User login
Glatiramer Acetate–Induced Lobular Panniculitis and Skin Necrosis
Glatiramer acetate (GA), a synthetic polypeptide that is injected subcutaneously, has proven effective in the treatment of relapsing-remitting multiple sclerosis (RRMS) and is now considered a first-line agent in the treatment of this condition. Adverse effects associated with GA primarily include local injection-site reactions (LISRs)(eg, erythema, pruritus, burning, pain, inflammation). Transient acute systemic reactions such as flushing and dyspnea also are commonly reported. Lipoatrophy at the injection site frequently has been reported in the literature as a cutaneous adverse effect of GA, but lobular panniculitis and necrosis at the site of injection rarely have been noted.
We report the case of a 36-year-old woman who experienced a severe adverse reaction to a single injection of GA after nearly 1 year of daily use to control symptoms of RRMS. Review of the current literature revealed few reports of the severe reaction of panniculitis and necrosis occurring at the injection site of GA.
Case Report
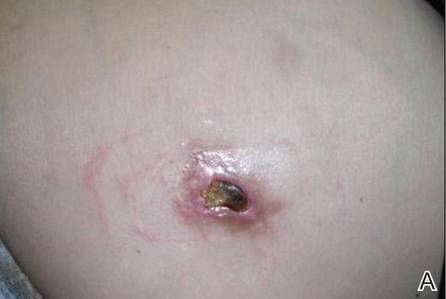
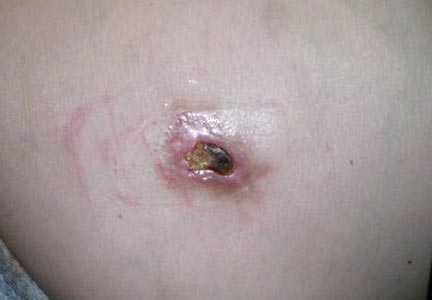
A 36-year-old woman was referred by her neurologist to the emergency department of our institution’s allergy and immunology clinic for treatment of an allergic reaction to a 20-mg GA injection, which she had been receiving daily for nearly 1 year as therapy for RRMS. A nodule immediately formed at the injection site and eventually became ulcerated. The patient also reported intense chest tightness, shortness of breath, and flushing following the injection. Physical examination revealed a large 8- to 9-cm erythematous area at the injection site on the left buttock. Necrosis and eschar formation also were evident (Figure 1).
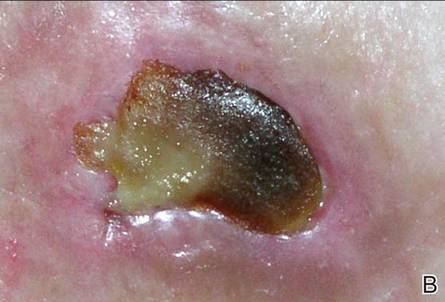
Figure 1. Panniculitis with central ulceration and necrosis at the site of a glatiramer acetate injection on the left buttock (A). Closer view of an irregularly shaped necrotic lesion with surrounding erythema (B). |
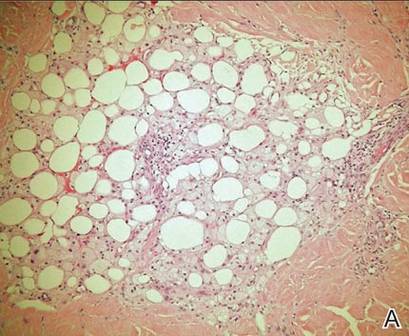
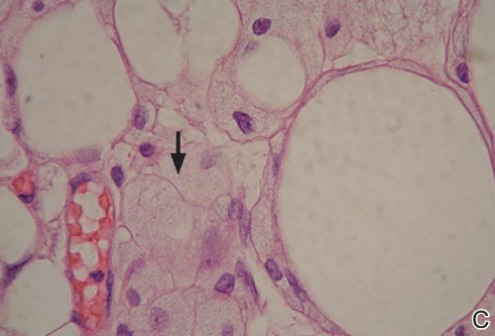
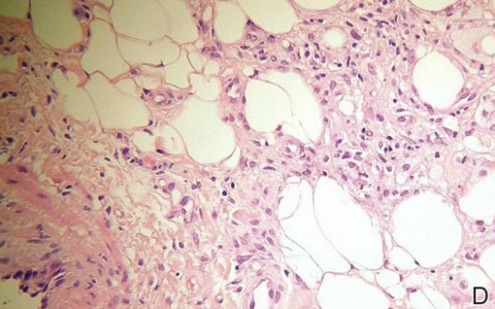
A punch biopsy from the edge of the lesion revealed predominantly lobular panniculitis (Figure 2A) with fat necrosis and numerous foamy macrophages (Figures 2B and 2C). Scattered lymphocytes also were present but no neutrophils or eosinophils were noted (Figure 2B). Interlobular septa were widened secondary to fibrosis (Figure 2A). No lymphoid follicles were identified. A subcutaneous artery was sampled but was negative for vasculitis (Figure 2D).
