User login
Smoldering myeloma progressed more rapidly in patients with elevated BMIs
An elevated body mass index appears to be a risk factor for progression of smoldering multiple myeloma, according to Wilson I. Gonsalves, MD, and his colleagues at Mayo Clinic, Rochester, Minn.
The findings, based on median follow up data of 106 months from 306 patients diagnosed with smoldering multiple myeloma from 2000-2010 at the Mayo Clinic, need to be confirmed in larger studies. Nevertheless, the results imply that patient weight is a potentially modifiable risk factor for progression from smoldering disease to multiple myeloma, Dr. Gonsalves and his colleagues wrote.
At initial evaluation, 28% of patients had myeloma defining events, such as a serum free light chain ratio greater than 100 or over 60% clonal bone marrow plasma cells. Myeloma defining events were present in 17% of patients with normal BMIs and 33% of patients with elevated BMIs, a statistically significant difference (P = .011).
When the analysis was limited to the 187 patients without myeloma-defining events at initial evaluation, the 2-year rate of progression to symptomatic multiple myeloma was 15% in those with a normal BMI and 33% in those with an elevated BMI (P = .013).
In a multivariable model, only elevated BMI (P = .004) and increasing clonal bone marrow plasma cells (P = .001) were statistically significant in predicting 2-year progression to multiple myeloma.
At last follow-up, 66% of patients had progressed to symptomatic multiple myeloma.
Dr. Gonsalves had no relationships to disclose.
The impact of body mass index on the risk of early progression of smoldering multiple myeloma to symptomatic myeloma. 2017 ASCO annual meeting. Abstract No: 8032.
mdales@frontlinemedcom.com
On Twitter @maryjodales
An elevated body mass index appears to be a risk factor for progression of smoldering multiple myeloma, according to Wilson I. Gonsalves, MD, and his colleagues at Mayo Clinic, Rochester, Minn.
The findings, based on median follow up data of 106 months from 306 patients diagnosed with smoldering multiple myeloma from 2000-2010 at the Mayo Clinic, need to be confirmed in larger studies. Nevertheless, the results imply that patient weight is a potentially modifiable risk factor for progression from smoldering disease to multiple myeloma, Dr. Gonsalves and his colleagues wrote.
At initial evaluation, 28% of patients had myeloma defining events, such as a serum free light chain ratio greater than 100 or over 60% clonal bone marrow plasma cells. Myeloma defining events were present in 17% of patients with normal BMIs and 33% of patients with elevated BMIs, a statistically significant difference (P = .011).
When the analysis was limited to the 187 patients without myeloma-defining events at initial evaluation, the 2-year rate of progression to symptomatic multiple myeloma was 15% in those with a normal BMI and 33% in those with an elevated BMI (P = .013).
In a multivariable model, only elevated BMI (P = .004) and increasing clonal bone marrow plasma cells (P = .001) were statistically significant in predicting 2-year progression to multiple myeloma.
At last follow-up, 66% of patients had progressed to symptomatic multiple myeloma.
Dr. Gonsalves had no relationships to disclose.
The impact of body mass index on the risk of early progression of smoldering multiple myeloma to symptomatic myeloma. 2017 ASCO annual meeting. Abstract No: 8032.
mdales@frontlinemedcom.com
On Twitter @maryjodales
An elevated body mass index appears to be a risk factor for progression of smoldering multiple myeloma, according to Wilson I. Gonsalves, MD, and his colleagues at Mayo Clinic, Rochester, Minn.
The findings, based on median follow up data of 106 months from 306 patients diagnosed with smoldering multiple myeloma from 2000-2010 at the Mayo Clinic, need to be confirmed in larger studies. Nevertheless, the results imply that patient weight is a potentially modifiable risk factor for progression from smoldering disease to multiple myeloma, Dr. Gonsalves and his colleagues wrote.
At initial evaluation, 28% of patients had myeloma defining events, such as a serum free light chain ratio greater than 100 or over 60% clonal bone marrow plasma cells. Myeloma defining events were present in 17% of patients with normal BMIs and 33% of patients with elevated BMIs, a statistically significant difference (P = .011).
When the analysis was limited to the 187 patients without myeloma-defining events at initial evaluation, the 2-year rate of progression to symptomatic multiple myeloma was 15% in those with a normal BMI and 33% in those with an elevated BMI (P = .013).
In a multivariable model, only elevated BMI (P = .004) and increasing clonal bone marrow plasma cells (P = .001) were statistically significant in predicting 2-year progression to multiple myeloma.
At last follow-up, 66% of patients had progressed to symptomatic multiple myeloma.
Dr. Gonsalves had no relationships to disclose.
The impact of body mass index on the risk of early progression of smoldering multiple myeloma to symptomatic myeloma. 2017 ASCO annual meeting. Abstract No: 8032.
mdales@frontlinemedcom.com
On Twitter @maryjodales
FROM ASCO 2017
Key clinical point:
Major finding: The 2-year rate of progression from smoldering disease to symptomatic multiple myeloma was 16% in patients with a normal BMI and 42% in patients with an elevated BMI (P less than 0.0001).
Data source: Median follow up data of 106 months from 306 patients diagnosed with smoldering multiple myeloma during 2000-2010 at the Mayo Clinic.
Disclosures: Dr. Gonsalves had no relationships to disclose.
Citation: The impact of body mass index on the risk of early progression of smoldering multiple myeloma to symptomatic myeloma. 2017 ASCO annual meeting. Abstract No: 8032.
Myeloma patients who get solid tumor cancers do as well as other cancer patients
With improved treatment, patients with multiple myeloma are surviving long enough to develop other cancers, Jorge J. Castillo, MD, and Adam J. Olszewski, MD, reported in a poster to be presented at the annual meeting of the American Society of Clinical Oncology.
The good news is that myeloma patients, when diagnosed with a subsequent solid tumor, are just as likely to respond to treatment and do just as well as patients without myeloma, according to Dr. Castillo of Dana-Farber Cancer Institute, Boston, and Dr. Olszewski of Alpert Medical School of Brown University, Providence, R.I.
They based their conclusion on Surveillance, Epidemiology, and End Results (SEER) data for patients diagnosed with six common cancers from 2004-2013.
“Among them, we identified [nearly 1,300] myeloma survivors, and we matched each to 50 randomly sampled controls with the same cancer by age, sex, race, and year of diagnosis. We then compared [cancer specific survival], cumulative incidence function (CIF) for death from the non-myeloma index cancer, and whether patients had surgery for non-metastastic, stage-matched tumors only,” the researchers wrote in their abstract.
They did analyses for breast, lung, prostate, colorectal, melanoma, and bladder cancers. The median time from diagnosis of myeloma to diagnosis of the second ranged from 35 months (bladder [133 myeloma patients] and lung [286 myeloma patients] cancers) to 50 months (melanoma [140 myeloma patients]). The median time after myeloma diagnosis was 40 months for those patients who developed breast, prostate, or colorectal cancers.
In the comparisons, myeloma survivors were significantly older (P less than .001) than patients initially diagnosed with the same respective cancers. In the case-control analysis, breast (P = .002) and lung cancers (P = .003) were more often diagnosed at an early stage among myeloma survivors.
The hazard ratio (HR) for cancer-specific survival for 189 myeloma patients diagnosed with breast cancer as compared to other breast cancer patients, for example, was 0.99, 95% confidence interval (CI) 0.61-1.61. The HR for the cumulative incidence function of cancer death was 0.82, 95% CI 0.50-1.35.
Myeloma patients were no less likely than were case-control subjects to have surgery for their cancers, with the exception of the 330 myeloma patients who developed prostate cancer (odds ratio, 0.59, 95% CI, 0.44-0.81).
Cancer-specific survival significantly differed (P less than .05) only for lung cancer, and was better among the 286 myeloma patients with lung cancer even when stratified by stage (HR 0.64, 95% CI 0.54-0.75). For cumulative incidence function of cancer death for lung cancer, the hazard ratio was 0.52 (95% CI 0.44-0.61). Better outcomes in lung cancer are not fully explained by earlier detection, suggesting a biological difference, the researchers reported.
Cumulative incidence function of cancer death was significantly lower for myeloma patients with lung and colorectal cancers.
Dr. Castillo disclosed honoraria from Celgene and Janssen; a consulting or advisory role with Biogen, Otsuka, and Pharmacyclics; and institutional research funding from Abbvie, Gilead Sciences, Millennium, and Pharmacyclics. Dr. Olszewski disclosed institutional research funding from Genentech, Incyte, and TG Therapeutics.
