User login
Warfarin therapy: Tips and tools for better control
• INR testing by an anticoagulation management service or private clinician can be reduced to intervals of as long as 4 weeks, but should be more frequent when dosing adjustments occur. B
• Weekly patient self-testing is associated with comparable clinical outcomes to high-quality clinic-based anticoagulation management. A
• Patients who self-test (and report their results) weekly should test more frequently when a change in medication (including herbal remedies and dietary supplements) or diet or an illness occurs. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 4 million Americans are receiving longterm oral anticoagulation therapy to reduce the risk of primary and secondary thromboembolism.1,2 And, as the population ages, the number of patients on lifelong therapy with warfarin—the only oral anticoagulant available in the United States until dabigatran was approved by the US Food and Drug Administration late last year3—is expected to grow.4
Such patients present a challenge for family physicians. Warfarin is notorious for having both a narrow therapeutic index and numerous drug and dietary interactions.5,6 To safeguard patients on warfarin therapy, frequent, and diligent, monitoring is required.
Engaging patients as participants in their own care can help you decrease the hazards. With that in mind, this article features warfarin treatment tips and tools for both physicians and patients, along with a review of some basic safeguards.
Warfarin therapy: Keeping it safe
Warfarin, a vitamin K antagonist, is used to prevent systemic embolism in patients with prosthetic heart valves, atrial fibrillation, or inherited/acquired thrombophilic disorders; as an adjunct in the prophylaxis of systemic embolism after myocardial infarction (MI); and to reduce the risk of recurrent MI, as well as venous thromboembolism.4,7 Because there is a small but definite risk (1%-2% per year)8 of severe bleeding associated with warfarin, however, therapy should be initiated only when the potential benefits clearly outweigh the risks.
A major contraindication for warfarin therapy is early pregnancy. The anticoagulant is a teratogen, causing deformations of the face (depressed nasal bridge) and bones (stippled epiphyses), neonatal seizures, and spontaneous abortion. If a woman in the first trimester of pregnancy requires anticoagulation, low-molecular-weight heparin should be substituted instead.9
In fact, warfarin is not recommended in the second or third trimesters either, as the use of vitamin K antagonists increases the risk of miscarriages, structural defects, and other adverse outcomes. Nor is warfarin recommended for women who are planning to become pregnant.
Warfarin is also contraindicated in patients for whom the risk of major bleeding outweighs the benefits. Risk factors for warfarin-associated bleeding include renal insufficiency and concomitant antiplatelet therapy, and physicians can use published clinical prediction rules to estimate bleeding risk.10
Dosing considerations
When you start a patient on warfarin therapy, it is important to ensure that therapeutic concentrations are achieved in a timely manner—and that the risk of supra- and subtherapeutic international normalized ratio (INR) values—≥4.0 and <2.0, respectively—is minimized.6
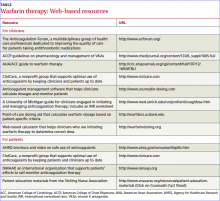
Factors to consider in determining the starting dose include patient-specifi c measures such as age, height, and weight; concomitant medications; and comorbidities. Increasing age, female sex, and a low body mass index all indicate a need for a lower dose.11 A number of Web-based dosing calculators (TABLE) can help clinicians estimate the therapeutic dose in patients who are new to warfarin.
Thyroid activity also affects warfarin dosing requirements.12 Hypothyroidism makes people less responsive to warfarin,13 while hyperthyroidism boosts the anticoagulant effect.14 Several mechanisms have been proposed for this effect, including changes in the rate of breakdown of clotting factors and in the metabolism of warfarin.15,16
Frequency of monitoring. Regardless of the initiation dose, INR values of outpatients should be monitored at least 2 to 3 times a week for the fi rst 7 to 10 days of therapy, or until a stable value is achieved. (In an inpatient setting, INR monitoring is usually performed daily until the therapeutic range has been maintained for ≥2 days.) The target INR level varies from case to case depending on the clinical indicators, but tends to be between 2 and 3 for most patients and between 2.5 and 3.5 for those with mechanical heart valves.17
After stabilization, testing can be reduced to intervals of as long as 4 weeks, althoughevidence suggests that more frequent testing leads to greater time-in-therapeutic range (TTR).18,19 When dosing adjustments are required, the cycle of more frequent monitoring should be repeated until a stable dose response can again be achieved.
Benefits of patient involvement
Patients on warfarin may be managed in one or more of the following 3 methods: (1) with usual care, provided by the patient’s personal physician; (2) by anticoagulation management services (AMSs), specialized programs overseen by physicians, pharmacists, and/or nurses; or (3) by self-testing/self-management, with the help of point-of-care devices that allow patients to monitor their own INR levels and adjust their anticoagulation dose, within certain limits, in consultation with a clinician.4
Many nonrandomized retrospective studies have reported better outcomes in patients whose anticoagulant therapy is managed by an AMS vs management by a primary care physician or specialist alone.7 Compared with usual care, AMS programs have been shown to greatly improve patients’ TTR, thereby reducing hemorrhage or thrombosis as a consequence of excessive or subtherapeutic anticoagulation.4,20,21
Self-testing/self-management—which depends on adequate patient training—has similar benefits: Self-care facilitates more frequent monitoring and empowers patients, and may be a major factor in patient compliance.4 Individuals using their own portable INR monitors and managing their own care have been found to have improved TTRs and a lower frequency of major hemorrhage or thrombosis compared with patients receiving usual care.7,18 The recent THINRS trial randomized 2922 patients to perform weekly self-testing or receive monthly clinic-based testing at an institution with a system for providing anticoagulant care. The study confirmed that patient self-testing is feasible for most warfarin-treated individuals and that weekly home monitoring is as safe and effective as high-quality clinic-based testing.22
Who’s a candidate for self-management?
