User login
Lack of Significant Anti-inflammatory Activity With Clindamycin in the Treatment of Rosacea: Results of 2 Randomized, Vehicle-Controlled Trials
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
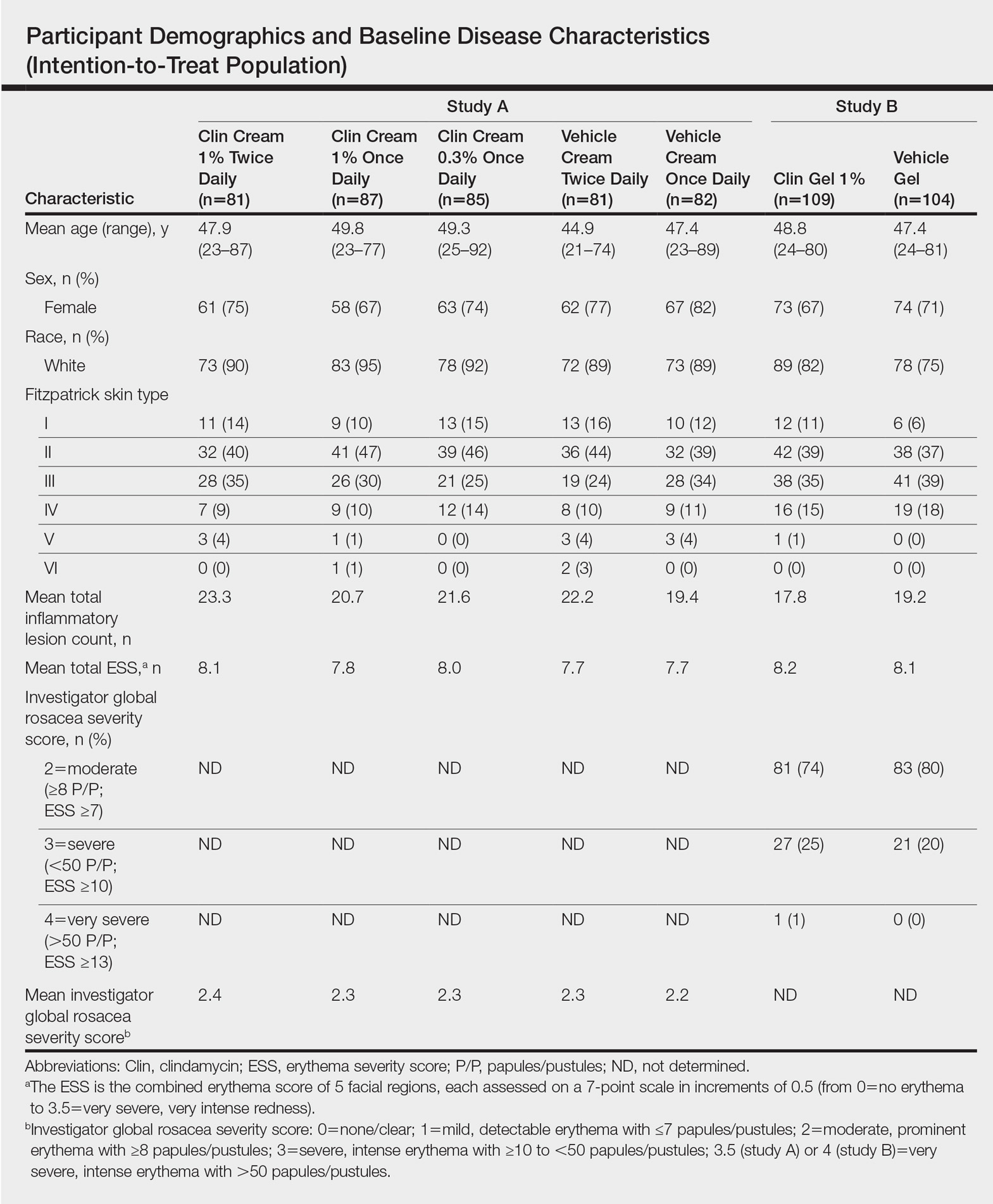
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.
