User login
Skin in the Game: Inadequate Photoprotection Among Olympic Athletes
The XXXIII Olympic Summer Games will take place in Paris, France, from July 26 to August 11, 2024, and a variety of outdoor sporting events (eg, surfing, cycling, beach volleyball) will be included. Participation in the Olympic Games is a distinct honor for athletes selected to compete at the highest level in their sports.
Because of their training regimens and lifestyles, Olympic athletes face unique health risks. One such risk appears to be skin cancer, a substantial contributor to the global burden of disease. Taken together, basal cell carcinoma, squamous cell carcinoma, and melanoma account for 6.7 million cases of skin cancer worldwide. Squamous cell carcinoma and malignant skin melanoma were attributed to 1.2 million and 1.7 million life-years lost to disability, respectively.1
Olympic athletes are at increased risk for sunburn from UVA and UVB radiation, placing them at higher risk for both melanoma and nonmelanoma skin cancers.2,3 Sweating increases skin photosensitivity, sportswear often offers inadequate sun protection, and sustained high-intensity exercise itself has an immunosuppressive effect. Athletes competing in skiing and snowboarding events also receive radiation reflected off snow and ice at high altitudes.3 In fact, skiing without sunscreen at 11,000-feet above sea level can induce sunburn after only 6 minutes of exposure.4 Moreover, sweat, water immersion, and friction can decrease the effectiveness of topical sunscreens.5
World-class athletes appear to be exposed to UV radiation to a substantially higher degree than the general public. In an analysis of 144 events at the 2020 XXXII Olympic Summer Games in Tokyo, Japan, the highest exposure assessments were for women’s tennis, men’s golf, and men’s road cycling.6 In a 2020 study (N=240), the rates of sunburn were as high as 76.7% among Olympic sailors, elite surfers, and windsurfers, with more than one-quarter of athletes reporting sunburn that lasted longer than 24 hours.7 An earlier study reported that professional cyclists were exposed to UV radiation during a single race that exceeded the personal exposure limit by 30 times.8
Regrettably, the high level of sun exposure experienced by elite athletes is compounded by their low rate of sunscreen use. In a 2020 survey of 95 Olympians and super sprint triathletes, approximately half rarely used sunscreen, with 1 in 5 athletes never using sunscreen during training.9 In another study of 246 elite athletes in surfing, windsurfing, and sailing, nearly half used inadequate sun protection and nearly one-quarter reported never using sunscreen.10 Surprisingly, as many as 90% of Olympic athletes and super sprint competitors understood the importance of using sunscreen.9
What can we learn from these findings?
First, elite athletes remain at high risk for skin cancer because of training regimens, occupational environmental hazards, and other requirements of their sport. Second, despite awareness of the risks of UV radiation exposure, Olympic athletes utilize inadequate photoprotection. Athletes with darker skin are still at risk for skin cancer, photoaging, and pigmentation disorders—indicating a need for photoprotective behaviors in athletes of all skin types.11
Therefore, efforts to promote adequate sunscreen use and understanding of the consequences of UV radiation may need to be prioritized earlier in athletes’ careers and implemented according to evidence-based guidelines. For example, the Stanford University Network for Sun Protection, Outreach, Research and Teamwork (Sunsport) provided information about skin cancer risk and prevention by educating student-athletes, coaches, and trainers in the National Collegiate Athletic Association in the United States. The Sunsport initiative led to a dramatic increase in sunscreen use by student-athletes as well as increased knowledge and discussion of skin cancer risk.12
- Zhang W, Zeng W, Jiang A, et al. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: an analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021;10:4905-4922. doi:10.1002/cam4.4046
- De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer Olympics: what a sports medicine physician should know. Sports Med. 2012;42:399-413. doi:10.2165/11599050-000000000-00000
- Moehrle M. Outdoor sports and skin cancer. Clin Dermatol. 2008;26:12-15. doi:10.1016/j.clindermatol.2007.10.001
- Rigel DS, Rigel EG, Rigel AC. Effects of altitude and latitude on ambient UVB radiation. J Am Acad Dermatol. 1999;40:114-116. doi:10.1016/s0190-9622(99)70542-6
- Harrison SC, Bergfeld WF. Ultraviolet light and skin cancer in athletes. Sports Health. 2009;1:335-340. doi:10.1177/19417381093338923
- Downs NJ, Axelsen T, Schouten P, et al. Biologically effective solar ultraviolet exposures and the potential skin cancer risk for individual gold medalists of the 2020 Tokyo Summer Olympic Games. Temperature (Austin). 2019;7:89-108. doi:10.1080/23328940.2019.1581427
- De Castro-Maqueda G, Gutierrez-Manzanedo JV, Ponce-González JG, et al. Sun protection habits and sunburn in elite aquatics athletes: surfers, windsurfers and Olympic sailors. J Cancer Educ. 2020;35:312-320. doi:10.1007/s13187-018-1466-x
- Moehrle M, Heinrich L, Schmid A, et al. Extreme UV exposure of professional cyclists. Dermatology. 2000;201:44-45. doi:10.1159/000018428
- Buljan M, Kolic´ M, Šitum M, et al. Do athletes practicing outdoors know and care enough about the importance of photoprotection? Acta Dermatovenerol Croat. 2020;28:41-42.
- De Castro-Maqueda G, Gutierrez-Manzanedo JV, Lagares-Franco C. Sun exposure during water sports: do elite athletes adequately protect their skin against skin cancer? Int J Environ Res Public Health. 2021;18:800. doi:10.3390/ijerph18020800
- Tsai J, Chien AL. Photoprotection for skin of color. Am J Clin Dermatol. 2022;23:195-205. doi:10.1007/s40257-021-00670-z
- Ally MS, Swetter SM, Hirotsu KE, et al. Promoting sunscreen use and sun-protective practices in NCAA athletes: impact of SUNSPORT educational intervention for student-athletes, athletic trainers, and coaches. J Am Acad Dermatol. 2018;78:289-292.e2. doi:10.1016/j.jaad.2017.08.050
The XXXIII Olympic Summer Games will take place in Paris, France, from July 26 to August 11, 2024, and a variety of outdoor sporting events (eg, surfing, cycling, beach volleyball) will be included. Participation in the Olympic Games is a distinct honor for athletes selected to compete at the highest level in their sports.
Because of their training regimens and lifestyles, Olympic athletes face unique health risks. One such risk appears to be skin cancer, a substantial contributor to the global burden of disease. Taken together, basal cell carcinoma, squamous cell carcinoma, and melanoma account for 6.7 million cases of skin cancer worldwide. Squamous cell carcinoma and malignant skin melanoma were attributed to 1.2 million and 1.7 million life-years lost to disability, respectively.1
Olympic athletes are at increased risk for sunburn from UVA and UVB radiation, placing them at higher risk for both melanoma and nonmelanoma skin cancers.2,3 Sweating increases skin photosensitivity, sportswear often offers inadequate sun protection, and sustained high-intensity exercise itself has an immunosuppressive effect. Athletes competing in skiing and snowboarding events also receive radiation reflected off snow and ice at high altitudes.3 In fact, skiing without sunscreen at 11,000-feet above sea level can induce sunburn after only 6 minutes of exposure.4 Moreover, sweat, water immersion, and friction can decrease the effectiveness of topical sunscreens.5
World-class athletes appear to be exposed to UV radiation to a substantially higher degree than the general public. In an analysis of 144 events at the 2020 XXXII Olympic Summer Games in Tokyo, Japan, the highest exposure assessments were for women’s tennis, men’s golf, and men’s road cycling.6 In a 2020 study (N=240), the rates of sunburn were as high as 76.7% among Olympic sailors, elite surfers, and windsurfers, with more than one-quarter of athletes reporting sunburn that lasted longer than 24 hours.7 An earlier study reported that professional cyclists were exposed to UV radiation during a single race that exceeded the personal exposure limit by 30 times.8
Regrettably, the high level of sun exposure experienced by elite athletes is compounded by their low rate of sunscreen use. In a 2020 survey of 95 Olympians and super sprint triathletes, approximately half rarely used sunscreen, with 1 in 5 athletes never using sunscreen during training.9 In another study of 246 elite athletes in surfing, windsurfing, and sailing, nearly half used inadequate sun protection and nearly one-quarter reported never using sunscreen.10 Surprisingly, as many as 90% of Olympic athletes and super sprint competitors understood the importance of using sunscreen.9
What can we learn from these findings?