|
The necrotic lesion on the left buttock was present for more than 2 months before complete healing occurred. The patient had a history of intolerance or unresponsiveness to all prior medications for RRMS. Several years prior she responded well to treatment with GA for a few months and had been responding well to the injections over the last year. Incremental challenge testing with GA for desensitization was offered to the patient, but she declined treatment out of fear of a recurrent episode, particularly the severe systemic symptoms she had experienced. Unfortunately, she was lost to follow-up.
Comment
Glatiramer acetate, formerly known as copolymer-1, is a first-line treatment of patients with RRMS.1 Daily administration of subcutaneous injections of GA (20 mg/mL) has proven effective in relapse rate reduction and reduced morbidity in patients with RRMS.2 Long-term studies support a relapse rate reduction of more than 50% in patients using GA.3 The most common adverse effects are LISRs.2 Systemic reactions following GA injection also are common. A much less common reaction is panniculitis followed by lipoatrophy and/or skin necrosis. Only a few instances of panniculitis-associated necrosis have been reported.
The occurrence of LISRs was reported in 20% to 90% of patients using GA to control RRMS.2,4 Local injection-site reactions typically resolve within hours to days and have been reported to decrease in frequency over time.5 Acute systemic reactions (eg, anxiety, flushing, palpitations, dyspnea) to GA injection are described in approximately 15% of patients.6 Systemic reactions usually resolve in 5 to 15 minutes but can last for more than 1 hour.5 These reactions are mostly benign and generally are not considered to be allergic or anaphylactic in nature. True systemic anaphylaxis associated with administration of GA is extremely rare.7
Lipoatrophy, or localized loss of subcutaneous adipose tissue without evidence of inflammation, has been reported fairly frequently in association with GA (up to 45% of patients receiving GA injections).2,6,8,9 Lipoatrophy also has been seen following subcutaneous injection of many other drugs, including steroids and insulin. Unlike LISRs, the likelihood of developing lipoatrophy at the injection site increases with longer durations of GA injections.5 Lipoatrophy also develops following panniculitis at the site of GA injection.
Based on a search of the MeSH (Medical Subject Headings) database using the terms panniculitis and glatiramer acetate, there only are 10 reported cases of panniculitis as an adverse effect of GA injections.2,6,10 Lesions were described as either subcutaneous erythematous nodules or atrophic areas that demonstrated panniculitis on histologic examination. Injections preceding the development of panniculitis often were described as remarkably painful.4 Residual lipoatrophy and/or hyperpigmentation at the site of panniculitis development is common.2 It has been suggested that GA-induced panniculitis may be an early underlying mechanism for the development of lipoatrophy, and thus may be more common than originally suspected.10
Histopathologic examination of GA-induced panniculitis typically reveals a localized, mostly lobular, panniculitis with lipophagic granulomas, lymphocytes, and thickened septa. The lipophagic granulomas (a characteristic finding in panniculitis) form from local macrophages that engulf the lipids released from necrotic adipocytes.11 A large, pale, granular or vacuolated cytoplasm typically can be observed on microscopic examination of the macrophages (Figure 2C). Connective tissue septa typically are widened with cell infiltrates, usually lymphocytes. Other cell types, including macrophages, eosinophils, and neutrophils, also have been identified in both the septa and fat lobules. These histologic elements may change and evolve over time.
Necrosis in association with panniculitis, as seen in our patient, rarely has been reported.4,12 All of the necrotic reactions described occurred after at least 1 year of GA therapy and took several weeks to resolve.4,12 When presented with the development of skin necrosis at the site of GA injection, it is essential to distinguish between an adverse effect associated with the drug itself and Nicolau syndrome (embolia cutis medicamentosa).13 Necrosis at multiple injection sites or recurrence with later injections supports a GA-specific effect.12
Nicolau syndrome is a well-known traumatic reaction that leads to microembolization and resultant vasospasm as well as necrosis throughout the skin and possibly the underlying muscular layer.14 Although more commonly associated with intramuscular injections, Nicolau syndrome has been described with subcutaneous injections of GA in a few rare instances.13,15 Because of the associated severe systemic reaction as well as the histologic examination (Figure 2D), we believe the skin necrosis seen in our patient was from a reaction to GA rather than Nicolau syndrome. Our patient was not interested in restarting GA therapy; therefore, it is unknown if this reaction would have recurred, but we suspect high probability of recurrence without desensitization attempts.
