User login
Are you ready for CHEST 2023 in Hawai’i?
Just a few weeks ahead of CHEST 2023, we’re sharing the can’t-miss opportunities available on site at the meeting.
Beyond the top-tier education, CHEST 2023 has a lot to offer attendees in the way of networking, development, and unique experiences that will all make for a memorable meeting.
We’re sharing a preview of the many opportunities that will be available over the 4 days of the meeting. For more specifics on these events, including locations, visit the CHEST 2023 website. You can also download the CHEST 2023 mobile app, which will be available in mid-September.
Networking and development
• For those who want to get more involved with the CHEST community, the Networks Mixer (Monday, October 9, 4 PM HST) is open to all who’d like to learn more about the seven CHEST Networks and the 21 clinically-focused Sections within them.
• The annual Women in Chest Medicine Luncheon (Monday, October 9, 12:45 PM HST) will feature a panel of three women speaking about their experiences, their advice, how to support other women in the field, and more. This event is free, but preregistration is required. Scan the QR code to sign up.
• The first-ever Ohana Mixer (Tuesday, October 10, 6 PM HST) is an opportunity for CHEST attendees to celebrate the spirit of community that unites us across our differences. Attendees can network with each other, meet the members of our newly formed Interest Groups – including the leaders of our Women in Chest Medicine Interest Group and Respiratory Care Interest Group – and socialize with presenters from our three local CHEST Community Connections organizations.
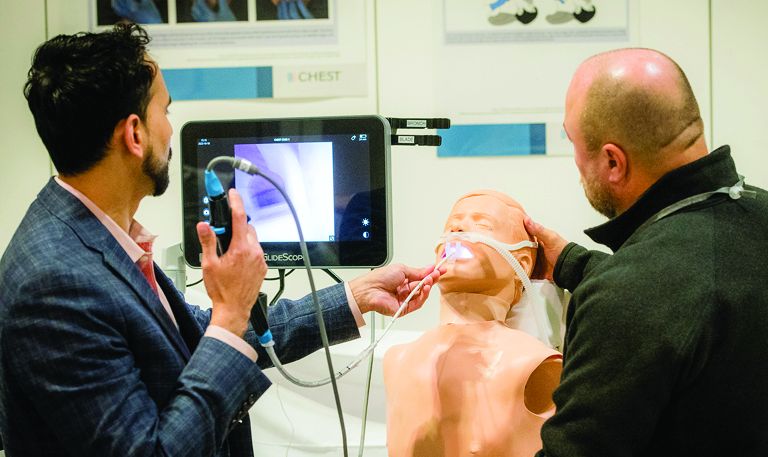
• The Trainee Lounge will feature activities like speed mentoring, a lunch and learn with the Keynote Speaker, Dr. Cedric “Jamie” Rutland, financial wellness presentations, and more.
CHEST experiences
• The Opening Session (Sunday, October 8, 3:15 PM HST) will showcase traditional Hawaiian performances and the Keynote Address from Dr. Rutland. Immediately following, the CHEST Welcome Reception will feature live music and a traditional Hawaiian luau.
• For the second year, CHEST After Hours (Monday, October 9, 3 PM HST) will feature clinicians sharing stories of their personal triumphs, tribulations, and more experiences within medicine.
• Each year, the CHEST Challenge Championship (Tuesday, October 10, 7 PM HST) gives pulmonary and critical care medicine fellows-in-training an opportunity to compete in a live Jeopardy-style game – with bragging rights and cash prizes on the line.
• The Wellness Zone has a packed schedule of events, including beachy workouts, food demonstrations, meditation, and more.
Exhibit hall activities
• Opportunities to network with and hear presentations from local Hawaiian organizations, such as the Waianae Coast Comprehensive Health Center
• Hands-on, experiential education escape rooms
• Live educational games, including Hocus POCUS Diagnosis, PulmMemory, Peer Pressure, and more
• Simulation experiences, including Aspirated and Need for Speed – Airway Bleed
Mark your calendars now to participate in all that CHEST 2023 has to offer. We’ll see you in Hawai’i!
Just a few weeks ahead of CHEST 2023, we’re sharing the can’t-miss opportunities available on site at the meeting.
Beyond the top-tier education, CHEST 2023 has a lot to offer attendees in the way of networking, development, and unique experiences that will all make for a memorable meeting.
We’re sharing a preview of the many opportunities that will be available over the 4 days of the meeting. For more specifics on these events, including locations, visit the CHEST 2023 website. You can also download the CHEST 2023 mobile app, which will be available in mid-September.
Networking and development
• For those who want to get more involved with the CHEST community, the Networks Mixer (Monday, October 9, 4 PM HST) is open to all who’d like to learn more about the seven CHEST Networks and the 21 clinically-focused Sections within them.
• The annual Women in Chest Medicine Luncheon (Monday, October 9, 12:45 PM HST) will feature a panel of three women speaking about their experiences, their advice, how to support other women in the field, and more. This event is free, but preregistration is required. Scan the QR code to sign up.
• The first-ever Ohana Mixer (Tuesday, October 10, 6 PM HST) is an opportunity for CHEST attendees to celebrate the spirit of community that unites us across our differences. Attendees can network with each other, meet the members of our newly formed Interest Groups – including the leaders of our Women in Chest Medicine Interest Group and Respiratory Care Interest Group – and socialize with presenters from our three local CHEST Community Connections organizations.
• The Trainee Lounge will feature activities like speed mentoring, a lunch and learn with the Keynote Speaker, Dr. Cedric “Jamie” Rutland, financial wellness presentations, and more.
CHEST experiences
• The Opening Session (Sunday, October 8, 3:15 PM HST) will showcase traditional Hawaiian performances and the Keynote Address from Dr. Rutland. Immediately following, the CHEST Welcome Reception will feature live music and a traditional Hawaiian luau.
• For the second year, CHEST After Hours (Monday, October 9, 3 PM HST) will feature clinicians sharing stories of their personal triumphs, tribulations, and more experiences within medicine.
• Each year, the CHEST Challenge Championship (Tuesday, October 10, 7 PM HST) gives pulmonary and critical care medicine fellows-in-training an opportunity to compete in a live Jeopardy-style game – with bragging rights and cash prizes on the line.
• The Wellness Zone has a packed schedule of events, including beachy workouts, food demonstrations, meditation, and more.
