User login
Crisis in Medicine: Have We Traded Technology for Our Six Senses?
Technology creates change, and change is moving fast and is relentless. Physicians, on the other hand, are generally slow to change. Wisely, we question change—we observe it, we study it, and we try to ensure our patients will benefit from it over time. Maybe as a result of this or as a consequence of our often myopic view of the world, we mistakenly let others lead the way and dictate how we must change and what our practices must absorb. We must turn this around and be the agents of change for our profession so we can appropriately use the available technology and create systems for managing the demands of a society that expects instant answers with fewer doctor resources devoted to the answer. The insurance industry is encouraging a wholesale dismantling of the classic patient visit to be replaced by nonphysician interactions, virtual diagnostics, and electronic medical records. We must not allow this and must ensure that we safeguard our profession by employing traditional skills, utilizing our 5 senses, and incorporating technology as a tool for better diagnosis and treatment but not as a substitute for the same.
Great doctors are often described as having a sixth sense—an intuition that guides them in diagnosing and treating patients. It is assumed, therefore, that the good doctor will have the benefit of 5 senses: sight, sound, touch, smell, and taste. Sound: What does the patient tell or neglect to tell the doctor? What sounds does a joint produce when it moves? Sight: How does the patient present? Are they weary from pain or chronic disease? Touch: What does the joint feel like? How does it move? What is the patient’s response to stabilization of a joint? Smell: Is there an odor that helps detect the presence of infection or decay? Is the patient coming into contact with a substance causing harm or preventing healing?
A good doctor must employ these senses first to understand the patient’s needs and then to treat the patient. The sixth sense is a gift, one that comes from years of experience, an attention to detail, and a commitment to the craft of medicine. A recent trend toward virtual medicine is a dangerous path that must be walked with care and discretion so that the 6 senses are maintained and nurtured. Technology must be used to enhance and not limit these senses. The patient cannot be reduced to a 2-dimensional version of his/herself so that the doctor’s powers of diagnosis and healing are similarly limited.
Change in the office has occurred with mandates for electronic medical records and work-hour restrictions for residents. Data do not support that either change has resulted in a net benefit to patients. We are mandated to invest scarce capital to support new technology, resulting in increased pressure to recoup investment. Where there is a cap on revenue, the only way to increase net profit is to increase volume and decrease services. Physician time is the variable and can be streamlined by performing video conferences or smartphone consultations. Change may bring higher order, as the English philosopher John Locke said, but it is time for all of us as physicians to step back and question that this type of change is the path we must take. An office with a schedule of 80 patients seen at 5-minute intervals by physician assistants has no place in medicine. The pressure imposed by the insurance industry or hospital administrators to meet quotas has gotten out of hand and the time is now to say with a strong but fair voice a resounding NO!
The office visit with a history and physical examination is the most exciting and effective time to meet, console, and relate to our patients. The use of the 5 senses is critical. We must not let technological advancements (eg, smartphones, the Internet, and electronic medical records) destroy what was created and taught to us all through our training. The reward that is accomplished by placing one’s hand on a patient’s knee to understand its warmth and swelling, the tactile feeling of a fluid wave, or performing carefully with compassion a provocative maneuver that gives by sight a grimace of discomfort can tell so much more than a status update on the phone. We must not allow ourselves to be replaced by ancillary services for so-called efficiency and cost saving. Rather, we must be innovative and sharp. We must find the way to use the wonders of the virtual world without giving up the human consult.
Imagine that you are able to travel to Iguazu Falls, South America, to see one of the wonders of our world. You sit in that life raft moving upstream to feel the heat from the water as it crushes the rocks below, and you feel the mist on your face. You see the majesty and hear the screams and breadth of excitement of those around you, while you listen to the deafening sounds created by this waterfall. Now imagine you are required to report on this same experience through a video or some form of technology that the world has convinced us is the best and far cheaper substitute. This is our electronic medical record. A tool we are forced to use, and while it has a purpose, it is a sterile tool that fails to provide information that will give clues to awaken the sixth sense. It is a checklist that could allow for completion of a task—like how to fix a leaky faucet.
How then do we accomplish walking the fine line of working with nonphysicians and technology and yet delivering pinnacle care? The answer isn’t simple but it must include education and a commitment to the profession. We must make the public aware that we are one of the few professions that dedicate our lives to others by promoting health and advancing research. My colleagues, the pendulum has swung too far; it is time to take back our great profession through education of ourselves and the public. While technology may help the world connect, it has a limited role unless we first use our 6 senses to help our patients. We must not submit to a compassionless and callous approach that is the inevitable outcome of virtual medicine done with speed. We must maintain our dignity and let the public understand how many years of sacrifice has taken place to earn a sixth sense and not allow a third party to take it away. We are the only source of protection for our patients and we need each one of our senses to perform this task.
Advancing research has been a cornerstone for the orthopedic surgeon. Position statements through meta-analyses and systematic reviews of the literature have recently been utilized with increasing frequency. Combining data of potentially flawed studies can often lead to erroneous conclusions and may stray away from best practices. Is this where we want evidence-based medicine to go? The end result is that decisions are made by insurance companies who rely on these flawed studies to force clinical decisions on the physician, as was most recently seen by the investigation of viscosupplementation for knee osteoarthritis.1
In a 2007 study published in JAMA (The Journal of the American Medical Association), only 62% of residents could appropriately interpret a P value.2 How can we expect young clinicians to evaluate, interpret, and apply the multitude of evidence in the literature to everyday practice? We must marry the use of best evidence with our expertise to make the most informed decision while managing the expectations of our patients. In order to achieve that balance, we must rely on our intuition, our sixth sense. There is too much patient individuality and complexity surrounding each individual’s situation for a one-size-fits-all approach and for wholesale reliance on research to address each unique situation.
If Nathan Davis in 1845 was able to convince the New York Medical Society to establish a nationwide professional association to assist in regulating the practice of medicine, then it is time for all of us to stand up and insist on a code of ethics that is unrelenting and uncompromising. Our wise leaders of the American Orthopaedic Association (AOA) who founded the formation of orthopedics in America knew guidelines were needed to “foster advances in the care of patients, improve the teaching of orthopaedic surgery in medical schools and formal orthopaedic training, and to promote orthopaedic surgery as a surgical discipline worldwide.”3 It is now our turn to renew the guidelines and encourage our leaders to help educate ourselves and patients as we work with technology and administrators, nurses and physician assistants to deliver pinnacle care. We must reform medical education and the practice of medicine so that technology is used as a companion but not a substitute for our 6 senses.
The next time a patient comes into the exam room, sit down, look the patient in the eye, listen, touch, console anxiety, make a human connection, and form a lasting relationship. By all means apologize to your patients as you fill out the electronic medical record and insurance forms. Discuss how we are in the same crisis together and ask for their help as they have come to you for yours.
1. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576.
2. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010-1022.
3. DeRosa GP. 75 Years of Doing the Right Thing: A History of the American Board of Orthopaedic Surgery. American Board of Orthopaedic Surgery; 2009.
Technology creates change, and change is moving fast and is relentless. Physicians, on the other hand, are generally slow to change. Wisely, we question change—we observe it, we study it, and we try to ensure our patients will benefit from it over time. Maybe as a result of this or as a consequence of our often myopic view of the world, we mistakenly let others lead the way and dictate how we must change and what our practices must absorb. We must turn this around and be the agents of change for our profession so we can appropriately use the available technology and create systems for managing the demands of a society that expects instant answers with fewer doctor resources devoted to the answer. The insurance industry is encouraging a wholesale dismantling of the classic patient visit to be replaced by nonphysician interactions, virtual diagnostics, and electronic medical records. We must not allow this and must ensure that we safeguard our profession by employing traditional skills, utilizing our 5 senses, and incorporating technology as a tool for better diagnosis and treatment but not as a substitute for the same.
Great doctors are often described as having a sixth sense—an intuition that guides them in diagnosing and treating patients. It is assumed, therefore, that the good doctor will have the benefit of 5 senses: sight, sound, touch, smell, and taste. Sound: What does the patient tell or neglect to tell the doctor? What sounds does a joint produce when it moves? Sight: How does the patient present? Are they weary from pain or chronic disease? Touch: What does the joint feel like? How does it move? What is the patient’s response to stabilization of a joint? Smell: Is there an odor that helps detect the presence of infection or decay? Is the patient coming into contact with a substance causing harm or preventing healing?
A good doctor must employ these senses first to understand the patient’s needs and then to treat the patient. The sixth sense is a gift, one that comes from years of experience, an attention to detail, and a commitment to the craft of medicine. A recent trend toward virtual medicine is a dangerous path that must be walked with care and discretion so that the 6 senses are maintained and nurtured. Technology must be used to enhance and not limit these senses. The patient cannot be reduced to a 2-dimensional version of his/herself so that the doctor’s powers of diagnosis and healing are similarly limited.
Change in the office has occurred with mandates for electronic medical records and work-hour restrictions for residents. Data do not support that either change has resulted in a net benefit to patients. We are mandated to invest scarce capital to support new technology, resulting in increased pressure to recoup investment. Where there is a cap on revenue, the only way to increase net profit is to increase volume and decrease services. Physician time is the variable and can be streamlined by performing video conferences or smartphone consultations. Change may bring higher order, as the English philosopher John Locke said, but it is time for all of us as physicians to step back and question that this type of change is the path we must take. An office with a schedule of 80 patients seen at 5-minute intervals by physician assistants has no place in medicine. The pressure imposed by the insurance industry or hospital administrators to meet quotas has gotten out of hand and the time is now to say with a strong but fair voice a resounding NO!
The office visit with a history and physical examination is the most exciting and effective time to meet, console, and relate to our patients. The use of the 5 senses is critical. We must not let technological advancements (eg, smartphones, the Internet, and electronic medical records) destroy what was created and taught to us all through our training. The reward that is accomplished by placing one’s hand on a patient’s knee to understand its warmth and swelling, the tactile feeling of a fluid wave, or performing carefully with compassion a provocative maneuver that gives by sight a grimace of discomfort can tell so much more than a status update on the phone. We must not allow ourselves to be replaced by ancillary services for so-called efficiency and cost saving. Rather, we must be innovative and sharp. We must find the way to use the wonders of the virtual world without giving up the human consult.
Imagine that you are able to travel to Iguazu Falls, South America, to see one of the wonders of our world. You sit in that life raft moving upstream to feel the heat from the water as it crushes the rocks below, and you feel the mist on your face. You see the majesty and hear the screams and breadth of excitement of those around you, while you listen to the deafening sounds created by this waterfall. Now imagine you are required to report on this same experience through a video or some form of technology that the world has convinced us is the best and far cheaper substitute. This is our electronic medical record. A tool we are forced to use, and while it has a purpose, it is a sterile tool that fails to provide information that will give clues to awaken the sixth sense. It is a checklist that could allow for completion of a task—like how to fix a leaky faucet.
How then do we accomplish walking the fine line of working with nonphysicians and technology and yet delivering pinnacle care? The answer isn’t simple but it must include education and a commitment to the profession. We must make the public aware that we are one of the few professions that dedicate our lives to others by promoting health and advancing research. My colleagues, the pendulum has swung too far; it is time to take back our great profession through education of ourselves and the public. While technology may help the world connect, it has a limited role unless we first use our 6 senses to help our patients. We must not submit to a compassionless and callous approach that is the inevitable outcome of virtual medicine done with speed. We must maintain our dignity and let the public understand how many years of sacrifice has taken place to earn a sixth sense and not allow a third party to take it away. We are the only source of protection for our patients and we need each one of our senses to perform this task.
Advancing research has been a cornerstone for the orthopedic surgeon. Position statements through meta-analyses and systematic reviews of the literature have recently been utilized with increasing frequency. Combining data of potentially flawed studies can often lead to erroneous conclusions and may stray away from best practices. Is this where we want evidence-based medicine to go? The end result is that decisions are made by insurance companies who rely on these flawed studies to force clinical decisions on the physician, as was most recently seen by the investigation of viscosupplementation for knee osteoarthritis.1
In a 2007 study published in JAMA (The Journal of the American Medical Association), only 62% of residents could appropriately interpret a P value.2 How can we expect young clinicians to evaluate, interpret, and apply the multitude of evidence in the literature to everyday practice? We must marry the use of best evidence with our expertise to make the most informed decision while managing the expectations of our patients. In order to achieve that balance, we must rely on our intuition, our sixth sense. There is too much patient individuality and complexity surrounding each individual’s situation for a one-size-fits-all approach and for wholesale reliance on research to address each unique situation.
If Nathan Davis in 1845 was able to convince the New York Medical Society to establish a nationwide professional association to assist in regulating the practice of medicine, then it is time for all of us to stand up and insist on a code of ethics that is unrelenting and uncompromising. Our wise leaders of the American Orthopaedic Association (AOA) who founded the formation of orthopedics in America knew guidelines were needed to “foster advances in the care of patients, improve the teaching of orthopaedic surgery in medical schools and formal orthopaedic training, and to promote orthopaedic surgery as a surgical discipline worldwide.”3 It is now our turn to renew the guidelines and encourage our leaders to help educate ourselves and patients as we work with technology and administrators, nurses and physician assistants to deliver pinnacle care. We must reform medical education and the practice of medicine so that technology is used as a companion but not a substitute for our 6 senses.
The next time a patient comes into the exam room, sit down, look the patient in the eye, listen, touch, console anxiety, make a human connection, and form a lasting relationship. By all means apologize to your patients as you fill out the electronic medical record and insurance forms. Discuss how we are in the same crisis together and ask for their help as they have come to you for yours.
Technology creates change, and change is moving fast and is relentless. Physicians, on the other hand, are generally slow to change. Wisely, we question change—we observe it, we study it, and we try to ensure our patients will benefit from it over time. Maybe as a result of this or as a consequence of our often myopic view of the world, we mistakenly let others lead the way and dictate how we must change and what our practices must absorb. We must turn this around and be the agents of change for our profession so we can appropriately use the available technology and create systems for managing the demands of a society that expects instant answers with fewer doctor resources devoted to the answer. The insurance industry is encouraging a wholesale dismantling of the classic patient visit to be replaced by nonphysician interactions, virtual diagnostics, and electronic medical records. We must not allow this and must ensure that we safeguard our profession by employing traditional skills, utilizing our 5 senses, and incorporating technology as a tool for better diagnosis and treatment but not as a substitute for the same.
Great doctors are often described as having a sixth sense—an intuition that guides them in diagnosing and treating patients. It is assumed, therefore, that the good doctor will have the benefit of 5 senses: sight, sound, touch, smell, and taste. Sound: What does the patient tell or neglect to tell the doctor? What sounds does a joint produce when it moves? Sight: How does the patient present? Are they weary from pain or chronic disease? Touch: What does the joint feel like? How does it move? What is the patient’s response to stabilization of a joint? Smell: Is there an odor that helps detect the presence of infection or decay? Is the patient coming into contact with a substance causing harm or preventing healing?
A good doctor must employ these senses first to understand the patient’s needs and then to treat the patient. The sixth sense is a gift, one that comes from years of experience, an attention to detail, and a commitment to the craft of medicine. A recent trend toward virtual medicine is a dangerous path that must be walked with care and discretion so that the 6 senses are maintained and nurtured. Technology must be used to enhance and not limit these senses. The patient cannot be reduced to a 2-dimensional version of his/herself so that the doctor’s powers of diagnosis and healing are similarly limited.
Change in the office has occurred with mandates for electronic medical records and work-hour restrictions for residents. Data do not support that either change has resulted in a net benefit to patients. We are mandated to invest scarce capital to support new technology, resulting in increased pressure to recoup investment. Where there is a cap on revenue, the only way to increase net profit is to increase volume and decrease services. Physician time is the variable and can be streamlined by performing video conferences or smartphone consultations. Change may bring higher order, as the English philosopher John Locke said, but it is time for all of us as physicians to step back and question that this type of change is the path we must take. An office with a schedule of 80 patients seen at 5-minute intervals by physician assistants has no place in medicine. The pressure imposed by the insurance industry or hospital administrators to meet quotas has gotten out of hand and the time is now to say with a strong but fair voice a resounding NO!
The office visit with a history and physical examination is the most exciting and effective time to meet, console, and relate to our patients. The use of the 5 senses is critical. We must not let technological advancements (eg, smartphones, the Internet, and electronic medical records) destroy what was created and taught to us all through our training. The reward that is accomplished by placing one’s hand on a patient’s knee to understand its warmth and swelling, the tactile feeling of a fluid wave, or performing carefully with compassion a provocative maneuver that gives by sight a grimace of discomfort can tell so much more than a status update on the phone. We must not allow ourselves to be replaced by ancillary services for so-called efficiency and cost saving. Rather, we must be innovative and sharp. We must find the way to use the wonders of the virtual world without giving up the human consult.
Imagine that you are able to travel to Iguazu Falls, South America, to see one of the wonders of our world. You sit in that life raft moving upstream to feel the heat from the water as it crushes the rocks below, and you feel the mist on your face. You see the majesty and hear the screams and breadth of excitement of those around you, while you listen to the deafening sounds created by this waterfall. Now imagine you are required to report on this same experience through a video or some form of technology that the world has convinced us is the best and far cheaper substitute. This is our electronic medical record. A tool we are forced to use, and while it has a purpose, it is a sterile tool that fails to provide information that will give clues to awaken the sixth sense. It is a checklist that could allow for completion of a task—like how to fix a leaky faucet.
How then do we accomplish walking the fine line of working with nonphysicians and technology and yet delivering pinnacle care? The answer isn’t simple but it must include education and a commitment to the profession. We must make the public aware that we are one of the few professions that dedicate our lives to others by promoting health and advancing research. My colleagues, the pendulum has swung too far; it is time to take back our great profession through education of ourselves and the public. While technology may help the world connect, it has a limited role unless we first use our 6 senses to help our patients. We must not submit to a compassionless and callous approach that is the inevitable outcome of virtual medicine done with speed. We must maintain our dignity and let the public understand how many years of sacrifice has taken place to earn a sixth sense and not allow a third party to take it away. We are the only source of protection for our patients and we need each one of our senses to perform this task.
