User login
Maximizing Efficiency in the Operating Room for Total Joint Arthroplasty
Developing a high-efficiency operating room (OR) is both a challenging and rewarding goal for any healthcare system. The OR is traditionally a high-cost/high-revenue environment1 and operative efficacy has been correlated with low complication rates and surgical success.2 An efficient OR is one that maximizes utilization while providing safe, reproducible, cost-effective, high-quality care. Total joint arthroplasty (TJA) has occupied the center stage for OR efficiency research, in part due to increasing demands from our aging population3 and economic pressures related to high implant costs, decreased reimbursement, and competition for market shares when OR time and space are limited.
A PubMed search on OR efficiency in TJA shows a disproportionately high focus on surgical technique, such as use of patient-specific instrumentation (PSI), computer-assisted surgery (CAS), minimally invasive surgery, and closure with barbed suture. In a retrospective review of 352 TKA patients who had PSI vs conventional instrumentation, DeHaan and colleagues4 found that PSI was associated with significantly decreased operative and room turnover times (20.4 minutes and 6.4 minutes, respectively). In another prospective multicenter study, Mont and colleagues5 showed a reduction in surgical time by 8.90 min for navigated total knee arthroplasty (TKA) performed with single-use instruments, cutting blocks, and trials. Other investigators compared PSI to CAS in TKA and found PSI to be 1.45 times more profitable than CAS, with 3 PSI cases performed in an 8-hour OR day compared to 2 CAS cases.6
There is no question that improved surgical technique can enhance OR efficiency. However, this model, while promising, is difficult to implement on a wide scale due to surgeon preferences, vendor limitations, and added costs related to the advanced preoperative imaging studies, manufacturing of the custom guides, and maintenance of navigation equipment. In addition, while interventions such as the use of barbed suture have the potential for speeding closure time, the time saved (4.7 minutes in one randomized trial)7 may not be enough to affect major utilization differences per OR per day. These technologies are also frequently employed by high-volume surgeons with high-volume teams and institutions.
Ideally, we need investment in the human capital and a collective change in work cultures to produce high-quality, well-choreographed, easily reproducible routines. An efficient OR requires the synchronous involvement of a large team of individuals, including hospital administrators, surgery schedulers, surgeons, anesthesiologists, preoperative holding area staff, OR nurses, surgical attendants, sterile processing personnel, and recovery room nurses. Case schedulers should match allocated block time with time required for surgery based on the historical performance of the individual surgeon, preferably scheduling similar cases on the same day. Preoperative work-up and medical clearance should be completed prior to scheduling to avoid last-minute cancellations. Patient reminders and accommodations for those traveling from long distances can further minimize late arrivals. Prompt initiation of the perioperative clinical pathway upon a patient’s check-in is important. The surgical site should be marked and the anesthesia plan confirmed upon arrival in the preoperative holding area. Necessary products need to be ready and/or administrated in time for transfer to the OR. These include prophylactic antibiotics, coagulation factors (eg, tranexamic acid), and blood products as indicated. Spinal anesthesia, regional nerve blocks, and intravenous (IV) lines should be completed before transfer to the OR. A “block room” close to the OR can allow concurrent induction of anesthesia and has been shown to increase the number of surgical cases performed during a regular workday.8 Hair clipping within the surgical site and pre-scrubbing of the operative extremity should also be performed prior to transfer to the OR in order to minimize micro-organisms and dispersal of loose hair onto the sterile field.
Upon arrival of the patient to the OR, instrument tables based on the surgeon preference cards should be opened, instrument count and implant templating completed, necessary imaging displayed, and OR staff ready with specific responsibilities assigned to each member. Small and colleagues9 showed that using dedicated orthopedic staff familiar with the surgical routine decreased operative time by 19 minutes per procedure, or 1.25 hours for a surgeon performing 4 primary TJAs per day. Practices such as routine placement of a urinary catheter should be seriously scrutinized. In a randomized prospective study of patients undergoing total hip arthroplasty under spinal anesthesia, Miller and colleagues10 found no benefit for indwelling catheters in preventing urinary retention. In another randomized prospective study, Huang and colleagues11 found the prevalence of urinary tract infections was significantly higher in TJA patients who received indwelling urinary catheters.
A scrub nurse familiar with the instruments, their assembly, and the sequence of events can ensure efficient surgical flow. The scrub nurse needs to anticipate missing or defective tools and call for them, ideally before the incision is made. Direct comparison studies are needed to assess the efficacy of routine intraoperative imaging vs commercially available universal cup alignment guides or clinical examinations in determining acceptable component positioning and limb length. Following component implantation and before wound closure, the circulating nurse should initiate the process of acquisition of a recovery room bed, make sure dressing supplies and necessary equipment are available, and call for surgical attendants. Lack of surgical attendants, delayed transfer from the OR table to hospital bed, and prolonged acquisition of a recovery room bed have been identified as major OR inefficiencies in a retrospective study by Attarian and colleagues.12
In summary, time is the OR’s most valuable resource.13 We believe that a consistent, almost automated attitude to the above procedures decreases variability and improves efficiency. By providing clear communication of the surgical needs with the team, having consistent anesthesia and nursing staff, implementing consistent perioperative protocols, and insuring that all necessary instruments and modalities are available prior to starting the procedure, we were able to sustainably increase OR throughput in a large teaching hospital.9,14 This process, however, requires constant review to identify and eliminate new gaps, with each member of the team sharing a frank desire to improve. In this regard, hospital administrators share the duty to facilitate the implementation of any necessary changes, allocation of needed resources, and rewarding good effort, which could ultimately increase staff satisfaction and retention. Because efficiency is the ratio of benefits (eg, revenue, safety, etc.) to investment (eg, implant costs, wages, etc.), raises the question: what would be the effect of transitioning from hourly-wage to a salary-based system for key support staff? Unlike hourly-wage personnel, who have no incentive for productivity, a salaried employee assigned to a high-efficiency OR will inherently strive for improvement, employing higher organizational skills to accomplish a common goal. To our knowledge, there is no published data on this topic.
1. Krupka DC, Sandberg WS. Operating room design and its impact on operating room economics. Curr Opin Anaesthesiol. 2006;19(2):185-191.
2. Scott WN, Booth RE Jr, Dalury DF, Healy WL, Lonner JH. Efficiency and economics in joint arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 5:33-36.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29(11):2065-2069.
5. Mont MA, McElroy MJ, Johnson AJ, Pivec R; Single-Use Multicenter Trial Group Writing Group. Single-use instruments, cutting blocks, and trials increase efficiency in the operating room during total knee arthroplasty: a prospective comparison of navigated and non-navigated cases. J Arthroplasty. 2013;28(7):1135-1140.
6. Lionberger DR, Crocker CL, Chen V. Patient specific instrumentation. J Arthroplasty. 2014;29(9):1699-1704.
7. Sah AP. Is there an advantage to knotless barbed suture in TKA wound closure? A randomized trial in simultaneous bilateral TKAs. Clin Orthop Relat Res. 2015;473(6):2019-2027.
8. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvelä OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103(2):401-405.
9. Small TJ, Gad BV, Klika AK, Mounir-Soliman LS, Gerritsen RL, Barsoum WK. Dedicated orthopedic operating room unit improves operating room efficiency. J Arthroplasty. 2013;28(7):1066-1071.e2.
10. Miller AG, McKenzie J, Greenky M, et al. Spinal anesthesia: should everyone receive a urinary catheter?: a randomized, prospective study of patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2013;95(16):1498-1503.
11. Huang Z, Ma J, Shen B, Pei F. General anesthesia: to catheterize or not? A prospective randomized controlled study of patients undergoing total knee arthroplasty. J Arthroplasty. 2015;30(3):502-506.
12. Attarian DE, Wahl JE, Wellman SS, Bolognesi MP. Developing a high-efficiency operating room for total joint arthroplasty in an academic setting. Clin Orthop Relat Res. 2013;471(6):1832-1836.
13. Gamble M. 6 cornerstones of operating room efficiency: best practices for each. Becker’s Hospital Review Web site. http://www.beckershospitalreview.com/or-efficiencies/6-cornerstones-of-operating-room-efficiency-best-practices-for-each.html. Updated January 18, 2013. Accessed September 3, 2015.
14. Smith MP, Sandberg WS, Foss J, et al. High-throughput operating room system for joint arthroplasties durably outperforms routine processes. Anesthesiology. 2008;109(1):25-35.
Developing a high-efficiency operating room (OR) is both a challenging and rewarding goal for any healthcare system. The OR is traditionally a high-cost/high-revenue environment1 and operative efficacy has been correlated with low complication rates and surgical success.2 An efficient OR is one that maximizes utilization while providing safe, reproducible, cost-effective, high-quality care. Total joint arthroplasty (TJA) has occupied the center stage for OR efficiency research, in part due to increasing demands from our aging population3 and economic pressures related to high implant costs, decreased reimbursement, and competition for market shares when OR time and space are limited.
A PubMed search on OR efficiency in TJA shows a disproportionately high focus on surgical technique, such as use of patient-specific instrumentation (PSI), computer-assisted surgery (CAS), minimally invasive surgery, and closure with barbed suture. In a retrospective review of 352 TKA patients who had PSI vs conventional instrumentation, DeHaan and colleagues4 found that PSI was associated with significantly decreased operative and room turnover times (20.4 minutes and 6.4 minutes, respectively). In another prospective multicenter study, Mont and colleagues5 showed a reduction in surgical time by 8.90 min for navigated total knee arthroplasty (TKA) performed with single-use instruments, cutting blocks, and trials. Other investigators compared PSI to CAS in TKA and found PSI to be 1.45 times more profitable than CAS, with 3 PSI cases performed in an 8-hour OR day compared to 2 CAS cases.6
There is no question that improved surgical technique can enhance OR efficiency. However, this model, while promising, is difficult to implement on a wide scale due to surgeon preferences, vendor limitations, and added costs related to the advanced preoperative imaging studies, manufacturing of the custom guides, and maintenance of navigation equipment. In addition, while interventions such as the use of barbed suture have the potential for speeding closure time, the time saved (4.7 minutes in one randomized trial)7 may not be enough to affect major utilization differences per OR per day. These technologies are also frequently employed by high-volume surgeons with high-volume teams and institutions.
Ideally, we need investment in the human capital and a collective change in work cultures to produce high-quality, well-choreographed, easily reproducible routines. An efficient OR requires the synchronous involvement of a large team of individuals, including hospital administrators, surgery schedulers, surgeons, anesthesiologists, preoperative holding area staff, OR nurses, surgical attendants, sterile processing personnel, and recovery room nurses. Case schedulers should match allocated block time with time required for surgery based on the historical performance of the individual surgeon, preferably scheduling similar cases on the same day. Preoperative work-up and medical clearance should be completed prior to scheduling to avoid last-minute cancellations. Patient reminders and accommodations for those traveling from long distances can further minimize late arrivals. Prompt initiation of the perioperative clinical pathway upon a patient’s check-in is important. The surgical site should be marked and the anesthesia plan confirmed upon arrival in the preoperative holding area. Necessary products need to be ready and/or administrated in time for transfer to the OR. These include prophylactic antibiotics, coagulation factors (eg, tranexamic acid), and blood products as indicated. Spinal anesthesia, regional nerve blocks, and intravenous (IV) lines should be completed before transfer to the OR. A “block room” close to the OR can allow concurrent induction of anesthesia and has been shown to increase the number of surgical cases performed during a regular workday.8 Hair clipping within the surgical site and pre-scrubbing of the operative extremity should also be performed prior to transfer to the OR in order to minimize micro-organisms and dispersal of loose hair onto the sterile field.