Various studies have found that, as with insulin-dependent diabetes, most patients who are independent and self-supporting are, in principle, capable of self-management of oral anticoagulation, regardless of education or social status.23,24 The only intellectual requirement is that the patient (or caregiver) grasp the concept of anticoagulant therapy and understand the potential risks. (For more help in determining whether your patient is eligible for self-management, see “Self-monitoring—for which patients?” on page 74.)
The patient must also be willing to actively participate in his or her own care and have sufficient manual dexterity and visual acuity. No previous experience in self-testing or monitoring is necessary.7
INR monitors for patients and physicians
Since the late 1980s, point-of-care devices that measure INR values have made it possible for an increasing number of patients to monitor the anticoagulant eff ects of warfarin without repeat visits to a health care facility. Of the 4 million US residents on warfarin, approximately 60,000 (1.6%) engage in self-testing, according to the International Self-Monitoring Association of Oral Anticoagulated Patients (www.ismaap.org).
One reason may be the cost. Portable monitors are available for approximately $2495, according to Alere Inc., a health management company—a price that may include supplies and training. The expense may not be covered by private insurers. However, in 2008, Medicare began covering the cost of INR monitors (and the testing materials required for their use) for seniors receiving anticoagulation therapy associated with mechanical heart valves, chronic atrial fibrillation, or venous thromboembolism.25 Portable monitoring devices include the following:
CoaguChek (http://www.coaguchek.com). The CoaguChek brand, now in its third generation, features both a monitor (CoaguChek XS) for patient use and a system (CoaguChek XS Plus) for health care professionals. CoaguChek has extended quality control and data management options.
INRatio2 PT/INR Monitor (www.hemosense.com). The HemoSense INRatio2 is a new whole-blood patient monitoring system. The device is well suited for use by both health care professionals and patients.
ProTime PT/INR Monitor (www.protimesystem.com). The ProTime Microcoagulation System is a portable, batteryoperated testing tool designed for both professionals and patients. There are also companies that sell or loan the devices to patients and provide the supplies, training, and support for enrollees engaged in self-testing, including Philips (http://www.inrselftest.com/content) and Roche (https://www.poc.roche.com/poc/home.do).
Preparing patients for self-management In addition to acquiring a monitor, patients interested in self-testing and management need to be aware that the risk of bleeding rises steeply when the INR exceeds 4.0—and the risk of thrombosis increases when INR values fall below 2.0.7
Guard against interactions. Emphasize that numerous environmental factors, such as drugs, diet, alcohol, and various disease states, can alter the pharmacokinetics of warfarin.26 Consequently, INR values need to be measured more frequently than the usual 4-week intervals when a patient taking warfarin adds (orstops taking) virtually any drug, dietary supplement, or herbal remedy, or significantly alters his or her vitamin K intake. Illnesses with a fever, such as infl uenza, or diarrhea and vomiting lasting more than one day, can also aff ect INR levels, and call for more frequent testing and possible adjustments in warfarin dosing.27
Explain that some drugs reduce warfarin’s anticoagulant eff ect by reducing its absorption or enhancing its clearance, while others—including many commonly used antibiotics—enhance the drug’s anticoagulant eff ect by inhibiting its clearance.6,7 Remind patients that the risk of bleeding is high when warfarin is combined with antiplatelet agents such as clopidogrel, aspirin, or nonsteroidal anti-infl ammatory drugs, among other medications.27 And caution them that excessive use of alcohol aff ects the metabolism of warfarin and can elevate the INR.26 (See Patient on warfarin? Steer clear of these drugs, in "Avoiding drug interactions: Here’s help," J Fam Pract. 2010; 59: 322-329.)
Seek medical attention. Patients engaged in self-testing and monitoring also need to be aware of the importance of obtaining treatment for dangerously high or low INR levels and being alert to early indicators of bleeding or other significant adverse effects. Similarly, family physicians who care for such patients need to establish a system to ensure that these individuals are not lost to followup. Whether INR results are transmitted by fax, phone, or e-mail, a patient who leaves a message reporting an INR of 5.6, for example, requires a callback without delay.
Advise patients to watch for signs of warfarin-induced skin necrosis—a rare but serious complication of oral anticoagulant therapy characterized by dusky skin discoloration and pain, typically in an area with significant subcutaneous fat (eg, the breast or abdominal wall). Warfarin necrosis is estimated to occur in 0.01% to 0.1% of patients—primarily women—mostly in the first week of therapy.15 Other serious adverse effects are osteoporosis and purple toe syndrome.1
Patients—and their family members—should also be advised that if the patient is hospitalized, it is critical to let the health care team know that he or she is taking warfarin. Patients should be encouraged to wear a medic alert bracelet, as well.
Warfarin’s effects can be reversed with vitamin K. (See “What to do when warfarin therapy goes too far,” J Fam Pract. 2009;58:346-352.) However, reversal may take 24 hours.7 In patients with life-threatening bleeding (eg, intracranial hemorrhage) and elevated INR, regardless of the magnitude of the elevation, INR should be normalized urgently with fresh frozen plasma, prothrombin complex concentrate, or recombinant factor VIIa supplemented with vitamin K10 mg by slow intravenous infusion.7
CORRESPONDENCE
Michael J. Schwartz, MD, 5 Sunnydale Circle, Swannanoa, NC 28778; ms112@columbia.edu
1. International Self-Monitoring Association of Oral Anticoagulated Patients. We motivate patients to take control of their own oral anticoagulation therapy. Available at: http://www.ismaap.org. Accessed January 12, 2011.