Advancing research has been a cornerstone for the orthopedic surgeon. Position statements through meta-analyses and systematic reviews of the literature have recently been utilized with increasing frequency. Combining data of potentially flawed studies can often lead to erroneous conclusions and may stray away from best practices. Is this where we want evidence-based medicine to go? The end result is that decisions are made by insurance companies who rely on these flawed studies to force clinical decisions on the physician, as was most recently seen by the investigation of viscosupplementation for knee osteoarthritis.1
In a 2007 study published in JAMA (The Journal of the American Medical Association), only 62% of residents could appropriately interpret a P value.2 How can we expect young clinicians to evaluate, interpret, and apply the multitude of evidence in the literature to everyday practice? We must marry the use of best evidence with our expertise to make the most informed decision while managing the expectations of our patients. In order to achieve that balance, we must rely on our intuition, our sixth sense. There is too much patient individuality and complexity surrounding each individual’s situation for a one-size-fits-all approach and for wholesale reliance on research to address each unique situation.
If Nathan Davis in 1845 was able to convince the New York Medical Society to establish a nationwide professional association to assist in regulating the practice of medicine, then it is time for all of us to stand up and insist on a code of ethics that is unrelenting and uncompromising. Our wise leaders of the American Orthopaedic Association (AOA) who founded the formation of orthopedics in America knew guidelines were needed to “foster advances in the care of patients, improve the teaching of orthopaedic surgery in medical schools and formal orthopaedic training, and to promote orthopaedic surgery as a surgical discipline worldwide.”3 It is now our turn to renew the guidelines and encourage our leaders to help educate ourselves and patients as we work with technology and administrators, nurses and physician assistants to deliver pinnacle care. We must reform medical education and the practice of medicine so that technology is used as a companion but not a substitute for our 6 senses.
The next time a patient comes into the exam room, sit down, look the patient in the eye, listen, touch, console anxiety, make a human connection, and form a lasting relationship. By all means apologize to your patients as you fill out the electronic medical record and insurance forms. Discuss how we are in the same crisis together and ask for their help as they have come to you for yours.
1. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576.
2. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010-1022.
3. DeRosa GP. 75 Years of Doing the Right Thing: A History of the American Board of Orthopaedic Surgery. American Board of Orthopaedic Surgery; 2009.
1. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576.
2. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010-1022.
3. DeRosa GP. 75 Years of Doing the Right Thing: A History of the American Board of Orthopaedic Surgery. American Board of Orthopaedic Surgery; 2009.
Dynamic Magnetic Resonance Imaging of Partial-Thickness Retearing of Distal Biceps Tendon After Endobutton Repair
Retearing after repair of the distal biceps tendon is rare.1 Heterotopic ossification (HO) is also considered uncommon, though reported rates in the literature vary widely, depending on repair and follow-up methods.1-3
In this article, we report a case of ruptured distal biceps tendon repaired with a 1-incision Endobutton technique with longitudinal clinical and imaging follow-up, and we discuss the potential biomechanical and rehabilitative implications of clinically occult retearing after repair.
This case is unique in that the patient was a physician who procured multiple magnetic resonance imaging (MRI) examinations during the postoperative period and again at 1-year follow-up. We witnessed formation of a small focus of HO, which entered and significantly narrowed the radioulnar space on forearm pronation on dynamic MRI. There was no obvious clinical evidence for retearing; high-grade partial-thickness tendon retearing was diagnosed on MRI. This prompted a gentler rehabilitation protocol. Subsequent scar formation and tendon remodeling allowed the patient to return to full activity by 1-year follow-up, confirming recent reports that intrasubstance signal abnormalities4 and even rerupture on MRI are not correlated with symptoms.5 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy right-hand–dominant 32-year-old man was rock climbing when he heard a pop and felt sudden weakness in his right elbow. The injury occurred during eccentric contraction, while he was climbing a 45° overhanging wall with his right elbow fully extended and forearm maximally pronated. Immediately after injury, he noticed obvious deformity in the right arm. Before this incident, there was no history of elbow symptoms or any medication use.
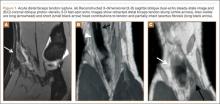
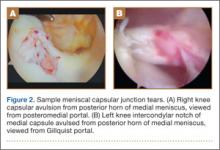
Physical examination revealed distortion of the biceps with a palpable defect in the right elbow consistent with a complete biceps tendon rupture. This was confirmed on MRI, which showed avulsion of the distal biceps tendon from its insertion on the radius. There was 4 cm of proximal retraction of the tendon, which was kept at the level of the joint line by a partially intact lacertus fibrosis (Figure 1).
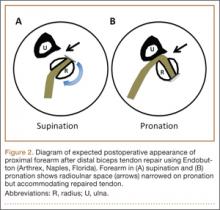
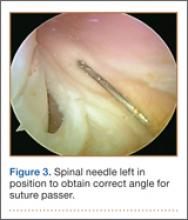
As the patient was physically active, operative treatment was chosen with the expectation of restoration to full function and strength. Six days after injury, surgery was performed using a 1-incision anterior approach with an Endobutton technique, as first described by Bain and colleagues6 and subsequently detailed by other authors.7 The diameter of the distal biceps tendon after attachment to the Endobutton (Arthrex, Naples, Florida) was measured, and a corresponding 7-mm unicortical tunnel was drilled into the radial tuberosity. During surgery, there was full range of motion (ROM) at the elbow and forearm. Before closure, the wound was copiously irrigated to minimize the potential of HO. In our practice, we do not routinely administer prophylactic anti-inflammatory drugs to low-risk patients because of the theoretical risks for delayed tendon–bone healing8 and inferior healing strength.9 The theoretical, expected postoperative appearance is illustrated in Figure 2.
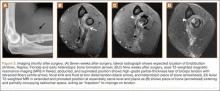
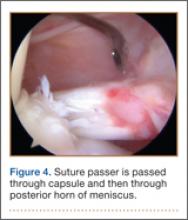
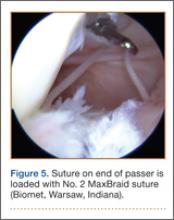
For 7 days after surgery, the patient wore a posterior elbow splint in a flexed, supinated position. Afterward, rehabilitation initially consisted of passive ROM progressing to active ROM at postoperative week 4. Pronation was slow to return, but essentially full ROM was regained by 7 weeks after surgery. Seven weeks after surgery, a radiograph showed a small amount of HO near the radial tuberosity (Figure 3A). However, the patient was clinically progressing well, and by 9 weeks was comfortably performing slow, controlled arm curls with a 10-lb weight. Despite the clinical improvements, MRI 9 weeks after surgery showed high-grade partial-thickness retearing of the distal biceps tendon without significant retraction. With dynamic MRI, it was evident that the focus of HO near but external to the distal tendon entered the radioulnar space on pronation (Figures 3B–3D). On axial images of the center of the cortical tunnel, the short-axis diameter of the heterotopic bone measured 2.5 mm, and the bone clearly was occupying part of the radioulnar space during pronation. As the patient was not having pain and was increasing in strength, the clinical team resumed rehabilitation, albeit at a gentler pace.
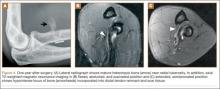
By 1-year follow-up, the patient had returned to preinjury activity levels, which included rock climbing and weightlifting without pain or loss of strength. One year after surgery, radiographs and MRI showed maturation of heterotopic bone, which was incorporated with scar tissue along the remodeled distal biceps tendon remnant (Figures 4A-4C).
Discussion
Distal biceps tendon ruptures historically have been considered relatively rare injuries. Postrepair complications are uncommon but well known. HO has been described with all distal biceps tendon repair techniques, but rates vary depending on follow-up method. Given the data reported, HO is thought to have a higher incidence with the 2-incision technique than with the 1-incision technique.10 The literature includes fewer reports of HO with the Endobutton technique11,12 than with the suture anchor technique.3 Incidence of HO after distal biceps tendon repair has been reported to be as high as 50%, with Marnitz and colleagues5 suggesting that its presence does not necessarily affect clinical outcome. This was confirmed in our patient’s case.
A much rarer complication of repair is rerupture, which can be asymptomatic or symptomatic.5 The most common failure site, discovered during surgery, is the fixation site.2,13 The true incidence of rerupture is unknown, as MRI typically is not obtained for asymptomatic patients. However, Marnitz and colleagues5 recently found increased intratendinous signal and thickness of repaired tendons in the majority of intact postoperative cases and no significant correlation between any MRI features, including tendon rerupture, and clinical measures. This was confirmed in our patient’s case, in which the MRI-based diagnosis of partial retear was not correlated with adverse clinical outcome at 1-year follow-up. Marnitz and colleagues5 hypothesized that the increased thickness of the repaired tendon would predispose the patient to impingement.
Our patient had no demonstrable loss of motion during surgery. However, postoperative dynamic MRI clearly showed insufficient room in the pronated radioulnar space for both heterotopic bone and repaired biceps tendon. It is possible that a space-occupying peritendinous hematoma or HO soon after surgery caused early loss of pronation. In a study of 10 volunteers, mean radioulnar distance was 4.0 mm (range, 2.1-6.0 mm) at its minimum in pronation.14 We used the same technique to measure our patient’s radioulnar space in active semipronation: 7 mm. This diameter was the same as that of the distal biceps tendon during surgery (Figure 3D). Had our patient been in maximum pronation during imaging, we would have expected a further decrease in radioulnar distance. Given the insufficient room in this case, it is possible that, during the attempt to regain full pronation, attritional wear of the repaired biceps tendon occurred with a corresponding maturation of the focus of heterotopic bone. Supporting this theory is the patient’s lack of history of traumatic loading, which would have suggested tensile failure of the repair. By 1-year follow-up, scar-tissue maturation and remodeling had occurred, and there was sufficient overall biomechanical strength to withstand return to normal activity.
The literature includes multiple reports of in vitro biomechanical studies of various types of distal biceps tendon fixation,15-17 and multiple authors have demonstrated the superior pullout strength of cortical fixation buttons,18,19 such as the Endobutton. It is important to note that all biomechanical tests are performed in cadaveric specimens and are therefore likely applicable only at time zero, after in vivo repair. In part stemming from the results of these cadaveric biomechanical tests, earlier and more aggressive rehabilitation protocols have been developed with the assumption that time zero is the weakest point.20 If in fact the native repaired biceps tendon is retorn and remodeled, there will exist a nadir in strength because of the high concentration of biomechanically inferior type III collagen in scar tissue (as opposed to the very strong type I collagen in native tendons).21 In the absence of complete rerupture, biomechanical strength would continue to improve during scar maturation and continued healing, leading to the typical excellent clinical result, as seen in our case.
This case report illustrates the dynamic MRI appearance of a small focus of HO after distal biceps tendon repair and adds to the time-zero cadaveric data of distal biceps tendon repair. The small focus of HO near the repaired distal tendon may have caused tendon impingement in pronation because of its space-occupying nature and possible attritional tendon wear. A gentler rehabilitation protocol for this pattern of HO, during a period in which biomechanically inferior scar tissue is maturing, may be warranted. Despite the high rates of clinical success with distal biceps tendon repair, there is lack of agreement between ex vivo cadaveric studies and the in vivo scenario. A prospective study involving a larger series of patients with postoperative dynamic MRI examinations would be useful to better understand the true in vivo course of distal biceps tendon repair.
1. Cohen MS. Complications of distal biceps tendon repairs. Sports Med Arthrosc. 2008;16(3):148-153.
2. Bisson L, Moyer M, Lanighan K, Marzo J. Complications associated with repair of a distal biceps rupture using the modified two-incision technique. J Shoulder Elbow Surg. 2008;17(1 suppl):67S-71S.
3. Gallinet D, Dietsch E, Barbier-Brion B, Lerais JM, Obert L. Suture anchor reinsertion of distal biceps rupture: clinical results and radiological assessment of tendon healing. Orthop Traumatol Surg Res. 2011;97(3):252-259.
4. Schmidt CC, Diaz VA, Weir DM, Latona CR, Miller MC. Repaired distal biceps magnetic resonance imaging anatomy compared with outcome. J Shoulder Elbow Surg. 2012;21(12):1623-1631.
5. Marnitz T, Spiegel D, Hug K, et al. MR imaging findings in flexed abducted supinated (FABS) position and clinical presentation following refixation of distal biceps tendon rupture using bioabsorbable suture anchors. Rofo. 2012;184(5):432-436.
6. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
7. King J, Bollier M. Repair of distal biceps tendon ruptures using the Endobutton. J Am Acad Orthop Surg. 2008;16(8):490-494.
8. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
9. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35(8):1326-1333.
10. Miyamoto RG, Elser F, Millett PJ. Distal biceps tendon injuries. J Bone Joint Surg Am. 2010;92(11):2128-2138.
11. Dillon MT, Lepore DJ. Heterotopic ossification after single-incision distal biceps tendon repair with an Endobutton. J Surg Orthop Adv. 2011;20(3):198-201.
12. Peeters T, Ching-Soon NG, Jansen N, Sneyers C, Declercq G, Verstreken F. Functional outcome after repair of distal biceps tendon ruptures using the Endobutton technique. J Shoulder Elbow Surg. 2009;18(2):283-287.
13. Katolik LI, Fernandez J, Cohen MS. Acute failure of distal biceps reconstruction: a case report. J Shoulder Elbow Surg. 2007;16(5):e10-e12.
14. Seiler JG 3rd, Parker LM, Chamberland PD, Sherbourne GM, Carpenter WA. The distal biceps tendon. Two potential mechanisms involved in its rupture: arterial supply and mechanical impingement. J Shoulder Elbow Surg. 1995;4(3):149-156.
15. Siebenlist S, Lenich A, Buchholz A, et al. Biomechanical in vitro validation of intramedullary cortical button fixation for distal biceps tendon repair: a new technique. Am J Sports Med. 2011;39(8):1762-1768.
16. Pereira DS, Kvitne RS, Liang M, Giacobetti FB, Ebramzadeh E. Surgical repair of distal biceps tendon ruptures: a biomechanical comparison of two techniques. Am J Sports Med. 2002;30(3):432-436.
17. Lemos SE, Ebramzedeh E, Kvitne RS. A new technique: in vitro suture anchor fixation has superior yield strength to bone tunnel fixation for distal biceps tendon repair. Am J Sports Med. 2004;32(2):406-410.
18. Kettler M, Lunger J, Kuhn V, Mutschler W, Tingart MJ. Failure strengths in distal biceps tendon repair. Am J Sports Med. 2007;35(9):1544-1548.
19. Mazzocca AD, Burton KJ, Romeo AA, Santangelo S, Adams DA, Arciero RA. Biomechanical evaluation of 4 techniques of distal biceps brachii tendon repair. Am J Sports Med. 2007;35(2):252-258.
20. Spencer EE Jr, Tisdale A, Kostka K, Ivy RE. Is therapy necessary after distal biceps tendon repair? Hand (N Y). 2008;3(4):316-319.
21. Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. Am J Sports Med. 2000;28(4):499-505.
Retearing after repair of the distal biceps tendon is rare.1 Heterotopic ossification (HO) is also considered uncommon, though reported rates in the literature vary widely, depending on repair and follow-up methods.1-3
In this article, we report a case of ruptured distal biceps tendon repaired with a 1-incision Endobutton technique with longitudinal clinical and imaging follow-up, and we discuss the potential biomechanical and rehabilitative implications of clinically occult retearing after repair.
This case is unique in that the patient was a physician who procured multiple magnetic resonance imaging (MRI) examinations during the postoperative period and again at 1-year follow-up. We witnessed formation of a small focus of HO, which entered and significantly narrowed the radioulnar space on forearm pronation on dynamic MRI. There was no obvious clinical evidence for retearing; high-grade partial-thickness tendon retearing was diagnosed on MRI. This prompted a gentler rehabilitation protocol. Subsequent scar formation and tendon remodeling allowed the patient to return to full activity by 1-year follow-up, confirming recent reports that intrasubstance signal abnormalities4 and even rerupture on MRI are not correlated with symptoms.5 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy right-hand–dominant 32-year-old man was rock climbing when he heard a pop and felt sudden weakness in his right elbow. The injury occurred during eccentric contraction, while he was climbing a 45° overhanging wall with his right elbow fully extended and forearm maximally pronated. Immediately after injury, he noticed obvious deformity in the right arm. Before this incident, there was no history of elbow symptoms or any medication use.
Physical examination revealed distortion of the biceps with a palpable defect in the right elbow consistent with a complete biceps tendon rupture. This was confirmed on MRI, which showed avulsion of the distal biceps tendon from its insertion on the radius. There was 4 cm of proximal retraction of the tendon, which was kept at the level of the joint line by a partially intact lacertus fibrosis (Figure 1).
As the patient was physically active, operative treatment was chosen with the expectation of restoration to full function and strength. Six days after injury, surgery was performed using a 1-incision anterior approach with an Endobutton technique, as first described by Bain and colleagues6 and subsequently detailed by other authors.7 The diameter of the distal biceps tendon after attachment to the Endobutton (Arthrex, Naples, Florida) was measured, and a corresponding 7-mm unicortical tunnel was drilled into the radial tuberosity. During surgery, there was full range of motion (ROM) at the elbow and forearm. Before closure, the wound was copiously irrigated to minimize the potential of HO. In our practice, we do not routinely administer prophylactic anti-inflammatory drugs to low-risk patients because of the theoretical risks for delayed tendon–bone healing8 and inferior healing strength.9 The theoretical, expected postoperative appearance is illustrated in Figure 2.
