User login
Product Update: Vistara; Ultravision trocar; CompuFlo Epidural; and Philips ultrasound
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
2018 Update on gynecologic cancer
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
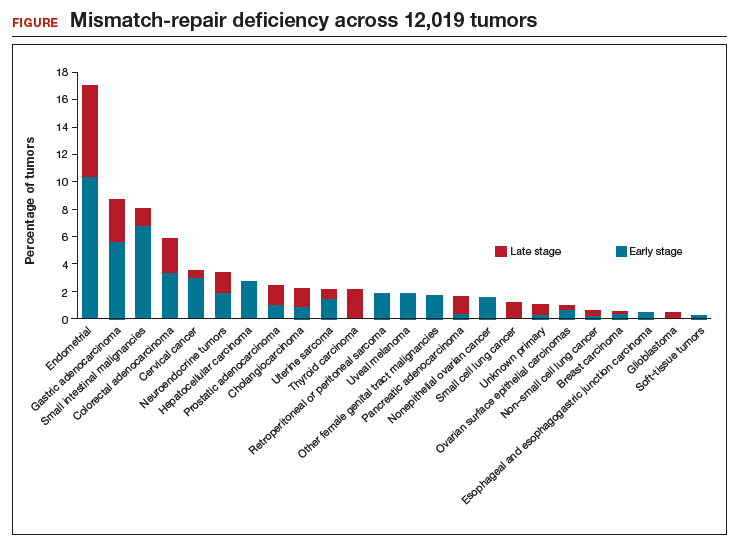
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
Hidradenitis suppurativa: An underdiagnosed skin problem of women
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
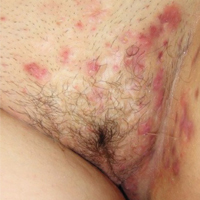
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9
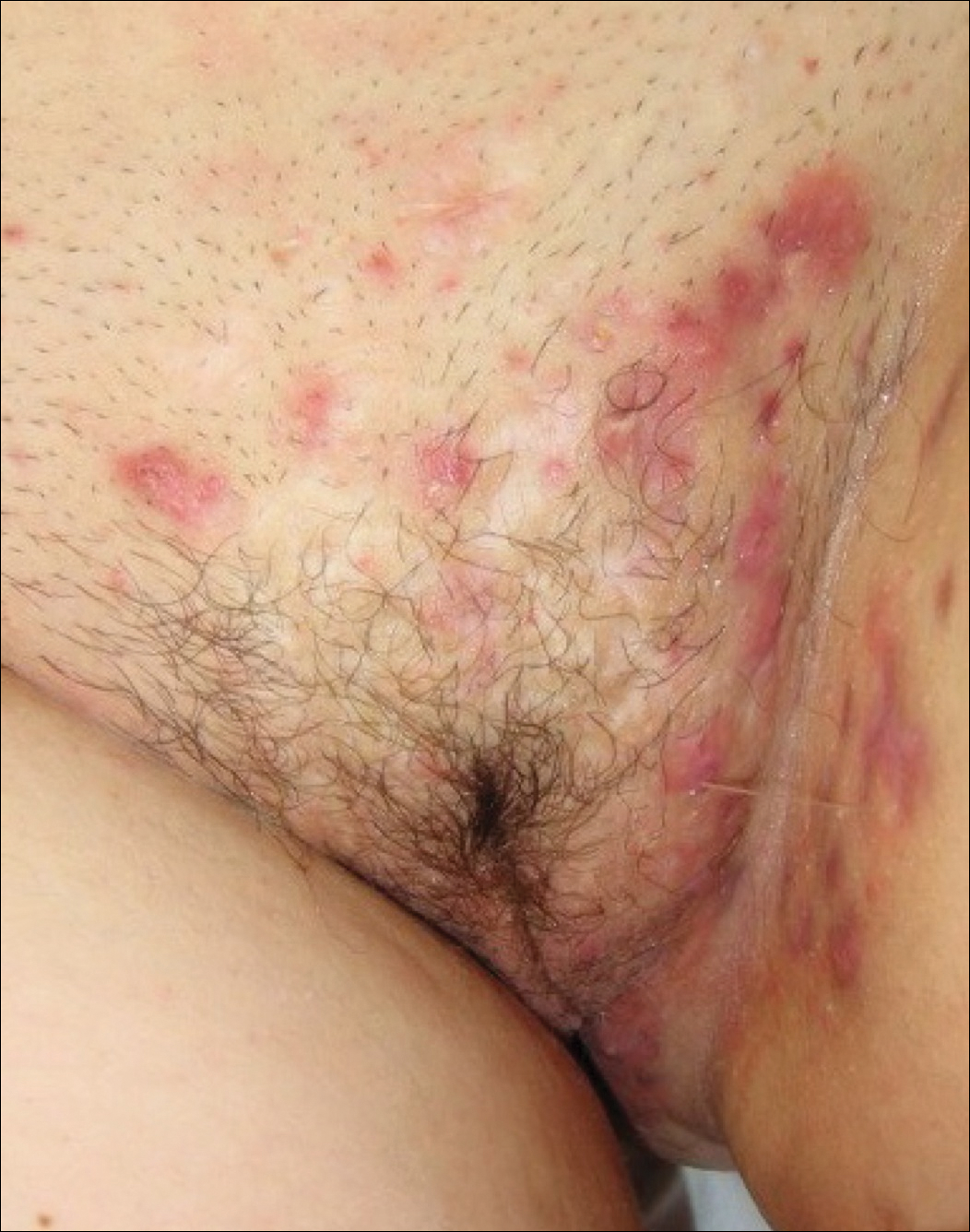
HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9

HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9

HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
ACOG app and applets: Tools to augment your practice
The American College of Obstetricians and Gynecologists (ACOG) is a nonprofit organization of women’s health care physicians advocating the highest standards of practice, continuing member education, and public awareness of women’s health care issues.1 The organization has long recognized the impact that social media and mobile technology would have for itself as well as its membership. ACOG published a Social Media Guide in 2012, featuring a section on how to use apps in ObGyn practice and provided a list of apps for ObGyns and their patients.2
ACOG introduced its own app 4 years ago and has since updated the app several times, most recently on December 6, 2017. The ACOG app has a useful search function, a home button, and a place for users to email feedback (TABLE 1). The app most importantly contains several applets (small applications designed to perform a specific function within the main application). These applets encompass 3 types of apps for health care providers: clinical decision-making apps (Practice Bulletins, Committee Opinions, an Estimated Due Date Calculator that was featured in a prior review,3 Indicated Delivery, and Immunize) (TABLE 2), reference and information gathering apps (Today’s Headlines), and member support apps (ACOG Contacts, Careers, Annual Meeting, Districts, Council on Resident Education in Obstetrics and Gynecology [CREOG], and Website).4
This review will focus on the main ACOG app, which is evaluated by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).5 In addition, the clinical decision-making applets will be highlighted in a second table. I commend ACOG for developing these useful tools to augment their members’ practices. Of note, for the Practice Bulletins and Indicated Delivery applets, users will need to input their ACOG log-in access information.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- The American College of Obstetricians and Gynecologists web site. https://www.acog.org/About-ACOG. Updated 2017. Accessed February 12, 2018.
