User login
Transthyretin (Prealbumin) and the Ambiguous Nature of Malnutrition
Lacy and colleagues identify an important “Thing We Do For No Reason”—prealbumin testing to diagnose malnutrition in hospitalized patients.1 They highlight the frequency and costs of ordering prealbumin tests although prealbumin is neither specific nor sensitive as a “marker of nutritional status,” shows
The term is also used to mean a condition where evidence shows better patient outcomes when improved nutrition is provided. Distinguishing between these two meanings is essential, as numerous patients with inflammatory illness will present abnormal “markers” when good evidence shows that they cannot benefit from nutritional support.
For example, a patient with advanced untreated human immunodeficiency virus (HIV) is likely to be considered malnourished because all of her “markers of nutritional status” are abnormal. She barely eats, has lost weight, and has low anthropometric, immunologic, and serologic measures, poor functional status, extreme vulnerability, and very poor prognosis. In this way she resembles a person in a famine situation. However, the patient is not malnourished in the sense that improved nutrient intake will lead to better patient outcomes. A Cochrane review of “nutritional interventions for reducing morbidity and mortality in people with HIV” found “no evidence that such supplementation translates into reductions in disease progression or HIV‐related complications, such as opportunistic infections or death.”2 The patient is dying of an inflammatory, cachectic illness. The same is true in managing patients with advanced cancer or several other serious illnesses.
Low prealbumin measures are associated with poor outcomes, which are then attributed to “malnutrition.” However, as Lacy and colleagues argue, prealbumin is a negative acute phase reactant and is thus a marker of the inflammatory effects of sickness/injury; it also responds variably to nutritional support. Citing Koretz, they note that “even when changes in nutritional markers are seen with nutritional support, the ‘changes in nutritional markers do not predict clinical outcomes.’”1,3 We know of no evidence from randomized controlled trials that prealbumin measurements help identify patients who can benefit from nutrition support.
By contrast, we and our colleagues have shown that in people who barely eat but show no inflammatory disease, eg, prison hunger-strikers and patients with anorexia nervosa, prealbumin level remains normal down to a body mass index below 13. The same is generally true for albumin.4 These measures fail to identify “malnutrition” in people who are starving.
Despite the complete lack of clinical trial evidence of benefit, prealbumin is widely used as an indicator of malnutrition. The National Institutes of Health’s Medline Plus website for the general public lists low prealbumin levels as a possible sign of malnutrition, for example, and advises that the prealbumin test may be used to “find out if you are getting enough nutrients, especially protein, in your diet” and to “check to see if you are getting enough nutrition if you are in the hospital.”5 Unjustified assertions such as these contribute to the dramatic overuse of nutritional interventions.
However, as a rule, things do occur for a reason. Using the term “prealbumin” conjures a certain relationship, perhaps as a precursor, to albumin, a venerable (but valueless) “marker of nutrition status.” In fact, the term refers only to a difference in electrophoretic mobility (prealbumin migrates faster). If prealbumin were called it by its proper name, transthyretin, it would probably have languished in obscurity among serum proteins until, in recent years, drug suppression of transthyretin synthesis has been shown to benefit patients with hereditary transthyretin amyloidosis.6 Using a name that references albumin, this protein has found the limelight as a marker of nutritional status.
The close similarity in appearance between starvation and wasting illness enables the strong, largely evidence-free7 emphasis on nutrition support. Many families and individuals suffer when a loved one loses weight. As a prominent reminder of serious illness, this wasted appearance can be painful to bear. Several caregivers may fear that they will be judged as neglectful by outside observers. Other individuals also wish to maintain their body weight for social reasons (as weight loss may be interpreted as a sign of illness, especially HIV). Nutrition maintains a special status in various contexts during the care of sick patients, and the drive to provide food to individuals who appear undernourished seems fundamental in humans.
A third reason for the frivolous, widespread overdiagnosis of “malnutrition” is that it leads directly to favorable consequences for the multibillion-dollar nutritional support industry. A consistent rational approach to the use of nutritional support products for sick people would lead to multibillion-dollar harm for that industry. For now, however, no self-respecting clinician could fail to provide nutritional support to a patient diagnosed as “malnourished” regardless of evidence.
The consistent rational approach in caring for patients is to search for good evidence of benefit before initiating a treatment course. Although sending blood tests for “nutritional markers” to diagnose nutritional needs may be easier and more popular, we caution against such over-simplification. Using prealbumin as a marker for malnutrition could lead to overlooking potentially treatable inflammatory or infectious illness. On the other hand, the use of prealbumin could also lead to unnecessary and potentially dangerous treatments, such as feeding tube placement and/or total parental nutrition. Thus, with one small amendment, we fully support Lacy and colleagues’ conclusion that prealbumin testing to identify malnutrition in hospitalized patients is a “Thing We Do For No (good) Reason.”
Disclosures
Drs. Lee and Finucane declare no financial conflicts of interest. Dr. Finucane discloses that he serves the pharmacy committee of an insurance company.
1. Lacy M, Roesch J, Langsjoen J. Things we do for no reason: prealbumin testing to diagnose malnutrition in the hospitalized patient. J Hosp Med. 2019;14(4):239-241. PubMed
2. Grobler L, Siegfried N, Visser ME, Mahlungulu SSN, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database Syst Rev. 2013;28(2):CD004536. PubMed
3. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
4. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-e22. PubMed
5. Prealbumin Blood Test. https://medlineplus.gov/lab-tests/prealbumin-blood-test/, updated June 14, 2018. Accessed November 12, 2018.
6. Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22-31. PubMed
7. U.S. dietary guidelines: an evidence-free zone. Ann Intern Med. 2016;164(8):558-559. PubMed
Lacy and colleagues identify an important “Thing We Do For No Reason”—prealbumin testing to diagnose malnutrition in hospitalized patients.1 They highlight the frequency and costs of ordering prealbumin tests although prealbumin is neither specific nor sensitive as a “marker of nutritional status,” shows
The term is also used to mean a condition where evidence shows better patient outcomes when improved nutrition is provided. Distinguishing between these two meanings is essential, as numerous patients with inflammatory illness will present abnormal “markers” when good evidence shows that they cannot benefit from nutritional support.
For example, a patient with advanced untreated human immunodeficiency virus (HIV) is likely to be considered malnourished because all of her “markers of nutritional status” are abnormal. She barely eats, has lost weight, and has low anthropometric, immunologic, and serologic measures, poor functional status, extreme vulnerability, and very poor prognosis. In this way she resembles a person in a famine situation. However, the patient is not malnourished in the sense that improved nutrient intake will lead to better patient outcomes. A Cochrane review of “nutritional interventions for reducing morbidity and mortality in people with HIV” found “no evidence that such supplementation translates into reductions in disease progression or HIV‐related complications, such as opportunistic infections or death.”2 The patient is dying of an inflammatory, cachectic illness. The same is true in managing patients with advanced cancer or several other serious illnesses.
Low prealbumin measures are associated with poor outcomes, which are then attributed to “malnutrition.” However, as Lacy and colleagues argue, prealbumin is a negative acute phase reactant and is thus a marker of the inflammatory effects of sickness/injury; it also responds variably to nutritional support. Citing Koretz, they note that “even when changes in nutritional markers are seen with nutritional support, the ‘changes in nutritional markers do not predict clinical outcomes.’”1,3 We know of no evidence from randomized controlled trials that prealbumin measurements help identify patients who can benefit from nutrition support.
By contrast, we and our colleagues have shown that in people who barely eat but show no inflammatory disease, eg, prison hunger-strikers and patients with anorexia nervosa, prealbumin level remains normal down to a body mass index below 13. The same is generally true for albumin.4 These measures fail to identify “malnutrition” in people who are starving.
Despite the complete lack of clinical trial evidence of benefit, prealbumin is widely used as an indicator of malnutrition. The National Institutes of Health’s Medline Plus website for the general public lists low prealbumin levels as a possible sign of malnutrition, for example, and advises that the prealbumin test may be used to “find out if you are getting enough nutrients, especially protein, in your diet” and to “check to see if you are getting enough nutrition if you are in the hospital.”5 Unjustified assertions such as these contribute to the dramatic overuse of nutritional interventions.
However, as a rule, things do occur for a reason. Using the term “prealbumin” conjures a certain relationship, perhaps as a precursor, to albumin, a venerable (but valueless) “marker of nutrition status.” In fact, the term refers only to a difference in electrophoretic mobility (prealbumin migrates faster). If prealbumin were called it by its proper name, transthyretin, it would probably have languished in obscurity among serum proteins until, in recent years, drug suppression of transthyretin synthesis has been shown to benefit patients with hereditary transthyretin amyloidosis.6 Using a name that references albumin, this protein has found the limelight as a marker of nutritional status.
The close similarity in appearance between starvation and wasting illness enables the strong, largely evidence-free7 emphasis on nutrition support. Many families and individuals suffer when a loved one loses weight. As a prominent reminder of serious illness, this wasted appearance can be painful to bear. Several caregivers may fear that they will be judged as neglectful by outside observers. Other individuals also wish to maintain their body weight for social reasons (as weight loss may be interpreted as a sign of illness, especially HIV). Nutrition maintains a special status in various contexts during the care of sick patients, and the drive to provide food to individuals who appear undernourished seems fundamental in humans.
A third reason for the frivolous, widespread overdiagnosis of “malnutrition” is that it leads directly to favorable consequences for the multibillion-dollar nutritional support industry. A consistent rational approach to the use of nutritional support products for sick people would lead to multibillion-dollar harm for that industry. For now, however, no self-respecting clinician could fail to provide nutritional support to a patient diagnosed as “malnourished” regardless of evidence.
The consistent rational approach in caring for patients is to search for good evidence of benefit before initiating a treatment course. Although sending blood tests for “nutritional markers” to diagnose nutritional needs may be easier and more popular, we caution against such over-simplification. Using prealbumin as a marker for malnutrition could lead to overlooking potentially treatable inflammatory or infectious illness. On the other hand, the use of prealbumin could also lead to unnecessary and potentially dangerous treatments, such as feeding tube placement and/or total parental nutrition. Thus, with one small amendment, we fully support Lacy and colleagues’ conclusion that prealbumin testing to identify malnutrition in hospitalized patients is a “Thing We Do For No (good) Reason.”
Disclosures
Drs. Lee and Finucane declare no financial conflicts of interest. Dr. Finucane discloses that he serves the pharmacy committee of an insurance company.
Lacy and colleagues identify an important “Thing We Do For No Reason”—prealbumin testing to diagnose malnutrition in hospitalized patients.1 They highlight the frequency and costs of ordering prealbumin tests although prealbumin is neither specific nor sensitive as a “marker of nutritional status,” shows
The term is also used to mean a condition where evidence shows better patient outcomes when improved nutrition is provided. Distinguishing between these two meanings is essential, as numerous patients with inflammatory illness will present abnormal “markers” when good evidence shows that they cannot benefit from nutritional support.
For example, a patient with advanced untreated human immunodeficiency virus (HIV) is likely to be considered malnourished because all of her “markers of nutritional status” are abnormal. She barely eats, has lost weight, and has low anthropometric, immunologic, and serologic measures, poor functional status, extreme vulnerability, and very poor prognosis. In this way she resembles a person in a famine situation. However, the patient is not malnourished in the sense that improved nutrient intake will lead to better patient outcomes. A Cochrane review of “nutritional interventions for reducing morbidity and mortality in people with HIV” found “no evidence that such supplementation translates into reductions in disease progression or HIV‐related complications, such as opportunistic infections or death.”2 The patient is dying of an inflammatory, cachectic illness. The same is true in managing patients with advanced cancer or several other serious illnesses.
Low prealbumin measures are associated with poor outcomes, which are then attributed to “malnutrition.” However, as Lacy and colleagues argue, prealbumin is a negative acute phase reactant and is thus a marker of the inflammatory effects of sickness/injury; it also responds variably to nutritional support. Citing Koretz, they note that “even when changes in nutritional markers are seen with nutritional support, the ‘changes in nutritional markers do not predict clinical outcomes.’”1,3 We know of no evidence from randomized controlled trials that prealbumin measurements help identify patients who can benefit from nutrition support.
By contrast, we and our colleagues have shown that in people who barely eat but show no inflammatory disease, eg, prison hunger-strikers and patients with anorexia nervosa, prealbumin level remains normal down to a body mass index below 13. The same is generally true for albumin.4 These measures fail to identify “malnutrition” in people who are starving.
Despite the complete lack of clinical trial evidence of benefit, prealbumin is widely used as an indicator of malnutrition. The National Institutes of Health’s Medline Plus website for the general public lists low prealbumin levels as a possible sign of malnutrition, for example, and advises that the prealbumin test may be used to “find out if you are getting enough nutrients, especially protein, in your diet” and to “check to see if you are getting enough nutrition if you are in the hospital.”5 Unjustified assertions such as these contribute to the dramatic overuse of nutritional interventions.
However, as a rule, things do occur for a reason. Using the term “prealbumin” conjures a certain relationship, perhaps as a precursor, to albumin, a venerable (but valueless) “marker of nutrition status.” In fact, the term refers only to a difference in electrophoretic mobility (prealbumin migrates faster). If prealbumin were called it by its proper name, transthyretin, it would probably have languished in obscurity among serum proteins until, in recent years, drug suppression of transthyretin synthesis has been shown to benefit patients with hereditary transthyretin amyloidosis.6 Using a name that references albumin, this protein has found the limelight as a marker of nutritional status.
The close similarity in appearance between starvation and wasting illness enables the strong, largely evidence-free7 emphasis on nutrition support. Many families and individuals suffer when a loved one loses weight. As a prominent reminder of serious illness, this wasted appearance can be painful to bear. Several caregivers may fear that they will be judged as neglectful by outside observers. Other individuals also wish to maintain their body weight for social reasons (as weight loss may be interpreted as a sign of illness, especially HIV). Nutrition maintains a special status in various contexts during the care of sick patients, and the drive to provide food to individuals who appear undernourished seems fundamental in humans.
A third reason for the frivolous, widespread overdiagnosis of “malnutrition” is that it leads directly to favorable consequences for the multibillion-dollar nutritional support industry. A consistent rational approach to the use of nutritional support products for sick people would lead to multibillion-dollar harm for that industry. For now, however, no self-respecting clinician could fail to provide nutritional support to a patient diagnosed as “malnourished” regardless of evidence.
The consistent rational approach in caring for patients is to search for good evidence of benefit before initiating a treatment course. Although sending blood tests for “nutritional markers” to diagnose nutritional needs may be easier and more popular, we caution against such over-simplification. Using prealbumin as a marker for malnutrition could lead to overlooking potentially treatable inflammatory or infectious illness. On the other hand, the use of prealbumin could also lead to unnecessary and potentially dangerous treatments, such as feeding tube placement and/or total parental nutrition. Thus, with one small amendment, we fully support Lacy and colleagues’ conclusion that prealbumin testing to identify malnutrition in hospitalized patients is a “Thing We Do For No (good) Reason.”
Disclosures
Drs. Lee and Finucane declare no financial conflicts of interest. Dr. Finucane discloses that he serves the pharmacy committee of an insurance company.
1. Lacy M, Roesch J, Langsjoen J. Things we do for no reason: prealbumin testing to diagnose malnutrition in the hospitalized patient. J Hosp Med. 2019;14(4):239-241. PubMed
2. Grobler L, Siegfried N, Visser ME, Mahlungulu SSN, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database Syst Rev. 2013;28(2):CD004536. PubMed
3. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
4. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-e22. PubMed
5. Prealbumin Blood Test. https://medlineplus.gov/lab-tests/prealbumin-blood-test/, updated June 14, 2018. Accessed November 12, 2018.
6. Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22-31. PubMed
7. U.S. dietary guidelines: an evidence-free zone. Ann Intern Med. 2016;164(8):558-559. PubMed
1. Lacy M, Roesch J, Langsjoen J. Things we do for no reason: prealbumin testing to diagnose malnutrition in the hospitalized patient. J Hosp Med. 2019;14(4):239-241. PubMed
2. Grobler L, Siegfried N, Visser ME, Mahlungulu SSN, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database Syst Rev. 2013;28(2):CD004536. PubMed
3. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
4. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-e22. PubMed
5. Prealbumin Blood Test. https://medlineplus.gov/lab-tests/prealbumin-blood-test/, updated June 14, 2018. Accessed November 12, 2018.
6. Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22-31. PubMed
7. U.S. dietary guidelines: an evidence-free zone. Ann Intern Med. 2016;164(8):558-559. PubMed
© 2019 Society of Hospital Medicine
Things We Do for No Reason: Routine Echocardiography in Hemodynamically Stable Patients with Acute Pulmonary Embolism
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
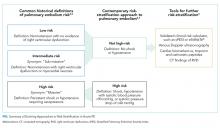
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
© 2019 Society of Hospital Medicine
The Right Frame
A 65-year-old man was transferred to a tertiary academic medical center with one week of progressive shortness of breath, dry cough, and fevers. He reported no weight loss or night sweats but had experienced mild right upper quadrant pain and anorexia for the preceding three weeks. Several years had passed since he had consulted a physician, and he did not take any medications. He immigrated to the United States from Mexico four decades prior. He traveled back frequently to visit his family, most recently one month before his presentation. He worked as a farming supervisor in the Central Valley of California. He smoked tobacco and had a 30 pack-year history. He drank alcohol occasionally and denied any drug use.
Causes of subacute cough and dyspnea include bronchitis, pneumonia, heart failure, and asthma. Pneumonia and heart failure might cause right upper quadrant pain from diaphragmatic irritation and hepatic congestion, respectively. Metastatic cancer or infection may lead to synchronous pulmonary and hepatic involvement. The patient is at increased risk of lung cancer, given his extensive smoking history.
The patient’s place of residence in the Southwestern United States places him at risk of respiratory illness from coccidioidomycosis. His exact involvement with animals and their products should be further explored. For example, consumption of unpasteurized milk might result in pneumonia, hepatitis, or both from M. bovis, Brucella species, or C. burnetii. His travel to Mexico prompts consideration of tuberculosis, histoplasmosis, and paracoccidiomycosis as causes of respiratory and possible hepatic illness.
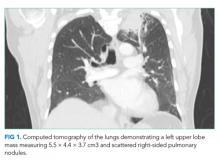
Two weeks prior, the patient had initially presented to another hospital with one week of intermittent right upper quadrant pain unrelated to eating. An abdominal ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were normal. Computed tomography (CT) of the chest, abdomen, and pelvis with contrast demonstrated a left upper lobe lung mass measuring 5.5 × 4.4 × 3.7 cm3 and scattered right-sided pulmonary nodules (Figure 1). He underwent CT-guided biopsy of the mass and was discharged with a presumed diagnosis of primary pulmonary malignancy with plans for outpatient follow-up.
Over the next four days, the patient developed progressive dyspnea with cough and subjective fevers. The patient was readmitted with a diagnosis of postobstructive pneumonia and acute kidney injury (creatinine increased from 0.7 mg/dL to 2.9 mg/dL between admissions), and this finding was attributed to contrast-induced nephropathy from his recent CT scan. He was treated with vancomycin and piperacillin/tazobactam for two days but wished to transfer to a tertiary care hospital for a second opinion.
Postobstructive pneumonia, pulmonary embolism, and pleural effusion are common causes of dyspnea in patients with lung cancer. The patient’s travel and occupational history, lung nodules, acute renal insufficiency, and rapidly progressive respiratory symptoms prompt consideration for radiographic mimickers of lung cancer. Tuberculosis might present as a lung mass (pulmonary tuberculoma) during primary infection or reactivation. Noninfectious causes of pulmonary masses and nodules include metastatic cancer (eg, colon cancer), sarcoidosis, IgG4-related disease, and granulomatous polyangiitis (GPA).
Contrast-induced nephropathy is unusual in patients with normal renal function. More probable explanations include hypovolemia or acute tubular necrosis (ATN) from underlying inflammation. The patient’s CT-negative right upper quadrant pain may be a distinct process or represent another facet of a disseminated illness such as hepatic infiltration from lymphoma.
Upon arrival, the patient’s temperature was 38°C, heart rate (HR) 107 beats per minute, blood pressure (BP) 159/89 mm Hg, respiratory rate 25 breaths per minute, and oxygen saturation 92% on 2 L of oxygen per minute. He showed no signs of distress. Mild scleral icterus was noted. The cardiac exam was normal. Auscultation revealed scattered wheezes and crackles in the left upper lobe. Mild right upper quadrant tenderness without hepatosplenomegaly was noted on the abdominal exam. The patient’s lower extremities exhibited bilateral trace edema. No rash was observed, and his neurologic exam was normal.
The white blood cell (WBC) count was 28,300 per cubic millimeter (87% neutrophils, 3.6% lymphocytes, and 0.03% eosinophils), hemoglobin 11.1 g per deciliter, and platelet count 789,000 per cubic millimeter. Sodium was 127 mmol per liter, potassium 4.6 mmol per liter, chloride 101 mmol per liter, bicarbonate 13 mmol per liter, blood urea nitrogen 60 mg per deciliter, and creatinine 3.4 mg per deciliter. Aspartate aminotransferase and alanine aminotransferase levels were normal. Alkaline phosphatase was 283 units per liter (normal range, 31-95), and total bilirubin was 4.5 mg per deciliter (normal range, 0.2-1.3) with a direct bilirubin of 2.7 mg per deciliter. Urinalysis demonstrated urine protein of 30 mg/dL, specific gravity of 1.013, negative nitrites, 10-21 white cells per high-powered field (normal, < 5), and 21-50 red cells per high-powered field (normal, < 3). Urine microscopy revealed muddy brown casts but no cellular casts or dysmorphic red cells. A chest radiograph (CXR) showed patchy consolidations in the bilateral upper lobes and left lower lobe along with Kerley B lines, a small left pleural effusion, and thickened right horizontal fissure; the left upper lobe mass was re-demonstrated. Vancomycin, piperacillin-tazobactam, and azithromycin were administered.
