User login
Modular Versus Nonmodular Femoral Necks for Primary Total Hip Arthroplasty
Femoral stem modularity in total hip arthroplasty (THA) has a checkered past. Developments such as the modular head–trunnion interface, which allows for placement of femoral heads of different sizes and offsets, and the modular midstem, which allows for version adjustments independent of patient anatomy (S-ROM, Depuy) and for bypassing proximal bone defects in the revision setting (Restoration Modular, Stryker; ZMR-XL, Zimmer), have proved very successful.1-10 However, even these successful advances have been associated with failures at the modular junction.11-13 Proximal femoral neck–stem modularity (PFNSM) has had mixed results, with notable failures and recalls associated with the neck–stem junction.14,15 Failures at this junction have occurred secondary to corrosion and breakage of the modular neck.16-18 Nevertheless, proximal modular stems remain available for implantation. One such system, the M/L Taper stem with Kinectiv technology (Zimmer), is an all-titanium construct that allows for adjustment of several variables (length, offset, version), providing numerous combinations beyond those of the original M/L Taper offerings. Advantages of these offerings include closer reconstruction of patient anatomy, stability improvements, and easing of the process of revision in polyethylene/femoral head exchanges or in infections in which single-staged irrigation and débridement and polyethylene/head exchange are chosen.
These theoretic advantages must be judged in the context of the possible disadvantages of the modular neck junction. The mechanical environment of the junction places it at risk for failure as well as for metallosis from fretting, crevice corrosion, and recurrent repassivation.19 Although the titanium necks are at less risk for degradation than their cobalt-chromium counterparts, they are at higher risk for breakage.13,19 For one of the surgeons in our practice, the M/L Taper stem with Kinectiv technology is the stem of choice for primary THA.
We conducted a study to determine, in the setting of primary THA, how often a neck–stem combination choice resulted in a reconstructive geometry that would not have been possible had the surgeon opted for the traditional M/L Taper stem. Every Kinectiv stem has numerous neck options with a head center position that would not be possible with the nonmodular M/L Taper. However, in a high-volume community practice, how often is a modular neck that results in an otherwise unavailable head center being used for the reconstruction?
Materials and Methods
This study was approved by our local institutional review board. The Kinectiv stem is used by 1 of the 4 high-volume joint replacement surgeons in our practice (not one of the authors). From our community practice joint registry, we identified every stem–neck combination used since the Kinectiv stem became available in 2006.20 Each case was performed using a posterior approach. A trabecular metal acetabular component (Zimmer) secured with 2 screws was used, and an M/L Taper stem with Kinectiv technology was implanted in each case.
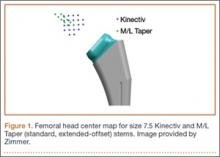
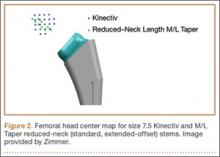
Once the neck–stem combination was determined, its position on the head centers map was compared with that of the standard M/L Taper head centers (Figures 1, 2) for each stem size as the relationship of the Kinectiv head center varies with each stem size compared with the head center of the M/L Taper stems. If the head centers were in contact on the map, the geometry was considered identical. If the head centers were not in contact, we noted where the nearest standard M/L Taper head center lay in terms of length and offset. As the head centers are laid out in regular, 4-mm increments, this estimation was relatively easy. Any anteverted or retroverted neck was considered to have no adequate substitution in the standard M/L Taper stem offerings. This initial evaluation was performed by Dr. Carothers.
We then reviewed the head center comparisons independently. For every Kinectiv head center that did not contact an M/L Taper counterpart, the difference between those head centers was reviewed. Each of us noted whether the difference between the head centers was clinically relevant, as many of the head center positions are extremely close. The head centers that were so close as to be deemed clinically irrelevant were recorded.
Results
Between January 2008 and October 2013, 463 primary THAs were performed using the M/L Taper femoral stem with Kinectiv technology. Of the neck options used, 205 (44%) had a head center identical to that of a nonmodular M/L Taper stem. In another 56 cases (12%), all 3 reviewing surgeons agreed that the M/L Taper head center was so close to the Kinectiv head center as to be clinically indistinguishable. Of these 56 cases, 54 had a head center difference of less than 1 mm in length or offset; the other 2 had a 2-mm difference in offset.
Thus, a total of 261 stems (56%) had a standard M/L Taper option that offered an identical head center or one so close as to be clinically indistinguishable. Interestingly, in the group of 202 stems that did not have an identical head center and were not clinically indistinguishable, 132 (65%) of these modular stems were within 4 mm in length and 2 mm of offset of the closest Kinectiv head center. A verted neck was used in 12 cases (11 anteverted, 1 retroverted).
Nine of the 463 cases required revision surgery, 3 for recurrent instability. In 1 of these 3 cases, the acetabulum was revised for malposition, and the neck was converted from standard offset, +0 mm length (head center identical to nonmodular stem), to extended offset, +4 mm length (2 mm shorter with 1 mm less offset than closest nonmodular head center). The second case had complete deficiency of the abductor tendons and was converted to a constrained liner, though at the time of the THA a head center identical to that of the nonmodular stem was used. The third case was revised to convert a standard offset, +0 mm length, straight neck (head center identical to nonmodular stem), to extended offset, +4 mm length, anteverted neck (anteversion making this a unique head center position). Of the other 6 cases, 1 was treated for corrosion at the head–neck junction by changing the head from cobalt-chromium to ceramic (the junction was noted to be pristine), 1 underwent revision of the acetabular component for loosening, 2 femoral stems were revised for periprosthetic femur fracture, and 2 cases underwent 2-stage revision for late infection. There were no failures secondary to metallosis at the neck–stem junction and no modular breakages. The 3 cases of recurrent instability had no dislocation episodes after revision.
