User login
FDA approves rituximab + hyaluronidase human for FL, DLBCL, and CLL
The Food and Drug Administration has approved rituximab plus hyaluronidase human for adult patients with follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), and chronic lymphocytic leukemia (CLL).
The combination product, to be marketed as Rituxan Hycela, is administered subcutaneously, shortening administration time to 5 to 7 minutes as compared with the several hours needed for intravenous infusion, the FDA said in a statement.
Approval was based on noninferior rituximab trough concentrations for the subcutaneously administered combination, compared with intravenous rituximab, and comparable efficacy and safety results as shown in multiple randomized clinical trials.
The most common adverse events seen with the combination in patients with FL included infections, neutropenia, nausea, constipation, cough, and fatigue. In patients with DLBCL, the most common adverse events were infections, neutropenia, alopecia, nausea, and anemia; in CLL patients, infections, neutropenia, nausea, thrombocytopenia, pyrexia, vomiting, and injection site erythema occurred most commonly.
The combination is indicated for the following previously approved indications for rituximab:
- Relapsed or refractory FL as a single agent.
- Previously untreated FL in combination with first line chemotherapy and, in patients achieving a complete or partial response to rituximab in combination with chemotherapy, as single-agent maintenance therapy.
- Nonprogressing (including stable disease) FL as a single agent after first-line cyclophosphamide, vincristine, and prednisone chemotherapy.
- Previously untreated DLBCL in combination with cyclophosphamide, doxorubicin, vincristine, prednisone or other anthracycline-based chemotherapy regimens.
- Previously untreated and previously treated CLL in combination with fludarabine and cyclophosphamide.
The recommended doses are 1,400 mg rituximab and 23,400 units hyaluronidase human for FL and DLBCL and 1,600 mg rituximab and 26,800 units hyaluronidase human for CLL. The combination treatment should be initiated only after patients have received at least one full dose of a rituximab product by intravenous infusion, according to the prescribing information.
Rituxan Hycela is marketed by Genentech.
The Food and Drug Administration has approved rituximab plus hyaluronidase human for adult patients with follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), and chronic lymphocytic leukemia (CLL).
The combination product, to be marketed as Rituxan Hycela, is administered subcutaneously, shortening administration time to 5 to 7 minutes as compared with the several hours needed for intravenous infusion, the FDA said in a statement.
Approval was based on noninferior rituximab trough concentrations for the subcutaneously administered combination, compared with intravenous rituximab, and comparable efficacy and safety results as shown in multiple randomized clinical trials.
The most common adverse events seen with the combination in patients with FL included infections, neutropenia, nausea, constipation, cough, and fatigue. In patients with DLBCL, the most common adverse events were infections, neutropenia, alopecia, nausea, and anemia; in CLL patients, infections, neutropenia, nausea, thrombocytopenia, pyrexia, vomiting, and injection site erythema occurred most commonly.
The combination is indicated for the following previously approved indications for rituximab:
- Relapsed or refractory FL as a single agent.
- Previously untreated FL in combination with first line chemotherapy and, in patients achieving a complete or partial response to rituximab in combination with chemotherapy, as single-agent maintenance therapy.
- Nonprogressing (including stable disease) FL as a single agent after first-line cyclophosphamide, vincristine, and prednisone chemotherapy.
- Previously untreated DLBCL in combination with cyclophosphamide, doxorubicin, vincristine, prednisone or other anthracycline-based chemotherapy regimens.
- Previously untreated and previously treated CLL in combination with fludarabine and cyclophosphamide.
The recommended doses are 1,400 mg rituximab and 23,400 units hyaluronidase human for FL and DLBCL and 1,600 mg rituximab and 26,800 units hyaluronidase human for CLL. The combination treatment should be initiated only after patients have received at least one full dose of a rituximab product by intravenous infusion, according to the prescribing information.
Rituxan Hycela is marketed by Genentech.
The Food and Drug Administration has approved rituximab plus hyaluronidase human for adult patients with follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), and chronic lymphocytic leukemia (CLL).
The combination product, to be marketed as Rituxan Hycela, is administered subcutaneously, shortening administration time to 5 to 7 minutes as compared with the several hours needed for intravenous infusion, the FDA said in a statement.
Approval was based on noninferior rituximab trough concentrations for the subcutaneously administered combination, compared with intravenous rituximab, and comparable efficacy and safety results as shown in multiple randomized clinical trials.
The most common adverse events seen with the combination in patients with FL included infections, neutropenia, nausea, constipation, cough, and fatigue. In patients with DLBCL, the most common adverse events were infections, neutropenia, alopecia, nausea, and anemia; in CLL patients, infections, neutropenia, nausea, thrombocytopenia, pyrexia, vomiting, and injection site erythema occurred most commonly.
The combination is indicated for the following previously approved indications for rituximab:
- Relapsed or refractory FL as a single agent.
- Previously untreated FL in combination with first line chemotherapy and, in patients achieving a complete or partial response to rituximab in combination with chemotherapy, as single-agent maintenance therapy.
- Nonprogressing (including stable disease) FL as a single agent after first-line cyclophosphamide, vincristine, and prednisone chemotherapy.
- Previously untreated DLBCL in combination with cyclophosphamide, doxorubicin, vincristine, prednisone or other anthracycline-based chemotherapy regimens.
- Previously untreated and previously treated CLL in combination with fludarabine and cyclophosphamide.
The recommended doses are 1,400 mg rituximab and 23,400 units hyaluronidase human for FL and DLBCL and 1,600 mg rituximab and 26,800 units hyaluronidase human for CLL. The combination treatment should be initiated only after patients have received at least one full dose of a rituximab product by intravenous infusion, according to the prescribing information.
Rituxan Hycela is marketed by Genentech.
Bendamustine plus rituximab may have edge for treating indolent NHL, MCL
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
mdales@frontlinemedcom.com
On Twitter @maryjodales
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
mdales@frontlinemedcom.com
On Twitter @maryjodales
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
mdales@frontlinemedcom.com
On Twitter @maryjodales
AT ASCO 2017
Key clinical point:
Major finding: For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively.
Data source: In BRIGHT, 224 treatment-naive patients with indolent non-Hodgkin lymphoma or mantle cell lymphoma were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients).
