User login
Pneumatic Tube-Induced Reverse Pseudohyperkalemia in a Patient With Chronic Lymphocytic Leukemia
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
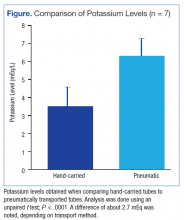
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.
Potential therapeutic target for leukemia, other cancers

Photo by Thomas Semkow
Preclinical research indicates that a member of the Mediator protein complex plays a key role in hematopoiesis.
Investigators found that MED12 was required for the survival of hematopoietic stem and progenitor cells (HSPCs).
The team said this finding, along with the fact that MED12 mutations have been linked to leukemia and solid tumor malignancies, suggests that targeting MED12 hyperactivity might be a useful strategy for treating cancers.
“Because MED12 appears to be so essential to hematopoiesis, our study points to it as a possible target for future anticancer therapies for both chronic and acute forms of leukemia,” said Iannis Aifantis, PhD, of NYU Langone Medical Center in New York.
“Our study also suggests that MED12 hyperactivation or loss of control is a possible explanation for what factors may trigger these cancers and other solid tumors.”
Dr Aifantis and his colleagues described their study in Cell Stem Cell.
The investigators first analyzed the effects of MED12 deletion in mice. Mice bred to lack MED12 died within 2 weeks of birth and showed evidence of aberrant hematopoiesis—namely, a “severe reduction of bone marrow and thymus cellularity.”
Adult mice that were engineered to lose expression of MED12 after the injection of an activating molecule experienced a “rapid” reduction in bone marrow cellularity, as well as reductions in spleen and thymus size. These mice also had low white blood cell and platelet counts and died within 3 weeks of MED12 deletion.
Subsequent analyses of the animals’ bone marrow showed that estimates of HSPCs in each mouse fell from nearly 150,000 to 15,000 within 4 days of injection. Within 10 days, there were no HSPCs left.
Deleting MED12 was also lethal for human HSPCs. Colonies of CD34+ cells dropped from an average of 25 per plate to 5 per plate within 10 days of MED12 deletion.
On the other hand, MED12 did not affect the survival of other cell types. For example, MED12 deletion did not impact mouse embryonic fibroblasts, embryonic stem cells, or hair follicle stem cells.
In addition, deleting members of the Mediator kinase module besides MED12—MED13, CDK8, or CYCLIN C—did not have a significant effect on HSPCs and did not kill mice. The investigators said this provides further evidence that MED12—by loss of its function alone—is essential for hematopoiesis.
The team found that MED12 deletion destabilizes P300 binding at lineage-specific enhancers, which results in H3K27Ac depletion, enhancer de-activation, and the consequent loss of hematopoietic stem cell gene expression signatures.
As a next step, the investigators plan to screen blood samples from cancer patients for signs of MED12 mutations and uncontrolled HSPC development.
The team also hopes to determine the biological mechanisms involved in MED12 hyperactivation and identify drug molecules that could block MED12 hyperactivity and serve as potential MED12 inhibitors. ![]()

Photo by Thomas Semkow
Preclinical research indicates that a member of the Mediator protein complex plays a key role in hematopoiesis.
Investigators found that MED12 was required for the survival of hematopoietic stem and progenitor cells (HSPCs).
The team said this finding, along with the fact that MED12 mutations have been linked to leukemia and solid tumor malignancies, suggests that targeting MED12 hyperactivity might be a useful strategy for treating cancers.
“Because MED12 appears to be so essential to hematopoiesis, our study points to it as a possible target for future anticancer therapies for both chronic and acute forms of leukemia,” said Iannis Aifantis, PhD, of NYU Langone Medical Center in New York.
“Our study also suggests that MED12 hyperactivation or loss of control is a possible explanation for what factors may trigger these cancers and other solid tumors.”
Dr Aifantis and his colleagues described their study in Cell Stem Cell.
The investigators first analyzed the effects of MED12 deletion in mice. Mice bred to lack MED12 died within 2 weeks of birth and showed evidence of aberrant hematopoiesis—namely, a “severe reduction of bone marrow and thymus cellularity.”
Adult mice that were engineered to lose expression of MED12 after the injection of an activating molecule experienced a “rapid” reduction in bone marrow cellularity, as well as reductions in spleen and thymus size. These mice also had low white blood cell and platelet counts and died within 3 weeks of MED12 deletion.
Subsequent analyses of the animals’ bone marrow showed that estimates of HSPCs in each mouse fell from nearly 150,000 to 15,000 within 4 days of injection. Within 10 days, there were no HSPCs left.
Deleting MED12 was also lethal for human HSPCs. Colonies of CD34+ cells dropped from an average of 25 per plate to 5 per plate within 10 days of MED12 deletion.
On the other hand, MED12 did not affect the survival of other cell types. For example, MED12 deletion did not impact mouse embryonic fibroblasts, embryonic stem cells, or hair follicle stem cells.
In addition, deleting members of the Mediator kinase module besides MED12—MED13, CDK8, or CYCLIN C—did not have a significant effect on HSPCs and did not kill mice. The investigators said this provides further evidence that MED12—by loss of its function alone—is essential for hematopoiesis.
The team found that MED12 deletion destabilizes P300 binding at lineage-specific enhancers, which results in H3K27Ac depletion, enhancer de-activation, and the consequent loss of hematopoietic stem cell gene expression signatures.
As a next step, the investigators plan to screen blood samples from cancer patients for signs of MED12 mutations and uncontrolled HSPC development.
The team also hopes to determine the biological mechanisms involved in MED12 hyperactivation and identify drug molecules that could block MED12 hyperactivity and serve as potential MED12 inhibitors. ![]()

