User login
Blood sample storage may hinder leukemia research

Credit: Graham Colm
Storing blood samples at room temperature can induce changes that may cloud research findings, according to a group of investigators.
The team initially found that blood samples from leukemia patients had high levels of malformed RNA, a discovery they believed could explain leukemia’s origins.
But additional research showed this abnormality was a result of storing blood samples at room temperature for hours, or even days, prior to processing.
Heidi Dvinge, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in PNAS.
The investigators had searched databases to collect genomic information on leukemia patients and healthy control subjects. In all but one of the datasets they analyzed, the team found high levels of abnormal RNA in leukemia cells.
Another finding was that samples from pediatric leukemia patients had the highest levels of abnormal RNA. And this led the researchers to speculate about the cause.
They realized that pediatric leukemias are rare, so blood samples can be difficult to obtain. Therefore, the samples in the databases were collected at facilities throughout the world and shipped to where they were needed. So the samples could be stored at room temperature for days at a time.
To confirm that this practice can affect blood samples, the investigators conducted an experiment. They collected samples from 4 healthy subjects (2 men and 2 women) and looked for differences between samples that were processed immediately and samples that sat in the lab at room temperature for up to 48 hours.
Sure enough, the team observed changes in the stored samples, even if they were only stored for 4 hours. Stored samples exhibited biased activation of biological pathways and upregulation of pseudogenes, antisense RNAs, and unannotated coding isoforms.
Storage affected a number of genes that play roles in biological pathways relevant to leukemia, including cytokine production, NF-κB signaling, chromatin modification, and RNA splicing. Additionally, storage inhibited RNA surveillance, leading to the genome-wide expression of normally degraded RNAs.
The researchers said these findings coincide with the database findings, as they observed inhibited RNA surveillance in all but one of the datasets they analyzed.
The samples from that dataset were processed immediately after collection. And samples from healthy subjects were processed immediately, which explains why those samples were normal as well.
The team also noted that they did not observe inhibited RNA surveillance in lymphoma or solid tumor datasets.
These results suggest previous research utilizing these types of databases may contain errors, and future work making use of these databases could be affected as well.
Fortunately, the investigators found that putting blood samples on ice can prevent the negative effects they observed. The group also identified biomarkers that indicate prolonged storage, so researchers can look for those biomarkers if chilling blood samples is not an option.
In addition to describing these findings in PNAS, Dr Dvinge and her colleagues are planning to present their work at the upcoming ASH Annual Meeting. ![]()

Credit: Graham Colm
Storing blood samples at room temperature can induce changes that may cloud research findings, according to a group of investigators.
The team initially found that blood samples from leukemia patients had high levels of malformed RNA, a discovery they believed could explain leukemia’s origins.
But additional research showed this abnormality was a result of storing blood samples at room temperature for hours, or even days, prior to processing.
Heidi Dvinge, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in PNAS.
The investigators had searched databases to collect genomic information on leukemia patients and healthy control subjects. In all but one of the datasets they analyzed, the team found high levels of abnormal RNA in leukemia cells.
Another finding was that samples from pediatric leukemia patients had the highest levels of abnormal RNA. And this led the researchers to speculate about the cause.
They realized that pediatric leukemias are rare, so blood samples can be difficult to obtain. Therefore, the samples in the databases were collected at facilities throughout the world and shipped to where they were needed. So the samples could be stored at room temperature for days at a time.
To confirm that this practice can affect blood samples, the investigators conducted an experiment. They collected samples from 4 healthy subjects (2 men and 2 women) and looked for differences between samples that were processed immediately and samples that sat in the lab at room temperature for up to 48 hours.
Sure enough, the team observed changes in the stored samples, even if they were only stored for 4 hours. Stored samples exhibited biased activation of biological pathways and upregulation of pseudogenes, antisense RNAs, and unannotated coding isoforms.
Storage affected a number of genes that play roles in biological pathways relevant to leukemia, including cytokine production, NF-κB signaling, chromatin modification, and RNA splicing. Additionally, storage inhibited RNA surveillance, leading to the genome-wide expression of normally degraded RNAs.
The researchers said these findings coincide with the database findings, as they observed inhibited RNA surveillance in all but one of the datasets they analyzed.
The samples from that dataset were processed immediately after collection. And samples from healthy subjects were processed immediately, which explains why those samples were normal as well.
The team also noted that they did not observe inhibited RNA surveillance in lymphoma or solid tumor datasets.
These results suggest previous research utilizing these types of databases may contain errors, and future work making use of these databases could be affected as well.
Fortunately, the investigators found that putting blood samples on ice can prevent the negative effects they observed. The group also identified biomarkers that indicate prolonged storage, so researchers can look for those biomarkers if chilling blood samples is not an option.
In addition to describing these findings in PNAS, Dr Dvinge and her colleagues are planning to present their work at the upcoming ASH Annual Meeting. ![]()

Credit: Graham Colm
Storing blood samples at room temperature can induce changes that may cloud research findings, according to a group of investigators.
The team initially found that blood samples from leukemia patients had high levels of malformed RNA, a discovery they believed could explain leukemia’s origins.
But additional research showed this abnormality was a result of storing blood samples at room temperature for hours, or even days, prior to processing.
Heidi Dvinge, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in PNAS.
The investigators had searched databases to collect genomic information on leukemia patients and healthy control subjects. In all but one of the datasets they analyzed, the team found high levels of abnormal RNA in leukemia cells.
Another finding was that samples from pediatric leukemia patients had the highest levels of abnormal RNA. And this led the researchers to speculate about the cause.
They realized that pediatric leukemias are rare, so blood samples can be difficult to obtain. Therefore, the samples in the databases were collected at facilities throughout the world and shipped to where they were needed. So the samples could be stored at room temperature for days at a time.
To confirm that this practice can affect blood samples, the investigators conducted an experiment. They collected samples from 4 healthy subjects (2 men and 2 women) and looked for differences between samples that were processed immediately and samples that sat in the lab at room temperature for up to 48 hours.
Sure enough, the team observed changes in the stored samples, even if they were only stored for 4 hours. Stored samples exhibited biased activation of biological pathways and upregulation of pseudogenes, antisense RNAs, and unannotated coding isoforms.
Storage affected a number of genes that play roles in biological pathways relevant to leukemia, including cytokine production, NF-κB signaling, chromatin modification, and RNA splicing. Additionally, storage inhibited RNA surveillance, leading to the genome-wide expression of normally degraded RNAs.
The researchers said these findings coincide with the database findings, as they observed inhibited RNA surveillance in all but one of the datasets they analyzed.
The samples from that dataset were processed immediately after collection. And samples from healthy subjects were processed immediately, which explains why those samples were normal as well.
The team also noted that they did not observe inhibited RNA surveillance in lymphoma or solid tumor datasets.
These results suggest previous research utilizing these types of databases may contain errors, and future work making use of these databases could be affected as well.
Fortunately, the investigators found that putting blood samples on ice can prevent the negative effects they observed. The group also identified biomarkers that indicate prolonged storage, so researchers can look for those biomarkers if chilling blood samples is not an option.
In addition to describing these findings in PNAS, Dr Dvinge and her colleagues are planning to present their work at the upcoming ASH Annual Meeting. ![]()
Hospice cuts cost and use of care for cancer patients

Credit: CDC
Patients with advanced cancer receive less aggressive care and have lower healthcare costs during their last year of life if they use hospice care, according to research published in JAMA.
Patients who entered hospice had significantly lower rates of hospitalization, intensive care unit admissions, and invasive procedures, compared to patients who did not enter hospice.
Furthermore, patients who chose hospice were about 5 times less likely to die in hospitals and nursing homes.
“Our study shows very clearly that hospice matters,” said Ziad Obermeyer, MD, of Brigham and Women’s Hospital in Boston.
“Hospice and non-hospice patients had very similar patterns of healthcare utilization right up until the week of hospice enrollment. Then, the care started to look very different. Patients who didn’t enroll in hospice ended up with far more aggressive care in their last year of life, most of it related to acute complications like infections and organ failure, and not directly related to their cancer diagnosis.”
To conduct this study, Dr Obermeyer and his colleagues used data from Medicare beneficiaries with poor-prognosis cancers, including hematologic malignancies. The study included a nationally representative 20% sample of Medicare fee-for-service beneficiaries who died in 2011.
Among 86,851 patients, 51,924 (60%) entered hospice before death. Matching patients based on various criteria produced hospice and non-hospice groups, each with 18,165 patients. The median hospice duration was 11 days.
The researchers found that non-hospice patients had significantly greater healthcare utilization, largely for acute conditions not directly related to cancer.
This included rates of hospitalization (65% vs 42%), intensive care unit admissions (36% vs 15%), invasive procedures (51% vs 27%), and death in a hospital or nursing facility (74% vs 14%).
The costs of care for hospice and non-hospice patients were not significantly different before hospice care began. But they diverged sharply thereafter, contributing to a significant difference in total costs of $8697 over the last year of life—$71,517 for non-hospice patients and $62,819 for hospice patients.
“These findings highlight the importance of honest discussions between doctors and patients about our patients’ goals of their care at the end of life, relating to treatment decisions and quality of life,” Dr Obermeyer said. “This is of particular importance now, in light of the ongoing policy discussions around reimbursing providers for advance-care planning.” ![]()

