User login
Some Peripartum Cardiomyopathy Can Be Deadly
STOCKHOLM – Healthy women who are first-degree relatives of patients with known familial dilated cardiomyopathy should be followed closely during pregnancy and the first 5 months post partum for the emergence of life-threatening peripartum cardiomyopathy.
A significant subset of peripartum cardiomyopathy is the initial manifestation of familial dilated cardiomyopathy. Thus, when women with peripartum cardiomyopathy don't fully recover left ventricular function, their first-degree family members should undergo screening for presymptomatic dilated cardiomyopathy, Dr. Karin Y. van Spaendonck-Zwarts said.
Dr. van Spaendonck-Zwarts offered three lines of evidence in support of the hypothesis that a subset of peripartum cardiomyopathy is actually a first manifestation of familial dilated cardiomyopathy rather than, as previously assumed, a sporadic event.
One, in reviewing a Dutch cohort of 90 proven dilated cardiomyopathy families, she and her coinvestigators discovered five families, or 6%, had members with peripartum cardiomyopathy. This is far too high a figure to be due to chance.
Peripartum cardiomyopathy is a rare disorder, with an estimated incidence in the United States of 1 case in 4,075 live births, noted Dr. van Spaendonck-Zwarts of University Medical Center, Groningen, the Netherlands.
Second, when the Dutch team reviewed their 10 most recent cases of peripartum cardiomyopathy, they found 3 of the 10 women did not show a full recovery of left ventricular ejection fraction and dimensions. Cardiologic screening of all first-degree relatives of these three women revealed previously undiagnosed cases of dilated cardiomyopathy in all three families.
Third, an in-depth genetic analysis conducted in one of the dilated cardiomyopathy families with a member who had peripartum cardiomyopathy revealed a mutation in TNNC1, the gene encoding cardiac troponin C. This supports the genetic nature of the disease in this particular case of peripartum cardiomyopathy.
“Together, these findings strongly suggest that a subset of peripartum cardiomyopathy is part of the spectrum of familial dilated cardiomyopathy presenting in the peripartum period,” said Dr. van Spaendonck-Zwarts.
If the Dutch findings are confirmed elsewhere, the European Society of Cardiology classification of the cardiomyopathies may need to be amended. The ESC characterizes peripartum cardiomyopathy as a nonfamilial, nongenetic form of dilated cardiomyopathy, she observed.
Dr. van Spaendonck-Zwarts declared she has no relevant financial interests.
'A subset of peripartum cardiomyopathy is part of the spectrum of familial dilated cardiomyopathy.'
Source DR. VAN SPAENDONCK-ZWARTS
STOCKHOLM – Healthy women who are first-degree relatives of patients with known familial dilated cardiomyopathy should be followed closely during pregnancy and the first 5 months post partum for the emergence of life-threatening peripartum cardiomyopathy.
A significant subset of peripartum cardiomyopathy is the initial manifestation of familial dilated cardiomyopathy. Thus, when women with peripartum cardiomyopathy don't fully recover left ventricular function, their first-degree family members should undergo screening for presymptomatic dilated cardiomyopathy, Dr. Karin Y. van Spaendonck-Zwarts said.
Dr. van Spaendonck-Zwarts offered three lines of evidence in support of the hypothesis that a subset of peripartum cardiomyopathy is actually a first manifestation of familial dilated cardiomyopathy rather than, as previously assumed, a sporadic event.
One, in reviewing a Dutch cohort of 90 proven dilated cardiomyopathy families, she and her coinvestigators discovered five families, or 6%, had members with peripartum cardiomyopathy. This is far too high a figure to be due to chance.
Peripartum cardiomyopathy is a rare disorder, with an estimated incidence in the United States of 1 case in 4,075 live births, noted Dr. van Spaendonck-Zwarts of University Medical Center, Groningen, the Netherlands.
Second, when the Dutch team reviewed their 10 most recent cases of peripartum cardiomyopathy, they found 3 of the 10 women did not show a full recovery of left ventricular ejection fraction and dimensions. Cardiologic screening of all first-degree relatives of these three women revealed previously undiagnosed cases of dilated cardiomyopathy in all three families.
Third, an in-depth genetic analysis conducted in one of the dilated cardiomyopathy families with a member who had peripartum cardiomyopathy revealed a mutation in TNNC1, the gene encoding cardiac troponin C. This supports the genetic nature of the disease in this particular case of peripartum cardiomyopathy.
“Together, these findings strongly suggest that a subset of peripartum cardiomyopathy is part of the spectrum of familial dilated cardiomyopathy presenting in the peripartum period,” said Dr. van Spaendonck-Zwarts.
If the Dutch findings are confirmed elsewhere, the European Society of Cardiology classification of the cardiomyopathies may need to be amended. The ESC characterizes peripartum cardiomyopathy as a nonfamilial, nongenetic form of dilated cardiomyopathy, she observed.
Dr. van Spaendonck-Zwarts declared she has no relevant financial interests.
'A subset of peripartum cardiomyopathy is part of the spectrum of familial dilated cardiomyopathy.'
Source DR. VAN SPAENDONCK-ZWARTS
STOCKHOLM – Healthy women who are first-degree relatives of patients with known familial dilated cardiomyopathy should be followed closely during pregnancy and the first 5 months post partum for the emergence of life-threatening peripartum cardiomyopathy.
A significant subset of peripartum cardiomyopathy is the initial manifestation of familial dilated cardiomyopathy. Thus, when women with peripartum cardiomyopathy don't fully recover left ventricular function, their first-degree family members should undergo screening for presymptomatic dilated cardiomyopathy, Dr. Karin Y. van Spaendonck-Zwarts said.
Dr. van Spaendonck-Zwarts offered three lines of evidence in support of the hypothesis that a subset of peripartum cardiomyopathy is actually a first manifestation of familial dilated cardiomyopathy rather than, as previously assumed, a sporadic event.
One, in reviewing a Dutch cohort of 90 proven dilated cardiomyopathy families, she and her coinvestigators discovered five families, or 6%, had members with peripartum cardiomyopathy. This is far too high a figure to be due to chance.
Peripartum cardiomyopathy is a rare disorder, with an estimated incidence in the United States of 1 case in 4,075 live births, noted Dr. van Spaendonck-Zwarts of University Medical Center, Groningen, the Netherlands.
Second, when the Dutch team reviewed their 10 most recent cases of peripartum cardiomyopathy, they found 3 of the 10 women did not show a full recovery of left ventricular ejection fraction and dimensions. Cardiologic screening of all first-degree relatives of these three women revealed previously undiagnosed cases of dilated cardiomyopathy in all three families.
Third, an in-depth genetic analysis conducted in one of the dilated cardiomyopathy families with a member who had peripartum cardiomyopathy revealed a mutation in TNNC1, the gene encoding cardiac troponin C. This supports the genetic nature of the disease in this particular case of peripartum cardiomyopathy.
“Together, these findings strongly suggest that a subset of peripartum cardiomyopathy is part of the spectrum of familial dilated cardiomyopathy presenting in the peripartum period,” said Dr. van Spaendonck-Zwarts.
If the Dutch findings are confirmed elsewhere, the European Society of Cardiology classification of the cardiomyopathies may need to be amended. The ESC characterizes peripartum cardiomyopathy as a nonfamilial, nongenetic form of dilated cardiomyopathy, she observed.
Dr. van Spaendonck-Zwarts declared she has no relevant financial interests.
'A subset of peripartum cardiomyopathy is part of the spectrum of familial dilated cardiomyopathy.'
Source DR. VAN SPAENDONCK-ZWARTS
From the Annual Congress of the European Society Of Cardiology
Buprenorphine Is Alternative to Methadone During Pregnancy
For opioid-dependent women who are pregnant, buprenorphine appears to offer an effective, safe, first-line alternative to methadone.
In a randomized clinical trial comparing pregnancy outcomes among women seeking treatment for opioid dependence, infants exposed in utero to buprenorphine developed significantly less severe neonatal abstinence syndrome than did infants exposed in utero to methadone, said Hendrée E. Jones, Ph.D., of Johns Hopkins University, Baltimore, and her associates.
The study involved 175 women aged 18–41 years who were at 6–30 weeks' gestation when they entered treatment for opioid dependence at eight sites in the United States, Austria, and Canada. Eighty-six were randomly assigned to receive oral buprenorphine and 89 to receive oral methadone in a double-blind fashion.
After delivery, their neonates were assessed for signs and symptoms of neonatal abstinence syndrome (NAS) twice a day for at least 10 days.
There were five primary neonatal outcomes. Three of these – percentage of neonates requiring NAS treatment, peak NAS scores, and head circumference – did not differ between the two study groups. However, two of the five primary outcomes – amount of morphine required to treat NAS and length of hospital stay – favored the infants in the buprenorphine group. On average, infants exposed to buprenorphine required 89% less morphine and spent 43% less time in the hospital (10 days vs. 17.5 days) than did infants exposed to methadone.
“The benefits of buprenorphine in reducing the severity of NAS among neonates with this complication suggest that it should be considered a first-line treatment option in pregnancy,” they said (N. Engl. J. Med. 2010;363:2320-31).
Despite the comparable or even superior efficacy and safety of buprenorphine, there was one important drawback with the therapy: Women were more likely to discontinue treatment for opioid dependency with buprenorphine (33%) than with methadone (18%).
Most (71%) of the women in the buprenorphine group who discontinued treatment cited “dissatisfaction” with the drug as their reason, while only 13% of those in the methadone group did so.
Future research should focus on reducing this “dissatisfaction” as well as on identifying “subpopulations of pregnant patients who are more likely to have a response to one medication than to the other,” they added.
This study was funded by grants from the National Institute on Drug Abuse. Buprenorphine tablets and the associated placebo were supplied by Reckitt Benckiser Healthcare. Dr. Jones's associates reported ties to numerous drug companies.
For opioid-dependent women who are pregnant, buprenorphine appears to offer an effective, safe, first-line alternative to methadone.
In a randomized clinical trial comparing pregnancy outcomes among women seeking treatment for opioid dependence, infants exposed in utero to buprenorphine developed significantly less severe neonatal abstinence syndrome than did infants exposed in utero to methadone, said Hendrée E. Jones, Ph.D., of Johns Hopkins University, Baltimore, and her associates.
The study involved 175 women aged 18–41 years who were at 6–30 weeks' gestation when they entered treatment for opioid dependence at eight sites in the United States, Austria, and Canada. Eighty-six were randomly assigned to receive oral buprenorphine and 89 to receive oral methadone in a double-blind fashion.
After delivery, their neonates were assessed for signs and symptoms of neonatal abstinence syndrome (NAS) twice a day for at least 10 days.
There were five primary neonatal outcomes. Three of these – percentage of neonates requiring NAS treatment, peak NAS scores, and head circumference – did not differ between the two study groups. However, two of the five primary outcomes – amount of morphine required to treat NAS and length of hospital stay – favored the infants in the buprenorphine group. On average, infants exposed to buprenorphine required 89% less morphine and spent 43% less time in the hospital (10 days vs. 17.5 days) than did infants exposed to methadone.