At this point, the most likely source of sepsis is multifocal pneumonia. The patient is at risk for S. aureus and P. aeruginosa given his recent hospitalization. A severe form of leptospirosis (Weil’s disease) is associated with pulmonary disease, hyperbilirubinemia, and renal failure. Repeat abdominal imaging is necessary to evaluate for cholangitis given the patient’s right upper quadrant pain, fever, and jaundice. It would also help categorize his cholestatic pattern of liver injury as intrahepatic or extrahepatic (eg, stricture). An infiltrative disease such as sarcoidosis may cause both intrahepatic cholestasis and parenchymal lung disease, although the pleural pathology is uncommon.
His normal cardiac exam does not exclude cardiogenic pulmonary edema, a common cause of interstitial edema and pleural effusion. In this setting of systemic inflammation (neutrophilia, thrombocytosis, and hypoalbuminemia), the thickened right horizontal fissure and interlobular septa might represent an infiltrative process, such as lymphangitic carcinomatosis, lymphoma, or sarcoidosis.
Muddy brown casts are characteristic of ATN. The patient’s risk factors for ATN include sepsis and previously administered iodinated contrast. Fluid retention from oliguric renal failure is likely contributing to his hyponatremia and lower extremity edema. Pathology isolated to the tubules, however, would not cause hematuria and pyuria and suggests glomerular or interstitial disease. The lack of cellular casts on a single urinary specimen does not eliminate the likelihood of either disease. Hematuria and diffuse parenchymal lung disease prompt consideration of pulmonary-renal syndromes, such as anti-glomerular basement membrane disease, GPA, and systemic lupus erythematosus, which can all be triggered by infection.
On the night of transfer, the patient experienced acute respiratory distress. Heart rate was 130 beats per minute, BP 170/95 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation 88% on six liters of supplemental oxygen by nasal cannula. His arterial blood gas demonstrated a pH of 7.23, PaCO2 of 32 mm Hg, and PaO2 of 65 mm Hg. He was emergently intubated for progressive hypoxemic respiratory failure. A small amount of blood was noted in the endotracheal tube. A noncontrast CT of the chest demonstrated multifocal airspace opacities and bilateral pleural effusions. The previously noted left upper lobe mass was unchanged.
Rapid respiratory decline and diffuse alveolar disease commonly result from aspiration, flash pulmonary edema, and acute respiratory distress syndrome (ARDS). Necrotizing pneumonia (eg, S. aureus) and trauma during intubation are possible causes of blood in his endotracheal tube. However, in the setting of multifocal airspace opacity, renal insufficiency, hematuria, and rapid respiratory decline, the blood might represent diffuse alveolar hemorrhage (DAH). Bronchoscopy with bronchioalveolar lavage to evaluate for pulmonary edema, infection, and hemorrhage would be indicated.
The patient subsequently developed oliguria, requiring continuous renal replacement therapy. An echocardiogram demonstrated impaired left ventricular relaxation and a reduced ejection fraction of 45% without segmental wall motion abnormalities or valvular disease, and a right ventricular systolic pressure of 36 mm Hg. Over the next 12 hours, his respiratory status improved, and he was extubated to 15 L per minute of supplemental oxygen by high-flow nasal cannula (HFNC).
The pathology report of the lung biopsy from the other hospital disclosed chronic inflammation and fibrosis with ill-defined areas of necrosis and myxoid degeneration surrounded by nuclear palisading suggestive of granulomatous inflammation. Staining for acid-fast bacilli (AFB) and fungal organisms was negative.
The rapid pulmonary recovery is inconsistent with multifocal pneumonia or ARDS. Flash pulmonary edema might result in sudden hypoxemic respiratory failure that resolves with positive pressure ventilation and ultrafiltration. However, this condition would not explain the biopsy results. Granulomatous lung pathology often results from mycobacterial or fungal disease. Tuberculosis and fungal pneumonia are not excluded with negative staining alone. However, neither would cause self-limited respiratory failure. Histologic evidence of necrosis lessens the likelihood of sarcoidosis, which rarely causes fulminant pulmonary disease. Lymphoma can result in granulomatous inflammation but would not cause transient pulmonary disease. GPA, a cause of necrotizing granulomatous lung disease, might result in a lung mass and worsened hypoxemia through DAH.
The patient continued to require 15 L of oxygen per minute by HFNC. He had persistent bilateral perihilar alveolar and interstitial opacities on CXR. Repeat WBC count was 29,200 per cubic millimeter, hemoglobin 7.8 g per deciliter, and platelets 656,000 per cubic millimeter. The C-reactive protein was 300 mg per L (normal range, <6.3) and erythrocyte sedimentation rate 100 mm per hour (normal range, <10). Legionella urinary antigen, serum immunodiffusion for Coccidiodes imitus, human immunodeficiency virus antibody, respiratory viral panel, and beta-D glucan were negative. Rare acid-fast bacilli were visualized in one out of three concentrated AFB sputum smears. He was started on empiric antituberculous therapy with rifampin, isoniazid, pyrazinamide, and ethambutol.
The sputum sample is suggestive of pulmonary tuberculosis. The salient features of this case include systemic inflammation, pulmonary nodules and mass, necrotizing granulomatous lung pathology, renal insufficiency, and hematuria. Disseminated tuberculosis might explain all these findings. However, a positive AFB smear may signal the presence of a nontuberculous mycobacteria, which is less likely to cause this clinical syndrome.
M. tuberculosis complex polymerase chain reaction (MTB PCR) assay returned negative for M. tuberculosis. Antiproteinase 3 antibody was 1,930 units (normal range, <20). Antimyeloperoxidase and antiglomerular basement membrane antibodies were negative.
Tuberculosis and GPA share several overlapping features, such as necrotizing lung pathology and less commonly antineutrophil cytoplasmic autoantibody (ANCA)-associated antibodies. However, the lung mass, acute renal and respiratory failure, hematuria, and the degree of anti-proteinase 3 level elevation are highly suggestive of GPA. The negative MTB PCR raises the possibility that a nontuberculous mycobacterium was detected on the sputum smear. Nevertheless, continued treatment until finalization of culture results is appropriate given that tuberculosis is endemic in Mexico.
The patient’s presenting features of right upper quadrant tenderness, jaundice, and cholestatic hepatitis remain poorly explained by either of these diagnoses. Neither tuberculosis nor GPA commonly presents with accompanying hepatic involvement, though both have been occasionally described as causing hepatitis. As the greatest concern in this patient remains his progressive renal failure and accompanying pulmonary hemorrhage, a renal biopsy to assess for glomerulonephritis associated with GPA is warranted before further investigation into the cause of his cholestatic hepatitis.
A core renal biopsy demonstrated pauci-immune focal crescentic and necrotizing glomerulonephritis with mixed tubulointerstitial inflammation (Figure 2). In conjunction with the pulmonary syndrome and positive antiproteinase 3 serology, a diagnosis of granulomatosis with polyangiitis was made. The patient was treated with pulse dose steroids, rituximab, and plasma exchange. Two weeks later, the sputum mycobacterial culture returned positive for Mycobacterium llatzerense and anti-tuberculous treatment was discontinued.
Over the following weeks, the patient improved and was transitioned off dialysis prior to hospital discharge. By six months later, he had resolution of his hemoptysis, shortness of breath, liver biochemical test abnormalities, and significant improvement in his renal function. Repeat sputum mycobacterial cultures were negative.
DISCUSSION
A 65-year-old man from Mexico with a significant smoking history presented with an apical lung mass and cough, prioritizing tuberculosis and pulmonary malignancy. As the case unfolded, renal failure, multifocal lung opacities, conflicting tuberculosis test results, positive anti-proteinase 3 antibody, and ultimately a renal biopsy led to the diagnosis of granulomatosis with polyangiitis (GPA).
The correct interpretation of occasionally conflicting mycobacterial testing is crucial. Mycobacterial cultures remain the gold standard for diagnosing tuberculosis. However, results take weeks to return. Rapid tests include acid-fast bacilli (AFB) smear microscopy and nucleic acid-amplification tests (NAAT) of sputum or bronchoalveolar samples.1 When three sputum smears are performed, the sensitivity of AFB smear microscopy for tuberculosis in immunocompetent hosts is 70%.1 The AFB smear does not distinguish between different mycobacterial organisms. Thus, a positive result must be interpreted with the relative prevalence of tuberculosis and nontuberculous mycobacteria (NTM) in mind. The addition of NAAT-based assays has allowed for enhanced sensitivity and specificity in the diagnosis of tuberculosis, such that a negative NAAT in a patient with a positive AFB smear strongly argues for the presence of a NTM.2-4
NTM are widely prevalent environmental microbes, with over 140 species described, and careful consideration is required to determine if an isolate is pathogenic.5 Given their ubiquitous nature, a high rate of asymptomatic respiratory and cutaneous colonization occurs. Correspondingly, the diagnosis of NTM disease requires multiple positive cultures or pathologic features on tissue biopsy, compatible clinical findings, and diligent exclusion of other causes.5 A retrospective study of all NTM isolates in Oregon from 2005-2006 revealed that only 47% of patients met the guideline criteria for having symptomatic NTM disease.6 In our case, the patient’s sputum grew M. llatzerense, an aerobic, nonfermenting mycobacterium found in water sources that has only infrequently been implicated as a human pathogen.7,8 Subsequent AFB sputum cultures were negative, and serial imaging showed resolution of the pulmonary findings without additional antimycobacterial therapy, suggesting that this organism was not responsible for the disease process.
Along with microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), GPA is an antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis that predominantly affects small to medium sized vessels. Although it can occur at any age, GPA most commonly afflicts older adults, with men and women being diagnosed at roughly equal rates.9 GPA is a multisystem disease with a wide array of clinical manifestations. The most frequently involved sites of disease are the respiratory tract and kidneys, although virtually any organ can be affected. Sino-nasal disease, such as destructive sinusitis, or ear involvement are nearly universal. Lower respiratory manifestations occur in 60% of patients, but are highly diverse and reflect the inherent difficulty in diagnosing this condition.9-11 Additionally, GPA is a frequent cause of the pulmonary-renal syndromes, with glomerulonephritis occurring in 80% of patients.9
The diagnosis of GPA in this case was delayed, in part, due to features suggestive of malignancy and pulmonary tuberculosis. While sino-nasal disease was not noted during this hospitalization, the patient had many different respiratory manifestations, including a dominant pulmonary mass, diffuse nodules, and hypoxemic respiratory failure due to suspected diffuse alveolar hemorrhage (DAH), all of which have been reported in GPA.12 Dysmorphic red cells and red blood cell casts are not sensitive for renal involvement in GPA; their absence does not exclude the possibility of an ANCA-associated vasculitis.13 Hematuria and rapid progression to oliguric renal failure are characteristic of a vasculitic process and should sway clinicians away from a working diagnosis of ATN.
The diagnosis of GPA involves the synthesis of clinical data, radiographic findings, serologic testing, and histopathology. ANCA testing is an essential step in the diagnosis of GPA but has limitations. Patients with GPA more commonly have ANCAs targeting the enzyme proteinase-3 (PR3-ANCA), with MPA being more closely associated with myeloperoxidase (MPO-ANCA), although cross-reactivity and antibody-negative disease can occur.14 Although 90% of patients with GPA with multiorgan involvement will have a positive ANCA, a negative test is more common in localized upper airway disease, where only 50% have a positive ANCA.15 A number of drugs, medications, infections, and nonvasculitic autoimmune diseases have been associated with positive ANCA serologies in the absence of systemic vasculitis.14,16,17 As such, pathologic demonstration of vasculitis is necessary for establishing the diagnosis. Typical sites for biopsy include the kidneys and lungs.9
This case illustrates how clinicians often find themselves at a diagnostic crossroads—being forced to choose which clinical elements to prioritize. At various points, our patient’s presentation could have been framed as “a man from a Tb-endemic country with hemoptysis and an apical opacity,” “an elderly man with extensive smoking history and lung mass,” or “a patient with elevated inflammatory markers and pulmonary-renal syndrome”. In such situations, it is incumbent on the clinician to evaluate how well a given problem representation encompasses or highlights the salient features of a case. As with painting or photography, an essential aspect of appreciating the whole picture involves carefully selecting the right frame.
KEY TEACHING POINTS
- The diagnosis of tuberculosis relies on smear microscopy, nucleic-acid amplification testing (NAAT), and cultures. A positive AFB smear with negative NAAT suggests the presence of a nontuberculous mycobacteria (NTM).
- NTM are common environmental organisms and often exist as nonpathogenic colonizers.6 The diagnosis of NTM disease requires exclusion of other causes and careful clinical, microbiologic, and radiographic correlation.
- Granulomatosis with polyangiitis is a multisystem disease often involving the respiratory track and kidney. Pulmonary disease can present with airway involvement, parenchymal nodules, opacities, pleural findings, and diffuse alveolar hemorrhage.12
Disclosures
Drs. Minter, Geha, Boslett, Chung, and Ramani have no disclosures. Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME).
1. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. PubMed
2. Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;(1):Cd009593. PubMed
3. Luetkemeyer AF, Firnhaber C, Kendall MA, et al. Evaluation of Xpert MTB/RIF versus afb smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis. 2016;62(9):1081-1088. PubMed
4. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015. PubMed
5. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416. PubMed
6. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977-982. PubMed
7. Teixeira L, Avery RK, Iseman M, et al. Mycobacterium llatzerense lung infection in a liver transplant recipient: case report and review of the literature. Am J Transplant. 2013;13(8):2198-2200. PubMed
8. Cárdenas AM, Gomila M, Lalucat J, Edelstein PH. Abdominal abscess caused by Mycobacterium llatzerense. J Clin Microbiol. 2014;52(4):1287-1289. PubMed
9. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512-1523. PubMed
10. Mahr A, Katsahian S, Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72(6):1003-1010. PubMed
11. Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63(1):257-266. PubMed
12. Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912. PubMed
13. Hamadah AM, Gharaibeh K, Mara KC, et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells. Nephrol Dial Transplant. 2018;33(8):1397-1403. PubMed
14. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10(8):463-473. PubMed
15. Borner U, Landis BN, Banz Y, et al. Diagnostic value of biopsies in identifying cytoplasmic antineutrophil cytoplasmic antibody-negative localized Wegener’s granulomatosis presenting primarily with sinonasal disease. Am J Rhinol Allergy. 2012;26(6):475-480. PubMed
16. Mahr A, Batteux F, Tubiana S, et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 2014;66(6):1672-1677. PubMed
17. Sherkat R, Mostafavizadeh K, Zeydabadi L, Shoaei P, Rostami S. Antineutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis. Iran J Immunol. 2011;8(1):52-57. PubMed
A 65-year-old man was transferred to a tertiary academic medical center with one week of progressive shortness of breath, dry cough, and fevers. He reported no weight loss or night sweats but had experienced mild right upper quadrant pain and anorexia for the preceding three weeks. Several years had passed since he had consulted a physician, and he did not take any medications. He immigrated to the United States from Mexico four decades prior. He traveled back frequently to visit his family, most recently one month before his presentation. He worked as a farming supervisor in the Central Valley of California. He smoked tobacco and had a 30 pack-year history. He drank alcohol occasionally and denied any drug use.
Causes of subacute cough and dyspnea include bronchitis, pneumonia, heart failure, and asthma. Pneumonia and heart failure might cause right upper quadrant pain from diaphragmatic irritation and hepatic congestion, respectively. Metastatic cancer or infection may lead to synchronous pulmonary and hepatic involvement. The patient is at increased risk of lung cancer, given his extensive smoking history.
The patient’s place of residence in the Southwestern United States places him at risk of respiratory illness from coccidioidomycosis. His exact involvement with animals and their products should be further explored. For example, consumption of unpasteurized milk might result in pneumonia, hepatitis, or both from M. bovis, Brucella species, or C. burnetii. His travel to Mexico prompts consideration of tuberculosis, histoplasmosis, and paracoccidiomycosis as causes of respiratory and possible hepatic illness.
Two weeks prior, the patient had initially presented to another hospital with one week of intermittent right upper quadrant pain unrelated to eating. An abdominal ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were normal. Computed tomography (CT) of the chest, abdomen, and pelvis with contrast demonstrated a left upper lobe lung mass measuring 5.5 × 4.4 × 3.7 cm3 and scattered right-sided pulmonary nodules (Figure 1). He underwent CT-guided biopsy of the mass and was discharged with a presumed diagnosis of primary pulmonary malignancy with plans for outpatient follow-up.
Over the next four days, the patient developed progressive dyspnea with cough and subjective fevers. The patient was readmitted with a diagnosis of postobstructive pneumonia and acute kidney injury (creatinine increased from 0.7 mg/dL to 2.9 mg/dL between admissions), and this finding was attributed to contrast-induced nephropathy from his recent CT scan. He was treated with vancomycin and piperacillin/tazobactam for two days but wished to transfer to a tertiary care hospital for a second opinion.
Postobstructive pneumonia, pulmonary embolism, and pleural effusion are common causes of dyspnea in patients with lung cancer. The patient’s travel and occupational history, lung nodules, acute renal insufficiency, and rapidly progressive respiratory symptoms prompt consideration for radiographic mimickers of lung cancer. Tuberculosis might present as a lung mass (pulmonary tuberculoma) during primary infection or reactivation. Noninfectious causes of pulmonary masses and nodules include metastatic cancer (eg, colon cancer), sarcoidosis, IgG4-related disease, and granulomatous polyangiitis (GPA).
Contrast-induced nephropathy is unusual in patients with normal renal function. More probable explanations include hypovolemia or acute tubular necrosis (ATN) from underlying inflammation. The patient’s CT-negative right upper quadrant pain may be a distinct process or represent another facet of a disseminated illness such as hepatic infiltration from lymphoma.
Upon arrival, the patient’s temperature was 38°C, heart rate (HR) 107 beats per minute, blood pressure (BP) 159/89 mm Hg, respiratory rate 25 breaths per minute, and oxygen saturation 92% on 2 L of oxygen per minute. He showed no signs of distress. Mild scleral icterus was noted. The cardiac exam was normal. Auscultation revealed scattered wheezes and crackles in the left upper lobe. Mild right upper quadrant tenderness without hepatosplenomegaly was noted on the abdominal exam. The patient’s lower extremities exhibited bilateral trace edema. No rash was observed, and his neurologic exam was normal.
The white blood cell (WBC) count was 28,300 per cubic millimeter (87% neutrophils, 3.6% lymphocytes, and 0.03% eosinophils), hemoglobin 11.1 g per deciliter, and platelet count 789,000 per cubic millimeter. Sodium was 127 mmol per liter, potassium 4.6 mmol per liter, chloride 101 mmol per liter, bicarbonate 13 mmol per liter, blood urea nitrogen 60 mg per deciliter, and creatinine 3.4 mg per deciliter. Aspartate aminotransferase and alanine aminotransferase levels were normal. Alkaline phosphatase was 283 units per liter (normal range, 31-95), and total bilirubin was 4.5 mg per deciliter (normal range, 0.2-1.3) with a direct bilirubin of 2.7 mg per deciliter. Urinalysis demonstrated urine protein of 30 mg/dL, specific gravity of 1.013, negative nitrites, 10-21 white cells per high-powered field (normal, < 5), and 21-50 red cells per high-powered field (normal, < 3). Urine microscopy revealed muddy brown casts but no cellular casts or dysmorphic red cells. A chest radiograph (CXR) showed patchy consolidations in the bilateral upper lobes and left lower lobe along with Kerley B lines, a small left pleural effusion, and thickened right horizontal fissure; the left upper lobe mass was re-demonstrated. Vancomycin, piperacillin-tazobactam, and azithromycin were administered.
At this point, the most likely source of sepsis is multifocal pneumonia. The patient is at risk for S. aureus and P. aeruginosa given his recent hospitalization. A severe form of leptospirosis (Weil’s disease) is associated with pulmonary disease, hyperbilirubinemia, and renal failure. Repeat abdominal imaging is necessary to evaluate for cholangitis given the patient’s right upper quadrant pain, fever, and jaundice. It would also help categorize his cholestatic pattern of liver injury as intrahepatic or extrahepatic (eg, stricture). An infiltrative disease such as sarcoidosis may cause both intrahepatic cholestasis and parenchymal lung disease, although the pleural pathology is uncommon.
His normal cardiac exam does not exclude cardiogenic pulmonary edema, a common cause of interstitial edema and pleural effusion. In this setting of systemic inflammation (neutrophilia, thrombocytosis, and hypoalbuminemia), the thickened right horizontal fissure and interlobular septa might represent an infiltrative process, such as lymphangitic carcinomatosis, lymphoma, or sarcoidosis.
Muddy brown casts are characteristic of ATN. The patient’s risk factors for ATN include sepsis and previously administered iodinated contrast. Fluid retention from oliguric renal failure is likely contributing to his hyponatremia and lower extremity edema. Pathology isolated to the tubules, however, would not cause hematuria and pyuria and suggests glomerular or interstitial disease. The lack of cellular casts on a single urinary specimen does not eliminate the likelihood of either disease. Hematuria and diffuse parenchymal lung disease prompt consideration of pulmonary-renal syndromes, such as anti-glomerular basement membrane disease, GPA, and systemic lupus erythematosus, which can all be triggered by infection.
On the night of transfer, the patient experienced acute respiratory distress. Heart rate was 130 beats per minute, BP 170/95 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation 88% on six liters of supplemental oxygen by nasal cannula. His arterial blood gas demonstrated a pH of 7.23, PaCO2 of 32 mm Hg, and PaO2 of 65 mm Hg. He was emergently intubated for progressive hypoxemic respiratory failure. A small amount of blood was noted in the endotracheal tube. A noncontrast CT of the chest demonstrated multifocal airspace opacities and bilateral pleural effusions. The previously noted left upper lobe mass was unchanged.
Rapid respiratory decline and diffuse alveolar disease commonly result from aspiration, flash pulmonary edema, and acute respiratory distress syndrome (ARDS). Necrotizing pneumonia (eg, S. aureus) and trauma during intubation are possible causes of blood in his endotracheal tube. However, in the setting of multifocal airspace opacity, renal insufficiency, hematuria, and rapid respiratory decline, the blood might represent diffuse alveolar hemorrhage (DAH). Bronchoscopy with bronchioalveolar lavage to evaluate for pulmonary edema, infection, and hemorrhage would be indicated.