Exhibit hall activities
• Opportunities to network with and hear presentations from local Hawaiian organizations, such as the Waianae Coast Comprehensive Health Center
• Hands-on, experiential education escape rooms
• Live educational games, including Hocus POCUS Diagnosis, PulmMemory, Peer Pressure, and more
• Simulation experiences, including Aspirated and Need for Speed – Airway Bleed
Mark your calendars now to participate in all that CHEST 2023 has to offer. We’ll see you in Hawai’i!
Just a few weeks ahead of CHEST 2023, we’re sharing the can’t-miss opportunities available on site at the meeting.
Beyond the top-tier education, CHEST 2023 has a lot to offer attendees in the way of networking, development, and unique experiences that will all make for a memorable meeting.
We’re sharing a preview of the many opportunities that will be available over the 4 days of the meeting. For more specifics on these events, including locations, visit the CHEST 2023 website. You can also download the CHEST 2023 mobile app, which will be available in mid-September.
Networking and development
• For those who want to get more involved with the CHEST community, the Networks Mixer (Monday, October 9, 4 PM HST) is open to all who’d like to learn more about the seven CHEST Networks and the 21 clinically-focused Sections within them.
• The annual Women in Chest Medicine Luncheon (Monday, October 9, 12:45 PM HST) will feature a panel of three women speaking about their experiences, their advice, how to support other women in the field, and more. This event is free, but preregistration is required. Scan the QR code to sign up.
• The first-ever Ohana Mixer (Tuesday, October 10, 6 PM HST) is an opportunity for CHEST attendees to celebrate the spirit of community that unites us across our differences. Attendees can network with each other, meet the members of our newly formed Interest Groups – including the leaders of our Women in Chest Medicine Interest Group and Respiratory Care Interest Group – and socialize with presenters from our three local CHEST Community Connections organizations.
• The Trainee Lounge will feature activities like speed mentoring, a lunch and learn with the Keynote Speaker, Dr. Cedric “Jamie” Rutland, financial wellness presentations, and more.
CHEST experiences
• The Opening Session (Sunday, October 8, 3:15 PM HST) will showcase traditional Hawaiian performances and the Keynote Address from Dr. Rutland. Immediately following, the CHEST Welcome Reception will feature live music and a traditional Hawaiian luau.
• For the second year, CHEST After Hours (Monday, October 9, 3 PM HST) will feature clinicians sharing stories of their personal triumphs, tribulations, and more experiences within medicine.
• Each year, the CHEST Challenge Championship (Tuesday, October 10, 7 PM HST) gives pulmonary and critical care medicine fellows-in-training an opportunity to compete in a live Jeopardy-style game – with bragging rights and cash prizes on the line.
• The Wellness Zone has a packed schedule of events, including beachy workouts, food demonstrations, meditation, and more.
Exhibit hall activities
• Opportunities to network with and hear presentations from local Hawaiian organizations, such as the Waianae Coast Comprehensive Health Center
• Hands-on, experiential education escape rooms
• Live educational games, including Hocus POCUS Diagnosis, PulmMemory, Peer Pressure, and more
• Simulation experiences, including Aspirated and Need for Speed – Airway Bleed
Mark your calendars now to participate in all that CHEST 2023 has to offer. We’ll see you in Hawai’i!
Environmental and occupational risk factors for lung cancer
Thoracic Oncology And Chest Imaging Network
Lung Cancer Section
Lung cancer is the third most prevalent cancer in United States, with the highest mortality (Oliver, 2022)(Siegel et al, 2023). The factors contributing to its occurrence have become more complex due to increased industrialization and worsening environmental pollution. Air pollution is a well-established environmental risk factor for lung cancer (Lu et al. 2019). On average, a full-time worker spends around 90,000 hours at work over their lifetime. It is crucial to control environmental and occupational exposures to decrease the risk of developing lung cancer. Occupations like asbestos-related work, mining, and transportation are well-known to be at risk for lung cancer (Li et al. 2021). With worsening air pollution, occupations such as firefighters, outdoor delivery workers, and forest rangers are facing an increased risk as well. Many of these carcinogens independently increase lung cancer risk (Li et al. 2021). Smoking combined with these exposures, causes a synergistic effect on lung cancer incidence. They also have a cell subtype differential risk favoring squamous and small cell lung cancer (Christiani, 2020). It is essential for workers in these high-risk occupations to use proper PPE, have regular check-ups and screenings and follow occupational safety regulations and guidelines. As air pollution continues to worsen, individuals living in these areas should reduce outdoor activities during AQI alerts, and use air purifiers and masks. Public health efforts to decrease air pollution with cleaner transportation and energy production, and better local and national air quality regulations will decrease risk in the general population (Rice et al. 2021).
Amaraja Kanitkar, MD, MBBSGuest Author
References
Christiani DC. Occupational exposures and lung cancer. Am J Respir Crit Care Med. 2020;202(3):317-19.
Li N, et al. Association of 13 occupational carcinogens in patients with cancer, individually and collectively, 1990-2017. JAMA Netw Open. 2021;4(2):e2037530.
Lu T, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943-53.
Oliver AL. Lung cancer: Epidemiology and screening. Surg Clin North Am. 2022;102(3):335-44.
Rice MB, et al. Respiratory impacts of wildland fire smoke: Future challenges and policy opportunities an official American thoracic society workshop report. Ann Am Thorac Soc. 2021;18(6):921-30.
Siegel RL, et al. Cancer statistics: 2023. CA Cancer J Clin. 2023;73(1):17-48.
Thoracic Oncology And Chest Imaging Network
Lung Cancer Section
Lung cancer is the third most prevalent cancer in United States, with the highest mortality (Oliver, 2022)(Siegel et al, 2023). The factors contributing to its occurrence have become more complex due to increased industrialization and worsening environmental pollution. Air pollution is a well-established environmental risk factor for lung cancer (Lu et al. 2019). On average, a full-time worker spends around 90,000 hours at work over their lifetime. It is crucial to control environmental and occupational exposures to decrease the risk of developing lung cancer. Occupations like asbestos-related work, mining, and transportation are well-known to be at risk for lung cancer (Li et al. 2021). With worsening air pollution, occupations such as firefighters, outdoor delivery workers, and forest rangers are facing an increased risk as well. Many of these carcinogens independently increase lung cancer risk (Li et al. 2021). Smoking combined with these exposures, causes a synergistic effect on lung cancer incidence. They also have a cell subtype differential risk favoring squamous and small cell lung cancer (Christiani, 2020). It is essential for workers in these high-risk occupations to use proper PPE, have regular check-ups and screenings and follow occupational safety regulations and guidelines. As air pollution continues to worsen, individuals living in these areas should reduce outdoor activities during AQI alerts, and use air purifiers and masks. Public health efforts to decrease air pollution with cleaner transportation and energy production, and better local and national air quality regulations will decrease risk in the general population (Rice et al. 2021).