Citation: Outcomes of secondary cancers among myeloma survivors. 2017 ASCO annual meeting. Abstract No. 8043.
mdales@frontlinemedcom.com
On Twitter @maryjodales
With improved treatment, patients with multiple myeloma are surviving long enough to develop other cancers, Jorge J. Castillo, MD, and Adam J. Olszewski, MD, reported in a poster to be presented at the annual meeting of the American Society of Clinical Oncology.
The good news is that myeloma patients, when diagnosed with a subsequent solid tumor, are just as likely to respond to treatment and do just as well as patients without myeloma, according to Dr. Castillo of Dana-Farber Cancer Institute, Boston, and Dr. Olszewski of Alpert Medical School of Brown University, Providence, R.I.
They based their conclusion on Surveillance, Epidemiology, and End Results (SEER) data for patients diagnosed with six common cancers from 2004-2013.
“Among them, we identified [nearly 1,300] myeloma survivors, and we matched each to 50 randomly sampled controls with the same cancer by age, sex, race, and year of diagnosis. We then compared [cancer specific survival], cumulative incidence function (CIF) for death from the non-myeloma index cancer, and whether patients had surgery for non-metastastic, stage-matched tumors only,” the researchers wrote in their abstract.
They did analyses for breast, lung, prostate, colorectal, melanoma, and bladder cancers. The median time from diagnosis of myeloma to diagnosis of the second ranged from 35 months (bladder [133 myeloma patients] and lung [286 myeloma patients] cancers) to 50 months (melanoma [140 myeloma patients]). The median time after myeloma diagnosis was 40 months for those patients who developed breast, prostate, or colorectal cancers.
In the comparisons, myeloma survivors were significantly older (P less than .001) than patients initially diagnosed with the same respective cancers. In the case-control analysis, breast (P = .002) and lung cancers (P = .003) were more often diagnosed at an early stage among myeloma survivors.
The hazard ratio (HR) for cancer-specific survival for 189 myeloma patients diagnosed with breast cancer as compared to other breast cancer patients, for example, was 0.99, 95% confidence interval (CI) 0.61-1.61. The HR for the cumulative incidence function of cancer death was 0.82, 95% CI 0.50-1.35.
Myeloma patients were no less likely than were case-control subjects to have surgery for their cancers, with the exception of the 330 myeloma patients who developed prostate cancer (odds ratio, 0.59, 95% CI, 0.44-0.81).
Cancer-specific survival significantly differed (P less than .05) only for lung cancer, and was better among the 286 myeloma patients with lung cancer even when stratified by stage (HR 0.64, 95% CI 0.54-0.75). For cumulative incidence function of cancer death for lung cancer, the hazard ratio was 0.52 (95% CI 0.44-0.61). Better outcomes in lung cancer are not fully explained by earlier detection, suggesting a biological difference, the researchers reported.
Cumulative incidence function of cancer death was significantly lower for myeloma patients with lung and colorectal cancers.
Dr. Castillo disclosed honoraria from Celgene and Janssen; a consulting or advisory role with Biogen, Otsuka, and Pharmacyclics; and institutional research funding from Abbvie, Gilead Sciences, Millennium, and Pharmacyclics. Dr. Olszewski disclosed institutional research funding from Genentech, Incyte, and TG Therapeutics.
Citation: Outcomes of secondary cancers among myeloma survivors. 2017 ASCO annual meeting. Abstract No. 8043.
mdales@frontlinemedcom.com
On Twitter @maryjodales
With improved treatment, patients with multiple myeloma are surviving long enough to develop other cancers, Jorge J. Castillo, MD, and Adam J. Olszewski, MD, reported in a poster to be presented at the annual meeting of the American Society of Clinical Oncology.
The good news is that myeloma patients, when diagnosed with a subsequent solid tumor, are just as likely to respond to treatment and do just as well as patients without myeloma, according to Dr. Castillo of Dana-Farber Cancer Institute, Boston, and Dr. Olszewski of Alpert Medical School of Brown University, Providence, R.I.
They based their conclusion on Surveillance, Epidemiology, and End Results (SEER) data for patients diagnosed with six common cancers from 2004-2013.
“Among them, we identified [nearly 1,300] myeloma survivors, and we matched each to 50 randomly sampled controls with the same cancer by age, sex, race, and year of diagnosis. We then compared [cancer specific survival], cumulative incidence function (CIF) for death from the non-myeloma index cancer, and whether patients had surgery for non-metastastic, stage-matched tumors only,” the researchers wrote in their abstract.
They did analyses for breast, lung, prostate, colorectal, melanoma, and bladder cancers. The median time from diagnosis of myeloma to diagnosis of the second ranged from 35 months (bladder [133 myeloma patients] and lung [286 myeloma patients] cancers) to 50 months (melanoma [140 myeloma patients]). The median time after myeloma diagnosis was 40 months for those patients who developed breast, prostate, or colorectal cancers.
In the comparisons, myeloma survivors were significantly older (P less than .001) than patients initially diagnosed with the same respective cancers. In the case-control analysis, breast (P = .002) and lung cancers (P = .003) were more often diagnosed at an early stage among myeloma survivors.
The hazard ratio (HR) for cancer-specific survival for 189 myeloma patients diagnosed with breast cancer as compared to other breast cancer patients, for example, was 0.99, 95% confidence interval (CI) 0.61-1.61. The HR for the cumulative incidence function of cancer death was 0.82, 95% CI 0.50-1.35.
Myeloma patients were no less likely than were case-control subjects to have surgery for their cancers, with the exception of the 330 myeloma patients who developed prostate cancer (odds ratio, 0.59, 95% CI, 0.44-0.81).
Cancer-specific survival significantly differed (P less than .05) only for lung cancer, and was better among the 286 myeloma patients with lung cancer even when stratified by stage (HR 0.64, 95% CI 0.54-0.75). For cumulative incidence function of cancer death for lung cancer, the hazard ratio was 0.52 (95% CI 0.44-0.61). Better outcomes in lung cancer are not fully explained by earlier detection, suggesting a biological difference, the researchers reported.
Cumulative incidence function of cancer death was significantly lower for myeloma patients with lung and colorectal cancers.
Dr. Castillo disclosed honoraria from Celgene and Janssen; a consulting or advisory role with Biogen, Otsuka, and Pharmacyclics; and institutional research funding from Abbvie, Gilead Sciences, Millennium, and Pharmacyclics. Dr. Olszewski disclosed institutional research funding from Genentech, Incyte, and TG Therapeutics.
Citation: Outcomes of secondary cancers among myeloma survivors. 2017 ASCO annual meeting. Abstract No. 8043.
mdales@frontlinemedcom.com
On Twitter @maryjodales
FROM 2017 ASCO ANNUAL MEETING
Key clinical point:
Major finding: Cancer-specific survival significantly differed (P less than .05) only for lung cancer, and was better among the 286 myeloma patients with lung cancer even when stratified by stage (HR, 0.64, 95% CI 0.54-0.75).
Data source: Surveillance, Epidemiology, and End Results (SEER) data for patients diagnosed with six common cancers from 2004-2013.
Disclosures: Dr. Castillo disclosed honoraria from Celgene and Janssen; a consulting or advisory role with Biogen, Otsuka, and Pharmacyclics; and institutional research funding from Abbvie, Gilead Sciences, Millennium, and Pharmacyclics. Dr. Olszewski disclosed institutional research funding from Genentech, Incyte, and TG Therapeutics.
Citation: Outcomes of secondary cancers among myeloma survivors. 2017 ASCO annual meeting. Abstract No. 8043.
Lenalidomide maintenance boosted depth of response in myeloma patients
Lenalidomide maintenance therapy further improved depth of response in newly diagnosed, transplant-eligible patients with multiple myeloma in the EMN02/HO95 trial, based on the abstract of a poster to be presented at the annual meeting of the American Society of Clinical Oncology.
The study results also show that using multiparameter flow cytometry to monitor minimal residual disease (MRD) was predictive of patient outcome. A high-risk cytogenetic profile – defined as having del17, translocation (4;14), or translocation (14;16) – was the most important prognostic factor in MRD-positive patients, according to Stefania Oliva, MD, of the University of Torino [Italy] and her colleagues.
At 3 years, progression-free survival was 50% in MRD-positive patients and 77% in MRD-negative patients (hazard ratio, 2.87; P less than .001). High-risk cytogenetics was the most important risk factor (HR, 9.87; interaction-P = .001). Further, 48% of patients who had MRD before maintenance therapy and had a second evaluation for minimal residual disease after at least 1 year of lenalidomide therapy became MRD negative.
The trial (NCT01208766) participants were no older than age 65 years and received received bortezomib-cyclophosphamide-dexamethasone (VCD) induction therapy, then bortezomib-melphalan-prednisone (VMP) or high-dose melphalan intensification therapy followed by stem cell transplant, and subsequently bortezomib-lenalidomide-dexamethasone (VRD) consolidation therapy or no consolidation therapy, followed by lenalidomide maintenance therapy.
