User login
Inhibitor shows preclinical promise in leukemia, MM

Credit: VCU
The experimental drug dinaciclib could potentially improve the treatment of multiple myeloma (MM) and myeloid leukemias, according to preclinical research published in Molecular Cancer Therapeutics.
The study showed that dinaciclib disrupts a cell survival mechanism known as the unfolded protein response (UPR).
And without the UPR, MM and myeloid leukemia cells were unable to combat damage caused by anticancer agents that induce stress in the endoplasmic reticulum (ER).
“Although dinaciclib has shown promising preclinical activity against a variety of tumor cells and is currently undergoing phase 1/2 clinical trials in several malignancies, the mechanisms responsible for its antitumor activity are not fully understood,” said study author Steven Grant, MD, of the Virginia Commonwealth University Massey Cancer Center.
“Our research highlights a potentially new mechanism of dinaciclib action and raises the possibility that this agent could be a useful addition to current multiple myeloma and myeloid leukemia therapies.”
Dinaciclib is a cyclin-dependent kinase (CDK) inhibitor. CDKs are overactive in many cancers, which results in unregulated proliferation of cancer cells.
Observations from this study suggest that 2 specific CDKs, CDK1 and CDK5, play key roles in regulating the UPR by helping to control the production and accumulation of X-box binding pretein-1 (XBP-1). The spliced form of XBP-1 (XBP-1s) helps regulate the expression of genes critical to cellular stress responses.
External stressors, including certain anticancer agents, can cause misfolded proteins to accumulate in the ER. These stressors can also cause XBP-1s to accumulate in the cell’s nucleus, which promotes the UPR and helps cells withstand the damaging effects of misfolded proteins.
This research showed that dinaciclib, by interfering with UPR activation, caused MM and myeloid leukemia cells to initiate apoptosis when exposed to thapsigargin and tunicamycin—2 agents that induce ER stress.
And single-agent dinaciclib treatment significantly decreased tumor growth in mouse models of MM, when compared to vehicle control.
“These findings build on a long history of work in our laboratory investigating mechanisms by which cancer cells respond to environmental stresses,” Dr Grant said.
“We intend to continue investigating ways in which dinaciclib and other CDK inhibitors might be used to disrupt the UPR and potentially improve the effectiveness of certain agents for the treatment of multiple myeloma or myeloid leukemia.” ![]()

Credit: VCU
The experimental drug dinaciclib could potentially improve the treatment of multiple myeloma (MM) and myeloid leukemias, according to preclinical research published in Molecular Cancer Therapeutics.
The study showed that dinaciclib disrupts a cell survival mechanism known as the unfolded protein response (UPR).
And without the UPR, MM and myeloid leukemia cells were unable to combat damage caused by anticancer agents that induce stress in the endoplasmic reticulum (ER).
“Although dinaciclib has shown promising preclinical activity against a variety of tumor cells and is currently undergoing phase 1/2 clinical trials in several malignancies, the mechanisms responsible for its antitumor activity are not fully understood,” said study author Steven Grant, MD, of the Virginia Commonwealth University Massey Cancer Center.
“Our research highlights a potentially new mechanism of dinaciclib action and raises the possibility that this agent could be a useful addition to current multiple myeloma and myeloid leukemia therapies.”
Dinaciclib is a cyclin-dependent kinase (CDK) inhibitor. CDKs are overactive in many cancers, which results in unregulated proliferation of cancer cells.
Observations from this study suggest that 2 specific CDKs, CDK1 and CDK5, play key roles in regulating the UPR by helping to control the production and accumulation of X-box binding pretein-1 (XBP-1). The spliced form of XBP-1 (XBP-1s) helps regulate the expression of genes critical to cellular stress responses.
External stressors, including certain anticancer agents, can cause misfolded proteins to accumulate in the ER. These stressors can also cause XBP-1s to accumulate in the cell’s nucleus, which promotes the UPR and helps cells withstand the damaging effects of misfolded proteins.
This research showed that dinaciclib, by interfering with UPR activation, caused MM and myeloid leukemia cells to initiate apoptosis when exposed to thapsigargin and tunicamycin—2 agents that induce ER stress.
And single-agent dinaciclib treatment significantly decreased tumor growth in mouse models of MM, when compared to vehicle control.
“These findings build on a long history of work in our laboratory investigating mechanisms by which cancer cells respond to environmental stresses,” Dr Grant said.
“We intend to continue investigating ways in which dinaciclib and other CDK inhibitors might be used to disrupt the UPR and potentially improve the effectiveness of certain agents for the treatment of multiple myeloma or myeloid leukemia.” ![]()

Credit: VCU
The experimental drug dinaciclib could potentially improve the treatment of multiple myeloma (MM) and myeloid leukemias, according to preclinical research published in Molecular Cancer Therapeutics.
The study showed that dinaciclib disrupts a cell survival mechanism known as the unfolded protein response (UPR).
And without the UPR, MM and myeloid leukemia cells were unable to combat damage caused by anticancer agents that induce stress in the endoplasmic reticulum (ER).
“Although dinaciclib has shown promising preclinical activity against a variety of tumor cells and is currently undergoing phase 1/2 clinical trials in several malignancies, the mechanisms responsible for its antitumor activity are not fully understood,” said study author Steven Grant, MD, of the Virginia Commonwealth University Massey Cancer Center.
“Our research highlights a potentially new mechanism of dinaciclib action and raises the possibility that this agent could be a useful addition to current multiple myeloma and myeloid leukemia therapies.”
Dinaciclib is a cyclin-dependent kinase (CDK) inhibitor. CDKs are overactive in many cancers, which results in unregulated proliferation of cancer cells.
Observations from this study suggest that 2 specific CDKs, CDK1 and CDK5, play key roles in regulating the UPR by helping to control the production and accumulation of X-box binding pretein-1 (XBP-1). The spliced form of XBP-1 (XBP-1s) helps regulate the expression of genes critical to cellular stress responses.
External stressors, including certain anticancer agents, can cause misfolded proteins to accumulate in the ER. These stressors can also cause XBP-1s to accumulate in the cell’s nucleus, which promotes the UPR and helps cells withstand the damaging effects of misfolded proteins.
This research showed that dinaciclib, by interfering with UPR activation, caused MM and myeloid leukemia cells to initiate apoptosis when exposed to thapsigargin and tunicamycin—2 agents that induce ER stress.
And single-agent dinaciclib treatment significantly decreased tumor growth in mouse models of MM, when compared to vehicle control.
“These findings build on a long history of work in our laboratory investigating mechanisms by which cancer cells respond to environmental stresses,” Dr Grant said.
“We intend to continue investigating ways in which dinaciclib and other CDK inhibitors might be used to disrupt the UPR and potentially improve the effectiveness of certain agents for the treatment of multiple myeloma or myeloid leukemia.” ![]()
Making K-Ras cancers druggable

Credit: PNAS
New findings suggest drugs can effectively fight K-Ras-mutant cancers—if they have a little help.
Experiments in human cancer cells showed that K-Ras-mutant tumor growth was highly dependent on the cells’ constant need to check and mend their DNA.
However, inhibiting the activity of H-Ras and N-Ras prevented the DNA damage response. And this made the cells more vulnerable to treatment.
“Our finding suggests that K-Ras cancers can be made more susceptible to existing therapies by interfering with their DNA repair mechanisms,” said Dafna Bar-Sagi, PhD, of the New York University School of Medicine.
“What some researchers have described as therapeutic ‘mission impossible’ may now become a ‘mission doable.’”
Dr Bar-Sagi and her colleagues reported this discovery in Cancer Cell.
The group’s research began with experiments to determine how Ras signaling leads to the uncontrolled growth of cancer cells. The team found that downregulation of wild-type H-Ras and N-Ras in mutant K-Ras cells caused the buildup of damaged DNA and slowed cell growth.
In the absence of H-Ras and N-Ras, K-Ras-mutant cancer cells failed to repair their DNA at the G2 phase of cell division. And this defect was caused by failure to properly activate Chk1.
With this in mind, the researchers decided to test the effects of H-Ras or N-Ras knockdown on treatment with DNA-damaging agents.
Knockdown of H-Ras or N-Ras sensitized K-Ras-mutant cancer cells to SN38 and oxaliplatin in vitro. But the same effect did not occur when H-Ras or N-Ras was knocked down in K-Ras-wild-type cancer cells.
K-Ras-mutant cancer cells were also sensitive to treatment with the Chk1/Chk2 inhibitor AZD7726 when H-Ras or N-Ras was knocked down in vitro.
To further support these findings, the researchers conducted experiments in mice with K-Ras-mutant tumors. Mice with H-Ras knockdown experienced tumor growth similar to controls.
But when the mice with H-Ras-suppressed tumors received the chemotherapy drug irinotecan, they experienced tumor regression that lasted up to 18 days post-treatment. On the other hand, mice without H-Ras suppression experienced modest tumor growth after treatment with irinotecan.
“Discovering more about how these different forms of Ras act on one another—including how they control DNA damage repair at Chk1 in combination with chemotherapy—could help us design drugs that greatly stall disease progression,” said study author Elda Grabocka, PhD, also of the New York University School of Medicine.
The researchers are now planning additional experiments on the biological interdependency of Ras proteins and what other chemotherapies might be involved in slowing cancer growth. Their goal is to map the Ras signaling pathways and identify as many therapeutic targets as possible. ![]()