Amaraja Kanitkar, MD, MBBSGuest Author
References
Christiani DC. Occupational exposures and lung cancer. Am J Respir Crit Care Med. 2020;202(3):317-19.
Li N, et al. Association of 13 occupational carcinogens in patients with cancer, individually and collectively, 1990-2017. JAMA Netw Open. 2021;4(2):e2037530.
Lu T, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943-53.
Oliver AL. Lung cancer: Epidemiology and screening. Surg Clin North Am. 2022;102(3):335-44.
Rice MB, et al. Respiratory impacts of wildland fire smoke: Future challenges and policy opportunities an official American thoracic society workshop report. Ann Am Thorac Soc. 2021;18(6):921-30.
Siegel RL, et al. Cancer statistics: 2023. CA Cancer J Clin. 2023;73(1):17-48.
Thoracic Oncology And Chest Imaging Network
Lung Cancer Section
Lung cancer is the third most prevalent cancer in United States, with the highest mortality (Oliver, 2022)(Siegel et al, 2023). The factors contributing to its occurrence have become more complex due to increased industrialization and worsening environmental pollution. Air pollution is a well-established environmental risk factor for lung cancer (Lu et al. 2019). On average, a full-time worker spends around 90,000 hours at work over their lifetime. It is crucial to control environmental and occupational exposures to decrease the risk of developing lung cancer. Occupations like asbestos-related work, mining, and transportation are well-known to be at risk for lung cancer (Li et al. 2021). With worsening air pollution, occupations such as firefighters, outdoor delivery workers, and forest rangers are facing an increased risk as well. Many of these carcinogens independently increase lung cancer risk (Li et al. 2021). Smoking combined with these exposures, causes a synergistic effect on lung cancer incidence. They also have a cell subtype differential risk favoring squamous and small cell lung cancer (Christiani, 2020). It is essential for workers in these high-risk occupations to use proper PPE, have regular check-ups and screenings and follow occupational safety regulations and guidelines. As air pollution continues to worsen, individuals living in these areas should reduce outdoor activities during AQI alerts, and use air purifiers and masks. Public health efforts to decrease air pollution with cleaner transportation and energy production, and better local and national air quality regulations will decrease risk in the general population (Rice et al. 2021).
Amaraja Kanitkar, MD, MBBSGuest Author
References
Christiani DC. Occupational exposures and lung cancer. Am J Respir Crit Care Med. 2020;202(3):317-19.
Li N, et al. Association of 13 occupational carcinogens in patients with cancer, individually and collectively, 1990-2017. JAMA Netw Open. 2021;4(2):e2037530.
Lu T, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943-53.
Oliver AL. Lung cancer: Epidemiology and screening. Surg Clin North Am. 2022;102(3):335-44.
Rice MB, et al. Respiratory impacts of wildland fire smoke: Future challenges and policy opportunities an official American thoracic society workshop report. Ann Am Thorac Soc. 2021;18(6):921-30.
Siegel RL, et al. Cancer statistics: 2023. CA Cancer J Clin. 2023;73(1):17-48.
PalliPulm: Time to expand our arsenal
Critical Care Network
Palliative and End-of-Life Section
Symptoms at the end of life in patients with COPD are just as severe as in patients with advanced cancer (Solano JP, et al. J Pain Symptom Manage. 2006;31[1]:58-69). However, despite the high symptom burden, palliative care is less common in patients with COPD (Gore J, et al. Thorax. 2000;55[12]:1000-6).
Palliative care is associated with a number of benefits, including improved symptom burden, quality of life, and patient satisfaction (Vermylen JH, et al. Int J Chron Obstruct Pulmon Dis. 2015;10:1543-51). The majority of pulmonologists report that palliative care for patients with COPD is desirable, but about half of pulmonologists indicate that they do not use the palliative care guidelines and many were not even aware they existed (Duenk RG, et al. Int J Chron Obstruct Pulmon Dis. 2017;12:299-311). Patients with COPD often have unmet needs, and the majority of patients with COPD do not have access to palliative care at their end of life (Gore JM, et al). Unfortunately, the supply of palliative care specialists is too low to meet demand, especially in outpatient settings (Kamal AH, et al. Am J Med. 2017;130:113-4).
The ATS released a multisociety policy statement in 2022 that established a framework for early palliative care in the care in patients with respiratory illnesses (Sullivan DR, et al. Am J Respir Crit Care Med. 2022;206[6]:e44-e69). However, given the paucity of specialists and the aging population, the needs of patients and their loved ones cannot be met exclusively by palliative care specialists. Pulmonologists must expand their practice to include guideline-based palliative care in order to truly serve our patients to the best of our abilities. It is incumbent on training programs to train future pulmonologists with these palliative skills, and upon medical organizations to supply time and resources to ensure the pulmonologist is able to use these skills.
Gretchen Winter, MD
Section Member-at-Large
Critical Care Network
Palliative and End-of-Life Section
Symptoms at the end of life in patients with COPD are just as severe as in patients with advanced cancer (Solano JP, et al. J Pain Symptom Manage. 2006;31[1]:58-69). However, despite the high symptom burden, palliative care is less common in patients with COPD (Gore J, et al. Thorax. 2000;55[12]:1000-6).
Palliative care is associated with a number of benefits, including improved symptom burden, quality of life, and patient satisfaction (Vermylen JH, et al. Int J Chron Obstruct Pulmon Dis. 2015;10:1543-51). The majority of pulmonologists report that palliative care for patients with COPD is desirable, but about half of pulmonologists indicate that they do not use the palliative care guidelines and many were not even aware they existed (Duenk RG, et al. Int J Chron Obstruct Pulmon Dis. 2017;12:299-311). Patients with COPD often have unmet needs, and the majority of patients with COPD do not have access to palliative care at their end of life (Gore JM, et al). Unfortunately, the supply of palliative care specialists is too low to meet demand, especially in outpatient settings (Kamal AH, et al. Am J Med. 2017;130:113-4).