Preventative measures can be taken to decrease the risk for LISRs, and patients should be educated on these techniques. Applying ice to the injection site for at least 30 seconds before cleaning the skin for injection may reduce local adverse effects.4 Proper instruction on injection techniques should be provided by a knowledgeable health care professional and topical anesthetics and/or steroids may be offered to reduce pain associated with injection. There have been no proven measures for prevention of lipoatrophy, panniculitis, or necrosis, and these adverse effects are not thought to be attributed to improper injection techniques.14 Rotation of injection sites is the only suggested means of decreasing the potential risk for more severely and permanently disfiguring local reactions.
If panniculitis following GA injection is suspected, a large biopsy that encompasses the entire subcutaneous fat layer is necessary for proper dermatopathologic classification.11 Glatiramer acetate injections should be stopped immediately. These reactions disappear when the injections are stopped but recur when restarting treatment.2 The efficacy of GA in the treatment of RRMS has led to the possible use of this drug in the treatment of other autoimmune diseases.16 Thus, it is important for clinicians to be aware of all adverse effects of subcutaneous injections of GA, including the rare occurrence of panniculitis and necrosis, and when discontinuation of therapy is indicated.
Conclusion
Daily subcutaneous injection of GA for the treatment of RRMS can result in the rare but characteristic development of localized panniculitis and necrosis. Glatiramer acetate is a common and highly effective therapy used for the treatment of RRMS. Common adverse effects include LISRs and transient acute systemic reactions. Less commonly observed but characteristic of GA injections is localized lipoatrophy and mostly lobular panniculitis. Necrosis rarely can develop in association with these cutaneous reactions. It is essential to differentiate between necrosis secondary to Nicolau syndrome and skin necrosis as a unique reaction to GA; the latter is an indication for discontinuation of GA injections. Dermatologists should be made aware of adverse cutaneous reactions seen with GA therapy, especially with the potential for expansion of the use of GA to treat other autoimmune processes. Further research is needed regarding the histopathologic evolution and mechanisms behind the development of lipoatrophy, panniculitis, and necrosis at the site of GA injection.
1. Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257:1917-1923.
2. Soares Almeida LM, Requena L, Kutzner H, et al. Localized panniculitis secondary to subcutaneous glatiramer acetate injections for the treatment of multiple sclerosis: a clinicopathologic and immunohistochemical study. J Am Acad Dermatol. 2006;55:968-974.
3. Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler. 2006;12:309-320.
4. Frohman EM, Brannon K, Alexander S, et al. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004;10:302-307.
5. Ziemssen T, Neuhaus O, Hohlfeld R. Risk-benefit assessment of glatiramer acetate in multiple sclerosis. Drug Saf. 2001;24:979-990.
6. Ball NJ, Cowan BJ, Moore GR, et al. Lobular panniculitis at the site of glatiramer acetate injections for the treatment of relapsing-remitting multiple sclerosis. a report of two cases. J Cutan Pathol. 2008;35:407-410.
7. Rauschka H, Farina C, Sator P, et al. Severe anaphylactic reaction to glatiramer acetate with specific IgE. Neurology. 2005;64:1481-1482.
8. Hwang L, Orengo I. Lipoatrophy associated with glatiramer acetate injections for the treatment of multiple sclerosis. Cutis. 2001;68:287-288.
9. Edgar CM, Brunet DG, Fenton P, et al. Lipoatrophy in patients with multiple sclerosis on glatiramer acetate. Can J Neurol Sci. 2004;31:58-63.
10. Soós N, Shakery K, Mrowietz U. Localized panniculitis and subsequent lipoatrophy with subcutaneous glatiramer acetate (Copaxone) injection for the treatment of multiple sclerosis. Am J Clin Dermatol. 2004;5:357-359.
11. Segura S, Requena L. Anatomy and histology of normal subcutaneous fat, necrosis of adipocytes, and classification of the panniculitides. Dermatol Clin. 2008;26:419-424, v.
12. Bosca I, Bosca M, Belenguer A, et al. Necrotising cutaneous lesions as a side effect of glatiramer acetate. J Neurol. 2006;253:1370-1371.
13. Feldmann R, Schierl M, Rauschka H, et al. Necrotizing skin lesions with involvement of muscle tissue after subcutaneous injection of glatiramer acetate. Eur J Dermatol. 2009;19:385.
14. Kluger N, Thouvenot E, Camu W, et al. Cutaneous adverse events related to glatiramer acetate injection (copolymer-1, Copaxone). J Eur Acad Dermatol Venereol. 2009;23:1332-1333.
