progression, which left 198 patients.
Results still showed a dramatic progression-free survival benefit for olaparib over placebo for those with a BRCA mutation (12.4 vs. 4.4 months; hazard ratio, 0.14). But now, olaparib also conferred a significant overall survival benefit, halving the risk of death (34.9 vs. 26.6 months; hazard ratio, 0.52), reported Dr. Matulonis, who disclosed that she has received research funding and speakers bureau remuneration from AstraZeneca.
“This change in overall survival hazard ratio may suggest a confounding influence that reduced the beneficial impact of olaparib,” she said. “There remains a degree of uncertainty owing to small sample sizes and lack of data maturity, so further analysis on more mature data is required to confirm these findings.”
Analysis confirms efficacy, safety in heavily pretreated patients
In the second study, Dr. Matulonis and colleagues performed a pooled analysis to assess the impact of olaparib in patients with advanced relapsed ovarian cancer having a germline BRCA mutation. Results were based on 273 patients given olaparib monotherapy in six AstraZeneca-sponsored phase I and II trials.
In the entire cohort, the overall response rate was 36% and the median duration of response was 7.4 months. The corresponding values were 31% and 7.8 months in the three-fourths of patients who had received at least three prior lines of chemotherapy.
Benefit was seen whether patients’ disease was platinum sensitive, resistant, or unknown, Dr. Matulonis reported. However, the response rate fell as the number of previous lines of therapy increased.
The rate of grade 3 or worse adverse events was 50% in the total population and 54% in the subset who received three or more previous therapies, and the rate of serious adverse events was 30% and 34%, respectively. Eight patients (all in the heavily pretreated group) had a serious adverse event leading to death, but none were considered causally related to olaparib.
“Olaparib treatment benefits were observed in all the patient subgroups,” Dr. Matulonis said. “The safety profile of olaparib was acceptable in patients who had received three or more prior lines of therapy.”
She noted that an ongoing series of phase III trials of monotherapy in patients with germline BRCA mutations – the Studies of Olaparib in Ovarian Cancer (SOLO) 1, 2, and 3 trials – should provide more information on use of the drug in various settings.
Invited discussant Dr. Elizabeth Swisher, medical director of the breast and ovarian cancer prevention program at the Seattle Cancer Care Alliance and professor in the gynecologic oncology division at the University of Washington, noted the uncertainty surrounding the optimal use of PARP inhibitors in ovarian cancer and mentioned that olaparib is approved as maintenance therapy in Europe.
“Many of you have probably been in the same situation that I have, where I have a patient in front of me and have to say, ‘Yes, this would be a good drug for you, but you need to fail a couple more lines of chemotherapy first,’ ” she said. “And we all know that later in the disease course, GI symptoms become more prominent and an oral drug may not be tolerated, so we might lose the opportunity to treat these patients with these drugs.”
Therefore, the field should address some key unanswered questions about PARP inhibitor therapy, according to Dr. Swisher: “Really, what is the best time point for using it – is it at maintenance, or is it at the time of relapse? And if we use it as maintenance, is it in the primary setting or the recurrent setting?” she elaborated.
She noted that women with somatic BRCA mutations as opposed to germline ones seem to benefit similarly from olaparib, but as insurance companies in the United States often resist covering tumor testing, this subset of women is often missed. “Predictors of response and resistance other than BRCA mutations are needed,” she added.
Models suggest maintenance therapy is not cost-effective
In the third study, investigators led by Dr. Haller J. Smith, a resident in obstetrics and gynecology at the University of Alabama, Birmingham, constructed models to assess the cost-effectiveness of olaparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer.
They used as a target population 5,549 women who had a complete response to primary therapy, experienced a recurrence, and then had at least a partial response to second-line chemotherapy. Analyses assumed a germline BRCA mutation prevalence of 20%.
In the model, patients received six cycles of chemotherapy, followed by either observation or olaparib maintenance therapy. The cost of olaparib was set at $7,000 per month, while the cost of observation was based on national guidelines and Medicare reimbursement rates. “The cost of adverse events was not included in the model as the majority of these in the phase II trial were grade 1 or 2,” Dr. Smith noted.
Results of the base case analysis showed that among patients with a BRCA mutation, the incremental cost-effectiveness ratio (ICER) for olaparib relative to observation was $135,672 per progression-free life-year saved – more than double the $50,000 threshold the investigators considered to be cost-effective, Dr. Smith reported. Among patients with wild type for BRCA, the ICER was sharply higher, at $315,840.
Sensitivity analyses showed that olaparib therapy was still not cost-effective when the prevalence of BRCA mutations and progression-free survival were varied. But the ICER fell to $97,404 when the cost of the drug was reduced to $5,000 and fell to $49,584 when it was reduced to $2,500.
“In order to achieve an ICER of less than $50,000, the cost of olaparib would have to be $2,500 or less per month,” Dr. Smith said. However, “in the era of molecular targeted therapies, an ICER of less than $100,000 would be considered by many to be cost-effective,” she acknowledged.
“While PARP inhibitors and other molecular targeted therapies represent exciting new therapeutic options for our patients, the costs associated with these drugs remain a significant concern. As health care costs continue to increase, cost-effectiveness studies are likely to become a more important part of the drug development and approval process,” she concluded.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
NASPAG: Hormonal add-back prevents GnRH-A–related bone loss in adolescents
ORLANDO – Hormonal add-back therapy using combination norethindrone acetate and conjugated equine estrogens was effective and better than norethindrone acetate plus placebo for preserving bone health and improving quality of life in adolescents receiving treatment with gonadotropin-releasing hormone agonists (GnRH-A) for endometriosis in a randomized, double-blind, placebo-controlled study.
Of 51 adolescents aged 15-22 years who were initiating GnRH-A therapy for endometriosis, 25 were randomized to receive add-back therapy with 5 mg daily oral norethindrone acetate (NA) plus 0.625 mg oral daily conjugated equine estrogens (CEE), and 26 were randomized to receive NA plus placebo; 18 and 16, respectively, completed the study.