For 7 days after surgery, the patient wore a posterior elbow splint in a flexed, supinated position. Afterward, rehabilitation initially consisted of passive ROM progressing to active ROM at postoperative week 4. Pronation was slow to return, but essentially full ROM was regained by 7 weeks after surgery. Seven weeks after surgery, a radiograph showed a small amount of HO near the radial tuberosity (Figure 3A). However, the patient was clinically progressing well, and by 9 weeks was comfortably performing slow, controlled arm curls with a 10-lb weight. Despite the clinical improvements, MRI 9 weeks after surgery showed high-grade partial-thickness retearing of the distal biceps tendon without significant retraction. With dynamic MRI, it was evident that the focus of HO near but external to the distal tendon entered the radioulnar space on pronation (Figures 3B–3D). On axial images of the center of the cortical tunnel, the short-axis diameter of the heterotopic bone measured 2.5 mm, and the bone clearly was occupying part of the radioulnar space during pronation. As the patient was not having pain and was increasing in strength, the clinical team resumed rehabilitation, albeit at a gentler pace.
By 1-year follow-up, the patient had returned to preinjury activity levels, which included rock climbing and weightlifting without pain or loss of strength. One year after surgery, radiographs and MRI showed maturation of heterotopic bone, which was incorporated with scar tissue along the remodeled distal biceps tendon remnant (Figures 4A-4C).
Discussion
Distal biceps tendon ruptures historically have been considered relatively rare injuries. Postrepair complications are uncommon but well known. HO has been described with all distal biceps tendon repair techniques, but rates vary depending on follow-up method. Given the data reported, HO is thought to have a higher incidence with the 2-incision technique than with the 1-incision technique.10 The literature includes fewer reports of HO with the Endobutton technique11,12 than with the suture anchor technique.3 Incidence of HO after distal biceps tendon repair has been reported to be as high as 50%, with Marnitz and colleagues5 suggesting that its presence does not necessarily affect clinical outcome. This was confirmed in our patient’s case.
A much rarer complication of repair is rerupture, which can be asymptomatic or symptomatic.5 The most common failure site, discovered during surgery, is the fixation site.2,13 The true incidence of rerupture is unknown, as MRI typically is not obtained for asymptomatic patients. However, Marnitz and colleagues5 recently found increased intratendinous signal and thickness of repaired tendons in the majority of intact postoperative cases and no significant correlation between any MRI features, including tendon rerupture, and clinical measures. This was confirmed in our patient’s case, in which the MRI-based diagnosis of partial retear was not correlated with adverse clinical outcome at 1-year follow-up. Marnitz and colleagues5 hypothesized that the increased thickness of the repaired tendon would predispose the patient to impingement.
Our patient had no demonstrable loss of motion during surgery. However, postoperative dynamic MRI clearly showed insufficient room in the pronated radioulnar space for both heterotopic bone and repaired biceps tendon. It is possible that a space-occupying peritendinous hematoma or HO soon after surgery caused early loss of pronation. In a study of 10 volunteers, mean radioulnar distance was 4.0 mm (range, 2.1-6.0 mm) at its minimum in pronation.14 We used the same technique to measure our patient’s radioulnar space in active semipronation: 7 mm. This diameter was the same as that of the distal biceps tendon during surgery (Figure 3D). Had our patient been in maximum pronation during imaging, we would have expected a further decrease in radioulnar distance. Given the insufficient room in this case, it is possible that, during the attempt to regain full pronation, attritional wear of the repaired biceps tendon occurred with a corresponding maturation of the focus of heterotopic bone. Supporting this theory is the patient’s lack of history of traumatic loading, which would have suggested tensile failure of the repair. By 1-year follow-up, scar-tissue maturation and remodeling had occurred, and there was sufficient overall biomechanical strength to withstand return to normal activity.
The literature includes multiple reports of in vitro biomechanical studies of various types of distal biceps tendon fixation,15-17 and multiple authors have demonstrated the superior pullout strength of cortical fixation buttons,18,19 such as the Endobutton. It is important to note that all biomechanical tests are performed in cadaveric specimens and are therefore likely applicable only at time zero, after in vivo repair. In part stemming from the results of these cadaveric biomechanical tests, earlier and more aggressive rehabilitation protocols have been developed with the assumption that time zero is the weakest point.20 If in fact the native repaired biceps tendon is retorn and remodeled, there will exist a nadir in strength because of the high concentration of biomechanically inferior type III collagen in scar tissue (as opposed to the very strong type I collagen in native tendons).21 In the absence of complete rerupture, biomechanical strength would continue to improve during scar maturation and continued healing, leading to the typical excellent clinical result, as seen in our case.
This case report illustrates the dynamic MRI appearance of a small focus of HO after distal biceps tendon repair and adds to the time-zero cadaveric data of distal biceps tendon repair. The small focus of HO near the repaired distal tendon may have caused tendon impingement in pronation because of its space-occupying nature and possible attritional tendon wear. A gentler rehabilitation protocol for this pattern of HO, during a period in which biomechanically inferior scar tissue is maturing, may be warranted. Despite the high rates of clinical success with distal biceps tendon repair, there is lack of agreement between ex vivo cadaveric studies and the in vivo scenario. A prospective study involving a larger series of patients with postoperative dynamic MRI examinations would be useful to better understand the true in vivo course of distal biceps tendon repair.
Retearing after repair of the distal biceps tendon is rare.1 Heterotopic ossification (HO) is also considered uncommon, though reported rates in the literature vary widely, depending on repair and follow-up methods.1-3
In this article, we report a case of ruptured distal biceps tendon repaired with a 1-incision Endobutton technique with longitudinal clinical and imaging follow-up, and we discuss the potential biomechanical and rehabilitative implications of clinically occult retearing after repair.
This case is unique in that the patient was a physician who procured multiple magnetic resonance imaging (MRI) examinations during the postoperative period and again at 1-year follow-up. We witnessed formation of a small focus of HO, which entered and significantly narrowed the radioulnar space on forearm pronation on dynamic MRI. There was no obvious clinical evidence for retearing; high-grade partial-thickness tendon retearing was diagnosed on MRI. This prompted a gentler rehabilitation protocol. Subsequent scar formation and tendon remodeling allowed the patient to return to full activity by 1-year follow-up, confirming recent reports that intrasubstance signal abnormalities4 and even rerupture on MRI are not correlated with symptoms.5 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy right-hand–dominant 32-year-old man was rock climbing when he heard a pop and felt sudden weakness in his right elbow. The injury occurred during eccentric contraction, while he was climbing a 45° overhanging wall with his right elbow fully extended and forearm maximally pronated. Immediately after injury, he noticed obvious deformity in the right arm. Before this incident, there was no history of elbow symptoms or any medication use.
Physical examination revealed distortion of the biceps with a palpable defect in the right elbow consistent with a complete biceps tendon rupture. This was confirmed on MRI, which showed avulsion of the distal biceps tendon from its insertion on the radius. There was 4 cm of proximal retraction of the tendon, which was kept at the level of the joint line by a partially intact lacertus fibrosis (Figure 1).
As the patient was physically active, operative treatment was chosen with the expectation of restoration to full function and strength. Six days after injury, surgery was performed using a 1-incision anterior approach with an Endobutton technique, as first described by Bain and colleagues6 and subsequently detailed by other authors.7 The diameter of the distal biceps tendon after attachment to the Endobutton (Arthrex, Naples, Florida) was measured, and a corresponding 7-mm unicortical tunnel was drilled into the radial tuberosity. During surgery, there was full range of motion (ROM) at the elbow and forearm. Before closure, the wound was copiously irrigated to minimize the potential of HO. In our practice, we do not routinely administer prophylactic anti-inflammatory drugs to low-risk patients because of the theoretical risks for delayed tendon–bone healing8 and inferior healing strength.9 The theoretical, expected postoperative appearance is illustrated in Figure 2.
For 7 days after surgery, the patient wore a posterior elbow splint in a flexed, supinated position. Afterward, rehabilitation initially consisted of passive ROM progressing to active ROM at postoperative week 4. Pronation was slow to return, but essentially full ROM was regained by 7 weeks after surgery. Seven weeks after surgery, a radiograph showed a small amount of HO near the radial tuberosity (Figure 3A). However, the patient was clinically progressing well, and by 9 weeks was comfortably performing slow, controlled arm curls with a 10-lb weight. Despite the clinical improvements, MRI 9 weeks after surgery showed high-grade partial-thickness retearing of the distal biceps tendon without significant retraction. With dynamic MRI, it was evident that the focus of HO near but external to the distal tendon entered the radioulnar space on pronation (Figures 3B–3D). On axial images of the center of the cortical tunnel, the short-axis diameter of the heterotopic bone measured 2.5 mm, and the bone clearly was occupying part of the radioulnar space during pronation. As the patient was not having pain and was increasing in strength, the clinical team resumed rehabilitation, albeit at a gentler pace.
By 1-year follow-up, the patient had returned to preinjury activity levels, which included rock climbing and weightlifting without pain or loss of strength. One year after surgery, radiographs and MRI showed maturation of heterotopic bone, which was incorporated with scar tissue along the remodeled distal biceps tendon remnant (Figures 4A-4C).
Discussion
Distal biceps tendon ruptures historically have been considered relatively rare injuries. Postrepair complications are uncommon but well known. HO has been described with all distal biceps tendon repair techniques, but rates vary depending on follow-up method. Given the data reported, HO is thought to have a higher incidence with the 2-incision technique than with the 1-incision technique.10 The literature includes fewer reports of HO with the Endobutton technique11,12 than with the suture anchor technique.3 Incidence of HO after distal biceps tendon repair has been reported to be as high as 50%, with Marnitz and colleagues5 suggesting that its presence does not necessarily affect clinical outcome. This was confirmed in our patient’s case.
A much rarer complication of repair is rerupture, which can be asymptomatic or symptomatic.5 The most common failure site, discovered during surgery, is the fixation site.2,13 The true incidence of rerupture is unknown, as MRI typically is not obtained for asymptomatic patients. However, Marnitz and colleagues5 recently found increased intratendinous signal and thickness of repaired tendons in the majority of intact postoperative cases and no significant correlation between any MRI features, including tendon rerupture, and clinical measures. This was confirmed in our patient’s case, in which the MRI-based diagnosis of partial retear was not correlated with adverse clinical outcome at 1-year follow-up. Marnitz and colleagues5 hypothesized that the increased thickness of the repaired tendon would predispose the patient to impingement.
Our patient had no demonstrable loss of motion during surgery. However, postoperative dynamic MRI clearly showed insufficient room in the pronated radioulnar space for both heterotopic bone and repaired biceps tendon. It is possible that a space-occupying peritendinous hematoma or HO soon after surgery caused early loss of pronation. In a study of 10 volunteers, mean radioulnar distance was 4.0 mm (range, 2.1-6.0 mm) at its minimum in pronation.14 We used the same technique to measure our patient’s radioulnar space in active semipronation: 7 mm. This diameter was the same as that of the distal biceps tendon during surgery (Figure 3D). Had our patient been in maximum pronation during imaging, we would have expected a further decrease in radioulnar distance. Given the insufficient room in this case, it is possible that, during the attempt to regain full pronation, attritional wear of the repaired biceps tendon occurred with a corresponding maturation of the focus of heterotopic bone. Supporting this theory is the patient’s lack of history of traumatic loading, which would have suggested tensile failure of the repair. By 1-year follow-up, scar-tissue maturation and remodeling had occurred, and there was sufficient overall biomechanical strength to withstand return to normal activity.
The literature includes multiple reports of in vitro biomechanical studies of various types of distal biceps tendon fixation,15-17 and multiple authors have demonstrated the superior pullout strength of cortical fixation buttons,18,19 such as the Endobutton. It is important to note that all biomechanical tests are performed in cadaveric specimens and are therefore likely applicable only at time zero, after in vivo repair. In part stemming from the results of these cadaveric biomechanical tests, earlier and more aggressive rehabilitation protocols have been developed with the assumption that time zero is the weakest point.20 If in fact the native repaired biceps tendon is retorn and remodeled, there will exist a nadir in strength because of the high concentration of biomechanically inferior type III collagen in scar tissue (as opposed to the very strong type I collagen in native tendons).21 In the absence of complete rerupture, biomechanical strength would continue to improve during scar maturation and continued healing, leading to the typical excellent clinical result, as seen in our case.
This case report illustrates the dynamic MRI appearance of a small focus of HO after distal biceps tendon repair and adds to the time-zero cadaveric data of distal biceps tendon repair. The small focus of HO near the repaired distal tendon may have caused tendon impingement in pronation because of its space-occupying nature and possible attritional tendon wear. A gentler rehabilitation protocol for this pattern of HO, during a period in which biomechanically inferior scar tissue is maturing, may be warranted. Despite the high rates of clinical success with distal biceps tendon repair, there is lack of agreement between ex vivo cadaveric studies and the in vivo scenario. A prospective study involving a larger series of patients with postoperative dynamic MRI examinations would be useful to better understand the true in vivo course of distal biceps tendon repair.
1. Cohen MS. Complications of distal biceps tendon repairs. Sports Med Arthrosc. 2008;16(3):148-153.
2. Bisson L, Moyer M, Lanighan K, Marzo J. Complications associated with repair of a distal biceps rupture using the modified two-incision technique. J Shoulder Elbow Surg. 2008;17(1 suppl):67S-71S.
3. Gallinet D, Dietsch E, Barbier-Brion B, Lerais JM, Obert L. Suture anchor reinsertion of distal biceps rupture: clinical results and radiological assessment of tendon healing. Orthop Traumatol Surg Res. 2011;97(3):252-259.
4. Schmidt CC, Diaz VA, Weir DM, Latona CR, Miller MC. Repaired distal biceps magnetic resonance imaging anatomy compared with outcome. J Shoulder Elbow Surg. 2012;21(12):1623-1631.
5. Marnitz T, Spiegel D, Hug K, et al. MR imaging findings in flexed abducted supinated (FABS) position and clinical presentation following refixation of distal biceps tendon rupture using bioabsorbable suture anchors. Rofo. 2012;184(5):432-436.
6. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
7. King J, Bollier M. Repair of distal biceps tendon ruptures using the Endobutton. J Am Acad Orthop Surg. 2008;16(8):490-494.
8. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
9. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35(8):1326-1333.
10. Miyamoto RG, Elser F, Millett PJ. Distal biceps tendon injuries. J Bone Joint Surg Am. 2010;92(11):2128-2138.
11. Dillon MT, Lepore DJ. Heterotopic ossification after single-incision distal biceps tendon repair with an Endobutton. J Surg Orthop Adv. 2011;20(3):198-201.
12. Peeters T, Ching-Soon NG, Jansen N, Sneyers C, Declercq G, Verstreken F. Functional outcome after repair of distal biceps tendon ruptures using the Endobutton technique. J Shoulder Elbow Surg. 2009;18(2):283-287.
13. Katolik LI, Fernandez J, Cohen MS. Acute failure of distal biceps reconstruction: a case report. J Shoulder Elbow Surg. 2007;16(5):e10-e12.
14. Seiler JG 3rd, Parker LM, Chamberland PD, Sherbourne GM, Carpenter WA. The distal biceps tendon. Two potential mechanisms involved in its rupture: arterial supply and mechanical impingement. J Shoulder Elbow Surg. 1995;4(3):149-156.
15. Siebenlist S, Lenich A, Buchholz A, et al. Biomechanical in vitro validation of intramedullary cortical button fixation for distal biceps tendon repair: a new technique. Am J Sports Med. 2011;39(8):1762-1768.
16. Pereira DS, Kvitne RS, Liang M, Giacobetti FB, Ebramzadeh E. Surgical repair of distal biceps tendon ruptures: a biomechanical comparison of two techniques. Am J Sports Med. 2002;30(3):432-436.
17. Lemos SE, Ebramzedeh E, Kvitne RS. A new technique: in vitro suture anchor fixation has superior yield strength to bone tunnel fixation for distal biceps tendon repair. Am J Sports Med. 2004;32(2):406-410.
18. Kettler M, Lunger J, Kuhn V, Mutschler W, Tingart MJ. Failure strengths in distal biceps tendon repair. Am J Sports Med. 2007;35(9):1544-1548.
19. Mazzocca AD, Burton KJ, Romeo AA, Santangelo S, Adams DA, Arciero RA. Biomechanical evaluation of 4 techniques of distal biceps brachii tendon repair. Am J Sports Med. 2007;35(2):252-258.
20. Spencer EE Jr, Tisdale A, Kostka K, Ivy RE. Is therapy necessary after distal biceps tendon repair? Hand (N Y). 2008;3(4):316-319.
21. Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. Am J Sports Med. 2000;28(4):499-505.
1. Cohen MS. Complications of distal biceps tendon repairs. Sports Med Arthrosc. 2008;16(3):148-153.
2. Bisson L, Moyer M, Lanighan K, Marzo J. Complications associated with repair of a distal biceps rupture using the modified two-incision technique. J Shoulder Elbow Surg. 2008;17(1 suppl):67S-71S.
3. Gallinet D, Dietsch E, Barbier-Brion B, Lerais JM, Obert L. Suture anchor reinsertion of distal biceps rupture: clinical results and radiological assessment of tendon healing. Orthop Traumatol Surg Res. 2011;97(3):252-259.
4. Schmidt CC, Diaz VA, Weir DM, Latona CR, Miller MC. Repaired distal biceps magnetic resonance imaging anatomy compared with outcome. J Shoulder Elbow Surg. 2012;21(12):1623-1631.
5. Marnitz T, Spiegel D, Hug K, et al. MR imaging findings in flexed abducted supinated (FABS) position and clinical presentation following refixation of distal biceps tendon rupture using bioabsorbable suture anchors. Rofo. 2012;184(5):432-436.
6. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
7. King J, Bollier M. Repair of distal biceps tendon ruptures using the Endobutton. J Am Acad Orthop Surg. 2008;16(8):490-494.
8. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
9. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35(8):1326-1333.
10. Miyamoto RG, Elser F, Millett PJ. Distal biceps tendon injuries. J Bone Joint Surg Am. 2010;92(11):2128-2138.
11. Dillon MT, Lepore DJ. Heterotopic ossification after single-incision distal biceps tendon repair with an Endobutton. J Surg Orthop Adv. 2011;20(3):198-201.
12. Peeters T, Ching-Soon NG, Jansen N, Sneyers C, Declercq G, Verstreken F. Functional outcome after repair of distal biceps tendon ruptures using the Endobutton technique. J Shoulder Elbow Surg. 2009;18(2):283-287.
