User login
Quantity, Quality, or Neither–Measuring the Effectiveness of Rounds
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
© 2019 Society of Hospital Medicine
Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy
Polypharmacy, the practice of taking multiple medications to manage health conditions, is common for children. Many children today have a higher burden chronic illness and an increasing number of pharmaceuticals—often delivered in various doses throughout the day. Polypharmacy has been linked to a variety of pediatric and adult outcomes, including medication errors and readmission.1-3 Consequently, the Society of Hospital Medicine recognizes polypharmacy as a risk factor for readmission for adult populations.4 These adverse outcomes are related to both the human elements of polypharmacy (eg, cognitive burden, adherence) and the pharmacologic elements, including drug–drug interactions. For many children, the safety implications of polypharmacy may be more consequential due to the reliance of multiple caregivers to administer medications, which requires additional coordination to ensure that medications are administered and not duplicated. Dual administration of the same medication by both parents is the most common reason for pediatric calls to Poison Control Centers.5 Yet, there is a paucity of research in this area, with most of the pediatric literature focusing on the outpatient setting and specific populations, including epilepsy and mental health.6-8
How providers, patients, and families translate medication lists to counts of medications—and hence the burden of polypharmacy—is not clearly or consistently described. Often in studies of polypharmacy, researchers utilize medication claims data to count the number of medications a patient has filled from the pharmacy. However, in routine clinical practice, clinicians rarely have access to medication claims and thus rely on patient or family report, which may or may not match the list of medications in the patients’ medical records.
Therefore, linking polypharmacy research to the pragmatic complexities of clinical care requires greater clarity and consistent application of concepts. At hospital discharge, families receive a list of medications to take, including home medications to resume as well as newly prescribed medications. However, not all medications are equally essential to patients’ care regarding importance of administration (eg, hydrocortisone ointment versus an anticonvulsant medication). Patients, parents, and caregivers are ultimately responsible for determining which medications to prioritize and administer.
Although there is no standard numerical definition for how to identify polypharmacy, five medications is commonly considered the threshold for polypharmacy.9 A recent review of the pediatric polypharmacy literature suggested a lower threshold, with any two concurrent medications for at least a day.7 Yet, the best approach to “count” medications at hospital discharge is unclear. The simplest method is to tally the number of medications listed in the discharge summary. However, medications are sometimes listed twice due to different dosages administered at different times. Frequently, medications are prescribed on an as-needed basis; these medications could be administered routinely or very infrequently (eg, epinephrine for anaphylaxis). Over-the-counter medications are also sometimes included in discharge summaries and consideration should be given as to whether these medications count toward measures of polypharmacy. Over-the-counter medications would not be counted by a polypharmacy measure that relies on claims data if those medications are not paid by the insurer.
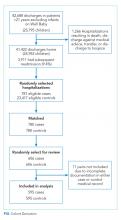
We sought consensus on how to count discharge medications through a series of informal interviews with hospitalists, nurses, and parents. We asked the seemingly simple question, “How many medications is this child on?” across a variety of scenarios (Figure). For panel A, all stakeholders agreed that this medication list includes two medications. All other scenarios elicited disagreement. For panel B, many people responded three medications, but others (often physicians) counted only clindamycin and therefore responded one medication.
For panel C, stakeholders were split between one (only topiramate), two (topiramate and rectal diazepam), and three medications (two different doses of topiramate, which counted as two different medications, plus rectal diazepam). Interestingly, one parent reflected that they would count panel C differently, depending on with whom they were discussing the medications. If the parent were speaking with a physician, they would consider the two different doses of topiramate as a single medication; however, if they were conveying a list of medications to a babysitter, they would consider them as two different medications. Finally, panel D also split stakeholders between counting one and two medications, with some parents expressing confusion as to why the child would be prescribed the same medication at different times.
While our informal conversations with physicians, nurses, and families should not be construed as rigorous qualitative research, we are concerned about the lack of a shared mental model about the best way to count discharge polypharmacy. In reviewing the comments that we collected, the family voice stands out—physicians do not know how a parent or a caregiver will prioritize the medications to give to their child; physicians do not know whether families will count medications as a group or as separate entities. Although providers, patients, and families share a list of medications at discharge, this list may contain items not considered as “medications” by physicians.10 Nevertheless, the medication list provided at discharge is what the family must navigate once home. One way to consider discharge polypharmacy would be to count all the medications in the discharge summary, regardless of clinicians’ perceptions of necessity or importance. Electronic health record based tools should sum medications counts. Ultimately, further research is needed to understand the cognitive and care burden discharge polypharmacy places on families as well as understand this burden’s relationship to safety and transition outcomes.
Disclosures
Dr. Auger has nothing to disclose. Dr. Shah is the Editor-in-Chief of the Journal of Hospital Medicine. Dr. Davis has nothing to disclose. Dr. Brady reports grants from Agency for Healthcare Research and Quality, outside the submitted work.
Funding
This project is supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1).
1. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
2. Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135(1):e99-e108. https://doi.org/10.1542/peds.2014-2015.
3. Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014(10):CD008165. https://doi.org/10.1002/14651858.CD008165.pub3.
4. Society of Hospital Medicine. Project BOOST: better outcomes for older adults through safe transitions—implementation guide to improve care transitions.
5. Smith MD, Spiller HA, Casavant MJ, Chounthirath T, Brophy TJ, Xiang H. Out-of-hospital medication errors among young children in the United States, 2002-2012. Pediatrics. 2014;134(5):867-876. https://doi.org/10.1542/peds.2014-0309.
6. Baker C, Feinstein JA, Ma X, et al. Variation of the prevalence of pediatric polypharmacy: a scoping review. Pharmacoepidemiol Drug Saf. 2019;28(3):275-287. https://doi.org/10.1002/pds.4719.
7. Bakaki PM, Horace A, Dawson N, et al. Defining pediatric polypharmacy: a scoping review. PLoS One. 2018;13(11):e0208047. https://doi.org/10.1371/journal.pone.0208047.
8. Horace AE, Ahmed F. Polypharmacy in pediatric patients and opportunities for pharmacists’ involvement. Integr Pharm Res Pract. 2015;4:113-126. https://doi.org/10.2147/IPRP.S64535.
9. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. https://doi.org/10.1186/s12877-017-0621-2.
10. Auger KA, Shah SS, Huang B, et al. Discharge Medical Complexity, Change in Medical Complexity and Pediatric Thirty-day Readmission. J Hosp Med. 2019;14(8):474-481. https://doi.org/10.12788/jhm.3222.
11. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. Jama. 2018;320(18):1889-1898. https://doi.org/10.1001/jama.2018.16131.
12. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. https://doi.org/10.1111/bcp.12975.
Polypharmacy, the practice of taking multiple medications to manage health conditions, is common for children. Many children today have a higher burden chronic illness and an increasing number of pharmaceuticals—often delivered in various doses throughout the day. Polypharmacy has been linked to a variety of pediatric and adult outcomes, including medication errors and readmission.1-3 Consequently, the Society of Hospital Medicine recognizes polypharmacy as a risk factor for readmission for adult populations.4 These adverse outcomes are related to both the human elements of polypharmacy (eg, cognitive burden, adherence) and the pharmacologic elements, including drug–drug interactions. For many children, the safety implications of polypharmacy may be more consequential due to the reliance of multiple caregivers to administer medications, which requires additional coordination to ensure that medications are administered and not duplicated. Dual administration of the same medication by both parents is the most common reason for pediatric calls to Poison Control Centers.5 Yet, there is a paucity of research in this area, with most of the pediatric literature focusing on the outpatient setting and specific populations, including epilepsy and mental health.6-8
How providers, patients, and families translate medication lists to counts of medications—and hence the burden of polypharmacy—is not clearly or consistently described. Often in studies of polypharmacy, researchers utilize medication claims data to count the number of medications a patient has filled from the pharmacy. However, in routine clinical practice, clinicians rarely have access to medication claims and thus rely on patient or family report, which may or may not match the list of medications in the patients’ medical records.
Therefore, linking polypharmacy research to the pragmatic complexities of clinical care requires greater clarity and consistent application of concepts. At hospital discharge, families receive a list of medications to take, including home medications to resume as well as newly prescribed medications. However, not all medications are equally essential to patients’ care regarding importance of administration (eg, hydrocortisone ointment versus an anticonvulsant medication). Patients, parents, and caregivers are ultimately responsible for determining which medications to prioritize and administer.
Although there is no standard numerical definition for how to identify polypharmacy, five medications is commonly considered the threshold for polypharmacy.9 A recent review of the pediatric polypharmacy literature suggested a lower threshold, with any two concurrent medications for at least a day.7 Yet, the best approach to “count” medications at hospital discharge is unclear. The simplest method is to tally the number of medications listed in the discharge summary. However, medications are sometimes listed twice due to different dosages administered at different times. Frequently, medications are prescribed on an as-needed basis; these medications could be administered routinely or very infrequently (eg, epinephrine for anaphylaxis). Over-the-counter medications are also sometimes included in discharge summaries and consideration should be given as to whether these medications count toward measures of polypharmacy. Over-the-counter medications would not be counted by a polypharmacy measure that relies on claims data if those medications are not paid by the insurer.
We sought consensus on how to count discharge medications through a series of informal interviews with hospitalists, nurses, and parents. We asked the seemingly simple question, “How many medications is this child on?” across a variety of scenarios (Figure). For panel A, all stakeholders agreed that this medication list includes two medications. All other scenarios elicited disagreement. For panel B, many people responded three medications, but others (often physicians) counted only clindamycin and therefore responded one medication.
For panel C, stakeholders were split between one (only topiramate), two (topiramate and rectal diazepam), and three medications (two different doses of topiramate, which counted as two different medications, plus rectal diazepam). Interestingly, one parent reflected that they would count panel C differently, depending on with whom they were discussing the medications. If the parent were speaking with a physician, they would consider the two different doses of topiramate as a single medication; however, if they were conveying a list of medications to a babysitter, they would consider them as two different medications. Finally, panel D also split stakeholders between counting one and two medications, with some parents expressing confusion as to why the child would be prescribed the same medication at different times.
While our informal conversations with physicians, nurses, and families should not be construed as rigorous qualitative research, we are concerned about the lack of a shared mental model about the best way to count discharge polypharmacy. In reviewing the comments that we collected, the family voice stands out—physicians do not know how a parent or a caregiver will prioritize the medications to give to their child; physicians do not know whether families will count medications as a group or as separate entities. Although providers, patients, and families share a list of medications at discharge, this list may contain items not considered as “medications” by physicians.10 Nevertheless, the medication list provided at discharge is what the family must navigate once home. One way to consider discharge polypharmacy would be to count all the medications in the discharge summary, regardless of clinicians’ perceptions of necessity or importance. Electronic health record based tools should sum medications counts. Ultimately, further research is needed to understand the cognitive and care burden discharge polypharmacy places on families as well as understand this burden’s relationship to safety and transition outcomes.
Disclosures
Dr. Auger has nothing to disclose. Dr. Shah is the Editor-in-Chief of the Journal of Hospital Medicine. Dr. Davis has nothing to disclose. Dr. Brady reports grants from Agency for Healthcare Research and Quality, outside the submitted work.
Funding
This project is supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1).
Polypharmacy, the practice of taking multiple medications to manage health conditions, is common for children. Many children today have a higher burden chronic illness and an increasing number of pharmaceuticals—often delivered in various doses throughout the day. Polypharmacy has been linked to a variety of pediatric and adult outcomes, including medication errors and readmission.1-3 Consequently, the Society of Hospital Medicine recognizes polypharmacy as a risk factor for readmission for adult populations.4 These adverse outcomes are related to both the human elements of polypharmacy (eg, cognitive burden, adherence) and the pharmacologic elements, including drug–drug interactions. For many children, the safety implications of polypharmacy may be more consequential due to the reliance of multiple caregivers to administer medications, which requires additional coordination to ensure that medications are administered and not duplicated. Dual administration of the same medication by both parents is the most common reason for pediatric calls to Poison Control Centers.5 Yet, there is a paucity of research in this area, with most of the pediatric literature focusing on the outpatient setting and specific populations, including epilepsy and mental health.6-8
How providers, patients, and families translate medication lists to counts of medications—and hence the burden of polypharmacy—is not clearly or consistently described. Often in studies of polypharmacy, researchers utilize medication claims data to count the number of medications a patient has filled from the pharmacy. However, in routine clinical practice, clinicians rarely have access to medication claims and thus rely on patient or family report, which may or may not match the list of medications in the patients’ medical records.
Therefore, linking polypharmacy research to the pragmatic complexities of clinical care requires greater clarity and consistent application of concepts. At hospital discharge, families receive a list of medications to take, including home medications to resume as well as newly prescribed medications. However, not all medications are equally essential to patients’ care regarding importance of administration (eg, hydrocortisone ointment versus an anticonvulsant medication). Patients, parents, and caregivers are ultimately responsible for determining which medications to prioritize and administer.
Although there is no standard numerical definition for how to identify polypharmacy, five medications is commonly considered the threshold for polypharmacy.9 A recent review of the pediatric polypharmacy literature suggested a lower threshold, with any two concurrent medications for at least a day.7 Yet, the best approach to “count” medications at hospital discharge is unclear. The simplest method is to tally the number of medications listed in the discharge summary. However, medications are sometimes listed twice due to different dosages administered at different times. Frequently, medications are prescribed on an as-needed basis; these medications could be administered routinely or very infrequently (eg, epinephrine for anaphylaxis). Over-the-counter medications are also sometimes included in discharge summaries and consideration should be given as to whether these medications count toward measures of polypharmacy. Over-the-counter medications would not be counted by a polypharmacy measure that relies on claims data if those medications are not paid by the insurer.
We sought consensus on how to count discharge medications through a series of informal interviews with hospitalists, nurses, and parents. We asked the seemingly simple question, “How many medications is this child on?” across a variety of scenarios (Figure). For panel A, all stakeholders agreed that this medication list includes two medications. All other scenarios elicited disagreement. For panel B, many people responded three medications, but others (often physicians) counted only clindamycin and therefore responded one medication.