“The benefits of buprenorphine in reducing the severity of NAS among neonates with this complication suggest that it should be considered a first-line treatment option in pregnancy,” they said (N. Engl. J. Med. 2010;363:2320-31).
Despite the comparable or even superior efficacy and safety of buprenorphine, there was one important drawback with the therapy: Women were more likely to discontinue treatment for opioid dependency with buprenorphine (33%) than with methadone (18%).
Most (71%) of the women in the buprenorphine group who discontinued treatment cited “dissatisfaction” with the drug as their reason, while only 13% of those in the methadone group did so.
Future research should focus on reducing this “dissatisfaction” as well as on identifying “subpopulations of pregnant patients who are more likely to have a response to one medication than to the other,” they added.
This study was funded by grants from the National Institute on Drug Abuse. Buprenorphine tablets and the associated placebo were supplied by Reckitt Benckiser Healthcare. Dr. Jones's associates reported ties to numerous drug companies.
For opioid-dependent women who are pregnant, buprenorphine appears to offer an effective, safe, first-line alternative to methadone.
In a randomized clinical trial comparing pregnancy outcomes among women seeking treatment for opioid dependence, infants exposed in utero to buprenorphine developed significantly less severe neonatal abstinence syndrome than did infants exposed in utero to methadone, said Hendrée E. Jones, Ph.D., of Johns Hopkins University, Baltimore, and her associates.
The study involved 175 women aged 18–41 years who were at 6–30 weeks' gestation when they entered treatment for opioid dependence at eight sites in the United States, Austria, and Canada. Eighty-six were randomly assigned to receive oral buprenorphine and 89 to receive oral methadone in a double-blind fashion.
After delivery, their neonates were assessed for signs and symptoms of neonatal abstinence syndrome (NAS) twice a day for at least 10 days.
There were five primary neonatal outcomes. Three of these – percentage of neonates requiring NAS treatment, peak NAS scores, and head circumference – did not differ between the two study groups. However, two of the five primary outcomes – amount of morphine required to treat NAS and length of hospital stay – favored the infants in the buprenorphine group. On average, infants exposed to buprenorphine required 89% less morphine and spent 43% less time in the hospital (10 days vs. 17.5 days) than did infants exposed to methadone.
“The benefits of buprenorphine in reducing the severity of NAS among neonates with this complication suggest that it should be considered a first-line treatment option in pregnancy,” they said (N. Engl. J. Med. 2010;363:2320-31).
Despite the comparable or even superior efficacy and safety of buprenorphine, there was one important drawback with the therapy: Women were more likely to discontinue treatment for opioid dependency with buprenorphine (33%) than with methadone (18%).
Most (71%) of the women in the buprenorphine group who discontinued treatment cited “dissatisfaction” with the drug as their reason, while only 13% of those in the methadone group did so.
Future research should focus on reducing this “dissatisfaction” as well as on identifying “subpopulations of pregnant patients who are more likely to have a response to one medication than to the other,” they added.
This study was funded by grants from the National Institute on Drug Abuse. Buprenorphine tablets and the associated placebo were supplied by Reckitt Benckiser Healthcare. Dr. Jones's associates reported ties to numerous drug companies.
From the New England Journal Of Medicine
Prenatal Spinal Surgery Improves Outcomes
Major Finding: Death or the need for a shunt was significantly less likely in the prenatal surgery group, compared with the postnatal surgery group (68% vs. 98%).
Data Source: In the Management of Myelomeningocele Study (MOMS), 183 volunteer women with singleton pregnancies were randomized to prenatal surgery before the 26th week of pregnancy or surgery for their infants after birth.
Disclosures: The study was sponsored by the National Institutes of Health.
Prenatal surgery to repair myelomeningoceles significantly reduced the need for shunts at 1 year of age and improved children's motor function at age 30 months, compared with children who had surgery after birth, based on data from a randomized trial of 183 pregnant women.
The results reflect data from 158 children who were evaluated at 12 months of age and 134 children evaluated at 30 months. Data collection is ongoing.
The surgery to repair the opening in the spine is usually performed after birth, but data from animal studies suggest that prenatal surgery could result in fewer complications, said Dr. N. Scott Adzick of the Children's Hospital of Philadelphia and his colleagues.
In the Management of Myelomeningocele Study (MOMS), 183 volunteer women with singleton pregnancies were randomized to prenatal surgery before the 26th week of pregnancy or surgery for their infants after birth (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1014379]).
The children were examined for two primary outcomes. The first outcome, at age 12 months, was patient death or the need for a shunt. The second outcome, at age 30 months, was a composite score of motor function and brain development. The score was based on the Bayley Scales of Infant Development II (BSID-II) Mental Development Index and the difference between each child's actual ability and his or her expected motor function based on the severity of the spinal defects.
Death or the need for a shunt was significantly less likely in the prenatal surgery group, compared with the postnatal surgery group (68% vs. 98%). The rates of shunt placement were significantly lower in the prenatal surgery group, compared with the postnatal surgery group (40% vs. 82%).
All the fetuses in the study suffered from hindbrain herniation, in which the base of the brain is pulled into the spinal canal. But at 12 months, 36% of the children in the prenatal surgery group had no evidence of hindbrain herniation, compared with 4% in the postnatal surgery group. In addition, infants in the prenatal surgery group had lower rates of moderate or severe hindbrain herniation than did the postnatal surgery group (25% vs. 67%).
In addition, infants in the prenatal surgery group scored an average of 21% higher on measures of mental and motor function, compared with the postnatal surgery group, with primary outcome scores of 149 vs. 123, respectively.
Infants who underwent prenatal surgery were born at a mean 34.1 weeks of pregnancy, compared with a mean 37.3 weeks of pregnancy for the postnatal surgery group. Significantly more infants in the prenatal surgery group had respiratory distress syndrome, compared with the postnatal surgery group (21% vs. 6%).
In terms of secondary outcomes, children in the prenatal surgery group were more likely to be able to walk without crutches or other orthotic devices, compared with the postnatal surgery group (21% vs. 42%).
The mean age of the pregnant women was 29 years. Each fetus had a myelomeningocele located between the T1 and S1 vertebrae, evidence of hindbrain herniation, and a gestational age of 19.0-25.9 weeks. Exclusion criteria included body mass index of 35 kg/m
Approximately one-third of the women in the prenatal surgery group showed uterine thinning or an area of dehiscence at the time of delivery. Women undergoing prenatal surgery must understand that they will require a cesarean delivery for the current pregnancy and any future pregnancies, the researchers added.
Myelomeningocele, a severe form of spina bifida in which the backbone and spinal canal do not close completely before birth, occurs in approximately 4 of every 10,000 births in the United States, Dr. Diana L. Farmer, division chief of pediatric surgery at the University of California, San Francisco, said in a teleconference. Dr. Farmer was one of several researchers on the study who took part in a teleconference to present the study findings.
The study was not large enough to show an impact of gestational age on the results, but data collection is ongoing. “This is a priceless cohort of patients that we will follow for a longer period of time,” Dr. Farmer said. She noted that the National Institutes of Health has agreed to fund follow-up of the patients until age 6–9 years. Future studies will include whether the children in the prenatal surgery group remain free of shunts, maintain improved motor function, and require fewer procedures, compared with the postnatal group.
Although the surgery is highly specialized and more research is needed, the results suggest that ob.gyns. can recommend the procedure to appropriate patients at this time, Dr. Farmer said.
“At the present time, it would be responsible to inform families that this represents an additional option in care that they could consider. The decision to undergo fetal surgery is quite individual and different for every patient, but I think families need to know that this is one option in the armamentarium.”
View on The News
Early Results Promising
Although the results are promising, it is important to be cautious in generalizing the success of prenatal surgery for myelomeningoceles to a wider population, Dr. Joe Leigh Simpson and Dr. Michael F. Greene said.
“The study by Adzick and colleagues. is “a major step in the right direction, but the still suboptimal rates of poor neonatal outcome and high maternal risk necessitate the use of less invasive approaches if such procedures are to be widely implemented,” they said.
Results might be less successful for patients treated in centers that are not as experienced in the procedure, Dr. Simpson and Dr. Greene noted.
In addition, more research is needed to determine which fetuses are more likely to benefit from the surgery, and whether performing the procedure earlier in gestation would yield even better outcomes, they added.
DR. SIMPSON is at Florida International University in Miami, and DR. GREENE is at Massachusetts General Hospital in Boston. They made their comments in an accompanying editorial (N. Engl. J. Med. 2011 [doi:10.1056/NEJMe1101228]). Dr. Simpson disclosed that he serves on the advisory boards for Rarecells Diagnostics, Novartis, BioDx, and Bayer HealthCare. Dr. Greene is an associate editor for the New England Journal of Medicine.
Major Finding: Death or the need for a shunt was significantly less likely in the prenatal surgery group, compared with the postnatal surgery group (68% vs. 98%).
Data Source: In the Management of Myelomeningocele Study (MOMS), 183 volunteer women with singleton pregnancies were randomized to prenatal surgery before the 26th week of pregnancy or surgery for their infants after birth.
Disclosures: The study was sponsored by the National Institutes of Health.
Prenatal surgery to repair myelomeningoceles significantly reduced the need for shunts at 1 year of age and improved children's motor function at age 30 months, compared with children who had surgery after birth, based on data from a randomized trial of 183 pregnant women.
The results reflect data from 158 children who were evaluated at 12 months of age and 134 children evaluated at 30 months. Data collection is ongoing.
The surgery to repair the opening in the spine is usually performed after birth, but data from animal studies suggest that prenatal surgery could result in fewer complications, said Dr. N. Scott Adzick of the Children's Hospital of Philadelphia and his colleagues.
In the Management of Myelomeningocele Study (MOMS), 183 volunteer women with singleton pregnancies were randomized to prenatal surgery before the 26th week of pregnancy or surgery for their infants after birth (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1014379]).
The children were examined for two primary outcomes. The first outcome, at age 12 months, was patient death or the need for a shunt. The second outcome, at age 30 months, was a composite score of motor function and brain development. The score was based on the Bayley Scales of Infant Development II (BSID-II) Mental Development Index and the difference between each child's actual ability and his or her expected motor function based on the severity of the spinal defects.
Death or the need for a shunt was significantly less likely in the prenatal surgery group, compared with the postnatal surgery group (68% vs. 98%). The rates of shunt placement were significantly lower in the prenatal surgery group, compared with the postnatal surgery group (40% vs. 82%).
All the fetuses in the study suffered from hindbrain herniation, in which the base of the brain is pulled into the spinal canal. But at 12 months, 36% of the children in the prenatal surgery group had no evidence of hindbrain herniation, compared with 4% in the postnatal surgery group. In addition, infants in the prenatal surgery group had lower rates of moderate or severe hindbrain herniation than did the postnatal surgery group (25% vs. 67%).