13. Katolik LI, Fernandez J, Cohen MS. Acute failure of distal biceps reconstruction: a case report. J Shoulder Elbow Surg. 2007;16(5):e10-e12.
14. Seiler JG 3rd, Parker LM, Chamberland PD, Sherbourne GM, Carpenter WA. The distal biceps tendon. Two potential mechanisms involved in its rupture: arterial supply and mechanical impingement. J Shoulder Elbow Surg. 1995;4(3):149-156.
15. Siebenlist S, Lenich A, Buchholz A, et al. Biomechanical in vitro validation of intramedullary cortical button fixation for distal biceps tendon repair: a new technique. Am J Sports Med. 2011;39(8):1762-1768.
16. Pereira DS, Kvitne RS, Liang M, Giacobetti FB, Ebramzadeh E. Surgical repair of distal biceps tendon ruptures: a biomechanical comparison of two techniques. Am J Sports Med. 2002;30(3):432-436.
17. Lemos SE, Ebramzedeh E, Kvitne RS. A new technique: in vitro suture anchor fixation has superior yield strength to bone tunnel fixation for distal biceps tendon repair. Am J Sports Med. 2004;32(2):406-410.
18. Kettler M, Lunger J, Kuhn V, Mutschler W, Tingart MJ. Failure strengths in distal biceps tendon repair. Am J Sports Med. 2007;35(9):1544-1548.
19. Mazzocca AD, Burton KJ, Romeo AA, Santangelo S, Adams DA, Arciero RA. Biomechanical evaluation of 4 techniques of distal biceps brachii tendon repair. Am J Sports Med. 2007;35(2):252-258.
20. Spencer EE Jr, Tisdale A, Kostka K, Ivy RE. Is therapy necessary after distal biceps tendon repair? Hand (N Y). 2008;3(4):316-319.
21. Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. Am J Sports Med. 2000;28(4):499-505.
Lower Extremity Injuries in Snowboarders
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
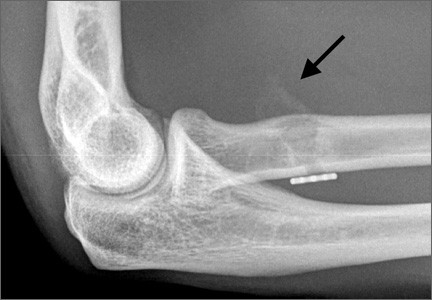
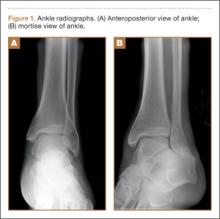
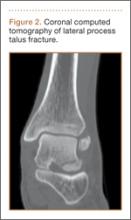
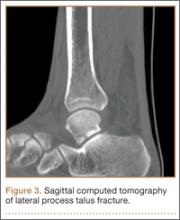
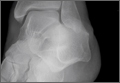
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
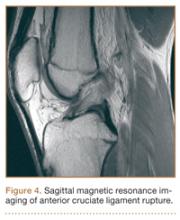
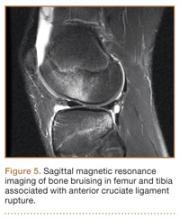
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.
Visualization and Reduction of a Meniscal Capsular Junction Tear in the Knee: An Arthroscopic Surgical Technique
The annual incidence of anterior cruciate ligament (ACL) injury in the general US population is estimated at 1 in 3000, or approximately 100,000 ACL injuries per year.1 The incidence of meniscal injuries after ACL tears ranges from 34% to 92%,2 with peripheral posterior horn tears of the medial meniscus accounting for 40% of the meniscal pathology.3
Although several meniscal tear patterns and their treatments have been described in the literature, posterior medial meniscal capsular junction (MCJ) tears have not been adequately addressed. Thijn4 found the accuracy of routine anterior portal arthroscopy in identifying medial meniscus tears was only 81%. Gillies and Seligson5 found a 25% arthroscopic false-negative rate caused by failure to detect peripheral tears in the posterior horn of the medial meniscus.
We reviewed 781 (517 male, 264 female) patients who underwent ACL reconstruction at our clinic and found a 12.3% incidence of MCJ tear with primary ACL injury and a 23.6% incidence of MCJ tear with revision ACL reconstruction. We believe this is a specific injury pattern. If not looked for during arthroscopy, it can be missed. Whether this tear pattern behaves differently from a posterior medial meniscus tear is yet to be determined.
To address such tear patterns, with or without ACL reconstruction, we use an arthroscopic repair technique that shows direct visualization of the tear and its reduction.
Materials and Methods
The standard anterior medial and lateral arthroscopic portals are established. A 30° scope is placed in the anterior lateral portal, and an arthroscopic shaver is used to débride the ACL remnants, including the footprint and the femoral insertion site. The camera is then adjusted to look straight down. Next, it is placed between the posterior cruciate ligament (PCL) and the medial femoral condyle and advanced toward the posterior capsule. It is then adjusted to view medially (Figure 1). If there is a tear (Figures 2A, 2B), a posterior medial portal (described by Gillquist and colleagues6) is established using an 18-gauge spinal needle for localization followed by a small stab incision through the skin. The spinal needle is left in position to obtain the correct angle for the suture passer (Figure 3). A 70° Hewson suture passer (Smith & Nephew, Memphis, Tennessee) is passed through the posterior medial portal.
Once inside the joint, the suture passer is passed through the capsule and then through the posterior horn of the meniscus (Figure 4). A loop grasper is used to grab the suture on the end of the passer and then is brought out the posterior medial portal and loaded with a No. 2 MaxBraid suture (Biomet, Warsaw, Indiana) (Figure 5). In some cases, the suture passer’s wire goes out the notch toward the anterior aspect of the knee. If this occurs, the loop grasper can be used to grab this wire from the anterior medial portal and load with the MaxBraid suture.
Standard arthroscopic knot-tying techniques are used under direct visualization showing the reduction of the capsule to the meniscus (Figure 6). This is done from the posterior medial portal. The excess suture is cut with an arthroscopic suture cutter in the standard fashion. In the rare case of an intact ACL with this same tear pattern, the same technique can be used. If there is difficulty moving past the intact ACL and PCL, a posterior lateral portal can be used as another accessory portal. The arthroscope can then be placed in the posterior lateral portal, while the posterior medial portal can be used as the working portal. Care must be taken in either technique to avoid soft-tissue bridges.
Discussion
Previous biomechanical studies have shown the meniscus to be important to knee stability. In an ACL-deficient knee, the posterior medial meniscus is important as a secondary stabilizer, and for that reason it is crucial to identify and repair tears there to avoid risking extra force on the ACL graft.7,8 We think an MCJ tear can potentially compromise knee stability as well, so there is a need to examine the posterior aspect of the knee during every knee arthroscopy. However, biomechanical studies must be performed to validate this theory.
To assess whether orthopedists in general are aware of and concerned about MCJ tears, a survey was e-mailed to members of the Arthroscopy Association of North America (AANA) and the American Sports Medicine Fellowship Society (ASMFS). Sixty-seven orthopedic surgeons who perform ACL reconstruction surgeries responded to some or all of the following questions. Nearly half (48%) of the surgeons said they always assess the posteromedial MCJ by placing the camera between the PCL and the medial femoral condyle. Only 25% said MCJ tears should be repaired always, but another 64% said these tears should be repaired sometimes. Thus, 89% responded that at least some MCJ tears should be repaired. Most (88%) said these tears could sometimes or always be a source of chronic pain. Also, 92% said these tears could sometimes or always change the contact pressures in the knee, and 66% said these tears could sometimes or always change the rotational stability of the knee. Finally, 60% said MCJ tears could sometimes or always affect ACL graft failure. These data show a need to determine an appropriate surgical technique that will help treat MCJ tears.
There is a vast amount of literature about the meniscus, but there are few current studies on the specific entity of MCJ tears. We think these tears act similarly to posterior meniscus tears and should be addressed similarly. MCJ tears are easily missed on anterior arthroscopy. In every knee arthroscopy, the posterior aspect of the knee should be checked for these injuries, particularly in ACL-deficient knees. A lesion found within the capsule can be repaired with the technique we have described.
1. Fu FH, Cohen SB. Current Concepts in ACL Reconstruction. Thorofare, NJ: Slack; 2008.
2. Simonian PT, Cole BJ, Bach BR. Sports Injuries of the Knee: Surgical Approaches. New York, NY: Thieme; 2006.
3. Smith JP 3rd, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415-419.
4. Thijn CJ. Accuracy of double-contrast arthrography and arthroscopy of the knee joint. Skeletal Radiol. 1982;8(3):187-192.
5. Gillies H, Seligson D. Precision in the diagnosis of meniscal lesions: a comparison of clinical evaluation, arthrography, and arthroscopy. J Bone Joint Surg Am. 1979;61(3):343-346.
6. Gillquist J, Hagberg G, Oretorp N. Arthroscopic examination of the posteromedial compartment of the knee joint. Int Orthop. 1979;3(1):13-18.
7. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883-888.
8. Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament–deficient knee. J Orthop Res. 2000;18(1):109-115.
The annual incidence of anterior cruciate ligament (ACL) injury in the general US population is estimated at 1 in 3000, or approximately 100,000 ACL injuries per year.1 The incidence of meniscal injuries after ACL tears ranges from 34% to 92%,2 with peripheral posterior horn tears of the medial meniscus accounting for 40% of the meniscal pathology.3
Although several meniscal tear patterns and their treatments have been described in the literature, posterior medial meniscal capsular junction (MCJ) tears have not been adequately addressed. Thijn4 found the accuracy of routine anterior portal arthroscopy in identifying medial meniscus tears was only 81%. Gillies and Seligson5 found a 25% arthroscopic false-negative rate caused by failure to detect peripheral tears in the posterior horn of the medial meniscus.
We reviewed 781 (517 male, 264 female) patients who underwent ACL reconstruction at our clinic and found a 12.3% incidence of MCJ tear with primary ACL injury and a 23.6% incidence of MCJ tear with revision ACL reconstruction. We believe this is a specific injury pattern. If not looked for during arthroscopy, it can be missed. Whether this tear pattern behaves differently from a posterior medial meniscus tear is yet to be determined.
To address such tear patterns, with or without ACL reconstruction, we use an arthroscopic repair technique that shows direct visualization of the tear and its reduction.
Materials and Methods
The standard anterior medial and lateral arthroscopic portals are established. A 30° scope is placed in the anterior lateral portal, and an arthroscopic shaver is used to débride the ACL remnants, including the footprint and the femoral insertion site. The camera is then adjusted to look straight down. Next, it is placed between the posterior cruciate ligament (PCL) and the medial femoral condyle and advanced toward the posterior capsule. It is then adjusted to view medially (Figure 1). If there is a tear (Figures 2A, 2B), a posterior medial portal (described by Gillquist and colleagues6) is established using an 18-gauge spinal needle for localization followed by a small stab incision through the skin. The spinal needle is left in position to obtain the correct angle for the suture passer (Figure 3). A 70° Hewson suture passer (Smith & Nephew, Memphis, Tennessee) is passed through the posterior medial portal.
Once inside the joint, the suture passer is passed through the capsule and then through the posterior horn of the meniscus (Figure 4). A loop grasper is used to grab the suture on the end of the passer and then is brought out the posterior medial portal and loaded with a No. 2 MaxBraid suture (Biomet, Warsaw, Indiana) (Figure 5). In some cases, the suture passer’s wire goes out the notch toward the anterior aspect of the knee. If this occurs, the loop grasper can be used to grab this wire from the anterior medial portal and load with the MaxBraid suture.
Standard arthroscopic knot-tying techniques are used under direct visualization showing the reduction of the capsule to the meniscus (Figure 6). This is done from the posterior medial portal. The excess suture is cut with an arthroscopic suture cutter in the standard fashion. In the rare case of an intact ACL with this same tear pattern, the same technique can be used. If there is difficulty moving past the intact ACL and PCL, a posterior lateral portal can be used as another accessory portal. The arthroscope can then be placed in the posterior lateral portal, while the posterior medial portal can be used as the working portal. Care must be taken in either technique to avoid soft-tissue bridges.
Discussion
Previous biomechanical studies have shown the meniscus to be important to knee stability. In an ACL-deficient knee, the posterior medial meniscus is important as a secondary stabilizer, and for that reason it is crucial to identify and repair tears there to avoid risking extra force on the ACL graft.7,8 We think an MCJ tear can potentially compromise knee stability as well, so there is a need to examine the posterior aspect of the knee during every knee arthroscopy. However, biomechanical studies must be performed to validate this theory.
To assess whether orthopedists in general are aware of and concerned about MCJ tears, a survey was e-mailed to members of the Arthroscopy Association of North America (AANA) and the American Sports Medicine Fellowship Society (ASMFS). Sixty-seven orthopedic surgeons who perform ACL reconstruction surgeries responded to some or all of the following questions. Nearly half (48%) of the surgeons said they always assess the posteromedial MCJ by placing the camera between the PCL and the medial femoral condyle. Only 25% said MCJ tears should be repaired always, but another 64% said these tears should be repaired sometimes. Thus, 89% responded that at least some MCJ tears should be repaired. Most (88%) said these tears could sometimes or always be a source of chronic pain. Also, 92% said these tears could sometimes or always change the contact pressures in the knee, and 66% said these tears could sometimes or always change the rotational stability of the knee. Finally, 60% said MCJ tears could sometimes or always affect ACL graft failure. These data show a need to determine an appropriate surgical technique that will help treat MCJ tears.
There is a vast amount of literature about the meniscus, but there are few current studies on the specific entity of MCJ tears. We think these tears act similarly to posterior meniscus tears and should be addressed similarly. MCJ tears are easily missed on anterior arthroscopy. In every knee arthroscopy, the posterior aspect of the knee should be checked for these injuries, particularly in ACL-deficient knees. A lesion found within the capsule can be repaired with the technique we have described.
The annual incidence of anterior cruciate ligament (ACL) injury in the general US population is estimated at 1 in 3000, or approximately 100,000 ACL injuries per year.1 The incidence of meniscal injuries after ACL tears ranges from 34% to 92%,2 with peripheral posterior horn tears of the medial meniscus accounting for 40% of the meniscal pathology.3
Although several meniscal tear patterns and their treatments have been described in the literature, posterior medial meniscal capsular junction (MCJ) tears have not been adequately addressed. Thijn4 found the accuracy of routine anterior portal arthroscopy in identifying medial meniscus tears was only 81%. Gillies and Seligson5 found a 25% arthroscopic false-negative rate caused by failure to detect peripheral tears in the posterior horn of the medial meniscus.
We reviewed 781 (517 male, 264 female) patients who underwent ACL reconstruction at our clinic and found a 12.3% incidence of MCJ tear with primary ACL injury and a 23.6% incidence of MCJ tear with revision ACL reconstruction. We believe this is a specific injury pattern. If not looked for during arthroscopy, it can be missed. Whether this tear pattern behaves differently from a posterior medial meniscus tear is yet to be determined.
To address such tear patterns, with or without ACL reconstruction, we use an arthroscopic repair technique that shows direct visualization of the tear and its reduction.
Materials and Methods
The standard anterior medial and lateral arthroscopic portals are established. A 30° scope is placed in the anterior lateral portal, and an arthroscopic shaver is used to débride the ACL remnants, including the footprint and the femoral insertion site. The camera is then adjusted to look straight down. Next, it is placed between the posterior cruciate ligament (PCL) and the medial femoral condyle and advanced toward the posterior capsule. It is then adjusted to view medially (Figure 1). If there is a tear (Figures 2A, 2B), a posterior medial portal (described by Gillquist and colleagues6) is established using an 18-gauge spinal needle for localization followed by a small stab incision through the skin. The spinal needle is left in position to obtain the correct angle for the suture passer (Figure 3). A 70° Hewson suture passer (Smith & Nephew, Memphis, Tennessee) is passed through the posterior medial portal.
Once inside the joint, the suture passer is passed through the capsule and then through the posterior horn of the meniscus (Figure 4). A loop grasper is used to grab the suture on the end of the passer and then is brought out the posterior medial portal and loaded with a No. 2 MaxBraid suture (Biomet, Warsaw, Indiana) (Figure 5). In some cases, the suture passer’s wire goes out the notch toward the anterior aspect of the knee. If this occurs, the loop grasper can be used to grab this wire from the anterior medial portal and load with the MaxBraid suture.
Standard arthroscopic knot-tying techniques are used under direct visualization showing the reduction of the capsule to the meniscus (Figure 6). This is done from the posterior medial portal. The excess suture is cut with an arthroscopic suture cutter in the standard fashion. In the rare case of an intact ACL with this same tear pattern, the same technique can be used. If there is difficulty moving past the intact ACL and PCL, a posterior lateral portal can be used as another accessory portal. The arthroscope can then be placed in the posterior lateral portal, while the posterior medial portal can be used as the working portal. Care must be taken in either technique to avoid soft-tissue bridges.
Discussion
Previous biomechanical studies have shown the meniscus to be important to knee stability. In an ACL-deficient knee, the posterior medial meniscus is important as a secondary stabilizer, and for that reason it is crucial to identify and repair tears there to avoid risking extra force on the ACL graft.7,8 We think an MCJ tear can potentially compromise knee stability as well, so there is a need to examine the posterior aspect of the knee during every knee arthroscopy. However, biomechanical studies must be performed to validate this theory.
To assess whether orthopedists in general are aware of and concerned about MCJ tears, a survey was e-mailed to members of the Arthroscopy Association of North America (AANA) and the American Sports Medicine Fellowship Society (ASMFS). Sixty-seven orthopedic surgeons who perform ACL reconstruction surgeries responded to some or all of the following questions. Nearly half (48%) of the surgeons said they always assess the posteromedial MCJ by placing the camera between the PCL and the medial femoral condyle. Only 25% said MCJ tears should be repaired always, but another 64% said these tears should be repaired sometimes. Thus, 89% responded that at least some MCJ tears should be repaired. Most (88%) said these tears could sometimes or always be a source of chronic pain. Also, 92% said these tears could sometimes or always change the contact pressures in the knee, and 66% said these tears could sometimes or always change the rotational stability of the knee. Finally, 60% said MCJ tears could sometimes or always affect ACL graft failure. These data show a need to determine an appropriate surgical technique that will help treat MCJ tears.