2. Alere Introducing InRatio 2. Available at: www.hemosense. com. Accessed January 25, 2009.
3. US Food and Drug Administration. FDA approves Pradaxa to prevent stroke in people with atrial fibrillation [press release]. October 19, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm230241.htm. Accessed January 12, 2011.
4. Garcia DA, Witt DM, Hyleck E, et al. Delivery of optimized anticoagulant therapy consensus statement from the Anticoagulant Forum. Ann Pharmacother. 2008;42:979-988.
5. Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):204S-233S.
6. Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(6 suppl):257S-299S.
9. Lip GY, Frison L, Halperin JL, et al. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) Score. J Am Coll Cardiol. 2011;57:173-180.
10. Beckmann CR. Obstetrics and Gynecology. 4th ed. Baltimore, Md: Lippincott Williams & Wilkins; 2002: 58.
11. Garcia D, Regan S, Crowther M, et al. Warfarin maintenance dosing patterns in clinical practice: implications for safer anticoagulation in the elderly population. Chest. 2005;127:2049-2056.
12. Kurnick D, Loebstein R, Farfel Z, et al. Complex drug-drug-disease interactions between amiodarone, warfarin and the thyroid gland. Medicine. 2004;83:107-113.
13. Stephens MA, Self TH, Lancaster D, et al. Hypothyroidism: effect on warfarin anticoagulation. South Med J. 1989;82:1585-1586.
14. Chute JP, Ryan CP, Sladek G, et al. Exacerbation of warfarin-induced anticoagulation by hyperthyroidism. Endocr Pract. 1997;3:77-79.
15. Kennedy M, Armanious C, Costa M. Dermatologic manifestations of hematologic disease. Emedicine web site. Updated June 25, 2009. Available at: http://emedicine.medscape.com/article/1096183-overview. Accessed January 14, 2011.
16. Kellett HA, Sawers JS, Boulton FE, et al. Problems of anticoagulation with warfarin in hyperthyroidism. Q J Med. 1986;58:43-51.
17. Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition–2005 update. Br J Haematol. 2006;132:277-285.
18. Horstkotte D, Piper C, Wiemer M. Optimal frequency of patient monitoring and intensity of oral anticoagulation therapy in valvular heart disease. J Thromb Thrombolysis. 1998;5(suppl):19-24.
19. Samsa GP, Matchar DB. Relationship between test frequency and outcomes of anticoagulation: a literature review and commentary with implications for the design of randomized trials of patient self-management. J Thromb Thrombolysis. 2000;9:283-292.
20. Ansell JE, Buttaro ML, Thomas OV, et al. Consensus guidelines for coordinated outpatient oral anticoagulation therapy management. Ann Pharmacother. 1997;31:604-615.
21. Palareti G, Legnani C, Guazzaloca G, et al. Risk factors for highly unstable response to oral anticoagulation: a case-control study. Br J Haematol. 2005;129:72-78.
22. Matchar DB, Jacobson A, Dolor R, et al. for the THINRS Executive Committee and Site Investigators. Effect of home testing of international normalized ratio on clinical events. N Engl J Med. 2010;363:1608-1620.
23. Cromheecke ME, Levi M, Colly LP, et al. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomized cross-over comparison. Lancet. 2000;356:97-102.
24. Heidinger KS, KS Bernardo A, Taborski U, et al. Clinical outcome of self-management of oral anticoagulation in patients with atrial fibrillation or deep vein thrombosis. Thromb Res. 2000;98:287-293.
25. Centers for Medicare and Medicaid Services. CMS manual system. Pub 100-04 Medicare claims processing. Transmittal 1562. July 25, 2008. Available at: http://www.cms.gov/transmittals/downloads/R1562CP.pdf. Accessed January 14, 2011.
26. Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999;23:40-54.
27. Delaney JA, Opatrny L, Brophy JM, et al. Drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. 2007;177:347-351.
28. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Stroke. 2005;36:1588-1593.
29. Philips Which patients qualify for PT/INR self-testing? Available at: http://www.inrselftest.com/content/clinicians/which-patients-qualify. Accessed January 13, 2011.
• INR testing by an anticoagulation management service or private clinician can be reduced to intervals of as long as 4 weeks, but should be more frequent when dosing adjustments occur. B
• Weekly patient self-testing is associated with comparable clinical outcomes to high-quality clinic-based anticoagulation management. A
• Patients who self-test (and report their results) weekly should test more frequently when a change in medication (including herbal remedies and dietary supplements) or diet or an illness occurs. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 4 million Americans are receiving longterm oral anticoagulation therapy to reduce the risk of primary and secondary thromboembolism.1,2 And, as the population ages, the number of patients on lifelong therapy with warfarin—the only oral anticoagulant available in the United States until dabigatran was approved by the US Food and Drug Administration late last year3—is expected to grow.4
Such patients present a challenge for family physicians. Warfarin is notorious for having both a narrow therapeutic index and numerous drug and dietary interactions.5,6 To safeguard patients on warfarin therapy, frequent, and diligent, monitoring is required.
Engaging patients as participants in their own care can help you decrease the hazards. With that in mind, this article features warfarin treatment tips and tools for both physicians and patients, along with a review of some basic safeguards.
Warfarin therapy: Keeping it safe
Warfarin, a vitamin K antagonist, is used to prevent systemic embolism in patients with prosthetic heart valves, atrial fibrillation, or inherited/acquired thrombophilic disorders; as an adjunct in the prophylaxis of systemic embolism after myocardial infarction (MI); and to reduce the risk of recurrent MI, as well as venous thromboembolism.4,7 Because there is a small but definite risk (1%-2% per year)8 of severe bleeding associated with warfarin, however, therapy should be initiated only when the potential benefits clearly outweigh the risks.
