User login
Maintaining Weight Loss With GLP-1s Needs Lifestyle Changes
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
To Hold or Not to Hold GLP-1s Before Surgery
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
Patient and treatment perspectives: Revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk
Type 2 diabetes mellitus (T2DM), excess weight, and obesity are increasing in prevalence at alarming rates.1–3 Concurrent with the increased prevalence is increased risk of morbidity and mortality. A healthy diet and exercise in conjunction with antidiabetes medications can help lower glucose concentration in patients with T2DM. Because these patients are at increased risk of cardiovascular (CV) morbidity and mortality, however, treatment strategies should address the CV risk factors, including blood pressure (BP), lipids, and body weight, as well as glycemic aspects of the disease.
To help clinicians manage the complex issues in treating patients with T2DM, this article presents an overview of patient and treatment perspectives relevant to overweight/obesity and CV disease (CVD). It includes an examination of the latest guidelines and algorithms for the management of T2DM, which continue to be updated and modified.
T2DM, WEIGHT GAIN OR OBESITY, AND CV RISK: A CHALLENGING TRIAD
Despite therapeutic advances in the diagnosis and treatment of diabetes and CVD over the last decade, the estimated number of persons in the United States older than 35 years with self-reported diabetes (with T2DM accounting for 90% to 95% of diagnosed cases) and CVD has increased from 4.2 million in 1997 to 5.7 million in 2005.3,4 The CV risk for patients with T2DM who have not had a CV event such as a myocardial infarction (MI) is similar to that of individuals without diabetes who have had a prior MI.5 Patients with T2DM have nearly double the mortality of those without the disease.6 Adding to their risk, about 80% of patients with T2DM are overweight or obese, conditions associated with worsened insulin resistance and increased CV risk and disease burden.7,8 Even a modest weight gain (5 kg) may increase the risk of coronary heart disease (CHD) by 30%, while associated changes in lipids and BP can increase the risk by another 20%.9
It is as important to control CV risk factors as it is to control glycemia in patients with T2DM, and both are difficult to achieve. Data from a recent nationwide Norwegian survey showed that only 13% of patients with T2DM achieved study-defined target levels; ie, glycosylated hemoglobin (HbA1c) less than 7.5%, BP less than 140/85 mm Hg, and total cholesterol/high-density lipoprotein (HDL-C) ratio less than 4.0.10
BENEFITS OF MANAGING GLYCEMIA, WEIGHT REDUCTION, AND CV RISK FACTORS
Several large studies, many ongoing, are generating data on the relationships among glycemia, weight reduction, and CV risk. It is well established that individuals with T2DM need aggressive risk factor reduction (glucose control, blood pressure management, and treatment of dyslipidemia) to optimize outcomes. However, characterization of the benefits of various components of risk factor reduction, particularly over many years, is only now occurring.
Results from the United Kingdom Prospective Diabetes Studies (UKPDS) showed the benefits and risks of pharmacologic glycemic control—essentially monotherapy with insulin or a sulfonylurea—compared with conventional dietary therapy in reducing diabetic complications in patients with newly diagnosed T2DM. In UKPDS 33, both insulin and sulfonylureas (intensive treatment) reduced the risk of microvascular end points (retinopathy, nephropathy) in patients whose median HbA1c was lowered to 7.0% at 10 years of follow-up, compared with patients who reached an HbA1c of 7.9%. However, intensive glycemic control did not translate into a statistically significant reduction in macrovascular complications, including MI, stroke, CVD, and death. Additionally, patients assigned to insulin had greater weight gain (+4.0 kg) than did patients assigned to receive the sulfonylurea chlorpropamide (+2.6 kg) or glyburide (+1.7 kg) (P < .01).11
The UKPDS showed that intensive treatment with metformin reduced the risk of T2DM-related end points compared with conventional treatment (primarily diet alone) in overweight patients.12 Although there were fewer patients in the metformin-treated subset (n = 342) than in the conventional treatment cohort, a secondary analysis showed that metformin was associated with less weight gain and fewer hypoglycemic episodes than either insulin or sulfonylurea therapy.12 Since HbA1c levels in the treatment groups were equal, the additional benefits seen with metformin in overweight patients with T2DM were not based solely on glycemic control.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial involved 10,000 individuals with T2DM. The primary outcome measure was a composite of CV events. The intensively treated group was controlled to a target HbA1c of less than 6.0%, with most patients receiving insulin. The trial was terminated early because an increased risk of sudden death was observed.13 A similar study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), evaluated more than 11,000 patients with T2DM, starting with a sulfonylurea-based regimen. In this study, there was no reduction in macrovascular events, but there was a reduction in nephropathy in the intensively treated group.14 In both studies, hypoglycemia and weight gain were more frequent in intensively treated patients; and in ACCORD, there were more episodes of severe hypoglycemia in the intensive-treatment group.13,14
The Veterans Affairs Diabetes Trial (VADT) evaluated the effect of intensive glucose control on CVD in 1,791 patients (mean age, 60 years) with poorly controlled T2DM (average duration, 11.5 years). The primary end points included MI, stroke, new or worsening congestive heart failure (CHF), limb amputation, and invasive intervention for coronary or peripheral arterial disease. The hazard ratio for these end points in the intensive-treatment group was 0.88 (95% confidence interval [CI], 0.74 to 1.05).15,16 Specifically, the following beneficial effects were achieved:
- HbA1c reduced by –1.0% to –2.5% in absolute units,
- systolic BP (SBP) reduced by –4 to –7 mm Hg,
- diastolic BP (DBP) reduced by –7 to –8 mm Hg,
- low-density lipoprotein cholesterol (LDL-C) reduced by –27 to –28 mg/dL,
- triglycerides reduced by –44 to –50 mg/dL, and
- HDL-C increased by 4 to 5 mg/dL.
Despite these benefits, body weight increased approximately 9 to 18 lb (4 to 8 kg) during therapy.15
Since overweight and obesity are independent risk factors for CHD and CVD in patients with T2DM,17 weight management is an integral component in treatment. In the Action for Health in Diabetes (Look AHEAD) trial, an intensive exercise and weight-loss program resulted in clinically significant (P < .001) weight loss at 1 year in patients who had T2DM and a body mass index (BMI) greater than 25 kg2/m (> 27 kg2/m if receiving insulin).18 When compared with patients who received less structured, infrequent support and minimal education about diabetes, participants in the intensive program showed more weight loss, improved glucose control, decreased CV events, and reduced medicine use. The Look AHEAD trial is currently evaluating whether these improvements will continue to result in lower CV risk.
PATIENT ADHERENCE AND SATISFACTION
It is often challenging for patients with T2DM to adhere to their treatment regimens. The Diabetes Attitude, Wishes, and Needs (DAWN) study examined psychosocial barriers to self-care in patients with diabetes and found that while 78% of patients with T2DM adhered to their medications, only 39% achieved complete success in at least two-thirds of their self-care domains.19 A multicenter, randomized, clinical trial examined the correlates of treatment satisfaction, including body weight, on patients’ appraisal of treatment satisfaction with injectable insulin. The 14.5% of patients who experienced a reduction in BMI reported systematic improvement in treatment satisfaction.20 Similarly, a cross-sectionally designed study (n = 99) that analyzed the interrelation of adherence, BMI, and depression in adults with T2DM found that patients with higher BMI and poor adherence also had depression, which was mediated by lower self-efficacy perceptions and increased diabetes symptoms.21 The results from these studies show a clear relationship between adherence with treatment regimens and achievement of HbA1c goals.22
RECENT DEVELOPMENTS IN T2DM MANAGEMENT: STRATEGIES TO REDUCE CV RISK
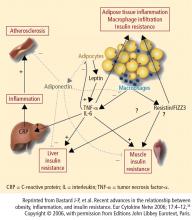
Because excess weight and obesity are prominent features of T2DM, it is important to use an antidiabetes agent that does not induce unnecessary weight gain (particularly central weight gain, which is thought to be most atherogenic).23 Metformin, considered the first-line agent for treatment of T2DM, is generally weight neutral with a low level of hypoglycemia.24,25 Sulfonylureas, insulin, and thiazolidinediones (TZDs) are all associated with weight gain, although newer-analogue insulins may cause less weight gain than older agents. TZDs, especially pioglitazone, are associated with improvements in long-term beta-cell function and CV risk factors despite weight gain.26,27
The newer antidiabetes agents belong to the dipeptidyl peptidase–4 (DPP-4) inhibitor and the glucagon-like peptide–1 (GLP-1) receptor agonist therapeutic classes and have been shown to be either weight neutral (DPP-4 inhibitors) or to cause weight loss (GLP-1 receptor agonists).28
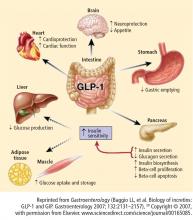
Obesity and the incretin effect
Two recent studies showed that surgically induced weight loss enhances the physiologic “incretin effect.” In one study, obese individuals with T2DM whose weight loss was secondary to bariatric surgery combined with caloric restriction showed improved insulin sensitivity, improved carbohydrate metabolism, and elevated levels of adiponectin and GLP-1, all of which may reduce the incidence of T2DM.36 In the other study, bariatric surgery in morbidly obese individuals with T2DM improved insulin secretion and ameliorated insulin resistance.37
DPP-4 inhibitors
DPP-4 inhibitors such as sitagliptin and saxagliptin inhibit the enzymatic activity of DPP-4 and increase endogenous concentrations of GLP-1.28 Sitagliptin has been compared with placebo as monotherapy and has been studied in combination with other therapies.