The ATS released a multisociety policy statement in 2022 that established a framework for early palliative care in the care in patients with respiratory illnesses (Sullivan DR, et al. Am J Respir Crit Care Med. 2022;206[6]:e44-e69). However, given the paucity of specialists and the aging population, the needs of patients and their loved ones cannot be met exclusively by palliative care specialists. Pulmonologists must expand their practice to include guideline-based palliative care in order to truly serve our patients to the best of our abilities. It is incumbent on training programs to train future pulmonologists with these palliative skills, and upon medical organizations to supply time and resources to ensure the pulmonologist is able to use these skills.
Gretchen Winter, MD
Section Member-at-Large
Critical Care Network
Palliative and End-of-Life Section
Symptoms at the end of life in patients with COPD are just as severe as in patients with advanced cancer (Solano JP, et al. J Pain Symptom Manage. 2006;31[1]:58-69). However, despite the high symptom burden, palliative care is less common in patients with COPD (Gore J, et al. Thorax. 2000;55[12]:1000-6).
Palliative care is associated with a number of benefits, including improved symptom burden, quality of life, and patient satisfaction (Vermylen JH, et al. Int J Chron Obstruct Pulmon Dis. 2015;10:1543-51). The majority of pulmonologists report that palliative care for patients with COPD is desirable, but about half of pulmonologists indicate that they do not use the palliative care guidelines and many were not even aware they existed (Duenk RG, et al. Int J Chron Obstruct Pulmon Dis. 2017;12:299-311). Patients with COPD often have unmet needs, and the majority of patients with COPD do not have access to palliative care at their end of life (Gore JM, et al). Unfortunately, the supply of palliative care specialists is too low to meet demand, especially in outpatient settings (Kamal AH, et al. Am J Med. 2017;130:113-4).
The ATS released a multisociety policy statement in 2022 that established a framework for early palliative care in the care in patients with respiratory illnesses (Sullivan DR, et al. Am J Respir Crit Care Med. 2022;206[6]:e44-e69). However, given the paucity of specialists and the aging population, the needs of patients and their loved ones cannot be met exclusively by palliative care specialists. Pulmonologists must expand their practice to include guideline-based palliative care in order to truly serve our patients to the best of our abilities. It is incumbent on training programs to train future pulmonologists with these palliative skills, and upon medical organizations to supply time and resources to ensure the pulmonologist is able to use these skills.
Gretchen Winter, MD
Section Member-at-Large
Hot or cold – impact on asthma and COPD
Airways Disorders Network
Asthma & COPD Section
Earlier works investigating effects of temperature and humidity changes on the airway in patients with asthma are insightful (Strauss, et al. 1978). Heat can irritate asthmatic airways that are already hyperreactive. Cold air can remove airway moisture. Similar mechanisms with warm/hot air can affect airway inflammation in COPD. In addition, poor air quality often occurs during extreme heat events and can affect patients with COPD.
Seasonal variation in COPD exacerbations was demonstrated by the TORCH study, where a two-fold increase in COPD exacerbations and hospitalizations was noted during the winter months in both northern and southern regions of the world. This trend was not observed in tropical countries with average annual temperatures of >18 °C (64 °F). Factors accounting for this variation may include greater risk of viral infections, increased host susceptibility, and more time spent indoors, along with impact of temperature variation on lung function (Jenkins, et al. 2012). This effect was accompanied by variation in the treatment choices with antibiotics alone or in combination with steroids. A trend towards combined antibiotics and steroids was noted during winters.
Ideal conditions for patients with COPD to minimize risk for exacerbation would be home humidity between 30% and 50% with indoor temperature of 21°C at least 9 hours per day in living areas (Osman, et al. 2008).
Outdoor activities during extreme temperatures should be avoided. Air conditioning and/or humidifiers can be helpful in modifying influences.
Maria Azhar, MD
Section Fellow-in-Training
Richard George Barbers, MD, FCCP
Section Chair
References
Jenkins CR, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39(1):38-45.
Osman LM, et al. Home warmth and health status of COPD patients. Eur J Public Health. 2008;18(4):399-405.
Strauss RH, et al. Influence of heat and humidity on the airway obstruction induced by exercise in asthma. J Clin Invest. 1978;61(2):433-40.
Airways Disorders Network
Asthma & COPD Section
Earlier works investigating effects of temperature and humidity changes on the airway in patients with asthma are insightful (Strauss, et al. 1978). Heat can irritate asthmatic airways that are already hyperreactive. Cold air can remove airway moisture. Similar mechanisms with warm/hot air can affect airway inflammation in COPD. In addition, poor air quality often occurs during extreme heat events and can affect patients with COPD.
Seasonal variation in COPD exacerbations was demonstrated by the TORCH study, where a two-fold increase in COPD exacerbations and hospitalizations was noted during the winter months in both northern and southern regions of the world. This trend was not observed in tropical countries with average annual temperatures of >18 °C (64 °F). Factors accounting for this variation may include greater risk of viral infections, increased host susceptibility, and more time spent indoors, along with impact of temperature variation on lung function (Jenkins, et al. 2012). This effect was accompanied by variation in the treatment choices with antibiotics alone or in combination with steroids. A trend towards combined antibiotics and steroids was noted during winters.
Ideal conditions for patients with COPD to minimize risk for exacerbation would be home humidity between 30% and 50% with indoor temperature of 21°C at least 9 hours per day in living areas (Osman, et al. 2008).
Outdoor activities during extreme temperatures should be avoided. Air conditioning and/or humidifiers can be helpful in modifying influences.
Maria Azhar, MD
Section Fellow-in-Training
Richard George Barbers, MD, FCCP
Section Chair
References
Jenkins CR, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39(1):38-45.
Osman LM, et al. Home warmth and health status of COPD patients. Eur J Public Health. 2008;18(4):399-405.
Strauss RH, et al. Influence of heat and humidity on the airway obstruction induced by exercise in asthma. J Clin Invest. 1978;61(2):433-40.
Airways Disorders Network
Asthma & COPD Section
Earlier works investigating effects of temperature and humidity changes on the airway in patients with asthma are insightful (Strauss, et al. 1978). Heat can irritate asthmatic airways that are already hyperreactive. Cold air can remove airway moisture. Similar mechanisms with warm/hot air can affect airway inflammation in COPD. In addition, poor air quality often occurs during extreme heat events and can affect patients with COPD.