Photo by Thomas Semkow
Preclinical research indicates that a member of the Mediator protein complex plays a key role in hematopoiesis.
Investigators found that MED12 was required for the survival of hematopoietic stem and progenitor cells (HSPCs).
The team said this finding, along with the fact that MED12 mutations have been linked to leukemia and solid tumor malignancies, suggests that targeting MED12 hyperactivity might be a useful strategy for treating cancers.
“Because MED12 appears to be so essential to hematopoiesis, our study points to it as a possible target for future anticancer therapies for both chronic and acute forms of leukemia,” said Iannis Aifantis, PhD, of NYU Langone Medical Center in New York.
“Our study also suggests that MED12 hyperactivation or loss of control is a possible explanation for what factors may trigger these cancers and other solid tumors.”
Dr Aifantis and his colleagues described their study in Cell Stem Cell.
The investigators first analyzed the effects of MED12 deletion in mice. Mice bred to lack MED12 died within 2 weeks of birth and showed evidence of aberrant hematopoiesis—namely, a “severe reduction of bone marrow and thymus cellularity.”
Adult mice that were engineered to lose expression of MED12 after the injection of an activating molecule experienced a “rapid” reduction in bone marrow cellularity, as well as reductions in spleen and thymus size. These mice also had low white blood cell and platelet counts and died within 3 weeks of MED12 deletion.
Subsequent analyses of the animals’ bone marrow showed that estimates of HSPCs in each mouse fell from nearly 150,000 to 15,000 within 4 days of injection. Within 10 days, there were no HSPCs left.
Deleting MED12 was also lethal for human HSPCs. Colonies of CD34+ cells dropped from an average of 25 per plate to 5 per plate within 10 days of MED12 deletion.
On the other hand, MED12 did not affect the survival of other cell types. For example, MED12 deletion did not impact mouse embryonic fibroblasts, embryonic stem cells, or hair follicle stem cells.
In addition, deleting members of the Mediator kinase module besides MED12—MED13, CDK8, or CYCLIN C—did not have a significant effect on HSPCs and did not kill mice. The investigators said this provides further evidence that MED12—by loss of its function alone—is essential for hematopoiesis.
The team found that MED12 deletion destabilizes P300 binding at lineage-specific enhancers, which results in H3K27Ac depletion, enhancer de-activation, and the consequent loss of hematopoietic stem cell gene expression signatures.
As a next step, the investigators plan to screen blood samples from cancer patients for signs of MED12 mutations and uncontrolled HSPC development.
The team also hopes to determine the biological mechanisms involved in MED12 hyperactivation and identify drug molecules that could block MED12 hyperactivity and serve as potential MED12 inhibitors. ![]()
NICE approves bosutinib for routine NHS use
The National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending that bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML), be made available through the National Health Service (NHS).
This means patients will no longer have to apply to the Cancer Drugs Fund (CDF) to obtain bosutinib.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
NICE previously considered making bosutinib available through the NHS in 2013 but decided the drug was not cost-effective. So bosutinib was made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib. ![]()

The National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending that bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML), be made available through the National Health Service (NHS).
This means patients will no longer have to apply to the Cancer Drugs Fund (CDF) to obtain bosutinib.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
NICE previously considered making bosutinib available through the NHS in 2013 but decided the drug was not cost-effective. So bosutinib was made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib. ![]()

The National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending that bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML), be made available through the National Health Service (NHS).
This means patients will no longer have to apply to the Cancer Drugs Fund (CDF) to obtain bosutinib.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
NICE previously considered making bosutinib available through the NHS in 2013 but decided the drug was not cost-effective. So bosutinib was made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib. ![]()
FDA grants drug orphan designation for CLL