Those in the combination-therapy group experienced significant increases over 12 months in both total body bone mineral content (BMC) and bone mineral density (BMD), compared with those who received NA plus placebo. BMC increased by nearly 40 g/unit scanned in the combination-therapy group, compared with about 12 g in the NA plus placebo group, and BMD increased more than 0.01 g/cm2, compared with no change in the NA plus placebo group, Dr. Amy D. DiVasta reported at the North American Society for Pediatric and Adolescent Gynecology annual meeting.
No losses of total hip or lumbar spine BMC or BMD occurred, said Dr. DiVasta of Boston Children’s Hospital.
Further, lean mass increased in the combination-therapy group at 12 months – by about 1.4 kg, compared with no change in the placebo group. No differences were seen between the groups in change in fat mass.
As for quality of life measures, overall physical health scores at baseline were significantly below the U.S. mean in both groups, and overall mental health scores were above the U.S. mean in both groups. Both groups improved over time on both measures; the increase in the Physical Component Summary scale scores on the SF-36 (Short Form 36 Health Survey) was significantly greater with combination-therapy group (increase from about 40 to 50 out of 100 ), compared with NA plus placebo (increase from about 40 to 45), but the increases on the Mental Component Summary scale scores were not statistically significant in either group, or between the groups.
The study subjects, who were treated for 12 months between 2008 and 2012, were at least 2 years post menarche at baseline and had a surgical diagnosis of endometriosis. The two treatment groups were similar with respect to baseline characteristics, including age, BMD Z score, and disease severity. No significant side effects were observed in either the combination or NA plus placebo group, Dr. DiVasta said, noting that laboratory tests included liver function tests and lipid levels.
Areal BMD, BMC, and body composition were measured by dual-energy X-ray absorptiometry (DXA) at baseline, 6 months, and 12 months. Anthropometrics, quality of life measures, and laboratory studies were conducted at 1, 3, 6, and 12 months.
GnRH-A are commonly utilized for endometriosis patients who fail primary therapy, such as NSAIDs or combined oral contraceptives, but long-term use is associated with deleterious effects on bone mineralization; adults have been shown to lose 5%-8% of BMD after 3-6 months of treatment, Dr. DiVasta noted.
Hormonal add-back therapy is a promising adjunct to counteract these effects and could be an important tool for protecting adolescents, who are at the greatest risk for the deleterious effects of GnRH-A therapy, she said.
In the current study, hormonal add-back did successfully protect bone health and improve quality of life for adolescents with endometriosis who were treated with 12 months of GnRH-A. Combination therapy with NA and CEE appears to be more effective for increasing total body BMC and BMD, lean mass, and physical health-related quality of life, as compared with NA monotherapy, she said.
The findings are limited by the inclusion of only skeletally mature young women, as the results may not be generalizable to growing girls. Also, DXA measures provide two-dimensional measures of bone mineral density, and do not yield information regarding skeletal strength or bone microarchitecture, she noted.
“Given the prevalence of endometriosis, our data suggest norethindrone plus estrogens to be a useful adjunctive therapy to prevent bone loss in these young women while they receive appropriate medical treatment for their underlying disease,” she concluded, noting that future work will explore the effects of add-back on the peripheral skeleton and bone strength.
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the McCarthy Family Foundation, Thrasher Research Fund, and Boston Children’s Hospital department of medicine. Medications were provided by Abbott, Duramed Pharmaceuticals, and Wyeth Pharmaceuticals. Dr. DiVasta reported having no relevant financial disclosures.
ORLANDO – Hormonal add-back therapy using combination norethindrone acetate and conjugated equine estrogens was effective and better than norethindrone acetate plus placebo for preserving bone health and improving quality of life in adolescents receiving treatment with gonadotropin-releasing hormone agonists (GnRH-A) for endometriosis in a randomized, double-blind, placebo-controlled study.
Of 51 adolescents aged 15-22 years who were initiating GnRH-A therapy for endometriosis, 25 were randomized to receive add-back therapy with 5 mg daily oral norethindrone acetate (NA) plus 0.625 mg oral daily conjugated equine estrogens (CEE), and 26 were randomized to receive NA plus placebo; 18 and 16, respectively, completed the study.
Those in the combination-therapy group experienced significant increases over 12 months in both total body bone mineral content (BMC) and bone mineral density (BMD), compared with those who received NA plus placebo. BMC increased by nearly 40 g/unit scanned in the combination-therapy group, compared with about 12 g in the NA plus placebo group, and BMD increased more than 0.01 g/cm2, compared with no change in the NA plus placebo group, Dr. Amy D. DiVasta reported at the North American Society for Pediatric and Adolescent Gynecology annual meeting.
No losses of total hip or lumbar spine BMC or BMD occurred, said Dr. DiVasta of Boston Children’s Hospital.
Further, lean mass increased in the combination-therapy group at 12 months – by about 1.4 kg, compared with no change in the placebo group. No differences were seen between the groups in change in fat mass.
As for quality of life measures, overall physical health scores at baseline were significantly below the U.S. mean in both groups, and overall mental health scores were above the U.S. mean in both groups. Both groups improved over time on both measures; the increase in the Physical Component Summary scale scores on the SF-36 (Short Form 36 Health Survey) was significantly greater with combination-therapy group (increase from about 40 to 50 out of 100 ), compared with NA plus placebo (increase from about 40 to 45), but the increases on the Mental Component Summary scale scores were not statistically significant in either group, or between the groups.
The study subjects, who were treated for 12 months between 2008 and 2012, were at least 2 years post menarche at baseline and had a surgical diagnosis of endometriosis. The two treatment groups were similar with respect to baseline characteristics, including age, BMD Z score, and disease severity. No significant side effects were observed in either the combination or NA plus placebo group, Dr. DiVasta said, noting that laboratory tests included liver function tests and lipid levels.
Areal BMD, BMC, and body composition were measured by dual-energy X-ray absorptiometry (DXA) at baseline, 6 months, and 12 months. Anthropometrics, quality of life measures, and laboratory studies were conducted at 1, 3, 6, and 12 months.