Credit: PNAS
New findings suggest drugs can effectively fight K-Ras-mutant cancers—if they have a little help.
Experiments in human cancer cells showed that K-Ras-mutant tumor growth was highly dependent on the cells’ constant need to check and mend their DNA.
However, inhibiting the activity of H-Ras and N-Ras prevented the DNA damage response. And this made the cells more vulnerable to treatment.
“Our finding suggests that K-Ras cancers can be made more susceptible to existing therapies by interfering with their DNA repair mechanisms,” said Dafna Bar-Sagi, PhD, of the New York University School of Medicine.
“What some researchers have described as therapeutic ‘mission impossible’ may now become a ‘mission doable.’”
Dr Bar-Sagi and her colleagues reported this discovery in Cancer Cell.
The group’s research began with experiments to determine how Ras signaling leads to the uncontrolled growth of cancer cells. The team found that downregulation of wild-type H-Ras and N-Ras in mutant K-Ras cells caused the buildup of damaged DNA and slowed cell growth.
In the absence of H-Ras and N-Ras, K-Ras-mutant cancer cells failed to repair their DNA at the G2 phase of cell division. And this defect was caused by failure to properly activate Chk1.
With this in mind, the researchers decided to test the effects of H-Ras or N-Ras knockdown on treatment with DNA-damaging agents.
Knockdown of H-Ras or N-Ras sensitized K-Ras-mutant cancer cells to SN38 and oxaliplatin in vitro. But the same effect did not occur when H-Ras or N-Ras was knocked down in K-Ras-wild-type cancer cells.
K-Ras-mutant cancer cells were also sensitive to treatment with the Chk1/Chk2 inhibitor AZD7726 when H-Ras or N-Ras was knocked down in vitro.
To further support these findings, the researchers conducted experiments in mice with K-Ras-mutant tumors. Mice with H-Ras knockdown experienced tumor growth similar to controls.
But when the mice with H-Ras-suppressed tumors received the chemotherapy drug irinotecan, they experienced tumor regression that lasted up to 18 days post-treatment. On the other hand, mice without H-Ras suppression experienced modest tumor growth after treatment with irinotecan.
“Discovering more about how these different forms of Ras act on one another—including how they control DNA damage repair at Chk1 in combination with chemotherapy—could help us design drugs that greatly stall disease progression,” said study author Elda Grabocka, PhD, also of the New York University School of Medicine.
The researchers are now planning additional experiments on the biological interdependency of Ras proteins and what other chemotherapies might be involved in slowing cancer growth. Their goal is to map the Ras signaling pathways and identify as many therapeutic targets as possible. ![]()

Credit: PNAS
New findings suggest drugs can effectively fight K-Ras-mutant cancers—if they have a little help.
Experiments in human cancer cells showed that K-Ras-mutant tumor growth was highly dependent on the cells’ constant need to check and mend their DNA.
However, inhibiting the activity of H-Ras and N-Ras prevented the DNA damage response. And this made the cells more vulnerable to treatment.
“Our finding suggests that K-Ras cancers can be made more susceptible to existing therapies by interfering with their DNA repair mechanisms,” said Dafna Bar-Sagi, PhD, of the New York University School of Medicine.
“What some researchers have described as therapeutic ‘mission impossible’ may now become a ‘mission doable.’”
Dr Bar-Sagi and her colleagues reported this discovery in Cancer Cell.
The group’s research began with experiments to determine how Ras signaling leads to the uncontrolled growth of cancer cells. The team found that downregulation of wild-type H-Ras and N-Ras in mutant K-Ras cells caused the buildup of damaged DNA and slowed cell growth.
In the absence of H-Ras and N-Ras, K-Ras-mutant cancer cells failed to repair their DNA at the G2 phase of cell division. And this defect was caused by failure to properly activate Chk1.
With this in mind, the researchers decided to test the effects of H-Ras or N-Ras knockdown on treatment with DNA-damaging agents.
Knockdown of H-Ras or N-Ras sensitized K-Ras-mutant cancer cells to SN38 and oxaliplatin in vitro. But the same effect did not occur when H-Ras or N-Ras was knocked down in K-Ras-wild-type cancer cells.
K-Ras-mutant cancer cells were also sensitive to treatment with the Chk1/Chk2 inhibitor AZD7726 when H-Ras or N-Ras was knocked down in vitro.
To further support these findings, the researchers conducted experiments in mice with K-Ras-mutant tumors. Mice with H-Ras knockdown experienced tumor growth similar to controls.
But when the mice with H-Ras-suppressed tumors received the chemotherapy drug irinotecan, they experienced tumor regression that lasted up to 18 days post-treatment. On the other hand, mice without H-Ras suppression experienced modest tumor growth after treatment with irinotecan.
“Discovering more about how these different forms of Ras act on one another—including how they control DNA damage repair at Chk1 in combination with chemotherapy—could help us design drugs that greatly stall disease progression,” said study author Elda Grabocka, PhD, also of the New York University School of Medicine.
The researchers are now planning additional experiments on the biological interdependency of Ras proteins and what other chemotherapies might be involved in slowing cancer growth. Their goal is to map the Ras signaling pathways and identify as many therapeutic targets as possible. ![]()
Health Canada approves pomalidomide for MM

Credit: CDC
Health Canada has approved pomalidomide (Pomalyst) for use in combination with dexamethasone to treat patients with relapsed or refractory multiple myeloma (MM).
Patients must have received at least 2 prior therapies, failed treatment with lenalidomide and bortezomib, and experienced disease progression while on their last treatment regimen.
Health Canada had given pomalidomide priority review status due to the unmet need of effective therapies for patients with aggressive MM.
“Until now, there have been no approved options for patients whose disease has progressed despite available treatments,” said Donna E. Reece, MD, of the Princess Margaret Cancer Centre in Toronto.
“With Pomalyst, we have a new option that extends periods of remission, is generally well-tolerated, and can be taken in the convenience of a patient’s home.”
Trial prompts approval
Health Canada based its approval of pomalidomide on findings from the MM-003 trial. Regulatory agencies in the United States and European Union, both of which approved pomalidomide last year, based their decisions on the results of this study as well.
The phase 3 trial included 455 patients with relapsed or refractory MM who had received a median of 5 prior treatment regimens.
Patients were randomized to receive pomalidomide plus low-dose dexamethasone (POM-LoDEX, n=302) or high-dose dexamethasone alone (HiDEX, n=153). The median follow-up was 10 months.
Researchers found that response and survival rates were superior in the POM-LoDEX arm, and rates of adverse events were largely similar between the 2 arms.
The overall response rate was 31% (n=95) in the POM-LoDEX arm and 10% (n=15) in the HiDEX arm. The median duration of response was 7.0 months and 6.1 months, respectively.
The median progression-free survival was 4.0 months in the POM-LoDEX arm and 1.9 months in the HiDEX arm (P<0.001). And the median overall survival was 12.7 months in the POM-LoDEX arm and 8.1 months in the HiDEX arm (P=0.028).
Patients in the POM-LoDEX arm experienced more grade 3/4 neutropenia than patients in the HiDEX arm. But rates of grade 3/4 anemia and thrombocytopenia were similar.
Rates of grade 3/4 non-hematologic toxicities were also comparable and included infection, pneumonia, hemorrhage, glucose intolerance, and fatigue. Other adverse events of note included venous thromboembolism and peripheral neuropathy, which occurred at similar rates in both arms.
These results were presented at the 2013 ASCO Annual Meeting and published in The Lancet Oncology in October.
Drug availability
Pomalidomide is expected to be commercially available in Canada in March.
The drug will be distributed through a risk-management program called RevAid, which was developed in 2008. By adding pomalidomide to the program, regulators are aiming to prevent fetal exposure to the drug because of its structural similarities to thalidomide, a known human teratogen.
Under the program, only prescribers and pharmacists registered with RevAid are able to prescribe and dispense pomalidomide. In addition, only those patients who are registered and meet all the conditions of the RevAid program will receive the drug. ![]()

Credit: CDC
Health Canada has approved pomalidomide (Pomalyst) for use in combination with dexamethasone to treat patients with relapsed or refractory multiple myeloma (MM).
Patients must have received at least 2 prior therapies, failed treatment with lenalidomide and bortezomib, and experienced disease progression while on their last treatment regimen.
Health Canada had given pomalidomide priority review status due to the unmet need of effective therapies for patients with aggressive MM.
“Until now, there have been no approved options for patients whose disease has progressed despite available treatments,” said Donna E. Reece, MD, of the Princess Margaret Cancer Centre in Toronto.
“With Pomalyst, we have a new option that extends periods of remission, is generally well-tolerated, and can be taken in the convenience of a patient’s home.”
Trial prompts approval
Health Canada based its approval of pomalidomide on findings from the MM-003 trial. Regulatory agencies in the United States and European Union, both of which approved pomalidomide last year, based their decisions on the results of this study as well.
The phase 3 trial included 455 patients with relapsed or refractory MM who had received a median of 5 prior treatment regimens.
Patients were randomized to receive pomalidomide plus low-dose dexamethasone (POM-LoDEX, n=302) or high-dose dexamethasone alone (HiDEX, n=153). The median follow-up was 10 months.
Researchers found that response and survival rates were superior in the POM-LoDEX arm, and rates of adverse events were largely similar between the 2 arms.
The overall response rate was 31% (n=95) in the POM-LoDEX arm and 10% (n=15) in the HiDEX arm. The median duration of response was 7.0 months and 6.1 months, respectively.
The median progression-free survival was 4.0 months in the POM-LoDEX arm and 1.9 months in the HiDEX arm (P<0.001). And the median overall survival was 12.7 months in the POM-LoDEX arm and 8.1 months in the HiDEX arm (P=0.028).
Patients in the POM-LoDEX arm experienced more grade 3/4 neutropenia than patients in the HiDEX arm. But rates of grade 3/4 anemia and thrombocytopenia were similar.
Rates of grade 3/4 non-hematologic toxicities were also comparable and included infection, pneumonia, hemorrhage, glucose intolerance, and fatigue. Other adverse events of note included venous thromboembolism and peripheral neuropathy, which occurred at similar rates in both arms.
These results were presented at the 2013 ASCO Annual Meeting and published in The Lancet Oncology in October.
Drug availability
Pomalidomide is expected to be commercially available in Canada in March.
The drug will be distributed through a risk-management program called RevAid, which was developed in 2008. By adding pomalidomide to the program, regulators are aiming to prevent fetal exposure to the drug because of its structural similarities to thalidomide, a known human teratogen.
Under the program, only prescribers and pharmacists registered with RevAid are able to prescribe and dispense pomalidomide. In addition, only those patients who are registered and meet all the conditions of the RevAid program will receive the drug. ![]()