A major contraindication for warfarin therapy is early pregnancy. The anticoagulant is a teratogen, causing deformations of the face (depressed nasal bridge) and bones (stippled epiphyses), neonatal seizures, and spontaneous abortion. If a woman in the first trimester of pregnancy requires anticoagulation, low-molecular-weight heparin should be substituted instead.9
In fact, warfarin is not recommended in the second or third trimesters either, as the use of vitamin K antagonists increases the risk of miscarriages, structural defects, and other adverse outcomes. Nor is warfarin recommended for women who are planning to become pregnant.
Warfarin is also contraindicated in patients for whom the risk of major bleeding outweighs the benefits. Risk factors for warfarin-associated bleeding include renal insufficiency and concomitant antiplatelet therapy, and physicians can use published clinical prediction rules to estimate bleeding risk.10
Dosing considerations
When you start a patient on warfarin therapy, it is important to ensure that therapeutic concentrations are achieved in a timely manner—and that the risk of supra- and subtherapeutic international normalized ratio (INR) values—≥4.0 and <2.0, respectively—is minimized.6
Factors to consider in determining the starting dose include patient-specifi c measures such as age, height, and weight; concomitant medications; and comorbidities. Increasing age, female sex, and a low body mass index all indicate a need for a lower dose.11 A number of Web-based dosing calculators (TABLE) can help clinicians estimate the therapeutic dose in patients who are new to warfarin.
Thyroid activity also affects warfarin dosing requirements.12 Hypothyroidism makes people less responsive to warfarin,13 while hyperthyroidism boosts the anticoagulant effect.14 Several mechanisms have been proposed for this effect, including changes in the rate of breakdown of clotting factors and in the metabolism of warfarin.15,16
Frequency of monitoring. Regardless of the initiation dose, INR values of outpatients should be monitored at least 2 to 3 times a week for the fi rst 7 to 10 days of therapy, or until a stable value is achieved. (In an inpatient setting, INR monitoring is usually performed daily until the therapeutic range has been maintained for ≥2 days.) The target INR level varies from case to case depending on the clinical indicators, but tends to be between 2 and 3 for most patients and between 2.5 and 3.5 for those with mechanical heart valves.17
After stabilization, testing can be reduced to intervals of as long as 4 weeks, althoughevidence suggests that more frequent testing leads to greater time-in-therapeutic range (TTR).18,19 When dosing adjustments are required, the cycle of more frequent monitoring should be repeated until a stable dose response can again be achieved.
Benefits of patient involvement
Patients on warfarin may be managed in one or more of the following 3 methods: (1) with usual care, provided by the patient’s personal physician; (2) by anticoagulation management services (AMSs), specialized programs overseen by physicians, pharmacists, and/or nurses; or (3) by self-testing/self-management, with the help of point-of-care devices that allow patients to monitor their own INR levels and adjust their anticoagulation dose, within certain limits, in consultation with a clinician.4
Many nonrandomized retrospective studies have reported better outcomes in patients whose anticoagulant therapy is managed by an AMS vs management by a primary care physician or specialist alone.7 Compared with usual care, AMS programs have been shown to greatly improve patients’ TTR, thereby reducing hemorrhage or thrombosis as a consequence of excessive or subtherapeutic anticoagulation.4,20,21
Self-testing/self-management—which depends on adequate patient training—has similar benefits: Self-care facilitates more frequent monitoring and empowers patients, and may be a major factor in patient compliance.4 Individuals using their own portable INR monitors and managing their own care have been found to have improved TTRs and a lower frequency of major hemorrhage or thrombosis compared with patients receiving usual care.7,18 The recent THINRS trial randomized 2922 patients to perform weekly self-testing or receive monthly clinic-based testing at an institution with a system for providing anticoagulant care. The study confirmed that patient self-testing is feasible for most warfarin-treated individuals and that weekly home monitoring is as safe and effective as high-quality clinic-based testing.22
Who’s a candidate for self-management?
Various studies have found that, as with insulin-dependent diabetes, most patients who are independent and self-supporting are, in principle, capable of self-management of oral anticoagulation, regardless of education or social status.23,24 The only intellectual requirement is that the patient (or caregiver) grasp the concept of anticoagulant therapy and understand the potential risks. (For more help in determining whether your patient is eligible for self-management, see “Self-monitoring—for which patients?” on page 74.)
The patient must also be willing to actively participate in his or her own care and have sufficient manual dexterity and visual acuity. No previous experience in self-testing or monitoring is necessary.7
INR monitors for patients and physicians
Since the late 1980s, point-of-care devices that measure INR values have made it possible for an increasing number of patients to monitor the anticoagulant eff ects of warfarin without repeat visits to a health care facility. Of the 4 million US residents on warfarin, approximately 60,000 (1.6%) engage in self-testing, according to the International Self-Monitoring Association of Oral Anticoagulated Patients (www.ismaap.org).
One reason may be the cost. Portable monitors are available for approximately $2495, according to Alere Inc., a health management company—a price that may include supplies and training. The expense may not be covered by private insurers. However, in 2008, Medicare began covering the cost of INR monitors (and the testing materials required for their use) for seniors receiving anticoagulation therapy associated with mechanical heart valves, chronic atrial fibrillation, or venous thromboembolism.25 Portable monitoring devices include the following:
CoaguChek (http://www.coaguchek.com). The CoaguChek brand, now in its third generation, features both a monitor (CoaguChek XS) for patient use and a system (CoaguChek XS Plus) for health care professionals. CoaguChek has extended quality control and data management options.
INRatio2 PT/INR Monitor (www.hemosense.com). The HemoSense INRatio2 is a new whole-blood patient monitoring system. The device is well suited for use by both health care professionals and patients.