Credit: CDC
Patients with advanced cancer receive less aggressive care and have lower healthcare costs during their last year of life if they use hospice care, according to research published in JAMA.
Patients who entered hospice had significantly lower rates of hospitalization, intensive care unit admissions, and invasive procedures, compared to patients who did not enter hospice.
Furthermore, patients who chose hospice were about 5 times less likely to die in hospitals and nursing homes.
“Our study shows very clearly that hospice matters,” said Ziad Obermeyer, MD, of Brigham and Women’s Hospital in Boston.
“Hospice and non-hospice patients had very similar patterns of healthcare utilization right up until the week of hospice enrollment. Then, the care started to look very different. Patients who didn’t enroll in hospice ended up with far more aggressive care in their last year of life, most of it related to acute complications like infections and organ failure, and not directly related to their cancer diagnosis.”
To conduct this study, Dr Obermeyer and his colleagues used data from Medicare beneficiaries with poor-prognosis cancers, including hematologic malignancies. The study included a nationally representative 20% sample of Medicare fee-for-service beneficiaries who died in 2011.
Among 86,851 patients, 51,924 (60%) entered hospice before death. Matching patients based on various criteria produced hospice and non-hospice groups, each with 18,165 patients. The median hospice duration was 11 days.
The researchers found that non-hospice patients had significantly greater healthcare utilization, largely for acute conditions not directly related to cancer.
This included rates of hospitalization (65% vs 42%), intensive care unit admissions (36% vs 15%), invasive procedures (51% vs 27%), and death in a hospital or nursing facility (74% vs 14%).
The costs of care for hospice and non-hospice patients were not significantly different before hospice care began. But they diverged sharply thereafter, contributing to a significant difference in total costs of $8697 over the last year of life—$71,517 for non-hospice patients and $62,819 for hospice patients.
“These findings highlight the importance of honest discussions between doctors and patients about our patients’ goals of their care at the end of life, relating to treatment decisions and quality of life,” Dr Obermeyer said. “This is of particular importance now, in light of the ongoing policy discussions around reimbursing providers for advance-care planning.” ![]()

Credit: CDC
Patients with advanced cancer receive less aggressive care and have lower healthcare costs during their last year of life if they use hospice care, according to research published in JAMA.
Patients who entered hospice had significantly lower rates of hospitalization, intensive care unit admissions, and invasive procedures, compared to patients who did not enter hospice.
Furthermore, patients who chose hospice were about 5 times less likely to die in hospitals and nursing homes.
“Our study shows very clearly that hospice matters,” said Ziad Obermeyer, MD, of Brigham and Women’s Hospital in Boston.
“Hospice and non-hospice patients had very similar patterns of healthcare utilization right up until the week of hospice enrollment. Then, the care started to look very different. Patients who didn’t enroll in hospice ended up with far more aggressive care in their last year of life, most of it related to acute complications like infections and organ failure, and not directly related to their cancer diagnosis.”
To conduct this study, Dr Obermeyer and his colleagues used data from Medicare beneficiaries with poor-prognosis cancers, including hematologic malignancies. The study included a nationally representative 20% sample of Medicare fee-for-service beneficiaries who died in 2011.
Among 86,851 patients, 51,924 (60%) entered hospice before death. Matching patients based on various criteria produced hospice and non-hospice groups, each with 18,165 patients. The median hospice duration was 11 days.
The researchers found that non-hospice patients had significantly greater healthcare utilization, largely for acute conditions not directly related to cancer.
This included rates of hospitalization (65% vs 42%), intensive care unit admissions (36% vs 15%), invasive procedures (51% vs 27%), and death in a hospital or nursing facility (74% vs 14%).
The costs of care for hospice and non-hospice patients were not significantly different before hospice care began. But they diverged sharply thereafter, contributing to a significant difference in total costs of $8697 over the last year of life—$71,517 for non-hospice patients and $62,819 for hospice patients.
“These findings highlight the importance of honest discussions between doctors and patients about our patients’ goals of their care at the end of life, relating to treatment decisions and quality of life,” Dr Obermeyer said. “This is of particular importance now, in light of the ongoing policy discussions around reimbursing providers for advance-care planning.” ![]()
Compounds could treat CML more effectively

Credit: Darren Baker
A handful of newly identified compounds may be able to treat chronic myelogenous leukemia (CML) more effectively than the tyrosine kinase inhibitors (TKIs) currently on the market, new research suggests.
Investigators identified 7 molecules that exhibited the potential to be more potent than approved TKIs.
Experiments suggested that 5 of the compounds—DB07107, DB06977, ST013616, DB04200, and ST007180—were more effective at inhibiting T315I-mutant BCR-ABL than wild-type BCR-ABL.
And 2 of them—ST019342 and DB01172—were effective only against mutant BCR-ABL.
Hemanth Naick Banavath, a PhD student at Pondicherry University-India, and his colleagues detailed these results in Nature: Scientific Reports.
To identify compounds to treat CML, the investigators screened several small molecule databases and docked against wild-type and drug-resistant T315I-mutant BCR-ABL.
The team also docked the TKIs ponatinib, bosutinib, bafetinib, dasatinib, nilotinib, and imatinib.
They identified 7 lead molecules—DB07107, DB06977, ST013616, DB04200, ST007180, ST019342, and DB01172—with better binding affinity and higher binding free energy than the TKIs.
Molecular dynamics simulations showed the dynamic behavior of protein-ligand complexes.
The protein backbone and ligand backbone of DB07107, DB06977, ST013616, DB04200, ST007180, and DB01172 were stable throughout the simulation period.
But the backbone of ST019342 showed anomalous fluctuations, and DB01172 showed instability with wild-type BCR-ABL.
A hydrogen bond analysis revealed that DB01172 has 6 hydrogens on average. However, in wild-type, it has 2 to 3 hydrogens on average. The investigators said this suggests low efficacy toward wild-type BCR-ABL.
Taking these findings together, the team said they cannot recommend ST019342 and DB01172 as potential treatments for CML.
On the other hand, they can endorse DB07107, DB06977, ST013616, DB04200, and ST007180, which showed “remarkable results.” ![]()

Credit: Darren Baker
A handful of newly identified compounds may be able to treat chronic myelogenous leukemia (CML) more effectively than the tyrosine kinase inhibitors (TKIs) currently on the market, new research suggests.
Investigators identified 7 molecules that exhibited the potential to be more potent than approved TKIs.
Experiments suggested that 5 of the compounds—DB07107, DB06977, ST013616, DB04200, and ST007180—were more effective at inhibiting T315I-mutant BCR-ABL than wild-type BCR-ABL.
And 2 of them—ST019342 and DB01172—were effective only against mutant BCR-ABL.
Hemanth Naick Banavath, a PhD student at Pondicherry University-India, and his colleagues detailed these results in Nature: Scientific Reports.
To identify compounds to treat CML, the investigators screened several small molecule databases and docked against wild-type and drug-resistant T315I-mutant BCR-ABL.
The team also docked the TKIs ponatinib, bosutinib, bafetinib, dasatinib, nilotinib, and imatinib.
They identified 7 lead molecules—DB07107, DB06977, ST013616, DB04200, ST007180, ST019342, and DB01172—with better binding affinity and higher binding free energy than the TKIs.
Molecular dynamics simulations showed the dynamic behavior of protein-ligand complexes.
The protein backbone and ligand backbone of DB07107, DB06977, ST013616, DB04200, ST007180, and DB01172 were stable throughout the simulation period.
But the backbone of ST019342 showed anomalous fluctuations, and DB01172 showed instability with wild-type BCR-ABL.
A hydrogen bond analysis revealed that DB01172 has 6 hydrogens on average. However, in wild-type, it has 2 to 3 hydrogens on average. The investigators said this suggests low efficacy toward wild-type BCR-ABL.
Taking these findings together, the team said they cannot recommend ST019342 and DB01172 as potential treatments for CML.
On the other hand, they can endorse DB07107, DB06977, ST013616, DB04200, and ST007180, which showed “remarkable results.” ![]()

