User login
Early Cutaneous Metastasis of Adenoid Cystic Carcinoma of the Salivary Gland
Adenoid cystic carcinoma (ACC) is the second most common malignant neoplasm of the major and minor salivary glands.1 The disease course generally is characterized by slow yet relentless progression with a poor long-term prognosis. The presence of distant metastasis heralds a poor prognosis in patients with ACC of the head and neck, with a median survival of 4.3 to 7.3 months.2 Twenty-five percent to 50% of ACCs of the salivary glands result in distant metastasis, primarily involving the lungs, bones,3-5 liver, and brain.4,5 Cutaneous metastasis of ACCs of the salivary glands is rare and usually is limited to the head and neck regions. We describe a case of early cutaneous metastasis of ACC on the head and neck, driving the identification of systemic diffusion of the neoplasm. Our case underscores the importance of conducting a complete skin examination in oncologic patients, especially focusing on cutaneous and subcutaneous nodules and papules that are eruptive or show rapid development.
Case Report
A 61-year-old man presented to the dermatology unit with a rapidly progressive mass on the right submandibular region. Five years prior he underwent wide excision of the lymph nodes of the right axilla because of the presence of a subcutaneous nodule, which revealed the absence of atypical histologic features; annual computed tomography (CT) scans were subsequently performed with no remarkable results. One year later, the presence of a small and stable submandibular nodule was noted; all biopsies performed were negative for cancer.
Wide excision of the submandibular mass was performed after clinical evaluation. Histopathologic examination revealed an ACC of the submandibular salivary gland. One week later, a dissection of the right side of the neck was performed with total sialectomy, right laterocervical lymphadenectomy (level 1-2 ab-3, Robbins), revealing nodal diffusion of the tumor cells (3 of 28 nodes were positive). Surgical treatment was immediately followed by radiotherapy (4800–6000 Gy) of the tumor region and lymphatic neck regions. At 6 months’ follow-up, clinical examination and a CT scan were negative for metastasis.
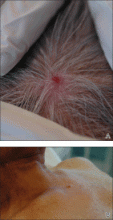
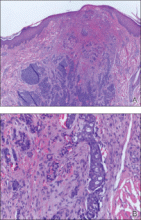
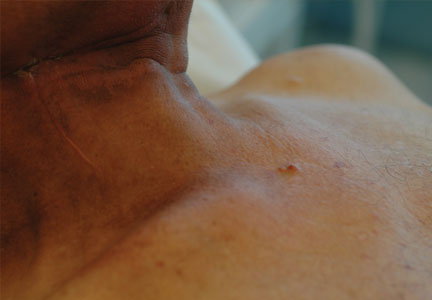
The patient was referred to our dermatology clinic 2 months later for evaluation of a nonspecific dermatitis that had developed at the surgical site on the right side of the neck. Physical examination revealed radiodermatitis on the scar. Additionally, slightly erythematous monomorphic and asymptomatic eruptive papules were noted on the scalp (Figure 1A) and on the left side of the clavicle (Figure 1B). The lesions had developed gradually over 10 days to a diameter of 5 mm. No lymphadenopathy was noted. Due to the characteristics of the lesions (morphology and rapid eruption) and the patient’s medical history, a skin biopsy was performed with a suspected diagnosis of cutaneous metastasis of ACC. The diagnosis was confirmed on histopathologic evaluation, which showed the characteristic features of metastatic ACC with solid areas as shown in Figure 2. Subsequent CT scans of the head, neck, and chest revealed lung and bone metastasis, which had not been present on prior investigation. A few days earlier the patient had developed a productive cough, hemoptysis, and mild dyspnea, which confirmed clinical progression of the disease. The patient was started on palliative chemotherapy and radiotherapy treatment.
Comment
Adenoid cystic carcinoma is a malignant salivary gland tumor characterized by slow yet relentless progression that is plagued by local recurrence, late metastases, and ultimately fatal outcomes.6 It represents approximately 7.5% of salivary gland malignancies.7 Few cases of cutaneous metastases of ACC have been reported on the head and neck as well as the abdomen,8-10 accounting for 1% to 2% of all cutaneous metastases,11 with typical distribution on the head and neck. The presence of distant metastases heralds a poor prognosis in head and neck cancers, with a median survival of 4.3 to 7.3 months.2 Treatment of this patient population usually is palliative. Patients with malignant salivary gland tumors should have a chest radiograph or CT scan at their initial assessment to exclude the possibility of lung metastasis. A higher risk for developing distant metastasis is associated with high-grade tumors such as ACC; salivary duct carcinomas; high-grade mucoepidermoid carcinomas; and tumors located in the submandibular gland, posterior tongue, and pharynx.12 The 3 major growth patterns are tubular, cribriform, and solid. The quantity of solid components is considered to be the most notable indication of a poor prognosis.13-15 In our patient, the site of the tumor—the submandibular region—as well as the presence of histopathologic solid structures suggested a poor prognosis. Our patient presented with eruptive papules on the scalp and on the left side of the clavicle, which was on the opposite side of the primary tumor. In many patients, cutaneous diffusion of the tumor precedes systemic diffusion and therefore can be considered as an early prognostic sign of metastasis.