Upon arrival of the patient to the OR, instrument tables based on the surgeon preference cards should be opened, instrument count and implant templating completed, necessary imaging displayed, and OR staff ready with specific responsibilities assigned to each member. Small and colleagues9 showed that using dedicated orthopedic staff familiar with the surgical routine decreased operative time by 19 minutes per procedure, or 1.25 hours for a surgeon performing 4 primary TJAs per day. Practices such as routine placement of a urinary catheter should be seriously scrutinized. In a randomized prospective study of patients undergoing total hip arthroplasty under spinal anesthesia, Miller and colleagues10 found no benefit for indwelling catheters in preventing urinary retention. In another randomized prospective study, Huang and colleagues11 found the prevalence of urinary tract infections was significantly higher in TJA patients who received indwelling urinary catheters.
A scrub nurse familiar with the instruments, their assembly, and the sequence of events can ensure efficient surgical flow. The scrub nurse needs to anticipate missing or defective tools and call for them, ideally before the incision is made. Direct comparison studies are needed to assess the efficacy of routine intraoperative imaging vs commercially available universal cup alignment guides or clinical examinations in determining acceptable component positioning and limb length. Following component implantation and before wound closure, the circulating nurse should initiate the process of acquisition of a recovery room bed, make sure dressing supplies and necessary equipment are available, and call for surgical attendants. Lack of surgical attendants, delayed transfer from the OR table to hospital bed, and prolonged acquisition of a recovery room bed have been identified as major OR inefficiencies in a retrospective study by Attarian and colleagues.12
In summary, time is the OR’s most valuable resource.13 We believe that a consistent, almost automated attitude to the above procedures decreases variability and improves efficiency. By providing clear communication of the surgical needs with the team, having consistent anesthesia and nursing staff, implementing consistent perioperative protocols, and insuring that all necessary instruments and modalities are available prior to starting the procedure, we were able to sustainably increase OR throughput in a large teaching hospital.9,14 This process, however, requires constant review to identify and eliminate new gaps, with each member of the team sharing a frank desire to improve. In this regard, hospital administrators share the duty to facilitate the implementation of any necessary changes, allocation of needed resources, and rewarding good effort, which could ultimately increase staff satisfaction and retention. Because efficiency is the ratio of benefits (eg, revenue, safety, etc.) to investment (eg, implant costs, wages, etc.), raises the question: what would be the effect of transitioning from hourly-wage to a salary-based system for key support staff? Unlike hourly-wage personnel, who have no incentive for productivity, a salaried employee assigned to a high-efficiency OR will inherently strive for improvement, employing higher organizational skills to accomplish a common goal. To our knowledge, there is no published data on this topic.
Developing a high-efficiency operating room (OR) is both a challenging and rewarding goal for any healthcare system. The OR is traditionally a high-cost/high-revenue environment1 and operative efficacy has been correlated with low complication rates and surgical success.2 An efficient OR is one that maximizes utilization while providing safe, reproducible, cost-effective, high-quality care. Total joint arthroplasty (TJA) has occupied the center stage for OR efficiency research, in part due to increasing demands from our aging population3 and economic pressures related to high implant costs, decreased reimbursement, and competition for market shares when OR time and space are limited.
A PubMed search on OR efficiency in TJA shows a disproportionately high focus on surgical technique, such as use of patient-specific instrumentation (PSI), computer-assisted surgery (CAS), minimally invasive surgery, and closure with barbed suture. In a retrospective review of 352 TKA patients who had PSI vs conventional instrumentation, DeHaan and colleagues4 found that PSI was associated with significantly decreased operative and room turnover times (20.4 minutes and 6.4 minutes, respectively). In another prospective multicenter study, Mont and colleagues5 showed a reduction in surgical time by 8.90 min for navigated total knee arthroplasty (TKA) performed with single-use instruments, cutting blocks, and trials. Other investigators compared PSI to CAS in TKA and found PSI to be 1.45 times more profitable than CAS, with 3 PSI cases performed in an 8-hour OR day compared to 2 CAS cases.6
There is no question that improved surgical technique can enhance OR efficiency. However, this model, while promising, is difficult to implement on a wide scale due to surgeon preferences, vendor limitations, and added costs related to the advanced preoperative imaging studies, manufacturing of the custom guides, and maintenance of navigation equipment. In addition, while interventions such as the use of barbed suture have the potential for speeding closure time, the time saved (4.7 minutes in one randomized trial)7 may not be enough to affect major utilization differences per OR per day. These technologies are also frequently employed by high-volume surgeons with high-volume teams and institutions.
Ideally, we need investment in the human capital and a collective change in work cultures to produce high-quality, well-choreographed, easily reproducible routines. An efficient OR requires the synchronous involvement of a large team of individuals, including hospital administrators, surgery schedulers, surgeons, anesthesiologists, preoperative holding area staff, OR nurses, surgical attendants, sterile processing personnel, and recovery room nurses. Case schedulers should match allocated block time with time required for surgery based on the historical performance of the individual surgeon, preferably scheduling similar cases on the same day. Preoperative work-up and medical clearance should be completed prior to scheduling to avoid last-minute cancellations. Patient reminders and accommodations for those traveling from long distances can further minimize late arrivals. Prompt initiation of the perioperative clinical pathway upon a patient’s check-in is important. The surgical site should be marked and the anesthesia plan confirmed upon arrival in the preoperative holding area. Necessary products need to be ready and/or administrated in time for transfer to the OR. These include prophylactic antibiotics, coagulation factors (eg, tranexamic acid), and blood products as indicated. Spinal anesthesia, regional nerve blocks, and intravenous (IV) lines should be completed before transfer to the OR. A “block room” close to the OR can allow concurrent induction of anesthesia and has been shown to increase the number of surgical cases performed during a regular workday.8 Hair clipping within the surgical site and pre-scrubbing of the operative extremity should also be performed prior to transfer to the OR in order to minimize micro-organisms and dispersal of loose hair onto the sterile field.
Upon arrival of the patient to the OR, instrument tables based on the surgeon preference cards should be opened, instrument count and implant templating completed, necessary imaging displayed, and OR staff ready with specific responsibilities assigned to each member. Small and colleagues9 showed that using dedicated orthopedic staff familiar with the surgical routine decreased operative time by 19 minutes per procedure, or 1.25 hours for a surgeon performing 4 primary TJAs per day. Practices such as routine placement of a urinary catheter should be seriously scrutinized. In a randomized prospective study of patients undergoing total hip arthroplasty under spinal anesthesia, Miller and colleagues10 found no benefit for indwelling catheters in preventing urinary retention. In another randomized prospective study, Huang and colleagues11 found the prevalence of urinary tract infections was significantly higher in TJA patients who received indwelling urinary catheters.
A scrub nurse familiar with the instruments, their assembly, and the sequence of events can ensure efficient surgical flow. The scrub nurse needs to anticipate missing or defective tools and call for them, ideally before the incision is made. Direct comparison studies are needed to assess the efficacy of routine intraoperative imaging vs commercially available universal cup alignment guides or clinical examinations in determining acceptable component positioning and limb length. Following component implantation and before wound closure, the circulating nurse should initiate the process of acquisition of a recovery room bed, make sure dressing supplies and necessary equipment are available, and call for surgical attendants. Lack of surgical attendants, delayed transfer from the OR table to hospital bed, and prolonged acquisition of a recovery room bed have been identified as major OR inefficiencies in a retrospective study by Attarian and colleagues.12
In summary, time is the OR’s most valuable resource.13 We believe that a consistent, almost automated attitude to the above procedures decreases variability and improves efficiency. By providing clear communication of the surgical needs with the team, having consistent anesthesia and nursing staff, implementing consistent perioperative protocols, and insuring that all necessary instruments and modalities are available prior to starting the procedure, we were able to sustainably increase OR throughput in a large teaching hospital.9,14 This process, however, requires constant review to identify and eliminate new gaps, with each member of the team sharing a frank desire to improve. In this regard, hospital administrators share the duty to facilitate the implementation of any necessary changes, allocation of needed resources, and rewarding good effort, which could ultimately increase staff satisfaction and retention. Because efficiency is the ratio of benefits (eg, revenue, safety, etc.) to investment (eg, implant costs, wages, etc.), raises the question: what would be the effect of transitioning from hourly-wage to a salary-based system for key support staff? Unlike hourly-wage personnel, who have no incentive for productivity, a salaried employee assigned to a high-efficiency OR will inherently strive for improvement, employing higher organizational skills to accomplish a common goal. To our knowledge, there is no published data on this topic.
1. Krupka DC, Sandberg WS. Operating room design and its impact on operating room economics. Curr Opin Anaesthesiol. 2006;19(2):185-191.
2. Scott WN, Booth RE Jr, Dalury DF, Healy WL, Lonner JH. Efficiency and economics in joint arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 5:33-36.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29(11):2065-2069.
5. Mont MA, McElroy MJ, Johnson AJ, Pivec R; Single-Use Multicenter Trial Group Writing Group. Single-use instruments, cutting blocks, and trials increase efficiency in the operating room during total knee arthroplasty: a prospective comparison of navigated and non-navigated cases. J Arthroplasty. 2013;28(7):1135-1140.
6. Lionberger DR, Crocker CL, Chen V. Patient specific instrumentation. J Arthroplasty. 2014;29(9):1699-1704.
7. Sah AP. Is there an advantage to knotless barbed suture in TKA wound closure? A randomized trial in simultaneous bilateral TKAs. Clin Orthop Relat Res. 2015;473(6):2019-2027.
8. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvelä OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103(2):401-405.
9. Small TJ, Gad BV, Klika AK, Mounir-Soliman LS, Gerritsen RL, Barsoum WK. Dedicated orthopedic operating room unit improves operating room efficiency. J Arthroplasty. 2013;28(7):1066-1071.e2.
10. Miller AG, McKenzie J, Greenky M, et al. Spinal anesthesia: should everyone receive a urinary catheter?: a randomized, prospective study of patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2013;95(16):1498-1503.
11. Huang Z, Ma J, Shen B, Pei F. General anesthesia: to catheterize or not? A prospective randomized controlled study of patients undergoing total knee arthroplasty. J Arthroplasty. 2015;30(3):502-506.
12. Attarian DE, Wahl JE, Wellman SS, Bolognesi MP. Developing a high-efficiency operating room for total joint arthroplasty in an academic setting. Clin Orthop Relat Res. 2013;471(6):1832-1836.
13. Gamble M. 6 cornerstones of operating room efficiency: best practices for each. Becker’s Hospital Review Web site. http://www.beckershospitalreview.com/or-efficiencies/6-cornerstones-of-operating-room-efficiency-best-practices-for-each.html. Updated January 18, 2013. Accessed September 3, 2015.