Credit: Darren Baker
A handful of newly identified compounds may be able to treat chronic myelogenous leukemia (CML) more effectively than the tyrosine kinase inhibitors (TKIs) currently on the market, new research suggests.
Investigators identified 7 molecules that exhibited the potential to be more potent than approved TKIs.
Experiments suggested that 5 of the compounds—DB07107, DB06977, ST013616, DB04200, and ST007180—were more effective at inhibiting T315I-mutant BCR-ABL than wild-type BCR-ABL.
And 2 of them—ST019342 and DB01172—were effective only against mutant BCR-ABL.
Hemanth Naick Banavath, a PhD student at Pondicherry University-India, and his colleagues detailed these results in Nature: Scientific Reports.
To identify compounds to treat CML, the investigators screened several small molecule databases and docked against wild-type and drug-resistant T315I-mutant BCR-ABL.
The team also docked the TKIs ponatinib, bosutinib, bafetinib, dasatinib, nilotinib, and imatinib.
They identified 7 lead molecules—DB07107, DB06977, ST013616, DB04200, ST007180, ST019342, and DB01172—with better binding affinity and higher binding free energy than the TKIs.
Molecular dynamics simulations showed the dynamic behavior of protein-ligand complexes.
The protein backbone and ligand backbone of DB07107, DB06977, ST013616, DB04200, ST007180, and DB01172 were stable throughout the simulation period.
But the backbone of ST019342 showed anomalous fluctuations, and DB01172 showed instability with wild-type BCR-ABL.
A hydrogen bond analysis revealed that DB01172 has 6 hydrogens on average. However, in wild-type, it has 2 to 3 hydrogens on average. The investigators said this suggests low efficacy toward wild-type BCR-ABL.
Taking these findings together, the team said they cannot recommend ST019342 and DB01172 as potential treatments for CML.
On the other hand, they can endorse DB07107, DB06977, ST013616, DB04200, and ST007180, which showed “remarkable results.” ![]()
Group develops cancer health literacy tool

patient and her father
Credit: Rhoda Baer
A new tool can identify patients with limited cancer health literacy, according to research published in the Journal of Health Communications.
The study authors noted that cancer patients are expected to play an active role in their care by adhering to medication regimens, distinguishing between scientifically credible medical evidence and misconceptions, and communicating with their providers about treatment decisions, risks, and survival rates.
However, patients with limited cancer health literacy can struggle with these responsibilities and potentially jeopardize their health by making uninformed decisions.
So Levent Dumenci, PhD, of Virginia Commonwealth University in Richmond, and his colleagues developed a tool to assess health literacy among cancer patients.
“Before now, there were only instruments that measured a particular aspect of general health literacy,” Dr Dumenci said. “It is important to have a tool that is specific to cancer because of the complex treatment options that cancer patients face, along with the increased demand for self-care.”
To meet that need, he and his colleagues developed the Cancer Health Literacy Test (CHLT)-30. The test, which is given via a touch-screen device, includes 30
questions about cancer treatment, medication side effects, and other cancer-related issues.
Six of the questions (CHLT-6) were specifically designed to quickly identify individuals with limited cancer health literacy.
The researchers tested the CHLT-30 in 1306 African American and Caucasian patients who were treated at oncology clinics in Virginia.
The team said the test accurately measured literacy and quickly identified patients with limited cancer health literacy. Eighteen percent of cancer patients had limited literacy, with an overrepresentation of African American, undereducated, and low-income patients.
“Using this tool, it takes just 1 to 2 minutes in the doctor’s office waiting room to identify patients with limited [cancer health literacy],” Dr Dumenci said. “Then, this information can be digitally communicated to the doctors prior to seeing the patients, so that they are prepared to talk with the patients in terms they can understand. This simple change could lead to big improvements in health outcomes.”
In future studies, the researchers hope to evaluate the cancer health literacy of Hispanic patients because prior research has pointed to disparities in this population’s health literacy. ![]()

patient and her father
Credit: Rhoda Baer
A new tool can identify patients with limited cancer health literacy, according to research published in the Journal of Health Communications.
The study authors noted that cancer patients are expected to play an active role in their care by adhering to medication regimens, distinguishing between scientifically credible medical evidence and misconceptions, and communicating with their providers about treatment decisions, risks, and survival rates.
However, patients with limited cancer health literacy can struggle with these responsibilities and potentially jeopardize their health by making uninformed decisions.
So Levent Dumenci, PhD, of Virginia Commonwealth University in Richmond, and his colleagues developed a tool to assess health literacy among cancer patients.
“Before now, there were only instruments that measured a particular aspect of general health literacy,” Dr Dumenci said. “It is important to have a tool that is specific to cancer because of the complex treatment options that cancer patients face, along with the increased demand for self-care.”
To meet that need, he and his colleagues developed the Cancer Health Literacy Test (CHLT)-30. The test, which is given via a touch-screen device, includes 30
questions about cancer treatment, medication side effects, and other cancer-related issues.
Six of the questions (CHLT-6) were specifically designed to quickly identify individuals with limited cancer health literacy.
The researchers tested the CHLT-30 in 1306 African American and Caucasian patients who were treated at oncology clinics in Virginia.
The team said the test accurately measured literacy and quickly identified patients with limited cancer health literacy. Eighteen percent of cancer patients had limited literacy, with an overrepresentation of African American, undereducated, and low-income patients.
“Using this tool, it takes just 1 to 2 minutes in the doctor’s office waiting room to identify patients with limited [cancer health literacy],” Dr Dumenci said. “Then, this information can be digitally communicated to the doctors prior to seeing the patients, so that they are prepared to talk with the patients in terms they can understand. This simple change could lead to big improvements in health outcomes.”
In future studies, the researchers hope to evaluate the cancer health literacy of Hispanic patients because prior research has pointed to disparities in this population’s health literacy. ![]()

patient and her father
Credit: Rhoda Baer
A new tool can identify patients with limited cancer health literacy, according to research published in the Journal of Health Communications.
The study authors noted that cancer patients are expected to play an active role in their care by adhering to medication regimens, distinguishing between scientifically credible medical evidence and misconceptions, and communicating with their providers about treatment decisions, risks, and survival rates.
However, patients with limited cancer health literacy can struggle with these responsibilities and potentially jeopardize their health by making uninformed decisions.
So Levent Dumenci, PhD, of Virginia Commonwealth University in Richmond, and his colleagues developed a tool to assess health literacy among cancer patients.
“Before now, there were only instruments that measured a particular aspect of general health literacy,” Dr Dumenci said. “It is important to have a tool that is specific to cancer because of the complex treatment options that cancer patients face, along with the increased demand for self-care.”
To meet that need, he and his colleagues developed the Cancer Health Literacy Test (CHLT)-30. The test, which is given via a touch-screen device, includes 30
questions about cancer treatment, medication side effects, and other cancer-related issues.
Six of the questions (CHLT-6) were specifically designed to quickly identify individuals with limited cancer health literacy.
The researchers tested the CHLT-30 in 1306 African American and Caucasian patients who were treated at oncology clinics in Virginia.
The team said the test accurately measured literacy and quickly identified patients with limited cancer health literacy. Eighteen percent of cancer patients had limited literacy, with an overrepresentation of African American, undereducated, and low-income patients.
“Using this tool, it takes just 1 to 2 minutes in the doctor’s office waiting room to identify patients with limited [cancer health literacy],” Dr Dumenci said. “Then, this information can be digitally communicated to the doctors prior to seeing the patients, so that they are prepared to talk with the patients in terms they can understand. This simple change could lead to big improvements in health outcomes.”
In future studies, the researchers hope to evaluate the cancer health literacy of Hispanic patients because prior research has pointed to disparities in this population’s health literacy. ![]()
Hematology drugs on the fast track