The patient subsequently developed oliguria, requiring continuous renal replacement therapy. An echocardiogram demonstrated impaired left ventricular relaxation and a reduced ejection fraction of 45% without segmental wall motion abnormalities or valvular disease, and a right ventricular systolic pressure of 36 mm Hg. Over the next 12 hours, his respiratory status improved, and he was extubated to 15 L per minute of supplemental oxygen by high-flow nasal cannula (HFNC).
The pathology report of the lung biopsy from the other hospital disclosed chronic inflammation and fibrosis with ill-defined areas of necrosis and myxoid degeneration surrounded by nuclear palisading suggestive of granulomatous inflammation. Staining for acid-fast bacilli (AFB) and fungal organisms was negative.
The rapid pulmonary recovery is inconsistent with multifocal pneumonia or ARDS. Flash pulmonary edema might result in sudden hypoxemic respiratory failure that resolves with positive pressure ventilation and ultrafiltration. However, this condition would not explain the biopsy results. Granulomatous lung pathology often results from mycobacterial or fungal disease. Tuberculosis and fungal pneumonia are not excluded with negative staining alone. However, neither would cause self-limited respiratory failure. Histologic evidence of necrosis lessens the likelihood of sarcoidosis, which rarely causes fulminant pulmonary disease. Lymphoma can result in granulomatous inflammation but would not cause transient pulmonary disease. GPA, a cause of necrotizing granulomatous lung disease, might result in a lung mass and worsened hypoxemia through DAH.
The patient continued to require 15 L of oxygen per minute by HFNC. He had persistent bilateral perihilar alveolar and interstitial opacities on CXR. Repeat WBC count was 29,200 per cubic millimeter, hemoglobin 7.8 g per deciliter, and platelets 656,000 per cubic millimeter. The C-reactive protein was 300 mg per L (normal range, <6.3) and erythrocyte sedimentation rate 100 mm per hour (normal range, <10). Legionella urinary antigen, serum immunodiffusion for Coccidiodes imitus, human immunodeficiency virus antibody, respiratory viral panel, and beta-D glucan were negative. Rare acid-fast bacilli were visualized in one out of three concentrated AFB sputum smears. He was started on empiric antituberculous therapy with rifampin, isoniazid, pyrazinamide, and ethambutol.
The sputum sample is suggestive of pulmonary tuberculosis. The salient features of this case include systemic inflammation, pulmonary nodules and mass, necrotizing granulomatous lung pathology, renal insufficiency, and hematuria. Disseminated tuberculosis might explain all these findings. However, a positive AFB smear may signal the presence of a nontuberculous mycobacteria, which is less likely to cause this clinical syndrome.
M. tuberculosis complex polymerase chain reaction (MTB PCR) assay returned negative for M. tuberculosis. Antiproteinase 3 antibody was 1,930 units (normal range, <20). Antimyeloperoxidase and antiglomerular basement membrane antibodies were negative.
Tuberculosis and GPA share several overlapping features, such as necrotizing lung pathology and less commonly antineutrophil cytoplasmic autoantibody (ANCA)-associated antibodies. However, the lung mass, acute renal and respiratory failure, hematuria, and the degree of anti-proteinase 3 level elevation are highly suggestive of GPA. The negative MTB PCR raises the possibility that a nontuberculous mycobacterium was detected on the sputum smear. Nevertheless, continued treatment until finalization of culture results is appropriate given that tuberculosis is endemic in Mexico.
The patient’s presenting features of right upper quadrant tenderness, jaundice, and cholestatic hepatitis remain poorly explained by either of these diagnoses. Neither tuberculosis nor GPA commonly presents with accompanying hepatic involvement, though both have been occasionally described as causing hepatitis. As the greatest concern in this patient remains his progressive renal failure and accompanying pulmonary hemorrhage, a renal biopsy to assess for glomerulonephritis associated with GPA is warranted before further investigation into the cause of his cholestatic hepatitis.
A core renal biopsy demonstrated pauci-immune focal crescentic and necrotizing glomerulonephritis with mixed tubulointerstitial inflammation (Figure 2). In conjunction with the pulmonary syndrome and positive antiproteinase 3 serology, a diagnosis of granulomatosis with polyangiitis was made. The patient was treated with pulse dose steroids, rituximab, and plasma exchange. Two weeks later, the sputum mycobacterial culture returned positive for Mycobacterium llatzerense and anti-tuberculous treatment was discontinued.
Over the following weeks, the patient improved and was transitioned off dialysis prior to hospital discharge. By six months later, he had resolution of his hemoptysis, shortness of breath, liver biochemical test abnormalities, and significant improvement in his renal function. Repeat sputum mycobacterial cultures were negative.
DISCUSSION
A 65-year-old man from Mexico with a significant smoking history presented with an apical lung mass and cough, prioritizing tuberculosis and pulmonary malignancy. As the case unfolded, renal failure, multifocal lung opacities, conflicting tuberculosis test results, positive anti-proteinase 3 antibody, and ultimately a renal biopsy led to the diagnosis of granulomatosis with polyangiitis (GPA).
The correct interpretation of occasionally conflicting mycobacterial testing is crucial. Mycobacterial cultures remain the gold standard for diagnosing tuberculosis. However, results take weeks to return. Rapid tests include acid-fast bacilli (AFB) smear microscopy and nucleic acid-amplification tests (NAAT) of sputum or bronchoalveolar samples.1 When three sputum smears are performed, the sensitivity of AFB smear microscopy for tuberculosis in immunocompetent hosts is 70%.1 The AFB smear does not distinguish between different mycobacterial organisms. Thus, a positive result must be interpreted with the relative prevalence of tuberculosis and nontuberculous mycobacteria (NTM) in mind. The addition of NAAT-based assays has allowed for enhanced sensitivity and specificity in the diagnosis of tuberculosis, such that a negative NAAT in a patient with a positive AFB smear strongly argues for the presence of a NTM.2-4
NTM are widely prevalent environmental microbes, with over 140 species described, and careful consideration is required to determine if an isolate is pathogenic.5 Given their ubiquitous nature, a high rate of asymptomatic respiratory and cutaneous colonization occurs. Correspondingly, the diagnosis of NTM disease requires multiple positive cultures or pathologic features on tissue biopsy, compatible clinical findings, and diligent exclusion of other causes.5 A retrospective study of all NTM isolates in Oregon from 2005-2006 revealed that only 47% of patients met the guideline criteria for having symptomatic NTM disease.6 In our case, the patient’s sputum grew M. llatzerense, an aerobic, nonfermenting mycobacterium found in water sources that has only infrequently been implicated as a human pathogen.7,8 Subsequent AFB sputum cultures were negative, and serial imaging showed resolution of the pulmonary findings without additional antimycobacterial therapy, suggesting that this organism was not responsible for the disease process.
Along with microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), GPA is an antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis that predominantly affects small to medium sized vessels. Although it can occur at any age, GPA most commonly afflicts older adults, with men and women being diagnosed at roughly equal rates.9 GPA is a multisystem disease with a wide array of clinical manifestations. The most frequently involved sites of disease are the respiratory tract and kidneys, although virtually any organ can be affected. Sino-nasal disease, such as destructive sinusitis, or ear involvement are nearly universal. Lower respiratory manifestations occur in 60% of patients, but are highly diverse and reflect the inherent difficulty in diagnosing this condition.9-11 Additionally, GPA is a frequent cause of the pulmonary-renal syndromes, with glomerulonephritis occurring in 80% of patients.9
The diagnosis of GPA in this case was delayed, in part, due to features suggestive of malignancy and pulmonary tuberculosis. While sino-nasal disease was not noted during this hospitalization, the patient had many different respiratory manifestations, including a dominant pulmonary mass, diffuse nodules, and hypoxemic respiratory failure due to suspected diffuse alveolar hemorrhage (DAH), all of which have been reported in GPA.12 Dysmorphic red cells and red blood cell casts are not sensitive for renal involvement in GPA; their absence does not exclude the possibility of an ANCA-associated vasculitis.13 Hematuria and rapid progression to oliguric renal failure are characteristic of a vasculitic process and should sway clinicians away from a working diagnosis of ATN.
The diagnosis of GPA involves the synthesis of clinical data, radiographic findings, serologic testing, and histopathology. ANCA testing is an essential step in the diagnosis of GPA but has limitations. Patients with GPA more commonly have ANCAs targeting the enzyme proteinase-3 (PR3-ANCA), with MPA being more closely associated with myeloperoxidase (MPO-ANCA), although cross-reactivity and antibody-negative disease can occur.14 Although 90% of patients with GPA with multiorgan involvement will have a positive ANCA, a negative test is more common in localized upper airway disease, where only 50% have a positive ANCA.15 A number of drugs, medications, infections, and nonvasculitic autoimmune diseases have been associated with positive ANCA serologies in the absence of systemic vasculitis.14,16,17 As such, pathologic demonstration of vasculitis is necessary for establishing the diagnosis. Typical sites for biopsy include the kidneys and lungs.9
This case illustrates how clinicians often find themselves at a diagnostic crossroads—being forced to choose which clinical elements to prioritize. At various points, our patient’s presentation could have been framed as “a man from a Tb-endemic country with hemoptysis and an apical opacity,” “an elderly man with extensive smoking history and lung mass,” or “a patient with elevated inflammatory markers and pulmonary-renal syndrome”. In such situations, it is incumbent on the clinician to evaluate how well a given problem representation encompasses or highlights the salient features of a case. As with painting or photography, an essential aspect of appreciating the whole picture involves carefully selecting the right frame.
KEY TEACHING POINTS
- The diagnosis of tuberculosis relies on smear microscopy, nucleic-acid amplification testing (NAAT), and cultures. A positive AFB smear with negative NAAT suggests the presence of a nontuberculous mycobacteria (NTM).
- NTM are common environmental organisms and often exist as nonpathogenic colonizers.6 The diagnosis of NTM disease requires exclusion of other causes and careful clinical, microbiologic, and radiographic correlation.
- Granulomatosis with polyangiitis is a multisystem disease often involving the respiratory track and kidney. Pulmonary disease can present with airway involvement, parenchymal nodules, opacities, pleural findings, and diffuse alveolar hemorrhage.12
Disclosures
Drs. Minter, Geha, Boslett, Chung, and Ramani have no disclosures. Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME).
A 65-year-old man was transferred to a tertiary academic medical center with one week of progressive shortness of breath, dry cough, and fevers. He reported no weight loss or night sweats but had experienced mild right upper quadrant pain and anorexia for the preceding three weeks. Several years had passed since he had consulted a physician, and he did not take any medications. He immigrated to the United States from Mexico four decades prior. He traveled back frequently to visit his family, most recently one month before his presentation. He worked as a farming supervisor in the Central Valley of California. He smoked tobacco and had a 30 pack-year history. He drank alcohol occasionally and denied any drug use.
Causes of subacute cough and dyspnea include bronchitis, pneumonia, heart failure, and asthma. Pneumonia and heart failure might cause right upper quadrant pain from diaphragmatic irritation and hepatic congestion, respectively. Metastatic cancer or infection may lead to synchronous pulmonary and hepatic involvement. The patient is at increased risk of lung cancer, given his extensive smoking history.
The patient’s place of residence in the Southwestern United States places him at risk of respiratory illness from coccidioidomycosis. His exact involvement with animals and their products should be further explored. For example, consumption of unpasteurized milk might result in pneumonia, hepatitis, or both from M. bovis, Brucella species, or C. burnetii. His travel to Mexico prompts consideration of tuberculosis, histoplasmosis, and paracoccidiomycosis as causes of respiratory and possible hepatic illness.
Two weeks prior, the patient had initially presented to another hospital with one week of intermittent right upper quadrant pain unrelated to eating. An abdominal ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were normal. Computed tomography (CT) of the chest, abdomen, and pelvis with contrast demonstrated a left upper lobe lung mass measuring 5.5 × 4.4 × 3.7 cm3 and scattered right-sided pulmonary nodules (Figure 1). He underwent CT-guided biopsy of the mass and was discharged with a presumed diagnosis of primary pulmonary malignancy with plans for outpatient follow-up.
Over the next four days, the patient developed progressive dyspnea with cough and subjective fevers. The patient was readmitted with a diagnosis of postobstructive pneumonia and acute kidney injury (creatinine increased from 0.7 mg/dL to 2.9 mg/dL between admissions), and this finding was attributed to contrast-induced nephropathy from his recent CT scan. He was treated with vancomycin and piperacillin/tazobactam for two days but wished to transfer to a tertiary care hospital for a second opinion.
Postobstructive pneumonia, pulmonary embolism, and pleural effusion are common causes of dyspnea in patients with lung cancer. The patient’s travel and occupational history, lung nodules, acute renal insufficiency, and rapidly progressive respiratory symptoms prompt consideration for radiographic mimickers of lung cancer. Tuberculosis might present as a lung mass (pulmonary tuberculoma) during primary infection or reactivation. Noninfectious causes of pulmonary masses and nodules include metastatic cancer (eg, colon cancer), sarcoidosis, IgG4-related disease, and granulomatous polyangiitis (GPA).
Contrast-induced nephropathy is unusual in patients with normal renal function. More probable explanations include hypovolemia or acute tubular necrosis (ATN) from underlying inflammation. The patient’s CT-negative right upper quadrant pain may be a distinct process or represent another facet of a disseminated illness such as hepatic infiltration from lymphoma.
Upon arrival, the patient’s temperature was 38°C, heart rate (HR) 107 beats per minute, blood pressure (BP) 159/89 mm Hg, respiratory rate 25 breaths per minute, and oxygen saturation 92% on 2 L of oxygen per minute. He showed no signs of distress. Mild scleral icterus was noted. The cardiac exam was normal. Auscultation revealed scattered wheezes and crackles in the left upper lobe. Mild right upper quadrant tenderness without hepatosplenomegaly was noted on the abdominal exam. The patient’s lower extremities exhibited bilateral trace edema. No rash was observed, and his neurologic exam was normal.
The white blood cell (WBC) count was 28,300 per cubic millimeter (87% neutrophils, 3.6% lymphocytes, and 0.03% eosinophils), hemoglobin 11.1 g per deciliter, and platelet count 789,000 per cubic millimeter. Sodium was 127 mmol per liter, potassium 4.6 mmol per liter, chloride 101 mmol per liter, bicarbonate 13 mmol per liter, blood urea nitrogen 60 mg per deciliter, and creatinine 3.4 mg per deciliter. Aspartate aminotransferase and alanine aminotransferase levels were normal. Alkaline phosphatase was 283 units per liter (normal range, 31-95), and total bilirubin was 4.5 mg per deciliter (normal range, 0.2-1.3) with a direct bilirubin of 2.7 mg per deciliter. Urinalysis demonstrated urine protein of 30 mg/dL, specific gravity of 1.013, negative nitrites, 10-21 white cells per high-powered field (normal, < 5), and 21-50 red cells per high-powered field (normal, < 3). Urine microscopy revealed muddy brown casts but no cellular casts or dysmorphic red cells. A chest radiograph (CXR) showed patchy consolidations in the bilateral upper lobes and left lower lobe along with Kerley B lines, a small left pleural effusion, and thickened right horizontal fissure; the left upper lobe mass was re-demonstrated. Vancomycin, piperacillin-tazobactam, and azithromycin were administered.
At this point, the most likely source of sepsis is multifocal pneumonia. The patient is at risk for S. aureus and P. aeruginosa given his recent hospitalization. A severe form of leptospirosis (Weil’s disease) is associated with pulmonary disease, hyperbilirubinemia, and renal failure. Repeat abdominal imaging is necessary to evaluate for cholangitis given the patient’s right upper quadrant pain, fever, and jaundice. It would also help categorize his cholestatic pattern of liver injury as intrahepatic or extrahepatic (eg, stricture). An infiltrative disease such as sarcoidosis may cause both intrahepatic cholestasis and parenchymal lung disease, although the pleural pathology is uncommon.
His normal cardiac exam does not exclude cardiogenic pulmonary edema, a common cause of interstitial edema and pleural effusion. In this setting of systemic inflammation (neutrophilia, thrombocytosis, and hypoalbuminemia), the thickened right horizontal fissure and interlobular septa might represent an infiltrative process, such as lymphangitic carcinomatosis, lymphoma, or sarcoidosis.
Muddy brown casts are characteristic of ATN. The patient’s risk factors for ATN include sepsis and previously administered iodinated contrast. Fluid retention from oliguric renal failure is likely contributing to his hyponatremia and lower extremity edema. Pathology isolated to the tubules, however, would not cause hematuria and pyuria and suggests glomerular or interstitial disease. The lack of cellular casts on a single urinary specimen does not eliminate the likelihood of either disease. Hematuria and diffuse parenchymal lung disease prompt consideration of pulmonary-renal syndromes, such as anti-glomerular basement membrane disease, GPA, and systemic lupus erythematosus, which can all be triggered by infection.
On the night of transfer, the patient experienced acute respiratory distress. Heart rate was 130 beats per minute, BP 170/95 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation 88% on six liters of supplemental oxygen by nasal cannula. His arterial blood gas demonstrated a pH of 7.23, PaCO2 of 32 mm Hg, and PaO2 of 65 mm Hg. He was emergently intubated for progressive hypoxemic respiratory failure. A small amount of blood was noted in the endotracheal tube. A noncontrast CT of the chest demonstrated multifocal airspace opacities and bilateral pleural effusions. The previously noted left upper lobe mass was unchanged.
Rapid respiratory decline and diffuse alveolar disease commonly result from aspiration, flash pulmonary edema, and acute respiratory distress syndrome (ARDS). Necrotizing pneumonia (eg, S. aureus) and trauma during intubation are possible causes of blood in his endotracheal tube. However, in the setting of multifocal airspace opacity, renal insufficiency, hematuria, and rapid respiratory decline, the blood might represent diffuse alveolar hemorrhage (DAH). Bronchoscopy with bronchioalveolar lavage to evaluate for pulmonary edema, infection, and hemorrhage would be indicated.
The patient subsequently developed oliguria, requiring continuous renal replacement therapy. An echocardiogram demonstrated impaired left ventricular relaxation and a reduced ejection fraction of 45% without segmental wall motion abnormalities or valvular disease, and a right ventricular systolic pressure of 36 mm Hg. Over the next 12 hours, his respiratory status improved, and he was extubated to 15 L per minute of supplemental oxygen by high-flow nasal cannula (HFNC).
The pathology report of the lung biopsy from the other hospital disclosed chronic inflammation and fibrosis with ill-defined areas of necrosis and myxoid degeneration surrounded by nuclear palisading suggestive of granulomatous inflammation. Staining for acid-fast bacilli (AFB) and fungal organisms was negative.
The rapid pulmonary recovery is inconsistent with multifocal pneumonia or ARDS. Flash pulmonary edema might result in sudden hypoxemic respiratory failure that resolves with positive pressure ventilation and ultrafiltration. However, this condition would not explain the biopsy results. Granulomatous lung pathology often results from mycobacterial or fungal disease. Tuberculosis and fungal pneumonia are not excluded with negative staining alone. However, neither would cause self-limited respiratory failure. Histologic evidence of necrosis lessens the likelihood of sarcoidosis, which rarely causes fulminant pulmonary disease. Lymphoma can result in granulomatous inflammation but would not cause transient pulmonary disease. GPA, a cause of necrotizing granulomatous lung disease, might result in a lung mass and worsened hypoxemia through DAH.
The patient continued to require 15 L of oxygen per minute by HFNC. He had persistent bilateral perihilar alveolar and interstitial opacities on CXR. Repeat WBC count was 29,200 per cubic millimeter, hemoglobin 7.8 g per deciliter, and platelets 656,000 per cubic millimeter. The C-reactive protein was 300 mg per L (normal range, <6.3) and erythrocyte sedimentation rate 100 mm per hour (normal range, <10). Legionella urinary antigen, serum immunodiffusion for Coccidiodes imitus, human immunodeficiency virus antibody, respiratory viral panel, and beta-D glucan were negative. Rare acid-fast bacilli were visualized in one out of three concentrated AFB sputum smears. He was started on empiric antituberculous therapy with rifampin, isoniazid, pyrazinamide, and ethambutol.
The sputum sample is suggestive of pulmonary tuberculosis. The salient features of this case include systemic inflammation, pulmonary nodules and mass, necrotizing granulomatous lung pathology, renal insufficiency, and hematuria. Disseminated tuberculosis might explain all these findings. However, a positive AFB smear may signal the presence of a nontuberculous mycobacteria, which is less likely to cause this clinical syndrome.
M. tuberculosis complex polymerase chain reaction (MTB PCR) assay returned negative for M. tuberculosis. Antiproteinase 3 antibody was 1,930 units (normal range, <20). Antimyeloperoxidase and antiglomerular basement membrane antibodies were negative.
Tuberculosis and GPA share several overlapping features, such as necrotizing lung pathology and less commonly antineutrophil cytoplasmic autoantibody (ANCA)-associated antibodies. However, the lung mass, acute renal and respiratory failure, hematuria, and the degree of anti-proteinase 3 level elevation are highly suggestive of GPA. The negative MTB PCR raises the possibility that a nontuberculous mycobacterium was detected on the sputum smear. Nevertheless, continued treatment until finalization of culture results is appropriate given that tuberculosis is endemic in Mexico.
The patient’s presenting features of right upper quadrant tenderness, jaundice, and cholestatic hepatitis remain poorly explained by either of these diagnoses. Neither tuberculosis nor GPA commonly presents with accompanying hepatic involvement, though both have been occasionally described as causing hepatitis. As the greatest concern in this patient remains his progressive renal failure and accompanying pulmonary hemorrhage, a renal biopsy to assess for glomerulonephritis associated with GPA is warranted before further investigation into the cause of his cholestatic hepatitis.