Disclosures: Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
Inhibitor elicits responses in heavily pretreated FL, DLBCL
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
New frontline treatments needed for Hodgkin lymphoma
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
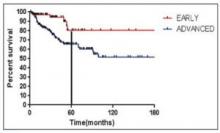
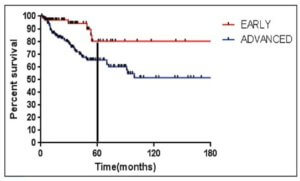
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
Lenalidomide-rituximab induces high CR rate in untreated follicular lymphoma
LUGANO, SWITZERLAND – The chemotherapy-free combination of lenalidomide (Revlimid) and rituximab was highly active as frontline therapy for patients with low- and intermediate-risk follicular lymphoma in a multicenter phase II trial.
Among 66 patients with previously untreated follicular lymphoma, the overall response rate to the combination was 95%, including complete responses in 72% of patients. The 5-year progression-free survival (PFS) rate was 70%, reported John P. Leonard, MD, of Weill Cornell Medicine, New York.
“I think this overall is a useful validation – confirmation of the single-center data that showed that this [combination] in a multicenter setting can be a highly effective and reasonably well-tolerated treatment approach for patients with untreated follicular lymphoma,” he said at the International Conference on Malignant Lymphoma, on behalf of colleagues in the National Cancer Institute Alliance for Clinical Trials in Oncology and Cancer and Leukemia Group B (CALGB) 50803 trial.
In the 50803 study, the investigators enrolled 66 treatment-naive patients. The median age was 53 years (range 32-79). Patients were eligible if they had grade 1-3a, stage 3-4 or bulky stage 2 untreated follicular lymphoma, with Follicular Lymphoma International Prognostic Index (FLIPI) scores of 0-2.
They received lenalidomide 20 mg/day on days 1 through 21 of each 28-day cycle for 12 cycles, plus rituximab administered once weekly for each week of cycle 1, and on the first day of cycles 4, 6, 8 and 10.
The investigators also evaluated polymorphisms in the Fc fragment of immunoglobulin G receptor IIa and IIIa (FcGR2A/FcGR3A).
One of the 66 patients never started treatment, leaving 65 for the response analysis.
As noted, the overall response rate was 95%, including 94% of 21 patients with FLIPI 0 or 1 disease, and 96% of 44 patients with FLIPI 2 or 3. There were no associations between FLIPI score and likelihood of achieving a complete response, and no associations between FcR polymorphism or change in angiogenic markers and either complete responses or PFS, Dr. Leonard said.
Complete responses, the primary endpoint, were seen in 15 of the 21 (71%) of patients with FLIPI 0-1, and in 32 of the 44 (73%) with FLIPI 2-3, for an overall complete response rate of 72%.
Partial responses occurred in 5 patients (23%) with FLIPI 0-1 and 10 patients (23%) with FLIPI 2-3, for an overall PR rate of 23%.
Respective rates of stable disease were 0, 2%, and 2%. One patient in each FLIPI group was not evaluated because of adverse events.
After a median follow-up of 5 years, the progression free survival rate was 70%.
The most common grade 3 or 4 adverse events were neutropenia, seen in 21% of patients, and infections, seen in 40% (including one grade 3 febrile neutropenia).
Grade 1-2 fatigue was reported by 51 patients, and grade 3 fatigue was reported by 4.
Other grade 3 or 4 events seen in more than 5% of patients included rash in 9%, and hyperglycemia, hypophosphatemia, or hypertension, each in 6% of patients.
Celgene and Genentech supported the study. Dr. Leonard has served as an adviser/consultant to Celgene and other companies.
LUGANO, SWITZERLAND – The chemotherapy-free combination of lenalidomide (Revlimid) and rituximab was highly active as frontline therapy for patients with low- and intermediate-risk follicular lymphoma in a multicenter phase II trial.
Among 66 patients with previously untreated follicular lymphoma, the overall response rate to the combination was 95%, including complete responses in 72% of patients. The 5-year progression-free survival (PFS) rate was 70%, reported John P. Leonard, MD, of Weill Cornell Medicine, New York.
“I think this overall is a useful validation – confirmation of the single-center data that showed that this [combination] in a multicenter setting can be a highly effective and reasonably well-tolerated treatment approach for patients with untreated follicular lymphoma,” he said at the International Conference on Malignant Lymphoma, on behalf of colleagues in the National Cancer Institute Alliance for Clinical Trials in Oncology and Cancer and Leukemia Group B (CALGB) 50803 trial.
In the 50803 study, the investigators enrolled 66 treatment-naive patients. The median age was 53 years (range 32-79). Patients were eligible if they had grade 1-3a, stage 3-4 or bulky stage 2 untreated follicular lymphoma, with Follicular Lymphoma International Prognostic Index (FLIPI) scores of 0-2.
They received lenalidomide 20 mg/day on days 1 through 21 of each 28-day cycle for 12 cycles, plus rituximab administered once weekly for each week of cycle 1, and on the first day of cycles 4, 6, 8 and 10.
The investigators also evaluated polymorphisms in the Fc fragment of immunoglobulin G receptor IIa and IIIa (FcGR2A/FcGR3A).
One of the 66 patients never started treatment, leaving 65 for the response analysis.
As noted, the overall response rate was 95%, including 94% of 21 patients with FLIPI 0 or 1 disease, and 96% of 44 patients with FLIPI 2 or 3. There were no associations between FLIPI score and likelihood of achieving a complete response, and no associations between FcR polymorphism or change in angiogenic markers and either complete responses or PFS, Dr. Leonard said.
Complete responses, the primary endpoint, were seen in 15 of the 21 (71%) of patients with FLIPI 0-1, and in 32 of the 44 (73%) with FLIPI 2-3, for an overall complete response rate of 72%.
Partial responses occurred in 5 patients (23%) with FLIPI 0-1 and 10 patients (23%) with FLIPI 2-3, for an overall PR rate of 23%.
Respective rates of stable disease were 0, 2%, and 2%. One patient in each FLIPI group was not evaluated because of adverse events.
After a median follow-up of 5 years, the progression free survival rate was 70%.
The most common grade 3 or 4 adverse events were neutropenia, seen in 21% of patients, and infections, seen in 40% (including one grade 3 febrile neutropenia).
Grade 1-2 fatigue was reported by 51 patients, and grade 3 fatigue was reported by 4.
Other grade 3 or 4 events seen in more than 5% of patients included rash in 9%, and hyperglycemia, hypophosphatemia, or hypertension, each in 6% of patients.
Celgene and Genentech supported the study. Dr. Leonard has served as an adviser/consultant to Celgene and other companies.