Credit: Bill Branson
The US Food and Drug Administration (FDA) has granted fast track designation to two hematology drugs: the monoclonal antibody MOR208 to treat relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and the antifibrotic agent PRM-151 to treat myelofibrosis (MF).
The FDA’s fast track program aims to expedite the development and review of drugs that have the potential to fill an unmet medical need in serious or life-threatening conditions.
MOR208
MOR208 is a humanized monoclonal antibody targeting CD19. It is under development by MorphoSys AG to treat B-cell malignancies. The program is in phase 2 clinical development in chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), and non-Hodgkin lymphoma.
Preclinical research with MOR208 revealed that it can trigger natural killer cell-mediated lysis of ALL cells. The drug had lytic activity against ALL cells from both adult and pediatric patients.
In a phase 1 study, MOR208 exhibited preliminary efficacy in patients with high-risk, heavily pretreated CLL, prompting responses in 67% of patients. Researchers said toxicity was acceptable, but infusion reactions were common.
“First results of our ongoing phase 2 trial, which we will present at this year’s ASH conference in December, have helped to identify diffuse large B-cell lymphoma as a valuable development opportunity for MOR208,” said Arndt Schottelius, chief development officer of MorphoSys AG.
“We are therefore delighted to have received the fast track designation for further development of MOR208 in DLBCL. The more frequent interactions with the FDA that this enables will help us to expedite the development of MOR208 in this particular subset of non-Hodgkin’s lymphoma patients.”
PRM-151
PRM-151 is a recombinant form of an endogenous human protein, pentraxin-2, that is specifically active at the site of tissue damage. PRM-151 is an agonist that acts as a monocyte/macrophage differentiation factor to prevent and potentially reverse fibrosis.
The drug has shown broad anti-fibrotic activity in preclinical models of fibrotic disease, including pulmonary fibrosis, acute and chronic nephropathy, liver fibrosis, and age-related macular degeneration.
PRM-151 has orphan designation in the US for MF and in both the US and European Union for the treatment of idiopathic pulmonary fibrosis.
The FDA’s fast track designation for PRM-151 covers primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF.
“This designation validates our perspective that there is a clear and compelling need for a novel mechanism for the treatment of myelofibrosis that specifically targets the underlying fibrotic processes of the disease,” said Beth Tréhu, MD, FACP, chief medical officer of Promedior Inc., the company developing PRM-151.
“We will continue to work expeditiously to advance this program through the clinic and look forward to presenting the full data set from the first stage of our phase 2 study later this year.”
Preliminary data from the phase 2 study of PRM-151 demonstrated benefits across all clinically relevant measures of MF, including decreases in bone marrow fibrosis, symptom responses, improvements in hemoglobin and platelets, and reductions in spleen size.
The drug also appeared to be well-tolerated and did not prompt myelosuppression. ![]()

Credit: Bill Branson
The US Food and Drug Administration (FDA) has granted fast track designation to two hematology drugs: the monoclonal antibody MOR208 to treat relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and the antifibrotic agent PRM-151 to treat myelofibrosis (MF).
The FDA’s fast track program aims to expedite the development and review of drugs that have the potential to fill an unmet medical need in serious or life-threatening conditions.
MOR208
MOR208 is a humanized monoclonal antibody targeting CD19. It is under development by MorphoSys AG to treat B-cell malignancies. The program is in phase 2 clinical development in chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), and non-Hodgkin lymphoma.
Preclinical research with MOR208 revealed that it can trigger natural killer cell-mediated lysis of ALL cells. The drug had lytic activity against ALL cells from both adult and pediatric patients.
In a phase 1 study, MOR208 exhibited preliminary efficacy in patients with high-risk, heavily pretreated CLL, prompting responses in 67% of patients. Researchers said toxicity was acceptable, but infusion reactions were common.
“First results of our ongoing phase 2 trial, which we will present at this year’s ASH conference in December, have helped to identify diffuse large B-cell lymphoma as a valuable development opportunity for MOR208,” said Arndt Schottelius, chief development officer of MorphoSys AG.
“We are therefore delighted to have received the fast track designation for further development of MOR208 in DLBCL. The more frequent interactions with the FDA that this enables will help us to expedite the development of MOR208 in this particular subset of non-Hodgkin’s lymphoma patients.”
PRM-151
PRM-151 is a recombinant form of an endogenous human protein, pentraxin-2, that is specifically active at the site of tissue damage. PRM-151 is an agonist that acts as a monocyte/macrophage differentiation factor to prevent and potentially reverse fibrosis.
The drug has shown broad anti-fibrotic activity in preclinical models of fibrotic disease, including pulmonary fibrosis, acute and chronic nephropathy, liver fibrosis, and age-related macular degeneration.
PRM-151 has orphan designation in the US for MF and in both the US and European Union for the treatment of idiopathic pulmonary fibrosis.
The FDA’s fast track designation for PRM-151 covers primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF.
“This designation validates our perspective that there is a clear and compelling need for a novel mechanism for the treatment of myelofibrosis that specifically targets the underlying fibrotic processes of the disease,” said Beth Tréhu, MD, FACP, chief medical officer of Promedior Inc., the company developing PRM-151.
“We will continue to work expeditiously to advance this program through the clinic and look forward to presenting the full data set from the first stage of our phase 2 study later this year.”
Preliminary data from the phase 2 study of PRM-151 demonstrated benefits across all clinically relevant measures of MF, including decreases in bone marrow fibrosis, symptom responses, improvements in hemoglobin and platelets, and reductions in spleen size.
The drug also appeared to be well-tolerated and did not prompt myelosuppression. ![]()