Discussion
PFNSM was developed to help more closely reconstruct patient anatomy. PFNSM allows for individualization of offset, length, and version—and thus for optimization of component interaction to avoid impingement and dislocation while promoting range of motion and normal gait.21 These benefits must be judged in light of the disadvantages of proximal stem modularity, including corrosion and breakage of the modular neck.14-18
In the present study, conducted in a high-volume private practice setting, 44% of necks used in a proximally modular construct had a head center identical to that of a nonmodular alternative. In the opinion of the 3 authors (high-volume hip surgeons), an additional 12% of the modular stems had a head center so close to that of the nonmodular stem as to be clinically indistinguishable. In addition, 132 of the modular necks had a femoral head center within 4 mm in length and 2 mm of offset of the nonmodular stem. These findings call into question the theoretical benefits of regular use of this modular femoral stem for primary THA. Certainly there are extreme femoral neck–shaft angle cases in which the standard nonmodular stem may be inadequate and this proximal modularity would be helpful, but our study showed such cases are relatively less frequent. We caution against routine use of this proximal modularity in primary THA and suggest restricting it to cases in which the standard stem offerings are unacceptable. These findings are not surprising given that the standard M/L Taper stem is based on a historically successful model with neck angle and length options designed to meet the goals of restoring length, offset, range of motion, and stability. We would expect that a well-designed stem will meet these goals in the majority of cases.
Of our 463 cases with the modular neck, 9 required revision surgery. Of these 9 revisions, 2 were for recurrent dislocation in which the modular neck was revised to one that enhanced stability, and there were no further dislocations. The ability to change the geometry of the proximal femur resulted in a stability solution that avoided revision of the entire femoral component, as might otherwise be required. One case of acetabular loosening and 1 case that required placement of a constrained liner were potentially benefited by the modular neck in that the surgeries may have been expedited by being able to remove the neck to ease exposure for placement of the acetabular components. The other 5 revisions—2 for periprosthetic femur fracture, 2 two-stage revisions for infection, and 1 femoral head exchange for metallosis at the head–neck junction—saw no benefit from the modularity in the revision setting.
This study had several limitations. First, as it was primarily an evaluation of use of a modular femoral system, there was no attempt to account for the fact that acetabular component orientation can affect stability and, thus, the perceived need for additional offset or changes in version. The habit of all 3 of the reviewing surgeons is to consider the position of the acetabular component and to reposition the component, if necessary, to achieve appropriate stability. Therefore, the need for the modularity may be even less than suggested by this study. In addition, the idea that (in 12 cases) no standard stem option would be acceptable because of the use of a verted neck ignores the possibility that cup repositioning could have obviated the need for additional version. Furthermore, use of a 36-mm head results in an additional 3.5 mm of offset in the polyethylene liner, and this study did not account for the option of increasing head size—and for the potential increase in stability from a larger head and the increased offset gained from the liner.
A second limitation is that a significant number of Kinectiv stems (132) had a head center within 4 mm in length and 2 mm of offset of the nearest M/L Taper stem. We carefully template every primary THA to determine the plan that will optimize component size and position and restore length and offset. More options for achieving these goals are available when templating with the intention of using the Kinectiv modular neck. The neck cut and position of the stem proximally or distally in the proximal femur may not need to be so exact, as the additional options may be able to accommodate minor inaccuracies. Thus, the reported percentage of clinically indistinguishable head centers (12%) may underestimate the actual number of modular stems that could have been replaced with a nonmodular stem.
Third, this study did not evaluate the effect of the modular junction on ease of irrigation and débridement with head/neck and polyethylene exchange in cases of infection, or on ease of head/neck and polyethylene exchange for revision. In addition, the study did not evaluate other cases of instability involving a nonmodular stem that otherwise could have been solved with simple revision of the head/neck combination, avoiding revision of the entire stem and/or the acetabular component. We reported revisions for infection and for instability, but comprehensive assessment and comparison were beyond the scope of this study. Certainly ease of revision of the head and neck is a factor that could favor use of the modularity.
Fourth, this was not a clinical outcome study comparing 2 different femoral stems. We sought only to determine how often a modular neck was chosen that resulted in a head center that would have been unavailable to the non-modular stem suggesting that the patient was receiving a reconstructive benefit in exchange for the modularity. However, 2 recent reports have noted no clinical benefit at 2-year follow-up with use of the modular neck compared with the nonmodular stem.22,23
Though the M/L Taper with Kinectiv technology has, thus far, performed well, PFNSM should be used with caution in light of recently reported failures at the neck–stem junction.14,16-18 Our study results suggest that most (≥56%) of the modular stems used could have been reconstructed as acceptably with a nonmodular stem, and therefore a reconstructive benefit was not realized in trade for the potential risks of proximal modularity. Only 2 of the 9 revision cases saw a clear advantage in being able to change the modular neck geometry in the revision setting. Given the recently reported failures and the high-profile recall of a modular stem,14 we recommend restricting the modular stem to cases that cannot be adequately reconstructed with the nonmodular option.
1. Barrack RL. Modularity of prosthetic implants. J Am Acad Orthop Surg. 1994;2(1):16-25.
2. Cameron HU. Modularity in primary total hip arthroplasty. J Arthroplasty. 1996;11(3):332-334.
3. Hozack WJ, Mesa JJ, Rothman RF. Head–neck modularity for total hip arthroplasty. Is it necessary? J Arthroplasty. 1996;11(4):397-399.
4. Holt GE, Christie MJ, Schwartz HS. Trabecular metal endoprosthetic limb salvage reconstruction of the lower limb. J Arthroplasty. 2009;24(7):1079-1089.