LUGANO, SWITZERLAND – The chemotherapy-free combination of lenalidomide (Revlimid) and rituximab was highly active as frontline therapy for patients with low- and intermediate-risk follicular lymphoma in a multicenter phase II trial.
Among 66 patients with previously untreated follicular lymphoma, the overall response rate to the combination was 95%, including complete responses in 72% of patients. The 5-year progression-free survival (PFS) rate was 70%, reported John P. Leonard, MD, of Weill Cornell Medicine, New York.
“I think this overall is a useful validation – confirmation of the single-center data that showed that this [combination] in a multicenter setting can be a highly effective and reasonably well-tolerated treatment approach for patients with untreated follicular lymphoma,” he said at the International Conference on Malignant Lymphoma, on behalf of colleagues in the National Cancer Institute Alliance for Clinical Trials in Oncology and Cancer and Leukemia Group B (CALGB) 50803 trial.
In the 50803 study, the investigators enrolled 66 treatment-naive patients. The median age was 53 years (range 32-79). Patients were eligible if they had grade 1-3a, stage 3-4 or bulky stage 2 untreated follicular lymphoma, with Follicular Lymphoma International Prognostic Index (FLIPI) scores of 0-2.
They received lenalidomide 20 mg/day on days 1 through 21 of each 28-day cycle for 12 cycles, plus rituximab administered once weekly for each week of cycle 1, and on the first day of cycles 4, 6, 8 and 10.
The investigators also evaluated polymorphisms in the Fc fragment of immunoglobulin G receptor IIa and IIIa (FcGR2A/FcGR3A).
One of the 66 patients never started treatment, leaving 65 for the response analysis.
As noted, the overall response rate was 95%, including 94% of 21 patients with FLIPI 0 or 1 disease, and 96% of 44 patients with FLIPI 2 or 3. There were no associations between FLIPI score and likelihood of achieving a complete response, and no associations between FcR polymorphism or change in angiogenic markers and either complete responses or PFS, Dr. Leonard said.
Complete responses, the primary endpoint, were seen in 15 of the 21 (71%) of patients with FLIPI 0-1, and in 32 of the 44 (73%) with FLIPI 2-3, for an overall complete response rate of 72%.
Partial responses occurred in 5 patients (23%) with FLIPI 0-1 and 10 patients (23%) with FLIPI 2-3, for an overall PR rate of 23%.
Respective rates of stable disease were 0, 2%, and 2%. One patient in each FLIPI group was not evaluated because of adverse events.
After a median follow-up of 5 years, the progression free survival rate was 70%.
The most common grade 3 or 4 adverse events were neutropenia, seen in 21% of patients, and infections, seen in 40% (including one grade 3 febrile neutropenia).
Grade 1-2 fatigue was reported by 51 patients, and grade 3 fatigue was reported by 4.
Other grade 3 or 4 events seen in more than 5% of patients included rash in 9%, and hyperglycemia, hypophosphatemia, or hypertension, each in 6% of patients.
Celgene and Genentech supported the study. Dr. Leonard has served as an adviser/consultant to Celgene and other companies.
AT 14-ICML
Key clinical point: The combination of lenalidomide and rituximab was highly active against previously untreated follicular lymphoma.
Major finding: The overall response rate was 95%; 5-year progression-free survival was 70%.
Data source: Open-label prospective study of lenalidomide-rituximab in 66 patients with previously untreated follicular lymphoma.
Disclosures: Celgene and Genentech supported the study. Dr. Leonard has served as an adviser/consultant to Celgene and other companies.
GALEN safe and effective in relapsed and refractory follicular lymphoma
LUGANO, SWITZERLAND – For patients with relapsed or refractory follicular lymphoma, a pairing of lenalidomide (Revlimid) and obinutuzumab (Gazyva) appeared to be especially useful among patients who had disease progression within 24 months, based on results from a Lymphoma Academic Research Organisation trial.
Among 86 patients who were enrolled in a phase II trial and were assessable for efficacy, overall response rates (ORR) with the combination therapy, nicknamed “GALEN,” were 80.2% by 1999 International Working Group criteria, and 74.4% according to the 2007 IWG criteria, reported Franck Morschhauser, MD, PhD, of the University of Lille, France.
The rationale for this combination is the known synergy between lenalidomide and rituximab in relapsed refractory non-Hodgkin lymphomas and in the frontline setting for patients with follicular lymphoma. Obinutuzumab, a follow-on to rituximab, is a unique type II glycoengineered monoclonal antibody directed against CD20, but with increased antibody-dependent cell-mediated cytotoxicity and increased direct cytotoxicity, compared with rituximab, he explained.
In the phase Ib part of the study, researchers settled on a dose of obinutuzumab 1000 mg and lenalidomide 20 mg. Obinutuzumab was administered on days 8, 15 and 22 and lenalidomide on days 1 to 21 of each 28 day cycle. Patients were evaluated for response after three cycles and at the end of induction (after completion of 4 to 6 cycles).
The maintenance phase consisted of obinutuzumab on day 1 of every other cycle beginning with cycle 1, and lenalidomide on days 1 through 22 for cycles 7 through 18. From cycles 19 through 24, obinutuzumab was given alone on the first day of every 56-day cycle.
The overall response rate (ORR) at the end of induction according to the IWG 1999 criteria, the primary endpoint, was 80.2%, including 39.5% complete or unconfirmed complete responses.
When the same patients were assessed according to 2007 IWG criteria, the ORR rate was slightly lower, at 74.4%, but the complete or unconfirmed complete response rate was slightly higher, at 44.2%.
An analysis of responses by time to relapse showed that the ORR among 24 patients with disease progression within 24 months was 70.8%, including 33.3% complete or unconfirmed complete responses by the 1999 criteria, and 66.7% with 54.2% complete or unconfirmed complete responses by the 2007 criteria.
ORR among the 64 patients with disease progression after more than 24 months was 83.9% with 41.9% complete or unconfirmed complete responses by 1999 criteria, and 77.4% with 40.3% complete or unconfirmed complete responses by 2007 criteria. The differences between the groups with disease progression within 24 months and later relapse groups were not significant.
A subanalysis by refractory status, however, showed that the 63 nonrefractory patients fared significantly better, with 87.3% ORR and 41.3% complete or unconfirmed complete responses by 1999 criteria, and 81.0% with 49.2% complete or unconfirmed complete response rate by 2007 determinations, compared with respective rates among 23 refractory patients of 60.9%/34.8% complete or unconfirmed complete response rate and 56.5% with 30.4% complete or unconfirmed complete responses (P = .0212 by 1999 criteria, and P = .022 by 2007 criteria).