Conclusion
Cutaneous metastasis represents an important sign of disease progression, allowing for the early discovery of systemic diffusion of the tumor as well as adjustment of the therapeutic approach. Our patient not only highlights a rare case of cutaneous metastases in this type of tumor but also the importance of conducting complete skin examinations in oncologic patients. Even minor findings should not be ignored in this patient population. In fact, if correctly interpreted the early discovery of cutaneous metastasis in patients with salivary gland tumors may determine a dramatic change in diagnosis, prognosis, and treatment.
1. Boukheris H, Curtis RE, Land CE, et al. Incidence of carcinoma of the major salivary glands according to WHO classification, 1992 to 2006: a population-based study in the United States [published online ahead of print October 27, 2009]. Cancer Epidemiol Biomarkers Prev. 2009;18:2899-2906.
2. Elnayal A, Moran CA, Fox PS, et al. Primary salivary gland-type lung cancer: imaging and clinical predictors of outcome. AJR Am J Roentgenol. 2013;201:W57-W63.
3. Perez DE, Magrin J, Almeida OP, et al. Multiple cutaneous metastases from a parotid adenoid cystic carcinoma. Pathol Oncol Res. 2007;13:167-169.
4. Khan AJ, DiGiovanna MP, Ross DA, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96:149-158.
5. Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495-498.
6. El-Naggar AK, Huvos AG. Adenoid cystic carcinoma. In: Barnes EL, Kleihues P, Sobin LH, et al, eds. Pathology and Genetics of Head and Neck Tumours. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2005:221-222.
7. Ellis GL, Auclair PL. Tumors of the Salivary Glands. 4th ed. Washington, DC: Armed Forces Institute of Pathology; 1996.
8. Chang CH, Liao YL, Hong HS. Cutaneous metastasis from adenoid cystic carcinoma of the parotid gland. Dermatol Surg. 2003;29:775-779.
9. Nakamura M, Miyachi Y. Cutaneous metastasis from an adenoid cystic carcinoma of the lacrimal gland. Br J Dermatol. 1999;141:373-374.
10. Nascimento AG, Amaral AL, Prado LA, et al. Adenoid cystic carcinoma of salivary glands: a study of 61 cases with clinicopathologic correlation. Cancer. 1986;57:312-319.
11. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33 (2, pt 1):161-182; quiz 183-186.
12. Schwentner I, Obrist P, Thumfart W, et al. Distant metastasis of parotid gland tumors. Acta Otolaryngol. 2006;126:340-345.
13. Da Cruz Perez DE, de Abreu Alves F, Nobuko Nishimoto I, et al. Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol. 2006;42:139-146.
14. Perzin KH, Gullane P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978;42:265-282.
15. Szanto PA, Luna MA, Tortoledo ME, et al. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54:1062-1069.
Adenoid cystic carcinoma (ACC) is the second most common malignant neoplasm of the major and minor salivary glands.1 The disease course generally is characterized by slow yet relentless progression with a poor long-term prognosis. The presence of distant metastasis heralds a poor prognosis in patients with ACC of the head and neck, with a median survival of 4.3 to 7.3 months.2 Twenty-five percent to 50% of ACCs of the salivary glands result in distant metastasis, primarily involving the lungs, bones,3-5 liver, and brain.4,5 Cutaneous metastasis of ACCs of the salivary glands is rare and usually is limited to the head and neck regions. We describe a case of early cutaneous metastasis of ACC on the head and neck, driving the identification of systemic diffusion of the neoplasm. Our case underscores the importance of conducting a complete skin examination in oncologic patients, especially focusing on cutaneous and subcutaneous nodules and papules that are eruptive or show rapid development.