14. Smith MP, Sandberg WS, Foss J, et al. High-throughput operating room system for joint arthroplasties durably outperforms routine processes. Anesthesiology. 2008;109(1):25-35.
1. Krupka DC, Sandberg WS. Operating room design and its impact on operating room economics. Curr Opin Anaesthesiol. 2006;19(2):185-191.
2. Scott WN, Booth RE Jr, Dalury DF, Healy WL, Lonner JH. Efficiency and economics in joint arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 5:33-36.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29(11):2065-2069.
5. Mont MA, McElroy MJ, Johnson AJ, Pivec R; Single-Use Multicenter Trial Group Writing Group. Single-use instruments, cutting blocks, and trials increase efficiency in the operating room during total knee arthroplasty: a prospective comparison of navigated and non-navigated cases. J Arthroplasty. 2013;28(7):1135-1140.
6. Lionberger DR, Crocker CL, Chen V. Patient specific instrumentation. J Arthroplasty. 2014;29(9):1699-1704.
7. Sah AP. Is there an advantage to knotless barbed suture in TKA wound closure? A randomized trial in simultaneous bilateral TKAs. Clin Orthop Relat Res. 2015;473(6):2019-2027.
8. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvelä OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103(2):401-405.
9. Small TJ, Gad BV, Klika AK, Mounir-Soliman LS, Gerritsen RL, Barsoum WK. Dedicated orthopedic operating room unit improves operating room efficiency. J Arthroplasty. 2013;28(7):1066-1071.e2.
10. Miller AG, McKenzie J, Greenky M, et al. Spinal anesthesia: should everyone receive a urinary catheter?: a randomized, prospective study of patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2013;95(16):1498-1503.
11. Huang Z, Ma J, Shen B, Pei F. General anesthesia: to catheterize or not? A prospective randomized controlled study of patients undergoing total knee arthroplasty. J Arthroplasty. 2015;30(3):502-506.
12. Attarian DE, Wahl JE, Wellman SS, Bolognesi MP. Developing a high-efficiency operating room for total joint arthroplasty in an academic setting. Clin Orthop Relat Res. 2013;471(6):1832-1836.
13. Gamble M. 6 cornerstones of operating room efficiency: best practices for each. Becker’s Hospital Review Web site. http://www.beckershospitalreview.com/or-efficiencies/6-cornerstones-of-operating-room-efficiency-best-practices-for-each.html. Updated January 18, 2013. Accessed September 3, 2015.
14. Smith MP, Sandberg WS, Foss J, et al. High-throughput operating room system for joint arthroplasties durably outperforms routine processes. Anesthesiology. 2008;109(1):25-35.
Optimizing Outcomes of Total Joint Arthroplasty Under the Comprehensive Care for Joint Replacement
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
Evaluation of Wound Healing After Direct Anterior Total Hip Arthroplasty With Use of a Novel Retraction Device
It is thought that, by placing more emphasis on soft-tissue preservation, minimally invasive surgery total hip arthroplasty (MIS-THA) results in less soft-tissue trauma, less blood loss, and earlier recovery.1-3 Despite these improvements over standard methods, there is a concern that the vigorous retraction needed for proper visualization through smaller incisions could injure soft tissues.4-7 Single-incision direct anterior THA (DA-THA) has gained in popularity because of the true intermuscular/internervous plane through which the procedure can be performed with relatively minimal muscle dissection using MIS techniques.8,9 This approach may offer quicker recovery and superior stability in comparison with nonintermuscular methods, which unavoidably cause more muscle damage.10-12
Although the evidence of these early gains is encouraging, several studies have found high complication rates with DA-THA.8,13-17 Noted disadvantages include a steep learning curve, lateral femoral cutaneous neurapraxia, need for a specialized table, and higher fracture and wound complication rates. Not surprisingly, with increased surgeon experience, the complication rate decreased substantially.14,15 However, wound-related complications remained steady, with 2 recent large studies reporting rates of 4.6% and 2.1%.14,15 The thin anterior skin, high tensional forces along the groin crease and perpendicular to the typical DA incision, and less resilient soft-tissue envelope are postulated reasons for wound-related issues, which are likely magnified in patients who are more obese.15,16
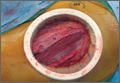
A novel device designed to lessen tissue damage is the ring retractor (Figure 1). Used initially in general surgery and obstetrics, it consists of 2 semirigid polymer rings connected by a flexible cylindrical polymer membrane.18-20 The lower ring is tucked and anchored underneath the wound edge, and then the upper ring is rolled down and cinched onto the skin. The resultant tension on the polymer sleeve—imparted by the rigidity of the ring—provides strong, evenly distributed wound-edge retraction. It also provides a physical barrier between the wound edge and the rest of the operative field. Proponents of the ring retractor claim increased wound-edge moisture, less bruising, and reduced local trauma compared with standard metal retractors alone.
Wound-edge retractor forces are doubled during MIS-THA compared with conventional THA.14-20 This may explain reports of worse scar cosmesis with MIS-THA. Given the theoretical benefits of minimized wound-edge trauma, the ring retractor may improve scar appearance compared with standard retraction alone. Any clinically relevant effect on cosmesis should be readily apparent to justify use of the retractor in this regard. Although some surgeons routinely use the device for primary THA, it has not been the subject of any recent orthopedic studies.
In the present study, we prospectively investigated wound cosmesis with and without use of the ring retractor in patients undergoing DA-THA.
Materials and Methods
This prospective, single-center, randomized study was reviewed and approved by the institutional review board at our facility. Consent was obtained from all participating patients.
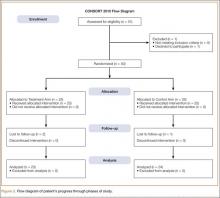
We evaluated 50 surgical incisions in 48 patients. Eligible participants were over age 18 years and undergoing primary DA-THA. Exclusion criteria included previous surgery on the affected hip, a pathological hip condition requiring an extensile exposure, systemic inflammatory illness, chronic corticosteroid use, and dermatologic abnormality of the incisional area. One patient was having simultaneous bilateral THAs, and another was having staged bilateral THAs. Each hip in these patients was given its own case number and treated separately. Of the 49 patients who met all the inclusion criteria, only 1 decided not to participate (Figure 2).
Stratified randomization with permuted block size (sex, body mass index [BMI]) was used to assign patients in a 1:1 ratio to either the treatment group or the control group. In the treatment group, the Protractor Incision Protector and Retractor (Gyrus ACMI, Southborough, Massachusetts) was used with standard metal retractors. In the control group, only standard metal retractors were used. Patients were blinded to their group assignments, and surgeons were informed about each assignment only after the initial incision was made.
Clinical research investigators were blinded to the groups’ prospectively collected data. Collection time points were preoperative clinical visit, day of surgery through discharge, and 2-, 6-, and 12-week postoperative follow-ups. Day-of-surgery data included estimated intraoperative blood loss, operative side, operative time, intraoperative complications, and American Society of Anesthesiologists (ASA) physical status classification. Total length of stay, pain scores (range, 0-10), estimated drain output, and blood-transfusion data were also recorded. To evaluate whether the device had any effect on short-term functional outcome, we collected Harris Hip Scores (HHS) and Short Form–12 (SF-12, Version 2) scores at the preoperative and 6-week postoperative visits. We also documented any wound-healing-related issues or complications that occurred up until the final visit.
To account for any effect of nutrition status on wound healing, we obtained pre-albumin and albumin levels and absolute lymphocyte counts from the preoperative electronic records. We used an albumin level under 3.5 g/dL and an absolute lymphocyte count under 1500/µL for our analysis, as these cutoffs have been associated with wound complications after primary THA.21 There is no similarly established threshold for pre-albumin level, so we used values under 20 mg/L based on comparable literature.22,23
At each postoperative visit, standardized high-resolution images were obtained. At the 12-week visit, patients completed 2 Likert scales regarding their overall opinion of their scars and how their scars compared with their expectations. They also ranked 5 separate THA-related outcomes in order of importance (Appendix).
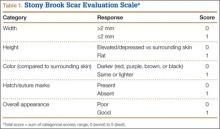
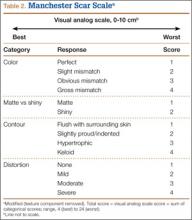
Photographs were evaluated by 2 blinded plastic surgeons (Dr. Friedman and Dr. Jack) using 2 grading systems—the Stony Brook Scar Evaluation Scale (SBSES)24 (Table 1) and a modified Manchester Scar Scale (MSS)25 (Table 2). We used these systems because they were photograph-based, psychometrically studied, and specifically designed to assess surgical incision healing with established validity and reliability.24-27 A particular advantage, strictly related to cosmetic outcome, is their validity in scoring scars from high-definition photographs in a different place or time. The SBSES, an ordinal wound evaluation scale that measures short-term cosmetic outcomes, consists of 6 items, each receiving 1 or 0 point, yielding a total score between 0 (worst) and 5 (best). The modified MSS includes a visual analog scale (VAS), which has a vertical hash marked on a 10-cm line and is scored between 0 (excellent) and 10 (poor) to 1 decimal point.26,28 This value is added to grades on color, surface appearance, contour, and distortion, resulting in a score between 4 (best) and 24 (worst). The primary outcome measures were Likert-scale responses obtained at final visit and SBSES/MSS scores for each visit; 12-week scores were the primary end point.
Operative Procedure
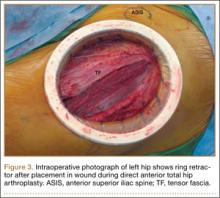
Experienced fellowship-trained orthopedic surgeons performed all procedures. A modified Hueter approach was used for exposure.9 Mean incision length was about 12 cm. For the treatment group, the ring retractor was inserted at the level of the tensor fascia, with the inferior ring resting between the fascia and the subcutaneous layer and the superior ring cinched over the skin (Figure 3). The device is made in 4 different sizes for incisions from 2.5 to 17 cm; our study population required only 1 size. Otherwise, the surgical protocol was based on that described by Matta and colleagues.8 Wound closure (over a drain) was performed according to a standardized protocol—running No. 1 Vicryl suture for the superficial tensor fascia, interrupted 2-0 Vicryl for the deep dermal layer, and subcutaneous 4-0 Monocryl for the skin followed by application of Dermabond (Ethicon, Somerville, New Jersey) and Tegaderm +Pad (3M, St. Paul, Minnesota) for outer dressing, which was replaced on postoperative day 2 and removed at the 2-week visit.
Statistical Methods
An a priori sample-size calculation was performed. Power performed in a base of a prior study that evaluated anterolateral and posterolateral THA scars using a VAS, a component of the MSS, suggested a sample size of 16 per group to detect the minimal clinically important difference of 1.5 cm: SD (σ) = 1.5 cm, α = 0.05, β = 0.20.29,30 In addition, a general estimate for detecting a 1-unit change on an ordinal scale (σ = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively decided to enroll 25 patients per arm in case of larger true variance.