First, elite athletes remain at high risk for skin cancer because of training regimens, occupational environmental hazards, and other requirements of their sport. Second, despite awareness of the risks of UV radiation exposure, Olympic athletes utilize inadequate photoprotection. Athletes with darker skin are still at risk for skin cancer, photoaging, and pigmentation disorders—indicating a need for photoprotective behaviors in athletes of all skin types.11
Therefore, efforts to promote adequate sunscreen use and understanding of the consequences of UV radiation may need to be prioritized earlier in athletes’ careers and implemented according to evidence-based guidelines. For example, the Stanford University Network for Sun Protection, Outreach, Research and Teamwork (Sunsport) provided information about skin cancer risk and prevention by educating student-athletes, coaches, and trainers in the National Collegiate Athletic Association in the United States. The Sunsport initiative led to a dramatic increase in sunscreen use by student-athletes as well as increased knowledge and discussion of skin cancer risk.12
The XXXIII Olympic Summer Games will take place in Paris, France, from July 26 to August 11, 2024, and a variety of outdoor sporting events (eg, surfing, cycling, beach volleyball) will be included. Participation in the Olympic Games is a distinct honor for athletes selected to compete at the highest level in their sports.
Because of their training regimens and lifestyles, Olympic athletes face unique health risks. One such risk appears to be skin cancer, a substantial contributor to the global burden of disease. Taken together, basal cell carcinoma, squamous cell carcinoma, and melanoma account for 6.7 million cases of skin cancer worldwide. Squamous cell carcinoma and malignant skin melanoma were attributed to 1.2 million and 1.7 million life-years lost to disability, respectively.1
Olympic athletes are at increased risk for sunburn from UVA and UVB radiation, placing them at higher risk for both melanoma and nonmelanoma skin cancers.2,3 Sweating increases skin photosensitivity, sportswear often offers inadequate sun protection, and sustained high-intensity exercise itself has an immunosuppressive effect. Athletes competing in skiing and snowboarding events also receive radiation reflected off snow and ice at high altitudes.3 In fact, skiing without sunscreen at 11,000-feet above sea level can induce sunburn after only 6 minutes of exposure.4 Moreover, sweat, water immersion, and friction can decrease the effectiveness of topical sunscreens.5
World-class athletes appear to be exposed to UV radiation to a substantially higher degree than the general public. In an analysis of 144 events at the 2020 XXXII Olympic Summer Games in Tokyo, Japan, the highest exposure assessments were for women’s tennis, men’s golf, and men’s road cycling.6 In a 2020 study (N=240), the rates of sunburn were as high as 76.7% among Olympic sailors, elite surfers, and windsurfers, with more than one-quarter of athletes reporting sunburn that lasted longer than 24 hours.7 An earlier study reported that professional cyclists were exposed to UV radiation during a single race that exceeded the personal exposure limit by 30 times.8
Regrettably, the high level of sun exposure experienced by elite athletes is compounded by their low rate of sunscreen use. In a 2020 survey of 95 Olympians and super sprint triathletes, approximately half rarely used sunscreen, with 1 in 5 athletes never using sunscreen during training.9 In another study of 246 elite athletes in surfing, windsurfing, and sailing, nearly half used inadequate sun protection and nearly one-quarter reported never using sunscreen.10 Surprisingly, as many as 90% of Olympic athletes and super sprint competitors understood the importance of using sunscreen.9
What can we learn from these findings?
First, elite athletes remain at high risk for skin cancer because of training regimens, occupational environmental hazards, and other requirements of their sport. Second, despite awareness of the risks of UV radiation exposure, Olympic athletes utilize inadequate photoprotection. Athletes with darker skin are still at risk for skin cancer, photoaging, and pigmentation disorders—indicating a need for photoprotective behaviors in athletes of all skin types.11
Therefore, efforts to promote adequate sunscreen use and understanding of the consequences of UV radiation may need to be prioritized earlier in athletes’ careers and implemented according to evidence-based guidelines. For example, the Stanford University Network for Sun Protection, Outreach, Research and Teamwork (Sunsport) provided information about skin cancer risk and prevention by educating student-athletes, coaches, and trainers in the National Collegiate Athletic Association in the United States. The Sunsport initiative led to a dramatic increase in sunscreen use by student-athletes as well as increased knowledge and discussion of skin cancer risk.12
- Zhang W, Zeng W, Jiang A, et al. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: an analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021;10:4905-4922. doi:10.1002/cam4.4046
- De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer Olympics: what a sports medicine physician should know. Sports Med. 2012;42:399-413. doi:10.2165/11599050-000000000-00000
- Moehrle M. Outdoor sports and skin cancer. Clin Dermatol. 2008;26:12-15. doi:10.1016/j.clindermatol.2007.10.001
- Rigel DS, Rigel EG, Rigel AC. Effects of altitude and latitude on ambient UVB radiation. J Am Acad Dermatol. 1999;40:114-116. doi:10.1016/s0190-9622(99)70542-6
- Harrison SC, Bergfeld WF. Ultraviolet light and skin cancer in athletes. Sports Health. 2009;1:335-340. doi:10.1177/19417381093338923
- Downs NJ, Axelsen T, Schouten P, et al. Biologically effective solar ultraviolet exposures and the potential skin cancer risk for individual gold medalists of the 2020 Tokyo Summer Olympic Games. Temperature (Austin). 2019;7:89-108. doi:10.1080/23328940.2019.1581427
- De Castro-Maqueda G, Gutierrez-Manzanedo JV, Ponce-González JG, et al. Sun protection habits and sunburn in elite aquatics athletes: surfers, windsurfers and Olympic sailors. J Cancer Educ. 2020;35:312-320. doi:10.1007/s13187-018-1466-x
- Moehrle M, Heinrich L, Schmid A, et al. Extreme UV exposure of professional cyclists. Dermatology. 2000;201:44-45. doi:10.1159/000018428
- Buljan M, Kolic´ M, Šitum M, et al. Do athletes practicing outdoors know and care enough about the importance of photoprotection? Acta Dermatovenerol Croat. 2020;28:41-42.
- De Castro-Maqueda G, Gutierrez-Manzanedo JV, Lagares-Franco C. Sun exposure during water sports: do elite athletes adequately protect their skin against skin cancer? Int J Environ Res Public Health. 2021;18:800. doi:10.3390/ijerph18020800
- Tsai J, Chien AL. Photoprotection for skin of color. Am J Clin Dermatol. 2022;23:195-205. doi:10.1007/s40257-021-00670-z
- Ally MS, Swetter SM, Hirotsu KE, et al. Promoting sunscreen use and sun-protective practices in NCAA athletes: impact of SUNSPORT educational intervention for student-athletes, athletic trainers, and coaches. J Am Acad Dermatol. 2018;78:289-292.e2. doi:10.1016/j.jaad.2017.08.050
- Zhang W, Zeng W, Jiang A, et al. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: an analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021;10:4905-4922. doi:10.1002/cam4.4046
- De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer Olympics: what a sports medicine physician should know. Sports Med. 2012;42:399-413. doi:10.2165/11599050-000000000-00000
- Moehrle M. Outdoor sports and skin cancer. Clin Dermatol. 2008;26:12-15. doi:10.1016/j.clindermatol.2007.10.001
- Rigel DS, Rigel EG, Rigel AC. Effects of altitude and latitude on ambient UVB radiation. J Am Acad Dermatol. 1999;40:114-116. doi:10.1016/s0190-9622(99)70542-6
- Harrison SC, Bergfeld WF. Ultraviolet light and skin cancer in athletes. Sports Health. 2009;1:335-340. doi:10.1177/19417381093338923
- Downs NJ, Axelsen T, Schouten P, et al. Biologically effective solar ultraviolet exposures and the potential skin cancer risk for individual gold medalists of the 2020 Tokyo Summer Olympic Games. Temperature (Austin). 2019;7:89-108. doi:10.1080/23328940.2019.1581427
- De Castro-Maqueda G, Gutierrez-Manzanedo JV, Ponce-González JG, et al. Sun protection habits and sunburn in elite aquatics athletes: surfers, windsurfers and Olympic sailors. J Cancer Educ. 2020;35:312-320. doi:10.1007/s13187-018-1466-x
- Moehrle M, Heinrich L, Schmid A, et al. Extreme UV exposure of professional cyclists. Dermatology. 2000;201:44-45. doi:10.1159/000018428
- Buljan M, Kolic´ M, Šitum M, et al. Do athletes practicing outdoors know and care enough about the importance of photoprotection? Acta Dermatovenerol Croat. 2020;28:41-42.