The US Food and Drug Administration (FDA) has granted orphan drug designation to the PI3K delta inhibitor TGR-1202 for the treatment of chronic lymphocytic leukemia (CLL).
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases.
This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved.
About TGR-1202
TG Therapeutics, Inc. is developing TGR-1202 as a treatment for hematologic malignancies.
The drug is currently being evaluated in the phase 3 UNITY-CLL trial (NCT02612311), which includes patients with previously treated and untreated CLL. Patients are receiving TGR-1202 plus ublituximab, obinutuzumab plus chlorambucil, ublituximab alone, or TGR-1202 alone.
At EHA 2016, researchers reported preliminary results of a phase 1/1b study (NCT02268851) of TGR-1202 in combination with ibrutinib in patients with relapsed/refractory CLL or mantle cell lymphoma.
At ASCO 2016, researchers reported long-term follow-up of 2 studies of TGR-1202.
The first (TGR-1202-101, NCT01767766) is a phase 1 study of TGR-1202 in patients with relapsed or refractory hematologic malignancies.
The second (UTX-TGR-103, NCT02006485) is a phase 1/1b trial evaluating the combination of ublituximab and TGR-1202 in patients with relapsed or refractory non-Hodgkin lymphoma or CLL.
At ASH 2015, researchers reported results of a phase 1 trial (TGR-GA-106, NCT02100852) of TGR-1202 in combination with obinutuzumab and chlorambucil in patients with CLL. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation to the PI3K delta inhibitor TGR-1202 for the treatment of chronic lymphocytic leukemia (CLL).
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases.
This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved.
About TGR-1202
TG Therapeutics, Inc. is developing TGR-1202 as a treatment for hematologic malignancies.
The drug is currently being evaluated in the phase 3 UNITY-CLL trial (NCT02612311), which includes patients with previously treated and untreated CLL. Patients are receiving TGR-1202 plus ublituximab, obinutuzumab plus chlorambucil, ublituximab alone, or TGR-1202 alone.
At EHA 2016, researchers reported preliminary results of a phase 1/1b study (NCT02268851) of TGR-1202 in combination with ibrutinib in patients with relapsed/refractory CLL or mantle cell lymphoma.
At ASCO 2016, researchers reported long-term follow-up of 2 studies of TGR-1202.
The first (TGR-1202-101, NCT01767766) is a phase 1 study of TGR-1202 in patients with relapsed or refractory hematologic malignancies.
The second (UTX-TGR-103, NCT02006485) is a phase 1/1b trial evaluating the combination of ublituximab and TGR-1202 in patients with relapsed or refractory non-Hodgkin lymphoma or CLL.
At ASH 2015, researchers reported results of a phase 1 trial (TGR-GA-106, NCT02100852) of TGR-1202 in combination with obinutuzumab and chlorambucil in patients with CLL. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation to the PI3K delta inhibitor TGR-1202 for the treatment of chronic lymphocytic leukemia (CLL).
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases.
This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved.
About TGR-1202
TG Therapeutics, Inc. is developing TGR-1202 as a treatment for hematologic malignancies.
The drug is currently being evaluated in the phase 3 UNITY-CLL trial (NCT02612311), which includes patients with previously treated and untreated CLL. Patients are receiving TGR-1202 plus ublituximab, obinutuzumab plus chlorambucil, ublituximab alone, or TGR-1202 alone.
At EHA 2016, researchers reported preliminary results of a phase 1/1b study (NCT02268851) of TGR-1202 in combination with ibrutinib in patients with relapsed/refractory CLL or mantle cell lymphoma.
At ASCO 2016, researchers reported long-term follow-up of 2 studies of TGR-1202.
The first (TGR-1202-101, NCT01767766) is a phase 1 study of TGR-1202 in patients with relapsed or refractory hematologic malignancies.
The second (UTX-TGR-103, NCT02006485) is a phase 1/1b trial evaluating the combination of ublituximab and TGR-1202 in patients with relapsed or refractory non-Hodgkin lymphoma or CLL.
At ASH 2015, researchers reported results of a phase 1 trial (TGR-GA-106, NCT02100852) of TGR-1202 in combination with obinutuzumab and chlorambucil in patients with CLL. ![]()
Massage therapy seems to benefit cancer patients

Photo courtesy of Barbara
E. Carver/New York College
of Health Professions
Massage therapy can reduce pain, fatigue, and anxiety in cancer patients, according to a review and meta-analysis published in Pain Medicine.
Massage therapy is commonly used among people seeking pain management, and research has generally supported its use.
However, there has been no published, rigorous review of the available research and evidence for massage therapy’s efficacy for pain populations, especially for cancer populations.
So Courtney Boyd, of the Samueli Institute in Alexandria, Virginia, and her colleagues set out to conduct just such a review.
The team assessed the quality of massage therapy research and evidence for its efficacy in treating pain, function-related quality of life, and health-related quality of life in cancer patients.
The researchers reviewed data from 12 high-quality studies and 4 low-quality studies.
The team said they could not assess health-related quality of life, emotional stress, or activity outcomes because too few of the studies examined these outcomes.
However, the data suggested that massage therapy can effectively reduce pain intensity or severity when compared to no treatment (standardized mean difference [SMD]=-0.20) or active comparators (SMD=-0.55).
Massage therapy also proved effective for reducing fatigue (SMD=-1.06) and anxiety (SMD=-1.24) when compared to active comparators.
Based on these results, the researchers concluded that massage therapy may have beneficial effects in cancer patients, so they should consider it as an option.
However, before definitive clinical conclusions and recommendations can be made at a policy level, specific factors surrounding the massage protocol, as well as selection of appropriate controls and standard outcomes, need to be well-understood. ![]()

Photo courtesy of Barbara
E. Carver/New York College
of Health Professions
Massage therapy can reduce pain, fatigue, and anxiety in cancer patients, according to a review and meta-analysis published in Pain Medicine.
Massage therapy is commonly used among people seeking pain management, and research has generally supported its use.
However, there has been no published, rigorous review of the available research and evidence for massage therapy’s efficacy for pain populations, especially for cancer populations.
So Courtney Boyd, of the Samueli Institute in Alexandria, Virginia, and her colleagues set out to conduct just such a review.
The team assessed the quality of massage therapy research and evidence for its efficacy in treating pain, function-related quality of life, and health-related quality of life in cancer patients.
The researchers reviewed data from 12 high-quality studies and 4 low-quality studies.
The team said they could not assess health-related quality of life, emotional stress, or activity outcomes because too few of the studies examined these outcomes.
However, the data suggested that massage therapy can effectively reduce pain intensity or severity when compared to no treatment (standardized mean difference [SMD]=-0.20) or active comparators (SMD=-0.55).
Massage therapy also proved effective for reducing fatigue (SMD=-1.06) and anxiety (SMD=-1.24) when compared to active comparators.
Based on these results, the researchers concluded that massage therapy may have beneficial effects in cancer patients, so they should consider it as an option.
However, before definitive clinical conclusions and recommendations can be made at a policy level, specific factors surrounding the massage protocol, as well as selection of appropriate controls and standard outcomes, need to be well-understood. ![]()