In an 18-week study, sitagliptin monotherapy, 100 and 200 mg QD, significantly reduced HbA1c compared with placebo (placebo-subtracted HbA1c reduction, –0.60% and –0.48%, respectively) in patients with T2DM. Sitagliptin also significantly decreased fasting plasma glucose (FPG) concentration relative to placebo.38 Twelve weeks of sitagliptin monotherapy at dosages of 5, 12.5, 25, and 50 mg BID led to significant (P < .001) reductions in HbA1c compared with placebo. Sitagliptin also produced significant reductions in FPG and mean daily glucose concentrations across the doses studied.39 Similar results were reported in other 12-week studies: 50 mg BID and 100 mg QD sitagliptin monotherapy significantly (P < .05) reduced HbA1c –0.39% to –0.56% and FPG concentration –11.0 to –17.2 mg/dL compared with placebo40; sitagliptin 100 mg QD compared with placebo produced a least-squares mean change from baseline HbA1c of –0.65% versus 0.41% (P < .001) and FPG of –22.5 versus 9.4 mg/dL (P < .001).41
Sitagliptin also has been studied in combination with other therapies. After 24 weeks, sitagliptin combined with pioglitazone significantly reduced HbA1c by –0.70% and FPG by –17.7 mg/dL (P < .001 for both) compared with placebo.42 In another 24-week study, 100 mg sitagliptin QD significantly improved glycemic control and beta-cell function (P < .05 for both) in patients with T2DM who had inadequate glycemic control with glimepiride or glimepiride plus metformin.43
In addition to significantly reducing HbA1c, sitagliptin 100 and 200 mg QD produced only small differences in body weight relative to placebo: least-squares mean change from baseline for sitagliptin 100 mg was –0.7 kg (95% CI, –1.3 to –0.1) and for 200 mg was –0.6 kg (95% CI, –1.0 to –0.2); for placebo it was –0.2 kg (95% CI, –0.7 to 0.2).38 These findings were consistent with those from another 24-week monotherapy study where sitagliptin produced weight loss of up to –0.2 kg44 and a 30-week study of sitagliptin added to ongoing metformin therapy. In the latter study, both sitagliptin and placebo resulted in weight reductions of –0.5 kg.45
The effects of sitagliptin on lipids and BP have been reported in clinical studies in patients with and without T2DM. In one study of patients with T2DM, the addition of sitagliptin to metformin increased total cholesterol (+8.1 mg/dL), LDL-C (+9.2 mg/dL), and HDL-C (+1.8 mg/dL) but lowered triglyceride (–14.5 mg/dL) after 18 weeks of treatment (24-week data).46 Data from a small (n = 19) study in nondiabetic patients with mild to moderate hypertension showed that sitagliptin produced small reductions (–2 to –3 mm Hg) in 24-hour ambulatory BP measurements.47
Another DPP-4 inhibitor, saxagliptin, with efficacy similar to that described for sitagliptin, was recently approved by the US Food and Drug Administration (FDA) for treatment of T2DM.48
GLP-1 receptor agonists
Many of the GLP-1 receptor agonists developed or under development have glucoregulatory effects similar to GLP-1 but are resistant to degradation by DPP-4.28 Exenatide, an exendin-4 receptor agonist, has compared favorably with sitagliptin and with insulin analogues. Long-acting (once-weekly and once-daily) GLP-1 receptor agonists are under development.
In a 2-week, head-to-head study in metformin-treated patients with T2DM, exenatide had a greater effect than sitagliptin in lowering PPG and was more potent in increasing insulin secretion and reducing postprandial glucagon secretion. In contrast to sitagliptin, exenatide slowed gastric emptying and reduced caloric intake.49
In two studies of patients treated with exenatide, on a background of either metformin alone or metformin plus a sulfonylurea, patients who received metformin lost more weight (–1.6 to –2.8 kg; P ≤ .01) and experienced more significant decreases from baseline HbA1c (–0.4% to –0.8%; P < .002) at 30 weeks than did patients who received placebo.50,51 In a 16-week trial of exenatide in patients previously treated with a TZD with or without metformin, exenatide reduced HbA1c –0.98%, fasting blood glucose –1.69 mmol/L, and body weight –1.51 kg.52
When compared with insulin analogues, exenatide has been associated with weight loss (~ –3 kg) while the insulin analogues were associated with weight gain (~ +3 kg).53 After 26 weeks, body weight decreased –2.3 kg with exenatide and increased +1.8 kg with insulin glargine.54 Similar results were found in a crossover noninferiority trial, where the least-squares mean difference in weight change was significantly (P < .001) different (2.2 kg) between the treatments.55 When exenatide was compared with insulin aspart in an open-label, noninferiority trial, there was a between-group difference in weight of –5.4 kg after 52 weeks.32
Exenatide has also demonstrated these benefits in open-label extension studies. After 2 years, mean HbA1c reductions of –1.1% from baseline were sustained (P < .05), and weight loss of –4.7 kg was maintained (P < .001).56 After 82 weeks, similar HbA1c decreases (–1.1%) and weight loss (–4.4 kg) were exhibited.57 Even after 3 years, these benefits were maintained in patients who remained on the drug (HbA1c reduction from baseline, –1.0%; weight loss, –5.3 kg [P < .0001 for both]).58
Long-acting formulations of GLP-1 receptor agonists are in clinical development; two of these are once-weekly exenatide and once-daily liraglutide. Exenatide once weekly has the advantage of less frequent dosing and has elicited greater reductions in HbA1c than exenatide BID. After 15 weeks of once-weekly administration, the 0.8-mg formulation reduced HbA1c –1.4% and the 2-mg formulation reduced it –1.7% (P < .0001 for both compared with placebo). Body weight was lowered –3.8 kg (P < .05 compared with placebo) with the 2-mg formulation.59 Compared with exenatide BID, exenatide 2 mg once weekly showed greater reductions in HbA1c (–1.9% vs –1.5%; P = .0023) after 30 weeks of therapy.60 In a 1-year noncomparative trial, treatment with exenatide once weekly improved HbA1c (–2.0%) and weight (–4.1 kg), as well as BP and lipid profiles compared with baseline.61
Liraglutide, a once-daily human analogue GLP-1 receptor agonist, is under review by the FDA.28 In a 26-week study of patients with T2DM, liraglutide was associated with reductions in HbA1c (mean, –1.04%; P = 0.067 compared with insulin) and body weight (mean, –2.5 kg; P < .001 compared with insulin) at dosages of 0.6 to 1.8 mg/day SC. Liraglutide produced a decline in SBP from 0.6 to 3 mm Hg but was not associated with a decrease in DBP.62 In a 52-week study comparing liraglutide with glimepiride monotherapy, liraglutide 1.2 mg was associated with an HbA1c reduction of –0.84% (P = .0014) and the 1.8-mg dose with a reduction of –1.14% (P < .0001) compared with –0.51% for glimepiride. SBP decreased –0.7 mm Hg with glimepiride compared with –2.1 mm Hg for liraglutide 1.2 mg (P = .2912) and –3.6 mm Hg for liraglutide 1.8 mg (P < .0118). Mean DBP fell slightly but not significantly in all treatment groups.63 No effects on lipid parameters were reported in these two liraglutide studies.