Case Report
A 61-year-old man presented to the dermatology unit with a rapidly progressive mass on the right submandibular region. Five years prior he underwent wide excision of the lymph nodes of the right axilla because of the presence of a subcutaneous nodule, which revealed the absence of atypical histologic features; annual computed tomography (CT) scans were subsequently performed with no remarkable results. One year later, the presence of a small and stable submandibular nodule was noted; all biopsies performed were negative for cancer.
Wide excision of the submandibular mass was performed after clinical evaluation. Histopathologic examination revealed an ACC of the submandibular salivary gland. One week later, a dissection of the right side of the neck was performed with total sialectomy, right laterocervical lymphadenectomy (level 1-2 ab-3, Robbins), revealing nodal diffusion of the tumor cells (3 of 28 nodes were positive). Surgical treatment was immediately followed by radiotherapy (4800–6000 Gy) of the tumor region and lymphatic neck regions. At 6 months’ follow-up, clinical examination and a CT scan were negative for metastasis.
The patient was referred to our dermatology clinic 2 months later for evaluation of a nonspecific dermatitis that had developed at the surgical site on the right side of the neck. Physical examination revealed radiodermatitis on the scar. Additionally, slightly erythematous monomorphic and asymptomatic eruptive papules were noted on the scalp (Figure 1A) and on the left side of the clavicle (Figure 1B). The lesions had developed gradually over 10 days to a diameter of 5 mm. No lymphadenopathy was noted. Due to the characteristics of the lesions (morphology and rapid eruption) and the patient’s medical history, a skin biopsy was performed with a suspected diagnosis of cutaneous metastasis of ACC. The diagnosis was confirmed on histopathologic evaluation, which showed the characteristic features of metastatic ACC with solid areas as shown in Figure 2. Subsequent CT scans of the head, neck, and chest revealed lung and bone metastasis, which had not been present on prior investigation. A few days earlier the patient had developed a productive cough, hemoptysis, and mild dyspnea, which confirmed clinical progression of the disease. The patient was started on palliative chemotherapy and radiotherapy treatment.
Comment
Adenoid cystic carcinoma is a malignant salivary gland tumor characterized by slow yet relentless progression that is plagued by local recurrence, late metastases, and ultimately fatal outcomes.6 It represents approximately 7.5% of salivary gland malignancies.7 Few cases of cutaneous metastases of ACC have been reported on the head and neck as well as the abdomen,8-10 accounting for 1% to 2% of all cutaneous metastases,11 with typical distribution on the head and neck. The presence of distant metastases heralds a poor prognosis in head and neck cancers, with a median survival of 4.3 to 7.3 months.2 Treatment of this patient population usually is palliative. Patients with malignant salivary gland tumors should have a chest radiograph or CT scan at their initial assessment to exclude the possibility of lung metastasis. A higher risk for developing distant metastasis is associated with high-grade tumors such as ACC; salivary duct carcinomas; high-grade mucoepidermoid carcinomas; and tumors located in the submandibular gland, posterior tongue, and pharynx.12 The 3 major growth patterns are tubular, cribriform, and solid. The quantity of solid components is considered to be the most notable indication of a poor prognosis.13-15 In our patient, the site of the tumor—the submandibular region—as well as the presence of histopathologic solid structures suggested a poor prognosis. Our patient presented with eruptive papules on the scalp and on the left side of the clavicle, which was on the opposite side of the primary tumor. In many patients, cutaneous diffusion of the tumor precedes systemic diffusion and therefore can be considered as an early prognostic sign of metastasis.
Conclusion
Cutaneous metastasis represents an important sign of disease progression, allowing for the early discovery of systemic diffusion of the tumor as well as adjustment of the therapeutic approach. Our patient not only highlights a rare case of cutaneous metastases in this type of tumor but also the importance of conducting complete skin examinations in oncologic patients. Even minor findings should not be ignored in this patient population. In fact, if correctly interpreted the early discovery of cutaneous metastasis in patients with salivary gland tumors may determine a dramatic change in diagnosis, prognosis, and treatment.
Adenoid cystic carcinoma (ACC) is the second most common malignant neoplasm of the major and minor salivary glands.1 The disease course generally is characterized by slow yet relentless progression with a poor long-term prognosis. The presence of distant metastasis heralds a poor prognosis in patients with ACC of the head and neck, with a median survival of 4.3 to 7.3 months.2 Twenty-five percent to 50% of ACCs of the salivary glands result in distant metastasis, primarily involving the lungs, bones,3-5 liver, and brain.4,5 Cutaneous metastasis of ACCs of the salivary glands is rare and usually is limited to the head and neck regions. We describe a case of early cutaneous metastasis of ACC on the head and neck, driving the identification of systemic diffusion of the neoplasm. Our case underscores the importance of conducting a complete skin examination in oncologic patients, especially focusing on cutaneous and subcutaneous nodules and papules that are eruptive or show rapid development.