5. Sporer SM, Obar RJ, Bernini PM. Primary total hip arthroplasty using a modular proximally coated prosthesis in patients older than 70: two to eight year results. J Arthroplasty. 2004;19(2):197-203.
6. Spitzer AI. The S-ROM cementless femoral stem: history and literature review. Orthopedics. 2005;28(9 suppl):s1117-s1124.
7. Mumme T, Müller-Rath R, Andereya S, Wirtz DC. Uncemented femoral revision arthroplasty using the modular revision prosthesis MRP-TITAN revision stem. Oper Orthop Traumatol. 2007;19(1):56-77.
8. Wirtz DC, Heller KD, Holzwarth U, et al. A modular femoral implant for uncemented stem revision in THR. Int Orthop. 2000;24(3):134-138.
9. Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Revision total hip arthroplasty with a porous-coated modular stem: 5 to 10 years follow-up. Clin Orthop Relat Res. 2010;468(5):1310-1315.
10. Bolognesi MP, Pietrobon R, Clifford PE, Vail TP. Comparison of a hydroxyapatite-coated sleeve and a porous-coated sleeve with a modular revision hip stem. A prospective, randomized study. J Bone Joint Surg Am. 2004;86(12):2720-2725.
11. Huot Carlson JC, Van Citters DW, Currier JH, Bryant AM, Mayor MB, Collier JP. Femoral stem fracture and in vivo corrosion of retrieved modular femoral hips. J Arthroplasty. 2012;27(7):1389-1396.e1.
12. Gilbert JL, Mehta M, Pinder B. Fretting crevice corrosion of stainless steel stem–CoCr femoral head connections: comparisons of materials, initial moisture, and offset length. J Biomed Mater Res B Appl Biomater. 2009;88(1):162-173.
13. Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—at what cost? An implant retrieval study. Clin Orthop Relat Res. 2012;470(7):1885-1894.
14. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck–body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
15. Vundelinckx BJ, Verhelst LA, De Schepper J. Taper corrosion in modular hip prostheses: analysis of serum metal ions in 19 patients. J Arthroplasty. 2013;28(7):1218-1223.
16. Kouzelis A, Georgiou CS, Megas P. Dissociation of modular total hip arthroplasty at the neck–stem interface without dislocation. J Orthop Traumatol. 2012;13(4):221-224.
17. Sotereanos NG, Sauber TJ, Tupis TT. Modular femoral neck fracture after primary total hip arthroplasty. J Arthroplasty. 2013;28(1):196.e7-e9.
18. Wodecki P, Sabbah D, Kermarrec G, Semaan I. New type of hip arthroplasty failure related to modular femoral components: breakage at the neck–stem junction. Orthop Traumatol Surg Res. 2013;99(6):741-744.
19. Dorn U, Neumann D, Frank M. Corrosion behavior of tantalum-coated cobalt-chromium modular necks compared to titanium modular necks in a simulator test. J Arthroplasty. 2014;29(4):831-835.
20. Carothers JT, White RE, Tripuraneni KR, Hattab MW, Archibeck MJ. Lessons learned from managing a prospective, private practice joint replacement registry: a 25-year experience. Clin Orthop Relat Res. 2013;471(2):537-543.
21. Archibeck MJ, Cummins T, Carothers J, Junick DW, White RE Jr. A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin Orthop Rel Res. 2011;469(2):443-446.
22. Duwelius PJ, Hartzband MA, Burkhart R, et al. Clinical results of a modular neck hip system: hitting the “bull’s-eye” more accurately. Am J Orthop. 2010;39(10 suppl):2-6.
23. Duwelius PJ, Burkhart B, Carnahan C, et al. Modular versus nonmodular neck femoral implants in primary total hip arthroplasty: which is better? Clin Orthop Relat Res. 2014;472(4):1240-1245.
Femoral stem modularity in total hip arthroplasty (THA) has a checkered past. Developments such as the modular head–trunnion interface, which allows for placement of femoral heads of different sizes and offsets, and the modular midstem, which allows for version adjustments independent of patient anatomy (S-ROM, Depuy) and for bypassing proximal bone defects in the revision setting (Restoration Modular, Stryker; ZMR-XL, Zimmer), have proved very successful.1-10 However, even these successful advances have been associated with failures at the modular junction.11-13 Proximal femoral neck–stem modularity (PFNSM) has had mixed results, with notable failures and recalls associated with the neck–stem junction.14,15 Failures at this junction have occurred secondary to corrosion and breakage of the modular neck.16-18 Nevertheless, proximal modular stems remain available for implantation. One such system, the M/L Taper stem with Kinectiv technology (Zimmer), is an all-titanium construct that allows for adjustment of several variables (length, offset, version), providing numerous combinations beyond those of the original M/L Taper offerings. Advantages of these offerings include closer reconstruction of patient anatomy, stability improvements, and easing of the process of revision in polyethylene/femoral head exchanges or in infections in which single-staged irrigation and débridement and polyethylene/head exchange are chosen.
These theoretic advantages must be judged in the context of the possible disadvantages of the modular neck junction. The mechanical environment of the junction places it at risk for failure as well as for metallosis from fretting, crevice corrosion, and recurrent repassivation.19 Although the titanium necks are at less risk for degradation than their cobalt-chromium counterparts, they are at higher risk for breakage.13,19 For one of the surgeons in our practice, the M/L Taper stem with Kinectiv technology is the stem of choice for primary THA.
We conducted a study to determine, in the setting of primary THA, how often a neck–stem combination choice resulted in a reconstructive geometry that would not have been possible had the surgeon opted for the traditional M/L Taper stem. Every Kinectiv stem has numerous neck options with a head center position that would not be possible with the nonmodular M/L Taper. However, in a high-volume community practice, how often is a modular neck that results in an otherwise unavailable head center being used for the reconstruction?