15. Harde V, Schwarz T. Embolia cutis medicamentosa following subcutaneous injection of glatiramer acetate [in English, German]. J Dtsch Dermatol Ges. 2007;5:1122-1123.
16. Racke MK, Lovett-Racke AE. Glatiramer acetate treatment of multiple sclerosis: an immunological perspective. J Immunol. 2011;186:1887-1890.
Glatiramer acetate (GA), a synthetic polypeptide that is injected subcutaneously, has proven effective in the treatment of relapsing-remitting multiple sclerosis (RRMS) and is now considered a first-line agent in the treatment of this condition. Adverse effects associated with GA primarily include local injection-site reactions (LISRs)(eg, erythema, pruritus, burning, pain, inflammation). Transient acute systemic reactions such as flushing and dyspnea also are commonly reported. Lipoatrophy at the injection site frequently has been reported in the literature as a cutaneous adverse effect of GA, but lobular panniculitis and necrosis at the site of injection rarely have been noted.
We report the case of a 36-year-old woman who experienced a severe adverse reaction to a single injection of GA after nearly 1 year of daily use to control symptoms of RRMS. Review of the current literature revealed few reports of the severe reaction of panniculitis and necrosis occurring at the injection site of GA.
Case Report
A 36-year-old woman was referred by her neurologist to the emergency department of our institution’s allergy and immunology clinic for treatment of an allergic reaction to a 20-mg GA injection, which she had been receiving daily for nearly 1 year as therapy for RRMS. A nodule immediately formed at the injection site and eventually became ulcerated. The patient also reported intense chest tightness, shortness of breath, and flushing following the injection. Physical examination revealed a large 8- to 9-cm erythematous area at the injection site on the left buttock. Necrosis and eschar formation also were evident (Figure 1).
Figure 1. Panniculitis with central ulceration and necrosis at the site of a glatiramer acetate injection on the left buttock (A). Closer view of an irregularly shaped necrotic lesion with surrounding erythema (B). |
A punch biopsy from the edge of the lesion revealed predominantly lobular panniculitis (Figure 2A) with fat necrosis and numerous foamy macrophages (Figures 2B and 2C). Scattered lymphocytes also were present but no neutrophils or eosinophils were noted (Figure 2B). Interlobular septa were widened secondary to fibrosis (Figure 2A). No lymphoid follicles were identified. A subcutaneous artery was sampled but was negative for vasculitis (Figure 2D).
|
The necrotic lesion on the left buttock was present for more than 2 months before complete healing occurred. The patient had a history of intolerance or unresponsiveness to all prior medications for RRMS. Several years prior she responded well to treatment with GA for a few months and had been responding well to the injections over the last year. Incremental challenge testing with GA for desensitization was offered to the patient, but she declined treatment out of fear of a recurrent episode, particularly the severe systemic symptoms she had experienced. Unfortunately, she was lost to follow-up.
Comment
Glatiramer acetate, formerly known as copolymer-1, is a first-line treatment of patients with RRMS.1 Daily administration of subcutaneous injections of GA (20 mg/mL) has proven effective in relapse rate reduction and reduced morbidity in patients with RRMS.2 Long-term studies support a relapse rate reduction of more than 50% in patients using GA.3 The most common adverse effects are LISRs.2 Systemic reactions following GA injection also are common. A much less common reaction is panniculitis followed by lipoatrophy and/or skin necrosis. Only a few instances of panniculitis-associated necrosis have been reported.
The occurrence of LISRs was reported in 20% to 90% of patients using GA to control RRMS.2,4 Local injection-site reactions typically resolve within hours to days and have been reported to decrease in frequency over time.5 Acute systemic reactions (eg, anxiety, flushing, palpitations, dyspnea) to GA injection are described in approximately 15% of patients.6 Systemic reactions usually resolve in 5 to 15 minutes but can last for more than 1 hour.5 These reactions are mostly benign and generally are not considered to be allergic or anaphylactic in nature. True systemic anaphylaxis associated with administration of GA is extremely rare.7
Lipoatrophy, or localized loss of subcutaneous adipose tissue without evidence of inflammation, has been reported fairly frequently in association with GA (up to 45% of patients receiving GA injections).2,6,8,9 Lipoatrophy also has been seen following subcutaneous injection of many other drugs, including steroids and insulin. Unlike LISRs, the likelihood of developing lipoatrophy at the injection site increases with longer durations of GA injections.5 Lipoatrophy also develops following panniculitis at the site of GA injection.
