User login
VIDEO: Romosozumab bested teriparatide in hip BMD transitioning from bisphosphonates
LONDON – Bone mineral density (BMD) and estimated bone strength in the hip significantly increased during 1 year of treatment with the investigational monoclonal antibody romosozumab in comparison with teriparatide in women with postmenopausal osteoporosis who had previous fracture history and had been taking bisphosphonates for at least the previous 3 years in an open-label, randomized trial.
The investigators in the phase III, international, multicenter STRUCTURE trial sought to compare the monoclonal anti-sclerostin antibody romosozumab, which works dually to increase bone formation and decrease bone resorption, against the bone-forming agent teriparatide (Forteo) to see if it would increase total hip bone mineral density to a significantly greater extent by 12 months. Teriparatide is known to take longer to build bone at the hip following bisphosphonate use.
“These results in our minds suggest that romosozumab may offer a unique benefit to patients at high risk for fracture, transitioning from bisphosphonates,” first author Bente L. Langdahl, Ph.D., of Aarhus (Denmark) University Hospital said at the European Congress of Rheumatology. She discussed the study results in this video interview.
When Dr. Langdahl was asked after her presentation about the foreseen role of the drug in clinical practice, she noted that ongoing phase III trials looking at fracture endpoints should indicate whether it works faster at preventing fractures. Because there does not seem to be a restriction to the repeated use of romosozumab, she suggested it might be given initially for 12 months to build bone, followed by bisphosphonates to stabilize gains made, and then it could be used again if needed in a treat-to-target fashion.
The trial randomized 436 patients to receive either subcutaneous romosozumab 210 mg once monthly or subcutaneous teriparatide 20 mcg once daily for 12 months. All patients received a loading dose of 50,000-60,000 IU vitamin D3 to make sure they were vitamin D replete. The patients were postmenopausal, aged 55-90 years, and required to have taken oral bisphosphonate therapy at a dose equivalent to 70 mg weekly of alendronate for at least 3 years before enrollment (with at least 1 year of alendronate therapy prior to enrollment). They had a T score of –2.5 or less in total hip, lumbar spine, or femoral neck BMD and a history of a nonvertebral fracture after 50 years of age or a vertebral fracture at any age.
At 12 months, the primary endpoint of total hip BMD percentage change from baseline, which was calculated by averaging dual-energy x-ray absorptiometry (DXA) measurements at 6 and 12 months, increased significantly by 2.6% in the romosozumab group, compared with a loss of –1.6% in the teriparatide group.
“Why did we choose this rather unusual endpoint? It’s because we wanted to capture what happened throughout the first year,” Dr. Langdahl said.
The respective changes from baseline total hip BMD at month 12 were 2.9% vs. –0.5%; in the femoral neck, the 12-month change was 3.2% vs. –0.2%; and in the lumbar spine, the change was 9.8% vs. 5.4%.
The trial participants had a mean age of about 71 years, and T scores ranging from –2.87 to –2.83 in the lumbar spine, –2.27 to –2.21 in total hip, and –2.49 to –2.43 in the femoral neck.
Secondary endpoints of volumetric BMD measured by quantitative CT at 12 months supported the results for DXA measurements at the total hip: integral BMD changed 3.4% with romosozumab vs. –0.2% with teriparatide, cortical BMD changed 1.1% with romosozumab vs. –3.6% with teriparatide, and trabecular BMD increased 15.6% vs. 9.9%, respectively. Total hip strength at 12 months as estimated by finite element analysis rose by 2.5% with romosozumab, compared with a loss of –0.7% with teriparatide. The loss in estimated bone strength at the hip occurred mainly in the first 6 months of treatment with teriparatide and had started to recover by 12 months.
The main difference between the two treatments in total hip BMD came from romosozumab’s bone-building effect on cortical bone, Dr. Langdahl said. The significant loss of total hip cortical BMD observed with teriparatide matches what has been seen in other studies, and it occurs “because the way that teriparatide works initially on cortical bone is to open up the remodeling space; therefore, a decrease in BMD is seen.”
While romosozumab’s speed in increasing BMD and building bone strength is likely to be clinically meaningful for patients at high risk of fracture, the short duration of the trial likely left out the possibility of seeing the same sort of late increases in hip BMD and bone strength during the second year of treatment with teriparatide that have been observed in other studies. Dr. Langdahl suggested that the decline in total hip cortical bone volumetric BMD may have increased had there been more time for measurement in the study; after bisphosphonate treatment, teriparatide’s mechanism of action is thought to open up the bone remodeling space by stimulating bone resorption and formation, particularly in cortical bone.
“You will see an increased porosity in the cortical bone, and I think that is what explains the initial decrease. You’ll also see that it’s kind of catching up [to romosozumab]. This is only a 12-month study, unfortunately. I’m sure if it had been continued for 24 months, there would be an increase in strength because that has been demonstrated in other studies.”
The trial’s comparison of romosozumab versus teriparatide alone also leaves open the question of whether adding teriparatide to bisphosphonate or switching to a combination of teriparatide plus denosumab or teriparatide plus zoledronic acid might have yielded results comparable with romosozumab alone.
Furthermore, The study is not large enough to determine the overall side-effect profile of romosozumab and will have to wait until the larger, ongoing phase III studies are completed, Dr. Langdahl said. In the STRUCTURE study, the overall side effect profile was well balanced between the two groups. More women who received teriparatide developed hypercalcemia, and patients who received romosozumab developed antibodies, although they did not neutralize the agent’s effects.
The study was sponsored by UCB Pharma and Amgen. One investigator is an employee of UCB, and one is an employee of Amgen. Dr. Langdahl reported receiving research grants, consulting fees, and/or honoraria from Amgen, UCB, Eli Lilly, Merck, and Orkla. Most other investigators also reported financial relationships to Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Bone mineral density (BMD) and estimated bone strength in the hip significantly increased during 1 year of treatment with the investigational monoclonal antibody romosozumab in comparison with teriparatide in women with postmenopausal osteoporosis who had previous fracture history and had been taking bisphosphonates for at least the previous 3 years in an open-label, randomized trial.
The investigators in the phase III, international, multicenter STRUCTURE trial sought to compare the monoclonal anti-sclerostin antibody romosozumab, which works dually to increase bone formation and decrease bone resorption, against the bone-forming agent teriparatide (Forteo) to see if it would increase total hip bone mineral density to a significantly greater extent by 12 months. Teriparatide is known to take longer to build bone at the hip following bisphosphonate use.
“These results in our minds suggest that romosozumab may offer a unique benefit to patients at high risk for fracture, transitioning from bisphosphonates,” first author Bente L. Langdahl, Ph.D., of Aarhus (Denmark) University Hospital said at the European Congress of Rheumatology. She discussed the study results in this video interview.
When Dr. Langdahl was asked after her presentation about the foreseen role of the drug in clinical practice, she noted that ongoing phase III trials looking at fracture endpoints should indicate whether it works faster at preventing fractures. Because there does not seem to be a restriction to the repeated use of romosozumab, she suggested it might be given initially for 12 months to build bone, followed by bisphosphonates to stabilize gains made, and then it could be used again if needed in a treat-to-target fashion.
The trial randomized 436 patients to receive either subcutaneous romosozumab 210 mg once monthly or subcutaneous teriparatide 20 mcg once daily for 12 months. All patients received a loading dose of 50,000-60,000 IU vitamin D3 to make sure they were vitamin D replete. The patients were postmenopausal, aged 55-90 years, and required to have taken oral bisphosphonate therapy at a dose equivalent to 70 mg weekly of alendronate for at least 3 years before enrollment (with at least 1 year of alendronate therapy prior to enrollment). They had a T score of –2.5 or less in total hip, lumbar spine, or femoral neck BMD and a history of a nonvertebral fracture after 50 years of age or a vertebral fracture at any age.
At 12 months, the primary endpoint of total hip BMD percentage change from baseline, which was calculated by averaging dual-energy x-ray absorptiometry (DXA) measurements at 6 and 12 months, increased significantly by 2.6% in the romosozumab group, compared with a loss of –1.6% in the teriparatide group.
“Why did we choose this rather unusual endpoint? It’s because we wanted to capture what happened throughout the first year,” Dr. Langdahl said.
The respective changes from baseline total hip BMD at month 12 were 2.9% vs. –0.5%; in the femoral neck, the 12-month change was 3.2% vs. –0.2%; and in the lumbar spine, the change was 9.8% vs. 5.4%.
The trial participants had a mean age of about 71 years, and T scores ranging from –2.87 to –2.83 in the lumbar spine, –2.27 to –2.21 in total hip, and –2.49 to –2.43 in the femoral neck.
Secondary endpoints of volumetric BMD measured by quantitative CT at 12 months supported the results for DXA measurements at the total hip: integral BMD changed 3.4% with romosozumab vs. –0.2% with teriparatide, cortical BMD changed 1.1% with romosozumab vs. –3.6% with teriparatide, and trabecular BMD increased 15.6% vs. 9.9%, respectively. Total hip strength at 12 months as estimated by finite element analysis rose by 2.5% with romosozumab, compared with a loss of –0.7% with teriparatide. The loss in estimated bone strength at the hip occurred mainly in the first 6 months of treatment with teriparatide and had started to recover by 12 months.
The main difference between the two treatments in total hip BMD came from romosozumab’s bone-building effect on cortical bone, Dr. Langdahl said. The significant loss of total hip cortical BMD observed with teriparatide matches what has been seen in other studies, and it occurs “because the way that teriparatide works initially on cortical bone is to open up the remodeling space; therefore, a decrease in BMD is seen.”
While romosozumab’s speed in increasing BMD and building bone strength is likely to be clinically meaningful for patients at high risk of fracture, the short duration of the trial likely left out the possibility of seeing the same sort of late increases in hip BMD and bone strength during the second year of treatment with teriparatide that have been observed in other studies. Dr. Langdahl suggested that the decline in total hip cortical bone volumetric BMD may have increased had there been more time for measurement in the study; after bisphosphonate treatment, teriparatide’s mechanism of action is thought to open up the bone remodeling space by stimulating bone resorption and formation, particularly in cortical bone.
“You will see an increased porosity in the cortical bone, and I think that is what explains the initial decrease. You’ll also see that it’s kind of catching up [to romosozumab]. This is only a 12-month study, unfortunately. I’m sure if it had been continued for 24 months, there would be an increase in strength because that has been demonstrated in other studies.”
The trial’s comparison of romosozumab versus teriparatide alone also leaves open the question of whether adding teriparatide to bisphosphonate or switching to a combination of teriparatide plus denosumab or teriparatide plus zoledronic acid might have yielded results comparable with romosozumab alone.
Furthermore, The study is not large enough to determine the overall side-effect profile of romosozumab and will have to wait until the larger, ongoing phase III studies are completed, Dr. Langdahl said. In the STRUCTURE study, the overall side effect profile was well balanced between the two groups. More women who received teriparatide developed hypercalcemia, and patients who received romosozumab developed antibodies, although they did not neutralize the agent’s effects.