The Wilcoxon rank sum test was used for comparisons of continuous data between groups. Differences between means were analyzed with 2-sided t tests. Categorical data were compared with the Pearson χ2 test or the Fisher exact test, as indicated. Ordinal ranking scores were compared with the Mantel-Haenszel test. Multivariate logistic regression was applied to identify the significant independent predictors of better scar grades for each surgeon by considering candidate variables with Ps < .20 in the univariate analysis.
Results
We found no differences in demographic or perioperative characteristics between treatment and control groups (Tables 3, 4). The groups showed similar mean improvements in their respective 6-week HHS (38.7 and 36.4 points; P = .65), SF-12 physical component summary scores (11.8 and 14.5 points; P = .37), and SF-12 mental component summary scores (5.1 and 3.7; P = .70).
Patient questionnaire outcomes are listed in Table 5. For the control group, 25/25 image sets were obtained at the 2-week visit, 25/25 at the 6-week visit, and 24/25 at the 12-week visit. For the treatment group, there were 23/25, 24/25, and 23/25 images sets, respectively.
When surgeon scoring was analyzed separately, SBSES and MSS scores were similar between treatment and control groups, with 1 exception: 2-week MSS scores were better for the treatment group according to surgeon A (P = .026). When grades were averaged, SBSES scores were again similar at all time points (Figure 4A); MSS scores were better for the treatment group at 2 weeks (P = .036) and equivalent at all other time points (Figure 4B). For the SBSES, Spearman correlation coefficient ρ with 95% confidence interval (CI) was 0.37
(95% CI, 0.08-0.66) at 2 weeks, 0.48 (95% CI, 0.20-0.76) at 6 weeks, and 0.62 (95% CI, 0.33-0.91) at 12 weeks. Following the same pattern for the MSS, ρ was 0.20 (95% CI, –0.09 to 0.49), 0.51 (95% CI, 0.23-0.79), and 0.32 (95% CI, 0.03-0.61).
Independent multivariate analysis revealed that age over 65 years was a significant predictor of worse scores. On SBSES, the odds ratio (OR) was 1.15 (95% CI, 1.07-1.24) for surgeon A and 1.11 (95% CI, 1.05-1.18) for surgeon B. On MSS, the OR was 0.89 (95% CI, 0.84-0.94) for surgeon A and 0.95 (95% CI, 0.91-0.99) for surgeon B. The likelihood of having worse SBSES scores according to surgeon A was 4.72 times higher if the pre-albumin level was under 20 mg/L (95% CI, 1.15-19.36). Albumin level under 3.5 g/dL and absolute lymphocyte count under 1500 cells/µL were not found to be independent predictors of poorer scores.
Patients’ overall opinion (P = .63) and assessment of their scars relative to expectations (P = .25) on the Likert scales were not different between groups. More scars exceeded patients’ expectations and had more excellent ratings in the control group. The 2 groups were similar with regard to relative importance of various patient-related outcomes. Factors most important to overall outcome were relief of hip pain, followed by implant longevity and length of recovery. Least important were incision-related variables.
There were only 3 minor noninfectious wound complications (6%), 2 in the treatment group and 1 in the control group. In the treatment group, a 67-year-old man with diabetes (ASA class III; BMI, 32.1 kg/m2; received transfusion) had 2 small areas (<5 mm) of superficial ulceration at 6-week follow-up—one at the proximal aspect of the incision and the other near the midpoint along the flexion crease. Both lesions resolved by 12-week follow-up. Also in the treatment group, a 77-year-old woman (ASA class II; BMI, 24.9 kg/m2; received transfusion) at 6 weeks had a spitting suture, which was removed in clinic without further issue. In the control group, a 55-year-old woman (ASA class II; BMI, 27.4 kg/m2) had a suture reaction near the proximal aspect of her incision 3 weeks after surgery. This reaction, which presented as a mild, localized erythema without pain, tenderness, or drainage, resolved by 6-week follow-up. None of these wound complications required intervention beyond observation.
Discussion
This study was designed to provide a bipartisan measure of wound-healing cosmesis after DA-THA. Scar evaluation by blinded plastic surgeons served as a standardized, clinical assessment, whereas the patient questionnaire offered a more subjective appraisal. The modified MMS25 and the SBSES24 are the only 2 wound-grading systems designed and validated for photographic assessment of postsurgical scars. Most scar evaluation schemes pertain to burn or traumatic scars.26,27,31 As a result, many earlier studies intending to compare incisional scars used poorly suited evaluation systems.
The current literature includes reports on 3 studies with scoring-based scar assessment in THA; all used grading systems designed for either burns or traumatic wounds, but 2 also used a VAS.32-34 VASs have been validated for measuring wound cosmesis but are entirely subjective and without structure and provide no feedback as to why a scar was rated good or bad.24 Mow and colleagues32 prospectively compared scars after standard posterior or MIS approaches and found no differences according to a scoring system intended for burn scars. In our study population, we found no group differences in patients’ cosmesis of their scars.
Although scars can take a year or longer to fully mature, researchers from the University of Michigan discovered that scar appearance at 1 year correlates highly with cosmesis 12 weeks after closure, though poorly with cosmesis 10 days after closure.35 Therefore, any observed differences in scar cosmesis between groups at 12-week follow-up would likely persist, whereas differences at 2-week follow-up would have little bearing on ultimate appearance. For this reason, our primary outcome measure was healing process and cosmesis at 12 weeks. High wound complication rates have been reported for MIS-DA-THA.8,14-16 Jewett and Collis15 noted a 4.6% wound complication rate (3% noninfectious ulcerative dehiscence, 1.6% superficial infection), which is comparable to the 6% rate found in this study. However, there likely is some variability across studies in what constitutes a wound complication or superficial infection. Of our 3 wound complications—stitch reaction, spitting suture, small noninfectious ulceration—only the ulceration was of a severity similar to that reported by Jewett and Collis.15 Matta and colleagues8 reported only 3 wound complications (in 494 patients), all severe enough to require operative intervention. One explanation for this low complication rate is use of a ring retractor, as it is routinely depicted in their technique paper. However, no specific reference is made to gauge how often the device was used.
Rates of superficial infection after DA-THA range from 0.6% to 1.6% in 3 large observational studies (combined deep infection rate, 0.43%).8,14,15 In 2 of these studies, all patients with superficial infection underwent formal débridement, though none developed deep infection. A prospective randomized study of 221 patients who underwent colorectal surgery—where perioperative infectious morbidity ranges from 25% to 50%—found that ring retractor use significantly reduced superficial wound infection rates (8.1% vs 0%). A significant reduction in wound infection was shown in a similarly designed study involving 48 patients who had open appendectomy (14.6% vs 1.6%). The device had no effect on deep infection in either general surgery study. The wound infection rates reported in these general surgery studies are markedly higher than those in our study population. As a result, the effect of the ring retractor on wound infection in DA-THA may be less. Regardless of the effect on deep infection, fewer superficial infections, which often require operative intervention, would be of considerable benefit.
Below-threshold albumin level and absolute lymphocyte count have been associated with wound-healing complications after hip replacement.21 In the present study, pre-albumin level under 20 mg/L was the only nutritional marker predictive of poor wound appearance, but this finding was seen only in SBSES scores from surgeon A. Subgroup analysis did not reveal any relationship between wound appearance and any of the recorded demographic or perioperative variables, but for a small predictive influence with age over 65 years.
This study had some limitations. Our findings cannot be generalized to all patients who undergo THA, as only DA incisions were studied. Results also may not be generalizable to non-fellowship-trained orthopedists. In addition, selection bias likely resulted from including patients already selected for the DA approach. Using digital images for evaluation (vs real-life evaluation) may have affected reliability as well. Last, by not incorporating texture, we omitted a potentially informative feature from scoring.
It is paramount that surgeons undergo diligent training before undertaking this approach for minimizing unwanted results; furthermore, higher early complication rates level off with increased surgeon experience.14,36,37 We recommend meticulous soft-tissue handling, cautious retraction, and careful patient selection (relative contraindication for patients with an abdominal pannus overlying the incision) as primary measures for minimizing incisional trauma and potential wound-healing complications.38 Preservation of the tensor fascia is also crucial,39 as it is the only closable layer separating deep and superficial compartments. Without good closure of the tensor fascia, there is no containment or tamponade of deep bleeding, which can facilitate hematoma formation.
In the population studied, we found no significant long-term differences in cosmetic appearance (based on clinician or patient evaluation) between wounds managed with and without the ring retractor. Our data do not support routine use of the ring retractor, during DA-THA, for improved wound cosmesis. Whether the device has any significant role in reducing the number of wound complications in THA is yet to be determined. Last, the ring retractor may have a role in other areas of orthopedic surgery, such as hip fractures in the elderly or orthopedic oncology. In situations like these, where adequate nutrition and immunocompetency may be lacking, the added protection provided by the device may translate into a more notable benefit than in elective THA.
1. Laffosse JM, Chiron P, Tricoire JL, Giordano G, Molinier F, Puget J. Prospective and comparative study of minimally invasive posterior approach versus standard posterior approach in total hip replacement [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(3):228-237.
2. Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop. 2011;35(2):173-184.
3. Wright JM, Crockett HC, Delgado S, Lyman S, Madsen M, Sculco TP. Mini-incision for total hip arthroplasty: a prospective, controlled investigation with 5-year follow-up evaluation. J Arthroplasty. 2004;19(5):538-545.
4. Mardones R, Pagnano MW, Nemanich JP, Trousdale RT. The Frank Stinchfield Award: muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop. 2005;(441):63-67.
5. Müller M, Tohtz S, Dewey M, Springer I, Perka C. Age-related appearance of muscle trauma in primary total hip arthroplasty and the benefit of a minimally invasive approach for patients older than 70 years. Int Orthop. 2011;35(2):165-171.
6. Noble PC, Johnston JD, Alexander JA, et al. Making minimally invasive THR safe: conclusions from biomechanical simulation and analysis. Int Orthop. 2007;31(suppl 1):S25-S28.
7. Bremer AK, Kalberer F, Pfirrmann CW, Dora C. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93(7):886-889.
8. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
9. Rachbauer F, Kain MSH, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320.
10. Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392-1398.
11. Mayr E, Nogler M, Benedetti MG, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech. 2009;24(10):812-818.
12. Meneghini RM, Pagnano MW, Trousdale RT, Hozack WJ. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop. 2006;(453):293-298.
13. Sculco TP. Anterior approach in THA improves outcomes: opposes. Orthopedics. 2011;34(9):e459-e461.
14. Bhandari M, Matta JM, Dodgin D, et al; Anterior Total Hip Arthroplasty Collaborative Investigators. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am. 2009;40(3):329-342.
15. Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop. 2011;469(2):503-507.
16. Barton C, Kim PR. Complications of the direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):371-375.
17. Bender B, Nogler M, Hozack WJ. Direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):321-328.
18. Pelosi MA 2nd, Pelosi MA 3rd. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96(5, pt 1):775-778.
19. Lee P, Waxman K, Taylor B, Yim S. Use of wound-protection system and postoperative wound-infection rates in open appendectomy: a randomized prospective trial. Arch Surg. 2009;144(9):872-875.
