User login
The family physician’s role in long COVID management
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; Nicole_Mayo@URMC.Rochester.edu
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; Nicole_Mayo@URMC.Rochester.edu
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; Nicole_Mayo@URMC.Rochester.edu
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
PRACTICE RECOMMENDATIONS
› Acknowledge and address the persistence of COVID-19 symptoms when meeting with patients. C
› Continue to monitor persistent, fluctuating symptoms of COVID-19 well after hospital discharge or apparent resolution of initial symptoms. C
› Provide psychological support and resources for mental health care to patients regarding their ongoing fears and frustrations with persistent COVID-19 symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to overcome hesitancy for COVID-19 and other vaccines
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
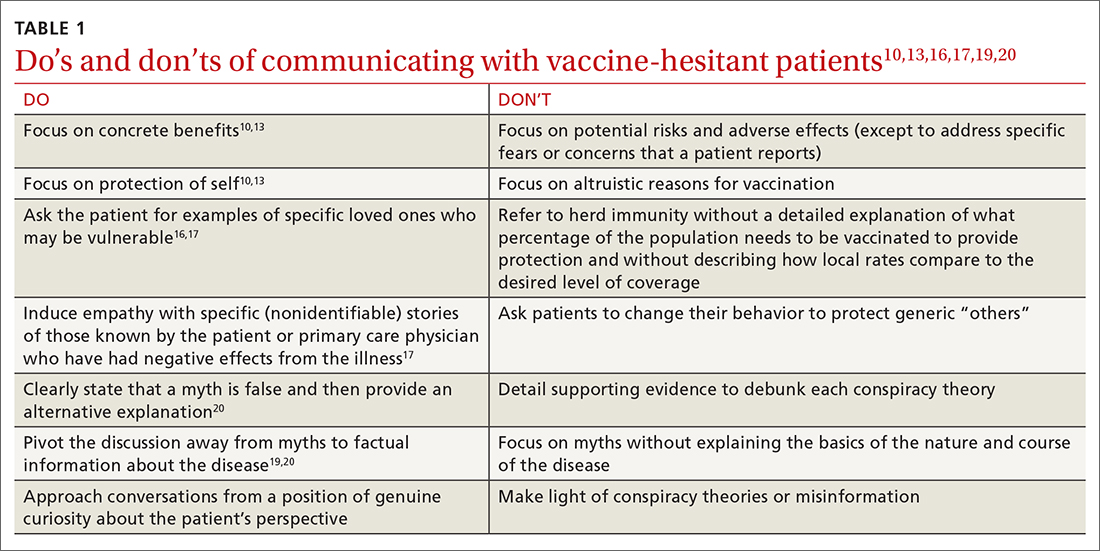
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.
Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.
Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; Nicole_Mayo@URMC.Rochester.edu
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.
Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.
Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; Nicole_Mayo@URMC.Rochester.edu
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.
Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.
Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; Nicole_Mayo@URMC.Rochester.edu
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
PRACTICE RECOMMENDATIONS
› Focus on personal benefits of vaccination with patients who express strong hesitancy and endorse vaccine myths; refocus the conversation away from myths and back to disease facts. C
› Emphasize personal and collective benefit to patients who are uncertain about vaccination; provide education about herd immunity and local vaccine coverage. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series