The Liraglutide Effect and Action in Diabetes (LEAD-6) trial was undertaken to compare exenatide (10 mg BID SC) and liraglutide (1.8 mg/day SC) as add-on therapy to metformin, a sulfonylurea, or a combination of both in 464 patients with T2DM. After 26 weeks of treatment, liraglutide was associated with a significant reduction in HbA1c of –1.12%, compared with –0.79% with exenatide (P < .0001). Patients treated with liraglutide lost –3.2 kg while those on exenatide lost –2.9 kg. Among patients previously treated with metformin alone, there was a 1-kg difference in favor of liraglutide (P = NS).64
Safety profile
All of the drugs discussed have potential adverse effects. Metformin continues to have a black box warning for lactic acidosis.65 Sulfonylureas and insulin can cause hypoglycemia. TZDs can cause fluid retention and, in rare cases, CHF (for which these drugs also carry a black box warning).66,67 TZDs also increase the risk of distal fracture.66,67 The most common side effects of exenatide are gastrointestinal, but there have been reported cases of pancreatitis, some of which have been fatal.68,69 It has been difficult to prove whether exenatide increases the risk of pancreatitis, as patients with T2DM are already at an increased (three- to fourfold) risk for this condition compared with persons who do not have T2DM.69 Exenatide should not be used in patients with severe renal impairment or end-stage renal disease; it should be used with caution in patients who have undergone renal transplantation and in patients with moderate renal impairment.
The prescribing information for sitagliptin includes pancreatitis among the adverse reactions identified during the drug’s postapproval use.70 As with exenatide, it is not fully known whether a true association exists between the agent and pancreatitis. However, since pancreatitis can occur in this patient population, it is recommended that abdominal pain be fully evaluated to rule out pancreatitis. Continued postmarketing surveillance is important for all of these agents.
THE ROLE OF GUIDELINES
The American Association of Clinical Endocrinologists (AACE),26 the American Diabetes Association (ADA),71 and the ADA in conjunction with the European Association for the Study of Diabetes (EASD)24 have recently revised their recommendations for the management of patients with diabetes. The guidelines are unanimous in setting a glycemic goal (HbA1c < 7.0% for the ADA, HbA1c ≤ 6.5% for the AACE) and advocating individualized care for a treatment goal of HbA1c lower than 6.0% in patients who stand to benefit from near euglycemia without inducing severe hypoglycemia.24,26,71
CVD is the major cause of morbidity and mortality associated with T2DM and is a source of increasing concern.5 Accordingly, special consideration should be given to patients with coexisting CV risk factors, including hypertension and dyslipidemia. The ADA and the EASD advocate lifestyle modification to decrease body weight and the concurrent initiation of metformin as first-line therapy.24 If that strategy is insufficient, then two tiers of treatment guide the choice of next steps24:
- Tier 1, in addition to metformin, includes the sulfonylureas and insulin. Although these are excellent glucose-lowering drugs, they are associated with weight gain, hypoglycemia, and no improvement in BP or lipid levels. They are relatively low in cost and have been used for many years. Their main drawback is evidence that despite their use, beta-cell failure continues unabated over time.
- Tier 2 treatments include pioglitazone and the GLP-1 receptor agonist exenatide. Consideration may be given to the use of pioglitazone or exenatide when hypoglycemia is of concern, with exenatide being preferred when weight loss is a major objective and HbA1c is close to target (< 8.0%).24 Additionally, both the TZDs and exenatide probably help slow the rate of beta-cell failure, particularly if they are used early in the course of the disease.72,73 The AACE recommends different pharmacologic approaches based on HbA1c at diagnosis.26
The American Heart Association and the ADA have issued a joint scientific statement on the primary prevention of CVD in patients with diabetes.74 They advocate lifestyle management of body weight, nutrition, and physical activity.74 In addition, they stress the need for attention to BP, lipid levels, and smoking status, and the use of antiplatelet agents in patients at increased CV risk (> 40 years of age and a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria).
CONCLUSION
T2DM, weight gain/obesity, and CV risk present a continuing challenge to patients and clinicians. Antidiabetes agents have varying degrees of evidence to support their effects on HbA1c, body weight, BP, and lipid levels. A better understanding of the pathophysiology of T2DM has led to the development of newer antidiabetes agents that target the fundamental defects of the disease. Evidence continues to accumulate for the improved benefits of glycemic control and weight loss in T2DM with GLP-1 receptor agonists such as exenatide currently having robust data in terms of beneficial effects on weight and CV risk factors. As clinicians continue to incorporate this knowledge into their practice patterns, patient adherence and clinical outcomes are expected to improve. Newer agents, such as incretin-based therapies, address T2DM as well as other factors that increase cardiometabolic risk through their effects not only on glycemic control but on body weight, BP, and lipids.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of self-reported cardiovascular disease among persons aged >35 years with diabetes—United States, 1997–2005. MMWR Morb Mortal Wkly Rep 2007; 56:1129–1132.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Bonora E, Targher G, Formentini G, et al. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabet Med 2004; 21:52–58.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Jenssen TG, Tonstad S, Claudi T, Midthejell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005; 22:1379–1385.
- Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes 2007; 5:8.
- Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: the mediational role of diabetes symptoms and self-efficacy. Health Psychol 2007; 26:693–700.
- Ruelas V, Roybal GM, Lu Y, Goldman D, Peters A. Clinical and behavioral correlates of achieving and maintaining glycemic targets in an underserved population with type 2 diabetes. Diabetes Care 2009; 32:54–56.
- Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 2003; 52:172–179.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007; 30:1127–1142.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 2001; 86:3853–3860.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17:4–12.
- de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 2009; 19:313–320.
- Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis 2008; 18:574–579.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Hanefeld M, Herman G, Wu M, Mickel C, Sanchez M, Stein PP; for the Sitagliptin Study 014 Investigators. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007; 23:1329–1339.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol 2008; 48:592–598.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29:139–153.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Garber A, Henry R. Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Fortamet [package insert]. Ft. Lauderdale, FL: Watson Laboratories; 2008.
- Actos. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008; 3077–3082.
- Avandia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2009; 1351–1359.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Idris I. News and views: FDA reviews incidences of acute pancreatitis in patients taking Byetta. Diabetes Obes Metab 2008; 10:96–98.
- Januvia [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2006, 2007.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292:E871–E883.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007; 30:162–172.
Type 2 diabetes mellitus (T2DM), excess weight, and obesity are increasing in prevalence at alarming rates.1–3 Concurrent with the increased prevalence is increased risk of morbidity and mortality. A healthy diet and exercise in conjunction with antidiabetes medications can help lower glucose concentration in patients with T2DM. Because these patients are at increased risk of cardiovascular (CV) morbidity and mortality, however, treatment strategies should address the CV risk factors, including blood pressure (BP), lipids, and body weight, as well as glycemic aspects of the disease.
To help clinicians manage the complex issues in treating patients with T2DM, this article presents an overview of patient and treatment perspectives relevant to overweight/obesity and CV disease (CVD). It includes an examination of the latest guidelines and algorithms for the management of T2DM, which continue to be updated and modified.
T2DM, WEIGHT GAIN OR OBESITY, AND CV RISK: A CHALLENGING TRIAD
Despite therapeutic advances in the diagnosis and treatment of diabetes and CVD over the last decade, the estimated number of persons in the United States older than 35 years with self-reported diabetes (with T2DM accounting for 90% to 95% of diagnosed cases) and CVD has increased from 4.2 million in 1997 to 5.7 million in 2005.3,4 The CV risk for patients with T2DM who have not had a CV event such as a myocardial infarction (MI) is similar to that of individuals without diabetes who have had a prior MI.5 Patients with T2DM have nearly double the mortality of those without the disease.6 Adding to their risk, about 80% of patients with T2DM are overweight or obese, conditions associated with worsened insulin resistance and increased CV risk and disease burden.7,8 Even a modest weight gain (5 kg) may increase the risk of coronary heart disease (CHD) by 30%, while associated changes in lipids and BP can increase the risk by another 20%.9
It is as important to control CV risk factors as it is to control glycemia in patients with T2DM, and both are difficult to achieve. Data from a recent nationwide Norwegian survey showed that only 13% of patients with T2DM achieved study-defined target levels; ie, glycosylated hemoglobin (HbA1c) less than 7.5%, BP less than 140/85 mm Hg, and total cholesterol/high-density lipoprotein (HDL-C) ratio less than 4.0.10
BENEFITS OF MANAGING GLYCEMIA, WEIGHT REDUCTION, AND CV RISK FACTORS
Several large studies, many ongoing, are generating data on the relationships among glycemia, weight reduction, and CV risk. It is well established that individuals with T2DM need aggressive risk factor reduction (glucose control, blood pressure management, and treatment of dyslipidemia) to optimize outcomes. However, characterization of the benefits of various components of risk factor reduction, particularly over many years, is only now occurring.