GnRH-A are commonly utilized for endometriosis patients who fail primary therapy, such as NSAIDs or combined oral contraceptives, but long-term use is associated with deleterious effects on bone mineralization; adults have been shown to lose 5%-8% of BMD after 3-6 months of treatment, Dr. DiVasta noted.
Hormonal add-back therapy is a promising adjunct to counteract these effects and could be an important tool for protecting adolescents, who are at the greatest risk for the deleterious effects of GnRH-A therapy, she said.
In the current study, hormonal add-back did successfully protect bone health and improve quality of life for adolescents with endometriosis who were treated with 12 months of GnRH-A. Combination therapy with NA and CEE appears to be more effective for increasing total body BMC and BMD, lean mass, and physical health-related quality of life, as compared with NA monotherapy, she said.
The findings are limited by the inclusion of only skeletally mature young women, as the results may not be generalizable to growing girls. Also, DXA measures provide two-dimensional measures of bone mineral density, and do not yield information regarding skeletal strength or bone microarchitecture, she noted.
“Given the prevalence of endometriosis, our data suggest norethindrone plus estrogens to be a useful adjunctive therapy to prevent bone loss in these young women while they receive appropriate medical treatment for their underlying disease,” she concluded, noting that future work will explore the effects of add-back on the peripheral skeleton and bone strength.
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the McCarthy Family Foundation, Thrasher Research Fund, and Boston Children’s Hospital department of medicine. Medications were provided by Abbott, Duramed Pharmaceuticals, and Wyeth Pharmaceuticals. Dr. DiVasta reported having no relevant financial disclosures.
ORLANDO – Hormonal add-back therapy using combination norethindrone acetate and conjugated equine estrogens was effective and better than norethindrone acetate plus placebo for preserving bone health and improving quality of life in adolescents receiving treatment with gonadotropin-releasing hormone agonists (GnRH-A) for endometriosis in a randomized, double-blind, placebo-controlled study.
Of 51 adolescents aged 15-22 years who were initiating GnRH-A therapy for endometriosis, 25 were randomized to receive add-back therapy with 5 mg daily oral norethindrone acetate (NA) plus 0.625 mg oral daily conjugated equine estrogens (CEE), and 26 were randomized to receive NA plus placebo; 18 and 16, respectively, completed the study.
Those in the combination-therapy group experienced significant increases over 12 months in both total body bone mineral content (BMC) and bone mineral density (BMD), compared with those who received NA plus placebo. BMC increased by nearly 40 g/unit scanned in the combination-therapy group, compared with about 12 g in the NA plus placebo group, and BMD increased more than 0.01 g/cm2, compared with no change in the NA plus placebo group, Dr. Amy D. DiVasta reported at the North American Society for Pediatric and Adolescent Gynecology annual meeting.
No losses of total hip or lumbar spine BMC or BMD occurred, said Dr. DiVasta of Boston Children’s Hospital.
Further, lean mass increased in the combination-therapy group at 12 months – by about 1.4 kg, compared with no change in the placebo group. No differences were seen between the groups in change in fat mass.
As for quality of life measures, overall physical health scores at baseline were significantly below the U.S. mean in both groups, and overall mental health scores were above the U.S. mean in both groups. Both groups improved over time on both measures; the increase in the Physical Component Summary scale scores on the SF-36 (Short Form 36 Health Survey) was significantly greater with combination-therapy group (increase from about 40 to 50 out of 100 ), compared with NA plus placebo (increase from about 40 to 45), but the increases on the Mental Component Summary scale scores were not statistically significant in either group, or between the groups.
The study subjects, who were treated for 12 months between 2008 and 2012, were at least 2 years post menarche at baseline and had a surgical diagnosis of endometriosis. The two treatment groups were similar with respect to baseline characteristics, including age, BMD Z score, and disease severity. No significant side effects were observed in either the combination or NA plus placebo group, Dr. DiVasta said, noting that laboratory tests included liver function tests and lipid levels.
Areal BMD, BMC, and body composition were measured by dual-energy X-ray absorptiometry (DXA) at baseline, 6 months, and 12 months. Anthropometrics, quality of life measures, and laboratory studies were conducted at 1, 3, 6, and 12 months.
GnRH-A are commonly utilized for endometriosis patients who fail primary therapy, such as NSAIDs or combined oral contraceptives, but long-term use is associated with deleterious effects on bone mineralization; adults have been shown to lose 5%-8% of BMD after 3-6 months of treatment, Dr. DiVasta noted.
Hormonal add-back therapy is a promising adjunct to counteract these effects and could be an important tool for protecting adolescents, who are at the greatest risk for the deleterious effects of GnRH-A therapy, she said.
In the current study, hormonal add-back did successfully protect bone health and improve quality of life for adolescents with endometriosis who were treated with 12 months of GnRH-A. Combination therapy with NA and CEE appears to be more effective for increasing total body BMC and BMD, lean mass, and physical health-related quality of life, as compared with NA monotherapy, she said.
The findings are limited by the inclusion of only skeletally mature young women, as the results may not be generalizable to growing girls. Also, DXA measures provide two-dimensional measures of bone mineral density, and do not yield information regarding skeletal strength or bone microarchitecture, she noted.
“Given the prevalence of endometriosis, our data suggest norethindrone plus estrogens to be a useful adjunctive therapy to prevent bone loss in these young women while they receive appropriate medical treatment for their underlying disease,” she concluded, noting that future work will explore the effects of add-back on the peripheral skeleton and bone strength.
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the McCarthy Family Foundation, Thrasher Research Fund, and Boston Children’s Hospital department of medicine. Medications were provided by Abbott, Duramed Pharmaceuticals, and Wyeth Pharmaceuticals. Dr. DiVasta reported having no relevant financial disclosures.
AT THE NASPAG ANNUAL MEETING
Key clinical point: Add-back therapy could allow for safe long-term GnRH-A therapy in adolescents with endometriosis.
Major finding: Bone mineral content increased nearly 40 g/unit scanned in the combination therapy group vs. about 12 g in the norethindrone acetate plus placebo group.
Data source: A randomized, placebo-controlled study of 51 adolescents.