Efficacy
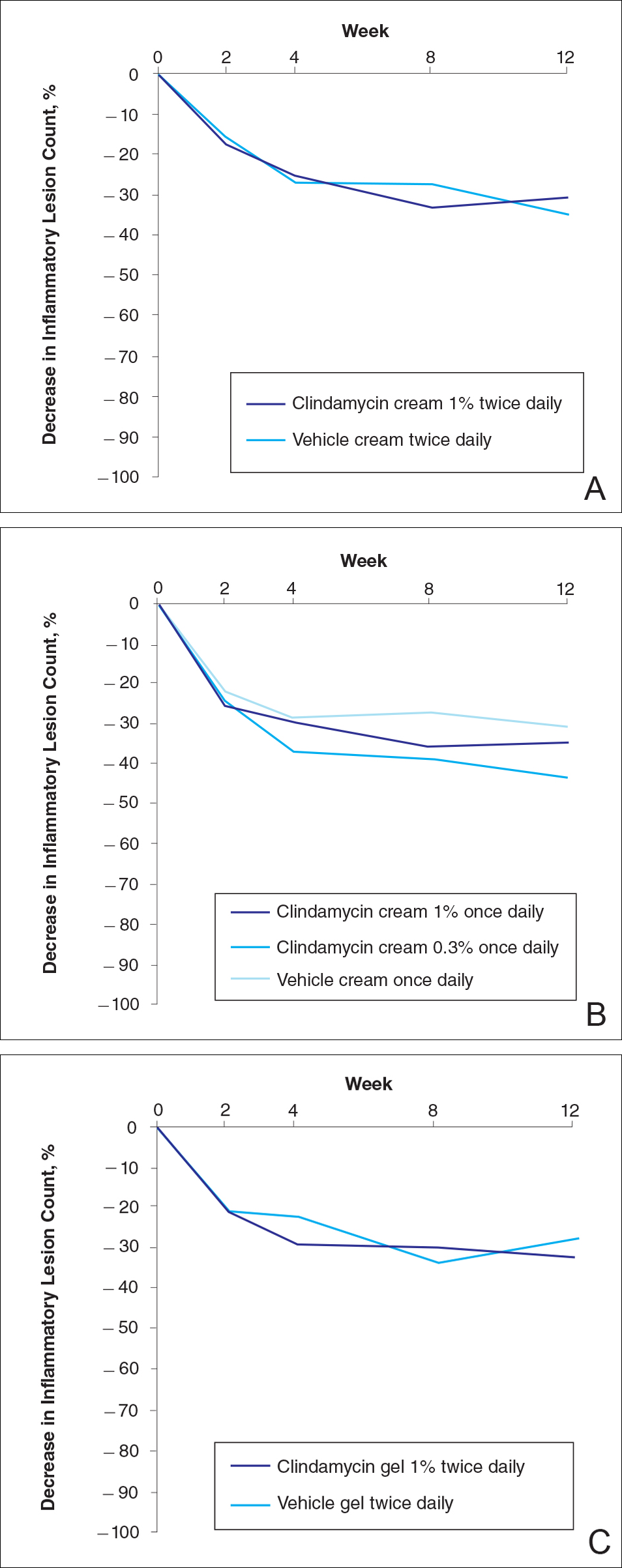
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).
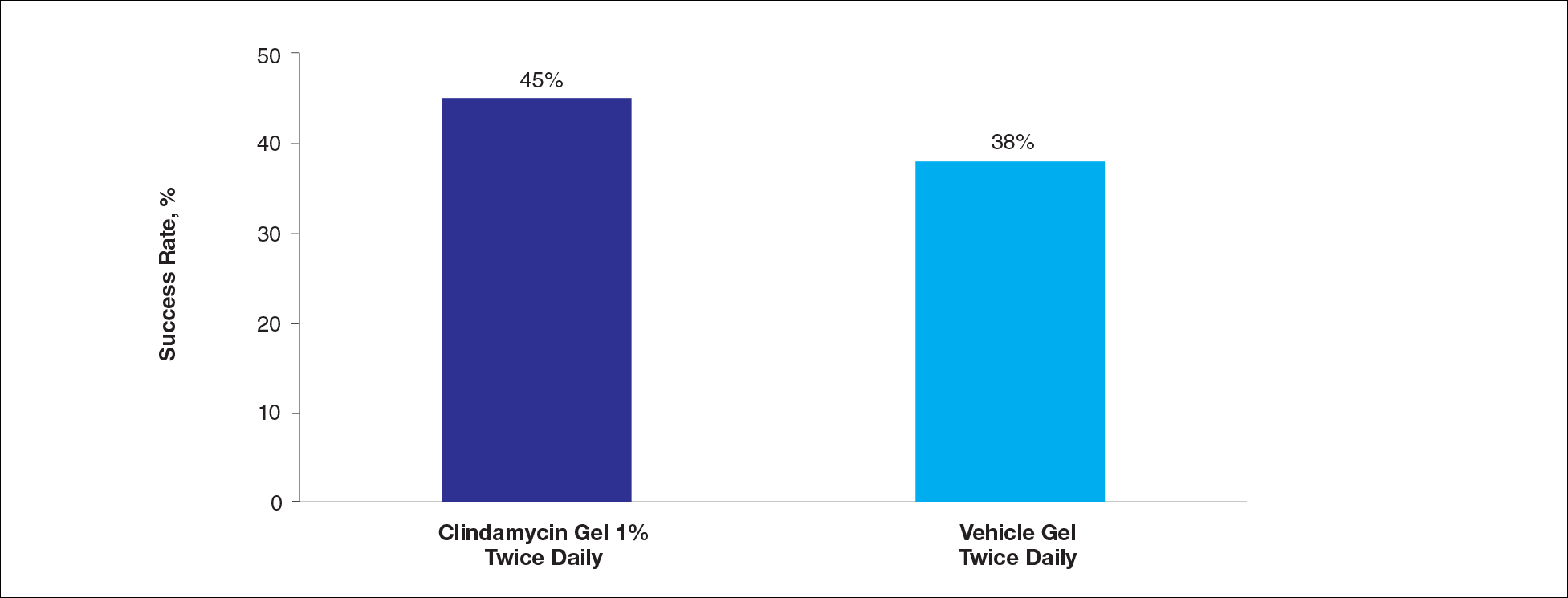
At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).