- ACOG today. The American College of Obstetricians and Gynecologists https://www.acog.org/-/media/ACOG-Today /acogToday201211.pdf. Published November 2012. Accessed February 12, 2018.
- Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29(1):45–46.
- Ventola CL. Mobile devices and apps for health care professionals: Uses and benefits. P T. 2014;39(5):356–364.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
The American College of Obstetricians and Gynecologists (ACOG) is a nonprofit organization of women’s health care physicians advocating the highest standards of practice, continuing member education, and public awareness of women’s health care issues.1 The organization has long recognized the impact that social media and mobile technology would have for itself as well as its membership. ACOG published a Social Media Guide in 2012, featuring a section on how to use apps in ObGyn practice and provided a list of apps for ObGyns and their patients.2
ACOG introduced its own app 4 years ago and has since updated the app several times, most recently on December 6, 2017. The ACOG app has a useful search function, a home button, and a place for users to email feedback (TABLE 1). The app most importantly contains several applets (small applications designed to perform a specific function within the main application). These applets encompass 3 types of apps for health care providers: clinical decision-making apps (Practice Bulletins, Committee Opinions, an Estimated Due Date Calculator that was featured in a prior review,3 Indicated Delivery, and Immunize) (TABLE 2), reference and information gathering apps (Today’s Headlines), and member support apps (ACOG Contacts, Careers, Annual Meeting, Districts, Council on Resident Education in Obstetrics and Gynecology [CREOG], and Website).4
This review will focus on the main ACOG app, which is evaluated by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).5 In addition, the clinical decision-making applets will be highlighted in a second table. I commend ACOG for developing these useful tools to augment their members’ practices. Of note, for the Practice Bulletins and Indicated Delivery applets, users will need to input their ACOG log-in access information.