Of 316 patients who were evaluable before maintenance therapy, 18% had International Staging System stage III disease (beta-2 microglobulin of 5.5 mg/L or greater) and 22% had a high-risk cytogenetic profile.
For intensification therapy, 63% had received high-dose melphalan and 37% got VMP; thereafter 51% had received VRD. Nearly two-thirds of the 76% of patients who were MRD negative got high-dose melphalan, with a median follow-up of 30 months from MRD enrollment.
Patients who had at least a very good partial response underwent minimal residual disease evaluation before starting maintenance therapy and then every 6-12 months during maintenance therapy. Multiparameter flow cytometry was performed on bone marrow according to Euroflow-based methods (eight colors, two tubes) with a sensitivity of 10-5, and quality checks were performed to compare sensitivity and to show correlation between protocols.
Dr. Oliva disclosed receiving honoraria from Celgene and Takeda.
Citation: Minimal residual disease (MRD) monitoring by multiparameter flow cytometry (MFC) in newly diagnosed transplant eligible multiple myeloma (MM) patients: Results from the EMN02/HO95 phase 3 trial. 2017 ASCO annual meeting. Abstract No: 8011
mdales@frontlinemedcom.com
On Twitter @maryjodales
Lenalidomide maintenance therapy further improved depth of response in newly diagnosed, transplant-eligible patients with multiple myeloma in the EMN02/HO95 trial, based on the abstract of a poster to be presented at the annual meeting of the American Society of Clinical Oncology.
The study results also show that using multiparameter flow cytometry to monitor minimal residual disease (MRD) was predictive of patient outcome. A high-risk cytogenetic profile – defined as having del17, translocation (4;14), or translocation (14;16) – was the most important prognostic factor in MRD-positive patients, according to Stefania Oliva, MD, of the University of Torino [Italy] and her colleagues.
At 3 years, progression-free survival was 50% in MRD-positive patients and 77% in MRD-negative patients (hazard ratio, 2.87; P less than .001). High-risk cytogenetics was the most important risk factor (HR, 9.87; interaction-P = .001). Further, 48% of patients who had MRD before maintenance therapy and had a second evaluation for minimal residual disease after at least 1 year of lenalidomide therapy became MRD negative.
The trial (NCT01208766) participants were no older than age 65 years and received received bortezomib-cyclophosphamide-dexamethasone (VCD) induction therapy, then bortezomib-melphalan-prednisone (VMP) or high-dose melphalan intensification therapy followed by stem cell transplant, and subsequently bortezomib-lenalidomide-dexamethasone (VRD) consolidation therapy or no consolidation therapy, followed by lenalidomide maintenance therapy.
Of 316 patients who were evaluable before maintenance therapy, 18% had International Staging System stage III disease (beta-2 microglobulin of 5.5 mg/L or greater) and 22% had a high-risk cytogenetic profile.
For intensification therapy, 63% had received high-dose melphalan and 37% got VMP; thereafter 51% had received VRD. Nearly two-thirds of the 76% of patients who were MRD negative got high-dose melphalan, with a median follow-up of 30 months from MRD enrollment.
Patients who had at least a very good partial response underwent minimal residual disease evaluation before starting maintenance therapy and then every 6-12 months during maintenance therapy. Multiparameter flow cytometry was performed on bone marrow according to Euroflow-based methods (eight colors, two tubes) with a sensitivity of 10-5, and quality checks were performed to compare sensitivity and to show correlation between protocols.
Dr. Oliva disclosed receiving honoraria from Celgene and Takeda.
Citation: Minimal residual disease (MRD) monitoring by multiparameter flow cytometry (MFC) in newly diagnosed transplant eligible multiple myeloma (MM) patients: Results from the EMN02/HO95 phase 3 trial. 2017 ASCO annual meeting. Abstract No: 8011
mdales@frontlinemedcom.com
On Twitter @maryjodales
Lenalidomide maintenance therapy further improved depth of response in newly diagnosed, transplant-eligible patients with multiple myeloma in the EMN02/HO95 trial, based on the abstract of a poster to be presented at the annual meeting of the American Society of Clinical Oncology.
The study results also show that using multiparameter flow cytometry to monitor minimal residual disease (MRD) was predictive of patient outcome. A high-risk cytogenetic profile – defined as having del17, translocation (4;14), or translocation (14;16) – was the most important prognostic factor in MRD-positive patients, according to Stefania Oliva, MD, of the University of Torino [Italy] and her colleagues.
At 3 years, progression-free survival was 50% in MRD-positive patients and 77% in MRD-negative patients (hazard ratio, 2.87; P less than .001). High-risk cytogenetics was the most important risk factor (HR, 9.87; interaction-P = .001). Further, 48% of patients who had MRD before maintenance therapy and had a second evaluation for minimal residual disease after at least 1 year of lenalidomide therapy became MRD negative.
The trial (NCT01208766) participants were no older than age 65 years and received received bortezomib-cyclophosphamide-dexamethasone (VCD) induction therapy, then bortezomib-melphalan-prednisone (VMP) or high-dose melphalan intensification therapy followed by stem cell transplant, and subsequently bortezomib-lenalidomide-dexamethasone (VRD) consolidation therapy or no consolidation therapy, followed by lenalidomide maintenance therapy.
Of 316 patients who were evaluable before maintenance therapy, 18% had International Staging System stage III disease (beta-2 microglobulin of 5.5 mg/L or greater) and 22% had a high-risk cytogenetic profile.
For intensification therapy, 63% had received high-dose melphalan and 37% got VMP; thereafter 51% had received VRD. Nearly two-thirds of the 76% of patients who were MRD negative got high-dose melphalan, with a median follow-up of 30 months from MRD enrollment.
Patients who had at least a very good partial response underwent minimal residual disease evaluation before starting maintenance therapy and then every 6-12 months during maintenance therapy. Multiparameter flow cytometry was performed on bone marrow according to Euroflow-based methods (eight colors, two tubes) with a sensitivity of 10-5, and quality checks were performed to compare sensitivity and to show correlation between protocols.
Dr. Oliva disclosed receiving honoraria from Celgene and Takeda.
Citation: Minimal residual disease (MRD) monitoring by multiparameter flow cytometry (MFC) in newly diagnosed transplant eligible multiple myeloma (MM) patients: Results from the EMN02/HO95 phase 3 trial. 2017 ASCO annual meeting. Abstract No: 8011
mdales@frontlinemedcom.com
On Twitter @maryjodales
FROM 2017 ASCO ANNUAL MEETING
Key clinical point:
Major finding: 48% of patients who had minimal residual disease before maintenance therapy and had a second evaluation for MRD after at least 1 year of lenalidomide therapy became MRD negative.
Data source: A 3-year study of 316 patients who were evaluable before maintenance therapy in the EMN02/HO95 trial.
Disclosures: Dr. Oliva disclosed receiving honoraria from Celgene and Takeda.
Citation: Minimal residual disease (MRD) monitoring by multiparameter flow cytometry (MFC) in newly diagnosed transplant eligible multiple myeloma (MM) patients: Results from the EMN02/HO95 phase 3 trial. 2017 ASCO annual meeting. Abstract No: 8011
FDA grants priority review to NDA for copanlisib
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for copanlisib, an intravenous PI3K inhibitor.
The NDA is for copanlisib as a treatment for patients with relapsed or refractory follicular lymphoma (FL) who have received at least 2 prior therapies.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The application for copanlisib is supported by data from the CHRONOS-1 trial. This phase 2 trial enrolled 141 patients with relapsed/refractory, indolent non-Hodgkin lymphoma. Most of these patients had FL (n=104).
In all patients, copanlisib produced an objective response rate of 59.2%, with a complete response rate of 12%. The median duration of response exceeded 98 weeks.
In the FL subset, copanlisib produced an overall response rate of 58.7%, with a complete response rate of 14.4%. The median duration of response exceeded 52 weeks.
In the entire cohort, there were 3 deaths considered related to copanlisib.
The most common treatment-related adverse events were transient hyperglycemia (all grades: 49%/grade 3-4: 40%) and hypertension (all grades: 29%/grade 3: 23%).
“Patients with relapsed or refractory follicular lymphoma have a poor prognosis, and new treatment options which are well tolerated and effective are needed to prolong progression-free survival and improve quality of life for these patients,” said Martin Dreyling, MD, a professor at the University of Munich Hospital (Grosshadern) in Germany and lead investigator of the CHRONOS-1 study.
“Based on the CHRONOS-1 results, where copanlisib showed durable efficacy with a manageable and distinct safety profile, the compound may have the potential to address this unmet medical need.”
