User login
Intragestational injection of methotrexate
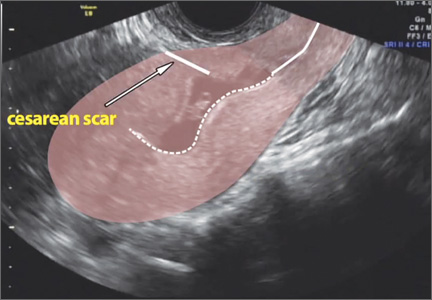
The presentation of a cesarean scar ectopic pregnancy can at times be daunting, especially without familiarity regarding its management. Women with cesarean scar ectopic pregnancy most often have no symptoms, although vaginal bleeding and abdominal pain can present. Upon visual diagnosis with transabdominal or transvaginal ultrasound, the preferred treatment method is direct injection of methotrexate into the gestational sac within the cesarean scar.
In this video, my colleagues review the indications and contraindications for direct injection of methotrexate as well as alternative treatment methods for this type of nonviable pregnancy that is increasing in frequency (given the US cesarean delivery rate). Demonstrated is the technique for methotrexate injection in the case of a 34-year-old woman (G6P0232) with ultrasound and beta−human chorionic gonadotropin confirmation of cesarean scar ectopic pregnancy.
We hope this video serves as a useful reference in your practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The presentation of a cesarean scar ectopic pregnancy can at times be daunting, especially without familiarity regarding its management. Women with cesarean scar ectopic pregnancy most often have no symptoms, although vaginal bleeding and abdominal pain can present. Upon visual diagnosis with transabdominal or transvaginal ultrasound, the preferred treatment method is direct injection of methotrexate into the gestational sac within the cesarean scar.
In this video, my colleagues review the indications and contraindications for direct injection of methotrexate as well as alternative treatment methods for this type of nonviable pregnancy that is increasing in frequency (given the US cesarean delivery rate). Demonstrated is the technique for methotrexate injection in the case of a 34-year-old woman (G6P0232) with ultrasound and beta−human chorionic gonadotropin confirmation of cesarean scar ectopic pregnancy.
We hope this video serves as a useful reference in your practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The presentation of a cesarean scar ectopic pregnancy can at times be daunting, especially without familiarity regarding its management. Women with cesarean scar ectopic pregnancy most often have no symptoms, although vaginal bleeding and abdominal pain can present. Upon visual diagnosis with transabdominal or transvaginal ultrasound, the preferred treatment method is direct injection of methotrexate into the gestational sac within the cesarean scar.
In this video, my colleagues review the indications and contraindications for direct injection of methotrexate as well as alternative treatment methods for this type of nonviable pregnancy that is increasing in frequency (given the US cesarean delivery rate). Demonstrated is the technique for methotrexate injection in the case of a 34-year-old woman (G6P0232) with ultrasound and beta−human chorionic gonadotropin confirmation of cesarean scar ectopic pregnancy.
We hope this video serves as a useful reference in your practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Benefits vs Risks of Cancer Screening
The VA is conducting a limited lung cancer screening pilot at 8 VA hospitals to better understand how to implement screening nationwide. Through this pilot, VA is attempting to identify the right physicians to offer screening during the course of routine care; determine the uptake of screening and what resources will be needed to meet the demand; establish appropriate screening and follow-up with accuracy, efficiency, and safety; and answer whether the overall outcomes of patients with early-stage cancer (especially those who are older with comorbid conditions) benefit from early detection.
Among many of the World Health Organization’s screening principles, Michael J. Kelley, MD, called attention to the organization’s concern that the natural history of the condition should be adequately understood. “I think the key word there is adequately,” said Dr. Kelley. “When you look at the development of disease from precursor lesions, do we really understand what is going to become a cancer that causes disease as opposed to that which is a cancer only to the pathologist?”
The VA is conducting a limited lung cancer screening pilot at 8 VA hospitals to better understand how to implement screening nationwide. Through this pilot, VA is attempting to identify the right physicians to offer screening during the course of routine care; determine the uptake of screening and what resources will be needed to meet the demand; establish appropriate screening and follow-up with accuracy, efficiency, and safety; and answer whether the overall outcomes of patients with early-stage cancer (especially those who are older with comorbid conditions) benefit from early detection.
Among many of the World Health Organization’s screening principles, Michael J. Kelley, MD, called attention to the organization’s concern that the natural history of the condition should be adequately understood. “I think the key word there is adequately,” said Dr. Kelley. “When you look at the development of disease from precursor lesions, do we really understand what is going to become a cancer that causes disease as opposed to that which is a cancer only to the pathologist?”
The VA is conducting a limited lung cancer screening pilot at 8 VA hospitals to better understand how to implement screening nationwide. Through this pilot, VA is attempting to identify the right physicians to offer screening during the course of routine care; determine the uptake of screening and what resources will be needed to meet the demand; establish appropriate screening and follow-up with accuracy, efficiency, and safety; and answer whether the overall outcomes of patients with early-stage cancer (especially those who are older with comorbid conditions) benefit from early detection.