Photo courtesy of Barbara
E. Carver/New York College
of Health Professions
Massage therapy can reduce pain, fatigue, and anxiety in cancer patients, according to a review and meta-analysis published in Pain Medicine.
Massage therapy is commonly used among people seeking pain management, and research has generally supported its use.
However, there has been no published, rigorous review of the available research and evidence for massage therapy’s efficacy for pain populations, especially for cancer populations.
So Courtney Boyd, of the Samueli Institute in Alexandria, Virginia, and her colleagues set out to conduct just such a review.
The team assessed the quality of massage therapy research and evidence for its efficacy in treating pain, function-related quality of life, and health-related quality of life in cancer patients.
The researchers reviewed data from 12 high-quality studies and 4 low-quality studies.
The team said they could not assess health-related quality of life, emotional stress, or activity outcomes because too few of the studies examined these outcomes.
However, the data suggested that massage therapy can effectively reduce pain intensity or severity when compared to no treatment (standardized mean difference [SMD]=-0.20) or active comparators (SMD=-0.55).
Massage therapy also proved effective for reducing fatigue (SMD=-1.06) and anxiety (SMD=-1.24) when compared to active comparators.
Based on these results, the researchers concluded that massage therapy may have beneficial effects in cancer patients, so they should consider it as an option.
However, before definitive clinical conclusions and recommendations can be made at a policy level, specific factors surrounding the massage protocol, as well as selection of appropriate controls and standard outcomes, need to be well-understood. ![]()
Drug granted breakthrough designation for BPDCN

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for SL-401 in the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies.
SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
The drug is under development by Stemline Therapeutics, Inc.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 2 trial of SL-401
The breakthrough designation for SL-401 was supported by data from a phase 2 trial of patients with BPDCN or acute myeloid leukemia. Results observed in the BPDCN patients were presented at the 2016 EHA Congress (abstract S812).
The study consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
At the data cut-off, 18 BPDCN patients had received SL-401 at 7 μg/kg (n=3, stage 1) or 12 μg/kg (n=15, 6 in stage 1, 9 in stage 2).
Fifteen of these patients were evaluable for response. The overall response rate was 87% (13/15). All 10 previously untreated BPDCN patients achieved a response, including 7 complete responses (CRs).
All 8 previously untreated BPDCN patients treated at the optimal dose (12 μg/kg) achieved a response, including 6 CRs. Four of these patients were still on SL-401 and in CR at the data cutoff, and 2 had gone on to stem cell transplant.
The most common treatment-related adverse events in the BPDCN patients were transient transaminase elevation (57%), hypoalbuminemia (40%), and transient thrombocytopenia (15%).
In stage 1, two patients had capillary leak syndrome (CLS)—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS. There have been no cases of severe CLS since then. ![]()

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for SL-401 in the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies.
SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
The drug is under development by Stemline Therapeutics, Inc.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 2 trial of SL-401
The breakthrough designation for SL-401 was supported by data from a phase 2 trial of patients with BPDCN or acute myeloid leukemia. Results observed in the BPDCN patients were presented at the 2016 EHA Congress (abstract S812).
The study consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
At the data cut-off, 18 BPDCN patients had received SL-401 at 7 μg/kg (n=3, stage 1) or 12 μg/kg (n=15, 6 in stage 1, 9 in stage 2).
Fifteen of these patients were evaluable for response. The overall response rate was 87% (13/15). All 10 previously untreated BPDCN patients achieved a response, including 7 complete responses (CRs).
All 8 previously untreated BPDCN patients treated at the optimal dose (12 μg/kg) achieved a response, including 6 CRs. Four of these patients were still on SL-401 and in CR at the data cutoff, and 2 had gone on to stem cell transplant.
The most common treatment-related adverse events in the BPDCN patients were transient transaminase elevation (57%), hypoalbuminemia (40%), and transient thrombocytopenia (15%).
In stage 1, two patients had capillary leak syndrome (CLS)—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS. There have been no cases of severe CLS since then. ![]()

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for SL-401 in the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies.
SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
The drug is under development by Stemline Therapeutics, Inc.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 2 trial of SL-401
The breakthrough designation for SL-401 was supported by data from a phase 2 trial of patients with BPDCN or acute myeloid leukemia. Results observed in the BPDCN patients were presented at the 2016 EHA Congress (abstract S812).
The study consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
At the data cut-off, 18 BPDCN patients had received SL-401 at 7 μg/kg (n=3, stage 1) or 12 μg/kg (n=15, 6 in stage 1, 9 in stage 2).
Fifteen of these patients were evaluable for response. The overall response rate was 87% (13/15). All 10 previously untreated BPDCN patients achieved a response, including 7 complete responses (CRs).
All 8 previously untreated BPDCN patients treated at the optimal dose (12 μg/kg) achieved a response, including 6 CRs. Four of these patients were still on SL-401 and in CR at the data cutoff, and 2 had gone on to stem cell transplant.
The most common treatment-related adverse events in the BPDCN patients were transient transaminase elevation (57%), hypoalbuminemia (40%), and transient thrombocytopenia (15%).
In stage 1, two patients had capillary leak syndrome (CLS)—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS. There have been no cases of severe CLS since then. ![]()
Pretransplantation mogamulizumab for ATLL raises risk of GVHD
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma was associated with GVHD and increased mortality.
Major finding: Grade 3-4 acute GVHD developed in 17.2% of patients who didn’t receive mogamulizumab, compared with 30.9% who did, for a relative risk of 1.80.
Data source: A retrospective cohort study involving 996 patients with adult T-cell leukemia/lymphoma in Japan.
Disclosures: This study was supported in part by Practical Research for Innovative Cancer Control and the Japan Agency for Medical Research and Development. Dr. Fuji and one associate reported receiving honoraria from Kyowa Hakko Kirin; another associate reported ties to numerous industry sources.
Predicting outcomes in relapsed BCP-ALL