Data from CHRONOS-1 were presented at the AACR Annual Meeting 2017.
Data from the FL subset of the trial are scheduled to be presented at the 2017 ASCO Annual Meeting in June.
Copanlisib is being developed by Bayer. The compound has fast track and orphan drug designations from the FDA.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the NDA or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for copanlisib, an intravenous PI3K inhibitor.
The NDA is for copanlisib as a treatment for patients with relapsed or refractory follicular lymphoma (FL) who have received at least 2 prior therapies.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The application for copanlisib is supported by data from the CHRONOS-1 trial. This phase 2 trial enrolled 141 patients with relapsed/refractory, indolent non-Hodgkin lymphoma. Most of these patients had FL (n=104).
In all patients, copanlisib produced an objective response rate of 59.2%, with a complete response rate of 12%. The median duration of response exceeded 98 weeks.
In the FL subset, copanlisib produced an overall response rate of 58.7%, with a complete response rate of 14.4%. The median duration of response exceeded 52 weeks.
In the entire cohort, there were 3 deaths considered related to copanlisib.
The most common treatment-related adverse events were transient hyperglycemia (all grades: 49%/grade 3-4: 40%) and hypertension (all grades: 29%/grade 3: 23%).
“Patients with relapsed or refractory follicular lymphoma have a poor prognosis, and new treatment options which are well tolerated and effective are needed to prolong progression-free survival and improve quality of life for these patients,” said Martin Dreyling, MD, a professor at the University of Munich Hospital (Grosshadern) in Germany and lead investigator of the CHRONOS-1 study.
“Based on the CHRONOS-1 results, where copanlisib showed durable efficacy with a manageable and distinct safety profile, the compound may have the potential to address this unmet medical need.”
Data from CHRONOS-1 were presented at the AACR Annual Meeting 2017.
Data from the FL subset of the trial are scheduled to be presented at the 2017 ASCO Annual Meeting in June.
Copanlisib is being developed by Bayer. The compound has fast track and orphan drug designations from the FDA.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the NDA or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for copanlisib, an intravenous PI3K inhibitor.
The NDA is for copanlisib as a treatment for patients with relapsed or refractory follicular lymphoma (FL) who have received at least 2 prior therapies.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The application for copanlisib is supported by data from the CHRONOS-1 trial. This phase 2 trial enrolled 141 patients with relapsed/refractory, indolent non-Hodgkin lymphoma. Most of these patients had FL (n=104).
In all patients, copanlisib produced an objective response rate of 59.2%, with a complete response rate of 12%. The median duration of response exceeded 98 weeks.
In the FL subset, copanlisib produced an overall response rate of 58.7%, with a complete response rate of 14.4%. The median duration of response exceeded 52 weeks.
In the entire cohort, there were 3 deaths considered related to copanlisib.
The most common treatment-related adverse events were transient hyperglycemia (all grades: 49%/grade 3-4: 40%) and hypertension (all grades: 29%/grade 3: 23%).
“Patients with relapsed or refractory follicular lymphoma have a poor prognosis, and new treatment options which are well tolerated and effective are needed to prolong progression-free survival and improve quality of life for these patients,” said Martin Dreyling, MD, a professor at the University of Munich Hospital (Grosshadern) in Germany and lead investigator of the CHRONOS-1 study.
“Based on the CHRONOS-1 results, where copanlisib showed durable efficacy with a manageable and distinct safety profile, the compound may have the potential to address this unmet medical need.”
Data from CHRONOS-1 were presented at the AACR Annual Meeting 2017.
Data from the FL subset of the trial are scheduled to be presented at the 2017 ASCO Annual Meeting in June.
Copanlisib is being developed by Bayer. The compound has fast track and orphan drug designations from the FDA.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the NDA or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
Compound could treat lymphoma, myeloma
A nucleoside analog has shown potential for treating primary effusion lymphoma (PEL) and multiple myeloma (MM), according to researchers.
The compound, 6-ethylthioinosine (6-ETI), killed both PEL and MM cells in vitro.
6-ETI also reduced tumor burden and prolonged survival in mouse models of MM and PEL.
Ethel Cesarman, MD, PhD, of Weill Cornell Medicine in New York, New York, and her colleagues conducted this research and disclosed their results in the Journal of Clinical Investigation.
The researchers identified 6-ETI via high-throughput screening, and they initially tested the compound in PEL cell lines. 6-ETI induced necrosis, apoptosis, and autophagy in these cells.
In a xenograft model of PEL, mice treated with 6-ETI experienced “striking and immediate regression of the implanted xenograft within 3 days of treatment,” according to the researchers.
In addition, mice that received 6-ETI had significantly longer progression-free and overall survival than control mice (P<0.0001 for both), and the researchers said there were no obvious toxicities from the treatment.
Looking into the mechanism of 6-ETI, the researchers found that adenosine kinase (ADK) is required to phosphorylate and activate the compound, and PEL cells have high levels of ADK.
Because PEL cells closely resemble plasma cells, the researchers theorized that MM might produce high levels of ADK as well. Experiments in MM cells proved this theory correct.
So the researchers tested 6-ETI in MM cell lines and samples from MM patients. The compound induced apoptosis and autophagy, and it activated a DNA damage response in MM cells.
In a mouse model of MM, 6-ETI treatment significantly reduced tumor burden but did not result in weight loss. And treated mice had significantly longer overall survival than control mice (P<0.005).
Dr Cesarman and her colleagues are now trying to better understand how 6-ETI works and determine what other cancers expressing high levels of ADK might respond to the drug.
“This compound could provide a much-needed approach to treat people with some forms of plasma malignancies as well as other cancers that express ADK,” Dr Cesarman said. ![]()
A nucleoside analog has shown potential for treating primary effusion lymphoma (PEL) and multiple myeloma (MM), according to researchers.
The compound, 6-ethylthioinosine (6-ETI), killed both PEL and MM cells in vitro.
6-ETI also reduced tumor burden and prolonged survival in mouse models of MM and PEL.
Ethel Cesarman, MD, PhD, of Weill Cornell Medicine in New York, New York, and her colleagues conducted this research and disclosed their results in the Journal of Clinical Investigation.
The researchers identified 6-ETI via high-throughput screening, and they initially tested the compound in PEL cell lines. 6-ETI induced necrosis, apoptosis, and autophagy in these cells.
In a xenograft model of PEL, mice treated with 6-ETI experienced “striking and immediate regression of the implanted xenograft within 3 days of treatment,” according to the researchers.
In addition, mice that received 6-ETI had significantly longer progression-free and overall survival than control mice (P<0.0001 for both), and the researchers said there were no obvious toxicities from the treatment.
Looking into the mechanism of 6-ETI, the researchers found that adenosine kinase (ADK) is required to phosphorylate and activate the compound, and PEL cells have high levels of ADK.
Because PEL cells closely resemble plasma cells, the researchers theorized that MM might produce high levels of ADK as well. Experiments in MM cells proved this theory correct.
So the researchers tested 6-ETI in MM cell lines and samples from MM patients. The compound induced apoptosis and autophagy, and it activated a DNA damage response in MM cells.
In a mouse model of MM, 6-ETI treatment significantly reduced tumor burden but did not result in weight loss. And treated mice had significantly longer overall survival than control mice (P<0.005).
Dr Cesarman and her colleagues are now trying to better understand how 6-ETI works and determine what other cancers expressing high levels of ADK might respond to the drug.
“This compound could provide a much-needed approach to treat people with some forms of plasma malignancies as well as other cancers that express ADK,” Dr Cesarman said. ![]()
A nucleoside analog has shown potential for treating primary effusion lymphoma (PEL) and multiple myeloma (MM), according to researchers.
The compound, 6-ethylthioinosine (6-ETI), killed both PEL and MM cells in vitro.
6-ETI also reduced tumor burden and prolonged survival in mouse models of MM and PEL.
Ethel Cesarman, MD, PhD, of Weill Cornell Medicine in New York, New York, and her colleagues conducted this research and disclosed their results in the Journal of Clinical Investigation.
The researchers identified 6-ETI via high-throughput screening, and they initially tested the compound in PEL cell lines. 6-ETI induced necrosis, apoptosis, and autophagy in these cells.
In a xenograft model of PEL, mice treated with 6-ETI experienced “striking and immediate regression of the implanted xenograft within 3 days of treatment,” according to the researchers.
In addition, mice that received 6-ETI had significantly longer progression-free and overall survival than control mice (P<0.0001 for both), and the researchers said there were no obvious toxicities from the treatment.
Looking into the mechanism of 6-ETI, the researchers found that adenosine kinase (ADK) is required to phosphorylate and activate the compound, and PEL cells have high levels of ADK.
