User login
The Skin Microbiome in Rosacea: Mechanisms, Gut-Skin Interactions, and Therapeutic Implications
The Skin Microbiome in Rosacea: Mechanisms, Gut-Skin Interactions, and Therapeutic Implications
Rosacea is a chronic inflammatory skin condition affecting the central face—including the cheeks, nose, chin, and forehead—that causes considerable discomfort.1 Its pathogenesis involves immune dysregulation, genetic predisposition, and microbial dysbiosis.2 While immune and environmental factors are known triggers of rosacea, recent research highlights the roles of the gut and skin microbiomes in disease progression. While the skin microbiome interacts directly with the immune system to regulate inflammation and skin homeostasis, the gut microbiome also influences cutaneous inflammation, emphasizing the need to address both topical and internal microbiome imbalances.3 In this article, we review gut and skin microbial alterations in rosacea, focusing on the skin microbiome and including the gut-skin axis implications as well as therapeutic strategies aimed at microbiome balance to enhance patient outcomes.
Skin Microbiome Alterations in Rosacea
The human skin microbiome interacts with the immune system, and microbial imbalances have been shown to contribute to immune dysregulation. Several key microbial species have been identified as playing a large role in rosacea, including Demodex folliculorum, Staphylococcus epidermidis, Bacillus oleronius, and Cutibacterium acnes (Figure).
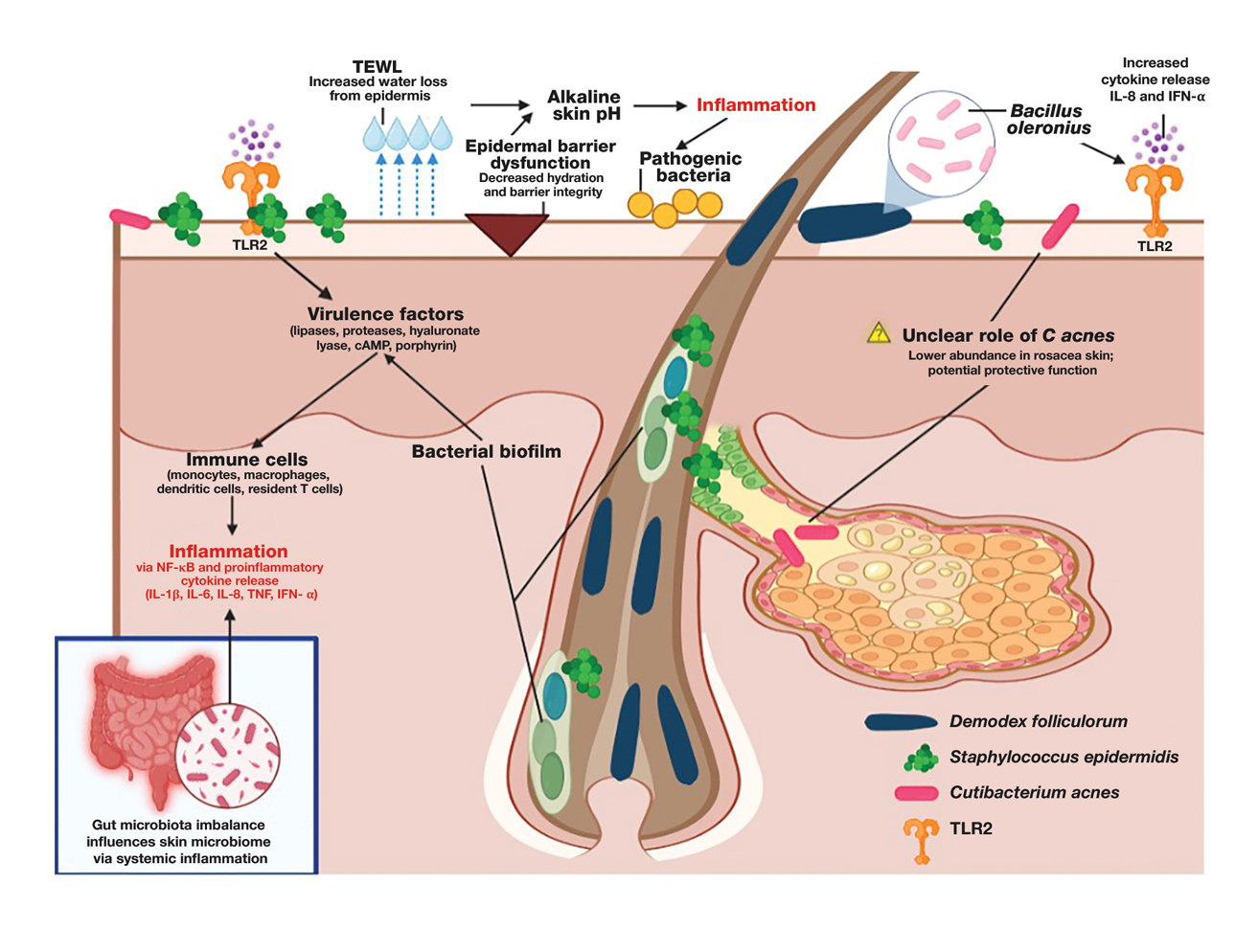
Demodex folliculorum is a microscopic mite is found in hair follicles and sebaceous glands. Patients with rosacea have higher densities of D folliculorum, which trigger follicular occlusion and immune activation.1Bacillus oleronius be isolated from D folliculorum and can further activate toll-like receptor 2, leading to cytokine production and immune cell infiltration.3,4 Increased propagation of this mite correlates with shifts in skin microbiome composition, demonstrating increased inflammatory microbial populations.3
Staphylococcus epidermidis normally is commensal but can become pathogenic (pathobiont) in rosacea due to disruptions in the skin microenvironment, where it can form biofilms and produce virulence factors, particularly in papulopustular rosacea.5
Bacillus oleronius has been isolated from D folliculorum mites and provokes inflammatory responses in patients with rosacea by triggering toll-like receptor 2 activation and cytokine secretion.6
Cutibacterium acnes commonly is associated with acne vulgaris. Its role in rosacea is unclear, but recent research suggests it may have a protective effect. A single-arm trial investigated the effects of minocycline on rosacea and found that treatment significantly reduced C acnes but increased microbial species diversity, improving inflammation.7 One longitudinal cohort study of 12 patients with rosacea found that C acnes levels were lower in those older than 60 years. Rosacea severity increased with age and correlated with a decline in C acnes, suggesting that it may confer some protective effect in rosacea.8 This finding is supported by studies that have shown a reduction in C acnes levels in patients with rosacea compared to controls.4,8
Important mechanisms in rosacea include epidermal barrier dysfunction, transepidermal water loss, and decreased stratum corneum hydration, particularly in erythematotelangiectatic and papulopustular subtypes. The resulting alkaline skin pH contributes to barrier instability and heightened inflammation, permitting pathogenic bacteria to proliferate and disrupt skin microbial homeostasis.9 A recent study identified metabolic changes in the skin microbiome of patients with rosacea, showing that increased heme and hydrogen sulfide in rosacea skin microbiomes likely drive inflammation, while healthy skin microbiomes produce more anti-inflammatory adenosylcobalamin, thiazole, and L-isoleucine.1 These findings highlight the link between microbial imbalances and inflammation in rosacea.