- De Castro-Maqueda G, Gutierrez-Manzanedo JV, Lagares-Franco C. Sun exposure during water sports: do elite athletes adequately protect their skin against skin cancer? Int J Environ Res Public Health. 2021;18:800. doi:10.3390/ijerph18020800
- Tsai J, Chien AL. Photoprotection for skin of color. Am J Clin Dermatol. 2022;23:195-205. doi:10.1007/s40257-021-00670-z
- Ally MS, Swetter SM, Hirotsu KE, et al. Promoting sunscreen use and sun-protective practices in NCAA athletes: impact of SUNSPORT educational intervention for student-athletes, athletic trainers, and coaches. J Am Acad Dermatol. 2018;78:289-292.e2. doi:10.1016/j.jaad.2017.08.050
Practice Points
- Providers should further investigate how patients spend their time outside to assess cancer risk and appropriately guide patients.
- Many athletes typically train for hours outside; therefore, these patients should be educated on the importance of sunscreen reapplication and protective clothing.
Prurigo nodularis: Picking the right treatment
› Start with topical corticosteroids under occlusion and periodically substitute with steroid-sparing agents (calcipotriol ointment or pimecrolimus 1% cream) for localized prurigo nodularis. B
› Consider adding oral antihistamines or montelukast to the initial regimen if a pruritic cause is suspected; alternatively, consider adding these agents if topical therapies alone do not effectively treat the prurigo nodules. C
› Turn to oral naltrexone, gabapentin, or pregabalin for more widespread or treatment-resistant cases. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 43-year-old woman arrives at your office with persistent itching on her arms and legs. For some time, she has used moisturizing lotions and herbal preparations suggested by her mother, but they have provided no relief. You note multiple 0.5- to 2-cm firm, excoriated nodules symmetrically distributed on her elbows and knees bilaterally. She has seasonal allergies and a history of childhood asthma. How would you care for this patient?
Treating prurigo nodularis (PN) can be a daunting task for even the most experienced clinician. Prurigo nodules are cutaneous lesions often produced by repetitive scratching—hence the nickname “picker’s nodules”—which may occur as sequelae of chronic pruritus or neurotic excoriations. Thus, PN can be classified as a subtype of neurodermatitis. The nodules can be intensely pruritic, resulting in an itch-scratch cycle that can be difficult to break.1,2 In this review, we examine evidence-based therapies for PN.
Key findings with prurigo nodularis
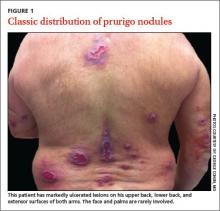
Typically, prurigo nodules are firm, hyperkeratotic, pruritic papules or nodules that range in diameter from a few millimeters to several centimeters. The lesions usually have eroded or ulcerated components secondary to repeated excoriation, which can eventually lead to scarring and changes in pigmentation. Patients can have one nodule or hundreds of lesions, depending on disease severity. The lesions tend to be distributed symmetrically and have a predilection for the extensor surfaces of the upper and lower limbs. The abdomen, posterior neck, upper and lower back, and buttocks are also commonly affected, whereas the face, palms, and flexural areas are rarely involved2-5 (FIGURE 1).
The differential diagnosis for PN includes dermatitis herpetiformis, scabies, lichen simplex chronicus, hypertrophic lichen planus, perforating disorders, atopic dermatitis, allergic contact dermatitis, neurotic excoriations, and multiple keratoacanthomas.4,5
PN prevalence and etiology are unknown. Although PN can occur at any age, the typical age range is 20 to 60 years, with middle-aged women most commonly affected. Patients who develop PN at a younger age are more likely to have an atopic diathesis.3,4
There is ongoing debate regarding whether PN is a primary cutaneous disease or a response to repetitive scratching provoked by a separate cause. PN has been associated with a variety of diseases, such as psychiatric disorders, atopic dermatitis, chronic renal failure, hyperthyroidism, iron-deficiency anemia, obstructive biliary disease, gastric malignancy, lymphoma, leukemia, human immunodeficiency virus (HIV), hepatitis B, and hepatitis C.2,3
Use the diagnostic work-up to focus on management decisions
When taking the history, first determine why patients are picking or scratching. If the lesions are pruritic or painful, look for a potential underlying cause of pruritic symptoms.6 If you identify an underlying dermatologic or systemic condition, treat that disorder first.1 For example, adequately treating a patient’s atopic dermatitis or hyperthyroidism may quell the pruritic symptoms and potentially make the prurigo nodules more responsive to symptomatic treatment or even obviate the need for such measures.
If treating the underlying cause of PN does not provide adequate relief, or if no cause for pruritic nodules can be found, the nodules may yet respond to symptomatic treatments targeted at decreasing pruritus and inflammation. In contrast, with patients who habitually scratch lesions they describe as non-pruritic, neurotic excoriations could be the source of PN, making the nodules less likely to respond to antipruritic therapies.4,7
Patient insights. Assessing whether patients have insight into their condition is also important. Some patients may be unaware that they are repetitively picking and scratching the affected areas and causing the development and perpetuation of the nodules. In cases associated with an underlying psychiatric component, such as delusional parasitosis, patients often lack insight into their condition and thus may benefit from treatment of psychiatric comorbidities.4,7
On physical exam, try to find lesions that have not been traumatized by patients. They can be useful in uncovering a primary cause, such as scabies, atopic dermatitis, lichenoid drug eruption, or simple xerosis.
If a diagnosis cannot be made clinically, consider obtaining a biopsy of a nontraumatized lesion. Traumatized lesions are typically unrevealing on histopathology. If the clinical assessment of pruritic lesions is indeterminate, laboratory tests that may prove helpful include, but are not limited to, thyroid-stimulating hormone levels, liver function tests, kidney function, a hepatitis panel, and HIV screening.
With severe refractory pruritus in which a primary cutaneous or systemic cause cannot be determined, evaluate for malignancy—especially polycythemia, lymphoma, or multiple myeloma—by ordering liver function tests (including lactate dehydrogenase), a complete blood count with differential, a basic metabolic panel, a chest x-ray, and possibly a serum protein electrophoresis.7
Available treatments
If the patient’s pruritic symptoms do not resolve and an underlying cause cannot be determined, direct treatment at decreasing pruritus either locally or systemically. Topical therapies, typically associated with fewer adverse effects, are preferable in localized cases of PN. In more severe, widespread, or recalcitrant disease, systemic agents may be necessary. Typical first-line treatments for PN aimed at decreasing pruritic symptoms include:
- topical antipruritics, such as ointments containing menthol or camphor; topical corticosteroids, with increased efficacy under occlusion as seen with flurandrenolide tape (Cordran tape)
- oral antihistamines, such as promethazine hydrochloride; oral antidepressants, such as doxepin
- intralesional corticosteroids—eg, triamcinolone acetonide (the concentration
used depends on the thickness of the lesion and how well the lesion responded to prior injections) - a short course of systemic corticosteroids, unless the patient has a comorbid condition that could be exacerbated by rapid tapering of corticosteroids (eg, psoriasis).
For patients with concomitant depression or anxiety, treatment with a selective serotonin reuptake inhibitor or anxiolytic, respectively, may be indicated.2-4 With the exception of topical corticosteroids8,9 and oral antihistamines,10 the aforementioned first-line treatments for PN are mostly based on clinical experience and anecdotal success with no studies to support their use.3 Furthermore, these treatments may be ineffective for many patients.11,12 We present our review of several studies in the literature examining potential therapies for PN.
Topical therapies
Calcipotriol vs betamethasone. A prospective, randomized, double-blind study that ran right/left comparisons of calcipotriol ointment (a vitamin D3 analog) and betamethasone ointment as treatment for PN in 9 patients showed that calcipotriol and betamethasone were both effective. However, calcipotriol ointment 50 mcg/g was more effective in reducing the number and size of nodules compared with 0.1% betamethasone valerate ointment.8
Topical corticosteroids have long been viewed as a first-line therapy for PN.2 However, given their potential for adverse effects with long-term use, such as skin atrophy, steroidsparing agents are preferred. Calcipotriol ointment can be useful as both a steroid-sparing and a keratolytic agent, as it inhibits keratinocyte proliferation.4,13 Corticosteroids and calcipotriol possess anti-inflammatory and antipruritic properties, likely explaining their efficacy in treating PN.4
Pimecrolimus and tacrolimus. The topical calcineurin inhibitors pimecrolimus and tacrolimus have been used successfully as steroid-sparing agents in treating atopic dermatitis.14 Their antipruritic effect, likely related to their influence on cutaneous sensory nerve fibers and inhibition of inflammatory cytokines, could also explain their efficacy in treating PN.15,16
A randomized, hydrocortisone-controlled, double-blind phase II trial sponsored by Novartis was designed as a right/left comparison study between pimecrolimus 1% cream and hydrocortisone 1% cream in 30 patients with non-atopic PN. When applied twice daily, each agent decreased pruritic symptoms and resolved scratch lesions to degrees that were statistically significant. However, an intention-to-treat analysis revealed no significant differences between pimecrolimus and hydrocortisone.15 In a prospective case series of 11 patients with PN, 2 out of 4 patients (50%) receiving tacrolimus 0.1% ointment and 5 out of 7 patients (71%) using pimecrolimus 1% cream experienced a reduction in pruritic symptoms and improvement of lesions by 50% or greater with twice daily application of their assigned calcineurin inhibitor.16
Before prescribing topical calcineurin inhibitors, inform patients of the black-box warning issued by the US Food and Drug Administration (FDA) regarding the theoretical increased risk of developing cutaneous malignancy and lymphoma. This warning is controversial because in clinical databases, the incidences of malignancy and lymphoma associated with topical calcineurin inhibitors are less than those observed in the general population.14
Capsaicin. Based on a prospective study of 33 patients with PN, topical capsaicin may be an effective treatment if administered 4 to 6 times daily for at least 2 weeks and up to 10 months.17 Patients may require up to 0.3% concentration for total resolution of pruritus. Importantly, capsaicin use may be limited by the high application frequency.