In addition, infants in the prenatal surgery group scored an average of 21% higher on measures of mental and motor function, compared with the postnatal surgery group, with primary outcome scores of 149 vs. 123, respectively.
Infants who underwent prenatal surgery were born at a mean 34.1 weeks of pregnancy, compared with a mean 37.3 weeks of pregnancy for the postnatal surgery group. Significantly more infants in the prenatal surgery group had respiratory distress syndrome, compared with the postnatal surgery group (21% vs. 6%).
In terms of secondary outcomes, children in the prenatal surgery group were more likely to be able to walk without crutches or other orthotic devices, compared with the postnatal surgery group (21% vs. 42%).
The mean age of the pregnant women was 29 years. Each fetus had a myelomeningocele located between the T1 and S1 vertebrae, evidence of hindbrain herniation, and a gestational age of 19.0-25.9 weeks. Exclusion criteria included body mass index of 35 kg/m
Approximately one-third of the women in the prenatal surgery group showed uterine thinning or an area of dehiscence at the time of delivery. Women undergoing prenatal surgery must understand that they will require a cesarean delivery for the current pregnancy and any future pregnancies, the researchers added.
Myelomeningocele, a severe form of spina bifida in which the backbone and spinal canal do not close completely before birth, occurs in approximately 4 of every 10,000 births in the United States, Dr. Diana L. Farmer, division chief of pediatric surgery at the University of California, San Francisco, said in a teleconference. Dr. Farmer was one of several researchers on the study who took part in a teleconference to present the study findings.
The study was not large enough to show an impact of gestational age on the results, but data collection is ongoing. “This is a priceless cohort of patients that we will follow for a longer period of time,” Dr. Farmer said. She noted that the National Institutes of Health has agreed to fund follow-up of the patients until age 6–9 years. Future studies will include whether the children in the prenatal surgery group remain free of shunts, maintain improved motor function, and require fewer procedures, compared with the postnatal group.
Although the surgery is highly specialized and more research is needed, the results suggest that ob.gyns. can recommend the procedure to appropriate patients at this time, Dr. Farmer said.
“At the present time, it would be responsible to inform families that this represents an additional option in care that they could consider. The decision to undergo fetal surgery is quite individual and different for every patient, but I think families need to know that this is one option in the armamentarium.”
View on The News
Early Results Promising
Although the results are promising, it is important to be cautious in generalizing the success of prenatal surgery for myelomeningoceles to a wider population, Dr. Joe Leigh Simpson and Dr. Michael F. Greene said.
“The study by Adzick and colleagues. is “a major step in the right direction, but the still suboptimal rates of poor neonatal outcome and high maternal risk necessitate the use of less invasive approaches if such procedures are to be widely implemented,” they said.
Results might be less successful for patients treated in centers that are not as experienced in the procedure, Dr. Simpson and Dr. Greene noted.
In addition, more research is needed to determine which fetuses are more likely to benefit from the surgery, and whether performing the procedure earlier in gestation would yield even better outcomes, they added.
DR. SIMPSON is at Florida International University in Miami, and DR. GREENE is at Massachusetts General Hospital in Boston. They made their comments in an accompanying editorial (N. Engl. J. Med. 2011 [doi:10.1056/NEJMe1101228]). Dr. Simpson disclosed that he serves on the advisory boards for Rarecells Diagnostics, Novartis, BioDx, and Bayer HealthCare. Dr. Greene is an associate editor for the New England Journal of Medicine.
Major Finding: Death or the need for a shunt was significantly less likely in the prenatal surgery group, compared with the postnatal surgery group (68% vs. 98%).
Data Source: In the Management of Myelomeningocele Study (MOMS), 183 volunteer women with singleton pregnancies were randomized to prenatal surgery before the 26th week of pregnancy or surgery for their infants after birth.
Disclosures: The study was sponsored by the National Institutes of Health.
Prenatal surgery to repair myelomeningoceles significantly reduced the need for shunts at 1 year of age and improved children's motor function at age 30 months, compared with children who had surgery after birth, based on data from a randomized trial of 183 pregnant women.
The results reflect data from 158 children who were evaluated at 12 months of age and 134 children evaluated at 30 months. Data collection is ongoing.
The surgery to repair the opening in the spine is usually performed after birth, but data from animal studies suggest that prenatal surgery could result in fewer complications, said Dr. N. Scott Adzick of the Children's Hospital of Philadelphia and his colleagues.
In the Management of Myelomeningocele Study (MOMS), 183 volunteer women with singleton pregnancies were randomized to prenatal surgery before the 26th week of pregnancy or surgery for their infants after birth (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1014379]).
The children were examined for two primary outcomes. The first outcome, at age 12 months, was patient death or the need for a shunt. The second outcome, at age 30 months, was a composite score of motor function and brain development. The score was based on the Bayley Scales of Infant Development II (BSID-II) Mental Development Index and the difference between each child's actual ability and his or her expected motor function based on the severity of the spinal defects.
Death or the need for a shunt was significantly less likely in the prenatal surgery group, compared with the postnatal surgery group (68% vs. 98%). The rates of shunt placement were significantly lower in the prenatal surgery group, compared with the postnatal surgery group (40% vs. 82%).
All the fetuses in the study suffered from hindbrain herniation, in which the base of the brain is pulled into the spinal canal. But at 12 months, 36% of the children in the prenatal surgery group had no evidence of hindbrain herniation, compared with 4% in the postnatal surgery group. In addition, infants in the prenatal surgery group had lower rates of moderate or severe hindbrain herniation than did the postnatal surgery group (25% vs. 67%).
In addition, infants in the prenatal surgery group scored an average of 21% higher on measures of mental and motor function, compared with the postnatal surgery group, with primary outcome scores of 149 vs. 123, respectively.
Infants who underwent prenatal surgery were born at a mean 34.1 weeks of pregnancy, compared with a mean 37.3 weeks of pregnancy for the postnatal surgery group. Significantly more infants in the prenatal surgery group had respiratory distress syndrome, compared with the postnatal surgery group (21% vs. 6%).
In terms of secondary outcomes, children in the prenatal surgery group were more likely to be able to walk without crutches or other orthotic devices, compared with the postnatal surgery group (21% vs. 42%).
The mean age of the pregnant women was 29 years. Each fetus had a myelomeningocele located between the T1 and S1 vertebrae, evidence of hindbrain herniation, and a gestational age of 19.0-25.9 weeks. Exclusion criteria included body mass index of 35 kg/m
Approximately one-third of the women in the prenatal surgery group showed uterine thinning or an area of dehiscence at the time of delivery. Women undergoing prenatal surgery must understand that they will require a cesarean delivery for the current pregnancy and any future pregnancies, the researchers added.
Myelomeningocele, a severe form of spina bifida in which the backbone and spinal canal do not close completely before birth, occurs in approximately 4 of every 10,000 births in the United States, Dr. Diana L. Farmer, division chief of pediatric surgery at the University of California, San Francisco, said in a teleconference. Dr. Farmer was one of several researchers on the study who took part in a teleconference to present the study findings.
The study was not large enough to show an impact of gestational age on the results, but data collection is ongoing. “This is a priceless cohort of patients that we will follow for a longer period of time,” Dr. Farmer said. She noted that the National Institutes of Health has agreed to fund follow-up of the patients until age 6–9 years. Future studies will include whether the children in the prenatal surgery group remain free of shunts, maintain improved motor function, and require fewer procedures, compared with the postnatal group.
Although the surgery is highly specialized and more research is needed, the results suggest that ob.gyns. can recommend the procedure to appropriate patients at this time, Dr. Farmer said.
“At the present time, it would be responsible to inform families that this represents an additional option in care that they could consider. The decision to undergo fetal surgery is quite individual and different for every patient, but I think families need to know that this is one option in the armamentarium.”
View on The News
Early Results Promising
Although the results are promising, it is important to be cautious in generalizing the success of prenatal surgery for myelomeningoceles to a wider population, Dr. Joe Leigh Simpson and Dr. Michael F. Greene said.
“The study by Adzick and colleagues. is “a major step in the right direction, but the still suboptimal rates of poor neonatal outcome and high maternal risk necessitate the use of less invasive approaches if such procedures are to be widely implemented,” they said.
Results might be less successful for patients treated in centers that are not as experienced in the procedure, Dr. Simpson and Dr. Greene noted.
In addition, more research is needed to determine which fetuses are more likely to benefit from the surgery, and whether performing the procedure earlier in gestation would yield even better outcomes, they added.
DR. SIMPSON is at Florida International University in Miami, and DR. GREENE is at Massachusetts General Hospital in Boston. They made their comments in an accompanying editorial (N. Engl. J. Med. 2011 [doi:10.1056/NEJMe1101228]). Dr. Simpson disclosed that he serves on the advisory boards for Rarecells Diagnostics, Novartis, BioDx, and Bayer HealthCare. Dr. Greene is an associate editor for the New England Journal of Medicine.
From the New England Journal Of Medicine
Probing Postpartum Depression
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
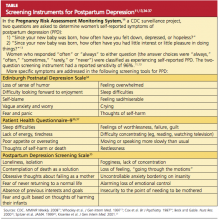
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.
Does the rate of postcesarean maternal infection vary by uterine closure technique?
More than one million cesarean deliveries are performed each year in the United States. That’s more than two cesarean deliveries every minute. Clearly, performing this most common of major surgeries safely and effectively is of the utmost importance.
The much-anticipated CAESAR study is a large randomized, controlled trial that assesses three technical aspects of cesarean delivery:
- single-layer versus double-layer uterine closure. The investigators define the former as approximation of both edges of the uterine incision using a single layer of sutures. Double-layer closure involves one set of sutures at the endometrial layer and an additional set of sutures at the serosal layer
- closure versus nonclosure of the pelvic peritoneum
- liberal versus restricted use of a subrectus sheath drain.
The primary outcome of this study is maternal infectious morbidity. Women who participated in the trial were all undergoing their first cesarean delivery, which was performed through the lower uterine segment. In addition, none of the women had a clear indication for any of the techniques explored in this study.
As in any trial, the findings of the CAESAR study should be interpreted in view of the totality of the literature. Other aspects of cesarean delivery have been summarized previously. 1
In the short term, the type of uterine closure doesn’t seem to matter
In the CAESAR trial, single-layer closure of the uterine incision was not associated with any effect on maternal infectious morbidity, compared with double-layer closure. In earlier randomized, controlled trials, single-layer closure was associated with shorter operative time, less blood loss, and less pain. 2
An important issue is the long-term effect of single-layer uterine closure, especially the incidence of uterine rupture in subsequent trials of labor, compared with double-layer closure. The CAESAR study did not report this outcome, but we hope that it will in the future, as it is one of the largest trials to explore uterine closure.
