User login
Nonconsecutive Pars Interarticularis Defects
Spondylolysis is a bone defect of the pars interarticularis. It is usually seen in adolescents who participate in sporting activities that involve repetitive axial loads to a hyperextended lower back, such as football offensive lineman, throwing athletes, and gymnasts. It occurs frequently in the L5 pars and can be unilateral or bilateral. The majority of reported multiple-level spondylolysis is at consecutive lumbar segments.1-6 Rarely, it affects noncontiguous levels. Most patients respond well to conservative treatment in the form of activity modification and orthosis.7 Surgical intervention is considered if 6 months of conservative management fails, spondylolisthesis develops, or intractable neurologic symptoms arise.
This case report presents an 18-year-old man with noncontiguous spondylolysis at L2 and L5 who was successfully treated with a 1-level pars repair at L2 after failed conservative management. This unique case highlights the importance of using single-photon emission computed tomography (SPECT) scan and diagnostic pars block when planning for surgical treatment in the rare cases of noncontiguous spondylolysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
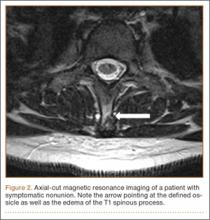
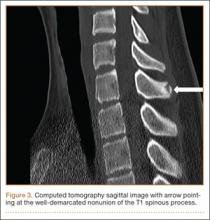
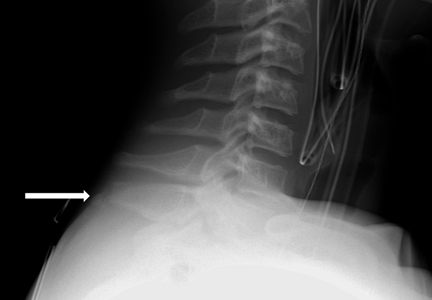
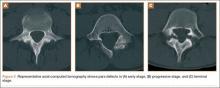
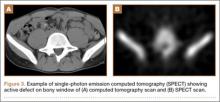
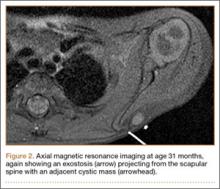
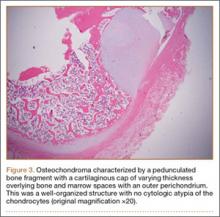
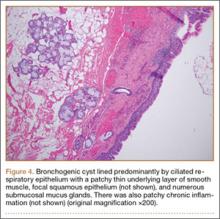
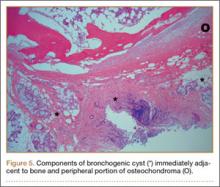
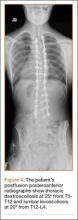
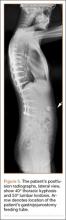
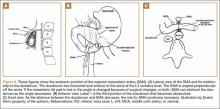
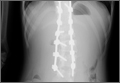
An 18-year-old man presented to the clinic with worsening lower back pain for the previous 4 weeks. He was playing high school baseball and stated the pain was worse when he swung his bat. He had no history of trauma or back pain. Rest was the only alleviating factor, and he was beginning to experience pain when he stood after sitting. He denied any radicular pain. On examination, he had midline tenderness along the upper lumbar spine and pain with lumbar spine extension. His neurologic examination showed normal sensation with 5/5 strength in all key muscle groups. Plain radiograph of the lumbar spine showed an L5 pars defect (Figures 1A, 1B). A SPECT scan showed increased uptake at L2 pars bilaterally; the L5 pars did not show increased uptake (Figures 2A, 2B). A computed tomography (CT) scan confirmed bilateral L2 pars fractures and a left L5 pars fracture (Figures 3A, 3B). Bony changes in the form of marginal sclerosis at the L5, but not the L2, pars suggested that the L2 fracture was acute while the L5 fracture was chronic (Figures 4A, 4B).
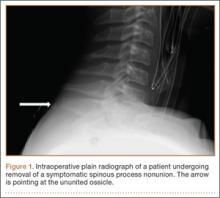
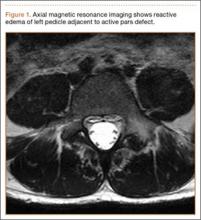
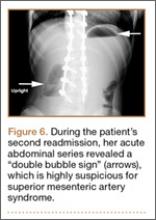
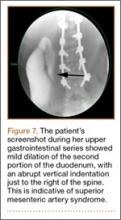
The patient had conservative management for 6 months in the form of lumbosacral orthosis (LSO), cessation of sports activities, and physical therapy. The patient was initially treated with an LSO brace for 3 months, after which he had physical therapy. At 6 month follow-up, he reported continuing, significant back pain. A repeat CT scan of the lumbar spine showed no interval healing of the bilateral L2 or the unilateral L5 pars fractures. As a result of the patient’s noncontiguous pars fractures, a diagnostic CT-guided block of L2 pars was performed to identify which level was his main pain generator (Figure 5). He reported a brief period of complete pain relief after the L2 pars block. With failure of 6 months’ conservative management and positive SPECT scan and diagnostic block, surgical treatment was recommended. Prior to surgical intervention, magnetic resonance imaging was obtained to rule out pathology (eg, disc degeneration, infection, or tumor) other than the pars defect that could require fusion instead of pars repair (Figures 6A, 6B). Because of the patient’s young age, bilateral L2 pars repair rather than fusion was indicated. After 8 months of persistent back pain, he underwent bilateral L2 pars repair with iliac crest autograft, pedicle screws, and sublaminar hook fixation (Figures 7A, 7B). The patient had an uneventful immediate postoperative course. A 6-month postoperative CT scan showed bridging callus at the L2 pars; however, the left L5 pars fracture was still visible (Figures 8A-8C). Over a 6-month postoperative period, the patient had continued improvement in his back pain, advanced his activity as tolerated without problem, and was allowed to resume his sports activities. At 2-year follow-up, he was playing baseball and basketball, and denied any back pain.