For panel C, stakeholders were split between one (only topiramate), two (topiramate and rectal diazepam), and three medications (two different doses of topiramate, which counted as two different medications, plus rectal diazepam). Interestingly, one parent reflected that they would count panel C differently, depending on with whom they were discussing the medications. If the parent were speaking with a physician, they would consider the two different doses of topiramate as a single medication; however, if they were conveying a list of medications to a babysitter, they would consider them as two different medications. Finally, panel D also split stakeholders between counting one and two medications, with some parents expressing confusion as to why the child would be prescribed the same medication at different times.
While our informal conversations with physicians, nurses, and families should not be construed as rigorous qualitative research, we are concerned about the lack of a shared mental model about the best way to count discharge polypharmacy. In reviewing the comments that we collected, the family voice stands out—physicians do not know how a parent or a caregiver will prioritize the medications to give to their child; physicians do not know whether families will count medications as a group or as separate entities. Although providers, patients, and families share a list of medications at discharge, this list may contain items not considered as “medications” by physicians.10 Nevertheless, the medication list provided at discharge is what the family must navigate once home. One way to consider discharge polypharmacy would be to count all the medications in the discharge summary, regardless of clinicians’ perceptions of necessity or importance. Electronic health record based tools should sum medications counts. Ultimately, further research is needed to understand the cognitive and care burden discharge polypharmacy places on families as well as understand this burden’s relationship to safety and transition outcomes.
Disclosures
Dr. Auger has nothing to disclose. Dr. Shah is the Editor-in-Chief of the Journal of Hospital Medicine. Dr. Davis has nothing to disclose. Dr. Brady reports grants from Agency for Healthcare Research and Quality, outside the submitted work.
Funding
This project is supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1).
1. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
2. Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135(1):e99-e108. https://doi.org/10.1542/peds.2014-2015.
3. Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014(10):CD008165. https://doi.org/10.1002/14651858.CD008165.pub3.
4. Society of Hospital Medicine. Project BOOST: better outcomes for older adults through safe transitions—implementation guide to improve care transitions.
5. Smith MD, Spiller HA, Casavant MJ, Chounthirath T, Brophy TJ, Xiang H. Out-of-hospital medication errors among young children in the United States, 2002-2012. Pediatrics. 2014;134(5):867-876. https://doi.org/10.1542/peds.2014-0309.
6. Baker C, Feinstein JA, Ma X, et al. Variation of the prevalence of pediatric polypharmacy: a scoping review. Pharmacoepidemiol Drug Saf. 2019;28(3):275-287. https://doi.org/10.1002/pds.4719.
7. Bakaki PM, Horace A, Dawson N, et al. Defining pediatric polypharmacy: a scoping review. PLoS One. 2018;13(11):e0208047. https://doi.org/10.1371/journal.pone.0208047.
8. Horace AE, Ahmed F. Polypharmacy in pediatric patients and opportunities for pharmacists’ involvement. Integr Pharm Res Pract. 2015;4:113-126. https://doi.org/10.2147/IPRP.S64535.
9. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. https://doi.org/10.1186/s12877-017-0621-2.
10. Auger KA, Shah SS, Huang B, et al. Discharge Medical Complexity, Change in Medical Complexity and Pediatric Thirty-day Readmission. J Hosp Med. 2019;14(8):474-481. https://doi.org/10.12788/jhm.3222.
11. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. Jama. 2018;320(18):1889-1898. https://doi.org/10.1001/jama.2018.16131.
12. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. https://doi.org/10.1111/bcp.12975.
1. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
2. Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135(1):e99-e108. https://doi.org/10.1542/peds.2014-2015.
3. Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014(10):CD008165. https://doi.org/10.1002/14651858.CD008165.pub3.
4. Society of Hospital Medicine. Project BOOST: better outcomes for older adults through safe transitions—implementation guide to improve care transitions.
5. Smith MD, Spiller HA, Casavant MJ, Chounthirath T, Brophy TJ, Xiang H. Out-of-hospital medication errors among young children in the United States, 2002-2012. Pediatrics. 2014;134(5):867-876. https://doi.org/10.1542/peds.2014-0309.
6. Baker C, Feinstein JA, Ma X, et al. Variation of the prevalence of pediatric polypharmacy: a scoping review. Pharmacoepidemiol Drug Saf. 2019;28(3):275-287. https://doi.org/10.1002/pds.4719.
7. Bakaki PM, Horace A, Dawson N, et al. Defining pediatric polypharmacy: a scoping review. PLoS One. 2018;13(11):e0208047. https://doi.org/10.1371/journal.pone.0208047.
8. Horace AE, Ahmed F. Polypharmacy in pediatric patients and opportunities for pharmacists’ involvement. Integr Pharm Res Pract. 2015;4:113-126. https://doi.org/10.2147/IPRP.S64535.
9. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. https://doi.org/10.1186/s12877-017-0621-2.
10. Auger KA, Shah SS, Huang B, et al. Discharge Medical Complexity, Change in Medical Complexity and Pediatric Thirty-day Readmission. J Hosp Med. 2019;14(8):474-481. https://doi.org/10.12788/jhm.3222.
11. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. Jama. 2018;320(18):1889-1898. https://doi.org/10.1001/jama.2018.16131.
12. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. https://doi.org/10.1111/bcp.12975.
© 2019 Society of Hospital Medicine
The Management of Anticoagulation for Venous Thromboembolism in the Hospitalized Adult
Anticoagulation for patients with venous thromboembolism (VTE) is associated not only with considerable benefits, including prevention of pulmonary embolus and thrombus extension, but also with potential significant risks, such as life-threatening bleeding.1 Hospitalized patients may require anticoagulation to treat new VTE or for secondary prevention of prior events. Hospital admission is a high-risk time for anticoagulation control.2 Additionally, anticoagulation has become an increasingly complex decision as the number of therapeutic agents on the market has significantly increased, coupled with medication interactions and dosing intricacies. Management is multifaceted and associated with wide variation in practice patterns.3 Thus, further evidence-based guidance for providers is necessary for the care of the hospitalized patient with VTE.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
The following are 16 selected guideline recommendations most relevant to adult hospitalists.4 Recommendations were graded as “strong” if most individuals should follow the recommended course of action and “conditional” if different choices are appropriate for different patients.
Initial Anticoagulant Dosing, Monitoring, and Medication Interactions
(for all recommendations–evidence quality: low certainty; recommendation strength: conditional)
Recommendation 1. In obese patients receiving low molecular weight heparin (LMWH), determine the initial dose based on actual body weight rather than a fixed or “capped” maximum dose.
Recommendation 2. For obese patients or those with renal dysfunction receiving LMWH, avoid dosing based on serum antifactor Xa levels. Instead, adjust dosing based on product labeling, with appropriate dose reduction in patients with chronic kidney disease.
Recommendation 3. For patients receiving direct oral anticoagulant (DOAC) therapy, avoid measuring the anticoagulation effect during management of bleeding as there is no evidence to support a beneficial effect, and it may result in a delay in treatment.
Recommendation 4. For patients requiring administration of inhibitors or inducers of P-glycoprotein or cytochrome P450 enzymes, use LMWH or vitamin K antagonists (VKA) rather than a DOAC.
Recommendation 5. When transitioning from a DOAC to a VKA, the medications should overlap until the international normalized ratio (INR) is therapeutic instead of bridging with a heparin agent.
Recommendations for Ongoing Outpatient Monitoring upon Discharge from the Hospital
Recommendation 6. Use point-of-care INR testing by patients at home, with self-adjustment of VKA dose (evidence quality: low certainty; recommendation strength: strong).
Recommendation 7. Patients should be referred for specialized anticoagulation management rather than to their primary care provider (PCP) (evidence quality: very low certainty; recommendation strength: conditional).
Recommendation 8. Supplementary education, in addition to basic education, should be made available to patients to help improve outcomes (evidence quality: very low certainty; recommendation strength: conditional).
Hospitalists are often responsible for the coordination of care upon discharge from the hospital, including discharge teaching, subspecialty referrals, and determination of patient suitability for home monitoring and dose adjustment. The follow-up plan may depend on local systems and access. A PCP can manage anticoagulation if performed in a systematic and coordinated fashion.5
Recommendations for Patients on Anticoagulation Undergoing Procedures
Recommendation 9. For patients with a low or moderate risk of recurrent VTE on VKA therapy undergoing procedures, periprocedural bridging with heparin or LMWH should be avoided. This excludes patients at high risk for recurrent VTE, defined as those with recent VTE (<3 months); having a known thrombophilic abnormality such as antiphospholipid syndrome, protein C/S deficiency, or antithrombin deficiency; or high-risk patient populations by expert consensus and practice guidelines4,6 (evidence quality: moderate certainty; recommendation strength: strong).
Recommendation 10. For patients on DOACs undergoing procedures, measurement of the anticoagulation effect of the DOAC should be avoided (evidence quality: very low certainty; recommendation strength: conditional).
Recommendations for Patients on Anticoagulation Suffering from Supratherapeutic Levels or Bleeding Complications
(for all recommendations–evidence quality: very low certainty; recommendation strength: conditional)
Recommendation 11. If a patient on VKA therapy has an INR between 4.5 and 10 without clinically relevant bleeding, the use of vitamin K therapy can be avoided in favor of temporary cessation of VKA alone.
Recommendation 12. If a patient on VKA therapy has life-threatening bleeding, four-factor prothrombin complex concentrate (PCC) should be used in addition to the cessation of VKA therapy and initiation of vitamin K therapy, over the use of fresh frozen plaza, because of the ease of administration and minimal risk of volume overload.
Recommendation 13. If a patient has life-threatening bleeding on a Xa inhibitor, the panel recommends discontinuation of the medication and the option to administer either PCC or recombinant coagulation factor Xa, as there have been no studies comparing these two strategies.
Recommendation 14. If life-threatening bleeding occurs in a patient on dabigatran, idarucizumab should be administered, if available.
Recommendation 15. In patients with bleeding while on heparin or LMWH, protamine should be administered.
Recommendation 16. Following an episode of life-threatening bleeding, anticoagulation should be resumed within 90 days, provided that the patient is at moderate to high risk for recurrent VTE, is not at high risk for recurrent bleeding, and is willing to continue anticoagulation.
CRITIQUE
Methods in Preparing Guidelines
The panel was funded by the American Society of Hematology (ASH), a nonprofit medical specialty society.4 The panel is multidisciplinary, including physicians and providers as well as patient representatives, and is supported by the McMaster University GRADE Center, which conducted new and updated systematic reviews of the evidence according to the “Cochrane Handbook for Systematic Reviews of Interventions.” The panel members agreed on 25 recommendations and two good practice statements. The recommendations were made available to external review by stakeholders and addressed. Comments made by 10 individuals or organizations were subsequently incorporated.
Sources of Potential Conflict of Interest
Panel members, other than patient representatives, did not receive funding, and the majority of the panel had no conflicts of interest to report. Given the minimal influence of outside parties including pharmaceutical companies, and the wide diversity of opinions sought in the creation of the guidelines, concern for conflict of interest is low.
Generalizability
These guidelines assume that the decision to anticoagulate a patient, and which agent to use, has already been made and thus do not offer further guidance on this decision. These guidelines also do not address optimal choices for anticoagulation in specific patient populations, such as patients with cancer. They are limited in scope to exclude the treatment of specific thromboembolic disease processes such as subsegmental pulmonary emboli, superficial venous thrombus, or distal vein thrombosis. Unfortunately, challenging decisions made by hospitalists frequently fall into one of these categories. Coincident with these guidelines, ASH introduced comprehensive guidelines to support basic diagnostic decisions.7
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand optimal monitoring practices for patients on anticoagulation therapy, including the ideal INR monitoring frequency for patients on VKA therapy. Additionally, there is a need to better understand the difference in clinical outcomes and resources utilization when care is provided by an anticoagulation specialist as compared with a PCP. Finally, while guidelines suggest that anticoagulation should be resumed within 90 days of a life-threatening bleed, there is a need to better understand the optimal timing of a restart, as well as the patient factors to be considered in this decision.
Disclosures
The authors have nothing to disclose.
Funding
There was no funding support in the creation of this manuscript.
1. Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A. Pharmacology of anticoagulants used in the treatment of venous thromboembolism [published correction appears in J Thromb Thrombolysis. 2016;42(2):296-311]. J Thromb Thrombolysis. 2016;41(1):15-31. https://doi.org/10.1007/s11239-015-1314-3.
2. van Walraven C, Austin PC, Oake N, Wells PS, Mamdani M, Forster AJ. The influence of hospitalization on oral anticoagulation control: a population-based study. Thromb Res. 2007;119(6):705-714. PubMed
3. Rodwin BA, Salami JA, Spatz ES, et al. Variation in the use of warfarin and direct oral anticoagulants in atrial fibrillation and associated cost implications. Am J Med. 2019:132(1):61-70. https://doi.org/10.1016/j.amjmed.2018.09.026.
4. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-3291. https://doi.org/10.1182/bloodadvances.2018024893.
5. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012;142(6):1698-1704]. Chest. 2012;141(2 suppl):e419S-e496S. https://doi.org/10.1378/chest.11-2301.
6. Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):299S-339S. https://doi.org/10.1378/chest.08-0675.
7. Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2(22):3226-3256. https://doi.org/10.1182/bloodadvances.2018024828.
Anticoagulation for patients with venous thromboembolism (VTE) is associated not only with considerable benefits, including prevention of pulmonary embolus and thrombus extension, but also with potential significant risks, such as life-threatening bleeding.1 Hospitalized patients may require anticoagulation to treat new VTE or for secondary prevention of prior events. Hospital admission is a high-risk time for anticoagulation control.2 Additionally, anticoagulation has become an increasingly complex decision as the number of therapeutic agents on the market has significantly increased, coupled with medication interactions and dosing intricacies. Management is multifaceted and associated with wide variation in practice patterns.3 Thus, further evidence-based guidance for providers is necessary for the care of the hospitalized patient with VTE.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
The following are 16 selected guideline recommendations most relevant to adult hospitalists.4 Recommendations were graded as “strong” if most individuals should follow the recommended course of action and “conditional” if different choices are appropriate for different patients.
Initial Anticoagulant Dosing, Monitoring, and Medication Interactions
(for all recommendations–evidence quality: low certainty; recommendation strength: conditional)
Recommendation 1. In obese patients receiving low molecular weight heparin (LMWH), determine the initial dose based on actual body weight rather than a fixed or “capped” maximum dose.
