User login
Blood Conservation Program Successful
COLORADO SPRINGS – Implementation of a comprehensive blood conservation algorithm in a community hospital cardiac surgery program led to a 41% reduction in total blood product usage with no adverse impact on safety, a study has shown.
The blood conservation strategy included lowering the postoperative hemoglobin threshold for transfusion to less than 7 g/dL, utilizing a miniature low prime perfusion circuit in patients on cardiopulmonary bypass, intraoperative point-of-care testing to avoid inappropriate RBC and component transfusion, and routine blood withdrawal and storage before bypass and transfusion after protamine administration, Dr. Steve Xydas explained at the annual meeting of the Western Thoracic Surgical Association.
Blood transfusions in patients undergoing cardiac surgery use 15%-20% of the nation’s blood supply.
At present the indications for transfusion aren’t standardized, and there is wide disparity in transfusion rates among cardiac surgery patients, noted Dr. Xydas of Morristown (N.J.) Medical Center (formerly Morristown Memorial Hospital).
For these reasons, he and the other three cardiac surgeons at the hospital decided to push for implementation of a comprehensive blood conservation program.
They prospectively collected data on 481 patients who underwent cardiac surgery during the 6 months prior to introduction of the program. Then, following a 3-month introductory program implementation period, they collected data for 6 months on the 557 patients whose surgery was performed under the new blood transfusion strategy.
Fifty-seven percent of the 1,038 patients underwent isolated coronary artery bypass grafting, 25% had isolated valve surgery, and 18% had both.
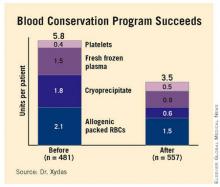
Total blood product usage (defined as the sum of blood, platelets, cryoprecipitate or fresh frozen plasma units) dropped from an average of 5.8 U per patient during the baseline period to 3.5 U per patient following introduction of the program.
Overall 30-day mortality was 1.3%, with no significant difference between the two groups. Nor were there differences in any major morbidity end points, including stroke, reoperation for bleeding, new-onset atrial fibrillation, acute MI, renal failure, or sternal wound infection. Length of stay was similar in the two groups as well.
Discussant Dr. James M. Maxwell of the International Heart Institute of Montana in Missoula observed that so much evidence has accumulated regarding the multiple harmful effects of transfusion that the current thrust is a search for meaningful evidence to support transfusion in the absence of life-threatening hemorrhage.
Because of transfusion’s harmful effects, he’d have expected to see significantly better outcomes in the group operated on under the blood conservation strategy. Dr. Maxwell attributed the lack of a significant difference in outcome between the two groups to the excellent surgical results the Morristown group obtained even before introducing the strategy.
In order to overcome the natural tendency to slide back into old habits, Dr. Xydas said he and his surgical colleagues make a point of sharing their updated results on a quarterly basis with staff cardiologists, pulmonologists, intensivists, and nurses.
Were there any barriers to implementation of the blood conservation program? he was asked. Dr. Xydas replied that this was a cardiac surgeon–led project; he and his surgical colleagues laid the groundwork by leading grand rounds for nonsurgeons in order to convince them the program was a good idea. There was occasional early resistance based on a physician’s anecdotal experience – for example, having encountered a single case of ischemic optic neuritis several decades earlier – but such reservations were easily overcome by presenting hard data on the harmful effects of transfusion, he said.
Dr. Xydas said he had no relevant financial disclosures.
COLORADO SPRINGS – Implementation of a comprehensive blood conservation algorithm in a community hospital cardiac surgery program led to a 41% reduction in total blood product usage with no adverse impact on safety, a study has shown.
The blood conservation strategy included lowering the postoperative hemoglobin threshold for transfusion to less than 7 g/dL, utilizing a miniature low prime perfusion circuit in patients on cardiopulmonary bypass, intraoperative point-of-care testing to avoid inappropriate RBC and component transfusion, and routine blood withdrawal and storage before bypass and transfusion after protamine administration, Dr. Steve Xydas explained at the annual meeting of the Western Thoracic Surgical Association.
Blood transfusions in patients undergoing cardiac surgery use 15%-20% of the nation’s blood supply.
At present the indications for transfusion aren’t standardized, and there is wide disparity in transfusion rates among cardiac surgery patients, noted Dr. Xydas of Morristown (N.J.) Medical Center (formerly Morristown Memorial Hospital).
For these reasons, he and the other three cardiac surgeons at the hospital decided to push for implementation of a comprehensive blood conservation program.
They prospectively collected data on 481 patients who underwent cardiac surgery during the 6 months prior to introduction of the program. Then, following a 3-month introductory program implementation period, they collected data for 6 months on the 557 patients whose surgery was performed under the new blood transfusion strategy.
Fifty-seven percent of the 1,038 patients underwent isolated coronary artery bypass grafting, 25% had isolated valve surgery, and 18% had both.
Total blood product usage (defined as the sum of blood, platelets, cryoprecipitate or fresh frozen plasma units) dropped from an average of 5.8 U per patient during the baseline period to 3.5 U per patient following introduction of the program.
Overall 30-day mortality was 1.3%, with no significant difference between the two groups. Nor were there differences in any major morbidity end points, including stroke, reoperation for bleeding, new-onset atrial fibrillation, acute MI, renal failure, or sternal wound infection. Length of stay was similar in the two groups as well.
Discussant Dr. James M. Maxwell of the International Heart Institute of Montana in Missoula observed that so much evidence has accumulated regarding the multiple harmful effects of transfusion that the current thrust is a search for meaningful evidence to support transfusion in the absence of life-threatening hemorrhage.
Because of transfusion’s harmful effects, he’d have expected to see significantly better outcomes in the group operated on under the blood conservation strategy. Dr. Maxwell attributed the lack of a significant difference in outcome between the two groups to the excellent surgical results the Morristown group obtained even before introducing the strategy.
In order to overcome the natural tendency to slide back into old habits, Dr. Xydas said he and his surgical colleagues make a point of sharing their updated results on a quarterly basis with staff cardiologists, pulmonologists, intensivists, and nurses.
Were there any barriers to implementation of the blood conservation program? he was asked. Dr. Xydas replied that this was a cardiac surgeon–led project; he and his surgical colleagues laid the groundwork by leading grand rounds for nonsurgeons in order to convince them the program was a good idea. There was occasional early resistance based on a physician’s anecdotal experience – for example, having encountered a single case of ischemic optic neuritis several decades earlier – but such reservations were easily overcome by presenting hard data on the harmful effects of transfusion, he said.
Dr. Xydas said he had no relevant financial disclosures.
COLORADO SPRINGS – Implementation of a comprehensive blood conservation algorithm in a community hospital cardiac surgery program led to a 41% reduction in total blood product usage with no adverse impact on safety, a study has shown.
The blood conservation strategy included lowering the postoperative hemoglobin threshold for transfusion to less than 7 g/dL, utilizing a miniature low prime perfusion circuit in patients on cardiopulmonary bypass, intraoperative point-of-care testing to avoid inappropriate RBC and component transfusion, and routine blood withdrawal and storage before bypass and transfusion after protamine administration, Dr. Steve Xydas explained at the annual meeting of the Western Thoracic Surgical Association.
Blood transfusions in patients undergoing cardiac surgery use 15%-20% of the nation’s blood supply.
At present the indications for transfusion aren’t standardized, and there is wide disparity in transfusion rates among cardiac surgery patients, noted Dr. Xydas of Morristown (N.J.) Medical Center (formerly Morristown Memorial Hospital).
For these reasons, he and the other three cardiac surgeons at the hospital decided to push for implementation of a comprehensive blood conservation program.
They prospectively collected data on 481 patients who underwent cardiac surgery during the 6 months prior to introduction of the program. Then, following a 3-month introductory program implementation period, they collected data for 6 months on the 557 patients whose surgery was performed under the new blood transfusion strategy.
Fifty-seven percent of the 1,038 patients underwent isolated coronary artery bypass grafting, 25% had isolated valve surgery, and 18% had both.
Total blood product usage (defined as the sum of blood, platelets, cryoprecipitate or fresh frozen plasma units) dropped from an average of 5.8 U per patient during the baseline period to 3.5 U per patient following introduction of the program.
Overall 30-day mortality was 1.3%, with no significant difference between the two groups. Nor were there differences in any major morbidity end points, including stroke, reoperation for bleeding, new-onset atrial fibrillation, acute MI, renal failure, or sternal wound infection. Length of stay was similar in the two groups as well.
Discussant Dr. James M. Maxwell of the International Heart Institute of Montana in Missoula observed that so much evidence has accumulated regarding the multiple harmful effects of transfusion that the current thrust is a search for meaningful evidence to support transfusion in the absence of life-threatening hemorrhage.
Because of transfusion’s harmful effects, he’d have expected to see significantly better outcomes in the group operated on under the blood conservation strategy. Dr. Maxwell attributed the lack of a significant difference in outcome between the two groups to the excellent surgical results the Morristown group obtained even before introducing the strategy.
In order to overcome the natural tendency to slide back into old habits, Dr. Xydas said he and his surgical colleagues make a point of sharing their updated results on a quarterly basis with staff cardiologists, pulmonologists, intensivists, and nurses.
Were there any barriers to implementation of the blood conservation program? he was asked. Dr. Xydas replied that this was a cardiac surgeon–led project; he and his surgical colleagues laid the groundwork by leading grand rounds for nonsurgeons in order to convince them the program was a good idea. There was occasional early resistance based on a physician’s anecdotal experience – for example, having encountered a single case of ischemic optic neuritis several decades earlier – but such reservations were easily overcome by presenting hard data on the harmful effects of transfusion, he said.
Dr. Xydas said he had no relevant financial disclosures.
Major Finding: Use of a blood conservation algorithm reduced total blood product usage from an average of 5.8 U per patient at baseline to 3.5 U per patient, without compromising safety.
Data Source: Prospective study of 1,038 patients undergoing isolated coronary artery bypass grafting.
Disclosures: Dr. Xydas said he had no relevant financial disclosures.
Rigid Plating Speeds Sternal Healing
COLORADO SPRINGS – Rigid fixation with sternal plates resulted in superior sternal bone healing post sternotomy, compared with conventional wire cerclage in a randomized trial.
This was the first-ever randomized study to utilize CT scans to objectively assess sternal union. The results at 6 months of follow-up showed a striking advantage for rigid plate fixation using the proprietary SternaLock system, Dr. Jaishankar Raman said at the annual meeting of the Western Thoracic Surgical Association.
Most cardiac surgeons will be surprised, as was he, at how low the sternal union rates were 3 months poststernotomy. In fact, the sternal union rate then with conventional wire closure was zero, while for sternal plating it was 17%, added Dr. Raman of the University of Chicago.
He reported on 141 randomized cardiac surgery patients at six centers in the United States and Germany. All were at high risk for sternal wound complications, mostly due to multiple comorbid diseases. Their multilevel CT scans obtained at 3 or 6 months post surgery were independently scored in structured fashion by two independent radiologists at a core imaging center.
At 6 months, sternal union, or osteosynthesis – as defined by a CT score of 3 or more – was achieved in 70% of the rigid plate fixation group, compared with 20% of those with conventional wire cerclage.
There were no significant differences between the two study arms in rates of wound dehiscence or other adverse events.
Asked about the cost of SternaLock fixation, Dr. Raman replied, "That’s the biggest stumbling block in developing this technology."