Seasonal variation in COPD exacerbations was demonstrated by the TORCH study, where a two-fold increase in COPD exacerbations and hospitalizations was noted during the winter months in both northern and southern regions of the world. This trend was not observed in tropical countries with average annual temperatures of >18 °C (64 °F). Factors accounting for this variation may include greater risk of viral infections, increased host susceptibility, and more time spent indoors, along with impact of temperature variation on lung function (Jenkins, et al. 2012). This effect was accompanied by variation in the treatment choices with antibiotics alone or in combination with steroids. A trend towards combined antibiotics and steroids was noted during winters.
Ideal conditions for patients with COPD to minimize risk for exacerbation would be home humidity between 30% and 50% with indoor temperature of 21°C at least 9 hours per day in living areas (Osman, et al. 2008).
Outdoor activities during extreme temperatures should be avoided. Air conditioning and/or humidifiers can be helpful in modifying influences.
Maria Azhar, MD
Section Fellow-in-Training
Richard George Barbers, MD, FCCP
Section Chair
References
Jenkins CR, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39(1):38-45.
Osman LM, et al. Home warmth and health status of COPD patients. Eur J Public Health. 2008;18(4):399-405.
Strauss RH, et al. Influence of heat and humidity on the airway obstruction induced by exercise in asthma. J Clin Invest. 1978;61(2):433-40.
Sepsis-induced cardiomyopathy: Is it time to establish a standard of care?
Pulmonary Vascular & Cardiovascular Network
Cardiovascular Medicine and Surgery Section
Sepsis and septic shock still carry high morbidity and mortality in ICU patients despite recent improvements in care. Sepsis-induced cardiomyopathy (SICM), which complicates greater than 10% of sepsis and septic shock cases, carries a worse prognosis and is often underrecognized. Unfortunately, no universal definition of SICM exists, making diagnosis and evaluation of novel therapeutic options difficult. Initially described in the 1980s, common fundamental features of SICM include an acute and reversible decline in LVEF with typical resolution in days to weeks; RV, LV, or BiV dysfunction; LV dilation; diminished response to fluid resuscitation or catecholamines; and absence of acute coronary syndrome (L’Heureux, Sternberg et al, 2020). A definition of SICM based solely on LVEF is incomplete due to its reliance on cardiac loading conditions. Diagnostic advances using pulse contour analysis and echocardiographic measure of longitudinal strain hold promise in better characterizing cardiac dysfunction in sepsis (Beesley et al, 2018). SICM should further be distinguished from stress-induced cardiomyopathy or Takotsubo cardiomyopathy, which can also complicate cases of sepsis and is characterized by regional wall motion abnormalities, classically LV apical ballooning with preserved contractility of the basal segments. A movement toward a standard definition of SICM would allow a more rigorous evaluation of risk factors and future directions for therapy, including a potential role for mechanical circulatory support in patients who fail to improve with inotropic support.
Looking for more information on sepsis? Visit CHEST’s Sepsis Topic Collection Page at chestnet.org/Topic-Collections/Sepsis for research, infographics, and more developed by the CHEST Sepsis Resources Steering Committee.
Tarun Kapoor, MD: Section Fellow-in-Training
Andrew Petrilli, MD
Pulmonary Vascular & Cardiovascular Network
Cardiovascular Medicine and Surgery Section
Sepsis and septic shock still carry high morbidity and mortality in ICU patients despite recent improvements in care. Sepsis-induced cardiomyopathy (SICM), which complicates greater than 10% of sepsis and septic shock cases, carries a worse prognosis and is often underrecognized. Unfortunately, no universal definition of SICM exists, making diagnosis and evaluation of novel therapeutic options difficult. Initially described in the 1980s, common fundamental features of SICM include an acute and reversible decline in LVEF with typical resolution in days to weeks; RV, LV, or BiV dysfunction; LV dilation; diminished response to fluid resuscitation or catecholamines; and absence of acute coronary syndrome (L’Heureux, Sternberg et al, 2020). A definition of SICM based solely on LVEF is incomplete due to its reliance on cardiac loading conditions. Diagnostic advances using pulse contour analysis and echocardiographic measure of longitudinal strain hold promise in better characterizing cardiac dysfunction in sepsis (Beesley et al, 2018). SICM should further be distinguished from stress-induced cardiomyopathy or Takotsubo cardiomyopathy, which can also complicate cases of sepsis and is characterized by regional wall motion abnormalities, classically LV apical ballooning with preserved contractility of the basal segments. A movement toward a standard definition of SICM would allow a more rigorous evaluation of risk factors and future directions for therapy, including a potential role for mechanical circulatory support in patients who fail to improve with inotropic support.
Looking for more information on sepsis? Visit CHEST’s Sepsis Topic Collection Page at chestnet.org/Topic-Collections/Sepsis for research, infographics, and more developed by the CHEST Sepsis Resources Steering Committee.
Tarun Kapoor, MD: Section Fellow-in-Training
Andrew Petrilli, MD
Pulmonary Vascular & Cardiovascular Network
Cardiovascular Medicine and Surgery Section
Sepsis and septic shock still carry high morbidity and mortality in ICU patients despite recent improvements in care. Sepsis-induced cardiomyopathy (SICM), which complicates greater than 10% of sepsis and septic shock cases, carries a worse prognosis and is often underrecognized. Unfortunately, no universal definition of SICM exists, making diagnosis and evaluation of novel therapeutic options difficult. Initially described in the 1980s, common fundamental features of SICM include an acute and reversible decline in LVEF with typical resolution in days to weeks; RV, LV, or BiV dysfunction; LV dilation; diminished response to fluid resuscitation or catecholamines; and absence of acute coronary syndrome (L’Heureux, Sternberg et al, 2020). A definition of SICM based solely on LVEF is incomplete due to its reliance on cardiac loading conditions. Diagnostic advances using pulse contour analysis and echocardiographic measure of longitudinal strain hold promise in better characterizing cardiac dysfunction in sepsis (Beesley et al, 2018). SICM should further be distinguished from stress-induced cardiomyopathy or Takotsubo cardiomyopathy, which can also complicate cases of sepsis and is characterized by regional wall motion abnormalities, classically LV apical ballooning with preserved contractility of the basal segments. A movement toward a standard definition of SICM would allow a more rigorous evaluation of risk factors and future directions for therapy, including a potential role for mechanical circulatory support in patients who fail to improve with inotropic support.
Looking for more information on sepsis? Visit CHEST’s Sepsis Topic Collection Page at chestnet.org/Topic-Collections/Sepsis for research, infographics, and more developed by the CHEST Sepsis Resources Steering Committee.