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The American College of Obstetricians and Gynecologists (ACOG) is a nonprofit organization of women’s health care physicians advocating the highest standards of practice, continuing member education, and public awareness of women’s health care issues.1 The organization has long recognized the impact that social media and mobile technology would have for itself as well as its membership. ACOG published a Social Media Guide in 2012, featuring a section on how to use apps in ObGyn practice and provided a list of apps for ObGyns and their patients.2
ACOG introduced its own app 4 years ago and has since updated the app several times, most recently on December 6, 2017. The ACOG app has a useful search function, a home button, and a place for users to email feedback (TABLE 1). The app most importantly contains several applets (small applications designed to perform a specific function within the main application). These applets encompass 3 types of apps for health care providers: clinical decision-making apps (Practice Bulletins, Committee Opinions, an Estimated Due Date Calculator that was featured in a prior review,3 Indicated Delivery, and Immunize) (TABLE 2), reference and information gathering apps (Today’s Headlines), and member support apps (ACOG Contacts, Careers, Annual Meeting, Districts, Council on Resident Education in Obstetrics and Gynecology [CREOG], and Website).4
This review will focus on the main ACOG app, which is evaluated by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).5 In addition, the clinical decision-making applets will be highlighted in a second table. I commend ACOG for developing these useful tools to augment their members’ practices. Of note, for the Practice Bulletins and Indicated Delivery applets, users will need to input their ACOG log-in access information.


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- The American College of Obstetricians and Gynecologists web site. https://www.acog.org/About-ACOG. Updated 2017. Accessed February 12, 2018.
- ACOG today. The American College of Obstetricians and Gynecologists https://www.acog.org/-/media/ACOG-Today /acogToday201211.pdf. Published November 2012. Accessed February 12, 2018.
- Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29(1):45–46.
- Ventola CL. Mobile devices and apps for health care professionals: Uses and benefits. P T. 2014;39(5):356–364.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
- The American College of Obstetricians and Gynecologists web site. https://www.acog.org/About-ACOG. Updated 2017. Accessed February 12, 2018.
- ACOG today. The American College of Obstetricians and Gynecologists https://www.acog.org/-/media/ACOG-Today /acogToday201211.pdf. Published November 2012. Accessed February 12, 2018.
- Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29(1):45–46.
- Ventola CL. Mobile devices and apps for health care professionals: Uses and benefits. P T. 2014;39(5):356–364.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
An either/or choice is not a good strategy for pain
An either/or choice is not a good strategy for pain
I found Dr. Barbieri’s editorial on postpartum opioid use and breastfeeding interesting, but one key issue was not addressed: Following this guidance means that new mothers have to choose between breastfeeding and pain control. You may explain to a patient with 2-day cesarean delivery pain, “If you take pain medicine while breastfeeding, it can adversely affect the baby. So we will give you acetaminophen.” While some moms will deal with it, others will stop breastfeeding. With the increasing pressure to advocate for breastfeeding, this strategy is likely not realistic.
R. Lee Toler, DO
Bolivia, North Carolina
My pain management protocol
While presently in an office-based setting, back in my inpatient practice days I would order oxycodone plus acetaminophen for 1 to 2 days postoperative cesarean delivery, and only 1 day after normal spontaneous delivery if the patient had a large perineal repair or multiparous involution pain. Otherwise, it was ibuprofen 800 mg, then 400 to 600 mg on discharge home.
Gabrielle Long, CNM
Mohegan Lake, New York
Respect women’s postsurgical pain management needs
There is a real disrespect for pain control for women, such as after a cesarean delivery. I would like to see any male have major surgery through a large muscle like the uterus and not need significant pain control options!