A core renal biopsy demonstrated pauci-immune focal crescentic and necrotizing glomerulonephritis with mixed tubulointerstitial inflammation (Figure 2). In conjunction with the pulmonary syndrome and positive antiproteinase 3 serology, a diagnosis of granulomatosis with polyangiitis was made. The patient was treated with pulse dose steroids, rituximab, and plasma exchange. Two weeks later, the sputum mycobacterial culture returned positive for Mycobacterium llatzerense and anti-tuberculous treatment was discontinued.
Over the following weeks, the patient improved and was transitioned off dialysis prior to hospital discharge. By six months later, he had resolution of his hemoptysis, shortness of breath, liver biochemical test abnormalities, and significant improvement in his renal function. Repeat sputum mycobacterial cultures were negative.
DISCUSSION
A 65-year-old man from Mexico with a significant smoking history presented with an apical lung mass and cough, prioritizing tuberculosis and pulmonary malignancy. As the case unfolded, renal failure, multifocal lung opacities, conflicting tuberculosis test results, positive anti-proteinase 3 antibody, and ultimately a renal biopsy led to the diagnosis of granulomatosis with polyangiitis (GPA).
The correct interpretation of occasionally conflicting mycobacterial testing is crucial. Mycobacterial cultures remain the gold standard for diagnosing tuberculosis. However, results take weeks to return. Rapid tests include acid-fast bacilli (AFB) smear microscopy and nucleic acid-amplification tests (NAAT) of sputum or bronchoalveolar samples.1 When three sputum smears are performed, the sensitivity of AFB smear microscopy for tuberculosis in immunocompetent hosts is 70%.1 The AFB smear does not distinguish between different mycobacterial organisms. Thus, a positive result must be interpreted with the relative prevalence of tuberculosis and nontuberculous mycobacteria (NTM) in mind. The addition of NAAT-based assays has allowed for enhanced sensitivity and specificity in the diagnosis of tuberculosis, such that a negative NAAT in a patient with a positive AFB smear strongly argues for the presence of a NTM.2-4
NTM are widely prevalent environmental microbes, with over 140 species described, and careful consideration is required to determine if an isolate is pathogenic.5 Given their ubiquitous nature, a high rate of asymptomatic respiratory and cutaneous colonization occurs. Correspondingly, the diagnosis of NTM disease requires multiple positive cultures or pathologic features on tissue biopsy, compatible clinical findings, and diligent exclusion of other causes.5 A retrospective study of all NTM isolates in Oregon from 2005-2006 revealed that only 47% of patients met the guideline criteria for having symptomatic NTM disease.6 In our case, the patient’s sputum grew M. llatzerense, an aerobic, nonfermenting mycobacterium found in water sources that has only infrequently been implicated as a human pathogen.7,8 Subsequent AFB sputum cultures were negative, and serial imaging showed resolution of the pulmonary findings without additional antimycobacterial therapy, suggesting that this organism was not responsible for the disease process.
Along with microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), GPA is an antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis that predominantly affects small to medium sized vessels. Although it can occur at any age, GPA most commonly afflicts older adults, with men and women being diagnosed at roughly equal rates.9 GPA is a multisystem disease with a wide array of clinical manifestations. The most frequently involved sites of disease are the respiratory tract and kidneys, although virtually any organ can be affected. Sino-nasal disease, such as destructive sinusitis, or ear involvement are nearly universal. Lower respiratory manifestations occur in 60% of patients, but are highly diverse and reflect the inherent difficulty in diagnosing this condition.9-11 Additionally, GPA is a frequent cause of the pulmonary-renal syndromes, with glomerulonephritis occurring in 80% of patients.9
The diagnosis of GPA in this case was delayed, in part, due to features suggestive of malignancy and pulmonary tuberculosis. While sino-nasal disease was not noted during this hospitalization, the patient had many different respiratory manifestations, including a dominant pulmonary mass, diffuse nodules, and hypoxemic respiratory failure due to suspected diffuse alveolar hemorrhage (DAH), all of which have been reported in GPA.12 Dysmorphic red cells and red blood cell casts are not sensitive for renal involvement in GPA; their absence does not exclude the possibility of an ANCA-associated vasculitis.13 Hematuria and rapid progression to oliguric renal failure are characteristic of a vasculitic process and should sway clinicians away from a working diagnosis of ATN.
The diagnosis of GPA involves the synthesis of clinical data, radiographic findings, serologic testing, and histopathology. ANCA testing is an essential step in the diagnosis of GPA but has limitations. Patients with GPA more commonly have ANCAs targeting the enzyme proteinase-3 (PR3-ANCA), with MPA being more closely associated with myeloperoxidase (MPO-ANCA), although cross-reactivity and antibody-negative disease can occur.14 Although 90% of patients with GPA with multiorgan involvement will have a positive ANCA, a negative test is more common in localized upper airway disease, where only 50% have a positive ANCA.15 A number of drugs, medications, infections, and nonvasculitic autoimmune diseases have been associated with positive ANCA serologies in the absence of systemic vasculitis.14,16,17 As such, pathologic demonstration of vasculitis is necessary for establishing the diagnosis. Typical sites for biopsy include the kidneys and lungs.9
This case illustrates how clinicians often find themselves at a diagnostic crossroads—being forced to choose which clinical elements to prioritize. At various points, our patient’s presentation could have been framed as “a man from a Tb-endemic country with hemoptysis and an apical opacity,” “an elderly man with extensive smoking history and lung mass,” or “a patient with elevated inflammatory markers and pulmonary-renal syndrome”. In such situations, it is incumbent on the clinician to evaluate how well a given problem representation encompasses or highlights the salient features of a case. As with painting or photography, an essential aspect of appreciating the whole picture involves carefully selecting the right frame.
KEY TEACHING POINTS
- The diagnosis of tuberculosis relies on smear microscopy, nucleic-acid amplification testing (NAAT), and cultures. A positive AFB smear with negative NAAT suggests the presence of a nontuberculous mycobacteria (NTM).
- NTM are common environmental organisms and often exist as nonpathogenic colonizers.6 The diagnosis of NTM disease requires exclusion of other causes and careful clinical, microbiologic, and radiographic correlation.
- Granulomatosis with polyangiitis is a multisystem disease often involving the respiratory track and kidney. Pulmonary disease can present with airway involvement, parenchymal nodules, opacities, pleural findings, and diffuse alveolar hemorrhage.12
Disclosures
Drs. Minter, Geha, Boslett, Chung, and Ramani have no disclosures. Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME).
1. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. PubMed
2. Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;(1):Cd009593. PubMed
3. Luetkemeyer AF, Firnhaber C, Kendall MA, et al. Evaluation of Xpert MTB/RIF versus afb smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis. 2016;62(9):1081-1088. PubMed
4. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015. PubMed
5. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416. PubMed
6. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977-982. PubMed
7. Teixeira L, Avery RK, Iseman M, et al. Mycobacterium llatzerense lung infection in a liver transplant recipient: case report and review of the literature. Am J Transplant. 2013;13(8):2198-2200. PubMed
8. Cárdenas AM, Gomila M, Lalucat J, Edelstein PH. Abdominal abscess caused by Mycobacterium llatzerense. J Clin Microbiol. 2014;52(4):1287-1289. PubMed
9. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512-1523. PubMed
10. Mahr A, Katsahian S, Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72(6):1003-1010. PubMed
11. Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63(1):257-266. PubMed
12. Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912. PubMed
13. Hamadah AM, Gharaibeh K, Mara KC, et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells. Nephrol Dial Transplant. 2018;33(8):1397-1403. PubMed
14. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10(8):463-473. PubMed
15. Borner U, Landis BN, Banz Y, et al. Diagnostic value of biopsies in identifying cytoplasmic antineutrophil cytoplasmic antibody-negative localized Wegener’s granulomatosis presenting primarily with sinonasal disease. Am J Rhinol Allergy. 2012;26(6):475-480. PubMed
16. Mahr A, Batteux F, Tubiana S, et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 2014;66(6):1672-1677. PubMed
17. Sherkat R, Mostafavizadeh K, Zeydabadi L, Shoaei P, Rostami S. Antineutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis. Iran J Immunol. 2011;8(1):52-57. PubMed
1. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. PubMed
2. Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;(1):Cd009593. PubMed
3. Luetkemeyer AF, Firnhaber C, Kendall MA, et al. Evaluation of Xpert MTB/RIF versus afb smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis. 2016;62(9):1081-1088. PubMed
4. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015. PubMed
5. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416. PubMed
6. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977-982. PubMed
7. Teixeira L, Avery RK, Iseman M, et al. Mycobacterium llatzerense lung infection in a liver transplant recipient: case report and review of the literature. Am J Transplant. 2013;13(8):2198-2200. PubMed
8. Cárdenas AM, Gomila M, Lalucat J, Edelstein PH. Abdominal abscess caused by Mycobacterium llatzerense. J Clin Microbiol. 2014;52(4):1287-1289. PubMed
9. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512-1523. PubMed
10. Mahr A, Katsahian S, Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72(6):1003-1010. PubMed
11. Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63(1):257-266. PubMed
12. Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912. PubMed
13. Hamadah AM, Gharaibeh K, Mara KC, et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells. Nephrol Dial Transplant. 2018;33(8):1397-1403. PubMed
14. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10(8):463-473. PubMed
15. Borner U, Landis BN, Banz Y, et al. Diagnostic value of biopsies in identifying cytoplasmic antineutrophil cytoplasmic antibody-negative localized Wegener’s granulomatosis presenting primarily with sinonasal disease. Am J Rhinol Allergy. 2012;26(6):475-480. PubMed
16. Mahr A, Batteux F, Tubiana S, et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 2014;66(6):1672-1677. PubMed
17. Sherkat R, Mostafavizadeh K, Zeydabadi L, Shoaei P, Rostami S. Antineutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis. Iran J Immunol. 2011;8(1):52-57. PubMed
© 2019 Society of Hospital Medicine
Reimagining Inpatient Care in Canadian Teaching Hospitals: Bold Initiatives or Tinkering at the Margins?
Canada’s 17 medical schools and their affiliated teaching hospitals are instrumental in serving local communities and providing regional and national access to specialized therapies. Akin to many other countries, patients in Canadian teaching hospitals typically receive care from trainees supervised by attending physicians on teams that Canadians refer to as clinical teaching units (CTUs).1 For more than 50 years, the CTU model has served trainees, attendings, and patients well.2 The success of the CTU model has been dependent on several factors including the crucial balance between the number of trainees and volume of patients. However, Canadian teaching hospitals are increasingly challenged by an imbalance in the trainee-to-patient volume equilibrium spurred by increasing patient volumes and declining house staff availability. The challenges we are facing today in Canada are similar to those teaching hospitals in the United States have faced and adapted to over the last 15 years. Can we build a new, sustainable model of inpatient care through attending-directed inpatient services much as has happened in the US?
Canada’s population of 36 million people is growing by approximately 1% per year, largely driven by immigration.3 At the same time, Canada’s population is aging and becoming increasingly medically complex; the percentage of Canadians age 65 years and older is anticipated to rise from approximately 17% today to 25% in 2035.4 Canada’s healthcare system historically functioned with relatively few inpatient beds, encouraging efficiency particularly with respect to which patients require hospital admission and which do not.5 Although data suggest that the number of hospital admissions declined in Canada between 1980 and 1995, recent data documented that General Internal Medicine admissions increased by 32% between 2010 and 2015 and accounted for 24% of total hospital bed days.6,7 The effects of population growth and aging on admission volumes might be mitigated to some extent by innovations in healthcare delivery such as improved access to primary care (largely family physicians in Canada). However, even with these innovations, a growing and aging population is likely to have a disproportionate effect on the types of undifferentiated illnesses that are typically admitted to General Internal Medicine in Canadian teaching hospitals.
Increasing volumes and complexity are occurring at the same time that residency training in Canada is undergoing an extraordinary shift, mirroring trends in other countries.8 CTUs in Canada typically have a census of 20 or more patients and are staffed by an attending, one senior resident, two to three junior residents, and medical students. Recognition that physician fatigue is associated with patient safety events and physician burnout has led to shorter resident shifts, though Canadian hospitals typically operate without concrete work hour limits or “hard” caps on team size.8 To fulfill accreditation standards set by the Royal College of Physicians and Surgeons of Canada, residency programs have required increases in formal teaching sessions during working hours, further reducing resident presence at the bedside. Many specialty training programs (eg, anesthesiology and ophthalmology) that traditionally required trainees to rotate through General Medicine have eliminated this requirement. Moreover, postgraduate training now requires additional time be spent in ambulatory and community hospital settings to better prepare residents for practice.9 There is little enthusiasm for increasing the number of residents, as postgraduate training spots increased by 85% between 2000 and 2013, before stabilizing in recent years.10
These factors are leading to a substantial decline in resident availability on CTUs, shifting increasing amounts of direct patient care to attending physicians in Canadian teaching hospitals across virtually all specialties. Unsurprisingly, increased rates of burnout and decreases in job satisfactio
Canadian teaching hospitals currently find themselves facing a confluence of factors nearly identical to those faced by teaching hospitals in the United States during 2003 when the Accreditation Council for Graduate Medical Education instituted resident duty hour restrictions to address concerns over trainee wellness, shift length, and patient safety.8 Instantly, hundreds of US teaching hospitals faced uncertainty over who would provide patient care when residents were unavailable. Virtually all US teaching hospitals responded with a creativity and speed that we are unaccustomed to in academic medicine. Hospitals reallocated money to finance attending-directed services where patient care was provided directly by attending physicians often working without trainees12 but frequently supported by nurse practitioners or physician assistants.13 Despite the differences between US and Canadian healthcare, 15 years later, we in Canada can and should learn from the US experience.14
Attending-directed services offer several advantages. First, attending-directed services offer patient outcomes including ICU transfer, mortality, readmissions, and satisfaction that are similar, if not modestly improved, when compared with traditional teaching services.15 Results also suggest potential reductions in hospital length of stay and diagnostic testing.16 Attending-directed services can enhance trainee education by insuring attending physician presence and oversight in-hospital 24-hours per day.17 Although not well studied, attending-directed services may reduce variation in CTU patient census so that excess volumes can be absorbed by attending-directed teams even with seasonal surges (eg, influenza). Recognizing that many specialties were experiencing the same challenges as General Medicine in 2003, attending-directed services in the US have been designed to care for a wide spectrum of patients drawn from an array of different specialties with evidence of improved outcomes.12 Building attending-directed services in Canadian teaching hospitals may expand to include patients from multiple specialties and subspecialties (surgery, orthopedics, and cardiology) where patient volumes are increasing and resident coverage is increasingly scarce.
The challenges that accompany the implementation of attending-directed teams must be acknowledged. First, while attending-directed teams solve many problems for teaching hospitals, physician billings may not generate sufficient income to be self-sustaining and require additional financial support.18 Without investment from hospitals or government, attending-directed models cannot flourish in teaching hospitals. US hospitals typically provide substantial financial support ($50,000-$100,000 per physician) to hospitalist programs, but Canadian teaching hospitals have been reluctant to follow suit.
Second, attending-directed services require a sustainable workforce. In Canada, inpatient care is provided predominately by family physician hospitalists in community hospitals, whereas internists typically fulfill these roles in teaching hospitals.19 Family physician hospitalists are commonly represented by the Canadian Society for Hospital Medicine, which is the Canadian branch of the Society of Hospital Medicine. Hospital medicine in Canada is typically organized around physician training (family physician vs internist) rather than clinical focus (outpatient vs inpatient). Collaborative models of care that unite hospitalists from all training streams (family physician, internist, and pediatrics) are only just emerging in Canadian teaching hospitals. How these programs are developed will be critical to the successful growth of attending-directed services. Third, if attending-directed services expand in teaching hospitals, the physicians who staff these services must come from somewhere. Either the “production” of physicians will need to increase or physicians will migrate to attending-directed services from outpatient practice or from community hospitals.20 Canadian teaching hospitals can also explore nurse practitioners and physician assistants, a previously underutilized resource. Though the costs of such programs can be significant,21 the payoff in safety, quality, and efficiency may be worth it—as demonstrated in the US system. Fourth, teaching hospitals and medical schools must create academic homes to support and mentor the physicians working on attending-directed services. Although physicians hired for attending-directed services primarily provide direct patient care, few will join academic medical centers solely for this purpose. Teaching hospitals and medical schools need to carefully consider job descriptions, mentoring, and career advancement opportunities as they build attending-directed services. Finally, the interactions between teaching and attending-directed services are complex. There is an inevitable learning curve as clinical operations and protocols are built and developed. For example, decisions need to be made about how patients are divided between services and whether nocturnists are responsible for teaching overnight residents.17 Successful programs have the potential to benefit hospitals, patients, learners, and faculty alike.
The risks associated with the status quo in Canada must also be addressed. Patient volumes and complexity in Canada are likely to continue to slowly increase, while the number of trainees in Canadian teaching hospitals will remain stable at best. Forcing more patients onto already overtaxed teaching services is likely to worsen hospital efficiency, patient outcomes, and educational experiences.22 Forcing additional patient care onto overstretched faculty will slowly erode the academic work (teaching and research) that has characterized excellence in Canadian medicine.
The changes we propose to overcome the challenges facing Canadian teaching hospitals are neither cheap nor easy (Table). We expect resistance on many fronts. Implementing them will likely require concerted advocacy from a diverse group of champions shining a bright spotlight on the sizable challenges Canadian teaching hospitals are confronting. We believe that each challenge maps to a discrete group of champions with discrete targets within hospital leadership, medical school administration, and government who will need to be engaged. In our opinion, organizing around these challenges offers the best opportunity to overcome the perpetual resistance around costs. Canadian teaching hospitals and their CTUs are under unprecedented pressure. Do we act boldly and embrace attending-directed models of care or continue tinkering at the margins?
Acknowledgments
The authors thank Chaim Bell for his advice and suggestions.
Disclosures
The authors have nothing to disclose.
1. Schrewe B, Pratt DD, McKellin WH. Adapting the forms of yesterday to the functions of today and the needs of tomorrow: a genealogical case study of clinical teaching units in Canada. Adv Health Sci Educ Theory Pract. 2016;21(2):475-499. PubMed
2. Maudsley RF. The clinical teaching unit in transition. CMAJ. 1993;148(9):1564-1566. PubMed
3. Statistics Canada. Recent Changes in Demographic Trends in Canada. Ottawa: Ontario, 2015. https://www150.statcan.gc.ca/n1/pub/75-006-x/2015001/article/14240-eng.htm. Accessed December 9, 2018
4. Statistics Canada. Census, Age and Sex. Ottawa: Ontario, 2016. https://www12.statcan.gc.ca/census-recensement/2016/rt-td/as-eng.cfm. Accessed December 10, 2018.
5. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. PubMed
6. van Walraven C. Trends in 1-year survival of people admitted to hospital in Ontario, 1994-2009. CMAJ. 2013;185(16):E755-E762. PubMed
7. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: the General Medicine Inpatient Initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5(4):E842-E849. PubMed
8. Pattani R, Wu PE, Dhalla IA. Resident duty hours in Canada: past, present and future. CMAJ. 2014;186(10):761-765. PubMed
9. Royal College of Physicians and Surgeons. Specialty Training Requirements in Internal medicine 2015. http://www.royalcollege.ca/cs/groups/public/documents/document/mdaw/mdg4/~edisp/088402.pdf. Accessed December 12, 2018.
10. Freeman TR, Petterson S, Finnegan S, Bazemore A. Shifting tides in the emigration patterns of Canadian physicians to the United States: a cross-sectional secondary data analysis. BMC Health Serv Res. 2016;16(1):678. PubMed
11. Wong BM, Imrie K. Why resident duty hours regulations must address attending physicians’ workload. Acad Med. 2013;88(9):1209-1211. PubMed
12. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. PubMed
13. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. PubMed
14. Ivers N, Brown AD, Detsky AS. Lessons from the Canadian experience with single-payer health insurance: just comfortable enough with the status quo. JAMA Intern Med. 2018;178(9):1250-1255. PubMed
15. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
16. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. PubMed
17. Farnan JM, Burger A, Boonyasai RT, et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521-523. PubMed
18. Gonzalo JD, Kuperman EF, Chuang CH, Lehman E, Glasser F, Abendroth T. Impact of an overnight internal medicine academic hospitalist program on patient outcomes. J Gen Intern Med. 2015;30(12):1795-1802. PubMed
19. Soong C, Fan E, Howell EE, et al. Characteristics of Hospitalists and Hospitalist Programs in the United States and Canada 2009. J Clin Outcomes Meas. 2009; 16 (2): 69-74.
20. Yousefi V, Maslowski R. Health system drivers of hospital medicine in Canada: systematic review. Can Fam Phys Med Fam Can. 2013;59(7):762-767. PubMed
21. Nuckols TK, Escarce JJ. Cost implications of ACGME’s 2011 changes to resident duty hours and the training environment. J Gen Intern Med. 2012;27(2):241-249. PubMed
22. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. PubMed
Canada’s 17 medical schools and their affiliated teaching hospitals are instrumental in serving local communities and providing regional and national access to specialized therapies. Akin to many other countries, patients in Canadian teaching hospitals typically receive care from trainees supervised by attending physicians on teams that Canadians refer to as clinical teaching units (CTUs).1 For more than 50 years, the CTU model has served trainees, attendings, and patients well.2 The success of the CTU model has been dependent on several factors including the crucial balance between the number of trainees and volume of patients. However, Canadian teaching hospitals are increasingly challenged by an imbalance in the trainee-to-patient volume equilibrium spurred by increasing patient volumes and declining house staff availability. The challenges we are facing today in Canada are similar to those teaching hospitals in the United States have faced and adapted to over the last 15 years. Can we build a new, sustainable model of inpatient care through attending-directed inpatient services much as has happened in the US?