20. Horiuchi T, Tanishima H, Tamagawa K, et al. Randomized, controlled investigation of the anti-infective properties of the Alexis retractor/protector of incision sites. J Trauma. 2007;62(1):212-215.
21. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
22. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
23. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostaticagent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
24. Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE. Development and validation of a novel scar evaluation scale. Plast Reconstr Surg. 2007;120(7):1892-1897.
25. Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954-1961.
26. Durani P, McGrouther DA, Ferguson MW. Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg. 2009;62(6):713-720.
27. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
28. Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118(4):909-918.
29. Quinn JV, Wells GA. An assessment of clinical wound evaluation scales. Acad Emerg Med. 1998;5(6):583-586.
30. Livesey C, Wylde V, Descamps S, et al. Skin closure after total hip replacement: a randomised controlled trial of skin adhesive versus surgical staples. J Bone Joint Surg Br. 2009;91(6):725-729.
31. Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31(5):468-492.
32. Mow CS, Woolson ST, Ngarmukos SG, Park EH, Lorenz HP. Comparison of scars from total hip replacements done with a standard or a mini-incision. Clin Orthop. 2005;(441):80-85.
33. Khan RJ, Fick D, Yao F, et al. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. J Bone Joint Surg Br. 2006;88(2):238-242.
34. Goldstein WM, Ali R, Branson JJ, Berland KA. Comparison of patient satisfaction with incision cosmesis after standard and minimally invasive total hip arthroplasty. Orthopedics. 2008;31(4):368.
35. Quinn J, Wells G, Sutcliffe T, et al. Tissue adhesive versus suture wound repair at 1 year: randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann Emerg Med. 1998;32(6):645-649.
36. Alberti LR, Petroianu A, Zac RI, Andrade JC Jr. The effect of surgical procedures on serum albumin concentration. Chirurgia (Bucur). 2008;103(1):39-43.
37. Berend KR, Lombardi AV Jr, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(suppl 6):107-120.
38. Mutnal A, Patel P, Cardona L, Suarez J. Periprosthetic Propionibacterium granulosum joint infection after direct anterior total hip arthroplasty: a case report. JBJS Case Connector. 2011;1(2):e10.
39. Alvarez AM, Suarez JC, Patel P, Benton EG. Fluoroscopic imaging of acetabular cup position during THA through a direct anterior approach. Orthopedics. 2013;36(10):776-777. Erratum in: Orthopedics. 2014;37(1):16.
It is thought that, by placing more emphasis on soft-tissue preservation, minimally invasive surgery total hip arthroplasty (MIS-THA) results in less soft-tissue trauma, less blood loss, and earlier recovery.1-3 Despite these improvements over standard methods, there is a concern that the vigorous retraction needed for proper visualization through smaller incisions could injure soft tissues.4-7 Single-incision direct anterior THA (DA-THA) has gained in popularity because of the true intermuscular/internervous plane through which the procedure can be performed with relatively minimal muscle dissection using MIS techniques.8,9 This approach may offer quicker recovery and superior stability in comparison with nonintermuscular methods, which unavoidably cause more muscle damage.10-12
Although the evidence of these early gains is encouraging, several studies have found high complication rates with DA-THA.8,13-17 Noted disadvantages include a steep learning curve, lateral femoral cutaneous neurapraxia, need for a specialized table, and higher fracture and wound complication rates. Not surprisingly, with increased surgeon experience, the complication rate decreased substantially.14,15 However, wound-related complications remained steady, with 2 recent large studies reporting rates of 4.6% and 2.1%.14,15 The thin anterior skin, high tensional forces along the groin crease and perpendicular to the typical DA incision, and less resilient soft-tissue envelope are postulated reasons for wound-related issues, which are likely magnified in patients who are more obese.15,16
A novel device designed to lessen tissue damage is the ring retractor (Figure 1). Used initially in general surgery and obstetrics, it consists of 2 semirigid polymer rings connected by a flexible cylindrical polymer membrane.18-20 The lower ring is tucked and anchored underneath the wound edge, and then the upper ring is rolled down and cinched onto the skin. The resultant tension on the polymer sleeve—imparted by the rigidity of the ring—provides strong, evenly distributed wound-edge retraction. It also provides a physical barrier between the wound edge and the rest of the operative field. Proponents of the ring retractor claim increased wound-edge moisture, less bruising, and reduced local trauma compared with standard metal retractors alone.
Wound-edge retractor forces are doubled during MIS-THA compared with conventional THA.14-20 This may explain reports of worse scar cosmesis with MIS-THA. Given the theoretical benefits of minimized wound-edge trauma, the ring retractor may improve scar appearance compared with standard retraction alone. Any clinically relevant effect on cosmesis should be readily apparent to justify use of the retractor in this regard. Although some surgeons routinely use the device for primary THA, it has not been the subject of any recent orthopedic studies.
In the present study, we prospectively investigated wound cosmesis with and without use of the ring retractor in patients undergoing DA-THA.
Materials and Methods
This prospective, single-center, randomized study was reviewed and approved by the institutional review board at our facility. Consent was obtained from all participating patients.
We evaluated 50 surgical incisions in 48 patients. Eligible participants were over age 18 years and undergoing primary DA-THA. Exclusion criteria included previous surgery on the affected hip, a pathological hip condition requiring an extensile exposure, systemic inflammatory illness, chronic corticosteroid use, and dermatologic abnormality of the incisional area. One patient was having simultaneous bilateral THAs, and another was having staged bilateral THAs. Each hip in these patients was given its own case number and treated separately. Of the 49 patients who met all the inclusion criteria, only 1 decided not to participate (Figure 2).
Stratified randomization with permuted block size (sex, body mass index [BMI]) was used to assign patients in a 1:1 ratio to either the treatment group or the control group. In the treatment group, the Protractor Incision Protector and Retractor (Gyrus ACMI, Southborough, Massachusetts) was used with standard metal retractors. In the control group, only standard metal retractors were used. Patients were blinded to their group assignments, and surgeons were informed about each assignment only after the initial incision was made.
Clinical research investigators were blinded to the groups’ prospectively collected data. Collection time points were preoperative clinical visit, day of surgery through discharge, and 2-, 6-, and 12-week postoperative follow-ups. Day-of-surgery data included estimated intraoperative blood loss, operative side, operative time, intraoperative complications, and American Society of Anesthesiologists (ASA) physical status classification. Total length of stay, pain scores (range, 0-10), estimated drain output, and blood-transfusion data were also recorded. To evaluate whether the device had any effect on short-term functional outcome, we collected Harris Hip Scores (HHS) and Short Form–12 (SF-12, Version 2) scores at the preoperative and 6-week postoperative visits. We also documented any wound-healing-related issues or complications that occurred up until the final visit.
To account for any effect of nutrition status on wound healing, we obtained pre-albumin and albumin levels and absolute lymphocyte counts from the preoperative electronic records. We used an albumin level under 3.5 g/dL and an absolute lymphocyte count under 1500/µL for our analysis, as these cutoffs have been associated with wound complications after primary THA.21 There is no similarly established threshold for pre-albumin level, so we used values under 20 mg/L based on comparable literature.22,23
At each postoperative visit, standardized high-resolution images were obtained. At the 12-week visit, patients completed 2 Likert scales regarding their overall opinion of their scars and how their scars compared with their expectations. They also ranked 5 separate THA-related outcomes in order of importance (Appendix).
Photographs were evaluated by 2 blinded plastic surgeons (Dr. Friedman and Dr. Jack) using 2 grading systems—the Stony Brook Scar Evaluation Scale (SBSES)24 (Table 1) and a modified Manchester Scar Scale (MSS)25 (Table 2). We used these systems because they were photograph-based, psychometrically studied, and specifically designed to assess surgical incision healing with established validity and reliability.24-27 A particular advantage, strictly related to cosmetic outcome, is their validity in scoring scars from high-definition photographs in a different place or time. The SBSES, an ordinal wound evaluation scale that measures short-term cosmetic outcomes, consists of 6 items, each receiving 1 or 0 point, yielding a total score between 0 (worst) and 5 (best). The modified MSS includes a visual analog scale (VAS), which has a vertical hash marked on a 10-cm line and is scored between 0 (excellent) and 10 (poor) to 1 decimal point.26,28 This value is added to grades on color, surface appearance, contour, and distortion, resulting in a score between 4 (best) and 24 (worst). The primary outcome measures were Likert-scale responses obtained at final visit and SBSES/MSS scores for each visit; 12-week scores were the primary end point.
Operative Procedure
Experienced fellowship-trained orthopedic surgeons performed all procedures. A modified Hueter approach was used for exposure.9 Mean incision length was about 12 cm. For the treatment group, the ring retractor was inserted at the level of the tensor fascia, with the inferior ring resting between the fascia and the subcutaneous layer and the superior ring cinched over the skin (Figure 3). The device is made in 4 different sizes for incisions from 2.5 to 17 cm; our study population required only 1 size. Otherwise, the surgical protocol was based on that described by Matta and colleagues.8 Wound closure (over a drain) was performed according to a standardized protocol—running No. 1 Vicryl suture for the superficial tensor fascia, interrupted 2-0 Vicryl for the deep dermal layer, and subcutaneous 4-0 Monocryl for the skin followed by application of Dermabond (Ethicon, Somerville, New Jersey) and Tegaderm +Pad (3M, St. Paul, Minnesota) for outer dressing, which was replaced on postoperative day 2 and removed at the 2-week visit.
Statistical Methods
An a priori sample-size calculation was performed. Power performed in a base of a prior study that evaluated anterolateral and posterolateral THA scars using a VAS, a component of the MSS, suggested a sample size of 16 per group to detect the minimal clinically important difference of 1.5 cm: SD (σ) = 1.5 cm, α = 0.05, β = 0.20.29,30 In addition, a general estimate for detecting a 1-unit change on an ordinal scale (σ = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively decided to enroll 25 patients per arm in case of larger true variance.
The Wilcoxon rank sum test was used for comparisons of continuous data between groups. Differences between means were analyzed with 2-sided t tests. Categorical data were compared with the Pearson χ2 test or the Fisher exact test, as indicated. Ordinal ranking scores were compared with the Mantel-Haenszel test. Multivariate logistic regression was applied to identify the significant independent predictors of better scar grades for each surgeon by considering candidate variables with Ps < .20 in the univariate analysis.
Results
We found no differences in demographic or perioperative characteristics between treatment and control groups (Tables 3, 4). The groups showed similar mean improvements in their respective 6-week HHS (38.7 and 36.4 points; P = .65), SF-12 physical component summary scores (11.8 and 14.5 points; P = .37), and SF-12 mental component summary scores (5.1 and 3.7; P = .70).
Patient questionnaire outcomes are listed in Table 5. For the control group, 25/25 image sets were obtained at the 2-week visit, 25/25 at the 6-week visit, and 24/25 at the 12-week visit. For the treatment group, there were 23/25, 24/25, and 23/25 images sets, respectively.