Anne V. Hale, MD
El Paso, Texas
Dr. Barbieri responds
I agree with Ms. Long that most postpartum patients, including many who have had a cesarean delivery, can achieve adequate pain control with the use of parenteral and oral nonsteroidal anti-inflammatory drugs (NSAIDs) and oral acetaminophen. Drs. Toler and Hale are concerned that postpartum pain control might be suboptimal if opioids are underprescribed. However, in many developed countries obstetricians do not use opioid pain medicine for postpartum pain management, relying on NSAIDs and acetaminophen. Given the success of this approach, I think we can significantly reduce the use of opioids by postpartum women in the United States by optimizing our use of nonopioid medications.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
An either/or choice is not a good strategy for pain
I found Dr. Barbieri’s editorial on postpartum opioid use and breastfeeding interesting, but one key issue was not addressed: Following this guidance means that new mothers have to choose between breastfeeding and pain control. You may explain to a patient with 2-day cesarean delivery pain, “If you take pain medicine while breastfeeding, it can adversely affect the baby. So we will give you acetaminophen.” While some moms will deal with it, others will stop breastfeeding. With the increasing pressure to advocate for breastfeeding, this strategy is likely not realistic.
R. Lee Toler, DO
Bolivia, North Carolina
My pain management protocol
While presently in an office-based setting, back in my inpatient practice days I would order oxycodone plus acetaminophen for 1 to 2 days postoperative cesarean delivery, and only 1 day after normal spontaneous delivery if the patient had a large perineal repair or multiparous involution pain. Otherwise, it was ibuprofen 800 mg, then 400 to 600 mg on discharge home.
Gabrielle Long, CNM
Mohegan Lake, New York
Respect women’s postsurgical pain management needs
There is a real disrespect for pain control for women, such as after a cesarean delivery. I would like to see any male have major surgery through a large muscle like the uterus and not need significant pain control options!
Anne V. Hale, MD
El Paso, Texas
Dr. Barbieri responds
I agree with Ms. Long that most postpartum patients, including many who have had a cesarean delivery, can achieve adequate pain control with the use of parenteral and oral nonsteroidal anti-inflammatory drugs (NSAIDs) and oral acetaminophen. Drs. Toler and Hale are concerned that postpartum pain control might be suboptimal if opioids are underprescribed. However, in many developed countries obstetricians do not use opioid pain medicine for postpartum pain management, relying on NSAIDs and acetaminophen. Given the success of this approach, I think we can significantly reduce the use of opioids by postpartum women in the United States by optimizing our use of nonopioid medications.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
An either/or choice is not a good strategy for pain
I found Dr. Barbieri’s editorial on postpartum opioid use and breastfeeding interesting, but one key issue was not addressed: Following this guidance means that new mothers have to choose between breastfeeding and pain control. You may explain to a patient with 2-day cesarean delivery pain, “If you take pain medicine while breastfeeding, it can adversely affect the baby. So we will give you acetaminophen.” While some moms will deal with it, others will stop breastfeeding. With the increasing pressure to advocate for breastfeeding, this strategy is likely not realistic.
R. Lee Toler, DO
Bolivia, North Carolina
My pain management protocol
While presently in an office-based setting, back in my inpatient practice days I would order oxycodone plus acetaminophen for 1 to 2 days postoperative cesarean delivery, and only 1 day after normal spontaneous delivery if the patient had a large perineal repair or multiparous involution pain. Otherwise, it was ibuprofen 800 mg, then 400 to 600 mg on discharge home.
Gabrielle Long, CNM
Mohegan Lake, New York
Respect women’s postsurgical pain management needs
There is a real disrespect for pain control for women, such as after a cesarean delivery. I would like to see any male have major surgery through a large muscle like the uterus and not need significant pain control options!
Anne V. Hale, MD
El Paso, Texas
Dr. Barbieri responds
I agree with Ms. Long that most postpartum patients, including many who have had a cesarean delivery, can achieve adequate pain control with the use of parenteral and oral nonsteroidal anti-inflammatory drugs (NSAIDs) and oral acetaminophen. Drs. Toler and Hale are concerned that postpartum pain control might be suboptimal if opioids are underprescribed. However, in many developed countries obstetricians do not use opioid pain medicine for postpartum pain management, relying on NSAIDs and acetaminophen. Given the success of this approach, I think we can significantly reduce the use of opioids by postpartum women in the United States by optimizing our use of nonopioid medications.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Consider thalassemia traits in patients with iron deficiency
Consider thalassemia traits in patients with iron deficiency
The editorial is an excellent review of iron deficiency as an associated finding with adverse health and pregnancy outcomes. However, one genetic issue appears to have escaped comment. In Florida, our African American patients hav
Your recommendation to routinely screen for ferritin deficit is laudable as a general health care practice. If the screening result is normal, however, consider thalassemia carrier states as a secondary explanation as well as a genetic issue requiring partner testing. Aggressive iron loading of a nondeficient anemic patient can risk excess absorption, storage, and ultimate organ compromise in later life if continued indefinitely.