Image by Vashi Donsk
Screening for genetic abnormalities can provide a more accurate prediction of outcomes in children with relapsed B-cell precursor acute lymphoblastic leukemia (BCP-ALL), according to a study published in Blood.
Researchers found that mutations or deletions in TP53, NR3C1, BTG1, and NRAS were associated with inferior outcomes in relapsed BCP-ALL.
And screening for these abnormalities could improve upon the predictive accuracy of clinical risk factors.
“Current methods used to guide treatment for relapsed leukemia are not accurate enough, with some children believed to have a good chance of survival actually responding very poorly to chemotherapy,” said study author Anthony Moorman, PhD, of Newcastle University in Newcastle upon Tyne, UK.
“Screening patients at relapse for key genetic abnormalities that influence outcome will ensure that treatment can be personalized, thereby improving their chances of survival.”
For this study, Dr Moorman and his colleagues analyzed cytogenetic data from 427 children with relapsed BCP-ALL and screened 238 patients with a marrow relapse for certain copy number alterations and mutations.
According to univariate analysis, alterations in TP53, NR3C1 deletions, and BTG1 deletions were significantly associated with patient outcomes.
Patients with TP53 alterations had a higher risk of progression (hazard ratio [HR]=2.36, P<0.001) and death (HR=2.56, P<0.001), as did patients with deletions in NR3C1 and BTG1.
Because both NR3C1 and BTG1 are implicated in resistance to glucocorticoids and the deletions are mutually exclusive, the researchers considered the effect of the deletions together. So for patients with NR3C1 and BTG1 deletions, the HR for progression was 2.15 (P=0.002), and the HR for death was 1.91 (P=0.015).
Patients with NRAS mutations had an increased risk of progression and death as well, but this did not reach statistical significance.
The researchers also found that patients who were standard risk according to clinical characteristics but, at the time of relapse, had one or more high-risk genetic abnormalities had poorer outcomes.
Standard-risk patients with a TP53 alteration had an increased risk of death (HR=2.56, P<0.001), as did standard-risk patients with NR3C1 and BTG1 deletions (HR=1.91, P=0.015).
Standard-risk patients with NRAS mutations and high hyperdiploidy had an increased risk of progression (HR=3.17, P=0.026) and death (HR=3.41, P=0.032).
The researchers concluded that the outcomes of clinical standard-risk patients with high-risk cytogenetics were equivalent to outcomes of clinical high-risk patients.
The team therefore believes that screening BCP-ALL patients for the aforementioned genetic abnormalities at relapse will improve patient stratification and outcomes. ![]()

Image by Vashi Donsk
Screening for genetic abnormalities can provide a more accurate prediction of outcomes in children with relapsed B-cell precursor acute lymphoblastic leukemia (BCP-ALL), according to a study published in Blood.
Researchers found that mutations or deletions in TP53, NR3C1, BTG1, and NRAS were associated with inferior outcomes in relapsed BCP-ALL.
And screening for these abnormalities could improve upon the predictive accuracy of clinical risk factors.
“Current methods used to guide treatment for relapsed leukemia are not accurate enough, with some children believed to have a good chance of survival actually responding very poorly to chemotherapy,” said study author Anthony Moorman, PhD, of Newcastle University in Newcastle upon Tyne, UK.
“Screening patients at relapse for key genetic abnormalities that influence outcome will ensure that treatment can be personalized, thereby improving their chances of survival.”
For this study, Dr Moorman and his colleagues analyzed cytogenetic data from 427 children with relapsed BCP-ALL and screened 238 patients with a marrow relapse for certain copy number alterations and mutations.
According to univariate analysis, alterations in TP53, NR3C1 deletions, and BTG1 deletions were significantly associated with patient outcomes.
Patients with TP53 alterations had a higher risk of progression (hazard ratio [HR]=2.36, P<0.001) and death (HR=2.56, P<0.001), as did patients with deletions in NR3C1 and BTG1.
Because both NR3C1 and BTG1 are implicated in resistance to glucocorticoids and the deletions are mutually exclusive, the researchers considered the effect of the deletions together. So for patients with NR3C1 and BTG1 deletions, the HR for progression was 2.15 (P=0.002), and the HR for death was 1.91 (P=0.015).
Patients with NRAS mutations had an increased risk of progression and death as well, but this did not reach statistical significance.
The researchers also found that patients who were standard risk according to clinical characteristics but, at the time of relapse, had one or more high-risk genetic abnormalities had poorer outcomes.
Standard-risk patients with a TP53 alteration had an increased risk of death (HR=2.56, P<0.001), as did standard-risk patients with NR3C1 and BTG1 deletions (HR=1.91, P=0.015).
Standard-risk patients with NRAS mutations and high hyperdiploidy had an increased risk of progression (HR=3.17, P=0.026) and death (HR=3.41, P=0.032).
The researchers concluded that the outcomes of clinical standard-risk patients with high-risk cytogenetics were equivalent to outcomes of clinical high-risk patients.
The team therefore believes that screening BCP-ALL patients for the aforementioned genetic abnormalities at relapse will improve patient stratification and outcomes. ![]()