Systemic therapies
Fexofenadine and montelukast. Oral antihistamines have long been used as a first-line treatment for PN. Although clinical experience and anecdotal success support the use of various antihistamines, evidence-based literature exists only for fexofenadine and the leukotriene receptor antagonist montelukast. These oral agents also avoid potential unwanted effects of topical antihistamines, which may sensitize skin and increase the risk of developing allergic contact dermatitis.1
Whereas antihistamines exert their antipruritic effect by blocking histamine H1-receptors, montelukast decreases pruritic symptoms by antagonizing leukotriene receptors.10 In a prospective study of 12 patients with PN receiving fexofenadine 240 mg twice daily and montelukast 10 mg daily for 4 weeks, 9 of the 12 patients (75%) reported some degree of improvement.10 However, 5 of these 9 patients (56%) achieved only slight improvement. Level of improvement was based on how well the agents reduced the pruritus and lesion number.
Naltrexone. As an opioid antagonist, naltrexone is able to block endogenous opiates from binding to central opioid receptors and causing the sensation of pruritus. Accordingly, oral naltrexone can be used to treat PN, as shown in an open-label clinical trial in which 9 out of 17 patients (53%) achieved high antipruritic effect, defined as a reduction of pruritic symptoms by at least half.18
When selecting naltrexone to treat PN, prescribe a daily dose of 50 mg for an average of 4.7 months; up to 20 months of treatment may be required. If tachyphylaxis occurs, consider increasing the dose to 50 mg twice a day. Most patients should notice some level of antipruritic efficacy and varying degrees of lesion flattening, softening, or healing. However, exacerbation after therapy discontinuation may occur in 41% of patients. Adverse medication effects include fatigue, nausea, and dizziness.18
Gabapentin and pregabalin. In response to a report of a case series in which 4 patients with PN responded well to gabapentin,19 Mazza et al20 conducted a prospective study of pregabalin treatment for 30 patients with PN. Both gabapentin and pregabalin inhibit calcium influx and subsequent excitatory neurotransmitter release, the mechanism by which they likely decrease pruritus in patients with PN.20 In the pregabalin study, 23 out of 30 patients (77%) experienced complete resolution of pruritic symptoms and a reduction of prurigo nodules in number or flattening. The recommended dosage of pregabalin is 25 mg 3 times daily for 3 months, after which time clinical progress is assessed. If a patient is not lesion-free, continue pregabalin at a maintenance dose of 50 mg/d for up to 2 years. Adverse effects typically include headache, sedation, and dizziness.20
When to refer a dermatologist
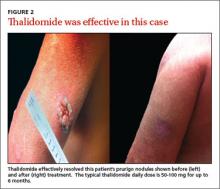
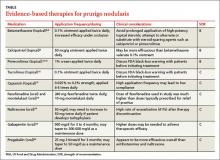
Refer patients to a dermatologist if initial clinical findings suggest a need for further work-up to rule out primary cutaneous diseases, or if the therapies discussed (TABLE8-10,15-20) yield unsatisfactory results. Dermatologists can provide more advanced treatments that require close monitoring, such as phototherapy,21-24 cyclosporine,25 or thalidomide.26-28 Based on multiple case series and case reports, as well as our own personal experience (FIGURE 2), thalidomide is efficacious in treating PN. However, thalidomide is typically reserved for cases that are severe and treatment-recalcitrant due to the drug’s high cost, teratogenicity (pregnancy category X), and potentially irreversible peripheral neuropathy.29
Putting Tx options into practice
In addition to ruling out potential causes of pruritus and determining the best treatment for each individual with PN, assess for and appropriately treat any psychiatric comorbidities, which are often a psychological component of PN.
Localized PN. Start with topical corticosteroids under occlusion for localized PN. To avoid complications of long-term topical corticosteroid use, including dermal atrophy, periodically switch to a steroid-sparing agent, such as calcipotriol ointment or topical pimecrolimus. Less evidence is available to support the efficacy of tacrolimus ointment in PN treatment. Topical capsaicin is not as practical as other topical treatments since it needs to be applied 4 to 6 times daily. Oral antihistamines and montelukast may be added to the therapeutic regimen if there is a chronic pruritic component related to the lesions themselves or an underlying atopic diathesis fueling the itch-scratch cycle.
Widespread or treatment-resistant PN. Prescribe naltrexone, gabapentin, or pregabalin for more widespread disease or lesions resistant to conservative therapies. If you suspect a primary cutaneous disease as the underlying cause of pruritus or if topical and oral therapies do not achieve the desired therapeutic effect, refer to a dermatologist for further work-up and treatment.
How we would manage the case presented in the introduction. We would start the 43-year-old on topical corticosteroids under occlusion and periodically substitute calcipotriol ointment. (Given the unease that some patients might feel with the black-box warning on topical calcineurin inhibitors, we would likely try calcipotriol ointment as a courtesy before suggesting topical calcineurin inhibitors.) We would also prescribe an oral antihistamine at the start, given that her history of seasonal allergies and childhood asthma increases her chances of having an atopic component causing or exacerbating her disease. However, assessing her response to topical therapies before initiating an oral antihistamine would also be an appropriate strategy.
Unfortunately, PN is typically a chronic and often treatment-resistant disease with disappointing recurrence rates. As we learn more about the pathophysiology of PN, more effective therapies will hopefully emerge to improve the quality of life for these patients.
CORRESPONDENCE
Michael Saco, MD, Department of Dermatology & Cutaneous Surgery, University of South Florida, 13330 Laurel Drive, Tampa, FL 33612; ms20142018@aol.com
1. Moses S. Pruritus. Am Fam Phys. 2003;68:1135-1142.
2. Jorizzo JL, Gatti S, Smith EB. Prurigo: a clinical review. J Am Acad Dermatol. 1981;4:723-728.
3. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-218.
4. Wallengren J. Prurigo: diagnosis and management. Am J Clin Dermatol. 2004;5:85-95.
5. Accioly-Filho LW, Nogueira A, Ramos-e-Silva M. Prurigo nodularis of Hyde: an update. J Eur Acad Dermatol Venereol. 2000;14:75-82.
6. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
7. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450.
8. Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of Prurigo nodularis. Arch Dermatol. 2000;136:807-808.
9. Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366.
10. Shintani T, Ohata C, Koga H, et al. Combination therapy of fexofenadine and montelukast is effective in prurigo nodularis and pemphigoid nodularis. Dermatol Ther. 2014;27:135-139.
11. Paghdal KV, Schwartz R. Thalidomide and its dermatologic uses. Acta Dermatovenerol Croat. 2007;15:39-44.
12. Alfadley A, Al-Hawsawi K, Thestrup-Pedersen K, et al. Treatment of prurigo nodularis with thalidomide: a case report and review of the literature. Int J Dermatol. 2003;42:372-375.
13. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
14. Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
15. Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, doubleblind phase II trial. Dermatology. 2013;227:353-360.
16. Ständer S, Schürmeyer-Horst F, Luger TA, et al. Treatment of pruritic diseases with topical calcineurin inhibitors. Ther Clin Risk Manag. 2006;2:213-218.
17. Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478.
18. Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
19. Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23:194-198.
20. Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18.
21. Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803.
22. Rombold S, Lobisch K, Katzer K, et al. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed. 2008;24:19-23.
23. Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695.
24. Saraceno R, Nisticò SP, Capriotti E, et al. Monochromatic excimer light (308 nm) in the treatment of prurigo nodularis. Photodermatol Photoimmunol Photomed. 2008;24:43-45.