ProTime PT/INR Monitor (www.protimesystem.com). The ProTime Microcoagulation System is a portable, batteryoperated testing tool designed for both professionals and patients. There are also companies that sell or loan the devices to patients and provide the supplies, training, and support for enrollees engaged in self-testing, including Philips (http://www.inrselftest.com/content) and Roche (https://www.poc.roche.com/poc/home.do).
Preparing patients for self-management In addition to acquiring a monitor, patients interested in self-testing and management need to be aware that the risk of bleeding rises steeply when the INR exceeds 4.0—and the risk of thrombosis increases when INR values fall below 2.0.7
Guard against interactions. Emphasize that numerous environmental factors, such as drugs, diet, alcohol, and various disease states, can alter the pharmacokinetics of warfarin.26 Consequently, INR values need to be measured more frequently than the usual 4-week intervals when a patient taking warfarin adds (orstops taking) virtually any drug, dietary supplement, or herbal remedy, or significantly alters his or her vitamin K intake. Illnesses with a fever, such as infl uenza, or diarrhea and vomiting lasting more than one day, can also aff ect INR levels, and call for more frequent testing and possible adjustments in warfarin dosing.27
Explain that some drugs reduce warfarin’s anticoagulant eff ect by reducing its absorption or enhancing its clearance, while others—including many commonly used antibiotics—enhance the drug’s anticoagulant eff ect by inhibiting its clearance.6,7 Remind patients that the risk of bleeding is high when warfarin is combined with antiplatelet agents such as clopidogrel, aspirin, or nonsteroidal anti-infl ammatory drugs, among other medications.27 And caution them that excessive use of alcohol aff ects the metabolism of warfarin and can elevate the INR.26 (See Patient on warfarin? Steer clear of these drugs, in "Avoiding drug interactions: Here’s help," J Fam Pract. 2010; 59: 322-329.)
Seek medical attention. Patients engaged in self-testing and monitoring also need to be aware of the importance of obtaining treatment for dangerously high or low INR levels and being alert to early indicators of bleeding or other significant adverse effects. Similarly, family physicians who care for such patients need to establish a system to ensure that these individuals are not lost to followup. Whether INR results are transmitted by fax, phone, or e-mail, a patient who leaves a message reporting an INR of 5.6, for example, requires a callback without delay.
Advise patients to watch for signs of warfarin-induced skin necrosis—a rare but serious complication of oral anticoagulant therapy characterized by dusky skin discoloration and pain, typically in an area with significant subcutaneous fat (eg, the breast or abdominal wall). Warfarin necrosis is estimated to occur in 0.01% to 0.1% of patients—primarily women—mostly in the first week of therapy.15 Other serious adverse effects are osteoporosis and purple toe syndrome.1
Patients—and their family members—should also be advised that if the patient is hospitalized, it is critical to let the health care team know that he or she is taking warfarin. Patients should be encouraged to wear a medic alert bracelet, as well.
Warfarin’s effects can be reversed with vitamin K. (See “What to do when warfarin therapy goes too far,” J Fam Pract. 2009;58:346-352.) However, reversal may take 24 hours.7 In patients with life-threatening bleeding (eg, intracranial hemorrhage) and elevated INR, regardless of the magnitude of the elevation, INR should be normalized urgently with fresh frozen plasma, prothrombin complex concentrate, or recombinant factor VIIa supplemented with vitamin K10 mg by slow intravenous infusion.7
CORRESPONDENCE
Michael J. Schwartz, MD, 5 Sunnydale Circle, Swannanoa, NC 28778; ms112@columbia.edu
• INR testing by an anticoagulation management service or private clinician can be reduced to intervals of as long as 4 weeks, but should be more frequent when dosing adjustments occur. B
• Weekly patient self-testing is associated with comparable clinical outcomes to high-quality clinic-based anticoagulation management. A
• Patients who self-test (and report their results) weekly should test more frequently when a change in medication (including herbal remedies and dietary supplements) or diet or an illness occurs. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 4 million Americans are receiving longterm oral anticoagulation therapy to reduce the risk of primary and secondary thromboembolism.1,2 And, as the population ages, the number of patients on lifelong therapy with warfarin—the only oral anticoagulant available in the United States until dabigatran was approved by the US Food and Drug Administration late last year3—is expected to grow.4
Such patients present a challenge for family physicians. Warfarin is notorious for having both a narrow therapeutic index and numerous drug and dietary interactions.5,6 To safeguard patients on warfarin therapy, frequent, and diligent, monitoring is required.
Engaging patients as participants in their own care can help you decrease the hazards. With that in mind, this article features warfarin treatment tips and tools for both physicians and patients, along with a review of some basic safeguards.
Warfarin therapy: Keeping it safe
Warfarin, a vitamin K antagonist, is used to prevent systemic embolism in patients with prosthetic heart valves, atrial fibrillation, or inherited/acquired thrombophilic disorders; as an adjunct in the prophylaxis of systemic embolism after myocardial infarction (MI); and to reduce the risk of recurrent MI, as well as venous thromboembolism.4,7 Because there is a small but definite risk (1%-2% per year)8 of severe bleeding associated with warfarin, however, therapy should be initiated only when the potential benefits clearly outweigh the risks.
A major contraindication for warfarin therapy is early pregnancy. The anticoagulant is a teratogen, causing deformations of the face (depressed nasal bridge) and bones (stippled epiphyses), neonatal seizures, and spontaneous abortion. If a woman in the first trimester of pregnancy requires anticoagulation, low-molecular-weight heparin should be substituted instead.9
In fact, warfarin is not recommended in the second or third trimesters either, as the use of vitamin K antagonists increases the risk of miscarriages, structural defects, and other adverse outcomes. Nor is warfarin recommended for women who are planning to become pregnant.