Disclosures: This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the McCarthy Family Foundation, Thrasher Research Fund, and Boston Children’s Hospital department of medicine. Medications were provided by Abbott, Duramed Pharmaceuticals, and Wyeth Pharmaceuticals. Dr. DiVasta reported having no relevant financial disclosures.
VIDEO: Patients with female genital cutting experience inadequate care
CHICAGO– Inappropriate treatment by physicians of women who have undergone female genital cutting (FGC) can block access to further care and harm patients psychologically, according to Dr. Nawal M. Nour, director of the ambulatory obstetrics practice at Brigham and Women’s Hospital and founder of the African Women’s Health Center in Boston.
Doctors who are unfamiliar with patients who have FGC can say or react to patients in ways that harms, rather than helps such women, Dr. Nour said at the annual meeting of the American Medical Women’s Association. Dr. Nour is the lead author of Female Genital Cutting: Clinical Management of Circumcised Women, published by the American Congress of Obstetricians and Gynecologists.
During her presentation, Dr. Nour shared cases in which doctors made teaching examples out of FGC patients, provided inaccurate information about vaginal deliveries, and focused on the FGC procedure, rather the woman’s reason for seeking medical attention.
In this video, Dr. Nour shares some common ways that physicians inappropriately respond to women who have undergone FGC, and how doctors can act more sensitively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
CHICAGO– Inappropriate treatment by physicians of women who have undergone female genital cutting (FGC) can block access to further care and harm patients psychologically, according to Dr. Nawal M. Nour, director of the ambulatory obstetrics practice at Brigham and Women’s Hospital and founder of the African Women’s Health Center in Boston.
Doctors who are unfamiliar with patients who have FGC can say or react to patients in ways that harms, rather than helps such women, Dr. Nour said at the annual meeting of the American Medical Women’s Association. Dr. Nour is the lead author of Female Genital Cutting: Clinical Management of Circumcised Women, published by the American Congress of Obstetricians and Gynecologists.
During her presentation, Dr. Nour shared cases in which doctors made teaching examples out of FGC patients, provided inaccurate information about vaginal deliveries, and focused on the FGC procedure, rather the woman’s reason for seeking medical attention.
In this video, Dr. Nour shares some common ways that physicians inappropriately respond to women who have undergone FGC, and how doctors can act more sensitively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
CHICAGO– Inappropriate treatment by physicians of women who have undergone female genital cutting (FGC) can block access to further care and harm patients psychologically, according to Dr. Nawal M. Nour, director of the ambulatory obstetrics practice at Brigham and Women’s Hospital and founder of the African Women’s Health Center in Boston.
Doctors who are unfamiliar with patients who have FGC can say or react to patients in ways that harms, rather than helps such women, Dr. Nour said at the annual meeting of the American Medical Women’s Association. Dr. Nour is the lead author of Female Genital Cutting: Clinical Management of Circumcised Women, published by the American Congress of Obstetricians and Gynecologists.
During her presentation, Dr. Nour shared cases in which doctors made teaching examples out of FGC patients, provided inaccurate information about vaginal deliveries, and focused on the FGC procedure, rather the woman’s reason for seeking medical attention.
In this video, Dr. Nour shares some common ways that physicians inappropriately respond to women who have undergone FGC, and how doctors can act more sensitively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
AT THE AMWA ANNUAL MEETING
Circulating tumor cells prognostic in advanced cervical cancer
CHICAGO – Circulating tumor cells are a prognostic biomarker for overall survival in women with advanced cervical cancer who are receiving chemotherapy with or without bevacizumab, according to an analysis of the Gynecologic Oncology Group’s 240 trial.
The risk of death among patients given cisplatin-paclitaxel plus bevacizumab was reduced by 10% for those who had a higher pretreatment level of circulating tumor cells (CTCs) and by 13% for those who had a greater treatment-related decline in CTCs, Dr. Krishnansu S. Tewari reported at the annual meeting of the Society of Gynecologic Oncology.
“We can predict that leaky vasculature resulting from tumor angiogenesis may permit systemic distribution of CTCs through intratumoral vascular shunting, and the vulnerability to anti-angiogenesis therapies may be due to this increased vascularization,” said Dr. Tewari of UC Irvine Health in Orange, Calif.
In comments provided by e-mail, session comoderator Dr. Charles N. Landen Jr. of the University of Virginia, Charlottesville, said that the study is important in that it demonstrates the feasibility of using CTCs as a biomarker in this setting, but its results are not practice changing.
“If you had a decrease in CTCs, you were more likely to have a good response to therapy. However, this was not any better at detecting response than conventional methods such as a CT scan,” he explained.
Giving some background to the research, Dr. Tewari said that “it’s difficult to monitor response in patients with advanced cervical cancer. Doing imaging studies after every two cycles is expensive, and there are no validated serum tumor markers in this disease. In addition, tumor is not often accessible for interrogation to help guide subsequent therapy when patients ultimately progress on anti-VEGF [vascular endothelial growth factor] therapy. Finally, predictive biomarkers are lacking in advanced cervical cancer.”
“CTCs can be seen as minimally invasive liquid biopsies, and their presence in breast cancer, prostate cancer, and non–small cell lung cancer has been correlated with survival,” he noted. “However, all three of those solid tumors are known to spread hematogenously. While hematogenous spread can occur in cervical cancer, it is felt to be rare.”
The phase III trial enrolled women with recurrent, persistent, or metastatic cervical cancer and tested the addition of bevacizumab (Avastin, manufactured by Genentech/Roche) to doublet chemotherapy (cisplatin-paclitaxel or topotecan-paclitaxel) given on 21-day cycles. Main results showed a significant gain in overall survival from the addition of bevacizumab (N. Engl. J. Med. 2014;370:734-43), leading to regulatory approval of combination therapy in the United States and elsewhere.
The new analysis focused on the 174 women (39%) in the trial who had 8.5 mL of whole blood collected for CTC measurement before starting the first cycle of therapy and, in most cases, again 36 days after the first cycle.
The investigators were able to identify CTCs for 97% of patients in the pretreatment sample and for 81% in the post-treatment sample, Dr. Tewari noted.