Among many of the World Health Organization’s screening principles, Michael J. Kelley, MD, called attention to the organization’s concern that the natural history of the condition should be adequately understood. “I think the key word there is adequately,” said Dr. Kelley. “When you look at the development of disease from precursor lesions, do we really understand what is going to become a cancer that causes disease as opposed to that which is a cancer only to the pathologist?”
What do you do when clozapine fails?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A survey of liability claims against obstetric providers highlights major areas of contention
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common allegationsby patients1:
- a delay in the treatment of fetal distress (22%). The term “fetal distress” remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
Where are the really big malpractice awards?
Everything may be bigger in Texas, but New York is the biggest in at least 1 area: large medical malpractice payments. New York had more than 3 times as many $1 million-plus malpractice awards as any other state in 2014, according to data from the National Practitioner Data Bank (NPDB).1
New York physicians had 210 malpractice payments of $1 million or more reported to the NPDB last year, compared with 61 for Illinois, the next-highest state. Rounding out the top 5 were Massachusetts with 49, followed by California with 43, and New Jersey with 41, the NPDB data show.
After taking population into account, New York was still the leader with 10.66 large awards per million residents. Next in this category was the New England trio of Rhode Island, which had 9.42 such payments per 1 million population; Massachusetts (7.26); and Connecticut (6.39).
In 2014, there were 4 states that had no malpractice payments of at least $1 million reported to the NPDB: Alaska, Kansas, North Dakota, and Nebraska, with Kansas having the largest population. In states with at least one $1 million-plus malpractice payment, Texas physicians had the lowest rate per million population, 0.22—just 6 awards from a population of 27 million.
Reference
1. NPDB Research Statistics. National Practitioner Data Bank. http://www.npdb.hrsa.gov/resources/npdbstats/npdbStatistics.jsp. Accessed
July 17, 2015.
Copyright © 2015 Ob.Gyn. News Digital Network, Frontline Medical Communications. Available at: http://www.obgynews.com/?id=11146&tx_ttnews[tt_news]=417377&cHash=5cc8cd69fa7c8a1186aaeec0e814e4e4
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD,a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco (see “Frequent sources of malpractice claims” below).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
Frequent sources of malpractice claims
Communication breakdowns and treatment delays are frequent sources of malpractice claims. Susan Mann, MD, spokesperson for The Doctors Company, the nation’s largest physician-owned medical malpractice insurer, discusses the underlying practice vulnerabilities revealed by the Obstetrics Closed Claims Study.
Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims1:
- Selection and management of therapy(34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
- Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
- Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
- Communication problems among providers (17%).
- Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
- Insufficient notes or a lack of documentation (14%).
- Communication problems between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”1 See “Tips for reducing malpractice claims in obstetrics” below.
Tips for reducing malpractice claims in obstetrics1
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.1
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
1. The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/Pa tient Safety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common allegationsby patients1:
- a delay in the treatment of fetal distress (22%). The term “fetal distress” remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
Where are the really big malpractice awards?
Everything may be bigger in Texas, but New York is the biggest in at least 1 area: large medical malpractice payments. New York had more than 3 times as many $1 million-plus malpractice awards as any other state in 2014, according to data from the National Practitioner Data Bank (NPDB).1
New York physicians had 210 malpractice payments of $1 million or more reported to the NPDB last year, compared with 61 for Illinois, the next-highest state. Rounding out the top 5 were Massachusetts with 49, followed by California with 43, and New Jersey with 41, the NPDB data show.
After taking population into account, New York was still the leader with 10.66 large awards per million residents. Next in this category was the New England trio of Rhode Island, which had 9.42 such payments per 1 million population; Massachusetts (7.26); and Connecticut (6.39).
In 2014, there were 4 states that had no malpractice payments of at least $1 million reported to the NPDB: Alaska, Kansas, North Dakota, and Nebraska, with Kansas having the largest population. In states with at least one $1 million-plus malpractice payment, Texas physicians had the lowest rate per million population, 0.22—just 6 awards from a population of 27 million.
Reference
1. NPDB Research Statistics. National Practitioner Data Bank. http://www.npdb.hrsa.gov/resources/npdbstats/npdbStatistics.jsp. Accessed
July 17, 2015.
Copyright © 2015 Ob.Gyn. News Digital Network, Frontline Medical Communications. Available at: http://www.obgynews.com/?id=11146&tx_ttnews[tt_news]=417377&cHash=5cc8cd69fa7c8a1186aaeec0e814e4e4
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD,a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco (see “Frequent sources of malpractice claims” below).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
Frequent sources of malpractice claims
Communication breakdowns and treatment delays are frequent sources of malpractice claims. Susan Mann, MD, spokesperson for The Doctors Company, the nation’s largest physician-owned medical malpractice insurer, discusses the underlying practice vulnerabilities revealed by the Obstetrics Closed Claims Study.
Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims1:
- Selection and management of therapy(34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
- Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
- Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
- Communication problems among providers (17%).
- Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
- Insufficient notes or a lack of documentation (14%).