There is a vast amount of literature about the meniscus, but there are few current studies on the specific entity of MCJ tears. We think these tears act similarly to posterior meniscus tears and should be addressed similarly. MCJ tears are easily missed on anterior arthroscopy. In every knee arthroscopy, the posterior aspect of the knee should be checked for these injuries, particularly in ACL-deficient knees. A lesion found within the capsule can be repaired with the technique we have described.
1. Fu FH, Cohen SB. Current Concepts in ACL Reconstruction. Thorofare, NJ: Slack; 2008.
2. Simonian PT, Cole BJ, Bach BR. Sports Injuries of the Knee: Surgical Approaches. New York, NY: Thieme; 2006.
3. Smith JP 3rd, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415-419.
4. Thijn CJ. Accuracy of double-contrast arthrography and arthroscopy of the knee joint. Skeletal Radiol. 1982;8(3):187-192.
5. Gillies H, Seligson D. Precision in the diagnosis of meniscal lesions: a comparison of clinical evaluation, arthrography, and arthroscopy. J Bone Joint Surg Am. 1979;61(3):343-346.
6. Gillquist J, Hagberg G, Oretorp N. Arthroscopic examination of the posteromedial compartment of the knee joint. Int Orthop. 1979;3(1):13-18.
7. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883-888.
8. Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament–deficient knee. J Orthop Res. 2000;18(1):109-115.
1. Fu FH, Cohen SB. Current Concepts in ACL Reconstruction. Thorofare, NJ: Slack; 2008.
2. Simonian PT, Cole BJ, Bach BR. Sports Injuries of the Knee: Surgical Approaches. New York, NY: Thieme; 2006.
3. Smith JP 3rd, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415-419.
4. Thijn CJ. Accuracy of double-contrast arthrography and arthroscopy of the knee joint. Skeletal Radiol. 1982;8(3):187-192.
5. Gillies H, Seligson D. Precision in the diagnosis of meniscal lesions: a comparison of clinical evaluation, arthrography, and arthroscopy. J Bone Joint Surg Am. 1979;61(3):343-346.
6. Gillquist J, Hagberg G, Oretorp N. Arthroscopic examination of the posteromedial compartment of the knee joint. Int Orthop. 1979;3(1):13-18.
7. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883-888.
8. Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament–deficient knee. J Orthop Res. 2000;18(1):109-115.
RA patients’ readmission rates after joint replacement are rising
Rheumatoid arthritis patients who have undergone a hip or knee replacement are more likely to be readmitted to a hospital than are patients with osteoarthritis, according to findings from a large prospective registry study.
The analysis revealed an increasing trend in the incidence of 90-day readmissions in the rheumatoid arthritis (RA) patients by year, at 5.8%, 8.9%, and 10.6% for 2009, 2010, and 2011, respectively, Dr. Jasvinder Singh of the Birmingham (Ala.) VA Medical Center and his colleagues reported (Arthritis Care Res. 2014 Oct. 9 [doi:10.1002/acr.22497]).
For osteoarthritis (OA) patients, the 90-day readmission rates were similar by year at 6.7%, 6.7%, and 6.8%, respectively. After accounting for differences, including the risk by year, the adjusted risk for 90-day readmission in RA patients was 0.89 (95% confidence interval, 0.46-1.71) in 2009, 1.34 (95% CI, 0.69-2.61) in 2010, and 1.74 (95% CI, 1.16-2.60) in 2011, compared with OA patients.
Readmission after an elective hip or knee replacement is a problem of significant public health proportions, the study authors noted. A 90-day readmission rate of 6.8% translates to more than 70,000 admissions annually in the United States, they said.
The investigators analyzed 34,311 joint replacement procedures during the 3-year period – 33,815 performed in OA patients and 496 in patients with RA.
Overall, 42 RA patients were readmitted over the 3-year period, and the two most common reasons for readmission were joint prosthesis infection (10.2%) and septicemia (10.2%). For the 2,277 OA patients who were readmitted, the most common reasons were joint prosthesis infection (5.7%) and other postoperative infections.
The finding of an increasing 90-day readmission over a 3-year period in RA patients was a particular concern. “We considered several patient, procedure, surgeon, and hospital variables as important covariates and adjusted for those that were significant (age, gender, American Society of Anesthesiologists category, and iron deficiency anemia) in our multivariable-adjusted model, indicating that the increasing readmission rate in RA patients is not explained by these variables,” they wrote.
The effects of medications and pre- and postoperative rehabilitation programs could have played a role in readmission rates in RA patients, but the authors did not have the information to analyze the impact of these factors.
No conflicts of interest were declared.
Rheumatoid arthritis patients who have undergone a hip or knee replacement are more likely to be readmitted to a hospital than are patients with osteoarthritis, according to findings from a large prospective registry study.
The analysis revealed an increasing trend in the incidence of 90-day readmissions in the rheumatoid arthritis (RA) patients by year, at 5.8%, 8.9%, and 10.6% for 2009, 2010, and 2011, respectively, Dr. Jasvinder Singh of the Birmingham (Ala.) VA Medical Center and his colleagues reported (Arthritis Care Res. 2014 Oct. 9 [doi:10.1002/acr.22497]).
For osteoarthritis (OA) patients, the 90-day readmission rates were similar by year at 6.7%, 6.7%, and 6.8%, respectively. After accounting for differences, including the risk by year, the adjusted risk for 90-day readmission in RA patients was 0.89 (95% confidence interval, 0.46-1.71) in 2009, 1.34 (95% CI, 0.69-2.61) in 2010, and 1.74 (95% CI, 1.16-2.60) in 2011, compared with OA patients.
Readmission after an elective hip or knee replacement is a problem of significant public health proportions, the study authors noted. A 90-day readmission rate of 6.8% translates to more than 70,000 admissions annually in the United States, they said.
The investigators analyzed 34,311 joint replacement procedures during the 3-year period – 33,815 performed in OA patients and 496 in patients with RA.
Overall, 42 RA patients were readmitted over the 3-year period, and the two most common reasons for readmission were joint prosthesis infection (10.2%) and septicemia (10.2%). For the 2,277 OA patients who were readmitted, the most common reasons were joint prosthesis infection (5.7%) and other postoperative infections.
The finding of an increasing 90-day readmission over a 3-year period in RA patients was a particular concern. “We considered several patient, procedure, surgeon, and hospital variables as important covariates and adjusted for those that were significant (age, gender, American Society of Anesthesiologists category, and iron deficiency anemia) in our multivariable-adjusted model, indicating that the increasing readmission rate in RA patients is not explained by these variables,” they wrote.
The effects of medications and pre- and postoperative rehabilitation programs could have played a role in readmission rates in RA patients, but the authors did not have the information to analyze the impact of these factors.
No conflicts of interest were declared.
Rheumatoid arthritis patients who have undergone a hip or knee replacement are more likely to be readmitted to a hospital than are patients with osteoarthritis, according to findings from a large prospective registry study.
The analysis revealed an increasing trend in the incidence of 90-day readmissions in the rheumatoid arthritis (RA) patients by year, at 5.8%, 8.9%, and 10.6% for 2009, 2010, and 2011, respectively, Dr. Jasvinder Singh of the Birmingham (Ala.) VA Medical Center and his colleagues reported (Arthritis Care Res. 2014 Oct. 9 [doi:10.1002/acr.22497]).
For osteoarthritis (OA) patients, the 90-day readmission rates were similar by year at 6.7%, 6.7%, and 6.8%, respectively. After accounting for differences, including the risk by year, the adjusted risk for 90-day readmission in RA patients was 0.89 (95% confidence interval, 0.46-1.71) in 2009, 1.34 (95% CI, 0.69-2.61) in 2010, and 1.74 (95% CI, 1.16-2.60) in 2011, compared with OA patients.
Readmission after an elective hip or knee replacement is a problem of significant public health proportions, the study authors noted. A 90-day readmission rate of 6.8% translates to more than 70,000 admissions annually in the United States, they said.
The investigators analyzed 34,311 joint replacement procedures during the 3-year period – 33,815 performed in OA patients and 496 in patients with RA.
Overall, 42 RA patients were readmitted over the 3-year period, and the two most common reasons for readmission were joint prosthesis infection (10.2%) and septicemia (10.2%). For the 2,277 OA patients who were readmitted, the most common reasons were joint prosthesis infection (5.7%) and other postoperative infections.
The finding of an increasing 90-day readmission over a 3-year period in RA patients was a particular concern. “We considered several patient, procedure, surgeon, and hospital variables as important covariates and adjusted for those that were significant (age, gender, American Society of Anesthesiologists category, and iron deficiency anemia) in our multivariable-adjusted model, indicating that the increasing readmission rate in RA patients is not explained by these variables,” they wrote.
The effects of medications and pre- and postoperative rehabilitation programs could have played a role in readmission rates in RA patients, but the authors did not have the information to analyze the impact of these factors.
No conflicts of interest were declared.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: The 90-day risk of readmission after hip or knee replacement surgery is higher in patients with RA, compared with patients with OA.
Major finding: Readmissions for RA patients climbed significantly each year studied, compared with OA patients.
Data source: A prospective analysis of data from a total joint replacement registry of adults with OA and RA.
Disclosures: No conflicts of interest were declared.
Imaging Use in Focal Rhabdomyolysis of the Left Shoulder
Rhabdomyolysis involves the breakdown of skeletal muscle with the release of intracellular contents into the extracellular space and circulation.1 Diffuse rhabdomyolysis has been found in athletes due to overexertion. However, focal rhabdomyolysis is rare.2,3 The clinical presentation of focal rhabdomyolysis is subtle and nonspecific, with swelling, vague pain, weakness, fatigue, and tea-colored urine.
Early recognition and prompt management are crucial to prevent complications such as compression syndrome, acute renal failure, disseminated intravascular coagulation, cardiac dysrhythmia, or even cardiac arrest. Sonography and magnetic resonance imaging (MRI) can, therefore, be a complementary part of the diagnosis and assessment of the extent of rhabdomyolysis.4-7
Case History
The patient was a 34-year-old white man with a history of polysubstance abuse who presented to the emergency department (ED) with numbness and weakness in the left arm and hand, pain in the left side of his neck, and 3 days of intermittent amnesia with confusion. He had used IV heroin about 2 weeks prior to admission and used tobacco and alcohol daily. He reported no current medications or known allergies. The patient was in a monogamous relationship with a same-sex partner.
On physical examination, vital signs were within normal limits. He was in distress, confused, and disoriented as to time and place. An extremity examination revealed 1/5 strength in the extensors of the left elbow, left wrist, and left fingers with normal strength noted in the right upper extremity as well as the lower extremities. No sensory deficits were noted. The patient’s skin was warm and dry. Remarkable laboratory findings included creatine kinase (CK) 1,744 U/L, creatinine (Cr) 1.9 mg/dL, ALT 1,065 U/L, AST 319 U/L, ALP 159 U/L. A urine toxicology screen was positive for cocaine and opiates, and the urine analysis dip was negative for red blood cells, white blood cells, and protein. A differential diagnosis favored a left arm inflammatory reaction to IV drugs, although rhabdomyolysis was questioned.
A neurology consult was obtained, and a bedside electroencephalography test was performed in the ED by the neurologist, showing mild left occipital slow wave abnormality with no epileptiform discharges. A chest X-ray and computed tomography (CT) scan of the head and cervical spine were unremarkable, other than incidental mild prominence of the ventricles.
Over the next 24 hours, the patient was hydrated with IV normal saline without bicarbonate. His altered mental status, urine output, and biochemical abnormalities returned to normal, except for the serum CK, which decreased to 917 U/L. He had minimal improvement in his left upper extremity nerve palsy symptoms; however, he was deemed to be stable for discharge with follow-up in the clinic.
Instead of a clinic follow-up, the patient returned to the ED 7 days later, with progressive weakness of the left arm, forearm, and wrist. The patient noted that his weakness was so significant that he had to move his left arm with his right arm. He also reported extremity swelling and increasing paresthesias involving the lateral aspect of his left arm and hand, dizziness, and left neck pain. A physical examination revealed 3/5 strength at the left deltoid and left triceps, and 0/5 strength in the left fingers and grip. Remeasurement of CK was 54 U/L and Cr was 0.9 mg/dL. Compartment pressures were not measured.
Magnetic resonance imaging using multiplanar spin echo T1 and fast spin T2 weighted and post-IV 16cc Omniscan contrast sequences of the left shoulder were performed, showing multiple patchy T2 hyperintense focal areas with peripheral enhancement in the muscles of the posterior shoulder and in the tissues adjacent to the brachial plexus in the neck and shoulder (Figures 1A, 1B, and 1C). Sonography with matrix array linear 6-15 MH3 transducer was performed, which demonstrated patchy focal hypoechoic areas of muscle with enlarged, thickened, and disrupted muscle, representing devitalized muscle without any drainable fluid collection or abscess (Figures 2A, 2B, 2C, and 2D).
Magnetic resonance imaging and magnetic resonance angiogram scans of the brain and cervical spine with and without contrast were unremarkable. At that time, a definitive diagnosis was made of focal rhabdomyolysis and compressive neuropathy of the brachial plexus posterior cord, leading to brachial plexopathy of the left shoulder.
The patient was treated with hydration, a left arm sling, elevated left arm, and ibuprofen 600 mg qid to reduce inflammation. His swelling decreased markedly, and there was a slight improvement in pain and mobility at a 2-week neurology clinic follow-up. The patient lost contact after that.
Discussion
Rhabdomyolysis is caused by diverse etiologies. Most commonly, it is generalized and occurs due to overexertion, crush injury, steroid use, metabolic abnormalities, and certain medications and illicit drugs.1,2 The most likely etiology of rhabdomyolysis in patients presenting to the ED without significant trauma is of substance abuse, especially with ethanol, heroin, amphetamines, cocaine, and other sedatives or stimulants.1-3 The patient presented in this case study had a history of drug abuse, with a positive urine toxicology screen for cocaine and opiates. He had been intermittently confused and amnesic for 3 days prior to presentation, during which he may have been lying on his shoulder for a prolonged period.
Focal rhabdomyolysis and acute compression at the posterior shoulder leading to compressive brachial plexopathy is rare, with only 3 cases reported in the literature, all occurring with IV drug use.1-3 This patient had compression of the brachial plexus posterior cord from rhabdomyolysis and prolonged immobilization. Intravenous drug abusers may delay medical care due to perceived illicit drug effects and frequently present to the ED confused, agitated, or obtunded. Acute extremity swelling, a palpable lump, and pain can be due to various etiologies, such as trauma, fluid collection, muscle tear, myopathy, venous thrombosis, neoplasm, or rhabdomyolysis.
Diagnosis of nontraumatic rhabdomyolysis depends on clinical history and biochemical tests, such as serum CK and urine myoglobin.1,8 Creatine kinase is present in large quantities in the myocytes and is 100% sensitive as a marker for rhabdomyolysis.1,8 Creatine kinase may increase acutely > 1,000 U/L, suggesting muscle lysis and necrosis as etiology for pain as opposed to other causes such as hematomas, abscesses, or venous thrombi.1,9 Serum CK decreases rapidly at a rate of 39% per day, and it may normalize by the time a patient presents for medical care.1,10-12 Imaging plays a significant complimentary role. During the patient’s second ED presentation, the CK was normal at 54 U/L, whereas ultrasound and MRI findings were suggestive of focal muscle abnormalities.
Although there are diverse etiologies of rhabdomyolysis, the ultimate consequences of rhabdomyolysis are muscle cell membrane injury, metabolism malfunction, and destruction of the myofibril, resulting in inflammatory changes, such as muscle edema, hemorrhage, and myonecrosis and disruption of muscle fibers.1,2,8,9,13 This may cause an alteration in muscle size, shape, and echogenicity on sonography and abnormal signal intensity on MRI.13 The sensitivity of MRI in the detection of muscle involvement is higher than that of CT or ultrasound due to the high soft tissue contrast.4,13,14 Specificity of all 3 modalities is low and not reported.
Although the sensitivity of ultrasound is lower than that of MRI, use of ultrasound in neuromuscular evaluation has been increasing recently due to technical refinements. Ultrasound can be effectively used as a first-line screening modality, especially in an emergency.5 Magnetic resonance imaging best assesses the distribution and extension of the affected muscles, especially when fasciotomy is considered for treatment, and initially reveals edema, inflammation, and findings of myonecrosis; muscle atrophy and fatty degeneration occur later.4,13-15 Typical MRI findings include increased signal intensity on T2-weighted and STIR (short-tau inversion recovery) sequences and variable enhancement on T1 postcontrast images, as was seen in this case, which indicated edema, inflammation, and necrosis of the muscle tissue.
Shintani and colleagues described the reversibility of the MRI findings, showing that the high-intensity lesions seen on T2-weighted images resolved in parallel with the clinical course.14,16 Lu and colleagues investigated 10 patients with rhabdomyolysis and found 2 distinct imaging types: Type 1 shows homogenous signal changes and enhancement in the affected muscles, and Type 2 shows rim enhancement on contrast-enhanced MRI, a “stipple sign” indicating areas of myonecrosis.17 Magnetic resonance imaging signal alterations in the musculature can be nonspecific and overlap with those of inflammatory myopathies such as polymyositis, connective tissue diseases with inflammatory myositis, muscle infection, muscle infarction such as diabetic myonecrosis, muscle contusion, drug-induced myotoxicity, corticosteroids use, and use of cholesterol-lowering agents.18,19
Sonography is a useful screening modality for pain and swelling of the extremity, because it can detect a muscle tear, muscle sprain, and fluid collection, especially in emergent cases. There is scant literature about sonographic findings in rhabdomyolysis and compression nerve entrapment. The sonographic findings of rhabdomyolysis are local disorganization of the damaged muscle, decreased muscle echogenicity, and enlargement of the muscle, with preservation of the muscle boundaries.5-7
Intramuscular hyperechoic areas are seen due to hypercontractility of injured muscle. In this case, noted findings included patchy, irregular, hypoechoic areas, enlargement of the muscles and tendons, and irregular hyperechoic areas without focal defects. These findings differentiated an abnormality from a muscle tear or rupture, as these often show a focal muscle gap and focal defect, signifying the ruptured muscle retracting.