Closure of the peritoneum
In earlier randomized, controlled trials, non-closure of the pelvic peritoneum has been associated with shorter operative time and hospitalization, a lower rate of fever, and less need for analgesia. 3
In the CAESAR trial, nonclosure of the peritoneum was not associated with any effect on maternal infectious morbidity, compared with closure.
Use of a drain is best limited
In the CAESAR trial, restricted use of a subrectus sheath drain was not associated with any effect on maternal infectious morbidity, compared with liberal use. In earlier trials, drainage was not associated with any bene-fit. 4 Therefore, it seems preferable to limit use of these drains during cesarean delivery.
It is too soon for us to know the long-term effects of these cesarean delivery techniques, but neither single-layer nor double-layer uterine closure appears to affect the rate of maternal postoperative infection.
Nonclosure of the peritoneum is preferred to closure, based on Level I literature on this issue.
Liberal use of a subrectus sheath drain is of little benefit. Its use should be limited. —VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607-1617.
2. Dodd JM, Anderson ER, Gates S. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst Rev. 2008;(3):CD004732.-
3. Bamigboye AA, Hofmeyr JG. Closure versus non-closure of the peritoneum at caesarean section. Cochrane Database Syst Rev. 1998;(1):CD000163.-doi: 10.1002/14651858. CD000163.
4. Gates S, Anderson ER. Wound drainage for caesarean section. Cochrane Database Syst Rev. 2005;(1):CD004549.-doi: 10.1002/14651858.CD004549.pub2.
More than one million cesarean deliveries are performed each year in the United States. That’s more than two cesarean deliveries every minute. Clearly, performing this most common of major surgeries safely and effectively is of the utmost importance.
The much-anticipated CAESAR study is a large randomized, controlled trial that assesses three technical aspects of cesarean delivery:
- single-layer versus double-layer uterine closure. The investigators define the former as approximation of both edges of the uterine incision using a single layer of sutures. Double-layer closure involves one set of sutures at the endometrial layer and an additional set of sutures at the serosal layer
- closure versus nonclosure of the pelvic peritoneum
- liberal versus restricted use of a subrectus sheath drain.
The primary outcome of this study is maternal infectious morbidity. Women who participated in the trial were all undergoing their first cesarean delivery, which was performed through the lower uterine segment. In addition, none of the women had a clear indication for any of the techniques explored in this study.
As in any trial, the findings of the CAESAR study should be interpreted in view of the totality of the literature. Other aspects of cesarean delivery have been summarized previously. 1
In the short term, the type of uterine closure doesn’t seem to matter
In the CAESAR trial, single-layer closure of the uterine incision was not associated with any effect on maternal infectious morbidity, compared with double-layer closure. In earlier randomized, controlled trials, single-layer closure was associated with shorter operative time, less blood loss, and less pain. 2
An important issue is the long-term effect of single-layer uterine closure, especially the incidence of uterine rupture in subsequent trials of labor, compared with double-layer closure. The CAESAR study did not report this outcome, but we hope that it will in the future, as it is one of the largest trials to explore uterine closure.
Closure of the peritoneum
In earlier randomized, controlled trials, non-closure of the pelvic peritoneum has been associated with shorter operative time and hospitalization, a lower rate of fever, and less need for analgesia. 3
In the CAESAR trial, nonclosure of the peritoneum was not associated with any effect on maternal infectious morbidity, compared with closure.
Use of a drain is best limited
In the CAESAR trial, restricted use of a subrectus sheath drain was not associated with any effect on maternal infectious morbidity, compared with liberal use. In earlier trials, drainage was not associated with any bene-fit. 4 Therefore, it seems preferable to limit use of these drains during cesarean delivery.
It is too soon for us to know the long-term effects of these cesarean delivery techniques, but neither single-layer nor double-layer uterine closure appears to affect the rate of maternal postoperative infection.
Nonclosure of the peritoneum is preferred to closure, based on Level I literature on this issue.
Liberal use of a subrectus sheath drain is of little benefit. Its use should be limited. —VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
More than one million cesarean deliveries are performed each year in the United States. That’s more than two cesarean deliveries every minute. Clearly, performing this most common of major surgeries safely and effectively is of the utmost importance.
The much-anticipated CAESAR study is a large randomized, controlled trial that assesses three technical aspects of cesarean delivery:
- single-layer versus double-layer uterine closure. The investigators define the former as approximation of both edges of the uterine incision using a single layer of sutures. Double-layer closure involves one set of sutures at the endometrial layer and an additional set of sutures at the serosal layer
- closure versus nonclosure of the pelvic peritoneum
- liberal versus restricted use of a subrectus sheath drain.
The primary outcome of this study is maternal infectious morbidity. Women who participated in the trial were all undergoing their first cesarean delivery, which was performed through the lower uterine segment. In addition, none of the women had a clear indication for any of the techniques explored in this study.
As in any trial, the findings of the CAESAR study should be interpreted in view of the totality of the literature. Other aspects of cesarean delivery have been summarized previously. 1
In the short term, the type of uterine closure doesn’t seem to matter
In the CAESAR trial, single-layer closure of the uterine incision was not associated with any effect on maternal infectious morbidity, compared with double-layer closure. In earlier randomized, controlled trials, single-layer closure was associated with shorter operative time, less blood loss, and less pain. 2
An important issue is the long-term effect of single-layer uterine closure, especially the incidence of uterine rupture in subsequent trials of labor, compared with double-layer closure. The CAESAR study did not report this outcome, but we hope that it will in the future, as it is one of the largest trials to explore uterine closure.
Closure of the peritoneum
In earlier randomized, controlled trials, non-closure of the pelvic peritoneum has been associated with shorter operative time and hospitalization, a lower rate of fever, and less need for analgesia. 3
In the CAESAR trial, nonclosure of the peritoneum was not associated with any effect on maternal infectious morbidity, compared with closure.
Use of a drain is best limited
In the CAESAR trial, restricted use of a subrectus sheath drain was not associated with any effect on maternal infectious morbidity, compared with liberal use. In earlier trials, drainage was not associated with any bene-fit. 4 Therefore, it seems preferable to limit use of these drains during cesarean delivery.
It is too soon for us to know the long-term effects of these cesarean delivery techniques, but neither single-layer nor double-layer uterine closure appears to affect the rate of maternal postoperative infection.
Nonclosure of the peritoneum is preferred to closure, based on Level I literature on this issue.
Liberal use of a subrectus sheath drain is of little benefit. Its use should be limited. —VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607-1617.
2. Dodd JM, Anderson ER, Gates S. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst Rev. 2008;(3):CD004732.-
3. Bamigboye AA, Hofmeyr JG. Closure versus non-closure of the peritoneum at caesarean section. Cochrane Database Syst Rev. 1998;(1):CD000163.-doi: 10.1002/14651858. CD000163.
4. Gates S, Anderson ER. Wound drainage for caesarean section. Cochrane Database Syst Rev. 2005;(1):CD004549.-doi: 10.1002/14651858.CD004549.pub2.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607-1617.
2. Dodd JM, Anderson ER, Gates S. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst Rev. 2008;(3):CD004732.-
3. Bamigboye AA, Hofmeyr JG. Closure versus non-closure of the peritoneum at caesarean section. Cochrane Database Syst Rev. 1998;(1):CD000163.-doi: 10.1002/14651858. CD000163.
4. Gates S, Anderson ER. Wound drainage for caesarean section. Cochrane Database Syst Rev. 2005;(1):CD004549.-doi: 10.1002/14651858.CD004549.pub2.
Telbivudine Appears Safe in Pregnancy
BOSTON – Limited use of the antiviral drug telbivudine is safe and effective for pregnant women with active hepatitis B virus infection and reduces perinatal transmission of the virus to their infants, Dr. Calvin Pan reported at the meeting.
In the first open-label case-control trial investigating the safety and efficacy of telbivudine during the second to third trimester of pregnancy, the synthetic nucleoside analogue led to significant increases in rates of complete virologic response and normalization of alanine aminotransferase (ALT) levels in treated vs. untreated pregnant women with hepatitis B virus (HBV) infection, reported Dr. Pan of Mount Sinai School of Medicine in New York.
Additionally, there were no congenital defects identified up to 28 weeks post partum in the infants born to treated mothers.
Dr. Pan and his colleagues at the Second Affiliated Hospital of the Southeast University in Nanjing, China, where the study was conducted, enrolled 88 pregnant women who screened positive for hepatitis B e antigen (HBeAg) between gestational weeks 12 and 23 and who had serum HBV DNA levels greater than 6 log10 copies/mL and elevated ALT levels up to 10 times the upper limit of normal (40 IU/mL). Of the 88 patients, 53 opted to take telbivudine, and the remaining 35 served as the study's control arm, he said.
Women in the treatment arm received 600 mg/day of telbivudine beginning sometime between 20 and 32 weeks' gestation and continuing until a minimum of 4 weeks post partum, and infants born to women in both groups received hepatitis B immunoglobulin within 24 hours of birth and the HBV vaccine at birth, 1 month, and 6 months.
The mean duration of telbivudine treatment was 15.5 weeks, and all of the women who took the drug remained in the study through at least postpartum week 4, compared with 92% of the controls, said Dr. Pan. Physical and laboratory examinations were conducted at baseline, at the time of delivery, and at 4 weeks post partum, he said, noting that a second study looked at infant outcomes through 28 weeks.
At the time of delivery and 4 weeks post partum, a complete virologic response was observed in 53% and 62% of the treated patients, respectively, while no patients in the control arm achieved a complete virologic response at either time point, Dr. Pan reported. “Both the control arm and the telbivudine arm had HBV DNA of approximately 8 logs. At the time of delivery, this was reduced substantially [to 2.35 log10 copies/mL] in the mothers in the treatment arm.”
With respect to ALT levels, 77% of the treatment group achieved normalization compared with 29% of the control group, Dr. Pan said. Also, the levels of HBeAg dropped by 98% in the treatment group, which was a significantly greater decrease than the 60% drop observed in the control group; the latter was likely the result of natural viral clearance, he noted.
An evaluation of newborn outcomes showed no congenital deformities nor any differences in gestational age, infant height and weight, or Apgar scores between the two groups, said Dr. Pan. Significantly fewer babies born to telbivudine-treated mothers vs. untreated mothers had hepatitis B surface antigens or detectable levels of HBV RNA at birth (4% vs. 23%).
Telbivudine appeared to be well tolerated as there were no adverse event–related treatment discontinuations, said Dr. Pan. Also, none of the patients experienced virologic breakthrough.
Despite concerns regarding antiviral treatment during pregnancy because of the potential risks to the fetus, the findings from this study suggest that limited treatment with telbivudine can improve maternal and child outcomes, Dr. Pan concluded.
The study was funded by the Chinese Department of Health with no commercial support. Dr. Pan said he had no relevant financial disclosures.
BOSTON – Limited use of the antiviral drug telbivudine is safe and effective for pregnant women with active hepatitis B virus infection and reduces perinatal transmission of the virus to their infants, Dr. Calvin Pan reported at the meeting.