Recommendation 2. For obese patients or those with renal dysfunction receiving LMWH, avoid dosing based on serum antifactor Xa levels. Instead, adjust dosing based on product labeling, with appropriate dose reduction in patients with chronic kidney disease.
Recommendation 3. For patients receiving direct oral anticoagulant (DOAC) therapy, avoid measuring the anticoagulation effect during management of bleeding as there is no evidence to support a beneficial effect, and it may result in a delay in treatment.
Recommendation 4. For patients requiring administration of inhibitors or inducers of P-glycoprotein or cytochrome P450 enzymes, use LMWH or vitamin K antagonists (VKA) rather than a DOAC.
Recommendation 5. When transitioning from a DOAC to a VKA, the medications should overlap until the international normalized ratio (INR) is therapeutic instead of bridging with a heparin agent.
Recommendations for Ongoing Outpatient Monitoring upon Discharge from the Hospital
Recommendation 6. Use point-of-care INR testing by patients at home, with self-adjustment of VKA dose (evidence quality: low certainty; recommendation strength: strong).
Recommendation 7. Patients should be referred for specialized anticoagulation management rather than to their primary care provider (PCP) (evidence quality: very low certainty; recommendation strength: conditional).
Recommendation 8. Supplementary education, in addition to basic education, should be made available to patients to help improve outcomes (evidence quality: very low certainty; recommendation strength: conditional).
Hospitalists are often responsible for the coordination of care upon discharge from the hospital, including discharge teaching, subspecialty referrals, and determination of patient suitability for home monitoring and dose adjustment. The follow-up plan may depend on local systems and access. A PCP can manage anticoagulation if performed in a systematic and coordinated fashion.5
Recommendations for Patients on Anticoagulation Undergoing Procedures
Recommendation 9. For patients with a low or moderate risk of recurrent VTE on VKA therapy undergoing procedures, periprocedural bridging with heparin or LMWH should be avoided. This excludes patients at high risk for recurrent VTE, defined as those with recent VTE (<3 months); having a known thrombophilic abnormality such as antiphospholipid syndrome, protein C/S deficiency, or antithrombin deficiency; or high-risk patient populations by expert consensus and practice guidelines4,6 (evidence quality: moderate certainty; recommendation strength: strong).
Recommendation 10. For patients on DOACs undergoing procedures, measurement of the anticoagulation effect of the DOAC should be avoided (evidence quality: very low certainty; recommendation strength: conditional).
Recommendations for Patients on Anticoagulation Suffering from Supratherapeutic Levels or Bleeding Complications
(for all recommendations–evidence quality: very low certainty; recommendation strength: conditional)
Recommendation 11. If a patient on VKA therapy has an INR between 4.5 and 10 without clinically relevant bleeding, the use of vitamin K therapy can be avoided in favor of temporary cessation of VKA alone.
Recommendation 12. If a patient on VKA therapy has life-threatening bleeding, four-factor prothrombin complex concentrate (PCC) should be used in addition to the cessation of VKA therapy and initiation of vitamin K therapy, over the use of fresh frozen plaza, because of the ease of administration and minimal risk of volume overload.
Recommendation 13. If a patient has life-threatening bleeding on a Xa inhibitor, the panel recommends discontinuation of the medication and the option to administer either PCC or recombinant coagulation factor Xa, as there have been no studies comparing these two strategies.
Recommendation 14. If life-threatening bleeding occurs in a patient on dabigatran, idarucizumab should be administered, if available.
Recommendation 15. In patients with bleeding while on heparin or LMWH, protamine should be administered.
Recommendation 16. Following an episode of life-threatening bleeding, anticoagulation should be resumed within 90 days, provided that the patient is at moderate to high risk for recurrent VTE, is not at high risk for recurrent bleeding, and is willing to continue anticoagulation.
CRITIQUE
Methods in Preparing Guidelines
The panel was funded by the American Society of Hematology (ASH), a nonprofit medical specialty society.4 The panel is multidisciplinary, including physicians and providers as well as patient representatives, and is supported by the McMaster University GRADE Center, which conducted new and updated systematic reviews of the evidence according to the “Cochrane Handbook for Systematic Reviews of Interventions.” The panel members agreed on 25 recommendations and two good practice statements. The recommendations were made available to external review by stakeholders and addressed. Comments made by 10 individuals or organizations were subsequently incorporated.
Sources of Potential Conflict of Interest
Panel members, other than patient representatives, did not receive funding, and the majority of the panel had no conflicts of interest to report. Given the minimal influence of outside parties including pharmaceutical companies, and the wide diversity of opinions sought in the creation of the guidelines, concern for conflict of interest is low.
Generalizability
These guidelines assume that the decision to anticoagulate a patient, and which agent to use, has already been made and thus do not offer further guidance on this decision. These guidelines also do not address optimal choices for anticoagulation in specific patient populations, such as patients with cancer. They are limited in scope to exclude the treatment of specific thromboembolic disease processes such as subsegmental pulmonary emboli, superficial venous thrombus, or distal vein thrombosis. Unfortunately, challenging decisions made by hospitalists frequently fall into one of these categories. Coincident with these guidelines, ASH introduced comprehensive guidelines to support basic diagnostic decisions.7
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand optimal monitoring practices for patients on anticoagulation therapy, including the ideal INR monitoring frequency for patients on VKA therapy. Additionally, there is a need to better understand the difference in clinical outcomes and resources utilization when care is provided by an anticoagulation specialist as compared with a PCP. Finally, while guidelines suggest that anticoagulation should be resumed within 90 days of a life-threatening bleed, there is a need to better understand the optimal timing of a restart, as well as the patient factors to be considered in this decision.
Disclosures
The authors have nothing to disclose.
Funding
There was no funding support in the creation of this manuscript.
Anticoagulation for patients with venous thromboembolism (VTE) is associated not only with considerable benefits, including prevention of pulmonary embolus and thrombus extension, but also with potential significant risks, such as life-threatening bleeding.1 Hospitalized patients may require anticoagulation to treat new VTE or for secondary prevention of prior events. Hospital admission is a high-risk time for anticoagulation control.2 Additionally, anticoagulation has become an increasingly complex decision as the number of therapeutic agents on the market has significantly increased, coupled with medication interactions and dosing intricacies. Management is multifaceted and associated with wide variation in practice patterns.3 Thus, further evidence-based guidance for providers is necessary for the care of the hospitalized patient with VTE.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
The following are 16 selected guideline recommendations most relevant to adult hospitalists.4 Recommendations were graded as “strong” if most individuals should follow the recommended course of action and “conditional” if different choices are appropriate for different patients.
Initial Anticoagulant Dosing, Monitoring, and Medication Interactions
(for all recommendations–evidence quality: low certainty; recommendation strength: conditional)
Recommendation 1. In obese patients receiving low molecular weight heparin (LMWH), determine the initial dose based on actual body weight rather than a fixed or “capped” maximum dose.
Recommendation 2. For obese patients or those with renal dysfunction receiving LMWH, avoid dosing based on serum antifactor Xa levels. Instead, adjust dosing based on product labeling, with appropriate dose reduction in patients with chronic kidney disease.
Recommendation 3. For patients receiving direct oral anticoagulant (DOAC) therapy, avoid measuring the anticoagulation effect during management of bleeding as there is no evidence to support a beneficial effect, and it may result in a delay in treatment.
Recommendation 4. For patients requiring administration of inhibitors or inducers of P-glycoprotein or cytochrome P450 enzymes, use LMWH or vitamin K antagonists (VKA) rather than a DOAC.
Recommendation 5. When transitioning from a DOAC to a VKA, the medications should overlap until the international normalized ratio (INR) is therapeutic instead of bridging with a heparin agent.
Recommendations for Ongoing Outpatient Monitoring upon Discharge from the Hospital
Recommendation 6. Use point-of-care INR testing by patients at home, with self-adjustment of VKA dose (evidence quality: low certainty; recommendation strength: strong).
Recommendation 7. Patients should be referred for specialized anticoagulation management rather than to their primary care provider (PCP) (evidence quality: very low certainty; recommendation strength: conditional).
Recommendation 8. Supplementary education, in addition to basic education, should be made available to patients to help improve outcomes (evidence quality: very low certainty; recommendation strength: conditional).
Hospitalists are often responsible for the coordination of care upon discharge from the hospital, including discharge teaching, subspecialty referrals, and determination of patient suitability for home monitoring and dose adjustment. The follow-up plan may depend on local systems and access. A PCP can manage anticoagulation if performed in a systematic and coordinated fashion.5
Recommendations for Patients on Anticoagulation Undergoing Procedures
Recommendation 9. For patients with a low or moderate risk of recurrent VTE on VKA therapy undergoing procedures, periprocedural bridging with heparin or LMWH should be avoided. This excludes patients at high risk for recurrent VTE, defined as those with recent VTE (<3 months); having a known thrombophilic abnormality such as antiphospholipid syndrome, protein C/S deficiency, or antithrombin deficiency; or high-risk patient populations by expert consensus and practice guidelines4,6 (evidence quality: moderate certainty; recommendation strength: strong).
Recommendation 10. For patients on DOACs undergoing procedures, measurement of the anticoagulation effect of the DOAC should be avoided (evidence quality: very low certainty; recommendation strength: conditional).
Recommendations for Patients on Anticoagulation Suffering from Supratherapeutic Levels or Bleeding Complications
(for all recommendations–evidence quality: very low certainty; recommendation strength: conditional)
Recommendation 11. If a patient on VKA therapy has an INR between 4.5 and 10 without clinically relevant bleeding, the use of vitamin K therapy can be avoided in favor of temporary cessation of VKA alone.
Recommendation 12. If a patient on VKA therapy has life-threatening bleeding, four-factor prothrombin complex concentrate (PCC) should be used in addition to the cessation of VKA therapy and initiation of vitamin K therapy, over the use of fresh frozen plaza, because of the ease of administration and minimal risk of volume overload.
Recommendation 13. If a patient has life-threatening bleeding on a Xa inhibitor, the panel recommends discontinuation of the medication and the option to administer either PCC or recombinant coagulation factor Xa, as there have been no studies comparing these two strategies.
Recommendation 14. If life-threatening bleeding occurs in a patient on dabigatran, idarucizumab should be administered, if available.
Recommendation 15. In patients with bleeding while on heparin or LMWH, protamine should be administered.
Recommendation 16. Following an episode of life-threatening bleeding, anticoagulation should be resumed within 90 days, provided that the patient is at moderate to high risk for recurrent VTE, is not at high risk for recurrent bleeding, and is willing to continue anticoagulation.
CRITIQUE
Methods in Preparing Guidelines
The panel was funded by the American Society of Hematology (ASH), a nonprofit medical specialty society.4 The panel is multidisciplinary, including physicians and providers as well as patient representatives, and is supported by the McMaster University GRADE Center, which conducted new and updated systematic reviews of the evidence according to the “Cochrane Handbook for Systematic Reviews of Interventions.” The panel members agreed on 25 recommendations and two good practice statements. The recommendations were made available to external review by stakeholders and addressed. Comments made by 10 individuals or organizations were subsequently incorporated.
Sources of Potential Conflict of Interest
Panel members, other than patient representatives, did not receive funding, and the majority of the panel had no conflicts of interest to report. Given the minimal influence of outside parties including pharmaceutical companies, and the wide diversity of opinions sought in the creation of the guidelines, concern for conflict of interest is low.
Generalizability
These guidelines assume that the decision to anticoagulate a patient, and which agent to use, has already been made and thus do not offer further guidance on this decision. These guidelines also do not address optimal choices for anticoagulation in specific patient populations, such as patients with cancer. They are limited in scope to exclude the treatment of specific thromboembolic disease processes such as subsegmental pulmonary emboli, superficial venous thrombus, or distal vein thrombosis. Unfortunately, challenging decisions made by hospitalists frequently fall into one of these categories. Coincident with these guidelines, ASH introduced comprehensive guidelines to support basic diagnostic decisions.7
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand optimal monitoring practices for patients on anticoagulation therapy, including the ideal INR monitoring frequency for patients on VKA therapy. Additionally, there is a need to better understand the difference in clinical outcomes and resources utilization when care is provided by an anticoagulation specialist as compared with a PCP. Finally, while guidelines suggest that anticoagulation should be resumed within 90 days of a life-threatening bleed, there is a need to better understand the optimal timing of a restart, as well as the patient factors to be considered in this decision.
Disclosures
The authors have nothing to disclose.
Funding
There was no funding support in the creation of this manuscript.
1. Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A. Pharmacology of anticoagulants used in the treatment of venous thromboembolism [published correction appears in J Thromb Thrombolysis. 2016;42(2):296-311]. J Thromb Thrombolysis. 2016;41(1):15-31. https://doi.org/10.1007/s11239-015-1314-3.
2. van Walraven C, Austin PC, Oake N, Wells PS, Mamdani M, Forster AJ. The influence of hospitalization on oral anticoagulation control: a population-based study. Thromb Res. 2007;119(6):705-714. PubMed
3. Rodwin BA, Salami JA, Spatz ES, et al. Variation in the use of warfarin and direct oral anticoagulants in atrial fibrillation and associated cost implications. Am J Med. 2019:132(1):61-70. https://doi.org/10.1016/j.amjmed.2018.09.026.
4. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-3291. https://doi.org/10.1182/bloodadvances.2018024893.
5. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012;142(6):1698-1704]. Chest. 2012;141(2 suppl):e419S-e496S. https://doi.org/10.1378/chest.11-2301.
6. Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):299S-339S. https://doi.org/10.1378/chest.08-0675.
7. Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2(22):3226-3256. https://doi.org/10.1182/bloodadvances.2018024828.
1. Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A. Pharmacology of anticoagulants used in the treatment of venous thromboembolism [published correction appears in J Thromb Thrombolysis. 2016;42(2):296-311]. J Thromb Thrombolysis. 2016;41(1):15-31. https://doi.org/10.1007/s11239-015-1314-3.
2. van Walraven C, Austin PC, Oake N, Wells PS, Mamdani M, Forster AJ. The influence of hospitalization on oral anticoagulation control: a population-based study. Thromb Res. 2007;119(6):705-714. PubMed
3. Rodwin BA, Salami JA, Spatz ES, et al. Variation in the use of warfarin and direct oral anticoagulants in atrial fibrillation and associated cost implications. Am J Med. 2019:132(1):61-70. https://doi.org/10.1016/j.amjmed.2018.09.026.
4. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-3291. https://doi.org/10.1182/bloodadvances.2018024893.
5. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012;142(6):1698-1704]. Chest. 2012;141(2 suppl):e419S-e496S. https://doi.org/10.1378/chest.11-2301.
6. Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):299S-339S. https://doi.org/10.1378/chest.08-0675.
7. Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2(22):3226-3256. https://doi.org/10.1182/bloodadvances.2018024828.
© 2019 Society of Hospital Medicine
Treatment of Pediatric Venous Thromboembolism
Venous thromboembolism (VTE) occurs uncommonly in pediatrics, affecting 0.07-0.14 per 10,000 children.1,2 Yet, in the last 20 years, the incidence of VTE in hospitalized children has increased dramatically to approximately 58 per 10,000 admissions.3 This increase may be attributed to improved survival of very ill children, better diagnostic imaging modalities, and heightened awareness by managing physicians.3 Randomized controlled trials are lacking in pediatric thrombosis, and clinical care is based on extrapolation of adult data and expert consensus guidelines.4,5 In 2014, the American Society of Hematology (ASH) sought to develop comprehensive guidelines on thrombosis. The pediatric VTE treatment guideline is one of six published to date.
RECOMMENDATIONS FOR THE HOSPITALIST
The following are five selected guideline recommendations thought most relevant to pediatric hospitalists. Three focus on the central venous access device (CVAD), since it is the most common risk factor for pediatric VTE.1 Recommendations were graded as “strong” if most providers, patients, and policy makers agreed with the intervention and if it was supported by credible research. Conditional recommendations had less uniform agreement with an emphasis on individualized care and weighing patients’ values and preferences.6
Recommendation 1. It is recommended that pediatric patients receive anticoagulation, versus no anticoagulation, for symptomatic VTE (evidence quality: low certainty; recommendation strength: strong).
There is strong indirect data in adults that symptomatic VTE requires treatment, with limited direct evidence in children. As VTE occurs most commonly in ill, hospitalized children with the potential for VTE to be life threatening, the benefit was felt to justify the strong recommendation despite low-quality evidence.
The primary benefit of anticoagulation in children with symptomatic VTE is the prevention of progressive or recurrent thrombosis with high morbidity and the prevention of life-threatening VTE. The greatest potential harm from the use of anticoagulation, particularly in very ill children, is the risk for major bleeding.4Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
The panel focused on the unique features of pediatric VTE related to the heterogeneity in both the site and pathophysiology of VTE in children, such as age, presence of a CVAD, and comorbidities. There is little certainty that treating asymptomatic VTE is beneficial in the same way that treating symptomatic VTE would be in preventing recurrent thrombosis and embolization.
Until better evidence is available to guide care, the primary benefit of this recommendation is individualization of care related to each patient’s risk-benefit profile and parental preferences.
Potential problems with using this recommendation include the cost of anticoagulant drugs and major bleeding if anticoagulation is used. Potential problems with not using anticoagulation would be progressive or recurrent thromboembolism. Close monitoring of children with VTE—regardless of whether anticoagulation is prescribed—is warranted.
Pediatric Patients with Symptomatic CVAD-Related Thrombosis
Recommendations three through five pertain to CVAD-associated thrombosis, so they are reviewed together.
Recommendation 3. No removal of a functioning CVAD is suggested if venous access is still required (evidence quality: low certainty; recommendation strength: conditional).Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).
Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
CVAD is the most common precipitating factor for pediatric VTE, particularly in neonates and older children.1 Based on limited direct and indirect observational studies, there is low evidence of benefit for CVAD removal, but high-quality indirect evidence of harm and high cost, which the panel felt justified the strong recommendation for removing an unneeded or nonfunctioning line. If ongoing care can be safely administered without central access, removing the thrombosis stimulus is recommended. The guideline suggests keeping a functioning CVAD in a patient who requires ongoing venous access and placing high value on avoiding new line insertion when access sites may be limited to avoid the potential thrombogenic effect of new line placement.
In the limited direct and indirect observational studies identified, the optimal timing of CVAD removal is uncertain. Given the potential risk of emboli leading to pulmonary embolism or stroke, prior publications have suggested delaying removal until after three to five days of anticoagulation, particularly in children with known or potential right-to-left shunts.4 The risk of infection and bleeding with anticoagulation prior to CVAD removal was considered small by the panel. This recommendation is primarily based on the panel’s anecdotal experience and first principles, which is a limitation.
CRITIQUE
Methods in Preparing Guideline. The panel included pediatric experts with clinical and research expertise in the guideline topic, including nine hematologists, one intensivist, one cardiologist, one hematology pharmacist, and one anticoagulation nurse practitioner. It also included two methodologists with evidence appraisal and guideline development expertise, as well as two patient representatives.
The panel brainstormed and prioritized questions to be addressed and selected outcomes of interest for each question. The McMaster University GRADE Centre vetted and retained researchers to conduct or update systematic evidence reviews and coordinate the guideline development using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.6 For each guideline question, the results of systematic reviews were summarized in GRADE Evidence-to-Decision tables. The evidence quality was categorized into four levels ranging from ver
Draft recommendations were made available online for review by stakeholders, including allied organizations, medical professionals, patients, and the public. Revisions were made to address pertinent submitted comments, but the recommendations were not changed. After approval by ASH, the guideline was subjected to peer review by Blood Advances.
Sources of Potential Conflict of Interest or Bias. The guideline was developed and funded by ASH. All participants’ conflicts of interest were managed according to ASH policies based on recommendations of the Institute of Medicine and the Guideline International Network. A majority of the guideline panel had no conflicts. During deliberations, panelists with direct financial interests were recused from making judgments about relevant recommendations. The McMaster University-affiliated researchers had no conflicts.Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
AREAS IN NEED OF FUTURE STUDY
Although there is increasing interest in pediatric VTE prevention and risk assessment,7 there is currently limited evidence on the best ways to mitigate VTE risk or anticoagulation-associated major bleeding in hospitalized children. The relatively low incidence of VTE in children makes large randomized controlled trials difficult, but several are ongoing. The Evaluation of the Duration of Therapy for Thrombosis in Children (Kids-DOTT) multicenter, randomized trial will inform care on the optimal duration of anticoagulation in children with a transient provoking factor,8 and several phase III studies are investigating the safety and efficacy of direct oral anticoagulants in children (NCT02234843, NCT02464969, NCT01895777, NCT02234843). These and future trials will better inform therapy in pediatric VTE.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding
No funding was secured for this study.
1. Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian registry of VTE. Blood. 1994;83(5):1251-1257. PubMed
2. van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in the Netherlands. J Pediatr. 2001;139(5):676-681. https://doi.org/10.1067/mpd.2001.118192.
3. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. https://doi.org/10.1542/peds.2009-0768.
4. Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e737S-e801S. https://doi.org/10.1378/chest.11-2308.
5. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786.
6. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. https://doi.org/10.1136/bmj.39489.470347.AD.
7. Faustino EV, Raffini LJ. Prevention of hospital-acquired venous thromboembolism in children: a review of published guidelines. Front Pediatr. 2017;5(9):1597-605. https://doi.org/10.3389/fped.2017.00009.8. Goldenberg NA, Abshire T, Blatchford PJ, et al. Multicenter randomized controlled trial on Duration of Therapy for Thrombosis in Children and Young Adults (the Kids-DOTT trial): pilot/feasibility phase findings. J Thromb Haemost. 2015;13(9):1597-1605. https://doi.org/10.1111/jth.13038.
Venous thromboembolism (VTE) occurs uncommonly in pediatrics, affecting 0.07-0.14 per 10,000 children.1,2 Yet, in the last 20 years, the incidence of VTE in hospitalized children has increased dramatically to approximately 58 per 10,000 admissions.3 This increase may be attributed to improved survival of very ill children, better diagnostic imaging modalities, and heightened awareness by managing physicians.3 Randomized controlled trials are lacking in pediatric thrombosis, and clinical care is based on extrapolation of adult data and expert consensus guidelines.4,5 In 2014, the American Society of Hematology (ASH) sought to develop comprehensive guidelines on thrombosis. The pediatric VTE treatment guideline is one of six published to date.
RECOMMENDATIONS FOR THE HOSPITALIST
The following are five selected guideline recommendations thought most relevant to pediatric hospitalists. Three focus on the central venous access device (CVAD), since it is the most common risk factor for pediatric VTE.1 Recommendations were graded as “strong” if most providers, patients, and policy makers agreed with the intervention and if it was supported by credible research. Conditional recommendations had less uniform agreement with an emphasis on individualized care and weighing patients’ values and preferences.6
Recommendation 1. It is recommended that pediatric patients receive anticoagulation, versus no anticoagulation, for symptomatic VTE (evidence quality: low certainty; recommendation strength: strong).
There is strong indirect data in adults that symptomatic VTE requires treatment, with limited direct evidence in children. As VTE occurs most commonly in ill, hospitalized children with the potential for VTE to be life threatening, the benefit was felt to justify the strong recommendation despite low-quality evidence.
The primary benefit of anticoagulation in children with symptomatic VTE is the prevention of progressive or recurrent thrombosis with high morbidity and the prevention of life-threatening VTE. The greatest potential harm from the use of anticoagulation, particularly in very ill children, is the risk for major bleeding.4Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
The panel focused on the unique features of pediatric VTE related to the heterogeneity in both the site and pathophysiology of VTE in children, such as age, presence of a CVAD, and comorbidities. There is little certainty that treating asymptomatic VTE is beneficial in the same way that treating symptomatic VTE would be in preventing recurrent thrombosis and embolization.
Until better evidence is available to guide care, the primary benefit of this recommendation is individualization of care related to each patient’s risk-benefit profile and parental preferences.
Potential problems with using this recommendation include the cost of anticoagulant drugs and major bleeding if anticoagulation is used. Potential problems with not using anticoagulation would be progressive or recurrent thromboembolism. Close monitoring of children with VTE—regardless of whether anticoagulation is prescribed—is warranted.
Pediatric Patients with Symptomatic CVAD-Related Thrombosis
Recommendations three through five pertain to CVAD-associated thrombosis, so they are reviewed together.
Recommendation 3. No removal of a functioning CVAD is suggested if venous access is still required (evidence quality: low certainty; recommendation strength: conditional).Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).
Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
CVAD is the most common precipitating factor for pediatric VTE, particularly in neonates and older children.1 Based on limited direct and indirect observational studies, there is low evidence of benefit for CVAD removal, but high-quality indirect evidence of harm and high cost, which the panel felt justified the strong recommendation for removing an unneeded or nonfunctioning line. If ongoing care can be safely administered without central access, removing the thrombosis stimulus is recommended. The guideline suggests keeping a functioning CVAD in a patient who requires ongoing venous access and placing high value on avoiding new line insertion when access sites may be limited to avoid the potential thrombogenic effect of new line placement.
In the limited direct and indirect observational studies identified, the optimal timing of CVAD removal is uncertain. Given the potential risk of emboli leading to pulmonary embolism or stroke, prior publications have suggested delaying removal until after three to five days of anticoagulation, particularly in children with known or potential right-to-left shunts.4 The risk of infection and bleeding with anticoagulation prior to CVAD removal was considered small by the panel. This recommendation is primarily based on the panel’s anecdotal experience and first principles, which is a limitation.
CRITIQUE
Methods in Preparing Guideline. The panel included pediatric experts with clinical and research expertise in the guideline topic, including nine hematologists, one intensivist, one cardiologist, one hematology pharmacist, and one anticoagulation nurse practitioner. It also included two methodologists with evidence appraisal and guideline development expertise, as well as two patient representatives.
The panel brainstormed and prioritized questions to be addressed and selected outcomes of interest for each question. The McMaster University GRADE Centre vetted and retained researchers to conduct or update systematic evidence reviews and coordinate the guideline development using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.6 For each guideline question, the results of systematic reviews were summarized in GRADE Evidence-to-Decision tables. The evidence quality was categorized into four levels ranging from ver
Draft recommendations were made available online for review by stakeholders, including allied organizations, medical professionals, patients, and the public. Revisions were made to address pertinent submitted comments, but the recommendations were not changed. After approval by ASH, the guideline was subjected to peer review by Blood Advances.
Sources of Potential Conflict of Interest or Bias. The guideline was developed and funded by ASH. All participants’ conflicts of interest were managed according to ASH policies based on recommendations of the Institute of Medicine and the Guideline International Network. A majority of the guideline panel had no conflicts. During deliberations, panelists with direct financial interests were recused from making judgments about relevant recommendations. The McMaster University-affiliated researchers had no conflicts.Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
AREAS IN NEED OF FUTURE STUDY
Although there is increasing interest in pediatric VTE prevention and risk assessment,7 there is currently limited evidence on the best ways to mitigate VTE risk or anticoagulation-associated major bleeding in hospitalized children. The relatively low incidence of VTE in children makes large randomized controlled trials difficult, but several are ongoing. The Evaluation of the Duration of Therapy for Thrombosis in Children (Kids-DOTT) multicenter, randomized trial will inform care on the optimal duration of anticoagulation in children with a transient provoking factor,8 and several phase III studies are investigating the safety and efficacy of direct oral anticoagulants in children (NCT02234843, NCT02464969, NCT01895777, NCT02234843). These and future trials will better inform therapy in pediatric VTE.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding
No funding was secured for this study.
Venous thromboembolism (VTE) occurs uncommonly in pediatrics, affecting 0.07-0.14 per 10,000 children.1,2 Yet, in the last 20 years, the incidence of VTE in hospitalized children has increased dramatically to approximately 58 per 10,000 admissions.3 This increase may be attributed to improved survival of very ill children, better diagnostic imaging modalities, and heightened awareness by managing physicians.3 Randomized controlled trials are lacking in pediatric thrombosis, and clinical care is based on extrapolation of adult data and expert consensus guidelines.4,5 In 2014, the American Society of Hematology (ASH) sought to develop comprehensive guidelines on thrombosis. The pediatric VTE treatment guideline is one of six published to date.