The study was sponsored by UCB Pharma and Amgen. One investigator is an employee of UCB, and one is an employee of Amgen. Dr. Langdahl reported receiving research grants, consulting fees, and/or honoraria from Amgen, UCB, Eli Lilly, Merck, and Orkla. Most other investigators also reported financial relationships to Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Bone mineral density (BMD) and estimated bone strength in the hip significantly increased during 1 year of treatment with the investigational monoclonal antibody romosozumab in comparison with teriparatide in women with postmenopausal osteoporosis who had previous fracture history and had been taking bisphosphonates for at least the previous 3 years in an open-label, randomized trial.
The investigators in the phase III, international, multicenter STRUCTURE trial sought to compare the monoclonal anti-sclerostin antibody romosozumab, which works dually to increase bone formation and decrease bone resorption, against the bone-forming agent teriparatide (Forteo) to see if it would increase total hip bone mineral density to a significantly greater extent by 12 months. Teriparatide is known to take longer to build bone at the hip following bisphosphonate use.
“These results in our minds suggest that romosozumab may offer a unique benefit to patients at high risk for fracture, transitioning from bisphosphonates,” first author Bente L. Langdahl, Ph.D., of Aarhus (Denmark) University Hospital said at the European Congress of Rheumatology. She discussed the study results in this video interview.
When Dr. Langdahl was asked after her presentation about the foreseen role of the drug in clinical practice, she noted that ongoing phase III trials looking at fracture endpoints should indicate whether it works faster at preventing fractures. Because there does not seem to be a restriction to the repeated use of romosozumab, she suggested it might be given initially for 12 months to build bone, followed by bisphosphonates to stabilize gains made, and then it could be used again if needed in a treat-to-target fashion.
The trial randomized 436 patients to receive either subcutaneous romosozumab 210 mg once monthly or subcutaneous teriparatide 20 mcg once daily for 12 months. All patients received a loading dose of 50,000-60,000 IU vitamin D3 to make sure they were vitamin D replete. The patients were postmenopausal, aged 55-90 years, and required to have taken oral bisphosphonate therapy at a dose equivalent to 70 mg weekly of alendronate for at least 3 years before enrollment (with at least 1 year of alendronate therapy prior to enrollment). They had a T score of –2.5 or less in total hip, lumbar spine, or femoral neck BMD and a history of a nonvertebral fracture after 50 years of age or a vertebral fracture at any age.
At 12 months, the primary endpoint of total hip BMD percentage change from baseline, which was calculated by averaging dual-energy x-ray absorptiometry (DXA) measurements at 6 and 12 months, increased significantly by 2.6% in the romosozumab group, compared with a loss of –1.6% in the teriparatide group.
“Why did we choose this rather unusual endpoint? It’s because we wanted to capture what happened throughout the first year,” Dr. Langdahl said.
The respective changes from baseline total hip BMD at month 12 were 2.9% vs. –0.5%; in the femoral neck, the 12-month change was 3.2% vs. –0.2%; and in the lumbar spine, the change was 9.8% vs. 5.4%.
The trial participants had a mean age of about 71 years, and T scores ranging from –2.87 to –2.83 in the lumbar spine, –2.27 to –2.21 in total hip, and –2.49 to –2.43 in the femoral neck.
Secondary endpoints of volumetric BMD measured by quantitative CT at 12 months supported the results for DXA measurements at the total hip: integral BMD changed 3.4% with romosozumab vs. –0.2% with teriparatide, cortical BMD changed 1.1% with romosozumab vs. –3.6% with teriparatide, and trabecular BMD increased 15.6% vs. 9.9%, respectively. Total hip strength at 12 months as estimated by finite element analysis rose by 2.5% with romosozumab, compared with a loss of –0.7% with teriparatide. The loss in estimated bone strength at the hip occurred mainly in the first 6 months of treatment with teriparatide and had started to recover by 12 months.
The main difference between the two treatments in total hip BMD came from romosozumab’s bone-building effect on cortical bone, Dr. Langdahl said. The significant loss of total hip cortical BMD observed with teriparatide matches what has been seen in other studies, and it occurs “because the way that teriparatide works initially on cortical bone is to open up the remodeling space; therefore, a decrease in BMD is seen.”
While romosozumab’s speed in increasing BMD and building bone strength is likely to be clinically meaningful for patients at high risk of fracture, the short duration of the trial likely left out the possibility of seeing the same sort of late increases in hip BMD and bone strength during the second year of treatment with teriparatide that have been observed in other studies. Dr. Langdahl suggested that the decline in total hip cortical bone volumetric BMD may have increased had there been more time for measurement in the study; after bisphosphonate treatment, teriparatide’s mechanism of action is thought to open up the bone remodeling space by stimulating bone resorption and formation, particularly in cortical bone.
“You will see an increased porosity in the cortical bone, and I think that is what explains the initial decrease. You’ll also see that it’s kind of catching up [to romosozumab]. This is only a 12-month study, unfortunately. I’m sure if it had been continued for 24 months, there would be an increase in strength because that has been demonstrated in other studies.”
The trial’s comparison of romosozumab versus teriparatide alone also leaves open the question of whether adding teriparatide to bisphosphonate or switching to a combination of teriparatide plus denosumab or teriparatide plus zoledronic acid might have yielded results comparable with romosozumab alone.
Furthermore, The study is not large enough to determine the overall side-effect profile of romosozumab and will have to wait until the larger, ongoing phase III studies are completed, Dr. Langdahl said. In the STRUCTURE study, the overall side effect profile was well balanced between the two groups. More women who received teriparatide developed hypercalcemia, and patients who received romosozumab developed antibodies, although they did not neutralize the agent’s effects.
The study was sponsored by UCB Pharma and Amgen. One investigator is an employee of UCB, and one is an employee of Amgen. Dr. Langdahl reported receiving research grants, consulting fees, and/or honoraria from Amgen, UCB, Eli Lilly, Merck, and Orkla. Most other investigators also reported financial relationships to Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2016 CONGRESS
Key clinical point: Romosozumab raised total hip BMD to a significantly greater extent than did teriparatide after 12 months in postmenopausal women with a history of fracture who were transitioning from bisphosphonate therapy.
Major finding: At 12 months, the primary endpoint of total hip BMD percentage change from baseline averaged from dual x-ray absorptiometry measurements at 6 and 12 months increased significantly by 2.6% in the romosozumab group, compared with –1.6% in the teriparatide group.
Data source: The multicenter, randomized, open-label STRUCTURE trial of romosozumab vs. teriparatide in postmenopausal women who had a previous history of nonvertebral or vertebral fracture and 3 or more previous years of bisphosphonate treatment.
Disclosures: The study was sponsored by UCB Pharma and Amgen. One investigator is an employee of UCB, and one is an employee of Amgen. Dr. Langdahl reported receiving research grants, consulting fees, and/or honoraria from Amgen, UCB, Eli Lilly, Merck, and Orkla. Most other investigators also reported financial relationships to Amgen and other pharmaceutical companies.
VIDEO: EULAR guidance on DMARD use in RA made ‘more concise’
LONDON – The European League Against Rheumatism guidelines on the use of disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis have been updated in line with current evidence and made more concise.
Dr. Josef S. Smolen of the department of rheumatology at the Medical University of Vienna who presented the 2016 guidelines at the European Congress of Rheumatology, noted that they now consist of 12 rather than the 14 recommendations that were included in the 2013 update (Ann Rheum Dis. 2014;73:492-509) and the 15 recommendations that were in the original 2010 version.
These 12 recommendations cover treatment targets and general approaches in the management of rheumatoid arthritis that incorporate disease-modifying antirheumatic drugs (DMARDs) and the use of glucocorticoids, and present treatment options as a hierarchy to help guide clinicians through appropriate procedures when initial and subsequent treatment fails. All DMARDs are considered in the recommendations, from the long-standing conventional synthetic (cs)DMARDs, such as methotrexate, sulfasalazine, and leflunomide, and the newer biologic DMARDs, such as the anti–tumor necrosis factor (TNF)–targeting drugs, to the newer biosimilar DMARDs, and targeted synthetic (ts)DMARDS, such as the Janus kinase (JAK) inhibitors tofacitinib and baricitinib.
The recommendations have been developed in accordance with EULAR’s standard operating procedure for the development of guidelines, Dr. Smolen observed, and involved three systematic literature reviews and expert opinion garnered from a task force of 50 experts and patients.
“This was the largest task force I have ever convened,” Dr. Smolen said, noting that rheumatologists from outside Europe had been invited to contribute their expertise and knowledge for the first time. Altogether 42 rheumatologists, three clinical fellows, two health professionals, and three patients were involved in revising the recommendations.
There are now four rather than three overarching principles, two of which are shared with early inflammatory arthritis recommendations that were also presented at the congress. The first two principles state that shared decision making is key to optimizing care and that rheumatologists should be the primary specialists looking after patients. The third principle recognizes the high burden that RA can have not only on an individual level but also on health care systems and society in general, which rheumatologists should be aware of. The fourth and final principle states that treatment decisions should be based on patients’ disease activity but that other factors, such as patients’ age, risk for progression, coexisting disease, and likely tolerance of treatment should also be kept in mind.
In an interview, Dr. Smolen highlighted that the EULAR recommendations cover three main phases of DMARD treatment: First is the DMARD-naive group of patients, who may be at an early or late stage of their disease. Second is the group in whom initial treatment has failed, and third is the group for whom subsequent treatment has not worked.
“In all these phases, we have some changes,” Dr. Smolen said. As an example, he noted that in the DMARD-naive setting, the use of csDMARDs has always been recommended but that the prior advice to consider combination csDMARD treatment has been edited out.
“We now say methotrexate should be part of the first treatment strategy, and the treatment strategy encompasses the use of additional, at least conventional synthetic, DMARDs.” Glucocorticoids are also more strongly recommended as part of the initial treatment strategy in combination with methotrexate, he said, although there is the proviso to use these for as short a time as possible.
In situations where patients do not respond to methotrexate plus glucocorticoids or they cannot tolerate methotrexate, then the recommendations advise stratifying patients into two groups. Those with poor prognostic factors might be switched to a biologic therapy, such as an anti-TNF agent or a tsDMARD. In regard to the latter, there is now more evidence behind the use of JAK inhibitors, notably tofacitinib, Dr. Smolen observed. Biologic DMARDs should be combined with csDMARDs, but if the latter is not tolerated then there is the option to use an IL-6 pathway inhibitor.
“There is now compelling evidence that all biologic DMARDs, including tocilizumab, convey better clinical, functional, and structural outcomes in combination with conventional synthetic DMARDs, especially methotrexate,” Dr. Smolen observed during his presentation of the recommendations. This may not be the case for the JAK inhibitors based on the current evidence.
When asked how the EULAR recommendations match up to those issued earlier this year by the American College of Rheumatology (Arthritis Care Res. 2016;68:1-25), Dr. Smolen observed that the two had become “much closer.” There remain differences in recommendations on glucocorticoid use, which are “somewhat clearer” in the European than in the American guidelines, and EULAR proposes combining biologic DMARDs with csDMARDs rather than using them as monotherapy. The EULAR recommendations also do not distinguish patients by disease duration but by treatment phase, and use prognostic factors for stratification.