Because PEL cells closely resemble plasma cells, the researchers theorized that MM might produce high levels of ADK as well. Experiments in MM cells proved this theory correct.
So the researchers tested 6-ETI in MM cell lines and samples from MM patients. The compound induced apoptosis and autophagy, and it activated a DNA damage response in MM cells.
In a mouse model of MM, 6-ETI treatment significantly reduced tumor burden but did not result in weight loss. And treated mice had significantly longer overall survival than control mice (P<0.005).
Dr Cesarman and her colleagues are now trying to better understand how 6-ETI works and determine what other cancers expressing high levels of ADK might respond to the drug.
“This compound could provide a much-needed approach to treat people with some forms of plasma malignancies as well as other cancers that express ADK,” Dr Cesarman said. ![]()
Intensive chemo upfront means DHL patients can skip HSCT
A new study suggests that patients with double-hit lymphoma (DHL) in first remission only benefit from an autologous hematopoietic stem cell transplant (auto-HSCT) if they received standard frontline chemotherapy.
Researchers looked at long-term outcomes for DHL patients who achieved remission and, overall, found that auto-HSCT did not significantly prolong remission or survival.
However, patients who received standard chemotherapy as frontline treatment did appear to benefit from auto-HSCT, as these patients had worse outcomes than patients who received intensive frontline chemotherapy.
This finding led the researchers to recommend that DHL patients receive intensive chemotherapy upfront and forgo subsequent auto-HSCT.
Daniel J. Landsburg, MD, of the University of Pennsylvania in Philadelphia, and his colleagues made these recommendations in the Journal of Clinical Oncology.
“A major dilemma for oncologists who treat [DHL] was whether or not to recommend the potentially harmful therapy of auto-[H]SCT to patients with this disease as a strategy to help keep them in remission,” Dr Landsburg said.
To gain some insight into the issue, Dr Landsburg and his colleagues looked at data on 159 patients from 19 academic medical centers across the US.
Patients were diagnosed with DHL between 2006 and 2015, and all achieved remission following frontline chemotherapy.
Thirty-five patients received standard frontline therapy—R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
The remaining patients received intensive frontline chemotherapy:
- 81 received DA-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab)
- 32 received R-hyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine)
- 11 received R-CODOX-M/IVAC (rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate/ifosfamide, etoposide, high-dose cytarabine).
Sixty-two patients underwent auto-HSCT, and 97 patients did not. There were no significant differences between these 2 patient groups at baseline.
“Our result is not explained by differences in patients’ overall health or disease features,” Dr Landsburg said. “The transplant and non-transplant arms of this study were very well-matched.”
Relapse and survival
For the entire patient cohort, the 3-year relapse-free survival (RFS) rate was 80%, and the 3-year overall survival (OS) rate was 87%.
There was no significant difference in RFS or OS between patients who underwent auto-HSCT and those who did not.
The RFS rate was 89% in patients who underwent auto-HSCT and 75% in patients who did not (P=0.12). The OS rate was 91% and 85%, respectively (P=0.74).
“Once these patients achieve remission, the data show they are likely to stay in remission,” Dr Landsburg said.
“In the absence of a large, randomized, controlled trial, which would be very challenging to carry out in this case, this is the best evidence we have, and it shows there’s no clear benefit to these patients undergoing auto-[H]SCT.”
Impact of frontline therapy
Patients who received R-CHOP upfront had worse RFS and OS than those who received intensive chemotherapy, although the OS difference was not significant.
RFS rates were 56% in patients who received R-CHOP, 88% in those who received DA-EPOCH-R, 87% in those who received R-hyperCVAD, and 91% in those who received R-CODOX-M/IVAC (P=0.003).
OS rates were 77% in patients who received R-CHOP, 87% in those who received DA-EPOCH-R, 90% in those who received R-hyperCVAD, and 100% in those who received R-CODOX-M/IVAC, respectively (P=0.36).
When the 3 intensive regimens were combined, the RFS rate was 88% (vs 56% for R-CHOP, P=0.002), and the OS rate was 90% (vs 77% for R-CHOP, P=0.13).
Frontline therapy and HSCT
Patients who received R-CHOP upfront benefited from auto-HSCT, but patients who received intensive chemotherapy did not.
The RFS was 51% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 75% for patients who received R-CHOP followed by auto-HSCT.
The OS was 75% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 83% for patients who received R-CHOP followed by auto-HSCT.
The RFS was 86% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 91% for patients who received intensive chemotherapy followed by auto-HSCT.
The OS was 89% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 92% for patients who received intensive chemotherapy followed by auto-HSCT.
An intergroup comparison showed a significant difference in RFS (P=0.003), which was driven by a significantly lower rate of RFS for patients who received R-CHOP without auto-HSCT, compared with patients who received intensive chemotherapy without auto-HSCT (P=0.003) or intensive chemotherapy with auto-HSCT (P=0.001).
“[I]f patients do go into remission with R-CHOP, it appears to be less durable, so, in these cases, going forward with auto-[H]SCT may still make sense,” Dr Landsburg said.
On the other hand, there was no significant difference between the groups with regard to OS (P=0.50).
Dr Landsburg said the next step for this research will be to study features of patients who don’t go into remission in order to understand why their disease is resistant to therapy and if that can be overcome with different treatment strategies. He also said it’s important to try to find more effective therapies for DHL patients who relapse. ![]()
A new study suggests that patients with double-hit lymphoma (DHL) in first remission only benefit from an autologous hematopoietic stem cell transplant (auto-HSCT) if they received standard frontline chemotherapy.
Researchers looked at long-term outcomes for DHL patients who achieved remission and, overall, found that auto-HSCT did not significantly prolong remission or survival.
However, patients who received standard chemotherapy as frontline treatment did appear to benefit from auto-HSCT, as these patients had worse outcomes than patients who received intensive frontline chemotherapy.
This finding led the researchers to recommend that DHL patients receive intensive chemotherapy upfront and forgo subsequent auto-HSCT.
Daniel J. Landsburg, MD, of the University of Pennsylvania in Philadelphia, and his colleagues made these recommendations in the Journal of Clinical Oncology.
“A major dilemma for oncologists who treat [DHL] was whether or not to recommend the potentially harmful therapy of auto-[H]SCT to patients with this disease as a strategy to help keep them in remission,” Dr Landsburg said.
To gain some insight into the issue, Dr Landsburg and his colleagues looked at data on 159 patients from 19 academic medical centers across the US.
Patients were diagnosed with DHL between 2006 and 2015, and all achieved remission following frontline chemotherapy.
Thirty-five patients received standard frontline therapy—R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
The remaining patients received intensive frontline chemotherapy:
- 81 received DA-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab)
- 32 received R-hyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine)
- 11 received R-CODOX-M/IVAC (rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate/ifosfamide, etoposide, high-dose cytarabine).
Sixty-two patients underwent auto-HSCT, and 97 patients did not. There were no significant differences between these 2 patient groups at baseline.
“Our result is not explained by differences in patients’ overall health or disease features,” Dr Landsburg said. “The transplant and non-transplant arms of this study were very well-matched.”
Relapse and survival
For the entire patient cohort, the 3-year relapse-free survival (RFS) rate was 80%, and the 3-year overall survival (OS) rate was 87%.
There was no significant difference in RFS or OS between patients who underwent auto-HSCT and those who did not.
The RFS rate was 89% in patients who underwent auto-HSCT and 75% in patients who did not (P=0.12). The OS rate was 91% and 85%, respectively (P=0.74).
“Once these patients achieve remission, the data show they are likely to stay in remission,” Dr Landsburg said.
“In the absence of a large, randomized, controlled trial, which would be very challenging to carry out in this case, this is the best evidence we have, and it shows there’s no clear benefit to these patients undergoing auto-[H]SCT.”
Impact of frontline therapy
Patients who received R-CHOP upfront had worse RFS and OS than those who received intensive chemotherapy, although the OS difference was not significant.
RFS rates were 56% in patients who received R-CHOP, 88% in those who received DA-EPOCH-R, 87% in those who received R-hyperCVAD, and 91% in those who received R-CODOX-M/IVAC (P=0.003).
OS rates were 77% in patients who received R-CHOP, 87% in those who received DA-EPOCH-R, 90% in those who received R-hyperCVAD, and 100% in those who received R-CODOX-M/IVAC, respectively (P=0.36).
When the 3 intensive regimens were combined, the RFS rate was 88% (vs 56% for R-CHOP, P=0.002), and the OS rate was 90% (vs 77% for R-CHOP, P=0.13).
Frontline therapy and HSCT
Patients who received R-CHOP upfront benefited from auto-HSCT, but patients who received intensive chemotherapy did not.