Image by Vashi Donsk
Screening for genetic abnormalities can provide a more accurate prediction of outcomes in children with relapsed B-cell precursor acute lymphoblastic leukemia (BCP-ALL), according to a study published in Blood.
Researchers found that mutations or deletions in TP53, NR3C1, BTG1, and NRAS were associated with inferior outcomes in relapsed BCP-ALL.
And screening for these abnormalities could improve upon the predictive accuracy of clinical risk factors.
“Current methods used to guide treatment for relapsed leukemia are not accurate enough, with some children believed to have a good chance of survival actually responding very poorly to chemotherapy,” said study author Anthony Moorman, PhD, of Newcastle University in Newcastle upon Tyne, UK.
“Screening patients at relapse for key genetic abnormalities that influence outcome will ensure that treatment can be personalized, thereby improving their chances of survival.”
For this study, Dr Moorman and his colleagues analyzed cytogenetic data from 427 children with relapsed BCP-ALL and screened 238 patients with a marrow relapse for certain copy number alterations and mutations.
According to univariate analysis, alterations in TP53, NR3C1 deletions, and BTG1 deletions were significantly associated with patient outcomes.
Patients with TP53 alterations had a higher risk of progression (hazard ratio [HR]=2.36, P<0.001) and death (HR=2.56, P<0.001), as did patients with deletions in NR3C1 and BTG1.
Because both NR3C1 and BTG1 are implicated in resistance to glucocorticoids and the deletions are mutually exclusive, the researchers considered the effect of the deletions together. So for patients with NR3C1 and BTG1 deletions, the HR for progression was 2.15 (P=0.002), and the HR for death was 1.91 (P=0.015).
Patients with NRAS mutations had an increased risk of progression and death as well, but this did not reach statistical significance.
The researchers also found that patients who were standard risk according to clinical characteristics but, at the time of relapse, had one or more high-risk genetic abnormalities had poorer outcomes.
Standard-risk patients with a TP53 alteration had an increased risk of death (HR=2.56, P<0.001), as did standard-risk patients with NR3C1 and BTG1 deletions (HR=1.91, P=0.015).
Standard-risk patients with NRAS mutations and high hyperdiploidy had an increased risk of progression (HR=3.17, P=0.026) and death (HR=3.41, P=0.032).
The researchers concluded that the outcomes of clinical standard-risk patients with high-risk cytogenetics were equivalent to outcomes of clinical high-risk patients.
The team therefore believes that screening BCP-ALL patients for the aforementioned genetic abnormalities at relapse will improve patient stratification and outcomes. ![]()
Cancer survivors have ‘normal’ sex lives, survey says

receiving treatment
Photo by Rhoda Baer
A new study suggests cancer survivors and the general population have comparable sex lives, although cancer survivors don’t realize it.
According to a survey of more than 6500 people, cancer survivors over the age of 49 have just as much sex and similar levels of sexual function as individuals of the same age who never had cancer.
However, the cancer survivors were more likely to report being dissatisfied with their sex lives.
“We hope our findings will put cancer survivors’ concerns to rest—showing that they are just as sexually active and function just as well as others their age,” said Sarah Jackson, PhD, of University College London in the UK.
“The next stage of our research will look at why cancer patients feel less satisfied with their sex lives.”
Dr Jackson and her colleagues reported their current findings in Cancer.
The researchers set out to explore differences in sexual activity and function, as well as concerns about sex, between cancer survivors and cancer-free controls in a population-based study.
The team surveyed 3708 women (341 cancer survivors and 3367 controls) and 2982 men (220 cancer survivors and 2762 controls) aged 50 and older. Male and female cancer survivors were significantly older than controls (P<0.001 for both) and reported more comorbidities (P=0.003 for both).
Frequency
There were no significant differences in levels of sexual activity between cancer survivors and controls of either sex.
Among women, 58.2% of cancer survivors and 55.5% of controls reported having any sexual activity in the last year. Among men, the rates were 76.0% and 78.5%, respectively.
Overall, about half of the people surveyed reported having “frequent” sexual intercourse, which was defined as 2 to 3 times a month or more.
This included 49.1% of female cancer survivors, 50.1% of female controls, 49% of male cancer survivors, and 48% of male controls.
Function
The incidence of sexual problems was similar in cancer survivors and controls—both male and female.
For example, around a third of the women said they had problems becoming aroused (31.4% of cancer survivors and 31.8% of controls), and about 40% of the men had erectile dysfunction (40.3% of cancer survivors and 39.3% of controls).
Satisfaction
Despite similar levels of sexual activity and function, cancer survivors were more likely than controls to report feeling dissatisfied with their sex lives.
Among the women, 18.2% of cancer survivors and 11.8% of controls reported dissatisfaction (P=0.034). Among the men, the rates were 30.9% and 19.8%, respectively (P=0.023).
In addition, female cancer survivors were more likely to be concerned about their libido than female controls—10.2% and 7.1%, respectively (P=0.006). But there was no significant difference for the men.
Time from cancer diagnosis
The researchers also found the amount of time from cancer diagnosis was a factor affecting sexual function and concern among women but not men.
Females diagnosed with cancer less than 5 years from the time they were surveyed were more likely than female controls to report difficulty becoming aroused (55.4% and 31.8%, respectively, P=0.016) and achieving orgasm (60.6% and 28.3%, respectively, P<0.001).
The recently diagnosed females were also more likely than controls to be concerned about sexual desire (14.8% and 7.1%, respectively, P=0.007) and orgasmic experience (17.6% and 7.1%, respectively, P=0.042).
“Although some cancer treatments are known to impact on sexual function, this study suggests that the majority of cancer patients have similar sexual function and activity as the general population,” said Martin Ledwick, of Cancer Research UK, which sponsored this study.
“However, cancer patients in the study were more likely to be dissatisfied with their sex lives . . . . This highlights the need for health professionals to make sure they talk about sex with all patients—not just the ones whose sexual function is likely to be affected by their cancer or its treatment.” ![]()