Richard P. Perkins, MD
Fort Myers, Florida
Patient education is key to managing iron deficiency
Forty years ago, my professors expounded on how some people could not absorb iron and that the answer was intravenous iron infusion. After writing a few prescriptions, however, I found that I no longer had patients with absorptive problems once I learned to carefully, and with visual aids, explain the iron story and meticulously monitor compliance. I have been through the “slow Fe” and the “prenatal vitamins have iron” nonsense. Ferrous sulfate is about as good as anything. I have explained the theory of vitamin C−assist and found that telling people to avoid taking iron with meals is folly.
I suggest that the iron story is complete. Rather than wasting money on further research, we should spend funds on teaching young physicians to educate patients and monitor compliance. In recent years, I have found that a daily text message to the patient frequently is very helpful.
Robert W. Jackson, MD
Washougal, Washington
Dr. Barbieri responds
I thank Drs. Perkins and Jackson for their helpful recommendations for the management of iron deficiency anemia. I agree with Dr. Perkins that screening for thalassemia is an important part of preconception and prenatal care. In the editorial’s table on page 10 discussing the differential diagnosis of anemia, we mentioned the importance of hemoglobin electrophoresis and measurement of vitamin B12 and folate levels to identify cases of anemia caused by thalassemia or vitamin deficiency. I agree with Dr. Jackson that oral iron supplementation along with patient education can resolve most cases of iron deficiency in early and mid-pregnancy. However, in the last few weeks of pregnancy there may not be sufficient time for oral iron supplementation to be effective in resolving iron deficiency anemia. In this situation and in patients at high risk for malabsorption, including women with prior gastric bypass, intravenous iron might be the best approach to resolving the anemia.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Consider thalassemia traits in patients with iron deficiency
The editorial is an excellent review of iron deficiency as an associated finding with adverse health and pregnancy outcomes. However, one genetic issue appears to have escaped comment. In Florida, our African American patients hav
Your recommendation to routinely screen for ferritin deficit is laudable as a general health care practice. If the screening result is normal, however, consider thalassemia carrier states as a secondary explanation as well as a genetic issue requiring partner testing. Aggressive iron loading of a nondeficient anemic patient can risk excess absorption, storage, and ultimate organ compromise in later life if continued indefinitely.
Richard P. Perkins, MD
Fort Myers, Florida
Patient education is key to managing iron deficiency
Forty years ago, my professors expounded on how some people could not absorb iron and that the answer was intravenous iron infusion. After writing a few prescriptions, however, I found that I no longer had patients with absorptive problems once I learned to carefully, and with visual aids, explain the iron story and meticulously monitor compliance. I have been through the “slow Fe” and the “prenatal vitamins have iron” nonsense. Ferrous sulfate is about as good as anything. I have explained the theory of vitamin C−assist and found that telling people to avoid taking iron with meals is folly.
I suggest that the iron story is complete. Rather than wasting money on further research, we should spend funds on teaching young physicians to educate patients and monitor compliance. In recent years, I have found that a daily text message to the patient frequently is very helpful.
Robert W. Jackson, MD
Washougal, Washington
Dr. Barbieri responds
I thank Drs. Perkins and Jackson for their helpful recommendations for the management of iron deficiency anemia. I agree with Dr. Perkins that screening for thalassemia is an important part of preconception and prenatal care. In the editorial’s table on page 10 discussing the differential diagnosis of anemia, we mentioned the importance of hemoglobin electrophoresis and measurement of vitamin B12 and folate levels to identify cases of anemia caused by thalassemia or vitamin deficiency. I agree with Dr. Jackson that oral iron supplementation along with patient education can resolve most cases of iron deficiency in early and mid-pregnancy. However, in the last few weeks of pregnancy there may not be sufficient time for oral iron supplementation to be effective in resolving iron deficiency anemia. In this situation and in patients at high risk for malabsorption, including women with prior gastric bypass, intravenous iron might be the best approach to resolving the anemia.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Consider thalassemia traits in patients with iron deficiency
The editorial is an excellent review of iron deficiency as an associated finding with adverse health and pregnancy outcomes. However, one genetic issue appears to have escaped comment. In Florida, our African American patients hav
Your recommendation to routinely screen for ferritin deficit is laudable as a general health care practice. If the screening result is normal, however, consider thalassemia carrier states as a secondary explanation as well as a genetic issue requiring partner testing. Aggressive iron loading of a nondeficient anemic patient can risk excess absorption, storage, and ultimate organ compromise in later life if continued indefinitely.