Canada’s population of 36 million people is growing by approximately 1% per year, largely driven by immigration.3 At the same time, Canada’s population is aging and becoming increasingly medically complex; the percentage of Canadians age 65 years and older is anticipated to rise from approximately 17% today to 25% in 2035.4 Canada’s healthcare system historically functioned with relatively few inpatient beds, encouraging efficiency particularly with respect to which patients require hospital admission and which do not.5 Although data suggest that the number of hospital admissions declined in Canada between 1980 and 1995, recent data documented that General Internal Medicine admissions increased by 32% between 2010 and 2015 and accounted for 24% of total hospital bed days.6,7 The effects of population growth and aging on admission volumes might be mitigated to some extent by innovations in healthcare delivery such as improved access to primary care (largely family physicians in Canada). However, even with these innovations, a growing and aging population is likely to have a disproportionate effect on the types of undifferentiated illnesses that are typically admitted to General Internal Medicine in Canadian teaching hospitals.
Increasing volumes and complexity are occurring at the same time that residency training in Canada is undergoing an extraordinary shift, mirroring trends in other countries.8 CTUs in Canada typically have a census of 20 or more patients and are staffed by an attending, one senior resident, two to three junior residents, and medical students. Recognition that physician fatigue is associated with patient safety events and physician burnout has led to shorter resident shifts, though Canadian hospitals typically operate without concrete work hour limits or “hard” caps on team size.8 To fulfill accreditation standards set by the Royal College of Physicians and Surgeons of Canada, residency programs have required increases in formal teaching sessions during working hours, further reducing resident presence at the bedside. Many specialty training programs (eg, anesthesiology and ophthalmology) that traditionally required trainees to rotate through General Medicine have eliminated this requirement. Moreover, postgraduate training now requires additional time be spent in ambulatory and community hospital settings to better prepare residents for practice.9 There is little enthusiasm for increasing the number of residents, as postgraduate training spots increased by 85% between 2000 and 2013, before stabilizing in recent years.10
These factors are leading to a substantial decline in resident availability on CTUs, shifting increasing amounts of direct patient care to attending physicians in Canadian teaching hospitals across virtually all specialties. Unsurprisingly, increased rates of burnout and decreases in job satisfactio
Canadian teaching hospitals currently find themselves facing a confluence of factors nearly identical to those faced by teaching hospitals in the United States during 2003 when the Accreditation Council for Graduate Medical Education instituted resident duty hour restrictions to address concerns over trainee wellness, shift length, and patient safety.8 Instantly, hundreds of US teaching hospitals faced uncertainty over who would provide patient care when residents were unavailable. Virtually all US teaching hospitals responded with a creativity and speed that we are unaccustomed to in academic medicine. Hospitals reallocated money to finance attending-directed services where patient care was provided directly by attending physicians often working without trainees12 but frequently supported by nurse practitioners or physician assistants.13 Despite the differences between US and Canadian healthcare, 15 years later, we in Canada can and should learn from the US experience.14
Attending-directed services offer several advantages. First, attending-directed services offer patient outcomes including ICU transfer, mortality, readmissions, and satisfaction that are similar, if not modestly improved, when compared with traditional teaching services.15 Results also suggest potential reductions in hospital length of stay and diagnostic testing.16 Attending-directed services can enhance trainee education by insuring attending physician presence and oversight in-hospital 24-hours per day.17 Although not well studied, attending-directed services may reduce variation in CTU patient census so that excess volumes can be absorbed by attending-directed teams even with seasonal surges (eg, influenza). Recognizing that many specialties were experiencing the same challenges as General Medicine in 2003, attending-directed services in the US have been designed to care for a wide spectrum of patients drawn from an array of different specialties with evidence of improved outcomes.12 Building attending-directed services in Canadian teaching hospitals may expand to include patients from multiple specialties and subspecialties (surgery, orthopedics, and cardiology) where patient volumes are increasing and resident coverage is increasingly scarce.
The challenges that accompany the implementation of attending-directed teams must be acknowledged. First, while attending-directed teams solve many problems for teaching hospitals, physician billings may not generate sufficient income to be self-sustaining and require additional financial support.18 Without investment from hospitals or government, attending-directed models cannot flourish in teaching hospitals. US hospitals typically provide substantial financial support ($50,000-$100,000 per physician) to hospitalist programs, but Canadian teaching hospitals have been reluctant to follow suit.
Second, attending-directed services require a sustainable workforce. In Canada, inpatient care is provided predominately by family physician hospitalists in community hospitals, whereas internists typically fulfill these roles in teaching hospitals.19 Family physician hospitalists are commonly represented by the Canadian Society for Hospital Medicine, which is the Canadian branch of the Society of Hospital Medicine. Hospital medicine in Canada is typically organized around physician training (family physician vs internist) rather than clinical focus (outpatient vs inpatient). Collaborative models of care that unite hospitalists from all training streams (family physician, internist, and pediatrics) are only just emerging in Canadian teaching hospitals. How these programs are developed will be critical to the successful growth of attending-directed services. Third, if attending-directed services expand in teaching hospitals, the physicians who staff these services must come from somewhere. Either the “production” of physicians will need to increase or physicians will migrate to attending-directed services from outpatient practice or from community hospitals.20 Canadian teaching hospitals can also explore nurse practitioners and physician assistants, a previously underutilized resource. Though the costs of such programs can be significant,21 the payoff in safety, quality, and efficiency may be worth it—as demonstrated in the US system. Fourth, teaching hospitals and medical schools must create academic homes to support and mentor the physicians working on attending-directed services. Although physicians hired for attending-directed services primarily provide direct patient care, few will join academic medical centers solely for this purpose. Teaching hospitals and medical schools need to carefully consider job descriptions, mentoring, and career advancement opportunities as they build attending-directed services. Finally, the interactions between teaching and attending-directed services are complex. There is an inevitable learning curve as clinical operations and protocols are built and developed. For example, decisions need to be made about how patients are divided between services and whether nocturnists are responsible for teaching overnight residents.17 Successful programs have the potential to benefit hospitals, patients, learners, and faculty alike.
The risks associated with the status quo in Canada must also be addressed. Patient volumes and complexity in Canada are likely to continue to slowly increase, while the number of trainees in Canadian teaching hospitals will remain stable at best. Forcing more patients onto already overtaxed teaching services is likely to worsen hospital efficiency, patient outcomes, and educational experiences.22 Forcing additional patient care onto overstretched faculty will slowly erode the academic work (teaching and research) that has characterized excellence in Canadian medicine.
The changes we propose to overcome the challenges facing Canadian teaching hospitals are neither cheap nor easy (Table). We expect resistance on many fronts. Implementing them will likely require concerted advocacy from a diverse group of champions shining a bright spotlight on the sizable challenges Canadian teaching hospitals are confronting. We believe that each challenge maps to a discrete group of champions with discrete targets within hospital leadership, medical school administration, and government who will need to be engaged. In our opinion, organizing around these challenges offers the best opportunity to overcome the perpetual resistance around costs. Canadian teaching hospitals and their CTUs are under unprecedented pressure. Do we act boldly and embrace attending-directed models of care or continue tinkering at the margins?
Acknowledgments
The authors thank Chaim Bell for his advice and suggestions.
Disclosures
The authors have nothing to disclose.
Canada’s 17 medical schools and their affiliated teaching hospitals are instrumental in serving local communities and providing regional and national access to specialized therapies. Akin to many other countries, patients in Canadian teaching hospitals typically receive care from trainees supervised by attending physicians on teams that Canadians refer to as clinical teaching units (CTUs).1 For more than 50 years, the CTU model has served trainees, attendings, and patients well.2 The success of the CTU model has been dependent on several factors including the crucial balance between the number of trainees and volume of patients. However, Canadian teaching hospitals are increasingly challenged by an imbalance in the trainee-to-patient volume equilibrium spurred by increasing patient volumes and declining house staff availability. The challenges we are facing today in Canada are similar to those teaching hospitals in the United States have faced and adapted to over the last 15 years. Can we build a new, sustainable model of inpatient care through attending-directed inpatient services much as has happened in the US?
Canada’s population of 36 million people is growing by approximately 1% per year, largely driven by immigration.3 At the same time, Canada’s population is aging and becoming increasingly medically complex; the percentage of Canadians age 65 years and older is anticipated to rise from approximately 17% today to 25% in 2035.4 Canada’s healthcare system historically functioned with relatively few inpatient beds, encouraging efficiency particularly with respect to which patients require hospital admission and which do not.5 Although data suggest that the number of hospital admissions declined in Canada between 1980 and 1995, recent data documented that General Internal Medicine admissions increased by 32% between 2010 and 2015 and accounted for 24% of total hospital bed days.6,7 The effects of population growth and aging on admission volumes might be mitigated to some extent by innovations in healthcare delivery such as improved access to primary care (largely family physicians in Canada). However, even with these innovations, a growing and aging population is likely to have a disproportionate effect on the types of undifferentiated illnesses that are typically admitted to General Internal Medicine in Canadian teaching hospitals.
Increasing volumes and complexity are occurring at the same time that residency training in Canada is undergoing an extraordinary shift, mirroring trends in other countries.8 CTUs in Canada typically have a census of 20 or more patients and are staffed by an attending, one senior resident, two to three junior residents, and medical students. Recognition that physician fatigue is associated with patient safety events and physician burnout has led to shorter resident shifts, though Canadian hospitals typically operate without concrete work hour limits or “hard” caps on team size.8 To fulfill accreditation standards set by the Royal College of Physicians and Surgeons of Canada, residency programs have required increases in formal teaching sessions during working hours, further reducing resident presence at the bedside. Many specialty training programs (eg, anesthesiology and ophthalmology) that traditionally required trainees to rotate through General Medicine have eliminated this requirement. Moreover, postgraduate training now requires additional time be spent in ambulatory and community hospital settings to better prepare residents for practice.9 There is little enthusiasm for increasing the number of residents, as postgraduate training spots increased by 85% between 2000 and 2013, before stabilizing in recent years.10
These factors are leading to a substantial decline in resident availability on CTUs, shifting increasing amounts of direct patient care to attending physicians in Canadian teaching hospitals across virtually all specialties. Unsurprisingly, increased rates of burnout and decreases in job satisfactio
Canadian teaching hospitals currently find themselves facing a confluence of factors nearly identical to those faced by teaching hospitals in the United States during 2003 when the Accreditation Council for Graduate Medical Education instituted resident duty hour restrictions to address concerns over trainee wellness, shift length, and patient safety.8 Instantly, hundreds of US teaching hospitals faced uncertainty over who would provide patient care when residents were unavailable. Virtually all US teaching hospitals responded with a creativity and speed that we are unaccustomed to in academic medicine. Hospitals reallocated money to finance attending-directed services where patient care was provided directly by attending physicians often working without trainees12 but frequently supported by nurse practitioners or physician assistants.13 Despite the differences between US and Canadian healthcare, 15 years later, we in Canada can and should learn from the US experience.14
Attending-directed services offer several advantages. First, attending-directed services offer patient outcomes including ICU transfer, mortality, readmissions, and satisfaction that are similar, if not modestly improved, when compared with traditional teaching services.15 Results also suggest potential reductions in hospital length of stay and diagnostic testing.16 Attending-directed services can enhance trainee education by insuring attending physician presence and oversight in-hospital 24-hours per day.17 Although not well studied, attending-directed services may reduce variation in CTU patient census so that excess volumes can be absorbed by attending-directed teams even with seasonal surges (eg, influenza). Recognizing that many specialties were experiencing the same challenges as General Medicine in 2003, attending-directed services in the US have been designed to care for a wide spectrum of patients drawn from an array of different specialties with evidence of improved outcomes.12 Building attending-directed services in Canadian teaching hospitals may expand to include patients from multiple specialties and subspecialties (surgery, orthopedics, and cardiology) where patient volumes are increasing and resident coverage is increasingly scarce.
The challenges that accompany the implementation of attending-directed teams must be acknowledged. First, while attending-directed teams solve many problems for teaching hospitals, physician billings may not generate sufficient income to be self-sustaining and require additional financial support.18 Without investment from hospitals or government, attending-directed models cannot flourish in teaching hospitals. US hospitals typically provide substantial financial support ($50,000-$100,000 per physician) to hospitalist programs, but Canadian teaching hospitals have been reluctant to follow suit.
Second, attending-directed services require a sustainable workforce. In Canada, inpatient care is provided predominately by family physician hospitalists in community hospitals, whereas internists typically fulfill these roles in teaching hospitals.19 Family physician hospitalists are commonly represented by the Canadian Society for Hospital Medicine, which is the Canadian branch of the Society of Hospital Medicine. Hospital medicine in Canada is typically organized around physician training (family physician vs internist) rather than clinical focus (outpatient vs inpatient). Collaborative models of care that unite hospitalists from all training streams (family physician, internist, and pediatrics) are only just emerging in Canadian teaching hospitals. How these programs are developed will be critical to the successful growth of attending-directed services. Third, if attending-directed services expand in teaching hospitals, the physicians who staff these services must come from somewhere. Either the “production” of physicians will need to increase or physicians will migrate to attending-directed services from outpatient practice or from community hospitals.20 Canadian teaching hospitals can also explore nurse practitioners and physician assistants, a previously underutilized resource. Though the costs of such programs can be significant,21 the payoff in safety, quality, and efficiency may be worth it—as demonstrated in the US system. Fourth, teaching hospitals and medical schools must create academic homes to support and mentor the physicians working on attending-directed services. Although physicians hired for attending-directed services primarily provide direct patient care, few will join academic medical centers solely for this purpose. Teaching hospitals and medical schools need to carefully consider job descriptions, mentoring, and career advancement opportunities as they build attending-directed services. Finally, the interactions between teaching and attending-directed services are complex. There is an inevitable learning curve as clinical operations and protocols are built and developed. For example, decisions need to be made about how patients are divided between services and whether nocturnists are responsible for teaching overnight residents.17 Successful programs have the potential to benefit hospitals, patients, learners, and faculty alike.
The risks associated with the status quo in Canada must also be addressed. Patient volumes and complexity in Canada are likely to continue to slowly increase, while the number of trainees in Canadian teaching hospitals will remain stable at best. Forcing more patients onto already overtaxed teaching services is likely to worsen hospital efficiency, patient outcomes, and educational experiences.22 Forcing additional patient care onto overstretched faculty will slowly erode the academic work (teaching and research) that has characterized excellence in Canadian medicine.
The changes we propose to overcome the challenges facing Canadian teaching hospitals are neither cheap nor easy (Table). We expect resistance on many fronts. Implementing them will likely require concerted advocacy from a diverse group of champions shining a bright spotlight on the sizable challenges Canadian teaching hospitals are confronting. We believe that each challenge maps to a discrete group of champions with discrete targets within hospital leadership, medical school administration, and government who will need to be engaged. In our opinion, organizing around these challenges offers the best opportunity to overcome the perpetual resistance around costs. Canadian teaching hospitals and their CTUs are under unprecedented pressure. Do we act boldly and embrace attending-directed models of care or continue tinkering at the margins?
Acknowledgments
The authors thank Chaim Bell for his advice and suggestions.
Disclosures
The authors have nothing to disclose.
1. Schrewe B, Pratt DD, McKellin WH. Adapting the forms of yesterday to the functions of today and the needs of tomorrow: a genealogical case study of clinical teaching units in Canada. Adv Health Sci Educ Theory Pract. 2016;21(2):475-499. PubMed
2. Maudsley RF. The clinical teaching unit in transition. CMAJ. 1993;148(9):1564-1566. PubMed
3. Statistics Canada. Recent Changes in Demographic Trends in Canada. Ottawa: Ontario, 2015. https://www150.statcan.gc.ca/n1/pub/75-006-x/2015001/article/14240-eng.htm. Accessed December 9, 2018
4. Statistics Canada. Census, Age and Sex. Ottawa: Ontario, 2016. https://www12.statcan.gc.ca/census-recensement/2016/rt-td/as-eng.cfm. Accessed December 10, 2018.
5. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. PubMed
6. van Walraven C. Trends in 1-year survival of people admitted to hospital in Ontario, 1994-2009. CMAJ. 2013;185(16):E755-E762. PubMed
7. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: the General Medicine Inpatient Initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5(4):E842-E849. PubMed
8. Pattani R, Wu PE, Dhalla IA. Resident duty hours in Canada: past, present and future. CMAJ. 2014;186(10):761-765. PubMed
9. Royal College of Physicians and Surgeons. Specialty Training Requirements in Internal medicine 2015. http://www.royalcollege.ca/cs/groups/public/documents/document/mdaw/mdg4/~edisp/088402.pdf. Accessed December 12, 2018.
10. Freeman TR, Petterson S, Finnegan S, Bazemore A. Shifting tides in the emigration patterns of Canadian physicians to the United States: a cross-sectional secondary data analysis. BMC Health Serv Res. 2016;16(1):678. PubMed
11. Wong BM, Imrie K. Why resident duty hours regulations must address attending physicians’ workload. Acad Med. 2013;88(9):1209-1211. PubMed
12. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. PubMed
13. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. PubMed
14. Ivers N, Brown AD, Detsky AS. Lessons from the Canadian experience with single-payer health insurance: just comfortable enough with the status quo. JAMA Intern Med. 2018;178(9):1250-1255. PubMed
15. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
16. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. PubMed
17. Farnan JM, Burger A, Boonyasai RT, et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521-523. PubMed
18. Gonzalo JD, Kuperman EF, Chuang CH, Lehman E, Glasser F, Abendroth T. Impact of an overnight internal medicine academic hospitalist program on patient outcomes. J Gen Intern Med. 2015;30(12):1795-1802. PubMed
19. Soong C, Fan E, Howell EE, et al. Characteristics of Hospitalists and Hospitalist Programs in the United States and Canada 2009. J Clin Outcomes Meas. 2009; 16 (2): 69-74.
20. Yousefi V, Maslowski R. Health system drivers of hospital medicine in Canada: systematic review. Can Fam Phys Med Fam Can. 2013;59(7):762-767. PubMed
21. Nuckols TK, Escarce JJ. Cost implications of ACGME’s 2011 changes to resident duty hours and the training environment. J Gen Intern Med. 2012;27(2):241-249. PubMed
22. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. PubMed
1. Schrewe B, Pratt DD, McKellin WH. Adapting the forms of yesterday to the functions of today and the needs of tomorrow: a genealogical case study of clinical teaching units in Canada. Adv Health Sci Educ Theory Pract. 2016;21(2):475-499. PubMed
2. Maudsley RF. The clinical teaching unit in transition. CMAJ. 1993;148(9):1564-1566. PubMed
3. Statistics Canada. Recent Changes in Demographic Trends in Canada. Ottawa: Ontario, 2015. https://www150.statcan.gc.ca/n1/pub/75-006-x/2015001/article/14240-eng.htm. Accessed December 9, 2018
4. Statistics Canada. Census, Age and Sex. Ottawa: Ontario, 2016. https://www12.statcan.gc.ca/census-recensement/2016/rt-td/as-eng.cfm. Accessed December 10, 2018.
5. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. PubMed
6. van Walraven C. Trends in 1-year survival of people admitted to hospital in Ontario, 1994-2009. CMAJ. 2013;185(16):E755-E762. PubMed
7. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: the General Medicine Inpatient Initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5(4):E842-E849. PubMed
8. Pattani R, Wu PE, Dhalla IA. Resident duty hours in Canada: past, present and future. CMAJ. 2014;186(10):761-765. PubMed
9. Royal College of Physicians and Surgeons. Specialty Training Requirements in Internal medicine 2015. http://www.royalcollege.ca/cs/groups/public/documents/document/mdaw/mdg4/~edisp/088402.pdf. Accessed December 12, 2018.
10. Freeman TR, Petterson S, Finnegan S, Bazemore A. Shifting tides in the emigration patterns of Canadian physicians to the United States: a cross-sectional secondary data analysis. BMC Health Serv Res. 2016;16(1):678. PubMed
11. Wong BM, Imrie K. Why resident duty hours regulations must address attending physicians’ workload. Acad Med. 2013;88(9):1209-1211. PubMed
12. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. PubMed
13. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. PubMed
14. Ivers N, Brown AD, Detsky AS. Lessons from the Canadian experience with single-payer health insurance: just comfortable enough with the status quo. JAMA Intern Med. 2018;178(9):1250-1255. PubMed
15. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
16. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. PubMed
17. Farnan JM, Burger A, Boonyasai RT, et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521-523. PubMed
18. Gonzalo JD, Kuperman EF, Chuang CH, Lehman E, Glasser F, Abendroth T. Impact of an overnight internal medicine academic hospitalist program on patient outcomes. J Gen Intern Med. 2015;30(12):1795-1802. PubMed
19. Soong C, Fan E, Howell EE, et al. Characteristics of Hospitalists and Hospitalist Programs in the United States and Canada 2009. J Clin Outcomes Meas. 2009; 16 (2): 69-74.
20. Yousefi V, Maslowski R. Health system drivers of hospital medicine in Canada: systematic review. Can Fam Phys Med Fam Can. 2013;59(7):762-767. PubMed
21. Nuckols TK, Escarce JJ. Cost implications of ACGME’s 2011 changes to resident duty hours and the training environment. J Gen Intern Med. 2012;27(2):241-249. PubMed
22. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. PubMed
© 2019 Society of Hospital Medicine
Leadership & Professional Development: Be the Change You Want to See
“…a truly strong, powerful man isn’t threatened by a strong, powerful woman. Instead, he is challenged by her, he is inspired by her, he is pleased to relate to her as an equal.”
—Michelle Obama
Mentorship is essential to success in hospital medicine and may be particularly important for women. Cross-gender mentorship is especially salient since roughly equal proportions of women and men enter the medical pipeline, but men occupy over 75% of senior leadership roles in healthcare companies.
Cross-gender mentorship poses challenges but can be done successfully.1 We’ve made cross-gender mentoring work well in our own mentoring relationship. We describe three practices for effective mentoring that are especially important for men who mentor women given how common the female mentee-male mentor dyad is in medicine. We make generalizations that don’t apply universally but illustrate the social context in which such mentorship resides.