Results showed that the median CTC count in the sample fell from seven cells pretreatment to four cells post-treatment (P < .0001). Of note, there was no correlation with the response to treatment according to RECIST (Response Evaluation Criteria in Solid Tumors).
Patients with high (above-median) pretreatment levels of CTCs had significantly better median progression-free survival with bevacizumab than without it (10.8 vs. 6.9 months; hazard ratio [HR], 0.59). In contrast, there was no significant benefit for patients with low (below-median) pretreatment levels of CTCs (7.3 and 6.2 months).
Among patients given cisplatin-paclitaxel plus bevacizumab, the higher the pretreatment CTC level, the lower the risk of death (HR, 0.90). Among patients given cisplatin-paclitaxel overall, the higher the post-treatment CTC level, the greater the risk of death (HR, 1.12), Dr. Tewari reported.
Finally, the greater the decline in CTCs from before to after treatment in patients given cisplatin-paclitaxel plus bevacizumab, the lower the risk of death (HR, 0.87), he said.
Dr. Tewari disclosed that he is a consultant to Genentech/Roche, Advaxis, and Caris Life Sciences; receives honoraria/reimbursement or grants from Genentech/Roche; and performs research for Caris Life Sciences.
CHICAGO – Circulating tumor cells are a prognostic biomarker for overall survival in women with advanced cervical cancer who are receiving chemotherapy with or without bevacizumab, according to an analysis of the Gynecologic Oncology Group’s 240 trial.
The risk of death among patients given cisplatin-paclitaxel plus bevacizumab was reduced by 10% for those who had a higher pretreatment level of circulating tumor cells (CTCs) and by 13% for those who had a greater treatment-related decline in CTCs, Dr. Krishnansu S. Tewari reported at the annual meeting of the Society of Gynecologic Oncology.
“We can predict that leaky vasculature resulting from tumor angiogenesis may permit systemic distribution of CTCs through intratumoral vascular shunting, and the vulnerability to anti-angiogenesis therapies may be due to this increased vascularization,” said Dr. Tewari of UC Irvine Health in Orange, Calif.
In comments provided by e-mail, session comoderator Dr. Charles N. Landen Jr. of the University of Virginia, Charlottesville, said that the study is important in that it demonstrates the feasibility of using CTCs as a biomarker in this setting, but its results are not practice changing.
“If you had a decrease in CTCs, you were more likely to have a good response to therapy. However, this was not any better at detecting response than conventional methods such as a CT scan,” he explained.
Giving some background to the research, Dr. Tewari said that “it’s difficult to monitor response in patients with advanced cervical cancer. Doing imaging studies after every two cycles is expensive, and there are no validated serum tumor markers in this disease. In addition, tumor is not often accessible for interrogation to help guide subsequent therapy when patients ultimately progress on anti-VEGF [vascular endothelial growth factor] therapy. Finally, predictive biomarkers are lacking in advanced cervical cancer.”
“CTCs can be seen as minimally invasive liquid biopsies, and their presence in breast cancer, prostate cancer, and non–small cell lung cancer has been correlated with survival,” he noted. “However, all three of those solid tumors are known to spread hematogenously. While hematogenous spread can occur in cervical cancer, it is felt to be rare.”
The phase III trial enrolled women with recurrent, persistent, or metastatic cervical cancer and tested the addition of bevacizumab (Avastin, manufactured by Genentech/Roche) to doublet chemotherapy (cisplatin-paclitaxel or topotecan-paclitaxel) given on 21-day cycles. Main results showed a significant gain in overall survival from the addition of bevacizumab (N. Engl. J. Med. 2014;370:734-43), leading to regulatory approval of combination therapy in the United States and elsewhere.
The new analysis focused on the 174 women (39%) in the trial who had 8.5 mL of whole blood collected for CTC measurement before starting the first cycle of therapy and, in most cases, again 36 days after the first cycle.
The investigators were able to identify CTCs for 97% of patients in the pretreatment sample and for 81% in the post-treatment sample, Dr. Tewari noted.
Results showed that the median CTC count in the sample fell from seven cells pretreatment to four cells post-treatment (P < .0001). Of note, there was no correlation with the response to treatment according to RECIST (Response Evaluation Criteria in Solid Tumors).
Patients with high (above-median) pretreatment levels of CTCs had significantly better median progression-free survival with bevacizumab than without it (10.8 vs. 6.9 months; hazard ratio [HR], 0.59). In contrast, there was no significant benefit for patients with low (below-median) pretreatment levels of CTCs (7.3 and 6.2 months).
Among patients given cisplatin-paclitaxel plus bevacizumab, the higher the pretreatment CTC level, the lower the risk of death (HR, 0.90). Among patients given cisplatin-paclitaxel overall, the higher the post-treatment CTC level, the greater the risk of death (HR, 1.12), Dr. Tewari reported.
Finally, the greater the decline in CTCs from before to after treatment in patients given cisplatin-paclitaxel plus bevacizumab, the lower the risk of death (HR, 0.87), he said.
Dr. Tewari disclosed that he is a consultant to Genentech/Roche, Advaxis, and Caris Life Sciences; receives honoraria/reimbursement or grants from Genentech/Roche; and performs research for Caris Life Sciences.
CHICAGO – Circulating tumor cells are a prognostic biomarker for overall survival in women with advanced cervical cancer who are receiving chemotherapy with or without bevacizumab, according to an analysis of the Gynecologic Oncology Group’s 240 trial.
The risk of death among patients given cisplatin-paclitaxel plus bevacizumab was reduced by 10% for those who had a higher pretreatment level of circulating tumor cells (CTCs) and by 13% for those who had a greater treatment-related decline in CTCs, Dr. Krishnansu S. Tewari reported at the annual meeting of the Society of Gynecologic Oncology.
“We can predict that leaky vasculature resulting from tumor angiogenesis may permit systemic distribution of CTCs through intratumoral vascular shunting, and the vulnerability to anti-angiogenesis therapies may be due to this increased vascularization,” said Dr. Tewari of UC Irvine Health in Orange, Calif.