The recommendations are currently in draft format and once finalized they will be published in Annals of the Rheumatic Diseases and also made freely available via the EULAR website, joining the organization’s many other recommendations for the management of rheumatic diseases. Dr. Smolen noted that these are intended as a template to provide national societies, health systems, and regulatory bodies a guide to the best evidence-based use of DMARDS in RA throughout Europe.
Dr. Smolen has received grant support and/or honoraria for consultations and/or for presentations from: AbbVie, Amgen, AstraZeneca, Astro-Pharma, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, ILTOO Pharma, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – The European League Against Rheumatism guidelines on the use of disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis have been updated in line with current evidence and made more concise.
Dr. Josef S. Smolen of the department of rheumatology at the Medical University of Vienna who presented the 2016 guidelines at the European Congress of Rheumatology, noted that they now consist of 12 rather than the 14 recommendations that were included in the 2013 update (Ann Rheum Dis. 2014;73:492-509) and the 15 recommendations that were in the original 2010 version.
These 12 recommendations cover treatment targets and general approaches in the management of rheumatoid arthritis that incorporate disease-modifying antirheumatic drugs (DMARDs) and the use of glucocorticoids, and present treatment options as a hierarchy to help guide clinicians through appropriate procedures when initial and subsequent treatment fails. All DMARDs are considered in the recommendations, from the long-standing conventional synthetic (cs)DMARDs, such as methotrexate, sulfasalazine, and leflunomide, and the newer biologic DMARDs, such as the anti–tumor necrosis factor (TNF)–targeting drugs, to the newer biosimilar DMARDs, and targeted synthetic (ts)DMARDS, such as the Janus kinase (JAK) inhibitors tofacitinib and baricitinib.
The recommendations have been developed in accordance with EULAR’s standard operating procedure for the development of guidelines, Dr. Smolen observed, and involved three systematic literature reviews and expert opinion garnered from a task force of 50 experts and patients.
“This was the largest task force I have ever convened,” Dr. Smolen said, noting that rheumatologists from outside Europe had been invited to contribute their expertise and knowledge for the first time. Altogether 42 rheumatologists, three clinical fellows, two health professionals, and three patients were involved in revising the recommendations.
There are now four rather than three overarching principles, two of which are shared with early inflammatory arthritis recommendations that were also presented at the congress. The first two principles state that shared decision making is key to optimizing care and that rheumatologists should be the primary specialists looking after patients. The third principle recognizes the high burden that RA can have not only on an individual level but also on health care systems and society in general, which rheumatologists should be aware of. The fourth and final principle states that treatment decisions should be based on patients’ disease activity but that other factors, such as patients’ age, risk for progression, coexisting disease, and likely tolerance of treatment should also be kept in mind.
In an interview, Dr. Smolen highlighted that the EULAR recommendations cover three main phases of DMARD treatment: First is the DMARD-naive group of patients, who may be at an early or late stage of their disease. Second is the group in whom initial treatment has failed, and third is the group for whom subsequent treatment has not worked.
“In all these phases, we have some changes,” Dr. Smolen said. As an example, he noted that in the DMARD-naive setting, the use of csDMARDs has always been recommended but that the prior advice to consider combination csDMARD treatment has been edited out.
“We now say methotrexate should be part of the first treatment strategy, and the treatment strategy encompasses the use of additional, at least conventional synthetic, DMARDs.” Glucocorticoids are also more strongly recommended as part of the initial treatment strategy in combination with methotrexate, he said, although there is the proviso to use these for as short a time as possible.
In situations where patients do not respond to methotrexate plus glucocorticoids or they cannot tolerate methotrexate, then the recommendations advise stratifying patients into two groups. Those with poor prognostic factors might be switched to a biologic therapy, such as an anti-TNF agent or a tsDMARD. In regard to the latter, there is now more evidence behind the use of JAK inhibitors, notably tofacitinib, Dr. Smolen observed. Biologic DMARDs should be combined with csDMARDs, but if the latter is not tolerated then there is the option to use an IL-6 pathway inhibitor.
“There is now compelling evidence that all biologic DMARDs, including tocilizumab, convey better clinical, functional, and structural outcomes in combination with conventional synthetic DMARDs, especially methotrexate,” Dr. Smolen observed during his presentation of the recommendations. This may not be the case for the JAK inhibitors based on the current evidence.
When asked how the EULAR recommendations match up to those issued earlier this year by the American College of Rheumatology (Arthritis Care Res. 2016;68:1-25), Dr. Smolen observed that the two had become “much closer.” There remain differences in recommendations on glucocorticoid use, which are “somewhat clearer” in the European than in the American guidelines, and EULAR proposes combining biologic DMARDs with csDMARDs rather than using them as monotherapy. The EULAR recommendations also do not distinguish patients by disease duration but by treatment phase, and use prognostic factors for stratification.
The recommendations are currently in draft format and once finalized they will be published in Annals of the Rheumatic Diseases and also made freely available via the EULAR website, joining the organization’s many other recommendations for the management of rheumatic diseases. Dr. Smolen noted that these are intended as a template to provide national societies, health systems, and regulatory bodies a guide to the best evidence-based use of DMARDS in RA throughout Europe.
Dr. Smolen has received grant support and/or honoraria for consultations and/or for presentations from: AbbVie, Amgen, AstraZeneca, Astro-Pharma, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, ILTOO Pharma, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – The European League Against Rheumatism guidelines on the use of disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis have been updated in line with current evidence and made more concise.
Dr. Josef S. Smolen of the department of rheumatology at the Medical University of Vienna who presented the 2016 guidelines at the European Congress of Rheumatology, noted that they now consist of 12 rather than the 14 recommendations that were included in the 2013 update (Ann Rheum Dis. 2014;73:492-509) and the 15 recommendations that were in the original 2010 version.
These 12 recommendations cover treatment targets and general approaches in the management of rheumatoid arthritis that incorporate disease-modifying antirheumatic drugs (DMARDs) and the use of glucocorticoids, and present treatment options as a hierarchy to help guide clinicians through appropriate procedures when initial and subsequent treatment fails. All DMARDs are considered in the recommendations, from the long-standing conventional synthetic (cs)DMARDs, such as methotrexate, sulfasalazine, and leflunomide, and the newer biologic DMARDs, such as the anti–tumor necrosis factor (TNF)–targeting drugs, to the newer biosimilar DMARDs, and targeted synthetic (ts)DMARDS, such as the Janus kinase (JAK) inhibitors tofacitinib and baricitinib.
The recommendations have been developed in accordance with EULAR’s standard operating procedure for the development of guidelines, Dr. Smolen observed, and involved three systematic literature reviews and expert opinion garnered from a task force of 50 experts and patients.
“This was the largest task force I have ever convened,” Dr. Smolen said, noting that rheumatologists from outside Europe had been invited to contribute their expertise and knowledge for the first time. Altogether 42 rheumatologists, three clinical fellows, two health professionals, and three patients were involved in revising the recommendations.
There are now four rather than three overarching principles, two of which are shared with early inflammatory arthritis recommendations that were also presented at the congress. The first two principles state that shared decision making is key to optimizing care and that rheumatologists should be the primary specialists looking after patients. The third principle recognizes the high burden that RA can have not only on an individual level but also on health care systems and society in general, which rheumatologists should be aware of. The fourth and final principle states that treatment decisions should be based on patients’ disease activity but that other factors, such as patients’ age, risk for progression, coexisting disease, and likely tolerance of treatment should also be kept in mind.
In an interview, Dr. Smolen highlighted that the EULAR recommendations cover three main phases of DMARD treatment: First is the DMARD-naive group of patients, who may be at an early or late stage of their disease. Second is the group in whom initial treatment has failed, and third is the group for whom subsequent treatment has not worked.
“In all these phases, we have some changes,” Dr. Smolen said. As an example, he noted that in the DMARD-naive setting, the use of csDMARDs has always been recommended but that the prior advice to consider combination csDMARD treatment has been edited out.
“We now say methotrexate should be part of the first treatment strategy, and the treatment strategy encompasses the use of additional, at least conventional synthetic, DMARDs.” Glucocorticoids are also more strongly recommended as part of the initial treatment strategy in combination with methotrexate, he said, although there is the proviso to use these for as short a time as possible.
In situations where patients do not respond to methotrexate plus glucocorticoids or they cannot tolerate methotrexate, then the recommendations advise stratifying patients into two groups. Those with poor prognostic factors might be switched to a biologic therapy, such as an anti-TNF agent or a tsDMARD. In regard to the latter, there is now more evidence behind the use of JAK inhibitors, notably tofacitinib, Dr. Smolen observed. Biologic DMARDs should be combined with csDMARDs, but if the latter is not tolerated then there is the option to use an IL-6 pathway inhibitor.
“There is now compelling evidence that all biologic DMARDs, including tocilizumab, convey better clinical, functional, and structural outcomes in combination with conventional synthetic DMARDs, especially methotrexate,” Dr. Smolen observed during his presentation of the recommendations. This may not be the case for the JAK inhibitors based on the current evidence.
When asked how the EULAR recommendations match up to those issued earlier this year by the American College of Rheumatology (Arthritis Care Res. 2016;68:1-25), Dr. Smolen observed that the two had become “much closer.” There remain differences in recommendations on glucocorticoid use, which are “somewhat clearer” in the European than in the American guidelines, and EULAR proposes combining biologic DMARDs with csDMARDs rather than using them as monotherapy. The EULAR recommendations also do not distinguish patients by disease duration but by treatment phase, and use prognostic factors for stratification.
The recommendations are currently in draft format and once finalized they will be published in Annals of the Rheumatic Diseases and also made freely available via the EULAR website, joining the organization’s many other recommendations for the management of rheumatic diseases. Dr. Smolen noted that these are intended as a template to provide national societies, health systems, and regulatory bodies a guide to the best evidence-based use of DMARDS in RA throughout Europe.
Dr. Smolen has received grant support and/or honoraria for consultations and/or for presentations from: AbbVie, Amgen, AstraZeneca, Astro-Pharma, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, ILTOO Pharma, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2016 CONGRESS
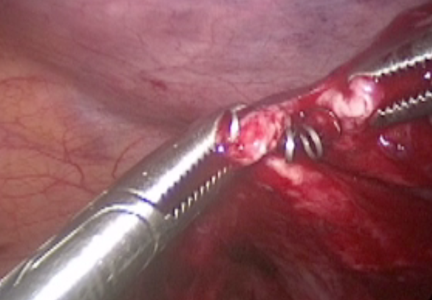
Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
This video is brought to you by ![]()
VIDEO: Telehealth program provides weight management support to rural clinics
MINNEAPOLIS – Can telehealth help obese patients lose weight?
Weight management experts at the Medical University of South Carolina, Charleston, sought to find out through a unique program to provide practice support to rural health care providers.
The telehealth program gives health clinics in rural South Carolina access to teams of weight management experts and support through mHealth applications linking providers and clinical faculty.
The project includes biweekly group patient sessions led by a psychologist, registered dietitian, and exercise physiologist. The program uses videoconferencing systems and a provider-focused mobile app that captures weight and blood pressure data from wireless peripherals, while allowing for manual input of data.