BE MINDFUL OF GENDER SCRIPTS
Gender scripts refer to social norms relating to gender identities and behaviors. Archetypal scripts include the father/daughter relationship and the knight/damsel-in-distress. Gender scripts often frame women as powerless—waiting to be rescued. By unconsciously activating a gender script, a mentor may reinforce a stereotype that women need rescuing (eg, “She’s really upset—I’ll email her Division Chief and help fix it for her”) or underestimate a mentee’s readiness for independence (eg, “She’s written four papers on this, but she’s still not ready to be senior author”). Astute mentors use reflection to combat gender scripts, asking themselves, “Am I allowing latent biases to affect my judgement?” They also consider when to intervene and when to let the mentee “rescue” herself (eg, “This is challenging, but I trust your judgement. What do you think you should do next?”).
PROMOTE RECIPROCAL LEARNING
Many women value collaborative behaviors and gravitate towards egalitarian learning environments at odds with a traditional, “top-down” mentorship model. Additionally, women may be penalized for demonstrating competitive behaviors, while identical behaviors are chalked up to confidence in men. A critical task, then, is for mentors to coach women to hone their natural leadership style, whether it be more commanding or more communal. A mentor can provide key feedback to the mentee about how her approach might be perceived and how to tweak it for optimal success. Mentors may wish to share missteps and even ask the mentee for advice. Pointing to her competence promotes “relational mentoring” and reciprocal learning, where mentor and mentee can learn positive behaviors from each other.
BE THE CHANGE YOU WANT TO SEE
Mentors will ideally wield their social capital to advance policies that promote gender equity—including fair recruiting, promotion, salary, paid leave, and breastfeeding policies. Exceptional mentors recognize that women may generally have less social capital than men in many organizations, and they proactively make women’s accomplishments more visible.2 They broadcast women’s strengths and nominate women for talks, national committees, honorific societies, and leadership positions. Effective mentors recognize that 30% of female medical faculty report experiencing sexual harassment at work,3 and thus maintain extremely high standards for professional integrity, for both themselves and others who interact with their mentees. They call out sexist remarks in the workplace as unacceptable, making it clear that such behavior won’t be tolerated. As Mohandas Gandhi said: “Be the change that you wish to see in the world.”
Cross-gender mentorship is critical to get right—nearly half our medical workforce depends on it. Men who mentor women help their organizations and gain satisfaction from playing a pivotal role in women’s advancement. When women succeed, we all do.
Disclosures
Dr. Moniz and Dr. Saint have nothing to disclose.
1. Byerley JS. Mentoring in the Era of #MeToo. JAMA. 2018;319(12):1199-1200. PubMed
2. Chopra V, Arora VM, Saint S. Will You Be My Mentor?-Four Archetypes to Help Mentees Succeed in Academic Medicine. JAMA Intern Med. 2018;178(2):175-176. PubMed
3. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual Harassment and Discrimination Experiences of Academic Medical Faculty. JAMA. 2016;315(19):2120-2121. PubMed
“…a truly strong, powerful man isn’t threatened by a strong, powerful woman. Instead, he is challenged by her, he is inspired by her, he is pleased to relate to her as an equal.”
—Michelle Obama
Mentorship is essential to success in hospital medicine and may be particularly important for women. Cross-gender mentorship is especially salient since roughly equal proportions of women and men enter the medical pipeline, but men occupy over 75% of senior leadership roles in healthcare companies.
Cross-gender mentorship poses challenges but can be done successfully.1 We’ve made cross-gender mentoring work well in our own mentoring relationship. We describe three practices for effective mentoring that are especially important for men who mentor women given how common the female mentee-male mentor dyad is in medicine. We make generalizations that don’t apply universally but illustrate the social context in which such mentorship resides.
BE MINDFUL OF GENDER SCRIPTS
Gender scripts refer to social norms relating to gender identities and behaviors. Archetypal scripts include the father/daughter relationship and the knight/damsel-in-distress. Gender scripts often frame women as powerless—waiting to be rescued. By unconsciously activating a gender script, a mentor may reinforce a stereotype that women need rescuing (eg, “She’s really upset—I’ll email her Division Chief and help fix it for her”) or underestimate a mentee’s readiness for independence (eg, “She’s written four papers on this, but she’s still not ready to be senior author”). Astute mentors use reflection to combat gender scripts, asking themselves, “Am I allowing latent biases to affect my judgement?” They also consider when to intervene and when to let the mentee “rescue” herself (eg, “This is challenging, but I trust your judgement. What do you think you should do next?”).
PROMOTE RECIPROCAL LEARNING
Many women value collaborative behaviors and gravitate towards egalitarian learning environments at odds with a traditional, “top-down” mentorship model. Additionally, women may be penalized for demonstrating competitive behaviors, while identical behaviors are chalked up to confidence in men. A critical task, then, is for mentors to coach women to hone their natural leadership style, whether it be more commanding or more communal. A mentor can provide key feedback to the mentee about how her approach might be perceived and how to tweak it for optimal success. Mentors may wish to share missteps and even ask the mentee for advice. Pointing to her competence promotes “relational mentoring” and reciprocal learning, where mentor and mentee can learn positive behaviors from each other.
BE THE CHANGE YOU WANT TO SEE
Mentors will ideally wield their social capital to advance policies that promote gender equity—including fair recruiting, promotion, salary, paid leave, and breastfeeding policies. Exceptional mentors recognize that women may generally have less social capital than men in many organizations, and they proactively make women’s accomplishments more visible.2 They broadcast women’s strengths and nominate women for talks, national committees, honorific societies, and leadership positions. Effective mentors recognize that 30% of female medical faculty report experiencing sexual harassment at work,3 and thus maintain extremely high standards for professional integrity, for both themselves and others who interact with their mentees. They call out sexist remarks in the workplace as unacceptable, making it clear that such behavior won’t be tolerated. As Mohandas Gandhi said: “Be the change that you wish to see in the world.”
Cross-gender mentorship is critical to get right—nearly half our medical workforce depends on it. Men who mentor women help their organizations and gain satisfaction from playing a pivotal role in women’s advancement. When women succeed, we all do.
Disclosures
Dr. Moniz and Dr. Saint have nothing to disclose.
“…a truly strong, powerful man isn’t threatened by a strong, powerful woman. Instead, he is challenged by her, he is inspired by her, he is pleased to relate to her as an equal.”
—Michelle Obama
Mentorship is essential to success in hospital medicine and may be particularly important for women. Cross-gender mentorship is especially salient since roughly equal proportions of women and men enter the medical pipeline, but men occupy over 75% of senior leadership roles in healthcare companies.
Cross-gender mentorship poses challenges but can be done successfully.1 We’ve made cross-gender mentoring work well in our own mentoring relationship. We describe three practices for effective mentoring that are especially important for men who mentor women given how common the female mentee-male mentor dyad is in medicine. We make generalizations that don’t apply universally but illustrate the social context in which such mentorship resides.
BE MINDFUL OF GENDER SCRIPTS
Gender scripts refer to social norms relating to gender identities and behaviors. Archetypal scripts include the father/daughter relationship and the knight/damsel-in-distress. Gender scripts often frame women as powerless—waiting to be rescued. By unconsciously activating a gender script, a mentor may reinforce a stereotype that women need rescuing (eg, “She’s really upset—I’ll email her Division Chief and help fix it for her”) or underestimate a mentee’s readiness for independence (eg, “She’s written four papers on this, but she’s still not ready to be senior author”). Astute mentors use reflection to combat gender scripts, asking themselves, “Am I allowing latent biases to affect my judgement?” They also consider when to intervene and when to let the mentee “rescue” herself (eg, “This is challenging, but I trust your judgement. What do you think you should do next?”).
PROMOTE RECIPROCAL LEARNING
Many women value collaborative behaviors and gravitate towards egalitarian learning environments at odds with a traditional, “top-down” mentorship model. Additionally, women may be penalized for demonstrating competitive behaviors, while identical behaviors are chalked up to confidence in men. A critical task, then, is for mentors to coach women to hone their natural leadership style, whether it be more commanding or more communal. A mentor can provide key feedback to the mentee about how her approach might be perceived and how to tweak it for optimal success. Mentors may wish to share missteps and even ask the mentee for advice. Pointing to her competence promotes “relational mentoring” and reciprocal learning, where mentor and mentee can learn positive behaviors from each other.
BE THE CHANGE YOU WANT TO SEE
Mentors will ideally wield their social capital to advance policies that promote gender equity—including fair recruiting, promotion, salary, paid leave, and breastfeeding policies. Exceptional mentors recognize that women may generally have less social capital than men in many organizations, and they proactively make women’s accomplishments more visible.2 They broadcast women’s strengths and nominate women for talks, national committees, honorific societies, and leadership positions. Effective mentors recognize that 30% of female medical faculty report experiencing sexual harassment at work,3 and thus maintain extremely high standards for professional integrity, for both themselves and others who interact with their mentees. They call out sexist remarks in the workplace as unacceptable, making it clear that such behavior won’t be tolerated. As Mohandas Gandhi said: “Be the change that you wish to see in the world.”
Cross-gender mentorship is critical to get right—nearly half our medical workforce depends on it. Men who mentor women help their organizations and gain satisfaction from playing a pivotal role in women’s advancement. When women succeed, we all do.
Disclosures
Dr. Moniz and Dr. Saint have nothing to disclose.
1. Byerley JS. Mentoring in the Era of #MeToo. JAMA. 2018;319(12):1199-1200. PubMed
2. Chopra V, Arora VM, Saint S. Will You Be My Mentor?-Four Archetypes to Help Mentees Succeed in Academic Medicine. JAMA Intern Med. 2018;178(2):175-176. PubMed
3. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual Harassment and Discrimination Experiences of Academic Medical Faculty. JAMA. 2016;315(19):2120-2121. PubMed
1. Byerley JS. Mentoring in the Era of #MeToo. JAMA. 2018;319(12):1199-1200. PubMed
2. Chopra V, Arora VM, Saint S. Will You Be My Mentor?-Four Archetypes to Help Mentees Succeed in Academic Medicine. JAMA Intern Med. 2018;178(2):175-176. PubMed
3. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual Harassment and Discrimination Experiences of Academic Medical Faculty. JAMA. 2016;315(19):2120-2121. PubMed
© 2019 Society of Hospital Medicine
In Reply to: “Practical Application of Pediatric Hospital Medicine Workforce Data: In Reference to ‘Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey’”
We appreciate the query by Drs. Douglas and Wilson. We hereby supply additional information that is critical for creating and administering sustainable staffing models.
For programs with a census cap, the majority cited 16 or fewer patients as the trigger for that cap. Nearly all programs with back-up used a census of 16 or fewer. Over 80% of programs cited a “safe 7
Regarding clinical weighting of nights, nighttime shifts were often more heavily weighted than day shifts, but approaches to weighting varied and have not been validated. Alternate staffing models for overnight pager calls varied greatly by individual program.
This is a time of significant growth for pediatric hospital medicine, and national workforce data are essential to hospitalists, administrators, and most importantly, patients. Our study1 provides pediatric hospital medicine leaders with data for discussions regarding appropriate FTE and staffing model considerations. The insights generated by our study are particularly relevant in expanding programs and solving problems related to recruitment and retention.
Disclosures
The authors have nothing to disclose.
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. PubMed
We appreciate the query by Drs. Douglas and Wilson. We hereby supply additional information that is critical for creating and administering sustainable staffing models.
For programs with a census cap, the majority cited 16 or fewer patients as the trigger for that cap. Nearly all programs with back-up used a census of 16 or fewer. Over 80% of programs cited a “safe 7
Regarding clinical weighting of nights, nighttime shifts were often more heavily weighted than day shifts, but approaches to weighting varied and have not been validated. Alternate staffing models for overnight pager calls varied greatly by individual program.
This is a time of significant growth for pediatric hospital medicine, and national workforce data are essential to hospitalists, administrators, and most importantly, patients. Our study1 provides pediatric hospital medicine leaders with data for discussions regarding appropriate FTE and staffing model considerations. The insights generated by our study are particularly relevant in expanding programs and solving problems related to recruitment and retention.
Disclosures
The authors have nothing to disclose.
We appreciate the query by Drs. Douglas and Wilson. We hereby supply additional information that is critical for creating and administering sustainable staffing models.
For programs with a census cap, the majority cited 16 or fewer patients as the trigger for that cap. Nearly all programs with back-up used a census of 16 or fewer. Over 80% of programs cited a “safe 7
Regarding clinical weighting of nights, nighttime shifts were often more heavily weighted than day shifts, but approaches to weighting varied and have not been validated. Alternate staffing models for overnight pager calls varied greatly by individual program.
This is a time of significant growth for pediatric hospital medicine, and national workforce data are essential to hospitalists, administrators, and most importantly, patients. Our study1 provides pediatric hospital medicine leaders with data for discussions regarding appropriate FTE and staffing model considerations. The insights generated by our study are particularly relevant in expanding programs and solving problems related to recruitment and retention.
Disclosures
The authors have nothing to disclose.
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. PubMed
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. PubMed
© 2019 Society of Hospital Medicine
Practical Application of Pediatric Hospital Medicine Workforce Data. In Reference to: “Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey”
As leaders of a new Pediatric Hospital Medicine program in New York City, we were pleased to read the Brief Report from Dr. Fromme and colleagues, “Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey.”
Although the study has greatly assisted us in developing our program, the manuscript lacked some data necessary for workforce planning. The authors report census caps for a majority of programs, but neither the actual number of patients in each cap nor whether programs with caps reported an association with patient safety or program sustainability. In addition, although overnight pager calls were calculated in median hours, there were no data on whether nights were weighted or alternate staffing models were used for overnight pager calls.
While the article will help guide our field’s continued understanding of our workforce, without additional detailed data, we found that we were unable to apply staffing models practically in the real world to our new program. Pediatric Hospital Medicine is one of the fastest growing fields in medicine; however, support of new programs and sustainability of existing ones, require benchmark details to create proposals that are acceptable to
Disclosures
Drs. Douglas and Wilson have nothing to disclose.
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey. J Hosp Med. 2018. 13:702-705. PubMed
As leaders of a new Pediatric Hospital Medicine program in New York City, we were pleased to read the Brief Report from Dr. Fromme and colleagues, “Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey.”
Although the study has greatly assisted us in developing our program, the manuscript lacked some data necessary for workforce planning. The authors report census caps for a majority of programs, but neither the actual number of patients in each cap nor whether programs with caps reported an association with patient safety or program sustainability. In addition, although overnight pager calls were calculated in median hours, there were no data on whether nights were weighted or alternate staffing models were used for overnight pager calls.
While the article will help guide our field’s continued understanding of our workforce, without additional detailed data, we found that we were unable to apply staffing models practically in the real world to our new program. Pediatric Hospital Medicine is one of the fastest growing fields in medicine; however, support of new programs and sustainability of existing ones, require benchmark details to create proposals that are acceptable to
Disclosures
Drs. Douglas and Wilson have nothing to disclose.
As leaders of a new Pediatric Hospital Medicine program in New York City, we were pleased to read the Brief Report from Dr. Fromme and colleagues, “Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey.”
Although the study has greatly assisted us in developing our program, the manuscript lacked some data necessary for workforce planning. The authors report census caps for a majority of programs, but neither the actual number of patients in each cap nor whether programs with caps reported an association with patient safety or program sustainability. In addition, although overnight pager calls were calculated in median hours, there were no data on whether nights were weighted or alternate staffing models were used for overnight pager calls.
While the article will help guide our field’s continued understanding of our workforce, without additional detailed data, we found that we were unable to apply staffing models practically in the real world to our new program. Pediatric Hospital Medicine is one of the fastest growing fields in medicine; however, support of new programs and sustainability of existing ones, require benchmark details to create proposals that are acceptable to
Disclosures
Drs. Douglas and Wilson have nothing to disclose.
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey. J Hosp Med. 2018. 13:702-705. PubMed
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey. J Hosp Med. 2018. 13:702-705. PubMed
© 2019 Society of Hospital Medicine DOI 10.12788/jhm.3149
Frequently Hospitalized Patients’ Perceptions of Factors Contributing to High Hospital Use
In recent years, hospitals have made considerable efforts to improve transitions of care, in part due to financial incentives from the Medicare Hospital Readmission Reduction Program (HRRP).1 Initially focusing on three medical conditions, the HRRP has been associated with significant reductions in readmission rates.2 Importantly, a small proportion of patients accounts for a very large proportion of hospital readmissions and hospital use.3,4 Frequently hospitalized patients often have multiple chronic conditions and unique needs which may not be met by conventional approaches to healthcare delivery, including those influenced by the HRRP.4-6 In light of this challenge, some hospitals have developed programs specifically focused on frequently hospitalized patients. A recent systematic review of these programs found relatively few studies of high quality, providing only limited insight in designing interventions to support this population.7 Moreover, no studies appear to have incorporated the patients’ perspectives into the design or adaptation of the model. Members of our research team developed and implemented the Complex High Admission Management Program (CHAMP) in January 2016 to address the needs of frequently hospitalized patients in our hospital. To enhance CHAMP and inform the design of programs serving similar populations in other health systems, we sought to identify factors associated with the onset and continuation of high hospital use. Our research question was, from the patients’ perspective, what factors contribute to patients’ becoming and continuing to be high users of hospital care.
METHODS
Setting, Study Design, and Participants
This qualitative study took place at Northwestern Memorial Hospital (NMH), an 894-bed urban academic hospital located in Chicago, Illinois. Between December 2016 and September 2017, we recruited adult patients admitted to the general medicine services. Eligible participants were identified with the assistance of a daily Northwestern Medicine Electronic Data Warehouse (EDW) search and included patients with two unplanned 30-day inpatient readmissions to NMH within the prior 12 months, in addition to one or more of the following criteria: (1) at least one readmission in the last six months; (2) a referral from one of the patient’s medical providers; or (3) at least three observation visits. We excluded patients whose preferred language was not English and those disoriented to person, place, or time. Considering NMH data showing that approximately one-third of high-utilizer patients have sickle cell disease, we used purposive sampling with the goal to compare findings within and between two groups of participants; those with and those without sickle cell disease. Our study was deemed exempt by the Northwestern University Institutional Review Board.
Participant Enrollment and Data Collection
We created an interview guide based on the research team’s experience with this population, a literature review, and our research question (See Appendix).8,9 A research coordinator approached eligible participants during their hospital stay. The coordinator explained the study to eligible participants and obtained verbal consent for participation. The research coordinator then conducted one-on-one semi-structured interviews. Interviews were audio recorded for subsequent transcription and coding. Each interview lasted approximately 45 minutes. Participants were compensated with a $20 gift card for their time.
Analysis
Digital audio recordings from interviews were transcribed verbatim, deidentified, and analyzed using an iterative inductive team-based approach to coding.10 In our first cycle coding, all coders (KJO, SF, MMC, LO, KAC) independently reviewed and coded three transcripts using descriptive coding and subcoding to generate a preliminary codebook with code definitions.10,11 Following the meetings to compare and compile our initial coding, each researcher then independently recoded the three transcripts with the developed codebook. The researchers met again to triangulate perspectives and reach a consensus on the final codebook. Using multiple coders is a standard process to control for subjective bias that one coder could bring to the coding process.12 Following this meeting, the coders split into two teams of two (KJO, SF, and MMC, LO) to complete the coding of the remaining transcripts. Each team member independently coded the assigned transcripts and reconciled their codes with their counterpart; any discrepancies were resolved through discussion. Using this strategy, every transcript was coded by at least two team members. Our second coding cycle utilized pattern coding and involved identifying consistency both within and between transcripts; discovering associations between codes.10,11,13 Constant comparison was used to compare responses among all participants, as well as between sickle-cell and nonsickle-cell participants.13,14 Following team coding and reconciling, the analyses were presented to a broader research team for additional feedback and critique. All analyses were conducted using Dedoose version 8.0.35 (Los Angeles, California). Participant recruitment, interviews, and analysis of the transcripts continued until no new codes emerged and thematic saturation was achieved.
RESULTS
Participant Characteristics
Overall, we invited 34 patients to be interviewed; 26 consented and completed interviews (76.5%). Six (17.6%) patients declined participation, one (2.9%) was unable to complete the interview before hospital discharge, and one (2.9%) was excluded due to disorientation. Demographic characteristics of the 26 participants are shown in Table 1.
Four main themes emerged from our analysis. Table 2 summarizes these themes, subthemes, and provides representative quotes.
Major Medical Problem(s) are Universal, but High Hospital Use Varies in Onset
Not surprisingly, all participants described having at least one major medical problem. Some participants, such as those with genetic disorders, had experienced periods of high hospital use throughout their entire lifetime, while other participants experienced an onset of high hospital use as an adult after being previously healthy. Though most participants with genetic disorders had sickle cell anemia; one had a rare genetic disorder which caused chronic gastrointestinal symptoms. Participants typically described having a significant medical condition as well as other medical problems or complications from past surgery. Some participants described having a major medical problem which did not require frequent hospitalization until a complication or other medical problem arose, suggesting these new issues pushed them over a threshold beyond which self-management at home was less successful.
Course Fluctuates over Time and is Related to Psychological, Social, and Economic Factors
Participants identified psychological stress, social support, and financial constraints as factors which influence the course of their illness over time. Deaths in the family, breakups, and concerns about other family members were mentioned as specific forms of psychological stress and directly linked by participants to worsening of symptoms. Social support was present for most, but not all, participants, with no appreciable difference based on whether the participant had sickle cell disease. Social support was generally perceived as helpful, and several participants indicated a benefit to their own health when providing social support to others. Financial pressures also served as stressors and often impeded care due to lack of access to medications, other treatments, and housing.
Onset and Progression of Episodes Vary, but Generally Seem Uncontrollable
Regarding the onset of illness episodes, some participants described the sudden, unpredictable onset of symptoms, others described a more gradual onset which allowed them to attempt self-management. Regardless of the timing, episodes of illness were often perceived as spontaneous or triggered by factors outside of the participant’s control. Several participants, especially those with sickle cell disease, mentioned a relationship between their symptoms and the weather. Participants also noted the inconsistency in factors which may trigger an episode (ie, sometimes the factor exacerbated symptoms, while other times it did not). Participants also described having a high symptom burden with significant limitations in activities of daily living during episodes of illness. Pain was a very common component of symptoms regardless of whether or not the participant had sickle cell disease.