Based on a search of the MeSH (Medical Subject Headings) database using the terms panniculitis and glatiramer acetate, there only are 10 reported cases of panniculitis as an adverse effect of GA injections.2,6,10 Lesions were described as either subcutaneous erythematous nodules or atrophic areas that demonstrated panniculitis on histologic examination. Injections preceding the development of panniculitis often were described as remarkably painful.4 Residual lipoatrophy and/or hyperpigmentation at the site of panniculitis development is common.2 It has been suggested that GA-induced panniculitis may be an early underlying mechanism for the development of lipoatrophy, and thus may be more common than originally suspected.10
Histopathologic examination of GA-induced panniculitis typically reveals a localized, mostly lobular, panniculitis with lipophagic granulomas, lymphocytes, and thickened septa. The lipophagic granulomas (a characteristic finding in panniculitis) form from local macrophages that engulf the lipids released from necrotic adipocytes.11 A large, pale, granular or vacuolated cytoplasm typically can be observed on microscopic examination of the macrophages (Figure 2C). Connective tissue septa typically are widened with cell infiltrates, usually lymphocytes. Other cell types, including macrophages, eosinophils, and neutrophils, also have been identified in both the septa and fat lobules. These histologic elements may change and evolve over time.
Necrosis in association with panniculitis, as seen in our patient, rarely has been reported.4,12 All of the necrotic reactions described occurred after at least 1 year of GA therapy and took several weeks to resolve.4,12 When presented with the development of skin necrosis at the site of GA injection, it is essential to distinguish between an adverse effect associated with the drug itself and Nicolau syndrome (embolia cutis medicamentosa).13 Necrosis at multiple injection sites or recurrence with later injections supports a GA-specific effect.12
Nicolau syndrome is a well-known traumatic reaction that leads to microembolization and resultant vasospasm as well as necrosis throughout the skin and possibly the underlying muscular layer.14 Although more commonly associated with intramuscular injections, Nicolau syndrome has been described with subcutaneous injections of GA in a few rare instances.13,15 Because of the associated severe systemic reaction as well as the histologic examination (Figure 2D), we believe the skin necrosis seen in our patient was from a reaction to GA rather than Nicolau syndrome. Our patient was not interested in restarting GA therapy; therefore, it is unknown if this reaction would have recurred, but we suspect high probability of recurrence without desensitization attempts.
Preventative measures can be taken to decrease the risk for LISRs, and patients should be educated on these techniques. Applying ice to the injection site for at least 30 seconds before cleaning the skin for injection may reduce local adverse effects.4 Proper instruction on injection techniques should be provided by a knowledgeable health care professional and topical anesthetics and/or steroids may be offered to reduce pain associated with injection. There have been no proven measures for prevention of lipoatrophy, panniculitis, or necrosis, and these adverse effects are not thought to be attributed to improper injection techniques.14 Rotation of injection sites is the only suggested means of decreasing the potential risk for more severely and permanently disfiguring local reactions.
If panniculitis following GA injection is suspected, a large biopsy that encompasses the entire subcutaneous fat layer is necessary for proper dermatopathologic classification.11 Glatiramer acetate injections should be stopped immediately. These reactions disappear when the injections are stopped but recur when restarting treatment.2 The efficacy of GA in the treatment of RRMS has led to the possible use of this drug in the treatment of other autoimmune diseases.16 Thus, it is important for clinicians to be aware of all adverse effects of subcutaneous injections of GA, including the rare occurrence of panniculitis and necrosis, and when discontinuation of therapy is indicated.
Conclusion
Daily subcutaneous injection of GA for the treatment of RRMS can result in the rare but characteristic development of localized panniculitis and necrosis. Glatiramer acetate is a common and highly effective therapy used for the treatment of RRMS. Common adverse effects include LISRs and transient acute systemic reactions. Less commonly observed but characteristic of GA injections is localized lipoatrophy and mostly lobular panniculitis. Necrosis rarely can develop in association with these cutaneous reactions. It is essential to differentiate between necrosis secondary to Nicolau syndrome and skin necrosis as a unique reaction to GA; the latter is an indication for discontinuation of GA injections. Dermatologists should be made aware of adverse cutaneous reactions seen with GA therapy, especially with the potential for expansion of the use of GA to treat other autoimmune processes. Further research is needed regarding the histopathologic evolution and mechanisms behind the development of lipoatrophy, panniculitis, and necrosis at the site of GA injection.
Glatiramer acetate (GA), a synthetic polypeptide that is injected subcutaneously, has proven effective in the treatment of relapsing-remitting multiple sclerosis (RRMS) and is now considered a first-line agent in the treatment of this condition. Adverse effects associated with GA primarily include local injection-site reactions (LISRs)(eg, erythema, pruritus, burning, pain, inflammation). Transient acute systemic reactions such as flushing and dyspnea also are commonly reported. Lipoatrophy at the injection site frequently has been reported in the literature as a cutaneous adverse effect of GA, but lobular panniculitis and necrosis at the site of injection rarely have been noted.