Results from the United Kingdom Prospective Diabetes Studies (UKPDS) showed the benefits and risks of pharmacologic glycemic control—essentially monotherapy with insulin or a sulfonylurea—compared with conventional dietary therapy in reducing diabetic complications in patients with newly diagnosed T2DM. In UKPDS 33, both insulin and sulfonylureas (intensive treatment) reduced the risk of microvascular end points (retinopathy, nephropathy) in patients whose median HbA1c was lowered to 7.0% at 10 years of follow-up, compared with patients who reached an HbA1c of 7.9%. However, intensive glycemic control did not translate into a statistically significant reduction in macrovascular complications, including MI, stroke, CVD, and death. Additionally, patients assigned to insulin had greater weight gain (+4.0 kg) than did patients assigned to receive the sulfonylurea chlorpropamide (+2.6 kg) or glyburide (+1.7 kg) (P < .01).11
The UKPDS showed that intensive treatment with metformin reduced the risk of T2DM-related end points compared with conventional treatment (primarily diet alone) in overweight patients.12 Although there were fewer patients in the metformin-treated subset (n = 342) than in the conventional treatment cohort, a secondary analysis showed that metformin was associated with less weight gain and fewer hypoglycemic episodes than either insulin or sulfonylurea therapy.12 Since HbA1c levels in the treatment groups were equal, the additional benefits seen with metformin in overweight patients with T2DM were not based solely on glycemic control.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial involved 10,000 individuals with T2DM. The primary outcome measure was a composite of CV events. The intensively treated group was controlled to a target HbA1c of less than 6.0%, with most patients receiving insulin. The trial was terminated early because an increased risk of sudden death was observed.13 A similar study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), evaluated more than 11,000 patients with T2DM, starting with a sulfonylurea-based regimen. In this study, there was no reduction in macrovascular events, but there was a reduction in nephropathy in the intensively treated group.14 In both studies, hypoglycemia and weight gain were more frequent in intensively treated patients; and in ACCORD, there were more episodes of severe hypoglycemia in the intensive-treatment group.13,14
The Veterans Affairs Diabetes Trial (VADT) evaluated the effect of intensive glucose control on CVD in 1,791 patients (mean age, 60 years) with poorly controlled T2DM (average duration, 11.5 years). The primary end points included MI, stroke, new or worsening congestive heart failure (CHF), limb amputation, and invasive intervention for coronary or peripheral arterial disease. The hazard ratio for these end points in the intensive-treatment group was 0.88 (95% confidence interval [CI], 0.74 to 1.05).15,16 Specifically, the following beneficial effects were achieved:
- HbA1c reduced by –1.0% to –2.5% in absolute units,
- systolic BP (SBP) reduced by –4 to –7 mm Hg,
- diastolic BP (DBP) reduced by –7 to –8 mm Hg,
- low-density lipoprotein cholesterol (LDL-C) reduced by –27 to –28 mg/dL,
- triglycerides reduced by –44 to –50 mg/dL, and
- HDL-C increased by 4 to 5 mg/dL.
Despite these benefits, body weight increased approximately 9 to 18 lb (4 to 8 kg) during therapy.15
Since overweight and obesity are independent risk factors for CHD and CVD in patients with T2DM,17 weight management is an integral component in treatment. In the Action for Health in Diabetes (Look AHEAD) trial, an intensive exercise and weight-loss program resulted in clinically significant (P < .001) weight loss at 1 year in patients who had T2DM and a body mass index (BMI) greater than 25 kg2/m (> 27 kg2/m if receiving insulin).18 When compared with patients who received less structured, infrequent support and minimal education about diabetes, participants in the intensive program showed more weight loss, improved glucose control, decreased CV events, and reduced medicine use. The Look AHEAD trial is currently evaluating whether these improvements will continue to result in lower CV risk.
PATIENT ADHERENCE AND SATISFACTION
It is often challenging for patients with T2DM to adhere to their treatment regimens. The Diabetes Attitude, Wishes, and Needs (DAWN) study examined psychosocial barriers to self-care in patients with diabetes and found that while 78% of patients with T2DM adhered to their medications, only 39% achieved complete success in at least two-thirds of their self-care domains.19 A multicenter, randomized, clinical trial examined the correlates of treatment satisfaction, including body weight, on patients’ appraisal of treatment satisfaction with injectable insulin. The 14.5% of patients who experienced a reduction in BMI reported systematic improvement in treatment satisfaction.20 Similarly, a cross-sectionally designed study (n = 99) that analyzed the interrelation of adherence, BMI, and depression in adults with T2DM found that patients with higher BMI and poor adherence also had depression, which was mediated by lower self-efficacy perceptions and increased diabetes symptoms.21 The results from these studies show a clear relationship between adherence with treatment regimens and achievement of HbA1c goals.22
RECENT DEVELOPMENTS IN T2DM MANAGEMENT: STRATEGIES TO REDUCE CV RISK
Because excess weight and obesity are prominent features of T2DM, it is important to use an antidiabetes agent that does not induce unnecessary weight gain (particularly central weight gain, which is thought to be most atherogenic).23 Metformin, considered the first-line agent for treatment of T2DM, is generally weight neutral with a low level of hypoglycemia.24,25 Sulfonylureas, insulin, and thiazolidinediones (TZDs) are all associated with weight gain, although newer-analogue insulins may cause less weight gain than older agents. TZDs, especially pioglitazone, are associated with improvements in long-term beta-cell function and CV risk factors despite weight gain.26,27
The newer antidiabetes agents belong to the dipeptidyl peptidase–4 (DPP-4) inhibitor and the glucagon-like peptide–1 (GLP-1) receptor agonist therapeutic classes and have been shown to be either weight neutral (DPP-4 inhibitors) or to cause weight loss (GLP-1 receptor agonists).28
Obesity and the incretin effect
Two recent studies showed that surgically induced weight loss enhances the physiologic “incretin effect.” In one study, obese individuals with T2DM whose weight loss was secondary to bariatric surgery combined with caloric restriction showed improved insulin sensitivity, improved carbohydrate metabolism, and elevated levels of adiponectin and GLP-1, all of which may reduce the incidence of T2DM.36 In the other study, bariatric surgery in morbidly obese individuals with T2DM improved insulin secretion and ameliorated insulin resistance.37
DPP-4 inhibitors
DPP-4 inhibitors such as sitagliptin and saxagliptin inhibit the enzymatic activity of DPP-4 and increase endogenous concentrations of GLP-1.28 Sitagliptin has been compared with placebo as monotherapy and has been studied in combination with other therapies.
In an 18-week study, sitagliptin monotherapy, 100 and 200 mg QD, significantly reduced HbA1c compared with placebo (placebo-subtracted HbA1c reduction, –0.60% and –0.48%, respectively) in patients with T2DM. Sitagliptin also significantly decreased fasting plasma glucose (FPG) concentration relative to placebo.38 Twelve weeks of sitagliptin monotherapy at dosages of 5, 12.5, 25, and 50 mg BID led to significant (P < .001) reductions in HbA1c compared with placebo. Sitagliptin also produced significant reductions in FPG and mean daily glucose concentrations across the doses studied.39 Similar results were reported in other 12-week studies: 50 mg BID and 100 mg QD sitagliptin monotherapy significantly (P < .05) reduced HbA1c –0.39% to –0.56% and FPG concentration –11.0 to –17.2 mg/dL compared with placebo40; sitagliptin 100 mg QD compared with placebo produced a least-squares mean change from baseline HbA1c of –0.65% versus 0.41% (P < .001) and FPG of –22.5 versus 9.4 mg/dL (P < .001).41
Sitagliptin also has been studied in combination with other therapies. After 24 weeks, sitagliptin combined with pioglitazone significantly reduced HbA1c by –0.70% and FPG by –17.7 mg/dL (P < .001 for both) compared with placebo.42 In another 24-week study, 100 mg sitagliptin QD significantly improved glycemic control and beta-cell function (P < .05 for both) in patients with T2DM who had inadequate glycemic control with glimepiride or glimepiride plus metformin.43
In addition to significantly reducing HbA1c, sitagliptin 100 and 200 mg QD produced only small differences in body weight relative to placebo: least-squares mean change from baseline for sitagliptin 100 mg was –0.7 kg (95% CI, –1.3 to –0.1) and for 200 mg was –0.6 kg (95% CI, –1.0 to –0.2); for placebo it was –0.2 kg (95% CI, –0.7 to 0.2).38 These findings were consistent with those from another 24-week monotherapy study where sitagliptin produced weight loss of up to –0.2 kg44 and a 30-week study of sitagliptin added to ongoing metformin therapy. In the latter study, both sitagliptin and placebo resulted in weight reductions of –0.5 kg.45
The effects of sitagliptin on lipids and BP have been reported in clinical studies in patients with and without T2DM. In one study of patients with T2DM, the addition of sitagliptin to metformin increased total cholesterol (+8.1 mg/dL), LDL-C (+9.2 mg/dL), and HDL-C (+1.8 mg/dL) but lowered triglyceride (–14.5 mg/dL) after 18 weeks of treatment (24-week data).46 Data from a small (n = 19) study in nondiabetic patients with mild to moderate hypertension showed that sitagliptin produced small reductions (–2 to –3 mm Hg) in 24-hour ambulatory BP measurements.47
Another DPP-4 inhibitor, saxagliptin, with efficacy similar to that described for sitagliptin, was recently approved by the US Food and Drug Administration (FDA) for treatment of T2DM.48
GLP-1 receptor agonists
Many of the GLP-1 receptor agonists developed or under development have glucoregulatory effects similar to GLP-1 but are resistant to degradation by DPP-4.28 Exenatide, an exendin-4 receptor agonist, has compared favorably with sitagliptin and with insulin analogues. Long-acting (once-weekly and once-daily) GLP-1 receptor agonists are under development.