Case Report
A 61-year-old man presented to the dermatology unit with a rapidly progressive mass on the right submandibular region. Five years prior he underwent wide excision of the lymph nodes of the right axilla because of the presence of a subcutaneous nodule, which revealed the absence of atypical histologic features; annual computed tomography (CT) scans were subsequently performed with no remarkable results. One year later, the presence of a small and stable submandibular nodule was noted; all biopsies performed were negative for cancer.
Wide excision of the submandibular mass was performed after clinical evaluation. Histopathologic examination revealed an ACC of the submandibular salivary gland. One week later, a dissection of the right side of the neck was performed with total sialectomy, right laterocervical lymphadenectomy (level 1-2 ab-3, Robbins), revealing nodal diffusion of the tumor cells (3 of 28 nodes were positive). Surgical treatment was immediately followed by radiotherapy (4800–6000 Gy) of the tumor region and lymphatic neck regions. At 6 months’ follow-up, clinical examination and a CT scan were negative for metastasis.
The patient was referred to our dermatology clinic 2 months later for evaluation of a nonspecific dermatitis that had developed at the surgical site on the right side of the neck. Physical examination revealed radiodermatitis on the scar. Additionally, slightly erythematous monomorphic and asymptomatic eruptive papules were noted on the scalp (Figure 1A) and on the left side of the clavicle (Figure 1B). The lesions had developed gradually over 10 days to a diameter of 5 mm. No lymphadenopathy was noted. Due to the characteristics of the lesions (morphology and rapid eruption) and the patient’s medical history, a skin biopsy was performed with a suspected diagnosis of cutaneous metastasis of ACC. The diagnosis was confirmed on histopathologic evaluation, which showed the characteristic features of metastatic ACC with solid areas as shown in Figure 2. Subsequent CT scans of the head, neck, and chest revealed lung and bone metastasis, which had not been present on prior investigation. A few days earlier the patient had developed a productive cough, hemoptysis, and mild dyspnea, which confirmed clinical progression of the disease. The patient was started on palliative chemotherapy and radiotherapy treatment.
Comment
Adenoid cystic carcinoma is a malignant salivary gland tumor characterized by slow yet relentless progression that is plagued by local recurrence, late metastases, and ultimately fatal outcomes.6 It represents approximately 7.5% of salivary gland malignancies.7 Few cases of cutaneous metastases of ACC have been reported on the head and neck as well as the abdomen,8-10 accounting for 1% to 2% of all cutaneous metastases,11 with typical distribution on the head and neck. The presence of distant metastases heralds a poor prognosis in head and neck cancers, with a median survival of 4.3 to 7.3 months.2 Treatment of this patient population usually is palliative. Patients with malignant salivary gland tumors should have a chest radiograph or CT scan at their initial assessment to exclude the possibility of lung metastasis. A higher risk for developing distant metastasis is associated with high-grade tumors such as ACC; salivary duct carcinomas; high-grade mucoepidermoid carcinomas; and tumors located in the submandibular gland, posterior tongue, and pharynx.12 The 3 major growth patterns are tubular, cribriform, and solid. The quantity of solid components is considered to be the most notable indication of a poor prognosis.13-15 In our patient, the site of the tumor—the submandibular region—as well as the presence of histopathologic solid structures suggested a poor prognosis. Our patient presented with eruptive papules on the scalp and on the left side of the clavicle, which was on the opposite side of the primary tumor. In many patients, cutaneous diffusion of the tumor precedes systemic diffusion and therefore can be considered as an early prognostic sign of metastasis.
Conclusion
Cutaneous metastasis represents an important sign of disease progression, allowing for the early discovery of systemic diffusion of the tumor as well as adjustment of the therapeutic approach. Our patient not only highlights a rare case of cutaneous metastases in this type of tumor but also the importance of conducting complete skin examinations in oncologic patients. Even minor findings should not be ignored in this patient population. In fact, if correctly interpreted the early discovery of cutaneous metastasis in patients with salivary gland tumors may determine a dramatic change in diagnosis, prognosis, and treatment.