After a median follow-up of 18 months, 1-year progression-free survival (PFS) among all patients was 75.5%, and 1-year overall survival (OS) was 88.8%.
There were no significant differences in either progression-free survival or overall survival by time to relapse. Although there appeared to be a nonsignificant trend toward worse outcomes among patients with refractory vs. nonrefractory disease, there was a significantly lower 1-year overall survival rate among refractory patients, at 71.5% compared with 95% for nonrefractory patients (censored logrank P = .0098).
Dr. Morschhauser said that the combination had no unexpected toxicities. Hematologic toxicities of grade 3 or greater included neutropenia in 28.4%, thrombocytopenia in 11.4%, and anemia and lymphopenia in 3.4% each.
The most common nonhematologic toxicities of all grades included infections in 62.5% of patients (grade 3 or greater in 6.8%), and asthenia in 52.3% of patients (grade 3 or greater in 2.3%). The only other grade 3 or greater toxicities were peripheral neuropathy in 1.1%, and infusion related rash in 3.4%.
Additional follow-up will be need for evaluation of the full impact of maintenance on outcomes, he added.
The study was funded by Celgene and Roche. Dr. Morschhauser disclosed receiving honoraria from and serving on advisory boards for both companies.
LUGANO, SWITZERLAND – For patients with relapsed or refractory follicular lymphoma, a pairing of lenalidomide (Revlimid) and obinutuzumab (Gazyva) appeared to be especially useful among patients who had disease progression within 24 months, based on results from a Lymphoma Academic Research Organisation trial.
Among 86 patients who were enrolled in a phase II trial and were assessable for efficacy, overall response rates (ORR) with the combination therapy, nicknamed “GALEN,” were 80.2% by 1999 International Working Group criteria, and 74.4% according to the 2007 IWG criteria, reported Franck Morschhauser, MD, PhD, of the University of Lille, France.
The rationale for this combination is the known synergy between lenalidomide and rituximab in relapsed refractory non-Hodgkin lymphomas and in the frontline setting for patients with follicular lymphoma. Obinutuzumab, a follow-on to rituximab, is a unique type II glycoengineered monoclonal antibody directed against CD20, but with increased antibody-dependent cell-mediated cytotoxicity and increased direct cytotoxicity, compared with rituximab, he explained.
In the phase Ib part of the study, researchers settled on a dose of obinutuzumab 1000 mg and lenalidomide 20 mg. Obinutuzumab was administered on days 8, 15 and 22 and lenalidomide on days 1 to 21 of each 28 day cycle. Patients were evaluated for response after three cycles and at the end of induction (after completion of 4 to 6 cycles).
The maintenance phase consisted of obinutuzumab on day 1 of every other cycle beginning with cycle 1, and lenalidomide on days 1 through 22 for cycles 7 through 18. From cycles 19 through 24, obinutuzumab was given alone on the first day of every 56-day cycle.
The overall response rate (ORR) at the end of induction according to the IWG 1999 criteria, the primary endpoint, was 80.2%, including 39.5% complete or unconfirmed complete responses.
When the same patients were assessed according to 2007 IWG criteria, the ORR rate was slightly lower, at 74.4%, but the complete or unconfirmed complete response rate was slightly higher, at 44.2%.
An analysis of responses by time to relapse showed that the ORR among 24 patients with disease progression within 24 months was 70.8%, including 33.3% complete or unconfirmed complete responses by the 1999 criteria, and 66.7% with 54.2% complete or unconfirmed complete responses by the 2007 criteria.
ORR among the 64 patients with disease progression after more than 24 months was 83.9% with 41.9% complete or unconfirmed complete responses by 1999 criteria, and 77.4% with 40.3% complete or unconfirmed complete responses by 2007 criteria. The differences between the groups with disease progression within 24 months and later relapse groups were not significant.
A subanalysis by refractory status, however, showed that the 63 nonrefractory patients fared significantly better, with 87.3% ORR and 41.3% complete or unconfirmed complete responses by 1999 criteria, and 81.0% with 49.2% complete or unconfirmed complete response rate by 2007 determinations, compared with respective rates among 23 refractory patients of 60.9%/34.8% complete or unconfirmed complete response rate and 56.5% with 30.4% complete or unconfirmed complete responses (P = .0212 by 1999 criteria, and P = .022 by 2007 criteria).
After a median follow-up of 18 months, 1-year progression-free survival (PFS) among all patients was 75.5%, and 1-year overall survival (OS) was 88.8%.
There were no significant differences in either progression-free survival or overall survival by time to relapse. Although there appeared to be a nonsignificant trend toward worse outcomes among patients with refractory vs. nonrefractory disease, there was a significantly lower 1-year overall survival rate among refractory patients, at 71.5% compared with 95% for nonrefractory patients (censored logrank P = .0098).
Dr. Morschhauser said that the combination had no unexpected toxicities. Hematologic toxicities of grade 3 or greater included neutropenia in 28.4%, thrombocytopenia in 11.4%, and anemia and lymphopenia in 3.4% each.
The most common nonhematologic toxicities of all grades included infections in 62.5% of patients (grade 3 or greater in 6.8%), and asthenia in 52.3% of patients (grade 3 or greater in 2.3%). The only other grade 3 or greater toxicities were peripheral neuropathy in 1.1%, and infusion related rash in 3.4%.
Additional follow-up will be need for evaluation of the full impact of maintenance on outcomes, he added.
The study was funded by Celgene and Roche. Dr. Morschhauser disclosed receiving honoraria from and serving on advisory boards for both companies.
LUGANO, SWITZERLAND – For patients with relapsed or refractory follicular lymphoma, a pairing of lenalidomide (Revlimid) and obinutuzumab (Gazyva) appeared to be especially useful among patients who had disease progression within 24 months, based on results from a Lymphoma Academic Research Organisation trial.
Among 86 patients who were enrolled in a phase II trial and were assessable for efficacy, overall response rates (ORR) with the combination therapy, nicknamed “GALEN,” were 80.2% by 1999 International Working Group criteria, and 74.4% according to the 2007 IWG criteria, reported Franck Morschhauser, MD, PhD, of the University of Lille, France.
The rationale for this combination is the known synergy between lenalidomide and rituximab in relapsed refractory non-Hodgkin lymphomas and in the frontline setting for patients with follicular lymphoma. Obinutuzumab, a follow-on to rituximab, is a unique type II glycoengineered monoclonal antibody directed against CD20, but with increased antibody-dependent cell-mediated cytotoxicity and increased direct cytotoxicity, compared with rituximab, he explained.