Credit: CDC
Health Canada has approved pomalidomide (Pomalyst) for use in combination with dexamethasone to treat patients with relapsed or refractory multiple myeloma (MM).
Patients must have received at least 2 prior therapies, failed treatment with lenalidomide and bortezomib, and experienced disease progression while on their last treatment regimen.
Health Canada had given pomalidomide priority review status due to the unmet need of effective therapies for patients with aggressive MM.
“Until now, there have been no approved options for patients whose disease has progressed despite available treatments,” said Donna E. Reece, MD, of the Princess Margaret Cancer Centre in Toronto.
“With Pomalyst, we have a new option that extends periods of remission, is generally well-tolerated, and can be taken in the convenience of a patient’s home.”
Trial prompts approval
Health Canada based its approval of pomalidomide on findings from the MM-003 trial. Regulatory agencies in the United States and European Union, both of which approved pomalidomide last year, based their decisions on the results of this study as well.
The phase 3 trial included 455 patients with relapsed or refractory MM who had received a median of 5 prior treatment regimens.
Patients were randomized to receive pomalidomide plus low-dose dexamethasone (POM-LoDEX, n=302) or high-dose dexamethasone alone (HiDEX, n=153). The median follow-up was 10 months.
Researchers found that response and survival rates were superior in the POM-LoDEX arm, and rates of adverse events were largely similar between the 2 arms.
The overall response rate was 31% (n=95) in the POM-LoDEX arm and 10% (n=15) in the HiDEX arm. The median duration of response was 7.0 months and 6.1 months, respectively.
The median progression-free survival was 4.0 months in the POM-LoDEX arm and 1.9 months in the HiDEX arm (P<0.001). And the median overall survival was 12.7 months in the POM-LoDEX arm and 8.1 months in the HiDEX arm (P=0.028).
Patients in the POM-LoDEX arm experienced more grade 3/4 neutropenia than patients in the HiDEX arm. But rates of grade 3/4 anemia and thrombocytopenia were similar.
Rates of grade 3/4 non-hematologic toxicities were also comparable and included infection, pneumonia, hemorrhage, glucose intolerance, and fatigue. Other adverse events of note included venous thromboembolism and peripheral neuropathy, which occurred at similar rates in both arms.
These results were presented at the 2013 ASCO Annual Meeting and published in The Lancet Oncology in October.
Drug availability
Pomalidomide is expected to be commercially available in Canada in March.
The drug will be distributed through a risk-management program called RevAid, which was developed in 2008. By adding pomalidomide to the program, regulators are aiming to prevent fetal exposure to the drug because of its structural similarities to thalidomide, a known human teratogen.
Under the program, only prescribers and pharmacists registered with RevAid are able to prescribe and dispense pomalidomide. In addition, only those patients who are registered and meet all the conditions of the RevAid program will receive the drug. ![]()
Improving the efficacy of etoposide

after treatment with etoposide
Credit: CNIO
A compound that interferes with the cell cycle can increase the antineoplastic effects of etoposide, according to research published in Cell Reports.
Etoposide works by inhibiting topoisomerase II (TOP2), a protein needed for DNA repair during cell division.
Researchers discovered a relationship between TOP2 and Cdh1, a protein that (along with Cdc20) controls cell division by activating the anaphase-promoting complex/cyclosome (APC/C).
So the team hypothesized that combining etoposide with a compound that inhibits Cdh1 might improve etoposide’s antineoplastic effects. Experiments in cancer cell lines confirmed this theory.
Marcos Malumbres, PhD, of the Spanish National Cancer Research Centre (CNIO) in Madrid, and his colleagues began this research by investigating Cdh1 in vitro and in mouse models.
The team found that a decrease in Cdh1 activity increases cells’ TOP2 levels. So they decided to combine etoposide with a Cdh1 inhibitor and evaluate the effect on cancer cells, which divide more than normal cells and therefore have a greater dependency on TOP2 to maintain DNA integrity.
The researchers tested proTAME, a small molecule that targets APC/C-Cdh1 and APC/C-Cdc20, in combination with etoposide. And they found the drugs had a synergistic effect against cancer cells.
In experiments with a lung cancer cell line (A549) and 2 breast cancer cell lines (HeLa and MCF7), administering proTAME and etoposide together proved more effective than administering either compound alone.
The researchers believe these findings could apply to other malignancies as well. Etoposide has demonstrated activity against a number of cancers, including leukemias, lymphomas, and multiple myeloma.
The team said their next step is to study the etoposide-proTAME combination in patients and investigate the malignancies in which this therapeutic strategy would be most effective.
The researchers also noted that previous studies have shown Cdh1 is inactive in some patients due to various oncogenic mutations. So stratifying patients according to their tumor’s Cdh1 status could optimize treatment with etoposide. ![]()

after treatment with etoposide
Credit: CNIO
A compound that interferes with the cell cycle can increase the antineoplastic effects of etoposide, according to research published in Cell Reports.
Etoposide works by inhibiting topoisomerase II (TOP2), a protein needed for DNA repair during cell division.
Researchers discovered a relationship between TOP2 and Cdh1, a protein that (along with Cdc20) controls cell division by activating the anaphase-promoting complex/cyclosome (APC/C).
So the team hypothesized that combining etoposide with a compound that inhibits Cdh1 might improve etoposide’s antineoplastic effects. Experiments in cancer cell lines confirmed this theory.
Marcos Malumbres, PhD, of the Spanish National Cancer Research Centre (CNIO) in Madrid, and his colleagues began this research by investigating Cdh1 in vitro and in mouse models.
The team found that a decrease in Cdh1 activity increases cells’ TOP2 levels. So they decided to combine etoposide with a Cdh1 inhibitor and evaluate the effect on cancer cells, which divide more than normal cells and therefore have a greater dependency on TOP2 to maintain DNA integrity.
The researchers tested proTAME, a small molecule that targets APC/C-Cdh1 and APC/C-Cdc20, in combination with etoposide. And they found the drugs had a synergistic effect against cancer cells.
In experiments with a lung cancer cell line (A549) and 2 breast cancer cell lines (HeLa and MCF7), administering proTAME and etoposide together proved more effective than administering either compound alone.
The researchers believe these findings could apply to other malignancies as well. Etoposide has demonstrated activity against a number of cancers, including leukemias, lymphomas, and multiple myeloma.
The team said their next step is to study the etoposide-proTAME combination in patients and investigate the malignancies in which this therapeutic strategy would be most effective.
The researchers also noted that previous studies have shown Cdh1 is inactive in some patients due to various oncogenic mutations. So stratifying patients according to their tumor’s Cdh1 status could optimize treatment with etoposide. ![]()

after treatment with etoposide
Credit: CNIO
A compound that interferes with the cell cycle can increase the antineoplastic effects of etoposide, according to research published in Cell Reports.
Etoposide works by inhibiting topoisomerase II (TOP2), a protein needed for DNA repair during cell division.
Researchers discovered a relationship between TOP2 and Cdh1, a protein that (along with Cdc20) controls cell division by activating the anaphase-promoting complex/cyclosome (APC/C).
So the team hypothesized that combining etoposide with a compound that inhibits Cdh1 might improve etoposide’s antineoplastic effects. Experiments in cancer cell lines confirmed this theory.
Marcos Malumbres, PhD, of the Spanish National Cancer Research Centre (CNIO) in Madrid, and his colleagues began this research by investigating Cdh1 in vitro and in mouse models.
The team found that a decrease in Cdh1 activity increases cells’ TOP2 levels. So they decided to combine etoposide with a Cdh1 inhibitor and evaluate the effect on cancer cells, which divide more than normal cells and therefore have a greater dependency on TOP2 to maintain DNA integrity.
The researchers tested proTAME, a small molecule that targets APC/C-Cdh1 and APC/C-Cdc20, in combination with etoposide. And they found the drugs had a synergistic effect against cancer cells.
In experiments with a lung cancer cell line (A549) and 2 breast cancer cell lines (HeLa and MCF7), administering proTAME and etoposide together proved more effective than administering either compound alone.
The researchers believe these findings could apply to other malignancies as well. Etoposide has demonstrated activity against a number of cancers, including leukemias, lymphomas, and multiple myeloma.
The team said their next step is to study the etoposide-proTAME combination in patients and investigate the malignancies in which this therapeutic strategy would be most effective.
The researchers also noted that previous studies have shown Cdh1 is inactive in some patients due to various oncogenic mutations. So stratifying patients according to their tumor’s Cdh1 status could optimize treatment with etoposide. ![]()
Unique protein found in MM patients