We report the case of a 36-year-old woman who experienced a severe adverse reaction to a single injection of GA after nearly 1 year of daily use to control symptoms of RRMS. Review of the current literature revealed few reports of the severe reaction of panniculitis and necrosis occurring at the injection site of GA.
Case Report
A 36-year-old woman was referred by her neurologist to the emergency department of our institution’s allergy and immunology clinic for treatment of an allergic reaction to a 20-mg GA injection, which she had been receiving daily for nearly 1 year as therapy for RRMS. A nodule immediately formed at the injection site and eventually became ulcerated. The patient also reported intense chest tightness, shortness of breath, and flushing following the injection. Physical examination revealed a large 8- to 9-cm erythematous area at the injection site on the left buttock. Necrosis and eschar formation also were evident (Figure 1).
Figure 1. Panniculitis with central ulceration and necrosis at the site of a glatiramer acetate injection on the left buttock (A). Closer view of an irregularly shaped necrotic lesion with surrounding erythema (B). |
A punch biopsy from the edge of the lesion revealed predominantly lobular panniculitis (Figure 2A) with fat necrosis and numerous foamy macrophages (Figures 2B and 2C). Scattered lymphocytes also were present but no neutrophils or eosinophils were noted (Figure 2B). Interlobular septa were widened secondary to fibrosis (Figure 2A). No lymphoid follicles were identified. A subcutaneous artery was sampled but was negative for vasculitis (Figure 2D).
|
The necrotic lesion on the left buttock was present for more than 2 months before complete healing occurred. The patient had a history of intolerance or unresponsiveness to all prior medications for RRMS. Several years prior she responded well to treatment with GA for a few months and had been responding well to the injections over the last year. Incremental challenge testing with GA for desensitization was offered to the patient, but she declined treatment out of fear of a recurrent episode, particularly the severe systemic symptoms she had experienced. Unfortunately, she was lost to follow-up.
Comment
Glatiramer acetate, formerly known as copolymer-1, is a first-line treatment of patients with RRMS.1 Daily administration of subcutaneous injections of GA (20 mg/mL) has proven effective in relapse rate reduction and reduced morbidity in patients with RRMS.2 Long-term studies support a relapse rate reduction of more than 50% in patients using GA.3 The most common adverse effects are LISRs.2 Systemic reactions following GA injection also are common. A much less common reaction is panniculitis followed by lipoatrophy and/or skin necrosis. Only a few instances of panniculitis-associated necrosis have been reported.
The occurrence of LISRs was reported in 20% to 90% of patients using GA to control RRMS.2,4 Local injection-site reactions typically resolve within hours to days and have been reported to decrease in frequency over time.5 Acute systemic reactions (eg, anxiety, flushing, palpitations, dyspnea) to GA injection are described in approximately 15% of patients.6 Systemic reactions usually resolve in 5 to 15 minutes but can last for more than 1 hour.5 These reactions are mostly benign and generally are not considered to be allergic or anaphylactic in nature. True systemic anaphylaxis associated with administration of GA is extremely rare.7
Lipoatrophy, or localized loss of subcutaneous adipose tissue without evidence of inflammation, has been reported fairly frequently in association with GA (up to 45% of patients receiving GA injections).2,6,8,9 Lipoatrophy also has been seen following subcutaneous injection of many other drugs, including steroids and insulin. Unlike LISRs, the likelihood of developing lipoatrophy at the injection site increases with longer durations of GA injections.5 Lipoatrophy also develops following panniculitis at the site of GA injection.
Based on a search of the MeSH (Medical Subject Headings) database using the terms panniculitis and glatiramer acetate, there only are 10 reported cases of panniculitis as an adverse effect of GA injections.2,6,10 Lesions were described as either subcutaneous erythematous nodules or atrophic areas that demonstrated panniculitis on histologic examination. Injections preceding the development of panniculitis often were described as remarkably painful.4 Residual lipoatrophy and/or hyperpigmentation at the site of panniculitis development is common.2 It has been suggested that GA-induced panniculitis may be an early underlying mechanism for the development of lipoatrophy, and thus may be more common than originally suspected.10
Histopathologic examination of GA-induced panniculitis typically reveals a localized, mostly lobular, panniculitis with lipophagic granulomas, lymphocytes, and thickened septa. The lipophagic granulomas (a characteristic finding in panniculitis) form from local macrophages that engulf the lipids released from necrotic adipocytes.11 A large, pale, granular or vacuolated cytoplasm typically can be observed on microscopic examination of the macrophages (Figure 2C). Connective tissue septa typically are widened with cell infiltrates, usually lymphocytes. Other cell types, including macrophages, eosinophils, and neutrophils, also have been identified in both the septa and fat lobules. These histologic elements may change and evolve over time.