Materials and Methods
This study was approved by our local institutional review board. The Kinectiv stem is used by 1 of the 4 high-volume joint replacement surgeons in our practice (not one of the authors). From our community practice joint registry, we identified every stem–neck combination used since the Kinectiv stem became available in 2006.20 Each case was performed using a posterior approach. A trabecular metal acetabular component (Zimmer) secured with 2 screws was used, and an M/L Taper stem with Kinectiv technology was implanted in each case.
Once the neck–stem combination was determined, its position on the head centers map was compared with that of the standard M/L Taper head centers (Figures 1, 2) for each stem size as the relationship of the Kinectiv head center varies with each stem size compared with the head center of the M/L Taper stems. If the head centers were in contact on the map, the geometry was considered identical. If the head centers were not in contact, we noted where the nearest standard M/L Taper head center lay in terms of length and offset. As the head centers are laid out in regular, 4-mm increments, this estimation was relatively easy. Any anteverted or retroverted neck was considered to have no adequate substitution in the standard M/L Taper stem offerings. This initial evaluation was performed by Dr. Carothers.
We then reviewed the head center comparisons independently. For every Kinectiv head center that did not contact an M/L Taper counterpart, the difference between those head centers was reviewed. Each of us noted whether the difference between the head centers was clinically relevant, as many of the head center positions are extremely close. The head centers that were so close as to be deemed clinically irrelevant were recorded.
Results
Between January 2008 and October 2013, 463 primary THAs were performed using the M/L Taper femoral stem with Kinectiv technology. Of the neck options used, 205 (44%) had a head center identical to that of a nonmodular M/L Taper stem. In another 56 cases (12%), all 3 reviewing surgeons agreed that the M/L Taper head center was so close to the Kinectiv head center as to be clinically indistinguishable. Of these 56 cases, 54 had a head center difference of less than 1 mm in length or offset; the other 2 had a 2-mm difference in offset.
Thus, a total of 261 stems (56%) had a standard M/L Taper option that offered an identical head center or one so close as to be clinically indistinguishable. Interestingly, in the group of 202 stems that did not have an identical head center and were not clinically indistinguishable, 132 (65%) of these modular stems were within 4 mm in length and 2 mm of offset of the closest Kinectiv head center. A verted neck was used in 12 cases (11 anteverted, 1 retroverted).
Nine of the 463 cases required revision surgery, 3 for recurrent instability. In 1 of these 3 cases, the acetabulum was revised for malposition, and the neck was converted from standard offset, +0 mm length (head center identical to nonmodular stem), to extended offset, +4 mm length (2 mm shorter with 1 mm less offset than closest nonmodular head center). The second case had complete deficiency of the abductor tendons and was converted to a constrained liner, though at the time of the THA a head center identical to that of the nonmodular stem was used. The third case was revised to convert a standard offset, +0 mm length, straight neck (head center identical to nonmodular stem), to extended offset, +4 mm length, anteverted neck (anteversion making this a unique head center position). Of the other 6 cases, 1 was treated for corrosion at the head–neck junction by changing the head from cobalt-chromium to ceramic (the junction was noted to be pristine), 1 underwent revision of the acetabular component for loosening, 2 femoral stems were revised for periprosthetic femur fracture, and 2 cases underwent 2-stage revision for late infection. There were no failures secondary to metallosis at the neck–stem junction and no modular breakages. The 3 cases of recurrent instability had no dislocation episodes after revision.
Discussion
PFNSM was developed to help more closely reconstruct patient anatomy. PFNSM allows for individualization of offset, length, and version—and thus for optimization of component interaction to avoid impingement and dislocation while promoting range of motion and normal gait.21 These benefits must be judged in light of the disadvantages of proximal stem modularity, including corrosion and breakage of the modular neck.14-18
In the present study, conducted in a high-volume private practice setting, 44% of necks used in a proximally modular construct had a head center identical to that of a nonmodular alternative. In the opinion of the 3 authors (high-volume hip surgeons), an additional 12% of the modular stems had a head center so close to that of the nonmodular stem as to be clinically indistinguishable. In addition, 132 of the modular necks had a femoral head center within 4 mm in length and 2 mm of offset of the nonmodular stem. These findings call into question the theoretical benefits of regular use of this modular femoral stem for primary THA. Certainly there are extreme femoral neck–shaft angle cases in which the standard nonmodular stem may be inadequate and this proximal modularity would be helpful, but our study showed such cases are relatively less frequent. We caution against routine use of this proximal modularity in primary THA and suggest restricting it to cases in which the standard stem offerings are unacceptable. These findings are not surprising given that the standard M/L Taper stem is based on a historically successful model with neck angle and length options designed to meet the goals of restoring length, offset, range of motion, and stability. We would expect that a well-designed stem will meet these goals in the majority of cases.
Of our 463 cases with the modular neck, 9 required revision surgery. Of these 9 revisions, 2 were for recurrent dislocation in which the modular neck was revised to one that enhanced stability, and there were no further dislocations. The ability to change the geometry of the proximal femur resulted in a stability solution that avoided revision of the entire femoral component, as might otherwise be required. One case of acetabular loosening and 1 case that required placement of a constrained liner were potentially benefited by the modular neck in that the surgeries may have been expedited by being able to remove the neck to ease exposure for placement of the acetabular components. The other 5 revisions—2 for periprosthetic femur fracture, 2 two-stage revisions for infection, and 1 femoral head exchange for metallosis at the head–neck junction—saw no benefit from the modularity in the revision setting.