Credit: Rhoda Baer
Researchers say they have discovered a bacterial protein that attaches to virtually any antibody and prevents it from binding to its target.
It appears that this molecule, called mycoplasma protein (or protein M), helps some bacteria evade the immune response and establish long-term infections.
The researchers discovered protein M in samples from multiple myeloma (MM) patients.
And the team believes the protein could be engineered to target cancerous B cells in patients with MM and other B-cell malignancies.
Protein M might also become a target for new antibacterial drugs, and it could prove useful for preparing highly pure antibodies for research and drug manufacturing.
Richard A. Lerner, MD, of The Scripps Research Institute (TSRI) in La Jolla, California, and his colleagues described their discovery of protein M in Science.
The discovery originated from an effort to understand the origin of MM. Clonal B-cell proliferation, as well as MM and lymphomas, can result from chronic infections by organisms such as Escherichia coli, Helicobacter pylori, and hepatitis C virus.
To better understand this process, Dr Lerner and his colleagues investigated mycoplasma, a parasite that infects people chronically and is largely confined to the cell surface.
In a search for factors associated with long-term mycoplasma infection, the team tested samples of antibodies from MM patients’ blood against a variety of mycoplasma species. One of the proteins recognized by the antibodies was from Mycoplasma genitalium, which causes sexually transmitted infections.
To the researchers’ surprise, every antibody sample tested showed reactivity to this protein. But further tests made it clear that these antibody reactions were not in response to mass infection with M genitalium.
Instead, the M genitalium protein, which the team named protein M, appeared to have evolved simply to bind to any antibody it encounters.
“It binds to every antibody generically—capable of hijacking the entire diversity of antibody repertoire—but, at the same time, it blocks the specific interaction between that antibody and its intended biomolecular target,” said Rajesh Grover, PhD, of TSRI.
To better understand how protein M works, the researchers took a structural biology approach. Using X-ray crystallography and other techniques, the team determined the protein’s 3D atomic structure while it was bound to various human antibodies.
Compared to thousands of known structures in the Protein Data Bank, the worldwide structure database, protein M appeared to be unique. The data also revealed that protein M binds to a small, conserved region at the outer tip of every antibody’s antigen-binding arm.
“It likely extends the other end of itself, like a tail, over the antibody’s main antigen-binding region,” said Xueyong Zhu, PhD, of TSRI.
The team is now studying protein M’s function during M genitalium infections. It seems likely that the protein evolved to help M genitalium cope with the immune response, as it has one of the smallest bacterial genomes in nature.
“It appears to represent an elegant evolutionary solution to the special problem that mycoplasma have in evading the adaptive immune system,” Dr Grover said. “The smallest parasitic [bacterium] on planet Earth seems to have evolved the most sophisticated invading molecular machine.”
If protein M is confirmed as a universal “decoy” for antibodies, it will become a target for new drugs, the researchers said. This could make it easier to treat chronic, sometimes silent, infections by M genitalium and any other microbes that have evolved a similar antibody-thwarting defense.
In principle, protein M also could be engineered to target specific groups of B cells, delivering cytotoxic agents to cancerous B cells in patients with MM and lymphomas.
But the most immediate use of protein M, according to the researchers, is likely to be as a tool for grabbing antibodies in test tubes and cell cultures. This would allow the preparation of highly pure antibodies for research and drug manufacturing. Other generic antibody-binding proteins have been put to use in this way, but, so far, it appears that none does the job quite as well as protein M.
“It may be the most useful antibody purification device ever found,” said Dr Lerner, who is now working to commercialize the protein. ![]()

Credit: Rhoda Baer
Researchers say they have discovered a bacterial protein that attaches to virtually any antibody and prevents it from binding to its target.
It appears that this molecule, called mycoplasma protein (or protein M), helps some bacteria evade the immune response and establish long-term infections.
The researchers discovered protein M in samples from multiple myeloma (MM) patients.
And the team believes the protein could be engineered to target cancerous B cells in patients with MM and other B-cell malignancies.
Protein M might also become a target for new antibacterial drugs, and it could prove useful for preparing highly pure antibodies for research and drug manufacturing.
Richard A. Lerner, MD, of The Scripps Research Institute (TSRI) in La Jolla, California, and his colleagues described their discovery of protein M in Science.
The discovery originated from an effort to understand the origin of MM. Clonal B-cell proliferation, as well as MM and lymphomas, can result from chronic infections by organisms such as Escherichia coli, Helicobacter pylori, and hepatitis C virus.
To better understand this process, Dr Lerner and his colleagues investigated mycoplasma, a parasite that infects people chronically and is largely confined to the cell surface.
In a search for factors associated with long-term mycoplasma infection, the team tested samples of antibodies from MM patients’ blood against a variety of mycoplasma species. One of the proteins recognized by the antibodies was from Mycoplasma genitalium, which causes sexually transmitted infections.
To the researchers’ surprise, every antibody sample tested showed reactivity to this protein. But further tests made it clear that these antibody reactions were not in response to mass infection with M genitalium.
Instead, the M genitalium protein, which the team named protein M, appeared to have evolved simply to bind to any antibody it encounters.
“It binds to every antibody generically—capable of hijacking the entire diversity of antibody repertoire—but, at the same time, it blocks the specific interaction between that antibody and its intended biomolecular target,” said Rajesh Grover, PhD, of TSRI.
To better understand how protein M works, the researchers took a structural biology approach. Using X-ray crystallography and other techniques, the team determined the protein’s 3D atomic structure while it was bound to various human antibodies.
Compared to thousands of known structures in the Protein Data Bank, the worldwide structure database, protein M appeared to be unique. The data also revealed that protein M binds to a small, conserved region at the outer tip of every antibody’s antigen-binding arm.
“It likely extends the other end of itself, like a tail, over the antibody’s main antigen-binding region,” said Xueyong Zhu, PhD, of TSRI.
The team is now studying protein M’s function during M genitalium infections. It seems likely that the protein evolved to help M genitalium cope with the immune response, as it has one of the smallest bacterial genomes in nature.
“It appears to represent an elegant evolutionary solution to the special problem that mycoplasma have in evading the adaptive immune system,” Dr Grover said. “The smallest parasitic [bacterium] on planet Earth seems to have evolved the most sophisticated invading molecular machine.”
If protein M is confirmed as a universal “decoy” for antibodies, it will become a target for new drugs, the researchers said. This could make it easier to treat chronic, sometimes silent, infections by M genitalium and any other microbes that have evolved a similar antibody-thwarting defense.
In principle, protein M also could be engineered to target specific groups of B cells, delivering cytotoxic agents to cancerous B cells in patients with MM and lymphomas.
But the most immediate use of protein M, according to the researchers, is likely to be as a tool for grabbing antibodies in test tubes and cell cultures. This would allow the preparation of highly pure antibodies for research and drug manufacturing. Other generic antibody-binding proteins have been put to use in this way, but, so far, it appears that none does the job quite as well as protein M.
“It may be the most useful antibody purification device ever found,” said Dr Lerner, who is now working to commercialize the protein. ![]()

Credit: Rhoda Baer
Researchers say they have discovered a bacterial protein that attaches to virtually any antibody and prevents it from binding to its target.
It appears that this molecule, called mycoplasma protein (or protein M), helps some bacteria evade the immune response and establish long-term infections.
The researchers discovered protein M in samples from multiple myeloma (MM) patients.
And the team believes the protein could be engineered to target cancerous B cells in patients with MM and other B-cell malignancies.
Protein M might also become a target for new antibacterial drugs, and it could prove useful for preparing highly pure antibodies for research and drug manufacturing.
Richard A. Lerner, MD, of The Scripps Research Institute (TSRI) in La Jolla, California, and his colleagues described their discovery of protein M in Science.
The discovery originated from an effort to understand the origin of MM. Clonal B-cell proliferation, as well as MM and lymphomas, can result from chronic infections by organisms such as Escherichia coli, Helicobacter pylori, and hepatitis C virus.
To better understand this process, Dr Lerner and his colleagues investigated mycoplasma, a parasite that infects people chronically and is largely confined to the cell surface.
In a search for factors associated with long-term mycoplasma infection, the team tested samples of antibodies from MM patients’ blood against a variety of mycoplasma species. One of the proteins recognized by the antibodies was from Mycoplasma genitalium, which causes sexually transmitted infections.
To the researchers’ surprise, every antibody sample tested showed reactivity to this protein. But further tests made it clear that these antibody reactions were not in response to mass infection with M genitalium.
Instead, the M genitalium protein, which the team named protein M, appeared to have evolved simply to bind to any antibody it encounters.
“It binds to every antibody generically—capable of hijacking the entire diversity of antibody repertoire—but, at the same time, it blocks the specific interaction between that antibody and its intended biomolecular target,” said Rajesh Grover, PhD, of TSRI.
To better understand how protein M works, the researchers took a structural biology approach. Using X-ray crystallography and other techniques, the team determined the protein’s 3D atomic structure while it was bound to various human antibodies.
Compared to thousands of known structures in the Protein Data Bank, the worldwide structure database, protein M appeared to be unique. The data also revealed that protein M binds to a small, conserved region at the outer tip of every antibody’s antigen-binding arm.
“It likely extends the other end of itself, like a tail, over the antibody’s main antigen-binding region,” said Xueyong Zhu, PhD, of TSRI.
The team is now studying protein M’s function during M genitalium infections. It seems likely that the protein evolved to help M genitalium cope with the immune response, as it has one of the smallest bacterial genomes in nature.
“It appears to represent an elegant evolutionary solution to the special problem that mycoplasma have in evading the adaptive immune system,” Dr Grover said. “The smallest parasitic [bacterium] on planet Earth seems to have evolved the most sophisticated invading molecular machine.”
If protein M is confirmed as a universal “decoy” for antibodies, it will become a target for new drugs, the researchers said. This could make it easier to treat chronic, sometimes silent, infections by M genitalium and any other microbes that have evolved a similar antibody-thwarting defense.
In principle, protein M also could be engineered to target specific groups of B cells, delivering cytotoxic agents to cancerous B cells in patients with MM and lymphomas.
But the most immediate use of protein M, according to the researchers, is likely to be as a tool for grabbing antibodies in test tubes and cell cultures. This would allow the preparation of highly pure antibodies for research and drug manufacturing. Other generic antibody-binding proteins have been put to use in this way, but, so far, it appears that none does the job quite as well as protein M.
“It may be the most useful antibody purification device ever found,” said Dr Lerner, who is now working to commercialize the protein. ![]()
Physical activity may cut death risk in male cancer survivors