In a video interview at the American Telemedicine Association annual conference, Ragan Aleise DuBose-Morris, Ph.D., director of telehealth education for the Medical University of South Carolina, and Joshua Brown, Ph.D., director of clinical services at the university’s Weight Management Center, discussed the weight management initiative and its effectiveness. Dr. DuBose-Morris and Dr. Brown also explained how the initiative was designed, and how the effort has impacted the weight of obese patients in the state.
agallegos@frontlinemedcom.com On Twitter @legal_med
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MINNEAPOLIS – Can telehealth help obese patients lose weight?
Weight management experts at the Medical University of South Carolina, Charleston, sought to find out through a unique program to provide practice support to rural health care providers.
The telehealth program gives health clinics in rural South Carolina access to teams of weight management experts and support through mHealth applications linking providers and clinical faculty.
The project includes biweekly group patient sessions led by a psychologist, registered dietitian, and exercise physiologist. The program uses videoconferencing systems and a provider-focused mobile app that captures weight and blood pressure data from wireless peripherals, while allowing for manual input of data.
In a video interview at the American Telemedicine Association annual conference, Ragan Aleise DuBose-Morris, Ph.D., director of telehealth education for the Medical University of South Carolina, and Joshua Brown, Ph.D., director of clinical services at the university’s Weight Management Center, discussed the weight management initiative and its effectiveness. Dr. DuBose-Morris and Dr. Brown also explained how the initiative was designed, and how the effort has impacted the weight of obese patients in the state.
agallegos@frontlinemedcom.com On Twitter @legal_med
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MINNEAPOLIS – Can telehealth help obese patients lose weight?
Weight management experts at the Medical University of South Carolina, Charleston, sought to find out through a unique program to provide practice support to rural health care providers.
The telehealth program gives health clinics in rural South Carolina access to teams of weight management experts and support through mHealth applications linking providers and clinical faculty.
The project includes biweekly group patient sessions led by a psychologist, registered dietitian, and exercise physiologist. The program uses videoconferencing systems and a provider-focused mobile app that captures weight and blood pressure data from wireless peripherals, while allowing for manual input of data.
In a video interview at the American Telemedicine Association annual conference, Ragan Aleise DuBose-Morris, Ph.D., director of telehealth education for the Medical University of South Carolina, and Joshua Brown, Ph.D., director of clinical services at the university’s Weight Management Center, discussed the weight management initiative and its effectiveness. Dr. DuBose-Morris and Dr. Brown also explained how the initiative was designed, and how the effort has impacted the weight of obese patients in the state.
agallegos@frontlinemedcom.com On Twitter @legal_med
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM ATA 2016
VIDEO: Early caffeine did not help, may harm preemies
BALTIMORE – Early initiation of caffeine treatment in premature neonates on mechanical ventilation did not cut the time to when these babies could successfully wean off the ventilator, according to findings of a single-center, randomized controlled study of 83 children.
The results also showed an “unexpected” trend toward increased mortality among the neonates who received early caffeine treatment, Dr. Cynthia M. Amaro reported at the annual meeting of the Pediatric Academic Societies. This signal of elevated mortality with caffeine treatment prompted the study’s data and safety monitoring board to prematurely stop the trial, limiting enrollment to just 75% of the number originally planned in the study’s design, thereby raising questions about the reliability of the primary-endpoint finding that early caffeine treatment did not result in the benefit of a reduced time to extubation.
Dr. Amaro said that she and her associates ran the study to address what had emerged as a significant area of doubt in routine U.S. practice on how to best use caffeine treatment in this neonatal population following publication of findings from the landmark Caffeine for Apnea of Prematurity (CAP) Trial (N Engl J Med. 2006 May 18;354[20]:2112-21). Results from the CAP Trial had shown in nearly 2,000 randomized, premature infants that treatment with caffeine led to significantly fewer episodes of bronchopulmonary dysplasia as well as quicker time to extubation of mechanical ventilation. Caffeine or other methylxanthines stimulate an infant’s respiratory center to allow faster extubation.
Ever since that publication a decade ago, “clinicians have been using caffeine earlier and more liberally, without really good data to support its early use in mechanically-ventilated preterm babies,” explained Dr. Amaro, a neonatologist at the University of Miami and Holtz Children’s Hospital in Miami.
Based on the new findings from the study she reported, “we are now not routinely initiating caffeine in mechanically ventilated preterm babies and just using caffeine immediately before extubation to treat apnea of prematurity. This returns caffeine treatment to the way it was used in the CAP Trial,” she said. “Further studies are needed before we can say what is best for early treatment of these preterm babies,” Dr. Amaro said in a video interview.
Her report led to a flurry of comments during the question period, with several pediatricians voicing concern about the reliability of results from a study that followed only 83 patients because of its premature termination.
“The data and safety monitoring board’s decision is a big issue,” said Dr. Carl E. Hunt, a pediatrician at the Uniformed Services University of the Health Sciences in Bethesda, Md. “There is a literature that shows results of studies can be very different when they stop early. It’s unfortunate because we don’t have other prospective data, and it may now be hard to do a large randomized, controlled trial” of early caffeine treatment, Dr. Hunt said.
While Dr. Amaro conceded that premature termination limited her study’s size, she also asserted that her analyses confirmed the validity of the finding of no benefit from early caffeine treatment. “We projected to full enrollment, and there still was no difference in the time to first successful extubation,” she said.
Her study enrolled preterm infants during January 2013–December 2015 born at 23-30 weeks’ gestation who required mechanical ventilation during their first 5 days. Randomization assigned 41 infants to receive a 20-mg/kg bolus of caffeine, followed by a maintenance dosage of 5 mg/kg that continued until extubation, while 42 patients received placebo and did not get caffeine until just before attempted extubation. The bolus and maintenance caffeine dosages tested were identical to those used in the CAP Trial.
The researchers defined successful extubation as keeping a child off restart of mechanical ventilation for more than 24 hours. The average gestational age of the enrolled neonates was 26 weeks, their average weight was 700 g, and intubation started an average of 3 hours after delivery.
The study’s primary endpoint, age at first successful extubation, was an average of 24 days among the neonates treated with caffeine and 20 days in those on placebo, Dr. Amaro reported. Mortality occurred at an average of 30 days after delivery in the caffeine recipients and after an average of 10 days in the controls. The incidence of death was 22% in those on early caffeine and 12% among those in the placebo group, an excess of four deaths in the intervention arm that was not statically significant.
A recent review of more than 29,000 matched very-low-birth-weight infants managed in routine practice showed that neonates who received early caffeine had an adjusted mortality risk that was 23% higher than that of matched infants not receiving early caffeine, Dr. Amaro noted (J Pediatrics. 2014 May;164[5]:992-8).
The incidence of bronchopulmonary dysplasia also did not show a statistically significant difference between the two study arms, 46% among those on early caffeine and 53% in the placebo group. Patients on early caffeine also had higher rates of necrotizing enterocolitis, more episodes of necrotizing enterocolitis requiring surgery, and more intraventricular hemorrhages, but none of these differences reached statistical significance.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Dr. Susan Millard, FCCP, comments: Apnea of prematurity is a very common occurrence. We are excited to have new data but I am in agreement that a larger multicenter study is extremely important before instituting a protocol change.
Dr. Susan Millard, FCCP, comments: Apnea of prematurity is a very common occurrence. We are excited to have new data but I am in agreement that a larger multicenter study is extremely important before instituting a protocol change.
Dr. Susan Millard, FCCP, comments: Apnea of prematurity is a very common occurrence. We are excited to have new data but I am in agreement that a larger multicenter study is extremely important before instituting a protocol change.
BALTIMORE – Early initiation of caffeine treatment in premature neonates on mechanical ventilation did not cut the time to when these babies could successfully wean off the ventilator, according to findings of a single-center, randomized controlled study of 83 children.
The results also showed an “unexpected” trend toward increased mortality among the neonates who received early caffeine treatment, Dr. Cynthia M. Amaro reported at the annual meeting of the Pediatric Academic Societies. This signal of elevated mortality with caffeine treatment prompted the study’s data and safety monitoring board to prematurely stop the trial, limiting enrollment to just 75% of the number originally planned in the study’s design, thereby raising questions about the reliability of the primary-endpoint finding that early caffeine treatment did not result in the benefit of a reduced time to extubation.
Dr. Amaro said that she and her associates ran the study to address what had emerged as a significant area of doubt in routine U.S. practice on how to best use caffeine treatment in this neonatal population following publication of findings from the landmark Caffeine for Apnea of Prematurity (CAP) Trial (N Engl J Med. 2006 May 18;354[20]:2112-21). Results from the CAP Trial had shown in nearly 2,000 randomized, premature infants that treatment with caffeine led to significantly fewer episodes of bronchopulmonary dysplasia as well as quicker time to extubation of mechanical ventilation. Caffeine or other methylxanthines stimulate an infant’s respiratory center to allow faster extubation.
Ever since that publication a decade ago, “clinicians have been using caffeine earlier and more liberally, without really good data to support its early use in mechanically-ventilated preterm babies,” explained Dr. Amaro, a neonatologist at the University of Miami and Holtz Children’s Hospital in Miami.
Based on the new findings from the study she reported, “we are now not routinely initiating caffeine in mechanically ventilated preterm babies and just using caffeine immediately before extubation to treat apnea of prematurity. This returns caffeine treatment to the way it was used in the CAP Trial,” she said. “Further studies are needed before we can say what is best for early treatment of these preterm babies,” Dr. Amaro said in a video interview.
Her report led to a flurry of comments during the question period, with several pediatricians voicing concern about the reliability of results from a study that followed only 83 patients because of its premature termination.
“The data and safety monitoring board’s decision is a big issue,” said Dr. Carl E. Hunt, a pediatrician at the Uniformed Services University of the Health Sciences in Bethesda, Md. “There is a literature that shows results of studies can be very different when they stop early. It’s unfortunate because we don’t have other prospective data, and it may now be hard to do a large randomized, controlled trial” of early caffeine treatment, Dr. Hunt said.
While Dr. Amaro conceded that premature termination limited her study’s size, she also asserted that her analyses confirmed the validity of the finding of no benefit from early caffeine treatment. “We projected to full enrollment, and there still was no difference in the time to first successful extubation,” she said.
Her study enrolled preterm infants during January 2013–December 2015 born at 23-30 weeks’ gestation who required mechanical ventilation during their first 5 days. Randomization assigned 41 infants to receive a 20-mg/kg bolus of caffeine, followed by a maintenance dosage of 5 mg/kg that continued until extubation, while 42 patients received placebo and did not get caffeine until just before attempted extubation. The bolus and maintenance caffeine dosages tested were identical to those used in the CAP Trial.
The researchers defined successful extubation as keeping a child off restart of mechanical ventilation for more than 24 hours. The average gestational age of the enrolled neonates was 26 weeks, their average weight was 700 g, and intubation started an average of 3 hours after delivery.