Necrosis in association with panniculitis, as seen in our patient, rarely has been reported.4,12 All of the necrotic reactions described occurred after at least 1 year of GA therapy and took several weeks to resolve.4,12 When presented with the development of skin necrosis at the site of GA injection, it is essential to distinguish between an adverse effect associated with the drug itself and Nicolau syndrome (embolia cutis medicamentosa).13 Necrosis at multiple injection sites or recurrence with later injections supports a GA-specific effect.12
Nicolau syndrome is a well-known traumatic reaction that leads to microembolization and resultant vasospasm as well as necrosis throughout the skin and possibly the underlying muscular layer.14 Although more commonly associated with intramuscular injections, Nicolau syndrome has been described with subcutaneous injections of GA in a few rare instances.13,15 Because of the associated severe systemic reaction as well as the histologic examination (Figure 2D), we believe the skin necrosis seen in our patient was from a reaction to GA rather than Nicolau syndrome. Our patient was not interested in restarting GA therapy; therefore, it is unknown if this reaction would have recurred, but we suspect high probability of recurrence without desensitization attempts.
Preventative measures can be taken to decrease the risk for LISRs, and patients should be educated on these techniques. Applying ice to the injection site for at least 30 seconds before cleaning the skin for injection may reduce local adverse effects.4 Proper instruction on injection techniques should be provided by a knowledgeable health care professional and topical anesthetics and/or steroids may be offered to reduce pain associated with injection. There have been no proven measures for prevention of lipoatrophy, panniculitis, or necrosis, and these adverse effects are not thought to be attributed to improper injection techniques.14 Rotation of injection sites is the only suggested means of decreasing the potential risk for more severely and permanently disfiguring local reactions.
If panniculitis following GA injection is suspected, a large biopsy that encompasses the entire subcutaneous fat layer is necessary for proper dermatopathologic classification.11 Glatiramer acetate injections should be stopped immediately. These reactions disappear when the injections are stopped but recur when restarting treatment.2 The efficacy of GA in the treatment of RRMS has led to the possible use of this drug in the treatment of other autoimmune diseases.16 Thus, it is important for clinicians to be aware of all adverse effects of subcutaneous injections of GA, including the rare occurrence of panniculitis and necrosis, and when discontinuation of therapy is indicated.
Conclusion
Daily subcutaneous injection of GA for the treatment of RRMS can result in the rare but characteristic development of localized panniculitis and necrosis. Glatiramer acetate is a common and highly effective therapy used for the treatment of RRMS. Common adverse effects include LISRs and transient acute systemic reactions. Less commonly observed but characteristic of GA injections is localized lipoatrophy and mostly lobular panniculitis. Necrosis rarely can develop in association with these cutaneous reactions. It is essential to differentiate between necrosis secondary to Nicolau syndrome and skin necrosis as a unique reaction to GA; the latter is an indication for discontinuation of GA injections. Dermatologists should be made aware of adverse cutaneous reactions seen with GA therapy, especially with the potential for expansion of the use of GA to treat other autoimmune processes. Further research is needed regarding the histopathologic evolution and mechanisms behind the development of lipoatrophy, panniculitis, and necrosis at the site of GA injection.
1. Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257:1917-1923.
2. Soares Almeida LM, Requena L, Kutzner H, et al. Localized panniculitis secondary to subcutaneous glatiramer acetate injections for the treatment of multiple sclerosis: a clinicopathologic and immunohistochemical study. J Am Acad Dermatol. 2006;55:968-974.
3. Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler. 2006;12:309-320.
4. Frohman EM, Brannon K, Alexander S, et al. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004;10:302-307.
5. Ziemssen T, Neuhaus O, Hohlfeld R. Risk-benefit assessment of glatiramer acetate in multiple sclerosis. Drug Saf. 2001;24:979-990.
6. Ball NJ, Cowan BJ, Moore GR, et al. Lobular panniculitis at the site of glatiramer acetate injections for the treatment of relapsing-remitting multiple sclerosis. a report of two cases. J Cutan Pathol. 2008;35:407-410.
7. Rauschka H, Farina C, Sator P, et al. Severe anaphylactic reaction to glatiramer acetate with specific IgE. Neurology. 2005;64:1481-1482.
8. Hwang L, Orengo I. Lipoatrophy associated with glatiramer acetate injections for the treatment of multiple sclerosis. Cutis. 2001;68:287-288.
9. Edgar CM, Brunet DG, Fenton P, et al. Lipoatrophy in patients with multiple sclerosis on glatiramer acetate. Can J Neurol Sci. 2004;31:58-63.