Richard P. Perkins, MD
Fort Myers, Florida
Patient education is key to managing iron deficiency
Forty years ago, my professors expounded on how some people could not absorb iron and that the answer was intravenous iron infusion. After writing a few prescriptions, however, I found that I no longer had patients with absorptive problems once I learned to carefully, and with visual aids, explain the iron story and meticulously monitor compliance. I have been through the “slow Fe” and the “prenatal vitamins have iron” nonsense. Ferrous sulfate is about as good as anything. I have explained the theory of vitamin C−assist and found that telling people to avoid taking iron with meals is folly.
I suggest that the iron story is complete. Rather than wasting money on further research, we should spend funds on teaching young physicians to educate patients and monitor compliance. In recent years, I have found that a daily text message to the patient frequently is very helpful.
Robert W. Jackson, MD
Washougal, Washington
Dr. Barbieri responds
I thank Drs. Perkins and Jackson for their helpful recommendations for the management of iron deficiency anemia. I agree with Dr. Perkins that screening for thalassemia is an important part of preconception and prenatal care. In the editorial’s table on page 10 discussing the differential diagnosis of anemia, we mentioned the importance of hemoglobin electrophoresis and measurement of vitamin B12 and folate levels to identify cases of anemia caused by thalassemia or vitamin deficiency. I agree with Dr. Jackson that oral iron supplementation along with patient education can resolve most cases of iron deficiency in early and mid-pregnancy. However, in the last few weeks of pregnancy there may not be sufficient time for oral iron supplementation to be effective in resolving iron deficiency anemia. In this situation and in patients at high risk for malabsorption, including women with prior gastric bypass, intravenous iron might be the best approach to resolving the anemia.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
FDA approves new HPV assay
Manufacturer Becton Dickinson announced on Feb. 13 that it had received premarket approval from the U.S. Food and Drug Administration for a human papillomavirus (HPV) assay.
The Onclarity assay detects 14 types of high-risk HPV from specimens collected from cervical cancer screening via a SurePath liquid-based Pap test. It has previously been approved in Europe, Canada, and Japan. The bench-top molecular testing platform used for the assay has received prior FDA approval for chlamydia and gonorrhea infection testing.
The assay in particular identifies the HPV genotypes 16, 18, and 45, which are associated with more than two-thirds of cervical cancers and precancerous cervical lesions and as many as 94% of glandular cervical cancer cases.
Manufacturer Becton Dickinson announced on Feb. 13 that it had received premarket approval from the U.S. Food and Drug Administration for a human papillomavirus (HPV) assay.
The Onclarity assay detects 14 types of high-risk HPV from specimens collected from cervical cancer screening via a SurePath liquid-based Pap test. It has previously been approved in Europe, Canada, and Japan. The bench-top molecular testing platform used for the assay has received prior FDA approval for chlamydia and gonorrhea infection testing.
The assay in particular identifies the HPV genotypes 16, 18, and 45, which are associated with more than two-thirds of cervical cancers and precancerous cervical lesions and as many as 94% of glandular cervical cancer cases.
Manufacturer Becton Dickinson announced on Feb. 13 that it had received premarket approval from the U.S. Food and Drug Administration for a human papillomavirus (HPV) assay.
The Onclarity assay detects 14 types of high-risk HPV from specimens collected from cervical cancer screening via a SurePath liquid-based Pap test. It has previously been approved in Europe, Canada, and Japan. The bench-top molecular testing platform used for the assay has received prior FDA approval for chlamydia and gonorrhea infection testing.
The assay in particular identifies the HPV genotypes 16, 18, and 45, which are associated with more than two-thirds of cervical cancers and precancerous cervical lesions and as many as 94% of glandular cervical cancer cases.
Video roundtable–Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management
How to avoid and manage complications when placing ports and docking



Endometriosis surgery on a young woman: $483,351 award
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.