25. Siepmann D, Luger TA, Ständer S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: results of a case series. J Dtsch Dermatol Ges. 2008;6:941-946.
26. Orlando A, Renna S, Cottone M. Prurigo nodularis of Hyde treated with low-dose thalidomide. Eur Rev Med Pharmacol Sci. 2009;13:141-145.
27. Lan CC, Lin CL, Wu CS, et al. Treatment of idiopathic prurigo nodularis in Taiwanese patients with low-dose thalidomide. J Dermatol. 2007;34:237-242.
28. Taefehnorooz H, Truchetet F, Barbaud A, et al. Efficacy of thalidomide in the treatment of prurigo nodularis. Acta Derm Venereol. 2011;91:344-345.
29. Wu JJ, Huang DB, Pang KR, et al. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254-273.
› Start with topical corticosteroids under occlusion and periodically substitute with steroid-sparing agents (calcipotriol ointment or pimecrolimus 1% cream) for localized prurigo nodularis. B
› Consider adding oral antihistamines or montelukast to the initial regimen if a pruritic cause is suspected; alternatively, consider adding these agents if topical therapies alone do not effectively treat the prurigo nodules. C
› Turn to oral naltrexone, gabapentin, or pregabalin for more widespread or treatment-resistant cases. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 43-year-old woman arrives at your office with persistent itching on her arms and legs. For some time, she has used moisturizing lotions and herbal preparations suggested by her mother, but they have provided no relief. You note multiple 0.5- to 2-cm firm, excoriated nodules symmetrically distributed on her elbows and knees bilaterally. She has seasonal allergies and a history of childhood asthma. How would you care for this patient?
Treating prurigo nodularis (PN) can be a daunting task for even the most experienced clinician. Prurigo nodules are cutaneous lesions often produced by repetitive scratching—hence the nickname “picker’s nodules”—which may occur as sequelae of chronic pruritus or neurotic excoriations. Thus, PN can be classified as a subtype of neurodermatitis. The nodules can be intensely pruritic, resulting in an itch-scratch cycle that can be difficult to break.1,2 In this review, we examine evidence-based therapies for PN.
Key findings with prurigo nodularis
Typically, prurigo nodules are firm, hyperkeratotic, pruritic papules or nodules that range in diameter from a few millimeters to several centimeters. The lesions usually have eroded or ulcerated components secondary to repeated excoriation, which can eventually lead to scarring and changes in pigmentation. Patients can have one nodule or hundreds of lesions, depending on disease severity. The lesions tend to be distributed symmetrically and have a predilection for the extensor surfaces of the upper and lower limbs. The abdomen, posterior neck, upper and lower back, and buttocks are also commonly affected, whereas the face, palms, and flexural areas are rarely involved2-5 (FIGURE 1).
The differential diagnosis for PN includes dermatitis herpetiformis, scabies, lichen simplex chronicus, hypertrophic lichen planus, perforating disorders, atopic dermatitis, allergic contact dermatitis, neurotic excoriations, and multiple keratoacanthomas.4,5
PN prevalence and etiology are unknown. Although PN can occur at any age, the typical age range is 20 to 60 years, with middle-aged women most commonly affected. Patients who develop PN at a younger age are more likely to have an atopic diathesis.3,4
There is ongoing debate regarding whether PN is a primary cutaneous disease or a response to repetitive scratching provoked by a separate cause. PN has been associated with a variety of diseases, such as psychiatric disorders, atopic dermatitis, chronic renal failure, hyperthyroidism, iron-deficiency anemia, obstructive biliary disease, gastric malignancy, lymphoma, leukemia, human immunodeficiency virus (HIV), hepatitis B, and hepatitis C.2,3
Use the diagnostic work-up to focus on management decisions
When taking the history, first determine why patients are picking or scratching. If the lesions are pruritic or painful, look for a potential underlying cause of pruritic symptoms.6 If you identify an underlying dermatologic or systemic condition, treat that disorder first.1 For example, adequately treating a patient’s atopic dermatitis or hyperthyroidism may quell the pruritic symptoms and potentially make the prurigo nodules more responsive to symptomatic treatment or even obviate the need for such measures.
If treating the underlying cause of PN does not provide adequate relief, or if no cause for pruritic nodules can be found, the nodules may yet respond to symptomatic treatments targeted at decreasing pruritus and inflammation. In contrast, with patients who habitually scratch lesions they describe as non-pruritic, neurotic excoriations could be the source of PN, making the nodules less likely to respond to antipruritic therapies.4,7
Patient insights. Assessing whether patients have insight into their condition is also important. Some patients may be unaware that they are repetitively picking and scratching the affected areas and causing the development and perpetuation of the nodules. In cases associated with an underlying psychiatric component, such as delusional parasitosis, patients often lack insight into their condition and thus may benefit from treatment of psychiatric comorbidities.4,7
On physical exam, try to find lesions that have not been traumatized by patients. They can be useful in uncovering a primary cause, such as scabies, atopic dermatitis, lichenoid drug eruption, or simple xerosis.
If a diagnosis cannot be made clinically, consider obtaining a biopsy of a nontraumatized lesion. Traumatized lesions are typically unrevealing on histopathology. If the clinical assessment of pruritic lesions is indeterminate, laboratory tests that may prove helpful include, but are not limited to, thyroid-stimulating hormone levels, liver function tests, kidney function, a hepatitis panel, and HIV screening.
With severe refractory pruritus in which a primary cutaneous or systemic cause cannot be determined, evaluate for malignancy—especially polycythemia, lymphoma, or multiple myeloma—by ordering liver function tests (including lactate dehydrogenase), a complete blood count with differential, a basic metabolic panel, a chest x-ray, and possibly a serum protein electrophoresis.7
Available treatments
If the patient’s pruritic symptoms do not resolve and an underlying cause cannot be determined, direct treatment at decreasing pruritus either locally or systemically. Topical therapies, typically associated with fewer adverse effects, are preferable in localized cases of PN. In more severe, widespread, or recalcitrant disease, systemic agents may be necessary. Typical first-line treatments for PN aimed at decreasing pruritic symptoms include:
- topical antipruritics, such as ointments containing menthol or camphor; topical corticosteroids, with increased efficacy under occlusion as seen with flurandrenolide tape (Cordran tape)
- oral antihistamines, such as promethazine hydrochloride; oral antidepressants, such as doxepin
- intralesional corticosteroids—eg, triamcinolone acetonide (the concentration
used depends on the thickness of the lesion and how well the lesion responded to prior injections) - a short course of systemic corticosteroids, unless the patient has a comorbid condition that could be exacerbated by rapid tapering of corticosteroids (eg, psoriasis).
For patients with concomitant depression or anxiety, treatment with a selective serotonin reuptake inhibitor or anxiolytic, respectively, may be indicated.2-4 With the exception of topical corticosteroids8,9 and oral antihistamines,10 the aforementioned first-line treatments for PN are mostly based on clinical experience and anecdotal success with no studies to support their use.3 Furthermore, these treatments may be ineffective for many patients.11,12 We present our review of several studies in the literature examining potential therapies for PN.
Topical therapies
Calcipotriol vs betamethasone. A prospective, randomized, double-blind study that ran right/left comparisons of calcipotriol ointment (a vitamin D3 analog) and betamethasone ointment as treatment for PN in 9 patients showed that calcipotriol and betamethasone were both effective. However, calcipotriol ointment 50 mcg/g was more effective in reducing the number and size of nodules compared with 0.1% betamethasone valerate ointment.8
Topical corticosteroids have long been viewed as a first-line therapy for PN.2 However, given their potential for adverse effects with long-term use, such as skin atrophy, steroidsparing agents are preferred. Calcipotriol ointment can be useful as both a steroid-sparing and a keratolytic agent, as it inhibits keratinocyte proliferation.4,13 Corticosteroids and calcipotriol possess anti-inflammatory and antipruritic properties, likely explaining their efficacy in treating PN.4
Pimecrolimus and tacrolimus. The topical calcineurin inhibitors pimecrolimus and tacrolimus have been used successfully as steroid-sparing agents in treating atopic dermatitis.14 Their antipruritic effect, likely related to their influence on cutaneous sensory nerve fibers and inhibition of inflammatory cytokines, could also explain their efficacy in treating PN.15,16
A randomized, hydrocortisone-controlled, double-blind phase II trial sponsored by Novartis was designed as a right/left comparison study between pimecrolimus 1% cream and hydrocortisone 1% cream in 30 patients with non-atopic PN. When applied twice daily, each agent decreased pruritic symptoms and resolved scratch lesions to degrees that were statistically significant. However, an intention-to-treat analysis revealed no significant differences between pimecrolimus and hydrocortisone.15 In a prospective case series of 11 patients with PN, 2 out of 4 patients (50%) receiving tacrolimus 0.1% ointment and 5 out of 7 patients (71%) using pimecrolimus 1% cream experienced a reduction in pruritic symptoms and improvement of lesions by 50% or greater with twice daily application of their assigned calcineurin inhibitor.16
Before prescribing topical calcineurin inhibitors, inform patients of the black-box warning issued by the US Food and Drug Administration (FDA) regarding the theoretical increased risk of developing cutaneous malignancy and lymphoma. This warning is controversial because in clinical databases, the incidences of malignancy and lymphoma associated with topical calcineurin inhibitors are less than those observed in the general population.14
Capsaicin. Based on a prospective study of 33 patients with PN, topical capsaicin may be an effective treatment if administered 4 to 6 times daily for at least 2 weeks and up to 10 months.17 Patients may require up to 0.3% concentration for total resolution of pruritus. Importantly, capsaicin use may be limited by the high application frequency.