Discussion
Lumbar spondylolysis is commonly seen at the fourth and fifth lumbar vertebrae, and accounts for more than 95% of spondylolysis cases.8 Multiple-level spondylolysis is a relatively rare finding with an incidence varying between 1.2% and 5.6%. The majority of the reported multiple-level cases are adjacent.1-3,6 Adolescents often present with a history of insidious-onset low back pain without radicular symptoms that is exacerbated by activity. Occasionally, an acute injury may elicit the onset of pain. A thorough history with emphasis on pain in relation to activity and sports involvement is beneficial. The patient in the current study was a throwing athlete and presented with 4 weeks of back pain that worsened with activity; he had no history of trauma.
Radiographic assessment using standing anteroposterior, lateral, and oblique radiographs of the thoracolumbar spine is useful in the initial assessment. A SPECT scan of the lumbosacral spine is highly sensitive for identifying spondylolytic defects when plain radiographs are within normal limits, yet a high index of suspicion remains given the patient’s history and physical examination findings.9,10 Increased radionuclide uptake within the pars indicates a stress reaction and, possibly, a more acute pathology. The plain radiographs of the patient showed only L5 spondylolysis. However, a SPECT scan showed only increased uptake in L2 pars on both sides. These findings suggested chronic L5 and acute L2 pars defects. A thin-cut CT scan gives the best visualization of pars defect and can help in differentiating chronic defect with sclerotic margins versus acute defect with hazy irregular margins. In the current case, the CT scan showed changes consistent with unilateral chronic L5 and bilateral acute L2 pars defects.
The origin of the pain in spondylolysis is from the tissues rich in nociceptive nerve endings in the loose posterior arch. A CT-guided pars block is a very useful diagnostic preoperative tool that confirms the symptomatic level in cases of multilevel pars defect, especially if they are noncontiguous. In this case, the diagnostic preoperative bilateral L2 pars block confirmed that the pain generator was the acute L2 rather than the chronic L5 pars defect. This step assured that surgical treatment involving only the L2 level would be beneficial in alleviating the patient’s back pain after the failure of 6 months of conservative treatment.
Most patients with single-level spondylolysis respond to conservative treatment, especially after early diagnosis and treatment. The traditional nonoperative treatment of children and adolescents with a symptomatic spondylolytic lesion was a period of rest and progressive increased activity with physical therapy. Immobilization with an LSO was reserved for individuals who did not respond to rest and physical therapy.11 However, multiple studies revealed early immobilization achieved results superior to activity restriction alone, and individuals who underwent a period of activity restriction prior to bracing were more likely to experience persistent symptoms.12-14 Our patient underwent conservative treatment for 6 months, in the form of LSO, cessation of sport activities, and physical therapy, which failed to give him relief of his back pain.
Surgical intervention is warranted for adolescents with persistent, debilitating pain intractable to at least a 6-month period of nonoperative management. Additional indications for surgical management are those individuals who present with neurologic deficits and isthmic spondylolisthesis. Surgical treatment involves direct pars repair with iliac crest bone graft and use of a sublaminar hook/pedicle screw construct, cerclage wire, or pars screw.15-18
In contrast to single-level pars defects that respond well to conservative treatment, there are conflicting reports regarding the management of multiple-level pars fractures; a few reports suggest good outcome with conservative management, but the majority state that surgery is often required and conservative measures are rarely useful.1-4,6 Nayeemuddin and colleagues19 reported a case of a 16-year-old football player who presented with a 4-month history of constant low back pain related to bilateral L3 and L5 pars defects that responded to 1 year of conservative management, when the more acute fractures at L3 showed complete bony union and the patient had symptomatic pain relief and was able to return to full sporting activity.
Chang and colleagues2 reported 10 patients with adjacent 2-level bilateral spondylolysis treated successfully using a pedicle screw–hook construct with autogenous bone grafting. Ogawa and colleagues5 reported adjacent 2-level spondylolysis in 5 patients and 3-level spondylolysis in 2 patients, who were treated successfully by segmental wire fixation and bone grafting. Ivanic and colleagues15 retrospectively reviewed 113 patients with spondylolysis who were treated with direct repair using a hook-screw construct and showed a pseudoarthrosis rate of 13.3%. Superior fusion rates were observed in patients 14 years and younger compared with older patients, particularly those 20 years and older.15 Roca and colleagues16 prospectively analyzed 19 consecutive cases of spondylolysis that were repaired using a hook-screw construct. Twelve of 13 patients (92%) who were 20 years or younger at the time of the study (average age, 17.2 years) had fusion, whereas, in 6 patients 21 years and older (average age, 27.5 years), no cases of fusion were observed. The patients 20 years or younger had significantly better clinical results than those obtained in the patients 21 years and older. The authors concluded that pedicle screw–hook fixation is a useful alternative in the treatment of spondylolysis in adolescents, but did not recommend this procedure in patients older than 20 years.16
Conclusion
The current case demonstrates a unique example of rare noncontiguous pars defects successfully treated with primary repair of 1 level when conservative management failed and the symptomatic defect was isolated. It also highlights the importance of investigating the entirety of the lumbar spine when diagnosis of L5 spondylolysis rules out noncontiguous pars defects. The treatment of noncontiguous pars defects is not well defined; this case showed the importance of using a SPECT scan and a diagnostic pars block to help isolate the symptomatic level when surgical management is considered after a failure of conservative treatment. This case shows 2 possible results: the chronic unilateral L5 defect responded to nonsurgical treatment with asymptomatic fibrous nonunion, while the more acute bilateral L2 defect responded to pars repair with pedicle screw–hook fixation and iliac crest bone graft.
1. Al-Sebai MW, Al-Khawashki H. Spondyloptosis and multiple-level spondylolysis. Eur Spine J. 1999;8(1):75-77.
2. Chang JH, Lee CH, Wu SS, Lin LC, et al. Management of multiple level spondylolysis of the lumbar spine in young males: a report of six cases. J Formos Med Assoc. 2001;100(7)2:497-502.
3. Eingorn D, Pizzutillo PD. Pars interarticularis fusion of multiple levels of lumbar spondylolysis. A case report. Spine. 1985;10(3):250-252.