The wire for conventional cerclage costs less than $50 per patient. In contrast, the SternaLock system costs about $1,000 per patient. It could be argued that if rigid plate fixation reduces the incidence of sternal wound complications in high-risk patients, the advanced technology would be cost effective. However, the randomized trial wasn’t powered to show a significant difference in such complications.
Dr. Raman observed that wire cerclage has been accepted as the time-honored and most widely employed means of sternal closure ever since the modern era of cardiac surgery began back in the 1950s, even though it is a crude method.
"Even though we may not like it, we in cardiac surgery do more bone approximation and bone fixation than most other bone-handling surgeons. Yet all other bone-handling surgeons have moved on to plate and screw fixation. Biomechanical studies show that plate fixation is significantly better than wire closure," according to the surgeon.
Discussant Dr. Matthew S. Slater, clinical director of adult cardiac surgery at Oregon Health & Sciences Center, Portland, said that based on the assumption that rigid plate fixation is a better method than conventional wire cerclage, what he’d really like to see next is a comparative study pitting the SternaLock system against other novel fixation technologies on the market, such as KLS Martin’s Sternal Talon and ACUTE Innovations’ AcuTie.
Dr. Raman agreed that would be a logical next step, adding that it’s his strong impression the industry is not interested in funding comparative trials.
He declared that he received a research grant from Biomet Microfixation, which markets the SternaLock and funded the randomized trial.
COLORADO SPRINGS – Rigid fixation with sternal plates resulted in superior sternal bone healing post sternotomy, compared with conventional wire cerclage in a randomized trial.
This was the first-ever randomized study to utilize CT scans to objectively assess sternal union. The results at 6 months of follow-up showed a striking advantage for rigid plate fixation using the proprietary SternaLock system, Dr. Jaishankar Raman said at the annual meeting of the Western Thoracic Surgical Association.
Most cardiac surgeons will be surprised, as was he, at how low the sternal union rates were 3 months poststernotomy. In fact, the sternal union rate then with conventional wire closure was zero, while for sternal plating it was 17%, added Dr. Raman of the University of Chicago.
He reported on 141 randomized cardiac surgery patients at six centers in the United States and Germany. All were at high risk for sternal wound complications, mostly due to multiple comorbid diseases. Their multilevel CT scans obtained at 3 or 6 months post surgery were independently scored in structured fashion by two independent radiologists at a core imaging center.
At 6 months, sternal union, or osteosynthesis – as defined by a CT score of 3 or more – was achieved in 70% of the rigid plate fixation group, compared with 20% of those with conventional wire cerclage.
There were no significant differences between the two study arms in rates of wound dehiscence or other adverse events.
Asked about the cost of SternaLock fixation, Dr. Raman replied, "That’s the biggest stumbling block in developing this technology."
The wire for conventional cerclage costs less than $50 per patient. In contrast, the SternaLock system costs about $1,000 per patient. It could be argued that if rigid plate fixation reduces the incidence of sternal wound complications in high-risk patients, the advanced technology would be cost effective. However, the randomized trial wasn’t powered to show a significant difference in such complications.
Dr. Raman observed that wire cerclage has been accepted as the time-honored and most widely employed means of sternal closure ever since the modern era of cardiac surgery began back in the 1950s, even though it is a crude method.
"Even though we may not like it, we in cardiac surgery do more bone approximation and bone fixation than most other bone-handling surgeons. Yet all other bone-handling surgeons have moved on to plate and screw fixation. Biomechanical studies show that plate fixation is significantly better than wire closure," according to the surgeon.
Discussant Dr. Matthew S. Slater, clinical director of adult cardiac surgery at Oregon Health & Sciences Center, Portland, said that based on the assumption that rigid plate fixation is a better method than conventional wire cerclage, what he’d really like to see next is a comparative study pitting the SternaLock system against other novel fixation technologies on the market, such as KLS Martin’s Sternal Talon and ACUTE Innovations’ AcuTie.
Dr. Raman agreed that would be a logical next step, adding that it’s his strong impression the industry is not interested in funding comparative trials.
He declared that he received a research grant from Biomet Microfixation, which markets the SternaLock and funded the randomized trial.
COLORADO SPRINGS – Rigid fixation with sternal plates resulted in superior sternal bone healing post sternotomy, compared with conventional wire cerclage in a randomized trial.
This was the first-ever randomized study to utilize CT scans to objectively assess sternal union. The results at 6 months of follow-up showed a striking advantage for rigid plate fixation using the proprietary SternaLock system, Dr. Jaishankar Raman said at the annual meeting of the Western Thoracic Surgical Association.
Most cardiac surgeons will be surprised, as was he, at how low the sternal union rates were 3 months poststernotomy. In fact, the sternal union rate then with conventional wire closure was zero, while for sternal plating it was 17%, added Dr. Raman of the University of Chicago.
He reported on 141 randomized cardiac surgery patients at six centers in the United States and Germany. All were at high risk for sternal wound complications, mostly due to multiple comorbid diseases. Their multilevel CT scans obtained at 3 or 6 months post surgery were independently scored in structured fashion by two independent radiologists at a core imaging center.
At 6 months, sternal union, or osteosynthesis – as defined by a CT score of 3 or more – was achieved in 70% of the rigid plate fixation group, compared with 20% of those with conventional wire cerclage.
There were no significant differences between the two study arms in rates of wound dehiscence or other adverse events.
Asked about the cost of SternaLock fixation, Dr. Raman replied, "That’s the biggest stumbling block in developing this technology."
The wire for conventional cerclage costs less than $50 per patient. In contrast, the SternaLock system costs about $1,000 per patient. It could be argued that if rigid plate fixation reduces the incidence of sternal wound complications in high-risk patients, the advanced technology would be cost effective. However, the randomized trial wasn’t powered to show a significant difference in such complications.
Dr. Raman observed that wire cerclage has been accepted as the time-honored and most widely employed means of sternal closure ever since the modern era of cardiac surgery began back in the 1950s, even though it is a crude method.
"Even though we may not like it, we in cardiac surgery do more bone approximation and bone fixation than most other bone-handling surgeons. Yet all other bone-handling surgeons have moved on to plate and screw fixation. Biomechanical studies show that plate fixation is significantly better than wire closure," according to the surgeon.
Discussant Dr. Matthew S. Slater, clinical director of adult cardiac surgery at Oregon Health & Sciences Center, Portland, said that based on the assumption that rigid plate fixation is a better method than conventional wire cerclage, what he’d really like to see next is a comparative study pitting the SternaLock system against other novel fixation technologies on the market, such as KLS Martin’s Sternal Talon and ACUTE Innovations’ AcuTie.
Dr. Raman agreed that would be a logical next step, adding that it’s his strong impression the industry is not interested in funding comparative trials.
He declared that he received a research grant from Biomet Microfixation, which markets the SternaLock and funded the randomized trial.
Major Finding: At 6 months, sternal union, or osteosynthesis – as defined by a CT score of 3 or more – was achieved in 70% of the rigid plate fixation group, compared with 20% who had conventional wire cerclage.
Data Source: A 141-patient multicenter randomized trial.
Disclosures: Dr. Raman received a research grant from Biomet Microfixation, which markets the SternaLock and funded the randomized trial.
Aggressive Nonmelanoma Skin Cancers Often Misdiagnosed
LISBON – Two of 10 nonmelanoma skin cancers are misdiagnosed as being of a nonaggressive tumor subtype at initial biopsy, according to Dr. Nathalie Zeitouni.
This raises concern that a substantial number of biopsied squamous and basal cell carcinomas are being treated suboptimally, Dr. Zeitouni said at the congress.
She presented a consecutive series of 513 patients referred for Mohs micrographic surgery for biopsy-proven BCC or SCC. Based upon routine Mohs intraoperative evaluation of all histologic tumor layers, 21.1% of the cancers were of aggressive subtypes that went undiagnosed on initial biopsy.
Aggressive subtypes of nonmelanoma skin cancer include basosquamous carcinoma, invasive SCC, and morpheaform, infiltrating, keratinizing, and micronodular BCC. Nonaggressive subtypes include follicular, nodular, adenoid cystic, and superficial BCC, as well as SCC in situ, according to Dr. Zeitouni, chief of dermatologic surgery at the Roswell Park Cancer Institute, Buffalo, N.Y.
In only 51% of cases was there concordance between the preoperative and the definitive intraoperative diagnosis of a nonmelanoma skin cancer as being of an aggressive or nonaggressive subtype.
In 21% of cases the intraoperative evaluation showed no residual tumor present, only scar. In 5.5% of cases, intraoperative histologic tumor layer evaluation resulted in downgrading of the nonmelanoma skin cancer from an aggressive to a nonaggressive subtype.
Dr. Zeitouni stressed that dermatologists need to have a low threshold for suspecting that a nonmelanoma skin cancer is of an undiagnosed aggressive subtype. If the lesion is clinically atypical or it responds poorly to standard excision or simple destructive measures, that possibility becomes distinctly more likely. Aggressive subtypes, she added, are best managed by Mohs surgery.
She said she had no relevant financial disclosures.
LISBON – Two of 10 nonmelanoma skin cancers are misdiagnosed as being of a nonaggressive tumor subtype at initial biopsy, according to Dr. Nathalie Zeitouni.
This raises concern that a substantial number of biopsied squamous and basal cell carcinomas are being treated suboptimally, Dr. Zeitouni said at the congress.
She presented a consecutive series of 513 patients referred for Mohs micrographic surgery for biopsy-proven BCC or SCC. Based upon routine Mohs intraoperative evaluation of all histologic tumor layers, 21.1% of the cancers were of aggressive subtypes that went undiagnosed on initial biopsy.
Aggressive subtypes of nonmelanoma skin cancer include basosquamous carcinoma, invasive SCC, and morpheaform, infiltrating, keratinizing, and micronodular BCC. Nonaggressive subtypes include follicular, nodular, adenoid cystic, and superficial BCC, as well as SCC in situ, according to Dr. Zeitouni, chief of dermatologic surgery at the Roswell Park Cancer Institute, Buffalo, N.Y.
In only 51% of cases was there concordance between the preoperative and the definitive intraoperative diagnosis of a nonmelanoma skin cancer as being of an aggressive or nonaggressive subtype.
In 21% of cases the intraoperative evaluation showed no residual tumor present, only scar. In 5.5% of cases, intraoperative histologic tumor layer evaluation resulted in downgrading of the nonmelanoma skin cancer from an aggressive to a nonaggressive subtype.
Dr. Zeitouni stressed that dermatologists need to have a low threshold for suspecting that a nonmelanoma skin cancer is of an undiagnosed aggressive subtype. If the lesion is clinically atypical or it responds poorly to standard excision or simple destructive measures, that possibility becomes distinctly more likely. Aggressive subtypes, she added, are best managed by Mohs surgery.
She said she had no relevant financial disclosures.
LISBON – Two of 10 nonmelanoma skin cancers are misdiagnosed as being of a nonaggressive tumor subtype at initial biopsy, according to Dr. Nathalie Zeitouni.
This raises concern that a substantial number of biopsied squamous and basal cell carcinomas are being treated suboptimally, Dr. Zeitouni said at the congress.
She presented a consecutive series of 513 patients referred for Mohs micrographic surgery for biopsy-proven BCC or SCC. Based upon routine Mohs intraoperative evaluation of all histologic tumor layers, 21.1% of the cancers were of aggressive subtypes that went undiagnosed on initial biopsy.