When surgeon scoring was analyzed separately, SBSES and MSS scores were similar between treatment and control groups, with 1 exception: 2-week MSS scores were better for the treatment group according to surgeon A (P = .026). When grades were averaged, SBSES scores were again similar at all time points (Figure 4A); MSS scores were better for the treatment group at 2 weeks (P = .036) and equivalent at all other time points (Figure 4B). For the SBSES, Spearman correlation coefficient ρ with 95% confidence interval (CI) was 0.37
(95% CI, 0.08-0.66) at 2 weeks, 0.48 (95% CI, 0.20-0.76) at 6 weeks, and 0.62 (95% CI, 0.33-0.91) at 12 weeks. Following the same pattern for the MSS, ρ was 0.20 (95% CI, –0.09 to 0.49), 0.51 (95% CI, 0.23-0.79), and 0.32 (95% CI, 0.03-0.61).
Independent multivariate analysis revealed that age over 65 years was a significant predictor of worse scores. On SBSES, the odds ratio (OR) was 1.15 (95% CI, 1.07-1.24) for surgeon A and 1.11 (95% CI, 1.05-1.18) for surgeon B. On MSS, the OR was 0.89 (95% CI, 0.84-0.94) for surgeon A and 0.95 (95% CI, 0.91-0.99) for surgeon B. The likelihood of having worse SBSES scores according to surgeon A was 4.72 times higher if the pre-albumin level was under 20 mg/L (95% CI, 1.15-19.36). Albumin level under 3.5 g/dL and absolute lymphocyte count under 1500 cells/µL were not found to be independent predictors of poorer scores.
Patients’ overall opinion (P = .63) and assessment of their scars relative to expectations (P = .25) on the Likert scales were not different between groups. More scars exceeded patients’ expectations and had more excellent ratings in the control group. The 2 groups were similar with regard to relative importance of various patient-related outcomes. Factors most important to overall outcome were relief of hip pain, followed by implant longevity and length of recovery. Least important were incision-related variables.
There were only 3 minor noninfectious wound complications (6%), 2 in the treatment group and 1 in the control group. In the treatment group, a 67-year-old man with diabetes (ASA class III; BMI, 32.1 kg/m2; received transfusion) had 2 small areas (<5 mm) of superficial ulceration at 6-week follow-up—one at the proximal aspect of the incision and the other near the midpoint along the flexion crease. Both lesions resolved by 12-week follow-up. Also in the treatment group, a 77-year-old woman (ASA class II; BMI, 24.9 kg/m2; received transfusion) at 6 weeks had a spitting suture, which was removed in clinic without further issue. In the control group, a 55-year-old woman (ASA class II; BMI, 27.4 kg/m2) had a suture reaction near the proximal aspect of her incision 3 weeks after surgery. This reaction, which presented as a mild, localized erythema without pain, tenderness, or drainage, resolved by 6-week follow-up. None of these wound complications required intervention beyond observation.
Discussion
This study was designed to provide a bipartisan measure of wound-healing cosmesis after DA-THA. Scar evaluation by blinded plastic surgeons served as a standardized, clinical assessment, whereas the patient questionnaire offered a more subjective appraisal. The modified MMS25 and the SBSES24 are the only 2 wound-grading systems designed and validated for photographic assessment of postsurgical scars. Most scar evaluation schemes pertain to burn or traumatic scars.26,27,31 As a result, many earlier studies intending to compare incisional scars used poorly suited evaluation systems.
The current literature includes reports on 3 studies with scoring-based scar assessment in THA; all used grading systems designed for either burns or traumatic wounds, but 2 also used a VAS.32-34 VASs have been validated for measuring wound cosmesis but are entirely subjective and without structure and provide no feedback as to why a scar was rated good or bad.24 Mow and colleagues32 prospectively compared scars after standard posterior or MIS approaches and found no differences according to a scoring system intended for burn scars. In our study population, we found no group differences in patients’ cosmesis of their scars.
Although scars can take a year or longer to fully mature, researchers from the University of Michigan discovered that scar appearance at 1 year correlates highly with cosmesis 12 weeks after closure, though poorly with cosmesis 10 days after closure.35 Therefore, any observed differences in scar cosmesis between groups at 12-week follow-up would likely persist, whereas differences at 2-week follow-up would have little bearing on ultimate appearance. For this reason, our primary outcome measure was healing process and cosmesis at 12 weeks. High wound complication rates have been reported for MIS-DA-THA.8,14-16 Jewett and Collis15 noted a 4.6% wound complication rate (3% noninfectious ulcerative dehiscence, 1.6% superficial infection), which is comparable to the 6% rate found in this study. However, there likely is some variability across studies in what constitutes a wound complication or superficial infection. Of our 3 wound complications—stitch reaction, spitting suture, small noninfectious ulceration—only the ulceration was of a severity similar to that reported by Jewett and Collis.15 Matta and colleagues8 reported only 3 wound complications (in 494 patients), all severe enough to require operative intervention. One explanation for this low complication rate is use of a ring retractor, as it is routinely depicted in their technique paper. However, no specific reference is made to gauge how often the device was used.
Rates of superficial infection after DA-THA range from 0.6% to 1.6% in 3 large observational studies (combined deep infection rate, 0.43%).8,14,15 In 2 of these studies, all patients with superficial infection underwent formal débridement, though none developed deep infection. A prospective randomized study of 221 patients who underwent colorectal surgery—where perioperative infectious morbidity ranges from 25% to 50%—found that ring retractor use significantly reduced superficial wound infection rates (8.1% vs 0%). A significant reduction in wound infection was shown in a similarly designed study involving 48 patients who had open appendectomy (14.6% vs 1.6%). The device had no effect on deep infection in either general surgery study. The wound infection rates reported in these general surgery studies are markedly higher than those in our study population. As a result, the effect of the ring retractor on wound infection in DA-THA may be less. Regardless of the effect on deep infection, fewer superficial infections, which often require operative intervention, would be of considerable benefit.
Below-threshold albumin level and absolute lymphocyte count have been associated with wound-healing complications after hip replacement.21 In the present study, pre-albumin level under 20 mg/L was the only nutritional marker predictive of poor wound appearance, but this finding was seen only in SBSES scores from surgeon A. Subgroup analysis did not reveal any relationship between wound appearance and any of the recorded demographic or perioperative variables, but for a small predictive influence with age over 65 years.
This study had some limitations. Our findings cannot be generalized to all patients who undergo THA, as only DA incisions were studied. Results also may not be generalizable to non-fellowship-trained orthopedists. In addition, selection bias likely resulted from including patients already selected for the DA approach. Using digital images for evaluation (vs real-life evaluation) may have affected reliability as well. Last, by not incorporating texture, we omitted a potentially informative feature from scoring.
It is paramount that surgeons undergo diligent training before undertaking this approach for minimizing unwanted results; furthermore, higher early complication rates level off with increased surgeon experience.14,36,37 We recommend meticulous soft-tissue handling, cautious retraction, and careful patient selection (relative contraindication for patients with an abdominal pannus overlying the incision) as primary measures for minimizing incisional trauma and potential wound-healing complications.38 Preservation of the tensor fascia is also crucial,39 as it is the only closable layer separating deep and superficial compartments. Without good closure of the tensor fascia, there is no containment or tamponade of deep bleeding, which can facilitate hematoma formation.
In the population studied, we found no significant long-term differences in cosmetic appearance (based on clinician or patient evaluation) between wounds managed with and without the ring retractor. Our data do not support routine use of the ring retractor, during DA-THA, for improved wound cosmesis. Whether the device has any significant role in reducing the number of wound complications in THA is yet to be determined. Last, the ring retractor may have a role in other areas of orthopedic surgery, such as hip fractures in the elderly or orthopedic oncology. In situations like these, where adequate nutrition and immunocompetency may be lacking, the added protection provided by the device may translate into a more notable benefit than in elective THA.
It is thought that, by placing more emphasis on soft-tissue preservation, minimally invasive surgery total hip arthroplasty (MIS-THA) results in less soft-tissue trauma, less blood loss, and earlier recovery.1-3 Despite these improvements over standard methods, there is a concern that the vigorous retraction needed for proper visualization through smaller incisions could injure soft tissues.4-7 Single-incision direct anterior THA (DA-THA) has gained in popularity because of the true intermuscular/internervous plane through which the procedure can be performed with relatively minimal muscle dissection using MIS techniques.8,9 This approach may offer quicker recovery and superior stability in comparison with nonintermuscular methods, which unavoidably cause more muscle damage.10-12
Although the evidence of these early gains is encouraging, several studies have found high complication rates with DA-THA.8,13-17 Noted disadvantages include a steep learning curve, lateral femoral cutaneous neurapraxia, need for a specialized table, and higher fracture and wound complication rates. Not surprisingly, with increased surgeon experience, the complication rate decreased substantially.14,15 However, wound-related complications remained steady, with 2 recent large studies reporting rates of 4.6% and 2.1%.14,15 The thin anterior skin, high tensional forces along the groin crease and perpendicular to the typical DA incision, and less resilient soft-tissue envelope are postulated reasons for wound-related issues, which are likely magnified in patients who are more obese.15,16
A novel device designed to lessen tissue damage is the ring retractor (Figure 1). Used initially in general surgery and obstetrics, it consists of 2 semirigid polymer rings connected by a flexible cylindrical polymer membrane.18-20 The lower ring is tucked and anchored underneath the wound edge, and then the upper ring is rolled down and cinched onto the skin. The resultant tension on the polymer sleeve—imparted by the rigidity of the ring—provides strong, evenly distributed wound-edge retraction. It also provides a physical barrier between the wound edge and the rest of the operative field. Proponents of the ring retractor claim increased wound-edge moisture, less bruising, and reduced local trauma compared with standard metal retractors alone.
Wound-edge retractor forces are doubled during MIS-THA compared with conventional THA.14-20 This may explain reports of worse scar cosmesis with MIS-THA. Given the theoretical benefits of minimized wound-edge trauma, the ring retractor may improve scar appearance compared with standard retraction alone. Any clinically relevant effect on cosmesis should be readily apparent to justify use of the retractor in this regard. Although some surgeons routinely use the device for primary THA, it has not been the subject of any recent orthopedic studies.
In the present study, we prospectively investigated wound cosmesis with and without use of the ring retractor in patients undergoing DA-THA.
Materials and Methods
This prospective, single-center, randomized study was reviewed and approved by the institutional review board at our facility. Consent was obtained from all participating patients.
We evaluated 50 surgical incisions in 48 patients. Eligible participants were over age 18 years and undergoing primary DA-THA. Exclusion criteria included previous surgery on the affected hip, a pathological hip condition requiring an extensile exposure, systemic inflammatory illness, chronic corticosteroid use, and dermatologic abnormality of the incisional area. One patient was having simultaneous bilateral THAs, and another was having staged bilateral THAs. Each hip in these patients was given its own case number and treated separately. Of the 49 patients who met all the inclusion criteria, only 1 decided not to participate (Figure 2).
Stratified randomization with permuted block size (sex, body mass index [BMI]) was used to assign patients in a 1:1 ratio to either the treatment group or the control group. In the treatment group, the Protractor Incision Protector and Retractor (Gyrus ACMI, Southborough, Massachusetts) was used with standard metal retractors. In the control group, only standard metal retractors were used. Patients were blinded to their group assignments, and surgeons were informed about each assignment only after the initial incision was made.