Credit: Bill Branson
The US Food and Drug Administration (FDA) has granted fast track designation to two hematology drugs: the monoclonal antibody MOR208 to treat relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and the antifibrotic agent PRM-151 to treat myelofibrosis (MF).
The FDA’s fast track program aims to expedite the development and review of drugs that have the potential to fill an unmet medical need in serious or life-threatening conditions.
MOR208
MOR208 is a humanized monoclonal antibody targeting CD19. It is under development by MorphoSys AG to treat B-cell malignancies. The program is in phase 2 clinical development in chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), and non-Hodgkin lymphoma.
Preclinical research with MOR208 revealed that it can trigger natural killer cell-mediated lysis of ALL cells. The drug had lytic activity against ALL cells from both adult and pediatric patients.
In a phase 1 study, MOR208 exhibited preliminary efficacy in patients with high-risk, heavily pretreated CLL, prompting responses in 67% of patients. Researchers said toxicity was acceptable, but infusion reactions were common.
“First results of our ongoing phase 2 trial, which we will present at this year’s ASH conference in December, have helped to identify diffuse large B-cell lymphoma as a valuable development opportunity for MOR208,” said Arndt Schottelius, chief development officer of MorphoSys AG.
“We are therefore delighted to have received the fast track designation for further development of MOR208 in DLBCL. The more frequent interactions with the FDA that this enables will help us to expedite the development of MOR208 in this particular subset of non-Hodgkin’s lymphoma patients.”
PRM-151
PRM-151 is a recombinant form of an endogenous human protein, pentraxin-2, that is specifically active at the site of tissue damage. PRM-151 is an agonist that acts as a monocyte/macrophage differentiation factor to prevent and potentially reverse fibrosis.
The drug has shown broad anti-fibrotic activity in preclinical models of fibrotic disease, including pulmonary fibrosis, acute and chronic nephropathy, liver fibrosis, and age-related macular degeneration.
PRM-151 has orphan designation in the US for MF and in both the US and European Union for the treatment of idiopathic pulmonary fibrosis.
The FDA’s fast track designation for PRM-151 covers primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF.
“This designation validates our perspective that there is a clear and compelling need for a novel mechanism for the treatment of myelofibrosis that specifically targets the underlying fibrotic processes of the disease,” said Beth Tréhu, MD, FACP, chief medical officer of Promedior Inc., the company developing PRM-151.
“We will continue to work expeditiously to advance this program through the clinic and look forward to presenting the full data set from the first stage of our phase 2 study later this year.”
Preliminary data from the phase 2 study of PRM-151 demonstrated benefits across all clinically relevant measures of MF, including decreases in bone marrow fibrosis, symptom responses, improvements in hemoglobin and platelets, and reductions in spleen size.
The drug also appeared to be well-tolerated and did not prompt myelosuppression. ![]()
FDA panel votes against panobinostat as add-on therapy for multiple myeloma
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel agreed that the risk-benefit profile of panobinostat as add-on treatment to bortezomib and dexamethasone did not support approval as a treatment for multiple myeloma at this time, citing issues that included toxicity and marginal progression-free survival results.
At a meeting on Nov. 6, the FDA’s Oncologic Drugs Advisory Committee voted 5-2 that the benefits of panobinostat, combined with the proteasome inhibitor bortezomib (Velcade) and dexamethasone, did not outweigh the risks for treating patients with multiple myeloma who had received at least one prior therapy, the indication proposed by the manufacturer, Novartis Pharmaceuticals. However, those voting against approval agreed that there was evidence that panobinostat had biologic activity against multiple myeloma and had an effect on progression-free survival (PFS), and said they hoped the company would continue to study the drug in patients with the disease.
Like other panelists voting no on the risk-benefit question, Dr. James Liebmann, of the division of hematology-oncology at the University of Massachusetts, Worcester, said the vote was a difficult decision “because I do think that this probably has some activity in this disease and probably can be useful.” But in the study presented by the company, “the toxicity outweighed the marginal benefit in progression-free survival,” he added.
Panobinostat, in a capsule formulation, is a histone deacetylase (DAC) inhibitor. It inhibits class I, II, and IV histone DACs, which “are epigenetic modulators and important cancer targets due to the dysregulation of these enzymes in many types of tumors,” and as an epigenetic regulator, the drug “may help restore cell programming in multiple myeloma,” according to Novartis. If approved, it would be the first drug in this class to be approved for multiple myeloma.
In the pivotal phase III, international PANORAMA-1 study, 768 patients with relapsed or relapsed-refractory multiple myeloma were randomized to treatment with panobinostat or placebo, added to bortezomib and dexamethasone. Treatment was given in two 24-week phases (3-week cycles for the first phase and 6-week cycles for the second phase, with a 2 weeks on, 1 week off schedule), with 20 mg of panobinostat three times a week during both phases, and bortezomib (1.3 mg/m2) administered twice a week during the first phase and once a week during the second phase.
The primary endpoint, median PFS assessed by the investigator, was 12 months in the panobinostat arm, compared with 8.1 months in the placebo arm, a 3.9-month difference (hazard ratio, 0.63) that was statistically significant. When an independent review committee conducted an assessment – a prespecified sensitivity analysis – the median PFS benefit was 1.9 months. Among those on panobinostat, the overall response rate was numerically higher (61% vs. 55%), and there was a trend toward an improvement in overall survival, a secondary endpoint (a difference of about 3 months in the median overall survival).
There were more deaths that were not due to progressive disease among those in the panobinostat arm – 7%, compared with 3.5% in the placebo arm – and serious adverse events associated with treatment included pneumonia, diarrhea, thrombocytopenia, sepsis, and fatigue. Treatment had to be interrupted or the dose had to be modified because of an adverse event in 89% of those on panobinostat, compared with 76% of those in the placebo arm. Diarrhea was the most common reason for discontinuation in the panobinostat group: About 25% had grade 3/4 diarrhea, compared with almost 8% of those in the placebo arm. The company had no data showing that quality of life was better among those in the panobinostat arm.
The main issue raised by the FDA was the large amount of missing and censored data, and the effect this had on determining the magnitude of the benefit, weighed against the toxicity, which limited the FDA’s confidence in the results.
“This is a drug that has some activity, but progression-free survival alone, particularly when there are issues of missing data and a high rate of censoring” is a concern, said the panel chair, Dr. Deborah Armstrong, professor of oncology at Johns Hopkins University, Baltimore. She added that she expects that the drug will play a role in the treatment of multiple myeloma, “but potentially in a more narrow population of patients.”
Another panelist, Dr. Tito Fojo, director of the medical oncology fellowship program at the National Cancer Institute, Bethesda, Md., added, “I think this is a drug that likely has activity in this disease and could benefit these patients, but not in this combination.”
Among other concerns raised by panelists were the age of the patients in the study, which was a median of 63 years at enrollment, about 10 years younger than the median age of patients at diagnosis, since toxicity could be worse in older patients, they pointed out.
“While we are disappointed with the committee’s recommendation, we will continue to work with the FDA as it evaluates the application,” Bruno Strigini, Pharm.D., president of Novartis Oncology, said in a statement issued after the vote. The drug is also under review in the European Union and Japan.
The FDA usually follows the recommendations of its advisory panels. The FDA is expected to make the decision this month. No panel members had conflicts of interest to disclose.
Bortezomib was approved as third-line and second-line therapy for multiple myeloma in 2003 and 2005, respectively, and in 2008 it was approved for previously untreated multiple myeloma.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel agreed that the risk-benefit profile of panobinostat as add-on treatment to bortezomib and dexamethasone did not support approval as a treatment for multiple myeloma at this time, citing issues that included toxicity and marginal progression-free survival results.
At a meeting on Nov. 6, the FDA’s Oncologic Drugs Advisory Committee voted 5-2 that the benefits of panobinostat, combined with the proteasome inhibitor bortezomib (Velcade) and dexamethasone, did not outweigh the risks for treating patients with multiple myeloma who had received at least one prior therapy, the indication proposed by the manufacturer, Novartis Pharmaceuticals. However, those voting against approval agreed that there was evidence that panobinostat had biologic activity against multiple myeloma and had an effect on progression-free survival (PFS), and said they hoped the company would continue to study the drug in patients with the disease.
Like other panelists voting no on the risk-benefit question, Dr. James Liebmann, of the division of hematology-oncology at the University of Massachusetts, Worcester, said the vote was a difficult decision “because I do think that this probably has some activity in this disease and probably can be useful.” But in the study presented by the company, “the toxicity outweighed the marginal benefit in progression-free survival,” he added.
Panobinostat, in a capsule formulation, is a histone deacetylase (DAC) inhibitor. It inhibits class I, II, and IV histone DACs, which “are epigenetic modulators and important cancer targets due to the dysregulation of these enzymes in many types of tumors,” and as an epigenetic regulator, the drug “may help restore cell programming in multiple myeloma,” according to Novartis. If approved, it would be the first drug in this class to be approved for multiple myeloma.
In the pivotal phase III, international PANORAMA-1 study, 768 patients with relapsed or relapsed-refractory multiple myeloma were randomized to treatment with panobinostat or placebo, added to bortezomib and dexamethasone. Treatment was given in two 24-week phases (3-week cycles for the first phase and 6-week cycles for the second phase, with a 2 weeks on, 1 week off schedule), with 20 mg of panobinostat three times a week during both phases, and bortezomib (1.3 mg/m2) administered twice a week during the first phase and once a week during the second phase.
The primary endpoint, median PFS assessed by the investigator, was 12 months in the panobinostat arm, compared with 8.1 months in the placebo arm, a 3.9-month difference (hazard ratio, 0.63) that was statistically significant. When an independent review committee conducted an assessment – a prespecified sensitivity analysis – the median PFS benefit was 1.9 months. Among those on panobinostat, the overall response rate was numerically higher (61% vs. 55%), and there was a trend toward an improvement in overall survival, a secondary endpoint (a difference of about 3 months in the median overall survival).
There were more deaths that were not due to progressive disease among those in the panobinostat arm – 7%, compared with 3.5% in the placebo arm – and serious adverse events associated with treatment included pneumonia, diarrhea, thrombocytopenia, sepsis, and fatigue. Treatment had to be interrupted or the dose had to be modified because of an adverse event in 89% of those on panobinostat, compared with 76% of those in the placebo arm. Diarrhea was the most common reason for discontinuation in the panobinostat group: About 25% had grade 3/4 diarrhea, compared with almost 8% of those in the placebo arm. The company had no data showing that quality of life was better among those in the panobinostat arm.
The main issue raised by the FDA was the large amount of missing and censored data, and the effect this had on determining the magnitude of the benefit, weighed against the toxicity, which limited the FDA’s confidence in the results.
“This is a drug that has some activity, but progression-free survival alone, particularly when there are issues of missing data and a high rate of censoring” is a concern, said the panel chair, Dr. Deborah Armstrong, professor of oncology at Johns Hopkins University, Baltimore. She added that she expects that the drug will play a role in the treatment of multiple myeloma, “but potentially in a more narrow population of patients.”
Another panelist, Dr. Tito Fojo, director of the medical oncology fellowship program at the National Cancer Institute, Bethesda, Md., added, “I think this is a drug that likely has activity in this disease and could benefit these patients, but not in this combination.”
Among other concerns raised by panelists were the age of the patients in the study, which was a median of 63 years at enrollment, about 10 years younger than the median age of patients at diagnosis, since toxicity could be worse in older patients, they pointed out.
“While we are disappointed with the committee’s recommendation, we will continue to work with the FDA as it evaluates the application,” Bruno Strigini, Pharm.D., president of Novartis Oncology, said in a statement issued after the vote. The drug is also under review in the European Union and Japan.
The FDA usually follows the recommendations of its advisory panels. The FDA is expected to make the decision this month. No panel members had conflicts of interest to disclose.
Bortezomib was approved as third-line and second-line therapy for multiple myeloma in 2003 and 2005, respectively, and in 2008 it was approved for previously untreated multiple myeloma.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel agreed that the risk-benefit profile of panobinostat as add-on treatment to bortezomib and dexamethasone did not support approval as a treatment for multiple myeloma at this time, citing issues that included toxicity and marginal progression-free survival results.
At a meeting on Nov. 6, the FDA’s Oncologic Drugs Advisory Committee voted 5-2 that the benefits of panobinostat, combined with the proteasome inhibitor bortezomib (Velcade) and dexamethasone, did not outweigh the risks for treating patients with multiple myeloma who had received at least one prior therapy, the indication proposed by the manufacturer, Novartis Pharmaceuticals. However, those voting against approval agreed that there was evidence that panobinostat had biologic activity against multiple myeloma and had an effect on progression-free survival (PFS), and said they hoped the company would continue to study the drug in patients with the disease.
Like other panelists voting no on the risk-benefit question, Dr. James Liebmann, of the division of hematology-oncology at the University of Massachusetts, Worcester, said the vote was a difficult decision “because I do think that this probably has some activity in this disease and probably can be useful.” But in the study presented by the company, “the toxicity outweighed the marginal benefit in progression-free survival,” he added.
Panobinostat, in a capsule formulation, is a histone deacetylase (DAC) inhibitor. It inhibits class I, II, and IV histone DACs, which “are epigenetic modulators and important cancer targets due to the dysregulation of these enzymes in many types of tumors,” and as an epigenetic regulator, the drug “may help restore cell programming in multiple myeloma,” according to Novartis. If approved, it would be the first drug in this class to be approved for multiple myeloma.
In the pivotal phase III, international PANORAMA-1 study, 768 patients with relapsed or relapsed-refractory multiple myeloma were randomized to treatment with panobinostat or placebo, added to bortezomib and dexamethasone. Treatment was given in two 24-week phases (3-week cycles for the first phase and 6-week cycles for the second phase, with a 2 weeks on, 1 week off schedule), with 20 mg of panobinostat three times a week during both phases, and bortezomib (1.3 mg/m2) administered twice a week during the first phase and once a week during the second phase.
The primary endpoint, median PFS assessed by the investigator, was 12 months in the panobinostat arm, compared with 8.1 months in the placebo arm, a 3.9-month difference (hazard ratio, 0.63) that was statistically significant. When an independent review committee conducted an assessment – a prespecified sensitivity analysis – the median PFS benefit was 1.9 months. Among those on panobinostat, the overall response rate was numerically higher (61% vs. 55%), and there was a trend toward an improvement in overall survival, a secondary endpoint (a difference of about 3 months in the median overall survival).
There were more deaths that were not due to progressive disease among those in the panobinostat arm – 7%, compared with 3.5% in the placebo arm – and serious adverse events associated with treatment included pneumonia, diarrhea, thrombocytopenia, sepsis, and fatigue. Treatment had to be interrupted or the dose had to be modified because of an adverse event in 89% of those on panobinostat, compared with 76% of those in the placebo arm. Diarrhea was the most common reason for discontinuation in the panobinostat group: About 25% had grade 3/4 diarrhea, compared with almost 8% of those in the placebo arm. The company had no data showing that quality of life was better among those in the panobinostat arm.
The main issue raised by the FDA was the large amount of missing and censored data, and the effect this had on determining the magnitude of the benefit, weighed against the toxicity, which limited the FDA’s confidence in the results.
“This is a drug that has some activity, but progression-free survival alone, particularly when there are issues of missing data and a high rate of censoring” is a concern, said the panel chair, Dr. Deborah Armstrong, professor of oncology at Johns Hopkins University, Baltimore. She added that she expects that the drug will play a role in the treatment of multiple myeloma, “but potentially in a more narrow population of patients.”
Another panelist, Dr. Tito Fojo, director of the medical oncology fellowship program at the National Cancer Institute, Bethesda, Md., added, “I think this is a drug that likely has activity in this disease and could benefit these patients, but not in this combination.”
Among other concerns raised by panelists were the age of the patients in the study, which was a median of 63 years at enrollment, about 10 years younger than the median age of patients at diagnosis, since toxicity could be worse in older patients, they pointed out.
“While we are disappointed with the committee’s recommendation, we will continue to work with the FDA as it evaluates the application,” Bruno Strigini, Pharm.D., president of Novartis Oncology, said in a statement issued after the vote. The drug is also under review in the European Union and Japan.
The FDA usually follows the recommendations of its advisory panels. The FDA is expected to make the decision this month. No panel members had conflicts of interest to disclose.
Bortezomib was approved as third-line and second-line therapy for multiple myeloma in 2003 and 2005, respectively, and in 2008 it was approved for previously untreated multiple myeloma.
AT AN FDA ADVISORY COMMITTEE MEETING
RNAi therapy may eliminate resistance in B-ALL
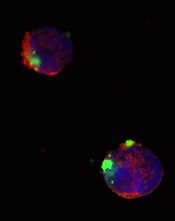
CD22ΔE12-siRNA nanoparticle
Credit: Fatih Uckun
Researchers have identified a molecular target for RNA interference (RNAi) therapy, and they believe that, by targeting this molecular lesion, they may be able to eliminate drug resistance in B-lineage acute lymphoblastic leukemia (ALL).
“We knew that we could kill chemotherapy-resistant leukemia cells if we only knew what made them so resistant,” said study author Fatih Uckun, MD, PhD, of the Children’s Hospital Los Angeles in California.
“Once we determined the mechanism, the next step was obvious—to rationally design a drug that would take out that specific target.”
The target is a defective gene that results in the production of abnormal CD22—CD22ΔE12. The researchers found that pediatric and adult B-lineage lymphoid malignancies are characterized by a high incidence of CD22ΔE12.
The group’s experiments revealed a causal link between CD22ΔE12 and the aggressive biology of B-ALL cells. When the researchers knocked down CD22ΔE12 in primary B-ALL cells using small interfering RNA (siRNA), they observed inhibition of clonogenicity.
The team therefore theorized that the siRNA-mediated depletion of CD22ΔE12 might be useful for treating B-ALL.
So they designed a nanoparticle formulation of CD22ΔE12-siRNA in which a polypeptide functions as the delivery system. The particle has a diameter of 100 nanometers.
Experiments showed that the nanoparticle could deliver its siRNA cargo into the cytosol of ALL-1 cells, thereby depleting CD22ΔE12 and inhibiting leukemic cell growth in vitro.
The researchers said that further development of this nanoparticle or other nanoformulation platforms for CD22ΔE12-siRNA could facilitate an effective therapeutic RNAi strategy to treat aggressive or chemotherapy-resistant B-lineage lymphoid malignancies.
“The goal is to translate our recent research discoveries in nanotechnology and biotherapy into effective patient-tailored treatment programs for the most common form of childhood cancer,” Dr Uckun said.
He and his colleagues described this research in EBioMedicine. ![]()