Individuals Seek Care after Self-Management Fails and Prefer to Avoid Hospitalization
Participants tried to control their symptoms with medications and typically sought care only when it was clear that this approach was not working, or they ran out of medications. This finding was consistent across both groups of participants (ie, those with and those without sickle cell disease). Many participants described very strong preferences not to come to the hospital; no participant described being in the hospital as a favorable or positive experience. Some participants mentioned that they had spent major holidays in the hospital and that they missed their family. No participant had a desire to come to the hospital.
DISCUSSION
In this study of frequently hospitalized patients, we found four major themes that illuminate patient perspectives about factors that contribute to high hospital use. While some of our findings corroborate those of previous studies, other emerging patterns were novel. Herein, we summarize key findings, provide context, and describe implications for the design of models of care for frequently hospitalized patients.
Similar to the findings of previous quantitative research, participants in our study described having a significant medical condition and typically had multiple medical conditions or complications.4-6 Importantly, some participants described having a major medical problem which did not require frequent hospitalization until another medical problem or complication arose. This finding suggests that there may be an opportunity to identify patients with significant medical problems who are at elevated risk before the onset of high hospital use. Early identification of these high-risk patients could allow for the provision of additional support to prevent potential complications or address other factors which may contribute to the need for frequent hospitalization.
Participants in our study directly linked psychological stress to fluctuations in their course of illness. Previous research by Mautner and colleagues queried participants about childhood experiences and early life stressors and reported that early life instabilities and traumas were prevalent among patients with high levels of emergency and hospital-based healthcare utilization.15 Our participants identified more recent traumatic events (eg, the death of a loved one and breakups) when reflecting on factors contributing to illness exacerbations; early life trauma did not emerge as an identified contributor. Of note, unlike Mautner et al., we did not ask participants to reflect on childhood determinants of disease and illness specifically. Our findings suggest that psychological stress contributes to illness exacerbation, even for those patients without other significant psychiatric conditions (eg, depressive disorder, schizophrenia). Incorporating mental health professionals into programs for this patient population may improve health by teaching specific coping strategies, including cognitive-behavioral therapy for an acute stress disorder.16,17
Social support was also a factor related to illness fluctuations over time. Notably, several participants indicated a benefit to their own health when providing social support to others, suggesting a role for peer support that may be reciprocally beneficial. This approach is supported by the literature. Williams and colleagues found that patients with sickle cell anemia experienced symptom improvement with peer support;18 while Johnson and colleagues recently reported a reduction in readmissions to acute care with the use of peer support for patients with severe mental illness.19
Financial constraints impeded care for some patients and served as a barrier to accessing medications, other treatments, and housing. Similar to the findings of prior quantitative research, our frequently hospitalized patients had a high proportion of patients with Medicaid and low proportion with private insurance, suggesting low socioeconomic status.9,20 We did not formally collect data on income or economic status. Interestingly, prior qualitative studies have not identified financial constraints as a major theme, though this may be explained by differences in study populations and the overall objectives of the studies.15,21 Importantly, the overwhelming majority of programs for frequently hospitalized patients identified in a recent systematic review included social workers.7 Our findings support the need to address financial constraints and the use of social workers in models of care for frequently hospitalized patients.
Many participants in our study felt that the factors contributing to exacerbations of illness were either inconsistent in their effect or out of their control. These findings have similarities to those from a qualitative study by Liu and colleagues in which they interviewed 20 “hospital-dependent” patients over 65 years of age.21 Though not explicitly focused on factors contributing to exacerbations, participants in their study felt that hospitalizations were generally inevitable. In our study, participants with sickle cell disease often identified changes in the weather as contributing to illness exacerbations. The relationship between weather and sickle cell disease remains incompletely understood, with an inconsistent association found in prior studies.22
Participants in our study strongly desired to avoid hospitalization and typically sought hospital care when symptoms could not be controlled at home. This finding is in contrast to that from the study by Liu and colleagues where they found that hospital-dependent patients over 65 years had favorable perspectives of hospitalization because they felt safer and more secure in the hospital.21 Our participants were younger than those from the study by Liu and colleagues, had a high symptom burden, and may have been more concerned about control of those symptoms than the risk for clinical deterioration. Programs should aim to strengthen their support of patients’ self-management efforts early in the episode of illness and potentially offer home visits or a day hospital to avoid hospitalization. A recent systematic review found evidence that alternatives to inpatient care (eg, hospital-at-home) for low risk medical patients can achieve comparable outcomes at lower costs.23 Similarly, some health systems have implemented day hospitals to treat low risk patients with uncomplicated sickle cell pain.24,25
The heavy symptom burden experienced by participants in our study is notable. Pain was especially common. Programs may wish to partner with palliative care and addiction specialists to balance symptom relief with the simultaneous need to address comorbid substance and opioid use disorders when they are present.4,9
Our study has several limitations. First, participants were recruited from the medicine service at a single academic hospital using criteria we developed to identify frequently hospitalized patients. Populations differ across hospitals and definitions of frequently hospitalized patients vary, limiting the generalizability of our findings. Second, we excluded patients whose preferred language was not English, as well as those disoriented to person, place, or time. It is possible that factors contributing to high hospital use differ for non-English speaking patients and those with cognitive deficits.
CONCLUSION
In this qualitative study, we identified factors associated with the onset and continuation of high hospital use. Emergent themes pointed to factors which influence patients’ onset of high hospital use, fluctuations in their illness over time, and triggers to seek care during an illness episode. These findings represent an important contribution to the literature because they allow patients’ perspectives to be incorporated into the design and adaptation of programs serving similar populations in other health systems. Programs that integrate patients’ perspectives into their design are likely to be better prepared to address patients’ needs and improve patient outcomes.
Acknowledgments
The authors thank the participants for their time and willingness to share their stories. The authors also thank Claire A. Knoten PhD and Erin Lambers PhD, former research team members who helped in the initial stages of the study.
Disclosures
The authors have nothing to disclose.
Funding
This project was funded by Northwestern Memorial Hospital and the Northwestern Medical Group.
1. Centers for Medicare & Medicaid Services. Readmissions Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed September 17, 2018.
2. Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2016;166(5):324-331. https://doi.org/10.7326/m16-0185.
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients-an urgent priority. N Engl J Med. 2016;375(10):909-911. https://doi.org/10.1056/nejmp1608511.
4. Szekendi MK, Williams MV, Carrier D, Hensley L, Thomas S, Cerese J. The characteristics of patients frequently admitted to academic medical centers in the United States. J Hosp Med. 2015;10(9):563-568. https://doi.org/10.1002/jhm.2375.
5. Dastidar JG, Jiang M. Characterization, categorization, and 5-year mortality of medicine high utilizer inpatients. J Palliat Care. 2018;33(3):167-174. https://doi.org/10.1177/0825859718769095.
6. Mudge AM, Kasper K, Clair A, et al. Recurrent readmissions in medical patients: a prospective study. J Hosp Med. 2010;6(2):61-67. https://doi.org/10.1002/jhm.811.
7. Goodwin A, Henschen BL, Odwyer LC, Nichols N, Oleary KJ. Interventions for frequently hospitalized patients and their effect on outcomes: a systematic review. J Hosp Med. 2018;13(12):853-859. https://doi.org/10.12788/jhm.3090.
8. Gelberg L, Andersen RM, Leake BD. The behavioral model for vulnerable populations: application to medical care use and outcomes for homeless people. Health Serv Res. 2000;34(6):1273-1302. PubMed
9. Rinehart DJ, Oronce C, Durfee MJ, et al. Identifying subgroups of adult superutilizers in an urban safety-net system using latent class analysis. Med Care. 2018;56(1):e1-e9. https://doi.org/10.1097/mlr.0000000000000628.
10. Miles MB, Huberman M, Saldana J. Qualitative Data Analysis. 3rd ed. Thousand Oaks, California: SAGE Publications; 2014.
11. Saldana J. The Coding Manual for Qualitative Researchers. Thousand Oaks, California: SAGE publications; 2013.
12. Lincoln YS, Guba EG. Naturalistic Inquiry. 1 ed. Beverly Hills, California: SAGE Publications; 1985.
13. Kolb SM. Grounded theory and the constant comparative method: valid research strategies for educators. J Emerging Trends Educ Res Policy Stud. 2012;3(1):83-86.
14. Glasser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York: Taylor and Francis Group; 2017.
15. Mautner DB, Pang H, Brenner JC, et al. Generating hypotheses about care needs of high utilizers: lessons from patient interviews. Popul Health Manag. 2013;16(Suppl 1):S26-S33. https://doi.org/10.1089/pop.2013.0033.
16. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depres Anxiety. 2018;35(6):502-514. https://doi.org/10.1002/da.22728.
17. Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Systematic review and meta-analysis of multiple-session early interventions following traumatic events. Am J Psychiatry. 2009;166(3):293-301. https://doi.org/10.1176/appi.ajp.2008.08040590.
18. Williams H, Tanabe P. Sickle cell disease: a review of nonpharmacological approaches for pain. J Pain Symptom Manag. 2016;51(2):163-177. doi: 10.1016/j.jpainsymman.2015.10.017.
19. Johnson S, Lamb D, Marston L, et al. Peer-supported self-management for people discharged from a mental health crisis team: a randomised controlled trial. Lancet. 2018;392(10145):409-418.https://doi.org/10.1016/s0140-6736(18)31470-3.
20. Mercer T, Bae J, Kipnes J, Velazquez M, Thomas S, Setji N. The highest utilizers of care: individualized care plans to coordinate care, improve healthcare service utilization, and reduce costs at an academic tertiary care center. J Hosp Med. 2015;10(7):419-424. https://doi.org/10.1002/jhm.2351.
21. Liu T, Kiwak E, Tinetti ME. Perceptions of hospital-dependent patients on their needs for hospitalization. J Hosp Med. 2017;12(6):450-453. https://doi.org/10.12788/jhm.2756.
22. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. https://doi.org/10.1056/nejmra1510865.
23. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. https://doi.org/10.1001/jamainternmed.2016.5974.
24. Adewoye AH, Nolan V, McMahon L, Ma Q, Steinberg MH. Effectiveness of a dedicated day hospital for management of acute sickle cell pain. Haematologica. 2007;92(6):854-855. https://doi.org/10.3324/haematol.10757.
25. Benjamin LJ, Swinson GI, Nagel RL. Sickle cell anemia day hospital: an approach for the management of uncomplicated painful crises. Blood. 2000;95(4):1130-1136. PubMed
In recent years, hospitals have made considerable efforts to improve transitions of care, in part due to financial incentives from the Medicare Hospital Readmission Reduction Program (HRRP).1 Initially focusing on three medical conditions, the HRRP has been associated with significant reductions in readmission rates.2 Importantly, a small proportion of patients accounts for a very large proportion of hospital readmissions and hospital use.3,4 Frequently hospitalized patients often have multiple chronic conditions and unique needs which may not be met by conventional approaches to healthcare delivery, including those influenced by the HRRP.4-6 In light of this challenge, some hospitals have developed programs specifically focused on frequently hospitalized patients. A recent systematic review of these programs found relatively few studies of high quality, providing only limited insight in designing interventions to support this population.7 Moreover, no studies appear to have incorporated the patients’ perspectives into the design or adaptation of the model. Members of our research team developed and implemented the Complex High Admission Management Program (CHAMP) in January 2016 to address the needs of frequently hospitalized patients in our hospital. To enhance CHAMP and inform the design of programs serving similar populations in other health systems, we sought to identify factors associated with the onset and continuation of high hospital use. Our research question was, from the patients’ perspective, what factors contribute to patients’ becoming and continuing to be high users of hospital care.
METHODS
Setting, Study Design, and Participants
This qualitative study took place at Northwestern Memorial Hospital (NMH), an 894-bed urban academic hospital located in Chicago, Illinois. Between December 2016 and September 2017, we recruited adult patients admitted to the general medicine services. Eligible participants were identified with the assistance of a daily Northwestern Medicine Electronic Data Warehouse (EDW) search and included patients with two unplanned 30-day inpatient readmissions to NMH within the prior 12 months, in addition to one or more of the following criteria: (1) at least one readmission in the last six months; (2) a referral from one of the patient’s medical providers; or (3) at least three observation visits. We excluded patients whose preferred language was not English and those disoriented to person, place, or time. Considering NMH data showing that approximately one-third of high-utilizer patients have sickle cell disease, we used purposive sampling with the goal to compare findings within and between two groups of participants; those with and those without sickle cell disease. Our study was deemed exempt by the Northwestern University Institutional Review Board.
Participant Enrollment and Data Collection
We created an interview guide based on the research team’s experience with this population, a literature review, and our research question (See Appendix).8,9 A research coordinator approached eligible participants during their hospital stay. The coordinator explained the study to eligible participants and obtained verbal consent for participation. The research coordinator then conducted one-on-one semi-structured interviews. Interviews were audio recorded for subsequent transcription and coding. Each interview lasted approximately 45 minutes. Participants were compensated with a $20 gift card for their time.
Analysis
Digital audio recordings from interviews were transcribed verbatim, deidentified, and analyzed using an iterative inductive team-based approach to coding.10 In our first cycle coding, all coders (KJO, SF, MMC, LO, KAC) independently reviewed and coded three transcripts using descriptive coding and subcoding to generate a preliminary codebook with code definitions.10,11 Following the meetings to compare and compile our initial coding, each researcher then independently recoded the three transcripts with the developed codebook. The researchers met again to triangulate perspectives and reach a consensus on the final codebook. Using multiple coders is a standard process to control for subjective bias that one coder could bring to the coding process.12 Following this meeting, the coders split into two teams of two (KJO, SF, and MMC, LO) to complete the coding of the remaining transcripts. Each team member independently coded the assigned transcripts and reconciled their codes with their counterpart; any discrepancies were resolved through discussion. Using this strategy, every transcript was coded by at least two team members. Our second coding cycle utilized pattern coding and involved identifying consistency both within and between transcripts; discovering associations between codes.10,11,13 Constant comparison was used to compare responses among all participants, as well as between sickle-cell and nonsickle-cell participants.13,14 Following team coding and reconciling, the analyses were presented to a broader research team for additional feedback and critique. All analyses were conducted using Dedoose version 8.0.35 (Los Angeles, California). Participant recruitment, interviews, and analysis of the transcripts continued until no new codes emerged and thematic saturation was achieved.
RESULTS
Participant Characteristics
Overall, we invited 34 patients to be interviewed; 26 consented and completed interviews (76.5%). Six (17.6%) patients declined participation, one (2.9%) was unable to complete the interview before hospital discharge, and one (2.9%) was excluded due to disorientation. Demographic characteristics of the 26 participants are shown in Table 1.
Four main themes emerged from our analysis. Table 2 summarizes these themes, subthemes, and provides representative quotes.
Major Medical Problem(s) are Universal, but High Hospital Use Varies in Onset
Not surprisingly, all participants described having at least one major medical problem. Some participants, such as those with genetic disorders, had experienced periods of high hospital use throughout their entire lifetime, while other participants experienced an onset of high hospital use as an adult after being previously healthy. Though most participants with genetic disorders had sickle cell anemia; one had a rare genetic disorder which caused chronic gastrointestinal symptoms. Participants typically described having a significant medical condition as well as other medical problems or complications from past surgery. Some participants described having a major medical problem which did not require frequent hospitalization until a complication or other medical problem arose, suggesting these new issues pushed them over a threshold beyond which self-management at home was less successful.
Course Fluctuates over Time and is Related to Psychological, Social, and Economic Factors
Participants identified psychological stress, social support, and financial constraints as factors which influence the course of their illness over time. Deaths in the family, breakups, and concerns about other family members were mentioned as specific forms of psychological stress and directly linked by participants to worsening of symptoms. Social support was present for most, but not all, participants, with no appreciable difference based on whether the participant had sickle cell disease. Social support was generally perceived as helpful, and several participants indicated a benefit to their own health when providing social support to others. Financial pressures also served as stressors and often impeded care due to lack of access to medications, other treatments, and housing.
Onset and Progression of Episodes Vary, but Generally Seem Uncontrollable
Regarding the onset of illness episodes, some participants described the sudden, unpredictable onset of symptoms, others described a more gradual onset which allowed them to attempt self-management. Regardless of the timing, episodes of illness were often perceived as spontaneous or triggered by factors outside of the participant’s control. Several participants, especially those with sickle cell disease, mentioned a relationship between their symptoms and the weather. Participants also noted the inconsistency in factors which may trigger an episode (ie, sometimes the factor exacerbated symptoms, while other times it did not). Participants also described having a high symptom burden with significant limitations in activities of daily living during episodes of illness. Pain was a very common component of symptoms regardless of whether or not the participant had sickle cell disease.
Individuals Seek Care after Self-Management Fails and Prefer to Avoid Hospitalization
Participants tried to control their symptoms with medications and typically sought care only when it was clear that this approach was not working, or they ran out of medications. This finding was consistent across both groups of participants (ie, those with and those without sickle cell disease). Many participants described very strong preferences not to come to the hospital; no participant described being in the hospital as a favorable or positive experience. Some participants mentioned that they had spent major holidays in the hospital and that they missed their family. No participant had a desire to come to the hospital.
DISCUSSION
In this study of frequently hospitalized patients, we found four major themes that illuminate patient perspectives about factors that contribute to high hospital use. While some of our findings corroborate those of previous studies, other emerging patterns were novel. Herein, we summarize key findings, provide context, and describe implications for the design of models of care for frequently hospitalized patients.
Similar to the findings of previous quantitative research, participants in our study described having a significant medical condition and typically had multiple medical conditions or complications.4-6 Importantly, some participants described having a major medical problem which did not require frequent hospitalization until another medical problem or complication arose. This finding suggests that there may be an opportunity to identify patients with significant medical problems who are at elevated risk before the onset of high hospital use. Early identification of these high-risk patients could allow for the provision of additional support to prevent potential complications or address other factors which may contribute to the need for frequent hospitalization.
Participants in our study directly linked psychological stress to fluctuations in their course of illness. Previous research by Mautner and colleagues queried participants about childhood experiences and early life stressors and reported that early life instabilities and traumas were prevalent among patients with high levels of emergency and hospital-based healthcare utilization.15 Our participants identified more recent traumatic events (eg, the death of a loved one and breakups) when reflecting on factors contributing to illness exacerbations; early life trauma did not emerge as an identified contributor. Of note, unlike Mautner et al., we did not ask participants to reflect on childhood determinants of disease and illness specifically. Our findings suggest that psychological stress contributes to illness exacerbation, even for those patients without other significant psychiatric conditions (eg, depressive disorder, schizophrenia). Incorporating mental health professionals into programs for this patient population may improve health by teaching specific coping strategies, including cognitive-behavioral therapy for an acute stress disorder.16,17
Social support was also a factor related to illness fluctuations over time. Notably, several participants indicated a benefit to their own health when providing social support to others, suggesting a role for peer support that may be reciprocally beneficial. This approach is supported by the literature. Williams and colleagues found that patients with sickle cell anemia experienced symptom improvement with peer support;18 while Johnson and colleagues recently reported a reduction in readmissions to acute care with the use of peer support for patients with severe mental illness.19
Financial constraints impeded care for some patients and served as a barrier to accessing medications, other treatments, and housing. Similar to the findings of prior quantitative research, our frequently hospitalized patients had a high proportion of patients with Medicaid and low proportion with private insurance, suggesting low socioeconomic status.9,20 We did not formally collect data on income or economic status. Interestingly, prior qualitative studies have not identified financial constraints as a major theme, though this may be explained by differences in study populations and the overall objectives of the studies.15,21 Importantly, the overwhelming majority of programs for frequently hospitalized patients identified in a recent systematic review included social workers.7 Our findings support the need to address financial constraints and the use of social workers in models of care for frequently hospitalized patients.
Many participants in our study felt that the factors contributing to exacerbations of illness were either inconsistent in their effect or out of their control. These findings have similarities to those from a qualitative study by Liu and colleagues in which they interviewed 20 “hospital-dependent” patients over 65 years of age.21 Though not explicitly focused on factors contributing to exacerbations, participants in their study felt that hospitalizations were generally inevitable. In our study, participants with sickle cell disease often identified changes in the weather as contributing to illness exacerbations. The relationship between weather and sickle cell disease remains incompletely understood, with an inconsistent association found in prior studies.22
Participants in our study strongly desired to avoid hospitalization and typically sought hospital care when symptoms could not be controlled at home. This finding is in contrast to that from the study by Liu and colleagues where they found that hospital-dependent patients over 65 years had favorable perspectives of hospitalization because they felt safer and more secure in the hospital.21 Our participants were younger than those from the study by Liu and colleagues, had a high symptom burden, and may have been more concerned about control of those symptoms than the risk for clinical deterioration. Programs should aim to strengthen their support of patients’ self-management efforts early in the episode of illness and potentially offer home visits or a day hospital to avoid hospitalization. A recent systematic review found evidence that alternatives to inpatient care (eg, hospital-at-home) for low risk medical patients can achieve comparable outcomes at lower costs.23 Similarly, some health systems have implemented day hospitals to treat low risk patients with uncomplicated sickle cell pain.24,25
The heavy symptom burden experienced by participants in our study is notable. Pain was especially common. Programs may wish to partner with palliative care and addiction specialists to balance symptom relief with the simultaneous need to address comorbid substance and opioid use disorders when they are present.4,9
Our study has several limitations. First, participants were recruited from the medicine service at a single academic hospital using criteria we developed to identify frequently hospitalized patients. Populations differ across hospitals and definitions of frequently hospitalized patients vary, limiting the generalizability of our findings. Second, we excluded patients whose preferred language was not English, as well as those disoriented to person, place, or time. It is possible that factors contributing to high hospital use differ for non-English speaking patients and those with cognitive deficits.