In the phase Ib part of the study, researchers settled on a dose of obinutuzumab 1000 mg and lenalidomide 20 mg. Obinutuzumab was administered on days 8, 15 and 22 and lenalidomide on days 1 to 21 of each 28 day cycle. Patients were evaluated for response after three cycles and at the end of induction (after completion of 4 to 6 cycles).
The maintenance phase consisted of obinutuzumab on day 1 of every other cycle beginning with cycle 1, and lenalidomide on days 1 through 22 for cycles 7 through 18. From cycles 19 through 24, obinutuzumab was given alone on the first day of every 56-day cycle.
The overall response rate (ORR) at the end of induction according to the IWG 1999 criteria, the primary endpoint, was 80.2%, including 39.5% complete or unconfirmed complete responses.
When the same patients were assessed according to 2007 IWG criteria, the ORR rate was slightly lower, at 74.4%, but the complete or unconfirmed complete response rate was slightly higher, at 44.2%.
An analysis of responses by time to relapse showed that the ORR among 24 patients with disease progression within 24 months was 70.8%, including 33.3% complete or unconfirmed complete responses by the 1999 criteria, and 66.7% with 54.2% complete or unconfirmed complete responses by the 2007 criteria.
ORR among the 64 patients with disease progression after more than 24 months was 83.9% with 41.9% complete or unconfirmed complete responses by 1999 criteria, and 77.4% with 40.3% complete or unconfirmed complete responses by 2007 criteria. The differences between the groups with disease progression within 24 months and later relapse groups were not significant.
A subanalysis by refractory status, however, showed that the 63 nonrefractory patients fared significantly better, with 87.3% ORR and 41.3% complete or unconfirmed complete responses by 1999 criteria, and 81.0% with 49.2% complete or unconfirmed complete response rate by 2007 determinations, compared with respective rates among 23 refractory patients of 60.9%/34.8% complete or unconfirmed complete response rate and 56.5% with 30.4% complete or unconfirmed complete responses (P = .0212 by 1999 criteria, and P = .022 by 2007 criteria).
After a median follow-up of 18 months, 1-year progression-free survival (PFS) among all patients was 75.5%, and 1-year overall survival (OS) was 88.8%.
There were no significant differences in either progression-free survival or overall survival by time to relapse. Although there appeared to be a nonsignificant trend toward worse outcomes among patients with refractory vs. nonrefractory disease, there was a significantly lower 1-year overall survival rate among refractory patients, at 71.5% compared with 95% for nonrefractory patients (censored logrank P = .0098).
Dr. Morschhauser said that the combination had no unexpected toxicities. Hematologic toxicities of grade 3 or greater included neutropenia in 28.4%, thrombocytopenia in 11.4%, and anemia and lymphopenia in 3.4% each.
The most common nonhematologic toxicities of all grades included infections in 62.5% of patients (grade 3 or greater in 6.8%), and asthenia in 52.3% of patients (grade 3 or greater in 2.3%). The only other grade 3 or greater toxicities were peripheral neuropathy in 1.1%, and infusion related rash in 3.4%.
Additional follow-up will be need for evaluation of the full impact of maintenance on outcomes, he added.
The study was funded by Celgene and Roche. Dr. Morschhauser disclosed receiving honoraria from and serving on advisory boards for both companies.
AT 14-ICML
Key clinical point: A combination of lenalidomide and obinutuzumab was safe and effective in patients with relapsed/refractory follicular lymphoma.
Major finding: The overall response rate according to 1999 International Working Group criteria was 80.2%, including 39.5% CR/CRu.
Data source: Single-arm phase II study with 86 patients evaluable for efficacy and 88 evaluable for safety.
Disclosures: The study was funded by Celgene and Roche. Dr. Morschhauser disclosed receiving honoraria from and serving on advisory boards for both companies.
Len plus anti-CD19 Mab MOR208 active against advanced DLBCL
LUGANO, SWITZERLAND – Combining lenalidomide (Revlimid) with an anti-CD19 monoclonal antibody labeled MOR208 showed promising activity in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who were ineligible for stem cell transplant and had poor prognosis, early interim results from a clinical study indicate.
Among 34 patients evaluable for response, the preliminary objective response rate (ORR) was 56%, including complete responses in 32% of patients, reported Gilles Salles, MD, PhD, of the University of Lyon, France.
MOR208 is a humanized anti-CD19 monoclonal antibody with the Fc-antibody region enhanced to improve cytotoxicity. Its mechanisms of action include natural killer cell–mediated antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and direct cytotoxicity.
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro, Dr. Salles said.
In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
In an ongoing phase II, open-label study, Dr. Salles and his colleagues are enrolling transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other NHL histological subtypes, or central nervous system lymphoma involvement are excluded.
Patients receive MOR208 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally is delivered on days 1-21 of each cycle. Patients who have stable disease or better at the end of 12 cycles can be maintained on MOR208 at the same dose on days 1 and 15.
As of the data cutoff on March 6, 2017, 44 patients had been enrolled, and 34 were evaluable for response. The median patient age was 73 years (range, 47-82 years).
At the time of the data presentation, ORR, the primary endpoint, was 56%, consisting of 32% complete responses (11 patients), 24% partial responses (8), 12% stable disease (4), and 32% of patients who either had disease progression or had not yet had a postbaseline response assessment.
The median time to response was 1.8 months, with a median time to complete response of 3.4 months. Of 19 responders, 16 continue to have a response, including 10 of 11 patients with complete responses.
The most common grade 3 or 4 hematologic toxicities were neutropenia, anemia, and thrombocytopenia. Nonhematologic toxicities of any grade included rashes in 20% of patients, pyrexia in 16%, diarrhea in 16%, asthenia in 14%, and pneumonia, bronchitis, and nausea in 11% each.
There were no reported infusion-related reactions with the antibody. In all, 27% of patients required a lenalidomide dose reduction – to 20 mg/day in 20% of patients and to 15 mg/day in 7%.
Study accrual, follow-up of patients on therapy, investigations of cell origin, and subgroup analyses are ongoing.
MorphoSys is sponsoring the study. Dr. Salles has received honoraria from Amgen, BMS, Celgene, Gilead, Janssen, Roche/Genentech, and Servier and is an advisor/consultant to many of the same companies.
LUGANO, SWITZERLAND – Combining lenalidomide (Revlimid) with an anti-CD19 monoclonal antibody labeled MOR208 showed promising activity in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who were ineligible for stem cell transplant and had poor prognosis, early interim results from a clinical study indicate.