CD22ΔE12-siRNA nanoparticle
Credit: Fatih Uckun
Researchers have identified a molecular target for RNA interference (RNAi) therapy, and they believe that, by targeting this molecular lesion, they may be able to eliminate drug resistance in B-lineage acute lymphoblastic leukemia (ALL).
“We knew that we could kill chemotherapy-resistant leukemia cells if we only knew what made them so resistant,” said study author Fatih Uckun, MD, PhD, of the Children’s Hospital Los Angeles in California.
“Once we determined the mechanism, the next step was obvious—to rationally design a drug that would take out that specific target.”
The target is a defective gene that results in the production of abnormal CD22—CD22ΔE12. The researchers found that pediatric and adult B-lineage lymphoid malignancies are characterized by a high incidence of CD22ΔE12.
The group’s experiments revealed a causal link between CD22ΔE12 and the aggressive biology of B-ALL cells. When the researchers knocked down CD22ΔE12 in primary B-ALL cells using small interfering RNA (siRNA), they observed inhibition of clonogenicity.
The team therefore theorized that the siRNA-mediated depletion of CD22ΔE12 might be useful for treating B-ALL.
So they designed a nanoparticle formulation of CD22ΔE12-siRNA in which a polypeptide functions as the delivery system. The particle has a diameter of 100 nanometers.
Experiments showed that the nanoparticle could deliver its siRNA cargo into the cytosol of ALL-1 cells, thereby depleting CD22ΔE12 and inhibiting leukemic cell growth in vitro.
The researchers said that further development of this nanoparticle or other nanoformulation platforms for CD22ΔE12-siRNA could facilitate an effective therapeutic RNAi strategy to treat aggressive or chemotherapy-resistant B-lineage lymphoid malignancies.
“The goal is to translate our recent research discoveries in nanotechnology and biotherapy into effective patient-tailored treatment programs for the most common form of childhood cancer,” Dr Uckun said.
He and his colleagues described this research in EBioMedicine. ![]()