Systemic therapies
Fexofenadine and montelukast. Oral antihistamines have long been used as a first-line treatment for PN. Although clinical experience and anecdotal success support the use of various antihistamines, evidence-based literature exists only for fexofenadine and the leukotriene receptor antagonist montelukast. These oral agents also avoid potential unwanted effects of topical antihistamines, which may sensitize skin and increase the risk of developing allergic contact dermatitis.1
Whereas antihistamines exert their antipruritic effect by blocking histamine H1-receptors, montelukast decreases pruritic symptoms by antagonizing leukotriene receptors.10 In a prospective study of 12 patients with PN receiving fexofenadine 240 mg twice daily and montelukast 10 mg daily for 4 weeks, 9 of the 12 patients (75%) reported some degree of improvement.10 However, 5 of these 9 patients (56%) achieved only slight improvement. Level of improvement was based on how well the agents reduced the pruritus and lesion number.
Naltrexone. As an opioid antagonist, naltrexone is able to block endogenous opiates from binding to central opioid receptors and causing the sensation of pruritus. Accordingly, oral naltrexone can be used to treat PN, as shown in an open-label clinical trial in which 9 out of 17 patients (53%) achieved high antipruritic effect, defined as a reduction of pruritic symptoms by at least half.18
When selecting naltrexone to treat PN, prescribe a daily dose of 50 mg for an average of 4.7 months; up to 20 months of treatment may be required. If tachyphylaxis occurs, consider increasing the dose to 50 mg twice a day. Most patients should notice some level of antipruritic efficacy and varying degrees of lesion flattening, softening, or healing. However, exacerbation after therapy discontinuation may occur in 41% of patients. Adverse medication effects include fatigue, nausea, and dizziness.18
Gabapentin and pregabalin. In response to a report of a case series in which 4 patients with PN responded well to gabapentin,19 Mazza et al20 conducted a prospective study of pregabalin treatment for 30 patients with PN. Both gabapentin and pregabalin inhibit calcium influx and subsequent excitatory neurotransmitter release, the mechanism by which they likely decrease pruritus in patients with PN.20 In the pregabalin study, 23 out of 30 patients (77%) experienced complete resolution of pruritic symptoms and a reduction of prurigo nodules in number or flattening. The recommended dosage of pregabalin is 25 mg 3 times daily for 3 months, after which time clinical progress is assessed. If a patient is not lesion-free, continue pregabalin at a maintenance dose of 50 mg/d for up to 2 years. Adverse effects typically include headache, sedation, and dizziness.20
When to refer a dermatologist
Refer patients to a dermatologist if initial clinical findings suggest a need for further work-up to rule out primary cutaneous diseases, or if the therapies discussed (TABLE8-10,15-20) yield unsatisfactory results. Dermatologists can provide more advanced treatments that require close monitoring, such as phototherapy,21-24 cyclosporine,25 or thalidomide.26-28 Based on multiple case series and case reports, as well as our own personal experience (FIGURE 2), thalidomide is efficacious in treating PN. However, thalidomide is typically reserved for cases that are severe and treatment-recalcitrant due to the drug’s high cost, teratogenicity (pregnancy category X), and potentially irreversible peripheral neuropathy.29
Putting Tx options into practice
In addition to ruling out potential causes of pruritus and determining the best treatment for each individual with PN, assess for and appropriately treat any psychiatric comorbidities, which are often a psychological component of PN.
Localized PN. Start with topical corticosteroids under occlusion for localized PN. To avoid complications of long-term topical corticosteroid use, including dermal atrophy, periodically switch to a steroid-sparing agent, such as calcipotriol ointment or topical pimecrolimus. Less evidence is available to support the efficacy of tacrolimus ointment in PN treatment. Topical capsaicin is not as practical as other topical treatments since it needs to be applied 4 to 6 times daily. Oral antihistamines and montelukast may be added to the therapeutic regimen if there is a chronic pruritic component related to the lesions themselves or an underlying atopic diathesis fueling the itch-scratch cycle.
Widespread or treatment-resistant PN. Prescribe naltrexone, gabapentin, or pregabalin for more widespread disease or lesions resistant to conservative therapies. If you suspect a primary cutaneous disease as the underlying cause of pruritus or if topical and oral therapies do not achieve the desired therapeutic effect, refer to a dermatologist for further work-up and treatment.
How we would manage the case presented in the introduction. We would start the 43-year-old on topical corticosteroids under occlusion and periodically substitute calcipotriol ointment. (Given the unease that some patients might feel with the black-box warning on topical calcineurin inhibitors, we would likely try calcipotriol ointment as a courtesy before suggesting topical calcineurin inhibitors.) We would also prescribe an oral antihistamine at the start, given that her history of seasonal allergies and childhood asthma increases her chances of having an atopic component causing or exacerbating her disease. However, assessing her response to topical therapies before initiating an oral antihistamine would also be an appropriate strategy.
Unfortunately, PN is typically a chronic and often treatment-resistant disease with disappointing recurrence rates. As we learn more about the pathophysiology of PN, more effective therapies will hopefully emerge to improve the quality of life for these patients.
CORRESPONDENCE
Michael Saco, MD, Department of Dermatology & Cutaneous Surgery, University of South Florida, 13330 Laurel Drive, Tampa, FL 33612; ms20142018@aol.com
› Start with topical corticosteroids under occlusion and periodically substitute with steroid-sparing agents (calcipotriol ointment or pimecrolimus 1% cream) for localized prurigo nodularis. B
› Consider adding oral antihistamines or montelukast to the initial regimen if a pruritic cause is suspected; alternatively, consider adding these agents if topical therapies alone do not effectively treat the prurigo nodules. C
› Turn to oral naltrexone, gabapentin, or pregabalin for more widespread or treatment-resistant cases. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 43-year-old woman arrives at your office with persistent itching on her arms and legs. For some time, she has used moisturizing lotions and herbal preparations suggested by her mother, but they have provided no relief. You note multiple 0.5- to 2-cm firm, excoriated nodules symmetrically distributed on her elbows and knees bilaterally. She has seasonal allergies and a history of childhood asthma. How would you care for this patient?
Treating prurigo nodularis (PN) can be a daunting task for even the most experienced clinician. Prurigo nodules are cutaneous lesions often produced by repetitive scratching—hence the nickname “picker’s nodules”—which may occur as sequelae of chronic pruritus or neurotic excoriations. Thus, PN can be classified as a subtype of neurodermatitis. The nodules can be intensely pruritic, resulting in an itch-scratch cycle that can be difficult to break.1,2 In this review, we examine evidence-based therapies for PN.
Key findings with prurigo nodularis
Typically, prurigo nodules are firm, hyperkeratotic, pruritic papules or nodules that range in diameter from a few millimeters to several centimeters. The lesions usually have eroded or ulcerated components secondary to repeated excoriation, which can eventually lead to scarring and changes in pigmentation. Patients can have one nodule or hundreds of lesions, depending on disease severity. The lesions tend to be distributed symmetrically and have a predilection for the extensor surfaces of the upper and lower limbs. The abdomen, posterior neck, upper and lower back, and buttocks are also commonly affected, whereas the face, palms, and flexural areas are rarely involved2-5 (FIGURE 1).