Among 34 patients evaluable for response, the preliminary objective response rate (ORR) was 56%, including complete responses in 32% of patients, reported Gilles Salles, MD, PhD, of the University of Lyon, France.
MOR208 is a humanized anti-CD19 monoclonal antibody with the Fc-antibody region enhanced to improve cytotoxicity. Its mechanisms of action include natural killer cell–mediated antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and direct cytotoxicity.
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro, Dr. Salles said.
In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
In an ongoing phase II, open-label study, Dr. Salles and his colleagues are enrolling transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other NHL histological subtypes, or central nervous system lymphoma involvement are excluded.
Patients receive MOR208 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally is delivered on days 1-21 of each cycle. Patients who have stable disease or better at the end of 12 cycles can be maintained on MOR208 at the same dose on days 1 and 15.
As of the data cutoff on March 6, 2017, 44 patients had been enrolled, and 34 were evaluable for response. The median patient age was 73 years (range, 47-82 years).
At the time of the data presentation, ORR, the primary endpoint, was 56%, consisting of 32% complete responses (11 patients), 24% partial responses (8), 12% stable disease (4), and 32% of patients who either had disease progression or had not yet had a postbaseline response assessment.
The median time to response was 1.8 months, with a median time to complete response of 3.4 months. Of 19 responders, 16 continue to have a response, including 10 of 11 patients with complete responses.
The most common grade 3 or 4 hematologic toxicities were neutropenia, anemia, and thrombocytopenia. Nonhematologic toxicities of any grade included rashes in 20% of patients, pyrexia in 16%, diarrhea in 16%, asthenia in 14%, and pneumonia, bronchitis, and nausea in 11% each.
There were no reported infusion-related reactions with the antibody. In all, 27% of patients required a lenalidomide dose reduction – to 20 mg/day in 20% of patients and to 15 mg/day in 7%.
Study accrual, follow-up of patients on therapy, investigations of cell origin, and subgroup analyses are ongoing.
MorphoSys is sponsoring the study. Dr. Salles has received honoraria from Amgen, BMS, Celgene, Gilead, Janssen, Roche/Genentech, and Servier and is an advisor/consultant to many of the same companies.
LUGANO, SWITZERLAND – Combining lenalidomide (Revlimid) with an anti-CD19 monoclonal antibody labeled MOR208 showed promising activity in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who were ineligible for stem cell transplant and had poor prognosis, early interim results from a clinical study indicate.
Among 34 patients evaluable for response, the preliminary objective response rate (ORR) was 56%, including complete responses in 32% of patients, reported Gilles Salles, MD, PhD, of the University of Lyon, France.
MOR208 is a humanized anti-CD19 monoclonal antibody with the Fc-antibody region enhanced to improve cytotoxicity. Its mechanisms of action include natural killer cell–mediated antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and direct cytotoxicity.
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro, Dr. Salles said.
In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
In an ongoing phase II, open-label study, Dr. Salles and his colleagues are enrolling transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other NHL histological subtypes, or central nervous system lymphoma involvement are excluded.
Patients receive MOR208 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally is delivered on days 1-21 of each cycle. Patients who have stable disease or better at the end of 12 cycles can be maintained on MOR208 at the same dose on days 1 and 15.
As of the data cutoff on March 6, 2017, 44 patients had been enrolled, and 34 were evaluable for response. The median patient age was 73 years (range, 47-82 years).
At the time of the data presentation, ORR, the primary endpoint, was 56%, consisting of 32% complete responses (11 patients), 24% partial responses (8), 12% stable disease (4), and 32% of patients who either had disease progression or had not yet had a postbaseline response assessment.
The median time to response was 1.8 months, with a median time to complete response of 3.4 months. Of 19 responders, 16 continue to have a response, including 10 of 11 patients with complete responses.
The most common grade 3 or 4 hematologic toxicities were neutropenia, anemia, and thrombocytopenia. Nonhematologic toxicities of any grade included rashes in 20% of patients, pyrexia in 16%, diarrhea in 16%, asthenia in 14%, and pneumonia, bronchitis, and nausea in 11% each.
There were no reported infusion-related reactions with the antibody. In all, 27% of patients required a lenalidomide dose reduction – to 20 mg/day in 20% of patients and to 15 mg/day in 7%.
Study accrual, follow-up of patients on therapy, investigations of cell origin, and subgroup analyses are ongoing.
MorphoSys is sponsoring the study. Dr. Salles has received honoraria from Amgen, BMS, Celgene, Gilead, Janssen, Roche/Genentech, and Servier and is an advisor/consultant to many of the same companies.
AT 14-ICML
Key clinical point: A combination of the anti-CD19 monoclonal antibody MOR208 and the immunomodulator lenalidomide has shown good activity against relapsed/refractory diffuse large B-cell lymphoma.
Major finding: The preliminary objective response rate was 56%, including 32% complete responses.
Data source: An ongoing open-label phase II study with 44 patients out of a planned 80 enrolled.
Disclosures: MorphoSys is sponsoring the study. Dr. Salles has received honoraria from Amgen, BMS, Celgene, Gilead, Janssen, Roche/Genentech, and Servier and is an advisor or consultant to many of the same companies.
Hitting BTK, PI3K pays off in B-cell malignancies
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
AT 14-ICML
Key clinical point:
Major finding: The objective response rate to the combination was 94% in 18 patients with chronic lymphocytic leukemia and 79% in 14 patients with mantle cell lymphoma.
Data source: A phase I/IB dose-escalation study.
Disclosures: The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
Biosimilar rituximab approved in Europe
The European Commission (EC) has approved the Sandoz biosimilar rituximab (Rixathon®) for use in the European Economic Area.
Rixathon is approved for all indications of the reference medicine, MabThera®, including follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and immunologic diseases such as rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis.
This approval allows Rixathon to be marketed in the member states of the European Union and Iceland, Liechtenstein, and Norway, members of the European Free Trade Association.
The approval “represents a big win for patients in Europe with blood cancers or immunological diseases,” according to Carol Lynch, global head of Biopharmaceuticals at Sandoz.
“Rixathon will be one of the 5 major launches we plan in the next 4 years,” she said.
Earlier in the year, the European Medicines Agency’s Committee for Medicinal Products for Human Use had recommended marketing authorization for Rixathon.
The EC based its approval on a comprehensive development program generating analytical, preclinical, and clinical data. Clinical studies included ASSIST-RA and ASSIST-FL.