In comments provided by e-mail, session comoderator Dr. Charles N. Landen Jr. of the University of Virginia, Charlottesville, said that the study is important in that it demonstrates the feasibility of using CTCs as a biomarker in this setting, but its results are not practice changing.
“If you had a decrease in CTCs, you were more likely to have a good response to therapy. However, this was not any better at detecting response than conventional methods such as a CT scan,” he explained.
Giving some background to the research, Dr. Tewari said that “it’s difficult to monitor response in patients with advanced cervical cancer. Doing imaging studies after every two cycles is expensive, and there are no validated serum tumor markers in this disease. In addition, tumor is not often accessible for interrogation to help guide subsequent therapy when patients ultimately progress on anti-VEGF [vascular endothelial growth factor] therapy. Finally, predictive biomarkers are lacking in advanced cervical cancer.”
“CTCs can be seen as minimally invasive liquid biopsies, and their presence in breast cancer, prostate cancer, and non–small cell lung cancer has been correlated with survival,” he noted. “However, all three of those solid tumors are known to spread hematogenously. While hematogenous spread can occur in cervical cancer, it is felt to be rare.”
The phase III trial enrolled women with recurrent, persistent, or metastatic cervical cancer and tested the addition of bevacizumab (Avastin, manufactured by Genentech/Roche) to doublet chemotherapy (cisplatin-paclitaxel or topotecan-paclitaxel) given on 21-day cycles. Main results showed a significant gain in overall survival from the addition of bevacizumab (N. Engl. J. Med. 2014;370:734-43), leading to regulatory approval of combination therapy in the United States and elsewhere.
The new analysis focused on the 174 women (39%) in the trial who had 8.5 mL of whole blood collected for CTC measurement before starting the first cycle of therapy and, in most cases, again 36 days after the first cycle.
The investigators were able to identify CTCs for 97% of patients in the pretreatment sample and for 81% in the post-treatment sample, Dr. Tewari noted.
Results showed that the median CTC count in the sample fell from seven cells pretreatment to four cells post-treatment (P < .0001). Of note, there was no correlation with the response to treatment according to RECIST (Response Evaluation Criteria in Solid Tumors).
Patients with high (above-median) pretreatment levels of CTCs had significantly better median progression-free survival with bevacizumab than without it (10.8 vs. 6.9 months; hazard ratio [HR], 0.59). In contrast, there was no significant benefit for patients with low (below-median) pretreatment levels of CTCs (7.3 and 6.2 months).
Among patients given cisplatin-paclitaxel plus bevacizumab, the higher the pretreatment CTC level, the lower the risk of death (HR, 0.90). Among patients given cisplatin-paclitaxel overall, the higher the post-treatment CTC level, the greater the risk of death (HR, 1.12), Dr. Tewari reported.
Finally, the greater the decline in CTCs from before to after treatment in patients given cisplatin-paclitaxel plus bevacizumab, the lower the risk of death (HR, 0.87), he said.
Dr. Tewari disclosed that he is a consultant to Genentech/Roche, Advaxis, and Caris Life Sciences; receives honoraria/reimbursement or grants from Genentech/Roche; and performs research for Caris Life Sciences.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: CTCs provide prognostic information in women being treated for advanced cervical cancer.
Major finding: Risk of death for patients given cisplatin-paclitaxel plus bevacizumab was reduced by 10% for those who had a high pretreatment level of CTCs and by 13% for those who had a greater treatment-related decline in CTCs.
Data source: Analysis of 174 women with advanced cervical cancer given chemotherapy with or without bevacizumab in a phase III trial.
Disclosures: Dr. Tewari disclosed that he is a consultant to Genentech/Roche, Advaxis, and Caris Life Sciences; receives honoraria/reimbursement or grants from Genentech/Roche; and performs research for Caris Life Sciences.
Sentinel node mapping adequately detects nodal spread of endometrial cancer
CHICAGO – Sentinel lymph node mapping is a safe, less extensive alternative to lymph node dissection in women with endometrial cancer regardless of the clinically suspected risk of metastases, suggest a pair of retrospective cohort studies reported at the annual meeting of the Society of Gynecologic Oncology.
“The role and extent of surgical staging in endometrial carcinoma is controversial. The various strategies range from no lymphadenectomy to a full lymphadenectomy dissection up to the renal vessels,” said Dr. Ane Gerda Zahl Eriksson, a surgical fellow in gynecologic oncology at the Memorial Sloan Kettering Cancer Center, New York.
“The use of SLN [sentinel lymph node] mapping is emerging as an acceptable approach for nodal assessment in endometrial carcinoma. However, as always when introducing a novel management strategy, we must be mindful not to compromise oncologic outcome or otherwise inflict harm on our patients by our approach,” she added.
Dr. Eriksson and colleagues assessed clinicopathologic outcomes according to nodal assessment approach among women with low-risk endometrial cancer, defined as that with endometrioid histology of any grade with myometrial invasion of less than 50%.
They compared 493 women who had selective lymph node dissection (LND) at the Mayo Clinic between 2004 and 2008 according to an institutional algorithm (complete pelvic and para-aortic lymphadenectomy to the renal veins in cases deemed at risk for nodal metastasis) with 642 women who had SLN mapping at the Memorial Sloan Kettering Cancer Center between 2006 and 2013 according to an institutional algorithm. The SLNs were evaluated by pathologic ultrastaging and were considered positive whether they had macrometastases, micrometastases, or isolated tumor cells.
Results showed that patients in the SLN cohort were more likely to have pelvic nodes excised (93% vs. 58%) and less likely to have para-aortic nodes excised (14% vs. 50%), Dr. Eriksson reported.
Markedly fewer lymph nodes were removed per patient with the SLN algorithm, yet it yielded a higher detection rate of stage IIIC1 disease (4.8% vs. 1.8%) and similar detection rates of stage IIIA/B and stage IIIC2 disease.
Patients in the SLN cohort were more than twice as likely to receive adjuvant therapy (27% vs. 12%). (Dr. Eriksson noted that patients in the SLN cohort who had positive nodes were offered the same adjuvant therapy options regardless of the amount of metastases found in the nodes.)