4. Nozawa S, Shimizu K, Miyamoto K, Tanaka M. Repair of pars interarticularis defect by segmental wire fixation in young athletes with spondylolysis. Am J Sports Med. 2003;31(3):359-364.
5. Ogawa H, Nishimoto H, Hosoe H, Suzuki N, Kanamori Y, Shimizu K. Clinical outcome after segmental wire fixation and bone grafting for repair of the defects in multiple level lumbar spondylolysis. J Spinal Disord Tech. 2007;20(7):521-525.
6. Ravichandran G. Multiple lumbar spondylolyses. Spine. 1980;5(6):552-557.
7. Sys J, Michielsen J, Bracke P, Martens M, Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10(6):498-504.
8. Saraste H. Spondylolysis and spondylolisthesis. Acta Orthop Scand Suppl. 1993;251:84-86.
9. Anderson K, Sarwark JF, Conway JJ, Logue ES, Schafer MS. Quantitative assessment with SPECT imaging of stress injuries of the pars interarticularis and response to bracing. J Pediatr Orthop. 2000;20(1):28-33.
10. Bodner RJ, Heyman S, Drummond DS, Gregg JR. The use of single photon emission computed tomography (SPECT) in the diagnosis of low-back pain in young patients. Spine. 1988;13(10):1155-1160.
11. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
12. Blanda J, Bethem D, Moats W, Lew M. Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. J Spinal Disord. 1993;6(5):406-411.
13. Kurd MF, Patel D, Norton R, Picetti G, Friel B, Vaccaro AR. Nonoperative treatment of symptomatic spondylolysis. J Spinal Disord Tech. 2007;20(8):560-564.
14. Pizzutillo PD, Hummer CD 3rd. Nonoperative treatment for painful adolescent spondylolysis or spondylolisthesis. J Pediatr Orthop. 1989;9(5):538-540.
15. Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook screw: mean 11-year follow-up period for 113 patients. Spine. 2003;28(3):255-259.
16. Roca J, Iborra M, Cavanilles-Walker JM, Alberti G. Direct repair of spondylolysis using a new pedicle screw hook fixation: clinical and CT-assessed study: an analysis of 19 patients. J Spinal Disord Tech. 2005;18(suppl):S82-S89.
17. Schlenzka D, Remes V, Helenius I, et al. Direct repair for treatment of symptomatic spondylolysis and low-grade isthmic spondylolisthesis in young patients: no benefit in comparison to segmental fusion after a mean follow-up of 14.8 years. Eur Spine J. 2006;15(10):1437-1447.
18. Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br. 1970;52(3):432-437.
19. Nayeemuddin M, Richards PJ, Ahmed EB. The imaging and management of nonconsecutive pars interarticularis defects: a case report and review of literature. Spine J. 2011;11(12):1157-1163.
Spondylolysis is a bone defect of the pars interarticularis. It is usually seen in adolescents who participate in sporting activities that involve repetitive axial loads to a hyperextended lower back, such as football offensive lineman, throwing athletes, and gymnasts. It occurs frequently in the L5 pars and can be unilateral or bilateral. The majority of reported multiple-level spondylolysis is at consecutive lumbar segments.1-6 Rarely, it affects noncontiguous levels. Most patients respond well to conservative treatment in the form of activity modification and orthosis.7 Surgical intervention is considered if 6 months of conservative management fails, spondylolisthesis develops, or intractable neurologic symptoms arise.
This case report presents an 18-year-old man with noncontiguous spondylolysis at L2 and L5 who was successfully treated with a 1-level pars repair at L2 after failed conservative management. This unique case highlights the importance of using single-photon emission computed tomography (SPECT) scan and diagnostic pars block when planning for surgical treatment in the rare cases of noncontiguous spondylolysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented to the clinic with worsening lower back pain for the previous 4 weeks. He was playing high school baseball and stated the pain was worse when he swung his bat. He had no history of trauma or back pain. Rest was the only alleviating factor, and he was beginning to experience pain when he stood after sitting. He denied any radicular pain. On examination, he had midline tenderness along the upper lumbar spine and pain with lumbar spine extension. His neurologic examination showed normal sensation with 5/5 strength in all key muscle groups. Plain radiograph of the lumbar spine showed an L5 pars defect (Figures 1A, 1B). A SPECT scan showed increased uptake at L2 pars bilaterally; the L5 pars did not show increased uptake (Figures 2A, 2B). A computed tomography (CT) scan confirmed bilateral L2 pars fractures and a left L5 pars fracture (Figures 3A, 3B). Bony changes in the form of marginal sclerosis at the L5, but not the L2, pars suggested that the L2 fracture was acute while the L5 fracture was chronic (Figures 4A, 4B).
The patient had conservative management for 6 months in the form of lumbosacral orthosis (LSO), cessation of sports activities, and physical therapy. The patient was initially treated with an LSO brace for 3 months, after which he had physical therapy. At 6 month follow-up, he reported continuing, significant back pain. A repeat CT scan of the lumbar spine showed no interval healing of the bilateral L2 or the unilateral L5 pars fractures. As a result of the patient’s noncontiguous pars fractures, a diagnostic CT-guided block of L2 pars was performed to identify which level was his main pain generator (Figure 5). He reported a brief period of complete pain relief after the L2 pars block. With failure of 6 months’ conservative management and positive SPECT scan and diagnostic block, surgical treatment was recommended. Prior to surgical intervention, magnetic resonance imaging was obtained to rule out pathology (eg, disc degeneration, infection, or tumor) other than the pars defect that could require fusion instead of pars repair (Figures 6A, 6B). Because of the patient’s young age, bilateral L2 pars repair rather than fusion was indicated. After 8 months of persistent back pain, he underwent bilateral L2 pars repair with iliac crest autograft, pedicle screws, and sublaminar hook fixation (Figures 7A, 7B). The patient had an uneventful immediate postoperative course. A 6-month postoperative CT scan showed bridging callus at the L2 pars; however, the left L5 pars fracture was still visible (Figures 8A-8C). Over a 6-month postoperative period, the patient had continued improvement in his back pain, advanced his activity as tolerated without problem, and was allowed to resume his sports activities. At 2-year follow-up, he was playing baseball and basketball, and denied any back pain.