CD22ΔE12-siRNA nanoparticle
Credit: Fatih Uckun
Researchers have identified a molecular target for RNA interference (RNAi) therapy, and they believe that, by targeting this molecular lesion, they may be able to eliminate drug resistance in B-lineage acute lymphoblastic leukemia (ALL).
“We knew that we could kill chemotherapy-resistant leukemia cells if we only knew what made them so resistant,” said study author Fatih Uckun, MD, PhD, of the Children’s Hospital Los Angeles in California.
“Once we determined the mechanism, the next step was obvious—to rationally design a drug that would take out that specific target.”
The target is a defective gene that results in the production of abnormal CD22—CD22ΔE12. The researchers found that pediatric and adult B-lineage lymphoid malignancies are characterized by a high incidence of CD22ΔE12.
The group’s experiments revealed a causal link between CD22ΔE12 and the aggressive biology of B-ALL cells. When the researchers knocked down CD22ΔE12 in primary B-ALL cells using small interfering RNA (siRNA), they observed inhibition of clonogenicity.
The team therefore theorized that the siRNA-mediated depletion of CD22ΔE12 might be useful for treating B-ALL.
So they designed a nanoparticle formulation of CD22ΔE12-siRNA in which a polypeptide functions as the delivery system. The particle has a diameter of 100 nanometers.
Experiments showed that the nanoparticle could deliver its siRNA cargo into the cytosol of ALL-1 cells, thereby depleting CD22ΔE12 and inhibiting leukemic cell growth in vitro.
The researchers said that further development of this nanoparticle or other nanoformulation platforms for CD22ΔE12-siRNA could facilitate an effective therapeutic RNAi strategy to treat aggressive or chemotherapy-resistant B-lineage lymphoid malignancies.
“The goal is to translate our recent research discoveries in nanotechnology and biotherapy into effective patient-tailored treatment programs for the most common form of childhood cancer,” Dr Uckun said.
He and his colleagues described this research in EBioMedicine. ![]()
NICE recommends ofatumumab in CLL

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations.
Obesity affects toxicity of immunotherapy
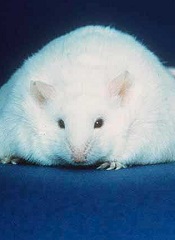
Immunotherapy that can be effective against tumors in young, thin mice can be lethal to obese ones, according to research published in The Journal of Experimental Medicine.
Investigators conducted experiments in mouse models to determine if there is a subset of cancer patients for whom certain immunotherapies might be especially toxic.
The group found a potential link between body fat and the risk of toxicity from some types of immunotherapy.
“Cancer is primarily considered a disease of the aged, and yet preclinical studies generally use young, lean animal models that may not be reflective of the ‘typical’ cancer patient,” said study author Annie Mirsoian, a PhD candidate at the University of California, Davis in Sacramento.
“Aging is a dynamic process that is characterized by increases in inflammatory factors, as well as a shift in body composition where there is a gradual loss of lean muscle mass and an increase in fat accumulation, which effect how the immune system functions.”
Mirsoian and her colleagues sought to determine if, by adjusting the mouse model to more closely reflect the cancer patient phenotype (advanced age and overweight), they could better understand the discrepancies between animal study outcomes and those in patients in the clinic.
So the team examined aged mice on standard diets and compared those to aged mice that were calorie-restricted throughout their lives.
The investigators found that calorie restriction plays a protective role against toxicity. When aged mice ate their standard diet freely throughout life, they became obese and ultimately experienced lethal adverse reactions after receiving a systemic immunotherapy regimen.
“We know that people who are obese in general are at higher risk for complications from surgery, radiation, and chemotherapy,” said study author Arta Monjazeb, MD, PhD, of the University of California, Davis.
“We know that obese people have higher levels of inflammatory markers in their blood, but there is a lack of data examining the effects of obesity on cancer treatment outcomes.”
In follow-up experiments, the investigators found that young mice that are obese also endure similar toxic consequences, demonstrating that fat is a critical factor in toxic responses to stimulatory anticancer immunotherapy regimens.
“It is important to note, however, that the aged mice on standard diets succumbed to lethality at a quicker rate than young obese mice,” Mirsoian said. “Although our data demonstrate that obesity plays a central role in the development of adverse effects, future studies will focus on examining the aged immune system and cellular characteristics that may have enhanced the sensitivity of these mice to inflammation.”
This study follows an earlier paper, which demonstrated that while young, lean mice tolerate immunotherapy regimens without toxicity, the same regimen for an aged cohort resulted in lethal consequences. The new paper takes a deeper look into how fat deposition throughout aging can be critical in determining treatment tolerance and efficacy.
“Obesity has become an epidemic in our society and is now also affecting younger populations,” Mirsoian said. “Therefore, it’s likely that what the ‘typical’ cancer patient looks like will change. Our findings demonstrate the importance of having preclinical animal models that reflect the clinical scenario.”
“Changing the characteristics of our mouse models allowed for a more accurate determination of possible adverse reactions to therapy and more closely modeled what has been reported in the clinic with stimulatory immunotherapies.”
The investigators said factors like age, fat content, and the types of infections experienced throughout life together shape how the immune system reacts, and they will continue to work on improving their modeling system to reflect these changes.
They project that improvements in mouse modeling may help produce data that can better modulate treatment choices for patients, as well as identify early which patients would benefit from the inclusion of drugs to prevent adverse reactions while maintaining anticancer efficacy.

Immunotherapy that can be effective against tumors in young, thin mice can be lethal to obese ones, according to research published in The Journal of Experimental Medicine.
Investigators conducted experiments in mouse models to determine if there is a subset of cancer patients for whom certain immunotherapies might be especially toxic.
The group found a potential link between body fat and the risk of toxicity from some types of immunotherapy.
“Cancer is primarily considered a disease of the aged, and yet preclinical studies generally use young, lean animal models that may not be reflective of the ‘typical’ cancer patient,” said study author Annie Mirsoian, a PhD candidate at the University of California, Davis in Sacramento.
“Aging is a dynamic process that is characterized by increases in inflammatory factors, as well as a shift in body composition where there is a gradual loss of lean muscle mass and an increase in fat accumulation, which effect how the immune system functions.”
Mirsoian and her colleagues sought to determine if, by adjusting the mouse model to more closely reflect the cancer patient phenotype (advanced age and overweight), they could better understand the discrepancies between animal study outcomes and those in patients in the clinic.
So the team examined aged mice on standard diets and compared those to aged mice that were calorie-restricted throughout their lives.
The investigators found that calorie restriction plays a protective role against toxicity. When aged mice ate their standard diet freely throughout life, they became obese and ultimately experienced lethal adverse reactions after receiving a systemic immunotherapy regimen.
“We know that people who are obese in general are at higher risk for complications from surgery, radiation, and chemotherapy,” said study author Arta Monjazeb, MD, PhD, of the University of California, Davis.
“We know that obese people have higher levels of inflammatory markers in their blood, but there is a lack of data examining the effects of obesity on cancer treatment outcomes.”
In follow-up experiments, the investigators found that young mice that are obese also endure similar toxic consequences, demonstrating that fat is a critical factor in toxic responses to stimulatory anticancer immunotherapy regimens.
“It is important to note, however, that the aged mice on standard diets succumbed to lethality at a quicker rate than young obese mice,” Mirsoian said. “Although our data demonstrate that obesity plays a central role in the development of adverse effects, future studies will focus on examining the aged immune system and cellular characteristics that may have enhanced the sensitivity of these mice to inflammation.”
This study follows an earlier paper, which demonstrated that while young, lean mice tolerate immunotherapy regimens without toxicity, the same regimen for an aged cohort resulted in lethal consequences. The new paper takes a deeper look into how fat deposition throughout aging can be critical in determining treatment tolerance and efficacy.
“Obesity has become an epidemic in our society and is now also affecting younger populations,” Mirsoian said. “Therefore, it’s likely that what the ‘typical’ cancer patient looks like will change. Our findings demonstrate the importance of having preclinical animal models that reflect the clinical scenario.”
“Changing the characteristics of our mouse models allowed for a more accurate determination of possible adverse reactions to therapy and more closely modeled what has been reported in the clinic with stimulatory immunotherapies.”
The investigators said factors like age, fat content, and the types of infections experienced throughout life together shape how the immune system reacts, and they will continue to work on improving their modeling system to reflect these changes.
They project that improvements in mouse modeling may help produce data that can better modulate treatment choices for patients, as well as identify early which patients would benefit from the inclusion of drugs to prevent adverse reactions while maintaining anticancer efficacy.