RECOMMENDATIONS FOR THE HOSPITALIST
The following are five selected guideline recommendations thought most relevant to pediatric hospitalists. Three focus on the central venous access device (CVAD), since it is the most common risk factor for pediatric VTE.1 Recommendations were graded as “strong” if most providers, patients, and policy makers agreed with the intervention and if it was supported by credible research. Conditional recommendations had less uniform agreement with an emphasis on individualized care and weighing patients’ values and preferences.6
Recommendation 1. It is recommended that pediatric patients receive anticoagulation, versus no anticoagulation, for symptomatic VTE (evidence quality: low certainty; recommendation strength: strong).
There is strong indirect data in adults that symptomatic VTE requires treatment, with limited direct evidence in children. As VTE occurs most commonly in ill, hospitalized children with the potential for VTE to be life threatening, the benefit was felt to justify the strong recommendation despite low-quality evidence.
The primary benefit of anticoagulation in children with symptomatic VTE is the prevention of progressive or recurrent thrombosis with high morbidity and the prevention of life-threatening VTE. The greatest potential harm from the use of anticoagulation, particularly in very ill children, is the risk for major bleeding.4Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
The panel focused on the unique features of pediatric VTE related to the heterogeneity in both the site and pathophysiology of VTE in children, such as age, presence of a CVAD, and comorbidities. There is little certainty that treating asymptomatic VTE is beneficial in the same way that treating symptomatic VTE would be in preventing recurrent thrombosis and embolization.
Until better evidence is available to guide care, the primary benefit of this recommendation is individualization of care related to each patient’s risk-benefit profile and parental preferences.
Potential problems with using this recommendation include the cost of anticoagulant drugs and major bleeding if anticoagulation is used. Potential problems with not using anticoagulation would be progressive or recurrent thromboembolism. Close monitoring of children with VTE—regardless of whether anticoagulation is prescribed—is warranted.
Pediatric Patients with Symptomatic CVAD-Related Thrombosis
Recommendations three through five pertain to CVAD-associated thrombosis, so they are reviewed together.
Recommendation 3. No removal of a functioning CVAD is suggested if venous access is still required (evidence quality: low certainty; recommendation strength: conditional).Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).
Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
CVAD is the most common precipitating factor for pediatric VTE, particularly in neonates and older children.1 Based on limited direct and indirect observational studies, there is low evidence of benefit for CVAD removal, but high-quality indirect evidence of harm and high cost, which the panel felt justified the strong recommendation for removing an unneeded or nonfunctioning line. If ongoing care can be safely administered without central access, removing the thrombosis stimulus is recommended. The guideline suggests keeping a functioning CVAD in a patient who requires ongoing venous access and placing high value on avoiding new line insertion when access sites may be limited to avoid the potential thrombogenic effect of new line placement.
In the limited direct and indirect observational studies identified, the optimal timing of CVAD removal is uncertain. Given the potential risk of emboli leading to pulmonary embolism or stroke, prior publications have suggested delaying removal until after three to five days of anticoagulation, particularly in children with known or potential right-to-left shunts.4 The risk of infection and bleeding with anticoagulation prior to CVAD removal was considered small by the panel. This recommendation is primarily based on the panel’s anecdotal experience and first principles, which is a limitation.
CRITIQUE
Methods in Preparing Guideline. The panel included pediatric experts with clinical and research expertise in the guideline topic, including nine hematologists, one intensivist, one cardiologist, one hematology pharmacist, and one anticoagulation nurse practitioner. It also included two methodologists with evidence appraisal and guideline development expertise, as well as two patient representatives.
The panel brainstormed and prioritized questions to be addressed and selected outcomes of interest for each question. The McMaster University GRADE Centre vetted and retained researchers to conduct or update systematic evidence reviews and coordinate the guideline development using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.6 For each guideline question, the results of systematic reviews were summarized in GRADE Evidence-to-Decision tables. The evidence quality was categorized into four levels ranging from ver
Draft recommendations were made available online for review by stakeholders, including allied organizations, medical professionals, patients, and the public. Revisions were made to address pertinent submitted comments, but the recommendations were not changed. After approval by ASH, the guideline was subjected to peer review by Blood Advances.
Sources of Potential Conflict of Interest or Bias. The guideline was developed and funded by ASH. All participants’ conflicts of interest were managed according to ASH policies based on recommendations of the Institute of Medicine and the Guideline International Network. A majority of the guideline panel had no conflicts. During deliberations, panelists with direct financial interests were recused from making judgments about relevant recommendations. The McMaster University-affiliated researchers had no conflicts.Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
AREAS IN NEED OF FUTURE STUDY
Although there is increasing interest in pediatric VTE prevention and risk assessment,7 there is currently limited evidence on the best ways to mitigate VTE risk or anticoagulation-associated major bleeding in hospitalized children. The relatively low incidence of VTE in children makes large randomized controlled trials difficult, but several are ongoing. The Evaluation of the Duration of Therapy for Thrombosis in Children (Kids-DOTT) multicenter, randomized trial will inform care on the optimal duration of anticoagulation in children with a transient provoking factor,8 and several phase III studies are investigating the safety and efficacy of direct oral anticoagulants in children (NCT02234843, NCT02464969, NCT01895777, NCT02234843). These and future trials will better inform therapy in pediatric VTE.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding
No funding was secured for this study.
1. Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian registry of VTE. Blood. 1994;83(5):1251-1257. PubMed
2. van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in the Netherlands. J Pediatr. 2001;139(5):676-681. https://doi.org/10.1067/mpd.2001.118192.
3. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. https://doi.org/10.1542/peds.2009-0768.
4. Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e737S-e801S. https://doi.org/10.1378/chest.11-2308.
5. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786.
6. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. https://doi.org/10.1136/bmj.39489.470347.AD.
7. Faustino EV, Raffini LJ. Prevention of hospital-acquired venous thromboembolism in children: a review of published guidelines. Front Pediatr. 2017;5(9):1597-605. https://doi.org/10.3389/fped.2017.00009.8. Goldenberg NA, Abshire T, Blatchford PJ, et al. Multicenter randomized controlled trial on Duration of Therapy for Thrombosis in Children and Young Adults (the Kids-DOTT trial): pilot/feasibility phase findings. J Thromb Haemost. 2015;13(9):1597-1605. https://doi.org/10.1111/jth.13038.
1. Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian registry of VTE. Blood. 1994;83(5):1251-1257. PubMed
2. van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in the Netherlands. J Pediatr. 2001;139(5):676-681. https://doi.org/10.1067/mpd.2001.118192.
3. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. https://doi.org/10.1542/peds.2009-0768.
4. Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e737S-e801S. https://doi.org/10.1378/chest.11-2308.
5. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786.
6. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. https://doi.org/10.1136/bmj.39489.470347.AD.
7. Faustino EV, Raffini LJ. Prevention of hospital-acquired venous thromboembolism in children: a review of published guidelines. Front Pediatr. 2017;5(9):1597-605. https://doi.org/10.3389/fped.2017.00009.8. Goldenberg NA, Abshire T, Blatchford PJ, et al. Multicenter randomized controlled trial on Duration of Therapy for Thrombosis in Children and Young Adults (the Kids-DOTT trial): pilot/feasibility phase findings. J Thromb Haemost. 2015;13(9):1597-1605. https://doi.org/10.1111/jth.13038.
© 2019 Society of Hospital Medicine
Mission-Driven Criteria for Life and Career
“I think healthcare is more about love than most other things”
—Don Berwick
Dr. Berwick speaks of the relationship between the doctor and the patient and family. I believe this relationship is sacred. My job as CEO of Blue Cross North Carolina is hard. But it was so much harder on a recent weekend to give a new diagnosis of a certainly fatal disease of a less than 1-year old child to her parents and discuss palliative care options. I cried and they cried. Being a leader, particularly in healthcare, requires us to maintain sight of what is important and return to those things often as we le
Growing up, my parents stressed two things: service and education. I decided early on that I wanted to improve our health care system. I have had a sometimes-winding path to this goal - including work as a consultant, medical school and residency, an RWJ Clinical Scholar, clinical work as a pediatric hospitalist and two tours through government as a White House Fellow, the Centers for Medicare and Medicaid Services (CMS) as Chief Medical Officer, Deputy Administrator and leader of the CMS Innovation Center. With each step I have used five criteria that have allowed me to consider decisions while staying true to myself and my mission.
First, Family. My wife and I have four children, age 10 and under. I put them first as I make decisions.
Second, Impact. Better quality, lower costs, and exceptional experience for populations of people. The triple aim, as we better know it.
Third, People. In the beginning, I took jobs to work with specific mentors. Now, I look carefully at the people and culture where I serve to assess fit and how I could uniquely add value.
Fourth, Learning. How much will I learn every day? When I interviewed for my current job, I told them that they could hire an insurance executive who would be better on day one than me, but if they wanted someone who would improve every day and try to make a model of health transformation and a model health plan for the nation, then they should choose me.
Fifth, Joy in Work. Self-explanatory.
We also have a family mission statement, which was my wife’s good idea. We wrote it together right after we were married. It is too personal to share in detail, but it talks about family, public service, commitment to community, life balance, faith, etc. It is short but to the point and has guided us well.
At some point, you will have someone more senior than you who says you must do A before B and then C. My advice: ignore them. Choose your own path. During my journey, I was encouraged to go down a traditional academic path. I did not do it. Yet, somehow, I was elected to the National Academy of Medicine before I turned 40. It was poignant because it was almost the only accomplishment that my father (a PhD scientist), who passed away before I was elected, would have understood.
So please, decide on your criteria and mission for career and life. Write them down, share them if you wish. Then follow them! Passionately! When things are going well, review them. Are you still aligned with what is important to you? When you are at a crossroads to make a decision, review them again. They should help guide your choice.
I often get asked “what keeps me up at night?” Honestly, nothing as I fall asleep in 10 seconds or less. But if something did, it is the fact that I am always worried that someone is falling through the cracks and getting suboptimal care. We must continue to strive to build a more highly reliable health system that delivers better quality, lower costs, and exceptional experience to all people. We cannot do that without great leaders. So, choose your own path, use your mission as a guide and lead focused on a better health system for all!
Disclosures
Dr. Conway has nothing to disclose.
“I think healthcare is more about love than most other things”
—Don Berwick
Dr. Berwick speaks of the relationship between the doctor and the patient and family. I believe this relationship is sacred. My job as CEO of Blue Cross North Carolina is hard. But it was so much harder on a recent weekend to give a new diagnosis of a certainly fatal disease of a less than 1-year old child to her parents and discuss palliative care options. I cried and they cried. Being a leader, particularly in healthcare, requires us to maintain sight of what is important and return to those things often as we le
Growing up, my parents stressed two things: service and education. I decided early on that I wanted to improve our health care system. I have had a sometimes-winding path to this goal - including work as a consultant, medical school and residency, an RWJ Clinical Scholar, clinical work as a pediatric hospitalist and two tours through government as a White House Fellow, the Centers for Medicare and Medicaid Services (CMS) as Chief Medical Officer, Deputy Administrator and leader of the CMS Innovation Center. With each step I have used five criteria that have allowed me to consider decisions while staying true to myself and my mission.
First, Family. My wife and I have four children, age 10 and under. I put them first as I make decisions.
Second, Impact. Better quality, lower costs, and exceptional experience for populations of people. The triple aim, as we better know it.
Third, People. In the beginning, I took jobs to work with specific mentors. Now, I look carefully at the people and culture where I serve to assess fit and how I could uniquely add value.
Fourth, Learning. How much will I learn every day? When I interviewed for my current job, I told them that they could hire an insurance executive who would be better on day one than me, but if they wanted someone who would improve every day and try to make a model of health transformation and a model health plan for the nation, then they should choose me.
Fifth, Joy in Work. Self-explanatory.
We also have a family mission statement, which was my wife’s good idea. We wrote it together right after we were married. It is too personal to share in detail, but it talks about family, public service, commitment to community, life balance, faith, etc. It is short but to the point and has guided us well.
At some point, you will have someone more senior than you who says you must do A before B and then C. My advice: ignore them. Choose your own path. During my journey, I was encouraged to go down a traditional academic path. I did not do it. Yet, somehow, I was elected to the National Academy of Medicine before I turned 40. It was poignant because it was almost the only accomplishment that my father (a PhD scientist), who passed away before I was elected, would have understood.
So please, decide on your criteria and mission for career and life. Write them down, share them if you wish. Then follow them! Passionately! When things are going well, review them. Are you still aligned with what is important to you? When you are at a crossroads to make a decision, review them again. They should help guide your choice.
I often get asked “what keeps me up at night?” Honestly, nothing as I fall asleep in 10 seconds or less. But if something did, it is the fact that I am always worried that someone is falling through the cracks and getting suboptimal care. We must continue to strive to build a more highly reliable health system that delivers better quality, lower costs, and exceptional experience to all people. We cannot do that without great leaders. So, choose your own path, use your mission as a guide and lead focused on a better health system for all!
Disclosures
Dr. Conway has nothing to disclose.
“I think healthcare is more about love than most other things”
—Don Berwick
Dr. Berwick speaks of the relationship between the doctor and the patient and family. I believe this relationship is sacred. My job as CEO of Blue Cross North Carolina is hard. But it was so much harder on a recent weekend to give a new diagnosis of a certainly fatal disease of a less than 1-year old child to her parents and discuss palliative care options. I cried and they cried. Being a leader, particularly in healthcare, requires us to maintain sight of what is important and return to those things often as we le
Growing up, my parents stressed two things: service and education. I decided early on that I wanted to improve our health care system. I have had a sometimes-winding path to this goal - including work as a consultant, medical school and residency, an RWJ Clinical Scholar, clinical work as a pediatric hospitalist and two tours through government as a White House Fellow, the Centers for Medicare and Medicaid Services (CMS) as Chief Medical Officer, Deputy Administrator and leader of the CMS Innovation Center. With each step I have used five criteria that have allowed me to consider decisions while staying true to myself and my mission.
First, Family. My wife and I have four children, age 10 and under. I put them first as I make decisions.
Second, Impact. Better quality, lower costs, and exceptional experience for populations of people. The triple aim, as we better know it.
Third, People. In the beginning, I took jobs to work with specific mentors. Now, I look carefully at the people and culture where I serve to assess fit and how I could uniquely add value.
Fourth, Learning. How much will I learn every day? When I interviewed for my current job, I told them that they could hire an insurance executive who would be better on day one than me, but if they wanted someone who would improve every day and try to make a model of health transformation and a model health plan for the nation, then they should choose me.