Discussion
Lumbar spondylolysis is commonly seen at the fourth and fifth lumbar vertebrae, and accounts for more than 95% of spondylolysis cases.8 Multiple-level spondylolysis is a relatively rare finding with an incidence varying between 1.2% and 5.6%. The majority of the reported multiple-level cases are adjacent.1-3,6 Adolescents often present with a history of insidious-onset low back pain without radicular symptoms that is exacerbated by activity. Occasionally, an acute injury may elicit the onset of pain. A thorough history with emphasis on pain in relation to activity and sports involvement is beneficial. The patient in the current study was a throwing athlete and presented with 4 weeks of back pain that worsened with activity; he had no history of trauma.
Radiographic assessment using standing anteroposterior, lateral, and oblique radiographs of the thoracolumbar spine is useful in the initial assessment. A SPECT scan of the lumbosacral spine is highly sensitive for identifying spondylolytic defects when plain radiographs are within normal limits, yet a high index of suspicion remains given the patient’s history and physical examination findings.9,10 Increased radionuclide uptake within the pars indicates a stress reaction and, possibly, a more acute pathology. The plain radiographs of the patient showed only L5 spondylolysis. However, a SPECT scan showed only increased uptake in L2 pars on both sides. These findings suggested chronic L5 and acute L2 pars defects. A thin-cut CT scan gives the best visualization of pars defect and can help in differentiating chronic defect with sclerotic margins versus acute defect with hazy irregular margins. In the current case, the CT scan showed changes consistent with unilateral chronic L5 and bilateral acute L2 pars defects.
The origin of the pain in spondylolysis is from the tissues rich in nociceptive nerve endings in the loose posterior arch. A CT-guided pars block is a very useful diagnostic preoperative tool that confirms the symptomatic level in cases of multilevel pars defect, especially if they are noncontiguous. In this case, the diagnostic preoperative bilateral L2 pars block confirmed that the pain generator was the acute L2 rather than the chronic L5 pars defect. This step assured that surgical treatment involving only the L2 level would be beneficial in alleviating the patient’s back pain after the failure of 6 months of conservative treatment.
Most patients with single-level spondylolysis respond to conservative treatment, especially after early diagnosis and treatment. The traditional nonoperative treatment of children and adolescents with a symptomatic spondylolytic lesion was a period of rest and progressive increased activity with physical therapy. Immobilization with an LSO was reserved for individuals who did not respond to rest and physical therapy.11 However, multiple studies revealed early immobilization achieved results superior to activity restriction alone, and individuals who underwent a period of activity restriction prior to bracing were more likely to experience persistent symptoms.12-14 Our patient underwent conservative treatment for 6 months, in the form of LSO, cessation of sport activities, and physical therapy, which failed to give him relief of his back pain.
Surgical intervention is warranted for adolescents with persistent, debilitating pain intractable to at least a 6-month period of nonoperative management. Additional indications for surgical management are those individuals who present with neurologic deficits and isthmic spondylolisthesis. Surgical treatment involves direct pars repair with iliac crest bone graft and use of a sublaminar hook/pedicle screw construct, cerclage wire, or pars screw.15-18
In contrast to single-level pars defects that respond well to conservative treatment, there are conflicting reports regarding the management of multiple-level pars fractures; a few reports suggest good outcome with conservative management, but the majority state that surgery is often required and conservative measures are rarely useful.1-4,6 Nayeemuddin and colleagues19 reported a case of a 16-year-old football player who presented with a 4-month history of constant low back pain related to bilateral L3 and L5 pars defects that responded to 1 year of conservative management, when the more acute fractures at L3 showed complete bony union and the patient had symptomatic pain relief and was able to return to full sporting activity.
Chang and colleagues2 reported 10 patients with adjacent 2-level bilateral spondylolysis treated successfully using a pedicle screw–hook construct with autogenous bone grafting. Ogawa and colleagues5 reported adjacent 2-level spondylolysis in 5 patients and 3-level spondylolysis in 2 patients, who were treated successfully by segmental wire fixation and bone grafting. Ivanic and colleagues15 retrospectively reviewed 113 patients with spondylolysis who were treated with direct repair using a hook-screw construct and showed a pseudoarthrosis rate of 13.3%. Superior fusion rates were observed in patients 14 years and younger compared with older patients, particularly those 20 years and older.15 Roca and colleagues16 prospectively analyzed 19 consecutive cases of spondylolysis that were repaired using a hook-screw construct. Twelve of 13 patients (92%) who were 20 years or younger at the time of the study (average age, 17.2 years) had fusion, whereas, in 6 patients 21 years and older (average age, 27.5 years), no cases of fusion were observed. The patients 20 years or younger had significantly better clinical results than those obtained in the patients 21 years and older. The authors concluded that pedicle screw–hook fixation is a useful alternative in the treatment of spondylolysis in adolescents, but did not recommend this procedure in patients older than 20 years.16
Conclusion
The current case demonstrates a unique example of rare noncontiguous pars defects successfully treated with primary repair of 1 level when conservative management failed and the symptomatic defect was isolated. It also highlights the importance of investigating the entirety of the lumbar spine when diagnosis of L5 spondylolysis rules out noncontiguous pars defects. The treatment of noncontiguous pars defects is not well defined; this case showed the importance of using a SPECT scan and a diagnostic pars block to help isolate the symptomatic level when surgical management is considered after a failure of conservative treatment. This case shows 2 possible results: the chronic unilateral L5 defect responded to nonsurgical treatment with asymptomatic fibrous nonunion, while the more acute bilateral L2 defect responded to pars repair with pedicle screw–hook fixation and iliac crest bone graft.