The study’s primary endpoint, age at first successful extubation, was an average of 24 days among the neonates treated with caffeine and 20 days in those on placebo, Dr. Amaro reported. Mortality occurred at an average of 30 days after delivery in the caffeine recipients and after an average of 10 days in the controls. The incidence of death was 22% in those on early caffeine and 12% among those in the placebo group, an excess of four deaths in the intervention arm that was not statically significant.
A recent review of more than 29,000 matched very-low-birth-weight infants managed in routine practice showed that neonates who received early caffeine had an adjusted mortality risk that was 23% higher than that of matched infants not receiving early caffeine, Dr. Amaro noted (J Pediatrics. 2014 May;164[5]:992-8).
The incidence of bronchopulmonary dysplasia also did not show a statistically significant difference between the two study arms, 46% among those on early caffeine and 53% in the placebo group. Patients on early caffeine also had higher rates of necrotizing enterocolitis, more episodes of necrotizing enterocolitis requiring surgery, and more intraventricular hemorrhages, but none of these differences reached statistical significance.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
BALTIMORE – Early initiation of caffeine treatment in premature neonates on mechanical ventilation did not cut the time to when these babies could successfully wean off the ventilator, according to findings of a single-center, randomized controlled study of 83 children.
The results also showed an “unexpected” trend toward increased mortality among the neonates who received early caffeine treatment, Dr. Cynthia M. Amaro reported at the annual meeting of the Pediatric Academic Societies. This signal of elevated mortality with caffeine treatment prompted the study’s data and safety monitoring board to prematurely stop the trial, limiting enrollment to just 75% of the number originally planned in the study’s design, thereby raising questions about the reliability of the primary-endpoint finding that early caffeine treatment did not result in the benefit of a reduced time to extubation.
Dr. Amaro said that she and her associates ran the study to address what had emerged as a significant area of doubt in routine U.S. practice on how to best use caffeine treatment in this neonatal population following publication of findings from the landmark Caffeine for Apnea of Prematurity (CAP) Trial (N Engl J Med. 2006 May 18;354[20]:2112-21). Results from the CAP Trial had shown in nearly 2,000 randomized, premature infants that treatment with caffeine led to significantly fewer episodes of bronchopulmonary dysplasia as well as quicker time to extubation of mechanical ventilation. Caffeine or other methylxanthines stimulate an infant’s respiratory center to allow faster extubation.
Ever since that publication a decade ago, “clinicians have been using caffeine earlier and more liberally, without really good data to support its early use in mechanically-ventilated preterm babies,” explained Dr. Amaro, a neonatologist at the University of Miami and Holtz Children’s Hospital in Miami.
Based on the new findings from the study she reported, “we are now not routinely initiating caffeine in mechanically ventilated preterm babies and just using caffeine immediately before extubation to treat apnea of prematurity. This returns caffeine treatment to the way it was used in the CAP Trial,” she said. “Further studies are needed before we can say what is best for early treatment of these preterm babies,” Dr. Amaro said in a video interview.
Her report led to a flurry of comments during the question period, with several pediatricians voicing concern about the reliability of results from a study that followed only 83 patients because of its premature termination.
“The data and safety monitoring board’s decision is a big issue,” said Dr. Carl E. Hunt, a pediatrician at the Uniformed Services University of the Health Sciences in Bethesda, Md. “There is a literature that shows results of studies can be very different when they stop early. It’s unfortunate because we don’t have other prospective data, and it may now be hard to do a large randomized, controlled trial” of early caffeine treatment, Dr. Hunt said.
While Dr. Amaro conceded that premature termination limited her study’s size, she also asserted that her analyses confirmed the validity of the finding of no benefit from early caffeine treatment. “We projected to full enrollment, and there still was no difference in the time to first successful extubation,” she said.
Her study enrolled preterm infants during January 2013–December 2015 born at 23-30 weeks’ gestation who required mechanical ventilation during their first 5 days. Randomization assigned 41 infants to receive a 20-mg/kg bolus of caffeine, followed by a maintenance dosage of 5 mg/kg that continued until extubation, while 42 patients received placebo and did not get caffeine until just before attempted extubation. The bolus and maintenance caffeine dosages tested were identical to those used in the CAP Trial.
The researchers defined successful extubation as keeping a child off restart of mechanical ventilation for more than 24 hours. The average gestational age of the enrolled neonates was 26 weeks, their average weight was 700 g, and intubation started an average of 3 hours after delivery.
The study’s primary endpoint, age at first successful extubation, was an average of 24 days among the neonates treated with caffeine and 20 days in those on placebo, Dr. Amaro reported. Mortality occurred at an average of 30 days after delivery in the caffeine recipients and after an average of 10 days in the controls. The incidence of death was 22% in those on early caffeine and 12% among those in the placebo group, an excess of four deaths in the intervention arm that was not statically significant.
A recent review of more than 29,000 matched very-low-birth-weight infants managed in routine practice showed that neonates who received early caffeine had an adjusted mortality risk that was 23% higher than that of matched infants not receiving early caffeine, Dr. Amaro noted (J Pediatrics. 2014 May;164[5]:992-8).
The incidence of bronchopulmonary dysplasia also did not show a statistically significant difference between the two study arms, 46% among those on early caffeine and 53% in the placebo group. Patients on early caffeine also had higher rates of necrotizing enterocolitis, more episodes of necrotizing enterocolitis requiring surgery, and more intraventricular hemorrhages, but none of these differences reached statistical significance.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
VIDEO: Locum Tenens in Hospital Medicine
Dr. Geeta Arora is a locum tenens hospitalist; James Levy is a PA who hires locums as the VP of Human Resources for Indigo Health Partners in Northern Michigan. They share their experiences navigating "freelance hospital medicine," from both the medical practice and business perspective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Geeta Arora is a locum tenens hospitalist; James Levy is a PA who hires locums as the VP of Human Resources for Indigo Health Partners in Northern Michigan. They share their experiences navigating "freelance hospital medicine," from both the medical practice and business perspective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Geeta Arora is a locum tenens hospitalist; James Levy is a PA who hires locums as the VP of Human Resources for Indigo Health Partners in Northern Michigan. They share their experiences navigating "freelance hospital medicine," from both the medical practice and business perspective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Smoking, excess weight hinder sustained remission in early RA
LONDON – Tobacco use and excess weight can make it harder to achieve sustained remission in the treatment of early rheumatoid arthritis, according to findings from more than 1,000 patients in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study.
Aggressive treatment that starts soon after diagnosis of rheumatoid arthritis (RA) is important for the absence of disease activity, which is the hallmark of sustained remission. But the reality is a success rate of less than 50% in the first 3 years with physical deterioration continuing thereafter. “Excess weight and smoking are two risk factors for developing RA. We were interested in seeing if they might also affect how well people responded to treatment,” said Susan Bartlett, Ph.D., a clinical psychologist at McGill University in Montreal.
At the annual European Congress of Rheumatology, Dr. Bartlett and colleagues reported on a cohort of 1,008 early RA patients who were enrolled in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study and followed from around the time of diagnosis through the first 3 years of treatment to estimate the time it took until they achieved sustained remission, defined as having a 28-joint Disease Activity Score less than 2.6 for two consecutive visits.
Mean age of the patients (72% female, 81% white) was early 50s. Overall, 30% of females and 47% of males were overweight, one-third of both genders were obese, and 15%-20% smoked. Treatment at entry included methotrexate in mono- or combination therapy in about three-quarters of the patients, with steroids used in about half and biologics used sparingly.
The proportion of patients in sustained remission was 38% at 3 years, with a median time to remission of 11.3 months. “That finding wasn’t surprising because that is generally what is found in most studies of early RA. However, when we looked more closely at who was and wasn’t achieving remission, we found that people who smoked and those who were overweight or obese were much less likely than their nonsmoking, normal-weight peers to be in sustained remission,” Dr. Bartlett said in a pre-congress interview.
After adjustment for factors that could affect response to treatment – including age, race, disability status, pain, and early medications used – smoking (P = .046) and excess weight (P = .003) were associated with a poorer likelihood of achieving sustained remission. While more men than women were overweight or obese, the effects of weight and smoking appeared to be more problematic for women (P = .02).
An average nonsmoking male with a healthy body mass index (BMI; 25 kg/m2 or less) had about a 41% probability of achieving sustained remission within 3 years, compared with 15% for an obese male smoker. A nonsmoking female with a healthy BMI had a 27% probability of achieving sustained remission within 3 years, compared with 10% for an obese female smoker. Probabilities of sustained remission were also lower for overweight men and women, Dr. Bartlett reported.
Smoking and obesity have already been linked with an increased likelihood of developing RA, which in turn increases the risk of cardiovascular disease and premature death. The latest data suggest that both smoking and extra weight – including overweight and obese as defined by BMI – may also independently influence the success of treatment. “Our data suggest that if you have RA, it’s important to take the medications that your doctor has prescribed. If you smoke, you need to stop. And if you’re carrying extra weight, not only is that placing a greater demand on already vulnerable joints, it may also be making your RA treatment less effective,” Dr. Bartlett said.
These lifestyle modifications can be challenging for some people with RA, she said in the interview. Clinicians can help by considering lifestyle behaviors that lead to chronic diseases and poorer outcomes in addition to their more traditional view of diagnosis and treatment, she said, adding that patients and clinicians should know that even a small amount of weight loss can improve health and may improve response to therapy.
Well controlled clinical trials will be needed to better understand the benefits of weight control and smoking cessation on response to RA treatment. Also, why women who smoke and are overweight are at more of a disadvantage than their male counterparts is unknown. “As we begin putting these pieces together, we may learn valuable information that helps us to better control and ultimately cure RA,” said Dr. Bartlett.
The researchers had no conflicts of interest to declare.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Tobacco use and excess weight can make it harder to achieve sustained remission in the treatment of early rheumatoid arthritis, according to findings from more than 1,000 patients in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study.
Aggressive treatment that starts soon after diagnosis of rheumatoid arthritis (RA) is important for the absence of disease activity, which is the hallmark of sustained remission. But the reality is a success rate of less than 50% in the first 3 years with physical deterioration continuing thereafter. “Excess weight and smoking are two risk factors for developing RA. We were interested in seeing if they might also affect how well people responded to treatment,” said Susan Bartlett, Ph.D., a clinical psychologist at McGill University in Montreal.
At the annual European Congress of Rheumatology, Dr. Bartlett and colleagues reported on a cohort of 1,008 early RA patients who were enrolled in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study and followed from around the time of diagnosis through the first 3 years of treatment to estimate the time it took until they achieved sustained remission, defined as having a 28-joint Disease Activity Score less than 2.6 for two consecutive visits.
Mean age of the patients (72% female, 81% white) was early 50s. Overall, 30% of females and 47% of males were overweight, one-third of both genders were obese, and 15%-20% smoked. Treatment at entry included methotrexate in mono- or combination therapy in about three-quarters of the patients, with steroids used in about half and biologics used sparingly.
The proportion of patients in sustained remission was 38% at 3 years, with a median time to remission of 11.3 months. “That finding wasn’t surprising because that is generally what is found in most studies of early RA. However, when we looked more closely at who was and wasn’t achieving remission, we found that people who smoked and those who were overweight or obese were much less likely than their nonsmoking, normal-weight peers to be in sustained remission,” Dr. Bartlett said in a pre-congress interview.