The Gut-Skin Axis in Rosacea
Gut microbiota play a critical role in managing systemic inflammation, and microbial dysbiosis in the intestine can influence the skin microbiome in rosacea. Patients with rosacea who have gastrointestinal conditions such as small intestinal bacterial overgrowth and Helicobacter pylori infection experience more severe rosacea symptoms.3,10
Patients with rosacea have distinctive gut microbiota compositions, with an increased prevalence of proinflammatory bacterial species, potentially affecting the skin microbiome.8,11 Systemic antibiotics have been shown to modulate the gut microbiome, indirectly influencing the skin microbiome.11 A recent study demonstrated that doxycycline treatment in patients with rosacea altered skin microbial diversity, reducing C acnes while increasing Weissella confusa—highlighting the complicated relationship between systemic antibiotics and the gut-skin axis.8
Specific probiotics, such as Escherichia coli Nissle, when given orally shifted gut microbial balance to protective microbiota with increased Lactobacillus and Bifidobacteria species and decreased pathogenic bacteria. This improved rosacea symptoms, normalized immunoglobulin A levels, and suppressed cytokine interleukin 8 levels.10 Recent studies also suggest oral sarecycline, a narrow-spectrum antibiotic, may improve papulopustular rosacea symptoms through its anti-inflammatory effects while having minimal impact on gut microbiota diversity.11,12
Gut-derived short-chain fatty acids, which are known to regulate immune function, also have been shown to influence the composition of skin microbiota, suggesting a direct link between gut dysbiosis and skin microbial imbalances. Notably, antibiotic and probiotic treatments targeting the gut microbiome (eg, rifaximin for small intestinal bacterial overgrowth) have been associated with improvements in rosacea symptoms, further underscoring the interconnectedness of the gut-skin axis.13 Understanding how gut-derived inflammation alters the skin microbiome may provide new therapeutic avenues for restoring microbial balance and reducing rosacea severity.
Immune Dysregulation and Inflammatory Pathways
Mechanisms of microbiome-driven inflammation via the innate immune system contribute to rosacea pathogenesis. Toll-like receptor 2 is upregulated in rosacea, producing increased peptides including cathelicidins.13 When abnormally processed, cathelicidins produce proinflammatory peptides and worsen rosacea symptoms such as erythema, telangiectasias, and neutrophilic infiltration by dysregulating the immune system and the skin barrier.6
Heightened levels of cytokines interleukin 8 and interferon α have been identified in patients with rosacea. These cytokines are involved in rosacea pathogenesis, including leukocyte recruitment, angiogenesis, and tissue remodeling and further activate the inflammatory cascade.8,14
Mendelian randomization studies have provided confirmation of a causal link between skin microbiota alterations and inflammatory skin diseases including rosacea.2 Specific alterations in bacteria such as Cutibacterium and Staphylococcus microbial species have been associated with shifts in host immune gene expression, potentially predisposing individuals to abnormal immune activation and inflammation.2,8 These studies show the potential of leveraging precision medicine to design therapies that target pathways that improve microbial imbalances seen in rosacea.
Environmental and Lifestyle Factors Affecting the Skin Microbiome
Individuals with rosacea often have increased sensitivity to environmental and lifestyle stressors such as high temperatures, UV exposure, and sugar and alcohol consumption. These factors influence the composition of the skin microbiome and potentially contribute to rosacea development and disease exacerbation; therefore, trigger avoidance is an important way to manage rosacea.
High temperatures and UV exposure—Demodex activity increases in response to heat exposure and subsequently worsens rosacea symptoms, while exposure to UV radiation can change the composition of the skin microbiome by encouraging inflammatory responses such as oxidative stress reactions.4 This effect on the skin microbiome is driven partly by the increased presence of certain skin microbial species, such as S epidermidis, which secrete virulence factors at higher temperatures and further contribute to inflammation.1,4
High-glycemic diet and alcohol consumption—High-glycemic diets and alcohol intake have been associated with gut dysbiosis and increased disease severity in rosacea. Processed foods and high sugar consumption can promote proinflammatory reactions that cause skin dysbiosis and exacerbate symptoms.15 Increased consumption of anti-inflammatory foods or consumption of probiotics and prebiotics can improve microbial balance.
Therapeutic Implications
The influence of the skin and gut microbiome on rosacea have been well described in the medical literature; therefore, many therapeutic strategies aim to address microbiome dysbiosis, including the use of antibiotics, anthelmintics, and a range of topical agents as well as probiotics, microbiome-friendly skin care products, and dietary modifications.