This study had several limitations. First, as it was primarily an evaluation of use of a modular femoral system, there was no attempt to account for the fact that acetabular component orientation can affect stability and, thus, the perceived need for additional offset or changes in version. The habit of all 3 of the reviewing surgeons is to consider the position of the acetabular component and to reposition the component, if necessary, to achieve appropriate stability. Therefore, the need for the modularity may be even less than suggested by this study. In addition, the idea that (in 12 cases) no standard stem option would be acceptable because of the use of a verted neck ignores the possibility that cup repositioning could have obviated the need for additional version. Furthermore, use of a 36-mm head results in an additional 3.5 mm of offset in the polyethylene liner, and this study did not account for the option of increasing head size—and for the potential increase in stability from a larger head and the increased offset gained from the liner.
A second limitation is that a significant number of Kinectiv stems (132) had a head center within 4 mm in length and 2 mm of offset of the nearest M/L Taper stem. We carefully template every primary THA to determine the plan that will optimize component size and position and restore length and offset. More options for achieving these goals are available when templating with the intention of using the Kinectiv modular neck. The neck cut and position of the stem proximally or distally in the proximal femur may not need to be so exact, as the additional options may be able to accommodate minor inaccuracies. Thus, the reported percentage of clinically indistinguishable head centers (12%) may underestimate the actual number of modular stems that could have been replaced with a nonmodular stem.
Third, this study did not evaluate the effect of the modular junction on ease of irrigation and débridement with head/neck and polyethylene exchange in cases of infection, or on ease of head/neck and polyethylene exchange for revision. In addition, the study did not evaluate other cases of instability involving a nonmodular stem that otherwise could have been solved with simple revision of the head/neck combination, avoiding revision of the entire stem and/or the acetabular component. We reported revisions for infection and for instability, but comprehensive assessment and comparison were beyond the scope of this study. Certainly ease of revision of the head and neck is a factor that could favor use of the modularity.
Fourth, this was not a clinical outcome study comparing 2 different femoral stems. We sought only to determine how often a modular neck was chosen that resulted in a head center that would have been unavailable to the non-modular stem suggesting that the patient was receiving a reconstructive benefit in exchange for the modularity. However, 2 recent reports have noted no clinical benefit at 2-year follow-up with use of the modular neck compared with the nonmodular stem.22,23
Though the M/L Taper with Kinectiv technology has, thus far, performed well, PFNSM should be used with caution in light of recently reported failures at the neck–stem junction.14,16-18 Our study results suggest that most (≥56%) of the modular stems used could have been reconstructed as acceptably with a nonmodular stem, and therefore a reconstructive benefit was not realized in trade for the potential risks of proximal modularity. Only 2 of the 9 revision cases saw a clear advantage in being able to change the modular neck geometry in the revision setting. Given the recently reported failures and the high-profile recall of a modular stem,14 we recommend restricting the modular stem to cases that cannot be adequately reconstructed with the nonmodular option.
Femoral stem modularity in total hip arthroplasty (THA) has a checkered past. Developments such as the modular head–trunnion interface, which allows for placement of femoral heads of different sizes and offsets, and the modular midstem, which allows for version adjustments independent of patient anatomy (S-ROM, Depuy) and for bypassing proximal bone defects in the revision setting (Restoration Modular, Stryker; ZMR-XL, Zimmer), have proved very successful.1-10 However, even these successful advances have been associated with failures at the modular junction.11-13 Proximal femoral neck–stem modularity (PFNSM) has had mixed results, with notable failures and recalls associated with the neck–stem junction.14,15 Failures at this junction have occurred secondary to corrosion and breakage of the modular neck.16-18 Nevertheless, proximal modular stems remain available for implantation. One such system, the M/L Taper stem with Kinectiv technology (Zimmer), is an all-titanium construct that allows for adjustment of several variables (length, offset, version), providing numerous combinations beyond those of the original M/L Taper offerings. Advantages of these offerings include closer reconstruction of patient anatomy, stability improvements, and easing of the process of revision in polyethylene/femoral head exchanges or in infections in which single-staged irrigation and débridement and polyethylene/head exchange are chosen.
These theoretic advantages must be judged in the context of the possible disadvantages of the modular neck junction. The mechanical environment of the junction places it at risk for failure as well as for metallosis from fretting, crevice corrosion, and recurrent repassivation.19 Although the titanium necks are at less risk for degradation than their cobalt-chromium counterparts, they are at higher risk for breakage.13,19 For one of the surgeons in our practice, the M/L Taper stem with Kinectiv technology is the stem of choice for primary THA.
We conducted a study to determine, in the setting of primary THA, how often a neck–stem combination choice resulted in a reconstructive geometry that would not have been possible had the surgeon opted for the traditional M/L Taper stem. Every Kinectiv stem has numerous neck options with a head center position that would not be possible with the nonmodular M/L Taper. However, in a high-volume community practice, how often is a modular neck that results in an otherwise unavailable head center being used for the reconstruction?
Materials and Methods
This study was approved by our local institutional review board. The Kinectiv stem is used by 1 of the 4 high-volume joint replacement surgeons in our practice (not one of the authors). From our community practice joint registry, we identified every stem–neck combination used since the Kinectiv stem became available in 2006.20 Each case was performed using a posterior approach. A trabecular metal acetabular component (Zimmer) secured with 2 screws was used, and an M/L Taper stem with Kinectiv technology was implanted in each case.
Once the neck–stem combination was determined, its position on the head centers map was compared with that of the standard M/L Taper head centers (Figures 1, 2) for each stem size as the relationship of the Kinectiv head center varies with each stem size compared with the head center of the M/L Taper stems. If the head centers were in contact on the map, the geometry was considered identical. If the head centers were not in contact, we noted where the nearest standard M/L Taper head center lay in terms of length and offset. As the head centers are laid out in regular, 4-mm increments, this estimation was relatively easy. Any anteverted or retroverted neck was considered to have no adequate substitution in the standard M/L Taper stem offerings. This initial evaluation was performed by Dr. Carothers.