Tarun Kapoor, MD: Section Fellow-in-Training
Andrew Petrilli, MD
Landmark obesity legislation reintroduced in Congress
The AGA Government Affairs Committee is pleased to announce the Senate and House have reintroduced the bipartisan Treat and Reduce Obesity Act (TROA) (H.R. 4818/S. 2407). This legislation is a vital first step in expanding access to obesity treatment. If passed, the bill would expand Medicare coverage to include screening and treatment of obesity by health care providers who provide obesity care. The bill also includes coverage of behavioral counseling, prescription drugs for long-term weight management, and other prevention and treatment options.
The passage of TROA could lead to improved obesity care options for all Americans since many private insurance companies model their covered health benefits to reflect Medicare.
The AGA Government Affairs Committee is pleased to announce the Senate and House have reintroduced the bipartisan Treat and Reduce Obesity Act (TROA) (H.R. 4818/S. 2407). This legislation is a vital first step in expanding access to obesity treatment. If passed, the bill would expand Medicare coverage to include screening and treatment of obesity by health care providers who provide obesity care. The bill also includes coverage of behavioral counseling, prescription drugs for long-term weight management, and other prevention and treatment options.
The passage of TROA could lead to improved obesity care options for all Americans since many private insurance companies model their covered health benefits to reflect Medicare.
The AGA Government Affairs Committee is pleased to announce the Senate and House have reintroduced the bipartisan Treat and Reduce Obesity Act (TROA) (H.R. 4818/S. 2407). This legislation is a vital first step in expanding access to obesity treatment. If passed, the bill would expand Medicare coverage to include screening and treatment of obesity by health care providers who provide obesity care. The bill also includes coverage of behavioral counseling, prescription drugs for long-term weight management, and other prevention and treatment options.
The passage of TROA could lead to improved obesity care options for all Americans since many private insurance companies model their covered health benefits to reflect Medicare.
AGA invests in virtual care clinic Oshi Health
specializing in treating patients with gastrointestinal disorders that has been named a recipient of funding through the AGA Center for GI Innovation & Technology’s GI Opportunity Fund.
Launched in 2020, Oshi Health works with employers, health insurance partners, health systems, and community GI practices to scale access to multidisciplinary care, reduce health care costs, and help improve outcomes for patients.
Research shows that a whole-person, multidisciplinary GI care model – which for Oshi includes nutrition and diet support, health coaching, behavioral and mental health services – is highly effective in mitigating symptoms. For example, a 2020 literature review published in the Journal of the Canadian Association of Gastroenterology documented far more advantages with integrated care models, as compared with the GI specialist model of care. The study found that integrated care teams were better equipped to meet the needs of patients with inflammatory bowel disease (IBD), patient outcomes and satisfaction were better, overall direct and indirect costs were lower, and psychological health needs were better addressed.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition, and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process.
For more information about Oshi Health, visit https://oshihealth.com.
specializing in treating patients with gastrointestinal disorders that has been named a recipient of funding through the AGA Center for GI Innovation & Technology’s GI Opportunity Fund.
Launched in 2020, Oshi Health works with employers, health insurance partners, health systems, and community GI practices to scale access to multidisciplinary care, reduce health care costs, and help improve outcomes for patients.
Research shows that a whole-person, multidisciplinary GI care model – which for Oshi includes nutrition and diet support, health coaching, behavioral and mental health services – is highly effective in mitigating symptoms. For example, a 2020 literature review published in the Journal of the Canadian Association of Gastroenterology documented far more advantages with integrated care models, as compared with the GI specialist model of care. The study found that integrated care teams were better equipped to meet the needs of patients with inflammatory bowel disease (IBD), patient outcomes and satisfaction were better, overall direct and indirect costs were lower, and psychological health needs were better addressed.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition, and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process.
For more information about Oshi Health, visit https://oshihealth.com.
specializing in treating patients with gastrointestinal disorders that has been named a recipient of funding through the AGA Center for GI Innovation & Technology’s GI Opportunity Fund.
Launched in 2020, Oshi Health works with employers, health insurance partners, health systems, and community GI practices to scale access to multidisciplinary care, reduce health care costs, and help improve outcomes for patients.
Research shows that a whole-person, multidisciplinary GI care model – which for Oshi includes nutrition and diet support, health coaching, behavioral and mental health services – is highly effective in mitigating symptoms. For example, a 2020 literature review published in the Journal of the Canadian Association of Gastroenterology documented far more advantages with integrated care models, as compared with the GI specialist model of care. The study found that integrated care teams were better equipped to meet the needs of patients with inflammatory bowel disease (IBD), patient outcomes and satisfaction were better, overall direct and indirect costs were lower, and psychological health needs were better addressed.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition, and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process.
For more information about Oshi Health, visit https://oshihealth.com.
AGA president Barbara Jung asks UHC to cease advance notification
for gastrointestinal endoscopy procedures, which took effect June 1, and UnitedHealthcare’s proposed “Gold Card” prior authorization program planned for 2024.
Dr. Jung made three requests: A request for UnitedHealthcare’s deidentified aggregate data on which the Advance Notification program is based.
She asked for clarification in regards to gastroenterologists who opt not to participate in the Advance Notification program. Will they be automatically subject to prior authorization when UnitedHealthcare implements the 2024 Gold Card program?
And, how will information gathered through the Advance Notification program shape the GI Gold Card prior authorization program that UnitedHealthcare plans to implement in 2024?
Dr. Jung asked for a written response to each of these three issues and a meeting to discuss concerns and questions.
She stated that the Advance Notification Program was launched without adequate communication to gastroenterologists, plus the AGA, the American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy have had questions and concerns that haven’t yet been addressed.
“Despite multiple requests, you have not shared any UnitedHealthcare-specific overuse or variation data on a code-by-code basis that would warrant such a burdensome process. Please share the deidentified aggregate data. Absent data, there is no rationale for such a policy. The Advance Notification program directly contradicts UnitedHealthcare’s publicly stated goals of reducing administrative burden and streamlining access to care – goals we support and encourage you to work toward in the gastroenterological specialty. In contrast, Advance Notification is imposing significant administrative burdens on physician practices, which will negatively impact patient access to timely, medically necessary care,” she wrote.