Spondylolysis is a bone defect of the pars interarticularis. It is usually seen in adolescents who participate in sporting activities that involve repetitive axial loads to a hyperextended lower back, such as football offensive lineman, throwing athletes, and gymnasts. It occurs frequently in the L5 pars and can be unilateral or bilateral. The majority of reported multiple-level spondylolysis is at consecutive lumbar segments.1-6 Rarely, it affects noncontiguous levels. Most patients respond well to conservative treatment in the form of activity modification and orthosis.7 Surgical intervention is considered if 6 months of conservative management fails, spondylolisthesis develops, or intractable neurologic symptoms arise.
This case report presents an 18-year-old man with noncontiguous spondylolysis at L2 and L5 who was successfully treated with a 1-level pars repair at L2 after failed conservative management. This unique case highlights the importance of using single-photon emission computed tomography (SPECT) scan and diagnostic pars block when planning for surgical treatment in the rare cases of noncontiguous spondylolysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented to the clinic with worsening lower back pain for the previous 4 weeks. He was playing high school baseball and stated the pain was worse when he swung his bat. He had no history of trauma or back pain. Rest was the only alleviating factor, and he was beginning to experience pain when he stood after sitting. He denied any radicular pain. On examination, he had midline tenderness along the upper lumbar spine and pain with lumbar spine extension. His neurologic examination showed normal sensation with 5/5 strength in all key muscle groups. Plain radiograph of the lumbar spine showed an L5 pars defect (Figures 1A, 1B). A SPECT scan showed increased uptake at L2 pars bilaterally; the L5 pars did not show increased uptake (Figures 2A, 2B). A computed tomography (CT) scan confirmed bilateral L2 pars fractures and a left L5 pars fracture (Figures 3A, 3B). Bony changes in the form of marginal sclerosis at the L5, but not the L2, pars suggested that the L2 fracture was acute while the L5 fracture was chronic (Figures 4A, 4B).
The patient had conservative management for 6 months in the form of lumbosacral orthosis (LSO), cessation of sports activities, and physical therapy. The patient was initially treated with an LSO brace for 3 months, after which he had physical therapy. At 6 month follow-up, he reported continuing, significant back pain. A repeat CT scan of the lumbar spine showed no interval healing of the bilateral L2 or the unilateral L5 pars fractures. As a result of the patient’s noncontiguous pars fractures, a diagnostic CT-guided block of L2 pars was performed to identify which level was his main pain generator (Figure 5). He reported a brief period of complete pain relief after the L2 pars block. With failure of 6 months’ conservative management and positive SPECT scan and diagnostic block, surgical treatment was recommended. Prior to surgical intervention, magnetic resonance imaging was obtained to rule out pathology (eg, disc degeneration, infection, or tumor) other than the pars defect that could require fusion instead of pars repair (Figures 6A, 6B). Because of the patient’s young age, bilateral L2 pars repair rather than fusion was indicated. After 8 months of persistent back pain, he underwent bilateral L2 pars repair with iliac crest autograft, pedicle screws, and sublaminar hook fixation (Figures 7A, 7B). The patient had an uneventful immediate postoperative course. A 6-month postoperative CT scan showed bridging callus at the L2 pars; however, the left L5 pars fracture was still visible (Figures 8A-8C). Over a 6-month postoperative period, the patient had continued improvement in his back pain, advanced his activity as tolerated without problem, and was allowed to resume his sports activities. At 2-year follow-up, he was playing baseball and basketball, and denied any back pain.
Discussion
Lumbar spondylolysis is commonly seen at the fourth and fifth lumbar vertebrae, and accounts for more than 95% of spondylolysis cases.8 Multiple-level spondylolysis is a relatively rare finding with an incidence varying between 1.2% and 5.6%. The majority of the reported multiple-level cases are adjacent.1-3,6 Adolescents often present with a history of insidious-onset low back pain without radicular symptoms that is exacerbated by activity. Occasionally, an acute injury may elicit the onset of pain. A thorough history with emphasis on pain in relation to activity and sports involvement is beneficial. The patient in the current study was a throwing athlete and presented with 4 weeks of back pain that worsened with activity; he had no history of trauma.
Radiographic assessment using standing anteroposterior, lateral, and oblique radiographs of the thoracolumbar spine is useful in the initial assessment. A SPECT scan of the lumbosacral spine is highly sensitive for identifying spondylolytic defects when plain radiographs are within normal limits, yet a high index of suspicion remains given the patient’s history and physical examination findings.9,10 Increased radionuclide uptake within the pars indicates a stress reaction and, possibly, a more acute pathology. The plain radiographs of the patient showed only L5 spondylolysis. However, a SPECT scan showed only increased uptake in L2 pars on both sides. These findings suggested chronic L5 and acute L2 pars defects. A thin-cut CT scan gives the best visualization of pars defect and can help in differentiating chronic defect with sclerotic margins versus acute defect with hazy irregular margins. In the current case, the CT scan showed changes consistent with unilateral chronic L5 and bilateral acute L2 pars defects.
The origin of the pain in spondylolysis is from the tissues rich in nociceptive nerve endings in the loose posterior arch. A CT-guided pars block is a very useful diagnostic preoperative tool that confirms the symptomatic level in cases of multilevel pars defect, especially if they are noncontiguous. In this case, the diagnostic preoperative bilateral L2 pars block confirmed that the pain generator was the acute L2 rather than the chronic L5 pars defect. This step assured that surgical treatment involving only the L2 level would be beneficial in alleviating the patient’s back pain after the failure of 6 months of conservative treatment.
Most patients with single-level spondylolysis respond to conservative treatment, especially after early diagnosis and treatment. The traditional nonoperative treatment of children and adolescents with a symptomatic spondylolytic lesion was a period of rest and progressive increased activity with physical therapy. Immobilization with an LSO was reserved for individuals who did not respond to rest and physical therapy.11 However, multiple studies revealed early immobilization achieved results superior to activity restriction alone, and individuals who underwent a period of activity restriction prior to bracing were more likely to experience persistent symptoms.12-14 Our patient underwent conservative treatment for 6 months, in the form of LSO, cessation of sport activities, and physical therapy, which failed to give him relief of his back pain.