- Communication problems between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”1 See “Tips for reducing malpractice claims in obstetrics” below.
Tips for reducing malpractice claims in obstetrics1
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.1
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common allegationsby patients1:
- a delay in the treatment of fetal distress (22%). The term “fetal distress” remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
Where are the really big malpractice awards?
Everything may be bigger in Texas, but New York is the biggest in at least 1 area: large medical malpractice payments. New York had more than 3 times as many $1 million-plus malpractice awards as any other state in 2014, according to data from the National Practitioner Data Bank (NPDB).1
New York physicians had 210 malpractice payments of $1 million or more reported to the NPDB last year, compared with 61 for Illinois, the next-highest state. Rounding out the top 5 were Massachusetts with 49, followed by California with 43, and New Jersey with 41, the NPDB data show.
After taking population into account, New York was still the leader with 10.66 large awards per million residents. Next in this category was the New England trio of Rhode Island, which had 9.42 such payments per 1 million population; Massachusetts (7.26); and Connecticut (6.39).
In 2014, there were 4 states that had no malpractice payments of at least $1 million reported to the NPDB: Alaska, Kansas, North Dakota, and Nebraska, with Kansas having the largest population. In states with at least one $1 million-plus malpractice payment, Texas physicians had the lowest rate per million population, 0.22—just 6 awards from a population of 27 million.
Reference
1. NPDB Research Statistics. National Practitioner Data Bank. http://www.npdb.hrsa.gov/resources/npdbstats/npdbStatistics.jsp. Accessed
July 17, 2015.
Copyright © 2015 Ob.Gyn. News Digital Network, Frontline Medical Communications. Available at: http://www.obgynews.com/?id=11146&tx_ttnews[tt_news]=417377&cHash=5cc8cd69fa7c8a1186aaeec0e814e4e4
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD,a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco (see “Frequent sources of malpractice claims” below).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
Frequent sources of malpractice claims
Communication breakdowns and treatment delays are frequent sources of malpractice claims. Susan Mann, MD, spokesperson for The Doctors Company, the nation’s largest physician-owned medical malpractice insurer, discusses the underlying practice vulnerabilities revealed by the Obstetrics Closed Claims Study.
Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims1:
- Selection and management of therapy(34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
- Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
- Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
- Communication problems among providers (17%).
- Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
- Insufficient notes or a lack of documentation (14%).
- Communication problems between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”1 See “Tips for reducing malpractice claims in obstetrics” below.
Tips for reducing malpractice claims in obstetrics1
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.1
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
1. The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/Pa tient Safety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
Reference
1. The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/Pa tient Safety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
In this Article
- Tips for reducing malpractice claims in obstetrics
- Where are the really big malpractice awards?
- Top 7 factors contributing topatient injury
VIDEO: Keeping the Alzheimer’s big picture in mind as antiamyloid trials continue
WASHINGTON – Amyloid-beta has been the Alzheimer’s research darling for more than a decade, and finally, after many failures, some small successes may be appearing in antiamyloid trials.
Experts suggest that these encouraging early results may lead companies to retest failed drugs under new conditions – for example, by using patients who test positive for amyloid-beta on brain PET scans, who have prodromal disease, or who are asymptomatic.
But questions remain, and some are fundamental. The functional nature of amyloid remains unknown. There are still questions about which form is the most neurotoxic. Little is understood about the way it interacts with tau as symptoms emerge.
Will these lines of investigation fall to the wayside if companies and amyloid-centric researchers put too many eggs into the antiamyloid basket? Dr. Michael Wolfe, Ph.D., of Brigham and Women’s Hospital, Boston, discusses these issues in an interview.
On Twitter @alz_gal
WASHINGTON – Amyloid-beta has been the Alzheimer’s research darling for more than a decade, and finally, after many failures, some small successes may be appearing in antiamyloid trials.
Experts suggest that these encouraging early results may lead companies to retest failed drugs under new conditions – for example, by using patients who test positive for amyloid-beta on brain PET scans, who have prodromal disease, or who are asymptomatic.
But questions remain, and some are fundamental. The functional nature of amyloid remains unknown. There are still questions about which form is the most neurotoxic. Little is understood about the way it interacts with tau as symptoms emerge.
Will these lines of investigation fall to the wayside if companies and amyloid-centric researchers put too many eggs into the antiamyloid basket? Dr. Michael Wolfe, Ph.D., of Brigham and Women’s Hospital, Boston, discusses these issues in an interview.
On Twitter @alz_gal
WASHINGTON – Amyloid-beta has been the Alzheimer’s research darling for more than a decade, and finally, after many failures, some small successes may be appearing in antiamyloid trials.
Experts suggest that these encouraging early results may lead companies to retest failed drugs under new conditions – for example, by using patients who test positive for amyloid-beta on brain PET scans, who have prodromal disease, or who are asymptomatic.
But questions remain, and some are fundamental. The functional nature of amyloid remains unknown. There are still questions about which form is the most neurotoxic. Little is understood about the way it interacts with tau as symptoms emerge.