Aggressive subtypes of nonmelanoma skin cancer include basosquamous carcinoma, invasive SCC, and morpheaform, infiltrating, keratinizing, and micronodular BCC. Nonaggressive subtypes include follicular, nodular, adenoid cystic, and superficial BCC, as well as SCC in situ, according to Dr. Zeitouni, chief of dermatologic surgery at the Roswell Park Cancer Institute, Buffalo, N.Y.
In only 51% of cases was there concordance between the preoperative and the definitive intraoperative diagnosis of a nonmelanoma skin cancer as being of an aggressive or nonaggressive subtype.
In 21% of cases the intraoperative evaluation showed no residual tumor present, only scar. In 5.5% of cases, intraoperative histologic tumor layer evaluation resulted in downgrading of the nonmelanoma skin cancer from an aggressive to a nonaggressive subtype.
Dr. Zeitouni stressed that dermatologists need to have a low threshold for suspecting that a nonmelanoma skin cancer is of an undiagnosed aggressive subtype. If the lesion is clinically atypical or it responds poorly to standard excision or simple destructive measures, that possibility becomes distinctly more likely. Aggressive subtypes, she added, are best managed by Mohs surgery.
She said she had no relevant financial disclosures.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: In only 51% of cases was there concordance between the preoperative and the definitive intraoperative diagnosis of a nonmelanoma skin cancer as being of an aggressive or nonaggressive subtype.
Data Source: A retrospective analysis of 513 consecutive patients with biopsy-proven basal or squamous cell carcinoma treated with Mohs micrographic surgery.
Disclosures: Dr. Zeitouni reported having no relevant financial disclosures.
Atopy May Protect Against Skin Cancer
LISBON – In the latest installment in an ongoing debate, dermatologist Thomas Kornek reported that atopic dermatitis was found to provide protection against skin cancer in patients in a large German study.
Standardized, onsite, dermatologist-conducted skin screenings of 90,880 employees in more than 300 German businesses demonstrated that the prevalence of nonmelanoma skin cancer among the 1.3% of workers with atopic dermatitis was less than half of that in coworkers without atopic dermatitis, Dr. Kornek reported at the congress.
Moreover, even though the subgroup with atopic dermatitis had significantly higher rates of well-established risk factors for melanoma, their actual prevalence of the malignancy was identical to that in workers without atopic dermatitis, implying a protective effect against melanoma as well, added Dr. Kornek of the University Medical Center Hamburg, Germany.
Participants in the workplace skin screening program averaged 43 years of age, and 53% were men. The prevalence of clinically diagnosed nonmelanoma skin cancer among patients with atopic dermatitis was 0.4%, compared with 0.9% in participants without atopy (P less than .05).
Premalignant skin lesions were identified in 1.7% of workers with atopic dermatitis and 2.1% of controls, a nonsignificant difference.
Melanomas were detected in 0.2% of participants with or without atopic dermatitis. Yet the prevalences of Fitzpatrick skin type I, more than 40 nevi, and a history of severe sunburns in childhood – all of which are risk factors for melanoma – were significantly greater in the atopic dermatitis subgroup, which in theory should have translated into more melanomas, said Dr. Kornek.
Two warring schools of thought have clashed with regard to the relationship between atopic dermatitis and cancer. One holds that the hyperreactive immune system that defines atopy in the form of asthma, hay fever, or atopic dermatitis ought to protect against carcinoma. The other school holds that the chronic immune stimulation present in individuals with atopic dermatitis ought to result in elevated risk. An additional consideration in patients with atopic dermatitis is that local and systemic immunotherapy could potentially boost the risk of developing skin cancer, noted Dr. Kornek.
Each side can point to supporting epidemiologic studies. For example, researchers in Sweden found that the risk of developing melanoma in 6,280 atopic dermatitis patients followed for more than 230,000 person-years was half that in the general Swedish population (J. Eur. Acad. Dermatol. Venereol. 2008;22:1423-8).
Dermatologists in Stockholm used the Swedish Cancer Registry to determine that a large cohort of atopic patients had no increased risk of nonmelanoma skin cancer, lymphoma, or cancer of the lung, pancreas, or cervix (Allergy 2005;60:1116-20).
Another group of investigators in Sweden reported modest but significantly increased risks of cancer of the pancreas, esophagus, lung, and brain, as well as lymphoma, in 15,666 patients earlier hospitalized for atopic dermatitis. There was a nonsignificant trend for more nonmelanoma skin cancers in the group, but no increase in melanoma (Arch. Dermatol. 2005;141:1123-7).
A literature review by dermatologists in Germany found mixed results regarding a possible association between atopy and cancer, although the investigators summed up the findings by noting that "the emerging picture from most of the currently available epidemiological data indicates that atopic disease is associated with a reduced risk of cancer" (Allergy 2005;60:1098-11).
Dr. Kornek speculated that one possible explanation for the reduced risk of skin cancer noted in the German workplace study is because individuals with atopic dermatitis are more aware of their skin and more alert to the rise of abnormal lesions than nonatopic persons.
He declared having no financial conflicts of interest.
LISBON – In the latest installment in an ongoing debate, dermatologist Thomas Kornek reported that atopic dermatitis was found to provide protection against skin cancer in patients in a large German study.
Standardized, onsite, dermatologist-conducted skin screenings of 90,880 employees in more than 300 German businesses demonstrated that the prevalence of nonmelanoma skin cancer among the 1.3% of workers with atopic dermatitis was less than half of that in coworkers without atopic dermatitis, Dr. Kornek reported at the congress.
Moreover, even though the subgroup with atopic dermatitis had significantly higher rates of well-established risk factors for melanoma, their actual prevalence of the malignancy was identical to that in workers without atopic dermatitis, implying a protective effect against melanoma as well, added Dr. Kornek of the University Medical Center Hamburg, Germany.
Participants in the workplace skin screening program averaged 43 years of age, and 53% were men. The prevalence of clinically diagnosed nonmelanoma skin cancer among patients with atopic dermatitis was 0.4%, compared with 0.9% in participants without atopy (P less than .05).
Premalignant skin lesions were identified in 1.7% of workers with atopic dermatitis and 2.1% of controls, a nonsignificant difference.
Melanomas were detected in 0.2% of participants with or without atopic dermatitis. Yet the prevalences of Fitzpatrick skin type I, more than 40 nevi, and a history of severe sunburns in childhood – all of which are risk factors for melanoma – were significantly greater in the atopic dermatitis subgroup, which in theory should have translated into more melanomas, said Dr. Kornek.
Two warring schools of thought have clashed with regard to the relationship between atopic dermatitis and cancer. One holds that the hyperreactive immune system that defines atopy in the form of asthma, hay fever, or atopic dermatitis ought to protect against carcinoma. The other school holds that the chronic immune stimulation present in individuals with atopic dermatitis ought to result in elevated risk. An additional consideration in patients with atopic dermatitis is that local and systemic immunotherapy could potentially boost the risk of developing skin cancer, noted Dr. Kornek.
Each side can point to supporting epidemiologic studies. For example, researchers in Sweden found that the risk of developing melanoma in 6,280 atopic dermatitis patients followed for more than 230,000 person-years was half that in the general Swedish population (J. Eur. Acad. Dermatol. Venereol. 2008;22:1423-8).
Dermatologists in Stockholm used the Swedish Cancer Registry to determine that a large cohort of atopic patients had no increased risk of nonmelanoma skin cancer, lymphoma, or cancer of the lung, pancreas, or cervix (Allergy 2005;60:1116-20).
Another group of investigators in Sweden reported modest but significantly increased risks of cancer of the pancreas, esophagus, lung, and brain, as well as lymphoma, in 15,666 patients earlier hospitalized for atopic dermatitis. There was a nonsignificant trend for more nonmelanoma skin cancers in the group, but no increase in melanoma (Arch. Dermatol. 2005;141:1123-7).
A literature review by dermatologists in Germany found mixed results regarding a possible association between atopy and cancer, although the investigators summed up the findings by noting that "the emerging picture from most of the currently available epidemiological data indicates that atopic disease is associated with a reduced risk of cancer" (Allergy 2005;60:1098-11).
Dr. Kornek speculated that one possible explanation for the reduced risk of skin cancer noted in the German workplace study is because individuals with atopic dermatitis are more aware of their skin and more alert to the rise of abnormal lesions than nonatopic persons.
He declared having no financial conflicts of interest.
LISBON – In the latest installment in an ongoing debate, dermatologist Thomas Kornek reported that atopic dermatitis was found to provide protection against skin cancer in patients in a large German study.
Standardized, onsite, dermatologist-conducted skin screenings of 90,880 employees in more than 300 German businesses demonstrated that the prevalence of nonmelanoma skin cancer among the 1.3% of workers with atopic dermatitis was less than half of that in coworkers without atopic dermatitis, Dr. Kornek reported at the congress.
Moreover, even though the subgroup with atopic dermatitis had significantly higher rates of well-established risk factors for melanoma, their actual prevalence of the malignancy was identical to that in workers without atopic dermatitis, implying a protective effect against melanoma as well, added Dr. Kornek of the University Medical Center Hamburg, Germany.
Participants in the workplace skin screening program averaged 43 years of age, and 53% were men. The prevalence of clinically diagnosed nonmelanoma skin cancer among patients with atopic dermatitis was 0.4%, compared with 0.9% in participants without atopy (P less than .05).
Premalignant skin lesions were identified in 1.7% of workers with atopic dermatitis and 2.1% of controls, a nonsignificant difference.
Melanomas were detected in 0.2% of participants with or without atopic dermatitis. Yet the prevalences of Fitzpatrick skin type I, more than 40 nevi, and a history of severe sunburns in childhood – all of which are risk factors for melanoma – were significantly greater in the atopic dermatitis subgroup, which in theory should have translated into more melanomas, said Dr. Kornek.
Two warring schools of thought have clashed with regard to the relationship between atopic dermatitis and cancer. One holds that the hyperreactive immune system that defines atopy in the form of asthma, hay fever, or atopic dermatitis ought to protect against carcinoma. The other school holds that the chronic immune stimulation present in individuals with atopic dermatitis ought to result in elevated risk. An additional consideration in patients with atopic dermatitis is that local and systemic immunotherapy could potentially boost the risk of developing skin cancer, noted Dr. Kornek.
Each side can point to supporting epidemiologic studies. For example, researchers in Sweden found that the risk of developing melanoma in 6,280 atopic dermatitis patients followed for more than 230,000 person-years was half that in the general Swedish population (J. Eur. Acad. Dermatol. Venereol. 2008;22:1423-8).
Dermatologists in Stockholm used the Swedish Cancer Registry to determine that a large cohort of atopic patients had no increased risk of nonmelanoma skin cancer, lymphoma, or cancer of the lung, pancreas, or cervix (Allergy 2005;60:1116-20).
Another group of investigators in Sweden reported modest but significantly increased risks of cancer of the pancreas, esophagus, lung, and brain, as well as lymphoma, in 15,666 patients earlier hospitalized for atopic dermatitis. There was a nonsignificant trend for more nonmelanoma skin cancers in the group, but no increase in melanoma (Arch. Dermatol. 2005;141:1123-7).
A literature review by dermatologists in Germany found mixed results regarding a possible association between atopy and cancer, although the investigators summed up the findings by noting that "the emerging picture from most of the currently available epidemiological data indicates that atopic disease is associated with a reduced risk of cancer" (Allergy 2005;60:1098-11).
Dr. Kornek speculated that one possible explanation for the reduced risk of skin cancer noted in the German workplace study is because individuals with atopic dermatitis are more aware of their skin and more alert to the rise of abnormal lesions than nonatopic persons.