Immunotherapy that can be effective against tumors in young, thin mice can be lethal to obese ones, according to research published in The Journal of Experimental Medicine.
Investigators conducted experiments in mouse models to determine if there is a subset of cancer patients for whom certain immunotherapies might be especially toxic.
The group found a potential link between body fat and the risk of toxicity from some types of immunotherapy.
“Cancer is primarily considered a disease of the aged, and yet preclinical studies generally use young, lean animal models that may not be reflective of the ‘typical’ cancer patient,” said study author Annie Mirsoian, a PhD candidate at the University of California, Davis in Sacramento.
“Aging is a dynamic process that is characterized by increases in inflammatory factors, as well as a shift in body composition where there is a gradual loss of lean muscle mass and an increase in fat accumulation, which effect how the immune system functions.”
Mirsoian and her colleagues sought to determine if, by adjusting the mouse model to more closely reflect the cancer patient phenotype (advanced age and overweight), they could better understand the discrepancies between animal study outcomes and those in patients in the clinic.
So the team examined aged mice on standard diets and compared those to aged mice that were calorie-restricted throughout their lives.
The investigators found that calorie restriction plays a protective role against toxicity. When aged mice ate their standard diet freely throughout life, they became obese and ultimately experienced lethal adverse reactions after receiving a systemic immunotherapy regimen.
“We know that people who are obese in general are at higher risk for complications from surgery, radiation, and chemotherapy,” said study author Arta Monjazeb, MD, PhD, of the University of California, Davis.
“We know that obese people have higher levels of inflammatory markers in their blood, but there is a lack of data examining the effects of obesity on cancer treatment outcomes.”
In follow-up experiments, the investigators found that young mice that are obese also endure similar toxic consequences, demonstrating that fat is a critical factor in toxic responses to stimulatory anticancer immunotherapy regimens.
“It is important to note, however, that the aged mice on standard diets succumbed to lethality at a quicker rate than young obese mice,” Mirsoian said. “Although our data demonstrate that obesity plays a central role in the development of adverse effects, future studies will focus on examining the aged immune system and cellular characteristics that may have enhanced the sensitivity of these mice to inflammation.”
This study follows an earlier paper, which demonstrated that while young, lean mice tolerate immunotherapy regimens without toxicity, the same regimen for an aged cohort resulted in lethal consequences. The new paper takes a deeper look into how fat deposition throughout aging can be critical in determining treatment tolerance and efficacy.
“Obesity has become an epidemic in our society and is now also affecting younger populations,” Mirsoian said. “Therefore, it’s likely that what the ‘typical’ cancer patient looks like will change. Our findings demonstrate the importance of having preclinical animal models that reflect the clinical scenario.”
“Changing the characteristics of our mouse models allowed for a more accurate determination of possible adverse reactions to therapy and more closely modeled what has been reported in the clinic with stimulatory immunotherapies.”
The investigators said factors like age, fat content, and the types of infections experienced throughout life together shape how the immune system reacts, and they will continue to work on improving their modeling system to reflect these changes.
They project that improvements in mouse modeling may help produce data that can better modulate treatment choices for patients, as well as identify early which patients would benefit from the inclusion of drugs to prevent adverse reactions while maintaining anticancer efficacy.
Air pollution not to blame for childhood leukemia, study suggests

The increased risk of leukemia reported among children born close to overhead power lines is likely not a result of alterations in air pollution, researchers have reported in the Journal of Radiological Protection.
The group found little evidence to support the “corona-ion hypothesis” which has been cited as a possible explanation for the excess of childhood leukemia cases close to high-voltage overhead power lines in the UK prior to the 1980s.
The hypothesis is based on the fact that high-voltage overhead power lines create charged particles in the surrounding air.
These ionized particles, known as corona ions, can be blown away by the wind and attach to air pollutants, such as those from traffic or smoking.
The corona-ion hypothesis suggests these electrically charged pollutants are more likely to be retained in the airways or lungs, and this could lead to serious health effects, including childhood leukemia.
The researchers previously showed that, on average, there has been no increased risk of leukemia among children born near high-voltage power lines in recent decades. However, the same piece of research confirmed an increased risk prior to the 1980s, which has yet to be explained.
To investigate this theory, John Swanson, of National Grid in London, and his colleagues used data from 7347 children in England and Wales who were born and diagnosed with leukemia between 1968 and 2008, and who lived within 600 m of a high-voltage overhead power line.
The researchers calculated the exposure of each of the subjects to corona ions using a model based on: the voltage of the power line; the distance from the line; how the concentration of corona ions varied with distance from the power lines; and, using data from various meteorological stations, the amount of time and speed that wind blew in each direction around the power lines.
The results did not suggest that exposure to corona ions explained the pattern of increased leukemia rates close to high-voltage overhead power lines previously found in earlier decades.
“We found in earlier studies that, for previous decades, childhood leukemia rates were higher near power lines,” said Kathryn Bunch, of the University of Oxford.
“This new paper seems to show that this wasn’t caused by corona ions, but it leaves us still searching for the true cause, and we are undertaking further investigations of the variation in risk over time.”

The increased risk of leukemia reported among children born close to overhead power lines is likely not a result of alterations in air pollution, researchers have reported in the Journal of Radiological Protection.
The group found little evidence to support the “corona-ion hypothesis” which has been cited as a possible explanation for the excess of childhood leukemia cases close to high-voltage overhead power lines in the UK prior to the 1980s.
The hypothesis is based on the fact that high-voltage overhead power lines create charged particles in the surrounding air.
These ionized particles, known as corona ions, can be blown away by the wind and attach to air pollutants, such as those from traffic or smoking.
The corona-ion hypothesis suggests these electrically charged pollutants are more likely to be retained in the airways or lungs, and this could lead to serious health effects, including childhood leukemia.
The researchers previously showed that, on average, there has been no increased risk of leukemia among children born near high-voltage power lines in recent decades. However, the same piece of research confirmed an increased risk prior to the 1980s, which has yet to be explained.
To investigate this theory, John Swanson, of National Grid in London, and his colleagues used data from 7347 children in England and Wales who were born and diagnosed with leukemia between 1968 and 2008, and who lived within 600 m of a high-voltage overhead power line.
The researchers calculated the exposure of each of the subjects to corona ions using a model based on: the voltage of the power line; the distance from the line; how the concentration of corona ions varied with distance from the power lines; and, using data from various meteorological stations, the amount of time and speed that wind blew in each direction around the power lines.
The results did not suggest that exposure to corona ions explained the pattern of increased leukemia rates close to high-voltage overhead power lines previously found in earlier decades.
“We found in earlier studies that, for previous decades, childhood leukemia rates were higher near power lines,” said Kathryn Bunch, of the University of Oxford.
“This new paper seems to show that this wasn’t caused by corona ions, but it leaves us still searching for the true cause, and we are undertaking further investigations of the variation in risk over time.”

The increased risk of leukemia reported among children born close to overhead power lines is likely not a result of alterations in air pollution, researchers have reported in the Journal of Radiological Protection.
The group found little evidence to support the “corona-ion hypothesis” which has been cited as a possible explanation for the excess of childhood leukemia cases close to high-voltage overhead power lines in the UK prior to the 1980s.
The hypothesis is based on the fact that high-voltage overhead power lines create charged particles in the surrounding air.
These ionized particles, known as corona ions, can be blown away by the wind and attach to air pollutants, such as those from traffic or smoking.
The corona-ion hypothesis suggests these electrically charged pollutants are more likely to be retained in the airways or lungs, and this could lead to serious health effects, including childhood leukemia.
The researchers previously showed that, on average, there has been no increased risk of leukemia among children born near high-voltage power lines in recent decades. However, the same piece of research confirmed an increased risk prior to the 1980s, which has yet to be explained.
To investigate this theory, John Swanson, of National Grid in London, and his colleagues used data from 7347 children in England and Wales who were born and diagnosed with leukemia between 1968 and 2008, and who lived within 600 m of a high-voltage overhead power line.
The researchers calculated the exposure of each of the subjects to corona ions using a model based on: the voltage of the power line; the distance from the line; how the concentration of corona ions varied with distance from the power lines; and, using data from various meteorological stations, the amount of time and speed that wind blew in each direction around the power lines.
The results did not suggest that exposure to corona ions explained the pattern of increased leukemia rates close to high-voltage overhead power lines previously found in earlier decades.
“We found in earlier studies that, for previous decades, childhood leukemia rates were higher near power lines,” said Kathryn Bunch, of the University of Oxford.
“This new paper seems to show that this wasn’t caused by corona ions, but it leaves us still searching for the true cause, and we are undertaking further investigations of the variation in risk over time.”