Antibiotics and Anthelmintics—Topical and oral antibiotics such as metronidazole and doxycycline reduce microbial load and inflammation.5,7,8 Ivermectin, an anthelmintic, has demonstrated efficacy in decreasing Demodex colonization and associated inflammation by interfering with mite survival and reducing bacterial interactions on the skin.5 Recent literature also has explored next-generation antibiotics that disrupt biofilm production by bacteria, which could positively affect outcomes while safeguarding antibiotic stewardship.15 Given its targeted antimicrobial activity and low propensity for microbial resistance, sarecycline represents a promising therapeutic option for managing rosacea symptoms with reduced risk for microbiome-related adverse events.12,16
Probiotics and Skin Care Interventions—Probiotics, prebiotics, and postbiotics have emerged as promising approaches to improve rosacea outcomes. Topical probiotics have been shown to maintain skin microbiome homeostasis, reduce inflammation, and enhance epidermal barrier function, making them a promising adjunctive therapy for rosacea.17,18 Physiological pH cleansers and moisturizers formulated with microbiome-friendly ingredients may reduce transepidermal water loss and improve skin hydration, which are critical in microbial equilibrium.9 Oral administration of E coli Nissle, Lactobacillus, and Bifidobacterium have shown potential in improving microbial balance and reducing disease severity.10
Other Topical Therapies—Azelaic acid and benzoyl peroxide can improve rosacea symptoms by decreasing inflammation and also may shift the skin microbiome.19,20 Formulations of topical therapies, including microencapsulated benzoyl peroxide, show improved efficacy in targeting pathogenic bacteria while maintaining tolerability.19
Dietary Modifications—Avoiding triggers such as alcohol and high-glycemic foods can help reduce gut and skin dysbiosis.13 Polyphenol-rich foods and prebiotic fiber may promote beneficial gut and skin microbial composition and currently are being studied.13
Emerging Therapies—Long-pulsed alexandrite laser therapy has been shown to reduce facial erythema and modulate skin microbiota.21 Patients with treatment-resistant rosacea may benefit from advanced precision targeted antimicrobials.
The future of rosacea treatment may involve integrating established and emerging microbiome-targeted treatment strategies to improve short- and long-term patient outcomes in rosacea.
Conclusion
As our understanding of rosacea, its pathogenesis, and the role of the skin microbiome continues to grow, so does our ability to develop increasingly effective and well-tolerated treatments. Future research should focus on how changes to the skin microbiome can influence disease progression and treatment responses as well as potential therapies targeting the skin microbiome. Integrating precision treatments that restore microbial balance alongside more traditional therapies may improve outcomes by addressing both inflammation and epidermal barrier dysfunction. Additionally, strategies that support a healthy skin microbiome, such as microbiome-friendly skin care and topical probiotics, should be further explored to enhance long-term disease management. There remains a dearth of literature addressing how the skin microbiome of patients with rosacea can be optimized to maximize treatment, highlighting the need for more research into these interventions.
- Joura MI, Jobbágy A, Dunai ZA, et al. Characteristics of the stool, blood and skin microbiome in rosacea patients. Microorganisms. 2024;12:2667. doi:10.3390/microorganisms12122667
- Li X, Chen S, Chen S, et al. Skin microbiome and causal relationships in three dermatological diseases: evidence from Mendelian randomization and Bayesian weighting. Skin Res Technol. 2024;30:E70035. doi:10.1111/srt.70035
- GulbasC aran F, Sar.mustafa S, Ozbag. c.van O, et al. Investigation of factors associated with gut microbiota in Demodex-associated skin conditions. Turkiye Parazitol Derg. 2024;48:171-177. doi:10.4274 /tpd.galenos.2024.93064
- Xiong J, Chen S, Wang P, et al. Characterisation of the bacterial microbiome in patients with rosacea and healthy controls. Eur J Dermatol. 2023;33:612-617. doi:10.1684/ejd.2023.4619
- Nakatsuji T, Cheng JY, Butcher A, et al. Topical ivermectin treatment of rosacea changes the bacterial microbiome of the skin. J Invest Dermatol. Published online October 29, 2024. doi:10.1016 /j.jid.2024.10.592
- Mylonas A, Hawerkamp HC, Wang Y, et al. Type I IFNs link skin-associated dysbiotic commensal bacteria to pathogenic inflammation and angiogenesis in rosacea. JCI Insight. 2023;8:e151846. doi:10.1172/jci.insight.151846
- Zhang Y, Zhou Y, Humbert P, et al. Effect on the skin microbiota of oral minocycline for rosacea. Acta Derm Venereol. 2023;103:adv10331. doi:10.2340/actadv.v103.10331
- Woo YR, Lee SH, Cho SH, et al. Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J Clin Med. 2020;9:185. doi:10.3390/jcm9010185
- Marson J, Bhatia N, Graber E, et al. Supplement article: the role of epidermal barrier dysfunction and cutaneous microbiome dysbiosis in the pathogenesis and management of acne vulgaris and rosacea. J Drugs Dermatol. 2022;21:SF3502915-SF35029114. doi:10.36849 /JDD.m0922
- Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol. 2016;22:5415-5421. doi:10.3748 /wjg.v22.i23.5415
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424. doi:10.1007/s12325-021-01624-x
- del Rosso JQ, Draelos ZD, Effron C, et al. Oral sarecycline for treatment of papulopustular rosacea: results of a pilot study of effectiveness and safety. J Drugs Dermatol. 2021;20:426-431. doi:10.36849 /JDD.2021.5923
- Qi X, Xiao Y, Zhang X, et al. Probiotics suppress LL37-generated rosacea-like skin inflammation by modulating the TLR2/MyD88 /NF-êB signaling pathway. Food Funct. 2024;15:8916-8934. doi:10.1039 /d4fo03083d
- Pan L, Li C, Liang Z, et al. Exploring the association between skin microbiota and inflammatory skin diseases: a two-sample Mendelian randomization analysis. Arch Dermatol Res. 2024;316:677. doi:10.1007/s00403-024-03433-y
- Sánchez-Pellicer P, Eguren-Michelena C, García-Gavín J, et al. Rosacea, microbiome and probiotics: the gut-skin axis. Front Microbiol. 2024;14:1323644. doi:10.3389/fmicb.2023.1323644
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911. doi:10.3389/fmicb.2022.901911
- Habeebuddin M, Karnati RK, Shiroorkar PN, et al. Topical probiotics: more than a skin deep. Pharmaceutics. 2022;14:557. doi:10.3390/pharmaceutics14030557
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020; 29:15-21. doi:10.1111/exd.14032
- Nong Y, Sugarman J, York JP, et al. Effect of topical microencapsulated benzoyl peroxide on the skin microbiome in rosacea: a randomized, double-blind, crossover, vehicle-controlled clinical trial. J Clin Aesthet Dermatol. 2024;17:19-26.
- Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient—a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321-330. doi:10.1093/jac/34.3.321
- Park S, Jang H, Seong SH, et al. The effects of long-pulsed alexandrite laser therapy on facial redness and skin microbiota compositions in rosacea: a prospective, multicentre, single-arm clinical trial. Photodermatol Photoimmunol Photomed. 2024;40:10.1111/phpp.12921. doi:10.1111/phpp.12921
Rosacea is a chronic inflammatory skin condition affecting the central face—including the cheeks, nose, chin, and forehead—that causes considerable discomfort.1 Its pathogenesis involves immune dysregulation, genetic predisposition, and microbial dysbiosis.2 While immune and environmental factors are known triggers of rosacea, recent research highlights the roles of the gut and skin microbiomes in disease progression. While the skin microbiome interacts directly with the immune system to regulate inflammation and skin homeostasis, the gut microbiome also influences cutaneous inflammation, emphasizing the need to address both topical and internal microbiome imbalances.3 In this article, we review gut and skin microbial alterations in rosacea, focusing on the skin microbiome and including the gut-skin axis implications as well as therapeutic strategies aimed at microbiome balance to enhance patient outcomes.
Skin Microbiome Alterations in Rosacea
The human skin microbiome interacts with the immune system, and microbial imbalances have been shown to contribute to immune dysregulation. Several key microbial species have been identified as playing a large role in rosacea, including Demodex folliculorum, Staphylococcus epidermidis, Bacillus oleronius, and Cutibacterium acnes (Figure).

Demodex folliculorum is a microscopic mite is found in hair follicles and sebaceous glands. Patients with rosacea have higher densities of D folliculorum, which trigger follicular occlusion and immune activation.1Bacillus oleronius be isolated from D folliculorum and can further activate toll-like receptor 2, leading to cytokine production and immune cell infiltration.3,4 Increased propagation of this mite correlates with shifts in skin microbiome composition, demonstrating increased inflammatory microbial populations.3
Staphylococcus epidermidis normally is commensal but can become pathogenic (pathobiont) in rosacea due to disruptions in the skin microenvironment, where it can form biofilms and produce virulence factors, particularly in papulopustular rosacea.5
Bacillus oleronius has been isolated from D folliculorum mites and provokes inflammatory responses in patients with rosacea by triggering toll-like receptor 2 activation and cytokine secretion.6
Cutibacterium acnes commonly is associated with acne vulgaris. Its role in rosacea is unclear, but recent research suggests it may have a protective effect. A single-arm trial investigated the effects of minocycline on rosacea and found that treatment significantly reduced C acnes but increased microbial species diversity, improving inflammation.7 One longitudinal cohort study of 12 patients with rosacea found that C acnes levels were lower in those older than 60 years. Rosacea severity increased with age and correlated with a decline in C acnes, suggesting that it may confer some protective effect in rosacea.8 This finding is supported by studies that have shown a reduction in C acnes levels in patients with rosacea compared to controls.4,8
Important mechanisms in rosacea include epidermal barrier dysfunction, transepidermal water loss, and decreased stratum corneum hydration, particularly in erythematotelangiectatic and papulopustular subtypes. The resulting alkaline skin pH contributes to barrier instability and heightened inflammation, permitting pathogenic bacteria to proliferate and disrupt skin microbial homeostasis.9 A recent study identified metabolic changes in the skin microbiome of patients with rosacea, showing that increased heme and hydrogen sulfide in rosacea skin microbiomes likely drive inflammation, while healthy skin microbiomes produce more anti-inflammatory adenosylcobalamin, thiazole, and L-isoleucine.1 These findings highlight the link between microbial imbalances and inflammation in rosacea.
The Gut-Skin Axis in Rosacea
Gut microbiota play a critical role in managing systemic inflammation, and microbial dysbiosis in the intestine can influence the skin microbiome in rosacea. Patients with rosacea who have gastrointestinal conditions such as small intestinal bacterial overgrowth and Helicobacter pylori infection experience more severe rosacea symptoms.3,10
Patients with rosacea have distinctive gut microbiota compositions, with an increased prevalence of proinflammatory bacterial species, potentially affecting the skin microbiome.8,11 Systemic antibiotics have been shown to modulate the gut microbiome, indirectly influencing the skin microbiome.11 A recent study demonstrated that doxycycline treatment in patients with rosacea altered skin microbial diversity, reducing C acnes while increasing Weissella confusa—highlighting the complicated relationship between systemic antibiotics and the gut-skin axis.8
Specific probiotics, such as Escherichia coli Nissle, when given orally shifted gut microbial balance to protective microbiota with increased Lactobacillus and Bifidobacteria species and decreased pathogenic bacteria. This improved rosacea symptoms, normalized immunoglobulin A levels, and suppressed cytokine interleukin 8 levels.10 Recent studies also suggest oral sarecycline, a narrow-spectrum antibiotic, may improve papulopustular rosacea symptoms through its anti-inflammatory effects while having minimal impact on gut microbiota diversity.11,12
Gut-derived short-chain fatty acids, which are known to regulate immune function, also have been shown to influence the composition of skin microbiota, suggesting a direct link between gut dysbiosis and skin microbial imbalances. Notably, antibiotic and probiotic treatments targeting the gut microbiome (eg, rifaximin for small intestinal bacterial overgrowth) have been associated with improvements in rosacea symptoms, further underscoring the interconnectedness of the gut-skin axis.13 Understanding how gut-derived inflammation alters the skin microbiome may provide new therapeutic avenues for restoring microbial balance and reducing rosacea severity.
Immune Dysregulation and Inflammatory Pathways
Mechanisms of microbiome-driven inflammation via the innate immune system contribute to rosacea pathogenesis. Toll-like receptor 2 is upregulated in rosacea, producing increased peptides including cathelicidins.13 When abnormally processed, cathelicidins produce proinflammatory peptides and worsen rosacea symptoms such as erythema, telangiectasias, and neutrophilic infiltration by dysregulating the immune system and the skin barrier.6
Heightened levels of cytokines interleukin 8 and interferon α have been identified in patients with rosacea. These cytokines are involved in rosacea pathogenesis, including leukocyte recruitment, angiogenesis, and tissue remodeling and further activate the inflammatory cascade.8,14
Mendelian randomization studies have provided confirmation of a causal link between skin microbiota alterations and inflammatory skin diseases including rosacea.2 Specific alterations in bacteria such as Cutibacterium and Staphylococcus microbial species have been associated with shifts in host immune gene expression, potentially predisposing individuals to abnormal immune activation and inflammation.2,8 These studies show the potential of leveraging precision medicine to design therapies that target pathways that improve microbial imbalances seen in rosacea.