Surgical intervention is warranted for adolescents with persistent, debilitating pain intractable to at least a 6-month period of nonoperative management. Additional indications for surgical management are those individuals who present with neurologic deficits and isthmic spondylolisthesis. Surgical treatment involves direct pars repair with iliac crest bone graft and use of a sublaminar hook/pedicle screw construct, cerclage wire, or pars screw.15-18
In contrast to single-level pars defects that respond well to conservative treatment, there are conflicting reports regarding the management of multiple-level pars fractures; a few reports suggest good outcome with conservative management, but the majority state that surgery is often required and conservative measures are rarely useful.1-4,6 Nayeemuddin and colleagues19 reported a case of a 16-year-old football player who presented with a 4-month history of constant low back pain related to bilateral L3 and L5 pars defects that responded to 1 year of conservative management, when the more acute fractures at L3 showed complete bony union and the patient had symptomatic pain relief and was able to return to full sporting activity.
Chang and colleagues2 reported 10 patients with adjacent 2-level bilateral spondylolysis treated successfully using a pedicle screw–hook construct with autogenous bone grafting. Ogawa and colleagues5 reported adjacent 2-level spondylolysis in 5 patients and 3-level spondylolysis in 2 patients, who were treated successfully by segmental wire fixation and bone grafting. Ivanic and colleagues15 retrospectively reviewed 113 patients with spondylolysis who were treated with direct repair using a hook-screw construct and showed a pseudoarthrosis rate of 13.3%. Superior fusion rates were observed in patients 14 years and younger compared with older patients, particularly those 20 years and older.15 Roca and colleagues16 prospectively analyzed 19 consecutive cases of spondylolysis that were repaired using a hook-screw construct. Twelve of 13 patients (92%) who were 20 years or younger at the time of the study (average age, 17.2 years) had fusion, whereas, in 6 patients 21 years and older (average age, 27.5 years), no cases of fusion were observed. The patients 20 years or younger had significantly better clinical results than those obtained in the patients 21 years and older. The authors concluded that pedicle screw–hook fixation is a useful alternative in the treatment of spondylolysis in adolescents, but did not recommend this procedure in patients older than 20 years.16
Conclusion
The current case demonstrates a unique example of rare noncontiguous pars defects successfully treated with primary repair of 1 level when conservative management failed and the symptomatic defect was isolated. It also highlights the importance of investigating the entirety of the lumbar spine when diagnosis of L5 spondylolysis rules out noncontiguous pars defects. The treatment of noncontiguous pars defects is not well defined; this case showed the importance of using a SPECT scan and a diagnostic pars block to help isolate the symptomatic level when surgical management is considered after a failure of conservative treatment. This case shows 2 possible results: the chronic unilateral L5 defect responded to nonsurgical treatment with asymptomatic fibrous nonunion, while the more acute bilateral L2 defect responded to pars repair with pedicle screw–hook fixation and iliac crest bone graft.
1. Al-Sebai MW, Al-Khawashki H. Spondyloptosis and multiple-level spondylolysis. Eur Spine J. 1999;8(1):75-77.
2. Chang JH, Lee CH, Wu SS, Lin LC, et al. Management of multiple level spondylolysis of the lumbar spine in young males: a report of six cases. J Formos Med Assoc. 2001;100(7)2:497-502.
3. Eingorn D, Pizzutillo PD. Pars interarticularis fusion of multiple levels of lumbar spondylolysis. A case report. Spine. 1985;10(3):250-252.
4. Nozawa S, Shimizu K, Miyamoto K, Tanaka M. Repair of pars interarticularis defect by segmental wire fixation in young athletes with spondylolysis. Am J Sports Med. 2003;31(3):359-364.
5. Ogawa H, Nishimoto H, Hosoe H, Suzuki N, Kanamori Y, Shimizu K. Clinical outcome after segmental wire fixation and bone grafting for repair of the defects in multiple level lumbar spondylolysis. J Spinal Disord Tech. 2007;20(7):521-525.
6. Ravichandran G. Multiple lumbar spondylolyses. Spine. 1980;5(6):552-557.
7. Sys J, Michielsen J, Bracke P, Martens M, Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10(6):498-504.
8. Saraste H. Spondylolysis and spondylolisthesis. Acta Orthop Scand Suppl. 1993;251:84-86.
9. Anderson K, Sarwark JF, Conway JJ, Logue ES, Schafer MS. Quantitative assessment with SPECT imaging of stress injuries of the pars interarticularis and response to bracing. J Pediatr Orthop. 2000;20(1):28-33.
10. Bodner RJ, Heyman S, Drummond DS, Gregg JR. The use of single photon emission computed tomography (SPECT) in the diagnosis of low-back pain in young patients. Spine. 1988;13(10):1155-1160.
11. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
12. Blanda J, Bethem D, Moats W, Lew M. Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. J Spinal Disord. 1993;6(5):406-411.
13. Kurd MF, Patel D, Norton R, Picetti G, Friel B, Vaccaro AR. Nonoperative treatment of symptomatic spondylolysis. J Spinal Disord Tech. 2007;20(8):560-564.
14. Pizzutillo PD, Hummer CD 3rd. Nonoperative treatment for painful adolescent spondylolysis or spondylolisthesis. J Pediatr Orthop. 1989;9(5):538-540.
15. Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook screw: mean 11-year follow-up period for 113 patients. Spine. 2003;28(3):255-259.
16. Roca J, Iborra M, Cavanilles-Walker JM, Alberti G. Direct repair of spondylolysis using a new pedicle screw hook fixation: clinical and CT-assessed study: an analysis of 19 patients. J Spinal Disord Tech. 2005;18(suppl):S82-S89.
17. Schlenzka D, Remes V, Helenius I, et al. Direct repair for treatment of symptomatic spondylolysis and low-grade isthmic spondylolisthesis in young patients: no benefit in comparison to segmental fusion after a mean follow-up of 14.8 years. Eur Spine J. 2006;15(10):1437-1447.
18. Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br. 1970;52(3):432-437.