The RFS was 51% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 75% for patients who received R-CHOP followed by auto-HSCT.
The OS was 75% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 83% for patients who received R-CHOP followed by auto-HSCT.
The RFS was 86% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 91% for patients who received intensive chemotherapy followed by auto-HSCT.
The OS was 89% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 92% for patients who received intensive chemotherapy followed by auto-HSCT.
An intergroup comparison showed a significant difference in RFS (P=0.003), which was driven by a significantly lower rate of RFS for patients who received R-CHOP without auto-HSCT, compared with patients who received intensive chemotherapy without auto-HSCT (P=0.003) or intensive chemotherapy with auto-HSCT (P=0.001).
“[I]f patients do go into remission with R-CHOP, it appears to be less durable, so, in these cases, going forward with auto-[H]SCT may still make sense,” Dr Landsburg said.
On the other hand, there was no significant difference between the groups with regard to OS (P=0.50).
Dr Landsburg said the next step for this research will be to study features of patients who don’t go into remission in order to understand why their disease is resistant to therapy and if that can be overcome with different treatment strategies. He also said it’s important to try to find more effective therapies for DHL patients who relapse. ![]()
A new study suggests that patients with double-hit lymphoma (DHL) in first remission only benefit from an autologous hematopoietic stem cell transplant (auto-HSCT) if they received standard frontline chemotherapy.
Researchers looked at long-term outcomes for DHL patients who achieved remission and, overall, found that auto-HSCT did not significantly prolong remission or survival.
However, patients who received standard chemotherapy as frontline treatment did appear to benefit from auto-HSCT, as these patients had worse outcomes than patients who received intensive frontline chemotherapy.
This finding led the researchers to recommend that DHL patients receive intensive chemotherapy upfront and forgo subsequent auto-HSCT.
Daniel J. Landsburg, MD, of the University of Pennsylvania in Philadelphia, and his colleagues made these recommendations in the Journal of Clinical Oncology.
“A major dilemma for oncologists who treat [DHL] was whether or not to recommend the potentially harmful therapy of auto-[H]SCT to patients with this disease as a strategy to help keep them in remission,” Dr Landsburg said.
To gain some insight into the issue, Dr Landsburg and his colleagues looked at data on 159 patients from 19 academic medical centers across the US.
Patients were diagnosed with DHL between 2006 and 2015, and all achieved remission following frontline chemotherapy.
Thirty-five patients received standard frontline therapy—R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
The remaining patients received intensive frontline chemotherapy:
- 81 received DA-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab)
- 32 received R-hyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine)
- 11 received R-CODOX-M/IVAC (rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate/ifosfamide, etoposide, high-dose cytarabine).
Sixty-two patients underwent auto-HSCT, and 97 patients did not. There were no significant differences between these 2 patient groups at baseline.
“Our result is not explained by differences in patients’ overall health or disease features,” Dr Landsburg said. “The transplant and non-transplant arms of this study were very well-matched.”
Relapse and survival
For the entire patient cohort, the 3-year relapse-free survival (RFS) rate was 80%, and the 3-year overall survival (OS) rate was 87%.
There was no significant difference in RFS or OS between patients who underwent auto-HSCT and those who did not.
The RFS rate was 89% in patients who underwent auto-HSCT and 75% in patients who did not (P=0.12). The OS rate was 91% and 85%, respectively (P=0.74).
“Once these patients achieve remission, the data show they are likely to stay in remission,” Dr Landsburg said.
“In the absence of a large, randomized, controlled trial, which would be very challenging to carry out in this case, this is the best evidence we have, and it shows there’s no clear benefit to these patients undergoing auto-[H]SCT.”
Impact of frontline therapy
Patients who received R-CHOP upfront had worse RFS and OS than those who received intensive chemotherapy, although the OS difference was not significant.
RFS rates were 56% in patients who received R-CHOP, 88% in those who received DA-EPOCH-R, 87% in those who received R-hyperCVAD, and 91% in those who received R-CODOX-M/IVAC (P=0.003).
OS rates were 77% in patients who received R-CHOP, 87% in those who received DA-EPOCH-R, 90% in those who received R-hyperCVAD, and 100% in those who received R-CODOX-M/IVAC, respectively (P=0.36).
When the 3 intensive regimens were combined, the RFS rate was 88% (vs 56% for R-CHOP, P=0.002), and the OS rate was 90% (vs 77% for R-CHOP, P=0.13).
Frontline therapy and HSCT
Patients who received R-CHOP upfront benefited from auto-HSCT, but patients who received intensive chemotherapy did not.
The RFS was 51% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 75% for patients who received R-CHOP followed by auto-HSCT.
The OS was 75% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 83% for patients who received R-CHOP followed by auto-HSCT.
The RFS was 86% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 91% for patients who received intensive chemotherapy followed by auto-HSCT.
The OS was 89% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 92% for patients who received intensive chemotherapy followed by auto-HSCT.
An intergroup comparison showed a significant difference in RFS (P=0.003), which was driven by a significantly lower rate of RFS for patients who received R-CHOP without auto-HSCT, compared with patients who received intensive chemotherapy without auto-HSCT (P=0.003) or intensive chemotherapy with auto-HSCT (P=0.001).
“[I]f patients do go into remission with R-CHOP, it appears to be less durable, so, in these cases, going forward with auto-[H]SCT may still make sense,” Dr Landsburg said.
On the other hand, there was no significant difference between the groups with regard to OS (P=0.50).
Dr Landsburg said the next step for this research will be to study features of patients who don’t go into remission in order to understand why their disease is resistant to therapy and if that can be overcome with different treatment strategies. He also said it’s important to try to find more effective therapies for DHL patients who relapse. ![]()
Study: Most oncologists don’t discuss exercise with patients
Results of a small, single-center study suggest oncologists may not provide cancer patients with adequate guidance on exercise.
A majority of the patients and oncologists surveyed for this study placed importance on exercise during cancer care, but most of the oncologists failed to give patients recommendations on exercise.
“Our results indicate that exercise is perceived as important to patients with cancer, both from a patient and physician perspective,” said study author Agnes Smaradottir, MD, of Gundersen Health System in La Crosse, Wisconsin.
“However, physicians are reluctant to consistently include [physical activity] recommendations in their patient discussions.”
Dr Smaradottir and her colleagues reported these findings in JNCCN.
The researchers surveyed 20 cancer patients and 9 oncologists for this study.
The patients’ mean age was 64. Ten patients had stage I-III non-metastatic cancer after adjuvant therapy, and 10 had stage IV metastatic disease and were undergoing palliative treatment. Most patients had solid tumor malignancies, but 1 had chronic lymphocytic leukemia.
The oncologists’ mean age was 45, 56% were male, and they had a mean of 12 years of practice. Most (89%) said they exercise on a regular basis.
Discussions
Nineteen (95%) of the patients surveyed felt they benefited from exercise during treatment, but only 3 of the patients recalled being instructed to exercise.
Exercise was felt to be an equally important part of treatment and well-being for patients with early stage cancer treated with curative intent as well as patients receiving palliative therapy.
Although all the oncologists noted that exercise can benefit cancer patients, only 1 of the 9 surveyed documented discussion of exercise in patient charts.
Preferences and concerns
More than 80% of the patients said they would prefer a home-based exercise regimen that could be performed in alignment with their personal schedules and symptoms.
Patients also noted a preference that exercise recommendations come from their oncologists, as they have an established relationship and feel their oncologists best understand the complexities of their personalized treatment plans.
The oncologists, on the other hand, wanted to refer patients to specialist care for exercise recommendations. Reasons for this included the oncologists’ mounting clinic schedules and a lack of education about appropriate physical activity recommendations for patients.
The oncologists also expressed concern about asking patients to be more physically active during chemotherapy and radiation and expressed trepidation about prescribing exercise to frail patients with limited mobility.
“We were surprised by the gap in expectations regarding exercise recommendation between patients and providers,” Dr Smaradottir said. “Many providers, ourselves included, thought patients would prefer to be referred to an exercise center, but they clearly preferred to have a home-based program recommended by their oncologist.”
“Our findings highlight the value of examining both patient and provider attitudes and behavioral intentions. While we uncovered barriers to exercise recommendations, questions remain on how to bridge the gap between patient and provider preferences.” ![]()
Results of a small, single-center study suggest oncologists may not provide cancer patients with adequate guidance on exercise.
A majority of the patients and oncologists surveyed for this study placed importance on exercise during cancer care, but most of the oncologists failed to give patients recommendations on exercise.
“Our results indicate that exercise is perceived as important to patients with cancer, both from a patient and physician perspective,” said study author Agnes Smaradottir, MD, of Gundersen Health System in La Crosse, Wisconsin.