He declared having no financial conflicts of interest.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: German workers with atopic dermatitis had a 56% lower prevalence of nonmelanoma skin cancer than their coworkers without atopy.
Data Source: A dermatologist-conducted skin screening program involving nearly 91,000 employees at more than 300 German companies.
Disclosures: Dr. Kornek had no conflicts of interest.
Adding Methotrexate to Etanercept Boosts Psoriasis Efficacy
LISBON – Adding methotrexate to etanercept in patients with moderate to severe plaque psoriasis brings a bump in efficacy with no increase in serious adverse events, according to a large, double-blind, randomized clinical trial.
The multicenter trial included 478 patients on etanercept (Enbrel) at 50 mg twice weekly for 12 weeks, followed by once-weekly treatment thereafter. Participants were randomized to methotrexate or placebo from the outset. Methotrexate was dosed at 7.5 mg/week for the first 2 weeks, 10 mg/week for the next 2 weeks, and 15 mg/week after that.
The primary study end point was the proportion of patients attaining a PASI 75 response – that is, at least a 75% improvement over baseline scores on the Psoriasis Area and Severity Index – at week 24. The PASI 75 rate was 77.3% with dual therapy, significantly better than the 60.3% rate with etanercept alone, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
These results with combination therapy are in line with the enhanced efficacy seen with ustekinumab (Stelara) and other biologics targeting interleukin-23/Th17. But the combination therapy relies upon familiar medications that have been around a long time and have well-understood safety profiles, she noted.
The 24-week PASI 90 rate was also markedly better with dual therapy: 53.8%, compared with 34.2%. Seventy-two percent of patients on methotrexate plus etanercept were rated clear or almost clear on Physician’s Global Assessment, as were 53% on etanercept alone, according to Dr. Gottlieb, professor and chair of dermatology at Tufts Medical Center, Boston.
Adding methotrexate accelerated the time course of improvement. By week 12, 70% of patients on methotrexate plus etanercept had achieved a PASI 75 response, compared with 54% of those on etanercept alone.
Three serious adverse events occurred in each study arm. In the dual-therapy arm, there was one case each of bacterial pneumonia, lumbar spinal stenosis, and synovial cyst. In the etanercept-only arm, there was a case of acute MI, cholecystitis, and asthma.
Dr. Gottlieb disclosed that she serves as a consultant to Amgen, which sponsored the trial. In addition, she is a consultant and/or advisory board member for numerous other pharmaceutical companies.
LISBON – Adding methotrexate to etanercept in patients with moderate to severe plaque psoriasis brings a bump in efficacy with no increase in serious adverse events, according to a large, double-blind, randomized clinical trial.
The multicenter trial included 478 patients on etanercept (Enbrel) at 50 mg twice weekly for 12 weeks, followed by once-weekly treatment thereafter. Participants were randomized to methotrexate or placebo from the outset. Methotrexate was dosed at 7.5 mg/week for the first 2 weeks, 10 mg/week for the next 2 weeks, and 15 mg/week after that.
The primary study end point was the proportion of patients attaining a PASI 75 response – that is, at least a 75% improvement over baseline scores on the Psoriasis Area and Severity Index – at week 24. The PASI 75 rate was 77.3% with dual therapy, significantly better than the 60.3% rate with etanercept alone, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
These results with combination therapy are in line with the enhanced efficacy seen with ustekinumab (Stelara) and other biologics targeting interleukin-23/Th17. But the combination therapy relies upon familiar medications that have been around a long time and have well-understood safety profiles, she noted.
The 24-week PASI 90 rate was also markedly better with dual therapy: 53.8%, compared with 34.2%. Seventy-two percent of patients on methotrexate plus etanercept were rated clear or almost clear on Physician’s Global Assessment, as were 53% on etanercept alone, according to Dr. Gottlieb, professor and chair of dermatology at Tufts Medical Center, Boston.
Adding methotrexate accelerated the time course of improvement. By week 12, 70% of patients on methotrexate plus etanercept had achieved a PASI 75 response, compared with 54% of those on etanercept alone.
Three serious adverse events occurred in each study arm. In the dual-therapy arm, there was one case each of bacterial pneumonia, lumbar spinal stenosis, and synovial cyst. In the etanercept-only arm, there was a case of acute MI, cholecystitis, and asthma.
Dr. Gottlieb disclosed that she serves as a consultant to Amgen, which sponsored the trial. In addition, she is a consultant and/or advisory board member for numerous other pharmaceutical companies.
LISBON – Adding methotrexate to etanercept in patients with moderate to severe plaque psoriasis brings a bump in efficacy with no increase in serious adverse events, according to a large, double-blind, randomized clinical trial.
The multicenter trial included 478 patients on etanercept (Enbrel) at 50 mg twice weekly for 12 weeks, followed by once-weekly treatment thereafter. Participants were randomized to methotrexate or placebo from the outset. Methotrexate was dosed at 7.5 mg/week for the first 2 weeks, 10 mg/week for the next 2 weeks, and 15 mg/week after that.
The primary study end point was the proportion of patients attaining a PASI 75 response – that is, at least a 75% improvement over baseline scores on the Psoriasis Area and Severity Index – at week 24. The PASI 75 rate was 77.3% with dual therapy, significantly better than the 60.3% rate with etanercept alone, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
These results with combination therapy are in line with the enhanced efficacy seen with ustekinumab (Stelara) and other biologics targeting interleukin-23/Th17. But the combination therapy relies upon familiar medications that have been around a long time and have well-understood safety profiles, she noted.
The 24-week PASI 90 rate was also markedly better with dual therapy: 53.8%, compared with 34.2%. Seventy-two percent of patients on methotrexate plus etanercept were rated clear or almost clear on Physician’s Global Assessment, as were 53% on etanercept alone, according to Dr. Gottlieb, professor and chair of dermatology at Tufts Medical Center, Boston.
Adding methotrexate accelerated the time course of improvement. By week 12, 70% of patients on methotrexate plus etanercept had achieved a PASI 75 response, compared with 54% of those on etanercept alone.
Three serious adverse events occurred in each study arm. In the dual-therapy arm, there was one case each of bacterial pneumonia, lumbar spinal stenosis, and synovial cyst. In the etanercept-only arm, there was a case of acute MI, cholecystitis, and asthma.
Dr. Gottlieb disclosed that she serves as a consultant to Amgen, which sponsored the trial. In addition, she is a consultant and/or advisory board member for numerous other pharmaceutical companies.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Adding methotrexate to etanercept resulted in a 77% PASI 75 response rate at 24 weeks, compared with 60% with etanercept alone.
Data Source: A double-blind, randomized, placebo-controlled clinical trial involving 478 patients with moderate to severe plaque psoriasis.
Disclosures: Dr. Gottlieb disclosed that she serves as a consultant to Amgen, which sponsored the trial. In addition, she is a consultant and/or advisory board member for numerous other pharmaceutical companies.
Overweight Female Teens More Likely to Have Acne
LISBON – Overweight or obesity is independently associated with a twofold increased prevalence of moderate to severe acne in adolescent girls, according to a large population-based Norwegian study.
No such association was apparent in adolescent boys, Dr. Jon A. Halvorsen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional questionnaire study of 3,774 Oslo teenagers aged 18-19 years. He and his coworkers undertook the study because the relationship between acne and body mass index has seldom been examined. The only prior study addressing the issue dates back to 1956; it found no association between acne and overweight in a group of young male soldiers (Br. Med. J. 1956;1:1268-70).
In the new Oslo study, overweight or obesity marked by a body mass index (BMI) of at least 25 kg/m2 was present in 9.5% of girls and 15.4% of boys. Self-reported moderate or severe acne, as defined using validated criteria, was present in 13.1% of girls and 14% of boys.
In the multivariate logistic regression analysis adjusted for mental health issues, sociodemographic factors, and diet, the prevalence of moderate or severe acne in overweight or obese girls was 18.5%, compared with less than 12% in the normal-weight comparison group of girls with a BMI of 18.5 to less than 23, reported Dr. Halvorsen of the University of Oslo. Girls with a BMI of 23 to less than 25 were 30% more likely than the comparison group to have significant acne, a trend that didn’t reach significance.
The situation was quite different in boys. The highest prevalence of moderate or severe acne (18%) occurred in underweight boys having a BMI less than 18.5. Normal-weight boys had less than a 12% prevalence of significant acne, while in overweight or obese boys the prevalence was slightly greater than 13%.
Dr. Halvorsen stressed that since these findings come from a cross-sectional study, they don’t establish a causal relationship between acne and BMI. But the data do raise questions worthy of further investigation.
"Can overweight cause acne? How important is inflammation in the pathogenesis of acne? How important are hormonal factors in adolescent acne? What about physical activity: Is it related to acne prevalence? How important are lifestyle factors?" he asked.
A lack of hormonal measurements is an important limitation of the Norwegian study. It’s an issue because polycystic ovary syndrome is known to be a risk factor for both overweight and acne, he noted.
The dietary variables incorporated in the analysis included rates of consumption of soft drinks, fatty junk foods, and raw vegetables. An audience member observed that milk consumption has previously been linked to acne; however, milk intake wasn’t recorded in the study.
Dr. Halvorsen reported having no financial conflicts of interest.
LISBON – Overweight or obesity is independently associated with a twofold increased prevalence of moderate to severe acne in adolescent girls, according to a large population-based Norwegian study.
No such association was apparent in adolescent boys, Dr. Jon A. Halvorsen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional questionnaire study of 3,774 Oslo teenagers aged 18-19 years. He and his coworkers undertook the study because the relationship between acne and body mass index has seldom been examined. The only prior study addressing the issue dates back to 1956; it found no association between acne and overweight in a group of young male soldiers (Br. Med. J. 1956;1:1268-70).
In the new Oslo study, overweight or obesity marked by a body mass index (BMI) of at least 25 kg/m2 was present in 9.5% of girls and 15.4% of boys. Self-reported moderate or severe acne, as defined using validated criteria, was present in 13.1% of girls and 14% of boys.
In the multivariate logistic regression analysis adjusted for mental health issues, sociodemographic factors, and diet, the prevalence of moderate or severe acne in overweight or obese girls was 18.5%, compared with less than 12% in the normal-weight comparison group of girls with a BMI of 18.5 to less than 23, reported Dr. Halvorsen of the University of Oslo. Girls with a BMI of 23 to less than 25 were 30% more likely than the comparison group to have significant acne, a trend that didn’t reach significance.
The situation was quite different in boys. The highest prevalence of moderate or severe acne (18%) occurred in underweight boys having a BMI less than 18.5. Normal-weight boys had less than a 12% prevalence of significant acne, while in overweight or obese boys the prevalence was slightly greater than 13%.
Dr. Halvorsen stressed that since these findings come from a cross-sectional study, they don’t establish a causal relationship between acne and BMI. But the data do raise questions worthy of further investigation.
"Can overweight cause acne? How important is inflammation in the pathogenesis of acne? How important are hormonal factors in adolescent acne? What about physical activity: Is it related to acne prevalence? How important are lifestyle factors?" he asked.
A lack of hormonal measurements is an important limitation of the Norwegian study. It’s an issue because polycystic ovary syndrome is known to be a risk factor for both overweight and acne, he noted.
The dietary variables incorporated in the analysis included rates of consumption of soft drinks, fatty junk foods, and raw vegetables. An audience member observed that milk consumption has previously been linked to acne; however, milk intake wasn’t recorded in the study.
Dr. Halvorsen reported having no financial conflicts of interest.
LISBON – Overweight or obesity is independently associated with a twofold increased prevalence of moderate to severe acne in adolescent girls, according to a large population-based Norwegian study.