In the first open-label case-control trial investigating the safety and efficacy of telbivudine during the second to third trimester of pregnancy, the synthetic nucleoside analogue led to significant increases in rates of complete virologic response and normalization of alanine aminotransferase (ALT) levels in treated vs. untreated pregnant women with hepatitis B virus (HBV) infection, reported Dr. Pan of Mount Sinai School of Medicine in New York.
Additionally, there were no congenital defects identified up to 28 weeks post partum in the infants born to treated mothers.
Dr. Pan and his colleagues at the Second Affiliated Hospital of the Southeast University in Nanjing, China, where the study was conducted, enrolled 88 pregnant women who screened positive for hepatitis B e antigen (HBeAg) between gestational weeks 12 and 23 and who had serum HBV DNA levels greater than 6 log10 copies/mL and elevated ALT levels up to 10 times the upper limit of normal (40 IU/mL). Of the 88 patients, 53 opted to take telbivudine, and the remaining 35 served as the study's control arm, he said.
Women in the treatment arm received 600 mg/day of telbivudine beginning sometime between 20 and 32 weeks' gestation and continuing until a minimum of 4 weeks post partum, and infants born to women in both groups received hepatitis B immunoglobulin within 24 hours of birth and the HBV vaccine at birth, 1 month, and 6 months.
The mean duration of telbivudine treatment was 15.5 weeks, and all of the women who took the drug remained in the study through at least postpartum week 4, compared with 92% of the controls, said Dr. Pan. Physical and laboratory examinations were conducted at baseline, at the time of delivery, and at 4 weeks post partum, he said, noting that a second study looked at infant outcomes through 28 weeks.
At the time of delivery and 4 weeks post partum, a complete virologic response was observed in 53% and 62% of the treated patients, respectively, while no patients in the control arm achieved a complete virologic response at either time point, Dr. Pan reported. “Both the control arm and the telbivudine arm had HBV DNA of approximately 8 logs. At the time of delivery, this was reduced substantially [to 2.35 log10 copies/mL] in the mothers in the treatment arm.”
With respect to ALT levels, 77% of the treatment group achieved normalization compared with 29% of the control group, Dr. Pan said. Also, the levels of HBeAg dropped by 98% in the treatment group, which was a significantly greater decrease than the 60% drop observed in the control group; the latter was likely the result of natural viral clearance, he noted.
An evaluation of newborn outcomes showed no congenital deformities nor any differences in gestational age, infant height and weight, or Apgar scores between the two groups, said Dr. Pan. Significantly fewer babies born to telbivudine-treated mothers vs. untreated mothers had hepatitis B surface antigens or detectable levels of HBV RNA at birth (4% vs. 23%).
Telbivudine appeared to be well tolerated as there were no adverse event–related treatment discontinuations, said Dr. Pan. Also, none of the patients experienced virologic breakthrough.
Despite concerns regarding antiviral treatment during pregnancy because of the potential risks to the fetus, the findings from this study suggest that limited treatment with telbivudine can improve maternal and child outcomes, Dr. Pan concluded.
The study was funded by the Chinese Department of Health with no commercial support. Dr. Pan said he had no relevant financial disclosures.
BOSTON – Limited use of the antiviral drug telbivudine is safe and effective for pregnant women with active hepatitis B virus infection and reduces perinatal transmission of the virus to their infants, Dr. Calvin Pan reported at the meeting.
In the first open-label case-control trial investigating the safety and efficacy of telbivudine during the second to third trimester of pregnancy, the synthetic nucleoside analogue led to significant increases in rates of complete virologic response and normalization of alanine aminotransferase (ALT) levels in treated vs. untreated pregnant women with hepatitis B virus (HBV) infection, reported Dr. Pan of Mount Sinai School of Medicine in New York.
Additionally, there were no congenital defects identified up to 28 weeks post partum in the infants born to treated mothers.
Dr. Pan and his colleagues at the Second Affiliated Hospital of the Southeast University in Nanjing, China, where the study was conducted, enrolled 88 pregnant women who screened positive for hepatitis B e antigen (HBeAg) between gestational weeks 12 and 23 and who had serum HBV DNA levels greater than 6 log10 copies/mL and elevated ALT levels up to 10 times the upper limit of normal (40 IU/mL). Of the 88 patients, 53 opted to take telbivudine, and the remaining 35 served as the study's control arm, he said.
Women in the treatment arm received 600 mg/day of telbivudine beginning sometime between 20 and 32 weeks' gestation and continuing until a minimum of 4 weeks post partum, and infants born to women in both groups received hepatitis B immunoglobulin within 24 hours of birth and the HBV vaccine at birth, 1 month, and 6 months.
The mean duration of telbivudine treatment was 15.5 weeks, and all of the women who took the drug remained in the study through at least postpartum week 4, compared with 92% of the controls, said Dr. Pan. Physical and laboratory examinations were conducted at baseline, at the time of delivery, and at 4 weeks post partum, he said, noting that a second study looked at infant outcomes through 28 weeks.
At the time of delivery and 4 weeks post partum, a complete virologic response was observed in 53% and 62% of the treated patients, respectively, while no patients in the control arm achieved a complete virologic response at either time point, Dr. Pan reported. “Both the control arm and the telbivudine arm had HBV DNA of approximately 8 logs. At the time of delivery, this was reduced substantially [to 2.35 log10 copies/mL] in the mothers in the treatment arm.”
With respect to ALT levels, 77% of the treatment group achieved normalization compared with 29% of the control group, Dr. Pan said. Also, the levels of HBeAg dropped by 98% in the treatment group, which was a significantly greater decrease than the 60% drop observed in the control group; the latter was likely the result of natural viral clearance, he noted.
An evaluation of newborn outcomes showed no congenital deformities nor any differences in gestational age, infant height and weight, or Apgar scores between the two groups, said Dr. Pan. Significantly fewer babies born to telbivudine-treated mothers vs. untreated mothers had hepatitis B surface antigens or detectable levels of HBV RNA at birth (4% vs. 23%).
Telbivudine appeared to be well tolerated as there were no adverse event–related treatment discontinuations, said Dr. Pan. Also, none of the patients experienced virologic breakthrough.
Despite concerns regarding antiviral treatment during pregnancy because of the potential risks to the fetus, the findings from this study suggest that limited treatment with telbivudine can improve maternal and child outcomes, Dr. Pan concluded.
The study was funded by the Chinese Department of Health with no commercial support. Dr. Pan said he had no relevant financial disclosures.
From the Annual Meeting of the American Association for the Study of Liver Diseases
Gestational Diabetes, Low SES Ups ADHD Risk : There was a ninefold increased risk of ADHD diagnosis in 6-year-olds.
Major Finding: Six-year-old children from low socioeconomic families and born to mothers who had gestational diabetes had a ninefold increased rate of diagnosis for attention-deficit/hyperactivity disorder, compared with children from higher socioeconomic families whose mothers did not have gestational diabetes.
Data Source: Multicenter study of 216 children, 9 of whom came from low socioeconomic families and were born to mothers who had gestational diabetes.
Disclosures: Ms. Jordan had no relevant financial disclosures.
NEW YORK – Children born to mothers with gestational diabetes during pregnancy had a significantly increased risk of developing attention-deficit/hyperactivity disorder when they reached 6 years old, based on a study with 216 children.
The risk was even greater in children of families with low socioeconomic status. The combined effect of gestational diabetes and low socioeconomic status was linked with a statistically significant, ninefold increased rate of attention-deficit/hyperactivity disorder (ADHD) in children when they reached age 6 years, Alexandra S. Jordan reported in a poster at the meeting.
The findings “raise the possibility that manifestations of ADHD are not simply genetically mediated. Rather, susceptibility may increase as a function of the uterine environment (as with gestational diabetes) and may be further aggravated as a result of socioeconomic hardship during childhood,” Ms. Jordan and her associates reported in their poster.
Prior published reports had identified both gestational diabetes and low socioeconomic status as risk factors for the subsequent development of ADHD in young children.
But in this study, when the researchers looked at the impact of both factors together, “something bizarre happened.” The risk increased “way beyond the expected impact,” Ms. Jordan said in an interview. She and her colleagues do not currently have an explanation for this apparent synergistic interaction.
The study assessed 216 unselected children for ADHD symptoms at age 4 years and for a diagnosis of ADHD at age 6 years. The mothers of 21 of the children had gestational diabetes during pregnancy, and this subgroup had a 2.19-fold increased risk for having a diagnosis of ADHD at age 6 years, compared with the children born to mothers who never had gestational diabetes.
In addition, 104 of the children came from low socioeconomic status households, and these children had a 2.05-fold increased rate of having ADHD, compared with the other children from higher socioeconomic families, said Ms. Jordan, a researcher in the department of counseling and clinical psychology at Columbia University in New York.
The group included nine children whose mother had gestational diabetes and who came from low socioeconomic family.
In this subgroup, the prevalence of the ADHD diagnosis at age 6 years was 9.23-fold higher than it was for the children whose mothers did not have gestational diabetes and who came from families with higher socioeconomic status.
The apparent effect of gestational diabetes and low socioeconomic status on ADHD prevalence remained statistically significant after researchers adjusted for whether one or both parents had ADHD.
“These findings may be useful in educating women considering pregnancy, particularly those in low socioeconomic environments, about the potential lingering effects of gestational diabetes on offspring into childhood,” the researchers said in their poster.
“This information may encourage women to control gestational diabetes symptoms during pregnancy.” In addition, it may help “educate health care providers on the importance of assessment and control of gestational diabetes symptoms throughout pregnancy and on ADHD's etiologic link to gestational diabetes.” The findings might also help “target early interventions to those low socioeconomic status families who are most vulnerable” to this interaction with gestational diabetes, they said.
Major Finding: Six-year-old children from low socioeconomic families and born to mothers who had gestational diabetes had a ninefold increased rate of diagnosis for attention-deficit/hyperactivity disorder, compared with children from higher socioeconomic families whose mothers did not have gestational diabetes.
Data Source: Multicenter study of 216 children, 9 of whom came from low socioeconomic families and were born to mothers who had gestational diabetes.
Disclosures: Ms. Jordan had no relevant financial disclosures.
NEW YORK – Children born to mothers with gestational diabetes during pregnancy had a significantly increased risk of developing attention-deficit/hyperactivity disorder when they reached 6 years old, based on a study with 216 children.
The risk was even greater in children of families with low socioeconomic status. The combined effect of gestational diabetes and low socioeconomic status was linked with a statistically significant, ninefold increased rate of attention-deficit/hyperactivity disorder (ADHD) in children when they reached age 6 years, Alexandra S. Jordan reported in a poster at the meeting.
The findings “raise the possibility that manifestations of ADHD are not simply genetically mediated. Rather, susceptibility may increase as a function of the uterine environment (as with gestational diabetes) and may be further aggravated as a result of socioeconomic hardship during childhood,” Ms. Jordan and her associates reported in their poster.