“However, physicians are reluctant to consistently include [physical activity] recommendations in their patient discussions.”
Dr Smaradottir and her colleagues reported these findings in JNCCN.
The researchers surveyed 20 cancer patients and 9 oncologists for this study.
The patients’ mean age was 64. Ten patients had stage I-III non-metastatic cancer after adjuvant therapy, and 10 had stage IV metastatic disease and were undergoing palliative treatment. Most patients had solid tumor malignancies, but 1 had chronic lymphocytic leukemia.
The oncologists’ mean age was 45, 56% were male, and they had a mean of 12 years of practice. Most (89%) said they exercise on a regular basis.
Discussions
Nineteen (95%) of the patients surveyed felt they benefited from exercise during treatment, but only 3 of the patients recalled being instructed to exercise.
Exercise was felt to be an equally important part of treatment and well-being for patients with early stage cancer treated with curative intent as well as patients receiving palliative therapy.
Although all the oncologists noted that exercise can benefit cancer patients, only 1 of the 9 surveyed documented discussion of exercise in patient charts.
Preferences and concerns
More than 80% of the patients said they would prefer a home-based exercise regimen that could be performed in alignment with their personal schedules and symptoms.
Patients also noted a preference that exercise recommendations come from their oncologists, as they have an established relationship and feel their oncologists best understand the complexities of their personalized treatment plans.
The oncologists, on the other hand, wanted to refer patients to specialist care for exercise recommendations. Reasons for this included the oncologists’ mounting clinic schedules and a lack of education about appropriate physical activity recommendations for patients.
The oncologists also expressed concern about asking patients to be more physically active during chemotherapy and radiation and expressed trepidation about prescribing exercise to frail patients with limited mobility.
“We were surprised by the gap in expectations regarding exercise recommendation between patients and providers,” Dr Smaradottir said. “Many providers, ourselves included, thought patients would prefer to be referred to an exercise center, but they clearly preferred to have a home-based program recommended by their oncologist.”
“Our findings highlight the value of examining both patient and provider attitudes and behavioral intentions. While we uncovered barriers to exercise recommendations, questions remain on how to bridge the gap between patient and provider preferences.” ![]()
Results of a small, single-center study suggest oncologists may not provide cancer patients with adequate guidance on exercise.
A majority of the patients and oncologists surveyed for this study placed importance on exercise during cancer care, but most of the oncologists failed to give patients recommendations on exercise.
“Our results indicate that exercise is perceived as important to patients with cancer, both from a patient and physician perspective,” said study author Agnes Smaradottir, MD, of Gundersen Health System in La Crosse, Wisconsin.
“However, physicians are reluctant to consistently include [physical activity] recommendations in their patient discussions.”
Dr Smaradottir and her colleagues reported these findings in JNCCN.
The researchers surveyed 20 cancer patients and 9 oncologists for this study.
The patients’ mean age was 64. Ten patients had stage I-III non-metastatic cancer after adjuvant therapy, and 10 had stage IV metastatic disease and were undergoing palliative treatment. Most patients had solid tumor malignancies, but 1 had chronic lymphocytic leukemia.
The oncologists’ mean age was 45, 56% were male, and they had a mean of 12 years of practice. Most (89%) said they exercise on a regular basis.
Discussions
Nineteen (95%) of the patients surveyed felt they benefited from exercise during treatment, but only 3 of the patients recalled being instructed to exercise.
Exercise was felt to be an equally important part of treatment and well-being for patients with early stage cancer treated with curative intent as well as patients receiving palliative therapy.
Although all the oncologists noted that exercise can benefit cancer patients, only 1 of the 9 surveyed documented discussion of exercise in patient charts.
Preferences and concerns
More than 80% of the patients said they would prefer a home-based exercise regimen that could be performed in alignment with their personal schedules and symptoms.
Patients also noted a preference that exercise recommendations come from their oncologists, as they have an established relationship and feel their oncologists best understand the complexities of their personalized treatment plans.
The oncologists, on the other hand, wanted to refer patients to specialist care for exercise recommendations. Reasons for this included the oncologists’ mounting clinic schedules and a lack of education about appropriate physical activity recommendations for patients.
The oncologists also expressed concern about asking patients to be more physically active during chemotherapy and radiation and expressed trepidation about prescribing exercise to frail patients with limited mobility.
“We were surprised by the gap in expectations regarding exercise recommendation between patients and providers,” Dr Smaradottir said. “Many providers, ourselves included, thought patients would prefer to be referred to an exercise center, but they clearly preferred to have a home-based program recommended by their oncologist.”
“Our findings highlight the value of examining both patient and provider attitudes and behavioral intentions. While we uncovered barriers to exercise recommendations, questions remain on how to bridge the gap between patient and provider preferences.” ![]()
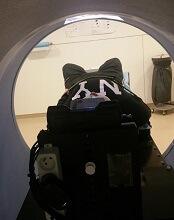
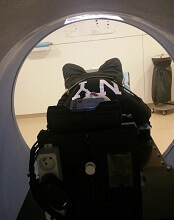
Videos reduce need for anesthesia in kids undergoing radiotherapy
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious. ![]()
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious. ![]()
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious. ![]()
HL survivors should be screened for CAD after chest irradiation
VIENNA, AUSTRIA—Hodgkin lymphoma (HL) survivors who received chest irradiation should be screened for coronary artery disease (CAD), according to researchers.
The team evaluated HL survivors who underwent mediastinal irradiation 20 years prior to study initiation.
These individuals were more likely to have CAD and to have more severe CAD than matched control subjects.
The researchers presented these findings at ICNC 2017, the International Conference on Nuclear Cardiology and Cardiac CT (abstract P118).
“Patients with Hodgkin lymphoma receive high-dose mediastinal irradiation at a young age as part of their treatment,” said Alexander van Rosendael, MD, of Leiden University Medical Centre in the Netherlands.
“There is an ongoing debate about whether to screen patients who get chest irradiation for coronary artery disease.”
Therefore, Dr van Rosendael and his colleagues assessed the extent, severity, and location of CAD in HL survivors who had received chest irradiation.
The study included 79 patients who had been free of HL for at least 10 years and had received mediastinal irradiation 20 years ago, plus 273 control subjects without HL or irradiation.
CAD was assessed using coronary computed tomography angiography (CTA). To assess differences in CAD between patients and controls, they were matched in a 1:3 fashion by age, gender, diabetes, hypertension, hypercholesterolemia, family history of CAD, and smoking status.
Patients were 45 years old, on average, and the presence of cardiovascular risk factors was low overall.
Forty-two percent of patients had no atherosclerosis on coronary CTA, compared to 64% of controls (P<0.001).
Regarding the extent and severity of CAD, HL patients had significantly more multi-vessel CAD than controls. Ten percent of patients had 2-vessel disease, and 24% had 3-vessel disease, compared to 6% and 9% of controls, respectively (P=0.001).
The segment involvement score (which measures overall coronary plaque distribution) and the segment stenosis score (which measures overall coronary plaque extent and severity) were significantly higher for patients than for controls (P<0.001 and P=0.034, respectively).
Regarding the location of CAD, patients had significantly more coronary plaques in the left main (17% vs 6%, P=0.004), proximal left anterior descending (30% vs 16%, P=0.004), proximal right coronary artery (25% vs 10%, P<0.001), and proximal left circumflex (14% vs 6%, P=0.022), but not in non-proximal coronary segments.
Patients had about a 4-fold higher risk of proximal plaque and about 3-fold higher risk of proximal obstructive stenosis compared to controls (odds ratios, 4.1 and 2.9, respectively; P values, <0.001 and 0.025, respectively).
“Hodgkin patients who have chest irradiation have much more CAD than people of the same age who did not have irradiation,” Dr van Rosendael said.
“The CAD occurred at a young age—patients were 45 years old, on average—and was probably caused by the irradiation. The CTA was done about 20 years after chest irradiation, so there was time for CAD to develop.”
“What was remarkable was that irradiated patients had all the features of high-risk CAD, including high stenosis severity, proximal location, and extensive disease. We know that the proximal location of the disease is much riskier, and this may explain why Hodgkin patients have such poor cardiovascular outcomes when they get older.”
Dr van Rosendael explained that irradiation of the chest can cause inflammation of the coronary arteries, making patients more vulnerable to developing CAD. But it is not known why the CAD in irradiated patients tends to be proximally located.
He said the finding of more, and more severe, CAD in irradiated patients supports the argument for screening.
“When you see CAD in patients who received chest irradiation, it is high-risk CAD,” he said. “Such patients should be screened at regular intervals after irradiation so that CAD can be spotted early and early treatment can be initiated.”