Will these lines of investigation fall to the wayside if companies and amyloid-centric researchers put too many eggs into the antiamyloid basket? Dr. Michael Wolfe, Ph.D., of Brigham and Women’s Hospital, Boston, discusses these issues in an interview.
On Twitter @alz_gal
AT AAIC 2015
VIDEO: Alzheimer’s disease drug development targets disease modification
WASHINGTON – Finding a drug therapy for patients with Alzheimer’s disease that not only improves symptoms but also slows or stops the underlying disease process and results in disease modification will be a major challenge.
Disease modification “indicates the drug is attacking the underlying biology of the disease. The medications we have now affect only symptoms and are palliative,” Dr. David S. Knopman said in an interview during the Alzheimer’s Association International Conference 2015.
Designing trials capable of identifying disease-modifying drugs “turns out to be very challenging. In principle, a drug with disease-modifying effects would have bigger and more enduring effects, could be started earlier in the disease, and would ultimately be of greater benefit to patients and to society,” said Dr. Knopman, a professor of neurology at the Mayo Clinic in Rochester, Minn. He participated in a session at the meeting focused on the potential design of trials that could test a drug’s disease-modifying effect.
The most likely design that researchers seem ready to use is a “delayed-start” trial, in which placebo-treated patients who serve as controls in the initial, blinded, and randomized phase of an efficacy trial then cross over to open-label treatment once the first segment primary-endpoint stage is finished. In most trials “the open-label, long-term extension will occur anyway,” so adding a delayed-start element following the end of an efficacy trial “does not add a lot of complication to the design,” he said.
Two factors make a delayed-start analysis challenging. First, the drug needs to show efficacy during the initial, double-blinded phase. “Only if you see both a cognitive and some sort of functional-outcome benefit can you engage in the delayed-start analysis, to see if the effect is enduring,” Dr. Knopman said. The second limitation is patient drop out. “An open-label, long-term extension over another 1, 2, 3 years will invariably lead to subjects dropping out because of health issues or social matters and that makes the statistical analysis more complicated.” During one trial that was discussed in depth at the session, about 40% of patients who entered the delayed-start phase had left the study by the time this stage finished 2 years later.
Despite these issues, adding a delayed-start phase to drug trials likely will become increasingly common, Dr. Knopman predicted. Drug developers “will probably include this as a secondary analysis because it doesn’t add much expense or added burden on participants, so it seems like a win-win.” Plus, representatives from the Food and Drug Administration who participated in the session seemed to endorse the general concept, he noted. But the most important caveat remains, he stressed: “You have to first show primary-outcome results. Only then you can talk about disease modification.”
The delayed-start trial design was developed by Eli Lilly. Dr. Knopman received an honorarium from Lilly for chairing the data and safety-monitoring committee for two of their trials through 2012, but since then he has not had a financial relationship with the company. He currently is an investigator in a trial sponsored by Lilly. He said he has no other disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
WASHINGTON – Finding a drug therapy for patients with Alzheimer’s disease that not only improves symptoms but also slows or stops the underlying disease process and results in disease modification will be a major challenge.
Disease modification “indicates the drug is attacking the underlying biology of the disease. The medications we have now affect only symptoms and are palliative,” Dr. David S. Knopman said in an interview during the Alzheimer’s Association International Conference 2015.
Designing trials capable of identifying disease-modifying drugs “turns out to be very challenging. In principle, a drug with disease-modifying effects would have bigger and more enduring effects, could be started earlier in the disease, and would ultimately be of greater benefit to patients and to society,” said Dr. Knopman, a professor of neurology at the Mayo Clinic in Rochester, Minn. He participated in a session at the meeting focused on the potential design of trials that could test a drug’s disease-modifying effect.
The most likely design that researchers seem ready to use is a “delayed-start” trial, in which placebo-treated patients who serve as controls in the initial, blinded, and randomized phase of an efficacy trial then cross over to open-label treatment once the first segment primary-endpoint stage is finished. In most trials “the open-label, long-term extension will occur anyway,” so adding a delayed-start element following the end of an efficacy trial “does not add a lot of complication to the design,” he said.
Two factors make a delayed-start analysis challenging. First, the drug needs to show efficacy during the initial, double-blinded phase. “Only if you see both a cognitive and some sort of functional-outcome benefit can you engage in the delayed-start analysis, to see if the effect is enduring,” Dr. Knopman said. The second limitation is patient drop out. “An open-label, long-term extension over another 1, 2, 3 years will invariably lead to subjects dropping out because of health issues or social matters and that makes the statistical analysis more complicated.” During one trial that was discussed in depth at the session, about 40% of patients who entered the delayed-start phase had left the study by the time this stage finished 2 years later.
Despite these issues, adding a delayed-start phase to drug trials likely will become increasingly common, Dr. Knopman predicted. Drug developers “will probably include this as a secondary analysis because it doesn’t add much expense or added burden on participants, so it seems like a win-win.” Plus, representatives from the Food and Drug Administration who participated in the session seemed to endorse the general concept, he noted. But the most important caveat remains, he stressed: “You have to first show primary-outcome results. Only then you can talk about disease modification.”