In a 2-week, head-to-head study in metformin-treated patients with T2DM, exenatide had a greater effect than sitagliptin in lowering PPG and was more potent in increasing insulin secretion and reducing postprandial glucagon secretion. In contrast to sitagliptin, exenatide slowed gastric emptying and reduced caloric intake.49
In two studies of patients treated with exenatide, on a background of either metformin alone or metformin plus a sulfonylurea, patients who received metformin lost more weight (–1.6 to –2.8 kg; P ≤ .01) and experienced more significant decreases from baseline HbA1c (–0.4% to –0.8%; P < .002) at 30 weeks than did patients who received placebo.50,51 In a 16-week trial of exenatide in patients previously treated with a TZD with or without metformin, exenatide reduced HbA1c –0.98%, fasting blood glucose –1.69 mmol/L, and body weight –1.51 kg.52
When compared with insulin analogues, exenatide has been associated with weight loss (~ –3 kg) while the insulin analogues were associated with weight gain (~ +3 kg).53 After 26 weeks, body weight decreased –2.3 kg with exenatide and increased +1.8 kg with insulin glargine.54 Similar results were found in a crossover noninferiority trial, where the least-squares mean difference in weight change was significantly (P < .001) different (2.2 kg) between the treatments.55 When exenatide was compared with insulin aspart in an open-label, noninferiority trial, there was a between-group difference in weight of –5.4 kg after 52 weeks.32
Exenatide has also demonstrated these benefits in open-label extension studies. After 2 years, mean HbA1c reductions of –1.1% from baseline were sustained (P < .05), and weight loss of –4.7 kg was maintained (P < .001).56 After 82 weeks, similar HbA1c decreases (–1.1%) and weight loss (–4.4 kg) were exhibited.57 Even after 3 years, these benefits were maintained in patients who remained on the drug (HbA1c reduction from baseline, –1.0%; weight loss, –5.3 kg [P < .0001 for both]).58
Long-acting formulations of GLP-1 receptor agonists are in clinical development; two of these are once-weekly exenatide and once-daily liraglutide. Exenatide once weekly has the advantage of less frequent dosing and has elicited greater reductions in HbA1c than exenatide BID. After 15 weeks of once-weekly administration, the 0.8-mg formulation reduced HbA1c –1.4% and the 2-mg formulation reduced it –1.7% (P < .0001 for both compared with placebo). Body weight was lowered –3.8 kg (P < .05 compared with placebo) with the 2-mg formulation.59 Compared with exenatide BID, exenatide 2 mg once weekly showed greater reductions in HbA1c (–1.9% vs –1.5%; P = .0023) after 30 weeks of therapy.60 In a 1-year noncomparative trial, treatment with exenatide once weekly improved HbA1c (–2.0%) and weight (–4.1 kg), as well as BP and lipid profiles compared with baseline.61
Liraglutide, a once-daily human analogue GLP-1 receptor agonist, is under review by the FDA.28 In a 26-week study of patients with T2DM, liraglutide was associated with reductions in HbA1c (mean, –1.04%; P = 0.067 compared with insulin) and body weight (mean, –2.5 kg; P < .001 compared with insulin) at dosages of 0.6 to 1.8 mg/day SC. Liraglutide produced a decline in SBP from 0.6 to 3 mm Hg but was not associated with a decrease in DBP.62 In a 52-week study comparing liraglutide with glimepiride monotherapy, liraglutide 1.2 mg was associated with an HbA1c reduction of –0.84% (P = .0014) and the 1.8-mg dose with a reduction of –1.14% (P < .0001) compared with –0.51% for glimepiride. SBP decreased –0.7 mm Hg with glimepiride compared with –2.1 mm Hg for liraglutide 1.2 mg (P = .2912) and –3.6 mm Hg for liraglutide 1.8 mg (P < .0118). Mean DBP fell slightly but not significantly in all treatment groups.63 No effects on lipid parameters were reported in these two liraglutide studies.
The Liraglutide Effect and Action in Diabetes (LEAD-6) trial was undertaken to compare exenatide (10 mg BID SC) and liraglutide (1.8 mg/day SC) as add-on therapy to metformin, a sulfonylurea, or a combination of both in 464 patients with T2DM. After 26 weeks of treatment, liraglutide was associated with a significant reduction in HbA1c of –1.12%, compared with –0.79% with exenatide (P < .0001). Patients treated with liraglutide lost –3.2 kg while those on exenatide lost –2.9 kg. Among patients previously treated with metformin alone, there was a 1-kg difference in favor of liraglutide (P = NS).64
Safety profile
All of the drugs discussed have potential adverse effects. Metformin continues to have a black box warning for lactic acidosis.65 Sulfonylureas and insulin can cause hypoglycemia. TZDs can cause fluid retention and, in rare cases, CHF (for which these drugs also carry a black box warning).66,67 TZDs also increase the risk of distal fracture.66,67 The most common side effects of exenatide are gastrointestinal, but there have been reported cases of pancreatitis, some of which have been fatal.68,69 It has been difficult to prove whether exenatide increases the risk of pancreatitis, as patients with T2DM are already at an increased (three- to fourfold) risk for this condition compared with persons who do not have T2DM.69 Exenatide should not be used in patients with severe renal impairment or end-stage renal disease; it should be used with caution in patients who have undergone renal transplantation and in patients with moderate renal impairment.
The prescribing information for sitagliptin includes pancreatitis among the adverse reactions identified during the drug’s postapproval use.70 As with exenatide, it is not fully known whether a true association exists between the agent and pancreatitis. However, since pancreatitis can occur in this patient population, it is recommended that abdominal pain be fully evaluated to rule out pancreatitis. Continued postmarketing surveillance is important for all of these agents.
THE ROLE OF GUIDELINES
The American Association of Clinical Endocrinologists (AACE),26 the American Diabetes Association (ADA),71 and the ADA in conjunction with the European Association for the Study of Diabetes (EASD)24 have recently revised their recommendations for the management of patients with diabetes. The guidelines are unanimous in setting a glycemic goal (HbA1c < 7.0% for the ADA, HbA1c ≤ 6.5% for the AACE) and advocating individualized care for a treatment goal of HbA1c lower than 6.0% in patients who stand to benefit from near euglycemia without inducing severe hypoglycemia.24,26,71
CVD is the major cause of morbidity and mortality associated with T2DM and is a source of increasing concern.5 Accordingly, special consideration should be given to patients with coexisting CV risk factors, including hypertension and dyslipidemia. The ADA and the EASD advocate lifestyle modification to decrease body weight and the concurrent initiation of metformin as first-line therapy.24 If that strategy is insufficient, then two tiers of treatment guide the choice of next steps24:
- Tier 1, in addition to metformin, includes the sulfonylureas and insulin. Although these are excellent glucose-lowering drugs, they are associated with weight gain, hypoglycemia, and no improvement in BP or lipid levels. They are relatively low in cost and have been used for many years. Their main drawback is evidence that despite their use, beta-cell failure continues unabated over time.
- Tier 2 treatments include pioglitazone and the GLP-1 receptor agonist exenatide. Consideration may be given to the use of pioglitazone or exenatide when hypoglycemia is of concern, with exenatide being preferred when weight loss is a major objective and HbA1c is close to target (< 8.0%).24 Additionally, both the TZDs and exenatide probably help slow the rate of beta-cell failure, particularly if they are used early in the course of the disease.72,73 The AACE recommends different pharmacologic approaches based on HbA1c at diagnosis.26
The American Heart Association and the ADA have issued a joint scientific statement on the primary prevention of CVD in patients with diabetes.74 They advocate lifestyle management of body weight, nutrition, and physical activity.74 In addition, they stress the need for attention to BP, lipid levels, and smoking status, and the use of antiplatelet agents in patients at increased CV risk (> 40 years of age and a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria).