A study by Su and colleagues used the large number of crush injuries after an earthquake in China.5 The characteristic sonographic findings were edema and thickened disrupted striated muscle, good overall muscle continuity, vague muscle texture, and enhanced cloudy or ground-glass-like echo. There was no blood flow signal in the hypoechoic areas.6 Ultrasound was deemed a cost-effective, easily available modality by the authors.
Conclusion
Nontraumatic, focal rhabdomyolysis is rare and should be detected and differentiated from other causes of swelling, lump, pain, or other muscle disorders to prevent late complications. Sonography is an important screening diagnostic modality. MRI is used for assessment of the extent and distribution of injury. Awareness and familiarity with imaging findings can play a significant role, along with clinical and laboratory findings in the diagnosis and management of rhabdomyolysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
:
1. Richards JR. Rhabdomyolysis and drugs of abuse. J Emerg Med. 2000;19(1):51-56.
2. Farkash U, Shabshin N, Pritsch Perry M. Rhabdomyolysis of the deltoid muscle in a bodybuilder using anabolic-androgenic steroids: A case report. J Athl Train. 2009;44(1):98-100.
3. Mubarak SJ, Owen CA, Hargens AR, Garetto LP, Akeson WH. Acute compartment syndromes: Diagnosis and treatment with the aid of the wick catheter. J Bone Joint Surg Am. 1978;60(8):1091-1095.
4. Lamminen AE, Hekali PE, Tiula E, Suramo I, Korhola OA. Acute rhabdomyolysis: Evaluation with magnetic resonance imaging compared with computed tomography and ultrasonography. Br J Radiol. 1989;62(736):326-330.
5. Su BH, Qui L, Fu P, Luo Y, Tao Y, Peng YL. Ultrasonic appearance of rhabdomyolysis in patients with crush injury in the Wenchuan earthquake. Chin Med J (Engl). 2009;122(16):1872-1876.
6. Chiu Y-N, Wang T-G, Hsu C-Y, et al. Sonographic diagnosis of rhabdomyolysis. J Med Ultrasound. 2008;16(2):158-162.
7. Kaplan GN. Ultrasonic appearance of rhabdomyolysis. AJR Am J Roentgenol. 1980;134(2):375-377.
8. Spector R, Choudhury A, Cancilla P, Lakin R. Alcohol myopathy. Diagnosis by alcohol challenge. JAMA. 1979;242(15):1648-1649.
9. Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore). 1982;61(3):141-152.
10. Knochel JP. Mechanisms of rhabdomyolysis. Curr Opin Rheumatol. 1993;5(6):725-731.
11. Cadnapaphornchai P, Taher S, McDonald FD. Acute drug-association rhabdomyolysis: An examination of its diverse renal manifestations and complications. Am J Med Sci. 1980;280(2):66-72.
12. Curry SC, Chang D, Connor D. Drug and toxin-induced rhabdomyolysis. Ann Emerg Med. 1989;18(10):1068-1084.
13. May D, Disler DG, Jones EA, Balkissoon AA, Manaster BJ. Abnormal signal intensity in skeletal muscle at MR imaging: Patterns, pearls, and pitfalls. RadioGraphics. 2000;20(spec no):S295-S315.
14. Moratalla MB, Braun P, Fornas GM. Importance of MRI in the diagnosis and treatment of rhabdomyolysis. Eur J Radiol. 2008;65(2):311-315.
15. Beltran J, Rosenberg ZS. Diagnosis of compressive and entrapment neurorpathies of the upper extremity: Value of MR imaging. AJR Am J Roentgenol. 1994;163(3):525-531.
16. Shintani S, Shiigai T. Repeat MRI in acute rhabdomyolysis: Correlation with clinicopathological findings. J Comput Assist Tomogr. 1993;17(5):786-791.
17. Lu CH, Tsang YM, Yu CW, et al. Rhabdomyolysis: Magnetic resonance imaging and computed tomography findings. J Comput Assist Tomogr. 2007;31(3):368-374.
18. Schulze M, Kötter I, Ernemann U, et al. MRI findings in inflammatory muscle diseases and their noninflammatory mimics. AJR Am J Roentgenol. 2009;192(6):1708-1716.
19. Adams EM, Chow CK, Premkumar A, Plotz PH. The idiopathic inflammatory myopathies: Spectrum of MR imaging findings. Radiographics. 1995;15(3):563-574.
Rhabdomyolysis involves the breakdown of skeletal muscle with the release of intracellular contents into the extracellular space and circulation.1 Diffuse rhabdomyolysis has been found in athletes due to overexertion. However, focal rhabdomyolysis is rare.2,3 The clinical presentation of focal rhabdomyolysis is subtle and nonspecific, with swelling, vague pain, weakness, fatigue, and tea-colored urine.
Early recognition and prompt management are crucial to prevent complications such as compression syndrome, acute renal failure, disseminated intravascular coagulation, cardiac dysrhythmia, or even cardiac arrest. Sonography and magnetic resonance imaging (MRI) can, therefore, be a complementary part of the diagnosis and assessment of the extent of rhabdomyolysis.4-7
Case History
The patient was a 34-year-old white man with a history of polysubstance abuse who presented to the emergency department (ED) with numbness and weakness in the left arm and hand, pain in the left side of his neck, and 3 days of intermittent amnesia with confusion. He had used IV heroin about 2 weeks prior to admission and used tobacco and alcohol daily. He reported no current medications or known allergies. The patient was in a monogamous relationship with a same-sex partner.
On physical examination, vital signs were within normal limits. He was in distress, confused, and disoriented as to time and place. An extremity examination revealed 1/5 strength in the extensors of the left elbow, left wrist, and left fingers with normal strength noted in the right upper extremity as well as the lower extremities. No sensory deficits were noted. The patient’s skin was warm and dry. Remarkable laboratory findings included creatine kinase (CK) 1,744 U/L, creatinine (Cr) 1.9 mg/dL, ALT 1,065 U/L, AST 319 U/L, ALP 159 U/L. A urine toxicology screen was positive for cocaine and opiates, and the urine analysis dip was negative for red blood cells, white blood cells, and protein. A differential diagnosis favored a left arm inflammatory reaction to IV drugs, although rhabdomyolysis was questioned.
A neurology consult was obtained, and a bedside electroencephalography test was performed in the ED by the neurologist, showing mild left occipital slow wave abnormality with no epileptiform discharges. A chest X-ray and computed tomography (CT) scan of the head and cervical spine were unremarkable, other than incidental mild prominence of the ventricles.
Over the next 24 hours, the patient was hydrated with IV normal saline without bicarbonate. His altered mental status, urine output, and biochemical abnormalities returned to normal, except for the serum CK, which decreased to 917 U/L. He had minimal improvement in his left upper extremity nerve palsy symptoms; however, he was deemed to be stable for discharge with follow-up in the clinic.
Instead of a clinic follow-up, the patient returned to the ED 7 days later, with progressive weakness of the left arm, forearm, and wrist. The patient noted that his weakness was so significant that he had to move his left arm with his right arm. He also reported extremity swelling and increasing paresthesias involving the lateral aspect of his left arm and hand, dizziness, and left neck pain. A physical examination revealed 3/5 strength at the left deltoid and left triceps, and 0/5 strength in the left fingers and grip. Remeasurement of CK was 54 U/L and Cr was 0.9 mg/dL. Compartment pressures were not measured.
Magnetic resonance imaging using multiplanar spin echo T1 and fast spin T2 weighted and post-IV 16cc Omniscan contrast sequences of the left shoulder were performed, showing multiple patchy T2 hyperintense focal areas with peripheral enhancement in the muscles of the posterior shoulder and in the tissues adjacent to the brachial plexus in the neck and shoulder (Figures 1A, 1B, and 1C). Sonography with matrix array linear 6-15 MH3 transducer was performed, which demonstrated patchy focal hypoechoic areas of muscle with enlarged, thickened, and disrupted muscle, representing devitalized muscle without any drainable fluid collection or abscess (Figures 2A, 2B, 2C, and 2D).
Magnetic resonance imaging and magnetic resonance angiogram scans of the brain and cervical spine with and without contrast were unremarkable. At that time, a definitive diagnosis was made of focal rhabdomyolysis and compressive neuropathy of the brachial plexus posterior cord, leading to brachial plexopathy of the left shoulder.
The patient was treated with hydration, a left arm sling, elevated left arm, and ibuprofen 600 mg qid to reduce inflammation. His swelling decreased markedly, and there was a slight improvement in pain and mobility at a 2-week neurology clinic follow-up. The patient lost contact after that.
Discussion
Rhabdomyolysis is caused by diverse etiologies. Most commonly, it is generalized and occurs due to overexertion, crush injury, steroid use, metabolic abnormalities, and certain medications and illicit drugs.1,2 The most likely etiology of rhabdomyolysis in patients presenting to the ED without significant trauma is of substance abuse, especially with ethanol, heroin, amphetamines, cocaine, and other sedatives or stimulants.1-3 The patient presented in this case study had a history of drug abuse, with a positive urine toxicology screen for cocaine and opiates. He had been intermittently confused and amnesic for 3 days prior to presentation, during which he may have been lying on his shoulder for a prolonged period.
Focal rhabdomyolysis and acute compression at the posterior shoulder leading to compressive brachial plexopathy is rare, with only 3 cases reported in the literature, all occurring with IV drug use.1-3 This patient had compression of the brachial plexus posterior cord from rhabdomyolysis and prolonged immobilization. Intravenous drug abusers may delay medical care due to perceived illicit drug effects and frequently present to the ED confused, agitated, or obtunded. Acute extremity swelling, a palpable lump, and pain can be due to various etiologies, such as trauma, fluid collection, muscle tear, myopathy, venous thrombosis, neoplasm, or rhabdomyolysis.
Diagnosis of nontraumatic rhabdomyolysis depends on clinical history and biochemical tests, such as serum CK and urine myoglobin.1,8 Creatine kinase is present in large quantities in the myocytes and is 100% sensitive as a marker for rhabdomyolysis.1,8 Creatine kinase may increase acutely > 1,000 U/L, suggesting muscle lysis and necrosis as etiology for pain as opposed to other causes such as hematomas, abscesses, or venous thrombi.1,9 Serum CK decreases rapidly at a rate of 39% per day, and it may normalize by the time a patient presents for medical care.1,10-12 Imaging plays a significant complimentary role. During the patient’s second ED presentation, the CK was normal at 54 U/L, whereas ultrasound and MRI findings were suggestive of focal muscle abnormalities.
Although there are diverse etiologies of rhabdomyolysis, the ultimate consequences of rhabdomyolysis are muscle cell membrane injury, metabolism malfunction, and destruction of the myofibril, resulting in inflammatory changes, such as muscle edema, hemorrhage, and myonecrosis and disruption of muscle fibers.1,2,8,9,13 This may cause an alteration in muscle size, shape, and echogenicity on sonography and abnormal signal intensity on MRI.13 The sensitivity of MRI in the detection of muscle involvement is higher than that of CT or ultrasound due to the high soft tissue contrast.4,13,14 Specificity of all 3 modalities is low and not reported.
Although the sensitivity of ultrasound is lower than that of MRI, use of ultrasound in neuromuscular evaluation has been increasing recently due to technical refinements. Ultrasound can be effectively used as a first-line screening modality, especially in an emergency.5 Magnetic resonance imaging best assesses the distribution and extension of the affected muscles, especially when fasciotomy is considered for treatment, and initially reveals edema, inflammation, and findings of myonecrosis; muscle atrophy and fatty degeneration occur later.4,13-15 Typical MRI findings include increased signal intensity on T2-weighted and STIR (short-tau inversion recovery) sequences and variable enhancement on T1 postcontrast images, as was seen in this case, which indicated edema, inflammation, and necrosis of the muscle tissue.
Shintani and colleagues described the reversibility of the MRI findings, showing that the high-intensity lesions seen on T2-weighted images resolved in parallel with the clinical course.14,16 Lu and colleagues investigated 10 patients with rhabdomyolysis and found 2 distinct imaging types: Type 1 shows homogenous signal changes and enhancement in the affected muscles, and Type 2 shows rim enhancement on contrast-enhanced MRI, a “stipple sign” indicating areas of myonecrosis.17 Magnetic resonance imaging signal alterations in the musculature can be nonspecific and overlap with those of inflammatory myopathies such as polymyositis, connective tissue diseases with inflammatory myositis, muscle infection, muscle infarction such as diabetic myonecrosis, muscle contusion, drug-induced myotoxicity, corticosteroids use, and use of cholesterol-lowering agents.18,19
Sonography is a useful screening modality for pain and swelling of the extremity, because it can detect a muscle tear, muscle sprain, and fluid collection, especially in emergent cases. There is scant literature about sonographic findings in rhabdomyolysis and compression nerve entrapment. The sonographic findings of rhabdomyolysis are local disorganization of the damaged muscle, decreased muscle echogenicity, and enlargement of the muscle, with preservation of the muscle boundaries.5-7
Intramuscular hyperechoic areas are seen due to hypercontractility of injured muscle. In this case, noted findings included patchy, irregular, hypoechoic areas, enlargement of the muscles and tendons, and irregular hyperechoic areas without focal defects. These findings differentiated an abnormality from a muscle tear or rupture, as these often show a focal muscle gap and focal defect, signifying the ruptured muscle retracting.
A study by Su and colleagues used the large number of crush injuries after an earthquake in China.5 The characteristic sonographic findings were edema and thickened disrupted striated muscle, good overall muscle continuity, vague muscle texture, and enhanced cloudy or ground-glass-like echo. There was no blood flow signal in the hypoechoic areas.6 Ultrasound was deemed a cost-effective, easily available modality by the authors.
Conclusion
Nontraumatic, focal rhabdomyolysis is rare and should be detected and differentiated from other causes of swelling, lump, pain, or other muscle disorders to prevent late complications. Sonography is an important screening diagnostic modality. MRI is used for assessment of the extent and distribution of injury. Awareness and familiarity with imaging findings can play a significant role, along with clinical and laboratory findings in the diagnosis and management of rhabdomyolysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Rhabdomyolysis involves the breakdown of skeletal muscle with the release of intracellular contents into the extracellular space and circulation.1 Diffuse rhabdomyolysis has been found in athletes due to overexertion. However, focal rhabdomyolysis is rare.2,3 The clinical presentation of focal rhabdomyolysis is subtle and nonspecific, with swelling, vague pain, weakness, fatigue, and tea-colored urine.
Early recognition and prompt management are crucial to prevent complications such as compression syndrome, acute renal failure, disseminated intravascular coagulation, cardiac dysrhythmia, or even cardiac arrest. Sonography and magnetic resonance imaging (MRI) can, therefore, be a complementary part of the diagnosis and assessment of the extent of rhabdomyolysis.4-7
Case History
The patient was a 34-year-old white man with a history of polysubstance abuse who presented to the emergency department (ED) with numbness and weakness in the left arm and hand, pain in the left side of his neck, and 3 days of intermittent amnesia with confusion. He had used IV heroin about 2 weeks prior to admission and used tobacco and alcohol daily. He reported no current medications or known allergies. The patient was in a monogamous relationship with a same-sex partner.
On physical examination, vital signs were within normal limits. He was in distress, confused, and disoriented as to time and place. An extremity examination revealed 1/5 strength in the extensors of the left elbow, left wrist, and left fingers with normal strength noted in the right upper extremity as well as the lower extremities. No sensory deficits were noted. The patient’s skin was warm and dry. Remarkable laboratory findings included creatine kinase (CK) 1,744 U/L, creatinine (Cr) 1.9 mg/dL, ALT 1,065 U/L, AST 319 U/L, ALP 159 U/L. A urine toxicology screen was positive for cocaine and opiates, and the urine analysis dip was negative for red blood cells, white blood cells, and protein. A differential diagnosis favored a left arm inflammatory reaction to IV drugs, although rhabdomyolysis was questioned.
A neurology consult was obtained, and a bedside electroencephalography test was performed in the ED by the neurologist, showing mild left occipital slow wave abnormality with no epileptiform discharges. A chest X-ray and computed tomography (CT) scan of the head and cervical spine were unremarkable, other than incidental mild prominence of the ventricles.
Over the next 24 hours, the patient was hydrated with IV normal saline without bicarbonate. His altered mental status, urine output, and biochemical abnormalities returned to normal, except for the serum CK, which decreased to 917 U/L. He had minimal improvement in his left upper extremity nerve palsy symptoms; however, he was deemed to be stable for discharge with follow-up in the clinic.
Instead of a clinic follow-up, the patient returned to the ED 7 days later, with progressive weakness of the left arm, forearm, and wrist. The patient noted that his weakness was so significant that he had to move his left arm with his right arm. He also reported extremity swelling and increasing paresthesias involving the lateral aspect of his left arm and hand, dizziness, and left neck pain. A physical examination revealed 3/5 strength at the left deltoid and left triceps, and 0/5 strength in the left fingers and grip. Remeasurement of CK was 54 U/L and Cr was 0.9 mg/dL. Compartment pressures were not measured.
Magnetic resonance imaging using multiplanar spin echo T1 and fast spin T2 weighted and post-IV 16cc Omniscan contrast sequences of the left shoulder were performed, showing multiple patchy T2 hyperintense focal areas with peripheral enhancement in the muscles of the posterior shoulder and in the tissues adjacent to the brachial plexus in the neck and shoulder (Figures 1A, 1B, and 1C). Sonography with matrix array linear 6-15 MH3 transducer was performed, which demonstrated patchy focal hypoechoic areas of muscle with enlarged, thickened, and disrupted muscle, representing devitalized muscle without any drainable fluid collection or abscess (Figures 2A, 2B, 2C, and 2D).