The delayed-start trial design was developed by Eli Lilly. Dr. Knopman received an honorarium from Lilly for chairing the data and safety-monitoring committee for two of their trials through 2012, but since then he has not had a financial relationship with the company. He currently is an investigator in a trial sponsored by Lilly. He said he has no other disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
WASHINGTON – Finding a drug therapy for patients with Alzheimer’s disease that not only improves symptoms but also slows or stops the underlying disease process and results in disease modification will be a major challenge.
Disease modification “indicates the drug is attacking the underlying biology of the disease. The medications we have now affect only symptoms and are palliative,” Dr. David S. Knopman said in an interview during the Alzheimer’s Association International Conference 2015.
Designing trials capable of identifying disease-modifying drugs “turns out to be very challenging. In principle, a drug with disease-modifying effects would have bigger and more enduring effects, could be started earlier in the disease, and would ultimately be of greater benefit to patients and to society,” said Dr. Knopman, a professor of neurology at the Mayo Clinic in Rochester, Minn. He participated in a session at the meeting focused on the potential design of trials that could test a drug’s disease-modifying effect.
The most likely design that researchers seem ready to use is a “delayed-start” trial, in which placebo-treated patients who serve as controls in the initial, blinded, and randomized phase of an efficacy trial then cross over to open-label treatment once the first segment primary-endpoint stage is finished. In most trials “the open-label, long-term extension will occur anyway,” so adding a delayed-start element following the end of an efficacy trial “does not add a lot of complication to the design,” he said.
Two factors make a delayed-start analysis challenging. First, the drug needs to show efficacy during the initial, double-blinded phase. “Only if you see both a cognitive and some sort of functional-outcome benefit can you engage in the delayed-start analysis, to see if the effect is enduring,” Dr. Knopman said. The second limitation is patient drop out. “An open-label, long-term extension over another 1, 2, 3 years will invariably lead to subjects dropping out because of health issues or social matters and that makes the statistical analysis more complicated.” During one trial that was discussed in depth at the session, about 40% of patients who entered the delayed-start phase had left the study by the time this stage finished 2 years later.
Despite these issues, adding a delayed-start phase to drug trials likely will become increasingly common, Dr. Knopman predicted. Drug developers “will probably include this as a secondary analysis because it doesn’t add much expense or added burden on participants, so it seems like a win-win.” Plus, representatives from the Food and Drug Administration who participated in the session seemed to endorse the general concept, he noted. But the most important caveat remains, he stressed: “You have to first show primary-outcome results. Only then you can talk about disease modification.”
The delayed-start trial design was developed by Eli Lilly. Dr. Knopman received an honorarium from Lilly for chairing the data and safety-monitoring committee for two of their trials through 2012, but since then he has not had a financial relationship with the company. He currently is an investigator in a trial sponsored by Lilly. He said he has no other disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
AT AAIC 2015
VIDEO: Postoperative cognitive decline hits women hardest
WASHINGTON – Postoperative cognitive decline, which occurs in roughly 20% of elderly patients who undergo major surgery, strikes older women with greater severity than it does similarly aged men, according to a retrospective analysis of data collected from 527 older Americans.
“We looked at the sex difference in postoperative cognitive decline and Alzheimer’s disease because of the sex difference in Alzheimer’s disease, where about two-thirds of patients are women,” Dr. Katie J. Schenning said in an interview during the Alzheimer’s Association International Conference 2015. “We know that a lot of the pathologic changes that happen to the brain after anesthesia and surgery are similar to the changes that happen in Alzheimer’s disease patients. But at this point that is all we can say about a link between the two. It is currently unknown whether there is a clear relationship between postoperative cognitive decline and Alzheimer’s disease,” said Dr. Schenning, an anesthesiologist at Oregon Health & Science University in Portland.
She and her associates studied data collected longitudinally from two cohorts, the Oregon Brain Aging Study and the Intelligent Systems for Assessment of Aging Changes. At baseline, the average age of the enrollees in the combined group was 83 years, and just under two-thirds were women. During follow-up, 182 of the participants underwent a total of 331 major surgeries, with some undergoing more than one surgery. The most common form of surgery was orthopedic, done in one-third of the patients, followed by general surgery, in a quarter.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The researchers running both studies collected data annually from participants using a battery of neuropsychological evaluations, brain MRIs, and information on their general health. Retrospective analysis of the data showed that following surgery, people showed evidence of statistically significant and clinically meaningful declines in several measures, compared with those who did not undergo surgery, including deficits measured by the Mini-Mental State Examination, instrumental activities of daily living, and logical memory delayed recall. The trajectory of these declines was significantly steeper in women following surgery, compared with men following surgery, Dr. Schenning reported in a poster at the meeting. In addition, the MRI scans showed ventricular enlargement in the postsurgical women but not in men, a change that is characteristic of neuropathology.