Clinical research investigators were blinded to the groups’ prospectively collected data. Collection time points were preoperative clinical visit, day of surgery through discharge, and 2-, 6-, and 12-week postoperative follow-ups. Day-of-surgery data included estimated intraoperative blood loss, operative side, operative time, intraoperative complications, and American Society of Anesthesiologists (ASA) physical status classification. Total length of stay, pain scores (range, 0-10), estimated drain output, and blood-transfusion data were also recorded. To evaluate whether the device had any effect on short-term functional outcome, we collected Harris Hip Scores (HHS) and Short Form–12 (SF-12, Version 2) scores at the preoperative and 6-week postoperative visits. We also documented any wound-healing-related issues or complications that occurred up until the final visit.
To account for any effect of nutrition status on wound healing, we obtained pre-albumin and albumin levels and absolute lymphocyte counts from the preoperative electronic records. We used an albumin level under 3.5 g/dL and an absolute lymphocyte count under 1500/µL for our analysis, as these cutoffs have been associated with wound complications after primary THA.21 There is no similarly established threshold for pre-albumin level, so we used values under 20 mg/L based on comparable literature.22,23
At each postoperative visit, standardized high-resolution images were obtained. At the 12-week visit, patients completed 2 Likert scales regarding their overall opinion of their scars and how their scars compared with their expectations. They also ranked 5 separate THA-related outcomes in order of importance (Appendix).
Photographs were evaluated by 2 blinded plastic surgeons (Dr. Friedman and Dr. Jack) using 2 grading systems—the Stony Brook Scar Evaluation Scale (SBSES)24 (Table 1) and a modified Manchester Scar Scale (MSS)25 (Table 2). We used these systems because they were photograph-based, psychometrically studied, and specifically designed to assess surgical incision healing with established validity and reliability.24-27 A particular advantage, strictly related to cosmetic outcome, is their validity in scoring scars from high-definition photographs in a different place or time. The SBSES, an ordinal wound evaluation scale that measures short-term cosmetic outcomes, consists of 6 items, each receiving 1 or 0 point, yielding a total score between 0 (worst) and 5 (best). The modified MSS includes a visual analog scale (VAS), which has a vertical hash marked on a 10-cm line and is scored between 0 (excellent) and 10 (poor) to 1 decimal point.26,28 This value is added to grades on color, surface appearance, contour, and distortion, resulting in a score between 4 (best) and 24 (worst). The primary outcome measures were Likert-scale responses obtained at final visit and SBSES/MSS scores for each visit; 12-week scores were the primary end point.
Operative Procedure
Experienced fellowship-trained orthopedic surgeons performed all procedures. A modified Hueter approach was used for exposure.9 Mean incision length was about 12 cm. For the treatment group, the ring retractor was inserted at the level of the tensor fascia, with the inferior ring resting between the fascia and the subcutaneous layer and the superior ring cinched over the skin (Figure 3). The device is made in 4 different sizes for incisions from 2.5 to 17 cm; our study population required only 1 size. Otherwise, the surgical protocol was based on that described by Matta and colleagues.8 Wound closure (over a drain) was performed according to a standardized protocol—running No. 1 Vicryl suture for the superficial tensor fascia, interrupted 2-0 Vicryl for the deep dermal layer, and subcutaneous 4-0 Monocryl for the skin followed by application of Dermabond (Ethicon, Somerville, New Jersey) and Tegaderm +Pad (3M, St. Paul, Minnesota) for outer dressing, which was replaced on postoperative day 2 and removed at the 2-week visit.
Statistical Methods
An a priori sample-size calculation was performed. Power performed in a base of a prior study that evaluated anterolateral and posterolateral THA scars using a VAS, a component of the MSS, suggested a sample size of 16 per group to detect the minimal clinically important difference of 1.5 cm: SD (σ) = 1.5 cm, α = 0.05, β = 0.20.29,30 In addition, a general estimate for detecting a 1-unit change on an ordinal scale (σ = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively decided to enroll 25 patients per arm in case of larger true variance.
The Wilcoxon rank sum test was used for comparisons of continuous data between groups. Differences between means were analyzed with 2-sided t tests. Categorical data were compared with the Pearson χ2 test or the Fisher exact test, as indicated. Ordinal ranking scores were compared with the Mantel-Haenszel test. Multivariate logistic regression was applied to identify the significant independent predictors of better scar grades for each surgeon by considering candidate variables with Ps < .20 in the univariate analysis.
Results
We found no differences in demographic or perioperative characteristics between treatment and control groups (Tables 3, 4). The groups showed similar mean improvements in their respective 6-week HHS (38.7 and 36.4 points; P = .65), SF-12 physical component summary scores (11.8 and 14.5 points; P = .37), and SF-12 mental component summary scores (5.1 and 3.7; P = .70).
Patient questionnaire outcomes are listed in Table 5. For the control group, 25/25 image sets were obtained at the 2-week visit, 25/25 at the 6-week visit, and 24/25 at the 12-week visit. For the treatment group, there were 23/25, 24/25, and 23/25 images sets, respectively.
When surgeon scoring was analyzed separately, SBSES and MSS scores were similar between treatment and control groups, with 1 exception: 2-week MSS scores were better for the treatment group according to surgeon A (P = .026). When grades were averaged, SBSES scores were again similar at all time points (Figure 4A); MSS scores were better for the treatment group at 2 weeks (P = .036) and equivalent at all other time points (Figure 4B). For the SBSES, Spearman correlation coefficient ρ with 95% confidence interval (CI) was 0.37
(95% CI, 0.08-0.66) at 2 weeks, 0.48 (95% CI, 0.20-0.76) at 6 weeks, and 0.62 (95% CI, 0.33-0.91) at 12 weeks. Following the same pattern for the MSS, ρ was 0.20 (95% CI, –0.09 to 0.49), 0.51 (95% CI, 0.23-0.79), and 0.32 (95% CI, 0.03-0.61).
Independent multivariate analysis revealed that age over 65 years was a significant predictor of worse scores. On SBSES, the odds ratio (OR) was 1.15 (95% CI, 1.07-1.24) for surgeon A and 1.11 (95% CI, 1.05-1.18) for surgeon B. On MSS, the OR was 0.89 (95% CI, 0.84-0.94) for surgeon A and 0.95 (95% CI, 0.91-0.99) for surgeon B. The likelihood of having worse SBSES scores according to surgeon A was 4.72 times higher if the pre-albumin level was under 20 mg/L (95% CI, 1.15-19.36). Albumin level under 3.5 g/dL and absolute lymphocyte count under 1500 cells/µL were not found to be independent predictors of poorer scores.
Patients’ overall opinion (P = .63) and assessment of their scars relative to expectations (P = .25) on the Likert scales were not different between groups. More scars exceeded patients’ expectations and had more excellent ratings in the control group. The 2 groups were similar with regard to relative importance of various patient-related outcomes. Factors most important to overall outcome were relief of hip pain, followed by implant longevity and length of recovery. Least important were incision-related variables.
There were only 3 minor noninfectious wound complications (6%), 2 in the treatment group and 1 in the control group. In the treatment group, a 67-year-old man with diabetes (ASA class III; BMI, 32.1 kg/m2; received transfusion) had 2 small areas (<5 mm) of superficial ulceration at 6-week follow-up—one at the proximal aspect of the incision and the other near the midpoint along the flexion crease. Both lesions resolved by 12-week follow-up. Also in the treatment group, a 77-year-old woman (ASA class II; BMI, 24.9 kg/m2; received transfusion) at 6 weeks had a spitting suture, which was removed in clinic without further issue. In the control group, a 55-year-old woman (ASA class II; BMI, 27.4 kg/m2) had a suture reaction near the proximal aspect of her incision 3 weeks after surgery. This reaction, which presented as a mild, localized erythema without pain, tenderness, or drainage, resolved by 6-week follow-up. None of these wound complications required intervention beyond observation.
Discussion
This study was designed to provide a bipartisan measure of wound-healing cosmesis after DA-THA. Scar evaluation by blinded plastic surgeons served as a standardized, clinical assessment, whereas the patient questionnaire offered a more subjective appraisal. The modified MMS25 and the SBSES24 are the only 2 wound-grading systems designed and validated for photographic assessment of postsurgical scars. Most scar evaluation schemes pertain to burn or traumatic scars.26,27,31 As a result, many earlier studies intending to compare incisional scars used poorly suited evaluation systems.
The current literature includes reports on 3 studies with scoring-based scar assessment in THA; all used grading systems designed for either burns or traumatic wounds, but 2 also used a VAS.32-34 VASs have been validated for measuring wound cosmesis but are entirely subjective and without structure and provide no feedback as to why a scar was rated good or bad.24 Mow and colleagues32 prospectively compared scars after standard posterior or MIS approaches and found no differences according to a scoring system intended for burn scars. In our study population, we found no group differences in patients’ cosmesis of their scars.
Although scars can take a year or longer to fully mature, researchers from the University of Michigan discovered that scar appearance at 1 year correlates highly with cosmesis 12 weeks after closure, though poorly with cosmesis 10 days after closure.35 Therefore, any observed differences in scar cosmesis between groups at 12-week follow-up would likely persist, whereas differences at 2-week follow-up would have little bearing on ultimate appearance. For this reason, our primary outcome measure was healing process and cosmesis at 12 weeks. High wound complication rates have been reported for MIS-DA-THA.8,14-16 Jewett and Collis15 noted a 4.6% wound complication rate (3% noninfectious ulcerative dehiscence, 1.6% superficial infection), which is comparable to the 6% rate found in this study. However, there likely is some variability across studies in what constitutes a wound complication or superficial infection. Of our 3 wound complications—stitch reaction, spitting suture, small noninfectious ulceration—only the ulceration was of a severity similar to that reported by Jewett and Collis.15 Matta and colleagues8 reported only 3 wound complications (in 494 patients), all severe enough to require operative intervention. One explanation for this low complication rate is use of a ring retractor, as it is routinely depicted in their technique paper. However, no specific reference is made to gauge how often the device was used.
Rates of superficial infection after DA-THA range from 0.6% to 1.6% in 3 large observational studies (combined deep infection rate, 0.43%).8,14,15 In 2 of these studies, all patients with superficial infection underwent formal débridement, though none developed deep infection. A prospective randomized study of 221 patients who underwent colorectal surgery—where perioperative infectious morbidity ranges from 25% to 50%—found that ring retractor use significantly reduced superficial wound infection rates (8.1% vs 0%). A significant reduction in wound infection was shown in a similarly designed study involving 48 patients who had open appendectomy (14.6% vs 1.6%). The device had no effect on deep infection in either general surgery study. The wound infection rates reported in these general surgery studies are markedly higher than those in our study population. As a result, the effect of the ring retractor on wound infection in DA-THA may be less. Regardless of the effect on deep infection, fewer superficial infections, which often require operative intervention, would be of considerable benefit.
Below-threshold albumin level and absolute lymphocyte count have been associated with wound-healing complications after hip replacement.21 In the present study, pre-albumin level under 20 mg/L was the only nutritional marker predictive of poor wound appearance, but this finding was seen only in SBSES scores from surgeon A. Subgroup analysis did not reveal any relationship between wound appearance and any of the recorded demographic or perioperative variables, but for a small predictive influence with age over 65 years.