Credit: Jason E. Miller
Physical activity may reduce the risk of mortality in male cancer survivors, according to research published in the Journal of Physical Activity & Health.
In a study of more than 1000 male cancer survivors, participants who were most active—expending more than 12,600 kilojoules per week in physical activity—cut their risk of death roughly in half.
This was in comparison to the least active cancer survivors—those who burned fewer than 2100 kilojoules per week.
Kathleen Y. Wolin, PhD, of Loyola University Chicago Stritch School of Medicine, and her colleagues conducted this research using data from the Harvard Alumni Health Study, an ongoing study of men who entered Harvard as undergraduates between 1916 and 1950.
The researchers looked at 1021 men, with an average age of 71, who had been diagnosed with cancers.
In 1988, the men completed questionnaires reporting their physical activities, including walking, stair-climbing, and participation in sports and recreational activities. Their physical activities were updated in 1993, and researchers followed the men until 2008.
In all, 777 of the men died—337 from cancer, 190 from cardiovascular disease, 228 from other causes, and 22 from unknown causes.
Compared with men who expended fewer than 2100 kilojoules per week in physical activity, men who expended more than 12,600 kilojoules per week were 48% less likely to die of any cause during the follow-up period. (Expending 12,600 kilojoules can be achieved with about 6 to 8 hours of moderate-intensity physical activity.)
This finding was adjusted for age, smoking habits, body mass index, early parental mortality, and dietary variables.
When the researchers tried to adjust for cancer severity and treatment, they were only able to collect data for 70 men. But the results were not very different from the prior analysis. The most active men were 49% less likely to die of any cause than the least active men.
The team also decided to analyze men who were diagnosed with cancer at least 5 years before baseline (n=421). And among these men, the most active were 52% less likely than the least active to die.
Similarly, among men diagnosed at least 10 years before baseline (n=262), the most active cancer survivors were 63% less likely to die of any cause than the least active survivors.
The researchers also obtained similar results when they assessed mortality from cancer and cardiovascular disease. The most physically active cancer survivors were 38% less likely to die of cancer and 49% less likely to die of cardiovascular disease during follow-up. ![]()

Credit: Jason E. Miller
Physical activity may reduce the risk of mortality in male cancer survivors, according to research published in the Journal of Physical Activity & Health.
In a study of more than 1000 male cancer survivors, participants who were most active—expending more than 12,600 kilojoules per week in physical activity—cut their risk of death roughly in half.
This was in comparison to the least active cancer survivors—those who burned fewer than 2100 kilojoules per week.
Kathleen Y. Wolin, PhD, of Loyola University Chicago Stritch School of Medicine, and her colleagues conducted this research using data from the Harvard Alumni Health Study, an ongoing study of men who entered Harvard as undergraduates between 1916 and 1950.
The researchers looked at 1021 men, with an average age of 71, who had been diagnosed with cancers.
In 1988, the men completed questionnaires reporting their physical activities, including walking, stair-climbing, and participation in sports and recreational activities. Their physical activities were updated in 1993, and researchers followed the men until 2008.
In all, 777 of the men died—337 from cancer, 190 from cardiovascular disease, 228 from other causes, and 22 from unknown causes.
Compared with men who expended fewer than 2100 kilojoules per week in physical activity, men who expended more than 12,600 kilojoules per week were 48% less likely to die of any cause during the follow-up period. (Expending 12,600 kilojoules can be achieved with about 6 to 8 hours of moderate-intensity physical activity.)
This finding was adjusted for age, smoking habits, body mass index, early parental mortality, and dietary variables.
When the researchers tried to adjust for cancer severity and treatment, they were only able to collect data for 70 men. But the results were not very different from the prior analysis. The most active men were 49% less likely to die of any cause than the least active men.
The team also decided to analyze men who were diagnosed with cancer at least 5 years before baseline (n=421). And among these men, the most active were 52% less likely than the least active to die.
Similarly, among men diagnosed at least 10 years before baseline (n=262), the most active cancer survivors were 63% less likely to die of any cause than the least active survivors.
The researchers also obtained similar results when they assessed mortality from cancer and cardiovascular disease. The most physically active cancer survivors were 38% less likely to die of cancer and 49% less likely to die of cardiovascular disease during follow-up. ![]()

Credit: Jason E. Miller
Physical activity may reduce the risk of mortality in male cancer survivors, according to research published in the Journal of Physical Activity & Health.
In a study of more than 1000 male cancer survivors, participants who were most active—expending more than 12,600 kilojoules per week in physical activity—cut their risk of death roughly in half.
This was in comparison to the least active cancer survivors—those who burned fewer than 2100 kilojoules per week.
Kathleen Y. Wolin, PhD, of Loyola University Chicago Stritch School of Medicine, and her colleagues conducted this research using data from the Harvard Alumni Health Study, an ongoing study of men who entered Harvard as undergraduates between 1916 and 1950.
The researchers looked at 1021 men, with an average age of 71, who had been diagnosed with cancers.
In 1988, the men completed questionnaires reporting their physical activities, including walking, stair-climbing, and participation in sports and recreational activities. Their physical activities were updated in 1993, and researchers followed the men until 2008.
In all, 777 of the men died—337 from cancer, 190 from cardiovascular disease, 228 from other causes, and 22 from unknown causes.
Compared with men who expended fewer than 2100 kilojoules per week in physical activity, men who expended more than 12,600 kilojoules per week were 48% less likely to die of any cause during the follow-up period. (Expending 12,600 kilojoules can be achieved with about 6 to 8 hours of moderate-intensity physical activity.)
This finding was adjusted for age, smoking habits, body mass index, early parental mortality, and dietary variables.
When the researchers tried to adjust for cancer severity and treatment, they were only able to collect data for 70 men. But the results were not very different from the prior analysis. The most active men were 49% less likely to die of any cause than the least active men.
The team also decided to analyze men who were diagnosed with cancer at least 5 years before baseline (n=421). And among these men, the most active were 52% less likely than the least active to die.
Similarly, among men diagnosed at least 10 years before baseline (n=262), the most active cancer survivors were 63% less likely to die of any cause than the least active survivors.
The researchers also obtained similar results when they assessed mortality from cancer and cardiovascular disease. The most physically active cancer survivors were 38% less likely to die of cancer and 49% less likely to die of cardiovascular disease during follow-up. ![]()
Technique could improve assessment of MM patients

the WB-DWI scans
Photo courtesy of the
Institute of Cancer Research
A novel imaging technique could improve care for patients with multiple myeloma (MM) and reduce physicians’ reliance on bone marrow biopsies, according to researchers.
The group found that whole-body, diffusion-weighted imaging (WB-DWI) scans accurately showed the spread of MM throughout patients’ bone marrow.
And, most of the time, doctors were able to accurately determine which patients were responding to treatment by consulting the scans.
The investigators reported these results in Radiology.
They first performed WB-DWI on 8 healthy volunteers and 7 patients with MM, to assess the repeatability of quantitative apparent diffusion coefficient (ADC) estimates. ADC records how restricted water movement is within tissues.
The researchers found that ADC measurement was highly repeatable. The mean coefficient of variation was 3.8% in healthy volunteers and 2.8% in MM patients.
The team also performed pre-treatment WB-DWI scans on an additional 34 MM patients. Twenty-six of these patients had a post-treatment scan as well.
Physicians trained in imaging could pinpoint the exact sites of MM with WB-DWI, as the scans could show MM in nearly all bones. The skull remained difficult to image, however, partly because of the frequency of metal dental implants and fillings.
“This is the first time we’ve been able to obtain information from all the bones in the entire body for myeloma in 1 scan without having to rely on individual bone X-rays,” said study author Nandita deSouza, MD, of The Royal Marsden NHS Foundation Trust in the UK. “It enables us to measure the involvement of individual bones and follow their response to treatment.”
In 86% of cases, doctors were able to correctly identify whether patients responded to treatment. The physicians identified non-responders 80% of the time.
The investigators also assessed the visible changes on these scans using ADC. Changes in ADC correctly identified treatment response for 24 of 25 MM patients.
The mean ADC increased in 95% of responding patients and decreased in all non-responders (P=0.002). A 3.3% increase in ADC allowed the researchers to identify responding patients with 90% sensitivity and 100% specificity. An 8% increase in ADC yielded 70% sensitivity and 100% specificity.
The investigators said WB-DWI was suitable for more patients than conventional tests. For example, 7 patients had bone marrow biopsies, but their samples were inadequate for analysis.
“The scan is better than blood tests, which don’t tell us in which bones the cancer is located,” Dr deSouza said. “It also reduces the need for uncomfortable biopsies, which don’t reveal the extent or severity of the disease.”
The researchers did note that this study was conducted in a small number of patients. So the team plans to test the technology in more patients and refine the technique. ![]()