CONCLUSION
T2DM, weight gain/obesity, and CV risk present a continuing challenge to patients and clinicians. Antidiabetes agents have varying degrees of evidence to support their effects on HbA1c, body weight, BP, and lipid levels. A better understanding of the pathophysiology of T2DM has led to the development of newer antidiabetes agents that target the fundamental defects of the disease. Evidence continues to accumulate for the improved benefits of glycemic control and weight loss in T2DM with GLP-1 receptor agonists such as exenatide currently having robust data in terms of beneficial effects on weight and CV risk factors. As clinicians continue to incorporate this knowledge into their practice patterns, patient adherence and clinical outcomes are expected to improve. Newer agents, such as incretin-based therapies, address T2DM as well as other factors that increase cardiometabolic risk through their effects not only on glycemic control but on body weight, BP, and lipids.
Type 2 diabetes mellitus (T2DM), excess weight, and obesity are increasing in prevalence at alarming rates.1–3 Concurrent with the increased prevalence is increased risk of morbidity and mortality. A healthy diet and exercise in conjunction with antidiabetes medications can help lower glucose concentration in patients with T2DM. Because these patients are at increased risk of cardiovascular (CV) morbidity and mortality, however, treatment strategies should address the CV risk factors, including blood pressure (BP), lipids, and body weight, as well as glycemic aspects of the disease.
To help clinicians manage the complex issues in treating patients with T2DM, this article presents an overview of patient and treatment perspectives relevant to overweight/obesity and CV disease (CVD). It includes an examination of the latest guidelines and algorithms for the management of T2DM, which continue to be updated and modified.
T2DM, WEIGHT GAIN OR OBESITY, AND CV RISK: A CHALLENGING TRIAD
Despite therapeutic advances in the diagnosis and treatment of diabetes and CVD over the last decade, the estimated number of persons in the United States older than 35 years with self-reported diabetes (with T2DM accounting for 90% to 95% of diagnosed cases) and CVD has increased from 4.2 million in 1997 to 5.7 million in 2005.3,4 The CV risk for patients with T2DM who have not had a CV event such as a myocardial infarction (MI) is similar to that of individuals without diabetes who have had a prior MI.5 Patients with T2DM have nearly double the mortality of those without the disease.6 Adding to their risk, about 80% of patients with T2DM are overweight or obese, conditions associated with worsened insulin resistance and increased CV risk and disease burden.7,8 Even a modest weight gain (5 kg) may increase the risk of coronary heart disease (CHD) by 30%, while associated changes in lipids and BP can increase the risk by another 20%.9
It is as important to control CV risk factors as it is to control glycemia in patients with T2DM, and both are difficult to achieve. Data from a recent nationwide Norwegian survey showed that only 13% of patients with T2DM achieved study-defined target levels; ie, glycosylated hemoglobin (HbA1c) less than 7.5%, BP less than 140/85 mm Hg, and total cholesterol/high-density lipoprotein (HDL-C) ratio less than 4.0.10
BENEFITS OF MANAGING GLYCEMIA, WEIGHT REDUCTION, AND CV RISK FACTORS
Several large studies, many ongoing, are generating data on the relationships among glycemia, weight reduction, and CV risk. It is well established that individuals with T2DM need aggressive risk factor reduction (glucose control, blood pressure management, and treatment of dyslipidemia) to optimize outcomes. However, characterization of the benefits of various components of risk factor reduction, particularly over many years, is only now occurring.
Results from the United Kingdom Prospective Diabetes Studies (UKPDS) showed the benefits and risks of pharmacologic glycemic control—essentially monotherapy with insulin or a sulfonylurea—compared with conventional dietary therapy in reducing diabetic complications in patients with newly diagnosed T2DM. In UKPDS 33, both insulin and sulfonylureas (intensive treatment) reduced the risk of microvascular end points (retinopathy, nephropathy) in patients whose median HbA1c was lowered to 7.0% at 10 years of follow-up, compared with patients who reached an HbA1c of 7.9%. However, intensive glycemic control did not translate into a statistically significant reduction in macrovascular complications, including MI, stroke, CVD, and death. Additionally, patients assigned to insulin had greater weight gain (+4.0 kg) than did patients assigned to receive the sulfonylurea chlorpropamide (+2.6 kg) or glyburide (+1.7 kg) (P < .01).11
The UKPDS showed that intensive treatment with metformin reduced the risk of T2DM-related end points compared with conventional treatment (primarily diet alone) in overweight patients.12 Although there were fewer patients in the metformin-treated subset (n = 342) than in the conventional treatment cohort, a secondary analysis showed that metformin was associated with less weight gain and fewer hypoglycemic episodes than either insulin or sulfonylurea therapy.12 Since HbA1c levels in the treatment groups were equal, the additional benefits seen with metformin in overweight patients with T2DM were not based solely on glycemic control.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial involved 10,000 individuals with T2DM. The primary outcome measure was a composite of CV events. The intensively treated group was controlled to a target HbA1c of less than 6.0%, with most patients receiving insulin. The trial was terminated early because an increased risk of sudden death was observed.13 A similar study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), evaluated more than 11,000 patients with T2DM, starting with a sulfonylurea-based regimen. In this study, there was no reduction in macrovascular events, but there was a reduction in nephropathy in the intensively treated group.14 In both studies, hypoglycemia and weight gain were more frequent in intensively treated patients; and in ACCORD, there were more episodes of severe hypoglycemia in the intensive-treatment group.13,14
The Veterans Affairs Diabetes Trial (VADT) evaluated the effect of intensive glucose control on CVD in 1,791 patients (mean age, 60 years) with poorly controlled T2DM (average duration, 11.5 years). The primary end points included MI, stroke, new or worsening congestive heart failure (CHF), limb amputation, and invasive intervention for coronary or peripheral arterial disease. The hazard ratio for these end points in the intensive-treatment group was 0.88 (95% confidence interval [CI], 0.74 to 1.05).15,16 Specifically, the following beneficial effects were achieved:
- HbA1c reduced by –1.0% to –2.5% in absolute units,
- systolic BP (SBP) reduced by –4 to –7 mm Hg,
- diastolic BP (DBP) reduced by –7 to –8 mm Hg,
- low-density lipoprotein cholesterol (LDL-C) reduced by –27 to –28 mg/dL,
- triglycerides reduced by –44 to –50 mg/dL, and
- HDL-C increased by 4 to 5 mg/dL.
Despite these benefits, body weight increased approximately 9 to 18 lb (4 to 8 kg) during therapy.15
Since overweight and obesity are independent risk factors for CHD and CVD in patients with T2DM,17 weight management is an integral component in treatment. In the Action for Health in Diabetes (Look AHEAD) trial, an intensive exercise and weight-loss program resulted in clinically significant (P < .001) weight loss at 1 year in patients who had T2DM and a body mass index (BMI) greater than 25 kg2/m (> 27 kg2/m if receiving insulin).18 When compared with patients who received less structured, infrequent support and minimal education about diabetes, participants in the intensive program showed more weight loss, improved glucose control, decreased CV events, and reduced medicine use. The Look AHEAD trial is currently evaluating whether these improvements will continue to result in lower CV risk.