“These patients are around 45 years old, and they are almost all asymptomatic. If you see a severe left main stenosis by screening with CTA (which occurred in 4%), then you can start statin therapy and perform revascularization, which may improve outcome. We know such treatment reduces the risk of events in non-irradiated patients, so it seems likely that it would benefit Hodgkin patients.” ![]()
VIENNA, AUSTRIA—Hodgkin lymphoma (HL) survivors who received chest irradiation should be screened for coronary artery disease (CAD), according to researchers.
The team evaluated HL survivors who underwent mediastinal irradiation 20 years prior to study initiation.
These individuals were more likely to have CAD and to have more severe CAD than matched control subjects.
The researchers presented these findings at ICNC 2017, the International Conference on Nuclear Cardiology and Cardiac CT (abstract P118).
“Patients with Hodgkin lymphoma receive high-dose mediastinal irradiation at a young age as part of their treatment,” said Alexander van Rosendael, MD, of Leiden University Medical Centre in the Netherlands.
“There is an ongoing debate about whether to screen patients who get chest irradiation for coronary artery disease.”
Therefore, Dr van Rosendael and his colleagues assessed the extent, severity, and location of CAD in HL survivors who had received chest irradiation.
The study included 79 patients who had been free of HL for at least 10 years and had received mediastinal irradiation 20 years ago, plus 273 control subjects without HL or irradiation.
CAD was assessed using coronary computed tomography angiography (CTA). To assess differences in CAD between patients and controls, they were matched in a 1:3 fashion by age, gender, diabetes, hypertension, hypercholesterolemia, family history of CAD, and smoking status.
Patients were 45 years old, on average, and the presence of cardiovascular risk factors was low overall.
Forty-two percent of patients had no atherosclerosis on coronary CTA, compared to 64% of controls (P<0.001).
Regarding the extent and severity of CAD, HL patients had significantly more multi-vessel CAD than controls. Ten percent of patients had 2-vessel disease, and 24% had 3-vessel disease, compared to 6% and 9% of controls, respectively (P=0.001).
The segment involvement score (which measures overall coronary plaque distribution) and the segment stenosis score (which measures overall coronary plaque extent and severity) were significantly higher for patients than for controls (P<0.001 and P=0.034, respectively).
Regarding the location of CAD, patients had significantly more coronary plaques in the left main (17% vs 6%, P=0.004), proximal left anterior descending (30% vs 16%, P=0.004), proximal right coronary artery (25% vs 10%, P<0.001), and proximal left circumflex (14% vs 6%, P=0.022), but not in non-proximal coronary segments.
Patients had about a 4-fold higher risk of proximal plaque and about 3-fold higher risk of proximal obstructive stenosis compared to controls (odds ratios, 4.1 and 2.9, respectively; P values, <0.001 and 0.025, respectively).
“Hodgkin patients who have chest irradiation have much more CAD than people of the same age who did not have irradiation,” Dr van Rosendael said.
“The CAD occurred at a young age—patients were 45 years old, on average—and was probably caused by the irradiation. The CTA was done about 20 years after chest irradiation, so there was time for CAD to develop.”
“What was remarkable was that irradiated patients had all the features of high-risk CAD, including high stenosis severity, proximal location, and extensive disease. We know that the proximal location of the disease is much riskier, and this may explain why Hodgkin patients have such poor cardiovascular outcomes when they get older.”
Dr van Rosendael explained that irradiation of the chest can cause inflammation of the coronary arteries, making patients more vulnerable to developing CAD. But it is not known why the CAD in irradiated patients tends to be proximally located.
He said the finding of more, and more severe, CAD in irradiated patients supports the argument for screening.
“When you see CAD in patients who received chest irradiation, it is high-risk CAD,” he said. “Such patients should be screened at regular intervals after irradiation so that CAD can be spotted early and early treatment can be initiated.”
“These patients are around 45 years old, and they are almost all asymptomatic. If you see a severe left main stenosis by screening with CTA (which occurred in 4%), then you can start statin therapy and perform revascularization, which may improve outcome. We know such treatment reduces the risk of events in non-irradiated patients, so it seems likely that it would benefit Hodgkin patients.” ![]()
VIENNA, AUSTRIA—Hodgkin lymphoma (HL) survivors who received chest irradiation should be screened for coronary artery disease (CAD), according to researchers.
The team evaluated HL survivors who underwent mediastinal irradiation 20 years prior to study initiation.
These individuals were more likely to have CAD and to have more severe CAD than matched control subjects.
The researchers presented these findings at ICNC 2017, the International Conference on Nuclear Cardiology and Cardiac CT (abstract P118).
“Patients with Hodgkin lymphoma receive high-dose mediastinal irradiation at a young age as part of their treatment,” said Alexander van Rosendael, MD, of Leiden University Medical Centre in the Netherlands.
“There is an ongoing debate about whether to screen patients who get chest irradiation for coronary artery disease.”
Therefore, Dr van Rosendael and his colleagues assessed the extent, severity, and location of CAD in HL survivors who had received chest irradiation.
The study included 79 patients who had been free of HL for at least 10 years and had received mediastinal irradiation 20 years ago, plus 273 control subjects without HL or irradiation.
CAD was assessed using coronary computed tomography angiography (CTA). To assess differences in CAD between patients and controls, they were matched in a 1:3 fashion by age, gender, diabetes, hypertension, hypercholesterolemia, family history of CAD, and smoking status.
Patients were 45 years old, on average, and the presence of cardiovascular risk factors was low overall.
Forty-two percent of patients had no atherosclerosis on coronary CTA, compared to 64% of controls (P<0.001).
Regarding the extent and severity of CAD, HL patients had significantly more multi-vessel CAD than controls. Ten percent of patients had 2-vessel disease, and 24% had 3-vessel disease, compared to 6% and 9% of controls, respectively (P=0.001).
The segment involvement score (which measures overall coronary plaque distribution) and the segment stenosis score (which measures overall coronary plaque extent and severity) were significantly higher for patients than for controls (P<0.001 and P=0.034, respectively).
Regarding the location of CAD, patients had significantly more coronary plaques in the left main (17% vs 6%, P=0.004), proximal left anterior descending (30% vs 16%, P=0.004), proximal right coronary artery (25% vs 10%, P<0.001), and proximal left circumflex (14% vs 6%, P=0.022), but not in non-proximal coronary segments.
Patients had about a 4-fold higher risk of proximal plaque and about 3-fold higher risk of proximal obstructive stenosis compared to controls (odds ratios, 4.1 and 2.9, respectively; P values, <0.001 and 0.025, respectively).
“Hodgkin patients who have chest irradiation have much more CAD than people of the same age who did not have irradiation,” Dr van Rosendael said.
“The CAD occurred at a young age—patients were 45 years old, on average—and was probably caused by the irradiation. The CTA was done about 20 years after chest irradiation, so there was time for CAD to develop.”
“What was remarkable was that irradiated patients had all the features of high-risk CAD, including high stenosis severity, proximal location, and extensive disease. We know that the proximal location of the disease is much riskier, and this may explain why Hodgkin patients have such poor cardiovascular outcomes when they get older.”
Dr van Rosendael explained that irradiation of the chest can cause inflammation of the coronary arteries, making patients more vulnerable to developing CAD. But it is not known why the CAD in irradiated patients tends to be proximally located.
He said the finding of more, and more severe, CAD in irradiated patients supports the argument for screening.
“When you see CAD in patients who received chest irradiation, it is high-risk CAD,” he said. “Such patients should be screened at regular intervals after irradiation so that CAD can be spotted early and early treatment can be initiated.”
“These patients are around 45 years old, and they are almost all asymptomatic. If you see a severe left main stenosis by screening with CTA (which occurred in 4%), then you can start statin therapy and perform revascularization, which may improve outcome. We know such treatment reduces the risk of events in non-irradiated patients, so it seems likely that it would benefit Hodgkin patients.” ![]()
Subcutaneous rituximab safe, effective for follicular lymphoma
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
FROM LANCET HAEMATOLOGY
Key clinical point: Subcutaneous rituximab had efficacy and safety profiles similar to those of standard IV rituximab when given as first-line therapy to adults with follicular lymphoma.
Major finding: The primary efficacy end point – overall response rate at the end of induction – was 84.9% with IV and 84.4% with subcutaneous rituximab.
Data source: An international randomized controlled phase III trial involving 410 adults followed for 3 years.
Disclosures: This trial was funded by Hoffmann-La Roche, maker of the subcutaneous formulation of rituximab. The pharmaceutical company also was involved in the design and conduct of the trial, collection and interpretation of the data, and writing of the results. Dr. Davies reported ties to Hoffmann-La Roche and numerous other drug companies.