ASSIST-RA demonstrated that the biosimilar product has equivalent pharmacokinetic and pharmacodynamic profiles to the reference medicine, with no clinically meaningful differences in safety, tolerability, or immunogenicity in patients with rheumatoid arthritis.
ASSIST-FL was a phase 3 study confirming efficacy and safety. The study met its primary endpoint of equivalence in overall response rate between the biosimilar product and the reference medicine after 6 months.
ASSIST-FL also confirmed the comparable safety profiles of the 2 medicines.
Sandoz is a division of the Swiss pharmaceutical company Novartis. MabThera is a registered trademark of F. Hoffmann-La-Roche AG.
Another Sandoz biosimilar rituximab has been approved in the EU as Riximyo® under a duplicate marketing authorization. ![]()
The European Commission (EC) has approved the Sandoz biosimilar rituximab (Rixathon®) for use in the European Economic Area.
Rixathon is approved for all indications of the reference medicine, MabThera®, including follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and immunologic diseases such as rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis.
This approval allows Rixathon to be marketed in the member states of the European Union and Iceland, Liechtenstein, and Norway, members of the European Free Trade Association.
The approval “represents a big win for patients in Europe with blood cancers or immunological diseases,” according to Carol Lynch, global head of Biopharmaceuticals at Sandoz.
“Rixathon will be one of the 5 major launches we plan in the next 4 years,” she said.
Earlier in the year, the European Medicines Agency’s Committee for Medicinal Products for Human Use had recommended marketing authorization for Rixathon.
The EC based its approval on a comprehensive development program generating analytical, preclinical, and clinical data. Clinical studies included ASSIST-RA and ASSIST-FL.
ASSIST-RA demonstrated that the biosimilar product has equivalent pharmacokinetic and pharmacodynamic profiles to the reference medicine, with no clinically meaningful differences in safety, tolerability, or immunogenicity in patients with rheumatoid arthritis.
ASSIST-FL was a phase 3 study confirming efficacy and safety. The study met its primary endpoint of equivalence in overall response rate between the biosimilar product and the reference medicine after 6 months.
ASSIST-FL also confirmed the comparable safety profiles of the 2 medicines.
Sandoz is a division of the Swiss pharmaceutical company Novartis. MabThera is a registered trademark of F. Hoffmann-La-Roche AG.
Another Sandoz biosimilar rituximab has been approved in the EU as Riximyo® under a duplicate marketing authorization. ![]()
The European Commission (EC) has approved the Sandoz biosimilar rituximab (Rixathon®) for use in the European Economic Area.
Rixathon is approved for all indications of the reference medicine, MabThera®, including follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and immunologic diseases such as rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis.
This approval allows Rixathon to be marketed in the member states of the European Union and Iceland, Liechtenstein, and Norway, members of the European Free Trade Association.
The approval “represents a big win for patients in Europe with blood cancers or immunological diseases,” according to Carol Lynch, global head of Biopharmaceuticals at Sandoz.
“Rixathon will be one of the 5 major launches we plan in the next 4 years,” she said.
Earlier in the year, the European Medicines Agency’s Committee for Medicinal Products for Human Use had recommended marketing authorization for Rixathon.
The EC based its approval on a comprehensive development program generating analytical, preclinical, and clinical data. Clinical studies included ASSIST-RA and ASSIST-FL.
ASSIST-RA demonstrated that the biosimilar product has equivalent pharmacokinetic and pharmacodynamic profiles to the reference medicine, with no clinically meaningful differences in safety, tolerability, or immunogenicity in patients with rheumatoid arthritis.
ASSIST-FL was a phase 3 study confirming efficacy and safety. The study met its primary endpoint of equivalence in overall response rate between the biosimilar product and the reference medicine after 6 months.
ASSIST-FL also confirmed the comparable safety profiles of the 2 medicines.
Sandoz is a division of the Swiss pharmaceutical company Novartis. MabThera is a registered trademark of F. Hoffmann-La-Roche AG.
Another Sandoz biosimilar rituximab has been approved in the EU as Riximyo® under a duplicate marketing authorization. ![]()
CAR T cells plus ibrutinib induce CLL remissions
CHICAGO—Chimeric antigen receptor (CAR) T cells combined with ibrutinib enhance T-cell function and can induce complete remission (CR) in patients with chronic lymphocytic leukemia (CLL), researchers report.
Many CLL patients receive ibrutinib treatment, which is well tolerated, but few patients achieve CR.
Immunotherapy with anti-CD19 CAR T cells has induced CR in 25% - 45% of patients with CLL, Saar Gill, MD, of the University of Pennsylvania in Philadelphia, told Hematology Times, and these CRs tend to be durable.
So investigators conducted a pilot trial in 10 patients to test whether combining anti-CD19 CAR T cells with ibrutinib would enhance the CR rate.
Dr Gill reported the findings of the pilot trial at the ASCO 2017 Annual Meeting (abstract 7509).
The patients must have failed at least 1 regimen before ibrutinib, unless they had del(17)(p13.1) or a TP53 mutation.
T cells were lentivirally transduced to express a CAR that included humanized anti-CD19.
Patients were lymphodepleted 1 week before infusion, and ibrutinib was continued throughout the trial.
After a median follow-up of 6 months, 8 of the 9 evaluable patients show absence of CLL in the bone marrow by flow cytometry or minimal residual disease (MRD) negative, and all remain in marrow CR at last follow-up, Dr Gill said.
Radiologic responses are less clear-cut and may require longer follow-up.
“All but 1 patient achieved MRD with deep sequencing. We have deep response in the bone marrow,” Dr Gill said. He also noted that the treatment was well tolerated.
Cytokine release syndrome (CRS) developed in 9 patients: grade 1 in 2 patients, grade 2 in 6 patients, and grade 3 in 1 patient. One patient developed grade 4 tumor lysis syndrome. Treatment of CRS with the IL-6 receptor antagonist tocilizumab was not required.
There was modest residual splenomegaly in 3 of 5 patients, and adenopathy resolved in 4 of 6 patients, with progression in 1 patient.
Ibrutinib reduced CRS apparently by blocking cytokine production by T cells, said Dr Gill, adding, “The combination led to improved efficacy without increased toxicity.”
Ibrutinib may make CAR T-cell therapy more feasible.
Patients who receive ibrutinib for 6 months have a better T-cell response.
“This opens up future discussions of bringing CAR T-cell therapy earlier into CLL treatment,” said Dr Gill.