With a median follow-up of 2.1 years in the SLN cohort and 3.5 years in the LND cohort, the cohorts had statistically indistinguishable 3-year rates of freedom from isolated nodal recurrence (99.6% in each) and disease-free survival (94.9% and 96.8%).
“The application of the SLN algorithm does not appear to compromise oncologic outcome in this short follow-up. The value of SLN dissection or selective lymphadenectomy in patients with tumors equal to or less than 2 cm remains controversial,” Dr. Eriksson commented. “The clinical significance of disease detected on ultrastaging and the role of adjuvant therapy in these patients remains to be determined.”
“Prospective assessment of the SLN algorithm is needed and underway,” she concluded. “Our findings support the use of either strategy for endometrial cancer staging with no apparent detriment to the SLN algorithm.”
In the second study, investigators led by Dr. Jennifer A. Ducie, also a surgical fellow in gynecologic oncology at the Memorial Sloan Kettering Cancer Center, performed a similar analysis among women with intermediate-risk endometrial cancer, defined as that having endometrioid histology with any grade and at least 50% myometrial invasion, and high-risk endometrial cancer, defined as serous or clear cell carcinoma.
In the intermediate-risk group, there were 82 patients in the SLN cohort and 107 patients in the LND cohort. The groups had a similar detection rate of stage IIIC disease overall (35% and 28%), but the SLN cohort had a higher rate of detection of stage IIIC1 disease (32% vs. 11%) and the LND cohort had a higher rate of detection of stage IIIC2 disease (17% vs. 4%), Dr. Ducie reported.
In the high-risk group, there were 120 patients in the SLN cohort and 103 patients in the LND cohort. Patients in the SLN cohort were more likely to have pelvic nodes removed (98% vs. 85%) but had fewer nodes removed (11 vs. 30). Among patients who had para-aortic nodes removed, the SLN cohort was similarly as likely as the LND cohort to have positive nodes, but had a smaller median number positive (two vs. five). The rate of detection of stage IIIC disease overall (23% vs. 19%) and of the substages was statistically indistinguishable.
“Though both strategies yield similar detection rates of stage IIIC disease, it remains to be determined if removal of more normal-appearing lymph nodes will improve survival,” Dr. Ducie concluded. “A limitation of this portion of our collaborative study is that we don’t address adjuvant therapy or outcomes, but these will be addressed in future analyses.”
CHICAGO – Sentinel lymph node mapping is a safe, less extensive alternative to lymph node dissection in women with endometrial cancer regardless of the clinically suspected risk of metastases, suggest a pair of retrospective cohort studies reported at the annual meeting of the Society of Gynecologic Oncology.
“The role and extent of surgical staging in endometrial carcinoma is controversial. The various strategies range from no lymphadenectomy to a full lymphadenectomy dissection up to the renal vessels,” said Dr. Ane Gerda Zahl Eriksson, a surgical fellow in gynecologic oncology at the Memorial Sloan Kettering Cancer Center, New York.
“The use of SLN [sentinel lymph node] mapping is emerging as an acceptable approach for nodal assessment in endometrial carcinoma. However, as always when introducing a novel management strategy, we must be mindful not to compromise oncologic outcome or otherwise inflict harm on our patients by our approach,” she added.
Dr. Eriksson and colleagues assessed clinicopathologic outcomes according to nodal assessment approach among women with low-risk endometrial cancer, defined as that with endometrioid histology of any grade with myometrial invasion of less than 50%.
They compared 493 women who had selective lymph node dissection (LND) at the Mayo Clinic between 2004 and 2008 according to an institutional algorithm (complete pelvic and para-aortic lymphadenectomy to the renal veins in cases deemed at risk for nodal metastasis) with 642 women who had SLN mapping at the Memorial Sloan Kettering Cancer Center between 2006 and 2013 according to an institutional algorithm. The SLNs were evaluated by pathologic ultrastaging and were considered positive whether they had macrometastases, micrometastases, or isolated tumor cells.
Results showed that patients in the SLN cohort were more likely to have pelvic nodes excised (93% vs. 58%) and less likely to have para-aortic nodes excised (14% vs. 50%), Dr. Eriksson reported.
Markedly fewer lymph nodes were removed per patient with the SLN algorithm, yet it yielded a higher detection rate of stage IIIC1 disease (4.8% vs. 1.8%) and similar detection rates of stage IIIA/B and stage IIIC2 disease.
Patients in the SLN cohort were more than twice as likely to receive adjuvant therapy (27% vs. 12%). (Dr. Eriksson noted that patients in the SLN cohort who had positive nodes were offered the same adjuvant therapy options regardless of the amount of metastases found in the nodes.)
With a median follow-up of 2.1 years in the SLN cohort and 3.5 years in the LND cohort, the cohorts had statistically indistinguishable 3-year rates of freedom from isolated nodal recurrence (99.6% in each) and disease-free survival (94.9% and 96.8%).
“The application of the SLN algorithm does not appear to compromise oncologic outcome in this short follow-up. The value of SLN dissection or selective lymphadenectomy in patients with tumors equal to or less than 2 cm remains controversial,” Dr. Eriksson commented. “The clinical significance of disease detected on ultrastaging and the role of adjuvant therapy in these patients remains to be determined.”
“Prospective assessment of the SLN algorithm is needed and underway,” she concluded. “Our findings support the use of either strategy for endometrial cancer staging with no apparent detriment to the SLN algorithm.”
In the second study, investigators led by Dr. Jennifer A. Ducie, also a surgical fellow in gynecologic oncology at the Memorial Sloan Kettering Cancer Center, performed a similar analysis among women with intermediate-risk endometrial cancer, defined as that having endometrioid histology with any grade and at least 50% myometrial invasion, and high-risk endometrial cancer, defined as serous or clear cell carcinoma.
In the intermediate-risk group, there were 82 patients in the SLN cohort and 107 patients in the LND cohort. The groups had a similar detection rate of stage IIIC disease overall (35% and 28%), but the SLN cohort had a higher rate of detection of stage IIIC1 disease (32% vs. 11%) and the LND cohort had a higher rate of detection of stage IIIC2 disease (17% vs. 4%), Dr. Ducie reported.