the WB-DWI scans
Photo courtesy of the
Institute of Cancer Research
A novel imaging technique could improve care for patients with multiple myeloma (MM) and reduce physicians’ reliance on bone marrow biopsies, according to researchers.
The group found that whole-body, diffusion-weighted imaging (WB-DWI) scans accurately showed the spread of MM throughout patients’ bone marrow.
And, most of the time, doctors were able to accurately determine which patients were responding to treatment by consulting the scans.
The investigators reported these results in Radiology.
They first performed WB-DWI on 8 healthy volunteers and 7 patients with MM, to assess the repeatability of quantitative apparent diffusion coefficient (ADC) estimates. ADC records how restricted water movement is within tissues.
The researchers found that ADC measurement was highly repeatable. The mean coefficient of variation was 3.8% in healthy volunteers and 2.8% in MM patients.
The team also performed pre-treatment WB-DWI scans on an additional 34 MM patients. Twenty-six of these patients had a post-treatment scan as well.
Physicians trained in imaging could pinpoint the exact sites of MM with WB-DWI, as the scans could show MM in nearly all bones. The skull remained difficult to image, however, partly because of the frequency of metal dental implants and fillings.
“This is the first time we’ve been able to obtain information from all the bones in the entire body for myeloma in 1 scan without having to rely on individual bone X-rays,” said study author Nandita deSouza, MD, of The Royal Marsden NHS Foundation Trust in the UK. “It enables us to measure the involvement of individual bones and follow their response to treatment.”
In 86% of cases, doctors were able to correctly identify whether patients responded to treatment. The physicians identified non-responders 80% of the time.
The investigators also assessed the visible changes on these scans using ADC. Changes in ADC correctly identified treatment response for 24 of 25 MM patients.
The mean ADC increased in 95% of responding patients and decreased in all non-responders (P=0.002). A 3.3% increase in ADC allowed the researchers to identify responding patients with 90% sensitivity and 100% specificity. An 8% increase in ADC yielded 70% sensitivity and 100% specificity.
The investigators said WB-DWI was suitable for more patients than conventional tests. For example, 7 patients had bone marrow biopsies, but their samples were inadequate for analysis.
“The scan is better than blood tests, which don’t tell us in which bones the cancer is located,” Dr deSouza said. “It also reduces the need for uncomfortable biopsies, which don’t reveal the extent or severity of the disease.”
The researchers did note that this study was conducted in a small number of patients. So the team plans to test the technology in more patients and refine the technique. ![]()

the WB-DWI scans
Photo courtesy of the
Institute of Cancer Research
A novel imaging technique could improve care for patients with multiple myeloma (MM) and reduce physicians’ reliance on bone marrow biopsies, according to researchers.
The group found that whole-body, diffusion-weighted imaging (WB-DWI) scans accurately showed the spread of MM throughout patients’ bone marrow.
And, most of the time, doctors were able to accurately determine which patients were responding to treatment by consulting the scans.
The investigators reported these results in Radiology.
They first performed WB-DWI on 8 healthy volunteers and 7 patients with MM, to assess the repeatability of quantitative apparent diffusion coefficient (ADC) estimates. ADC records how restricted water movement is within tissues.
The researchers found that ADC measurement was highly repeatable. The mean coefficient of variation was 3.8% in healthy volunteers and 2.8% in MM patients.
The team also performed pre-treatment WB-DWI scans on an additional 34 MM patients. Twenty-six of these patients had a post-treatment scan as well.
Physicians trained in imaging could pinpoint the exact sites of MM with WB-DWI, as the scans could show MM in nearly all bones. The skull remained difficult to image, however, partly because of the frequency of metal dental implants and fillings.
“This is the first time we’ve been able to obtain information from all the bones in the entire body for myeloma in 1 scan without having to rely on individual bone X-rays,” said study author Nandita deSouza, MD, of The Royal Marsden NHS Foundation Trust in the UK. “It enables us to measure the involvement of individual bones and follow their response to treatment.”
In 86% of cases, doctors were able to correctly identify whether patients responded to treatment. The physicians identified non-responders 80% of the time.
The investigators also assessed the visible changes on these scans using ADC. Changes in ADC correctly identified treatment response for 24 of 25 MM patients.
The mean ADC increased in 95% of responding patients and decreased in all non-responders (P=0.002). A 3.3% increase in ADC allowed the researchers to identify responding patients with 90% sensitivity and 100% specificity. An 8% increase in ADC yielded 70% sensitivity and 100% specificity.
The investigators said WB-DWI was suitable for more patients than conventional tests. For example, 7 patients had bone marrow biopsies, but their samples were inadequate for analysis.
“The scan is better than blood tests, which don’t tell us in which bones the cancer is located,” Dr deSouza said. “It also reduces the need for uncomfortable biopsies, which don’t reveal the extent or severity of the disease.”
The researchers did note that this study was conducted in a small number of patients. So the team plans to test the technology in more patients and refine the technique.
ACA exchanges limiting for patients with blood cancers, report suggests

Credit: CDC
A new report suggests that many health plans in the insurance exchanges mandated by the Affordable Care Act (ACA) will impose high out-of-pocket costs for patients with hematologic malignancies and provide limited access to specialty treatment centers.
Furthermore, although the plans analyzed appear to provide adequate coverage of hematology/oncology drugs, most require prior authorization.
In other words, the insurer must be notified and may not approve the purchase of a drug based on medical evidence or other criteria.
This report, “2014 Individual Exchange Policies in Four States: An Early Look for Patients with Blood Cancers,” was commissioned by the Leukemia & Lymphoma Society and prepared by Milliman, Inc.
It provides a look at the 2014 individual benefit designs, coverage benefits, and premiums for policies sold on 4 state health insurance exchanges—California, New York, Florida, and Texas—with a focus on items of interest for patients with hematologic malignancies.
“[W]hile many new rules under ACA make obtaining insurance easier for people with blood cancers, such as prohibiting companies from turning away patients with pre-existing conditions and eliminating lifetime coverage limitations, the Milliman report identifies several areas of concern that we want cancer patients to be aware of and policymakers to address,” said Mark Velleca, MD, PhD, chief policy and advocacy officer of the Leukemia & Lymphoma Society.
Premium costs
To compare monthly premium rates, the report’s authors captured rates for a 50-year-old non-smoker with an annual income of $90,000 residing in Houston, Los Angeles, Miami, or New York City.
They found considerable variation according to plan type and location, but overall, plans were cheapest in Houston. Monthly premiums for Houston ranged from $234 to $520. The range was $274 to $566 for Los Angeles, $277 to $635 for Miami, and $307 to $896 for New York.
The ranges reflect the costs according to plan type. Each insurer offers 4 different health plans: Platinum (about 10% cost-sharing), Gold (roughly 20%), Silver (roughly 30%), and Bronze (roughly 40%).
Cost-sharing
The authors noted that the lower-tier Bronze and Silver plans require significant cost-sharing for patients. The report revealed high deductibles in the health plans, sometimes nearly as high as the out-of-pocket ceiling.
Deductibles for the Silver and Bronze plans are often at least $2000 and $4000, respectively, for individuals. The maximum out-of-pocket limits set for 2014 are $6350 for an individual policy and $12,799 for a family policy.
Some insurers offer plans in some states with lower out-of-pocket limits. However, the out-of-pocket limit does not apply to non-covered drugs or treatment centers.
Drug coverage
When analyzing drug coverage, the authors decided to look at 3 drugs used to treat chronic myeloid leukemia—imatinib (Gleevec), nilotinib (Tasigna), and dasatinib (Sprycel)—and 5 drugs used to treat multiple myeloma—thalidomide (Thalomid), lenalidomide (Revlimid), pomalidomide (Pomalyst), cyclophosphamide (Cytoxan), and melphalan (Alkeran).
Most of the insurers require prior authorization for these drugs, but most of them cover all 3 chronic myeloid leukemia drugs and a majority of the myeloma drugs. Pomalyst and Cytoxan are often not covered, although most insurers do cover generic cyclophosphamide.
Network adequacy
Most of the insurers studied do not cover all NCI-designated cancer and transplant centers, and a few do not cover any of these centers. The authors said this could discourage patient enrollment in these plans or mean that a patient’s recommended treatment is not covered.
And since it is unlikely that any out-of-network expenses will count toward a patient’s out-of-pocket maximum, cancer patients could accumulate thousands of dollars of medical expenses and never reach their out-of-pocket maximum.
The authors did note, however, that satisfactory cancer care can be provided outside of NCI-designated cancer and transplant centers.
For more details, see the full report.