Practice burden
“The chaotic rollout of the new data reporting requirements led to widespread confusion throughout the gastroenterological community and has since forced physicians and staff to spend multiple hours every day completing reporting requirements for data that UnitedHealthcare already has through claims forms. This is a serious drain on gastroenterology practices’ time, staff, and resources – which should be entirely focused on patient care, not reams of paperwork,” Dr. Jung wrote.
AGA members have stated that:
- Most local UHC representatives are unaware of the Advance Notification Program for GI endoscopy program and are unable to advise them regarding concerns or problems.
- Local UHC representatives have no information on the Gold Card program and how it might operate.
Many practices report they have not received any follow-up from UHC requesting additional records via the Advance Notification Program.
Some large GI practices report it takes their staff 5-7 minutes per patient to enter the required data. Others quantify the additional work of participating in the advance notification program as 25%-35% more work than before the program was implemented.
Practices with large UHC volume report having to divert multiple staff to work full-time on UHC accounts.
All practices report that they are required to input/upload the same clinical information as other UHC prior authorizations. Some practices additionally take a screenshot of the statement that the procedure does not require precertification and place it in the patient notes as a precaution in case issues arise in the future.
GI practices that have tried to use the telephone number to report a change in procedure report spending an average of an hour on hold per case.
Dr. Jung said that “given these challenges, many practices are not participating” in the advance notification program.
“AGA is troubled by the serious lack of specific details about the Gold Card prior authorization program to date. With less than 6 months until 2024, UnitedHealthcare has not issued any details about eligibility criteria, participation, or what new prior authorization requirements may be implemented for practices that do not qualify for a Gold Card. We resolutely oppose the implementation of any type of preauthorization requirements for colonoscopies and endoscopies. We are medical practitioners who have years of training and experience treating patients. Our medical decisions are evidence-based, which no prior authorization policy can claim.
“As you recognized when announcing UnitedHealthcare will slash prior authorization requirements by 20% earlier this spring, requiring physicians to apply for and receive preapproval before being able to deliver medically necessary care is not just frustrating – it is disruptive and dangerous for patients’ health. This is particularly true when it comes to performing colonoscopies and endoscopies, which are vital for detecting and monitoring diseases such as inflammatory bowel disease and colorectal cancer, the second deadliest form of cancer in the United States.
“AGA stands ready to partner with UnitedHealthcare on mutually beneficial educational initiatives to promote appropriate use of endoscopy procedures. However, we reiterate our call for UnitedHealthcare to halt the confusing and burdensome Advance Notification Program – and scrap plans to implement a Gold Card prior authorization program as planned in 2024. Instead, we invite UnitedHealthcare to work collaboratively with us to develop programs that improve quality of care without creating barriers to treatment for patients and unnecessary and inappropriate administrative burdens for physicians. We urge you to stop the Advance Notification and any prior authorization programs impacting GI endoscopy and directly engage with AGA to ensure patients’ continued access to high-value, patient-centered endoscopy care. Please contact Leslie Narramore at lnarramore@gastro.org at your earliest convenience so we can resume our dialogue.”
Dr. Jung closed the letter urging UHC to stop the advance notification program and planned prior authorization programs and instead engage in a dialogue with AGA about the issues.
To read Dr. Jung’s letter in full, see https://shorturl.at/dhjyH.
for gastrointestinal endoscopy procedures, which took effect June 1, and UnitedHealthcare’s proposed “Gold Card” prior authorization program planned for 2024.
Dr. Jung made three requests: A request for UnitedHealthcare’s deidentified aggregate data on which the Advance Notification program is based.
She asked for clarification in regards to gastroenterologists who opt not to participate in the Advance Notification program. Will they be automatically subject to prior authorization when UnitedHealthcare implements the 2024 Gold Card program?
And, how will information gathered through the Advance Notification program shape the GI Gold Card prior authorization program that UnitedHealthcare plans to implement in 2024?
Dr. Jung asked for a written response to each of these three issues and a meeting to discuss concerns and questions.
She stated that the Advance Notification Program was launched without adequate communication to gastroenterologists, plus the AGA, the American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy have had questions and concerns that haven’t yet been addressed.
“Despite multiple requests, you have not shared any UnitedHealthcare-specific overuse or variation data on a code-by-code basis that would warrant such a burdensome process. Please share the deidentified aggregate data. Absent data, there is no rationale for such a policy. The Advance Notification program directly contradicts UnitedHealthcare’s publicly stated goals of reducing administrative burden and streamlining access to care – goals we support and encourage you to work toward in the gastroenterological specialty. In contrast, Advance Notification is imposing significant administrative burdens on physician practices, which will negatively impact patient access to timely, medically necessary care,” she wrote.
Practice burden
“The chaotic rollout of the new data reporting requirements led to widespread confusion throughout the gastroenterological community and has since forced physicians and staff to spend multiple hours every day completing reporting requirements for data that UnitedHealthcare already has through claims forms. This is a serious drain on gastroenterology practices’ time, staff, and resources – which should be entirely focused on patient care, not reams of paperwork,” Dr. Jung wrote.
AGA members have stated that:
- Most local UHC representatives are unaware of the Advance Notification Program for GI endoscopy program and are unable to advise them regarding concerns or problems.
- Local UHC representatives have no information on the Gold Card program and how it might operate.
Many practices report they have not received any follow-up from UHC requesting additional records via the Advance Notification Program.
Some large GI practices report it takes their staff 5-7 minutes per patient to enter the required data. Others quantify the additional work of participating in the advance notification program as 25%-35% more work than before the program was implemented.
Practices with large UHC volume report having to divert multiple staff to work full-time on UHC accounts.
All practices report that they are required to input/upload the same clinical information as other UHC prior authorizations. Some practices additionally take a screenshot of the statement that the procedure does not require precertification and place it in the patient notes as a precaution in case issues arise in the future.
GI practices that have tried to use the telephone number to report a change in procedure report spending an average of an hour on hold per case.
Dr. Jung said that “given these challenges, many practices are not participating” in the advance notification program.
“AGA is troubled by the serious lack of specific details about the Gold Card prior authorization program to date. With less than 6 months until 2024, UnitedHealthcare has not issued any details about eligibility criteria, participation, or what new prior authorization requirements may be implemented for practices that do not qualify for a Gold Card. We resolutely oppose the implementation of any type of preauthorization requirements for colonoscopies and endoscopies. We are medical practitioners who have years of training and experience treating patients. Our medical decisions are evidence-based, which no prior authorization policy can claim.