CONCLUSION
In this qualitative study, we identified factors associated with the onset and continuation of high hospital use. Emergent themes pointed to factors which influence patients’ onset of high hospital use, fluctuations in their illness over time, and triggers to seek care during an illness episode. These findings represent an important contribution to the literature because they allow patients’ perspectives to be incorporated into the design and adaptation of programs serving similar populations in other health systems. Programs that integrate patients’ perspectives into their design are likely to be better prepared to address patients’ needs and improve patient outcomes.
Acknowledgments
The authors thank the participants for their time and willingness to share their stories. The authors also thank Claire A. Knoten PhD and Erin Lambers PhD, former research team members who helped in the initial stages of the study.
Disclosures
The authors have nothing to disclose.
Funding
This project was funded by Northwestern Memorial Hospital and the Northwestern Medical Group.
In recent years, hospitals have made considerable efforts to improve transitions of care, in part due to financial incentives from the Medicare Hospital Readmission Reduction Program (HRRP).1 Initially focusing on three medical conditions, the HRRP has been associated with significant reductions in readmission rates.2 Importantly, a small proportion of patients accounts for a very large proportion of hospital readmissions and hospital use.3,4 Frequently hospitalized patients often have multiple chronic conditions and unique needs which may not be met by conventional approaches to healthcare delivery, including those influenced by the HRRP.4-6 In light of this challenge, some hospitals have developed programs specifically focused on frequently hospitalized patients. A recent systematic review of these programs found relatively few studies of high quality, providing only limited insight in designing interventions to support this population.7 Moreover, no studies appear to have incorporated the patients’ perspectives into the design or adaptation of the model. Members of our research team developed and implemented the Complex High Admission Management Program (CHAMP) in January 2016 to address the needs of frequently hospitalized patients in our hospital. To enhance CHAMP and inform the design of programs serving similar populations in other health systems, we sought to identify factors associated with the onset and continuation of high hospital use. Our research question was, from the patients’ perspective, what factors contribute to patients’ becoming and continuing to be high users of hospital care.
METHODS
Setting, Study Design, and Participants
This qualitative study took place at Northwestern Memorial Hospital (NMH), an 894-bed urban academic hospital located in Chicago, Illinois. Between December 2016 and September 2017, we recruited adult patients admitted to the general medicine services. Eligible participants were identified with the assistance of a daily Northwestern Medicine Electronic Data Warehouse (EDW) search and included patients with two unplanned 30-day inpatient readmissions to NMH within the prior 12 months, in addition to one or more of the following criteria: (1) at least one readmission in the last six months; (2) a referral from one of the patient’s medical providers; or (3) at least three observation visits. We excluded patients whose preferred language was not English and those disoriented to person, place, or time. Considering NMH data showing that approximately one-third of high-utilizer patients have sickle cell disease, we used purposive sampling with the goal to compare findings within and between two groups of participants; those with and those without sickle cell disease. Our study was deemed exempt by the Northwestern University Institutional Review Board.
Participant Enrollment and Data Collection
We created an interview guide based on the research team’s experience with this population, a literature review, and our research question (See Appendix).8,9 A research coordinator approached eligible participants during their hospital stay. The coordinator explained the study to eligible participants and obtained verbal consent for participation. The research coordinator then conducted one-on-one semi-structured interviews. Interviews were audio recorded for subsequent transcription and coding. Each interview lasted approximately 45 minutes. Participants were compensated with a $20 gift card for their time.
Analysis
Digital audio recordings from interviews were transcribed verbatim, deidentified, and analyzed using an iterative inductive team-based approach to coding.10 In our first cycle coding, all coders (KJO, SF, MMC, LO, KAC) independently reviewed and coded three transcripts using descriptive coding and subcoding to generate a preliminary codebook with code definitions.10,11 Following the meetings to compare and compile our initial coding, each researcher then independently recoded the three transcripts with the developed codebook. The researchers met again to triangulate perspectives and reach a consensus on the final codebook. Using multiple coders is a standard process to control for subjective bias that one coder could bring to the coding process.12 Following this meeting, the coders split into two teams of two (KJO, SF, and MMC, LO) to complete the coding of the remaining transcripts. Each team member independently coded the assigned transcripts and reconciled their codes with their counterpart; any discrepancies were resolved through discussion. Using this strategy, every transcript was coded by at least two team members. Our second coding cycle utilized pattern coding and involved identifying consistency both within and between transcripts; discovering associations between codes.10,11,13 Constant comparison was used to compare responses among all participants, as well as between sickle-cell and nonsickle-cell participants.13,14 Following team coding and reconciling, the analyses were presented to a broader research team for additional feedback and critique. All analyses were conducted using Dedoose version 8.0.35 (Los Angeles, California). Participant recruitment, interviews, and analysis of the transcripts continued until no new codes emerged and thematic saturation was achieved.
RESULTS
Participant Characteristics
Overall, we invited 34 patients to be interviewed; 26 consented and completed interviews (76.5%). Six (17.6%) patients declined participation, one (2.9%) was unable to complete the interview before hospital discharge, and one (2.9%) was excluded due to disorientation. Demographic characteristics of the 26 participants are shown in Table 1.
Four main themes emerged from our analysis. Table 2 summarizes these themes, subthemes, and provides representative quotes.
Major Medical Problem(s) are Universal, but High Hospital Use Varies in Onset
Not surprisingly, all participants described having at least one major medical problem. Some participants, such as those with genetic disorders, had experienced periods of high hospital use throughout their entire lifetime, while other participants experienced an onset of high hospital use as an adult after being previously healthy. Though most participants with genetic disorders had sickle cell anemia; one had a rare genetic disorder which caused chronic gastrointestinal symptoms. Participants typically described having a significant medical condition as well as other medical problems or complications from past surgery. Some participants described having a major medical problem which did not require frequent hospitalization until a complication or other medical problem arose, suggesting these new issues pushed them over a threshold beyond which self-management at home was less successful.
Course Fluctuates over Time and is Related to Psychological, Social, and Economic Factors
Participants identified psychological stress, social support, and financial constraints as factors which influence the course of their illness over time. Deaths in the family, breakups, and concerns about other family members were mentioned as specific forms of psychological stress and directly linked by participants to worsening of symptoms. Social support was present for most, but not all, participants, with no appreciable difference based on whether the participant had sickle cell disease. Social support was generally perceived as helpful, and several participants indicated a benefit to their own health when providing social support to others. Financial pressures also served as stressors and often impeded care due to lack of access to medications, other treatments, and housing.
Onset and Progression of Episodes Vary, but Generally Seem Uncontrollable
Regarding the onset of illness episodes, some participants described the sudden, unpredictable onset of symptoms, others described a more gradual onset which allowed them to attempt self-management. Regardless of the timing, episodes of illness were often perceived as spontaneous or triggered by factors outside of the participant’s control. Several participants, especially those with sickle cell disease, mentioned a relationship between their symptoms and the weather. Participants also noted the inconsistency in factors which may trigger an episode (ie, sometimes the factor exacerbated symptoms, while other times it did not). Participants also described having a high symptom burden with significant limitations in activities of daily living during episodes of illness. Pain was a very common component of symptoms regardless of whether or not the participant had sickle cell disease.
Individuals Seek Care after Self-Management Fails and Prefer to Avoid Hospitalization
Participants tried to control their symptoms with medications and typically sought care only when it was clear that this approach was not working, or they ran out of medications. This finding was consistent across both groups of participants (ie, those with and those without sickle cell disease). Many participants described very strong preferences not to come to the hospital; no participant described being in the hospital as a favorable or positive experience. Some participants mentioned that they had spent major holidays in the hospital and that they missed their family. No participant had a desire to come to the hospital.
DISCUSSION
In this study of frequently hospitalized patients, we found four major themes that illuminate patient perspectives about factors that contribute to high hospital use. While some of our findings corroborate those of previous studies, other emerging patterns were novel. Herein, we summarize key findings, provide context, and describe implications for the design of models of care for frequently hospitalized patients.
Similar to the findings of previous quantitative research, participants in our study described having a significant medical condition and typically had multiple medical conditions or complications.4-6 Importantly, some participants described having a major medical problem which did not require frequent hospitalization until another medical problem or complication arose. This finding suggests that there may be an opportunity to identify patients with significant medical problems who are at elevated risk before the onset of high hospital use. Early identification of these high-risk patients could allow for the provision of additional support to prevent potential complications or address other factors which may contribute to the need for frequent hospitalization.
Participants in our study directly linked psychological stress to fluctuations in their course of illness. Previous research by Mautner and colleagues queried participants about childhood experiences and early life stressors and reported that early life instabilities and traumas were prevalent among patients with high levels of emergency and hospital-based healthcare utilization.15 Our participants identified more recent traumatic events (eg, the death of a loved one and breakups) when reflecting on factors contributing to illness exacerbations; early life trauma did not emerge as an identified contributor. Of note, unlike Mautner et al., we did not ask participants to reflect on childhood determinants of disease and illness specifically. Our findings suggest that psychological stress contributes to illness exacerbation, even for those patients without other significant psychiatric conditions (eg, depressive disorder, schizophrenia). Incorporating mental health professionals into programs for this patient population may improve health by teaching specific coping strategies, including cognitive-behavioral therapy for an acute stress disorder.16,17
Social support was also a factor related to illness fluctuations over time. Notably, several participants indicated a benefit to their own health when providing social support to others, suggesting a role for peer support that may be reciprocally beneficial. This approach is supported by the literature. Williams and colleagues found that patients with sickle cell anemia experienced symptom improvement with peer support;18 while Johnson and colleagues recently reported a reduction in readmissions to acute care with the use of peer support for patients with severe mental illness.19
Financial constraints impeded care for some patients and served as a barrier to accessing medications, other treatments, and housing. Similar to the findings of prior quantitative research, our frequently hospitalized patients had a high proportion of patients with Medicaid and low proportion with private insurance, suggesting low socioeconomic status.9,20 We did not formally collect data on income or economic status. Interestingly, prior qualitative studies have not identified financial constraints as a major theme, though this may be explained by differences in study populations and the overall objectives of the studies.15,21 Importantly, the overwhelming majority of programs for frequently hospitalized patients identified in a recent systematic review included social workers.7 Our findings support the need to address financial constraints and the use of social workers in models of care for frequently hospitalized patients.
Many participants in our study felt that the factors contributing to exacerbations of illness were either inconsistent in their effect or out of their control. These findings have similarities to those from a qualitative study by Liu and colleagues in which they interviewed 20 “hospital-dependent” patients over 65 years of age.21 Though not explicitly focused on factors contributing to exacerbations, participants in their study felt that hospitalizations were generally inevitable. In our study, participants with sickle cell disease often identified changes in the weather as contributing to illness exacerbations. The relationship between weather and sickle cell disease remains incompletely understood, with an inconsistent association found in prior studies.22
Participants in our study strongly desired to avoid hospitalization and typically sought hospital care when symptoms could not be controlled at home. This finding is in contrast to that from the study by Liu and colleagues where they found that hospital-dependent patients over 65 years had favorable perspectives of hospitalization because they felt safer and more secure in the hospital.21 Our participants were younger than those from the study by Liu and colleagues, had a high symptom burden, and may have been more concerned about control of those symptoms than the risk for clinical deterioration. Programs should aim to strengthen their support of patients’ self-management efforts early in the episode of illness and potentially offer home visits or a day hospital to avoid hospitalization. A recent systematic review found evidence that alternatives to inpatient care (eg, hospital-at-home) for low risk medical patients can achieve comparable outcomes at lower costs.23 Similarly, some health systems have implemented day hospitals to treat low risk patients with uncomplicated sickle cell pain.24,25
The heavy symptom burden experienced by participants in our study is notable. Pain was especially common. Programs may wish to partner with palliative care and addiction specialists to balance symptom relief with the simultaneous need to address comorbid substance and opioid use disorders when they are present.4,9
Our study has several limitations. First, participants were recruited from the medicine service at a single academic hospital using criteria we developed to identify frequently hospitalized patients. Populations differ across hospitals and definitions of frequently hospitalized patients vary, limiting the generalizability of our findings. Second, we excluded patients whose preferred language was not English, as well as those disoriented to person, place, or time. It is possible that factors contributing to high hospital use differ for non-English speaking patients and those with cognitive deficits.
CONCLUSION
In this qualitative study, we identified factors associated with the onset and continuation of high hospital use. Emergent themes pointed to factors which influence patients’ onset of high hospital use, fluctuations in their illness over time, and triggers to seek care during an illness episode. These findings represent an important contribution to the literature because they allow patients’ perspectives to be incorporated into the design and adaptation of programs serving similar populations in other health systems. Programs that integrate patients’ perspectives into their design are likely to be better prepared to address patients’ needs and improve patient outcomes.
Acknowledgments
The authors thank the participants for their time and willingness to share their stories. The authors also thank Claire A. Knoten PhD and Erin Lambers PhD, former research team members who helped in the initial stages of the study.
Disclosures
The authors have nothing to disclose.
Funding
This project was funded by Northwestern Memorial Hospital and the Northwestern Medical Group.
1. Centers for Medicare & Medicaid Services. Readmissions Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed September 17, 2018.
2. Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2016;166(5):324-331. https://doi.org/10.7326/m16-0185.
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients-an urgent priority. N Engl J Med. 2016;375(10):909-911. https://doi.org/10.1056/nejmp1608511.
4. Szekendi MK, Williams MV, Carrier D, Hensley L, Thomas S, Cerese J. The characteristics of patients frequently admitted to academic medical centers in the United States. J Hosp Med. 2015;10(9):563-568. https://doi.org/10.1002/jhm.2375.
5. Dastidar JG, Jiang M. Characterization, categorization, and 5-year mortality of medicine high utilizer inpatients. J Palliat Care. 2018;33(3):167-174. https://doi.org/10.1177/0825859718769095.
6. Mudge AM, Kasper K, Clair A, et al. Recurrent readmissions in medical patients: a prospective study. J Hosp Med. 2010;6(2):61-67. https://doi.org/10.1002/jhm.811.
7. Goodwin A, Henschen BL, Odwyer LC, Nichols N, Oleary KJ. Interventions for frequently hospitalized patients and their effect on outcomes: a systematic review. J Hosp Med. 2018;13(12):853-859. https://doi.org/10.12788/jhm.3090.
8. Gelberg L, Andersen RM, Leake BD. The behavioral model for vulnerable populations: application to medical care use and outcomes for homeless people. Health Serv Res. 2000;34(6):1273-1302. PubMed
9. Rinehart DJ, Oronce C, Durfee MJ, et al. Identifying subgroups of adult superutilizers in an urban safety-net system using latent class analysis. Med Care. 2018;56(1):e1-e9. https://doi.org/10.1097/mlr.0000000000000628.
10. Miles MB, Huberman M, Saldana J. Qualitative Data Analysis. 3rd ed. Thousand Oaks, California: SAGE Publications; 2014.
11. Saldana J. The Coding Manual for Qualitative Researchers. Thousand Oaks, California: SAGE publications; 2013.
12. Lincoln YS, Guba EG. Naturalistic Inquiry. 1 ed. Beverly Hills, California: SAGE Publications; 1985.
13. Kolb SM. Grounded theory and the constant comparative method: valid research strategies for educators. J Emerging Trends Educ Res Policy Stud. 2012;3(1):83-86.
14. Glasser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York: Taylor and Francis Group; 2017.
15. Mautner DB, Pang H, Brenner JC, et al. Generating hypotheses about care needs of high utilizers: lessons from patient interviews. Popul Health Manag. 2013;16(Suppl 1):S26-S33. https://doi.org/10.1089/pop.2013.0033.
16. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depres Anxiety. 2018;35(6):502-514. https://doi.org/10.1002/da.22728.
17. Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Systematic review and meta-analysis of multiple-session early interventions following traumatic events. Am J Psychiatry. 2009;166(3):293-301. https://doi.org/10.1176/appi.ajp.2008.08040590.
18. Williams H, Tanabe P. Sickle cell disease: a review of nonpharmacological approaches for pain. J Pain Symptom Manag. 2016;51(2):163-177. doi: 10.1016/j.jpainsymman.2015.10.017.
19. Johnson S, Lamb D, Marston L, et al. Peer-supported self-management for people discharged from a mental health crisis team: a randomised controlled trial. Lancet. 2018;392(10145):409-418.https://doi.org/10.1016/s0140-6736(18)31470-3.
20. Mercer T, Bae J, Kipnes J, Velazquez M, Thomas S, Setji N. The highest utilizers of care: individualized care plans to coordinate care, improve healthcare service utilization, and reduce costs at an academic tertiary care center. J Hosp Med. 2015;10(7):419-424. https://doi.org/10.1002/jhm.2351.
21. Liu T, Kiwak E, Tinetti ME. Perceptions of hospital-dependent patients on their needs for hospitalization. J Hosp Med. 2017;12(6):450-453. https://doi.org/10.12788/jhm.2756.
22. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. https://doi.org/10.1056/nejmra1510865.
23. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. https://doi.org/10.1001/jamainternmed.2016.5974.
24. Adewoye AH, Nolan V, McMahon L, Ma Q, Steinberg MH. Effectiveness of a dedicated day hospital for management of acute sickle cell pain. Haematologica. 2007;92(6):854-855. https://doi.org/10.3324/haematol.10757.
25. Benjamin LJ, Swinson GI, Nagel RL. Sickle cell anemia day hospital: an approach for the management of uncomplicated painful crises. Blood. 2000;95(4):1130-1136. PubMed
1. Centers for Medicare & Medicaid Services. Readmissions Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed September 17, 2018.
2. Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2016;166(5):324-331. https://doi.org/10.7326/m16-0185.
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients-an urgent priority. N Engl J Med. 2016;375(10):909-911. https://doi.org/10.1056/nejmp1608511.
4. Szekendi MK, Williams MV, Carrier D, Hensley L, Thomas S, Cerese J. The characteristics of patients frequently admitted to academic medical centers in the United States. J Hosp Med. 2015;10(9):563-568. https://doi.org/10.1002/jhm.2375.
5. Dastidar JG, Jiang M. Characterization, categorization, and 5-year mortality of medicine high utilizer inpatients. J Palliat Care. 2018;33(3):167-174. https://doi.org/10.1177/0825859718769095.
6. Mudge AM, Kasper K, Clair A, et al. Recurrent readmissions in medical patients: a prospective study. J Hosp Med. 2010;6(2):61-67. https://doi.org/10.1002/jhm.811.
7. Goodwin A, Henschen BL, Odwyer LC, Nichols N, Oleary KJ. Interventions for frequently hospitalized patients and their effect on outcomes: a systematic review. J Hosp Med. 2018;13(12):853-859. https://doi.org/10.12788/jhm.3090.
8. Gelberg L, Andersen RM, Leake BD. The behavioral model for vulnerable populations: application to medical care use and outcomes for homeless people. Health Serv Res. 2000;34(6):1273-1302. PubMed
9. Rinehart DJ, Oronce C, Durfee MJ, et al. Identifying subgroups of adult superutilizers in an urban safety-net system using latent class analysis. Med Care. 2018;56(1):e1-e9. https://doi.org/10.1097/mlr.0000000000000628.
10. Miles MB, Huberman M, Saldana J. Qualitative Data Analysis. 3rd ed. Thousand Oaks, California: SAGE Publications; 2014.
11. Saldana J. The Coding Manual for Qualitative Researchers. Thousand Oaks, California: SAGE publications; 2013.
12. Lincoln YS, Guba EG. Naturalistic Inquiry. 1 ed. Beverly Hills, California: SAGE Publications; 1985.
13. Kolb SM. Grounded theory and the constant comparative method: valid research strategies for educators. J Emerging Trends Educ Res Policy Stud. 2012;3(1):83-86.
14. Glasser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York: Taylor and Francis Group; 2017.
15. Mautner DB, Pang H, Brenner JC, et al. Generating hypotheses about care needs of high utilizers: lessons from patient interviews. Popul Health Manag. 2013;16(Suppl 1):S26-S33. https://doi.org/10.1089/pop.2013.0033.
16. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depres Anxiety. 2018;35(6):502-514. https://doi.org/10.1002/da.22728.
17. Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Systematic review and meta-analysis of multiple-session early interventions following traumatic events. Am J Psychiatry. 2009;166(3):293-301. https://doi.org/10.1176/appi.ajp.2008.08040590.
18. Williams H, Tanabe P. Sickle cell disease: a review of nonpharmacological approaches for pain. J Pain Symptom Manag. 2016;51(2):163-177. doi: 10.1016/j.jpainsymman.2015.10.017.
19. Johnson S, Lamb D, Marston L, et al. Peer-supported self-management for people discharged from a mental health crisis team: a randomised controlled trial. Lancet. 2018;392(10145):409-418.https://doi.org/10.1016/s0140-6736(18)31470-3.
20. Mercer T, Bae J, Kipnes J, Velazquez M, Thomas S, Setji N. The highest utilizers of care: individualized care plans to coordinate care, improve healthcare service utilization, and reduce costs at an academic tertiary care center. J Hosp Med. 2015;10(7):419-424. https://doi.org/10.1002/jhm.2351.
21. Liu T, Kiwak E, Tinetti ME. Perceptions of hospital-dependent patients on their needs for hospitalization. J Hosp Med. 2017;12(6):450-453. https://doi.org/10.12788/jhm.2756.
22. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. https://doi.org/10.1056/nejmra1510865.
23. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. https://doi.org/10.1001/jamainternmed.2016.5974.
24. Adewoye AH, Nolan V, McMahon L, Ma Q, Steinberg MH. Effectiveness of a dedicated day hospital for management of acute sickle cell pain. Haematologica. 2007;92(6):854-855. https://doi.org/10.3324/haematol.10757.
25. Benjamin LJ, Swinson GI, Nagel RL. Sickle cell anemia day hospital: an approach for the management of uncomplicated painful crises. Blood. 2000;95(4):1130-1136. PubMed
© 2019 Society of Hospital Medicine
Frequency of Ethical Issues on a Hospitalist Teaching Service at an Urban, Tertiary Care Center
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
© 2019 Society of Hospital Medicine
Do Hospitals Participating in Accountable Care Organizations Discharge Patients to Higher Quality Nursing Homes?
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
© 2019 Society of Hospital Medicine