Environmental and Lifestyle Factors Affecting the Skin Microbiome
Individuals with rosacea often have increased sensitivity to environmental and lifestyle stressors such as high temperatures, UV exposure, and sugar and alcohol consumption. These factors influence the composition of the skin microbiome and potentially contribute to rosacea development and disease exacerbation; therefore, trigger avoidance is an important way to manage rosacea.
High temperatures and UV exposure—Demodex activity increases in response to heat exposure and subsequently worsens rosacea symptoms, while exposure to UV radiation can change the composition of the skin microbiome by encouraging inflammatory responses such as oxidative stress reactions.4 This effect on the skin microbiome is driven partly by the increased presence of certain skin microbial species, such as S epidermidis, which secrete virulence factors at higher temperatures and further contribute to inflammation.1,4
High-glycemic diet and alcohol consumption—High-glycemic diets and alcohol intake have been associated with gut dysbiosis and increased disease severity in rosacea. Processed foods and high sugar consumption can promote proinflammatory reactions that cause skin dysbiosis and exacerbate symptoms.15 Increased consumption of anti-inflammatory foods or consumption of probiotics and prebiotics can improve microbial balance.
Therapeutic Implications
The influence of the skin and gut microbiome on rosacea have been well described in the medical literature; therefore, many therapeutic strategies aim to address microbiome dysbiosis, including the use of antibiotics, anthelmintics, and a range of topical agents as well as probiotics, microbiome-friendly skin care products, and dietary modifications.
Antibiotics and Anthelmintics—Topical and oral antibiotics such as metronidazole and doxycycline reduce microbial load and inflammation.5,7,8 Ivermectin, an anthelmintic, has demonstrated efficacy in decreasing Demodex colonization and associated inflammation by interfering with mite survival and reducing bacterial interactions on the skin.5 Recent literature also has explored next-generation antibiotics that disrupt biofilm production by bacteria, which could positively affect outcomes while safeguarding antibiotic stewardship.15 Given its targeted antimicrobial activity and low propensity for microbial resistance, sarecycline represents a promising therapeutic option for managing rosacea symptoms with reduced risk for microbiome-related adverse events.12,16
Probiotics and Skin Care Interventions—Probiotics, prebiotics, and postbiotics have emerged as promising approaches to improve rosacea outcomes. Topical probiotics have been shown to maintain skin microbiome homeostasis, reduce inflammation, and enhance epidermal barrier function, making them a promising adjunctive therapy for rosacea.17,18 Physiological pH cleansers and moisturizers formulated with microbiome-friendly ingredients may reduce transepidermal water loss and improve skin hydration, which are critical in microbial equilibrium.9 Oral administration of E coli Nissle, Lactobacillus, and Bifidobacterium have shown potential in improving microbial balance and reducing disease severity.10
Other Topical Therapies—Azelaic acid and benzoyl peroxide can improve rosacea symptoms by decreasing inflammation and also may shift the skin microbiome.19,20 Formulations of topical therapies, including microencapsulated benzoyl peroxide, show improved efficacy in targeting pathogenic bacteria while maintaining tolerability.19
Dietary Modifications—Avoiding triggers such as alcohol and high-glycemic foods can help reduce gut and skin dysbiosis.13 Polyphenol-rich foods and prebiotic fiber may promote beneficial gut and skin microbial composition and currently are being studied.13
Emerging Therapies—Long-pulsed alexandrite laser therapy has been shown to reduce facial erythema and modulate skin microbiota.21 Patients with treatment-resistant rosacea may benefit from advanced precision targeted antimicrobials.
The future of rosacea treatment may involve integrating established and emerging microbiome-targeted treatment strategies to improve short- and long-term patient outcomes in rosacea.
Conclusion
As our understanding of rosacea, its pathogenesis, and the role of the skin microbiome continues to grow, so does our ability to develop increasingly effective and well-tolerated treatments. Future research should focus on how changes to the skin microbiome can influence disease progression and treatment responses as well as potential therapies targeting the skin microbiome. Integrating precision treatments that restore microbial balance alongside more traditional therapies may improve outcomes by addressing both inflammation and epidermal barrier dysfunction. Additionally, strategies that support a healthy skin microbiome, such as microbiome-friendly skin care and topical probiotics, should be further explored to enhance long-term disease management. There remains a dearth of literature addressing how the skin microbiome of patients with rosacea can be optimized to maximize treatment, highlighting the need for more research into these interventions.
Rosacea is a chronic inflammatory skin condition affecting the central face—including the cheeks, nose, chin, and forehead—that causes considerable discomfort.1 Its pathogenesis involves immune dysregulation, genetic predisposition, and microbial dysbiosis.2 While immune and environmental factors are known triggers of rosacea, recent research highlights the roles of the gut and skin microbiomes in disease progression. While the skin microbiome interacts directly with the immune system to regulate inflammation and skin homeostasis, the gut microbiome also influences cutaneous inflammation, emphasizing the need to address both topical and internal microbiome imbalances.3 In this article, we review gut and skin microbial alterations in rosacea, focusing on the skin microbiome and including the gut-skin axis implications as well as therapeutic strategies aimed at microbiome balance to enhance patient outcomes.
Skin Microbiome Alterations in Rosacea
The human skin microbiome interacts with the immune system, and microbial imbalances have been shown to contribute to immune dysregulation. Several key microbial species have been identified as playing a large role in rosacea, including Demodex folliculorum, Staphylococcus epidermidis, Bacillus oleronius, and Cutibacterium acnes (Figure).