PATIENT ADHERENCE AND SATISFACTION
It is often challenging for patients with T2DM to adhere to their treatment regimens. The Diabetes Attitude, Wishes, and Needs (DAWN) study examined psychosocial barriers to self-care in patients with diabetes and found that while 78% of patients with T2DM adhered to their medications, only 39% achieved complete success in at least two-thirds of their self-care domains.19 A multicenter, randomized, clinical trial examined the correlates of treatment satisfaction, including body weight, on patients’ appraisal of treatment satisfaction with injectable insulin. The 14.5% of patients who experienced a reduction in BMI reported systematic improvement in treatment satisfaction.20 Similarly, a cross-sectionally designed study (n = 99) that analyzed the interrelation of adherence, BMI, and depression in adults with T2DM found that patients with higher BMI and poor adherence also had depression, which was mediated by lower self-efficacy perceptions and increased diabetes symptoms.21 The results from these studies show a clear relationship between adherence with treatment regimens and achievement of HbA1c goals.22
RECENT DEVELOPMENTS IN T2DM MANAGEMENT: STRATEGIES TO REDUCE CV RISK
Because excess weight and obesity are prominent features of T2DM, it is important to use an antidiabetes agent that does not induce unnecessary weight gain (particularly central weight gain, which is thought to be most atherogenic).23 Metformin, considered the first-line agent for treatment of T2DM, is generally weight neutral with a low level of hypoglycemia.24,25 Sulfonylureas, insulin, and thiazolidinediones (TZDs) are all associated with weight gain, although newer-analogue insulins may cause less weight gain than older agents. TZDs, especially pioglitazone, are associated with improvements in long-term beta-cell function and CV risk factors despite weight gain.26,27
The newer antidiabetes agents belong to the dipeptidyl peptidase–4 (DPP-4) inhibitor and the glucagon-like peptide–1 (GLP-1) receptor agonist therapeutic classes and have been shown to be either weight neutral (DPP-4 inhibitors) or to cause weight loss (GLP-1 receptor agonists).28
Obesity and the incretin effect
Two recent studies showed that surgically induced weight loss enhances the physiologic “incretin effect.” In one study, obese individuals with T2DM whose weight loss was secondary to bariatric surgery combined with caloric restriction showed improved insulin sensitivity, improved carbohydrate metabolism, and elevated levels of adiponectin and GLP-1, all of which may reduce the incidence of T2DM.36 In the other study, bariatric surgery in morbidly obese individuals with T2DM improved insulin secretion and ameliorated insulin resistance.37
DPP-4 inhibitors
DPP-4 inhibitors such as sitagliptin and saxagliptin inhibit the enzymatic activity of DPP-4 and increase endogenous concentrations of GLP-1.28 Sitagliptin has been compared with placebo as monotherapy and has been studied in combination with other therapies.
In an 18-week study, sitagliptin monotherapy, 100 and 200 mg QD, significantly reduced HbA1c compared with placebo (placebo-subtracted HbA1c reduction, –0.60% and –0.48%, respectively) in patients with T2DM. Sitagliptin also significantly decreased fasting plasma glucose (FPG) concentration relative to placebo.38 Twelve weeks of sitagliptin monotherapy at dosages of 5, 12.5, 25, and 50 mg BID led to significant (P < .001) reductions in HbA1c compared with placebo. Sitagliptin also produced significant reductions in FPG and mean daily glucose concentrations across the doses studied.39 Similar results were reported in other 12-week studies: 50 mg BID and 100 mg QD sitagliptin monotherapy significantly (P < .05) reduced HbA1c –0.39% to –0.56% and FPG concentration –11.0 to –17.2 mg/dL compared with placebo40; sitagliptin 100 mg QD compared with placebo produced a least-squares mean change from baseline HbA1c of –0.65% versus 0.41% (P < .001) and FPG of –22.5 versus 9.4 mg/dL (P < .001).41
Sitagliptin also has been studied in combination with other therapies. After 24 weeks, sitagliptin combined with pioglitazone significantly reduced HbA1c by –0.70% and FPG by –17.7 mg/dL (P < .001 for both) compared with placebo.42 In another 24-week study, 100 mg sitagliptin QD significantly improved glycemic control and beta-cell function (P < .05 for both) in patients with T2DM who had inadequate glycemic control with glimepiride or glimepiride plus metformin.43
In addition to significantly reducing HbA1c, sitagliptin 100 and 200 mg QD produced only small differences in body weight relative to placebo: least-squares mean change from baseline for sitagliptin 100 mg was –0.7 kg (95% CI, –1.3 to –0.1) and for 200 mg was –0.6 kg (95% CI, –1.0 to –0.2); for placebo it was –0.2 kg (95% CI, –0.7 to 0.2).38 These findings were consistent with those from another 24-week monotherapy study where sitagliptin produced weight loss of up to –0.2 kg44 and a 30-week study of sitagliptin added to ongoing metformin therapy. In the latter study, both sitagliptin and placebo resulted in weight reductions of –0.5 kg.45
The effects of sitagliptin on lipids and BP have been reported in clinical studies in patients with and without T2DM. In one study of patients with T2DM, the addition of sitagliptin to metformin increased total cholesterol (+8.1 mg/dL), LDL-C (+9.2 mg/dL), and HDL-C (+1.8 mg/dL) but lowered triglyceride (–14.5 mg/dL) after 18 weeks of treatment (24-week data).46 Data from a small (n = 19) study in nondiabetic patients with mild to moderate hypertension showed that sitagliptin produced small reductions (–2 to –3 mm Hg) in 24-hour ambulatory BP measurements.47
Another DPP-4 inhibitor, saxagliptin, with efficacy similar to that described for sitagliptin, was recently approved by the US Food and Drug Administration (FDA) for treatment of T2DM.48
GLP-1 receptor agonists
Many of the GLP-1 receptor agonists developed or under development have glucoregulatory effects similar to GLP-1 but are resistant to degradation by DPP-4.28 Exenatide, an exendin-4 receptor agonist, has compared favorably with sitagliptin and with insulin analogues. Long-acting (once-weekly and once-daily) GLP-1 receptor agonists are under development.
In a 2-week, head-to-head study in metformin-treated patients with T2DM, exenatide had a greater effect than sitagliptin in lowering PPG and was more potent in increasing insulin secretion and reducing postprandial glucagon secretion. In contrast to sitagliptin, exenatide slowed gastric emptying and reduced caloric intake.49
In two studies of patients treated with exenatide, on a background of either metformin alone or metformin plus a sulfonylurea, patients who received metformin lost more weight (–1.6 to –2.8 kg; P ≤ .01) and experienced more significant decreases from baseline HbA1c (–0.4% to –0.8%; P < .002) at 30 weeks than did patients who received placebo.50,51 In a 16-week trial of exenatide in patients previously treated with a TZD with or without metformin, exenatide reduced HbA1c –0.98%, fasting blood glucose –1.69 mmol/L, and body weight –1.51 kg.52
When compared with insulin analogues, exenatide has been associated with weight loss (~ –3 kg) while the insulin analogues were associated with weight gain (~ +3 kg).53 After 26 weeks, body weight decreased –2.3 kg with exenatide and increased +1.8 kg with insulin glargine.54 Similar results were found in a crossover noninferiority trial, where the least-squares mean difference in weight change was significantly (P < .001) different (2.2 kg) between the treatments.55 When exenatide was compared with insulin aspart in an open-label, noninferiority trial, there was a between-group difference in weight of –5.4 kg after 52 weeks.32
Exenatide has also demonstrated these benefits in open-label extension studies. After 2 years, mean HbA1c reductions of –1.1% from baseline were sustained (P < .05), and weight loss of –4.7 kg was maintained (P < .001).56 After 82 weeks, similar HbA1c decreases (–1.1%) and weight loss (–4.4 kg) were exhibited.57 Even after 3 years, these benefits were maintained in patients who remained on the drug (HbA1c reduction from baseline, –1.0%; weight loss, –5.3 kg [P < .0001 for both]).58
Long-acting formulations of GLP-1 receptor agonists are in clinical development; two of these are once-weekly exenatide and once-daily liraglutide. Exenatide once weekly has the advantage of less frequent dosing and has elicited greater reductions in HbA1c than exenatide BID. After 15 weeks of once-weekly administration, the 0.8-mg formulation reduced HbA1c –1.4% and the 2-mg formulation reduced it –1.7% (P < .0001 for both compared with placebo). Body weight was lowered –3.8 kg (P < .05 compared with placebo) with the 2-mg formulation.59 Compared with exenatide BID, exenatide 2 mg once weekly showed greater reductions in HbA1c (–1.9% vs –1.5%; P = .0023) after 30 weeks of therapy.60 In a 1-year noncomparative trial, treatment with exenatide once weekly improved HbA1c (–2.0%) and weight (–4.1 kg), as well as BP and lipid profiles compared with baseline.61
Liraglutide, a once-daily human analogue GLP-1 receptor agonist, is under review by the FDA.28 In a 26-week study of patients with T2DM, liraglutide was associated with reductions in HbA1c (mean, –1.04%; P = 0.067 compared with insulin) and body weight (mean, –2.5 kg; P < .001 compared with insulin) at dosages of 0.6 to 1.8 mg/day SC. Liraglutide produced a decline in SBP from 0.6 to 3 mm Hg but was not associated with a decrease in DBP.62 In a 52-week study comparing liraglutide with glimepiride monotherapy, liraglutide 1.2 mg was associated with an HbA1c reduction of –0.84% (P = .0014) and the 1.8-mg dose with a reduction of –1.14% (P < .0001) compared with –0.51% for glimepiride. SBP decreased –0.7 mm Hg with glimepiride compared with –2.1 mm Hg for liraglutide 1.2 mg (P = .2912) and –3.6 mm Hg for liraglutide 1.8 mg (P < .0118). Mean DBP fell slightly but not significantly in all treatment groups.63 No effects on lipid parameters were reported in these two liraglutide studies.