Magnetic resonance imaging and magnetic resonance angiogram scans of the brain and cervical spine with and without contrast were unremarkable. At that time, a definitive diagnosis was made of focal rhabdomyolysis and compressive neuropathy of the brachial plexus posterior cord, leading to brachial plexopathy of the left shoulder.
The patient was treated with hydration, a left arm sling, elevated left arm, and ibuprofen 600 mg qid to reduce inflammation. His swelling decreased markedly, and there was a slight improvement in pain and mobility at a 2-week neurology clinic follow-up. The patient lost contact after that.
Discussion
Rhabdomyolysis is caused by diverse etiologies. Most commonly, it is generalized and occurs due to overexertion, crush injury, steroid use, metabolic abnormalities, and certain medications and illicit drugs.1,2 The most likely etiology of rhabdomyolysis in patients presenting to the ED without significant trauma is of substance abuse, especially with ethanol, heroin, amphetamines, cocaine, and other sedatives or stimulants.1-3 The patient presented in this case study had a history of drug abuse, with a positive urine toxicology screen for cocaine and opiates. He had been intermittently confused and amnesic for 3 days prior to presentation, during which he may have been lying on his shoulder for a prolonged period.
Focal rhabdomyolysis and acute compression at the posterior shoulder leading to compressive brachial plexopathy is rare, with only 3 cases reported in the literature, all occurring with IV drug use.1-3 This patient had compression of the brachial plexus posterior cord from rhabdomyolysis and prolonged immobilization. Intravenous drug abusers may delay medical care due to perceived illicit drug effects and frequently present to the ED confused, agitated, or obtunded. Acute extremity swelling, a palpable lump, and pain can be due to various etiologies, such as trauma, fluid collection, muscle tear, myopathy, venous thrombosis, neoplasm, or rhabdomyolysis.
Diagnosis of nontraumatic rhabdomyolysis depends on clinical history and biochemical tests, such as serum CK and urine myoglobin.1,8 Creatine kinase is present in large quantities in the myocytes and is 100% sensitive as a marker for rhabdomyolysis.1,8 Creatine kinase may increase acutely > 1,000 U/L, suggesting muscle lysis and necrosis as etiology for pain as opposed to other causes such as hematomas, abscesses, or venous thrombi.1,9 Serum CK decreases rapidly at a rate of 39% per day, and it may normalize by the time a patient presents for medical care.1,10-12 Imaging plays a significant complimentary role. During the patient’s second ED presentation, the CK was normal at 54 U/L, whereas ultrasound and MRI findings were suggestive of focal muscle abnormalities.
Although there are diverse etiologies of rhabdomyolysis, the ultimate consequences of rhabdomyolysis are muscle cell membrane injury, metabolism malfunction, and destruction of the myofibril, resulting in inflammatory changes, such as muscle edema, hemorrhage, and myonecrosis and disruption of muscle fibers.1,2,8,9,13 This may cause an alteration in muscle size, shape, and echogenicity on sonography and abnormal signal intensity on MRI.13 The sensitivity of MRI in the detection of muscle involvement is higher than that of CT or ultrasound due to the high soft tissue contrast.4,13,14 Specificity of all 3 modalities is low and not reported.
Although the sensitivity of ultrasound is lower than that of MRI, use of ultrasound in neuromuscular evaluation has been increasing recently due to technical refinements. Ultrasound can be effectively used as a first-line screening modality, especially in an emergency.5 Magnetic resonance imaging best assesses the distribution and extension of the affected muscles, especially when fasciotomy is considered for treatment, and initially reveals edema, inflammation, and findings of myonecrosis; muscle atrophy and fatty degeneration occur later.4,13-15 Typical MRI findings include increased signal intensity on T2-weighted and STIR (short-tau inversion recovery) sequences and variable enhancement on T1 postcontrast images, as was seen in this case, which indicated edema, inflammation, and necrosis of the muscle tissue.
Shintani and colleagues described the reversibility of the MRI findings, showing that the high-intensity lesions seen on T2-weighted images resolved in parallel with the clinical course.14,16 Lu and colleagues investigated 10 patients with rhabdomyolysis and found 2 distinct imaging types: Type 1 shows homogenous signal changes and enhancement in the affected muscles, and Type 2 shows rim enhancement on contrast-enhanced MRI, a “stipple sign” indicating areas of myonecrosis.17 Magnetic resonance imaging signal alterations in the musculature can be nonspecific and overlap with those of inflammatory myopathies such as polymyositis, connective tissue diseases with inflammatory myositis, muscle infection, muscle infarction such as diabetic myonecrosis, muscle contusion, drug-induced myotoxicity, corticosteroids use, and use of cholesterol-lowering agents.18,19
Sonography is a useful screening modality for pain and swelling of the extremity, because it can detect a muscle tear, muscle sprain, and fluid collection, especially in emergent cases. There is scant literature about sonographic findings in rhabdomyolysis and compression nerve entrapment. The sonographic findings of rhabdomyolysis are local disorganization of the damaged muscle, decreased muscle echogenicity, and enlargement of the muscle, with preservation of the muscle boundaries.5-7
Intramuscular hyperechoic areas are seen due to hypercontractility of injured muscle. In this case, noted findings included patchy, irregular, hypoechoic areas, enlargement of the muscles and tendons, and irregular hyperechoic areas without focal defects. These findings differentiated an abnormality from a muscle tear or rupture, as these often show a focal muscle gap and focal defect, signifying the ruptured muscle retracting.
A study by Su and colleagues used the large number of crush injuries after an earthquake in China.5 The characteristic sonographic findings were edema and thickened disrupted striated muscle, good overall muscle continuity, vague muscle texture, and enhanced cloudy or ground-glass-like echo. There was no blood flow signal in the hypoechoic areas.6 Ultrasound was deemed a cost-effective, easily available modality by the authors.
Conclusion
Nontraumatic, focal rhabdomyolysis is rare and should be detected and differentiated from other causes of swelling, lump, pain, or other muscle disorders to prevent late complications. Sonography is an important screening diagnostic modality. MRI is used for assessment of the extent and distribution of injury. Awareness and familiarity with imaging findings can play a significant role, along with clinical and laboratory findings in the diagnosis and management of rhabdomyolysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
:
1. Richards JR. Rhabdomyolysis and drugs of abuse. J Emerg Med. 2000;19(1):51-56.
2. Farkash U, Shabshin N, Pritsch Perry M. Rhabdomyolysis of the deltoid muscle in a bodybuilder using anabolic-androgenic steroids: A case report. J Athl Train. 2009;44(1):98-100.
3. Mubarak SJ, Owen CA, Hargens AR, Garetto LP, Akeson WH. Acute compartment syndromes: Diagnosis and treatment with the aid of the wick catheter. J Bone Joint Surg Am. 1978;60(8):1091-1095.
4. Lamminen AE, Hekali PE, Tiula E, Suramo I, Korhola OA. Acute rhabdomyolysis: Evaluation with magnetic resonance imaging compared with computed tomography and ultrasonography. Br J Radiol. 1989;62(736):326-330.
5. Su BH, Qui L, Fu P, Luo Y, Tao Y, Peng YL. Ultrasonic appearance of rhabdomyolysis in patients with crush injury in the Wenchuan earthquake. Chin Med J (Engl). 2009;122(16):1872-1876.
6. Chiu Y-N, Wang T-G, Hsu C-Y, et al. Sonographic diagnosis of rhabdomyolysis. J Med Ultrasound. 2008;16(2):158-162.
7. Kaplan GN. Ultrasonic appearance of rhabdomyolysis. AJR Am J Roentgenol. 1980;134(2):375-377.
8. Spector R, Choudhury A, Cancilla P, Lakin R. Alcohol myopathy. Diagnosis by alcohol challenge. JAMA. 1979;242(15):1648-1649.
9. Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore). 1982;61(3):141-152.
10. Knochel JP. Mechanisms of rhabdomyolysis. Curr Opin Rheumatol. 1993;5(6):725-731.
11. Cadnapaphornchai P, Taher S, McDonald FD. Acute drug-association rhabdomyolysis: An examination of its diverse renal manifestations and complications. Am J Med Sci. 1980;280(2):66-72.
12. Curry SC, Chang D, Connor D. Drug and toxin-induced rhabdomyolysis. Ann Emerg Med. 1989;18(10):1068-1084.
13. May D, Disler DG, Jones EA, Balkissoon AA, Manaster BJ. Abnormal signal intensity in skeletal muscle at MR imaging: Patterns, pearls, and pitfalls. RadioGraphics. 2000;20(spec no):S295-S315.
14. Moratalla MB, Braun P, Fornas GM. Importance of MRI in the diagnosis and treatment of rhabdomyolysis. Eur J Radiol. 2008;65(2):311-315.
15. Beltran J, Rosenberg ZS. Diagnosis of compressive and entrapment neurorpathies of the upper extremity: Value of MR imaging. AJR Am J Roentgenol. 1994;163(3):525-531.
16. Shintani S, Shiigai T. Repeat MRI in acute rhabdomyolysis: Correlation with clinicopathological findings. J Comput Assist Tomogr. 1993;17(5):786-791.
17. Lu CH, Tsang YM, Yu CW, et al. Rhabdomyolysis: Magnetic resonance imaging and computed tomography findings. J Comput Assist Tomogr. 2007;31(3):368-374.
18. Schulze M, Kötter I, Ernemann U, et al. MRI findings in inflammatory muscle diseases and their noninflammatory mimics. AJR Am J Roentgenol. 2009;192(6):1708-1716.
19. Adams EM, Chow CK, Premkumar A, Plotz PH. The idiopathic inflammatory myopathies: Spectrum of MR imaging findings. Radiographics. 1995;15(3):563-574.
:
1. Richards JR. Rhabdomyolysis and drugs of abuse. J Emerg Med. 2000;19(1):51-56.
2. Farkash U, Shabshin N, Pritsch Perry M. Rhabdomyolysis of the deltoid muscle in a bodybuilder using anabolic-androgenic steroids: A case report. J Athl Train. 2009;44(1):98-100.
3. Mubarak SJ, Owen CA, Hargens AR, Garetto LP, Akeson WH. Acute compartment syndromes: Diagnosis and treatment with the aid of the wick catheter. J Bone Joint Surg Am. 1978;60(8):1091-1095.
4. Lamminen AE, Hekali PE, Tiula E, Suramo I, Korhola OA. Acute rhabdomyolysis: Evaluation with magnetic resonance imaging compared with computed tomography and ultrasonography. Br J Radiol. 1989;62(736):326-330.
5. Su BH, Qui L, Fu P, Luo Y, Tao Y, Peng YL. Ultrasonic appearance of rhabdomyolysis in patients with crush injury in the Wenchuan earthquake. Chin Med J (Engl). 2009;122(16):1872-1876.
6. Chiu Y-N, Wang T-G, Hsu C-Y, et al. Sonographic diagnosis of rhabdomyolysis. J Med Ultrasound. 2008;16(2):158-162.
7. Kaplan GN. Ultrasonic appearance of rhabdomyolysis. AJR Am J Roentgenol. 1980;134(2):375-377.
8. Spector R, Choudhury A, Cancilla P, Lakin R. Alcohol myopathy. Diagnosis by alcohol challenge. JAMA. 1979;242(15):1648-1649.
9. Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore). 1982;61(3):141-152.
10. Knochel JP. Mechanisms of rhabdomyolysis. Curr Opin Rheumatol. 1993;5(6):725-731.
11. Cadnapaphornchai P, Taher S, McDonald FD. Acute drug-association rhabdomyolysis: An examination of its diverse renal manifestations and complications. Am J Med Sci. 1980;280(2):66-72.
12. Curry SC, Chang D, Connor D. Drug and toxin-induced rhabdomyolysis. Ann Emerg Med. 1989;18(10):1068-1084.
13. May D, Disler DG, Jones EA, Balkissoon AA, Manaster BJ. Abnormal signal intensity in skeletal muscle at MR imaging: Patterns, pearls, and pitfalls. RadioGraphics. 2000;20(spec no):S295-S315.
14. Moratalla MB, Braun P, Fornas GM. Importance of MRI in the diagnosis and treatment of rhabdomyolysis. Eur J Radiol. 2008;65(2):311-315.
15. Beltran J, Rosenberg ZS. Diagnosis of compressive and entrapment neurorpathies of the upper extremity: Value of MR imaging. AJR Am J Roentgenol. 1994;163(3):525-531.
16. Shintani S, Shiigai T. Repeat MRI in acute rhabdomyolysis: Correlation with clinicopathological findings. J Comput Assist Tomogr. 1993;17(5):786-791.
17. Lu CH, Tsang YM, Yu CW, et al. Rhabdomyolysis: Magnetic resonance imaging and computed tomography findings. J Comput Assist Tomogr. 2007;31(3):368-374.
18. Schulze M, Kötter I, Ernemann U, et al. MRI findings in inflammatory muscle diseases and their noninflammatory mimics. AJR Am J Roentgenol. 2009;192(6):1708-1716.
19. Adams EM, Chow CK, Premkumar A, Plotz PH. The idiopathic inflammatory myopathies: Spectrum of MR imaging findings. Radiographics. 1995;15(3):563-574.
‘Prehabilitation’ cut postoperative care costs for total hip and knee replacements
Physical therapy before joint replacement surgery cut the predicted use of postoperative care by 29%, saving an estimated $1,215 in health care costs per patient, according to a Medicare claims analysis.
“These data are clinically relevant and can be used in the development of cost-effective and value-based total joint replacement programs,” said Dr. Richard Snow at OhioHealth in Columbus and his associates. The study is the first to evaluate the real-world link between preoperative physical therapy and use of postoperative care, the researchers said.
Numbers of total hip and knee replacements are projected to increase by 1.7 and 6.7 times, respectively, in the United States between 2005 and 2030, Dr. Snow and his coauthors noted. And while average length of hospital stay after these surgeries has dropped by more than 50%, there has been a substantial rise in per-patient costs of skilled nursing facilities, home health agencies, and inpatient rehabilitation, they said (J. Bone Joint Surg. 2014 Oct. 1 [doi:10.2106/JBJS.M.01285]).
The researchers analyzed 4,733 hip and knee replacement cases within a 39-county cluster of Medicare referral hospitals, and looked at the association between preoperative physical therapy (or “prehabilitation”) and use of postoperative care services in the 90 days after hospital discharge. Because of the skewed distribution of payments, the investigators looked only at cases that fell below the 95th percentile (or $41,113) for cost of care, they said.
In all, 79.7% of patients who did not undergo prehabilitation used acute care services after surgery, compared with 54.2% of patients who did (P < .0001). After controlling for comorbidities and demographic variables, prehabilitation was linked to an absolute reduction of 29% from the predicted to the observed rate of post-acute care use, saving an estimated average of $1,215 per patient, they said. Reductions in use of skilled nursing facilities, home health care services, and inpatient rehabilitation were the main drivers of the cost reduction, the researchers reported.
The findings support prior work indicating that prehabilitation can improve the value of care for patients undergoing total joint replacements, said Dr. Snow and his associates. “As the volume of arthroplasties expands within the framework of increasing health care costs, providers are under mounting pressure to identify the most cost-effective method of delivering high-quality, value-based health care,” they said.
Future studies should look at optimal ways to balance preoperative and postoperative care in specific populations of patients, the investigators said.
The research was partially supported by OhioHealth Research Institute. One or more authors reported financial relationships with biomedical entities deemed to possibly influence the research.
Physical therapy before joint replacement surgery cut the predicted use of postoperative care by 29%, saving an estimated $1,215 in health care costs per patient, according to a Medicare claims analysis.
“These data are clinically relevant and can be used in the development of cost-effective and value-based total joint replacement programs,” said Dr. Richard Snow at OhioHealth in Columbus and his associates. The study is the first to evaluate the real-world link between preoperative physical therapy and use of postoperative care, the researchers said.
Numbers of total hip and knee replacements are projected to increase by 1.7 and 6.7 times, respectively, in the United States between 2005 and 2030, Dr. Snow and his coauthors noted. And while average length of hospital stay after these surgeries has dropped by more than 50%, there has been a substantial rise in per-patient costs of skilled nursing facilities, home health agencies, and inpatient rehabilitation, they said (J. Bone Joint Surg. 2014 Oct. 1 [doi:10.2106/JBJS.M.01285]).
The researchers analyzed 4,733 hip and knee replacement cases within a 39-county cluster of Medicare referral hospitals, and looked at the association between preoperative physical therapy (or “prehabilitation”) and use of postoperative care services in the 90 days after hospital discharge. Because of the skewed distribution of payments, the investigators looked only at cases that fell below the 95th percentile (or $41,113) for cost of care, they said.
In all, 79.7% of patients who did not undergo prehabilitation used acute care services after surgery, compared with 54.2% of patients who did (P < .0001). After controlling for comorbidities and demographic variables, prehabilitation was linked to an absolute reduction of 29% from the predicted to the observed rate of post-acute care use, saving an estimated average of $1,215 per patient, they said. Reductions in use of skilled nursing facilities, home health care services, and inpatient rehabilitation were the main drivers of the cost reduction, the researchers reported.
The findings support prior work indicating that prehabilitation can improve the value of care for patients undergoing total joint replacements, said Dr. Snow and his associates. “As the volume of arthroplasties expands within the framework of increasing health care costs, providers are under mounting pressure to identify the most cost-effective method of delivering high-quality, value-based health care,” they said.
Future studies should look at optimal ways to balance preoperative and postoperative care in specific populations of patients, the investigators said.
The research was partially supported by OhioHealth Research Institute. One or more authors reported financial relationships with biomedical entities deemed to possibly influence the research.
Physical therapy before joint replacement surgery cut the predicted use of postoperative care by 29%, saving an estimated $1,215 in health care costs per patient, according to a Medicare claims analysis.