No such association was apparent in adolescent boys, Dr. Jon A. Halvorsen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional questionnaire study of 3,774 Oslo teenagers aged 18-19 years. He and his coworkers undertook the study because the relationship between acne and body mass index has seldom been examined. The only prior study addressing the issue dates back to 1956; it found no association between acne and overweight in a group of young male soldiers (Br. Med. J. 1956;1:1268-70).
In the new Oslo study, overweight or obesity marked by a body mass index (BMI) of at least 25 kg/m2 was present in 9.5% of girls and 15.4% of boys. Self-reported moderate or severe acne, as defined using validated criteria, was present in 13.1% of girls and 14% of boys.
In the multivariate logistic regression analysis adjusted for mental health issues, sociodemographic factors, and diet, the prevalence of moderate or severe acne in overweight or obese girls was 18.5%, compared with less than 12% in the normal-weight comparison group of girls with a BMI of 18.5 to less than 23, reported Dr. Halvorsen of the University of Oslo. Girls with a BMI of 23 to less than 25 were 30% more likely than the comparison group to have significant acne, a trend that didn’t reach significance.
The situation was quite different in boys. The highest prevalence of moderate or severe acne (18%) occurred in underweight boys having a BMI less than 18.5. Normal-weight boys had less than a 12% prevalence of significant acne, while in overweight or obese boys the prevalence was slightly greater than 13%.
Dr. Halvorsen stressed that since these findings come from a cross-sectional study, they don’t establish a causal relationship between acne and BMI. But the data do raise questions worthy of further investigation.
"Can overweight cause acne? How important is inflammation in the pathogenesis of acne? How important are hormonal factors in adolescent acne? What about physical activity: Is it related to acne prevalence? How important are lifestyle factors?" he asked.
A lack of hormonal measurements is an important limitation of the Norwegian study. It’s an issue because polycystic ovary syndrome is known to be a risk factor for both overweight and acne, he noted.
The dietary variables incorporated in the analysis included rates of consumption of soft drinks, fatty junk foods, and raw vegetables. An audience member observed that milk consumption has previously been linked to acne; however, milk intake wasn’t recorded in the study.
Dr. Halvorsen reported having no financial conflicts of interest.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: The prevalence of moderate or severe acne in overweight or obese girls was 18.5%, compared with less than 12% in the normal-weight comparison group of girls.
Data Source: A population-based, cross-sectional, questionnaire study of 3,774 patients aged 18-19 years.
Disclosures: Dr. Halvorsen reported having no financial conflicts of interest.
Steroid Use Ups Fractures After Renal Transplant : Early corticosteroid withdrawal regimens can reduce fracture rate.
Major Finding: Discharge of kidney transplant recipients on corticosteroid-based immunosuppression was independently associated with a 45% increased risk in major fractures over the next 4 years compared to patients managed with early corticosteroid withdrawal while still in-hospital.
Data Source: Retrospective analysis of nearly 78,000 kidney transplant recipients in the United States Renal System database.
Disclosures: Dr. Nikkel declared having no financial conflicts.
SAN DIEGO – Early corticosteroid withdrawal after kidney transplantation is associated with a marked reduction in major fracture rate, according to an analysis of the United States Renal Data System database.
Indeed, discharge on corticosteroids was independently associated with a 45% increased risk of major fractures requiring hospitalization during a median follow-up of about 4 years, Dr. Lucas Nikkel reported at the meeting.
Early corticosteroid withdrawal regimens are gaining popularity at renal transplant centers as a means of reducing a wide range of immunosuppression-related side effects. The documented major clinical advantages include reduced rates of hyperlipidemia, posttransplant diabetes, cardiovascular events, infections, and cancer. Until now, however, no study had examined whether early corticosteroid withdrawal regimens are also effective in reducing fracture incidence, noted Dr. Nikkel, who earned the ASBMR Young Investigator Award for his work.
Early corticosteroid withdrawal regimens consist of 4-7 days of high-dose methylprednisolone administered at the time of transplantation, followed by withdrawal of the drug in favor of long-term immunosuppression with calcineurin inhibitors and mycophenolic acid. More than 30% of kidney transplant recipients in the United States are now managed using such protocols.
Dr. Nikkel identified 77,625 adults in the USRDS database who received a kidney transplant during 2000-2006. Pretransplant fracture rates were similar in the 11,178 patients not on steroids at discharge and the 66,447 who were.
The incidence of fractures requiring hospitalization during the follow-up period was 1.7% in patients with early corticosteroid withdrawal, significantly less than the 3.3% for those discharged on corticosteroid-based immunosuppression. The rate was 5.8 major fractures per 1,000 patient-years in the early steroid withdrawal group, compared with 8.0 per 1,000 patient-years in those on corticosteroid-based immunosuppression. The increased fracture risk in patients discharged on steroids became apparent at 1 year of follow-up and significant at 2 years.
“It's noteworthy that patients in the youngest age group discharged on corticosteroids had a similar risk of fractures compared to the oldest patients discharged without corticosteroids,” observed Dr. Nikkel, who is with Columbia University, New York.
In addition to discharge on steroids, other fracture risk factors identified in the study included older age, female gender, white race, pretransplant diabetes, a positive fracture history, and being on dialysis.
Since most fractures are treated on an outpatient basis, it is likely that these study results greatly underestimate the true fracture burden that is associated with kidney transplantation, he added.
Dr. Nikkel pointed out that his study was a retrospective analysis of observational data and said that prospective, long-term studies are needed to confirm the results.
Each year, more than 17,000 kidney transplants are performed in the United States, and more than 68,000 worldwide. Bone loss is high in these patients, especially in the first 18 months after transplantation.
Kidney transplant recipients have a 4.5-fold greater fracture risk than do the general population and a 30% greater risk than do hemodialysis patients during the first 3 years post transplant.
Audience members said that there is evidence to suggest calcineurin inhibitors adversely affect bone health as well, and they urged Dr. Nikkel to extend his follow-up to monitor this situation.
Younger patients given steroids had a risk similar to older patients discharged without corticosteroids.
Source DR. NIKKEL
Major Finding: Discharge of kidney transplant recipients on corticosteroid-based immunosuppression was independently associated with a 45% increased risk in major fractures over the next 4 years compared to patients managed with early corticosteroid withdrawal while still in-hospital.
Data Source: Retrospective analysis of nearly 78,000 kidney transplant recipients in the United States Renal System database.
Disclosures: Dr. Nikkel declared having no financial conflicts.
SAN DIEGO – Early corticosteroid withdrawal after kidney transplantation is associated with a marked reduction in major fracture rate, according to an analysis of the United States Renal Data System database.
Indeed, discharge on corticosteroids was independently associated with a 45% increased risk of major fractures requiring hospitalization during a median follow-up of about 4 years, Dr. Lucas Nikkel reported at the meeting.
Early corticosteroid withdrawal regimens are gaining popularity at renal transplant centers as a means of reducing a wide range of immunosuppression-related side effects. The documented major clinical advantages include reduced rates of hyperlipidemia, posttransplant diabetes, cardiovascular events, infections, and cancer. Until now, however, no study had examined whether early corticosteroid withdrawal regimens are also effective in reducing fracture incidence, noted Dr. Nikkel, who earned the ASBMR Young Investigator Award for his work.
Early corticosteroid withdrawal regimens consist of 4-7 days of high-dose methylprednisolone administered at the time of transplantation, followed by withdrawal of the drug in favor of long-term immunosuppression with calcineurin inhibitors and mycophenolic acid. More than 30% of kidney transplant recipients in the United States are now managed using such protocols.
Dr. Nikkel identified 77,625 adults in the USRDS database who received a kidney transplant during 2000-2006. Pretransplant fracture rates were similar in the 11,178 patients not on steroids at discharge and the 66,447 who were.
The incidence of fractures requiring hospitalization during the follow-up period was 1.7% in patients with early corticosteroid withdrawal, significantly less than the 3.3% for those discharged on corticosteroid-based immunosuppression. The rate was 5.8 major fractures per 1,000 patient-years in the early steroid withdrawal group, compared with 8.0 per 1,000 patient-years in those on corticosteroid-based immunosuppression. The increased fracture risk in patients discharged on steroids became apparent at 1 year of follow-up and significant at 2 years.
“It's noteworthy that patients in the youngest age group discharged on corticosteroids had a similar risk of fractures compared to the oldest patients discharged without corticosteroids,” observed Dr. Nikkel, who is with Columbia University, New York.
In addition to discharge on steroids, other fracture risk factors identified in the study included older age, female gender, white race, pretransplant diabetes, a positive fracture history, and being on dialysis.
Since most fractures are treated on an outpatient basis, it is likely that these study results greatly underestimate the true fracture burden that is associated with kidney transplantation, he added.
Dr. Nikkel pointed out that his study was a retrospective analysis of observational data and said that prospective, long-term studies are needed to confirm the results.
Each year, more than 17,000 kidney transplants are performed in the United States, and more than 68,000 worldwide. Bone loss is high in these patients, especially in the first 18 months after transplantation.
Kidney transplant recipients have a 4.5-fold greater fracture risk than do the general population and a 30% greater risk than do hemodialysis patients during the first 3 years post transplant.
Audience members said that there is evidence to suggest calcineurin inhibitors adversely affect bone health as well, and they urged Dr. Nikkel to extend his follow-up to monitor this situation.
Younger patients given steroids had a risk similar to older patients discharged without corticosteroids.
Source DR. NIKKEL
Major Finding: Discharge of kidney transplant recipients on corticosteroid-based immunosuppression was independently associated with a 45% increased risk in major fractures over the next 4 years compared to patients managed with early corticosteroid withdrawal while still in-hospital.
Data Source: Retrospective analysis of nearly 78,000 kidney transplant recipients in the United States Renal System database.
Disclosures: Dr. Nikkel declared having no financial conflicts.
SAN DIEGO – Early corticosteroid withdrawal after kidney transplantation is associated with a marked reduction in major fracture rate, according to an analysis of the United States Renal Data System database.
Indeed, discharge on corticosteroids was independently associated with a 45% increased risk of major fractures requiring hospitalization during a median follow-up of about 4 years, Dr. Lucas Nikkel reported at the meeting.
Early corticosteroid withdrawal regimens are gaining popularity at renal transplant centers as a means of reducing a wide range of immunosuppression-related side effects. The documented major clinical advantages include reduced rates of hyperlipidemia, posttransplant diabetes, cardiovascular events, infections, and cancer. Until now, however, no study had examined whether early corticosteroid withdrawal regimens are also effective in reducing fracture incidence, noted Dr. Nikkel, who earned the ASBMR Young Investigator Award for his work.
Early corticosteroid withdrawal regimens consist of 4-7 days of high-dose methylprednisolone administered at the time of transplantation, followed by withdrawal of the drug in favor of long-term immunosuppression with calcineurin inhibitors and mycophenolic acid. More than 30% of kidney transplant recipients in the United States are now managed using such protocols.
Dr. Nikkel identified 77,625 adults in the USRDS database who received a kidney transplant during 2000-2006. Pretransplant fracture rates were similar in the 11,178 patients not on steroids at discharge and the 66,447 who were.
The incidence of fractures requiring hospitalization during the follow-up period was 1.7% in patients with early corticosteroid withdrawal, significantly less than the 3.3% for those discharged on corticosteroid-based immunosuppression. The rate was 5.8 major fractures per 1,000 patient-years in the early steroid withdrawal group, compared with 8.0 per 1,000 patient-years in those on corticosteroid-based immunosuppression. The increased fracture risk in patients discharged on steroids became apparent at 1 year of follow-up and significant at 2 years.