Fifth, Joy in Work. Self-explanatory.
We also have a family mission statement, which was my wife’s good idea. We wrote it together right after we were married. It is too personal to share in detail, but it talks about family, public service, commitment to community, life balance, faith, etc. It is short but to the point and has guided us well.
At some point, you will have someone more senior than you who says you must do A before B and then C. My advice: ignore them. Choose your own path. During my journey, I was encouraged to go down a traditional academic path. I did not do it. Yet, somehow, I was elected to the National Academy of Medicine before I turned 40. It was poignant because it was almost the only accomplishment that my father (a PhD scientist), who passed away before I was elected, would have understood.
So please, decide on your criteria and mission for career and life. Write them down, share them if you wish. Then follow them! Passionately! When things are going well, review them. Are you still aligned with what is important to you? When you are at a crossroads to make a decision, review them again. They should help guide your choice.
I often get asked “what keeps me up at night?” Honestly, nothing as I fall asleep in 10 seconds or less. But if something did, it is the fact that I am always worried that someone is falling through the cracks and getting suboptimal care. We must continue to strive to build a more highly reliable health system that delivers better quality, lower costs, and exceptional experience to all people. We cannot do that without great leaders. So, choose your own path, use your mission as a guide and lead focused on a better health system for all!
Disclosures
Dr. Conway has nothing to disclose.
Discharge Medical Complexity, Change in Medical Complexity and Pediatric 30-day Readmission
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
© 2019 Society of Hospital Medicine
Waiting for Godot: The Quest to Promote Scholarship in Hospital Medicine
Twenty years into the hospitalist movement, the proven formula for developing high-quality scholarly output in a hospital medicine group remains elusive. In this issue of the Journal of Hospital Medicine, McKinney et al. describe a new model in which an academic research coach—a PhD-trained researcher with 50% protected time to assist with hospitalist scholarly activities—is utilized to support scholarship.1 Built on the premise that most hospitalist faculty do not have research training and many are embarking on their first academic project, the research coach was available to engage hospitalists at any stage of scholarship from conceptualizing an idea, to submitting one’s first IRB, to data analysis, and grant and manuscript submission. This innovation (and the financial investment required) provides an opportunity to consider how to facilitate scholarship and measure its value in hospital medicine groups.
Academic institutions are built on the premise that scholarship—and research in particular—is of equal value to clinical care and teaching; a perspective that is commonly enshrined in promotion criteria that require scholarship for career advancement. While hospitalists are competent to begin clinical practice and transfer their knowledge to others at the conclusion of their residency, most are not prepared to lead research programs or create academic products from their clinical innovations, quality improvement, or medical education work. Yet, particularly for hospitalists who choose to practice in an academic setting, the leadership of their Section, Division, or Department may naturally expect scholarship to occur, similar to other clinical disciplines. In our experience as the directors of research and faculty development in our hospital medicine group, meeting this expectation requires recognizing that faculty development and scholarship development are intertwined and there must be an investment in both.
We believe that faculty development is required—but not sufficient—for the development of high-quality scholarship. In order for hospitalists to generate new knowledge in clinical, educational, quality improvement, and research domains, they must acquire a new skill set after residency training. These skills can be gained in different formats and time frames such as dedicated hospital medicine fellowships, internal faculty development programs, external programs (eg, Academic Hospitalist Academy), and/or individual mentorship. Descriptions of internal faculty development programs have unfortunately been limited to a single institutions with uncertain generalizability.2,3 One could argue that faculty development may even be more important in hospital medicine than in clinical subspecialties given the relative youth of the field and the experience level of the entry-level faculty. Pediatric hospital medicine may be farthest along in faculty development and scholarship development after becoming a distinct subspecialty recognized by the American Board of Pediatrics and American Board of Medical Specialties; pediatric hospitalists must now complete fellowship training after residency before independent practice.4 Importantly, completion of a scholarly product that advances the field is a required component of the pediatric hospital medicine fellowship curricular framework.5 Regardless of what infrastructure a hospital medicine group chooses to build, there is a growing realization that faculty development must be firmly in place in order for scholarship to flourish.
In addition to junior faculty development, there is also a need for scholarship development to translate new skills into products of scholarship. For example, a well-published senior faculty member still may need statistical assistance and a midcareer hospitalist who leads quality improvement may struggle to write an effective manuscript to disseminate their findings. McKinney et al.’s innovation seems intended to meet this need, and the just-in-time and menu-style nature of the academic research coach resource is unique and novel. One can imagine how this approach to increasing scholarship productivity could be effective and utilized by busy junior, midcareer, and senior hospitalists alike. As the authors point out, this model attempts to mitigate the drawbacks that other models for enhancing hospitalist scholarship have faced, such as relying on physician scientists as mentors, holding works-in-progress or research seminars, or funding a consulting statistician. A well-trained scientist who meets hospitalists “where they are” is appealing when placed in the context of an effective faculty development program that enables faculty to take advantage of this resource. We hope that future evaluations of this promising innovation will include a comparison group to measure the effect of the academic research coach and demonstrate a return on the financial investment supporting the academic research coach.
Measuring return on investment requires defining the value of scholarship in hospital medicine. Some things that are easy to measure and have valence for traditional academic productivity are captured in the McKinney manuscript: the number of abstracts, papers, and grants.
Measuring the value of scholarship in hospital medicine touches very near to the core of the value proposition of hospital medicine overall as a specialty. Without high-quality scholarship that demonstrates the influence of hospitalists in improving systems, leading change, educating learners, and advocating for the needs of our patients, why continue to invest in this model? We are struck every year at the Society of Hospital Medicine national conference about how much innovation hospitalists are leading – and how little is systematically evaluated or disseminated. In Beckett’s “Waiting for Godot,” Vladimir and Estragon talk about life and wait for Godot who, of course, never arrives. Instead of patiently waiting for more scholarship to arrive, we suggest that hospital medicine leaders follow the lead of McKinney et al. and take action by investing in it.
Disclosures
The views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Funding
Dr. Burke is funded by a VA HSR&D Career Development Award.
1. McKinney CM, Mookherjee S, Fihn SD, Gallagher TH. An academic research coach: an innovative approach to increasing scholarly productivity in medicine. J Hosp Med. 2019;14(8):457-461. https://doi.org/10.12788/jhm.3194.
2. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845.
3. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: Insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: a proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
5. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
6. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
Twenty years into the hospitalist movement, the proven formula for developing high-quality scholarly output in a hospital medicine group remains elusive. In this issue of the Journal of Hospital Medicine, McKinney et al. describe a new model in which an academic research coach—a PhD-trained researcher with 50% protected time to assist with hospitalist scholarly activities—is utilized to support scholarship.1 Built on the premise that most hospitalist faculty do not have research training and many are embarking on their first academic project, the research coach was available to engage hospitalists at any stage of scholarship from conceptualizing an idea, to submitting one’s first IRB, to data analysis, and grant and manuscript submission. This innovation (and the financial investment required) provides an opportunity to consider how to facilitate scholarship and measure its value in hospital medicine groups.
Academic institutions are built on the premise that scholarship—and research in particular—is of equal value to clinical care and teaching; a perspective that is commonly enshrined in promotion criteria that require scholarship for career advancement. While hospitalists are competent to begin clinical practice and transfer their knowledge to others at the conclusion of their residency, most are not prepared to lead research programs or create academic products from their clinical innovations, quality improvement, or medical education work. Yet, particularly for hospitalists who choose to practice in an academic setting, the leadership of their Section, Division, or Department may naturally expect scholarship to occur, similar to other clinical disciplines. In our experience as the directors of research and faculty development in our hospital medicine group, meeting this expectation requires recognizing that faculty development and scholarship development are intertwined and there must be an investment in both.
We believe that faculty development is required—but not sufficient—for the development of high-quality scholarship. In order for hospitalists to generate new knowledge in clinical, educational, quality improvement, and research domains, they must acquire a new skill set after residency training. These skills can be gained in different formats and time frames such as dedicated hospital medicine fellowships, internal faculty development programs, external programs (eg, Academic Hospitalist Academy), and/or individual mentorship. Descriptions of internal faculty development programs have unfortunately been limited to a single institutions with uncertain generalizability.2,3 One could argue that faculty development may even be more important in hospital medicine than in clinical subspecialties given the relative youth of the field and the experience level of the entry-level faculty. Pediatric hospital medicine may be farthest along in faculty development and scholarship development after becoming a distinct subspecialty recognized by the American Board of Pediatrics and American Board of Medical Specialties; pediatric hospitalists must now complete fellowship training after residency before independent practice.4 Importantly, completion of a scholarly product that advances the field is a required component of the pediatric hospital medicine fellowship curricular framework.5 Regardless of what infrastructure a hospital medicine group chooses to build, there is a growing realization that faculty development must be firmly in place in order for scholarship to flourish.
In addition to junior faculty development, there is also a need for scholarship development to translate new skills into products of scholarship. For example, a well-published senior faculty member still may need statistical assistance and a midcareer hospitalist who leads quality improvement may struggle to write an effective manuscript to disseminate their findings. McKinney et al.’s innovation seems intended to meet this need, and the just-in-time and menu-style nature of the academic research coach resource is unique and novel. One can imagine how this approach to increasing scholarship productivity could be effective and utilized by busy junior, midcareer, and senior hospitalists alike. As the authors point out, this model attempts to mitigate the drawbacks that other models for enhancing hospitalist scholarship have faced, such as relying on physician scientists as mentors, holding works-in-progress or research seminars, or funding a consulting statistician. A well-trained scientist who meets hospitalists “where they are” is appealing when placed in the context of an effective faculty development program that enables faculty to take advantage of this resource. We hope that future evaluations of this promising innovation will include a comparison group to measure the effect of the academic research coach and demonstrate a return on the financial investment supporting the academic research coach.
Measuring return on investment requires defining the value of scholarship in hospital medicine. Some things that are easy to measure and have valence for traditional academic productivity are captured in the McKinney manuscript: the number of abstracts, papers, and grants.
Measuring the value of scholarship in hospital medicine touches very near to the core of the value proposition of hospital medicine overall as a specialty. Without high-quality scholarship that demonstrates the influence of hospitalists in improving systems, leading change, educating learners, and advocating for the needs of our patients, why continue to invest in this model? We are struck every year at the Society of Hospital Medicine national conference about how much innovation hospitalists are leading – and how little is systematically evaluated or disseminated. In Beckett’s “Waiting for Godot,” Vladimir and Estragon talk about life and wait for Godot who, of course, never arrives. Instead of patiently waiting for more scholarship to arrive, we suggest that hospital medicine leaders follow the lead of McKinney et al. and take action by investing in it.
Disclosures
The views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Funding
Dr. Burke is funded by a VA HSR&D Career Development Award.
Twenty years into the hospitalist movement, the proven formula for developing high-quality scholarly output in a hospital medicine group remains elusive. In this issue of the Journal of Hospital Medicine, McKinney et al. describe a new model in which an academic research coach—a PhD-trained researcher with 50% protected time to assist with hospitalist scholarly activities—is utilized to support scholarship.1 Built on the premise that most hospitalist faculty do not have research training and many are embarking on their first academic project, the research coach was available to engage hospitalists at any stage of scholarship from conceptualizing an idea, to submitting one’s first IRB, to data analysis, and grant and manuscript submission. This innovation (and the financial investment required) provides an opportunity to consider how to facilitate scholarship and measure its value in hospital medicine groups.
Academic institutions are built on the premise that scholarship—and research in particular—is of equal value to clinical care and teaching; a perspective that is commonly enshrined in promotion criteria that require scholarship for career advancement. While hospitalists are competent to begin clinical practice and transfer their knowledge to others at the conclusion of their residency, most are not prepared to lead research programs or create academic products from their clinical innovations, quality improvement, or medical education work. Yet, particularly for hospitalists who choose to practice in an academic setting, the leadership of their Section, Division, or Department may naturally expect scholarship to occur, similar to other clinical disciplines. In our experience as the directors of research and faculty development in our hospital medicine group, meeting this expectation requires recognizing that faculty development and scholarship development are intertwined and there must be an investment in both.
We believe that faculty development is required—but not sufficient—for the development of high-quality scholarship. In order for hospitalists to generate new knowledge in clinical, educational, quality improvement, and research domains, they must acquire a new skill set after residency training. These skills can be gained in different formats and time frames such as dedicated hospital medicine fellowships, internal faculty development programs, external programs (eg, Academic Hospitalist Academy), and/or individual mentorship. Descriptions of internal faculty development programs have unfortunately been limited to a single institutions with uncertain generalizability.2,3 One could argue that faculty development may even be more important in hospital medicine than in clinical subspecialties given the relative youth of the field and the experience level of the entry-level faculty. Pediatric hospital medicine may be farthest along in faculty development and scholarship development after becoming a distinct subspecialty recognized by the American Board of Pediatrics and American Board of Medical Specialties; pediatric hospitalists must now complete fellowship training after residency before independent practice.4 Importantly, completion of a scholarly product that advances the field is a required component of the pediatric hospital medicine fellowship curricular framework.5 Regardless of what infrastructure a hospital medicine group chooses to build, there is a growing realization that faculty development must be firmly in place in order for scholarship to flourish.
In addition to junior faculty development, there is also a need for scholarship development to translate new skills into products of scholarship. For example, a well-published senior faculty member still may need statistical assistance and a midcareer hospitalist who leads quality improvement may struggle to write an effective manuscript to disseminate their findings. McKinney et al.’s innovation seems intended to meet this need, and the just-in-time and menu-style nature of the academic research coach resource is unique and novel. One can imagine how this approach to increasing scholarship productivity could be effective and utilized by busy junior, midcareer, and senior hospitalists alike. As the authors point out, this model attempts to mitigate the drawbacks that other models for enhancing hospitalist scholarship have faced, such as relying on physician scientists as mentors, holding works-in-progress or research seminars, or funding a consulting statistician. A well-trained scientist who meets hospitalists “where they are” is appealing when placed in the context of an effective faculty development program that enables faculty to take advantage of this resource. We hope that future evaluations of this promising innovation will include a comparison group to measure the effect of the academic research coach and demonstrate a return on the financial investment supporting the academic research coach.
Measuring return on investment requires defining the value of scholarship in hospital medicine. Some things that are easy to measure and have valence for traditional academic productivity are captured in the McKinney manuscript: the number of abstracts, papers, and grants.