After adjustment for factors that could affect response to treatment – including age, race, disability status, pain, and early medications used – smoking (P = .046) and excess weight (P = .003) were associated with a poorer likelihood of achieving sustained remission. While more men than women were overweight or obese, the effects of weight and smoking appeared to be more problematic for women (P = .02).
An average nonsmoking male with a healthy body mass index (BMI; 25 kg/m2 or less) had about a 41% probability of achieving sustained remission within 3 years, compared with 15% for an obese male smoker. A nonsmoking female with a healthy BMI had a 27% probability of achieving sustained remission within 3 years, compared with 10% for an obese female smoker. Probabilities of sustained remission were also lower for overweight men and women, Dr. Bartlett reported.
Smoking and obesity have already been linked with an increased likelihood of developing RA, which in turn increases the risk of cardiovascular disease and premature death. The latest data suggest that both smoking and extra weight – including overweight and obese as defined by BMI – may also independently influence the success of treatment. “Our data suggest that if you have RA, it’s important to take the medications that your doctor has prescribed. If you smoke, you need to stop. And if you’re carrying extra weight, not only is that placing a greater demand on already vulnerable joints, it may also be making your RA treatment less effective,” Dr. Bartlett said.
These lifestyle modifications can be challenging for some people with RA, she said in the interview. Clinicians can help by considering lifestyle behaviors that lead to chronic diseases and poorer outcomes in addition to their more traditional view of diagnosis and treatment, she said, adding that patients and clinicians should know that even a small amount of weight loss can improve health and may improve response to therapy.
Well controlled clinical trials will be needed to better understand the benefits of weight control and smoking cessation on response to RA treatment. Also, why women who smoke and are overweight are at more of a disadvantage than their male counterparts is unknown. “As we begin putting these pieces together, we may learn valuable information that helps us to better control and ultimately cure RA,” said Dr. Bartlett.
The researchers had no conflicts of interest to declare.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Tobacco use and excess weight can make it harder to achieve sustained remission in the treatment of early rheumatoid arthritis, according to findings from more than 1,000 patients in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study.
Aggressive treatment that starts soon after diagnosis of rheumatoid arthritis (RA) is important for the absence of disease activity, which is the hallmark of sustained remission. But the reality is a success rate of less than 50% in the first 3 years with physical deterioration continuing thereafter. “Excess weight and smoking are two risk factors for developing RA. We were interested in seeing if they might also affect how well people responded to treatment,” said Susan Bartlett, Ph.D., a clinical psychologist at McGill University in Montreal.
At the annual European Congress of Rheumatology, Dr. Bartlett and colleagues reported on a cohort of 1,008 early RA patients who were enrolled in the Canadian Early Arthritis Cohort (CATCH) multicenter, prospective study and followed from around the time of diagnosis through the first 3 years of treatment to estimate the time it took until they achieved sustained remission, defined as having a 28-joint Disease Activity Score less than 2.6 for two consecutive visits.
Mean age of the patients (72% female, 81% white) was early 50s. Overall, 30% of females and 47% of males were overweight, one-third of both genders were obese, and 15%-20% smoked. Treatment at entry included methotrexate in mono- or combination therapy in about three-quarters of the patients, with steroids used in about half and biologics used sparingly.
The proportion of patients in sustained remission was 38% at 3 years, with a median time to remission of 11.3 months. “That finding wasn’t surprising because that is generally what is found in most studies of early RA. However, when we looked more closely at who was and wasn’t achieving remission, we found that people who smoked and those who were overweight or obese were much less likely than their nonsmoking, normal-weight peers to be in sustained remission,” Dr. Bartlett said in a pre-congress interview.
After adjustment for factors that could affect response to treatment – including age, race, disability status, pain, and early medications used – smoking (P = .046) and excess weight (P = .003) were associated with a poorer likelihood of achieving sustained remission. While more men than women were overweight or obese, the effects of weight and smoking appeared to be more problematic for women (P = .02).
An average nonsmoking male with a healthy body mass index (BMI; 25 kg/m2 or less) had about a 41% probability of achieving sustained remission within 3 years, compared with 15% for an obese male smoker. A nonsmoking female with a healthy BMI had a 27% probability of achieving sustained remission within 3 years, compared with 10% for an obese female smoker. Probabilities of sustained remission were also lower for overweight men and women, Dr. Bartlett reported.
Smoking and obesity have already been linked with an increased likelihood of developing RA, which in turn increases the risk of cardiovascular disease and premature death. The latest data suggest that both smoking and extra weight – including overweight and obese as defined by BMI – may also independently influence the success of treatment. “Our data suggest that if you have RA, it’s important to take the medications that your doctor has prescribed. If you smoke, you need to stop. And if you’re carrying extra weight, not only is that placing a greater demand on already vulnerable joints, it may also be making your RA treatment less effective,” Dr. Bartlett said.
These lifestyle modifications can be challenging for some people with RA, she said in the interview. Clinicians can help by considering lifestyle behaviors that lead to chronic diseases and poorer outcomes in addition to their more traditional view of diagnosis and treatment, she said, adding that patients and clinicians should know that even a small amount of weight loss can improve health and may improve response to therapy.
Well controlled clinical trials will be needed to better understand the benefits of weight control and smoking cessation on response to RA treatment. Also, why women who smoke and are overweight are at more of a disadvantage than their male counterparts is unknown. “As we begin putting these pieces together, we may learn valuable information that helps us to better control and ultimately cure RA,” said Dr. Bartlett.
The researchers had no conflicts of interest to declare.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR CONGRESS 2016
Key clinical point: The latest data suggest that both smoking and extra weight – including overweight and obese as defined by BMI – may independently influence the success of treatment for rheumatoid arthritis.
Major finding: A nonsmoking female with a healthy BMI had a 27% probability of achieving sustained remission within 3 years, compared with 10% for an obese female smoker.
Data source: A prospective study of 1,008 patients with early RA in the prospective, multicenter CATCH study.
Disclosures: The researchers had no conflicts of interest to declare.
Distress or depression in a 12-year-old girl?
A 12-year-old girl presents for a well-child visit but is withdrawn, and her mother reports recent behavioral changes. Symptoms suggest a possible depressive disorder. The physician did not anticipate a mental health concern and is behind schedule. What are the next steps?
In this edition of Mental Health Consult, join our expert panelists for their analysis of this case, and their recommendations for assessing similar patients and addressing their mental health needs within the context of a busy primary care practice. Our panel includes Dr. David Pickar, a psychiatrist and former intramural research director for the National Institute of Mental Health; Dr. Lee Savio Beers of Children’s National Health System, Washington; and Dr. Lorenzo Norris, medical director of psychiatric and behavioral services at George Washington University Hospital, Washington.
“This is a case that … really gets at the heart of the idea of what is normal versus abnormal in terms of clinical depression,” says Dr. Norris. Watch the video to hear their perspectives on when and how to perform screening interventions, decide on referrals, handle emergent situations, and decide how practice models drive decisions and reimbursement.
Click here for a PDF of the case study.
A 12-year-old girl presents for a well-child visit but is withdrawn, and her mother reports recent behavioral changes. Symptoms suggest a possible depressive disorder. The physician did not anticipate a mental health concern and is behind schedule. What are the next steps?
In this edition of Mental Health Consult, join our expert panelists for their analysis of this case, and their recommendations for assessing similar patients and addressing their mental health needs within the context of a busy primary care practice. Our panel includes Dr. David Pickar, a psychiatrist and former intramural research director for the National Institute of Mental Health; Dr. Lee Savio Beers of Children’s National Health System, Washington; and Dr. Lorenzo Norris, medical director of psychiatric and behavioral services at George Washington University Hospital, Washington.
“This is a case that … really gets at the heart of the idea of what is normal versus abnormal in terms of clinical depression,” says Dr. Norris. Watch the video to hear their perspectives on when and how to perform screening interventions, decide on referrals, handle emergent situations, and decide how practice models drive decisions and reimbursement.
Click here for a PDF of the case study.
A 12-year-old girl presents for a well-child visit but is withdrawn, and her mother reports recent behavioral changes. Symptoms suggest a possible depressive disorder. The physician did not anticipate a mental health concern and is behind schedule. What are the next steps?
In this edition of Mental Health Consult, join our expert panelists for their analysis of this case, and their recommendations for assessing similar patients and addressing their mental health needs within the context of a busy primary care practice. Our panel includes Dr. David Pickar, a psychiatrist and former intramural research director for the National Institute of Mental Health; Dr. Lee Savio Beers of Children’s National Health System, Washington; and Dr. Lorenzo Norris, medical director of psychiatric and behavioral services at George Washington University Hospital, Washington.
“This is a case that … really gets at the heart of the idea of what is normal versus abnormal in terms of clinical depression,” says Dr. Norris. Watch the video to hear their perspectives on when and how to perform screening interventions, decide on referrals, handle emergent situations, and decide how practice models drive decisions and reimbursement.
Click here for a PDF of the case study.
VIDEO: Lobectomy quality requires linking outcomes to process change
BALTIMORE – An “introspective” analysis connecting patient outcomes with process changes may lead to significant surgical quality improvement, according to a study presented at the 2016 annual meeting of the American Association for Thoracic Surgery.
The case study detailed the University of Alabama at Birmingham School of Medicine’s attempt to identify the metrics used for the Society of Thoracic Surgeons lobectomy ranking, and show how the institution used root cause analysis with “lean” and process improvements to improve outcomes from Jan. 2006 until July 2014 in order to achieve a three star STS ranking.
UAB researchers found that their most common root cause analysis was failure to escalate care. The institution implemented process improvements such as increasing pulmonary rehabilitation prior to surgery, adding a respiratory therapist, eliminating (lean) non-valued steps, favoring stereotactic radiotherapy and segmentectomy instead of lobectomy for marginal patients, and using minimally invasive lobectomy. They ultimately achieved a three-star STS ranking.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Stephen D. Cassivi, professor of surgery at the Mayo Clinic in Rochester, Minn., and a discussant on the paper at AATS 2016, said in an interview that the research was important because it encourages surgeons to discuss and reevaluate quality improvement measures. He noted that early phases of surgical quality improvement was based on process measures, specifically around the idea that if surgeons were attentive to process measures, their outcome measures would improve. But over time, the emphasis on process measures has dissipated in favor of outcomes-focused analysis.
“Now that we have more robust [outcomes] data... we can examine our practices in a more thoughtful, data-driven, evidence-based way,” Dr. Cassivi said. He added that the shift from process measures to outcome measures is important in that surgeons can easily interpret and compare outcomes data across facilities. But he noted that there is a downside: If an institution’s outcome measures are not up to standard, it is sometimes difficult to determine why.
“The current way that the [outcomes] data are reported and processed is not easily interpretable into which processes we need to adapt,” Dr. Cassivi said. “There is still work that needs to be done, but [this paper] is a first step.”
Dr. Cassivi reported no relevant financial disclosures.
On Twitter @richpizzi
BALTIMORE – An “introspective” analysis connecting patient outcomes with process changes may lead to significant surgical quality improvement, according to a study presented at the 2016 annual meeting of the American Association for Thoracic Surgery.