Credit: CDC
A new report suggests that many health plans in the insurance exchanges mandated by the Affordable Care Act (ACA) will impose high out-of-pocket costs for patients with hematologic malignancies and provide limited access to specialty treatment centers.
Furthermore, although the plans analyzed appear to provide adequate coverage of hematology/oncology drugs, most require prior authorization.
In other words, the insurer must be notified and may not approve the purchase of a drug based on medical evidence or other criteria.
This report, “2014 Individual Exchange Policies in Four States: An Early Look for Patients with Blood Cancers,” was commissioned by the Leukemia & Lymphoma Society and prepared by Milliman, Inc.
It provides a look at the 2014 individual benefit designs, coverage benefits, and premiums for policies sold on 4 state health insurance exchanges—California, New York, Florida, and Texas—with a focus on items of interest for patients with hematologic malignancies.
“[W]hile many new rules under ACA make obtaining insurance easier for people with blood cancers, such as prohibiting companies from turning away patients with pre-existing conditions and eliminating lifetime coverage limitations, the Milliman report identifies several areas of concern that we want cancer patients to be aware of and policymakers to address,” said Mark Velleca, MD, PhD, chief policy and advocacy officer of the Leukemia & Lymphoma Society.
Premium costs
To compare monthly premium rates, the report’s authors captured rates for a 50-year-old non-smoker with an annual income of $90,000 residing in Houston, Los Angeles, Miami, or New York City.
They found considerable variation according to plan type and location, but overall, plans were cheapest in Houston. Monthly premiums for Houston ranged from $234 to $520. The range was $274 to $566 for Los Angeles, $277 to $635 for Miami, and $307 to $896 for New York.
The ranges reflect the costs according to plan type. Each insurer offers 4 different health plans: Platinum (about 10% cost-sharing), Gold (roughly 20%), Silver (roughly 30%), and Bronze (roughly 40%).
Cost-sharing
The authors noted that the lower-tier Bronze and Silver plans require significant cost-sharing for patients. The report revealed high deductibles in the health plans, sometimes nearly as high as the out-of-pocket ceiling.
Deductibles for the Silver and Bronze plans are often at least $2000 and $4000, respectively, for individuals. The maximum out-of-pocket limits set for 2014 are $6350 for an individual policy and $12,799 for a family policy.
Some insurers offer plans in some states with lower out-of-pocket limits. However, the out-of-pocket limit does not apply to non-covered drugs or treatment centers.
Drug coverage
When analyzing drug coverage, the authors decided to look at 3 drugs used to treat chronic myeloid leukemia—imatinib (Gleevec), nilotinib (Tasigna), and dasatinib (Sprycel)—and 5 drugs used to treat multiple myeloma—thalidomide (Thalomid), lenalidomide (Revlimid), pomalidomide (Pomalyst), cyclophosphamide (Cytoxan), and melphalan (Alkeran).
Most of the insurers require prior authorization for these drugs, but most of them cover all 3 chronic myeloid leukemia drugs and a majority of the myeloma drugs. Pomalyst and Cytoxan are often not covered, although most insurers do cover generic cyclophosphamide.
Network adequacy
Most of the insurers studied do not cover all NCI-designated cancer and transplant centers, and a few do not cover any of these centers. The authors said this could discourage patient enrollment in these plans or mean that a patient’s recommended treatment is not covered.
And since it is unlikely that any out-of-network expenses will count toward a patient’s out-of-pocket maximum, cancer patients could accumulate thousands of dollars of medical expenses and never reach their out-of-pocket maximum.
The authors did note, however, that satisfactory cancer care can be provided outside of NCI-designated cancer and transplant centers.
For more details, see the full report.

Credit: CDC
A new report suggests that many health plans in the insurance exchanges mandated by the Affordable Care Act (ACA) will impose high out-of-pocket costs for patients with hematologic malignancies and provide limited access to specialty treatment centers.
Furthermore, although the plans analyzed appear to provide adequate coverage of hematology/oncology drugs, most require prior authorization.
In other words, the insurer must be notified and may not approve the purchase of a drug based on medical evidence or other criteria.
This report, “2014 Individual Exchange Policies in Four States: An Early Look for Patients with Blood Cancers,” was commissioned by the Leukemia & Lymphoma Society and prepared by Milliman, Inc.
It provides a look at the 2014 individual benefit designs, coverage benefits, and premiums for policies sold on 4 state health insurance exchanges—California, New York, Florida, and Texas—with a focus on items of interest for patients with hematologic malignancies.
“[W]hile many new rules under ACA make obtaining insurance easier for people with blood cancers, such as prohibiting companies from turning away patients with pre-existing conditions and eliminating lifetime coverage limitations, the Milliman report identifies several areas of concern that we want cancer patients to be aware of and policymakers to address,” said Mark Velleca, MD, PhD, chief policy and advocacy officer of the Leukemia & Lymphoma Society.
Premium costs
To compare monthly premium rates, the report’s authors captured rates for a 50-year-old non-smoker with an annual income of $90,000 residing in Houston, Los Angeles, Miami, or New York City.
They found considerable variation according to plan type and location, but overall, plans were cheapest in Houston. Monthly premiums for Houston ranged from $234 to $520. The range was $274 to $566 for Los Angeles, $277 to $635 for Miami, and $307 to $896 for New York.
The ranges reflect the costs according to plan type. Each insurer offers 4 different health plans: Platinum (about 10% cost-sharing), Gold (roughly 20%), Silver (roughly 30%), and Bronze (roughly 40%).
Cost-sharing
The authors noted that the lower-tier Bronze and Silver plans require significant cost-sharing for patients. The report revealed high deductibles in the health plans, sometimes nearly as high as the out-of-pocket ceiling.
Deductibles for the Silver and Bronze plans are often at least $2000 and $4000, respectively, for individuals. The maximum out-of-pocket limits set for 2014 are $6350 for an individual policy and $12,799 for a family policy.
Some insurers offer plans in some states with lower out-of-pocket limits. However, the out-of-pocket limit does not apply to non-covered drugs or treatment centers.
Drug coverage
When analyzing drug coverage, the authors decided to look at 3 drugs used to treat chronic myeloid leukemia—imatinib (Gleevec), nilotinib (Tasigna), and dasatinib (Sprycel)—and 5 drugs used to treat multiple myeloma—thalidomide (Thalomid), lenalidomide (Revlimid), pomalidomide (Pomalyst), cyclophosphamide (Cytoxan), and melphalan (Alkeran).
Most of the insurers require prior authorization for these drugs, but most of them cover all 3 chronic myeloid leukemia drugs and a majority of the myeloma drugs. Pomalyst and Cytoxan are often not covered, although most insurers do cover generic cyclophosphamide.
Network adequacy
Most of the insurers studied do not cover all NCI-designated cancer and transplant centers, and a few do not cover any of these centers. The authors said this could discourage patient enrollment in these plans or mean that a patient’s recommended treatment is not covered.
And since it is unlikely that any out-of-network expenses will count toward a patient’s out-of-pocket maximum, cancer patients could accumulate thousands of dollars of medical expenses and never reach their out-of-pocket maximum.
The authors did note, however, that satisfactory cancer care can be provided outside of NCI-designated cancer and transplant centers.
For more details, see the full report.
Compound active against a range of cancers
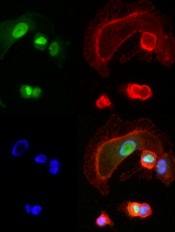
expressing NFAT3c-GFP
A little-studied chemical compound has “wide and potent” anticancer activity, investigators have reported in Cancer Cell.
The compound, BMH-21, works by inhibiting the RNA polymerase transcription pathway (Pol I), thereby preventing cancer cell communication and replication.
“Without this transcription machinery, cancer cells cannot function,” said study author Marikki Laiho, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
She and her colleagues homed in on BMH-21 by screening a library of chemical compounds thought to have potential for anticancer activity.
Specifically, the team looked at the compounds’ ability to interfere with transcription in the National Cancer Institute’s collection of 60 human tumor cell lines (known as NCI-60).
BMH-21 demonstrated activity against all 9 cancer types studied—leukemia and melanoma, as well as breast, CNS, colon, lung, ovarian, prostate, and renal cancers.
The drug also repressed tumor growth in mouse models of colon cancer and melanoma.
Additional analyses showed that BMH-21 inhibited Pol I transcription and caused disintegration of the nucleolus. The drug activated loss of the Pol I catalytic subunit RPA194, which led to disassembly of the Pol I holocomplex from the ribosomal DNA.
And the loss of RPA194, which was a result of increased proteasome-mediated turnover, was associated with decreased cancer cell viability.
Dr Laiho and her colleagues are continuing studies of BMH-21 in animal models to confirm the drug’s anticancer activity, identify any toxicities associated with the compound, and determine the optimal dose.
And because Pol I activity is frequently deregulated in cancers, the investigators believe BMH-21 could have therapeutic potential for many malignancies.
Dr Laiho is currently collaborating with experts in multiple myeloma, medullary thyroid cancer, and prostate cancer to explore the drug’s activity in these malignancies.

expressing NFAT3c-GFP
A little-studied chemical compound has “wide and potent” anticancer activity, investigators have reported in Cancer Cell.
The compound, BMH-21, works by inhibiting the RNA polymerase transcription pathway (Pol I), thereby preventing cancer cell communication and replication.
“Without this transcription machinery, cancer cells cannot function,” said study author Marikki Laiho, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
She and her colleagues homed in on BMH-21 by screening a library of chemical compounds thought to have potential for anticancer activity.
Specifically, the team looked at the compounds’ ability to interfere with transcription in the National Cancer Institute’s collection of 60 human tumor cell lines (known as NCI-60).
BMH-21 demonstrated activity against all 9 cancer types studied—leukemia and melanoma, as well as breast, CNS, colon, lung, ovarian, prostate, and renal cancers.
The drug also repressed tumor growth in mouse models of colon cancer and melanoma.
Additional analyses showed that BMH-21 inhibited Pol I transcription and caused disintegration of the nucleolus. The drug activated loss of the Pol I catalytic subunit RPA194, which led to disassembly of the Pol I holocomplex from the ribosomal DNA.
And the loss of RPA194, which was a result of increased proteasome-mediated turnover, was associated with decreased cancer cell viability.
Dr Laiho and her colleagues are continuing studies of BMH-21 in animal models to confirm the drug’s anticancer activity, identify any toxicities associated with the compound, and determine the optimal dose.
And because Pol I activity is frequently deregulated in cancers, the investigators believe BMH-21 could have therapeutic potential for many malignancies.
Dr Laiho is currently collaborating with experts in multiple myeloma, medullary thyroid cancer, and prostate cancer to explore the drug’s activity in these malignancies.