Measuring the value of scholarship in hospital medicine touches very near to the core of the value proposition of hospital medicine overall as a specialty. Without high-quality scholarship that demonstrates the influence of hospitalists in improving systems, leading change, educating learners, and advocating for the needs of our patients, why continue to invest in this model? We are struck every year at the Society of Hospital Medicine national conference about how much innovation hospitalists are leading – and how little is systematically evaluated or disseminated. In Beckett’s “Waiting for Godot,” Vladimir and Estragon talk about life and wait for Godot who, of course, never arrives. Instead of patiently waiting for more scholarship to arrive, we suggest that hospital medicine leaders follow the lead of McKinney et al. and take action by investing in it.
Disclosures
The views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Funding
Dr. Burke is funded by a VA HSR&D Career Development Award.
1. McKinney CM, Mookherjee S, Fihn SD, Gallagher TH. An academic research coach: an innovative approach to increasing scholarly productivity in medicine. J Hosp Med. 2019;14(8):457-461. https://doi.org/10.12788/jhm.3194.
2. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845.
3. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: Insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: a proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
5. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
6. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
1. McKinney CM, Mookherjee S, Fihn SD, Gallagher TH. An academic research coach: an innovative approach to increasing scholarly productivity in medicine. J Hosp Med. 2019;14(8):457-461. https://doi.org/10.12788/jhm.3194.
2. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845.
3. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: Insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: a proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
5. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
6. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
© 2019 Society of Hospital Medicine
Top Qualifications Hospitalist Leaders Seek in Candidates: Results from a National Survey
Hospital Medicine (HM) is medicine’s fastest growing specialty.1 Rapid expansion of the field has been met with rising interest by young physicians, many of whom are first-time job seekers and may desire information on best practices for applying and interviewing in HM.2-4 However, no prior work has examined HM-specific candidate qualifications and qualities that may be most valued in the hiring process.
As members of the Society of Hospital Medicine (SHM) Physicians in Training Committee, a group charged with “prepar[ing] trainees and early career hospitalists in their transition into hospital medicine,” we aimed to fill this knowledge gap around the HM-specific hiring process.
METHODS
Survey Instrument
The authors developed the survey based on expertise as HM interviewers (JAD, AH, CD, EE, BK, DS, and SM) and local and national interview workshop leaders (JAD, CD, BK, SM). The questionnaire focused on objective applicant qualifications, qualities and attributes displayed during interviews (Appendix 1). Content, length, and reliability of physician understanding were assessed via feedback from local HM group leaders.
Respondents were asked to provide nonidentifying demographics and their role in their HM group’s hiring process. If they reported no role, the survey was terminated. Subsequent standardized HM group demographic questions were adapted from the Society of Hospital Medicine (SHM) State of Hospital Medicine Report.5
Survey questions were multiple choice, ranking and free-response aimed at understanding how respondents assess HM candidate attributes, skills, and behavior. For ranking questions, answer choice order was randomized to reduce answer order-based bias. One free-response question asked the respondent to provide a unique interview question they use that “reveals the most about a hospitalist candidate.” Responses were then individually inserted into the list of choices for a subsequent ranking question regarding the most important qualities a candidate must demonstrate.
Respondents were asked four open-ended questions designed to understand the approach to candidate assessment: (1) use of unique interview questions (as above); (2) identification of “red flags” during interviews; (3) distinctions between assessment of long-term (LT) career hospitalist candidates versus short-term (ST) candidates (eg, those seeking positions prior to fellowship); and (4) key qualifications of ST candidates.
Survey Administration
Survey recipients were identified via SHM administrative rosters. Surveys were distributed electronically via SHM to all current nontrainee physician members who reported a United States mailing address. The survey was determined to not constitute human subjects research by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
Data Analysis
Multiple-choice responses were analyzed descriptively. For ranking-type questions, answers were weighted based on ranking order.
Responses to all open-ended survey questions were analyzed using thematic analysis. We used an iterative process to develop and refine codes identifying key concepts that emerged from the data. Three authors independently coded survey responses. As a group, research team members established the coding framework and resolved discrepancies via discussion to achieve consensus.
RESULTS
Survey links were sent to 8,398 e-mail addresses, of which 7,306 were undeliverable or unopened, leaving 1,092 total eligible respondents. Of these, 347 (31.8%) responded.
A total of 236 respondents reported having a formal role in HM hiring. Of these roles, 79.0% were one-on-one interviewers, 49.6% group interviewers, 45.5% telephone/videoconference interviewers, 41.5% participated on a selection committee, and 32.1% identified as the ultimate decision-maker. Regarding graduate medical education teaching status, 42.0% of respondents identified their primary workplace as a community/affiliated teaching hospital, 33.05% as a university-based teaching hospital, and 23.0% as a nonteaching hospital. Additional characteristics are reported in Appendix 2.
Quantitative Analysis
Respondents ranked the top five qualifications of HM candidates and the top five qualities a candidate should demonstrate on the interview day to be considered for hiring (Table 1).
When asked to rate agreement with the statement “I evaluate and consider all hospital medicine candidates similarly, regardless of whether they articulate an interest in hospital medicine as a long-term career or as a short-term position before fellowship,” 99 (57.23%) respondents disagreed.
Qualitative Analysis
Thematic analysis of responses to open-ended survey questions identified several “red flag” themes (Table 2). Negative interactions with current providers or staff were commonly noted. Additional red flags were a lack of knowledge or interest in the specific HM group, an inability to articulate career goals, or abnormalities in employment history or application materials. Respondents identified an overly strong focus on lifestyle or salary as factors that might limit a candidate’s chance of advancing in the hiring process.
Responses to free-text questions additionally highlighted preferred questioning techniques and approaches to HM candidate assessment (Appendix 3). Many interview questions addressed candidate interest in a particular HM program and candidate responses to challenging scenarios they had encountered. Other questions explored career development. Respondents wanted LT candidates to have specific HM career goals, while they expected ST candidates to demonstrate commitment to and appreciation of HM as a discipline.
Some respondents described their approach to candidate assessment in terms of investment and risk. LT candidates were often viewed as investments in stability and performance; they were evaluated on current abilities and future potential as related to group-specific goals. Some respondents viewed hiring ST candidates as more risky given concerns that they might be less engaged or integrated with the group. Others viewed the hiring of LT candidates as comparably more risky, relating the longer time commitment to the potential for higher impact on the group and patient care. Accordingly, these respondents viewed ST candidate hiring as less risky, estimating their shorter time commitment as having less of a positive or negative impact, with the benefit of addressing urgent staffing issues or unfilled less desirable positions. One respondent summarized: “If they plan to be a career candidate, I care more about them as people and future coworkers. Short term folks are great if we are in a pinch and can deal with personality issues for a short period of time.”
Respondents also described how valued candidate qualities could help mitigate the risk inherent in hiring, especially for ST hires. Strong interpersonal and teamwork skills were highlighted, as well as a demonstrated record of clinical excellence, evidenced by strong training backgrounds and superlative references. A key factor aiding in ST hiring decisions was prior knowledge of the candidate, such as residents or moonlighters previously working in the respondent’s institution. This allowed for familiarity with the candidate’s clinical acumen as well as perceived ease of onboarding and knowledge of the system.
DISCUSSION
We present the results of a national survey of hospitalists identifying candidate attributes, skills, and behaviors viewed most favorably by those involved in the HM hiring process. To our knowledge, this is the first research to be published on the topic of evaluating HM candidates.
Survey respondents identified demonstrable HM candidate clinical skills and experience as highly important, consistent with prior research identifying clinical skills as being among those that hospitalists most value.6 Based on these responses, job seekers should be prepared to discuss objective measures of clinical experience when appropriate, such as number of cases seen or procedures performed. HM groups may accordingly consider the use of hiring rubrics or scoring systems to standardize these measures and reduce bias.
Respondents also highly valued more subjective assessments of HM applicants’ candidacy. The most highly ranked action item was a candidate’s ability to meaningfully respond to a respondent’s customized interview question. There was also a preference for candidates who were knowledgeable about and interested in the specifics of a particular HM group. The high value placed on these elements may suggest the need for formalized coaching or interview preparation for HM candidates. Similarly, interviewer emphasis on customized questions may also highlight an opportunity for HM groups to internally standardize how to best approach subjective components of the interview.
Our heterogeneous findings on the distinctions between ST and LT candidate hiring practices support the need for additional research on the ST HM job market. Until then, our findings reinforce the importance of applicant transparency about ST versus LT career goals. Although many programs may prefer LT candidates over ST candidates, our results suggest ST candidates may benefit from targeting groups with ST needs and using the application process as an opportunity to highlight certain mitigating strengths.
Our study has limitations. While our population included diverse national representation, the response rate and demographics of our respondents may limit generalizability beyond our study population. Respondents represented multiple perspectives within the HM hiring process and were not limited to those making the final hiring decisions. For questions with prespecified multiple-choice answers, answer choices may have influenced participant responses. Our conclusions are based on the reported preferences of those involved in the HM hiring process and not actual hiring behavior. Future research should attempt to identify factors (eg, region, graduate medical education status, practice setting type) that may be responsible for some of the heterogeneous themes we observed in our analysis.
Our research represents introductory work into the previously unpublished topic of HM-specific hiring practices. These findings may provide relevant insight for trainees considering careers in HM, hospitalists reentering the job market, and those involved in career advising, professional development and the HM hiring process.
Acknowledgments
The authors would like to acknowledge current and former members of SHM’s Physicians in Training Committee whose feedback and leadership helped to inspire this project, as well as those students, residents, and hospitalists who have participated in our Hospital Medicine Annual Meeting interview workshop.
Disclosures
The authors have no conflicts of interest to disclose.
1. Wachter RM, Goldman L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
3. Ratelle JT, Dupras DM, Alguire P, Masters P, Weissman A, West CP. Hospitalist career decisions among internal medicine residents. J Gen Intern Med. 2014;29(7):1026-1030. doi: 10.1007/s11606-014-2811-3.
4. Sweigart JR, Tad-Y D, Kneeland P, Williams MV, Glasheen JJ. Hospital medicine resident training tracks: developing the hospital medicine pipeline. J Hosp Med. 2017;12(3):173-176. doi: 10.12788/jhm.2703.
5. 2016 State of Hospital Medicine Report. 2016. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/. Accessed 7/1/2017.
6. Plauth WH, 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Emerg Med. 2001;111(3):247-254. doi: https://doi.org/10.1016/S0002-9343(01)00837-3.
Hospital Medicine (HM) is medicine’s fastest growing specialty.1 Rapid expansion of the field has been met with rising interest by young physicians, many of whom are first-time job seekers and may desire information on best practices for applying and interviewing in HM.2-4 However, no prior work has examined HM-specific candidate qualifications and qualities that may be most valued in the hiring process.
As members of the Society of Hospital Medicine (SHM) Physicians in Training Committee, a group charged with “prepar[ing] trainees and early career hospitalists in their transition into hospital medicine,” we aimed to fill this knowledge gap around the HM-specific hiring process.
METHODS
Survey Instrument
The authors developed the survey based on expertise as HM interviewers (JAD, AH, CD, EE, BK, DS, and SM) and local and national interview workshop leaders (JAD, CD, BK, SM). The questionnaire focused on objective applicant qualifications, qualities and attributes displayed during interviews (Appendix 1). Content, length, and reliability of physician understanding were assessed via feedback from local HM group leaders.
Respondents were asked to provide nonidentifying demographics and their role in their HM group’s hiring process. If they reported no role, the survey was terminated. Subsequent standardized HM group demographic questions were adapted from the Society of Hospital Medicine (SHM) State of Hospital Medicine Report.5
Survey questions were multiple choice, ranking and free-response aimed at understanding how respondents assess HM candidate attributes, skills, and behavior. For ranking questions, answer choice order was randomized to reduce answer order-based bias. One free-response question asked the respondent to provide a unique interview question they use that “reveals the most about a hospitalist candidate.” Responses were then individually inserted into the list of choices for a subsequent ranking question regarding the most important qualities a candidate must demonstrate.
Respondents were asked four open-ended questions designed to understand the approach to candidate assessment: (1) use of unique interview questions (as above); (2) identification of “red flags” during interviews; (3) distinctions between assessment of long-term (LT) career hospitalist candidates versus short-term (ST) candidates (eg, those seeking positions prior to fellowship); and (4) key qualifications of ST candidates.
Survey Administration
Survey recipients were identified via SHM administrative rosters. Surveys were distributed electronically via SHM to all current nontrainee physician members who reported a United States mailing address. The survey was determined to not constitute human subjects research by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
Data Analysis
Multiple-choice responses were analyzed descriptively. For ranking-type questions, answers were weighted based on ranking order.
Responses to all open-ended survey questions were analyzed using thematic analysis. We used an iterative process to develop and refine codes identifying key concepts that emerged from the data. Three authors independently coded survey responses. As a group, research team members established the coding framework and resolved discrepancies via discussion to achieve consensus.
RESULTS
Survey links were sent to 8,398 e-mail addresses, of which 7,306 were undeliverable or unopened, leaving 1,092 total eligible respondents. Of these, 347 (31.8%) responded.
A total of 236 respondents reported having a formal role in HM hiring. Of these roles, 79.0% were one-on-one interviewers, 49.6% group interviewers, 45.5% telephone/videoconference interviewers, 41.5% participated on a selection committee, and 32.1% identified as the ultimate decision-maker. Regarding graduate medical education teaching status, 42.0% of respondents identified their primary workplace as a community/affiliated teaching hospital, 33.05% as a university-based teaching hospital, and 23.0% as a nonteaching hospital. Additional characteristics are reported in Appendix 2.
Quantitative Analysis
Respondents ranked the top five qualifications of HM candidates and the top five qualities a candidate should demonstrate on the interview day to be considered for hiring (Table 1).
When asked to rate agreement with the statement “I evaluate and consider all hospital medicine candidates similarly, regardless of whether they articulate an interest in hospital medicine as a long-term career or as a short-term position before fellowship,” 99 (57.23%) respondents disagreed.