“Women who underwent surgery had a more rapid rate of decline in measures of cognition and function than women who did not have surgery, and it affected women in more categories than in men,” Dr. Schenning said.
The risk for postoperative cognitive decline “is one of the things that patients should take into consideration before undergoing elective surgery, especially if they are older or have pre-existing cognitive impairment,” Dr. Schenning suggested. The enhanced risk for postsurgical cognitive decline faced by older women and even the somewhat lesser risk that exists for older men “is certainly something that patients should discuss with their surgeon, anesthesiologist, and family members,” she said.
Dr. Schenning had no disclosures.
On Twitter @mitchelzoler
WASHINGTON – Postoperative cognitive decline, which occurs in roughly 20% of elderly patients who undergo major surgery, strikes older women with greater severity than it does similarly aged men, according to a retrospective analysis of data collected from 527 older Americans.
“We looked at the sex difference in postoperative cognitive decline and Alzheimer’s disease because of the sex difference in Alzheimer’s disease, where about two-thirds of patients are women,” Dr. Katie J. Schenning said in an interview during the Alzheimer’s Association International Conference 2015. “We know that a lot of the pathologic changes that happen to the brain after anesthesia and surgery are similar to the changes that happen in Alzheimer’s disease patients. But at this point that is all we can say about a link between the two. It is currently unknown whether there is a clear relationship between postoperative cognitive decline and Alzheimer’s disease,” said Dr. Schenning, an anesthesiologist at Oregon Health & Science University in Portland.
She and her associates studied data collected longitudinally from two cohorts, the Oregon Brain Aging Study and the Intelligent Systems for Assessment of Aging Changes. At baseline, the average age of the enrollees in the combined group was 83 years, and just under two-thirds were women. During follow-up, 182 of the participants underwent a total of 331 major surgeries, with some undergoing more than one surgery. The most common form of surgery was orthopedic, done in one-third of the patients, followed by general surgery, in a quarter.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The researchers running both studies collected data annually from participants using a battery of neuropsychological evaluations, brain MRIs, and information on their general health. Retrospective analysis of the data showed that following surgery, people showed evidence of statistically significant and clinically meaningful declines in several measures, compared with those who did not undergo surgery, including deficits measured by the Mini-Mental State Examination, instrumental activities of daily living, and logical memory delayed recall. The trajectory of these declines was significantly steeper in women following surgery, compared with men following surgery, Dr. Schenning reported in a poster at the meeting. In addition, the MRI scans showed ventricular enlargement in the postsurgical women but not in men, a change that is characteristic of neuropathology.
“Women who underwent surgery had a more rapid rate of decline in measures of cognition and function than women who did not have surgery, and it affected women in more categories than in men,” Dr. Schenning said.
The risk for postoperative cognitive decline “is one of the things that patients should take into consideration before undergoing elective surgery, especially if they are older or have pre-existing cognitive impairment,” Dr. Schenning suggested. The enhanced risk for postsurgical cognitive decline faced by older women and even the somewhat lesser risk that exists for older men “is certainly something that patients should discuss with their surgeon, anesthesiologist, and family members,” she said.
Dr. Schenning had no disclosures.
On Twitter @mitchelzoler
WASHINGTON – Postoperative cognitive decline, which occurs in roughly 20% of elderly patients who undergo major surgery, strikes older women with greater severity than it does similarly aged men, according to a retrospective analysis of data collected from 527 older Americans.
“We looked at the sex difference in postoperative cognitive decline and Alzheimer’s disease because of the sex difference in Alzheimer’s disease, where about two-thirds of patients are women,” Dr. Katie J. Schenning said in an interview during the Alzheimer’s Association International Conference 2015. “We know that a lot of the pathologic changes that happen to the brain after anesthesia and surgery are similar to the changes that happen in Alzheimer’s disease patients. But at this point that is all we can say about a link between the two. It is currently unknown whether there is a clear relationship between postoperative cognitive decline and Alzheimer’s disease,” said Dr. Schenning, an anesthesiologist at Oregon Health & Science University in Portland.
She and her associates studied data collected longitudinally from two cohorts, the Oregon Brain Aging Study and the Intelligent Systems for Assessment of Aging Changes. At baseline, the average age of the enrollees in the combined group was 83 years, and just under two-thirds were women. During follow-up, 182 of the participants underwent a total of 331 major surgeries, with some undergoing more than one surgery. The most common form of surgery was orthopedic, done in one-third of the patients, followed by general surgery, in a quarter.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The researchers running both studies collected data annually from participants using a battery of neuropsychological evaluations, brain MRIs, and information on their general health. Retrospective analysis of the data showed that following surgery, people showed evidence of statistically significant and clinically meaningful declines in several measures, compared with those who did not undergo surgery, including deficits measured by the Mini-Mental State Examination, instrumental activities of daily living, and logical memory delayed recall. The trajectory of these declines was significantly steeper in women following surgery, compared with men following surgery, Dr. Schenning reported in a poster at the meeting. In addition, the MRI scans showed ventricular enlargement in the postsurgical women but not in men, a change that is characteristic of neuropathology.