19. Nayeemuddin M, Richards PJ, Ahmed EB. The imaging and management of nonconsecutive pars interarticularis defects: a case report and review of literature. Spine J. 2011;11(12):1157-1163.
1. Al-Sebai MW, Al-Khawashki H. Spondyloptosis and multiple-level spondylolysis. Eur Spine J. 1999;8(1):75-77.
2. Chang JH, Lee CH, Wu SS, Lin LC, et al. Management of multiple level spondylolysis of the lumbar spine in young males: a report of six cases. J Formos Med Assoc. 2001;100(7)2:497-502.
3. Eingorn D, Pizzutillo PD. Pars interarticularis fusion of multiple levels of lumbar spondylolysis. A case report. Spine. 1985;10(3):250-252.
4. Nozawa S, Shimizu K, Miyamoto K, Tanaka M. Repair of pars interarticularis defect by segmental wire fixation in young athletes with spondylolysis. Am J Sports Med. 2003;31(3):359-364.
5. Ogawa H, Nishimoto H, Hosoe H, Suzuki N, Kanamori Y, Shimizu K. Clinical outcome after segmental wire fixation and bone grafting for repair of the defects in multiple level lumbar spondylolysis. J Spinal Disord Tech. 2007;20(7):521-525.
6. Ravichandran G. Multiple lumbar spondylolyses. Spine. 1980;5(6):552-557.
7. Sys J, Michielsen J, Bracke P, Martens M, Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10(6):498-504.
8. Saraste H. Spondylolysis and spondylolisthesis. Acta Orthop Scand Suppl. 1993;251:84-86.
9. Anderson K, Sarwark JF, Conway JJ, Logue ES, Schafer MS. Quantitative assessment with SPECT imaging of stress injuries of the pars interarticularis and response to bracing. J Pediatr Orthop. 2000;20(1):28-33.
10. Bodner RJ, Heyman S, Drummond DS, Gregg JR. The use of single photon emission computed tomography (SPECT) in the diagnosis of low-back pain in young patients. Spine. 1988;13(10):1155-1160.
11. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
12. Blanda J, Bethem D, Moats W, Lew M. Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. J Spinal Disord. 1993;6(5):406-411.
13. Kurd MF, Patel D, Norton R, Picetti G, Friel B, Vaccaro AR. Nonoperative treatment of symptomatic spondylolysis. J Spinal Disord Tech. 2007;20(8):560-564.
14. Pizzutillo PD, Hummer CD 3rd. Nonoperative treatment for painful adolescent spondylolysis or spondylolisthesis. J Pediatr Orthop. 1989;9(5):538-540.
15. Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook screw: mean 11-year follow-up period for 113 patients. Spine. 2003;28(3):255-259.
16. Roca J, Iborra M, Cavanilles-Walker JM, Alberti G. Direct repair of spondylolysis using a new pedicle screw hook fixation: clinical and CT-assessed study: an analysis of 19 patients. J Spinal Disord Tech. 2005;18(suppl):S82-S89.
17. Schlenzka D, Remes V, Helenius I, et al. Direct repair for treatment of symptomatic spondylolysis and low-grade isthmic spondylolisthesis in young patients: no benefit in comparison to segmental fusion after a mean follow-up of 14.8 years. Eur Spine J. 2006;15(10):1437-1447.
18. Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br. 1970;52(3):432-437.
19. Nayeemuddin M, Richards PJ, Ahmed EB. The imaging and management of nonconsecutive pars interarticularis defects: a case report and review of literature. Spine J. 2011;11(12):1157-1163.
Surgical Management of Gorham-Stout Disease of the Pelvis Refractory to Medical and Radiation Therapy
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
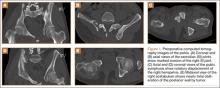
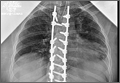
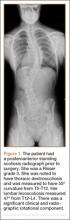
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
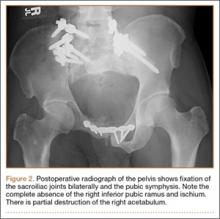
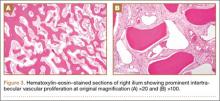
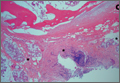
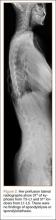
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
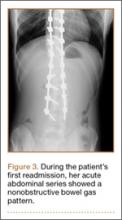
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Posterior Reversible Encephalopathy Syndrome: Temporary Visual Loss After Spinal Deformity Surgery
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
Medicaid Insurance Is Associated With Larger Curves in Patients Who Require Scoliosis Surgery
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
Excision of Symptomatic Spinous Process Nonunion in Adolescent Athletes
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
Characteristics Associated With Active Defects in Juvenile Spondylolysis
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Significant Differences in Health Care Costs for Spine Surgery
Costs for spinal fusion surgery in the United States vary substantially by region, with costs being lowest in the Midwest and highest in the Northeast, according to a study published in the September 1 issue of Spine.
The researchers analyzed 2012 Medicare data on the costs of anterior cervical discectomy and fusion (ACDF) and posterior lumbar fusion (PLF). For comparison, the costs of total knee arthroplasty (TKA) also were assessed. The analysis focused on direct costs, defined as the amount reimbursed to health care providers by Medicare or other payers. Most previous economic analyses of spinal surgery have focused on the amount billed by providers to payers.
Average national costs were about $14,000 for a single-level ACDF procedure and $26,000 for a single-level PLF. These total figures reflected combined professional and facility costs. Average cost for TKA was about $13,000, increasing to $22,000 for TKA in patients with other accompanying major medical conditions.
“Each procedure had a significant range in cost across the country,” said William Ryan Spiker, MD, Assistant Professor at the University of Utah in Salt Lake City. “This data sheds light on the actual cost of common surgeries throughout the United States, and will allow further progress towards the development of cost effective, value driven care.”
Costs for ACDF ranged from about $11,000 to $25,000, while PLF costs ranged from $20,000 to $37,000. For TKA patients without major medical conditions, the range was from about $11,000 to $19,000.