Qualitative Analysis
Thematic analysis of responses to open-ended survey questions identified several “red flag” themes (Table 2). Negative interactions with current providers or staff were commonly noted. Additional red flags were a lack of knowledge or interest in the specific HM group, an inability to articulate career goals, or abnormalities in employment history or application materials. Respondents identified an overly strong focus on lifestyle or salary as factors that might limit a candidate’s chance of advancing in the hiring process.
Responses to free-text questions additionally highlighted preferred questioning techniques and approaches to HM candidate assessment (Appendix 3). Many interview questions addressed candidate interest in a particular HM program and candidate responses to challenging scenarios they had encountered. Other questions explored career development. Respondents wanted LT candidates to have specific HM career goals, while they expected ST candidates to demonstrate commitment to and appreciation of HM as a discipline.
Some respondents described their approach to candidate assessment in terms of investment and risk. LT candidates were often viewed as investments in stability and performance; they were evaluated on current abilities and future potential as related to group-specific goals. Some respondents viewed hiring ST candidates as more risky given concerns that they might be less engaged or integrated with the group. Others viewed the hiring of LT candidates as comparably more risky, relating the longer time commitment to the potential for higher impact on the group and patient care. Accordingly, these respondents viewed ST candidate hiring as less risky, estimating their shorter time commitment as having less of a positive or negative impact, with the benefit of addressing urgent staffing issues or unfilled less desirable positions. One respondent summarized: “If they plan to be a career candidate, I care more about them as people and future coworkers. Short term folks are great if we are in a pinch and can deal with personality issues for a short period of time.”
Respondents also described how valued candidate qualities could help mitigate the risk inherent in hiring, especially for ST hires. Strong interpersonal and teamwork skills were highlighted, as well as a demonstrated record of clinical excellence, evidenced by strong training backgrounds and superlative references. A key factor aiding in ST hiring decisions was prior knowledge of the candidate, such as residents or moonlighters previously working in the respondent’s institution. This allowed for familiarity with the candidate’s clinical acumen as well as perceived ease of onboarding and knowledge of the system.
DISCUSSION
We present the results of a national survey of hospitalists identifying candidate attributes, skills, and behaviors viewed most favorably by those involved in the HM hiring process. To our knowledge, this is the first research to be published on the topic of evaluating HM candidates.
Survey respondents identified demonstrable HM candidate clinical skills and experience as highly important, consistent with prior research identifying clinical skills as being among those that hospitalists most value.6 Based on these responses, job seekers should be prepared to discuss objective measures of clinical experience when appropriate, such as number of cases seen or procedures performed. HM groups may accordingly consider the use of hiring rubrics or scoring systems to standardize these measures and reduce bias.
Respondents also highly valued more subjective assessments of HM applicants’ candidacy. The most highly ranked action item was a candidate’s ability to meaningfully respond to a respondent’s customized interview question. There was also a preference for candidates who were knowledgeable about and interested in the specifics of a particular HM group. The high value placed on these elements may suggest the need for formalized coaching or interview preparation for HM candidates. Similarly, interviewer emphasis on customized questions may also highlight an opportunity for HM groups to internally standardize how to best approach subjective components of the interview.
Our heterogeneous findings on the distinctions between ST and LT candidate hiring practices support the need for additional research on the ST HM job market. Until then, our findings reinforce the importance of applicant transparency about ST versus LT career goals. Although many programs may prefer LT candidates over ST candidates, our results suggest ST candidates may benefit from targeting groups with ST needs and using the application process as an opportunity to highlight certain mitigating strengths.
Our study has limitations. While our population included diverse national representation, the response rate and demographics of our respondents may limit generalizability beyond our study population. Respondents represented multiple perspectives within the HM hiring process and were not limited to those making the final hiring decisions. For questions with prespecified multiple-choice answers, answer choices may have influenced participant responses. Our conclusions are based on the reported preferences of those involved in the HM hiring process and not actual hiring behavior. Future research should attempt to identify factors (eg, region, graduate medical education status, practice setting type) that may be responsible for some of the heterogeneous themes we observed in our analysis.
Our research represents introductory work into the previously unpublished topic of HM-specific hiring practices. These findings may provide relevant insight for trainees considering careers in HM, hospitalists reentering the job market, and those involved in career advising, professional development and the HM hiring process.
Acknowledgments
The authors would like to acknowledge current and former members of SHM’s Physicians in Training Committee whose feedback and leadership helped to inspire this project, as well as those students, residents, and hospitalists who have participated in our Hospital Medicine Annual Meeting interview workshop.
Disclosures
The authors have no conflicts of interest to disclose.
Hospital Medicine (HM) is medicine’s fastest growing specialty.1 Rapid expansion of the field has been met with rising interest by young physicians, many of whom are first-time job seekers and may desire information on best practices for applying and interviewing in HM.2-4 However, no prior work has examined HM-specific candidate qualifications and qualities that may be most valued in the hiring process.
As members of the Society of Hospital Medicine (SHM) Physicians in Training Committee, a group charged with “prepar[ing] trainees and early career hospitalists in their transition into hospital medicine,” we aimed to fill this knowledge gap around the HM-specific hiring process.
METHODS
Survey Instrument
The authors developed the survey based on expertise as HM interviewers (JAD, AH, CD, EE, BK, DS, and SM) and local and national interview workshop leaders (JAD, CD, BK, SM). The questionnaire focused on objective applicant qualifications, qualities and attributes displayed during interviews (Appendix 1). Content, length, and reliability of physician understanding were assessed via feedback from local HM group leaders.
Respondents were asked to provide nonidentifying demographics and their role in their HM group’s hiring process. If they reported no role, the survey was terminated. Subsequent standardized HM group demographic questions were adapted from the Society of Hospital Medicine (SHM) State of Hospital Medicine Report.5
Survey questions were multiple choice, ranking and free-response aimed at understanding how respondents assess HM candidate attributes, skills, and behavior. For ranking questions, answer choice order was randomized to reduce answer order-based bias. One free-response question asked the respondent to provide a unique interview question they use that “reveals the most about a hospitalist candidate.” Responses were then individually inserted into the list of choices for a subsequent ranking question regarding the most important qualities a candidate must demonstrate.
Respondents were asked four open-ended questions designed to understand the approach to candidate assessment: (1) use of unique interview questions (as above); (2) identification of “red flags” during interviews; (3) distinctions between assessment of long-term (LT) career hospitalist candidates versus short-term (ST) candidates (eg, those seeking positions prior to fellowship); and (4) key qualifications of ST candidates.
Survey Administration
Survey recipients were identified via SHM administrative rosters. Surveys were distributed electronically via SHM to all current nontrainee physician members who reported a United States mailing address. The survey was determined to not constitute human subjects research by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
Data Analysis
Multiple-choice responses were analyzed descriptively. For ranking-type questions, answers were weighted based on ranking order.
Responses to all open-ended survey questions were analyzed using thematic analysis. We used an iterative process to develop and refine codes identifying key concepts that emerged from the data. Three authors independently coded survey responses. As a group, research team members established the coding framework and resolved discrepancies via discussion to achieve consensus.
RESULTS
Survey links were sent to 8,398 e-mail addresses, of which 7,306 were undeliverable or unopened, leaving 1,092 total eligible respondents. Of these, 347 (31.8%) responded.
A total of 236 respondents reported having a formal role in HM hiring. Of these roles, 79.0% were one-on-one interviewers, 49.6% group interviewers, 45.5% telephone/videoconference interviewers, 41.5% participated on a selection committee, and 32.1% identified as the ultimate decision-maker. Regarding graduate medical education teaching status, 42.0% of respondents identified their primary workplace as a community/affiliated teaching hospital, 33.05% as a university-based teaching hospital, and 23.0% as a nonteaching hospital. Additional characteristics are reported in Appendix 2.
Quantitative Analysis
Respondents ranked the top five qualifications of HM candidates and the top five qualities a candidate should demonstrate on the interview day to be considered for hiring (Table 1).
When asked to rate agreement with the statement “I evaluate and consider all hospital medicine candidates similarly, regardless of whether they articulate an interest in hospital medicine as a long-term career or as a short-term position before fellowship,” 99 (57.23%) respondents disagreed.
Qualitative Analysis
Thematic analysis of responses to open-ended survey questions identified several “red flag” themes (Table 2). Negative interactions with current providers or staff were commonly noted. Additional red flags were a lack of knowledge or interest in the specific HM group, an inability to articulate career goals, or abnormalities in employment history or application materials. Respondents identified an overly strong focus on lifestyle or salary as factors that might limit a candidate’s chance of advancing in the hiring process.
Responses to free-text questions additionally highlighted preferred questioning techniques and approaches to HM candidate assessment (Appendix 3). Many interview questions addressed candidate interest in a particular HM program and candidate responses to challenging scenarios they had encountered. Other questions explored career development. Respondents wanted LT candidates to have specific HM career goals, while they expected ST candidates to demonstrate commitment to and appreciation of HM as a discipline.
Some respondents described their approach to candidate assessment in terms of investment and risk. LT candidates were often viewed as investments in stability and performance; they were evaluated on current abilities and future potential as related to group-specific goals. Some respondents viewed hiring ST candidates as more risky given concerns that they might be less engaged or integrated with the group. Others viewed the hiring of LT candidates as comparably more risky, relating the longer time commitment to the potential for higher impact on the group and patient care. Accordingly, these respondents viewed ST candidate hiring as less risky, estimating their shorter time commitment as having less of a positive or negative impact, with the benefit of addressing urgent staffing issues or unfilled less desirable positions. One respondent summarized: “If they plan to be a career candidate, I care more about them as people and future coworkers. Short term folks are great if we are in a pinch and can deal with personality issues for a short period of time.”
Respondents also described how valued candidate qualities could help mitigate the risk inherent in hiring, especially for ST hires. Strong interpersonal and teamwork skills were highlighted, as well as a demonstrated record of clinical excellence, evidenced by strong training backgrounds and superlative references. A key factor aiding in ST hiring decisions was prior knowledge of the candidate, such as residents or moonlighters previously working in the respondent’s institution. This allowed for familiarity with the candidate’s clinical acumen as well as perceived ease of onboarding and knowledge of the system.
DISCUSSION
We present the results of a national survey of hospitalists identifying candidate attributes, skills, and behaviors viewed most favorably by those involved in the HM hiring process. To our knowledge, this is the first research to be published on the topic of evaluating HM candidates.
Survey respondents identified demonstrable HM candidate clinical skills and experience as highly important, consistent with prior research identifying clinical skills as being among those that hospitalists most value.6 Based on these responses, job seekers should be prepared to discuss objective measures of clinical experience when appropriate, such as number of cases seen or procedures performed. HM groups may accordingly consider the use of hiring rubrics or scoring systems to standardize these measures and reduce bias.
Respondents also highly valued more subjective assessments of HM applicants’ candidacy. The most highly ranked action item was a candidate’s ability to meaningfully respond to a respondent’s customized interview question. There was also a preference for candidates who were knowledgeable about and interested in the specifics of a particular HM group. The high value placed on these elements may suggest the need for formalized coaching or interview preparation for HM candidates. Similarly, interviewer emphasis on customized questions may also highlight an opportunity for HM groups to internally standardize how to best approach subjective components of the interview.
Our heterogeneous findings on the distinctions between ST and LT candidate hiring practices support the need for additional research on the ST HM job market. Until then, our findings reinforce the importance of applicant transparency about ST versus LT career goals. Although many programs may prefer LT candidates over ST candidates, our results suggest ST candidates may benefit from targeting groups with ST needs and using the application process as an opportunity to highlight certain mitigating strengths.
Our study has limitations. While our population included diverse national representation, the response rate and demographics of our respondents may limit generalizability beyond our study population. Respondents represented multiple perspectives within the HM hiring process and were not limited to those making the final hiring decisions. For questions with prespecified multiple-choice answers, answer choices may have influenced participant responses. Our conclusions are based on the reported preferences of those involved in the HM hiring process and not actual hiring behavior. Future research should attempt to identify factors (eg, region, graduate medical education status, practice setting type) that may be responsible for some of the heterogeneous themes we observed in our analysis.
Our research represents introductory work into the previously unpublished topic of HM-specific hiring practices. These findings may provide relevant insight for trainees considering careers in HM, hospitalists reentering the job market, and those involved in career advising, professional development and the HM hiring process.
Acknowledgments
The authors would like to acknowledge current and former members of SHM’s Physicians in Training Committee whose feedback and leadership helped to inspire this project, as well as those students, residents, and hospitalists who have participated in our Hospital Medicine Annual Meeting interview workshop.
Disclosures
The authors have no conflicts of interest to disclose.
1. Wachter RM, Goldman L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
3. Ratelle JT, Dupras DM, Alguire P, Masters P, Weissman A, West CP. Hospitalist career decisions among internal medicine residents. J Gen Intern Med. 2014;29(7):1026-1030. doi: 10.1007/s11606-014-2811-3.
4. Sweigart JR, Tad-Y D, Kneeland P, Williams MV, Glasheen JJ. Hospital medicine resident training tracks: developing the hospital medicine pipeline. J Hosp Med. 2017;12(3):173-176. doi: 10.12788/jhm.2703.
5. 2016 State of Hospital Medicine Report. 2016. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/. Accessed 7/1/2017.
6. Plauth WH, 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Emerg Med. 2001;111(3):247-254. doi: https://doi.org/10.1016/S0002-9343(01)00837-3.
1. Wachter RM, Goldman L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
3. Ratelle JT, Dupras DM, Alguire P, Masters P, Weissman A, West CP. Hospitalist career decisions among internal medicine residents. J Gen Intern Med. 2014;29(7):1026-1030. doi: 10.1007/s11606-014-2811-3.
4. Sweigart JR, Tad-Y D, Kneeland P, Williams MV, Glasheen JJ. Hospital medicine resident training tracks: developing the hospital medicine pipeline. J Hosp Med. 2017;12(3):173-176. doi: 10.12788/jhm.2703.
5. 2016 State of Hospital Medicine Report. 2016. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/. Accessed 7/1/2017.
6. Plauth WH, 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Emerg Med. 2001;111(3):247-254. doi: https://doi.org/10.1016/S0002-9343(01)00837-3.
© 2019 Society of Hospital Medicine
Discrepant Advanced Directives and Code Status Orders: A Preventable Medical Error
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
© 2019 Society of Hospital Medicine
Pain in the United States: Time for a Culture Shift in Expectations, Messaging, and Management
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
© 2019 Society of Hospital Medicine