“These data are clinically relevant and can be used in the development of cost-effective and value-based total joint replacement programs,” said Dr. Richard Snow at OhioHealth in Columbus and his associates. The study is the first to evaluate the real-world link between preoperative physical therapy and use of postoperative care, the researchers said.
Numbers of total hip and knee replacements are projected to increase by 1.7 and 6.7 times, respectively, in the United States between 2005 and 2030, Dr. Snow and his coauthors noted. And while average length of hospital stay after these surgeries has dropped by more than 50%, there has been a substantial rise in per-patient costs of skilled nursing facilities, home health agencies, and inpatient rehabilitation, they said (J. Bone Joint Surg. 2014 Oct. 1 [doi:10.2106/JBJS.M.01285]).
The researchers analyzed 4,733 hip and knee replacement cases within a 39-county cluster of Medicare referral hospitals, and looked at the association between preoperative physical therapy (or “prehabilitation”) and use of postoperative care services in the 90 days after hospital discharge. Because of the skewed distribution of payments, the investigators looked only at cases that fell below the 95th percentile (or $41,113) for cost of care, they said.
In all, 79.7% of patients who did not undergo prehabilitation used acute care services after surgery, compared with 54.2% of patients who did (P < .0001). After controlling for comorbidities and demographic variables, prehabilitation was linked to an absolute reduction of 29% from the predicted to the observed rate of post-acute care use, saving an estimated average of $1,215 per patient, they said. Reductions in use of skilled nursing facilities, home health care services, and inpatient rehabilitation were the main drivers of the cost reduction, the researchers reported.
The findings support prior work indicating that prehabilitation can improve the value of care for patients undergoing total joint replacements, said Dr. Snow and his associates. “As the volume of arthroplasties expands within the framework of increasing health care costs, providers are under mounting pressure to identify the most cost-effective method of delivering high-quality, value-based health care,” they said.
Future studies should look at optimal ways to balance preoperative and postoperative care in specific populations of patients, the investigators said.
The research was partially supported by OhioHealth Research Institute. One or more authors reported financial relationships with biomedical entities deemed to possibly influence the research.
FROM JOURNAL OF BONE & JOINT SURGERY
Key clinical point: Physical therapy before total knee or total hip replacement surgeries can improve the value of care.
Major finding: In all, 79.7% of patients who did not undergo presurgical physical therapy used acute care services after surgery, compared with 54.2% of patients who underwent “prehabilitation” (P < .0001).
Data source: Medicare claims analysis of 4,733 total hip or knee replacement surgeries.
Disclosures: The research was partially supported by OhioHealth Research Institute. One or more authors reported financial relationships with biomedical entities deemed to possibly influence the research.
Sleep Disturbances Linked to Pain and Depression In Patients with Osteoarthritis
New research confirms that sleep disturbances are linked to pain and depression, but not disability, among patients with osteoarthritis (OA). Study results published online ahead of print October 6 in Arthritis Care & Research found that poor sleep increases depression and disability, but does not worsen pain over time.
“Sleep disturbance is a common complaint among those with pain, particularly among those with OA,” said Patricia A. Parmelee, PhD, Director, Center for Mental Health & Aging, Professor, Department of Psychology at The University of Alabama in Tuscaloosa. “Our research is unique as we investigate the complex relationships among sleep, OA-related pain, disability and depressed mood simultaneously in a single study.”
For the study, 288 patients with knee OA provided information on pain, sleep disturbances, functional limitations, and depressive symptoms. Researchers recruited participants from diverse settings to gather a broad representation of OA subjects. Sleep disturbances at the start of the study were used to predict changes in pain, disability, and depression after a one-year period.
Findings indicated that sleep was independently associated with pain and depression at baseline. Disability was not linked to baseline sleep disturbances. In individuals with high pain levels, the combination of poor sleep and pain exacerbated depression. Sleep disturbance at baseline predicted increased depression and disability, but not pain at one-year follow-up.
“This study shows that depression plays a strong role in the sleep-pain connection, particularly with severe pain,” Dr. Parmelee and colleagues said. Further investigation of sleep in disability progression may lead to new interventions that disrupt the cycle of OA distress.”
Suggested Reading
Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in osteoarthritis: linkages with pain, disability and depressive symptoms. Arthritis Care Res (Hoboken). 2014 Oct 6. [Epub ahead of print]
New research confirms that sleep disturbances are linked to pain and depression, but not disability, among patients with osteoarthritis (OA). Study results published online ahead of print October 6 in Arthritis Care & Research found that poor sleep increases depression and disability, but does not worsen pain over time.
“Sleep disturbance is a common complaint among those with pain, particularly among those with OA,” said Patricia A. Parmelee, PhD, Director, Center for Mental Health & Aging, Professor, Department of Psychology at The University of Alabama in Tuscaloosa. “Our research is unique as we investigate the complex relationships among sleep, OA-related pain, disability and depressed mood simultaneously in a single study.”
For the study, 288 patients with knee OA provided information on pain, sleep disturbances, functional limitations, and depressive symptoms. Researchers recruited participants from diverse settings to gather a broad representation of OA subjects. Sleep disturbances at the start of the study were used to predict changes in pain, disability, and depression after a one-year period.
Findings indicated that sleep was independently associated with pain and depression at baseline. Disability was not linked to baseline sleep disturbances. In individuals with high pain levels, the combination of poor sleep and pain exacerbated depression. Sleep disturbance at baseline predicted increased depression and disability, but not pain at one-year follow-up.
“This study shows that depression plays a strong role in the sleep-pain connection, particularly with severe pain,” Dr. Parmelee and colleagues said. Further investigation of sleep in disability progression may lead to new interventions that disrupt the cycle of OA distress.”
New research confirms that sleep disturbances are linked to pain and depression, but not disability, among patients with osteoarthritis (OA). Study results published online ahead of print October 6 in Arthritis Care & Research found that poor sleep increases depression and disability, but does not worsen pain over time.
“Sleep disturbance is a common complaint among those with pain, particularly among those with OA,” said Patricia A. Parmelee, PhD, Director, Center for Mental Health & Aging, Professor, Department of Psychology at The University of Alabama in Tuscaloosa. “Our research is unique as we investigate the complex relationships among sleep, OA-related pain, disability and depressed mood simultaneously in a single study.”
For the study, 288 patients with knee OA provided information on pain, sleep disturbances, functional limitations, and depressive symptoms. Researchers recruited participants from diverse settings to gather a broad representation of OA subjects. Sleep disturbances at the start of the study were used to predict changes in pain, disability, and depression after a one-year period.
Findings indicated that sleep was independently associated with pain and depression at baseline. Disability was not linked to baseline sleep disturbances. In individuals with high pain levels, the combination of poor sleep and pain exacerbated depression. Sleep disturbance at baseline predicted increased depression and disability, but not pain at one-year follow-up.
“This study shows that depression plays a strong role in the sleep-pain connection, particularly with severe pain,” Dr. Parmelee and colleagues said. Further investigation of sleep in disability progression may lead to new interventions that disrupt the cycle of OA distress.”
Suggested Reading
Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in osteoarthritis: linkages with pain, disability and depressive symptoms. Arthritis Care Res (Hoboken). 2014 Oct 6. [Epub ahead of print]
Suggested Reading
Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in osteoarthritis: linkages with pain, disability and depressive symptoms. Arthritis Care Res (Hoboken). 2014 Oct 6. [Epub ahead of print]
MRI Measures of Joint’s Geometry Suggest Role in Athletes Severe Knee Injuries
Several recent studies, which include a controlled study of first-time anterior cruciate ligament (ACL) injuries in Vermont high school and college athletic team members—conducted by Bruce Beynnon, PhD, University of Vermont McClure Professor of Musculoskeletal Research, Director of Research, Department of Orthopaedics and Rehabilitation and research colleagues—have examined multiple factors such as the size of the femoral notch to explain why some people are at greater risk for injury than others.
In a study published in the August issue of American Journal of Sports Medicine, Dr. Beynnon and colleagues have “very accurately characterized the incidence rate and magnitude of this problem in Vermont.”
The investigators examined 88 student athletes (27 male and 61 female) who suffered first-time, noncontact ACL injuries during the study and compared their measurements, which were taken using MRI, to a non-injured control group of 88 athletes (same male-female breakdown) from the same teams, with the same extrinsic factors (eg, environment, playing surface, training, footwear, level of competition, and coaching). One of the findings that study authors discovered is that the risk of injury increased as the size of the femoral notch and ACL decreases.
In a parallel five-year epidemiological study, also published in same issue, researchers reported on the effects of level of competition, sport, and sex on the incidence of first-time noncontact ACL injuries.
For this study, Dr. Beynnon and colleagues collected data from 38 institutions located throughout Vermont. Colleges reported 48 ACL injuries during the sport seasons studied, while high schools reported 53 injuries. The research team learned that college athletes had a significantly higher ACL injury risk than high school athletes and that female athletes were two times more at risk for ACL injuries than males. In comparison to athletes taking part in Lacrosse, risk of ACL injury was significantly greater among those participating in soccer and rugby.
“An athlete’s risk of having a first-time noncontact ACL injury is independently influenced by level of competition, the participant’s sex, and type of sport they participate in, and there are no interactions between their effects. Female college athletes have the highest risk of having a first-time noncontact ACL injury among the groups studied,” Dr. Beynnon and colleagues stated.
Suggested Reading
Whitney DC, Sturnick DR, Vacek PM, et al. Relationship between the risk of suffering a first-time noncontact ACL injury and geometry of the femoral notch and ACL: a prospective cohort study with a nested case-control analysis. Am J Sports Med. 42(8):1796-1805.
Several recent studies, which include a controlled study of first-time anterior cruciate ligament (ACL) injuries in Vermont high school and college athletic team members—conducted by Bruce Beynnon, PhD, University of Vermont McClure Professor of Musculoskeletal Research, Director of Research, Department of Orthopaedics and Rehabilitation and research colleagues—have examined multiple factors such as the size of the femoral notch to explain why some people are at greater risk for injury than others.
In a study published in the August issue of American Journal of Sports Medicine, Dr. Beynnon and colleagues have “very accurately characterized the incidence rate and magnitude of this problem in Vermont.”
The investigators examined 88 student athletes (27 male and 61 female) who suffered first-time, noncontact ACL injuries during the study and compared their measurements, which were taken using MRI, to a non-injured control group of 88 athletes (same male-female breakdown) from the same teams, with the same extrinsic factors (eg, environment, playing surface, training, footwear, level of competition, and coaching). One of the findings that study authors discovered is that the risk of injury increased as the size of the femoral notch and ACL decreases.
In a parallel five-year epidemiological study, also published in same issue, researchers reported on the effects of level of competition, sport, and sex on the incidence of first-time noncontact ACL injuries.
For this study, Dr. Beynnon and colleagues collected data from 38 institutions located throughout Vermont. Colleges reported 48 ACL injuries during the sport seasons studied, while high schools reported 53 injuries. The research team learned that college athletes had a significantly higher ACL injury risk than high school athletes and that female athletes were two times more at risk for ACL injuries than males. In comparison to athletes taking part in Lacrosse, risk of ACL injury was significantly greater among those participating in soccer and rugby.
“An athlete’s risk of having a first-time noncontact ACL injury is independently influenced by level of competition, the participant’s sex, and type of sport they participate in, and there are no interactions between their effects. Female college athletes have the highest risk of having a first-time noncontact ACL injury among the groups studied,” Dr. Beynnon and colleagues stated.
Several recent studies, which include a controlled study of first-time anterior cruciate ligament (ACL) injuries in Vermont high school and college athletic team members—conducted by Bruce Beynnon, PhD, University of Vermont McClure Professor of Musculoskeletal Research, Director of Research, Department of Orthopaedics and Rehabilitation and research colleagues—have examined multiple factors such as the size of the femoral notch to explain why some people are at greater risk for injury than others.
In a study published in the August issue of American Journal of Sports Medicine, Dr. Beynnon and colleagues have “very accurately characterized the incidence rate and magnitude of this problem in Vermont.”
The investigators examined 88 student athletes (27 male and 61 female) who suffered first-time, noncontact ACL injuries during the study and compared their measurements, which were taken using MRI, to a non-injured control group of 88 athletes (same male-female breakdown) from the same teams, with the same extrinsic factors (eg, environment, playing surface, training, footwear, level of competition, and coaching). One of the findings that study authors discovered is that the risk of injury increased as the size of the femoral notch and ACL decreases.
In a parallel five-year epidemiological study, also published in same issue, researchers reported on the effects of level of competition, sport, and sex on the incidence of first-time noncontact ACL injuries.
For this study, Dr. Beynnon and colleagues collected data from 38 institutions located throughout Vermont. Colleges reported 48 ACL injuries during the sport seasons studied, while high schools reported 53 injuries. The research team learned that college athletes had a significantly higher ACL injury risk than high school athletes and that female athletes were two times more at risk for ACL injuries than males. In comparison to athletes taking part in Lacrosse, risk of ACL injury was significantly greater among those participating in soccer and rugby.
“An athlete’s risk of having a first-time noncontact ACL injury is independently influenced by level of competition, the participant’s sex, and type of sport they participate in, and there are no interactions between their effects. Female college athletes have the highest risk of having a first-time noncontact ACL injury among the groups studied,” Dr. Beynnon and colleagues stated.
Suggested Reading
Whitney DC, Sturnick DR, Vacek PM, et al. Relationship between the risk of suffering a first-time noncontact ACL injury and geometry of the femoral notch and ACL: a prospective cohort study with a nested case-control analysis. Am J Sports Med. 42(8):1796-1805.
Suggested Reading
Whitney DC, Sturnick DR, Vacek PM, et al. Relationship between the risk of suffering a first-time noncontact ACL injury and geometry of the femoral notch and ACL: a prospective cohort study with a nested case-control analysis. Am J Sports Med. 42(8):1796-1805.
Surgeon Uses Cadaver Meniscus to Reconstruct Finger Joints
Joost van Oss, an artist, was chopping wood years ago when he injured the middle knuckle on his right hand. The intense pain and swelling that followed the accident forced him to give up activities he enjoyed, such as sailing and cooking. This mishap also nearly ended his career as a sculptor and painter. He turned to David A. Kulber, MD, FACS, Clinical Chief and Director of the Cedars-Sinai Center for Plastic and Reconstructive Surgery in Los Angeles, California.
Dr. Kulber performed surgery on van Oss by using knee meniscus from a cadaver to reconstruct van Oss’ finger joint, which is a departure from the conventional technique of inserting a silicone implant into a joint.
According to Dr. Kulber, silicone implants are imperfect because they can become infected or break over time, leaving patients with lasting pain or in need of follow-up surgeries. Because the meniscus is malleable, it fits neatly into the joint, merging into the finger as new blood flows through it, Dr. Kulber said.
The patient regained mobility in his finger and just nine months after surgery he can now carry-on with the activities that he once did, all without pain. “You don’t realize what you’re missing when you have pain,” van Oss said. “Once it’s gone, all of the possibilities that were once there show up again.”
Dr. Kulber pioneered joint reconstruction with cadaver meniscus in the hope of achieving better outcomes for patients like van Oss who suffer from damaged finger joints or arthritis. “This is a very exciting approach to a problem that has defied reliable solutions,” said Dr. Kulber. “It’s a promising option because the meniscus becomes part of the finger.”
Joost van Oss, an artist, was chopping wood years ago when he injured the middle knuckle on his right hand. The intense pain and swelling that followed the accident forced him to give up activities he enjoyed, such as sailing and cooking. This mishap also nearly ended his career as a sculptor and painter. He turned to David A. Kulber, MD, FACS, Clinical Chief and Director of the Cedars-Sinai Center for Plastic and Reconstructive Surgery in Los Angeles, California.
Dr. Kulber performed surgery on van Oss by using knee meniscus from a cadaver to reconstruct van Oss’ finger joint, which is a departure from the conventional technique of inserting a silicone implant into a joint.
According to Dr. Kulber, silicone implants are imperfect because they can become infected or break over time, leaving patients with lasting pain or in need of follow-up surgeries. Because the meniscus is malleable, it fits neatly into the joint, merging into the finger as new blood flows through it, Dr. Kulber said.
The patient regained mobility in his finger and just nine months after surgery he can now carry-on with the activities that he once did, all without pain. “You don’t realize what you’re missing when you have pain,” van Oss said. “Once it’s gone, all of the possibilities that were once there show up again.”
Dr. Kulber pioneered joint reconstruction with cadaver meniscus in the hope of achieving better outcomes for patients like van Oss who suffer from damaged finger joints or arthritis. “This is a very exciting approach to a problem that has defied reliable solutions,” said Dr. Kulber. “It’s a promising option because the meniscus becomes part of the finger.”
Joost van Oss, an artist, was chopping wood years ago when he injured the middle knuckle on his right hand. The intense pain and swelling that followed the accident forced him to give up activities he enjoyed, such as sailing and cooking. This mishap also nearly ended his career as a sculptor and painter. He turned to David A. Kulber, MD, FACS, Clinical Chief and Director of the Cedars-Sinai Center for Plastic and Reconstructive Surgery in Los Angeles, California.
Dr. Kulber performed surgery on van Oss by using knee meniscus from a cadaver to reconstruct van Oss’ finger joint, which is a departure from the conventional technique of inserting a silicone implant into a joint.
According to Dr. Kulber, silicone implants are imperfect because they can become infected or break over time, leaving patients with lasting pain or in need of follow-up surgeries. Because the meniscus is malleable, it fits neatly into the joint, merging into the finger as new blood flows through it, Dr. Kulber said.
The patient regained mobility in his finger and just nine months after surgery he can now carry-on with the activities that he once did, all without pain. “You don’t realize what you’re missing when you have pain,” van Oss said. “Once it’s gone, all of the possibilities that were once there show up again.”
Dr. Kulber pioneered joint reconstruction with cadaver meniscus in the hope of achieving better outcomes for patients like van Oss who suffer from damaged finger joints or arthritis. “This is a very exciting approach to a problem that has defied reliable solutions,” said Dr. Kulber. “It’s a promising option because the meniscus becomes part of the finger.”