In the high-risk group, there were 120 patients in the SLN cohort and 103 patients in the LND cohort. Patients in the SLN cohort were more likely to have pelvic nodes removed (98% vs. 85%) but had fewer nodes removed (11 vs. 30). Among patients who had para-aortic nodes removed, the SLN cohort was similarly as likely as the LND cohort to have positive nodes, but had a smaller median number positive (two vs. five). The rate of detection of stage IIIC disease overall (23% vs. 19%) and of the substages was statistically indistinguishable.
“Though both strategies yield similar detection rates of stage IIIC disease, it remains to be determined if removal of more normal-appearing lymph nodes will improve survival,” Dr. Ducie concluded. “A limitation of this portion of our collaborative study is that we don’t address adjuvant therapy or outcomes, but these will be addressed in future analyses.”
CHICAGO – Sentinel lymph node mapping is a safe, less extensive alternative to lymph node dissection in women with endometrial cancer regardless of the clinically suspected risk of metastases, suggest a pair of retrospective cohort studies reported at the annual meeting of the Society of Gynecologic Oncology.
“The role and extent of surgical staging in endometrial carcinoma is controversial. The various strategies range from no lymphadenectomy to a full lymphadenectomy dissection up to the renal vessels,” said Dr. Ane Gerda Zahl Eriksson, a surgical fellow in gynecologic oncology at the Memorial Sloan Kettering Cancer Center, New York.
“The use of SLN [sentinel lymph node] mapping is emerging as an acceptable approach for nodal assessment in endometrial carcinoma. However, as always when introducing a novel management strategy, we must be mindful not to compromise oncologic outcome or otherwise inflict harm on our patients by our approach,” she added.
Dr. Eriksson and colleagues assessed clinicopathologic outcomes according to nodal assessment approach among women with low-risk endometrial cancer, defined as that with endometrioid histology of any grade with myometrial invasion of less than 50%.
They compared 493 women who had selective lymph node dissection (LND) at the Mayo Clinic between 2004 and 2008 according to an institutional algorithm (complete pelvic and para-aortic lymphadenectomy to the renal veins in cases deemed at risk for nodal metastasis) with 642 women who had SLN mapping at the Memorial Sloan Kettering Cancer Center between 2006 and 2013 according to an institutional algorithm. The SLNs were evaluated by pathologic ultrastaging and were considered positive whether they had macrometastases, micrometastases, or isolated tumor cells.
Results showed that patients in the SLN cohort were more likely to have pelvic nodes excised (93% vs. 58%) and less likely to have para-aortic nodes excised (14% vs. 50%), Dr. Eriksson reported.
Markedly fewer lymph nodes were removed per patient with the SLN algorithm, yet it yielded a higher detection rate of stage IIIC1 disease (4.8% vs. 1.8%) and similar detection rates of stage IIIA/B and stage IIIC2 disease.
Patients in the SLN cohort were more than twice as likely to receive adjuvant therapy (27% vs. 12%). (Dr. Eriksson noted that patients in the SLN cohort who had positive nodes were offered the same adjuvant therapy options regardless of the amount of metastases found in the nodes.)
With a median follow-up of 2.1 years in the SLN cohort and 3.5 years in the LND cohort, the cohorts had statistically indistinguishable 3-year rates of freedom from isolated nodal recurrence (99.6% in each) and disease-free survival (94.9% and 96.8%).
“The application of the SLN algorithm does not appear to compromise oncologic outcome in this short follow-up. The value of SLN dissection or selective lymphadenectomy in patients with tumors equal to or less than 2 cm remains controversial,” Dr. Eriksson commented. “The clinical significance of disease detected on ultrastaging and the role of adjuvant therapy in these patients remains to be determined.”
“Prospective assessment of the SLN algorithm is needed and underway,” she concluded. “Our findings support the use of either strategy for endometrial cancer staging with no apparent detriment to the SLN algorithm.”
In the second study, investigators led by Dr. Jennifer A. Ducie, also a surgical fellow in gynecologic oncology at the Memorial Sloan Kettering Cancer Center, performed a similar analysis among women with intermediate-risk endometrial cancer, defined as that having endometrioid histology with any grade and at least 50% myometrial invasion, and high-risk endometrial cancer, defined as serous or clear cell carcinoma.
In the intermediate-risk group, there were 82 patients in the SLN cohort and 107 patients in the LND cohort. The groups had a similar detection rate of stage IIIC disease overall (35% and 28%), but the SLN cohort had a higher rate of detection of stage IIIC1 disease (32% vs. 11%) and the LND cohort had a higher rate of detection of stage IIIC2 disease (17% vs. 4%), Dr. Ducie reported.
In the high-risk group, there were 120 patients in the SLN cohort and 103 patients in the LND cohort. Patients in the SLN cohort were more likely to have pelvic nodes removed (98% vs. 85%) but had fewer nodes removed (11 vs. 30). Among patients who had para-aortic nodes removed, the SLN cohort was similarly as likely as the LND cohort to have positive nodes, but had a smaller median number positive (two vs. five). The rate of detection of stage IIIC disease overall (23% vs. 19%) and of the substages was statistically indistinguishable.
“Though both strategies yield similar detection rates of stage IIIC disease, it remains to be determined if removal of more normal-appearing lymph nodes will improve survival,” Dr. Ducie concluded. “A limitation of this portion of our collaborative study is that we don’t address adjuvant therapy or outcomes, but these will be addressed in future analyses.”
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: SLN mapping performs similarly to LND for detecting stage IIIC disease in women with endometrial cancer.
Major finding: SLN mapping had a higher detection rate of stage IIIC1 disease in low-risk disease (4.8% vs. 1.8%) and a similar detection rate of stage IIIC disease overall in intermediate- and high-risk disease.
Data source: Two retrospective cohort studies of 1,135 women with low-risk endometrial cancer and 412 women with intermediate- or high-risk endometrial cancer.
Disclosures: Dr. Eriksson disclosed that she had no relevant conflicts of interest. Dr. Ducie disclosed that she had no relevant conflicts of interest.