“As you recognized when announcing UnitedHealthcare will slash prior authorization requirements by 20% earlier this spring, requiring physicians to apply for and receive preapproval before being able to deliver medically necessary care is not just frustrating – it is disruptive and dangerous for patients’ health. This is particularly true when it comes to performing colonoscopies and endoscopies, which are vital for detecting and monitoring diseases such as inflammatory bowel disease and colorectal cancer, the second deadliest form of cancer in the United States.
“AGA stands ready to partner with UnitedHealthcare on mutually beneficial educational initiatives to promote appropriate use of endoscopy procedures. However, we reiterate our call for UnitedHealthcare to halt the confusing and burdensome Advance Notification Program – and scrap plans to implement a Gold Card prior authorization program as planned in 2024. Instead, we invite UnitedHealthcare to work collaboratively with us to develop programs that improve quality of care without creating barriers to treatment for patients and unnecessary and inappropriate administrative burdens for physicians. We urge you to stop the Advance Notification and any prior authorization programs impacting GI endoscopy and directly engage with AGA to ensure patients’ continued access to high-value, patient-centered endoscopy care. Please contact Leslie Narramore at lnarramore@gastro.org at your earliest convenience so we can resume our dialogue.”
Dr. Jung closed the letter urging UHC to stop the advance notification program and planned prior authorization programs and instead engage in a dialogue with AGA about the issues.
To read Dr. Jung’s letter in full, see https://shorturl.at/dhjyH.
for gastrointestinal endoscopy procedures, which took effect June 1, and UnitedHealthcare’s proposed “Gold Card” prior authorization program planned for 2024.
Dr. Jung made three requests: A request for UnitedHealthcare’s deidentified aggregate data on which the Advance Notification program is based.
She asked for clarification in regards to gastroenterologists who opt not to participate in the Advance Notification program. Will they be automatically subject to prior authorization when UnitedHealthcare implements the 2024 Gold Card program?
And, how will information gathered through the Advance Notification program shape the GI Gold Card prior authorization program that UnitedHealthcare plans to implement in 2024?
Dr. Jung asked for a written response to each of these three issues and a meeting to discuss concerns and questions.
She stated that the Advance Notification Program was launched without adequate communication to gastroenterologists, plus the AGA, the American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy have had questions and concerns that haven’t yet been addressed.
“Despite multiple requests, you have not shared any UnitedHealthcare-specific overuse or variation data on a code-by-code basis that would warrant such a burdensome process. Please share the deidentified aggregate data. Absent data, there is no rationale for such a policy. The Advance Notification program directly contradicts UnitedHealthcare’s publicly stated goals of reducing administrative burden and streamlining access to care – goals we support and encourage you to work toward in the gastroenterological specialty. In contrast, Advance Notification is imposing significant administrative burdens on physician practices, which will negatively impact patient access to timely, medically necessary care,” she wrote.
Practice burden
“The chaotic rollout of the new data reporting requirements led to widespread confusion throughout the gastroenterological community and has since forced physicians and staff to spend multiple hours every day completing reporting requirements for data that UnitedHealthcare already has through claims forms. This is a serious drain on gastroenterology practices’ time, staff, and resources – which should be entirely focused on patient care, not reams of paperwork,” Dr. Jung wrote.
AGA members have stated that:
- Most local UHC representatives are unaware of the Advance Notification Program for GI endoscopy program and are unable to advise them regarding concerns or problems.
- Local UHC representatives have no information on the Gold Card program and how it might operate.
Many practices report they have not received any follow-up from UHC requesting additional records via the Advance Notification Program.
Some large GI practices report it takes their staff 5-7 minutes per patient to enter the required data. Others quantify the additional work of participating in the advance notification program as 25%-35% more work than before the program was implemented.
Practices with large UHC volume report having to divert multiple staff to work full-time on UHC accounts.
All practices report that they are required to input/upload the same clinical information as other UHC prior authorizations. Some practices additionally take a screenshot of the statement that the procedure does not require precertification and place it in the patient notes as a precaution in case issues arise in the future.
GI practices that have tried to use the telephone number to report a change in procedure report spending an average of an hour on hold per case.
Dr. Jung said that “given these challenges, many practices are not participating” in the advance notification program.
“AGA is troubled by the serious lack of specific details about the Gold Card prior authorization program to date. With less than 6 months until 2024, UnitedHealthcare has not issued any details about eligibility criteria, participation, or what new prior authorization requirements may be implemented for practices that do not qualify for a Gold Card. We resolutely oppose the implementation of any type of preauthorization requirements for colonoscopies and endoscopies. We are medical practitioners who have years of training and experience treating patients. Our medical decisions are evidence-based, which no prior authorization policy can claim.
“As you recognized when announcing UnitedHealthcare will slash prior authorization requirements by 20% earlier this spring, requiring physicians to apply for and receive preapproval before being able to deliver medically necessary care is not just frustrating – it is disruptive and dangerous for patients’ health. This is particularly true when it comes to performing colonoscopies and endoscopies, which are vital for detecting and monitoring diseases such as inflammatory bowel disease and colorectal cancer, the second deadliest form of cancer in the United States.
“AGA stands ready to partner with UnitedHealthcare on mutually beneficial educational initiatives to promote appropriate use of endoscopy procedures. However, we reiterate our call for UnitedHealthcare to halt the confusing and burdensome Advance Notification Program – and scrap plans to implement a Gold Card prior authorization program as planned in 2024. Instead, we invite UnitedHealthcare to work collaboratively with us to develop programs that improve quality of care without creating barriers to treatment for patients and unnecessary and inappropriate administrative burdens for physicians. We urge you to stop the Advance Notification and any prior authorization programs impacting GI endoscopy and directly engage with AGA to ensure patients’ continued access to high-value, patient-centered endoscopy care. Please contact Leslie Narramore at lnarramore@gastro.org at your earliest convenience so we can resume our dialogue.”
Dr. Jung closed the letter urging UHC to stop the advance notification program and planned prior authorization programs and instead engage in a dialogue with AGA about the issues.
To read Dr. Jung’s letter in full, see https://shorturl.at/dhjyH.
Use the SCAI stages to identify and treat cardiogenic shock
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Add hands-on and interactive learning opportunities to your CHEST 2023 schedule
Explore the many ticketed sessions, and sign up early in case they sell out.
Simulation sessions
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”
Explore the many ticketed sessions, and sign up early in case they sell out.
Simulation sessions
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”
Explore the many ticketed sessions, and sign up early in case they sell out.
Simulation sessions
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”