receiving treatment
Photo by Rhoda Baer
A new study suggests cancer survivors and the general population have comparable sex lives, although cancer survivors don’t realize it.
According to a survey of more than 6500 people, cancer survivors over the age of 49 have just as much sex and similar levels of sexual function as individuals of the same age who never had cancer.
However, the cancer survivors were more likely to report being dissatisfied with their sex lives.
“We hope our findings will put cancer survivors’ concerns to rest—showing that they are just as sexually active and function just as well as others their age,” said Sarah Jackson, PhD, of University College London in the UK.
“The next stage of our research will look at why cancer patients feel less satisfied with their sex lives.”
Dr Jackson and her colleagues reported their current findings in Cancer.
The researchers set out to explore differences in sexual activity and function, as well as concerns about sex, between cancer survivors and cancer-free controls in a population-based study.
The team surveyed 3708 women (341 cancer survivors and 3367 controls) and 2982 men (220 cancer survivors and 2762 controls) aged 50 and older. Male and female cancer survivors were significantly older than controls (P<0.001 for both) and reported more comorbidities (P=0.003 for both).
Frequency
There were no significant differences in levels of sexual activity between cancer survivors and controls of either sex.
Among women, 58.2% of cancer survivors and 55.5% of controls reported having any sexual activity in the last year. Among men, the rates were 76.0% and 78.5%, respectively.
Overall, about half of the people surveyed reported having “frequent” sexual intercourse, which was defined as 2 to 3 times a month or more.
This included 49.1% of female cancer survivors, 50.1% of female controls, 49% of male cancer survivors, and 48% of male controls.
Function
The incidence of sexual problems was similar in cancer survivors and controls—both male and female.
For example, around a third of the women said they had problems becoming aroused (31.4% of cancer survivors and 31.8% of controls), and about 40% of the men had erectile dysfunction (40.3% of cancer survivors and 39.3% of controls).
Satisfaction
Despite similar levels of sexual activity and function, cancer survivors were more likely than controls to report feeling dissatisfied with their sex lives.
Among the women, 18.2% of cancer survivors and 11.8% of controls reported dissatisfaction (P=0.034). Among the men, the rates were 30.9% and 19.8%, respectively (P=0.023).
In addition, female cancer survivors were more likely to be concerned about their libido than female controls—10.2% and 7.1%, respectively (P=0.006). But there was no significant difference for the men.
Time from cancer diagnosis
The researchers also found the amount of time from cancer diagnosis was a factor affecting sexual function and concern among women but not men.
Females diagnosed with cancer less than 5 years from the time they were surveyed were more likely than female controls to report difficulty becoming aroused (55.4% and 31.8%, respectively, P=0.016) and achieving orgasm (60.6% and 28.3%, respectively, P<0.001).
The recently diagnosed females were also more likely than controls to be concerned about sexual desire (14.8% and 7.1%, respectively, P=0.007) and orgasmic experience (17.6% and 7.1%, respectively, P=0.042).
“Although some cancer treatments are known to impact on sexual function, this study suggests that the majority of cancer patients have similar sexual function and activity as the general population,” said Martin Ledwick, of Cancer Research UK, which sponsored this study.
“However, cancer patients in the study were more likely to be dissatisfied with their sex lives . . . . This highlights the need for health professionals to make sure they talk about sex with all patients—not just the ones whose sexual function is likely to be affected by their cancer or its treatment.” ![]()

receiving treatment
Photo by Rhoda Baer
A new study suggests cancer survivors and the general population have comparable sex lives, although cancer survivors don’t realize it.
According to a survey of more than 6500 people, cancer survivors over the age of 49 have just as much sex and similar levels of sexual function as individuals of the same age who never had cancer.
However, the cancer survivors were more likely to report being dissatisfied with their sex lives.
“We hope our findings will put cancer survivors’ concerns to rest—showing that they are just as sexually active and function just as well as others their age,” said Sarah Jackson, PhD, of University College London in the UK.
“The next stage of our research will look at why cancer patients feel less satisfied with their sex lives.”
Dr Jackson and her colleagues reported their current findings in Cancer.
The researchers set out to explore differences in sexual activity and function, as well as concerns about sex, between cancer survivors and cancer-free controls in a population-based study.
The team surveyed 3708 women (341 cancer survivors and 3367 controls) and 2982 men (220 cancer survivors and 2762 controls) aged 50 and older. Male and female cancer survivors were significantly older than controls (P<0.001 for both) and reported more comorbidities (P=0.003 for both).
Frequency
There were no significant differences in levels of sexual activity between cancer survivors and controls of either sex.
Among women, 58.2% of cancer survivors and 55.5% of controls reported having any sexual activity in the last year. Among men, the rates were 76.0% and 78.5%, respectively.
Overall, about half of the people surveyed reported having “frequent” sexual intercourse, which was defined as 2 to 3 times a month or more.
This included 49.1% of female cancer survivors, 50.1% of female controls, 49% of male cancer survivors, and 48% of male controls.
Function
The incidence of sexual problems was similar in cancer survivors and controls—both male and female.
For example, around a third of the women said they had problems becoming aroused (31.4% of cancer survivors and 31.8% of controls), and about 40% of the men had erectile dysfunction (40.3% of cancer survivors and 39.3% of controls).
Satisfaction
Despite similar levels of sexual activity and function, cancer survivors were more likely than controls to report feeling dissatisfied with their sex lives.
Among the women, 18.2% of cancer survivors and 11.8% of controls reported dissatisfaction (P=0.034). Among the men, the rates were 30.9% and 19.8%, respectively (P=0.023).
In addition, female cancer survivors were more likely to be concerned about their libido than female controls—10.2% and 7.1%, respectively (P=0.006). But there was no significant difference for the men.
Time from cancer diagnosis
The researchers also found the amount of time from cancer diagnosis was a factor affecting sexual function and concern among women but not men.
Females diagnosed with cancer less than 5 years from the time they were surveyed were more likely than female controls to report difficulty becoming aroused (55.4% and 31.8%, respectively, P=0.016) and achieving orgasm (60.6% and 28.3%, respectively, P<0.001).
The recently diagnosed females were also more likely than controls to be concerned about sexual desire (14.8% and 7.1%, respectively, P=0.007) and orgasmic experience (17.6% and 7.1%, respectively, P=0.042).
“Although some cancer treatments are known to impact on sexual function, this study suggests that the majority of cancer patients have similar sexual function and activity as the general population,” said Martin Ledwick, of Cancer Research UK, which sponsored this study.
“However, cancer patients in the study were more likely to be dissatisfied with their sex lives . . . . This highlights the need for health professionals to make sure they talk about sex with all patients—not just the ones whose sexual function is likely to be affected by their cancer or its treatment.”
Music may alleviate cancer patients’ symptoms