1. Boukheris H, Curtis RE, Land CE, et al. Incidence of carcinoma of the major salivary glands according to WHO classification, 1992 to 2006: a population-based study in the United States [published online ahead of print October 27, 2009]. Cancer Epidemiol Biomarkers Prev. 2009;18:2899-2906.
2. Elnayal A, Moran CA, Fox PS, et al. Primary salivary gland-type lung cancer: imaging and clinical predictors of outcome. AJR Am J Roentgenol. 2013;201:W57-W63.
3. Perez DE, Magrin J, Almeida OP, et al. Multiple cutaneous metastases from a parotid adenoid cystic carcinoma. Pathol Oncol Res. 2007;13:167-169.
4. Khan AJ, DiGiovanna MP, Ross DA, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96:149-158.
5. Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495-498.
6. El-Naggar AK, Huvos AG. Adenoid cystic carcinoma. In: Barnes EL, Kleihues P, Sobin LH, et al, eds. Pathology and Genetics of Head and Neck Tumours. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2005:221-222.
7. Ellis GL, Auclair PL. Tumors of the Salivary Glands. 4th ed. Washington, DC: Armed Forces Institute of Pathology; 1996.
8. Chang CH, Liao YL, Hong HS. Cutaneous metastasis from adenoid cystic carcinoma of the parotid gland. Dermatol Surg. 2003;29:775-779.
9. Nakamura M, Miyachi Y. Cutaneous metastasis from an adenoid cystic carcinoma of the lacrimal gland. Br J Dermatol. 1999;141:373-374.
10. Nascimento AG, Amaral AL, Prado LA, et al. Adenoid cystic carcinoma of salivary glands: a study of 61 cases with clinicopathologic correlation. Cancer. 1986;57:312-319.
11. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33 (2, pt 1):161-182; quiz 183-186.
12. Schwentner I, Obrist P, Thumfart W, et al. Distant metastasis of parotid gland tumors. Acta Otolaryngol. 2006;126:340-345.
13. Da Cruz Perez DE, de Abreu Alves F, Nobuko Nishimoto I, et al. Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol. 2006;42:139-146.
14. Perzin KH, Gullane P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978;42:265-282.
15. Szanto PA, Luna MA, Tortoledo ME, et al. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54:1062-1069.
1. Boukheris H, Curtis RE, Land CE, et al. Incidence of carcinoma of the major salivary glands according to WHO classification, 1992 to 2006: a population-based study in the United States [published online ahead of print October 27, 2009]. Cancer Epidemiol Biomarkers Prev. 2009;18:2899-2906.
2. Elnayal A, Moran CA, Fox PS, et al. Primary salivary gland-type lung cancer: imaging and clinical predictors of outcome. AJR Am J Roentgenol. 2013;201:W57-W63.
3. Perez DE, Magrin J, Almeida OP, et al. Multiple cutaneous metastases from a parotid adenoid cystic carcinoma. Pathol Oncol Res. 2007;13:167-169.
4. Khan AJ, DiGiovanna MP, Ross DA, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96:149-158.
5. Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495-498.
6. El-Naggar AK, Huvos AG. Adenoid cystic carcinoma. In: Barnes EL, Kleihues P, Sobin LH, et al, eds. Pathology and Genetics of Head and Neck Tumours. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2005:221-222.
7. Ellis GL, Auclair PL. Tumors of the Salivary Glands. 4th ed. Washington, DC: Armed Forces Institute of Pathology; 1996.
8. Chang CH, Liao YL, Hong HS. Cutaneous metastasis from adenoid cystic carcinoma of the parotid gland. Dermatol Surg. 2003;29:775-779.
9. Nakamura M, Miyachi Y. Cutaneous metastasis from an adenoid cystic carcinoma of the lacrimal gland. Br J Dermatol. 1999;141:373-374.
10. Nascimento AG, Amaral AL, Prado LA, et al. Adenoid cystic carcinoma of salivary glands: a study of 61 cases with clinicopathologic correlation. Cancer. 1986;57:312-319.
11. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33 (2, pt 1):161-182; quiz 183-186.
12. Schwentner I, Obrist P, Thumfart W, et al. Distant metastasis of parotid gland tumors. Acta Otolaryngol. 2006;126:340-345.
13. Da Cruz Perez DE, de Abreu Alves F, Nobuko Nishimoto I, et al. Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol. 2006;42:139-146.
14. Perzin KH, Gullane P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978;42:265-282.
15. Szanto PA, Luna MA, Tortoledo ME, et al. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54:1062-1069.