Demodex folliculorum is a microscopic mite is found in hair follicles and sebaceous glands. Patients with rosacea have higher densities of D folliculorum, which trigger follicular occlusion and immune activation.1Bacillus oleronius be isolated from D folliculorum and can further activate toll-like receptor 2, leading to cytokine production and immune cell infiltration.3,4 Increased propagation of this mite correlates with shifts in skin microbiome composition, demonstrating increased inflammatory microbial populations.3
Staphylococcus epidermidis normally is commensal but can become pathogenic (pathobiont) in rosacea due to disruptions in the skin microenvironment, where it can form biofilms and produce virulence factors, particularly in papulopustular rosacea.5
Bacillus oleronius has been isolated from D folliculorum mites and provokes inflammatory responses in patients with rosacea by triggering toll-like receptor 2 activation and cytokine secretion.6
Cutibacterium acnes commonly is associated with acne vulgaris. Its role in rosacea is unclear, but recent research suggests it may have a protective effect. A single-arm trial investigated the effects of minocycline on rosacea and found that treatment significantly reduced C acnes but increased microbial species diversity, improving inflammation.7 One longitudinal cohort study of 12 patients with rosacea found that C acnes levels were lower in those older than 60 years. Rosacea severity increased with age and correlated with a decline in C acnes, suggesting that it may confer some protective effect in rosacea.8 This finding is supported by studies that have shown a reduction in C acnes levels in patients with rosacea compared to controls.4,8
Important mechanisms in rosacea include epidermal barrier dysfunction, transepidermal water loss, and decreased stratum corneum hydration, particularly in erythematotelangiectatic and papulopustular subtypes. The resulting alkaline skin pH contributes to barrier instability and heightened inflammation, permitting pathogenic bacteria to proliferate and disrupt skin microbial homeostasis.9 A recent study identified metabolic changes in the skin microbiome of patients with rosacea, showing that increased heme and hydrogen sulfide in rosacea skin microbiomes likely drive inflammation, while healthy skin microbiomes produce more anti-inflammatory adenosylcobalamin, thiazole, and L-isoleucine.1 These findings highlight the link between microbial imbalances and inflammation in rosacea.
The Gut-Skin Axis in Rosacea
Gut microbiota play a critical role in managing systemic inflammation, and microbial dysbiosis in the intestine can influence the skin microbiome in rosacea. Patients with rosacea who have gastrointestinal conditions such as small intestinal bacterial overgrowth and Helicobacter pylori infection experience more severe rosacea symptoms.3,10
Patients with rosacea have distinctive gut microbiota compositions, with an increased prevalence of proinflammatory bacterial species, potentially affecting the skin microbiome.8,11 Systemic antibiotics have been shown to modulate the gut microbiome, indirectly influencing the skin microbiome.11 A recent study demonstrated that doxycycline treatment in patients with rosacea altered skin microbial diversity, reducing C acnes while increasing Weissella confusa—highlighting the complicated relationship between systemic antibiotics and the gut-skin axis.8
Specific probiotics, such as Escherichia coli Nissle, when given orally shifted gut microbial balance to protective microbiota with increased Lactobacillus and Bifidobacteria species and decreased pathogenic bacteria. This improved rosacea symptoms, normalized immunoglobulin A levels, and suppressed cytokine interleukin 8 levels.10 Recent studies also suggest oral sarecycline, a narrow-spectrum antibiotic, may improve papulopustular rosacea symptoms through its anti-inflammatory effects while having minimal impact on gut microbiota diversity.11,12
Gut-derived short-chain fatty acids, which are known to regulate immune function, also have been shown to influence the composition of skin microbiota, suggesting a direct link between gut dysbiosis and skin microbial imbalances. Notably, antibiotic and probiotic treatments targeting the gut microbiome (eg, rifaximin for small intestinal bacterial overgrowth) have been associated with improvements in rosacea symptoms, further underscoring the interconnectedness of the gut-skin axis.13 Understanding how gut-derived inflammation alters the skin microbiome may provide new therapeutic avenues for restoring microbial balance and reducing rosacea severity.
Immune Dysregulation and Inflammatory Pathways
Mechanisms of microbiome-driven inflammation via the innate immune system contribute to rosacea pathogenesis. Toll-like receptor 2 is upregulated in rosacea, producing increased peptides including cathelicidins.13 When abnormally processed, cathelicidins produce proinflammatory peptides and worsen rosacea symptoms such as erythema, telangiectasias, and neutrophilic infiltration by dysregulating the immune system and the skin barrier.6
Heightened levels of cytokines interleukin 8 and interferon α have been identified in patients with rosacea. These cytokines are involved in rosacea pathogenesis, including leukocyte recruitment, angiogenesis, and tissue remodeling and further activate the inflammatory cascade.8,14
Mendelian randomization studies have provided confirmation of a causal link between skin microbiota alterations and inflammatory skin diseases including rosacea.2 Specific alterations in bacteria such as Cutibacterium and Staphylococcus microbial species have been associated with shifts in host immune gene expression, potentially predisposing individuals to abnormal immune activation and inflammation.2,8 These studies show the potential of leveraging precision medicine to design therapies that target pathways that improve microbial imbalances seen in rosacea.
Environmental and Lifestyle Factors Affecting the Skin Microbiome
Individuals with rosacea often have increased sensitivity to environmental and lifestyle stressors such as high temperatures, UV exposure, and sugar and alcohol consumption. These factors influence the composition of the skin microbiome and potentially contribute to rosacea development and disease exacerbation; therefore, trigger avoidance is an important way to manage rosacea.
High temperatures and UV exposure—Demodex activity increases in response to heat exposure and subsequently worsens rosacea symptoms, while exposure to UV radiation can change the composition of the skin microbiome by encouraging inflammatory responses such as oxidative stress reactions.4 This effect on the skin microbiome is driven partly by the increased presence of certain skin microbial species, such as S epidermidis, which secrete virulence factors at higher temperatures and further contribute to inflammation.1,4
High-glycemic diet and alcohol consumption—High-glycemic diets and alcohol intake have been associated with gut dysbiosis and increased disease severity in rosacea. Processed foods and high sugar consumption can promote proinflammatory reactions that cause skin dysbiosis and exacerbate symptoms.15 Increased consumption of anti-inflammatory foods or consumption of probiotics and prebiotics can improve microbial balance.
Therapeutic Implications
The influence of the skin and gut microbiome on rosacea have been well described in the medical literature; therefore, many therapeutic strategies aim to address microbiome dysbiosis, including the use of antibiotics, anthelmintics, and a range of topical agents as well as probiotics, microbiome-friendly skin care products, and dietary modifications.