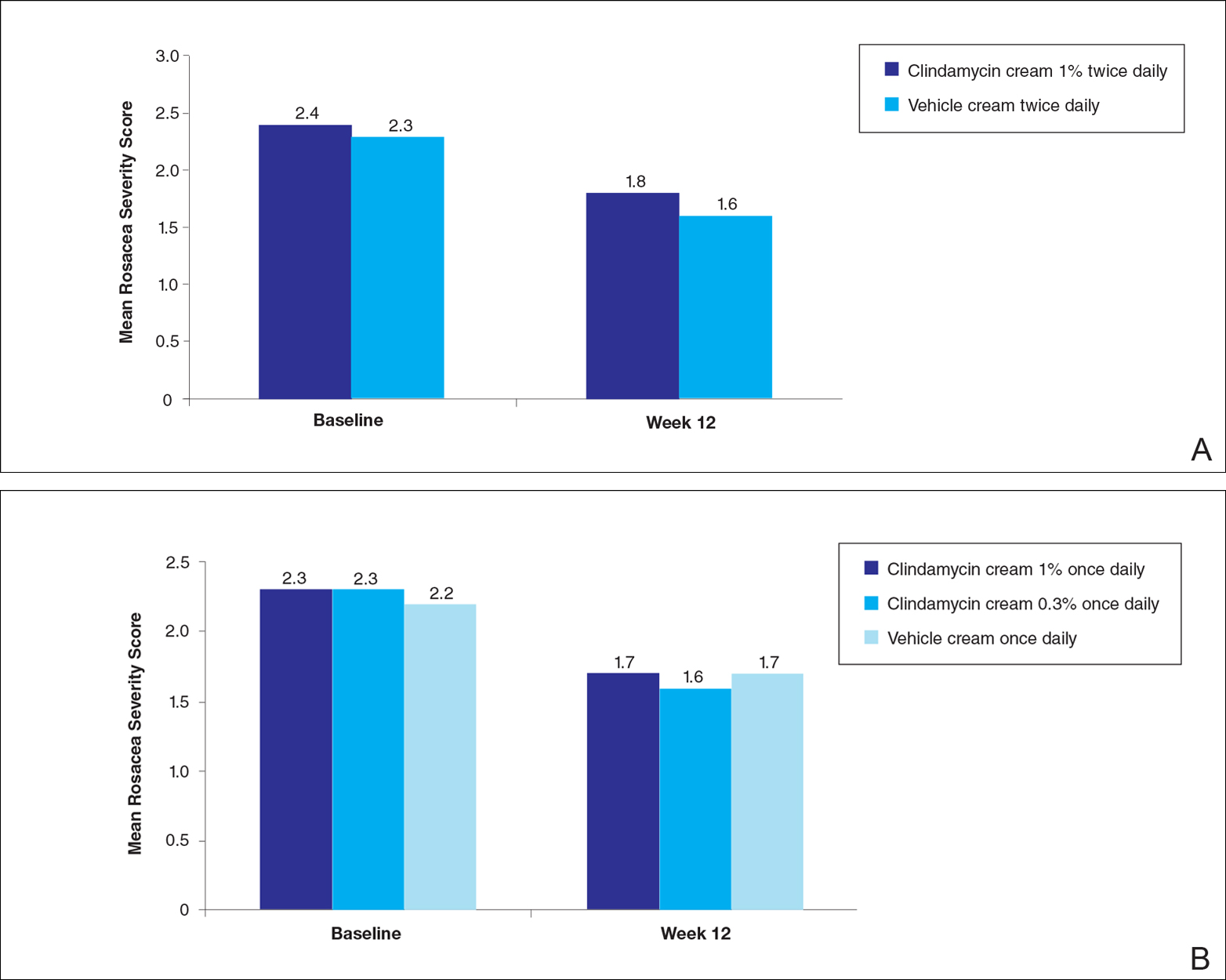
For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).
There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.
Efficacy
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).

At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).
For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).

There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.
Efficacy
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).

At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).
For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).

There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
Practice Points
- Clindamycin cream 0.3% and 1% and clindamycin gel 1% were no more effective than their respective vehicles in the treatment of moderate to severe rosacea.
- Clindamycin may have no intrinsic anti-inflammatory activity in rosacea.