Warfarin is also contraindicated in patients for whom the risk of major bleeding outweighs the benefits. Risk factors for warfarin-associated bleeding include renal insufficiency and concomitant antiplatelet therapy, and physicians can use published clinical prediction rules to estimate bleeding risk.10
Dosing considerations
When you start a patient on warfarin therapy, it is important to ensure that therapeutic concentrations are achieved in a timely manner—and that the risk of supra- and subtherapeutic international normalized ratio (INR) values—≥4.0 and <2.0, respectively—is minimized.6
Factors to consider in determining the starting dose include patient-specifi c measures such as age, height, and weight; concomitant medications; and comorbidities. Increasing age, female sex, and a low body mass index all indicate a need for a lower dose.11 A number of Web-based dosing calculators (TABLE) can help clinicians estimate the therapeutic dose in patients who are new to warfarin.
Thyroid activity also affects warfarin dosing requirements.12 Hypothyroidism makes people less responsive to warfarin,13 while hyperthyroidism boosts the anticoagulant effect.14 Several mechanisms have been proposed for this effect, including changes in the rate of breakdown of clotting factors and in the metabolism of warfarin.15,16
Frequency of monitoring. Regardless of the initiation dose, INR values of outpatients should be monitored at least 2 to 3 times a week for the fi rst 7 to 10 days of therapy, or until a stable value is achieved. (In an inpatient setting, INR monitoring is usually performed daily until the therapeutic range has been maintained for ≥2 days.) The target INR level varies from case to case depending on the clinical indicators, but tends to be between 2 and 3 for most patients and between 2.5 and 3.5 for those with mechanical heart valves.17
After stabilization, testing can be reduced to intervals of as long as 4 weeks, althoughevidence suggests that more frequent testing leads to greater time-in-therapeutic range (TTR).18,19 When dosing adjustments are required, the cycle of more frequent monitoring should be repeated until a stable dose response can again be achieved.
Benefits of patient involvement
Patients on warfarin may be managed in one or more of the following 3 methods: (1) with usual care, provided by the patient’s personal physician; (2) by anticoagulation management services (AMSs), specialized programs overseen by physicians, pharmacists, and/or nurses; or (3) by self-testing/self-management, with the help of point-of-care devices that allow patients to monitor their own INR levels and adjust their anticoagulation dose, within certain limits, in consultation with a clinician.4
Many nonrandomized retrospective studies have reported better outcomes in patients whose anticoagulant therapy is managed by an AMS vs management by a primary care physician or specialist alone.7 Compared with usual care, AMS programs have been shown to greatly improve patients’ TTR, thereby reducing hemorrhage or thrombosis as a consequence of excessive or subtherapeutic anticoagulation.4,20,21
Self-testing/self-management—which depends on adequate patient training—has similar benefits: Self-care facilitates more frequent monitoring and empowers patients, and may be a major factor in patient compliance.4 Individuals using their own portable INR monitors and managing their own care have been found to have improved TTRs and a lower frequency of major hemorrhage or thrombosis compared with patients receiving usual care.7,18 The recent THINRS trial randomized 2922 patients to perform weekly self-testing or receive monthly clinic-based testing at an institution with a system for providing anticoagulant care. The study confirmed that patient self-testing is feasible for most warfarin-treated individuals and that weekly home monitoring is as safe and effective as high-quality clinic-based testing.22
Who’s a candidate for self-management?
Various studies have found that, as with insulin-dependent diabetes, most patients who are independent and self-supporting are, in principle, capable of self-management of oral anticoagulation, regardless of education or social status.23,24 The only intellectual requirement is that the patient (or caregiver) grasp the concept of anticoagulant therapy and understand the potential risks. (For more help in determining whether your patient is eligible for self-management, see “Self-monitoring—for which patients?” on page 74.)
The patient must also be willing to actively participate in his or her own care and have sufficient manual dexterity and visual acuity. No previous experience in self-testing or monitoring is necessary.7
INR monitors for patients and physicians
Since the late 1980s, point-of-care devices that measure INR values have made it possible for an increasing number of patients to monitor the anticoagulant eff ects of warfarin without repeat visits to a health care facility. Of the 4 million US residents on warfarin, approximately 60,000 (1.6%) engage in self-testing, according to the International Self-Monitoring Association of Oral Anticoagulated Patients (www.ismaap.org).
One reason may be the cost. Portable monitors are available for approximately $2495, according to Alere Inc., a health management company—a price that may include supplies and training. The expense may not be covered by private insurers. However, in 2008, Medicare began covering the cost of INR monitors (and the testing materials required for their use) for seniors receiving anticoagulation therapy associated with mechanical heart valves, chronic atrial fibrillation, or venous thromboembolism.25 Portable monitoring devices include the following:
CoaguChek (http://www.coaguchek.com). The CoaguChek brand, now in its third generation, features both a monitor (CoaguChek XS) for patient use and a system (CoaguChek XS Plus) for health care professionals. CoaguChek has extended quality control and data management options.
INRatio2 PT/INR Monitor (www.hemosense.com). The HemoSense INRatio2 is a new whole-blood patient monitoring system. The device is well suited for use by both health care professionals and patients.
ProTime PT/INR Monitor (www.protimesystem.com). The ProTime Microcoagulation System is a portable, batteryoperated testing tool designed for both professionals and patients. There are also companies that sell or loan the devices to patients and provide the supplies, training, and support for enrollees engaged in self-testing, including Philips (http://www.inrselftest.com/content) and Roche (https://www.poc.roche.com/poc/home.do).