This study had some limitations. Our findings cannot be generalized to all patients who undergo THA, as only DA incisions were studied. Results also may not be generalizable to non-fellowship-trained orthopedists. In addition, selection bias likely resulted from including patients already selected for the DA approach. Using digital images for evaluation (vs real-life evaluation) may have affected reliability as well. Last, by not incorporating texture, we omitted a potentially informative feature from scoring.
It is paramount that surgeons undergo diligent training before undertaking this approach for minimizing unwanted results; furthermore, higher early complication rates level off with increased surgeon experience.14,36,37 We recommend meticulous soft-tissue handling, cautious retraction, and careful patient selection (relative contraindication for patients with an abdominal pannus overlying the incision) as primary measures for minimizing incisional trauma and potential wound-healing complications.38 Preservation of the tensor fascia is also crucial,39 as it is the only closable layer separating deep and superficial compartments. Without good closure of the tensor fascia, there is no containment or tamponade of deep bleeding, which can facilitate hematoma formation.
In the population studied, we found no significant long-term differences in cosmetic appearance (based on clinician or patient evaluation) between wounds managed with and without the ring retractor. Our data do not support routine use of the ring retractor, during DA-THA, for improved wound cosmesis. Whether the device has any significant role in reducing the number of wound complications in THA is yet to be determined. Last, the ring retractor may have a role in other areas of orthopedic surgery, such as hip fractures in the elderly or orthopedic oncology. In situations like these, where adequate nutrition and immunocompetency may be lacking, the added protection provided by the device may translate into a more notable benefit than in elective THA.
1. Laffosse JM, Chiron P, Tricoire JL, Giordano G, Molinier F, Puget J. Prospective and comparative study of minimally invasive posterior approach versus standard posterior approach in total hip replacement [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(3):228-237.
2. Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop. 2011;35(2):173-184.
3. Wright JM, Crockett HC, Delgado S, Lyman S, Madsen M, Sculco TP. Mini-incision for total hip arthroplasty: a prospective, controlled investigation with 5-year follow-up evaluation. J Arthroplasty. 2004;19(5):538-545.
4. Mardones R, Pagnano MW, Nemanich JP, Trousdale RT. The Frank Stinchfield Award: muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop. 2005;(441):63-67.
5. Müller M, Tohtz S, Dewey M, Springer I, Perka C. Age-related appearance of muscle trauma in primary total hip arthroplasty and the benefit of a minimally invasive approach for patients older than 70 years. Int Orthop. 2011;35(2):165-171.
6. Noble PC, Johnston JD, Alexander JA, et al. Making minimally invasive THR safe: conclusions from biomechanical simulation and analysis. Int Orthop. 2007;31(suppl 1):S25-S28.
7. Bremer AK, Kalberer F, Pfirrmann CW, Dora C. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93(7):886-889.
8. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
9. Rachbauer F, Kain MSH, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320.
10. Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392-1398.
11. Mayr E, Nogler M, Benedetti MG, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech. 2009;24(10):812-818.
12. Meneghini RM, Pagnano MW, Trousdale RT, Hozack WJ. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop. 2006;(453):293-298.
13. Sculco TP. Anterior approach in THA improves outcomes: opposes. Orthopedics. 2011;34(9):e459-e461.
14. Bhandari M, Matta JM, Dodgin D, et al; Anterior Total Hip Arthroplasty Collaborative Investigators. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am. 2009;40(3):329-342.
15. Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop. 2011;469(2):503-507.
16. Barton C, Kim PR. Complications of the direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):371-375.
17. Bender B, Nogler M, Hozack WJ. Direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):321-328.
18. Pelosi MA 2nd, Pelosi MA 3rd. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96(5, pt 1):775-778.
19. Lee P, Waxman K, Taylor B, Yim S. Use of wound-protection system and postoperative wound-infection rates in open appendectomy: a randomized prospective trial. Arch Surg. 2009;144(9):872-875.
20. Horiuchi T, Tanishima H, Tamagawa K, et al. Randomized, controlled investigation of the anti-infective properties of the Alexis retractor/protector of incision sites. J Trauma. 2007;62(1):212-215.
21. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
22. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
23. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostaticagent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
24. Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE. Development and validation of a novel scar evaluation scale. Plast Reconstr Surg. 2007;120(7):1892-1897.
25. Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954-1961.
26. Durani P, McGrouther DA, Ferguson MW. Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg. 2009;62(6):713-720.
27. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
28. Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118(4):909-918.
29. Quinn JV, Wells GA. An assessment of clinical wound evaluation scales. Acad Emerg Med. 1998;5(6):583-586.
30. Livesey C, Wylde V, Descamps S, et al. Skin closure after total hip replacement: a randomised controlled trial of skin adhesive versus surgical staples. J Bone Joint Surg Br. 2009;91(6):725-729.
31. Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31(5):468-492.
32. Mow CS, Woolson ST, Ngarmukos SG, Park EH, Lorenz HP. Comparison of scars from total hip replacements done with a standard or a mini-incision. Clin Orthop. 2005;(441):80-85.
33. Khan RJ, Fick D, Yao F, et al. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. J Bone Joint Surg Br. 2006;88(2):238-242.
34. Goldstein WM, Ali R, Branson JJ, Berland KA. Comparison of patient satisfaction with incision cosmesis after standard and minimally invasive total hip arthroplasty. Orthopedics. 2008;31(4):368.
35. Quinn J, Wells G, Sutcliffe T, et al. Tissue adhesive versus suture wound repair at 1 year: randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann Emerg Med. 1998;32(6):645-649.
36. Alberti LR, Petroianu A, Zac RI, Andrade JC Jr. The effect of surgical procedures on serum albumin concentration. Chirurgia (Bucur). 2008;103(1):39-43.
37. Berend KR, Lombardi AV Jr, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(suppl 6):107-120.
38. Mutnal A, Patel P, Cardona L, Suarez J. Periprosthetic Propionibacterium granulosum joint infection after direct anterior total hip arthroplasty: a case report. JBJS Case Connector. 2011;1(2):e10.
39. Alvarez AM, Suarez JC, Patel P, Benton EG. Fluoroscopic imaging of acetabular cup position during THA through a direct anterior approach. Orthopedics. 2013;36(10):776-777. Erratum in: Orthopedics. 2014;37(1):16.
1. Laffosse JM, Chiron P, Tricoire JL, Giordano G, Molinier F, Puget J. Prospective and comparative study of minimally invasive posterior approach versus standard posterior approach in total hip replacement [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(3):228-237.
2. Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop. 2011;35(2):173-184.
3. Wright JM, Crockett HC, Delgado S, Lyman S, Madsen M, Sculco TP. Mini-incision for total hip arthroplasty: a prospective, controlled investigation with 5-year follow-up evaluation. J Arthroplasty. 2004;19(5):538-545.
4. Mardones R, Pagnano MW, Nemanich JP, Trousdale RT. The Frank Stinchfield Award: muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop. 2005;(441):63-67.
5. Müller M, Tohtz S, Dewey M, Springer I, Perka C. Age-related appearance of muscle trauma in primary total hip arthroplasty and the benefit of a minimally invasive approach for patients older than 70 years. Int Orthop. 2011;35(2):165-171.
6. Noble PC, Johnston JD, Alexander JA, et al. Making minimally invasive THR safe: conclusions from biomechanical simulation and analysis. Int Orthop. 2007;31(suppl 1):S25-S28.
7. Bremer AK, Kalberer F, Pfirrmann CW, Dora C. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93(7):886-889.
8. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
9. Rachbauer F, Kain MSH, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320.
10. Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392-1398.
11. Mayr E, Nogler M, Benedetti MG, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech. 2009;24(10):812-818.
12. Meneghini RM, Pagnano MW, Trousdale RT, Hozack WJ. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop. 2006;(453):293-298.
13. Sculco TP. Anterior approach in THA improves outcomes: opposes. Orthopedics. 2011;34(9):e459-e461.
14. Bhandari M, Matta JM, Dodgin D, et al; Anterior Total Hip Arthroplasty Collaborative Investigators. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am. 2009;40(3):329-342.
15. Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop. 2011;469(2):503-507.
16. Barton C, Kim PR. Complications of the direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):371-375.
17. Bender B, Nogler M, Hozack WJ. Direct anterior approach for total hip arthroplasty. Orthop Clin North Am. 2009;40(3):321-328.
18. Pelosi MA 2nd, Pelosi MA 3rd. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96(5, pt 1):775-778.
19. Lee P, Waxman K, Taylor B, Yim S. Use of wound-protection system and postoperative wound-infection rates in open appendectomy: a randomized prospective trial. Arch Surg. 2009;144(9):872-875.
20. Horiuchi T, Tanishima H, Tamagawa K, et al. Randomized, controlled investigation of the anti-infective properties of the Alexis retractor/protector of incision sites. J Trauma. 2007;62(1):212-215.
21. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
22. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
23. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostaticagent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
24. Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE. Development and validation of a novel scar evaluation scale. Plast Reconstr Surg. 2007;120(7):1892-1897.
25. Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954-1961.
26. Durani P, McGrouther DA, Ferguson MW. Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg. 2009;62(6):713-720.
27. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
28. Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118(4):909-918.
29. Quinn JV, Wells GA. An assessment of clinical wound evaluation scales. Acad Emerg Med. 1998;5(6):583-586.
30. Livesey C, Wylde V, Descamps S, et al. Skin closure after total hip replacement: a randomised controlled trial of skin adhesive versus surgical staples. J Bone Joint Surg Br. 2009;91(6):725-729.
31. Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31(5):468-492.
32. Mow CS, Woolson ST, Ngarmukos SG, Park EH, Lorenz HP. Comparison of scars from total hip replacements done with a standard or a mini-incision. Clin Orthop. 2005;(441):80-85.
33. Khan RJ, Fick D, Yao F, et al. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. J Bone Joint Surg Br. 2006;88(2):238-242.
34. Goldstein WM, Ali R, Branson JJ, Berland KA. Comparison of patient satisfaction with incision cosmesis after standard and minimally invasive total hip arthroplasty. Orthopedics. 2008;31(4):368.
35. Quinn J, Wells G, Sutcliffe T, et al. Tissue adhesive versus suture wound repair at 1 year: randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann Emerg Med. 1998;32(6):645-649.
36. Alberti LR, Petroianu A, Zac RI, Andrade JC Jr. The effect of surgical procedures on serum albumin concentration. Chirurgia (Bucur). 2008;103(1):39-43.
37. Berend KR, Lombardi AV Jr, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(suppl 6):107-120.
38. Mutnal A, Patel P, Cardona L, Suarez J. Periprosthetic Propionibacterium granulosum joint infection after direct anterior total hip arthroplasty: a case report. JBJS Case Connector. 2011;1(2):e10.
39. Alvarez AM, Suarez JC, Patel P, Benton EG. Fluoroscopic imaging of acetabular cup position during THA through a direct anterior approach. Orthopedics. 2013;36(10):776-777. Erratum in: Orthopedics. 2014;37(1):16.