Prior published reports had identified both gestational diabetes and low socioeconomic status as risk factors for the subsequent development of ADHD in young children.
But in this study, when the researchers looked at the impact of both factors together, “something bizarre happened.” The risk increased “way beyond the expected impact,” Ms. Jordan said in an interview. She and her colleagues do not currently have an explanation for this apparent synergistic interaction.
The study assessed 216 unselected children for ADHD symptoms at age 4 years and for a diagnosis of ADHD at age 6 years. The mothers of 21 of the children had gestational diabetes during pregnancy, and this subgroup had a 2.19-fold increased risk for having a diagnosis of ADHD at age 6 years, compared with the children born to mothers who never had gestational diabetes.
In addition, 104 of the children came from low socioeconomic status households, and these children had a 2.05-fold increased rate of having ADHD, compared with the other children from higher socioeconomic families, said Ms. Jordan, a researcher in the department of counseling and clinical psychology at Columbia University in New York.
The group included nine children whose mother had gestational diabetes and who came from low socioeconomic family.
In this subgroup, the prevalence of the ADHD diagnosis at age 6 years was 9.23-fold higher than it was for the children whose mothers did not have gestational diabetes and who came from families with higher socioeconomic status.
The apparent effect of gestational diabetes and low socioeconomic status on ADHD prevalence remained statistically significant after researchers adjusted for whether one or both parents had ADHD.
“These findings may be useful in educating women considering pregnancy, particularly those in low socioeconomic environments, about the potential lingering effects of gestational diabetes on offspring into childhood,” the researchers said in their poster.
“This information may encourage women to control gestational diabetes symptoms during pregnancy.” In addition, it may help “educate health care providers on the importance of assessment and control of gestational diabetes symptoms throughout pregnancy and on ADHD's etiologic link to gestational diabetes.” The findings might also help “target early interventions to those low socioeconomic status families who are most vulnerable” to this interaction with gestational diabetes, they said.
Major Finding: Six-year-old children from low socioeconomic families and born to mothers who had gestational diabetes had a ninefold increased rate of diagnosis for attention-deficit/hyperactivity disorder, compared with children from higher socioeconomic families whose mothers did not have gestational diabetes.
Data Source: Multicenter study of 216 children, 9 of whom came from low socioeconomic families and were born to mothers who had gestational diabetes.
Disclosures: Ms. Jordan had no relevant financial disclosures.
NEW YORK – Children born to mothers with gestational diabetes during pregnancy had a significantly increased risk of developing attention-deficit/hyperactivity disorder when they reached 6 years old, based on a study with 216 children.
The risk was even greater in children of families with low socioeconomic status. The combined effect of gestational diabetes and low socioeconomic status was linked with a statistically significant, ninefold increased rate of attention-deficit/hyperactivity disorder (ADHD) in children when they reached age 6 years, Alexandra S. Jordan reported in a poster at the meeting.
The findings “raise the possibility that manifestations of ADHD are not simply genetically mediated. Rather, susceptibility may increase as a function of the uterine environment (as with gestational diabetes) and may be further aggravated as a result of socioeconomic hardship during childhood,” Ms. Jordan and her associates reported in their poster.
Prior published reports had identified both gestational diabetes and low socioeconomic status as risk factors for the subsequent development of ADHD in young children.
But in this study, when the researchers looked at the impact of both factors together, “something bizarre happened.” The risk increased “way beyond the expected impact,” Ms. Jordan said in an interview. She and her colleagues do not currently have an explanation for this apparent synergistic interaction.
The study assessed 216 unselected children for ADHD symptoms at age 4 years and for a diagnosis of ADHD at age 6 years. The mothers of 21 of the children had gestational diabetes during pregnancy, and this subgroup had a 2.19-fold increased risk for having a diagnosis of ADHD at age 6 years, compared with the children born to mothers who never had gestational diabetes.
In addition, 104 of the children came from low socioeconomic status households, and these children had a 2.05-fold increased rate of having ADHD, compared with the other children from higher socioeconomic families, said Ms. Jordan, a researcher in the department of counseling and clinical psychology at Columbia University in New York.
The group included nine children whose mother had gestational diabetes and who came from low socioeconomic family.
In this subgroup, the prevalence of the ADHD diagnosis at age 6 years was 9.23-fold higher than it was for the children whose mothers did not have gestational diabetes and who came from families with higher socioeconomic status.
The apparent effect of gestational diabetes and low socioeconomic status on ADHD prevalence remained statistically significant after researchers adjusted for whether one or both parents had ADHD.
“These findings may be useful in educating women considering pregnancy, particularly those in low socioeconomic environments, about the potential lingering effects of gestational diabetes on offspring into childhood,” the researchers said in their poster.
“This information may encourage women to control gestational diabetes symptoms during pregnancy.” In addition, it may help “educate health care providers on the importance of assessment and control of gestational diabetes symptoms throughout pregnancy and on ADHD's etiologic link to gestational diabetes.” The findings might also help “target early interventions to those low socioeconomic status families who are most vulnerable” to this interaction with gestational diabetes, they said.
From the Annual Meeting of the American Academy of Child & Adolescent Psychiatry
Shedding Light on Unintended Pregnancy
Major Finding: Having been in a physically abusive relationship within the past year was associated with a 2.8-fold increased risk of unintended pregnancy at 2-year schools and a 4.5-fold elevated risk at 4-year colleges.
Data Source: Data from the Spring 2007 National College Health Assessment.
Disclosures: Dr. Lindley said she had no relevant financial conflicts.
DENVER – Unintended pregnancies are threefold more frequent among unmarried sexually active female college students at 2-year as opposed to 4-year institutions.
Data from the Spring 2007 National College Health Assessment showed that, overall, 2.6% of unmarried sexually active female students aged 18-24 years at western U.S. colleges experienced an unwanted pregnancy during the past year.
The rate was 5.3% among 1,433 attendees at 2-year colleges and 1.8% in 4,732 at 4-year schools, Lisa L. Lindley, Dr. P.H., reported at the meeting.
A multivariate logistic regression analysis identified 9 risk factors associated with unintended pregnancy in young women attending 2-year institutions and 11 risk factors operative at 4-year schools. Some of these risk factors were common to both types of schools, while others were unique to one or the other.
Among the most striking shared risk factors was having been in a physically abusive relationship within the past year. This was associated with a 2.8-fold increased risk of unintended pregnancy at 2-year schools and a 4.5-fold elevated risk at 4-year colleges, according to Dr. Lindley of George Mason University, Fairfax, Va.
Use of the emergency contraceptive pill within the previous year was another risk factor for unintended pregnancy common to students at both types of colleges. It was associated with a 2.6-fold increased risk in unmarried sexually active students at 2-year schools and a 2.8-fold risk in those at 4-year schools.
Having a C or lower grade average was associated with a 2.2-fold increased likelihood of unintended pregnancy in women attending a 4-year college, but was not a significant risk factor at 2-year colleges. On the other hand, having unprotected sex in the past year as a consequence of drinking alcohol was a significant risk factor for unintended pregnancy at 2-year, but not 4-year institutions. Having had a gynecologic exam during the past year was associated with a 2.7-fold increased likelihood of unintended pregnancy among women at 2-year schools but wasn't a significant factor at 4-year colleges.
Race was not a significant predictor of unintended pregnancy among the college students. This is an intriguing finding in light of the marked racial disparity in pregnancy rates among young women as a whole in the United States, Dr. Lindley said. For example, in 2004 the pregnancy rates among 20- to 24-year-old black and Hispanic women were 259 and 245 per 1,000 population, respectively, compared with 123 per 1,000 in similarly aged white women.
Dr. Lindley explained that the college study was undertaken in an effort to better understand the phenomenon of unintended pregnancy. Half of all pregnancies in the United States are unintended. Among young unmarried women this figure is far higher: 73% among 20- to 24-year-olds, and 86% in those under age 20.
Solid progress has been made in reducing the national pregnancy rate among 15- to 17-year-olds. But pregnancy rates among 18- to 24-year-olds remain excessive, she said.
A key lesson from the college study lies in the importance of addressing multiple risk factors at once for unwanted pregnancy, including abusive relationships – physical, emotional, and sexual – as well as alcohol use and proper use of the emergency contraceptive pill, Dr. Lindley added.
Major Finding: Having been in a physically abusive relationship within the past year was associated with a 2.8-fold increased risk of unintended pregnancy at 2-year schools and a 4.5-fold elevated risk at 4-year colleges.
Data Source: Data from the Spring 2007 National College Health Assessment.
Disclosures: Dr. Lindley said she had no relevant financial conflicts.
DENVER – Unintended pregnancies are threefold more frequent among unmarried sexually active female college students at 2-year as opposed to 4-year institutions.
Data from the Spring 2007 National College Health Assessment showed that, overall, 2.6% of unmarried sexually active female students aged 18-24 years at western U.S. colleges experienced an unwanted pregnancy during the past year.
The rate was 5.3% among 1,433 attendees at 2-year colleges and 1.8% in 4,732 at 4-year schools, Lisa L. Lindley, Dr. P.H., reported at the meeting.
A multivariate logistic regression analysis identified 9 risk factors associated with unintended pregnancy in young women attending 2-year institutions and 11 risk factors operative at 4-year schools. Some of these risk factors were common to both types of schools, while others were unique to one or the other.
Among the most striking shared risk factors was having been in a physically abusive relationship within the past year. This was associated with a 2.8-fold increased risk of unintended pregnancy at 2-year schools and a 4.5-fold elevated risk at 4-year colleges, according to Dr. Lindley of George Mason University, Fairfax, Va.
Use of the emergency contraceptive pill within the previous year was another risk factor for unintended pregnancy common to students at both types of colleges. It was associated with a 2.6-fold increased risk in unmarried sexually active students at 2-year schools and a 2.8-fold risk in those at 4-year schools.
Having a C or lower grade average was associated with a 2.2-fold increased likelihood of unintended pregnancy in women attending a 4-year college, but was not a significant risk factor at 2-year colleges. On the other hand, having unprotected sex in the past year as a consequence of drinking alcohol was a significant risk factor for unintended pregnancy at 2-year, but not 4-year institutions. Having had a gynecologic exam during the past year was associated with a 2.7-fold increased likelihood of unintended pregnancy among women at 2-year schools but wasn't a significant factor at 4-year colleges.
Race was not a significant predictor of unintended pregnancy among the college students. This is an intriguing finding in light of the marked racial disparity in pregnancy rates among young women as a whole in the United States, Dr. Lindley said. For example, in 2004 the pregnancy rates among 20- to 24-year-old black and Hispanic women were 259 and 245 per 1,000 population, respectively, compared with 123 per 1,000 in similarly aged white women.
Dr. Lindley explained that the college study was undertaken in an effort to better understand the phenomenon of unintended pregnancy. Half of all pregnancies in the United States are unintended. Among young unmarried women this figure is far higher: 73% among 20- to 24-year-olds, and 86% in those under age 20.