Preparing patients for self-management In addition to acquiring a monitor, patients interested in self-testing and management need to be aware that the risk of bleeding rises steeply when the INR exceeds 4.0—and the risk of thrombosis increases when INR values fall below 2.0.7
Guard against interactions. Emphasize that numerous environmental factors, such as drugs, diet, alcohol, and various disease states, can alter the pharmacokinetics of warfarin.26 Consequently, INR values need to be measured more frequently than the usual 4-week intervals when a patient taking warfarin adds (orstops taking) virtually any drug, dietary supplement, or herbal remedy, or significantly alters his or her vitamin K intake. Illnesses with a fever, such as infl uenza, or diarrhea and vomiting lasting more than one day, can also aff ect INR levels, and call for more frequent testing and possible adjustments in warfarin dosing.27
Explain that some drugs reduce warfarin’s anticoagulant eff ect by reducing its absorption or enhancing its clearance, while others—including many commonly used antibiotics—enhance the drug’s anticoagulant eff ect by inhibiting its clearance.6,7 Remind patients that the risk of bleeding is high when warfarin is combined with antiplatelet agents such as clopidogrel, aspirin, or nonsteroidal anti-infl ammatory drugs, among other medications.27 And caution them that excessive use of alcohol aff ects the metabolism of warfarin and can elevate the INR.26 (See Patient on warfarin? Steer clear of these drugs, in "Avoiding drug interactions: Here’s help," J Fam Pract. 2010; 59: 322-329.)
Seek medical attention. Patients engaged in self-testing and monitoring also need to be aware of the importance of obtaining treatment for dangerously high or low INR levels and being alert to early indicators of bleeding or other significant adverse effects. Similarly, family physicians who care for such patients need to establish a system to ensure that these individuals are not lost to followup. Whether INR results are transmitted by fax, phone, or e-mail, a patient who leaves a message reporting an INR of 5.6, for example, requires a callback without delay.
Advise patients to watch for signs of warfarin-induced skin necrosis—a rare but serious complication of oral anticoagulant therapy characterized by dusky skin discoloration and pain, typically in an area with significant subcutaneous fat (eg, the breast or abdominal wall). Warfarin necrosis is estimated to occur in 0.01% to 0.1% of patients—primarily women—mostly in the first week of therapy.15 Other serious adverse effects are osteoporosis and purple toe syndrome.1
Patients—and their family members—should also be advised that if the patient is hospitalized, it is critical to let the health care team know that he or she is taking warfarin. Patients should be encouraged to wear a medic alert bracelet, as well.
Warfarin’s effects can be reversed with vitamin K. (See “What to do when warfarin therapy goes too far,” J Fam Pract. 2009;58:346-352.) However, reversal may take 24 hours.7 In patients with life-threatening bleeding (eg, intracranial hemorrhage) and elevated INR, regardless of the magnitude of the elevation, INR should be normalized urgently with fresh frozen plasma, prothrombin complex concentrate, or recombinant factor VIIa supplemented with vitamin K10 mg by slow intravenous infusion.7
CORRESPONDENCE
Michael J. Schwartz, MD, 5 Sunnydale Circle, Swannanoa, NC 28778; ms112@columbia.edu
1. International Self-Monitoring Association of Oral Anticoagulated Patients. We motivate patients to take control of their own oral anticoagulation therapy. Available at: http://www.ismaap.org. Accessed January 12, 2011.
2. Alere Introducing InRatio 2. Available at: www.hemosense. com. Accessed January 25, 2009.
3. US Food and Drug Administration. FDA approves Pradaxa to prevent stroke in people with atrial fibrillation [press release]. October 19, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm230241.htm. Accessed January 12, 2011.
4. Garcia DA, Witt DM, Hyleck E, et al. Delivery of optimized anticoagulant therapy consensus statement from the Anticoagulant Forum. Ann Pharmacother. 2008;42:979-988.
5. Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):204S-233S.
6. Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(6 suppl):257S-299S.
9. Lip GY, Frison L, Halperin JL, et al. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) Score. J Am Coll Cardiol. 2011;57:173-180.
10. Beckmann CR. Obstetrics and Gynecology. 4th ed. Baltimore, Md: Lippincott Williams & Wilkins; 2002: 58.
11. Garcia D, Regan S, Crowther M, et al. Warfarin maintenance dosing patterns in clinical practice: implications for safer anticoagulation in the elderly population. Chest. 2005;127:2049-2056.
12. Kurnick D, Loebstein R, Farfel Z, et al. Complex drug-drug-disease interactions between amiodarone, warfarin and the thyroid gland. Medicine. 2004;83:107-113.
13. Stephens MA, Self TH, Lancaster D, et al. Hypothyroidism: effect on warfarin anticoagulation. South Med J. 1989;82:1585-1586.
14. Chute JP, Ryan CP, Sladek G, et al. Exacerbation of warfarin-induced anticoagulation by hyperthyroidism. Endocr Pract. 1997;3:77-79.
15. Kennedy M, Armanious C, Costa M. Dermatologic manifestations of hematologic disease. Emedicine web site. Updated June 25, 2009. Available at: http://emedicine.medscape.com/article/1096183-overview. Accessed January 14, 2011.
16. Kellett HA, Sawers JS, Boulton FE, et al. Problems of anticoagulation with warfarin in hyperthyroidism. Q J Med. 1986;58:43-51.
17. Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition–2005 update. Br J Haematol. 2006;132:277-285.
18. Horstkotte D, Piper C, Wiemer M. Optimal frequency of patient monitoring and intensity of oral anticoagulation therapy in valvular heart disease. J Thromb Thrombolysis. 1998;5(suppl):19-24.
19. Samsa GP, Matchar DB. Relationship between test frequency and outcomes of anticoagulation: a literature review and commentary with implications for the design of randomized trials of patient self-management. J Thromb Thrombolysis. 2000;9:283-292.
20. Ansell JE, Buttaro ML, Thomas OV, et al. Consensus guidelines for coordinated outpatient oral anticoagulation therapy management. Ann Pharmacother. 1997;31:604-615.