“Women who underwent surgery had a more rapid rate of decline in measures of cognition and function than women who did not have surgery, and it affected women in more categories than in men,” Dr. Schenning said.
The risk for postoperative cognitive decline “is one of the things that patients should take into consideration before undergoing elective surgery, especially if they are older or have pre-existing cognitive impairment,” Dr. Schenning suggested. The enhanced risk for postsurgical cognitive decline faced by older women and even the somewhat lesser risk that exists for older men “is certainly something that patients should discuss with their surgeon, anesthesiologist, and family members,” she said.
Dr. Schenning had no disclosures.
On Twitter @mitchelzoler
AT AAIC 2015
VIDEO: Investigating the role of plasmalogens in Alzheimer’s disease
WASHINGTON – Could a ubiquitous lipid – manufactured in the liver – be one of the root causes of Alzheimer’s disease? Plasmalogens are important in maintaining cell membrane permeability and effective neurotransmission. They also appear to influence the production of toxic forms of amyloid-beta by affecting the activity of alpha-secretase. What are they, and how strong could their influence in Alzheimer’s be? Dayan Goodenowe, Ph.D., explains at the Alzheimer’s Association International Conference 2015.
Dr. Goodenowe is the founder and CEO of Phenomenome Discoveries, which holds patents on measuring plasmalogen levels and is developing a plasmalogen therapeutic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
WASHINGTON – Could a ubiquitous lipid – manufactured in the liver – be one of the root causes of Alzheimer’s disease? Plasmalogens are important in maintaining cell membrane permeability and effective neurotransmission. They also appear to influence the production of toxic forms of amyloid-beta by affecting the activity of alpha-secretase. What are they, and how strong could their influence in Alzheimer’s be? Dayan Goodenowe, Ph.D., explains at the Alzheimer’s Association International Conference 2015.
Dr. Goodenowe is the founder and CEO of Phenomenome Discoveries, which holds patents on measuring plasmalogen levels and is developing a plasmalogen therapeutic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
WASHINGTON – Could a ubiquitous lipid – manufactured in the liver – be one of the root causes of Alzheimer’s disease? Plasmalogens are important in maintaining cell membrane permeability and effective neurotransmission. They also appear to influence the production of toxic forms of amyloid-beta by affecting the activity of alpha-secretase. What are they, and how strong could their influence in Alzheimer’s be? Dayan Goodenowe, Ph.D., explains at the Alzheimer’s Association International Conference 2015.
Dr. Goodenowe is the founder and CEO of Phenomenome Discoveries, which holds patents on measuring plasmalogen levels and is developing a plasmalogen therapeutic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
AT AAIC 2015
VIDEO: Dementia risk doubled in type 1 diabetes patients
WASHINGTON – Improved management of patients with type 1 diabetes and the resulting increased longevity it has fostered has produced a new medical concern that few patients faced in the past: their risk for developing dementia as they age into their 60s, 70s, and beyond.
That risk, which turns out to be nearly double that of people without diabetes, closely matches the higher risk faced by patients with type 2 diabetes, based on analysis of data collected during 2002-2014 from more than 490,000 people aged 60 years or older enrolled in the Kaiser Permanente Medical Care Program of Northern California, Rachel Whitmer, Ph.D. reported at the Alzheimer’s Association International Conference 2015.
Her study tracked the dementia incidence in 334 of these Kaiser Permanente enrollees with type 1 diabetes and no dementia at baseline and found the rate ran 73% higher when compared with controls without any type of diabetes in the cohort after adjustment for sex, race, and vascular complications such as hypertension, stroke, and peripheral vascular disease. The patients with type 1 diabetes averaged 71 years old at baseline and their follow-up averaged 7 years. This is the first study to look at the incidence of dementia in elderly patients with type 1 diabetes, Dr. Whitmer said in an interview during the meeting.
Physicians who care for patients with type 1 diabetes should be aware of this increased risk, be on the lookout for signs of developing dementia in these patients, and use the increased risk to help motivate type 1 diabetes patients to be vigilant in controlling their disease to help avoid the microvascular complications that likely contribute to their dementia risk. Patients with type 1 diabetes are especially vulnerable to the consequences of cognitive impairment as they must maintain complex self-management routines of blood glucose monitoring, insulin administration, and keeping close tabs on their diet and exercise, Dr. Whitmer noted.
In 2013, she and her associates published a simple and easy-to-use tool for clinicians to assess the dementia risk in individual patients with type 2 diabetes (Lancet Diabetes Endocrinol. 2013;3:183-90). Further study of the risk factors that contribute to dementia onset in patients with type 1 diabetes will hopefully lead to creation of a similar risk-assessment tool for use in type 1 patients, said Dr. Whitmer, an epidemiologist at the Kaiser Permanente Northern California Division of Research in Oakland.
Dr. Whitmer had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
WASHINGTON – Improved management of patients with type 1 diabetes and the resulting increased longevity it has fostered has produced a new medical concern that few patients faced in the past: their risk for developing dementia as they age into their 60s, 70s, and beyond.