“It's noteworthy that patients in the youngest age group discharged on corticosteroids had a similar risk of fractures compared to the oldest patients discharged without corticosteroids,” observed Dr. Nikkel, who is with Columbia University, New York.
In addition to discharge on steroids, other fracture risk factors identified in the study included older age, female gender, white race, pretransplant diabetes, a positive fracture history, and being on dialysis.
Since most fractures are treated on an outpatient basis, it is likely that these study results greatly underestimate the true fracture burden that is associated with kidney transplantation, he added.
Dr. Nikkel pointed out that his study was a retrospective analysis of observational data and said that prospective, long-term studies are needed to confirm the results.
Each year, more than 17,000 kidney transplants are performed in the United States, and more than 68,000 worldwide. Bone loss is high in these patients, especially in the first 18 months after transplantation.
Kidney transplant recipients have a 4.5-fold greater fracture risk than do the general population and a 30% greater risk than do hemodialysis patients during the first 3 years post transplant.
Audience members said that there is evidence to suggest calcineurin inhibitors adversely affect bone health as well, and they urged Dr. Nikkel to extend his follow-up to monitor this situation.
Younger patients given steroids had a risk similar to older patients discharged without corticosteroids.
Source DR. NIKKEL
From the Annual Meeting of the American Society for Bone and Mineral Research
Folic Acid, B Vitamins Fail to Cut Fracture Risk in Women
Major Finding: During an average 7.3 years of treatment and follow-up, the overall nonvertebral fracture incidence was 7.6% in the supplementation group, compared with 6.9% in placebo-treated controls.
Data Source: Secondary analysis of the 5,442-subject randomized, double-blind, placebo-controlled Women's Antioxidant and Folic Acid Cardiovascular Study.
Disclosures: WAFACS was funded by the National Heart, Lung, and Blood Institute. Dr. Bauer reported having no financial conflicts.
SAN DIEGO – Combined daily supplementation with folic acid and vitamins B6 and B12 proved to be a bust for reduction of nonvertebral fracture risk in a large, randomized, double-blind clinical trial conducted in women with known cardiovascular disease or multiple risk factors.
A secondary analysis of fracture outcomes in 5,442 female health professionals over age 42 years who participated in the Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS) showed an overall nonvertebral fracture incidence of 7.6% during an average 7.3 years of treatment and follow-up in the supplementation group and a similar 6.9% rate in placebo-treated controls, Dr. Douglas C. Bauer reported at the meeting.
Participants in WAFACS had known cardiovascular disease or at least three cardiovascular risk factors. The previously reported primary study end points were cardiovascular event rates and all-cause mortality, where supplements had no effect (JAMA 2008;299:2027-36).
The new retrospective secondary analysis of WAFACS was undertaken because some prior observational studies had concluded that elevated homocysteine and low vitamin B12 levels are associated with increased fracture risk. The supplement regimen utilized in the trial was designed to lower homocysteine and boost vitamin B12 levels. It consisted of daily folic acid at 2.5 mg, vitamin B6 at 50 mg, and vitamin B12 at 1 mg.
In all, 80% of participants reported greater than 66% compliance with therapy. Rates of hip, wrist, and total nonspine fractures were similar in both high-compliance subgroups, noted Dr. Bauer of the University of California, San Francisco.
Major Finding: During an average 7.3 years of treatment and follow-up, the overall nonvertebral fracture incidence was 7.6% in the supplementation group, compared with 6.9% in placebo-treated controls.
Data Source: Secondary analysis of the 5,442-subject randomized, double-blind, placebo-controlled Women's Antioxidant and Folic Acid Cardiovascular Study.
Disclosures: WAFACS was funded by the National Heart, Lung, and Blood Institute. Dr. Bauer reported having no financial conflicts.
SAN DIEGO – Combined daily supplementation with folic acid and vitamins B6 and B12 proved to be a bust for reduction of nonvertebral fracture risk in a large, randomized, double-blind clinical trial conducted in women with known cardiovascular disease or multiple risk factors.
A secondary analysis of fracture outcomes in 5,442 female health professionals over age 42 years who participated in the Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS) showed an overall nonvertebral fracture incidence of 7.6% during an average 7.3 years of treatment and follow-up in the supplementation group and a similar 6.9% rate in placebo-treated controls, Dr. Douglas C. Bauer reported at the meeting.
Participants in WAFACS had known cardiovascular disease or at least three cardiovascular risk factors. The previously reported primary study end points were cardiovascular event rates and all-cause mortality, where supplements had no effect (JAMA 2008;299:2027-36).
The new retrospective secondary analysis of WAFACS was undertaken because some prior observational studies had concluded that elevated homocysteine and low vitamin B12 levels are associated with increased fracture risk. The supplement regimen utilized in the trial was designed to lower homocysteine and boost vitamin B12 levels. It consisted of daily folic acid at 2.5 mg, vitamin B6 at 50 mg, and vitamin B12 at 1 mg.
In all, 80% of participants reported greater than 66% compliance with therapy. Rates of hip, wrist, and total nonspine fractures were similar in both high-compliance subgroups, noted Dr. Bauer of the University of California, San Francisco.
Major Finding: During an average 7.3 years of treatment and follow-up, the overall nonvertebral fracture incidence was 7.6% in the supplementation group, compared with 6.9% in placebo-treated controls.
Data Source: Secondary analysis of the 5,442-subject randomized, double-blind, placebo-controlled Women's Antioxidant and Folic Acid Cardiovascular Study.
Disclosures: WAFACS was funded by the National Heart, Lung, and Blood Institute. Dr. Bauer reported having no financial conflicts.
SAN DIEGO – Combined daily supplementation with folic acid and vitamins B6 and B12 proved to be a bust for reduction of nonvertebral fracture risk in a large, randomized, double-blind clinical trial conducted in women with known cardiovascular disease or multiple risk factors.
A secondary analysis of fracture outcomes in 5,442 female health professionals over age 42 years who participated in the Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS) showed an overall nonvertebral fracture incidence of 7.6% during an average 7.3 years of treatment and follow-up in the supplementation group and a similar 6.9% rate in placebo-treated controls, Dr. Douglas C. Bauer reported at the meeting.
Participants in WAFACS had known cardiovascular disease or at least three cardiovascular risk factors. The previously reported primary study end points were cardiovascular event rates and all-cause mortality, where supplements had no effect (JAMA 2008;299:2027-36).
The new retrospective secondary analysis of WAFACS was undertaken because some prior observational studies had concluded that elevated homocysteine and low vitamin B12 levels are associated with increased fracture risk. The supplement regimen utilized in the trial was designed to lower homocysteine and boost vitamin B12 levels. It consisted of daily folic acid at 2.5 mg, vitamin B6 at 50 mg, and vitamin B12 at 1 mg.
In all, 80% of participants reported greater than 66% compliance with therapy. Rates of hip, wrist, and total nonspine fractures were similar in both high-compliance subgroups, noted Dr. Bauer of the University of California, San Francisco.
From the Annual Meeting of the American Society for Bone and Mineral Research
Teriparatide Now a Preferred Drug for GIOP
SAN DIEGO – Teriparatide has received a boost in status as a preferentially effective treatment for glucocorticoid-induced osteoporosis in the form of a second, confirmatory, randomized double-blind trial demonstrating the anabolic agent achieves substantially greater increases in bone mineral density compared to a bisphosphonate.
One of these studies also included fracture rates as a preplanned secondary end point; the trial showed a significant reduction in vertebral fractures with teriparatide (Forteo) as compared to alendronate (Fosamax).
These positive study findings are bolstered by a biologically plausible mechanism of benefit, observed Dr. Kenneth G. Saag. “Based upon the pathogenesis of glucocorticoid-induced osteoporosis [GIOP], we think that an anabolic agent makes some sense. It may be beneficial to stimulate osteoblasts to promote new bone formation,” said Dr. Saag, professor of medicine and epidemiology at the University of Alabama at Birmingham.
Steroids are known to have apoptosis-mediated deleterious effects on both osteocytes and osteoblasts that lead to decline in bone function and an abrupt increase in fracture risk independent of bone mineral density (BMD). Osteoclasts are also unfavorably impacted because of cross talk and the indirect effects of sex steroids, insulin-like growth factor, and a modest effect of secondary hyperparathyroidism mediated through altered calcium absorption. In addition, higher-dose steroids have adverse effects upon muscle that can independently lead to a higher fracture rate, he explained.
The Food and Drug Administration has approved alendronate, zoledronic acid (Reclast), risedronate (Actonel), and teriparatide (Forteo) for treatment of GIOP. More recently, raloxifene (Evista) has joined the ranks of agents shown to increase BMD in patients on long-term steroids; this came in the form of a double-blind, placebo-controlled, 12-month study (Ann. Rheum. Dis. 2011;70:778-84).
Subcutaneous daily teriparatide for up to 2 years also has the approval of the FDA for use in adults at high fracture risk because they are on sustained systemic steroid therapy.
There is no definitive study demonstrating that any of these agents actually prevents fractures in patients with GIOP.
However, data from a study in which Dr. Saag was lead investigator showed that teriparatide-treated patients had significantly fewer vertebral fractures than those assigned to alendronate. The study was a 36-month randomized, double-blind, clinical trial that assessed fracture rates as a preplanned secondary end point. The radiographic and clinical vertebral fracture rates in 169 alendronate-treated patients were 7.7% and 2.4%, respectively, compared with 1.7% and 0% in 173 teriparatide-treated patients (P = .007 and .037). No significant difference in nonvertebral fractures was found between the two treatment arms.
The primary study end point was the change in BMD from baseline. Here again teriparatide proved significantly more effective than the bisphosphonate, with a mean 11% increase at the lumbar spine, compared to 5.3% with alendronate.
Teriparatide-treated patients also had a 5.2% increase in BMD at the total hip and a 6.3% boost at the femoral neck, compared to 2.7% and 3.4%, respectively, with alendronate (Arthritis Rheum. 2009;60:3346-55).
Confirmation of teriparatide's superior BMD-building effect came from the EuroGIOPs trial presented by Dr. Claus C. Glüer at the ASBMR meeting.
EuroGIOPS was an 18-month, open-label, phase III clinical trial in which 92 men with GIOP were randomized to teriparatide or risedronate.
At 6 months the mean increase in lumbar spine BMD was 5.7% in the teriparatide group, compared with 3.3% in the risedronate arm.
At 18 months – the primary study end point – the teriparatide group averaged a 16.3% BMD increase over baseline, while the risedronate arm had a 3.8% rise.
Intriguingly, new clinical fractures occurred during 18 months of therapy in five patients on risedronate and none on teriparatide, a difference that came within a hair of statistical significance (P = .056).
Bone strength as formally measured in terms of anterior bending, axial compression, and axial torsion was also significantly greater in the teriparatide group, according to Dr. Glüer, who is professor of medical physics at the department of diagnostic radiology, University Hospital Schleswig-Holstein in Kiel, Germany.
The 2010 update of the American College of Rheumatology guidelines for the prevention and treatment of GIOP recommend bisphosphonates but not teriparatide for older adults at high risk of fracture who are taking less than 5 mg/day of prednisone for less than 1 month (Arthritis Care Res. 2010;62:1515-26).