21. Palareti G, Legnani C, Guazzaloca G, et al. Risk factors for highly unstable response to oral anticoagulation: a case-control study. Br J Haematol. 2005;129:72-78.
22. Matchar DB, Jacobson A, Dolor R, et al. for the THINRS Executive Committee and Site Investigators. Effect of home testing of international normalized ratio on clinical events. N Engl J Med. 2010;363:1608-1620.
23. Cromheecke ME, Levi M, Colly LP, et al. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomized cross-over comparison. Lancet. 2000;356:97-102.
24. Heidinger KS, KS Bernardo A, Taborski U, et al. Clinical outcome of self-management of oral anticoagulation in patients with atrial fibrillation or deep vein thrombosis. Thromb Res. 2000;98:287-293.
25. Centers for Medicare and Medicaid Services. CMS manual system. Pub 100-04 Medicare claims processing. Transmittal 1562. July 25, 2008. Available at: http://www.cms.gov/transmittals/downloads/R1562CP.pdf. Accessed January 14, 2011.
26. Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999;23:40-54.
27. Delaney JA, Opatrny L, Brophy JM, et al. Drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. 2007;177:347-351.
28. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Stroke. 2005;36:1588-1593.
29. Philips Which patients qualify for PT/INR self-testing? Available at: http://www.inrselftest.com/content/clinicians/which-patients-qualify. Accessed January 13, 2011.
1. International Self-Monitoring Association of Oral Anticoagulated Patients. We motivate patients to take control of their own oral anticoagulation therapy. Available at: http://www.ismaap.org. Accessed January 12, 2011.
2. Alere Introducing InRatio 2. Available at: www.hemosense. com. Accessed January 25, 2009.
3. US Food and Drug Administration. FDA approves Pradaxa to prevent stroke in people with atrial fibrillation [press release]. October 19, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm230241.htm. Accessed January 12, 2011.
4. Garcia DA, Witt DM, Hyleck E, et al. Delivery of optimized anticoagulant therapy consensus statement from the Anticoagulant Forum. Ann Pharmacother. 2008;42:979-988.
5. Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):204S-233S.
6. Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(6 suppl):257S-299S.
9. Lip GY, Frison L, Halperin JL, et al. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) Score. J Am Coll Cardiol. 2011;57:173-180.
10. Beckmann CR. Obstetrics and Gynecology. 4th ed. Baltimore, Md: Lippincott Williams & Wilkins; 2002: 58.
11. Garcia D, Regan S, Crowther M, et al. Warfarin maintenance dosing patterns in clinical practice: implications for safer anticoagulation in the elderly population. Chest. 2005;127:2049-2056.
12. Kurnick D, Loebstein R, Farfel Z, et al. Complex drug-drug-disease interactions between amiodarone, warfarin and the thyroid gland. Medicine. 2004;83:107-113.
13. Stephens MA, Self TH, Lancaster D, et al. Hypothyroidism: effect on warfarin anticoagulation. South Med J. 1989;82:1585-1586.
14. Chute JP, Ryan CP, Sladek G, et al. Exacerbation of warfarin-induced anticoagulation by hyperthyroidism. Endocr Pract. 1997;3:77-79.
15. Kennedy M, Armanious C, Costa M. Dermatologic manifestations of hematologic disease. Emedicine web site. Updated June 25, 2009. Available at: http://emedicine.medscape.com/article/1096183-overview. Accessed January 14, 2011.
16. Kellett HA, Sawers JS, Boulton FE, et al. Problems of anticoagulation with warfarin in hyperthyroidism. Q J Med. 1986;58:43-51.
17. Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition–2005 update. Br J Haematol. 2006;132:277-285.
18. Horstkotte D, Piper C, Wiemer M. Optimal frequency of patient monitoring and intensity of oral anticoagulation therapy in valvular heart disease. J Thromb Thrombolysis. 1998;5(suppl):19-24.
19. Samsa GP, Matchar DB. Relationship between test frequency and outcomes of anticoagulation: a literature review and commentary with implications for the design of randomized trials of patient self-management. J Thromb Thrombolysis. 2000;9:283-292.
20. Ansell JE, Buttaro ML, Thomas OV, et al. Consensus guidelines for coordinated outpatient oral anticoagulation therapy management. Ann Pharmacother. 1997;31:604-615.
21. Palareti G, Legnani C, Guazzaloca G, et al. Risk factors for highly unstable response to oral anticoagulation: a case-control study. Br J Haematol. 2005;129:72-78.
22. Matchar DB, Jacobson A, Dolor R, et al. for the THINRS Executive Committee and Site Investigators. Effect of home testing of international normalized ratio on clinical events. N Engl J Med. 2010;363:1608-1620.
23. Cromheecke ME, Levi M, Colly LP, et al. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomized cross-over comparison. Lancet. 2000;356:97-102.
24. Heidinger KS, KS Bernardo A, Taborski U, et al. Clinical outcome of self-management of oral anticoagulation in patients with atrial fibrillation or deep vein thrombosis. Thromb Res. 2000;98:287-293.
25. Centers for Medicare and Medicaid Services. CMS manual system. Pub 100-04 Medicare claims processing. Transmittal 1562. July 25, 2008. Available at: http://www.cms.gov/transmittals/downloads/R1562CP.pdf. Accessed January 14, 2011.
26. Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999;23:40-54.
27. Delaney JA, Opatrny L, Brophy JM, et al. Drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. 2007;177:347-351.
28. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Stroke. 2005;36:1588-1593.
29. Philips Which patients qualify for PT/INR self-testing? Available at: http://www.inrselftest.com/content/clinicians/which-patients-qualify. Accessed January 13, 2011.