The case study detailed the University of Alabama at Birmingham School of Medicine’s attempt to identify the metrics used for the Society of Thoracic Surgeons lobectomy ranking, and show how the institution used root cause analysis with “lean” and process improvements to improve outcomes from Jan. 2006 until July 2014 in order to achieve a three star STS ranking.
UAB researchers found that their most common root cause analysis was failure to escalate care. The institution implemented process improvements such as increasing pulmonary rehabilitation prior to surgery, adding a respiratory therapist, eliminating (lean) non-valued steps, favoring stereotactic radiotherapy and segmentectomy instead of lobectomy for marginal patients, and using minimally invasive lobectomy. They ultimately achieved a three-star STS ranking.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Stephen D. Cassivi, professor of surgery at the Mayo Clinic in Rochester, Minn., and a discussant on the paper at AATS 2016, said in an interview that the research was important because it encourages surgeons to discuss and reevaluate quality improvement measures. He noted that early phases of surgical quality improvement was based on process measures, specifically around the idea that if surgeons were attentive to process measures, their outcome measures would improve. But over time, the emphasis on process measures has dissipated in favor of outcomes-focused analysis.
“Now that we have more robust [outcomes] data... we can examine our practices in a more thoughtful, data-driven, evidence-based way,” Dr. Cassivi said. He added that the shift from process measures to outcome measures is important in that surgeons can easily interpret and compare outcomes data across facilities. But he noted that there is a downside: If an institution’s outcome measures are not up to standard, it is sometimes difficult to determine why.
“The current way that the [outcomes] data are reported and processed is not easily interpretable into which processes we need to adapt,” Dr. Cassivi said. “There is still work that needs to be done, but [this paper] is a first step.”
Dr. Cassivi reported no relevant financial disclosures.
On Twitter @richpizzi
BALTIMORE – An “introspective” analysis connecting patient outcomes with process changes may lead to significant surgical quality improvement, according to a study presented at the 2016 annual meeting of the American Association for Thoracic Surgery.
The case study detailed the University of Alabama at Birmingham School of Medicine’s attempt to identify the metrics used for the Society of Thoracic Surgeons lobectomy ranking, and show how the institution used root cause analysis with “lean” and process improvements to improve outcomes from Jan. 2006 until July 2014 in order to achieve a three star STS ranking.
UAB researchers found that their most common root cause analysis was failure to escalate care. The institution implemented process improvements such as increasing pulmonary rehabilitation prior to surgery, adding a respiratory therapist, eliminating (lean) non-valued steps, favoring stereotactic radiotherapy and segmentectomy instead of lobectomy for marginal patients, and using minimally invasive lobectomy. They ultimately achieved a three-star STS ranking.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Stephen D. Cassivi, professor of surgery at the Mayo Clinic in Rochester, Minn., and a discussant on the paper at AATS 2016, said in an interview that the research was important because it encourages surgeons to discuss and reevaluate quality improvement measures. He noted that early phases of surgical quality improvement was based on process measures, specifically around the idea that if surgeons were attentive to process measures, their outcome measures would improve. But over time, the emphasis on process measures has dissipated in favor of outcomes-focused analysis.
“Now that we have more robust [outcomes] data... we can examine our practices in a more thoughtful, data-driven, evidence-based way,” Dr. Cassivi said. He added that the shift from process measures to outcome measures is important in that surgeons can easily interpret and compare outcomes data across facilities. But he noted that there is a downside: If an institution’s outcome measures are not up to standard, it is sometimes difficult to determine why.
“The current way that the [outcomes] data are reported and processed is not easily interpretable into which processes we need to adapt,” Dr. Cassivi said. “There is still work that needs to be done, but [this paper] is a first step.”
Dr. Cassivi reported no relevant financial disclosures.
On Twitter @richpizzi
AT THE AATS ANNUAL MEETING
Secukinumab may slow structural ankylosing spondylitis progression
LONDON – Long-term treatment with the interleukin 17A inhibitor secukinumab showed suggestive evidence of inhibiting structural progression of spinal disease in 168 patients with ankylosing spondylitis, the first time any evidence for an effect like this has been seen with a biologic drug or any other agent used to treat ankylosing spondylitis.
However, the effect occurred in uncontrolled, 2-year open-label treatment of patients originally enrolled in one of the secukinumab pivotal trials, and the analysis did not include comparison against a historical control group, caveats that demand confirmation of this effect in additional studies, Dr. Jürgen Braun said at the European Congress of Rheumatology.
In a second, unrelated study, the oral Janus kinase inhibitor tofacitinib showed promising efficacy for controlling clinical symptoms in patients with active ankylosing spondylitis (AS) during 12 weeks of treatment in a placebo-controlled, dose-ranging phase II study.
The open-label secukinumab extension study involved patients who had been enrolled in the MEASURE 1 study, one of the pivotal trials that had established secukinumab as safe and effective for improving the clinical status of patients with active AS. The primary endpoint of MEASURE 1 had been the percentage of patients achieving at least a 20% improvement in their Assessment of Spondyloarthritis international Society (ASAS20) response after 16 weeks of treatment (New Engl J Med. 2015 Dec 24;373[26]:2534-48).
Based in part on these data the Food and Drug Administration approved secukinumab (Cosentyx) for the treatment of ankylosing spondylitis in January 2016. The new data reported by Dr. Braun assessed the level of spinal pathology in a subgroup of the MEASURE 1 patients when measured by radiography using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) at baseline and after 104 weeks on secukinumab treatment.
Patients in MEASURE 1 who began on active treatment received 10 mg/kg intravenous secukinumab for 4 weeks, followed by subcutaneous dosages of either 75 mg or 150 mg every 4 weeks for 104 weeks. His analysis also included some patients who entered MEASURE 1 in the placebo group and then switched to open-label, subcutaneous secukinumab treatment after 16 or 24 weeks on placebo.
Analysis of 168 patients who started on intravenous secukinumab and later received any subcutaneous secukinumab treatment out to 104 weeks showed an average increase in mSASSS of 0.30 after 104 weeks when compared against their baseline scores, reported Dr. Braun, professor and medical director of the Ruhr Rheumatology Center of the University of Bochum, Germany.
Among an additional 89 patients who began in the placebo group and then switched to subcutaneous secukinumab, the average change in mSASSS from baseline to 104 weeks was 0.54. By comparison, Dr. Braun noted that AS patients treated with a tumor necrosis factor inhibitor have shown 2-year progression in their mSASSS of about 0.8-0.9, and AS patients not treated with an active biologic drug have shown 2-year mSASSS progression of about 1.0.
A second analysis of the data reported by Dr. Braun showed that among the 168 patients treated for the full 104 weeks with secukinumab about 80% showed no mSASSS progression, but about 20% did have some level of detectable mSASSS progression.
The phase II study of tofacitinib (Xeljanz) randomized 208 patients with active AS to either tofacitinib at daily dosages of 2 mg bid, 5 mg bid, 10 mg bid, or placebo. The study’s primary endpoint was their ASAS20 response after 12 weeks that underwent a Bayesian Emax model analysis to estimate incremental efficacy when compared against placebo.
The primary efficacy analysis showed the greatest EmaxASAS20 response among patients treated with 5 mg bid daily, 63%, which was about 23% above the placebo level, reportedDr. Désirée van der Heijde, professor of rheumatology at the Leiden University Medical Center, The Netherlands. The absolute ASAS20 response rate of the 52 patients randomized to this tofacitinib dosage was about 81%, about 40% higher than the response rate seen in the 51 patients in the placebo arm.
All dosages of tofacitinib tested were well tolerated, with safety data similar to what has previously been shown for tofacitinib, a drug that has Food and Drug Administration approval for treating rheumatoid arthritis.
On Twitter @mitchelzoler
The results on radiographic progression in ankylosing spondylitis patients who continued on secukinumab treatment for 104 weeks suggest for the first time that a biologic drug can reduce radiographic progression of ankylosing spondylitis. This effect has not been seen in patients treated with a tumor necrosis factor inhibitor. The results showed that roughly 80% of the patients maintained for 2 years on secukinumab did not have radiographic progression, although the results also showed that about 20% of these patients did have detectable radiographic progression.
This analysis has several limitations and caveats. The study did not include a control group, not even a historical control group, and it involved open-label treatment. In addition, the treatment effect observed was very close to the level of a measurement error. It would help to compare these results with a historic control group, or to run a new study that compares the effect of secukinumab on long-term radiographic progression directly with the effect of treatment with a tumor necrosis factor inhibitor.
Because of these limitations the results of this analysis are of limited immediate value. Prior results have shown that clinically the efficacy of secukinumab for treating ankylosing spondylitis is more or less the same as the efficacy of various tumor necrosis factor inhibitors. If an additional effect from secukinumab on slowing radiographic progression in these patients were proven, it would be of clear added value, but further study is needed to show this.
The phase II study assessing the impact of tofacitinib, an oral Janus kinase inhibitor, on the clinical activity of ankylosing spondylitis shows promise for this drug in this setting. Currently, the only biologic drugs with proven activity in patients with ankylosing spondylitis are tumor necrosis factor inhibitors and the interleukin 17A inhibitor secukinumab. The data reported by Dr. van der Heijde show that tofacitinib is a good candidate to move to a phase III trial. In this phase II trial the activity seen with 5 mg bid of tofacitinib for reducing disease activity was more or less the same as has been seen with other active biologic drugs.
Dr. Denis Poddubnyy is a professor of rheumatology and head of rheumatology at the Benjamin Franklin campus of Charité Medical University in Berlin. He made these comments in an interview. He has been a consultant to Novartis and to Pfizer and to several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results on radiographic progression in ankylosing spondylitis patients who continued on secukinumab treatment for 104 weeks suggest for the first time that a biologic drug can reduce radiographic progression of ankylosing spondylitis. This effect has not been seen in patients treated with a tumor necrosis factor inhibitor. The results showed that roughly 80% of the patients maintained for 2 years on secukinumab did not have radiographic progression, although the results also showed that about 20% of these patients did have detectable radiographic progression.
This analysis has several limitations and caveats. The study did not include a control group, not even a historical control group, and it involved open-label treatment. In addition, the treatment effect observed was very close to the level of a measurement error. It would help to compare these results with a historic control group, or to run a new study that compares the effect of secukinumab on long-term radiographic progression directly with the effect of treatment with a tumor necrosis factor inhibitor.
Because of these limitations the results of this analysis are of limited immediate value. Prior results have shown that clinically the efficacy of secukinumab for treating ankylosing spondylitis is more or less the same as the efficacy of various tumor necrosis factor inhibitors. If an additional effect from secukinumab on slowing radiographic progression in these patients were proven, it would be of clear added value, but further study is needed to show this.
The phase II study assessing the impact of tofacitinib, an oral Janus kinase inhibitor, on the clinical activity of ankylosing spondylitis shows promise for this drug in this setting. Currently, the only biologic drugs with proven activity in patients with ankylosing spondylitis are tumor necrosis factor inhibitors and the interleukin 17A inhibitor secukinumab. The data reported by Dr. van der Heijde show that tofacitinib is a good candidate to move to a phase III trial. In this phase II trial the activity seen with 5 mg bid of tofacitinib for reducing disease activity was more or less the same as has been seen with other active biologic drugs.