The ACR guidelines recommend reserving use of teriparatide for high-risk patients – that is, those with a prevalent fracture or a World Health Organization Fracture Risk Assessment Tool (FRAX) score indicative of a greater than 20% 10-year risk of a major osteoporotic fracture – who are on at least 6 mg/day of prednisone for less than 1 month or on any dose of glucocorticoids for longer than 1 month.
Although Dr. Saag was a coauthor of the ACR guidelines, he was pleased to see a new commentary on the ACR guidelines published by the Professional Practice Committee of the ASBMR. The review recommends teriparatide or any of the bisphosphonates for high-risk patients, period.
Dr. Saag, who was not involved in the ASBMR review, recommended it as useful reading both for its areas of agreement with the ACR guidelines as well as for raising several patient scenarios in which the ASBMR committee believes the ACR recommendations either do not apply or might be improved upon (J. Bone Miner. Res. 2011;26:1989-96).
Dr. Glüer declared having no financial conflicts regarding the Eli Lilly–funded EuroGIOPs trial. Dr. Saag disclosed that he has received research grants from and serves as a paid consultant to Amgen, Eli Lilly, Merck, and Novartis.
Dr. Kenneth G. Saag noted that an anabolic agent such as teriparatide stimulates osteoblasts to promote new bone formation.
Source Courtesy University of Alabama
SAN DIEGO – Teriparatide has received a boost in status as a preferentially effective treatment for glucocorticoid-induced osteoporosis in the form of a second, confirmatory, randomized double-blind trial demonstrating the anabolic agent achieves substantially greater increases in bone mineral density compared to a bisphosphonate.
One of these studies also included fracture rates as a preplanned secondary end point; the trial showed a significant reduction in vertebral fractures with teriparatide (Forteo) as compared to alendronate (Fosamax).
These positive study findings are bolstered by a biologically plausible mechanism of benefit, observed Dr. Kenneth G. Saag. “Based upon the pathogenesis of glucocorticoid-induced osteoporosis [GIOP], we think that an anabolic agent makes some sense. It may be beneficial to stimulate osteoblasts to promote new bone formation,” said Dr. Saag, professor of medicine and epidemiology at the University of Alabama at Birmingham.
Steroids are known to have apoptosis-mediated deleterious effects on both osteocytes and osteoblasts that lead to decline in bone function and an abrupt increase in fracture risk independent of bone mineral density (BMD). Osteoclasts are also unfavorably impacted because of cross talk and the indirect effects of sex steroids, insulin-like growth factor, and a modest effect of secondary hyperparathyroidism mediated through altered calcium absorption. In addition, higher-dose steroids have adverse effects upon muscle that can independently lead to a higher fracture rate, he explained.
The Food and Drug Administration has approved alendronate, zoledronic acid (Reclast), risedronate (Actonel), and teriparatide (Forteo) for treatment of GIOP. More recently, raloxifene (Evista) has joined the ranks of agents shown to increase BMD in patients on long-term steroids; this came in the form of a double-blind, placebo-controlled, 12-month study (Ann. Rheum. Dis. 2011;70:778-84).
Subcutaneous daily teriparatide for up to 2 years also has the approval of the FDA for use in adults at high fracture risk because they are on sustained systemic steroid therapy.
There is no definitive study demonstrating that any of these agents actually prevents fractures in patients with GIOP.
However, data from a study in which Dr. Saag was lead investigator showed that teriparatide-treated patients had significantly fewer vertebral fractures than those assigned to alendronate. The study was a 36-month randomized, double-blind, clinical trial that assessed fracture rates as a preplanned secondary end point. The radiographic and clinical vertebral fracture rates in 169 alendronate-treated patients were 7.7% and 2.4%, respectively, compared with 1.7% and 0% in 173 teriparatide-treated patients (P = .007 and .037). No significant difference in nonvertebral fractures was found between the two treatment arms.
The primary study end point was the change in BMD from baseline. Here again teriparatide proved significantly more effective than the bisphosphonate, with a mean 11% increase at the lumbar spine, compared to 5.3% with alendronate.
Teriparatide-treated patients also had a 5.2% increase in BMD at the total hip and a 6.3% boost at the femoral neck, compared to 2.7% and 3.4%, respectively, with alendronate (Arthritis Rheum. 2009;60:3346-55).
Confirmation of teriparatide's superior BMD-building effect came from the EuroGIOPs trial presented by Dr. Claus C. Glüer at the ASBMR meeting.
EuroGIOPS was an 18-month, open-label, phase III clinical trial in which 92 men with GIOP were randomized to teriparatide or risedronate.
At 6 months the mean increase in lumbar spine BMD was 5.7% in the teriparatide group, compared with 3.3% in the risedronate arm.
At 18 months – the primary study end point – the teriparatide group averaged a 16.3% BMD increase over baseline, while the risedronate arm had a 3.8% rise.
Intriguingly, new clinical fractures occurred during 18 months of therapy in five patients on risedronate and none on teriparatide, a difference that came within a hair of statistical significance (P = .056).
Bone strength as formally measured in terms of anterior bending, axial compression, and axial torsion was also significantly greater in the teriparatide group, according to Dr. Glüer, who is professor of medical physics at the department of diagnostic radiology, University Hospital Schleswig-Holstein in Kiel, Germany.
The 2010 update of the American College of Rheumatology guidelines for the prevention and treatment of GIOP recommend bisphosphonates but not teriparatide for older adults at high risk of fracture who are taking less than 5 mg/day of prednisone for less than 1 month (Arthritis Care Res. 2010;62:1515-26).
The ACR guidelines recommend reserving use of teriparatide for high-risk patients – that is, those with a prevalent fracture or a World Health Organization Fracture Risk Assessment Tool (FRAX) score indicative of a greater than 20% 10-year risk of a major osteoporotic fracture – who are on at least 6 mg/day of prednisone for less than 1 month or on any dose of glucocorticoids for longer than 1 month.
Although Dr. Saag was a coauthor of the ACR guidelines, he was pleased to see a new commentary on the ACR guidelines published by the Professional Practice Committee of the ASBMR. The review recommends teriparatide or any of the bisphosphonates for high-risk patients, period.
Dr. Saag, who was not involved in the ASBMR review, recommended it as useful reading both for its areas of agreement with the ACR guidelines as well as for raising several patient scenarios in which the ASBMR committee believes the ACR recommendations either do not apply or might be improved upon (J. Bone Miner. Res. 2011;26:1989-96).
Dr. Glüer declared having no financial conflicts regarding the Eli Lilly–funded EuroGIOPs trial. Dr. Saag disclosed that he has received research grants from and serves as a paid consultant to Amgen, Eli Lilly, Merck, and Novartis.
Dr. Kenneth G. Saag noted that an anabolic agent such as teriparatide stimulates osteoblasts to promote new bone formation.
Source Courtesy University of Alabama
SAN DIEGO – Teriparatide has received a boost in status as a preferentially effective treatment for glucocorticoid-induced osteoporosis in the form of a second, confirmatory, randomized double-blind trial demonstrating the anabolic agent achieves substantially greater increases in bone mineral density compared to a bisphosphonate.
One of these studies also included fracture rates as a preplanned secondary end point; the trial showed a significant reduction in vertebral fractures with teriparatide (Forteo) as compared to alendronate (Fosamax).
These positive study findings are bolstered by a biologically plausible mechanism of benefit, observed Dr. Kenneth G. Saag. “Based upon the pathogenesis of glucocorticoid-induced osteoporosis [GIOP], we think that an anabolic agent makes some sense. It may be beneficial to stimulate osteoblasts to promote new bone formation,” said Dr. Saag, professor of medicine and epidemiology at the University of Alabama at Birmingham.
Steroids are known to have apoptosis-mediated deleterious effects on both osteocytes and osteoblasts that lead to decline in bone function and an abrupt increase in fracture risk independent of bone mineral density (BMD). Osteoclasts are also unfavorably impacted because of cross talk and the indirect effects of sex steroids, insulin-like growth factor, and a modest effect of secondary hyperparathyroidism mediated through altered calcium absorption. In addition, higher-dose steroids have adverse effects upon muscle that can independently lead to a higher fracture rate, he explained.
The Food and Drug Administration has approved alendronate, zoledronic acid (Reclast), risedronate (Actonel), and teriparatide (Forteo) for treatment of GIOP. More recently, raloxifene (Evista) has joined the ranks of agents shown to increase BMD in patients on long-term steroids; this came in the form of a double-blind, placebo-controlled, 12-month study (Ann. Rheum. Dis. 2011;70:778-84).
Subcutaneous daily teriparatide for up to 2 years also has the approval of the FDA for use in adults at high fracture risk because they are on sustained systemic steroid therapy.
There is no definitive study demonstrating that any of these agents actually prevents fractures in patients with GIOP.
However, data from a study in which Dr. Saag was lead investigator showed that teriparatide-treated patients had significantly fewer vertebral fractures than those assigned to alendronate. The study was a 36-month randomized, double-blind, clinical trial that assessed fracture rates as a preplanned secondary end point. The radiographic and clinical vertebral fracture rates in 169 alendronate-treated patients were 7.7% and 2.4%, respectively, compared with 1.7% and 0% in 173 teriparatide-treated patients (P = .007 and .037). No significant difference in nonvertebral fractures was found between the two treatment arms.
The primary study end point was the change in BMD from baseline. Here again teriparatide proved significantly more effective than the bisphosphonate, with a mean 11% increase at the lumbar spine, compared to 5.3% with alendronate.
Teriparatide-treated patients also had a 5.2% increase in BMD at the total hip and a 6.3% boost at the femoral neck, compared to 2.7% and 3.4%, respectively, with alendronate (Arthritis Rheum. 2009;60:3346-55).
Confirmation of teriparatide's superior BMD-building effect came from the EuroGIOPs trial presented by Dr. Claus C. Glüer at the ASBMR meeting.
EuroGIOPS was an 18-month, open-label, phase III clinical trial in which 92 men with GIOP were randomized to teriparatide or risedronate.
At 6 months the mean increase in lumbar spine BMD was 5.7% in the teriparatide group, compared with 3.3% in the risedronate arm.
At 18 months – the primary study end point – the teriparatide group averaged a 16.3% BMD increase over baseline, while the risedronate arm had a 3.8% rise.
Intriguingly, new clinical fractures occurred during 18 months of therapy in five patients on risedronate and none on teriparatide, a difference that came within a hair of statistical significance (P = .056).
Bone strength as formally measured in terms of anterior bending, axial compression, and axial torsion was also significantly greater in the teriparatide group, according to Dr. Glüer, who is professor of medical physics at the department of diagnostic radiology, University Hospital Schleswig-Holstein in Kiel, Germany.
The 2010 update of the American College of Rheumatology guidelines for the prevention and treatment of GIOP recommend bisphosphonates but not teriparatide for older adults at high risk of fracture who are taking less than 5 mg/day of prednisone for less than 1 month (Arthritis Care Res. 2010;62:1515-26).
The ACR guidelines recommend reserving use of teriparatide for high-risk patients – that is, those with a prevalent fracture or a World Health Organization Fracture Risk Assessment Tool (FRAX) score indicative of a greater than 20% 10-year risk of a major osteoporotic fracture – who are on at least 6 mg/day of prednisone for less than 1 month or on any dose of glucocorticoids for longer than 1 month.
Although Dr. Saag was a coauthor of the ACR guidelines, he was pleased to see a new commentary on the ACR guidelines published by the Professional Practice Committee of the ASBMR. The review recommends teriparatide or any of the bisphosphonates for high-risk patients, period.