We then reviewed the head center comparisons independently. For every Kinectiv head center that did not contact an M/L Taper counterpart, the difference between those head centers was reviewed. Each of us noted whether the difference between the head centers was clinically relevant, as many of the head center positions are extremely close. The head centers that were so close as to be deemed clinically irrelevant were recorded.
Results
Between January 2008 and October 2013, 463 primary THAs were performed using the M/L Taper femoral stem with Kinectiv technology. Of the neck options used, 205 (44%) had a head center identical to that of a nonmodular M/L Taper stem. In another 56 cases (12%), all 3 reviewing surgeons agreed that the M/L Taper head center was so close to the Kinectiv head center as to be clinically indistinguishable. Of these 56 cases, 54 had a head center difference of less than 1 mm in length or offset; the other 2 had a 2-mm difference in offset.
Thus, a total of 261 stems (56%) had a standard M/L Taper option that offered an identical head center or one so close as to be clinically indistinguishable. Interestingly, in the group of 202 stems that did not have an identical head center and were not clinically indistinguishable, 132 (65%) of these modular stems were within 4 mm in length and 2 mm of offset of the closest Kinectiv head center. A verted neck was used in 12 cases (11 anteverted, 1 retroverted).
Nine of the 463 cases required revision surgery, 3 for recurrent instability. In 1 of these 3 cases, the acetabulum was revised for malposition, and the neck was converted from standard offset, +0 mm length (head center identical to nonmodular stem), to extended offset, +4 mm length (2 mm shorter with 1 mm less offset than closest nonmodular head center). The second case had complete deficiency of the abductor tendons and was converted to a constrained liner, though at the time of the THA a head center identical to that of the nonmodular stem was used. The third case was revised to convert a standard offset, +0 mm length, straight neck (head center identical to nonmodular stem), to extended offset, +4 mm length, anteverted neck (anteversion making this a unique head center position). Of the other 6 cases, 1 was treated for corrosion at the head–neck junction by changing the head from cobalt-chromium to ceramic (the junction was noted to be pristine), 1 underwent revision of the acetabular component for loosening, 2 femoral stems were revised for periprosthetic femur fracture, and 2 cases underwent 2-stage revision for late infection. There were no failures secondary to metallosis at the neck–stem junction and no modular breakages. The 3 cases of recurrent instability had no dislocation episodes after revision.
Discussion
PFNSM was developed to help more closely reconstruct patient anatomy. PFNSM allows for individualization of offset, length, and version—and thus for optimization of component interaction to avoid impingement and dislocation while promoting range of motion and normal gait.21 These benefits must be judged in light of the disadvantages of proximal stem modularity, including corrosion and breakage of the modular neck.14-18
In the present study, conducted in a high-volume private practice setting, 44% of necks used in a proximally modular construct had a head center identical to that of a nonmodular alternative. In the opinion of the 3 authors (high-volume hip surgeons), an additional 12% of the modular stems had a head center so close to that of the nonmodular stem as to be clinically indistinguishable. In addition, 132 of the modular necks had a femoral head center within 4 mm in length and 2 mm of offset of the nonmodular stem. These findings call into question the theoretical benefits of regular use of this modular femoral stem for primary THA. Certainly there are extreme femoral neck–shaft angle cases in which the standard nonmodular stem may be inadequate and this proximal modularity would be helpful, but our study showed such cases are relatively less frequent. We caution against routine use of this proximal modularity in primary THA and suggest restricting it to cases in which the standard stem offerings are unacceptable. These findings are not surprising given that the standard M/L Taper stem is based on a historically successful model with neck angle and length options designed to meet the goals of restoring length, offset, range of motion, and stability. We would expect that a well-designed stem will meet these goals in the majority of cases.
Of our 463 cases with the modular neck, 9 required revision surgery. Of these 9 revisions, 2 were for recurrent dislocation in which the modular neck was revised to one that enhanced stability, and there were no further dislocations. The ability to change the geometry of the proximal femur resulted in a stability solution that avoided revision of the entire femoral component, as might otherwise be required. One case of acetabular loosening and 1 case that required placement of a constrained liner were potentially benefited by the modular neck in that the surgeries may have been expedited by being able to remove the neck to ease exposure for placement of the acetabular components. The other 5 revisions—2 for periprosthetic femur fracture, 2 two-stage revisions for infection, and 1 femoral head exchange for metallosis at the head–neck junction—saw no benefit from the modularity in the revision setting.
This study had several limitations. First, as it was primarily an evaluation of use of a modular femoral system, there was no attempt to account for the fact that acetabular component orientation can affect stability and, thus, the perceived need for additional offset or changes in version. The habit of all 3 of the reviewing surgeons is to consider the position of the acetabular component and to reposition the component, if necessary, to achieve appropriate stability. Therefore, the need for the modularity may be even less than suggested by this study. In addition, the idea that (in 12 cases) no standard stem option would be acceptable because of the use of a verted neck ignores the possibility that cup repositioning could have obviated the need for additional version. Furthermore, use of a 36-mm head results in an additional 3.5 mm of offset in the polyethylene liner, and this study did not account for the option of increasing head size—and for the potential increase in stability from a larger head and the increased offset gained from the liner.