The differential diagnosis for PN includes dermatitis herpetiformis, scabies, lichen simplex chronicus, hypertrophic lichen planus, perforating disorders, atopic dermatitis, allergic contact dermatitis, neurotic excoriations, and multiple keratoacanthomas.4,5
PN prevalence and etiology are unknown. Although PN can occur at any age, the typical age range is 20 to 60 years, with middle-aged women most commonly affected. Patients who develop PN at a younger age are more likely to have an atopic diathesis.3,4
There is ongoing debate regarding whether PN is a primary cutaneous disease or a response to repetitive scratching provoked by a separate cause. PN has been associated with a variety of diseases, such as psychiatric disorders, atopic dermatitis, chronic renal failure, hyperthyroidism, iron-deficiency anemia, obstructive biliary disease, gastric malignancy, lymphoma, leukemia, human immunodeficiency virus (HIV), hepatitis B, and hepatitis C.2,3
Use the diagnostic work-up to focus on management decisions
When taking the history, first determine why patients are picking or scratching. If the lesions are pruritic or painful, look for a potential underlying cause of pruritic symptoms.6 If you identify an underlying dermatologic or systemic condition, treat that disorder first.1 For example, adequately treating a patient’s atopic dermatitis or hyperthyroidism may quell the pruritic symptoms and potentially make the prurigo nodules more responsive to symptomatic treatment or even obviate the need for such measures.
If treating the underlying cause of PN does not provide adequate relief, or if no cause for pruritic nodules can be found, the nodules may yet respond to symptomatic treatments targeted at decreasing pruritus and inflammation. In contrast, with patients who habitually scratch lesions they describe as non-pruritic, neurotic excoriations could be the source of PN, making the nodules less likely to respond to antipruritic therapies.4,7
Patient insights. Assessing whether patients have insight into their condition is also important. Some patients may be unaware that they are repetitively picking and scratching the affected areas and causing the development and perpetuation of the nodules. In cases associated with an underlying psychiatric component, such as delusional parasitosis, patients often lack insight into their condition and thus may benefit from treatment of psychiatric comorbidities.4,7
On physical exam, try to find lesions that have not been traumatized by patients. They can be useful in uncovering a primary cause, such as scabies, atopic dermatitis, lichenoid drug eruption, or simple xerosis.
If a diagnosis cannot be made clinically, consider obtaining a biopsy of a nontraumatized lesion. Traumatized lesions are typically unrevealing on histopathology. If the clinical assessment of pruritic lesions is indeterminate, laboratory tests that may prove helpful include, but are not limited to, thyroid-stimulating hormone levels, liver function tests, kidney function, a hepatitis panel, and HIV screening.
With severe refractory pruritus in which a primary cutaneous or systemic cause cannot be determined, evaluate for malignancy—especially polycythemia, lymphoma, or multiple myeloma—by ordering liver function tests (including lactate dehydrogenase), a complete blood count with differential, a basic metabolic panel, a chest x-ray, and possibly a serum protein electrophoresis.7
Available treatments
If the patient’s pruritic symptoms do not resolve and an underlying cause cannot be determined, direct treatment at decreasing pruritus either locally or systemically. Topical therapies, typically associated with fewer adverse effects, are preferable in localized cases of PN. In more severe, widespread, or recalcitrant disease, systemic agents may be necessary. Typical first-line treatments for PN aimed at decreasing pruritic symptoms include:
- topical antipruritics, such as ointments containing menthol or camphor; topical corticosteroids, with increased efficacy under occlusion as seen with flurandrenolide tape (Cordran tape)
- oral antihistamines, such as promethazine hydrochloride; oral antidepressants, such as doxepin
- intralesional corticosteroids—eg, triamcinolone acetonide (the concentration
used depends on the thickness of the lesion and how well the lesion responded to prior injections) - a short course of systemic corticosteroids, unless the patient has a comorbid condition that could be exacerbated by rapid tapering of corticosteroids (eg, psoriasis).
For patients with concomitant depression or anxiety, treatment with a selective serotonin reuptake inhibitor or anxiolytic, respectively, may be indicated.2-4 With the exception of topical corticosteroids8,9 and oral antihistamines,10 the aforementioned first-line treatments for PN are mostly based on clinical experience and anecdotal success with no studies to support their use.3 Furthermore, these treatments may be ineffective for many patients.11,12 We present our review of several studies in the literature examining potential therapies for PN.
Topical therapies
Calcipotriol vs betamethasone. A prospective, randomized, double-blind study that ran right/left comparisons of calcipotriol ointment (a vitamin D3 analog) and betamethasone ointment as treatment for PN in 9 patients showed that calcipotriol and betamethasone were both effective. However, calcipotriol ointment 50 mcg/g was more effective in reducing the number and size of nodules compared with 0.1% betamethasone valerate ointment.8
Topical corticosteroids have long been viewed as a first-line therapy for PN.2 However, given their potential for adverse effects with long-term use, such as skin atrophy, steroidsparing agents are preferred. Calcipotriol ointment can be useful as both a steroid-sparing and a keratolytic agent, as it inhibits keratinocyte proliferation.4,13 Corticosteroids and calcipotriol possess anti-inflammatory and antipruritic properties, likely explaining their efficacy in treating PN.4
Pimecrolimus and tacrolimus. The topical calcineurin inhibitors pimecrolimus and tacrolimus have been used successfully as steroid-sparing agents in treating atopic dermatitis.14 Their antipruritic effect, likely related to their influence on cutaneous sensory nerve fibers and inhibition of inflammatory cytokines, could also explain their efficacy in treating PN.15,16
A randomized, hydrocortisone-controlled, double-blind phase II trial sponsored by Novartis was designed as a right/left comparison study between pimecrolimus 1% cream and hydrocortisone 1% cream in 30 patients with non-atopic PN. When applied twice daily, each agent decreased pruritic symptoms and resolved scratch lesions to degrees that were statistically significant. However, an intention-to-treat analysis revealed no significant differences between pimecrolimus and hydrocortisone.15 In a prospective case series of 11 patients with PN, 2 out of 4 patients (50%) receiving tacrolimus 0.1% ointment and 5 out of 7 patients (71%) using pimecrolimus 1% cream experienced a reduction in pruritic symptoms and improvement of lesions by 50% or greater with twice daily application of their assigned calcineurin inhibitor.16
Before prescribing topical calcineurin inhibitors, inform patients of the black-box warning issued by the US Food and Drug Administration (FDA) regarding the theoretical increased risk of developing cutaneous malignancy and lymphoma. This warning is controversial because in clinical databases, the incidences of malignancy and lymphoma associated with topical calcineurin inhibitors are less than those observed in the general population.14
Capsaicin. Based on a prospective study of 33 patients with PN, topical capsaicin may be an effective treatment if administered 4 to 6 times daily for at least 2 weeks and up to 10 months.17 Patients may require up to 0.3% concentration for total resolution of pruritus. Importantly, capsaicin use may be limited by the high application frequency.
Systemic therapies
Fexofenadine and montelukast. Oral antihistamines have long been used as a first-line treatment for PN. Although clinical experience and anecdotal success support the use of various antihistamines, evidence-based literature exists only for fexofenadine and the leukotriene receptor antagonist montelukast. These oral agents also avoid potential unwanted effects of topical antihistamines, which may sensitize skin and increase the risk of developing allergic contact dermatitis.1
Whereas antihistamines exert their antipruritic effect by blocking histamine H1-receptors, montelukast decreases pruritic symptoms by antagonizing leukotriene receptors.10 In a prospective study of 12 patients with PN receiving fexofenadine 240 mg twice daily and montelukast 10 mg daily for 4 weeks, 9 of the 12 patients (75%) reported some degree of improvement.10 However, 5 of these 9 patients (56%) achieved only slight improvement. Level of improvement was based on how well the agents reduced the pruritus and lesion number.