Dr. Saag, who was not involved in the ASBMR review, recommended it as useful reading both for its areas of agreement with the ACR guidelines as well as for raising several patient scenarios in which the ASBMR committee believes the ACR recommendations either do not apply or might be improved upon (J. Bone Miner. Res. 2011;26:1989-96).
Dr. Glüer declared having no financial conflicts regarding the Eli Lilly–funded EuroGIOPs trial. Dr. Saag disclosed that he has received research grants from and serves as a paid consultant to Amgen, Eli Lilly, Merck, and Novartis.
Dr. Kenneth G. Saag noted that an anabolic agent such as teriparatide stimulates osteoblasts to promote new bone formation.
Source Courtesy University of Alabama
Expert Analysis from the Annual Meeting of the American Society for Bone and Mineral Research
Current Guidelines Provide More Realistic Exercise Goals
KEYSTONE, COLO. – Contemporary exercise guidelines for type 2 diabetes patients take a kinder, gentler approach than previous versions did.
This stance is based partly on scientific advances, but there's also a greater common-sense recognition among health care providers that type 2 diabetes patients find it tough to embark on an exercise program and even harder to stick with it. Current guidelines aim to remove barriers to doing so, Judith G. Regensteiner, Ph.D., said.
“Once you get them started, a minority will love it and will continue to exercise for life, but the majority will struggle. If you can't make it fun for them, they're not going to persist with it. Exercising with a group, with your family, your children or grandchildren – we have to keep working on this behavioral piece,” explained Dr. Regensteiner, professor of medicine and director of the center for women's health at the University of Colorado at Denver.
One major barrier to exercising is a still-widespread misconception that activity must be vigorous to provide health benefits. It's an idea dating back to the pre-1995 American Heart Association and American College of Sports Medicine exercise guidelines, which called for at least 20 minutes of vigorous exercise continuously three or more times a week.
“This was the 'no pain/no gain' era, and it did us a lot of damage, I think. That's where people got the idea that if they weren't running a marathon or a 10-K or doing something equally vigorous, they weren't helping themselves. – We did ourselves harm by saying it because we scared people off in droves,” she said at the meeting, which was sponsored by the Children's Diabetes Foundation at Denver and the university.
“We know very well from reams of data that walking – simple walking – is effective for the aerobic component of an exercise program. We want people to walk because it's the easiest exercise to do,” Dr. Regensteiner added.
That core message is contained in two recent sets of exercise guidelines that she coauthored. One is the comprehensive Physical Activity Guidelines Advisory Committee Report 2008 to the Secretary of Health and Human Services. The report reviews in detail the strong evidence for all of the medical conditions in which exercise has been shown to be beneficial, including colon and breast cancer, depression, and numerous other diseases in addition to type 2 diabetes. Dr. Regensteiner summed up the 683-page tome in three words: “Exercise is medicine.”
The HHS guidelines state that even though any amount of physical activity provides some health benefit, 150 minutes per week of moderate-intensity aerobic activity – such as brisk walking – substantially reduce the risk of many chronic diseases. Moving up to 5 hours per week provides additional health benefits. A 20-minute session devoted to strengthening all the major muscle groups is recommended on 2 or more days per week.
More recently, Dr. Regensteiner coauthored an American College of Sports Medicine/American Diabetes Association joint position statement on exercise and type 2 diabetes (Med. Sci. Sports Exerc. 2010;42;2282-303; Diabetes Care 2010;33:e147-67). The detailed document knocks down another major barrier to exercise that was a fixture in older exercise guidelines: the recommendation that everybody who has type 2 diabetes or who is at least 40 years old, with multiple cardiovascular risk factors, should get an exercise stress test before starting an exercise program.
“This is completely unrealistic. It's not going to happen. What it did was provide people with another reason not to exercise,” she said.
The joint position statement declares, “Before undertaking exercise more intensive than brisk walking, sedentary persons with type 2 diabetes will likely benefit from an evaluation by a physician. Electrocardiogram exercise stress testing for asymptomatic individuals at low risk for [coronary artery disease] is not recommended but may be indicated for higher risk.”
What that means, Dr. Regensteiner explained, is that many sedentary patients with type 2 diabetes don't need a stress test if they simply want to start a walking program.
“We want people to get out there and walk, so don't put a barrier up,” she said.
Dr. Regensteiner reported having no financial conflicts.
'People got the idea that if they weren't running a marathon or a 10-K – they weren't helping themselves.'
Source DR. REGENSTEINER
KEYSTONE, COLO. – Contemporary exercise guidelines for type 2 diabetes patients take a kinder, gentler approach than previous versions did.
This stance is based partly on scientific advances, but there's also a greater common-sense recognition among health care providers that type 2 diabetes patients find it tough to embark on an exercise program and even harder to stick with it. Current guidelines aim to remove barriers to doing so, Judith G. Regensteiner, Ph.D., said.
“Once you get them started, a minority will love it and will continue to exercise for life, but the majority will struggle. If you can't make it fun for them, they're not going to persist with it. Exercising with a group, with your family, your children or grandchildren – we have to keep working on this behavioral piece,” explained Dr. Regensteiner, professor of medicine and director of the center for women's health at the University of Colorado at Denver.
One major barrier to exercising is a still-widespread misconception that activity must be vigorous to provide health benefits. It's an idea dating back to the pre-1995 American Heart Association and American College of Sports Medicine exercise guidelines, which called for at least 20 minutes of vigorous exercise continuously three or more times a week.
“This was the 'no pain/no gain' era, and it did us a lot of damage, I think. That's where people got the idea that if they weren't running a marathon or a 10-K or doing something equally vigorous, they weren't helping themselves. – We did ourselves harm by saying it because we scared people off in droves,” she said at the meeting, which was sponsored by the Children's Diabetes Foundation at Denver and the university.
“We know very well from reams of data that walking – simple walking – is effective for the aerobic component of an exercise program. We want people to walk because it's the easiest exercise to do,” Dr. Regensteiner added.
That core message is contained in two recent sets of exercise guidelines that she coauthored. One is the comprehensive Physical Activity Guidelines Advisory Committee Report 2008 to the Secretary of Health and Human Services. The report reviews in detail the strong evidence for all of the medical conditions in which exercise has been shown to be beneficial, including colon and breast cancer, depression, and numerous other diseases in addition to type 2 diabetes. Dr. Regensteiner summed up the 683-page tome in three words: “Exercise is medicine.”
The HHS guidelines state that even though any amount of physical activity provides some health benefit, 150 minutes per week of moderate-intensity aerobic activity – such as brisk walking – substantially reduce the risk of many chronic diseases. Moving up to 5 hours per week provides additional health benefits. A 20-minute session devoted to strengthening all the major muscle groups is recommended on 2 or more days per week.
More recently, Dr. Regensteiner coauthored an American College of Sports Medicine/American Diabetes Association joint position statement on exercise and type 2 diabetes (Med. Sci. Sports Exerc. 2010;42;2282-303; Diabetes Care 2010;33:e147-67). The detailed document knocks down another major barrier to exercise that was a fixture in older exercise guidelines: the recommendation that everybody who has type 2 diabetes or who is at least 40 years old, with multiple cardiovascular risk factors, should get an exercise stress test before starting an exercise program.
“This is completely unrealistic. It's not going to happen. What it did was provide people with another reason not to exercise,” she said.
The joint position statement declares, “Before undertaking exercise more intensive than brisk walking, sedentary persons with type 2 diabetes will likely benefit from an evaluation by a physician. Electrocardiogram exercise stress testing for asymptomatic individuals at low risk for [coronary artery disease] is not recommended but may be indicated for higher risk.”
What that means, Dr. Regensteiner explained, is that many sedentary patients with type 2 diabetes don't need a stress test if they simply want to start a walking program.
“We want people to get out there and walk, so don't put a barrier up,” she said.
Dr. Regensteiner reported having no financial conflicts.
'People got the idea that if they weren't running a marathon or a 10-K – they weren't helping themselves.'
Source DR. REGENSTEINER
KEYSTONE, COLO. – Contemporary exercise guidelines for type 2 diabetes patients take a kinder, gentler approach than previous versions did.
This stance is based partly on scientific advances, but there's also a greater common-sense recognition among health care providers that type 2 diabetes patients find it tough to embark on an exercise program and even harder to stick with it. Current guidelines aim to remove barriers to doing so, Judith G. Regensteiner, Ph.D., said.
“Once you get them started, a minority will love it and will continue to exercise for life, but the majority will struggle. If you can't make it fun for them, they're not going to persist with it. Exercising with a group, with your family, your children or grandchildren – we have to keep working on this behavioral piece,” explained Dr. Regensteiner, professor of medicine and director of the center for women's health at the University of Colorado at Denver.
One major barrier to exercising is a still-widespread misconception that activity must be vigorous to provide health benefits. It's an idea dating back to the pre-1995 American Heart Association and American College of Sports Medicine exercise guidelines, which called for at least 20 minutes of vigorous exercise continuously three or more times a week.
“This was the 'no pain/no gain' era, and it did us a lot of damage, I think. That's where people got the idea that if they weren't running a marathon or a 10-K or doing something equally vigorous, they weren't helping themselves. – We did ourselves harm by saying it because we scared people off in droves,” she said at the meeting, which was sponsored by the Children's Diabetes Foundation at Denver and the university.
“We know very well from reams of data that walking – simple walking – is effective for the aerobic component of an exercise program. We want people to walk because it's the easiest exercise to do,” Dr. Regensteiner added.
That core message is contained in two recent sets of exercise guidelines that she coauthored. One is the comprehensive Physical Activity Guidelines Advisory Committee Report 2008 to the Secretary of Health and Human Services. The report reviews in detail the strong evidence for all of the medical conditions in which exercise has been shown to be beneficial, including colon and breast cancer, depression, and numerous other diseases in addition to type 2 diabetes. Dr. Regensteiner summed up the 683-page tome in three words: “Exercise is medicine.”
The HHS guidelines state that even though any amount of physical activity provides some health benefit, 150 minutes per week of moderate-intensity aerobic activity – such as brisk walking – substantially reduce the risk of many chronic diseases. Moving up to 5 hours per week provides additional health benefits. A 20-minute session devoted to strengthening all the major muscle groups is recommended on 2 or more days per week.
More recently, Dr. Regensteiner coauthored an American College of Sports Medicine/American Diabetes Association joint position statement on exercise and type 2 diabetes (Med. Sci. Sports Exerc. 2010;42;2282-303; Diabetes Care 2010;33:e147-67). The detailed document knocks down another major barrier to exercise that was a fixture in older exercise guidelines: the recommendation that everybody who has type 2 diabetes or who is at least 40 years old, with multiple cardiovascular risk factors, should get an exercise stress test before starting an exercise program.
“This is completely unrealistic. It's not going to happen. What it did was provide people with another reason not to exercise,” she said.
The joint position statement declares, “Before undertaking exercise more intensive than brisk walking, sedentary persons with type 2 diabetes will likely benefit from an evaluation by a physician. Electrocardiogram exercise stress testing for asymptomatic individuals at low risk for [coronary artery disease] is not recommended but may be indicated for higher risk.”
What that means, Dr. Regensteiner explained, is that many sedentary patients with type 2 diabetes don't need a stress test if they simply want to start a walking program.
“We want people to get out there and walk, so don't put a barrier up,” she said.
Dr. Regensteiner reported having no financial conflicts.
'People got the idea that if they weren't running a marathon or a 10-K – they weren't helping themselves.'
Source DR. REGENSTEINER
Expert Analysis from a Conference on Practical Ways to Achieve Targets in Diabetes Care