Dr. Denis Poddubnyy is a professor of rheumatology and head of rheumatology at the Benjamin Franklin campus of Charité Medical University in Berlin. He made these comments in an interview. He has been a consultant to Novartis and to Pfizer and to several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results on radiographic progression in ankylosing spondylitis patients who continued on secukinumab treatment for 104 weeks suggest for the first time that a biologic drug can reduce radiographic progression of ankylosing spondylitis. This effect has not been seen in patients treated with a tumor necrosis factor inhibitor. The results showed that roughly 80% of the patients maintained for 2 years on secukinumab did not have radiographic progression, although the results also showed that about 20% of these patients did have detectable radiographic progression.
This analysis has several limitations and caveats. The study did not include a control group, not even a historical control group, and it involved open-label treatment. In addition, the treatment effect observed was very close to the level of a measurement error. It would help to compare these results with a historic control group, or to run a new study that compares the effect of secukinumab on long-term radiographic progression directly with the effect of treatment with a tumor necrosis factor inhibitor.
Because of these limitations the results of this analysis are of limited immediate value. Prior results have shown that clinically the efficacy of secukinumab for treating ankylosing spondylitis is more or less the same as the efficacy of various tumor necrosis factor inhibitors. If an additional effect from secukinumab on slowing radiographic progression in these patients were proven, it would be of clear added value, but further study is needed to show this.
The phase II study assessing the impact of tofacitinib, an oral Janus kinase inhibitor, on the clinical activity of ankylosing spondylitis shows promise for this drug in this setting. Currently, the only biologic drugs with proven activity in patients with ankylosing spondylitis are tumor necrosis factor inhibitors and the interleukin 17A inhibitor secukinumab. The data reported by Dr. van der Heijde show that tofacitinib is a good candidate to move to a phase III trial. In this phase II trial the activity seen with 5 mg bid of tofacitinib for reducing disease activity was more or less the same as has been seen with other active biologic drugs.
Dr. Denis Poddubnyy is a professor of rheumatology and head of rheumatology at the Benjamin Franklin campus of Charité Medical University in Berlin. He made these comments in an interview. He has been a consultant to Novartis and to Pfizer and to several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Long-term treatment with the interleukin 17A inhibitor secukinumab showed suggestive evidence of inhibiting structural progression of spinal disease in 168 patients with ankylosing spondylitis, the first time any evidence for an effect like this has been seen with a biologic drug or any other agent used to treat ankylosing spondylitis.
However, the effect occurred in uncontrolled, 2-year open-label treatment of patients originally enrolled in one of the secukinumab pivotal trials, and the analysis did not include comparison against a historical control group, caveats that demand confirmation of this effect in additional studies, Dr. Jürgen Braun said at the European Congress of Rheumatology.
In a second, unrelated study, the oral Janus kinase inhibitor tofacitinib showed promising efficacy for controlling clinical symptoms in patients with active ankylosing spondylitis (AS) during 12 weeks of treatment in a placebo-controlled, dose-ranging phase II study.
The open-label secukinumab extension study involved patients who had been enrolled in the MEASURE 1 study, one of the pivotal trials that had established secukinumab as safe and effective for improving the clinical status of patients with active AS. The primary endpoint of MEASURE 1 had been the percentage of patients achieving at least a 20% improvement in their Assessment of Spondyloarthritis international Society (ASAS20) response after 16 weeks of treatment (New Engl J Med. 2015 Dec 24;373[26]:2534-48).
Based in part on these data the Food and Drug Administration approved secukinumab (Cosentyx) for the treatment of ankylosing spondylitis in January 2016. The new data reported by Dr. Braun assessed the level of spinal pathology in a subgroup of the MEASURE 1 patients when measured by radiography using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) at baseline and after 104 weeks on secukinumab treatment.
Patients in MEASURE 1 who began on active treatment received 10 mg/kg intravenous secukinumab for 4 weeks, followed by subcutaneous dosages of either 75 mg or 150 mg every 4 weeks for 104 weeks. His analysis also included some patients who entered MEASURE 1 in the placebo group and then switched to open-label, subcutaneous secukinumab treatment after 16 or 24 weeks on placebo.
Analysis of 168 patients who started on intravenous secukinumab and later received any subcutaneous secukinumab treatment out to 104 weeks showed an average increase in mSASSS of 0.30 after 104 weeks when compared against their baseline scores, reported Dr. Braun, professor and medical director of the Ruhr Rheumatology Center of the University of Bochum, Germany.
Among an additional 89 patients who began in the placebo group and then switched to subcutaneous secukinumab, the average change in mSASSS from baseline to 104 weeks was 0.54. By comparison, Dr. Braun noted that AS patients treated with a tumor necrosis factor inhibitor have shown 2-year progression in their mSASSS of about 0.8-0.9, and AS patients not treated with an active biologic drug have shown 2-year mSASSS progression of about 1.0.
A second analysis of the data reported by Dr. Braun showed that among the 168 patients treated for the full 104 weeks with secukinumab about 80% showed no mSASSS progression, but about 20% did have some level of detectable mSASSS progression.
The phase II study of tofacitinib (Xeljanz) randomized 208 patients with active AS to either tofacitinib at daily dosages of 2 mg bid, 5 mg bid, 10 mg bid, or placebo. The study’s primary endpoint was their ASAS20 response after 12 weeks that underwent a Bayesian Emax model analysis to estimate incremental efficacy when compared against placebo.
The primary efficacy analysis showed the greatest EmaxASAS20 response among patients treated with 5 mg bid daily, 63%, which was about 23% above the placebo level, reportedDr. Désirée van der Heijde, professor of rheumatology at the Leiden University Medical Center, The Netherlands. The absolute ASAS20 response rate of the 52 patients randomized to this tofacitinib dosage was about 81%, about 40% higher than the response rate seen in the 51 patients in the placebo arm.
All dosages of tofacitinib tested were well tolerated, with safety data similar to what has previously been shown for tofacitinib, a drug that has Food and Drug Administration approval for treating rheumatoid arthritis.
On Twitter @mitchelzoler
LONDON – Long-term treatment with the interleukin 17A inhibitor secukinumab showed suggestive evidence of inhibiting structural progression of spinal disease in 168 patients with ankylosing spondylitis, the first time any evidence for an effect like this has been seen with a biologic drug or any other agent used to treat ankylosing spondylitis.
However, the effect occurred in uncontrolled, 2-year open-label treatment of patients originally enrolled in one of the secukinumab pivotal trials, and the analysis did not include comparison against a historical control group, caveats that demand confirmation of this effect in additional studies, Dr. Jürgen Braun said at the European Congress of Rheumatology.
In a second, unrelated study, the oral Janus kinase inhibitor tofacitinib showed promising efficacy for controlling clinical symptoms in patients with active ankylosing spondylitis (AS) during 12 weeks of treatment in a placebo-controlled, dose-ranging phase II study.
The open-label secukinumab extension study involved patients who had been enrolled in the MEASURE 1 study, one of the pivotal trials that had established secukinumab as safe and effective for improving the clinical status of patients with active AS. The primary endpoint of MEASURE 1 had been the percentage of patients achieving at least a 20% improvement in their Assessment of Spondyloarthritis international Society (ASAS20) response after 16 weeks of treatment (New Engl J Med. 2015 Dec 24;373[26]:2534-48).
Based in part on these data the Food and Drug Administration approved secukinumab (Cosentyx) for the treatment of ankylosing spondylitis in January 2016. The new data reported by Dr. Braun assessed the level of spinal pathology in a subgroup of the MEASURE 1 patients when measured by radiography using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) at baseline and after 104 weeks on secukinumab treatment.
Patients in MEASURE 1 who began on active treatment received 10 mg/kg intravenous secukinumab for 4 weeks, followed by subcutaneous dosages of either 75 mg or 150 mg every 4 weeks for 104 weeks. His analysis also included some patients who entered MEASURE 1 in the placebo group and then switched to open-label, subcutaneous secukinumab treatment after 16 or 24 weeks on placebo.
Analysis of 168 patients who started on intravenous secukinumab and later received any subcutaneous secukinumab treatment out to 104 weeks showed an average increase in mSASSS of 0.30 after 104 weeks when compared against their baseline scores, reported Dr. Braun, professor and medical director of the Ruhr Rheumatology Center of the University of Bochum, Germany.
Among an additional 89 patients who began in the placebo group and then switched to subcutaneous secukinumab, the average change in mSASSS from baseline to 104 weeks was 0.54. By comparison, Dr. Braun noted that AS patients treated with a tumor necrosis factor inhibitor have shown 2-year progression in their mSASSS of about 0.8-0.9, and AS patients not treated with an active biologic drug have shown 2-year mSASSS progression of about 1.0.
A second analysis of the data reported by Dr. Braun showed that among the 168 patients treated for the full 104 weeks with secukinumab about 80% showed no mSASSS progression, but about 20% did have some level of detectable mSASSS progression.
The phase II study of tofacitinib (Xeljanz) randomized 208 patients with active AS to either tofacitinib at daily dosages of 2 mg bid, 5 mg bid, 10 mg bid, or placebo. The study’s primary endpoint was their ASAS20 response after 12 weeks that underwent a Bayesian Emax model analysis to estimate incremental efficacy when compared against placebo.
The primary efficacy analysis showed the greatest EmaxASAS20 response among patients treated with 5 mg bid daily, 63%, which was about 23% above the placebo level, reportedDr. Désirée van der Heijde, professor of rheumatology at the Leiden University Medical Center, The Netherlands. The absolute ASAS20 response rate of the 52 patients randomized to this tofacitinib dosage was about 81%, about 40% higher than the response rate seen in the 51 patients in the placebo arm.
All dosages of tofacitinib tested were well tolerated, with safety data similar to what has previously been shown for tofacitinib, a drug that has Food and Drug Administration approval for treating rheumatoid arthritis.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: Ankylosing spondylitis patients maintained on open-label secukinumab treatment for 2 years showed a low level of structural spinal progression in an uncontrolled study. Also, in a placebo-controlled phase II study, 12 weeks’ treatment of patients with active ankylosing spondylitis with 5 mg tofacitinib bid led to significant clinical responses, compared with placebo.
Major finding: Little to no radiographic progression occurred in about 80% of ankylosing spondylitis patients maintained on secukinumab for 104 weeks.
Data source: The secukinumab study involved an open-label, nonrandomized extension of treatment in 168 of the 371 patients originally enrolled in MEASURE 1. The tofacitinib study included 208 patients.
Disclosures: Patients in the secukinumab study had been enrolled in MEASURE 1, a study sponsored by Novartis, the company that markets secukinumab (Cosentyx). Dr. Braun has been a consultant to Novartis, and several other drug companies, and three of his coauthors are Novartis employees. The tofacitinib study was sponsored by Pfizer, the company that markets tofacitinib (Xeljanz). Dr. van der Heijde has been a consultant to Pfizer and several other drug companies, and five of her coauthors are Pfizer employees.