expressing NFAT3c-GFP
A little-studied chemical compound has “wide and potent” anticancer activity, investigators have reported in Cancer Cell.
The compound, BMH-21, works by inhibiting the RNA polymerase transcription pathway (Pol I), thereby preventing cancer cell communication and replication.
“Without this transcription machinery, cancer cells cannot function,” said study author Marikki Laiho, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
She and her colleagues homed in on BMH-21 by screening a library of chemical compounds thought to have potential for anticancer activity.
Specifically, the team looked at the compounds’ ability to interfere with transcription in the National Cancer Institute’s collection of 60 human tumor cell lines (known as NCI-60).
BMH-21 demonstrated activity against all 9 cancer types studied—leukemia and melanoma, as well as breast, CNS, colon, lung, ovarian, prostate, and renal cancers.
The drug also repressed tumor growth in mouse models of colon cancer and melanoma.
Additional analyses showed that BMH-21 inhibited Pol I transcription and caused disintegration of the nucleolus. The drug activated loss of the Pol I catalytic subunit RPA194, which led to disassembly of the Pol I holocomplex from the ribosomal DNA.
And the loss of RPA194, which was a result of increased proteasome-mediated turnover, was associated with decreased cancer cell viability.
Dr Laiho and her colleagues are continuing studies of BMH-21 in animal models to confirm the drug’s anticancer activity, identify any toxicities associated with the compound, and determine the optimal dose.
And because Pol I activity is frequently deregulated in cancers, the investigators believe BMH-21 could have therapeutic potential for many malignancies.
Dr Laiho is currently collaborating with experts in multiple myeloma, medullary thyroid cancer, and prostate cancer to explore the drug’s activity in these malignancies.
Study reveals ‘widespread’ genetic heterogeneity in MM
different colored cells
Credit: Lauren Solomon
Results of a new study suggest the genetic landscape of multiple myeloma (MM) may be more complex than we thought.
The research revealed “widespread” heterogeneity in samples from more than 200 MM patients.
In some cases, a single patient had multiple mutations in the same pathway. And most of the patients harbored at least 3 detectable subclonal mutations.
The researchers said these findings, published in Cancer Cell, might explain why targeted therapies are not always effective in MM and why some patients relapse after treatment.
“What this new work shows us is that when we treat an individual patient with multiple myeloma, it’s possible that we’re not just looking at one disease, but at many,” said study author Todd Golub, MD, of the Dana-Farber Cancer Institute in Cambridge, Massachusetts.
“In the same person, there could be cancer cells with different genetic make-ups. These findings indicate a need to identify the extent of genetic diversity within a tumor as we move toward precision cancer medicine and genome-based diagnostics.”
Dr Golub and his colleagues studied samples from 203 MM patients and identified frequent mutations in genes known to play an important role in MM, including KRAS, NRAS, and BRAF.
But many of these telltale mutations were not present in all MM cells. Instead, they were often observed only in a subclonal population.
This suggests targeted therapies may have limitations in patients whose tumors are made up of these subclonal populations, the researchers said.
To explore the therapeutic implications of this research, the team performed follow-up experiments looking specifically at BRAF, a gene for which several inhibitors exist.
Previous studies indicated that roughly 4% of MM patients may have mutations in this gene. And a recent report on a single MM patient treated with drugs targeting BRAF showed promising results.
However, Dr Golub and his colleagues found evidence that treating a tumor harboring subclonal BRAF mutations with one of these agents may, at best, kill a fraction of the cells and, at worst, stimulate another cancer cell subpopulation to grow.
“There’s clearly potential for these drugs in some patients with multiple myeloma, but we show that there are also potential problems for others,” said study author Jens Lohr, MD, PhD, also of Dana-Farber.
“If a patient has a BRAF mutation in less than 100% of his cells, or if he has mutations in KRAS or NRAS at the same time, [it] may influence the response to an inhibitor.”
This suggests subclonal populations could be one of the reasons many patients suffer relapse after treatment, the researchers said.
“Matching the right drug to the right patient may not be as easy as finding a mutation and having a drug that targets it,” Dr Lohr said. “We have to keep this additional parameter of heterogeneity in mind and keep exploring what it means for therapy.”

different colored cells
Credit: Lauren Solomon
Results of a new study suggest the genetic landscape of multiple myeloma (MM) may be more complex than we thought.
The research revealed “widespread” heterogeneity in samples from more than 200 MM patients.
In some cases, a single patient had multiple mutations in the same pathway. And most of the patients harbored at least 3 detectable subclonal mutations.
The researchers said these findings, published in Cancer Cell, might explain why targeted therapies are not always effective in MM and why some patients relapse after treatment.
“What this new work shows us is that when we treat an individual patient with multiple myeloma, it’s possible that we’re not just looking at one disease, but at many,” said study author Todd Golub, MD, of the Dana-Farber Cancer Institute in Cambridge, Massachusetts.
“In the same person, there could be cancer cells with different genetic make-ups. These findings indicate a need to identify the extent of genetic diversity within a tumor as we move toward precision cancer medicine and genome-based diagnostics.”
Dr Golub and his colleagues studied samples from 203 MM patients and identified frequent mutations in genes known to play an important role in MM, including KRAS, NRAS, and BRAF.
But many of these telltale mutations were not present in all MM cells. Instead, they were often observed only in a subclonal population.
This suggests targeted therapies may have limitations in patients whose tumors are made up of these subclonal populations, the researchers said.
To explore the therapeutic implications of this research, the team performed follow-up experiments looking specifically at BRAF, a gene for which several inhibitors exist.
Previous studies indicated that roughly 4% of MM patients may have mutations in this gene. And a recent report on a single MM patient treated with drugs targeting BRAF showed promising results.
However, Dr Golub and his colleagues found evidence that treating a tumor harboring subclonal BRAF mutations with one of these agents may, at best, kill a fraction of the cells and, at worst, stimulate another cancer cell subpopulation to grow.
“There’s clearly potential for these drugs in some patients with multiple myeloma, but we show that there are also potential problems for others,” said study author Jens Lohr, MD, PhD, also of Dana-Farber.
“If a patient has a BRAF mutation in less than 100% of his cells, or if he has mutations in KRAS or NRAS at the same time, [it] may influence the response to an inhibitor.”
This suggests subclonal populations could be one of the reasons many patients suffer relapse after treatment, the researchers said.
“Matching the right drug to the right patient may not be as easy as finding a mutation and having a drug that targets it,” Dr Lohr said. “We have to keep this additional parameter of heterogeneity in mind and keep exploring what it means for therapy.”

different colored cells
Credit: Lauren Solomon
Results of a new study suggest the genetic landscape of multiple myeloma (MM) may be more complex than we thought.
The research revealed “widespread” heterogeneity in samples from more than 200 MM patients.
In some cases, a single patient had multiple mutations in the same pathway. And most of the patients harbored at least 3 detectable subclonal mutations.
The researchers said these findings, published in Cancer Cell, might explain why targeted therapies are not always effective in MM and why some patients relapse after treatment.
“What this new work shows us is that when we treat an individual patient with multiple myeloma, it’s possible that we’re not just looking at one disease, but at many,” said study author Todd Golub, MD, of the Dana-Farber Cancer Institute in Cambridge, Massachusetts.
“In the same person, there could be cancer cells with different genetic make-ups. These findings indicate a need to identify the extent of genetic diversity within a tumor as we move toward precision cancer medicine and genome-based diagnostics.”
Dr Golub and his colleagues studied samples from 203 MM patients and identified frequent mutations in genes known to play an important role in MM, including KRAS, NRAS, and BRAF.
But many of these telltale mutations were not present in all MM cells. Instead, they were often observed only in a subclonal population.
This suggests targeted therapies may have limitations in patients whose tumors are made up of these subclonal populations, the researchers said.
To explore the therapeutic implications of this research, the team performed follow-up experiments looking specifically at BRAF, a gene for which several inhibitors exist.
Previous studies indicated that roughly 4% of MM patients may have mutations in this gene. And a recent report on a single MM patient treated with drugs targeting BRAF showed promising results.
However, Dr Golub and his colleagues found evidence that treating a tumor harboring subclonal BRAF mutations with one of these agents may, at best, kill a fraction of the cells and, at worst, stimulate another cancer cell subpopulation to grow.
“There’s clearly potential for these drugs in some patients with multiple myeloma, but we show that there are also potential problems for others,” said study author Jens Lohr, MD, PhD, also of Dana-Farber.
“If a patient has a BRAF mutation in less than 100% of his cells, or if he has mutations in KRAS or NRAS at the same time, [it] may influence the response to an inhibitor.”
This suggests subclonal populations could be one of the reasons many patients suffer relapse after treatment, the researchers said.
“Matching the right drug to the right patient may not be as easy as finding a mutation and having a drug that targets it,” Dr Lohr said. “We have to keep this additional parameter of heterogeneity in mind and keep exploring what it means for therapy.”