Solid progress has been made in reducing the national pregnancy rate among 15- to 17-year-olds. But pregnancy rates among 18- to 24-year-olds remain excessive, she said.
A key lesson from the college study lies in the importance of addressing multiple risk factors at once for unwanted pregnancy, including abusive relationships – physical, emotional, and sexual – as well as alcohol use and proper use of the emergency contraceptive pill, Dr. Lindley added.
Major Finding: Having been in a physically abusive relationship within the past year was associated with a 2.8-fold increased risk of unintended pregnancy at 2-year schools and a 4.5-fold elevated risk at 4-year colleges.
Data Source: Data from the Spring 2007 National College Health Assessment.
Disclosures: Dr. Lindley said she had no relevant financial conflicts.
DENVER – Unintended pregnancies are threefold more frequent among unmarried sexually active female college students at 2-year as opposed to 4-year institutions.
Data from the Spring 2007 National College Health Assessment showed that, overall, 2.6% of unmarried sexually active female students aged 18-24 years at western U.S. colleges experienced an unwanted pregnancy during the past year.
The rate was 5.3% among 1,433 attendees at 2-year colleges and 1.8% in 4,732 at 4-year schools, Lisa L. Lindley, Dr. P.H., reported at the meeting.
A multivariate logistic regression analysis identified 9 risk factors associated with unintended pregnancy in young women attending 2-year institutions and 11 risk factors operative at 4-year schools. Some of these risk factors were common to both types of schools, while others were unique to one or the other.
Among the most striking shared risk factors was having been in a physically abusive relationship within the past year. This was associated with a 2.8-fold increased risk of unintended pregnancy at 2-year schools and a 4.5-fold elevated risk at 4-year colleges, according to Dr. Lindley of George Mason University, Fairfax, Va.
Use of the emergency contraceptive pill within the previous year was another risk factor for unintended pregnancy common to students at both types of colleges. It was associated with a 2.6-fold increased risk in unmarried sexually active students at 2-year schools and a 2.8-fold risk in those at 4-year schools.
Having a C or lower grade average was associated with a 2.2-fold increased likelihood of unintended pregnancy in women attending a 4-year college, but was not a significant risk factor at 2-year colleges. On the other hand, having unprotected sex in the past year as a consequence of drinking alcohol was a significant risk factor for unintended pregnancy at 2-year, but not 4-year institutions. Having had a gynecologic exam during the past year was associated with a 2.7-fold increased likelihood of unintended pregnancy among women at 2-year schools but wasn't a significant factor at 4-year colleges.
Race was not a significant predictor of unintended pregnancy among the college students. This is an intriguing finding in light of the marked racial disparity in pregnancy rates among young women as a whole in the United States, Dr. Lindley said. For example, in 2004 the pregnancy rates among 20- to 24-year-old black and Hispanic women were 259 and 245 per 1,000 population, respectively, compared with 123 per 1,000 in similarly aged white women.
Dr. Lindley explained that the college study was undertaken in an effort to better understand the phenomenon of unintended pregnancy. Half of all pregnancies in the United States are unintended. Among young unmarried women this figure is far higher: 73% among 20- to 24-year-olds, and 86% in those under age 20.
Solid progress has been made in reducing the national pregnancy rate among 15- to 17-year-olds. But pregnancy rates among 18- to 24-year-olds remain excessive, she said.
A key lesson from the college study lies in the importance of addressing multiple risk factors at once for unwanted pregnancy, including abusive relationships – physical, emotional, and sexual – as well as alcohol use and proper use of the emergency contraceptive pill, Dr. Lindley added.
From the Annual Meeting of the American Public Health Association
Lysteda Has QOL Benefits in Menorrhagia
Major Finding: Six of eight SF-36 categories and domains improved significantly at cycle 15 compared with baseline. Scores on the AMCOQ indicated patients experienced a consistent improvement in daily activities that are impacted by cyclic heavy menstrual bleeding.
Data Source: An open-label study of quality of life in 723 women who took oral tranexamic acid at 1.3 g three times per day for a maximum of 5 days per menstrual cycle, starting with the onset of heavy menstrual bleeding.
Disclosures: The study was funded by Xanodyne Pharmaceuticals and Ferring Pharmaceuticals, which acquired global marketing rights to Lysteda last May. Dr. Muse disclosed that he has received research grants and served as an adviser to Xanodyne.
DENVER – A novel oral formulation of tranexamic acid provided immediate and enduring improvement in two quality of life measures among women with menorrhagia in a large open-label study.
The improved quality of life benefits were noted during the first menstrual cycle after initiating treatment.
Also, the benefits were maintained throughout the 15-cycle study, Dr. Ken Muse reported at the meeting.
Tranexamic acid is a lysine analogue that acts as a competitive plasmin inhibitor and an antifibrinolytic agent. The oral formulation was approved by the Food and Drug Administration late last year for the treatment of cyclic heavy menstrual bleeding and is marketed under the trade name Lysteda.
It's a valuable alternative to surgical or hormonal treatments for this disorder, which affects up to 22 million Americans, said Dr. Muse of the University of Kentucky, Lexington.
Oral tranexamic acid won marketing approval on the strength of studies showing it reduced menstrual flow by nearly 40%.
The quality of life study was conducted because what drives many women to consult a physician regarding menorrhagia is the disorder's negative impact on their daily activities.
The study involved 723 women who took oral tranexamic acid at 1.3 g three times per day for a maximum of 5 days per menstrual cycle, starting with the onset of heavy menstrual bleeding.
General quality of life was assessed using the 36-Item Short Form Health Survey. The Aberdeen Menorrhagia Clinical Outcome Questionnaire (AMCOQ) was used as a disease-specific measure of the impact of treatment.
Significant improvements were noted in six of eight SF-36 categories and domains at cycle 15 compared with baseline.
The greatest improvements were in vitality, with a 13.5% gain, and social functioning, with an 8.3% gain.
Patients also showed significant long-term improvements in bodily pain, mental health, role-physical, and role-emotional items. Only physical functioning and general health weren't significantly different at cycle 15 compared with baseline.
Women on oral tranexamic acid had a mean 5.4% long-term improvement in the mental health component of the SF-36, and a less robust but nonetheless significant 1.7% gain in the physical functioning component, according to Dr. Muse.
Oral tranexamic acid was well tolerated. Adverse events deemed by investigators to be probably or definitely treatment related occurred in 7.3% of subjects; these were mild to moderate in nature and most often consisted of headache, menstrual discomfort, or back pain. There were few gastrointestinal side effects, and no thrombotic or thromboembolic events.
Scores on the AMCOQ indicated patients had a consistent improvement in daily activities that are affected by cyclic heavy menstrual bleeding.
Major Finding: Six of eight SF-36 categories and domains improved significantly at cycle 15 compared with baseline. Scores on the AMCOQ indicated patients experienced a consistent improvement in daily activities that are impacted by cyclic heavy menstrual bleeding.
Data Source: An open-label study of quality of life in 723 women who took oral tranexamic acid at 1.3 g three times per day for a maximum of 5 days per menstrual cycle, starting with the onset of heavy menstrual bleeding.
Disclosures: The study was funded by Xanodyne Pharmaceuticals and Ferring Pharmaceuticals, which acquired global marketing rights to Lysteda last May. Dr. Muse disclosed that he has received research grants and served as an adviser to Xanodyne.
DENVER – A novel oral formulation of tranexamic acid provided immediate and enduring improvement in two quality of life measures among women with menorrhagia in a large open-label study.
The improved quality of life benefits were noted during the first menstrual cycle after initiating treatment.
Also, the benefits were maintained throughout the 15-cycle study, Dr. Ken Muse reported at the meeting.
Tranexamic acid is a lysine analogue that acts as a competitive plasmin inhibitor and an antifibrinolytic agent. The oral formulation was approved by the Food and Drug Administration late last year for the treatment of cyclic heavy menstrual bleeding and is marketed under the trade name Lysteda.
It's a valuable alternative to surgical or hormonal treatments for this disorder, which affects up to 22 million Americans, said Dr. Muse of the University of Kentucky, Lexington.
Oral tranexamic acid won marketing approval on the strength of studies showing it reduced menstrual flow by nearly 40%.
The quality of life study was conducted because what drives many women to consult a physician regarding menorrhagia is the disorder's negative impact on their daily activities.
The study involved 723 women who took oral tranexamic acid at 1.3 g three times per day for a maximum of 5 days per menstrual cycle, starting with the onset of heavy menstrual bleeding.
General quality of life was assessed using the 36-Item Short Form Health Survey. The Aberdeen Menorrhagia Clinical Outcome Questionnaire (AMCOQ) was used as a disease-specific measure of the impact of treatment.
Significant improvements were noted in six of eight SF-36 categories and domains at cycle 15 compared with baseline.
The greatest improvements were in vitality, with a 13.5% gain, and social functioning, with an 8.3% gain.
Patients also showed significant long-term improvements in bodily pain, mental health, role-physical, and role-emotional items. Only physical functioning and general health weren't significantly different at cycle 15 compared with baseline.
Women on oral tranexamic acid had a mean 5.4% long-term improvement in the mental health component of the SF-36, and a less robust but nonetheless significant 1.7% gain in the physical functioning component, according to Dr. Muse.
Oral tranexamic acid was well tolerated. Adverse events deemed by investigators to be probably or definitely treatment related occurred in 7.3% of subjects; these were mild to moderate in nature and most often consisted of headache, menstrual discomfort, or back pain. There were few gastrointestinal side effects, and no thrombotic or thromboembolic events.
Scores on the AMCOQ indicated patients had a consistent improvement in daily activities that are affected by cyclic heavy menstrual bleeding.
Major Finding: Six of eight SF-36 categories and domains improved significantly at cycle 15 compared with baseline. Scores on the AMCOQ indicated patients experienced a consistent improvement in daily activities that are impacted by cyclic heavy menstrual bleeding.
Data Source: An open-label study of quality of life in 723 women who took oral tranexamic acid at 1.3 g three times per day for a maximum of 5 days per menstrual cycle, starting with the onset of heavy menstrual bleeding.
Disclosures: The study was funded by Xanodyne Pharmaceuticals and Ferring Pharmaceuticals, which acquired global marketing rights to Lysteda last May. Dr. Muse disclosed that he has received research grants and served as an adviser to Xanodyne.
DENVER – A novel oral formulation of tranexamic acid provided immediate and enduring improvement in two quality of life measures among women with menorrhagia in a large open-label study.
The improved quality of life benefits were noted during the first menstrual cycle after initiating treatment.
Also, the benefits were maintained throughout the 15-cycle study, Dr. Ken Muse reported at the meeting.
Tranexamic acid is a lysine analogue that acts as a competitive plasmin inhibitor and an antifibrinolytic agent. The oral formulation was approved by the Food and Drug Administration late last year for the treatment of cyclic heavy menstrual bleeding and is marketed under the trade name Lysteda.