All procedures except ACDF also showed significant variations on the regional level, with the lowest costs in the Midwest and highest costs in the Northeast. For PLF, costs ranged from $24,000 in the Midwest to $28,000 in the Northeast. The figures were $12,000 versus $14,000 for primary TKA, and $21,000 versus $25,000 for TKA with major medical conditions.
On the state level, total costs for all 4 procedures were significantly correlated with the state’s cost of living index, but not with state population.
Spinal fusion procedures such as ACDF and PLF are a major source of costs for Medicare and other payers. From 2001 to 2010, an estimated 3.6 million spinal fusions were performed in the United States, with total charges of more than $287 billion.
The study does not show what’s behind the variations in cost, although state cost-of-living index is one related factor. Dr. Spiker and coauthors said, “In the pursuit of cost optimization, and the broader pursuit of value driven health care, it may prove valuable to study the factors that allow these states to deliver care at a lower cost.”
Suggested Reading
Goz V, Rane A, Abtahi AM, et al. Geographic variations in the cost of spine surgery. Spine (Phila Pa 1976). 2015;40(17):1380-1389.
Costs for spinal fusion surgery in the United States vary substantially by region, with costs being lowest in the Midwest and highest in the Northeast, according to a study published in the September 1 issue of Spine.
The researchers analyzed 2012 Medicare data on the costs of anterior cervical discectomy and fusion (ACDF) and posterior lumbar fusion (PLF). For comparison, the costs of total knee arthroplasty (TKA) also were assessed. The analysis focused on direct costs, defined as the amount reimbursed to health care providers by Medicare or other payers. Most previous economic analyses of spinal surgery have focused on the amount billed by providers to payers.
Average national costs were about $14,000 for a single-level ACDF procedure and $26,000 for a single-level PLF. These total figures reflected combined professional and facility costs. Average cost for TKA was about $13,000, increasing to $22,000 for TKA in patients with other accompanying major medical conditions.
“Each procedure had a significant range in cost across the country,” said William Ryan Spiker, MD, Assistant Professor at the University of Utah in Salt Lake City. “This data sheds light on the actual cost of common surgeries throughout the United States, and will allow further progress towards the development of cost effective, value driven care.”
Costs for ACDF ranged from about $11,000 to $25,000, while PLF costs ranged from $20,000 to $37,000. For TKA patients without major medical conditions, the range was from about $11,000 to $19,000.
All procedures except ACDF also showed significant variations on the regional level, with the lowest costs in the Midwest and highest costs in the Northeast. For PLF, costs ranged from $24,000 in the Midwest to $28,000 in the Northeast. The figures were $12,000 versus $14,000 for primary TKA, and $21,000 versus $25,000 for TKA with major medical conditions.
On the state level, total costs for all 4 procedures were significantly correlated with the state’s cost of living index, but not with state population.
Spinal fusion procedures such as ACDF and PLF are a major source of costs for Medicare and other payers. From 2001 to 2010, an estimated 3.6 million spinal fusions were performed in the United States, with total charges of more than $287 billion.
The study does not show what’s behind the variations in cost, although state cost-of-living index is one related factor. Dr. Spiker and coauthors said, “In the pursuit of cost optimization, and the broader pursuit of value driven health care, it may prove valuable to study the factors that allow these states to deliver care at a lower cost.”
Costs for spinal fusion surgery in the United States vary substantially by region, with costs being lowest in the Midwest and highest in the Northeast, according to a study published in the September 1 issue of Spine.
The researchers analyzed 2012 Medicare data on the costs of anterior cervical discectomy and fusion (ACDF) and posterior lumbar fusion (PLF). For comparison, the costs of total knee arthroplasty (TKA) also were assessed. The analysis focused on direct costs, defined as the amount reimbursed to health care providers by Medicare or other payers. Most previous economic analyses of spinal surgery have focused on the amount billed by providers to payers.
Average national costs were about $14,000 for a single-level ACDF procedure and $26,000 for a single-level PLF. These total figures reflected combined professional and facility costs. Average cost for TKA was about $13,000, increasing to $22,000 for TKA in patients with other accompanying major medical conditions.
“Each procedure had a significant range in cost across the country,” said William Ryan Spiker, MD, Assistant Professor at the University of Utah in Salt Lake City. “This data sheds light on the actual cost of common surgeries throughout the United States, and will allow further progress towards the development of cost effective, value driven care.”
Costs for ACDF ranged from about $11,000 to $25,000, while PLF costs ranged from $20,000 to $37,000. For TKA patients without major medical conditions, the range was from about $11,000 to $19,000.
All procedures except ACDF also showed significant variations on the regional level, with the lowest costs in the Midwest and highest costs in the Northeast. For PLF, costs ranged from $24,000 in the Midwest to $28,000 in the Northeast. The figures were $12,000 versus $14,000 for primary TKA, and $21,000 versus $25,000 for TKA with major medical conditions.
On the state level, total costs for all 4 procedures were significantly correlated with the state’s cost of living index, but not with state population.
Spinal fusion procedures such as ACDF and PLF are a major source of costs for Medicare and other payers. From 2001 to 2010, an estimated 3.6 million spinal fusions were performed in the United States, with total charges of more than $287 billion.
The study does not show what’s behind the variations in cost, although state cost-of-living index is one related factor. Dr. Spiker and coauthors said, “In the pursuit of cost optimization, and the broader pursuit of value driven health care, it may prove valuable to study the factors that allow these states to deliver care at a lower cost.”
Suggested Reading
Goz V, Rane A, Abtahi AM, et al. Geographic variations in the cost of spine surgery. Spine (Phila Pa 1976). 2015;40(17):1380-1389.
Suggested Reading
Goz V, Rane A, Abtahi AM, et al. Geographic variations in the cost of spine surgery. Spine (Phila Pa 1976). 2015;40(17):1380-1389.
Osteochondroma With Contiguous Bronchogenic Cyst of the Scapula
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
A Rare Cause of Postoperative Abdominal Pain in a Spinal Fusion Patient
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.
Mortality Rates Associated With Odontoid and Subaxial Cervical Spine Fractures
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.