Naltrexone. As an opioid antagonist, naltrexone is able to block endogenous opiates from binding to central opioid receptors and causing the sensation of pruritus. Accordingly, oral naltrexone can be used to treat PN, as shown in an open-label clinical trial in which 9 out of 17 patients (53%) achieved high antipruritic effect, defined as a reduction of pruritic symptoms by at least half.18
When selecting naltrexone to treat PN, prescribe a daily dose of 50 mg for an average of 4.7 months; up to 20 months of treatment may be required. If tachyphylaxis occurs, consider increasing the dose to 50 mg twice a day. Most patients should notice some level of antipruritic efficacy and varying degrees of lesion flattening, softening, or healing. However, exacerbation after therapy discontinuation may occur in 41% of patients. Adverse medication effects include fatigue, nausea, and dizziness.18
Gabapentin and pregabalin. In response to a report of a case series in which 4 patients with PN responded well to gabapentin,19 Mazza et al20 conducted a prospective study of pregabalin treatment for 30 patients with PN. Both gabapentin and pregabalin inhibit calcium influx and subsequent excitatory neurotransmitter release, the mechanism by which they likely decrease pruritus in patients with PN.20 In the pregabalin study, 23 out of 30 patients (77%) experienced complete resolution of pruritic symptoms and a reduction of prurigo nodules in number or flattening. The recommended dosage of pregabalin is 25 mg 3 times daily for 3 months, after which time clinical progress is assessed. If a patient is not lesion-free, continue pregabalin at a maintenance dose of 50 mg/d for up to 2 years. Adverse effects typically include headache, sedation, and dizziness.20
When to refer a dermatologist
Refer patients to a dermatologist if initial clinical findings suggest a need for further work-up to rule out primary cutaneous diseases, or if the therapies discussed (TABLE8-10,15-20) yield unsatisfactory results. Dermatologists can provide more advanced treatments that require close monitoring, such as phototherapy,21-24 cyclosporine,25 or thalidomide.26-28 Based on multiple case series and case reports, as well as our own personal experience (FIGURE 2), thalidomide is efficacious in treating PN. However, thalidomide is typically reserved for cases that are severe and treatment-recalcitrant due to the drug’s high cost, teratogenicity (pregnancy category X), and potentially irreversible peripheral neuropathy.29
Putting Tx options into practice
In addition to ruling out potential causes of pruritus and determining the best treatment for each individual with PN, assess for and appropriately treat any psychiatric comorbidities, which are often a psychological component of PN.
Localized PN. Start with topical corticosteroids under occlusion for localized PN. To avoid complications of long-term topical corticosteroid use, including dermal atrophy, periodically switch to a steroid-sparing agent, such as calcipotriol ointment or topical pimecrolimus. Less evidence is available to support the efficacy of tacrolimus ointment in PN treatment. Topical capsaicin is not as practical as other topical treatments since it needs to be applied 4 to 6 times daily. Oral antihistamines and montelukast may be added to the therapeutic regimen if there is a chronic pruritic component related to the lesions themselves or an underlying atopic diathesis fueling the itch-scratch cycle.
Widespread or treatment-resistant PN. Prescribe naltrexone, gabapentin, or pregabalin for more widespread disease or lesions resistant to conservative therapies. If you suspect a primary cutaneous disease as the underlying cause of pruritus or if topical and oral therapies do not achieve the desired therapeutic effect, refer to a dermatologist for further work-up and treatment.
How we would manage the case presented in the introduction. We would start the 43-year-old on topical corticosteroids under occlusion and periodically substitute calcipotriol ointment. (Given the unease that some patients might feel with the black-box warning on topical calcineurin inhibitors, we would likely try calcipotriol ointment as a courtesy before suggesting topical calcineurin inhibitors.) We would also prescribe an oral antihistamine at the start, given that her history of seasonal allergies and childhood asthma increases her chances of having an atopic component causing or exacerbating her disease. However, assessing her response to topical therapies before initiating an oral antihistamine would also be an appropriate strategy.
Unfortunately, PN is typically a chronic and often treatment-resistant disease with disappointing recurrence rates. As we learn more about the pathophysiology of PN, more effective therapies will hopefully emerge to improve the quality of life for these patients.
CORRESPONDENCE
Michael Saco, MD, Department of Dermatology & Cutaneous Surgery, University of South Florida, 13330 Laurel Drive, Tampa, FL 33612; ms20142018@aol.com
1. Moses S. Pruritus. Am Fam Phys. 2003;68:1135-1142.
2. Jorizzo JL, Gatti S, Smith EB. Prurigo: a clinical review. J Am Acad Dermatol. 1981;4:723-728.
3. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-218.
4. Wallengren J. Prurigo: diagnosis and management. Am J Clin Dermatol. 2004;5:85-95.
5. Accioly-Filho LW, Nogueira A, Ramos-e-Silva M. Prurigo nodularis of Hyde: an update. J Eur Acad Dermatol Venereol. 2000;14:75-82.
6. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
7. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450.
8. Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of Prurigo nodularis. Arch Dermatol. 2000;136:807-808.
9. Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366.
10. Shintani T, Ohata C, Koga H, et al. Combination therapy of fexofenadine and montelukast is effective in prurigo nodularis and pemphigoid nodularis. Dermatol Ther. 2014;27:135-139.
11. Paghdal KV, Schwartz R. Thalidomide and its dermatologic uses. Acta Dermatovenerol Croat. 2007;15:39-44.
12. Alfadley A, Al-Hawsawi K, Thestrup-Pedersen K, et al. Treatment of prurigo nodularis with thalidomide: a case report and review of the literature. Int J Dermatol. 2003;42:372-375.
13. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
14. Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
15. Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, doubleblind phase II trial. Dermatology. 2013;227:353-360.
16. Ständer S, Schürmeyer-Horst F, Luger TA, et al. Treatment of pruritic diseases with topical calcineurin inhibitors. Ther Clin Risk Manag. 2006;2:213-218.
17. Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478.
18. Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
19. Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23:194-198.
20. Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18.
21. Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803.
22. Rombold S, Lobisch K, Katzer K, et al. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed. 2008;24:19-23.
23. Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695.
24. Saraceno R, Nisticò SP, Capriotti E, et al. Monochromatic excimer light (308 nm) in the treatment of prurigo nodularis. Photodermatol Photoimmunol Photomed. 2008;24:43-45.
25. Siepmann D, Luger TA, Ständer S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: results of a case series. J Dtsch Dermatol Ges. 2008;6:941-946.
26. Orlando A, Renna S, Cottone M. Prurigo nodularis of Hyde treated with low-dose thalidomide. Eur Rev Med Pharmacol Sci. 2009;13:141-145.
27. Lan CC, Lin CL, Wu CS, et al. Treatment of idiopathic prurigo nodularis in Taiwanese patients with low-dose thalidomide. J Dermatol. 2007;34:237-242.
28. Taefehnorooz H, Truchetet F, Barbaud A, et al. Efficacy of thalidomide in the treatment of prurigo nodularis. Acta Derm Venereol. 2011;91:344-345.
29. Wu JJ, Huang DB, Pang KR, et al. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254-273.
1. Moses S. Pruritus. Am Fam Phys. 2003;68:1135-1142.
2. Jorizzo JL, Gatti S, Smith EB. Prurigo: a clinical review. J Am Acad Dermatol. 1981;4:723-728.
3. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-218.
4. Wallengren J. Prurigo: diagnosis and management. Am J Clin Dermatol. 2004;5:85-95.
5. Accioly-Filho LW, Nogueira A, Ramos-e-Silva M. Prurigo nodularis of Hyde: an update. J Eur Acad Dermatol Venereol. 2000;14:75-82.
6. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
7. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450.
8. Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of Prurigo nodularis. Arch Dermatol. 2000;136:807-808.
9. Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366.
10. Shintani T, Ohata C, Koga H, et al. Combination therapy of fexofenadine and montelukast is effective in prurigo nodularis and pemphigoid nodularis. Dermatol Ther. 2014;27:135-139.
11. Paghdal KV, Schwartz R. Thalidomide and its dermatologic uses. Acta Dermatovenerol Croat. 2007;15:39-44.
12. Alfadley A, Al-Hawsawi K, Thestrup-Pedersen K, et al. Treatment of prurigo nodularis with thalidomide: a case report and review of the literature. Int J Dermatol. 2003;42:372-375.
13. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
14. Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
15. Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, doubleblind phase II trial. Dermatology. 2013;227:353-360.
16. Ständer S, Schürmeyer-Horst F, Luger TA, et al. Treatment of pruritic diseases with topical calcineurin inhibitors. Ther Clin Risk Manag. 2006;2:213-218.
17. Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478.
18. Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
19. Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23:194-198.
20. Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18.
21. Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803.
22. Rombold S, Lobisch K, Katzer K, et al. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed. 2008;24:19-23.
23. Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695.
24. Saraceno R, Nisticò SP, Capriotti E, et al. Monochromatic excimer light (308 nm) in the treatment of prurigo nodularis. Photodermatol Photoimmunol Photomed. 2008;24:43-45.
25. Siepmann D, Luger TA, Ständer S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: results of a case series. J Dtsch Dermatol Ges. 2008;6:941-946.
26. Orlando A, Renna S, Cottone M. Prurigo nodularis of Hyde treated with low-dose thalidomide. Eur Rev Med Pharmacol Sci. 2009;13:141-145.
27. Lan CC, Lin CL, Wu CS, et al. Treatment of idiopathic prurigo nodularis in Taiwanese patients with low-dose thalidomide. J Dermatol. 2007;34:237-242.
28. Taefehnorooz H, Truchetet F, Barbaud A, et al. Efficacy of thalidomide in the treatment of prurigo nodularis. Acta Derm Venereol. 2011;91:344-345.
29. Wu JJ, Huang DB, Pang KR, et al. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254-273.