It's a valuable alternative to surgical or hormonal treatments for this disorder, which affects up to 22 million Americans, said Dr. Muse of the University of Kentucky, Lexington.
Oral tranexamic acid won marketing approval on the strength of studies showing it reduced menstrual flow by nearly 40%.
The quality of life study was conducted because what drives many women to consult a physician regarding menorrhagia is the disorder's negative impact on their daily activities.
The study involved 723 women who took oral tranexamic acid at 1.3 g three times per day for a maximum of 5 days per menstrual cycle, starting with the onset of heavy menstrual bleeding.
General quality of life was assessed using the 36-Item Short Form Health Survey. The Aberdeen Menorrhagia Clinical Outcome Questionnaire (AMCOQ) was used as a disease-specific measure of the impact of treatment.
Significant improvements were noted in six of eight SF-36 categories and domains at cycle 15 compared with baseline.
The greatest improvements were in vitality, with a 13.5% gain, and social functioning, with an 8.3% gain.
Patients also showed significant long-term improvements in bodily pain, mental health, role-physical, and role-emotional items. Only physical functioning and general health weren't significantly different at cycle 15 compared with baseline.
Women on oral tranexamic acid had a mean 5.4% long-term improvement in the mental health component of the SF-36, and a less robust but nonetheless significant 1.7% gain in the physical functioning component, according to Dr. Muse.
Oral tranexamic acid was well tolerated. Adverse events deemed by investigators to be probably or definitely treatment related occurred in 7.3% of subjects; these were mild to moderate in nature and most often consisted of headache, menstrual discomfort, or back pain. There were few gastrointestinal side effects, and no thrombotic or thromboembolic events.
Scores on the AMCOQ indicated patients had a consistent improvement in daily activities that are affected by cyclic heavy menstrual bleeding.
From the Annual Meeting of the American Society for Reproductive Medicine
Monitor TSH Suppression in Cancer Survivors
Major Finding: Among 36 pregnancies, 14 subjects needed no additional thyroxine to maintain adequate suppression; 22 needed dosage increases and, of those, 4 were still not adequately suppressed during the entire pregnancy.
Data Source: Prospective study of thyroid function testing at 4-6 weeks throughout 36 pregnancies in 28 women.
Disclosures: Dr. Besic had no relevant financial disclosures.
PARIS – Frequent thyroid function tests during pregnancy can ensure that thyroid-stimulating hormone levels are optimally suppressed in women with a history of thyroid cancer.
In a small prospective study, TSH was adequately suppressed without dose adjustments in 39% of 36 pregnancies in 28 women. In the other pregnancies, additional thyroxine was needed as pregnancy progressed. This variability suggests that women who have undergone a total thyroidectomy experience a wide range of changes in TSH concentration throughout pregnancy, and argues for frequent monitoring and thyroxine dosage adjustment, according to Dr. Nikola Besic.
“This is why we perform thyroid function tests every 4-6 weeks in these women,” said Dr. Besic of the Institute of Oncology, Ljubljana, Slovenia. “This gives us the opportunity to change doses quickly and prevent elevation of the TSH.”
All 28 women in the study had undergone a total thyroidectomy followed by radioiodine ablation, and all were on suppressive doses of levothyroxine.
Thyroid function tests were performed before pregnancy, every 6-8 weeks during pregnancy, and after pregnancy. Adequate suppression was considered to be a TSH of 0.01-0.29 mU/L and a free T3 in the normal range.
Before conception, the group's mean TSH level was 0.9 mU/L. With pregnancy, the mean level rose to 2.01 mU/L in the second trimester, 1.46 mU/L in the third, and fell to 0.05 mU/L after delivery.
Before conception, the mean levothyroxine dose was 139 mcg daily. TSH remained adequately suppressed on the woman's baseline dosage in 14 pregnancies (39%); these women were taking a mean daily dose of 139 mcg.
In the remaining 22 pregnancies (61%), thyroxine dose had to be increased over time. The mean dose was 141 mcg in the first trimester, 150 mcg in the second, and 171 mcg in the third. After delivery, the mean dose was 156 mcg daily. The mean dose change necessary to maintain adequate TSH suppression throughout pregnancy was 31 mcg.
Dosing changes did not ensure adequate suppression at all times; 23% of pregnancies were adequately suppressed at all times during gestation, Dr. Besic said. “In these 22 pregnancies, TSH concentration remained suppressed in 4 pregnancies, in the normal range in 13, and elevated in 5.”
During the discussion period, panel chairman Dr. Bryan McIver of the Mayo Clinic, Rochester, Minn., asked about the common practice of automatically giving a 25- to 30-mcg increase in levothyroxine dose after conception, rather than performing consecutive thyroid function tests throughout pregnancy in women with a history of thyroid cancer.
Dr Besic said that more than one-third of the patients in his study did not need an increase in their dosage to maintain suppression. “However, TSH should not be elevated at all during pregnancy, and in our patients, despite our attempt to increase the dosage, some still had elevated levels.”
Major Finding: Among 36 pregnancies, 14 subjects needed no additional thyroxine to maintain adequate suppression; 22 needed dosage increases and, of those, 4 were still not adequately suppressed during the entire pregnancy.
Data Source: Prospective study of thyroid function testing at 4-6 weeks throughout 36 pregnancies in 28 women.
Disclosures: Dr. Besic had no relevant financial disclosures.
PARIS – Frequent thyroid function tests during pregnancy can ensure that thyroid-stimulating hormone levels are optimally suppressed in women with a history of thyroid cancer.
In a small prospective study, TSH was adequately suppressed without dose adjustments in 39% of 36 pregnancies in 28 women. In the other pregnancies, additional thyroxine was needed as pregnancy progressed. This variability suggests that women who have undergone a total thyroidectomy experience a wide range of changes in TSH concentration throughout pregnancy, and argues for frequent monitoring and thyroxine dosage adjustment, according to Dr. Nikola Besic.
“This is why we perform thyroid function tests every 4-6 weeks in these women,” said Dr. Besic of the Institute of Oncology, Ljubljana, Slovenia. “This gives us the opportunity to change doses quickly and prevent elevation of the TSH.”
All 28 women in the study had undergone a total thyroidectomy followed by radioiodine ablation, and all were on suppressive doses of levothyroxine.
Thyroid function tests were performed before pregnancy, every 6-8 weeks during pregnancy, and after pregnancy. Adequate suppression was considered to be a TSH of 0.01-0.29 mU/L and a free T3 in the normal range.
Before conception, the group's mean TSH level was 0.9 mU/L. With pregnancy, the mean level rose to 2.01 mU/L in the second trimester, 1.46 mU/L in the third, and fell to 0.05 mU/L after delivery.
Before conception, the mean levothyroxine dose was 139 mcg daily. TSH remained adequately suppressed on the woman's baseline dosage in 14 pregnancies (39%); these women were taking a mean daily dose of 139 mcg.
In the remaining 22 pregnancies (61%), thyroxine dose had to be increased over time. The mean dose was 141 mcg in the first trimester, 150 mcg in the second, and 171 mcg in the third. After delivery, the mean dose was 156 mcg daily. The mean dose change necessary to maintain adequate TSH suppression throughout pregnancy was 31 mcg.
Dosing changes did not ensure adequate suppression at all times; 23% of pregnancies were adequately suppressed at all times during gestation, Dr. Besic said. “In these 22 pregnancies, TSH concentration remained suppressed in 4 pregnancies, in the normal range in 13, and elevated in 5.”
During the discussion period, panel chairman Dr. Bryan McIver of the Mayo Clinic, Rochester, Minn., asked about the common practice of automatically giving a 25- to 30-mcg increase in levothyroxine dose after conception, rather than performing consecutive thyroid function tests throughout pregnancy in women with a history of thyroid cancer.
Dr Besic said that more than one-third of the patients in his study did not need an increase in their dosage to maintain suppression. “However, TSH should not be elevated at all during pregnancy, and in our patients, despite our attempt to increase the dosage, some still had elevated levels.”
Major Finding: Among 36 pregnancies, 14 subjects needed no additional thyroxine to maintain adequate suppression; 22 needed dosage increases and, of those, 4 were still not adequately suppressed during the entire pregnancy.
Data Source: Prospective study of thyroid function testing at 4-6 weeks throughout 36 pregnancies in 28 women.
Disclosures: Dr. Besic had no relevant financial disclosures.
PARIS – Frequent thyroid function tests during pregnancy can ensure that thyroid-stimulating hormone levels are optimally suppressed in women with a history of thyroid cancer.
In a small prospective study, TSH was adequately suppressed without dose adjustments in 39% of 36 pregnancies in 28 women. In the other pregnancies, additional thyroxine was needed as pregnancy progressed. This variability suggests that women who have undergone a total thyroidectomy experience a wide range of changes in TSH concentration throughout pregnancy, and argues for frequent monitoring and thyroxine dosage adjustment, according to Dr. Nikola Besic.
“This is why we perform thyroid function tests every 4-6 weeks in these women,” said Dr. Besic of the Institute of Oncology, Ljubljana, Slovenia. “This gives us the opportunity to change doses quickly and prevent elevation of the TSH.”
All 28 women in the study had undergone a total thyroidectomy followed by radioiodine ablation, and all were on suppressive doses of levothyroxine.
Thyroid function tests were performed before pregnancy, every 6-8 weeks during pregnancy, and after pregnancy. Adequate suppression was considered to be a TSH of 0.01-0.29 mU/L and a free T3 in the normal range.
Before conception, the group's mean TSH level was 0.9 mU/L. With pregnancy, the mean level rose to 2.01 mU/L in the second trimester, 1.46 mU/L in the third, and fell to 0.05 mU/L after delivery.
Before conception, the mean levothyroxine dose was 139 mcg daily. TSH remained adequately suppressed on the woman's baseline dosage in 14 pregnancies (39%); these women were taking a mean daily dose of 139 mcg.
In the remaining 22 pregnancies (61%), thyroxine dose had to be increased over time. The mean dose was 141 mcg in the first trimester, 150 mcg in the second, and 171 mcg in the third. After delivery, the mean dose was 156 mcg daily. The mean dose change necessary to maintain adequate TSH suppression throughout pregnancy was 31 mcg.
Dosing changes did not ensure adequate suppression at all times; 23% of pregnancies were adequately suppressed at all times during gestation, Dr. Besic said. “In these 22 pregnancies, TSH concentration remained suppressed in 4 pregnancies, in the normal range in 13, and elevated in 5.”
During the discussion period, panel chairman Dr. Bryan McIver of the Mayo Clinic, Rochester, Minn., asked about the common practice of automatically giving a 25- to 30-mcg increase in levothyroxine dose after conception, rather than performing consecutive thyroid function tests throughout pregnancy in women with a history of thyroid cancer.
Dr Besic said that more than one-third of the patients in his study did not need an increase in their dosage to maintain suppression. “However, TSH should not be elevated at all during pregnancy, and in our patients, despite our attempt to increase the dosage, some still had elevated levels.”
From the International Thyroid Congress