The Liraglutide Effect and Action in Diabetes (LEAD-6) trial was undertaken to compare exenatide (10 mg BID SC) and liraglutide (1.8 mg/day SC) as add-on therapy to metformin, a sulfonylurea, or a combination of both in 464 patients with T2DM. After 26 weeks of treatment, liraglutide was associated with a significant reduction in HbA1c of –1.12%, compared with –0.79% with exenatide (P < .0001). Patients treated with liraglutide lost –3.2 kg while those on exenatide lost –2.9 kg. Among patients previously treated with metformin alone, there was a 1-kg difference in favor of liraglutide (P = NS).64
Safety profile
All of the drugs discussed have potential adverse effects. Metformin continues to have a black box warning for lactic acidosis.65 Sulfonylureas and insulin can cause hypoglycemia. TZDs can cause fluid retention and, in rare cases, CHF (for which these drugs also carry a black box warning).66,67 TZDs also increase the risk of distal fracture.66,67 The most common side effects of exenatide are gastrointestinal, but there have been reported cases of pancreatitis, some of which have been fatal.68,69 It has been difficult to prove whether exenatide increases the risk of pancreatitis, as patients with T2DM are already at an increased (three- to fourfold) risk for this condition compared with persons who do not have T2DM.69 Exenatide should not be used in patients with severe renal impairment or end-stage renal disease; it should be used with caution in patients who have undergone renal transplantation and in patients with moderate renal impairment.
The prescribing information for sitagliptin includes pancreatitis among the adverse reactions identified during the drug’s postapproval use.70 As with exenatide, it is not fully known whether a true association exists between the agent and pancreatitis. However, since pancreatitis can occur in this patient population, it is recommended that abdominal pain be fully evaluated to rule out pancreatitis. Continued postmarketing surveillance is important for all of these agents.
THE ROLE OF GUIDELINES
The American Association of Clinical Endocrinologists (AACE),26 the American Diabetes Association (ADA),71 and the ADA in conjunction with the European Association for the Study of Diabetes (EASD)24 have recently revised their recommendations for the management of patients with diabetes. The guidelines are unanimous in setting a glycemic goal (HbA1c < 7.0% for the ADA, HbA1c ≤ 6.5% for the AACE) and advocating individualized care for a treatment goal of HbA1c lower than 6.0% in patients who stand to benefit from near euglycemia without inducing severe hypoglycemia.24,26,71
CVD is the major cause of morbidity and mortality associated with T2DM and is a source of increasing concern.5 Accordingly, special consideration should be given to patients with coexisting CV risk factors, including hypertension and dyslipidemia. The ADA and the EASD advocate lifestyle modification to decrease body weight and the concurrent initiation of metformin as first-line therapy.24 If that strategy is insufficient, then two tiers of treatment guide the choice of next steps24:
- Tier 1, in addition to metformin, includes the sulfonylureas and insulin. Although these are excellent glucose-lowering drugs, they are associated with weight gain, hypoglycemia, and no improvement in BP or lipid levels. They are relatively low in cost and have been used for many years. Their main drawback is evidence that despite their use, beta-cell failure continues unabated over time.
- Tier 2 treatments include pioglitazone and the GLP-1 receptor agonist exenatide. Consideration may be given to the use of pioglitazone or exenatide when hypoglycemia is of concern, with exenatide being preferred when weight loss is a major objective and HbA1c is close to target (< 8.0%).24 Additionally, both the TZDs and exenatide probably help slow the rate of beta-cell failure, particularly if they are used early in the course of the disease.72,73 The AACE recommends different pharmacologic approaches based on HbA1c at diagnosis.26
The American Heart Association and the ADA have issued a joint scientific statement on the primary prevention of CVD in patients with diabetes.74 They advocate lifestyle management of body weight, nutrition, and physical activity.74 In addition, they stress the need for attention to BP, lipid levels, and smoking status, and the use of antiplatelet agents in patients at increased CV risk (> 40 years of age and a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria).
CONCLUSION
T2DM, weight gain/obesity, and CV risk present a continuing challenge to patients and clinicians. Antidiabetes agents have varying degrees of evidence to support their effects on HbA1c, body weight, BP, and lipid levels. A better understanding of the pathophysiology of T2DM has led to the development of newer antidiabetes agents that target the fundamental defects of the disease. Evidence continues to accumulate for the improved benefits of glycemic control and weight loss in T2DM with GLP-1 receptor agonists such as exenatide currently having robust data in terms of beneficial effects on weight and CV risk factors. As clinicians continue to incorporate this knowledge into their practice patterns, patient adherence and clinical outcomes are expected to improve. Newer agents, such as incretin-based therapies, address T2DM as well as other factors that increase cardiometabolic risk through their effects not only on glycemic control but on body weight, BP, and lipids.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of self-reported cardiovascular disease among persons aged >35 years with diabetes—United States, 1997–2005. MMWR Morb Mortal Wkly Rep 2007; 56:1129–1132.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Bonora E, Targher G, Formentini G, et al. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabet Med 2004; 21:52–58.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Jenssen TG, Tonstad S, Claudi T, Midthejell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005; 22:1379–1385.
- Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes 2007; 5:8.
- Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: the mediational role of diabetes symptoms and self-efficacy. Health Psychol 2007; 26:693–700.
- Ruelas V, Roybal GM, Lu Y, Goldman D, Peters A. Clinical and behavioral correlates of achieving and maintaining glycemic targets in an underserved population with type 2 diabetes. Diabetes Care 2009; 32:54–56.
- Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 2003; 52:172–179.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007; 30:1127–1142.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 2001; 86:3853–3860.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17:4–12.
- de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 2009; 19:313–320.
- Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis 2008; 18:574–579.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Hanefeld M, Herman G, Wu M, Mickel C, Sanchez M, Stein PP; for the Sitagliptin Study 014 Investigators. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007; 23:1329–1339.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol 2008; 48:592–598.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29:139–153.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Garber A, Henry R. Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Fortamet [package insert]. Ft. Lauderdale, FL: Watson Laboratories; 2008.
- Actos. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008; 3077–3082.
- Avandia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2009; 1351–1359.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Idris I. News and views: FDA reviews incidences of acute pancreatitis in patients taking Byetta. Diabetes Obes Metab 2008; 10:96–98.
- Januvia [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2006, 2007.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292:E871–E883.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007; 30:162–172.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of self-reported cardiovascular disease among persons aged >35 years with diabetes—United States, 1997–2005. MMWR Morb Mortal Wkly Rep 2007; 56:1129–1132.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Bonora E, Targher G, Formentini G, et al. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabet Med 2004; 21:52–58.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Jenssen TG, Tonstad S, Claudi T, Midthejell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005; 22:1379–1385.
- Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes 2007; 5:8.
- Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: the mediational role of diabetes symptoms and self-efficacy. Health Psychol 2007; 26:693–700.
- Ruelas V, Roybal GM, Lu Y, Goldman D, Peters A. Clinical and behavioral correlates of achieving and maintaining glycemic targets in an underserved population with type 2 diabetes. Diabetes Care 2009; 32:54–56.
- Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 2003; 52:172–179.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007; 30:1127–1142.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 2001; 86:3853–3860.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17:4–12.
- de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 2009; 19:313–320.
- Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis 2008; 18:574–579.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Hanefeld M, Herman G, Wu M, Mickel C, Sanchez M, Stein PP; for the Sitagliptin Study 014 Investigators. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007; 23:1329–1339.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol 2008; 48:592–598.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29:139–153.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Garber A, Henry R. Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Fortamet [package insert]. Ft. Lauderdale, FL: Watson Laboratories; 2008.
- Actos. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008; 3077–3082.
- Avandia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2009; 1351–1359.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Idris I. News and views: FDA reviews incidences of acute pancreatitis in patients taking Byetta. Diabetes Obes Metab 2008; 10:96–98.
- Januvia [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2006, 2007.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292:E871–E883.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007; 30:162–172.
KEY POINTS
- Control of cardiovascular risk factors is as important as glycemic control in patients with T2DM.
- Intensive glucose control has shown mixed results in terms of correlation with improved cardiovascular risk factors.
- Newer agents target the fundamental pathophysiologic defects of T2DM, with beneficial effects on weight and other cardiovascular risk factors.