A second limitation is that a significant number of Kinectiv stems (132) had a head center within 4 mm in length and 2 mm of offset of the nearest M/L Taper stem. We carefully template every primary THA to determine the plan that will optimize component size and position and restore length and offset. More options for achieving these goals are available when templating with the intention of using the Kinectiv modular neck. The neck cut and position of the stem proximally or distally in the proximal femur may not need to be so exact, as the additional options may be able to accommodate minor inaccuracies. Thus, the reported percentage of clinically indistinguishable head centers (12%) may underestimate the actual number of modular stems that could have been replaced with a nonmodular stem.
Third, this study did not evaluate the effect of the modular junction on ease of irrigation and débridement with head/neck and polyethylene exchange in cases of infection, or on ease of head/neck and polyethylene exchange for revision. In addition, the study did not evaluate other cases of instability involving a nonmodular stem that otherwise could have been solved with simple revision of the head/neck combination, avoiding revision of the entire stem and/or the acetabular component. We reported revisions for infection and for instability, but comprehensive assessment and comparison were beyond the scope of this study. Certainly ease of revision of the head and neck is a factor that could favor use of the modularity.
Fourth, this was not a clinical outcome study comparing 2 different femoral stems. We sought only to determine how often a modular neck was chosen that resulted in a head center that would have been unavailable to the non-modular stem suggesting that the patient was receiving a reconstructive benefit in exchange for the modularity. However, 2 recent reports have noted no clinical benefit at 2-year follow-up with use of the modular neck compared with the nonmodular stem.22,23
Though the M/L Taper with Kinectiv technology has, thus far, performed well, PFNSM should be used with caution in light of recently reported failures at the neck–stem junction.14,16-18 Our study results suggest that most (≥56%) of the modular stems used could have been reconstructed as acceptably with a nonmodular stem, and therefore a reconstructive benefit was not realized in trade for the potential risks of proximal modularity. Only 2 of the 9 revision cases saw a clear advantage in being able to change the modular neck geometry in the revision setting. Given the recently reported failures and the high-profile recall of a modular stem,14 we recommend restricting the modular stem to cases that cannot be adequately reconstructed with the nonmodular option.
1. Barrack RL. Modularity of prosthetic implants. J Am Acad Orthop Surg. 1994;2(1):16-25.
2. Cameron HU. Modularity in primary total hip arthroplasty. J Arthroplasty. 1996;11(3):332-334.
3. Hozack WJ, Mesa JJ, Rothman RF. Head–neck modularity for total hip arthroplasty. Is it necessary? J Arthroplasty. 1996;11(4):397-399.
4. Holt GE, Christie MJ, Schwartz HS. Trabecular metal endoprosthetic limb salvage reconstruction of the lower limb. J Arthroplasty. 2009;24(7):1079-1089.
5. Sporer SM, Obar RJ, Bernini PM. Primary total hip arthroplasty using a modular proximally coated prosthesis in patients older than 70: two to eight year results. J Arthroplasty. 2004;19(2):197-203.
6. Spitzer AI. The S-ROM cementless femoral stem: history and literature review. Orthopedics. 2005;28(9 suppl):s1117-s1124.
7. Mumme T, Müller-Rath R, Andereya S, Wirtz DC. Uncemented femoral revision arthroplasty using the modular revision prosthesis MRP-TITAN revision stem. Oper Orthop Traumatol. 2007;19(1):56-77.
8. Wirtz DC, Heller KD, Holzwarth U, et al. A modular femoral implant for uncemented stem revision in THR. Int Orthop. 2000;24(3):134-138.
9. Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Revision total hip arthroplasty with a porous-coated modular stem: 5 to 10 years follow-up. Clin Orthop Relat Res. 2010;468(5):1310-1315.
10. Bolognesi MP, Pietrobon R, Clifford PE, Vail TP. Comparison of a hydroxyapatite-coated sleeve and a porous-coated sleeve with a modular revision hip stem. A prospective, randomized study. J Bone Joint Surg Am. 2004;86(12):2720-2725.
11. Huot Carlson JC, Van Citters DW, Currier JH, Bryant AM, Mayor MB, Collier JP. Femoral stem fracture and in vivo corrosion of retrieved modular femoral hips. J Arthroplasty. 2012;27(7):1389-1396.e1.
12. Gilbert JL, Mehta M, Pinder B. Fretting crevice corrosion of stainless steel stem–CoCr femoral head connections: comparisons of materials, initial moisture, and offset length. J Biomed Mater Res B Appl Biomater. 2009;88(1):162-173.
13. Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—at what cost? An implant retrieval study. Clin Orthop Relat Res. 2012;470(7):1885-1894.
14. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck–body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
15. Vundelinckx BJ, Verhelst LA, De Schepper J. Taper corrosion in modular hip prostheses: analysis of serum metal ions in 19 patients. J Arthroplasty. 2013;28(7):1218-1223.
16. Kouzelis A, Georgiou CS, Megas P. Dissociation of modular total hip arthroplasty at the neck–stem interface without dislocation. J Orthop Traumatol. 2012;13(4):221-224.
17. Sotereanos NG, Sauber TJ, Tupis TT. Modular femoral neck fracture after primary total hip arthroplasty. J Arthroplasty. 2013;28(1):196.e7-e9.
18. Wodecki P, Sabbah D, Kermarrec G, Semaan I. New type of hip arthroplasty failure related to modular femoral components: breakage at the neck–stem junction. Orthop Traumatol Surg Res. 2013;99(6):741-744.
19. Dorn U, Neumann D, Frank M. Corrosion behavior of tantalum-coated cobalt-chromium modular necks compared to titanium modular necks in a simulator test. J Arthroplasty. 2014;29(4):831-835.
20. Carothers JT, White RE, Tripuraneni KR, Hattab MW, Archibeck MJ. Lessons learned from managing a prospective, private practice joint replacement registry: a 25-year experience. Clin Orthop Relat Res. 2013;471(2):537-543.