He envisions patients receiving ibrutinib for 6 months, which would allow time to manufacture T cells, and then have a T-cell infusion.
“Once patients achieve MRD, then we can discuss the possibility of stopping ibrutinib therapy,” he said.
“Most patients remain on ibrutinib, but longer follow-up may show whether remissions are sustained off ibrutinib.”
The researchers have ongoing plans to treat 25 patients with CTL19 plus ibrutinib in a continuation of this trial.
Dr Gill said longer follow-up will reveal the durability of these results “and could support evaluation of a first-line combination approach in an attempt to obviate the need for chronic therapy.” ![]()
CHICAGO—Chimeric antigen receptor (CAR) T cells combined with ibrutinib enhance T-cell function and can induce complete remission (CR) in patients with chronic lymphocytic leukemia (CLL), researchers report.
Many CLL patients receive ibrutinib treatment, which is well tolerated, but few patients achieve CR.
Immunotherapy with anti-CD19 CAR T cells has induced CR in 25% - 45% of patients with CLL, Saar Gill, MD, of the University of Pennsylvania in Philadelphia, told Hematology Times, and these CRs tend to be durable.
So investigators conducted a pilot trial in 10 patients to test whether combining anti-CD19 CAR T cells with ibrutinib would enhance the CR rate.
Dr Gill reported the findings of the pilot trial at the ASCO 2017 Annual Meeting (abstract 7509).
The patients must have failed at least 1 regimen before ibrutinib, unless they had del(17)(p13.1) or a TP53 mutation.
T cells were lentivirally transduced to express a CAR that included humanized anti-CD19.
Patients were lymphodepleted 1 week before infusion, and ibrutinib was continued throughout the trial.
After a median follow-up of 6 months, 8 of the 9 evaluable patients show absence of CLL in the bone marrow by flow cytometry or minimal residual disease (MRD) negative, and all remain in marrow CR at last follow-up, Dr Gill said.
Radiologic responses are less clear-cut and may require longer follow-up.
“All but 1 patient achieved MRD with deep sequencing. We have deep response in the bone marrow,” Dr Gill said. He also noted that the treatment was well tolerated.
Cytokine release syndrome (CRS) developed in 9 patients: grade 1 in 2 patients, grade 2 in 6 patients, and grade 3 in 1 patient. One patient developed grade 4 tumor lysis syndrome. Treatment of CRS with the IL-6 receptor antagonist tocilizumab was not required.
There was modest residual splenomegaly in 3 of 5 patients, and adenopathy resolved in 4 of 6 patients, with progression in 1 patient.
Ibrutinib reduced CRS apparently by blocking cytokine production by T cells, said Dr Gill, adding, “The combination led to improved efficacy without increased toxicity.”
Ibrutinib may make CAR T-cell therapy more feasible.
Patients who receive ibrutinib for 6 months have a better T-cell response.
“This opens up future discussions of bringing CAR T-cell therapy earlier into CLL treatment,” said Dr Gill.
He envisions patients receiving ibrutinib for 6 months, which would allow time to manufacture T cells, and then have a T-cell infusion.
“Once patients achieve MRD, then we can discuss the possibility of stopping ibrutinib therapy,” he said.
“Most patients remain on ibrutinib, but longer follow-up may show whether remissions are sustained off ibrutinib.”
The researchers have ongoing plans to treat 25 patients with CTL19 plus ibrutinib in a continuation of this trial.
Dr Gill said longer follow-up will reveal the durability of these results “and could support evaluation of a first-line combination approach in an attempt to obviate the need for chronic therapy.” ![]()
CHICAGO—Chimeric antigen receptor (CAR) T cells combined with ibrutinib enhance T-cell function and can induce complete remission (CR) in patients with chronic lymphocytic leukemia (CLL), researchers report.
Many CLL patients receive ibrutinib treatment, which is well tolerated, but few patients achieve CR.
Immunotherapy with anti-CD19 CAR T cells has induced CR in 25% - 45% of patients with CLL, Saar Gill, MD, of the University of Pennsylvania in Philadelphia, told Hematology Times, and these CRs tend to be durable.
So investigators conducted a pilot trial in 10 patients to test whether combining anti-CD19 CAR T cells with ibrutinib would enhance the CR rate.
Dr Gill reported the findings of the pilot trial at the ASCO 2017 Annual Meeting (abstract 7509).
The patients must have failed at least 1 regimen before ibrutinib, unless they had del(17)(p13.1) or a TP53 mutation.
T cells were lentivirally transduced to express a CAR that included humanized anti-CD19.
Patients were lymphodepleted 1 week before infusion, and ibrutinib was continued throughout the trial.
After a median follow-up of 6 months, 8 of the 9 evaluable patients show absence of CLL in the bone marrow by flow cytometry or minimal residual disease (MRD) negative, and all remain in marrow CR at last follow-up, Dr Gill said.
Radiologic responses are less clear-cut and may require longer follow-up.
“All but 1 patient achieved MRD with deep sequencing. We have deep response in the bone marrow,” Dr Gill said. He also noted that the treatment was well tolerated.
Cytokine release syndrome (CRS) developed in 9 patients: grade 1 in 2 patients, grade 2 in 6 patients, and grade 3 in 1 patient. One patient developed grade 4 tumor lysis syndrome. Treatment of CRS with the IL-6 receptor antagonist tocilizumab was not required.
There was modest residual splenomegaly in 3 of 5 patients, and adenopathy resolved in 4 of 6 patients, with progression in 1 patient.
Ibrutinib reduced CRS apparently by blocking cytokine production by T cells, said Dr Gill, adding, “The combination led to improved efficacy without increased toxicity.”
Ibrutinib may make CAR T-cell therapy more feasible.
Patients who receive ibrutinib for 6 months have a better T-cell response.
“This opens up future discussions of bringing CAR T-cell therapy earlier into CLL treatment,” said Dr Gill.
He envisions patients receiving ibrutinib for 6 months, which would allow time to manufacture T cells, and then have a T-cell infusion.
“Once patients achieve MRD, then we can discuss the possibility of stopping ibrutinib therapy,” he said.
“Most patients remain on ibrutinib, but longer follow-up may show whether remissions are sustained off ibrutinib.”
The researchers have ongoing plans to treat 25 patients with CTL19 plus ibrutinib in a continuation of this trial.
Dr Gill said longer follow-up will reveal the durability of these results “and could support evaluation of a first-line combination approach in an attempt to obviate the need for chronic therapy.” ![]()