Antibiotics and Anthelmintics—Topical and oral antibiotics such as metronidazole and doxycycline reduce microbial load and inflammation.5,7,8 Ivermectin, an anthelmintic, has demonstrated efficacy in decreasing Demodex colonization and associated inflammation by interfering with mite survival and reducing bacterial interactions on the skin.5 Recent literature also has explored next-generation antibiotics that disrupt biofilm production by bacteria, which could positively affect outcomes while safeguarding antibiotic stewardship.15 Given its targeted antimicrobial activity and low propensity for microbial resistance, sarecycline represents a promising therapeutic option for managing rosacea symptoms with reduced risk for microbiome-related adverse events.12,16
Probiotics and Skin Care Interventions—Probiotics, prebiotics, and postbiotics have emerged as promising approaches to improve rosacea outcomes. Topical probiotics have been shown to maintain skin microbiome homeostasis, reduce inflammation, and enhance epidermal barrier function, making them a promising adjunctive therapy for rosacea.17,18 Physiological pH cleansers and moisturizers formulated with microbiome-friendly ingredients may reduce transepidermal water loss and improve skin hydration, which are critical in microbial equilibrium.9 Oral administration of E coli Nissle, Lactobacillus, and Bifidobacterium have shown potential in improving microbial balance and reducing disease severity.10
Other Topical Therapies—Azelaic acid and benzoyl peroxide can improve rosacea symptoms by decreasing inflammation and also may shift the skin microbiome.19,20 Formulations of topical therapies, including microencapsulated benzoyl peroxide, show improved efficacy in targeting pathogenic bacteria while maintaining tolerability.19
Dietary Modifications—Avoiding triggers such as alcohol and high-glycemic foods can help reduce gut and skin dysbiosis.13 Polyphenol-rich foods and prebiotic fiber may promote beneficial gut and skin microbial composition and currently are being studied.13
Emerging Therapies—Long-pulsed alexandrite laser therapy has been shown to reduce facial erythema and modulate skin microbiota.21 Patients with treatment-resistant rosacea may benefit from advanced precision targeted antimicrobials.
The future of rosacea treatment may involve integrating established and emerging microbiome-targeted treatment strategies to improve short- and long-term patient outcomes in rosacea.
Conclusion
As our understanding of rosacea, its pathogenesis, and the role of the skin microbiome continues to grow, so does our ability to develop increasingly effective and well-tolerated treatments. Future research should focus on how changes to the skin microbiome can influence disease progression and treatment responses as well as potential therapies targeting the skin microbiome. Integrating precision treatments that restore microbial balance alongside more traditional therapies may improve outcomes by addressing both inflammation and epidermal barrier dysfunction. Additionally, strategies that support a healthy skin microbiome, such as microbiome-friendly skin care and topical probiotics, should be further explored to enhance long-term disease management. There remains a dearth of literature addressing how the skin microbiome of patients with rosacea can be optimized to maximize treatment, highlighting the need for more research into these interventions.
- Joura MI, Jobbágy A, Dunai ZA, et al. Characteristics of the stool, blood and skin microbiome in rosacea patients. Microorganisms. 2024;12:2667. doi:10.3390/microorganisms12122667
- Li X, Chen S, Chen S, et al. Skin microbiome and causal relationships in three dermatological diseases: evidence from Mendelian randomization and Bayesian weighting. Skin Res Technol. 2024;30:E70035. doi:10.1111/srt.70035
- GulbasC aran F, Sar.mustafa S, Ozbag. c.van O, et al. Investigation of factors associated with gut microbiota in Demodex-associated skin conditions. Turkiye Parazitol Derg. 2024;48:171-177. doi:10.4274 /tpd.galenos.2024.93064
- Xiong J, Chen S, Wang P, et al. Characterisation of the bacterial microbiome in patients with rosacea and healthy controls. Eur J Dermatol. 2023;33:612-617. doi:10.1684/ejd.2023.4619
- Nakatsuji T, Cheng JY, Butcher A, et al. Topical ivermectin treatment of rosacea changes the bacterial microbiome of the skin. J Invest Dermatol. Published online October 29, 2024. doi:10.1016 /j.jid.2024.10.592
- Mylonas A, Hawerkamp HC, Wang Y, et al. Type I IFNs link skin-associated dysbiotic commensal bacteria to pathogenic inflammation and angiogenesis in rosacea. JCI Insight. 2023;8:e151846. doi:10.1172/jci.insight.151846
- Zhang Y, Zhou Y, Humbert P, et al. Effect on the skin microbiota of oral minocycline for rosacea. Acta Derm Venereol. 2023;103:adv10331. doi:10.2340/actadv.v103.10331
- Woo YR, Lee SH, Cho SH, et al. Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J Clin Med. 2020;9:185. doi:10.3390/jcm9010185
- Marson J, Bhatia N, Graber E, et al. Supplement article: the role of epidermal barrier dysfunction and cutaneous microbiome dysbiosis in the pathogenesis and management of acne vulgaris and rosacea. J Drugs Dermatol. 2022;21:SF3502915-SF35029114. doi:10.36849 /JDD.m0922
- Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol. 2016;22:5415-5421. doi:10.3748 /wjg.v22.i23.5415
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424. doi:10.1007/s12325-021-01624-x
- del Rosso JQ, Draelos ZD, Effron C, et al. Oral sarecycline for treatment of papulopustular rosacea: results of a pilot study of effectiveness and safety. J Drugs Dermatol. 2021;20:426-431. doi:10.36849 /JDD.2021.5923
- Qi X, Xiao Y, Zhang X, et al. Probiotics suppress LL37-generated rosacea-like skin inflammation by modulating the TLR2/MyD88 /NF-êB signaling pathway. Food Funct. 2024;15:8916-8934. doi:10.1039 /d4fo03083d
- Pan L, Li C, Liang Z, et al. Exploring the association between skin microbiota and inflammatory skin diseases: a two-sample Mendelian randomization analysis. Arch Dermatol Res. 2024;316:677. doi:10.1007/s00403-024-03433-y
- Sánchez-Pellicer P, Eguren-Michelena C, García-Gavín J, et al. Rosacea, microbiome and probiotics: the gut-skin axis. Front Microbiol. 2024;14:1323644. doi:10.3389/fmicb.2023.1323644
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911. doi:10.3389/fmicb.2022.901911
- Habeebuddin M, Karnati RK, Shiroorkar PN, et al. Topical probiotics: more than a skin deep. Pharmaceutics. 2022;14:557. doi:10.3390/pharmaceutics14030557
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020; 29:15-21. doi:10.1111/exd.14032
- Nong Y, Sugarman J, York JP, et al. Effect of topical microencapsulated benzoyl peroxide on the skin microbiome in rosacea: a randomized, double-blind, crossover, vehicle-controlled clinical trial. J Clin Aesthet Dermatol. 2024;17:19-26.
- Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient—a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321-330. doi:10.1093/jac/34.3.321
- Park S, Jang H, Seong SH, et al. The effects of long-pulsed alexandrite laser therapy on facial redness and skin microbiota compositions in rosacea: a prospective, multicentre, single-arm clinical trial. Photodermatol Photoimmunol Photomed. 2024;40:10.1111/phpp.12921. doi:10.1111/phpp.12921
- Joura MI, Jobbágy A, Dunai ZA, et al. Characteristics of the stool, blood and skin microbiome in rosacea patients. Microorganisms. 2024;12:2667. doi:10.3390/microorganisms12122667
- Li X, Chen S, Chen S, et al. Skin microbiome and causal relationships in three dermatological diseases: evidence from Mendelian randomization and Bayesian weighting. Skin Res Technol. 2024;30:E70035. doi:10.1111/srt.70035
- GulbasC aran F, Sar.mustafa S, Ozbag. c.van O, et al. Investigation of factors associated with gut microbiota in Demodex-associated skin conditions. Turkiye Parazitol Derg. 2024;48:171-177. doi:10.4274 /tpd.galenos.2024.93064
- Xiong J, Chen S, Wang P, et al. Characterisation of the bacterial microbiome in patients with rosacea and healthy controls. Eur J Dermatol. 2023;33:612-617. doi:10.1684/ejd.2023.4619
- Nakatsuji T, Cheng JY, Butcher A, et al. Topical ivermectin treatment of rosacea changes the bacterial microbiome of the skin. J Invest Dermatol. Published online October 29, 2024. doi:10.1016 /j.jid.2024.10.592
- Mylonas A, Hawerkamp HC, Wang Y, et al. Type I IFNs link skin-associated dysbiotic commensal bacteria to pathogenic inflammation and angiogenesis in rosacea. JCI Insight. 2023;8:e151846. doi:10.1172/jci.insight.151846
- Zhang Y, Zhou Y, Humbert P, et al. Effect on the skin microbiota of oral minocycline for rosacea. Acta Derm Venereol. 2023;103:adv10331. doi:10.2340/actadv.v103.10331
- Woo YR, Lee SH, Cho SH, et al. Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J Clin Med. 2020;9:185. doi:10.3390/jcm9010185
- Marson J, Bhatia N, Graber E, et al. Supplement article: the role of epidermal barrier dysfunction and cutaneous microbiome dysbiosis in the pathogenesis and management of acne vulgaris and rosacea. J Drugs Dermatol. 2022;21:SF3502915-SF35029114. doi:10.36849 /JDD.m0922
- Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol. 2016;22:5415-5421. doi:10.3748 /wjg.v22.i23.5415
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424. doi:10.1007/s12325-021-01624-x
- del Rosso JQ, Draelos ZD, Effron C, et al. Oral sarecycline for treatment of papulopustular rosacea: results of a pilot study of effectiveness and safety. J Drugs Dermatol. 2021;20:426-431. doi:10.36849 /JDD.2021.5923
- Qi X, Xiao Y, Zhang X, et al. Probiotics suppress LL37-generated rosacea-like skin inflammation by modulating the TLR2/MyD88 /NF-êB signaling pathway. Food Funct. 2024;15:8916-8934. doi:10.1039 /d4fo03083d
- Pan L, Li C, Liang Z, et al. Exploring the association between skin microbiota and inflammatory skin diseases: a two-sample Mendelian randomization analysis. Arch Dermatol Res. 2024;316:677. doi:10.1007/s00403-024-03433-y
- Sánchez-Pellicer P, Eguren-Michelena C, García-Gavín J, et al. Rosacea, microbiome and probiotics: the gut-skin axis. Front Microbiol. 2024;14:1323644. doi:10.3389/fmicb.2023.1323644
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911. doi:10.3389/fmicb.2022.901911
- Habeebuddin M, Karnati RK, Shiroorkar PN, et al. Topical probiotics: more than a skin deep. Pharmaceutics. 2022;14:557. doi:10.3390/pharmaceutics14030557
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020; 29:15-21. doi:10.1111/exd.14032
- Nong Y, Sugarman J, York JP, et al. Effect of topical microencapsulated benzoyl peroxide on the skin microbiome in rosacea: a randomized, double-blind, crossover, vehicle-controlled clinical trial. J Clin Aesthet Dermatol. 2024;17:19-26.
- Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient—a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321-330. doi:10.1093/jac/34.3.321
- Park S, Jang H, Seong SH, et al. The effects of long-pulsed alexandrite laser therapy on facial redness and skin microbiota compositions in rosacea: a prospective, multicentre, single-arm clinical trial. Photodermatol Photoimmunol Photomed. 2024;40:10.1111/phpp.12921. doi:10.1111/phpp.12921
The Skin Microbiome in Rosacea: Mechanisms, Gut-Skin Interactions, and Therapeutic Implications
The Skin Microbiome in Rosacea: Mechanisms, Gut-Skin Interactions, and Therapeutic Implications
PRACTICE POINTS:
- It is important to assess both the gut and skin microbiomes in patients with rosacea (eg, incorporate evaluation of Demodex folliculorum density, take a gut-health history).
- Narrow-spectrum antibiotics such as sarecycline or anthelmintics such as topical ivermectin target pathogens while preserving beneficial flora.
- Patients with rosacea should be counseled on trigger avoidance as well as pH-balanced, microbiomefriendly skin care and lifestyle tips to strengthen the skin barrier.