Photo by Lars Frantzen
Results of a systematic review suggest music can help alleviate symptoms of anxiety, pain, and fatigue in cancer patients.
The review included more than 50 studies investigating the impact of music therapy—a personalized music experience offered by trained music therapists—and music medicine—listening to pre-recorded music provided by a doctor or nurse—on psychological and physical outcomes in people with cancer.
“We found that music therapy interventions specifically help improve patients’ quality of life,” said study author Joke Bradt, PhD, of Drexel University in Philadelphia, Pennsylvania.
“These are important findings, as these outcomes play an important role in patients’ overall well-being.”
Dr Bradt and her colleagues reported their findings in Cochrane Database of Systematic Reviews.
The researchers examined 52 trials including 3731 cancer patients. The music interventions were classified as music therapy in 23 of the trials and as music medicine in 29 trials.
Analyses suggested that both types of music interventions positively impacted patients. The interventions had a moderate-to-strong effect on anxiety, a strong effect on pain reduction, and a small-to-moderate effect on fatigue.
Small reductions in heart and respiratory rates, as well as lowered blood pressure, were linked to the interventions as well.
In addition, the researchers observed a moderate increase in patients’ quality of life with music therapy but not music medicine.
The team could not determine the effect of music interventions on depression due to the low quality of evidence. And there was no evidence that the interventions improve mood, distress, or physical functioning, but there were few trials investigating these outcomes.
Similarly, the researchers said they could not draw any conclusions about the effect of music interventions on immunologic functioning, coping, resilience, or communication because there were not enough trials evaluating these outcomes.
Still, the researchers hope music interventions will become more widely used, in light of the potential benefits to cancer patients.
“We hope that the findings of this review will encourage healthcare providers in medical settings to seriously consider the use of music therapy in the psychosocial care of people with cancer,” Dr Bradt said.

Photo by Lars Frantzen
Results of a systematic review suggest music can help alleviate symptoms of anxiety, pain, and fatigue in cancer patients.
The review included more than 50 studies investigating the impact of music therapy—a personalized music experience offered by trained music therapists—and music medicine—listening to pre-recorded music provided by a doctor or nurse—on psychological and physical outcomes in people with cancer.
“We found that music therapy interventions specifically help improve patients’ quality of life,” said study author Joke Bradt, PhD, of Drexel University in Philadelphia, Pennsylvania.
“These are important findings, as these outcomes play an important role in patients’ overall well-being.”
Dr Bradt and her colleagues reported their findings in Cochrane Database of Systematic Reviews.
The researchers examined 52 trials including 3731 cancer patients. The music interventions were classified as music therapy in 23 of the trials and as music medicine in 29 trials.
Analyses suggested that both types of music interventions positively impacted patients. The interventions had a moderate-to-strong effect on anxiety, a strong effect on pain reduction, and a small-to-moderate effect on fatigue.
Small reductions in heart and respiratory rates, as well as lowered blood pressure, were linked to the interventions as well.
In addition, the researchers observed a moderate increase in patients’ quality of life with music therapy but not music medicine.
The team could not determine the effect of music interventions on depression due to the low quality of evidence. And there was no evidence that the interventions improve mood, distress, or physical functioning, but there were few trials investigating these outcomes.
Similarly, the researchers said they could not draw any conclusions about the effect of music interventions on immunologic functioning, coping, resilience, or communication because there were not enough trials evaluating these outcomes.
Still, the researchers hope music interventions will become more widely used, in light of the potential benefits to cancer patients.
“We hope that the findings of this review will encourage healthcare providers in medical settings to seriously consider the use of music therapy in the psychosocial care of people with cancer,” Dr Bradt said.

Photo by Lars Frantzen
Results of a systematic review suggest music can help alleviate symptoms of anxiety, pain, and fatigue in cancer patients.
The review included more than 50 studies investigating the impact of music therapy—a personalized music experience offered by trained music therapists—and music medicine—listening to pre-recorded music provided by a doctor or nurse—on psychological and physical outcomes in people with cancer.
“We found that music therapy interventions specifically help improve patients’ quality of life,” said study author Joke Bradt, PhD, of Drexel University in Philadelphia, Pennsylvania.
“These are important findings, as these outcomes play an important role in patients’ overall well-being.”
Dr Bradt and her colleagues reported their findings in Cochrane Database of Systematic Reviews.
The researchers examined 52 trials including 3731 cancer patients. The music interventions were classified as music therapy in 23 of the trials and as music medicine in 29 trials.
Analyses suggested that both types of music interventions positively impacted patients. The interventions had a moderate-to-strong effect on anxiety, a strong effect on pain reduction, and a small-to-moderate effect on fatigue.
Small reductions in heart and respiratory rates, as well as lowered blood pressure, were linked to the interventions as well.
In addition, the researchers observed a moderate increase in patients’ quality of life with music therapy but not music medicine.
The team could not determine the effect of music interventions on depression due to the low quality of evidence. And there was no evidence that the interventions improve mood, distress, or physical functioning, but there were few trials investigating these outcomes.
Similarly, the researchers said they could not draw any conclusions about the effect of music interventions on immunologic functioning, coping, resilience, or communication because there were not enough trials evaluating these outcomes.
Still, the researchers hope music interventions will become more widely used, in light of the potential benefits to cancer patients.
“We hope that the findings of this review will encourage healthcare providers in medical settings to seriously consider the use of music therapy in the psychosocial care of people with cancer,” Dr Bradt said.