21. Archibeck MJ, Cummins T, Carothers J, Junick DW, White RE Jr. A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin Orthop Rel Res. 2011;469(2):443-446.
22. Duwelius PJ, Hartzband MA, Burkhart R, et al. Clinical results of a modular neck hip system: hitting the “bull’s-eye” more accurately. Am J Orthop. 2010;39(10 suppl):2-6.
23. Duwelius PJ, Burkhart B, Carnahan C, et al. Modular versus nonmodular neck femoral implants in primary total hip arthroplasty: which is better? Clin Orthop Relat Res. 2014;472(4):1240-1245.
1. Barrack RL. Modularity of prosthetic implants. J Am Acad Orthop Surg. 1994;2(1):16-25.
2. Cameron HU. Modularity in primary total hip arthroplasty. J Arthroplasty. 1996;11(3):332-334.
3. Hozack WJ, Mesa JJ, Rothman RF. Head–neck modularity for total hip arthroplasty. Is it necessary? J Arthroplasty. 1996;11(4):397-399.
4. Holt GE, Christie MJ, Schwartz HS. Trabecular metal endoprosthetic limb salvage reconstruction of the lower limb. J Arthroplasty. 2009;24(7):1079-1089.
5. Sporer SM, Obar RJ, Bernini PM. Primary total hip arthroplasty using a modular proximally coated prosthesis in patients older than 70: two to eight year results. J Arthroplasty. 2004;19(2):197-203.
6. Spitzer AI. The S-ROM cementless femoral stem: history and literature review. Orthopedics. 2005;28(9 suppl):s1117-s1124.
7. Mumme T, Müller-Rath R, Andereya S, Wirtz DC. Uncemented femoral revision arthroplasty using the modular revision prosthesis MRP-TITAN revision stem. Oper Orthop Traumatol. 2007;19(1):56-77.
8. Wirtz DC, Heller KD, Holzwarth U, et al. A modular femoral implant for uncemented stem revision in THR. Int Orthop. 2000;24(3):134-138.
9. Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Revision total hip arthroplasty with a porous-coated modular stem: 5 to 10 years follow-up. Clin Orthop Relat Res. 2010;468(5):1310-1315.
10. Bolognesi MP, Pietrobon R, Clifford PE, Vail TP. Comparison of a hydroxyapatite-coated sleeve and a porous-coated sleeve with a modular revision hip stem. A prospective, randomized study. J Bone Joint Surg Am. 2004;86(12):2720-2725.
11. Huot Carlson JC, Van Citters DW, Currier JH, Bryant AM, Mayor MB, Collier JP. Femoral stem fracture and in vivo corrosion of retrieved modular femoral hips. J Arthroplasty. 2012;27(7):1389-1396.e1.
12. Gilbert JL, Mehta M, Pinder B. Fretting crevice corrosion of stainless steel stem–CoCr femoral head connections: comparisons of materials, initial moisture, and offset length. J Biomed Mater Res B Appl Biomater. 2009;88(1):162-173.
13. Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—at what cost? An implant retrieval study. Clin Orthop Relat Res. 2012;470(7):1885-1894.
14. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck–body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
15. Vundelinckx BJ, Verhelst LA, De Schepper J. Taper corrosion in modular hip prostheses: analysis of serum metal ions in 19 patients. J Arthroplasty. 2013;28(7):1218-1223.
16. Kouzelis A, Georgiou CS, Megas P. Dissociation of modular total hip arthroplasty at the neck–stem interface without dislocation. J Orthop Traumatol. 2012;13(4):221-224.
17. Sotereanos NG, Sauber TJ, Tupis TT. Modular femoral neck fracture after primary total hip arthroplasty. J Arthroplasty. 2013;28(1):196.e7-e9.
18. Wodecki P, Sabbah D, Kermarrec G, Semaan I. New type of hip arthroplasty failure related to modular femoral components: breakage at the neck–stem junction. Orthop Traumatol Surg Res. 2013;99(6):741-744.
19. Dorn U, Neumann D, Frank M. Corrosion behavior of tantalum-coated cobalt-chromium modular necks compared to titanium modular necks in a simulator test. J Arthroplasty. 2014;29(4):831-835.
20. Carothers JT, White RE, Tripuraneni KR, Hattab MW, Archibeck MJ. Lessons learned from managing a prospective, private practice joint replacement registry: a 25-year experience. Clin Orthop Relat Res. 2013;471(2):537-543.
21. Archibeck MJ, Cummins T, Carothers J, Junick DW, White RE Jr. A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin Orthop Rel Res. 2011;469(2):443-446.
22. Duwelius PJ, Hartzband MA, Burkhart R, et al. Clinical results of a modular neck hip system: hitting the “bull’s-eye” more accurately. Am J Orthop. 2010;39(10 suppl):2-6.
23. Duwelius PJ, Burkhart B, Carnahan C, et al. Modular versus nonmodular neck femoral implants in primary total hip arthroplasty: which is better? Clin Orthop Relat Res. 2014;472(4):1240-1245.
Failure of Total Hip Arthroplasty Secondary to Infection Caused by Brucella abortus and the Risk of Transmission to Operative Staff
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
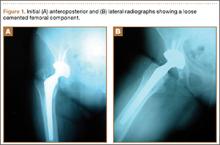
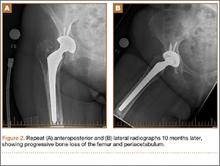
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
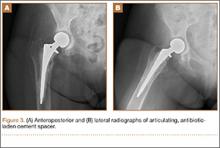
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
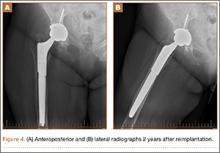
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.