10. Soós N, Shakery K, Mrowietz U. Localized panniculitis and subsequent lipoatrophy with subcutaneous glatiramer acetate (Copaxone) injection for the treatment of multiple sclerosis. Am J Clin Dermatol. 2004;5:357-359.
11. Segura S, Requena L. Anatomy and histology of normal subcutaneous fat, necrosis of adipocytes, and classification of the panniculitides. Dermatol Clin. 2008;26:419-424, v.
12. Bosca I, Bosca M, Belenguer A, et al. Necrotising cutaneous lesions as a side effect of glatiramer acetate. J Neurol. 2006;253:1370-1371.
13. Feldmann R, Schierl M, Rauschka H, et al. Necrotizing skin lesions with involvement of muscle tissue after subcutaneous injection of glatiramer acetate. Eur J Dermatol. 2009;19:385.
14. Kluger N, Thouvenot E, Camu W, et al. Cutaneous adverse events related to glatiramer acetate injection (copolymer-1, Copaxone). J Eur Acad Dermatol Venereol. 2009;23:1332-1333.
15. Harde V, Schwarz T. Embolia cutis medicamentosa following subcutaneous injection of glatiramer acetate [in English, German]. J Dtsch Dermatol Ges. 2007;5:1122-1123.
16. Racke MK, Lovett-Racke AE. Glatiramer acetate treatment of multiple sclerosis: an immunological perspective. J Immunol. 2011;186:1887-1890.
1. Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257:1917-1923.
2. Soares Almeida LM, Requena L, Kutzner H, et al. Localized panniculitis secondary to subcutaneous glatiramer acetate injections for the treatment of multiple sclerosis: a clinicopathologic and immunohistochemical study. J Am Acad Dermatol. 2006;55:968-974.
3. Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler. 2006;12:309-320.
4. Frohman EM, Brannon K, Alexander S, et al. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004;10:302-307.
5. Ziemssen T, Neuhaus O, Hohlfeld R. Risk-benefit assessment of glatiramer acetate in multiple sclerosis. Drug Saf. 2001;24:979-990.
6. Ball NJ, Cowan BJ, Moore GR, et al. Lobular panniculitis at the site of glatiramer acetate injections for the treatment of relapsing-remitting multiple sclerosis. a report of two cases. J Cutan Pathol. 2008;35:407-410.
7. Rauschka H, Farina C, Sator P, et al. Severe anaphylactic reaction to glatiramer acetate with specific IgE. Neurology. 2005;64:1481-1482.
8. Hwang L, Orengo I. Lipoatrophy associated with glatiramer acetate injections for the treatment of multiple sclerosis. Cutis. 2001;68:287-288.
9. Edgar CM, Brunet DG, Fenton P, et al. Lipoatrophy in patients with multiple sclerosis on glatiramer acetate. Can J Neurol Sci. 2004;31:58-63.
10. Soós N, Shakery K, Mrowietz U. Localized panniculitis and subsequent lipoatrophy with subcutaneous glatiramer acetate (Copaxone) injection for the treatment of multiple sclerosis. Am J Clin Dermatol. 2004;5:357-359.
11. Segura S, Requena L. Anatomy and histology of normal subcutaneous fat, necrosis of adipocytes, and classification of the panniculitides. Dermatol Clin. 2008;26:419-424, v.
12. Bosca I, Bosca M, Belenguer A, et al. Necrotising cutaneous lesions as a side effect of glatiramer acetate. J Neurol. 2006;253:1370-1371.
13. Feldmann R, Schierl M, Rauschka H, et al. Necrotizing skin lesions with involvement of muscle tissue after subcutaneous injection of glatiramer acetate. Eur J Dermatol. 2009;19:385.
14. Kluger N, Thouvenot E, Camu W, et al. Cutaneous adverse events related to glatiramer acetate injection (copolymer-1, Copaxone). J Eur Acad Dermatol Venereol. 2009;23:1332-1333.
15. Harde V, Schwarz T. Embolia cutis medicamentosa following subcutaneous injection of glatiramer acetate [in English, German]. J Dtsch Dermatol Ges. 2007;5:1122-1123.
16. Racke MK, Lovett-Racke AE. Glatiramer acetate treatment of multiple sclerosis: an immunological perspective. J Immunol. 2011;186:1887-1890.
Practice Points
- Glatiramer acetate is a common and highly effective therapy administered subcutaneously for the treatment of relapsing-remitting multiple sclerosis.
- Common adverse effects include local injection-site reactions and transient acute systemic reactions.
- Rarely, localized lipoatrophy and mostly lobular panniculitis with occasional necrosis can be observed at the site of glatiramer acetate injections. This reaction is specific to the medication and can recur with subsequent injections.