That risk, which turns out to be nearly double that of people without diabetes, closely matches the higher risk faced by patients with type 2 diabetes, based on analysis of data collected during 2002-2014 from more than 490,000 people aged 60 years or older enrolled in the Kaiser Permanente Medical Care Program of Northern California, Rachel Whitmer, Ph.D. reported at the Alzheimer’s Association International Conference 2015.
Her study tracked the dementia incidence in 334 of these Kaiser Permanente enrollees with type 1 diabetes and no dementia at baseline and found the rate ran 73% higher when compared with controls without any type of diabetes in the cohort after adjustment for sex, race, and vascular complications such as hypertension, stroke, and peripheral vascular disease. The patients with type 1 diabetes averaged 71 years old at baseline and their follow-up averaged 7 years. This is the first study to look at the incidence of dementia in elderly patients with type 1 diabetes, Dr. Whitmer said in an interview during the meeting.
Physicians who care for patients with type 1 diabetes should be aware of this increased risk, be on the lookout for signs of developing dementia in these patients, and use the increased risk to help motivate type 1 diabetes patients to be vigilant in controlling their disease to help avoid the microvascular complications that likely contribute to their dementia risk. Patients with type 1 diabetes are especially vulnerable to the consequences of cognitive impairment as they must maintain complex self-management routines of blood glucose monitoring, insulin administration, and keeping close tabs on their diet and exercise, Dr. Whitmer noted.
In 2013, she and her associates published a simple and easy-to-use tool for clinicians to assess the dementia risk in individual patients with type 2 diabetes (Lancet Diabetes Endocrinol. 2013;3:183-90). Further study of the risk factors that contribute to dementia onset in patients with type 1 diabetes will hopefully lead to creation of a similar risk-assessment tool for use in type 1 patients, said Dr. Whitmer, an epidemiologist at the Kaiser Permanente Northern California Division of Research in Oakland.
Dr. Whitmer had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
WASHINGTON – Improved management of patients with type 1 diabetes and the resulting increased longevity it has fostered has produced a new medical concern that few patients faced in the past: their risk for developing dementia as they age into their 60s, 70s, and beyond.
That risk, which turns out to be nearly double that of people without diabetes, closely matches the higher risk faced by patients with type 2 diabetes, based on analysis of data collected during 2002-2014 from more than 490,000 people aged 60 years or older enrolled in the Kaiser Permanente Medical Care Program of Northern California, Rachel Whitmer, Ph.D. reported at the Alzheimer’s Association International Conference 2015.
Her study tracked the dementia incidence in 334 of these Kaiser Permanente enrollees with type 1 diabetes and no dementia at baseline and found the rate ran 73% higher when compared with controls without any type of diabetes in the cohort after adjustment for sex, race, and vascular complications such as hypertension, stroke, and peripheral vascular disease. The patients with type 1 diabetes averaged 71 years old at baseline and their follow-up averaged 7 years. This is the first study to look at the incidence of dementia in elderly patients with type 1 diabetes, Dr. Whitmer said in an interview during the meeting.
Physicians who care for patients with type 1 diabetes should be aware of this increased risk, be on the lookout for signs of developing dementia in these patients, and use the increased risk to help motivate type 1 diabetes patients to be vigilant in controlling their disease to help avoid the microvascular complications that likely contribute to their dementia risk. Patients with type 1 diabetes are especially vulnerable to the consequences of cognitive impairment as they must maintain complex self-management routines of blood glucose monitoring, insulin administration, and keeping close tabs on their diet and exercise, Dr. Whitmer noted.
In 2013, she and her associates published a simple and easy-to-use tool for clinicians to assess the dementia risk in individual patients with type 2 diabetes (Lancet Diabetes Endocrinol. 2013;3:183-90). Further study of the risk factors that contribute to dementia onset in patients with type 1 diabetes will hopefully lead to creation of a similar risk-assessment tool for use in type 1 patients, said Dr. Whitmer, an epidemiologist at the Kaiser Permanente Northern California Division of Research in Oakland.
Dr. Whitmer had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT AAIC 2015
Carrier Screening for Duchenne Muscular Dystrophy
In this video, genetic counselor Barbara Petterson discusses the latest crucial advances in the population-wide carrier screening of duchenne muscular dystrophy (DMD). DMD, an X-linked condition, is the most common muscular dystrophy in children and affects families of all ethnicities.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To read the supplement on the pivotal findings discussed in this video, click here.
In this video, genetic counselor Barbara Petterson discusses the latest crucial advances in the population-wide carrier screening of duchenne muscular dystrophy (DMD). DMD, an X-linked condition, is the most common muscular dystrophy in children and affects families of all ethnicities.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To read the supplement on the pivotal findings discussed in this video, click here.
In this video, genetic counselor Barbara Petterson discusses the latest crucial advances in the population-wide carrier screening of duchenne muscular dystrophy (DMD). DMD, an X-linked condition, is the most common muscular dystrophy in children and affects families of all ethnicities.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To read the supplement on the pivotal findings discussed in this video, click here.